User login
New Antibiotic Promising for Complicated UTIs
TOPLINE:
, according to a study published in The New England Journal of Medicine.
METHODOLOGY:
- Cefepime-taniborbactam is an antibiotic currently being explored as a treatment for antibiotic-resistant bacteria.
- The phase 3, double-blind, randomized trial included participants from 15 countries, including a safety group of 657 patients who were studied for adverse events and 436 in the micro intention-to-treat group who were studied for drug effectiveness.
- Each drug’s efficacy was measured as a combination of reduced bacteria levels and a resolution of symptoms and signs of infection.
- Patients in the study were over age 18; had a diagnosis of either complicated UTI or acute pyelonephritis; and had pyuria, at least one systemic sign, and at least one local sign or symptom. People were excluded if they had already received antibacterial drug therapy for more than 24 hours before randomization or had an infection with a meropenem-resistant pathogen.
TAKEAWAY:
- At days 19-23, 70.6% of patients in the cefepime-taniborbactam group showed a successful reduction in bacteria and symptoms compared with 58.0% in the meropenem group.
- Cefepime-taniborbactam was more effective than meropenem during follow-up, with 89.1% efficacy less than 24 hours after the last dose, compared to meropenem’s 86%. Cefepime-taniborbactam continued to have 63.8% efficacy up to 35 days after starting treatment, while meropenem was 51.7% during that timeframe.
- In the cefepime-taniborbactam group, 35.5% of patients experienced adverse effects that were mild to moderate, including headache, diarrhea, constipation, hypertension, and nausea, compared to 29% in the meropenem group.
- Overall, 3% of participants discontinued cefepime-taniborbactam and 1.8% discontinued meropenem, but reasons were heterogeneous.
IN PRACTICE:
“Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTI that included acute pyelonephritis, with a safety profile similar to that of meropenem,” the study authors wrote.
SOURCE:
Paul McGovern, MD, infectious disease specialist and senior vice president of Venatorx Pharmaceuticals, was the corresponding author of the study.
LIMITATIONS:
The authors reported no limitations.
DISCLOSURES:
The study was funded by Venatorx Pharmaceuticals, which received funding from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, the Biomedical Advanced Research and Development Authority, the Global Antibiotic Research and Development Partnership, and Everest Medicines.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a study published in The New England Journal of Medicine.
METHODOLOGY:
- Cefepime-taniborbactam is an antibiotic currently being explored as a treatment for antibiotic-resistant bacteria.
- The phase 3, double-blind, randomized trial included participants from 15 countries, including a safety group of 657 patients who were studied for adverse events and 436 in the micro intention-to-treat group who were studied for drug effectiveness.
- Each drug’s efficacy was measured as a combination of reduced bacteria levels and a resolution of symptoms and signs of infection.
- Patients in the study were over age 18; had a diagnosis of either complicated UTI or acute pyelonephritis; and had pyuria, at least one systemic sign, and at least one local sign or symptom. People were excluded if they had already received antibacterial drug therapy for more than 24 hours before randomization or had an infection with a meropenem-resistant pathogen.
TAKEAWAY:
- At days 19-23, 70.6% of patients in the cefepime-taniborbactam group showed a successful reduction in bacteria and symptoms compared with 58.0% in the meropenem group.
- Cefepime-taniborbactam was more effective than meropenem during follow-up, with 89.1% efficacy less than 24 hours after the last dose, compared to meropenem’s 86%. Cefepime-taniborbactam continued to have 63.8% efficacy up to 35 days after starting treatment, while meropenem was 51.7% during that timeframe.
- In the cefepime-taniborbactam group, 35.5% of patients experienced adverse effects that were mild to moderate, including headache, diarrhea, constipation, hypertension, and nausea, compared to 29% in the meropenem group.
- Overall, 3% of participants discontinued cefepime-taniborbactam and 1.8% discontinued meropenem, but reasons were heterogeneous.
IN PRACTICE:
“Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTI that included acute pyelonephritis, with a safety profile similar to that of meropenem,” the study authors wrote.
SOURCE:
Paul McGovern, MD, infectious disease specialist and senior vice president of Venatorx Pharmaceuticals, was the corresponding author of the study.
LIMITATIONS:
The authors reported no limitations.
DISCLOSURES:
The study was funded by Venatorx Pharmaceuticals, which received funding from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, the Biomedical Advanced Research and Development Authority, the Global Antibiotic Research and Development Partnership, and Everest Medicines.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a study published in The New England Journal of Medicine.
METHODOLOGY:
- Cefepime-taniborbactam is an antibiotic currently being explored as a treatment for antibiotic-resistant bacteria.
- The phase 3, double-blind, randomized trial included participants from 15 countries, including a safety group of 657 patients who were studied for adverse events and 436 in the micro intention-to-treat group who were studied for drug effectiveness.
- Each drug’s efficacy was measured as a combination of reduced bacteria levels and a resolution of symptoms and signs of infection.
- Patients in the study were over age 18; had a diagnosis of either complicated UTI or acute pyelonephritis; and had pyuria, at least one systemic sign, and at least one local sign or symptom. People were excluded if they had already received antibacterial drug therapy for more than 24 hours before randomization or had an infection with a meropenem-resistant pathogen.
TAKEAWAY:
- At days 19-23, 70.6% of patients in the cefepime-taniborbactam group showed a successful reduction in bacteria and symptoms compared with 58.0% in the meropenem group.
