User login
FDA approves pembrolizumab/lenvatinib combo for advanced endometrial carcinoma
The Food and Drug Administration has granted accelerated approval to a pembrolizumab (Keytruda) plus lenvatinib (Lenvima) combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The approval was based on results of KEYNOTE-146, a single-arm, multicenter, open-label, multicohort trial with 108 patients with metastatic endometrial carcinoma; 94 of these were not microsatellite instability high or mismatch repair deficient. The objective response rate in those 94 patients was 38.3% (95% confidence interval, 29%-49%), with 10 complete responses and 26 partial responses. The median duration of response was not reached over the trial period, and 69% of those who responded had a response duration of at least 6 months.
The most common adverse events reported during the trial were fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash.
The recommended dosage is lenvatinib 20 mg orally once daily with pembrolizumab 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks, according to the FDA.
The Food and Drug Administration has granted accelerated approval to a pembrolizumab (Keytruda) plus lenvatinib (Lenvima) combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The approval was based on results of KEYNOTE-146, a single-arm, multicenter, open-label, multicohort trial with 108 patients with metastatic endometrial carcinoma; 94 of these were not microsatellite instability high or mismatch repair deficient. The objective response rate in those 94 patients was 38.3% (95% confidence interval, 29%-49%), with 10 complete responses and 26 partial responses. The median duration of response was not reached over the trial period, and 69% of those who responded had a response duration of at least 6 months.
The most common adverse events reported during the trial were fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash.
The recommended dosage is lenvatinib 20 mg orally once daily with pembrolizumab 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks, according to the FDA.
The Food and Drug Administration has granted accelerated approval to a pembrolizumab (Keytruda) plus lenvatinib (Lenvima) combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The approval was based on results of KEYNOTE-146, a single-arm, multicenter, open-label, multicohort trial with 108 patients with metastatic endometrial carcinoma; 94 of these were not microsatellite instability high or mismatch repair deficient. The objective response rate in those 94 patients was 38.3% (95% confidence interval, 29%-49%), with 10 complete responses and 26 partial responses. The median duration of response was not reached over the trial period, and 69% of those who responded had a response duration of at least 6 months.
The most common adverse events reported during the trial were fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash.
The recommended dosage is lenvatinib 20 mg orally once daily with pembrolizumab 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks, according to the FDA.
Rivaroxaban bests combo therapy in post-PCI AFib
PARIS – Rivaroxaban monotherapy bested combination therapy with rivaroxaban and an antiplatelet agent for patients with atrial fibrillation and stable coronary artery disease, with significantly more deaths and bleeding events seen with combination therapy.
The pronounced imbalance in all-cause and cardiovascular mortality (the hazard ratio favoring rivaroxaban monotherapy was 9.72) came as a surprise, and led to early cessation of the multisite Japanese trial, lead investigator Satoshi Yasuda, MD, said at the annual congress of the European Society of Cardiology.
Several previous clinical trials had studied a reduced antithrombotic regimen for patients with atrial fibrillation (AFib) after percutaneous coronary intervention (PCI), said Dr. Yasuda, professor of medicine at Tohoku University, Sendai, Japan. Current guidelines recommend triple therapy with an oral anticoagulant plus aspirin and a P2Y12 inhibitor for the shortest duration possible, with combination therapy of an anticoagulant plus a P2Y12 inhibitor for up to 12 months. Once the 1-year post-PCI mark is reached, current European and American guidelines or consensus documents recommend monotherapy with an oral anticoagulant if AFib persists and the patient has stable coronary artery disease (CAD), explained Dr. Yasuda. “However, this approach has yet to be supported by evidence from randomized, controlled trials,” he said, adding “substantial numbers of patients in this situation continue to be treated with combination therapy, which indicates a gap between guidelines and clinical practice.”
The Atrial Fibrillation and Ischemic events with Rivaroxaban in Patients With Stable Coronary Artery Disease Study (AFIRE), he said, was designed to address this practice gap, randomizing 2,200 individuals to receive monotherapy with rivaroxaban or combination therapy. A total of 1,973 patients completed follow-up.
Patients were included in the randomized, open-label, parallel-group trial if they had AFib and stable CAD and were more than 1 year out from revascularization, or if they had angiographically confirmed CAD that did not need revascularization. All 294 AFIRE study sites were in Japan.
The study’s primary endpoint for efficacy was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, and all-cause death.
Most of the patients (79%) were male, and the mean age was 74 years. About 70% of patients in each treatment arm had received prior PCI, and 11% had undergone previous coronary artery bypass grafting (CABG).
The monotherapy arm received rivaroxaban 10 or 15 mg once daily depending on renal status. Patients in the combination therapy arm received rivaroxaban, plus a single antiplatelet drug. This could be 81 or 100 mg aspirin daily, clopidogrel at 50 or 75 mg/day, or prasugrel at 2.5 or 3.5 mg/day.
On the recommendation of the data and safety monitoring committee, the trial was terminated about 3 months early because significantly more all-cause deaths were being seen in the combination therapy group, said Dr. Yasuda. In the end, patients were treated under the study protocol for a median 23 months and followed up for a median 24.1 months.
Kaplan-Meier estimates for the first occurrence of the composite efficacy endpoint showed that monotherapy had a rate of 4.14% per patient-year, while combination therapy had a rate of occurrence for the efficacy endpoint of 5.75% per patient-year.
These figures yielded a statistically significant hazard ratio (HR) of 9.72 favoring monotherapy (P less than .001) for the prespecified noninferiority endpoint. In a post hoc analysis, rivaroxaban monotherapy achieved superiority over dual therapy (P = .02).
Breaking down the composite efficacy endpoint into its constituents, deaths by any cause and cardiovascular deaths primarily drove the difference in treatment arms. Seventy-three patients in the combo therapy arm and 41 in the rivaroxaban arm died of any cause, and the cause of death was cardiovascular for 43 combination therapy patients and 26 monotherapy patients. This yielded HRs favoring rivaroxaban of 0.55 for all-cause mortality and 0.59 for cardiovascular deaths.
Hazard ratios for individual cardiovascular events were not statistically significantly different between treatment arms, except for hemorrhagic stroke, which was seen in 13 patients receiving dual therapy and 4 receiving rivaroxaban alone, for a hazard ratio of 0.30.
Rivaroxaban monotherapy also bested dual therapy in safety: The HR was 0.59 for the incidence of a major bleed on rivaroxaban versus combination therapy, using International Society on Thrombosis and Haemostasis–established criteria for major bleeding. In the dual therapy arm, 58 individuals experienced major bleeding – the study’s primary safety endpoint – compared with 35 in the monotherapy arm, for a hazard ratio of 0.59; nonmajor bleeding occurred in 198 dual therapy patients and 121 monotherapy patients, yielding a hazard ratio of 0.58.
The Kaplan-Meier estimate for major bleeding on monotherapy was 1.62% per patient-year, compared with 2.76% per patient-year for those on combination therapy. These findings, said Dr. Yasuda, were “generally” consistent across prespecified subgroups that included participant stratification by age, sex, and bleeding risk, among others.
Dr. Yasuda acknowledged the many limitations of the trial. First, early termination introduced the possibility of overestimating the benefit of rivaroxaban monotherapy. Indeed, said Dr. Yasuda, “the reductions in rate of ischemic events and death from any cause with rivaroxaban monotherapy were unanticipated and are difficult to explain.”
Furthermore, the open-label trial design could be a source of bias and the use of both aspirin and P2Y12 inhibitors for antiplatelet therapy “makes it uncertain whether the benefit of rivaroxaban monotherapy applies equally to the two combination regimens,” said Dr. Yasuda.
Rivaroxaban dosing in AFIRE was tailored to the Japanese study population, noted Dr. Yasuda. This means that the study is not immediately generalizable to non-Asian populations, needing replication before fully closing the knowledge gap about best long-term management of patients with AFib and stable CAD in the United States and Western Europe.
However, Dr. Yasuda pointed out, serum rivaroxaban levels in Japanese patients taking the 10- or 15-mg dose are generally similar to those seen in white patients taking a 20-mg rivaroxaban dose.
Freek Verheugt, MD, of Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, was the discussant for the presentation. He raised an additional concern: “East Asian patients are poor metabolizers of clopidogrel, which may have resulted also in underestimation of bleeding.” He cautioned that the AFIRE results may not be applicable to patients on a novel anticoagulant other than clopidogrel, or on vitamin K antagonists.
In his detailed critique of the AFIRE results, Dr. Verheugt cited the OAC ALONE trial, which used a similar study design and was also conducted in Japan. For OAC ALONE, Dr. Verheugt pointed out that “You can see ... that it was not harmful in this 700-patient study to stop aspirin therapy 1 year after an intervention.” However, he said, “the net clinical benefit is not very different, either” between treatment arms in the OAC ALONE trial. “Given the low number of patients and the low number of events, this trial was not conclusive whatsoever” he added, so AFIRE’s findings were needed.
The safety data from AFIRE, with a study population triple that of OAC ALONE, makes the safety argument for monotherapy “a very easy winner,” said Dr. Verheugt.
Dr. Verheugt was not mystified by the lower all-cause and cardiovascular death rate in the monotherapy group. “What are the mechanisms that if you stop antiplatelet therapy you have a better ischemic outcome? How come?” asked Dr. Verheugt.
“Very likely, it is the bleeding ... that you prevent if you stop antiplatelet therapy,” he said, adding that it’s known from previous studies in individuals with acute coronary syndromes and AFib that “bleeding is correlated with mortality, and that’s also proven here.”
Though Dr. Verheugt joined Dr. Yasuda in calling for replication of the results in a non-Asian population, he concurred that the AFIRE results validate current practice for anticoagulation in AFib with stable CAD. “Stopping at 1 year is safer than continuation and, most of all, it saves lives,” he said.
Full results of AFIRE were published online at the time of Dr. Yasuda’s presentation (N Engl J Med. 2019 Sep 2. doi: 10.1056/NEJMoa1904143).
The study was funded by the Japanese Cardiovascular Research Foundation. Dr. Yasuda reported financial relationships with Abbott, Bristol-Myers, Daiichi-Sankyo, and Takeda. Dr. Verheugt reported financial relationships with BayerHealthcare, BMS/Pfizer, Boehringer-Ingelheim, and Daiichi-Sankyo.
SOURCE: Yasuda S. et al. ESC 2019, Hot Line Session 3, Abstract 3175.
PARIS – Rivaroxaban monotherapy bested combination therapy with rivaroxaban and an antiplatelet agent for patients with atrial fibrillation and stable coronary artery disease, with significantly more deaths and bleeding events seen with combination therapy.
The pronounced imbalance in all-cause and cardiovascular mortality (the hazard ratio favoring rivaroxaban monotherapy was 9.72) came as a surprise, and led to early cessation of the multisite Japanese trial, lead investigator Satoshi Yasuda, MD, said at the annual congress of the European Society of Cardiology.
Several previous clinical trials had studied a reduced antithrombotic regimen for patients with atrial fibrillation (AFib) after percutaneous coronary intervention (PCI), said Dr. Yasuda, professor of medicine at Tohoku University, Sendai, Japan. Current guidelines recommend triple therapy with an oral anticoagulant plus aspirin and a P2Y12 inhibitor for the shortest duration possible, with combination therapy of an anticoagulant plus a P2Y12 inhibitor for up to 12 months. Once the 1-year post-PCI mark is reached, current European and American guidelines or consensus documents recommend monotherapy with an oral anticoagulant if AFib persists and the patient has stable coronary artery disease (CAD), explained Dr. Yasuda. “However, this approach has yet to be supported by evidence from randomized, controlled trials,” he said, adding “substantial numbers of patients in this situation continue to be treated with combination therapy, which indicates a gap between guidelines and clinical practice.”
The Atrial Fibrillation and Ischemic events with Rivaroxaban in Patients With Stable Coronary Artery Disease Study (AFIRE), he said, was designed to address this practice gap, randomizing 2,200 individuals to receive monotherapy with rivaroxaban or combination therapy. A total of 1,973 patients completed follow-up.
Patients were included in the randomized, open-label, parallel-group trial if they had AFib and stable CAD and were more than 1 year out from revascularization, or if they had angiographically confirmed CAD that did not need revascularization. All 294 AFIRE study sites were in Japan.
The study’s primary endpoint for efficacy was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, and all-cause death.
Most of the patients (79%) were male, and the mean age was 74 years. About 70% of patients in each treatment arm had received prior PCI, and 11% had undergone previous coronary artery bypass grafting (CABG).
The monotherapy arm received rivaroxaban 10 or 15 mg once daily depending on renal status. Patients in the combination therapy arm received rivaroxaban, plus a single antiplatelet drug. This could be 81 or 100 mg aspirin daily, clopidogrel at 50 or 75 mg/day, or prasugrel at 2.5 or 3.5 mg/day.
On the recommendation of the data and safety monitoring committee, the trial was terminated about 3 months early because significantly more all-cause deaths were being seen in the combination therapy group, said Dr. Yasuda. In the end, patients were treated under the study protocol for a median 23 months and followed up for a median 24.1 months.
Kaplan-Meier estimates for the first occurrence of the composite efficacy endpoint showed that monotherapy had a rate of 4.14% per patient-year, while combination therapy had a rate of occurrence for the efficacy endpoint of 5.75% per patient-year.
These figures yielded a statistically significant hazard ratio (HR) of 9.72 favoring monotherapy (P less than .001) for the prespecified noninferiority endpoint. In a post hoc analysis, rivaroxaban monotherapy achieved superiority over dual therapy (P = .02).
Breaking down the composite efficacy endpoint into its constituents, deaths by any cause and cardiovascular deaths primarily drove the difference in treatment arms. Seventy-three patients in the combo therapy arm and 41 in the rivaroxaban arm died of any cause, and the cause of death was cardiovascular for 43 combination therapy patients and 26 monotherapy patients. This yielded HRs favoring rivaroxaban of 0.55 for all-cause mortality and 0.59 for cardiovascular deaths.
Hazard ratios for individual cardiovascular events were not statistically significantly different between treatment arms, except for hemorrhagic stroke, which was seen in 13 patients receiving dual therapy and 4 receiving rivaroxaban alone, for a hazard ratio of 0.30.
Rivaroxaban monotherapy also bested dual therapy in safety: The HR was 0.59 for the incidence of a major bleed on rivaroxaban versus combination therapy, using International Society on Thrombosis and Haemostasis–established criteria for major bleeding. In the dual therapy arm, 58 individuals experienced major bleeding – the study’s primary safety endpoint – compared with 35 in the monotherapy arm, for a hazard ratio of 0.59; nonmajor bleeding occurred in 198 dual therapy patients and 121 monotherapy patients, yielding a hazard ratio of 0.58.
The Kaplan-Meier estimate for major bleeding on monotherapy was 1.62% per patient-year, compared with 2.76% per patient-year for those on combination therapy. These findings, said Dr. Yasuda, were “generally” consistent across prespecified subgroups that included participant stratification by age, sex, and bleeding risk, among others.
Dr. Yasuda acknowledged the many limitations of the trial. First, early termination introduced the possibility of overestimating the benefit of rivaroxaban monotherapy. Indeed, said Dr. Yasuda, “the reductions in rate of ischemic events and death from any cause with rivaroxaban monotherapy were unanticipated and are difficult to explain.”
Furthermore, the open-label trial design could be a source of bias and the use of both aspirin and P2Y12 inhibitors for antiplatelet therapy “makes it uncertain whether the benefit of rivaroxaban monotherapy applies equally to the two combination regimens,” said Dr. Yasuda.
Rivaroxaban dosing in AFIRE was tailored to the Japanese study population, noted Dr. Yasuda. This means that the study is not immediately generalizable to non-Asian populations, needing replication before fully closing the knowledge gap about best long-term management of patients with AFib and stable CAD in the United States and Western Europe.
However, Dr. Yasuda pointed out, serum rivaroxaban levels in Japanese patients taking the 10- or 15-mg dose are generally similar to those seen in white patients taking a 20-mg rivaroxaban dose.
Freek Verheugt, MD, of Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, was the discussant for the presentation. He raised an additional concern: “East Asian patients are poor metabolizers of clopidogrel, which may have resulted also in underestimation of bleeding.” He cautioned that the AFIRE results may not be applicable to patients on a novel anticoagulant other than clopidogrel, or on vitamin K antagonists.
In his detailed critique of the AFIRE results, Dr. Verheugt cited the OAC ALONE trial, which used a similar study design and was also conducted in Japan. For OAC ALONE, Dr. Verheugt pointed out that “You can see ... that it was not harmful in this 700-patient study to stop aspirin therapy 1 year after an intervention.” However, he said, “the net clinical benefit is not very different, either” between treatment arms in the OAC ALONE trial. “Given the low number of patients and the low number of events, this trial was not conclusive whatsoever” he added, so AFIRE’s findings were needed.
The safety data from AFIRE, with a study population triple that of OAC ALONE, makes the safety argument for monotherapy “a very easy winner,” said Dr. Verheugt.
Dr. Verheugt was not mystified by the lower all-cause and cardiovascular death rate in the monotherapy group. “What are the mechanisms that if you stop antiplatelet therapy you have a better ischemic outcome? How come?” asked Dr. Verheugt.
“Very likely, it is the bleeding ... that you prevent if you stop antiplatelet therapy,” he said, adding that it’s known from previous studies in individuals with acute coronary syndromes and AFib that “bleeding is correlated with mortality, and that’s also proven here.”
Though Dr. Verheugt joined Dr. Yasuda in calling for replication of the results in a non-Asian population, he concurred that the AFIRE results validate current practice for anticoagulation in AFib with stable CAD. “Stopping at 1 year is safer than continuation and, most of all, it saves lives,” he said.
Full results of AFIRE were published online at the time of Dr. Yasuda’s presentation (N Engl J Med. 2019 Sep 2. doi: 10.1056/NEJMoa1904143).
The study was funded by the Japanese Cardiovascular Research Foundation. Dr. Yasuda reported financial relationships with Abbott, Bristol-Myers, Daiichi-Sankyo, and Takeda. Dr. Verheugt reported financial relationships with BayerHealthcare, BMS/Pfizer, Boehringer-Ingelheim, and Daiichi-Sankyo.
SOURCE: Yasuda S. et al. ESC 2019, Hot Line Session 3, Abstract 3175.
PARIS – Rivaroxaban monotherapy bested combination therapy with rivaroxaban and an antiplatelet agent for patients with atrial fibrillation and stable coronary artery disease, with significantly more deaths and bleeding events seen with combination therapy.
