User login
Cardiac failure due to left atrial angiosarcoma
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
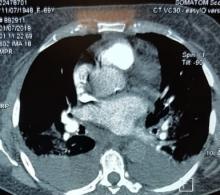
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
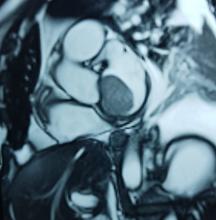
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
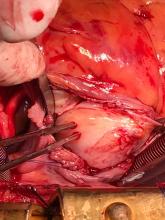
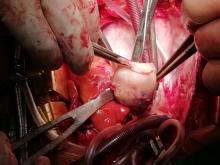
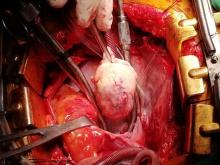
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
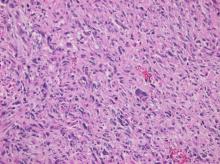
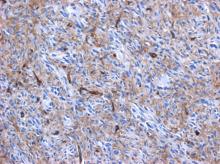
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: [email protected]
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: [email protected]
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: [email protected]
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
Texas judge strikes down ACA putting law in peril — again
The future of the Affordable Care Act is threatened – again – this time by a ruling Friday from a federal district court judge in Texas.
Judge Reed C. O’Connor struck down the law, siding with a group of 18 Republican state attorneys general and two GOP governors who brought the case. Judge O’Connor said the tax bill passed by Congress in December 2017 effectively rendered the entire health law unconstitutional.
That tax measure eliminated the penalty for not having insurance. An earlier Supreme Court decision upheld the ACA based on the view that the penalty was a tax and thus the law was valid because it relied on appropriate power allowed Congress under the Constitution. Judge O’Connor’s decision said that without that penalty, the law no longer met that constitutional test.
“In some ways, the question before the court involves the intent of both the 2010 and 2017 Congresses,” Judge O’Connor wrote in his 55-page decision. “The former enacted the ACA. The latter sawed off the last leg it stood on.”
The decision came just hours before the end of open enrollment for ACA plans in most states that use the federal HealthCare.gov insurance exchange. It is not expected that the ruling will impact the coverage for those people – the final decision will likely not come until the case reaches the Supreme Court again.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, which oversees those insurance exchanges, said in a tweet: “The recent federal court decision is still moving through the courts, and the exchanges are still open for business and we will continue with open enrollment. There is no impact to current coverage or coverage in a 2019 plan.”
The 16 Democratic state attorneys general who intervened in the case to defend the health law immediately vowed to appeal.
“The ACA has already survived more than 70 unsuccessful repeal attempts and withstood scrutiny in the Supreme Court,” said a statement from California Attorney General Xavier Becerra. “Today’s misguided ruling will not deter us: our coalition will continue to fight in court for the health and wellbeing of all Americans.”
It is all but certain the case will become the third time the Supreme Court decides a constitutional question related to the ACA. In addition to upholding the law in 2012, the court rejected another challenge to the law in 2015.
It is hard to overstate what would happen to the nation’s health care system if the decision is ultimately upheld. The Affordable Care Act touched almost every aspect of health care, from Medicare and Medicaid to generic biologic drugs, the Indian Health Service, and public health changes like calorie counts on menus.
The case, Texas v. United States, was filed in February. The plaintiffs argued that because the Supreme Court upheld the ACA in 2012 as a constitutional use of its taxing power, the elimination of the tax makes the rest of the law unconstitutional.
In June, the Justice Department announced it would not fully defend the law in court. While the Trump administration said it did not agree with the plaintiffs that the tax law meant the entire ACA was unconstitutional, it said that the provisions of the law guaranteeing that people with preexisting health conditions could purchase coverage at the same price as everyone else were so inextricably linked to the tax penalty that they should be struck.
The administration urged the court to declare those provisions invalid beginning Jan. 1, 2019. That is the day the tax penalty for not having insurance disappears.
The protections for people with preexisting conditions was one of the top health issues in the midterm elections in November. While the issue mostly played to the advantage of Democrats, one of the Republican plaintiffs, Missouri Attorney General Josh Hawley, defeated Democratic incumbent Sen. Claire McCaskill. Another plaintiff, West Virginia Attorney General Patrick Morrisey, lost to Democratic incumbent Sen. Joe Manchin.
President Donald Trump was quick to take a victory lap, and pressed Senate Majority Leader Mitch McConnell (R-Ky.) and presumed incoming House Speaker Nancy Pelosi (D-Calif.) to fix the problem. He tweeted Friday night that “As I predicted all along, Obamacare has been struck down as an UNCONSTITUTIONAL disaster! Now Congress must pass a STRONG law that provides GREAT healthcare and protects pre-existing conditions. Mitch and Nancy, get it done!”
But congressional leaders were quick to point out that the suit is far from over.
“The ruling seems to be based on faulty legal reasoning and hopefully it will be overturned,” said a statement from Senate Minority Leader Chuck Schumer (D-N.Y.).
Many legal experts agreed with that. “This is insanity in print, and it will not stand up on appeal,” tweeted University of Michigan Law School professor Nicholas Bagley, an expert in health law.
Even some conservatives were left scratching their heads. “Congress acted last year to repeal the mandate, but leave everything else in place and the courts should have deferred to that,” tweeted former congressional GOP aide Chris Jacobs.
AGA believes that Congress must include provisions to ensure patient access to specialty care and other essential patient protections in any new health care legislation. Read more at http://ow.ly/kzIz30n1cBo.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
The future of the Affordable Care Act is threatened – again – this time by a ruling Friday from a federal district court judge in Texas.
Judge Reed C. O’Connor struck down the law, siding with a group of 18 Republican state attorneys general and two GOP governors who brought the case. Judge O’Connor said the tax bill passed by Congress in December 2017 effectively rendered the entire health law unconstitutional.
That tax measure eliminated the penalty for not having insurance. An earlier Supreme Court decision upheld the ACA based on the view that the penalty was a tax and thus the law was valid because it relied on appropriate power allowed Congress under the Constitution. Judge O’Connor’s decision said that without that penalty, the law no longer met that constitutional test.
“In some ways, the question before the court involves the intent of both the 2010 and 2017 Congresses,” Judge O’Connor wrote in his 55-page decision. “The former enacted the ACA. The latter sawed off the last leg it stood on.”
The decision came just hours before the end of open enrollment for ACA plans in most states that use the federal HealthCare.gov insurance exchange. It is not expected that the ruling will impact the coverage for those people – the final decision will likely not come until the case reaches the Supreme Court again.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, which oversees those insurance exchanges, said in a tweet: “The recent federal court decision is still moving through the courts, and the exchanges are still open for business and we will continue with open enrollment. There is no impact to current coverage or coverage in a 2019 plan.”
The 16 Democratic state attorneys general who intervened in the case to defend the health law immediately vowed to appeal.
“The ACA has already survived more than 70 unsuccessful repeal attempts and withstood scrutiny in the Supreme Court,” said a statement from California Attorney General Xavier Becerra. “Today’s misguided ruling will not deter us: our coalition will continue to fight in court for the health and wellbeing of all Americans.”
It is all but certain the case will become the third time the Supreme Court decides a constitutional question related to the ACA. In addition to upholding the law in 2012, the court rejected another challenge to the law in 2015.
It is hard to overstate what would happen to the nation’s health care system if the decision is ultimately upheld. The Affordable Care Act touched almost every aspect of health care, from Medicare and Medicaid to generic biologic drugs, the Indian Health Service, and public health changes like calorie counts on menus.
The case, Texas v. United States, was filed in February. The plaintiffs argued that because the Supreme Court upheld the ACA in 2012 as a constitutional use of its taxing power, the elimination of the tax makes the rest of the law unconstitutional.
In June, the Justice Department announced it would not fully defend the law in court. While the Trump administration said it did not agree with the plaintiffs that the tax law meant the entire ACA was unconstitutional, it said that the provisions of the law guaranteeing that people with preexisting health conditions could purchase coverage at the same price as everyone else were so inextricably linked to the tax penalty that they should be struck.
The administration urged the court to declare those provisions invalid beginning Jan. 1, 2019. That is the day the tax penalty for not having insurance disappears.
The protections for people with preexisting conditions was one of the top health issues in the midterm elections in November. While the issue mostly played to the advantage of Democrats, one of the Republican plaintiffs, Missouri Attorney General Josh Hawley, defeated Democratic incumbent Sen. Claire McCaskill. Another plaintiff, West Virginia Attorney General Patrick Morrisey, lost to Democratic incumbent Sen. Joe Manchin.
President Donald Trump was quick to take a victory lap, and pressed Senate Majority Leader Mitch McConnell (R-Ky.) and presumed incoming House Speaker Nancy Pelosi (D-Calif.) to fix the problem. He tweeted Friday night that “As I predicted all along, Obamacare has been struck down as an UNCONSTITUTIONAL disaster! Now Congress must pass a STRONG law that provides GREAT healthcare and protects pre-existing conditions. Mitch and Nancy, get it done!”
But congressional leaders were quick to point out that the suit is far from over.
“The ruling seems to be based on faulty legal reasoning and hopefully it will be overturned,” said a statement from Senate Minority Leader Chuck Schumer (D-N.Y.).
Many legal experts agreed with that. “This is insanity in print, and it will not stand up on appeal,” tweeted University of Michigan Law School professor Nicholas Bagley, an expert in health law.
Even some conservatives were left scratching their heads. “Congress acted last year to repeal the mandate, but leave everything else in place and the courts should have deferred to that,” tweeted former congressional GOP aide Chris Jacobs.
AGA believes that Congress must include provisions to ensure patient access to specialty care and other essential patient protections in any new health care legislation. Read more at http://ow.ly/kzIz30n1cBo.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
The future of the Affordable Care Act is threatened – again – this time by a ruling Friday from a federal district court judge in Texas.
Judge Reed C. O’Connor struck down the law, siding with a group of 18 Republican state attorneys general and two GOP governors who brought the case. Judge O’Connor said the tax bill passed by Congress in December 2017 effectively rendered the entire health law unconstitutional.
That tax measure eliminated the penalty for not having insurance. An earlier Supreme Court decision upheld the ACA based on the view that the penalty was a tax and thus the law was valid because it relied on appropriate power allowed Congress under the Constitution. Judge O’Connor’s decision said that without that penalty, the law no longer met that constitutional test.
“In some ways, the question before the court involves the intent of both the 2010 and 2017 Congresses,” Judge O’Connor wrote in his 55-page decision. “The former enacted the ACA. The latter sawed off the last leg it stood on.”
The decision came just hours before the end of open enrollment for ACA plans in most states that use the federal HealthCare.gov insurance exchange. It is not expected that the ruling will impact the coverage for those people – the final decision will likely not come until the case reaches the Supreme Court again.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services, which oversees those insurance exchanges, said in a tweet: “The recent federal court decision is still moving through the courts, and the exchanges are still open for business and we will continue with open enrollment. There is no impact to current coverage or coverage in a 2019 plan.”
The 16 Democratic state attorneys general who intervened in the case to defend the health law immediately vowed to appeal.
“The ACA has already survived more than 70 unsuccessful repeal attempts and withstood scrutiny in the Supreme Court,” said a statement from California Attorney General Xavier Becerra. “Today’s misguided ruling will not deter us: our coalition will continue to fight in court for the health and wellbeing of all Americans.”
It is all but certain the case will become the third time the Supreme Court decides a constitutional question related to the ACA. In addition to upholding the law in 2012, the court rejected another challenge to the law in 2015.
It is hard to overstate what would happen to the nation’s health care system if the decision is ultimately upheld. The Affordable Care Act touched almost every aspect of health care, from Medicare and Medicaid to generic biologic drugs, the Indian Health Service, and public health changes like calorie counts on menus.
The case, Texas v. United States, was filed in February. The plaintiffs argued that because the Supreme Court upheld the ACA in 2012 as a constitutional use of its taxing power, the elimination of the tax makes the rest of the law unconstitutional.
In June, the Justice Department announced it would not fully defend the law in court. While the Trump administration said it did not agree with the plaintiffs that the tax law meant the entire ACA was unconstitutional, it said that the provisions of the law guaranteeing that people with preexisting health conditions could purchase coverage at the same price as everyone else were so inextricably linked to the tax penalty that they should be struck.
The administration urged the court to declare those provisions invalid beginning Jan. 1, 2019. That is the day the tax penalty for not having insurance disappears.
