User login
Patellofemoral Instability in the Skeletally Immature Patient: A Review and Technical Description of Medial Patellofemoral Ligament Reconstruction in Patients with Open Physes
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
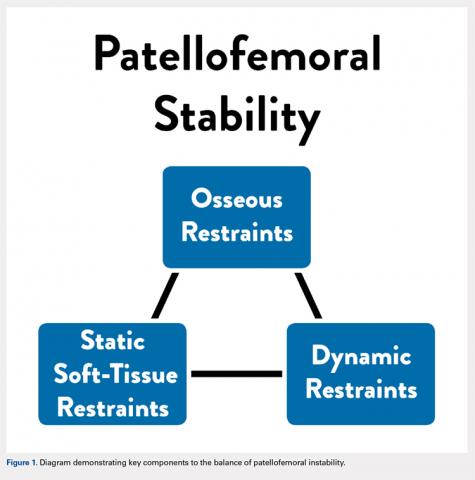
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
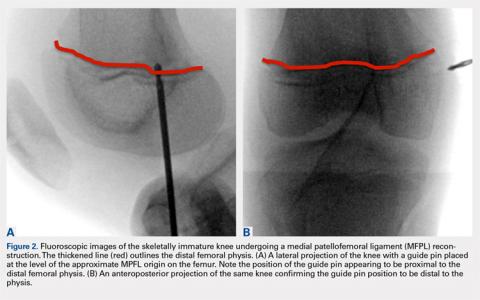
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
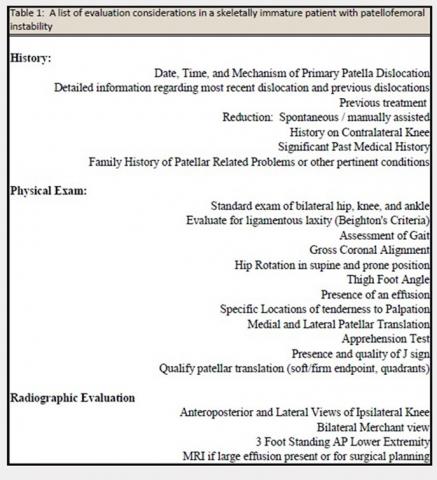
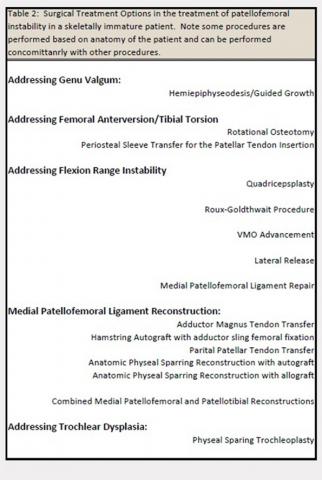
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
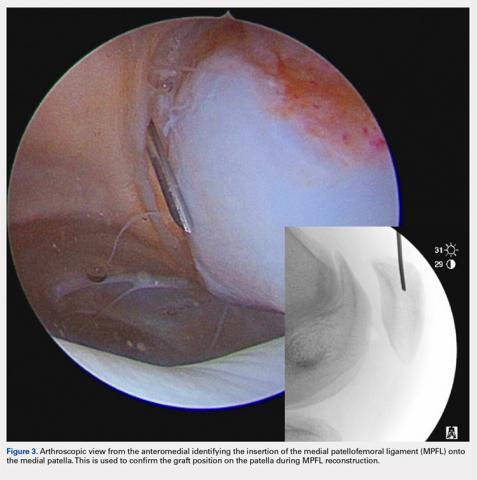
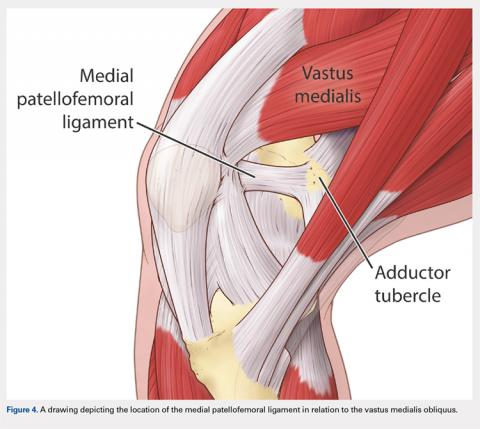
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
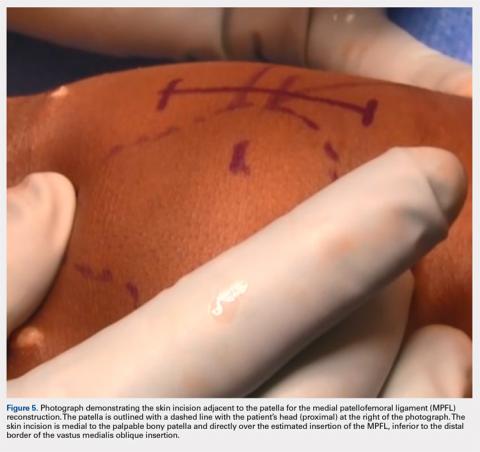
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
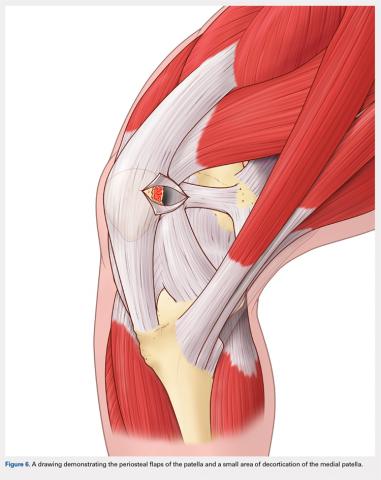
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
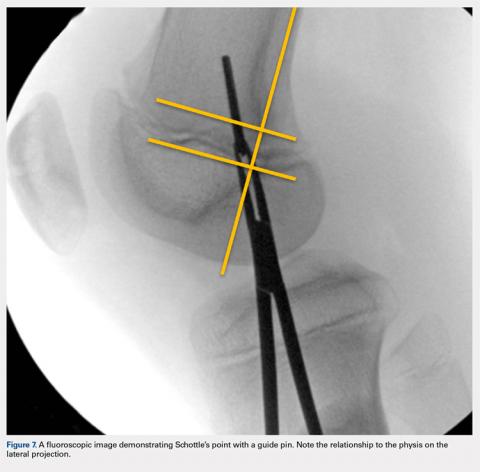
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
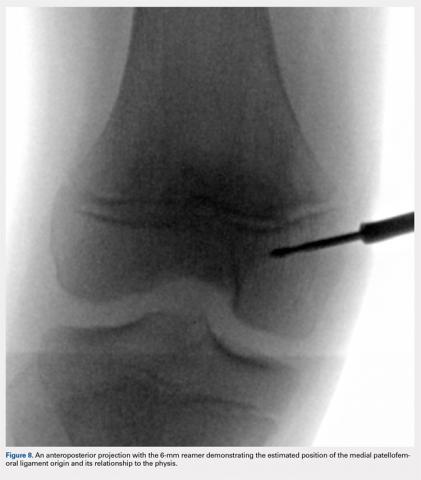
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
TAKE-HOME POINTS
- Patellofemoral instability is common in the skeletally immature age group.
- Many treatment options exist for patellofemoral instability; however, a medial patellofemoral ligament (MPFL) reconstruction is an option in a skeletally immature patient.
- Allograft or autografts may be utilized for the MPFL reconstruction.
- Three options exist to confirm location of the MPFL origin on the patella, but only one drill tunnel is recommended in pediatrics.
- During identification of MPFL origin, special views in the coronal plane should be considered to avoid injury or damage to the growth plate.
Opioid benefit small in chronic noncancer pain
Compared with placebo, opioids provide very modest improvements in chronic noncancer pain and physical functioning that decrease over time, according to the authors of a systematic review and meta-analysis of nearly 100 randomized clinical trials.
There was little difference in pain control between opioids and nonopioid alternatives such as NSAIDs in a subset of nine such comparative trials, reported the authors, led by Jason W. Busse, DC, PhD, of the department of anesthesia at McMaster University, Hamilton, Ont.
Pain benefits of opioids decreased over time in longer trials, possibly because of opioid tolerance or hyperalgesia, a condition marked by hypersensitivity to pain. “A reduced association with benefit over time might lead to prescription of higher opioid doses and consequent harms,” Dr. Busse and his coauthors wrote in JAMA.
The meta-analysis included 96 randomized clinical trials including 26,169 patients with chronic noncancer pain.
Opioid treatment did significantly improve pain and physical function versus placebo, though the magnitude of benefit was small, according to the investigators. The reduction in pain was –0.69 cm on a 10-cm visual analog scale (P less than .001), based on high-quality evidence from 42 randomized, controlled trials that followed patients for at least 3 months.
The improvement in physical functioning was likewise significant but small at 2.04 out of 100 points on the SF-36 physical component score (P less than .001). Emotional and role functioning were not significantly improved by opioid use.
Opioid use was linked to increased vomiting incidence versus placebo, with a relative risk of 4.12 (95% CI, 3.34-5.07; P less than .001) for patients in “nonenrichment” trials – those studies that included all patients regardless of whether or not they reported lack of improvement or had substantial adverse events during a study run-in period.
Nausea, constipation, dizziness, drowsiness, pruritus, and dry mouth were also linked to opioid use as compared with placebo, Dr. Busse and his colleagues reported.
The benefit of opioids and nonopioid alternatives appeared to be similar in this meta-analysis, though the available evidence from comparative studies was of low to moderate quality, the authors advised.
In moderate-quality evidence from nine clinical trials of opioids versus NSAIDs including 1,431 patients, there was no difference in pain relief between the two interventions, the investigators said. Moreover, comparisons of physician functioning also suggested no difference, while opioids were associated with more vomiting.
Both tricyclic antidepressants and synthetic cannabinoids offered similar pain relief, compared with opioids, based on low-quality clinical trial evidence, they added, while moderate-quality evidence suggested opioids offered superior pain relief, compared with anticonvulsants.
Support for the study came from the Canadian Institutes of Health Research and Health Canada. One study coauthor reported receiving personal fees from Purdue Pharma and the Nova Scotia College of Physicians and Surgeons.
SOURCE: Busse JW et al. JAMA. 2018;320(23):2448-60.
This meta-analysis suggests that most patients receiving opioids for chronic noncancer pain will not benefit from them, according to Michael A. Ashburn, MD, MPH, and Lee A. Fleisher, MD.
Outcomes of the study, which suggest opioids produce modest benefits over placebo in pain and physical functioning, and no difference in pain relief versus NSAIDs, are likely to represent the best case scenario, the authors wrote.
