User login
Researchers seek more sickle cell drug research
BETHESDA, MD – While there are experimental treatments such as sevuparin and gene therapy in testing for sickle cell disease (SCD), researchers said this field is lagging because of a lack of funding.
Yogen Saunthararajah, MD, of the Cleveland Clinic, described what he called a “paltry” landscape of new drugs for SCD at Sickle Cell in Focus, a conference held by the National Institutes of Health.
There are only four main approaches taken by drugs now in clinical testing for addressing the root causes of SCD, despite decades’ worth of research of the genetic and mechanistic underpinnings of this disease, he said.
“It’s pretty sad,” Dr. Saunthararajah said, referring to the quantity of efforts, not their quality.
Within days of his presentation at the conference, one of the drugs he highlighted had officially fallen out of contention. An Oct. 26 post on NIH’s Clinicaltrials.gov site said Incyte had terminated its phase 1 study of INCB059872 in SCD “due to a business decision” not to pursue this indication. Incyte confirmed that it dropped development of INCB059872 for SCD but will continue testing it for other indications, including acute myeloid leukemia (AML).
Dr. Saunthararajah said that work on another approach, using decitabine (Dacogen), for which he has done a phase 1 study, is “struggling,” because of the search for funding.
Two other approaches that Dr. Saunthararajah cited in his presentation appear to remain on track. These are gene therapy and the once-daily voxelotor treatment from Global Blood Therapeutics.
In the field of gene therapy, Sangamo Therapeutics and Sanofi’s Bioverativ in May said the Food and Drug Administration had cleared the way for them to start a phase 1/2 clinical trial for the BIVV003 product that they are developing together. This uses zinc finger nuclease (ZFN) gene-editing technology to modifying a short sequence of the BCL11A gene, with the aim of reactivating fetal hemoglobin.
Bluebird Bio’s LentiGlobin gene therapy has advanced as far as phase 3 for transfusion-dependent beta-thalassemia and phase 1/2 for SCD. In October, the European Medicines Agency accepted the company’s application for approval of LentiGlobin gene therapy for the treatment of adolescents and adults with transfusion-dependent beta-thalassemia (TDT) and a non-beta0/beta0 genotype. The company has not said when it expects to file with the FDA for approval of this treatment.
In the field of oral therapies, Global Blood Therapeutics has said it’s in discussions with the FDA about a potential accelerated approval of voxelotor. The tablet is meant to inhibit the underlying mechanism that causes sickling of red blood cells. In June, the company completed a planned review of early data from its phase 3 trial, known as the HOPE study.
“On the primary endpoint (the proportion of patients with greater than 1 g/dL increase in hemoglobin versus baseline), a statistically significant increase was demonstrated with voxelotor at both the 1,500-mg and 900-mg doses after 12 weeks of treatment versus placebo,” Global Blood Therapeutics said in an August regulatory filing with the Securities and Exchange Commission.
The company has said that voxelotor may meet the FDA’s standard for accelerated approval under Subpart H program.
In his presentation at the NIH conference, Tom Williams, MD, PhD, of KEMRI/Wellcome Trust Research Programme highlighted a recent review of experimental SCD treatments by Marilyn Jo Telen, MD, of Duke University, Durham, N.C. (Blood. 2016;127[7]:810-9).
Also among the drugs being tested for SCD is Modus Therapeutics’ sevuparin, which Dr. Williams described as being “a heparin-like molecule without the anticoagulant complications.”
The compound originated as a “passion project” of Mats Wahlgren, MD, PhD, of the Karolinska Institute, Stockholm, who developed it for the treatment of malaria, Dr. Williams said. The company that’s developing sevuparin, Modus Therapeutics, is moving it forward first as a treatment for SCD for commercial reasons, Dr. Williams said.
“They think it’s a better first target,” he said.
An ongoing study of sevuparin for painful crisis is expected to be completed in December, with data then expected to be released in the middle of 2019, Ellen K. Donnelly, PhD, chief executive officer of Modus Therapeutics, said in an interview. She cited a mix of scientific, medical and commercial reasons for her company’s decision to advance sevuparin in SCD.
“First, and most importantly, there is proof of clinical benefit of a similar molecule (the low-molecular-weight heparin called tinzaparin) in patients with sickle cell disease,” Dr. Donnelly said. “Unfortunately, there is a bleeding risk with tinzaparin that limits use of the agent for the treatment of sickle cell disease. Sevuparin does not have the bleeding risk and thus is a strong candidate for SCD.”
Dr. Donnelly also noted the emphasis that the FDA’s Division of Hematology Products has put on development of therapeutics for SCD as a reason for proceeding first with this indication. The agency and the American Society of Hematology in early October held a workshop of experts, physicians, patients, and industry collaborators focused on identifying new endpoints for clinical studies.
Still, she noted that commercial reasons did factor into this decision.
“[I]t is possible for a small company like Modus to take an asset all the way to market when developing a therapeutic for a rare disease given the need for fewer patients and smaller trials,” Dr. Donnelly wrote. “As you can see from www.clinicaltrials.gov, we were able to run our phase 2 study with a small number of sites. In addition, in SCD it is possible to get approval with only one or two confirmatory studies.”
Financial interests
Other drugs in testing for SCD include rivipansel from GlycoMimetics, for which Pfizer is leading development. The sponsors expect to complete the so-called RESET trial by 2019, according to the clinicaltrials.gov. In this study, which is intended to enroll 350 participants, patients who have vaso-occlusive crises are randomly selected for treatment with either rivipansel or placebo.
Speaking during a question-and-answer session at the NIH conference, Robert Swift, PhD, said there’s a need for inexpensive oral drugs to treat SCD. Many other options will remain beyond the finances of people living in poor countries, he said.
“We need to focus not only on the root cause, but on something that is oral and inexpensive to solve the greater sickle cell problem,” Dr. Swift said.
Large drugmakers already have hospital-based sales forces, making SCD drugs administered in this setting attractive to them, he said.
“This is partly about where money is. The drug companies are going where the money is. It’s not oral drugs to treat everybody, it’s something else,” Dr. Swift said. “So someone else is going to have to fund the basic research” into treatments that could be more broadly used.
Dr. Swift said in an interview that he has received NIH funding for developing SCD-101, an oral drug, for which a placebo-controlled crossover study is underway.
Presenters at the NIH conference, including Dr. Saunthararajah, expressed frustration about what they see as relatively little work being done on SCD despite decades of knowledge about the root causes. Like Dr. Swift, he criticized the approach taken in selecting which treatments advance in this field.
“It’s not being driven by what is the most cost effective, what the patients need the most,” Dr. Saunthararajah said. “It’s driven by what will make the most money, not just for [the] drug company, but also for the hospital and also for the physicians.”
Dr. Saunthararajah reported having patents and patent applications around decitabine/tetrahydrouridine, 5-azacytidine/tetrahydrouridine, and differentiation therapy for oncology. He has also been a consultant for EpiDestiny, Novo Nordisk, and Takeda Oncology. Dr. Williams reported having no relevant financial disclosures. Dr. Swift is a managing member of Invenux and reported equity in Mast Therapeutics and SCD Development.
This article was updated on 11/9/2018.
BETHESDA, MD – While there are experimental treatments such as sevuparin and gene therapy in testing for sickle cell disease (SCD), researchers said this field is lagging because of a lack of funding.
Yogen Saunthararajah, MD, of the Cleveland Clinic, described what he called a “paltry” landscape of new drugs for SCD at Sickle Cell in Focus, a conference held by the National Institutes of Health.
There are only four main approaches taken by drugs now in clinical testing for addressing the root causes of SCD, despite decades’ worth of research of the genetic and mechanistic underpinnings of this disease, he said.
“It’s pretty sad,” Dr. Saunthararajah said, referring to the quantity of efforts, not their quality.
Within days of his presentation at the conference, one of the drugs he highlighted had officially fallen out of contention. An Oct. 26 post on NIH’s Clinicaltrials.gov site said Incyte had terminated its phase 1 study of INCB059872 in SCD “due to a business decision” not to pursue this indication. Incyte confirmed that it dropped development of INCB059872 for SCD but will continue testing it for other indications, including acute myeloid leukemia (AML).
Dr. Saunthararajah said that work on another approach, using decitabine (Dacogen), for which he has done a phase 1 study, is “struggling,” because of the search for funding.
Two other approaches that Dr. Saunthararajah cited in his presentation appear to remain on track. These are gene therapy and the once-daily voxelotor treatment from Global Blood Therapeutics.
In the field of gene therapy, Sangamo Therapeutics and Sanofi’s Bioverativ in May said the Food and Drug Administration had cleared the way for them to start a phase 1/2 clinical trial for the BIVV003 product that they are developing together. This uses zinc finger nuclease (ZFN) gene-editing technology to modifying a short sequence of the BCL11A gene, with the aim of reactivating fetal hemoglobin.
Bluebird Bio’s LentiGlobin gene therapy has advanced as far as phase 3 for transfusion-dependent beta-thalassemia and phase 1/2 for SCD. In October, the European Medicines Agency accepted the company’s application for approval of LentiGlobin gene therapy for the treatment of adolescents and adults with transfusion-dependent beta-thalassemia (TDT) and a non-beta0/beta0 genotype. The company has not said when it expects to file with the FDA for approval of this treatment.
In the field of oral therapies, Global Blood Therapeutics has said it’s in discussions with the FDA about a potential accelerated approval of voxelotor. The tablet is meant to inhibit the underlying mechanism that causes sickling of red blood cells. In June, the company completed a planned review of early data from its phase 3 trial, known as the HOPE study.
“On the primary endpoint (the proportion of patients with greater than 1 g/dL increase in hemoglobin versus baseline), a statistically significant increase was demonstrated with voxelotor at both the 1,500-mg and 900-mg doses after 12 weeks of treatment versus placebo,” Global Blood Therapeutics said in an August regulatory filing with the Securities and Exchange Commission.
The company has said that voxelotor may meet the FDA’s standard for accelerated approval under Subpart H program.
In his presentation at the NIH conference, Tom Williams, MD, PhD, of KEMRI/Wellcome Trust Research Programme highlighted a recent review of experimental SCD treatments by Marilyn Jo Telen, MD, of Duke University, Durham, N.C. (Blood. 2016;127[7]:810-9).
Also among the drugs being tested for SCD is Modus Therapeutics’ sevuparin, which Dr. Williams described as being “a heparin-like molecule without the anticoagulant complications.”
The compound originated as a “passion project” of Mats Wahlgren, MD, PhD, of the Karolinska Institute, Stockholm, who developed it for the treatment of malaria, Dr. Williams said. The company that’s developing sevuparin, Modus Therapeutics, is moving it forward first as a treatment for SCD for commercial reasons, Dr. Williams said.
“They think it’s a better first target,” he said.
An ongoing study of sevuparin for painful crisis is expected to be completed in December, with data then expected to be released in the middle of 2019, Ellen K. Donnelly, PhD, chief executive officer of Modus Therapeutics, said in an interview. She cited a mix of scientific, medical and commercial reasons for her company’s decision to advance sevuparin in SCD.
“First, and most importantly, there is proof of clinical benefit of a similar molecule (the low-molecular-weight heparin called tinzaparin) in patients with sickle cell disease,” Dr. Donnelly said. “Unfortunately, there is a bleeding risk with tinzaparin that limits use of the agent for the treatment of sickle cell disease. Sevuparin does not have the bleeding risk and thus is a strong candidate for SCD.”
Dr. Donnelly also noted the emphasis that the FDA’s Division of Hematology Products has put on development of therapeutics for SCD as a reason for proceeding first with this indication. The agency and the American Society of Hematology in early October held a workshop of experts, physicians, patients, and industry collaborators focused on identifying new endpoints for clinical studies.
Still, she noted that commercial reasons did factor into this decision.
“[I]t is possible for a small company like Modus to take an asset all the way to market when developing a therapeutic for a rare disease given the need for fewer patients and smaller trials,” Dr. Donnelly wrote. “As you can see from www.clinicaltrials.gov, we were able to run our phase 2 study with a small number of sites. In addition, in SCD it is possible to get approval with only one or two confirmatory studies.”
