User login
Hydrogen Peroxide as a Hemostatic Agent During Dermatologic Surgery
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
Updates in Our Understanding of Central Centrifugal Cicatricial Alopecia
It has been more than 50 years since central centrifugal cicatricial alopecia (CCCA) was first defined by LoPresti and colleagues1 as hot comb alopecia. Fifty years later, we are only just starting to understand the pathogenesis of CCCA and its systemic implications.
Then and Now
The use of hot combs, a metal device used to straighten naturally curly hair, was ubiquitous in the households of black women in the 1960s. It is no surprise then that this styling process was labeled as the culprit of this disease that affects black women almost exclusively. As the use of hot combs waned but the prevalence of CCCA persisted, its name evolved to chemically induced alopecia—an ode to the popular styling product of the 1990s, the chemical relaxer—and eventually CCCA, a name that reflects its clinical progression and histologic findings.2
Since then, research has explored the association with systemic diseases, some noting increased rates of type 2 diabetes mellitus and thyroid disease, and more recently, an increased rate of fibroids in affected patients.3,4
Clues to Pathogenesis
Compared to other primary cicatricial alopecias, CCCA is unique in that active progression is difficult to detect. Symptoms, such as pruritus, often are minimal or absent, rendering clinical assessment quite difficult.5 Unlike other forms of scarring hair loss, fibrosis, not inflammation, is the predominant clinical feature. The clinical presentation is not unlike a group of disorders termed fibroproliferative disorders, which includes systemic sclerosis, uterine fibroids, atherosclerosis, and keloids, among others. It has been postulated that diseases of aberrant scarring are more common in black individuals due to the protective effect profibrotic alleles have against endemic helminthic infections of sub-Saharan infections, including oncocerciasis.6
A recent study showed an increased expression of fibroproliferative genes, particularly those implicated in other fibroproliferative disorders, in affected scalp of patients with CCCA.7 Most notably, an expression in gene overlap was noted between fibroids and CCCA in this study, though the relationship between these two diseases needs to be further explored.
Gene Variants Identified in CCCA
More recently, a new study has identified a gene variant of peptidyl arginine deiminase 3, PADI3, that is present in approximately one-quarter of studied patients with CCCA.8PADI3 plays a role in hair shaft formation and has been implicated in another hair disorder, uncombable hair syndrome, though the latter presents in children, improves with age, and is not associated with a scarring phenotype.9 However, this study has provided greater insight into our understanding of CCCA by establishing a possible genetic predisposition in patients affected with this disease.8
What’s Next for CCCA?
For years, many patients with CCCA have been turned away with few answers and left thinking that it is their own styling habits that have led to their hair loss,
The future is bright for CCCA. Although our understanding of CCCA is still in its infancy, it is my hope that with greater understanding of this disease will come greater empathy for our patients.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234-238.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909-914.
- Dina Y, Okoye GA, Aguh C. Association of uterine leiomyomas with central centrifugal cicatricial alopecia. JAMA Dermatol. 2018;154:213-214.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21:268-278.
- Hellwege JN, Torstenson ES, Russell SB, et al. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12:e0182791. doi:10.1371/journal.pone.0182791.
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904.e1-912.e1.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Matis WL, Baden H, Green R, et al. Uncombable-hair syndrome. Pediatr Dermatol. 1987;4:215-219.
It has been more than 50 years since central centrifugal cicatricial alopecia (CCCA) was first defined by LoPresti and colleagues1 as hot comb alopecia. Fifty years later, we are only just starting to understand the pathogenesis of CCCA and its systemic implications.
Then and Now
The use of hot combs, a metal device used to straighten naturally curly hair, was ubiquitous in the households of black women in the 1960s. It is no surprise then that this styling process was labeled as the culprit of this disease that affects black women almost exclusively. As the use of hot combs waned but the prevalence of CCCA persisted, its name evolved to chemically induced alopecia—an ode to the popular styling product of the 1990s, the chemical relaxer—and eventually CCCA, a name that reflects its clinical progression and histologic findings.2
Since then, research has explored the association with systemic diseases, some noting increased rates of type 2 diabetes mellitus and thyroid disease, and more recently, an increased rate of fibroids in affected patients.3,4
Clues to Pathogenesis
Compared to other primary cicatricial alopecias, CCCA is unique in that active progression is difficult to detect. Symptoms, such as pruritus, often are minimal or absent, rendering clinical assessment quite difficult.5 Unlike other forms of scarring hair loss, fibrosis, not inflammation, is the predominant clinical feature. The clinical presentation is not unlike a group of disorders termed fibroproliferative disorders, which includes systemic sclerosis, uterine fibroids, atherosclerosis, and keloids, among others. It has been postulated that diseases of aberrant scarring are more common in black individuals due to the protective effect profibrotic alleles have against endemic helminthic infections of sub-Saharan infections, including oncocerciasis.6
A recent study showed an increased expression of fibroproliferative genes, particularly those implicated in other fibroproliferative disorders, in affected scalp of patients with CCCA.7 Most notably, an expression in gene overlap was noted between fibroids and CCCA in this study, though the relationship between these two diseases needs to be further explored.
Gene Variants Identified in CCCA
More recently, a new study has identified a gene variant of peptidyl arginine deiminase 3, PADI3, that is present in approximately one-quarter of studied patients with CCCA.8PADI3 plays a role in hair shaft formation and has been implicated in another hair disorder, uncombable hair syndrome, though the latter presents in children, improves with age, and is not associated with a scarring phenotype.9 However, this study has provided greater insight into our understanding of CCCA by establishing a possible genetic predisposition in patients affected with this disease.8
What’s Next for CCCA?
For years, many patients with CCCA have been turned away with few answers and left thinking that it is their own styling habits that have led to their hair loss,
The future is bright for CCCA. Although our understanding of CCCA is still in its infancy, it is my hope that with greater understanding of this disease will come greater empathy for our patients.
It has been more than 50 years since central centrifugal cicatricial alopecia (CCCA) was first defined by LoPresti and colleagues1 as hot comb alopecia. Fifty years later, we are only just starting to understand the pathogenesis of CCCA and its systemic implications.
Then and Now
The use of hot combs, a metal device used to straighten naturally curly hair, was ubiquitous in the households of black women in the 1960s. It is no surprise then that this styling process was labeled as the culprit of this disease that affects black women almost exclusively. As the use of hot combs waned but the prevalence of CCCA persisted, its name evolved to chemically induced alopecia—an ode to the popular styling product of the 1990s, the chemical relaxer—and eventually CCCA, a name that reflects its clinical progression and histologic findings.2
Since then, research has explored the association with systemic diseases, some noting increased rates of type 2 diabetes mellitus and thyroid disease, and more recently, an increased rate of fibroids in affected patients.3,4
Clues to Pathogenesis
Compared to other primary cicatricial alopecias, CCCA is unique in that active progression is difficult to detect. Symptoms, such as pruritus, often are minimal or absent, rendering clinical assessment quite difficult.5 Unlike other forms of scarring hair loss, fibrosis, not inflammation, is the predominant clinical feature. The clinical presentation is not unlike a group of disorders termed fibroproliferative disorders, which includes systemic sclerosis, uterine fibroids, atherosclerosis, and keloids, among others. It has been postulated that diseases of aberrant scarring are more common in black individuals due to the protective effect profibrotic alleles have against endemic helminthic infections of sub-Saharan infections, including oncocerciasis.6
A recent study showed an increased expression of fibroproliferative genes, particularly those implicated in other fibroproliferative disorders, in affected scalp of patients with CCCA.7 Most notably, an expression in gene overlap was noted between fibroids and CCCA in this study, though the relationship between these two diseases needs to be further explored.
Gene Variants Identified in CCCA
More recently, a new study has identified a gene variant of peptidyl arginine deiminase 3, PADI3, that is present in approximately one-quarter of studied patients with CCCA.8PADI3 plays a role in hair shaft formation and has been implicated in another hair disorder, uncombable hair syndrome, though the latter presents in children, improves with age, and is not associated with a scarring phenotype.9 However, this study has provided greater insight into our understanding of CCCA by establishing a possible genetic predisposition in patients affected with this disease.8
What’s Next for CCCA?
For years, many patients with CCCA have been turned away with few answers and left thinking that it is their own styling habits that have led to their hair loss,
The future is bright for CCCA. Although our understanding of CCCA is still in its infancy, it is my hope that with greater understanding of this disease will come greater empathy for our patients.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234-238.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909-914.
- Dina Y, Okoye GA, Aguh C. Association of uterine leiomyomas with central centrifugal cicatricial alopecia. JAMA Dermatol. 2018;154:213-214.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21:268-278.
- Hellwege JN, Torstenson ES, Russell SB, et al. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12:e0182791. doi:10.1371/journal.pone.0182791.
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904.e1-912.e1.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Matis WL, Baden H, Green R, et al. Uncombable-hair syndrome. Pediatr Dermatol. 1987;4:215-219.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98:234-238.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol. 2011;147:909-914.
- Dina Y, Okoye GA, Aguh C. Association of uterine leiomyomas with central centrifugal cicatricial alopecia. JAMA Dermatol. 2018;154:213-214.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21:268-278.
- Hellwege JN, Torstenson ES, Russell SB, et al. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12:e0182791. doi:10.1371/journal.pone.0182791.
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904.e1-912.e1.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Matis WL, Baden H, Green R, et al. Uncombable-hair syndrome. Pediatr Dermatol. 1987;4:215-219.
