User login
Button batteries that pass to the stomach may warrant rapid endoscopic removal
SAN DIEGO – A button battery lodged in a child’s esophagus is an acknowledged emergency, but there is less evidence about retrieval of button batteries that have passed to the stomach. Observation alone has been recommended when an x-ray determines that the button battery has passed to the stomach within 2 hours of ingestion, when the battery is less than 20 mm, and the child is aged at least 5 years.
At the annual Digestive Disease Week, Racha Khalaf, MD, and Thomas Walker, MD, both of Children’s Hospital Colorado, Aurora, presented data that call this approach into question. Their retrospective cohort study of 4 years’ worth of records from four pediatric centers in the United States identified 68 cases in which a pediatric gastroenterologist had endoscopically removed the button battery. In 60% of those cases, the battery had already caused mucosal damage varying from minor to deep necrosis and perforation.
Further, the degree of injury was not correlated with symptoms, strengthening the recommendation for retrieving the button battery from the stomach.
In our exclusive video interview, Dr. Khalaf and Dr. Walker discussed the impact of their findings for guidelines for pediatric gastroenterologists and Poison Control Center advice to parents about ingestion of button batteries.
Their study was partly supported by a Cystic Fibrosis Foundational Grant Award and by National Institutes of Health Training Grants.
SAN DIEGO – A button battery lodged in a child’s esophagus is an acknowledged emergency, but there is less evidence about retrieval of button batteries that have passed to the stomach. Observation alone has been recommended when an x-ray determines that the button battery has passed to the stomach within 2 hours of ingestion, when the battery is less than 20 mm, and the child is aged at least 5 years.
At the annual Digestive Disease Week, Racha Khalaf, MD, and Thomas Walker, MD, both of Children’s Hospital Colorado, Aurora, presented data that call this approach into question. Their retrospective cohort study of 4 years’ worth of records from four pediatric centers in the United States identified 68 cases in which a pediatric gastroenterologist had endoscopically removed the button battery. In 60% of those cases, the battery had already caused mucosal damage varying from minor to deep necrosis and perforation.
Further, the degree of injury was not correlated with symptoms, strengthening the recommendation for retrieving the button battery from the stomach.
In our exclusive video interview, Dr. Khalaf and Dr. Walker discussed the impact of their findings for guidelines for pediatric gastroenterologists and Poison Control Center advice to parents about ingestion of button batteries.
Their study was partly supported by a Cystic Fibrosis Foundational Grant Award and by National Institutes of Health Training Grants.
SAN DIEGO – A button battery lodged in a child’s esophagus is an acknowledged emergency, but there is less evidence about retrieval of button batteries that have passed to the stomach. Observation alone has been recommended when an x-ray determines that the button battery has passed to the stomach within 2 hours of ingestion, when the battery is less than 20 mm, and the child is aged at least 5 years.
At the annual Digestive Disease Week, Racha Khalaf, MD, and Thomas Walker, MD, both of Children’s Hospital Colorado, Aurora, presented data that call this approach into question. Their retrospective cohort study of 4 years’ worth of records from four pediatric centers in the United States identified 68 cases in which a pediatric gastroenterologist had endoscopically removed the button battery. In 60% of those cases, the battery had already caused mucosal damage varying from minor to deep necrosis and perforation.
Further, the degree of injury was not correlated with symptoms, strengthening the recommendation for retrieving the button battery from the stomach.
In our exclusive video interview, Dr. Khalaf and Dr. Walker discussed the impact of their findings for guidelines for pediatric gastroenterologists and Poison Control Center advice to parents about ingestion of button batteries.
Their study was partly supported by a Cystic Fibrosis Foundational Grant Award and by National Institutes of Health Training Grants.
REPORTING FROM DDW 2019
Rifaximin effective for uncomplicated diverticulitis in real-life study
SAN DIEGO – Rifaximin relieves symptoms and reduces the risk of disease-related complications in patients with symptomatic uncomplicated diverticular disease (SUDD) of the colon, results from a retrospective study showed.

“The majority of studies published on this topic are not exactly the picture of real life, because they’re conducted on a selected sample of patients queued into the hospital,” lead study author Francesco Di Mario, MD, said at the annual Digestive Disease Week. Dr. Di Mario sought long-term data “from general practitioners – data from real life.”
For an 8-year follow-up study, Dr. Di Mario, professor of gastroenterology at the University of Parma (Italy), and colleagues at several general physician practices in Italy enrolled two groups of patients. The study group (group A) consisted of 346 SUDD patients who were treated with rifaximin 800 mg/day for 7 days every month. Their median age was 64 years, and 63% were female. The control group (group B) included 470 SUDD patients who were taking spasmolithics or any other treatment on demand. Their median age was 65 years, and 61% were female.
The researchers administered a 10-point visual analog scale (VAS) to assess left lower abdominal pain and bloating, with a score of 10 representing the most severe symptoms. Daily bowel movements were also reported.
The median baseline VAS score for pain was 6 in groups A and B. After 8 years of follow-up, the VAS scores for the two groups were 3 and 6, respectively (P less than .0001), and both bloating and daily bowel movements were significantly reduced in group A (P less than .0001).
As for the impact of rifaximin on other outcomes, acute diverticulitis occurred in 9 patients in group A (2.6%) and in 21 patients in group B (4.5%), a difference that reached statistical significance (P = .155). In addition, four patients (1.2%) in group A and nine patients (1.9%) in group B had surgery (P = .432). No disease-related deaths occurred in group A, but two patients in group B died (0.4%; P = .239). No side effects were recorded during the entire study period.
The researchers reported having no financial disclosures.
SAN DIEGO – Rifaximin relieves symptoms and reduces the risk of disease-related complications in patients with symptomatic uncomplicated diverticular disease (SUDD) of the colon, results from a retrospective study showed.

“The majority of studies published on this topic are not exactly the picture of real life, because they’re conducted on a selected sample of patients queued into the hospital,” lead study author Francesco Di Mario, MD, said at the annual Digestive Disease Week. Dr. Di Mario sought long-term data “from general practitioners – data from real life.”
For an 8-year follow-up study, Dr. Di Mario, professor of gastroenterology at the University of Parma (Italy), and colleagues at several general physician practices in Italy enrolled two groups of patients. The study group (group A) consisted of 346 SUDD patients who were treated with rifaximin 800 mg/day for 7 days every month. Their median age was 64 years, and 63% were female. The control group (group B) included 470 SUDD patients who were taking spasmolithics or any other treatment on demand. Their median age was 65 years, and 61% were female.
The researchers administered a 10-point visual analog scale (VAS) to assess left lower abdominal pain and bloating, with a score of 10 representing the most severe symptoms. Daily bowel movements were also reported.
The median baseline VAS score for pain was 6 in groups A and B. After 8 years of follow-up, the VAS scores for the two groups were 3 and 6, respectively (P less than .0001), and both bloating and daily bowel movements were significantly reduced in group A (P less than .0001).
As for the impact of rifaximin on other outcomes, acute diverticulitis occurred in 9 patients in group A (2.6%) and in 21 patients in group B (4.5%), a difference that reached statistical significance (P = .155). In addition, four patients (1.2%) in group A and nine patients (1.9%) in group B had surgery (P = .432). No disease-related deaths occurred in group A, but two patients in group B died (0.4%; P = .239). No side effects were recorded during the entire study period.
The researchers reported having no financial disclosures.
SAN DIEGO – Rifaximin relieves symptoms and reduces the risk of disease-related complications in patients with symptomatic uncomplicated diverticular disease (SUDD) of the colon, results from a retrospective study showed.

“The majority of studies published on this topic are not exactly the picture of real life, because they’re conducted on a selected sample of patients queued into the hospital,” lead study author Francesco Di Mario, MD, said at the annual Digestive Disease Week. Dr. Di Mario sought long-term data “from general practitioners – data from real life.”
