User login
‘Trigger zone’ resection ups seizure-free odds in temporal lobe epilepsy
Resection of a brain area implicated in seizure modulation improves the odds of being seizure free in patients with temporal lobe epilepsy, according to results of a recent multicenter analysis.
Patients with long-term postoperative freedom from seizures had a larger proportion of the piriform cortex resected versus patients who were not seizure free. Removing at least half the piriform cortex was associated with a 1500% increase in odds of seizure freedom, first author Marian Galovic, MD, of the department of clinical and experimental epilepsy at the University College London’s Queen Square Institute of Neurology and his colleagues reported in JAMA Neurology.
“If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection,” Dr. Galovic and his coinvestigators wrote.
The area tempestas in the piriform cortex has been identified as an epileptic trigger zone in animal studies, but to date, evidence of a human epileptic trigger zone in this area remain limited, according to the investigators.
To evaluate the impact of resection in this area, Dr. Galovic and his colleagues evaluated 107 patients with temporal lobe epilepsy from an ongoing, single-center, prospective study, and validated their findings with 31 patients from two other independent cohorts.
Of the 107 patients in the main cohort, 46% were completely seizure free for a median of 5 years after epilepsy surgery, with results of voxel-based morphometry showing that those patients had a more pronounced loss of gray matter in the ipsilateral piriform, compared with non–seizure-free patients.
The seizure-free patients had a median of 83% of the piriform cortex resected, compared with 52% for the non–seizure-free patients (P less than .001), results of a volumetric analysis confirmed.
Anxiety or psychosis outcomes were not influenced by the extent of piriform cortex resection, the investigators wrote, adding that poor verbal memory outcome was linked to the extent of resection of other brain regions, but not the piriform cortex.
The investigators confirmed these findings in the 31 patients of the validation cohort, with significant associations between extent of piriform cortex resection and postsurgical outcomes.
Resecting at least half of the region increased odds of being seizure free by a factor of 16 (95% CI, 5-47; P less than .001), Dr. Galovic and his colleagues added.
“Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe,” they wrote in a discussion of their results.
The findings, if confirmed, could have implications not only for surgical practice, they wrote, but also for the understanding of the mechanisms underlying epileptic networks, which could lead to new drug and nondrug interventions to mitigate seizure activity.
Dr. Galovic reported receiving a grant from the Medical Research Council. His coauthors reported disclosures with the Medical Research Council, Wellcome Trust, Medtronic, Neuropace, Nevro, Eisai, UCB, and Mallinckrodt, among other entities.
SOURCE: Galovic M et al. JAMA Neurol. 2019 Mar 11. doi: 10.1001/jamaneurol.2019.0204.
Resection of a brain area implicated in seizure modulation improves the odds of being seizure free in patients with temporal lobe epilepsy, according to results of a recent multicenter analysis.
Patients with long-term postoperative freedom from seizures had a larger proportion of the piriform cortex resected versus patients who were not seizure free. Removing at least half the piriform cortex was associated with a 1500% increase in odds of seizure freedom, first author Marian Galovic, MD, of the department of clinical and experimental epilepsy at the University College London’s Queen Square Institute of Neurology and his colleagues reported in JAMA Neurology.
“If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection,” Dr. Galovic and his coinvestigators wrote.
The area tempestas in the piriform cortex has been identified as an epileptic trigger zone in animal studies, but to date, evidence of a human epileptic trigger zone in this area remain limited, according to the investigators.
To evaluate the impact of resection in this area, Dr. Galovic and his colleagues evaluated 107 patients with temporal lobe epilepsy from an ongoing, single-center, prospective study, and validated their findings with 31 patients from two other independent cohorts.
Of the 107 patients in the main cohort, 46% were completely seizure free for a median of 5 years after epilepsy surgery, with results of voxel-based morphometry showing that those patients had a more pronounced loss of gray matter in the ipsilateral piriform, compared with non–seizure-free patients.
The seizure-free patients had a median of 83% of the piriform cortex resected, compared with 52% for the non–seizure-free patients (P less than .001), results of a volumetric analysis confirmed.
Anxiety or psychosis outcomes were not influenced by the extent of piriform cortex resection, the investigators wrote, adding that poor verbal memory outcome was linked to the extent of resection of other brain regions, but not the piriform cortex.
The investigators confirmed these findings in the 31 patients of the validation cohort, with significant associations between extent of piriform cortex resection and postsurgical outcomes.
Resecting at least half of the region increased odds of being seizure free by a factor of 16 (95% CI, 5-47; P less than .001), Dr. Galovic and his colleagues added.
“Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe,” they wrote in a discussion of their results.
The findings, if confirmed, could have implications not only for surgical practice, they wrote, but also for the understanding of the mechanisms underlying epileptic networks, which could lead to new drug and nondrug interventions to mitigate seizure activity.
Dr. Galovic reported receiving a grant from the Medical Research Council. His coauthors reported disclosures with the Medical Research Council, Wellcome Trust, Medtronic, Neuropace, Nevro, Eisai, UCB, and Mallinckrodt, among other entities.
SOURCE: Galovic M et al. JAMA Neurol. 2019 Mar 11. doi: 10.1001/jamaneurol.2019.0204.
Resection of a brain area implicated in seizure modulation improves the odds of being seizure free in patients with temporal lobe epilepsy, according to results of a recent multicenter analysis.
Patients with long-term postoperative freedom from seizures had a larger proportion of the piriform cortex resected versus patients who were not seizure free. Removing at least half the piriform cortex was associated with a 1500% increase in odds of seizure freedom, first author Marian Galovic, MD, of the department of clinical and experimental epilepsy at the University College London’s Queen Square Institute of Neurology and his colleagues reported in JAMA Neurology.
“If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection,” Dr. Galovic and his coinvestigators wrote.
The area tempestas in the piriform cortex has been identified as an epileptic trigger zone in animal studies, but to date, evidence of a human epileptic trigger zone in this area remain limited, according to the investigators.
To evaluate the impact of resection in this area, Dr. Galovic and his colleagues evaluated 107 patients with temporal lobe epilepsy from an ongoing, single-center, prospective study, and validated their findings with 31 patients from two other independent cohorts.
Of the 107 patients in the main cohort, 46% were completely seizure free for a median of 5 years after epilepsy surgery, with results of voxel-based morphometry showing that those patients had a more pronounced loss of gray matter in the ipsilateral piriform, compared with non–seizure-free patients.
The seizure-free patients had a median of 83% of the piriform cortex resected, compared with 52% for the non–seizure-free patients (P less than .001), results of a volumetric analysis confirmed.
Anxiety or psychosis outcomes were not influenced by the extent of piriform cortex resection, the investigators wrote, adding that poor verbal memory outcome was linked to the extent of resection of other brain regions, but not the piriform cortex.
The investigators confirmed these findings in the 31 patients of the validation cohort, with significant associations between extent of piriform cortex resection and postsurgical outcomes.
Resecting at least half of the region increased odds of being seizure free by a factor of 16 (95% CI, 5-47; P less than .001), Dr. Galovic and his colleagues added.
“Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe,” they wrote in a discussion of their results.
The findings, if confirmed, could have implications not only for surgical practice, they wrote, but also for the understanding of the mechanisms underlying epileptic networks, which could lead to new drug and nondrug interventions to mitigate seizure activity.
Dr. Galovic reported receiving a grant from the Medical Research Council. His coauthors reported disclosures with the Medical Research Council, Wellcome Trust, Medtronic, Neuropace, Nevro, Eisai, UCB, and Mallinckrodt, among other entities.
SOURCE: Galovic M et al. JAMA Neurol. 2019 Mar 11. doi: 10.1001/jamaneurol.2019.0204.
FROM JAMA NEUROLOGY
Teriflunomide transmission can occur in female partners of men taking the drug
DALLAS – Low or undetectable levels of teriflunomide (Aubagio) occur in women who are sexually active with men taking the drug for relapsing multiple sclerosis, results from a small study demonstrated.
“One of the issues with this particular drug is that it carries a strong pregnancy warning because in animal studies the drug has been teratogenic,” Joseph B. Guarnaccia, MD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other issue is that it remains detectable in the body for some time. The issue of females taking this drug and conception are well known. There are strong warnings that, if a woman wants to become pregnant, the drug should be removed quickly from the system. But if their male partner is on the drug, does that pose a risk to their female partner? That question has never been addressed in a human study.”
