User login
Four-drug combo shows durable responses in relapsed/refractory lymphomas
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Seven patients responded, and three patients had responses lasting more than 24 months.
Study details: Phase 1 trial of 15 patients.
Disclosures: This research was supported by Celgene. The presenter reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
TAPs top epidurals in ventral hernia repair
, according to a review of 246 cases at the Greenville Health System, in South Carolina.
“Regional anesthesia using TAP block[s] provides an effective alternative to epidural analgesia or opioid use alone for perioperative pain control ... In light of these findings, use of TAP block should be strongly considered as an adjunct to abdominal surgery,” wrote investigators led by general surgeon Jeremy Warren, MD, of the University of South Carolina School of Medicine, Greenville, in the Journal of the American College of Surgeons.
Prompted by favorable reports in the literature, the team switched from epidural analgesia to TAP blocks in early 2017. To see how it’s worked out, they reviewed all patients who had ventral hernia repairs at the Greenville Health System from Feb. 2015 to March 2018. They were all mesh cases, without parastomal hernias or enterostomy reversal.
Seventy-four patients had TAP blocks, which were placed in the OR after anesthesia induction and consisted of 200 mg ropivacaine, 100 mcg epinephrine, and 100 mcg clonidine in 60 ml saline, with 30 ml injected on each side under ultrasound guidance.
Their outcomes were compared with 172 patients who received epidurals, which were placed preoperatively and consisted of 0.125% bupivacaine initiated shortly before patients came out of anesthesia, at a rate of 8-12 ml/hr.
Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1, and 36.1 versus 52.5 MME on postop day 2 (P = .018).
There were no differences in the rates of surgical site infections or other wound complications. The mean duration of epidural infusion was 49.5 hours.
The shorter length of stay with TAP block was probably related to side effects of epidurals, which can include leg paresthesias, hypotension, and urinary retention, all of which get in the way of early ambulation. “Additionally, the decision of when to discontinue epidural analgesia in our series was left to the judgment of the pain management and surgical team based on reporting of patient pain, rather than duration determined by a protocol,” which may have also played a role, the study team said.
Overall, the results mirror outcomes from previous TAP block studies, but there were caveats. Epidural patients had wider hernias (median 10.8 cm versus 8.8 cm); required more myofascial releases; and had longer operative times, “indicating a higher degree of complexity that may influence the need for longer hospitalization and greater opioid use,” the investigators said.
Also, a greater number of TAP block patients received non-opioid pain killers, including ketorolac and acetaminophen.
The study was conducted within the health system’s enhanced recovery after surgery protocol, which includes a preoperative cocktail of pregabalin 75 mg, celecoxib 400 mg, and acetaminophen 1,000 mg, given within 1-2 hours of surgery. Post-operative management includes intravenous ketamine infusions at sub-anesthetic doses, NSAIDs, and acetaminophen, among other measures. The approach has pretty much eliminated patient-controlled analgesia.
There were slightly more men than women in the review. Study participants, on average, were in their late 50s. There were no significant differences in comorbidities.
No funding was reported, and the investigators didn’t have any relevant disclosures.
SOURCE: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
, according to a review of 246 cases at the Greenville Health System, in South Carolina.
“Regional anesthesia using TAP block[s] provides an effective alternative to epidural analgesia or opioid use alone for perioperative pain control ... In light of these findings, use of TAP block should be strongly considered as an adjunct to abdominal surgery,” wrote investigators led by general surgeon Jeremy Warren, MD, of the University of South Carolina School of Medicine, Greenville, in the Journal of the American College of Surgeons.
Prompted by favorable reports in the literature, the team switched from epidural analgesia to TAP blocks in early 2017. To see how it’s worked out, they reviewed all patients who had ventral hernia repairs at the Greenville Health System from Feb. 2015 to March 2018. They were all mesh cases, without parastomal hernias or enterostomy reversal.
Seventy-four patients had TAP blocks, which were placed in the OR after anesthesia induction and consisted of 200 mg ropivacaine, 100 mcg epinephrine, and 100 mcg clonidine in 60 ml saline, with 30 ml injected on each side under ultrasound guidance.
Their outcomes were compared with 172 patients who received epidurals, which were placed preoperatively and consisted of 0.125% bupivacaine initiated shortly before patients came out of anesthesia, at a rate of 8-12 ml/hr.
Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1, and 36.1 versus 52.5 MME on postop day 2 (P = .018).
There were no differences in the rates of surgical site infections or other wound complications. The mean duration of epidural infusion was 49.5 hours.
The shorter length of stay with TAP block was probably related to side effects of epidurals, which can include leg paresthesias, hypotension, and urinary retention, all of which get in the way of early ambulation. “Additionally, the decision of when to discontinue epidural analgesia in our series was left to the judgment of the pain management and surgical team based on reporting of patient pain, rather than duration determined by a protocol,” which may have also played a role, the study team said.
Overall, the results mirror outcomes from previous TAP block studies, but there were caveats. Epidural patients had wider hernias (median 10.8 cm versus 8.8 cm); required more myofascial releases; and had longer operative times, “indicating a higher degree of complexity that may influence the need for longer hospitalization and greater opioid use,” the investigators said.
Also, a greater number of TAP block patients received non-opioid pain killers, including ketorolac and acetaminophen.
The study was conducted within the health system’s enhanced recovery after surgery protocol, which includes a preoperative cocktail of pregabalin 75 mg, celecoxib 400 mg, and acetaminophen 1,000 mg, given within 1-2 hours of surgery. Post-operative management includes intravenous ketamine infusions at sub-anesthetic doses, NSAIDs, and acetaminophen, among other measures. The approach has pretty much eliminated patient-controlled analgesia.
There were slightly more men than women in the review. Study participants, on average, were in their late 50s. There were no significant differences in comorbidities.
No funding was reported, and the investigators didn’t have any relevant disclosures.
SOURCE: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
, according to a review of 246 cases at the Greenville Health System, in South Carolina.
“Regional anesthesia using TAP block[s] provides an effective alternative to epidural analgesia or opioid use alone for perioperative pain control ... In light of these findings, use of TAP block should be strongly considered as an adjunct to abdominal surgery,” wrote investigators led by general surgeon Jeremy Warren, MD, of the University of South Carolina School of Medicine, Greenville, in the Journal of the American College of Surgeons.
Prompted by favorable reports in the literature, the team switched from epidural analgesia to TAP blocks in early 2017. To see how it’s worked out, they reviewed all patients who had ventral hernia repairs at the Greenville Health System from Feb. 2015 to March 2018. They were all mesh cases, without parastomal hernias or enterostomy reversal.
Seventy-four patients had TAP blocks, which were placed in the OR after anesthesia induction and consisted of 200 mg ropivacaine, 100 mcg epinephrine, and 100 mcg clonidine in 60 ml saline, with 30 ml injected on each side under ultrasound guidance.
Their outcomes were compared with 172 patients who received epidurals, which were placed preoperatively and consisted of 0.125% bupivacaine initiated shortly before patients came out of anesthesia, at a rate of 8-12 ml/hr.
Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1, and 36.1 versus 52.5 MME on postop day 2 (P = .018).
There were no differences in the rates of surgical site infections or other wound complications. The mean duration of epidural infusion was 49.5 hours.
The shorter length of stay with TAP block was probably related to side effects of epidurals, which can include leg paresthesias, hypotension, and urinary retention, all of which get in the way of early ambulation. “Additionally, the decision of when to discontinue epidural analgesia in our series was left to the judgment of the pain management and surgical team based on reporting of patient pain, rather than duration determined by a protocol,” which may have also played a role, the study team said.
Overall, the results mirror outcomes from previous TAP block studies, but there were caveats. Epidural patients had wider hernias (median 10.8 cm versus 8.8 cm); required more myofascial releases; and had longer operative times, “indicating a higher degree of complexity that may influence the need for longer hospitalization and greater opioid use,” the investigators said.
Also, a greater number of TAP block patients received non-opioid pain killers, including ketorolac and acetaminophen.
The study was conducted within the health system’s enhanced recovery after surgery protocol, which includes a preoperative cocktail of pregabalin 75 mg, celecoxib 400 mg, and acetaminophen 1,000 mg, given within 1-2 hours of surgery. Post-operative management includes intravenous ketamine infusions at sub-anesthetic doses, NSAIDs, and acetaminophen, among other measures. The approach has pretty much eliminated patient-controlled analgesia.
There were slightly more men than women in the review. Study participants, on average, were in their late 50s. There were no significant differences in comorbidities.
No funding was reported, and the investigators didn’t have any relevant disclosures.
SOURCE: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: TAP blocks are better than epidurals for pain control after ventral hernia repair.
Major finding: Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1.
Study details: Review of 246 repairs
Disclosures: No funding was reported, and the investigators didn’t have any relevant disclosures.