For an 8-year follow-up study, Dr. Di Mario, professor of gastroenterology at the University of Parma (Italy), and colleagues at several general physician practices in Italy enrolled two groups of patients. The study group (group A) consisted of 346 SUDD patients who were treated with rifaximin 800 mg/day for 7 days every month. Their median age was 64 years, and 63% were female. The control group (group B) included 470 SUDD patients who were taking spasmolithics or any other treatment on demand. Their median age was 65 years, and 61% were female.
The researchers administered a 10-point visual analog scale (VAS) to assess left lower abdominal pain and bloating, with a score of 10 representing the most severe symptoms. Daily bowel movements were also reported.
The median baseline VAS score for pain was 6 in groups A and B. After 8 years of follow-up, the VAS scores for the two groups were 3 and 6, respectively (P less than .0001), and both bloating and daily bowel movements were significantly reduced in group A (P less than .0001).
As for the impact of rifaximin on other outcomes, acute diverticulitis occurred in 9 patients in group A (2.6%) and in 21 patients in group B (4.5%), a difference that reached statistical significance (P = .155). In addition, four patients (1.2%) in group A and nine patients (1.9%) in group B had surgery (P = .432). No disease-related deaths occurred in group A, but two patients in group B died (0.4%; P = .239). No side effects were recorded during the entire study period.
The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Key clinical point: “Real-life” data show a benefit of rifaximin on symptoms and complications experienced by patients with symptomatic uncomplicated diverticular disease of the colon.
Major finding: Acute diverticulitis occurred in 9 patients in the rifaximin group (2.6%) and in 21 patients who did not receive rifaximin (4.5%), a difference that reached statistical significance (P = .155).
Study details: A retrospective study of 816 patients with symptomatic uncomplicated diverticular disease.
Disclosures: The researchers reported having no financial disclosures.
Multiple Eruptive Syringomas on the Penis
To the Editor:
Syringomas are small, benign, asymptomatic eccrine or apocrine tumors that present as multiple discrete flesh-colored papules. They are more common in females than males.1 The etiology of eruptive syringomas is unclear, though an inflammatory process has been implicated in the abnormal proliferation of sweat glands.2 However, a minority of tumors have been known to have an autosomal-dominant mode of transmission. Multiple or eruptive syringomas are associated with Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and Blau syndrome.3 The clear cell variant has been found to be associated with diabetes mellitus.4 Syringomas most commonly appear on the lower eyelids, upper cheeks, neck, and upper chest; presentation on the penis is rare.5 We report a case of multiple eruptive syringomas located exclusively on the penis mimicking a sexually transmitted condition.
A 53-year-old man who was otherwise healthy presented with multiple flesh-colored papules on the penis that initially began to develop 30 years prior, but increased crops of lesions appeared 4 to 6 weeks prior to presentation. The patient described the lesions as rashlike, nonpruritic, and sensitive to the touch. He denied any discharge, oozing, crusting, or bleeding from the lesions. He did not report any high-risk sexual behaviors and stated that he was in a monogamous relationship with his wife. He had a medical history of molluscum contagiosum that was diagnosed and treated with cryotherapy 30 years prior; however, he did not have a history of any other sexually transmitted diseases. He also did not have a history of diabetes mellitus or thyroid disease.
Physical examination revealed multiple pink papules on the dorsal and ventral shaft of the penis, measuring 2 to 4 mm in diameter, with koebnerization (Figure 1). Based on clinical examination, the differential included condyloma, inflamed seborrheic keratosis, bowenoid papulosis, atypical molluscum contagiosum, or lichen planus. Consequently, a punch biopsy of the penile shaft was performed and histopathologic examination revealed proliferation of ducts focally that were tadpole shaped and embedded in a sclerotic stroma. The lining of the ducts was composed of cuboidal cells, some with clear cell change. The microscopic findings were consistent with penile syringomas (Figure 2). Laboratory results revealed the patient was negative for human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. The patient was given topical hydrocortisone butyrate and tacrolimus for symptomatic treatment. He declined further aggressive treatment.
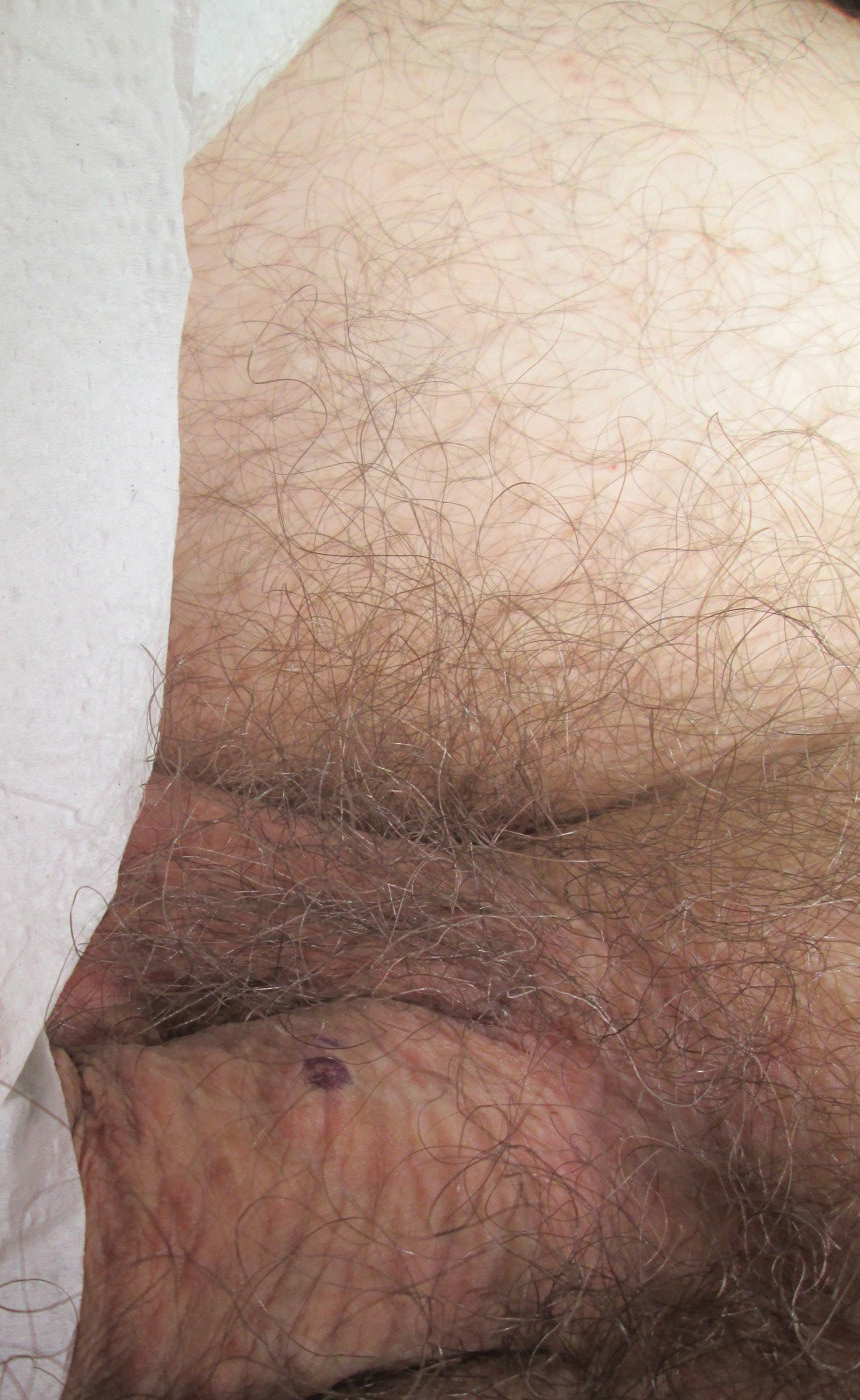

Due to the rarity of syringomas on the penis, presentation of these benign eccrine tumors can be commonly mistaken for lichen planus, molluscum contagiosum, genital warts, or bowenoid papulosis.5 The characteristic histopathology of syringomas consists of multiple, small, tadpole or paisley tie–shaped ducts within an eosinophilic stroma. Often, the findings can be histologically confused with desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma. Although the histopathology of our patient’s biopsy showed clear cell change, the patient did not report a history of diabetes mellitus, which is a disease that can be associated with the clear cell variant of syringoma. Because syringomas are benign tumors, treatment is not medically necessary unless the lesions are symptomatic. Treatment often is regarded as challenging, as lesions often recur and scarring is a consideration. Possible treatments for removal of the benign papules include surgical excision, electrodesiccation and curettage, shave removal, chemical peels, liquid nitrogen cryotherapy, and CO2 laser vaporization.6
To prevent misdiagnosis and unnecessary treatment, it is important to have syringomas as part of the differential diagnosis when patients present with multiple small flesh-colored papules on the penis. The lesions should be biopsied for accurate diagnosis and to provide reassurance to patients who usually come in for evaluation for fear of having acquired a sexually transmitted disease.
