User login
Helping adolescents get enough quality sleep
NEW ORLEANS – Social media and electronics aren’t the only barriers to a good night’s sleep for teens, according to Adiaha I. A. Spinks-Franklin, MD, MPH, a pediatrician at Texas Children’s Hospital in Houston.
Another half-dozen “sleep enemies” interfere with adolescents’ sleep and can contribute to insomnia or other sleep disorders, she told attendees at the annual meeting of the American Academy of Pediatrics.
Knowing what normal sleep physiology looks like in youth and understanding the most common sleep enemies and sleep-behavior problems can help you use effective interventions to help your patients get the sleep they need, she said.
Infants need the most sleep, about 12-16 hours each 24-hour period, including naps, for those aged 4-12 months. As they grow into toddlerhood and preschool age, children gradually need less: Children aged 1-2 years need 11-14 hours and children aged 3-5 years need 10-13 hours, including naps. By the time children are in school, ages 6-12, they should have dropped their naps and need 9-12 hours a night.
In fact, 75% of high school seniors get less than 8 hours of sleep a day and live with a chronic sleep debt, Dr. Spinks-Franklin said.
Although social media use and electronics in the bedroom – TVs, computers, cell phones, and video games – can certainly contribute to inadequate sleep, a heavy academic load and extracurricular activities can be just as problematic, Dr. Spinks-Franklin said. Teens who work after school also may have difficulty getting enough sleep, especially if they also have to balance a heavier academic load or even one or two extracurricular activities.
Socializing with friends also can interfere with sleep, especially when get-togethers run late; drinking caffeinated drinks in the afternoon onward can make it difficult for adolescents to get the sleep they need as well. Less-modifiable contributors to too little sleep are stress and early school start times, Dr. Spinks-Franklin said.
The two most common sleep problems seen in teens are insomnia and delayed sleep phase syndrome. Addressing these is important because the effects of chronic insufficient sleep can have far-reaching consequences. Obesity and related chronic health conditions are associated with inadequate sleep, as are poor academic performance, poor judgment, poor executive functioning, and mental health disorders like depression.
Short-term effects of insufficient sleep also can be problematic and can exacerbate existing sleep problems, such as sleeping in on the weekends to “catch up” on sleep or drinking more caffeine to try to stay awake during the day. Increased caffeine intake can interfere with non-REM deep sleep, Dr. Spinks-Franklin said, and therefore reduce the quality of sleep even if the person gets the total hours they need.
Insomnia in adolescents
Insomnia can refer to difficulty falling asleep, staying asleep, sleeping for long enough, or getting enough sleep in one period of time even when the opportunity is there. Some people may have no trouble falling asleep, but they wake up too early – before they have had gotten the sleep they need – and cannot return to sleep.
To be insomnia, the problem must occur “despite having enough time available for sleep,” Dr. Spinks-Franklin said. “Patient who restrict the amount of time for sleep due to work or social commitments may have trouble sleeping and daytime sleepiness but do not have insomnia.”
Daytime impairment also is part of the American Academy of Sleep Medicine’s definition of insomnia. The rare teen who doesn’t need as much sleep as average and functions without difficulty during the day does not necessarily have insomnia.
But the impairment may not necessarily just be fatigue or sleepiness. In fact, many of the symptoms are the same as those seen with ADHD.
Daytime consequences of insomnia can include the following:
- Depression, feeling sad or “blue,” or emotional hypersensitivity.
- Mood swings, crankiness, or irritability.
- Difficulty concentrating or paying attention, poor memory, mind wandering, or even inability to sit still.
- Job or school problems, such as not being able to finish homework, not finishing tasks they start, or forgetfulness.
- Difficulty in social situations, such as discomfort with others or problems with friends.
- Daytime sleepiness, even when unable to actually take a nap.
- Behavioral problems, such as hyperactivity, impulsivity, or aggression.
- Frequent mistakes, especially at work, at school, or while driving (often “errors of omission,” such as not seeing a street sign or not hearing an instruction).
- Lower levels of motivation or initiative, feeling less energetic.
- Excessive worry about sleep.
Evaluation of insomnia can be framed with “the three-factor model,” which includes predisposing factors, precipitating factors, and perpetuating factors.
Predisposing factors – those that indicate a person already may be at risk for insomnia – include potential genetic influences as well as their typical response to stress. “Do they sleep more or less?” Dr. Spinks-Franklin said. Even teens predisposed to insomnia may not develop it, however, without a precipitating trigger.
These triggers could include stress, anxiety, poor initial sleep hygiene that becomes a pattern, dietary intake or behaviors (such as drinking caffeine or eating too much or too late in the evening), changes to their schedule, or side effects of medications.
Once insomnia begins, various factors can then perpetuate the cycle, including some of those that triggered it, such as anxiety or a school or work schedule. Sometimes it can be difficult to pinpoint the factor prolonging insomnia, such as the unconscious reward of going to work or school late with few or no consequences.
Delayed sleep phase syndrome
Delayed sleep phase syndrome occurs when someone has a delayed onset of melatonin secretion that pushes back the time when they can fall asleep. Melatonin is the neurotransmitter produced by the pineal gland that signals the start of nighttime. Although it has a hereditary component, delayed sleep phase syndrome also can result from a pattern of poor sleep onset and sleeping in on the weekends.
Dr. Spinks-Franklin described the typical cycle: A teen doesn’t go to sleep until after midnight and then wants to sleep in later in the morning. Because they have to wake up early for school, they sleep in on the weekends to try to regain the sleep they lost. Sleeping in pushes their circadian rhythm even later, perpetuating the problem.
Interventions for sleep disorders
The recommended treatment for insomnia is cognitive-behavior therapy for insomnia, for which strong evidence exists. Before seeking cognitive-behavior therapy, however, families can work to improve sleep hygiene and reduce stimuli that contribute to insomnia.
Teens should avoid screens for at least 1 hour before bedtime and avoid caffeine and exercise for at least 4 hours before going to bed. They also need to develop a schedule with a consistent bedtime and wake-up time, including on the weekends. They should avoid sleeping in on the weekends or taking naps during the day, Dr. Spinks-Franklin said.
Delayed sleep phase syndrome is more resistant to treatment and has a high recurrence rate, she said, and it requires commitment from the parent and their child to address it successfully. Teens with this condition also can start with sleep hygiene practices: a consistent wake-up time that they maintain on the weekends and no daytime naps. Phototherapy in the morning can be added to hopefully induce an earlier onset of melatonin release in the evening.
The next step is making changes to the youth’s schedule, particularly evening and/or weekend activities. They can try to gradually advance their biological clock by changing their sleep schedule.
Dr. Spinks-Franklin also briefly addressed the use of over-the-counter melatonin supplements for treating sleep problems. Melatonin can be effective for treating insomnia by improving sleep onset and sleep quality, particularly in children and teens with autism spectrum disorder or ADHD.
Dr. Spinks-Franklin had no disclosures, and her presentation used no outside funding.
NEW ORLEANS – Social media and electronics aren’t the only barriers to a good night’s sleep for teens, according to Adiaha I. A. Spinks-Franklin, MD, MPH, a pediatrician at Texas Children’s Hospital in Houston.
Another half-dozen “sleep enemies” interfere with adolescents’ sleep and can contribute to insomnia or other sleep disorders, she told attendees at the annual meeting of the American Academy of Pediatrics.