The pronounced imbalance in all-cause and cardiovascular mortality (the hazard ratio favoring rivaroxaban monotherapy was 9.72) came as a surprise, and led to early cessation of the multisite Japanese trial, lead investigator Satoshi Yasuda, MD, said at the annual congress of the European Society of Cardiology.
Several previous clinical trials had studied a reduced antithrombotic regimen for patients with atrial fibrillation (AFib) after percutaneous coronary intervention (PCI), said Dr. Yasuda, professor of medicine at Tohoku University, Sendai, Japan. Current guidelines recommend triple therapy with an oral anticoagulant plus aspirin and a P2Y12 inhibitor for the shortest duration possible, with combination therapy of an anticoagulant plus a P2Y12 inhibitor for up to 12 months. Once the 1-year post-PCI mark is reached, current European and American guidelines or consensus documents recommend monotherapy with an oral anticoagulant if AFib persists and the patient has stable coronary artery disease (CAD), explained Dr. Yasuda. “However, this approach has yet to be supported by evidence from randomized, controlled trials,” he said, adding “substantial numbers of patients in this situation continue to be treated with combination therapy, which indicates a gap between guidelines and clinical practice.”
The Atrial Fibrillation and Ischemic events with Rivaroxaban in Patients With Stable Coronary Artery Disease Study (AFIRE), he said, was designed to address this practice gap, randomizing 2,200 individuals to receive monotherapy with rivaroxaban or combination therapy. A total of 1,973 patients completed follow-up.
Patients were included in the randomized, open-label, parallel-group trial if they had AFib and stable CAD and were more than 1 year out from revascularization, or if they had angiographically confirmed CAD that did not need revascularization. All 294 AFIRE study sites were in Japan.
The study’s primary endpoint for efficacy was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, and all-cause death.
Most of the patients (79%) were male, and the mean age was 74 years. About 70% of patients in each treatment arm had received prior PCI, and 11% had undergone previous coronary artery bypass grafting (CABG).
The monotherapy arm received rivaroxaban 10 or 15 mg once daily depending on renal status. Patients in the combination therapy arm received rivaroxaban, plus a single antiplatelet drug. This could be 81 or 100 mg aspirin daily, clopidogrel at 50 or 75 mg/day, or prasugrel at 2.5 or 3.5 mg/day.
On the recommendation of the data and safety monitoring committee, the trial was terminated about 3 months early because significantly more all-cause deaths were being seen in the combination therapy group, said Dr. Yasuda. In the end, patients were treated under the study protocol for a median 23 months and followed up for a median 24.1 months.
Kaplan-Meier estimates for the first occurrence of the composite efficacy endpoint showed that monotherapy had a rate of 4.14% per patient-year, while combination therapy had a rate of occurrence for the efficacy endpoint of 5.75% per patient-year.
These figures yielded a statistically significant hazard ratio (HR) of 9.72 favoring monotherapy (P less than .001) for the prespecified noninferiority endpoint. In a post hoc analysis, rivaroxaban monotherapy achieved superiority over dual therapy (P = .02).
Breaking down the composite efficacy endpoint into its constituents, deaths by any cause and cardiovascular deaths primarily drove the difference in treatment arms. Seventy-three patients in the combo therapy arm and 41 in the rivaroxaban arm died of any cause, and the cause of death was cardiovascular for 43 combination therapy patients and 26 monotherapy patients. This yielded HRs favoring rivaroxaban of 0.55 for all-cause mortality and 0.59 for cardiovascular deaths.
Hazard ratios for individual cardiovascular events were not statistically significantly different between treatment arms, except for hemorrhagic stroke, which was seen in 13 patients receiving dual therapy and 4 receiving rivaroxaban alone, for a hazard ratio of 0.30.
Rivaroxaban monotherapy also bested dual therapy in safety: The HR was 0.59 for the incidence of a major bleed on rivaroxaban versus combination therapy, using International Society on Thrombosis and Haemostasis–established criteria for major bleeding. In the dual therapy arm, 58 individuals experienced major bleeding – the study’s primary safety endpoint – compared with 35 in the monotherapy arm, for a hazard ratio of 0.59; nonmajor bleeding occurred in 198 dual therapy patients and 121 monotherapy patients, yielding a hazard ratio of 0.58.
The Kaplan-Meier estimate for major bleeding on monotherapy was 1.62% per patient-year, compared with 2.76% per patient-year for those on combination therapy. These findings, said Dr. Yasuda, were “generally” consistent across prespecified subgroups that included participant stratification by age, sex, and bleeding risk, among others.
Dr. Yasuda acknowledged the many limitations of the trial. First, early termination introduced the possibility of overestimating the benefit of rivaroxaban monotherapy. Indeed, said Dr. Yasuda, “the reductions in rate of ischemic events and death from any cause with rivaroxaban monotherapy were unanticipated and are difficult to explain.”
Furthermore, the open-label trial design could be a source of bias and the use of both aspirin and P2Y12 inhibitors for antiplatelet therapy “makes it uncertain whether the benefit of rivaroxaban monotherapy applies equally to the two combination regimens,” said Dr. Yasuda.
Rivaroxaban dosing in AFIRE was tailored to the Japanese study population, noted Dr. Yasuda. This means that the study is not immediately generalizable to non-Asian populations, needing replication before fully closing the knowledge gap about best long-term management of patients with AFib and stable CAD in the United States and Western Europe.
However, Dr. Yasuda pointed out, serum rivaroxaban levels in Japanese patients taking the 10- or 15-mg dose are generally similar to those seen in white patients taking a 20-mg rivaroxaban dose.
Freek Verheugt, MD, of Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, was the discussant for the presentation. He raised an additional concern: “East Asian patients are poor metabolizers of clopidogrel, which may have resulted also in underestimation of bleeding.” He cautioned that the AFIRE results may not be applicable to patients on a novel anticoagulant other than clopidogrel, or on vitamin K antagonists.
In his detailed critique of the AFIRE results, Dr. Verheugt cited the OAC ALONE trial, which used a similar study design and was also conducted in Japan. For OAC ALONE, Dr. Verheugt pointed out that “You can see ... that it was not harmful in this 700-patient study to stop aspirin therapy 1 year after an intervention.” However, he said, “the net clinical benefit is not very different, either” between treatment arms in the OAC ALONE trial. “Given the low number of patients and the low number of events, this trial was not conclusive whatsoever” he added, so AFIRE’s findings were needed.
The safety data from AFIRE, with a study population triple that of OAC ALONE, makes the safety argument for monotherapy “a very easy winner,” said Dr. Verheugt.
Dr. Verheugt was not mystified by the lower all-cause and cardiovascular death rate in the monotherapy group. “What are the mechanisms that if you stop antiplatelet therapy you have a better ischemic outcome? How come?” asked Dr. Verheugt.
“Very likely, it is the bleeding ... that you prevent if you stop antiplatelet therapy,” he said, adding that it’s known from previous studies in individuals with acute coronary syndromes and AFib that “bleeding is correlated with mortality, and that’s also proven here.”
Though Dr. Verheugt joined Dr. Yasuda in calling for replication of the results in a non-Asian population, he concurred that the AFIRE results validate current practice for anticoagulation in AFib with stable CAD. “Stopping at 1 year is safer than continuation and, most of all, it saves lives,” he said.
Full results of AFIRE were published online at the time of Dr. Yasuda’s presentation (N Engl J Med. 2019 Sep 2. doi: 10.1056/NEJMoa1904143).
The study was funded by the Japanese Cardiovascular Research Foundation. Dr. Yasuda reported financial relationships with Abbott, Bristol-Myers, Daiichi-Sankyo, and Takeda. Dr. Verheugt reported financial relationships with BayerHealthcare, BMS/Pfizer, Boehringer-Ingelheim, and Daiichi-Sankyo.
SOURCE: Yasuda S. et al. ESC 2019, Hot Line Session 3, Abstract 3175.
AT THE ESC CONGRESS 2019
ISAR-REACT 5: Prasugrel superior to ticagrelor in ACS
PARIS – Prasugrel proved superior to ticagrelor in patients with acute coronary syndrome (ACS) in what was hailed as “a landmark study” presented at the annual congress of the European Society of Cardiology.
The results of ISAR-REACT 5 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5) were unequivocal: “In ACS patients with or without ST-segment elevation, treatment with prasugrel as compared with ticagrelor significantly reduced the composite rate of death, myocardial infarction, or stroke at 1 year without an increase in major bleeding,” declared first author Stephanie Schuepke, MD, a cardiologist at the German Heart Center in Munich.
The study outcome was totally unexpected. Indeed, the result didn’t merely turn heads, it no doubt caused numerous wrenched necks caused by strenuous double-takes on the part of interventional cardiologists at the congress. That’s because, even though both ticagrelor and prasugrel enjoy a class I recommendation for use in ACS in the ESC guidelines, it has been widely assumed – based on previous evidence plus the fact that ticagrelor is the more potent platelet inhibitor – that ticagrelor is the superior drug in this setting. It turns out, however, that those earlier studies weren’t germane to the issue directly addressed in ISAR-REACT 5, the first-ever direct head-to-head comparison of the two potent P2Y12 inhibitors in the setting of ACS with a planned invasive strategy.
“Obviously, very surprising results,” commented Roxana Mehran, MD, professor of medicine and director of interventional cardiology research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York, who cochaired a press conference where Dr. Schuepke shared the study findings.
“We were surprised as well,” confessed Dr. Schuepke. “We assumed that ticagrelor is superior to prasugrel in terms of clinical outcomes in patients with ACS with a planned invasive strategy. But the results show us that the opposite is true.”
ISAR-REACT 5 was an open-label, investigator-initiated randomized trial conducted at 23 centers in Germany and Italy. It included 4,018 participants with ST-elevation segment MI (STEMI), without STEMI, or with unstable angina, all with scheduled coronary angiography. Participants were randomized to ticagrelor or prasugrel and were expected to remain on their assigned potent antiplatelet agent plus aspirin for 1 year of dual-antiplatelet therapy.
The primary outcome was the composite of death, MI, or stroke at 1 year of follow-up. This endpoint occurred in 9.3% of the ticagrelor group and 6.9% of patients in the prasugrel group, for a highly significant 36% increased relative risk in the ticagrelor-treated patients. Prasugrel had a numeric advantage in each of the individual components of the endpoint: the 1-year rate of all-cause mortality was 4.5% with ticagrelor versus 3.7% with prasugrel; for MI, the incidence was 4.8% with ticagrelor and 3.0% with prasugrel, a statistically significant difference; and for stroke, 1.1% versus 1.0%.
Major bleeding as defined by the Bleeding Academic Research Consortium scale occurred in 5.4% of patients in the ticagrelor arm, and was similar at 4.8% in the prasugrel group, Dr. Schuepke continued.
Definite or probable stent thrombosis occurred in 1.3% of the ticagrelor group and 1.0% of patients assigned to prasugrel.
The mechanism for prasugrel’s superior results is unclear, she said. Possibilities include the fact that it’s a once-daily drug, compared with twice-daily ticagrelor, which could affect adherence; a differential profile in terms of drug interactions; and prasugrel’s reversibility of action and capacity for step-down dosing in patients at high bleeding risk.
Discussant Gilles Montalescot, MD, PhD, called ISAR-REACT 5 a “fascinating” study. He elaborated: “It is a pragmatic study to answer a pragmatic question. It’s not a drug trial; really, it’s more a strategy trial, with a comparison of two drugs and two strategies.”
In ISAR-REACT 5, ticagrelor was utilized in standard fashion: Patients, regardless of their ACS type, received a 180-mg loading dose as soon as possible after randomization, typically about 3 hours after presentation. That was also the protocol in the prasugrel arm, but only in patients with STEMI, who quickly got a 60-mg loading dose. In contrast, patients without STEMI or with unstable angina didn’t get a loading dose of prasugrel until they’d undergone coronary angiography and their coronary anatomy was known. That was the ISAR-REACT 5 strategy in light of an earlier study, which concluded that giving prasugrel prior to angiography in such patients led to increased bleeding without any improvement in clinical outcomes.
The essence of ISAR-REACT 5 lies in that difference in treatment strategies and the impact it had on outcomes. “The one-size-fits-all strategy – here, with ticagrelor – was inferior to an individualized strategy, here with prasugrel,” observed Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Paris-Salpetriere Hospital.
The study results were notably consistent, favoring prasugrel over ticagrelor numerically across the board regardless of whether a patient presented with STEMI, without STEMI, or with unstable angina. Particularly noteworthy was the finding that this was also true in terms of the individual components of the 1-year composite endpoint. Dr. Montalescot drew special attention to the large subset of patients who had presented with STEMI and thus received the same treatment strategy involving a loading dose of their assigned P2Y12 inhibitor prior to angiography. This allowed for a direct head-to-head comparison of the clinical efficacy of the two antiplatelet agents in STEMI patients. The clear winner here was prasugrel, as the composite event rate was 10.1% in the ticagrelor group, compared with 7.9% in the prasugrel group.
“ISAR-REACT 5 is a landmark study which is going to impact our practice and the next set of guidelines to come in 2020,” the interventional cardiologist predicted.
Dr. Schuepke reported having no financial conflicts regarding the ISAR-REACT 5 study, sponsored by the German Heart Center and the German Center for Cardiovascular Research.
Simultaneous with her presentation of ISAR-REACT 5, the study results were published online (N Engl J Med. 2019 Sep 1. doi: 10.1056/NEJMoa1908973).
PARIS – Prasugrel proved superior to ticagrelor in patients with acute coronary syndrome (ACS) in what was hailed as “a landmark study” presented at the annual congress of the European Society of Cardiology.
The results of ISAR-REACT 5 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5) were unequivocal: “In ACS patients with or without ST-segment elevation, treatment with prasugrel as compared with ticagrelor significantly reduced the composite rate of death, myocardial infarction, or stroke at 1 year without an increase in major bleeding,” declared first author Stephanie Schuepke, MD, a cardiologist at the German Heart Center in Munich.
The study outcome was totally unexpected. Indeed, the result didn’t merely turn heads, it no doubt caused numerous wrenched necks caused by strenuous double-takes on the part of interventional cardiologists at the congress. That’s because, even though both ticagrelor and prasugrel enjoy a class I recommendation for use in ACS in the ESC guidelines, it has been widely assumed – based on previous evidence plus the fact that ticagrelor is the more potent platelet inhibitor – that ticagrelor is the superior drug in this setting. It turns out, however, that those earlier studies weren’t germane to the issue directly addressed in ISAR-REACT 5, the first-ever direct head-to-head comparison of the two potent P2Y12 inhibitors in the setting of ACS with a planned invasive strategy.
“Obviously, very surprising results,” commented Roxana Mehran, MD, professor of medicine and director of interventional cardiology research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York, who cochaired a press conference where Dr. Schuepke shared the study findings.
“We were surprised as well,” confessed Dr. Schuepke. “We assumed that ticagrelor is superior to prasugrel in terms of clinical outcomes in patients with ACS with a planned invasive strategy. But the results show us that the opposite is true.”
ISAR-REACT 5 was an open-label, investigator-initiated randomized trial conducted at 23 centers in Germany and Italy. It included 4,018 participants with ST-elevation segment MI (STEMI), without STEMI, or with unstable angina, all with scheduled coronary angiography. Participants were randomized to ticagrelor or prasugrel and were expected to remain on their assigned potent antiplatelet agent plus aspirin for 1 year of dual-antiplatelet therapy.
The primary outcome was the composite of death, MI, or stroke at 1 year of follow-up. This endpoint occurred in 9.3% of the ticagrelor group and 6.9% of patients in the prasugrel group, for a highly significant 36% increased relative risk in the ticagrelor-treated patients. Prasugrel had a numeric advantage in each of the individual components of the endpoint: the 1-year rate of all-cause mortality was 4.5% with ticagrelor versus 3.7% with prasugrel; for MI, the incidence was 4.8% with ticagrelor and 3.0% with prasugrel, a statistically significant difference; and for stroke, 1.1% versus 1.0%.
Major bleeding as defined by the Bleeding Academic Research Consortium scale occurred in 5.4% of patients in the ticagrelor arm, and was similar at 4.8% in the prasugrel group, Dr. Schuepke continued.
Definite or probable stent thrombosis occurred in 1.3% of the ticagrelor group and 1.0% of patients assigned to prasugrel.
The mechanism for prasugrel’s superior results is unclear, she said. Possibilities include the fact that it’s a once-daily drug, compared with twice-daily ticagrelor, which could affect adherence; a differential profile in terms of drug interactions; and prasugrel’s reversibility of action and capacity for step-down dosing in patients at high bleeding risk.
Discussant Gilles Montalescot, MD, PhD, called ISAR-REACT 5 a “fascinating” study. He elaborated: “It is a pragmatic study to answer a pragmatic question. It’s not a drug trial; really, it’s more a strategy trial, with a comparison of two drugs and two strategies.”
In ISAR-REACT 5, ticagrelor was utilized in standard fashion: Patients, regardless of their ACS type, received a 180-mg loading dose as soon as possible after randomization, typically about 3 hours after presentation. That was also the protocol in the prasugrel arm, but only in patients with STEMI, who quickly got a 60-mg loading dose. In contrast, patients without STEMI or with unstable angina didn’t get a loading dose of prasugrel until they’d undergone coronary angiography and their coronary anatomy was known. That was the ISAR-REACT 5 strategy in light of an earlier study, which concluded that giving prasugrel prior to angiography in such patients led to increased bleeding without any improvement in clinical outcomes.
The essence of ISAR-REACT 5 lies in that difference in treatment strategies and the impact it had on outcomes. “The one-size-fits-all strategy – here, with ticagrelor – was inferior to an individualized strategy, here with prasugrel,” observed Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Paris-Salpetriere Hospital.
The study results were notably consistent, favoring prasugrel over ticagrelor numerically across the board regardless of whether a patient presented with STEMI, without STEMI, or with unstable angina. Particularly noteworthy was the finding that this was also true in terms of the individual components of the 1-year composite endpoint. Dr. Montalescot drew special attention to the large subset of patients who had presented with STEMI and thus received the same treatment strategy involving a loading dose of their assigned P2Y12 inhibitor prior to angiography. This allowed for a direct head-to-head comparison of the clinical efficacy of the two antiplatelet agents in STEMI patients. The clear winner here was prasugrel, as the composite event rate was 10.1% in the ticagrelor group, compared with 7.9% in the prasugrel group.
