User login
FDA approves Tecentriq plus Abraxane in breast cancer
The Food and Drug Administration has granted accelerated approval for the combination of atezolizumab (Tecentriq) plus nanoparticle albumin–bound paclitaxel (nab-paclitaxel; Abraxane) for the treatment of adults with unresectable locally advanced or metastatic programmed death-ligand 1 (PD-L1)–positive triple-negative breast cancer (TNBC).
This conditional approval is granted to medicines that fill an unmet medical need for serious or life-threatening diseases or conditions, but the FDA may require confirmatory trials to provide verification and description of clinical benefit to allow continued approval.
The approval is based on the phase 3 IMpassion130 trial (NCT02425891), which enrolled 902 patients with unresectable, locally advanced or metastatic TNBC who had not received prior lines of chemo for metastatic disease, according to Genentech.
The multicenter, randomized, double-blind study has been evaluating the drug combination’s efficacy, safety, and pharmacokinetics. Compared with placebo plus nab-paclitaxel, atezolizumab/nab-paclitaxel demonstrated significantly superior progression-free survival (median PFS, 7.4 months vs. 4.8 months; hazard ratio, 0.60; 95% confidence interval, 0.48-0.77; P less than .0001).
The overall survival data for the intention-to-treat population remains immature, but further data will be shared with the FDA in the future, according to Genentech.
No new safety signals were seen in the atezolizumab/nab-paclitaxel arm, and the combination’s safety appeared consistent with the known safety profiles of each medicine individually.
The most common grade 3-4 events (occurring in more than 2% of patients) in the combination arm included low red blood cells, low white blood cells, feeling tired, low blood potassium level, and pneumonia.
The most common side effects (occurring in more than 20% of patients) in the combination arm included hair loss, tingling, nausea, diarrhea, headache, low red blood cells, low white blood cells, and decreased appetite.
Atezolizumab is a monoclonal antibody that binds to the PD-L1 receptor, which could possibly lead to the reactivation of T cells; however, atezolizumab also may interact with other cells in the body. Nab-paclitaxel is an injectable suspension of the common chemotherapy drug.
The Food and Drug Administration has granted accelerated approval for the combination of atezolizumab (Tecentriq) plus nanoparticle albumin–bound paclitaxel (nab-paclitaxel; Abraxane) for the treatment of adults with unresectable locally advanced or metastatic programmed death-ligand 1 (PD-L1)–positive triple-negative breast cancer (TNBC).
This conditional approval is granted to medicines that fill an unmet medical need for serious or life-threatening diseases or conditions, but the FDA may require confirmatory trials to provide verification and description of clinical benefit to allow continued approval.
The approval is based on the phase 3 IMpassion130 trial (NCT02425891), which enrolled 902 patients with unresectable, locally advanced or metastatic TNBC who had not received prior lines of chemo for metastatic disease, according to Genentech.
The multicenter, randomized, double-blind study has been evaluating the drug combination’s efficacy, safety, and pharmacokinetics. Compared with placebo plus nab-paclitaxel, atezolizumab/nab-paclitaxel demonstrated significantly superior progression-free survival (median PFS, 7.4 months vs. 4.8 months; hazard ratio, 0.60; 95% confidence interval, 0.48-0.77; P less than .0001).
The overall survival data for the intention-to-treat population remains immature, but further data will be shared with the FDA in the future, according to Genentech.
No new safety signals were seen in the atezolizumab/nab-paclitaxel arm, and the combination’s safety appeared consistent with the known safety profiles of each medicine individually.
The most common grade 3-4 events (occurring in more than 2% of patients) in the combination arm included low red blood cells, low white blood cells, feeling tired, low blood potassium level, and pneumonia.
The most common side effects (occurring in more than 20% of patients) in the combination arm included hair loss, tingling, nausea, diarrhea, headache, low red blood cells, low white blood cells, and decreased appetite.
Atezolizumab is a monoclonal antibody that binds to the PD-L1 receptor, which could possibly lead to the reactivation of T cells; however, atezolizumab also may interact with other cells in the body. Nab-paclitaxel is an injectable suspension of the common chemotherapy drug.
The Food and Drug Administration has granted accelerated approval for the combination of atezolizumab (Tecentriq) plus nanoparticle albumin–bound paclitaxel (nab-paclitaxel; Abraxane) for the treatment of adults with unresectable locally advanced or metastatic programmed death-ligand 1 (PD-L1)–positive triple-negative breast cancer (TNBC).
This conditional approval is granted to medicines that fill an unmet medical need for serious or life-threatening diseases or conditions, but the FDA may require confirmatory trials to provide verification and description of clinical benefit to allow continued approval.
The approval is based on the phase 3 IMpassion130 trial (NCT02425891), which enrolled 902 patients with unresectable, locally advanced or metastatic TNBC who had not received prior lines of chemo for metastatic disease, according to Genentech.
The multicenter, randomized, double-blind study has been evaluating the drug combination’s efficacy, safety, and pharmacokinetics. Compared with placebo plus nab-paclitaxel, atezolizumab/nab-paclitaxel demonstrated significantly superior progression-free survival (median PFS, 7.4 months vs. 4.8 months; hazard ratio, 0.60; 95% confidence interval, 0.48-0.77; P less than .0001).
The overall survival data for the intention-to-treat population remains immature, but further data will be shared with the FDA in the future, according to Genentech.
No new safety signals were seen in the atezolizumab/nab-paclitaxel arm, and the combination’s safety appeared consistent with the known safety profiles of each medicine individually.
The most common grade 3-4 events (occurring in more than 2% of patients) in the combination arm included low red blood cells, low white blood cells, feeling tired, low blood potassium level, and pneumonia.
The most common side effects (occurring in more than 20% of patients) in the combination arm included hair loss, tingling, nausea, diarrhea, headache, low red blood cells, low white blood cells, and decreased appetite.
Atezolizumab is a monoclonal antibody that binds to the PD-L1 receptor, which could possibly lead to the reactivation of T cells; however, atezolizumab also may interact with other cells in the body. Nab-paclitaxel is an injectable suspension of the common chemotherapy drug.
February CHEST Physician story on lung screening complication risk: Further reflections
We received several emails from our engaged readership about one of our front-page stories from the February issue. In brief, there were concerns raised about how CHEST Physician characterized the findings of the recent study by Huo et al in JAMA Internal Medicine. On my repeat review of our story and the Huo manuscript, as well as several conversations with content experts both within and outside of CHEST, I agree that we did mischaracterize the findings in our write-up. While the study was not necessarily poorly conducted, there were some methodological concerns that deserved more careful consideration before putting the findings into our publication. CHEST Physician Editorial Board member M. Patricia Rivera, MD, FCCP, and past CHEST President Gerard Silvestri, MD, MS, FCCP, have kindly put together a brief discussion of the potential problems with this paper; while we will further address this in our next issue to go to print (and will likely host further conversations about this manuscript down the road), I wanted to make this expert opinion available to the readership as soon as possible.
For those of you who took the time to write in, thanks so very much!
David A. Schulman, MD, FCCP
Editor in Chief, CHEST Physician
The cover story of the February 2019 edition of CHEST Physician titled “In real-world setting, LDCT screen is linked to high complication risk” erroneously interpreted a study by Huo and colleagues recently published in JAMA Internal Medicine. The cover story states that “the study included 174,702 individuals who underwent an invasive diagnostic procedure as a result of abnormal findings on lung cancer screening and 169,808 control subjects,” “the rates of complications associated with diagnostic procedures following LDCT for lung cancer screening were substantially higher than the rates reported in clinical trials of LDCT” and that “the findings emphasize the importance of discussing the risk of adverse events and cost as part of the shared decision-making process before LDCT screening.”
One wonders if the data reported by Huo and colleagues was skewed by the lens it was presented through or by the lens through which it was interpreted. Let us first elucidate that the study by Huo and colleagues titled “Complication Rates and Downstream Medical Costs Associated with Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting” was NOT a study of patients who underwent LDCT for lung cancer screening but rather a retrospective, database cohort study from 2008-2013 of patients within the age eligible for screening (age 55 to 77) WITHOUT lung cancer, who underwent similar invasive diagnostic procedures as those performed in the NLST in non–protocol-driven community practices.