- Yalisove B, Stolar EEH, Williams CM. Multiple penile papules. syringoma. Arch Dermatol. 1987;123:1391-1396.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Yoshimi N, Kurokawa I, Kakuno A, et al. Case of generalized eruptive clear cell syringoma with diabetes mellitus. J Dermatol. 2012;39:744-745.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Wu CY. Multifocal penile syringoma masquerading as genital warts. Clin Exp Dermatol. 2009;34:e290-e291.
- Lipshutz RL, Kantor GR, Vonderheid EC. Multiple penile syringomas mimicking verrucae. Int J Dermatol. 1991;30:69.
To the Editor:
Syringomas are small, benign, asymptomatic eccrine or apocrine tumors that present as multiple discrete flesh-colored papules. They are more common in females than males.1 The etiology of eruptive syringomas is unclear, though an inflammatory process has been implicated in the abnormal proliferation of sweat glands.2 However, a minority of tumors have been known to have an autosomal-dominant mode of transmission. Multiple or eruptive syringomas are associated with Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and Blau syndrome.3 The clear cell variant has been found to be associated with diabetes mellitus.4 Syringomas most commonly appear on the lower eyelids, upper cheeks, neck, and upper chest; presentation on the penis is rare.5 We report a case of multiple eruptive syringomas located exclusively on the penis mimicking a sexually transmitted condition.
A 53-year-old man who was otherwise healthy presented with multiple flesh-colored papules on the penis that initially began to develop 30 years prior, but increased crops of lesions appeared 4 to 6 weeks prior to presentation. The patient described the lesions as rashlike, nonpruritic, and sensitive to the touch. He denied any discharge, oozing, crusting, or bleeding from the lesions. He did not report any high-risk sexual behaviors and stated that he was in a monogamous relationship with his wife. He had a medical history of molluscum contagiosum that was diagnosed and treated with cryotherapy 30 years prior; however, he did not have a history of any other sexually transmitted diseases. He also did not have a history of diabetes mellitus or thyroid disease.
Physical examination revealed multiple pink papules on the dorsal and ventral shaft of the penis, measuring 2 to 4 mm in diameter, with koebnerization (Figure 1). Based on clinical examination, the differential included condyloma, inflamed seborrheic keratosis, bowenoid papulosis, atypical molluscum contagiosum, or lichen planus. Consequently, a punch biopsy of the penile shaft was performed and histopathologic examination revealed proliferation of ducts focally that were tadpole shaped and embedded in a sclerotic stroma. The lining of the ducts was composed of cuboidal cells, some with clear cell change. The microscopic findings were consistent with penile syringomas (Figure 2). Laboratory results revealed the patient was negative for human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. The patient was given topical hydrocortisone butyrate and tacrolimus for symptomatic treatment. He declined further aggressive treatment.


Due to the rarity of syringomas on the penis, presentation of these benign eccrine tumors can be commonly mistaken for lichen planus, molluscum contagiosum, genital warts, or bowenoid papulosis.5 The characteristic histopathology of syringomas consists of multiple, small, tadpole or paisley tie–shaped ducts within an eosinophilic stroma. Often, the findings can be histologically confused with desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma. Although the histopathology of our patient’s biopsy showed clear cell change, the patient did not report a history of diabetes mellitus, which is a disease that can be associated with the clear cell variant of syringoma. Because syringomas are benign tumors, treatment is not medically necessary unless the lesions are symptomatic. Treatment often is regarded as challenging, as lesions often recur and scarring is a consideration. Possible treatments for removal of the benign papules include surgical excision, electrodesiccation and curettage, shave removal, chemical peels, liquid nitrogen cryotherapy, and CO2 laser vaporization.6
To prevent misdiagnosis and unnecessary treatment, it is important to have syringomas as part of the differential diagnosis when patients present with multiple small flesh-colored papules on the penis. The lesions should be biopsied for accurate diagnosis and to provide reassurance to patients who usually come in for evaluation for fear of having acquired a sexually transmitted disease.
To the Editor:
Syringomas are small, benign, asymptomatic eccrine or apocrine tumors that present as multiple discrete flesh-colored papules. They are more common in females than males.1 The etiology of eruptive syringomas is unclear, though an inflammatory process has been implicated in the abnormal proliferation of sweat glands.2 However, a minority of tumors have been known to have an autosomal-dominant mode of transmission. Multiple or eruptive syringomas are associated with Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and Blau syndrome.3 The clear cell variant has been found to be associated with diabetes mellitus.4 Syringomas most commonly appear on the lower eyelids, upper cheeks, neck, and upper chest; presentation on the penis is rare.5 We report a case of multiple eruptive syringomas located exclusively on the penis mimicking a sexually transmitted condition.
A 53-year-old man who was otherwise healthy presented with multiple flesh-colored papules on the penis that initially began to develop 30 years prior, but increased crops of lesions appeared 4 to 6 weeks prior to presentation. The patient described the lesions as rashlike, nonpruritic, and sensitive to the touch. He denied any discharge, oozing, crusting, or bleeding from the lesions. He did not report any high-risk sexual behaviors and stated that he was in a monogamous relationship with his wife. He had a medical history of molluscum contagiosum that was diagnosed and treated with cryotherapy 30 years prior; however, he did not have a history of any other sexually transmitted diseases. He also did not have a history of diabetes mellitus or thyroid disease.
Physical examination revealed multiple pink papules on the dorsal and ventral shaft of the penis, measuring 2 to 4 mm in diameter, with koebnerization (Figure 1). Based on clinical examination, the differential included condyloma, inflamed seborrheic keratosis, bowenoid papulosis, atypical molluscum contagiosum, or lichen planus. Consequently, a punch biopsy of the penile shaft was performed and histopathologic examination revealed proliferation of ducts focally that were tadpole shaped and embedded in a sclerotic stroma. The lining of the ducts was composed of cuboidal cells, some with clear cell change. The microscopic findings were consistent with penile syringomas (Figure 2). Laboratory results revealed the patient was negative for human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. The patient was given topical hydrocortisone butyrate and tacrolimus for symptomatic treatment. He declined further aggressive treatment.


Due to the rarity of syringomas on the penis, presentation of these benign eccrine tumors can be commonly mistaken for lichen planus, molluscum contagiosum, genital warts, or bowenoid papulosis.5 The characteristic histopathology of syringomas consists of multiple, small, tadpole or paisley tie–shaped ducts within an eosinophilic stroma. Often, the findings can be histologically confused with desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma. Although the histopathology of our patient’s biopsy showed clear cell change, the patient did not report a history of diabetes mellitus, which is a disease that can be associated with the clear cell variant of syringoma. Because syringomas are benign tumors, treatment is not medically necessary unless the lesions are symptomatic. Treatment often is regarded as challenging, as lesions often recur and scarring is a consideration. Possible treatments for removal of the benign papules include surgical excision, electrodesiccation and curettage, shave removal, chemical peels, liquid nitrogen cryotherapy, and CO2 laser vaporization.6
To prevent misdiagnosis and unnecessary treatment, it is important to have syringomas as part of the differential diagnosis when patients present with multiple small flesh-colored papules on the penis. The lesions should be biopsied for accurate diagnosis and to provide reassurance to patients who usually come in for evaluation for fear of having acquired a sexually transmitted disease.
