User login
Higher HDL Tied to Prediabetes Reversion — Up to a Point
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
10 Reasons to Refer Your Patient to an Endocrinologist
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
Does Eliminating Alcohol Intake Lower Cancer Risk?
Dry January has come to an end — at least for those who jumped on the trendy post-holiday no-booze wagon.
The benefits of drinking less alcohol are well documented. A systematic review of 63 studies, for example, found that reducing or giving up alcohol reduced people’s risk for hospitalization, injuries, and death. The lifestyle change also improved people’s physical and mental health as well as their quality of life.
When it comes to cancer risk, however, the benefits of quitting or cutting back on alcohol remain much less clear, according to a new report from the cancer agency of the World Health Organization (WHO).
After reviewing dozens of studies, the International Agency for Research on Cancer (IARC) concluded that, for most alcohol-related cancers, there is limited evidence to support a link between eliminating or reducing alcohol consumption and lowering of cancer risk.
More specifically, the IARC Working Group, which included 15 scientists from eight countries, reported “limited” evidence on this association for laryngeal, colorectal (CRC), and breast cancer as well as «inadequate» evidence for pharyngeal and liver cancer.
The report did highlight two exceptions: Reducing or quitting alcohol was associated with a lower risk for both oral and esophageal cancer. The IARC working group based this conclusion on large studies of long-term alcohol cessation in these cancer types.
Still, the authors noted, “significant scientific gaps” exist for most alcohol-related cancers.
Take the data on CRC. Two studies found that reducing alcohol consumption did appear to lower CRC risk, while two others — which focused on the duration of quitting — did not suggest a reduced risk for CRC.
“Given the inconsistencies among studies and the few studies on duration of cessation, the Working Group concluded that there was limited evidence that alcohol reduction or cessation reduces colorectal cancer risk,” the authors wrote.
For liver cancer, the experts did note an association between quitting alcohol and lower cancer risk, but that cohort study only included individuals with alcohol-related liver disease. Outside of this study, the IARC group found no clear association between quitting drinking and liver cancer among people without alcohol-related liver disease in the other 11 studies evaluated.
For pharyngeal cancer, the evidence was limited overall, but one analysis looking at long-term cessation and oropharyngeal or hypopharyngeal cancer found a 26% lower risk (95% CI, 0.50-1.09). That association went away, however, after adjusting for detailed smoking history (odds ratio, 0.95; 95% CI, 0.56-1.61), and the working group concluded, overall, that «there was inadequate evidence that alcohol reduction or cessation reduces pharyngeal cancer risk.”
The IARC working group did find sufficient evidence linking drinking cessation and reduced risk for oral and esophageal cancers.
For instance, an international pooled analysis, which included 12 studies assessing a link between quitting smoking and alcohol and oral cancer risk, found that longer duration since quitting was associated with lower risk. Not drinking for up to 4 years was associated with a 19% lower risk for oral cancer, quitting for 5-9 years was associated with a 23% lower risk, while quitting for 20 years was associated with 55% lower risk.
“Given the consistent evidence of a reduced risk of oral cancer associated with long-term alcohol cessation,” the IARC working group concluded that there was “sufficient evidence that alcohol reduction or cessation reduces oral cancer risk.”
The working group also found “sufficient evidence from mechanistic studies that alcohol cessation reduces alcohol-related carcinogenesis.” In other words, quitting drinking appeared to reverse certain cancer-promoting biological mechanisms.
Outside the recent IARC report, some individual studies have suggested that quitting or cutting back on alcohol can reduce the risk for certain cancers.
For example, a large population-based study of about 4.5 million individuals in Korea found a lower risk for alcohol-related cancers among mild drinkers who quit (adjusted hazard ratio [aHR], 0.96) and heavy drinkers who reduced their drinking levels to mild (aHR, 0.92) or moderate (aHR, 0.91). These findings, however, may not be generalizable beyond East Asian populations.
Addressing the existing evidence gaps could help “support alcohol-control measures to reduce consumption,” the IARC working group concluded.
The Case for Limiting Alcohol
While the evidence linking reducing or stopping drinking and lower cancer risk remains limited, the opposite association is well-established — greater alcohol consumption does increase cancer risk.
A previous IARC analysis estimated that alcohol consumption accounts for about 4% of newly diagnosed cancers worldwide, most commonly esophagus, liver, and breast cancer. The IARC has even classified alcohol as a group 1 carcinogen, highlighting the strong evidence demonstrating that alcohol can cause cancer in humans.
Experts also recommend following existing guidelines for alcohol intake. Guidelines from the American Cancer Society and from the US Department of Agriculture and Department of Health and Human Services specify limiting alcohol intake to one drink or less for women and two drinks or less for men on any given day.
In a January 9, 2023, blog post, National Institute on Alcohol Abuse and Alcoholism director George F. Koob, PhD, touted the known benefits of limiting drinking.
“Research shows that even small amounts of alcohol can carry health risks, including for certain cancers and cardiovascular issues,” Dr. Koob said.
A version of this article appeared on Medscape.com.
Dry January has come to an end — at least for those who jumped on the trendy post-holiday no-booze wagon.
The benefits of drinking less alcohol are well documented. A systematic review of 63 studies, for example, found that reducing or giving up alcohol reduced people’s risk for hospitalization, injuries, and death. The lifestyle change also improved people’s physical and mental health as well as their quality of life.
When it comes to cancer risk, however, the benefits of quitting or cutting back on alcohol remain much less clear, according to a new report from the cancer agency of the World Health Organization (WHO).
After reviewing dozens of studies, the International Agency for Research on Cancer (IARC) concluded that, for most alcohol-related cancers, there is limited evidence to support a link between eliminating or reducing alcohol consumption and lowering of cancer risk.
More specifically, the IARC Working Group, which included 15 scientists from eight countries, reported “limited” evidence on this association for laryngeal, colorectal (CRC), and breast cancer as well as «inadequate» evidence for pharyngeal and liver cancer.
The report did highlight two exceptions: Reducing or quitting alcohol was associated with a lower risk for both oral and esophageal cancer. The IARC working group based this conclusion on large studies of long-term alcohol cessation in these cancer types.
Still, the authors noted, “significant scientific gaps” exist for most alcohol-related cancers.
Take the data on CRC. Two studies found that reducing alcohol consumption did appear to lower CRC risk, while two others — which focused on the duration of quitting — did not suggest a reduced risk for CRC.
“Given the inconsistencies among studies and the few studies on duration of cessation, the Working Group concluded that there was limited evidence that alcohol reduction or cessation reduces colorectal cancer risk,” the authors wrote.
For liver cancer, the experts did note an association between quitting alcohol and lower cancer risk, but that cohort study only included individuals with alcohol-related liver disease. Outside of this study, the IARC group found no clear association between quitting drinking and liver cancer among people without alcohol-related liver disease in the other 11 studies evaluated.
For pharyngeal cancer, the evidence was limited overall, but one analysis looking at long-term cessation and oropharyngeal or hypopharyngeal cancer found a 26% lower risk (95% CI, 0.50-1.09). That association went away, however, after adjusting for detailed smoking history (odds ratio, 0.95; 95% CI, 0.56-1.61), and the working group concluded, overall, that «there was inadequate evidence that alcohol reduction or cessation reduces pharyngeal cancer risk.”
The IARC working group did find sufficient evidence linking drinking cessation and reduced risk for oral and esophageal cancers.
For instance, an international pooled analysis, which included 12 studies assessing a link between quitting smoking and alcohol and oral cancer risk, found that longer duration since quitting was associated with lower risk. Not drinking for up to 4 years was associated with a 19% lower risk for oral cancer, quitting for 5-9 years was associated with a 23% lower risk, while quitting for 20 years was associated with 55% lower risk.
“Given the consistent evidence of a reduced risk of oral cancer associated with long-term alcohol cessation,” the IARC working group concluded that there was “sufficient evidence that alcohol reduction or cessation reduces oral cancer risk.”
The working group also found “sufficient evidence from mechanistic studies that alcohol cessation reduces alcohol-related carcinogenesis.” In other words, quitting drinking appeared to reverse certain cancer-promoting biological mechanisms.
Outside the recent IARC report, some individual studies have suggested that quitting or cutting back on alcohol can reduce the risk for certain cancers.