- Cefepime-taniborbactam was more effective than meropenem during follow-up, with 89.1% efficacy less than 24 hours after the last dose, compared to meropenem’s 86%. Cefepime-taniborbactam continued to have 63.8% efficacy up to 35 days after starting treatment, while meropenem was 51.7% during that timeframe.
- In the cefepime-taniborbactam group, 35.5% of patients experienced adverse effects that were mild to moderate, including headache, diarrhea, constipation, hypertension, and nausea, compared to 29% in the meropenem group.
- Overall, 3% of participants discontinued cefepime-taniborbactam and 1.8% discontinued meropenem, but reasons were heterogeneous.
IN PRACTICE:
“Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTI that included acute pyelonephritis, with a safety profile similar to that of meropenem,” the study authors wrote.
SOURCE:
Paul McGovern, MD, infectious disease specialist and senior vice president of Venatorx Pharmaceuticals, was the corresponding author of the study.
LIMITATIONS:
The authors reported no limitations.
DISCLOSURES:
The study was funded by Venatorx Pharmaceuticals, which received funding from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, the Biomedical Advanced Research and Development Authority, the Global Antibiotic Research and Development Partnership, and Everest Medicines.
A version of this article appeared on Medscape.com.
FDA Approves First Cellular Therapy for Metastatic Melanoma
The US Food and Drug Administration (FDA) has approved lifileucel (Amtagvi, Iovance Biotherapeutics) for the treatment of certain adults with unresectable or metastatic melanoma, marking the first approval of a cellular therapy in the solid tumor setting.
Specifically, the tumor-derived autologous T-cell immunotherapy is indicated for adult patients previously treated with a programmed cell death protein 1 (PD-1)–blocking antibody, and if BRAF V600–positive, a BRAF inhibitor with or without an MEK inhibitor.
,” Samantha R. Guild, JD, president, AIM at Melanoma Foundation, stated in a press release. “This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
The approval was based on findings from the open-label single-arm global C-144-01 clinical trial, which showed an objective response rate of 31.5% in 73 patients treated within the recommended dosing rage of 7.5 x 109 to 72 x 109 viable cells. Complete responses occurred in three patients (4.1%) and partial responses occurred in 20 patients (27.4%)
Median duration of response was not reached at 18.6 months of follow-up. The median time to initial response to the therapy was 1.5 months, according to an FDA press release.
“Unresectable or metastatic melanoma is an aggressive form of cancer that can be fatal,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research stated in the FDA release. “The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options.”
“The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval,” Allison Betof Warner, MD, PhD, director of Melanoma Medical Oncology at Stanford Medicine, wrote on X. “We are very excited to bring this life-saving therapy to patients ASAP! Available immediately at @StanfordCancer!!!”
For the C-144-01 trial, lifileucel was administered after a lymphodepletion regimen of 60 mg/kg/d of cyclophosphamide for 2 days followed by 25 mg/m2/d of fludarabine for 5 days. Between 3 and 34 hours after infusion, patients received 600,000 IU/Kg of the interleukin 2 aldesleukin every 8-12 hours for up to six doses to support cell expansion in vivo.
The full prescribing information for lifileucel contains a boxed warning for treatment-related mortality, prolonged severe cytopenia, severe infection, cardiopulmonary, and renal impairment. The most common adverse reactions, which occurred in at least 20% of patients, were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash hypotension, alopecia, infection, hypoxia, and dyspnea.
“Patients receiving this product should be closely monitored before and after infusion for signs and symptoms of adverse reactions. Treatment should be withheld or discontinued in the presence of these symptoms, as indicated,” according to the FDA statement.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved lifileucel (Amtagvi, Iovance Biotherapeutics) for the treatment of certain adults with unresectable or metastatic melanoma, marking the first approval of a cellular therapy in the solid tumor setting.
Specifically, the tumor-derived autologous T-cell immunotherapy is indicated for adult patients previously treated with a programmed cell death protein 1 (PD-1)–blocking antibody, and if BRAF V600–positive, a BRAF inhibitor with or without an MEK inhibitor.
,” Samantha R. Guild, JD, president, AIM at Melanoma Foundation, stated in a press release. “This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
The approval was based on findings from the open-label single-arm global C-144-01 clinical trial, which showed an objective response rate of 31.5% in 73 patients treated within the recommended dosing rage of 7.5 x 109 to 72 x 109 viable cells. Complete responses occurred in three patients (4.1%) and partial responses occurred in 20 patients (27.4%)
Median duration of response was not reached at 18.6 months of follow-up. The median time to initial response to the therapy was 1.5 months, according to an FDA press release.
“Unresectable or metastatic melanoma is an aggressive form of cancer that can be fatal,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research stated in the FDA release. “The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options.”
“The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval,” Allison Betof Warner, MD, PhD, director of Melanoma Medical Oncology at Stanford Medicine, wrote on X. “We are very excited to bring this life-saving therapy to patients ASAP! Available immediately at @StanfordCancer!!!”
For the C-144-01 trial, lifileucel was administered after a lymphodepletion regimen of 60 mg/kg/d of cyclophosphamide for 2 days followed by 25 mg/m2/d of fludarabine for 5 days. Between 3 and 34 hours after infusion, patients received 600,000 IU/Kg of the interleukin 2 aldesleukin every 8-12 hours for up to six doses to support cell expansion in vivo.