Clinical Characterization of Leukemia Cutis Presentation
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
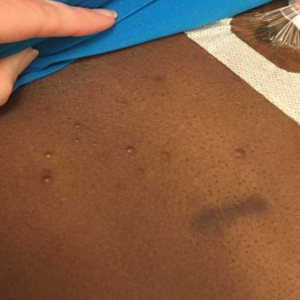
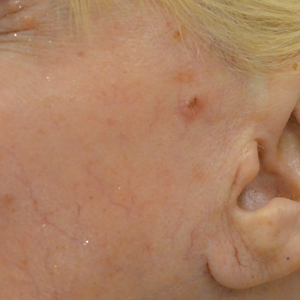
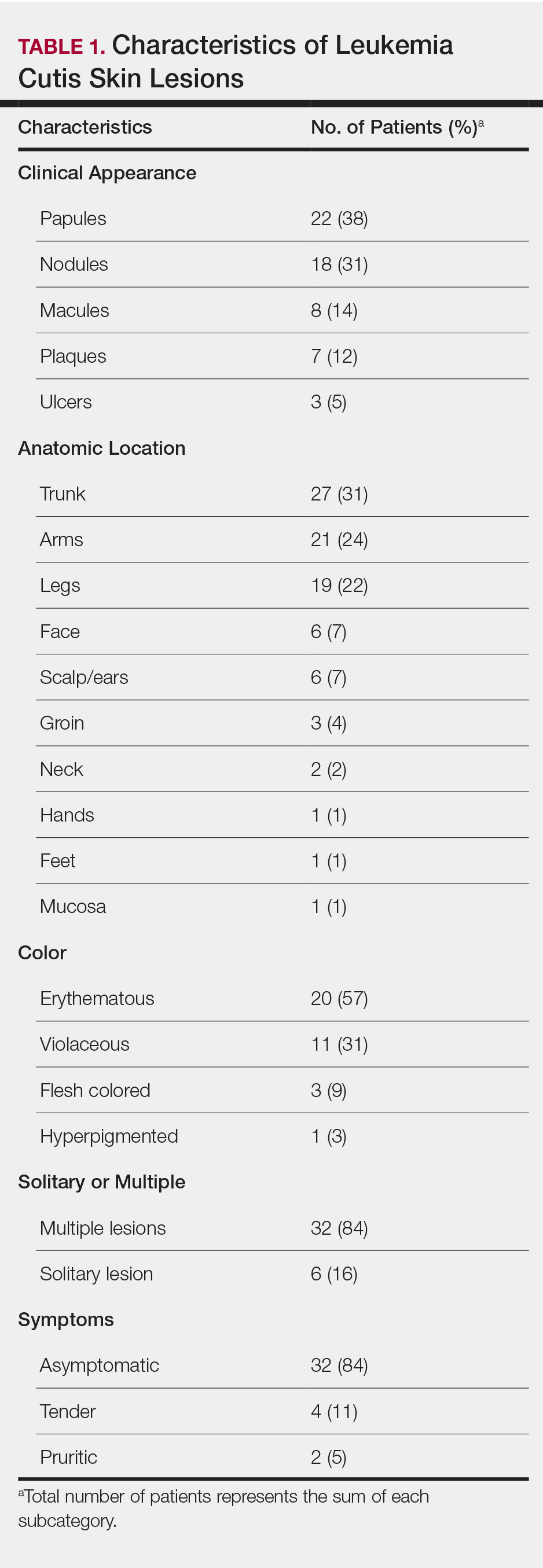
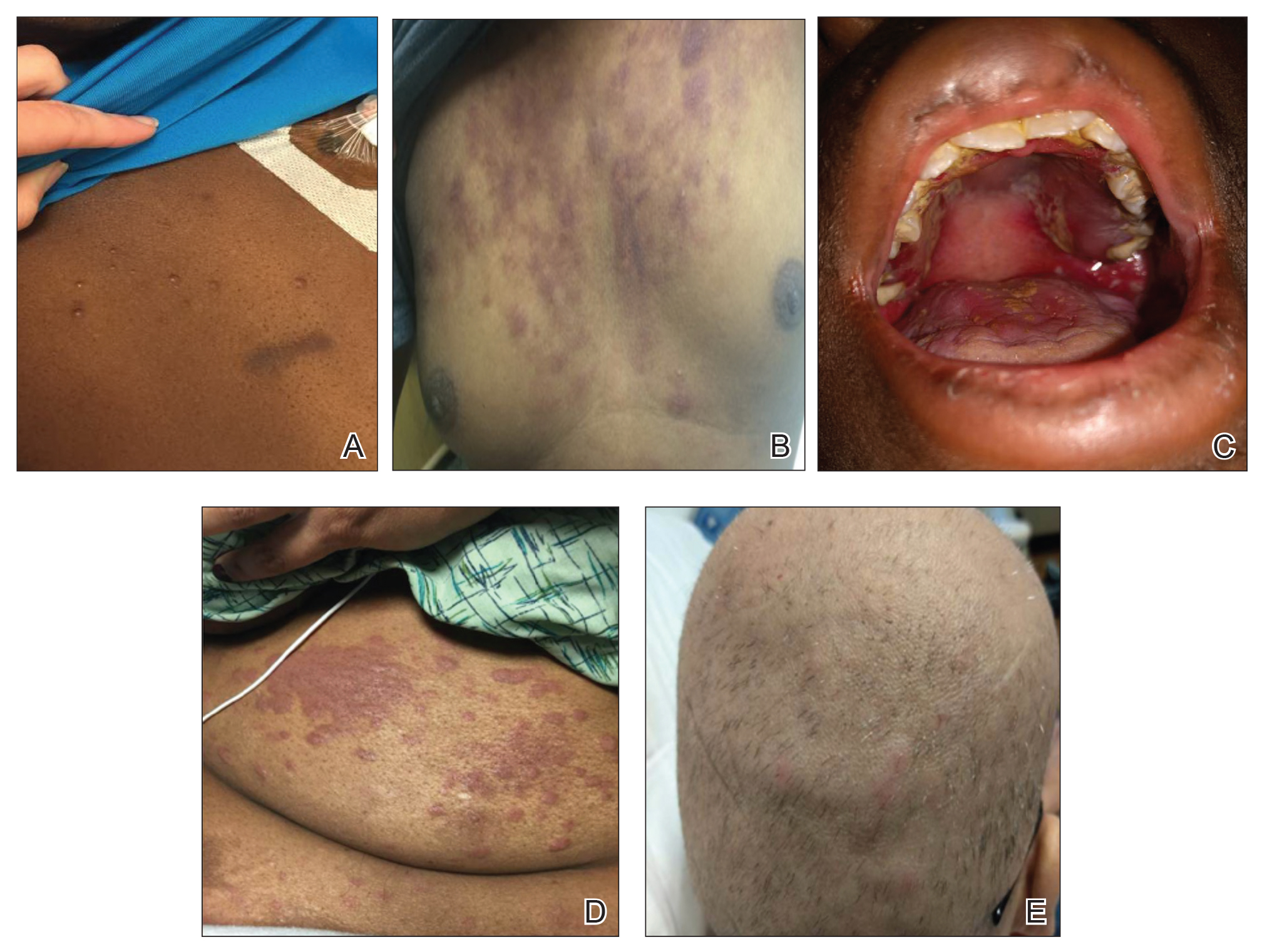
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.
Interval Between Leukemia Diagnosis and LC Diagnosis
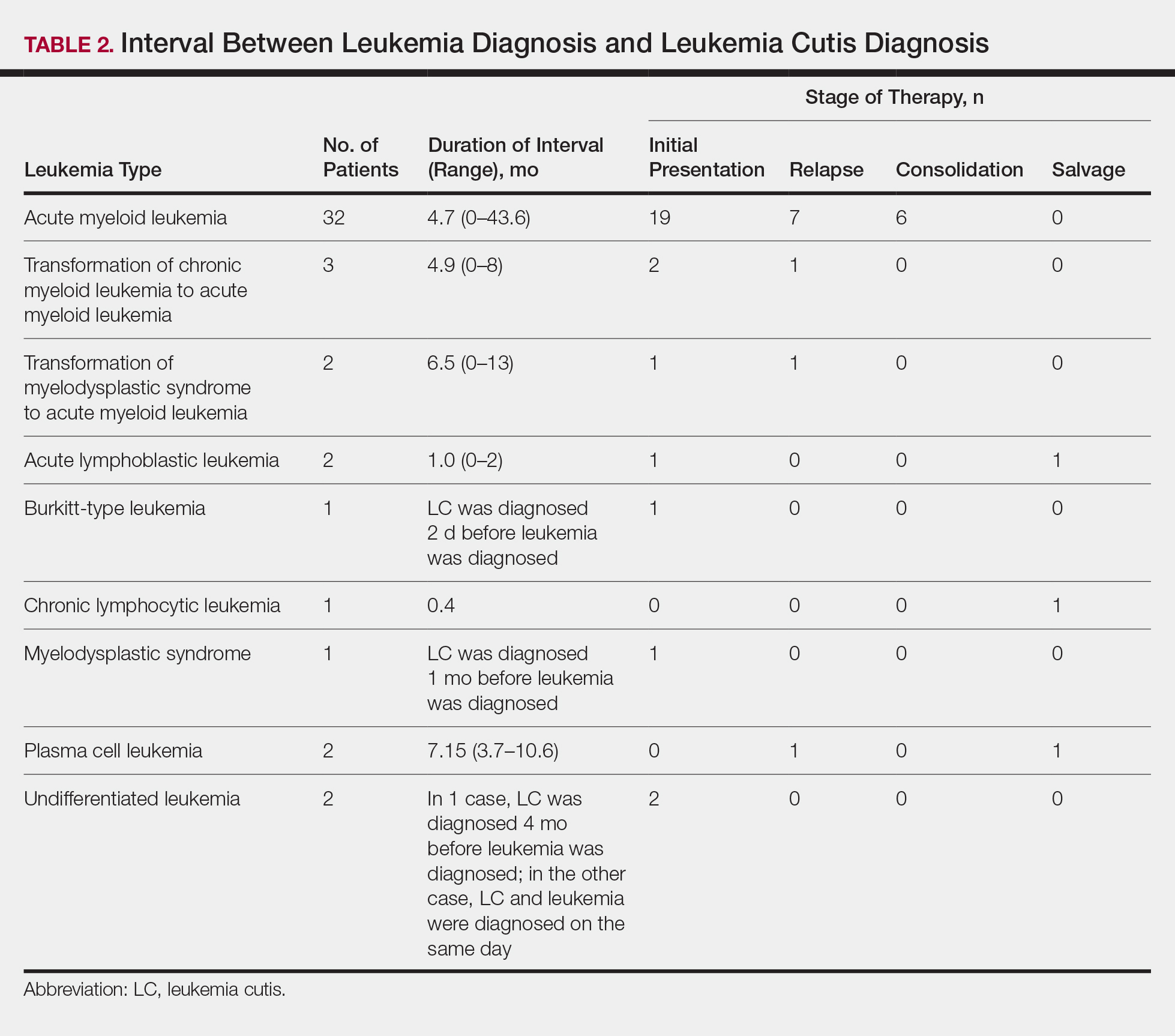
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.
