User login
ACIP approves child and adolescent vaccination schedule for 2020
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
PHM 19: PREP yourself for the PHM boards
Get ready for the first-ever ABP PHM exam
Presenters
Jared Austin, MD, FAAP
Ryan Bode, MD, FAAP
Jeremy Kern, MD, FAAP
Mary Ottolini, MD, MPH, MEd, FAAP
Stacy Pierson, MD, FAAP
Mary Rocha, MD, MPH, FAAP
Susan Walley, MD, CTTS, FAAP
Session summary
Professional development sessions at the Pediatric Hospital Medicine 2019 conference intended to further educate pediatric hospitalists and advance their careers. In November 2019, many pediatric hospitalists will be taking subspecialty PHM boards for the very first time. This PHM19 session had clear objectives: to describe the American Board of Pediatrics (ABP) PHM board content areas, to analyze common knowledge gaps in PREP PHM, and to examine different approaches to clinical management of PHM patients.
The session opened with a brief history of a vision of PHM and the story of its realization. In 2016, a group of eight stalwart writers, four new writers, and three editors created PREP 2018 and 2019 questions that were released in full prior to November 2019. The ABP will offer the board exam in 2019, 2021, and 2023
The exam content domains include the following:
- Medical conditions.
- Behavioral and mental health conditions.
- Newborn care.
- Children with medical complexity.
- Medical procedures.
- Patient and family centered care.
- Transitions of care.
- Quality improvement, patient safety and system based improvement.
- Evidence-based, high-value care.
- Advocacy and leadership.
- Ethics, legal issues, and human rights.
- Teaching and education.
- Core knowledge in scholarly activities.
Each question consists of a case vignette, question, response choices, critiques, PREP PEARLs, and references. There are also additional PREP Ponder Points that intend to prompt reflection on practice change.
For the remainder of the session presenters reviewed the PHM PREP questions that were most frequently answered incorrectly. Some of the topics included: asthma vs. anaphylaxis, venous thromboembolism prophylaxis in surgical patients, postoperative feeding regimens, transmission-based precautions, febrile neonates, Ebola, medical child abuse, absolute indications for intubation, toxic megacolon, palivizumab prophylaxis guidelines, key driver diagrams, and infantile hemangiomas.
Key takeaway
Pediatric hospitalists all over the United States will for the first time ever take PHM boards in November 2019. The exam content domains were demonstrated in detail, and several often incorrectly answered PREP questions were presented and discussed.
Dr. Giordano is assistant professor in pediatrics at Columbia University Medical Center, New York.
Get ready for the first-ever ABP PHM exam
Get ready for the first-ever ABP PHM exam
Presenters
Jared Austin, MD, FAAP
Ryan Bode, MD, FAAP
Jeremy Kern, MD, FAAP
Mary Ottolini, MD, MPH, MEd, FAAP
Stacy Pierson, MD, FAAP
Mary Rocha, MD, MPH, FAAP
Susan Walley, MD, CTTS, FAAP
Session summary
Professional development sessions at the Pediatric Hospital Medicine 2019 conference intended to further educate pediatric hospitalists and advance their careers. In November 2019, many pediatric hospitalists will be taking subspecialty PHM boards for the very first time. This PHM19 session had clear objectives: to describe the American Board of Pediatrics (ABP) PHM board content areas, to analyze common knowledge gaps in PREP PHM, and to examine different approaches to clinical management of PHM patients.
The session opened with a brief history of a vision of PHM and the story of its realization. In 2016, a group of eight stalwart writers, four new writers, and three editors created PREP 2018 and 2019 questions that were released in full prior to November 2019. The ABP will offer the board exam in 2019, 2021, and 2023
The exam content domains include the following:
- Medical conditions.
- Behavioral and mental health conditions.
- Newborn care.
- Children with medical complexity.
- Medical procedures.
- Patient and family centered care.
- Transitions of care.
- Quality improvement, patient safety and system based improvement.
- Evidence-based, high-value care.
- Advocacy and leadership.
- Ethics, legal issues, and human rights.
- Teaching and education.
- Core knowledge in scholarly activities.
Each question consists of a case vignette, question, response choices, critiques, PREP PEARLs, and references. There are also additional PREP Ponder Points that intend to prompt reflection on practice change.
For the remainder of the session presenters reviewed the PHM PREP questions that were most frequently answered incorrectly. Some of the topics included: asthma vs. anaphylaxis, venous thromboembolism prophylaxis in surgical patients, postoperative feeding regimens, transmission-based precautions, febrile neonates, Ebola, medical child abuse, absolute indications for intubation, toxic megacolon, palivizumab prophylaxis guidelines, key driver diagrams, and infantile hemangiomas.
Key takeaway
Pediatric hospitalists all over the United States will for the first time ever take PHM boards in November 2019. The exam content domains were demonstrated in detail, and several often incorrectly answered PREP questions were presented and discussed.
Dr. Giordano is assistant professor in pediatrics at Columbia University Medical Center, New York.
Presenters
Jared Austin, MD, FAAP
Ryan Bode, MD, FAAP
Jeremy Kern, MD, FAAP
Mary Ottolini, MD, MPH, MEd, FAAP
Stacy Pierson, MD, FAAP
Mary Rocha, MD, MPH, FAAP
Susan Walley, MD, CTTS, FAAP
Session summary
Professional development sessions at the Pediatric Hospital Medicine 2019 conference intended to further educate pediatric hospitalists and advance their careers. In November 2019, many pediatric hospitalists will be taking subspecialty PHM boards for the very first time. This PHM19 session had clear objectives: to describe the American Board of Pediatrics (ABP) PHM board content areas, to analyze common knowledge gaps in PREP PHM, and to examine different approaches to clinical management of PHM patients.
The session opened with a brief history of a vision of PHM and the story of its realization. In 2016, a group of eight stalwart writers, four new writers, and three editors created PREP 2018 and 2019 questions that were released in full prior to November 2019. The ABP will offer the board exam in 2019, 2021, and 2023
The exam content domains include the following:
- Medical conditions.
- Behavioral and mental health conditions.
- Newborn care.
- Children with medical complexity.
- Medical procedures.
- Patient and family centered care.
- Transitions of care.
- Quality improvement, patient safety and system based improvement.
- Evidence-based, high-value care.
- Advocacy and leadership.
- Ethics, legal issues, and human rights.
- Teaching and education.
- Core knowledge in scholarly activities.
Each question consists of a case vignette, question, response choices, critiques, PREP PEARLs, and references. There are also additional PREP Ponder Points that intend to prompt reflection on practice change.
For the remainder of the session presenters reviewed the PHM PREP questions that were most frequently answered incorrectly. Some of the topics included: asthma vs. anaphylaxis, venous thromboembolism prophylaxis in surgical patients, postoperative feeding regimens, transmission-based precautions, febrile neonates, Ebola, medical child abuse, absolute indications for intubation, toxic megacolon, palivizumab prophylaxis guidelines, key driver diagrams, and infantile hemangiomas.
Key takeaway
Pediatric hospitalists all over the United States will for the first time ever take PHM boards in November 2019. The exam content domains were demonstrated in detail, and several often incorrectly answered PREP questions were presented and discussed.
Dr. Giordano is assistant professor in pediatrics at Columbia University Medical Center, New York.
FDA approves onabotulinumtoxinA for pediatric lower limb spasticity
The Food and Drug Administration has approved onabotulinumtoxinA (Botox) for treatment of pediatric lower limb spasticity in patients aged 2-17 years, excluding those in whom it is associated with cerebral palsy, according to an announcement from Allergan.
