User login
Systemic Epstein-Barr Virus–Positive T-cell Lymphoma of Childhood
Case Report
A 7-year-old Chinese boy presented with multiple painful oral and tongue ulcers of 2 weeks’ duration as well as acute onset of moderate to high fever (highest temperature, 39.3°C) for 5 days. The fever was reported to have run a relapsing course, accompanied by rigors but without convulsions or cognitive changes. At times, the patient had nasal congestion, nasal discharge, and cough. He also had a transient eruption on the back and hands as well as an indurated red nodule on the left forearm.
Before the patient was hospitalized, antibiotic therapy was administered by other physicians, but the condition of fever and oral ulcers did not improve. After the patient was hospitalized, new tender nodules emerged on the scalp, buttocks, and lower extremities. New ulcers also appeared on the palate.
History
Two months earlier, the patient had presented with a painful perioral skin ulcer that resolved after being treated as contagious eczema. Another dermatologist previously had considered a diagnosis of hand-foot-and-mouth disease.
The patient was born by normal spontaneous vaginal delivery, without abnormality. He was breastfed; feeding, growth, and the developmental history showed no abnormality. He was the family’s eldest child, with a healthy brother and sister. There was no history of familial illness. He received bacillus Calmette-Guérin and poliomyelitis vaccines after birth; the rest of the vaccine history was unclear. There was no history of immunologic abnormality.
Physical Examination
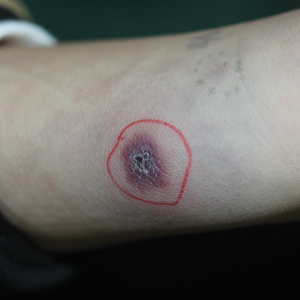
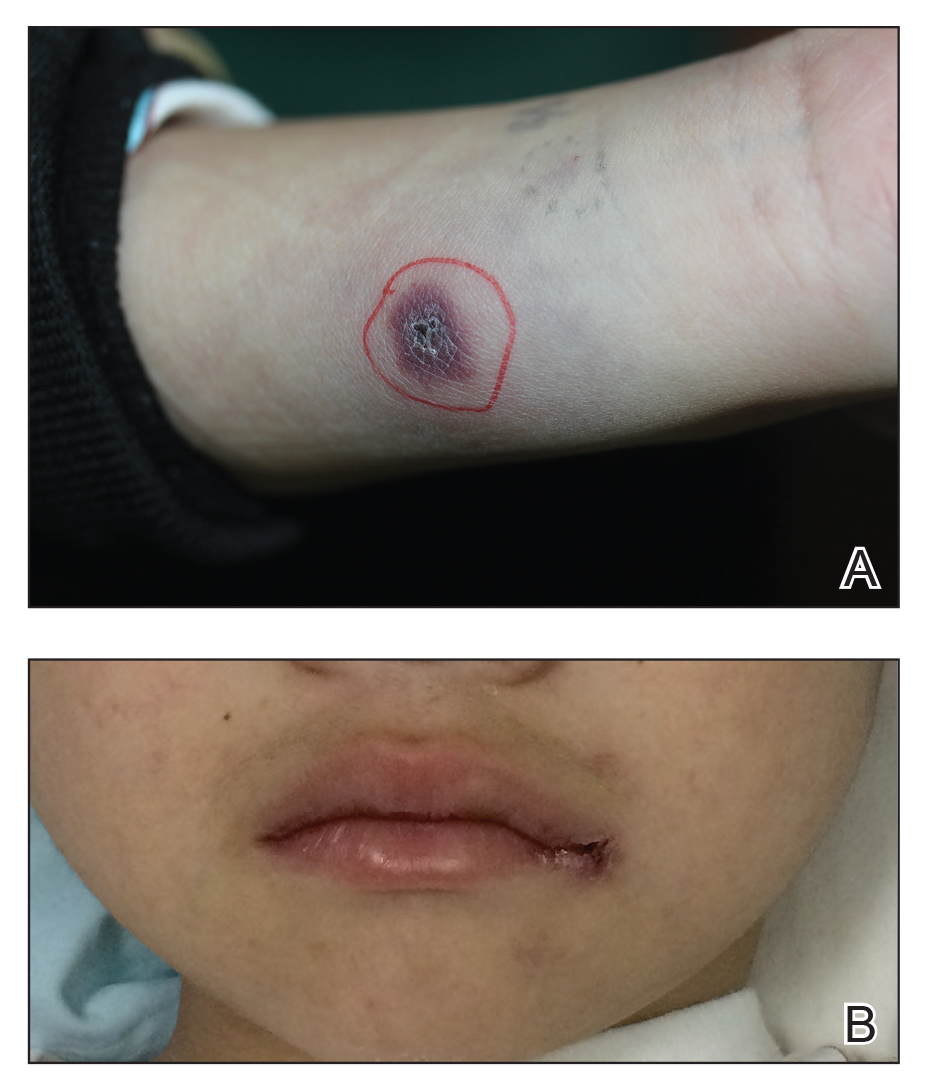
A 1.5×1.5-cm, warm, red nodule with a central black crust was noted on the left forearm (Figure 1A). Several similar lesions were noted on the buttocks, scalp, and lower extremities. Multiple ulcers, as large as 1 cm, were present on the tongue, palate, and left angle of the mouth (Figure 1B). The pharynx was congested, and the tonsils were mildly enlarged. Multiple enlarged, movable, nontender lymph nodes could be palpated in the cervical basins, axillae, and groin. No purpura or ecchymosis was detected.
Laboratory Results
Laboratory testing revealed a normal total white blood cell count (4.26×109/L [reference range, 4.0–12.0×109/L]), with normal neutrophils (1.36×109/L [reference range, 1.32–7.90×109/L]), lymphocytes (2.77×109/L [reference range, 1.20–6.00×109/L]), and monocytes (0.13×109/L [reference range, 0.08–0.80×109/L]); a mildly decreased hemoglobin level (115 g/L [reference range, 120–160 g/L]); a normal platelet count (102×109/L [reference range, 100–380×109/L]); an elevated lactate dehydrogenase level (614 U/L [reference range, 110–330 U/L]); an elevated α-hydroxybutyrate dehydrogenase level (483 U/L [reference range, 120–270 U/L]); elevated prothrombin time (15.3 s [reference range, 9–14 s]); elevated activated partial thromboplastin time (59.8 s [reference range, 20.6–39.6 s]); and an elevated D-dimer level (1.51 mg/L [reference range, <0.73 mg/L]). In addition, autoantibody testing revealed a positive antinuclear antibody titer of 1:320 and a strong positive anti–Ro-52 level.
The peripheral blood lymphocyte classification demonstrated a prominent elevated percentage of T lymphocytes, with predominantly CD8+ cells (CD3, 94.87%; CD8, 71.57%; CD4, 24.98%; CD4:CD8 ratio, 0.35) and a diminished percentage of B lymphocytes and natural killer (NK) cells. Epstein-Barr virus (EBV) antibody testing was positive for anti–viral capsid antigen (VCA) IgG and negative for anti-VCA IgM.
Smears of the ulcer on the tongue demonstrated gram-positive cocci, gram-negative bacilli, and diplococci. Culture of sputum showed methicillin-resistant Staphylococcus aureus. Inspection for acid-fast bacilli in sputum yielded negative results 3 times. A purified protein derivative skin test for Mycobacterium tuberculosis infection was negative.
Imaging and Other Studies
Computed tomography of the chest and abdomen demonstrated 2 nodular opacities on the lower right lung; spotted opacities on the upper right lung; floccular opacities on the rest area of the lung; mild pleural effusion; enlargement of lymph nodes on the mediastinum, the bilateral hilum of the lung, and mesentery; and hepatosplenomegaly. Electrocardiography showed sinus tachycardia. Nasal cavity endoscopy showed sinusitis. Fundus examination showed vasculopathy of the left retina. A colonoscopy showed normal mucosa.
Histopathology
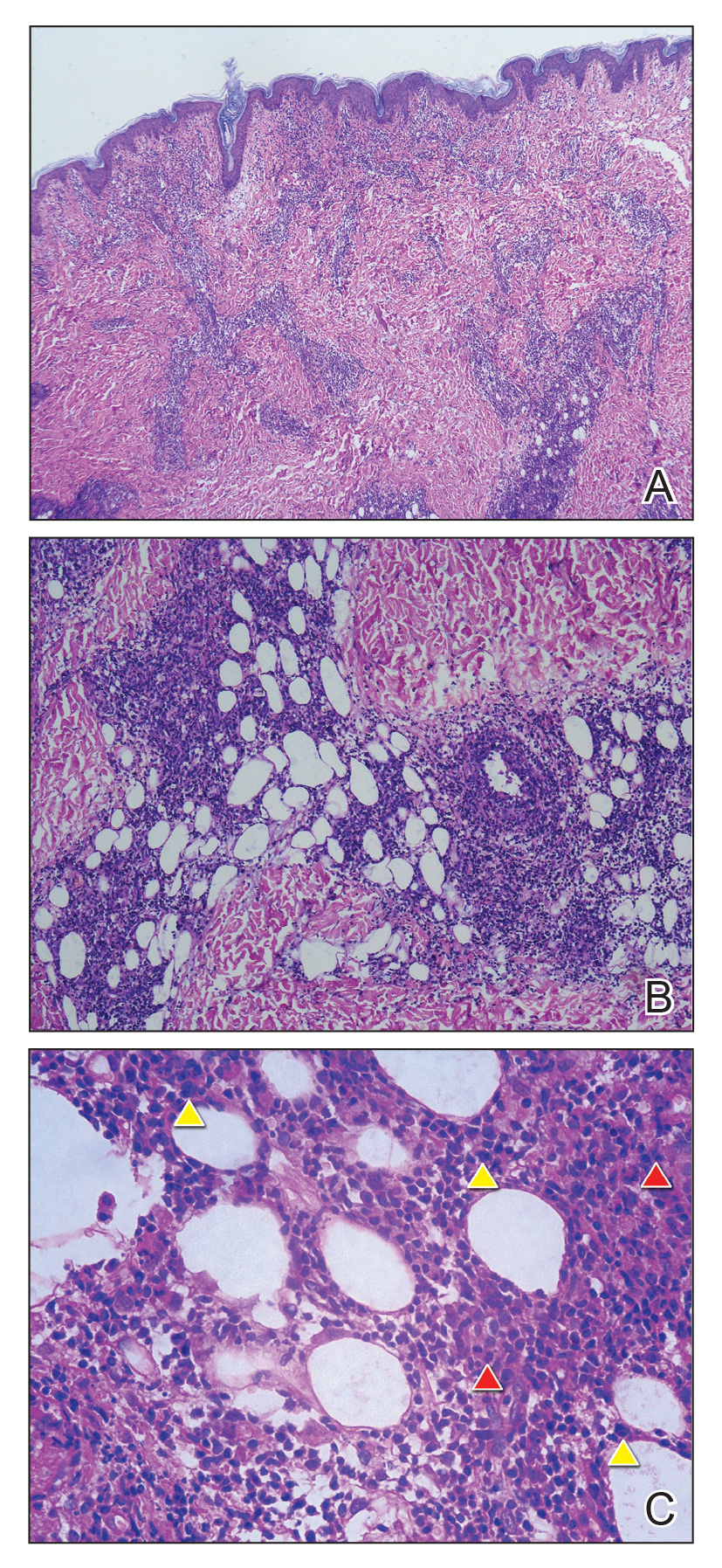
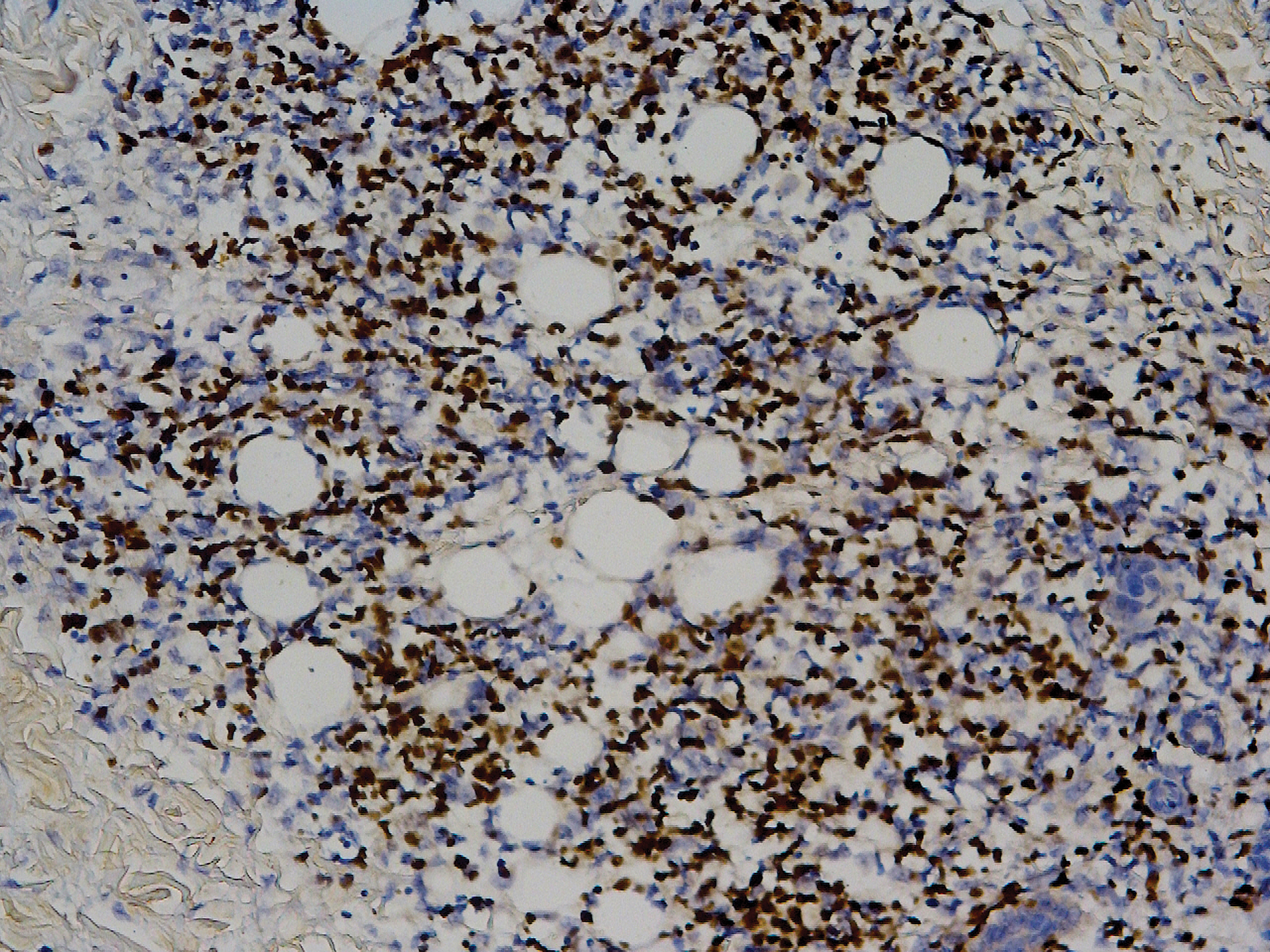
Biopsy of the nodule on the left arm showed dense, superficial to deep perivascular, periadnexal, perineural, and panniculitislike lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells (Figure 2). Infiltrative atypical lymphoid cells expressed CD3 and CD7 and were mostly CD8+, with a few CD4+ cells and most cells negative for CD5, CD20, CD30, CD56, and anaplastic lymphoma kinase. Cytotoxic markers granzyme B and T-cell intracellular antigen protein 1 were scattered positive. Immunostaining for Ki-67 protein highlighted an increased proliferative rate of 80% in malignant cells. In situ hybridization for EBV-encoded RNA (EBER) demonstrated EBV-positive atypical lymphoid cells (Figure 3). Analysis for T-cell receptor (TCR) γ gene rearrangement revealed a monoclonal pattern. Bone marrow aspirate showed proliferation of the 3 cell lines. The percentage of T lymphocytes was increased (20% of all nucleated cells). No hemophagocytic activity was found.
Diagnosis
A diagnosis of systemic EBV-positive T-cell lymphoma was made. Before the final diagnosis was made, the patient was treated by rheumatologists with antibiotics, antiviral drugs, nonsteroidal anti-inflammatory drugs, and other symptomatic treatments. Following antibiotic therapy, a sputum culture reverted to normal flora, the coagulation index (ie, prothrombin time, activated partial thromboplastin time) returned to normal, and the D-dimer level decreased to 1.19 mg/L.
The patient’s parents refused to accept chemotherapy for him. Instead, they chose herbal therapy only; 5 months later, they reported that all of his symptoms had resolved; however, the disease suddenly relapsed after another 7 months, with multiple skin nodules and fever. The patient died, even with chemotherapy in another hospital.
Comment
Prevalence and Presentation
Epstein-Barr virus is a ubiquitous γ-herpesvirus with tropism for B cells, affecting more than 90% of the adult population worldwide. In addition to infecting B cells, EBV is capable of infecting T and NK cells, leading to various EBV-related lymphoproliferative disorders (LPDs). The frequency and clinical presentation of infection varies based on the type of EBV-infected cells and the state of host immunity.1-3
Primary infection usually is asymptomatic and occurs early in life; when symptomatic, the disease usually presents as infectious mononucleosis (IM), characterized by polyclonal expansion of infected B cells and subsequent cytotoxic T-cell response. A diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against VCA, early antigens, and EBV nuclear antigen proteins.3,4
Associated LPDs
Although most symptoms associated with IM resolve within weeks or months, persistent or recurrent IM-like symptoms or even lasting disease occasionally occur, particularly in children and young adults. This complication is known as chronic active EBV infection (CAEBV), frequently associated with EBV-infected T-cell or NK-cell proliferation, especially in East Asian populations.3,5
Epstein-Barr virus–positive T-cell and NK-cell LPDs of childhood include CAEBV infection of T-cell and NK-cell types and systemic EBV-positive T-cell lymphoma of childhood. The former includes hydroa vacciniforme–like LPD and severe mosquito bite allergy.3
Systemic EBV-Positive T-cell Lymphoma of Childhood
This entity occurs not only in children but also in adolescents and young adults. A fulminant illness characterized by clonal proliferation of EBV-infected cytotoxic T cells, it can develop shortly after primary EBV infection or is linked to CAEBV infection. The disorder is rare and has a racial predilection for Asian (ie, Japanese, Chinese, Korean) populations and indigenous populations of Mexico and Central and South America.6-8
Complications
Systemic EBV-positive T-cell lymphoma of childhood is often complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. Other signs and symptoms include high fever, rash, jaundice, diarrhea, pancytopenia, and hepatosplenomegaly. The liver, spleen, lymph nodes, and bone marrow are commonly involved, and the disease can involve skin, the heart, and the lungs.9,10
Diagnosis
When systemic EBV-positive T-cell lymphoma occurs shortly after IM, serology shows low or absent anti-VCA IgM and positive anti-VCA IgG. Infiltrating T cells usually are small and lack cytologic atypia; however, cases with pleomorphic, medium to large lymphoid cells, irregular nuclei, and frequent mitoses have been described. Hemophagocytosis can be seen in the liver, spleen, and bone marrow.3,11
The most typical phenotype of systemic EBV-positive T-cell lymphoma is CD2+CD3+CD8+CD20−CD56−, with expression of the cytotoxic granules known as T-cell intracellular antigen 1 and granzyme B. Rare cases of CD4+ and mixed CD4+/CD8+ phenotypes have been described, usually in the setting of CAEBV infection.3,12 Neoplastic cells have monoclonally rearranged TCR-γ genes and consistent EBER positivity with in situ hybridization.13 A final diagnosis is based on a comprehensive analysis of clinical, morphological, immunohistochemical, and molecular biological aspects.
Clinical Course and Prognosis
Most patients with systemic EBV-positive T-cell lymphoma have an aggressive clinical course with high mortality. In a few cases, patients were reported to respond to a regimen of etoposide and dexamethasone, followed by allogeneic hematopoietic stem cell transplantation.3
In recognition of the aggressive clinical behavior and desire to clearly distinguish systemic EBV-positive T-cell lymphoma from CAEBV infection, the older term systemic EBV-positive T-cell LPD of childhood, which had been introduced in 2008 to the World Health Organization classification, was changed to systemic EBV-positive T-cell lymphoma of childhood in the revised 2016 World Health Organization classification.6,12 However, Kim et al14 reported a case with excellent response to corticosteroid administration, suggesting that systemic EBV-positive T-cell lymphoma of childhood may be more heterogeneous in terms of prognosis.
Our patient presented with acute IM-like symptoms, including high fever, tonsillar enlargement, lymphadenopathy, and hepatosplenomegaly, as well as uncommon oral ulcers and skin lesions, including indurated nodules. Histopathologic changes in the skin nodule, proliferation in bone marrow, immunohistochemical phenotype, and positivity of EBER and TCR-γ monoclonal rearrangement were all consistent with systemic EBV-positive T-cell lymphoma of childhood. The patient was positive for VCA IgG and negative for VCA IgM, compatible with systemic EBV-positive T-cell lymphoma of childhood occurring shortly after IM. Neither pancytopenia, hemophagocytic syndrome, nor multiorgan failure occurred during the course.
Differential Diagnosis
It is important to distinguish IM from systemic EBV-positive T-cell lymphoma of childhood and CAEBV infection. Detection of anti–VCA IgM in the early stage, its disappearance during the clinical course, and appearance of anti-EBV–determined nuclear antigen is useful to distinguish IM from the neoplasms, as systemic EBV-positive T-cell lymphoma of childhood is negative for anti-EBV–determined nuclear antigen. Carefully following the clinical course also is important.3,15
Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis can occur in association with systemic EBV-positive T-cell lymphoma of childhood and might represent a continuum of disease rather than distinct entities.14 The most useful marker for differentiating EBV-associated hemophagocytic lymphohistiocytosis and systemic EBV-positive T-cell lymphoma of childhood is an abnormal karyotype rather than molecular clonality.16
Outcome
Mortality risk in EBV-associated T-cell and NK-cell LPD is not primarily dependent on whether the lesion has progressed to lymphoma but instead is related to associated complications.17
Conclusion
Although systemic EBV-positive T-cell lymphoma of childhood is a rare disorder and has race predilection, dermatologists should be aware due to the aggressive clinical source and poor prognosis. Histopathology and in situ hybridization for EBER and TCR gene rearrangements are critical for final diagnosis. Although rare cases can show temporary resolution, the final outcome of this disease is not optimistic.
- Ameli F, Ghafourian F, Masir N. Systematic Epstein-Barr virus-positive T-cell lymphoproliferative disease presenting as a persistent fever and cough: a case report. J Med Case Rep. 2014;8:288.
- Kim HJ, Ko YH, Kim JE, et al. Epstein-Barr virus-associated lympho-proliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51:352-358.
- Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts [published online March 7, 2018]. Pathogens. doi:10.3390/pathogens7010028.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
- Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71.
- Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137-147.
- Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443-451.
- Chen G, Chen L, Qin X, et al. Systemic Epstein-Barr virus positive T-cell lymphoproliferative disease of childhood with hemophagocytic syndrome. Int J Clin Exp Pathol. 2014;7:7110-7113.
- Grywalska E, Rolinski J. Epstein-Barr virus-associated lymphomas. Semin Oncol. 2015;42:291-303.
- Huang W, Lv N, Ying J, et al. Clinicopathological characteristics of four cases of EBV positive T-cell lymphoproliferative disorders of childhood in China. Int J Clin Exp Pathol. 2014;7:4991-4999.
- Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011;5:218.
- Kim DH, Kim M, Kim Y, et al. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood with good response to steroid therapy. J Pediatr Hematol Oncol. 2017;39:e497-e500.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671-675.
- Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738-5749.
- Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53-63.
Case Report
A 7-year-old Chinese boy presented with multiple painful oral and tongue ulcers of 2 weeks’ duration as well as acute onset of moderate to high fever (highest temperature, 39.3°C) for 5 days. The fever was reported to have run a relapsing course, accompanied by rigors but without convulsions or cognitive changes. At times, the patient had nasal congestion, nasal discharge, and cough. He also had a transient eruption on the back and hands as well as an indurated red nodule on the left forearm.
Before the patient was hospitalized, antibiotic therapy was administered by other physicians, but the condition of fever and oral ulcers did not improve. After the patient was hospitalized, new tender nodules emerged on the scalp, buttocks, and lower extremities. New ulcers also appeared on the palate.
History
Two months earlier, the patient had presented with a painful perioral skin ulcer that resolved after being treated as contagious eczema. Another dermatologist previously had considered a diagnosis of hand-foot-and-mouth disease.
The patient was born by normal spontaneous vaginal delivery, without abnormality. He was breastfed; feeding, growth, and the developmental history showed no abnormality. He was the family’s eldest child, with a healthy brother and sister. There was no history of familial illness. He received bacillus Calmette-Guérin and poliomyelitis vaccines after birth; the rest of the vaccine history was unclear. There was no history of immunologic abnormality.
Physical Examination
A 1.5×1.5-cm, warm, red nodule with a central black crust was noted on the left forearm (Figure 1A). Several similar lesions were noted on the buttocks, scalp, and lower extremities. Multiple ulcers, as large as 1 cm, were present on the tongue, palate, and left angle of the mouth (Figure 1B). The pharynx was congested, and the tonsils were mildly enlarged. Multiple enlarged, movable, nontender lymph nodes could be palpated in the cervical basins, axillae, and groin. No purpura or ecchymosis was detected.