Interval Between LC Diagnosis and Death
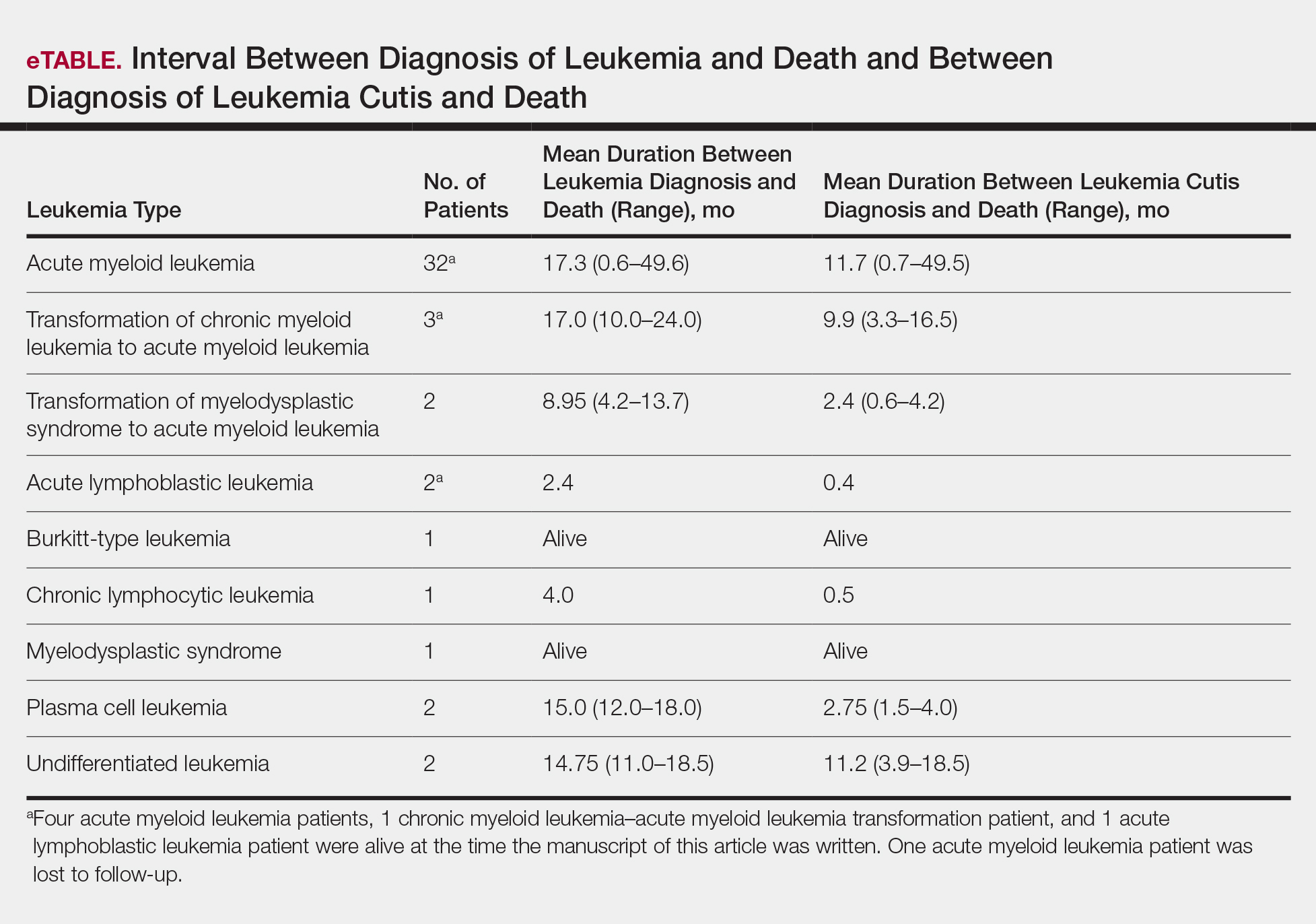
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).
Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.


Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.

Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).

Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.


Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.

Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).

Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
Practice Points
- Complete and comprehensive skin examination is important in leukemia patients, as leukemia cutis (LC) lesions can present in all body sites including ocular and oral mucosa as well as the groin.
- Given the wide variability in appearance, symptoms, distribution, and stage of leukemia at presentation, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy.
- Performing thorough skin examination on leukemia patients throughout the course of their disease may help identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
Systemic Contact Dermatitis: Sometimes It Is the Food
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
Practice Points
- Although most cases of allergic contact dermatitis are from direct skin contact, systemic contact dermatitis (SCD) can occur from ingesting certain allergens.
- Systemic contact dermatitis tends to present as a symmetric pruritic eruption, which may involve the flexural or intertriginous surfaces, eyelids, hands, or genital skin.
- Allergens known to cause SCD include certain plants, fragrances, metals, preservatives, and medications.
Patient-Driven Management Using Same-Day Noninvasive Diagnosis and Complete Laser Treatment of Basal Cell Carcinomas: A Pilot Study
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.
This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.
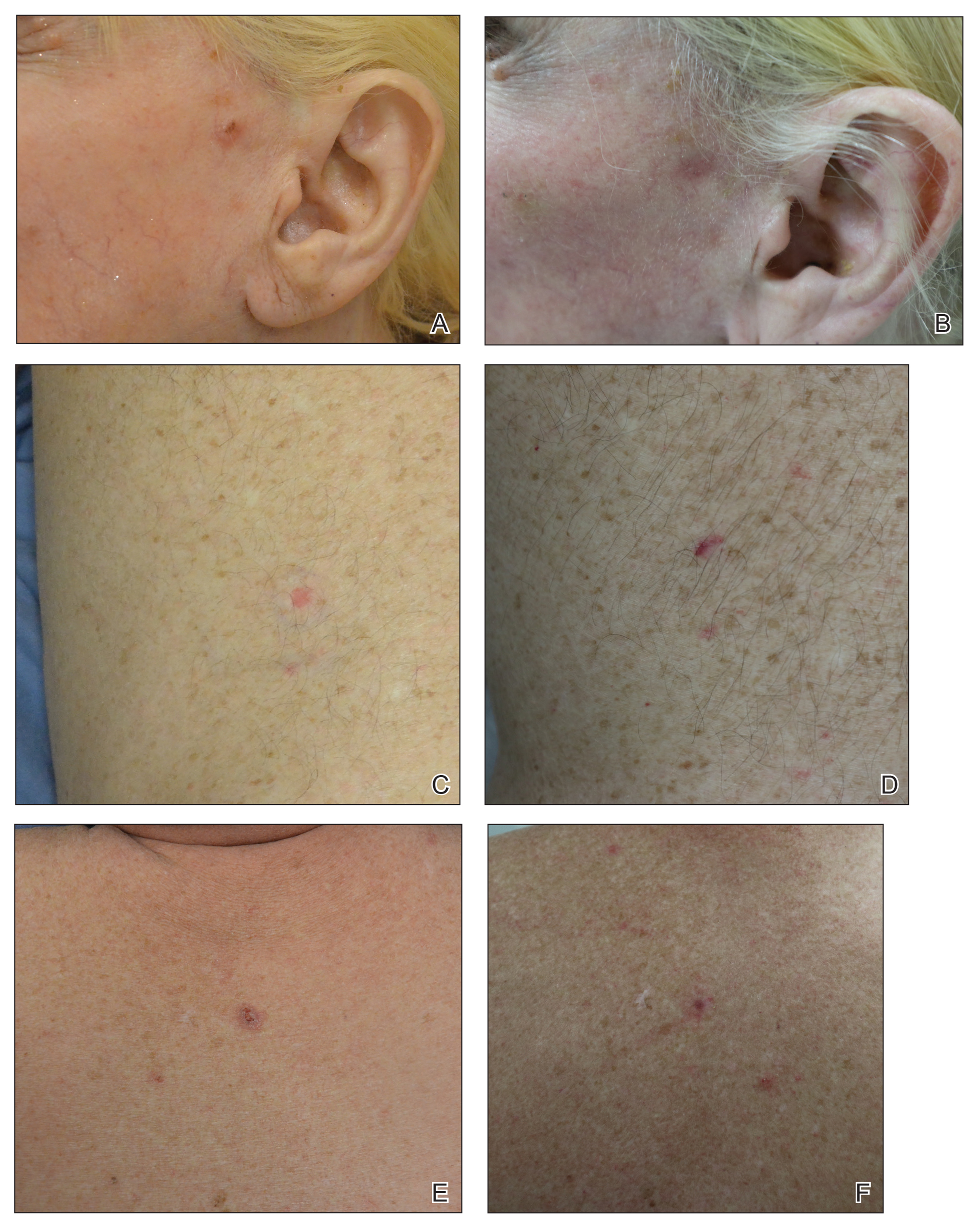
The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.

This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.

The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.

This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.

The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
Practice Points
- Novel imaging modalities such as reflectance confocal microscopy and optical coherence tomography can be used to diagnose, monitor treatment response, and confirm clearance of basal cell carcinoma.