Huo et al. hypothesized that the rates of complications after invasive diagnostic procedures observed among screen-eligible patients in the general population would be higher than those reported in the NLST and tested their hypothesis by estimating the complication rate of common invasive diagnostic procedures using data from a database of procedure codes. The database did not however, provide the clinical condition or indication for the procedures, define the number of procedures required to achieve a diagnosis, or define what was the most invasive procedure performed. The authors followed patients for 1 year after their procedure and reported any complication that occurred during that period as related to that procedure. This is not the standard in reporting complications from diagnostic bronchoscopic or radiologic procedures (usually occur within 24-48 hours, or maybe days) or thoracic surgery (30-90 days). As a significant number of the complications reported in the NLST were cardiac, it would be atypical to consider a cardiac complication occurring 1 year after an invasive diagnostic procedure as a complication related to the procedure.
Although the results of the study by Huo and colleagues may not be representative of complications from invasive diagnostic procedures in patients undergoing lung cancer screening, they do show that diagnostic procedures performed in the inpatient and outpatient setting for any pulmonary abnormalities (nodules, masses, adenopathy, infiltrates) are associated with a high risk of complications. In an era of advanced technologies and an increasing aging and chronic critically-ill population, clinicians need to carefully appraise the risks that may be incurred following a diagnostic procedure for a pulmonary lesion and equally, the benefit and diagnostic yield of the procedure. Multidisciplinary discussions, particularly in high-risk patients, can provide guidance to clinical decision-making regarding which procedure will be the least invasive, safest, and most likely to render a diagnosis for the individual patient. Furthermore, we need to take into account that complication rates following procedures are likely higher in centers with a low volume of diagnostic procedures or the inability to provide a less-invasive procedure that can still provide a diagnosis. While it is easy to be critical of large database analyses because of the inherent limitations associated with constructing cohorts that can provide meaningful data, we should not ignore the trends outlined in this article, particularly as the size of the cohort is substantial.
One cannot argue about the importance of discussing the risk of potential complications and cost as part of the shared decision-making process before LDCT screening, but the increased rate of complications reported by Huo et al. should not be interpreted as the complication rate from lung cancer screening in real-world setting, for this is inaccurate and has potential to create additional barriers in lung cancer screening, already beset by barriers on multiple levels. Moreover, we must emphasize that discussions of potential risks and cost from diagnostic pulmonary procedures should not be isolated to lung cancer screening.
M. Patricia Rivera, MD, FCCP
Professor of Medicine
Division of Pulmonary and Critical Care Medicine
Co-Director, Multidisciplinary Thoracic Oncology Program
Director, Multidisciplinary Lung Cancer Screening Program
Medical Director Bronchoscopy and PFT Laboratory
University of North Carolina at Chapel Hill
Chapel Hill, NC
Gerard A. Silvestri, MD, MS, FCCP
Hillenbrand Professor of Thoracic Oncology
Vice-Chair of Medicine for Faculty Development
Division of Pulmonary and Critical Care Medicine
Medical University of South Carolina
Charleston, SC
We received several emails from our engaged readership about one of our front-page stories from the February issue. In brief, there were concerns raised about how CHEST Physician characterized the findings of the recent study by Huo et al in JAMA Internal Medicine. On my repeat review of our story and the Huo manuscript, as well as several conversations with content experts both within and outside of CHEST, I agree that we did mischaracterize the findings in our write-up. While the study was not necessarily poorly conducted, there were some methodological concerns that deserved more careful consideration before putting the findings into our publication. CHEST Physician Editorial Board member M. Patricia Rivera, MD, FCCP, and past CHEST President Gerard Silvestri, MD, MS, FCCP, have kindly put together a brief discussion of the potential problems with this paper; while we will further address this in our next issue to go to print (and will likely host further conversations about this manuscript down the road), I wanted to make this expert opinion available to the readership as soon as possible.
For those of you who took the time to write in, thanks so very much!
David A. Schulman, MD, FCCP
Editor in Chief, CHEST Physician
The cover story of the February 2019 edition of CHEST Physician titled “In real-world setting, LDCT screen is linked to high complication risk” erroneously interpreted a study by Huo and colleagues recently published in JAMA Internal Medicine. The cover story states that “the study included 174,702 individuals who underwent an invasive diagnostic procedure as a result of abnormal findings on lung cancer screening and 169,808 control subjects,” “the rates of complications associated with diagnostic procedures following LDCT for lung cancer screening were substantially higher than the rates reported in clinical trials of LDCT” and that “the findings emphasize the importance of discussing the risk of adverse events and cost as part of the shared decision-making process before LDCT screening.”
One wonders if the data reported by Huo and colleagues was skewed by the lens it was presented through or by the lens through which it was interpreted. Let us first elucidate that the study by Huo and colleagues titled “Complication Rates and Downstream Medical Costs Associated with Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting” was NOT a study of patients who underwent LDCT for lung cancer screening but rather a retrospective, database cohort study from 2008-2013 of patients within the age eligible for screening (age 55 to 77) WITHOUT lung cancer, who underwent similar invasive diagnostic procedures as those performed in the NLST in non–protocol-driven community practices.
Huo et al. hypothesized that the rates of complications after invasive diagnostic procedures observed among screen-eligible patients in the general population would be higher than those reported in the NLST and tested their hypothesis by estimating the complication rate of common invasive diagnostic procedures using data from a database of procedure codes. The database did not however, provide the clinical condition or indication for the procedures, define the number of procedures required to achieve a diagnosis, or define what was the most invasive procedure performed. The authors followed patients for 1 year after their procedure and reported any complication that occurred during that period as related to that procedure. This is not the standard in reporting complications from diagnostic bronchoscopic or radiologic procedures (usually occur within 24-48 hours, or maybe days) or thoracic surgery (30-90 days). As a significant number of the complications reported in the NLST were cardiac, it would be atypical to consider a cardiac complication occurring 1 year after an invasive diagnostic procedure as a complication related to the procedure.
Although the results of the study by Huo and colleagues may not be representative of complications from invasive diagnostic procedures in patients undergoing lung cancer screening, they do show that diagnostic procedures performed in the inpatient and outpatient setting for any pulmonary abnormalities (nodules, masses, adenopathy, infiltrates) are associated with a high risk of complications. In an era of advanced technologies and an increasing aging and chronic critically-ill population, clinicians need to carefully appraise the risks that may be incurred following a diagnostic procedure for a pulmonary lesion and equally, the benefit and diagnostic yield of the procedure. Multidisciplinary discussions, particularly in high-risk patients, can provide guidance to clinical decision-making regarding which procedure will be the least invasive, safest, and most likely to render a diagnosis for the individual patient. Furthermore, we need to take into account that complication rates following procedures are likely higher in centers with a low volume of diagnostic procedures or the inability to provide a less-invasive procedure that can still provide a diagnosis. While it is easy to be critical of large database analyses because of the inherent limitations associated with constructing cohorts that can provide meaningful data, we should not ignore the trends outlined in this article, particularly as the size of the cohort is substantial.
One cannot argue about the importance of discussing the risk of potential complications and cost as part of the shared decision-making process before LDCT screening, but the increased rate of complications reported by Huo et al. should not be interpreted as the complication rate from lung cancer screening in real-world setting, for this is inaccurate and has potential to create additional barriers in lung cancer screening, already beset by barriers on multiple levels. Moreover, we must emphasize that discussions of potential risks and cost from diagnostic pulmonary procedures should not be isolated to lung cancer screening.