Financial interests
Other drugs in testing for SCD include rivipansel from GlycoMimetics, for which Pfizer is leading development. The sponsors expect to complete the so-called RESET trial by 2019, according to the clinicaltrials.gov. In this study, which is intended to enroll 350 participants, patients who have vaso-occlusive crises are randomly selected for treatment with either rivipansel or placebo.
Speaking during a question-and-answer session at the NIH conference, Robert Swift, PhD, said there’s a need for inexpensive oral drugs to treat SCD. Many other options will remain beyond the finances of people living in poor countries, he said.
“We need to focus not only on the root cause, but on something that is oral and inexpensive to solve the greater sickle cell problem,” Dr. Swift said.
Large drugmakers already have hospital-based sales forces, making SCD drugs administered in this setting attractive to them, he said.
“This is partly about where money is. The drug companies are going where the money is. It’s not oral drugs to treat everybody, it’s something else,” Dr. Swift said. “So someone else is going to have to fund the basic research” into treatments that could be more broadly used.
Dr. Swift said in an interview that he has received NIH funding for developing SCD-101, an oral drug, for which a placebo-controlled crossover study is underway.
Presenters at the NIH conference, including Dr. Saunthararajah, expressed frustration about what they see as relatively little work being done on SCD despite decades of knowledge about the root causes. Like Dr. Swift, he criticized the approach taken in selecting which treatments advance in this field.
“It’s not being driven by what is the most cost effective, what the patients need the most,” Dr. Saunthararajah said. “It’s driven by what will make the most money, not just for [the] drug company, but also for the hospital and also for the physicians.”
Dr. Saunthararajah reported having patents and patent applications around decitabine/tetrahydrouridine, 5-azacytidine/tetrahydrouridine, and differentiation therapy for oncology. He has also been a consultant for EpiDestiny, Novo Nordisk, and Takeda Oncology. Dr. Williams reported having no relevant financial disclosures. Dr. Swift is a managing member of Invenux and reported equity in Mast Therapeutics and SCD Development.
This article was updated on 11/9/2018.
BETHESDA, MD – While there are experimental treatments such as sevuparin and gene therapy in testing for sickle cell disease (SCD), researchers said this field is lagging because of a lack of funding.
Yogen Saunthararajah, MD, of the Cleveland Clinic, described what he called a “paltry” landscape of new drugs for SCD at Sickle Cell in Focus, a conference held by the National Institutes of Health.
There are only four main approaches taken by drugs now in clinical testing for addressing the root causes of SCD, despite decades’ worth of research of the genetic and mechanistic underpinnings of this disease, he said.
“It’s pretty sad,” Dr. Saunthararajah said, referring to the quantity of efforts, not their quality.
Within days of his presentation at the conference, one of the drugs he highlighted had officially fallen out of contention. An Oct. 26 post on NIH’s Clinicaltrials.gov site said Incyte had terminated its phase 1 study of INCB059872 in SCD “due to a business decision” not to pursue this indication. Incyte confirmed that it dropped development of INCB059872 for SCD but will continue testing it for other indications, including acute myeloid leukemia (AML).
Dr. Saunthararajah said that work on another approach, using decitabine (Dacogen), for which he has done a phase 1 study, is “struggling,” because of the search for funding.
Two other approaches that Dr. Saunthararajah cited in his presentation appear to remain on track. These are gene therapy and the once-daily voxelotor treatment from Global Blood Therapeutics.
In the field of gene therapy, Sangamo Therapeutics and Sanofi’s Bioverativ in May said the Food and Drug Administration had cleared the way for them to start a phase 1/2 clinical trial for the BIVV003 product that they are developing together. This uses zinc finger nuclease (ZFN) gene-editing technology to modifying a short sequence of the BCL11A gene, with the aim of reactivating fetal hemoglobin.
Bluebird Bio’s LentiGlobin gene therapy has advanced as far as phase 3 for transfusion-dependent beta-thalassemia and phase 1/2 for SCD. In October, the European Medicines Agency accepted the company’s application for approval of LentiGlobin gene therapy for the treatment of adolescents and adults with transfusion-dependent beta-thalassemia (TDT) and a non-beta0/beta0 genotype. The company has not said when it expects to file with the FDA for approval of this treatment.
In the field of oral therapies, Global Blood Therapeutics has said it’s in discussions with the FDA about a potential accelerated approval of voxelotor. The tablet is meant to inhibit the underlying mechanism that causes sickling of red blood cells. In June, the company completed a planned review of early data from its phase 3 trial, known as the HOPE study.
“On the primary endpoint (the proportion of patients with greater than 1 g/dL increase in hemoglobin versus baseline), a statistically significant increase was demonstrated with voxelotor at both the 1,500-mg and 900-mg doses after 12 weeks of treatment versus placebo,” Global Blood Therapeutics said in an August regulatory filing with the Securities and Exchange Commission.
The company has said that voxelotor may meet the FDA’s standard for accelerated approval under Subpart H program.
In his presentation at the NIH conference, Tom Williams, MD, PhD, of KEMRI/Wellcome Trust Research Programme highlighted a recent review of experimental SCD treatments by Marilyn Jo Telen, MD, of Duke University, Durham, N.C. (Blood. 2016;127[7]:810-9).
Also among the drugs being tested for SCD is Modus Therapeutics’ sevuparin, which Dr. Williams described as being “a heparin-like molecule without the anticoagulant complications.”
The compound originated as a “passion project” of Mats Wahlgren, MD, PhD, of the Karolinska Institute, Stockholm, who developed it for the treatment of malaria, Dr. Williams said. The company that’s developing sevuparin, Modus Therapeutics, is moving it forward first as a treatment for SCD for commercial reasons, Dr. Williams said.
“They think it’s a better first target,” he said.
An ongoing study of sevuparin for painful crisis is expected to be completed in December, with data then expected to be released in the middle of 2019, Ellen K. Donnelly, PhD, chief executive officer of Modus Therapeutics, said in an interview. She cited a mix of scientific, medical and commercial reasons for her company’s decision to advance sevuparin in SCD.
“First, and most importantly, there is proof of clinical benefit of a similar molecule (the low-molecular-weight heparin called tinzaparin) in patients with sickle cell disease,” Dr. Donnelly said. “Unfortunately, there is a bleeding risk with tinzaparin that limits use of the agent for the treatment of sickle cell disease. Sevuparin does not have the bleeding risk and thus is a strong candidate for SCD.”
Dr. Donnelly also noted the emphasis that the FDA’s Division of Hematology Products has put on development of therapeutics for SCD as a reason for proceeding first with this indication. The agency and the American Society of Hematology in early October held a workshop of experts, physicians, patients, and industry collaborators focused on identifying new endpoints for clinical studies.
Still, she noted that commercial reasons did factor into this decision.
“[I]t is possible for a small company like Modus to take an asset all the way to market when developing a therapeutic for a rare disease given the need for fewer patients and smaller trials,” Dr. Donnelly wrote. “As you can see from www.clinicaltrials.gov, we were able to run our phase 2 study with a small number of sites. In addition, in SCD it is possible to get approval with only one or two confirmatory studies.”
Financial interests
Other drugs in testing for SCD include rivipansel from GlycoMimetics, for which Pfizer is leading development. The sponsors expect to complete the so-called RESET trial by 2019, according to the clinicaltrials.gov. In this study, which is intended to enroll 350 participants, patients who have vaso-occlusive crises are randomly selected for treatment with either rivipansel or placebo.
Speaking during a question-and-answer session at the NIH conference, Robert Swift, PhD, said there’s a need for inexpensive oral drugs to treat SCD. Many other options will remain beyond the finances of people living in poor countries, he said.
“We need to focus not only on the root cause, but on something that is oral and inexpensive to solve the greater sickle cell problem,” Dr. Swift said.
Large drugmakers already have hospital-based sales forces, making SCD drugs administered in this setting attractive to them, he said.
“This is partly about where money is. The drug companies are going where the money is. It’s not oral drugs to treat everybody, it’s something else,” Dr. Swift said. “So someone else is going to have to fund the basic research” into treatments that could be more broadly used.
Dr. Swift said in an interview that he has received NIH funding for developing SCD-101, an oral drug, for which a placebo-controlled crossover study is underway.
Presenters at the NIH conference, including Dr. Saunthararajah, expressed frustration about what they see as relatively little work being done on SCD despite decades of knowledge about the root causes. Like Dr. Swift, he criticized the approach taken in selecting which treatments advance in this field.
“It’s not being driven by what is the most cost effective, what the patients need the most,” Dr. Saunthararajah said. “It’s driven by what will make the most money, not just for [the] drug company, but also for the hospital and also for the physicians.”
Dr. Saunthararajah reported having patents and patent applications around decitabine/tetrahydrouridine, 5-azacytidine/tetrahydrouridine, and differentiation therapy for oncology. He has also been a consultant for EpiDestiny, Novo Nordisk, and Takeda Oncology. Dr. Williams reported having no relevant financial disclosures. Dr. Swift is a managing member of Invenux and reported equity in Mast Therapeutics and SCD Development.
This article was updated on 11/9/2018.
REPORTING FROM SICKLE CELL IN FOCUS
Bradycardia guidelines, hypertension’s risk in young adults, and more
This week on Cardiocast, guidelines for bradycardia set a new bar for shared decision-making in pacemaker placement, young adults with hypertension may be at higher CVD risk, how sleep quality affects cardiovascular risk, and a strong showing for exercise in patients with both heart failure and sleep apnea.
Tune in next Friday for the most exciting news from the scientific sessions of the American Heart Association, as told by the reporters who cover it.
Subscribe to Cardiocast wherever you get your podcasts.
This week on Cardiocast, guidelines for bradycardia set a new bar for shared decision-making in pacemaker placement, young adults with hypertension may be at higher CVD risk, how sleep quality affects cardiovascular risk, and a strong showing for exercise in patients with both heart failure and sleep apnea.
Tune in next Friday for the most exciting news from the scientific sessions of the American Heart Association, as told by the reporters who cover it.
Subscribe to Cardiocast wherever you get your podcasts.
This week on Cardiocast, guidelines for bradycardia set a new bar for shared decision-making in pacemaker placement, young adults with hypertension may be at higher CVD risk, how sleep quality affects cardiovascular risk, and a strong showing for exercise in patients with both heart failure and sleep apnea.
Tune in next Friday for the most exciting news from the scientific sessions of the American Heart Association, as told by the reporters who cover it.
Subscribe to Cardiocast wherever you get your podcasts.
Trump administration rule erodes ACA contraceptive mandate
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
Zanubrutinib looks ‘robust’ in Waldenström macroglobulinemia
The BTK inhibitor zanubrutinib has demonstrated “robust activity” and “good tolerability” in patients with Waldenström macroglobulinemia (WM), according to an investigator.
In a phase 1 trial, zanubrutinib produced an overall response rate (ORR) of 92%, and the estimated 12-month progression-free survival (PFS) rate was 89%.
Most adverse events (AEs) in this trial were grade 1 or 2 in severity, although the incidence of serious AEs was 42%.
Constantine Tam, MD, of the Peter MacCallum Cancer Center in Victoria, Australia, presented these results at the 10th International Workshop on Waldenström’s Macroglobulinemia.
The trial is sponsored by BeiGene, the company developing zanubrutinib.
The trial (NCT02343120) includes patients with WM and other B-cell malignancies. As of July 24, 2018, 77 patients with treatment-naive or relapsed/refractory WM had been enrolled. Of those patients, 73 were evaluable for efficacy in this analysis, and the median follow-up time was 22.5 months.
At the time of the data cutoff, 62 patients remained on study treatment. Four patients (3%) discontinued treatment because of disease progression, and one patient remains on treatment after progression.
The median time to response was 85 days. The ORR was 92%, and the major response rate (MRR) was 82%. Of patients in the analysis, 41% achieved a very good partial response (VGPR), defined as a greater than 90% reduction in baseline immunoglobulin M (IgM) levels and improvement of extramedullary disease by computed tomography.