- Leveraging new imaging technologies can enable a streamlined patient experience, with same-day diagnosis and management of skin cancer.
- For patients who do not desire surgical management of nonmelanoma skin cancer, laser treatment with Nd:YAG is a promising emerging therapeutic option.
Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective
Another Match Day is approaching. Students find themselves paying more each year to apply to one of the most competitive fields, while program directors struggle to sort through hundreds of stellar applications to invite a handful of candidates for interviews. Estimates place the cost of the application process at $5 million in total for all medical school seniors, or roughly $10,000 per applicant.1 Approximately 60% of these costs occur during the interview process.1,2 In an era in which students routinely graduate medical school with hundreds of thousands of dollars of debt, these costs must be addressed as soon as possible.
This problem is not unique to dermatology; otolaryngology, another especially competitive field, has considered various changes to the match process based on applicants’ feedback.3 As an applicant during the 2018-2019 match cycle for dermatology, I offer 2 solutions that are a starting point aimed at streamlining the application process for both applicants and program directors: regional interview coordination and a cap on the number of residency applications.
Regional Interview Coordination
Regional interview coordination would reduce travel costs and facilitate greater predictability in scheduling clinical rotations. In the current climate, it is not uncommon for applicants to make multiple cross-country round trips in the same week, especially given that the interview season for dermatology, including interviews for preliminary programs, now ranges from mid-October to early February. Although affluent applicants may not be concerned with financial costs of frequent travel, all applicants face travel inconveniences that could be mitigated through regional coordination. For example, an applicant invited to multiple interviews in the New York City area could reserve a room in a single hotel over a period of several days. During each interview day, he/she could travel back and forth from that accommodation to each institution without needing to bring luggage, worrying about reaching the airport on time, or missing a pre-interview dinner at a program in a faraway city.
Given the amount of coordination required among programs, it may lead to more positive working relationships among regional dermatology programs. One limitation of this approach is that competitive programs may be unwilling to cooperate. If even one program deviates from the interview time frame, it reduces the incentive for others to participate. Programs must be willing to sacrifice short-term autonomy in interview scheduling for their long-term shared interest in reducing the application burden for students, which is known as a commitment problem in game theory, and could be addressed through joint decision-making that incorporates the time frame preferences of all programs as well as binding commitments on interview dates that are decided before the process begins.4 Another limitation is that inclement weather could affect all regional programs simultaneously. In this case, offering interviews via video conference for affected students may be a solution.
Capping the Number of Applications
A second method of reducing interview costs would be capping the number of applications. Although matched seniors applied to a median of 72 programs, the Association of American Medical Colleges suggests that dermatology applicants can maximize their return on investment (ie, ratio of interviews to applications) by sending 35 to 55 applications depending on US Medical Licensing Examination scores. Attending more than 10 interviews does not meaningfully improve the chance of matching.5,6
Programs have limited capacity for interviews and must judiciously allocate invitations based solely on the information provided through the Electronic Residency Application Service (ERAS). Given the competitiveness of dermatology, applicants usually will accept every interview invitation. Therefore, applicants who are not genuinely interested in a program may crowd out others who are interested. In a survey of otolaryngology applicants (N=150), 90.6% of respondents admitted applying to programs in which they had no specific interest, simply to increase their chance of matching.3 Capping application numbers would force students to apply more selectively and enable residencies to gauge students’ true interest more effectively. In contrast to regional interview coordination, this policy change would be easy to enforce. It also may be popular; nearly two-thirds of otolaryngology applicants agreed to a hypothetical cap on residency applications to reduce the burden on students and programs.3
An alternative to a hard cap on applications could be restructuring the ERAS application fee to incentivize students to apply to fewer programs. For example, a flat fee might cover application numbers up to the point of diminishing returns, after which the price per application could increase exponentially. This approach would have a similar effect of a hard cap and cause many students to apply to fewer programs; however, one notable drawback is that highly affluent applicants would simply absorb the extra cost and still gain a competitive advantage in applying to more programs, which might further decrease the number of lower-income individuals successfully matching into dermatology.
A benefit of decreased application numbers to program directors would be giving them more time to conduct a holistic review of applicants, rather than attempting to weed out candidates through arbitrary cutoffs for US Medical Licensing Examination scores or Alpha Omega Alpha Honor Medical Society membership. The ERAS could allow applicants the option of stating preferences for geographic regions, desired fellowships, areas of research interest, and other intangible metrics. Selection committees could filter their candidate search by different variables and then look at each candidate holistically.
Limitations of capping application numbers include the risk that such a cap would harm less-competitive applicants while failing to address the primary cost drivers (ie, travel costs). The specific cap number would be controversial and may need to be adjusted higher for special cases such as couples matching and international applicants, thus making a cap seem arbitrary.
Final Thoughts
The dermatology residency match can be streamlined to the benefit of both applicants and selection committees. Regional interview coordination would reduce both financial and logistical barriers for applicants but may be difficult to enforce without cooperation from multiple programs. Capping the number of applications, either through a hard cap or an increased financial barrier, would be relatively easy to enforce and might empower selection committees to conduct more detailed, holistic reviews of applicants; however, certain types of applicants may find the application limits detrimental to their chances of matching. These policy recommendations are meant to be a starting point for discussion. Streamlining the application process is critical to improving the diversity of dermatology residencies.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
- Tichy AL, Peng DH, Lane AT. Applying for dermatology residency is difficult and expensive. J Am Acad Dermatol. 2012;66:696-697.
- Ward M, Pingree C, Laury AM, et al. Applicant perspectives on the otolaryngology residency application process. JAMA Otolaryngol Head Neck Surg. 2017;143:782-787.
- North DC. Institutions and credible commitment. J Inst Theor Econ. 1993;149:11-23.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed November 12, 2019.
- Charting Outcomes in the Match: Characteristics of U.S. Allopathic Seniors Who Matched to Their Preferred Specialty in the 2018 Main Residency Match. 2nd ed. Washington, DC: National Resident Matching Program; July 2018. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed November 11, 2019.
Another Match Day is approaching. Students find themselves paying more each year to apply to one of the most competitive fields, while program directors struggle to sort through hundreds of stellar applications to invite a handful of candidates for interviews. Estimates place the cost of the application process at $5 million in total for all medical school seniors, or roughly $10,000 per applicant.1 Approximately 60% of these costs occur during the interview process.1,2 In an era in which students routinely graduate medical school with hundreds of thousands of dollars of debt, these costs must be addressed as soon as possible.
This problem is not unique to dermatology; otolaryngology, another especially competitive field, has considered various changes to the match process based on applicants’ feedback.3 As an applicant during the 2018-2019 match cycle for dermatology, I offer 2 solutions that are a starting point aimed at streamlining the application process for both applicants and program directors: regional interview coordination and a cap on the number of residency applications.
Regional Interview Coordination
Regional interview coordination would reduce travel costs and facilitate greater predictability in scheduling clinical rotations. In the current climate, it is not uncommon for applicants to make multiple cross-country round trips in the same week, especially given that the interview season for dermatology, including interviews for preliminary programs, now ranges from mid-October to early February. Although affluent applicants may not be concerned with financial costs of frequent travel, all applicants face travel inconveniences that could be mitigated through regional coordination. For example, an applicant invited to multiple interviews in the New York City area could reserve a room in a single hotel over a period of several days. During each interview day, he/she could travel back and forth from that accommodation to each institution without needing to bring luggage, worrying about reaching the airport on time, or missing a pre-interview dinner at a program in a faraway city.
Given the amount of coordination required among programs, it may lead to more positive working relationships among regional dermatology programs. One limitation of this approach is that competitive programs may be unwilling to cooperate. If even one program deviates from the interview time frame, it reduces the incentive for others to participate. Programs must be willing to sacrifice short-term autonomy in interview scheduling for their long-term shared interest in reducing the application burden for students, which is known as a commitment problem in game theory, and could be addressed through joint decision-making that incorporates the time frame preferences of all programs as well as binding commitments on interview dates that are decided before the process begins.4 Another limitation is that inclement weather could affect all regional programs simultaneously. In this case, offering interviews via video conference for affected students may be a solution.
Capping the Number of Applications
A second method of reducing interview costs would be capping the number of applications. Although matched seniors applied to a median of 72 programs, the Association of American Medical Colleges suggests that dermatology applicants can maximize their return on investment (ie, ratio of interviews to applications) by sending 35 to 55 applications depending on US Medical Licensing Examination scores. Attending more than 10 interviews does not meaningfully improve the chance of matching.5,6
Programs have limited capacity for interviews and must judiciously allocate invitations based solely on the information provided through the Electronic Residency Application Service (ERAS). Given the competitiveness of dermatology, applicants usually will accept every interview invitation. Therefore, applicants who are not genuinely interested in a program may crowd out others who are interested. In a survey of otolaryngology applicants (N=150), 90.6% of respondents admitted applying to programs in which they had no specific interest, simply to increase their chance of matching.3 Capping application numbers would force students to apply more selectively and enable residencies to gauge students’ true interest more effectively. In contrast to regional interview coordination, this policy change would be easy to enforce. It also may be popular; nearly two-thirds of otolaryngology applicants agreed to a hypothetical cap on residency applications to reduce the burden on students and programs.3
An alternative to a hard cap on applications could be restructuring the ERAS application fee to incentivize students to apply to fewer programs. For example, a flat fee might cover application numbers up to the point of diminishing returns, after which the price per application could increase exponentially. This approach would have a similar effect of a hard cap and cause many students to apply to fewer programs; however, one notable drawback is that highly affluent applicants would simply absorb the extra cost and still gain a competitive advantage in applying to more programs, which might further decrease the number of lower-income individuals successfully matching into dermatology.
A benefit of decreased application numbers to program directors would be giving them more time to conduct a holistic review of applicants, rather than attempting to weed out candidates through arbitrary cutoffs for US Medical Licensing Examination scores or Alpha Omega Alpha Honor Medical Society membership. The ERAS could allow applicants the option of stating preferences for geographic regions, desired fellowships, areas of research interest, and other intangible metrics. Selection committees could filter their candidate search by different variables and then look at each candidate holistically.
