User login
Baseline subtypes predict dementia and death in patients with Parkinson’s disease
ST. LOUIS – , according to the results of a longitudinal study of 162 patients at Washington University, St. Louis.
Parkinson’s disease is often only staged as mild, moderate, or severe, and sometimes cognitive and psychiatric symptoms are not assessed. The St. Louis team wanted to see if there are actual subtypes, and if they have clinical relevance, said lead investigator Peter Myers, PhD, a postdoctoral research associate at Washington University.
The magnitude of the findings was “surprising. ... We don’t think we are seeing stages” because the different symptom patterns were apparent at baseline. Instead, “we are seeing really distinct clinical subtypes that could inform patient prognosis and help prepare families and caregivers. It is important that you assess more than just motor symptoms,” he said at the annual meeting of the American Neurological Association.
Some of the subjects were newly diagnosed and others diagnosed years earlier. At baseline, they had symptoms for an average of 6 years, and none had dementia.
After a battery of cognitive, psychiatric, and movement tests, Dr. Myers and his colleagues found that their subjects fell into three patterns: motor symptoms only, with normal cognitive and psychiatric performance (63 subjects); motor symptoms plus prominent anxiety or depression (17); and motor symptoms plus cognitive deficits (82).
A total of 42 patients developed dementia – a score of at least 1 on the Clinical Dementia Rating evaluation – including three in the motor-only group (5%), two in the psychiatric/motor group (12%), and 37 in the cognitive/motor group (45%).
After controlling for age, sex, and symptom duration, the risk of dementia conversion was far higher in the cognitive/motor group (relative risk, 4.16, 95% confidence interval, 1.18-14.65) and psychiatric/motor group (RR, 3.08; 95% CI, 0.72-13.28), compared with motor-only subjects.
Thirty-eight patients died, the majority because of Parkinson’s disease-related causes, including five in the motor-only group (8%), two in the psychiatric/motor group (12%), and 31 in the cognitive/motor group (38%).
The risk of death, relative to motor-only patients, was higher with both the cognitive/motor (RR, 4.06; 95% CI, 1.37-12.03) and psychiatric/motor subtypes (RR, 4.17; 95% CI, 0.70-24.91).
It is unclear what leads to the progression differences, but the researchers’ hypothesis is that the broader scope of symptoms indicates a greater extent of brain pathology. The St. Louis team is looking at brain-imaging data to see if that is true, and if the subtypes can be distinguished on imaging. They are also interested in seeing if genetics plays a role in susceptibility to the different subtypes, and if the baseline subtypes are stable over time or if patients convert between them.
Subjects were, on average, 66 years old, and 62% were men. The mean levodopa-equivalent daily dose was 613 mg in the motor-only group, 1,004 mg in the psychiatric/motor group, and 783 mg in the cognitive/motor group (P = .03). Mean symptom duration was shorter in motor-only patients (5.1 years) versus the psychiatric/motor group (7.2 years) and cognitive/motor group (6.9 years, P less than .01).
Although relative risks crossed 1 in the psychiatric/motor group, it was probably because there were only 17 patients. “We do think there is a very strong likelihood that” the psychiatric/motor findings, like the cognitive/motor findings, “are valid,” Dr. Myer said.
“I think [the findings] could be real,” said Clair Henchcliffe, MD, DPhil, vice chair for clinical research in the department of neurology at Weill Cornell Medical College in New York.
“Other publications have shown that people who have cognitive symptoms at onset are more likely to go on to develop dementia down the line. This is much in line with that,” she said when asked for comment.
The field has “always been interested in finding data that help us personalize treatment, and a lot of people are looking to subtype Parkinson’s disease to help us plan with our patients.” It could also help with frequency of follow-up, trial participation, and maybe someday treatment selection. “We are always thinking a few years ahead” with Parkinson’s disease, she said.
There was no industry funding, and the investigators did not have any relevant disclosures.
ST. LOUIS – , according to the results of a longitudinal study of 162 patients at Washington University, St. Louis.
Parkinson’s disease is often only staged as mild, moderate, or severe, and sometimes cognitive and psychiatric symptoms are not assessed. The St. Louis team wanted to see if there are actual subtypes, and if they have clinical relevance, said lead investigator Peter Myers, PhD, a postdoctoral research associate at Washington University.
The magnitude of the findings was “surprising. ... We don’t think we are seeing stages” because the different symptom patterns were apparent at baseline. Instead, “we are seeing really distinct clinical subtypes that could inform patient prognosis and help prepare families and caregivers. It is important that you assess more than just motor symptoms,” he said at the annual meeting of the American Neurological Association.
Some of the subjects were newly diagnosed and others diagnosed years earlier. At baseline, they had symptoms for an average of 6 years, and none had dementia.
After a battery of cognitive, psychiatric, and movement tests, Dr. Myers and his colleagues found that their subjects fell into three patterns: motor symptoms only, with normal cognitive and psychiatric performance (63 subjects); motor symptoms plus prominent anxiety or depression (17); and motor symptoms plus cognitive deficits (82).
A total of 42 patients developed dementia – a score of at least 1 on the Clinical Dementia Rating evaluation – including three in the motor-only group (5%), two in the psychiatric/motor group (12%), and 37 in the cognitive/motor group (45%).
After controlling for age, sex, and symptom duration, the risk of dementia conversion was far higher in the cognitive/motor group (relative risk, 4.16, 95% confidence interval, 1.18-14.65) and psychiatric/motor group (RR, 3.08; 95% CI, 0.72-13.28), compared with motor-only subjects.
Thirty-eight patients died, the majority because of Parkinson’s disease-related causes, including five in the motor-only group (8%), two in the psychiatric/motor group (12%), and 31 in the cognitive/motor group (38%).
The risk of death, relative to motor-only patients, was higher with both the cognitive/motor (RR, 4.06; 95% CI, 1.37-12.03) and psychiatric/motor subtypes (RR, 4.17; 95% CI, 0.70-24.91).
It is unclear what leads to the progression differences, but the researchers’ hypothesis is that the broader scope of symptoms indicates a greater extent of brain pathology. The St. Louis team is looking at brain-imaging data to see if that is true, and if the subtypes can be distinguished on imaging. They are also interested in seeing if genetics plays a role in susceptibility to the different subtypes, and if the baseline subtypes are stable over time or if patients convert between them.
Subjects were, on average, 66 years old, and 62% were men. The mean levodopa-equivalent daily dose was 613 mg in the motor-only group, 1,004 mg in the psychiatric/motor group, and 783 mg in the cognitive/motor group (P = .03). Mean symptom duration was shorter in motor-only patients (5.1 years) versus the psychiatric/motor group (7.2 years) and cognitive/motor group (6.9 years, P less than .01).
Although relative risks crossed 1 in the psychiatric/motor group, it was probably because there were only 17 patients. “We do think there is a very strong likelihood that” the psychiatric/motor findings, like the cognitive/motor findings, “are valid,” Dr. Myer said.
“I think [the findings] could be real,” said Clair Henchcliffe, MD, DPhil, vice chair for clinical research in the department of neurology at Weill Cornell Medical College in New York.
“Other publications have shown that people who have cognitive symptoms at onset are more likely to go on to develop dementia down the line. This is much in line with that,” she said when asked for comment.
The field has “always been interested in finding data that help us personalize treatment, and a lot of people are looking to subtype Parkinson’s disease to help us plan with our patients.” It could also help with frequency of follow-up, trial participation, and maybe someday treatment selection. “We are always thinking a few years ahead” with Parkinson’s disease, she said.
There was no industry funding, and the investigators did not have any relevant disclosures.
ST. LOUIS – , according to the results of a longitudinal study of 162 patients at Washington University, St. Louis.
Parkinson’s disease is often only staged as mild, moderate, or severe, and sometimes cognitive and psychiatric symptoms are not assessed. The St. Louis team wanted to see if there are actual subtypes, and if they have clinical relevance, said lead investigator Peter Myers, PhD, a postdoctoral research associate at Washington University.
The magnitude of the findings was “surprising. ... We don’t think we are seeing stages” because the different symptom patterns were apparent at baseline. Instead, “we are seeing really distinct clinical subtypes that could inform patient prognosis and help prepare families and caregivers. It is important that you assess more than just motor symptoms,” he said at the annual meeting of the American Neurological Association.
Some of the subjects were newly diagnosed and others diagnosed years earlier. At baseline, they had symptoms for an average of 6 years, and none had dementia.
After a battery of cognitive, psychiatric, and movement tests, Dr. Myers and his colleagues found that their subjects fell into three patterns: motor symptoms only, with normal cognitive and psychiatric performance (63 subjects); motor symptoms plus prominent anxiety or depression (17); and motor symptoms plus cognitive deficits (82).
A total of 42 patients developed dementia – a score of at least 1 on the Clinical Dementia Rating evaluation – including three in the motor-only group (5%), two in the psychiatric/motor group (12%), and 37 in the cognitive/motor group (45%).
After controlling for age, sex, and symptom duration, the risk of dementia conversion was far higher in the cognitive/motor group (relative risk, 4.16, 95% confidence interval, 1.18-14.65) and psychiatric/motor group (RR, 3.08; 95% CI, 0.72-13.28), compared with motor-only subjects.
Thirty-eight patients died, the majority because of Parkinson’s disease-related causes, including five in the motor-only group (8%), two in the psychiatric/motor group (12%), and 31 in the cognitive/motor group (38%).
The risk of death, relative to motor-only patients, was higher with both the cognitive/motor (RR, 4.06; 95% CI, 1.37-12.03) and psychiatric/motor subtypes (RR, 4.17; 95% CI, 0.70-24.91).
It is unclear what leads to the progression differences, but the researchers’ hypothesis is that the broader scope of symptoms indicates a greater extent of brain pathology. The St. Louis team is looking at brain-imaging data to see if that is true, and if the subtypes can be distinguished on imaging. They are also interested in seeing if genetics plays a role in susceptibility to the different subtypes, and if the baseline subtypes are stable over time or if patients convert between them.
Subjects were, on average, 66 years old, and 62% were men. The mean levodopa-equivalent daily dose was 613 mg in the motor-only group, 1,004 mg in the psychiatric/motor group, and 783 mg in the cognitive/motor group (P = .03). Mean symptom duration was shorter in motor-only patients (5.1 years) versus the psychiatric/motor group (7.2 years) and cognitive/motor group (6.9 years, P less than .01).
Although relative risks crossed 1 in the psychiatric/motor group, it was probably because there were only 17 patients. “We do think there is a very strong likelihood that” the psychiatric/motor findings, like the cognitive/motor findings, “are valid,” Dr. Myer said.
“I think [the findings] could be real,” said Clair Henchcliffe, MD, DPhil, vice chair for clinical research in the department of neurology at Weill Cornell Medical College in New York.
“Other publications have shown that people who have cognitive symptoms at onset are more likely to go on to develop dementia down the line. This is much in line with that,” she said when asked for comment.
The field has “always been interested in finding data that help us personalize treatment, and a lot of people are looking to subtype Parkinson’s disease to help us plan with our patients.” It could also help with frequency of follow-up, trial participation, and maybe someday treatment selection. “We are always thinking a few years ahead” with Parkinson’s disease, she said.
There was no industry funding, and the investigators did not have any relevant disclosures.
REPORTING FROM ANA 2019
Atopic dermatitis acts differently in certain populations
LAS VEGAS – Eczema is eczema is eczema, right? Maybe not. “Atopic dermatitis might not be one disease,” a dermatologist told colleagues, and treatments may need to be adjusted to reflect the age and ethnicity of patients.
More research is needed, Kenneth B. Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, said during a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “We’re probably just on the tip of the iceberg of understanding the physiology of atopic dermatitis. Hopefully, it will lead to the therapeutic advances we’ve seen in psoriasis.”