The Food and Drug Administration prescribing information recommends that men wishing to father a child should discontinue use of teriflunomide and undergo the accelerated elimination procedure. It also recommends that female partners wishing to become pregnant should discontinue the drug and undergo an accelerated elimination procedure to verify that the plasma teriflunomide concentration is less than 0.020 mcg/mL.
In an effort to test the risk of female exposure to potentially teratogenic levels of teriflunomide through sexual intercourse, Dr. Guarnaccia, a neurologist with the Multiple Sclerosis Treatment Center at Griffin Hospital in Derby, Conn., and his colleagues recruited 10 couples and compared serum levels of teriflunomide in men with relapsing multiple sclerosis with those of their female partners. Enrollment criteria for men included a diagnosis of relapsing multiple sclerosis, age between 18 and 55 years, treatment with teriflunomide for at least 2 months prior to study entry, and frequency of sexual intercourse with their female partners at least twice a month. Pregnancy was excluded in females, and couples could not use barrier or withdrawal methods of contraception. The couples completed a brief questionnaire and underwent a one-time blood draw for teriflunomide levels either at the investigator’s office or at a LabCorp facility.
The mean age of study participants was 47 years and the mean frequency of intercourse was seven episodes per month. The mean teriflunomide concentration in men was 42.30 mcg/mL (ranged from 10.07 to 142.84 mcg/mL). Six women had teriflunomide below detection levels (0.020 mcg/mL). However, four women had detectable levels that averaged 0.045 mcg/mL (ranging from 0.022 to 0.077 mcg/mL).
“This small study demonstrates that low or undetectable levels of teriflunomide occur in females who are sexually active with males taking teriflunomide for relapsing multiple sclerosis,” the researchers wrote in their poster. They found that women who had low detectable levels of teriflunomide, compared with women with undetectable levels, did not engage in more frequent sexual intercourse nor were their levels associated with higher levels of teriflunomide in their male partners.
“Indeed, one might have expected a positive correlation between serum levels of teriflunomide in females and the frequency or concentration of inoculation in semen from their partners,” the researchers wrote. “While semen levels of teriflunomide were not measured in this study, it might be assumed that serum and semen concentrations of small molecules like teriflunomide are similar.”
The study was supported by a investigator-sponsored research grant from Sanofi-Genzyme. Dr. Guarnaccia reported that he has received speaking honoraria and educational grants from Sanofi-Genzyme, Biogen, Teva, Acorda Therapeutics, Bayer, EMD Serono, and Genentech.
SOURCE: Guarnaccia JB et al. ACTRIMS Forum 2019, Poster 115.
DALLAS – Low or undetectable levels of teriflunomide (Aubagio) occur in women who are sexually active with men taking the drug for relapsing multiple sclerosis, results from a small study demonstrated.
“One of the issues with this particular drug is that it carries a strong pregnancy warning because in animal studies the drug has been teratogenic,” Joseph B. Guarnaccia, MD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other issue is that it remains detectable in the body for some time. The issue of females taking this drug and conception are well known. There are strong warnings that, if a woman wants to become pregnant, the drug should be removed quickly from the system. But if their male partner is on the drug, does that pose a risk to their female partner? That question has never been addressed in a human study.”
The Food and Drug Administration prescribing information recommends that men wishing to father a child should discontinue use of teriflunomide and undergo the accelerated elimination procedure. It also recommends that female partners wishing to become pregnant should discontinue the drug and undergo an accelerated elimination procedure to verify that the plasma teriflunomide concentration is less than 0.020 mcg/mL.
In an effort to test the risk of female exposure to potentially teratogenic levels of teriflunomide through sexual intercourse, Dr. Guarnaccia, a neurologist with the Multiple Sclerosis Treatment Center at Griffin Hospital in Derby, Conn., and his colleagues recruited 10 couples and compared serum levels of teriflunomide in men with relapsing multiple sclerosis with those of their female partners. Enrollment criteria for men included a diagnosis of relapsing multiple sclerosis, age between 18 and 55 years, treatment with teriflunomide for at least 2 months prior to study entry, and frequency of sexual intercourse with their female partners at least twice a month. Pregnancy was excluded in females, and couples could not use barrier or withdrawal methods of contraception. The couples completed a brief questionnaire and underwent a one-time blood draw for teriflunomide levels either at the investigator’s office or at a LabCorp facility.
The mean age of study participants was 47 years and the mean frequency of intercourse was seven episodes per month. The mean teriflunomide concentration in men was 42.30 mcg/mL (ranged from 10.07 to 142.84 mcg/mL). Six women had teriflunomide below detection levels (0.020 mcg/mL). However, four women had detectable levels that averaged 0.045 mcg/mL (ranging from 0.022 to 0.077 mcg/mL).
“This small study demonstrates that low or undetectable levels of teriflunomide occur in females who are sexually active with males taking teriflunomide for relapsing multiple sclerosis,” the researchers wrote in their poster. They found that women who had low detectable levels of teriflunomide, compared with women with undetectable levels, did not engage in more frequent sexual intercourse nor were their levels associated with higher levels of teriflunomide in their male partners.
“Indeed, one might have expected a positive correlation between serum levels of teriflunomide in females and the frequency or concentration of inoculation in semen from their partners,” the researchers wrote. “While semen levels of teriflunomide were not measured in this study, it might be assumed that serum and semen concentrations of small molecules like teriflunomide are similar.”
The study was supported by a investigator-sponsored research grant from Sanofi-Genzyme. Dr. Guarnaccia reported that he has received speaking honoraria and educational grants from Sanofi-Genzyme, Biogen, Teva, Acorda Therapeutics, Bayer, EMD Serono, and Genentech.
SOURCE: Guarnaccia JB et al. ACTRIMS Forum 2019, Poster 115.
DALLAS – Low or undetectable levels of teriflunomide (Aubagio) occur in women who are sexually active with men taking the drug for relapsing multiple sclerosis, results from a small study demonstrated.
“One of the issues with this particular drug is that it carries a strong pregnancy warning because in animal studies the drug has been teratogenic,” Joseph B. Guarnaccia, MD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other issue is that it remains detectable in the body for some time. The issue of females taking this drug and conception are well known. There are strong warnings that, if a woman wants to become pregnant, the drug should be removed quickly from the system. But if their male partner is on the drug, does that pose a risk to their female partner? That question has never been addressed in a human study.”
The Food and Drug Administration prescribing information recommends that men wishing to father a child should discontinue use of teriflunomide and undergo the accelerated elimination procedure. It also recommends that female partners wishing to become pregnant should discontinue the drug and undergo an accelerated elimination procedure to verify that the plasma teriflunomide concentration is less than 0.020 mcg/mL.
In an effort to test the risk of female exposure to potentially teratogenic levels of teriflunomide through sexual intercourse, Dr. Guarnaccia, a neurologist with the Multiple Sclerosis Treatment Center at Griffin Hospital in Derby, Conn., and his colleagues recruited 10 couples and compared serum levels of teriflunomide in men with relapsing multiple sclerosis with those of their female partners. Enrollment criteria for men included a diagnosis of relapsing multiple sclerosis, age between 18 and 55 years, treatment with teriflunomide for at least 2 months prior to study entry, and frequency of sexual intercourse with their female partners at least twice a month. Pregnancy was excluded in females, and couples could not use barrier or withdrawal methods of contraception. The couples completed a brief questionnaire and underwent a one-time blood draw for teriflunomide levels either at the investigator’s office or at a LabCorp facility.
The mean age of study participants was 47 years and the mean frequency of intercourse was seven episodes per month. The mean teriflunomide concentration in men was 42.30 mcg/mL (ranged from 10.07 to 142.84 mcg/mL). Six women had teriflunomide below detection levels (0.020 mcg/mL). However, four women had detectable levels that averaged 0.045 mcg/mL (ranging from 0.022 to 0.077 mcg/mL).
“This small study demonstrates that low or undetectable levels of teriflunomide occur in females who are sexually active with males taking teriflunomide for relapsing multiple sclerosis,” the researchers wrote in their poster. They found that women who had low detectable levels of teriflunomide, compared with women with undetectable levels, did not engage in more frequent sexual intercourse nor were their levels associated with higher levels of teriflunomide in their male partners.
“Indeed, one might have expected a positive correlation between serum levels of teriflunomide in females and the frequency or concentration of inoculation in semen from their partners,” the researchers wrote. “While semen levels of teriflunomide were not measured in this study, it might be assumed that serum and semen concentrations of small molecules like teriflunomide are similar.”