M. Patricia Rivera, MD, FCCP
Professor of Medicine
Division of Pulmonary and Critical Care Medicine
Co-Director, Multidisciplinary Thoracic Oncology Program
Director, Multidisciplinary Lung Cancer Screening Program
Medical Director Bronchoscopy and PFT Laboratory
University of North Carolina at Chapel Hill
Chapel Hill, NC
Gerard A. Silvestri, MD, MS, FCCP
Hillenbrand Professor of Thoracic Oncology
Vice-Chair of Medicine for Faculty Development
Division of Pulmonary and Critical Care Medicine
Medical University of South Carolina
Charleston, SC
We received several emails from our engaged readership about one of our front-page stories from the February issue. In brief, there were concerns raised about how CHEST Physician characterized the findings of the recent study by Huo et al in JAMA Internal Medicine. On my repeat review of our story and the Huo manuscript, as well as several conversations with content experts both within and outside of CHEST, I agree that we did mischaracterize the findings in our write-up. While the study was not necessarily poorly conducted, there were some methodological concerns that deserved more careful consideration before putting the findings into our publication. CHEST Physician Editorial Board member M. Patricia Rivera, MD, FCCP, and past CHEST President Gerard Silvestri, MD, MS, FCCP, have kindly put together a brief discussion of the potential problems with this paper; while we will further address this in our next issue to go to print (and will likely host further conversations about this manuscript down the road), I wanted to make this expert opinion available to the readership as soon as possible.
For those of you who took the time to write in, thanks so very much!
David A. Schulman, MD, FCCP
Editor in Chief, CHEST Physician
The cover story of the February 2019 edition of CHEST Physician titled “In real-world setting, LDCT screen is linked to high complication risk” erroneously interpreted a study by Huo and colleagues recently published in JAMA Internal Medicine. The cover story states that “the study included 174,702 individuals who underwent an invasive diagnostic procedure as a result of abnormal findings on lung cancer screening and 169,808 control subjects,” “the rates of complications associated with diagnostic procedures following LDCT for lung cancer screening were substantially higher than the rates reported in clinical trials of LDCT” and that “the findings emphasize the importance of discussing the risk of adverse events and cost as part of the shared decision-making process before LDCT screening.”
One wonders if the data reported by Huo and colleagues was skewed by the lens it was presented through or by the lens through which it was interpreted. Let us first elucidate that the study by Huo and colleagues titled “Complication Rates and Downstream Medical Costs Associated with Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting” was NOT a study of patients who underwent LDCT for lung cancer screening but rather a retrospective, database cohort study from 2008-2013 of patients within the age eligible for screening (age 55 to 77) WITHOUT lung cancer, who underwent similar invasive diagnostic procedures as those performed in the NLST in non–protocol-driven community practices.
Huo et al. hypothesized that the rates of complications after invasive diagnostic procedures observed among screen-eligible patients in the general population would be higher than those reported in the NLST and tested their hypothesis by estimating the complication rate of common invasive diagnostic procedures using data from a database of procedure codes. The database did not however, provide the clinical condition or indication for the procedures, define the number of procedures required to achieve a diagnosis, or define what was the most invasive procedure performed. The authors followed patients for 1 year after their procedure and reported any complication that occurred during that period as related to that procedure. This is not the standard in reporting complications from diagnostic bronchoscopic or radiologic procedures (usually occur within 24-48 hours, or maybe days) or thoracic surgery (30-90 days). As a significant number of the complications reported in the NLST were cardiac, it would be atypical to consider a cardiac complication occurring 1 year after an invasive diagnostic procedure as a complication related to the procedure.
Although the results of the study by Huo and colleagues may not be representative of complications from invasive diagnostic procedures in patients undergoing lung cancer screening, they do show that diagnostic procedures performed in the inpatient and outpatient setting for any pulmonary abnormalities (nodules, masses, adenopathy, infiltrates) are associated with a high risk of complications. In an era of advanced technologies and an increasing aging and chronic critically-ill population, clinicians need to carefully appraise the risks that may be incurred following a diagnostic procedure for a pulmonary lesion and equally, the benefit and diagnostic yield of the procedure. Multidisciplinary discussions, particularly in high-risk patients, can provide guidance to clinical decision-making regarding which procedure will be the least invasive, safest, and most likely to render a diagnosis for the individual patient. Furthermore, we need to take into account that complication rates following procedures are likely higher in centers with a low volume of diagnostic procedures or the inability to provide a less-invasive procedure that can still provide a diagnosis. While it is easy to be critical of large database analyses because of the inherent limitations associated with constructing cohorts that can provide meaningful data, we should not ignore the trends outlined in this article, particularly as the size of the cohort is substantial.
One cannot argue about the importance of discussing the risk of potential complications and cost as part of the shared decision-making process before LDCT screening, but the increased rate of complications reported by Huo et al. should not be interpreted as the complication rate from lung cancer screening in real-world setting, for this is inaccurate and has potential to create additional barriers in lung cancer screening, already beset by barriers on multiple levels. Moreover, we must emphasize that discussions of potential risks and cost from diagnostic pulmonary procedures should not be isolated to lung cancer screening.
M. Patricia Rivera, MD, FCCP
Professor of Medicine
Division of Pulmonary and Critical Care Medicine
Co-Director, Multidisciplinary Thoracic Oncology Program
Director, Multidisciplinary Lung Cancer Screening Program
Medical Director Bronchoscopy and PFT Laboratory
University of North Carolina at Chapel Hill
Chapel Hill, NC
Gerard A. Silvestri, MD, MS, FCCP
Hillenbrand Professor of Thoracic Oncology
Vice-Chair of Medicine for Faculty Development
Division of Pulmonary and Critical Care Medicine
Medical University of South Carolina
Charleston, SC
Anxiety, depression compromise believability of drug-allergy testing
SAN FRANCISCO – Less than 4% of people who undergo drug-allergy testing are positive and need to avoid the drug in the future, but many patients who undergo drug-allergy testing and have a negative result cling to their allergic status and struggle with letting go.
New findings suggest that preexisting anxiety or depression plays a role in some people who refuse to believe a negative drug-allergy result, which suggests that these people may need a more tailored intervention to drug-allergy testing and its aftermath, including some type of behavioral intervention.
“Underlying anxiety and depression may reduce the effectiveness of negative drug-allergy evaluation and functional delabeling,” Christine Rukasin, MD, said while presenting a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “In the future, tailored drug-allergy evaluation, behavioral interventions, targeted follow-up communication, and patient education appear necessary to improve the sustained effectiveness of a negative drug-allergy and functional delabeling,” said Dr. Rukasin, an allergy immunology physician at Vanderbilt University in Nashville, Tenn.
The results showed that some people who undergo drug allergy testing “have a high anxiety state and don’t feel comfortable regardless of their test result,” she said in an interview. “This is not where one size fits all. We usually perform a single, oral drug challenge and then pronounce the person free of allergy if the result was negative. We need to better anticipate how effective a drug evaluation will be for someone; will they believe the result?” Individual patients, especially those with diagnosed anxiety or depression, may need multiple challenge tests, both oral and skin, before they believe a negative result, and they may also need referral to a behavioral health specialist, she said.
Dr. Rukasin and her associates ran their study with 100 people who underwent assessment at the Vanderbilt drug-allergy clinic and completed a set of questionnaires. The range of suspected drug allergies included 40% with a suspected reaction to penicillin, 22% to a sulfa-containing drug, 17% to a cephalosporin, 8% to another antibiotic, 7% to an NSAID, and the remainder to other drugs. The 100 participants included 57 people without diagnosed anxiety or depression, 31 diagnosed with anxiety, and 33 diagnosed with depression; some patients had diagnoses for both anxiety and depression.
The questionnaire results from before and after drug-allergy testing showed an apparent association between anxiety, depression, and a decreased willingness to believe the results of a negative drug-allergy test. For example, when posed with the prospect of finding out they were not allergic to the tested drug, 24% of the people with anxiety and 20% of those with depression said that they still would not take the medication if it were prescribed to them, compared with 7% of those without anxiety or depression who gave this response.
