User login
Bariatric surgery found to be effective in IBD patients
SAN DIEGO – In carefully selected patients with well-controlled inflammatory bowel disease (IBD), bariatric surgery results in sustained weight loss over a 2-year period, results from a retrospective study suggest.
“Obesity is increasing in patients with inflammatory bowel disease at a rate similar to that seen in the general population,” the study’s primary author, Nicholas P. McKenna, MD, said in an interview in advance of the annual Digestive Disease Week. “While bariatric surgery is a well-accepted therapy for obesity in patients without IBD, its use in patients with IBD is less well studied.”
For the current study, Dr. McKenna, a resident in the department of surgery at the Mayo Clinic in Rochester, Minn., and colleagues collected data on 33 patients who underwent bariatric surgery with a pre- or postoperative diagnosis of IBD across three academic centers between August 2006 and December 2017. They evaluated IBD characteristics and medications; postoperative complications; the need for future IBD-related surgery; and weight loss at 6, 12, and 24 months.
The patients underwent 34 bariatric operations. Their median age was 51 years and their median duration of IBD was 13 years. Of the 33 patients, 16 underwent a Roux-en-Y gastric bypass procedure: 9 who had ulcerative colitis, 6 who had Crohn’s disease, and 1 who had indeterminate colitis. A total of 14 patients underwent sleeve gastrectomy: 7 who had ulcerative colitis and 7 who had Crohn’s disease. Four patients underwent a gastric band procedure, all of whom had ulcerative colitis. The mean body mass index of patients prior to their bariatric procedures was 42.7 kg/m2. A total of 31 patients had an existing diagnosis of IBD, and 2 were diagnosed with Crohn’s disease after Roux-en-Y gastric bypass. In addition, 9 patients were on preoperative immunosuppression for IBD, and 11 had undergone prior intestinal resection for IBD.
Dr. McKenna reported that the average hospitalization for all patients was 3.6 days and that only four 30-day infectious complications occurred: two superficial surgical site infections, one infected intra-abdominal hematoma, and one hepatic abscess. In the long term, seven patients required reoperation: three for failed gastric band, two for reduction of internal hernia, and two for cholelithiasis. The researchers found that the mean percentage of overall excess weight loss was 57.5% at 6 months, 63.3% at 12 months, and 58.6% at 24 months. During a mean follow-up of 3.4 years, no IBD flares requiring surgery were observed.
“Our hypothesis based on the existing literature was that bariatric surgery would be safe in carefully selected patients with IBD and result in sustained weight loss, so we were not surprised with these results,” Dr. McKenna said. “We were not sure if medication requirements would change after surgery as the literature is conflicted on this. We observed that most patients continued to require no immunosuppression for control of their IBD after surgery. Further, we did not observe that any patients required future surgery at the time of last follow-up for an IBD flare.”
He acknowledged certain limitations of the study, including its retrospective design. “Additionally, though it is a relatively large sample, compared to the existing literature on bariatric surgery in IBD, it is still only 33 patients. This limits the comparisons that can be performed between patients with ulcerative colitis and Crohn’s disease and between the operation choices.”
The study’s secondary author, Alaa Sada, MD, a surgery resident at Mayo, presented the findings at the meeting. The researchers reported having no financial disclosures.
SAN DIEGO – In carefully selected patients with well-controlled inflammatory bowel disease (IBD), bariatric surgery results in sustained weight loss over a 2-year period, results from a retrospective study suggest.
“Obesity is increasing in patients with inflammatory bowel disease at a rate similar to that seen in the general population,” the study’s primary author, Nicholas P. McKenna, MD, said in an interview in advance of the annual Digestive Disease Week. “While bariatric surgery is a well-accepted therapy for obesity in patients without IBD, its use in patients with IBD is less well studied.”
For the current study, Dr. McKenna, a resident in the department of surgery at the Mayo Clinic in Rochester, Minn., and colleagues collected data on 33 patients who underwent bariatric surgery with a pre- or postoperative diagnosis of IBD across three academic centers between August 2006 and December 2017. They evaluated IBD characteristics and medications; postoperative complications; the need for future IBD-related surgery; and weight loss at 6, 12, and 24 months.
The patients underwent 34 bariatric operations. Their median age was 51 years and their median duration of IBD was 13 years. Of the 33 patients, 16 underwent a Roux-en-Y gastric bypass procedure: 9 who had ulcerative colitis, 6 who had Crohn’s disease, and 1 who had indeterminate colitis. A total of 14 patients underwent sleeve gastrectomy: 7 who had ulcerative colitis and 7 who had Crohn’s disease. Four patients underwent a gastric band procedure, all of whom had ulcerative colitis. The mean body mass index of patients prior to their bariatric procedures was 42.7 kg/m2. A total of 31 patients had an existing diagnosis of IBD, and 2 were diagnosed with Crohn’s disease after Roux-en-Y gastric bypass. In addition, 9 patients were on preoperative immunosuppression for IBD, and 11 had undergone prior intestinal resection for IBD.
Dr. McKenna reported that the average hospitalization for all patients was 3.6 days and that only four 30-day infectious complications occurred: two superficial surgical site infections, one infected intra-abdominal hematoma, and one hepatic abscess. In the long term, seven patients required reoperation: three for failed gastric band, two for reduction of internal hernia, and two for cholelithiasis. The researchers found that the mean percentage of overall excess weight loss was 57.5% at 6 months, 63.3% at 12 months, and 58.6% at 24 months. During a mean follow-up of 3.4 years, no IBD flares requiring surgery were observed.
“Our hypothesis based on the existing literature was that bariatric surgery would be safe in carefully selected patients with IBD and result in sustained weight loss, so we were not surprised with these results,” Dr. McKenna said. “We were not sure if medication requirements would change after surgery as the literature is conflicted on this. We observed that most patients continued to require no immunosuppression for control of their IBD after surgery. Further, we did not observe that any patients required future surgery at the time of last follow-up for an IBD flare.”
He acknowledged certain limitations of the study, including its retrospective design. “Additionally, though it is a relatively large sample, compared to the existing literature on bariatric surgery in IBD, it is still only 33 patients. This limits the comparisons that can be performed between patients with ulcerative colitis and Crohn’s disease and between the operation choices.”
The study’s secondary author, Alaa Sada, MD, a surgery resident at Mayo, presented the findings at the meeting. The researchers reported having no financial disclosures.
SAN DIEGO – In carefully selected patients with well-controlled inflammatory bowel disease (IBD), bariatric surgery results in sustained weight loss over a 2-year period, results from a retrospective study suggest.
“Obesity is increasing in patients with inflammatory bowel disease at a rate similar to that seen in the general population,” the study’s primary author, Nicholas P. McKenna, MD, said in an interview in advance of the annual Digestive Disease Week. “While bariatric surgery is a well-accepted therapy for obesity in patients without IBD, its use in patients with IBD is less well studied.”
For the current study, Dr. McKenna, a resident in the department of surgery at the Mayo Clinic in Rochester, Minn., and colleagues collected data on 33 patients who underwent bariatric surgery with a pre- or postoperative diagnosis of IBD across three academic centers between August 2006 and December 2017. They evaluated IBD characteristics and medications; postoperative complications; the need for future IBD-related surgery; and weight loss at 6, 12, and 24 months.
The patients underwent 34 bariatric operations. Their median age was 51 years and their median duration of IBD was 13 years. Of the 33 patients, 16 underwent a Roux-en-Y gastric bypass procedure: 9 who had ulcerative colitis, 6 who had Crohn’s disease, and 1 who had indeterminate colitis. A total of 14 patients underwent sleeve gastrectomy: 7 who had ulcerative colitis and 7 who had Crohn’s disease. Four patients underwent a gastric band procedure, all of whom had ulcerative colitis. The mean body mass index of patients prior to their bariatric procedures was 42.7 kg/m2. A total of 31 patients had an existing diagnosis of IBD, and 2 were diagnosed with Crohn’s disease after Roux-en-Y gastric bypass. In addition, 9 patients were on preoperative immunosuppression for IBD, and 11 had undergone prior intestinal resection for IBD.
Dr. McKenna reported that the average hospitalization for all patients was 3.6 days and that only four 30-day infectious complications occurred: two superficial surgical site infections, one infected intra-abdominal hematoma, and one hepatic abscess. In the long term, seven patients required reoperation: three for failed gastric band, two for reduction of internal hernia, and two for cholelithiasis. The researchers found that the mean percentage of overall excess weight loss was 57.5% at 6 months, 63.3% at 12 months, and 58.6% at 24 months. During a mean follow-up of 3.4 years, no IBD flares requiring surgery were observed.
“Our hypothesis based on the existing literature was that bariatric surgery would be safe in carefully selected patients with IBD and result in sustained weight loss, so we were not surprised with these results,” Dr. McKenna said. “We were not sure if medication requirements would change after surgery as the literature is conflicted on this. We observed that most patients continued to require no immunosuppression for control of their IBD after surgery. Further, we did not observe that any patients required future surgery at the time of last follow-up for an IBD flare.”
He acknowledged certain limitations of the study, including its retrospective design. “Additionally, though it is a relatively large sample, compared to the existing literature on bariatric surgery in IBD, it is still only 33 patients. This limits the comparisons that can be performed between patients with ulcerative colitis and Crohn’s disease and between the operation choices.”