Knowing what normal sleep physiology looks like in youth and understanding the most common sleep enemies and sleep-behavior problems can help you use effective interventions to help your patients get the sleep they need, she said.
Infants need the most sleep, about 12-16 hours each 24-hour period, including naps, for those aged 4-12 months. As they grow into toddlerhood and preschool age, children gradually need less: Children aged 1-2 years need 11-14 hours and children aged 3-5 years need 10-13 hours, including naps. By the time children are in school, ages 6-12, they should have dropped their naps and need 9-12 hours a night.
In fact, 75% of high school seniors get less than 8 hours of sleep a day and live with a chronic sleep debt, Dr. Spinks-Franklin said.
Although social media use and electronics in the bedroom – TVs, computers, cell phones, and video games – can certainly contribute to inadequate sleep, a heavy academic load and extracurricular activities can be just as problematic, Dr. Spinks-Franklin said. Teens who work after school also may have difficulty getting enough sleep, especially if they also have to balance a heavier academic load or even one or two extracurricular activities.
Socializing with friends also can interfere with sleep, especially when get-togethers run late; drinking caffeinated drinks in the afternoon onward can make it difficult for adolescents to get the sleep they need as well. Less-modifiable contributors to too little sleep are stress and early school start times, Dr. Spinks-Franklin said.
The two most common sleep problems seen in teens are insomnia and delayed sleep phase syndrome. Addressing these is important because the effects of chronic insufficient sleep can have far-reaching consequences. Obesity and related chronic health conditions are associated with inadequate sleep, as are poor academic performance, poor judgment, poor executive functioning, and mental health disorders like depression.
Short-term effects of insufficient sleep also can be problematic and can exacerbate existing sleep problems, such as sleeping in on the weekends to “catch up” on sleep or drinking more caffeine to try to stay awake during the day. Increased caffeine intake can interfere with non-REM deep sleep, Dr. Spinks-Franklin said, and therefore reduce the quality of sleep even if the person gets the total hours they need.
Insomnia in adolescents
Insomnia can refer to difficulty falling asleep, staying asleep, sleeping for long enough, or getting enough sleep in one period of time even when the opportunity is there. Some people may have no trouble falling asleep, but they wake up too early – before they have had gotten the sleep they need – and cannot return to sleep.
To be insomnia, the problem must occur “despite having enough time available for sleep,” Dr. Spinks-Franklin said. “Patient who restrict the amount of time for sleep due to work or social commitments may have trouble sleeping and daytime sleepiness but do not have insomnia.”
Daytime impairment also is part of the American Academy of Sleep Medicine’s definition of insomnia. The rare teen who doesn’t need as much sleep as average and functions without difficulty during the day does not necessarily have insomnia.
But the impairment may not necessarily just be fatigue or sleepiness. In fact, many of the symptoms are the same as those seen with ADHD.
Daytime consequences of insomnia can include the following:
- Depression, feeling sad or “blue,” or emotional hypersensitivity.
- Mood swings, crankiness, or irritability.
- Difficulty concentrating or paying attention, poor memory, mind wandering, or even inability to sit still.
- Job or school problems, such as not being able to finish homework, not finishing tasks they start, or forgetfulness.
- Difficulty in social situations, such as discomfort with others or problems with friends.
- Daytime sleepiness, even when unable to actually take a nap.
- Behavioral problems, such as hyperactivity, impulsivity, or aggression.
- Frequent mistakes, especially at work, at school, or while driving (often “errors of omission,” such as not seeing a street sign or not hearing an instruction).
- Lower levels of motivation or initiative, feeling less energetic.
- Excessive worry about sleep.
Evaluation of insomnia can be framed with “the three-factor model,” which includes predisposing factors, precipitating factors, and perpetuating factors.
Predisposing factors – those that indicate a person already may be at risk for insomnia – include potential genetic influences as well as their typical response to stress. “Do they sleep more or less?” Dr. Spinks-Franklin said. Even teens predisposed to insomnia may not develop it, however, without a precipitating trigger.
These triggers could include stress, anxiety, poor initial sleep hygiene that becomes a pattern, dietary intake or behaviors (such as drinking caffeine or eating too much or too late in the evening), changes to their schedule, or side effects of medications.
Once insomnia begins, various factors can then perpetuate the cycle, including some of those that triggered it, such as anxiety or a school or work schedule. Sometimes it can be difficult to pinpoint the factor prolonging insomnia, such as the unconscious reward of going to work or school late with few or no consequences.
Delayed sleep phase syndrome
Delayed sleep phase syndrome occurs when someone has a delayed onset of melatonin secretion that pushes back the time when they can fall asleep. Melatonin is the neurotransmitter produced by the pineal gland that signals the start of nighttime. Although it has a hereditary component, delayed sleep phase syndrome also can result from a pattern of poor sleep onset and sleeping in on the weekends.
Dr. Spinks-Franklin described the typical cycle: A teen doesn’t go to sleep until after midnight and then wants to sleep in later in the morning. Because they have to wake up early for school, they sleep in on the weekends to try to regain the sleep they lost. Sleeping in pushes their circadian rhythm even later, perpetuating the problem.
Interventions for sleep disorders
The recommended treatment for insomnia is cognitive-behavior therapy for insomnia, for which strong evidence exists. Before seeking cognitive-behavior therapy, however, families can work to improve sleep hygiene and reduce stimuli that contribute to insomnia.
Teens should avoid screens for at least 1 hour before bedtime and avoid caffeine and exercise for at least 4 hours before going to bed. They also need to develop a schedule with a consistent bedtime and wake-up time, including on the weekends. They should avoid sleeping in on the weekends or taking naps during the day, Dr. Spinks-Franklin said.
Delayed sleep phase syndrome is more resistant to treatment and has a high recurrence rate, she said, and it requires commitment from the parent and their child to address it successfully. Teens with this condition also can start with sleep hygiene practices: a consistent wake-up time that they maintain on the weekends and no daytime naps. Phototherapy in the morning can be added to hopefully induce an earlier onset of melatonin release in the evening.
The next step is making changes to the youth’s schedule, particularly evening and/or weekend activities. They can try to gradually advance their biological clock by changing their sleep schedule.
Dr. Spinks-Franklin also briefly addressed the use of over-the-counter melatonin supplements for treating sleep problems. Melatonin can be effective for treating insomnia by improving sleep onset and sleep quality, particularly in children and teens with autism spectrum disorder or ADHD.
Dr. Spinks-Franklin had no disclosures, and her presentation used no outside funding.
NEW ORLEANS – Social media and electronics aren’t the only barriers to a good night’s sleep for teens, according to Adiaha I. A. Spinks-Franklin, MD, MPH, a pediatrician at Texas Children’s Hospital in Houston.
Another half-dozen “sleep enemies” interfere with adolescents’ sleep and can contribute to insomnia or other sleep disorders, she told attendees at the annual meeting of the American Academy of Pediatrics.
Knowing what normal sleep physiology looks like in youth and understanding the most common sleep enemies and sleep-behavior problems can help you use effective interventions to help your patients get the sleep they need, she said.
Infants need the most sleep, about 12-16 hours each 24-hour period, including naps, for those aged 4-12 months. As they grow into toddlerhood and preschool age, children gradually need less: Children aged 1-2 years need 11-14 hours and children aged 3-5 years need 10-13 hours, including naps. By the time children are in school, ages 6-12, they should have dropped their naps and need 9-12 hours a night.