Laboratory Results
Laboratory testing revealed a normal total white blood cell count (4.26×109/L [reference range, 4.0–12.0×109/L]), with normal neutrophils (1.36×109/L [reference range, 1.32–7.90×109/L]), lymphocytes (2.77×109/L [reference range, 1.20–6.00×109/L]), and monocytes (0.13×109/L [reference range, 0.08–0.80×109/L]); a mildly decreased hemoglobin level (115 g/L [reference range, 120–160 g/L]); a normal platelet count (102×109/L [reference range, 100–380×109/L]); an elevated lactate dehydrogenase level (614 U/L [reference range, 110–330 U/L]); an elevated α-hydroxybutyrate dehydrogenase level (483 U/L [reference range, 120–270 U/L]); elevated prothrombin time (15.3 s [reference range, 9–14 s]); elevated activated partial thromboplastin time (59.8 s [reference range, 20.6–39.6 s]); and an elevated D-dimer level (1.51 mg/L [reference range, <0.73 mg/L]). In addition, autoantibody testing revealed a positive antinuclear antibody titer of 1:320 and a strong positive anti–Ro-52 level.
The peripheral blood lymphocyte classification demonstrated a prominent elevated percentage of T lymphocytes, with predominantly CD8+ cells (CD3, 94.87%; CD8, 71.57%; CD4, 24.98%; CD4:CD8 ratio, 0.35) and a diminished percentage of B lymphocytes and natural killer (NK) cells. Epstein-Barr virus (EBV) antibody testing was positive for anti–viral capsid antigen (VCA) IgG and negative for anti-VCA IgM.
Smears of the ulcer on the tongue demonstrated gram-positive cocci, gram-negative bacilli, and diplococci. Culture of sputum showed methicillin-resistant Staphylococcus aureus. Inspection for acid-fast bacilli in sputum yielded negative results 3 times. A purified protein derivative skin test for Mycobacterium tuberculosis infection was negative.
Imaging and Other Studies
Computed tomography of the chest and abdomen demonstrated 2 nodular opacities on the lower right lung; spotted opacities on the upper right lung; floccular opacities on the rest area of the lung; mild pleural effusion; enlargement of lymph nodes on the mediastinum, the bilateral hilum of the lung, and mesentery; and hepatosplenomegaly. Electrocardiography showed sinus tachycardia. Nasal cavity endoscopy showed sinusitis. Fundus examination showed vasculopathy of the left retina. A colonoscopy showed normal mucosa.
Histopathology
Biopsy of the nodule on the left arm showed dense, superficial to deep perivascular, periadnexal, perineural, and panniculitislike lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells (Figure 2). Infiltrative atypical lymphoid cells expressed CD3 and CD7 and were mostly CD8+, with a few CD4+ cells and most cells negative for CD5, CD20, CD30, CD56, and anaplastic lymphoma kinase. Cytotoxic markers granzyme B and T-cell intracellular antigen protein 1 were scattered positive. Immunostaining for Ki-67 protein highlighted an increased proliferative rate of 80% in malignant cells. In situ hybridization for EBV-encoded RNA (EBER) demonstrated EBV-positive atypical lymphoid cells (Figure 3). Analysis for T-cell receptor (TCR) γ gene rearrangement revealed a monoclonal pattern. Bone marrow aspirate showed proliferation of the 3 cell lines. The percentage of T lymphocytes was increased (20% of all nucleated cells). No hemophagocytic activity was found.


Diagnosis
A diagnosis of systemic EBV-positive T-cell lymphoma was made. Before the final diagnosis was made, the patient was treated by rheumatologists with antibiotics, antiviral drugs, nonsteroidal anti-inflammatory drugs, and other symptomatic treatments. Following antibiotic therapy, a sputum culture reverted to normal flora, the coagulation index (ie, prothrombin time, activated partial thromboplastin time) returned to normal, and the D-dimer level decreased to 1.19 mg/L.
The patient’s parents refused to accept chemotherapy for him. Instead, they chose herbal therapy only; 5 months later, they reported that all of his symptoms had resolved; however, the disease suddenly relapsed after another 7 months, with multiple skin nodules and fever. The patient died, even with chemotherapy in another hospital.
Comment
Prevalence and Presentation
Epstein-Barr virus is a ubiquitous γ-herpesvirus with tropism for B cells, affecting more than 90% of the adult population worldwide. In addition to infecting B cells, EBV is capable of infecting T and NK cells, leading to various EBV-related lymphoproliferative disorders (LPDs). The frequency and clinical presentation of infection varies based on the type of EBV-infected cells and the state of host immunity.1-3
Primary infection usually is asymptomatic and occurs early in life; when symptomatic, the disease usually presents as infectious mononucleosis (IM), characterized by polyclonal expansion of infected B cells and subsequent cytotoxic T-cell response. A diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against VCA, early antigens, and EBV nuclear antigen proteins.3,4
Associated LPDs
Although most symptoms associated with IM resolve within weeks or months, persistent or recurrent IM-like symptoms or even lasting disease occasionally occur, particularly in children and young adults. This complication is known as chronic active EBV infection (CAEBV), frequently associated with EBV-infected T-cell or NK-cell proliferation, especially in East Asian populations.3,5
Epstein-Barr virus–positive T-cell and NK-cell LPDs of childhood include CAEBV infection of T-cell and NK-cell types and systemic EBV-positive T-cell lymphoma of childhood. The former includes hydroa vacciniforme–like LPD and severe mosquito bite allergy.3
Systemic EBV-Positive T-cell Lymphoma of Childhood
This entity occurs not only in children but also in adolescents and young adults. A fulminant illness characterized by clonal proliferation of EBV-infected cytotoxic T cells, it can develop shortly after primary EBV infection or is linked to CAEBV infection. The disorder is rare and has a racial predilection for Asian (ie, Japanese, Chinese, Korean) populations and indigenous populations of Mexico and Central and South America.6-8
Complications
Systemic EBV-positive T-cell lymphoma of childhood is often complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. Other signs and symptoms include high fever, rash, jaundice, diarrhea, pancytopenia, and hepatosplenomegaly. The liver, spleen, lymph nodes, and bone marrow are commonly involved, and the disease can involve skin, the heart, and the lungs.9,10
Diagnosis
When systemic EBV-positive T-cell lymphoma occurs shortly after IM, serology shows low or absent anti-VCA IgM and positive anti-VCA IgG. Infiltrating T cells usually are small and lack cytologic atypia; however, cases with pleomorphic, medium to large lymphoid cells, irregular nuclei, and frequent mitoses have been described. Hemophagocytosis can be seen in the liver, spleen, and bone marrow.3,11
The most typical phenotype of systemic EBV-positive T-cell lymphoma is CD2+CD3+CD8+CD20−CD56−, with expression of the cytotoxic granules known as T-cell intracellular antigen 1 and granzyme B. Rare cases of CD4+ and mixed CD4+/CD8+ phenotypes have been described, usually in the setting of CAEBV infection.3,12 Neoplastic cells have monoclonally rearranged TCR-γ genes and consistent EBER positivity with in situ hybridization.13 A final diagnosis is based on a comprehensive analysis of clinical, morphological, immunohistochemical, and molecular biological aspects.
Clinical Course and Prognosis
Most patients with systemic EBV-positive T-cell lymphoma have an aggressive clinical course with high mortality. In a few cases, patients were reported to respond to a regimen of etoposide and dexamethasone, followed by allogeneic hematopoietic stem cell transplantation.3
In recognition of the aggressive clinical behavior and desire to clearly distinguish systemic EBV-positive T-cell lymphoma from CAEBV infection, the older term systemic EBV-positive T-cell LPD of childhood, which had been introduced in 2008 to the World Health Organization classification, was changed to systemic EBV-positive T-cell lymphoma of childhood in the revised 2016 World Health Organization classification.6,12 However, Kim et al14 reported a case with excellent response to corticosteroid administration, suggesting that systemic EBV-positive T-cell lymphoma of childhood may be more heterogeneous in terms of prognosis.
Our patient presented with acute IM-like symptoms, including high fever, tonsillar enlargement, lymphadenopathy, and hepatosplenomegaly, as well as uncommon oral ulcers and skin lesions, including indurated nodules. Histopathologic changes in the skin nodule, proliferation in bone marrow, immunohistochemical phenotype, and positivity of EBER and TCR-γ monoclonal rearrangement were all consistent with systemic EBV-positive T-cell lymphoma of childhood. The patient was positive for VCA IgG and negative for VCA IgM, compatible with systemic EBV-positive T-cell lymphoma of childhood occurring shortly after IM. Neither pancytopenia, hemophagocytic syndrome, nor multiorgan failure occurred during the course.
Differential Diagnosis
It is important to distinguish IM from systemic EBV-positive T-cell lymphoma of childhood and CAEBV infection. Detection of anti–VCA IgM in the early stage, its disappearance during the clinical course, and appearance of anti-EBV–determined nuclear antigen is useful to distinguish IM from the neoplasms, as systemic EBV-positive T-cell lymphoma of childhood is negative for anti-EBV–determined nuclear antigen. Carefully following the clinical course also is important.3,15
Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis can occur in association with systemic EBV-positive T-cell lymphoma of childhood and might represent a continuum of disease rather than distinct entities.14 The most useful marker for differentiating EBV-associated hemophagocytic lymphohistiocytosis and systemic EBV-positive T-cell lymphoma of childhood is an abnormal karyotype rather than molecular clonality.16
Outcome
Mortality risk in EBV-associated T-cell and NK-cell LPD is not primarily dependent on whether the lesion has progressed to lymphoma but instead is related to associated complications.17
Conclusion
Although systemic EBV-positive T-cell lymphoma of childhood is a rare disorder and has race predilection, dermatologists should be aware due to the aggressive clinical source and poor prognosis. Histopathology and in situ hybridization for EBER and TCR gene rearrangements are critical for final diagnosis. Although rare cases can show temporary resolution, the final outcome of this disease is not optimistic.
Case Report
A 7-year-old Chinese boy presented with multiple painful oral and tongue ulcers of 2 weeks’ duration as well as acute onset of moderate to high fever (highest temperature, 39.3°C) for 5 days. The fever was reported to have run a relapsing course, accompanied by rigors but without convulsions or cognitive changes. At times, the patient had nasal congestion, nasal discharge, and cough. He also had a transient eruption on the back and hands as well as an indurated red nodule on the left forearm.
Before the patient was hospitalized, antibiotic therapy was administered by other physicians, but the condition of fever and oral ulcers did not improve. After the patient was hospitalized, new tender nodules emerged on the scalp, buttocks, and lower extremities. New ulcers also appeared on the palate.
History
Two months earlier, the patient had presented with a painful perioral skin ulcer that resolved after being treated as contagious eczema. Another dermatologist previously had considered a diagnosis of hand-foot-and-mouth disease.
The patient was born by normal spontaneous vaginal delivery, without abnormality. He was breastfed; feeding, growth, and the developmental history showed no abnormality. He was the family’s eldest child, with a healthy brother and sister. There was no history of familial illness. He received bacillus Calmette-Guérin and poliomyelitis vaccines after birth; the rest of the vaccine history was unclear. There was no history of immunologic abnormality.
Physical Examination
A 1.5×1.5-cm, warm, red nodule with a central black crust was noted on the left forearm (Figure 1A). Several similar lesions were noted on the buttocks, scalp, and lower extremities. Multiple ulcers, as large as 1 cm, were present on the tongue, palate, and left angle of the mouth (Figure 1B). The pharynx was congested, and the tonsils were mildly enlarged. Multiple enlarged, movable, nontender lymph nodes could be palpated in the cervical basins, axillae, and groin. No purpura or ecchymosis was detected.

Laboratory Results
Laboratory testing revealed a normal total white blood cell count (4.26×109/L [reference range, 4.0–12.0×109/L]), with normal neutrophils (1.36×109/L [reference range, 1.32–7.90×109/L]), lymphocytes (2.77×109/L [reference range, 1.20–6.00×109/L]), and monocytes (0.13×109/L [reference range, 0.08–0.80×109/L]); a mildly decreased hemoglobin level (115 g/L [reference range, 120–160 g/L]); a normal platelet count (102×109/L [reference range, 100–380×109/L]); an elevated lactate dehydrogenase level (614 U/L [reference range, 110–330 U/L]); an elevated α-hydroxybutyrate dehydrogenase level (483 U/L [reference range, 120–270 U/L]); elevated prothrombin time (15.3 s [reference range, 9–14 s]); elevated activated partial thromboplastin time (59.8 s [reference range, 20.6–39.6 s]); and an elevated D-dimer level (1.51 mg/L [reference range, <0.73 mg/L]). In addition, autoantibody testing revealed a positive antinuclear antibody titer of 1:320 and a strong positive anti–Ro-52 level.
The peripheral blood lymphocyte classification demonstrated a prominent elevated percentage of T lymphocytes, with predominantly CD8+ cells (CD3, 94.87%; CD8, 71.57%; CD4, 24.98%; CD4:CD8 ratio, 0.35) and a diminished percentage of B lymphocytes and natural killer (NK) cells. Epstein-Barr virus (EBV) antibody testing was positive for anti–viral capsid antigen (VCA) IgG and negative for anti-VCA IgM.
Smears of the ulcer on the tongue demonstrated gram-positive cocci, gram-negative bacilli, and diplococci. Culture of sputum showed methicillin-resistant Staphylococcus aureus. Inspection for acid-fast bacilli in sputum yielded negative results 3 times. A purified protein derivative skin test for Mycobacterium tuberculosis infection was negative.
Imaging and Other Studies
Computed tomography of the chest and abdomen demonstrated 2 nodular opacities on the lower right lung; spotted opacities on the upper right lung; floccular opacities on the rest area of the lung; mild pleural effusion; enlargement of lymph nodes on the mediastinum, the bilateral hilum of the lung, and mesentery; and hepatosplenomegaly. Electrocardiography showed sinus tachycardia. Nasal cavity endoscopy showed sinusitis. Fundus examination showed vasculopathy of the left retina. A colonoscopy showed normal mucosa.
Histopathology
Biopsy of the nodule on the left arm showed dense, superficial to deep perivascular, periadnexal, perineural, and panniculitislike lymphoid infiltrates, as well as a sparse interstitial infiltrate with irregular and pleomorphic medium to large nuclei. Lymphoid cells showed mild epidermotropism, with tagging to the basal layer. Some vessel walls were infiltrated by similar cells (Figure 2). Infiltrative atypical lymphoid cells expressed CD3 and CD7 and were mostly CD8+, with a few CD4+ cells and most cells negative for CD5, CD20, CD30, CD56, and anaplastic lymphoma kinase. Cytotoxic markers granzyme B and T-cell intracellular antigen protein 1 were scattered positive. Immunostaining for Ki-67 protein highlighted an increased proliferative rate of 80% in malignant cells. In situ hybridization for EBV-encoded RNA (EBER) demonstrated EBV-positive atypical lymphoid cells (Figure 3). Analysis for T-cell receptor (TCR) γ gene rearrangement revealed a monoclonal pattern. Bone marrow aspirate showed proliferation of the 3 cell lines. The percentage of T lymphocytes was increased (20% of all nucleated cells). No hemophagocytic activity was found.