Limitations of capping application numbers include the risk that such a cap would harm less-competitive applicants while failing to address the primary cost drivers (ie, travel costs). The specific cap number would be controversial and may need to be adjusted higher for special cases such as couples matching and international applicants, thus making a cap seem arbitrary.
Final Thoughts
The dermatology residency match can be streamlined to the benefit of both applicants and selection committees. Regional interview coordination would reduce both financial and logistical barriers for applicants but may be difficult to enforce without cooperation from multiple programs. Capping the number of applications, either through a hard cap or an increased financial barrier, would be relatively easy to enforce and might empower selection committees to conduct more detailed, holistic reviews of applicants; however, certain types of applicants may find the application limits detrimental to their chances of matching. These policy recommendations are meant to be a starting point for discussion. Streamlining the application process is critical to improving the diversity of dermatology residencies.
Another Match Day is approaching. Students find themselves paying more each year to apply to one of the most competitive fields, while program directors struggle to sort through hundreds of stellar applications to invite a handful of candidates for interviews. Estimates place the cost of the application process at $5 million in total for all medical school seniors, or roughly $10,000 per applicant.1 Approximately 60% of these costs occur during the interview process.1,2 In an era in which students routinely graduate medical school with hundreds of thousands of dollars of debt, these costs must be addressed as soon as possible.
This problem is not unique to dermatology; otolaryngology, another especially competitive field, has considered various changes to the match process based on applicants’ feedback.3 As an applicant during the 2018-2019 match cycle for dermatology, I offer 2 solutions that are a starting point aimed at streamlining the application process for both applicants and program directors: regional interview coordination and a cap on the number of residency applications.
Regional Interview Coordination
Regional interview coordination would reduce travel costs and facilitate greater predictability in scheduling clinical rotations. In the current climate, it is not uncommon for applicants to make multiple cross-country round trips in the same week, especially given that the interview season for dermatology, including interviews for preliminary programs, now ranges from mid-October to early February. Although affluent applicants may not be concerned with financial costs of frequent travel, all applicants face travel inconveniences that could be mitigated through regional coordination. For example, an applicant invited to multiple interviews in the New York City area could reserve a room in a single hotel over a period of several days. During each interview day, he/she could travel back and forth from that accommodation to each institution without needing to bring luggage, worrying about reaching the airport on time, or missing a pre-interview dinner at a program in a faraway city.
Given the amount of coordination required among programs, it may lead to more positive working relationships among regional dermatology programs. One limitation of this approach is that competitive programs may be unwilling to cooperate. If even one program deviates from the interview time frame, it reduces the incentive for others to participate. Programs must be willing to sacrifice short-term autonomy in interview scheduling for their long-term shared interest in reducing the application burden for students, which is known as a commitment problem in game theory, and could be addressed through joint decision-making that incorporates the time frame preferences of all programs as well as binding commitments on interview dates that are decided before the process begins.4 Another limitation is that inclement weather could affect all regional programs simultaneously. In this case, offering interviews via video conference for affected students may be a solution.
Capping the Number of Applications
A second method of reducing interview costs would be capping the number of applications. Although matched seniors applied to a median of 72 programs, the Association of American Medical Colleges suggests that dermatology applicants can maximize their return on investment (ie, ratio of interviews to applications) by sending 35 to 55 applications depending on US Medical Licensing Examination scores. Attending more than 10 interviews does not meaningfully improve the chance of matching.5,6
Programs have limited capacity for interviews and must judiciously allocate invitations based solely on the information provided through the Electronic Residency Application Service (ERAS). Given the competitiveness of dermatology, applicants usually will accept every interview invitation. Therefore, applicants who are not genuinely interested in a program may crowd out others who are interested. In a survey of otolaryngology applicants (N=150), 90.6% of respondents admitted applying to programs in which they had no specific interest, simply to increase their chance of matching.3 Capping application numbers would force students to apply more selectively and enable residencies to gauge students’ true interest more effectively. In contrast to regional interview coordination, this policy change would be easy to enforce. It also may be popular; nearly two-thirds of otolaryngology applicants agreed to a hypothetical cap on residency applications to reduce the burden on students and programs.3
An alternative to a hard cap on applications could be restructuring the ERAS application fee to incentivize students to apply to fewer programs. For example, a flat fee might cover application numbers up to the point of diminishing returns, after which the price per application could increase exponentially. This approach would have a similar effect of a hard cap and cause many students to apply to fewer programs; however, one notable drawback is that highly affluent applicants would simply absorb the extra cost and still gain a competitive advantage in applying to more programs, which might further decrease the number of lower-income individuals successfully matching into dermatology.
A benefit of decreased application numbers to program directors would be giving them more time to conduct a holistic review of applicants, rather than attempting to weed out candidates through arbitrary cutoffs for US Medical Licensing Examination scores or Alpha Omega Alpha Honor Medical Society membership. The ERAS could allow applicants the option of stating preferences for geographic regions, desired fellowships, areas of research interest, and other intangible metrics. Selection committees could filter their candidate search by different variables and then look at each candidate holistically.
Limitations of capping application numbers include the risk that such a cap would harm less-competitive applicants while failing to address the primary cost drivers (ie, travel costs). The specific cap number would be controversial and may need to be adjusted higher for special cases such as couples matching and international applicants, thus making a cap seem arbitrary.
Final Thoughts
The dermatology residency match can be streamlined to the benefit of both applicants and selection committees. Regional interview coordination would reduce both financial and logistical barriers for applicants but may be difficult to enforce without cooperation from multiple programs. Capping the number of applications, either through a hard cap or an increased financial barrier, would be relatively easy to enforce and might empower selection committees to conduct more detailed, holistic reviews of applicants; however, certain types of applicants may find the application limits detrimental to their chances of matching. These policy recommendations are meant to be a starting point for discussion. Streamlining the application process is critical to improving the diversity of dermatology residencies.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
- Tichy AL, Peng DH, Lane AT. Applying for dermatology residency is difficult and expensive. J Am Acad Dermatol. 2012;66:696-697.
- Ward M, Pingree C, Laury AM, et al. Applicant perspectives on the otolaryngology residency application process. JAMA Otolaryngol Head Neck Surg. 2017;143:782-787.
- North DC. Institutions and credible commitment. J Inst Theor Econ. 1993;149:11-23.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed November 12, 2019.
- Charting Outcomes in the Match: Characteristics of U.S. Allopathic Seniors Who Matched to Their Preferred Specialty in the 2018 Main Residency Match. 2nd ed. Washington, DC: National Resident Matching Program; July 2018. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed November 11, 2019.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
- Tichy AL, Peng DH, Lane AT. Applying for dermatology residency is difficult and expensive. J Am Acad Dermatol. 2012;66:696-697.
- Ward M, Pingree C, Laury AM, et al. Applicant perspectives on the otolaryngology residency application process. JAMA Otolaryngol Head Neck Surg. 2017;143:782-787.
- North DC. Institutions and credible commitment. J Inst Theor Econ. 1993;149:11-23.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed November 12, 2019.
- Charting Outcomes in the Match: Characteristics of U.S. Allopathic Seniors Who Matched to Their Preferred Specialty in the 2018 Main Residency Match. 2nd ed. Washington, DC: National Resident Matching Program; July 2018. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed November 11, 2019.
UC: Tofacitinib tied to modest, reversible lipid increases, infrequent CV events
according to results of an analysis including more than 1,000 patients.
Major adverse cardiac events (MACE) were “infrequent” following treatment, according to the authors of the analysis, with an incidence rate similar to what has been reported for tofacitinib in rheumatoid arthritis and for other agents in ulcerative colitis.
That said, the period of observation in the analysis is “relatively short,” and so may not provide an accurate risk estimate for MACE, noted the investigators, led by Bruce E. Sands, MD, of the division of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York.
“Longer-term studies, involving a larger number of patients, will be needed to further assess MACE risk in patients with ulcerative colitis,” Dr. Sands and coinvestigators wrote in their report on the observational analysis, which appears in Clinical Gastroenterology and Hepatology.
“It is noteworthy that no increase in MACE risk and no dose relationship with tofacitinib have been observed in a larger rheumatoid arthritis cohort with over 8.5 years of observation and more than 19,000 patient-years of collective exposure,” they continued.
The present analysis included 1,157 patients with ulcerative colitis who participated in 8-week phase 2 and 3 tofacitinib induction studies, a phase 3 maintenance study, and a long-term extension study that is ongoing.
Reversible and dose-dependent increases in both LDL cholesterol and HDL cholesterol were observed after 8 weeks of treatment with tofacitinib at the recommended induction dose of 10 mg twice daily, the investigators found.
Increases in LDL cholesterol, HDL cholesterol, and total cholesterol correlated with decreases in high-sensitivity C-reactive protein, suggesting any potential impact of lipid increases on cardiovascular events might be offset by reduced inflammation, according to the investigators.
“Previous studies in RA and inflammatory bowel disease have shown an inverse relationship between active inflammation and serum lipid profiles, suggesting that inflammation lowers lipid concentrations, and that treatment of the underlying inflammatory disease may, therefore, increase them,” Dr. Sands and colleagues wrote.
The lipid changes also correlated with increases in body mass index, possibly because of better nutrition, reduced protein loss, and less catabolism following tofacitinib treatment, along with the corticosteroid taper required in these studies of the drug, they added.
Lipid increases generally stayed elevated through 61 weeks of treatment, while in patients randomized to placebo after 8 weeks of tofacitinib treatment, lipid levels fell back toward baseline, which suggests a reversal of the increases after tofacitinib withdrawal, the investigators wrote.
