User login
Metformin associated with acidosis only in patients with eGFR 30 mL/min per 1.73 m 2
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Biopsy? What Biopsy?
This 80-year-old man has been complaining to health care providers about the asymptomatic lesion on his right forearm for at least 5 years—“maybe more,” he says. During that time, he has been given a variety of diagnoses, mostly “infection” of some sort, along with prescriptions for oral antibiotics (cephalexin, trimethoprim/sulfa), topical antibiotics (mupirocin, triple-antibiotic cream), and germicidal washes (povidone and others). None of these treatment attempts has achieved results.
Prior to now, there has been no referral to dermatology, nor has any provider suggested biopsy of the lesion. The patient has a history of skin cancer (basal cell or squamous cell carcinomas) on his face, back, and arms. These followed a lifetime of sun exposure due to his work in construction and roofing.
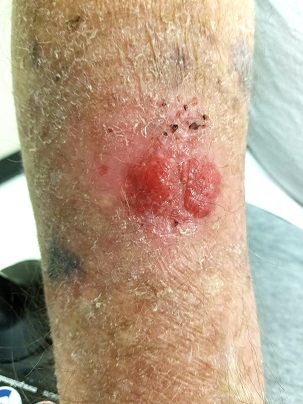
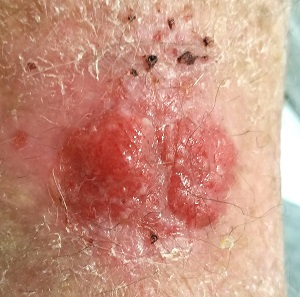
EXAMINATION
The lesion in question is located on the dorsal aspect of his mid right forearm. Measuring almost 4 cm in aggregate, the lesion is composed of 2 adjacent bright red half-moon friable nodules in a circular configuration. There is almost no erythema or edema in or around the lesion, which is neither warm nor tender to touch.
There are no palpable nodes in the adjacent epitrochlear or axillary nodal locations.
The surrounding skin of this arm, as well as that of the opposite arm, is quite thin, discolored, and scaly and is covered with stellate scars. Examination of all other sun-exposed areas reveals similar changes but no other notable lesions.
What’s the diagnosis?
DISCUSSION
It would be obvious to most readers that a biopsy is in order, given the likelihood that this lesion is either a basal cell or squamous cell carcinoma—an impression bolstered by the contextual findings of advanced sun damage and the history of skin cancer. And yet, this patient finds himself with a nonhealing friable lesion that is at least 5 years old.
Repeated misdiagnosis as infection is actually a common occurrence. One suspects that at least some of the providers making this mistake followed the patient’s lead: Many affected patients assume such lesions represent infection and therefore demand treatment appropriate for that diagnosis.
As a rule, however, bacterial infections don’t last years, especially in the absence of pain and swelling. Yes, there are nonbacterial infections (eg, those caused by acid-fast bacilli) that can be just as, if not more, indolent, but they too are apt to be painful and swollen.
Biopsy, the obvious missing step in this entire saga, would rule out any of these odd things (including melanoma, which has been known to present in this atypical morphologic manner). Other items in the differential include pyogenic granuloma and metastatic cancer (eg, breast, colon, lung).
In this case, biopsy confirmed the lesion was (as expected) a basal cell carcinoma. The lesion was excised in 2 stages over a 2.5-month period. And the patient was reminded that he will remain at extremely high risk for additional sun-caused skin cancers for the rest of his life.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be assumed to be skin cancer until proven otherwise, especially in sun-damaged individuals.
- Correct treatment is dictated by correct diagnosis; in this case, a biopsy was clearly indicated.
- Besides the more common cancers (eg, basal cell and squamous cell carcinoma), there were numerous other items in the differential, including melanoma and even metastatic cancer.
- Only rarely do skin infections smolder for years without pain or surrounding erythema.
This 80-year-old man has been complaining to health care providers about the asymptomatic lesion on his right forearm for at least 5 years—“maybe more,” he says. During that time, he has been given a variety of diagnoses, mostly “infection” of some sort, along with prescriptions for oral antibiotics (cephalexin, trimethoprim/sulfa), topical antibiotics (mupirocin, triple-antibiotic cream), and germicidal washes (povidone and others). None of these treatment attempts has achieved results.
Prior to now, there has been no referral to dermatology, nor has any provider suggested biopsy of the lesion. The patient has a history of skin cancer (basal cell or squamous cell carcinomas) on his face, back, and arms. These followed a lifetime of sun exposure due to his work in construction and roofing.

EXAMINATION
The lesion in question is located on the dorsal aspect of his mid right forearm. Measuring almost 4 cm in aggregate, the lesion is composed of 2 adjacent bright red half-moon friable nodules in a circular configuration. There is almost no erythema or edema in or around the lesion, which is neither warm nor tender to touch.
There are no palpable nodes in the adjacent epitrochlear or axillary nodal locations.
The surrounding skin of this arm, as well as that of the opposite arm, is quite thin, discolored, and scaly and is covered with stellate scars. Examination of all other sun-exposed areas reveals similar changes but no other notable lesions.
What’s the diagnosis?
DISCUSSION
It would be obvious to most readers that a biopsy is in order, given the likelihood that this lesion is either a basal cell or squamous cell carcinoma—an impression bolstered by the contextual findings of advanced sun damage and the history of skin cancer. And yet, this patient finds himself with a nonhealing friable lesion that is at least 5 years old.
Repeated misdiagnosis as infection is actually a common occurrence. One suspects that at least some of the providers making this mistake followed the patient’s lead: Many affected patients assume such lesions represent infection and therefore demand treatment appropriate for that diagnosis.
As a rule, however, bacterial infections don’t last years, especially in the absence of pain and swelling. Yes, there are nonbacterial infections (eg, those caused by acid-fast bacilli) that can be just as, if not more, indolent, but they too are apt to be painful and swollen.
Biopsy, the obvious missing step in this entire saga, would rule out any of these odd things (including melanoma, which has been known to present in this atypical morphologic manner). Other items in the differential include pyogenic granuloma and metastatic cancer (eg, breast, colon, lung).
In this case, biopsy confirmed the lesion was (as expected) a basal cell carcinoma. The lesion was excised in 2 stages over a 2.5-month period. And the patient was reminded that he will remain at extremely high risk for additional sun-caused skin cancers for the rest of his life.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be assumed to be skin cancer until proven otherwise, especially in sun-damaged individuals.
- Correct treatment is dictated by correct diagnosis; in this case, a biopsy was clearly indicated.
- Besides the more common cancers (eg, basal cell and squamous cell carcinoma), there were numerous other items in the differential, including melanoma and even metastatic cancer.
- Only rarely do skin infections smolder for years without pain or surrounding erythema.