The protections for people with preexisting conditions was one of the top health issues in the midterm elections in November. While the issue mostly played to the advantage of Democrats, one of the Republican plaintiffs, Missouri Attorney General Josh Hawley, defeated Democratic incumbent Sen. Claire McCaskill. Another plaintiff, West Virginia Attorney General Patrick Morrisey, lost to Democratic incumbent Sen. Joe Manchin.
President Donald Trump was quick to take a victory lap, and pressed Senate Majority Leader Mitch McConnell (R-Ky.) and presumed incoming House Speaker Nancy Pelosi (D-Calif.) to fix the problem. He tweeted Friday night that “As I predicted all along, Obamacare has been struck down as an UNCONSTITUTIONAL disaster! Now Congress must pass a STRONG law that provides GREAT healthcare and protects pre-existing conditions. Mitch and Nancy, get it done!”
But congressional leaders were quick to point out that the suit is far from over.
“The ruling seems to be based on faulty legal reasoning and hopefully it will be overturned,” said a statement from Senate Minority Leader Chuck Schumer (D-N.Y.).
Many legal experts agreed with that. “This is insanity in print, and it will not stand up on appeal,” tweeted University of Michigan Law School professor Nicholas Bagley, an expert in health law.
Even some conservatives were left scratching their heads. “Congress acted last year to repeal the mandate, but leave everything else in place and the courts should have deferred to that,” tweeted former congressional GOP aide Chris Jacobs.
AGA believes that Congress must include provisions to ensure patient access to specialty care and other essential patient protections in any new health care legislation. Read more at http://ow.ly/kzIz30n1cBo.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
Active migraine in women linked to lower risk of developing T2DM
Women with active migraines are less likely to have type 2 diabetes mellitus (T2DM) and show a decrease in migraine symptoms prior to diagnosis of T2DM, indicating an inverse relationship between hyperglycemia, hyperinsulinism, and migraines, according to recent research published in JAMA Neurology.
“Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease,” Guy Fagherazzi, PhD, at the Center for Research in Epidemiology and Population Health at the Gustave Roussy Institute in Villejuif, France, and his colleagues wrote in their study. “Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.”
The researchers used data from the prospective Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study, initiated in 1990 and identified 74,247 women (mean age, 61 years old) with self-reported migraine in a 2002 follow-up questionnaire who had 10-year follow-up data during 2004-2014. The women in the cohort were born during 1925-1950 and completed biennial questionnaires about their health, including migraine status and medications, since 1992. The participants were divided into groups based on no migraine (49,199 participants), active migraine (7,839 participants), or prior migraine history (17,209 participants), and patients with T2DM at baseline were excluded.
Dr. Fagherazzi and his colleagues found 2,372 cases of type 2 diabetes over the follow-up period. Women who had active migraine status were less likely to have T2DM (hazard ratio, 0.80; 95% confidence interval, 0.67-0.96) than were the participants who did not have migraines, and this inverse association persisted after the researchers adjusted for factors such as myocardial infarction, education level, family history of diabetes, body mass index, smoking status, hypertension, physical activity, oral contraceptive use, menopausal status, menopausal hormone therapy, handedness, antimigraine preparations, and other prescribed migraine drugs (HR, 0.70; 95% CI, 0.58-0.85).
In the participants who developed T2DM, the researchers also found that there was a decrease in the prevalence of active migraine in the 24 years prior to T2DM diagnosis from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) after adjusting for T2DM risk factors, which was then followed by an up to 22-year plateau in migraine prevalence of 11% for these participants.
“The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations,” Dr. Fagherazzi and his colleagues wrote. “The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.”
The researchers noted limitations in the study, such as self-reported migraine by participants in the cohort, exclusion of non–pharmacologically treated T2DM cases, observational nature of the study, and homogenized population in the E3N cohort consisting of mainly women in menopause who were teachers and belonged to the same health insurance plan.
This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the “Mutuelle Générale de l’Education Nationale,” European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided BMJ with editorial services.
SOURCE: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
Although it has been noted for some time in the clinical setting, researchers are still unsure why there is an inverse association between active migraine and type 2 diabetes mellitus, as noted by Fagherazzi et al. in a recent study.
One explanation is the presence of calcitonin gene–related peptide in both animal models of energy metabolism and the pathophysiology of migraine. It is possible that insulin resistance and hyperglycemia damage the sensory neurons that produce the peptide. If these damaged nerves are soothed, migraine may resolve.
Other silver linings associated with active migraine include an increased likelihood of having a healthy cardiovascular system and decreased alcohol consumption.
The epidemiology of migraine and findings like those in this study prompt the question: What is migraine good for?
Amy A. Gelfand, MD , of the University of California, San Francisco, and Elizabeth Loder, MD , MPH, of Harvard Medical School in Boston made these comments in an editorial accompanying Dr. Fagherazzi’s study. They disclosed a number of financial relationships with companies marketing treatments for migraine.
Although it has been noted for some time in the clinical setting, researchers are still unsure why there is an inverse association between active migraine and type 2 diabetes mellitus, as noted by Fagherazzi et al. in a recent study.
One explanation is the presence of calcitonin gene–related peptide in both animal models of energy metabolism and the pathophysiology of migraine. It is possible that insulin resistance and hyperglycemia damage the sensory neurons that produce the peptide. If these damaged nerves are soothed, migraine may resolve.
Other silver linings associated with active migraine include an increased likelihood of having a healthy cardiovascular system and decreased alcohol consumption.
The epidemiology of migraine and findings like those in this study prompt the question: What is migraine good for?
Amy A. Gelfand, MD , of the University of California, San Francisco, and Elizabeth Loder, MD , MPH, of Harvard Medical School in Boston made these comments in an editorial accompanying Dr. Fagherazzi’s study. They disclosed a number of financial relationships with companies marketing treatments for migraine.
Although it has been noted for some time in the clinical setting, researchers are still unsure why there is an inverse association between active migraine and type 2 diabetes mellitus, as noted by Fagherazzi et al. in a recent study.
One explanation is the presence of calcitonin gene–related peptide in both animal models of energy metabolism and the pathophysiology of migraine. It is possible that insulin resistance and hyperglycemia damage the sensory neurons that produce the peptide. If these damaged nerves are soothed, migraine may resolve.
Other silver linings associated with active migraine include an increased likelihood of having a healthy cardiovascular system and decreased alcohol consumption.
The epidemiology of migraine and findings like those in this study prompt the question: What is migraine good for?
Amy A. Gelfand, MD , of the University of California, San Francisco, and Elizabeth Loder, MD , MPH, of Harvard Medical School in Boston made these comments in an editorial accompanying Dr. Fagherazzi’s study. They disclosed a number of financial relationships with companies marketing treatments for migraine.
Women with active migraines are less likely to have type 2 diabetes mellitus (T2DM) and show a decrease in migraine symptoms prior to diagnosis of T2DM, indicating an inverse relationship between hyperglycemia, hyperinsulinism, and migraines, according to recent research published in JAMA Neurology.
“Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease,” Guy Fagherazzi, PhD, at the Center for Research in Epidemiology and Population Health at the Gustave Roussy Institute in Villejuif, France, and his colleagues wrote in their study. “Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.”
The researchers used data from the prospective Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study, initiated in 1990 and identified 74,247 women (mean age, 61 years old) with self-reported migraine in a 2002 follow-up questionnaire who had 10-year follow-up data during 2004-2014. The women in the cohort were born during 1925-1950 and completed biennial questionnaires about their health, including migraine status and medications, since 1992. The participants were divided into groups based on no migraine (49,199 participants), active migraine (7,839 participants), or prior migraine history (17,209 participants), and patients with T2DM at baseline were excluded.
Dr. Fagherazzi and his colleagues found 2,372 cases of type 2 diabetes over the follow-up period. Women who had active migraine status were less likely to have T2DM (hazard ratio, 0.80; 95% confidence interval, 0.67-0.96) than were the participants who did not have migraines, and this inverse association persisted after the researchers adjusted for factors such as myocardial infarction, education level, family history of diabetes, body mass index, smoking status, hypertension, physical activity, oral contraceptive use, menopausal status, menopausal hormone therapy, handedness, antimigraine preparations, and other prescribed migraine drugs (HR, 0.70; 95% CI, 0.58-0.85).
In the participants who developed T2DM, the researchers also found that there was a decrease in the prevalence of active migraine in the 24 years prior to T2DM diagnosis from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) after adjusting for T2DM risk factors, which was then followed by an up to 22-year plateau in migraine prevalence of 11% for these participants.
“The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations,” Dr. Fagherazzi and his colleagues wrote. “The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.”
The researchers noted limitations in the study, such as self-reported migraine by participants in the cohort, exclusion of non–pharmacologically treated T2DM cases, observational nature of the study, and homogenized population in the E3N cohort consisting of mainly women in menopause who were teachers and belonged to the same health insurance plan.
This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the “Mutuelle Générale de l’Education Nationale,” European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided BMJ with editorial services.
SOURCE: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
Women with active migraines are less likely to have type 2 diabetes mellitus (T2DM) and show a decrease in migraine symptoms prior to diagnosis of T2DM, indicating an inverse relationship between hyperglycemia, hyperinsulinism, and migraines, according to recent research published in JAMA Neurology.
“Because plasma glucose concentration rises with time up to the point of type 2 diabetes occurrence, the prevalence of migraine symptoms may decrease,” Guy Fagherazzi, PhD, at the Center for Research in Epidemiology and Population Health at the Gustave Roussy Institute in Villejuif, France, and his colleagues wrote in their study. “Consequently, tracking the evolution and especially the decrease of migraine frequency in individuals with migraine at high risk of diabetes, such as individuals with obesity, irrespective of age could be the sign of an emerging increased blood glucose levels, prediabetes, or type 2 diabetes.”
The researchers used data from the prospective Etude Epidémiologique Auprès des Femmes de la Mutuelle Générale de l’Education Nationale (E3N) study, initiated in 1990 and identified 74,247 women (mean age, 61 years old) with self-reported migraine in a 2002 follow-up questionnaire who had 10-year follow-up data during 2004-2014. The women in the cohort were born during 1925-1950 and completed biennial questionnaires about their health, including migraine status and medications, since 1992. The participants were divided into groups based on no migraine (49,199 participants), active migraine (7,839 participants), or prior migraine history (17,209 participants), and patients with T2DM at baseline were excluded.
Dr. Fagherazzi and his colleagues found 2,372 cases of type 2 diabetes over the follow-up period. Women who had active migraine status were less likely to have T2DM (hazard ratio, 0.80; 95% confidence interval, 0.67-0.96) than were the participants who did not have migraines, and this inverse association persisted after the researchers adjusted for factors such as myocardial infarction, education level, family history of diabetes, body mass index, smoking status, hypertension, physical activity, oral contraceptive use, menopausal status, menopausal hormone therapy, handedness, antimigraine preparations, and other prescribed migraine drugs (HR, 0.70; 95% CI, 0.58-0.85).
In the participants who developed T2DM, the researchers also found that there was a decrease in the prevalence of active migraine in the 24 years prior to T2DM diagnosis from 22% (95% CI, 16%-27%) to 11% (95% CI, 10%-12%) after adjusting for T2DM risk factors, which was then followed by an up to 22-year plateau in migraine prevalence of 11% for these participants.
“The linear decrease of migraine prevalence long before and the plateau long after type 2 diabetes diagnosis is novel and the association deserves to be studied in other populations,” Dr. Fagherazzi and his colleagues wrote. “The potential beneficial role of both hyperglycemia and hyperinsulinism on migraine occurrence needs to be further explored.”
The researchers noted limitations in the study, such as self-reported migraine by participants in the cohort, exclusion of non–pharmacologically treated T2DM cases, observational nature of the study, and homogenized population in the E3N cohort consisting of mainly women in menopause who were teachers and belonged to the same health insurance plan.
This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the “Mutuelle Générale de l’Education Nationale,” European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided BMJ with editorial services.
SOURCE: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
FROM JAMA NEUROLOGY
Key clinical point: There was an inverse association between active migraine and type 2 diabetes mellitus in women over 10 years of follow-up.
Major finding: Compared with women who had no history of active migraine, women with active migraine had a lower risk of developing type 2 diabetes (univariate hazard ratio, 0.80; 95% confidence interval, 0.67-0.96).
Study details: Results from a prospective, population-based study of 74,247 women with active migraines in the E3N cohort study in France.