Many patients who come to the drug-allergy clinic are scared and worried. “We want to dig deeper, to better help these patients,” Dr. Rukasin said. This is the first reported study to evaluate anxiety in the setting of drug-allergy testing. Further insight into ways to improve the effectiveness of drug-allergy testing hopefully will come from additional analysis of the findings.
Dr. Rukasin had no relevant financial disclosures.
SOURCE: Rukasin C et al. J Allergy Clin Immunol. 2019 Feb;143(2):AB428.
SAN FRANCISCO – Less than 4% of people who undergo drug-allergy testing are positive and need to avoid the drug in the future, but many patients who undergo drug-allergy testing and have a negative result cling to their allergic status and struggle with letting go.
New findings suggest that preexisting anxiety or depression plays a role in some people who refuse to believe a negative drug-allergy result, which suggests that these people may need a more tailored intervention to drug-allergy testing and its aftermath, including some type of behavioral intervention.
“Underlying anxiety and depression may reduce the effectiveness of negative drug-allergy evaluation and functional delabeling,” Christine Rukasin, MD, said while presenting a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “In the future, tailored drug-allergy evaluation, behavioral interventions, targeted follow-up communication, and patient education appear necessary to improve the sustained effectiveness of a negative drug-allergy and functional delabeling,” said Dr. Rukasin, an allergy immunology physician at Vanderbilt University in Nashville, Tenn.
The results showed that some people who undergo drug allergy testing “have a high anxiety state and don’t feel comfortable regardless of their test result,” she said in an interview. “This is not where one size fits all. We usually perform a single, oral drug challenge and then pronounce the person free of allergy if the result was negative. We need to better anticipate how effective a drug evaluation will be for someone; will they believe the result?” Individual patients, especially those with diagnosed anxiety or depression, may need multiple challenge tests, both oral and skin, before they believe a negative result, and they may also need referral to a behavioral health specialist, she said.
Dr. Rukasin and her associates ran their study with 100 people who underwent assessment at the Vanderbilt drug-allergy clinic and completed a set of questionnaires. The range of suspected drug allergies included 40% with a suspected reaction to penicillin, 22% to a sulfa-containing drug, 17% to a cephalosporin, 8% to another antibiotic, 7% to an NSAID, and the remainder to other drugs. The 100 participants included 57 people without diagnosed anxiety or depression, 31 diagnosed with anxiety, and 33 diagnosed with depression; some patients had diagnoses for both anxiety and depression.
The questionnaire results from before and after drug-allergy testing showed an apparent association between anxiety, depression, and a decreased willingness to believe the results of a negative drug-allergy test. For example, when posed with the prospect of finding out they were not allergic to the tested drug, 24% of the people with anxiety and 20% of those with depression said that they still would not take the medication if it were prescribed to them, compared with 7% of those without anxiety or depression who gave this response.
Many patients who come to the drug-allergy clinic are scared and worried. “We want to dig deeper, to better help these patients,” Dr. Rukasin said. This is the first reported study to evaluate anxiety in the setting of drug-allergy testing. Further insight into ways to improve the effectiveness of drug-allergy testing hopefully will come from additional analysis of the findings.
Dr. Rukasin had no relevant financial disclosures.
SOURCE: Rukasin C et al. J Allergy Clin Immunol. 2019 Feb;143(2):AB428.
SAN FRANCISCO – Less than 4% of people who undergo drug-allergy testing are positive and need to avoid the drug in the future, but many patients who undergo drug-allergy testing and have a negative result cling to their allergic status and struggle with letting go.
New findings suggest that preexisting anxiety or depression plays a role in some people who refuse to believe a negative drug-allergy result, which suggests that these people may need a more tailored intervention to drug-allergy testing and its aftermath, including some type of behavioral intervention.
“Underlying anxiety and depression may reduce the effectiveness of negative drug-allergy evaluation and functional delabeling,” Christine Rukasin, MD, said while presenting a poster at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “In the future, tailored drug-allergy evaluation, behavioral interventions, targeted follow-up communication, and patient education appear necessary to improve the sustained effectiveness of a negative drug-allergy and functional delabeling,” said Dr. Rukasin, an allergy immunology physician at Vanderbilt University in Nashville, Tenn.
The results showed that some people who undergo drug allergy testing “have a high anxiety state and don’t feel comfortable regardless of their test result,” she said in an interview. “This is not where one size fits all. We usually perform a single, oral drug challenge and then pronounce the person free of allergy if the result was negative. We need to better anticipate how effective a drug evaluation will be for someone; will they believe the result?” Individual patients, especially those with diagnosed anxiety or depression, may need multiple challenge tests, both oral and skin, before they believe a negative result, and they may also need referral to a behavioral health specialist, she said.
Dr. Rukasin and her associates ran their study with 100 people who underwent assessment at the Vanderbilt drug-allergy clinic and completed a set of questionnaires. The range of suspected drug allergies included 40% with a suspected reaction to penicillin, 22% to a sulfa-containing drug, 17% to a cephalosporin, 8% to another antibiotic, 7% to an NSAID, and the remainder to other drugs. The 100 participants included 57 people without diagnosed anxiety or depression, 31 diagnosed with anxiety, and 33 diagnosed with depression; some patients had diagnoses for both anxiety and depression.
The questionnaire results from before and after drug-allergy testing showed an apparent association between anxiety, depression, and a decreased willingness to believe the results of a negative drug-allergy test. For example, when posed with the prospect of finding out they were not allergic to the tested drug, 24% of the people with anxiety and 20% of those with depression said that they still would not take the medication if it were prescribed to them, compared with 7% of those without anxiety or depression who gave this response.
Many patients who come to the drug-allergy clinic are scared and worried. “We want to dig deeper, to better help these patients,” Dr. Rukasin said. This is the first reported study to evaluate anxiety in the setting of drug-allergy testing. Further insight into ways to improve the effectiveness of drug-allergy testing hopefully will come from additional analysis of the findings.
Dr. Rukasin had no relevant financial disclosures.
SOURCE: Rukasin C et al. J Allergy Clin Immunol. 2019 Feb;143(2):AB428.
REPORTING FROM AAAAI 2019
Sudden-onset rash on the trunk and limbs • morbid obesity • family history of diabetes mellitus • Dx?