Diagnosis
A diagnosis of systemic EBV-positive T-cell lymphoma was made. Before the final diagnosis was made, the patient was treated by rheumatologists with antibiotics, antiviral drugs, nonsteroidal anti-inflammatory drugs, and other symptomatic treatments. Following antibiotic therapy, a sputum culture reverted to normal flora, the coagulation index (ie, prothrombin time, activated partial thromboplastin time) returned to normal, and the D-dimer level decreased to 1.19 mg/L.
The patient’s parents refused to accept chemotherapy for him. Instead, they chose herbal therapy only; 5 months later, they reported that all of his symptoms had resolved; however, the disease suddenly relapsed after another 7 months, with multiple skin nodules and fever. The patient died, even with chemotherapy in another hospital.
Comment
Prevalence and Presentation
Epstein-Barr virus is a ubiquitous γ-herpesvirus with tropism for B cells, affecting more than 90% of the adult population worldwide. In addition to infecting B cells, EBV is capable of infecting T and NK cells, leading to various EBV-related lymphoproliferative disorders (LPDs). The frequency and clinical presentation of infection varies based on the type of EBV-infected cells and the state of host immunity.1-3
Primary infection usually is asymptomatic and occurs early in life; when symptomatic, the disease usually presents as infectious mononucleosis (IM), characterized by polyclonal expansion of infected B cells and subsequent cytotoxic T-cell response. A diagnosis of EBV infection can be made by testing for specific IgM and IgG antibodies against VCA, early antigens, and EBV nuclear antigen proteins.3,4
Associated LPDs
Although most symptoms associated with IM resolve within weeks or months, persistent or recurrent IM-like symptoms or even lasting disease occasionally occur, particularly in children and young adults. This complication is known as chronic active EBV infection (CAEBV), frequently associated with EBV-infected T-cell or NK-cell proliferation, especially in East Asian populations.3,5
Epstein-Barr virus–positive T-cell and NK-cell LPDs of childhood include CAEBV infection of T-cell and NK-cell types and systemic EBV-positive T-cell lymphoma of childhood. The former includes hydroa vacciniforme–like LPD and severe mosquito bite allergy.3
Systemic EBV-Positive T-cell Lymphoma of Childhood
This entity occurs not only in children but also in adolescents and young adults. A fulminant illness characterized by clonal proliferation of EBV-infected cytotoxic T cells, it can develop shortly after primary EBV infection or is linked to CAEBV infection. The disorder is rare and has a racial predilection for Asian (ie, Japanese, Chinese, Korean) populations and indigenous populations of Mexico and Central and South America.6-8
Complications
Systemic EBV-positive T-cell lymphoma of childhood is often complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. Other signs and symptoms include high fever, rash, jaundice, diarrhea, pancytopenia, and hepatosplenomegaly. The liver, spleen, lymph nodes, and bone marrow are commonly involved, and the disease can involve skin, the heart, and the lungs.9,10
Diagnosis
When systemic EBV-positive T-cell lymphoma occurs shortly after IM, serology shows low or absent anti-VCA IgM and positive anti-VCA IgG. Infiltrating T cells usually are small and lack cytologic atypia; however, cases with pleomorphic, medium to large lymphoid cells, irregular nuclei, and frequent mitoses have been described. Hemophagocytosis can be seen in the liver, spleen, and bone marrow.3,11
The most typical phenotype of systemic EBV-positive T-cell lymphoma is CD2+CD3+CD8+CD20−CD56−, with expression of the cytotoxic granules known as T-cell intracellular antigen 1 and granzyme B. Rare cases of CD4+ and mixed CD4+/CD8+ phenotypes have been described, usually in the setting of CAEBV infection.3,12 Neoplastic cells have monoclonally rearranged TCR-γ genes and consistent EBER positivity with in situ hybridization.13 A final diagnosis is based on a comprehensive analysis of clinical, morphological, immunohistochemical, and molecular biological aspects.
Clinical Course and Prognosis
Most patients with systemic EBV-positive T-cell lymphoma have an aggressive clinical course with high mortality. In a few cases, patients were reported to respond to a regimen of etoposide and dexamethasone, followed by allogeneic hematopoietic stem cell transplantation.3
In recognition of the aggressive clinical behavior and desire to clearly distinguish systemic EBV-positive T-cell lymphoma from CAEBV infection, the older term systemic EBV-positive T-cell LPD of childhood, which had been introduced in 2008 to the World Health Organization classification, was changed to systemic EBV-positive T-cell lymphoma of childhood in the revised 2016 World Health Organization classification.6,12 However, Kim et al14 reported a case with excellent response to corticosteroid administration, suggesting that systemic EBV-positive T-cell lymphoma of childhood may be more heterogeneous in terms of prognosis.
Our patient presented with acute IM-like symptoms, including high fever, tonsillar enlargement, lymphadenopathy, and hepatosplenomegaly, as well as uncommon oral ulcers and skin lesions, including indurated nodules. Histopathologic changes in the skin nodule, proliferation in bone marrow, immunohistochemical phenotype, and positivity of EBER and TCR-γ monoclonal rearrangement were all consistent with systemic EBV-positive T-cell lymphoma of childhood. The patient was positive for VCA IgG and negative for VCA IgM, compatible with systemic EBV-positive T-cell lymphoma of childhood occurring shortly after IM. Neither pancytopenia, hemophagocytic syndrome, nor multiorgan failure occurred during the course.
Differential Diagnosis
It is important to distinguish IM from systemic EBV-positive T-cell lymphoma of childhood and CAEBV infection. Detection of anti–VCA IgM in the early stage, its disappearance during the clinical course, and appearance of anti-EBV–determined nuclear antigen is useful to distinguish IM from the neoplasms, as systemic EBV-positive T-cell lymphoma of childhood is negative for anti-EBV–determined nuclear antigen. Carefully following the clinical course also is important.3,15
Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis can occur in association with systemic EBV-positive T-cell lymphoma of childhood and might represent a continuum of disease rather than distinct entities.14 The most useful marker for differentiating EBV-associated hemophagocytic lymphohistiocytosis and systemic EBV-positive T-cell lymphoma of childhood is an abnormal karyotype rather than molecular clonality.16
Outcome
Mortality risk in EBV-associated T-cell and NK-cell LPD is not primarily dependent on whether the lesion has progressed to lymphoma but instead is related to associated complications.17
Conclusion
Although systemic EBV-positive T-cell lymphoma of childhood is a rare disorder and has race predilection, dermatologists should be aware due to the aggressive clinical source and poor prognosis. Histopathology and in situ hybridization for EBER and TCR gene rearrangements are critical for final diagnosis. Although rare cases can show temporary resolution, the final outcome of this disease is not optimistic.
- Ameli F, Ghafourian F, Masir N. Systematic Epstein-Barr virus-positive T-cell lymphoproliferative disease presenting as a persistent fever and cough: a case report. J Med Case Rep. 2014;8:288.
- Kim HJ, Ko YH, Kim JE, et al. Epstein-Barr virus-associated lympho-proliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51:352-358.
- Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts [published online March 7, 2018]. Pathogens. doi:10.3390/pathogens7010028.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
- Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71.
- Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137-147.
- Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443-451.
- Chen G, Chen L, Qin X, et al. Systemic Epstein-Barr virus positive T-cell lymphoproliferative disease of childhood with hemophagocytic syndrome. Int J Clin Exp Pathol. 2014;7:7110-7113.
- Grywalska E, Rolinski J. Epstein-Barr virus-associated lymphomas. Semin Oncol. 2015;42:291-303.
- Huang W, Lv N, Ying J, et al. Clinicopathological characteristics of four cases of EBV positive T-cell lymphoproliferative disorders of childhood in China. Int J Clin Exp Pathol. 2014;7:4991-4999.
- Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011;5:218.
- Kim DH, Kim M, Kim Y, et al. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood with good response to steroid therapy. J Pediatr Hematol Oncol. 2017;39:e497-e500.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671-675.
- Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738-5749.
- Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53-63.
- Ameli F, Ghafourian F, Masir N. Systematic Epstein-Barr virus-positive T-cell lymphoproliferative disease presenting as a persistent fever and cough: a case report. J Med Case Rep. 2014;8:288.
- Kim HJ, Ko YH, Kim JE, et al. Epstein-Barr virus-associated lympho-proliferative disorders: review and update on 2016 WHO classification. J Pathol Transl Med. 2017;51:352-358.
- Dojcinov SD, Fend F, Quintanilla-Martinez L. EBV-positive lymphoproliferations of B- T- and NK-cell derivation in non-immunocompromised hosts [published online March 7, 2018]. Pathogens. doi:10.3390/pathogens7010028.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
- Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71.
- Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137-147.
- Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443-451.
- Chen G, Chen L, Qin X, et al. Systemic Epstein-Barr virus positive T-cell lymphoproliferative disease of childhood with hemophagocytic syndrome. Int J Clin Exp Pathol. 2014;7:7110-7113.
- Grywalska E, Rolinski J. Epstein-Barr virus-associated lymphomas. Semin Oncol. 2015;42:291-303.
- Huang W, Lv N, Ying J, et al. Clinicopathological characteristics of four cases of EBV positive T-cell lymphoproliferative disorders of childhood in China. Int J Clin Exp Pathol. 2014;7:4991-4999.
- Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011;5:218.
- Kim DH, Kim M, Kim Y, et al. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood with good response to steroid therapy. J Pediatr Hematol Oncol. 2017;39:e497-e500.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671-675.
- Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7:5738-5749.
- Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53-63.
Practice Points
- Systemic Epstein-Barr virus (EBV)–positive T-cell lymphoma of childhood is a fulminant illness with a predilection for Asians and indigenous populations from Mexico and Central and South America. In most patients, the disease has an aggressive clinical course with high mortality.
- The disease often is complicated by hemophagocytic syndrome, coagulopathy, sepsis, and multiorgan failure. When these severe complications are absent, the prognosis might be better.
- In situ hybridization for EBV-encoded RNA and for T-cell receptor gene rearrangements is an important tool to establish the diagnosis as well as for treatment options and predicting the prognosis.
Seborrhea Herpeticum: Cutaneous Herpes Simplex Virus Infection Within Infantile Seborrheic Dermatitis
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.
Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Practice Points
- Cutaneous herpes simplex virus may present in a seborrheic distribution within infantile seborrheic dermatitis, suggesting underlying dysfunction secondary to seborrheic dermatitis.
- Treatment of seborrhea herpeticum involves antiviral therapy to treat the secondary viral infection and gentle skin care precautions for the primary condition.
Pediatric Molluscum: An Update
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
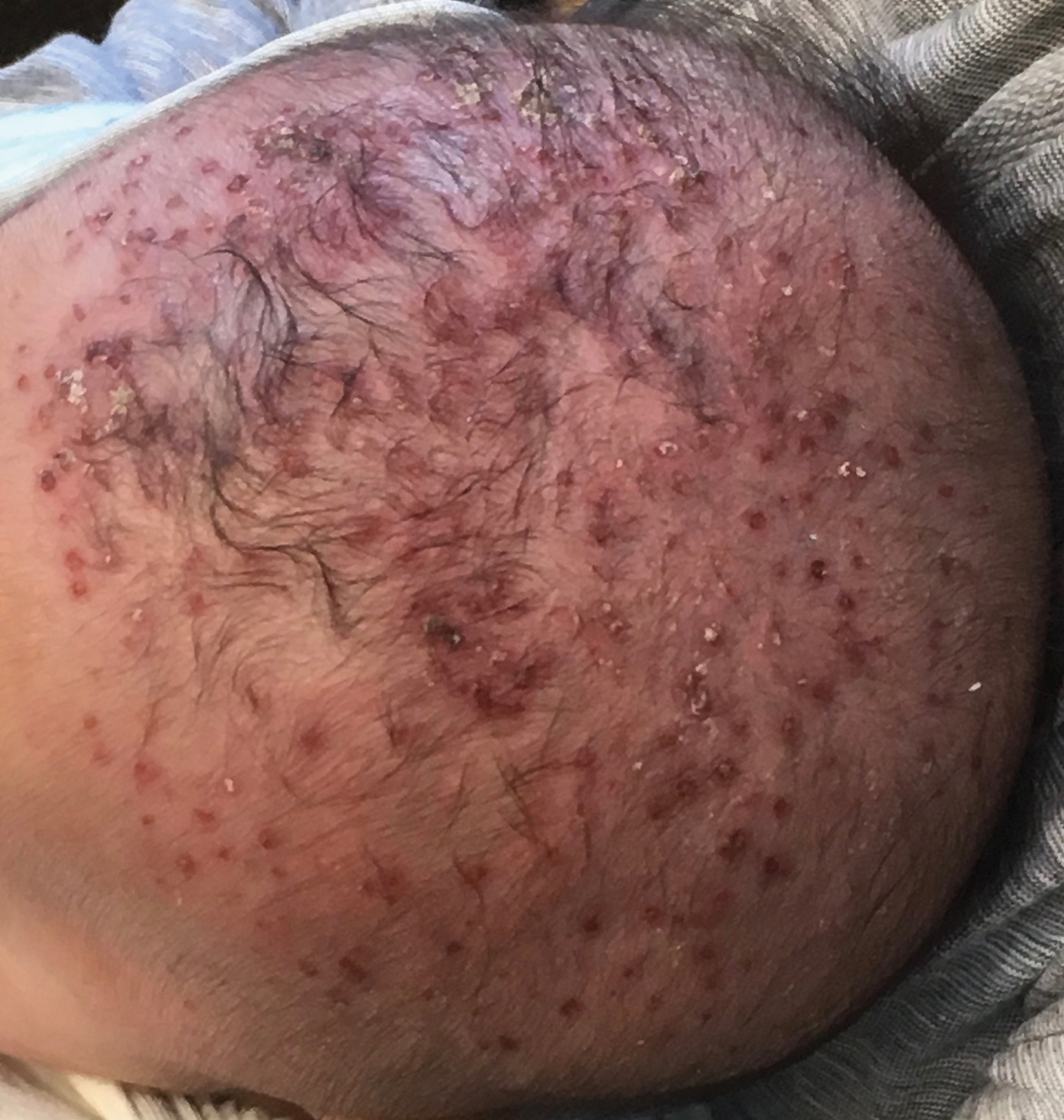
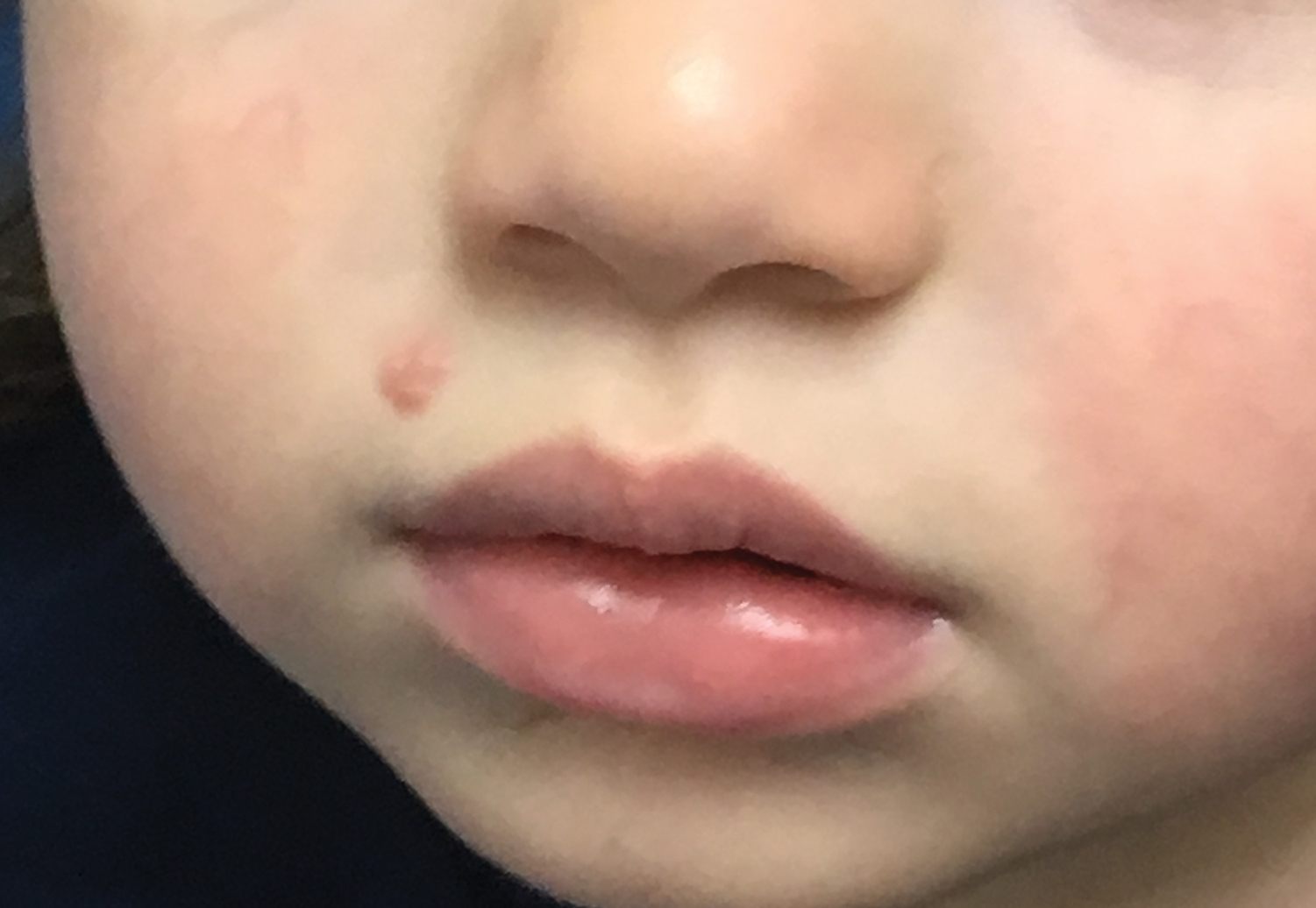
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38


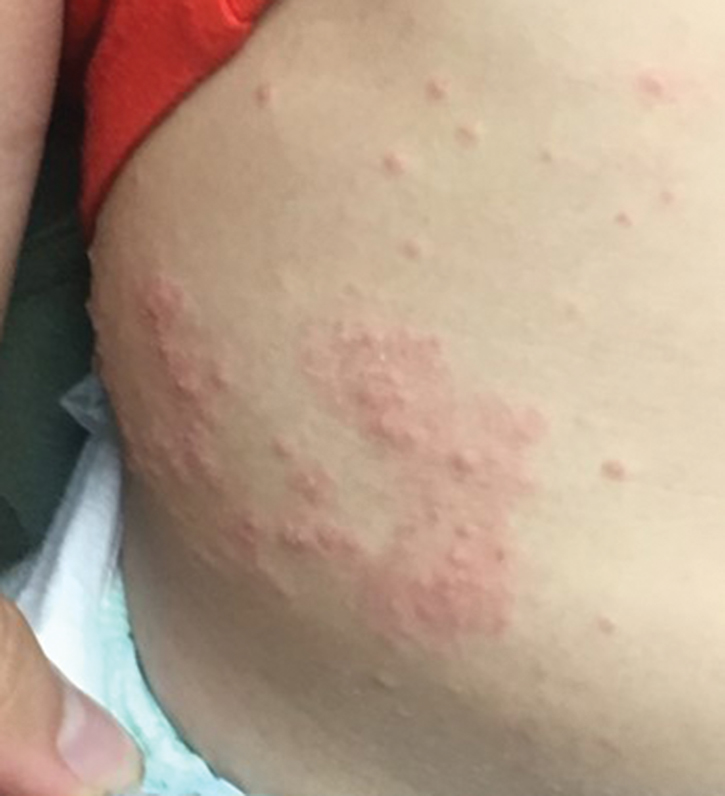
Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39

Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.

Interventional Therapy
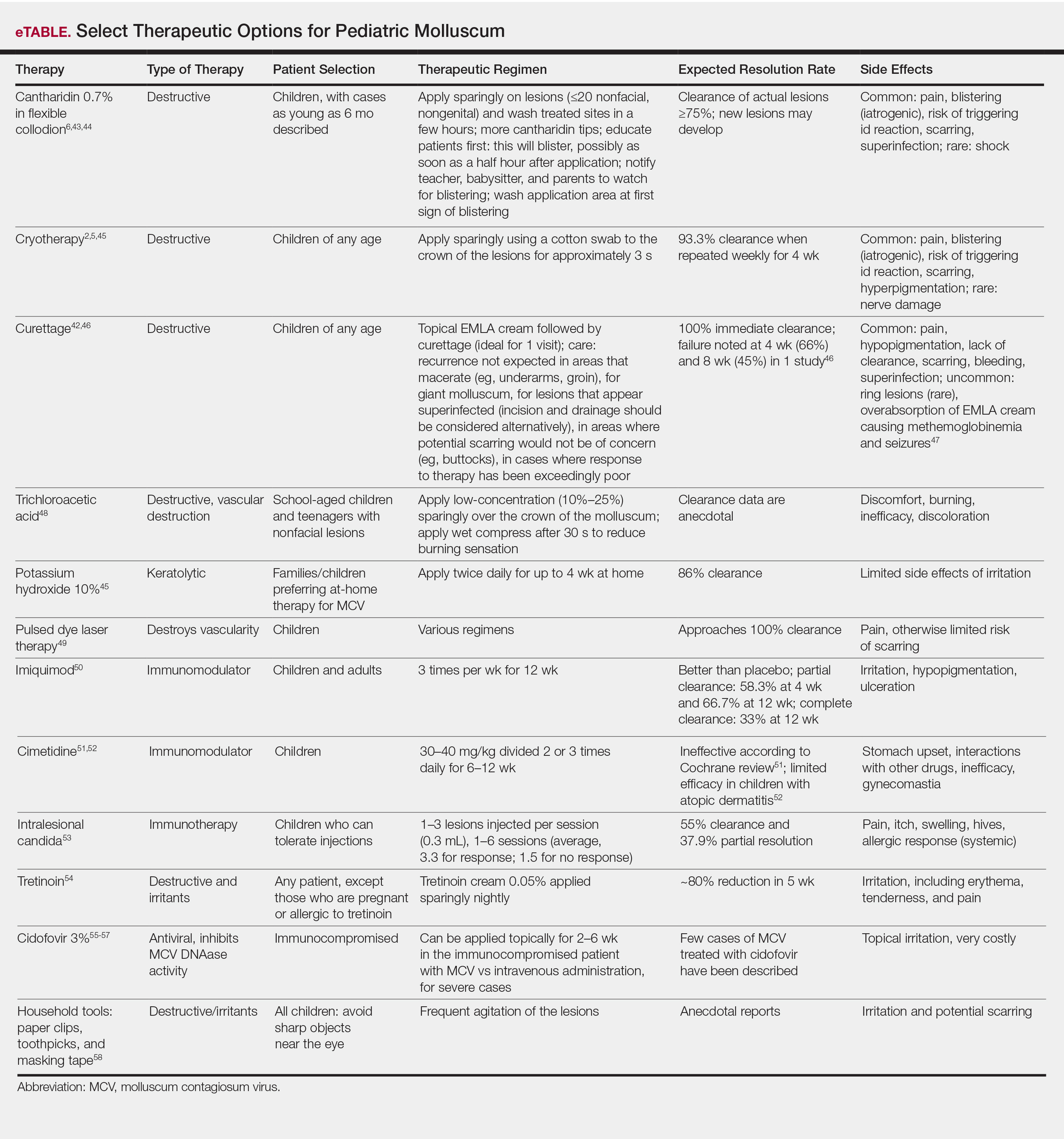
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42
Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38




Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39

Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.

Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42

Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38




Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39

Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.

Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42

Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
Practice Points
- Molluscum appears as pearly papules with a central dell (ie, umbilicated).
- Caused by a poxvirus, the disease is very contagious and transferred via skin-to-skin contact or fomites.
- One-third of children with molluscum will develop symptoms of local erythema, swelling, or pruritus.
- Diagnosis usually is clinical.
- Children are primarily managed through observation; however, cantharidin, cryotherapy, or curettage can be used for symptomatic or cosmetically concerning lesions.
Comment on “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences”
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
Hidradenitis Suppurativa for the Dermatologic Hospitalist
Hidradenitis suppurativa (HS) is a common chronic inflammatory skin disease characterized by purulent subcutaneous nodules, papules, abscesses, and fistula tracts that lead to scarring and fibrosis. Lesions develop primarily in the axilla, groin, and other intertriginous and hair-bearing areas.
The natural history of the disease is characterized by periods of disease flare, followed by periods of disease quiescence. Patients might have weeks or months of low disease activity but frequently develop multiple exacerbating episodes over the course of weeks or months. The condition primarily presents in adolescent and peripubescent years, continuing throughout adulthood. Some evidence suggests a bimodal disease distribution, with a second peak of incidence in middle-aged adults. Women and men are affected equally; however, the disease can be phenotypically different in men and women.
Patients frequently present in emergency and inpatient settings for evaluation because of the pain and severity of HS flares as well as associated systemic symptoms. Inpatient and emergency department (ED) care are unique opportunities for dermatologic hospitalist and dermatologic consultative services to educate other physicians about the condition and initiate aggressive treatments that are frequently necessary to control HS flares. This article aims to address best methods for treating HS in these settings.
Pathophysiology
Although the exact pathophysiology of the condition is unknown, HS is thought to begin with follicular occlusion with downstream inflammation mediating neutrophilic activity and scarring. Hyperplasia of the infundibular epithelium is observed on histology, and the resulting occlusion, contained keratin, and follicular rupture initiate robust downstream inflammation.1,2 Follicular occlusion might be initially androgen mediated3 or might occur in combination with friction4 and genetic or acquired factors involving Notch signaling. Although HS characteristically presents in areas of high apocrine density, apocrine glands are not thought to be the primary mediator of disease activity.5 IL-17, IL-23, tumor necrosis factor α, and IL-1β are implicated in the pathogenesis of HS, but it is unknown if these cytokines are the driving pathologic factor in HS or if they are merely secondary sequelae.6
Demographics and Prevalence in Hospitalized Patients
Although increasing treatment availability has brought more attention to HS, true prevalence is unknown. A prevalence of 1% has been reported in many European countries.7 Global prevalence has been more difficult to determine, with variable data suggesting a prevalence of 0.03% to 8%, depending on the population included.8 Most patients studied in a US-based claims database were aged 30 to 64 years, and the overall prevalence was 0.05%.9 Despite prevalence similar to psoriasis, utilization of high-cost emergency and inpatient admissions is notably higher among patients with HS. Recent claims data suggest that HS patients utilize the ED at a rate 3 times higher than psoriasis patients and are admitted as inpatients at a rate 5 times higher.10 Similar data suggest an associated increased cost of care for patients with HS vs other conditions, such as psoriasis, due to frequent ED and inpatient stays.11 Although HS frequently presents in the inpatient and emergency settings, there is little literature on best methods for managing patients in these settings.
Pearls for Inpatient and Emergency Evaluation and Management
Initial Evaluation
When dermatologic consultative services are asked to evaluate patients with HS, preliminary evaluation should reflect the acuity of the patient. Vital signs and toxicity should be reviewed to ensure that there is no evidence of severe infection necessitating critical or acute care.
History
History-taking should reflect assessment of the patient’s baseline disease, including date of initial onset; exacerbating factors, such as friction, smoking, pregnancy, and menses; and the current history of the patient’s flare. A history of antibiotics, immunosuppression, topical therapy, antiandrogen therapy, and vitamin A analog therapy also should be reviewed. If an initial diagnosis is made in the ED or inpatient setting, a family and personal history should focus on specific risk factors and disease associations, including inflammatory bowel disease,12 pilonidal cysts,13 polycystic ovary syndrome,14 and metabolic syndrome.15
Physical Examination
As with all dermatologic consultations, a full-body skin examination, with special attention to the axilla, inframammary skin, groin, buttocks, and perineum, should be undertaken. In addition to these common areas of disease progression, examination should focus on atypical sites for disease manifestation, including the posterior auricular scalp, skin folds in the pannus and back, and the beard area in men. Evaluation of axillary and gluteal hair should note features of folliculitis and hair removal, which can exacerbate HS. Examination also should include an investigation of cutaneous manifestations of comorbid conditions, including acanthosis nigricans, contiguous or metastatic cutaneous Crohn disease, erythema nodosum, and pilonidal cysts. Caution should be exercised when diagnosing pilonidal cysts, as isolated or evolving HS in the gluteal cleft often is misdiagnosed as a pilonidal cyst.
Laboratory Evaluation
Testing often is misleading in patients with HS, especially in the acute setting, because the condition is a chronic inflammatory process. The C-reactive protein level as well as the absolute white blood cell and neutrophil counts often are elevated, even in the absence of acute infection.16 In fact, although patients often are treated with intravenous antibiotics by inpatient and emergency teams in the setting of these 3 laboratory abnormalities, these findings often reflect disease activity, not frank infection. Fever, especially low-grade fever, also can reflect ongoing disease activity. Thrombocytosis and anemia also are anecdotally common, though these findings have not been reported specifically in the literature.
Bacterial Cultures
The role of lesional and perilesional bacterial cultures is controversial in HS. Prior studies have demonstrated that biofilm formation may be associated with the chronic inflammation seen in HS.17 However, most data to date suggest that infection is not the primary driver of HS disease flares, as demonstrated by the frequency of sterile cultures and the variable response of the disease to penicillin and related antibiotics.18
Imaging
Ultrasonography and magnetic resonance imaging can be conducted if there is concern about deeper abscesses that are not apparent on examination. When interpreted by nondermatologic practitioners, however, the findings of these modalities can result in unnecessary surgical intervention, given the concern for development of infectious abscess.19
Diagnosis
Many patients with HS experience a notable delay in time to diagnosis, living with symptoms for 7 years on average prior to being given a name for their condition.20 Often, patients seek ED care at initial presentation because lesions can present quickly and are associated with remarkable pain. Inpatient dermatologic evaluation can provide patients with definitive diagnosis, appropriate counseling that provides an overview of the natural history of the disease, lifestyle recommendations, and expedited connection to outpatient longitudinal care.
Diagnosis is made clinically by assessment for typical lesions, such as painful or tender papules, nodules, or abscesses in the axillae, inframammary region, groin, thighs, and perineal and perianal regions. Cordlike scarring often is seen in the absence of active inflammatory lesions.21 Double-headed open comedones and prominent follicular occlusion are seen in some phenotypes but are not required for diagnosis.22
Multiple scoring modalities are in use23; the Hurley staging system, initially developed for surgical staging, has become a commonly used method in the clinical setting24:
• Hurley stage I: isolated nodules or abscesses;
• Hurley stage II: widely separate lesions and sinus tracts or scarring are suggestive; and
• Hurley stage III: multiple lesions with near-diffuse involvement and formation of sinus tracts and scarring.
Other scoring modalities, such as the Hidradenitis Suppurativa Clinical Response (HiSCR), are more commonly used in the clinical trial setting and quantitatively capture lesion count improvement while the patient is being treated.25
Treatment
Evaluation in the ED might necessitate recommendations for inpatient admission. Dermatologic consultation can be helpful in providing ED physicians with context for interpretation of laboratory results and clinical findings. Specifically, dermatologic evaluation can help differentiate presentations consistent with a primary infection from a more common presentation of HS flaring and associated bacterial colonization. Indications for inpatient admission are severe pain; concern for systemic infection, including high fever or sepsis; and need for surgical intervention. Patients with severe disease who do not have a longitudinal care plan or who lack the ability to care for lesions at home also are candidates for inpatient admission, where they can receive more intensive nursing and wound care as well as outpatient logistical management.
Acute care should be aimed at treatments that work quickly and aggressively and have both anti-inflammatory and antimicrobial effects. Severe flares require aggressive initial treatment to ensure more long-term remission. Adalimumab, maintained at 40 mg/wk after a loading dose, is the mainstay of evidence-based treatment for moderate to severe HS in patients 12 years or older; however, this treatment might not be easy to initiate in the inpatient setting because of its cost and availability and the fact that it is not as fast acting as other therapies.26 For patients with severe disease flares, prednisone,27 infliximab,28 or cyclosporine29 can be used in combination with antimicrobial therapy in the inpatient setting to quickly control active flaring. Intravenous antimicrobial therapy might be necessary in severe disease and should include coverage of gram-positive30 and anaerobic organisms.31
Although management of acute flares is critical, especially for hospitalized patients, initiating longitudinal treatment modalities while the patient is an inpatient will help prevent future readmissions, facilitate better outcomes, and enable longer periods of disease-free progression. Specific treatments, stratified by disease severity, are listed in the Table.
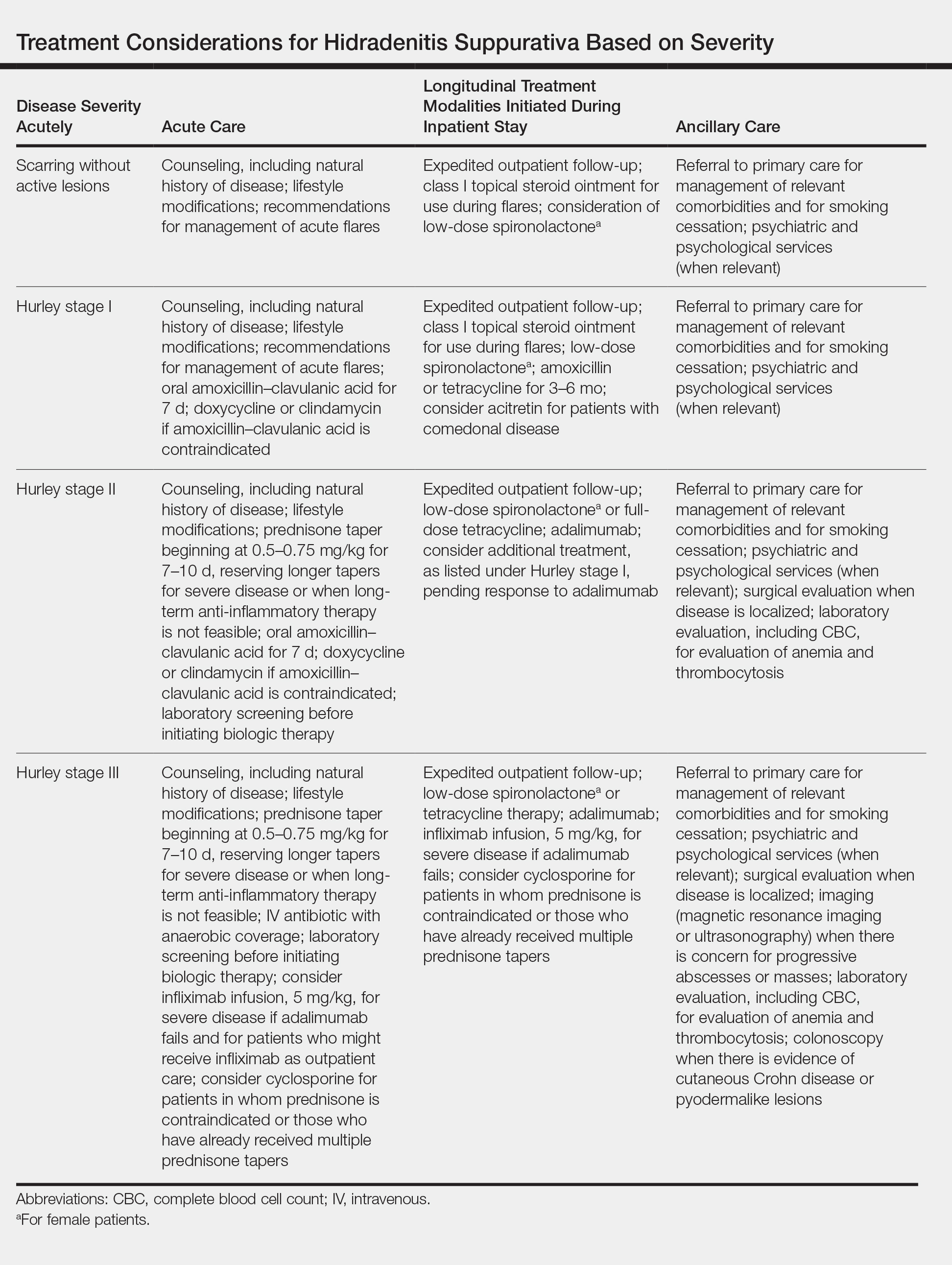
Postdischarge Lifestyle Modification
All disease management should include recommendations for lifestyle modification, including counseling on terminal hair removal (ie, avoid shaving, plucking, and waxing) and recommendations for daily and weekly decolonization with chlorhexidine or other antimicrobial soap, a weekly vinegar bath, and antiperspirant use in the groin and axilla. Avoiding tight clothes and humidity might also be helpful.
Other beneficial postdischarge strategies include smoking cessation and weight loss, which often are beneficial but difficult for many patients to achieve on their own; connecting patients with a primary care provider, which can facilitate better long-term outcomes; informing patients of the natural history of the disease and providing strategies for them to implement for acute flares to help avoid readmission and ED visits; and writing a “pill-in-pocket” prescription for a course of an antibiotic that provides good staphylococcal and anaerobic coverage, which can be helpful for patients who are prone to infrequent flares.
Lastly, appropriate postdischarge maintenance therapy also can be initiated during the inpatient stay, including maintenance antibiotic therapy, spironolactone32 for female patients, and acitretin33 for comedonal-predominant patients.
Final Thoughts
Hidradenitis suppurativa is a common dermatologic condition that frequently presents in emergency and inpatient settings, given its association with painful and acutely indurated lesions that often appear concerning for infection. Elevated inflammatory markers and fever are common in HS and are not necessarily suggestive of infection. As such, while antibiotics may be part of acute management of HS, care also should address the inflammatory component of the disease. Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting ED and inpatient care utilization.
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999.
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(suppl 1):S8-S11.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125:304-308.
- de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012;21:176-177.
- Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763-769.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601.
- Jemec GE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(suppl 1):S4-S7.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419.
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944.
- Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76:49-53.
- Benhadou F, Van der Zee HH, Pascual JC, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients [published online March 27, 2019]. Br J Dermatol. doi:10.1111/bjd.17927.
- Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol. 2018;138:1288-1292.
- Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:55-57.
- Hessam S, Sand M, Gambichler T, et al. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol. 2015;73:998-1005.
- Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993-1000.
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Wortsman X. Imaging of hidradenitis suppurativa. Dermatol Clin. 2016;34:59-68.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546-1549.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin. 2016;34:1-5.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506-1511.
- Porter ML, Kimball AB. Hidradenitis suppurativa scoring systems: can we choose just one? Cutis. 2017;99:156-157.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker, Inc; 1989:732-738.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75:1059-1062.
- Sullivan TP, Welsh E, Kerdel FA. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149:1046-1049.
- Anderson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa—a case series. J Dermatolog Treat. 2016;27:247-250.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Guet-Revillet H, Coignard-Biehler H, Jais JP, et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:114-119.
- Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170-174.
Hidradenitis suppurativa (HS) is a common chronic inflammatory skin disease characterized by purulent subcutaneous nodules, papules, abscesses, and fistula tracts that lead to scarring and fibrosis. Lesions develop primarily in the axilla, groin, and other intertriginous and hair-bearing areas.
The natural history of the disease is characterized by periods of disease flare, followed by periods of disease quiescence. Patients might have weeks or months of low disease activity but frequently develop multiple exacerbating episodes over the course of weeks or months. The condition primarily presents in adolescent and peripubescent years, continuing throughout adulthood. Some evidence suggests a bimodal disease distribution, with a second peak of incidence in middle-aged adults. Women and men are affected equally; however, the disease can be phenotypically different in men and women.
Patients frequently present in emergency and inpatient settings for evaluation because of the pain and severity of HS flares as well as associated systemic symptoms. Inpatient and emergency department (ED) care are unique opportunities for dermatologic hospitalist and dermatologic consultative services to educate other physicians about the condition and initiate aggressive treatments that are frequently necessary to control HS flares. This article aims to address best methods for treating HS in these settings.
Pathophysiology
Although the exact pathophysiology of the condition is unknown, HS is thought to begin with follicular occlusion with downstream inflammation mediating neutrophilic activity and scarring. Hyperplasia of the infundibular epithelium is observed on histology, and the resulting occlusion, contained keratin, and follicular rupture initiate robust downstream inflammation.1,2 Follicular occlusion might be initially androgen mediated3 or might occur in combination with friction4 and genetic or acquired factors involving Notch signaling. Although HS characteristically presents in areas of high apocrine density, apocrine glands are not thought to be the primary mediator of disease activity.5 IL-17, IL-23, tumor necrosis factor α, and IL-1β are implicated in the pathogenesis of HS, but it is unknown if these cytokines are the driving pathologic factor in HS or if they are merely secondary sequelae.6
Demographics and Prevalence in Hospitalized Patients
Although increasing treatment availability has brought more attention to HS, true prevalence is unknown. A prevalence of 1% has been reported in many European countries.7 Global prevalence has been more difficult to determine, with variable data suggesting a prevalence of 0.03% to 8%, depending on the population included.8 Most patients studied in a US-based claims database were aged 30 to 64 years, and the overall prevalence was 0.05%.9 Despite prevalence similar to psoriasis, utilization of high-cost emergency and inpatient admissions is notably higher among patients with HS. Recent claims data suggest that HS patients utilize the ED at a rate 3 times higher than psoriasis patients and are admitted as inpatients at a rate 5 times higher.10 Similar data suggest an associated increased cost of care for patients with HS vs other conditions, such as psoriasis, due to frequent ED and inpatient stays.11 Although HS frequently presents in the inpatient and emergency settings, there is little literature on best methods for managing patients in these settings.
Pearls for Inpatient and Emergency Evaluation and Management
Initial Evaluation
When dermatologic consultative services are asked to evaluate patients with HS, preliminary evaluation should reflect the acuity of the patient. Vital signs and toxicity should be reviewed to ensure that there is no evidence of severe infection necessitating critical or acute care.
History
History-taking should reflect assessment of the patient’s baseline disease, including date of initial onset; exacerbating factors, such as friction, smoking, pregnancy, and menses; and the current history of the patient’s flare. A history of antibiotics, immunosuppression, topical therapy, antiandrogen therapy, and vitamin A analog therapy also should be reviewed. If an initial diagnosis is made in the ED or inpatient setting, a family and personal history should focus on specific risk factors and disease associations, including inflammatory bowel disease,12 pilonidal cysts,13 polycystic ovary syndrome,14 and metabolic syndrome.15
Physical Examination
As with all dermatologic consultations, a full-body skin examination, with special attention to the axilla, inframammary skin, groin, buttocks, and perineum, should be undertaken. In addition to these common areas of disease progression, examination should focus on atypical sites for disease manifestation, including the posterior auricular scalp, skin folds in the pannus and back, and the beard area in men. Evaluation of axillary and gluteal hair should note features of folliculitis and hair removal, which can exacerbate HS. Examination also should include an investigation of cutaneous manifestations of comorbid conditions, including acanthosis nigricans, contiguous or metastatic cutaneous Crohn disease, erythema nodosum, and pilonidal cysts. Caution should be exercised when diagnosing pilonidal cysts, as isolated or evolving HS in the gluteal cleft often is misdiagnosed as a pilonidal cyst.
Laboratory Evaluation
Testing often is misleading in patients with HS, especially in the acute setting, because the condition is a chronic inflammatory process. The C-reactive protein level as well as the absolute white blood cell and neutrophil counts often are elevated, even in the absence of acute infection.16 In fact, although patients often are treated with intravenous antibiotics by inpatient and emergency teams in the setting of these 3 laboratory abnormalities, these findings often reflect disease activity, not frank infection. Fever, especially low-grade fever, also can reflect ongoing disease activity. Thrombocytosis and anemia also are anecdotally common, though these findings have not been reported specifically in the literature.
Bacterial Cultures
The role of lesional and perilesional bacterial cultures is controversial in HS. Prior studies have demonstrated that biofilm formation may be associated with the chronic inflammation seen in HS.17 However, most data to date suggest that infection is not the primary driver of HS disease flares, as demonstrated by the frequency of sterile cultures and the variable response of the disease to penicillin and related antibiotics.18
Imaging
Ultrasonography and magnetic resonance imaging can be conducted if there is concern about deeper abscesses that are not apparent on examination. When interpreted by nondermatologic practitioners, however, the findings of these modalities can result in unnecessary surgical intervention, given the concern for development of infectious abscess.19
Diagnosis
Many patients with HS experience a notable delay in time to diagnosis, living with symptoms for 7 years on average prior to being given a name for their condition.20 Often, patients seek ED care at initial presentation because lesions can present quickly and are associated with remarkable pain. Inpatient dermatologic evaluation can provide patients with definitive diagnosis, appropriate counseling that provides an overview of the natural history of the disease, lifestyle recommendations, and expedited connection to outpatient longitudinal care.
Diagnosis is made clinically by assessment for typical lesions, such as painful or tender papules, nodules, or abscesses in the axillae, inframammary region, groin, thighs, and perineal and perianal regions. Cordlike scarring often is seen in the absence of active inflammatory lesions.21 Double-headed open comedones and prominent follicular occlusion are seen in some phenotypes but are not required for diagnosis.22
Multiple scoring modalities are in use23; the Hurley staging system, initially developed for surgical staging, has become a commonly used method in the clinical setting24:
• Hurley stage I: isolated nodules or abscesses;
• Hurley stage II: widely separate lesions and sinus tracts or scarring are suggestive; and
• Hurley stage III: multiple lesions with near-diffuse involvement and formation of sinus tracts and scarring.
Other scoring modalities, such as the Hidradenitis Suppurativa Clinical Response (HiSCR), are more commonly used in the clinical trial setting and quantitatively capture lesion count improvement while the patient is being treated.25
Treatment
Evaluation in the ED might necessitate recommendations for inpatient admission. Dermatologic consultation can be helpful in providing ED physicians with context for interpretation of laboratory results and clinical findings. Specifically, dermatologic evaluation can help differentiate presentations consistent with a primary infection from a more common presentation of HS flaring and associated bacterial colonization. Indications for inpatient admission are severe pain; concern for systemic infection, including high fever or sepsis; and need for surgical intervention. Patients with severe disease who do not have a longitudinal care plan or who lack the ability to care for lesions at home also are candidates for inpatient admission, where they can receive more intensive nursing and wound care as well as outpatient logistical management.
Acute care should be aimed at treatments that work quickly and aggressively and have both anti-inflammatory and antimicrobial effects. Severe flares require aggressive initial treatment to ensure more long-term remission. Adalimumab, maintained at 40 mg/wk after a loading dose, is the mainstay of evidence-based treatment for moderate to severe HS in patients 12 years or older; however, this treatment might not be easy to initiate in the inpatient setting because of its cost and availability and the fact that it is not as fast acting as other therapies.26 For patients with severe disease flares, prednisone,27 infliximab,28 or cyclosporine29 can be used in combination with antimicrobial therapy in the inpatient setting to quickly control active flaring. Intravenous antimicrobial therapy might be necessary in severe disease and should include coverage of gram-positive30 and anaerobic organisms.31
Although management of acute flares is critical, especially for hospitalized patients, initiating longitudinal treatment modalities while the patient is an inpatient will help prevent future readmissions, facilitate better outcomes, and enable longer periods of disease-free progression. Specific treatments, stratified by disease severity, are listed in the Table.