The approval is based on a phase 3 study evaluating safety and efficacy in more than 300 patients with lower limb spasticity. Although patients with cerebral palsy were included in the study, they’re excluded from this indication. Orphan Drug Exclusivity prevents it from being indicated for lower limb spasticity in cerebral palsy because abobotulinumtoxinA (Dysport) already has marketing exclusivity for the indication. Botox also is indicated for children aged 2-17 years of age with upper limb spasticity, as well as nine other indications.
OnabotulinumtoxinA comes with warnings, including problems of swallowing, speaking, or breathing and even risk of spread of the toxin. It also may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours or weeks of administration. Serious and sometimes immediate allergic reactions have been reported. Patients and health care professionals should discuss various concerns before treatment, including whether the patient has recently received antibiotics by injection, or has taken muscle relaxants, allergy or cold medicine, sleep medicine, and aspirinlike products or blood thinners. It’s important to note that the dose of onabotulinumtoxinA is not the same as that for other botulinum toxin products. The full prescribing information is available on the Allergan website.
The Food and Drug Administration has approved onabotulinumtoxinA (Botox) for treatment of pediatric lower limb spasticity in patients aged 2-17 years, excluding those in whom it is associated with cerebral palsy, according to an announcement from Allergan.
The approval is based on a phase 3 study evaluating safety and efficacy in more than 300 patients with lower limb spasticity. Although patients with cerebral palsy were included in the study, they’re excluded from this indication. Orphan Drug Exclusivity prevents it from being indicated for lower limb spasticity in cerebral palsy because abobotulinumtoxinA (Dysport) already has marketing exclusivity for the indication. Botox also is indicated for children aged 2-17 years of age with upper limb spasticity, as well as nine other indications.
OnabotulinumtoxinA comes with warnings, including problems of swallowing, speaking, or breathing and even risk of spread of the toxin. It also may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours or weeks of administration. Serious and sometimes immediate allergic reactions have been reported. Patients and health care professionals should discuss various concerns before treatment, including whether the patient has recently received antibiotics by injection, or has taken muscle relaxants, allergy or cold medicine, sleep medicine, and aspirinlike products or blood thinners. It’s important to note that the dose of onabotulinumtoxinA is not the same as that for other botulinum toxin products. The full prescribing information is available on the Allergan website.
The Food and Drug Administration has approved onabotulinumtoxinA (Botox) for treatment of pediatric lower limb spasticity in patients aged 2-17 years, excluding those in whom it is associated with cerebral palsy, according to an announcement from Allergan.
The approval is based on a phase 3 study evaluating safety and efficacy in more than 300 patients with lower limb spasticity. Although patients with cerebral palsy were included in the study, they’re excluded from this indication. Orphan Drug Exclusivity prevents it from being indicated for lower limb spasticity in cerebral palsy because abobotulinumtoxinA (Dysport) already has marketing exclusivity for the indication. Botox also is indicated for children aged 2-17 years of age with upper limb spasticity, as well as nine other indications.
OnabotulinumtoxinA comes with warnings, including problems of swallowing, speaking, or breathing and even risk of spread of the toxin. It also may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours or weeks of administration. Serious and sometimes immediate allergic reactions have been reported. Patients and health care professionals should discuss various concerns before treatment, including whether the patient has recently received antibiotics by injection, or has taken muscle relaxants, allergy or cold medicine, sleep medicine, and aspirinlike products or blood thinners. It’s important to note that the dose of onabotulinumtoxinA is not the same as that for other botulinum toxin products. The full prescribing information is available on the Allergan website.
FDA proposes new breast implant labeling with a boxed warning
Breast implants sold in the United States may soon require a boxed warning in their label, along with other label changes proposed by the Food and Drug Administration aimed at better informing prospective patients and clinicians of the potential risks from breast implants.
Other elements of the proposed labeling changes include creation of a patient-decision checklist, new recommendations for follow-up imaging to monitor for implant rupture, inclusion of detailed and understandable information about materials in the device, and provision of a device card to each patient with details on the specific implant they received.
These labeling changes all stemmed from a breast implant hearing held by the agency’s General and Plastic Surgery Devices Panel in March 2019, according to the draft guidance document officially released by the FDA on Oct. 24.
The proposed labeling changes were generally welcomed by patient advocates and by clinicians as a reasonable response to the concerns discussed at the March hearing. In an earlier move to address issues brought up at the hearing, the FDA in July arranged for a recall for certain Allergan models of textured breast implants because of their link with the development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).
The boxed warning proposed by the FDA would highlight four specific facts that patients, physicians, and surgeons should know about breast implants: They are not considered lifetime devices, the chance of developing complications from implants increases over time, some complications require additional surgery, and placement of breast implants has been associated with development of BIA-ALCL and may also be associated with certain systemic symptoms.
The FDA also proposed four other notable labeling changes:
- Creation of a patient-decision checklist to better systematize the informed consent process and make sure that certain aspects of breast implant placement are clearly brought to patients’ attention. The FDA proposed that patients sign their checklist attesting to having read and understood the information and that patients receive a take-home copy for their future reference. Proposed elements of the checklist include situations to not use breast implants; considerations for successful implant recipients; the risks of breast implant surgery; the importance of appropriate physician education, training, and experience; the risk for developing BIA-ALCL or systemic symptoms; and discussion of options other than breast implants.
- A new scheme for systematically and serially using imaging to screen for implant rupture that designates for the first time that ultrasound is an acceptable alternative to MRI and relies on a schedule by which either method initially screens the implant 5-6 years post operatively and then every 2 years thereafter.
- Detailed and understandable information about each material component of the implant with further information on possible adverse health effects of these compounds.
- A device card that patients should receive after their surgery with the implant’s name, serial number, and other identifiers; the boxed warning information; and a web link for accessing more up-to-date information.
The patient group Breast Implant Victim Advocacy praised the draft guidance. “The March Advisory Committee meeting seems to have prompted a shift by the FDA, surgeons, and industry,” said Jamee Cook, cofounder of the group. “We are definitely seeing a change in patient engagement. The FDA has been cooperating with patients and listening to our concerns. We still have a long way to go in raising public awareness of breast implant issues, but progress over the past 1-2 years has been amazing.”
Diana Zuckerman, PhD, president of the National Center for Health Research in Washington, gave the draft guidance a mixed review. “The FDA’s draft includes the types of information that we had proposed to the FDA in recent months in our work with patient advocates and plastic surgeons,” she said. “However, it is not as informative as it should be in describing well-designed studies indicating a risk of systemic illnesses. Patients deserve to make better-informed decisions in the future than most women considering breast implants have been able to make” in the past.
Patricia McGuire, MD, a St. Louis plastic surgeon who specializes in breast surgery and has studied breast implant illness, declared the guidance to be “reasonable.”
“I think the changes address the concerns expressed by patients during the [March] hearing; I agree with everything the FDA proposed in the guidance document,” Dr. McGuire said. “The boxed warning is reasonable and needs to be part of the informed consent process. I also agree with the changes in screening implants postoperatively. Most patients do not get MRI examinations. High-resolution ultrasound is more convenient and cost effective.”
The boxed warning was rated as “reasonably strong” and “the most serious step the FDA can take short of taking a device off the market,” but in the case of breast implants, a wider recall of textured implants than what the FDA arranged last July would be even more appropriate, commented Sidney M. Wolfe, MD, founder and senior adviser to Public Citizen. He also faulted the agency for not taking quicker action in mandating inclusion of the proposed boxed warning.
Issuing the labeling changes as draft guidance “is a ministep forward,” but also a process that “guarantees delay” and “creeps along at a dangerously slow pace,” Dr. Wolfe said. “The FDA is delaying what should be inevitable. The agency could put the boxed warning in place right now if they had the guts to do it.”