In fact, 75% of high school seniors get less than 8 hours of sleep a day and live with a chronic sleep debt, Dr. Spinks-Franklin said.
Although social media use and electronics in the bedroom – TVs, computers, cell phones, and video games – can certainly contribute to inadequate sleep, a heavy academic load and extracurricular activities can be just as problematic, Dr. Spinks-Franklin said. Teens who work after school also may have difficulty getting enough sleep, especially if they also have to balance a heavier academic load or even one or two extracurricular activities.
Socializing with friends also can interfere with sleep, especially when get-togethers run late; drinking caffeinated drinks in the afternoon onward can make it difficult for adolescents to get the sleep they need as well. Less-modifiable contributors to too little sleep are stress and early school start times, Dr. Spinks-Franklin said.
The two most common sleep problems seen in teens are insomnia and delayed sleep phase syndrome. Addressing these is important because the effects of chronic insufficient sleep can have far-reaching consequences. Obesity and related chronic health conditions are associated with inadequate sleep, as are poor academic performance, poor judgment, poor executive functioning, and mental health disorders like depression.
Short-term effects of insufficient sleep also can be problematic and can exacerbate existing sleep problems, such as sleeping in on the weekends to “catch up” on sleep or drinking more caffeine to try to stay awake during the day. Increased caffeine intake can interfere with non-REM deep sleep, Dr. Spinks-Franklin said, and therefore reduce the quality of sleep even if the person gets the total hours they need.
Insomnia in adolescents
Insomnia can refer to difficulty falling asleep, staying asleep, sleeping for long enough, or getting enough sleep in one period of time even when the opportunity is there. Some people may have no trouble falling asleep, but they wake up too early – before they have had gotten the sleep they need – and cannot return to sleep.
To be insomnia, the problem must occur “despite having enough time available for sleep,” Dr. Spinks-Franklin said. “Patient who restrict the amount of time for sleep due to work or social commitments may have trouble sleeping and daytime sleepiness but do not have insomnia.”
Daytime impairment also is part of the American Academy of Sleep Medicine’s definition of insomnia. The rare teen who doesn’t need as much sleep as average and functions without difficulty during the day does not necessarily have insomnia.
But the impairment may not necessarily just be fatigue or sleepiness. In fact, many of the symptoms are the same as those seen with ADHD.
Daytime consequences of insomnia can include the following:
- Depression, feeling sad or “blue,” or emotional hypersensitivity.
- Mood swings, crankiness, or irritability.
- Difficulty concentrating or paying attention, poor memory, mind wandering, or even inability to sit still.
- Job or school problems, such as not being able to finish homework, not finishing tasks they start, or forgetfulness.
- Difficulty in social situations, such as discomfort with others or problems with friends.
- Daytime sleepiness, even when unable to actually take a nap.
- Behavioral problems, such as hyperactivity, impulsivity, or aggression.
- Frequent mistakes, especially at work, at school, or while driving (often “errors of omission,” such as not seeing a street sign or not hearing an instruction).
- Lower levels of motivation or initiative, feeling less energetic.
- Excessive worry about sleep.
Evaluation of insomnia can be framed with “the three-factor model,” which includes predisposing factors, precipitating factors, and perpetuating factors.
Predisposing factors – those that indicate a person already may be at risk for insomnia – include potential genetic influences as well as their typical response to stress. “Do they sleep more or less?” Dr. Spinks-Franklin said. Even teens predisposed to insomnia may not develop it, however, without a precipitating trigger.
These triggers could include stress, anxiety, poor initial sleep hygiene that becomes a pattern, dietary intake or behaviors (such as drinking caffeine or eating too much or too late in the evening), changes to their schedule, or side effects of medications.
Once insomnia begins, various factors can then perpetuate the cycle, including some of those that triggered it, such as anxiety or a school or work schedule. Sometimes it can be difficult to pinpoint the factor prolonging insomnia, such as the unconscious reward of going to work or school late with few or no consequences.
Delayed sleep phase syndrome
Delayed sleep phase syndrome occurs when someone has a delayed onset of melatonin secretion that pushes back the time when they can fall asleep. Melatonin is the neurotransmitter produced by the pineal gland that signals the start of nighttime. Although it has a hereditary component, delayed sleep phase syndrome also can result from a pattern of poor sleep onset and sleeping in on the weekends.
Dr. Spinks-Franklin described the typical cycle: A teen doesn’t go to sleep until after midnight and then wants to sleep in later in the morning. Because they have to wake up early for school, they sleep in on the weekends to try to regain the sleep they lost. Sleeping in pushes their circadian rhythm even later, perpetuating the problem.
Interventions for sleep disorders
The recommended treatment for insomnia is cognitive-behavior therapy for insomnia, for which strong evidence exists. Before seeking cognitive-behavior therapy, however, families can work to improve sleep hygiene and reduce stimuli that contribute to insomnia.
Teens should avoid screens for at least 1 hour before bedtime and avoid caffeine and exercise for at least 4 hours before going to bed. They also need to develop a schedule with a consistent bedtime and wake-up time, including on the weekends. They should avoid sleeping in on the weekends or taking naps during the day, Dr. Spinks-Franklin said.
Delayed sleep phase syndrome is more resistant to treatment and has a high recurrence rate, she said, and it requires commitment from the parent and their child to address it successfully. Teens with this condition also can start with sleep hygiene practices: a consistent wake-up time that they maintain on the weekends and no daytime naps. Phototherapy in the morning can be added to hopefully induce an earlier onset of melatonin release in the evening.
The next step is making changes to the youth’s schedule, particularly evening and/or weekend activities. They can try to gradually advance their biological clock by changing their sleep schedule.
Dr. Spinks-Franklin also briefly addressed the use of over-the-counter melatonin supplements for treating sleep problems. Melatonin can be effective for treating insomnia by improving sleep onset and sleep quality, particularly in children and teens with autism spectrum disorder or ADHD.
Dr. Spinks-Franklin had no disclosures, and her presentation used no outside funding.
EXPERT ANALYSIS FROM AAP 19
Stroke is diagnosed in about one-fifth of children with strokelike symptoms
CHARLOTTE, N.C. – (TIA), according to research presented at the annual meeting of the Child Neurology Society. Ischemic stroke and TIA were the second leading diagnoses among the stroke activations examined in the study, after seizure and Todd’s paralysis. “These data, in conjunction with previous studies, highlight the importance of developing protocols for early recognition and evaluation of children who present with strokelike symptoms,” said Tiffany Barkley, DO, a child neurology resident at Children’s Mercy Hospital in Kansas City, Mo., and colleagues.
Dr. Barkley and colleagues conducted their research to describe the demographic and other characteristics of patients who present with strokelike symptoms to their hospital. They undertook a descriptive, retrospective chart review of patients who came to Children’s Mercy Hospital from Sept. 1, 2016, to August 31, 2018, with concern for acute stroke. The investigators examined only patients for whom the Stroke Alert Process and power plan were activated.
“Power plans were created at Children’s Mercy Hospital to streamline and standardize care for children,” said Dr. Barkley. “While stroke order sets tend to be common practice in many adult hospitals, stroke order sets in pediatric hospitals are new.”