The study’s secondary author, Alaa Sada, MD, a surgery resident at Mayo, presented the findings at the meeting. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Chronic opioid use linked to low testosterone levels
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
REPORTING FROM ENDO 2019
FDA updates warning about Impella RP System
The Food and Drug Administration has reported that the higher postapproval mortality rates seen with Abiomed’s Impella RP System seem concentrated in a certain subgroup of patients only, according to a letter to health care providers.
The letter updates one from February regarding the observation of higher postapproval mortality rates with the temporary right heart pump.
This subgroup, which did not qualify for premarket clinical studies, was more likely to have been in cardiogenic shock for longer than 48 hours, experienced a cardiac arrest, or suffered a preimplant hypoxic or ischemic neurologic event prior to receiving the device, the FDA suggested in this new letter to health care providers. The 30-day survival rate in this subgroup within a postapproval study (PAS) was 10.7% (3 out of 28), while that among patients who would have qualified for the premarket clinical studies was 64.3% (9 of 14), according to the most recent interim results of that study. The rate among patients who would have qualified for premarket studies is similar to that seen among those premarket studies (73.4%); the overall 30-day survival rate in this PAS was 28.6%.
The FDA said that, based on these analyses, it still believes the benefits outweigh the risks when the Impella RP System is “used for the currently approved indication in appropriately selected patients.”
The FDA advises that health care providers review the device’s revised labeling, which now includes a checklist to help understand which patients could benefit the most. It also advises providers to promptly report any adverse events through MedWatch, which can help the FDA identify and understand the risks associated with the Impella RP System.
More information can be found in the FDA’s letter to health care providers, which is available on the FDA website.
The Food and Drug Administration has reported that the higher postapproval mortality rates seen with Abiomed’s Impella RP System seem concentrated in a certain subgroup of patients only, according to a letter to health care providers.
The letter updates one from February regarding the observation of higher postapproval mortality rates with the temporary right heart pump.
This subgroup, which did not qualify for premarket clinical studies, was more likely to have been in cardiogenic shock for longer than 48 hours, experienced a cardiac arrest, or suffered a preimplant hypoxic or ischemic neurologic event prior to receiving the device, the FDA suggested in this new letter to health care providers. The 30-day survival rate in this subgroup within a postapproval study (PAS) was 10.7% (3 out of 28), while that among patients who would have qualified for the premarket clinical studies was 64.3% (9 of 14), according to the most recent interim results of that study. The rate among patients who would have qualified for premarket studies is similar to that seen among those premarket studies (73.4%); the overall 30-day survival rate in this PAS was 28.6%.
The FDA said that, based on these analyses, it still believes the benefits outweigh the risks when the Impella RP System is “used for the currently approved indication in appropriately selected patients.”
The FDA advises that health care providers review the device’s revised labeling, which now includes a checklist to help understand which patients could benefit the most. It also advises providers to promptly report any adverse events through MedWatch, which can help the FDA identify and understand the risks associated with the Impella RP System.
More information can be found in the FDA’s letter to health care providers, which is available on the FDA website.
The Food and Drug Administration has reported that the higher postapproval mortality rates seen with Abiomed’s Impella RP System seem concentrated in a certain subgroup of patients only, according to a letter to health care providers.
The letter updates one from February regarding the observation of higher postapproval mortality rates with the temporary right heart pump.
This subgroup, which did not qualify for premarket clinical studies, was more likely to have been in cardiogenic shock for longer than 48 hours, experienced a cardiac arrest, or suffered a preimplant hypoxic or ischemic neurologic event prior to receiving the device, the FDA suggested in this new letter to health care providers. The 30-day survival rate in this subgroup within a postapproval study (PAS) was 10.7% (3 out of 28), while that among patients who would have qualified for the premarket clinical studies was 64.3% (9 of 14), according to the most recent interim results of that study. The rate among patients who would have qualified for premarket studies is similar to that seen among those premarket studies (73.4%); the overall 30-day survival rate in this PAS was 28.6%.
The FDA said that, based on these analyses, it still believes the benefits outweigh the risks when the Impella RP System is “used for the currently approved indication in appropriately selected patients.”
The FDA advises that health care providers review the device’s revised labeling, which now includes a checklist to help understand which patients could benefit the most. It also advises providers to promptly report any adverse events through MedWatch, which can help the FDA identify and understand the risks associated with the Impella RP System.
More information can be found in the FDA’s letter to health care providers, which is available on the FDA website.
Prior authorizations for dermatology care nearly doubled in the last 2 years at one center
CHICAGO – Prior authorizations for the delivery of care in dermatology may be increasing steeply, judging from the experience of one large academic dermatology practice.
“About the same number of patients were seen in 2018 as in 2016, but the number of prior authorizations required to serve those patients went up substantially,” reported Ryan P. Carlisle, a medical student who performed this analysis under the guidance of Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City.
Tracking of prior authorizations for the delivery of dermatologic care was initiated in 2016 at the University of Utah. By a number of measures, the burden of prior authorizations has been increasing steadily since that time, Mr. Carlisle said at the annual meeting of the Society for Investigative Dermatology.
As an example, one prior authorization was required for every 15 patient visits (6.7%) over a 30-day period in September 2016. In comparison, one prior authorization was required for every 9 patient visits (11.1%) in a comparable 30-day period in September 2018. Further, the number of clinic visits during this more recent period was 2.4% higher than in the earlier one (9,743 vs. 9,512), so the number of prior authorizations increased by 73.8% (1,088 vs. 626), Mr. Carlisle reported.
Two full-time staff and eight part-time staff at the University of Utah handle prior authorizations for 40 dermatologists and 10 nonphysician clinicians. The substantial unreimbursed costs incurred by this labor can be huge, he said. In one specific case, 81% of the reimbursed cost for a patient visit was consumed by seeking a prior authorization.
Of prior authorizations tracked at the University of Utah, 39.1% were for nonbiologic therapies, 21.6% were for excisions, 16% were for Moh’s surgery, 11% were for biologics, and the remainder was for an array of other procedures or therapies.
Of these, prior authorizations for biologics “were the most burdensome both in time and cost” on a per-visit basis, Mr. Carlisle reported.
The proportions of prior authorizations that were denied were relatively low. The highest proportion of denials was for nonbiologic medications (25%). The rate of denials for biologics over the study period was just 11%. Moreover, of denials that were appealed, 56% were granted.
Importantly, some prior authorizations were rarely denied. This includes a 0% denial rate for Moh’s surgery and a 1% denial rate for incisional procedures. Mr. Carlisle questioned whether the requirement for prior authorizations makes sense in these situations.
“Dermatology should partner with insurers to reduce unnecessary prior authorizations and appeals,” Mr. Carlisle suggested. It “is likely that these are affecting patient care” as well as ultimately, and perhaps unnecessarily, reducing reimbursement.
“The process to get prior authorizations completed and get patients their medications has just gotten to be too burdensome,” said Mr. Carlisle, summarizing the Utah experience.
SOURCE: Carlisle RP. SID 2019, Abstract 247.
CHICAGO – Prior authorizations for the delivery of care in dermatology may be increasing steeply, judging from the experience of one large academic dermatology practice.
“About the same number of patients were seen in 2018 as in 2016, but the number of prior authorizations required to serve those patients went up substantially,” reported Ryan P. Carlisle, a medical student who performed this analysis under the guidance of Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City.
Tracking of prior authorizations for the delivery of dermatologic care was initiated in 2016 at the University of Utah. By a number of measures, the burden of prior authorizations has been increasing steadily since that time, Mr. Carlisle said at the annual meeting of the Society for Investigative Dermatology.
As an example, one prior authorization was required for every 15 patient visits (6.7%) over a 30-day period in September 2016. In comparison, one prior authorization was required for every 9 patient visits (11.1%) in a comparable 30-day period in September 2018. Further, the number of clinic visits during this more recent period was 2.4% higher than in the earlier one (9,743 vs. 9,512), so the number of prior authorizations increased by 73.8% (1,088 vs. 626), Mr. Carlisle reported.
Two full-time staff and eight part-time staff at the University of Utah handle prior authorizations for 40 dermatologists and 10 nonphysician clinicians. The substantial unreimbursed costs incurred by this labor can be huge, he said. In one specific case, 81% of the reimbursed cost for a patient visit was consumed by seeking a prior authorization.
Of prior authorizations tracked at the University of Utah, 39.1% were for nonbiologic therapies, 21.6% were for excisions, 16% were for Moh’s surgery, 11% were for biologics, and the remainder was for an array of other procedures or therapies.
Of these, prior authorizations for biologics “were the most burdensome both in time and cost” on a per-visit basis, Mr. Carlisle reported.
The proportions of prior authorizations that were denied were relatively low. The highest proportion of denials was for nonbiologic medications (25%). The rate of denials for biologics over the study period was just 11%. Moreover, of denials that were appealed, 56% were granted.
Importantly, some prior authorizations were rarely denied. This includes a 0% denial rate for Moh’s surgery and a 1% denial rate for incisional procedures. Mr. Carlisle questioned whether the requirement for prior authorizations makes sense in these situations.
“Dermatology should partner with insurers to reduce unnecessary prior authorizations and appeals,” Mr. Carlisle suggested. It “is likely that these are affecting patient care” as well as ultimately, and perhaps unnecessarily, reducing reimbursement.
