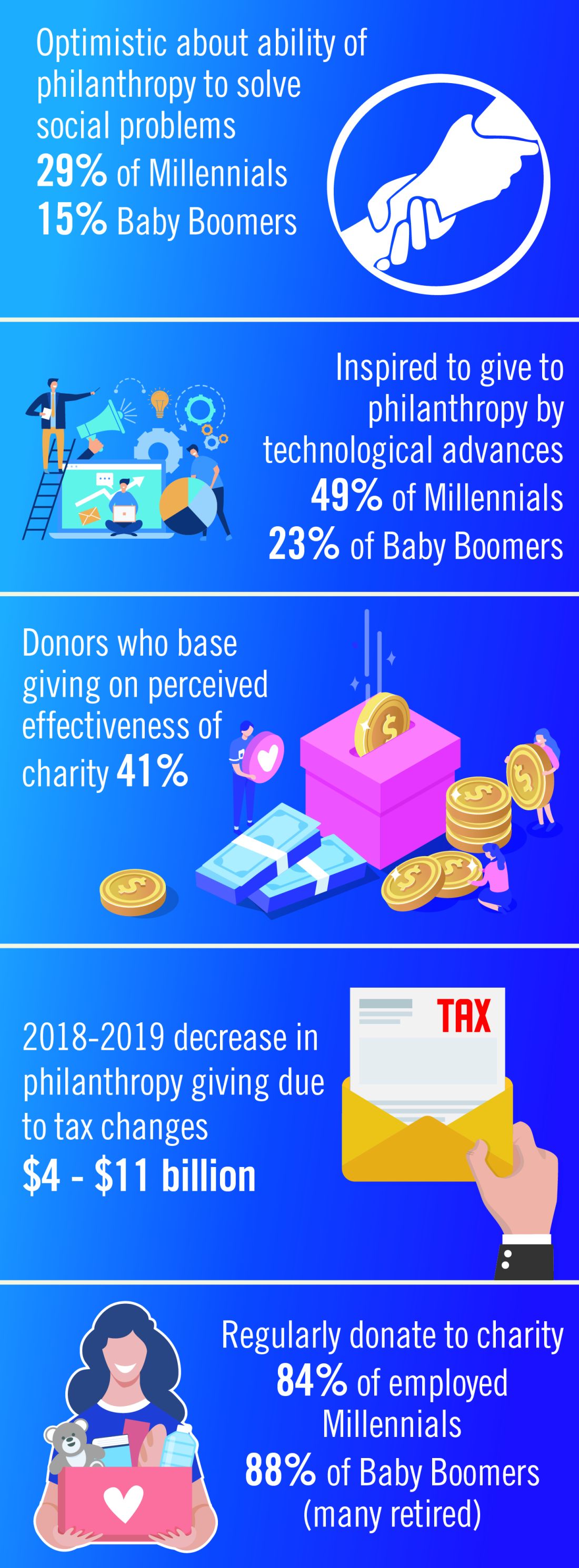
User login
SVS Members: You Have Options When It Comes to Disability Insurance
The Affinity Program of expanded benefits gives SVS members more options for disability insurance. Many members are part of a group plan in which the employer or group pays the premium. These plans may meet the needs of members, but sometimes they may not. With the options offered through the Affinity Program, members could avoid offsets that reduce tax-free benefits or ones that limit the time period in which they are covered. The program can connect interested members with three companies: Principal Life Insurance Company, Securian and Lloyd’s of London. These group plans are unlike many others, providing tax-free benefits that could potentially protect hundreds of thousands of dollars. Click here for details.
The Affinity Program of expanded benefits gives SVS members more options for disability insurance. Many members are part of a group plan in which the employer or group pays the premium. These plans may meet the needs of members, but sometimes they may not. With the options offered through the Affinity Program, members could avoid offsets that reduce tax-free benefits or ones that limit the time period in which they are covered. The program can connect interested members with three companies: Principal Life Insurance Company, Securian and Lloyd’s of London. These group plans are unlike many others, providing tax-free benefits that could potentially protect hundreds of thousands of dollars. Click here for details.
The Affinity Program of expanded benefits gives SVS members more options for disability insurance. Many members are part of a group plan in which the employer or group pays the premium. These plans may meet the needs of members, but sometimes they may not. With the options offered through the Affinity Program, members could avoid offsets that reduce tax-free benefits or ones that limit the time period in which they are covered. The program can connect interested members with three companies: Principal Life Insurance Company, Securian and Lloyd’s of London. These group plans are unlike many others, providing tax-free benefits that could potentially protect hundreds of thousands of dollars. Click here for details.
Study halted; ‘hyperprogression’ seen with nivolumab for R/R PTCL
ORLANDO – There is an urgent need for new therapies to treat relapsed or refractory peripheral T-cell lymphoma, but results of a phase 2 study suggest that monotherapy with the immune checkpoint inhibitor nivolumab (Opdivo) is not the hoped-for salvage treatment.
An interim analysis of data on 12 patients with peripheral T-cell lymphoma (PTCL) treated with nivolumab monotherapy showed an overall response rate of 33%, consisting of 2 complete responses and 2 partial responses. But the responses were short lived, and one patient had hyperprogressive disease – dramatic progression within one cycle of treatment – while two more had progression within two cycles, leading to a trial halt, reported N. Nora Bennani, MD, from the Mayo Clinic in Rochester, Minn.
“These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases,” she said at the annual meeting of the American Society of Hematology.
The rationale for using an immune checkpoint inhibitor directed against the programmed death–1 protein and its ligands (PD and PD-L1/2) is that malignant cells in PTCL induce a profoundly immunosuppressive tumor microenvironment. Checkpoint inhibitors have shown strong activity against relapsed Hodgkin lymphoma, and the Mayo Clinic researchers speculated that an anti-PD-1 agent could have a similar effect in PTCL.
They had originally planned to enroll 29 patients into a phase 2 trial with nivolumab delivered 240 mg every 2 weeks for eight cycles, followed by a dose of 480 mg given every 4 weeks until disease progression or intolerable toxicities.
Patients were eligible if they had biopsy-confirmed relapsed or refractory PTCL, measurable disease on cross-sectional imaging of at least 1.5 cm, and prior systemic chemoimmunotherapy and/or autologous stem cell transplantation.
The interim analysis included 12 patients who received at least one dose of nivolumab. Of the 12 patients, 6 had angioimmunoblastic T-cell lymphoma (AITL), 3 had PTCL not otherwise specified, and 1 each had ALK-negative anaplastic large cell lymphoma (ALK-ALCL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic gamma/delta T-cell lymphoma.
All patients had Ann Arbor stage III/IV disease, and 11 had extranodal involvement.
As noted, there were 4 responses among the 12 patients, consisting of 1 complete response in the patient with ALK-ALCL and 1 in a patient with AITL, and 2 partial responses – 1 in a patient with PTCL-NOS, and 1 in the patient with EATL.
The median progression-free survival for all 12 patients was short at 2.7 months, and the median overall survival was estimated at 6.7 months.
“It was staggering to see this: The duration of response was significantly short, less than 2 months,” Dr. Bennani said.
Nonhematologic toxicities were seen in 5 of the 12 patients (42%), and hematologic adverse events occurred in 3 (25%). All patients are now off treatment, 10 because of disease progression, 1 because of acute pancreatitis, and the aforementioned patient with hyperprogressive disease.
The patient with hyperprogressive disease had significant progression in tonsillar and cervical lymphadenopathy within 7-10 days of nivolumab infusion, with biopsy-proven AITL in the involved nodes.
“I believe that, in this patient population, combination therapies will be key. I think checkpoint blockers alone are not going to be sufficient to see meaningful outcomes in these patients,” Dr. Bennani said in an interview.
“An overall response rate of 33% is significant, because most other agents that were FDA approved in this patient population have response rates around 30%,” she said, adding that it’s possible that the patients with rapid progression had disease too advanced to be effectively treated with a checkpoint inhibitor.
“Ideally however, if we want to move forward, it will need to be with combinations of checkpoint inhibitors with HDAC [histone deacetylase] inhibitors, hypomethylating agents, or even PI3 kinase inhibitors,” she said.
The study was supported by Bristol-Myers Squibb. Dr. Bennani reported research funding and advisory board activities for Bristol-Myers Squibb and others.
SOURCE: Bennani NN et al. ASH 2019, Abstract 467.
ORLANDO – There is an urgent need for new therapies to treat relapsed or refractory peripheral T-cell lymphoma, but results of a phase 2 study suggest that monotherapy with the immune checkpoint inhibitor nivolumab (Opdivo) is not the hoped-for salvage treatment.