For example, a large population-based study of about 4.5 million individuals in Korea found a lower risk for alcohol-related cancers among mild drinkers who quit (adjusted hazard ratio [aHR], 0.96) and heavy drinkers who reduced their drinking levels to mild (aHR, 0.92) or moderate (aHR, 0.91). These findings, however, may not be generalizable beyond East Asian populations.
Addressing the existing evidence gaps could help “support alcohol-control measures to reduce consumption,” the IARC working group concluded.
The Case for Limiting Alcohol
While the evidence linking reducing or stopping drinking and lower cancer risk remains limited, the opposite association is well-established — greater alcohol consumption does increase cancer risk.
A previous IARC analysis estimated that alcohol consumption accounts for about 4% of newly diagnosed cancers worldwide, most commonly esophagus, liver, and breast cancer. The IARC has even classified alcohol as a group 1 carcinogen, highlighting the strong evidence demonstrating that alcohol can cause cancer in humans.
Experts also recommend following existing guidelines for alcohol intake. Guidelines from the American Cancer Society and from the US Department of Agriculture and Department of Health and Human Services specify limiting alcohol intake to one drink or less for women and two drinks or less for men on any given day.
In a January 9, 2023, blog post, National Institute on Alcohol Abuse and Alcoholism director George F. Koob, PhD, touted the known benefits of limiting drinking.
“Research shows that even small amounts of alcohol can carry health risks, including for certain cancers and cardiovascular issues,” Dr. Koob said.
A version of this article appeared on Medscape.com.
Dry January has come to an end — at least for those who jumped on the trendy post-holiday no-booze wagon.
The benefits of drinking less alcohol are well documented. A systematic review of 63 studies, for example, found that reducing or giving up alcohol reduced people’s risk for hospitalization, injuries, and death. The lifestyle change also improved people’s physical and mental health as well as their quality of life.
When it comes to cancer risk, however, the benefits of quitting or cutting back on alcohol remain much less clear, according to a new report from the cancer agency of the World Health Organization (WHO).
After reviewing dozens of studies, the International Agency for Research on Cancer (IARC) concluded that, for most alcohol-related cancers, there is limited evidence to support a link between eliminating or reducing alcohol consumption and lowering of cancer risk.
More specifically, the IARC Working Group, which included 15 scientists from eight countries, reported “limited” evidence on this association for laryngeal, colorectal (CRC), and breast cancer as well as «inadequate» evidence for pharyngeal and liver cancer.
The report did highlight two exceptions: Reducing or quitting alcohol was associated with a lower risk for both oral and esophageal cancer. The IARC working group based this conclusion on large studies of long-term alcohol cessation in these cancer types.
Still, the authors noted, “significant scientific gaps” exist for most alcohol-related cancers.
Take the data on CRC. Two studies found that reducing alcohol consumption did appear to lower CRC risk, while two others — which focused on the duration of quitting — did not suggest a reduced risk for CRC.
“Given the inconsistencies among studies and the few studies on duration of cessation, the Working Group concluded that there was limited evidence that alcohol reduction or cessation reduces colorectal cancer risk,” the authors wrote.
For liver cancer, the experts did note an association between quitting alcohol and lower cancer risk, but that cohort study only included individuals with alcohol-related liver disease. Outside of this study, the IARC group found no clear association between quitting drinking and liver cancer among people without alcohol-related liver disease in the other 11 studies evaluated.
For pharyngeal cancer, the evidence was limited overall, but one analysis looking at long-term cessation and oropharyngeal or hypopharyngeal cancer found a 26% lower risk (95% CI, 0.50-1.09). That association went away, however, after adjusting for detailed smoking history (odds ratio, 0.95; 95% CI, 0.56-1.61), and the working group concluded, overall, that «there was inadequate evidence that alcohol reduction or cessation reduces pharyngeal cancer risk.”
The IARC working group did find sufficient evidence linking drinking cessation and reduced risk for oral and esophageal cancers.
For instance, an international pooled analysis, which included 12 studies assessing a link between quitting smoking and alcohol and oral cancer risk, found that longer duration since quitting was associated with lower risk. Not drinking for up to 4 years was associated with a 19% lower risk for oral cancer, quitting for 5-9 years was associated with a 23% lower risk, while quitting for 20 years was associated with 55% lower risk.
“Given the consistent evidence of a reduced risk of oral cancer associated with long-term alcohol cessation,” the IARC working group concluded that there was “sufficient evidence that alcohol reduction or cessation reduces oral cancer risk.”
The working group also found “sufficient evidence from mechanistic studies that alcohol cessation reduces alcohol-related carcinogenesis.” In other words, quitting drinking appeared to reverse certain cancer-promoting biological mechanisms.
Outside the recent IARC report, some individual studies have suggested that quitting or cutting back on alcohol can reduce the risk for certain cancers.
For example, a large population-based study of about 4.5 million individuals in Korea found a lower risk for alcohol-related cancers among mild drinkers who quit (adjusted hazard ratio [aHR], 0.96) and heavy drinkers who reduced their drinking levels to mild (aHR, 0.92) or moderate (aHR, 0.91). These findings, however, may not be generalizable beyond East Asian populations.
Addressing the existing evidence gaps could help “support alcohol-control measures to reduce consumption,” the IARC working group concluded.
The Case for Limiting Alcohol
While the evidence linking reducing or stopping drinking and lower cancer risk remains limited, the opposite association is well-established — greater alcohol consumption does increase cancer risk.
A previous IARC analysis estimated that alcohol consumption accounts for about 4% of newly diagnosed cancers worldwide, most commonly esophagus, liver, and breast cancer. The IARC has even classified alcohol as a group 1 carcinogen, highlighting the strong evidence demonstrating that alcohol can cause cancer in humans.
Experts also recommend following existing guidelines for alcohol intake. Guidelines from the American Cancer Society and from the US Department of Agriculture and Department of Health and Human Services specify limiting alcohol intake to one drink or less for women and two drinks or less for men on any given day.
In a January 9, 2023, blog post, National Institute on Alcohol Abuse and Alcoholism director George F. Koob, PhD, touted the known benefits of limiting drinking.
“Research shows that even small amounts of alcohol can carry health risks, including for certain cancers and cardiovascular issues,” Dr. Koob said.
A version of this article appeared on Medscape.com.
While Rare, Periocular Melanoma May Be Slightly Increasing
SAN DIEGO — Every year, about 4000 patients in the United States are diagnosed with periocular melanoma, according to Geva Mannor, MD, MPH.
“Though rare, the incidence is thought to be slightly increasing, while the onset tends to occur in patients over age 40 years,” Dr. Mannor, an oculofacial plastic surgeon in the division of ophthalmology at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “It may be more common in males and welding is a risk factor. Pain and vision loss are late symptoms, and there are amelanotic variants. Gene expression profiling and other genetic testing can predict metastasis, especially expression of BRCA1-associated protein 1 (BAP1).”
. Extrapolating from the best available data, Dr. Mannor said that the annual incidence of choroidal melanoma in the United States is less than 2500, the annual incidence of eyelid melanoma is less than 750, and the annual incidence of conjunctival melanoma is less than 250 — much lower than for cutaneous melanomas. Put another way, the ratio between cutaneous and choroid melanoma is 80:1, the ratio between cutaneous and eyelid melanoma is 266:1, and the ratio between cutaneous and conjunctival melanoma is 800:1.
According to an article published in 2021 on the topic, risk factors for periocular melanoma include light eye color (blue/gray; relative risk, 1.75), fair skin (RR, 1.80), and inability to tan (RR, 1.64), but not blonde hair. A review of 210 patients with melanoma of the eyelid from 11 studies showed that 57% were located on the lower lid, 13% were on the upper lid (“I think because the brow protects sun exposure to the upper lid,” Dr. Mannor said), 12% were on the brow, 10% were on the lateral canthus, and 2% were on the medial canthus. In addition, 35% of the eyelid melanomas were superficial spreading cases, 31% were lentigo maligna, and 19% were nodular. The mean Breslow depth was 1.36 mm and the mortality rate was 4.9%.
Dr. Mannor said that cheek and brow melanomas can extend to the inside of the eyelid and conjunctiva. “Therefore, you want to examine the inside of upper and lower eyelids,” he said at the meeting, which was hosted by the Scripps Cancer Center. “Margin-control excision of lid melanoma is the standard treatment.”