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
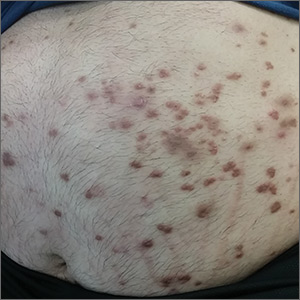
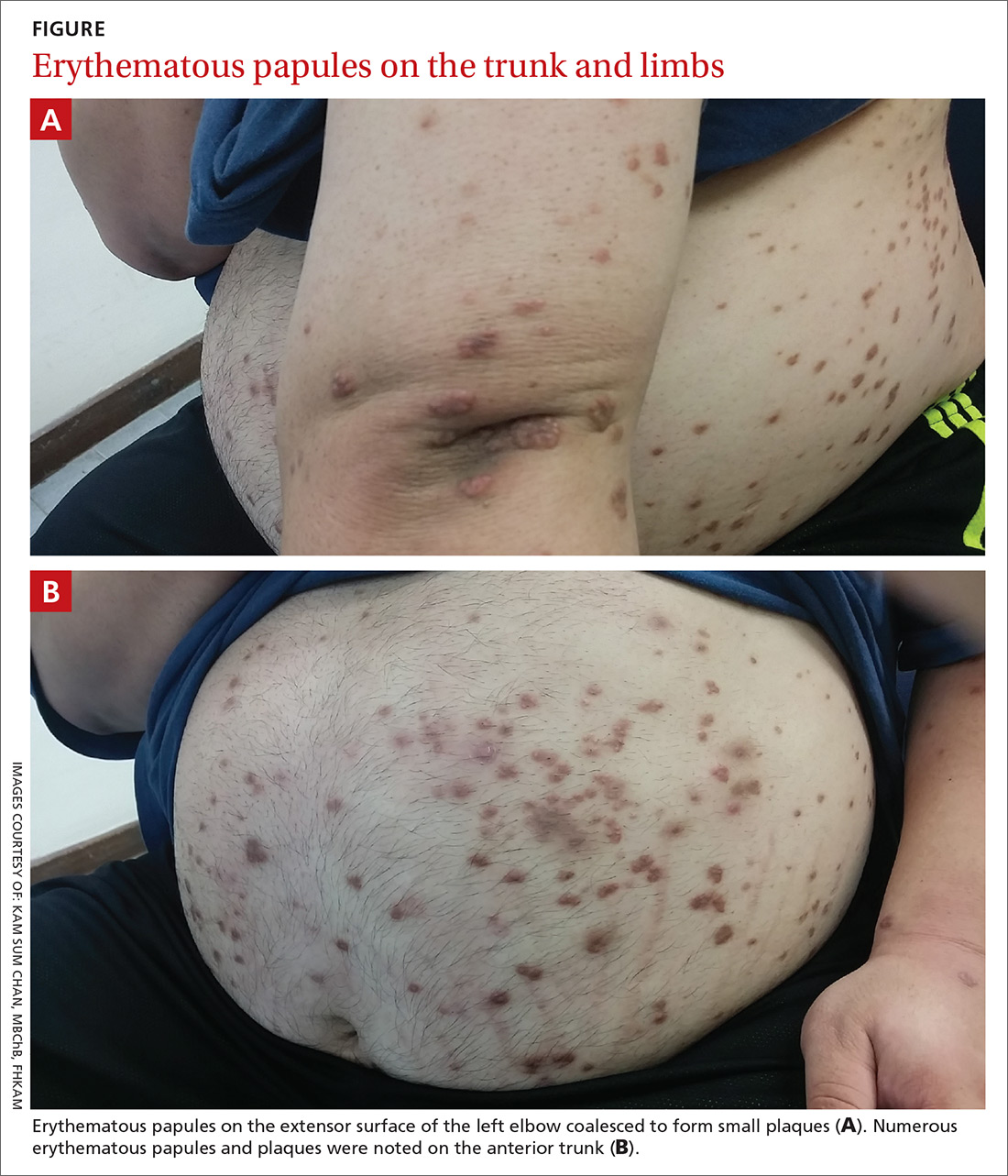
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
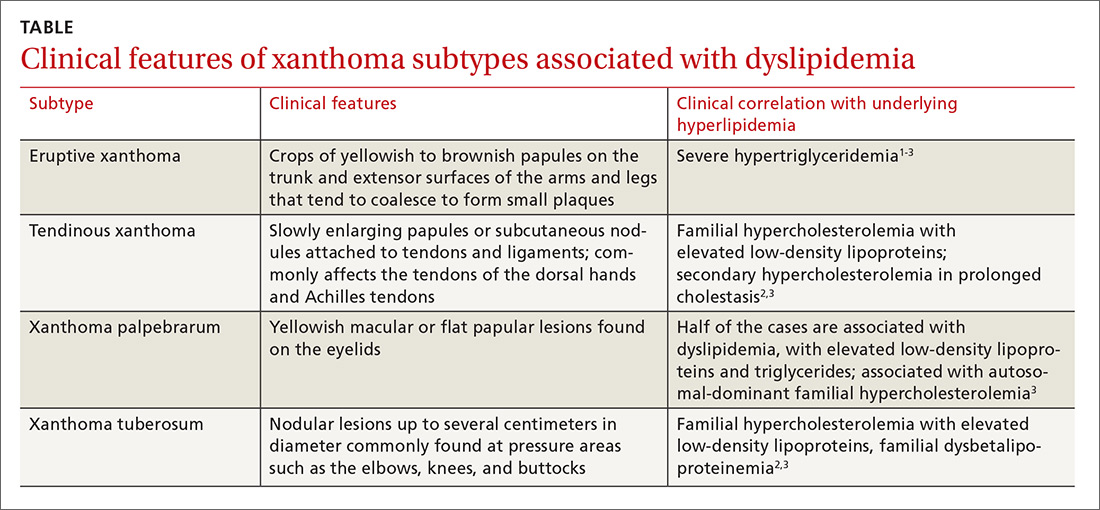
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; [email protected]
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.

THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION

Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; [email protected]
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.

THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION

Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; [email protected]
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
Bariatric surgery + medical therapy: Effective Tx for T2DM?
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
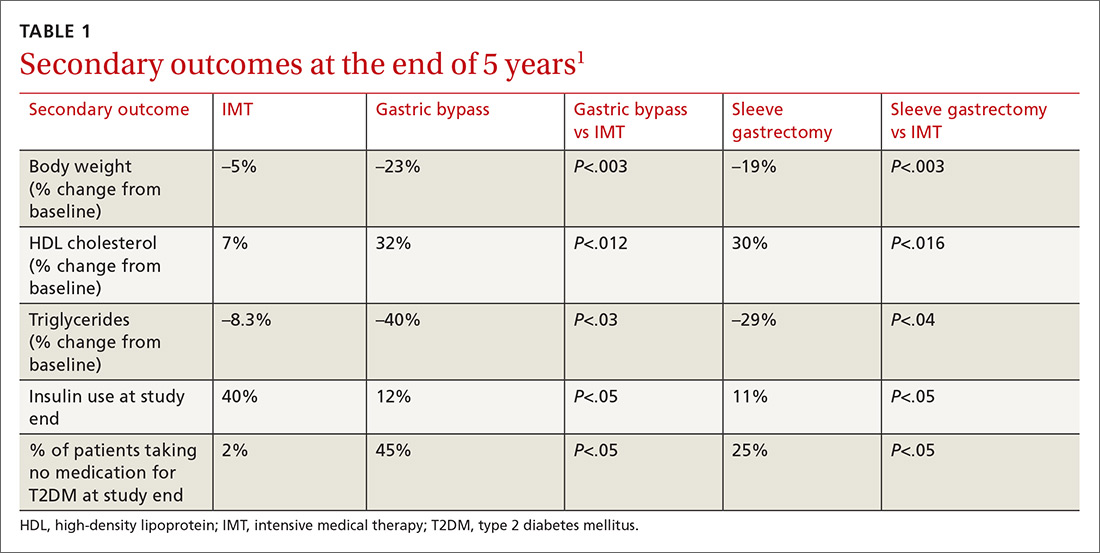
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.

WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.

WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
PRACTICE CHANGER
Consider bariatric surgery with medical therapy as a treatment option for adults with uncontrolled type 2 diabetes and a body mass index ≥27 kg/m2.1
STRENGTH OF RECOMMENDATION
B: Based on a nonblinded, single-center, randomized controlled trial.
Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
How do these 3 diabetes agents compare in reducing mortality?
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
PRACTICE CHANGER
Consider adding a sodium-glucose cotransporter 2 (SGLT-2) inhibitor or a glucagon-like peptide 1 (GLP-1) agonist to the treatment regimen of patients with poorly controlled type 2 diabetes—especially those with higher CV risk. Doing so can reduce all-cause and cardiovascular (CV) mortality 1
STRENGTH OF RECOMMENDATION
B: Based on a network meta-analysis of 236 randomized controlled trials.
Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
Rare Neurological Disease Special Report
The 5th annual Rare Neurological Disease Special Report covers a wide range of topics related to rare neurological diseases. Nearly 20 bylined articles include overviews of specific diseases, discussions of translational science, reports on trends in diagnosis and screening, advice on resources for health care professionals, snapshots of emerging therapies, and more. This year’s issue has a special focus on genetics and the potential for genetic therapy to transform the treatment of rare diseases.
The 5th annual Rare Neurological Disease Special Report covers a wide range of topics related to rare neurological diseases. Nearly 20 bylined articles include overviews of specific diseases, discussions of translational science, reports on trends in diagnosis and screening, advice on resources for health care professionals, snapshots of emerging therapies, and more. This year’s issue has a special focus on genetics and the potential for genetic therapy to transform the treatment of rare diseases.