The full prescribing information for lifileucel contains a boxed warning for treatment-related mortality, prolonged severe cytopenia, severe infection, cardiopulmonary, and renal impairment. The most common adverse reactions, which occurred in at least 20% of patients, were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash hypotension, alopecia, infection, hypoxia, and dyspnea.
“Patients receiving this product should be closely monitored before and after infusion for signs and symptoms of adverse reactions. Treatment should be withheld or discontinued in the presence of these symptoms, as indicated,” according to the FDA statement.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved lifileucel (Amtagvi, Iovance Biotherapeutics) for the treatment of certain adults with unresectable or metastatic melanoma, marking the first approval of a cellular therapy in the solid tumor setting.
Specifically, the tumor-derived autologous T-cell immunotherapy is indicated for adult patients previously treated with a programmed cell death protein 1 (PD-1)–blocking antibody, and if BRAF V600–positive, a BRAF inhibitor with or without an MEK inhibitor.
,” Samantha R. Guild, JD, president, AIM at Melanoma Foundation, stated in a press release. “This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
The approval was based on findings from the open-label single-arm global C-144-01 clinical trial, which showed an objective response rate of 31.5% in 73 patients treated within the recommended dosing rage of 7.5 x 109 to 72 x 109 viable cells. Complete responses occurred in three patients (4.1%) and partial responses occurred in 20 patients (27.4%)
Median duration of response was not reached at 18.6 months of follow-up. The median time to initial response to the therapy was 1.5 months, according to an FDA press release.
“Unresectable or metastatic melanoma is an aggressive form of cancer that can be fatal,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research stated in the FDA release. “The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options.”
“The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval,” Allison Betof Warner, MD, PhD, director of Melanoma Medical Oncology at Stanford Medicine, wrote on X. “We are very excited to bring this life-saving therapy to patients ASAP! Available immediately at @StanfordCancer!!!”
For the C-144-01 trial, lifileucel was administered after a lymphodepletion regimen of 60 mg/kg/d of cyclophosphamide for 2 days followed by 25 mg/m2/d of fludarabine for 5 days. Between 3 and 34 hours after infusion, patients received 600,000 IU/Kg of the interleukin 2 aldesleukin every 8-12 hours for up to six doses to support cell expansion in vivo.
The full prescribing information for lifileucel contains a boxed warning for treatment-related mortality, prolonged severe cytopenia, severe infection, cardiopulmonary, and renal impairment. The most common adverse reactions, which occurred in at least 20% of patients, were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash hypotension, alopecia, infection, hypoxia, and dyspnea.
“Patients receiving this product should be closely monitored before and after infusion for signs and symptoms of adverse reactions. Treatment should be withheld or discontinued in the presence of these symptoms, as indicated,” according to the FDA statement.
A version of this article appeared on Medscape.com.
FDA Approves Drug to Reduce Accidental Food Allergies
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
An 8-Year-Old Male With Asymptomatic Brown Rough Plaques on the Dorsum of the Right Hand and Fingers, Accompanied by Widening of the Knuckles
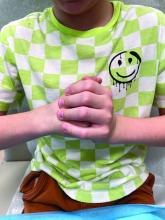
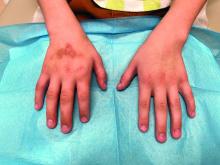
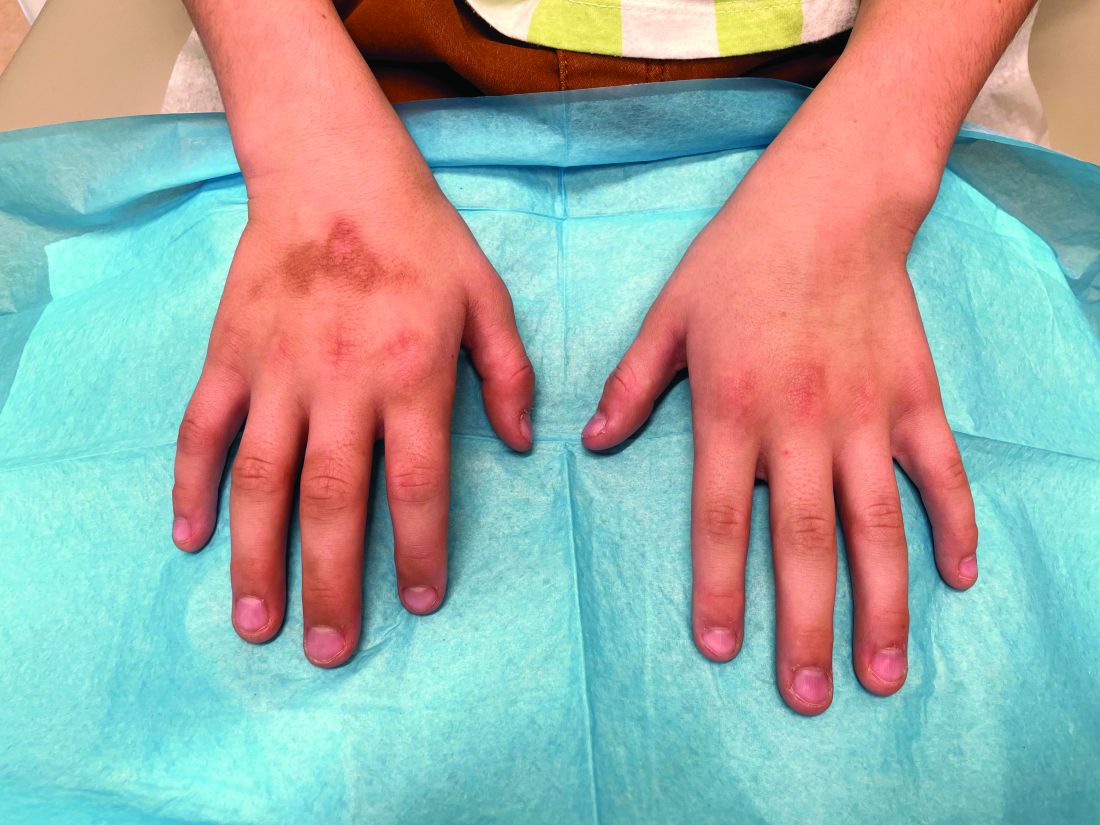
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
The patient was otherwise healthy, with no current medication intake, and he engaged in baseball and soccer activities. Upon physical examination, a hyperpigmented lichenified irregular plaque was observed on the dorsum of the right hand, along with irregular hyperpigmented macules and plaques on the fingers. Fusiform widening of the interphalangeal joints on the second, third, and fourth fingers of the right hand was noted, without associated pain, edema, or erythema.