In all, 61 stroke activations occurred during the study period. Twelve patients (20%) had a final diagnosis of ischemic stroke or TIA. Among the patients with a final diagnosis of ischemic stroke, the most common presenting symptom was unilateral weakness. Two of these patients were candidates for intervention with mechanical thrombectomy, and none received tissue plasminogen activator. The average age of patients in all activations was 14 years, and the average age of patients with a final diagnosis of ischemic stroke or TIA was 4 years. About 37 (61%) subjects of activations were female, and the most common racial demographic was Caucasian.
Ischemic stroke or TIA was the second most common diagnosis of all activations (12 patients; 20%). Seizure or Todd’s paralysis (14 patients; 23%) was the leading diagnosis. Other common diagnoses included migraine (18%), psychogenic or conversion disorder (15%), oncologic process (3.0%), and complications of meningitis or encephalitis (1.6%). Children who presented with ischemic stroke secondary to Moyamoya disease were classified separately (two patients or 3%). It can be difficult to distinguish between stroke and stroke mimics based on neurologic examination alone, and imaging such as MRI often is needed, said Dr. Barkley. The researchers did not identify any intracranial hemorrhages in this patient population.
These findings are consistent with current reported literature, said the researchers. “Our study is one of the first to look at the demographics of children who present with strokelike symptoms,” said Dr. Barkley. “We hope that our study will not only help identify children who present with symptoms concerning for stroke, but also help us identify children who may be at risk for ischemic stroke before the stroke happens.”
The investigators did not have funding for this study and did not report any disclosures.
SOURCE: Barkley T et al. CNS 2019. Abstract 235.
CHARLOTTE, N.C. – (TIA), according to research presented at the annual meeting of the Child Neurology Society. Ischemic stroke and TIA were the second leading diagnoses among the stroke activations examined in the study, after seizure and Todd’s paralysis. “These data, in conjunction with previous studies, highlight the importance of developing protocols for early recognition and evaluation of children who present with strokelike symptoms,” said Tiffany Barkley, DO, a child neurology resident at Children’s Mercy Hospital in Kansas City, Mo., and colleagues.
Dr. Barkley and colleagues conducted their research to describe the demographic and other characteristics of patients who present with strokelike symptoms to their hospital. They undertook a descriptive, retrospective chart review of patients who came to Children’s Mercy Hospital from Sept. 1, 2016, to August 31, 2018, with concern for acute stroke. The investigators examined only patients for whom the Stroke Alert Process and power plan were activated.
“Power plans were created at Children’s Mercy Hospital to streamline and standardize care for children,” said Dr. Barkley. “While stroke order sets tend to be common practice in many adult hospitals, stroke order sets in pediatric hospitals are new.”
In all, 61 stroke activations occurred during the study period. Twelve patients (20%) had a final diagnosis of ischemic stroke or TIA. Among the patients with a final diagnosis of ischemic stroke, the most common presenting symptom was unilateral weakness. Two of these patients were candidates for intervention with mechanical thrombectomy, and none received tissue plasminogen activator. The average age of patients in all activations was 14 years, and the average age of patients with a final diagnosis of ischemic stroke or TIA was 4 years. About 37 (61%) subjects of activations were female, and the most common racial demographic was Caucasian.
Ischemic stroke or TIA was the second most common diagnosis of all activations (12 patients; 20%). Seizure or Todd’s paralysis (14 patients; 23%) was the leading diagnosis. Other common diagnoses included migraine (18%), psychogenic or conversion disorder (15%), oncologic process (3.0%), and complications of meningitis or encephalitis (1.6%). Children who presented with ischemic stroke secondary to Moyamoya disease were classified separately (two patients or 3%). It can be difficult to distinguish between stroke and stroke mimics based on neurologic examination alone, and imaging such as MRI often is needed, said Dr. Barkley. The researchers did not identify any intracranial hemorrhages in this patient population.
These findings are consistent with current reported literature, said the researchers. “Our study is one of the first to look at the demographics of children who present with strokelike symptoms,” said Dr. Barkley. “We hope that our study will not only help identify children who present with symptoms concerning for stroke, but also help us identify children who may be at risk for ischemic stroke before the stroke happens.”
The investigators did not have funding for this study and did not report any disclosures.
SOURCE: Barkley T et al. CNS 2019. Abstract 235.
CHARLOTTE, N.C. – (TIA), according to research presented at the annual meeting of the Child Neurology Society. Ischemic stroke and TIA were the second leading diagnoses among the stroke activations examined in the study, after seizure and Todd’s paralysis. “These data, in conjunction with previous studies, highlight the importance of developing protocols for early recognition and evaluation of children who present with strokelike symptoms,” said Tiffany Barkley, DO, a child neurology resident at Children’s Mercy Hospital in Kansas City, Mo., and colleagues.
Dr. Barkley and colleagues conducted their research to describe the demographic and other characteristics of patients who present with strokelike symptoms to their hospital. They undertook a descriptive, retrospective chart review of patients who came to Children’s Mercy Hospital from Sept. 1, 2016, to August 31, 2018, with concern for acute stroke. The investigators examined only patients for whom the Stroke Alert Process and power plan were activated.
“Power plans were created at Children’s Mercy Hospital to streamline and standardize care for children,” said Dr. Barkley. “While stroke order sets tend to be common practice in many adult hospitals, stroke order sets in pediatric hospitals are new.”
In all, 61 stroke activations occurred during the study period. Twelve patients (20%) had a final diagnosis of ischemic stroke or TIA. Among the patients with a final diagnosis of ischemic stroke, the most common presenting symptom was unilateral weakness. Two of these patients were candidates for intervention with mechanical thrombectomy, and none received tissue plasminogen activator. The average age of patients in all activations was 14 years, and the average age of patients with a final diagnosis of ischemic stroke or TIA was 4 years. About 37 (61%) subjects of activations were female, and the most common racial demographic was Caucasian.
Ischemic stroke or TIA was the second most common diagnosis of all activations (12 patients; 20%). Seizure or Todd’s paralysis (14 patients; 23%) was the leading diagnosis. Other common diagnoses included migraine (18%), psychogenic or conversion disorder (15%), oncologic process (3.0%), and complications of meningitis or encephalitis (1.6%). Children who presented with ischemic stroke secondary to Moyamoya disease were classified separately (two patients or 3%). It can be difficult to distinguish between stroke and stroke mimics based on neurologic examination alone, and imaging such as MRI often is needed, said Dr. Barkley. The researchers did not identify any intracranial hemorrhages in this patient population.
These findings are consistent with current reported literature, said the researchers. “Our study is one of the first to look at the demographics of children who present with strokelike symptoms,” said Dr. Barkley. “We hope that our study will not only help identify children who present with symptoms concerning for stroke, but also help us identify children who may be at risk for ischemic stroke before the stroke happens.”
The investigators did not have funding for this study and did not report any disclosures.
SOURCE: Barkley T et al. CNS 2019. Abstract 235.
REPORTING FROM CNS 2019
Neoantigen vaccine appears safe and active in NSCLC
NATIONAL HARBOR, MD. – Trial results suggest a personalized vaccination approach is feasible and safe, and the vaccine can produce clinical responses in patients with non–small cell lung cancer (NSCLC).
The neoantigen vaccine produced only grade 1 adverse events, yielded responses in patients with epidermal growth factor receptor (EGFR) mutations, and proved particularly effective in patients who were also receiving an EGFR inhibitor.