That’s because most trials excluded patients with substance use disorder and nearly half excluded patients with mental illness or those taking psychotropic medications, they explained.
In the clinical setting, many patients will have depression, anxiety, sleep-disordered breathing, and other conditions that could increase the potential risk of harm with opioids, according to the authors.
That said, when proper monitoring is incorporated into care, opioid treatment can be safe and effective for selected patients. “Diligent opioid prescribing to carefully selected patients will lower the risk of harm to patients, their families, and the community,” the authors wrote in their editorial.
Dr. Ashburn and Dr. Fleisher are with the department of anesthesiology and critical care at the University of Pennsylvania, Philadelphia. Their editorial appears in JAMA. Dr. Ashburn reported receiving personal fees from Teva, the Department of Justice, the Attorney General for the State of Maryland, the Department of State for the Commonwealth of Pennsylvania, the Montgomery County District Attorney, and the Carolinas Pain Society. He also reported several patents related to drug delivery systems and methods.
This meta-analysis suggests that most patients receiving opioids for chronic noncancer pain will not benefit from them, according to Michael A. Ashburn, MD, MPH, and Lee A. Fleisher, MD.
Outcomes of the study, which suggest opioids produce modest benefits over placebo in pain and physical functioning, and no difference in pain relief versus NSAIDs, are likely to represent the best case scenario, the authors wrote.
That’s because most trials excluded patients with substance use disorder and nearly half excluded patients with mental illness or those taking psychotropic medications, they explained.
In the clinical setting, many patients will have depression, anxiety, sleep-disordered breathing, and other conditions that could increase the potential risk of harm with opioids, according to the authors.
That said, when proper monitoring is incorporated into care, opioid treatment can be safe and effective for selected patients. “Diligent opioid prescribing to carefully selected patients will lower the risk of harm to patients, their families, and the community,” the authors wrote in their editorial.
Dr. Ashburn and Dr. Fleisher are with the department of anesthesiology and critical care at the University of Pennsylvania, Philadelphia. Their editorial appears in JAMA. Dr. Ashburn reported receiving personal fees from Teva, the Department of Justice, the Attorney General for the State of Maryland, the Department of State for the Commonwealth of Pennsylvania, the Montgomery County District Attorney, and the Carolinas Pain Society. He also reported several patents related to drug delivery systems and methods.
This meta-analysis suggests that most patients receiving opioids for chronic noncancer pain will not benefit from them, according to Michael A. Ashburn, MD, MPH, and Lee A. Fleisher, MD.
Outcomes of the study, which suggest opioids produce modest benefits over placebo in pain and physical functioning, and no difference in pain relief versus NSAIDs, are likely to represent the best case scenario, the authors wrote.
That’s because most trials excluded patients with substance use disorder and nearly half excluded patients with mental illness or those taking psychotropic medications, they explained.
In the clinical setting, many patients will have depression, anxiety, sleep-disordered breathing, and other conditions that could increase the potential risk of harm with opioids, according to the authors.
That said, when proper monitoring is incorporated into care, opioid treatment can be safe and effective for selected patients. “Diligent opioid prescribing to carefully selected patients will lower the risk of harm to patients, their families, and the community,” the authors wrote in their editorial.
Dr. Ashburn and Dr. Fleisher are with the department of anesthesiology and critical care at the University of Pennsylvania, Philadelphia. Their editorial appears in JAMA. Dr. Ashburn reported receiving personal fees from Teva, the Department of Justice, the Attorney General for the State of Maryland, the Department of State for the Commonwealth of Pennsylvania, the Montgomery County District Attorney, and the Carolinas Pain Society. He also reported several patents related to drug delivery systems and methods.
Compared with placebo, opioids provide very modest improvements in chronic noncancer pain and physical functioning that decrease over time, according to the authors of a systematic review and meta-analysis of nearly 100 randomized clinical trials.
There was little difference in pain control between opioids and nonopioid alternatives such as NSAIDs in a subset of nine such comparative trials, reported the authors, led by Jason W. Busse, DC, PhD, of the department of anesthesia at McMaster University, Hamilton, Ont.
Pain benefits of opioids decreased over time in longer trials, possibly because of opioid tolerance or hyperalgesia, a condition marked by hypersensitivity to pain. “A reduced association with benefit over time might lead to prescription of higher opioid doses and consequent harms,” Dr. Busse and his coauthors wrote in JAMA.
The meta-analysis included 96 randomized clinical trials including 26,169 patients with chronic noncancer pain.
Opioid treatment did significantly improve pain and physical function versus placebo, though the magnitude of benefit was small, according to the investigators. The reduction in pain was –0.69 cm on a 10-cm visual analog scale (P less than .001), based on high-quality evidence from 42 randomized, controlled trials that followed patients for at least 3 months.
The improvement in physical functioning was likewise significant but small at 2.04 out of 100 points on the SF-36 physical component score (P less than .001). Emotional and role functioning were not significantly improved by opioid use.
Opioid use was linked to increased vomiting incidence versus placebo, with a relative risk of 4.12 (95% CI, 3.34-5.07; P less than .001) for patients in “nonenrichment” trials – those studies that included all patients regardless of whether or not they reported lack of improvement or had substantial adverse events during a study run-in period.
Nausea, constipation, dizziness, drowsiness, pruritus, and dry mouth were also linked to opioid use as compared with placebo, Dr. Busse and his colleagues reported.
The benefit of opioids and nonopioid alternatives appeared to be similar in this meta-analysis, though the available evidence from comparative studies was of low to moderate quality, the authors advised.
In moderate-quality evidence from nine clinical trials of opioids versus NSAIDs including 1,431 patients, there was no difference in pain relief between the two interventions, the investigators said. Moreover, comparisons of physician functioning also suggested no difference, while opioids were associated with more vomiting.
Both tricyclic antidepressants and synthetic cannabinoids offered similar pain relief, compared with opioids, based on low-quality clinical trial evidence, they added, while moderate-quality evidence suggested opioids offered superior pain relief, compared with anticonvulsants.
Support for the study came from the Canadian Institutes of Health Research and Health Canada. One study coauthor reported receiving personal fees from Purdue Pharma and the Nova Scotia College of Physicians and Surgeons.
SOURCE: Busse JW et al. JAMA. 2018;320(23):2448-60.
Compared with placebo, opioids provide very modest improvements in chronic noncancer pain and physical functioning that decrease over time, according to the authors of a systematic review and meta-analysis of nearly 100 randomized clinical trials.
There was little difference in pain control between opioids and nonopioid alternatives such as NSAIDs in a subset of nine such comparative trials, reported the authors, led by Jason W. Busse, DC, PhD, of the department of anesthesia at McMaster University, Hamilton, Ont.
Pain benefits of opioids decreased over time in longer trials, possibly because of opioid tolerance or hyperalgesia, a condition marked by hypersensitivity to pain. “A reduced association with benefit over time might lead to prescription of higher opioid doses and consequent harms,” Dr. Busse and his coauthors wrote in JAMA.
The meta-analysis included 96 randomized clinical trials including 26,169 patients with chronic noncancer pain.
Opioid treatment did significantly improve pain and physical function versus placebo, though the magnitude of benefit was small, according to the investigators. The reduction in pain was –0.69 cm on a 10-cm visual analog scale (P less than .001), based on high-quality evidence from 42 randomized, controlled trials that followed patients for at least 3 months.
The improvement in physical functioning was likewise significant but small at 2.04 out of 100 points on the SF-36 physical component score (P less than .001). Emotional and role functioning were not significantly improved by opioid use.
Opioid use was linked to increased vomiting incidence versus placebo, with a relative risk of 4.12 (95% CI, 3.34-5.07; P less than .001) for patients in “nonenrichment” trials – those studies that included all patients regardless of whether or not they reported lack of improvement or had substantial adverse events during a study run-in period.
Nausea, constipation, dizziness, drowsiness, pruritus, and dry mouth were also linked to opioid use as compared with placebo, Dr. Busse and his colleagues reported.
The benefit of opioids and nonopioid alternatives appeared to be similar in this meta-analysis, though the available evidence from comparative studies was of low to moderate quality, the authors advised.
In moderate-quality evidence from nine clinical trials of opioids versus NSAIDs including 1,431 patients, there was no difference in pain relief between the two interventions, the investigators said. Moreover, comparisons of physician functioning also suggested no difference, while opioids were associated with more vomiting.
Both tricyclic antidepressants and synthetic cannabinoids offered similar pain relief, compared with opioids, based on low-quality clinical trial evidence, they added, while moderate-quality evidence suggested opioids offered superior pain relief, compared with anticonvulsants.
Support for the study came from the Canadian Institutes of Health Research and Health Canada. One study coauthor reported receiving personal fees from Purdue Pharma and the Nova Scotia College of Physicians and Surgeons.
SOURCE: Busse JW et al. JAMA. 2018;320(23):2448-60.
FROM JAMA
Key clinical point: A meta-analysis showed that, in patients with chronic noncancer pain, opioids provided modest improvements versus placebo that receded with time, and comparable benefits versus nonopioid alternatives.
Major finding: The reduction in pain for opioids versus placebo was significant but small, at –0.69 cm on a 10-cm visual analog scale (P less than .001), in randomized, controlled trials following patients for at least 3 months.
Study details: A systematic review and meta-analysis of 96 randomized clinical trials for noncancer pain.
Disclosures: Support for the study came from the Canadian Institutes of Health Research and Health Canada. One study author reported receiving personal fees from Purdue Pharma and the Nova Scotia College of Physicians and Surgeons.
Source: Busse JW et al. JAMA. 2018;320(23):2448-60.
Young opioid users in NYC savvy about HCV
Assessing patient knowledge about hepatitis C virus (HCV) transmission knowledge is difficult given the lack of psychometrically tested measures available, according to researchers who developed and validated an HCV injection–risk knowledge scale.
The 5-item, validated HCV Injection-Risk Knowledge Scale may provide educators, care providers, and researchers with critical information for reducing HCV among people who inject drugs (PWID), according to their report.
The researchers analyzed data from 539 New York City opioid users aged 18-29 years who were recruited via respondent-driven sampling in 2014-2016, according to the study published in Drug and Alcohol Dependence.
Principal components analysis (PCA) of nine knowledge items answered true, false, or don’t know identified useful scale items. These were then evaluated for internal consistency and assessed by comparing knowledge levels with those from a previously validated general HCV knowledge scale and by comparing key subgroup knowledge levels.
PCA identified one component with five items that explained 45% of the total variance and had high internal consistency (alpha, 0.91). All of the component items referred to transmission through drug-injection equipment and practices: sharing materials such as cookers, cottons, diluting water, water containers, and cleaning syringes with water.