On a related note, he said that glaucoma eye drops that contain prostaglandin F2alpha induce cutaneous and iris pigmentation with varying rates depending on the specific type of prostaglandin. “There have been case reports of these eye drops causing periocular pigmentation that masquerades as suspicious, melanoma-like skin cancer, often necessitating a skin biopsy,” he said.
Dr. Mannor reported having no disclosures.
SAN DIEGO — Every year, about 4000 patients in the United States are diagnosed with periocular melanoma, according to Geva Mannor, MD, MPH.
“Though rare, the incidence is thought to be slightly increasing, while the onset tends to occur in patients over age 40 years,” Dr. Mannor, an oculofacial plastic surgeon in the division of ophthalmology at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “It may be more common in males and welding is a risk factor. Pain and vision loss are late symptoms, and there are amelanotic variants. Gene expression profiling and other genetic testing can predict metastasis, especially expression of BRCA1-associated protein 1 (BAP1).”
. Extrapolating from the best available data, Dr. Mannor said that the annual incidence of choroidal melanoma in the United States is less than 2500, the annual incidence of eyelid melanoma is less than 750, and the annual incidence of conjunctival melanoma is less than 250 — much lower than for cutaneous melanomas. Put another way, the ratio between cutaneous and choroid melanoma is 80:1, the ratio between cutaneous and eyelid melanoma is 266:1, and the ratio between cutaneous and conjunctival melanoma is 800:1.
According to an article published in 2021 on the topic, risk factors for periocular melanoma include light eye color (blue/gray; relative risk, 1.75), fair skin (RR, 1.80), and inability to tan (RR, 1.64), but not blonde hair. A review of 210 patients with melanoma of the eyelid from 11 studies showed that 57% were located on the lower lid, 13% were on the upper lid (“I think because the brow protects sun exposure to the upper lid,” Dr. Mannor said), 12% were on the brow, 10% were on the lateral canthus, and 2% were on the medial canthus. In addition, 35% of the eyelid melanomas were superficial spreading cases, 31% were lentigo maligna, and 19% were nodular. The mean Breslow depth was 1.36 mm and the mortality rate was 4.9%.
Dr. Mannor said that cheek and brow melanomas can extend to the inside of the eyelid and conjunctiva. “Therefore, you want to examine the inside of upper and lower eyelids,” he said at the meeting, which was hosted by the Scripps Cancer Center. “Margin-control excision of lid melanoma is the standard treatment.”
On a related note, he said that glaucoma eye drops that contain prostaglandin F2alpha induce cutaneous and iris pigmentation with varying rates depending on the specific type of prostaglandin. “There have been case reports of these eye drops causing periocular pigmentation that masquerades as suspicious, melanoma-like skin cancer, often necessitating a skin biopsy,” he said.
Dr. Mannor reported having no disclosures.
SAN DIEGO — Every year, about 4000 patients in the United States are diagnosed with periocular melanoma, according to Geva Mannor, MD, MPH.
“Though rare, the incidence is thought to be slightly increasing, while the onset tends to occur in patients over age 40 years,” Dr. Mannor, an oculofacial plastic surgeon in the division of ophthalmology at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “It may be more common in males and welding is a risk factor. Pain and vision loss are late symptoms, and there are amelanotic variants. Gene expression profiling and other genetic testing can predict metastasis, especially expression of BRCA1-associated protein 1 (BAP1).”
. Extrapolating from the best available data, Dr. Mannor said that the annual incidence of choroidal melanoma in the United States is less than 2500, the annual incidence of eyelid melanoma is less than 750, and the annual incidence of conjunctival melanoma is less than 250 — much lower than for cutaneous melanomas. Put another way, the ratio between cutaneous and choroid melanoma is 80:1, the ratio between cutaneous and eyelid melanoma is 266:1, and the ratio between cutaneous and conjunctival melanoma is 800:1.
According to an article published in 2021 on the topic, risk factors for periocular melanoma include light eye color (blue/gray; relative risk, 1.75), fair skin (RR, 1.80), and inability to tan (RR, 1.64), but not blonde hair. A review of 210 patients with melanoma of the eyelid from 11 studies showed that 57% were located on the lower lid, 13% were on the upper lid (“I think because the brow protects sun exposure to the upper lid,” Dr. Mannor said), 12% were on the brow, 10% were on the lateral canthus, and 2% were on the medial canthus. In addition, 35% of the eyelid melanomas were superficial spreading cases, 31% were lentigo maligna, and 19% were nodular. The mean Breslow depth was 1.36 mm and the mortality rate was 4.9%.
Dr. Mannor said that cheek and brow melanomas can extend to the inside of the eyelid and conjunctiva. “Therefore, you want to examine the inside of upper and lower eyelids,” he said at the meeting, which was hosted by the Scripps Cancer Center. “Margin-control excision of lid melanoma is the standard treatment.”
On a related note, he said that glaucoma eye drops that contain prostaglandin F2alpha induce cutaneous and iris pigmentation with varying rates depending on the specific type of prostaglandin. “There have been case reports of these eye drops causing periocular pigmentation that masquerades as suspicious, melanoma-like skin cancer, often necessitating a skin biopsy,” he said.
Dr. Mannor reported having no disclosures.
FROM MELANOMA 2024
A Neurotoxin, an Antidepressant, and More Emerging Options for Treating Rosacea
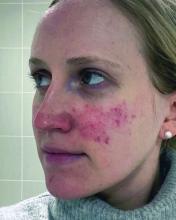
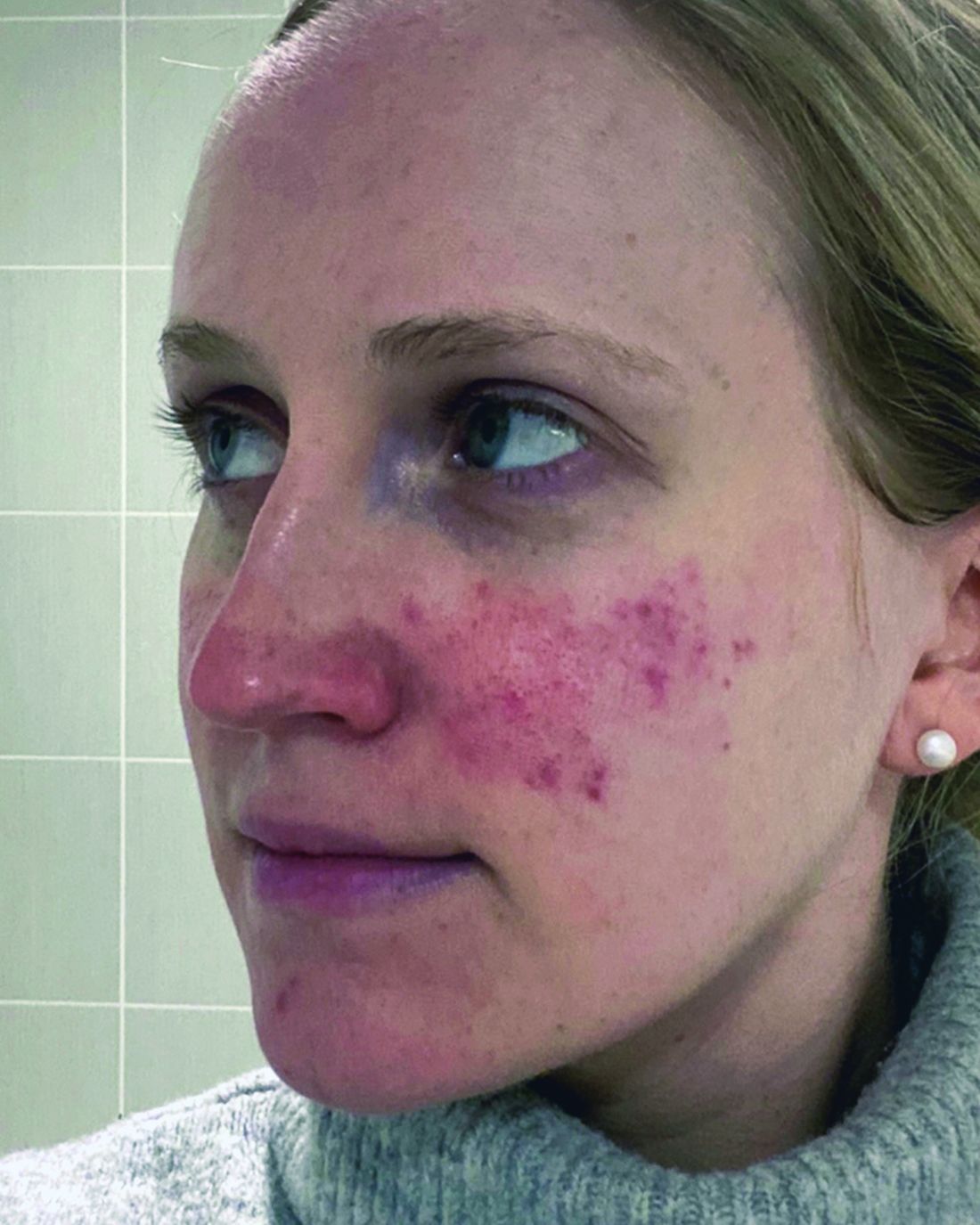
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
FROM ODAC 2024
RNA Vaccines: Risk for Heavy Menstrual Bleeding Clarified
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Automated ADR Software Shows Promise
, according to investigators.
