User login
FDA clears portable hematology analyzer
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for PixCell Medical’s HemoScreen™.
This portable hematology analyzer is used to perform a complete blood count at the point of care.
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100. This study was published in the Journal of Clinical Pathology in 2016.
“The HemoScreen delivers lab-accurate results,” said Avishay Bransky, PhD, chief executive officer of PixCell Medical.
He added that HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for PixCell Medical’s HemoScreen™.
This portable hematology analyzer is used to perform a complete blood count at the point of care.
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100. This study was published in the Journal of Clinical Pathology in 2016.
“The HemoScreen delivers lab-accurate results,” said Avishay Bransky, PhD, chief executive officer of PixCell Medical.
He added that HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for PixCell Medical’s HemoScreen™.
This portable hematology analyzer is used to perform a complete blood count at the point of care.
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100. This study was published in the Journal of Clinical Pathology in 2016.
“The HemoScreen delivers lab-accurate results,” said Avishay Bransky, PhD, chief executive officer of PixCell Medical.
He added that HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
Selinexor on fast track for DLBCL
The U.S. Food and Drug Administration (FDA) has granted fast track designation to selinexor for the treatment of diffuse large B-cell lymphoma (DLBCL).
The designation is for selinexor to treat DLBCL patients who have received at least two prior therapies and are not eligible for high-dose chemotherapy with stem cell rescue or chimeric antigen receptor T-cell therapy.
Selinexor is being studied in the phase 2b SADAL trial (NCT02227251), which is enrolling patients with relapsed or refractory DLBCL who have received two to five prior therapies and are not eligible for stem cell transplant.
Top-line results from this trial are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 1677).
Selinexor is an oral selective inhibitor of nuclear export (SINE) compound being developed by Karyopharm Therapeutics Inc.
The company previously received fast track designation for selinexor to treat patients with penta-refractory multiple myeloma who have received at least three prior lines of therapy.
The FDA says its fast track program is designed to facilitate the development and expedite the review of products that are intended to treat serious conditions and have the potential to address unmet medical needs.
Fast track designation provides developers with greater access to the FDA as well as eligibility for accelerated approval, priority review, and rolling review.
“The receipt of fast track designation from the FDA for selinexor in relapsed DLBCL underscores the great unmet medical need for this aggressive form of lymphoma,” said Sharon Shacham, PhD, founder, president, and chief scientific officer of Karyopharm.
“Pending positive results from the phase 2b SADAL study, we plan to submit a second NDA [new drug application] to the FDA in the first half of 2019, with a request for accelerated approval, for oral selinexor as a potential new treatment for patients with relapsed or refractory DLBCL.”
Last month, the FDA accepted a new drug application for selinexor as a treatment for penta-refractory multiple myeloma. The agency granted the application priority review and set an action date of April 6, 2019.
The U.S. Food and Drug Administration (FDA) has granted fast track designation to selinexor for the treatment of diffuse large B-cell lymphoma (DLBCL).
The designation is for selinexor to treat DLBCL patients who have received at least two prior therapies and are not eligible for high-dose chemotherapy with stem cell rescue or chimeric antigen receptor T-cell therapy.
Selinexor is being studied in the phase 2b SADAL trial (NCT02227251), which is enrolling patients with relapsed or refractory DLBCL who have received two to five prior therapies and are not eligible for stem cell transplant.
Top-line results from this trial are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 1677).
Selinexor is an oral selective inhibitor of nuclear export (SINE) compound being developed by Karyopharm Therapeutics Inc.
The company previously received fast track designation for selinexor to treat patients with penta-refractory multiple myeloma who have received at least three prior lines of therapy.
The FDA says its fast track program is designed to facilitate the development and expedite the review of products that are intended to treat serious conditions and have the potential to address unmet medical needs.
Fast track designation provides developers with greater access to the FDA as well as eligibility for accelerated approval, priority review, and rolling review.
“The receipt of fast track designation from the FDA for selinexor in relapsed DLBCL underscores the great unmet medical need for this aggressive form of lymphoma,” said Sharon Shacham, PhD, founder, president, and chief scientific officer of Karyopharm.
“Pending positive results from the phase 2b SADAL study, we plan to submit a second NDA [new drug application] to the FDA in the first half of 2019, with a request for accelerated approval, for oral selinexor as a potential new treatment for patients with relapsed or refractory DLBCL.”
Last month, the FDA accepted a new drug application for selinexor as a treatment for penta-refractory multiple myeloma. The agency granted the application priority review and set an action date of April 6, 2019.
The U.S. Food and Drug Administration (FDA) has granted fast track designation to selinexor for the treatment of diffuse large B-cell lymphoma (DLBCL).
The designation is for selinexor to treat DLBCL patients who have received at least two prior therapies and are not eligible for high-dose chemotherapy with stem cell rescue or chimeric antigen receptor T-cell therapy.
Selinexor is being studied in the phase 2b SADAL trial (NCT02227251), which is enrolling patients with relapsed or refractory DLBCL who have received two to five prior therapies and are not eligible for stem cell transplant.
Top-line results from this trial are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 1677).
Selinexor is an oral selective inhibitor of nuclear export (SINE) compound being developed by Karyopharm Therapeutics Inc.
The company previously received fast track designation for selinexor to treat patients with penta-refractory multiple myeloma who have received at least three prior lines of therapy.
The FDA says its fast track program is designed to facilitate the development and expedite the review of products that are intended to treat serious conditions and have the potential to address unmet medical needs.
Fast track designation provides developers with greater access to the FDA as well as eligibility for accelerated approval, priority review, and rolling review.
“The receipt of fast track designation from the FDA for selinexor in relapsed DLBCL underscores the great unmet medical need for this aggressive form of lymphoma,” said Sharon Shacham, PhD, founder, president, and chief scientific officer of Karyopharm.
“Pending positive results from the phase 2b SADAL study, we plan to submit a second NDA [new drug application] to the FDA in the first half of 2019, with a request for accelerated approval, for oral selinexor as a potential new treatment for patients with relapsed or refractory DLBCL.”
Last month, the FDA accepted a new drug application for selinexor as a treatment for penta-refractory multiple myeloma. The agency granted the application priority review and set an action date of April 6, 2019.