“The process to get prior authorizations completed and get patients their medications has just gotten to be too burdensome,” said Mr. Carlisle, summarizing the Utah experience.
SOURCE: Carlisle RP. SID 2019, Abstract 247.
CHICAGO – Prior authorizations for the delivery of care in dermatology may be increasing steeply, judging from the experience of one large academic dermatology practice.
“About the same number of patients were seen in 2018 as in 2016, but the number of prior authorizations required to serve those patients went up substantially,” reported Ryan P. Carlisle, a medical student who performed this analysis under the guidance of Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City.
Tracking of prior authorizations for the delivery of dermatologic care was initiated in 2016 at the University of Utah. By a number of measures, the burden of prior authorizations has been increasing steadily since that time, Mr. Carlisle said at the annual meeting of the Society for Investigative Dermatology.
As an example, one prior authorization was required for every 15 patient visits (6.7%) over a 30-day period in September 2016. In comparison, one prior authorization was required for every 9 patient visits (11.1%) in a comparable 30-day period in September 2018. Further, the number of clinic visits during this more recent period was 2.4% higher than in the earlier one (9,743 vs. 9,512), so the number of prior authorizations increased by 73.8% (1,088 vs. 626), Mr. Carlisle reported.
Two full-time staff and eight part-time staff at the University of Utah handle prior authorizations for 40 dermatologists and 10 nonphysician clinicians. The substantial unreimbursed costs incurred by this labor can be huge, he said. In one specific case, 81% of the reimbursed cost for a patient visit was consumed by seeking a prior authorization.
Of prior authorizations tracked at the University of Utah, 39.1% were for nonbiologic therapies, 21.6% were for excisions, 16% were for Moh’s surgery, 11% were for biologics, and the remainder was for an array of other procedures or therapies.
Of these, prior authorizations for biologics “were the most burdensome both in time and cost” on a per-visit basis, Mr. Carlisle reported.
The proportions of prior authorizations that were denied were relatively low. The highest proportion of denials was for nonbiologic medications (25%). The rate of denials for biologics over the study period was just 11%. Moreover, of denials that were appealed, 56% were granted.
Importantly, some prior authorizations were rarely denied. This includes a 0% denial rate for Moh’s surgery and a 1% denial rate for incisional procedures. Mr. Carlisle questioned whether the requirement for prior authorizations makes sense in these situations.
“Dermatology should partner with insurers to reduce unnecessary prior authorizations and appeals,” Mr. Carlisle suggested. It “is likely that these are affecting patient care” as well as ultimately, and perhaps unnecessarily, reducing reimbursement.
“The process to get prior authorizations completed and get patients their medications has just gotten to be too burdensome,” said Mr. Carlisle, summarizing the Utah experience.
SOURCE: Carlisle RP. SID 2019, Abstract 247.
REPORTING FROM SID 2019
Pilot program addresses social determinants of health
WASHINGTON –
The program, called SHAPE (Social Health Alliance to Promote Equity), was designed to screen patients across multiple social categories. In-house patient navigators, some bilingual, were trained on how to work with diverse populations and were able to address unmet patient needs through referrals to individualized resources. Physicians could refer patients to the following local community partners:
- The Child Center of NY.
- The INN – serving hungry & homeless Long Islanders.
- Maurice A. Deane School of Law – Hofstra Law.
- The Gitenstein Institute for Health Law and Policy.
The legal partners provided free assistance to patience with legal needs.
“By implementing a program where you address the nonmedical social needs, you will actually improve the overall health of the patient. You can’t just address the biomedical needs of your patients, you need to understand their home environment, their background, and social situations they’re going through to keep them healthy,” said Jane Lindahl at the annual meeting of the Society of General Internal Medicine. Ms. Lindahl is a research assistant at Cohen Children’s Medical Center at Northwell Health in New York.
The SHAPE program was conducted at two internal medicine and pediatric primary care clinics at Northwell Health, a large academic hospital system in New York. It was originally created in the pediatric practice in August 2016 and expanded to the internal medicine practice in June 2018. A medicolegal partnership was created as part of the program in October 2018.
The patient population comprised low-income, racially ethnic, primarily Medicaid and uninsured individuals, including a high number of documented and undocumented immigrants. While 927 patients were screened, 590 screened positive for social determinants of health (SDOH). Of those 590 patients, 190 patients connected with patient navigators for intake and accepted initial assistance and 74 patients were connected to resources.
Screening was based on patients’ completion of a one-page SDOH form in the waiting room of their physician’s office on the same day of their annual visit. There were 15 categories of social needs identified on the screen.
After the screening, the results were discussed with the patients and the necessary referrals were determined. The screening indicated that the largest needs for the patients were health/dental insurance (cited by 296 people), education (cited by 269 people), and health literacy (cited by 225 patients).
Those who had emergent social needs were referred to on-site social workers and providers to address such needs. The emergent social needs included being a victim of domestic violence, being homeless, having an imminent eviction, and having imminent deportation.
Those patients with nonemergent social needs received referral and follow-up processes within 48 hours.
After a referral was made, the patient navigator followed up every 2 weeks with the patient to check on the status of the referral and social needs. After this period, a final phone interview was conducted to get feedback on the patient’s experience and SDOH status.
Ms. Lindahl had no financial conflicts of interest to disclose. The program was funded by Robert Wood Johnson Foundation Clinical Scholars Grant, Health Leads, Collaborative to Advance Social Health Integration, N.Y. State Delivery System Reform Incentive Program, and United Hospital Foundation.
WASHINGTON –
The program, called SHAPE (Social Health Alliance to Promote Equity), was designed to screen patients across multiple social categories. In-house patient navigators, some bilingual, were trained on how to work with diverse populations and were able to address unmet patient needs through referrals to individualized resources. Physicians could refer patients to the following local community partners:
- The Child Center of NY.
- The INN – serving hungry & homeless Long Islanders.
- Maurice A. Deane School of Law – Hofstra Law.
- The Gitenstein Institute for Health Law and Policy.
The legal partners provided free assistance to patience with legal needs.
“By implementing a program where you address the nonmedical social needs, you will actually improve the overall health of the patient. You can’t just address the biomedical needs of your patients, you need to understand their home environment, their background, and social situations they’re going through to keep them healthy,” said Jane Lindahl at the annual meeting of the Society of General Internal Medicine. Ms. Lindahl is a research assistant at Cohen Children’s Medical Center at Northwell Health in New York.
The SHAPE program was conducted at two internal medicine and pediatric primary care clinics at Northwell Health, a large academic hospital system in New York. It was originally created in the pediatric practice in August 2016 and expanded to the internal medicine practice in June 2018. A medicolegal partnership was created as part of the program in October 2018.
The patient population comprised low-income, racially ethnic, primarily Medicaid and uninsured individuals, including a high number of documented and undocumented immigrants. While 927 patients were screened, 590 screened positive for social determinants of health (SDOH). Of those 590 patients, 190 patients connected with patient navigators for intake and accepted initial assistance and 74 patients were connected to resources.
Screening was based on patients’ completion of a one-page SDOH form in the waiting room of their physician’s office on the same day of their annual visit. There were 15 categories of social needs identified on the screen.
After the screening, the results were discussed with the patients and the necessary referrals were determined. The screening indicated that the largest needs for the patients were health/dental insurance (cited by 296 people), education (cited by 269 people), and health literacy (cited by 225 patients).
Those who had emergent social needs were referred to on-site social workers and providers to address such needs. The emergent social needs included being a victim of domestic violence, being homeless, having an imminent eviction, and having imminent deportation.
Those patients with nonemergent social needs received referral and follow-up processes within 48 hours.
After a referral was made, the patient navigator followed up every 2 weeks with the patient to check on the status of the referral and social needs. After this period, a final phone interview was conducted to get feedback on the patient’s experience and SDOH status.
Ms. Lindahl had no financial conflicts of interest to disclose. The program was funded by Robert Wood Johnson Foundation Clinical Scholars Grant, Health Leads, Collaborative to Advance Social Health Integration, N.Y. State Delivery System Reform Incentive Program, and United Hospital Foundation.
WASHINGTON –
The program, called SHAPE (Social Health Alliance to Promote Equity), was designed to screen patients across multiple social categories. In-house patient navigators, some bilingual, were trained on how to work with diverse populations and were able to address unmet patient needs through referrals to individualized resources. Physicians could refer patients to the following local community partners:
- The Child Center of NY.
- The INN – serving hungry & homeless Long Islanders.
- Maurice A. Deane School of Law – Hofstra Law.
- The Gitenstein Institute for Health Law and Policy.
The legal partners provided free assistance to patience with legal needs.
“By implementing a program where you address the nonmedical social needs, you will actually improve the overall health of the patient. You can’t just address the biomedical needs of your patients, you need to understand their home environment, their background, and social situations they’re going through to keep them healthy,” said Jane Lindahl at the annual meeting of the Society of General Internal Medicine. Ms. Lindahl is a research assistant at Cohen Children’s Medical Center at Northwell Health in New York.
The SHAPE program was conducted at two internal medicine and pediatric primary care clinics at Northwell Health, a large academic hospital system in New York. It was originally created in the pediatric practice in August 2016 and expanded to the internal medicine practice in June 2018. A medicolegal partnership was created as part of the program in October 2018.