The mean percent correct was 75%, and was moderately correlated with general HCV knowledge. Knowledge levels were highest for those previously tested for HCV, those with HCV antibody–positive status, PWID, and those who had received harm reduction information in various settings.
“The low percentage correct among those who had never injected [52% vs. 83% correct among injectors] is concerning given the possibility of their experimenting with drug injection as route of administration,” they added.
“These results suggest that primary care providers, drug treatment programs, and syringe exchange programs are important sources of HCV risk knowledge among at-risk populations, similar to other studies’ findings,” the researchers concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest.
SOURCE: Quinn K et al. Drug Alcohol Depend. 2019;194:453-9.
Assessing patient knowledge about hepatitis C virus (HCV) transmission knowledge is difficult given the lack of psychometrically tested measures available, according to researchers who developed and validated an HCV injection–risk knowledge scale.
The 5-item, validated HCV Injection-Risk Knowledge Scale may provide educators, care providers, and researchers with critical information for reducing HCV among people who inject drugs (PWID), according to their report.
The researchers analyzed data from 539 New York City opioid users aged 18-29 years who were recruited via respondent-driven sampling in 2014-2016, according to the study published in Drug and Alcohol Dependence.
Principal components analysis (PCA) of nine knowledge items answered true, false, or don’t know identified useful scale items. These were then evaluated for internal consistency and assessed by comparing knowledge levels with those from a previously validated general HCV knowledge scale and by comparing key subgroup knowledge levels.
PCA identified one component with five items that explained 45% of the total variance and had high internal consistency (alpha, 0.91). All of the component items referred to transmission through drug-injection equipment and practices: sharing materials such as cookers, cottons, diluting water, water containers, and cleaning syringes with water.
The mean percent correct was 75%, and was moderately correlated with general HCV knowledge. Knowledge levels were highest for those previously tested for HCV, those with HCV antibody–positive status, PWID, and those who had received harm reduction information in various settings.
“The low percentage correct among those who had never injected [52% vs. 83% correct among injectors] is concerning given the possibility of their experimenting with drug injection as route of administration,” they added.
“These results suggest that primary care providers, drug treatment programs, and syringe exchange programs are important sources of HCV risk knowledge among at-risk populations, similar to other studies’ findings,” the researchers concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest.
SOURCE: Quinn K et al. Drug Alcohol Depend. 2019;194:453-9.
Assessing patient knowledge about hepatitis C virus (HCV) transmission knowledge is difficult given the lack of psychometrically tested measures available, according to researchers who developed and validated an HCV injection–risk knowledge scale.
The 5-item, validated HCV Injection-Risk Knowledge Scale may provide educators, care providers, and researchers with critical information for reducing HCV among people who inject drugs (PWID), according to their report.
The researchers analyzed data from 539 New York City opioid users aged 18-29 years who were recruited via respondent-driven sampling in 2014-2016, according to the study published in Drug and Alcohol Dependence.
Principal components analysis (PCA) of nine knowledge items answered true, false, or don’t know identified useful scale items. These were then evaluated for internal consistency and assessed by comparing knowledge levels with those from a previously validated general HCV knowledge scale and by comparing key subgroup knowledge levels.
PCA identified one component with five items that explained 45% of the total variance and had high internal consistency (alpha, 0.91). All of the component items referred to transmission through drug-injection equipment and practices: sharing materials such as cookers, cottons, diluting water, water containers, and cleaning syringes with water.
The mean percent correct was 75%, and was moderately correlated with general HCV knowledge. Knowledge levels were highest for those previously tested for HCV, those with HCV antibody–positive status, PWID, and those who had received harm reduction information in various settings.
“The low percentage correct among those who had never injected [52% vs. 83% correct among injectors] is concerning given the possibility of their experimenting with drug injection as route of administration,” they added.
“These results suggest that primary care providers, drug treatment programs, and syringe exchange programs are important sources of HCV risk knowledge among at-risk populations, similar to other studies’ findings,” the researchers concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest.
SOURCE: Quinn K et al. Drug Alcohol Depend. 2019;194:453-9.
FROM DRUG AND ALCOHOL DEPENDENCE
Expert panel publishes consensus on robotic TME
in an effort to establish uniform training and education and improve outcomes for the robotic operations.
“The aim of this consensus study was to establish a detailed description of technical steps for robotic anterior resection and TME of the rectum as recommended by a representative group of established European expert surgeons,” wrote Danilo Miskovic, PhD, FRCS, of St. Mark’s Hospital in London, and his coauthors. The study, published in Colorectal Disease, provides a baseline for technical standards and structured training in robotic rectal surgery.
The consensus authors acted at the behest of the European Academy for Robotic Colorectal Surgery (EARCS), a nonprofit organization that provides training for robotic colorectal surgery. They cited evidence suggesting that the robotic approach to TME confers significant benefits in selected patients, compared with laparoscopic surgery (Dis Colon Rectum. 2014;57:570-7), but that the ROLARR trial found no benefit with robotic surgery (Int J Colorectal Dis. 2012;27:233-41).
“Notwithstanding the absence of evidence, robotic surgery continues to increase in popularity in many countries,” Dr. Miskovic and his coauthors wrote.
The consensus statement covers recommendations for the da Vinci Si and Xi robotic platforms in the following areas.
- Surgical setup, including patient positioning and port placement and docking.
- Colonic mobilization, including vascular pedicle dissection and splenic flexure mobilization.
- Pelvic dissection, including establishing operative planes and specimen extraction.
Dr. Miskovic and his coauthors arrived at the consensus statement by asking 24 EARCS faculty members to complete a 72-item questionnaire. The initial responses yielded an 87% agreement among the responses, but after suggested modifications, the average level of agreement for all items was 97%.
One of the limitations of the study that the investigators acknowledged is that it covers only two da Vinci robotic platforms; the recommendations may not apply to new robotic systems. Secondly, a selected group of experts provided input. “There may be other experienced surgeons who might disagree with some aspects of our recommendations,” the authors wrote. “This document should therefore be seen as a foundation for debate and modification in light of future technical developments.”
Future research should evaluate how training of technical standards within a group like EARCS impacts clinical outcomes, Dr. Miskovic and his coauthors noted.
The investigators had no financial relationships to report. Participating surgeons are members of the EARCS faculty and/or its scientific committee
SOURCE: Miskovic D et al. Colorectal Dis. 2018 Nov 29. doi: 10.1111/codi.14502.
in an effort to establish uniform training and education and improve outcomes for the robotic operations.
“The aim of this consensus study was to establish a detailed description of technical steps for robotic anterior resection and TME of the rectum as recommended by a representative group of established European expert surgeons,” wrote Danilo Miskovic, PhD, FRCS, of St. Mark’s Hospital in London, and his coauthors. The study, published in Colorectal Disease, provides a baseline for technical standards and structured training in robotic rectal surgery.
The consensus authors acted at the behest of the European Academy for Robotic Colorectal Surgery (EARCS), a nonprofit organization that provides training for robotic colorectal surgery. They cited evidence suggesting that the robotic approach to TME confers significant benefits in selected patients, compared with laparoscopic surgery (Dis Colon Rectum. 2014;57:570-7), but that the ROLARR trial found no benefit with robotic surgery (Int J Colorectal Dis. 2012;27:233-41).
“Notwithstanding the absence of evidence, robotic surgery continues to increase in popularity in many countries,” Dr. Miskovic and his coauthors wrote.
The consensus statement covers recommendations for the da Vinci Si and Xi robotic platforms in the following areas.
- Surgical setup, including patient positioning and port placement and docking.
- Colonic mobilization, including vascular pedicle dissection and splenic flexure mobilization.
- Pelvic dissection, including establishing operative planes and specimen extraction.
Dr. Miskovic and his coauthors arrived at the consensus statement by asking 24 EARCS faculty members to complete a 72-item questionnaire. The initial responses yielded an 87% agreement among the responses, but after suggested modifications, the average level of agreement for all items was 97%.
One of the limitations of the study that the investigators acknowledged is that it covers only two da Vinci robotic platforms; the recommendations may not apply to new robotic systems. Secondly, a selected group of experts provided input. “There may be other experienced surgeons who might disagree with some aspects of our recommendations,” the authors wrote. “This document should therefore be seen as a foundation for debate and modification in light of future technical developments.”
Future research should evaluate how training of technical standards within a group like EARCS impacts clinical outcomes, Dr. Miskovic and his coauthors noted.
The investigators had no financial relationships to report. Participating surgeons are members of the EARCS faculty and/or its scientific committee
SOURCE: Miskovic D et al. Colorectal Dis. 2018 Nov 29. doi: 10.1111/codi.14502.
in an effort to establish uniform training and education and improve outcomes for the robotic operations.
“The aim of this consensus study was to establish a detailed description of technical steps for robotic anterior resection and TME of the rectum as recommended by a representative group of established European expert surgeons,” wrote Danilo Miskovic, PhD, FRCS, of St. Mark’s Hospital in London, and his coauthors. The study, published in Colorectal Disease, provides a baseline for technical standards and structured training in robotic rectal surgery.
The consensus authors acted at the behest of the European Academy for Robotic Colorectal Surgery (EARCS), a nonprofit organization that provides training for robotic colorectal surgery. They cited evidence suggesting that the robotic approach to TME confers significant benefits in selected patients, compared with laparoscopic surgery (Dis Colon Rectum. 2014;57:570-7), but that the ROLARR trial found no benefit with robotic surgery (Int J Colorectal Dis. 2012;27:233-41).
“Notwithstanding the absence of evidence, robotic surgery continues to increase in popularity in many countries,” Dr. Miskovic and his coauthors wrote.
The consensus statement covers recommendations for the da Vinci Si and Xi robotic platforms in the following areas.
- Surgical setup, including patient positioning and port placement and docking.
- Colonic mobilization, including vascular pedicle dissection and splenic flexure mobilization.
- Pelvic dissection, including establishing operative planes and specimen extraction.
Dr. Miskovic and his coauthors arrived at the consensus statement by asking 24 EARCS faculty members to complete a 72-item questionnaire. The initial responses yielded an 87% agreement among the responses, but after suggested modifications, the average level of agreement for all items was 97%.
One of the limitations of the study that the investigators acknowledged is that it covers only two da Vinci robotic platforms; the recommendations may not apply to new robotic systems. Secondly, a selected group of experts provided input. “There may be other experienced surgeons who might disagree with some aspects of our recommendations,” the authors wrote. “This document should therefore be seen as a foundation for debate and modification in light of future technical developments.”
Future research should evaluate how training of technical standards within a group like EARCS impacts clinical outcomes, Dr. Miskovic and his coauthors noted.