The 5th annual Rare Neurological Disease Special Report covers a wide range of topics related to rare neurological diseases. Nearly 20 bylined articles include overviews of specific diseases, discussions of translational science, reports on trends in diagnosis and screening, advice on resources for health care professionals, snapshots of emerging therapies, and more. This year’s issue has a special focus on genetics and the potential for genetic therapy to transform the treatment of rare diseases.
Vaginal approach is the most cost-effective route
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
Five pitfalls in optimizing medical management of HFrEF
SNOWMASS, COLO. – Many of the abundant missed opportunities to optimize pharmacotherapy for heart failure with reduced ejection fraction revolve around not getting fully on board with the guideline-directed medical therapy shown to be highly effective at improving clinical outcomes, Akshay S. Desai, MD, asserted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“If you take nothing else away from this talk, is really quite profound,” declared Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, and a cardiologist at Harvard Medical School, Boston.
He highlighted five common traps or pitfalls for physicians with regard to medical therapy of patients with heart failure with reduced ejection fraction (HFrEF):
Underutilization of guideline-directed medical therapy
The current ACC/American Heart Association/Heart Failure Society of America guidelines on heart failure management (Circulation. 2017 Aug 8;136[6]:e137-61) reflect 20 years of impressive progress in improving heart failure outcomes through the use of increasingly effective guideline-directed medical therapy (GDMT). The magnitude of this improvement was nicely captured in a meta-analysis of 57 randomized controlled trials published during 1987-2015. The meta-analysis showed that, although ACE inhibitor therapy alone had no significant impact on all-cause mortality compared to placebo in patients with HFrEF, the sequential addition of guideline-directed drugs conferred stepwise improvements in survival. This approach culminated in a 56% reduction in all-cause mortality with the combination of an ACE inhibitor, beta-blocker, and mineralocorticoid receptor antagonist (MRA), compared with placebo, and a 63% reduction with an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker, and MRA (Circ Heart Fail. 2017 Jan;10(1). pii: e003529).
Moreover, the benefits of contemporary GDMT extend beyond reductions in all-cause mortality, death due to heart failure, and heart failure–related hospitalizations into areas where one wouldn’t necessarily have expected to see much benefit. For example, an analysis of data on more than 40,000 HFrEF patients in 12 clinical trials showed a sharp decline in the rate of sudden death over the years as new agents were incorporated into GDMT. The cumulative incidence of sudden death within 90 days after randomization plunged from 2.4% in the earliest trial to 1.0% in the most recent one (N Engl J Med. 2017 Jul 6;377[1]:41-51).
“We’re at the point where we now question whether routine use of implantable cardioverter-defibrillators in primary prevention patients with nonischemic heart failure is really worthwhile on the backdrop of effective medical therapy,” Dr. Desai observed.
But there’s a problem: “We don’t do a great job with GDMT, even with this incredible evidence base that we have,” the cardiologist said.
He cited a report from the CHAMP-HF registry that scrutinized the use of GDMT in more than 3,500 ambulatory HFrEF patients in 150 U.S. primary care and cardiology practices. It found that 67% of patients deemed eligible for an MRA weren’t on one. Neither were 33% with no contraindications to beta-blocker therapy and 27% who were eligible for an ACE inhibitor, angiotensin receptor blocker (ARB), or ARNI (J Am Coll Cardiol. 2018 Jul 24;72[4]:351-66).
“This highlights a huge opportunity for further guideline-directed optimization of therapy,” he said.
Underdosing of GDMT
The CHAMP-HF registry contained further disappointing news regarding the state of treatment of patients with HFrEF in ambulatory settings: Among those patients who were on GDMT, very few were receiving the recommended target doses of the medications as established in major clinical trials and specified in the guidelines. Only 14% of patients on an ARNI were on the target dose, as were 28% on a beta-blocker, and 17% of those on an ACE inhibitor or ARB. And among patients who were eligible for all classes of GDMT, just 1% were simultaneously on the target doses of an MRA, beta-blocker, and ARNI, ACE inhibitor, or ARB. This despite solid evidence that, although some benefit is derived from initiating these medications, incremental benefit comes from dose titration.
“Even for those of us who feel like we do this quite well, if we examine our practices systematically – and we’ve done this in our own practices at Brigham and Women’s – you see that a lot of eligible patients aren’t on optimal therapy. And you might argue that many of them have contraindications, but even when you do a deep dive into the literature or the electronic medical record and ask the question – Why is this patient with normal renal function and normal potassium with class II HFrEF not on an MRA? – sometimes it’s hard to establish why that’s the case,” said Dr. Desai.
Interrupting GDMT during hospitalizations
This is common practice. But in fact, continuation of GDMT is generally well tolerated in the setting of acute decompensated heart failure in the absence of severe hypotension and cardiogenic shock. Moreover, in-hospital discontinuation or dose reduction is associated with increased risks of readmission and mortality.
And in treatment-naive HFrEF patients, what better place to introduce a medication and assess its tolerability than the hospital? Plus, medications prescribed at discharge are more likely to be continued in the outpatient setting, he noted.
Being seduced by the illusion of stability
The guidelines state that patients with NYHA class II or III HFrEF who tolerate an ACE inhibitor or ARB should be transitioned to an ARNI to further reduce their risk of morbidity and mortality. Yet many physicians wait to make the switch until clinical decompensation occurs. That’s a mistake, as was demonstrated in the landmark PARADIGM-HF trial. Twenty percent of study participants without a prior hospitalization for heart failure experienced cardiovascular death or heart failure hospitalization during the follow-up period. Patients who were clinically stable as defined by no prior heart failure hospitalization or none within 3 months prior to enrollment were as likely to benefit from ARNI therapy with sacubitril/valsartan (Entresto) as were those with a recent decompensation (JACC Heart Fail. 2016 Oct;4[10]:816-22). “A key message is that stability is an illusion in patients with symptomatic heart failure,”said Dr. Desai. “In PARADIGM-HF, the first event for about half of patients was not heralded by a worsening of symptoms or a heart failure hospitalization, it was an abrupt death at home. This may mean that a missed opportunity to optimize treatment may not come back to you down the road, so waiting until patients get worse in order to optimize their therapy may not be the best strategy.”
Inadequate laboratory monitoring
The MRAs, spironolactone and eplerenone (Inspra), are the GDMT drugs for which laboratory surveillance takes on the greatest importance because of their potential to induce hyperkalemia. The guidelines are clear that a potassium level and measurement of renal function should be obtained within a week of initiating therapy with an MRA, again at 4 weeks, and periodically thereafter.
“In general, this is done in clinical practice almost never,” Dr. Desai stressed.
These agents should be avoided in patients with prior hyperkalemia or advanced chronic kidney disease, and used with care in groups known to be at increased risk for hyperkalemia, including the elderly and patients with diabetes.
He considers spironolactone equivalent to eplerenone so long as the dosing is adequate. He generally reserves eplerenone for patients with poorly tolerated antiandrogenic effects on spironolactone.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – Many of the abundant missed opportunities to optimize pharmacotherapy for heart failure with reduced ejection fraction revolve around not getting fully on board with the guideline-directed medical therapy shown to be highly effective at improving clinical outcomes, Akshay S. Desai, MD, asserted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“If you take nothing else away from this talk, is really quite profound,” declared Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, and a cardiologist at Harvard Medical School, Boston.
He highlighted five common traps or pitfalls for physicians with regard to medical therapy of patients with heart failure with reduced ejection fraction (HFrEF):
Underutilization of guideline-directed medical therapy
The current ACC/American Heart Association/Heart Failure Society of America guidelines on heart failure management (Circulation. 2017 Aug 8;136[6]:e137-61) reflect 20 years of impressive progress in improving heart failure outcomes through the use of increasingly effective guideline-directed medical therapy (GDMT). The magnitude of this improvement was nicely captured in a meta-analysis of 57 randomized controlled trials published during 1987-2015. The meta-analysis showed that, although ACE inhibitor therapy alone had no significant impact on all-cause mortality compared to placebo in patients with HFrEF, the sequential addition of guideline-directed drugs conferred stepwise improvements in survival. This approach culminated in a 56% reduction in all-cause mortality with the combination of an ACE inhibitor, beta-blocker, and mineralocorticoid receptor antagonist (MRA), compared with placebo, and a 63% reduction with an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker, and MRA (Circ Heart Fail. 2017 Jan;10(1). pii: e003529).