Dr. McGuire has been a consultant to Allergan, Establishment Labs, and Hans Biomed. Ms. Cook, Dr. Zuckerman, and Dr. Wolfe reported having no commercial disclosures.
Breast implants sold in the United States may soon require a boxed warning in their label, along with other label changes proposed by the Food and Drug Administration aimed at better informing prospective patients and clinicians of the potential risks from breast implants.
Other elements of the proposed labeling changes include creation of a patient-decision checklist, new recommendations for follow-up imaging to monitor for implant rupture, inclusion of detailed and understandable information about materials in the device, and provision of a device card to each patient with details on the specific implant they received.
These labeling changes all stemmed from a breast implant hearing held by the agency’s General and Plastic Surgery Devices Panel in March 2019, according to the draft guidance document officially released by the FDA on Oct. 24.
The proposed labeling changes were generally welcomed by patient advocates and by clinicians as a reasonable response to the concerns discussed at the March hearing. In an earlier move to address issues brought up at the hearing, the FDA in July arranged for a recall for certain Allergan models of textured breast implants because of their link with the development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).
The boxed warning proposed by the FDA would highlight four specific facts that patients, physicians, and surgeons should know about breast implants: They are not considered lifetime devices, the chance of developing complications from implants increases over time, some complications require additional surgery, and placement of breast implants has been associated with development of BIA-ALCL and may also be associated with certain systemic symptoms.
The FDA also proposed four other notable labeling changes:
- Creation of a patient-decision checklist to better systematize the informed consent process and make sure that certain aspects of breast implant placement are clearly brought to patients’ attention. The FDA proposed that patients sign their checklist attesting to having read and understood the information and that patients receive a take-home copy for their future reference. Proposed elements of the checklist include situations to not use breast implants; considerations for successful implant recipients; the risks of breast implant surgery; the importance of appropriate physician education, training, and experience; the risk for developing BIA-ALCL or systemic symptoms; and discussion of options other than breast implants.
- A new scheme for systematically and serially using imaging to screen for implant rupture that designates for the first time that ultrasound is an acceptable alternative to MRI and relies on a schedule by which either method initially screens the implant 5-6 years post operatively and then every 2 years thereafter.
- Detailed and understandable information about each material component of the implant with further information on possible adverse health effects of these compounds.
- A device card that patients should receive after their surgery with the implant’s name, serial number, and other identifiers; the boxed warning information; and a web link for accessing more up-to-date information.
The patient group Breast Implant Victim Advocacy praised the draft guidance. “The March Advisory Committee meeting seems to have prompted a shift by the FDA, surgeons, and industry,” said Jamee Cook, cofounder of the group. “We are definitely seeing a change in patient engagement. The FDA has been cooperating with patients and listening to our concerns. We still have a long way to go in raising public awareness of breast implant issues, but progress over the past 1-2 years has been amazing.”
Diana Zuckerman, PhD, president of the National Center for Health Research in Washington, gave the draft guidance a mixed review. “The FDA’s draft includes the types of information that we had proposed to the FDA in recent months in our work with patient advocates and plastic surgeons,” she said. “However, it is not as informative as it should be in describing well-designed studies indicating a risk of systemic illnesses. Patients deserve to make better-informed decisions in the future than most women considering breast implants have been able to make” in the past.
Patricia McGuire, MD, a St. Louis plastic surgeon who specializes in breast surgery and has studied breast implant illness, declared the guidance to be “reasonable.”
“I think the changes address the concerns expressed by patients during the [March] hearing; I agree with everything the FDA proposed in the guidance document,” Dr. McGuire said. “The boxed warning is reasonable and needs to be part of the informed consent process. I also agree with the changes in screening implants postoperatively. Most patients do not get MRI examinations. High-resolution ultrasound is more convenient and cost effective.”
The boxed warning was rated as “reasonably strong” and “the most serious step the FDA can take short of taking a device off the market,” but in the case of breast implants, a wider recall of textured implants than what the FDA arranged last July would be even more appropriate, commented Sidney M. Wolfe, MD, founder and senior adviser to Public Citizen. He also faulted the agency for not taking quicker action in mandating inclusion of the proposed boxed warning.
Issuing the labeling changes as draft guidance “is a ministep forward,” but also a process that “guarantees delay” and “creeps along at a dangerously slow pace,” Dr. Wolfe said. “The FDA is delaying what should be inevitable. The agency could put the boxed warning in place right now if they had the guts to do it.”
Dr. McGuire has been a consultant to Allergan, Establishment Labs, and Hans Biomed. Ms. Cook, Dr. Zuckerman, and Dr. Wolfe reported having no commercial disclosures.
Breast implants sold in the United States may soon require a boxed warning in their label, along with other label changes proposed by the Food and Drug Administration aimed at better informing prospective patients and clinicians of the potential risks from breast implants.
Other elements of the proposed labeling changes include creation of a patient-decision checklist, new recommendations for follow-up imaging to monitor for implant rupture, inclusion of detailed and understandable information about materials in the device, and provision of a device card to each patient with details on the specific implant they received.
These labeling changes all stemmed from a breast implant hearing held by the agency’s General and Plastic Surgery Devices Panel in March 2019, according to the draft guidance document officially released by the FDA on Oct. 24.
The proposed labeling changes were generally welcomed by patient advocates and by clinicians as a reasonable response to the concerns discussed at the March hearing. In an earlier move to address issues brought up at the hearing, the FDA in July arranged for a recall for certain Allergan models of textured breast implants because of their link with the development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).
The boxed warning proposed by the FDA would highlight four specific facts that patients, physicians, and surgeons should know about breast implants: They are not considered lifetime devices, the chance of developing complications from implants increases over time, some complications require additional surgery, and placement of breast implants has been associated with development of BIA-ALCL and may also be associated with certain systemic symptoms.
The FDA also proposed four other notable labeling changes:
- Creation of a patient-decision checklist to better systematize the informed consent process and make sure that certain aspects of breast implant placement are clearly brought to patients’ attention. The FDA proposed that patients sign their checklist attesting to having read and understood the information and that patients receive a take-home copy for their future reference. Proposed elements of the checklist include situations to not use breast implants; considerations for successful implant recipients; the risks of breast implant surgery; the importance of appropriate physician education, training, and experience; the risk for developing BIA-ALCL or systemic symptoms; and discussion of options other than breast implants.
- A new scheme for systematically and serially using imaging to screen for implant rupture that designates for the first time that ultrasound is an acceptable alternative to MRI and relies on a schedule by which either method initially screens the implant 5-6 years post operatively and then every 2 years thereafter.
- Detailed and understandable information about each material component of the implant with further information on possible adverse health effects of these compounds.
- A device card that patients should receive after their surgery with the implant’s name, serial number, and other identifiers; the boxed warning information; and a web link for accessing more up-to-date information.
The patient group Breast Implant Victim Advocacy praised the draft guidance. “The March Advisory Committee meeting seems to have prompted a shift by the FDA, surgeons, and industry,” said Jamee Cook, cofounder of the group. “We are definitely seeing a change in patient engagement. The FDA has been cooperating with patients and listening to our concerns. We still have a long way to go in raising public awareness of breast implant issues, but progress over the past 1-2 years has been amazing.”
Diana Zuckerman, PhD, president of the National Center for Health Research in Washington, gave the draft guidance a mixed review. “The FDA’s draft includes the types of information that we had proposed to the FDA in recent months in our work with patient advocates and plastic surgeons,” she said. “However, it is not as informative as it should be in describing well-designed studies indicating a risk of systemic illnesses. Patients deserve to make better-informed decisions in the future than most women considering breast implants have been able to make” in the past.