The investigators had no financial relationships to report. Participating surgeons are members of the EARCS faculty and/or its scientific committee
SOURCE: Miskovic D et al. Colorectal Dis. 2018 Nov 29. doi: 10.1111/codi.14502.
FROM COLORECTAL DISEASE
Digital alerts reduced AF-related stroke, MI rates
CHICAGO – and had significantly lower rates of death and other cardiovascular events, compared with patients on a standard admissions protocol, according to results of a randomized, controlled trial presented at the American Heart Association Scientific Sessions.
“Alert-based computerized decision support [CDS] increased the prescription of anticoagulation for stroke prevention in atrial fibrillation during hospitalization, at discharge, and at 90 days after randomization in high-risk patients,” said Gregory Piazza, MD, of Brigham and Women’s Hospital, Boston, in presenting results of the AF-ALERT trial. “The reductions in major cardiovascular events was attributable to reductions in MI and stroke/transient ischemic attack at 90 days in patients whose physicians received the alert.”
The trial evaluated 458 patients hospitalized for AF or flutter and with CHA2DS2-VASc scores of 1-8 randomly assigned to the alert (n = 258) or no-alert (n = 210) groups.
Dr. Piazza explained that for those in the alert group, the CDS system notified physicians when the patient’s CHA2DS2-VASc score increased. From there, the physician could choose to open an order template to prescribe evidence-based medications to prevent stroke, to elect to review evidence-based clinical practice guidelines, or to continue with the admissions order with an acknowledged reason for omitting anticoagulation (such as high bleeding risk, low stroke risk, high risk for falls, or patient refusal of anticoagulation).
“In patients for whom their providers were alerted, 35% elected to open the stroke-prevention order set, a very tiny percentage elected to read the AF guidelines, and about 64% exited but provided a rationale for omitting anticoagulation,” Dr. Piazza noted.
The alert group was far more likely to be prescribed anticoagulation during the hospitalization (25.8% vs. 9.5%; P less than .0001), at discharge (23.8% vs. 12.9%; P = .003), and at 90 days (27.7% vs. 17.1%; P = .007) than the control group. The alert resulted in a 55% relative risk reduction in a composite outcome of death, MI, cerebrovascular event, and systemic embolic event at 90 days (11.3% vs. 21.9%; P = .002). The alert group had an 87% lower incidence of MI at 90 days (1.2% vs. 8.6%, P = .0002) and 88% lower incidence of cerebrovascular events or systemic embolism at 90 days (0% vs. 2.4%; P = .02). Death at 90 days occurred in 10.1% in the alert group and 14.8% in the control group (P = .13).
One of the limitations of the study, Dr. Piazza noted, was that the most dramatic finding – reduction of major cardiovascular events – was a secondary, not a primary, endpoint. “CDS has the potential to be a powerful tool in prevention of cardiovascular events in patients with atrial fibrillation.”
Moderator Mintu Turakhia, MD, of Stanford (Calif.) University, questioned the low rate of anticoagulation in the study’s control arm – 9.5% – much lower than medians reported in many registries. He also asked Dr. Piazza to describe the mechanism of action for prescribing anticoagulation in these patients.
Dr. Piazza noted the study population was hospitalized patients whose providers had decided prior to their admissions not to prescribe anticoagulation; hence, the rate of anticoagulation in these patients was actually higher than expected.
Regarding the mechanism of action, “the electronic alert seems to preferentially increase the prescription of [direct oral anticoagulants] over warfarin, and that may have been one of the mechanisms,” Dr. Piazza said. Another explanation he offered were “off-target” effects whereby, if providers have a better idea of a patient’s risk for a stroke or MI, they’ll be more aggressive about managing other risk factors.
“There are a number of interventions that could be triggered if the alert prompted the provider to have a conversation with patients about their risk of stroke from AF,” he said. “This may have impact beyond what we can tell from this simple [Best Practice Advisory in the Epic EHR system]. I think we don’t have a great understanding of the full mechanisms of CDS.”
Dr. Piazza reported financial relationships with BTG, Janssen, Bristol-Myers Squibb, Daiichi Sankyo, Portola, and Bayer. Daiichi Sankyo funded the trial. Dr. Turakhia reported relationships with Apple, Janssen, AstraZeneca, VA, Boehringer Ingelheim, Cardiva Medical, Medtronic, Abbott, Precision Health Economics, iBeat, iRhythm, MyoKardia, Biotronik, and an ownership Interest in AliveCor.
CHICAGO – and had significantly lower rates of death and other cardiovascular events, compared with patients on a standard admissions protocol, according to results of a randomized, controlled trial presented at the American Heart Association Scientific Sessions.
“Alert-based computerized decision support [CDS] increased the prescription of anticoagulation for stroke prevention in atrial fibrillation during hospitalization, at discharge, and at 90 days after randomization in high-risk patients,” said Gregory Piazza, MD, of Brigham and Women’s Hospital, Boston, in presenting results of the AF-ALERT trial. “The reductions in major cardiovascular events was attributable to reductions in MI and stroke/transient ischemic attack at 90 days in patients whose physicians received the alert.”
The trial evaluated 458 patients hospitalized for AF or flutter and with CHA2DS2-VASc scores of 1-8 randomly assigned to the alert (n = 258) or no-alert (n = 210) groups.
Dr. Piazza explained that for those in the alert group, the CDS system notified physicians when the patient’s CHA2DS2-VASc score increased. From there, the physician could choose to open an order template to prescribe evidence-based medications to prevent stroke, to elect to review evidence-based clinical practice guidelines, or to continue with the admissions order with an acknowledged reason for omitting anticoagulation (such as high bleeding risk, low stroke risk, high risk for falls, or patient refusal of anticoagulation).
“In patients for whom their providers were alerted, 35% elected to open the stroke-prevention order set, a very tiny percentage elected to read the AF guidelines, and about 64% exited but provided a rationale for omitting anticoagulation,” Dr. Piazza noted.
The alert group was far more likely to be prescribed anticoagulation during the hospitalization (25.8% vs. 9.5%; P less than .0001), at discharge (23.8% vs. 12.9%; P = .003), and at 90 days (27.7% vs. 17.1%; P = .007) than the control group. The alert resulted in a 55% relative risk reduction in a composite outcome of death, MI, cerebrovascular event, and systemic embolic event at 90 days (11.3% vs. 21.9%; P = .002). The alert group had an 87% lower incidence of MI at 90 days (1.2% vs. 8.6%, P = .0002) and 88% lower incidence of cerebrovascular events or systemic embolism at 90 days (0% vs. 2.4%; P = .02). Death at 90 days occurred in 10.1% in the alert group and 14.8% in the control group (P = .13).
One of the limitations of the study, Dr. Piazza noted, was that the most dramatic finding – reduction of major cardiovascular events – was a secondary, not a primary, endpoint. “CDS has the potential to be a powerful tool in prevention of cardiovascular events in patients with atrial fibrillation.”
Moderator Mintu Turakhia, MD, of Stanford (Calif.) University, questioned the low rate of anticoagulation in the study’s control arm – 9.5% – much lower than medians reported in many registries. He also asked Dr. Piazza to describe the mechanism of action for prescribing anticoagulation in these patients.
Dr. Piazza noted the study population was hospitalized patients whose providers had decided prior to their admissions not to prescribe anticoagulation; hence, the rate of anticoagulation in these patients was actually higher than expected.
Regarding the mechanism of action, “the electronic alert seems to preferentially increase the prescription of [direct oral anticoagulants] over warfarin, and that may have been one of the mechanisms,” Dr. Piazza said. Another explanation he offered were “off-target” effects whereby, if providers have a better idea of a patient’s risk for a stroke or MI, they’ll be more aggressive about managing other risk factors.
“There are a number of interventions that could be triggered if the alert prompted the provider to have a conversation with patients about their risk of stroke from AF,” he said. “This may have impact beyond what we can tell from this simple [Best Practice Advisory in the Epic EHR system]. I think we don’t have a great understanding of the full mechanisms of CDS.”
Dr. Piazza reported financial relationships with BTG, Janssen, Bristol-Myers Squibb, Daiichi Sankyo, Portola, and Bayer. Daiichi Sankyo funded the trial. Dr. Turakhia reported relationships with Apple, Janssen, AstraZeneca, VA, Boehringer Ingelheim, Cardiva Medical, Medtronic, Abbott, Precision Health Economics, iBeat, iRhythm, MyoKardia, Biotronik, and an ownership Interest in AliveCor.
CHICAGO – and had significantly lower rates of death and other cardiovascular events, compared with patients on a standard admissions protocol, according to results of a randomized, controlled trial presented at the American Heart Association Scientific Sessions.
“Alert-based computerized decision support [CDS] increased the prescription of anticoagulation for stroke prevention in atrial fibrillation during hospitalization, at discharge, and at 90 days after randomization in high-risk patients,” said Gregory Piazza, MD, of Brigham and Women’s Hospital, Boston, in presenting results of the AF-ALERT trial. “The reductions in major cardiovascular events was attributable to reductions in MI and stroke/transient ischemic attack at 90 days in patients whose physicians received the alert.”
The trial evaluated 458 patients hospitalized for AF or flutter and with CHA2DS2-VASc scores of 1-8 randomly assigned to the alert (n = 258) or no-alert (n = 210) groups.
Dr. Piazza explained that for those in the alert group, the CDS system notified physicians when the patient’s CHA2DS2-VASc score increased. From there, the physician could choose to open an order template to prescribe evidence-based medications to prevent stroke, to elect to review evidence-based clinical practice guidelines, or to continue with the admissions order with an acknowledged reason for omitting anticoagulation (such as high bleeding risk, low stroke risk, high risk for falls, or patient refusal of anticoagulation).
“In patients for whom their providers were alerted, 35% elected to open the stroke-prevention order set, a very tiny percentage elected to read the AF guidelines, and about 64% exited but provided a rationale for omitting anticoagulation,” Dr. Piazza noted.
The alert group was far more likely to be prescribed anticoagulation during the hospitalization (25.8% vs. 9.5%; P less than .0001), at discharge (23.8% vs. 12.9%; P = .003), and at 90 days (27.7% vs. 17.1%; P = .007) than the control group. The alert resulted in a 55% relative risk reduction in a composite outcome of death, MI, cerebrovascular event, and systemic embolic event at 90 days (11.3% vs. 21.9%; P = .002). The alert group had an 87% lower incidence of MI at 90 days (1.2% vs. 8.6%, P = .0002) and 88% lower incidence of cerebrovascular events or systemic embolism at 90 days (0% vs. 2.4%; P = .02). Death at 90 days occurred in 10.1% in the alert group and 14.8% in the control group (P = .13).
One of the limitations of the study, Dr. Piazza noted, was that the most dramatic finding – reduction of major cardiovascular events – was a secondary, not a primary, endpoint. “CDS has the potential to be a powerful tool in prevention of cardiovascular events in patients with atrial fibrillation.”