“ISAR-REACT 5 is a landmark study which is going to impact our practice and the next set of guidelines to come in 2020,” the interventional cardiologist predicted.
Dr. Schuepke reported having no financial conflicts regarding the ISAR-REACT 5 study, sponsored by the German Heart Center and the German Center for Cardiovascular Research.
Simultaneous with her presentation of ISAR-REACT 5, the study results were published online (N Engl J Med. 2019 Sep 1. doi: 10.1056/NEJMoa1908973).
PARIS – Prasugrel proved superior to ticagrelor in patients with acute coronary syndrome (ACS) in what was hailed as “a landmark study” presented at the annual congress of the European Society of Cardiology.
The results of ISAR-REACT 5 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5) were unequivocal: “In ACS patients with or without ST-segment elevation, treatment with prasugrel as compared with ticagrelor significantly reduced the composite rate of death, myocardial infarction, or stroke at 1 year without an increase in major bleeding,” declared first author Stephanie Schuepke, MD, a cardiologist at the German Heart Center in Munich.
The study outcome was totally unexpected. Indeed, the result didn’t merely turn heads, it no doubt caused numerous wrenched necks caused by strenuous double-takes on the part of interventional cardiologists at the congress. That’s because, even though both ticagrelor and prasugrel enjoy a class I recommendation for use in ACS in the ESC guidelines, it has been widely assumed – based on previous evidence plus the fact that ticagrelor is the more potent platelet inhibitor – that ticagrelor is the superior drug in this setting. It turns out, however, that those earlier studies weren’t germane to the issue directly addressed in ISAR-REACT 5, the first-ever direct head-to-head comparison of the two potent P2Y12 inhibitors in the setting of ACS with a planned invasive strategy.
“Obviously, very surprising results,” commented Roxana Mehran, MD, professor of medicine and director of interventional cardiology research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York, who cochaired a press conference where Dr. Schuepke shared the study findings.
“We were surprised as well,” confessed Dr. Schuepke. “We assumed that ticagrelor is superior to prasugrel in terms of clinical outcomes in patients with ACS with a planned invasive strategy. But the results show us that the opposite is true.”
ISAR-REACT 5 was an open-label, investigator-initiated randomized trial conducted at 23 centers in Germany and Italy. It included 4,018 participants with ST-elevation segment MI (STEMI), without STEMI, or with unstable angina, all with scheduled coronary angiography. Participants were randomized to ticagrelor or prasugrel and were expected to remain on their assigned potent antiplatelet agent plus aspirin for 1 year of dual-antiplatelet therapy.
The primary outcome was the composite of death, MI, or stroke at 1 year of follow-up. This endpoint occurred in 9.3% of the ticagrelor group and 6.9% of patients in the prasugrel group, for a highly significant 36% increased relative risk in the ticagrelor-treated patients. Prasugrel had a numeric advantage in each of the individual components of the endpoint: the 1-year rate of all-cause mortality was 4.5% with ticagrelor versus 3.7% with prasugrel; for MI, the incidence was 4.8% with ticagrelor and 3.0% with prasugrel, a statistically significant difference; and for stroke, 1.1% versus 1.0%.
Major bleeding as defined by the Bleeding Academic Research Consortium scale occurred in 5.4% of patients in the ticagrelor arm, and was similar at 4.8% in the prasugrel group, Dr. Schuepke continued.
Definite or probable stent thrombosis occurred in 1.3% of the ticagrelor group and 1.0% of patients assigned to prasugrel.
The mechanism for prasugrel’s superior results is unclear, she said. Possibilities include the fact that it’s a once-daily drug, compared with twice-daily ticagrelor, which could affect adherence; a differential profile in terms of drug interactions; and prasugrel’s reversibility of action and capacity for step-down dosing in patients at high bleeding risk.
Discussant Gilles Montalescot, MD, PhD, called ISAR-REACT 5 a “fascinating” study. He elaborated: “It is a pragmatic study to answer a pragmatic question. It’s not a drug trial; really, it’s more a strategy trial, with a comparison of two drugs and two strategies.”
In ISAR-REACT 5, ticagrelor was utilized in standard fashion: Patients, regardless of their ACS type, received a 180-mg loading dose as soon as possible after randomization, typically about 3 hours after presentation. That was also the protocol in the prasugrel arm, but only in patients with STEMI, who quickly got a 60-mg loading dose. In contrast, patients without STEMI or with unstable angina didn’t get a loading dose of prasugrel until they’d undergone coronary angiography and their coronary anatomy was known. That was the ISAR-REACT 5 strategy in light of an earlier study, which concluded that giving prasugrel prior to angiography in such patients led to increased bleeding without any improvement in clinical outcomes.
The essence of ISAR-REACT 5 lies in that difference in treatment strategies and the impact it had on outcomes. “The one-size-fits-all strategy – here, with ticagrelor – was inferior to an individualized strategy, here with prasugrel,” observed Dr. Montalescot, professor of cardiology at the University of Paris VI and director of the cardiac care unit at Paris-Salpetriere Hospital.
The study results were notably consistent, favoring prasugrel over ticagrelor numerically across the board regardless of whether a patient presented with STEMI, without STEMI, or with unstable angina. Particularly noteworthy was the finding that this was also true in terms of the individual components of the 1-year composite endpoint. Dr. Montalescot drew special attention to the large subset of patients who had presented with STEMI and thus received the same treatment strategy involving a loading dose of their assigned P2Y12 inhibitor prior to angiography. This allowed for a direct head-to-head comparison of the clinical efficacy of the two antiplatelet agents in STEMI patients. The clear winner here was prasugrel, as the composite event rate was 10.1% in the ticagrelor group, compared with 7.9% in the prasugrel group.
“ISAR-REACT 5 is a landmark study which is going to impact our practice and the next set of guidelines to come in 2020,” the interventional cardiologist predicted.
Dr. Schuepke reported having no financial conflicts regarding the ISAR-REACT 5 study, sponsored by the German Heart Center and the German Center for Cardiovascular Research.
Simultaneous with her presentation of ISAR-REACT 5, the study results were published online (N Engl J Med. 2019 Sep 1. doi: 10.1056/NEJMoa1908973).
REPORTING FROM THE ESC CONGRESS 2019
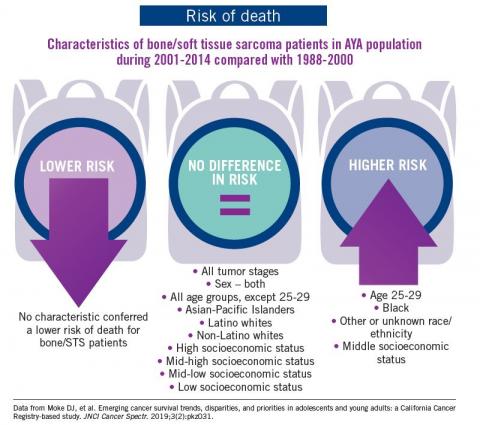
Adolescent and young adult (AYA) survival trends
The good news: AYA survival improvement was at least as large as in younger children and older adults comparing deaths in two time periods, 1988-2000 and 2001-2014, in a California Cancer Registry.
The bad news: There was no statistically significant difference in survival between time periods for patients with bone and soft tissue sarcoma.
The good news: AYA survival improvement was at least as large as in younger children and older adults comparing deaths in two time periods, 1988-2000 and 2001-2014, in a California Cancer Registry.
The bad news: There was no statistically significant difference in survival between time periods for patients with bone and soft tissue sarcoma.
The good news: AYA survival improvement was at least as large as in younger children and older adults comparing deaths in two time periods, 1988-2000 and 2001-2014, in a California Cancer Registry.
The bad news: There was no statistically significant difference in survival between time periods for patients with bone and soft tissue sarcoma.
Observation versus inpatient status
A dilemma for hospitalists and patients
A federal effort to reduce health care expenditures has left many older Medicare recipients experiencing the sticker shock of “observation status.” Patients who are not sick enough to meet inpatient admission criteria, however, still require hospitalization, and may be placed under Medicare observation care.
Seniors can get frustrated, confused, and anxious as their status can be changed while they are in the hospital, and they may receive large medical bills after they are discharged. The Centers for Medicare & Medicaid Services’ “3-day rule” mandates that Medicare will not pay for skilled nursing facility care unless the patient is admitted as an “inpatient” for at least 3 days. Observation days do not count towards this 3-day hospital stay.
There has been an increase in outpatient services over the years since 2006. The 2018 State of Hospital Medicine Report (SoHM) highlights the percentage of discharges based on hospitalists’ billed Current Procedural Terminology codes. Codes 99217 (observation discharge) and 99238-99239 (inpatient discharge) were used to calculate the percentages. 80.7% of adult medicine hospitalist discharges were coded using inpatient discharge codes, while 19.3% of patients were discharged with observation discharge codes.
In the 2016 SoHM report, the ratio was 76.0% inpatient and 21.1% observation codes and in the 2014 report we saw 80.3% inpatient and 16.1% observation discharges (see table 1). But in both of those surveys, same-day admission/discharge codes were also separately reported, which did not occur in 2018. That makes year-over-year comparison of the data challenging.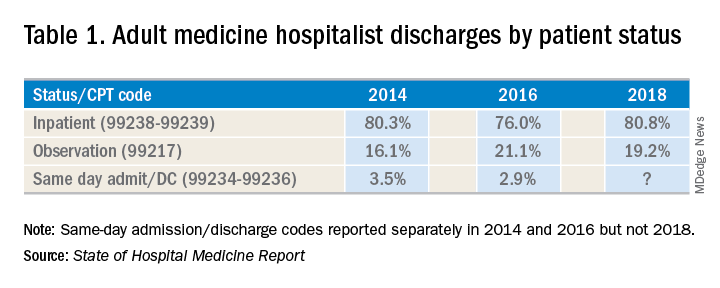
Interestingly, the 2017 CMS data on Evaluation and Management Codes by Specialty for the first time included separate data for hospitalists, based on hospitalists who credentialed with Medicare using the new C6 specialty code. Based on that data, when looking only at inpatient (99238-99239) and observation (99217) codes, 83% of the discharges were inpatient and 17% were observation.
Physicians feel the pressure of strained patient-physician relationships as a consequence of patients feeling the brunt of the financing gap related to observation status. Patients often feel they were not warned adequately about the financial ramifications of observation status. Even if Medicare beneficiaries have received the Medicare Outpatient Observation Notice, outlined by the Notice of Observation Treatment and Implication for Care Eligibility Act, they have no rights to appeal.
Currently Medicare beneficiaries admitted as inpatients only incur a Part A deductible; they are not liable for tests, procedures, and nursing care. On the other hand, in observation status all services are billed separately. For Medicare Part B services (which covers observation care) patients must pay 20% of services after the Part B deductible, which could result in a huge financial burden. Costs for skilled nursing facilities, when they are not covered by Medicare Part A, because of the 3-day rule, can easily go up to $20,000 or more. Medicare beneficiaries have no cap on costs for an observation stay. In some cases, hospitals have to apply a condition code 44 and retroactively change the stay to observation status.
I attended the 2019 Society of Hospital Medicine Annual Conference in Washington. Hospitalists from all parts of the country advocated on Capitol Hill against the “observation bill,” and “meet and greets” with congressional representatives increased their opposition to the bill. These efforts may work in favor of protecting patients from surprise medical bills. Hospital medicine physicians are on the front lines for providing health care in the hospital setting; they have demanded a fix to this legislative loophole which brings high out of pocket costs to our nation’s most vulnerable seniors. The observation status “2-midnight rule” utilized by CMS has increased financial barriers and decreased access to postacute care, affecting the provision of high-quality care for patients.
My hospital has a utilization review committee which reviews all cases to determine the appropriateness of an inpatient versus an observation designation. (An interesting question is whether the financial resources used to support this additional staff could be better assigned to provide high-quality care.) Distribution of these patients is determined on very specific criteria as outlined by Medicare. Observation is basically considered a billing method implemented by payers to decrease dollars paid to acute care hospitals for inpatient care. It pertains to admission status, not to the level of care provided in the hospital. Unfortunately, it is felt that no two payers define observation the same way. A few examples of common observation diagnoses are chest pain, abdominal pain, syncope, and migraine headache; in other words, patients with diagnoses where it is suspected that a less than 24-hour stay in the hospital could be sufficient.
Observation care is increasing and can sometimes contribute to work flow impediments and frustrations in hospitalists; thus, hospitalists are demanding reform. It has been proposed that observation could be eliminated altogether by creating a payment blend of inpatient/outpatient rates. Another option could be to assign lower Diagnosis Related Group coding to lower acuity disease processes, instead of separate observation reimbursement.
Patients and doctors lament that “Once you are in the hospital, you are admitted!” I don’t know the right answer that would solve the observation versus inpatient dilemma, but it is intriguing to consider changes in policy that might focus on the complete elimination of observation status.
Dr. Puri is a hospitalist at Lahey Hospital and Medical Center in Burlington, Mass.
A dilemma for hospitalists and patients
A dilemma for hospitalists and patients
A federal effort to reduce health care expenditures has left many older Medicare recipients experiencing the sticker shock of “observation status.” Patients who are not sick enough to meet inpatient admission criteria, however, still require hospitalization, and may be placed under Medicare observation care.
Seniors can get frustrated, confused, and anxious as their status can be changed while they are in the hospital, and they may receive large medical bills after they are discharged. The Centers for Medicare & Medicaid Services’ “3-day rule” mandates that Medicare will not pay for skilled nursing facility care unless the patient is admitted as an “inpatient” for at least 3 days. Observation days do not count towards this 3-day hospital stay.
There has been an increase in outpatient services over the years since 2006. The 2018 State of Hospital Medicine Report (SoHM) highlights the percentage of discharges based on hospitalists’ billed Current Procedural Terminology codes. Codes 99217 (observation discharge) and 99238-99239 (inpatient discharge) were used to calculate the percentages. 80.7% of adult medicine hospitalist discharges were coded using inpatient discharge codes, while 19.3% of patients were discharged with observation discharge codes.
In the 2016 SoHM report, the ratio was 76.0% inpatient and 21.1% observation codes and in the 2014 report we saw 80.3% inpatient and 16.1% observation discharges (see table 1). But in both of those surveys, same-day admission/discharge codes were also separately reported, which did not occur in 2018. That makes year-over-year comparison of the data challenging.
Interestingly, the 2017 CMS data on Evaluation and Management Codes by Specialty for the first time included separate data for hospitalists, based on hospitalists who credentialed with Medicare using the new C6 specialty code. Based on that data, when looking only at inpatient (99238-99239) and observation (99217) codes, 83% of the discharges were inpatient and 17% were observation.
Physicians feel the pressure of strained patient-physician relationships as a consequence of patients feeling the brunt of the financing gap related to observation status. Patients often feel they were not warned adequately about the financial ramifications of observation status. Even if Medicare beneficiaries have received the Medicare Outpatient Observation Notice, outlined by the Notice of Observation Treatment and Implication for Care Eligibility Act, they have no rights to appeal.
Currently Medicare beneficiaries admitted as inpatients only incur a Part A deductible; they are not liable for tests, procedures, and nursing care. On the other hand, in observation status all services are billed separately. For Medicare Part B services (which covers observation care) patients must pay 20% of services after the Part B deductible, which could result in a huge financial burden. Costs for skilled nursing facilities, when they are not covered by Medicare Part A, because of the 3-day rule, can easily go up to $20,000 or more. Medicare beneficiaries have no cap on costs for an observation stay. In some cases, hospitals have to apply a condition code 44 and retroactively change the stay to observation status.
I attended the 2019 Society of Hospital Medicine Annual Conference in Washington. Hospitalists from all parts of the country advocated on Capitol Hill against the “observation bill,” and “meet and greets” with congressional representatives increased their opposition to the bill. These efforts may work in favor of protecting patients from surprise medical bills. Hospital medicine physicians are on the front lines for providing health care in the hospital setting; they have demanded a fix to this legislative loophole which brings high out of pocket costs to our nation’s most vulnerable seniors. The observation status “2-midnight rule” utilized by CMS has increased financial barriers and decreased access to postacute care, affecting the provision of high-quality care for patients.
My hospital has a utilization review committee which reviews all cases to determine the appropriateness of an inpatient versus an observation designation. (An interesting question is whether the financial resources used to support this additional staff could be better assigned to provide high-quality care.) Distribution of these patients is determined on very specific criteria as outlined by Medicare. Observation is basically considered a billing method implemented by payers to decrease dollars paid to acute care hospitals for inpatient care. It pertains to admission status, not to the level of care provided in the hospital. Unfortunately, it is felt that no two payers define observation the same way. A few examples of common observation diagnoses are chest pain, abdominal pain, syncope, and migraine headache; in other words, patients with diagnoses where it is suspected that a less than 24-hour stay in the hospital could be sufficient.
Observation care is increasing and can sometimes contribute to work flow impediments and frustrations in hospitalists; thus, hospitalists are demanding reform. It has been proposed that observation could be eliminated altogether by creating a payment blend of inpatient/outpatient rates. Another option could be to assign lower Diagnosis Related Group coding to lower acuity disease processes, instead of separate observation reimbursement.
Patients and doctors lament that “Once you are in the hospital, you are admitted!” I don’t know the right answer that would solve the observation versus inpatient dilemma, but it is intriguing to consider changes in policy that might focus on the complete elimination of observation status.
Dr. Puri is a hospitalist at Lahey Hospital and Medical Center in Burlington, Mass.
A federal effort to reduce health care expenditures has left many older Medicare recipients experiencing the sticker shock of “observation status.” Patients who are not sick enough to meet inpatient admission criteria, however, still require hospitalization, and may be placed under Medicare observation care.
Seniors can get frustrated, confused, and anxious as their status can be changed while they are in the hospital, and they may receive large medical bills after they are discharged. The Centers for Medicare & Medicaid Services’ “3-day rule” mandates that Medicare will not pay for skilled nursing facility care unless the patient is admitted as an “inpatient” for at least 3 days. Observation days do not count towards this 3-day hospital stay.
There has been an increase in outpatient services over the years since 2006. The 2018 State of Hospital Medicine Report (SoHM) highlights the percentage of discharges based on hospitalists’ billed Current Procedural Terminology codes. Codes 99217 (observation discharge) and 99238-99239 (inpatient discharge) were used to calculate the percentages. 80.7% of adult medicine hospitalist discharges were coded using inpatient discharge codes, while 19.3% of patients were discharged with observation discharge codes.