Moreover, the benefits of contemporary GDMT extend beyond reductions in all-cause mortality, death due to heart failure, and heart failure–related hospitalizations into areas where one wouldn’t necessarily have expected to see much benefit. For example, an analysis of data on more than 40,000 HFrEF patients in 12 clinical trials showed a sharp decline in the rate of sudden death over the years as new agents were incorporated into GDMT. The cumulative incidence of sudden death within 90 days after randomization plunged from 2.4% in the earliest trial to 1.0% in the most recent one (N Engl J Med. 2017 Jul 6;377[1]:41-51).
“We’re at the point where we now question whether routine use of implantable cardioverter-defibrillators in primary prevention patients with nonischemic heart failure is really worthwhile on the backdrop of effective medical therapy,” Dr. Desai observed.
But there’s a problem: “We don’t do a great job with GDMT, even with this incredible evidence base that we have,” the cardiologist said.
He cited a report from the CHAMP-HF registry that scrutinized the use of GDMT in more than 3,500 ambulatory HFrEF patients in 150 U.S. primary care and cardiology practices. It found that 67% of patients deemed eligible for an MRA weren’t on one. Neither were 33% with no contraindications to beta-blocker therapy and 27% who were eligible for an ACE inhibitor, angiotensin receptor blocker (ARB), or ARNI (J Am Coll Cardiol. 2018 Jul 24;72[4]:351-66).
“This highlights a huge opportunity for further guideline-directed optimization of therapy,” he said.
Underdosing of GDMT
The CHAMP-HF registry contained further disappointing news regarding the state of treatment of patients with HFrEF in ambulatory settings: Among those patients who were on GDMT, very few were receiving the recommended target doses of the medications as established in major clinical trials and specified in the guidelines. Only 14% of patients on an ARNI were on the target dose, as were 28% on a beta-blocker, and 17% of those on an ACE inhibitor or ARB. And among patients who were eligible for all classes of GDMT, just 1% were simultaneously on the target doses of an MRA, beta-blocker, and ARNI, ACE inhibitor, or ARB. This despite solid evidence that, although some benefit is derived from initiating these medications, incremental benefit comes from dose titration.
“Even for those of us who feel like we do this quite well, if we examine our practices systematically – and we’ve done this in our own practices at Brigham and Women’s – you see that a lot of eligible patients aren’t on optimal therapy. And you might argue that many of them have contraindications, but even when you do a deep dive into the literature or the electronic medical record and ask the question – Why is this patient with normal renal function and normal potassium with class II HFrEF not on an MRA? – sometimes it’s hard to establish why that’s the case,” said Dr. Desai.
Interrupting GDMT during hospitalizations
This is common practice. But in fact, continuation of GDMT is generally well tolerated in the setting of acute decompensated heart failure in the absence of severe hypotension and cardiogenic shock. Moreover, in-hospital discontinuation or dose reduction is associated with increased risks of readmission and mortality.
And in treatment-naive HFrEF patients, what better place to introduce a medication and assess its tolerability than the hospital? Plus, medications prescribed at discharge are more likely to be continued in the outpatient setting, he noted.
Being seduced by the illusion of stability
The guidelines state that patients with NYHA class II or III HFrEF who tolerate an ACE inhibitor or ARB should be transitioned to an ARNI to further reduce their risk of morbidity and mortality. Yet many physicians wait to make the switch until clinical decompensation occurs. That’s a mistake, as was demonstrated in the landmark PARADIGM-HF trial. Twenty percent of study participants without a prior hospitalization for heart failure experienced cardiovascular death or heart failure hospitalization during the follow-up period. Patients who were clinically stable as defined by no prior heart failure hospitalization or none within 3 months prior to enrollment were as likely to benefit from ARNI therapy with sacubitril/valsartan (Entresto) as were those with a recent decompensation (JACC Heart Fail. 2016 Oct;4[10]:816-22). “A key message is that stability is an illusion in patients with symptomatic heart failure,”said Dr. Desai. “In PARADIGM-HF, the first event for about half of patients was not heralded by a worsening of symptoms or a heart failure hospitalization, it was an abrupt death at home. This may mean that a missed opportunity to optimize treatment may not come back to you down the road, so waiting until patients get worse in order to optimize their therapy may not be the best strategy.”
Inadequate laboratory monitoring
The MRAs, spironolactone and eplerenone (Inspra), are the GDMT drugs for which laboratory surveillance takes on the greatest importance because of their potential to induce hyperkalemia. The guidelines are clear that a potassium level and measurement of renal function should be obtained within a week of initiating therapy with an MRA, again at 4 weeks, and periodically thereafter.
“In general, this is done in clinical practice almost never,” Dr. Desai stressed.
These agents should be avoided in patients with prior hyperkalemia or advanced chronic kidney disease, and used with care in groups known to be at increased risk for hyperkalemia, including the elderly and patients with diabetes.
He considers spironolactone equivalent to eplerenone so long as the dosing is adequate. He generally reserves eplerenone for patients with poorly tolerated antiandrogenic effects on spironolactone.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – Many of the abundant missed opportunities to optimize pharmacotherapy for heart failure with reduced ejection fraction revolve around not getting fully on board with the guideline-directed medical therapy shown to be highly effective at improving clinical outcomes, Akshay S. Desai, MD, asserted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“If you take nothing else away from this talk, is really quite profound,” declared Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, and a cardiologist at Harvard Medical School, Boston.
He highlighted five common traps or pitfalls for physicians with regard to medical therapy of patients with heart failure with reduced ejection fraction (HFrEF):
Underutilization of guideline-directed medical therapy
The current ACC/American Heart Association/Heart Failure Society of America guidelines on heart failure management (Circulation. 2017 Aug 8;136[6]:e137-61) reflect 20 years of impressive progress in improving heart failure outcomes through the use of increasingly effective guideline-directed medical therapy (GDMT). The magnitude of this improvement was nicely captured in a meta-analysis of 57 randomized controlled trials published during 1987-2015. The meta-analysis showed that, although ACE inhibitor therapy alone had no significant impact on all-cause mortality compared to placebo in patients with HFrEF, the sequential addition of guideline-directed drugs conferred stepwise improvements in survival. This approach culminated in a 56% reduction in all-cause mortality with the combination of an ACE inhibitor, beta-blocker, and mineralocorticoid receptor antagonist (MRA), compared with placebo, and a 63% reduction with an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker, and MRA (Circ Heart Fail. 2017 Jan;10(1). pii: e003529).
Moreover, the benefits of contemporary GDMT extend beyond reductions in all-cause mortality, death due to heart failure, and heart failure–related hospitalizations into areas where one wouldn’t necessarily have expected to see much benefit. For example, an analysis of data on more than 40,000 HFrEF patients in 12 clinical trials showed a sharp decline in the rate of sudden death over the years as new agents were incorporated into GDMT. The cumulative incidence of sudden death within 90 days after randomization plunged from 2.4% in the earliest trial to 1.0% in the most recent one (N Engl J Med. 2017 Jul 6;377[1]:41-51).
“We’re at the point where we now question whether routine use of implantable cardioverter-defibrillators in primary prevention patients with nonischemic heart failure is really worthwhile on the backdrop of effective medical therapy,” Dr. Desai observed.
But there’s a problem: “We don’t do a great job with GDMT, even with this incredible evidence base that we have,” the cardiologist said.