Moderator Mintu Turakhia, MD, of Stanford (Calif.) University, questioned the low rate of anticoagulation in the study’s control arm – 9.5% – much lower than medians reported in many registries. He also asked Dr. Piazza to describe the mechanism of action for prescribing anticoagulation in these patients.
Dr. Piazza noted the study population was hospitalized patients whose providers had decided prior to their admissions not to prescribe anticoagulation; hence, the rate of anticoagulation in these patients was actually higher than expected.
Regarding the mechanism of action, “the electronic alert seems to preferentially increase the prescription of [direct oral anticoagulants] over warfarin, and that may have been one of the mechanisms,” Dr. Piazza said. Another explanation he offered were “off-target” effects whereby, if providers have a better idea of a patient’s risk for a stroke or MI, they’ll be more aggressive about managing other risk factors.
“There are a number of interventions that could be triggered if the alert prompted the provider to have a conversation with patients about their risk of stroke from AF,” he said. “This may have impact beyond what we can tell from this simple [Best Practice Advisory in the Epic EHR system]. I think we don’t have a great understanding of the full mechanisms of CDS.”
Dr. Piazza reported financial relationships with BTG, Janssen, Bristol-Myers Squibb, Daiichi Sankyo, Portola, and Bayer. Daiichi Sankyo funded the trial. Dr. Turakhia reported relationships with Apple, Janssen, AstraZeneca, VA, Boehringer Ingelheim, Cardiva Medical, Medtronic, Abbott, Precision Health Economics, iBeat, iRhythm, MyoKardia, Biotronik, and an ownership Interest in AliveCor.
REPORTING FROM AHA SCIENTIFIC SESSIONS
Key clinical point: A digital alert system led to improved outcomes in atrial fibrillation patients.
Major finding: Anticoagulation rates were 25.8% in the alert group versus 9.5% for controls.
Study details: AF-ALERT was a randomized, controlled trial of 458 high-risk patients with atrial fibrillation or flutter.
Disclosures: Dr. Piazza reported financial relationships with BTG, Janssen, Bristol-Myers Squibb, Daiichi Sankyo, Portola, and Bayer. Daiichi Sankyo provided funding for the study.
BMI compares favorably with body scanning for ID of cardiometabolic traits
Although body mass index is criticized for not distinguishing fat from lean mass, its ability to detect subclinical cardiometabolic abnormalities was on par with more sophisticated body scanning technology, according a recent analysis.
BMI and dual-energy x-ray absorptiometry (DXA) had similar associations with cardiometabolic traits associated with coronary heart disease in individuals evaluated at 10 and 18 years of age in a population-based birth cohort study, the study investigators said.
Changes over time in BMI and DXA also were strongly associated with changes in blood pressure, cholesterol, and other markers, according to Joshua A. Bell, PhD, of MRC Integrative Epidemiology Unit, University of Bristol, England, and his coinvestigators.
“Altogether, the results support abdominal fatness as a primary driver of cardiometabolic dysfunction and BMI as a useful tool for detecting its effects,” Dr. Bell and his colleagues said in a report on the study appearing in the Journal of the American College of Cardiology.
In their analysis, Dr. Bell and coinvestigators used Pearson correlation coefficients to compare BMI and total and regional fat indexes from DXA in offspring participants from ALSPAC, (the Avon Longitudinal Study of Parents and Children), in which BMI and DXA measurements were collected at 10 and 18 years of age.
Researchers identified a total of 2,840 participants with at least one measurement at each of those time points. The mean BMI was 17.5 kg/m2 at 10 years of age and 22.7 kg/m2 at 18 years of age, with greater than 10% of participants classified as obese at each of those time points.
High BMI and high total fat mass index were similarly associated with a variety of cardiometabolic traits, including systolic and diastolic blood pressure, higher low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) cholesterol levels and lower high-density lipoprotein (HDL) cholesterol levels, and more inflammation, investigators found.
BMI was strongly correlated with DXA total and regional fat indexes at 10 years of age, and again at 18 years of age, they reported.
Moreover, gains in BMI from 10 to 18 years of age were strongly associated with higher blood pressure, higher LDL and VLDL cholesterol, lower HDL cholesterol, and other cardiometabolic traits, while associations between DXA measurements and those traits closely tracked those of BMI in pattern and magnitude, investigators added.
Fatness is most often measured in populations using BMI, and causal analyses suggest linkage between higher BMI and coronary heart disease and its intermediates, including blood pressure, LDL and remnant cholesterol, and glucose; despite that, BMI is often disparaged as a tool for assessing cardiometabolic abnormalities because it does not distinguish fat from lean mass and cannot quantify fat distribution, investigators said.
However, based on results of this analysis, it is reasonable to depend on BMI to indirectly measure body and abdominal fatness in future studies, they said in their report.
Dr. Bell and his colleagues reported that they had no relationships relevant to the study publication.
SOURCE: Bell JA et al. J Am Coll Cardiol. 2018 Dec 18;72(24):3142-54.
This study reinforces fatness, quantified by body mass index, as the key modifiable factor for maintaining healthy metabolism in young people, according to Ville-Petteri Mäkinen, ScD.
“The good news is that a single BMI measurement may be enough to capture the same essential information as a detailed body scan and serial measurements,” Dr. Mäkinen wrote in an accompanying editorial.
One other important take-home message of the study is that fat gain is not beneficial in any body region; that finding is important with respect to changes in BMI and fat mass index observed in the second decade of life that were associated with cardiometabolic risk factors in the late teens, he noted.
However, the broader take-home message for society is that children are being exposed to an “adverse metabolic milieu” that predicts cardiovascular disease in adulthood, according to Dr. Mäkinen.
“Childhood obesity must not be seen as a phase that passes but as a looming public health crisis that needs to be addressed by all of us,” he concluded.
Dr. Mäkinen is with the South Australian Health and Medical Research Institute, Adelaide. He had no relationships relevant to the contents of his editorial (J Am College Cardiol. 2018 Dec 18;74[24]3155-7).
This study reinforces fatness, quantified by body mass index, as the key modifiable factor for maintaining healthy metabolism in young people, according to Ville-Petteri Mäkinen, ScD.
“The good news is that a single BMI measurement may be enough to capture the same essential information as a detailed body scan and serial measurements,” Dr. Mäkinen wrote in an accompanying editorial.
One other important take-home message of the study is that fat gain is not beneficial in any body region; that finding is important with respect to changes in BMI and fat mass index observed in the second decade of life that were associated with cardiometabolic risk factors in the late teens, he noted.
However, the broader take-home message for society is that children are being exposed to an “adverse metabolic milieu” that predicts cardiovascular disease in adulthood, according to Dr. Mäkinen.
“Childhood obesity must not be seen as a phase that passes but as a looming public health crisis that needs to be addressed by all of us,” he concluded.
Dr. Mäkinen is with the South Australian Health and Medical Research Institute, Adelaide. He had no relationships relevant to the contents of his editorial (J Am College Cardiol. 2018 Dec 18;74[24]3155-7).
This study reinforces fatness, quantified by body mass index, as the key modifiable factor for maintaining healthy metabolism in young people, according to Ville-Petteri Mäkinen, ScD.
“The good news is that a single BMI measurement may be enough to capture the same essential information as a detailed body scan and serial measurements,” Dr. Mäkinen wrote in an accompanying editorial.
One other important take-home message of the study is that fat gain is not beneficial in any body region; that finding is important with respect to changes in BMI and fat mass index observed in the second decade of life that were associated with cardiometabolic risk factors in the late teens, he noted.
However, the broader take-home message for society is that children are being exposed to an “adverse metabolic milieu” that predicts cardiovascular disease in adulthood, according to Dr. Mäkinen.
“Childhood obesity must not be seen as a phase that passes but as a looming public health crisis that needs to be addressed by all of us,” he concluded.
Dr. Mäkinen is with the South Australian Health and Medical Research Institute, Adelaide. He had no relationships relevant to the contents of his editorial (J Am College Cardiol. 2018 Dec 18;74[24]3155-7).
Although body mass index is criticized for not distinguishing fat from lean mass, its ability to detect subclinical cardiometabolic abnormalities was on par with more sophisticated body scanning technology, according a recent analysis.
BMI and dual-energy x-ray absorptiometry (DXA) had similar associations with cardiometabolic traits associated with coronary heart disease in individuals evaluated at 10 and 18 years of age in a population-based birth cohort study, the study investigators said.
Changes over time in BMI and DXA also were strongly associated with changes in blood pressure, cholesterol, and other markers, according to Joshua A. Bell, PhD, of MRC Integrative Epidemiology Unit, University of Bristol, England, and his coinvestigators.
“Altogether, the results support abdominal fatness as a primary driver of cardiometabolic dysfunction and BMI as a useful tool for detecting its effects,” Dr. Bell and his colleagues said in a report on the study appearing in the Journal of the American College of Cardiology.
In their analysis, Dr. Bell and coinvestigators used Pearson correlation coefficients to compare BMI and total and regional fat indexes from DXA in offspring participants from ALSPAC, (the Avon Longitudinal Study of Parents and Children), in which BMI and DXA measurements were collected at 10 and 18 years of age.
Researchers identified a total of 2,840 participants with at least one measurement at each of those time points. The mean BMI was 17.5 kg/m2 at 10 years of age and 22.7 kg/m2 at 18 years of age, with greater than 10% of participants classified as obese at each of those time points.
High BMI and high total fat mass index were similarly associated with a variety of cardiometabolic traits, including systolic and diastolic blood pressure, higher low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) cholesterol levels and lower high-density lipoprotein (HDL) cholesterol levels, and more inflammation, investigators found.
BMI was strongly correlated with DXA total and regional fat indexes at 10 years of age, and again at 18 years of age, they reported.
Moreover, gains in BMI from 10 to 18 years of age were strongly associated with higher blood pressure, higher LDL and VLDL cholesterol, lower HDL cholesterol, and other cardiometabolic traits, while associations between DXA measurements and those traits closely tracked those of BMI in pattern and magnitude, investigators added.
Fatness is most often measured in populations using BMI, and causal analyses suggest linkage between higher BMI and coronary heart disease and its intermediates, including blood pressure, LDL and remnant cholesterol, and glucose; despite that, BMI is often disparaged as a tool for assessing cardiometabolic abnormalities because it does not distinguish fat from lean mass and cannot quantify fat distribution, investigators said.
However, based on results of this analysis, it is reasonable to depend on BMI to indirectly measure body and abdominal fatness in future studies, they said in their report.
Dr. Bell and his colleagues reported that they had no relationships relevant to the study publication.