In the 2016 SoHM report, the ratio was 76.0% inpatient and 21.1% observation codes and in the 2014 report we saw 80.3% inpatient and 16.1% observation discharges (see table 1). But in both of those surveys, same-day admission/discharge codes were also separately reported, which did not occur in 2018. That makes year-over-year comparison of the data challenging.
Interestingly, the 2017 CMS data on Evaluation and Management Codes by Specialty for the first time included separate data for hospitalists, based on hospitalists who credentialed with Medicare using the new C6 specialty code. Based on that data, when looking only at inpatient (99238-99239) and observation (99217) codes, 83% of the discharges were inpatient and 17% were observation.
Physicians feel the pressure of strained patient-physician relationships as a consequence of patients feeling the brunt of the financing gap related to observation status. Patients often feel they were not warned adequately about the financial ramifications of observation status. Even if Medicare beneficiaries have received the Medicare Outpatient Observation Notice, outlined by the Notice of Observation Treatment and Implication for Care Eligibility Act, they have no rights to appeal.
Currently Medicare beneficiaries admitted as inpatients only incur a Part A deductible; they are not liable for tests, procedures, and nursing care. On the other hand, in observation status all services are billed separately. For Medicare Part B services (which covers observation care) patients must pay 20% of services after the Part B deductible, which could result in a huge financial burden. Costs for skilled nursing facilities, when they are not covered by Medicare Part A, because of the 3-day rule, can easily go up to $20,000 or more. Medicare beneficiaries have no cap on costs for an observation stay. In some cases, hospitals have to apply a condition code 44 and retroactively change the stay to observation status.
I attended the 2019 Society of Hospital Medicine Annual Conference in Washington. Hospitalists from all parts of the country advocated on Capitol Hill against the “observation bill,” and “meet and greets” with congressional representatives increased their opposition to the bill. These efforts may work in favor of protecting patients from surprise medical bills. Hospital medicine physicians are on the front lines for providing health care in the hospital setting; they have demanded a fix to this legislative loophole which brings high out of pocket costs to our nation’s most vulnerable seniors. The observation status “2-midnight rule” utilized by CMS has increased financial barriers and decreased access to postacute care, affecting the provision of high-quality care for patients.
My hospital has a utilization review committee which reviews all cases to determine the appropriateness of an inpatient versus an observation designation. (An interesting question is whether the financial resources used to support this additional staff could be better assigned to provide high-quality care.) Distribution of these patients is determined on very specific criteria as outlined by Medicare. Observation is basically considered a billing method implemented by payers to decrease dollars paid to acute care hospitals for inpatient care. It pertains to admission status, not to the level of care provided in the hospital. Unfortunately, it is felt that no two payers define observation the same way. A few examples of common observation diagnoses are chest pain, abdominal pain, syncope, and migraine headache; in other words, patients with diagnoses where it is suspected that a less than 24-hour stay in the hospital could be sufficient.
Observation care is increasing and can sometimes contribute to work flow impediments and frustrations in hospitalists; thus, hospitalists are demanding reform. It has been proposed that observation could be eliminated altogether by creating a payment blend of inpatient/outpatient rates. Another option could be to assign lower Diagnosis Related Group coding to lower acuity disease processes, instead of separate observation reimbursement.
Patients and doctors lament that “Once you are in the hospital, you are admitted!” I don’t know the right answer that would solve the observation versus inpatient dilemma, but it is intriguing to consider changes in policy that might focus on the complete elimination of observation status.
Dr. Puri is a hospitalist at Lahey Hospital and Medical Center in Burlington, Mass.
My patient tells me that they are transgender – now what?
I am privileged to work in a university hospital system where I have access to colleagues with expertise in LGBT health; however, medical providers in the community may not enjoy such resources. Many transgender and gender-diverse (TGD) youth now are seeking help from their community primary care providers to affirm their gender identity, but many community primary care providers do not have the luxury of referring these patients to an expert in gender-affirming care when their TGD patients express the desire to affirm their gender through medical and surgical means. This is even more difficult if the nearest referral center is hundreds of miles away. Nevertheless,
If a TGD youth discloses their gender identity to you, it is critical that you make the patient feel safe and supported. Showing support is important in maintaining rapport between you and the patient. Furthermore, you may be one of the very few adults in the child’s life whom they can trust.
First of all, thank them. For many TGD patients, disclosing their gender identity to a health care provider can be a herculean task. They may have spent many hours trying to find the right words to say to disclose an important aspect of themselves to you. They also probably spent a fair amount of time worrying about whether or not you would react positively to this disclosure. This fear is reasonable. About one-fifth of transgender people have reported being kicked out of a medical practice because of their disclosure of their gender identity.1
Secondly, assure the TGD patient that your treatment would be no different from the care provided for other patients. Discrimination from a health care provider has frequently been reported by TGD patients1 and is expected from this population.2 By emphasizing this, you have signaled to them that you are committed to treating them with dignity and respect. Furthermore, signal your commitment to this treatment by making the clinic a safe and welcoming place for LGBT youth. Several resources exist that can help with this. The American Medical Association provides a good example on how to draft a nondiscrimination statement that can be posted in waiting areas;3 the Fenway Institute has a good example of an intake form that is LGBT friendly.4
In addition, a good way to help affirm their gender identity is to tell them that being transgender or gender-diverse is normal and healthy. Many times, TGD youth will hear narratives that gender diversity is pathological or aberrant; however, hearing that they are healthy and normal, especially from a health care provider, can make a powerful impact on feeling supported and affirmed.
Furthermore, inform your TGD youth of their right to confidentiality. Many TGD youth may not be out to their parents, and you may be the first person to whom they disclosed their gender identity. This is especially helpful if you describe their right to and the limits of confidentiality (e.g., suicidality) at the beginning of the visit. Assurance of confidentiality is a vital reason adolescents and young adults seek health care from a medical provider,5 and the same can be said of TGD youth; however, keep in mind that if they do desire to transition using cross-sex hormones or surgery, parental permission is required.
If they are not out to their parents and they are planning to come out to their parents, offer to be there when they do. Having someone to support the child – someone who is a medical provider – can add to the sense of legitimacy that what the child is experiencing is normal and healthy. Providing guidance on how parents can support their TGD child is essential for successful affirmation, and some suggestions can be found in an LGBT Youth Consult column I wrote titled, “Guidance for parents of LGBT youth.”
If you practice in a location where the nearest expert in gender-affirming care can be hundreds of miles away, educate yourself on gender-affirming care. Several guidelines are available. The World Professional Society for Transgender Standards of Care (SOC) focuses on the mental health aspects of gender-affirming care. The SOC recommends, but no longer requires, letters from a mental health therapist to start gender-affirming medical treatments and does allow for a discussion between you and the patient on the risks, benefits, alternatives, unknowns, limitations of treatment, and risks of not treating (i.e., obtaining informed consent) as the threshold for hormone therapy.6 This approach, known as the “informed consent model,” can be helpful in expanding health care access for TGD youth. Furthermore, there’s the Endocrine Society Clinical Practice Guidelines7 and the University of California, San Francisco, Guidelines,8 which focus on the medical aspects of gender-affirming care, such as when to start pubertal blockers and dosing for cross-sex hormones. Finally, there are resources that allow providers to consult an expert remotely for more complicated cases. Transline is a transgender medical consultation service staffed by medical providers with expertise in gender-affirming care. Providers can learn more about this valuable service on the website: http://project-health.org/transline/.
Working in a major medical center is not necessary in providing gender-affirming care to TGD youth. Being respectful, supportive, and having the willingness to learn are the minimal requirements. Resources are available to help guide you on the more technical aspects of gender-affirming care. Maintaining a supportive environment and using these resources will help you expand health care access for this population.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
2. Psychol Bull. 2003 Sep;129(5):674-97.
3. “Creating an LGBTQ-friendly practice,” American Medical Association.
4. Fenway Health Client Registration Form.
5. JAMA. 1993 Mar 17;269(11):1404-7.
6. Int J Transgenderism 2012;13:165-232.
7. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
8. “Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People,” 2nd edition (San Francisco, CA: University of California, San Francisco, June 17, 2016).
I am privileged to work in a university hospital system where I have access to colleagues with expertise in LGBT health; however, medical providers in the community may not enjoy such resources. Many transgender and gender-diverse (TGD) youth now are seeking help from their community primary care providers to affirm their gender identity, but many community primary care providers do not have the luxury of referring these patients to an expert in gender-affirming care when their TGD patients express the desire to affirm their gender through medical and surgical means. This is even more difficult if the nearest referral center is hundreds of miles away. Nevertheless,
If a TGD youth discloses their gender identity to you, it is critical that you make the patient feel safe and supported. Showing support is important in maintaining rapport between you and the patient. Furthermore, you may be one of the very few adults in the child’s life whom they can trust.
First of all, thank them. For many TGD patients, disclosing their gender identity to a health care provider can be a herculean task. They may have spent many hours trying to find the right words to say to disclose an important aspect of themselves to you. They also probably spent a fair amount of time worrying about whether or not you would react positively to this disclosure. This fear is reasonable. About one-fifth of transgender people have reported being kicked out of a medical practice because of their disclosure of their gender identity.1
Secondly, assure the TGD patient that your treatment would be no different from the care provided for other patients. Discrimination from a health care provider has frequently been reported by TGD patients1 and is expected from this population.2 By emphasizing this, you have signaled to them that you are committed to treating them with dignity and respect. Furthermore, signal your commitment to this treatment by making the clinic a safe and welcoming place for LGBT youth. Several resources exist that can help with this. The American Medical Association provides a good example on how to draft a nondiscrimination statement that can be posted in waiting areas;3 the Fenway Institute has a good example of an intake form that is LGBT friendly.4
In addition, a good way to help affirm their gender identity is to tell them that being transgender or gender-diverse is normal and healthy. Many times, TGD youth will hear narratives that gender diversity is pathological or aberrant; however, hearing that they are healthy and normal, especially from a health care provider, can make a powerful impact on feeling supported and affirmed.
Furthermore, inform your TGD youth of their right to confidentiality. Many TGD youth may not be out to their parents, and you may be the first person to whom they disclosed their gender identity. This is especially helpful if you describe their right to and the limits of confidentiality (e.g., suicidality) at the beginning of the visit. Assurance of confidentiality is a vital reason adolescents and young adults seek health care from a medical provider,5 and the same can be said of TGD youth; however, keep in mind that if they do desire to transition using cross-sex hormones or surgery, parental permission is required.
If they are not out to their parents and they are planning to come out to their parents, offer to be there when they do. Having someone to support the child – someone who is a medical provider – can add to the sense of legitimacy that what the child is experiencing is normal and healthy. Providing guidance on how parents can support their TGD child is essential for successful affirmation, and some suggestions can be found in an LGBT Youth Consult column I wrote titled, “Guidance for parents of LGBT youth.”
If you practice in a location where the nearest expert in gender-affirming care can be hundreds of miles away, educate yourself on gender-affirming care. Several guidelines are available. The World Professional Society for Transgender Standards of Care (SOC) focuses on the mental health aspects of gender-affirming care. The SOC recommends, but no longer requires, letters from a mental health therapist to start gender-affirming medical treatments and does allow for a discussion between you and the patient on the risks, benefits, alternatives, unknowns, limitations of treatment, and risks of not treating (i.e., obtaining informed consent) as the threshold for hormone therapy.6 This approach, known as the “informed consent model,” can be helpful in expanding health care access for TGD youth. Furthermore, there’s the Endocrine Society Clinical Practice Guidelines7 and the University of California, San Francisco, Guidelines,8 which focus on the medical aspects of gender-affirming care, such as when to start pubertal blockers and dosing for cross-sex hormones. Finally, there are resources that allow providers to consult an expert remotely for more complicated cases. Transline is a transgender medical consultation service staffed by medical providers with expertise in gender-affirming care. Providers can learn more about this valuable service on the website: http://project-health.org/transline/.
Working in a major medical center is not necessary in providing gender-affirming care to TGD youth. Being respectful, supportive, and having the willingness to learn are the minimal requirements. Resources are available to help guide you on the more technical aspects of gender-affirming care. Maintaining a supportive environment and using these resources will help you expand health care access for this population.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
2. Psychol Bull. 2003 Sep;129(5):674-97.
3. “Creating an LGBTQ-friendly practice,” American Medical Association.
4. Fenway Health Client Registration Form.
5. JAMA. 1993 Mar 17;269(11):1404-7.
6. Int J Transgenderism 2012;13:165-232.
7. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
8. “Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People,” 2nd edition (San Francisco, CA: University of California, San Francisco, June 17, 2016).
I am privileged to work in a university hospital system where I have access to colleagues with expertise in LGBT health; however, medical providers in the community may not enjoy such resources. Many transgender and gender-diverse (TGD) youth now are seeking help from their community primary care providers to affirm their gender identity, but many community primary care providers do not have the luxury of referring these patients to an expert in gender-affirming care when their TGD patients express the desire to affirm their gender through medical and surgical means. This is even more difficult if the nearest referral center is hundreds of miles away. Nevertheless,
If a TGD youth discloses their gender identity to you, it is critical that you make the patient feel safe and supported. Showing support is important in maintaining rapport between you and the patient. Furthermore, you may be one of the very few adults in the child’s life whom they can trust.
First of all, thank them. For many TGD patients, disclosing their gender identity to a health care provider can be a herculean task. They may have spent many hours trying to find the right words to say to disclose an important aspect of themselves to you. They also probably spent a fair amount of time worrying about whether or not you would react positively to this disclosure. This fear is reasonable. About one-fifth of transgender people have reported being kicked out of a medical practice because of their disclosure of their gender identity.1
Secondly, assure the TGD patient that your treatment would be no different from the care provided for other patients. Discrimination from a health care provider has frequently been reported by TGD patients1 and is expected from this population.2 By emphasizing this, you have signaled to them that you are committed to treating them with dignity and respect. Furthermore, signal your commitment to this treatment by making the clinic a safe and welcoming place for LGBT youth. Several resources exist that can help with this. The American Medical Association provides a good example on how to draft a nondiscrimination statement that can be posted in waiting areas;3 the Fenway Institute has a good example of an intake form that is LGBT friendly.4
In addition, a good way to help affirm their gender identity is to tell them that being transgender or gender-diverse is normal and healthy. Many times, TGD youth will hear narratives that gender diversity is pathological or aberrant; however, hearing that they are healthy and normal, especially from a health care provider, can make a powerful impact on feeling supported and affirmed.
Furthermore, inform your TGD youth of their right to confidentiality. Many TGD youth may not be out to their parents, and you may be the first person to whom they disclosed their gender identity. This is especially helpful if you describe their right to and the limits of confidentiality (e.g., suicidality) at the beginning of the visit. Assurance of confidentiality is a vital reason adolescents and young adults seek health care from a medical provider,5 and the same can be said of TGD youth; however, keep in mind that if they do desire to transition using cross-sex hormones or surgery, parental permission is required.
If they are not out to their parents and they are planning to come out to their parents, offer to be there when they do. Having someone to support the child – someone who is a medical provider – can add to the sense of legitimacy that what the child is experiencing is normal and healthy. Providing guidance on how parents can support their TGD child is essential for successful affirmation, and some suggestions can be found in an LGBT Youth Consult column I wrote titled, “Guidance for parents of LGBT youth.”
If you practice in a location where the nearest expert in gender-affirming care can be hundreds of miles away, educate yourself on gender-affirming care. Several guidelines are available. The World Professional Society for Transgender Standards of Care (SOC) focuses on the mental health aspects of gender-affirming care. The SOC recommends, but no longer requires, letters from a mental health therapist to start gender-affirming medical treatments and does allow for a discussion between you and the patient on the risks, benefits, alternatives, unknowns, limitations of treatment, and risks of not treating (i.e., obtaining informed consent) as the threshold for hormone therapy.6 This approach, known as the “informed consent model,” can be helpful in expanding health care access for TGD youth. Furthermore, there’s the Endocrine Society Clinical Practice Guidelines7 and the University of California, San Francisco, Guidelines,8 which focus on the medical aspects of gender-affirming care, such as when to start pubertal blockers and dosing for cross-sex hormones. Finally, there are resources that allow providers to consult an expert remotely for more complicated cases. Transline is a transgender medical consultation service staffed by medical providers with expertise in gender-affirming care. Providers can learn more about this valuable service on the website: http://project-health.org/transline/.
Working in a major medical center is not necessary in providing gender-affirming care to TGD youth. Being respectful, supportive, and having the willingness to learn are the minimal requirements. Resources are available to help guide you on the more technical aspects of gender-affirming care. Maintaining a supportive environment and using these resources will help you expand health care access for this population.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
2. Psychol Bull. 2003 Sep;129(5):674-97.
3. “Creating an LGBTQ-friendly practice,” American Medical Association.
4. Fenway Health Client Registration Form.
5. JAMA. 1993 Mar 17;269(11):1404-7.
6. Int J Transgenderism 2012;13:165-232.
7. J Clin Endocrinol Metab. 2017 Nov 1;102(11):3869-903.
8. “Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People,” 2nd edition (San Francisco, CA: University of California, San Francisco, June 17, 2016).
Wildfire smoke has acute cardiorespiratory impact, but long-term effects still under study
The 2019 wildfire season is underway in many locales across the United States, exposing millions of individuals to smoky conditions that will have health consequences ranging from stinging eyes to scratchy throats to a trip to the ED for asthma or chronic obstructive pulmonary disease (COPD) exacerbation. Questions about long-term health impacts are on the minds of many, including physicians and their patients who live with cardiorespiratory conditions.

John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, suggested that the best available published literature points to “pretty strong evidence for acute effects of wildfire smoke on respiratory health, meaning people with preexisting asthma and COPD are at risk for exacerbations, and probably for respiratory tract infections as well.” He said, “It’s a little less clear, but there’s good biological plausibility for increased risk of respiratory tract infections because when your alveolar macrophages are overloaded with carbon particles that are toxic to those cells, they don’t function as well as a first line of defense against bacterial infection, for example.”
The new normal of wildfires
Warmer, drier summers in recent years in the western United States and many other regions, attributed by climate experts to global climate change, have produced catastrophic wildfires (PNAS;2016 Oct 18;113[42]11770-5; Science 2006 Aug 18;313:940-3). The Camp Fire in Northern California broke out in November 2018, took the lives of at least 85 people, and cost more than $16 billion in damage. Smoke from that blaze reached hazardous levels in San Francisco, Sacramento, Fresno, and many other smaller towns. Other forest fires in that year caused heavy smoke conditions in Portland, Seattle, Vancouver, and Anchorage. Such events are expected to be repeated often in the coming years (Int J Environ Res Public Health. 2019 Jul 6;16[13]).