An interim analysis of data on 12 patients with peripheral T-cell lymphoma (PTCL) treated with nivolumab monotherapy showed an overall response rate of 33%, consisting of 2 complete responses and 2 partial responses. But the responses were short lived, and one patient had hyperprogressive disease – dramatic progression within one cycle of treatment – while two more had progression within two cycles, leading to a trial halt, reported N. Nora Bennani, MD, from the Mayo Clinic in Rochester, Minn.
“These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases,” she said at the annual meeting of the American Society of Hematology.
The rationale for using an immune checkpoint inhibitor directed against the programmed death–1 protein and its ligands (PD and PD-L1/2) is that malignant cells in PTCL induce a profoundly immunosuppressive tumor microenvironment. Checkpoint inhibitors have shown strong activity against relapsed Hodgkin lymphoma, and the Mayo Clinic researchers speculated that an anti-PD-1 agent could have a similar effect in PTCL.
They had originally planned to enroll 29 patients into a phase 2 trial with nivolumab delivered 240 mg every 2 weeks for eight cycles, followed by a dose of 480 mg given every 4 weeks until disease progression or intolerable toxicities.
Patients were eligible if they had biopsy-confirmed relapsed or refractory PTCL, measurable disease on cross-sectional imaging of at least 1.5 cm, and prior systemic chemoimmunotherapy and/or autologous stem cell transplantation.
The interim analysis included 12 patients who received at least one dose of nivolumab. Of the 12 patients, 6 had angioimmunoblastic T-cell lymphoma (AITL), 3 had PTCL not otherwise specified, and 1 each had ALK-negative anaplastic large cell lymphoma (ALK-ALCL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic gamma/delta T-cell lymphoma.
All patients had Ann Arbor stage III/IV disease, and 11 had extranodal involvement.
As noted, there were 4 responses among the 12 patients, consisting of 1 complete response in the patient with ALK-ALCL and 1 in a patient with AITL, and 2 partial responses – 1 in a patient with PTCL-NOS, and 1 in the patient with EATL.
The median progression-free survival for all 12 patients was short at 2.7 months, and the median overall survival was estimated at 6.7 months.
“It was staggering to see this: The duration of response was significantly short, less than 2 months,” Dr. Bennani said.
Nonhematologic toxicities were seen in 5 of the 12 patients (42%), and hematologic adverse events occurred in 3 (25%). All patients are now off treatment, 10 because of disease progression, 1 because of acute pancreatitis, and the aforementioned patient with hyperprogressive disease.
The patient with hyperprogressive disease had significant progression in tonsillar and cervical lymphadenopathy within 7-10 days of nivolumab infusion, with biopsy-proven AITL in the involved nodes.
“I believe that, in this patient population, combination therapies will be key. I think checkpoint blockers alone are not going to be sufficient to see meaningful outcomes in these patients,” Dr. Bennani said in an interview.
“An overall response rate of 33% is significant, because most other agents that were FDA approved in this patient population have response rates around 30%,” she said, adding that it’s possible that the patients with rapid progression had disease too advanced to be effectively treated with a checkpoint inhibitor.
“Ideally however, if we want to move forward, it will need to be with combinations of checkpoint inhibitors with HDAC [histone deacetylase] inhibitors, hypomethylating agents, or even PI3 kinase inhibitors,” she said.
The study was supported by Bristol-Myers Squibb. Dr. Bennani reported research funding and advisory board activities for Bristol-Myers Squibb and others.
SOURCE: Bennani NN et al. ASH 2019, Abstract 467.
ORLANDO – There is an urgent need for new therapies to treat relapsed or refractory peripheral T-cell lymphoma, but results of a phase 2 study suggest that monotherapy with the immune checkpoint inhibitor nivolumab (Opdivo) is not the hoped-for salvage treatment.
An interim analysis of data on 12 patients with peripheral T-cell lymphoma (PTCL) treated with nivolumab monotherapy showed an overall response rate of 33%, consisting of 2 complete responses and 2 partial responses. But the responses were short lived, and one patient had hyperprogressive disease – dramatic progression within one cycle of treatment – while two more had progression within two cycles, leading to a trial halt, reported N. Nora Bennani, MD, from the Mayo Clinic in Rochester, Minn.
“These findings likely reflect the distinct biology of PTCL and should be considered when designing future studies using checkpoint inhibitors in these diseases,” she said at the annual meeting of the American Society of Hematology.
The rationale for using an immune checkpoint inhibitor directed against the programmed death–1 protein and its ligands (PD and PD-L1/2) is that malignant cells in PTCL induce a profoundly immunosuppressive tumor microenvironment. Checkpoint inhibitors have shown strong activity against relapsed Hodgkin lymphoma, and the Mayo Clinic researchers speculated that an anti-PD-1 agent could have a similar effect in PTCL.
They had originally planned to enroll 29 patients into a phase 2 trial with nivolumab delivered 240 mg every 2 weeks for eight cycles, followed by a dose of 480 mg given every 4 weeks until disease progression or intolerable toxicities.
Patients were eligible if they had biopsy-confirmed relapsed or refractory PTCL, measurable disease on cross-sectional imaging of at least 1.5 cm, and prior systemic chemoimmunotherapy and/or autologous stem cell transplantation.
The interim analysis included 12 patients who received at least one dose of nivolumab. Of the 12 patients, 6 had angioimmunoblastic T-cell lymphoma (AITL), 3 had PTCL not otherwise specified, and 1 each had ALK-negative anaplastic large cell lymphoma (ALK-ALCL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic gamma/delta T-cell lymphoma.
All patients had Ann Arbor stage III/IV disease, and 11 had extranodal involvement.
As noted, there were 4 responses among the 12 patients, consisting of 1 complete response in the patient with ALK-ALCL and 1 in a patient with AITL, and 2 partial responses – 1 in a patient with PTCL-NOS, and 1 in the patient with EATL.
The median progression-free survival for all 12 patients was short at 2.7 months, and the median overall survival was estimated at 6.7 months.
“It was staggering to see this: The duration of response was significantly short, less than 2 months,” Dr. Bennani said.
Nonhematologic toxicities were seen in 5 of the 12 patients (42%), and hematologic adverse events occurred in 3 (25%). All patients are now off treatment, 10 because of disease progression, 1 because of acute pancreatitis, and the aforementioned patient with hyperprogressive disease.
The patient with hyperprogressive disease had significant progression in tonsillar and cervical lymphadenopathy within 7-10 days of nivolumab infusion, with biopsy-proven AITL in the involved nodes.
“I believe that, in this patient population, combination therapies will be key. I think checkpoint blockers alone are not going to be sufficient to see meaningful outcomes in these patients,” Dr. Bennani said in an interview.
“An overall response rate of 33% is significant, because most other agents that were FDA approved in this patient population have response rates around 30%,” she said, adding that it’s possible that the patients with rapid progression had disease too advanced to be effectively treated with a checkpoint inhibitor.
“Ideally however, if we want to move forward, it will need to be with combinations of checkpoint inhibitors with HDAC [histone deacetylase] inhibitors, hypomethylating agents, or even PI3 kinase inhibitors,” she said.
The study was supported by Bristol-Myers Squibb. Dr. Bennani reported research funding and advisory board activities for Bristol-Myers Squibb and others.
SOURCE: Bennani NN et al. ASH 2019, Abstract 467.
REPORTING FROM ASH 2019
NAMDRC legislative initiatives take shape
Two priorities of NAMDRC have moved into the formal congressional arena. The issues focus on access to pulmonary rehabilitation and CMS’s move to include home mechanical ventilation in competitive bidding.
Pulmonary Rehabilitation – The Problem: One of the major concerns to CMS and Congress is the fact that different payment methodologies for the same service result in different payment amounts dependent upon the actual site of service. To address the phenomenon of hospitals purchasing certain physician practices to game the payment system, Congress included in the 2015 Budget Act a provision that would remove incentives for such hospital purchases by stating that new hospital outpatient services must be within 250 yards of the main hospital campus in order to receive payment based on the hospital outpatient prospective payment system methodology. If a hospital opens such services beyond that 250-yard threshold, the hospital would be reimbursed at the physician fee schedule amount for the same service. Likewise, if an off campus program moved its grandfathered location because of expansion, loss of lease, etc, the physician fee schedule would again kick in.
For pulmonary rehabilitation services, this is extremely problematic and is tying the hands of hospitals providing this service. The physician fee schedule payment for pulmonary rehabilitation is less than $30 for 1 hour of service, and it is, therefore, not surprising that the service is simply not provided in physician offices. In fact, Medicare data show that all physician specialties bill less than $1M for code G0424, and we believe that most of that is likely billing error. Pulmonologists bill less than $500K for code G0424, and putting that number in context, the entire Medicare program is approaching $700B in outlays.