SOURCE: Bell JA et al. J Am Coll Cardiol. 2018 Dec 18;72(24):3142-54.
Although body mass index is criticized for not distinguishing fat from lean mass, its ability to detect subclinical cardiometabolic abnormalities was on par with more sophisticated body scanning technology, according a recent analysis.
BMI and dual-energy x-ray absorptiometry (DXA) had similar associations with cardiometabolic traits associated with coronary heart disease in individuals evaluated at 10 and 18 years of age in a population-based birth cohort study, the study investigators said.
Changes over time in BMI and DXA also were strongly associated with changes in blood pressure, cholesterol, and other markers, according to Joshua A. Bell, PhD, of MRC Integrative Epidemiology Unit, University of Bristol, England, and his coinvestigators.
“Altogether, the results support abdominal fatness as a primary driver of cardiometabolic dysfunction and BMI as a useful tool for detecting its effects,” Dr. Bell and his colleagues said in a report on the study appearing in the Journal of the American College of Cardiology.
In their analysis, Dr. Bell and coinvestigators used Pearson correlation coefficients to compare BMI and total and regional fat indexes from DXA in offspring participants from ALSPAC, (the Avon Longitudinal Study of Parents and Children), in which BMI and DXA measurements were collected at 10 and 18 years of age.
Researchers identified a total of 2,840 participants with at least one measurement at each of those time points. The mean BMI was 17.5 kg/m2 at 10 years of age and 22.7 kg/m2 at 18 years of age, with greater than 10% of participants classified as obese at each of those time points.
High BMI and high total fat mass index were similarly associated with a variety of cardiometabolic traits, including systolic and diastolic blood pressure, higher low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) cholesterol levels and lower high-density lipoprotein (HDL) cholesterol levels, and more inflammation, investigators found.
BMI was strongly correlated with DXA total and regional fat indexes at 10 years of age, and again at 18 years of age, they reported.
Moreover, gains in BMI from 10 to 18 years of age were strongly associated with higher blood pressure, higher LDL and VLDL cholesterol, lower HDL cholesterol, and other cardiometabolic traits, while associations between DXA measurements and those traits closely tracked those of BMI in pattern and magnitude, investigators added.
Fatness is most often measured in populations using BMI, and causal analyses suggest linkage between higher BMI and coronary heart disease and its intermediates, including blood pressure, LDL and remnant cholesterol, and glucose; despite that, BMI is often disparaged as a tool for assessing cardiometabolic abnormalities because it does not distinguish fat from lean mass and cannot quantify fat distribution, investigators said.
However, based on results of this analysis, it is reasonable to depend on BMI to indirectly measure body and abdominal fatness in future studies, they said in their report.
Dr. Bell and his colleagues reported that they had no relationships relevant to the study publication.
SOURCE: Bell JA et al. J Am Coll Cardiol. 2018 Dec 18;72(24):3142-54.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Body mass index and dual-energy x-ray absorptiometry had similar associations with cardiometabolic abnormalities in individuals evaluated at 10 and 18 years of age.
Major finding: BMI was strongly correlated with DXA total and regional fat indexes at age 10 years (0.9) and age 18 years.
Study details: Analysis of 2,840 offspring participants in ALSPAC (the Avon Longitudinal Study of Parents and Children).
Disclosures: Study authors reported that they had no relationships relevant to the study or its publication.
Source: Bell JA et al. J Am Coll Cardiol. 2018 Dec 10;72(24):3142-54.
ACA in peril after Texas ruling
Also today, baracitinib findings have immediate clinical relevance for patients with RA, a look at how often epileptic patients have treatment delayed, and the FDA issues an alert after e-cigarette liquids were found containing erectile dysfunction medication.
Also today, baracitinib findings have immediate clinical relevance for patients with RA, a look at how often epileptic patients have treatment delayed, and the FDA issues an alert after e-cigarette liquids were found containing erectile dysfunction medication.
Also today, baracitinib findings have immediate clinical relevance for patients with RA, a look at how often epileptic patients have treatment delayed, and the FDA issues an alert after e-cigarette liquids were found containing erectile dysfunction medication.
Health Canada approves new indication for daratumumab
Health Canada has approved a third indication for daratumumab (Darzalex®) in multiple myeloma (MM).
The drug is now approved for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat patients with newly diagnosed MM who are ineligible for autologous stem cell transplant.
Health Canada previously approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat MM patients who have received at least one prior therapy.
Health Canada also approved daratumumab as monotherapy for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are refractory to both a proteasome inhibitor and an immunomodulatory agent.
For the product monograph and more information about daratumumab, visit https://www.janssen.com/canada/products.
Trial results
Health Canada’s latest approval of daratumumab is supported by data from the phase 3 ALCYONE study (NCT02195479), which were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The ALCYONE trial was sponsored by Janssen Research & Development, LLC.
Health Canada has approved a third indication for daratumumab (Darzalex®) in multiple myeloma (MM).
The drug is now approved for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat patients with newly diagnosed MM who are ineligible for autologous stem cell transplant.
Health Canada previously approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat MM patients who have received at least one prior therapy.
Health Canada also approved daratumumab as monotherapy for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are refractory to both a proteasome inhibitor and an immunomodulatory agent.
For the product monograph and more information about daratumumab, visit https://www.janssen.com/canada/products.
Trial results
Health Canada’s latest approval of daratumumab is supported by data from the phase 3 ALCYONE study (NCT02195479), which were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The ALCYONE trial was sponsored by Janssen Research & Development, LLC.
Health Canada has approved a third indication for daratumumab (Darzalex®) in multiple myeloma (MM).
The drug is now approved for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat patients with newly diagnosed MM who are ineligible for autologous stem cell transplant.
Health Canada previously approved daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat MM patients who have received at least one prior therapy.
Health Canada also approved daratumumab as monotherapy for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are refractory to both a proteasome inhibitor and an immunomodulatory agent.
For the product monograph and more information about daratumumab, visit https://www.janssen.com/canada/products.
Trial results
Health Canada’s latest approval of daratumumab is supported by data from the phase 3 ALCYONE study (NCT02195479), which were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The ALCYONE trial was sponsored by Janssen Research & Development, LLC.
CHMP recommends BV+AVD for Hodgkin lymphoma
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the marketing authorization for brentuximab vedotin (BV).
The CHMP has recommended approval for BV (Adcetris) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) to treat adults with previously untreated, CD30+, stage IV Hodgkin lymphoma (HL).
The CHMP’s recommendation will be reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of a CHMP recommendation.
BV is already EC-approved to treat adults with:
- CD30+ HL at increased risk of relapse or progression following autologous stem cell transplant (ASCT)
- Relapsed or refractory, CD30+ HL following ASCT or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- Relapsed or refractory systemic anaplastic large-cell lymphoma
- CD30+ cutaneous T-cell lymphoma after at least one prior systemic therapy.
Phase 3 trial
The CHMP’s recommendation to approve BV in combination with AVD is supported by the phase 3 ECHELON-1 trial (NCT01712490).
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
In this trial, researchers compared BV plus AVD (BV+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, BV+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the BV+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the BV+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the BV+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the BV+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with BV+AVD, while pulmonary toxicity was more common with ABVD.
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals, Inc. (a Takeda company) in collaboration with Seattle Genetics, Inc.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the marketing authorization for brentuximab vedotin (BV).
The CHMP has recommended approval for BV (Adcetris) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) to treat adults with previously untreated, CD30+, stage IV Hodgkin lymphoma (HL).
The CHMP’s recommendation will be reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of a CHMP recommendation.
BV is already EC-approved to treat adults with:
- CD30+ HL at increased risk of relapse or progression following autologous stem cell transplant (ASCT)
- Relapsed or refractory, CD30+ HL following ASCT or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- Relapsed or refractory systemic anaplastic large-cell lymphoma
- CD30+ cutaneous T-cell lymphoma after at least one prior systemic therapy.
Phase 3 trial
The CHMP’s recommendation to approve BV in combination with AVD is supported by the phase 3 ECHELON-1 trial (NCT01712490).
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
In this trial, researchers compared BV plus AVD (BV+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, BV+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the BV+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the BV+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the BV+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the BV+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with BV+AVD, while pulmonary toxicity was more common with ABVD.
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals, Inc. (a Takeda company) in collaboration with Seattle Genetics, Inc.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the marketing authorization for brentuximab vedotin (BV).
The CHMP has recommended approval for BV (Adcetris) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) to treat adults with previously untreated, CD30+, stage IV Hodgkin lymphoma (HL).
The CHMP’s recommendation will be reviewed by the European Commission (EC), which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The EC usually makes a decision within 67 days of a CHMP recommendation.
BV is already EC-approved to treat adults with:
- CD30+ HL at increased risk of relapse or progression following autologous stem cell transplant (ASCT)
- Relapsed or refractory, CD30+ HL following ASCT or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- Relapsed or refractory systemic anaplastic large-cell lymphoma
- CD30+ cutaneous T-cell lymphoma after at least one prior systemic therapy.
Phase 3 trial
The CHMP’s recommendation to approve BV in combination with AVD is supported by the phase 3 ECHELON-1 trial (NCT01712490).
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
In this trial, researchers compared BV plus AVD (BV+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, BV+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the BV+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the BV+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the BV+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the BV+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with BV+AVD, while pulmonary toxicity was more common with ABVD.
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals, Inc. (a Takeda company) in collaboration with Seattle Genetics, Inc.
Avoiding the Pitfalls of “Half-visits”
A 3-and-a-half-year-old girl presented to a pediatrician’s office with complaints of vomiting and high fever (103.3°). She was seen by a nurse practitioner, who diagnosed gastroenteritis, prescribed fluid replacement and acetaminophen, and sent the child home.
The NP did not chart the child’s blood pressure, pulse, or respiratory rate. She did note swollen lymph nodes and absence of diarrhea. The NP performed a flu screen but did not order a rapid strep test or urinalysis.
Several hours later, the child was taken to the emergency department with shortness of breath, cough, congestion, tachycardia, hypoxia, dehydration, and lethargy. She was admitted to the pediatric ICU with diagnoses of pneumonia, acute respiratory distress, hypoxemia, neutropenia, and sepsis. She was given IV antibiotics.
Several hours later, the decision was made to transfer the patient to a regional medical center. During transfer, she suffered cardiopulmonary arrest while being placed on a ventilator for transport. Upon arrival at the hospital, she arrested again and required resuscitation for several hours until spontaneous circulation could not be restored.
An autopsy concluded the child died of sepsis and shock from Group A beta-hemolytic streptococcal infection.