He cited a report from the CHAMP-HF registry that scrutinized the use of GDMT in more than 3,500 ambulatory HFrEF patients in 150 U.S. primary care and cardiology practices. It found that 67% of patients deemed eligible for an MRA weren’t on one. Neither were 33% with no contraindications to beta-blocker therapy and 27% who were eligible for an ACE inhibitor, angiotensin receptor blocker (ARB), or ARNI (J Am Coll Cardiol. 2018 Jul 24;72[4]:351-66).
“This highlights a huge opportunity for further guideline-directed optimization of therapy,” he said.
Underdosing of GDMT
The CHAMP-HF registry contained further disappointing news regarding the state of treatment of patients with HFrEF in ambulatory settings: Among those patients who were on GDMT, very few were receiving the recommended target doses of the medications as established in major clinical trials and specified in the guidelines. Only 14% of patients on an ARNI were on the target dose, as were 28% on a beta-blocker, and 17% of those on an ACE inhibitor or ARB. And among patients who were eligible for all classes of GDMT, just 1% were simultaneously on the target doses of an MRA, beta-blocker, and ARNI, ACE inhibitor, or ARB. This despite solid evidence that, although some benefit is derived from initiating these medications, incremental benefit comes from dose titration.
“Even for those of us who feel like we do this quite well, if we examine our practices systematically – and we’ve done this in our own practices at Brigham and Women’s – you see that a lot of eligible patients aren’t on optimal therapy. And you might argue that many of them have contraindications, but even when you do a deep dive into the literature or the electronic medical record and ask the question – Why is this patient with normal renal function and normal potassium with class II HFrEF not on an MRA? – sometimes it’s hard to establish why that’s the case,” said Dr. Desai.
Interrupting GDMT during hospitalizations
This is common practice. But in fact, continuation of GDMT is generally well tolerated in the setting of acute decompensated heart failure in the absence of severe hypotension and cardiogenic shock. Moreover, in-hospital discontinuation or dose reduction is associated with increased risks of readmission and mortality.
And in treatment-naive HFrEF patients, what better place to introduce a medication and assess its tolerability than the hospital? Plus, medications prescribed at discharge are more likely to be continued in the outpatient setting, he noted.
Being seduced by the illusion of stability
The guidelines state that patients with NYHA class II or III HFrEF who tolerate an ACE inhibitor or ARB should be transitioned to an ARNI to further reduce their risk of morbidity and mortality. Yet many physicians wait to make the switch until clinical decompensation occurs. That’s a mistake, as was demonstrated in the landmark PARADIGM-HF trial. Twenty percent of study participants without a prior hospitalization for heart failure experienced cardiovascular death or heart failure hospitalization during the follow-up period. Patients who were clinically stable as defined by no prior heart failure hospitalization or none within 3 months prior to enrollment were as likely to benefit from ARNI therapy with sacubitril/valsartan (Entresto) as were those with a recent decompensation (JACC Heart Fail. 2016 Oct;4[10]:816-22). “A key message is that stability is an illusion in patients with symptomatic heart failure,”said Dr. Desai. “In PARADIGM-HF, the first event for about half of patients was not heralded by a worsening of symptoms or a heart failure hospitalization, it was an abrupt death at home. This may mean that a missed opportunity to optimize treatment may not come back to you down the road, so waiting until patients get worse in order to optimize their therapy may not be the best strategy.”
Inadequate laboratory monitoring
The MRAs, spironolactone and eplerenone (Inspra), are the GDMT drugs for which laboratory surveillance takes on the greatest importance because of their potential to induce hyperkalemia. The guidelines are clear that a potassium level and measurement of renal function should be obtained within a week of initiating therapy with an MRA, again at 4 weeks, and periodically thereafter.
“In general, this is done in clinical practice almost never,” Dr. Desai stressed.
These agents should be avoided in patients with prior hyperkalemia or advanced chronic kidney disease, and used with care in groups known to be at increased risk for hyperkalemia, including the elderly and patients with diabetes.
He considers spironolactone equivalent to eplerenone so long as the dosing is adequate. He generally reserves eplerenone for patients with poorly tolerated antiandrogenic effects on spironolactone.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
REPORTING FROM ACC SNOWMASS 2019
Dupilumab to undergo FDA Priority Review for CRSwNP treatment
The Food and Drug Administration will conduct a Priority Review on the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent) as an add-on treatment for adults with inadequately controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP).
CRSwNP is a chronic disease of the upper airway in which patients can experience severe nasal obstruction with breathing difficulties, nasal discharge, reduction or loss of sense of smell and taste, and facial pain or pressure. There are currently no FDA-approved treatments for the disease, Regeneron said in the press release.
The sBLA is based on results from a pair of phase 3 trials in which patients with CRSwNP received either dupilumab plus a standard-of-care corticosteroid nasal spray or the standard-of-care spray alone. In results presented at the 2019 annual meeting of the American Academy of Allergy, Asthma, and Immunology, dupilumab plus the spray improved nasal polyp size, nasal congestion severity, chronic sinus disease, sense of smell, and comorbid asthma outcomes while reducing the need for corticosteroid use and nasal/sinus surgery.
Dupilumab is currently approved in the United States to treat moderate to severe atopic dermatitis in adults whose disease is poorly controlled with topical agents and as a maintenance treatment in combination with other asthma medications in patients aged 12 years and older whose disease is not controlled with their current prescription. The most common adverse events include injection-site reactions, oropharyngeal pain, and cold sores.
The target action date for the FDA decision is June 26, 2019, Regeneron said.
Find the full press release on the Regeneron website.
The Food and Drug Administration will conduct a Priority Review on the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent) as an add-on treatment for adults with inadequately controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP).
CRSwNP is a chronic disease of the upper airway in which patients can experience severe nasal obstruction with breathing difficulties, nasal discharge, reduction or loss of sense of smell and taste, and facial pain or pressure. There are currently no FDA-approved treatments for the disease, Regeneron said in the press release.
The sBLA is based on results from a pair of phase 3 trials in which patients with CRSwNP received either dupilumab plus a standard-of-care corticosteroid nasal spray or the standard-of-care spray alone. In results presented at the 2019 annual meeting of the American Academy of Allergy, Asthma, and Immunology, dupilumab plus the spray improved nasal polyp size, nasal congestion severity, chronic sinus disease, sense of smell, and comorbid asthma outcomes while reducing the need for corticosteroid use and nasal/sinus surgery.
Dupilumab is currently approved in the United States to treat moderate to severe atopic dermatitis in adults whose disease is poorly controlled with topical agents and as a maintenance treatment in combination with other asthma medications in patients aged 12 years and older whose disease is not controlled with their current prescription. The most common adverse events include injection-site reactions, oropharyngeal pain, and cold sores.
The target action date for the FDA decision is June 26, 2019, Regeneron said.
Find the full press release on the Regeneron website.
The Food and Drug Administration will conduct a Priority Review on the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent) as an add-on treatment for adults with inadequately controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP).
CRSwNP is a chronic disease of the upper airway in which patients can experience severe nasal obstruction with breathing difficulties, nasal discharge, reduction or loss of sense of smell and taste, and facial pain or pressure. There are currently no FDA-approved treatments for the disease, Regeneron said in the press release.
The sBLA is based on results from a pair of phase 3 trials in which patients with CRSwNP received either dupilumab plus a standard-of-care corticosteroid nasal spray or the standard-of-care spray alone. In results presented at the 2019 annual meeting of the American Academy of Allergy, Asthma, and Immunology, dupilumab plus the spray improved nasal polyp size, nasal congestion severity, chronic sinus disease, sense of smell, and comorbid asthma outcomes while reducing the need for corticosteroid use and nasal/sinus surgery.
Dupilumab is currently approved in the United States to treat moderate to severe atopic dermatitis in adults whose disease is poorly controlled with topical agents and as a maintenance treatment in combination with other asthma medications in patients aged 12 years and older whose disease is not controlled with their current prescription. The most common adverse events include injection-site reactions, oropharyngeal pain, and cold sores.
The target action date for the FDA decision is June 26, 2019, Regeneron said.
Find the full press release on the Regeneron website.