Pulmonary Rehabilitation – The Solution: As a solution to this problem, HR 4838 has been introduced in the House of Representatives. There is no specific reference to pulmonary rehabilitation in the bill as our approach is based not only on substance but political considerations, as well. Using CMS’ own acknowledgment of “unintended consequences,” this legislation would exempt all CPT codes from the restrictions imposed by Section 603 of the 2015 Budget Act when the physician billings for that code are under $2M for the most recent year for which data are available. CMS has signaled to us that such a limitation would apply only to pulmonary and cardiac rehab services, but others may be affected, as well. By putting a dollar limit rather than identifying a specific service for such a “carve out,” it is a more politically viable approach.
Bills such as this rarely see the light of day; however, such provisions are often attached to larger, more substantive bills. For nearly 2 decades, the common legislative vehicle for such provisions is a larger Medicare bill, often including “must pass Medicare extender” provisions that are slated to expire on a particular date. Our goal is to include HR 4838 in such a package of extenders some time between now and the end of this Congress in 2020.
Home Mechanical Ventilation – The Problem: CMS has proposed inclusion of home mechanical ventilation in competitive bidding for durable medical equipment. Such a regulatory proposal is fraught with downside risk, most notably that such a policy would follow the history of liquid oxygen. Liquid 02 has virtually disappeared from the marketplace since it was included in competitive bidding as suppliers simply refused to provide liquid oxygen systems as their own bidding dropped the price to prohibitively low levels. Also, because there is a statutory requirement that such payment be made on the basis of “frequent and substantial servicing,” and that stipulation could trigger wide variations in actual bidding because some states require involvement of respiratory therapists in such services, while others do not.
It is critical to understand that the driving force behind all of this is the reality that CMS’ own coverage policies for home mechanical ventilation are seriously flawed and outdated, creating perverse incentives for physicians to order easily accessible systems rather than clinically appropriate ones. NAMDRC and its sister societies have been pushing CMS to revise those policies with no success.
Home Mechanical Ventilation – The Solution: Our solution is twofold. HR 4945 bill was introduced on November 1, 2019. First, the proposed legislation would create a blanket exemption for home mechanical ventilation from competitive bidding. Second, it requires CMS to convene a technical expert panel to craft up-to-date policies for home mechanical ventilation.
The political strategy here is slightly different. While passage of the bill is certainly our first choice, we believe that introduction of the bill is a red flag signal to CMS for the need to revise its coverage policies as those policies are the root cause of the growth of home mechanical ventilation outlays.
Two priorities of NAMDRC have moved into the formal congressional arena. The issues focus on access to pulmonary rehabilitation and CMS’s move to include home mechanical ventilation in competitive bidding.
Pulmonary Rehabilitation – The Problem: One of the major concerns to CMS and Congress is the fact that different payment methodologies for the same service result in different payment amounts dependent upon the actual site of service. To address the phenomenon of hospitals purchasing certain physician practices to game the payment system, Congress included in the 2015 Budget Act a provision that would remove incentives for such hospital purchases by stating that new hospital outpatient services must be within 250 yards of the main hospital campus in order to receive payment based on the hospital outpatient prospective payment system methodology. If a hospital opens such services beyond that 250-yard threshold, the hospital would be reimbursed at the physician fee schedule amount for the same service. Likewise, if an off campus program moved its grandfathered location because of expansion, loss of lease, etc, the physician fee schedule would again kick in.
For pulmonary rehabilitation services, this is extremely problematic and is tying the hands of hospitals providing this service. The physician fee schedule payment for pulmonary rehabilitation is less than $30 for 1 hour of service, and it is, therefore, not surprising that the service is simply not provided in physician offices. In fact, Medicare data show that all physician specialties bill less than $1M for code G0424, and we believe that most of that is likely billing error. Pulmonologists bill less than $500K for code G0424, and putting that number in context, the entire Medicare program is approaching $700B in outlays.
Pulmonary Rehabilitation – The Solution: As a solution to this problem, HR 4838 has been introduced in the House of Representatives. There is no specific reference to pulmonary rehabilitation in the bill as our approach is based not only on substance but political considerations, as well. Using CMS’ own acknowledgment of “unintended consequences,” this legislation would exempt all CPT codes from the restrictions imposed by Section 603 of the 2015 Budget Act when the physician billings for that code are under $2M for the most recent year for which data are available. CMS has signaled to us that such a limitation would apply only to pulmonary and cardiac rehab services, but others may be affected, as well. By putting a dollar limit rather than identifying a specific service for such a “carve out,” it is a more politically viable approach.
Bills such as this rarely see the light of day; however, such provisions are often attached to larger, more substantive bills. For nearly 2 decades, the common legislative vehicle for such provisions is a larger Medicare bill, often including “must pass Medicare extender” provisions that are slated to expire on a particular date. Our goal is to include HR 4838 in such a package of extenders some time between now and the end of this Congress in 2020.
Home Mechanical Ventilation – The Problem: CMS has proposed inclusion of home mechanical ventilation in competitive bidding for durable medical equipment. Such a regulatory proposal is fraught with downside risk, most notably that such a policy would follow the history of liquid oxygen. Liquid 02 has virtually disappeared from the marketplace since it was included in competitive bidding as suppliers simply refused to provide liquid oxygen systems as their own bidding dropped the price to prohibitively low levels. Also, because there is a statutory requirement that such payment be made on the basis of “frequent and substantial servicing,” and that stipulation could trigger wide variations in actual bidding because some states require involvement of respiratory therapists in such services, while others do not.
It is critical to understand that the driving force behind all of this is the reality that CMS’ own coverage policies for home mechanical ventilation are seriously flawed and outdated, creating perverse incentives for physicians to order easily accessible systems rather than clinically appropriate ones. NAMDRC and its sister societies have been pushing CMS to revise those policies with no success.
Home Mechanical Ventilation – The Solution: Our solution is twofold. HR 4945 bill was introduced on November 1, 2019. First, the proposed legislation would create a blanket exemption for home mechanical ventilation from competitive bidding. Second, it requires CMS to convene a technical expert panel to craft up-to-date policies for home mechanical ventilation.
The political strategy here is slightly different. While passage of the bill is certainly our first choice, we believe that introduction of the bill is a red flag signal to CMS for the need to revise its coverage policies as those policies are the root cause of the growth of home mechanical ventilation outlays.
Two priorities of NAMDRC have moved into the formal congressional arena. The issues focus on access to pulmonary rehabilitation and CMS’s move to include home mechanical ventilation in competitive bidding.
Pulmonary Rehabilitation – The Problem: One of the major concerns to CMS and Congress is the fact that different payment methodologies for the same service result in different payment amounts dependent upon the actual site of service. To address the phenomenon of hospitals purchasing certain physician practices to game the payment system, Congress included in the 2015 Budget Act a provision that would remove incentives for such hospital purchases by stating that new hospital outpatient services must be within 250 yards of the main hospital campus in order to receive payment based on the hospital outpatient prospective payment system methodology. If a hospital opens such services beyond that 250-yard threshold, the hospital would be reimbursed at the physician fee schedule amount for the same service. Likewise, if an off campus program moved its grandfathered location because of expansion, loss of lease, etc, the physician fee schedule would again kick in.
For pulmonary rehabilitation services, this is extremely problematic and is tying the hands of hospitals providing this service. The physician fee schedule payment for pulmonary rehabilitation is less than $30 for 1 hour of service, and it is, therefore, not surprising that the service is simply not provided in physician offices. In fact, Medicare data show that all physician specialties bill less than $1M for code G0424, and we believe that most of that is likely billing error. Pulmonologists bill less than $500K for code G0424, and putting that number in context, the entire Medicare program is approaching $700B in outlays.
Pulmonary Rehabilitation – The Solution: As a solution to this problem, HR 4838 has been introduced in the House of Representatives. There is no specific reference to pulmonary rehabilitation in the bill as our approach is based not only on substance but political considerations, as well. Using CMS’ own acknowledgment of “unintended consequences,” this legislation would exempt all CPT codes from the restrictions imposed by Section 603 of the 2015 Budget Act when the physician billings for that code are under $2M for the most recent year for which data are available. CMS has signaled to us that such a limitation would apply only to pulmonary and cardiac rehab services, but others may be affected, as well. By putting a dollar limit rather than identifying a specific service for such a “carve out,” it is a more politically viable approach.
Bills such as this rarely see the light of day; however, such provisions are often attached to larger, more substantive bills. For nearly 2 decades, the common legislative vehicle for such provisions is a larger Medicare bill, often including “must pass Medicare extender” provisions that are slated to expire on a particular date. Our goal is to include HR 4838 in such a package of extenders some time between now and the end of this Congress in 2020.