“EGFR inhibitors seemed to reduce tumor immunosuppression barriers and may enhance antitumor immune responses before and during immunization, suggesting there may be a potential synergy of EGFR with immunotherapies,” Gregory A. Lizee, PhD, of University of Texas MD Anderson Cancer Center, Houston, said at the annual meeting of the Society for Immunotherapy of Cancer.
The research began with an elderly patient who had heavily pretreated NSCLC (Oncoimmunology. 2016;5[12]:e1238539). Dr. Lizee and colleagues used tumor mutational profiling and human leukocyte antigen (HLA) typing to develop a personalized peptide vaccine for the patient. He received the vaccine along with topical imiquimod and had multiple lung tumor nodules regress. However, the patient also had liver metastasis that remained refractory to treatment, and he ultimately died.
To investigate this treatment approach in a larger group, Dr. Lizee and colleagues began a phase 1b trial of patients with advanced NSCLC (ChiCTR-IIR-16009867). As with the prior patient, the researchers designed personalized peptide vaccines for the trial subjects based on mutational profiling of 508 cancer-associated genes and high-resolution HLA typing. The peptides were selected based on nonsynonymous somatic tumor–associated mutations with variant allele frequency greater than 0.04 and the highest predicted neoantigen peptide binding to each patient’s HLA class I and II molecules. The vaccines targeted up to eight independent somatic mutations (mean, 3.75 mutations).
In all, 31 patients provided lung tumor biopsies and peripheral blood for mutational and HLA analyses. The researchers designed 27 personalized neoantigen vaccines, and 24 patients were ultimately vaccinated. This translates to a vaccination rate of 77%, which suggests this treatment approach is feasible, Dr. Lizee said.
Of the 24 vaccinated patients, 18 had adenocarcinoma, and 6 had squamous cell carcinoma. All patients had received multiple prior therapies, including surgery, chemotherapy, radiation, and EGFR inhibitors.
Each patient was vaccinated with a personalized mixture of short and long neoantigen peptides (mean, 9.4 peptides) dissolved in isotonic saline. Patients received at least 12 weekly immunizations and had topical imiquimod applied over the injection site for costimulation through toll-like receptor 7. The 16 patients with EGFR mutations were given the option of continuing on an EGFR inhibitor, and 9 patients elected to do so.
Results
Dr. Lizee said this treatment approach was “very safe,” with only grade 1 treatment-related adverse events. The events were fatigue (n = 2), rash (n = 1), and fever (n = 1).
Seven patients achieved a response after vaccination, and one patient achieved a complete response. All seven responders had EGFR mutations, and four of them were receiving an EGFR inhibitor.
The patients on an EGFR inhibitor had significantly better overall survival than that of EGFR-mutated patients who had stopped taking an EGFR inhibitor – 13.8 months and 7.6 months, respectively (P = .038).
Immune profiling revealed that neoantigen-specific T-cell reactivity was associated with clinical responses. The researchers observed EGFR neoantigen-specific T-cell responses in five responders. In three responders, the strongest response was against a peptide encompassing the L858R driver mutation.
The researchers also found evidence of synergy between EGFR inhibitor therapy and the peptide vaccine. EGFR inhibition caused immunomodulatory pathways in EGFR-mutated cancer cells to favor immune-cell infiltration and HLA-mediated antigen presentation.
“Our mechanistic working model is that, in the circulation, the personalized vaccine increased the T-cell frequency,” Dr. Lizee said. “The EGFR inhibitor increased chemokines and antigen presentation at the tumor site, which then attracted those T cells to migrate to the tumor. Then, recognition of the antigen caused interferon gamma [to increase], which caused, potentially, a feed-forward loop by increasing chemokines and antigen presentation further.”
This research is sponsored by Tianjin Beichen Hospital and funded by Tianjin HengJia Biotechnology Development Co. Ltd. Dr. Lizee disclosed a consulting relationship with Tianjin HengJia Biotechnology Development Co. Ltd.
SOURCE: Lizee G et al. SITC 2019. Abstract O18.
NATIONAL HARBOR, MD. – Trial results suggest a personalized vaccination approach is feasible and safe, and the vaccine can produce clinical responses in patients with non–small cell lung cancer (NSCLC).
The neoantigen vaccine produced only grade 1 adverse events, yielded responses in patients with epidermal growth factor receptor (EGFR) mutations, and proved particularly effective in patients who were also receiving an EGFR inhibitor.
“EGFR inhibitors seemed to reduce tumor immunosuppression barriers and may enhance antitumor immune responses before and during immunization, suggesting there may be a potential synergy of EGFR with immunotherapies,” Gregory A. Lizee, PhD, of University of Texas MD Anderson Cancer Center, Houston, said at the annual meeting of the Society for Immunotherapy of Cancer.
The research began with an elderly patient who had heavily pretreated NSCLC (Oncoimmunology. 2016;5[12]:e1238539). Dr. Lizee and colleagues used tumor mutational profiling and human leukocyte antigen (HLA) typing to develop a personalized peptide vaccine for the patient. He received the vaccine along with topical imiquimod and had multiple lung tumor nodules regress. However, the patient also had liver metastasis that remained refractory to treatment, and he ultimately died.
To investigate this treatment approach in a larger group, Dr. Lizee and colleagues began a phase 1b trial of patients with advanced NSCLC (ChiCTR-IIR-16009867). As with the prior patient, the researchers designed personalized peptide vaccines for the trial subjects based on mutational profiling of 508 cancer-associated genes and high-resolution HLA typing. The peptides were selected based on nonsynonymous somatic tumor–associated mutations with variant allele frequency greater than 0.04 and the highest predicted neoantigen peptide binding to each patient’s HLA class I and II molecules. The vaccines targeted up to eight independent somatic mutations (mean, 3.75 mutations).
In all, 31 patients provided lung tumor biopsies and peripheral blood for mutational and HLA analyses. The researchers designed 27 personalized neoantigen vaccines, and 24 patients were ultimately vaccinated. This translates to a vaccination rate of 77%, which suggests this treatment approach is feasible, Dr. Lizee said.
Of the 24 vaccinated patients, 18 had adenocarcinoma, and 6 had squamous cell carcinoma. All patients had received multiple prior therapies, including surgery, chemotherapy, radiation, and EGFR inhibitors.
Each patient was vaccinated with a personalized mixture of short and long neoantigen peptides (mean, 9.4 peptides) dissolved in isotonic saline. Patients received at least 12 weekly immunizations and had topical imiquimod applied over the injection site for costimulation through toll-like receptor 7. The 16 patients with EGFR mutations were given the option of continuing on an EGFR inhibitor, and 9 patients elected to do so.
Results
Dr. Lizee said this treatment approach was “very safe,” with only grade 1 treatment-related adverse events. The events were fatigue (n = 2), rash (n = 1), and fever (n = 1).
Seven patients achieved a response after vaccination, and one patient achieved a complete response. All seven responders had EGFR mutations, and four of them were receiving an EGFR inhibitor.
The patients on an EGFR inhibitor had significantly better overall survival than that of EGFR-mutated patients who had stopped taking an EGFR inhibitor – 13.8 months and 7.6 months, respectively (P = .038).
Immune profiling revealed that neoantigen-specific T-cell reactivity was associated with clinical responses. The researchers observed EGFR neoantigen-specific T-cell responses in five responders. In three responders, the strongest response was against a peptide encompassing the L858R driver mutation.