The study was supported by a investigator-sponsored research grant from Sanofi-Genzyme. Dr. Guarnaccia reported that he has received speaking honoraria and educational grants from Sanofi-Genzyme, Biogen, Teva, Acorda Therapeutics, Bayer, EMD Serono, and Genentech.
SOURCE: Guarnaccia JB et al. ACTRIMS Forum 2019, Poster 115.
REPORTING FROM ACTRIMS FORUM 2019
Endoscopic full-thickness resection shows promise in CRC
“EFTR is an emerging technique for removal of nonlifting colorectal lesions but data on resection of malignant tumors are limited,” wrote Armin Kuellmer, MD, of the University of Freiburg (Germany), along with his colleagues.
The researchers retrospectively studied a cohort of 156 patients with histologically confirmed adenocarcinoma from 2015 to 2018 included in a German endoscopy registry. The most common indication for the procedure was removal of nonlifting lesions (n = 92), followed by resection of malignant polyps (n = 64). Dr. Kuellmer and his colleagues examined the efficacy, safety, and clinical significance of this novel technique.
The primary outcomes measured were technical success and R0 resection, which were defined as the macroscopic complete removal of the lesion and complete histologic removal with no evidence of malignant tissue in a tissue sample, respectively.
“Low-risk versus high-risk lesions are difficult to discriminate before resection as criteria are based on histological features,” the researchers wrote. “The study underlines the potential of EFTR to exactly discriminate between high-risk versus low-risk tumors to aid decision[s] for the optimal individual treatment strategy.”
After analysis, the investigators reported a technical success rate of 92.3% in patients who underwent the procedure and that the R0 resection rate was 71.8% in the same group.
“Reported technical success rates of EFTR for other indications [mainly nonlifting adenomas] range from 75% to 100% and are in line with our data,” they explained.
In a subgroup analysis, Dr. Kuellmer and his colleagues saw a higher R0 resection rate in patients who underwent the procedure for removal of malignant polyps (group 1) versus nonlifting lesions (group 2) (87.5% vs. 60.9%; P less than .001).
“This [difference] may be due to the fact that group 1 mainly consisted of low-risk tumors and small resection scars whereas the majority of lesions in group 2 proved to be more advanced tumors,” they added.
With respect to safety, 21 treatment-related adverse events were seen in the cohort, which were classified as either mild, moderate, or severe. Of these events, six were severe, defined as possibly life-threatening or requiring surgical intervention to resolve.
“The rate of major [adverse events] including perforation is in line with reported data on EFTR for other indications [0%-5%],” they wrote.
The authors acknowledged that a key limitation of the study was the retrospective cohort design. As a result, specific treatment strategies and procedural expertise could have varied across the participating centers. “We therefore cannot provide data on how much patients in total have been evaluated for EFTR,” they wrote.
Moving forward, the researchers highlighted the importance of conducting prospective studies to assess whether EFTR is a viable, safe, and effective treatment option for patients with early colorectal cancer.
“For patients with high-risk lesions unfit for surgery, [EFTR] might as well be a valuable option for local endoscopic treatment,” they concluded.
Funding sources and conflicts of interests were not reported.
SOURCE: Kuellmer A et al. Gastrointest Endosc. 2019 Jan 14. doi: 10.1016/j.gie.2018.12.025.
“EFTR is an emerging technique for removal of nonlifting colorectal lesions but data on resection of malignant tumors are limited,” wrote Armin Kuellmer, MD, of the University of Freiburg (Germany), along with his colleagues.
The researchers retrospectively studied a cohort of 156 patients with histologically confirmed adenocarcinoma from 2015 to 2018 included in a German endoscopy registry. The most common indication for the procedure was removal of nonlifting lesions (n = 92), followed by resection of malignant polyps (n = 64). Dr. Kuellmer and his colleagues examined the efficacy, safety, and clinical significance of this novel technique.
The primary outcomes measured were technical success and R0 resection, which were defined as the macroscopic complete removal of the lesion and complete histologic removal with no evidence of malignant tissue in a tissue sample, respectively.
“Low-risk versus high-risk lesions are difficult to discriminate before resection as criteria are based on histological features,” the researchers wrote. “The study underlines the potential of EFTR to exactly discriminate between high-risk versus low-risk tumors to aid decision[s] for the optimal individual treatment strategy.”
After analysis, the investigators reported a technical success rate of 92.3% in patients who underwent the procedure and that the R0 resection rate was 71.8% in the same group.
“Reported technical success rates of EFTR for other indications [mainly nonlifting adenomas] range from 75% to 100% and are in line with our data,” they explained.
In a subgroup analysis, Dr. Kuellmer and his colleagues saw a higher R0 resection rate in patients who underwent the procedure for removal of malignant polyps (group 1) versus nonlifting lesions (group 2) (87.5% vs. 60.9%; P less than .001).
“This [difference] may be due to the fact that group 1 mainly consisted of low-risk tumors and small resection scars whereas the majority of lesions in group 2 proved to be more advanced tumors,” they added.
With respect to safety, 21 treatment-related adverse events were seen in the cohort, which were classified as either mild, moderate, or severe. Of these events, six were severe, defined as possibly life-threatening or requiring surgical intervention to resolve.
“The rate of major [adverse events] including perforation is in line with reported data on EFTR for other indications [0%-5%],” they wrote.
The authors acknowledged that a key limitation of the study was the retrospective cohort design. As a result, specific treatment strategies and procedural expertise could have varied across the participating centers. “We therefore cannot provide data on how much patients in total have been evaluated for EFTR,” they wrote.
Moving forward, the researchers highlighted the importance of conducting prospective studies to assess whether EFTR is a viable, safe, and effective treatment option for patients with early colorectal cancer.
“For patients with high-risk lesions unfit for surgery, [EFTR] might as well be a valuable option for local endoscopic treatment,” they concluded.
Funding sources and conflicts of interests were not reported.
SOURCE: Kuellmer A et al. Gastrointest Endosc. 2019 Jan 14. doi: 10.1016/j.gie.2018.12.025.
“EFTR is an emerging technique for removal of nonlifting colorectal lesions but data on resection of malignant tumors are limited,” wrote Armin Kuellmer, MD, of the University of Freiburg (Germany), along with his colleagues.
The researchers retrospectively studied a cohort of 156 patients with histologically confirmed adenocarcinoma from 2015 to 2018 included in a German endoscopy registry. The most common indication for the procedure was removal of nonlifting lesions (n = 92), followed by resection of malignant polyps (n = 64). Dr. Kuellmer and his colleagues examined the efficacy, safety, and clinical significance of this novel technique.
The primary outcomes measured were technical success and R0 resection, which were defined as the macroscopic complete removal of the lesion and complete histologic removal with no evidence of malignant tissue in a tissue sample, respectively.
“Low-risk versus high-risk lesions are difficult to discriminate before resection as criteria are based on histological features,” the researchers wrote. “The study underlines the potential of EFTR to exactly discriminate between high-risk versus low-risk tumors to aid decision[s] for the optimal individual treatment strategy.”
After analysis, the investigators reported a technical success rate of 92.3% in patients who underwent the procedure and that the R0 resection rate was 71.8% in the same group.
“Reported technical success rates of EFTR for other indications [mainly nonlifting adenomas] range from 75% to 100% and are in line with our data,” they explained.
In a subgroup analysis, Dr. Kuellmer and his colleagues saw a higher R0 resection rate in patients who underwent the procedure for removal of malignant polyps (group 1) versus nonlifting lesions (group 2) (87.5% vs. 60.9%; P less than .001).
“This [difference] may be due to the fact that group 1 mainly consisted of low-risk tumors and small resection scars whereas the majority of lesions in group 2 proved to be more advanced tumors,” they added.
With respect to safety, 21 treatment-related adverse events were seen in the cohort, which were classified as either mild, moderate, or severe. Of these events, six were severe, defined as possibly life-threatening or requiring surgical intervention to resolve.
“The rate of major [adverse events] including perforation is in line with reported data on EFTR for other indications [0%-5%],” they wrote.
The authors acknowledged that a key limitation of the study was the retrospective cohort design. As a result, specific treatment strategies and procedural expertise could have varied across the participating centers. “We therefore cannot provide data on how much patients in total have been evaluated for EFTR,” they wrote.
Moving forward, the researchers highlighted the importance of conducting prospective studies to assess whether EFTR is a viable, safe, and effective treatment option for patients with early colorectal cancer.
“For patients with high-risk lesions unfit for surgery, [EFTR] might as well be a valuable option for local endoscopic treatment,” they concluded.