Inflammatory Arthritis Often Occurs with Systemic Sclerosis; Has Big Impact
TOPLINE:
Inflammatory arthritis (IA) occurred in one-third of patients with systemic sclerosis (SSc) in a large observational study and was significantly associated with poor quality of life and physical function, as well as diffuse disease, musculoskeletal manifestations, myositis, and sicca.
METHODOLOGY:
- Researchers reviewed data from 1717 adults with SSc who were enrolled in the Australian Cohort Study to identify those with IA, defined as the presence of synovitis in one or more joints on clinical examination documented by the treating physician.
- The primary outcome was health-related quality of life (HRQoL) based on patient reports using the Medical Outcomes Short Form 36 and Patient-Reported Outcomes Measurement Information System, and physical function measured with the Health Assessment Questionnaire.
TAKEAWAY:
- IA was identified in 33.3% of the study participants over a median of 4.3 years’ follow-up. IA occurred at a median age of about 60 years and after a median SSc disease duration of 7.9 years. No significant differences in baseline demographics appeared between patients with and without IA.
- Patients with IA had significantly increased risk for diffuse cutaneous SSc (odds ratio [OR], 1.33), concurrent musculoskeletal manifestations such as tendon friction rubs and joint contractures (OR, 1.70), myositis (OR, 2.11), and sicca symptoms (OR, 1.57), compared with those without.
- Patients with IA reported significantly lower HRQoL scores and significantly greater physical disability, compared with those who did not have IA (P < .001 for both).
- IA was significantly less common among patients with , compared with those without pulmonary arterial hypertension (7.2% vs 11.3%; P = .007).
IN PRACTICE:
“Recognizing the presence of IA in SSc is an important first step, as its treatment and monitoring may alleviate some of the associated morbidity,” the researchers wrote.
SOURCE:
The lead author of the study was Eric Schwender, a medical student at the Royal College of Surgeons in Ireland, Dublin, Ireland. The study was published online in Arthritis Care & Research.
LIMITATIONS:
The inability to assess distribution and severity of IA limited the results, as did the inability to assess the impact of disease-modifying antirheumatic drugs in patients with IA.
DISCLOSURES:
The study was supported by Scleroderma Australia, Arthritis Australia, Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, and Pfizer, as well as grants to several researchers from the National Health and Medical Research Council of Australia. Lead author Mr. Schwender had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Inflammatory arthritis (IA) occurred in one-third of patients with systemic sclerosis (SSc) in a large observational study and was significantly associated with poor quality of life and physical function, as well as diffuse disease, musculoskeletal manifestations, myositis, and sicca.
METHODOLOGY:
- Researchers reviewed data from 1717 adults with SSc who were enrolled in the Australian Cohort Study to identify those with IA, defined as the presence of synovitis in one or more joints on clinical examination documented by the treating physician.
- The primary outcome was health-related quality of life (HRQoL) based on patient reports using the Medical Outcomes Short Form 36 and Patient-Reported Outcomes Measurement Information System, and physical function measured with the Health Assessment Questionnaire.
TAKEAWAY:
- IA was identified in 33.3% of the study participants over a median of 4.3 years’ follow-up. IA occurred at a median age of about 60 years and after a median SSc disease duration of 7.9 years. No significant differences in baseline demographics appeared between patients with and without IA.
- Patients with IA had significantly increased risk for diffuse cutaneous SSc (odds ratio [OR], 1.33), concurrent musculoskeletal manifestations such as tendon friction rubs and joint contractures (OR, 1.70), myositis (OR, 2.11), and sicca symptoms (OR, 1.57), compared with those without.
- Patients with IA reported significantly lower HRQoL scores and significantly greater physical disability, compared with those who did not have IA (P < .001 for both).
- IA was significantly less common among patients with , compared with those without pulmonary arterial hypertension (7.2% vs 11.3%; P = .007).