Patricia McGuire, MD, a St. Louis plastic surgeon who specializes in breast surgery and has studied breast implant illness, declared the guidance to be “reasonable.”
“I think the changes address the concerns expressed by patients during the [March] hearing; I agree with everything the FDA proposed in the guidance document,” Dr. McGuire said. “The boxed warning is reasonable and needs to be part of the informed consent process. I also agree with the changes in screening implants postoperatively. Most patients do not get MRI examinations. High-resolution ultrasound is more convenient and cost effective.”
The boxed warning was rated as “reasonably strong” and “the most serious step the FDA can take short of taking a device off the market,” but in the case of breast implants, a wider recall of textured implants than what the FDA arranged last July would be even more appropriate, commented Sidney M. Wolfe, MD, founder and senior adviser to Public Citizen. He also faulted the agency for not taking quicker action in mandating inclusion of the proposed boxed warning.
Issuing the labeling changes as draft guidance “is a ministep forward,” but also a process that “guarantees delay” and “creeps along at a dangerously slow pace,” Dr. Wolfe said. “The FDA is delaying what should be inevitable. The agency could put the boxed warning in place right now if they had the guts to do it.”
Dr. McGuire has been a consultant to Allergan, Establishment Labs, and Hans Biomed. Ms. Cook, Dr. Zuckerman, and Dr. Wolfe reported having no commercial disclosures.
Acoustic pulse boosts laser tattoo removal
In tattoo removal, the impact of laser treatments in single office visits is limited because of the laser’s effects on the skin. Now, a new study suggests that a
“As a result,” the authors of the study wrote, “a lower total number of office visits will likely be required for complete tattoo removal leading to improved convenience and efficiency as well as increased satisfaction for both patients and clinicians.” The study, led by cosmetic surgeon Michael S. Kaminer, MD, of SkinCare Physicians in Chestnut Hill, Mass., appeared in Lasers in Surgery and Medicine.
In the study, he and his coauthors pointed out that tattoos are most frequently removed with short-pulse high-fluence lasers, such as a 1064-nm Nd:YAG Q‐switched (QS) laser. However, “the QS laser has a limited ability to affect the tattoo ink pigment particles in each treatment session due to shielding of the pigment particles caused by both the agglomeration of the pigment particles and laser‐induced epidermal and dermal vacuoles known as ‘whitening.’ ” Therefore, “use of the QS laser often requires 10 or more single‐pass office sessions to achieve acceptable fading results.”
The study evaluated whether the use of a rapid acoustic pulse (RAP) device could reduce the whitening effect by clearing vacuoles and make it possible to increase the number of potential laser passes. In the single-center, prospective trial, they treated 32 black-ink tattoos in 21 patients, dividing the tattoos into zones and treating them differently: One zone received at least three consecutive laser passes alternating with a minute of treatment with the RAP device, one received single-pass laser treatment, and one received no treatment.
Reviewers assessed the tattoos for fading at 12 weeks. Average percent fading was higher in the laser/RAP group, compared with the laser-only group (44% and 25%, respectively, P less than .01). The percentages of tattoos with more than 50% fading (38% vs. 9%, P less than .01) and more than 75% fading (22% vs. 3%, P less than .05) were also higher in the laser/RAP group, compared with the laser-only group.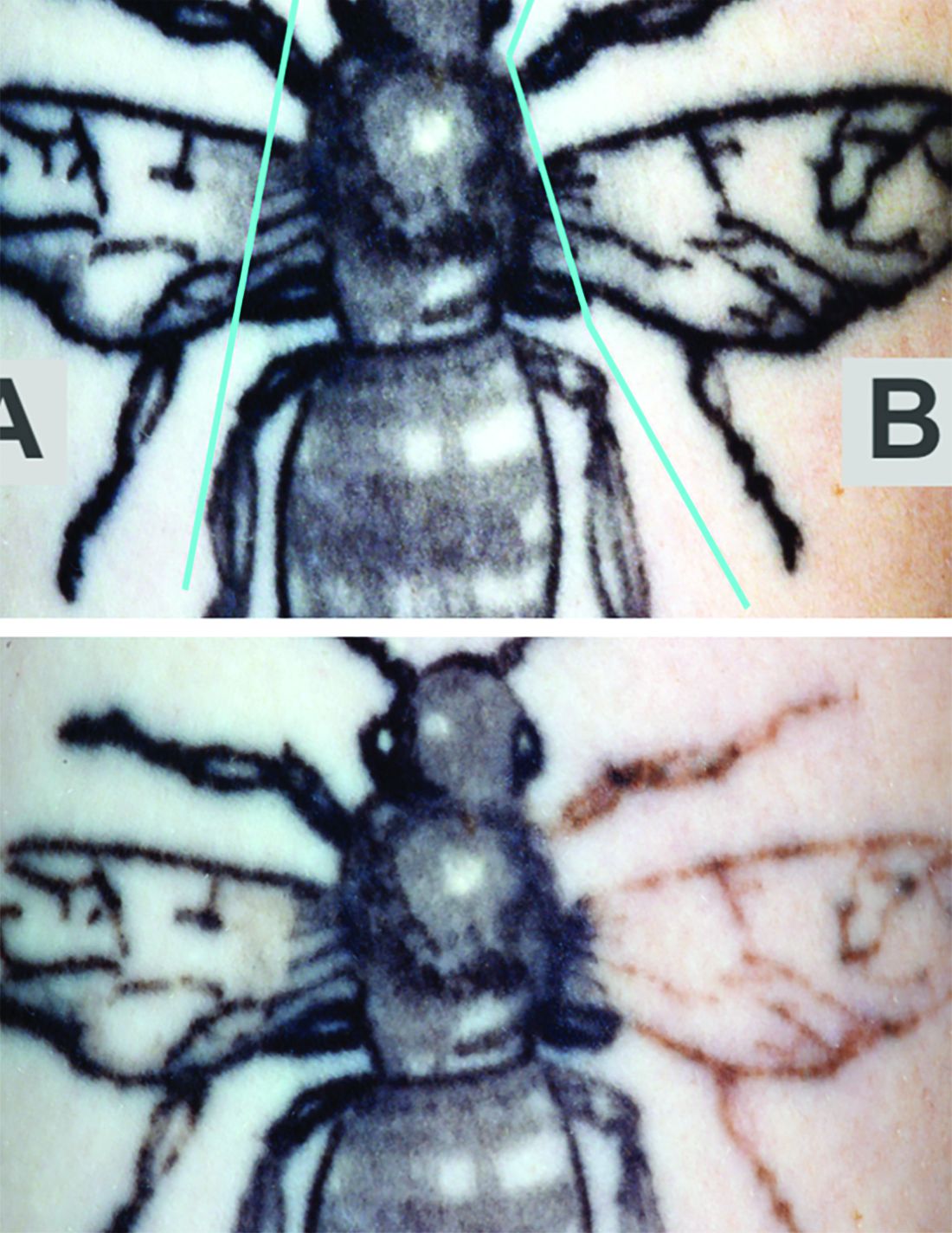
“Further clinical studies will be performed to investigate the broader applicability of the RAP device as an accessory device to reduce the number of laser tattoo removal sessions for other tattoo ink colors in a broader range of skin types,” the researchers commented, noting that the study included patients with Fitzpatrick skin types I-III.
The study was funded by Soliton, which provided the equipment. One of the six authors is an employee of the company The other authors reported no relevant disclosures.
SOURCE: Kaminer MS et al. Lasers Surg Med. 2019 Sep 19. doi: 10.1002/lsm.23163.