Home Mechanical Ventilation – The Problem: CMS has proposed inclusion of home mechanical ventilation in competitive bidding for durable medical equipment. Such a regulatory proposal is fraught with downside risk, most notably that such a policy would follow the history of liquid oxygen. Liquid 02 has virtually disappeared from the marketplace since it was included in competitive bidding as suppliers simply refused to provide liquid oxygen systems as their own bidding dropped the price to prohibitively low levels. Also, because there is a statutory requirement that such payment be made on the basis of “frequent and substantial servicing,” and that stipulation could trigger wide variations in actual bidding because some states require involvement of respiratory therapists in such services, while others do not.
It is critical to understand that the driving force behind all of this is the reality that CMS’ own coverage policies for home mechanical ventilation are seriously flawed and outdated, creating perverse incentives for physicians to order easily accessible systems rather than clinically appropriate ones. NAMDRC and its sister societies have been pushing CMS to revise those policies with no success.
Home Mechanical Ventilation – The Solution: Our solution is twofold. HR 4945 bill was introduced on November 1, 2019. First, the proposed legislation would create a blanket exemption for home mechanical ventilation from competitive bidding. Second, it requires CMS to convene a technical expert panel to craft up-to-date policies for home mechanical ventilation.
The political strategy here is slightly different. While passage of the bill is certainly our first choice, we believe that introduction of the bill is a red flag signal to CMS for the need to revise its coverage policies as those policies are the root cause of the growth of home mechanical ventilation outlays.
Mark J. Rosen, MD, Master FCCP Endowment
When most think of Mark J. Rosen, MD, Master FCCP, so many words come to mind: master educator, astute and caring clinician, researcher, mentor, leader. We recall his generosity, kindness, honesty, brilliance, and sense of humor.
Mark loved CHEST. He gave so much to the organization and was happy to do so. He was one of the rare Past Presidents who contributed even more after his presidency than during or before. Mark left an enormous footprint on CHEST’s educational programs, including the CHEST Annual Meeting, Pulmonary Board Review, and SEEK. He was instrumental in building our international educational programs and a key player in empowering our Chinese colleagues in establishing pulmonary fellowships in their country. Much of what we have all accomplished at CHEST and in pulmonary medicine is directly related to the wonderful mentors we have had in the organization, and Mark was certainly one of the most prominent.
Mark introduced many of us to so many friends and mentors. He especially did this for hundreds of trainees and junior faculty throughout his career. What made him most happy was seeing his trainees and mentees succeed – Mark was THE example of an outstanding mentor. After his passing, and in recognition of his work that can and will live on, the CHEST Foundation has established an endowment with a major focus that truly honors Mark’s most memorable traits – the Rosen International Scholarship Fund
Mark always believed the core strength of the college was education. The CHEST Foundation is endowing the Rosen International Scholarship and raising $100,000 to support deserving international clinicians. This endowed fund will directly support international CHEST members’ travel to the CHEST Annual Meeting affording CHEST’s world-class educational and mentorship opportunities to members who could not otherwise attend.
To support the Mark J. Rosen, MD, FCCP Endowment, his legacy, and international CHEST members, visit chestfoundation.org/donate today.
When most think of Mark J. Rosen, MD, Master FCCP, so many words come to mind: master educator, astute and caring clinician, researcher, mentor, leader. We recall his generosity, kindness, honesty, brilliance, and sense of humor.
Mark loved CHEST. He gave so much to the organization and was happy to do so. He was one of the rare Past Presidents who contributed even more after his presidency than during or before. Mark left an enormous footprint on CHEST’s educational programs, including the CHEST Annual Meeting, Pulmonary Board Review, and SEEK. He was instrumental in building our international educational programs and a key player in empowering our Chinese colleagues in establishing pulmonary fellowships in their country. Much of what we have all accomplished at CHEST and in pulmonary medicine is directly related to the wonderful mentors we have had in the organization, and Mark was certainly one of the most prominent.
Mark introduced many of us to so many friends and mentors. He especially did this for hundreds of trainees and junior faculty throughout his career. What made him most happy was seeing his trainees and mentees succeed – Mark was THE example of an outstanding mentor. After his passing, and in recognition of his work that can and will live on, the CHEST Foundation has established an endowment with a major focus that truly honors Mark’s most memorable traits – the Rosen International Scholarship Fund
Mark always believed the core strength of the college was education. The CHEST Foundation is endowing the Rosen International Scholarship and raising $100,000 to support deserving international clinicians. This endowed fund will directly support international CHEST members’ travel to the CHEST Annual Meeting affording CHEST’s world-class educational and mentorship opportunities to members who could not otherwise attend.
To support the Mark J. Rosen, MD, FCCP Endowment, his legacy, and international CHEST members, visit chestfoundation.org/donate today.
When most think of Mark J. Rosen, MD, Master FCCP, so many words come to mind: master educator, astute and caring clinician, researcher, mentor, leader. We recall his generosity, kindness, honesty, brilliance, and sense of humor.
Mark loved CHEST. He gave so much to the organization and was happy to do so. He was one of the rare Past Presidents who contributed even more after his presidency than during or before. Mark left an enormous footprint on CHEST’s educational programs, including the CHEST Annual Meeting, Pulmonary Board Review, and SEEK. He was instrumental in building our international educational programs and a key player in empowering our Chinese colleagues in establishing pulmonary fellowships in their country. Much of what we have all accomplished at CHEST and in pulmonary medicine is directly related to the wonderful mentors we have had in the organization, and Mark was certainly one of the most prominent.
Mark introduced many of us to so many friends and mentors. He especially did this for hundreds of trainees and junior faculty throughout his career. What made him most happy was seeing his trainees and mentees succeed – Mark was THE example of an outstanding mentor. After his passing, and in recognition of his work that can and will live on, the CHEST Foundation has established an endowment with a major focus that truly honors Mark’s most memorable traits – the Rosen International Scholarship Fund
Mark always believed the core strength of the college was education. The CHEST Foundation is endowing the Rosen International Scholarship and raising $100,000 to support deserving international clinicians. This endowed fund will directly support international CHEST members’ travel to the CHEST Annual Meeting affording CHEST’s world-class educational and mentorship opportunities to members who could not otherwise attend.
To support the Mark J. Rosen, MD, FCCP Endowment, his legacy, and international CHEST members, visit chestfoundation.org/donate today.
News From the Board of Regents: Highlights of ongoing successes
CHEST leadership recently met for its fall quarterly face to face meeting prior to the CHEST Annual Meeting in New Orleans this October. Like all of CHEST board meetings, the agenda was packed with important topics and a great deal of meaningful discussion. I left the meeting more energized about CHEST and its current and future offerings for our membership. Below are a few highlights from the meeting.
The meeting was opened with an update from the outgoing CHEST President Clayton Cowl, MD, MS, FCCP. He highlighted some of the organization’s major achievements over the past year, including: Confirming and signing a new contract with our new EVP/CEO Robert Musacchio, PhD; hiring a new Chief Learning Officer, a new Editor in Chief for the CHEST® journal, and a new Chief Legal Counsel; and expansion of the international strategy with CHEST Congress in Bangkok and a CHEST Regional meeting in Athens with plans for CHEST Congress 2020 in Bologna, Italy. In addition, CHEST convened a Digital Strategy Task Force, which made recommendations to improve how members, patients, and staff interact with our organization.
EVP/CEO Robert Musacchio reviewed some additional organizational accomplishments and areas of focus for the future. These included redefining the One CHEST operating model and a continued focus on international business development with plans for CHEST Congress 2020 in Italy and exploration of future meetings in Singapore and The Philippines. CHEST continues to develop an improved membership strategy focused on improving experiences for our membership and improving member engagement to help secure the future of CHEST. Finally, CHEST remains focused on innovation though new experiences for our members, including new games, virtual patient tours, and enduring activities and products. Many of these experiences were highlighted and on display at the recent CHEST annual meeting.
Doreen Addrizzo-Harris, MD, FCCP, the CHEST Foundation outgoing President, recapped a very successful year for the CHEST Foundation with plans for increasing the impact and visibility of the CHEST Foundation going forward.. John Howington, MD, FCCP, Chair of the Finance Committee, updated the board on the financial health of the organization. Overall, CHEST had a solid financial report based on budgeted revenue and strong expense management by our executive leadership team.
John Studdard, MD, FCCP, the immediate Past President and Chair of the Governance Committee, led the Governance Committee report and discussion. The Governance Committee is composed of members of both the College Board of Regents (BOR) and Foundation Board of Trustees (BOT) and is responsible for the overall health of both boards and ensuring that the boards are consistently performing at a high level. Dr. Studdard presented slates for 2019-20 Board of Trustees and Board of Regents for discussion and approval. Five new members for the Board of Regents and four new members for the Board of Trustees were approved for the upcoming year. In addition, David Schulman, MD, MS, FCCP, and Robert De Marco, MD, FCCP, were elected as President-Designate of the BOR and BOT, respectively.