A total of 4 MACEs were seen among the 1,157 patients in the analysis, for an incidence rate of 0.24 (95% confidence interval, 0.07-0.62), according to the report. Those events included an acute coronary syndrome, an MI, an aortic dissection, and a hemorrhagic stroke. All four occurred in tofacitinib-treated patients, though the investigators noted that the aortic dissection and hemorrhagic stroke are events typically associated with genetics or other nonlipid factors.
In any case, that MACE incidence rate was “similar” to infliximab (Remicade) for what has been observed in tumor necrosis factor antagonist treatment of ulcerative colitis within a U.S. claims database study. In that analysis, including patients treated with infliximab, golimumab, and adalimumab, the incidence rate was 0.51 (95% CI, 0.31-0.79), the investigators noted.
These findings, taken together, support recommendations in tofacitinib prescribing information that call for monitoring of lipid concentrations 4-8 weeks after treatment is started, according to Dr. Sands and coauthors.
Funding for the study came from Pfizer. The study authors disclosed potential conflicts of interest related to Pfizer, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, MedImmune (AstraZeneca), Millennium Pharmaceuticals, Prometheus Laboratories, Takeda, and 4D Pharma, among others.
SOURCE: Sands BE et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.059.
Tofacitinib has several well-described effects on lipid metabolism, increasing levels of LDL cholesterol, HDL cholesterol, and total cholesterol. The clinical consequences of these lipid changes remains uncertain in ulcerative colitis (UC), for which there may be an increased risk of cardiovascular events.
In this study, Sands and colleagues used the largest cohort to date to quantify the effect of tofacitinib-associated lipid profile changes, their association with inflammatory markers, and the risk of major adverse cardiovascular events (MACEs). Using pooled data from multiple controlled, open-label studies of tofacitinib in UC, the authors appreciated a significant association between the rise in HDL cholesterol, LDL cholesterol, and total cholesterol levels and declines in C-reactive protein. They noted only four MACEs, an incidence rate similar to that seen in prior anti–tumor necrosis factor trials, and no change in a commonly used risk score for cardiovascular events.
These results are an important initial step in quantifying the cardiovascular risk associated with tofacitinib, but should be interpreted with caution. A significant proportion of individuals evaluated were from induction studies, with only 8 weeks of exposure. Only one dose of tofacitinib was required for inclusion. The median age in the OCTAVE trials, which contributed the majority of the data for this cohort, was only 41 years, and the baseline cardiovascular risk was low. While these data and rheumatologic literature are reassuring, further research with longitudinal follow-up, the assessment of time-varying exposures, and stratification by baseline cardiovascular risk will be required to better understand the association between tofacitinib and MACEs.
Frank I. Scott, MD, MSCE, assistant professor of medicine, Crohn’s and Colitis Center, codirector of clinical research/DART, director of GI fellowship research, division of gastroenterology and hepatology, University of Colorado at Denver, Aurora. He has received research funding and consulting fees from Takeda, and Janssen, and consulting fees from Merck.
Tofacitinib has several well-described effects on lipid metabolism, increasing levels of LDL cholesterol, HDL cholesterol, and total cholesterol. The clinical consequences of these lipid changes remains uncertain in ulcerative colitis (UC), for which there may be an increased risk of cardiovascular events.
In this study, Sands and colleagues used the largest cohort to date to quantify the effect of tofacitinib-associated lipid profile changes, their association with inflammatory markers, and the risk of major adverse cardiovascular events (MACEs). Using pooled data from multiple controlled, open-label studies of tofacitinib in UC, the authors appreciated a significant association between the rise in HDL cholesterol, LDL cholesterol, and total cholesterol levels and declines in C-reactive protein. They noted only four MACEs, an incidence rate similar to that seen in prior anti–tumor necrosis factor trials, and no change in a commonly used risk score for cardiovascular events.
These results are an important initial step in quantifying the cardiovascular risk associated with tofacitinib, but should be interpreted with caution. A significant proportion of individuals evaluated were from induction studies, with only 8 weeks of exposure. Only one dose of tofacitinib was required for inclusion. The median age in the OCTAVE trials, which contributed the majority of the data for this cohort, was only 41 years, and the baseline cardiovascular risk was low. While these data and rheumatologic literature are reassuring, further research with longitudinal follow-up, the assessment of time-varying exposures, and stratification by baseline cardiovascular risk will be required to better understand the association between tofacitinib and MACEs.
Frank I. Scott, MD, MSCE, assistant professor of medicine, Crohn’s and Colitis Center, codirector of clinical research/DART, director of GI fellowship research, division of gastroenterology and hepatology, University of Colorado at Denver, Aurora. He has received research funding and consulting fees from Takeda, and Janssen, and consulting fees from Merck.
Tofacitinib has several well-described effects on lipid metabolism, increasing levels of LDL cholesterol, HDL cholesterol, and total cholesterol. The clinical consequences of these lipid changes remains uncertain in ulcerative colitis (UC), for which there may be an increased risk of cardiovascular events.
In this study, Sands and colleagues used the largest cohort to date to quantify the effect of tofacitinib-associated lipid profile changes, their association with inflammatory markers, and the risk of major adverse cardiovascular events (MACEs). Using pooled data from multiple controlled, open-label studies of tofacitinib in UC, the authors appreciated a significant association between the rise in HDL cholesterol, LDL cholesterol, and total cholesterol levels and declines in C-reactive protein. They noted only four MACEs, an incidence rate similar to that seen in prior anti–tumor necrosis factor trials, and no change in a commonly used risk score for cardiovascular events.
These results are an important initial step in quantifying the cardiovascular risk associated with tofacitinib, but should be interpreted with caution. A significant proportion of individuals evaluated were from induction studies, with only 8 weeks of exposure. Only one dose of tofacitinib was required for inclusion. The median age in the OCTAVE trials, which contributed the majority of the data for this cohort, was only 41 years, and the baseline cardiovascular risk was low. While these data and rheumatologic literature are reassuring, further research with longitudinal follow-up, the assessment of time-varying exposures, and stratification by baseline cardiovascular risk will be required to better understand the association between tofacitinib and MACEs.
Frank I. Scott, MD, MSCE, assistant professor of medicine, Crohn’s and Colitis Center, codirector of clinical research/DART, director of GI fellowship research, division of gastroenterology and hepatology, University of Colorado at Denver, Aurora. He has received research funding and consulting fees from Takeda, and Janssen, and consulting fees from Merck.
according to results of an analysis including more than 1,000 patients.
Major adverse cardiac events (MACE) were “infrequent” following treatment, according to the authors of the analysis, with an incidence rate similar to what has been reported for tofacitinib in rheumatoid arthritis and for other agents in ulcerative colitis.
That said, the period of observation in the analysis is “relatively short,” and so may not provide an accurate risk estimate for MACE, noted the investigators, led by Bruce E. Sands, MD, of the division of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York.
“Longer-term studies, involving a larger number of patients, will be needed to further assess MACE risk in patients with ulcerative colitis,” Dr. Sands and coinvestigators wrote in their report on the observational analysis, which appears in Clinical Gastroenterology and Hepatology.
“It is noteworthy that no increase in MACE risk and no dose relationship with tofacitinib have been observed in a larger rheumatoid arthritis cohort with over 8.5 years of observation and more than 19,000 patient-years of collective exposure,” they continued.
The present analysis included 1,157 patients with ulcerative colitis who participated in 8-week phase 2 and 3 tofacitinib induction studies, a phase 3 maintenance study, and a long-term extension study that is ongoing.
Reversible and dose-dependent increases in both LDL cholesterol and HDL cholesterol were observed after 8 weeks of treatment with tofacitinib at the recommended induction dose of 10 mg twice daily, the investigators found.
Increases in LDL cholesterol, HDL cholesterol, and total cholesterol correlated with decreases in high-sensitivity C-reactive protein, suggesting any potential impact of lipid increases on cardiovascular events might be offset by reduced inflammation, according to the investigators.
“Previous studies in RA and inflammatory bowel disease have shown an inverse relationship between active inflammation and serum lipid profiles, suggesting that inflammation lowers lipid concentrations, and that treatment of the underlying inflammatory disease may, therefore, increase them,” Dr. Sands and colleagues wrote.
The lipid changes also correlated with increases in body mass index, possibly because of better nutrition, reduced protein loss, and less catabolism following tofacitinib treatment, along with the corticosteroid taper required in these studies of the drug, they added.
Lipid increases generally stayed elevated through 61 weeks of treatment, while in patients randomized to placebo after 8 weeks of tofacitinib treatment, lipid levels fell back toward baseline, which suggests a reversal of the increases after tofacitinib withdrawal, the investigators wrote.
A total of 4 MACEs were seen among the 1,157 patients in the analysis, for an incidence rate of 0.24 (95% confidence interval, 0.07-0.62), according to the report. Those events included an acute coronary syndrome, an MI, an aortic dissection, and a hemorrhagic stroke. All four occurred in tofacitinib-treated patients, though the investigators noted that the aortic dissection and hemorrhagic stroke are events typically associated with genetics or other nonlipid factors.
In any case, that MACE incidence rate was “similar” to infliximab (Remicade) for what has been observed in tumor necrosis factor antagonist treatment of ulcerative colitis within a U.S. claims database study. In that analysis, including patients treated with infliximab, golimumab, and adalimumab, the incidence rate was 0.51 (95% CI, 0.31-0.79), the investigators noted.
These findings, taken together, support recommendations in tofacitinib prescribing information that call for monitoring of lipid concentrations 4-8 weeks after treatment is started, according to Dr. Sands and coauthors.
Funding for the study came from Pfizer. The study authors disclosed potential conflicts of interest related to Pfizer, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, MedImmune (AstraZeneca), Millennium Pharmaceuticals, Prometheus Laboratories, Takeda, and 4D Pharma, among others.
SOURCE: Sands BE et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.059.
according to results of an analysis including more than 1,000 patients.
Major adverse cardiac events (MACE) were “infrequent” following treatment, according to the authors of the analysis, with an incidence rate similar to what has been reported for tofacitinib in rheumatoid arthritis and for other agents in ulcerative colitis.