Wildfire smoke can contain a wide range of substances, chemicals, and gases with known and unknown cardiorespiratory implications. “Smoke is composed primarily of carbon dioxide, water vapor, carbon monoxide, particulate matter, hydrocarbons and other organic chemicals, nitrogen oxides, trace minerals and several thousand other compounds,” according to the U.S. Environmental Protection Agency (Wildfire smoke: A guide for public health officials 2019. Washington, D.C.: EPA, 2019). The EPA report noted, “Particles with diameters less than 10 mcm (particulate matter, or PM10) can be inhaled into the lungs and affect the lungs, heart, and blood vessels. The smallest particles, those less than 2.5 mcm in diameter (PM2.5), are the greatest risk to public health because they can reach deep into the lungs and may even make it into the bloodstream.”
Research on health impact
In early June of 2008, Wayne Cascio, MD, awoke in his Greenville, N.C., home to the stench of smoke emanating from a large peat fire burning some 65 miles away. By the time he reached the parking lot at East Carolina University in Greenville to begin his workday as chief of cardiology, the haze of smoke had thickened to the point where he could only see a few feet in front of him.
Over the next several weeks, the fire scorched 41,000 acres and produced haze and air pollution that far exceeded National Ambient Air Quality Standards for particulate matter and blanketed rural communities in the state’s eastern region. The price tag for management of the blaze reached $20 million. Because of his interest in the health effects of wildfire smoke and because of his relationship with investigators at the EPA, Dr. Cascio initiated an epidemiology study to investigate the effects of exposure on cardiorespiratory outcomes in the population affected by the fire (Environ Health Perspect. 2011 Oct;119[10]:1415-20).
By combining satellite data with syndromic surveillance drawn from hospital records in 41 counties contained in the North Carolina Disease Event Tracking and Epidemiologic Collection Tool, he and his colleagues found that exposure to the peat wildfire smoke led to increases in the cumulative risk ratio for asthma (relative risk, 1.65), chronic obstructive pulmonary disease (RR, 1.73), and pneumonia and acute bronchitis (RR, 1.59). ED visits related to cardiopulmonary symptoms and heart failure also were significantly increased (RR, 1.23 and 1.37, respectively). “That was really the first study to strongly identify a cardiac endpoint related to wildfire smoke exposure,” said Dr. Cascio, who now directs the EPA’s National Health and Environmental Effects Research Laboratory. “It really pointed out how little we knew about the health effects of wildfire up until that time.”
Those early findings have been replicated in subsequent research about the acute health effects of exposure to wildfire smoke, which contains PM2.5 and other toxic substances from structures, electronic devices, and automobiles destroyed in the path of flames, including heavy metals and asbestos. Most of the work has focused on smoke-related cardiovascular and respiratory ED visits and hospitalizations.
A study of the 2008 California wildfire impact on ED visits accounted for ozone levels in addition to PM2.5 in the smoke. During the active fire periods, PM2.5 was significantly associated with exacerbations of asthma and COPD and these effects remained after controlling for ozone levels. PM2.5 inhalation during the wildfires was associated with increased risk of an ED visit for asthma (RR, 1.112; 95% confidence interval, 1.087-1.138) for a 10 mcg/m3 increase in PM2.5 and COPD (RR, 1.05; 95% CI, 1.019-1.0825), as well as for combined respiratory visits (RR, 1.035; 95% CI, 1.023-1.046) (Environ Int. 2109 Aug;129:291-8).
Researchers who evaluated the health impacts of wildfires in California during the 2015 fire season found an increase in all-cause cardiovascular and respiratory ED visits, especially among those aged 65 years and older during smoke days. The population-based study included 1,196,233 ED visits during May 1–Sept. 30 that year. PM2.5 concentrations were categorized as light, medium, or dense. Relative risk rose with the amount of smoke in the air. Rates of all-cause cardiovascular ED visits were elevated across levels of smoke density, with the greatest increase on dense smoke days and among those aged 65 years or older (RR,1.15; 95% CI, 1.09-1.22). All-cause cerebrovascular visits were associated with dense smoke days, especially among those aged 65 years and older (RR, 1.22; 95% CI, 1.00-1.49). Respiratory conditions also were increased on dense smoke days (RR, 1.18; 95% CI, 1.08-1.28) (J Am Heart Assoc. 2018 Apr 11;7:e007492. doi: 10.1161/JAHA.117.007492).
Long-term effects unknown
When it comes to the long-term effects of wildfire smoke on human health outcomes, much less is known. In a recent literature review, Colleen E. Reid, PhD, and Melissa May Maestas, PhD, found only one study that investigated long-term respiratory health impacts of wildfire smoke, and only a few studies that have estimated future health impacts of wildfires under likely climate change scenarios (Curr Opin Pulm Med. 2019 Mar;25:179-87).
“We know that there are immediate respiratory health effects from wildfire smoke,” said Dr. Reid of the department of geography at the University of Colorado Boulder. “What’s less known is everything else. That’s challenging, because people want to know about the long-term health effects.”
Evidence from the scientific literature suggests that exposure to air pollution adversely affects cardiovascular health, but whether exposure to wildfire smoke confers a similar risk is less clear. “Until just a few years ago we haven’t been able to study wildfire exposure measures on a large scale,” said EPA scientist Ana G. Rappold, PhD, a statistician there in the environmental public health division of the National Health and Environmental Effects Research Laboratory. “It’s also hard to predict wildfires, so it’s hard to plan for an epidemiologic study if you don’t know where they’re going to occur.”
Dr. Rappold and colleagues examined cardiopulmonary hospitalizations among adults aged 65 years and older in 692 U.S. counties within 200 km of 123 large wildfires during 2008-2010 (Environ Health Perspect. 2019;127[3]:37006. doi: 10.1289/EHP3860). They observed that an increased risk of PM2.5-related cardiopulmonary hospitalizations was similar on smoke and nonsmoke days across multiple lags and exposure metrics, while risk for asthma-related hospitalizations was higher during smoke days. “One hypothesis is that this was an older study population, so naturally if you’re inhaling smoke, the first organ that’s impacted in an older population is the lungs,” Dr. Rappold said. “If you go to the hospital for asthma, wheezing, or bronchitis, you are taken out of the risk pool for cardiovascular and other diseases. That could explain why in other studies we don’t see a clear cardiovascular signal as we have for air pollution studies in general. Another aspect to this study is, the exposure metric was PM2.5, but smoke contains many other components, particularly gases, which are respiratory irritants. It could be that this triggers a higher risk for respiratory [effects] than regular episodes of high PM2.5 exposure, just because of the additional gases that people are exposed to.”
Another complicating factor is the paucity of data about solutions to long-term exposure to wildfire smoke. “If you’re impacted by high-exposure levels for 60 days, that is not something we have experienced before,” Dr. Rappold noted. “What are the solutions for that community? What works? Can we show that by implementing community-level resilience plans with HEPA [high-efficiency particulate air] filters or other interventions, do the overall outcomes improve? Doctors are the first ones to talk with their patients about their symptoms and about how to take care of their conditions. They can clearly make a difference in emphasizing reducing exposures in a way that fits their patients individually, either reducing the amount of time spent outside, the duration of exposure, and the level of exposure. Maybe change activities based on the intensity of exposure. Don’t go for a run outside when it’s smoky, because your ventilation rate is higher and you will breathe in more smoke. Become aware of those things.”
Advising vulnerable patients
While research in this field advances, the unforgiving wildfire season looms, assuring more destruction of property and threats to cardiorespiratory health. “There are a lot of questions that research will have an opportunity to address as we go forward, including the utility and the benefit of N95 masks, the utility of HEPA filters used in the house, and even with HVAC [heating, ventilation, and air conditioning] systems,” Dr. Cascio said. “Can we really clean up the indoor air well enough to protect us from wildfire smoke?”
The way he sees it, the time is ripe for clinicians and officials in public and private practice settings to refine how they distribute information to people living in areas affected by wildfire smoke. “We can’t force people do anything, but at least if they’re informed, then they understand they can make an informed decision about how they might want to affect what they do that would limit their exposure,” he said. “As a patient, my health care system sends text and email messages to me. So, why couldn’t the hospital send out a text message or an email to all of the patients with COPD, coronary disease, and heart failure when an area is impacted by smoke, saying, ‘Check your air quality and take action if air quality is poor?’ Physicians don’t have time to do this kind of education in the office for all of their patients. I know that from experience. But if one were to only focus on those at highest risk, and encourage them to follow our guidelines, which might include doing HEPA filter treatment in the home, we probably would reduce the number of clinical events in a cost-effective way.”
The 2019 wildfire season is underway in many locales across the United States, exposing millions of individuals to smoky conditions that will have health consequences ranging from stinging eyes to scratchy throats to a trip to the ED for asthma or chronic obstructive pulmonary disease (COPD) exacerbation. Questions about long-term health impacts are on the minds of many, including physicians and their patients who live with cardiorespiratory conditions.

John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, suggested that the best available published literature points to “pretty strong evidence for acute effects of wildfire smoke on respiratory health, meaning people with preexisting asthma and COPD are at risk for exacerbations, and probably for respiratory tract infections as well.” He said, “It’s a little less clear, but there’s good biological plausibility for increased risk of respiratory tract infections because when your alveolar macrophages are overloaded with carbon particles that are toxic to those cells, they don’t function as well as a first line of defense against bacterial infection, for example.”
The new normal of wildfires
Warmer, drier summers in recent years in the western United States and many other regions, attributed by climate experts to global climate change, have produced catastrophic wildfires (PNAS;2016 Oct 18;113[42]11770-5; Science 2006 Aug 18;313:940-3). The Camp Fire in Northern California broke out in November 2018, took the lives of at least 85 people, and cost more than $16 billion in damage. Smoke from that blaze reached hazardous levels in San Francisco, Sacramento, Fresno, and many other smaller towns. Other forest fires in that year caused heavy smoke conditions in Portland, Seattle, Vancouver, and Anchorage. Such events are expected to be repeated often in the coming years (Int J Environ Res Public Health. 2019 Jul 6;16[13]).

Wildfire smoke can contain a wide range of substances, chemicals, and gases with known and unknown cardiorespiratory implications. “Smoke is composed primarily of carbon dioxide, water vapor, carbon monoxide, particulate matter, hydrocarbons and other organic chemicals, nitrogen oxides, trace minerals and several thousand other compounds,” according to the U.S. Environmental Protection Agency (Wildfire smoke: A guide for public health officials 2019. Washington, D.C.: EPA, 2019). The EPA report noted, “Particles with diameters less than 10 mcm (particulate matter, or PM10) can be inhaled into the lungs and affect the lungs, heart, and blood vessels. The smallest particles, those less than 2.5 mcm in diameter (PM2.5), are the greatest risk to public health because they can reach deep into the lungs and may even make it into the bloodstream.”
Research on health impact
In early June of 2008, Wayne Cascio, MD, awoke in his Greenville, N.C., home to the stench of smoke emanating from a large peat fire burning some 65 miles away. By the time he reached the parking lot at East Carolina University in Greenville to begin his workday as chief of cardiology, the haze of smoke had thickened to the point where he could only see a few feet in front of him.
Over the next several weeks, the fire scorched 41,000 acres and produced haze and air pollution that far exceeded National Ambient Air Quality Standards for particulate matter and blanketed rural communities in the state’s eastern region. The price tag for management of the blaze reached $20 million. Because of his interest in the health effects of wildfire smoke and because of his relationship with investigators at the EPA, Dr. Cascio initiated an epidemiology study to investigate the effects of exposure on cardiorespiratory outcomes in the population affected by the fire (Environ Health Perspect. 2011 Oct;119[10]:1415-20).
By combining satellite data with syndromic surveillance drawn from hospital records in 41 counties contained in the North Carolina Disease Event Tracking and Epidemiologic Collection Tool, he and his colleagues found that exposure to the peat wildfire smoke led to increases in the cumulative risk ratio for asthma (relative risk, 1.65), chronic obstructive pulmonary disease (RR, 1.73), and pneumonia and acute bronchitis (RR, 1.59). ED visits related to cardiopulmonary symptoms and heart failure also were significantly increased (RR, 1.23 and 1.37, respectively). “That was really the first study to strongly identify a cardiac endpoint related to wildfire smoke exposure,” said Dr. Cascio, who now directs the EPA’s National Health and Environmental Effects Research Laboratory. “It really pointed out how little we knew about the health effects of wildfire up until that time.”
Those early findings have been replicated in subsequent research about the acute health effects of exposure to wildfire smoke, which contains PM2.5 and other toxic substances from structures, electronic devices, and automobiles destroyed in the path of flames, including heavy metals and asbestos. Most of the work has focused on smoke-related cardiovascular and respiratory ED visits and hospitalizations.
A study of the 2008 California wildfire impact on ED visits accounted for ozone levels in addition to PM2.5 in the smoke. During the active fire periods, PM2.5 was significantly associated with exacerbations of asthma and COPD and these effects remained after controlling for ozone levels. PM2.5 inhalation during the wildfires was associated with increased risk of an ED visit for asthma (RR, 1.112; 95% confidence interval, 1.087-1.138) for a 10 mcg/m3 increase in PM2.5 and COPD (RR, 1.05; 95% CI, 1.019-1.0825), as well as for combined respiratory visits (RR, 1.035; 95% CI, 1.023-1.046) (Environ Int. 2109 Aug;129:291-8).
Researchers who evaluated the health impacts of wildfires in California during the 2015 fire season found an increase in all-cause cardiovascular and respiratory ED visits, especially among those aged 65 years and older during smoke days. The population-based study included 1,196,233 ED visits during May 1–Sept. 30 that year. PM2.5 concentrations were categorized as light, medium, or dense. Relative risk rose with the amount of smoke in the air. Rates of all-cause cardiovascular ED visits were elevated across levels of smoke density, with the greatest increase on dense smoke days and among those aged 65 years or older (RR,1.15; 95% CI, 1.09-1.22). All-cause cerebrovascular visits were associated with dense smoke days, especially among those aged 65 years and older (RR, 1.22; 95% CI, 1.00-1.49). Respiratory conditions also were increased on dense smoke days (RR, 1.18; 95% CI, 1.08-1.28) (J Am Heart Assoc. 2018 Apr 11;7:e007492. doi: 10.1161/JAHA.117.007492).
Long-term effects unknown
When it comes to the long-term effects of wildfire smoke on human health outcomes, much less is known. In a recent literature review, Colleen E. Reid, PhD, and Melissa May Maestas, PhD, found only one study that investigated long-term respiratory health impacts of wildfire smoke, and only a few studies that have estimated future health impacts of wildfires under likely climate change scenarios (Curr Opin Pulm Med. 2019 Mar;25:179-87).
“We know that there are immediate respiratory health effects from wildfire smoke,” said Dr. Reid of the department of geography at the University of Colorado Boulder. “What’s less known is everything else. That’s challenging, because people want to know about the long-term health effects.”
Evidence from the scientific literature suggests that exposure to air pollution adversely affects cardiovascular health, but whether exposure to wildfire smoke confers a similar risk is less clear. “Until just a few years ago we haven’t been able to study wildfire exposure measures on a large scale,” said EPA scientist Ana G. Rappold, PhD, a statistician there in the environmental public health division of the National Health and Environmental Effects Research Laboratory. “It’s also hard to predict wildfires, so it’s hard to plan for an epidemiologic study if you don’t know where they’re going to occur.”
Dr. Rappold and colleagues examined cardiopulmonary hospitalizations among adults aged 65 years and older in 692 U.S. counties within 200 km of 123 large wildfires during 2008-2010 (Environ Health Perspect. 2019;127[3]:37006. doi: 10.1289/EHP3860). They observed that an increased risk of PM2.5-related cardiopulmonary hospitalizations was similar on smoke and nonsmoke days across multiple lags and exposure metrics, while risk for asthma-related hospitalizations was higher during smoke days. “One hypothesis is that this was an older study population, so naturally if you’re inhaling smoke, the first organ that’s impacted in an older population is the lungs,” Dr. Rappold said. “If you go to the hospital for asthma, wheezing, or bronchitis, you are taken out of the risk pool for cardiovascular and other diseases. That could explain why in other studies we don’t see a clear cardiovascular signal as we have for air pollution studies in general. Another aspect to this study is, the exposure metric was PM2.5, but smoke contains many other components, particularly gases, which are respiratory irritants. It could be that this triggers a higher risk for respiratory [effects] than regular episodes of high PM2.5 exposure, just because of the additional gases that people are exposed to.”
Another complicating factor is the paucity of data about solutions to long-term exposure to wildfire smoke. “If you’re impacted by high-exposure levels for 60 days, that is not something we have experienced before,” Dr. Rappold noted. “What are the solutions for that community? What works? Can we show that by implementing community-level resilience plans with HEPA [high-efficiency particulate air] filters or other interventions, do the overall outcomes improve? Doctors are the first ones to talk with their patients about their symptoms and about how to take care of their conditions. They can clearly make a difference in emphasizing reducing exposures in a way that fits their patients individually, either reducing the amount of time spent outside, the duration of exposure, and the level of exposure. Maybe change activities based on the intensity of exposure. Don’t go for a run outside when it’s smoky, because your ventilation rate is higher and you will breathe in more smoke. Become aware of those things.”
Advising vulnerable patients
While research in this field advances, the unforgiving wildfire season looms, assuring more destruction of property and threats to cardiorespiratory health. “There are a lot of questions that research will have an opportunity to address as we go forward, including the utility and the benefit of N95 masks, the utility of HEPA filters used in the house, and even with HVAC [heating, ventilation, and air conditioning] systems,” Dr. Cascio said. “Can we really clean up the indoor air well enough to protect us from wildfire smoke?”
The way he sees it, the time is ripe for clinicians and officials in public and private practice settings to refine how they distribute information to people living in areas affected by wildfire smoke. “We can’t force people do anything, but at least if they’re informed, then they understand they can make an informed decision about how they might want to affect what they do that would limit their exposure,” he said. “As a patient, my health care system sends text and email messages to me. So, why couldn’t the hospital send out a text message or an email to all of the patients with COPD, coronary disease, and heart failure when an area is impacted by smoke, saying, ‘Check your air quality and take action if air quality is poor?’ Physicians don’t have time to do this kind of education in the office for all of their patients. I know that from experience. But if one were to only focus on those at highest risk, and encourage them to follow our guidelines, which might include doing HEPA filter treatment in the home, we probably would reduce the number of clinical events in a cost-effective way.”