Several committees presented to the Board to review this year’s progress, future plans, and potential barriers to success. The Council of Global Governors continued to see growth in our international membership, though a potential ongoing barrier to future growth will be developing an efficient mode of communication between the Global Governors and our international members. Discussion around using the expertise within the Digital Strategy Task Force was offered as one method to improve international member communication and engagement. The Education Committee continues to be one of CHEST’s most popular committees with an unprecedented 130 nominations with exceptional credentials for the 2019-20 election cycle. The Education Committee has expanded the size of three of its subcommittees to better include and engage these individuals in CHEST education projects. The Training and Transitions Committee continues to see increased engagement from trainees and training programs. CHEST 2019 had an increased number of trainee submissions, as well as the number of accepted submissions to the meeting. The committee will continue to evolve their strategy for engaging trainees and early career professionals. Finally, Christopher Hergott, MD, FCCP, Chair of the Membership Committee, reviewed several strategies and recommendations to expand our membership offerings and improve the value that we bring to our all of our members.
Finally, it was time to say thank you and farewell to out our outgoing Board members. Clayton Cowl, MD, MS, FCCP, and Stephanie Levine, MD, FCCP, recognized the following board members for their service to CHEST over the last several years: Jack Buckley, MD, FCCP; John Studdard, MD, FCCP; David Zielinski, MD, FCCP; and Burt Lesnick, MD, FCCP.
CHEST leadership recently met for its fall quarterly face to face meeting prior to the CHEST Annual Meeting in New Orleans this October. Like all of CHEST board meetings, the agenda was packed with important topics and a great deal of meaningful discussion. I left the meeting more energized about CHEST and its current and future offerings for our membership. Below are a few highlights from the meeting.
The meeting was opened with an update from the outgoing CHEST President Clayton Cowl, MD, MS, FCCP. He highlighted some of the organization’s major achievements over the past year, including: Confirming and signing a new contract with our new EVP/CEO Robert Musacchio, PhD; hiring a new Chief Learning Officer, a new Editor in Chief for the CHEST® journal, and a new Chief Legal Counsel; and expansion of the international strategy with CHEST Congress in Bangkok and a CHEST Regional meeting in Athens with plans for CHEST Congress 2020 in Bologna, Italy. In addition, CHEST convened a Digital Strategy Task Force, which made recommendations to improve how members, patients, and staff interact with our organization.
EVP/CEO Robert Musacchio reviewed some additional organizational accomplishments and areas of focus for the future. These included redefining the One CHEST operating model and a continued focus on international business development with plans for CHEST Congress 2020 in Italy and exploration of future meetings in Singapore and The Philippines. CHEST continues to develop an improved membership strategy focused on improving experiences for our membership and improving member engagement to help secure the future of CHEST. Finally, CHEST remains focused on innovation though new experiences for our members, including new games, virtual patient tours, and enduring activities and products. Many of these experiences were highlighted and on display at the recent CHEST annual meeting.
Doreen Addrizzo-Harris, MD, FCCP, the CHEST Foundation outgoing President, recapped a very successful year for the CHEST Foundation with plans for increasing the impact and visibility of the CHEST Foundation going forward.. John Howington, MD, FCCP, Chair of the Finance Committee, updated the board on the financial health of the organization. Overall, CHEST had a solid financial report based on budgeted revenue and strong expense management by our executive leadership team.
John Studdard, MD, FCCP, the immediate Past President and Chair of the Governance Committee, led the Governance Committee report and discussion. The Governance Committee is composed of members of both the College Board of Regents (BOR) and Foundation Board of Trustees (BOT) and is responsible for the overall health of both boards and ensuring that the boards are consistently performing at a high level. Dr. Studdard presented slates for 2019-20 Board of Trustees and Board of Regents for discussion and approval. Five new members for the Board of Regents and four new members for the Board of Trustees were approved for the upcoming year. In addition, David Schulman, MD, MS, FCCP, and Robert De Marco, MD, FCCP, were elected as President-Designate of the BOR and BOT, respectively.
Several committees presented to the Board to review this year’s progress, future plans, and potential barriers to success. The Council of Global Governors continued to see growth in our international membership, though a potential ongoing barrier to future growth will be developing an efficient mode of communication between the Global Governors and our international members. Discussion around using the expertise within the Digital Strategy Task Force was offered as one method to improve international member communication and engagement. The Education Committee continues to be one of CHEST’s most popular committees with an unprecedented 130 nominations with exceptional credentials for the 2019-20 election cycle. The Education Committee has expanded the size of three of its subcommittees to better include and engage these individuals in CHEST education projects. The Training and Transitions Committee continues to see increased engagement from trainees and training programs. CHEST 2019 had an increased number of trainee submissions, as well as the number of accepted submissions to the meeting. The committee will continue to evolve their strategy for engaging trainees and early career professionals. Finally, Christopher Hergott, MD, FCCP, Chair of the Membership Committee, reviewed several strategies and recommendations to expand our membership offerings and improve the value that we bring to our all of our members.
Finally, it was time to say thank you and farewell to out our outgoing Board members. Clayton Cowl, MD, MS, FCCP, and Stephanie Levine, MD, FCCP, recognized the following board members for their service to CHEST over the last several years: Jack Buckley, MD, FCCP; John Studdard, MD, FCCP; David Zielinski, MD, FCCP; and Burt Lesnick, MD, FCCP.
CHEST leadership recently met for its fall quarterly face to face meeting prior to the CHEST Annual Meeting in New Orleans this October. Like all of CHEST board meetings, the agenda was packed with important topics and a great deal of meaningful discussion. I left the meeting more energized about CHEST and its current and future offerings for our membership. Below are a few highlights from the meeting.
The meeting was opened with an update from the outgoing CHEST President Clayton Cowl, MD, MS, FCCP. He highlighted some of the organization’s major achievements over the past year, including: Confirming and signing a new contract with our new EVP/CEO Robert Musacchio, PhD; hiring a new Chief Learning Officer, a new Editor in Chief for the CHEST® journal, and a new Chief Legal Counsel; and expansion of the international strategy with CHEST Congress in Bangkok and a CHEST Regional meeting in Athens with plans for CHEST Congress 2020 in Bologna, Italy. In addition, CHEST convened a Digital Strategy Task Force, which made recommendations to improve how members, patients, and staff interact with our organization.
EVP/CEO Robert Musacchio reviewed some additional organizational accomplishments and areas of focus for the future. These included redefining the One CHEST operating model and a continued focus on international business development with plans for CHEST Congress 2020 in Italy and exploration of future meetings in Singapore and The Philippines. CHEST continues to develop an improved membership strategy focused on improving experiences for our membership and improving member engagement to help secure the future of CHEST. Finally, CHEST remains focused on innovation though new experiences for our members, including new games, virtual patient tours, and enduring activities and products. Many of these experiences were highlighted and on display at the recent CHEST annual meeting.
Doreen Addrizzo-Harris, MD, FCCP, the CHEST Foundation outgoing President, recapped a very successful year for the CHEST Foundation with plans for increasing the impact and visibility of the CHEST Foundation going forward.. John Howington, MD, FCCP, Chair of the Finance Committee, updated the board on the financial health of the organization. Overall, CHEST had a solid financial report based on budgeted revenue and strong expense management by our executive leadership team.
John Studdard, MD, FCCP, the immediate Past President and Chair of the Governance Committee, led the Governance Committee report and discussion. The Governance Committee is composed of members of both the College Board of Regents (BOR) and Foundation Board of Trustees (BOT) and is responsible for the overall health of both boards and ensuring that the boards are consistently performing at a high level. Dr. Studdard presented slates for 2019-20 Board of Trustees and Board of Regents for discussion and approval. Five new members for the Board of Regents and four new members for the Board of Trustees were approved for the upcoming year. In addition, David Schulman, MD, MS, FCCP, and Robert De Marco, MD, FCCP, were elected as President-Designate of the BOR and BOT, respectively.
Several committees presented to the Board to review this year’s progress, future plans, and potential barriers to success. The Council of Global Governors continued to see growth in our international membership, though a potential ongoing barrier to future growth will be developing an efficient mode of communication between the Global Governors and our international members. Discussion around using the expertise within the Digital Strategy Task Force was offered as one method to improve international member communication and engagement. The Education Committee continues to be one of CHEST’s most popular committees with an unprecedented 130 nominations with exceptional credentials for the 2019-20 election cycle. The Education Committee has expanded the size of three of its subcommittees to better include and engage these individuals in CHEST education projects. The Training and Transitions Committee continues to see increased engagement from trainees and training programs. CHEST 2019 had an increased number of trainee submissions, as well as the number of accepted submissions to the meeting. The committee will continue to evolve their strategy for engaging trainees and early career professionals. Finally, Christopher Hergott, MD, FCCP, Chair of the Membership Committee, reviewed several strategies and recommendations to expand our membership offerings and improve the value that we bring to our all of our members.