The new software, which automatically integrates endoscopy and pathology reports across a variety of practice settings, delivered an ADR on par with manual review, supporting its accuracy and feasibility for real-world usage, reported Todd A. Brenner, MD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“ADR calculation is resource-intensive, often requiring manual collation of endoscopy and pathology data across multiple reporting modalities, making it an impractical tool for frequent quality audits at many centers,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
Although others have tried to streamline ADR calculation, most efforts have relied upon manual entry of pathology data, while approaches using artificial intelligence tend to be costly and clumsy to implement across different databases, according to the investigators.
“Thus, there is a substantial demand for a novel tool to extract and analyze colonoscopy indicators from text-based reports that provides accurate data extraction in a package that is easily implemented and modified by clinicians,” they wrote.
Dr. Brenner and colleagues developed a web-based platform to meet these goals.
Following colonoscopy, the system gathers procedural and histopathology results, extracts and classifies relevant data, then outputs ADR, along with cecal intubation rate, Boston Bowel Preparation Score (BBPS), and withdrawal time.
The software was evaluated using endoscopy and pathology reports from 3,809 colonoscopies performed at six centers over 3 months. Six months later, the investigators manually reviewed data from a validation cohort of 1384 colonoscopies conducted over a 1-month period.
Comparing the automated versus manual approach revealed high congruity, with an ADR of 45.1% for the automated system vs 44.3% for manual review. The software also correctly identified most ADR-qualifying screening colonoscopies (sensitivity, 0.918; specificity, 1.0).
“The discrepancy between manual and automated ADR calculations was exclusively attributable to missed (i.e., false negative) identification of ADR-qualifying procedures,” the investigators wrote.
Of these 43 mislabeled cases, about half involved pending pathology results or erroneous pathology sample entries, while the remainder were due to spelling and/or syntax issues that stumped the system.
Still, Dr. Brenner and colleagues suggested that additional programming can overcome these kinds of issues and allow for generalizability across institutions. They noted that search terms can be edited to match local practice patterns, while the web-based reporting platform can be customized to deliver desired quality metrics.
The publication includes a screenshot of one such dashboard, including a readout of ADR, a comparison of ADR across sexes, a pie chart of BBPS score distribution, and gauge charts for cecal intubation rate and mean withdrawal time.
“Further development of this Internet-based colonoscopy quality reporting platform will focus on integrating additional metrics, such as adenomas per colonoscopy, as well as novel metrics, such as a size-stratified ADR, location-stratified ADR, or ADR stratified by polyp histology,” the investigators wrote.
They predicted that automating data collection in this way could help determine which metrics provide clinically meaningful insights, potentially expanding the roster of standard performance benchmarks.
“We further intend to study the integration of this platform into colonoscopy quality improvement and transparency programs to better characterize the impact of frequent, on-demand ADR feedback on colonoscopy performance,” Dr. Brenner and colleagues concluded.The investigators disclosed relationships with Olympus, Medtronic, Apollo Endosurgery, and others.
Adenoma detection rate (ADR) has proven to be a useful metric for the evaluation of quality in screening colonoscopies. Outside of its proven inverse associations with interval colon cancer, ADR also can facilitate quality improvement interventions aimed at improving colonoscopy quality among low performing endoscopists. By focusing on this metric, healthcare providers can identify areas for improvement, ensuring a higher standard of care and ensuring maximum benefit of screening colonoscopies for patients.
Brenner and colleagues describe an automated system importing smart-phrase–based pathology reports into the endoscopy reporting software allowing for the subsequent calculation of an endoscopist-specific ADR. The automated reporting system provided a high level of agreement against manual review and correlated with average withdrawal time. Additional available quality metrics included cecal intubation rate and individual endoscopist procedural volumes.
The added methodology for developing endoscopist and site-specific ADR is an exciting and potentially more generalizable tool that will allow for widespread adoption of this quality metric. Site-specific data limitations and the use of smart-phrase–based reporting systems may limit the utility of this methodology, but it can also encourage more uniform reporting in pathologic and endoscopic reports. Regular service intervals may be required to inspect the quality of the reporting when initially implementing systems at a variety of practice settings.
Vijaya L. Rao, MD, is Assistant Professor of Medicine in the Division of Digestive Diseases & Nutrition at Rush University Medical Center, Chicago. She reports no conflicts of interest.
Adenoma detection rate (ADR) has proven to be a useful metric for the evaluation of quality in screening colonoscopies. Outside of its proven inverse associations with interval colon cancer, ADR also can facilitate quality improvement interventions aimed at improving colonoscopy quality among low performing endoscopists. By focusing on this metric, healthcare providers can identify areas for improvement, ensuring a higher standard of care and ensuring maximum benefit of screening colonoscopies for patients.
Brenner and colleagues describe an automated system importing smart-phrase–based pathology reports into the endoscopy reporting software allowing for the subsequent calculation of an endoscopist-specific ADR. The automated reporting system provided a high level of agreement against manual review and correlated with average withdrawal time. Additional available quality metrics included cecal intubation rate and individual endoscopist procedural volumes.
The added methodology for developing endoscopist and site-specific ADR is an exciting and potentially more generalizable tool that will allow for widespread adoption of this quality metric. Site-specific data limitations and the use of smart-phrase–based reporting systems may limit the utility of this methodology, but it can also encourage more uniform reporting in pathologic and endoscopic reports. Regular service intervals may be required to inspect the quality of the reporting when initially implementing systems at a variety of practice settings.
Vijaya L. Rao, MD, is Assistant Professor of Medicine in the Division of Digestive Diseases & Nutrition at Rush University Medical Center, Chicago. She reports no conflicts of interest.
Adenoma detection rate (ADR) has proven to be a useful metric for the evaluation of quality in screening colonoscopies. Outside of its proven inverse associations with interval colon cancer, ADR also can facilitate quality improvement interventions aimed at improving colonoscopy quality among low performing endoscopists. By focusing on this metric, healthcare providers can identify areas for improvement, ensuring a higher standard of care and ensuring maximum benefit of screening colonoscopies for patients.
Brenner and colleagues describe an automated system importing smart-phrase–based pathology reports into the endoscopy reporting software allowing for the subsequent calculation of an endoscopist-specific ADR. The automated reporting system provided a high level of agreement against manual review and correlated with average withdrawal time. Additional available quality metrics included cecal intubation rate and individual endoscopist procedural volumes.
The added methodology for developing endoscopist and site-specific ADR is an exciting and potentially more generalizable tool that will allow for widespread adoption of this quality metric. Site-specific data limitations and the use of smart-phrase–based reporting systems may limit the utility of this methodology, but it can also encourage more uniform reporting in pathologic and endoscopic reports. Regular service intervals may be required to inspect the quality of the reporting when initially implementing systems at a variety of practice settings.
Vijaya L. Rao, MD, is Assistant Professor of Medicine in the Division of Digestive Diseases & Nutrition at Rush University Medical Center, Chicago. She reports no conflicts of interest.
, according to investigators.
The new software, which automatically integrates endoscopy and pathology reports across a variety of practice settings, delivered an ADR on par with manual review, supporting its accuracy and feasibility for real-world usage, reported Todd A. Brenner, MD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“ADR calculation is resource-intensive, often requiring manual collation of endoscopy and pathology data across multiple reporting modalities, making it an impractical tool for frequent quality audits at many centers,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
Although others have tried to streamline ADR calculation, most efforts have relied upon manual entry of pathology data, while approaches using artificial intelligence tend to be costly and clumsy to implement across different databases, according to the investigators.