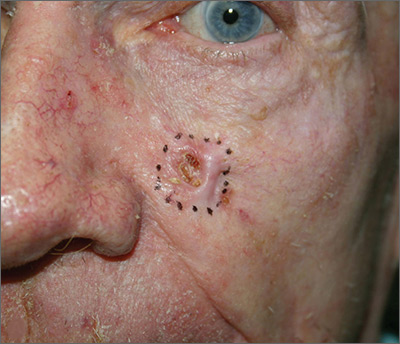
Growing lesion on cheek
Figure 1
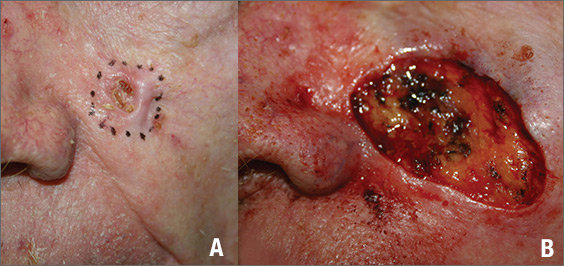
The FP suspected that this was a basal cell carcinoma (BCC) or squamous cell carcinoma. He leaned toward a BCC because of the pearly border on the edge, but knew that a biopsy diagnosis was needed before planning definitive treatment.
The FP recommended performing a shave biopsy that day. (See the Watch & Learn video on “Shave biopsy.”) After obtaining patient consent, he injected 1% lidocaine with epinephrine and waited for the epinephrine to work. He performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. The bleeding stopped, and the FP dressed the lesion with petrolatum and some gauze. Dermatopathology revealed a sclerosing BCC.
The FP realized this was an aggressive tumor and referred the patient for Mohs surgery. The surgery required 4 excisions to get clean margins (FIGURE 1B). The usual 4- to 5-mm margins with an elliptical excision would not have removed the full tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

The FP suspected that this was a basal cell carcinoma (BCC) or squamous cell carcinoma. He leaned toward a BCC because of the pearly border on the edge, but knew that a biopsy diagnosis was needed before planning definitive treatment.
The FP recommended performing a shave biopsy that day. (See the Watch & Learn video on “Shave biopsy.”) After obtaining patient consent, he injected 1% lidocaine with epinephrine and waited for the epinephrine to work. He performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. The bleeding stopped, and the FP dressed the lesion with petrolatum and some gauze. Dermatopathology revealed a sclerosing BCC.
The FP realized this was an aggressive tumor and referred the patient for Mohs surgery. The surgery required 4 excisions to get clean margins (FIGURE 1B). The usual 4- to 5-mm margins with an elliptical excision would not have removed the full tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Figure 1

The FP suspected that this was a basal cell carcinoma (BCC) or squamous cell carcinoma. He leaned toward a BCC because of the pearly border on the edge, but knew that a biopsy diagnosis was needed before planning definitive treatment.
The FP recommended performing a shave biopsy that day. (See the Watch & Learn video on “Shave biopsy.”) After obtaining patient consent, he injected 1% lidocaine with epinephrine and waited for the epinephrine to work. He performed the shave biopsy with a Dermablade, and used a cotton-tipped applicator to vigorously apply aluminum chloride to the site. He used a twisting motion and pressure to achieve hemostasis. The bleeding stopped, and the FP dressed the lesion with petrolatum and some gauze. Dermatopathology revealed a sclerosing BCC.
The FP realized this was an aggressive tumor and referred the patient for Mohs surgery. The surgery required 4 excisions to get clean margins (FIGURE 1B). The usual 4- to 5-mm margins with an elliptical excision would not have removed the full tumor.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
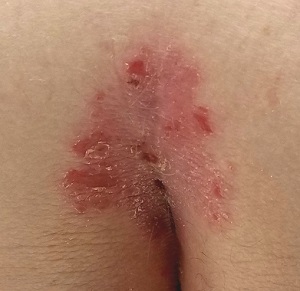
A Nuisance for the Newlyweds
Prompted by his new bride, who is concerned she might “catch something” from him, a 53-year-old man self-refers for evaluation of a slightly itchy intergluteal rash. He’s had it for years; it waxes and wanes but never fully resolves.
It has been previously diagnosed as a yeast infection, fungal infection, and even herpes. But none of the respective treatments have helped.
More history-taking reveals a family history of psoriasis (maternal grandmother), but the patient denies other areas of involvement or other skin changes. He also denies having arthritis.
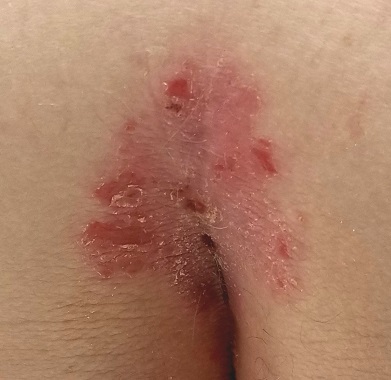
EXAMINATION
A salmon-pink, 7-cm, roughly round dry patch covered by white tenacious scale is located in the upper intergluteal/sacral interface. There is no increased warmth or tenderness on palpation.
A similar process is noted in the periumbilical area (a difficult area for this patient to see, due to his weight). Inspection of his fingernails reveals 3/10 with definite tiny pits.
What is the diagnosis?
DISCUSSION
The urge to call any and every rash occurring near the genitals a yeast infection is universally compelling among primary care providers. It is often so reflexive that even when anti-yeast medications fail, the provider hangs onto the diagnosis. This happens for one simple reason: Their differential is lacking.
This patient has psoriasis, albeit a somewhat unusual form, which demonstrates an important learning point: Psoriasis can present in any number of ways, not just in the standard “extensor surfaces of elbows and knees” distribution. It’s not unusual for psoriasis to zero in on one or two areas. I’ve seen it confined to the groin, the genitals, and the scalp. It can even involve the oral mucosa.
In these somewhat obscure cases, additional findings can be helpful to establish the diagnosis. The two areas of involvement in this case—the upper intergluteal area and the periumbilical region—may not fit the classic “knees and elbows” picture of psoriasis, but they are not atypical for the disease. Add the nail pits, the fixed nature of the problem, and the family history, and you’ve nailed the diagnosis.
Don’t forget that, occasionally, psoriatic arthropathy can precede the appearance of psoriasis, and that the severity of one does not predict the severity of the other.
Finally, in a fair number of cases, the diagnosis of psoriasis must be made by biopsy, which shows characteristic changes such as parakeratosis, epidermal thickening, and fusing of rete ridges. These “psoriasiform” changes seen microscopically must be corroborated by clinical findings, though, since many other papulosquamous diseases can exhibit similar changes.
TAKE-HOME LEARNING POINTS
- Psoriasis is one of the more common dermatoses in this country, which means you will see it with some frequency.
- Psoriasis can affect limited or atypical areas, but corroboration of the diagnosis can be sought in classic areas (nails, scalp, upper intergluteal and periumbilical areas).
- Strive to develop alternative diagnoses for similar rashes—in other words, build your differential for “yeast infection.”