The median IgM decreased from 32.7 g/L at baseline to 8.2 g/L. The median hemoglobin increased from 8.85 g/dL to 13.4 g/dL among 32 patients with hemoglobin less than 10 g/dL at baseline.
MYD88 genotype was known in 63 patients. In the subset known to have the MYD88L265P mutation (n = 54), the ORR was 94%, the MRR was 89%, and the VGPR rate was 46%. In the nine patients known to have wild-type MYD88 (a genotype that, historically, has had suboptimal response to BTK inhibition), the ORR was 89%, the MRR was 67%, and the VGPR rate was 22%.
The 12-month PFS was estimated to be 89%, and the median PFS had not been reached.
The most frequent AEs of any attribution were petechiae/purpura/contusion (43%), upper respiratory tract infection (42%), cough (17%), diarrhea (17%), constipation (16%), back pain (16%), and headache (16%). Grade 3-4 AEs of any attribution reported in three or more patients included neutropenia (9%), anemia (7%), hypertension (5%), basal cell carcinoma (5%), renal and urinary disorders (4%), and pneumonia (4%). Serious AEs were seen in 32 patients (42%). Events in five patients (7%) were considered possibly related to zanubrutinib treatment – febrile neutropenia, colitis, atrial fibrillation, hemothorax, and pneumonia. Atrial fibrillation/flutter occurred in four patients (5%), and major hemorrhage was observed in two patients (3%).
“We are encouraged that additional data on zanubrutinib in patients with WM confirms the initially reported experience with consistent demonstration of robust activity and good tolerability,” Dr. Tam said in a statement.
The trial is sponsored by BeiGene. Dr. Tam reported financial relationships with BeiGene and other companies.
The BTK inhibitor zanubrutinib has demonstrated “robust activity” and “good tolerability” in patients with Waldenström macroglobulinemia (WM), according to an investigator.
In a phase 1 trial, zanubrutinib produced an overall response rate (ORR) of 92%, and the estimated 12-month progression-free survival (PFS) rate was 89%.
Most adverse events (AEs) in this trial were grade 1 or 2 in severity, although the incidence of serious AEs was 42%.
Constantine Tam, MD, of the Peter MacCallum Cancer Center in Victoria, Australia, presented these results at the 10th International Workshop on Waldenström’s Macroglobulinemia.
The trial is sponsored by BeiGene, the company developing zanubrutinib.
The trial (NCT02343120) includes patients with WM and other B-cell malignancies. As of July 24, 2018, 77 patients with treatment-naive or relapsed/refractory WM had been enrolled. Of those patients, 73 were evaluable for efficacy in this analysis, and the median follow-up time was 22.5 months.
At the time of the data cutoff, 62 patients remained on study treatment. Four patients (3%) discontinued treatment because of disease progression, and one patient remains on treatment after progression.
The median time to response was 85 days. The ORR was 92%, and the major response rate (MRR) was 82%. Of patients in the analysis, 41% achieved a very good partial response (VGPR), defined as a greater than 90% reduction in baseline immunoglobulin M (IgM) levels and improvement of extramedullary disease by computed tomography.
The median IgM decreased from 32.7 g/L at baseline to 8.2 g/L. The median hemoglobin increased from 8.85 g/dL to 13.4 g/dL among 32 patients with hemoglobin less than 10 g/dL at baseline.
MYD88 genotype was known in 63 patients. In the subset known to have the MYD88L265P mutation (n = 54), the ORR was 94%, the MRR was 89%, and the VGPR rate was 46%. In the nine patients known to have wild-type MYD88 (a genotype that, historically, has had suboptimal response to BTK inhibition), the ORR was 89%, the MRR was 67%, and the VGPR rate was 22%.
The 12-month PFS was estimated to be 89%, and the median PFS had not been reached.
The most frequent AEs of any attribution were petechiae/purpura/contusion (43%), upper respiratory tract infection (42%), cough (17%), diarrhea (17%), constipation (16%), back pain (16%), and headache (16%). Grade 3-4 AEs of any attribution reported in three or more patients included neutropenia (9%), anemia (7%), hypertension (5%), basal cell carcinoma (5%), renal and urinary disorders (4%), and pneumonia (4%). Serious AEs were seen in 32 patients (42%). Events in five patients (7%) were considered possibly related to zanubrutinib treatment – febrile neutropenia, colitis, atrial fibrillation, hemothorax, and pneumonia. Atrial fibrillation/flutter occurred in four patients (5%), and major hemorrhage was observed in two patients (3%).
“We are encouraged that additional data on zanubrutinib in patients with WM confirms the initially reported experience with consistent demonstration of robust activity and good tolerability,” Dr. Tam said in a statement.
The trial is sponsored by BeiGene. Dr. Tam reported financial relationships with BeiGene and other companies.
The BTK inhibitor zanubrutinib has demonstrated “robust activity” and “good tolerability” in patients with Waldenström macroglobulinemia (WM), according to an investigator.
In a phase 1 trial, zanubrutinib produced an overall response rate (ORR) of 92%, and the estimated 12-month progression-free survival (PFS) rate was 89%.
Most adverse events (AEs) in this trial were grade 1 or 2 in severity, although the incidence of serious AEs was 42%.
Constantine Tam, MD, of the Peter MacCallum Cancer Center in Victoria, Australia, presented these results at the 10th International Workshop on Waldenström’s Macroglobulinemia.
The trial is sponsored by BeiGene, the company developing zanubrutinib.
The trial (NCT02343120) includes patients with WM and other B-cell malignancies. As of July 24, 2018, 77 patients with treatment-naive or relapsed/refractory WM had been enrolled. Of those patients, 73 were evaluable for efficacy in this analysis, and the median follow-up time was 22.5 months.
At the time of the data cutoff, 62 patients remained on study treatment. Four patients (3%) discontinued treatment because of disease progression, and one patient remains on treatment after progression.
The median time to response was 85 days. The ORR was 92%, and the major response rate (MRR) was 82%. Of patients in the analysis, 41% achieved a very good partial response (VGPR), defined as a greater than 90% reduction in baseline immunoglobulin M (IgM) levels and improvement of extramedullary disease by computed tomography.
The median IgM decreased from 32.7 g/L at baseline to 8.2 g/L. The median hemoglobin increased from 8.85 g/dL to 13.4 g/dL among 32 patients with hemoglobin less than 10 g/dL at baseline.
MYD88 genotype was known in 63 patients. In the subset known to have the MYD88L265P mutation (n = 54), the ORR was 94%, the MRR was 89%, and the VGPR rate was 46%. In the nine patients known to have wild-type MYD88 (a genotype that, historically, has had suboptimal response to BTK inhibition), the ORR was 89%, the MRR was 67%, and the VGPR rate was 22%.
The 12-month PFS was estimated to be 89%, and the median PFS had not been reached.
The most frequent AEs of any attribution were petechiae/purpura/contusion (43%), upper respiratory tract infection (42%), cough (17%), diarrhea (17%), constipation (16%), back pain (16%), and headache (16%). Grade 3-4 AEs of any attribution reported in three or more patients included neutropenia (9%), anemia (7%), hypertension (5%), basal cell carcinoma (5%), renal and urinary disorders (4%), and pneumonia (4%). Serious AEs were seen in 32 patients (42%). Events in five patients (7%) were considered possibly related to zanubrutinib treatment – febrile neutropenia, colitis, atrial fibrillation, hemothorax, and pneumonia. Atrial fibrillation/flutter occurred in four patients (5%), and major hemorrhage was observed in two patients (3%).
“We are encouraged that additional data on zanubrutinib in patients with WM confirms the initially reported experience with consistent demonstration of robust activity and good tolerability,” Dr. Tam said in a statement.
The trial is sponsored by BeiGene. Dr. Tam reported financial relationships with BeiGene and other companies.
Key clinical point:
Major finding: Zanubrutinib had an overall response rate of 92%, and the estimated 12-month progression-free survival rate was 89%.
Study details: A phase 1 study with 73 patients evaluable for efficacy in this analysis.
Disclosures: The trial is sponsored by BeiGene. Dr. Tam reported financial relationships with BeiGene and other companies.
Lay counseling effective for reducing late-life depression
Counseling delivered by trained lay community members can effectively treat depression and anxiety in older adults in low- and middle-income countries, a study shows.
“The [depression in later life] intervention, is to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in a [low- and middle-income country] and as such addresses a previously unmet need in global health,” wrote Amit Dias, MD, and his colleagues. The findings show that the intervention could be a viable prevention option for older people living in those countries, which often lack the resources to provide prevention services for this population.
The study randomized 181 adults aged 60 years and older with subsyndromal depressive symptoms who attended rural and urban primary care clinics in Goa, India, to an intervention arm (n = 91) or to usual care (n = 90), reported Dr. Dias and his colleagues. The intervention arm was delivered by lay counselors (LCs) who were members of the local community, aged over 30 years, and graduates of any nonhealth-related field. The LCs, who received training, had weekly supervision and support from experts in the United States via Skype, reported Dr. Dias, of the department of preventive and social medicine at Goa Medical College in Bambolim, India, and his colleagues.
People in the intervention group also were given assistance with accessing medical and social programs. Six sessions lasting 30-40 minutes were delivered either in the patients’ homes or at a local center over a 6-10 week period.
Patients randomized to the control group received care as usual together with the same outcome assessments as the intervention group. Depressive episodes were measured using the Mini-International Neuropsychiatric Interview.
Results showed that 4.4% of participants in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04), Dr. Dias and his colleagues wrote in JAMA Psychiatry. Kaplan-Meier estimates showed that 95.1% of patients in the intervention group were free of depression at 12 months, compared with 87.4% of those in the control arm.
The incidence of depression, as measured by General Health Questionnaire–12 scores, also was lower in the intervention group (12-month mean difference, –1.18; 95% CI, –2.03 to –0.31; P less than .001). The intervention also was associated with lower systolic blood pressure at 12 months (difference, –6.98; 95% CI, –11.96 to –2.01; group x time interaction, P less than 0.001) and a change in body mass index (difference, 0.23; 95% CI, –0.97 to 1.43; P = 0.04).
However, the intervention did not affect measures of functional status or cognition.
The researchers concluded that their findings extend earlier work (Lancet. 2010;376[9758]:2086-95)(Lancet. 2017:389[10065]:176-85), which also showed that LCs could effectively treat prevalent cases of depression and anxiety in primary care practice. “If the success of the [depression in later life] intervention in depression prevention can be replicated in other [low- and middle-income countries], then its utility and scalability would be further supported,” they concluded.
Dr. Dias and his colleagues cited several limitations. One is that people with mild cognitive impairment or dementia were excluded from the study.
The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
SOURCE: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychaitry.2018.3048.
Depression occurring later in life is the most common mental health issue in the elderly and has been shown to have a negative impact on comorbidities and contribute to the risk for dementia and mortality. There is no doubt later-life depression poses a significant public health challenge. Low-income countries with limited resources can experience those challenges at a deeper level.
The current study contributes to the existing evidence, which shows that interventions carried out by nonhealth care professionals can be effective for addressing mental health conditions in low-resource settings. In addition, previous studies have shown that task sharing as a method is effective in tackling other health conditions such as HIV, hypertension, and tuberculosis in such settings.
However, it should be noted that, in the current study, the intervention was delivered by workers who received regular support. A logical next step, therefore, would be to examine the efficacy of interventions delivered by public health workers. Organizations that currently provide counseling services should be encouraged to adopt a structured approach demonstrated in the current study.
Jagadisha Thirthalli, MD, Palanimuthu T. Sivakumar, MD, and Bangalore N. Gangadhar, MD, are affiliated with the department of psychiatry at the National Institute of Mental Health and Neurosciences in Bengaluru, India. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.2898). No conflicts of interest were reported.
Depression occurring later in life is the most common mental health issue in the elderly and has been shown to have a negative impact on comorbidities and contribute to the risk for dementia and mortality. There is no doubt later-life depression poses a significant public health challenge. Low-income countries with limited resources can experience those challenges at a deeper level.