Funding sources and conflicts of interests were not reported.
SOURCE: Kuellmer A et al. Gastrointest Endosc. 2019 Jan 14. doi: 10.1016/j.gie.2018.12.025.
FROM GASTROINTESTINAL ENDOSCOPY
Invasive cardiologists among top revenue-generating specialists
Physicians continue to be the major drivers of hospital revenue, and
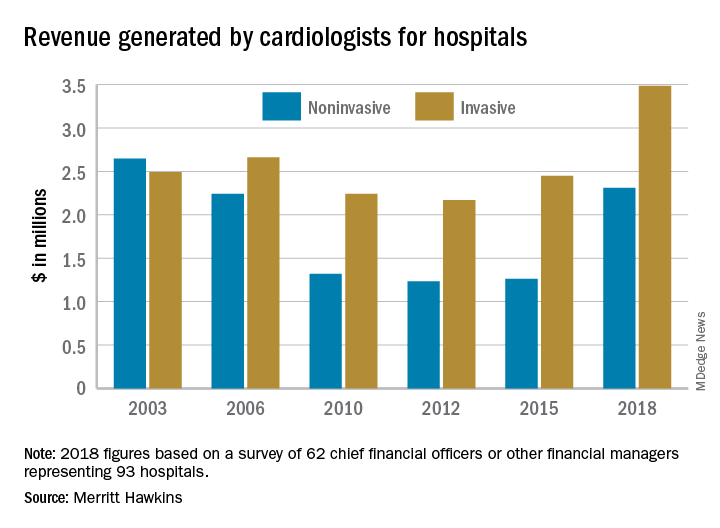
“The value of physician care is not only related to the quality of patient outcomes,” Travis Singleton, the company’s executive vice president, wrote in a statement. “Physicians continue to drive the financial health and viability of hospitals, even in a health care system that is evolving towards value-based payments.”
Invasive cardiologists generated an average of $3.48 million for their affiliated hospitals last year, second only to cardiovascular surgeons’ $3.70 million among the 19 specialties included in the survey. Average revenue generated by noninvasive cardiologists in 2018 came in at $2.31 million – that’s an 83% increase over the 3 years since the survey was last conducted, compared with the 42% rise seen by the invasive cardiologists over that time, Merritt Hawkins reported.
Specialist physicians as a group averaged over $2.45 million in revenue in 2018, compared with $2.13 million for primary care physicians, which “suggests that emerging value-based delivery models have yet to inhibit the revenue generating power of physician specialists,” the report said. Average revenue for all physicians in the survey was almost $2.38 million, an increase of 52% since Merritt Hawkins’ last survey.
The survey was conducted from October to December 2018 and is based on responses from 62 chief financial officers or other financial managers who represented 93 hospitals. Responses from smaller hospitals (0-99 beds) were “somewhat overrepresented in the survey” relative to their number nationwide, Merritt Hawkins said.
Physicians continue to be the major drivers of hospital revenue, and

“The value of physician care is not only related to the quality of patient outcomes,” Travis Singleton, the company’s executive vice president, wrote in a statement. “Physicians continue to drive the financial health and viability of hospitals, even in a health care system that is evolving towards value-based payments.”
Invasive cardiologists generated an average of $3.48 million for their affiliated hospitals last year, second only to cardiovascular surgeons’ $3.70 million among the 19 specialties included in the survey. Average revenue generated by noninvasive cardiologists in 2018 came in at $2.31 million – that’s an 83% increase over the 3 years since the survey was last conducted, compared with the 42% rise seen by the invasive cardiologists over that time, Merritt Hawkins reported.
Specialist physicians as a group averaged over $2.45 million in revenue in 2018, compared with $2.13 million for primary care physicians, which “suggests that emerging value-based delivery models have yet to inhibit the revenue generating power of physician specialists,” the report said. Average revenue for all physicians in the survey was almost $2.38 million, an increase of 52% since Merritt Hawkins’ last survey.
The survey was conducted from October to December 2018 and is based on responses from 62 chief financial officers or other financial managers who represented 93 hospitals. Responses from smaller hospitals (0-99 beds) were “somewhat overrepresented in the survey” relative to their number nationwide, Merritt Hawkins said.
Physicians continue to be the major drivers of hospital revenue, and

“The value of physician care is not only related to the quality of patient outcomes,” Travis Singleton, the company’s executive vice president, wrote in a statement. “Physicians continue to drive the financial health and viability of hospitals, even in a health care system that is evolving towards value-based payments.”
Invasive cardiologists generated an average of $3.48 million for their affiliated hospitals last year, second only to cardiovascular surgeons’ $3.70 million among the 19 specialties included in the survey. Average revenue generated by noninvasive cardiologists in 2018 came in at $2.31 million – that’s an 83% increase over the 3 years since the survey was last conducted, compared with the 42% rise seen by the invasive cardiologists over that time, Merritt Hawkins reported.
Specialist physicians as a group averaged over $2.45 million in revenue in 2018, compared with $2.13 million for primary care physicians, which “suggests that emerging value-based delivery models have yet to inhibit the revenue generating power of physician specialists,” the report said. Average revenue for all physicians in the survey was almost $2.38 million, an increase of 52% since Merritt Hawkins’ last survey.
The survey was conducted from October to December 2018 and is based on responses from 62 chief financial officers or other financial managers who represented 93 hospitals. Responses from smaller hospitals (0-99 beds) were “somewhat overrepresented in the survey” relative to their number nationwide, Merritt Hawkins said.
Myeloma risk score has treatment-planning potential
A proposed clinical scoring system using readily available laboratory data, rather than complex formulas, can be used to predict risk for overall survival and help with clinical decision making for patients with multiple myeloma who are ineligible for stem cell transplants, the system’s creators claim.
When applied to data from two clinical trials that enrolled patients with transplant-ineligible multiple myeloma, the UK Myeloma Research Alliance Risk Profile (MRP) was shown to accurately group patients into low-, medium- and high-risk categories and was prognostic of overall survival, reported Gordon Cook, PhD, of the University of Leeds, England, and his colleagues.
“The ability of clinical scoring systems, such as that proposed here, to predict whether a patient is likely to stop treatment early because of treatment intolerability, could enable preemptive, upfront dose adjustments in patients with multiple myeloma, preventing toxicity and potentially enabling patients to stay on therapy for longer,” they wrote in the Lancet Haematology.
The investigators used data on 1,852 newly diagnosed patients recruited to the non–intensive treatment pathway of the UK’s National Cancer Research Institute Myeloma XI study (NCRI-XI, ISRCTN49407852) for a training dataset and internal validation dataset, and 520 patients recruited into the Medical Research Council Myeloma IX study (MRC-IX, ISRCTN68454111) for the test dataset.
Patient characteristics, biochemical measurements, and hematological data were plugged into univariate and multivariate models to determine their potential as prognostic variables.
The final model for the test and validation datasets included World Health Organization performance status, the multiple myeloma International Staging System, patient age, and C-reactive protein concentrations.
As noted before, the scoring algorithm groups patients into low-, medium- and high-risk categories, with each of the prognostic variables increasing in severity across the three groups in both clinical trials.
In the NCRI-XI trial, median overall survival for patients in the MRP low-risk group was 60 months, compared with 44 months in the medium-risk group, and 25 months in the high-risk group.
Similarly, in the MRC-IX trial, the respective median overall survival was 49, 34, and 20 months.
The risk groups also were associated with progression-free survival in each trial, although not as robustly as the association with overall survival.
The investigators also found that, the higher the risk group, the greater the likelihood that the median percentage of protocol dose delivered would be lower, and both a decrease in protocol dose delivered and quality of life at baseline were associated with increased risk.
The MRP categories were prognostic in patients treated with various therapeutic regimens and in patients with high-risk cytogenetics.
“None of the risk scoring systems previously developed in myeloma are dynamic, making them unable to accommodate changes in disease-related frailty that might be minimized by effective anti-myeloma therapy. There is therefore scope to improve clinical risk scores by the addition of a suitable frailty biomarker, which is currently still in developmental stages,” Dr. Cook and his colleagues wrote.
The study was funded by the Medical Research Council, Novartis, Schering Health Care, Chugai, Pharmion, Celgene, Ortho Biotech, Cancer Research UK, Celgene, Merck Sharp & Dohme, and Amgen. Dr. Cook reported grants and nonfinancial support from Celgene, Amgen, and Merck Sharp & Dohme, during the conduct of the study and personal fees from other companies outside the submitted work.