The patient population comprised low-income, racially ethnic, primarily Medicaid and uninsured individuals, including a high number of documented and undocumented immigrants. While 927 patients were screened, 590 screened positive for social determinants of health (SDOH). Of those 590 patients, 190 patients connected with patient navigators for intake and accepted initial assistance and 74 patients were connected to resources.
Screening was based on patients’ completion of a one-page SDOH form in the waiting room of their physician’s office on the same day of their annual visit. There were 15 categories of social needs identified on the screen.
After the screening, the results were discussed with the patients and the necessary referrals were determined. The screening indicated that the largest needs for the patients were health/dental insurance (cited by 296 people), education (cited by 269 people), and health literacy (cited by 225 patients).
Those who had emergent social needs were referred to on-site social workers and providers to address such needs. The emergent social needs included being a victim of domestic violence, being homeless, having an imminent eviction, and having imminent deportation.
Those patients with nonemergent social needs received referral and follow-up processes within 48 hours.
After a referral was made, the patient navigator followed up every 2 weeks with the patient to check on the status of the referral and social needs. After this period, a final phone interview was conducted to get feedback on the patient’s experience and SDOH status.
Ms. Lindahl had no financial conflicts of interest to disclose. The program was funded by Robert Wood Johnson Foundation Clinical Scholars Grant, Health Leads, Collaborative to Advance Social Health Integration, N.Y. State Delivery System Reform Incentive Program, and United Hospital Foundation.
REPORTING FROM SGIM 2019
Multiple Subcutaneous Dermoid Cysts
To the Editor:
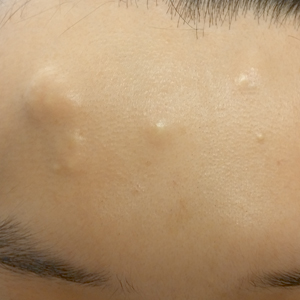
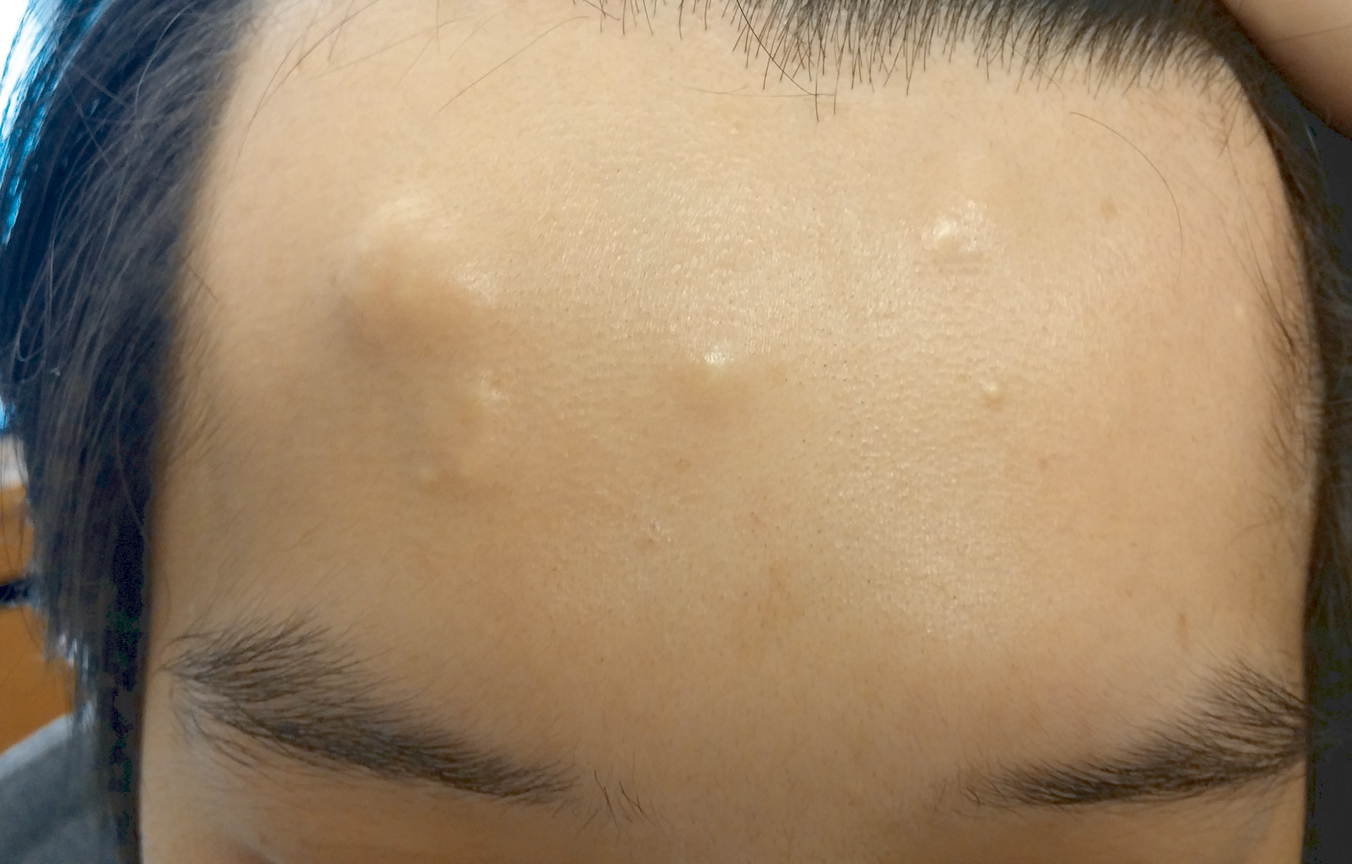
A 30-year-old man with no notable medical history presented to the dermatology clinic with multiple subcutaneous nodules on the forehead of 5 years’ duration. He reported no history of forehead trauma or manipulation of the lesions, and there was no accompanying pruritis, pain, erythema, or purulent discharge. There was no family history of skin or gastrointestinal tract tumors. On physical examination, the patient had 5 firm, flesh-colored to yellow nodules measuring approximately 0.2 to 1.5 cm in diameter without central punctae scattered over the central forehead (Figure 1). Due to cosmetic concerns, the patient elected to pursue surgical excision of the lesions, which occurred over several office visits. During surgical excision, the lesions were found to be smooth, encapsulated, and mobile, and they were excised without surgical complication. Histopathologic examination showed subcutaneous cysts lined by squamous epithelium with associated sebaceous glands (Figure 2A) and hair follicles in the cyst lumen (Figure 2B). These findings confirmed the diagnosis of multiple subcutaneous dermoid cysts.