That said, the period of observation in the analysis is “relatively short,” and so may not provide an accurate risk estimate for MACE, noted the investigators, led by Bruce E. Sands, MD, of the division of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York.
“Longer-term studies, involving a larger number of patients, will be needed to further assess MACE risk in patients with ulcerative colitis,” Dr. Sands and coinvestigators wrote in their report on the observational analysis, which appears in Clinical Gastroenterology and Hepatology.
“It is noteworthy that no increase in MACE risk and no dose relationship with tofacitinib have been observed in a larger rheumatoid arthritis cohort with over 8.5 years of observation and more than 19,000 patient-years of collective exposure,” they continued.
The present analysis included 1,157 patients with ulcerative colitis who participated in 8-week phase 2 and 3 tofacitinib induction studies, a phase 3 maintenance study, and a long-term extension study that is ongoing.
Reversible and dose-dependent increases in both LDL cholesterol and HDL cholesterol were observed after 8 weeks of treatment with tofacitinib at the recommended induction dose of 10 mg twice daily, the investigators found.
Increases in LDL cholesterol, HDL cholesterol, and total cholesterol correlated with decreases in high-sensitivity C-reactive protein, suggesting any potential impact of lipid increases on cardiovascular events might be offset by reduced inflammation, according to the investigators.
“Previous studies in RA and inflammatory bowel disease have shown an inverse relationship between active inflammation and serum lipid profiles, suggesting that inflammation lowers lipid concentrations, and that treatment of the underlying inflammatory disease may, therefore, increase them,” Dr. Sands and colleagues wrote.
The lipid changes also correlated with increases in body mass index, possibly because of better nutrition, reduced protein loss, and less catabolism following tofacitinib treatment, along with the corticosteroid taper required in these studies of the drug, they added.
Lipid increases generally stayed elevated through 61 weeks of treatment, while in patients randomized to placebo after 8 weeks of tofacitinib treatment, lipid levels fell back toward baseline, which suggests a reversal of the increases after tofacitinib withdrawal, the investigators wrote.
A total of 4 MACEs were seen among the 1,157 patients in the analysis, for an incidence rate of 0.24 (95% confidence interval, 0.07-0.62), according to the report. Those events included an acute coronary syndrome, an MI, an aortic dissection, and a hemorrhagic stroke. All four occurred in tofacitinib-treated patients, though the investigators noted that the aortic dissection and hemorrhagic stroke are events typically associated with genetics or other nonlipid factors.
In any case, that MACE incidence rate was “similar” to infliximab (Remicade) for what has been observed in tumor necrosis factor antagonist treatment of ulcerative colitis within a U.S. claims database study. In that analysis, including patients treated with infliximab, golimumab, and adalimumab, the incidence rate was 0.51 (95% CI, 0.31-0.79), the investigators noted.
These findings, taken together, support recommendations in tofacitinib prescribing information that call for monitoring of lipid concentrations 4-8 weeks after treatment is started, according to Dr. Sands and coauthors.
Funding for the study came from Pfizer. The study authors disclosed potential conflicts of interest related to Pfizer, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, MedImmune (AstraZeneca), Millennium Pharmaceuticals, Prometheus Laboratories, Takeda, and 4D Pharma, among others.
SOURCE: Sands BE et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.059.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
FDA approves infliximab-axxq for numerous indications
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
The Food and Drug Administration has approved the biosimilar infliximab-axxq (Avsola) for various indications, making it the fourth biosimilar of infliximab (Remicade) to be cleared for marketing by the agency.
The tumor necrosis factor inhibitor is indicated for patients with Crohn’s disease or ulcerative colitis who are aged 6 years and older, RA in combination with methotrexate, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The approval is based on numerous trials. The most common adverse reactions are infections, infusion-related reactions, headache, and abdominal pain.
Full prescribing information can be found on the FDA website, as can more information about biosimilars.
US rates of preterm birth
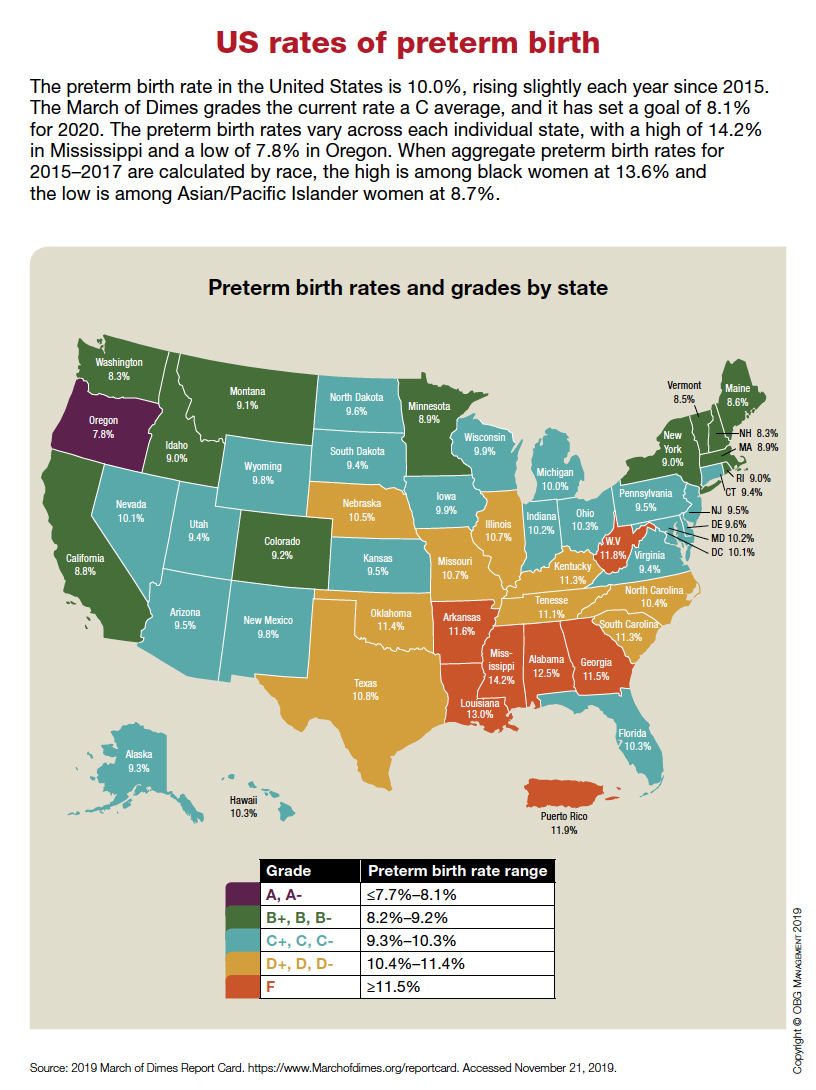


No clear-cut evidence of vedolizumab effect in retrospective study of primary sclerosing cholangitis
While initial case reports and series provided preliminarily encouraging results, a larger retrospective study has provided no clear-cut evidence of biochemical response to vedolizumab in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease, investigators report.
A subset of patients in the retrospective analysis did experience a substantial drop in alkaline phosphatase (ALP), according to investigators with the International Primary Sclerosing Cholangitis Study Group.
Overall, however, levels of that cholestasis marker rose by a small but statistically significant amount in this study, which included more than 100 patients with PSC and inflammatory bowel disease (IBD).
Responses were more likely in patients with cirrhosis and in those with elevated ALP at baseline, both of which are indicators of more aggressive disease, according to investigator Kate D. Lynch, MD, PhD, of the University of Oxford (England) and her coauthors.
The rate of liver outcomes was in line with the natural history of the disease, according to Dr. Lynch and coinvestigators, who added that most patients had an endoscopic IBD response, as might be expected based on studies of IBD-only patients treated with vedolizumab.
“Despite the disappointment with lack of a uniform response, further evaluation of vedolizumab as a beneficial treatment in PSC may be warranted in a subset of patients via a stratified randomized clinical trial,” Dr. Lynch and coauthors said in their report, which was published in Clinical Gastroenterology and Hepatology.
Vedolizumab, a monoclonal antibody against integrin alpha4beta7, is effective in Crohn’s disease and ulcerative colitis, according to investigators, who added that the “gut-homing pathway” it targets has also been implicated in the pathophysiology of PSC.
“It is possible that vedolizumab may play a role in reducing lymphocyte infiltration into the liver in patients with PSC and thereby in reducing hepatic and biliary inflammation, authors of the retrospective analysis said.
Their analysis included 102 patients with PSC and IBD at 20 centers in Europe and North America. All patients had received at least three doses of vedolizumab for their IBD, given according to the usual dosing schedule. Most of the patients were male (64 patients, or 62.8%) and about 90% had classical large-duct PSC. About one-fifth had cirrhosis, and the majority (about 65%) had ulcerative colitis. Patients were followed until death, liver transplant, or 56 days after the last vedolizumab dose.
The median ALP increased from 1.53 times the upper limit of normal at baseline to 1.64 times the upper limit of normal by the last follow-up, an increase that was statistically significant (P = .018) but not clinically significant, according to investigators. Likewise, they said, statistically significant increases were seen overall in median alanine transaminase and aspartate aminotransferase levels.
However, 21 patients (20.6%) had a drop in ALP of at least 20% from baseline to last follow-up, and another 39 patients (38.2%) had stable ALP over that period, data show, while the remaining 42 (41.2%) had an increase of 20% or more.
Cirrhosis was associated with a near fivefold odds of a 20% or greater ALP drop from baseline to follow-up (odds ratio, 4.70, 95% confidence interval, 1.61-13.76), according to results of univariate analysis, which investigators said were “reproduced” in multivariate analysis.
While no other variables were so clearly linked to a 20% or greater drop in ALP, Dr. Lynch and colleagues said there was a “trend toward an association” in patients with ALP raised at baseline, and in those who had Crohn’s disease or IBD-unspecified instead of ulcerative colitis.