The current study contributes to the existing evidence, which shows that interventions carried out by nonhealth care professionals can be effective for addressing mental health conditions in low-resource settings. In addition, previous studies have shown that task sharing as a method is effective in tackling other health conditions such as HIV, hypertension, and tuberculosis in such settings.
However, it should be noted that, in the current study, the intervention was delivered by workers who received regular support. A logical next step, therefore, would be to examine the efficacy of interventions delivered by public health workers. Organizations that currently provide counseling services should be encouraged to adopt a structured approach demonstrated in the current study.
Jagadisha Thirthalli, MD, Palanimuthu T. Sivakumar, MD, and Bangalore N. Gangadhar, MD, are affiliated with the department of psychiatry at the National Institute of Mental Health and Neurosciences in Bengaluru, India. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.2898). No conflicts of interest were reported.
Depression occurring later in life is the most common mental health issue in the elderly and has been shown to have a negative impact on comorbidities and contribute to the risk for dementia and mortality. There is no doubt later-life depression poses a significant public health challenge. Low-income countries with limited resources can experience those challenges at a deeper level.
The current study contributes to the existing evidence, which shows that interventions carried out by nonhealth care professionals can be effective for addressing mental health conditions in low-resource settings. In addition, previous studies have shown that task sharing as a method is effective in tackling other health conditions such as HIV, hypertension, and tuberculosis in such settings.
However, it should be noted that, in the current study, the intervention was delivered by workers who received regular support. A logical next step, therefore, would be to examine the efficacy of interventions delivered by public health workers. Organizations that currently provide counseling services should be encouraged to adopt a structured approach demonstrated in the current study.
Jagadisha Thirthalli, MD, Palanimuthu T. Sivakumar, MD, and Bangalore N. Gangadhar, MD, are affiliated with the department of psychiatry at the National Institute of Mental Health and Neurosciences in Bengaluru, India. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.2898). No conflicts of interest were reported.
Counseling delivered by trained lay community members can effectively treat depression and anxiety in older adults in low- and middle-income countries, a study shows.
“The [depression in later life] intervention, is to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in a [low- and middle-income country] and as such addresses a previously unmet need in global health,” wrote Amit Dias, MD, and his colleagues. The findings show that the intervention could be a viable prevention option for older people living in those countries, which often lack the resources to provide prevention services for this population.
The study randomized 181 adults aged 60 years and older with subsyndromal depressive symptoms who attended rural and urban primary care clinics in Goa, India, to an intervention arm (n = 91) or to usual care (n = 90), reported Dr. Dias and his colleagues. The intervention arm was delivered by lay counselors (LCs) who were members of the local community, aged over 30 years, and graduates of any nonhealth-related field. The LCs, who received training, had weekly supervision and support from experts in the United States via Skype, reported Dr. Dias, of the department of preventive and social medicine at Goa Medical College in Bambolim, India, and his colleagues.
People in the intervention group also were given assistance with accessing medical and social programs. Six sessions lasting 30-40 minutes were delivered either in the patients’ homes or at a local center over a 6-10 week period.
Patients randomized to the control group received care as usual together with the same outcome assessments as the intervention group. Depressive episodes were measured using the Mini-International Neuropsychiatric Interview.
Results showed that 4.4% of participants in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04), Dr. Dias and his colleagues wrote in JAMA Psychiatry. Kaplan-Meier estimates showed that 95.1% of patients in the intervention group were free of depression at 12 months, compared with 87.4% of those in the control arm.
The incidence of depression, as measured by General Health Questionnaire–12 scores, also was lower in the intervention group (12-month mean difference, –1.18; 95% CI, –2.03 to –0.31; P less than .001). The intervention also was associated with lower systolic blood pressure at 12 months (difference, –6.98; 95% CI, –11.96 to –2.01; group x time interaction, P less than 0.001) and a change in body mass index (difference, 0.23; 95% CI, –0.97 to 1.43; P = 0.04).
However, the intervention did not affect measures of functional status or cognition.
The researchers concluded that their findings extend earlier work (Lancet. 2010;376[9758]:2086-95)(Lancet. 2017:389[10065]:176-85), which also showed that LCs could effectively treat prevalent cases of depression and anxiety in primary care practice. “If the success of the [depression in later life] intervention in depression prevention can be replicated in other [low- and middle-income countries], then its utility and scalability would be further supported,” they concluded.
Dr. Dias and his colleagues cited several limitations. One is that people with mild cognitive impairment or dementia were excluded from the study.
The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
SOURCE: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychaitry.2018.3048.
Counseling delivered by trained lay community members can effectively treat depression and anxiety in older adults in low- and middle-income countries, a study shows.
“The [depression in later life] intervention, is to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in a [low- and middle-income country] and as such addresses a previously unmet need in global health,” wrote Amit Dias, MD, and his colleagues. The findings show that the intervention could be a viable prevention option for older people living in those countries, which often lack the resources to provide prevention services for this population.
The study randomized 181 adults aged 60 years and older with subsyndromal depressive symptoms who attended rural and urban primary care clinics in Goa, India, to an intervention arm (n = 91) or to usual care (n = 90), reported Dr. Dias and his colleagues. The intervention arm was delivered by lay counselors (LCs) who were members of the local community, aged over 30 years, and graduates of any nonhealth-related field. The LCs, who received training, had weekly supervision and support from experts in the United States via Skype, reported Dr. Dias, of the department of preventive and social medicine at Goa Medical College in Bambolim, India, and his colleagues.
People in the intervention group also were given assistance with accessing medical and social programs. Six sessions lasting 30-40 minutes were delivered either in the patients’ homes or at a local center over a 6-10 week period.
Patients randomized to the control group received care as usual together with the same outcome assessments as the intervention group. Depressive episodes were measured using the Mini-International Neuropsychiatric Interview.
Results showed that 4.4% of participants in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04), Dr. Dias and his colleagues wrote in JAMA Psychiatry. Kaplan-Meier estimates showed that 95.1% of patients in the intervention group were free of depression at 12 months, compared with 87.4% of those in the control arm.
The incidence of depression, as measured by General Health Questionnaire–12 scores, also was lower in the intervention group (12-month mean difference, –1.18; 95% CI, –2.03 to –0.31; P less than .001). The intervention also was associated with lower systolic blood pressure at 12 months (difference, –6.98; 95% CI, –11.96 to –2.01; group x time interaction, P less than 0.001) and a change in body mass index (difference, 0.23; 95% CI, –0.97 to 1.43; P = 0.04).
However, the intervention did not affect measures of functional status or cognition.
The researchers concluded that their findings extend earlier work (Lancet. 2010;376[9758]:2086-95)(Lancet. 2017:389[10065]:176-85), which also showed that LCs could effectively treat prevalent cases of depression and anxiety in primary care practice. “If the success of the [depression in later life] intervention in depression prevention can be replicated in other [low- and middle-income countries], then its utility and scalability would be further supported,” they concluded.
Dr. Dias and his colleagues cited several limitations. One is that people with mild cognitive impairment or dementia were excluded from the study.
The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
SOURCE: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychaitry.2018.3048.
FROM JAMA PSYCHIATRY
Key clinical point: Lay counseling can be an effective intervention in reducing late-life depression in low- and middle-income countries.
Major finding: More than 4% of those in the intervention group had a major depressive episode, compared with 14.4% of those in the usual care group (number needed to treat, 9.95; 95% confidence interval, 5.12-182.43; P = 0.04).
Study details: Overall, 181 adults aged over 60 years with subsyndromal depressive symptoms who attended a rural and urban primary care clinics in Goa, India, who were randomized to an intervention arm (n = 91) or to usual care (n = 90).
Disclosures: The study was supported by grants from the U.S. National Institute of Mental Health. The authors reported no conflicts of interest.
Source: Dias A et al. JAMA Psychiatry. 2018 Nov 7. doi: 10.1001/jamapsychiatry.2018.3048.
Venous Venous Venous @VEITHsymposium
The Venous Venous Venous @VEITHsymposium program has become a popular staple of the meeting. With a mixture of didactic sessions and workshops, the Venous Venous Venous program, which will be held on Thursday, Friday, and Saturday, will cover the full gamut of venous disorders and their treatments, surgical, endovascular, and medical.
The didactic Program J (Sessions 63-67) on Superficial Venous Disease will be held on Thursday morning and early afternoon and will detail the latest developments in venous clinical examinations and imaging, superficial vein strategies and techniques, thermal and non-thermal ablation, and there will be a special session on venous societal issues and governance. Moderated by Elna M. Masuda, MD, and Marc A. Passman, MD, this special session will feature discussions of the Centers for Medicare & Medicaid policy update on venous ablation, dealing with MACRA, the RUC, and the reevaluation of phlebectomy, and more.
The didactic Program N (Sessions 88-92) on Deep Venous Disease will be all day Friday and will cover pelvic venous disorders, femoro-iliocaval interventions, deep vein reflux, wounds, and endovascular and open solutions for inferior vena cava disorders, and more.
The didactic Program R (Sessions 109-114) on Superficial Venous Disease will be held on Thursday morning and early afternoon and will cover all aspects of venous disease, from venous imaging, thrombophilia, schelrotherapy, phlebectomy, and more.
This year’s workshops will be held on Thursday afternoon and early evening, Nov. 15, and will feature video case presentations, and lectures and demonstrations on managing venous disease by leading experts in the field. In addition, there will be hands-on work station opportunities for participants to work with trained professionals to hone their skills.Two workshop modules are being offered this year:
1) Thrombus Management, which will focus on thromolysis, thrombectomry, the latest in stents and filters, difficult recanalizations, and issues in anticoagulation.
2) Superficial Venous Disease and Compression Management, which will deal with venous ablation, phelebctomy, schlerotherapy, and the critical areas of lymphedema, lipedema, and venous edema treatment, as well as wound care and compression.
The Venous Venous Venous @VEITHsymposium program has become a popular staple of the meeting. With a mixture of didactic sessions and workshops, the Venous Venous Venous program, which will be held on Thursday, Friday, and Saturday, will cover the full gamut of venous disorders and their treatments, surgical, endovascular, and medical.
The didactic Program J (Sessions 63-67) on Superficial Venous Disease will be held on Thursday morning and early afternoon and will detail the latest developments in venous clinical examinations and imaging, superficial vein strategies and techniques, thermal and non-thermal ablation, and there will be a special session on venous societal issues and governance. Moderated by Elna M. Masuda, MD, and Marc A. Passman, MD, this special session will feature discussions of the Centers for Medicare & Medicaid policy update on venous ablation, dealing with MACRA, the RUC, and the reevaluation of phlebectomy, and more.
The didactic Program N (Sessions 88-92) on Deep Venous Disease will be all day Friday and will cover pelvic venous disorders, femoro-iliocaval interventions, deep vein reflux, wounds, and endovascular and open solutions for inferior vena cava disorders, and more.
The didactic Program R (Sessions 109-114) on Superficial Venous Disease will be held on Thursday morning and early afternoon and will cover all aspects of venous disease, from venous imaging, thrombophilia, schelrotherapy, phlebectomy, and more.
This year’s workshops will be held on Thursday afternoon and early evening, Nov. 15, and will feature video case presentations, and lectures and demonstrations on managing venous disease by leading experts in the field. In addition, there will be hands-on work station opportunities for participants to work with trained professionals to hone their skills.Two workshop modules are being offered this year:
1) Thrombus Management, which will focus on thromolysis, thrombectomry, the latest in stents and filters, difficult recanalizations, and issues in anticoagulation.
2) Superficial Venous Disease and Compression Management, which will deal with venous ablation, phelebctomy, schlerotherapy, and the critical areas of lymphedema, lipedema, and venous edema treatment, as well as wound care and compression.
The Venous Venous Venous @VEITHsymposium program has become a popular staple of the meeting. With a mixture of didactic sessions and workshops, the Venous Venous Venous program, which will be held on Thursday, Friday, and Saturday, will cover the full gamut of venous disorders and their treatments, surgical, endovascular, and medical.