SOURCE: Cook G et al. Lancet Haematol. 2019 Mar;6(3):e154-66.
A proposed clinical scoring system using readily available laboratory data, rather than complex formulas, can be used to predict risk for overall survival and help with clinical decision making for patients with multiple myeloma who are ineligible for stem cell transplants, the system’s creators claim.
When applied to data from two clinical trials that enrolled patients with transplant-ineligible multiple myeloma, the UK Myeloma Research Alliance Risk Profile (MRP) was shown to accurately group patients into low-, medium- and high-risk categories and was prognostic of overall survival, reported Gordon Cook, PhD, of the University of Leeds, England, and his colleagues.
“The ability of clinical scoring systems, such as that proposed here, to predict whether a patient is likely to stop treatment early because of treatment intolerability, could enable preemptive, upfront dose adjustments in patients with multiple myeloma, preventing toxicity and potentially enabling patients to stay on therapy for longer,” they wrote in the Lancet Haematology.
The investigators used data on 1,852 newly diagnosed patients recruited to the non–intensive treatment pathway of the UK’s National Cancer Research Institute Myeloma XI study (NCRI-XI, ISRCTN49407852) for a training dataset and internal validation dataset, and 520 patients recruited into the Medical Research Council Myeloma IX study (MRC-IX, ISRCTN68454111) for the test dataset.
Patient characteristics, biochemical measurements, and hematological data were plugged into univariate and multivariate models to determine their potential as prognostic variables.
The final model for the test and validation datasets included World Health Organization performance status, the multiple myeloma International Staging System, patient age, and C-reactive protein concentrations.
As noted before, the scoring algorithm groups patients into low-, medium- and high-risk categories, with each of the prognostic variables increasing in severity across the three groups in both clinical trials.
In the NCRI-XI trial, median overall survival for patients in the MRP low-risk group was 60 months, compared with 44 months in the medium-risk group, and 25 months in the high-risk group.
Similarly, in the MRC-IX trial, the respective median overall survival was 49, 34, and 20 months.
The risk groups also were associated with progression-free survival in each trial, although not as robustly as the association with overall survival.
The investigators also found that, the higher the risk group, the greater the likelihood that the median percentage of protocol dose delivered would be lower, and both a decrease in protocol dose delivered and quality of life at baseline were associated with increased risk.
The MRP categories were prognostic in patients treated with various therapeutic regimens and in patients with high-risk cytogenetics.
“None of the risk scoring systems previously developed in myeloma are dynamic, making them unable to accommodate changes in disease-related frailty that might be minimized by effective anti-myeloma therapy. There is therefore scope to improve clinical risk scores by the addition of a suitable frailty biomarker, which is currently still in developmental stages,” Dr. Cook and his colleagues wrote.
The study was funded by the Medical Research Council, Novartis, Schering Health Care, Chugai, Pharmion, Celgene, Ortho Biotech, Cancer Research UK, Celgene, Merck Sharp & Dohme, and Amgen. Dr. Cook reported grants and nonfinancial support from Celgene, Amgen, and Merck Sharp & Dohme, during the conduct of the study and personal fees from other companies outside the submitted work.
SOURCE: Cook G et al. Lancet Haematol. 2019 Mar;6(3):e154-66.
A proposed clinical scoring system using readily available laboratory data, rather than complex formulas, can be used to predict risk for overall survival and help with clinical decision making for patients with multiple myeloma who are ineligible for stem cell transplants, the system’s creators claim.
When applied to data from two clinical trials that enrolled patients with transplant-ineligible multiple myeloma, the UK Myeloma Research Alliance Risk Profile (MRP) was shown to accurately group patients into low-, medium- and high-risk categories and was prognostic of overall survival, reported Gordon Cook, PhD, of the University of Leeds, England, and his colleagues.
“The ability of clinical scoring systems, such as that proposed here, to predict whether a patient is likely to stop treatment early because of treatment intolerability, could enable preemptive, upfront dose adjustments in patients with multiple myeloma, preventing toxicity and potentially enabling patients to stay on therapy for longer,” they wrote in the Lancet Haematology.
The investigators used data on 1,852 newly diagnosed patients recruited to the non–intensive treatment pathway of the UK’s National Cancer Research Institute Myeloma XI study (NCRI-XI, ISRCTN49407852) for a training dataset and internal validation dataset, and 520 patients recruited into the Medical Research Council Myeloma IX study (MRC-IX, ISRCTN68454111) for the test dataset.
Patient characteristics, biochemical measurements, and hematological data were plugged into univariate and multivariate models to determine their potential as prognostic variables.
The final model for the test and validation datasets included World Health Organization performance status, the multiple myeloma International Staging System, patient age, and C-reactive protein concentrations.
As noted before, the scoring algorithm groups patients into low-, medium- and high-risk categories, with each of the prognostic variables increasing in severity across the three groups in both clinical trials.
In the NCRI-XI trial, median overall survival for patients in the MRP low-risk group was 60 months, compared with 44 months in the medium-risk group, and 25 months in the high-risk group.
Similarly, in the MRC-IX trial, the respective median overall survival was 49, 34, and 20 months.
The risk groups also were associated with progression-free survival in each trial, although not as robustly as the association with overall survival.
The investigators also found that, the higher the risk group, the greater the likelihood that the median percentage of protocol dose delivered would be lower, and both a decrease in protocol dose delivered and quality of life at baseline were associated with increased risk.
The MRP categories were prognostic in patients treated with various therapeutic regimens and in patients with high-risk cytogenetics.
“None of the risk scoring systems previously developed in myeloma are dynamic, making them unable to accommodate changes in disease-related frailty that might be minimized by effective anti-myeloma therapy. There is therefore scope to improve clinical risk scores by the addition of a suitable frailty biomarker, which is currently still in developmental stages,” Dr. Cook and his colleagues wrote.
The study was funded by the Medical Research Council, Novartis, Schering Health Care, Chugai, Pharmion, Celgene, Ortho Biotech, Cancer Research UK, Celgene, Merck Sharp & Dohme, and Amgen. Dr. Cook reported grants and nonfinancial support from Celgene, Amgen, and Merck Sharp & Dohme, during the conduct of the study and personal fees from other companies outside the submitted work.
SOURCE: Cook G et al. Lancet Haematol. 2019 Mar;6(3):e154-66.
FROM THE LANCET HAEMATOLOGY
Autism risk with in utero infections
Also today, for FPs, 2018 was a big year for generating hospital revenue, prenatal betamethasone is not linked to later adverse neurocognitive problems, and heart-harming toxins may hurt hookah smokers.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, for FPs, 2018 was a big year for generating hospital revenue, prenatal betamethasone is not linked to later adverse neurocognitive problems, and heart-harming toxins may hurt hookah smokers.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, for FPs, 2018 was a big year for generating hospital revenue, prenatal betamethasone is not linked to later adverse neurocognitive problems, and heart-harming toxins may hurt hookah smokers.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Bariatric surgery leads to less improvement in black patients
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
FROM JAMA SURGERY
Key clinical point: Black patients who underwent bariatric surgery suffered more overall complications and reported a lower quality of life than white patients.
Major finding: The rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02).
Study details: A matched cohort study of 14,210 patients, half black and half white, who underwent a primary bariatric operation in Michigan between June 2006 and January 2017.
Disclosures: The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the Michigan Bariatric Surgery Collaborative, and one other coauthor reported receiving an honorarium for being the collaborative’s executive committee chair.
Source: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
FDA extends Dupixent indication for 12- to 17-year-olds
The Food and Drug Administration has approved dupilumab for adolescents with moderate to severe atopic dermatitis (AD) that has been inadequately controlled with topical prescription treatments “or when those therapies are not advisable.”
Dupilumab (Dupixent), which inhibits interleukin-4 and interleukin-13 signaling, was initially approved in March 2017, for the same indication, becoming the first targeted biologic treatment for AD. The adolescent approval was announced by the manufacturer.
While there are several systemic medications used as second-line therapy for treatment of pediatric AD, dupilumab is the first FDA-approved biologic for treatment of the disease in adolescents aged 12-17 years, Dawn Marie R. Davis, MD, a pediatric dermatologist at the Mayo Clinic, Rochester (MN), and current president of the Society for Pediatric Dermatology, said in an interview.
FDA approval should decrease insurance barriers and the need for prior authorization, thus increasing access to the drug, she noted, adding, “I hope it will offer a successful alternative to other advanced therapies, as the medicine works through a different mechanism of action, compared to the current systemic medications available.”