In tattoo removal, the impact of laser treatments in single office visits is limited because of the laser’s effects on the skin. Now, a new study suggests that a
“As a result,” the authors of the study wrote, “a lower total number of office visits will likely be required for complete tattoo removal leading to improved convenience and efficiency as well as increased satisfaction for both patients and clinicians.” The study, led by cosmetic surgeon Michael S. Kaminer, MD, of SkinCare Physicians in Chestnut Hill, Mass., appeared in Lasers in Surgery and Medicine.
In the study, he and his coauthors pointed out that tattoos are most frequently removed with short-pulse high-fluence lasers, such as a 1064-nm Nd:YAG Q‐switched (QS) laser. However, “the QS laser has a limited ability to affect the tattoo ink pigment particles in each treatment session due to shielding of the pigment particles caused by both the agglomeration of the pigment particles and laser‐induced epidermal and dermal vacuoles known as ‘whitening.’ ” Therefore, “use of the QS laser often requires 10 or more single‐pass office sessions to achieve acceptable fading results.”
The study evaluated whether the use of a rapid acoustic pulse (RAP) device could reduce the whitening effect by clearing vacuoles and make it possible to increase the number of potential laser passes. In the single-center, prospective trial, they treated 32 black-ink tattoos in 21 patients, dividing the tattoos into zones and treating them differently: One zone received at least three consecutive laser passes alternating with a minute of treatment with the RAP device, one received single-pass laser treatment, and one received no treatment.
Reviewers assessed the tattoos for fading at 12 weeks. Average percent fading was higher in the laser/RAP group, compared with the laser-only group (44% and 25%, respectively, P less than .01). The percentages of tattoos with more than 50% fading (38% vs. 9%, P less than .01) and more than 75% fading (22% vs. 3%, P less than .05) were also higher in the laser/RAP group, compared with the laser-only group.
“Further clinical studies will be performed to investigate the broader applicability of the RAP device as an accessory device to reduce the number of laser tattoo removal sessions for other tattoo ink colors in a broader range of skin types,” the researchers commented, noting that the study included patients with Fitzpatrick skin types I-III.
The study was funded by Soliton, which provided the equipment. One of the six authors is an employee of the company The other authors reported no relevant disclosures.
SOURCE: Kaminer MS et al. Lasers Surg Med. 2019 Sep 19. doi: 10.1002/lsm.23163.
In tattoo removal, the impact of laser treatments in single office visits is limited because of the laser’s effects on the skin. Now, a new study suggests that a
“As a result,” the authors of the study wrote, “a lower total number of office visits will likely be required for complete tattoo removal leading to improved convenience and efficiency as well as increased satisfaction for both patients and clinicians.” The study, led by cosmetic surgeon Michael S. Kaminer, MD, of SkinCare Physicians in Chestnut Hill, Mass., appeared in Lasers in Surgery and Medicine.
In the study, he and his coauthors pointed out that tattoos are most frequently removed with short-pulse high-fluence lasers, such as a 1064-nm Nd:YAG Q‐switched (QS) laser. However, “the QS laser has a limited ability to affect the tattoo ink pigment particles in each treatment session due to shielding of the pigment particles caused by both the agglomeration of the pigment particles and laser‐induced epidermal and dermal vacuoles known as ‘whitening.’ ” Therefore, “use of the QS laser often requires 10 or more single‐pass office sessions to achieve acceptable fading results.”
The study evaluated whether the use of a rapid acoustic pulse (RAP) device could reduce the whitening effect by clearing vacuoles and make it possible to increase the number of potential laser passes. In the single-center, prospective trial, they treated 32 black-ink tattoos in 21 patients, dividing the tattoos into zones and treating them differently: One zone received at least three consecutive laser passes alternating with a minute of treatment with the RAP device, one received single-pass laser treatment, and one received no treatment.
Reviewers assessed the tattoos for fading at 12 weeks. Average percent fading was higher in the laser/RAP group, compared with the laser-only group (44% and 25%, respectively, P less than .01). The percentages of tattoos with more than 50% fading (38% vs. 9%, P less than .01) and more than 75% fading (22% vs. 3%, P less than .05) were also higher in the laser/RAP group, compared with the laser-only group.
“Further clinical studies will be performed to investigate the broader applicability of the RAP device as an accessory device to reduce the number of laser tattoo removal sessions for other tattoo ink colors in a broader range of skin types,” the researchers commented, noting that the study included patients with Fitzpatrick skin types I-III.
The study was funded by Soliton, which provided the equipment. One of the six authors is an employee of the company The other authors reported no relevant disclosures.
SOURCE: Kaminer MS et al. Lasers Surg Med. 2019 Sep 19. doi: 10.1002/lsm.23163.
FROM LASERS IN SURGERY AND MEDICINE
Smoking impairs cognition and shrinks brain volume in MS
STOCKHOLM – Ebtesam Alshehri, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We found that smokers have reduced Processing Speed Test scores compared with nonsmokers. They also have more brain atrophy. And we found that former smokers had intermediate results between current smokers and never smokers, suggesting that there is a benefit of smoking cessation early in the course of the disease,” explained Dr. Alshehri, a clinical neuroimmunology fellow at the Cleveland Clinic.
Dr. Alshehri and colleagues presented a cross-sectional study of 997 patients with MS. At the Cleveland Clinic, patients with MS routinely undergo the Processing Speed Test (PST) – an electronic version of the Symbol Digit Modalities Test – at each clinical visit as a means of assessing cognitive function. All 997 patients also underwent quantitative brain MRI within 90 days of their cognitive function test. The study population consisted of 520 never smokers, 335 ex-smokers, and 142 current smokers. Seventy-seven percent of the patients had relapsing-remitting MS, and most of the rest had progressive disease.
The impetus for this hypothesis-generating study was a recognition that while smoking has previously been reported to be a risk factor for MS and has also been associated with accelerated progression of disability in patients who already have the disease, the impact of smoking on cognition and brain atrophy in the MS population has been a murky area, according to Dr. Alshehri.
In a multivariate analysis adjusted for age, disease duration, and disease course, smoking status was an independent predictor of PST score. Former smokers scored an average of 3.6 points lower than never smokers, and current smokers scored 5.9 points lower than the never smokers. Similarly, former smokers appeared better off than current smokers in terms of brain atrophy as measured by whole brain function on MRI: The ex-smokers had significantly greater brain atrophy than that of never smokers, an effect that was magnified in current smokers.
For Dr. Alshehri, these findings contain an important message for physicians: “I think as clinicians we should ask our patients with MS about tobacco use at each visit, remind patients about the risks of smoking and the negative impact not only on their general health, but also the impact on disease progression and disability worsening, encourage patients to stop smoking, and provide them with this encouraging data.”
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
STOCKHOLM – Ebtesam Alshehri, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We found that smokers have reduced Processing Speed Test scores compared with nonsmokers. They also have more brain atrophy. And we found that former smokers had intermediate results between current smokers and never smokers, suggesting that there is a benefit of smoking cessation early in the course of the disease,” explained Dr. Alshehri, a clinical neuroimmunology fellow at the Cleveland Clinic.
Dr. Alshehri and colleagues presented a cross-sectional study of 997 patients with MS. At the Cleveland Clinic, patients with MS routinely undergo the Processing Speed Test (PST) – an electronic version of the Symbol Digit Modalities Test – at each clinical visit as a means of assessing cognitive function. All 997 patients also underwent quantitative brain MRI within 90 days of their cognitive function test. The study population consisted of 520 never smokers, 335 ex-smokers, and 142 current smokers. Seventy-seven percent of the patients had relapsing-remitting MS, and most of the rest had progressive disease.