Source: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
Survey: Barriers discourage diverse students from dermatology career
A .
Yssra S. Soliman of the division of dermatology, in the department of medicine, Albert Einstein College of Medicine, New York, and colleagues surveyed 155 students from 28 different medical schools between January and April 2018 about barriers to applying for a dermatology residency. Of total participants, 43% expressed an interest in applying for a dermatology residency. Of the 155 survey respondents, 58% were nonwhite.
Students of ethnic minorities and students with lower incomes cited lack of diversity in dermatology as a top barrier to applying for a dermatology residency, according to a research letter published in JAMA Dermatology.Other primary barriers reported by students of color were negative perceptions of minority students by residencies, such as lower performance expectations; socioeconomic factors, such as lack of loan forgiveness; and a lack of mentors. (Minorities in the study were defined as nonwhite students and lower-income students were defined as those with annual household incomes below $40,000.)
Study authors wrote that the results highlight the need to recruit and mentor students of all backgrounds. Furthermore, efforts should be made to increase minority students’ exposure to dermatology through curriculum, providing research opportunities, and reducing the cost of visiting electives by providing stipends, they concluded.
The survey results are not surprising, said Susan C. Taylor, MD, cofounder of the Skin of Color Center at St. Luke’s-Roosevelt Hospital in New York and a Philadelphia-based dermatologist. “I think that [the survey] is accurate and reflects the most common barriers for minority students,” Dr. Taylor said in an interview.
While recent progress has been made in discussing the subject of diversity in dermatology and identifying ways to improve, there has been little change in the actual numbers of diverse dermatologists, she noted.
Dermatology remains the least diverse medical field, after orthopedics. Blacks for example, compose 13% of the U.S. population and Hispanics compose 16% of the population, but each group represents less than 5% of dermatologists, according to the JAMA Dermatology article. At the same time, data show that race-concordant visits are longer and have higher ratings of patient satisfaction. In addition, minority physicians are more likely to care for patients of their own race or ethnic group, practice in underserved areas, care for poor patients and those with Medicaid, and treat patients who report poor health status, studies have found.
Amit Pandya, MD, chair of the American Academy of Dermatology membership committee, said that, while the response rate for the JAMA Dermatology survey was small, the results add important new information about the views of medical students regarding a career in dermatology.
“More research in a larger population of students is needed to learn more about the barriers to matching in dermatology, particularly for students from an under-represented minority background,” Dr. Pandya, professor of dermatology at the University of Texas Southwestern Medical Center, Dallas, said in an interview.
He added that AAD is making marked efforts to improve diversity in dermatology through its diversity committee. AAD diversity initiatives include an intersociety Work Group on Diversity in Dermatology, a diversity mentorship program for medical students, sponsorship of Nth Dimension bioskill workshops, and the Diversity Champion program, which promotes mentorship, volunteerism, and educational activities for students underrepresented in medicine.
It’s too early to tell if the efforts are working, Dr. Pandya said. It will take a few years to see if the efforts result in more minority applicants being interviewed or accepted to a dermatology residency program.
The AAD will hold a Diversity Champion conference in September in Chicago, which is open to “diversity champions” from dermatology residency programs across the United States. The conference is sponsored by the AAD, Association of Professors of Dermatology, Women’s Dermatologic Society, Skin of Color Society, and Society of Investigative Dermatology.
“It will be an annual conference with the goal of improving diversity in our field,” said Dr. Pandya, conference cochair.
SOURCE: Soliman YS et al. JAMA Dermatol. 2019 Jan 9. doi: 10.1001/jamadermatol.2018.4813.
A .
Yssra S. Soliman of the division of dermatology, in the department of medicine, Albert Einstein College of Medicine, New York, and colleagues surveyed 155 students from 28 different medical schools between January and April 2018 about barriers to applying for a dermatology residency. Of total participants, 43% expressed an interest in applying for a dermatology residency. Of the 155 survey respondents, 58% were nonwhite.
Students of ethnic minorities and students with lower incomes cited lack of diversity in dermatology as a top barrier to applying for a dermatology residency, according to a research letter published in JAMA Dermatology.Other primary barriers reported by students of color were negative perceptions of minority students by residencies, such as lower performance expectations; socioeconomic factors, such as lack of loan forgiveness; and a lack of mentors. (Minorities in the study were defined as nonwhite students and lower-income students were defined as those with annual household incomes below $40,000.)
Study authors wrote that the results highlight the need to recruit and mentor students of all backgrounds. Furthermore, efforts should be made to increase minority students’ exposure to dermatology through curriculum, providing research opportunities, and reducing the cost of visiting electives by providing stipends, they concluded.
The survey results are not surprising, said Susan C. Taylor, MD, cofounder of the Skin of Color Center at St. Luke’s-Roosevelt Hospital in New York and a Philadelphia-based dermatologist. “I think that [the survey] is accurate and reflects the most common barriers for minority students,” Dr. Taylor said in an interview.
While recent progress has been made in discussing the subject of diversity in dermatology and identifying ways to improve, there has been little change in the actual numbers of diverse dermatologists, she noted.
Dermatology remains the least diverse medical field, after orthopedics. Blacks for example, compose 13% of the U.S. population and Hispanics compose 16% of the population, but each group represents less than 5% of dermatologists, according to the JAMA Dermatology article. At the same time, data show that race-concordant visits are longer and have higher ratings of patient satisfaction. In addition, minority physicians are more likely to care for patients of their own race or ethnic group, practice in underserved areas, care for poor patients and those with Medicaid, and treat patients who report poor health status, studies have found.
Amit Pandya, MD, chair of the American Academy of Dermatology membership committee, said that, while the response rate for the JAMA Dermatology survey was small, the results add important new information about the views of medical students regarding a career in dermatology.
“More research in a larger population of students is needed to learn more about the barriers to matching in dermatology, particularly for students from an under-represented minority background,” Dr. Pandya, professor of dermatology at the University of Texas Southwestern Medical Center, Dallas, said in an interview.
He added that AAD is making marked efforts to improve diversity in dermatology through its diversity committee. AAD diversity initiatives include an intersociety Work Group on Diversity in Dermatology, a diversity mentorship program for medical students, sponsorship of Nth Dimension bioskill workshops, and the Diversity Champion program, which promotes mentorship, volunteerism, and educational activities for students underrepresented in medicine.
It’s too early to tell if the efforts are working, Dr. Pandya said. It will take a few years to see if the efforts result in more minority applicants being interviewed or accepted to a dermatology residency program.
The AAD will hold a Diversity Champion conference in September in Chicago, which is open to “diversity champions” from dermatology residency programs across the United States. The conference is sponsored by the AAD, Association of Professors of Dermatology, Women’s Dermatologic Society, Skin of Color Society, and Society of Investigative Dermatology.
“It will be an annual conference with the goal of improving diversity in our field,” said Dr. Pandya, conference cochair.
SOURCE: Soliman YS et al. JAMA Dermatol. 2019 Jan 9. doi: 10.1001/jamadermatol.2018.4813.
A .
Yssra S. Soliman of the division of dermatology, in the department of medicine, Albert Einstein College of Medicine, New York, and colleagues surveyed 155 students from 28 different medical schools between January and April 2018 about barriers to applying for a dermatology residency. Of total participants, 43% expressed an interest in applying for a dermatology residency. Of the 155 survey respondents, 58% were nonwhite.
Students of ethnic minorities and students with lower incomes cited lack of diversity in dermatology as a top barrier to applying for a dermatology residency, according to a research letter published in JAMA Dermatology.Other primary barriers reported by students of color were negative perceptions of minority students by residencies, such as lower performance expectations; socioeconomic factors, such as lack of loan forgiveness; and a lack of mentors. (Minorities in the study were defined as nonwhite students and lower-income students were defined as those with annual household incomes below $40,000.)
Study authors wrote that the results highlight the need to recruit and mentor students of all backgrounds. Furthermore, efforts should be made to increase minority students’ exposure to dermatology through curriculum, providing research opportunities, and reducing the cost of visiting electives by providing stipends, they concluded.
The survey results are not surprising, said Susan C. Taylor, MD, cofounder of the Skin of Color Center at St. Luke’s-Roosevelt Hospital in New York and a Philadelphia-based dermatologist. “I think that [the survey] is accurate and reflects the most common barriers for minority students,” Dr. Taylor said in an interview.
While recent progress has been made in discussing the subject of diversity in dermatology and identifying ways to improve, there has been little change in the actual numbers of diverse dermatologists, she noted.