The didactic Program J (Sessions 63-67) on Superficial Venous Disease will be held on Thursday morning and early afternoon and will detail the latest developments in venous clinical examinations and imaging, superficial vein strategies and techniques, thermal and non-thermal ablation, and there will be a special session on venous societal issues and governance. Moderated by Elna M. Masuda, MD, and Marc A. Passman, MD, this special session will feature discussions of the Centers for Medicare & Medicaid policy update on venous ablation, dealing with MACRA, the RUC, and the reevaluation of phlebectomy, and more.
The didactic Program N (Sessions 88-92) on Deep Venous Disease will be all day Friday and will cover pelvic venous disorders, femoro-iliocaval interventions, deep vein reflux, wounds, and endovascular and open solutions for inferior vena cava disorders, and more.
The didactic Program R (Sessions 109-114) on Superficial Venous Disease will be held on Thursday morning and early afternoon and will cover all aspects of venous disease, from venous imaging, thrombophilia, schelrotherapy, phlebectomy, and more.
This year’s workshops will be held on Thursday afternoon and early evening, Nov. 15, and will feature video case presentations, and lectures and demonstrations on managing venous disease by leading experts in the field. In addition, there will be hands-on work station opportunities for participants to work with trained professionals to hone their skills.Two workshop modules are being offered this year:
1) Thrombus Management, which will focus on thromolysis, thrombectomry, the latest in stents and filters, difficult recanalizations, and issues in anticoagulation.
2) Superficial Venous Disease and Compression Management, which will deal with venous ablation, phelebctomy, schlerotherapy, and the critical areas of lymphedema, lipedema, and venous edema treatment, as well as wound care and compression.
VEITHsymposium: Focusing on (clinical) trials and tribulations
A hallmark of the VEITHsymposium has always been its focus on the results of recent and ongoing clinical trials, and this year is no exception. These trials will be presented by experts in their various fields who will discuss how the results will affect your daily practice.
A plethora of such clinical trials take center stage throughout the week and Tuesday alone has its fair share of highlighted studies.
For example, Tuesday morning, Jan D. Blankensteijn, MD, will discuss how and why the late results of the Dutch Randomised Endovascular Aneurysm Management (DREAM) and the Standard Open Surgery Versus Endovascular Repair of Abdominal Aortic Aneurysm (OVER) randomized controlled trials did not show the same late survival benefit for open repair as for EVAR and will address the issue of whether EVAR should be the treatment of choice for all anatomically suitable AAA patients.
Intracranial treatments for stroke will be a key interest of three trial presentations: Colin P. Derdeyn, MD, will present new findings from the Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) randomized controlled trial comparing intracranial stenting to best medical treatments, highlighting the high incidence of in-stent restenosis causing strokes that were observed. Alejandro M. Spiotta, MD, will discuss how the COMPASS Trial: a Direct Aspiration First Pass Technique (COMPASS) shows that new aspiration systems are equivalent to stentrievers for removing intracranial clots to treat acute strokes, and address when they appear to be actually better. In addition, L. Nelson Hopkins, MD, will present an update on the value of intracranial clot removal for acute strokes, highlighting the question of when a longer window after symptom onset (up to 24 hours) is acceptable, as seen in recent trials such as the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) and Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke (EXTEND-IA TNK) studies.
Switching gears later in the day, the 1-year results of the Bare Metal Stent Versus Paclitaxel Eluting Stent in the Setting of Primary Stenting of Intermediate Length Femoropopliteal Lesions (BATTLE) multicenter randomized controlled trial, will be presented by Yann Gouëffic, MD, PhD.
Be sure to catch up with these trial results and others on Tuesday and the host of trials to be presented and discussed throughout the week at the 2018 VEITHsymposium.
A hallmark of the VEITHsymposium has always been its focus on the results of recent and ongoing clinical trials, and this year is no exception. These trials will be presented by experts in their various fields who will discuss how the results will affect your daily practice.
A plethora of such clinical trials take center stage throughout the week and Tuesday alone has its fair share of highlighted studies.
For example, Tuesday morning, Jan D. Blankensteijn, MD, will discuss how and why the late results of the Dutch Randomised Endovascular Aneurysm Management (DREAM) and the Standard Open Surgery Versus Endovascular Repair of Abdominal Aortic Aneurysm (OVER) randomized controlled trials did not show the same late survival benefit for open repair as for EVAR and will address the issue of whether EVAR should be the treatment of choice for all anatomically suitable AAA patients.
Intracranial treatments for stroke will be a key interest of three trial presentations: Colin P. Derdeyn, MD, will present new findings from the Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) randomized controlled trial comparing intracranial stenting to best medical treatments, highlighting the high incidence of in-stent restenosis causing strokes that were observed. Alejandro M. Spiotta, MD, will discuss how the COMPASS Trial: a Direct Aspiration First Pass Technique (COMPASS) shows that new aspiration systems are equivalent to stentrievers for removing intracranial clots to treat acute strokes, and address when they appear to be actually better. In addition, L. Nelson Hopkins, MD, will present an update on the value of intracranial clot removal for acute strokes, highlighting the question of when a longer window after symptom onset (up to 24 hours) is acceptable, as seen in recent trials such as the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) and Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke (EXTEND-IA TNK) studies.
Switching gears later in the day, the 1-year results of the Bare Metal Stent Versus Paclitaxel Eluting Stent in the Setting of Primary Stenting of Intermediate Length Femoropopliteal Lesions (BATTLE) multicenter randomized controlled trial, will be presented by Yann Gouëffic, MD, PhD.
Be sure to catch up with these trial results and others on Tuesday and the host of trials to be presented and discussed throughout the week at the 2018 VEITHsymposium.
A hallmark of the VEITHsymposium has always been its focus on the results of recent and ongoing clinical trials, and this year is no exception. These trials will be presented by experts in their various fields who will discuss how the results will affect your daily practice.
A plethora of such clinical trials take center stage throughout the week and Tuesday alone has its fair share of highlighted studies.
For example, Tuesday morning, Jan D. Blankensteijn, MD, will discuss how and why the late results of the Dutch Randomised Endovascular Aneurysm Management (DREAM) and the Standard Open Surgery Versus Endovascular Repair of Abdominal Aortic Aneurysm (OVER) randomized controlled trials did not show the same late survival benefit for open repair as for EVAR and will address the issue of whether EVAR should be the treatment of choice for all anatomically suitable AAA patients.
Intracranial treatments for stroke will be a key interest of three trial presentations: Colin P. Derdeyn, MD, will present new findings from the Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) randomized controlled trial comparing intracranial stenting to best medical treatments, highlighting the high incidence of in-stent restenosis causing strokes that were observed. Alejandro M. Spiotta, MD, will discuss how the COMPASS Trial: a Direct Aspiration First Pass Technique (COMPASS) shows that new aspiration systems are equivalent to stentrievers for removing intracranial clots to treat acute strokes, and address when they appear to be actually better. In addition, L. Nelson Hopkins, MD, will present an update on the value of intracranial clot removal for acute strokes, highlighting the question of when a longer window after symptom onset (up to 24 hours) is acceptable, as seen in recent trials such as the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) and Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke (EXTEND-IA TNK) studies.
Switching gears later in the day, the 1-year results of the Bare Metal Stent Versus Paclitaxel Eluting Stent in the Setting of Primary Stenting of Intermediate Length Femoropopliteal Lesions (BATTLE) multicenter randomized controlled trial, will be presented by Yann Gouëffic, MD, PhD.
Be sure to catch up with these trial results and others on Tuesday and the host of trials to be presented and discussed throughout the week at the 2018 VEITHsymposium.
Cigarette smoking at lowest level ever
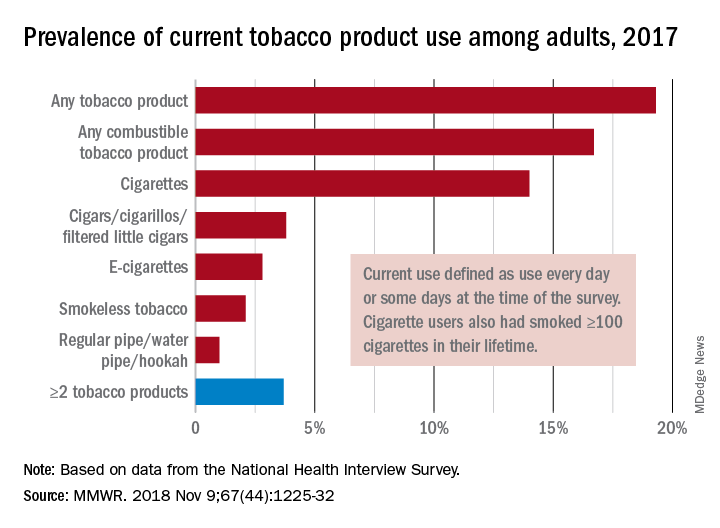
“This new all-time low in cigarette smoking among U.S. adults is a tremendous public health accomplishment, and it demonstrates the importance of continued proven strategies to reduce smoking,” CDC Director Robert Redfield said in a written statement.
In 2017, 19.3% of adults aged 18 years and older – approximately 47.4 million Americans – reported current use of some type of tobacco product, and current use of combustible tobacco was 16.7%, Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report. Current use was defined as use every day or some days, with an added requirement of at least 100 cigarettes in a lifetime added for cigarette smokers.
Data from the National Health Interview Survey showed that from 2016 to 2017, current use declined for any tobacco product, any combustible tobacco product, cigarettes, smokeless tobacco, and the combination of two or more tobacco products. The most common combination in 2017 was cigarettes and e-cigarettes, which was reported by 30.1% of the 9 million adults who used more than one product, Dr. Wang and her associates said.
Prevalence of current tobacco use was higher among men than women (24.8% vs. 14.2%), and adults aged 25-44 years (22.5%) had the highest level by age, followed by those aged 45-64 years (21.3%), 18-24 years (18.3%), and 65 years or older (11%). Use by race/ethnicity was highest among American Indian/Alaska Natives (29.8%), with the Midwest putting up the highest prevalence by region at 23.5%, they said.
“Although cigarette smoking among U.S. adults has declined considerably, tobacco products have evolved in recent years to include various combustible, noncombustible, and electronic products,” Dr. Wang and her associates wrote. “Implementation of evidence-based tobacco control interventions that address the diversity of tobacco products used by U.S. adults, in coordination with regulation of tobacco product manufacturing, marketing, and sales, can reduce tobacco-related disease and death in the United States.”
SOURCE: Wang TW et al. MMWR. 2018 Nov 9;67[44]:1225-32.

“This new all-time low in cigarette smoking among U.S. adults is a tremendous public health accomplishment, and it demonstrates the importance of continued proven strategies to reduce smoking,” CDC Director Robert Redfield said in a written statement.
In 2017, 19.3% of adults aged 18 years and older – approximately 47.4 million Americans – reported current use of some type of tobacco product, and current use of combustible tobacco was 16.7%, Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report. Current use was defined as use every day or some days, with an added requirement of at least 100 cigarettes in a lifetime added for cigarette smokers.
Data from the National Health Interview Survey showed that from 2016 to 2017, current use declined for any tobacco product, any combustible tobacco product, cigarettes, smokeless tobacco, and the combination of two or more tobacco products. The most common combination in 2017 was cigarettes and e-cigarettes, which was reported by 30.1% of the 9 million adults who used more than one product, Dr. Wang and her associates said.
Prevalence of current tobacco use was higher among men than women (24.8% vs. 14.2%), and adults aged 25-44 years (22.5%) had the highest level by age, followed by those aged 45-64 years (21.3%), 18-24 years (18.3%), and 65 years or older (11%). Use by race/ethnicity was highest among American Indian/Alaska Natives (29.8%), with the Midwest putting up the highest prevalence by region at 23.5%, they said.
“Although cigarette smoking among U.S. adults has declined considerably, tobacco products have evolved in recent years to include various combustible, noncombustible, and electronic products,” Dr. Wang and her associates wrote. “Implementation of evidence-based tobacco control interventions that address the diversity of tobacco products used by U.S. adults, in coordination with regulation of tobacco product manufacturing, marketing, and sales, can reduce tobacco-related disease and death in the United States.”
SOURCE: Wang TW et al. MMWR. 2018 Nov 9;67[44]:1225-32.