Postdischarge Lifestyle Modification
All disease management should include recommendations for lifestyle modification, including counseling on terminal hair removal (ie, avoid shaving, plucking, and waxing) and recommendations for daily and weekly decolonization with chlorhexidine or other antimicrobial soap, a weekly vinegar bath, and antiperspirant use in the groin and axilla. Avoiding tight clothes and humidity might also be helpful.
Other beneficial postdischarge strategies include smoking cessation and weight loss, which often are beneficial but difficult for many patients to achieve on their own; connecting patients with a primary care provider, which can facilitate better long-term outcomes; informing patients of the natural history of the disease and providing strategies for them to implement for acute flares to help avoid readmission and ED visits; and writing a “pill-in-pocket” prescription for a course of an antibiotic that provides good staphylococcal and anaerobic coverage, which can be helpful for patients who are prone to infrequent flares.
Lastly, appropriate postdischarge maintenance therapy also can be initiated during the inpatient stay, including maintenance antibiotic therapy, spironolactone32 for female patients, and acitretin33 for comedonal-predominant patients.
Final Thoughts
Hidradenitis suppurativa is a common dermatologic condition that frequently presents in emergency and inpatient settings, given its association with painful and acutely indurated lesions that often appear concerning for infection. Elevated inflammatory markers and fever are common in HS and are not necessarily suggestive of infection. As such, while antibiotics may be part of acute management of HS, care also should address the inflammatory component of the disease. Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting ED and inpatient care utilization.
Hidradenitis suppurativa (HS) is a common chronic inflammatory skin disease characterized by purulent subcutaneous nodules, papules, abscesses, and fistula tracts that lead to scarring and fibrosis. Lesions develop primarily in the axilla, groin, and other intertriginous and hair-bearing areas.
The natural history of the disease is characterized by periods of disease flare, followed by periods of disease quiescence. Patients might have weeks or months of low disease activity but frequently develop multiple exacerbating episodes over the course of weeks or months. The condition primarily presents in adolescent and peripubescent years, continuing throughout adulthood. Some evidence suggests a bimodal disease distribution, with a second peak of incidence in middle-aged adults. Women and men are affected equally; however, the disease can be phenotypically different in men and women.
Patients frequently present in emergency and inpatient settings for evaluation because of the pain and severity of HS flares as well as associated systemic symptoms. Inpatient and emergency department (ED) care are unique opportunities for dermatologic hospitalist and dermatologic consultative services to educate other physicians about the condition and initiate aggressive treatments that are frequently necessary to control HS flares. This article aims to address best methods for treating HS in these settings.
Pathophysiology
Although the exact pathophysiology of the condition is unknown, HS is thought to begin with follicular occlusion with downstream inflammation mediating neutrophilic activity and scarring. Hyperplasia of the infundibular epithelium is observed on histology, and the resulting occlusion, contained keratin, and follicular rupture initiate robust downstream inflammation.1,2 Follicular occlusion might be initially androgen mediated3 or might occur in combination with friction4 and genetic or acquired factors involving Notch signaling. Although HS characteristically presents in areas of high apocrine density, apocrine glands are not thought to be the primary mediator of disease activity.5 IL-17, IL-23, tumor necrosis factor α, and IL-1β are implicated in the pathogenesis of HS, but it is unknown if these cytokines are the driving pathologic factor in HS or if they are merely secondary sequelae.6
Demographics and Prevalence in Hospitalized Patients
Although increasing treatment availability has brought more attention to HS, true prevalence is unknown. A prevalence of 1% has been reported in many European countries.7 Global prevalence has been more difficult to determine, with variable data suggesting a prevalence of 0.03% to 8%, depending on the population included.8 Most patients studied in a US-based claims database were aged 30 to 64 years, and the overall prevalence was 0.05%.9 Despite prevalence similar to psoriasis, utilization of high-cost emergency and inpatient admissions is notably higher among patients with HS. Recent claims data suggest that HS patients utilize the ED at a rate 3 times higher than psoriasis patients and are admitted as inpatients at a rate 5 times higher.10 Similar data suggest an associated increased cost of care for patients with HS vs other conditions, such as psoriasis, due to frequent ED and inpatient stays.11 Although HS frequently presents in the inpatient and emergency settings, there is little literature on best methods for managing patients in these settings.
Pearls for Inpatient and Emergency Evaluation and Management
Initial Evaluation
When dermatologic consultative services are asked to evaluate patients with HS, preliminary evaluation should reflect the acuity of the patient. Vital signs and toxicity should be reviewed to ensure that there is no evidence of severe infection necessitating critical or acute care.
History
History-taking should reflect assessment of the patient’s baseline disease, including date of initial onset; exacerbating factors, such as friction, smoking, pregnancy, and menses; and the current history of the patient’s flare. A history of antibiotics, immunosuppression, topical therapy, antiandrogen therapy, and vitamin A analog therapy also should be reviewed. If an initial diagnosis is made in the ED or inpatient setting, a family and personal history should focus on specific risk factors and disease associations, including inflammatory bowel disease,12 pilonidal cysts,13 polycystic ovary syndrome,14 and metabolic syndrome.15
Physical Examination
As with all dermatologic consultations, a full-body skin examination, with special attention to the axilla, inframammary skin, groin, buttocks, and perineum, should be undertaken. In addition to these common areas of disease progression, examination should focus on atypical sites for disease manifestation, including the posterior auricular scalp, skin folds in the pannus and back, and the beard area in men. Evaluation of axillary and gluteal hair should note features of folliculitis and hair removal, which can exacerbate HS. Examination also should include an investigation of cutaneous manifestations of comorbid conditions, including acanthosis nigricans, contiguous or metastatic cutaneous Crohn disease, erythema nodosum, and pilonidal cysts. Caution should be exercised when diagnosing pilonidal cysts, as isolated or evolving HS in the gluteal cleft often is misdiagnosed as a pilonidal cyst.
Laboratory Evaluation
Testing often is misleading in patients with HS, especially in the acute setting, because the condition is a chronic inflammatory process. The C-reactive protein level as well as the absolute white blood cell and neutrophil counts often are elevated, even in the absence of acute infection.16 In fact, although patients often are treated with intravenous antibiotics by inpatient and emergency teams in the setting of these 3 laboratory abnormalities, these findings often reflect disease activity, not frank infection. Fever, especially low-grade fever, also can reflect ongoing disease activity. Thrombocytosis and anemia also are anecdotally common, though these findings have not been reported specifically in the literature.
Bacterial Cultures
The role of lesional and perilesional bacterial cultures is controversial in HS. Prior studies have demonstrated that biofilm formation may be associated with the chronic inflammation seen in HS.17 However, most data to date suggest that infection is not the primary driver of HS disease flares, as demonstrated by the frequency of sterile cultures and the variable response of the disease to penicillin and related antibiotics.18
Imaging
Ultrasonography and magnetic resonance imaging can be conducted if there is concern about deeper abscesses that are not apparent on examination. When interpreted by nondermatologic practitioners, however, the findings of these modalities can result in unnecessary surgical intervention, given the concern for development of infectious abscess.19
Diagnosis
Many patients with HS experience a notable delay in time to diagnosis, living with symptoms for 7 years on average prior to being given a name for their condition.20 Often, patients seek ED care at initial presentation because lesions can present quickly and are associated with remarkable pain. Inpatient dermatologic evaluation can provide patients with definitive diagnosis, appropriate counseling that provides an overview of the natural history of the disease, lifestyle recommendations, and expedited connection to outpatient longitudinal care.
Diagnosis is made clinically by assessment for typical lesions, such as painful or tender papules, nodules, or abscesses in the axillae, inframammary region, groin, thighs, and perineal and perianal regions. Cordlike scarring often is seen in the absence of active inflammatory lesions.21 Double-headed open comedones and prominent follicular occlusion are seen in some phenotypes but are not required for diagnosis.22
Multiple scoring modalities are in use23; the Hurley staging system, initially developed for surgical staging, has become a commonly used method in the clinical setting24:
• Hurley stage I: isolated nodules or abscesses;
• Hurley stage II: widely separate lesions and sinus tracts or scarring are suggestive; and
• Hurley stage III: multiple lesions with near-diffuse involvement and formation of sinus tracts and scarring.
Other scoring modalities, such as the Hidradenitis Suppurativa Clinical Response (HiSCR), are more commonly used in the clinical trial setting and quantitatively capture lesion count improvement while the patient is being treated.25
Treatment
Evaluation in the ED might necessitate recommendations for inpatient admission. Dermatologic consultation can be helpful in providing ED physicians with context for interpretation of laboratory results and clinical findings. Specifically, dermatologic evaluation can help differentiate presentations consistent with a primary infection from a more common presentation of HS flaring and associated bacterial colonization. Indications for inpatient admission are severe pain; concern for systemic infection, including high fever or sepsis; and need for surgical intervention. Patients with severe disease who do not have a longitudinal care plan or who lack the ability to care for lesions at home also are candidates for inpatient admission, where they can receive more intensive nursing and wound care as well as outpatient logistical management.
Acute care should be aimed at treatments that work quickly and aggressively and have both anti-inflammatory and antimicrobial effects. Severe flares require aggressive initial treatment to ensure more long-term remission. Adalimumab, maintained at 40 mg/wk after a loading dose, is the mainstay of evidence-based treatment for moderate to severe HS in patients 12 years or older; however, this treatment might not be easy to initiate in the inpatient setting because of its cost and availability and the fact that it is not as fast acting as other therapies.26 For patients with severe disease flares, prednisone,27 infliximab,28 or cyclosporine29 can be used in combination with antimicrobial therapy in the inpatient setting to quickly control active flaring. Intravenous antimicrobial therapy might be necessary in severe disease and should include coverage of gram-positive30 and anaerobic organisms.31
Although management of acute flares is critical, especially for hospitalized patients, initiating longitudinal treatment modalities while the patient is an inpatient will help prevent future readmissions, facilitate better outcomes, and enable longer periods of disease-free progression. Specific treatments, stratified by disease severity, are listed in the Table.

Postdischarge Lifestyle Modification
All disease management should include recommendations for lifestyle modification, including counseling on terminal hair removal (ie, avoid shaving, plucking, and waxing) and recommendations for daily and weekly decolonization with chlorhexidine or other antimicrobial soap, a weekly vinegar bath, and antiperspirant use in the groin and axilla. Avoiding tight clothes and humidity might also be helpful.
Other beneficial postdischarge strategies include smoking cessation and weight loss, which often are beneficial but difficult for many patients to achieve on their own; connecting patients with a primary care provider, which can facilitate better long-term outcomes; informing patients of the natural history of the disease and providing strategies for them to implement for acute flares to help avoid readmission and ED visits; and writing a “pill-in-pocket” prescription for a course of an antibiotic that provides good staphylococcal and anaerobic coverage, which can be helpful for patients who are prone to infrequent flares.
Lastly, appropriate postdischarge maintenance therapy also can be initiated during the inpatient stay, including maintenance antibiotic therapy, spironolactone32 for female patients, and acitretin33 for comedonal-predominant patients.
Final Thoughts
Hidradenitis suppurativa is a common dermatologic condition that frequently presents in emergency and inpatient settings, given its association with painful and acutely indurated lesions that often appear concerning for infection. Elevated inflammatory markers and fever are common in HS and are not necessarily suggestive of infection. As such, while antibiotics may be part of acute management of HS, care also should address the inflammatory component of the disease. Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting ED and inpatient care utilization.
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999.
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(suppl 1):S8-S11.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125:304-308.
- de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012;21:176-177.
- Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763-769.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601.
- Jemec GE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(suppl 1):S4-S7.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419.
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944.
- Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76:49-53.
- Benhadou F, Van der Zee HH, Pascual JC, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients [published online March 27, 2019]. Br J Dermatol. doi:10.1111/bjd.17927.
- Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol. 2018;138:1288-1292.
- Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:55-57.
- Hessam S, Sand M, Gambichler T, et al. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol. 2015;73:998-1005.
- Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993-1000.
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Wortsman X. Imaging of hidradenitis suppurativa. Dermatol Clin. 2016;34:59-68.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546-1549.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin. 2016;34:1-5.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506-1511.
- Porter ML, Kimball AB. Hidradenitis suppurativa scoring systems: can we choose just one? Cutis. 2017;99:156-157.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker, Inc; 1989:732-738.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75:1059-1062.
- Sullivan TP, Welsh E, Kerdel FA. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149:1046-1049.
- Anderson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa—a case series. J Dermatolog Treat. 2016;27:247-250.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Guet-Revillet H, Coignard-Biehler H, Jais JP, et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:114-119.
- Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170-174.
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999.
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(suppl 1):S8-S11.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125:304-308.
- de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012;21:176-177.
- Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763-769.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601.
- Jemec GE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(suppl 1):S4-S7.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419.
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944.
- Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76:49-53.
- Benhadou F, Van der Zee HH, Pascual JC, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients [published online March 27, 2019]. Br J Dermatol. doi:10.1111/bjd.17927.
- Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol. 2018;138:1288-1292.
- Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:55-57.
- Hessam S, Sand M, Gambichler T, et al. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol. 2015;73:998-1005.
- Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993-1000.
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Wortsman X. Imaging of hidradenitis suppurativa. Dermatol Clin. 2016;34:59-68.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546-1549.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin. 2016;34:1-5.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506-1511.
- Porter ML, Kimball AB. Hidradenitis suppurativa scoring systems: can we choose just one? Cutis. 2017;99:156-157.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker, Inc; 1989:732-738.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75:1059-1062.
- Sullivan TP, Welsh E, Kerdel FA. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149:1046-1049.
- Anderson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa—a case series. J Dermatolog Treat. 2016;27:247-250.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Guet-Revillet H, Coignard-Biehler H, Jais JP, et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:114-119.
- Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170-174.
Practice Points
- Hidradenitis suppurativa (HS) is a common dermatologic condition that frequently presents in emergency and inpatient settings.
- Anemia, leukocytosis, neutrophilia, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level are common markers of chronic inflammation in HS patients and might not signify infection.
- Acute management of HS should focus on anti-inflammatory and antibiotic regimens, with increasing severity dictating the need for more aggressive therapy.
- Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting emergency department and inpatient care utilization.
What’s Eating You? Dusky Pigmy Rattlesnake Envenomation and Management
Rattlesnakes are pit vipers with a rattle attached to the tip of the tail and facial pits located between the eyes and nose with a special organ that detects heat energy (infrared light) and is used for hunting prey. There are 2 genera of rattlesnakes, Sistrurus (3 species) and Crotalus (23 species). 1 The pigmy rattlesnake belongs to the Sistrurus miliarius species that is subdivided into 3 subspecies: the Carolina pigmy rattlesnake (Sistrurus miliarius miliarius), the Western pigmy rattlesnake (Sistrurus miliarius streckeri ), and the dusky pigmy rattlesnake (Sistrurus miliarius barbouri ). 1 The dusky pigmy rattlesnake is found in South Carolina, southern Georgia, southern Alabama, southeastern Mississippi, and Florida. 2 It is the most abundant venomous snake in Florida. 3 Its rattle is barely audible, and it is an aggressive small snake ranging in length from 38 to 56 cm. 4 Its venom is hemorrhagic, causing tissue damage but not containing neurotoxins. 4 Although bites can be painful, resulting in localized necrosis and rare loss of digits, it is unlikely for bites to be fatal given the snake’s small fangs, small size, and amount of envenomation. However, bites on children may require hospitalization. The venom contains proteins, polypeptides, and enzymes. 5 One such peptide, barbourin, inhibits a transmembrane receptor that plays a role in platelet aggregation. 6
We report a case of a 54-year-old man who was bitten on the left index finger by a dusky pigmy rattlesnake. We describe the clinical course and successful treatment with crotalidae polyvalent immune fab (CPIF) antivenom.
Case Report
A 54-year-old man presented to the emergency department with a rapidly swelling and erythematous left hand following a snakebite to the left index fingertip while weeding in his yard (Figure 1). The patient was able to kill the snake with a shovel and photograph it, which helped identify it as a dusky pigmy rattlesnake (Figure 2). Vitals on presentation included a blood pressure of 161/98, pulse oximeter of 99%, temperature of 36.4°C, pulse of 84 beats per minute, and respiratory rate of 16 breaths per minute.
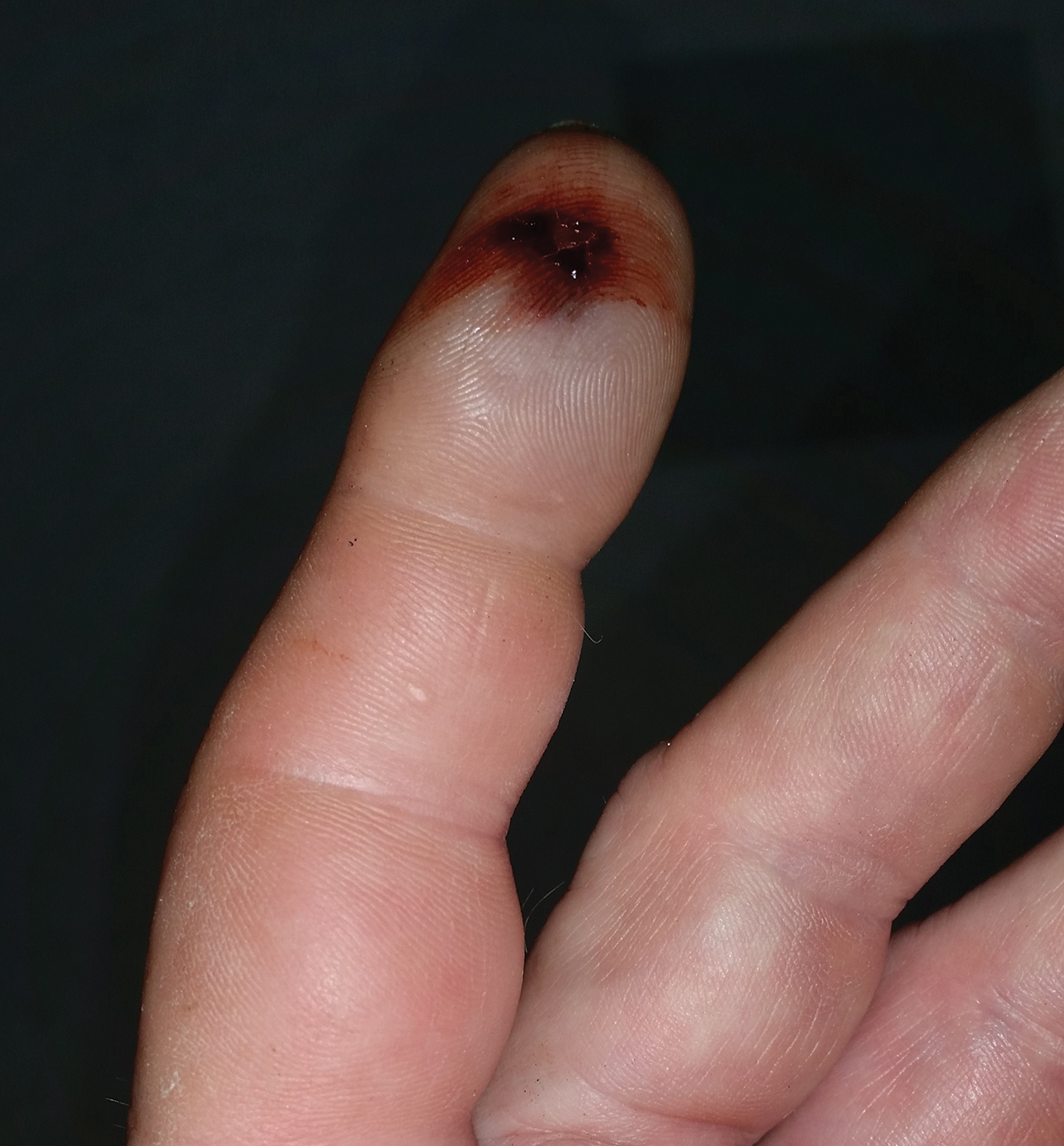