Disclosures: This study was funded by a grant from the French Research agency. The E3N cohort study was funded by the Mutuelle Générale de l’Education Nationale, European Community, French League against Cancer, Gustave Roussy, and French Institute of Health and Medical Research. Dr. Kurth is an advisory board member for CoLucid and has received funding for a research project from Amgen, honoraria from Lilly, lecture support from Novartis and Daiichi Sankyo, and travel support from the International Headache Society, as well as provided the BMJ with editorial services.
Source: Fagherazzi G et al. JAMA Neurol. 2018. doi: 10.1001/jamaneurol.2018.3960.
Soft Tissue Sarcoma Chemotherapy
Predicting response to chemotherapy
The prognostic nomogram called Sarculator was used effectively to define a high-risk subgroup of patients likely to benefit from adjuvant chemotherapy, Sandro Pasquali, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy and his colleagues reported at the meeting.
Perioperative chemotherapy was shown to afford no survival advantage over observation in the EORTC 62931 (European Organization for Research and Treatment of Cancer—62931) study of adjuvant doxorubicin plus ifosfamide (Lancet Oncol 2012;13:1045-54). However, subsequent analyses of that data attributed this finding to variations in treatment schedules and the inclusion of low-risk tumors, which may have diluted the effect of chemotherapy, the researchers said in their abstract.
Further, a recent interim report of the ISG-1001 trial showed a survival benefit for patients who received neoadjuvant epirubicin plus ifosfamide therapy for localized high-risk soft-tissue sarcoma of the extremities or trunk wall (Lancet Oncol 2017;18:812-822).
The researchers performed a retrospective analysis of individual data for 290 patients with extremity and trunk wall soft-tissue sarcomas in the EORTC-STBSG 62931 study. The Sarculator was used to calculate 10-year predicted probability of overall survival (pr-OS) for each patient.
Patients were grouped in two categories of predicted overall survival: high predicted survival (over 60%) and low predicted overall survival (60% or less). Overall survival and disease-free survival were calculated at 8 years, the study’s median follow-up.
The 8-year probability of overall survival and disease-free survival was 58% [95% confidence interval (CI): 52–63%] and 51% (95% CI: 46–57%), respectively. In the 290 patients with extremity and trunk wall soft tissue sarcomas, adjuvant chemotherapy was not associated with an overall survival benefit [Hazard ratio (HR) = 0.91, 95%CI 0.63–1.31]. The Sarcolator Nomogram detected 80 patients who were at greater risk of death compared to the 210 patients with higher predicted overall survival. The risk of death was significantly lower with adjuvant chemotherapy in the group with low predicted survival based on the Sarculator Nomogram (HR=0.50, 95%CI 0.30-0.90). Consistently, the risk of recurrence was significantly lower when adjuvant chemotherapy was used in the group with predicted overall survival of less than 60% (HR = 0.49, 95%CI 0.28-0.85) while this difference was not observed in patients with high predicted overall survival (HR = 0.95, 95%CI 0.62-1.44).
Doxorubicin plus dacarbazine deserve evaluation in prospective trials in leiomyosarcoma
Doxorubicin plus dacarbazine appeared to best the outcomes seen with doxorubicin plus ifosfamide and with doxorubicin alone in terms of overall response rate and progression free survival as first-line treatment in patients with advanced leiomyosarcomas, based on a retrospective analysis presented by Lorenzo D’Ambrosio, MD, of the Unitversity of Torino, Italy, and his associates.
As patients in the trial were not randomized to therapy, the researchers used a logistic regression model that accounted for histology, site of primary, age, gender, performance status, tumor extent, and tumor grade. Patients were then matched across the different groups by their propensity scores.The 303 patients, 216 of them women, were enrolled from 18 EORTC STBSG (European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group) sites. Doxorubicin plus dacarbazine was given to 117 patients (39%), doxorubicin plus ifosfamide was given to 71 (23%), and doxorubicin alone was given to 115 (38%). There were no significant differences among the regimens in terms of dose reductions of more than 10%, delays of greater than 72 hours, or granulocyte-colony stimulating factor use.
In the whole population, unadjusted median progression free survival was 9.4 months (95% CI 6.1-9.7 months) for those given doxorubicin plus dacarbazine, 6.8 months (4.5-9.5 months) for those given doxorubicin plus ifosfamide), and 5.4 months (3.8-6.8 months) for those given doxorubicin alone. The respective overall response rates for the three regimens were 36.8%, 21.5%, and 25.9%. When using propensity scores to adjust for lack of randomization, progression free survival was significantly longer with doxorubicin plus dacarbazine [median 9.2 months (95%CI 5.2-9.7 months) than with doxorubicin [median 4.8 months (2.3-6.0 months); HR 0.72 (0.52-0.99)]. The difference was not significant when compared to doxorubicin plus ifosfamide [8.2 months (5.2-10.1 months), HR 1.01 (0.68-1.50)]. Progression free survival did not differ significantly between doxorubicin plus ifosfamide, and doxorubicin [HR 0.71 (0.48-1.06)]. In the same matched population, overall response rates were 30.9%, 19.5%, and 25.6% for doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, and doxorubicin, respectively.
Overall survival comparisons were weakened by a shorter median follow-up in the doxorubicin plus dacarbazine groups (32 months) compared to the doxorubicin plus ifosfamide group (50 months) and the doxorubicin group (46 months). With this limit, patients in the doxorubicin plus dacarbazine arm had longer overall survival [median 36.8 (27.9-47.2) months] when compared to both doxorubicin plus ifosfamide [21.9 (16.7-33.4), HR 0.65 (0.40-1.06); and doxorubicin arms 30.3 (21.0-36.3) months, HR 0.66 (0.43-0.99).
Subsequent treatments were well balanced across arms. None of the selected factors for multivariate analysis (age, sex, ECOG performance status, histotype, site of primary tumor, tumor grade, and tumor extent) significantly affected the progression free survival and overall survival associated with the treatments.
Olaratumab in combination with doxorubicin plus ifosfamide
Olaratumab at 15 mg/kg has been shown to be safe in combination with doxorubicin plus ifosfamide in a Phase 1b study (NCT03283696), reported Sebastian Bauer, MD, of the West German Cancer Center, University of Duisburg-Essen, Essen, Germany, and his colleagues.
Given that 8 of 10 evaluable patients have completed the drug-limiting toxicity period without drug-limiting toxicities at the 15 mg/kg dose level of olaratumab, the study has proceeded to the next cohort. In those patients, an olaratumab loading dose of 20 mg/kg will be evaluated in cycle 1, followed by 15 mg/kg of olaratumab in subsequent cycles with the same doses of doxorubicin plus ifosfamide, the researchers wrote in their abstract.
The phase 1 trial enrolled 16 patients with advanced or metastatic soft tissue sarcomas and no prior lines of systemic therapy and ECOG performance status 0-1. Adequate follow up data was available for 10 patients.
Olaratumab, (Lartruvo), which binds platelet-derived growth factor receptor alpha (PDGFRα), was given at 15 mg/kg in combination with doxorubicin (75 mg/m2 on days 1-3) and ifosfamide (10 g/m2 on days 1-4) followed by mandatory granulocyte-colony-stimulating factor therapy in cycles 1-6 on a 21-day cycle. Doxorubicin was allowed to be administered by continuous infusion or bolus administration and with cardiac protection. Mesna dosing was at least 60% of the ifosfamide dose.
Two of the 10 patients had dose-limiting toxicities; one had Grade 4 febrile neutropenia and the other had Grade 3 febrile neutropenia and Grade 3 mucositis. Common related adverse events occurring in over 30% of patients included fatigue, anemia, neutropenia, thrombocytopenia, constipation, and nausea. One patient discontinued study treatment due to progressive disease, and all others were on study treatment as of data cutoff. Among 7 patients evaluated for tumor response assessment, 3 patients had a partial response according to RECIST and 3 further patients had stabilized disease as best overall response for a disease control rate of 86%.
Anthracycline-based regimen excels in FIGO-1 uterine leiomyosarcoma
Future trials to assess the efficacy of adjuvant chemotherapy in uterine leiomyosarcoma should incorporate anthracyclines, according to Roberta Sanfilippo, MD, of Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues.
Disease-free survival was extended in patients with uterine leiomyosarcomas treated with anthracycline-based regimens as compared to gemcitabine and docetaxel, based on a retrospective analysis reported at the meeting by Dr. Sanfilippo.
They reviewed all patients with FIGO stage I uterine leiomyosarcomas who underwent hysterectomy with or without oophorectomy and were treated with adjuvant chemotherapy with either anthracycline-based or gemcitabine-based chemotherapy at two Italian centers.
Of 145 patients, 97 were treated with an anthracycline-based regimen and 48 with gemcitabine and docetaxel. The median number of cycles of anthracycline based regimen received was 4 (range 2-6) and with gemcitabine and docetaxel was 5 (range 3-7). Disease free survival was 31 months in patients treated with anthracycline-based chemotherapy and 19 months in patients treated with gemcitabine and docetaxel.
Trabectedin and low-dose radiotherapy
Trabectedin concurrent with low-dose radiotherapy is being examined as an option for patients with pulmonary metastatic soft tissue sarcoma (NCT02275286).
In a phase 1 study, long-lasting dimensional responses were seen in 71% of the irradiated lesions showed. Based on those results, trabectedin (Yondelis) at 1.5 mg/m 2 will be the recommended dose for phase 2, according to Javier Martín-Broto, MD, of the Institute of Biomedicine Research (IBIS)-University Hospital Virgen del Rocio/CSIC/University of Seville, Spain, and his colleagues.
For the study, trabectedin was given along with radiotherapy (30 Gy) in 10 fractions (3 Gy/fraction). Three dose levels of trabectedin were administered: -1 (1.1 mg/m 2), 1 (1.3 mg/m 2) and 2 (1.5 mg/m 2). Dose-limiting toxicity was defined as grade 3 or greater events excluding grade 3/4 neutropenia lasting less than 5 days, grade 3 transaminitis if it did not lead to trabectedin delay, and grade 3/4 nausea/vomiting due to inadequate prophylaxis.
Ten of the 18 patients enrolled had synovial sarcoma; 3 had undifferentiated pleomorphic sarcomas and the other patients had either myxoid liposarcoma, dedifferentiated liposarcoma, G3 not otherwise specified sarcoma, leiomyosarcoma, and malignant peripheral nerve sheath tumor.
Patients received a median of 1 prior line of chemotherapy (range: 0-3). Twelve patients received trabectedin at dose level 1 and 6 patients at dose level 2. Grade 3/4 adverse events were neutropenia, seen in 8 patients; alanine aminotransferase (ALT) elevation, seen in 2 patients; gamma-glutamyl transferase (GGT) elevation, seen in 2 patients; anemia, seen in 2 patients; febrile neutropenia, seen in 1 patient; and pneumonitis, seen in 1 patient.
There were two dose-limiting toxicities: transient grade 4 ALT elevation at the level 1 dose and grade 4 neutropenia for more than 5 days at the level 2 dose.
Based on central radiological review of 17 evaluable patients, 2 patients achieved complete response, 3 had partial responses, 6 had stable disease, and 6 had progressive disease. The local review reported complete responses in 2 patients, partial responses in 5, stable disease in 4, and progressive disease in 6.
On the irradiated lesions, 4 had complete responses, 8 had partial responses, 4 had stable disease, and 1 had progressive disease. With a median follow-up of 18 months, median progression-free survival was 2.83 months (95%CI: 2.3-3.3 months). Thirteen patients have died, with a median overall survival of 8.77 months (95%CI: 3.6-13.9) and a 12-month overall survival rate of 48%.
Predicting response to chemotherapy
The prognostic nomogram called Sarculator was used effectively to define a high-risk subgroup of patients likely to benefit from adjuvant chemotherapy, Sandro Pasquali, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy and his colleagues reported at the meeting.
Perioperative chemotherapy was shown to afford no survival advantage over observation in the EORTC 62931 (European Organization for Research and Treatment of Cancer—62931) study of adjuvant doxorubicin plus ifosfamide (Lancet Oncol 2012;13:1045-54). However, subsequent analyses of that data attributed this finding to variations in treatment schedules and the inclusion of low-risk tumors, which may have diluted the effect of chemotherapy, the researchers said in their abstract.
Further, a recent interim report of the ISG-1001 trial showed a survival benefit for patients who received neoadjuvant epirubicin plus ifosfamide therapy for localized high-risk soft-tissue sarcoma of the extremities or trunk wall (Lancet Oncol 2017;18:812-822).