“This new all-time low in cigarette smoking among U.S. adults is a tremendous public health accomplishment, and it demonstrates the importance of continued proven strategies to reduce smoking,” CDC Director Robert Redfield said in a written statement.
In 2017, 19.3% of adults aged 18 years and older – approximately 47.4 million Americans – reported current use of some type of tobacco product, and current use of combustible tobacco was 16.7%, Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and her associates reported in the Morbidity and Mortality Weekly Report. Current use was defined as use every day or some days, with an added requirement of at least 100 cigarettes in a lifetime added for cigarette smokers.
Data from the National Health Interview Survey showed that from 2016 to 2017, current use declined for any tobacco product, any combustible tobacco product, cigarettes, smokeless tobacco, and the combination of two or more tobacco products. The most common combination in 2017 was cigarettes and e-cigarettes, which was reported by 30.1% of the 9 million adults who used more than one product, Dr. Wang and her associates said.
Prevalence of current tobacco use was higher among men than women (24.8% vs. 14.2%), and adults aged 25-44 years (22.5%) had the highest level by age, followed by those aged 45-64 years (21.3%), 18-24 years (18.3%), and 65 years or older (11%). Use by race/ethnicity was highest among American Indian/Alaska Natives (29.8%), with the Midwest putting up the highest prevalence by region at 23.5%, they said.
“Although cigarette smoking among U.S. adults has declined considerably, tobacco products have evolved in recent years to include various combustible, noncombustible, and electronic products,” Dr. Wang and her associates wrote. “Implementation of evidence-based tobacco control interventions that address the diversity of tobacco products used by U.S. adults, in coordination with regulation of tobacco product manufacturing, marketing, and sales, can reduce tobacco-related disease and death in the United States.”
SOURCE: Wang TW et al. MMWR. 2018 Nov 9;67[44]:1225-32.
FROM MMWR
Identifying and stopping a likely mass shooter: A case study
AUSTIN, TEX. – While the media relentlessly reports on every mass shooting that occurs, the public hears less often about the shootings that never happened – because people were paying attention and taking action, according to James L. Knoll, IV, MD, director of forensic psychiatry at the State University of New York, Syracuse.
“We’ve learned a lot about risk factors [for mass shootings], we’ve learned a lot about associations and correlations, and it’s gotten us so far,” Dr. Knoll told attendees at the annual meeting of the American Academy of Psychiatry and the Law. “I want to invite you to look at this from the angle of those shootings that were able to be prevented or disrupted.” (Dr. Knoll said he used the term “disrupted” because it’s impossible to ever know for certain that a shooting was thwarted.)
It is difficult to track mass homicides that would have occurred but were disrupted, but one study Dr. Knoll cited combed through news reports and identified 57 interrupted mass homicides (Aggress Viol Behav. 2016 Sep-Oct;30:88-93). Most of those (77%) had been interrupted by family and friends or the general public reporting suspicious behavior.
It was while Dr. Knoll was leading the threat assessment subcommittee of the Syracuse School Safety Task Force that a potential school shooting threat arose.
A 22-year-old Chinese international student named Xiaofeng “Lincoln” Zhan walked into AJ’s Archery/The Gun Shop on March 12, asking to buy an AR-15. The AR-15 is the semiautomatic weapon of choice for most mass shooters.
Mr. Zhan should have been barred from purchasing a gun because he was an international student on a temporary visa. Under U.S. code, it is “unlawful for any person to sell or otherwise dispose of any firearm or ammunition to any person knowing or having reasonable cause to believe that such person” is an alien who is “illegally or unlawfully in the United States” or “ has been admitted to the United States under a nonimmigrant visa.”
But the second provision was subject to certain exceptions, the first of which was that the person had been “admitted to the United States for lawful hunting or supporting purposes“ or was “in possession of a hunting license or permit lawfully issued in the United States.”
Mr. Zhan had a hunting license. He had taken a hunter safety course on March 11, the day before he entered the gun shop, and then bought a hunting license.
But the gun shop owner was not so easily persuaded. Mr. Zhan asked about “high-capacity shotguns” and said he belonged to a shooting club, yet he did not appear familiar with firearms. The gun shop owner was also skeptical because it didn’t make sense to use a high-capacity shotgun for hunting, and Mr. Zhan had just gotten his hunting license and didn’t know how to use the gun. Further, Mr. Zhan claimed that Syracuse University offered a class on how to use the gun – but the gun store owner knew that the university did not offer such a class.
The gun shop owner’s first thought was not that Mr. Zhan was a potential mass shooter but that he was a “secret shopper,” which Dr. Knoll defined as an undercover law enforcement officer who attempts to buy guns in a manner that should arouse suspicion in the store owner.
Ultimately, Zhan’s behavior was concerning and he made the owner feel uncomfortable. The owner captured Mr. Zhan’s information on U.S. ATF form 4473 and recorded his license plate. Then the gun shop owner contacted the Madison County Sheriff’s Office with the information.
The police opened an investigation that established that Mr. Zhan was a student enrolled at Syracuse University, which was on spring break at the time. The Syracuse Police Department arranged a joint meeting between the Onondaga County district attorney, Syracuse University Department of Public Safety, Onondaga County Sheriff’s Office, and the FBI to present their findings, including the fact that local high schools were planning walk-outs that might be potential targets.
Further investigation revealed that Mr. Zhan had been a student at Northeastern University in Boston in 2015, where he had asked a teacher how to get guns. The teacher emailed his supervisor, but the university police found no concerns.
Meanwhile, the police obtained a subpoena to get Mr. Zhan’s mental health records from Syracuse University. Mr. Zhan had sought psychiatric care at two facilities, Northeastern University in 2015 and Syracuse University in 2018. His mental health records revealed alcohol abuse, depression, suicidal thinking, anger problems, feelings of isolation and withdrawal, and his feeling that he might lose control or act violently, said Dr. Knoll, who is also professor of psychiatry at the university.
On March 13, the day after he had attempted to buy the gun, Syracuse University’s mental health services were contacted and briefed on Mr. Zhan. They filled out the paperwork for New York’s SAFE Act, which prevents people from buying a gun if a mental health professional makes the reasonable judgment that the individual might harm themselves or someone else.
The police investigation continued and found that Mr. Zhan had previously tried to buy an AR-15 at a Dick’s Sporting Goods store. He was denied because the SAFE Act prevents their sale.
Mr. Zhan, meanwhile, had gone to Mexico for the break and was due to return March 19. While he was away, an alarm allegedly went off in his apartment on March 16, leading the landlord to check on the apartment since he remembered previous police inquiries. He knocked on the door but there was no answer, so the landlord entered to do a safety check. He found ammunition and other concerning supplies.
The same day the landlord was checking Mr. Zhan’s apartment, students traveling with him in Mexico emailed Syracuse University about concerning behavior they observed in him. This behavior included signs of severe depression, verbalizing extremely negative thoughts, discussing suicide, drinking heavily, and making cuts to his forearms with the knife he possessed.
They also shared screenshots of messages they had seen him post in a social media group about feeling compelled to buy a gun and bulletproof vest and practice shooting.
Three days later, the police obtained a search warrant for Mr. Zhan’s apartment and vehicle. They found in his apartment high-powered optics, scopes, ammunition, targets from shooting ranges, receipts from shooting ranges, and similar equipment.
Ultimately, authorities revoked Mr. Zhan’s visa, enabling them to detain him at the airport when he returned from Mexico and deport him back to China.
After Mr. Zhan had returned to China, further investigation uncovered a series of texts between Mr. Zhan and his girlfriend in which he openly talks about wanting to shoot people.
“So, what went right here instead of what went wrong?” Dr. Knoll rhetorically asked. A lot of things: leakage of Mr. Zhan’s plans; fellow students seeing and reporting his electronic messages and concerning behaviors; the gun store owner’s skepticism and contact with the police; the landlord’s check on Mr. Zhan’s apartment; and the cooperation among local police, school authorities, and the school’s mental health services.
“There was also good communication among the threat assessment teams and law enforcement and the collaboration across disciplines,” Dr. Knoll said. Mass shootings have now “taken on more of a sociocultural phenomenon,” and “sociocultural problems require sociocultural solutions. I like these laws focusing on behaviors, not psychiatric diagnoses.”
He then reviewed potential interventions that might help identify or interfere with a planned incident or intent to commit one, including increased attention paid to suspicious behavior, third-party reporting of a potential shooter’s intent, and suicide prevention programs.
Dr. Knoll shared recent FBI research on 63 active shooters between 2000 and 2013 showing that the majority (77%) had been planning their attack for at least 1 week. Further, 46% have been preparing for 1 week before. The majority of those likely shooters also obtained their guns legally.
Although a quarter of those in the FBI study had some mental health diagnosis – predominantly depression or anxiety – the agency uncovered no significant correlation between mental illness and becoming a shooter.
The study concluded that,“absent specific evidence, careful consideration should be given to social and contextual factors that might interact with any mental health issue before concluding that an active shooting was ‘caused’ by a mental illness. In short, declarations that all active shooters must simply be mentally ill are misleading and unhelpful.”
Dr. Knoll reported no conflicts of interest.
AUSTIN, TEX. – While the media relentlessly reports on every mass shooting that occurs, the public hears less often about the shootings that never happened – because people were paying attention and taking action, according to James L. Knoll, IV, MD, director of forensic psychiatry at the State University of New York, Syracuse.
“We’ve learned a lot about risk factors [for mass shootings], we’ve learned a lot about associations and correlations, and it’s gotten us so far,” Dr. Knoll told attendees at the annual meeting of the American Academy of Psychiatry and the Law. “I want to invite you to look at this from the angle of those shootings that were able to be prevented or disrupted.” (Dr. Knoll said he used the term “disrupted” because it’s impossible to ever know for certain that a shooting was thwarted.)
It is difficult to track mass homicides that would have occurred but were disrupted, but one study Dr. Knoll cited combed through news reports and identified 57 interrupted mass homicides (Aggress Viol Behav. 2016 Sep-Oct;30:88-93). Most of those (77%) had been interrupted by family and friends or the general public reporting suspicious behavior.
It was while Dr. Knoll was leading the threat assessment subcommittee of the Syracuse School Safety Task Force that a potential school shooting threat arose.
A 22-year-old Chinese international student named Xiaofeng “Lincoln” Zhan walked into AJ’s Archery/The Gun Shop on March 12, asking to buy an AR-15. The AR-15 is the semiautomatic weapon of choice for most mass shooters.
Mr. Zhan should have been barred from purchasing a gun because he was an international student on a temporary visa. Under U.S. code, it is “unlawful for any person to sell or otherwise dispose of any firearm or ammunition to any person knowing or having reasonable cause to believe that such person” is an alien who is “illegally or unlawfully in the United States” or “ has been admitted to the United States under a nonimmigrant visa.”
But the second provision was subject to certain exceptions, the first of which was that the person had been “admitted to the United States for lawful hunting or supporting purposes“ or was “in possession of a hunting license or permit lawfully issued in the United States.”
Mr. Zhan had a hunting license. He had taken a hunter safety course on March 11, the day before he entered the gun shop, and then bought a hunting license.
But the gun shop owner was not so easily persuaded. Mr. Zhan asked about “high-capacity shotguns” and said he belonged to a shooting club, yet he did not appear familiar with firearms. The gun shop owner was also skeptical because it didn’t make sense to use a high-capacity shotgun for hunting, and Mr. Zhan had just gotten his hunting license and didn’t know how to use the gun. Further, Mr. Zhan claimed that Syracuse University offered a class on how to use the gun – but the gun store owner knew that the university did not offer such a class.
The gun shop owner’s first thought was not that Mr. Zhan was a potential mass shooter but that he was a “secret shopper,” which Dr. Knoll defined as an undercover law enforcement officer who attempts to buy guns in a manner that should arouse suspicion in the store owner.
Ultimately, Zhan’s behavior was concerning and he made the owner feel uncomfortable. The owner captured Mr. Zhan’s information on U.S. ATF form 4473 and recorded his license plate. Then the gun shop owner contacted the Madison County Sheriff’s Office with the information.