The researchers also found evidence of synergy between EGFR inhibitor therapy and the peptide vaccine. EGFR inhibition caused immunomodulatory pathways in EGFR-mutated cancer cells to favor immune-cell infiltration and HLA-mediated antigen presentation.
“Our mechanistic working model is that, in the circulation, the personalized vaccine increased the T-cell frequency,” Dr. Lizee said. “The EGFR inhibitor increased chemokines and antigen presentation at the tumor site, which then attracted those T cells to migrate to the tumor. Then, recognition of the antigen caused interferon gamma [to increase], which caused, potentially, a feed-forward loop by increasing chemokines and antigen presentation further.”
This research is sponsored by Tianjin Beichen Hospital and funded by Tianjin HengJia Biotechnology Development Co. Ltd. Dr. Lizee disclosed a consulting relationship with Tianjin HengJia Biotechnology Development Co. Ltd.
SOURCE: Lizee G et al. SITC 2019. Abstract O18.
NATIONAL HARBOR, MD. – Trial results suggest a personalized vaccination approach is feasible and safe, and the vaccine can produce clinical responses in patients with non–small cell lung cancer (NSCLC).
The neoantigen vaccine produced only grade 1 adverse events, yielded responses in patients with epidermal growth factor receptor (EGFR) mutations, and proved particularly effective in patients who were also receiving an EGFR inhibitor.
“EGFR inhibitors seemed to reduce tumor immunosuppression barriers and may enhance antitumor immune responses before and during immunization, suggesting there may be a potential synergy of EGFR with immunotherapies,” Gregory A. Lizee, PhD, of University of Texas MD Anderson Cancer Center, Houston, said at the annual meeting of the Society for Immunotherapy of Cancer.
The research began with an elderly patient who had heavily pretreated NSCLC (Oncoimmunology. 2016;5[12]:e1238539). Dr. Lizee and colleagues used tumor mutational profiling and human leukocyte antigen (HLA) typing to develop a personalized peptide vaccine for the patient. He received the vaccine along with topical imiquimod and had multiple lung tumor nodules regress. However, the patient also had liver metastasis that remained refractory to treatment, and he ultimately died.
To investigate this treatment approach in a larger group, Dr. Lizee and colleagues began a phase 1b trial of patients with advanced NSCLC (ChiCTR-IIR-16009867). As with the prior patient, the researchers designed personalized peptide vaccines for the trial subjects based on mutational profiling of 508 cancer-associated genes and high-resolution HLA typing. The peptides were selected based on nonsynonymous somatic tumor–associated mutations with variant allele frequency greater than 0.04 and the highest predicted neoantigen peptide binding to each patient’s HLA class I and II molecules. The vaccines targeted up to eight independent somatic mutations (mean, 3.75 mutations).
In all, 31 patients provided lung tumor biopsies and peripheral blood for mutational and HLA analyses. The researchers designed 27 personalized neoantigen vaccines, and 24 patients were ultimately vaccinated. This translates to a vaccination rate of 77%, which suggests this treatment approach is feasible, Dr. Lizee said.
Of the 24 vaccinated patients, 18 had adenocarcinoma, and 6 had squamous cell carcinoma. All patients had received multiple prior therapies, including surgery, chemotherapy, radiation, and EGFR inhibitors.
Each patient was vaccinated with a personalized mixture of short and long neoantigen peptides (mean, 9.4 peptides) dissolved in isotonic saline. Patients received at least 12 weekly immunizations and had topical imiquimod applied over the injection site for costimulation through toll-like receptor 7. The 16 patients with EGFR mutations were given the option of continuing on an EGFR inhibitor, and 9 patients elected to do so.
Results
Dr. Lizee said this treatment approach was “very safe,” with only grade 1 treatment-related adverse events. The events were fatigue (n = 2), rash (n = 1), and fever (n = 1).
Seven patients achieved a response after vaccination, and one patient achieved a complete response. All seven responders had EGFR mutations, and four of them were receiving an EGFR inhibitor.
The patients on an EGFR inhibitor had significantly better overall survival than that of EGFR-mutated patients who had stopped taking an EGFR inhibitor – 13.8 months and 7.6 months, respectively (P = .038).
Immune profiling revealed that neoantigen-specific T-cell reactivity was associated with clinical responses. The researchers observed EGFR neoantigen-specific T-cell responses in five responders. In three responders, the strongest response was against a peptide encompassing the L858R driver mutation.
The researchers also found evidence of synergy between EGFR inhibitor therapy and the peptide vaccine. EGFR inhibition caused immunomodulatory pathways in EGFR-mutated cancer cells to favor immune-cell infiltration and HLA-mediated antigen presentation.
“Our mechanistic working model is that, in the circulation, the personalized vaccine increased the T-cell frequency,” Dr. Lizee said. “The EGFR inhibitor increased chemokines and antigen presentation at the tumor site, which then attracted those T cells to migrate to the tumor. Then, recognition of the antigen caused interferon gamma [to increase], which caused, potentially, a feed-forward loop by increasing chemokines and antigen presentation further.”
This research is sponsored by Tianjin Beichen Hospital and funded by Tianjin HengJia Biotechnology Development Co. Ltd. Dr. Lizee disclosed a consulting relationship with Tianjin HengJia Biotechnology Development Co. Ltd.
SOURCE: Lizee G et al. SITC 2019. Abstract O18.
REPORTING FROM SITC 2019
CDC releases update of 2013 Antibiotic Resistance Threats Report
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
Pediatricians uniquely qualified to treat adolescents with opioid use disorder
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Opioid reduction works after minimally invasive gynecologic surgery
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Study detects lower CV risk in breast cancer survivors
Breast cancer survivors may have a lower prevalence of cardiac risk factors at the time of first myocardial infarction and better outcomes compared with those having a first MI from the general population, according to findings from a retrospective study.
In addition, women without breast cancer were younger at the time of first MI compared with survivors, wrote Srikanth Yandrapalli, MD, of New York Medical College, Valhalla, and colleagues. Their report is in the American Journal of Medicine.
The researchers identified 1,644,032 women with a first MI, 56,842 of whom were breast cancer survivors. The team evaluated differences in the prevalence of cardiac risk factors and related outcomes in breast cancer survivors in comparison to the general population.
At baseline, the mean age of subjects with a history of breast cancer was 77 years (range, 11 years), while the mean age of women without breast cancer was 71 years (range, 15 years).
Clinical data were collected from the United States National Inpatient Sample for January 2005 to September 2015. Other outcomes assessed were differences in baseline characteristics and the rate of in-hospital mortality in both groups.
After analysis, the researchers found that breast cancer survivors had a lower prevalence of diabetes mellitus (30.1% vs. 33.1%), obesity (9.4% vs. 13.0%), and smoking (24.1% vs. 27.0%), but higher rates of dyslipidemia (52.7% vs. 48.4%) and hypertension (73.6% vs. 68.1%), compared with women without breast cancer (All P less than .001).
With respect to age, women without breast cancer were 6 years younger than breast cancer survivors at the time of first acute MI (mean age, 71 vs. 77 years; P less than .001).
In addition, the rate of in-hospital mortality was higher in women without breast cancer (7.9%) compared with survivors (7.1%) (P less than .001). After risk adjustment, these results remained unchanged (odds ratio, 0.89; 95% confidence interval, 0.82-0.94).
“Breast cancer survivors in the U.S. are at least 6 years older than the general population of women without breast cancer, and they had a favorable cardiac risk factor profile at the time of first myocardial infarction,” Dr. Yandrapalli and colleagues explained. “The reason for these findings are unclear and hypothesis generating,” they added.