Dermatology remains the least diverse medical field, after orthopedics. Blacks for example, compose 13% of the U.S. population and Hispanics compose 16% of the population, but each group represents less than 5% of dermatologists, according to the JAMA Dermatology article. At the same time, data show that race-concordant visits are longer and have higher ratings of patient satisfaction. In addition, minority physicians are more likely to care for patients of their own race or ethnic group, practice in underserved areas, care for poor patients and those with Medicaid, and treat patients who report poor health status, studies have found.
Amit Pandya, MD, chair of the American Academy of Dermatology membership committee, said that, while the response rate for the JAMA Dermatology survey was small, the results add important new information about the views of medical students regarding a career in dermatology.
“More research in a larger population of students is needed to learn more about the barriers to matching in dermatology, particularly for students from an under-represented minority background,” Dr. Pandya, professor of dermatology at the University of Texas Southwestern Medical Center, Dallas, said in an interview.
He added that AAD is making marked efforts to improve diversity in dermatology through its diversity committee. AAD diversity initiatives include an intersociety Work Group on Diversity in Dermatology, a diversity mentorship program for medical students, sponsorship of Nth Dimension bioskill workshops, and the Diversity Champion program, which promotes mentorship, volunteerism, and educational activities for students underrepresented in medicine.
It’s too early to tell if the efforts are working, Dr. Pandya said. It will take a few years to see if the efforts result in more minority applicants being interviewed or accepted to a dermatology residency program.
The AAD will hold a Diversity Champion conference in September in Chicago, which is open to “diversity champions” from dermatology residency programs across the United States. The conference is sponsored by the AAD, Association of Professors of Dermatology, Women’s Dermatologic Society, Skin of Color Society, and Society of Investigative Dermatology.
“It will be an annual conference with the goal of improving diversity in our field,” said Dr. Pandya, conference cochair.
SOURCE: Soliman YS et al. JAMA Dermatol. 2019 Jan 9. doi: 10.1001/jamadermatol.2018.4813.
FDA: Benefits still outweigh risks from paclitaxel-coated devices for PAD
The Food and Drug Administration has issued a letter alerting health care providers that it is aware of and examining recent data on an increase in long-term mortality rates for patients receiving paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
“Currently, the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use,” William Maisel, MD, MPH, chief medical officer of the Center for Devices and Radiological Health at the FDA, wrote in a letter to health care providers.
The FDA letter was in response to a recent systematic review of paclitaxel-coated balloons and stents recently published in the Journal of the American Heart Association. Konstantinos Katsanos, MD, PhD, from Patras University Hospital in Rion, Greece, and colleagues performed the systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients who received paclitaxel-coated devices in the femoral and/or popliteal arteries and found similar 1-year risk of all-cause patient mortality (2.3%; risk ratio, 1.08; 95% confidence interval, 0.72-1.61). However, there was an increased risk of all-cause mortality for patients with paclitaxel-coated devices at 2 years (7.2% vs. 3.8%; RR, 1.68; 95% CI, 1.15-2.47) and at 5 years (14.7% vs. 8.1%; RR, 1.93; 95% CI, 1.27-2.93), compared with control groups. The number needed to harm at 2 years was 29 patients (95% CI, 19-59) and 14 patients (95% CI, 9-32) at 5 years. Their meta regression analysis found a significant link between paclitaxel exposure and absolute risk of death.
“Actual causes for this serious late side effect remain unknown, and further investigations with longer-term follow-up are urgently warranted,” Dr. Katsanos and colleagues wrote in their review.
The FDA told health care providers of patients with paclitaxel-coated balloons and paclitaxel-eluting stents to continue surveillance of these patients per standard of care, to discuss the risks and benefits of PAD treatment options with patients, and to report any adverse or suspected adverse events to MedWatch.
The FDA said they are currently evaluating long-term data on paclitaxel-coated products to determine whether the devices carry an increased risk of death or other long-term risks, and noted there were several paclitaxel-coated balloons or paclitaxel-eluting stents that have either been approved or are under study in the United States.
SOURCE: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
The Food and Drug Administration has issued a letter alerting health care providers that it is aware of and examining recent data on an increase in long-term mortality rates for patients receiving paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
“Currently, the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use,” William Maisel, MD, MPH, chief medical officer of the Center for Devices and Radiological Health at the FDA, wrote in a letter to health care providers.
The FDA letter was in response to a recent systematic review of paclitaxel-coated balloons and stents recently published in the Journal of the American Heart Association. Konstantinos Katsanos, MD, PhD, from Patras University Hospital in Rion, Greece, and colleagues performed the systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients who received paclitaxel-coated devices in the femoral and/or popliteal arteries and found similar 1-year risk of all-cause patient mortality (2.3%; risk ratio, 1.08; 95% confidence interval, 0.72-1.61). However, there was an increased risk of all-cause mortality for patients with paclitaxel-coated devices at 2 years (7.2% vs. 3.8%; RR, 1.68; 95% CI, 1.15-2.47) and at 5 years (14.7% vs. 8.1%; RR, 1.93; 95% CI, 1.27-2.93), compared with control groups. The number needed to harm at 2 years was 29 patients (95% CI, 19-59) and 14 patients (95% CI, 9-32) at 5 years. Their meta regression analysis found a significant link between paclitaxel exposure and absolute risk of death.
“Actual causes for this serious late side effect remain unknown, and further investigations with longer-term follow-up are urgently warranted,” Dr. Katsanos and colleagues wrote in their review.
The FDA told health care providers of patients with paclitaxel-coated balloons and paclitaxel-eluting stents to continue surveillance of these patients per standard of care, to discuss the risks and benefits of PAD treatment options with patients, and to report any adverse or suspected adverse events to MedWatch.
The FDA said they are currently evaluating long-term data on paclitaxel-coated products to determine whether the devices carry an increased risk of death or other long-term risks, and noted there were several paclitaxel-coated balloons or paclitaxel-eluting stents that have either been approved or are under study in the United States.
SOURCE: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
The Food and Drug Administration has issued a letter alerting health care providers that it is aware of and examining recent data on an increase in long-term mortality rates for patients receiving paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
“Currently, the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use,” William Maisel, MD, MPH, chief medical officer of the Center for Devices and Radiological Health at the FDA, wrote in a letter to health care providers.
The FDA letter was in response to a recent systematic review of paclitaxel-coated balloons and stents recently published in the Journal of the American Heart Association. Konstantinos Katsanos, MD, PhD, from Patras University Hospital in Rion, Greece, and colleagues performed the systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients who received paclitaxel-coated devices in the femoral and/or popliteal arteries and found similar 1-year risk of all-cause patient mortality (2.3%; risk ratio, 1.08; 95% confidence interval, 0.72-1.61). However, there was an increased risk of all-cause mortality for patients with paclitaxel-coated devices at 2 years (7.2% vs. 3.8%; RR, 1.68; 95% CI, 1.15-2.47) and at 5 years (14.7% vs. 8.1%; RR, 1.93; 95% CI, 1.27-2.93), compared with control groups. The number needed to harm at 2 years was 29 patients (95% CI, 19-59) and 14 patients (95% CI, 9-32) at 5 years. Their meta regression analysis found a significant link between paclitaxel exposure and absolute risk of death.
“Actual causes for this serious late side effect remain unknown, and further investigations with longer-term follow-up are urgently warranted,” Dr. Katsanos and colleagues wrote in their review.
The FDA told health care providers of patients with paclitaxel-coated balloons and paclitaxel-eluting stents to continue surveillance of these patients per standard of care, to discuss the risks and benefits of PAD treatment options with patients, and to report any adverse or suspected adverse events to MedWatch.
The FDA said they are currently evaluating long-term data on paclitaxel-coated products to determine whether the devices carry an increased risk of death or other long-term risks, and noted there were several paclitaxel-coated balloons or paclitaxel-eluting stents that have either been approved or are under study in the United States.
SOURCE: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
Key clinical point: In a letter to health care providers, FDA said it was investigating data from a recent meta-analysis of increased long-term mortality risk from paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
Major finding: All-cause mortality increased significantly after 2 years (7.2% vs. 3.8%) and 5 years (14.7% vs. 8.1%) compared with control groups.
Study details: A systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients.