Given the poisonous snakebite, the patient was admitted to the intensive care unit. Laboratory test results at admission revealed the following values: platelet count, 235,000/µL (reference range, 150,000–450,000/µL); fibrinogen, 226.1 mg/dL (reference range, 185–410 mg/dL); fibrin degradation products, less than 10 µg/mL (reference range, <10 µg/mL); glucose, 145 mg/dL (reference range, 74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. His blood type was O Rh+. Radiography of the left second digit did not show any fractures, dislocations, or foreign object.
After consulting with the Tampa General Hospital Florida Poison Information Center, 6 vials of CPIF antivenom in 250 mL of sodium chloride initially were infused intravenously, followed by 2 additional vials each at 6, 12, and 18 hours. Serial laboratory test results revealed white blood cell counts of 13,600, 10,000, 6800, 6100, and 6800/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Platelet counts were 222,000, 159,000, 116,000, 99,000, and 129,000/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. The hemoglobin level was 14.8, 13.1, 13.8, 13.7, and 14.3 g/dL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Other laboratory test results including prothrombin time (10.0 s), fibrinogen (226.1 mg/dL), and fibrin degradation products (<10 µg/mL) at 4 hours postadmission remained within reference range during serial monitoring.
The patient was hospitalized for 4 days until the erythema of the left arm receded and only involved the left second phalanx where he eventually experienced localized skin necrosis (Figures 3 and 4). His thrombocytopenia trended upward from a low of 99,000/µL to 121,000/µL at the time of discharge. During his hospital stay, the patient developed hypertension from which he remained asymptomatic and was treated with lisinopril. The patient was treated with intravenous cefazolin and discharged on oral cephalexin due to an elevation in his white blood cell count on admission (13,600/µL). His cultures remained negative, and on discharge his white blood cell count had normalized (6800/µL) and he was transitioned to oral antibiotics to complete his treatment course. Following a surgical consultation, it was decided that skin debridement of the localized area of necrosis of the index fingertip was not necessary. The area of skin necrosis sloughed uneventfully with no residual functional impairment; however, the patient was left with residual numbness of the left second digit (Figure 5). He did not experience recurrent coagulopathy.
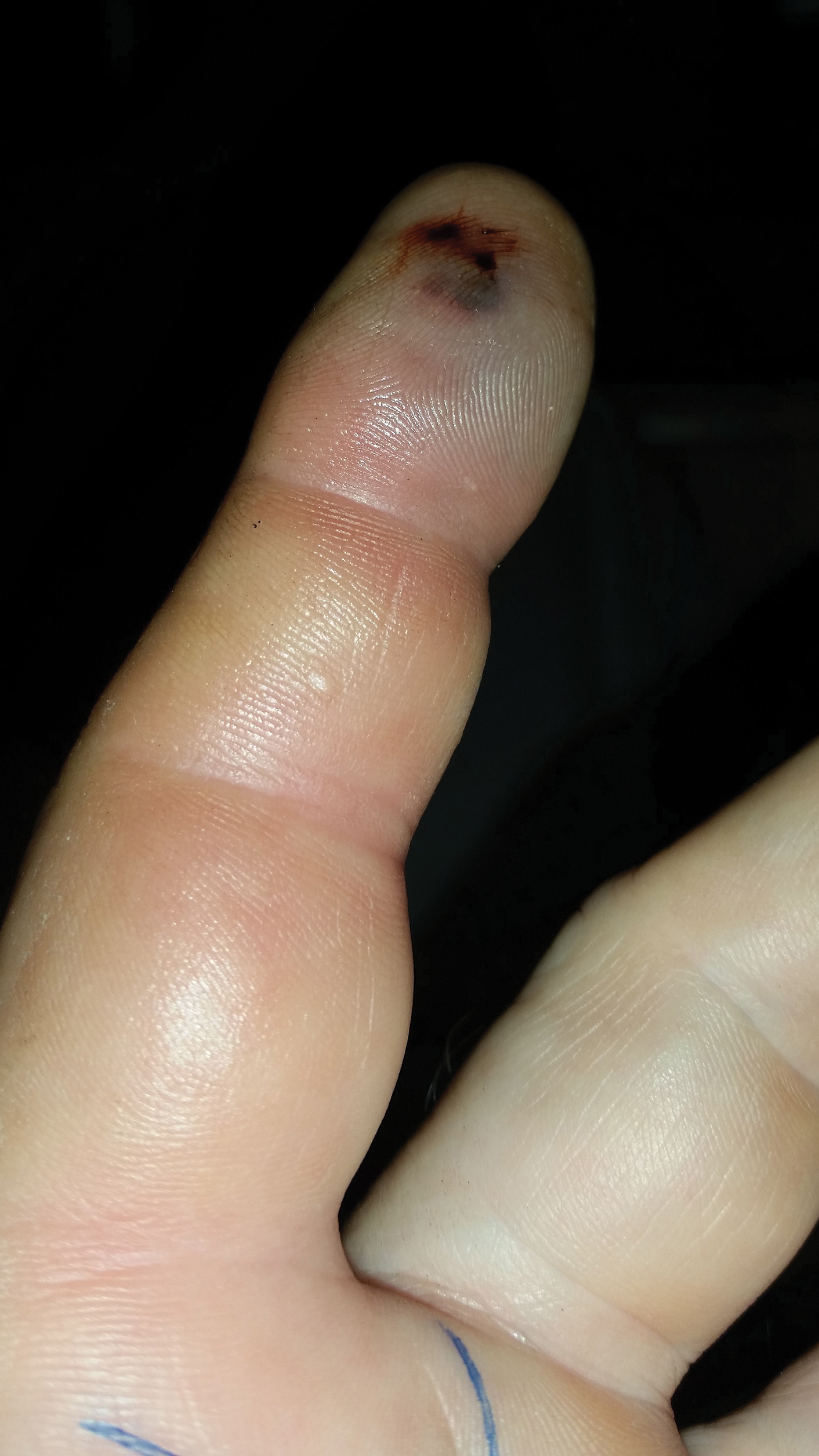
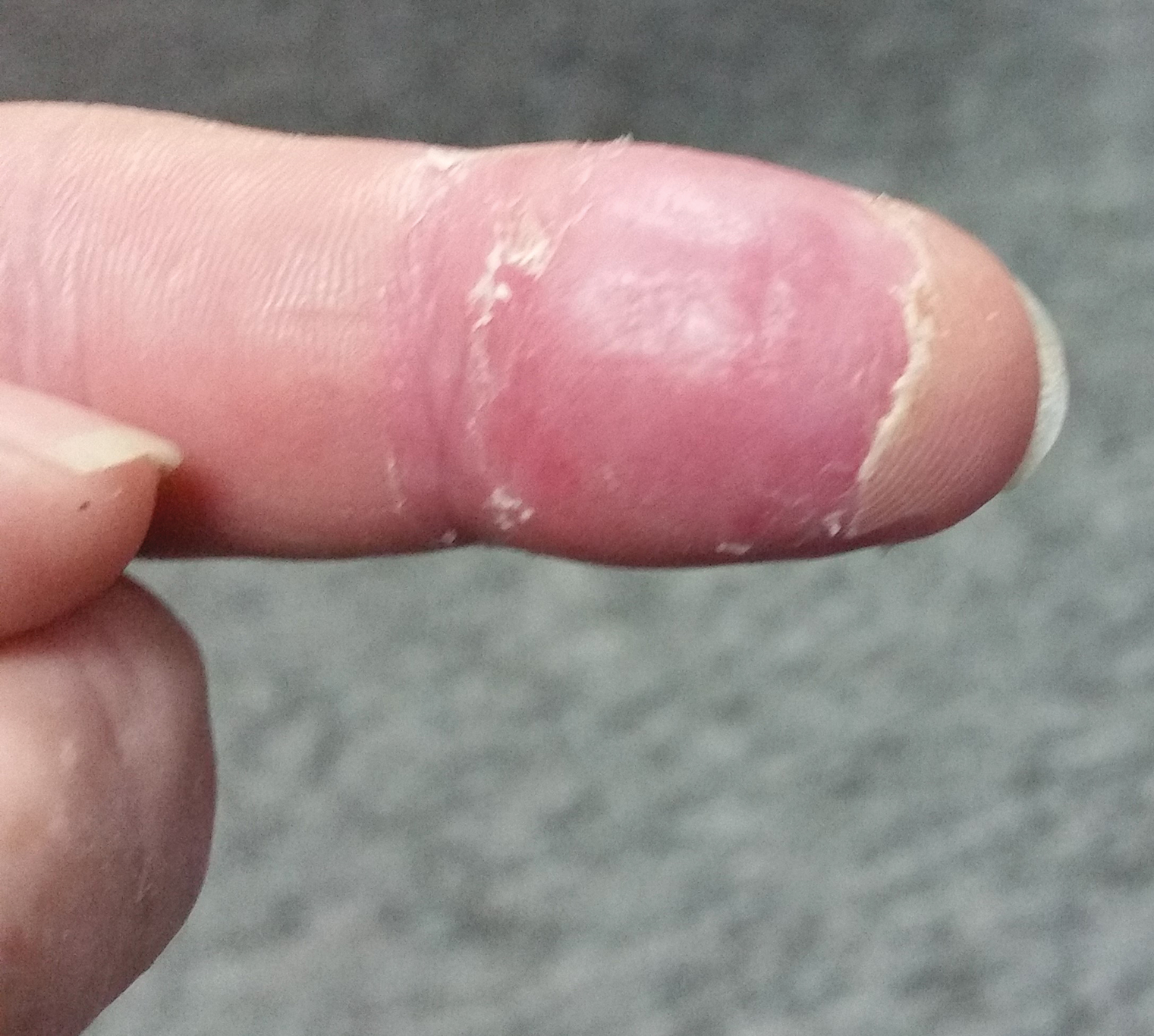
Comment
Envenomation
Snakebites and envenomation are a complex and broad subject beyond the scope of this article and further reading on this subject is highly encouraged. The clinical findings from snakebites range from mild local tissue reactions to severe systemic symptoms depending on the volume of venom injected, snake species, age and health of victim, and location of bite. Severe systemic symptoms include disseminated intravascular coagulation, acute renal failure, hypovolemic shock, and death.5 Venom can be hemotoxic and/or neurotoxic. Hemotoxic symptoms include pain, edema, swelling, ecchymoses, necrosis, and hemolysis. Neurotoxic symptoms may encompass diplopia, dysphagia, sweating, salivation, diaphoresis, respiratory depression, and paralysis.5 The pigmy rattlesnake venom is only hemotoxic, not neurotoxic.4 Eastern and western variety rattlesnakes account for most snake deaths due to their potent venom. Water moccasins (cottonmouths) have intermediate-potency venom, and copperhead snakes have the least-potent venom.5 Coral snakes are not pit vipers and require a different antivenom.
Management of Snakebites
Venomous snakes may bite a person without injecting venom. In fact, as many as 20% to 25% of all pit viper bites are dry.7 No attempts should be made to capture or kill the biting snake, but identifying it safely is helpful. Dead snakes should not be handled carelessly, as reflex biting after death has been reported.8 Cutting or suctioning the bite wound, nonsteroidal anti-inflammatory drugs, prophylactic antibiotics, tourniquets, prophylactic fasciotomy, and ice have not proven to be beneficial in the management of viper envenomation.5,9-11 The best management involves immobilizing the affected extremity, seeking immediate medical attention, and initiating antivenom therapy as soon as possible.
Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20
Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21
- The pigmy rattlesnake (Sistrurus miliarius). Stetson University website. http://www.stetson.edu/other/pigmy/pigmy-rattlesnake-information.php. Accessed October 18, 2019.
- Meadows A. Pigmy rattlesnake (Sistrurus miliarius)-Venomous. Savannah River Ecology Laboratory, University of Georgia website. https://srelherp.uga.edu/snakes/sismil.htm. Accessed October 21, 2019.
- Dusky pygmy rattlesnake. Central Florida Zoo & Botanical Gardens website. http://www.centralfloridazoo.org/animals/dusky-pygmy-rattlesnake/. Accessed October 21, 2019.
- Singha R. Facts about the pigmy rattlesnake that are sure to surprise you. AnimalSake website. https://animalsake.com/pygmy-rattlesnake. Updated August 1, 2017. Accessed October 21, 2019.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A spIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362.
- Johnson SA. Frequently asked questions about venomous snakes. UF Wildlife website. http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml. Accessed October 18, 2019.
- Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead. N Engl J Med. 1999;20:659-661.
- Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;37:175-180.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-373.
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586.
- Keating GM. Crotalidae polyvalent immune fab: in patients with North American crotaline envenomation. Bio Drugs. 2011;25:69-76.
- CroFab [prescribing information]. BTG International Inc; 2018.
- Consroe P, Egen NB, Russell FE, et al. Comparison of a new antigen binding fragment (FAB) antivenin for United States crotalidae with the commercial antivenin for protection against venom induced lethality in mice. Am J Trop Med Hyg. 1995;53:507-510.
- Quarre JP, Lecomte J, Lauwers D, et al. Allergy to latex and papain. J Allergy Clin Immunol. 1995;95:922.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2-15.
- Lavonas EJ, Khatri V, Daugherty C, et al. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71-78.
- Pizon AF, Riley BD, LoVecchio F, et al. Safety and efficacy of crotalidae polyvalent immune fab in pediatric crotaline envenomations. Acad Emerg Med. 2007;14:373-376.
- Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968-971.
- Centers for Disease Control and Prevention. Thimerosal in vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Updated October 25, 2015. Accessed October 21, 2019.
- King AM, Crim WS, Menke NB. Pigmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
Rattlesnakes are pit vipers with a rattle attached to the tip of the tail and facial pits located between the eyes and nose with a special organ that detects heat energy (infrared light) and is used for hunting prey. There are 2 genera of rattlesnakes, Sistrurus (3 species) and Crotalus (23 species). 1 The pigmy rattlesnake belongs to the Sistrurus miliarius species that is subdivided into 3 subspecies: the Carolina pigmy rattlesnake (Sistrurus miliarius miliarius), the Western pigmy rattlesnake (Sistrurus miliarius streckeri ), and the dusky pigmy rattlesnake (Sistrurus miliarius barbouri ). 1 The dusky pigmy rattlesnake is found in South Carolina, southern Georgia, southern Alabama, southeastern Mississippi, and Florida. 2 It is the most abundant venomous snake in Florida. 3 Its rattle is barely audible, and it is an aggressive small snake ranging in length from 38 to 56 cm. 4 Its venom is hemorrhagic, causing tissue damage but not containing neurotoxins. 4 Although bites can be painful, resulting in localized necrosis and rare loss of digits, it is unlikely for bites to be fatal given the snake’s small fangs, small size, and amount of envenomation. However, bites on children may require hospitalization. The venom contains proteins, polypeptides, and enzymes. 5 One such peptide, barbourin, inhibits a transmembrane receptor that plays a role in platelet aggregation. 6
We report a case of a 54-year-old man who was bitten on the left index finger by a dusky pigmy rattlesnake. We describe the clinical course and successful treatment with crotalidae polyvalent immune fab (CPIF) antivenom.
Case Report
A 54-year-old man presented to the emergency department with a rapidly swelling and erythematous left hand following a snakebite to the left index fingertip while weeding in his yard (Figure 1). The patient was able to kill the snake with a shovel and photograph it, which helped identify it as a dusky pigmy rattlesnake (Figure 2). Vitals on presentation included a blood pressure of 161/98, pulse oximeter of 99%, temperature of 36.4°C, pulse of 84 beats per minute, and respiratory rate of 16 breaths per minute.


Given the poisonous snakebite, the patient was admitted to the intensive care unit. Laboratory test results at admission revealed the following values: platelet count, 235,000/µL (reference range, 150,000–450,000/µL); fibrinogen, 226.1 mg/dL (reference range, 185–410 mg/dL); fibrin degradation products, less than 10 µg/mL (reference range, <10 µg/mL); glucose, 145 mg/dL (reference range, 74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. His blood type was O Rh+. Radiography of the left second digit did not show any fractures, dislocations, or foreign object.
After consulting with the Tampa General Hospital Florida Poison Information Center, 6 vials of CPIF antivenom in 250 mL of sodium chloride initially were infused intravenously, followed by 2 additional vials each at 6, 12, and 18 hours. Serial laboratory test results revealed white blood cell counts of 13,600, 10,000, 6800, 6100, and 6800/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Platelet counts were 222,000, 159,000, 116,000, 99,000, and 129,000/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. The hemoglobin level was 14.8, 13.1, 13.8, 13.7, and 14.3 g/dL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Other laboratory test results including prothrombin time (10.0 s), fibrinogen (226.1 mg/dL), and fibrin degradation products (<10 µg/mL) at 4 hours postadmission remained within reference range during serial monitoring.
The patient was hospitalized for 4 days until the erythema of the left arm receded and only involved the left second phalanx where he eventually experienced localized skin necrosis (Figures 3 and 4). His thrombocytopenia trended upward from a low of 99,000/µL to 121,000/µL at the time of discharge. During his hospital stay, the patient developed hypertension from which he remained asymptomatic and was treated with lisinopril. The patient was treated with intravenous cefazolin and discharged on oral cephalexin due to an elevation in his white blood cell count on admission (13,600/µL). His cultures remained negative, and on discharge his white blood cell count had normalized (6800/µL) and he was transitioned to oral antibiotics to complete his treatment course. Following a surgical consultation, it was decided that skin debridement of the localized area of necrosis of the index fingertip was not necessary. The area of skin necrosis sloughed uneventfully with no residual functional impairment; however, the patient was left with residual numbness of the left second digit (Figure 5). He did not experience recurrent coagulopathy.