- Yalisove B, Stolar EEH, Williams CM. Multiple penile papules. syringoma. Arch Dermatol. 1987;123:1391-1396.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Yoshimi N, Kurokawa I, Kakuno A, et al. Case of generalized eruptive clear cell syringoma with diabetes mellitus. J Dermatol. 2012;39:744-745.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Wu CY. Multifocal penile syringoma masquerading as genital warts. Clin Exp Dermatol. 2009;34:e290-e291.
- Lipshutz RL, Kantor GR, Vonderheid EC. Multiple penile syringomas mimicking verrucae. Int J Dermatol. 1991;30:69.
- Yalisove B, Stolar EEH, Williams CM. Multiple penile papules. syringoma. Arch Dermatol. 1987;123:1391-1396.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Yoshimi N, Kurokawa I, Kakuno A, et al. Case of generalized eruptive clear cell syringoma with diabetes mellitus. J Dermatol. 2012;39:744-745.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Wu CY. Multifocal penile syringoma masquerading as genital warts. Clin Exp Dermatol. 2009;34:e290-e291.
- Lipshutz RL, Kantor GR, Vonderheid EC. Multiple penile syringomas mimicking verrucae. Int J Dermatol. 1991;30:69.
Practice Points
- Penile syringoma can mimic sexually transmitted disease such as condyloma acuminatum or molluscum contagiosum.
- Penile syringomas can be long-standing and require biopsy to differentiate from other conditions.
Elderly concussion patients who used statins had lower dementia risk
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
FROM JAMA NEUROLOGY
Key clinical point: Older adults taking a statin within 90 days after a concussion had a lower rate of dementia.
Major finding: Statin use within 90 days of a concussion in older adults was associated with a 13% reduced risk of dementia (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Study details: A population-based double cohort study of 28,815 elderly patients who had a concussion between April 1993 and April 2013.
Disclosures: This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
Source: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
Members to Elect Secretary at VAM Meeting
At the Annual Business Meeting, 12 to 1:30 p.m. Saturday, June 15, SVS members (Active, Seniors and/or Distinguished Fellows) will be asked to elect individuals to fill the positions of Secretary and Vice President and then approve the full slate of SVS Officers for 2019-20. The Nominating Committee unanimously identified one person felt to be uniquely qualified to assume the position of Vice President; as in the past, that candidate will be announced at the meeting. Members will select among three highly qualified candidates for Secretary: Keith Calligaro, Michael Conte and Amy Reed. Find information from each of them here.
At the Annual Business Meeting, 12 to 1:30 p.m. Saturday, June 15, SVS members (Active, Seniors and/or Distinguished Fellows) will be asked to elect individuals to fill the positions of Secretary and Vice President and then approve the full slate of SVS Officers for 2019-20. The Nominating Committee unanimously identified one person felt to be uniquely qualified to assume the position of Vice President; as in the past, that candidate will be announced at the meeting. Members will select among three highly qualified candidates for Secretary: Keith Calligaro, Michael Conte and Amy Reed. Find information from each of them here.
At the Annual Business Meeting, 12 to 1:30 p.m. Saturday, June 15, SVS members (Active, Seniors and/or Distinguished Fellows) will be asked to elect individuals to fill the positions of Secretary and Vice President and then approve the full slate of SVS Officers for 2019-20. The Nominating Committee unanimously identified one person felt to be uniquely qualified to assume the position of Vice President; as in the past, that candidate will be announced at the meeting. Members will select among three highly qualified candidates for Secretary: Keith Calligaro, Michael Conte and Amy Reed. Find information from each of them here.
BMI in male teens predicts cardiomyopathy risk
and their risk increased as body mass index increased, according to the results of a nationwide, prospective, registry-based cohort study from Sweden.
The association was strongest for dilated cardiomyopathy, wrote Josefina Robertson, MD, and associates at the University of Gothenburg (Sweden). Over a median of 27 years of follow-up, the risk for dilated cardiomyopathy in adulthood was approximately 38% greater when adolescent body mass index was 22.5-25.0 kg/m2, using a lean but not underweight BMI (18.5-20.0 kg/m2) as the reference group. The increase in risk for dilated cardiomyopathy continued to rise with adolescent BMI and exceeded 700% at a BMI over 35.
The rate of hospitalizations for heart failure caused by cardiomyopathy more than doubled in Sweden from 1987 to 2006, the researchers noted. Adolescent obesity is strongly linked to early heart failure, but few studies have assessed whether adiposity as measured by BMI is associated with cardiomyopathy, and none have confirmed diagnostic validity or looked at subtypes of cardiomyopathy.
“The already marked importance of weight control in youth is further strengthened by [our] findings,” the researchers wrote, “as well as greater evidence for obesity as a potential important cause of adverse cardiac remodeling independent of clinically evident ischemic heart disease.”
The study included 1,668,893 male adolescents who had enlisted for military service in Sweden between 1969 and 2005, when compulsory enlistment ended. It excluded women and the small proportion of men lacking weight or height data. A total of 4,477 cases of cardiomyopathy were diagnosed during follow-up, 59% were dilated cardiomyopathy, 15% were hypertrophic cardiomyopathy, and 11% were alcohol or drug-related cardiomyopathy.
The link between even slightly elevated BMI and dilated cardiomyopathy did not depend on age, year, location, or baseline comorbidities. For each unit increase in BMI, the adjusted risk of dilated cardiomyopathy rose by approximately 15%, the risk of hypertrophic cardiomyopathy rose by 9%, and the risk for drug- or alcohol-related cardiomyopathy rose by 10%. Estimated risks were generally similar after controlling for blood pressure, cardiorespiratory fitness, muscle strength, parents’ level of education, and alcohol or substance use disorders.
Funders included the Swedish government; Swedish Research Council; Swedish Heart and Lung Foundation; and Swedish Council for Health, Working Life, and Welfare. The researchers reported having no conflicts of interest.
SOURCE: Robertson J et al. Circulation. 2019 May 20.
and their risk increased as body mass index increased, according to the results of a nationwide, prospective, registry-based cohort study from Sweden.
The association was strongest for dilated cardiomyopathy, wrote Josefina Robertson, MD, and associates at the University of Gothenburg (Sweden). Over a median of 27 years of follow-up, the risk for dilated cardiomyopathy in adulthood was approximately 38% greater when adolescent body mass index was 22.5-25.0 kg/m2, using a lean but not underweight BMI (18.5-20.0 kg/m2) as the reference group. The increase in risk for dilated cardiomyopathy continued to rise with adolescent BMI and exceeded 700% at a BMI over 35.
The rate of hospitalizations for heart failure caused by cardiomyopathy more than doubled in Sweden from 1987 to 2006, the researchers noted. Adolescent obesity is strongly linked to early heart failure, but few studies have assessed whether adiposity as measured by BMI is associated with cardiomyopathy, and none have confirmed diagnostic validity or looked at subtypes of cardiomyopathy.
“The already marked importance of weight control in youth is further strengthened by [our] findings,” the researchers wrote, “as well as greater evidence for obesity as a potential important cause of adverse cardiac remodeling independent of clinically evident ischemic heart disease.”
The study included 1,668,893 male adolescents who had enlisted for military service in Sweden between 1969 and 2005, when compulsory enlistment ended. It excluded women and the small proportion of men lacking weight or height data. A total of 4,477 cases of cardiomyopathy were diagnosed during follow-up, 59% were dilated cardiomyopathy, 15% were hypertrophic cardiomyopathy, and 11% were alcohol or drug-related cardiomyopathy.