Prompted by his new bride, who is concerned she might “catch something” from him, a 53-year-old man self-refers for evaluation of a slightly itchy intergluteal rash. He’s had it for years; it waxes and wanes but never fully resolves.
It has been previously diagnosed as a yeast infection, fungal infection, and even herpes. But none of the respective treatments have helped.
More history-taking reveals a family history of psoriasis (maternal grandmother), but the patient denies other areas of involvement or other skin changes. He also denies having arthritis.

EXAMINATION
A salmon-pink, 7-cm, roughly round dry patch covered by white tenacious scale is located in the upper intergluteal/sacral interface. There is no increased warmth or tenderness on palpation.
A similar process is noted in the periumbilical area (a difficult area for this patient to see, due to his weight). Inspection of his fingernails reveals 3/10 with definite tiny pits.
What is the diagnosis?
DISCUSSION
The urge to call any and every rash occurring near the genitals a yeast infection is universally compelling among primary care providers. It is often so reflexive that even when anti-yeast medications fail, the provider hangs onto the diagnosis. This happens for one simple reason: Their differential is lacking.
This patient has psoriasis, albeit a somewhat unusual form, which demonstrates an important learning point: Psoriasis can present in any number of ways, not just in the standard “extensor surfaces of elbows and knees” distribution. It’s not unusual for psoriasis to zero in on one or two areas. I’ve seen it confined to the groin, the genitals, and the scalp. It can even involve the oral mucosa.
In these somewhat obscure cases, additional findings can be helpful to establish the diagnosis. The two areas of involvement in this case—the upper intergluteal area and the periumbilical region—may not fit the classic “knees and elbows” picture of psoriasis, but they are not atypical for the disease. Add the nail pits, the fixed nature of the problem, and the family history, and you’ve nailed the diagnosis.
Don’t forget that, occasionally, psoriatic arthropathy can precede the appearance of psoriasis, and that the severity of one does not predict the severity of the other.
Finally, in a fair number of cases, the diagnosis of psoriasis must be made by biopsy, which shows characteristic changes such as parakeratosis, epidermal thickening, and fusing of rete ridges. These “psoriasiform” changes seen microscopically must be corroborated by clinical findings, though, since many other papulosquamous diseases can exhibit similar changes.
TAKE-HOME LEARNING POINTS
- Psoriasis is one of the more common dermatoses in this country, which means you will see it with some frequency.
- Psoriasis can affect limited or atypical areas, but corroboration of the diagnosis can be sought in classic areas (nails, scalp, upper intergluteal and periumbilical areas).
- Strive to develop alternative diagnoses for similar rashes—in other words, build your differential for “yeast infection.”
Prompted by his new bride, who is concerned she might “catch something” from him, a 53-year-old man self-refers for evaluation of a slightly itchy intergluteal rash. He’s had it for years; it waxes and wanes but never fully resolves.
It has been previously diagnosed as a yeast infection, fungal infection, and even herpes. But none of the respective treatments have helped.
More history-taking reveals a family history of psoriasis (maternal grandmother), but the patient denies other areas of involvement or other skin changes. He also denies having arthritis.

EXAMINATION
A salmon-pink, 7-cm, roughly round dry patch covered by white tenacious scale is located in the upper intergluteal/sacral interface. There is no increased warmth or tenderness on palpation.
A similar process is noted in the periumbilical area (a difficult area for this patient to see, due to his weight). Inspection of his fingernails reveals 3/10 with definite tiny pits.
What is the diagnosis?
DISCUSSION
The urge to call any and every rash occurring near the genitals a yeast infection is universally compelling among primary care providers. It is often so reflexive that even when anti-yeast medications fail, the provider hangs onto the diagnosis. This happens for one simple reason: Their differential is lacking.
This patient has psoriasis, albeit a somewhat unusual form, which demonstrates an important learning point: Psoriasis can present in any number of ways, not just in the standard “extensor surfaces of elbows and knees” distribution. It’s not unusual for psoriasis to zero in on one or two areas. I’ve seen it confined to the groin, the genitals, and the scalp. It can even involve the oral mucosa.
In these somewhat obscure cases, additional findings can be helpful to establish the diagnosis. The two areas of involvement in this case—the upper intergluteal area and the periumbilical region—may not fit the classic “knees and elbows” picture of psoriasis, but they are not atypical for the disease. Add the nail pits, the fixed nature of the problem, and the family history, and you’ve nailed the diagnosis.
Don’t forget that, occasionally, psoriatic arthropathy can precede the appearance of psoriasis, and that the severity of one does not predict the severity of the other.
Finally, in a fair number of cases, the diagnosis of psoriasis must be made by biopsy, which shows characteristic changes such as parakeratosis, epidermal thickening, and fusing of rete ridges. These “psoriasiform” changes seen microscopically must be corroborated by clinical findings, though, since many other papulosquamous diseases can exhibit similar changes.
TAKE-HOME LEARNING POINTS
- Psoriasis is one of the more common dermatoses in this country, which means you will see it with some frequency.
- Psoriasis can affect limited or atypical areas, but corroboration of the diagnosis can be sought in classic areas (nails, scalp, upper intergluteal and periumbilical areas).
- Strive to develop alternative diagnoses for similar rashes—in other words, build your differential for “yeast infection.”
The price we pay for trying to see more and more patients
In the October 2018 issue of Medscape Business of Medicine, the question was asked, “How can you practice quality medicine if you’re being asked to see patients every 15 minutes or less?”
I’m pretty sure the answer is, “you can’t.”
Yet, this is what many doctors are asked to do just to make ends meet. The majority of everyday medicine is, and always will be, a thinking game. It takes time to piece together the clues from a history and exam and decide what tests and/or treatment are the next step.
This ain’t easy. Even the shortest residencies require a combined 7 years of medical school and postgrad training. Experience and learning makes us all faster, but then the number of things that you can handle in 15 minutes is minimal. And that doesn’t even include the time needed to answer patient or family questions (which can be quite a lot) write up or transmit test orders or a prescription, and, inevitably, document the entire encounter in a meaningful way.
I don’t see patients at such a breakneck speed in my office, and yet I still end up doing most of my dictations after (or before) office hours.
In spite of lip service by politicians and administrators to correct the issue, medicine still continues to penalize those services that require thinking. And this task is the center of being a good doctor – and always has been.
Procedures are more lucrative, but imagine how my colleagues in neurosurgery would react if they were given a similar time limit on cases: A new patient has to be on the table every 15-30 minutes, and in that time you have to open, do the surgery, close, meet with family, and document the whole thing. Then get back in the OR (scrub, first) before the next case. Doesn’t matter whether you’re doing a lumbar fusion, glioma resection, or carotid endarterectomy. Those are the time limits. You get 30 minutes for lunch and to return calls. The administrator said so.