As Dr. Gordon explained, there’s a , he said, “and our medicines aren’t well understood.”
As for the disease itself, he said, “you might hear a renowned [expert] say, ‘This is how it works,’ and another say, ‘This is absolutely not how it works.’ ” One camp focused on the skin barrier, he said, while another camp highlighted inflammation in AD.
“Both the barrier and inflammation are important,” he said. “There are multiple cell types and cytokines that are important, but we don’t know yet the relative importance of them all. You have this cytokine soup, and we’re still trying to figure out the driving forces.”
What is clear, Dr. Gordon said, is that AD acts differently in certain patient populations. It’s not the same in pediatric versus adult patients, he said, and it’s not the same in white versus black versus Asian patients. Research, for example, suggests that Th2, Th22, and Th17 pathways appear to be important in pediatric AD, but not Th1, he said. In contrast, the Th1 pathway plays a role in white adults – but not in black adults
Different cytokines appear to play different roles in these populations, he said. “One of the key things moving forward is going to be figuring out which patients you apply these medications to,” he noted.
Dr. Gordon has multiple disclosures including honoraria or research support from Abbvie, Lilly, Novartis, Pfizer, UCB, and others. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Eczema is eczema is eczema, right? Maybe not. “Atopic dermatitis might not be one disease,” a dermatologist told colleagues, and treatments may need to be adjusted to reflect the age and ethnicity of patients.
More research is needed, Kenneth B. Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, said during a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “We’re probably just on the tip of the iceberg of understanding the physiology of atopic dermatitis. Hopefully, it will lead to the therapeutic advances we’ve seen in psoriasis.”
As Dr. Gordon explained, there’s a , he said, “and our medicines aren’t well understood.”
As for the disease itself, he said, “you might hear a renowned [expert] say, ‘This is how it works,’ and another say, ‘This is absolutely not how it works.’ ” One camp focused on the skin barrier, he said, while another camp highlighted inflammation in AD.
“Both the barrier and inflammation are important,” he said. “There are multiple cell types and cytokines that are important, but we don’t know yet the relative importance of them all. You have this cytokine soup, and we’re still trying to figure out the driving forces.”
What is clear, Dr. Gordon said, is that AD acts differently in certain patient populations. It’s not the same in pediatric versus adult patients, he said, and it’s not the same in white versus black versus Asian patients. Research, for example, suggests that Th2, Th22, and Th17 pathways appear to be important in pediatric AD, but not Th1, he said. In contrast, the Th1 pathway plays a role in white adults – but not in black adults
Different cytokines appear to play different roles in these populations, he said. “One of the key things moving forward is going to be figuring out which patients you apply these medications to,” he noted.
Dr. Gordon has multiple disclosures including honoraria or research support from Abbvie, Lilly, Novartis, Pfizer, UCB, and others. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Eczema is eczema is eczema, right? Maybe not. “Atopic dermatitis might not be one disease,” a dermatologist told colleagues, and treatments may need to be adjusted to reflect the age and ethnicity of patients.
More research is needed, Kenneth B. Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, said during a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “We’re probably just on the tip of the iceberg of understanding the physiology of atopic dermatitis. Hopefully, it will lead to the therapeutic advances we’ve seen in psoriasis.”
As Dr. Gordon explained, there’s a , he said, “and our medicines aren’t well understood.”
As for the disease itself, he said, “you might hear a renowned [expert] say, ‘This is how it works,’ and another say, ‘This is absolutely not how it works.’ ” One camp focused on the skin barrier, he said, while another camp highlighted inflammation in AD.
“Both the barrier and inflammation are important,” he said. “There are multiple cell types and cytokines that are important, but we don’t know yet the relative importance of them all. You have this cytokine soup, and we’re still trying to figure out the driving forces.”
What is clear, Dr. Gordon said, is that AD acts differently in certain patient populations. It’s not the same in pediatric versus adult patients, he said, and it’s not the same in white versus black versus Asian patients. Research, for example, suggests that Th2, Th22, and Th17 pathways appear to be important in pediatric AD, but not Th1, he said. In contrast, the Th1 pathway plays a role in white adults – but not in black adults
Different cytokines appear to play different roles in these populations, he said. “One of the key things moving forward is going to be figuring out which patients you apply these medications to,” he noted.
Dr. Gordon has multiple disclosures including honoraria or research support from Abbvie, Lilly, Novartis, Pfizer, UCB, and others. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Oral baricitinib performs well in phase 3 for atopic dermatitis
MADRID – Adding the oral Janus kinase (JAK) inhibitor baricitinib to standard atopic dermatitis therapy with low- and midpotency topical corticosteroids markedly improved disease severity and key patient-reported outcomes, compared with topical corticosteroids alone, in the phase 3, randomized, double-blind BREEZE-AD7 trial, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
in the phase 3 BREEZE-AD1 and -AD2 trials. But BREEZE-AD7 further advances the field because it’s the first phase 3 study testing the efficacy of a JAK inhibitor in combination with low- and midpotency topical steroids.
“I think this study is important because it looks into the situation that’s more like what happens in the real world, which is, as with dupilumab and other drugs, we use the systemic agent in combination with topical therapies and, in particular, with topical corticosteroids,” commented Dr. Reich, professor of dermatology at University Medical Center, Hamburg, and medical director at SCIderm, a scientific research company.
“This is what I think we can expect from existing and upcoming systemic therapies in atopic dermatitis: We will use them in combination with topical corticosteroids, and hopefully this will allow patients to dramatically reduce the concomitant use of topical corticosteroids, as shown here in BREEZE-AD7,” he added.
BREEZE-AD7 was a 16-week study that included 329 adults with moderate or severe atopic dermatitis who were randomized to low- and midpotency topical corticosteroids plus either baricitinib at 2 mg once daily, baricitinib at 4 mg once daily, or placebo. The group’s mean baseline Eczema Area and Severity Index (EASI) score was 29. Overall, 45% of participants had a baseline Investigator’s Global Assessment (IGA) of disease severity of 4 on a 0-4 scale.
The primary endpoint was achievement of an IGA of 0 or 1, meaning clear or almost clear, along with at least a 2-point IGA improvement from baseline at week 16. This was accomplished in 30.6% of those on 4 mg/day of baricitinib, 23.9% of patients in the 2-mg group, and 14.7% of controls.
The 4-mg dose of baricitinib was statistically superior to placebo; the 2-mg dose was not. However, Dr. Reich indicated he was untroubled by this because the primary endpoint was set at a high bar, and both doses of baricitinib proved to be significantly better than topical steroids plus placebo in terms of EASI 75 response rates, as well as reductions in itch, skin pain, and sleep problems, which aren’t captured in EASI scores (see graphic).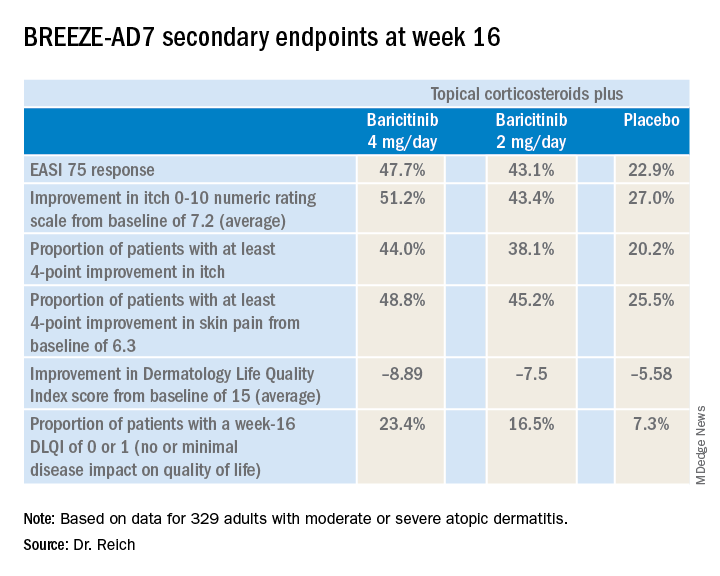
“One of my big learnings from this year’s EADV is that we have to rethink the dimensions of atopic dermatitis. I think we have underestimated the relevance of important symptoms such as itch, the impact atopic dermatitis has on pain, and the effect it has on sleeping problems,” the dermatologist said. “My feeling is that baricitinib is strongest in reducing itch, improving sleep, and reducing pain, but it also has good effects on the clinical signs of atopic dermatitis.”
The baricitinib-treated patients’ rapidity of improvement in the various endpoints was particularly impressive. Both doses of the JAK 1/2 inhibitor showed significant separation from the control group in the first week, and the majority of improvement occurred by week 4.
A key finding was that patients on baricitinib at 2 mg/day and 4 mg/day used a mean total of 162 g and 137 g of midpotency topical steroids, respectively, during the 16 weeks, compared with 225 g in the control group. The higher-dose baricitinib group was topical corticosteroid-free on 33% of study days, compared with 25% of days for the baricitinib 2 mg patients and 17% of days for controls.
In terms of safety, there was a case of pulmonary embolism in the higher-dose baricitinib group and an opportunistic toxoplasmosis eye infection in the control population. The frequency of oral herpes and herpes simplex virus infections was 2.8% in controls, 4.6% in the baricitinib 2-mg group, and 6.3% in the 4-mg group. There was also a signal of a dose-dependent increased risk of new-onset acne, with rates of 0.9% in controls and patients on baricitinib 2 mg, climbing to 3.6% with baricitinib 4 mg.
“In phase 2 results with upadacitinib [another oral JAK inhibitor], we saw that more than 10% of patients in the highest-dose group developed what was classified as acne. I cannot explain this, but it’s something we will monitor in the future,” Dr. Reich promised.
A fuller picture of baricitinib’s safety profile in the setting of atopic dermatitis clearly requires larger and longer-term studies, he added.
Baricitinib at the 2 mg daily dose is already marketed as Olumiant for the treatment of rheumatoid arthritis, with labeling that includes a boxed warning about serious infections, malignancy, and thrombosis. The Food and Drug Administration did not approve the 4-mg dose after determining that its higher safety hazard outweighed the efficacy advantage over the lower dose.
The BREEZE-AD7 study was sponsored by Eli Lilly. Dr. Reich reported serving as an adviser to, paid speaker for, and recipient of research grants from that pharmaceutical company and more than two dozen others.
MADRID – Adding the oral Janus kinase (JAK) inhibitor baricitinib to standard atopic dermatitis therapy with low- and midpotency topical corticosteroids markedly improved disease severity and key patient-reported outcomes, compared with topical corticosteroids alone, in the phase 3, randomized, double-blind BREEZE-AD7 trial, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
in the phase 3 BREEZE-AD1 and -AD2 trials. But BREEZE-AD7 further advances the field because it’s the first phase 3 study testing the efficacy of a JAK inhibitor in combination with low- and midpotency topical steroids.
“I think this study is important because it looks into the situation that’s more like what happens in the real world, which is, as with dupilumab and other drugs, we use the systemic agent in combination with topical therapies and, in particular, with topical corticosteroids,” commented Dr. Reich, professor of dermatology at University Medical Center, Hamburg, and medical director at SCIderm, a scientific research company.
“This is what I think we can expect from existing and upcoming systemic therapies in atopic dermatitis: We will use them in combination with topical corticosteroids, and hopefully this will allow patients to dramatically reduce the concomitant use of topical corticosteroids, as shown here in BREEZE-AD7,” he added.
BREEZE-AD7 was a 16-week study that included 329 adults with moderate or severe atopic dermatitis who were randomized to low- and midpotency topical corticosteroids plus either baricitinib at 2 mg once daily, baricitinib at 4 mg once daily, or placebo. The group’s mean baseline Eczema Area and Severity Index (EASI) score was 29. Overall, 45% of participants had a baseline Investigator’s Global Assessment (IGA) of disease severity of 4 on a 0-4 scale.