The link between even slightly elevated BMI and dilated cardiomyopathy did not depend on age, year, location, or baseline comorbidities. For each unit increase in BMI, the adjusted risk of dilated cardiomyopathy rose by approximately 15%, the risk of hypertrophic cardiomyopathy rose by 9%, and the risk for drug- or alcohol-related cardiomyopathy rose by 10%. Estimated risks were generally similar after controlling for blood pressure, cardiorespiratory fitness, muscle strength, parents’ level of education, and alcohol or substance use disorders.
Funders included the Swedish government; Swedish Research Council; Swedish Heart and Lung Foundation; and Swedish Council for Health, Working Life, and Welfare. The researchers reported having no conflicts of interest.
SOURCE: Robertson J et al. Circulation. 2019 May 20.
and their risk increased as body mass index increased, according to the results of a nationwide, prospective, registry-based cohort study from Sweden.
The association was strongest for dilated cardiomyopathy, wrote Josefina Robertson, MD, and associates at the University of Gothenburg (Sweden). Over a median of 27 years of follow-up, the risk for dilated cardiomyopathy in adulthood was approximately 38% greater when adolescent body mass index was 22.5-25.0 kg/m2, using a lean but not underweight BMI (18.5-20.0 kg/m2) as the reference group. The increase in risk for dilated cardiomyopathy continued to rise with adolescent BMI and exceeded 700% at a BMI over 35.
The rate of hospitalizations for heart failure caused by cardiomyopathy more than doubled in Sweden from 1987 to 2006, the researchers noted. Adolescent obesity is strongly linked to early heart failure, but few studies have assessed whether adiposity as measured by BMI is associated with cardiomyopathy, and none have confirmed diagnostic validity or looked at subtypes of cardiomyopathy.
“The already marked importance of weight control in youth is further strengthened by [our] findings,” the researchers wrote, “as well as greater evidence for obesity as a potential important cause of adverse cardiac remodeling independent of clinically evident ischemic heart disease.”
The study included 1,668,893 male adolescents who had enlisted for military service in Sweden between 1969 and 2005, when compulsory enlistment ended. It excluded women and the small proportion of men lacking weight or height data. A total of 4,477 cases of cardiomyopathy were diagnosed during follow-up, 59% were dilated cardiomyopathy, 15% were hypertrophic cardiomyopathy, and 11% were alcohol or drug-related cardiomyopathy.
The link between even slightly elevated BMI and dilated cardiomyopathy did not depend on age, year, location, or baseline comorbidities. For each unit increase in BMI, the adjusted risk of dilated cardiomyopathy rose by approximately 15%, the risk of hypertrophic cardiomyopathy rose by 9%, and the risk for drug- or alcohol-related cardiomyopathy rose by 10%. Estimated risks were generally similar after controlling for blood pressure, cardiorespiratory fitness, muscle strength, parents’ level of education, and alcohol or substance use disorders.
Funders included the Swedish government; Swedish Research Council; Swedish Heart and Lung Foundation; and Swedish Council for Health, Working Life, and Welfare. The researchers reported having no conflicts of interest.
SOURCE: Robertson J et al. Circulation. 2019 May 20.
FROM CIRCULATION
Key clinical point: Overweight in male teens predicts subsequent cardiomyopathy. The association increases with BMI and is strongest for dilated cardiomyopathy.
Major finding: Over a median of 27 years of follow-up, the hazard ratio for dilated cardiomyopathy in adulthood was 1.38 when adolescent body mass index was 22.5-25.0 kg/m2, using a BMI of 18.5-20.0 as the reference group. At a BMI over 35, the hazard ratio reached 8.11.
Study details: A nationwide, prospective registry cohort study of 1.67 million adolescent males in Sweden.
Disclosures: Funders included the Swedish government; Swedish Research Council; Swedish Heart and Lung Foundation; and Swedish Council for Health, Working Life and Welfare. The researchers reported having no conflicts of interest.
Local consolidative therapy shows benefit in oligometastatic NSCLC
Patients with oligometastatic non–small cell lung cancer (NSCLC) have better outcomes when treated with local consolidative therapy than with maintenance therapy or observation, based on updated results from a phase 2 trial.
The randomized study showed that both median progression-free and overall survival were better in patients who received radiotherapy or surgery instead of maintenance therapy or observation, reported lead author Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. These findings build on earlier results that showed the positive impact of local consolidative therapy (LCT), the investigators noted.
“The trial was closed early after it demonstrated an observed 8-month benefit in [progression-free survival] for patients who received LCT relative to patients who received maintenance therapy or observation,” the investigators wrote in Journal of Clinical Oncology.
After early closure, 49 patients remained in the dataset. All had metastatic NSCLC with three or fewer metastases that did not progress for at least 3 months after first-line systemic therapy. Most patients had adenocarcinoma (80%). Patients were randomly divided in a 1:1 ratio between radiotherapy or surgery (LCT) for all active disease sites or maintenance therapy/observation (MT/O). Progression-free survival was the primary endpoint. Overall survival and several other secondary endpoints were also evaluated.
Data analysis showed a clear benefit of LCT. Continuing the previously reported trend, median progression-free survival was extended in the LCT group, compared with the MT/O group (14.2 vs. 4.4 months; P = .022). Similarly, median overall survival showed a significant improvement (41.2 vs. 17.0 months; P = .017). Median time to appearance of new lesions also supported the advantage of LCT over MT/O, albeit with less statistical significance (14.2 vs. 6.0 months; P = .11).
The investigators suggested several mechanisms behind the efficacy of LCT, including elimination of treatment-resistant cells, potentiation of systemic therapy, and elimination of the residual tumor as a driver of distant micrometastatic disease. “Notably,” the investigators wrote, “these mechanisms are not mutually exclusive, and more than one could contribute to the benefits of LCT.”
“[A]lthough these data are compelling ... we emphasize that future studies should be supported to definitively assess the role of LCT in larger populations (e.g., phase III trials such as NRG-LU002) and in the context of novel systemic therapies,” the investigators concluded.
The study was funded by MD Anderson Cancer Center, The Mohaymen Sahebzadah Family Philanthropic Grant, and the National Cancer Institute, National Institutes of Health. The authors disclosed relationships with Merck, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Gomez et al. J Clin Oncol. 8 May 2019. doi:10.1200/JCO.19.00201.
Patients with oligometastatic non–small cell lung cancer (NSCLC) have better outcomes when treated with local consolidative therapy than with maintenance therapy or observation, based on updated results from a phase 2 trial.
The randomized study showed that both median progression-free and overall survival were better in patients who received radiotherapy or surgery instead of maintenance therapy or observation, reported lead author Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. These findings build on earlier results that showed the positive impact of local consolidative therapy (LCT), the investigators noted.
“The trial was closed early after it demonstrated an observed 8-month benefit in [progression-free survival] for patients who received LCT relative to patients who received maintenance therapy or observation,” the investigators wrote in Journal of Clinical Oncology.
After early closure, 49 patients remained in the dataset. All had metastatic NSCLC with three or fewer metastases that did not progress for at least 3 months after first-line systemic therapy. Most patients had adenocarcinoma (80%). Patients were randomly divided in a 1:1 ratio between radiotherapy or surgery (LCT) for all active disease sites or maintenance therapy/observation (MT/O). Progression-free survival was the primary endpoint. Overall survival and several other secondary endpoints were also evaluated.
Data analysis showed a clear benefit of LCT. Continuing the previously reported trend, median progression-free survival was extended in the LCT group, compared with the MT/O group (14.2 vs. 4.4 months; P = .022). Similarly, median overall survival showed a significant improvement (41.2 vs. 17.0 months; P = .017). Median time to appearance of new lesions also supported the advantage of LCT over MT/O, albeit with less statistical significance (14.2 vs. 6.0 months; P = .11).