And this is where medicine continues to go. Overhead costs keep rising, and, for most docs, the only way they know to keep up is to keep cramming more patients into the day. Nobody wants to practice shoddy, hurried medicine, but neither do they want to lose their jobs to the next hungry graduate or close down a practice they spent years building.
I wish I had an answer. In fact, I think most of us do, but not one that will make patients, administrators, and doctors all happy. So the spiral continues.
And that isn’t good for patients, the people at the center of this job.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the October 2018 issue of Medscape Business of Medicine, the question was asked, “How can you practice quality medicine if you’re being asked to see patients every 15 minutes or less?”
I’m pretty sure the answer is, “you can’t.”
Yet, this is what many doctors are asked to do just to make ends meet. The majority of everyday medicine is, and always will be, a thinking game. It takes time to piece together the clues from a history and exam and decide what tests and/or treatment are the next step.
This ain’t easy. Even the shortest residencies require a combined 7 years of medical school and postgrad training. Experience and learning makes us all faster, but then the number of things that you can handle in 15 minutes is minimal. And that doesn’t even include the time needed to answer patient or family questions (which can be quite a lot) write up or transmit test orders or a prescription, and, inevitably, document the entire encounter in a meaningful way.
I don’t see patients at such a breakneck speed in my office, and yet I still end up doing most of my dictations after (or before) office hours.
In spite of lip service by politicians and administrators to correct the issue, medicine still continues to penalize those services that require thinking. And this task is the center of being a good doctor – and always has been.
Procedures are more lucrative, but imagine how my colleagues in neurosurgery would react if they were given a similar time limit on cases: A new patient has to be on the table every 15-30 minutes, and in that time you have to open, do the surgery, close, meet with family, and document the whole thing. Then get back in the OR (scrub, first) before the next case. Doesn’t matter whether you’re doing a lumbar fusion, glioma resection, or carotid endarterectomy. Those are the time limits. You get 30 minutes for lunch and to return calls. The administrator said so.
And this is where medicine continues to go. Overhead costs keep rising, and, for most docs, the only way they know to keep up is to keep cramming more patients into the day. Nobody wants to practice shoddy, hurried medicine, but neither do they want to lose their jobs to the next hungry graduate or close down a practice they spent years building.
I wish I had an answer. In fact, I think most of us do, but not one that will make patients, administrators, and doctors all happy. So the spiral continues.
And that isn’t good for patients, the people at the center of this job.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the October 2018 issue of Medscape Business of Medicine, the question was asked, “How can you practice quality medicine if you’re being asked to see patients every 15 minutes or less?”
I’m pretty sure the answer is, “you can’t.”
Yet, this is what many doctors are asked to do just to make ends meet. The majority of everyday medicine is, and always will be, a thinking game. It takes time to piece together the clues from a history and exam and decide what tests and/or treatment are the next step.
This ain’t easy. Even the shortest residencies require a combined 7 years of medical school and postgrad training. Experience and learning makes us all faster, but then the number of things that you can handle in 15 minutes is minimal. And that doesn’t even include the time needed to answer patient or family questions (which can be quite a lot) write up or transmit test orders or a prescription, and, inevitably, document the entire encounter in a meaningful way.
I don’t see patients at such a breakneck speed in my office, and yet I still end up doing most of my dictations after (or before) office hours.
In spite of lip service by politicians and administrators to correct the issue, medicine still continues to penalize those services that require thinking. And this task is the center of being a good doctor – and always has been.
Procedures are more lucrative, but imagine how my colleagues in neurosurgery would react if they were given a similar time limit on cases: A new patient has to be on the table every 15-30 minutes, and in that time you have to open, do the surgery, close, meet with family, and document the whole thing. Then get back in the OR (scrub, first) before the next case. Doesn’t matter whether you’re doing a lumbar fusion, glioma resection, or carotid endarterectomy. Those are the time limits. You get 30 minutes for lunch and to return calls. The administrator said so.
And this is where medicine continues to go. Overhead costs keep rising, and, for most docs, the only way they know to keep up is to keep cramming more patients into the day. Nobody wants to practice shoddy, hurried medicine, but neither do they want to lose their jobs to the next hungry graduate or close down a practice they spent years building.
I wish I had an answer. In fact, I think most of us do, but not one that will make patients, administrators, and doctors all happy. So the spiral continues.
And that isn’t good for patients, the people at the center of this job.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Open enrollment: Slow first week at HealthCare.gov
About 372,000 people selected a health insurance plan during the first week of Affordable Care Act open enrollment for 2019, according to the Centers for Medicare & Medicaid Services. The first week was short, with only 3 days to select coverage.
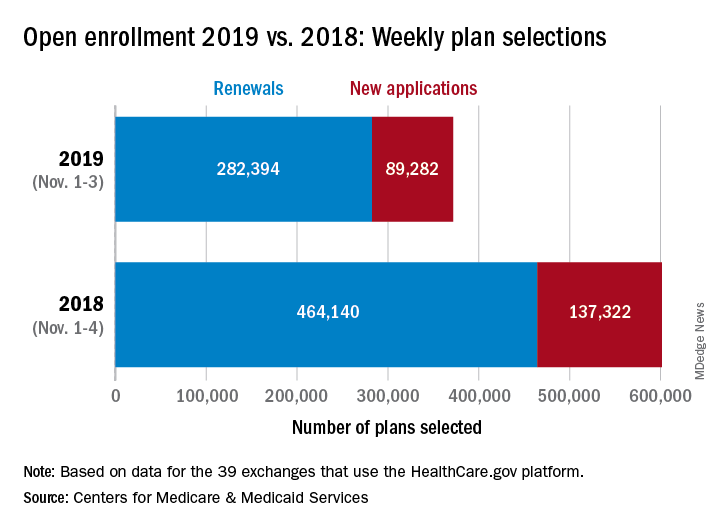
For Nov. 1-3, the exact number of plans selected was 371,676, which is about 38% less than last year’s first week, which was 4 days long, so the average number of plans selected per day was down by a little less than 18%, CMS data show.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS officials said, adding that the weekly data release “only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Open enrollment will continue for another 6 weeks, with Dec. 15 being the final day to enroll for 2019 coverage on the 39 state exchanges that use the HealthCare.gov platform.
About 372,000 people selected a health insurance plan during the first week of Affordable Care Act open enrollment for 2019, according to the Centers for Medicare & Medicaid Services. The first week was short, with only 3 days to select coverage.

For Nov. 1-3, the exact number of plans selected was 371,676, which is about 38% less than last year’s first week, which was 4 days long, so the average number of plans selected per day was down by a little less than 18%, CMS data show.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS officials said, adding that the weekly data release “only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Open enrollment will continue for another 6 weeks, with Dec. 15 being the final day to enroll for 2019 coverage on the 39 state exchanges that use the HealthCare.gov platform.