“Thus, there is a substantial demand for a novel tool to extract and analyze colonoscopy indicators from text-based reports that provides accurate data extraction in a package that is easily implemented and modified by clinicians,” they wrote.
Dr. Brenner and colleagues developed a web-based platform to meet these goals.
Following colonoscopy, the system gathers procedural and histopathology results, extracts and classifies relevant data, then outputs ADR, along with cecal intubation rate, Boston Bowel Preparation Score (BBPS), and withdrawal time.
The software was evaluated using endoscopy and pathology reports from 3,809 colonoscopies performed at six centers over 3 months. Six months later, the investigators manually reviewed data from a validation cohort of 1384 colonoscopies conducted over a 1-month period.
Comparing the automated versus manual approach revealed high congruity, with an ADR of 45.1% for the automated system vs 44.3% for manual review. The software also correctly identified most ADR-qualifying screening colonoscopies (sensitivity, 0.918; specificity, 1.0).
“The discrepancy between manual and automated ADR calculations was exclusively attributable to missed (i.e., false negative) identification of ADR-qualifying procedures,” the investigators wrote.
Of these 43 mislabeled cases, about half involved pending pathology results or erroneous pathology sample entries, while the remainder were due to spelling and/or syntax issues that stumped the system.
Still, Dr. Brenner and colleagues suggested that additional programming can overcome these kinds of issues and allow for generalizability across institutions. They noted that search terms can be edited to match local practice patterns, while the web-based reporting platform can be customized to deliver desired quality metrics.
The publication includes a screenshot of one such dashboard, including a readout of ADR, a comparison of ADR across sexes, a pie chart of BBPS score distribution, and gauge charts for cecal intubation rate and mean withdrawal time.
“Further development of this Internet-based colonoscopy quality reporting platform will focus on integrating additional metrics, such as adenomas per colonoscopy, as well as novel metrics, such as a size-stratified ADR, location-stratified ADR, or ADR stratified by polyp histology,” the investigators wrote.
They predicted that automating data collection in this way could help determine which metrics provide clinically meaningful insights, potentially expanding the roster of standard performance benchmarks.
“We further intend to study the integration of this platform into colonoscopy quality improvement and transparency programs to better characterize the impact of frequent, on-demand ADR feedback on colonoscopy performance,” Dr. Brenner and colleagues concluded.The investigators disclosed relationships with Olympus, Medtronic, Apollo Endosurgery, and others.
, according to investigators.
The new software, which automatically integrates endoscopy and pathology reports across a variety of practice settings, delivered an ADR on par with manual review, supporting its accuracy and feasibility for real-world usage, reported Todd A. Brenner, MD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“ADR calculation is resource-intensive, often requiring manual collation of endoscopy and pathology data across multiple reporting modalities, making it an impractical tool for frequent quality audits at many centers,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
Although others have tried to streamline ADR calculation, most efforts have relied upon manual entry of pathology data, while approaches using artificial intelligence tend to be costly and clumsy to implement across different databases, according to the investigators.
“Thus, there is a substantial demand for a novel tool to extract and analyze colonoscopy indicators from text-based reports that provides accurate data extraction in a package that is easily implemented and modified by clinicians,” they wrote.
Dr. Brenner and colleagues developed a web-based platform to meet these goals.
Following colonoscopy, the system gathers procedural and histopathology results, extracts and classifies relevant data, then outputs ADR, along with cecal intubation rate, Boston Bowel Preparation Score (BBPS), and withdrawal time.
The software was evaluated using endoscopy and pathology reports from 3,809 colonoscopies performed at six centers over 3 months. Six months later, the investigators manually reviewed data from a validation cohort of 1384 colonoscopies conducted over a 1-month period.
Comparing the automated versus manual approach revealed high congruity, with an ADR of 45.1% for the automated system vs 44.3% for manual review. The software also correctly identified most ADR-qualifying screening colonoscopies (sensitivity, 0.918; specificity, 1.0).
“The discrepancy between manual and automated ADR calculations was exclusively attributable to missed (i.e., false negative) identification of ADR-qualifying procedures,” the investigators wrote.
Of these 43 mislabeled cases, about half involved pending pathology results or erroneous pathology sample entries, while the remainder were due to spelling and/or syntax issues that stumped the system.
Still, Dr. Brenner and colleagues suggested that additional programming can overcome these kinds of issues and allow for generalizability across institutions. They noted that search terms can be edited to match local practice patterns, while the web-based reporting platform can be customized to deliver desired quality metrics.
The publication includes a screenshot of one such dashboard, including a readout of ADR, a comparison of ADR across sexes, a pie chart of BBPS score distribution, and gauge charts for cecal intubation rate and mean withdrawal time.
“Further development of this Internet-based colonoscopy quality reporting platform will focus on integrating additional metrics, such as adenomas per colonoscopy, as well as novel metrics, such as a size-stratified ADR, location-stratified ADR, or ADR stratified by polyp histology,” the investigators wrote.
They predicted that automating data collection in this way could help determine which metrics provide clinically meaningful insights, potentially expanding the roster of standard performance benchmarks.
“We further intend to study the integration of this platform into colonoscopy quality improvement and transparency programs to better characterize the impact of frequent, on-demand ADR feedback on colonoscopy performance,” Dr. Brenner and colleagues concluded.The investigators disclosed relationships with Olympus, Medtronic, Apollo Endosurgery, and others.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Expert Shares Tips for Diagnosing, Managing Spitz Nevi
SAN DIEGO —
“For a pigmented Spitz nevus, we were taught to look for a starburst pattern, a central area of homogeneous pigment, and peripheral symmetrical streaks or pseudopods,” Dr. Piggott, an adult and pediatric dermatologist at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “For Spitz nevi without pigment, we were taught to look for symmetric dotted vessels.”
However, results from a retrospective study published in 2015 gave her pause in relying on dermoscopy alone for assessing Spitz nevi. Researchers from Italy, Japan, and Brazil studied excision specimens of 384 Spitzoid-looking lesions in patients 12 years and older. On histology, 86.7% were diagnosed as benign Spitz nevi and 13.3% were diagnosed as melanoma.
When the researchers looked at the dermoscopic images, many cases of atypical Spitz nevi were indistinguishable from the benign Spitz nevi. Now, Dr. Piggott said, “I respect the dermoscopy criteria, but I don’t rely solely on it.”
If a child presents with Spitzoid-looking lesion, biopsy is generally preferred to observation. “The traditional belief was that punch biopsy was preferable, followed by shave biopsy,” she said. “This is always on a case-by-case basis.”
However, results from a retrospective study of the records of 123 cases of biopsy-proven Spitz nevi with incomplete removal on biopsy suggests that the method of biopsy matters. The researchers found that the presence of residual lesion in the re-excision specimen was significantly higher when the initial biopsy was done by punch biopsy (90.9%) when compared with shave biopsy (48.9%) and formal excision (62.5%; P < .05).
“This suggests that shave may better than punch for the initial biopsy, but the study was limited by its retrospective design,” Dr. Piggott said at the meeting, which was hosted by Scripps Cancer Center. “Even today it remains controversial whether you should do a shave or punch biopsy.”
Parameters for diagnosing Spitzoid tumors that pathologists look for under the microscope are asymmetry, Clark’s level IV/V, lack of maturation, solid growth, nuclear pleomorphism, high nuclear-cytoplasmic ratio, and mitoses that are atypical, deep, or that exceed 6 mm2 in size.
In terms of treatment recommendations for children with biopsy-proven Spitz nevi, Dr. Piggott said that there is no consensus among pediatric dermatologists. If the biopsy comes back as a benign Spitz nevus, the most reasonable approach is observation, “especially if there is no clinical residue — no pigment on exam, no papule left over in the scar,” she said. “You also want to educate the family about the rare potential for transformation down the line. Monitor for recurrence and consider re-excision if recurrence occurs.”
If the initial Spitz nevus biopsy reveals any degree of atypia, excision is preferred. “In young children, you have to weigh the risks of anesthesia for removal,” she said. “If you’re unable to excise the lesion, close observation is recommended at 6 months or 1 year.”