The police opened an investigation that established that Mr. Zhan was a student enrolled at Syracuse University, which was on spring break at the time. The Syracuse Police Department arranged a joint meeting between the Onondaga County district attorney, Syracuse University Department of Public Safety, Onondaga County Sheriff’s Office, and the FBI to present their findings, including the fact that local high schools were planning walk-outs that might be potential targets.
Further investigation revealed that Mr. Zhan had been a student at Northeastern University in Boston in 2015, where he had asked a teacher how to get guns. The teacher emailed his supervisor, but the university police found no concerns.
Meanwhile, the police obtained a subpoena to get Mr. Zhan’s mental health records from Syracuse University. Mr. Zhan had sought psychiatric care at two facilities, Northeastern University in 2015 and Syracuse University in 2018. His mental health records revealed alcohol abuse, depression, suicidal thinking, anger problems, feelings of isolation and withdrawal, and his feeling that he might lose control or act violently, said Dr. Knoll, who is also professor of psychiatry at the university.
On March 13, the day after he had attempted to buy the gun, Syracuse University’s mental health services were contacted and briefed on Mr. Zhan. They filled out the paperwork for New York’s SAFE Act, which prevents people from buying a gun if a mental health professional makes the reasonable judgment that the individual might harm themselves or someone else.
The police investigation continued and found that Mr. Zhan had previously tried to buy an AR-15 at a Dick’s Sporting Goods store. He was denied because the SAFE Act prevents their sale.
Mr. Zhan, meanwhile, had gone to Mexico for the break and was due to return March 19. While he was away, an alarm allegedly went off in his apartment on March 16, leading the landlord to check on the apartment since he remembered previous police inquiries. He knocked on the door but there was no answer, so the landlord entered to do a safety check. He found ammunition and other concerning supplies.
The same day the landlord was checking Mr. Zhan’s apartment, students traveling with him in Mexico emailed Syracuse University about concerning behavior they observed in him. This behavior included signs of severe depression, verbalizing extremely negative thoughts, discussing suicide, drinking heavily, and making cuts to his forearms with the knife he possessed.
They also shared screenshots of messages they had seen him post in a social media group about feeling compelled to buy a gun and bulletproof vest and practice shooting.
Three days later, the police obtained a search warrant for Mr. Zhan’s apartment and vehicle. They found in his apartment high-powered optics, scopes, ammunition, targets from shooting ranges, receipts from shooting ranges, and similar equipment.
Ultimately, authorities revoked Mr. Zhan’s visa, enabling them to detain him at the airport when he returned from Mexico and deport him back to China.
After Mr. Zhan had returned to China, further investigation uncovered a series of texts between Mr. Zhan and his girlfriend in which he openly talks about wanting to shoot people.
“So, what went right here instead of what went wrong?” Dr. Knoll rhetorically asked. A lot of things: leakage of Mr. Zhan’s plans; fellow students seeing and reporting his electronic messages and concerning behaviors; the gun store owner’s skepticism and contact with the police; the landlord’s check on Mr. Zhan’s apartment; and the cooperation among local police, school authorities, and the school’s mental health services.
“There was also good communication among the threat assessment teams and law enforcement and the collaboration across disciplines,” Dr. Knoll said. Mass shootings have now “taken on more of a sociocultural phenomenon,” and “sociocultural problems require sociocultural solutions. I like these laws focusing on behaviors, not psychiatric diagnoses.”
He then reviewed potential interventions that might help identify or interfere with a planned incident or intent to commit one, including increased attention paid to suspicious behavior, third-party reporting of a potential shooter’s intent, and suicide prevention programs.
Dr. Knoll shared recent FBI research on 63 active shooters between 2000 and 2013 showing that the majority (77%) had been planning their attack for at least 1 week. Further, 46% have been preparing for 1 week before. The majority of those likely shooters also obtained their guns legally.
Although a quarter of those in the FBI study had some mental health diagnosis – predominantly depression or anxiety – the agency uncovered no significant correlation between mental illness and becoming a shooter.
The study concluded that,“absent specific evidence, careful consideration should be given to social and contextual factors that might interact with any mental health issue before concluding that an active shooting was ‘caused’ by a mental illness. In short, declarations that all active shooters must simply be mentally ill are misleading and unhelpful.”
Dr. Knoll reported no conflicts of interest.
AUSTIN, TEX. – While the media relentlessly reports on every mass shooting that occurs, the public hears less often about the shootings that never happened – because people were paying attention and taking action, according to James L. Knoll, IV, MD, director of forensic psychiatry at the State University of New York, Syracuse.
“We’ve learned a lot about risk factors [for mass shootings], we’ve learned a lot about associations and correlations, and it’s gotten us so far,” Dr. Knoll told attendees at the annual meeting of the American Academy of Psychiatry and the Law. “I want to invite you to look at this from the angle of those shootings that were able to be prevented or disrupted.” (Dr. Knoll said he used the term “disrupted” because it’s impossible to ever know for certain that a shooting was thwarted.)
It is difficult to track mass homicides that would have occurred but were disrupted, but one study Dr. Knoll cited combed through news reports and identified 57 interrupted mass homicides (Aggress Viol Behav. 2016 Sep-Oct;30:88-93). Most of those (77%) had been interrupted by family and friends or the general public reporting suspicious behavior.
It was while Dr. Knoll was leading the threat assessment subcommittee of the Syracuse School Safety Task Force that a potential school shooting threat arose.
A 22-year-old Chinese international student named Xiaofeng “Lincoln” Zhan walked into AJ’s Archery/The Gun Shop on March 12, asking to buy an AR-15. The AR-15 is the semiautomatic weapon of choice for most mass shooters.
Mr. Zhan should have been barred from purchasing a gun because he was an international student on a temporary visa. Under U.S. code, it is “unlawful for any person to sell or otherwise dispose of any firearm or ammunition to any person knowing or having reasonable cause to believe that such person” is an alien who is “illegally or unlawfully in the United States” or “ has been admitted to the United States under a nonimmigrant visa.”
But the second provision was subject to certain exceptions, the first of which was that the person had been “admitted to the United States for lawful hunting or supporting purposes“ or was “in possession of a hunting license or permit lawfully issued in the United States.”
Mr. Zhan had a hunting license. He had taken a hunter safety course on March 11, the day before he entered the gun shop, and then bought a hunting license.
But the gun shop owner was not so easily persuaded. Mr. Zhan asked about “high-capacity shotguns” and said he belonged to a shooting club, yet he did not appear familiar with firearms. The gun shop owner was also skeptical because it didn’t make sense to use a high-capacity shotgun for hunting, and Mr. Zhan had just gotten his hunting license and didn’t know how to use the gun. Further, Mr. Zhan claimed that Syracuse University offered a class on how to use the gun – but the gun store owner knew that the university did not offer such a class.
The gun shop owner’s first thought was not that Mr. Zhan was a potential mass shooter but that he was a “secret shopper,” which Dr. Knoll defined as an undercover law enforcement officer who attempts to buy guns in a manner that should arouse suspicion in the store owner.
Ultimately, Zhan’s behavior was concerning and he made the owner feel uncomfortable. The owner captured Mr. Zhan’s information on U.S. ATF form 4473 and recorded his license plate. Then the gun shop owner contacted the Madison County Sheriff’s Office with the information.
The police opened an investigation that established that Mr. Zhan was a student enrolled at Syracuse University, which was on spring break at the time. The Syracuse Police Department arranged a joint meeting between the Onondaga County district attorney, Syracuse University Department of Public Safety, Onondaga County Sheriff’s Office, and the FBI to present their findings, including the fact that local high schools were planning walk-outs that might be potential targets.
Further investigation revealed that Mr. Zhan had been a student at Northeastern University in Boston in 2015, where he had asked a teacher how to get guns. The teacher emailed his supervisor, but the university police found no concerns.
Meanwhile, the police obtained a subpoena to get Mr. Zhan’s mental health records from Syracuse University. Mr. Zhan had sought psychiatric care at two facilities, Northeastern University in 2015 and Syracuse University in 2018. His mental health records revealed alcohol abuse, depression, suicidal thinking, anger problems, feelings of isolation and withdrawal, and his feeling that he might lose control or act violently, said Dr. Knoll, who is also professor of psychiatry at the university.
On March 13, the day after he had attempted to buy the gun, Syracuse University’s mental health services were contacted and briefed on Mr. Zhan. They filled out the paperwork for New York’s SAFE Act, which prevents people from buying a gun if a mental health professional makes the reasonable judgment that the individual might harm themselves or someone else.
The police investigation continued and found that Mr. Zhan had previously tried to buy an AR-15 at a Dick’s Sporting Goods store. He was denied because the SAFE Act prevents their sale.
Mr. Zhan, meanwhile, had gone to Mexico for the break and was due to return March 19. While he was away, an alarm allegedly went off in his apartment on March 16, leading the landlord to check on the apartment since he remembered previous police inquiries. He knocked on the door but there was no answer, so the landlord entered to do a safety check. He found ammunition and other concerning supplies.
The same day the landlord was checking Mr. Zhan’s apartment, students traveling with him in Mexico emailed Syracuse University about concerning behavior they observed in him. This behavior included signs of severe depression, verbalizing extremely negative thoughts, discussing suicide, drinking heavily, and making cuts to his forearms with the knife he possessed.
They also shared screenshots of messages they had seen him post in a social media group about feeling compelled to buy a gun and bulletproof vest and practice shooting.
Three days later, the police obtained a search warrant for Mr. Zhan’s apartment and vehicle. They found in his apartment high-powered optics, scopes, ammunition, targets from shooting ranges, receipts from shooting ranges, and similar equipment.
Ultimately, authorities revoked Mr. Zhan’s visa, enabling them to detain him at the airport when he returned from Mexico and deport him back to China.
After Mr. Zhan had returned to China, further investigation uncovered a series of texts between Mr. Zhan and his girlfriend in which he openly talks about wanting to shoot people.
“So, what went right here instead of what went wrong?” Dr. Knoll rhetorically asked. A lot of things: leakage of Mr. Zhan’s plans; fellow students seeing and reporting his electronic messages and concerning behaviors; the gun store owner’s skepticism and contact with the police; the landlord’s check on Mr. Zhan’s apartment; and the cooperation among local police, school authorities, and the school’s mental health services.
“There was also good communication among the threat assessment teams and law enforcement and the collaboration across disciplines,” Dr. Knoll said. Mass shootings have now “taken on more of a sociocultural phenomenon,” and “sociocultural problems require sociocultural solutions. I like these laws focusing on behaviors, not psychiatric diagnoses.”
He then reviewed potential interventions that might help identify or interfere with a planned incident or intent to commit one, including increased attention paid to suspicious behavior, third-party reporting of a potential shooter’s intent, and suicide prevention programs.
Dr. Knoll shared recent FBI research on 63 active shooters between 2000 and 2013 showing that the majority (77%) had been planning their attack for at least 1 week. Further, 46% have been preparing for 1 week before. The majority of those likely shooters also obtained their guns legally.
Although a quarter of those in the FBI study had some mental health diagnosis – predominantly depression or anxiety – the agency uncovered no significant correlation between mental illness and becoming a shooter.
The study concluded that,“absent specific evidence, careful consideration should be given to social and contextual factors that might interact with any mental health issue before concluding that an active shooting was ‘caused’ by a mental illness. In short, declarations that all active shooters must simply be mentally ill are misleading and unhelpful.”
Dr. Knoll reported no conflicts of interest.
EXPERT ANALYSIS FROM THE AAPL ANNUAL MEETING
Rate of STIs is rising, and many U.S. teens are sexually active
ORLANDO – Consider point-of-care testing and treat potentially infected partners when diagnosing and treating adolescents for STIs, Diane M. Straub, MD, MPH, said at the annual meeting of the American Academy of Pediatrics.
In addition, adolescents are sometimes reluctant to disclose their full sexual history to their health care provider, which can complicate diagnosis and treatment, noted Dr. Straub, professor of pediatrics at the University of South Florida, Tampa. “That sometimes takes a few questions,” but can be achieved by asking the same questions in different ways and emphasizing the clinical importance of testing.