The researchers acknowledged that a key limitation of the study was the retrospective design. As a result, the potential effects of residual confounding should be considered when interpreting the results.
“The favorable impact of health education and participation in cancer survivorship programs on these observed differences in breast cancer survivors should be further explored,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Yandrapalli S et al. Am J Med. 2019 Nov 9. doi: 10.1016/j.amjmed.2019.10.018.
Breast cancer survivors may have a lower prevalence of cardiac risk factors at the time of first myocardial infarction and better outcomes compared with those having a first MI from the general population, according to findings from a retrospective study.
In addition, women without breast cancer were younger at the time of first MI compared with survivors, wrote Srikanth Yandrapalli, MD, of New York Medical College, Valhalla, and colleagues. Their report is in the American Journal of Medicine.
The researchers identified 1,644,032 women with a first MI, 56,842 of whom were breast cancer survivors. The team evaluated differences in the prevalence of cardiac risk factors and related outcomes in breast cancer survivors in comparison to the general population.
At baseline, the mean age of subjects with a history of breast cancer was 77 years (range, 11 years), while the mean age of women without breast cancer was 71 years (range, 15 years).
Clinical data were collected from the United States National Inpatient Sample for January 2005 to September 2015. Other outcomes assessed were differences in baseline characteristics and the rate of in-hospital mortality in both groups.
After analysis, the researchers found that breast cancer survivors had a lower prevalence of diabetes mellitus (30.1% vs. 33.1%), obesity (9.4% vs. 13.0%), and smoking (24.1% vs. 27.0%), but higher rates of dyslipidemia (52.7% vs. 48.4%) and hypertension (73.6% vs. 68.1%), compared with women without breast cancer (All P less than .001).
With respect to age, women without breast cancer were 6 years younger than breast cancer survivors at the time of first acute MI (mean age, 71 vs. 77 years; P less than .001).
In addition, the rate of in-hospital mortality was higher in women without breast cancer (7.9%) compared with survivors (7.1%) (P less than .001). After risk adjustment, these results remained unchanged (odds ratio, 0.89; 95% confidence interval, 0.82-0.94).
“Breast cancer survivors in the U.S. are at least 6 years older than the general population of women without breast cancer, and they had a favorable cardiac risk factor profile at the time of first myocardial infarction,” Dr. Yandrapalli and colleagues explained. “The reason for these findings are unclear and hypothesis generating,” they added.
The researchers acknowledged that a key limitation of the study was the retrospective design. As a result, the potential effects of residual confounding should be considered when interpreting the results.
“The favorable impact of health education and participation in cancer survivorship programs on these observed differences in breast cancer survivors should be further explored,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Yandrapalli S et al. Am J Med. 2019 Nov 9. doi: 10.1016/j.amjmed.2019.10.018.
Breast cancer survivors may have a lower prevalence of cardiac risk factors at the time of first myocardial infarction and better outcomes compared with those having a first MI from the general population, according to findings from a retrospective study.
In addition, women without breast cancer were younger at the time of first MI compared with survivors, wrote Srikanth Yandrapalli, MD, of New York Medical College, Valhalla, and colleagues. Their report is in the American Journal of Medicine.
The researchers identified 1,644,032 women with a first MI, 56,842 of whom were breast cancer survivors. The team evaluated differences in the prevalence of cardiac risk factors and related outcomes in breast cancer survivors in comparison to the general population.
At baseline, the mean age of subjects with a history of breast cancer was 77 years (range, 11 years), while the mean age of women without breast cancer was 71 years (range, 15 years).
Clinical data were collected from the United States National Inpatient Sample for January 2005 to September 2015. Other outcomes assessed were differences in baseline characteristics and the rate of in-hospital mortality in both groups.
After analysis, the researchers found that breast cancer survivors had a lower prevalence of diabetes mellitus (30.1% vs. 33.1%), obesity (9.4% vs. 13.0%), and smoking (24.1% vs. 27.0%), but higher rates of dyslipidemia (52.7% vs. 48.4%) and hypertension (73.6% vs. 68.1%), compared with women without breast cancer (All P less than .001).
With respect to age, women without breast cancer were 6 years younger than breast cancer survivors at the time of first acute MI (mean age, 71 vs. 77 years; P less than .001).
In addition, the rate of in-hospital mortality was higher in women without breast cancer (7.9%) compared with survivors (7.1%) (P less than .001). After risk adjustment, these results remained unchanged (odds ratio, 0.89; 95% confidence interval, 0.82-0.94).
“Breast cancer survivors in the U.S. are at least 6 years older than the general population of women without breast cancer, and they had a favorable cardiac risk factor profile at the time of first myocardial infarction,” Dr. Yandrapalli and colleagues explained. “The reason for these findings are unclear and hypothesis generating,” they added.
The researchers acknowledged that a key limitation of the study was the retrospective design. As a result, the potential effects of residual confounding should be considered when interpreting the results.
“The favorable impact of health education and participation in cancer survivorship programs on these observed differences in breast cancer survivors should be further explored,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Yandrapalli S et al. Am J Med. 2019 Nov 9. doi: 10.1016/j.amjmed.2019.10.018.
FROM THE AMERICAN JOURNAL OF MEDICINE
Open enrollment 2020: HealthCare.gov activity down from last year
The federal health insurance exchange is now through the first 2 weeks of its 2020 open enrollment on the HealthCare.gov platform, and just over 930,000 plans have been selected, the Centers for Medicare & Medicaid Services reported.
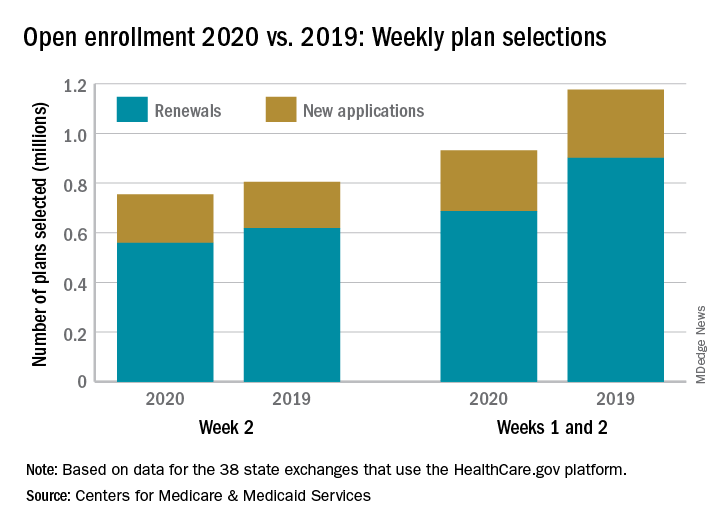
The majority of those selections – almost 690,000 – were made by consumers renewing their existing coverage, with the rest coming from new consumers who did not have exchange coverage for 2019, the CMS said in its weekly enrollment snapshot on Nov. 13.
The total number of plans selected through week 2 is down almost 21% from last year, when selections totaled almost 1.18 million. This year, however, week 1 of open enrollment was only 2 days long (Nov. 1-2), versus 3 days (Nov. 1-3) last year, and last year there were 39 states using HealthCare.gov, compared with 38 this year since Nevada now has its own state-based exchange.