IN PRACTICE:
“Recognizing the presence of IA in SSc is an important first step, as its treatment and monitoring may alleviate some of the associated morbidity,” the researchers wrote.
SOURCE:
The lead author of the study was Eric Schwender, a medical student at the Royal College of Surgeons in Ireland, Dublin, Ireland. The study was published online in Arthritis Care & Research.
LIMITATIONS:
The inability to assess distribution and severity of IA limited the results, as did the inability to assess the impact of disease-modifying antirheumatic drugs in patients with IA.
DISCLOSURES:
The study was supported by Scleroderma Australia, Arthritis Australia, Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, and Pfizer, as well as grants to several researchers from the National Health and Medical Research Council of Australia. Lead author Mr. Schwender had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Inflammatory arthritis (IA) occurred in one-third of patients with systemic sclerosis (SSc) in a large observational study and was significantly associated with poor quality of life and physical function, as well as diffuse disease, musculoskeletal manifestations, myositis, and sicca.
METHODOLOGY:
- Researchers reviewed data from 1717 adults with SSc who were enrolled in the Australian Cohort Study to identify those with IA, defined as the presence of synovitis in one or more joints on clinical examination documented by the treating physician.
- The primary outcome was health-related quality of life (HRQoL) based on patient reports using the Medical Outcomes Short Form 36 and Patient-Reported Outcomes Measurement Information System, and physical function measured with the Health Assessment Questionnaire.
TAKEAWAY:
- IA was identified in 33.3% of the study participants over a median of 4.3 years’ follow-up. IA occurred at a median age of about 60 years and after a median SSc disease duration of 7.9 years. No significant differences in baseline demographics appeared between patients with and without IA.
- Patients with IA had significantly increased risk for diffuse cutaneous SSc (odds ratio [OR], 1.33), concurrent musculoskeletal manifestations such as tendon friction rubs and joint contractures (OR, 1.70), myositis (OR, 2.11), and sicca symptoms (OR, 1.57), compared with those without.
- Patients with IA reported significantly lower HRQoL scores and significantly greater physical disability, compared with those who did not have IA (P < .001 for both).
- IA was significantly less common among patients with , compared with those without pulmonary arterial hypertension (7.2% vs 11.3%; P = .007).
IN PRACTICE:
“Recognizing the presence of IA in SSc is an important first step, as its treatment and monitoring may alleviate some of the associated morbidity,” the researchers wrote.
SOURCE:
The lead author of the study was Eric Schwender, a medical student at the Royal College of Surgeons in Ireland, Dublin, Ireland. The study was published online in Arthritis Care & Research.
LIMITATIONS:
The inability to assess distribution and severity of IA limited the results, as did the inability to assess the impact of disease-modifying antirheumatic drugs in patients with IA.
DISCLOSURES:
The study was supported by Scleroderma Australia, Arthritis Australia, Actelion Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, and Pfizer, as well as grants to several researchers from the National Health and Medical Research Council of Australia. Lead author Mr. Schwender had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Increased Burden of Headache and Migraine in PsA Patients
Key clinical point: Compared with control individuals without psoriatic arthritis (PsA), the burden of headache and migraine without aura was significantly higher in patients with PsA.
Major finding: The prevalence of headache (39.81% vs 26.44%; P = .028) and migraine without aura (18.52% vs 9.2%; P = .044) was significantly higher in patients with PsA vs control individuals without PsA. Patients with PsA who did vs did not have headaches also presented with a higher burden of comorbidities and prevalence of enthesitis (P = .02 for both).
Study details: This cross-sectional, observational cohort study included 216 patients with PsA, 70 patients with axial spondyloarthritis, and 87 gender-matched control individuals.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Marino A, Currado D, Altamura C, et al. Increased prevalence of headaches and migraine in patients with psoriatic arthritis and axial spondyloarthritis: Insights from an Italian cohort study. Biomedicines. 2024;12(2):371. doi: 10.3390/biomedicines12020371 Source
Key clinical point: Compared with control individuals without psoriatic arthritis (PsA), the burden of headache and migraine without aura was significantly higher in patients with PsA.
Major finding: The prevalence of headache (39.81% vs 26.44%; P = .028) and migraine without aura (18.52% vs 9.2%; P = .044) was significantly higher in patients with PsA vs control individuals without PsA. Patients with PsA who did vs did not have headaches also presented with a higher burden of comorbidities and prevalence of enthesitis (P = .02 for both).
Study details: This cross-sectional, observational cohort study included 216 patients with PsA, 70 patients with axial spondyloarthritis, and 87 gender-matched control individuals.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Marino A, Currado D, Altamura C, et al. Increased prevalence of headaches and migraine in patients with psoriatic arthritis and axial spondyloarthritis: Insights from an Italian cohort study. Biomedicines. 2024;12(2):371. doi: 10.3390/biomedicines12020371 Source
Key clinical point: Compared with control individuals without psoriatic arthritis (PsA), the burden of headache and migraine without aura was significantly higher in patients with PsA.
Major finding: The prevalence of headache (39.81% vs 26.44%; P = .028) and migraine without aura (18.52% vs 9.2%; P = .044) was significantly higher in patients with PsA vs control individuals without PsA. Patients with PsA who did vs did not have headaches also presented with a higher burden of comorbidities and prevalence of enthesitis (P = .02 for both).