The primary endpoint was achievement of an IGA of 0 or 1, meaning clear or almost clear, along with at least a 2-point IGA improvement from baseline at week 16. This was accomplished in 30.6% of those on 4 mg/day of baricitinib, 23.9% of patients in the 2-mg group, and 14.7% of controls.
The 4-mg dose of baricitinib was statistically superior to placebo; the 2-mg dose was not. However, Dr. Reich indicated he was untroubled by this because the primary endpoint was set at a high bar, and both doses of baricitinib proved to be significantly better than topical steroids plus placebo in terms of EASI 75 response rates, as well as reductions in itch, skin pain, and sleep problems, which aren’t captured in EASI scores (see graphic).
“One of my big learnings from this year’s EADV is that we have to rethink the dimensions of atopic dermatitis. I think we have underestimated the relevance of important symptoms such as itch, the impact atopic dermatitis has on pain, and the effect it has on sleeping problems,” the dermatologist said. “My feeling is that baricitinib is strongest in reducing itch, improving sleep, and reducing pain, but it also has good effects on the clinical signs of atopic dermatitis.”
The baricitinib-treated patients’ rapidity of improvement in the various endpoints was particularly impressive. Both doses of the JAK 1/2 inhibitor showed significant separation from the control group in the first week, and the majority of improvement occurred by week 4.
A key finding was that patients on baricitinib at 2 mg/day and 4 mg/day used a mean total of 162 g and 137 g of midpotency topical steroids, respectively, during the 16 weeks, compared with 225 g in the control group. The higher-dose baricitinib group was topical corticosteroid-free on 33% of study days, compared with 25% of days for the baricitinib 2 mg patients and 17% of days for controls.
In terms of safety, there was a case of pulmonary embolism in the higher-dose baricitinib group and an opportunistic toxoplasmosis eye infection in the control population. The frequency of oral herpes and herpes simplex virus infections was 2.8% in controls, 4.6% in the baricitinib 2-mg group, and 6.3% in the 4-mg group. There was also a signal of a dose-dependent increased risk of new-onset acne, with rates of 0.9% in controls and patients on baricitinib 2 mg, climbing to 3.6% with baricitinib 4 mg.
“In phase 2 results with upadacitinib [another oral JAK inhibitor], we saw that more than 10% of patients in the highest-dose group developed what was classified as acne. I cannot explain this, but it’s something we will monitor in the future,” Dr. Reich promised.
A fuller picture of baricitinib’s safety profile in the setting of atopic dermatitis clearly requires larger and longer-term studies, he added.
Baricitinib at the 2 mg daily dose is already marketed as Olumiant for the treatment of rheumatoid arthritis, with labeling that includes a boxed warning about serious infections, malignancy, and thrombosis. The Food and Drug Administration did not approve the 4-mg dose after determining that its higher safety hazard outweighed the efficacy advantage over the lower dose.
The BREEZE-AD7 study was sponsored by Eli Lilly. Dr. Reich reported serving as an adviser to, paid speaker for, and recipient of research grants from that pharmaceutical company and more than two dozen others.
MADRID – Adding the oral Janus kinase (JAK) inhibitor baricitinib to standard atopic dermatitis therapy with low- and midpotency topical corticosteroids markedly improved disease severity and key patient-reported outcomes, compared with topical corticosteroids alone, in the phase 3, randomized, double-blind BREEZE-AD7 trial, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
in the phase 3 BREEZE-AD1 and -AD2 trials. But BREEZE-AD7 further advances the field because it’s the first phase 3 study testing the efficacy of a JAK inhibitor in combination with low- and midpotency topical steroids.
“I think this study is important because it looks into the situation that’s more like what happens in the real world, which is, as with dupilumab and other drugs, we use the systemic agent in combination with topical therapies and, in particular, with topical corticosteroids,” commented Dr. Reich, professor of dermatology at University Medical Center, Hamburg, and medical director at SCIderm, a scientific research company.
“This is what I think we can expect from existing and upcoming systemic therapies in atopic dermatitis: We will use them in combination with topical corticosteroids, and hopefully this will allow patients to dramatically reduce the concomitant use of topical corticosteroids, as shown here in BREEZE-AD7,” he added.
BREEZE-AD7 was a 16-week study that included 329 adults with moderate or severe atopic dermatitis who were randomized to low- and midpotency topical corticosteroids plus either baricitinib at 2 mg once daily, baricitinib at 4 mg once daily, or placebo. The group’s mean baseline Eczema Area and Severity Index (EASI) score was 29. Overall, 45% of participants had a baseline Investigator’s Global Assessment (IGA) of disease severity of 4 on a 0-4 scale.
The primary endpoint was achievement of an IGA of 0 or 1, meaning clear or almost clear, along with at least a 2-point IGA improvement from baseline at week 16. This was accomplished in 30.6% of those on 4 mg/day of baricitinib, 23.9% of patients in the 2-mg group, and 14.7% of controls.
The 4-mg dose of baricitinib was statistically superior to placebo; the 2-mg dose was not. However, Dr. Reich indicated he was untroubled by this because the primary endpoint was set at a high bar, and both doses of baricitinib proved to be significantly better than topical steroids plus placebo in terms of EASI 75 response rates, as well as reductions in itch, skin pain, and sleep problems, which aren’t captured in EASI scores (see graphic).
“One of my big learnings from this year’s EADV is that we have to rethink the dimensions of atopic dermatitis. I think we have underestimated the relevance of important symptoms such as itch, the impact atopic dermatitis has on pain, and the effect it has on sleeping problems,” the dermatologist said. “My feeling is that baricitinib is strongest in reducing itch, improving sleep, and reducing pain, but it also has good effects on the clinical signs of atopic dermatitis.”
The baricitinib-treated patients’ rapidity of improvement in the various endpoints was particularly impressive. Both doses of the JAK 1/2 inhibitor showed significant separation from the control group in the first week, and the majority of improvement occurred by week 4.
A key finding was that patients on baricitinib at 2 mg/day and 4 mg/day used a mean total of 162 g and 137 g of midpotency topical steroids, respectively, during the 16 weeks, compared with 225 g in the control group. The higher-dose baricitinib group was topical corticosteroid-free on 33% of study days, compared with 25% of days for the baricitinib 2 mg patients and 17% of days for controls.
In terms of safety, there was a case of pulmonary embolism in the higher-dose baricitinib group and an opportunistic toxoplasmosis eye infection in the control population. The frequency of oral herpes and herpes simplex virus infections was 2.8% in controls, 4.6% in the baricitinib 2-mg group, and 6.3% in the 4-mg group. There was also a signal of a dose-dependent increased risk of new-onset acne, with rates of 0.9% in controls and patients on baricitinib 2 mg, climbing to 3.6% with baricitinib 4 mg.
“In phase 2 results with upadacitinib [another oral JAK inhibitor], we saw that more than 10% of patients in the highest-dose group developed what was classified as acne. I cannot explain this, but it’s something we will monitor in the future,” Dr. Reich promised.
A fuller picture of baricitinib’s safety profile in the setting of atopic dermatitis clearly requires larger and longer-term studies, he added.
Baricitinib at the 2 mg daily dose is already marketed as Olumiant for the treatment of rheumatoid arthritis, with labeling that includes a boxed warning about serious infections, malignancy, and thrombosis. The Food and Drug Administration did not approve the 4-mg dose after determining that its higher safety hazard outweighed the efficacy advantage over the lower dose.
The BREEZE-AD7 study was sponsored by Eli Lilly. Dr. Reich reported serving as an adviser to, paid speaker for, and recipient of research grants from that pharmaceutical company and more than two dozen others.
REPORTING FROM EADV CONGRESS
Key clinical point: The Janus kinase 1/2 inhibitor baricitinib shows promise as a novel oral treatment for moderate or severe atopic dermatitis.
Major finding: Among atopic dermatitis patients on concomitant topical corticosteroids, a 75% improvement on Eczema Area and Severity Index at 16 weeks was achieved in 48% of those on baricitinib at 4 mg/day, 43% with baricitinib at 2 mg/day, and 23% on placebo.
Study details: BREEZE-AD7 was a phase 3, multicenter, 16-week, double-blind, three-arm study including 329 adults with moderate or severe atopic dermatitis.
Disclosures: The BREEZE-AD7 study was sponsored by Eli Lilly. The presenter reported serving as an adviser to, paid speaker for, and/or recipient of research grants from that pharmaceutical company and more than two dozen others.
Source: Reich K. EADV Congress, late breaker.
The 2018 SoHM Report: Takeaways for pediatric hospitalists
Increased complexity in workforce staffing
In November 2019, more than 1,500 pediatric hospitalists will be first to take the subspecialty exam approved by the American Board of Pediatrics (ABP) for certification in pediatric hospital medicine (PHM). This landmark signifies the recognition of hospital medicine as an essential component of the health care landscape and further acknowledges the importance of our expanding field.
But recent controversy over the requirements set by the ABP to sit for the exam has highlighted the new considerations for practice management that will be associated with this change. The need to analyze and understand how PHM programs function has never been more important for hospital medicine groups that care for children. This information is essential if they are to remain nimble in their approach to the changes that will occur in the years ahead.
To understand the impact that the new subspecialty board exam will have on groups that care for children, we need to first understand the criteria for eligibility. As for all ABP subspecialty boards, applicants must be Pediatric Board certified. The ABP has established three pathways by which practitioners can attain eligibility to sit for the PHM exam.1 Most currently practicing hospitalists have applied to take the exam under the “practice pathway,” which will be available temporarily to allow candidates to apply for the certifying exam based on experience rather than fellowship training. This temporary period will span the first three examination cycles (2019, 2021, 2023). The requirements for inclusion via this pathway, recently modified by the ABP in response to concerns voiced by the PHM community at large,2 consist of the following:
1. Practice period of 4 years (with a start date of July 2015 to be eligible for the November 2019 exam.
2. Work hours for all PHM professional activities of more than 900-1000 hours/year.
3. Patient care hours in PHM of more than 450-500 hours per year, every year for the preceding 4 years.
4. Scope of practice covering the full range of hospitalized children.
5. Practice experience and hours acquired in the United States or Canada.
This set of criteria raises several questions about the eligibility of the physicians currently caring for children in the hospital setting. The State of Hospital Medicine Report is an excellent source of information about hospital medicine trends in staffing and much more. While the response to the survey is more robust from practices that care for adults only, important information can be gleaned from the participant groups that care for children.
Question 1: How many clinicians that care for children in the hospital are trained in pediatrics, thereby meeting the first criteria to sit for the boards?
Based on the 2018 State of Hospital Medicine Report, 100% of groups that treat only children had physicians trained in pediatrics, 41.7% employed physicians trained in med/peds, and 5.6% had clinicians trained in internal medicine.
In groups that treat both children and adults the variation in practitioner type was much broader. While 85.7% of groups reported employing physicians trained in internal medicine and 64.3% employed family medicine practitioners, only 35.7% reported employing physicians trained in pediatrics and 46.4% with training in med/peds. A smattering of other clinician types was also noted, most of which were not likely to be pediatrics trained.
If information based on this relatively small number of respondents is generalizable, it means that a large number of the practitioners currently caring for hospitalized children are not pediatrics board-certified and therefore will not be eligible to sit for the subspecialty exam.
Question 2: What portion of the current PHM new hires are fellowship trained?
The 2018 State of Hospital Medicine Report notes that over 50% of new physicians joining a group treating only children come directly from residency, while only 5.1% come from a hospital medicine fellowship. For groups that treat adults and children, this percentage is even more significant, with 63% coming directly from residency and only 2.2% coming from a fellowship program.