The investigators suggested several mechanisms behind the efficacy of LCT, including elimination of treatment-resistant cells, potentiation of systemic therapy, and elimination of the residual tumor as a driver of distant micrometastatic disease. “Notably,” the investigators wrote, “these mechanisms are not mutually exclusive, and more than one could contribute to the benefits of LCT.”
“[A]lthough these data are compelling ... we emphasize that future studies should be supported to definitively assess the role of LCT in larger populations (e.g., phase III trials such as NRG-LU002) and in the context of novel systemic therapies,” the investigators concluded.
The study was funded by MD Anderson Cancer Center, The Mohaymen Sahebzadah Family Philanthropic Grant, and the National Cancer Institute, National Institutes of Health. The authors disclosed relationships with Merck, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Gomez et al. J Clin Oncol. 8 May 2019. doi:10.1200/JCO.19.00201.
Patients with oligometastatic non–small cell lung cancer (NSCLC) have better outcomes when treated with local consolidative therapy than with maintenance therapy or observation, based on updated results from a phase 2 trial.
The randomized study showed that both median progression-free and overall survival were better in patients who received radiotherapy or surgery instead of maintenance therapy or observation, reported lead author Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. These findings build on earlier results that showed the positive impact of local consolidative therapy (LCT), the investigators noted.
“The trial was closed early after it demonstrated an observed 8-month benefit in [progression-free survival] for patients who received LCT relative to patients who received maintenance therapy or observation,” the investigators wrote in Journal of Clinical Oncology.
After early closure, 49 patients remained in the dataset. All had metastatic NSCLC with three or fewer metastases that did not progress for at least 3 months after first-line systemic therapy. Most patients had adenocarcinoma (80%). Patients were randomly divided in a 1:1 ratio between radiotherapy or surgery (LCT) for all active disease sites or maintenance therapy/observation (MT/O). Progression-free survival was the primary endpoint. Overall survival and several other secondary endpoints were also evaluated.
Data analysis showed a clear benefit of LCT. Continuing the previously reported trend, median progression-free survival was extended in the LCT group, compared with the MT/O group (14.2 vs. 4.4 months; P = .022). Similarly, median overall survival showed a significant improvement (41.2 vs. 17.0 months; P = .017). Median time to appearance of new lesions also supported the advantage of LCT over MT/O, albeit with less statistical significance (14.2 vs. 6.0 months; P = .11).
The investigators suggested several mechanisms behind the efficacy of LCT, including elimination of treatment-resistant cells, potentiation of systemic therapy, and elimination of the residual tumor as a driver of distant micrometastatic disease. “Notably,” the investigators wrote, “these mechanisms are not mutually exclusive, and more than one could contribute to the benefits of LCT.”
“[A]lthough these data are compelling ... we emphasize that future studies should be supported to definitively assess the role of LCT in larger populations (e.g., phase III trials such as NRG-LU002) and in the context of novel systemic therapies,” the investigators concluded.
The study was funded by MD Anderson Cancer Center, The Mohaymen Sahebzadah Family Philanthropic Grant, and the National Cancer Institute, National Institutes of Health. The authors disclosed relationships with Merck, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Gomez et al. J Clin Oncol. 8 May 2019. doi:10.1200/JCO.19.00201.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with oligometastatic non–small cell lung cancer (NSCLC) have better outcomes when treated with local consolidative therapy than with maintenance therapy or observation.
Major finding: Patients treated with local consolidative therapy had a median overall survival of 41.2 months, compared with 17.0 months among patients treated with maintenance therapy or observation (P = .017).
Study details: A phase 2 randomized trial involving 49 patients with stage IV non–small cell lung cancer who had three or fewer metastases.
Disclosures: The study was funded by MD Anderson Cancer Center, The Mohaymen Sahebzadah Family Philanthropic Grant, and the National Cancer Institute, National Institutes of Health. The authors disclosed relationships with Merck, Bristol-Myers Squibb, AstraZeneca, and others.
Source: Gomez et al. J Clin Oncol. 2019 May 8. doi: 10.1200/JCO.19.00201.
Active psoriatic arthritis, ankylosing spondylitis linked to increase in adverse pregnancy outcomes
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
FROM ARTHRITIS CARE & RESEARCH
For some MST survivors, VA hospitals can trigger PTSD
Alternative treatment settings could be ‘easier access point’
SAN FRANCISCO – Veterans who are survivors of military sexual trauma during their service face unique challenges in their treatment and recovery. They are often reluctant to report their experiences – and understandably so.
“Military sexual assault represents a huge violation of that trust and safety. That’s what makes it so toxic and hard for participants to [come] forward, because they’re accused of breaking cohesion of their unit and breaking morale, and yet they have been mistreated,” Niranjan Karnik, MD, PhD, associate dean for community behavioral health at Rush Medical College, Chicago, said in an interview.
Dr. Karnik moderated a session on the prevalence and treatment of military sexual assault at the annual meeting of the American Psychiatric Association. Although the Department of Veterans Affairs treats many survivors of sexual assault, not all of them feel comfortable in that environment. “A VA hospital has a quasi-military feel to it, and that’s a reflection of what it is and the people who are there. That can be an inhibition – and can even be a trigger for [PTSD] symptoms,” Dr. Karnik said.
Survivors may also worry about being labeled, or about adverse entries going into their official record and how that could affect them in the future. The issue is a stark contrast to veterans who are suffering from combat-related trauma.
“When a combat trauma survivor goes to the VA, they feel protected because their colleagues are there. With military sexual trauma, because of that violation of trust from their peers, it can really exacerbate things,” Dr. Karnik said.
Fortunately, there are alternatives, such as the Road Home* Program at Rush Hospital, which has a few military accoutrements but more closely resembles a civilian center. “It can be an easier access point. The VA is taking care of a large majority of patients. We are a boutique program for the vets who can’t or feel unable to go through the VA program,” Dr. Karnik said.
Overall, 52.5% of women and 8.9% of men in the military report sexual harassment, and 23.6% of women and 1.9% of men report being sexually assaulted. That amounts to 14,900 service members, 8,600 women, and 6,300 men who were assaulted in 2016, according to Neeral K. Sheth, DO, assistant professor of psychiatry at Rush Medical College, who also presented at the session. The frequency of assault is higher among LGBTQ individuals, and African American men and women are more likely to experience sexual harassment.
There are options for treatment of military sexual trauma (MST). The 3-week Road Home intensive outpatient treatment program at Rush Hospital combines group and individual cognitive-processing therapy, which is a cognitive-behavioral therapy that has been shown to improve PTSD resulting from MST. The program places combat trauma and MST trauma patients into separate cohorts, each containing individual and group components. Individual sessions closely follow a manualized protocol, while group sessions offer an opportunity to practice cognitive-processing therapy skills.
The team adapted the program to MST treatment by incorporating dialectical-behavioral therapy skills modules in the first week of the program, and implemented one-on-one skills consultation by request throughout the program.
An analysis of 191 subjects participating in 19 cohorts (12 combat, 9 MST cohorts) showed a 92% completion rate, which was similar, regardless of gender or cohort type. Both cohorts had significant reductions in PTSD severity as measured by the PTSD Checklist for DSM-5, and depression symptoms as measured by the Patient Health Questionnaire–9.
Another program, Families OverComing Under Stress, can also be adapted to MST. It is designed to build resiliency and wellness within families dealing with trauma or loss. It incorporates family assessment, psychoeducation tailored to the needs of the entire family, family-level resilience skills, and a narrative component.
An important element is the identification and management of stress reminders – triggers that remind the individual of a trauma and may cause a sudden shift in mood or behavior. A family member’s knowledge that the survivor is experiencing a stress reminder can reduce misunderstandings or unhelpful interpretations of behavior.