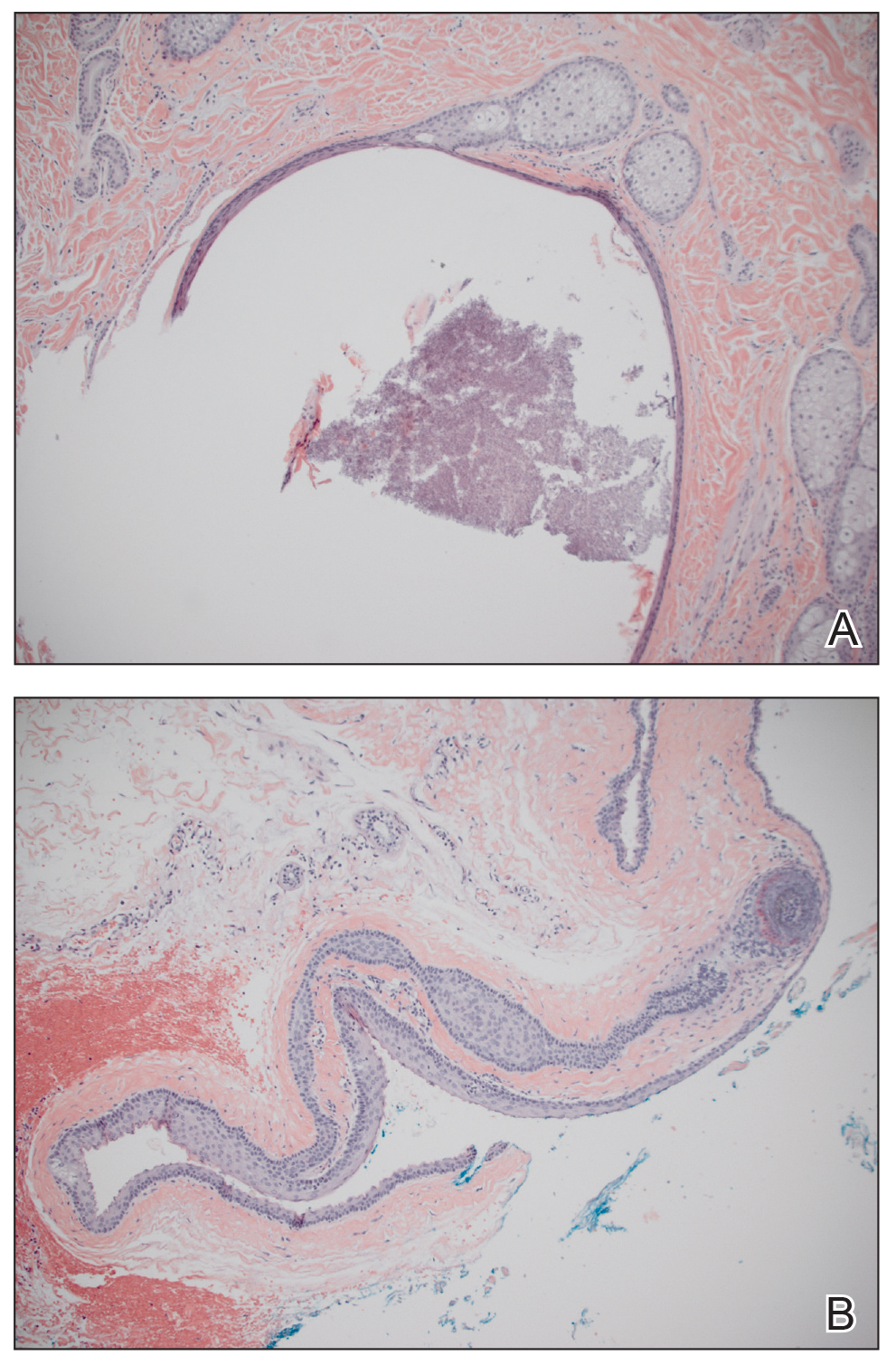
Dermoid cysts are relatively uncommon, benign tumors consisting of tissue derived from ectodermal and mesodermal germ cell layers. Dermoid cysts may be distinguished from teratomas, which may contain tissues derived from all 3 germ cell layers and typically consist of types of tissues foreign to the site of origin, such as dental, thyroid, gastrointestinal, or neural tissue.1,2 The majority of dermoid cysts are congenitally developed along the lines of embryologic fusion due to an error in the division of the ectoderm and mesoderm3,4; however, some dermoid cysts may be acquired from epidermal elements being traumatically implanted into the dermis.5
Our patient’s presentation with multiple dermoid cysts was atypical, as dermoid cysts are almost always solitary tumors. A similar case was reported in a 41-year-old man who developed multiple dermoid cysts on the forehead over a 20-year period.This patient also was otherwise healthy, denied prior trauma to the forehead, and reported no family history of skin or gastrointestinal tract tumors.5
Another unusual feature in our case was the location of the dermoid cysts on the central forehead. The most common location for dermoid cysts is the lateral third of the eyebrows (47%–70% of cases).1,4,6-10 These cysts occur because of sequestration of the surface ectoderm during fusion along the naso-optic groove.2 Dermoid cysts also have been noted in other anatomical areas such as the scalp, nose, anterior neck, and trunk.6
Dermoid cysts tend to be small, round, smooth, and slowly growing until sudden enlargement prompts surgical evaluation.4,6 During surgical excision, they often are fixed to the underlying bone but also may be freely mobile, as in our patient.6 Histopathologic examination reveals a stratified squamous epithelium with associated adnexal structures such as sebaceous glands or hair follicles.1 Smooth muscle fibers, prominent vascular stroma, small nerves, and collagen and elastic fibers also may be found within the lumen of dermoid cysts.2
In some cases, dermoid cysts may be invasive and carry the risk of bony erosion, intracranial extension, osteomyelitis, meningitis, or cerebral abscess. Imaging studies sometimes are needed to rule out intracranial or intraspinal extension, particularly for midline dermoid cysts.6 The standard of treatment for dermoid cysts is surgical excision and complete enucleation without disruption of the cyst wall; however, invasive dermoid cysts may require endoscopic excision, orbitotomy, or craniotomy.4,6
- Brownstein MH, Helwig EB. Subcutaneous dermoid cysts. Arch Dermatol. 1973;107:237-239.
- Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Yamaki T, Higuchi R, Sasaki K, et al. Multiple dermoid cysts on the forehead. case report. Scand J Plast Reconstr Surg Hand Surg. 1996;30:321-324.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Al-Khateeb TH, Al-Masri NM, Al-Zoubi F. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2009;67:52-57.
- McAvoy JM, Zuckerbraun L. Dermoid cysts of the head and neck in children. Arch Otolaryngol. 1976;102:529-531.
- Taylor BW, Erich JB, Dockerty MB. Dermoids of the head and neck. Minnesota Med. 1966;49:1535-1540.
- Golden BA, Zide MF. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2005;63:1613-1619.
To the Editor:
A 30-year-old man with no notable medical history presented to the dermatology clinic with multiple subcutaneous nodules on the forehead of 5 years’ duration. He reported no history of forehead trauma or manipulation of the lesions, and there was no accompanying pruritis, pain, erythema, or purulent discharge. There was no family history of skin or gastrointestinal tract tumors. On physical examination, the patient had 5 firm, flesh-colored to yellow nodules measuring approximately 0.2 to 1.5 cm in diameter without central punctae scattered over the central forehead (Figure 1). Due to cosmetic concerns, the patient elected to pursue surgical excision of the lesions, which occurred over several office visits. During surgical excision, the lesions were found to be smooth, encapsulated, and mobile, and they were excised without surgical complication. Histopathologic examination showed subcutaneous cysts lined by squamous epithelium with associated sebaceous glands (Figure 2A) and hair follicles in the cyst lumen (Figure 2B). These findings confirmed the diagnosis of multiple subcutaneous dermoid cysts.


Dermoid cysts are relatively uncommon, benign tumors consisting of tissue derived from ectodermal and mesodermal germ cell layers. Dermoid cysts may be distinguished from teratomas, which may contain tissues derived from all 3 germ cell layers and typically consist of types of tissues foreign to the site of origin, such as dental, thyroid, gastrointestinal, or neural tissue.1,2 The majority of dermoid cysts are congenitally developed along the lines of embryologic fusion due to an error in the division of the ectoderm and mesoderm3,4; however, some dermoid cysts may be acquired from epidermal elements being traumatically implanted into the dermis.5
Our patient’s presentation with multiple dermoid cysts was atypical, as dermoid cysts are almost always solitary tumors. A similar case was reported in a 41-year-old man who developed multiple dermoid cysts on the forehead over a 20-year period.This patient also was otherwise healthy, denied prior trauma to the forehead, and reported no family history of skin or gastrointestinal tract tumors.5
Another unusual feature in our case was the location of the dermoid cysts on the central forehead. The most common location for dermoid cysts is the lateral third of the eyebrows (47%–70% of cases).1,4,6-10 These cysts occur because of sequestration of the surface ectoderm during fusion along the naso-optic groove.2 Dermoid cysts also have been noted in other anatomical areas such as the scalp, nose, anterior neck, and trunk.6
Dermoid cysts tend to be small, round, smooth, and slowly growing until sudden enlargement prompts surgical evaluation.4,6 During surgical excision, they often are fixed to the underlying bone but also may be freely mobile, as in our patient.6 Histopathologic examination reveals a stratified squamous epithelium with associated adnexal structures such as sebaceous glands or hair follicles.1 Smooth muscle fibers, prominent vascular stroma, small nerves, and collagen and elastic fibers also may be found within the lumen of dermoid cysts.2
In some cases, dermoid cysts may be invasive and carry the risk of bony erosion, intracranial extension, osteomyelitis, meningitis, or cerebral abscess. Imaging studies sometimes are needed to rule out intracranial or intraspinal extension, particularly for midline dermoid cysts.6 The standard of treatment for dermoid cysts is surgical excision and complete enucleation without disruption of the cyst wall; however, invasive dermoid cysts may require endoscopic excision, orbitotomy, or craniotomy.4,6
To the Editor:
A 30-year-old man with no notable medical history presented to the dermatology clinic with multiple subcutaneous nodules on the forehead of 5 years’ duration. He reported no history of forehead trauma or manipulation of the lesions, and there was no accompanying pruritis, pain, erythema, or purulent discharge. There was no family history of skin or gastrointestinal tract tumors. On physical examination, the patient had 5 firm, flesh-colored to yellow nodules measuring approximately 0.2 to 1.5 cm in diameter without central punctae scattered over the central forehead (Figure 1). Due to cosmetic concerns, the patient elected to pursue surgical excision of the lesions, which occurred over several office visits. During surgical excision, the lesions were found to be smooth, encapsulated, and mobile, and they were excised without surgical complication. Histopathologic examination showed subcutaneous cysts lined by squamous epithelium with associated sebaceous glands (Figure 2A) and hair follicles in the cyst lumen (Figure 2B). These findings confirmed the diagnosis of multiple subcutaneous dermoid cysts.


Dermoid cysts are relatively uncommon, benign tumors consisting of tissue derived from ectodermal and mesodermal germ cell layers. Dermoid cysts may be distinguished from teratomas, which may contain tissues derived from all 3 germ cell layers and typically consist of types of tissues foreign to the site of origin, such as dental, thyroid, gastrointestinal, or neural tissue.1,2 The majority of dermoid cysts are congenitally developed along the lines of embryologic fusion due to an error in the division of the ectoderm and mesoderm3,4; however, some dermoid cysts may be acquired from epidermal elements being traumatically implanted into the dermis.5
Our patient’s presentation with multiple dermoid cysts was atypical, as dermoid cysts are almost always solitary tumors. A similar case was reported in a 41-year-old man who developed multiple dermoid cysts on the forehead over a 20-year period.This patient also was otherwise healthy, denied prior trauma to the forehead, and reported no family history of skin or gastrointestinal tract tumors.5
Another unusual feature in our case was the location of the dermoid cysts on the central forehead. The most common location for dermoid cysts is the lateral third of the eyebrows (47%–70% of cases).1,4,6-10 These cysts occur because of sequestration of the surface ectoderm during fusion along the naso-optic groove.2 Dermoid cysts also have been noted in other anatomical areas such as the scalp, nose, anterior neck, and trunk.6
Dermoid cysts tend to be small, round, smooth, and slowly growing until sudden enlargement prompts surgical evaluation.4,6 During surgical excision, they often are fixed to the underlying bone but also may be freely mobile, as in our patient.6 Histopathologic examination reveals a stratified squamous epithelium with associated adnexal structures such as sebaceous glands or hair follicles.1 Smooth muscle fibers, prominent vascular stroma, small nerves, and collagen and elastic fibers also may be found within the lumen of dermoid cysts.2
In some cases, dermoid cysts may be invasive and carry the risk of bony erosion, intracranial extension, osteomyelitis, meningitis, or cerebral abscess. Imaging studies sometimes are needed to rule out intracranial or intraspinal extension, particularly for midline dermoid cysts.6 The standard of treatment for dermoid cysts is surgical excision and complete enucleation without disruption of the cyst wall; however, invasive dermoid cysts may require endoscopic excision, orbitotomy, or craniotomy.4,6
- Brownstein MH, Helwig EB. Subcutaneous dermoid cysts. Arch Dermatol. 1973;107:237-239.
- Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Yamaki T, Higuchi R, Sasaki K, et al. Multiple dermoid cysts on the forehead. case report. Scand J Plast Reconstr Surg Hand Surg. 1996;30:321-324.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Al-Khateeb TH, Al-Masri NM, Al-Zoubi F. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2009;67:52-57.
- McAvoy JM, Zuckerbraun L. Dermoid cysts of the head and neck in children. Arch Otolaryngol. 1976;102:529-531.
- Taylor BW, Erich JB, Dockerty MB. Dermoids of the head and neck. Minnesota Med. 1966;49:1535-1540.
- Golden BA, Zide MF. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2005;63:1613-1619.
- Brownstein MH, Helwig EB. Subcutaneous dermoid cysts. Arch Dermatol. 1973;107:237-239.
- Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Yamaki T, Higuchi R, Sasaki K, et al. Multiple dermoid cysts on the forehead. case report. Scand J Plast Reconstr Surg Hand Surg. 1996;30:321-324.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Al-Khateeb TH, Al-Masri NM, Al-Zoubi F. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2009;67:52-57.
- McAvoy JM, Zuckerbraun L. Dermoid cysts of the head and neck in children. Arch Otolaryngol. 1976;102:529-531.
- Taylor BW, Erich JB, Dockerty MB. Dermoids of the head and neck. Minnesota Med. 1966;49:1535-1540.
- Golden BA, Zide MF. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2005;63:1613-1619.
Practice Points
- The majority of dermoid cysts are congenital; however, they may be acquired from traumatic implantation of epidermal elements into the dermis.
- The most common location for dermoid cysts is the lateral third of the eyebrows; however, they also may occur on the mid forehead, scalp, nose, anterior neck, and trunk.
- Imaging studies may be needed to rule out intracranial or intraspinal extension of dermoid cysts, particularly for those presenting in the midline.
Weight can block psychiatric facility admission
SAN FRANCISCO – Not too long ago, a 42-year-old homeless man could not leave the Los Angeles County Hospital because he was too heavy.
He had been admitted on a 72-hour involuntary psychiatric hold because he was suicidal; he had a history of severe depression and had made several attempts in the past. He had few social connections and had come in covered in lice after sleeping outdoors for months. He was mobile, but barely so, with the help of a walker.
He was cleaned up and medically stabilized at the hospital, but when it came time to transfer him to a psychiatric facility, no one would take him because he was morbidly obese, with a body mass index (BMI) of 53 mg/m2.
“We noticed this had happened with other patients. It wasn’t just this one case, so we” decided to take a closer look, said Katherine Camfield, MD, a psychiatric resident at Los Angeles County Hospital.
She and her colleagues called 43 inpatient psychiatric facilities in L.A. County. It turned out that 41, more than 60% of the total and including both public and private facilities, had weight restrictions. Staff members at the remaining two facilities were unsure.
For 2 hospitals, it was a BMI above 50; for 17, a weight above 350 pounds; and for 6, a weight above 300 pounds. Three had a limit of just 250 pounds. Others decided on a case-by-case basis. About 10% of the people put on psychiatric holds at the L.A. County Hospital weigh 250 pounds or more and therefore would not meet the weight limits at many facilities.
Some hospitals cited concerns about staff injuries from moving – or trying to calm – a large patient. Others said they did not have lifts and other specialized equipment or that their beds could not handle the weight. Others did not really give a reason.
County hospitals “are not a very therapeutic milieu; staff and nursing aren’t necessarily trained for unstable psychiatric patients,” Dr. Camfield said at the American Psychiatric Association annual meeting.
“Honestly, having a mental illness alone increases your risk of obesity, and then we give a lot of medications that increase the risk” even more. “It’s a vicious cycle,” and one that raises the issue of discrimination, although “whether these routine denials to access psychiatric hospitalization violate antidiscrimination laws is unclear,” she said.
She and her colleagues plan to take a deeper dive into the issue, to find out how widespread the problem is, and the reasons behind it. They are also interested in cost; “a county facility has limited resources; are they being misallocated because these patients are stuck in the hospital?” Dr. Camfield wondered.
The homeless man never made to a psychiatric facility. He was put on antidepressants and stayed in the county hospital for 20 days, until he was no longer suicidal. He then was transferred to skilled nursing facility.
There was no external funding, and Dr. Camfield had no disclosures.
SAN FRANCISCO – Not too long ago, a 42-year-old homeless man could not leave the Los Angeles County Hospital because he was too heavy.
He had been admitted on a 72-hour involuntary psychiatric hold because he was suicidal; he had a history of severe depression and had made several attempts in the past. He had few social connections and had come in covered in lice after sleeping outdoors for months. He was mobile, but barely so, with the help of a walker.
He was cleaned up and medically stabilized at the hospital, but when it came time to transfer him to a psychiatric facility, no one would take him because he was morbidly obese, with a body mass index (BMI) of 53 mg/m2.
“We noticed this had happened with other patients. It wasn’t just this one case, so we” decided to take a closer look, said Katherine Camfield, MD, a psychiatric resident at Los Angeles County Hospital.
She and her colleagues called 43 inpatient psychiatric facilities in L.A. County. It turned out that 41, more than 60% of the total and including both public and private facilities, had weight restrictions. Staff members at the remaining two facilities were unsure.
For 2 hospitals, it was a BMI above 50; for 17, a weight above 350 pounds; and for 6, a weight above 300 pounds. Three had a limit of just 250 pounds. Others decided on a case-by-case basis. About 10% of the people put on psychiatric holds at the L.A. County Hospital weigh 250 pounds or more and therefore would not meet the weight limits at many facilities.
Some hospitals cited concerns about staff injuries from moving – or trying to calm – a large patient. Others said they did not have lifts and other specialized equipment or that their beds could not handle the weight. Others did not really give a reason.
County hospitals “are not a very therapeutic milieu; staff and nursing aren’t necessarily trained for unstable psychiatric patients,” Dr. Camfield said at the American Psychiatric Association annual meeting.
“Honestly, having a mental illness alone increases your risk of obesity, and then we give a lot of medications that increase the risk” even more. “It’s a vicious cycle,” and one that raises the issue of discrimination, although “whether these routine denials to access psychiatric hospitalization violate antidiscrimination laws is unclear,” she said.
She and her colleagues plan to take a deeper dive into the issue, to find out how widespread the problem is, and the reasons behind it. They are also interested in cost; “a county facility has limited resources; are they being misallocated because these patients are stuck in the hospital?” Dr. Camfield wondered.
The homeless man never made to a psychiatric facility. He was put on antidepressants and stayed in the county hospital for 20 days, until he was no longer suicidal. He then was transferred to skilled nursing facility.
There was no external funding, and Dr. Camfield had no disclosures.
SAN FRANCISCO – Not too long ago, a 42-year-old homeless man could not leave the Los Angeles County Hospital because he was too heavy.
He had been admitted on a 72-hour involuntary psychiatric hold because he was suicidal; he had a history of severe depression and had made several attempts in the past. He had few social connections and had come in covered in lice after sleeping outdoors for months. He was mobile, but barely so, with the help of a walker.
He was cleaned up and medically stabilized at the hospital, but when it came time to transfer him to a psychiatric facility, no one would take him because he was morbidly obese, with a body mass index (BMI) of 53 mg/m2.
“We noticed this had happened with other patients. It wasn’t just this one case, so we” decided to take a closer look, said Katherine Camfield, MD, a psychiatric resident at Los Angeles County Hospital.
She and her colleagues called 43 inpatient psychiatric facilities in L.A. County. It turned out that 41, more than 60% of the total and including both public and private facilities, had weight restrictions. Staff members at the remaining two facilities were unsure.
For 2 hospitals, it was a BMI above 50; for 17, a weight above 350 pounds; and for 6, a weight above 300 pounds. Three had a limit of just 250 pounds. Others decided on a case-by-case basis. About 10% of the people put on psychiatric holds at the L.A. County Hospital weigh 250 pounds or more and therefore would not meet the weight limits at many facilities.
Some hospitals cited concerns about staff injuries from moving – or trying to calm – a large patient. Others said they did not have lifts and other specialized equipment or that their beds could not handle the weight. Others did not really give a reason.
County hospitals “are not a very therapeutic milieu; staff and nursing aren’t necessarily trained for unstable psychiatric patients,” Dr. Camfield said at the American Psychiatric Association annual meeting.
“Honestly, having a mental illness alone increases your risk of obesity, and then we give a lot of medications that increase the risk” even more. “It’s a vicious cycle,” and one that raises the issue of discrimination, although “whether these routine denials to access psychiatric hospitalization violate antidiscrimination laws is unclear,” she said.
She and her colleagues plan to take a deeper dive into the issue, to find out how widespread the problem is, and the reasons behind it. They are also interested in cost; “a county facility has limited resources; are they being misallocated because these patients are stuck in the hospital?” Dr. Camfield wondered.
The homeless man never made to a psychiatric facility. He was put on antidepressants and stayed in the county hospital for 20 days, until he was no longer suicidal. He then was transferred to skilled nursing facility.
There was no external funding, and Dr. Camfield had no disclosures.
REPORTING FROM APA 2019
Confronting physician depression and suicide

Please join us Monday, June 3, at 8:00 p.m. ET as we discuss depression in physicians and the resulting tragedies, as well as how to help physicians receive the treatment they need to prevent suicide.
Our special guests include physicians with expertise on the triggers of physician depression and solutions for fighting this epidemic, Sarah Candler, MD, (@sarahgcandler) and Elisabeth Poorman, MD, (@DrPoorman). We hope you will participate in our Twitter chat on June 3 at 8 p.m. ET. #MDedgeChats
Physicians have higher rates of depression and suicide, compared with the general population, according to the American Foundation for Suicide Prevention. Suicide rates for male physicians are 1.41 times higher than in the general population and, for female physicians, 2.27 times greater.
“But these numbers come from only those deaths that are reported publicly,” Dr. Poorman wrote in an article that was published by WBUR, Boston’s NPR News Station. In fact, as Dr. Poorman was writing this story, she learned of five separate deaths that were widely known to be suicides, but never publicly identified as such, she said.
One possible explanation for physicians’ higher likelihood of becoming depressed or dying by suicide, compared with other professionals, is their hesitance to receive mental health treatment, experts have suggested. Psychiatrist Michael F. Myers, MD, found anecdotal evidence of this tendency when he interviewed the loved ones, friends, and colleagues of physicians who took their own lives.
In this chat we will discuss this topic and other possible reasons for the high rate of depression and suicide among physicians.
Topics of conversation
Question 1: What are the causes of physician depression and suicide in training and practice?
Question 2: How can we end stigma against seeking mental health treatment among physicians?
Question 3: How can we prevent depression and suicide among medical students and physicians?
Question 4: Which institutions or programs are exemplars in providing resources to improve mental health?
Question 5: What organizational and political changes are likely to reduce physician suicides?

Resources
1. Getting Back to Medicine as a Community
2. What stops physicians from getting mental health care?
3. Risk considerations for suicidal physicians
4. Two more and counting: Suicide in medical trainees
5. How to talk with a struggling physician colleague
6. Healthcare industry takes on high physician suicide rates, mental health stigma
7. The Curbsiders episode #129 Depression and Suicide: Occupational Hazards of Practicing Medicine
8. Creating Joy in Medicine in St. Paul, MN: A Case Study
About Dr. Poorman
Dr. Poorman (@DrPoorman) is a primary care physician at the University of Washington in Seattle, a freelance journalist covering issues related to medicine, and a frequent speaker on the causes of and solutions to mental illness among providers.
Her writing has been featured in Self, The Guardian. Medpage Today’s KevinMD.com, and other publications. She produces the blog: https://www.drpoorman.org/what-we-do.
About Dr. Candler
Dr. Candler (@sarahgcandler) is a primary care physician in Houston. She is coeditor of the Annals Fresh Look blog, and a member of Internal Medicine News’ Editorial Advisory Board.
She is currently consulting for a tech firm and will later be joining Iora Health to start a Medicare Advantage clinic.

Please join us Monday, June 3, at 8:00 p.m. ET as we discuss depression in physicians and the resulting tragedies, as well as how to help physicians receive the treatment they need to prevent suicide.
Our special guests include physicians with expertise on the triggers of physician depression and solutions for fighting this epidemic, Sarah Candler, MD, (@sarahgcandler) and Elisabeth Poorman, MD, (@DrPoorman). We hope you will participate in our Twitter chat on June 3 at 8 p.m. ET. #MDedgeChats
Physicians have higher rates of depression and suicide, compared with the general population, according to the American Foundation for Suicide Prevention. Suicide rates for male physicians are 1.41 times higher than in the general population and, for female physicians, 2.27 times greater.
“But these numbers come from only those deaths that are reported publicly,” Dr. Poorman wrote in an article that was published by WBUR, Boston’s NPR News Station. In fact, as Dr. Poorman was writing this story, she learned of five separate deaths that were widely known to be suicides, but never publicly identified as such, she said.
One possible explanation for physicians’ higher likelihood of becoming depressed or dying by suicide, compared with other professionals, is their hesitance to receive mental health treatment, experts have suggested. Psychiatrist Michael F. Myers, MD, found anecdotal evidence of this tendency when he interviewed the loved ones, friends, and colleagues of physicians who took their own lives.
In this chat we will discuss this topic and other possible reasons for the high rate of depression and suicide among physicians.
Topics of conversation
Question 1: What are the causes of physician depression and suicide in training and practice?
Question 2: How can we end stigma against seeking mental health treatment among physicians?
Question 3: How can we prevent depression and suicide among medical students and physicians?
Question 4: Which institutions or programs are exemplars in providing resources to improve mental health?
Question 5: What organizational and political changes are likely to reduce physician suicides?

Resources
1. Getting Back to Medicine as a Community
2. What stops physicians from getting mental health care?
3. Risk considerations for suicidal physicians
4. Two more and counting: Suicide in medical trainees
5. How to talk with a struggling physician colleague
6. Healthcare industry takes on high physician suicide rates, mental health stigma
7. The Curbsiders episode #129 Depression and Suicide: Occupational Hazards of Practicing Medicine
8. Creating Joy in Medicine in St. Paul, MN: A Case Study
About Dr. Poorman
Dr. Poorman (@DrPoorman) is a primary care physician at the University of Washington in Seattle, a freelance journalist covering issues related to medicine, and a frequent speaker on the causes of and solutions to mental illness among providers.
Her writing has been featured in Self, The Guardian. Medpage Today’s KevinMD.com, and other publications. She produces the blog: https://www.drpoorman.org/what-we-do.
About Dr. Candler
Dr. Candler (@sarahgcandler) is a primary care physician in Houston. She is coeditor of the Annals Fresh Look blog, and a member of Internal Medicine News’ Editorial Advisory Board.
She is currently consulting for a tech firm and will later be joining Iora Health to start a Medicare Advantage clinic.

Please join us Monday, June 3, at 8:00 p.m. ET as we discuss depression in physicians and the resulting tragedies, as well as how to help physicians receive the treatment they need to prevent suicide.
Our special guests include physicians with expertise on the triggers of physician depression and solutions for fighting this epidemic, Sarah Candler, MD, (@sarahgcandler) and Elisabeth Poorman, MD, (@DrPoorman). We hope you will participate in our Twitter chat on June 3 at 8 p.m. ET. #MDedgeChats
Physicians have higher rates of depression and suicide, compared with the general population, according to the American Foundation for Suicide Prevention. Suicide rates for male physicians are 1.41 times higher than in the general population and, for female physicians, 2.27 times greater.
“But these numbers come from only those deaths that are reported publicly,” Dr. Poorman wrote in an article that was published by WBUR, Boston’s NPR News Station. In fact, as Dr. Poorman was writing this story, she learned of five separate deaths that were widely known to be suicides, but never publicly identified as such, she said.
One possible explanation for physicians’ higher likelihood of becoming depressed or dying by suicide, compared with other professionals, is their hesitance to receive mental health treatment, experts have suggested. Psychiatrist Michael F. Myers, MD, found anecdotal evidence of this tendency when he interviewed the loved ones, friends, and colleagues of physicians who took their own lives.
In this chat we will discuss this topic and other possible reasons for the high rate of depression and suicide among physicians.
Topics of conversation
Question 1: What are the causes of physician depression and suicide in training and practice?
Question 2: How can we end stigma against seeking mental health treatment among physicians?
Question 3: How can we prevent depression and suicide among medical students and physicians?
Question 4: Which institutions or programs are exemplars in providing resources to improve mental health?
Question 5: What organizational and political changes are likely to reduce physician suicides?