The residents who recently graduated in 2019 are the last to be eligible to meet the practice duration criteria (4 years) during the “practice pathway” temporary period, thereby allowing them to sit for the subspecialty board exam without completing a fellowship. Recent surveys have shown that over 10% of graduating residents in pediatrics plan to pursue a career in PHM (over 280 respondents), however only under 75 fellows graduate from PHM fellowships each year.3 As the current number of fellowship positions in PHM are not adequate to meet the demand of the rapidly expanding workforce, groups treating children will need to continue to fill staff vacancies with variably trained clinicians.
In the years to come, information from the State of Hospital Medicine Report will be increasingly important, as programs that care for children meet the challenge of blending their workforce to include members with variable board certification and eligibility.
Question 3: How do the “patient care hours” and “work hours for all PHM activities” requirements affect currently practicing hospitalists in terms of their board eligibility?
Because of rigorous ABP criteria to sit for the PHM subspecialty exam, especially those regarding the minimum clinical and overall work hours in the care of children, many part time and med-peds practitioners may find that they are not board eligible. Variations in clinical coverage needs at individual sites, as well as competing nonclinical tasks in the adult setting, may limit pediatric-specific work hours for med/peds trained hospitalists.
As noted above, in groups that treat only children and groups that treat both adults and children, the 2018 State of Hospital Medicine Report shows that over 40% had physicians trained in med-peds. These highly trained and capable physicians will continue to be assets to their group; however, they may wish to find other ways to achieve merit-based distinction. For these physicians, the Fellow designation through SHM may provide an alternate means of recognition.
With the increasing complexity of staffing a workforce for the treatment of children that the PHM board subspecialty exam brings, the SHM Practice Analysis Committee developed a task force of pediatric leaders from across the country to aid in the development of additional pediatric-specific questions for the 2020 version of the State of Hospital Medicine Report. The questions to be included in the 2020 version will request information about the number of clinical hours (rather than shifts) per year required for full-time faculty, the percentage of the workforce that is part time, and the percentage of personnel in each group that is board certified in pediatric hospital medicine.
It is our hope that all groups treating children will respond to the 2020 State of Hospital Medicine survey, as a robust response will provide meaningful information to direct the leaders of these groups in the changing days ahead.
Dr. Gage is associate division chief, department of hospital medicine, at Phoenix Children’s Hospital and clinical associate professor, University of Arizona, Phoenix. She is a member of the SHM Practice Analysis Committee.
References
1. American Board of Pediatrics. Pediatric Hospital Medicine Certification. 2019 Edition.
2. American Board of Pediatrics. ABP responds to pediatric hospital medicine petition. 2019 Aug 29.
3. Pediatric Hospital Medicine Fellows. 2019 Edition.
Increased complexity in workforce staffing
Increased complexity in workforce staffing
In November 2019, more than 1,500 pediatric hospitalists will be first to take the subspecialty exam approved by the American Board of Pediatrics (ABP) for certification in pediatric hospital medicine (PHM). This landmark signifies the recognition of hospital medicine as an essential component of the health care landscape and further acknowledges the importance of our expanding field.
But recent controversy over the requirements set by the ABP to sit for the exam has highlighted the new considerations for practice management that will be associated with this change. The need to analyze and understand how PHM programs function has never been more important for hospital medicine groups that care for children. This information is essential if they are to remain nimble in their approach to the changes that will occur in the years ahead.
To understand the impact that the new subspecialty board exam will have on groups that care for children, we need to first understand the criteria for eligibility. As for all ABP subspecialty boards, applicants must be Pediatric Board certified. The ABP has established three pathways by which practitioners can attain eligibility to sit for the PHM exam.1 Most currently practicing hospitalists have applied to take the exam under the “practice pathway,” which will be available temporarily to allow candidates to apply for the certifying exam based on experience rather than fellowship training. This temporary period will span the first three examination cycles (2019, 2021, 2023). The requirements for inclusion via this pathway, recently modified by the ABP in response to concerns voiced by the PHM community at large,2 consist of the following:
1. Practice period of 4 years (with a start date of July 2015 to be eligible for the November 2019 exam.
2. Work hours for all PHM professional activities of more than 900-1000 hours/year.
3. Patient care hours in PHM of more than 450-500 hours per year, every year for the preceding 4 years.
4. Scope of practice covering the full range of hospitalized children.
5. Practice experience and hours acquired in the United States or Canada.
This set of criteria raises several questions about the eligibility of the physicians currently caring for children in the hospital setting. The State of Hospital Medicine Report is an excellent source of information about hospital medicine trends in staffing and much more. While the response to the survey is more robust from practices that care for adults only, important information can be gleaned from the participant groups that care for children.
Question 1: How many clinicians that care for children in the hospital are trained in pediatrics, thereby meeting the first criteria to sit for the boards?
Based on the 2018 State of Hospital Medicine Report, 100% of groups that treat only children had physicians trained in pediatrics, 41.7% employed physicians trained in med/peds, and 5.6% had clinicians trained in internal medicine.
In groups that treat both children and adults the variation in practitioner type was much broader. While 85.7% of groups reported employing physicians trained in internal medicine and 64.3% employed family medicine practitioners, only 35.7% reported employing physicians trained in pediatrics and 46.4% with training in med/peds. A smattering of other clinician types was also noted, most of which were not likely to be pediatrics trained.
If information based on this relatively small number of respondents is generalizable, it means that a large number of the practitioners currently caring for hospitalized children are not pediatrics board-certified and therefore will not be eligible to sit for the subspecialty exam.
Question 2: What portion of the current PHM new hires are fellowship trained?
The 2018 State of Hospital Medicine Report notes that over 50% of new physicians joining a group treating only children come directly from residency, while only 5.1% come from a hospital medicine fellowship. For groups that treat adults and children, this percentage is even more significant, with 63% coming directly from residency and only 2.2% coming from a fellowship program.
The residents who recently graduated in 2019 are the last to be eligible to meet the practice duration criteria (4 years) during the “practice pathway” temporary period, thereby allowing them to sit for the subspecialty board exam without completing a fellowship. Recent surveys have shown that over 10% of graduating residents in pediatrics plan to pursue a career in PHM (over 280 respondents), however only under 75 fellows graduate from PHM fellowships each year.3 As the current number of fellowship positions in PHM are not adequate to meet the demand of the rapidly expanding workforce, groups treating children will need to continue to fill staff vacancies with variably trained clinicians.
In the years to come, information from the State of Hospital Medicine Report will be increasingly important, as programs that care for children meet the challenge of blending their workforce to include members with variable board certification and eligibility.
Question 3: How do the “patient care hours” and “work hours for all PHM activities” requirements affect currently practicing hospitalists in terms of their board eligibility?
Because of rigorous ABP criteria to sit for the PHM subspecialty exam, especially those regarding the minimum clinical and overall work hours in the care of children, many part time and med-peds practitioners may find that they are not board eligible. Variations in clinical coverage needs at individual sites, as well as competing nonclinical tasks in the adult setting, may limit pediatric-specific work hours for med/peds trained hospitalists.
As noted above, in groups that treat only children and groups that treat both adults and children, the 2018 State of Hospital Medicine Report shows that over 40% had physicians trained in med-peds. These highly trained and capable physicians will continue to be assets to their group; however, they may wish to find other ways to achieve merit-based distinction. For these physicians, the Fellow designation through SHM may provide an alternate means of recognition.
With the increasing complexity of staffing a workforce for the treatment of children that the PHM board subspecialty exam brings, the SHM Practice Analysis Committee developed a task force of pediatric leaders from across the country to aid in the development of additional pediatric-specific questions for the 2020 version of the State of Hospital Medicine Report. The questions to be included in the 2020 version will request information about the number of clinical hours (rather than shifts) per year required for full-time faculty, the percentage of the workforce that is part time, and the percentage of personnel in each group that is board certified in pediatric hospital medicine.
It is our hope that all groups treating children will respond to the 2020 State of Hospital Medicine survey, as a robust response will provide meaningful information to direct the leaders of these groups in the changing days ahead.
Dr. Gage is associate division chief, department of hospital medicine, at Phoenix Children’s Hospital and clinical associate professor, University of Arizona, Phoenix. She is a member of the SHM Practice Analysis Committee.
References
1. American Board of Pediatrics. Pediatric Hospital Medicine Certification. 2019 Edition.
2. American Board of Pediatrics. ABP responds to pediatric hospital medicine petition. 2019 Aug 29.
3. Pediatric Hospital Medicine Fellows. 2019 Edition.
In November 2019, more than 1,500 pediatric hospitalists will be first to take the subspecialty exam approved by the American Board of Pediatrics (ABP) for certification in pediatric hospital medicine (PHM). This landmark signifies the recognition of hospital medicine as an essential component of the health care landscape and further acknowledges the importance of our expanding field.
But recent controversy over the requirements set by the ABP to sit for the exam has highlighted the new considerations for practice management that will be associated with this change. The need to analyze and understand how PHM programs function has never been more important for hospital medicine groups that care for children. This information is essential if they are to remain nimble in their approach to the changes that will occur in the years ahead.
To understand the impact that the new subspecialty board exam will have on groups that care for children, we need to first understand the criteria for eligibility. As for all ABP subspecialty boards, applicants must be Pediatric Board certified. The ABP has established three pathways by which practitioners can attain eligibility to sit for the PHM exam.1 Most currently practicing hospitalists have applied to take the exam under the “practice pathway,” which will be available temporarily to allow candidates to apply for the certifying exam based on experience rather than fellowship training. This temporary period will span the first three examination cycles (2019, 2021, 2023). The requirements for inclusion via this pathway, recently modified by the ABP in response to concerns voiced by the PHM community at large,2 consist of the following:
1. Practice period of 4 years (with a start date of July 2015 to be eligible for the November 2019 exam.
2. Work hours for all PHM professional activities of more than 900-1000 hours/year.
3. Patient care hours in PHM of more than 450-500 hours per year, every year for the preceding 4 years.
4. Scope of practice covering the full range of hospitalized children.
5. Practice experience and hours acquired in the United States or Canada.
This set of criteria raises several questions about the eligibility of the physicians currently caring for children in the hospital setting. The State of Hospital Medicine Report is an excellent source of information about hospital medicine trends in staffing and much more. While the response to the survey is more robust from practices that care for adults only, important information can be gleaned from the participant groups that care for children.
Question 1: How many clinicians that care for children in the hospital are trained in pediatrics, thereby meeting the first criteria to sit for the boards?
Based on the 2018 State of Hospital Medicine Report, 100% of groups that treat only children had physicians trained in pediatrics, 41.7% employed physicians trained in med/peds, and 5.6% had clinicians trained in internal medicine.
In groups that treat both children and adults the variation in practitioner type was much broader. While 85.7% of groups reported employing physicians trained in internal medicine and 64.3% employed family medicine practitioners, only 35.7% reported employing physicians trained in pediatrics and 46.4% with training in med/peds. A smattering of other clinician types was also noted, most of which were not likely to be pediatrics trained.
If information based on this relatively small number of respondents is generalizable, it means that a large number of the practitioners currently caring for hospitalized children are not pediatrics board-certified and therefore will not be eligible to sit for the subspecialty exam.
Question 2: What portion of the current PHM new hires are fellowship trained?
The 2018 State of Hospital Medicine Report notes that over 50% of new physicians joining a group treating only children come directly from residency, while only 5.1% come from a hospital medicine fellowship. For groups that treat adults and children, this percentage is even more significant, with 63% coming directly from residency and only 2.2% coming from a fellowship program.