About 372,000 people selected a health insurance plan during the first week of Affordable Care Act open enrollment for 2019, according to the Centers for Medicare & Medicaid Services. The first week was short, with only 3 days to select coverage.

For Nov. 1-3, the exact number of plans selected was 371,676, which is about 38% less than last year’s first week, which was 4 days long, so the average number of plans selected per day was down by a little less than 18%, CMS data show.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS officials said, adding that the weekly data release “only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Open enrollment will continue for another 6 weeks, with Dec. 15 being the final day to enroll for 2019 coverage on the 39 state exchanges that use the HealthCare.gov platform.
New pregnancy, genetic testing guidance added to AAD’s melanoma guidelines
Pregnancy does not necessarily increase a woman’s risk for melanoma, nor is it clear that becoming pregnant affects melanoma’s disease course, according to current evidence. This guidance is among several updates added to newly released guidelines for managing patients with primary cutaneous melanoma.
also addressed the burgeoning field of genetic testing for cancer in the guidelines, which were published online on Nov. 1. Although there may be a hereditary component to some melanomas, genetic testing may not be appropriate for all patients, and any formal genetic testing should be carried out only after individualized education and counseling, according to the updates.
However, the guidelines make it clear that all patients whose family history includes melanoma should be counseled about their genetic risk.
As with genetic testing, counseling regarding future pregnancies for women with melanoma, or a history of melanoma, should be personalized and account for individual history and melanoma risk, according to the new guidelines. Since evidence is lacking that pregnancy affects the course of melanoma, physicians caring for pregnant women with melanoma should first look at patient and the disease characteristics. The addition of detailed guidance regarding pregnancy reflects research showing that CM is the most common malignancy seen in pregnancy, amounting to nearly one-third of the malignancies that arise in pregnancy. “Although the incidence of CM is generally higher in men, it is higher in younger women than in men, most notably during women’s reproductive years,” wrote Susan M. Swetter, MD, and her guideline coauthors.
“Melanoma is the deadliest form of skin cancer, and we hope these guidelines will help dermatologists and other physicians enhance their delivery of life-saving treatment to patients,” Dr. Swetter said in a press release announcing the guideline updates. Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, led the working group that developed the guidelines. “In order to provide the best possible resource for practitioners, we reviewed the latest scientific data and addressed certain topics that weren’t covered in the AAD’s previous melanoma guidelines,” she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. Adjuvant topical therapies or radiation, say the guidelines, can be considered as second-line care, but only in limited situations in which surgery is not feasible. Staged excision techniques, such as Mohs surgery, also may be considered for certain types of melanoma and in certain body areas.
In an interview, Dr. Swetter also said that is critical that the updated guidelines have been harmonized with changes made in the American Joint Committee on Cancer’s 8th edition of its melanoma staging manual. Key points for dermatologists to understand that reporting of Breslow thickness to the nearest 1/10th decimal point (over the nearest 1/100th), such that a melanoma measuring 0.75-0.84 mm in thickness would be reported as 0.8 mm depth and one between 0.95-1.04 mm would be rounded to 1 mm.
The main changes regarding staging of thin (T1) melanoma – that is less than or equal to 1 mm – is that the 0.8 mm thickness is the threshold for a T1a melanoma (now classified as less than 0.8 mm without ulceration), whereas T1b is now 0.8 – 1.0 mm thickness with or w/out ulceration or less than 0.8 mm thickness with ulceration. A T1a melanoma generally is not considered appropriate for staging of the regional lymph nodes with sentinel lymph node biopsy (with exceptions noted in the guideline), whereas a T1b melanoma may be considered for SLNB staging – though rates of SLN positivity remain relatively low in the T1b group.”
Dr. Swetter also emphasized that histologic ulceration of the primary tumor was affirmed as an indicator of worse prognosis; mitotic rate, although removed from T1 staging, is still tracked by pathologists and still seen as an independent predictor of worse prognosis, according to the 8th edition, she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. “Mohs micrographic surgery and other staged excision techniques can provide exhaustive peripheral margin histologic assessment for melanoma in situ, lentigo maligna type and tissue sparing in anatomically constrained sites,” Dr. Swetter said. “Current data are insufficient to recommend Mohs surgery for invasive cutaneous melanoma, in which the use of surgical margins less than 1 cm has not been adequately studied,” she cautioned.
Reinforcing the importance of surgery as the primary treatment for melanoma, Dr. Swetter clarified that “Nonsurgical approaches (imiquimod and traditional forms of radiation therapy) should be considered [only] if surgery is impractical or contraindicated, and only for melanoma in situ, lentigo maligna type, as cure rates are lower.”
In terms of other therapies, the guideline working group found insufficient evidence to recommend electronic brachytherapy for melanoma.
Assessment of novel diagnostic and molecular imaging modalities was not the primary focus of the AAD guidelines, Dr. Swetter pointed out. Looking to the future, though, she added that the hope is “that these prebiopsy modalities can one day reduce unnecessary biopsies from being done” in the clinic.
Other knowledge gaps cited by the working group included several related to pathology, including determination of appropriate margin control in some lesion types, and the quest to reduce inter-reader variability in histopathologic diagnosis of melanocytic tissue samples. However, noted Dr. Swetter and her coauthors, the rapid pace of genomic medicine advances “may make many of the aforementioned issues obsolete” before the next guideline update.
In the interview, Dr. Swetter said that the guidelines reflect evolving thinking about melanoma in the context of a rapidly growing field. “Only in the last year have effective, more tolerable adjuvant therapies been [Food and Drug Administration] approved for patients with resected stage III melanoma, including patients with regional lymph node disease detected via sentinel lymph node biopsy. The hope is that less invasive procedures for melanoma will be performed in the future, and replaced by better drugs and novel techniques.”
Dr. Swetter reported that she had no relevant financial disclosures; several working group members reported multiple financial relationships with pharmaceutical, diagnostic, and imaging companies. Working group members were recused from discussion of guidelines where their particular relationships might pose a conflict of interest.
SOURCE: Swetter S. et al. J Am Acad Dermatol. 2011 Nov;65(5):1032-47.
Pregnancy does not necessarily increase a woman’s risk for melanoma, nor is it clear that becoming pregnant affects melanoma’s disease course, according to current evidence. This guidance is among several updates added to newly released guidelines for managing patients with primary cutaneous melanoma.
also addressed the burgeoning field of genetic testing for cancer in the guidelines, which were published online on Nov. 1. Although there may be a hereditary component to some melanomas, genetic testing may not be appropriate for all patients, and any formal genetic testing should be carried out only after individualized education and counseling, according to the updates.