The total number of plans selected during a particular reporting period can change later if plans are modified or canceled, CMS noted, and “the weekly snapshot only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
The federal health insurance exchange is now through the first 2 weeks of its 2020 open enrollment on the HealthCare.gov platform, and just over 930,000 plans have been selected, the Centers for Medicare & Medicaid Services reported.

The majority of those selections – almost 690,000 – were made by consumers renewing their existing coverage, with the rest coming from new consumers who did not have exchange coverage for 2019, the CMS said in its weekly enrollment snapshot on Nov. 13.
The total number of plans selected through week 2 is down almost 21% from last year, when selections totaled almost 1.18 million. This year, however, week 1 of open enrollment was only 2 days long (Nov. 1-2), versus 3 days (Nov. 1-3) last year, and last year there were 39 states using HealthCare.gov, compared with 38 this year since Nevada now has its own state-based exchange.
The total number of plans selected during a particular reporting period can change later if plans are modified or canceled, CMS noted, and “the weekly snapshot only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
The federal health insurance exchange is now through the first 2 weeks of its 2020 open enrollment on the HealthCare.gov platform, and just over 930,000 plans have been selected, the Centers for Medicare & Medicaid Services reported.

The majority of those selections – almost 690,000 – were made by consumers renewing their existing coverage, with the rest coming from new consumers who did not have exchange coverage for 2019, the CMS said in its weekly enrollment snapshot on Nov. 13.
The total number of plans selected through week 2 is down almost 21% from last year, when selections totaled almost 1.18 million. This year, however, week 1 of open enrollment was only 2 days long (Nov. 1-2), versus 3 days (Nov. 1-3) last year, and last year there were 39 states using HealthCare.gov, compared with 38 this year since Nevada now has its own state-based exchange.
The total number of plans selected during a particular reporting period can change later if plans are modified or canceled, CMS noted, and “the weekly snapshot only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Surgical staging improves cervical cancer outcomes
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Going beyond the QI project
Role modeling for residents
Quality improvement (QI) education has become increasingly seen as core content in graduate medical education, said Brian Wong, MD, FRCPC, of the University of Toronto. One of the most common strategies for teaching QI is to have residents participate in a QI project, in which hospitalists often take a leading role.
“Given the investment made and time spent carrying out these projects, it is important to know whether or not the training has led to the desired outcome from both a learning and a project standpoint,” Dr. Wong said, which is why he coauthored a recent editorial on the subject in BMJ Quality and Safety. QI educators have long recognized that it’s difficult to know whether the education was successful.
“For example, if the project was not successful, does it matter if the residents learned key QI principles that they were able to apply to their project work?” Dr. Wong noted. “Our perspective extends this discussion by asking, ‘What does success look like in QI education?’ We argue that rather than focusing on whether the project was successful or not, our real goal should be to create QI educational experiences that will ensure that residents change their behaviors in future practice to embrace QI as an activity that is core to their everyday work.”
Hospitalists have an important role in that. “They can set the stage for learners to recognize just how important it is to incorporate QI into daily work. Through this role modeling, residents who carry out QI projects can see that the lessons learned contribute to lifelong engagement in QI.”
Dr. Wong’s hope is to focus on the type of QI experience that fosters long-term behavior changes.
“We want residents, when they graduate, to embrace QI, to volunteer to participate in organizational initiatives, to welcome practice data and reflect on it for the purposes of continuous improvement, to collaborate interprofessionally to make small iterative changes to the care delivery system to ensure that patients receive the highest quality of care possible,” he said. “My hope is that we can start to think differently about how we measure success in QI education.”
Reference
1. Myers JS, Wong BM. Measuring outcomes in quality improvement education: Success is in the eye of the beholder. BMJ Qual Saf. 2019 Mar 18. doi: 10.1136/bmjqs-2018-008305.
Role modeling for residents
Role modeling for residents
Quality improvement (QI) education has become increasingly seen as core content in graduate medical education, said Brian Wong, MD, FRCPC, of the University of Toronto. One of the most common strategies for teaching QI is to have residents participate in a QI project, in which hospitalists often take a leading role.
“Given the investment made and time spent carrying out these projects, it is important to know whether or not the training has led to the desired outcome from both a learning and a project standpoint,” Dr. Wong said, which is why he coauthored a recent editorial on the subject in BMJ Quality and Safety. QI educators have long recognized that it’s difficult to know whether the education was successful.
“For example, if the project was not successful, does it matter if the residents learned key QI principles that they were able to apply to their project work?” Dr. Wong noted. “Our perspective extends this discussion by asking, ‘What does success look like in QI education?’ We argue that rather than focusing on whether the project was successful or not, our real goal should be to create QI educational experiences that will ensure that residents change their behaviors in future practice to embrace QI as an activity that is core to their everyday work.”
Hospitalists have an important role in that. “They can set the stage for learners to recognize just how important it is to incorporate QI into daily work. Through this role modeling, residents who carry out QI projects can see that the lessons learned contribute to lifelong engagement in QI.”
Dr. Wong’s hope is to focus on the type of QI experience that fosters long-term behavior changes.
“We want residents, when they graduate, to embrace QI, to volunteer to participate in organizational initiatives, to welcome practice data and reflect on it for the purposes of continuous improvement, to collaborate interprofessionally to make small iterative changes to the care delivery system to ensure that patients receive the highest quality of care possible,” he said. “My hope is that we can start to think differently about how we measure success in QI education.”
Reference
1. Myers JS, Wong BM. Measuring outcomes in quality improvement education: Success is in the eye of the beholder. BMJ Qual Saf. 2019 Mar 18. doi: 10.1136/bmjqs-2018-008305.
Quality improvement (QI) education has become increasingly seen as core content in graduate medical education, said Brian Wong, MD, FRCPC, of the University of Toronto. One of the most common strategies for teaching QI is to have residents participate in a QI project, in which hospitalists often take a leading role.
“Given the investment made and time spent carrying out these projects, it is important to know whether or not the training has led to the desired outcome from both a learning and a project standpoint,” Dr. Wong said, which is why he coauthored a recent editorial on the subject in BMJ Quality and Safety. QI educators have long recognized that it’s difficult to know whether the education was successful.
“For example, if the project was not successful, does it matter if the residents learned key QI principles that they were able to apply to their project work?” Dr. Wong noted. “Our perspective extends this discussion by asking, ‘What does success look like in QI education?’ We argue that rather than focusing on whether the project was successful or not, our real goal should be to create QI educational experiences that will ensure that residents change their behaviors in future practice to embrace QI as an activity that is core to their everyday work.”
Hospitalists have an important role in that. “They can set the stage for learners to recognize just how important it is to incorporate QI into daily work. Through this role modeling, residents who carry out QI projects can see that the lessons learned contribute to lifelong engagement in QI.”
Dr. Wong’s hope is to focus on the type of QI experience that fosters long-term behavior changes.
“We want residents, when they graduate, to embrace QI, to volunteer to participate in organizational initiatives, to welcome practice data and reflect on it for the purposes of continuous improvement, to collaborate interprofessionally to make small iterative changes to the care delivery system to ensure that patients receive the highest quality of care possible,” he said. “My hope is that we can start to think differently about how we measure success in QI education.”
Reference
1. Myers JS, Wong BM. Measuring outcomes in quality improvement education: Success is in the eye of the beholder. BMJ Qual Saf. 2019 Mar 18. doi: 10.1136/bmjqs-2018-008305.