Finally, it was time to say thank you and farewell to out our outgoing Board members. Clayton Cowl, MD, MS, FCCP, and Stephanie Levine, MD, FCCP, recognized the following board members for their service to CHEST over the last several years: Jack Buckley, MD, FCCP; John Studdard, MD, FCCP; David Zielinski, MD, FCCP; and Burt Lesnick, MD, FCCP.
An update on the current standard for ultrasound education in fellowship
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
This month in the journal CHEST®
Editor’s Picks
Editorials
Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. By Drs. B. W. Bottiger and W. A. Wetsch.
Interstitial Lung Abnormalities: A Word of Caution.
By Drs. V. Tzilas and D. Bouros.
Original Research
Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry.
By Dr. F. Javaudin, et al.Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. By Dr. S-A. Whittaker Brown, et al.
Commentary
Publishing a Clinical Research Manuscript: Guidance for Early-Career Researchers With a Focus on Pulmonary and Critical Care Medicine.
By Dr. E. M. Viglianti, et al.
Editor’s Picks
Editor’s Picks
Editorials
Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. By Drs. B. W. Bottiger and W. A. Wetsch.
Interstitial Lung Abnormalities: A Word of Caution.
By Drs. V. Tzilas and D. Bouros.
Original Research
Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry.
By Dr. F. Javaudin, et al.Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. By Dr. S-A. Whittaker Brown, et al.
Commentary
Publishing a Clinical Research Manuscript: Guidance for Early-Career Researchers With a Focus on Pulmonary and Critical Care Medicine.
By Dr. E. M. Viglianti, et al.
Editorials
Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. By Drs. B. W. Bottiger and W. A. Wetsch.
Interstitial Lung Abnormalities: A Word of Caution.
By Drs. V. Tzilas and D. Bouros.
Original Research
Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry.
By Dr. F. Javaudin, et al.Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. By Dr. S-A. Whittaker Brown, et al.
Commentary
Publishing a Clinical Research Manuscript: Guidance for Early-Career Researchers With a Focus on Pulmonary and Critical Care Medicine.
By Dr. E. M. Viglianti, et al.
Nutrition support during adult critical illness
Many critically ill patients you care for cannot maintain volitional oral intake. Therefore, nutrition support, through enteral or parenteral routes, remains a cornerstone in ensuring our critically ill patients receive substrates like glucose and protein. To understand the supportive role of nutrition during critical illness, let’s identify and contextualize the different phases of critical illness.
Phases of critical illness
The European Society of Parenteral and Enteral Nutrition’s (ESPEN) 2018 critical care nutrition guideline incorporates stages of critical illness in making nutrition recommendations (Singer P et al. Clin Nutr. 2019;38:48-79). The first week of critical illness is the acute phase and hallmarked by catabolism and metabolic and hemodynamic instability. The late phase is thereafter and hallmarked by rehabilitation and anabolism or chronic critical illness. The acute phase is further divided into early (days 1-2) and late acute phase (days 3-7). The time-points are arbitrary and merely serve as placeholders. An objective marker to distinguish phases does not exist and transition periods will be different for each patient.
Acute phase
Critical illness defining conditions like circulatory shock, respiratory failure, and trauma are stressors and lead to two key acute phase perturbations that nutrition may have a role in altering:
The first is hypercatabolism. Critical illness defining conditions activate neuroendocrine, inflammatory/immune, adipokine, and GI tract hormone pathways that increase serum glucagon, cortisol, and catecholamines to promote glycogenolysis, gluconeogenesis, insulin resistance, protein catabolism, and restricted/impaired anabolism.
The second is gut dysfunction. During health, there is cross-talk signaling that occurs between commensal bacteria, epithelium, and the immune system, which maintains gut barrier functions, achieved, for example, by promoting tight junction protein production. Acute critical illness pathophysiology loosens epithelial tight junctions, and the gut barrier is breached, creating an opportunity for downstream migration of pancreatic enzymes and cytokines. Furthermore, the microbiome morphs into a virulent pathobiome, which induces gut-derived inflammation.
When, where, and how much should we feed critically ill patients?
Since the acute phase of critical illness begins a series of events leading to negative energy balance and gut dysfunction, you might find early nutrition provision intuitive. Indeed, the 2016 ASPEN/SCCM and 2018 ESPEN critical care nutrition guidelines recommend early (within 24-48 hours of ICU admission) enteral nutrition (EN), delivered into the stomach, for all critically ill patients unable to maintain volitional intake. Meta-analyses of randomized controlled trials (RCT) conducted between1979 and 2013 show early EN reduces both mortality and infectious complications, compared with no early nutrition (McClave SA et al. JPEN. 2016;40:159-211).
RCT level data do not show superiority of EN over parenteral nutrition (PN). Nonetheless, early EN is recommended over PN because it maintains epithelial barrier function and supports immunity.
What is the optimal nutrition dose? The 2016 ASPEN/SCCM guideline recommends getting to >80% estimated energy goal within 48-72 hours in patients with high nutrition risk while the 2018 ESPEN guideline suggests maintaining a hypocaloric, or not exceeding 70% of prescribed energy goal, during the early acute phase. The recommendation is based on meta-analyses of RCTs conducted between 2011 and 2017, which shows no mortality difference between hypocaloric and isocaloric nutrition.
Biologically plausible rationale for starting hypocaloric, as opposed to full dose nutrition, during the acute phase of critical illness includes: (a) the acute phase represents a period of hemodynamic instability and mitochondrial dysfunction, and full-dose EN may lead to feeding intolerance and lack of substrate utilization, respectively; (b) in those with risk factors (like pre-existing malnutrition), starting full dose nutrition may lead to refeeding syndrome; and (c) endogenous glucose production occurs during the acute phase, and full dose nutrition may worsen hyperglycemia.
Therefore, during the early acute phase of critical illness, hypocaloric feeding using an isosmotic formula, with a slow up-titration to goal rate thereafter, while monitoring for feeding intolerance and refeeding syndrome is a reasonable starting point.
What is the role of parenteral nutrition in critical illness?
PN can be exclusive or supplemental (in a patient receiving EN). Historically, providers may have been reluctant to utilize PN for fear of infectious morbidity; however, contemporary pragmatic-design RCTs demonstrate safety with exclusive PN (Harvey SE et al. N Engl J Med. 2014;371:1673-84). When your patient has a contraindication for EN or does not tolerate it despite a trial of small bowel feeding, meta-analyses have shown a mortality benefit of early exclusive PN in malnourished patients, as compared with no nutrition (Braunschweig C et al. Am J Clin Nutr.2001;74:534-42).
As for supplemental PN (SPN), the 2016 ASPEN/SCCM guideline does not recommend it until day 7 in all critically ill patients, while the 2018 ESPEN guideline recommends its use on a case-by-case basis. Since, two trials inform SPN use. The EAT-ICU trial showed no difference in 6-month physical function between EN group and early-goal-directed nutritiongroup, which included SPN to achieve estimated energy requirement during the first week of critical illness (Allingstrup MJ et al. Intensive Care Med. 2017;43:1637-47). The TOP-UP trial compared EN alone with EN plus SPN in nutritionally high risk patients (ie, those who stand to have more complications as a result of undernutrition) and found those with a BMI < 25 kg/m2 and those with a NUTRIC score >5 who received supplemental PN atop EN had improved 30-day mortality, as compared with EN alone (Wischmeyer P et al. Crit Care. 2017;21:142). Mortality was a secondary outcome, and further study of supplemental PN in nutritionally high-risk patients is warranted. Until further data are available, supplemental PN should probably be restricted during the acute phase of critical illness.
Protein may be the important substrate
Proteolysis is the rule during critical illness, and amino acids are liberated from skeletal muscle breakdown. Using ultrasound, Puthucheary et al found a 17.7% reduction in rectus femoris cross-sectional area in 63 critically ill adults and identified muscle cellular infiltration at ICU day 10, suggesting critical illness leads to quantitative and qualitative muscle defects (Puthucheary Z et al. JAMA. 2013;15:1591-1600).
Since survivorship from critical illness is increasing, acquired loss of muscle mass may contribute to post-ICU physical functioning impairments. Thus, protein may be the most important substrate to deliver during critical illness. The 2016 ASPEN/SCCM guideline recommends 1.2 – 2.0 g/kg actual body weight (ABW)/day in nonobese critically ill patients.
Unfortunately, the optimal protein dose and the timing of intake are unknown. Observational studies suggest benefit with lower and higher doses, which creates equipoise for protein dose. The signal may be lost in heterogeneity, and observational data suggest higher protein dose may benefit patients with high nutritional risk. In terms of timing, one observational study found lower (<0.8 g/kg/d) protein dose before day 3 followed by higher (>0.8 g/kg/d) dose thereafter was associated with mortality benefit (Koekkoek WAC et al. Clin Nutr.2019;38:883-890).