However, the guidelines make it clear that all patients whose family history includes melanoma should be counseled about their genetic risk.
As with genetic testing, counseling regarding future pregnancies for women with melanoma, or a history of melanoma, should be personalized and account for individual history and melanoma risk, according to the new guidelines. Since evidence is lacking that pregnancy affects the course of melanoma, physicians caring for pregnant women with melanoma should first look at patient and the disease characteristics. The addition of detailed guidance regarding pregnancy reflects research showing that CM is the most common malignancy seen in pregnancy, amounting to nearly one-third of the malignancies that arise in pregnancy. “Although the incidence of CM is generally higher in men, it is higher in younger women than in men, most notably during women’s reproductive years,” wrote Susan M. Swetter, MD, and her guideline coauthors.
“Melanoma is the deadliest form of skin cancer, and we hope these guidelines will help dermatologists and other physicians enhance their delivery of life-saving treatment to patients,” Dr. Swetter said in a press release announcing the guideline updates. Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, led the working group that developed the guidelines. “In order to provide the best possible resource for practitioners, we reviewed the latest scientific data and addressed certain topics that weren’t covered in the AAD’s previous melanoma guidelines,” she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. Adjuvant topical therapies or radiation, say the guidelines, can be considered as second-line care, but only in limited situations in which surgery is not feasible. Staged excision techniques, such as Mohs surgery, also may be considered for certain types of melanoma and in certain body areas.
In an interview, Dr. Swetter also said that is critical that the updated guidelines have been harmonized with changes made in the American Joint Committee on Cancer’s 8th edition of its melanoma staging manual. Key points for dermatologists to understand that reporting of Breslow thickness to the nearest 1/10th decimal point (over the nearest 1/100th), such that a melanoma measuring 0.75-0.84 mm in thickness would be reported as 0.8 mm depth and one between 0.95-1.04 mm would be rounded to 1 mm.
The main changes regarding staging of thin (T1) melanoma – that is less than or equal to 1 mm – is that the 0.8 mm thickness is the threshold for a T1a melanoma (now classified as less than 0.8 mm without ulceration), whereas T1b is now 0.8 – 1.0 mm thickness with or w/out ulceration or less than 0.8 mm thickness with ulceration. A T1a melanoma generally is not considered appropriate for staging of the regional lymph nodes with sentinel lymph node biopsy (with exceptions noted in the guideline), whereas a T1b melanoma may be considered for SLNB staging – though rates of SLN positivity remain relatively low in the T1b group.”
Dr. Swetter also emphasized that histologic ulceration of the primary tumor was affirmed as an indicator of worse prognosis; mitotic rate, although removed from T1 staging, is still tracked by pathologists and still seen as an independent predictor of worse prognosis, according to the 8th edition, she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. “Mohs micrographic surgery and other staged excision techniques can provide exhaustive peripheral margin histologic assessment for melanoma in situ, lentigo maligna type and tissue sparing in anatomically constrained sites,” Dr. Swetter said. “Current data are insufficient to recommend Mohs surgery for invasive cutaneous melanoma, in which the use of surgical margins less than 1 cm has not been adequately studied,” she cautioned.
Reinforcing the importance of surgery as the primary treatment for melanoma, Dr. Swetter clarified that “Nonsurgical approaches (imiquimod and traditional forms of radiation therapy) should be considered [only] if surgery is impractical or contraindicated, and only for melanoma in situ, lentigo maligna type, as cure rates are lower.”
In terms of other therapies, the guideline working group found insufficient evidence to recommend electronic brachytherapy for melanoma.
Assessment of novel diagnostic and molecular imaging modalities was not the primary focus of the AAD guidelines, Dr. Swetter pointed out. Looking to the future, though, she added that the hope is “that these prebiopsy modalities can one day reduce unnecessary biopsies from being done” in the clinic.
Other knowledge gaps cited by the working group included several related to pathology, including determination of appropriate margin control in some lesion types, and the quest to reduce inter-reader variability in histopathologic diagnosis of melanocytic tissue samples. However, noted Dr. Swetter and her coauthors, the rapid pace of genomic medicine advances “may make many of the aforementioned issues obsolete” before the next guideline update.
In the interview, Dr. Swetter said that the guidelines reflect evolving thinking about melanoma in the context of a rapidly growing field. “Only in the last year have effective, more tolerable adjuvant therapies been [Food and Drug Administration] approved for patients with resected stage III melanoma, including patients with regional lymph node disease detected via sentinel lymph node biopsy. The hope is that less invasive procedures for melanoma will be performed in the future, and replaced by better drugs and novel techniques.”
Dr. Swetter reported that she had no relevant financial disclosures; several working group members reported multiple financial relationships with pharmaceutical, diagnostic, and imaging companies. Working group members were recused from discussion of guidelines where their particular relationships might pose a conflict of interest.
SOURCE: Swetter S. et al. J Am Acad Dermatol. 2011 Nov;65(5):1032-47.
Pregnancy does not necessarily increase a woman’s risk for melanoma, nor is it clear that becoming pregnant affects melanoma’s disease course, according to current evidence. This guidance is among several updates added to newly released guidelines for managing patients with primary cutaneous melanoma.
also addressed the burgeoning field of genetic testing for cancer in the guidelines, which were published online on Nov. 1. Although there may be a hereditary component to some melanomas, genetic testing may not be appropriate for all patients, and any formal genetic testing should be carried out only after individualized education and counseling, according to the updates.
However, the guidelines make it clear that all patients whose family history includes melanoma should be counseled about their genetic risk.
As with genetic testing, counseling regarding future pregnancies for women with melanoma, or a history of melanoma, should be personalized and account for individual history and melanoma risk, according to the new guidelines. Since evidence is lacking that pregnancy affects the course of melanoma, physicians caring for pregnant women with melanoma should first look at patient and the disease characteristics. The addition of detailed guidance regarding pregnancy reflects research showing that CM is the most common malignancy seen in pregnancy, amounting to nearly one-third of the malignancies that arise in pregnancy. “Although the incidence of CM is generally higher in men, it is higher in younger women than in men, most notably during women’s reproductive years,” wrote Susan M. Swetter, MD, and her guideline coauthors.
“Melanoma is the deadliest form of skin cancer, and we hope these guidelines will help dermatologists and other physicians enhance their delivery of life-saving treatment to patients,” Dr. Swetter said in a press release announcing the guideline updates. Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford (Calif.) University Medical Center and Cancer Institute, led the working group that developed the guidelines. “In order to provide the best possible resource for practitioners, we reviewed the latest scientific data and addressed certain topics that weren’t covered in the AAD’s previous melanoma guidelines,” she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. Adjuvant topical therapies or radiation, say the guidelines, can be considered as second-line care, but only in limited situations in which surgery is not feasible. Staged excision techniques, such as Mohs surgery, also may be considered for certain types of melanoma and in certain body areas.