The residents who recently graduated in 2019 are the last to be eligible to meet the practice duration criteria (4 years) during the “practice pathway” temporary period, thereby allowing them to sit for the subspecialty board exam without completing a fellowship. Recent surveys have shown that over 10% of graduating residents in pediatrics plan to pursue a career in PHM (over 280 respondents), however only under 75 fellows graduate from PHM fellowships each year.3 As the current number of fellowship positions in PHM are not adequate to meet the demand of the rapidly expanding workforce, groups treating children will need to continue to fill staff vacancies with variably trained clinicians.
In the years to come, information from the State of Hospital Medicine Report will be increasingly important, as programs that care for children meet the challenge of blending their workforce to include members with variable board certification and eligibility.
Question 3: How do the “patient care hours” and “work hours for all PHM activities” requirements affect currently practicing hospitalists in terms of their board eligibility?
Because of rigorous ABP criteria to sit for the PHM subspecialty exam, especially those regarding the minimum clinical and overall work hours in the care of children, many part time and med-peds practitioners may find that they are not board eligible. Variations in clinical coverage needs at individual sites, as well as competing nonclinical tasks in the adult setting, may limit pediatric-specific work hours for med/peds trained hospitalists.
As noted above, in groups that treat only children and groups that treat both adults and children, the 2018 State of Hospital Medicine Report shows that over 40% had physicians trained in med-peds. These highly trained and capable physicians will continue to be assets to their group; however, they may wish to find other ways to achieve merit-based distinction. For these physicians, the Fellow designation through SHM may provide an alternate means of recognition.
With the increasing complexity of staffing a workforce for the treatment of children that the PHM board subspecialty exam brings, the SHM Practice Analysis Committee developed a task force of pediatric leaders from across the country to aid in the development of additional pediatric-specific questions for the 2020 version of the State of Hospital Medicine Report. The questions to be included in the 2020 version will request information about the number of clinical hours (rather than shifts) per year required for full-time faculty, the percentage of the workforce that is part time, and the percentage of personnel in each group that is board certified in pediatric hospital medicine.
It is our hope that all groups treating children will respond to the 2020 State of Hospital Medicine survey, as a robust response will provide meaningful information to direct the leaders of these groups in the changing days ahead.
Dr. Gage is associate division chief, department of hospital medicine, at Phoenix Children’s Hospital and clinical associate professor, University of Arizona, Phoenix. She is a member of the SHM Practice Analysis Committee.
References
1. American Board of Pediatrics. Pediatric Hospital Medicine Certification. 2019 Edition.
2. American Board of Pediatrics. ABP responds to pediatric hospital medicine petition. 2019 Aug 29.
3. Pediatric Hospital Medicine Fellows. 2019 Edition.
Poor OR posture a key cause of vascular burnout
NEW YORK – Career burnout is common is common among physicians and surgeons, but vascular surgeons might be able to lower their risk simply by taking steps to improve their posture in the operating room, according to data presented at a symposium on vascular and endovascular issues on an evolution that is already underway.
“We looked at physical pain and we were able to demonstrate a correlation with burnout. More pain, more burnout,” said Samuel R. Money, MD, division of vascular surgery, Mayo Clinic, Phoenix, Arizona.
Pain was a reasonable focus for efforts to identify causes of burnout because it is so common among vascular surgeons. In data recently published by Dr. Money and his coinvestigators, 78.3% reported moderate to severe physical pain at the end of a day of surgery (J Vasc Surg 2018;70:913-920).
“Forty percent of vascular surgeons have chronic pain,” Dr. Money said at the symposium sponsored by the Cleveland Clinic Foundation.
Physical pain is not the only cause of burnout, which affects 30% of vascular surgeons, according to data recently presented at the annual meeting of the Society of Vascular Surgery (J Vasc Surg 2019;69[6]:e97.). In that survey, physical pain was joined by work hours, documentation tasks, on-call frequency, and conflicts between work and personal life as significant factors.
“The average vascular surgeon in North America works 63 hours per week,” noted Dr. Money, adding that his survey found nearly 90% of surgeons operate on 3 or more days of every week. This amount of time in the operating room is relevant because almost all surgeons report some degree of pain after a procedure. In the survey, the proportion was greater than 95%.
Yet, risk of pain is modifiable.
“Body position matters,” said Dr. Money, citing studies showing that open procedures are most closely associated with neck pain whereas endovascular procedures are more likely to produce back pain. Although there is a high risk of either type of pain with these procedures, the types of predominant pain are consistent with the demands on body positioning.
“The more you lean forward, the more stress is placed on your neck and back. When standing straight, your head weighs 10-12 pounds, but leaning forward, it can put 60 pounds of pressure on your neck,” he said.
The relative stress can be measured objectively. Dr. Money cited work with a device that measures the body force in inertial measurement units (IMU). According to Dr. Money, the neck is in a high stress position about 75% of the time spent performing typical vascular surgery.
“The trunk is placed in a high stress position approximately 40% of the time, while the other parts of the body that were measured were not generally that bad,” Dr. Money said.
To avoid postural pain, which is not often stressed in surgical training, Dr. Money had specific recommendations. Some are obvious, such as positioning the operating table to minimize the amount of time the head is inclined. He also recommended positioning display monitors no more than 10-20 degrees below and no higher than eye level.
“If you sit down to perform tasks during the procedure, use an adjustable chair so that you can optimize the height,” he said.
He identified loupes as a risk factor for bad posture, and he stressed the importance of wearing lead garments only when necessary and adjusted properly.
“Padded floor mats? They really help,” Dr. Money said. He also recommended appropriate footwear and support stocking.
“Microbreaks are being used in a lot of professions. This means stopping for a moment to stretch every 15-30 minutes,” Dr. Money said.
As a first step, Dr. Money recommended simply developing posture awareness. Many surgeons are simply ignoring the risk and failing to optimize the ways they can increase their comfort during surgery.
Even before entering the surgical suite, regular exercise, yoga, and stretching are all strategies that have the potential to make a difference, according to Dr. Money.
The immediate goal is to reduce the physical pain that is an important occupational hazard for vascular surgeons, but the ultimate goal is to improve job satisfaction, an important defense against professional burnout.
NEW YORK – Career burnout is common is common among physicians and surgeons, but vascular surgeons might be able to lower their risk simply by taking steps to improve their posture in the operating room, according to data presented at a symposium on vascular and endovascular issues on an evolution that is already underway.
“We looked at physical pain and we were able to demonstrate a correlation with burnout. More pain, more burnout,” said Samuel R. Money, MD, division of vascular surgery, Mayo Clinic, Phoenix, Arizona.
Pain was a reasonable focus for efforts to identify causes of burnout because it is so common among vascular surgeons. In data recently published by Dr. Money and his coinvestigators, 78.3% reported moderate to severe physical pain at the end of a day of surgery (J Vasc Surg 2018;70:913-920).
“Forty percent of vascular surgeons have chronic pain,” Dr. Money said at the symposium sponsored by the Cleveland Clinic Foundation.
Physical pain is not the only cause of burnout, which affects 30% of vascular surgeons, according to data recently presented at the annual meeting of the Society of Vascular Surgery (J Vasc Surg 2019;69[6]:e97.). In that survey, physical pain was joined by work hours, documentation tasks, on-call frequency, and conflicts between work and personal life as significant factors.
“The average vascular surgeon in North America works 63 hours per week,” noted Dr. Money, adding that his survey found nearly 90% of surgeons operate on 3 or more days of every week. This amount of time in the operating room is relevant because almost all surgeons report some degree of pain after a procedure. In the survey, the proportion was greater than 95%.
Yet, risk of pain is modifiable.
“Body position matters,” said Dr. Money, citing studies showing that open procedures are most closely associated with neck pain whereas endovascular procedures are more likely to produce back pain. Although there is a high risk of either type of pain with these procedures, the types of predominant pain are consistent with the demands on body positioning.
“The more you lean forward, the more stress is placed on your neck and back. When standing straight, your head weighs 10-12 pounds, but leaning forward, it can put 60 pounds of pressure on your neck,” he said.
The relative stress can be measured objectively. Dr. Money cited work with a device that measures the body force in inertial measurement units (IMU). According to Dr. Money, the neck is in a high stress position about 75% of the time spent performing typical vascular surgery.
“The trunk is placed in a high stress position approximately 40% of the time, while the other parts of the body that were measured were not generally that bad,” Dr. Money said.
To avoid postural pain, which is not often stressed in surgical training, Dr. Money had specific recommendations. Some are obvious, such as positioning the operating table to minimize the amount of time the head is inclined. He also recommended positioning display monitors no more than 10-20 degrees below and no higher than eye level.
“If you sit down to perform tasks during the procedure, use an adjustable chair so that you can optimize the height,” he said.
He identified loupes as a risk factor for bad posture, and he stressed the importance of wearing lead garments only when necessary and adjusted properly.
“Padded floor mats? They really help,” Dr. Money said. He also recommended appropriate footwear and support stocking.
“Microbreaks are being used in a lot of professions. This means stopping for a moment to stretch every 15-30 minutes,” Dr. Money said.
As a first step, Dr. Money recommended simply developing posture awareness. Many surgeons are simply ignoring the risk and failing to optimize the ways they can increase their comfort during surgery.
Even before entering the surgical suite, regular exercise, yoga, and stretching are all strategies that have the potential to make a difference, according to Dr. Money.
The immediate goal is to reduce the physical pain that is an important occupational hazard for vascular surgeons, but the ultimate goal is to improve job satisfaction, an important defense against professional burnout.
NEW YORK – Career burnout is common is common among physicians and surgeons, but vascular surgeons might be able to lower their risk simply by taking steps to improve their posture in the operating room, according to data presented at a symposium on vascular and endovascular issues on an evolution that is already underway.
“We looked at physical pain and we were able to demonstrate a correlation with burnout. More pain, more burnout,” said Samuel R. Money, MD, division of vascular surgery, Mayo Clinic, Phoenix, Arizona.
Pain was a reasonable focus for efforts to identify causes of burnout because it is so common among vascular surgeons. In data recently published by Dr. Money and his coinvestigators, 78.3% reported moderate to severe physical pain at the end of a day of surgery (J Vasc Surg 2018;70:913-920).
“Forty percent of vascular surgeons have chronic pain,” Dr. Money said at the symposium sponsored by the Cleveland Clinic Foundation.
Physical pain is not the only cause of burnout, which affects 30% of vascular surgeons, according to data recently presented at the annual meeting of the Society of Vascular Surgery (J Vasc Surg 2019;69[6]:e97.). In that survey, physical pain was joined by work hours, documentation tasks, on-call frequency, and conflicts between work and personal life as significant factors.
“The average vascular surgeon in North America works 63 hours per week,” noted Dr. Money, adding that his survey found nearly 90% of surgeons operate on 3 or more days of every week. This amount of time in the operating room is relevant because almost all surgeons report some degree of pain after a procedure. In the survey, the proportion was greater than 95%.
Yet, risk of pain is modifiable.
“Body position matters,” said Dr. Money, citing studies showing that open procedures are most closely associated with neck pain whereas endovascular procedures are more likely to produce back pain. Although there is a high risk of either type of pain with these procedures, the types of predominant pain are consistent with the demands on body positioning.
“The more you lean forward, the more stress is placed on your neck and back. When standing straight, your head weighs 10-12 pounds, but leaning forward, it can put 60 pounds of pressure on your neck,” he said.
The relative stress can be measured objectively. Dr. Money cited work with a device that measures the body force in inertial measurement units (IMU). According to Dr. Money, the neck is in a high stress position about 75% of the time spent performing typical vascular surgery.
“The trunk is placed in a high stress position approximately 40% of the time, while the other parts of the body that were measured were not generally that bad,” Dr. Money said.
To avoid postural pain, which is not often stressed in surgical training, Dr. Money had specific recommendations. Some are obvious, such as positioning the operating table to minimize the amount of time the head is inclined. He also recommended positioning display monitors no more than 10-20 degrees below and no higher than eye level.