Comment
Envenomation
Snakebites and envenomation are a complex and broad subject beyond the scope of this article and further reading on this subject is highly encouraged. The clinical findings from snakebites range from mild local tissue reactions to severe systemic symptoms depending on the volume of venom injected, snake species, age and health of victim, and location of bite. Severe systemic symptoms include disseminated intravascular coagulation, acute renal failure, hypovolemic shock, and death.5 Venom can be hemotoxic and/or neurotoxic. Hemotoxic symptoms include pain, edema, swelling, ecchymoses, necrosis, and hemolysis. Neurotoxic symptoms may encompass diplopia, dysphagia, sweating, salivation, diaphoresis, respiratory depression, and paralysis.5 The pigmy rattlesnake venom is only hemotoxic, not neurotoxic.4 Eastern and western variety rattlesnakes account for most snake deaths due to their potent venom. Water moccasins (cottonmouths) have intermediate-potency venom, and copperhead snakes have the least-potent venom.5 Coral snakes are not pit vipers and require a different antivenom.
Management of Snakebites
Venomous snakes may bite a person without injecting venom. In fact, as many as 20% to 25% of all pit viper bites are dry.7 No attempts should be made to capture or kill the biting snake, but identifying it safely is helpful. Dead snakes should not be handled carelessly, as reflex biting after death has been reported.8 Cutting or suctioning the bite wound, nonsteroidal anti-inflammatory drugs, prophylactic antibiotics, tourniquets, prophylactic fasciotomy, and ice have not proven to be beneficial in the management of viper envenomation.5,9-11 The best management involves immobilizing the affected extremity, seeking immediate medical attention, and initiating antivenom therapy as soon as possible.
Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20

Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21
Rattlesnakes are pit vipers with a rattle attached to the tip of the tail and facial pits located between the eyes and nose with a special organ that detects heat energy (infrared light) and is used for hunting prey. There are 2 genera of rattlesnakes, Sistrurus (3 species) and Crotalus (23 species). 1 The pigmy rattlesnake belongs to the Sistrurus miliarius species that is subdivided into 3 subspecies: the Carolina pigmy rattlesnake (Sistrurus miliarius miliarius), the Western pigmy rattlesnake (Sistrurus miliarius streckeri ), and the dusky pigmy rattlesnake (Sistrurus miliarius barbouri ). 1 The dusky pigmy rattlesnake is found in South Carolina, southern Georgia, southern Alabama, southeastern Mississippi, and Florida. 2 It is the most abundant venomous snake in Florida. 3 Its rattle is barely audible, and it is an aggressive small snake ranging in length from 38 to 56 cm. 4 Its venom is hemorrhagic, causing tissue damage but not containing neurotoxins. 4 Although bites can be painful, resulting in localized necrosis and rare loss of digits, it is unlikely for bites to be fatal given the snake’s small fangs, small size, and amount of envenomation. However, bites on children may require hospitalization. The venom contains proteins, polypeptides, and enzymes. 5 One such peptide, barbourin, inhibits a transmembrane receptor that plays a role in platelet aggregation. 6
We report a case of a 54-year-old man who was bitten on the left index finger by a dusky pigmy rattlesnake. We describe the clinical course and successful treatment with crotalidae polyvalent immune fab (CPIF) antivenom.
Case Report
A 54-year-old man presented to the emergency department with a rapidly swelling and erythematous left hand following a snakebite to the left index fingertip while weeding in his yard (Figure 1). The patient was able to kill the snake with a shovel and photograph it, which helped identify it as a dusky pigmy rattlesnake (Figure 2). Vitals on presentation included a blood pressure of 161/98, pulse oximeter of 99%, temperature of 36.4°C, pulse of 84 beats per minute, and respiratory rate of 16 breaths per minute.


Given the poisonous snakebite, the patient was admitted to the intensive care unit. Laboratory test results at admission revealed the following values: platelet count, 235,000/µL (reference range, 150,000–450,000/µL); fibrinogen, 226.1 mg/dL (reference range, 185–410 mg/dL); fibrin degradation products, less than 10 µg/mL (reference range, <10 µg/mL); glucose, 145 mg/dL (reference range, 74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. His blood type was O Rh+. Radiography of the left second digit did not show any fractures, dislocations, or foreign object.
After consulting with the Tampa General Hospital Florida Poison Information Center, 6 vials of CPIF antivenom in 250 mL of sodium chloride initially were infused intravenously, followed by 2 additional vials each at 6, 12, and 18 hours. Serial laboratory test results revealed white blood cell counts of 13,600, 10,000, 6800, 6100, and 6800/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Platelet counts were 222,000, 159,000, 116,000, 99,000, and 129,000/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. The hemoglobin level was 14.8, 13.1, 13.8, 13.7, and 14.3 g/dL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Other laboratory test results including prothrombin time (10.0 s), fibrinogen (226.1 mg/dL), and fibrin degradation products (<10 µg/mL) at 4 hours postadmission remained within reference range during serial monitoring.
The patient was hospitalized for 4 days until the erythema of the left arm receded and only involved the left second phalanx where he eventually experienced localized skin necrosis (Figures 3 and 4). His thrombocytopenia trended upward from a low of 99,000/µL to 121,000/µL at the time of discharge. During his hospital stay, the patient developed hypertension from which he remained asymptomatic and was treated with lisinopril. The patient was treated with intravenous cefazolin and discharged on oral cephalexin due to an elevation in his white blood cell count on admission (13,600/µL). His cultures remained negative, and on discharge his white blood cell count had normalized (6800/µL) and he was transitioned to oral antibiotics to complete his treatment course. Following a surgical consultation, it was decided that skin debridement of the localized area of necrosis of the index fingertip was not necessary. The area of skin necrosis sloughed uneventfully with no residual functional impairment; however, the patient was left with residual numbness of the left second digit (Figure 5). He did not experience recurrent coagulopathy.



Comment
Envenomation
Snakebites and envenomation are a complex and broad subject beyond the scope of this article and further reading on this subject is highly encouraged. The clinical findings from snakebites range from mild local tissue reactions to severe systemic symptoms depending on the volume of venom injected, snake species, age and health of victim, and location of bite. Severe systemic symptoms include disseminated intravascular coagulation, acute renal failure, hypovolemic shock, and death.5 Venom can be hemotoxic and/or neurotoxic. Hemotoxic symptoms include pain, edema, swelling, ecchymoses, necrosis, and hemolysis. Neurotoxic symptoms may encompass diplopia, dysphagia, sweating, salivation, diaphoresis, respiratory depression, and paralysis.5 The pigmy rattlesnake venom is only hemotoxic, not neurotoxic.4 Eastern and western variety rattlesnakes account for most snake deaths due to their potent venom. Water moccasins (cottonmouths) have intermediate-potency venom, and copperhead snakes have the least-potent venom.5 Coral snakes are not pit vipers and require a different antivenom.
Management of Snakebites
Venomous snakes may bite a person without injecting venom. In fact, as many as 20% to 25% of all pit viper bites are dry.7 No attempts should be made to capture or kill the biting snake, but identifying it safely is helpful. Dead snakes should not be handled carelessly, as reflex biting after death has been reported.8 Cutting or suctioning the bite wound, nonsteroidal anti-inflammatory drugs, prophylactic antibiotics, tourniquets, prophylactic fasciotomy, and ice have not proven to be beneficial in the management of viper envenomation.5,9-11 The best management involves immobilizing the affected extremity, seeking immediate medical attention, and initiating antivenom therapy as soon as possible.
Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20

Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21
- The pigmy rattlesnake (Sistrurus miliarius). Stetson University website. http://www.stetson.edu/other/pigmy/pigmy-rattlesnake-information.php. Accessed October 18, 2019.
- Meadows A. Pigmy rattlesnake (Sistrurus miliarius)-Venomous. Savannah River Ecology Laboratory, University of Georgia website. https://srelherp.uga.edu/snakes/sismil.htm. Accessed October 21, 2019.
- Dusky pygmy rattlesnake. Central Florida Zoo & Botanical Gardens website. http://www.centralfloridazoo.org/animals/dusky-pygmy-rattlesnake/. Accessed October 21, 2019.
- Singha R. Facts about the pigmy rattlesnake that are sure to surprise you. AnimalSake website. https://animalsake.com/pygmy-rattlesnake. Updated August 1, 2017. Accessed October 21, 2019.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A spIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362.
- Johnson SA. Frequently asked questions about venomous snakes. UF Wildlife website. http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml. Accessed October 18, 2019.
- Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead. N Engl J Med. 1999;20:659-661.
- Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;37:175-180.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-373.
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586.
- Keating GM. Crotalidae polyvalent immune fab: in patients with North American crotaline envenomation. Bio Drugs. 2011;25:69-76.
- CroFab [prescribing information]. BTG International Inc; 2018.
- Consroe P, Egen NB, Russell FE, et al. Comparison of a new antigen binding fragment (FAB) antivenin for United States crotalidae with the commercial antivenin for protection against venom induced lethality in mice. Am J Trop Med Hyg. 1995;53:507-510.
- Quarre JP, Lecomte J, Lauwers D, et al. Allergy to latex and papain. J Allergy Clin Immunol. 1995;95:922.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2-15.
- Lavonas EJ, Khatri V, Daugherty C, et al. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71-78.
- Pizon AF, Riley BD, LoVecchio F, et al. Safety and efficacy of crotalidae polyvalent immune fab in pediatric crotaline envenomations. Acad Emerg Med. 2007;14:373-376.
- Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968-971.
- Centers for Disease Control and Prevention. Thimerosal in vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Updated October 25, 2015. Accessed October 21, 2019.
- King AM, Crim WS, Menke NB. Pigmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- The pigmy rattlesnake (Sistrurus miliarius). Stetson University website. http://www.stetson.edu/other/pigmy/pigmy-rattlesnake-information.php. Accessed October 18, 2019.
- Meadows A. Pigmy rattlesnake (Sistrurus miliarius)-Venomous. Savannah River Ecology Laboratory, University of Georgia website. https://srelherp.uga.edu/snakes/sismil.htm. Accessed October 21, 2019.
- Dusky pygmy rattlesnake. Central Florida Zoo & Botanical Gardens website. http://www.centralfloridazoo.org/animals/dusky-pygmy-rattlesnake/. Accessed October 21, 2019.
- Singha R. Facts about the pigmy rattlesnake that are sure to surprise you. AnimalSake website. https://animalsake.com/pygmy-rattlesnake. Updated August 1, 2017. Accessed October 21, 2019.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A spIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362.
- Johnson SA. Frequently asked questions about venomous snakes. UF Wildlife website. http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml. Accessed October 18, 2019.
- Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead. N Engl J Med. 1999;20:659-661.
- Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;37:175-180.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-373.
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586.
- Keating GM. Crotalidae polyvalent immune fab: in patients with North American crotaline envenomation. Bio Drugs. 2011;25:69-76.
- CroFab [prescribing information]. BTG International Inc; 2018.
- Consroe P, Egen NB, Russell FE, et al. Comparison of a new antigen binding fragment (FAB) antivenin for United States crotalidae with the commercial antivenin for protection against venom induced lethality in mice. Am J Trop Med Hyg. 1995;53:507-510.
- Quarre JP, Lecomte J, Lauwers D, et al. Allergy to latex and papain. J Allergy Clin Immunol. 1995;95:922.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2-15.
- Lavonas EJ, Khatri V, Daugherty C, et al. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71-78.
- Pizon AF, Riley BD, LoVecchio F, et al. Safety and efficacy of crotalidae polyvalent immune fab in pediatric crotaline envenomations. Acad Emerg Med. 2007;14:373-376.
- Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968-971.
- Centers for Disease Control and Prevention. Thimerosal in vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Updated October 25, 2015. Accessed October 21, 2019.
- King AM, Crim WS, Menke NB. Pigmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
Practice Points
- Avoid icing, cutting, and suctioning a snakebite wound or using tourniquets.
- Immobilize and elevate the affected extremity and seek medical attention immediately for early initiation of antivenom treatment.
- Remove rings or constrictive items in the event of swelling.
Patch Testing in Children: Not Just Little Adults
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
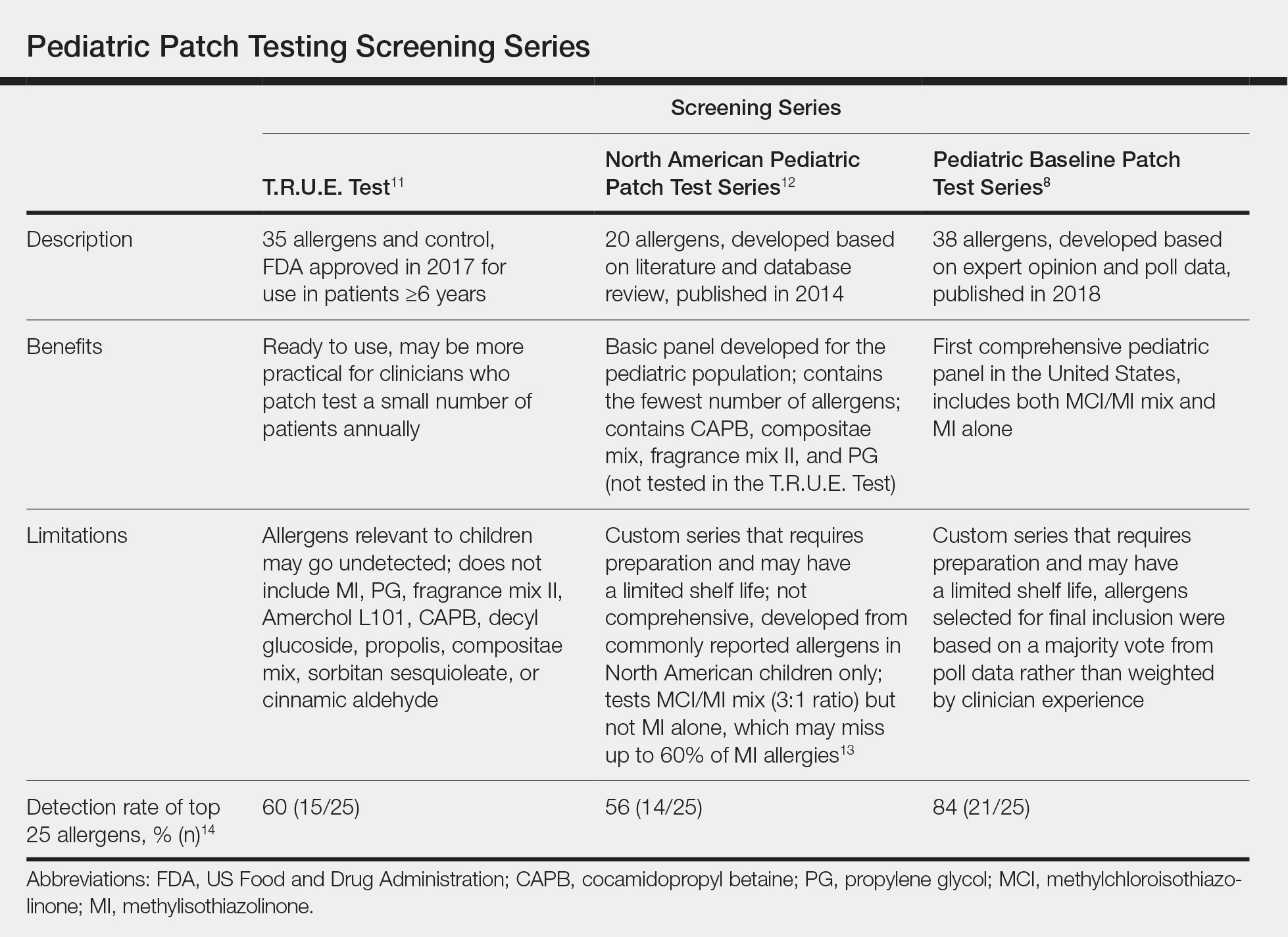
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).

The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).