In an interview, Dr. Swetter also said that is critical that the updated guidelines have been harmonized with changes made in the American Joint Committee on Cancer’s 8th edition of its melanoma staging manual. Key points for dermatologists to understand that reporting of Breslow thickness to the nearest 1/10th decimal point (over the nearest 1/100th), such that a melanoma measuring 0.75-0.84 mm in thickness would be reported as 0.8 mm depth and one between 0.95-1.04 mm would be rounded to 1 mm.
The main changes regarding staging of thin (T1) melanoma – that is less than or equal to 1 mm – is that the 0.8 mm thickness is the threshold for a T1a melanoma (now classified as less than 0.8 mm without ulceration), whereas T1b is now 0.8 – 1.0 mm thickness with or w/out ulceration or less than 0.8 mm thickness with ulceration. A T1a melanoma generally is not considered appropriate for staging of the regional lymph nodes with sentinel lymph node biopsy (with exceptions noted in the guideline), whereas a T1b melanoma may be considered for SLNB staging – though rates of SLN positivity remain relatively low in the T1b group.”
Dr. Swetter also emphasized that histologic ulceration of the primary tumor was affirmed as an indicator of worse prognosis; mitotic rate, although removed from T1 staging, is still tracked by pathologists and still seen as an independent predictor of worse prognosis, according to the 8th edition, she said.
A cornerstone of cutaneous melanoma care remains unchanged in the guidelines: Surgical excision is still the preferred method for treating melanoma. “Mohs micrographic surgery and other staged excision techniques can provide exhaustive peripheral margin histologic assessment for melanoma in situ, lentigo maligna type and tissue sparing in anatomically constrained sites,” Dr. Swetter said. “Current data are insufficient to recommend Mohs surgery for invasive cutaneous melanoma, in which the use of surgical margins less than 1 cm has not been adequately studied,” she cautioned.
Reinforcing the importance of surgery as the primary treatment for melanoma, Dr. Swetter clarified that “Nonsurgical approaches (imiquimod and traditional forms of radiation therapy) should be considered [only] if surgery is impractical or contraindicated, and only for melanoma in situ, lentigo maligna type, as cure rates are lower.”
In terms of other therapies, the guideline working group found insufficient evidence to recommend electronic brachytherapy for melanoma.
Assessment of novel diagnostic and molecular imaging modalities was not the primary focus of the AAD guidelines, Dr. Swetter pointed out. Looking to the future, though, she added that the hope is “that these prebiopsy modalities can one day reduce unnecessary biopsies from being done” in the clinic.
Other knowledge gaps cited by the working group included several related to pathology, including determination of appropriate margin control in some lesion types, and the quest to reduce inter-reader variability in histopathologic diagnosis of melanocytic tissue samples. However, noted Dr. Swetter and her coauthors, the rapid pace of genomic medicine advances “may make many of the aforementioned issues obsolete” before the next guideline update.
In the interview, Dr. Swetter said that the guidelines reflect evolving thinking about melanoma in the context of a rapidly growing field. “Only in the last year have effective, more tolerable adjuvant therapies been [Food and Drug Administration] approved for patients with resected stage III melanoma, including patients with regional lymph node disease detected via sentinel lymph node biopsy. The hope is that less invasive procedures for melanoma will be performed in the future, and replaced by better drugs and novel techniques.”
Dr. Swetter reported that she had no relevant financial disclosures; several working group members reported multiple financial relationships with pharmaceutical, diagnostic, and imaging companies. Working group members were recused from discussion of guidelines where their particular relationships might pose a conflict of interest.
SOURCE: Swetter S. et al. J Am Acad Dermatol. 2011 Nov;65(5):1032-47.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Variant not linked to CLL in Southeast Europe
DUBROVNIK, CROATIA – New research suggests there is no association between the PTPN22 R620W polymorphism and chronic lymphocytic leukemia (CLL) or autoimmune hematologic disorders in patients from the Republic of Macedonia.
Past studies have shown an association between the PTPN22 R620W variant and both CLL and autoimmune diseases in patients from Northwest Europe. However, a new study of Macedonian patients suggests there is no association between the variant and CLL, autoimmune hemolytic anemia (AIHA), or idiopathic thrombocytopenic purpura (ITP) for patients from Southeast Europe.
Irina Panovska-Stavridis, PhD, of Ss. Cyril and Methodius University in Skopje, Republic of Macedonia, and her colleagues presented this finding at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
“A lot of data from the literature suggests [the PTPN22 R620W variant ] has a role in developing multiple immune diseases, but it is validated just in patients from Northwest Europe,” Dr. Panovska-Stavridis noted.
She and her colleagues decided to assess the frequency of the PTPN22 R620W variant (C1858T, rs2476601) in individuals from Southeast Europe, particularly the Republic of Macedonia.
The researchers evaluated 320 patients – 168 with CLL, 66 with AIHA, and 86 with ITP – and 182 age- and sex-matched control subjects with no history of malignant or autoimmune disease.
The team found a similar frequency of the minor T allele and genotype distribution in control subjects and patients. For example, minor T allele was 0.107 in CLL, 0.067 in AIHA, 0.036 in ITP, and 0.05 in controls. Similarly, the frequency of the CC genotype was 0.809 in CLL, 0.166 in AIHA, 0.023 in ITP, and 0.901 in controls.
Dr. Panovska-Stavridis said these results suggest the PTPN22 R620W variant is not a risk factor for the development of CLL, AIHA, or ITP in patients from Southeast Europe.
She also said the results suggest the influence of the variant on lymphocytic homeostasis is affected by certain genetic and environmental factors, and the development of CLL and autoimmune diseases is influenced by race/ethnicity-based variations in the germline composition of the IGHV locus in correlation with environmental factors.
Dr. Panovska-Stavridis did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – New research suggests there is no association between the PTPN22 R620W polymorphism and chronic lymphocytic leukemia (CLL) or autoimmune hematologic disorders in patients from the Republic of Macedonia.
Past studies have shown an association between the PTPN22 R620W variant and both CLL and autoimmune diseases in patients from Northwest Europe. However, a new study of Macedonian patients suggests there is no association between the variant and CLL, autoimmune hemolytic anemia (AIHA), or idiopathic thrombocytopenic purpura (ITP) for patients from Southeast Europe.