Until stronger data are available to guide optimal protein dose and timing, it is reasonable to observe the 2016 ASPEN/SCCM guideline protein recommendation of at least 1.2 g/kg/day. The 2018 ESPEN guideline recommends a similar dose of 1.3 g/kg/day.
Future research and summary
Many questions remain unanswered and present opportunities for future research. Priorities for critical care nutrition research include studying the impact of combined nutrition and exercise in the acute and late phases of critical illness and identifying best tools to differentiate responses to caloric and protein intake.
In summary, critical illness has acute and late phases. The acute phase is a hypercatabolic state leading to negative energy and nitrogen balance and gut dysfunction. Take-home points for nutrition support in the acute phase of critical illness are:
1. It is reasonable to start early hypocaloric EN with an isosmotic formula with slow up-titration over the first week of critical illness while monitoring for refeeding syndrome and feeding intolerance.
2. Use exclusive PN in ICU patients with pre-existing malnutrition when EN is contraindicated or not tolerated.
3. Supplemental PN should probably be restricted during the acute phase of critical illness.
4. Optimal protein dose and timing are unknown. It is reasonable to start with at least 1.2 g/kg ABW/day in non-obese patients.
Dr. Patel is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin.
Dr. Rice is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Vanderbilt University, Nashville, Tennessee.
Many critically ill patients you care for cannot maintain volitional oral intake. Therefore, nutrition support, through enteral or parenteral routes, remains a cornerstone in ensuring our critically ill patients receive substrates like glucose and protein. To understand the supportive role of nutrition during critical illness, let’s identify and contextualize the different phases of critical illness.
Phases of critical illness
The European Society of Parenteral and Enteral Nutrition’s (ESPEN) 2018 critical care nutrition guideline incorporates stages of critical illness in making nutrition recommendations (Singer P et al. Clin Nutr. 2019;38:48-79). The first week of critical illness is the acute phase and hallmarked by catabolism and metabolic and hemodynamic instability. The late phase is thereafter and hallmarked by rehabilitation and anabolism or chronic critical illness. The acute phase is further divided into early (days 1-2) and late acute phase (days 3-7). The time-points are arbitrary and merely serve as placeholders. An objective marker to distinguish phases does not exist and transition periods will be different for each patient.
Acute phase
Critical illness defining conditions like circulatory shock, respiratory failure, and trauma are stressors and lead to two key acute phase perturbations that nutrition may have a role in altering:
The first is hypercatabolism. Critical illness defining conditions activate neuroendocrine, inflammatory/immune, adipokine, and GI tract hormone pathways that increase serum glucagon, cortisol, and catecholamines to promote glycogenolysis, gluconeogenesis, insulin resistance, protein catabolism, and restricted/impaired anabolism.
The second is gut dysfunction. During health, there is cross-talk signaling that occurs between commensal bacteria, epithelium, and the immune system, which maintains gut barrier functions, achieved, for example, by promoting tight junction protein production. Acute critical illness pathophysiology loosens epithelial tight junctions, and the gut barrier is breached, creating an opportunity for downstream migration of pancreatic enzymes and cytokines. Furthermore, the microbiome morphs into a virulent pathobiome, which induces gut-derived inflammation.
When, where, and how much should we feed critically ill patients?
Since the acute phase of critical illness begins a series of events leading to negative energy balance and gut dysfunction, you might find early nutrition provision intuitive. Indeed, the 2016 ASPEN/SCCM and 2018 ESPEN critical care nutrition guidelines recommend early (within 24-48 hours of ICU admission) enteral nutrition (EN), delivered into the stomach, for all critically ill patients unable to maintain volitional intake. Meta-analyses of randomized controlled trials (RCT) conducted between1979 and 2013 show early EN reduces both mortality and infectious complications, compared with no early nutrition (McClave SA et al. JPEN. 2016;40:159-211).
RCT level data do not show superiority of EN over parenteral nutrition (PN). Nonetheless, early EN is recommended over PN because it maintains epithelial barrier function and supports immunity.
What is the optimal nutrition dose? The 2016 ASPEN/SCCM guideline recommends getting to >80% estimated energy goal within 48-72 hours in patients with high nutrition risk while the 2018 ESPEN guideline suggests maintaining a hypocaloric, or not exceeding 70% of prescribed energy goal, during the early acute phase. The recommendation is based on meta-analyses of RCTs conducted between 2011 and 2017, which shows no mortality difference between hypocaloric and isocaloric nutrition.
Biologically plausible rationale for starting hypocaloric, as opposed to full dose nutrition, during the acute phase of critical illness includes: (a) the acute phase represents a period of hemodynamic instability and mitochondrial dysfunction, and full-dose EN may lead to feeding intolerance and lack of substrate utilization, respectively; (b) in those with risk factors (like pre-existing malnutrition), starting full dose nutrition may lead to refeeding syndrome; and (c) endogenous glucose production occurs during the acute phase, and full dose nutrition may worsen hyperglycemia.
Therefore, during the early acute phase of critical illness, hypocaloric feeding using an isosmotic formula, with a slow up-titration to goal rate thereafter, while monitoring for feeding intolerance and refeeding syndrome is a reasonable starting point.
What is the role of parenteral nutrition in critical illness?
PN can be exclusive or supplemental (in a patient receiving EN). Historically, providers may have been reluctant to utilize PN for fear of infectious morbidity; however, contemporary pragmatic-design RCTs demonstrate safety with exclusive PN (Harvey SE et al. N Engl J Med. 2014;371:1673-84). When your patient has a contraindication for EN or does not tolerate it despite a trial of small bowel feeding, meta-analyses have shown a mortality benefit of early exclusive PN in malnourished patients, as compared with no nutrition (Braunschweig C et al. Am J Clin Nutr.2001;74:534-42).
As for supplemental PN (SPN), the 2016 ASPEN/SCCM guideline does not recommend it until day 7 in all critically ill patients, while the 2018 ESPEN guideline recommends its use on a case-by-case basis. Since, two trials inform SPN use. The EAT-ICU trial showed no difference in 6-month physical function between EN group and early-goal-directed nutritiongroup, which included SPN to achieve estimated energy requirement during the first week of critical illness (Allingstrup MJ et al. Intensive Care Med. 2017;43:1637-47). The TOP-UP trial compared EN alone with EN plus SPN in nutritionally high risk patients (ie, those who stand to have more complications as a result of undernutrition) and found those with a BMI < 25 kg/m2 and those with a NUTRIC score >5 who received supplemental PN atop EN had improved 30-day mortality, as compared with EN alone (Wischmeyer P et al. Crit Care. 2017;21:142). Mortality was a secondary outcome, and further study of supplemental PN in nutritionally high-risk patients is warranted. Until further data are available, supplemental PN should probably be restricted during the acute phase of critical illness.
Protein may be the important substrate
Proteolysis is the rule during critical illness, and amino acids are liberated from skeletal muscle breakdown. Using ultrasound, Puthucheary et al found a 17.7% reduction in rectus femoris cross-sectional area in 63 critically ill adults and identified muscle cellular infiltration at ICU day 10, suggesting critical illness leads to quantitative and qualitative muscle defects (Puthucheary Z et al. JAMA. 2013;15:1591-1600).
Since survivorship from critical illness is increasing, acquired loss of muscle mass may contribute to post-ICU physical functioning impairments. Thus, protein may be the most important substrate to deliver during critical illness. The 2016 ASPEN/SCCM guideline recommends 1.2 – 2.0 g/kg actual body weight (ABW)/day in nonobese critically ill patients.
Unfortunately, the optimal protein dose and the timing of intake are unknown. Observational studies suggest benefit with lower and higher doses, which creates equipoise for protein dose. The signal may be lost in heterogeneity, and observational data suggest higher protein dose may benefit patients with high nutritional risk. In terms of timing, one observational study found lower (<0.8 g/kg/d) protein dose before day 3 followed by higher (>0.8 g/kg/d) dose thereafter was associated with mortality benefit (Koekkoek WAC et al. Clin Nutr.2019;38:883-890).
Until stronger data are available to guide optimal protein dose and timing, it is reasonable to observe the 2016 ASPEN/SCCM guideline protein recommendation of at least 1.2 g/kg/day. The 2018 ESPEN guideline recommends a similar dose of 1.3 g/kg/day.
Future research and summary
Many questions remain unanswered and present opportunities for future research. Priorities for critical care nutrition research include studying the impact of combined nutrition and exercise in the acute and late phases of critical illness and identifying best tools to differentiate responses to caloric and protein intake.