The 2019 wildfire season is underway in many locales across the United States, exposing millions of individuals to smoky conditions that will have health consequences ranging from stinging eyes to scratchy throats to a trip to the ED for asthma or chronic obstructive pulmonary disease (COPD) exacerbation. Questions about long-term health impacts are on the minds of many, including physicians and their patients who live with cardiorespiratory conditions.

John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, suggested that the best available published literature points to “pretty strong evidence for acute effects of wildfire smoke on respiratory health, meaning people with preexisting asthma and COPD are at risk for exacerbations, and probably for respiratory tract infections as well.” He said, “It’s a little less clear, but there’s good biological plausibility for increased risk of respiratory tract infections because when your alveolar macrophages are overloaded with carbon particles that are toxic to those cells, they don’t function as well as a first line of defense against bacterial infection, for example.”
The new normal of wildfires
Warmer, drier summers in recent years in the western United States and many other regions, attributed by climate experts to global climate change, have produced catastrophic wildfires (PNAS;2016 Oct 18;113[42]11770-5; Science 2006 Aug 18;313:940-3). The Camp Fire in Northern California broke out in November 2018, took the lives of at least 85 people, and cost more than $16 billion in damage. Smoke from that blaze reached hazardous levels in San Francisco, Sacramento, Fresno, and many other smaller towns. Other forest fires in that year caused heavy smoke conditions in Portland, Seattle, Vancouver, and Anchorage. Such events are expected to be repeated often in the coming years (Int J Environ Res Public Health. 2019 Jul 6;16[13]).

Wildfire smoke can contain a wide range of substances, chemicals, and gases with known and unknown cardiorespiratory implications. “Smoke is composed primarily of carbon dioxide, water vapor, carbon monoxide, particulate matter, hydrocarbons and other organic chemicals, nitrogen oxides, trace minerals and several thousand other compounds,” according to the U.S. Environmental Protection Agency (Wildfire smoke: A guide for public health officials 2019. Washington, D.C.: EPA, 2019). The EPA report noted, “Particles with diameters less than 10 mcm (particulate matter, or PM10) can be inhaled into the lungs and affect the lungs, heart, and blood vessels. The smallest particles, those less than 2.5 mcm in diameter (PM2.5), are the greatest risk to public health because they can reach deep into the lungs and may even make it into the bloodstream.”
Research on health impact
In early June of 2008, Wayne Cascio, MD, awoke in his Greenville, N.C., home to the stench of smoke emanating from a large peat fire burning some 65 miles away. By the time he reached the parking lot at East Carolina University in Greenville to begin his workday as chief of cardiology, the haze of smoke had thickened to the point where he could only see a few feet in front of him.
Over the next several weeks, the fire scorched 41,000 acres and produced haze and air pollution that far exceeded National Ambient Air Quality Standards for particulate matter and blanketed rural communities in the state’s eastern region. The price tag for management of the blaze reached $20 million. Because of his interest in the health effects of wildfire smoke and because of his relationship with investigators at the EPA, Dr. Cascio initiated an epidemiology study to investigate the effects of exposure on cardiorespiratory outcomes in the population affected by the fire (Environ Health Perspect. 2011 Oct;119[10]:1415-20).
By combining satellite data with syndromic surveillance drawn from hospital records in 41 counties contained in the North Carolina Disease Event Tracking and Epidemiologic Collection Tool, he and his colleagues found that exposure to the peat wildfire smoke led to increases in the cumulative risk ratio for asthma (relative risk, 1.65), chronic obstructive pulmonary disease (RR, 1.73), and pneumonia and acute bronchitis (RR, 1.59). ED visits related to cardiopulmonary symptoms and heart failure also were significantly increased (RR, 1.23 and 1.37, respectively). “That was really the first study to strongly identify a cardiac endpoint related to wildfire smoke exposure,” said Dr. Cascio, who now directs the EPA’s National Health and Environmental Effects Research Laboratory. “It really pointed out how little we knew about the health effects of wildfire up until that time.”
Those early findings have been replicated in subsequent research about the acute health effects of exposure to wildfire smoke, which contains PM2.5 and other toxic substances from structures, electronic devices, and automobiles destroyed in the path of flames, including heavy metals and asbestos. Most of the work has focused on smoke-related cardiovascular and respiratory ED visits and hospitalizations.
A study of the 2008 California wildfire impact on ED visits accounted for ozone levels in addition to PM2.5 in the smoke. During the active fire periods, PM2.5 was significantly associated with exacerbations of asthma and COPD and these effects remained after controlling for ozone levels. PM2.5 inhalation during the wildfires was associated with increased risk of an ED visit for asthma (RR, 1.112; 95% confidence interval, 1.087-1.138) for a 10 mcg/m3 increase in PM2.5 and COPD (RR, 1.05; 95% CI, 1.019-1.0825), as well as for combined respiratory visits (RR, 1.035; 95% CI, 1.023-1.046) (Environ Int. 2109 Aug;129:291-8).
Researchers who evaluated the health impacts of wildfires in California during the 2015 fire season found an increase in all-cause cardiovascular and respiratory ED visits, especially among those aged 65 years and older during smoke days. The population-based study included 1,196,233 ED visits during May 1–Sept. 30 that year. PM2.5 concentrations were categorized as light, medium, or dense. Relative risk rose with the amount of smoke in the air. Rates of all-cause cardiovascular ED visits were elevated across levels of smoke density, with the greatest increase on dense smoke days and among those aged 65 years or older (RR,1.15; 95% CI, 1.09-1.22). All-cause cerebrovascular visits were associated with dense smoke days, especially among those aged 65 years and older (RR, 1.22; 95% CI, 1.00-1.49). Respiratory conditions also were increased on dense smoke days (RR, 1.18; 95% CI, 1.08-1.28) (J Am Heart Assoc. 2018 Apr 11;7:e007492. doi: 10.1161/JAHA.117.007492).
Long-term effects unknown
When it comes to the long-term effects of wildfire smoke on human health outcomes, much less is known. In a recent literature review, Colleen E. Reid, PhD, and Melissa May Maestas, PhD, found only one study that investigated long-term respiratory health impacts of wildfire smoke, and only a few studies that have estimated future health impacts of wildfires under likely climate change scenarios (Curr Opin Pulm Med. 2019 Mar;25:179-87).
“We know that there are immediate respiratory health effects from wildfire smoke,” said Dr. Reid of the department of geography at the University of Colorado Boulder. “What’s less known is everything else. That’s challenging, because people want to know about the long-term health effects.”
Evidence from the scientific literature suggests that exposure to air pollution adversely affects cardiovascular health, but whether exposure to wildfire smoke confers a similar risk is less clear. “Until just a few years ago we haven’t been able to study wildfire exposure measures on a large scale,” said EPA scientist Ana G. Rappold, PhD, a statistician there in the environmental public health division of the National Health and Environmental Effects Research Laboratory. “It’s also hard to predict wildfires, so it’s hard to plan for an epidemiologic study if you don’t know where they’re going to occur.”
Dr. Rappold and colleagues examined cardiopulmonary hospitalizations among adults aged 65 years and older in 692 U.S. counties within 200 km of 123 large wildfires during 2008-2010 (Environ Health Perspect. 2019;127[3]:37006. doi: 10.1289/EHP3860). They observed that an increased risk of PM2.5-related cardiopulmonary hospitalizations was similar on smoke and nonsmoke days across multiple lags and exposure metrics, while risk for asthma-related hospitalizations was higher during smoke days. “One hypothesis is that this was an older study population, so naturally if you’re inhaling smoke, the first organ that’s impacted in an older population is the lungs,” Dr. Rappold said. “If you go to the hospital for asthma, wheezing, or bronchitis, you are taken out of the risk pool for cardiovascular and other diseases. That could explain why in other studies we don’t see a clear cardiovascular signal as we have for air pollution studies in general. Another aspect to this study is, the exposure metric was PM2.5, but smoke contains many other components, particularly gases, which are respiratory irritants. It could be that this triggers a higher risk for respiratory [effects] than regular episodes of high PM2.5 exposure, just because of the additional gases that people are exposed to.”
Another complicating factor is the paucity of data about solutions to long-term exposure to wildfire smoke. “If you’re impacted by high-exposure levels for 60 days, that is not something we have experienced before,” Dr. Rappold noted. “What are the solutions for that community? What works? Can we show that by implementing community-level resilience plans with HEPA [high-efficiency particulate air] filters or other interventions, do the overall outcomes improve? Doctors are the first ones to talk with their patients about their symptoms and about how to take care of their conditions. They can clearly make a difference in emphasizing reducing exposures in a way that fits their patients individually, either reducing the amount of time spent outside, the duration of exposure, and the level of exposure. Maybe change activities based on the intensity of exposure. Don’t go for a run outside when it’s smoky, because your ventilation rate is higher and you will breathe in more smoke. Become aware of those things.”
Advising vulnerable patients
While research in this field advances, the unforgiving wildfire season looms, assuring more destruction of property and threats to cardiorespiratory health. “There are a lot of questions that research will have an opportunity to address as we go forward, including the utility and the benefit of N95 masks, the utility of HEPA filters used in the house, and even with HVAC [heating, ventilation, and air conditioning] systems,” Dr. Cascio said. “Can we really clean up the indoor air well enough to protect us from wildfire smoke?”
The way he sees it, the time is ripe for clinicians and officials in public and private practice settings to refine how they distribute information to people living in areas affected by wildfire smoke. “We can’t force people do anything, but at least if they’re informed, then they understand they can make an informed decision about how they might want to affect what they do that would limit their exposure,” he said. “As a patient, my health care system sends text and email messages to me. So, why couldn’t the hospital send out a text message or an email to all of the patients with COPD, coronary disease, and heart failure when an area is impacted by smoke, saying, ‘Check your air quality and take action if air quality is poor?’ Physicians don’t have time to do this kind of education in the office for all of their patients. I know that from experience. But if one were to only focus on those at highest risk, and encourage them to follow our guidelines, which might include doing HEPA filter treatment in the home, we probably would reduce the number of clinical events in a cost-effective way.”
Study confirms prognostic impact of MYC partner gene in DLBCL
MYC rearrangement (MYC-R) has negative prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL) in relation to the MYC partner gene, according to a retrospective study.
The negative prognostic effect of MYC-R was largely limited to patients with MYC–double hit/MYC–triple hit status and an immunoglobulin (IG) partner within 24 months following diagnosis.
“The primary objective of the study was to validate the prognostic relevance of [MYC–single hit] and [MYC–double hit/MYC–triple hit] status within the context of the MYC translocation partner (MYC-IG v. MYC-non-IG) in patients with DLBCL morphology,” wrote Andreas Rosenwald, MD, of the University of Würzburg (Germany) and colleagues in the Journal of Clinical Oncology.
The researchers identified 5,117 patients who all received R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like immunochemotherapy, 2,383 of whom were evaluable for MYC-R and had complete clinical data. The cohort consisted of patients enrolled in various population-based registries and prospective clinical studies throughout North America and Europe.
The team used fluorescence in situ hybridization testing to identify MYC-R, in addition to BCL2 and/or BCL6 translocations if MYC-R was detected. Subsequently, these data were correlated with clinical endpoints.
After analysis, the researchers found that MYC-R was detected in 11% of patients. The presence of MYC-R was associated with significantly reduced survival, particularly within the initial 24 months following diagnosis.
Adverse prognostic implications were largely apparent in patients with accompanying rearrangement of BCL2 and/or BCL6 translocations (MYC–double-hit/MYC–triple hit status) and an immunoglobulin (IG) partner (hazard ratio, 2.4; 95% confidence interval, 1.6-3.6; P less than .001).
“These data suggest that little justification exists for altering initial therapeutic approaches in patients with DLBCL whose tumors carry an MYC translocation alone [MYC single hit],” they wrote. “However, for [MYC double hit/MYC triple hit] DLBCL, the major negative prognostic impact and 2-year effect support the practice of optimizing first-line treatment approaches to achieve maximum complete response rates because salvage treatment at relapse is not effective.”
Dr. Rosenwald and colleagues suggested that, in the future, diagnostic approaches should be implemented to detect patients in this high-risk group and that risk-modified treatment strategies should be further developed.
The study was supported by unrestricted grants to the Lunenburg Lymphoma Biomarker Consortium from several pharmaceutical companies and Bloodwise. Dr. Rosenwald reported having no conflicts of interest, but several coauthors reported relationships with industry.
SOURCE: : Rosenwald A et al. J Clin Oncol. 2019 Sep 9. doi: 10.1200/JCO.19.00743.
MYC rearrangement (MYC-R) has negative prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL) in relation to the MYC partner gene, according to a retrospective study.
The negative prognostic effect of MYC-R was largely limited to patients with MYC–double hit/MYC–triple hit status and an immunoglobulin (IG) partner within 24 months following diagnosis.
“The primary objective of the study was to validate the prognostic relevance of [MYC–single hit] and [MYC–double hit/MYC–triple hit] status within the context of the MYC translocation partner (MYC-IG v. MYC-non-IG) in patients with DLBCL morphology,” wrote Andreas Rosenwald, MD, of the University of Würzburg (Germany) and colleagues in the Journal of Clinical Oncology.
The researchers identified 5,117 patients who all received R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like immunochemotherapy, 2,383 of whom were evaluable for MYC-R and had complete clinical data. The cohort consisted of patients enrolled in various population-based registries and prospective clinical studies throughout North America and Europe.
The team used fluorescence in situ hybridization testing to identify MYC-R, in addition to BCL2 and/or BCL6 translocations if MYC-R was detected. Subsequently, these data were correlated with clinical endpoints.
After analysis, the researchers found that MYC-R was detected in 11% of patients. The presence of MYC-R was associated with significantly reduced survival, particularly within the initial 24 months following diagnosis.
Adverse prognostic implications were largely apparent in patients with accompanying rearrangement of BCL2 and/or BCL6 translocations (MYC–double-hit/MYC–triple hit status) and an immunoglobulin (IG) partner (hazard ratio, 2.4; 95% confidence interval, 1.6-3.6; P less than .001).
“These data suggest that little justification exists for altering initial therapeutic approaches in patients with DLBCL whose tumors carry an MYC translocation alone [MYC single hit],” they wrote. “However, for [MYC double hit/MYC triple hit] DLBCL, the major negative prognostic impact and 2-year effect support the practice of optimizing first-line treatment approaches to achieve maximum complete response rates because salvage treatment at relapse is not effective.”
Dr. Rosenwald and colleagues suggested that, in the future, diagnostic approaches should be implemented to detect patients in this high-risk group and that risk-modified treatment strategies should be further developed.
The study was supported by unrestricted grants to the Lunenburg Lymphoma Biomarker Consortium from several pharmaceutical companies and Bloodwise. Dr. Rosenwald reported having no conflicts of interest, but several coauthors reported relationships with industry.
SOURCE: : Rosenwald A et al. J Clin Oncol. 2019 Sep 9. doi: 10.1200/JCO.19.00743.
MYC rearrangement (MYC-R) has negative prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL) in relation to the MYC partner gene, according to a retrospective study.
The negative prognostic effect of MYC-R was largely limited to patients with MYC–double hit/MYC–triple hit status and an immunoglobulin (IG) partner within 24 months following diagnosis.
“The primary objective of the study was to validate the prognostic relevance of [MYC–single hit] and [MYC–double hit/MYC–triple hit] status within the context of the MYC translocation partner (MYC-IG v. MYC-non-IG) in patients with DLBCL morphology,” wrote Andreas Rosenwald, MD, of the University of Würzburg (Germany) and colleagues in the Journal of Clinical Oncology.
The researchers identified 5,117 patients who all received R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like immunochemotherapy, 2,383 of whom were evaluable for MYC-R and had complete clinical data. The cohort consisted of patients enrolled in various population-based registries and prospective clinical studies throughout North America and Europe.
The team used fluorescence in situ hybridization testing to identify MYC-R, in addition to BCL2 and/or BCL6 translocations if MYC-R was detected. Subsequently, these data were correlated with clinical endpoints.
After analysis, the researchers found that MYC-R was detected in 11% of patients. The presence of MYC-R was associated with significantly reduced survival, particularly within the initial 24 months following diagnosis.
Adverse prognostic implications were largely apparent in patients with accompanying rearrangement of BCL2 and/or BCL6 translocations (MYC–double-hit/MYC–triple hit status) and an immunoglobulin (IG) partner (hazard ratio, 2.4; 95% confidence interval, 1.6-3.6; P less than .001).
“These data suggest that little justification exists for altering initial therapeutic approaches in patients with DLBCL whose tumors carry an MYC translocation alone [MYC single hit],” they wrote. “However, for [MYC double hit/MYC triple hit] DLBCL, the major negative prognostic impact and 2-year effect support the practice of optimizing first-line treatment approaches to achieve maximum complete response rates because salvage treatment at relapse is not effective.”
Dr. Rosenwald and colleagues suggested that, in the future, diagnostic approaches should be implemented to detect patients in this high-risk group and that risk-modified treatment strategies should be further developed.
The study was supported by unrestricted grants to the Lunenburg Lymphoma Biomarker Consortium from several pharmaceutical companies and Bloodwise. Dr. Rosenwald reported having no conflicts of interest, but several coauthors reported relationships with industry.
SOURCE: : Rosenwald A et al. J Clin Oncol. 2019 Sep 9. doi: 10.1200/JCO.19.00743.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Flesh-Colored Papules on the Scrotum
The Diagnosis: Cutaneous Sarcoidosis
Histologic examination of the shave biopsy showed focal parakeratosis, irregular epidermal hyperplasia, and multiple noncaseating naked granulomas with occasional multinucleated giant cells in the dermis (Figure, A). The granulomas were surrounded by mild lymphocytic infiltration with rare eosinophils (Figure, B). Periodic acid-Schiff and Fite stains were negative for organisms, and polariscopic examination was negative; these findings confirmed the diagnosis of cutaneous sarcoidosis. Topical or intralesional steroids were recommended, but our patient declined treatment given that the lesions were asymptomatic.