With the expanded indication to include adolescents, “patients with more moderate to severe disease who aren’t well controlled with a topical therapy are going to get treatment that will change their lives for many years to come,” dupilumab investigator Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview. “On the whole, patients are likely being undertreated and suffering from the disease more than they need to be,” said Dr. Simpson, “With the advent of this new therapy and the new data, it’s going to change the risk benefit calculation for providers and for patients.”
Results from a phase 3 clinical trial of dupilumab in adolescents with moderate to severe AD were presented last fall at the European Academy of Dermatology and Venereology Congress in Paris. In that study, the proportion of patients who achieved a 75% or greater improvement in the Eczema Area and Severity Index at 16 weeks was 38.1% with monthly dupilumab, 41.5% with dupilumab every 2 weeks, and 8.2% with placebo. Dr. Simpson, the first author of this study, presented the results at that meeting.
Dr. Simpson said that he hopes dupilumab approval for adolescents and the clinical trial results will help providers recognize when patients are not in good control of their AD, and which patients qualify for a step-up in therapy when treatments such as topical therapy or prednisone are not effective. “There are so many patients out there who qualify for a step-up in therapy,” he commented. “I hope that provides comfort to both patients and providers, that it’s OK to take the next step, because the results show us that, not only it can improve your skin rash, but it can have dramatic effects on all the downstream effects of the condition.”
These downstream effects include not only quality of life and comorbidities of mental health but also the patient’s emotional state. Hopefully, dupilumab can reduce stigmatization of AD and feelings of embarrassment for adolescents at a time in life when “socialization, education, and activity is so important in creating your kind of identity in yourself and your sense of self-worth,” Dr. Simpson said.
“It is important to remember atopic dermatitis is a disease that impacts not only the skin, but the patient as a whole,” said Dr. Davis. “It is an exciting time to be caring for atopic dermatitis patients with the various new medications coming to market.”
The FDA had granted a priority review for the adolescent indication; previously the FDA had granted Breakthrough Therapy designation for dupilumab in 2016 for the treatment of moderate to severe AD in adolescents and severe AD in children aged 6 months to 11 years who are insufficiently controlled with topical medications
The dosing for adolescents is weight based; two doses are available, 200 mg and 300 mg, administered subcutaneously, every other week after a loading dose. The updated prescribing information is available at https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf.Dr. Simpson reports relationships with Sanofi and Regeneron Pharmaceuticals. Dr. Davis reports no relevant financial disclosures.
The Food and Drug Administration has approved dupilumab for adolescents with moderate to severe atopic dermatitis (AD) that has been inadequately controlled with topical prescription treatments “or when those therapies are not advisable.”
Dupilumab (Dupixent), which inhibits interleukin-4 and interleukin-13 signaling, was initially approved in March 2017, for the same indication, becoming the first targeted biologic treatment for AD. The adolescent approval was announced by the manufacturer.
While there are several systemic medications used as second-line therapy for treatment of pediatric AD, dupilumab is the first FDA-approved biologic for treatment of the disease in adolescents aged 12-17 years, Dawn Marie R. Davis, MD, a pediatric dermatologist at the Mayo Clinic, Rochester (MN), and current president of the Society for Pediatric Dermatology, said in an interview.
FDA approval should decrease insurance barriers and the need for prior authorization, thus increasing access to the drug, she noted, adding, “I hope it will offer a successful alternative to other advanced therapies, as the medicine works through a different mechanism of action, compared to the current systemic medications available.”
With the expanded indication to include adolescents, “patients with more moderate to severe disease who aren’t well controlled with a topical therapy are going to get treatment that will change their lives for many years to come,” dupilumab investigator Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview. “On the whole, patients are likely being undertreated and suffering from the disease more than they need to be,” said Dr. Simpson, “With the advent of this new therapy and the new data, it’s going to change the risk benefit calculation for providers and for patients.”
Results from a phase 3 clinical trial of dupilumab in adolescents with moderate to severe AD were presented last fall at the European Academy of Dermatology and Venereology Congress in Paris. In that study, the proportion of patients who achieved a 75% or greater improvement in the Eczema Area and Severity Index at 16 weeks was 38.1% with monthly dupilumab, 41.5% with dupilumab every 2 weeks, and 8.2% with placebo. Dr. Simpson, the first author of this study, presented the results at that meeting.
Dr. Simpson said that he hopes dupilumab approval for adolescents and the clinical trial results will help providers recognize when patients are not in good control of their AD, and which patients qualify for a step-up in therapy when treatments such as topical therapy or prednisone are not effective. “There are so many patients out there who qualify for a step-up in therapy,” he commented. “I hope that provides comfort to both patients and providers, that it’s OK to take the next step, because the results show us that, not only it can improve your skin rash, but it can have dramatic effects on all the downstream effects of the condition.”
These downstream effects include not only quality of life and comorbidities of mental health but also the patient’s emotional state. Hopefully, dupilumab can reduce stigmatization of AD and feelings of embarrassment for adolescents at a time in life when “socialization, education, and activity is so important in creating your kind of identity in yourself and your sense of self-worth,” Dr. Simpson said.
“It is important to remember atopic dermatitis is a disease that impacts not only the skin, but the patient as a whole,” said Dr. Davis. “It is an exciting time to be caring for atopic dermatitis patients with the various new medications coming to market.”
The FDA had granted a priority review for the adolescent indication; previously the FDA had granted Breakthrough Therapy designation for dupilumab in 2016 for the treatment of moderate to severe AD in adolescents and severe AD in children aged 6 months to 11 years who are insufficiently controlled with topical medications
The dosing for adolescents is weight based; two doses are available, 200 mg and 300 mg, administered subcutaneously, every other week after a loading dose. The updated prescribing information is available at https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf.Dr. Simpson reports relationships with Sanofi and Regeneron Pharmaceuticals. Dr. Davis reports no relevant financial disclosures.
The Food and Drug Administration has approved dupilumab for adolescents with moderate to severe atopic dermatitis (AD) that has been inadequately controlled with topical prescription treatments “or when those therapies are not advisable.”
Dupilumab (Dupixent), which inhibits interleukin-4 and interleukin-13 signaling, was initially approved in March 2017, for the same indication, becoming the first targeted biologic treatment for AD. The adolescent approval was announced by the manufacturer.
While there are several systemic medications used as second-line therapy for treatment of pediatric AD, dupilumab is the first FDA-approved biologic for treatment of the disease in adolescents aged 12-17 years, Dawn Marie R. Davis, MD, a pediatric dermatologist at the Mayo Clinic, Rochester (MN), and current president of the Society for Pediatric Dermatology, said in an interview.
FDA approval should decrease insurance barriers and the need for prior authorization, thus increasing access to the drug, she noted, adding, “I hope it will offer a successful alternative to other advanced therapies, as the medicine works through a different mechanism of action, compared to the current systemic medications available.”
With the expanded indication to include adolescents, “patients with more moderate to severe disease who aren’t well controlled with a topical therapy are going to get treatment that will change their lives for many years to come,” dupilumab investigator Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview. “On the whole, patients are likely being undertreated and suffering from the disease more than they need to be,” said Dr. Simpson, “With the advent of this new therapy and the new data, it’s going to change the risk benefit calculation for providers and for patients.”
Results from a phase 3 clinical trial of dupilumab in adolescents with moderate to severe AD were presented last fall at the European Academy of Dermatology and Venereology Congress in Paris. In that study, the proportion of patients who achieved a 75% or greater improvement in the Eczema Area and Severity Index at 16 weeks was 38.1% with monthly dupilumab, 41.5% with dupilumab every 2 weeks, and 8.2% with placebo. Dr. Simpson, the first author of this study, presented the results at that meeting.
Dr. Simpson said that he hopes dupilumab approval for adolescents and the clinical trial results will help providers recognize when patients are not in good control of their AD, and which patients qualify for a step-up in therapy when treatments such as topical therapy or prednisone are not effective. “There are so many patients out there who qualify for a step-up in therapy,” he commented. “I hope that provides comfort to both patients and providers, that it’s OK to take the next step, because the results show us that, not only it can improve your skin rash, but it can have dramatic effects on all the downstream effects of the condition.”
These downstream effects include not only quality of life and comorbidities of mental health but also the patient’s emotional state. Hopefully, dupilumab can reduce stigmatization of AD and feelings of embarrassment for adolescents at a time in life when “socialization, education, and activity is so important in creating your kind of identity in yourself and your sense of self-worth,” Dr. Simpson said.