Source: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
FDA approves third trastuzumab biosimilar
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
Choose your steps for treating chronic spontaneous urticaria
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Flu activity down for second consecutive week
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
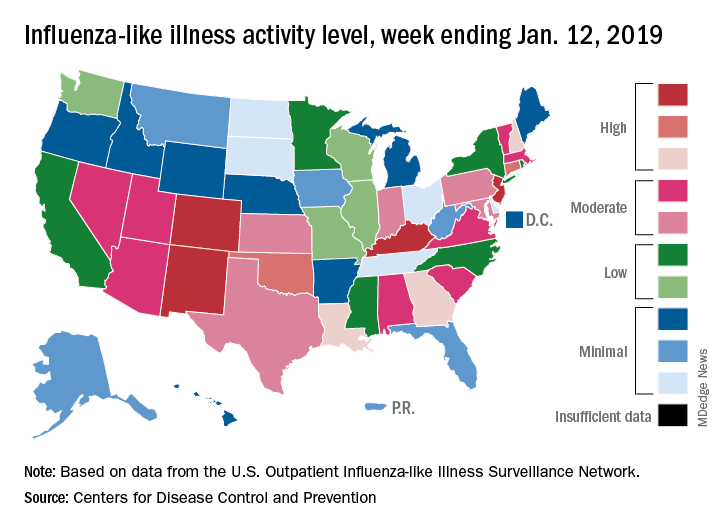
The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
Bidirectional relationship found between depression, vitiligo
Vitiligo and major depressive disorder have a bidirectional relationship, according to a new study that examined data from a cohort of more than 6 million people.
“Ultimately, this suggests that mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo, which in turn can increase the risk of MDD, especially in younger patients,” wrote Isabelle Vallerand, PhD, and her colleagues. The report is in the Journal of the American Academy of Dermatology.
Dr. Vallerand and her colleagues found that patients with major depressive disorder (MDD, n = 405,397) had a 64% increased risk of vitiligo, compared with a referent cohort (n = 5,739,048; 95% confidence interval, 1.43-1.87; P less than .0001). Conversely, patients who had vitiligo also were at an increased risk of MDD. Patients who were younger than 30 years old at diagnosis (n = 7,104) had a hazard ratio of 1.31 for MDD (P less than .0001), compared with 1.22 for patients aged 30 years and older (P = .001).
Individuals who took antidepressants, whether or not they also had an MDD diagnosis, had a decreased risk for vitiligo.
Though it’s known that vitiligo increases the risk of MDD, less clarity has been in the literature about whether the converse also might be true. “The question of whether vitiligo onset can be precipitated by MDD has received less attention, despite the notion that patients often ask their dermatologists if stress or depression may have contributed to their disease,” wrote Dr. Vallerand, an epidemiologist and medical student at the University of Calgary, Alberta, and her colleagues.
There is a biologic plausibility for a bidirectional relationship, said Dr. Vallerand and her colleagues, since depression can boost systemic inflammation, and the risk for autoimmune disease such as vitiligo can be increased by proinflammatory states.
Access to a large dataset gave Dr. Vallerand and her collaborators the numbers to look at the relationship between vitiligo and MDD in the context of potential confounders, and to correct for those in their statistical analysis. Using medical records from The Health Improvement Network (THIN) database in the United Kingdom, the investigators conducted two independent population-based cohort studies. Each looked at risk in one direction of the MDD-vitiligo association.
The first analysis looked at MDD as a risk factor for vitiligo, following all patients with an incident diagnostic code for MDD. Patients without the MDD diagnosis code were the referent cohort. Patients in each cohort were followed until they reached the outcome of interest – a diagnosis of vitiligo – or were censored. Patients who had a vitiligo diagnosis before receiving an MDD diagnosis were not included.
The second analysis examined whether vitiligo was a risk factor for MDD, with a similar design that used nonvitiligo patients as the referent cohort. This analysis followed all patients until a diagnosis of MDD was recorded, or patients were censored. Again, patients with MDD diagnoses that came before the vitiligo diagnosis were excluded.
For the analysis of risk of vitiligo, the investigators looked at the effects of multiple covariates, including age, sex, alcohol use and smoking status, socioeconomic status, medical comorbidities, and whether patients were taking antidepressants. The covariates included in the analysis of risk of MDD were age, sex, medical comorbidities, and type of vitiligo treatment.
After the researchers determined unadjusted hazard ratios, each covariate was removed one at a time to see where there were substantial changes to the HR. Two additional models, one unadjusted and one that fully adjusted for all covariates, also were built.
The sensitivity analyses showed “an overall protective effect of antidepressants among both cohorts,” wrote Dr. Vallerand and her colleagues. The incidence rate of vitiligo among patients with MDD using antidepressants was 19.7 per 100,000 person-years, compared with 27.5 among MDD patients not using antidepressants (P = .0053).
“Similarly, those in the referent cohort who used antidepressants had about half the risk of vitiligo,” compared with the nonusers in the referent group, the investigators said. Serotonin also is present in the skin, and neurons and melanocytes share embryonic ectodermal origins, Dr. Vallerand and her colleagues said. Though the exact mechanisms are not known, in the THIN cohorts, they noted.
Though younger patients with vitiligo were at higher risk for MDD than were those aged 30 years and older, the overall cohort of individuals with vitiligo still had an unadjusted elevated risk for MDD, compared with the referent cohort (HR 1.27; 95% confidence interval, 1.16-1.40; P less than .0001).
“Unexpectedly, the magnitude of the reciprocal association was highest with MDD being a risk factor for vitiligo,” wrote Dr. Vallerand and her colleagues. “This highlights the notion that mental health may have a greater impact on the body, specifically with dermatologic manifestations, than previously thought.”
Some misclassification of both conditions is likely in such a large dataset, the investigators acknowledged. Also, subclinical depression was not evaluated, and there was no way to track the severity of either depression or vitiligo, they noted. Still, the big data approach “renders this one of the largest studies on psychodermatology to date,” said Dr. Vallerand and her colleagues, and the independent bidirectional analyses support causality.
Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRX, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
SOURCE: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
Vitiligo and major depressive disorder have a bidirectional relationship, according to a new study that examined data from a cohort of more than 6 million people.
“Ultimately, this suggests that mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo, which in turn can increase the risk of MDD, especially in younger patients,” wrote Isabelle Vallerand, PhD, and her colleagues. The report is in the Journal of the American Academy of Dermatology.
Dr. Vallerand and her colleagues found that patients with major depressive disorder (MDD, n = 405,397) had a 64% increased risk of vitiligo, compared with a referent cohort (n = 5,739,048; 95% confidence interval, 1.43-1.87; P less than .0001). Conversely, patients who had vitiligo also were at an increased risk of MDD. Patients who were younger than 30 years old at diagnosis (n = 7,104) had a hazard ratio of 1.31 for MDD (P less than .0001), compared with 1.22 for patients aged 30 years and older (P = .001).
Individuals who took antidepressants, whether or not they also had an MDD diagnosis, had a decreased risk for vitiligo.
Though it’s known that vitiligo increases the risk of MDD, less clarity has been in the literature about whether the converse also might be true. “The question of whether vitiligo onset can be precipitated by MDD has received less attention, despite the notion that patients often ask their dermatologists if stress or depression may have contributed to their disease,” wrote Dr. Vallerand, an epidemiologist and medical student at the University of Calgary, Alberta, and her colleagues.
There is a biologic plausibility for a bidirectional relationship, said Dr. Vallerand and her colleagues, since depression can boost systemic inflammation, and the risk for autoimmune disease such as vitiligo can be increased by proinflammatory states.
Access to a large dataset gave Dr. Vallerand and her collaborators the numbers to look at the relationship between vitiligo and MDD in the context of potential confounders, and to correct for those in their statistical analysis. Using medical records from The Health Improvement Network (THIN) database in the United Kingdom, the investigators conducted two independent population-based cohort studies. Each looked at risk in one direction of the MDD-vitiligo association.
The first analysis looked at MDD as a risk factor for vitiligo, following all patients with an incident diagnostic code for MDD. Patients without the MDD diagnosis code were the referent cohort. Patients in each cohort were followed until they reached the outcome of interest – a diagnosis of vitiligo – or were censored. Patients who had a vitiligo diagnosis before receiving an MDD diagnosis were not included.
The second analysis examined whether vitiligo was a risk factor for MDD, with a similar design that used nonvitiligo patients as the referent cohort. This analysis followed all patients until a diagnosis of MDD was recorded, or patients were censored. Again, patients with MDD diagnoses that came before the vitiligo diagnosis were excluded.
For the analysis of risk of vitiligo, the investigators looked at the effects of multiple covariates, including age, sex, alcohol use and smoking status, socioeconomic status, medical comorbidities, and whether patients were taking antidepressants. The covariates included in the analysis of risk of MDD were age, sex, medical comorbidities, and type of vitiligo treatment.
After the researchers determined unadjusted hazard ratios, each covariate was removed one at a time to see where there were substantial changes to the HR. Two additional models, one unadjusted and one that fully adjusted for all covariates, also were built.
The sensitivity analyses showed “an overall protective effect of antidepressants among both cohorts,” wrote Dr. Vallerand and her colleagues. The incidence rate of vitiligo among patients with MDD using antidepressants was 19.7 per 100,000 person-years, compared with 27.5 among MDD patients not using antidepressants (P = .0053).