Treatment for borderline atypical Spitz tumor is excision, she continued, but no outcomes data exist that document a survival benefit with sentinel lymph node (SLN) biopsy. “The decision on whether to do a SLN biopsy is usually made on a case-by-case basis,” Dr. Piggott said. “Nodal metastases from atypical Spitzoid tumors are not uncommon, but death from widespread disease is rare. If the SLN biopsy is positive, complete lymphadenectomy is associated with increased risk of morbidity and no evidence of increased survival. If lymph node disease is found, we in pediatric dermatology would consider referral to a pediatric oncologist for consideration of systemic therapy such as interferon or a newer immunotherapy.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO —
“For a pigmented Spitz nevus, we were taught to look for a starburst pattern, a central area of homogeneous pigment, and peripheral symmetrical streaks or pseudopods,” Dr. Piggott, an adult and pediatric dermatologist at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “For Spitz nevi without pigment, we were taught to look for symmetric dotted vessels.”
However, results from a retrospective study published in 2015 gave her pause in relying on dermoscopy alone for assessing Spitz nevi. Researchers from Italy, Japan, and Brazil studied excision specimens of 384 Spitzoid-looking lesions in patients 12 years and older. On histology, 86.7% were diagnosed as benign Spitz nevi and 13.3% were diagnosed as melanoma.
When the researchers looked at the dermoscopic images, many cases of atypical Spitz nevi were indistinguishable from the benign Spitz nevi. Now, Dr. Piggott said, “I respect the dermoscopy criteria, but I don’t rely solely on it.”
If a child presents with Spitzoid-looking lesion, biopsy is generally preferred to observation. “The traditional belief was that punch biopsy was preferable, followed by shave biopsy,” she said. “This is always on a case-by-case basis.”
However, results from a retrospective study of the records of 123 cases of biopsy-proven Spitz nevi with incomplete removal on biopsy suggests that the method of biopsy matters. The researchers found that the presence of residual lesion in the re-excision specimen was significantly higher when the initial biopsy was done by punch biopsy (90.9%) when compared with shave biopsy (48.9%) and formal excision (62.5%; P < .05).
“This suggests that shave may better than punch for the initial biopsy, but the study was limited by its retrospective design,” Dr. Piggott said at the meeting, which was hosted by Scripps Cancer Center. “Even today it remains controversial whether you should do a shave or punch biopsy.”
Parameters for diagnosing Spitzoid tumors that pathologists look for under the microscope are asymmetry, Clark’s level IV/V, lack of maturation, solid growth, nuclear pleomorphism, high nuclear-cytoplasmic ratio, and mitoses that are atypical, deep, or that exceed 6 mm2 in size.
In terms of treatment recommendations for children with biopsy-proven Spitz nevi, Dr. Piggott said that there is no consensus among pediatric dermatologists. If the biopsy comes back as a benign Spitz nevus, the most reasonable approach is observation, “especially if there is no clinical residue — no pigment on exam, no papule left over in the scar,” she said. “You also want to educate the family about the rare potential for transformation down the line. Monitor for recurrence and consider re-excision if recurrence occurs.”
If the initial Spitz nevus biopsy reveals any degree of atypia, excision is preferred. “In young children, you have to weigh the risks of anesthesia for removal,” she said. “If you’re unable to excise the lesion, close observation is recommended at 6 months or 1 year.”
Treatment for borderline atypical Spitz tumor is excision, she continued, but no outcomes data exist that document a survival benefit with sentinel lymph node (SLN) biopsy. “The decision on whether to do a SLN biopsy is usually made on a case-by-case basis,” Dr. Piggott said. “Nodal metastases from atypical Spitzoid tumors are not uncommon, but death from widespread disease is rare. If the SLN biopsy is positive, complete lymphadenectomy is associated with increased risk of morbidity and no evidence of increased survival. If lymph node disease is found, we in pediatric dermatology would consider referral to a pediatric oncologist for consideration of systemic therapy such as interferon or a newer immunotherapy.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO —
“For a pigmented Spitz nevus, we were taught to look for a starburst pattern, a central area of homogeneous pigment, and peripheral symmetrical streaks or pseudopods,” Dr. Piggott, an adult and pediatric dermatologist at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “For Spitz nevi without pigment, we were taught to look for symmetric dotted vessels.”
However, results from a retrospective study published in 2015 gave her pause in relying on dermoscopy alone for assessing Spitz nevi. Researchers from Italy, Japan, and Brazil studied excision specimens of 384 Spitzoid-looking lesions in patients 12 years and older. On histology, 86.7% were diagnosed as benign Spitz nevi and 13.3% were diagnosed as melanoma.
When the researchers looked at the dermoscopic images, many cases of atypical Spitz nevi were indistinguishable from the benign Spitz nevi. Now, Dr. Piggott said, “I respect the dermoscopy criteria, but I don’t rely solely on it.”
If a child presents with Spitzoid-looking lesion, biopsy is generally preferred to observation. “The traditional belief was that punch biopsy was preferable, followed by shave biopsy,” she said. “This is always on a case-by-case basis.”
However, results from a retrospective study of the records of 123 cases of biopsy-proven Spitz nevi with incomplete removal on biopsy suggests that the method of biopsy matters. The researchers found that the presence of residual lesion in the re-excision specimen was significantly higher when the initial biopsy was done by punch biopsy (90.9%) when compared with shave biopsy (48.9%) and formal excision (62.5%; P < .05).
“This suggests that shave may better than punch for the initial biopsy, but the study was limited by its retrospective design,” Dr. Piggott said at the meeting, which was hosted by Scripps Cancer Center. “Even today it remains controversial whether you should do a shave or punch biopsy.”
Parameters for diagnosing Spitzoid tumors that pathologists look for under the microscope are asymmetry, Clark’s level IV/V, lack of maturation, solid growth, nuclear pleomorphism, high nuclear-cytoplasmic ratio, and mitoses that are atypical, deep, or that exceed 6 mm2 in size.
In terms of treatment recommendations for children with biopsy-proven Spitz nevi, Dr. Piggott said that there is no consensus among pediatric dermatologists. If the biopsy comes back as a benign Spitz nevus, the most reasonable approach is observation, “especially if there is no clinical residue — no pigment on exam, no papule left over in the scar,” she said. “You also want to educate the family about the rare potential for transformation down the line. Monitor for recurrence and consider re-excision if recurrence occurs.”
If the initial Spitz nevus biopsy reveals any degree of atypia, excision is preferred. “In young children, you have to weigh the risks of anesthesia for removal,” she said. “If you’re unable to excise the lesion, close observation is recommended at 6 months or 1 year.”
Treatment for borderline atypical Spitz tumor is excision, she continued, but no outcomes data exist that document a survival benefit with sentinel lymph node (SLN) biopsy. “The decision on whether to do a SLN biopsy is usually made on a case-by-case basis,” Dr. Piggott said. “Nodal metastases from atypical Spitzoid tumors are not uncommon, but death from widespread disease is rare. If the SLN biopsy is positive, complete lymphadenectomy is associated with increased risk of morbidity and no evidence of increased survival. If lymph node disease is found, we in pediatric dermatology would consider referral to a pediatric oncologist for consideration of systemic therapy such as interferon or a newer immunotherapy.”
Dr. Piggott reported having no relevant disclosures.
FROM MELANOMA 2024
Disparities Seen in Weight Loss Drug Prescriptions, Fills
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
GLP-1s for Obesity: Your Questions Answered
The arrival of GLP-1 receptor agonists has revolutionized treatment options for people with obesity and medical practice.
This news organization recently hosted a panel of experts across specialties — including endocrinology, gastroenterology, and obesity medicine — to discuss these potentially life-changing medications and to answer questions from the audience.
Because of the flood of queries from our audience, we asked our panelists to address some of the questions that didn’t make the recording. Their answers are below.
Beverly Tchang, MD, endocrinologist, Weill Cornell Medicine, New York City
Audience member: Can you initiate glucagon-like peptide-1 agonists (GLP-1 RAs) as a primary drug in a patient with obesity and newly diagnosed type 2 diabetes?
BT: We often prescribe GLP-1 RAs to individuals with type 2 diabetes as a first-line medication. Guidelines from the American Diabetes Association are really emphasizing a patient-centered approach, and metformin may not be the best first-line medication anymore.
Audience member:
BT: GLP-1 RAs do not need to be renally dosed, but I still recommend conferring with the patient’s nephrologist because the glomerular filtration rate might decrease in the setting of dehydration. Because GLP1s suppress the thirst, not just appetite, patients can go all day without drinking water and not feel thirsty.
Michael Camilleri, MD, gastroenterologist, Mayo Clinic, Rochester, Minnesota
Audience member: Should GLP-1 RAs be held for 1 week or 4 weeks prior to surgery to reduce the patient’s risk for aspiration? And is tapering required?
MC: For a patient taking liraglutide, I would hold the drug for 1 week prior to surgery. For patients taking other GLP-1 RAs, including extended exenatide, I advise holding for between 2 and 3 weeks before the procedure. It’s also important to make sure the patient’s diabetes is well-controlled with other medications — not GLP-1 RAs — during this period.
After surgery, you can restart GLP-1 RA therapy once there is recovery of oral food intake and normal bowel function.
Audience member: Is treatment with GLP-1 RAs appropriate for a patient with a family history of colon cancer but an otherwise unremarkable medical and family history?
MC: I have not seen a contraindication to receiving GLP-1 RAs based on a family history of colorectal cancer or other malignancies. An analysis of the French national healthcare insurance system database has suggested 1-3 years use of GLP-1 RAs (exenatide, liraglutide, and dulaglutide) may be linked with increased occurrence of thyroid cancer. Data from 37 randomized controlled trials and 19 real-world studies having 16,839 patients in placebo control group, 16,550 patients in active control group, and 13,330 patients in real-world studies were analyzed in a 2023 systematic review and meta-analysis. Compared to placebo or active control treatments, occurrence of pancreatic cancer, thyroid cancer, and all neoplasms — benign, malignant, and otherwise unspecified — were similar in the semaglutide group.
Toshi Iroku-Malize, MD, MPH, MBA, FAAFP, family physician, Zucker School of Medicine, Hempstead, New York
Audience member: What do you do about elevated liver functions after starting treatment with GLP-1 RAs, and what do you do when a patient has reached their weight loss goal?
TI-M: I recommend monitoring the liver function tests, evaluating for underlying causes, such as viral hepatitis, alcohol-related damage, or problems with other medications, and consulting a gastroenterologist or liver specialist if necessary. It’s also important to discuss the risk-benefit of continuing on the GP-1 RA for that particular patient.
Audience member: What effects will GLP-1 RAs have on sleep-disordered breathing/obstructive sleep apnea (OSA)? Are you aware of any ongoing trials addressing this subject?
TI-M: GLP-1 RAs may have beneficial effects on sleep-disordered breathing and OSA through weight loss, which can lead to a reduction in excess adipose tissue, and improvements in metabolic parameters. In terms of studies, a 2023 paper addressed this question, but more research is needed.
Audience member: Is it within a psychiatric provider’s scope of practice to prescribe GLP-1 agents for the reduction of weight gain associated with psychiatric medications?
TI-M: Obesity medicine is an interdisciplinary process. Numerous medications prescribed for mental health can contribute to obesity, and psychiatrists can play a role in collaborating with a patient’s primary care provider and/or obesity medicine specialist to determine which medications can be adjusted or replaced. It is important to remember that obesity management is not just about medications. It requires managing nutrition and activity in addition to behavioral health issues and social determinants of health. If the clinician has had the training to manage these pillars and is comfortable managing this chronic illness — similar to diabetes, hypertension, and other conditions — then this is a possibility. Otherwise, team-based care is appropriate.
Holly Lofton, MD, obesity medicine, NYU Langone Health, New York City
Audience member: Can we safely use them on patients who have had bariatric surgery and regularly develop dumping syndrome?
HL: These medications can be used after bariatric surgery in patients who meet the criteria for pharmacologic treatment. If a patient is having postoperative symptoms of dumping syndrome or excessive gastrointestinal losses from vomiting or diarrhea, dietary adjustments and other methods of managing the dumping syndrome in gastric bypass patients should be initiated before considering GLP-1 RAs because these patients do not have a functioning pylorus in their alimentary tract and these drugs are not indicated to treat dumping syndrome. The first-line approach typically involves reducing the patient’s intake of simple carbohydrates but can also include medications or surgical intervention when appropriate.
Audience member: Would teaching a patient to fast intermittently while they’re on GLP-1 RAs help them preserve weight loss if they choose to wean off the medication?
HL: Personally, I feel it is best to use the titration period and the time in which the patient is actively losing weight when on GLP-1 RAs. These are the best periods to help develop an individualized treatment plan, one that includes nutrition, activity, behavior modification, and resistance training. The patient’s lifestyle plan will likely change based on their environment and other factors. Intermittent fasting can be a part of such a plan. There is no consensus as to exactly which eating pattern will help patients maintain weight once they lose the physiologic benefit of the weight loss medications. However, studies have been published that demonstrate an average weight regain of 66% or greater when patients go from taking the maximum dose of a GLP-1 RA to taking none at all. Thus, patients should still be followed closely for weight regain when they discontinue a GLP-1 RA.
A version of this article appeared on Medscape.com.
The arrival of GLP-1 receptor agonists has revolutionized treatment options for people with obesity and medical practice.
This news organization recently hosted a panel of experts across specialties — including endocrinology, gastroenterology, and obesity medicine — to discuss these potentially life-changing medications and to answer questions from the audience.
Because of the flood of queries from our audience, we asked our panelists to address some of the questions that didn’t make the recording. Their answers are below.
Beverly Tchang, MD, endocrinologist, Weill Cornell Medicine, New York City
Audience member: Can you initiate glucagon-like peptide-1 agonists (GLP-1 RAs) as a primary drug in a patient with obesity and newly diagnosed type 2 diabetes?
BT: We often prescribe GLP-1 RAs to individuals with type 2 diabetes as a first-line medication. Guidelines from the American Diabetes Association are really emphasizing a patient-centered approach, and metformin may not be the best first-line medication anymore.
Audience member:
BT: GLP-1 RAs do not need to be renally dosed, but I still recommend conferring with the patient’s nephrologist because the glomerular filtration rate might decrease in the setting of dehydration. Because GLP1s suppress the thirst, not just appetite, patients can go all day without drinking water and not feel thirsty.
Michael Camilleri, MD, gastroenterologist, Mayo Clinic, Rochester, Minnesota
Audience member: Should GLP-1 RAs be held for 1 week or 4 weeks prior to surgery to reduce the patient’s risk for aspiration? And is tapering required?
MC: For a patient taking liraglutide, I would hold the drug for 1 week prior to surgery. For patients taking other GLP-1 RAs, including extended exenatide, I advise holding for between 2 and 3 weeks before the procedure. It’s also important to make sure the patient’s diabetes is well-controlled with other medications — not GLP-1 RAs — during this period.
After surgery, you can restart GLP-1 RA therapy once there is recovery of oral food intake and normal bowel function.
Audience member: Is treatment with GLP-1 RAs appropriate for a patient with a family history of colon cancer but an otherwise unremarkable medical and family history?
MC: I have not seen a contraindication to receiving GLP-1 RAs based on a family history of colorectal cancer or other malignancies. An analysis of the French national healthcare insurance system database has suggested 1-3 years use of GLP-1 RAs (exenatide, liraglutide, and dulaglutide) may be linked with increased occurrence of thyroid cancer. Data from 37 randomized controlled trials and 19 real-world studies having 16,839 patients in placebo control group, 16,550 patients in active control group, and 13,330 patients in real-world studies were analyzed in a 2023 systematic review and meta-analysis. Compared to placebo or active control treatments, occurrence of pancreatic cancer, thyroid cancer, and all neoplasms — benign, malignant, and otherwise unspecified — were similar in the semaglutide group.
Toshi Iroku-Malize, MD, MPH, MBA, FAAFP, family physician, Zucker School of Medicine, Hempstead, New York
Audience member: What do you do about elevated liver functions after starting treatment with GLP-1 RAs, and what do you do when a patient has reached their weight loss goal?
TI-M: I recommend monitoring the liver function tests, evaluating for underlying causes, such as viral hepatitis, alcohol-related damage, or problems with other medications, and consulting a gastroenterologist or liver specialist if necessary. It’s also important to discuss the risk-benefit of continuing on the GP-1 RA for that particular patient.
Audience member: What effects will GLP-1 RAs have on sleep-disordered breathing/obstructive sleep apnea (OSA)? Are you aware of any ongoing trials addressing this subject?
TI-M: GLP-1 RAs may have beneficial effects on sleep-disordered breathing and OSA through weight loss, which can lead to a reduction in excess adipose tissue, and improvements in metabolic parameters. In terms of studies, a 2023 paper addressed this question, but more research is needed.
Audience member: Is it within a psychiatric provider’s scope of practice to prescribe GLP-1 agents for the reduction of weight gain associated with psychiatric medications?
TI-M: Obesity medicine is an interdisciplinary process. Numerous medications prescribed for mental health can contribute to obesity, and psychiatrists can play a role in collaborating with a patient’s primary care provider and/or obesity medicine specialist to determine which medications can be adjusted or replaced. It is important to remember that obesity management is not just about medications. It requires managing nutrition and activity in addition to behavioral health issues and social determinants of health. If the clinician has had the training to manage these pillars and is comfortable managing this chronic illness — similar to diabetes, hypertension, and other conditions — then this is a possibility. Otherwise, team-based care is appropriate.
Holly Lofton, MD, obesity medicine, NYU Langone Health, New York City
Audience member: Can we safely use them on patients who have had bariatric surgery and regularly develop dumping syndrome?
HL: These medications can be used after bariatric surgery in patients who meet the criteria for pharmacologic treatment. If a patient is having postoperative symptoms of dumping syndrome or excessive gastrointestinal losses from vomiting or diarrhea, dietary adjustments and other methods of managing the dumping syndrome in gastric bypass patients should be initiated before considering GLP-1 RAs because these patients do not have a functioning pylorus in their alimentary tract and these drugs are not indicated to treat dumping syndrome. The first-line approach typically involves reducing the patient’s intake of simple carbohydrates but can also include medications or surgical intervention when appropriate.
Audience member: Would teaching a patient to fast intermittently while they’re on GLP-1 RAs help them preserve weight loss if they choose to wean off the medication?
HL: Personally, I feel it is best to use the titration period and the time in which the patient is actively losing weight when on GLP-1 RAs. These are the best periods to help develop an individualized treatment plan, one that includes nutrition, activity, behavior modification, and resistance training. The patient’s lifestyle plan will likely change based on their environment and other factors. Intermittent fasting can be a part of such a plan. There is no consensus as to exactly which eating pattern will help patients maintain weight once they lose the physiologic benefit of the weight loss medications. However, studies have been published that demonstrate an average weight regain of 66% or greater when patients go from taking the maximum dose of a GLP-1 RA to taking none at all. Thus, patients should still be followed closely for weight regain when they discontinue a GLP-1 RA.
A version of this article appeared on Medscape.com.
The arrival of GLP-1 receptor agonists has revolutionized treatment options for people with obesity and medical practice.
This news organization recently hosted a panel of experts across specialties — including endocrinology, gastroenterology, and obesity medicine — to discuss these potentially life-changing medications and to answer questions from the audience.
Because of the flood of queries from our audience, we asked our panelists to address some of the questions that didn’t make the recording. Their answers are below.
Beverly Tchang, MD, endocrinologist, Weill Cornell Medicine, New York City
Audience member: Can you initiate glucagon-like peptide-1 agonists (GLP-1 RAs) as a primary drug in a patient with obesity and newly diagnosed type 2 diabetes?
BT: We often prescribe GLP-1 RAs to individuals with type 2 diabetes as a first-line medication. Guidelines from the American Diabetes Association are really emphasizing a patient-centered approach, and metformin may not be the best first-line medication anymore.
Audience member:
BT: GLP-1 RAs do not need to be renally dosed, but I still recommend conferring with the patient’s nephrologist because the glomerular filtration rate might decrease in the setting of dehydration. Because GLP1s suppress the thirst, not just appetite, patients can go all day without drinking water and not feel thirsty.
Michael Camilleri, MD, gastroenterologist, Mayo Clinic, Rochester, Minnesota
Audience member: Should GLP-1 RAs be held for 1 week or 4 weeks prior to surgery to reduce the patient’s risk for aspiration? And is tapering required?
MC: For a patient taking liraglutide, I would hold the drug for 1 week prior to surgery. For patients taking other GLP-1 RAs, including extended exenatide, I advise holding for between 2 and 3 weeks before the procedure. It’s also important to make sure the patient’s diabetes is well-controlled with other medications — not GLP-1 RAs — during this period.
After surgery, you can restart GLP-1 RA therapy once there is recovery of oral food intake and normal bowel function.
Audience member: Is treatment with GLP-1 RAs appropriate for a patient with a family history of colon cancer but an otherwise unremarkable medical and family history?
MC: I have not seen a contraindication to receiving GLP-1 RAs based on a family history of colorectal cancer or other malignancies. An analysis of the French national healthcare insurance system database has suggested 1-3 years use of GLP-1 RAs (exenatide, liraglutide, and dulaglutide) may be linked with increased occurrence of thyroid cancer. Data from 37 randomized controlled trials and 19 real-world studies having 16,839 patients in placebo control group, 16,550 patients in active control group, and 13,330 patients in real-world studies were analyzed in a 2023 systematic review and meta-analysis. Compared to placebo or active control treatments, occurrence of pancreatic cancer, thyroid cancer, and all neoplasms — benign, malignant, and otherwise unspecified — were similar in the semaglutide group.
Toshi Iroku-Malize, MD, MPH, MBA, FAAFP, family physician, Zucker School of Medicine, Hempstead, New York
Audience member: What do you do about elevated liver functions after starting treatment with GLP-1 RAs, and what do you do when a patient has reached their weight loss goal?
TI-M: I recommend monitoring the liver function tests, evaluating for underlying causes, such as viral hepatitis, alcohol-related damage, or problems with other medications, and consulting a gastroenterologist or liver specialist if necessary. It’s also important to discuss the risk-benefit of continuing on the GP-1 RA for that particular patient.
Audience member: What effects will GLP-1 RAs have on sleep-disordered breathing/obstructive sleep apnea (OSA)? Are you aware of any ongoing trials addressing this subject?
TI-M: GLP-1 RAs may have beneficial effects on sleep-disordered breathing and OSA through weight loss, which can lead to a reduction in excess adipose tissue, and improvements in metabolic parameters. In terms of studies, a 2023 paper addressed this question, but more research is needed.
Audience member: Is it within a psychiatric provider’s scope of practice to prescribe GLP-1 agents for the reduction of weight gain associated with psychiatric medications?
TI-M: Obesity medicine is an interdisciplinary process. Numerous medications prescribed for mental health can contribute to obesity, and psychiatrists can play a role in collaborating with a patient’s primary care provider and/or obesity medicine specialist to determine which medications can be adjusted or replaced. It is important to remember that obesity management is not just about medications. It requires managing nutrition and activity in addition to behavioral health issues and social determinants of health. If the clinician has had the training to manage these pillars and is comfortable managing this chronic illness — similar to diabetes, hypertension, and other conditions — then this is a possibility. Otherwise, team-based care is appropriate.
Holly Lofton, MD, obesity medicine, NYU Langone Health, New York City
Audience member: Can we safely use them on patients who have had bariatric surgery and regularly develop dumping syndrome?
HL: These medications can be used after bariatric surgery in patients who meet the criteria for pharmacologic treatment. If a patient is having postoperative symptoms of dumping syndrome or excessive gastrointestinal losses from vomiting or diarrhea, dietary adjustments and other methods of managing the dumping syndrome in gastric bypass patients should be initiated before considering GLP-1 RAs because these patients do not have a functioning pylorus in their alimentary tract and these drugs are not indicated to treat dumping syndrome. The first-line approach typically involves reducing the patient’s intake of simple carbohydrates but can also include medications or surgical intervention when appropriate.
Audience member: Would teaching a patient to fast intermittently while they’re on GLP-1 RAs help them preserve weight loss if they choose to wean off the medication?
HL: Personally, I feel it is best to use the titration period and the time in which the patient is actively losing weight when on GLP-1 RAs. These are the best periods to help develop an individualized treatment plan, one that includes nutrition, activity, behavior modification, and resistance training. The patient’s lifestyle plan will likely change based on their environment and other factors. Intermittent fasting can be a part of such a plan. There is no consensus as to exactly which eating pattern will help patients maintain weight once they lose the physiologic benefit of the weight loss medications. However, studies have been published that demonstrate an average weight regain of 66% or greater when patients go from taking the maximum dose of a GLP-1 RA to taking none at all. Thus, patients should still be followed closely for weight regain when they discontinue a GLP-1 RA.
A version of this article appeared on Medscape.com.