The researchers performed a retrospective analysis of individual data for 290 patients with extremity and trunk wall soft-tissue sarcomas in the EORTC-STBSG 62931 study. The Sarculator was used to calculate 10-year predicted probability of overall survival (pr-OS) for each patient.
Patients were grouped in two categories of predicted overall survival: high predicted survival (over 60%) and low predicted overall survival (60% or less). Overall survival and disease-free survival were calculated at 8 years, the study’s median follow-up.
The 8-year probability of overall survival and disease-free survival was 58% [95% confidence interval (CI): 52–63%] and 51% (95% CI: 46–57%), respectively. In the 290 patients with extremity and trunk wall soft tissue sarcomas, adjuvant chemotherapy was not associated with an overall survival benefit [Hazard ratio (HR) = 0.91, 95%CI 0.63–1.31]. The Sarcolator Nomogram detected 80 patients who were at greater risk of death compared to the 210 patients with higher predicted overall survival. The risk of death was significantly lower with adjuvant chemotherapy in the group with low predicted survival based on the Sarculator Nomogram (HR=0.50, 95%CI 0.30-0.90). Consistently, the risk of recurrence was significantly lower when adjuvant chemotherapy was used in the group with predicted overall survival of less than 60% (HR = 0.49, 95%CI 0.28-0.85) while this difference was not observed in patients with high predicted overall survival (HR = 0.95, 95%CI 0.62-1.44).
Doxorubicin plus dacarbazine deserve evaluation in prospective trials in leiomyosarcoma
Doxorubicin plus dacarbazine appeared to best the outcomes seen with doxorubicin plus ifosfamide and with doxorubicin alone in terms of overall response rate and progression free survival as first-line treatment in patients with advanced leiomyosarcomas, based on a retrospective analysis presented by Lorenzo D’Ambrosio, MD, of the Unitversity of Torino, Italy, and his associates.
As patients in the trial were not randomized to therapy, the researchers used a logistic regression model that accounted for histology, site of primary, age, gender, performance status, tumor extent, and tumor grade. Patients were then matched across the different groups by their propensity scores.The 303 patients, 216 of them women, were enrolled from 18 EORTC STBSG (European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group) sites. Doxorubicin plus dacarbazine was given to 117 patients (39%), doxorubicin plus ifosfamide was given to 71 (23%), and doxorubicin alone was given to 115 (38%). There were no significant differences among the regimens in terms of dose reductions of more than 10%, delays of greater than 72 hours, or granulocyte-colony stimulating factor use.
In the whole population, unadjusted median progression free survival was 9.4 months (95% CI 6.1-9.7 months) for those given doxorubicin plus dacarbazine, 6.8 months (4.5-9.5 months) for those given doxorubicin plus ifosfamide), and 5.4 months (3.8-6.8 months) for those given doxorubicin alone. The respective overall response rates for the three regimens were 36.8%, 21.5%, and 25.9%. When using propensity scores to adjust for lack of randomization, progression free survival was significantly longer with doxorubicin plus dacarbazine [median 9.2 months (95%CI 5.2-9.7 months) than with doxorubicin [median 4.8 months (2.3-6.0 months); HR 0.72 (0.52-0.99)]. The difference was not significant when compared to doxorubicin plus ifosfamide [8.2 months (5.2-10.1 months), HR 1.01 (0.68-1.50)]. Progression free survival did not differ significantly between doxorubicin plus ifosfamide, and doxorubicin [HR 0.71 (0.48-1.06)]. In the same matched population, overall response rates were 30.9%, 19.5%, and 25.6% for doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, and doxorubicin, respectively.
Overall survival comparisons were weakened by a shorter median follow-up in the doxorubicin plus dacarbazine groups (32 months) compared to the doxorubicin plus ifosfamide group (50 months) and the doxorubicin group (46 months). With this limit, patients in the doxorubicin plus dacarbazine arm had longer overall survival [median 36.8 (27.9-47.2) months] when compared to both doxorubicin plus ifosfamide [21.9 (16.7-33.4), HR 0.65 (0.40-1.06); and doxorubicin arms 30.3 (21.0-36.3) months, HR 0.66 (0.43-0.99).
Subsequent treatments were well balanced across arms. None of the selected factors for multivariate analysis (age, sex, ECOG performance status, histotype, site of primary tumor, tumor grade, and tumor extent) significantly affected the progression free survival and overall survival associated with the treatments.
Olaratumab in combination with doxorubicin plus ifosfamide
Olaratumab at 15 mg/kg has been shown to be safe in combination with doxorubicin plus ifosfamide in a Phase 1b study (NCT03283696), reported Sebastian Bauer, MD, of the West German Cancer Center, University of Duisburg-Essen, Essen, Germany, and his colleagues.
Given that 8 of 10 evaluable patients have completed the drug-limiting toxicity period without drug-limiting toxicities at the 15 mg/kg dose level of olaratumab, the study has proceeded to the next cohort. In those patients, an olaratumab loading dose of 20 mg/kg will be evaluated in cycle 1, followed by 15 mg/kg of olaratumab in subsequent cycles with the same doses of doxorubicin plus ifosfamide, the researchers wrote in their abstract.
The phase 1 trial enrolled 16 patients with advanced or metastatic soft tissue sarcomas and no prior lines of systemic therapy and ECOG performance status 0-1. Adequate follow up data was available for 10 patients.
Olaratumab, (Lartruvo), which binds platelet-derived growth factor receptor alpha (PDGFRα), was given at 15 mg/kg in combination with doxorubicin (75 mg/m2 on days 1-3) and ifosfamide (10 g/m2 on days 1-4) followed by mandatory granulocyte-colony-stimulating factor therapy in cycles 1-6 on a 21-day cycle. Doxorubicin was allowed to be administered by continuous infusion or bolus administration and with cardiac protection. Mesna dosing was at least 60% of the ifosfamide dose.
Two of the 10 patients had dose-limiting toxicities; one had Grade 4 febrile neutropenia and the other had Grade 3 febrile neutropenia and Grade 3 mucositis. Common related adverse events occurring in over 30% of patients included fatigue, anemia, neutropenia, thrombocytopenia, constipation, and nausea. One patient discontinued study treatment due to progressive disease, and all others were on study treatment as of data cutoff. Among 7 patients evaluated for tumor response assessment, 3 patients had a partial response according to RECIST and 3 further patients had stabilized disease as best overall response for a disease control rate of 86%.
Anthracycline-based regimen excels in FIGO-1 uterine leiomyosarcoma
Future trials to assess the efficacy of adjuvant chemotherapy in uterine leiomyosarcoma should incorporate anthracyclines, according to Roberta Sanfilippo, MD, of Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues.
Disease-free survival was extended in patients with uterine leiomyosarcomas treated with anthracycline-based regimens as compared to gemcitabine and docetaxel, based on a retrospective analysis reported at the meeting by Dr. Sanfilippo.
They reviewed all patients with FIGO stage I uterine leiomyosarcomas who underwent hysterectomy with or without oophorectomy and were treated with adjuvant chemotherapy with either anthracycline-based or gemcitabine-based chemotherapy at two Italian centers.
Of 145 patients, 97 were treated with an anthracycline-based regimen and 48 with gemcitabine and docetaxel. The median number of cycles of anthracycline based regimen received was 4 (range 2-6) and with gemcitabine and docetaxel was 5 (range 3-7). Disease free survival was 31 months in patients treated with anthracycline-based chemotherapy and 19 months in patients treated with gemcitabine and docetaxel.
Trabectedin and low-dose radiotherapy
Trabectedin concurrent with low-dose radiotherapy is being examined as an option for patients with pulmonary metastatic soft tissue sarcoma (NCT02275286).
In a phase 1 study, long-lasting dimensional responses were seen in 71% of the irradiated lesions showed. Based on those results, trabectedin (Yondelis) at 1.5 mg/m 2 will be the recommended dose for phase 2, according to Javier Martín-Broto, MD, of the Institute of Biomedicine Research (IBIS)-University Hospital Virgen del Rocio/CSIC/University of Seville, Spain, and his colleagues.
For the study, trabectedin was given along with radiotherapy (30 Gy) in 10 fractions (3 Gy/fraction). Three dose levels of trabectedin were administered: -1 (1.1 mg/m 2), 1 (1.3 mg/m 2) and 2 (1.5 mg/m 2). Dose-limiting toxicity was defined as grade 3 or greater events excluding grade 3/4 neutropenia lasting less than 5 days, grade 3 transaminitis if it did not lead to trabectedin delay, and grade 3/4 nausea/vomiting due to inadequate prophylaxis.
Ten of the 18 patients enrolled had synovial sarcoma; 3 had undifferentiated pleomorphic sarcomas and the other patients had either myxoid liposarcoma, dedifferentiated liposarcoma, G3 not otherwise specified sarcoma, leiomyosarcoma, and malignant peripheral nerve sheath tumor.
Patients received a median of 1 prior line of chemotherapy (range: 0-3). Twelve patients received trabectedin at dose level 1 and 6 patients at dose level 2. Grade 3/4 adverse events were neutropenia, seen in 8 patients; alanine aminotransferase (ALT) elevation, seen in 2 patients; gamma-glutamyl transferase (GGT) elevation, seen in 2 patients; anemia, seen in 2 patients; febrile neutropenia, seen in 1 patient; and pneumonitis, seen in 1 patient.
There were two dose-limiting toxicities: transient grade 4 ALT elevation at the level 1 dose and grade 4 neutropenia for more than 5 days at the level 2 dose.
Based on central radiological review of 17 evaluable patients, 2 patients achieved complete response, 3 had partial responses, 6 had stable disease, and 6 had progressive disease. The local review reported complete responses in 2 patients, partial responses in 5, stable disease in 4, and progressive disease in 6.
On the irradiated lesions, 4 had complete responses, 8 had partial responses, 4 had stable disease, and 1 had progressive disease. With a median follow-up of 18 months, median progression-free survival was 2.83 months (95%CI: 2.3-3.3 months). Thirteen patients have died, with a median overall survival of 8.77 months (95%CI: 3.6-13.9) and a 12-month overall survival rate of 48%.
Predicting response to chemotherapy
The prognostic nomogram called Sarculator was used effectively to define a high-risk subgroup of patients likely to benefit from adjuvant chemotherapy, Sandro Pasquali, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy and his colleagues reported at the meeting.
Perioperative chemotherapy was shown to afford no survival advantage over observation in the EORTC 62931 (European Organization for Research and Treatment of Cancer—62931) study of adjuvant doxorubicin plus ifosfamide (Lancet Oncol 2012;13:1045-54). However, subsequent analyses of that data attributed this finding to variations in treatment schedules and the inclusion of low-risk tumors, which may have diluted the effect of chemotherapy, the researchers said in their abstract.
Further, a recent interim report of the ISG-1001 trial showed a survival benefit for patients who received neoadjuvant epirubicin plus ifosfamide therapy for localized high-risk soft-tissue sarcoma of the extremities or trunk wall (Lancet Oncol 2017;18:812-822).
The researchers performed a retrospective analysis of individual data for 290 patients with extremity and trunk wall soft-tissue sarcomas in the EORTC-STBSG 62931 study. The Sarculator was used to calculate 10-year predicted probability of overall survival (pr-OS) for each patient.
Patients were grouped in two categories of predicted overall survival: high predicted survival (over 60%) and low predicted overall survival (60% or less). Overall survival and disease-free survival were calculated at 8 years, the study’s median follow-up.
The 8-year probability of overall survival and disease-free survival was 58% [95% confidence interval (CI): 52–63%] and 51% (95% CI: 46–57%), respectively. In the 290 patients with extremity and trunk wall soft tissue sarcomas, adjuvant chemotherapy was not associated with an overall survival benefit [Hazard ratio (HR) = 0.91, 95%CI 0.63–1.31]. The Sarcolator Nomogram detected 80 patients who were at greater risk of death compared to the 210 patients with higher predicted overall survival. The risk of death was significantly lower with adjuvant chemotherapy in the group with low predicted survival based on the Sarculator Nomogram (HR=0.50, 95%CI 0.30-0.90). Consistently, the risk of recurrence was significantly lower when adjuvant chemotherapy was used in the group with predicted overall survival of less than 60% (HR = 0.49, 95%CI 0.28-0.85) while this difference was not observed in patients with high predicted overall survival (HR = 0.95, 95%CI 0.62-1.44).