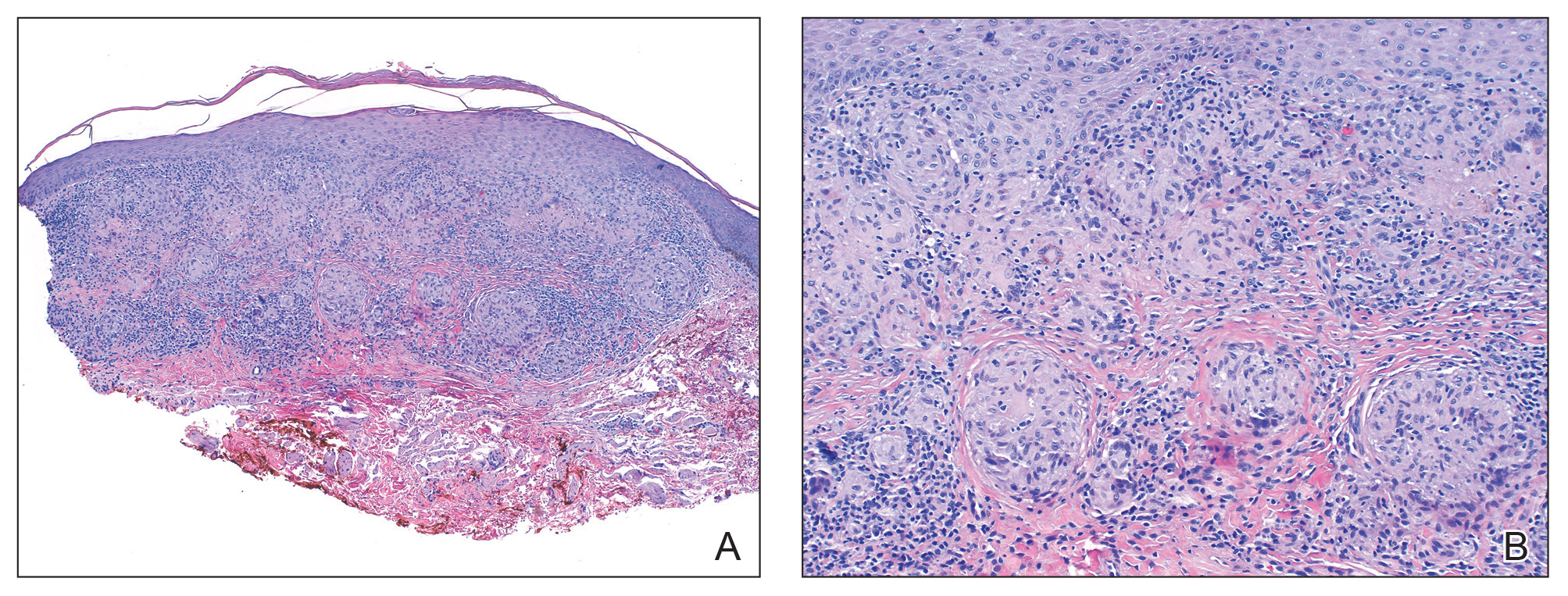
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that affects the skin in approximately 25% of patients.1 Cutaneous lesions manifest in 2 forms: specific and nonspecific. Noncaseating granulomas are considered specific. Nonspecific lesions include erythema nodosum, calcinosis cutis, Sweet syndrome, and nail clubbing. The most common sites of specific sarcoidosis lesions include the face, lips, neck, upper trunk, and extremities. Few cases have reported cutaneous sarcoidosis involving the genitalia; most reports describe vulvar cutaneous sarcoidosis.2-4 Although there have been reports of sarcoidosis involving the epididymis and testes, which presented as scrotal masses, cutaneous scrotal involvement with the skin as the primary site of involvement is rare.5,6 McLaughlin et al5 reported an extensive, pruritic, and eczematous eruption of the scrotum with associated edema and tenderness. Wei et al6 reported cutaneous sarcoidosis in the form of multiple indurated papules involving the penis and scrotum, similar to our case.
Comparing our patient to the case reported by Wei et al,6 both patients had Fitzpatrick skin type V or VI and systemic involvement including pulmonary disease. However, Wei et al6 did not clearly mention if the cutaneous manifestations preceded the diagnosis of systemic sarcoidosis or if they were present at the time of the diagnosis. Our patient developed cutaneous lesions 4 years after being diagnosed with systemic sarcoidosis from hilar lymphadenopathy. In addition to the scrotal lesions, he also had a lesion of lupus pernio presenting as a violaceous to brown plaque on the tip of the nose. Although both patients denied pruritus, the other patient's lesions were painful.6 Wei et al6 mentioned that treatment with topical, intralesional, and systemic steroids failed, and the patient's lesions continued to progress. Generally, topical and intralesional steroids are considered mainstay treatment of cutaneous sarcoidosis despite insufficient data to support their efficacy.7
The differential diagnosis of papules on the scrotum can be broad. Our provisional diagnoses for this particular morphology of small, flesh-colored, shiny, polygonal, flat-topped papules included condyloma acuminatum; lichen planus; idiopathic scrotal calcinosis; steatocystoma multiplex; and sarcoidosis (although uncommon for the site), given the history of pulmonary involvement. We considered a diagnosis of condyloma acuminatum, but the lesions were too shiny and smooth. On histology, condyloma acuminatum shows a hyperkeratotic and parakeratotic stratum corneum, an exophytic growth with marked acanthosis, and superficially located koilocytes. Morphologically, our patient's lesions resembled genital lichen planus. However, Wickham striae were absent, and our patient's lesions were asymptomatic while lesions of lichen planus usually are pruritic. Histologically, lichen planus is characterized by hyperkeratosis, hypergranulosis, sawtooth rete ridges, and lichenoid interface inflammation. Idiopathic scrotal calcinosis also could be included in the differential; however, the lesions would look whiter and firmer than those of our patient, and biopsy will clearly show calcium deposition. Steatocystoma multiplex is another condition that can affect the scrotum, along with the trunk, axillae, extremities, and neck. However, the lesions are expected to discharge oily material if squeezed and have a characteristic corrugated eosinophilic cuticle lining a cyst histologically.
Although it is undetermined if the risk for systemic involvement increases in patients with cutaneous sarcoidosis, evaluation for probable systemic involvement is necessary.1 Because cutaneous sarcoidosis generally can precede any systemic involvement, it would be reasonable to consider skin biopsies in patients who present with atypical wartlike lesions on the scrotum and penis to rule out sarcoidosis.
- Marcoval J, Mañá J, Rubio M. Specific cutaneous lesions in patients with systemic sarcoidosis: relationship to severity and chronicity of disease. Clin Exp Dermatol. 2011;36:739.
- Vera C, Funaro D, Bouffard D. Vulvar sarcoidosis: case report and review of the literature. J Cutan Med Surg. 2013;17:287-290.
- Watkins S, Ismail A, McKay K, et al. Systemic sarcoidosis with unique vulvar involvement. JAMA Dermatol. 2014;150:666-667.
- Pereira IB, Khan A. Sarcoidosis rare cutaneous manifestations: vulval and perianal involvement. J Obstet Gynaecol. 2017;6:1-2.
- McLaughlin SS, Linquist AM, Burnett JW. Cutaneous sarcoidosis of the scrotum: a rare manifestation of systemic disease. Acta Derm Venereol. 2002;82:216-217.
- Wei H, Friedman KA, Rudikoff D. Multiple indurated papules on penis and scrotum. J Cutan Med Surg. 2000;4:202-204.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361.
The Diagnosis: Cutaneous Sarcoidosis
Histologic examination of the shave biopsy showed focal parakeratosis, irregular epidermal hyperplasia, and multiple noncaseating naked granulomas with occasional multinucleated giant cells in the dermis (Figure, A). The granulomas were surrounded by mild lymphocytic infiltration with rare eosinophils (Figure, B). Periodic acid-Schiff and Fite stains were negative for organisms, and polariscopic examination was negative; these findings confirmed the diagnosis of cutaneous sarcoidosis. Topical or intralesional steroids were recommended, but our patient declined treatment given that the lesions were asymptomatic.

Sarcoidosis is a multisystem granulomatous disease of unknown etiology that affects the skin in approximately 25% of patients.1 Cutaneous lesions manifest in 2 forms: specific and nonspecific. Noncaseating granulomas are considered specific. Nonspecific lesions include erythema nodosum, calcinosis cutis, Sweet syndrome, and nail clubbing. The most common sites of specific sarcoidosis lesions include the face, lips, neck, upper trunk, and extremities. Few cases have reported cutaneous sarcoidosis involving the genitalia; most reports describe vulvar cutaneous sarcoidosis.2-4 Although there have been reports of sarcoidosis involving the epididymis and testes, which presented as scrotal masses, cutaneous scrotal involvement with the skin as the primary site of involvement is rare.5,6 McLaughlin et al5 reported an extensive, pruritic, and eczematous eruption of the scrotum with associated edema and tenderness. Wei et al6 reported cutaneous sarcoidosis in the form of multiple indurated papules involving the penis and scrotum, similar to our case.
Comparing our patient to the case reported by Wei et al,6 both patients had Fitzpatrick skin type V or VI and systemic involvement including pulmonary disease. However, Wei et al6 did not clearly mention if the cutaneous manifestations preceded the diagnosis of systemic sarcoidosis or if they were present at the time of the diagnosis. Our patient developed cutaneous lesions 4 years after being diagnosed with systemic sarcoidosis from hilar lymphadenopathy. In addition to the scrotal lesions, he also had a lesion of lupus pernio presenting as a violaceous to brown plaque on the tip of the nose. Although both patients denied pruritus, the other patient's lesions were painful.6 Wei et al6 mentioned that treatment with topical, intralesional, and systemic steroids failed, and the patient's lesions continued to progress. Generally, topical and intralesional steroids are considered mainstay treatment of cutaneous sarcoidosis despite insufficient data to support their efficacy.7
The differential diagnosis of papules on the scrotum can be broad. Our provisional diagnoses for this particular morphology of small, flesh-colored, shiny, polygonal, flat-topped papules included condyloma acuminatum; lichen planus; idiopathic scrotal calcinosis; steatocystoma multiplex; and sarcoidosis (although uncommon for the site), given the history of pulmonary involvement. We considered a diagnosis of condyloma acuminatum, but the lesions were too shiny and smooth. On histology, condyloma acuminatum shows a hyperkeratotic and parakeratotic stratum corneum, an exophytic growth with marked acanthosis, and superficially located koilocytes. Morphologically, our patient's lesions resembled genital lichen planus. However, Wickham striae were absent, and our patient's lesions were asymptomatic while lesions of lichen planus usually are pruritic. Histologically, lichen planus is characterized by hyperkeratosis, hypergranulosis, sawtooth rete ridges, and lichenoid interface inflammation. Idiopathic scrotal calcinosis also could be included in the differential; however, the lesions would look whiter and firmer than those of our patient, and biopsy will clearly show calcium deposition. Steatocystoma multiplex is another condition that can affect the scrotum, along with the trunk, axillae, extremities, and neck. However, the lesions are expected to discharge oily material if squeezed and have a characteristic corrugated eosinophilic cuticle lining a cyst histologically.
Although it is undetermined if the risk for systemic involvement increases in patients with cutaneous sarcoidosis, evaluation for probable systemic involvement is necessary.1 Because cutaneous sarcoidosis generally can precede any systemic involvement, it would be reasonable to consider skin biopsies in patients who present with atypical wartlike lesions on the scrotum and penis to rule out sarcoidosis.
The Diagnosis: Cutaneous Sarcoidosis
Histologic examination of the shave biopsy showed focal parakeratosis, irregular epidermal hyperplasia, and multiple noncaseating naked granulomas with occasional multinucleated giant cells in the dermis (Figure, A). The granulomas were surrounded by mild lymphocytic infiltration with rare eosinophils (Figure, B). Periodic acid-Schiff and Fite stains were negative for organisms, and polariscopic examination was negative; these findings confirmed the diagnosis of cutaneous sarcoidosis. Topical or intralesional steroids were recommended, but our patient declined treatment given that the lesions were asymptomatic.

Sarcoidosis is a multisystem granulomatous disease of unknown etiology that affects the skin in approximately 25% of patients.1 Cutaneous lesions manifest in 2 forms: specific and nonspecific. Noncaseating granulomas are considered specific. Nonspecific lesions include erythema nodosum, calcinosis cutis, Sweet syndrome, and nail clubbing. The most common sites of specific sarcoidosis lesions include the face, lips, neck, upper trunk, and extremities. Few cases have reported cutaneous sarcoidosis involving the genitalia; most reports describe vulvar cutaneous sarcoidosis.2-4 Although there have been reports of sarcoidosis involving the epididymis and testes, which presented as scrotal masses, cutaneous scrotal involvement with the skin as the primary site of involvement is rare.5,6 McLaughlin et al5 reported an extensive, pruritic, and eczematous eruption of the scrotum with associated edema and tenderness. Wei et al6 reported cutaneous sarcoidosis in the form of multiple indurated papules involving the penis and scrotum, similar to our case.
Comparing our patient to the case reported by Wei et al,6 both patients had Fitzpatrick skin type V or VI and systemic involvement including pulmonary disease. However, Wei et al6 did not clearly mention if the cutaneous manifestations preceded the diagnosis of systemic sarcoidosis or if they were present at the time of the diagnosis. Our patient developed cutaneous lesions 4 years after being diagnosed with systemic sarcoidosis from hilar lymphadenopathy. In addition to the scrotal lesions, he also had a lesion of lupus pernio presenting as a violaceous to brown plaque on the tip of the nose. Although both patients denied pruritus, the other patient's lesions were painful.6 Wei et al6 mentioned that treatment with topical, intralesional, and systemic steroids failed, and the patient's lesions continued to progress. Generally, topical and intralesional steroids are considered mainstay treatment of cutaneous sarcoidosis despite insufficient data to support their efficacy.7
The differential diagnosis of papules on the scrotum can be broad. Our provisional diagnoses for this particular morphology of small, flesh-colored, shiny, polygonal, flat-topped papules included condyloma acuminatum; lichen planus; idiopathic scrotal calcinosis; steatocystoma multiplex; and sarcoidosis (although uncommon for the site), given the history of pulmonary involvement. We considered a diagnosis of condyloma acuminatum, but the lesions were too shiny and smooth. On histology, condyloma acuminatum shows a hyperkeratotic and parakeratotic stratum corneum, an exophytic growth with marked acanthosis, and superficially located koilocytes. Morphologically, our patient's lesions resembled genital lichen planus. However, Wickham striae were absent, and our patient's lesions were asymptomatic while lesions of lichen planus usually are pruritic. Histologically, lichen planus is characterized by hyperkeratosis, hypergranulosis, sawtooth rete ridges, and lichenoid interface inflammation. Idiopathic scrotal calcinosis also could be included in the differential; however, the lesions would look whiter and firmer than those of our patient, and biopsy will clearly show calcium deposition. Steatocystoma multiplex is another condition that can affect the scrotum, along with the trunk, axillae, extremities, and neck. However, the lesions are expected to discharge oily material if squeezed and have a characteristic corrugated eosinophilic cuticle lining a cyst histologically.
Although it is undetermined if the risk for systemic involvement increases in patients with cutaneous sarcoidosis, evaluation for probable systemic involvement is necessary.1 Because cutaneous sarcoidosis generally can precede any systemic involvement, it would be reasonable to consider skin biopsies in patients who present with atypical wartlike lesions on the scrotum and penis to rule out sarcoidosis.
- Marcoval J, Mañá J, Rubio M. Specific cutaneous lesions in patients with systemic sarcoidosis: relationship to severity and chronicity of disease. Clin Exp Dermatol. 2011;36:739.
- Vera C, Funaro D, Bouffard D. Vulvar sarcoidosis: case report and review of the literature. J Cutan Med Surg. 2013;17:287-290.
- Watkins S, Ismail A, McKay K, et al. Systemic sarcoidosis with unique vulvar involvement. JAMA Dermatol. 2014;150:666-667.
- Pereira IB, Khan A. Sarcoidosis rare cutaneous manifestations: vulval and perianal involvement. J Obstet Gynaecol. 2017;6:1-2.
- McLaughlin SS, Linquist AM, Burnett JW. Cutaneous sarcoidosis of the scrotum: a rare manifestation of systemic disease. Acta Derm Venereol. 2002;82:216-217.
- Wei H, Friedman KA, Rudikoff D. Multiple indurated papules on penis and scrotum. J Cutan Med Surg. 2000;4:202-204.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361.
- Marcoval J, Mañá J, Rubio M. Specific cutaneous lesions in patients with systemic sarcoidosis: relationship to severity and chronicity of disease. Clin Exp Dermatol. 2011;36:739.
- Vera C, Funaro D, Bouffard D. Vulvar sarcoidosis: case report and review of the literature. J Cutan Med Surg. 2013;17:287-290.
- Watkins S, Ismail A, McKay K, et al. Systemic sarcoidosis with unique vulvar involvement. JAMA Dermatol. 2014;150:666-667.
- Pereira IB, Khan A. Sarcoidosis rare cutaneous manifestations: vulval and perianal involvement. J Obstet Gynaecol. 2017;6:1-2.
- McLaughlin SS, Linquist AM, Burnett JW. Cutaneous sarcoidosis of the scrotum: a rare manifestation of systemic disease. Acta Derm Venereol. 2002;82:216-217.
- Wei H, Friedman KA, Rudikoff D. Multiple indurated papules on penis and scrotum. J Cutan Med Surg. 2000;4:202-204.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361.