Study details: This cross-sectional, observational cohort study included 216 patients with PsA, 70 patients with axial spondyloarthritis, and 87 gender-matched control individuals.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Marino A, Currado D, Altamura C, et al. Increased prevalence of headaches and migraine in patients with psoriatic arthritis and axial spondyloarthritis: Insights from an Italian cohort study. Biomedicines. 2024;12(2):371. doi: 10.3390/biomedicines12020371 Source
Etanercept Effective and Safe in PsA Patients in the Real-World
Key clinical point: In patients with psoriatic arthritis (PsA), etanercept improved disease remission in routine clinical practice without showing any new safety signals.
Major finding: At week 12, 38.3% of patients with PsA achieved disease remission, with the proportion of patients achieving remission increasing to 51% and 54% at weeks 24 and 52, respectively. Among patients who achieved remission at week 12, 52.2% maintained it up to week 52. The most common treatment-emergent serious adverse events were aggravated condition, injury, poisoning, procedural complications, cardiac disorders, gastrointestinal disorders, and neoplasms.
Study details: Findings are from the prospective, non-interventional ADEQUATE study including patients with PsA (n = 254), axial spondyloarthritis (n = 305), or psoriasis (n = 70) who received etanercept for up to 52 weeks.
Disclosures: This study was funded by Pfizer Pharma GmbH. Three authors declared being employees and shareholders of Pfizer. Other authors declared receiving consulting fees, speaker fees, honoraria, or grants from or having other ties with various sources, including Pfizer.
Source: Feist E, Baraliakos X, Behrens F, et al. Etanercept in axial spondyloarthritis, psoriatic arthritis, and plaque psoriasis: Real-world outcome data from German non-interventional study ADEQUATE. Rheumatol Ther. 2024 (Feb 3). doi: 10.1007/s40744-023-00633-2 Source
Key clinical point: In patients with psoriatic arthritis (PsA), etanercept improved disease remission in routine clinical practice without showing any new safety signals.
Major finding: At week 12, 38.3% of patients with PsA achieved disease remission, with the proportion of patients achieving remission increasing to 51% and 54% at weeks 24 and 52, respectively. Among patients who achieved remission at week 12, 52.2% maintained it up to week 52. The most common treatment-emergent serious adverse events were aggravated condition, injury, poisoning, procedural complications, cardiac disorders, gastrointestinal disorders, and neoplasms.
Study details: Findings are from the prospective, non-interventional ADEQUATE study including patients with PsA (n = 254), axial spondyloarthritis (n = 305), or psoriasis (n = 70) who received etanercept for up to 52 weeks.
Disclosures: This study was funded by Pfizer Pharma GmbH. Three authors declared being employees and shareholders of Pfizer. Other authors declared receiving consulting fees, speaker fees, honoraria, or grants from or having other ties with various sources, including Pfizer.
Source: Feist E, Baraliakos X, Behrens F, et al. Etanercept in axial spondyloarthritis, psoriatic arthritis, and plaque psoriasis: Real-world outcome data from German non-interventional study ADEQUATE. Rheumatol Ther. 2024 (Feb 3). doi: 10.1007/s40744-023-00633-2 Source
Key clinical point: In patients with psoriatic arthritis (PsA), etanercept improved disease remission in routine clinical practice without showing any new safety signals.
Major finding: At week 12, 38.3% of patients with PsA achieved disease remission, with the proportion of patients achieving remission increasing to 51% and 54% at weeks 24 and 52, respectively. Among patients who achieved remission at week 12, 52.2% maintained it up to week 52. The most common treatment-emergent serious adverse events were aggravated condition, injury, poisoning, procedural complications, cardiac disorders, gastrointestinal disorders, and neoplasms.
Study details: Findings are from the prospective, non-interventional ADEQUATE study including patients with PsA (n = 254), axial spondyloarthritis (n = 305), or psoriasis (n = 70) who received etanercept for up to 52 weeks.
Disclosures: This study was funded by Pfizer Pharma GmbH. Three authors declared being employees and shareholders of Pfizer. Other authors declared receiving consulting fees, speaker fees, honoraria, or grants from or having other ties with various sources, including Pfizer.
Source: Feist E, Baraliakos X, Behrens F, et al. Etanercept in axial spondyloarthritis, psoriatic arthritis, and plaque psoriasis: Real-world outcome data from German non-interventional study ADEQUATE. Rheumatol Ther. 2024 (Feb 3). doi: 10.1007/s40744-023-00633-2 Source
Two or More Morbidities at Psoriasis Onset May Indicate Increased PsA Risk
Key clinical point: Patients with multimorbid psoriasis should be monitored for the potential development of psoriatic arthritis (PsA) as the presence of ≥2 morbidities at psoriasis incidence has been associated with an increased risk for PsA.
Major finding: The cumulative incidence of PsA in patients with psoriasis was 1.7%, 2.5%, and 2.9% at 5, 10, and 15 years after psoriasis incidence, respectively, with the risk for PsA being higher in those with ≥2 morbidities (hazard ratio 2.46; 95% CI 1.01-6.01).
Study details: Findings are from a retrospective cohort study including 817 patients with incident psoriasis and 849 age- and sex-matched comparator individuals without psoriasis; of these, 23 patients with psoriasis developed PsA during a median follow-up of 13.3 years.
Disclosures: This study was sponsored by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and other sources. Two authors declared serving as consultants for or receiving research support and grants from various sources.