Doxorubicin plus dacarbazine deserve evaluation in prospective trials in leiomyosarcoma
Doxorubicin plus dacarbazine appeared to best the outcomes seen with doxorubicin plus ifosfamide and with doxorubicin alone in terms of overall response rate and progression free survival as first-line treatment in patients with advanced leiomyosarcomas, based on a retrospective analysis presented by Lorenzo D’Ambrosio, MD, of the Unitversity of Torino, Italy, and his associates.
As patients in the trial were not randomized to therapy, the researchers used a logistic regression model that accounted for histology, site of primary, age, gender, performance status, tumor extent, and tumor grade. Patients were then matched across the different groups by their propensity scores.The 303 patients, 216 of them women, were enrolled from 18 EORTC STBSG (European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group) sites. Doxorubicin plus dacarbazine was given to 117 patients (39%), doxorubicin plus ifosfamide was given to 71 (23%), and doxorubicin alone was given to 115 (38%). There were no significant differences among the regimens in terms of dose reductions of more than 10%, delays of greater than 72 hours, or granulocyte-colony stimulating factor use.
In the whole population, unadjusted median progression free survival was 9.4 months (95% CI 6.1-9.7 months) for those given doxorubicin plus dacarbazine, 6.8 months (4.5-9.5 months) for those given doxorubicin plus ifosfamide), and 5.4 months (3.8-6.8 months) for those given doxorubicin alone. The respective overall response rates for the three regimens were 36.8%, 21.5%, and 25.9%. When using propensity scores to adjust for lack of randomization, progression free survival was significantly longer with doxorubicin plus dacarbazine [median 9.2 months (95%CI 5.2-9.7 months) than with doxorubicin [median 4.8 months (2.3-6.0 months); HR 0.72 (0.52-0.99)]. The difference was not significant when compared to doxorubicin plus ifosfamide [8.2 months (5.2-10.1 months), HR 1.01 (0.68-1.50)]. Progression free survival did not differ significantly between doxorubicin plus ifosfamide, and doxorubicin [HR 0.71 (0.48-1.06)]. In the same matched population, overall response rates were 30.9%, 19.5%, and 25.6% for doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, and doxorubicin, respectively.
Overall survival comparisons were weakened by a shorter median follow-up in the doxorubicin plus dacarbazine groups (32 months) compared to the doxorubicin plus ifosfamide group (50 months) and the doxorubicin group (46 months). With this limit, patients in the doxorubicin plus dacarbazine arm had longer overall survival [median 36.8 (27.9-47.2) months] when compared to both doxorubicin plus ifosfamide [21.9 (16.7-33.4), HR 0.65 (0.40-1.06); and doxorubicin arms 30.3 (21.0-36.3) months, HR 0.66 (0.43-0.99).
Subsequent treatments were well balanced across arms. None of the selected factors for multivariate analysis (age, sex, ECOG performance status, histotype, site of primary tumor, tumor grade, and tumor extent) significantly affected the progression free survival and overall survival associated with the treatments.
Olaratumab in combination with doxorubicin plus ifosfamide
Olaratumab at 15 mg/kg has been shown to be safe in combination with doxorubicin plus ifosfamide in a Phase 1b study (NCT03283696), reported Sebastian Bauer, MD, of the West German Cancer Center, University of Duisburg-Essen, Essen, Germany, and his colleagues.
Given that 8 of 10 evaluable patients have completed the drug-limiting toxicity period without drug-limiting toxicities at the 15 mg/kg dose level of olaratumab, the study has proceeded to the next cohort. In those patients, an olaratumab loading dose of 20 mg/kg will be evaluated in cycle 1, followed by 15 mg/kg of olaratumab in subsequent cycles with the same doses of doxorubicin plus ifosfamide, the researchers wrote in their abstract.
The phase 1 trial enrolled 16 patients with advanced or metastatic soft tissue sarcomas and no prior lines of systemic therapy and ECOG performance status 0-1. Adequate follow up data was available for 10 patients.
Olaratumab, (Lartruvo), which binds platelet-derived growth factor receptor alpha (PDGFRα), was given at 15 mg/kg in combination with doxorubicin (75 mg/m2 on days 1-3) and ifosfamide (10 g/m2 on days 1-4) followed by mandatory granulocyte-colony-stimulating factor therapy in cycles 1-6 on a 21-day cycle. Doxorubicin was allowed to be administered by continuous infusion or bolus administration and with cardiac protection. Mesna dosing was at least 60% of the ifosfamide dose.
Two of the 10 patients had dose-limiting toxicities; one had Grade 4 febrile neutropenia and the other had Grade 3 febrile neutropenia and Grade 3 mucositis. Common related adverse events occurring in over 30% of patients included fatigue, anemia, neutropenia, thrombocytopenia, constipation, and nausea. One patient discontinued study treatment due to progressive disease, and all others were on study treatment as of data cutoff. Among 7 patients evaluated for tumor response assessment, 3 patients had a partial response according to RECIST and 3 further patients had stabilized disease as best overall response for a disease control rate of 86%.
Anthracycline-based regimen excels in FIGO-1 uterine leiomyosarcoma
Future trials to assess the efficacy of adjuvant chemotherapy in uterine leiomyosarcoma should incorporate anthracyclines, according to Roberta Sanfilippo, MD, of Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues.
Disease-free survival was extended in patients with uterine leiomyosarcomas treated with anthracycline-based regimens as compared to gemcitabine and docetaxel, based on a retrospective analysis reported at the meeting by Dr. Sanfilippo.
They reviewed all patients with FIGO stage I uterine leiomyosarcomas who underwent hysterectomy with or without oophorectomy and were treated with adjuvant chemotherapy with either anthracycline-based or gemcitabine-based chemotherapy at two Italian centers.
Of 145 patients, 97 were treated with an anthracycline-based regimen and 48 with gemcitabine and docetaxel. The median number of cycles of anthracycline based regimen received was 4 (range 2-6) and with gemcitabine and docetaxel was 5 (range 3-7). Disease free survival was 31 months in patients treated with anthracycline-based chemotherapy and 19 months in patients treated with gemcitabine and docetaxel.
Trabectedin and low-dose radiotherapy
Trabectedin concurrent with low-dose radiotherapy is being examined as an option for patients with pulmonary metastatic soft tissue sarcoma (NCT02275286).
In a phase 1 study, long-lasting dimensional responses were seen in 71% of the irradiated lesions showed. Based on those results, trabectedin (Yondelis) at 1.5 mg/m 2 will be the recommended dose for phase 2, according to Javier Martín-Broto, MD, of the Institute of Biomedicine Research (IBIS)-University Hospital Virgen del Rocio/CSIC/University of Seville, Spain, and his colleagues.
For the study, trabectedin was given along with radiotherapy (30 Gy) in 10 fractions (3 Gy/fraction). Three dose levels of trabectedin were administered: -1 (1.1 mg/m 2), 1 (1.3 mg/m 2) and 2 (1.5 mg/m 2). Dose-limiting toxicity was defined as grade 3 or greater events excluding grade 3/4 neutropenia lasting less than 5 days, grade 3 transaminitis if it did not lead to trabectedin delay, and grade 3/4 nausea/vomiting due to inadequate prophylaxis.
Ten of the 18 patients enrolled had synovial sarcoma; 3 had undifferentiated pleomorphic sarcomas and the other patients had either myxoid liposarcoma, dedifferentiated liposarcoma, G3 not otherwise specified sarcoma, leiomyosarcoma, and malignant peripheral nerve sheath tumor.
Patients received a median of 1 prior line of chemotherapy (range: 0-3). Twelve patients received trabectedin at dose level 1 and 6 patients at dose level 2. Grade 3/4 adverse events were neutropenia, seen in 8 patients; alanine aminotransferase (ALT) elevation, seen in 2 patients; gamma-glutamyl transferase (GGT) elevation, seen in 2 patients; anemia, seen in 2 patients; febrile neutropenia, seen in 1 patient; and pneumonitis, seen in 1 patient.
There were two dose-limiting toxicities: transient grade 4 ALT elevation at the level 1 dose and grade 4 neutropenia for more than 5 days at the level 2 dose.
Based on central radiological review of 17 evaluable patients, 2 patients achieved complete response, 3 had partial responses, 6 had stable disease, and 6 had progressive disease. The local review reported complete responses in 2 patients, partial responses in 5, stable disease in 4, and progressive disease in 6.
On the irradiated lesions, 4 had complete responses, 8 had partial responses, 4 had stable disease, and 1 had progressive disease. With a median follow-up of 18 months, median progression-free survival was 2.83 months (95%CI: 2.3-3.3 months). Thirteen patients have died, with a median overall survival of 8.77 months (95%CI: 3.6-13.9) and a 12-month overall survival rate of 48%.
Uptick in adult syphilis means congenital syphilis may be lurking
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
Launching an HIV testing reminder
Trying a new tool to reduce infection rates
The world’s largest gay dating app, Grindr, changed its software earlier this year to create reminders for users to get regular HIV tests.
According to Grindr, 3.3 million users around the world visit the site daily; it sends those who opt into the service a reminder every 3-6 months to get a test. The message also directs them to the nearest testing site. Grindr also plans to give clinics, gay community centers, and other testing sites free advertising.
Among health care providers, the decision has been widely applauded. “This will ‘demedicalize’ testing and destigmatize it,” Perry N. Halkitis, PhD, dean of the Rutgers School of Public Health, in Newark, N.J., told the New York Times. “The more you make it normal, the more people are going to access it.”
Studies have shown that reminders by text or phone can triple or quadruple the chance that the recipient will get tested.
Reference
McNeil Jr. DG. Grindr App to Offer H.I.V. Test Reminders. The New York Times. March 26, 2018. Accessed April 5, 2018.
Trying a new tool to reduce infection rates
Trying a new tool to reduce infection rates
The world’s largest gay dating app, Grindr, changed its software earlier this year to create reminders for users to get regular HIV tests.
According to Grindr, 3.3 million users around the world visit the site daily; it sends those who opt into the service a reminder every 3-6 months to get a test. The message also directs them to the nearest testing site. Grindr also plans to give clinics, gay community centers, and other testing sites free advertising.
Among health care providers, the decision has been widely applauded. “This will ‘demedicalize’ testing and destigmatize it,” Perry N. Halkitis, PhD, dean of the Rutgers School of Public Health, in Newark, N.J., told the New York Times. “The more you make it normal, the more people are going to access it.”
Studies have shown that reminders by text or phone can triple or quadruple the chance that the recipient will get tested.
Reference
McNeil Jr. DG. Grindr App to Offer H.I.V. Test Reminders. The New York Times. March 26, 2018. Accessed April 5, 2018.
The world’s largest gay dating app, Grindr, changed its software earlier this year to create reminders for users to get regular HIV tests.
According to Grindr, 3.3 million users around the world visit the site daily; it sends those who opt into the service a reminder every 3-6 months to get a test. The message also directs them to the nearest testing site. Grindr also plans to give clinics, gay community centers, and other testing sites free advertising.
Among health care providers, the decision has been widely applauded. “This will ‘demedicalize’ testing and destigmatize it,” Perry N. Halkitis, PhD, dean of the Rutgers School of Public Health, in Newark, N.J., told the New York Times. “The more you make it normal, the more people are going to access it.”
Studies have shown that reminders by text or phone can triple or quadruple the chance that the recipient will get tested.
Reference
McNeil Jr. DG. Grindr App to Offer H.I.V. Test Reminders. The New York Times. March 26, 2018. Accessed April 5, 2018.
More rheumatology slots available, filled on Specialty Match Day, but numbers well below need
Although more fellowships slots in rheumatology were available and filled during the Specialty Match Day for the 2019 appointment year, the numbers are still well below what will be needed to fill the physician shortfall in this area.
For the 2019 appointment year, there were 236 fellowship positions available, with 233 of them filled, up from 221 positions available and 218 filled on the previous year’s Specialty Match Day, according to results released by the National Resident Matching Program.
Adding 10 or even 20 a year “is still not going to make a dent” in the expected rheumatologist shortfall, Beth Jonas, MD, director of the Rheumatology Fellowship Training Program at the University of North Carolina, Chapel Hill, said in an interview.
That being said, there are still positive messages to be taken away from the Specialty Match Day results.
“The rheumatology match was great this year,” Dr. Jonas said. “From the perspective of the quality of the applicant pool, it was very strong. The interest in rheumatology is very high. From the perspective of program directors, we had a lot of excellent applicants to interview and to choose from.”
She noted that in total there were 358 applicants who listed rheumatology as their preference for the 2019 appointment year, up from 313. Dr. Jonas also serves as chair of the American College of Rheumatology’s Committee on Rheumatology Training and Workforce Issues.