The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
Practice Points
- Pediatric allergic contact dermatitis (ACD) is common with children having unique product exposures.
- Children suspected to have ACD should be patch tested with customized panels based on history and exposure.
- Common pediatric allergens have been identified in personal care products, household products, and recreational gear and toys.
Fluoroscopic system can improve targeting of lung lesions
NEW ORLEANS – A novel according to an industry-sponsored, prospective study.
The system, which incorporates fluoroscopic navigation, increased the percentage of cases in which the target overlapped with the lesion, from 60% to 83%. The percentage of cases without any target overlap decreased from 32% to 5%.
“Tomosynthesis-based fluoroscopic navigation … improves the three-dimensional convergence between the virtual target and the actual target,” said Krish Bhadra, MD, of CHI Memorial Medical Group in Chattanooga, Tenn.
Dr. Bhadra presented results with this system at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a study of Medtronic’s superDimension navigation system (version 7.2), which provides real-time imaging with three-dimensional fluoroscopy. The system has a “local registration” feature, which uses fluoroscopy and an algorithm to update the virtual target location during the procedure. This allows the user to reposition the catheter based on the location of the lesion.
The researchers tested the system in 50 patients from two centers (NCT03585959). Patients’ lesions had to be larger than 10 mm, not visible endobronchially, and not reachable by convex endobronchial ultrasound. Lesions within 10 mm of the diaphragm were excluded.
The median lesion size was 17.0 mm, 61.2% were smaller than 20 mm, 65.3% were in the upper lobe, and 53.1% had a bronchus sign present. The median distance from lesion to pleura was 5.9 mm.
Dr. Bhadra said the system performed as designed in all cases, and the protocol-defined technical success rate was 95.9% (47/49). Local registration was attempted in 49 patients and was successful in 47 patients (95.9%). In the unsuccessful cases, local registration was not completed based on the system design because the correction distance was greater than 3.0 cm.
The study’s primary endpoint was three-dimensional overlap of the virtual target and the actual lesion, as confirmed by cone-beam computed tomography. Success was defined as greater than 0% overlap after location correction. Target overlap was achieved in 59.6% (28/47) of cases before local registration and 83.0% (39/47) of cases after.
There were six cases in which local registration was successful, but these subjects weren’t evaluable because of failed procedure recording. When those subjects were excluded, target overlap was achieved in 95.1% (39/41) of cases after local registration.
The median percent overlap between the virtual target and the actual lesion was 11.4% before local registration and 32.8% after. The percentage of cases without any target overlap decreased from 31.7% (13/41) before local registration to 4.9% (2/41) after.
Focusing on the two cases without target overlap, Dr. Bhadra noted that he was able to get a biopsy that proved a malignancy in one of those patients. In the other patient, Dr. Bhadra was able to identify features of organizing pneumonia.
“Even though we did not have overlap, we must have been close enough that we were able to get malignant tissue in one [patient] and features of organizing pneumonia in a patient who’s got no history of organizing pneumonia,” Dr. Bhadra said.
He and his colleagues did not evaluate diagnostic yield in this study, but they did assess complications up to 7 days after the procedure.
The team reported one case of pneumothorax, but the patient didn’t require a chest tube. Additionally, there were two cases of bronchopulmonary hemorrhage, but the patients didn’t require any interventions.
This study was sponsored by Medtronic. Dr. Bhadra disclosed relationships with Medtronic, Boston Scientific, BodyVision, Auris Surgical Robotics, Intuitive Surgical, Veracyte, Biodesix, Merit Medical Endotek, and Johnson & Johnson.
SOURCE: Bhadra K et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.314.
NEW ORLEANS – A novel according to an industry-sponsored, prospective study.
The system, which incorporates fluoroscopic navigation, increased the percentage of cases in which the target overlapped with the lesion, from 60% to 83%. The percentage of cases without any target overlap decreased from 32% to 5%.
“Tomosynthesis-based fluoroscopic navigation … improves the three-dimensional convergence between the virtual target and the actual target,” said Krish Bhadra, MD, of CHI Memorial Medical Group in Chattanooga, Tenn.
Dr. Bhadra presented results with this system at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a study of Medtronic’s superDimension navigation system (version 7.2), which provides real-time imaging with three-dimensional fluoroscopy. The system has a “local registration” feature, which uses fluoroscopy and an algorithm to update the virtual target location during the procedure. This allows the user to reposition the catheter based on the location of the lesion.
The researchers tested the system in 50 patients from two centers (NCT03585959). Patients’ lesions had to be larger than 10 mm, not visible endobronchially, and not reachable by convex endobronchial ultrasound. Lesions within 10 mm of the diaphragm were excluded.
The median lesion size was 17.0 mm, 61.2% were smaller than 20 mm, 65.3% were in the upper lobe, and 53.1% had a bronchus sign present. The median distance from lesion to pleura was 5.9 mm.
Dr. Bhadra said the system performed as designed in all cases, and the protocol-defined technical success rate was 95.9% (47/49). Local registration was attempted in 49 patients and was successful in 47 patients (95.9%). In the unsuccessful cases, local registration was not completed based on the system design because the correction distance was greater than 3.0 cm.
The study’s primary endpoint was three-dimensional overlap of the virtual target and the actual lesion, as confirmed by cone-beam computed tomography. Success was defined as greater than 0% overlap after location correction. Target overlap was achieved in 59.6% (28/47) of cases before local registration and 83.0% (39/47) of cases after.
There were six cases in which local registration was successful, but these subjects weren’t evaluable because of failed procedure recording. When those subjects were excluded, target overlap was achieved in 95.1% (39/41) of cases after local registration.
The median percent overlap between the virtual target and the actual lesion was 11.4% before local registration and 32.8% after. The percentage of cases without any target overlap decreased from 31.7% (13/41) before local registration to 4.9% (2/41) after.
Focusing on the two cases without target overlap, Dr. Bhadra noted that he was able to get a biopsy that proved a malignancy in one of those patients. In the other patient, Dr. Bhadra was able to identify features of organizing pneumonia.
“Even though we did not have overlap, we must have been close enough that we were able to get malignant tissue in one [patient] and features of organizing pneumonia in a patient who’s got no history of organizing pneumonia,” Dr. Bhadra said.
He and his colleagues did not evaluate diagnostic yield in this study, but they did assess complications up to 7 days after the procedure.
The team reported one case of pneumothorax, but the patient didn’t require a chest tube. Additionally, there were two cases of bronchopulmonary hemorrhage, but the patients didn’t require any interventions.
This study was sponsored by Medtronic. Dr. Bhadra disclosed relationships with Medtronic, Boston Scientific, BodyVision, Auris Surgical Robotics, Intuitive Surgical, Veracyte, Biodesix, Merit Medical Endotek, and Johnson & Johnson.
SOURCE: Bhadra K et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.314.
NEW ORLEANS – A novel according to an industry-sponsored, prospective study.
The system, which incorporates fluoroscopic navigation, increased the percentage of cases in which the target overlapped with the lesion, from 60% to 83%. The percentage of cases without any target overlap decreased from 32% to 5%.
“Tomosynthesis-based fluoroscopic navigation … improves the three-dimensional convergence between the virtual target and the actual target,” said Krish Bhadra, MD, of CHI Memorial Medical Group in Chattanooga, Tenn.
Dr. Bhadra presented results with this system at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a study of Medtronic’s superDimension navigation system (version 7.2), which provides real-time imaging with three-dimensional fluoroscopy. The system has a “local registration” feature, which uses fluoroscopy and an algorithm to update the virtual target location during the procedure. This allows the user to reposition the catheter based on the location of the lesion.
The researchers tested the system in 50 patients from two centers (NCT03585959). Patients’ lesions had to be larger than 10 mm, not visible endobronchially, and not reachable by convex endobronchial ultrasound. Lesions within 10 mm of the diaphragm were excluded.
The median lesion size was 17.0 mm, 61.2% were smaller than 20 mm, 65.3% were in the upper lobe, and 53.1% had a bronchus sign present. The median distance from lesion to pleura was 5.9 mm.
Dr. Bhadra said the system performed as designed in all cases, and the protocol-defined technical success rate was 95.9% (47/49). Local registration was attempted in 49 patients and was successful in 47 patients (95.9%). In the unsuccessful cases, local registration was not completed based on the system design because the correction distance was greater than 3.0 cm.
The study’s primary endpoint was three-dimensional overlap of the virtual target and the actual lesion, as confirmed by cone-beam computed tomography. Success was defined as greater than 0% overlap after location correction. Target overlap was achieved in 59.6% (28/47) of cases before local registration and 83.0% (39/47) of cases after.
There were six cases in which local registration was successful, but these subjects weren’t evaluable because of failed procedure recording. When those subjects were excluded, target overlap was achieved in 95.1% (39/41) of cases after local registration.
The median percent overlap between the virtual target and the actual lesion was 11.4% before local registration and 32.8% after. The percentage of cases without any target overlap decreased from 31.7% (13/41) before local registration to 4.9% (2/41) after.
Focusing on the two cases without target overlap, Dr. Bhadra noted that he was able to get a biopsy that proved a malignancy in one of those patients. In the other patient, Dr. Bhadra was able to identify features of organizing pneumonia.
“Even though we did not have overlap, we must have been close enough that we were able to get malignant tissue in one [patient] and features of organizing pneumonia in a patient who’s got no history of organizing pneumonia,” Dr. Bhadra said.
He and his colleagues did not evaluate diagnostic yield in this study, but they did assess complications up to 7 days after the procedure.
The team reported one case of pneumothorax, but the patient didn’t require a chest tube. Additionally, there were two cases of bronchopulmonary hemorrhage, but the patients didn’t require any interventions.
This study was sponsored by Medtronic. Dr. Bhadra disclosed relationships with Medtronic, Boston Scientific, BodyVision, Auris Surgical Robotics, Intuitive Surgical, Veracyte, Biodesix, Merit Medical Endotek, and Johnson & Johnson.
SOURCE: Bhadra K et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.314.
REPORTING FROM CHEST 2019
Fewer bloodstream infections with FMT for C. difficile
Treating Clostridioides difficile infection with fecal microbiota transplantation is associated with a lower risk of bloodstream infection and recurrence than treatment with antibiotics, new research has found.
A paper published in Annals of Internal Medicine presents outcomes of a prospective cohort study in 290 inpatients with recurrent C. difficile infection, 109 of whom were treated with fecal microbiota transplantation (FMT); the remainder were treated with antibiotics including metronidazole, vancomycin, and fidaxomicin.
While the FMT group had a higher mean number of previous C. difficile infections than the antibiotics group (2.82 vs. 1.23, respectively), a sustained cure was achieved in 97% of the FMT group, compared with 38% in the antibiotics group.
Blood cultures were done if patients developed a temperature above 30° C or showed symptoms that might be attributable to sepsis. Bloodstream infections were diagnosed in 5% (5 patients) of those treated with FMT, and 22% (40 patients) in the antibiotics group.
The patients in the FMT group with bloodstream infections all had bacterial infections – one of which was polymicrobial – and there were no cases of fungal bloodstream infections. In the antibiotics group, 28 patients (15%) had bacterial bloodstream infections – 11 of which were polymicrobial – and 12 (7%) had fungal bloodstream infections.
Bloodstream infections were particularly evident among the 11 patients whose C. difficile infection was treated with fidaxomicin, 4 of whom developed a bloodstream infection.
Overall, 27% of patients died during the 90-day follow-up, with 7% dying because of bloodstream infections, all of whom were in the antibiotic-treated cohort. Three patients in the FMT group died because of overwhelming C. difficile infection, compared with 12 in the antibiotic cohort.
Nearly three-quarters of deaths occurred within 30 days of the end of treatment; 5 of these deaths were in the FMT group, and 53 were in the antibiotics group.
“These findings suggest that the longer 90-day [overall survival] of patients in the FMT group is attributable to cure of [C. difficile infection] leading to an improvement in clinical condition,” wrote Gianluca Ianiro, MD, from the Catholic University of the Sacred Heart in Rome, and coauthors.
The 90-day overall survival rate was 92% in the FMT group and 61% in the antibiotic group. Patients treated with FMT also showed significantly shorter mean duration of hospital stay at 13.3 days, compared with 29.7 days in patients treated with antibiotics.
The authors noted the results should be interpreted with caution because of baseline differences between the two groups that were not entirely accounted for by using propensity matching. However, even in the propensity-matched cohort of 57 patients from each group, there was still a significantly higher overall survival at 90 days among patients treated with FMT.
One author declared grants from the pharmaceutical sector outside the submitted work. No funding or other conflicts of interest were reported.
SOURCE: Ianiro G et al. Ann Intern Med. 2019 Nov 4. doi: 10.7326/M18-3635.
Treating Clostridioides difficile infection with fecal microbiota transplantation is associated with a lower risk of bloodstream infection and recurrence than treatment with antibiotics, new research has found.
A paper published in Annals of Internal Medicine presents outcomes of a prospective cohort study in 290 inpatients with recurrent C. difficile infection, 109 of whom were treated with fecal microbiota transplantation (FMT); the remainder were treated with antibiotics including metronidazole, vancomycin, and fidaxomicin.
While the FMT group had a higher mean number of previous C. difficile infections than the antibiotics group (2.82 vs. 1.23, respectively), a sustained cure was achieved in 97% of the FMT group, compared with 38% in the antibiotics group.
Blood cultures were done if patients developed a temperature above 30° C or showed symptoms that might be attributable to sepsis. Bloodstream infections were diagnosed in 5% (5 patients) of those treated with FMT, and 22% (40 patients) in the antibiotics group.
The patients in the FMT group with bloodstream infections all had bacterial infections – one of which was polymicrobial – and there were no cases of fungal bloodstream infections. In the antibiotics group, 28 patients (15%) had bacterial bloodstream infections – 11 of which were polymicrobial – and 12 (7%) had fungal bloodstream infections.
Bloodstream infections were particularly evident among the 11 patients whose C. difficile infection was treated with fidaxomicin, 4 of whom developed a bloodstream infection.
Overall, 27% of patients died during the 90-day follow-up, with 7% dying because of bloodstream infections, all of whom were in the antibiotic-treated cohort. Three patients in the FMT group died because of overwhelming C. difficile infection, compared with 12 in the antibiotic cohort.
Nearly three-quarters of deaths occurred within 30 days of the end of treatment; 5 of these deaths were in the FMT group, and 53 were in the antibiotics group.
“These findings suggest that the longer 90-day [overall survival] of patients in the FMT group is attributable to cure of [C. difficile infection] leading to an improvement in clinical condition,” wrote Gianluca Ianiro, MD, from the Catholic University of the Sacred Heart in Rome, and coauthors.
The 90-day overall survival rate was 92% in the FMT group and 61% in the antibiotic group. Patients treated with FMT also showed significantly shorter mean duration of hospital stay at 13.3 days, compared with 29.7 days in patients treated with antibiotics.
The authors noted the results should be interpreted with caution because of baseline differences between the two groups that were not entirely accounted for by using propensity matching. However, even in the propensity-matched cohort of 57 patients from each group, there was still a significantly higher overall survival at 90 days among patients treated with FMT.
One author declared grants from the pharmaceutical sector outside the submitted work. No funding or other conflicts of interest were reported.
SOURCE: Ianiro G et al. Ann Intern Med. 2019 Nov 4. doi: 10.7326/M18-3635.
Treating Clostridioides difficile infection with fecal microbiota transplantation is associated with a lower risk of bloodstream infection and recurrence than treatment with antibiotics, new research has found.
A paper published in Annals of Internal Medicine presents outcomes of a prospective cohort study in 290 inpatients with recurrent C. difficile infection, 109 of whom were treated with fecal microbiota transplantation (FMT); the remainder were treated with antibiotics including metronidazole, vancomycin, and fidaxomicin.
While the FMT group had a higher mean number of previous C. difficile infections than the antibiotics group (2.82 vs. 1.23, respectively), a sustained cure was achieved in 97% of the FMT group, compared with 38% in the antibiotics group.
Blood cultures were done if patients developed a temperature above 30° C or showed symptoms that might be attributable to sepsis. Bloodstream infections were diagnosed in 5% (5 patients) of those treated with FMT, and 22% (40 patients) in the antibiotics group.
The patients in the FMT group with bloodstream infections all had bacterial infections – one of which was polymicrobial – and there were no cases of fungal bloodstream infections. In the antibiotics group, 28 patients (15%) had bacterial bloodstream infections – 11 of which were polymicrobial – and 12 (7%) had fungal bloodstream infections.
Bloodstream infections were particularly evident among the 11 patients whose C. difficile infection was treated with fidaxomicin, 4 of whom developed a bloodstream infection.
Overall, 27% of patients died during the 90-day follow-up, with 7% dying because of bloodstream infections, all of whom were in the antibiotic-treated cohort. Three patients in the FMT group died because of overwhelming C. difficile infection, compared with 12 in the antibiotic cohort.
Nearly three-quarters of deaths occurred within 30 days of the end of treatment; 5 of these deaths were in the FMT group, and 53 were in the antibiotics group.
“These findings suggest that the longer 90-day [overall survival] of patients in the FMT group is attributable to cure of [C. difficile infection] leading to an improvement in clinical condition,” wrote Gianluca Ianiro, MD, from the Catholic University of the Sacred Heart in Rome, and coauthors.
The 90-day overall survival rate was 92% in the FMT group and 61% in the antibiotic group. Patients treated with FMT also showed significantly shorter mean duration of hospital stay at 13.3 days, compared with 29.7 days in patients treated with antibiotics.
The authors noted the results should be interpreted with caution because of baseline differences between the two groups that were not entirely accounted for by using propensity matching. However, even in the propensity-matched cohort of 57 patients from each group, there was still a significantly higher overall survival at 90 days among patients treated with FMT.
One author declared grants from the pharmaceutical sector outside the submitted work. No funding or other conflicts of interest were reported.
SOURCE: Ianiro G et al. Ann Intern Med. 2019 Nov 4. doi: 10.7326/M18-3635.
FROM ANNALS OF INTERNAL MEDICINE
Signs of adult diabetes apparent in very young children
BARCELONA – Disturbed HDL cholesterol metabolism is one of the earliest features that may predispose individuals to the development of type 2 diabetes, according to data from a genetics and metabolomics study conducted in the United Kingdom.
Changes in HDL cholesterol metabolism were seen in children as young as 8 years, decades before the clinical onset of disease, Joshua Bell, PhD, a research fellow at the University of Bristol (England), reported at the annual meeting of the European Association for the Study of Diabetes.
“We know that type 2 diabetes certainly doesn’t develop overnight,” Dr. Bell said. Indeed, data exist showing that there are changes in glucose metabolism several years before a formal diagnosis may be made in adults. “What we don’t know is what the very earliest features of diabetes look like,” he added.
“The main assumption is that type 2 diabetes is a metabolic disease, and so disease features are visible in systemic metabolism,” explained Dr. Bell. What was not clear, however, was that if any metabolic features – seen mainly in observational studies and in adults – were caused by the disease itself or perhaps were independent causes of type 2 diabetes.To investigate, Dr. Bell and associates performed a study linking genetic liability with metabolomic data collected at four time points from 4,761 offspring from participants in the Avon Longitudinal Study of Parents and Children cohort, which is also known as the Children of the 90s cohort. More than 200 metabolic traits were considered, and a genetic risk score comprising more than 162 single nucleotide polymorphisms previously linked to adult type 2 diabetes was used.
The metabolomic traits considered included lipoprotein subclass-specific cholesterol and triglyceride content, amino and fatty acids, and inflammatory glycoprotein acetyls, which had been measured in childhood at the age of 8 years, in adolescence at 16 years, in young adulthood at 18 years, and in adulthood at 25 years.
Early metabolic features of type 2 diabetes liability were grouped together and one feature that stood out was the sizes of lipid particles. In particular, it was the size of HDL cholesterol particle subtypes in children at the age of 8 years. Before other types of changes in lipid particles were being seen, there were reductions in the lipid content of HDL cholesterol particle subtypes, notably those that were very large.
By age 16 years, strong associations remained with lower lipids in HDL cholesterol particle subtypes and type 2 diabetes liability, which became stronger with preglycemic traits, such as citrate, and with glycoprotein acetyls. By age 18 years, elevations were seen in branched amino acids, and by age 25, association had strengthened for the lipid content of very low–density lipoprotein cholesterol.
“Linking genetic liability to adult disease with traits measured much earlier in life can tell you something about how the disease activity unfolds over a lifetime,” Dr. Bell said, adding that the feature that was “most consistently tracked” could be evaluated and could help reveal whether or not an individual might go on to develop type 2 diabetes.
In a press release issued by the EASD, Dr. Bell observed: “It’s remarkable that we can see signs of adult diabetes in the blood from such a young age. Knowing what early features of type 2 diabetes look like, could help us to intervene much earlier to halt progression to full-blown diabetes and its complications.”
The study was funded by Diabetes U.K., Cancer Research U.K., the Elizabeth Blackwell Institute for Health Research, the Wellcome Trust, the Medical Research Council, and the University of Bristol. Dr. Bell said he had no conflicts of interest to declare.
SOURCE: Bell J et al. bioRxiv. 2019 Sep 17. doi: 10.1101/767756.
BARCELONA – Disturbed HDL cholesterol metabolism is one of the earliest features that may predispose individuals to the development of type 2 diabetes, according to data from a genetics and metabolomics study conducted in the United Kingdom.
Changes in HDL cholesterol metabolism were seen in children as young as 8 years, decades before the clinical onset of disease, Joshua Bell, PhD, a research fellow at the University of Bristol (England), reported at the annual meeting of the European Association for the Study of Diabetes.
“We know that type 2 diabetes certainly doesn’t develop overnight,” Dr. Bell said. Indeed, data exist showing that there are changes in glucose metabolism several years before a formal diagnosis may be made in adults. “What we don’t know is what the very earliest features of diabetes look like,” he added.
“The main assumption is that type 2 diabetes is a metabolic disease, and so disease features are visible in systemic metabolism,” explained Dr. Bell. What was not clear, however, was that if any metabolic features – seen mainly in observational studies and in adults – were caused by the disease itself or perhaps were independent causes of type 2 diabetes.To investigate, Dr. Bell and associates performed a study linking genetic liability with metabolomic data collected at four time points from 4,761 offspring from participants in the Avon Longitudinal Study of Parents and Children cohort, which is also known as the Children of the 90s cohort. More than 200 metabolic traits were considered, and a genetic risk score comprising more than 162 single nucleotide polymorphisms previously linked to adult type 2 diabetes was used.
The metabolomic traits considered included lipoprotein subclass-specific cholesterol and triglyceride content, amino and fatty acids, and inflammatory glycoprotein acetyls, which had been measured in childhood at the age of 8 years, in adolescence at 16 years, in young adulthood at 18 years, and in adulthood at 25 years.
Early metabolic features of type 2 diabetes liability were grouped together and one feature that stood out was the sizes of lipid particles. In particular, it was the size of HDL cholesterol particle subtypes in children at the age of 8 years. Before other types of changes in lipid particles were being seen, there were reductions in the lipid content of HDL cholesterol particle subtypes, notably those that were very large.
By age 16 years, strong associations remained with lower lipids in HDL cholesterol particle subtypes and type 2 diabetes liability, which became stronger with preglycemic traits, such as citrate, and with glycoprotein acetyls. By age 18 years, elevations were seen in branched amino acids, and by age 25, association had strengthened for the lipid content of very low–density lipoprotein cholesterol.
“Linking genetic liability to adult disease with traits measured much earlier in life can tell you something about how the disease activity unfolds over a lifetime,” Dr. Bell said, adding that the feature that was “most consistently tracked” could be evaluated and could help reveal whether or not an individual might go on to develop type 2 diabetes.
In a press release issued by the EASD, Dr. Bell observed: “It’s remarkable that we can see signs of adult diabetes in the blood from such a young age. Knowing what early features of type 2 diabetes look like, could help us to intervene much earlier to halt progression to full-blown diabetes and its complications.”
The study was funded by Diabetes U.K., Cancer Research U.K., the Elizabeth Blackwell Institute for Health Research, the Wellcome Trust, the Medical Research Council, and the University of Bristol. Dr. Bell said he had no conflicts of interest to declare.
SOURCE: Bell J et al. bioRxiv. 2019 Sep 17. doi: 10.1101/767756.
BARCELONA – Disturbed HDL cholesterol metabolism is one of the earliest features that may predispose individuals to the development of type 2 diabetes, according to data from a genetics and metabolomics study conducted in the United Kingdom.
Changes in HDL cholesterol metabolism were seen in children as young as 8 years, decades before the clinical onset of disease, Joshua Bell, PhD, a research fellow at the University of Bristol (England), reported at the annual meeting of the European Association for the Study of Diabetes.
“We know that type 2 diabetes certainly doesn’t develop overnight,” Dr. Bell said. Indeed, data exist showing that there are changes in glucose metabolism several years before a formal diagnosis may be made in adults. “What we don’t know is what the very earliest features of diabetes look like,” he added.
“The main assumption is that type 2 diabetes is a metabolic disease, and so disease features are visible in systemic metabolism,” explained Dr. Bell. What was not clear, however, was that if any metabolic features – seen mainly in observational studies and in adults – were caused by the disease itself or perhaps were independent causes of type 2 diabetes.To investigate, Dr. Bell and associates performed a study linking genetic liability with metabolomic data collected at four time points from 4,761 offspring from participants in the Avon Longitudinal Study of Parents and Children cohort, which is also known as the Children of the 90s cohort. More than 200 metabolic traits were considered, and a genetic risk score comprising more than 162 single nucleotide polymorphisms previously linked to adult type 2 diabetes was used.
The metabolomic traits considered included lipoprotein subclass-specific cholesterol and triglyceride content, amino and fatty acids, and inflammatory glycoprotein acetyls, which had been measured in childhood at the age of 8 years, in adolescence at 16 years, in young adulthood at 18 years, and in adulthood at 25 years.
Early metabolic features of type 2 diabetes liability were grouped together and one feature that stood out was the sizes of lipid particles. In particular, it was the size of HDL cholesterol particle subtypes in children at the age of 8 years. Before other types of changes in lipid particles were being seen, there were reductions in the lipid content of HDL cholesterol particle subtypes, notably those that were very large.
By age 16 years, strong associations remained with lower lipids in HDL cholesterol particle subtypes and type 2 diabetes liability, which became stronger with preglycemic traits, such as citrate, and with glycoprotein acetyls. By age 18 years, elevations were seen in branched amino acids, and by age 25, association had strengthened for the lipid content of very low–density lipoprotein cholesterol.
“Linking genetic liability to adult disease with traits measured much earlier in life can tell you something about how the disease activity unfolds over a lifetime,” Dr. Bell said, adding that the feature that was “most consistently tracked” could be evaluated and could help reveal whether or not an individual might go on to develop type 2 diabetes.
In a press release issued by the EASD, Dr. Bell observed: “It’s remarkable that we can see signs of adult diabetes in the blood from such a young age. Knowing what early features of type 2 diabetes look like, could help us to intervene much earlier to halt progression to full-blown diabetes and its complications.”
The study was funded by Diabetes U.K., Cancer Research U.K., the Elizabeth Blackwell Institute for Health Research, the Wellcome Trust, the Medical Research Council, and the University of Bristol. Dr. Bell said he had no conflicts of interest to declare.
SOURCE: Bell J et al. bioRxiv. 2019 Sep 17. doi: 10.1101/767756.
REPORTING FROM EASD 2019