Source: Karmacharya P, Chakradhar R, Hulshizer CA, et al. Multimorbidity in psoriasis as a risk factor for psoriatic arthritis: A population-based study. Rheumatology (Oxford). 2024 (Jan 30). doi: 10.1093/rheumatology/keae040 Source
Key clinical point: Patients with multimorbid psoriasis should be monitored for the potential development of psoriatic arthritis (PsA) as the presence of ≥2 morbidities at psoriasis incidence has been associated with an increased risk for PsA.
Major finding: The cumulative incidence of PsA in patients with psoriasis was 1.7%, 2.5%, and 2.9% at 5, 10, and 15 years after psoriasis incidence, respectively, with the risk for PsA being higher in those with ≥2 morbidities (hazard ratio 2.46; 95% CI 1.01-6.01).
Study details: Findings are from a retrospective cohort study including 817 patients with incident psoriasis and 849 age- and sex-matched comparator individuals without psoriasis; of these, 23 patients with psoriasis developed PsA during a median follow-up of 13.3 years.
Disclosures: This study was sponsored by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and other sources. Two authors declared serving as consultants for or receiving research support and grants from various sources.
Source: Karmacharya P, Chakradhar R, Hulshizer CA, et al. Multimorbidity in psoriasis as a risk factor for psoriatic arthritis: A population-based study. Rheumatology (Oxford). 2024 (Jan 30). doi: 10.1093/rheumatology/keae040 Source
Key clinical point: Patients with multimorbid psoriasis should be monitored for the potential development of psoriatic arthritis (PsA) as the presence of ≥2 morbidities at psoriasis incidence has been associated with an increased risk for PsA.
Major finding: The cumulative incidence of PsA in patients with psoriasis was 1.7%, 2.5%, and 2.9% at 5, 10, and 15 years after psoriasis incidence, respectively, with the risk for PsA being higher in those with ≥2 morbidities (hazard ratio 2.46; 95% CI 1.01-6.01).
Study details: Findings are from a retrospective cohort study including 817 patients with incident psoriasis and 849 age- and sex-matched comparator individuals without psoriasis; of these, 23 patients with psoriasis developed PsA during a median follow-up of 13.3 years.
Disclosures: This study was sponsored by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and other sources. Two authors declared serving as consultants for or receiving research support and grants from various sources.
Source: Karmacharya P, Chakradhar R, Hulshizer CA, et al. Multimorbidity in psoriasis as a risk factor for psoriatic arthritis: A population-based study. Rheumatology (Oxford). 2024 (Jan 30). doi: 10.1093/rheumatology/keae040 Source
Serum Biomarkers Identifying Guselkumab Responders Among TNFi-Inadequate Responders with PsA
Key clinical point: Elevated baseline levels of interleukin (IL)-22, IL-17A, and β-defensin (BD)-2 were linked to the achievement of robust skin response with guselkumab in difficult to treat patients with psoriatic arthritis (PsA) who showed inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Levels of IL-22 and BD-2 were elevated in TNFi-IR patients with PsA who did vs did not achieve a ≥90% improvement in Psoriasis Area and Severity Index with guselkumab (P < .05). Levels of IL-17A and BD-2 were similarly elevated in patients who did vs did not respond to Investigator’s Global Assessment 0/1 criteria with guselkumab (all P < .05).
Study details: This post hoc analysis of pooled data from DISCOVER-1, DISCOVER-2, and COSMOS studies included patients with active PsA who were biologic-naive (n = 251) or had TNFi-IR (n = 93) and who received 100 mg guselkumab every 8 weeks.
Disclosures: This study was sponsored by Janssen Research & Development. Ten authors declared being employees of Janssen or subsidiaries or owning stocks or stock options in Johnson & Johnson. Several authors declared other ties with various sources, including Janssen. Other authors had no conflicts of interest.
Source: Siebert S, Coates LC, Schett G, et al. Modulation of IL-23 signaling with guselkumab in biologic-naïve patients versus TNF inhibitor-inadequate responders with active psoriatic arthritis. Arthritis Rheumatol. 2024 (Jan 22). doi: 10.1002/art.42803 Source
Key clinical point: Elevated baseline levels of interleukin (IL)-22, IL-17A, and β-defensin (BD)-2 were linked to the achievement of robust skin response with guselkumab in difficult to treat patients with psoriatic arthritis (PsA) who showed inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Levels of IL-22 and BD-2 were elevated in TNFi-IR patients with PsA who did vs did not achieve a ≥90% improvement in Psoriasis Area and Severity Index with guselkumab (P < .05). Levels of IL-17A and BD-2 were similarly elevated in patients who did vs did not respond to Investigator’s Global Assessment 0/1 criteria with guselkumab (all P < .05).
Study details: This post hoc analysis of pooled data from DISCOVER-1, DISCOVER-2, and COSMOS studies included patients with active PsA who were biologic-naive (n = 251) or had TNFi-IR (n = 93) and who received 100 mg guselkumab every 8 weeks.
Disclosures: This study was sponsored by Janssen Research & Development. Ten authors declared being employees of Janssen or subsidiaries or owning stocks or stock options in Johnson & Johnson. Several authors declared other ties with various sources, including Janssen. Other authors had no conflicts of interest.