“The message here is that there are a lot more people who want to become rheumatologists than there are slots to train them,” she stated.
And the key reason more people aren’t being trained is money.
“There is capacity to train more people, but the challenge is funding those slots,” Dr. Jonas said, noting that the ACR is doing what it can to help fund fellowship slots, and the Arthritis Foundation has a new grant mechanism that has helped to fund new slots.
“But I think there needs to be other ways.”
She suggested that there should be more involvement from the federal government, perhaps through Medicare, and more involvement from local institutions.
Mona Singer, president and CEO of the National Resident Matching Program, highlighted rheumatology as a standout among the overall specialty matching programs for the 2019 program year.
“The specialty that has become more popular over the years is rheumatology, which I find interesting,” Ms. Singer said. “This isn’t the first year it has done well, but it has been on an upward trend.”
Overall, the programs that have been doing well, matching at a rate of 95% or better, have continued to see those high fill rates: allergy/immunology, cardiology, gastroenterology, hematology, oncology, pulmonary and critical care medicine, and endocrinology.
For the 2019 appointment year, there were a total of 5,881 applicants for 5,215 program slots, with 4,579 positions filled. The fill rate of 87.8% is unchanged from the previous year, when 5,491 applicants competed for 4,831 positions, with 4,242 slots filled.
Although more fellowships slots in rheumatology were available and filled during the Specialty Match Day for the 2019 appointment year, the numbers are still well below what will be needed to fill the physician shortfall in this area.
For the 2019 appointment year, there were 236 fellowship positions available, with 233 of them filled, up from 221 positions available and 218 filled on the previous year’s Specialty Match Day, according to results released by the National Resident Matching Program.
Adding 10 or even 20 a year “is still not going to make a dent” in the expected rheumatologist shortfall, Beth Jonas, MD, director of the Rheumatology Fellowship Training Program at the University of North Carolina, Chapel Hill, said in an interview.
That being said, there are still positive messages to be taken away from the Specialty Match Day results.
“The rheumatology match was great this year,” Dr. Jonas said. “From the perspective of the quality of the applicant pool, it was very strong. The interest in rheumatology is very high. From the perspective of program directors, we had a lot of excellent applicants to interview and to choose from.”
She noted that in total there were 358 applicants who listed rheumatology as their preference for the 2019 appointment year, up from 313. Dr. Jonas also serves as chair of the American College of Rheumatology’s Committee on Rheumatology Training and Workforce Issues.
“The message here is that there are a lot more people who want to become rheumatologists than there are slots to train them,” she stated.
And the key reason more people aren’t being trained is money.
“There is capacity to train more people, but the challenge is funding those slots,” Dr. Jonas said, noting that the ACR is doing what it can to help fund fellowship slots, and the Arthritis Foundation has a new grant mechanism that has helped to fund new slots.
“But I think there needs to be other ways.”
She suggested that there should be more involvement from the federal government, perhaps through Medicare, and more involvement from local institutions.
Mona Singer, president and CEO of the National Resident Matching Program, highlighted rheumatology as a standout among the overall specialty matching programs for the 2019 program year.
“The specialty that has become more popular over the years is rheumatology, which I find interesting,” Ms. Singer said. “This isn’t the first year it has done well, but it has been on an upward trend.”
Overall, the programs that have been doing well, matching at a rate of 95% or better, have continued to see those high fill rates: allergy/immunology, cardiology, gastroenterology, hematology, oncology, pulmonary and critical care medicine, and endocrinology.
For the 2019 appointment year, there were a total of 5,881 applicants for 5,215 program slots, with 4,579 positions filled. The fill rate of 87.8% is unchanged from the previous year, when 5,491 applicants competed for 4,831 positions, with 4,242 slots filled.
Although more fellowships slots in rheumatology were available and filled during the Specialty Match Day for the 2019 appointment year, the numbers are still well below what will be needed to fill the physician shortfall in this area.
For the 2019 appointment year, there were 236 fellowship positions available, with 233 of them filled, up from 221 positions available and 218 filled on the previous year’s Specialty Match Day, according to results released by the National Resident Matching Program.
Adding 10 or even 20 a year “is still not going to make a dent” in the expected rheumatologist shortfall, Beth Jonas, MD, director of the Rheumatology Fellowship Training Program at the University of North Carolina, Chapel Hill, said in an interview.
That being said, there are still positive messages to be taken away from the Specialty Match Day results.
“The rheumatology match was great this year,” Dr. Jonas said. “From the perspective of the quality of the applicant pool, it was very strong. The interest in rheumatology is very high. From the perspective of program directors, we had a lot of excellent applicants to interview and to choose from.”
She noted that in total there were 358 applicants who listed rheumatology as their preference for the 2019 appointment year, up from 313. Dr. Jonas also serves as chair of the American College of Rheumatology’s Committee on Rheumatology Training and Workforce Issues.
“The message here is that there are a lot more people who want to become rheumatologists than there are slots to train them,” she stated.
And the key reason more people aren’t being trained is money.
“There is capacity to train more people, but the challenge is funding those slots,” Dr. Jonas said, noting that the ACR is doing what it can to help fund fellowship slots, and the Arthritis Foundation has a new grant mechanism that has helped to fund new slots.
“But I think there needs to be other ways.”
She suggested that there should be more involvement from the federal government, perhaps through Medicare, and more involvement from local institutions.
Mona Singer, president and CEO of the National Resident Matching Program, highlighted rheumatology as a standout among the overall specialty matching programs for the 2019 program year.
“The specialty that has become more popular over the years is rheumatology, which I find interesting,” Ms. Singer said. “This isn’t the first year it has done well, but it has been on an upward trend.”
Overall, the programs that have been doing well, matching at a rate of 95% or better, have continued to see those high fill rates: allergy/immunology, cardiology, gastroenterology, hematology, oncology, pulmonary and critical care medicine, and endocrinology.
For the 2019 appointment year, there were a total of 5,881 applicants for 5,215 program slots, with 4,579 positions filled. The fill rate of 87.8% is unchanged from the previous year, when 5,491 applicants competed for 4,831 positions, with 4,242 slots filled.
Duodenoscopes contain more bacteria than expected
Reprocessed duodenoscopes are more contaminated than expected, with up to 3% of samples testing positive for disease-causing bacteria including Escherichia coli and Staphylococcus aureus, according to an updated safety communication issued by the Food and Drug Administration on December 10.
“Because of the higher-than-expected contamination rates and to help protect patients from bacterial infections associated with the use of duodenoscopes, we have included in today’s safety communication updated recommendations regarding steps that health care providers can take to enhance duodenoscope reprocessing,” Jeff Shuren, MD, director of the Center for Devices and Radiological Health, wrote in the statement.
The FDA advised clinicians to follow additional cleaning measures including microbiological culturing, sterilization, use of a liquid chemical sterilant processing system, and repeated high-level disinfection beyond what is recommended by duodenoscope manufacturers.
The interim data cited in the safety communication come from postmarket surveillance studies conducted by duodenoscope manufacturers at the FDA’s request as part of the agency’s ongoing efforts to prevent patient infections caused by contaminated duodenoscopes. In addition to the positive tests for disease-causing bacteria, up to 3% of properly collected samples contained more than 100 colony-forming units of other organisms unlikely to cause infection. However, the presence of such organisms further highlights the failure of the current reprocessing protocol to adequately clean the devices, according to the FDA.
Dr. Shuren emphasized that the risk of infection from a duodenoscope for an individual patient remains low and that infection rates have declined in recent years in response to the FDA’s enhanced safety measures and stated that the agency remains “committed to enhancing the safety margin of procedures with reprocessed medical devices.”
Read the full safety communication here: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm628020.htm.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process. Learn more at www.gastro.org/CGIT
Reprocessed duodenoscopes are more contaminated than expected, with up to 3% of samples testing positive for disease-causing bacteria including Escherichia coli and Staphylococcus aureus, according to an updated safety communication issued by the Food and Drug Administration on December 10.
“Because of the higher-than-expected contamination rates and to help protect patients from bacterial infections associated with the use of duodenoscopes, we have included in today’s safety communication updated recommendations regarding steps that health care providers can take to enhance duodenoscope reprocessing,” Jeff Shuren, MD, director of the Center for Devices and Radiological Health, wrote in the statement.
The FDA advised clinicians to follow additional cleaning measures including microbiological culturing, sterilization, use of a liquid chemical sterilant processing system, and repeated high-level disinfection beyond what is recommended by duodenoscope manufacturers.
The interim data cited in the safety communication come from postmarket surveillance studies conducted by duodenoscope manufacturers at the FDA’s request as part of the agency’s ongoing efforts to prevent patient infections caused by contaminated duodenoscopes. In addition to the positive tests for disease-causing bacteria, up to 3% of properly collected samples contained more than 100 colony-forming units of other organisms unlikely to cause infection. However, the presence of such organisms further highlights the failure of the current reprocessing protocol to adequately clean the devices, according to the FDA.
Dr. Shuren emphasized that the risk of infection from a duodenoscope for an individual patient remains low and that infection rates have declined in recent years in response to the FDA’s enhanced safety measures and stated that the agency remains “committed to enhancing the safety margin of procedures with reprocessed medical devices.”
Read the full safety communication here: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm628020.htm.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process. Learn more at www.gastro.org/CGIT
Reprocessed duodenoscopes are more contaminated than expected, with up to 3% of samples testing positive for disease-causing bacteria including Escherichia coli and Staphylococcus aureus, according to an updated safety communication issued by the Food and Drug Administration on December 10.
“Because of the higher-than-expected contamination rates and to help protect patients from bacterial infections associated with the use of duodenoscopes, we have included in today’s safety communication updated recommendations regarding steps that health care providers can take to enhance duodenoscope reprocessing,” Jeff Shuren, MD, director of the Center for Devices and Radiological Health, wrote in the statement.
The FDA advised clinicians to follow additional cleaning measures including microbiological culturing, sterilization, use of a liquid chemical sterilant processing system, and repeated high-level disinfection beyond what is recommended by duodenoscope manufacturers.
The interim data cited in the safety communication come from postmarket surveillance studies conducted by duodenoscope manufacturers at the FDA’s request as part of the agency’s ongoing efforts to prevent patient infections caused by contaminated duodenoscopes. In addition to the positive tests for disease-causing bacteria, up to 3% of properly collected samples contained more than 100 colony-forming units of other organisms unlikely to cause infection. However, the presence of such organisms further highlights the failure of the current reprocessing protocol to adequately clean the devices, according to the FDA.
Dr. Shuren emphasized that the risk of infection from a duodenoscope for an individual patient remains low and that infection rates have declined in recent years in response to the FDA’s enhanced safety measures and stated that the agency remains “committed to enhancing the safety margin of procedures with reprocessed medical devices.”
Read the full safety communication here: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm628020.htm.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process. Learn more at www.gastro.org/CGIT
Negative colonoscopy linked with lower risk for more than 10 years
A negative colonoscopy result is associated with a reduced risk of colorectal cancer for more than 12 years after the examination, compared with an unscreened population, new research has found.
In a retrospective cohort study published in JAMA Internal Medicine, researchers analyzed data from 1,251,318 individuals at average risk of colorectal cancer who were eligible to participate in screening over more than 9 million person-years of follow-up.
They found that screened individuals with a negative result had an adjusted 46%-95% lower risk of colorectal cancer and 29%-96% lower risk of colorectal cancer mortality than unscreened individuals across more than 12 years of follow-up.
At 10 years post colonoscopy, participants who had a negative colonoscopy result still had a significant 46% lower risk of colorectal cancer and 88% lower risk of colorectal cancer mortality. After more than 12 years, there was still a nonsignificant trend towards a lower risk of colorectal cancer incidence and mortality.
Jeffrey K. Lee, MD, of Kaiser Permanente San Francisco, and his coauthors suggested that their findings have implications for the timing of rescreening after a negative colonoscopy result.
“The current guideline-recommended 10-year rescreening interval is not based on a predetermined risk threshold, and while we observed a reduced risk of colorectal cancer and related deaths throughout the more than 12-year follow-up period, an examination of absolute risk [incidence] could provide another justification for the timing for rescreening,” they wrote. “Additional research is needed to evaluate the costs and benefits of earlier versus later rescreening, optimal rescreening tests following a negative colonoscopy result [e.g., another colonoscopy versus annual fecal immunochemical testing], and whether the benefits of rescreening vary between subgroups.”