In fact, family considerations are often what bring veterans in for help in the first place, according to Dr. Karnik. He or she may be concerned about behavioral problems in a child, which the VA cannot address because its federal funding dictates a sole focus on the veteran. “We will take care of the whole family,” Dr. Karnik said. “Often that’s the entry point, and that allows us to do some engagement with the veteran, and things start to get uncovered.”
Dr. Karnik has no relevant financial disclosures.
*CORRECTION, 5/21/2019
Alternative treatment settings could be ‘easier access point’
Alternative treatment settings could be ‘easier access point’
SAN FRANCISCO – Veterans who are survivors of military sexual trauma during their service face unique challenges in their treatment and recovery. They are often reluctant to report their experiences – and understandably so.
“Military sexual assault represents a huge violation of that trust and safety. That’s what makes it so toxic and hard for participants to [come] forward, because they’re accused of breaking cohesion of their unit and breaking morale, and yet they have been mistreated,” Niranjan Karnik, MD, PhD, associate dean for community behavioral health at Rush Medical College, Chicago, said in an interview.
Dr. Karnik moderated a session on the prevalence and treatment of military sexual assault at the annual meeting of the American Psychiatric Association. Although the Department of Veterans Affairs treats many survivors of sexual assault, not all of them feel comfortable in that environment. “A VA hospital has a quasi-military feel to it, and that’s a reflection of what it is and the people who are there. That can be an inhibition – and can even be a trigger for [PTSD] symptoms,” Dr. Karnik said.
Survivors may also worry about being labeled, or about adverse entries going into their official record and how that could affect them in the future. The issue is a stark contrast to veterans who are suffering from combat-related trauma.
“When a combat trauma survivor goes to the VA, they feel protected because their colleagues are there. With military sexual trauma, because of that violation of trust from their peers, it can really exacerbate things,” Dr. Karnik said.
Fortunately, there are alternatives, such as the Road Home* Program at Rush Hospital, which has a few military accoutrements but more closely resembles a civilian center. “It can be an easier access point. The VA is taking care of a large majority of patients. We are a boutique program for the vets who can’t or feel unable to go through the VA program,” Dr. Karnik said.
Overall, 52.5% of women and 8.9% of men in the military report sexual harassment, and 23.6% of women and 1.9% of men report being sexually assaulted. That amounts to 14,900 service members, 8,600 women, and 6,300 men who were assaulted in 2016, according to Neeral K. Sheth, DO, assistant professor of psychiatry at Rush Medical College, who also presented at the session. The frequency of assault is higher among LGBTQ individuals, and African American men and women are more likely to experience sexual harassment.
There are options for treatment of military sexual trauma (MST). The 3-week Road Home intensive outpatient treatment program at Rush Hospital combines group and individual cognitive-processing therapy, which is a cognitive-behavioral therapy that has been shown to improve PTSD resulting from MST. The program places combat trauma and MST trauma patients into separate cohorts, each containing individual and group components. Individual sessions closely follow a manualized protocol, while group sessions offer an opportunity to practice cognitive-processing therapy skills.
The team adapted the program to MST treatment by incorporating dialectical-behavioral therapy skills modules in the first week of the program, and implemented one-on-one skills consultation by request throughout the program.
An analysis of 191 subjects participating in 19 cohorts (12 combat, 9 MST cohorts) showed a 92% completion rate, which was similar, regardless of gender or cohort type. Both cohorts had significant reductions in PTSD severity as measured by the PTSD Checklist for DSM-5, and depression symptoms as measured by the Patient Health Questionnaire–9.
Another program, Families OverComing Under Stress, can also be adapted to MST. It is designed to build resiliency and wellness within families dealing with trauma or loss. It incorporates family assessment, psychoeducation tailored to the needs of the entire family, family-level resilience skills, and a narrative component.
An important element is the identification and management of stress reminders – triggers that remind the individual of a trauma and may cause a sudden shift in mood or behavior. A family member’s knowledge that the survivor is experiencing a stress reminder can reduce misunderstandings or unhelpful interpretations of behavior.
In fact, family considerations are often what bring veterans in for help in the first place, according to Dr. Karnik. He or she may be concerned about behavioral problems in a child, which the VA cannot address because its federal funding dictates a sole focus on the veteran. “We will take care of the whole family,” Dr. Karnik said. “Often that’s the entry point, and that allows us to do some engagement with the veteran, and things start to get uncovered.”
Dr. Karnik has no relevant financial disclosures.
*CORRECTION, 5/21/2019
SAN FRANCISCO – Veterans who are survivors of military sexual trauma during their service face unique challenges in their treatment and recovery. They are often reluctant to report their experiences – and understandably so.
“Military sexual assault represents a huge violation of that trust and safety. That’s what makes it so toxic and hard for participants to [come] forward, because they’re accused of breaking cohesion of their unit and breaking morale, and yet they have been mistreated,” Niranjan Karnik, MD, PhD, associate dean for community behavioral health at Rush Medical College, Chicago, said in an interview.
Dr. Karnik moderated a session on the prevalence and treatment of military sexual assault at the annual meeting of the American Psychiatric Association. Although the Department of Veterans Affairs treats many survivors of sexual assault, not all of them feel comfortable in that environment. “A VA hospital has a quasi-military feel to it, and that’s a reflection of what it is and the people who are there. That can be an inhibition – and can even be a trigger for [PTSD] symptoms,” Dr. Karnik said.
Survivors may also worry about being labeled, or about adverse entries going into their official record and how that could affect them in the future. The issue is a stark contrast to veterans who are suffering from combat-related trauma.
“When a combat trauma survivor goes to the VA, they feel protected because their colleagues are there. With military sexual trauma, because of that violation of trust from their peers, it can really exacerbate things,” Dr. Karnik said.
Fortunately, there are alternatives, such as the Road Home* Program at Rush Hospital, which has a few military accoutrements but more closely resembles a civilian center. “It can be an easier access point. The VA is taking care of a large majority of patients. We are a boutique program for the vets who can’t or feel unable to go through the VA program,” Dr. Karnik said.
Overall, 52.5% of women and 8.9% of men in the military report sexual harassment, and 23.6% of women and 1.9% of men report being sexually assaulted. That amounts to 14,900 service members, 8,600 women, and 6,300 men who were assaulted in 2016, according to Neeral K. Sheth, DO, assistant professor of psychiatry at Rush Medical College, who also presented at the session. The frequency of assault is higher among LGBTQ individuals, and African American men and women are more likely to experience sexual harassment.
There are options for treatment of military sexual trauma (MST). The 3-week Road Home intensive outpatient treatment program at Rush Hospital combines group and individual cognitive-processing therapy, which is a cognitive-behavioral therapy that has been shown to improve PTSD resulting from MST. The program places combat trauma and MST trauma patients into separate cohorts, each containing individual and group components. Individual sessions closely follow a manualized protocol, while group sessions offer an opportunity to practice cognitive-processing therapy skills.
The team adapted the program to MST treatment by incorporating dialectical-behavioral therapy skills modules in the first week of the program, and implemented one-on-one skills consultation by request throughout the program.
An analysis of 191 subjects participating in 19 cohorts (12 combat, 9 MST cohorts) showed a 92% completion rate, which was similar, regardless of gender or cohort type. Both cohorts had significant reductions in PTSD severity as measured by the PTSD Checklist for DSM-5, and depression symptoms as measured by the Patient Health Questionnaire–9.
Another program, Families OverComing Under Stress, can also be adapted to MST. It is designed to build resiliency and wellness within families dealing with trauma or loss. It incorporates family assessment, psychoeducation tailored to the needs of the entire family, family-level resilience skills, and a narrative component.
An important element is the identification and management of stress reminders – triggers that remind the individual of a trauma and may cause a sudden shift in mood or behavior. A family member’s knowledge that the survivor is experiencing a stress reminder can reduce misunderstandings or unhelpful interpretations of behavior.