Source: Siebert S, Coates LC, Schett G, et al. Modulation of IL-23 signaling with guselkumab in biologic-naïve patients versus TNF inhibitor-inadequate responders with active psoriatic arthritis. Arthritis Rheumatol. 2024 (Jan 22). doi: 10.1002/art.42803 Source
Key clinical point: Elevated baseline levels of interleukin (IL)-22, IL-17A, and β-defensin (BD)-2 were linked to the achievement of robust skin response with guselkumab in difficult to treat patients with psoriatic arthritis (PsA) who showed inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Levels of IL-22 and BD-2 were elevated in TNFi-IR patients with PsA who did vs did not achieve a ≥90% improvement in Psoriasis Area and Severity Index with guselkumab (P < .05). Levels of IL-17A and BD-2 were similarly elevated in patients who did vs did not respond to Investigator’s Global Assessment 0/1 criteria with guselkumab (all P < .05).
Study details: This post hoc analysis of pooled data from DISCOVER-1, DISCOVER-2, and COSMOS studies included patients with active PsA who were biologic-naive (n = 251) or had TNFi-IR (n = 93) and who received 100 mg guselkumab every 8 weeks.
Disclosures: This study was sponsored by Janssen Research & Development. Ten authors declared being employees of Janssen or subsidiaries or owning stocks or stock options in Johnson & Johnson. Several authors declared other ties with various sources, including Janssen. Other authors had no conflicts of interest.
Source: Siebert S, Coates LC, Schett G, et al. Modulation of IL-23 signaling with guselkumab in biologic-naïve patients versus TNF inhibitor-inadequate responders with active psoriatic arthritis. Arthritis Rheumatol. 2024 (Jan 22). doi: 10.1002/art.42803 Source
Lower Prevalence of Psychotic Disorders in Patients with PsA
Key clinical point: Compared with the general population, the prevalence of psychotic disorders was significantly lower in patients with psoriatic arthritis (PsA), but a co-diagnosis of PsA and psychotic disorders was associated with an increased mortality rate, that too at a lower age.
Major finding: The prevalence of psychotic disorders was significantly lower in patients with PsA vs the general population (0.61% vs 0.72%; P = .006). Patients with PsA who did vs did not have psychotic disorders had an increased likelihood of death (9.3% vs 4.9%; P = .005) and significantly lower age at death (64.7 years vs 74.5 years; P < .001).
Study details: This cohort study included 449,392 patients with psoriasis and 47,825 patients with PsA.
Disclosures: The lead author Emilie Brenaut declared receiving a postdoctoral fellowship from the French Society of Dermatology and the Collège des Enseignants en Dermatologie de France as well as personal fees and nonfinancial support from various sources. The other authors reported no conflicts of interest.
Source: Brenaut E, Godin O, Leboyer M, et al. Association between psychotic disorders and psoriasis or psoriatic arthritis: Cohort study of French health insurance database. J Invest Dermatol. 2024 (Jan 19). doi: 10.1016/j.jid.2024.01.005 Source
Key clinical point: Compared with the general population, the prevalence of psychotic disorders was significantly lower in patients with psoriatic arthritis (PsA), but a co-diagnosis of PsA and psychotic disorders was associated with an increased mortality rate, that too at a lower age.
Major finding: The prevalence of psychotic disorders was significantly lower in patients with PsA vs the general population (0.61% vs 0.72%; P = .006). Patients with PsA who did vs did not have psychotic disorders had an increased likelihood of death (9.3% vs 4.9%; P = .005) and significantly lower age at death (64.7 years vs 74.5 years; P < .001).
Study details: This cohort study included 449,392 patients with psoriasis and 47,825 patients with PsA.
Disclosures: The lead author Emilie Brenaut declared receiving a postdoctoral fellowship from the French Society of Dermatology and the Collège des Enseignants en Dermatologie de France as well as personal fees and nonfinancial support from various sources. The other authors reported no conflicts of interest.
Source: Brenaut E, Godin O, Leboyer M, et al. Association between psychotic disorders and psoriasis or psoriatic arthritis: Cohort study of French health insurance database. J Invest Dermatol. 2024 (Jan 19). doi: 10.1016/j.jid.2024.01.005 Source
Key clinical point: Compared with the general population, the prevalence of psychotic disorders was significantly lower in patients with psoriatic arthritis (PsA), but a co-diagnosis of PsA and psychotic disorders was associated with an increased mortality rate, that too at a lower age.
Major finding: The prevalence of psychotic disorders was significantly lower in patients with PsA vs the general population (0.61% vs 0.72%; P = .006). Patients with PsA who did vs did not have psychotic disorders had an increased likelihood of death (9.3% vs 4.9%; P = .005) and significantly lower age at death (64.7 years vs 74.5 years; P < .001).
Study details: This cohort study included 449,392 patients with psoriasis and 47,825 patients with PsA.
Disclosures: The lead author Emilie Brenaut declared receiving a postdoctoral fellowship from the French Society of Dermatology and the Collège des Enseignants en Dermatologie de France as well as personal fees and nonfinancial support from various sources. The other authors reported no conflicts of interest.
Source: Brenaut E, Godin O, Leboyer M, et al. Association between psychotic disorders and psoriasis or psoriatic arthritis: Cohort study of French health insurance database. J Invest Dermatol. 2024 (Jan 19). doi: 10.1016/j.jid.2024.01.005 Source