The study showed that the rate of repeat endoscopic procedures increased at year 10, largely because of screening colonoscopies which are recommended at 10-year intervals. However in a separate analysis, the authors excluded colonoscopies for a screening indication and still found a similar reduction in the risk of colorectal cancer, compared with the unscreened group.
The data also showed a 22%-87% lower risk of proximal colorectal cancer, a 50%-99% lower risk of distal cancer, a 31%-95% lower risk of early-stage colorectal cancer, and a 56%-96% reduced risk of advanced-stage colorectal cancer among those who had a negative result, compared with those who did not undergo screening.
The authors wrote that this pattern of greater risk reductions in the distal versus proximal cancer had been seen in previous studies and could be the result of incomplete examinations, inadequate bowel cleansing, challenges in identifying right colon polyps and sessile serrated adenomas, or differences in polyp biology in the proximal versus distal colon.
The incidence rates of colorectal cancer among those who had a negative result from colonoscopy ranged from 16.6 per 100,000 person-years at 1 year after screening to 133.2 per 100,000 person-years at the 10-year mark. In comparison, the incidence rates among the unscreened population increased from 62.9 per 100,000 person-years at year 1 to 224.8 after year 12.
Mortality rates from colorectal cancer at year 1 were 6.8 per 100,000 person-years in the negative results group and 10.5 in the unscreened cohort. At year 12, that figure was 92.2 per 100,000 person-years in the negative results cohort, while after year 12 in the unscreened cohort, colorectal cancer mortality rates increased to 192 per 100,000 person years.
While the study made use of a validated cancer registry to ensure they accurately captured cancers and mortality, the authors acknowledged that they weren’t able to adjust for residual confounding factors such as red-meat intake or smoking.
The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
SOURCE: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
A negative colonoscopy result is associated with a reduced risk of colorectal cancer for more than 12 years after the examination, compared with an unscreened population, new research has found.
In a retrospective cohort study published in JAMA Internal Medicine, researchers analyzed data from 1,251,318 individuals at average risk of colorectal cancer who were eligible to participate in screening over more than 9 million person-years of follow-up.
They found that screened individuals with a negative result had an adjusted 46%-95% lower risk of colorectal cancer and 29%-96% lower risk of colorectal cancer mortality than unscreened individuals across more than 12 years of follow-up.
At 10 years post colonoscopy, participants who had a negative colonoscopy result still had a significant 46% lower risk of colorectal cancer and 88% lower risk of colorectal cancer mortality. After more than 12 years, there was still a nonsignificant trend towards a lower risk of colorectal cancer incidence and mortality.
Jeffrey K. Lee, MD, of Kaiser Permanente San Francisco, and his coauthors suggested that their findings have implications for the timing of rescreening after a negative colonoscopy result.
“The current guideline-recommended 10-year rescreening interval is not based on a predetermined risk threshold, and while we observed a reduced risk of colorectal cancer and related deaths throughout the more than 12-year follow-up period, an examination of absolute risk [incidence] could provide another justification for the timing for rescreening,” they wrote. “Additional research is needed to evaluate the costs and benefits of earlier versus later rescreening, optimal rescreening tests following a negative colonoscopy result [e.g., another colonoscopy versus annual fecal immunochemical testing], and whether the benefits of rescreening vary between subgroups.”
The study showed that the rate of repeat endoscopic procedures increased at year 10, largely because of screening colonoscopies which are recommended at 10-year intervals. However in a separate analysis, the authors excluded colonoscopies for a screening indication and still found a similar reduction in the risk of colorectal cancer, compared with the unscreened group.
The data also showed a 22%-87% lower risk of proximal colorectal cancer, a 50%-99% lower risk of distal cancer, a 31%-95% lower risk of early-stage colorectal cancer, and a 56%-96% reduced risk of advanced-stage colorectal cancer among those who had a negative result, compared with those who did not undergo screening.
The authors wrote that this pattern of greater risk reductions in the distal versus proximal cancer had been seen in previous studies and could be the result of incomplete examinations, inadequate bowel cleansing, challenges in identifying right colon polyps and sessile serrated adenomas, or differences in polyp biology in the proximal versus distal colon.
The incidence rates of colorectal cancer among those who had a negative result from colonoscopy ranged from 16.6 per 100,000 person-years at 1 year after screening to 133.2 per 100,000 person-years at the 10-year mark. In comparison, the incidence rates among the unscreened population increased from 62.9 per 100,000 person-years at year 1 to 224.8 after year 12.
Mortality rates from colorectal cancer at year 1 were 6.8 per 100,000 person-years in the negative results group and 10.5 in the unscreened cohort. At year 12, that figure was 92.2 per 100,000 person-years in the negative results cohort, while after year 12 in the unscreened cohort, colorectal cancer mortality rates increased to 192 per 100,000 person years.
While the study made use of a validated cancer registry to ensure they accurately captured cancers and mortality, the authors acknowledged that they weren’t able to adjust for residual confounding factors such as red-meat intake or smoking.
The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
SOURCE: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
A negative colonoscopy result is associated with a reduced risk of colorectal cancer for more than 12 years after the examination, compared with an unscreened population, new research has found.
In a retrospective cohort study published in JAMA Internal Medicine, researchers analyzed data from 1,251,318 individuals at average risk of colorectal cancer who were eligible to participate in screening over more than 9 million person-years of follow-up.
They found that screened individuals with a negative result had an adjusted 46%-95% lower risk of colorectal cancer and 29%-96% lower risk of colorectal cancer mortality than unscreened individuals across more than 12 years of follow-up.
At 10 years post colonoscopy, participants who had a negative colonoscopy result still had a significant 46% lower risk of colorectal cancer and 88% lower risk of colorectal cancer mortality. After more than 12 years, there was still a nonsignificant trend towards a lower risk of colorectal cancer incidence and mortality.
Jeffrey K. Lee, MD, of Kaiser Permanente San Francisco, and his coauthors suggested that their findings have implications for the timing of rescreening after a negative colonoscopy result.
“The current guideline-recommended 10-year rescreening interval is not based on a predetermined risk threshold, and while we observed a reduced risk of colorectal cancer and related deaths throughout the more than 12-year follow-up period, an examination of absolute risk [incidence] could provide another justification for the timing for rescreening,” they wrote. “Additional research is needed to evaluate the costs and benefits of earlier versus later rescreening, optimal rescreening tests following a negative colonoscopy result [e.g., another colonoscopy versus annual fecal immunochemical testing], and whether the benefits of rescreening vary between subgroups.”
The study showed that the rate of repeat endoscopic procedures increased at year 10, largely because of screening colonoscopies which are recommended at 10-year intervals. However in a separate analysis, the authors excluded colonoscopies for a screening indication and still found a similar reduction in the risk of colorectal cancer, compared with the unscreened group.
The data also showed a 22%-87% lower risk of proximal colorectal cancer, a 50%-99% lower risk of distal cancer, a 31%-95% lower risk of early-stage colorectal cancer, and a 56%-96% reduced risk of advanced-stage colorectal cancer among those who had a negative result, compared with those who did not undergo screening.
The authors wrote that this pattern of greater risk reductions in the distal versus proximal cancer had been seen in previous studies and could be the result of incomplete examinations, inadequate bowel cleansing, challenges in identifying right colon polyps and sessile serrated adenomas, or differences in polyp biology in the proximal versus distal colon.
The incidence rates of colorectal cancer among those who had a negative result from colonoscopy ranged from 16.6 per 100,000 person-years at 1 year after screening to 133.2 per 100,000 person-years at the 10-year mark. In comparison, the incidence rates among the unscreened population increased from 62.9 per 100,000 person-years at year 1 to 224.8 after year 12.
Mortality rates from colorectal cancer at year 1 were 6.8 per 100,000 person-years in the negative results group and 10.5 in the unscreened cohort. At year 12, that figure was 92.2 per 100,000 person-years in the negative results cohort, while after year 12 in the unscreened cohort, colorectal cancer mortality rates increased to 192 per 100,000 person years.
While the study made use of a validated cancer registry to ensure they accurately captured cancers and mortality, the authors acknowledged that they weren’t able to adjust for residual confounding factors such as red-meat intake or smoking.
The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
SOURCE: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Reduced rates of colorectal cancer after a negative colonoscopy persist beyond 10 years.
Major finding: After 10 years, a negative colonoscopy is still associated with an 88% lower risk of colorectal cancer mortality, compared with the unscreened.
Study details: A retrospective cohort study in 1,251,318 screening-eligible individuals.
Disclosures: The study was supported by the National Cancer Institute, the American Gastroenterological Association, and the Sylvia Allison Kaplan Foundation. No conflicts of interest were reported.
Source: Lee JK et al. JAMA Intern Med. 2018 Dec 17. doi: 10.1001/jamainternmed.2018.5565.
“FAST” indices help to identify fibromyalgia in routine rheumatology care
CHICAGO – Fibromyalgia assessment screening tool (FAST) indices derived from the Multidimensional Health Assessment Questionnaire (MDHAQ) provide a simple and effective method for identifying fibromyalgia in routine care, according to findings from a series of more than 500 patients.
The indices are as accurate as the existing – and more complex – diagnostic criteria for fibromyalgia, Juan Schmukler, MD, reported at the annual meeting of the American College of Rheumatology.
For example, three FAST indices developed in the course of the study had better performance versus existing diagnostic criteria than did certain individual MDHAQ scales alone (area under the curve range, 0.924-0.937 vs. 0.829-0.889, respectively), said Dr. Schmukler, who conducted the research as a medical student at Rush Medical College, Chicago, and is now a rheumatology fellow at Mount Sinai Hospital in Chicago.
The findings are notable, because the two-page MDHAQ and a summary index (the RAPID3) derived from three of the MDHAQ scales are already used routinely in clinical care for diagnosing rheumatic diseases, and the new indices could easily – and with minimal work flow disruption – also be incorporated for each patient at each visit.
“Fibromyalgia is common in the general population and it is believed to be more common in patients with other rheumatic diagnoses,” Dr. Schmukler said. “Fibromyalgia may be easily recognized in many cases, but it can also be very subtle, particularly in patients who have other rheumatic diseases.”
ACR fibromyalgia classification criteria published in 1990 were based on tender point examination and non-ACR diagnostic criteria as revised in 2011 are based entirely on patient self-report.
A one-page fibromyalgia criteria questionnaire is available and is useful in clinical trials and other research, but is “rarely, if ever” used in routine care, he said, explaining that the questionnaire contains two domains: a symptom severity score (0-12 scale) and a widespread pain index (0-19 scale).
The MDHAQ/RAPID3 is informative in RA, and has also been shown to be “useful in all rheumatic diseases in which it has been studied,” and at least three prior reports have suggested that the MDHAQ may also provide clues to the presence of fibromyalgia, Dr. Schmukler said.
“And we know from prior reports that patients with fibromyalgia reported the highest RAPID3 scores, compared to other rheumatic disease,” he added, explaining that the goal of the present study was to develop FAST cumulative indices based on the routine MDHAQ scales and using the 2011 diagnostic criteria as a reference standard.
All patients with all diagnoses seen at the Rush Medical College rheumatology clinic in Chicago complete the MDHAQ at all visits in routine care, and between April and July 2017, the fibromyalgia criteria questionnaire was also administered in 502 consecutive patients.
Of those patients, 131 met the 2011 fibromyalgia diagnostic criteria.
MDHAQ scores were analyzed for agreement with the fibromyalgia criteria questionnaire according to receiver operator characteristic curves for AUC and were compiled into three different FAST indices that included various combinations of either three or four of the MDHAQ measures that had the best agreement with the diagnostic criteria questionnaire as identified by the highest AUC values. Those were the 60-symptom checklist (AUC, 0.889), painful joint count (AUC, 0.870), fatigue visual analog scale (VAS; AUC, 0.860), and pain VAS (AUC, 0.829), Dr. Schmukler said.
Proposed cut points that reflected the optimal trade-off between specificity and sensitivity for each of the scales were scores of 6 or greater for the pain VAS and fatigue VAS, and scores of 16 or greater for the symptom checklist and painful joint count measure.
In addition to the better performance of each FAST index versus the 2011 criteria, their performance was also better than that of the RAPID3 versus the 2011 criteria (AUC, 0.848), which many clinicians use in practice without using the full MDHAQ for patient assessment, he said.
Further, the “very easily calculated” FAST indices performed as well as a “very difficult to calculate” polysymptomatic distress continuous scale derived from the fibromyalgia questionnaire to assess the degree of fibromyalgia symptoms, which had an AUC of 0.929 versus the 2011 criteria. The latter index requires complex mathematical calculations for scale conversion and thus is impractical in clinical practice, he noted.