It was argued that the NP failed to diagnose and treat streptococcal toxic shock syndrome at the time of the child’s presentation. In support of this contention, it was argued that the NP had failed to perform basic follow-up when the child’s flu test came back negative and that the child’s swollen lymph nodes and lack of diarrhea both mitigated against the NP’s diagnosis of gastroenteritis.
VERDICT
The parties in this case reached a $950,000 settlement.
Continue to: DISCUSSION
DISCUSSION
Every headache and fever could be an early meningitis, every vague abdominal pain an early appendicitis. So how do we handle innocuous-appearing cases with early, nonspecific symptoms of a very serious illness about to unfold?
We must start by following the Miyagi rule. In The Karate Kid, Mr. Miyagi advised that walking on the left or the right side of the road was safe, but walking in the middle would result, sooner or later, in “squish, just like grape.” Although he related this premise to karate, we can also apply it to medicine: See a patient or do not see a patient; but if you see a patient “so-so,” you will be squished—by the patient, by a plaintiff’s attorney, and/or by your state’s medical board.
A case such as this one strikes fear in the heart of anyone who has seen patients in an ambulatory setting. The initial presentation was modest: a toddler with vomiting and fever. We do not know what the other vital signs were, and we do not know whether the child appeared toxic. The lack of vital signs or recorded vital signs represent half-measures. The patient’s vitals could have been normal, and the NP’s actions could have been fully defensible. The problem is, we don’t know—and the clinician is on the hook.
All patients require vital signs. They must be done; they must be complete, and they must be recorded. At a minimum, temperature, blood pressure, respiratory rate, and (generally) O2 saturation are required. Some specialties may have other requirements (eg, fingerstick glucose for patients with diabetes, visual acuity testing for those with eye complaints). A full list of data you should be obtaining is practice specific and beyond the scope of this article; the point is, decide on the relevant set of vitals and intake data and be sure it is recorded at every visit.
Failure to obtain and record vital signs—as seen in this case—is sloppy practice, difficult to defend, and sets up an inference of negligence. Even when the care is perfect (and without bad outcome), if the medical board reviews the record for any reason, you will be sanctioned for “failure to keep adequate and accurate medical records” and your license burdened. Here, we are told the defendant NP “did not chart the child’s blood pressure, pulse, or respiratory rate.” I am willing to bet the NP was not responsible for charting the values in the normal course of practice, but see how responsibility is parked with the clinician? If intake staff do not record vital signs, politely (yet firmly) insist they do so.
Continue to: Furthermore, the disposition of many child visits...
Furthermore, the disposition of many child visits turns on whether the patient “appeared toxic.” Any child’s condition could worsen after evaluation—and in litigation, parents, friends, and family will testify the patient was extremely ill, they “knew something was wrong,” and the clinician ignored their loved one. Thus, the jury will be invited to reconstruct how the child appeared.
When assessing children and the question of “toxic appearance” arises, don’t state a conclusion—paint a picture. Don’t merely state “child appeared nontoxic.” Use your powers of observation to record why they appear nontoxic: “Child sitting up, watching Moana on parent’s phone, smiling and laughing appropriately.” Get interactive; some pediatric providers carry a small vial of bubbles with them and record the child’s response to bubble-making (“Child appropriately reaching for bubbles, smiling, holding one on finger”). The cost is less than $1 for the bubbles, plus the documentation time. The benefit is that it paints a clear picture for the jury of a child responding appropriately. And if your observations suggest a child who is at least unwell—if the movie is poorly received or the bubbles prompt the child to scream or bury her face in her mom’s shoulder—you can consider oral antipyretics/analgesics, fluid, and re-observation.
Another way to create a strong and defensible record is to use patient quotations. These can be extremely helpful to your defense in a malpractice action; as an attorney, I have searched 8,000 pages of records in a medical malpractice case, hoping to find a clear description from a human (not a template) of how a patient looked. Make it clear by adding patient remarks to the chart—just remember that “the only thing that belongs in quotes is what comes out of the patient’s mouth.” Words from an 8-year-old boy— such as “My brother found a legendary scar [a reference to Fortnite] and almost won”—may seem silly, but this documentation itself could win your case.
With teenagers, you may have to ask more questions to glean something suitable; you could ask a 13-year-old her favorite sport and when her one-word answer is “Lacrosse,” ask why. Even if the response is “Because, I don’t know, it’s exciting. There are a lot of goals,” write that down exactly (along with any other observations, such as Teen texting on her phone). These notations tell the plaintiff’s attorney, the judge, and the jury that the patient was behaving normally and interacting with the environment. Should this teen later deteriorate with meningitis, the plaintiff will claim she was toxic in the office. The medical record, however, will show that the patient’s condition changed, and it was a departure from how she looked in your office.
Also, it never hurts to get backup. In any close call, ask the nurse to reevaluate the patient as to whether he or she is “toxic appearing” or is interacting normally with the environment. Have the nurse or medical assistant record facts, such as “patient trying to make a plane out of two tongue depressors, pretending to land it on sister’s leg.” This will create a strong and defensible record: two clinicians relaying two sets of detailed observations.
Continue to: Likewise, encourage intake staff to document...
Likewise, encourage intake staff to document what they see rather than what they conclude from it. Buzzwords (eg, listless, lethargic) should be avoided. If such characterizations find their way into the record, you must take active steps to address them. Either agree with the characterization and perform appropriate work-up, or establish why you do not agree using the methods described (detailed description, verification by another clinician).
Taking these steps will help to protect you in the event of a changing clinical course. But also be wary of those predictable circumstances that lead you into Mr. Miyagi’s middle of the road (what I call “half-visits”): a quick look at a sibling in the room during a patient’s appointment; a “curbside consult” on the medical assistant’s child; the neighborhood acquaintance who asks you to “just take a look.” Why are these dangerous? Because they remove the clinician from his or her usual routine: proper examination on a properly undressed patient, formal assessment of vital signs, and review of relevant history in the chart, among other things. (In this way, phone and email communications with patients require similar caution.) Skipping the routine leads to shortcuts, and shortcuts lead to bad medicine. And if that doesn’t worry you, remember: All these scenarios create a full legal duty and clinician/patient relationship—making them potential pathways to misdiagnosis and eventual loss of license.
IN SUMMARY
Don’t be party to a “half-visit”; insist on full vital signs and a complete visit following your usual routine. Use observational powers and patient quotations to paint a picture of how a patient looked, get backup from another clinician with similar observations. If you can’t document a reassuring record, protect the patient and make the required intervention.
A 3-and-a-half-year-old girl presented to a pediatrician’s office with complaints of vomiting and high fever (103.3°). She was seen by a nurse practitioner, who diagnosed gastroenteritis, prescribed fluid replacement and acetaminophen, and sent the child home.
The NP did not chart the child’s blood pressure, pulse, or respiratory rate. She did note swollen lymph nodes and absence of diarrhea. The NP performed a flu screen but did not order a rapid strep test or urinalysis.
Several hours later, the child was taken to the emergency department with shortness of breath, cough, congestion, tachycardia, hypoxia, dehydration, and lethargy. She was admitted to the pediatric ICU with diagnoses of pneumonia, acute respiratory distress, hypoxemia, neutropenia, and sepsis. She was given IV antibiotics.
Several hours later, the decision was made to transfer the patient to a regional medical center. During transfer, she suffered cardiopulmonary arrest while being placed on a ventilator for transport. Upon arrival at the hospital, she arrested again and required resuscitation for several hours until spontaneous circulation could not be restored.
An autopsy concluded the child died of sepsis and shock from Group A beta-hemolytic streptococcal infection.
It was argued that the NP failed to diagnose and treat streptococcal toxic shock syndrome at the time of the child’s presentation. In support of this contention, it was argued that the NP had failed to perform basic follow-up when the child’s flu test came back negative and that the child’s swollen lymph nodes and lack of diarrhea both mitigated against the NP’s diagnosis of gastroenteritis.
VERDICT
The parties in this case reached a $950,000 settlement.
Continue to: DISCUSSION
DISCUSSION
Every headache and fever could be an early meningitis, every vague abdominal pain an early appendicitis. So how do we handle innocuous-appearing cases with early, nonspecific symptoms of a very serious illness about to unfold?
We must start by following the Miyagi rule. In The Karate Kid, Mr. Miyagi advised that walking on the left or the right side of the road was safe, but walking in the middle would result, sooner or later, in “squish, just like grape.” Although he related this premise to karate, we can also apply it to medicine: See a patient or do not see a patient; but if you see a patient “so-so,” you will be squished—by the patient, by a plaintiff’s attorney, and/or by your state’s medical board.
A case such as this one strikes fear in the heart of anyone who has seen patients in an ambulatory setting. The initial presentation was modest: a toddler with vomiting and fever. We do not know what the other vital signs were, and we do not know whether the child appeared toxic. The lack of vital signs or recorded vital signs represent half-measures. The patient’s vitals could have been normal, and the NP’s actions could have been fully defensible. The problem is, we don’t know—and the clinician is on the hook.
All patients require vital signs. They must be done; they must be complete, and they must be recorded. At a minimum, temperature, blood pressure, respiratory rate, and (generally) O2 saturation are required. Some specialties may have other requirements (eg, fingerstick glucose for patients with diabetes, visual acuity testing for those with eye complaints). A full list of data you should be obtaining is practice specific and beyond the scope of this article; the point is, decide on the relevant set of vitals and intake data and be sure it is recorded at every visit.
Failure to obtain and record vital signs—as seen in this case—is sloppy practice, difficult to defend, and sets up an inference of negligence. Even when the care is perfect (and without bad outcome), if the medical board reviews the record for any reason, you will be sanctioned for “failure to keep adequate and accurate medical records” and your license burdened. Here, we are told the defendant NP “did not chart the child’s blood pressure, pulse, or respiratory rate.” I am willing to bet the NP was not responsible for charting the values in the normal course of practice, but see how responsibility is parked with the clinician? If intake staff do not record vital signs, politely (yet firmly) insist they do so.
Continue to: Furthermore, the disposition of many child visits...
Furthermore, the disposition of many child visits turns on whether the patient “appeared toxic.” Any child’s condition could worsen after evaluation—and in litigation, parents, friends, and family will testify the patient was extremely ill, they “knew something was wrong,” and the clinician ignored their loved one. Thus, the jury will be invited to reconstruct how the child appeared.
When assessing children and the question of “toxic appearance” arises, don’t state a conclusion—paint a picture. Don’t merely state “child appeared nontoxic.” Use your powers of observation to record why they appear nontoxic: “Child sitting up, watching Moana on parent’s phone, smiling and laughing appropriately.” Get interactive; some pediatric providers carry a small vial of bubbles with them and record the child’s response to bubble-making (“Child appropriately reaching for bubbles, smiling, holding one on finger”). The cost is less than $1 for the bubbles, plus the documentation time. The benefit is that it paints a clear picture for the jury of a child responding appropriately. And if your observations suggest a child who is at least unwell—if the movie is poorly received or the bubbles prompt the child to scream or bury her face in her mom’s shoulder—you can consider oral antipyretics/analgesics, fluid, and re-observation.