“Similarly, those in the referent cohort who used antidepressants had about half the risk of vitiligo,” compared with the nonusers in the referent group, the investigators said. Serotonin also is present in the skin, and neurons and melanocytes share embryonic ectodermal origins, Dr. Vallerand and her colleagues said. Though the exact mechanisms are not known, in the THIN cohorts, they noted.
Though younger patients with vitiligo were at higher risk for MDD than were those aged 30 years and older, the overall cohort of individuals with vitiligo still had an unadjusted elevated risk for MDD, compared with the referent cohort (HR 1.27; 95% confidence interval, 1.16-1.40; P less than .0001).
“Unexpectedly, the magnitude of the reciprocal association was highest with MDD being a risk factor for vitiligo,” wrote Dr. Vallerand and her colleagues. “This highlights the notion that mental health may have a greater impact on the body, specifically with dermatologic manifestations, than previously thought.”
Some misclassification of both conditions is likely in such a large dataset, the investigators acknowledged. Also, subclinical depression was not evaluated, and there was no way to track the severity of either depression or vitiligo, they noted. Still, the big data approach “renders this one of the largest studies on psychodermatology to date,” said Dr. Vallerand and her colleagues, and the independent bidirectional analyses support causality.
Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRX, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
SOURCE: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
Vitiligo and major depressive disorder have a bidirectional relationship, according to a new study that examined data from a cohort of more than 6 million people.
“Ultimately, this suggests that mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo, which in turn can increase the risk of MDD, especially in younger patients,” wrote Isabelle Vallerand, PhD, and her colleagues. The report is in the Journal of the American Academy of Dermatology.
Dr. Vallerand and her colleagues found that patients with major depressive disorder (MDD, n = 405,397) had a 64% increased risk of vitiligo, compared with a referent cohort (n = 5,739,048; 95% confidence interval, 1.43-1.87; P less than .0001). Conversely, patients who had vitiligo also were at an increased risk of MDD. Patients who were younger than 30 years old at diagnosis (n = 7,104) had a hazard ratio of 1.31 for MDD (P less than .0001), compared with 1.22 for patients aged 30 years and older (P = .001).
Individuals who took antidepressants, whether or not they also had an MDD diagnosis, had a decreased risk for vitiligo.
Though it’s known that vitiligo increases the risk of MDD, less clarity has been in the literature about whether the converse also might be true. “The question of whether vitiligo onset can be precipitated by MDD has received less attention, despite the notion that patients often ask their dermatologists if stress or depression may have contributed to their disease,” wrote Dr. Vallerand, an epidemiologist and medical student at the University of Calgary, Alberta, and her colleagues.
There is a biologic plausibility for a bidirectional relationship, said Dr. Vallerand and her colleagues, since depression can boost systemic inflammation, and the risk for autoimmune disease such as vitiligo can be increased by proinflammatory states.
Access to a large dataset gave Dr. Vallerand and her collaborators the numbers to look at the relationship between vitiligo and MDD in the context of potential confounders, and to correct for those in their statistical analysis. Using medical records from The Health Improvement Network (THIN) database in the United Kingdom, the investigators conducted two independent population-based cohort studies. Each looked at risk in one direction of the MDD-vitiligo association.
The first analysis looked at MDD as a risk factor for vitiligo, following all patients with an incident diagnostic code for MDD. Patients without the MDD diagnosis code were the referent cohort. Patients in each cohort were followed until they reached the outcome of interest – a diagnosis of vitiligo – or were censored. Patients who had a vitiligo diagnosis before receiving an MDD diagnosis were not included.
The second analysis examined whether vitiligo was a risk factor for MDD, with a similar design that used nonvitiligo patients as the referent cohort. This analysis followed all patients until a diagnosis of MDD was recorded, or patients were censored. Again, patients with MDD diagnoses that came before the vitiligo diagnosis were excluded.
For the analysis of risk of vitiligo, the investigators looked at the effects of multiple covariates, including age, sex, alcohol use and smoking status, socioeconomic status, medical comorbidities, and whether patients were taking antidepressants. The covariates included in the analysis of risk of MDD were age, sex, medical comorbidities, and type of vitiligo treatment.
After the researchers determined unadjusted hazard ratios, each covariate was removed one at a time to see where there were substantial changes to the HR. Two additional models, one unadjusted and one that fully adjusted for all covariates, also were built.
The sensitivity analyses showed “an overall protective effect of antidepressants among both cohorts,” wrote Dr. Vallerand and her colleagues. The incidence rate of vitiligo among patients with MDD using antidepressants was 19.7 per 100,000 person-years, compared with 27.5 among MDD patients not using antidepressants (P = .0053).
“Similarly, those in the referent cohort who used antidepressants had about half the risk of vitiligo,” compared with the nonusers in the referent group, the investigators said. Serotonin also is present in the skin, and neurons and melanocytes share embryonic ectodermal origins, Dr. Vallerand and her colleagues said. Though the exact mechanisms are not known, in the THIN cohorts, they noted.
Though younger patients with vitiligo were at higher risk for MDD than were those aged 30 years and older, the overall cohort of individuals with vitiligo still had an unadjusted elevated risk for MDD, compared with the referent cohort (HR 1.27; 95% confidence interval, 1.16-1.40; P less than .0001).
“Unexpectedly, the magnitude of the reciprocal association was highest with MDD being a risk factor for vitiligo,” wrote Dr. Vallerand and her colleagues. “This highlights the notion that mental health may have a greater impact on the body, specifically with dermatologic manifestations, than previously thought.”
Some misclassification of both conditions is likely in such a large dataset, the investigators acknowledged. Also, subclinical depression was not evaluated, and there was no way to track the severity of either depression or vitiligo, they noted. Still, the big data approach “renders this one of the largest studies on psychodermatology to date,” said Dr. Vallerand and her colleagues, and the independent bidirectional analyses support causality.
Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRX, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
SOURCE: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: The findings suggest that “mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo.”
Major finding: Patients with major depressive disorder had a 64% increased risk of vitiligo.
Study details: Retrospective records review of 405,397 patients with MDD and 5,738,048 patients in a referent cohort.
Disclosures: Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRx, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
Source: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
Long-term mogamulizumab appears safe, effective in CTCL
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Prolonged exposure to mogamulizumab can improve responses without compromising safety in patients with cutaneous T-cell lymphoma (CTCL), according to a post hoc analysis of the MAVORIC trial.
Investigators found that exposure to mogamulizumab correlated with response. The highest response rate — 75.6% — was observed in patients exposed to the drug for at least 351 days, and the lowest — 1.9% — was observed in patients exposed to mogamulizumab for less than 72 days.
On the other hand, rates of adverse events (AEs) were similar regardless of how long patients were treated with mogamulizumab.
Youn H. Kim, MD, of Stanford Cancer Institute at Stanford (Calif.) University, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The phase 3 MAVORIC trial (NCT01728805) included 372 adults with CTCL who had failed at least one systemic therapy. The patients were randomized to treatment with mogamulizumab or vorinostat.
Results from this comparison were previously reported at the 10th annual T-cell Lymphoma Forum.
At this year’s meeting, Dr. Kim and her colleagues reported results in 184 patients who were randomized to mogamulizumab — 105 of whom had mycosis fungoides (MF) and 79 of whom had Sézary syndrome (SS).
Patients were exposed to mogamulizumab for a mean of 275.2 days and a median of 170.0 days (range, 1-1,617 days).
The investigators divided patients into the following quartiles according to mogamulizumab exposure:
- Less than 72 days — 52 patients (28%)
- 72-170 days — 40 patients (22%)
- 171-351 days — 47 patients (26%)
- More than 351 days — 45 patients (24%).
Patients exposed to mogamulizumab for longer were more likely to have SS, stage III/IV disease, blood involvement, and a performance status of 0.
Dr. Kim said the SS patients “benefited a lot” from mogamulizumab and therefore remained on treatment longer.
Response
As expected, patients exposed to mogamulizumab for the longest period had the highest global response rates. Confirmed response rates according to drug exposure were as follows:
- Less than 72 days: 1.9% overall, 0% for SS, and 2.9% for MF
- 72-170 days: 10% overall, 18.8% for SS, and 4.2% for MF
- 171-351 days: 29.8% overall, 36.4% for SS, and 24% for MF
- More than 351 days: 75.6% overall, 83.3% for SS, and 66.7% for MF.
In addition, rates of complete response (CR) and partial response (PR) tended to increase with mogamulizumab exposure. Rates of CR, PR, and stable disease (SD) according to exposure time were as follows:
- Less than 72 days: 0% CR, 7.7% PR, and 38.5% SD
- 72-170 days: 2.5% CR, 20% PR, and 62.5% SD
- 171-351 days: 2.1% CR, 34% PR, and 57.4% SD
- More than 351 days: 6.7% CR, 71.1% PR, and 17.8% SD.
Safety
“The percentage of patients reporting adverse events was not different in the long-term treatment-exposure patients, compared to the short-term,” Dr. Kim said.
Percentages of treatment-emergent AEs (TEAEs) and serious AEs (SAEs) according to mogamulizumab exposure were as follows:
- Less than 72 days: 26.6% TEAEs and 6.5% SAEs
- 72-170 days: 18.5% TEAEs and 3.3% SAEs
- 171-351 days: 23.4% TEAEs and 6.0% SAEs
- More than 351 days: 21.7% TEAEs and 4.3% SAEs.
“The majority of the grade 3 events occurred in the first two quartiles, not later, which is important to show,” Dr. Kim said.
Most grade 3 AEs occurred within 170 days of treatment initiation, and the median time to a grade 3 or higher AE was 109 days.
The most common treatment-related AEs in the longest exposure cohort were drug eruption (20.0%), thrombocytopenia (11.1%), stomatitis (8.9%), and anemia (8.9%).
Of all patients in this analysis, 45 experienced drug eruption, which was defined as a skin rash possibly, probably, or definitely related to the study drug.
Nine drug eruption events were grade 3, and the rest were grade 1 or 2. The median time to drug eruption was 107 days.
While drug eruption “didn’t show up early,” there is no cumulative risk with longer exposure to mogamulizumab, Dr. Kim said. Likewise, she said, autoimmune AEs were not dose-cumulative events.
There were two patients with definite autoimmune disease — a 65-year-old man with Miller Fisher syndrome (occurring 199 days after mogamulizumab initiation) and a 40-year-old woman with myositis (151 days) and myocarditis (310 days).
The investigators also identified three patients with possible autoimmune disease, including:
- Pneumonitis (310 days) in a 74-year-old woman
- Polymyalgia rheumatica (209 days) and myopathy (not available) in an 84-year-old man
- Hepatitis (144 days), pneumonitis (about 174 days), and polymyositis (about 174 days) in a 73-year-old man.
Dr. Kim and her colleagues said these data suggest prolonged treatment with mogamulizumab is not associated with an increased safety risk in patients with MF or SS. And the manageable safety profile of mogamulizumab meant that patients who derived a clinical benefit could remain on the drug for an extended period of time.
The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Dr. Kim reported relationships with Merck, Portola Pharmaceuticals, Soligenix, Takeda, TetraLogic Pharmaceuticals, Kyowa Kirin, Seattle Genetics, Medivir, Neumedicines, Eisai, Innate Pharma, Galderma, Miragen Therapeutics, Forty Seven, and Horizon Pharma. Her coinvestigators reported relationships with several companies.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The highest response rate – 75.6% – was observed in patients exposed to mogamulizumab for at least 351 days.
Study details: A post hoc analysis of the MAVORIC trial, including 184 patients treated with mogamulizumab.
Disclosures: The MAVORIC trial was sponsored by Kyowa Hakko Kirin Pharma. Investigators disclosed relationships with several companies.
AGA News
AGA workshops/webcasts to give free advice on advancing your GI career
The free half-day workshops and webcasts in Columbus, Ohio, on Feb. 16, 2019, and in Boston on March 30, 2019, emphasize mastering basic business skills that can help advance your GI career.
Fellows and early-career GIs will have an opportunity to connect with seasoned GIs to gain real-world insights on successfully managing their careers at one of two upcoming American Gastroenterological Association’s Regional Practice Skills Workshops. Seasoned faculty will share their experiences and recommendations on:
- Measuring quality and delivering value-based care.
- Health care reform and the future of GI.
- Planning and managing finances, and much more.
Register and plan to join one of the upcoming workshops or webcasts:
If you’re in the Columbus or Boston area, attending the workshop in person is a great opportunity to ask questions of presenters and to network with faculty and peers. If you are not able to attend in person, you may still benefit from the valuable information by registering for the live webcast.
Open to AGA members and nonmembers, the workshops have been a hit with recent attendees who have called them an “eye opener,” “amazing and very informative,” and “phenomenal.” Take advantage of this free learning opportunity and register for one or both events/webcasts today.
Registration for all workshops and webcasts is required.
Rising microbiome investigator: Lea Ann Chen, MD
Dr. Chen, of New York University, talks about her research on gastrointestinal illnesses and what motivated her to focus on the gut microbiome.
We spoke with Dr. Chen, the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S et al. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives, including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
My experiences during AGA’s Advocacy Day: Facilitating change
BY YAMINI NATARAJAN, MD
The hospital is often the intersection between patients’ medical illness and their social and financial issues.
As physicians, it is important to recognize that patient care encompasses the prescribing of medications, the performing of procedures, as well as systems-based practice, and ensuring that social and financial barriers do not impede access to, and delivery of, care. Some of these barriers cannot be eliminated by any one individual health care professional (HCP); they can only be improved by working with government representatives and policymakers to make systemic changes. For gastroenterologists, advocacy involves educating patients, HCPs, and our government representatives about issues related to GI illnesses and the importance of ensuring access to GI specialty care and treatment for all the patients who require it.
AGA, via the Government Affairs Committee, facilitates advocacy in several ways. These include policy briefs, position statements, and facilitating meetings with our representatives and senators in home districts and in Washington. AGA hosted Advocacy Day in Washington on Sept. 14, 2018. Seventeen AGA members from 11 states visited 26 congressional offices. I am an assistant professor at the Baylor College of Medicine in Houston. During Advocacy Day, I visited the office of my congressional representative, Rep. Pete Olsen (R-Tex.), as well as health policy advisors for Sen. Ted Cruz (R-Tex.) and Sen. John Cornyn (R-Tex.). For the visits to the senators’ offices, I was joined by my colleagues from Baylor, Avinash Ketwaroo, MD, and Richard Robbins, MD, as well as Thomas Kerr, MD, PhD, of University of Texas, Dallas. During these visits, we discussed National Institutes of Health funding and barriers to effective care in digestive diseases such as copays for colonoscopy.
Academic institutions share the aim of conducting high-quality research to further advances in medicine. These research projects are often funded through NIH grant programs. Unfortunately, these programs are also often the target of budget cuts, which can affect primary research and also downstream economic growth. An analysis by United for Medical Research found that, for every dollar spent in NIH grants, $2 of economic output is generated.1 In 2016, these programs created 379,000 jobs and $64 billion in economic activity nationally. AGA calls for increased NIH funding to maintain pace with inflation.2
We also discussed how projects funded by NIH have led to important advances in gastroenterology in Texas. For example, NIH-funded research by Hashem El-Serag, MD, and Fasiha Kanwal, MD, has produced studies to evaluate biomarkers and improve screening techniques in hepatocellular carcinoma.3,4 Dr. Kerr discussed his experiences as a physician-scientist and the importance of basic science research as a foundation for clinical advances.
After the Affordable Care Act was passed, deductibles and coinsurance fees were waived for colorectal cancer screening tests that received an “A” or “B” grade from the U.S. Preventive Services Task Force. However, once a polyp is found and removed during a screening colonoscopy, the procedure is reclassified as a therapeutic procedure, meaning the patient will have to pay the coinsurance.5
Coinsurance costs can be 20%-25% of the Medicare-approved amount. In essence, patients may go into a procedure with the expectation that it will be 100% covered by insurance only to find out that they will receive a larger bill because polyps were removed. It puts gastroenterologists in a difficult position because they know that polyp removal will increase the cost to the patient; however, waiting for a repeat procedure would be redundant and lead to possible loss of follow-up care. The Removing Barriers to Colorectal Cancer Screening Act would correct this by waiving the coinsurance for a screening colonoscopy even if polyps were removed.6 We discussed the importance of this legislation to removing barriers to screening.
Use of biologics has advanced the treatment of many diseases, including inflammatory bowel disease (IBD). However, mandates by insurance companies can make it difficult to use these medications without first “stepping” through other less costly medications. We spoke with staffers regarding the Restoring the Patient’s Voice Act, which would remove unneeded barriers to prescribing appropriate therapy. It would also streamline the prior authorization/appeals process by requiring insurance companies to respond in a timely manner. We discussed the effects IBD has on the quality of life of our patients and shared our experiences in obtaining timely therapy.
As physicians, we are uniquely positioned to represent the needs of our patients. We appreciate AGA facilitating that voice by providing updates on legislation and coordinating meetings between senators, others members of Congress, and practicing gastroenterologists and GI fellows. AGA Advocacy Day is an important event to discuss our perspective as physicians and our experiences dealing with the health care system on a daily basis. Congressional staffers were very interested to hear our points of view as HCPs. They even shared their personal stories regarding friends and relatives with colon cancer and other digestive diseases. I strongly encourage other AGA members to take advantage of this important program. Other advocacy programs by AGA are discussed as follows.
Congressional Advocates Program
This is a grassroots program aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities that range from creating educational posts on social media to meeting with government representatives. Members are mentored by AGA leadership and staff for advocacy training. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
AGA PAC
The AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA and is the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections and access to specialty care, and support for federal funding of digestive disease research. If you are interested in learning more, contact AGA’s Government and Political Affairs Manager, Navneet Buttar, at [email protected] or 240-482-3221.
GovPredict
AGA’s online advocacy platform allows members to contact their members of Congress with just a few clicks. AGA develops messages on key pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have great effects on gastroenterology. The platform also allows AGA to track legislation, key votes, a legislator’s priority issues, and other key legislative activity. AGA can also track member activity with a legislator and their staff, a key component in building and maintaining relationships with key legislators.
References
1. Ehrlich E. United for Medical Research. NIH’S role in sustaining the U.S. economy. http://www.unitedformedicalresearch.com/advocacy_reports/nihs-role-in-sustaining-the-u-s-economy-2017-update/nih-role-in-the-economy-fy2016-2/#.XD9RafZFy5t.
2. AGA. AGA position statement on research funding. http://www.gastro.org/take-action/top-issues/research-funding.
3. El-Serag HB et al. Gastroenterology. 2014 May;146(5):1249-55.e1.
4. White DL et al. Gastroenterology. 2015 Dec;149(7):1986-7.
5. AGA. AGA position statement on patient cost sharing for screening colonoscopy. http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
6. Removing Barriers to Colorectal Cancer Screening Act of 2017. S. 479 U.S.C. (2017-2018).
Dr. Natarajan has received clinical trial support from Gilead and Allergan. Dr. Natarajan is a member of the AGA Government Affairs Committee. This feature originally appeared in AGA Perspectives.
AGA workshops/webcasts to give free advice on advancing your GI career
The free half-day workshops and webcasts in Columbus, Ohio, on Feb. 16, 2019, and in Boston on March 30, 2019, emphasize mastering basic business skills that can help advance your GI career.
Fellows and early-career GIs will have an opportunity to connect with seasoned GIs to gain real-world insights on successfully managing their careers at one of two upcoming American Gastroenterological Association’s Regional Practice Skills Workshops. Seasoned faculty will share their experiences and recommendations on:
- Measuring quality and delivering value-based care.
- Health care reform and the future of GI.
- Planning and managing finances, and much more.
Register and plan to join one of the upcoming workshops or webcasts:
If you’re in the Columbus or Boston area, attending the workshop in person is a great opportunity to ask questions of presenters and to network with faculty and peers. If you are not able to attend in person, you may still benefit from the valuable information by registering for the live webcast.
Open to AGA members and nonmembers, the workshops have been a hit with recent attendees who have called them an “eye opener,” “amazing and very informative,” and “phenomenal.” Take advantage of this free learning opportunity and register for one or both events/webcasts today.
Registration for all workshops and webcasts is required.
Rising microbiome investigator: Lea Ann Chen, MD
Dr. Chen, of New York University, talks about her research on gastrointestinal illnesses and what motivated her to focus on the gut microbiome.
We spoke with Dr. Chen, the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S et al. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives, including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
My experiences during AGA’s Advocacy Day: Facilitating change
BY YAMINI NATARAJAN, MD
The hospital is often the intersection between patients’ medical illness and their social and financial issues.
As physicians, it is important to recognize that patient care encompasses the prescribing of medications, the performing of procedures, as well as systems-based practice, and ensuring that social and financial barriers do not impede access to, and delivery of, care. Some of these barriers cannot be eliminated by any one individual health care professional (HCP); they can only be improved by working with government representatives and policymakers to make systemic changes. For gastroenterologists, advocacy involves educating patients, HCPs, and our government representatives about issues related to GI illnesses and the importance of ensuring access to GI specialty care and treatment for all the patients who require it.
AGA, via the Government Affairs Committee, facilitates advocacy in several ways. These include policy briefs, position statements, and facilitating meetings with our representatives and senators in home districts and in Washington. AGA hosted Advocacy Day in Washington on Sept. 14, 2018. Seventeen AGA members from 11 states visited 26 congressional offices. I am an assistant professor at the Baylor College of Medicine in Houston. During Advocacy Day, I visited the office of my congressional representative, Rep. Pete Olsen (R-Tex.), as well as health policy advisors for Sen. Ted Cruz (R-Tex.) and Sen. John Cornyn (R-Tex.). For the visits to the senators’ offices, I was joined by my colleagues from Baylor, Avinash Ketwaroo, MD, and Richard Robbins, MD, as well as Thomas Kerr, MD, PhD, of University of Texas, Dallas. During these visits, we discussed National Institutes of Health funding and barriers to effective care in digestive diseases such as copays for colonoscopy.
Academic institutions share the aim of conducting high-quality research to further advances in medicine. These research projects are often funded through NIH grant programs. Unfortunately, these programs are also often the target of budget cuts, which can affect primary research and also downstream economic growth. An analysis by United for Medical Research found that, for every dollar spent in NIH grants, $2 of economic output is generated.1 In 2016, these programs created 379,000 jobs and $64 billion in economic activity nationally. AGA calls for increased NIH funding to maintain pace with inflation.2
We also discussed how projects funded by NIH have led to important advances in gastroenterology in Texas. For example, NIH-funded research by Hashem El-Serag, MD, and Fasiha Kanwal, MD, has produced studies to evaluate biomarkers and improve screening techniques in hepatocellular carcinoma.3,4 Dr. Kerr discussed his experiences as a physician-scientist and the importance of basic science research as a foundation for clinical advances.
After the Affordable Care Act was passed, deductibles and coinsurance fees were waived for colorectal cancer screening tests that received an “A” or “B” grade from the U.S. Preventive Services Task Force. However, once a polyp is found and removed during a screening colonoscopy, the procedure is reclassified as a therapeutic procedure, meaning the patient will have to pay the coinsurance.5
Coinsurance costs can be 20%-25% of the Medicare-approved amount. In essence, patients may go into a procedure with the expectation that it will be 100% covered by insurance only to find out that they will receive a larger bill because polyps were removed. It puts gastroenterologists in a difficult position because they know that polyp removal will increase the cost to the patient; however, waiting for a repeat procedure would be redundant and lead to possible loss of follow-up care. The Removing Barriers to Colorectal Cancer Screening Act would correct this by waiving the coinsurance for a screening colonoscopy even if polyps were removed.6 We discussed the importance of this legislation to removing barriers to screening.
Use of biologics has advanced the treatment of many diseases, including inflammatory bowel disease (IBD). However, mandates by insurance companies can make it difficult to use these medications without first “stepping” through other less costly medications. We spoke with staffers regarding the Restoring the Patient’s Voice Act, which would remove unneeded barriers to prescribing appropriate therapy. It would also streamline the prior authorization/appeals process by requiring insurance companies to respond in a timely manner. We discussed the effects IBD has on the quality of life of our patients and shared our experiences in obtaining timely therapy.
As physicians, we are uniquely positioned to represent the needs of our patients. We appreciate AGA facilitating that voice by providing updates on legislation and coordinating meetings between senators, others members of Congress, and practicing gastroenterologists and GI fellows. AGA Advocacy Day is an important event to discuss our perspective as physicians and our experiences dealing with the health care system on a daily basis. Congressional staffers were very interested to hear our points of view as HCPs. They even shared their personal stories regarding friends and relatives with colon cancer and other digestive diseases. I strongly encourage other AGA members to take advantage of this important program. Other advocacy programs by AGA are discussed as follows.
Congressional Advocates Program
This is a grassroots program aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities that range from creating educational posts on social media to meeting with government representatives. Members are mentored by AGA leadership and staff for advocacy training. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
AGA PAC
The AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA and is the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections and access to specialty care, and support for federal funding of digestive disease research. If you are interested in learning more, contact AGA’s Government and Political Affairs Manager, Navneet Buttar, at [email protected] or 240-482-3221.
GovPredict
AGA’s online advocacy platform allows members to contact their members of Congress with just a few clicks. AGA develops messages on key pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have great effects on gastroenterology. The platform also allows AGA to track legislation, key votes, a legislator’s priority issues, and other key legislative activity. AGA can also track member activity with a legislator and their staff, a key component in building and maintaining relationships with key legislators.
References
1. Ehrlich E. United for Medical Research. NIH’S role in sustaining the U.S. economy. http://www.unitedformedicalresearch.com/advocacy_reports/nihs-role-in-sustaining-the-u-s-economy-2017-update/nih-role-in-the-economy-fy2016-2/#.XD9RafZFy5t.
2. AGA. AGA position statement on research funding. http://www.gastro.org/take-action/top-issues/research-funding.
3. El-Serag HB et al. Gastroenterology. 2014 May;146(5):1249-55.e1.
4. White DL et al. Gastroenterology. 2015 Dec;149(7):1986-7.
5. AGA. AGA position statement on patient cost sharing for screening colonoscopy. http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
6. Removing Barriers to Colorectal Cancer Screening Act of 2017. S. 479 U.S.C. (2017-2018).
Dr. Natarajan has received clinical trial support from Gilead and Allergan. Dr. Natarajan is a member of the AGA Government Affairs Committee. This feature originally appeared in AGA Perspectives.
AGA workshops/webcasts to give free advice on advancing your GI career
The free half-day workshops and webcasts in Columbus, Ohio, on Feb. 16, 2019, and in Boston on March 30, 2019, emphasize mastering basic business skills that can help advance your GI career.
Fellows and early-career GIs will have an opportunity to connect with seasoned GIs to gain real-world insights on successfully managing their careers at one of two upcoming American Gastroenterological Association’s Regional Practice Skills Workshops. Seasoned faculty will share their experiences and recommendations on:
- Measuring quality and delivering value-based care.
- Health care reform and the future of GI.
- Planning and managing finances, and much more.
Register and plan to join one of the upcoming workshops or webcasts:
If you’re in the Columbus or Boston area, attending the workshop in person is a great opportunity to ask questions of presenters and to network with faculty and peers. If you are not able to attend in person, you may still benefit from the valuable information by registering for the live webcast.
Open to AGA members and nonmembers, the workshops have been a hit with recent attendees who have called them an “eye opener,” “amazing and very informative,” and “phenomenal.” Take advantage of this free learning opportunity and register for one or both events/webcasts today.
Registration for all workshops and webcasts is required.
Rising microbiome investigator: Lea Ann Chen, MD
Dr. Chen, of New York University, talks about her research on gastrointestinal illnesses and what motivated her to focus on the gut microbiome.
We spoke with Dr. Chen, the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S et al. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives, including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
My experiences during AGA’s Advocacy Day: Facilitating change
BY YAMINI NATARAJAN, MD
The hospital is often the intersection between patients’ medical illness and their social and financial issues.
As physicians, it is important to recognize that patient care encompasses the prescribing of medications, the performing of procedures, as well as systems-based practice, and ensuring that social and financial barriers do not impede access to, and delivery of, care. Some of these barriers cannot be eliminated by any one individual health care professional (HCP); they can only be improved by working with government representatives and policymakers to make systemic changes. For gastroenterologists, advocacy involves educating patients, HCPs, and our government representatives about issues related to GI illnesses and the importance of ensuring access to GI specialty care and treatment for all the patients who require it.
AGA, via the Government Affairs Committee, facilitates advocacy in several ways. These include policy briefs, position statements, and facilitating meetings with our representatives and senators in home districts and in Washington. AGA hosted Advocacy Day in Washington on Sept. 14, 2018. Seventeen AGA members from 11 states visited 26 congressional offices. I am an assistant professor at the Baylor College of Medicine in Houston. During Advocacy Day, I visited the office of my congressional representative, Rep. Pete Olsen (R-Tex.), as well as health policy advisors for Sen. Ted Cruz (R-Tex.) and Sen. John Cornyn (R-Tex.). For the visits to the senators’ offices, I was joined by my colleagues from Baylor, Avinash Ketwaroo, MD, and Richard Robbins, MD, as well as Thomas Kerr, MD, PhD, of University of Texas, Dallas. During these visits, we discussed National Institutes of Health funding and barriers to effective care in digestive diseases such as copays for colonoscopy.
Academic institutions share the aim of conducting high-quality research to further advances in medicine. These research projects are often funded through NIH grant programs. Unfortunately, these programs are also often the target of budget cuts, which can affect primary research and also downstream economic growth. An analysis by United for Medical Research found that, for every dollar spent in NIH grants, $2 of economic output is generated.1 In 2016, these programs created 379,000 jobs and $64 billion in economic activity nationally. AGA calls for increased NIH funding to maintain pace with inflation.2
We also discussed how projects funded by NIH have led to important advances in gastroenterology in Texas. For example, NIH-funded research by Hashem El-Serag, MD, and Fasiha Kanwal, MD, has produced studies to evaluate biomarkers and improve screening techniques in hepatocellular carcinoma.3,4 Dr. Kerr discussed his experiences as a physician-scientist and the importance of basic science research as a foundation for clinical advances.
After the Affordable Care Act was passed, deductibles and coinsurance fees were waived for colorectal cancer screening tests that received an “A” or “B” grade from the U.S. Preventive Services Task Force. However, once a polyp is found and removed during a screening colonoscopy, the procedure is reclassified as a therapeutic procedure, meaning the patient will have to pay the coinsurance.5
Coinsurance costs can be 20%-25% of the Medicare-approved amount. In essence, patients may go into a procedure with the expectation that it will be 100% covered by insurance only to find out that they will receive a larger bill because polyps were removed. It puts gastroenterologists in a difficult position because they know that polyp removal will increase the cost to the patient; however, waiting for a repeat procedure would be redundant and lead to possible loss of follow-up care. The Removing Barriers to Colorectal Cancer Screening Act would correct this by waiving the coinsurance for a screening colonoscopy even if polyps were removed.6 We discussed the importance of this legislation to removing barriers to screening.
Use of biologics has advanced the treatment of many diseases, including inflammatory bowel disease (IBD). However, mandates by insurance companies can make it difficult to use these medications without first “stepping” through other less costly medications. We spoke with staffers regarding the Restoring the Patient’s Voice Act, which would remove unneeded barriers to prescribing appropriate therapy. It would also streamline the prior authorization/appeals process by requiring insurance companies to respond in a timely manner. We discussed the effects IBD has on the quality of life of our patients and shared our experiences in obtaining timely therapy.
As physicians, we are uniquely positioned to represent the needs of our patients. We appreciate AGA facilitating that voice by providing updates on legislation and coordinating meetings between senators, others members of Congress, and practicing gastroenterologists and GI fellows. AGA Advocacy Day is an important event to discuss our perspective as physicians and our experiences dealing with the health care system on a daily basis. Congressional staffers were very interested to hear our points of view as HCPs. They even shared their personal stories regarding friends and relatives with colon cancer and other digestive diseases. I strongly encourage other AGA members to take advantage of this important program. Other advocacy programs by AGA are discussed as follows.
Congressional Advocates Program
This is a grassroots program aimed at establishing a stronger foundation for our current and future advocacy initiatives by creating state teams to work on advocacy on the local, state, and national levels. Participation can include a wide variety of activities that range from creating educational posts on social media to meeting with government representatives. Members are mentored by AGA leadership and staff for advocacy training. Participating members receive an AGA Congressional Advocate Program Certificate, a Digestive Disease Week® (DDW) badge ribbon, policy badge on the AGA Community, and recognition on AGA’s website. Applications for the next cycle will be released in 2019.
AGA PAC
The AGA PAC is a voluntary, nonpartisan political organization affiliated with and supported by AGA and is the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions. AGA PAC supports candidates who support our policy priorities, such as fair reimbursement, cutting regulatory red tape, supporting patient protections and access to specialty care, and support for federal funding of digestive disease research. If you are interested in learning more, contact AGA’s Government and Political Affairs Manager, Navneet Buttar, at [email protected] or 240-482-3221.
GovPredict
AGA’s online advocacy platform allows members to contact their members of Congress with just a few clicks. AGA develops messages on key pieces of legislation, key efforts in Congress, or on issues being advanced by federal agencies that have great effects on gastroenterology. The platform also allows AGA to track legislation, key votes, a legislator’s priority issues, and other key legislative activity. AGA can also track member activity with a legislator and their staff, a key component in building and maintaining relationships with key legislators.
References
1. Ehrlich E. United for Medical Research. NIH’S role in sustaining the U.S. economy. http://www.unitedformedicalresearch.com/advocacy_reports/nihs-role-in-sustaining-the-u-s-economy-2017-update/nih-role-in-the-economy-fy2016-2/#.XD9RafZFy5t.
2. AGA. AGA position statement on research funding. http://www.gastro.org/take-action/top-issues/research-funding.
3. El-Serag HB et al. Gastroenterology. 2014 May;146(5):1249-55.e1.
4. White DL et al. Gastroenterology. 2015 Dec;149(7):1986-7.
5. AGA. AGA position statement on patient cost sharing for screening colonoscopy. http://www.gastro.org/take-action/top-issues/patient-cost-sharing-for-screening-colonoscopy.
6. Removing Barriers to Colorectal Cancer Screening Act of 2017. S. 479 U.S.C. (2017-2018).
Dr. Natarajan has received clinical trial support from Gilead and Allergan. Dr. Natarajan is a member of the AGA Government Affairs Committee. This feature originally appeared in AGA Perspectives.