The FAST indices also performed comparably with physician diagnoses as indicated in patient charts and with diagnoses based on tender point count as shown in prior studies.
The FAST index with the greatest sensitivity (85.5% at a cut point of 2 or greater on a scale of 1-3) was the FAST3-P, which includes pain VAS score of 6 or greater, symptom checklist score of 16 or greater, and painful joint count of 16 or greater. The FAST index with the greatest specificity (90.3% at a cut point of score of 3 or greater on a scale of 1-4) was the FAST4 index, which includes a pain VAS score of 6 or greater, fatigue VAS score of 6 or greater, symptom checklist score of 16 or greater, and painful joint count of 16 or greater.
Although the findings are limited by the lack of a gold standard for fibromyalgia diagnosis, changing diagnostic criteria, and a need for physician input for a fibromyalgia diagnosis, the study provides useful real-world data and supports findings from some prior studies with respect to the benefit of using these tools in routine practice.
With minimal extra physician time – if the MDHAQ is completed by patients at the time of registration – this approach can be used in all rheumatology patients to help identify fibromyalgia, he concluded.
Dr. Schmukler reported having no disclosures. One of his coauthors at Rush, Ted Pincus, MD, receives royalties and license fees for the copyright and trademark for the MDHAQ and RAPID3, all of which go to support clinical research.
SOURCE: Schmukler J et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 839.
CHICAGO – Fibromyalgia assessment screening tool (FAST) indices derived from the Multidimensional Health Assessment Questionnaire (MDHAQ) provide a simple and effective method for identifying fibromyalgia in routine care, according to findings from a series of more than 500 patients.
The indices are as accurate as the existing – and more complex – diagnostic criteria for fibromyalgia, Juan Schmukler, MD, reported at the annual meeting of the American College of Rheumatology.
For example, three FAST indices developed in the course of the study had better performance versus existing diagnostic criteria than did certain individual MDHAQ scales alone (area under the curve range, 0.924-0.937 vs. 0.829-0.889, respectively), said Dr. Schmukler, who conducted the research as a medical student at Rush Medical College, Chicago, and is now a rheumatology fellow at Mount Sinai Hospital in Chicago.
The findings are notable, because the two-page MDHAQ and a summary index (the RAPID3) derived from three of the MDHAQ scales are already used routinely in clinical care for diagnosing rheumatic diseases, and the new indices could easily – and with minimal work flow disruption – also be incorporated for each patient at each visit.
“Fibromyalgia is common in the general population and it is believed to be more common in patients with other rheumatic diagnoses,” Dr. Schmukler said. “Fibromyalgia may be easily recognized in many cases, but it can also be very subtle, particularly in patients who have other rheumatic diseases.”
ACR fibromyalgia classification criteria published in 1990 were based on tender point examination and non-ACR diagnostic criteria as revised in 2011 are based entirely on patient self-report.
A one-page fibromyalgia criteria questionnaire is available and is useful in clinical trials and other research, but is “rarely, if ever” used in routine care, he said, explaining that the questionnaire contains two domains: a symptom severity score (0-12 scale) and a widespread pain index (0-19 scale).
The MDHAQ/RAPID3 is informative in RA, and has also been shown to be “useful in all rheumatic diseases in which it has been studied,” and at least three prior reports have suggested that the MDHAQ may also provide clues to the presence of fibromyalgia, Dr. Schmukler said.
“And we know from prior reports that patients with fibromyalgia reported the highest RAPID3 scores, compared to other rheumatic disease,” he added, explaining that the goal of the present study was to develop FAST cumulative indices based on the routine MDHAQ scales and using the 2011 diagnostic criteria as a reference standard.
All patients with all diagnoses seen at the Rush Medical College rheumatology clinic in Chicago complete the MDHAQ at all visits in routine care, and between April and July 2017, the fibromyalgia criteria questionnaire was also administered in 502 consecutive patients.
Of those patients, 131 met the 2011 fibromyalgia diagnostic criteria.
MDHAQ scores were analyzed for agreement with the fibromyalgia criteria questionnaire according to receiver operator characteristic curves for AUC and were compiled into three different FAST indices that included various combinations of either three or four of the MDHAQ measures that had the best agreement with the diagnostic criteria questionnaire as identified by the highest AUC values. Those were the 60-symptom checklist (AUC, 0.889), painful joint count (AUC, 0.870), fatigue visual analog scale (VAS; AUC, 0.860), and pain VAS (AUC, 0.829), Dr. Schmukler said.
Proposed cut points that reflected the optimal trade-off between specificity and sensitivity for each of the scales were scores of 6 or greater for the pain VAS and fatigue VAS, and scores of 16 or greater for the symptom checklist and painful joint count measure.
In addition to the better performance of each FAST index versus the 2011 criteria, their performance was also better than that of the RAPID3 versus the 2011 criteria (AUC, 0.848), which many clinicians use in practice without using the full MDHAQ for patient assessment, he said.
Further, the “very easily calculated” FAST indices performed as well as a “very difficult to calculate” polysymptomatic distress continuous scale derived from the fibromyalgia questionnaire to assess the degree of fibromyalgia symptoms, which had an AUC of 0.929 versus the 2011 criteria. The latter index requires complex mathematical calculations for scale conversion and thus is impractical in clinical practice, he noted.
The FAST indices also performed comparably with physician diagnoses as indicated in patient charts and with diagnoses based on tender point count as shown in prior studies.
The FAST index with the greatest sensitivity (85.5% at a cut point of 2 or greater on a scale of 1-3) was the FAST3-P, which includes pain VAS score of 6 or greater, symptom checklist score of 16 or greater, and painful joint count of 16 or greater. The FAST index with the greatest specificity (90.3% at a cut point of score of 3 or greater on a scale of 1-4) was the FAST4 index, which includes a pain VAS score of 6 or greater, fatigue VAS score of 6 or greater, symptom checklist score of 16 or greater, and painful joint count of 16 or greater.
Although the findings are limited by the lack of a gold standard for fibromyalgia diagnosis, changing diagnostic criteria, and a need for physician input for a fibromyalgia diagnosis, the study provides useful real-world data and supports findings from some prior studies with respect to the benefit of using these tools in routine practice.
With minimal extra physician time – if the MDHAQ is completed by patients at the time of registration – this approach can be used in all rheumatology patients to help identify fibromyalgia, he concluded.
Dr. Schmukler reported having no disclosures. One of his coauthors at Rush, Ted Pincus, MD, receives royalties and license fees for the copyright and trademark for the MDHAQ and RAPID3, all of which go to support clinical research.
SOURCE: Schmukler J et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 839.
CHICAGO – Fibromyalgia assessment screening tool (FAST) indices derived from the Multidimensional Health Assessment Questionnaire (MDHAQ) provide a simple and effective method for identifying fibromyalgia in routine care, according to findings from a series of more than 500 patients.
The indices are as accurate as the existing – and more complex – diagnostic criteria for fibromyalgia, Juan Schmukler, MD, reported at the annual meeting of the American College of Rheumatology.
For example, three FAST indices developed in the course of the study had better performance versus existing diagnostic criteria than did certain individual MDHAQ scales alone (area under the curve range, 0.924-0.937 vs. 0.829-0.889, respectively), said Dr. Schmukler, who conducted the research as a medical student at Rush Medical College, Chicago, and is now a rheumatology fellow at Mount Sinai Hospital in Chicago.
The findings are notable, because the two-page MDHAQ and a summary index (the RAPID3) derived from three of the MDHAQ scales are already used routinely in clinical care for diagnosing rheumatic diseases, and the new indices could easily – and with minimal work flow disruption – also be incorporated for each patient at each visit.
“Fibromyalgia is common in the general population and it is believed to be more common in patients with other rheumatic diagnoses,” Dr. Schmukler said. “Fibromyalgia may be easily recognized in many cases, but it can also be very subtle, particularly in patients who have other rheumatic diseases.”
ACR fibromyalgia classification criteria published in 1990 were based on tender point examination and non-ACR diagnostic criteria as revised in 2011 are based entirely on patient self-report.
A one-page fibromyalgia criteria questionnaire is available and is useful in clinical trials and other research, but is “rarely, if ever” used in routine care, he said, explaining that the questionnaire contains two domains: a symptom severity score (0-12 scale) and a widespread pain index (0-19 scale).
The MDHAQ/RAPID3 is informative in RA, and has also been shown to be “useful in all rheumatic diseases in which it has been studied,” and at least three prior reports have suggested that the MDHAQ may also provide clues to the presence of fibromyalgia, Dr. Schmukler said.
“And we know from prior reports that patients with fibromyalgia reported the highest RAPID3 scores, compared to other rheumatic disease,” he added, explaining that the goal of the present study was to develop FAST cumulative indices based on the routine MDHAQ scales and using the 2011 diagnostic criteria as a reference standard.
All patients with all diagnoses seen at the Rush Medical College rheumatology clinic in Chicago complete the MDHAQ at all visits in routine care, and between April and July 2017, the fibromyalgia criteria questionnaire was also administered in 502 consecutive patients.
Of those patients, 131 met the 2011 fibromyalgia diagnostic criteria.
MDHAQ scores were analyzed for agreement with the fibromyalgia criteria questionnaire according to receiver operator characteristic curves for AUC and were compiled into three different FAST indices that included various combinations of either three or four of the MDHAQ measures that had the best agreement with the diagnostic criteria questionnaire as identified by the highest AUC values. Those were the 60-symptom checklist (AUC, 0.889), painful joint count (AUC, 0.870), fatigue visual analog scale (VAS; AUC, 0.860), and pain VAS (AUC, 0.829), Dr. Schmukler said.
Proposed cut points that reflected the optimal trade-off between specificity and sensitivity for each of the scales were scores of 6 or greater for the pain VAS and fatigue VAS, and scores of 16 or greater for the symptom checklist and painful joint count measure.
In addition to the better performance of each FAST index versus the 2011 criteria, their performance was also better than that of the RAPID3 versus the 2011 criteria (AUC, 0.848), which many clinicians use in practice without using the full MDHAQ for patient assessment, he said.
Further, the “very easily calculated” FAST indices performed as well as a “very difficult to calculate” polysymptomatic distress continuous scale derived from the fibromyalgia questionnaire to assess the degree of fibromyalgia symptoms, which had an AUC of 0.929 versus the 2011 criteria. The latter index requires complex mathematical calculations for scale conversion and thus is impractical in clinical practice, he noted.
The FAST indices also performed comparably with physician diagnoses as indicated in patient charts and with diagnoses based on tender point count as shown in prior studies.
The FAST index with the greatest sensitivity (85.5% at a cut point of 2 or greater on a scale of 1-3) was the FAST3-P, which includes pain VAS score of 6 or greater, symptom checklist score of 16 or greater, and painful joint count of 16 or greater. The FAST index with the greatest specificity (90.3% at a cut point of score of 3 or greater on a scale of 1-4) was the FAST4 index, which includes a pain VAS score of 6 or greater, fatigue VAS score of 6 or greater, symptom checklist score of 16 or greater, and painful joint count of 16 or greater.
Although the findings are limited by the lack of a gold standard for fibromyalgia diagnosis, changing diagnostic criteria, and a need for physician input for a fibromyalgia diagnosis, the study provides useful real-world data and supports findings from some prior studies with respect to the benefit of using these tools in routine practice.
With minimal extra physician time – if the MDHAQ is completed by patients at the time of registration – this approach can be used in all rheumatology patients to help identify fibromyalgia, he concluded.
Dr. Schmukler reported having no disclosures. One of his coauthors at Rush, Ted Pincus, MD, receives royalties and license fees for the copyright and trademark for the MDHAQ and RAPID3, all of which go to support clinical research.
SOURCE: Schmukler J et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 839.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Each fibromyalgia assessment screening tool index had better performance versus 2011 diagnostic criteria (area under the curve, 0.924-0.937) than did individual scales alone (AUCs, 0.829-0.889) and the RAPID3 summary index (AUC, 0.848).
Study details: An assessment of fibromyalgia assessment screening tool indices developed in a series of 502 patients.
Disclosures: Dr. Schmukler reported having no disclosures. One of his coauthors at Rush, Ted Pincus, MD, receives royalties and license fees for the copyright and trademark for the Multidimensional Health Assessment Questionnaire and RAPID3, all of which go to support clinical research.
Source: Schmukler J et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 839.