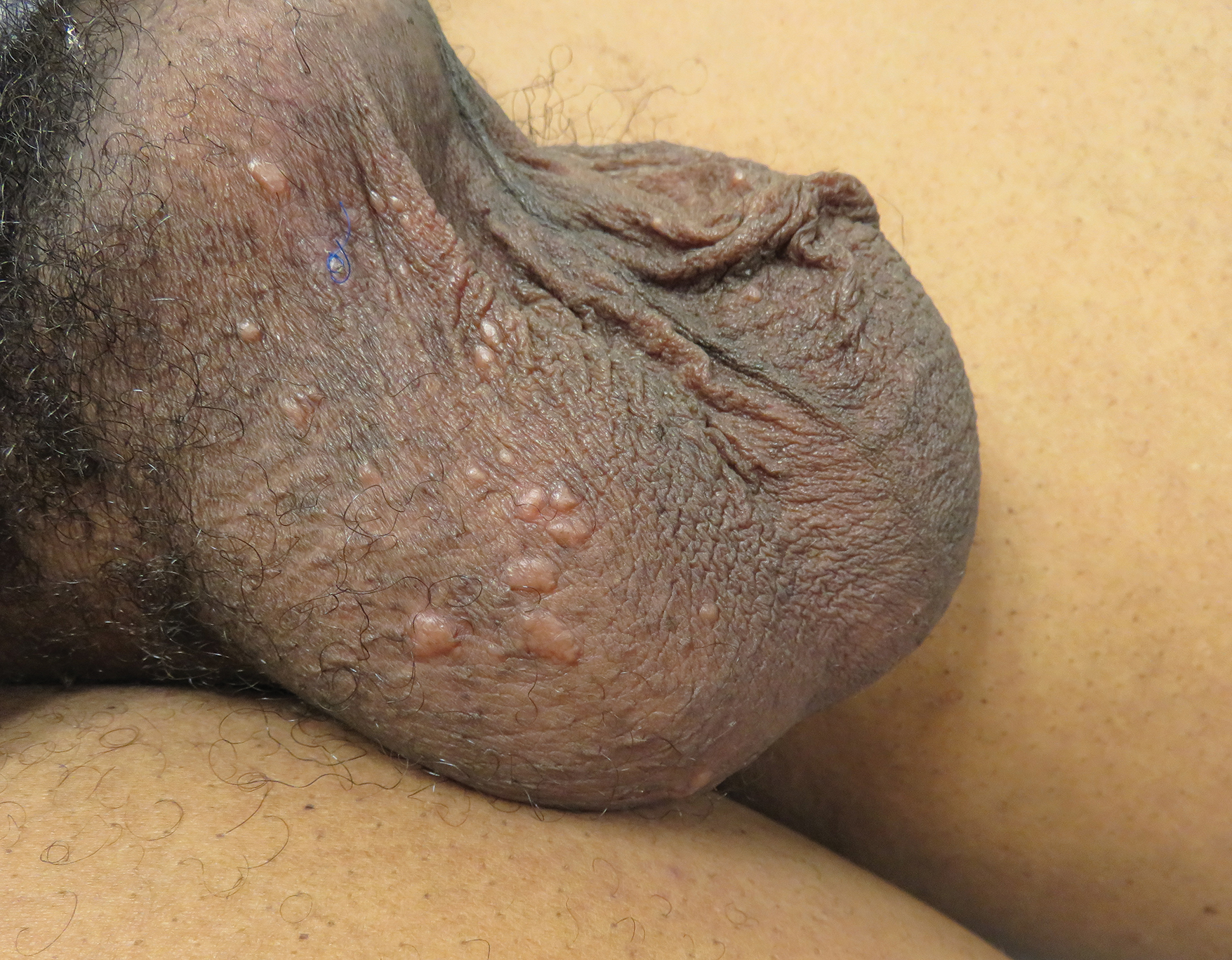
A 44-year-old black man presented with "bumps on the scrotum" of approximately 4 months' duration. They were asymptomatic and untreated. The patient denied extramarital sexual contacts or a history of any sexually transmitted infection. His medical history was notable for sarcoidosis diagnosed 4 years prior to presentation when hilar lymphadenopathy was incidentally found on routine screening. His condition was managed with regular follow-up without treatment. He also had a positive tuberculosis skin test in the past without radiologic evidence of active pulmonary disease. Physical examination revealed multiple 2- to 5-mm, flesh-colored, shiny, polygonal, flat-topped papules spread diffusely over the scrotum. A 1-cm, barely palpable, nonscaly, violaceous to brown plaque also was seen on the tip of the nose. A punch biopsy was taken from a lesion on the scrotum.
Clinical research in private practice? It can be done, and here’s how
Not every physician coming out of medical school wants to go down the path of conducting clinical research. But for those who do, the decision is a rewarding one that can make a real difference in patients’ lives.
I took a nontraditional route to get to my current role as the director of clinical research and education at the largest gastroenterology practice in the United States. I had a business degree coming out of college and worked for years in the business world prior to going to medical school. After graduation, I got an offer to join the pharmaceutical industry in medical affairs. There, I focused on inflammatory bowel disease (IBD), where my role was to share high-level scientific data and liaise with IBD disease state experts. Part of the role was working internally with clinical operations and externally with sites conducting research throughout the United States. In the next 6 years, I had the opportunity to observe how research was run in both the academic and community practice settings, noting those characteristics that allowed some to succeed and far more to stagnate or fail.
In 2014, I joined Texas Digestive Disease Consultants with the goal to ramp up the practice’s clinical research arm and create a department expressly for that purpose. To date, the department has become incredibly successful and ultimately sustainable. If you’re considering joining a practice based on its clinical research program or starting one in your current practice, here is what I’ve learned along the way:
Know the benefit to patients
You have to decide to conduct clinical research because of its benefit to the patient, not because you see it as an alternative revenue stream. It will never be sustainable if viewed as a profit center. Clinical research offers therapeutic advancements that will not be available to most for the next 5-10 years. Additionally, patients do not need insurance in order to participate in a study. The sponsor covers all study visits, procedures, and therapeutics.
Know the value to the practice
Having a clinical trials program brings both direct and indirect enterprise value to the practice. It may come in the form of referrals from other practices that don’t have the same capabilities. Patients may view your practice differently, knowing that it has the added value of research. There is a halo effect from having clinical trials capabilities in how you are viewed by patients and other physicians in the community.
Get the right people in place
First, you will need an enthusiastic primary investigator who can take a bit of time from practice to conduct clinical trials. But just as importantly, you need a knowledgeable clinical research coordinator. Without an effective coordinator, the program is doomed. A good coordinator should be well rounded in all aspects of research (e.g., regulatory, patient recruitment, quality assurance, contracts, budgets, running labs, conducting patient visits) and able to deal with the day-to-day intricacies of running clinical trials, which will allow a doctor to take care of the existing practice. They should also be versed in the requisite equipment (e.g., locking refrigerator, freezer, and ambient cabinet, temperature monitoring devices, EKG machine, and centrifuge) necessary to run a clinical study.
Know how to recruit patients
The database at Texas Digestive Disease Consultants/GI Alliance (TDDC/GIA) is vast, which makes identification of patients who may qualify for a trial an easier task. Many practices will have an electronic medical record that will aid in identification, but if that is not the case at your practice, there are a number of ways you can go about this. First, talk to physicians in your practice and in other practices – internists, family physicians, and other gastroenterologists come to mind – about what you can offer. Send a letter or an email out to physicians in the community with the inclusion/exclusion criteria for your study, and always direct them to https://clinicaltrials.gov/ for additional information. Patient advocacy organizations are another good source of referrals for clinical trials. And of course, pharmaceutical companies have recruitment services that they use and can help steer patients your way.
Understand the ethics
There are numerous ethical and legal considerations when it comes to running clinical trials. The principles of Good Clinical Practice (GCP) and Human Subject Protection (HSP) guide the conduct of clinical trials in the United States. Understanding the concepts and regulations around caring for patients in trials is not only “good practice,” it’s a necessity. There are free Good Clinical Practice training courses available, which are required every few years for everyone conducting clinical research. These courses are quite lengthy (3-6 hours), but provide a great overview of the Food and Drug Administration regulations, ethical considerations, and other advice on successfully operating a clinical trials program. Again, a capable clinical research coordinator will help guide you here.
Adopt standard operating procedures
Every clinical trials sponsor will require standard operating procedures for such facets of research as informed consent, reporting requirements, and safety monitoring. Here again, a seasoned clinical trials coordinator can put you on the right path. You may choose to purchase standardized templates or work with another group that already has them and would be willing to share.
Be smart about spending
While this may depend on the scale you want to grow your research, at TDDC/GIA we knew we wanted to build a sustainable program. We invested a lot of time and money into our infrastructure, as well as adopting a robust Clinical Trials Management System (CTMS). A CTMS system will provide the ability to measure productivity, finances, schedule patient visits, and pay patient stipends. Some systems even automate the regulatory process (E-Regulatory) or source process (E-source), which can cut down on the paper burden on a site.
Seek mentors
Look for someone to emulate who is already established in operating a clinical research program in private practice. Reach out to the practices or physicians that are already doing this work and doing it well. For physicians who are just starting out or still in training, look for opportunities to publish or be involved with the clinical trials program at your institution.
Contacts in the pharmaceutical industry are very important to getting a new program off the ground. Ask the pharma sales representatives who visit your clinic to put you in touch with their medical science liaison (MSL). The MSL can get you information about the clinical trials their company is currently running and those they have in the pipeline.
Dr. Chris Fourment, director of clinical research and education, Texas Digestive Disease Consultants/GI Alliance.
Not every physician coming out of medical school wants to go down the path of conducting clinical research. But for those who do, the decision is a rewarding one that can make a real difference in patients’ lives.
I took a nontraditional route to get to my current role as the director of clinical research and education at the largest gastroenterology practice in the United States. I had a business degree coming out of college and worked for years in the business world prior to going to medical school. After graduation, I got an offer to join the pharmaceutical industry in medical affairs. There, I focused on inflammatory bowel disease (IBD), where my role was to share high-level scientific data and liaise with IBD disease state experts. Part of the role was working internally with clinical operations and externally with sites conducting research throughout the United States. In the next 6 years, I had the opportunity to observe how research was run in both the academic and community practice settings, noting those characteristics that allowed some to succeed and far more to stagnate or fail.
In 2014, I joined Texas Digestive Disease Consultants with the goal to ramp up the practice’s clinical research arm and create a department expressly for that purpose. To date, the department has become incredibly successful and ultimately sustainable. If you’re considering joining a practice based on its clinical research program or starting one in your current practice, here is what I’ve learned along the way:
Know the benefit to patients
You have to decide to conduct clinical research because of its benefit to the patient, not because you see it as an alternative revenue stream. It will never be sustainable if viewed as a profit center. Clinical research offers therapeutic advancements that will not be available to most for the next 5-10 years. Additionally, patients do not need insurance in order to participate in a study. The sponsor covers all study visits, procedures, and therapeutics.
Know the value to the practice
Having a clinical trials program brings both direct and indirect enterprise value to the practice. It may come in the form of referrals from other practices that don’t have the same capabilities. Patients may view your practice differently, knowing that it has the added value of research. There is a halo effect from having clinical trials capabilities in how you are viewed by patients and other physicians in the community.
Get the right people in place
First, you will need an enthusiastic primary investigator who can take a bit of time from practice to conduct clinical trials. But just as importantly, you need a knowledgeable clinical research coordinator. Without an effective coordinator, the program is doomed. A good coordinator should be well rounded in all aspects of research (e.g., regulatory, patient recruitment, quality assurance, contracts, budgets, running labs, conducting patient visits) and able to deal with the day-to-day intricacies of running clinical trials, which will allow a doctor to take care of the existing practice. They should also be versed in the requisite equipment (e.g., locking refrigerator, freezer, and ambient cabinet, temperature monitoring devices, EKG machine, and centrifuge) necessary to run a clinical study.
Know how to recruit patients
The database at Texas Digestive Disease Consultants/GI Alliance (TDDC/GIA) is vast, which makes identification of patients who may qualify for a trial an easier task. Many practices will have an electronic medical record that will aid in identification, but if that is not the case at your practice, there are a number of ways you can go about this. First, talk to physicians in your practice and in other practices – internists, family physicians, and other gastroenterologists come to mind – about what you can offer. Send a letter or an email out to physicians in the community with the inclusion/exclusion criteria for your study, and always direct them to https://clinicaltrials.gov/ for additional information. Patient advocacy organizations are another good source of referrals for clinical trials. And of course, pharmaceutical companies have recruitment services that they use and can help steer patients your way.
Understand the ethics
There are numerous ethical and legal considerations when it comes to running clinical trials. The principles of Good Clinical Practice (GCP) and Human Subject Protection (HSP) guide the conduct of clinical trials in the United States. Understanding the concepts and regulations around caring for patients in trials is not only “good practice,” it’s a necessity. There are free Good Clinical Practice training courses available, which are required every few years for everyone conducting clinical research. These courses are quite lengthy (3-6 hours), but provide a great overview of the Food and Drug Administration regulations, ethical considerations, and other advice on successfully operating a clinical trials program. Again, a capable clinical research coordinator will help guide you here.
Adopt standard operating procedures
Every clinical trials sponsor will require standard operating procedures for such facets of research as informed consent, reporting requirements, and safety monitoring. Here again, a seasoned clinical trials coordinator can put you on the right path. You may choose to purchase standardized templates or work with another group that already has them and would be willing to share.
Be smart about spending
While this may depend on the scale you want to grow your research, at TDDC/GIA we knew we wanted to build a sustainable program. We invested a lot of time and money into our infrastructure, as well as adopting a robust Clinical Trials Management System (CTMS). A CTMS system will provide the ability to measure productivity, finances, schedule patient visits, and pay patient stipends. Some systems even automate the regulatory process (E-Regulatory) or source process (E-source), which can cut down on the paper burden on a site.
Seek mentors
Look for someone to emulate who is already established in operating a clinical research program in private practice. Reach out to the practices or physicians that are already doing this work and doing it well. For physicians who are just starting out or still in training, look for opportunities to publish or be involved with the clinical trials program at your institution.
Contacts in the pharmaceutical industry are very important to getting a new program off the ground. Ask the pharma sales representatives who visit your clinic to put you in touch with their medical science liaison (MSL). The MSL can get you information about the clinical trials their company is currently running and those they have in the pipeline.
Dr. Chris Fourment, director of clinical research and education, Texas Digestive Disease Consultants/GI Alliance.
Not every physician coming out of medical school wants to go down the path of conducting clinical research. But for those who do, the decision is a rewarding one that can make a real difference in patients’ lives.
I took a nontraditional route to get to my current role as the director of clinical research and education at the largest gastroenterology practice in the United States. I had a business degree coming out of college and worked for years in the business world prior to going to medical school. After graduation, I got an offer to join the pharmaceutical industry in medical affairs. There, I focused on inflammatory bowel disease (IBD), where my role was to share high-level scientific data and liaise with IBD disease state experts. Part of the role was working internally with clinical operations and externally with sites conducting research throughout the United States. In the next 6 years, I had the opportunity to observe how research was run in both the academic and community practice settings, noting those characteristics that allowed some to succeed and far more to stagnate or fail.
In 2014, I joined Texas Digestive Disease Consultants with the goal to ramp up the practice’s clinical research arm and create a department expressly for that purpose. To date, the department has become incredibly successful and ultimately sustainable. If you’re considering joining a practice based on its clinical research program or starting one in your current practice, here is what I’ve learned along the way:
Know the benefit to patients
You have to decide to conduct clinical research because of its benefit to the patient, not because you see it as an alternative revenue stream. It will never be sustainable if viewed as a profit center. Clinical research offers therapeutic advancements that will not be available to most for the next 5-10 years. Additionally, patients do not need insurance in order to participate in a study. The sponsor covers all study visits, procedures, and therapeutics.
Know the value to the practice
Having a clinical trials program brings both direct and indirect enterprise value to the practice. It may come in the form of referrals from other practices that don’t have the same capabilities. Patients may view your practice differently, knowing that it has the added value of research. There is a halo effect from having clinical trials capabilities in how you are viewed by patients and other physicians in the community.
Get the right people in place
First, you will need an enthusiastic primary investigator who can take a bit of time from practice to conduct clinical trials. But just as importantly, you need a knowledgeable clinical research coordinator. Without an effective coordinator, the program is doomed. A good coordinator should be well rounded in all aspects of research (e.g., regulatory, patient recruitment, quality assurance, contracts, budgets, running labs, conducting patient visits) and able to deal with the day-to-day intricacies of running clinical trials, which will allow a doctor to take care of the existing practice. They should also be versed in the requisite equipment (e.g., locking refrigerator, freezer, and ambient cabinet, temperature monitoring devices, EKG machine, and centrifuge) necessary to run a clinical study.
Know how to recruit patients
The database at Texas Digestive Disease Consultants/GI Alliance (TDDC/GIA) is vast, which makes identification of patients who may qualify for a trial an easier task. Many practices will have an electronic medical record that will aid in identification, but if that is not the case at your practice, there are a number of ways you can go about this. First, talk to physicians in your practice and in other practices – internists, family physicians, and other gastroenterologists come to mind – about what you can offer. Send a letter or an email out to physicians in the community with the inclusion/exclusion criteria for your study, and always direct them to https://clinicaltrials.gov/ for additional information. Patient advocacy organizations are another good source of referrals for clinical trials. And of course, pharmaceutical companies have recruitment services that they use and can help steer patients your way.
Understand the ethics
There are numerous ethical and legal considerations when it comes to running clinical trials. The principles of Good Clinical Practice (GCP) and Human Subject Protection (HSP) guide the conduct of clinical trials in the United States. Understanding the concepts and regulations around caring for patients in trials is not only “good practice,” it’s a necessity. There are free Good Clinical Practice training courses available, which are required every few years for everyone conducting clinical research. These courses are quite lengthy (3-6 hours), but provide a great overview of the Food and Drug Administration regulations, ethical considerations, and other advice on successfully operating a clinical trials program. Again, a capable clinical research coordinator will help guide you here.
Adopt standard operating procedures
Every clinical trials sponsor will require standard operating procedures for such facets of research as informed consent, reporting requirements, and safety monitoring. Here again, a seasoned clinical trials coordinator can put you on the right path. You may choose to purchase standardized templates or work with another group that already has them and would be willing to share.
Be smart about spending
While this may depend on the scale you want to grow your research, at TDDC/GIA we knew we wanted to build a sustainable program. We invested a lot of time and money into our infrastructure, as well as adopting a robust Clinical Trials Management System (CTMS). A CTMS system will provide the ability to measure productivity, finances, schedule patient visits, and pay patient stipends. Some systems even automate the regulatory process (E-Regulatory) or source process (E-source), which can cut down on the paper burden on a site.
Seek mentors
Look for someone to emulate who is already established in operating a clinical research program in private practice. Reach out to the practices or physicians that are already doing this work and doing it well. For physicians who are just starting out or still in training, look for opportunities to publish or be involved with the clinical trials program at your institution.
Contacts in the pharmaceutical industry are very important to getting a new program off the ground. Ask the pharma sales representatives who visit your clinic to put you in touch with their medical science liaison (MSL). The MSL can get you information about the clinical trials their company is currently running and those they have in the pipeline.
Dr. Chris Fourment, director of clinical research and education, Texas Digestive Disease Consultants/GI Alliance.