Another way to create a strong and defensible record is to use patient quotations. These can be extremely helpful to your defense in a malpractice action; as an attorney, I have searched 8,000 pages of records in a medical malpractice case, hoping to find a clear description from a human (not a template) of how a patient looked. Make it clear by adding patient remarks to the chart—just remember that “the only thing that belongs in quotes is what comes out of the patient’s mouth.” Words from an 8-year-old boy— such as “My brother found a legendary scar [a reference to Fortnite] and almost won”—may seem silly, but this documentation itself could win your case.
With teenagers, you may have to ask more questions to glean something suitable; you could ask a 13-year-old her favorite sport and when her one-word answer is “Lacrosse,” ask why. Even if the response is “Because, I don’t know, it’s exciting. There are a lot of goals,” write that down exactly (along with any other observations, such as Teen texting on her phone). These notations tell the plaintiff’s attorney, the judge, and the jury that the patient was behaving normally and interacting with the environment. Should this teen later deteriorate with meningitis, the plaintiff will claim she was toxic in the office. The medical record, however, will show that the patient’s condition changed, and it was a departure from how she looked in your office.
Also, it never hurts to get backup. In any close call, ask the nurse to reevaluate the patient as to whether he or she is “toxic appearing” or is interacting normally with the environment. Have the nurse or medical assistant record facts, such as “patient trying to make a plane out of two tongue depressors, pretending to land it on sister’s leg.” This will create a strong and defensible record: two clinicians relaying two sets of detailed observations.
Continue to: Likewise, encourage intake staff to document...
Likewise, encourage intake staff to document what they see rather than what they conclude from it. Buzzwords (eg, listless, lethargic) should be avoided. If such characterizations find their way into the record, you must take active steps to address them. Either agree with the characterization and perform appropriate work-up, or establish why you do not agree using the methods described (detailed description, verification by another clinician).
Taking these steps will help to protect you in the event of a changing clinical course. But also be wary of those predictable circumstances that lead you into Mr. Miyagi’s middle of the road (what I call “half-visits”): a quick look at a sibling in the room during a patient’s appointment; a “curbside consult” on the medical assistant’s child; the neighborhood acquaintance who asks you to “just take a look.” Why are these dangerous? Because they remove the clinician from his or her usual routine: proper examination on a properly undressed patient, formal assessment of vital signs, and review of relevant history in the chart, among other things. (In this way, phone and email communications with patients require similar caution.) Skipping the routine leads to shortcuts, and shortcuts lead to bad medicine. And if that doesn’t worry you, remember: All these scenarios create a full legal duty and clinician/patient relationship—making them potential pathways to misdiagnosis and eventual loss of license.
IN SUMMARY
Don’t be party to a “half-visit”; insist on full vital signs and a complete visit following your usual routine. Use observational powers and patient quotations to paint a picture of how a patient looked, get backup from another clinician with similar observations. If you can’t document a reassuring record, protect the patient and make the required intervention.
A 3-and-a-half-year-old girl presented to a pediatrician’s office with complaints of vomiting and high fever (103.3°). She was seen by a nurse practitioner, who diagnosed gastroenteritis, prescribed fluid replacement and acetaminophen, and sent the child home.
The NP did not chart the child’s blood pressure, pulse, or respiratory rate. She did note swollen lymph nodes and absence of diarrhea. The NP performed a flu screen but did not order a rapid strep test or urinalysis.
Several hours later, the child was taken to the emergency department with shortness of breath, cough, congestion, tachycardia, hypoxia, dehydration, and lethargy. She was admitted to the pediatric ICU with diagnoses of pneumonia, acute respiratory distress, hypoxemia, neutropenia, and sepsis. She was given IV antibiotics.
Several hours later, the decision was made to transfer the patient to a regional medical center. During transfer, she suffered cardiopulmonary arrest while being placed on a ventilator for transport. Upon arrival at the hospital, she arrested again and required resuscitation for several hours until spontaneous circulation could not be restored.
An autopsy concluded the child died of sepsis and shock from Group A beta-hemolytic streptococcal infection.
It was argued that the NP failed to diagnose and treat streptococcal toxic shock syndrome at the time of the child’s presentation. In support of this contention, it was argued that the NP had failed to perform basic follow-up when the child’s flu test came back negative and that the child’s swollen lymph nodes and lack of diarrhea both mitigated against the NP’s diagnosis of gastroenteritis.
VERDICT
The parties in this case reached a $950,000 settlement.
Continue to: DISCUSSION
DISCUSSION
Every headache and fever could be an early meningitis, every vague abdominal pain an early appendicitis. So how do we handle innocuous-appearing cases with early, nonspecific symptoms of a very serious illness about to unfold?
We must start by following the Miyagi rule. In The Karate Kid, Mr. Miyagi advised that walking on the left or the right side of the road was safe, but walking in the middle would result, sooner or later, in “squish, just like grape.” Although he related this premise to karate, we can also apply it to medicine: See a patient or do not see a patient; but if you see a patient “so-so,” you will be squished—by the patient, by a plaintiff’s attorney, and/or by your state’s medical board.
A case such as this one strikes fear in the heart of anyone who has seen patients in an ambulatory setting. The initial presentation was modest: a toddler with vomiting and fever. We do not know what the other vital signs were, and we do not know whether the child appeared toxic. The lack of vital signs or recorded vital signs represent half-measures. The patient’s vitals could have been normal, and the NP’s actions could have been fully defensible. The problem is, we don’t know—and the clinician is on the hook.
All patients require vital signs. They must be done; they must be complete, and they must be recorded. At a minimum, temperature, blood pressure, respiratory rate, and (generally) O2 saturation are required. Some specialties may have other requirements (eg, fingerstick glucose for patients with diabetes, visual acuity testing for those with eye complaints). A full list of data you should be obtaining is practice specific and beyond the scope of this article; the point is, decide on the relevant set of vitals and intake data and be sure it is recorded at every visit.
Failure to obtain and record vital signs—as seen in this case—is sloppy practice, difficult to defend, and sets up an inference of negligence. Even when the care is perfect (and without bad outcome), if the medical board reviews the record for any reason, you will be sanctioned for “failure to keep adequate and accurate medical records” and your license burdened. Here, we are told the defendant NP “did not chart the child’s blood pressure, pulse, or respiratory rate.” I am willing to bet the NP was not responsible for charting the values in the normal course of practice, but see how responsibility is parked with the clinician? If intake staff do not record vital signs, politely (yet firmly) insist they do so.
Continue to: Furthermore, the disposition of many child visits...
Furthermore, the disposition of many child visits turns on whether the patient “appeared toxic.” Any child’s condition could worsen after evaluation—and in litigation, parents, friends, and family will testify the patient was extremely ill, they “knew something was wrong,” and the clinician ignored their loved one. Thus, the jury will be invited to reconstruct how the child appeared.
When assessing children and the question of “toxic appearance” arises, don’t state a conclusion—paint a picture. Don’t merely state “child appeared nontoxic.” Use your powers of observation to record why they appear nontoxic: “Child sitting up, watching Moana on parent’s phone, smiling and laughing appropriately.” Get interactive; some pediatric providers carry a small vial of bubbles with them and record the child’s response to bubble-making (“Child appropriately reaching for bubbles, smiling, holding one on finger”). The cost is less than $1 for the bubbles, plus the documentation time. The benefit is that it paints a clear picture for the jury of a child responding appropriately. And if your observations suggest a child who is at least unwell—if the movie is poorly received or the bubbles prompt the child to scream or bury her face in her mom’s shoulder—you can consider oral antipyretics/analgesics, fluid, and re-observation.
Another way to create a strong and defensible record is to use patient quotations. These can be extremely helpful to your defense in a malpractice action; as an attorney, I have searched 8,000 pages of records in a medical malpractice case, hoping to find a clear description from a human (not a template) of how a patient looked. Make it clear by adding patient remarks to the chart—just remember that “the only thing that belongs in quotes is what comes out of the patient’s mouth.” Words from an 8-year-old boy— such as “My brother found a legendary scar [a reference to Fortnite] and almost won”—may seem silly, but this documentation itself could win your case.
With teenagers, you may have to ask more questions to glean something suitable; you could ask a 13-year-old her favorite sport and when her one-word answer is “Lacrosse,” ask why. Even if the response is “Because, I don’t know, it’s exciting. There are a lot of goals,” write that down exactly (along with any other observations, such as Teen texting on her phone). These notations tell the plaintiff’s attorney, the judge, and the jury that the patient was behaving normally and interacting with the environment. Should this teen later deteriorate with meningitis, the plaintiff will claim she was toxic in the office. The medical record, however, will show that the patient’s condition changed, and it was a departure from how she looked in your office.
Also, it never hurts to get backup. In any close call, ask the nurse to reevaluate the patient as to whether he or she is “toxic appearing” or is interacting normally with the environment. Have the nurse or medical assistant record facts, such as “patient trying to make a plane out of two tongue depressors, pretending to land it on sister’s leg.” This will create a strong and defensible record: two clinicians relaying two sets of detailed observations.
Continue to: Likewise, encourage intake staff to document...
Likewise, encourage intake staff to document what they see rather than what they conclude from it. Buzzwords (eg, listless, lethargic) should be avoided. If such characterizations find their way into the record, you must take active steps to address them. Either agree with the characterization and perform appropriate work-up, or establish why you do not agree using the methods described (detailed description, verification by another clinician).
Taking these steps will help to protect you in the event of a changing clinical course. But also be wary of those predictable circumstances that lead you into Mr. Miyagi’s middle of the road (what I call “half-visits”): a quick look at a sibling in the room during a patient’s appointment; a “curbside consult” on the medical assistant’s child; the neighborhood acquaintance who asks you to “just take a look.” Why are these dangerous? Because they remove the clinician from his or her usual routine: proper examination on a properly undressed patient, formal assessment of vital signs, and review of relevant history in the chart, among other things. (In this way, phone and email communications with patients require similar caution.) Skipping the routine leads to shortcuts, and shortcuts lead to bad medicine. And if that doesn’t worry you, remember: All these scenarios create a full legal duty and clinician/patient relationship—making them potential pathways to misdiagnosis and eventual loss of license.
IN SUMMARY
Don’t be party to a “half-visit”; insist on full vital signs and a complete visit following your usual routine. Use observational powers and patient quotations to paint a picture of how a patient looked, get backup from another clinician with similar observations. If you can’t document a reassuring record, protect the patient and make the required intervention.