“It is important to remember atopic dermatitis is a disease that impacts not only the skin, but the patient as a whole,” said Dr. Davis. “It is an exciting time to be caring for atopic dermatitis patients with the various new medications coming to market.”
The FDA had granted a priority review for the adolescent indication; previously the FDA had granted Breakthrough Therapy designation for dupilumab in 2016 for the treatment of moderate to severe AD in adolescents and severe AD in children aged 6 months to 11 years who are insufficiently controlled with topical medications
The dosing for adolescents is weight based; two doses are available, 200 mg and 300 mg, administered subcutaneously, every other week after a loading dose. The updated prescribing information is available at https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf.Dr. Simpson reports relationships with Sanofi and Regeneron Pharmaceuticals. Dr. Davis reports no relevant financial disclosures.
Measles now confirmed in 12 states
The number of new measles cases was down by more than half last week, but another state has been added to the list of those with reported cases in 2019, according to the Centers for Disease Control and Prevention.
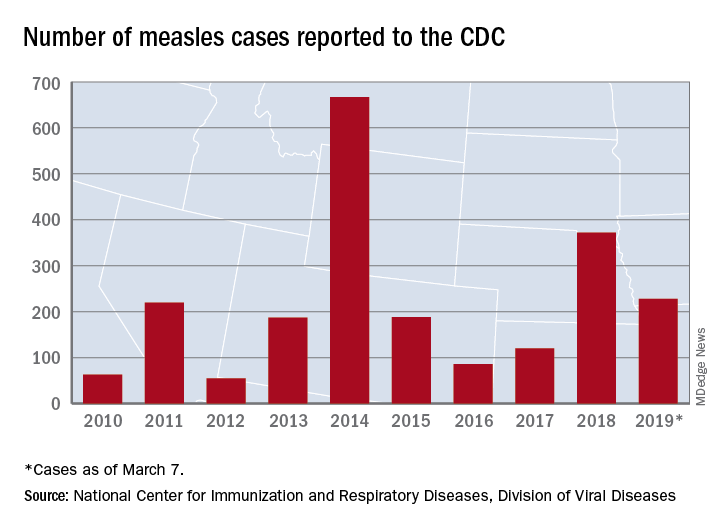
The total for the year is 228 cases, which moves 2019 ahead of 2011 for third place over the last decade, the CDC reported March 11. Going back even further in time, the 206 measles cases reported through January and February is the highest 2-month total in a quarter of a century, the Washington Post said.
New Hampshire became the 12th and latest state to report a case of measles this year, joining California, Colorado, Connecticut, Georgia, Illinois, Kentucky, New Jersey, New York, Oregon, Texas, and Washington. California’s situation is now considered an outbreak (defined as three or more cases), but one of the three outbreaks in New York has been taken off the list, so total outbreaks for 2019 remain at six, the CDC said.
For the third consecutive week, New York City produced the most measles cases, with Brooklyn’s Williamsburg neighborhood recording 11 of the U.S. total of 21. The outbreak in King County, Wash., – totaling 70 cases this year – may be winding down as only one new case was reported last week, and no new cases are being investigated, the county’s public health service reported.
The number of new measles cases was down by more than half last week, but another state has been added to the list of those with reported cases in 2019, according to the Centers for Disease Control and Prevention.

The total for the year is 228 cases, which moves 2019 ahead of 2011 for third place over the last decade, the CDC reported March 11. Going back even further in time, the 206 measles cases reported through January and February is the highest 2-month total in a quarter of a century, the Washington Post said.
New Hampshire became the 12th and latest state to report a case of measles this year, joining California, Colorado, Connecticut, Georgia, Illinois, Kentucky, New Jersey, New York, Oregon, Texas, and Washington. California’s situation is now considered an outbreak (defined as three or more cases), but one of the three outbreaks in New York has been taken off the list, so total outbreaks for 2019 remain at six, the CDC said.
For the third consecutive week, New York City produced the most measles cases, with Brooklyn’s Williamsburg neighborhood recording 11 of the U.S. total of 21. The outbreak in King County, Wash., – totaling 70 cases this year – may be winding down as only one new case was reported last week, and no new cases are being investigated, the county’s public health service reported.
The number of new measles cases was down by more than half last week, but another state has been added to the list of those with reported cases in 2019, according to the Centers for Disease Control and Prevention.

The total for the year is 228 cases, which moves 2019 ahead of 2011 for third place over the last decade, the CDC reported March 11. Going back even further in time, the 206 measles cases reported through January and February is the highest 2-month total in a quarter of a century, the Washington Post said.
New Hampshire became the 12th and latest state to report a case of measles this year, joining California, Colorado, Connecticut, Georgia, Illinois, Kentucky, New Jersey, New York, Oregon, Texas, and Washington. California’s situation is now considered an outbreak (defined as three or more cases), but one of the three outbreaks in New York has been taken off the list, so total outbreaks for 2019 remain at six, the CDC said.
For the third consecutive week, New York City produced the most measles cases, with Brooklyn’s Williamsburg neighborhood recording 11 of the U.S. total of 21. The outbreak in King County, Wash., – totaling 70 cases this year – may be winding down as only one new case was reported last week, and no new cases are being investigated, the county’s public health service reported.
Pembrolizumab/lenvatinib active against urothelial carcinoma
SAN FRANCISCO – A combination of a targeted therapy and an immune checkpoint inhibitor showed promising activity against advanced urothelial cancer in early data from a phase 1b/2 study.
In a cohort of 20 patients with urothelial carcinoma who were enrolled in a larger clinical trial testing the combination of the tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) and the checkpoint inhibitor pembrolizumab (Keytruda) against urinary tract and other solid malignancies, 5 had an objective response to the combination, including one complete and four partial responses, for an objective response rate of 25%, reported Nicholas J. Vogelzang, MD, from Comprehensive Cancers Centers of Nevada in Las Vegas.
“This response rate warrants further investigation. The lenvatinib plus pembrolizumab combination will be studied in a phase 3 trial in urothelial carcinoma,” he said at the American Society of Clinical Oncology (ASCO) - Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
Dr. Vogelzang noted that urothelial carcinomas account for more than 90% of all bladder cancers. Pembrolizumab monotherapy is approved for treatment of patients with urothelial carcinoma who are ineligible for cisplatin and whose tumors have a combined positive score (CPS) for programmed death-ligand 1 (PD-L1) of 10 or greater or who are ineligible for platinum-based chemotherapy regimens and, in the second line, for advanced or metastatic urothelial carcinoma.
Lenvatinib, a multikinase inhibitor, is approved as monotherapy for radioiodine-refractory differentiated thyroid cancer, unresectable hepatocellular carcinoma, and in combination with everolimus for advanced renal cell carcinoma (RCC) after one year of antiangiogenic therapy.
Dr. Vogelzang reported results of the urothelial cancer cohort from a multicohort study testing the combination.
Twenty patients with histologically confirmed metastatic urothelial cancer were enrolled. The patients all had no more than two prior systemic regimens, good performance status, and a life expectancy of at least 12 weeks. The patients received oral lenvatinib 20 mg daily and pembrolizumab 200 mg intravenously every 21 days. The median patient age was 72 years. The cohort included 14 men and six women.
The objective response rate (ORR) at 24 weeks, the primary endpoint, was 25%, comprising one complete and four partial responses. Nine patients had stable disease, two had disease progression, and four were not evaluable for efficacy. The results were identical according to immune-related Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST version 1.1. Of the 16 evaluable patients, 12 experienced tumor-size reductions from baseline.
“Although there were five objective responses, there were an additional seven patients or more who had minor regressions of disease – clearly an active regimen,” Dr. Vogelzang said. Four of the patients, including one with a PD-L1–positive tumor and three with PD-L1–negative tumors were still alive, with the longest survival past 80 weeks since the start of therapy. The majority of patients, however, had no objective response or disease progression within about 20 weeks.
After a median follow-up of 11.7 months, the median progression-free survival (PFS) was 5.4 months, and the 12-month PFS rate was 26%.
In all, 18 of the 20 patients (90%) experienced a treatment-related adverse event of any grade, 10 had grade 3 or 4 events, and 6 had serious adverse events including one death from gastrointestinal hemorrhage that Dr. Vogelzang said appeared to be related to lenvatinib. A total of four patients (20%) had a treatment-related event leading to withdrawal or discontinuation, seven had a dose reduction, and 12 had an interruption in therapy, primarily of lenvatinib. The most common toxicities were proteinuria, diarrhea, hypertension, fatigue, hypothyroidism, decreased appetite with nausea, pancreatitis with increased lipase, skin rash, vomiting, and dry mouth.