The impetus for this hypothesis-generating study was a recognition that while smoking has previously been reported to be a risk factor for MS and has also been associated with accelerated progression of disability in patients who already have the disease, the impact of smoking on cognition and brain atrophy in the MS population has been a murky area, according to Dr. Alshehri.
In a multivariate analysis adjusted for age, disease duration, and disease course, smoking status was an independent predictor of PST score. Former smokers scored an average of 3.6 points lower than never smokers, and current smokers scored 5.9 points lower than the never smokers. Similarly, former smokers appeared better off than current smokers in terms of brain atrophy as measured by whole brain function on MRI: The ex-smokers had significantly greater brain atrophy than that of never smokers, an effect that was magnified in current smokers.
For Dr. Alshehri, these findings contain an important message for physicians: “I think as clinicians we should ask our patients with MS about tobacco use at each visit, remind patients about the risks of smoking and the negative impact not only on their general health, but also the impact on disease progression and disability worsening, encourage patients to stop smoking, and provide them with this encouraging data.”
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
STOCKHOLM – Ebtesam Alshehri, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We found that smokers have reduced Processing Speed Test scores compared with nonsmokers. They also have more brain atrophy. And we found that former smokers had intermediate results between current smokers and never smokers, suggesting that there is a benefit of smoking cessation early in the course of the disease,” explained Dr. Alshehri, a clinical neuroimmunology fellow at the Cleveland Clinic.
Dr. Alshehri and colleagues presented a cross-sectional study of 997 patients with MS. At the Cleveland Clinic, patients with MS routinely undergo the Processing Speed Test (PST) – an electronic version of the Symbol Digit Modalities Test – at each clinical visit as a means of assessing cognitive function. All 997 patients also underwent quantitative brain MRI within 90 days of their cognitive function test. The study population consisted of 520 never smokers, 335 ex-smokers, and 142 current smokers. Seventy-seven percent of the patients had relapsing-remitting MS, and most of the rest had progressive disease.
The impetus for this hypothesis-generating study was a recognition that while smoking has previously been reported to be a risk factor for MS and has also been associated with accelerated progression of disability in patients who already have the disease, the impact of smoking on cognition and brain atrophy in the MS population has been a murky area, according to Dr. Alshehri.
In a multivariate analysis adjusted for age, disease duration, and disease course, smoking status was an independent predictor of PST score. Former smokers scored an average of 3.6 points lower than never smokers, and current smokers scored 5.9 points lower than the never smokers. Similarly, former smokers appeared better off than current smokers in terms of brain atrophy as measured by whole brain function on MRI: The ex-smokers had significantly greater brain atrophy than that of never smokers, an effect that was magnified in current smokers.
For Dr. Alshehri, these findings contain an important message for physicians: “I think as clinicians we should ask our patients with MS about tobacco use at each visit, remind patients about the risks of smoking and the negative impact not only on their general health, but also the impact on disease progression and disability worsening, encourage patients to stop smoking, and provide them with this encouraging data.”
She reported having no financial conflicts regarding this study, which was conducted free of commercial support.
REPORTING FROM ECTRIMS 2019
Vaping-linked injuries top 1,600 cases
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-four deaths have been confirmed.

E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Deaths have occurred in 24 states: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (2), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee, Texas, Utah, and Virginia. More deaths are under investigation.
The median age of deceased patients was 49 years and ranged from 17 to 75 years.
Data on age, sex, and substances used in e-cigarette, or vaping, products will be updated in the Morbidity and Mortality Weekly Report (MMWR) report being released on Friday, Oct. 25, 2019.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
REPORTING FROM THE CDC
SEER analysis reveals medication adherence factors in newly diagnosed myeloma
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
ACIP approves 2020 adult vaccination schedule
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
Policy help needed as rheumatology drug prices continue to rise
Rheumatology drugs, like most drugs, are seeing significant price hikes in recent years and, according to a new analysis, those hikes account for the majority of growth in spending.
From 2012 to 2016, annual spending on public-payer claims for 10 biologic disease-modifying antirheumatic drugs (bDMARDs) more than doubled (from $3.8 billion to $8.6 billion), with median drug price increases of 51% in the Medicare Part D prescription drug plans (mean, 54%) and 8% within Medicare Part B drugs administered within the physician office (mean, 21%).
“The prominent bDMARD drug cost increases observed in this study, which were substantially greater under Part D than Part B, even when accounting for rebates, represent a growing burden to taxpayers and beneficiaries,” first author Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital, Boston, and colleagues wrote in Arthritis & Rheumatology.
“The magnitude of these increases is independent from any assessment of value,” the authors continued.
Dr. McCormick and colleagues highlighted the limited effect that rebates negotiated by pharmacy benefit managers (PBMs) for Part D have on patient out-of-pocket costs.
Negotiations “may not necessarily result in lower price increases since pharmacy benefit managers, who negotiate on behalf of Part D plans, retain a percentage of the negotiated rebates as compensation, and higher list prices can achieve higher rebates,” they stated. “While these rebates accrue to insurance plans and pharmacy benefit managers, they do not directly impact Part D beneficiaries’ out-of-pocket costs, which are driven by prerebate prices. Thus, many patients end up paying higher out-of-pocket costs when the list price increases, creating financial barriers to use and adherence.”
They further noted that, for most Part D drugs individually, “drug prices accounted for approximately 50% or more of the increase in spending. Adalimumab and etanercept, two of the oldest bDMARDs, were prescribed to the largest number of Part D beneficiaries (more than 47,000 in 2016) and had the biggest 5-year price increases: 84% and 88%, respectively (72% and 75% respectively, accounting for rebates).”
It is not surprising that the analysis found that prices are rising and are a driving force in spending.
“We know that high launch prices of a new drug is an important consideration for patient access,” Jeromie Ballreich, PhD, a health economist at Johns Hopkins University, Baltimore, said in an interview. “But we also know that a number of these drugs, particularly Humira and Enbrel, have been on the market for a while. They have experienced a tremendous price increase over the past 10 years plus. That’s exactly what the authors found. So that is really not surprising. Analysis of drug spending changes over the time [shows that] it is indeed price increases that really have been driving increases in drug spending.”
Angus Worthing, MD, a rheumatologist at Arthritis & Rheumatism Associates in Washington, D.C., and current chair of the American College of Rheumatology’s Government Affairs Committee, said that the faster price hikes in Part D stood out in the analysis and the role PBMs played in it.
“I was a little surprised at just how much more PBMs were responsible for higher inflation, compared to the part B system,” he said in an interview. “In the McCormick article, drug prices went up 45% after rebates in the Part D space, but only 21% in the Part B space, and we had seen hints that drug prices were going up faster, we just didn’t know just how much faster, so more than double the increase is more than I expected, and it is very concerning to look at that and realize that PBMs are more than doubling an already high inflation rate in drug prices.”
He suggested that part of the rise in Part D bDMARD prices observed in the study might be because of the application of step therapy in Part D.
“In Medicare specifically, step therapy is allowed in Part D but not in Part B – except in Medicare Advantage plans starting in 2019 – and this important difference between Part B and Part D explains how Part D rebates, linked with step therapy, may be the main reason that bDMARD prices rose more in Part D compared to the open formulary system in Medicare Part B,” Dr. Worthing wrote in an editorial on Dr. McCormick’s study.
The Pharmaceutical Care Management Association, the lobby group representing PBMs, declined an interview request, but challenged the analysis’ portrayal of the role of PBMs in an email. It pointed to a Government Accountability Organization report from July 2019 that found that Part D rebates helped offset spending by about 20% and that PBM compensation was primarily derived from administrative fees and not from maintaining a portion of rebates that the PBM negotiated on behalf of the plan.