“If you sit down to perform tasks during the procedure, use an adjustable chair so that you can optimize the height,” he said.
He identified loupes as a risk factor for bad posture, and he stressed the importance of wearing lead garments only when necessary and adjusted properly.
“Padded floor mats? They really help,” Dr. Money said. He also recommended appropriate footwear and support stocking.
“Microbreaks are being used in a lot of professions. This means stopping for a moment to stretch every 15-30 minutes,” Dr. Money said.
As a first step, Dr. Money recommended simply developing posture awareness. Many surgeons are simply ignoring the risk and failing to optimize the ways they can increase their comfort during surgery.
Even before entering the surgical suite, regular exercise, yoga, and stretching are all strategies that have the potential to make a difference, according to Dr. Money.
The immediate goal is to reduce the physical pain that is an important occupational hazard for vascular surgeons, but the ultimate goal is to improve job satisfaction, an important defense against professional burnout.
REPORTING FROM VEITHsymposium
Differences in U.S. and European aneurysm guidelines called unavoidable
NEW YORK – Published 12 months apart, guidelines on management of abdominal aortic aneurysm (AAA) from the European Society for Vascular Surgery are similar but diverged in instructive ways from those of the Society for Vascular Surgery, according to a critical review at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “Some of the differences were almost unavoidable in the sense that the ESVS guidelines represent multiple idiosyncratic health care systems across Europe,” reported Ronald L. Dalman, MD, chief of vascular surgery, Stanford (Calif.) University.
As a result, the ESVS guidelines provide very little specificity about pharmacologic options because of the differences in availability of these treatments within specific health systems. In addition, both open and endovascular aneurysm repair (EVAR) are given similar emphasis because of the limited availability of EVAR in some parts of Europe.
“The ESVS guidelines specifically recommend repair of an aneurysm within 8 weeks when repair is indicated, but there are not many aneurysms that go 8 weeks in the U.S. without being fixed by a fee-for-service surgeon,” Dr. Dalman observed.
The SVS AAA guidelines were published in January 2018 (J Vasc Surg 2018;67:2-77) and the ESVS guidelines followed 1 year later (Eur J Vasc Surg 2019;57:8-93).
The differences in the guidelines, although modest, are interesting because each set of guidelines was based largely on the same set of trials and published studies, according to Dr. Dalman, who was a coauthor of the SVS guidelines and an external reviewer for the ESVS guidelines.
In the lag between completion of the two guidelines, new information led to three ESVS additions not found in the SVS guidelines, according to Dr. Dalman. They involved the importance of considering aneurysm diameter as a prognostic factor, new understanding of the limitations on endovascular aneurysm sealing (EVAS), and new information about how aneurysm size should affect frequency of surveillance.
Overall, the U.S. guidelines contain 111 recommendations based on 177 references, while the ESVS guidelines contain 125 guidelines based on 189 references. In retrospect, Dr. Dalman believes both sets of guidelines omitted some clinically meaningful information, such as the risk of large-diameter devices for causing endoleaks.
The authors of the ESVS guidelines did have an opportunity to review of a draft of the SVS guidelines, so differences can be interpreted as intentional. For example, the SVS guidelines recommend risk calculators, but Dr. Dalman suggested that the authors of the ESVS guidelines were less convinced that their utility was established.
The decision not to recommend a door-to-treatment time for ruptured aneurysms, as in the SVS recommendations, might have been in deference to disparate practice across European countries, Dr. Dalman suggested.
Ultimately, the guidelines are “substantially similar,” according to Dr. Dalman, but he expressed concerned that neither guideline is accompanied by a specific mechanism or recommended strategy to ensure implementation.
Many of the SVS recommendations are likely to be translated into quality metrics at U.S. institutions, but “there are implementation issues” for ensuring that each guideline is applied, Dr. Dalman said.
Given the agreement on the vast majority of the recommendations, Dr. Dalman suggested that “it might be time to consider global guidelines” for management of AAA and other vascular diseases. Some type of language might be required to accommodate divergent resources or practices across borders, but Dr. Dalman questioned the need to review the same literature to arrive at mostly the same conclusions.
NEW YORK – Published 12 months apart, guidelines on management of abdominal aortic aneurysm (AAA) from the European Society for Vascular Surgery are similar but diverged in instructive ways from those of the Society for Vascular Surgery, according to a critical review at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “Some of the differences were almost unavoidable in the sense that the ESVS guidelines represent multiple idiosyncratic health care systems across Europe,” reported Ronald L. Dalman, MD, chief of vascular surgery, Stanford (Calif.) University.
As a result, the ESVS guidelines provide very little specificity about pharmacologic options because of the differences in availability of these treatments within specific health systems. In addition, both open and endovascular aneurysm repair (EVAR) are given similar emphasis because of the limited availability of EVAR in some parts of Europe.
“The ESVS guidelines specifically recommend repair of an aneurysm within 8 weeks when repair is indicated, but there are not many aneurysms that go 8 weeks in the U.S. without being fixed by a fee-for-service surgeon,” Dr. Dalman observed.
The SVS AAA guidelines were published in January 2018 (J Vasc Surg 2018;67:2-77) and the ESVS guidelines followed 1 year later (Eur J Vasc Surg 2019;57:8-93).
The differences in the guidelines, although modest, are interesting because each set of guidelines was based largely on the same set of trials and published studies, according to Dr. Dalman, who was a coauthor of the SVS guidelines and an external reviewer for the ESVS guidelines.
In the lag between completion of the two guidelines, new information led to three ESVS additions not found in the SVS guidelines, according to Dr. Dalman. They involved the importance of considering aneurysm diameter as a prognostic factor, new understanding of the limitations on endovascular aneurysm sealing (EVAS), and new information about how aneurysm size should affect frequency of surveillance.
Overall, the U.S. guidelines contain 111 recommendations based on 177 references, while the ESVS guidelines contain 125 guidelines based on 189 references. In retrospect, Dr. Dalman believes both sets of guidelines omitted some clinically meaningful information, such as the risk of large-diameter devices for causing endoleaks.
The authors of the ESVS guidelines did have an opportunity to review of a draft of the SVS guidelines, so differences can be interpreted as intentional. For example, the SVS guidelines recommend risk calculators, but Dr. Dalman suggested that the authors of the ESVS guidelines were less convinced that their utility was established.
The decision not to recommend a door-to-treatment time for ruptured aneurysms, as in the SVS recommendations, might have been in deference to disparate practice across European countries, Dr. Dalman suggested.
Ultimately, the guidelines are “substantially similar,” according to Dr. Dalman, but he expressed concerned that neither guideline is accompanied by a specific mechanism or recommended strategy to ensure implementation.
Many of the SVS recommendations are likely to be translated into quality metrics at U.S. institutions, but “there are implementation issues” for ensuring that each guideline is applied, Dr. Dalman said.
Given the agreement on the vast majority of the recommendations, Dr. Dalman suggested that “it might be time to consider global guidelines” for management of AAA and other vascular diseases. Some type of language might be required to accommodate divergent resources or practices across borders, but Dr. Dalman questioned the need to review the same literature to arrive at mostly the same conclusions.
NEW YORK – Published 12 months apart, guidelines on management of abdominal aortic aneurysm (AAA) from the European Society for Vascular Surgery are similar but diverged in instructive ways from those of the Society for Vascular Surgery, according to a critical review at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “Some of the differences were almost unavoidable in the sense that the ESVS guidelines represent multiple idiosyncratic health care systems across Europe,” reported Ronald L. Dalman, MD, chief of vascular surgery, Stanford (Calif.) University.
As a result, the ESVS guidelines provide very little specificity about pharmacologic options because of the differences in availability of these treatments within specific health systems. In addition, both open and endovascular aneurysm repair (EVAR) are given similar emphasis because of the limited availability of EVAR in some parts of Europe.
“The ESVS guidelines specifically recommend repair of an aneurysm within 8 weeks when repair is indicated, but there are not many aneurysms that go 8 weeks in the U.S. without being fixed by a fee-for-service surgeon,” Dr. Dalman observed.
The SVS AAA guidelines were published in January 2018 (J Vasc Surg 2018;67:2-77) and the ESVS guidelines followed 1 year later (Eur J Vasc Surg 2019;57:8-93).
The differences in the guidelines, although modest, are interesting because each set of guidelines was based largely on the same set of trials and published studies, according to Dr. Dalman, who was a coauthor of the SVS guidelines and an external reviewer for the ESVS guidelines.
In the lag between completion of the two guidelines, new information led to three ESVS additions not found in the SVS guidelines, according to Dr. Dalman. They involved the importance of considering aneurysm diameter as a prognostic factor, new understanding of the limitations on endovascular aneurysm sealing (EVAS), and new information about how aneurysm size should affect frequency of surveillance.
Overall, the U.S. guidelines contain 111 recommendations based on 177 references, while the ESVS guidelines contain 125 guidelines based on 189 references. In retrospect, Dr. Dalman believes both sets of guidelines omitted some clinically meaningful information, such as the risk of large-diameter devices for causing endoleaks.
The authors of the ESVS guidelines did have an opportunity to review of a draft of the SVS guidelines, so differences can be interpreted as intentional. For example, the SVS guidelines recommend risk calculators, but Dr. Dalman suggested that the authors of the ESVS guidelines were less convinced that their utility was established.
The decision not to recommend a door-to-treatment time for ruptured aneurysms, as in the SVS recommendations, might have been in deference to disparate practice across European countries, Dr. Dalman suggested.
Ultimately, the guidelines are “substantially similar,” according to Dr. Dalman, but he expressed concerned that neither guideline is accompanied by a specific mechanism or recommended strategy to ensure implementation.
Many of the SVS recommendations are likely to be translated into quality metrics at U.S. institutions, but “there are implementation issues” for ensuring that each guideline is applied, Dr. Dalman said.
Given the agreement on the vast majority of the recommendations, Dr. Dalman suggested that “it might be time to consider global guidelines” for management of AAA and other vascular diseases. Some type of language might be required to accommodate divergent resources or practices across borders, but Dr. Dalman questioned the need to review the same literature to arrive at mostly the same conclusions.
REPORTING FROM VEITHsymposium
Key clinical point:
Major finding: Less emphasis on endovascular repair and specific drugs in Europe reflects accommodation of nationalized health systems.
Study details: Expert review.
Disclosures: Dr. Dalman reports no potential financial conflicts of interest relevant to this topic.
Source: Dalman RL et al. 46th VEITHsymposium.
Challenges outlined for teaching open surgery to Gen Z in endovascular era
NEW YORK – The dual challenges of teaching open vascular surgery techniques when few are performed and reaching a generation that has a different attitude to absorbing information requires new and innovative approaches, according to an academic surgeon speaking at symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“The see one, do one, teach one approach to surgical training is no longer possible,” explained R. Clement Darling III, MD, chief of the division of vascular surgery at Albany (N.Y.) Medical Center Hospital.
For open procedures, the problem is a rapidly declining number of cases in the era of endovascular surgery, but Dr. Darling also recommended adjusting training to the outlook and expectations of a new generation. First observed in the millennial generation, an attitude of firm work-related boundaries is also being seen in generation Z. Generation Z, characterized by birth after 1995, is just now beginning to reach residency programs.
“The approach to work-life balance is extremely different for these individuals than it was for my generation,” Dr. Darling said. While previous generations were often motivated by fear and pressure, newer generations appear to respond less well to anxiety.
“People learn differently. Some from their tactile sense, some intellectually, and some from fear or pressure, but mostly, particularly those who are younger, now learn from positive reinforcement,” Dr. Darling said.
For teaching open procedures at his own institution, Dr. Darling has switched from the traditional model of one-on-one instruction undertaken in the surgical suite to a group approach. The limited number of open cases was the impetus, but group instruction now extends beyond the operating room.