Resources
1. Getting Back to Medicine as a Community
2. What stops physicians from getting mental health care?
3. Risk considerations for suicidal physicians
4. Two more and counting: Suicide in medical trainees
5. How to talk with a struggling physician colleague
6. Healthcare industry takes on high physician suicide rates, mental health stigma
7. The Curbsiders episode #129 Depression and Suicide: Occupational Hazards of Practicing Medicine
8. Creating Joy in Medicine in St. Paul, MN: A Case Study
About Dr. Poorman
Dr. Poorman (@DrPoorman) is a primary care physician at the University of Washington in Seattle, a freelance journalist covering issues related to medicine, and a frequent speaker on the causes of and solutions to mental illness among providers.
Her writing has been featured in Self, The Guardian. Medpage Today’s KevinMD.com, and other publications. She produces the blog: https://www.drpoorman.org/what-we-do.
About Dr. Candler
Dr. Candler (@sarahgcandler) is a primary care physician in Houston. She is coeditor of the Annals Fresh Look blog, and a member of Internal Medicine News’ Editorial Advisory Board.
She is currently consulting for a tech firm and will later be joining Iora Health to start a Medicare Advantage clinic.
Posthospitalization thromboprophylaxis with rivaroxaban is unnecessary
Background: Anticoagulation for at-risk medical populations for posthospitalization thromboprophylaxis has been investigated in previous studies demonstrating a benefit in reducing risk of asymptomatic deep-vein thrombosis (DVT) development, but no studies have examined symptomatic DVTs.
Study design: Randomized, double-blind, placebo-controlled, multinational clinical trial.
Setting: 671 multinational hospitals.
Synopsis: 11,962 patients were identified as at-risk patients based on length of hospitalization (3-10 days), diagnosis, and additional risk factors identified by an IMPROVE risk score of greater than 4 or 2-3 with a D-dimer level more than twice the upper limit of normal. Patients were randomly assigned to receive rivaroxaban or placebo for 45 days. Primary outcome was composite of any symptomatic DVT or death related to VTE. Safety outcomes were principally related to bleeding. Symptomatic VTE or death from VTE occurred in 0.83% in the anticoagulation group and 1.1% in the placebo group (95% confidence interval, 0.52-1.09; P = .14). No significant difference was found in safety outcomes. The major limitation of the study was the low incidence of VTE and the need to include lower-risk patients (IMPROVE score 2/3 with elevated D-dimer), which may have decreased the effect of anticoagulation in the high-risk group (IMPROVE score 4 or greater).
Bottom line: No significant improvement in symptomatic VTE complications was found with posthospitalization thromboprophylaxis using rivaroxaban for an at-risk medical population.
Citation: Spyropoulos AC et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Eng J Med. 2018 Sep 20;379:1118-27.
Dr. Imber is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Anticoagulation for at-risk medical populations for posthospitalization thromboprophylaxis has been investigated in previous studies demonstrating a benefit in reducing risk of asymptomatic deep-vein thrombosis (DVT) development, but no studies have examined symptomatic DVTs.
Study design: Randomized, double-blind, placebo-controlled, multinational clinical trial.
Setting: 671 multinational hospitals.
Synopsis: 11,962 patients were identified as at-risk patients based on length of hospitalization (3-10 days), diagnosis, and additional risk factors identified by an IMPROVE risk score of greater than 4 or 2-3 with a D-dimer level more than twice the upper limit of normal. Patients were randomly assigned to receive rivaroxaban or placebo for 45 days. Primary outcome was composite of any symptomatic DVT or death related to VTE. Safety outcomes were principally related to bleeding. Symptomatic VTE or death from VTE occurred in 0.83% in the anticoagulation group and 1.1% in the placebo group (95% confidence interval, 0.52-1.09; P = .14). No significant difference was found in safety outcomes. The major limitation of the study was the low incidence of VTE and the need to include lower-risk patients (IMPROVE score 2/3 with elevated D-dimer), which may have decreased the effect of anticoagulation in the high-risk group (IMPROVE score 4 or greater).
Bottom line: No significant improvement in symptomatic VTE complications was found with posthospitalization thromboprophylaxis using rivaroxaban for an at-risk medical population.
Citation: Spyropoulos AC et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Eng J Med. 2018 Sep 20;379:1118-27.
Dr. Imber is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Anticoagulation for at-risk medical populations for posthospitalization thromboprophylaxis has been investigated in previous studies demonstrating a benefit in reducing risk of asymptomatic deep-vein thrombosis (DVT) development, but no studies have examined symptomatic DVTs.
Study design: Randomized, double-blind, placebo-controlled, multinational clinical trial.
Setting: 671 multinational hospitals.
Synopsis: 11,962 patients were identified as at-risk patients based on length of hospitalization (3-10 days), diagnosis, and additional risk factors identified by an IMPROVE risk score of greater than 4 or 2-3 with a D-dimer level more than twice the upper limit of normal. Patients were randomly assigned to receive rivaroxaban or placebo for 45 days. Primary outcome was composite of any symptomatic DVT or death related to VTE. Safety outcomes were principally related to bleeding. Symptomatic VTE or death from VTE occurred in 0.83% in the anticoagulation group and 1.1% in the placebo group (95% confidence interval, 0.52-1.09; P = .14). No significant difference was found in safety outcomes. The major limitation of the study was the low incidence of VTE and the need to include lower-risk patients (IMPROVE score 2/3 with elevated D-dimer), which may have decreased the effect of anticoagulation in the high-risk group (IMPROVE score 4 or greater).
Bottom line: No significant improvement in symptomatic VTE complications was found with posthospitalization thromboprophylaxis using rivaroxaban for an at-risk medical population.
Citation: Spyropoulos AC et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Eng J Med. 2018 Sep 20;379:1118-27.
Dr. Imber is an assistant professor in the division of hospital medicine, University of New Mexico.
Psychiatrists urged to raise awareness about human trafficking
SAN FRANCISCO – Psychiatrists see and interact with people who are being sex and labor trafficked “all the time” – and can learn more about how to identify these individuals, Rachel Robitz, MD, said at the annual meeting of the American Psychiatric Association.
In an exclusive video, Mollie Gordon, MD, interviewed Dr. Robitz about the intersection between trafficking and mental health. “What scares me the most is some of the statistics about self-harm,” said Dr. Robitz. “One study of sex-trafficked adults found that about 40% of them had a history of a suicide attempt. A study of sex-trafficked minors found that about 30% of them had a history of moderate to severe self-harm behavior.”
Dr. Robitz said. Other resources include those provided by the Department of Health & Human Services’s Office on Trafficking in Persons.
Dr. Robitz, who is double boarded in psychiatry and family medicine, is with the University of California, Davis. She previously worked for a program for homeless youth and for many programs aimed at helping adult and youth survivors of human trafficking. Dr. Robitz has no disclosures. Dr. Gordon is associate professor of psychiatry in the Menninger department of behavioral health at Baylor College of Medicine, Houston. She is a founding member of the Houston Area Human Trafficking Health Care Consortium. Dr. Gordon has no disclosures.
SAN FRANCISCO – Psychiatrists see and interact with people who are being sex and labor trafficked “all the time” – and can learn more about how to identify these individuals, Rachel Robitz, MD, said at the annual meeting of the American Psychiatric Association.
In an exclusive video, Mollie Gordon, MD, interviewed Dr. Robitz about the intersection between trafficking and mental health. “What scares me the most is some of the statistics about self-harm,” said Dr. Robitz. “One study of sex-trafficked adults found that about 40% of them had a history of a suicide attempt. A study of sex-trafficked minors found that about 30% of them had a history of moderate to severe self-harm behavior.”
Dr. Robitz said. Other resources include those provided by the Department of Health & Human Services’s Office on Trafficking in Persons.
Dr. Robitz, who is double boarded in psychiatry and family medicine, is with the University of California, Davis. She previously worked for a program for homeless youth and for many programs aimed at helping adult and youth survivors of human trafficking. Dr. Robitz has no disclosures. Dr. Gordon is associate professor of psychiatry in the Menninger department of behavioral health at Baylor College of Medicine, Houston. She is a founding member of the Houston Area Human Trafficking Health Care Consortium. Dr. Gordon has no disclosures.
SAN FRANCISCO – Psychiatrists see and interact with people who are being sex and labor trafficked “all the time” – and can learn more about how to identify these individuals, Rachel Robitz, MD, said at the annual meeting of the American Psychiatric Association.
In an exclusive video, Mollie Gordon, MD, interviewed Dr. Robitz about the intersection between trafficking and mental health. “What scares me the most is some of the statistics about self-harm,” said Dr. Robitz. “One study of sex-trafficked adults found that about 40% of them had a history of a suicide attempt. A study of sex-trafficked minors found that about 30% of them had a history of moderate to severe self-harm behavior.”
Dr. Robitz said. Other resources include those provided by the Department of Health & Human Services’s Office on Trafficking in Persons.
Dr. Robitz, who is double boarded in psychiatry and family medicine, is with the University of California, Davis. She previously worked for a program for homeless youth and for many programs aimed at helping adult and youth survivors of human trafficking. Dr. Robitz has no disclosures. Dr. Gordon is associate professor of psychiatry in the Menninger department of behavioral health at Baylor College of Medicine, Houston. She is a founding member of the Houston Area Human Trafficking Health Care Consortium. Dr. Gordon has no disclosures.
REPORTING FROM APA 2019