Dr. Worthing said in an interview that these bDMARD price hikes are causing access issues, particularly in Medicare where beneficiaries cannot take advantage of manufacturer programs, such as discount cards and copay coupons, and potentially face coinsurance payments based on the list price and not the net-of-rebate price.
From a policy standpoint, he advocated for a cap on out-of-pocket spending for Medicare Part D drugs, which is a feature of legislation (H.R. 3, Lower Drug Costs Now Act of 2019) that is moving through the House of Representatives, most recently passing the House Ways and Means Committee in a party-line vote of 24-17, with 1 Democrat not voting.
Dr. Worthing’s editorial covers a few other policy options, including more financial transparency in the role PBMs play in the drug supply chain, the elimination of rebates in favor of a flat fee for PBMs or requiring all rebates on drugs to be passed through to Part D beneficiaries, penalties on manufacturers for price increases that exceed inflation, and usage of a price index based on what foreign countries pay for the same drug.
Many of the suggestions from Dr. Worthing’s editorial are in H.R. 3 and face an uphill battle to get through the Senate.
Dr. Worthing said that tackling the rebate issue by removing incentives for manufacturers to raise prices in order to offer higher rebates and changing step-therapy rules are things Congress could probably get accomplished as it heads into an election year, but there is a chance it could be even more comprehensive.
“With such an important issue like high drug prices, it probably benefits both parties, and everybody facing an election will be able to go back to their constituents and say, ‘Look we got something done. I was part of this,’ and there is a better chance they will do it if they hear about it from their constituents,” he said.
Dr. Ballreich was less optimistic that H.R. 3 is going to be able to survive.
“The interesting thing with this bill is that a number of pieces of it have been endorsed by President Trump, and the question is, [is] that a meaningful endorsement? Is the Trump administration going to want to use the this plan as a win for him, as a win for showing potential voters that he can work in a bipartisan way, [that] he could take on ‘big drug companies’ – all those successful soundbites? Can then Trump use his power to push this bill through the Senate? I think that is a question mark,” he said, noting that it could take a lot of political capital to counter the powerful industry lobby. Ultimately, he expects that, if drug pricing does make it to the White House, it will go through some changes from where H.R. 3 is right now.
“I don’t think it will pass in its current iteration,” he said.
Each author of Dr. McCormick’s report had funding from different sources, though the funders had no role in the design and conduct of the study. No other disclosures were made.
SOURCES: McCormick N et al. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/ART.41138; Worthing A. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/art.41135.
Rheumatology drugs, like most drugs, are seeing significant price hikes in recent years and, according to a new analysis, those hikes account for the majority of growth in spending.
From 2012 to 2016, annual spending on public-payer claims for 10 biologic disease-modifying antirheumatic drugs (bDMARDs) more than doubled (from $3.8 billion to $8.6 billion), with median drug price increases of 51% in the Medicare Part D prescription drug plans (mean, 54%) and 8% within Medicare Part B drugs administered within the physician office (mean, 21%).
“The prominent bDMARD drug cost increases observed in this study, which were substantially greater under Part D than Part B, even when accounting for rebates, represent a growing burden to taxpayers and beneficiaries,” first author Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital, Boston, and colleagues wrote in Arthritis & Rheumatology.
“The magnitude of these increases is independent from any assessment of value,” the authors continued.
Dr. McCormick and colleagues highlighted the limited effect that rebates negotiated by pharmacy benefit managers (PBMs) for Part D have on patient out-of-pocket costs.
Negotiations “may not necessarily result in lower price increases since pharmacy benefit managers, who negotiate on behalf of Part D plans, retain a percentage of the negotiated rebates as compensation, and higher list prices can achieve higher rebates,” they stated. “While these rebates accrue to insurance plans and pharmacy benefit managers, they do not directly impact Part D beneficiaries’ out-of-pocket costs, which are driven by prerebate prices. Thus, many patients end up paying higher out-of-pocket costs when the list price increases, creating financial barriers to use and adherence.”
They further noted that, for most Part D drugs individually, “drug prices accounted for approximately 50% or more of the increase in spending. Adalimumab and etanercept, two of the oldest bDMARDs, were prescribed to the largest number of Part D beneficiaries (more than 47,000 in 2016) and had the biggest 5-year price increases: 84% and 88%, respectively (72% and 75% respectively, accounting for rebates).”
It is not surprising that the analysis found that prices are rising and are a driving force in spending.
“We know that high launch prices of a new drug is an important consideration for patient access,” Jeromie Ballreich, PhD, a health economist at Johns Hopkins University, Baltimore, said in an interview. “But we also know that a number of these drugs, particularly Humira and Enbrel, have been on the market for a while. They have experienced a tremendous price increase over the past 10 years plus. That’s exactly what the authors found. So that is really not surprising. Analysis of drug spending changes over the time [shows that] it is indeed price increases that really have been driving increases in drug spending.”
Angus Worthing, MD, a rheumatologist at Arthritis & Rheumatism Associates in Washington, D.C., and current chair of the American College of Rheumatology’s Government Affairs Committee, said that the faster price hikes in Part D stood out in the analysis and the role PBMs played in it.
“I was a little surprised at just how much more PBMs were responsible for higher inflation, compared to the part B system,” he said in an interview. “In the McCormick article, drug prices went up 45% after rebates in the Part D space, but only 21% in the Part B space, and we had seen hints that drug prices were going up faster, we just didn’t know just how much faster, so more than double the increase is more than I expected, and it is very concerning to look at that and realize that PBMs are more than doubling an already high inflation rate in drug prices.”
He suggested that part of the rise in Part D bDMARD prices observed in the study might be because of the application of step therapy in Part D.
“In Medicare specifically, step therapy is allowed in Part D but not in Part B – except in Medicare Advantage plans starting in 2019 – and this important difference between Part B and Part D explains how Part D rebates, linked with step therapy, may be the main reason that bDMARD prices rose more in Part D compared to the open formulary system in Medicare Part B,” Dr. Worthing wrote in an editorial on Dr. McCormick’s study.
The Pharmaceutical Care Management Association, the lobby group representing PBMs, declined an interview request, but challenged the analysis’ portrayal of the role of PBMs in an email. It pointed to a Government Accountability Organization report from July 2019 that found that Part D rebates helped offset spending by about 20% and that PBM compensation was primarily derived from administrative fees and not from maintaining a portion of rebates that the PBM negotiated on behalf of the plan.
Dr. Worthing said in an interview that these bDMARD price hikes are causing access issues, particularly in Medicare where beneficiaries cannot take advantage of manufacturer programs, such as discount cards and copay coupons, and potentially face coinsurance payments based on the list price and not the net-of-rebate price.
From a policy standpoint, he advocated for a cap on out-of-pocket spending for Medicare Part D drugs, which is a feature of legislation (H.R. 3, Lower Drug Costs Now Act of 2019) that is moving through the House of Representatives, most recently passing the House Ways and Means Committee in a party-line vote of 24-17, with 1 Democrat not voting.
Dr. Worthing’s editorial covers a few other policy options, including more financial transparency in the role PBMs play in the drug supply chain, the elimination of rebates in favor of a flat fee for PBMs or requiring all rebates on drugs to be passed through to Part D beneficiaries, penalties on manufacturers for price increases that exceed inflation, and usage of a price index based on what foreign countries pay for the same drug.
Many of the suggestions from Dr. Worthing’s editorial are in H.R. 3 and face an uphill battle to get through the Senate.
Dr. Worthing said that tackling the rebate issue by removing incentives for manufacturers to raise prices in order to offer higher rebates and changing step-therapy rules are things Congress could probably get accomplished as it heads into an election year, but there is a chance it could be even more comprehensive.