“We have a meeting before the case, when we go over the technical aspects,” Dr. Darling explained. Fellows are asked to envision and describe potential problems and potential solutions.
“We try to make them visualize as well as verbalize exactly what will be done in the operating room,” Dr. Darling said. The plans are outlined carefully “so no one does any thinking in the OR. All the thinking is done in advance.”
Videos and simulators are teaching aids, but a great deal of learning can be accomplished independent of doing, according to Dr. Darling. Moreover, understanding the anatomy, which comes before developing surgical skills, is the same for open and endovascular procedures, so each is relevant to the other.
After witnessing an open case, all of the trainees along with the nurses and attending physicians go through a debriefing to consider the potential lessons. At Dr. Darling’s center, open procedures increasingly involve sicker and older patients, conferring case analysis with a particularly vital learning function in the curriculum.
Because of the diminishing number of open cases and the diminishing open skills, even among experienced vascular surgeons, residents in an increasing number of training programs “graduate without any open experience, which is a little shocking,” Dr. Darling said.
Importantly, group instruction, although valuable and necessary for exposing residents and fellows to open vascular surgery, has its own lessons to impart even if it was born out of necessity.
“We always emphasize that it is not the sewing that counts, it is the setup that counts,” said Dr. Darling, indicating that this is a clear message when the group is assembled for case planning. The group planning also emphasizes that surgery is a team sport.
“All of us is smarter than one of us,” said Dr. Darling, articulating the implicit message of group training.
Although this is a departure from a bygone era where infallible surgeons ruled the OR, it is fits nicely with changing attitudes about the best attributes of a competent surgeon.
NEW YORK – The dual challenges of teaching open vascular surgery techniques when few are performed and reaching a generation that has a different attitude to absorbing information requires new and innovative approaches, according to an academic surgeon speaking at symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“The see one, do one, teach one approach to surgical training is no longer possible,” explained R. Clement Darling III, MD, chief of the division of vascular surgery at Albany (N.Y.) Medical Center Hospital.
For open procedures, the problem is a rapidly declining number of cases in the era of endovascular surgery, but Dr. Darling also recommended adjusting training to the outlook and expectations of a new generation. First observed in the millennial generation, an attitude of firm work-related boundaries is also being seen in generation Z. Generation Z, characterized by birth after 1995, is just now beginning to reach residency programs.
“The approach to work-life balance is extremely different for these individuals than it was for my generation,” Dr. Darling said. While previous generations were often motivated by fear and pressure, newer generations appear to respond less well to anxiety.
“People learn differently. Some from their tactile sense, some intellectually, and some from fear or pressure, but mostly, particularly those who are younger, now learn from positive reinforcement,” Dr. Darling said.
For teaching open procedures at his own institution, Dr. Darling has switched from the traditional model of one-on-one instruction undertaken in the surgical suite to a group approach. The limited number of open cases was the impetus, but group instruction now extends beyond the operating room.
“We have a meeting before the case, when we go over the technical aspects,” Dr. Darling explained. Fellows are asked to envision and describe potential problems and potential solutions.
“We try to make them visualize as well as verbalize exactly what will be done in the operating room,” Dr. Darling said. The plans are outlined carefully “so no one does any thinking in the OR. All the thinking is done in advance.”
Videos and simulators are teaching aids, but a great deal of learning can be accomplished independent of doing, according to Dr. Darling. Moreover, understanding the anatomy, which comes before developing surgical skills, is the same for open and endovascular procedures, so each is relevant to the other.
After witnessing an open case, all of the trainees along with the nurses and attending physicians go through a debriefing to consider the potential lessons. At Dr. Darling’s center, open procedures increasingly involve sicker and older patients, conferring case analysis with a particularly vital learning function in the curriculum.
Because of the diminishing number of open cases and the diminishing open skills, even among experienced vascular surgeons, residents in an increasing number of training programs “graduate without any open experience, which is a little shocking,” Dr. Darling said.
Importantly, group instruction, although valuable and necessary for exposing residents and fellows to open vascular surgery, has its own lessons to impart even if it was born out of necessity.
“We always emphasize that it is not the sewing that counts, it is the setup that counts,” said Dr. Darling, indicating that this is a clear message when the group is assembled for case planning. The group planning also emphasizes that surgery is a team sport.
“All of us is smarter than one of us,” said Dr. Darling, articulating the implicit message of group training.
Although this is a departure from a bygone era where infallible surgeons ruled the OR, it is fits nicely with changing attitudes about the best attributes of a competent surgeon.
NEW YORK – The dual challenges of teaching open vascular surgery techniques when few are performed and reaching a generation that has a different attitude to absorbing information requires new and innovative approaches, according to an academic surgeon speaking at symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“The see one, do one, teach one approach to surgical training is no longer possible,” explained R. Clement Darling III, MD, chief of the division of vascular surgery at Albany (N.Y.) Medical Center Hospital.
For open procedures, the problem is a rapidly declining number of cases in the era of endovascular surgery, but Dr. Darling also recommended adjusting training to the outlook and expectations of a new generation. First observed in the millennial generation, an attitude of firm work-related boundaries is also being seen in generation Z. Generation Z, characterized by birth after 1995, is just now beginning to reach residency programs.
“The approach to work-life balance is extremely different for these individuals than it was for my generation,” Dr. Darling said. While previous generations were often motivated by fear and pressure, newer generations appear to respond less well to anxiety.
“People learn differently. Some from their tactile sense, some intellectually, and some from fear or pressure, but mostly, particularly those who are younger, now learn from positive reinforcement,” Dr. Darling said.
For teaching open procedures at his own institution, Dr. Darling has switched from the traditional model of one-on-one instruction undertaken in the surgical suite to a group approach. The limited number of open cases was the impetus, but group instruction now extends beyond the operating room.
“We have a meeting before the case, when we go over the technical aspects,” Dr. Darling explained. Fellows are asked to envision and describe potential problems and potential solutions.
“We try to make them visualize as well as verbalize exactly what will be done in the operating room,” Dr. Darling said. The plans are outlined carefully “so no one does any thinking in the OR. All the thinking is done in advance.”
Videos and simulators are teaching aids, but a great deal of learning can be accomplished independent of doing, according to Dr. Darling. Moreover, understanding the anatomy, which comes before developing surgical skills, is the same for open and endovascular procedures, so each is relevant to the other.
After witnessing an open case, all of the trainees along with the nurses and attending physicians go through a debriefing to consider the potential lessons. At Dr. Darling’s center, open procedures increasingly involve sicker and older patients, conferring case analysis with a particularly vital learning function in the curriculum.
Because of the diminishing number of open cases and the diminishing open skills, even among experienced vascular surgeons, residents in an increasing number of training programs “graduate without any open experience, which is a little shocking,” Dr. Darling said.
Importantly, group instruction, although valuable and necessary for exposing residents and fellows to open vascular surgery, has its own lessons to impart even if it was born out of necessity.
“We always emphasize that it is not the sewing that counts, it is the setup that counts,” said Dr. Darling, indicating that this is a clear message when the group is assembled for case planning. The group planning also emphasizes that surgery is a team sport.
“All of us is smarter than one of us,” said Dr. Darling, articulating the implicit message of group training.
Although this is a departure from a bygone era where infallible surgeons ruled the OR, it is fits nicely with changing attitudes about the best attributes of a competent surgeon.
REPORTING FROM VEITHsymposium
Independent vascular surgery board called key to self-determination
NEW YORK – Numerous experts, including a past president of the Society for Clinical Vascular Surgery, lined up at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation to explain why there is a critical need for a separate vascular surgery board with membership on the American Board of Medical Specialties.
Not least of the reasons is, “We need to control our destiny by training more residents and fellows in vascular surgery,” said Alan M. Dietzek, MD, who served as SCVS president in 2018 and is an active vascular surgeon in Danbury, Conn.
According to Dr. Dietzek, the American Board of Surgeons recently rejected an application for a vascular resident review committee (RRC), which develops and controls training within a specialty.
“A vascular surgery RRC would facilitate more applications [for training] as well as innovations in specialized programs, which we could certainly use in aortic and venous fellowships,” Dr. Dietzek said. “All ABMS-recognized boards have their own RRC.”
Other speakers, including Timothy M. Sullivan, MD, a professor of surgery at the University of Minnesota, Minneapolis, made the same point. He also believes that a vascular surgery RRC is pivotal in establishing recognition for the specialty and what it offers.
From his perspective, O. William Brown, MD, a vascular surgeon from Bingham Falls, Minn., believes that creating a vascular surgery board will increase recognition in general. Like the others, he maintained that an independent board could draw attention to the specific skills of vascular surgeons, creating a basis for attracting patients, advocating for their needs, and lobbying for resources.
Many experts, including Dr. Brown, believe that the specialty of vascular surgery already meets the qualifications for creating an independent board. However, Dr. Dietzek said that membership in ABMS is dependent on support from the Society of Vascular Surgeons, “and we don’t have that yet.”
In the title of his talk on creating a vascular surgery board, Dr. Brown called for the SVS Executive Committee to “recognize this need and go after it with full force.”
Dr. Dietzek believes the SVS should survey the membership. If there is support for an independent board, it should move ahead with the appropriate support.
“Can we afford it? Other small boards have done just fine,” said Dr. Dietzek, citing the American Board of Colorectal Surgery and the American Board of Thoracic Surgery. He said both are doing well financially, and he provided estimates suggesting that a vascular surgery board would also achieve firm financial footing.
The value of an independent board in exercising control over training programs is part of a larger issue of self-determination, according to Dr. Dietzek. For example, vascular surgeons have “little or no control over the priorities or the budget” at most institutions where they work. An established and recognized vascular surgery board could help these specialists define their identity and separate from other surgical specialties to create their own divisions or departments.
Others who spoke on this topic agreed. Many expressed concern about marginalization by hospital administrators who are often unclear on what vascular surgeons do. A vascular surgery board has the potential to provide a degree of stature that is now lacking.
“We need to build relationships with hospital administrators, politicians, and the insurance industry. This is critical,” Dr. Dietzek said. He believes a vascular surgery board offers an opportunity to achieve these goals and “help us control our own destiny.”
Dr. Dietzek and the other participants report no financial conflicts of interest relevant to this topic.
NEW YORK – Numerous experts, including a past president of the Society for Clinical Vascular Surgery, lined up at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation to explain why there is a critical need for a separate vascular surgery board with membership on the American Board of Medical Specialties.
Not least of the reasons is, “We need to control our destiny by training more residents and fellows in vascular surgery,” said Alan M. Dietzek, MD, who served as SCVS president in 2018 and is an active vascular surgeon in Danbury, Conn.
According to Dr. Dietzek, the American Board of Surgeons recently rejected an application for a vascular resident review committee (RRC), which develops and controls training within a specialty.
“A vascular surgery RRC would facilitate more applications [for training] as well as innovations in specialized programs, which we could certainly use in aortic and venous fellowships,” Dr. Dietzek said. “All ABMS-recognized boards have their own RRC.”
Other speakers, including Timothy M. Sullivan, MD, a professor of surgery at the University of Minnesota, Minneapolis, made the same point. He also believes that a vascular surgery RRC is pivotal in establishing recognition for the specialty and what it offers.
From his perspective, O. William Brown, MD, a vascular surgeon from Bingham Falls, Minn., believes that creating a vascular surgery board will increase recognition in general. Like the others, he maintained that an independent board could draw attention to the specific skills of vascular surgeons, creating a basis for attracting patients, advocating for their needs, and lobbying for resources.
Many experts, including Dr. Brown, believe that the specialty of vascular surgery already meets the qualifications for creating an independent board. However, Dr. Dietzek said that membership in ABMS is dependent on support from the Society of Vascular Surgeons, “and we don’t have that yet.”