Irina Panovska-Stavridis, PhD, of Ss. Cyril and Methodius University in Skopje, Republic of Macedonia, and her colleagues presented this finding at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
“A lot of data from the literature suggests [the PTPN22 R620W variant ] has a role in developing multiple immune diseases, but it is validated just in patients from Northwest Europe,” Dr. Panovska-Stavridis noted.
She and her colleagues decided to assess the frequency of the PTPN22 R620W variant (C1858T, rs2476601) in individuals from Southeast Europe, particularly the Republic of Macedonia.
The researchers evaluated 320 patients – 168 with CLL, 66 with AIHA, and 86 with ITP – and 182 age- and sex-matched control subjects with no history of malignant or autoimmune disease.
The team found a similar frequency of the minor T allele and genotype distribution in control subjects and patients. For example, minor T allele was 0.107 in CLL, 0.067 in AIHA, 0.036 in ITP, and 0.05 in controls. Similarly, the frequency of the CC genotype was 0.809 in CLL, 0.166 in AIHA, 0.023 in ITP, and 0.901 in controls.
Dr. Panovska-Stavridis said these results suggest the PTPN22 R620W variant is not a risk factor for the development of CLL, AIHA, or ITP in patients from Southeast Europe.
She also said the results suggest the influence of the variant on lymphocytic homeostasis is affected by certain genetic and environmental factors, and the development of CLL and autoimmune diseases is influenced by race/ethnicity-based variations in the germline composition of the IGHV locus in correlation with environmental factors.
Dr. Panovska-Stavridis did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – New research suggests there is no association between the PTPN22 R620W polymorphism and chronic lymphocytic leukemia (CLL) or autoimmune hematologic disorders in patients from the Republic of Macedonia.
Past studies have shown an association between the PTPN22 R620W variant and both CLL and autoimmune diseases in patients from Northwest Europe. However, a new study of Macedonian patients suggests there is no association between the variant and CLL, autoimmune hemolytic anemia (AIHA), or idiopathic thrombocytopenic purpura (ITP) for patients from Southeast Europe.
Irina Panovska-Stavridis, PhD, of Ss. Cyril and Methodius University in Skopje, Republic of Macedonia, and her colleagues presented this finding at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
“A lot of data from the literature suggests [the PTPN22 R620W variant ] has a role in developing multiple immune diseases, but it is validated just in patients from Northwest Europe,” Dr. Panovska-Stavridis noted.
She and her colleagues decided to assess the frequency of the PTPN22 R620W variant (C1858T, rs2476601) in individuals from Southeast Europe, particularly the Republic of Macedonia.
The researchers evaluated 320 patients – 168 with CLL, 66 with AIHA, and 86 with ITP – and 182 age- and sex-matched control subjects with no history of malignant or autoimmune disease.
The team found a similar frequency of the minor T allele and genotype distribution in control subjects and patients. For example, minor T allele was 0.107 in CLL, 0.067 in AIHA, 0.036 in ITP, and 0.05 in controls. Similarly, the frequency of the CC genotype was 0.809 in CLL, 0.166 in AIHA, 0.023 in ITP, and 0.901 in controls.
Dr. Panovska-Stavridis said these results suggest the PTPN22 R620W variant is not a risk factor for the development of CLL, AIHA, or ITP in patients from Southeast Europe.
She also said the results suggest the influence of the variant on lymphocytic homeostasis is affected by certain genetic and environmental factors, and the development of CLL and autoimmune diseases is influenced by race/ethnicity-based variations in the germline composition of the IGHV locus in correlation with environmental factors.
Dr. Panovska-Stavridis did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
REPORTING FROM LEUKEMIA AND LYMPHOMA 2018
Key clinical point:
Major finding: The frequency of minor T allele was 0.107 in patients with CLL, 0.067 in patients with autoimmune hemolytic anemia, 0.036 in patients with idiopathic thrombocytopenic purpura, and 0.05 in controls.
Study details: An analysis of the frequency of the PTPN22 R620W variant in 320 individuals from the Republic of Macedonia.
Disclosures: Dr. Panovska-Stavridis did not declare any conflicts of interest.
ICYMI: Alirocumab improves cardiovascular outcomes
Among patients in the ODYSSEY Outcomes trial who’d had an acute coronary syndrome, only 9.5% of the alirocumab group versus 11.1% of the placebo group experienced composite primary endpoint events – death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. Furthermore, the incidence of adverse events in both groups was similar, although the alirocumab group experienced more local injection-site reactions.
The ODYSSEY Outcomes primary results were published in the New England Journal of Medicine (2018 Nov 7; doi: 10.1056/NEJMoa1801174).
We covered the story last March, from the American College of Cardiology scientific sessions. Find our coverage at the link below:
https://www.mdedge.com/ecardiologynews/article/160512/acc-conference-coverage/odyssey-outcomes-trial-redefines-secondary.
Among patients in the ODYSSEY Outcomes trial who’d had an acute coronary syndrome, only 9.5% of the alirocumab group versus 11.1% of the placebo group experienced composite primary endpoint events – death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. Furthermore, the incidence of adverse events in both groups was similar, although the alirocumab group experienced more local injection-site reactions.
The ODYSSEY Outcomes primary results were published in the New England Journal of Medicine (2018 Nov 7; doi: 10.1056/NEJMoa1801174).
We covered the story last March, from the American College of Cardiology scientific sessions. Find our coverage at the link below:
https://www.mdedge.com/ecardiologynews/article/160512/acc-conference-coverage/odyssey-outcomes-trial-redefines-secondary.
Among patients in the ODYSSEY Outcomes trial who’d had an acute coronary syndrome, only 9.5% of the alirocumab group versus 11.1% of the placebo group experienced composite primary endpoint events – death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. Furthermore, the incidence of adverse events in both groups was similar, although the alirocumab group experienced more local injection-site reactions.
The ODYSSEY Outcomes primary results were published in the New England Journal of Medicine (2018 Nov 7; doi: 10.1056/NEJMoa1801174).
We covered the story last March, from the American College of Cardiology scientific sessions. Find our coverage at the link below:
https://www.mdedge.com/ecardiologynews/article/160512/acc-conference-coverage/odyssey-outcomes-trial-redefines-secondary.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Single-dose zoliflodacin successfully treats uncomplicated urogenital gonorrhea
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Cure rates of 96% and 100% were reported for urethral/cervical and rectal infections, respectively, and ranged from 50% to 82% in pharyngeal infections.
Study details: A randomized, open-label, phase 2 study including 181 patients with uncomplicated urogenital gonorrhea who received zoliflodacin or ceftriaxone.
Disclosures: The study authors reported disclosures related to AstraZeneca (parent company of Entasis Therapeutics, which is developing zoliflodacin) and other pharmaceutical companies.
Source: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.