According to the 2017 Youth Risk Behavior Surveillance survey, 40% of adolescents reported ever having sexual intercourse, with 20% of 9th-grade, 36% of 10th-grade, 47% of 11th-grade, and 57% of 12th-grade students reporting they had sexual intercourse. By gender, 41% of adolescent males and 38% of adolescent females reported ever having sexual intercourse; by race, 39% of white, 41% of Hispanic, and 46% of black participants reported any sexual activity. Overall, 10% of adolescents said they had four or more partners, 3% said they had intercourse before age 13 years, 54% used a condom the last time they had intercourse, and 7% said they were raped.
The rate of STIs in the United States is rising. There has been a sharp increase in the number of combined diagnoses of gonorrhea, syphilis, and chlamydia, with an increase from 1.8 million in 2013 to 2.3 million cases in 2017, according to the Centers for Disease Control and Prevention. During that same time period, gonorrhea increased 67% from 333,004 to 555,608 cases, syphilis (primary and secondary) rose 76% from 17,375 to 30,644 cases, and chlamydia increased 22% to 1.7 million cases.
According to a 2013 CDC infographic shown by Dr. Straub, young people in the United States aged 15-24 years old represent 27% of the total sexually active population but account for 50% of new STI cases each year. Persons in this population account for 70% of gonorrhea cases, 63% of chlamydia cases, 49% of human papillomavirus (HPV) cases, 45% of genital herpes cases, and 20% of syphilis cases.
All sexually active females aged 25 years or younger should be screened for chlamydia and gonorrhea, as well as “at-risk” young men who have sex with men (YMSM), Dr. Straub said. All adolescent males and females aged over 13 years should be offered HIV screening, and HIV screening should be discussed “at least once.” And depending on how at risk each subpopulation is, health care providers should be have that conversation and offer screening multiple times.
Women who have sex with women (WSW) are a diverse population and should be treated based on their individual sexual identities, behaviors, and practices. “Most self-identified WSWs report having sex with men, so therefore adolescent WSWs and females with both male and female sex partners might be at increased risk for STIs, such as syphillis, chlamydia, and HPV as well as HIV, so you may want to adjust your screening accordingly,” she said.
Pregnant women, if at risk, should be screened for HIV, syphilis, hepatitis B, gonorrhea, and chlamydia.
YMSM should have annual screenings for syphilis and HIV, screenings for chlamydia and gonorrhea by infection site; also consider herpes simplex virus serology and anal cytology in these patients, Dr. Straub said. They also should be screened for hepatitis B surface antigen, vaccinated for hepatitis A, hepatitis B and, if using drugs, screened* for hepatitis C virus.
Dr. Straub recommended licensed health care professionals who may treat minor patients review their state’s laws on minors and their legal ability to consent to treatment of STIs without the involvement of their parent or guardian, including disclosure of positive results and in the case of HIV care.
In places where index insured are allowed to find out about any services a beneficiary receives on their insurance, “this is a little problematic, because in some states, this is in direct conflict with the explanation of benefits requirement,” she said. “There are certain ways to get around that, but it’s really important for you to know what the statutes are where you’re practicing and where the breaches of confidentiality [are].”
Expedited partner therapy, or treating one or multiple partners of patients with an STI, is recommended for certain patients and infections, such as male partners of female patients with chlamydia and gonorrhea. While this is recommended less for YMSM because of a higher rate of concurrent infection, “if you have a young person who has partners who are unlikely to have access to care and get treated, it’s recommended you give that treatment to your index patient and to then treat their partners,” Dr. Straub said.
A recent and frequently updated resource on STI treatment can be found at the CDC website.
Dr. Straub reported no relevant conflicts of interest.
*This article was updated 1/11/19.
ORLANDO – Consider point-of-care testing and treat potentially infected partners when diagnosing and treating adolescents for STIs, Diane M. Straub, MD, MPH, said at the annual meeting of the American Academy of Pediatrics.
In addition, adolescents are sometimes reluctant to disclose their full sexual history to their health care provider, which can complicate diagnosis and treatment, noted Dr. Straub, professor of pediatrics at the University of South Florida, Tampa. “That sometimes takes a few questions,” but can be achieved by asking the same questions in different ways and emphasizing the clinical importance of testing.
According to the 2017 Youth Risk Behavior Surveillance survey, 40% of adolescents reported ever having sexual intercourse, with 20% of 9th-grade, 36% of 10th-grade, 47% of 11th-grade, and 57% of 12th-grade students reporting they had sexual intercourse. By gender, 41% of adolescent males and 38% of adolescent females reported ever having sexual intercourse; by race, 39% of white, 41% of Hispanic, and 46% of black participants reported any sexual activity. Overall, 10% of adolescents said they had four or more partners, 3% said they had intercourse before age 13 years, 54% used a condom the last time they had intercourse, and 7% said they were raped.
The rate of STIs in the United States is rising. There has been a sharp increase in the number of combined diagnoses of gonorrhea, syphilis, and chlamydia, with an increase from 1.8 million in 2013 to 2.3 million cases in 2017, according to the Centers for Disease Control and Prevention. During that same time period, gonorrhea increased 67% from 333,004 to 555,608 cases, syphilis (primary and secondary) rose 76% from 17,375 to 30,644 cases, and chlamydia increased 22% to 1.7 million cases.
According to a 2013 CDC infographic shown by Dr. Straub, young people in the United States aged 15-24 years old represent 27% of the total sexually active population but account for 50% of new STI cases each year. Persons in this population account for 70% of gonorrhea cases, 63% of chlamydia cases, 49% of human papillomavirus (HPV) cases, 45% of genital herpes cases, and 20% of syphilis cases.
All sexually active females aged 25 years or younger should be screened for chlamydia and gonorrhea, as well as “at-risk” young men who have sex with men (YMSM), Dr. Straub said. All adolescent males and females aged over 13 years should be offered HIV screening, and HIV screening should be discussed “at least once.” And depending on how at risk each subpopulation is, health care providers should be have that conversation and offer screening multiple times.
Women who have sex with women (WSW) are a diverse population and should be treated based on their individual sexual identities, behaviors, and practices. “Most self-identified WSWs report having sex with men, so therefore adolescent WSWs and females with both male and female sex partners might be at increased risk for STIs, such as syphillis, chlamydia, and HPV as well as HIV, so you may want to adjust your screening accordingly,” she said.
Pregnant women, if at risk, should be screened for HIV, syphilis, hepatitis B, gonorrhea, and chlamydia.
YMSM should have annual screenings for syphilis and HIV, screenings for chlamydia and gonorrhea by infection site; also consider herpes simplex virus serology and anal cytology in these patients, Dr. Straub said. They also should be screened for hepatitis B surface antigen, vaccinated for hepatitis A, hepatitis B and, if using drugs, screened* for hepatitis C virus.
Dr. Straub recommended licensed health care professionals who may treat minor patients review their state’s laws on minors and their legal ability to consent to treatment of STIs without the involvement of their parent or guardian, including disclosure of positive results and in the case of HIV care.
In places where index insured are allowed to find out about any services a beneficiary receives on their insurance, “this is a little problematic, because in some states, this is in direct conflict with the explanation of benefits requirement,” she said. “There are certain ways to get around that, but it’s really important for you to know what the statutes are where you’re practicing and where the breaches of confidentiality [are].”
Expedited partner therapy, or treating one or multiple partners of patients with an STI, is recommended for certain patients and infections, such as male partners of female patients with chlamydia and gonorrhea. While this is recommended less for YMSM because of a higher rate of concurrent infection, “if you have a young person who has partners who are unlikely to have access to care and get treated, it’s recommended you give that treatment to your index patient and to then treat their partners,” Dr. Straub said.
A recent and frequently updated resource on STI treatment can be found at the CDC website.
Dr. Straub reported no relevant conflicts of interest.
*This article was updated 1/11/19.
ORLANDO – Consider point-of-care testing and treat potentially infected partners when diagnosing and treating adolescents for STIs, Diane M. Straub, MD, MPH, said at the annual meeting of the American Academy of Pediatrics.
In addition, adolescents are sometimes reluctant to disclose their full sexual history to their health care provider, which can complicate diagnosis and treatment, noted Dr. Straub, professor of pediatrics at the University of South Florida, Tampa. “That sometimes takes a few questions,” but can be achieved by asking the same questions in different ways and emphasizing the clinical importance of testing.
According to the 2017 Youth Risk Behavior Surveillance survey, 40% of adolescents reported ever having sexual intercourse, with 20% of 9th-grade, 36% of 10th-grade, 47% of 11th-grade, and 57% of 12th-grade students reporting they had sexual intercourse. By gender, 41% of adolescent males and 38% of adolescent females reported ever having sexual intercourse; by race, 39% of white, 41% of Hispanic, and 46% of black participants reported any sexual activity. Overall, 10% of adolescents said they had four or more partners, 3% said they had intercourse before age 13 years, 54% used a condom the last time they had intercourse, and 7% said they were raped.
The rate of STIs in the United States is rising. There has been a sharp increase in the number of combined diagnoses of gonorrhea, syphilis, and chlamydia, with an increase from 1.8 million in 2013 to 2.3 million cases in 2017, according to the Centers for Disease Control and Prevention. During that same time period, gonorrhea increased 67% from 333,004 to 555,608 cases, syphilis (primary and secondary) rose 76% from 17,375 to 30,644 cases, and chlamydia increased 22% to 1.7 million cases.
According to a 2013 CDC infographic shown by Dr. Straub, young people in the United States aged 15-24 years old represent 27% of the total sexually active population but account for 50% of new STI cases each year. Persons in this population account for 70% of gonorrhea cases, 63% of chlamydia cases, 49% of human papillomavirus (HPV) cases, 45% of genital herpes cases, and 20% of syphilis cases.
All sexually active females aged 25 years or younger should be screened for chlamydia and gonorrhea, as well as “at-risk” young men who have sex with men (YMSM), Dr. Straub said. All adolescent males and females aged over 13 years should be offered HIV screening, and HIV screening should be discussed “at least once.” And depending on how at risk each subpopulation is, health care providers should be have that conversation and offer screening multiple times.
Women who have sex with women (WSW) are a diverse population and should be treated based on their individual sexual identities, behaviors, and practices. “Most self-identified WSWs report having sex with men, so therefore adolescent WSWs and females with both male and female sex partners might be at increased risk for STIs, such as syphillis, chlamydia, and HPV as well as HIV, so you may want to adjust your screening accordingly,” she said.
Pregnant women, if at risk, should be screened for HIV, syphilis, hepatitis B, gonorrhea, and chlamydia.
YMSM should have annual screenings for syphilis and HIV, screenings for chlamydia and gonorrhea by infection site; also consider herpes simplex virus serology and anal cytology in these patients, Dr. Straub said. They also should be screened for hepatitis B surface antigen, vaccinated for hepatitis A, hepatitis B and, if using drugs, screened* for hepatitis C virus.
Dr. Straub recommended licensed health care professionals who may treat minor patients review their state’s laws on minors and their legal ability to consent to treatment of STIs without the involvement of their parent or guardian, including disclosure of positive results and in the case of HIV care.
In places where index insured are allowed to find out about any services a beneficiary receives on their insurance, “this is a little problematic, because in some states, this is in direct conflict with the explanation of benefits requirement,” she said. “There are certain ways to get around that, but it’s really important for you to know what the statutes are where you’re practicing and where the breaches of confidentiality [are].”
Expedited partner therapy, or treating one or multiple partners of patients with an STI, is recommended for certain patients and infections, such as male partners of female patients with chlamydia and gonorrhea. While this is recommended less for YMSM because of a higher rate of concurrent infection, “if you have a young person who has partners who are unlikely to have access to care and get treated, it’s recommended you give that treatment to your index patient and to then treat their partners,” Dr. Straub said.
A recent and frequently updated resource on STI treatment can be found at the CDC website.
Dr. Straub reported no relevant conflicts of interest.
*This article was updated 1/11/19.
EXPERT ANALYSIS FROM AAP 18