In the title of his talk on creating a vascular surgery board, Dr. Brown called for the SVS Executive Committee to “recognize this need and go after it with full force.”
Dr. Dietzek believes the SVS should survey the membership. If there is support for an independent board, it should move ahead with the appropriate support.
“Can we afford it? Other small boards have done just fine,” said Dr. Dietzek, citing the American Board of Colorectal Surgery and the American Board of Thoracic Surgery. He said both are doing well financially, and he provided estimates suggesting that a vascular surgery board would also achieve firm financial footing.
The value of an independent board in exercising control over training programs is part of a larger issue of self-determination, according to Dr. Dietzek. For example, vascular surgeons have “little or no control over the priorities or the budget” at most institutions where they work. An established and recognized vascular surgery board could help these specialists define their identity and separate from other surgical specialties to create their own divisions or departments.
Others who spoke on this topic agreed. Many expressed concern about marginalization by hospital administrators who are often unclear on what vascular surgeons do. A vascular surgery board has the potential to provide a degree of stature that is now lacking.
“We need to build relationships with hospital administrators, politicians, and the insurance industry. This is critical,” Dr. Dietzek said. He believes a vascular surgery board offers an opportunity to achieve these goals and “help us control our own destiny.”
Dr. Dietzek and the other participants report no financial conflicts of interest relevant to this topic.
NEW YORK – Numerous experts, including a past president of the Society for Clinical Vascular Surgery, lined up at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation to explain why there is a critical need for a separate vascular surgery board with membership on the American Board of Medical Specialties.
Not least of the reasons is, “We need to control our destiny by training more residents and fellows in vascular surgery,” said Alan M. Dietzek, MD, who served as SCVS president in 2018 and is an active vascular surgeon in Danbury, Conn.
According to Dr. Dietzek, the American Board of Surgeons recently rejected an application for a vascular resident review committee (RRC), which develops and controls training within a specialty.
“A vascular surgery RRC would facilitate more applications [for training] as well as innovations in specialized programs, which we could certainly use in aortic and venous fellowships,” Dr. Dietzek said. “All ABMS-recognized boards have their own RRC.”
Other speakers, including Timothy M. Sullivan, MD, a professor of surgery at the University of Minnesota, Minneapolis, made the same point. He also believes that a vascular surgery RRC is pivotal in establishing recognition for the specialty and what it offers.
From his perspective, O. William Brown, MD, a vascular surgeon from Bingham Falls, Minn., believes that creating a vascular surgery board will increase recognition in general. Like the others, he maintained that an independent board could draw attention to the specific skills of vascular surgeons, creating a basis for attracting patients, advocating for their needs, and lobbying for resources.
Many experts, including Dr. Brown, believe that the specialty of vascular surgery already meets the qualifications for creating an independent board. However, Dr. Dietzek said that membership in ABMS is dependent on support from the Society of Vascular Surgeons, “and we don’t have that yet.”
In the title of his talk on creating a vascular surgery board, Dr. Brown called for the SVS Executive Committee to “recognize this need and go after it with full force.”
Dr. Dietzek believes the SVS should survey the membership. If there is support for an independent board, it should move ahead with the appropriate support.
“Can we afford it? Other small boards have done just fine,” said Dr. Dietzek, citing the American Board of Colorectal Surgery and the American Board of Thoracic Surgery. He said both are doing well financially, and he provided estimates suggesting that a vascular surgery board would also achieve firm financial footing.
The value of an independent board in exercising control over training programs is part of a larger issue of self-determination, according to Dr. Dietzek. For example, vascular surgeons have “little or no control over the priorities or the budget” at most institutions where they work. An established and recognized vascular surgery board could help these specialists define their identity and separate from other surgical specialties to create their own divisions or departments.
Others who spoke on this topic agreed. Many expressed concern about marginalization by hospital administrators who are often unclear on what vascular surgeons do. A vascular surgery board has the potential to provide a degree of stature that is now lacking.
“We need to build relationships with hospital administrators, politicians, and the insurance industry. This is critical,” Dr. Dietzek said. He believes a vascular surgery board offers an opportunity to achieve these goals and “help us control our own destiny.”
Dr. Dietzek and the other participants report no financial conflicts of interest relevant to this topic.
REPORTING FROM VEITHsymposium
Blunted ventral striatal responses found in remitted bipolar I
People with remitted bipolar I disorder appear to show lower rather than higher activity in the ventral striatum in anticipation of monetary gains, compared with controls, results of a small study suggest. In addition, associations were found between positive urgency measure (PUM) and blunted activity in the nucleus accumbens in individuals with bipolar I.
“Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money),” wrote lead author Sheri L. Johnson, PhD, and associates. The study was published in Neuroimage: Clinical.
Dr. Johnson’s team recruited the participants through advertisements online and in the community. Their study included 24 people with bipolar I (14 men; mean age, 36.22 years) and 24 controls without bipolar (13 men; mean age, 33.92 years), reported Dr. Johnson, of the University of California, Berkeley, and associates. The investigators used a version of the Monetary Incentive Delay Task to elicit neural and behavioral responses and their outcomes during fMRI.
Whole brain analyses found that, compared with the control group, those in the bipolar group “showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right [nucleus accumbens] and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus,” the investigators wrote.
PUM, a dimensional trait, was designed to assess tendencies to act “regrettably” or “impulsively” during states of positive emotion. PUM scores have been correlated with outcomes such as lower functioning in behavior, decreased quality of life, and worse outcomes in bipolar disorder. The investigators hoped that, by including PUM to help evaluate group differences, they could account for inconsistent findings regarding associations of neural imaging and reward responses.
“The current findings imply that, in individuals with bipolar disorder, individual differences in PUM may also help account for blunted [nucleus accumbens] during reward anticipation.”
The study’s sample size limited the power to detect small effects or interactions. The researchers felt, though, that the sample was well matched and adequately powered for the primary variables of interest.
The authors had no conflicts of interest to disclose. The study was funded by a grant from the National Institute of Mental Health.
SOURCE: Johnson SL et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2019.102018.
People with remitted bipolar I disorder appear to show lower rather than higher activity in the ventral striatum in anticipation of monetary gains, compared with controls, results of a small study suggest. In addition, associations were found between positive urgency measure (PUM) and blunted activity in the nucleus accumbens in individuals with bipolar I.
“Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money),” wrote lead author Sheri L. Johnson, PhD, and associates. The study was published in Neuroimage: Clinical.
Dr. Johnson’s team recruited the participants through advertisements online and in the community. Their study included 24 people with bipolar I (14 men; mean age, 36.22 years) and 24 controls without bipolar (13 men; mean age, 33.92 years), reported Dr. Johnson, of the University of California, Berkeley, and associates. The investigators used a version of the Monetary Incentive Delay Task to elicit neural and behavioral responses and their outcomes during fMRI.
Whole brain analyses found that, compared with the control group, those in the bipolar group “showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right [nucleus accumbens] and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus,” the investigators wrote.
PUM, a dimensional trait, was designed to assess tendencies to act “regrettably” or “impulsively” during states of positive emotion. PUM scores have been correlated with outcomes such as lower functioning in behavior, decreased quality of life, and worse outcomes in bipolar disorder. The investigators hoped that, by including PUM to help evaluate group differences, they could account for inconsistent findings regarding associations of neural imaging and reward responses.
“The current findings imply that, in individuals with bipolar disorder, individual differences in PUM may also help account for blunted [nucleus accumbens] during reward anticipation.”
The study’s sample size limited the power to detect small effects or interactions. The researchers felt, though, that the sample was well matched and adequately powered for the primary variables of interest.
The authors had no conflicts of interest to disclose. The study was funded by a grant from the National Institute of Mental Health.
SOURCE: Johnson SL et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2019.102018.
People with remitted bipolar I disorder appear to show lower rather than higher activity in the ventral striatum in anticipation of monetary gains, compared with controls, results of a small study suggest. In addition, associations were found between positive urgency measure (PUM) and blunted activity in the nucleus accumbens in individuals with bipolar I.
“Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money),” wrote lead author Sheri L. Johnson, PhD, and associates. The study was published in Neuroimage: Clinical.
Dr. Johnson’s team recruited the participants through advertisements online and in the community. Their study included 24 people with bipolar I (14 men; mean age, 36.22 years) and 24 controls without bipolar (13 men; mean age, 33.92 years), reported Dr. Johnson, of the University of California, Berkeley, and associates. The investigators used a version of the Monetary Incentive Delay Task to elicit neural and behavioral responses and their outcomes during fMRI.
Whole brain analyses found that, compared with the control group, those in the bipolar group “showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right [nucleus accumbens] and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus,” the investigators wrote.
PUM, a dimensional trait, was designed to assess tendencies to act “regrettably” or “impulsively” during states of positive emotion. PUM scores have been correlated with outcomes such as lower functioning in behavior, decreased quality of life, and worse outcomes in bipolar disorder. The investigators hoped that, by including PUM to help evaluate group differences, they could account for inconsistent findings regarding associations of neural imaging and reward responses.
“The current findings imply that, in individuals with bipolar disorder, individual differences in PUM may also help account for blunted [nucleus accumbens] during reward anticipation.”
The study’s sample size limited the power to detect small effects or interactions. The researchers felt, though, that the sample was well matched and adequately powered for the primary variables of interest.
The authors had no conflicts of interest to disclose. The study was funded by a grant from the National Institute of Mental Health.
SOURCE: Johnson SL et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2019.102018.
FROM NEUROIMAGE: CLINICAL
FDA: Two repackagers issue voluntary ranitidine recall
The ranitidine recall saga continues as two more companies have issued voluntary recalls of their repackaged ranitidine products because of possibly unacceptable levels of N-nitrosodimethylamine (NDMA), according to the Food and Drug Administration.
Golden State Medical Supply has recalled 150-mg and 300-mg ranitidine capsules, manufactured by Novitium, and Precision Dose has recalled a ranitidine oral solution at 150 mg/mL, manufactured by Amneal Pharmaceuticals.
“FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 ng/day or 0.32 parts per million for ranitidine),” the FDA said. The agency has “posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing.”
The FDA added that consumers taking over-the-counter ranitidine can consider alternatives such as famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
Find the full press release and more information on other ranitidine recalls on the FDA website.
The ranitidine recall saga continues as two more companies have issued voluntary recalls of their repackaged ranitidine products because of possibly unacceptable levels of N-nitrosodimethylamine (NDMA), according to the Food and Drug Administration.
Golden State Medical Supply has recalled 150-mg and 300-mg ranitidine capsules, manufactured by Novitium, and Precision Dose has recalled a ranitidine oral solution at 150 mg/mL, manufactured by Amneal Pharmaceuticals.
“FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 ng/day or 0.32 parts per million for ranitidine),” the FDA said. The agency has “posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing.”
The FDA added that consumers taking over-the-counter ranitidine can consider alternatives such as famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
Find the full press release and more information on other ranitidine recalls on the FDA website.
The ranitidine recall saga continues as two more companies have issued voluntary recalls of their repackaged ranitidine products because of possibly unacceptable levels of N-nitrosodimethylamine (NDMA), according to the Food and Drug Administration.
Golden State Medical Supply has recalled 150-mg and 300-mg ranitidine capsules, manufactured by Novitium, and Precision Dose has recalled a ranitidine oral solution at 150 mg/mL, manufactured by Amneal Pharmaceuticals.
“FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 ng/day or 0.32 parts per million for ranitidine),” the FDA said. The agency has “posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing.”
The FDA added that consumers taking over-the-counter ranitidine can consider alternatives such as famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
Find the full press release and more information on other ranitidine recalls on the FDA website.