In fact, family considerations are often what bring veterans in for help in the first place, according to Dr. Karnik. He or she may be concerned about behavioral problems in a child, which the VA cannot address because its federal funding dictates a sole focus on the veteran. “We will take care of the whole family,” Dr. Karnik said. “Often that’s the entry point, and that allows us to do some engagement with the veteran, and things start to get uncovered.”
Dr. Karnik has no relevant financial disclosures.
*CORRECTION, 5/21/2019
REPORTING FROM APA 2019
NCCN and MD Anderson award funding to researchers
The National Comprehensive Cancer Network (NCCN) and the University of Texas MD Anderson Cancer Center have awarded funding to investigators conducting a range of research projects looking at everything from chimeric antigen receptor T-cell therapy in lung cancer to financial toxicity to fecal microbiota transplantation.
Four researchers won funding through the NCCN Young Investigator Awards, which are supported by the NCCN Foundation, Astra Zeneca, Merck & Co., Genentech, Pfizer, and Incyte.
Prasanna Ananth, MD, of Yale Cancer Center in New Haven, Conn., won funding for her work investigating benchmarks for high quality end-of-life care in children with cancer.
Jaehyuk Choi, MD, PhD, of Northwestern University in Chicago won for his research investigating genomic determinants of response to immunotherapy in Merkel cell carcinoma.
Kedar Kirtane, MD, of Moffitt Cancer Center in Tampa won for his work on a digitized peer-to-peer patient support system for patients with locally advanced head and neck cancer who are receiving chemoradiation.
Yanming Li, PhD, of the University of Michigan in Ann Arbor won for network genome-wide association studies for early detection of cancers.
Meanwhile, the University of Texas MD Anderson Cancer Center named eight researchers to this year’s class of Andrew Sabin Family Fellows. Each researcher will receive $100,000 in funding over 2 years through a $30-million endowment from the Andrew Sabin Family Foundation.
Lauren Averett Byers, MD, is conducting research on the first chimeric antigen receptor T-cell therapy for small cell lung cancer.
Florencia McAllister, MD, is studying the intratumoral bacteria detected in pancreatic cancer patients and the use of fecal microbial transplants to improve treatment outcomes.
Jose Alejandro Rauh-Hain, MD, is attempting to improve gynecologic genetic testing and risk-reduction interventions in underserved populations.
Grace Li Smith, MD, PhD, is researching financial toxicity in patients with early-stage breast cancer who are treated with short-course versus standard adjuvant radiation.
Ishwaria Mohan Subbiah, MD, is investigating a personalized, technology-enhanced symptom-management strategy to provide holistic care for patients on phase 1 trials.
Andrea Viale, MD, is studying the effects of transient inflammation and the role of epithelial memory during progression of pancreatic ductal adenocarcinoma.
Linghua Wang, PhD, is investigating TIM-3 as a potential target for treating peritoneal carcinomatosis in patients with advanced gastric cancer.
Yinghong Wang, MD, PhD, is researching fecal microbiota transplantation as an option for managing immune-mediated colitis following cancer immunotherapy.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
The National Comprehensive Cancer Network (NCCN) and the University of Texas MD Anderson Cancer Center have awarded funding to investigators conducting a range of research projects looking at everything from chimeric antigen receptor T-cell therapy in lung cancer to financial toxicity to fecal microbiota transplantation.
Four researchers won funding through the NCCN Young Investigator Awards, which are supported by the NCCN Foundation, Astra Zeneca, Merck & Co., Genentech, Pfizer, and Incyte.
Prasanna Ananth, MD, of Yale Cancer Center in New Haven, Conn., won funding for her work investigating benchmarks for high quality end-of-life care in children with cancer.
Jaehyuk Choi, MD, PhD, of Northwestern University in Chicago won for his research investigating genomic determinants of response to immunotherapy in Merkel cell carcinoma.
Kedar Kirtane, MD, of Moffitt Cancer Center in Tampa won for his work on a digitized peer-to-peer patient support system for patients with locally advanced head and neck cancer who are receiving chemoradiation.
Yanming Li, PhD, of the University of Michigan in Ann Arbor won for network genome-wide association studies for early detection of cancers.
Meanwhile, the University of Texas MD Anderson Cancer Center named eight researchers to this year’s class of Andrew Sabin Family Fellows. Each researcher will receive $100,000 in funding over 2 years through a $30-million endowment from the Andrew Sabin Family Foundation.
Lauren Averett Byers, MD, is conducting research on the first chimeric antigen receptor T-cell therapy for small cell lung cancer.
Florencia McAllister, MD, is studying the intratumoral bacteria detected in pancreatic cancer patients and the use of fecal microbial transplants to improve treatment outcomes.
Jose Alejandro Rauh-Hain, MD, is attempting to improve gynecologic genetic testing and risk-reduction interventions in underserved populations.
Grace Li Smith, MD, PhD, is researching financial toxicity in patients with early-stage breast cancer who are treated with short-course versus standard adjuvant radiation.
Ishwaria Mohan Subbiah, MD, is investigating a personalized, technology-enhanced symptom-management strategy to provide holistic care for patients on phase 1 trials.
Andrea Viale, MD, is studying the effects of transient inflammation and the role of epithelial memory during progression of pancreatic ductal adenocarcinoma.
Linghua Wang, PhD, is investigating TIM-3 as a potential target for treating peritoneal carcinomatosis in patients with advanced gastric cancer.
Yinghong Wang, MD, PhD, is researching fecal microbiota transplantation as an option for managing immune-mediated colitis following cancer immunotherapy.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
The National Comprehensive Cancer Network (NCCN) and the University of Texas MD Anderson Cancer Center have awarded funding to investigators conducting a range of research projects looking at everything from chimeric antigen receptor T-cell therapy in lung cancer to financial toxicity to fecal microbiota transplantation.
Four researchers won funding through the NCCN Young Investigator Awards, which are supported by the NCCN Foundation, Astra Zeneca, Merck & Co., Genentech, Pfizer, and Incyte.
Prasanna Ananth, MD, of Yale Cancer Center in New Haven, Conn., won funding for her work investigating benchmarks for high quality end-of-life care in children with cancer.
Jaehyuk Choi, MD, PhD, of Northwestern University in Chicago won for his research investigating genomic determinants of response to immunotherapy in Merkel cell carcinoma.
Kedar Kirtane, MD, of Moffitt Cancer Center in Tampa won for his work on a digitized peer-to-peer patient support system for patients with locally advanced head and neck cancer who are receiving chemoradiation.
Yanming Li, PhD, of the University of Michigan in Ann Arbor won for network genome-wide association studies for early detection of cancers.
Meanwhile, the University of Texas MD Anderson Cancer Center named eight researchers to this year’s class of Andrew Sabin Family Fellows. Each researcher will receive $100,000 in funding over 2 years through a $30-million endowment from the Andrew Sabin Family Foundation.
Lauren Averett Byers, MD, is conducting research on the first chimeric antigen receptor T-cell therapy for small cell lung cancer.
Florencia McAllister, MD, is studying the intratumoral bacteria detected in pancreatic cancer patients and the use of fecal microbial transplants to improve treatment outcomes.
Jose Alejandro Rauh-Hain, MD, is attempting to improve gynecologic genetic testing and risk-reduction interventions in underserved populations.
Grace Li Smith, MD, PhD, is researching financial toxicity in patients with early-stage breast cancer who are treated with short-course versus standard adjuvant radiation.
Ishwaria Mohan Subbiah, MD, is investigating a personalized, technology-enhanced symptom-management strategy to provide holistic care for patients on phase 1 trials.
Andrea Viale, MD, is studying the effects of transient inflammation and the role of epithelial memory during progression of pancreatic ductal adenocarcinoma.
Linghua Wang, PhD, is investigating TIM-3 as a potential target for treating peritoneal carcinomatosis in patients with advanced gastric cancer.
Yinghong Wang, MD, PhD, is researching fecal microbiota transplantation as an option for managing immune-mediated colitis following cancer immunotherapy.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.