In addition to the planned phase 3 trial of the combination in urothelial carcinoma, lenvatinib/pembrolizumab is also being studied for the treatment of RCC.
The study was supported by Eisai and Merck Sharp & Dohme. Dr. Vogelzang disclosed financial relationships with Caris Life Sciences, Pfizer, Up to Date, AstraZeneca, MedImmune, and other companies. Five coauthors are employees of Merck or Esai.
SOURCE: Vogelzang NJ et al. ASCO-SITC, Abstract 11.
SAN FRANCISCO – A combination of a targeted therapy and an immune checkpoint inhibitor showed promising activity against advanced urothelial cancer in early data from a phase 1b/2 study.
In a cohort of 20 patients with urothelial carcinoma who were enrolled in a larger clinical trial testing the combination of the tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) and the checkpoint inhibitor pembrolizumab (Keytruda) against urinary tract and other solid malignancies, 5 had an objective response to the combination, including one complete and four partial responses, for an objective response rate of 25%, reported Nicholas J. Vogelzang, MD, from Comprehensive Cancers Centers of Nevada in Las Vegas.
“This response rate warrants further investigation. The lenvatinib plus pembrolizumab combination will be studied in a phase 3 trial in urothelial carcinoma,” he said at the American Society of Clinical Oncology (ASCO) - Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
Dr. Vogelzang noted that urothelial carcinomas account for more than 90% of all bladder cancers. Pembrolizumab monotherapy is approved for treatment of patients with urothelial carcinoma who are ineligible for cisplatin and whose tumors have a combined positive score (CPS) for programmed death-ligand 1 (PD-L1) of 10 or greater or who are ineligible for platinum-based chemotherapy regimens and, in the second line, for advanced or metastatic urothelial carcinoma.
Lenvatinib, a multikinase inhibitor, is approved as monotherapy for radioiodine-refractory differentiated thyroid cancer, unresectable hepatocellular carcinoma, and in combination with everolimus for advanced renal cell carcinoma (RCC) after one year of antiangiogenic therapy.
Dr. Vogelzang reported results of the urothelial cancer cohort from a multicohort study testing the combination.
Twenty patients with histologically confirmed metastatic urothelial cancer were enrolled. The patients all had no more than two prior systemic regimens, good performance status, and a life expectancy of at least 12 weeks. The patients received oral lenvatinib 20 mg daily and pembrolizumab 200 mg intravenously every 21 days. The median patient age was 72 years. The cohort included 14 men and six women.
The objective response rate (ORR) at 24 weeks, the primary endpoint, was 25%, comprising one complete and four partial responses. Nine patients had stable disease, two had disease progression, and four were not evaluable for efficacy. The results were identical according to immune-related Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST version 1.1. Of the 16 evaluable patients, 12 experienced tumor-size reductions from baseline.
“Although there were five objective responses, there were an additional seven patients or more who had minor regressions of disease – clearly an active regimen,” Dr. Vogelzang said. Four of the patients, including one with a PD-L1–positive tumor and three with PD-L1–negative tumors were still alive, with the longest survival past 80 weeks since the start of therapy. The majority of patients, however, had no objective response or disease progression within about 20 weeks.
After a median follow-up of 11.7 months, the median progression-free survival (PFS) was 5.4 months, and the 12-month PFS rate was 26%.
In all, 18 of the 20 patients (90%) experienced a treatment-related adverse event of any grade, 10 had grade 3 or 4 events, and 6 had serious adverse events including one death from gastrointestinal hemorrhage that Dr. Vogelzang said appeared to be related to lenvatinib. A total of four patients (20%) had a treatment-related event leading to withdrawal or discontinuation, seven had a dose reduction, and 12 had an interruption in therapy, primarily of lenvatinib. The most common toxicities were proteinuria, diarrhea, hypertension, fatigue, hypothyroidism, decreased appetite with nausea, pancreatitis with increased lipase, skin rash, vomiting, and dry mouth.
In addition to the planned phase 3 trial of the combination in urothelial carcinoma, lenvatinib/pembrolizumab is also being studied for the treatment of RCC.
The study was supported by Eisai and Merck Sharp & Dohme. Dr. Vogelzang disclosed financial relationships with Caris Life Sciences, Pfizer, Up to Date, AstraZeneca, MedImmune, and other companies. Five coauthors are employees of Merck or Esai.
SOURCE: Vogelzang NJ et al. ASCO-SITC, Abstract 11.
SAN FRANCISCO – A combination of a targeted therapy and an immune checkpoint inhibitor showed promising activity against advanced urothelial cancer in early data from a phase 1b/2 study.
In a cohort of 20 patients with urothelial carcinoma who were enrolled in a larger clinical trial testing the combination of the tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) and the checkpoint inhibitor pembrolizumab (Keytruda) against urinary tract and other solid malignancies, 5 had an objective response to the combination, including one complete and four partial responses, for an objective response rate of 25%, reported Nicholas J. Vogelzang, MD, from Comprehensive Cancers Centers of Nevada in Las Vegas.
“This response rate warrants further investigation. The lenvatinib plus pembrolizumab combination will be studied in a phase 3 trial in urothelial carcinoma,” he said at the American Society of Clinical Oncology (ASCO) - Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
Dr. Vogelzang noted that urothelial carcinomas account for more than 90% of all bladder cancers. Pembrolizumab monotherapy is approved for treatment of patients with urothelial carcinoma who are ineligible for cisplatin and whose tumors have a combined positive score (CPS) for programmed death-ligand 1 (PD-L1) of 10 or greater or who are ineligible for platinum-based chemotherapy regimens and, in the second line, for advanced or metastatic urothelial carcinoma.
Lenvatinib, a multikinase inhibitor, is approved as monotherapy for radioiodine-refractory differentiated thyroid cancer, unresectable hepatocellular carcinoma, and in combination with everolimus for advanced renal cell carcinoma (RCC) after one year of antiangiogenic therapy.
Dr. Vogelzang reported results of the urothelial cancer cohort from a multicohort study testing the combination.
Twenty patients with histologically confirmed metastatic urothelial cancer were enrolled. The patients all had no more than two prior systemic regimens, good performance status, and a life expectancy of at least 12 weeks. The patients received oral lenvatinib 20 mg daily and pembrolizumab 200 mg intravenously every 21 days. The median patient age was 72 years. The cohort included 14 men and six women.
The objective response rate (ORR) at 24 weeks, the primary endpoint, was 25%, comprising one complete and four partial responses. Nine patients had stable disease, two had disease progression, and four were not evaluable for efficacy. The results were identical according to immune-related Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST version 1.1. Of the 16 evaluable patients, 12 experienced tumor-size reductions from baseline.
“Although there were five objective responses, there were an additional seven patients or more who had minor regressions of disease – clearly an active regimen,” Dr. Vogelzang said. Four of the patients, including one with a PD-L1–positive tumor and three with PD-L1–negative tumors were still alive, with the longest survival past 80 weeks since the start of therapy. The majority of patients, however, had no objective response or disease progression within about 20 weeks.
After a median follow-up of 11.7 months, the median progression-free survival (PFS) was 5.4 months, and the 12-month PFS rate was 26%.
In all, 18 of the 20 patients (90%) experienced a treatment-related adverse event of any grade, 10 had grade 3 or 4 events, and 6 had serious adverse events including one death from gastrointestinal hemorrhage that Dr. Vogelzang said appeared to be related to lenvatinib. A total of four patients (20%) had a treatment-related event leading to withdrawal or discontinuation, seven had a dose reduction, and 12 had an interruption in therapy, primarily of lenvatinib. The most common toxicities were proteinuria, diarrhea, hypertension, fatigue, hypothyroidism, decreased appetite with nausea, pancreatitis with increased lipase, skin rash, vomiting, and dry mouth.
In addition to the planned phase 3 trial of the combination in urothelial carcinoma, lenvatinib/pembrolizumab is also being studied for the treatment of RCC.
The study was supported by Eisai and Merck Sharp & Dohme. Dr. Vogelzang disclosed financial relationships with Caris Life Sciences, Pfizer, Up to Date, AstraZeneca, MedImmune, and other companies. Five coauthors are employees of Merck or Esai.
SOURCE: Vogelzang NJ et al. ASCO-SITC, Abstract 11.
REPORTING FROM ASCO-SITC