“With such an important issue like high drug prices, it probably benefits both parties, and everybody facing an election will be able to go back to their constituents and say, ‘Look we got something done. I was part of this,’ and there is a better chance they will do it if they hear about it from their constituents,” he said.
Dr. Ballreich was less optimistic that H.R. 3 is going to be able to survive.
“The interesting thing with this bill is that a number of pieces of it have been endorsed by President Trump, and the question is, [is] that a meaningful endorsement? Is the Trump administration going to want to use the this plan as a win for him, as a win for showing potential voters that he can work in a bipartisan way, [that] he could take on ‘big drug companies’ – all those successful soundbites? Can then Trump use his power to push this bill through the Senate? I think that is a question mark,” he said, noting that it could take a lot of political capital to counter the powerful industry lobby. Ultimately, he expects that, if drug pricing does make it to the White House, it will go through some changes from where H.R. 3 is right now.
“I don’t think it will pass in its current iteration,” he said.
Each author of Dr. McCormick’s report had funding from different sources, though the funders had no role in the design and conduct of the study. No other disclosures were made.
SOURCES: McCormick N et al. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/ART.41138; Worthing A. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/art.41135.
Rheumatology drugs, like most drugs, are seeing significant price hikes in recent years and, according to a new analysis, those hikes account for the majority of growth in spending.
From 2012 to 2016, annual spending on public-payer claims for 10 biologic disease-modifying antirheumatic drugs (bDMARDs) more than doubled (from $3.8 billion to $8.6 billion), with median drug price increases of 51% in the Medicare Part D prescription drug plans (mean, 54%) and 8% within Medicare Part B drugs administered within the physician office (mean, 21%).
“The prominent bDMARD drug cost increases observed in this study, which were substantially greater under Part D than Part B, even when accounting for rebates, represent a growing burden to taxpayers and beneficiaries,” first author Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital, Boston, and colleagues wrote in Arthritis & Rheumatology.
“The magnitude of these increases is independent from any assessment of value,” the authors continued.
Dr. McCormick and colleagues highlighted the limited effect that rebates negotiated by pharmacy benefit managers (PBMs) for Part D have on patient out-of-pocket costs.
Negotiations “may not necessarily result in lower price increases since pharmacy benefit managers, who negotiate on behalf of Part D plans, retain a percentage of the negotiated rebates as compensation, and higher list prices can achieve higher rebates,” they stated. “While these rebates accrue to insurance plans and pharmacy benefit managers, they do not directly impact Part D beneficiaries’ out-of-pocket costs, which are driven by prerebate prices. Thus, many patients end up paying higher out-of-pocket costs when the list price increases, creating financial barriers to use and adherence.”
They further noted that, for most Part D drugs individually, “drug prices accounted for approximately 50% or more of the increase in spending. Adalimumab and etanercept, two of the oldest bDMARDs, were prescribed to the largest number of Part D beneficiaries (more than 47,000 in 2016) and had the biggest 5-year price increases: 84% and 88%, respectively (72% and 75% respectively, accounting for rebates).”
It is not surprising that the analysis found that prices are rising and are a driving force in spending.
“We know that high launch prices of a new drug is an important consideration for patient access,” Jeromie Ballreich, PhD, a health economist at Johns Hopkins University, Baltimore, said in an interview. “But we also know that a number of these drugs, particularly Humira and Enbrel, have been on the market for a while. They have experienced a tremendous price increase over the past 10 years plus. That’s exactly what the authors found. So that is really not surprising. Analysis of drug spending changes over the time [shows that] it is indeed price increases that really have been driving increases in drug spending.”
Angus Worthing, MD, a rheumatologist at Arthritis & Rheumatism Associates in Washington, D.C., and current chair of the American College of Rheumatology’s Government Affairs Committee, said that the faster price hikes in Part D stood out in the analysis and the role PBMs played in it.
“I was a little surprised at just how much more PBMs were responsible for higher inflation, compared to the part B system,” he said in an interview. “In the McCormick article, drug prices went up 45% after rebates in the Part D space, but only 21% in the Part B space, and we had seen hints that drug prices were going up faster, we just didn’t know just how much faster, so more than double the increase is more than I expected, and it is very concerning to look at that and realize that PBMs are more than doubling an already high inflation rate in drug prices.”
He suggested that part of the rise in Part D bDMARD prices observed in the study might be because of the application of step therapy in Part D.
“In Medicare specifically, step therapy is allowed in Part D but not in Part B – except in Medicare Advantage plans starting in 2019 – and this important difference between Part B and Part D explains how Part D rebates, linked with step therapy, may be the main reason that bDMARD prices rose more in Part D compared to the open formulary system in Medicare Part B,” Dr. Worthing wrote in an editorial on Dr. McCormick’s study.
The Pharmaceutical Care Management Association, the lobby group representing PBMs, declined an interview request, but challenged the analysis’ portrayal of the role of PBMs in an email. It pointed to a Government Accountability Organization report from July 2019 that found that Part D rebates helped offset spending by about 20% and that PBM compensation was primarily derived from administrative fees and not from maintaining a portion of rebates that the PBM negotiated on behalf of the plan.
Dr. Worthing said in an interview that these bDMARD price hikes are causing access issues, particularly in Medicare where beneficiaries cannot take advantage of manufacturer programs, such as discount cards and copay coupons, and potentially face coinsurance payments based on the list price and not the net-of-rebate price.
From a policy standpoint, he advocated for a cap on out-of-pocket spending for Medicare Part D drugs, which is a feature of legislation (H.R. 3, Lower Drug Costs Now Act of 2019) that is moving through the House of Representatives, most recently passing the House Ways and Means Committee in a party-line vote of 24-17, with 1 Democrat not voting.
Dr. Worthing’s editorial covers a few other policy options, including more financial transparency in the role PBMs play in the drug supply chain, the elimination of rebates in favor of a flat fee for PBMs or requiring all rebates on drugs to be passed through to Part D beneficiaries, penalties on manufacturers for price increases that exceed inflation, and usage of a price index based on what foreign countries pay for the same drug.
Many of the suggestions from Dr. Worthing’s editorial are in H.R. 3 and face an uphill battle to get through the Senate.
Dr. Worthing said that tackling the rebate issue by removing incentives for manufacturers to raise prices in order to offer higher rebates and changing step-therapy rules are things Congress could probably get accomplished as it heads into an election year, but there is a chance it could be even more comprehensive.
“With such an important issue like high drug prices, it probably benefits both parties, and everybody facing an election will be able to go back to their constituents and say, ‘Look we got something done. I was part of this,’ and there is a better chance they will do it if they hear about it from their constituents,” he said.
Dr. Ballreich was less optimistic that H.R. 3 is going to be able to survive.
“The interesting thing with this bill is that a number of pieces of it have been endorsed by President Trump, and the question is, [is] that a meaningful endorsement? Is the Trump administration going to want to use the this plan as a win for him, as a win for showing potential voters that he can work in a bipartisan way, [that] he could take on ‘big drug companies’ – all those successful soundbites? Can then Trump use his power to push this bill through the Senate? I think that is a question mark,” he said, noting that it could take a lot of political capital to counter the powerful industry lobby. Ultimately, he expects that, if drug pricing does make it to the White House, it will go through some changes from where H.R. 3 is right now.
“I don’t think it will pass in its current iteration,” he said.
Each author of Dr. McCormick’s report had funding from different sources, though the funders had no role in the design and conduct of the study. No other disclosures were made.
SOURCES: McCormick N et al. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/ART.41138; Worthing A. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/art.41135.
FROM ARTHRITIS & RHEUMATOLOGY