In summary, critical illness has acute and late phases. The acute phase is a hypercatabolic state leading to negative energy and nitrogen balance and gut dysfunction. Take-home points for nutrition support in the acute phase of critical illness are:
1. It is reasonable to start early hypocaloric EN with an isosmotic formula with slow up-titration over the first week of critical illness while monitoring for refeeding syndrome and feeding intolerance.
2. Use exclusive PN in ICU patients with pre-existing malnutrition when EN is contraindicated or not tolerated.
3. Supplemental PN should probably be restricted during the acute phase of critical illness.
4. Optimal protein dose and timing are unknown. It is reasonable to start with at least 1.2 g/kg ABW/day in non-obese patients.
Dr. Patel is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin.
Dr. Rice is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Vanderbilt University, Nashville, Tennessee.
Many critically ill patients you care for cannot maintain volitional oral intake. Therefore, nutrition support, through enteral or parenteral routes, remains a cornerstone in ensuring our critically ill patients receive substrates like glucose and protein. To understand the supportive role of nutrition during critical illness, let’s identify and contextualize the different phases of critical illness.
Phases of critical illness
The European Society of Parenteral and Enteral Nutrition’s (ESPEN) 2018 critical care nutrition guideline incorporates stages of critical illness in making nutrition recommendations (Singer P et al. Clin Nutr. 2019;38:48-79). The first week of critical illness is the acute phase and hallmarked by catabolism and metabolic and hemodynamic instability. The late phase is thereafter and hallmarked by rehabilitation and anabolism or chronic critical illness. The acute phase is further divided into early (days 1-2) and late acute phase (days 3-7). The time-points are arbitrary and merely serve as placeholders. An objective marker to distinguish phases does not exist and transition periods will be different for each patient.
Acute phase
Critical illness defining conditions like circulatory shock, respiratory failure, and trauma are stressors and lead to two key acute phase perturbations that nutrition may have a role in altering:
The first is hypercatabolism. Critical illness defining conditions activate neuroendocrine, inflammatory/immune, adipokine, and GI tract hormone pathways that increase serum glucagon, cortisol, and catecholamines to promote glycogenolysis, gluconeogenesis, insulin resistance, protein catabolism, and restricted/impaired anabolism.
The second is gut dysfunction. During health, there is cross-talk signaling that occurs between commensal bacteria, epithelium, and the immune system, which maintains gut barrier functions, achieved, for example, by promoting tight junction protein production. Acute critical illness pathophysiology loosens epithelial tight junctions, and the gut barrier is breached, creating an opportunity for downstream migration of pancreatic enzymes and cytokines. Furthermore, the microbiome morphs into a virulent pathobiome, which induces gut-derived inflammation.
When, where, and how much should we feed critically ill patients?
Since the acute phase of critical illness begins a series of events leading to negative energy balance and gut dysfunction, you might find early nutrition provision intuitive. Indeed, the 2016 ASPEN/SCCM and 2018 ESPEN critical care nutrition guidelines recommend early (within 24-48 hours of ICU admission) enteral nutrition (EN), delivered into the stomach, for all critically ill patients unable to maintain volitional intake. Meta-analyses of randomized controlled trials (RCT) conducted between1979 and 2013 show early EN reduces both mortality and infectious complications, compared with no early nutrition (McClave SA et al. JPEN. 2016;40:159-211).
RCT level data do not show superiority of EN over parenteral nutrition (PN). Nonetheless, early EN is recommended over PN because it maintains epithelial barrier function and supports immunity.
What is the optimal nutrition dose? The 2016 ASPEN/SCCM guideline recommends getting to >80% estimated energy goal within 48-72 hours in patients with high nutrition risk while the 2018 ESPEN guideline suggests maintaining a hypocaloric, or not exceeding 70% of prescribed energy goal, during the early acute phase. The recommendation is based on meta-analyses of RCTs conducted between 2011 and 2017, which shows no mortality difference between hypocaloric and isocaloric nutrition.
Biologically plausible rationale for starting hypocaloric, as opposed to full dose nutrition, during the acute phase of critical illness includes: (a) the acute phase represents a period of hemodynamic instability and mitochondrial dysfunction, and full-dose EN may lead to feeding intolerance and lack of substrate utilization, respectively; (b) in those with risk factors (like pre-existing malnutrition), starting full dose nutrition may lead to refeeding syndrome; and (c) endogenous glucose production occurs during the acute phase, and full dose nutrition may worsen hyperglycemia.
Therefore, during the early acute phase of critical illness, hypocaloric feeding using an isosmotic formula, with a slow up-titration to goal rate thereafter, while monitoring for feeding intolerance and refeeding syndrome is a reasonable starting point.
What is the role of parenteral nutrition in critical illness?
PN can be exclusive or supplemental (in a patient receiving EN). Historically, providers may have been reluctant to utilize PN for fear of infectious morbidity; however, contemporary pragmatic-design RCTs demonstrate safety with exclusive PN (Harvey SE et al. N Engl J Med. 2014;371:1673-84). When your patient has a contraindication for EN or does not tolerate it despite a trial of small bowel feeding, meta-analyses have shown a mortality benefit of early exclusive PN in malnourished patients, as compared with no nutrition (Braunschweig C et al. Am J Clin Nutr.2001;74:534-42).
As for supplemental PN (SPN), the 2016 ASPEN/SCCM guideline does not recommend it until day 7 in all critically ill patients, while the 2018 ESPEN guideline recommends its use on a case-by-case basis. Since, two trials inform SPN use. The EAT-ICU trial showed no difference in 6-month physical function between EN group and early-goal-directed nutritiongroup, which included SPN to achieve estimated energy requirement during the first week of critical illness (Allingstrup MJ et al. Intensive Care Med. 2017;43:1637-47). The TOP-UP trial compared EN alone with EN plus SPN in nutritionally high risk patients (ie, those who stand to have more complications as a result of undernutrition) and found those with a BMI < 25 kg/m2 and those with a NUTRIC score >5 who received supplemental PN atop EN had improved 30-day mortality, as compared with EN alone (Wischmeyer P et al. Crit Care. 2017;21:142). Mortality was a secondary outcome, and further study of supplemental PN in nutritionally high-risk patients is warranted. Until further data are available, supplemental PN should probably be restricted during the acute phase of critical illness.
Protein may be the important substrate
Proteolysis is the rule during critical illness, and amino acids are liberated from skeletal muscle breakdown. Using ultrasound, Puthucheary et al found a 17.7% reduction in rectus femoris cross-sectional area in 63 critically ill adults and identified muscle cellular infiltration at ICU day 10, suggesting critical illness leads to quantitative and qualitative muscle defects (Puthucheary Z et al. JAMA. 2013;15:1591-1600).
Since survivorship from critical illness is increasing, acquired loss of muscle mass may contribute to post-ICU physical functioning impairments. Thus, protein may be the most important substrate to deliver during critical illness. The 2016 ASPEN/SCCM guideline recommends 1.2 – 2.0 g/kg actual body weight (ABW)/day in nonobese critically ill patients.
Unfortunately, the optimal protein dose and the timing of intake are unknown. Observational studies suggest benefit with lower and higher doses, which creates equipoise for protein dose. The signal may be lost in heterogeneity, and observational data suggest higher protein dose may benefit patients with high nutritional risk. In terms of timing, one observational study found lower (<0.8 g/kg/d) protein dose before day 3 followed by higher (>0.8 g/kg/d) dose thereafter was associated with mortality benefit (Koekkoek WAC et al. Clin Nutr.2019;38:883-890).
Until stronger data are available to guide optimal protein dose and timing, it is reasonable to observe the 2016 ASPEN/SCCM guideline protein recommendation of at least 1.2 g/kg/day. The 2018 ESPEN guideline recommends a similar dose of 1.3 g/kg/day.
Future research and summary
Many questions remain unanswered and present opportunities for future research. Priorities for critical care nutrition research include studying the impact of combined nutrition and exercise in the acute and late phases of critical illness and identifying best tools to differentiate responses to caloric and protein intake.
In summary, critical illness has acute and late phases. The acute phase is a hypercatabolic state leading to negative energy and nitrogen balance and gut dysfunction. Take-home points for nutrition support in the acute phase of critical illness are:
1. It is reasonable to start early hypocaloric EN with an isosmotic formula with slow up-titration over the first week of critical illness while monitoring for refeeding syndrome and feeding intolerance.
2. Use exclusive PN in ICU patients with pre-existing malnutrition when EN is contraindicated or not tolerated.
3. Supplemental PN should probably be restricted during the acute phase of critical illness.
4. Optimal protein dose and timing are unknown. It is reasonable to start with at least 1.2 g/kg ABW/day in non-obese patients.
Dr. Patel is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin.
Dr. Rice is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Vanderbilt University, Nashville, Tennessee.
Environmental Scan: Drivers of philanthropy
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Outcomes of epilepsy surgery at 1 year may be better among older patients
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
REPORTING FROM AES 2019