This 80-year-old man has been complaining to health care providers about the asymptomatic lesion on his right forearm for at least 5 years—“maybe more,” he says. During that time, he has been given a variety of diagnoses, mostly “infection” of some sort, along with prescriptions for oral antibiotics (cephalexin, trimethoprim/sulfa), topical antibiotics (mupirocin, triple-antibiotic cream), and germicidal washes (povidone and others). None of these treatment attempts has achieved results.
Prior to now, there has been no referral to dermatology, nor has any provider suggested biopsy of the lesion. The patient has a history of skin cancer (basal cell or squamous cell carcinomas) on his face, back, and arms. These followed a lifetime of sun exposure due to his work in construction and roofing.

EXAMINATION
The lesion in question is located on the dorsal aspect of his mid right forearm. Measuring almost 4 cm in aggregate, the lesion is composed of 2 adjacent bright red half-moon friable nodules in a circular configuration. There is almost no erythema or edema in or around the lesion, which is neither warm nor tender to touch.
There are no palpable nodes in the adjacent epitrochlear or axillary nodal locations.
The surrounding skin of this arm, as well as that of the opposite arm, is quite thin, discolored, and scaly and is covered with stellate scars. Examination of all other sun-exposed areas reveals similar changes but no other notable lesions.
What’s the diagnosis?
DISCUSSION
It would be obvious to most readers that a biopsy is in order, given the likelihood that this lesion is either a basal cell or squamous cell carcinoma—an impression bolstered by the contextual findings of advanced sun damage and the history of skin cancer. And yet, this patient finds himself with a nonhealing friable lesion that is at least 5 years old.
Repeated misdiagnosis as infection is actually a common occurrence. One suspects that at least some of the providers making this mistake followed the patient’s lead: Many affected patients assume such lesions represent infection and therefore demand treatment appropriate for that diagnosis.
As a rule, however, bacterial infections don’t last years, especially in the absence of pain and swelling. Yes, there are nonbacterial infections (eg, those caused by acid-fast bacilli) that can be just as, if not more, indolent, but they too are apt to be painful and swollen.
Biopsy, the obvious missing step in this entire saga, would rule out any of these odd things (including melanoma, which has been known to present in this atypical morphologic manner). Other items in the differential include pyogenic granuloma and metastatic cancer (eg, breast, colon, lung).
In this case, biopsy confirmed the lesion was (as expected) a basal cell carcinoma. The lesion was excised in 2 stages over a 2.5-month period. And the patient was reminded that he will remain at extremely high risk for additional sun-caused skin cancers for the rest of his life.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be assumed to be skin cancer until proven otherwise, especially in sun-damaged individuals.
- Correct treatment is dictated by correct diagnosis; in this case, a biopsy was clearly indicated.
- Besides the more common cancers (eg, basal cell and squamous cell carcinoma), there were numerous other items in the differential, including melanoma and even metastatic cancer.
- Only rarely do skin infections smolder for years without pain or surrounding erythema.
Lesions around anus
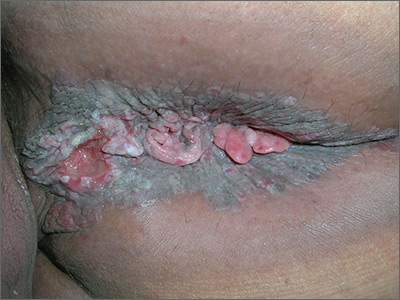
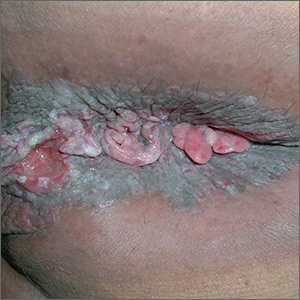
The FP suspected invasive squamous cell carcinoma (SCC) related to human papillomavirus. He recognized that the patient was at a higher risk of this secondary to the HIV/AIDS diagnosis.
The FP recommended a shave biopsy (See the Watch & Learn video on “Shave biopsy.”) of the ulcerated lesion, and the patient consented to this procedure. The FP used a surgical marker to mark an area at the edge of the ulcer including some tissue outside of the ulcer. (This is recommended for biopsy of any ulcerated skin lesion.) He then injected 1% lidocaine with epinephrine into the edge of the ulcer for anesthesia and to minimize bleeding during the shave biopsy. The shave was performed and aluminum chloride, along with electrosurgery, was used to stop the bleeding.
The pathology showed an invasive SCC. Due to the size and location of the lesions, the patient was referred to Colorectal Surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected invasive squamous cell carcinoma (SCC) related to human papillomavirus. He recognized that the patient was at a higher risk of this secondary to the HIV/AIDS diagnosis.
The FP recommended a shave biopsy (See the Watch & Learn video on “Shave biopsy.”) of the ulcerated lesion, and the patient consented to this procedure. The FP used a surgical marker to mark an area at the edge of the ulcer including some tissue outside of the ulcer. (This is recommended for biopsy of any ulcerated skin lesion.) He then injected 1% lidocaine with epinephrine into the edge of the ulcer for anesthesia and to minimize bleeding during the shave biopsy. The shave was performed and aluminum chloride, along with electrosurgery, was used to stop the bleeding.
The pathology showed an invasive SCC. Due to the size and location of the lesions, the patient was referred to Colorectal Surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected invasive squamous cell carcinoma (SCC) related to human papillomavirus. He recognized that the patient was at a higher risk of this secondary to the HIV/AIDS diagnosis.
The FP recommended a shave biopsy (See the Watch & Learn video on “Shave biopsy.”) of the ulcerated lesion, and the patient consented to this procedure. The FP used a surgical marker to mark an area at the edge of the ulcer including some tissue outside of the ulcer. (This is recommended for biopsy of any ulcerated skin lesion.) He then injected 1% lidocaine with epinephrine into the edge of the ulcer for anesthesia and to minimize bleeding during the shave biopsy. The shave was performed and aluminum chloride, along with electrosurgery, was used to stop the bleeding.
The pathology showed an invasive SCC. Due to the size and location of the lesions, the patient was referred to Colorectal Surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
FDA committee votes yes on romosozumab for osteoporosis
In an 18-1 vote, the FDA’s Bone, Reproductive, and Urologic Drugs Advisory Committee agreed that the risk-benefit profile of romosozumab, to be marketed by Amgen as Evenity, was favorable enough to support approval. Relative risk reductions of up to 75%, compared with placebo, and 36%, compared with alendronate, were seen in pivotal clinical trials.
A signal for increased major adverse cardiovascular events (MACE) among those receiving romosozumab had been seen in just one of the clinical trials, with a hazard ratio for MACE of 1.87 for those taking romosozumab, compared with those taking alendronate (95% confidence interval, 1.11-3.14).
Committee members were enthusiastic about the efficacy of the monoclonal antibody, which binds to sclerostin and prevents its inhibiting effect, allowing robust new bone formation. “I want to emphasize the remarkable skeletal efficacy of this drug; truly, it’s better than anything we’ve seen before,” said committee member Sundeep Khosla, MD, professor of medicine and physiology at the Mayo Clinic, Rochester, Minn.
In its application, the sponsor relied on two clinical trials. In the first, 7,180 women with osteoporosis aged 55-90 years were randomized 1:1 to receive romosozumab or placebo for 12 months in a double-blind trial. After this time, participants in each arm received follow-on treatment with denosumab (Prolia) for another 12 months. This study, dubbed Trial 337, followed morphometric vertebral fractures at 12 and 24 months. Morphometric fractures included both symptomatic and asymptomatic fractures.
Those treated with romosozumab had relative risk reductions of new vertebral fractures of 73% and 75%, compared with those given placebo at 12 and 24 months. Absolute risk reductions for vertebral fractures were 1.30% and 1.89% at 1 and 2 years (P less than .001 for both).
The second study, Trial 142, was a double-blind, active-controlled study that included 4,093 women aged 55-90 years with osteoporosis and a history of prior fragility fracture. Participants were randomized 1:1 to receive either romosozumab or alendronate for 12 months, with an additional variable period of alendronate follow-on of at least 12 months for both arms.
For Trial 142, one primary endpoint was morphometric vertebral fractures at month 24. An additional endpoint, clinical fracture, was a composite of symptomatic vertebral fractures and nonvertebral fractures. This second endpoint was assessed at the time of primary analysis, an event-driven cut point that occurred when at least 330 participants experienced a clinical fracture and all participants had completed the 24-month visit.
Hip fractures were less common among those given romosozumab, and bone mineral density increased significantly as well.
Vertebral fractures were reduced by 36% in the romosozumab group relative to the alendronate group, and clinical fractures by 27% (P less than .001 for both).
Overall, the number of adverse events for the more than 7,500 patients in the safety population was similar between those receiving romosozumab and either placebo or alendronate, said Scott Wasserman, MD, vice president of global development for Amgen.
However, in Trial 142, which included patients who were slightly older and on more cardiovascular medications at baseline than in Trial 337, MACE – defined as cardiovascular death, MI, and stroke – occurred more frequently among those taking romosozumab, driven primarily by increased cardiac and cerebral ischemic events occurring within the first 12 months of beginning the study drug. At 12 months, the romosozumab arm saw 41 instances of MACE, compared with 22 in the alendronate arm to produce the HR of 1.87 (2.0% vs. 1.1%).
With regard to the imbalance in MACE seen in Trial 142, both the FDA and presenters for the sponsor entertained the notion that alendronate may have been somewhat protective for cardiovascular events. Although there is some biologic plausibility for a cardioprotective event for bisphosphonates, alendronate is highly specific for bone activity and the preponderance of previous studies have not shown such cardioprotection, the FDA, sponsors, and committee members all agreed.
Marc Sabatine, MD, the Lewis Dexter, MD Distinguished Chair in Cardiovascular Medicine at Harvard Medical School, Boston, was available to answer questions on behalf of the study’s sponsor. He noted at several points during the meeting just how few total cardiovascular events were seen overall in the romosozumab trial. The overall small numbers, he said, made it very difficult to distinguish whether the smaller number of MACE seen in the alendronate arm of Trial 142 were a true safety signal or just “a play of chance.”
Almost all the committee’s questioning and discussion centered on this potential increased cardiovascular risk. Amgen, in discussion with the FDA, had agreed to a black box warning designed to wave off prescribing romosozumab to those at increased risk for cardiovascular disease, focusing on those with a history of MI or stroke.
Additionally, the sponsor proposed a postmarketing real-world observational study to track incidence of MACE in those receiving romosozumab, comparing them with those receiving standard of care for osteoporosis.
Several committee members pointed out a problem with the proposed safety mitigation scheme: By conducting a postmarketing observational study for a drug that has a black-box warning to exclude those at high risk for MACE, the chance of detecting an actual cardiovascular safety problem plummets. “This is the textbook example of when observational studies struggle or are virtually guaranteed to fail,” noted Tobias Gerhard, PhD, a pharmacoepidemiologist at Rutgers University, New Brunswick, N.J.
On the other hand, noted several committee members, pausing to conduct a premarketing randomized, controlled trial would keep a beneficial drug away from a population in need. A postmarketing randomized, controlled trial, even a simple trial, still presents challenges, some of the committee acknowledged. Dr. Khosla voiced the opinion that “A randomized, controlled trial is virtually impossible.”
The committee, which was charged with discussing, but not voting on, what additional data should be obtained – and when – to sort out the cardiovascular safety question, was approximately evenly divided in the matter of whether an observational or registry-based trial, or a controlled trial, would be the best path forward.
Committee member Robert A. Adler, MD, put a realistic frame around the debate. “As an endocrinologist, I deal with nuances every day. I really think the kind of clinician who is going to be using this drug is used to dealing with benefits and risks and trying to tailor treatment to a given patient,” said Dr. Adler, professor of internal medicine and epidemiology at Virginia Commonwealth University, Richmond.
In its proposed indication, Amgen defined the population of menopausal women at high risk of fracture as those with a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. Romosozumab would be given as a once-monthly subcutaneous injection of 210 mg for a period of 12 months, to be followed by antiresorptive therapy.
Most of the participants in the clinical trials resided outside the United States, primarily because of the difficulty of recruiting clinical trial participants in the United States, Amgen officials said during their presentation. However, neither the time to the first positively adjudicated MACE nor bone mineral density responses at month 12 differed significantly across the various geographic regions where clinical trial sites were located, said Rachel Wagman, MD, the executive medical director of global clinical development for Amgen. Still, several committee members called for postmarketing data to focus on U.S. patients.
Romosozumab was approved for marketing in Japan on Jan. 8, 2019; approval is also being sought in Europe .
The FDA usually follows the recommendations of its advisory panels.
In an 18-1 vote, the FDA’s Bone, Reproductive, and Urologic Drugs Advisory Committee agreed that the risk-benefit profile of romosozumab, to be marketed by Amgen as Evenity, was favorable enough to support approval. Relative risk reductions of up to 75%, compared with placebo, and 36%, compared with alendronate, were seen in pivotal clinical trials.
A signal for increased major adverse cardiovascular events (MACE) among those receiving romosozumab had been seen in just one of the clinical trials, with a hazard ratio for MACE of 1.87 for those taking romosozumab, compared with those taking alendronate (95% confidence interval, 1.11-3.14).
Committee members were enthusiastic about the efficacy of the monoclonal antibody, which binds to sclerostin and prevents its inhibiting effect, allowing robust new bone formation. “I want to emphasize the remarkable skeletal efficacy of this drug; truly, it’s better than anything we’ve seen before,” said committee member Sundeep Khosla, MD, professor of medicine and physiology at the Mayo Clinic, Rochester, Minn.
In its application, the sponsor relied on two clinical trials. In the first, 7,180 women with osteoporosis aged 55-90 years were randomized 1:1 to receive romosozumab or placebo for 12 months in a double-blind trial. After this time, participants in each arm received follow-on treatment with denosumab (Prolia) for another 12 months. This study, dubbed Trial 337, followed morphometric vertebral fractures at 12 and 24 months. Morphometric fractures included both symptomatic and asymptomatic fractures.
Those treated with romosozumab had relative risk reductions of new vertebral fractures of 73% and 75%, compared with those given placebo at 12 and 24 months. Absolute risk reductions for vertebral fractures were 1.30% and 1.89% at 1 and 2 years (P less than .001 for both).
The second study, Trial 142, was a double-blind, active-controlled study that included 4,093 women aged 55-90 years with osteoporosis and a history of prior fragility fracture. Participants were randomized 1:1 to receive either romosozumab or alendronate for 12 months, with an additional variable period of alendronate follow-on of at least 12 months for both arms.
For Trial 142, one primary endpoint was morphometric vertebral fractures at month 24. An additional endpoint, clinical fracture, was a composite of symptomatic vertebral fractures and nonvertebral fractures. This second endpoint was assessed at the time of primary analysis, an event-driven cut point that occurred when at least 330 participants experienced a clinical fracture and all participants had completed the 24-month visit.
Hip fractures were less common among those given romosozumab, and bone mineral density increased significantly as well.
Vertebral fractures were reduced by 36% in the romosozumab group relative to the alendronate group, and clinical fractures by 27% (P less than .001 for both).
Overall, the number of adverse events for the more than 7,500 patients in the safety population was similar between those receiving romosozumab and either placebo or alendronate, said Scott Wasserman, MD, vice president of global development for Amgen.
However, in Trial 142, which included patients who were slightly older and on more cardiovascular medications at baseline than in Trial 337, MACE – defined as cardiovascular death, MI, and stroke – occurred more frequently among those taking romosozumab, driven primarily by increased cardiac and cerebral ischemic events occurring within the first 12 months of beginning the study drug. At 12 months, the romosozumab arm saw 41 instances of MACE, compared with 22 in the alendronate arm to produce the HR of 1.87 (2.0% vs. 1.1%).
With regard to the imbalance in MACE seen in Trial 142, both the FDA and presenters for the sponsor entertained the notion that alendronate may have been somewhat protective for cardiovascular events. Although there is some biologic plausibility for a cardioprotective event for bisphosphonates, alendronate is highly specific for bone activity and the preponderance of previous studies have not shown such cardioprotection, the FDA, sponsors, and committee members all agreed.
Marc Sabatine, MD, the Lewis Dexter, MD Distinguished Chair in Cardiovascular Medicine at Harvard Medical School, Boston, was available to answer questions on behalf of the study’s sponsor. He noted at several points during the meeting just how few total cardiovascular events were seen overall in the romosozumab trial. The overall small numbers, he said, made it very difficult to distinguish whether the smaller number of MACE seen in the alendronate arm of Trial 142 were a true safety signal or just “a play of chance.”
Almost all the committee’s questioning and discussion centered on this potential increased cardiovascular risk. Amgen, in discussion with the FDA, had agreed to a black box warning designed to wave off prescribing romosozumab to those at increased risk for cardiovascular disease, focusing on those with a history of MI or stroke.
Additionally, the sponsor proposed a postmarketing real-world observational study to track incidence of MACE in those receiving romosozumab, comparing them with those receiving standard of care for osteoporosis.
Several committee members pointed out a problem with the proposed safety mitigation scheme: By conducting a postmarketing observational study for a drug that has a black-box warning to exclude those at high risk for MACE, the chance of detecting an actual cardiovascular safety problem plummets. “This is the textbook example of when observational studies struggle or are virtually guaranteed to fail,” noted Tobias Gerhard, PhD, a pharmacoepidemiologist at Rutgers University, New Brunswick, N.J.
On the other hand, noted several committee members, pausing to conduct a premarketing randomized, controlled trial would keep a beneficial drug away from a population in need. A postmarketing randomized, controlled trial, even a simple trial, still presents challenges, some of the committee acknowledged. Dr. Khosla voiced the opinion that “A randomized, controlled trial is virtually impossible.”
The committee, which was charged with discussing, but not voting on, what additional data should be obtained – and when – to sort out the cardiovascular safety question, was approximately evenly divided in the matter of whether an observational or registry-based trial, or a controlled trial, would be the best path forward.
Committee member Robert A. Adler, MD, put a realistic frame around the debate. “As an endocrinologist, I deal with nuances every day. I really think the kind of clinician who is going to be using this drug is used to dealing with benefits and risks and trying to tailor treatment to a given patient,” said Dr. Adler, professor of internal medicine and epidemiology at Virginia Commonwealth University, Richmond.
In its proposed indication, Amgen defined the population of menopausal women at high risk of fracture as those with a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. Romosozumab would be given as a once-monthly subcutaneous injection of 210 mg for a period of 12 months, to be followed by antiresorptive therapy.
Most of the participants in the clinical trials resided outside the United States, primarily because of the difficulty of recruiting clinical trial participants in the United States, Amgen officials said during their presentation. However, neither the time to the first positively adjudicated MACE nor bone mineral density responses at month 12 differed significantly across the various geographic regions where clinical trial sites were located, said Rachel Wagman, MD, the executive medical director of global clinical development for Amgen. Still, several committee members called for postmarketing data to focus on U.S. patients.
Romosozumab was approved for marketing in Japan on Jan. 8, 2019; approval is also being sought in Europe .
The FDA usually follows the recommendations of its advisory panels.
In an 18-1 vote, the FDA’s Bone, Reproductive, and Urologic Drugs Advisory Committee agreed that the risk-benefit profile of romosozumab, to be marketed by Amgen as Evenity, was favorable enough to support approval. Relative risk reductions of up to 75%, compared with placebo, and 36%, compared with alendronate, were seen in pivotal clinical trials.
A signal for increased major adverse cardiovascular events (MACE) among those receiving romosozumab had been seen in just one of the clinical trials, with a hazard ratio for MACE of 1.87 for those taking romosozumab, compared with those taking alendronate (95% confidence interval, 1.11-3.14).
Committee members were enthusiastic about the efficacy of the monoclonal antibody, which binds to sclerostin and prevents its inhibiting effect, allowing robust new bone formation. “I want to emphasize the remarkable skeletal efficacy of this drug; truly, it’s better than anything we’ve seen before,” said committee member Sundeep Khosla, MD, professor of medicine and physiology at the Mayo Clinic, Rochester, Minn.
In its application, the sponsor relied on two clinical trials. In the first, 7,180 women with osteoporosis aged 55-90 years were randomized 1:1 to receive romosozumab or placebo for 12 months in a double-blind trial. After this time, participants in each arm received follow-on treatment with denosumab (Prolia) for another 12 months. This study, dubbed Trial 337, followed morphometric vertebral fractures at 12 and 24 months. Morphometric fractures included both symptomatic and asymptomatic fractures.
Those treated with romosozumab had relative risk reductions of new vertebral fractures of 73% and 75%, compared with those given placebo at 12 and 24 months. Absolute risk reductions for vertebral fractures were 1.30% and 1.89% at 1 and 2 years (P less than .001 for both).
The second study, Trial 142, was a double-blind, active-controlled study that included 4,093 women aged 55-90 years with osteoporosis and a history of prior fragility fracture. Participants were randomized 1:1 to receive either romosozumab or alendronate for 12 months, with an additional variable period of alendronate follow-on of at least 12 months for both arms.
For Trial 142, one primary endpoint was morphometric vertebral fractures at month 24. An additional endpoint, clinical fracture, was a composite of symptomatic vertebral fractures and nonvertebral fractures. This second endpoint was assessed at the time of primary analysis, an event-driven cut point that occurred when at least 330 participants experienced a clinical fracture and all participants had completed the 24-month visit.
Hip fractures were less common among those given romosozumab, and bone mineral density increased significantly as well.
Vertebral fractures were reduced by 36% in the romosozumab group relative to the alendronate group, and clinical fractures by 27% (P less than .001 for both).
Overall, the number of adverse events for the more than 7,500 patients in the safety population was similar between those receiving romosozumab and either placebo or alendronate, said Scott Wasserman, MD, vice president of global development for Amgen.
However, in Trial 142, which included patients who were slightly older and on more cardiovascular medications at baseline than in Trial 337, MACE – defined as cardiovascular death, MI, and stroke – occurred more frequently among those taking romosozumab, driven primarily by increased cardiac and cerebral ischemic events occurring within the first 12 months of beginning the study drug. At 12 months, the romosozumab arm saw 41 instances of MACE, compared with 22 in the alendronate arm to produce the HR of 1.87 (2.0% vs. 1.1%).
With regard to the imbalance in MACE seen in Trial 142, both the FDA and presenters for the sponsor entertained the notion that alendronate may have been somewhat protective for cardiovascular events. Although there is some biologic plausibility for a cardioprotective event for bisphosphonates, alendronate is highly specific for bone activity and the preponderance of previous studies have not shown such cardioprotection, the FDA, sponsors, and committee members all agreed.
Marc Sabatine, MD, the Lewis Dexter, MD Distinguished Chair in Cardiovascular Medicine at Harvard Medical School, Boston, was available to answer questions on behalf of the study’s sponsor. He noted at several points during the meeting just how few total cardiovascular events were seen overall in the romosozumab trial. The overall small numbers, he said, made it very difficult to distinguish whether the smaller number of MACE seen in the alendronate arm of Trial 142 were a true safety signal or just “a play of chance.”
Almost all the committee’s questioning and discussion centered on this potential increased cardiovascular risk. Amgen, in discussion with the FDA, had agreed to a black box warning designed to wave off prescribing romosozumab to those at increased risk for cardiovascular disease, focusing on those with a history of MI or stroke.
Additionally, the sponsor proposed a postmarketing real-world observational study to track incidence of MACE in those receiving romosozumab, comparing them with those receiving standard of care for osteoporosis.
Several committee members pointed out a problem with the proposed safety mitigation scheme: By conducting a postmarketing observational study for a drug that has a black-box warning to exclude those at high risk for MACE, the chance of detecting an actual cardiovascular safety problem plummets. “This is the textbook example of when observational studies struggle or are virtually guaranteed to fail,” noted Tobias Gerhard, PhD, a pharmacoepidemiologist at Rutgers University, New Brunswick, N.J.
On the other hand, noted several committee members, pausing to conduct a premarketing randomized, controlled trial would keep a beneficial drug away from a population in need. A postmarketing randomized, controlled trial, even a simple trial, still presents challenges, some of the committee acknowledged. Dr. Khosla voiced the opinion that “A randomized, controlled trial is virtually impossible.”
The committee, which was charged with discussing, but not voting on, what additional data should be obtained – and when – to sort out the cardiovascular safety question, was approximately evenly divided in the matter of whether an observational or registry-based trial, or a controlled trial, would be the best path forward.
Committee member Robert A. Adler, MD, put a realistic frame around the debate. “As an endocrinologist, I deal with nuances every day. I really think the kind of clinician who is going to be using this drug is used to dealing with benefits and risks and trying to tailor treatment to a given patient,” said Dr. Adler, professor of internal medicine and epidemiology at Virginia Commonwealth University, Richmond.
In its proposed indication, Amgen defined the population of menopausal women at high risk of fracture as those with a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. Romosozumab would be given as a once-monthly subcutaneous injection of 210 mg for a period of 12 months, to be followed by antiresorptive therapy.
Most of the participants in the clinical trials resided outside the United States, primarily because of the difficulty of recruiting clinical trial participants in the United States, Amgen officials said during their presentation. However, neither the time to the first positively adjudicated MACE nor bone mineral density responses at month 12 differed significantly across the various geographic regions where clinical trial sites were located, said Rachel Wagman, MD, the executive medical director of global clinical development for Amgen. Still, several committee members called for postmarketing data to focus on U.S. patients.
Romosozumab was approved for marketing in Japan on Jan. 8, 2019; approval is also being sought in Europe .
The FDA usually follows the recommendations of its advisory panels.
More than 23% of antibiotic fills deemed unnecessary
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.
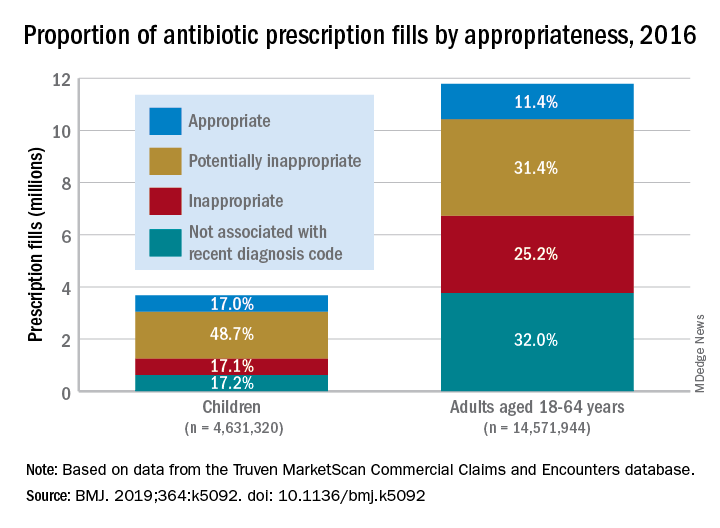
Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.

Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.

Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
FROM THE BMJ
FDA approves generic version of vigabatrin
The drug is approved for the adjunctive treatment of focal seizures in patients aged 10 years and older who have not had an adequate response to other therapies.
The approval was granted to Teva Pharmaceuticals.
An FDA announcement noted that the agency has prioritized the approval of generic versions of drugs to improve access to treatments and to lower drug costs. Vigabatrin had been included on an FDA list of off-patent, off-exclusivity branded drugs without approved generics. The approval of generic vigabatrin “demonstrates that there is an open pathway to approving products like this one,” said FDA Commissioner Scott Gottlieb, MD.
The label for vigabatrin tablets includes a boxed warning for permanent vision loss. The generic vigabatrin tablets are part of a single shared-system Risk Evaluation and Mitigation Strategy (REMS) program with other drug products containing vigabatrin.
The most common side effects associated with vigabatrin tablets include dizziness, fatigue, sleepiness, involuntary eye movement, tremor, blurred vision, memory impairment, weight gain, joint pain, upper respiratory tract infection, aggression, double vision, abnormal coordination, and a confused state. Serious side effects associated with vigabatrin tablets include permanent vision loss and risk of suicidal thoughts or actions.
The drug is approved for the adjunctive treatment of focal seizures in patients aged 10 years and older who have not had an adequate response to other therapies.
The approval was granted to Teva Pharmaceuticals.
An FDA announcement noted that the agency has prioritized the approval of generic versions of drugs to improve access to treatments and to lower drug costs. Vigabatrin had been included on an FDA list of off-patent, off-exclusivity branded drugs without approved generics. The approval of generic vigabatrin “demonstrates that there is an open pathway to approving products like this one,” said FDA Commissioner Scott Gottlieb, MD.
The label for vigabatrin tablets includes a boxed warning for permanent vision loss. The generic vigabatrin tablets are part of a single shared-system Risk Evaluation and Mitigation Strategy (REMS) program with other drug products containing vigabatrin.
The most common side effects associated with vigabatrin tablets include dizziness, fatigue, sleepiness, involuntary eye movement, tremor, blurred vision, memory impairment, weight gain, joint pain, upper respiratory tract infection, aggression, double vision, abnormal coordination, and a confused state. Serious side effects associated with vigabatrin tablets include permanent vision loss and risk of suicidal thoughts or actions.
The drug is approved for the adjunctive treatment of focal seizures in patients aged 10 years and older who have not had an adequate response to other therapies.
The approval was granted to Teva Pharmaceuticals.
An FDA announcement noted that the agency has prioritized the approval of generic versions of drugs to improve access to treatments and to lower drug costs. Vigabatrin had been included on an FDA list of off-patent, off-exclusivity branded drugs without approved generics. The approval of generic vigabatrin “demonstrates that there is an open pathway to approving products like this one,” said FDA Commissioner Scott Gottlieb, MD.
The label for vigabatrin tablets includes a boxed warning for permanent vision loss. The generic vigabatrin tablets are part of a single shared-system Risk Evaluation and Mitigation Strategy (REMS) program with other drug products containing vigabatrin.
The most common side effects associated with vigabatrin tablets include dizziness, fatigue, sleepiness, involuntary eye movement, tremor, blurred vision, memory impairment, weight gain, joint pain, upper respiratory tract infection, aggression, double vision, abnormal coordination, and a confused state. Serious side effects associated with vigabatrin tablets include permanent vision loss and risk of suicidal thoughts or actions.
Study supports need for less toxic therapies in FL
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The cumulative risk of mortality from lymphoma was 10.3% at 10 years for patients with follicular lymphoma.
Study details: A pooled cohort study of 1,654 patients with follicular lymphoma in the United States and France.
Disclosures: The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
Source: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Obinutuzumab-based regimens yield durable remissions in CLL
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
FROM BLOOD
Key clinical point:
Major finding: With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Study details: Long-term follow-up of the phase 1b GALTON trial, including 41 patients with CLL.
Disclosures: The study was sponsored by Genentech. The study authors reported disclosures related to Genentech/Roche and other companies.
Source: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Host stress response may be a factor in early-stage HCV clearance
The cellular stress response in hepatocytes may play a major role in controlling hepatitis C virus (HCV). This response may be an important host factor for virus clearance during the early stages of HCV infection, according to a review of acute and chronic HCV infection by W. Alfredo Ríos-Ocampo, MD, and his colleagues (Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
The reviewers examined the mechanisms of induction and modulation of oxidative stress and endoplasmic-reticular stress with regard to viral persistence and cell survival. The accumulated research indicates that the activation of the eIF2-alpha/ATF4 pathway and selective autophagy induction are involved in the elimination of harmful viral proteins after oxidative stress induction. This all suggests a negative role of autophagy upon HCV infection or a negative regulation of viral replication.
“We conclude from published studies and our own research that viral protein synthesis activates adaptive responses, including autophagy pathways, that act to limit viral protein load and thereby reduce oxidative stress and cell death. Exploitation of these pathways to reduce viral replication will be the next goal and might be a valuable addition to antiviral therapy,” the reviewers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ríos-Ocampo, WA, et al. Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
The cellular stress response in hepatocytes may play a major role in controlling hepatitis C virus (HCV). This response may be an important host factor for virus clearance during the early stages of HCV infection, according to a review of acute and chronic HCV infection by W. Alfredo Ríos-Ocampo, MD, and his colleagues (Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).

The reviewers examined the mechanisms of induction and modulation of oxidative stress and endoplasmic-reticular stress with regard to viral persistence and cell survival. The accumulated research indicates that the activation of the eIF2-alpha/ATF4 pathway and selective autophagy induction are involved in the elimination of harmful viral proteins after oxidative stress induction. This all suggests a negative role of autophagy upon HCV infection or a negative regulation of viral replication.
“We conclude from published studies and our own research that viral protein synthesis activates adaptive responses, including autophagy pathways, that act to limit viral protein load and thereby reduce oxidative stress and cell death. Exploitation of these pathways to reduce viral replication will be the next goal and might be a valuable addition to antiviral therapy,” the reviewers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ríos-Ocampo, WA, et al. Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
The cellular stress response in hepatocytes may play a major role in controlling hepatitis C virus (HCV). This response may be an important host factor for virus clearance during the early stages of HCV infection, according to a review of acute and chronic HCV infection by W. Alfredo Ríos-Ocampo, MD, and his colleagues (Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).

The reviewers examined the mechanisms of induction and modulation of oxidative stress and endoplasmic-reticular stress with regard to viral persistence and cell survival. The accumulated research indicates that the activation of the eIF2-alpha/ATF4 pathway and selective autophagy induction are involved in the elimination of harmful viral proteins after oxidative stress induction. This all suggests a negative role of autophagy upon HCV infection or a negative regulation of viral replication.
“We conclude from published studies and our own research that viral protein synthesis activates adaptive responses, including autophagy pathways, that act to limit viral protein load and thereby reduce oxidative stress and cell death. Exploitation of these pathways to reduce viral replication will be the next goal and might be a valuable addition to antiviral therapy,” the reviewers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ríos-Ocampo, WA, et al. Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
FROM VIRUS RESEARCH
Treat-to-target approach for CVD risk factors decreased atherosclerosis in RA patients
Researchers from the Netherlands found that a treat-to-target approach for cardiovascular risk factors in patients with rheumatoid arthritis was effective in reducing clinical and subclinical atherosclerosis; however, the researchers noted there was a “considerable” dropout in the study that could have limited the results, according to data recently published in Annals of the Rheumatic Diseases.
Benjamin Burggraaf, MD, and his associates at Franciscus Gasthuis & Vlietland Hospital in Rotterdam, the Netherlands, performed an open-label, randomized, controlled trial of 320 patients with rheumatoid arthritis (RA) who were younger than 70 years old (mean age, 52.4 years) without a prior history of cardiovascular disease (CVD) and diabetes mellitus. The patients received either usual care for traditional CVD risk factors or a treat-to-target approach using a prespecified protocol for treating hypertension, hyperlipidemia and hypertriglyceridemia, as well hemoglobin A1c greater than 48 mmol/mol. A total of 219 patients (68.4%) finished the study after 5 years. The two groups were similar at baseline, but the treat-to-target group had significantly more women (77.2%) compared with the usual care group (62.0%).
The researchers compared the progression of carotid intima media thickness (cIMT) at baseline and 5 years, with secondary outcomes of nonfatal myocardial infarction, nonfatal stroke, coronary artery bypass grafting, percutaneous coronary intervention, peripheral atherosclerotic arterial disease–related amputation, peripheral atherosclerotic arterial disease revascularization, and death due to cardiovascular causes.
“To account for the high dropout, all missing cIMT values after 5 years of follow-up were imputed with all available cIMT values (baseline, years 1-4) and treatment group as covariate,” the researchers said. “Assuming that unobserved cIMT values were missing at random, missing data were imputed with multiple imputation using the fully conditional specification method for seven cycles.”
Dr. Burggraaf and his colleagues found the treat-to-target group had lower mean cIMT progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up than did the group that received usual care for CVD risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028). At 5 years, there were no significant differences between treat-to-target and usual care groups regarding mean systolic blood pressure (124.6 mm Hg vs. 124.7 mm Hg, respectively; P = .97), treat-to-target treatment targets for blood pressure (72.4% for usual care vs. 75.9% for treat-to-target; P = .56) and mean HbA1c (37.6 mmol/mol for treat-to-target vs. 37.0 mmol/mol for usual care; P = .39). The treat-to-target group also had fewer cardiovascular events (two events, 1.3%) compared with the usual care group (seven events, 4.7%; P = .048). There were five patients in the treat-to-target group who died during the study (4.7%), compared with seven patients (3.2%) in the usual care group (P = .51).
“Although the difference in cIMT progression between the groups was relatively small in absolute terms, the relative reduction in progression was almost 50% in favor of the treat-to-target group,” they noted. “In light of the reduction of cardiovascular events, these effects are, in our opinion, clinically relevant.”
Other limitations of the study included the use of cIMT as a “soft endpoint” for modern cardiovascular trials and the use of unblinded cIMT progression measurement. In addition, the researchers noted the study was underpowered, and they used data from a type 2 diabetes mellitus cohort to perform the power calculation, which carried a 50% reduction in CVD risk factors. “We had doubts whether this high risk would apply to patients with RA and therefore used a more conservative target of 20% reduction for cIMT progression,” the researchers said.
This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
SOURCE: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
It is well recognized that traditional cardiovascular disease (CVD) risk factors contribute to atherogenesis and CVD events in rheumatoid arthritis (RA) patients at least as much as inflammatory risk factors, and traditional risk factors and inflammatory risk factors may interact (i.e. inflammatory risk factors have a greater impact in the setting of higher number of traditional risk factors).
There is documented reluctance among many rheumatologists to take on the additional burden of screening and managing hyperlipidemia, hypertension, diabetes, weight management, diet, and so on in addition to all the other aspects of managing these patients that take a lot of time. Research is needed on how to efficiently streamline measurement and management of CVD risk in these patients to optimize outcomes.
Jon T. Giles, MD , is a rheumatologist in the division of rheumatology at Columbia University in New York. He was not involved in the study by Burggraaf et al. and reported no relevant conflicts of interest.
It is well recognized that traditional cardiovascular disease (CVD) risk factors contribute to atherogenesis and CVD events in rheumatoid arthritis (RA) patients at least as much as inflammatory risk factors, and traditional risk factors and inflammatory risk factors may interact (i.e. inflammatory risk factors have a greater impact in the setting of higher number of traditional risk factors).
There is documented reluctance among many rheumatologists to take on the additional burden of screening and managing hyperlipidemia, hypertension, diabetes, weight management, diet, and so on in addition to all the other aspects of managing these patients that take a lot of time. Research is needed on how to efficiently streamline measurement and management of CVD risk in these patients to optimize outcomes.
Jon T. Giles, MD , is a rheumatologist in the division of rheumatology at Columbia University in New York. He was not involved in the study by Burggraaf et al. and reported no relevant conflicts of interest.
It is well recognized that traditional cardiovascular disease (CVD) risk factors contribute to atherogenesis and CVD events in rheumatoid arthritis (RA) patients at least as much as inflammatory risk factors, and traditional risk factors and inflammatory risk factors may interact (i.e. inflammatory risk factors have a greater impact in the setting of higher number of traditional risk factors).
There is documented reluctance among many rheumatologists to take on the additional burden of screening and managing hyperlipidemia, hypertension, diabetes, weight management, diet, and so on in addition to all the other aspects of managing these patients that take a lot of time. Research is needed on how to efficiently streamline measurement and management of CVD risk in these patients to optimize outcomes.
Jon T. Giles, MD , is a rheumatologist in the division of rheumatology at Columbia University in New York. He was not involved in the study by Burggraaf et al. and reported no relevant conflicts of interest.
Researchers from the Netherlands found that a treat-to-target approach for cardiovascular risk factors in patients with rheumatoid arthritis was effective in reducing clinical and subclinical atherosclerosis; however, the researchers noted there was a “considerable” dropout in the study that could have limited the results, according to data recently published in Annals of the Rheumatic Diseases.
Benjamin Burggraaf, MD, and his associates at Franciscus Gasthuis & Vlietland Hospital in Rotterdam, the Netherlands, performed an open-label, randomized, controlled trial of 320 patients with rheumatoid arthritis (RA) who were younger than 70 years old (mean age, 52.4 years) without a prior history of cardiovascular disease (CVD) and diabetes mellitus. The patients received either usual care for traditional CVD risk factors or a treat-to-target approach using a prespecified protocol for treating hypertension, hyperlipidemia and hypertriglyceridemia, as well hemoglobin A1c greater than 48 mmol/mol. A total of 219 patients (68.4%) finished the study after 5 years. The two groups were similar at baseline, but the treat-to-target group had significantly more women (77.2%) compared with the usual care group (62.0%).
The researchers compared the progression of carotid intima media thickness (cIMT) at baseline and 5 years, with secondary outcomes of nonfatal myocardial infarction, nonfatal stroke, coronary artery bypass grafting, percutaneous coronary intervention, peripheral atherosclerotic arterial disease–related amputation, peripheral atherosclerotic arterial disease revascularization, and death due to cardiovascular causes.
“To account for the high dropout, all missing cIMT values after 5 years of follow-up were imputed with all available cIMT values (baseline, years 1-4) and treatment group as covariate,” the researchers said. “Assuming that unobserved cIMT values were missing at random, missing data were imputed with multiple imputation using the fully conditional specification method for seven cycles.”
Dr. Burggraaf and his colleagues found the treat-to-target group had lower mean cIMT progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up than did the group that received usual care for CVD risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028). At 5 years, there were no significant differences between treat-to-target and usual care groups regarding mean systolic blood pressure (124.6 mm Hg vs. 124.7 mm Hg, respectively; P = .97), treat-to-target treatment targets for blood pressure (72.4% for usual care vs. 75.9% for treat-to-target; P = .56) and mean HbA1c (37.6 mmol/mol for treat-to-target vs. 37.0 mmol/mol for usual care; P = .39). The treat-to-target group also had fewer cardiovascular events (two events, 1.3%) compared with the usual care group (seven events, 4.7%; P = .048). There were five patients in the treat-to-target group who died during the study (4.7%), compared with seven patients (3.2%) in the usual care group (P = .51).
“Although the difference in cIMT progression between the groups was relatively small in absolute terms, the relative reduction in progression was almost 50% in favor of the treat-to-target group,” they noted. “In light of the reduction of cardiovascular events, these effects are, in our opinion, clinically relevant.”
Other limitations of the study included the use of cIMT as a “soft endpoint” for modern cardiovascular trials and the use of unblinded cIMT progression measurement. In addition, the researchers noted the study was underpowered, and they used data from a type 2 diabetes mellitus cohort to perform the power calculation, which carried a 50% reduction in CVD risk factors. “We had doubts whether this high risk would apply to patients with RA and therefore used a more conservative target of 20% reduction for cIMT progression,” the researchers said.
This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
SOURCE: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
Researchers from the Netherlands found that a treat-to-target approach for cardiovascular risk factors in patients with rheumatoid arthritis was effective in reducing clinical and subclinical atherosclerosis; however, the researchers noted there was a “considerable” dropout in the study that could have limited the results, according to data recently published in Annals of the Rheumatic Diseases.
Benjamin Burggraaf, MD, and his associates at Franciscus Gasthuis & Vlietland Hospital in Rotterdam, the Netherlands, performed an open-label, randomized, controlled trial of 320 patients with rheumatoid arthritis (RA) who were younger than 70 years old (mean age, 52.4 years) without a prior history of cardiovascular disease (CVD) and diabetes mellitus. The patients received either usual care for traditional CVD risk factors or a treat-to-target approach using a prespecified protocol for treating hypertension, hyperlipidemia and hypertriglyceridemia, as well hemoglobin A1c greater than 48 mmol/mol. A total of 219 patients (68.4%) finished the study after 5 years. The two groups were similar at baseline, but the treat-to-target group had significantly more women (77.2%) compared with the usual care group (62.0%).
The researchers compared the progression of carotid intima media thickness (cIMT) at baseline and 5 years, with secondary outcomes of nonfatal myocardial infarction, nonfatal stroke, coronary artery bypass grafting, percutaneous coronary intervention, peripheral atherosclerotic arterial disease–related amputation, peripheral atherosclerotic arterial disease revascularization, and death due to cardiovascular causes.
“To account for the high dropout, all missing cIMT values after 5 years of follow-up were imputed with all available cIMT values (baseline, years 1-4) and treatment group as covariate,” the researchers said. “Assuming that unobserved cIMT values were missing at random, missing data were imputed with multiple imputation using the fully conditional specification method for seven cycles.”
Dr. Burggraaf and his colleagues found the treat-to-target group had lower mean cIMT progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up than did the group that received usual care for CVD risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028). At 5 years, there were no significant differences between treat-to-target and usual care groups regarding mean systolic blood pressure (124.6 mm Hg vs. 124.7 mm Hg, respectively; P = .97), treat-to-target treatment targets for blood pressure (72.4% for usual care vs. 75.9% for treat-to-target; P = .56) and mean HbA1c (37.6 mmol/mol for treat-to-target vs. 37.0 mmol/mol for usual care; P = .39). The treat-to-target group also had fewer cardiovascular events (two events, 1.3%) compared with the usual care group (seven events, 4.7%; P = .048). There were five patients in the treat-to-target group who died during the study (4.7%), compared with seven patients (3.2%) in the usual care group (P = .51).
“Although the difference in cIMT progression between the groups was relatively small in absolute terms, the relative reduction in progression was almost 50% in favor of the treat-to-target group,” they noted. “In light of the reduction of cardiovascular events, these effects are, in our opinion, clinically relevant.”
Other limitations of the study included the use of cIMT as a “soft endpoint” for modern cardiovascular trials and the use of unblinded cIMT progression measurement. In addition, the researchers noted the study was underpowered, and they used data from a type 2 diabetes mellitus cohort to perform the power calculation, which carried a 50% reduction in CVD risk factors. “We had doubts whether this high risk would apply to patients with RA and therefore used a more conservative target of 20% reduction for cIMT progression,” the researchers said.
This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
SOURCE: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: The treat-to-target group had greater mean carotid intima media thickness progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up compared with the group that received usual care for cardiovascular disease risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028).
Study details: An open-label, randomized, controlled trial of 320 RA patients who received a treat-to-target intervention or usual care for cardiovascular disease risk factors.
Disclosures: This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
Source: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.