Endoscopic IBD responses were seen in 42 out of 74 patients (56.8%) for whom those data were available, investigators added.
A total of 22 patients (20.9%) had a liver-related outcome over median follow-up of 561 days; however, that outcome may be “slightly overrepresented” by an incidence of cholangitis in 8.8%, which in and of itself is not necessarily an indicator of advanced liver disease, said Dr. Lynch and coauthors in their report.
“This proportion of liver-related outcomes is consistent with the natural history of PSC and does not by itself indicate that vedolizumab treatment is harmful in PSC,” they said, adding that the findings were similar to a study of simtuzumab, a monoclonal antibody directed against lysyl oxidase-like 2, in patients with PSC, of whom 20.1% had a PSC-related event and the incidence of cholangitis was 13.2%.
The retrospective study was supported by the Birmingham National Institute for Health Research (NIHR) Biomedical Research Centre in the United Kingdom. Authors of the report provided disclosures related to Takeda, AbbVie, Dr. Falk Pharma, Intercept, MSD, Janssen, Vifor, Gilead, and Novartis, among others.
SOURCE: Lynch KD et al. Clin Gastroenterol Hepatol. 2019 May 14. doi: 10.1016/j.cgh.2019.05.013.
While initial case reports and series provided preliminarily encouraging results, a larger retrospective study has provided no clear-cut evidence of biochemical response to vedolizumab in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease, investigators report.
A subset of patients in the retrospective analysis did experience a substantial drop in alkaline phosphatase (ALP), according to investigators with the International Primary Sclerosing Cholangitis Study Group.
Overall, however, levels of that cholestasis marker rose by a small but statistically significant amount in this study, which included more than 100 patients with PSC and inflammatory bowel disease (IBD).
Responses were more likely in patients with cirrhosis and in those with elevated ALP at baseline, both of which are indicators of more aggressive disease, according to investigator Kate D. Lynch, MD, PhD, of the University of Oxford (England) and her coauthors.
The rate of liver outcomes was in line with the natural history of the disease, according to Dr. Lynch and coinvestigators, who added that most patients had an endoscopic IBD response, as might be expected based on studies of IBD-only patients treated with vedolizumab.
“Despite the disappointment with lack of a uniform response, further evaluation of vedolizumab as a beneficial treatment in PSC may be warranted in a subset of patients via a stratified randomized clinical trial,” Dr. Lynch and coauthors said in their report, which was published in Clinical Gastroenterology and Hepatology.
Vedolizumab, a monoclonal antibody against integrin alpha4beta7, is effective in Crohn’s disease and ulcerative colitis, according to investigators, who added that the “gut-homing pathway” it targets has also been implicated in the pathophysiology of PSC.
“It is possible that vedolizumab may play a role in reducing lymphocyte infiltration into the liver in patients with PSC and thereby in reducing hepatic and biliary inflammation, authors of the retrospective analysis said.
Their analysis included 102 patients with PSC and IBD at 20 centers in Europe and North America. All patients had received at least three doses of vedolizumab for their IBD, given according to the usual dosing schedule. Most of the patients were male (64 patients, or 62.8%) and about 90% had classical large-duct PSC. About one-fifth had cirrhosis, and the majority (about 65%) had ulcerative colitis. Patients were followed until death, liver transplant, or 56 days after the last vedolizumab dose.
The median ALP increased from 1.53 times the upper limit of normal at baseline to 1.64 times the upper limit of normal by the last follow-up, an increase that was statistically significant (P = .018) but not clinically significant, according to investigators. Likewise, they said, statistically significant increases were seen overall in median alanine transaminase and aspartate aminotransferase levels.
However, 21 patients (20.6%) had a drop in ALP of at least 20% from baseline to last follow-up, and another 39 patients (38.2%) had stable ALP over that period, data show, while the remaining 42 (41.2%) had an increase of 20% or more.
Cirrhosis was associated with a near fivefold odds of a 20% or greater ALP drop from baseline to follow-up (odds ratio, 4.70, 95% confidence interval, 1.61-13.76), according to results of univariate analysis, which investigators said were “reproduced” in multivariate analysis.
While no other variables were so clearly linked to a 20% or greater drop in ALP, Dr. Lynch and colleagues said there was a “trend toward an association” in patients with ALP raised at baseline, and in those who had Crohn’s disease or IBD-unspecified instead of ulcerative colitis.
Endoscopic IBD responses were seen in 42 out of 74 patients (56.8%) for whom those data were available, investigators added.
A total of 22 patients (20.9%) had a liver-related outcome over median follow-up of 561 days; however, that outcome may be “slightly overrepresented” by an incidence of cholangitis in 8.8%, which in and of itself is not necessarily an indicator of advanced liver disease, said Dr. Lynch and coauthors in their report.
“This proportion of liver-related outcomes is consistent with the natural history of PSC and does not by itself indicate that vedolizumab treatment is harmful in PSC,” they said, adding that the findings were similar to a study of simtuzumab, a monoclonal antibody directed against lysyl oxidase-like 2, in patients with PSC, of whom 20.1% had a PSC-related event and the incidence of cholangitis was 13.2%.
The retrospective study was supported by the Birmingham National Institute for Health Research (NIHR) Biomedical Research Centre in the United Kingdom. Authors of the report provided disclosures related to Takeda, AbbVie, Dr. Falk Pharma, Intercept, MSD, Janssen, Vifor, Gilead, and Novartis, among others.
SOURCE: Lynch KD et al. Clin Gastroenterol Hepatol. 2019 May 14. doi: 10.1016/j.cgh.2019.05.013.
While initial case reports and series provided preliminarily encouraging results, a larger retrospective study has provided no clear-cut evidence of biochemical response to vedolizumab in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease, investigators report.
A subset of patients in the retrospective analysis did experience a substantial drop in alkaline phosphatase (ALP), according to investigators with the International Primary Sclerosing Cholangitis Study Group.
Overall, however, levels of that cholestasis marker rose by a small but statistically significant amount in this study, which included more than 100 patients with PSC and inflammatory bowel disease (IBD).
Responses were more likely in patients with cirrhosis and in those with elevated ALP at baseline, both of which are indicators of more aggressive disease, according to investigator Kate D. Lynch, MD, PhD, of the University of Oxford (England) and her coauthors.
The rate of liver outcomes was in line with the natural history of the disease, according to Dr. Lynch and coinvestigators, who added that most patients had an endoscopic IBD response, as might be expected based on studies of IBD-only patients treated with vedolizumab.
“Despite the disappointment with lack of a uniform response, further evaluation of vedolizumab as a beneficial treatment in PSC may be warranted in a subset of patients via a stratified randomized clinical trial,” Dr. Lynch and coauthors said in their report, which was published in Clinical Gastroenterology and Hepatology.
Vedolizumab, a monoclonal antibody against integrin alpha4beta7, is effective in Crohn’s disease and ulcerative colitis, according to investigators, who added that the “gut-homing pathway” it targets has also been implicated in the pathophysiology of PSC.
“It is possible that vedolizumab may play a role in reducing lymphocyte infiltration into the liver in patients with PSC and thereby in reducing hepatic and biliary inflammation, authors of the retrospective analysis said.
Their analysis included 102 patients with PSC and IBD at 20 centers in Europe and North America. All patients had received at least three doses of vedolizumab for their IBD, given according to the usual dosing schedule. Most of the patients were male (64 patients, or 62.8%) and about 90% had classical large-duct PSC. About one-fifth had cirrhosis, and the majority (about 65%) had ulcerative colitis. Patients were followed until death, liver transplant, or 56 days after the last vedolizumab dose.
The median ALP increased from 1.53 times the upper limit of normal at baseline to 1.64 times the upper limit of normal by the last follow-up, an increase that was statistically significant (P = .018) but not clinically significant, according to investigators. Likewise, they said, statistically significant increases were seen overall in median alanine transaminase and aspartate aminotransferase levels.
However, 21 patients (20.6%) had a drop in ALP of at least 20% from baseline to last follow-up, and another 39 patients (38.2%) had stable ALP over that period, data show, while the remaining 42 (41.2%) had an increase of 20% or more.
Cirrhosis was associated with a near fivefold odds of a 20% or greater ALP drop from baseline to follow-up (odds ratio, 4.70, 95% confidence interval, 1.61-13.76), according to results of univariate analysis, which investigators said were “reproduced” in multivariate analysis.
While no other variables were so clearly linked to a 20% or greater drop in ALP, Dr. Lynch and colleagues said there was a “trend toward an association” in patients with ALP raised at baseline, and in those who had Crohn’s disease or IBD-unspecified instead of ulcerative colitis.
Endoscopic IBD responses were seen in 42 out of 74 patients (56.8%) for whom those data were available, investigators added.
A total of 22 patients (20.9%) had a liver-related outcome over median follow-up of 561 days; however, that outcome may be “slightly overrepresented” by an incidence of cholangitis in 8.8%, which in and of itself is not necessarily an indicator of advanced liver disease, said Dr. Lynch and coauthors in their report.
“This proportion of liver-related outcomes is consistent with the natural history of PSC and does not by itself indicate that vedolizumab treatment is harmful in PSC,” they said, adding that the findings were similar to a study of simtuzumab, a monoclonal antibody directed against lysyl oxidase-like 2, in patients with PSC, of whom 20.1% had a PSC-related event and the incidence of cholangitis was 13.2%.
The retrospective study was supported by the Birmingham National Institute for Health Research (NIHR) Biomedical Research Centre in the United Kingdom. Authors of the report provided disclosures related to Takeda, AbbVie, Dr. Falk Pharma, Intercept, MSD, Janssen, Vifor, Gilead, and Novartis, among others.
SOURCE: Lynch KD et al. Clin Gastroenterol Hepatol. 2019 May 14. doi: 10.1016/j.cgh.2019.05.013.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY