User login
New evidence informs discussions on FL treatment and breast screening
In this edition of “How I Will Treat My Next Patient,” I highlight two recent studies that reinforce prior diagnostic and treatment preferences among many oncologists and will certainly influence the discussions we all will have with our patients in the coming weeks and months.
Rituximab maintenance in follicular lymphoma
The largest trial addressing the role of maintenance CD20-targeted antibody therapy was the Primary Rituximab and Maintenance (PRIMA) phase 3, intergroup study in 1,018 advanced follicular lymphoma patients with an initial response to induction chemoimmunotherapy. The induction regimen could be either of three commonly used regimens plus rituximab. Importantly, none of the induction regimens included bendamustine.
Patients were randomized to 2 years of rituximab maintenance or observation. Prior interim analyses at 3 and 6 years showed improvements for the rituximab maintenance patients in progression-free survival and other secondary endpoints, but no improvement in overall survival. Emmanuel Bachy, MD, PhD, and colleagues published the final survival data after 9 years of follow-up, including a final safety analysis (J Clin Oncol. 2019 Nov 1;37[31]:2815-24).
Among the 607 patients consenting to extended follow-up, median progression-free survival was 10.5 years with rituximab maintenance, compared with 4.1 years for observation (hazard ratio, 0.61; P less than .001). There were more patients with progression-free survival at 3 years, complete response or unconfirmed complete response at 2 years, longer time to next antilymphoma treatment, and later time to next chemotherapy. But the 10-year overall survival was similar in the two groups, as were the quality of life ratings.
In all, 503 patients experienced disease progression. Overall survival after progression was shorter in the rituximab maintenance arm versus the observation arm. Among the approximately 4% of patients experiencing transformation from follicular lymphoma to a more aggressive histology, there was no difference observed in time to transformation. Results were independent of the induction regimen received, response to induction, Follicular Lymphoma International Prognostic Index score, and other clinical factors.
In the safety analysis, there were more grade 3-4 adverse events among the rituximab maintenance patients – primarily cytopenia and infections – more serious adverse events, and more adverse events overall. Fatal adverse events were low in both groups (1.6 vs. 0.6%).
How these results influence practice
Many years ago, a senior mentor of mine taught that “progression-free survival is an important endpoint since the quality of life for patients is generally superior before relapse than afterwards.”
With that mantra playing in my mind and with the reality that discussions about relapse and subsequent treatment are never easy, the results of PRIMA seem straightforward: Rituximab maintenance is beneficial, despite the absence of an overall survival benefit, in a disease with multiple options for subsequent therapy and a long natural history. In this 9-year, final analysis of PRIMA, rituximab maintenance seems to have achieved its goals of deepening responses with low (but not inconsequential) toxicity and delaying substantially those difficult conversations with patients about how to proceed after relapse.
The influence of the final analysis of PRIMA, however, may be more complicated that it would initially seem. Bendamustine-containing regimens were not employed, are commonly used now, and some studies with bendamustine have suggested higher nonrelapse mortality with rituximab maintenance.
Low-grade adverse events take on greater importance for patients on long-term therapy than they do for patients receiving abbreviated treatment, and with noncurative treatment, the higher adverse event profile with 2 years of rituximab maintenance needs to be taken seriously. Induction and maintenance regimens involving rituximab alone, lenalidomide, or obinutuzumab may be preferred for some patients. For all of those reasons, the discussion about rituximab with advanced follicular lymphoma patients remains a long one, demanding detailed descriptions of risks, benefits, limitations of the data, and multiple modern day alternatives to the treatments employed in PRIMA.
Supplemental MRI for dense breast tissue
In the DENSE trial, investigators assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to be offered either supplemental MRI or mammography screening every other year only. The women were 50-75 years old and were enrolled in the Dutch population-based digital mammography screening program. The primary outcome was the difference in the number of cancers that developed in the 2-year interval between mammograms (N Engl J Med 2019;381:2091-102).
The interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the “MRI-invitation” group (41% of whom did not actually agree to have an MRI), and 5 per 1,000 in the 32,312 women in the “mammography-only” group, a difference of 2.5 per 1,000 screenings (P less than .001).
Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the 59% of women who had undergone MRI (0.8 per 1,000 screenings). The remaining 16 were diagnosed in those who had declined an MRI (4.9 per 1,000 screenings) – virtually identical to the rate of interval cancers in the group that was not invited to have an MRI. This speaks against nonrandom allocation of patients between the mammography-only and the supplemental MRI groups.
Although supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings, there was a false positive rate of 8.0% (79.8 per 1,000 screenings). Of the women who underwent a breast biopsy after MRI, 73.7% did not have cancer. Among the women who had MRI-detected cancers, in general, the malignancies diagnosed were smaller, more likely node negative, better differentiated, and more often hormone receptor–positive, compared with those in the mammography-only group.
At the next screening mammogram, the cancer detection rate was lower in the MRI-screened group (2 per 1,000 screenings) than in the MRI–“offered but declined” (7.1 per 1,000 screenings) or mammogram-only groups (6 per 1,000 screenings).
How these results influence practice
American physicians are obligated to inform women with dense breast tissue about the limitations (for them) of conventional mammography, an unquantified, but elevated, risk of breast cancer in women with dense breast tissue, and the fact that there are no universally accepted recommended subsequent steps those women should take.
One side benefit of the requirement to disclose information about breast density, however, is that disclosure can prompt discussions about breast cancer risk reduction – an important discussion with multiple possible health-maintaining interventions.
The DENSE trial is very important news, with potential for even more value in future years. It is finite (2 years of study, with only one MRI scan mandated), narrow (Vopara or BI-RADs breast density grade 4 only), and oligo-institutional (eight participating centers in the Netherlands). Still, it provides randomized, rigorously gathered and analyzed data where there were previously none. Importantly, it answers the question, “So what can I do now, doctor?”
I have some concerns about whether we, as a medical community, have the discipline to restrict application of supplemental MRI screening beyond the population that was studied in the Netherlands. Will we be able to restrain ourselves from ordering MRI scans in women with heterogeneously dense – but not extremely dense – breasts? Will we truly manage patients whose MRI scans had BI-RADs readings of less than 4 in the rigorous, but conservative, fashion employed in the trial? Would we miss fewer significant cancers and subject fewer patients to potential expense and harm with annual mammography, instead of the biennial screening performed in the Netherlands? Does tomo-synthesis improve the interpretation of screening mammograms so much that the interval cancer rate is a lot closer to the rate obtained with supplemental MRI scans? Is the expense of MRI scanning, with the resultant subsequent tests and procedures, justified by the reduction in detecting relatively favorable breast cancers that may not materially impact overall survival?
The DENSE study promises to be a “gift that keeps on giving” as the investigators continue to assess a number of factors including: the value of ongoing supplemental MRI scans (compared with the one-time-only screening reported here); whether there will be a reduction in the advanced cancers and subsequent mortality benefit; the extent of over diagnosis, costs, and impact on quality-of-life; and the applicability of artificial intelligence techniques to reduce false positive MRI results.
While the study may not be practice changing in the United States at the present time, it may be just that as subsequent analyses emerge.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two recent studies that reinforce prior diagnostic and treatment preferences among many oncologists and will certainly influence the discussions we all will have with our patients in the coming weeks and months.
Rituximab maintenance in follicular lymphoma
The largest trial addressing the role of maintenance CD20-targeted antibody therapy was the Primary Rituximab and Maintenance (PRIMA) phase 3, intergroup study in 1,018 advanced follicular lymphoma patients with an initial response to induction chemoimmunotherapy. The induction regimen could be either of three commonly used regimens plus rituximab. Importantly, none of the induction regimens included bendamustine.
Patients were randomized to 2 years of rituximab maintenance or observation. Prior interim analyses at 3 and 6 years showed improvements for the rituximab maintenance patients in progression-free survival and other secondary endpoints, but no improvement in overall survival. Emmanuel Bachy, MD, PhD, and colleagues published the final survival data after 9 years of follow-up, including a final safety analysis (J Clin Oncol. 2019 Nov 1;37[31]:2815-24).
Among the 607 patients consenting to extended follow-up, median progression-free survival was 10.5 years with rituximab maintenance, compared with 4.1 years for observation (hazard ratio, 0.61; P less than .001). There were more patients with progression-free survival at 3 years, complete response or unconfirmed complete response at 2 years, longer time to next antilymphoma treatment, and later time to next chemotherapy. But the 10-year overall survival was similar in the two groups, as were the quality of life ratings.
In all, 503 patients experienced disease progression. Overall survival after progression was shorter in the rituximab maintenance arm versus the observation arm. Among the approximately 4% of patients experiencing transformation from follicular lymphoma to a more aggressive histology, there was no difference observed in time to transformation. Results were independent of the induction regimen received, response to induction, Follicular Lymphoma International Prognostic Index score, and other clinical factors.
In the safety analysis, there were more grade 3-4 adverse events among the rituximab maintenance patients – primarily cytopenia and infections – more serious adverse events, and more adverse events overall. Fatal adverse events were low in both groups (1.6 vs. 0.6%).
How these results influence practice
Many years ago, a senior mentor of mine taught that “progression-free survival is an important endpoint since the quality of life for patients is generally superior before relapse than afterwards.”
With that mantra playing in my mind and with the reality that discussions about relapse and subsequent treatment are never easy, the results of PRIMA seem straightforward: Rituximab maintenance is beneficial, despite the absence of an overall survival benefit, in a disease with multiple options for subsequent therapy and a long natural history. In this 9-year, final analysis of PRIMA, rituximab maintenance seems to have achieved its goals of deepening responses with low (but not inconsequential) toxicity and delaying substantially those difficult conversations with patients about how to proceed after relapse.
The influence of the final analysis of PRIMA, however, may be more complicated that it would initially seem. Bendamustine-containing regimens were not employed, are commonly used now, and some studies with bendamustine have suggested higher nonrelapse mortality with rituximab maintenance.
Low-grade adverse events take on greater importance for patients on long-term therapy than they do for patients receiving abbreviated treatment, and with noncurative treatment, the higher adverse event profile with 2 years of rituximab maintenance needs to be taken seriously. Induction and maintenance regimens involving rituximab alone, lenalidomide, or obinutuzumab may be preferred for some patients. For all of those reasons, the discussion about rituximab with advanced follicular lymphoma patients remains a long one, demanding detailed descriptions of risks, benefits, limitations of the data, and multiple modern day alternatives to the treatments employed in PRIMA.
Supplemental MRI for dense breast tissue
In the DENSE trial, investigators assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to be offered either supplemental MRI or mammography screening every other year only. The women were 50-75 years old and were enrolled in the Dutch population-based digital mammography screening program. The primary outcome was the difference in the number of cancers that developed in the 2-year interval between mammograms (N Engl J Med 2019;381:2091-102).
The interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the “MRI-invitation” group (41% of whom did not actually agree to have an MRI), and 5 per 1,000 in the 32,312 women in the “mammography-only” group, a difference of 2.5 per 1,000 screenings (P less than .001).
Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the 59% of women who had undergone MRI (0.8 per 1,000 screenings). The remaining 16 were diagnosed in those who had declined an MRI (4.9 per 1,000 screenings) – virtually identical to the rate of interval cancers in the group that was not invited to have an MRI. This speaks against nonrandom allocation of patients between the mammography-only and the supplemental MRI groups.
Although supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings, there was a false positive rate of 8.0% (79.8 per 1,000 screenings). Of the women who underwent a breast biopsy after MRI, 73.7% did not have cancer. Among the women who had MRI-detected cancers, in general, the malignancies diagnosed were smaller, more likely node negative, better differentiated, and more often hormone receptor–positive, compared with those in the mammography-only group.
At the next screening mammogram, the cancer detection rate was lower in the MRI-screened group (2 per 1,000 screenings) than in the MRI–“offered but declined” (7.1 per 1,000 screenings) or mammogram-only groups (6 per 1,000 screenings).
How these results influence practice
American physicians are obligated to inform women with dense breast tissue about the limitations (for them) of conventional mammography, an unquantified, but elevated, risk of breast cancer in women with dense breast tissue, and the fact that there are no universally accepted recommended subsequent steps those women should take.
One side benefit of the requirement to disclose information about breast density, however, is that disclosure can prompt discussions about breast cancer risk reduction – an important discussion with multiple possible health-maintaining interventions.
The DENSE trial is very important news, with potential for even more value in future years. It is finite (2 years of study, with only one MRI scan mandated), narrow (Vopara or BI-RADs breast density grade 4 only), and oligo-institutional (eight participating centers in the Netherlands). Still, it provides randomized, rigorously gathered and analyzed data where there were previously none. Importantly, it answers the question, “So what can I do now, doctor?”
I have some concerns about whether we, as a medical community, have the discipline to restrict application of supplemental MRI screening beyond the population that was studied in the Netherlands. Will we be able to restrain ourselves from ordering MRI scans in women with heterogeneously dense – but not extremely dense – breasts? Will we truly manage patients whose MRI scans had BI-RADs readings of less than 4 in the rigorous, but conservative, fashion employed in the trial? Would we miss fewer significant cancers and subject fewer patients to potential expense and harm with annual mammography, instead of the biennial screening performed in the Netherlands? Does tomo-synthesis improve the interpretation of screening mammograms so much that the interval cancer rate is a lot closer to the rate obtained with supplemental MRI scans? Is the expense of MRI scanning, with the resultant subsequent tests and procedures, justified by the reduction in detecting relatively favorable breast cancers that may not materially impact overall survival?
The DENSE study promises to be a “gift that keeps on giving” as the investigators continue to assess a number of factors including: the value of ongoing supplemental MRI scans (compared with the one-time-only screening reported here); whether there will be a reduction in the advanced cancers and subsequent mortality benefit; the extent of over diagnosis, costs, and impact on quality-of-life; and the applicability of artificial intelligence techniques to reduce false positive MRI results.
While the study may not be practice changing in the United States at the present time, it may be just that as subsequent analyses emerge.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two recent studies that reinforce prior diagnostic and treatment preferences among many oncologists and will certainly influence the discussions we all will have with our patients in the coming weeks and months.
Rituximab maintenance in follicular lymphoma
The largest trial addressing the role of maintenance CD20-targeted antibody therapy was the Primary Rituximab and Maintenance (PRIMA) phase 3, intergroup study in 1,018 advanced follicular lymphoma patients with an initial response to induction chemoimmunotherapy. The induction regimen could be either of three commonly used regimens plus rituximab. Importantly, none of the induction regimens included bendamustine.
Patients were randomized to 2 years of rituximab maintenance or observation. Prior interim analyses at 3 and 6 years showed improvements for the rituximab maintenance patients in progression-free survival and other secondary endpoints, but no improvement in overall survival. Emmanuel Bachy, MD, PhD, and colleagues published the final survival data after 9 years of follow-up, including a final safety analysis (J Clin Oncol. 2019 Nov 1;37[31]:2815-24).
Among the 607 patients consenting to extended follow-up, median progression-free survival was 10.5 years with rituximab maintenance, compared with 4.1 years for observation (hazard ratio, 0.61; P less than .001). There were more patients with progression-free survival at 3 years, complete response or unconfirmed complete response at 2 years, longer time to next antilymphoma treatment, and later time to next chemotherapy. But the 10-year overall survival was similar in the two groups, as were the quality of life ratings.
In all, 503 patients experienced disease progression. Overall survival after progression was shorter in the rituximab maintenance arm versus the observation arm. Among the approximately 4% of patients experiencing transformation from follicular lymphoma to a more aggressive histology, there was no difference observed in time to transformation. Results were independent of the induction regimen received, response to induction, Follicular Lymphoma International Prognostic Index score, and other clinical factors.
In the safety analysis, there were more grade 3-4 adverse events among the rituximab maintenance patients – primarily cytopenia and infections – more serious adverse events, and more adverse events overall. Fatal adverse events were low in both groups (1.6 vs. 0.6%).
How these results influence practice
Many years ago, a senior mentor of mine taught that “progression-free survival is an important endpoint since the quality of life for patients is generally superior before relapse than afterwards.”
With that mantra playing in my mind and with the reality that discussions about relapse and subsequent treatment are never easy, the results of PRIMA seem straightforward: Rituximab maintenance is beneficial, despite the absence of an overall survival benefit, in a disease with multiple options for subsequent therapy and a long natural history. In this 9-year, final analysis of PRIMA, rituximab maintenance seems to have achieved its goals of deepening responses with low (but not inconsequential) toxicity and delaying substantially those difficult conversations with patients about how to proceed after relapse.
The influence of the final analysis of PRIMA, however, may be more complicated that it would initially seem. Bendamustine-containing regimens were not employed, are commonly used now, and some studies with bendamustine have suggested higher nonrelapse mortality with rituximab maintenance.
Low-grade adverse events take on greater importance for patients on long-term therapy than they do for patients receiving abbreviated treatment, and with noncurative treatment, the higher adverse event profile with 2 years of rituximab maintenance needs to be taken seriously. Induction and maintenance regimens involving rituximab alone, lenalidomide, or obinutuzumab may be preferred for some patients. For all of those reasons, the discussion about rituximab with advanced follicular lymphoma patients remains a long one, demanding detailed descriptions of risks, benefits, limitations of the data, and multiple modern day alternatives to the treatments employed in PRIMA.
Supplemental MRI for dense breast tissue
In the DENSE trial, investigators assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to be offered either supplemental MRI or mammography screening every other year only. The women were 50-75 years old and were enrolled in the Dutch population-based digital mammography screening program. The primary outcome was the difference in the number of cancers that developed in the 2-year interval between mammograms (N Engl J Med 2019;381:2091-102).
The interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the “MRI-invitation” group (41% of whom did not actually agree to have an MRI), and 5 per 1,000 in the 32,312 women in the “mammography-only” group, a difference of 2.5 per 1,000 screenings (P less than .001).
Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the 59% of women who had undergone MRI (0.8 per 1,000 screenings). The remaining 16 were diagnosed in those who had declined an MRI (4.9 per 1,000 screenings) – virtually identical to the rate of interval cancers in the group that was not invited to have an MRI. This speaks against nonrandom allocation of patients between the mammography-only and the supplemental MRI groups.
Although supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings, there was a false positive rate of 8.0% (79.8 per 1,000 screenings). Of the women who underwent a breast biopsy after MRI, 73.7% did not have cancer. Among the women who had MRI-detected cancers, in general, the malignancies diagnosed were smaller, more likely node negative, better differentiated, and more often hormone receptor–positive, compared with those in the mammography-only group.
At the next screening mammogram, the cancer detection rate was lower in the MRI-screened group (2 per 1,000 screenings) than in the MRI–“offered but declined” (7.1 per 1,000 screenings) or mammogram-only groups (6 per 1,000 screenings).
How these results influence practice
American physicians are obligated to inform women with dense breast tissue about the limitations (for them) of conventional mammography, an unquantified, but elevated, risk of breast cancer in women with dense breast tissue, and the fact that there are no universally accepted recommended subsequent steps those women should take.
One side benefit of the requirement to disclose information about breast density, however, is that disclosure can prompt discussions about breast cancer risk reduction – an important discussion with multiple possible health-maintaining interventions.
The DENSE trial is very important news, with potential for even more value in future years. It is finite (2 years of study, with only one MRI scan mandated), narrow (Vopara or BI-RADs breast density grade 4 only), and oligo-institutional (eight participating centers in the Netherlands). Still, it provides randomized, rigorously gathered and analyzed data where there were previously none. Importantly, it answers the question, “So what can I do now, doctor?”
I have some concerns about whether we, as a medical community, have the discipline to restrict application of supplemental MRI screening beyond the population that was studied in the Netherlands. Will we be able to restrain ourselves from ordering MRI scans in women with heterogeneously dense – but not extremely dense – breasts? Will we truly manage patients whose MRI scans had BI-RADs readings of less than 4 in the rigorous, but conservative, fashion employed in the trial? Would we miss fewer significant cancers and subject fewer patients to potential expense and harm with annual mammography, instead of the biennial screening performed in the Netherlands? Does tomo-synthesis improve the interpretation of screening mammograms so much that the interval cancer rate is a lot closer to the rate obtained with supplemental MRI scans? Is the expense of MRI scanning, with the resultant subsequent tests and procedures, justified by the reduction in detecting relatively favorable breast cancers that may not materially impact overall survival?
The DENSE study promises to be a “gift that keeps on giving” as the investigators continue to assess a number of factors including: the value of ongoing supplemental MRI scans (compared with the one-time-only screening reported here); whether there will be a reduction in the advanced cancers and subsequent mortality benefit; the extent of over diagnosis, costs, and impact on quality-of-life; and the applicability of artificial intelligence techniques to reduce false positive MRI results.
While the study may not be practice changing in the United States at the present time, it may be just that as subsequent analyses emerge.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Widespread Skin Thickening
The Diagnosis: Scleromyxedema
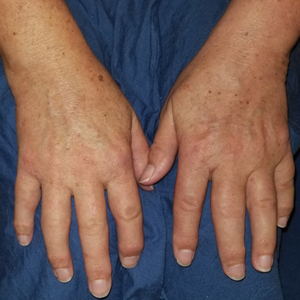
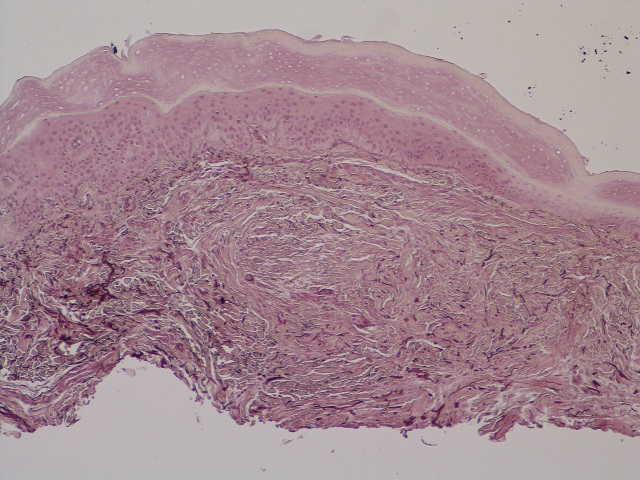
Scleromyxedema is a rare skin disorder characterized by a diffuse eruption of small waxy papules that are linearly arranged and closely spaced together. As the papular lesions coalesce, the skin thickens. Firm induration of the skin is widespread and--unlike the distribution in scleroderma and scleredema--amplified over the facial convexities, especially the glabella and ears. Histopathology reveals the classic triad of mucin accumulation, proliferation of fibroblasts, and collagen deposition with associated fibrotic changes.1
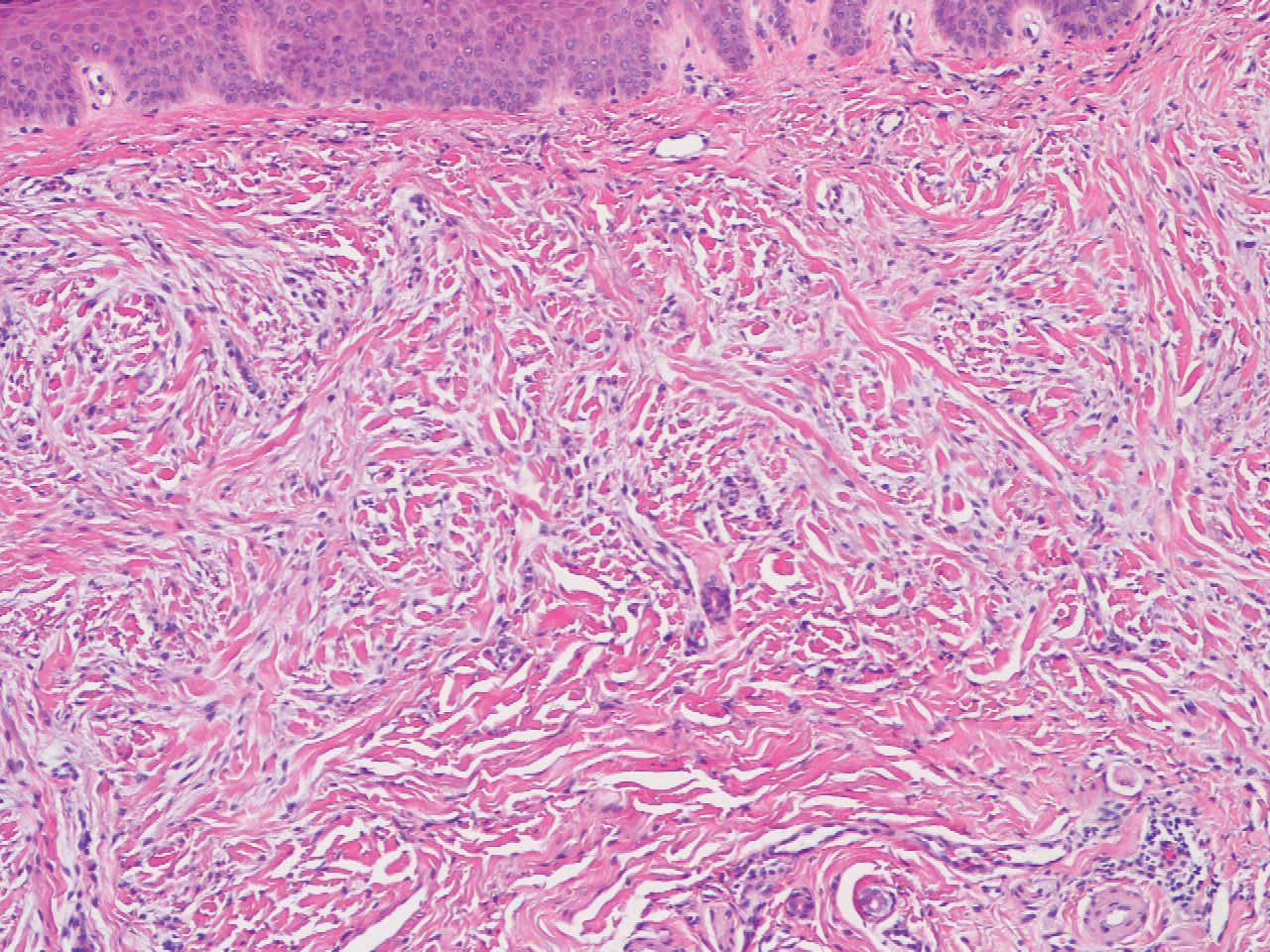
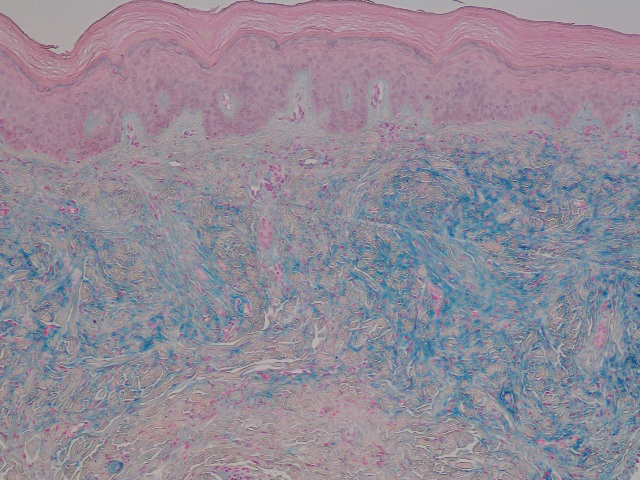
In this case, the patient exhibited the characteristic doughnut sign over the second interphalangeal joint of the right hand due to thickening and dimpling of the skin (quiz image). Histopathology from the right second finger showed the dermis with a spindled histiocytic infiltrate, fibroblasts, fibrosis, and increased interstitial mucin deposition highlighted with colloidal iron stain (Figures 1 and 2). Multinucleated giant cells also were seen in the dermis (Figure 3), and a Shikata orcein stain illustrated decreased elastic fibers (stained black) in the superficial dermis within areas of increased collagen deposition (Figure 4).
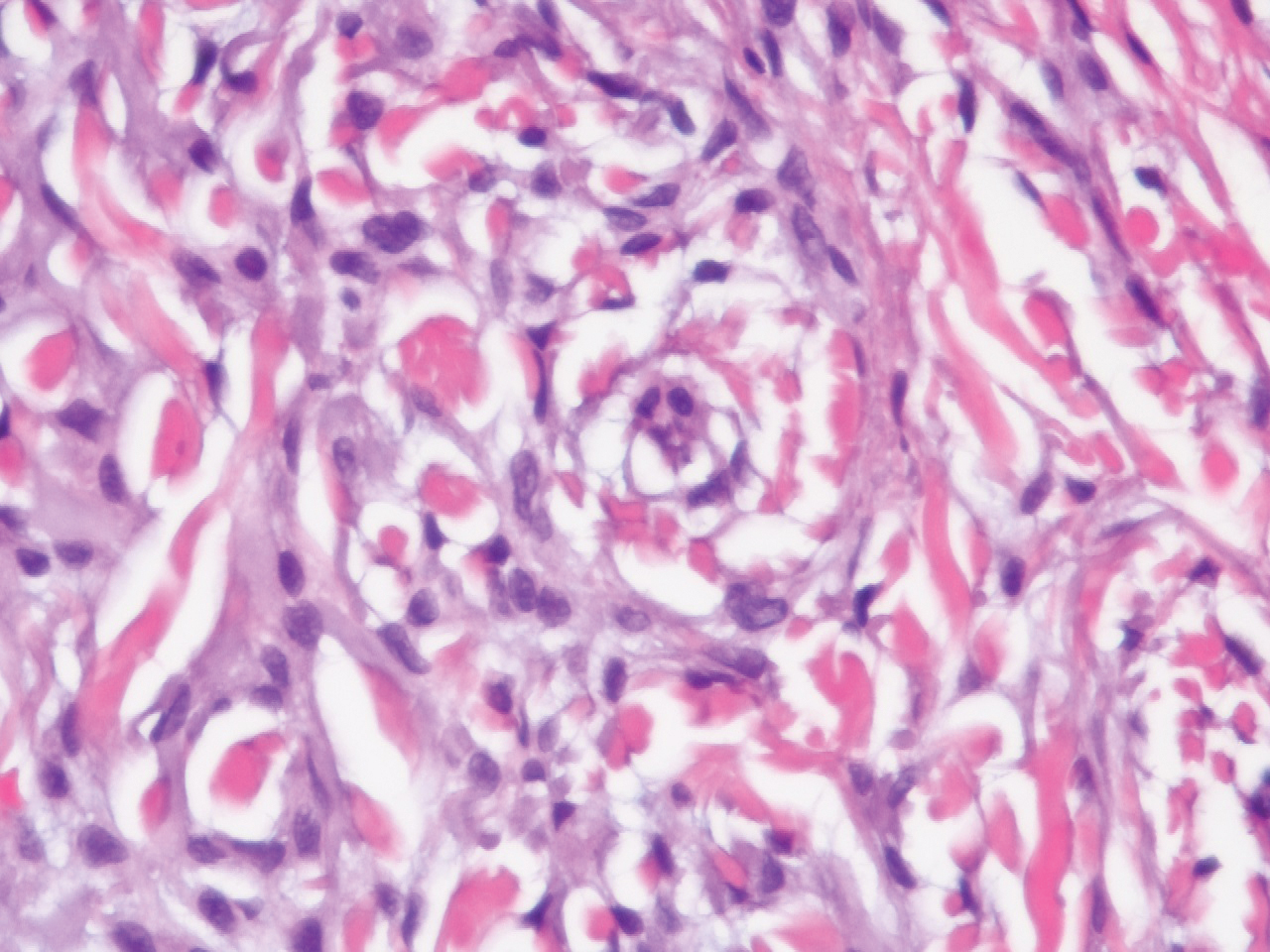
Scleromyxedema is strongly associated with a monoclonal gammopathy, which is generally mild and, in almost 80% of cases, related to λ IgG. However, the clinical implications are unclear.2,3 Scleromyxedema also is accompanied by systemic features, most commonly dysphagia, myalgias, and arthralgias, which distinguishes it from other cutaneous mucinoses. Notable morbidity and mortality are associated with involvement of the central nervous system, which can manifest as encephalopathy, seizures, or coma. Mucin deposition may be found in the myocardium and the coronary and pulmonary vasculature, resulting in rare cases of cardiac and pulmonary disease.4,5
Clinically, scleroderma also demonstrates progressive generalized skin tightening. Histopathology reveals hyalinization and thickening of the connective tissue of the deep dermis, subcutaneous fat, and muscular fascia. These changes are accompanied by a perivascular and focal interstitial lymphocytic and plasma cell infiltrate with prominent myofibroblast proliferation. In this case, the normal nail fold capillaries and absence of Raynaud phenomenon help to distinguish the diagnosis from scleroderma. In limited scleroderma, common findings include calcinosis, esophageal dysmotility, telangiectasia, and pulmonary hypertension, while systemic sclerosis can involve the internal organs, with the greatest morbidity stemming from interstitial lung disease.3,6,7
Nephrogenic systemic fibrosis is another disorder of skin thickening that involves the extremities and trunk. It is a rare complication of exposure to gadolinium contrast media in patients with advanced renal disease and those undergoing dialysis; use of gadolinium is now strictly avoided in patients with at least moderately impaired glomerular filtration rate.8 Although nephrogenic systemic fibrosis histologically looks similar to scleromyxedema, it involves deeper tissues. There is fibrosis with haphazard collagen bundle deposition in the deep dermis and subcutaneous septa. Fibroblasts and histiocytes are increased between the collagen bundles, with surrounding edema and mucin buildup. Multinucleated giant cells may or may not be present.9
Patients with scleredema present with nonpitting edema and dermal hardening over the neck, face, upper trunk, and arms. Lesional skin appears shiny and indurated. The skin is characteristically difficult to wrinkle, and the face may appear expressionless. Skin biopsy will show a thickened reticular dermis with mucin deposition and eccrine glands appearing in the upper third or mid dermis. Fibroblasts are normal in number. Scleredema generally is associated with poorly controlled diabetes, a monoclonal gammopathy, or as the aftermath of an acute infection, especially streptococcal pharyngitis. The condition may develop at any age, though nearly 50% of cases occur in children and adolescents.3,10
Interstitial granuloma annulare (GA) is a common, benign, and self-limiting dermatosis. Distinct from other skin-thickening disorders, GA is composed of smooth annular papules and plaques that do not result in skin hardening. Although GA may manifest in a generalized distribution, it is more frequently localized to the distal extremities.11 The presence of an inflammatory infiltrate surrounded by abundant mucin and collagen resembles scleromyxedema. However, GA is distinguished by palisading granulomas of histiocytes, fibroblasts, and lymphocytes lining a necrobiotic center of collagen, mucin, and fibrin.12
This patient's skin showed an immediate and marked response to intravenous immunoglobulin with dramatic softening. Such a response is well reported in the literature, and intravenous immunoglobulin is considered first-line therapy for scleromyxedema, typically in conjunction with steroids.5 Other immunosuppressive agents have been employed with reported efficacy, as have several treatments used for multiple myeloma, including thalidomide and lenalidomide.
Although spontaneous improvement infrequently occurs, a chronic progressive course is far more common with scleromyxedema. Studies are ongoing to elucidate the etiopathogenesis of scleromyxedema, scleroderma, and similar disorders to uncover the triggers that provoke the underlying dysregulation of dermal fibroblast activation and proliferation, which could offer a more precise target for effective treatments.5,13-15
- Crowe DR. Scleromyxedema. In: Crowe DR, Morgan M, Somach S, Trapp K, eds. Deadly Dermatologic Diseases: Clinicopathologic Atlas and Text. Cham, Switzerland: Springer International Publishing; 2016:139-142.
- Farmer ER, Hambrick GW Jr, Shulman LE. Papular mucinosis: a clinicopathologic study of four patients. Arch Dermatol. 1982;118:9-13.
- Rongioletti F. Mucinoses. In: Smoller BR, Rongioletti F, eds. Clinical and Pathological Aspects of Skin Diseases in Endocrine, Metabolic, Nutritional and Deposition Disease. New York, NY: Springer New York; 2010:139-152.
- Gabriel PH, Oleson GB, Bowles GA. Scleromyxoedema: a scleroderma-like disorder with systemic manifestations. Medicine. 1988;67:58-65.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
- Hamodat M. Scleroderma. PathologyOutlines.com. http://www.pathologyoutlines.com/topic/skinnontumorscleroderma.html. Published August 1, 2011. Updated March 29, 2019. Accessed December 11, 2019.
- Van Praet JT, Smith V, Haspeslagh M, et al. Histopathological cutaneous alterations in systemic sclerosis: a clinicopathological study. Arthritis Res Ther. 2011;13:R35.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol. 2008;66:191-199.
- Boin F, Hummers LK. Scleroderma-like fibrosing disorders. Rheum Dis Clin North Am. 2008;34:199-ix.
- Plaza JA, Prieto VG. Inflammatory Skin Conditions. In: Modern Surgical Pathology. 2nd ed. Philadelphia, PA: Saunders; 2009:1843-1889.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Blum M, Wigley FM, Hummers LK. Scleromyxedema: a case series highlighting long-term outcomes of treatment with intravenous immunoglobulin (IVIG). Medicine. 2008;87:10-20.
- Caradonna S, Jacobe H. Thalidomide as a potential treatment for scleromyxedema. Arch Dermatol. 2004;140:277-280.
- Yeung CK, Loong F, Kwong YL. Scleromyxoedema due to a plasma cell neoplasm: rapid remission with bortezomib, thalidomide and dexamethasone. Br J Haematol. 2012;157:411.
The Diagnosis: Scleromyxedema
Scleromyxedema is a rare skin disorder characterized by a diffuse eruption of small waxy papules that are linearly arranged and closely spaced together. As the papular lesions coalesce, the skin thickens. Firm induration of the skin is widespread and--unlike the distribution in scleroderma and scleredema--amplified over the facial convexities, especially the glabella and ears. Histopathology reveals the classic triad of mucin accumulation, proliferation of fibroblasts, and collagen deposition with associated fibrotic changes.1
In this case, the patient exhibited the characteristic doughnut sign over the second interphalangeal joint of the right hand due to thickening and dimpling of the skin (quiz image). Histopathology from the right second finger showed the dermis with a spindled histiocytic infiltrate, fibroblasts, fibrosis, and increased interstitial mucin deposition highlighted with colloidal iron stain (Figures 1 and 2). Multinucleated giant cells also were seen in the dermis (Figure 3), and a Shikata orcein stain illustrated decreased elastic fibers (stained black) in the superficial dermis within areas of increased collagen deposition (Figure 4).




Scleromyxedema is strongly associated with a monoclonal gammopathy, which is generally mild and, in almost 80% of cases, related to λ IgG. However, the clinical implications are unclear.2,3 Scleromyxedema also is accompanied by systemic features, most commonly dysphagia, myalgias, and arthralgias, which distinguishes it from other cutaneous mucinoses. Notable morbidity and mortality are associated with involvement of the central nervous system, which can manifest as encephalopathy, seizures, or coma. Mucin deposition may be found in the myocardium and the coronary and pulmonary vasculature, resulting in rare cases of cardiac and pulmonary disease.4,5
Clinically, scleroderma also demonstrates progressive generalized skin tightening. Histopathology reveals hyalinization and thickening of the connective tissue of the deep dermis, subcutaneous fat, and muscular fascia. These changes are accompanied by a perivascular and focal interstitial lymphocytic and plasma cell infiltrate with prominent myofibroblast proliferation. In this case, the normal nail fold capillaries and absence of Raynaud phenomenon help to distinguish the diagnosis from scleroderma. In limited scleroderma, common findings include calcinosis, esophageal dysmotility, telangiectasia, and pulmonary hypertension, while systemic sclerosis can involve the internal organs, with the greatest morbidity stemming from interstitial lung disease.3,6,7
Nephrogenic systemic fibrosis is another disorder of skin thickening that involves the extremities and trunk. It is a rare complication of exposure to gadolinium contrast media in patients with advanced renal disease and those undergoing dialysis; use of gadolinium is now strictly avoided in patients with at least moderately impaired glomerular filtration rate.8 Although nephrogenic systemic fibrosis histologically looks similar to scleromyxedema, it involves deeper tissues. There is fibrosis with haphazard collagen bundle deposition in the deep dermis and subcutaneous septa. Fibroblasts and histiocytes are increased between the collagen bundles, with surrounding edema and mucin buildup. Multinucleated giant cells may or may not be present.9
Patients with scleredema present with nonpitting edema and dermal hardening over the neck, face, upper trunk, and arms. Lesional skin appears shiny and indurated. The skin is characteristically difficult to wrinkle, and the face may appear expressionless. Skin biopsy will show a thickened reticular dermis with mucin deposition and eccrine glands appearing in the upper third or mid dermis. Fibroblasts are normal in number. Scleredema generally is associated with poorly controlled diabetes, a monoclonal gammopathy, or as the aftermath of an acute infection, especially streptococcal pharyngitis. The condition may develop at any age, though nearly 50% of cases occur in children and adolescents.3,10
Interstitial granuloma annulare (GA) is a common, benign, and self-limiting dermatosis. Distinct from other skin-thickening disorders, GA is composed of smooth annular papules and plaques that do not result in skin hardening. Although GA may manifest in a generalized distribution, it is more frequently localized to the distal extremities.11 The presence of an inflammatory infiltrate surrounded by abundant mucin and collagen resembles scleromyxedema. However, GA is distinguished by palisading granulomas of histiocytes, fibroblasts, and lymphocytes lining a necrobiotic center of collagen, mucin, and fibrin.12
This patient's skin showed an immediate and marked response to intravenous immunoglobulin with dramatic softening. Such a response is well reported in the literature, and intravenous immunoglobulin is considered first-line therapy for scleromyxedema, typically in conjunction with steroids.5 Other immunosuppressive agents have been employed with reported efficacy, as have several treatments used for multiple myeloma, including thalidomide and lenalidomide.
Although spontaneous improvement infrequently occurs, a chronic progressive course is far more common with scleromyxedema. Studies are ongoing to elucidate the etiopathogenesis of scleromyxedema, scleroderma, and similar disorders to uncover the triggers that provoke the underlying dysregulation of dermal fibroblast activation and proliferation, which could offer a more precise target for effective treatments.5,13-15
The Diagnosis: Scleromyxedema
Scleromyxedema is a rare skin disorder characterized by a diffuse eruption of small waxy papules that are linearly arranged and closely spaced together. As the papular lesions coalesce, the skin thickens. Firm induration of the skin is widespread and--unlike the distribution in scleroderma and scleredema--amplified over the facial convexities, especially the glabella and ears. Histopathology reveals the classic triad of mucin accumulation, proliferation of fibroblasts, and collagen deposition with associated fibrotic changes.1
In this case, the patient exhibited the characteristic doughnut sign over the second interphalangeal joint of the right hand due to thickening and dimpling of the skin (quiz image). Histopathology from the right second finger showed the dermis with a spindled histiocytic infiltrate, fibroblasts, fibrosis, and increased interstitial mucin deposition highlighted with colloidal iron stain (Figures 1 and 2). Multinucleated giant cells also were seen in the dermis (Figure 3), and a Shikata orcein stain illustrated decreased elastic fibers (stained black) in the superficial dermis within areas of increased collagen deposition (Figure 4).




Scleromyxedema is strongly associated with a monoclonal gammopathy, which is generally mild and, in almost 80% of cases, related to λ IgG. However, the clinical implications are unclear.2,3 Scleromyxedema also is accompanied by systemic features, most commonly dysphagia, myalgias, and arthralgias, which distinguishes it from other cutaneous mucinoses. Notable morbidity and mortality are associated with involvement of the central nervous system, which can manifest as encephalopathy, seizures, or coma. Mucin deposition may be found in the myocardium and the coronary and pulmonary vasculature, resulting in rare cases of cardiac and pulmonary disease.4,5
Clinically, scleroderma also demonstrates progressive generalized skin tightening. Histopathology reveals hyalinization and thickening of the connective tissue of the deep dermis, subcutaneous fat, and muscular fascia. These changes are accompanied by a perivascular and focal interstitial lymphocytic and plasma cell infiltrate with prominent myofibroblast proliferation. In this case, the normal nail fold capillaries and absence of Raynaud phenomenon help to distinguish the diagnosis from scleroderma. In limited scleroderma, common findings include calcinosis, esophageal dysmotility, telangiectasia, and pulmonary hypertension, while systemic sclerosis can involve the internal organs, with the greatest morbidity stemming from interstitial lung disease.3,6,7
Nephrogenic systemic fibrosis is another disorder of skin thickening that involves the extremities and trunk. It is a rare complication of exposure to gadolinium contrast media in patients with advanced renal disease and those undergoing dialysis; use of gadolinium is now strictly avoided in patients with at least moderately impaired glomerular filtration rate.8 Although nephrogenic systemic fibrosis histologically looks similar to scleromyxedema, it involves deeper tissues. There is fibrosis with haphazard collagen bundle deposition in the deep dermis and subcutaneous septa. Fibroblasts and histiocytes are increased between the collagen bundles, with surrounding edema and mucin buildup. Multinucleated giant cells may or may not be present.9
Patients with scleredema present with nonpitting edema and dermal hardening over the neck, face, upper trunk, and arms. Lesional skin appears shiny and indurated. The skin is characteristically difficult to wrinkle, and the face may appear expressionless. Skin biopsy will show a thickened reticular dermis with mucin deposition and eccrine glands appearing in the upper third or mid dermis. Fibroblasts are normal in number. Scleredema generally is associated with poorly controlled diabetes, a monoclonal gammopathy, or as the aftermath of an acute infection, especially streptococcal pharyngitis. The condition may develop at any age, though nearly 50% of cases occur in children and adolescents.3,10
Interstitial granuloma annulare (GA) is a common, benign, and self-limiting dermatosis. Distinct from other skin-thickening disorders, GA is composed of smooth annular papules and plaques that do not result in skin hardening. Although GA may manifest in a generalized distribution, it is more frequently localized to the distal extremities.11 The presence of an inflammatory infiltrate surrounded by abundant mucin and collagen resembles scleromyxedema. However, GA is distinguished by palisading granulomas of histiocytes, fibroblasts, and lymphocytes lining a necrobiotic center of collagen, mucin, and fibrin.12
This patient's skin showed an immediate and marked response to intravenous immunoglobulin with dramatic softening. Such a response is well reported in the literature, and intravenous immunoglobulin is considered first-line therapy for scleromyxedema, typically in conjunction with steroids.5 Other immunosuppressive agents have been employed with reported efficacy, as have several treatments used for multiple myeloma, including thalidomide and lenalidomide.
Although spontaneous improvement infrequently occurs, a chronic progressive course is far more common with scleromyxedema. Studies are ongoing to elucidate the etiopathogenesis of scleromyxedema, scleroderma, and similar disorders to uncover the triggers that provoke the underlying dysregulation of dermal fibroblast activation and proliferation, which could offer a more precise target for effective treatments.5,13-15
- Crowe DR. Scleromyxedema. In: Crowe DR, Morgan M, Somach S, Trapp K, eds. Deadly Dermatologic Diseases: Clinicopathologic Atlas and Text. Cham, Switzerland: Springer International Publishing; 2016:139-142.
- Farmer ER, Hambrick GW Jr, Shulman LE. Papular mucinosis: a clinicopathologic study of four patients. Arch Dermatol. 1982;118:9-13.
- Rongioletti F. Mucinoses. In: Smoller BR, Rongioletti F, eds. Clinical and Pathological Aspects of Skin Diseases in Endocrine, Metabolic, Nutritional and Deposition Disease. New York, NY: Springer New York; 2010:139-152.
- Gabriel PH, Oleson GB, Bowles GA. Scleromyxoedema: a scleroderma-like disorder with systemic manifestations. Medicine. 1988;67:58-65.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
- Hamodat M. Scleroderma. PathologyOutlines.com. http://www.pathologyoutlines.com/topic/skinnontumorscleroderma.html. Published August 1, 2011. Updated March 29, 2019. Accessed December 11, 2019.
- Van Praet JT, Smith V, Haspeslagh M, et al. Histopathological cutaneous alterations in systemic sclerosis: a clinicopathological study. Arthritis Res Ther. 2011;13:R35.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol. 2008;66:191-199.
- Boin F, Hummers LK. Scleroderma-like fibrosing disorders. Rheum Dis Clin North Am. 2008;34:199-ix.
- Plaza JA, Prieto VG. Inflammatory Skin Conditions. In: Modern Surgical Pathology. 2nd ed. Philadelphia, PA: Saunders; 2009:1843-1889.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Blum M, Wigley FM, Hummers LK. Scleromyxedema: a case series highlighting long-term outcomes of treatment with intravenous immunoglobulin (IVIG). Medicine. 2008;87:10-20.
- Caradonna S, Jacobe H. Thalidomide as a potential treatment for scleromyxedema. Arch Dermatol. 2004;140:277-280.
- Yeung CK, Loong F, Kwong YL. Scleromyxoedema due to a plasma cell neoplasm: rapid remission with bortezomib, thalidomide and dexamethasone. Br J Haematol. 2012;157:411.
- Crowe DR. Scleromyxedema. In: Crowe DR, Morgan M, Somach S, Trapp K, eds. Deadly Dermatologic Diseases: Clinicopathologic Atlas and Text. Cham, Switzerland: Springer International Publishing; 2016:139-142.
- Farmer ER, Hambrick GW Jr, Shulman LE. Papular mucinosis: a clinicopathologic study of four patients. Arch Dermatol. 1982;118:9-13.
- Rongioletti F. Mucinoses. In: Smoller BR, Rongioletti F, eds. Clinical and Pathological Aspects of Skin Diseases in Endocrine, Metabolic, Nutritional and Deposition Disease. New York, NY: Springer New York; 2010:139-152.
- Gabriel PH, Oleson GB, Bowles GA. Scleromyxoedema: a scleroderma-like disorder with systemic manifestations. Medicine. 1988;67:58-65.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
- Hamodat M. Scleroderma. PathologyOutlines.com. http://www.pathologyoutlines.com/topic/skinnontumorscleroderma.html. Published August 1, 2011. Updated March 29, 2019. Accessed December 11, 2019.
- Van Praet JT, Smith V, Haspeslagh M, et al. Histopathological cutaneous alterations in systemic sclerosis: a clinicopathological study. Arthritis Res Ther. 2011;13:R35.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol. 2008;66:191-199.
- Boin F, Hummers LK. Scleroderma-like fibrosing disorders. Rheum Dis Clin North Am. 2008;34:199-ix.
- Plaza JA, Prieto VG. Inflammatory Skin Conditions. In: Modern Surgical Pathology. 2nd ed. Philadelphia, PA: Saunders; 2009:1843-1889.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Blum M, Wigley FM, Hummers LK. Scleromyxedema: a case series highlighting long-term outcomes of treatment with intravenous immunoglobulin (IVIG). Medicine. 2008;87:10-20.
- Caradonna S, Jacobe H. Thalidomide as a potential treatment for scleromyxedema. Arch Dermatol. 2004;140:277-280.
- Yeung CK, Loong F, Kwong YL. Scleromyxoedema due to a plasma cell neoplasm: rapid remission with bortezomib, thalidomide and dexamethasone. Br J Haematol. 2012;157:411.

A 62-year-old woman presented with widespread skin thickening and tightness that progressed over 2 months. Physical examination showed generalized, nonpruritic, nonpainful, flesh-colored papules along the fingers, bilateral arms and legs, chest, neck, forehead, chin, and ears. She reported mild acid reflux on review of systems. She denied a history of Raynaud phenomenon and had normal-appearing nail beds on capillaroscopy. Laboratory studies including autoimmune serologies were normal, aside from a mildly elevated monoclonal IgG spike.
Verruciform Plaques Within a Tattoo of an HIV-Positive Patient
The Diagnosis: Lichenoid Reaction With Pseudoepitheliomatous Hyperplasia
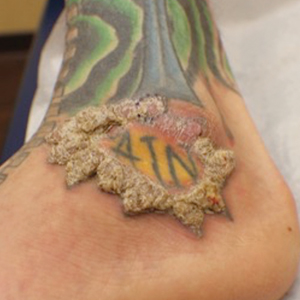
A shave biopsy of the left ankle and a punch biopsy of the left medial calf were performed and sent for histologic examination and acid-fast stain. Bacterial, fungal, and mycobacterial tissue cultures also were sent for testing. The findings from direct examination were negative, and tissue cultures exhibited no growth. The shave and punch biopsies and histology revealed pseudoepitheliomatous hyperplasia (PEH) with keratinocyte necrosis, satellitosis, and areas of acute folliculitis (Figure 1). A lichenoid hypersensitivity mixed infiltrate that included histiocytes admixed with anthracotic and red-orange pigment, lymphocytes, plasma cells, neutrophils, and rare eosinophils was noted in the dermis. Given these clinical and histopathologic findings, the patient was diagnosed with red-pigment tattoo lichenoid reaction with PEH.
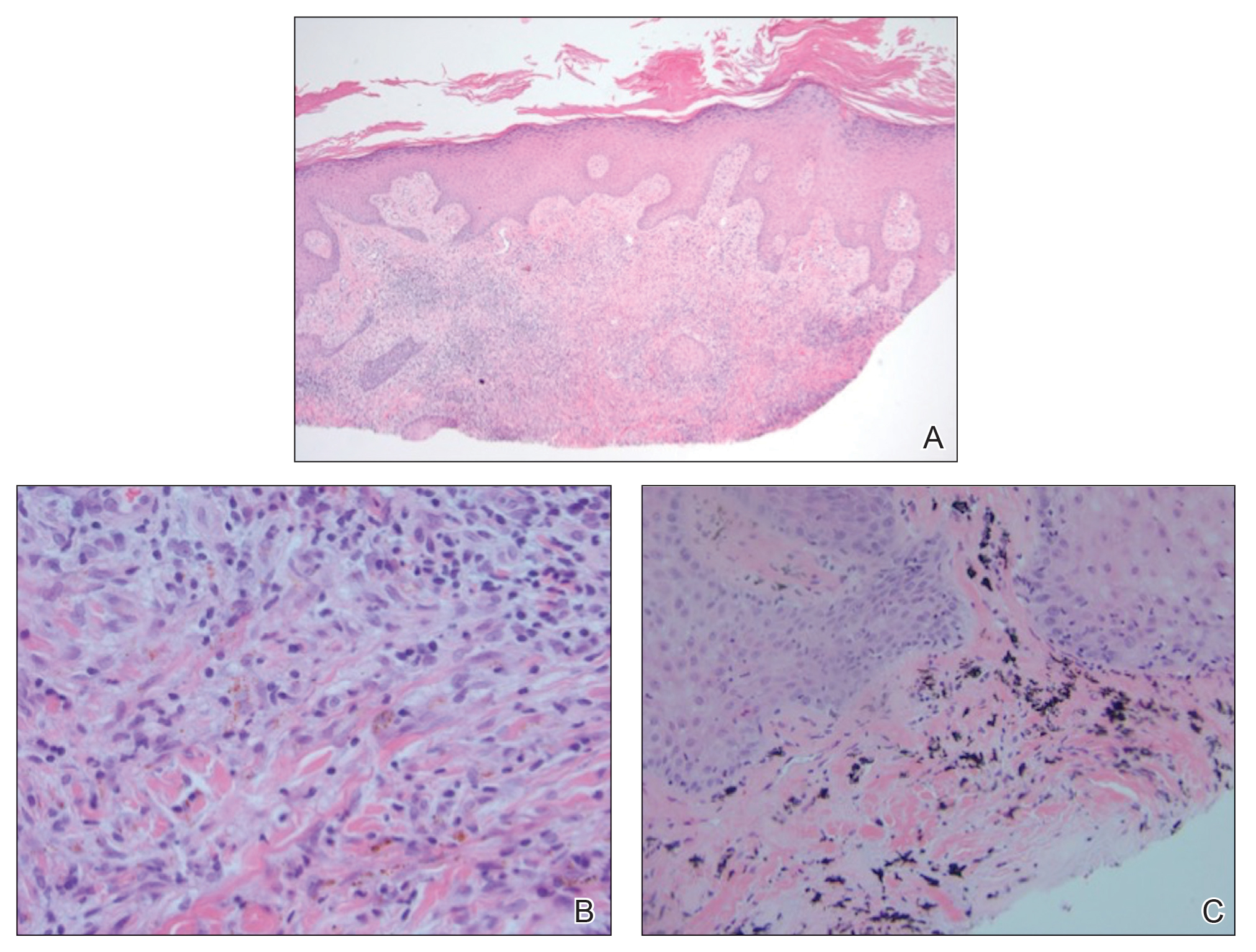
Tattoo-related inflammatory reactions can manifest clinically as allergic contact dermatitis, photodermatitis, infection, malignancy, foreign body granulomas, and delayed hypersensitivity reactions with myriad associated histopathologic patterns including spongiotic, psoriasiform, granulomatous, and lichenoid (as seen in our patient). Lichenoid tattoo reactions are the most common histopathologic variants of delayed hypersensitivity seen, mostly with cinnabar or red dye.1 However, there is a paucity of cases in the literature of PEH following tattooing with red dye. Interestingly, lichenoid tissue reaction accompanies PEH in all reported cases.2
Pseudoepitheliomatous hyperplasia can mimic squamous cell carcinoma and keratoacanthoma (KA) both clinically and histologically. All 3 conditions may exhibit epithelial hyperplasia with prominent dilated hyperplastic infundibula. In a case series of 11 presumed KAs within tattoos, Fraga and Prossick2 reported 82% (9/11) of the lesions were located strictly in areas with red pigment, and many were associated with a lichenoid tissue reaction. Kazlouskaya and Junkins-Hopkins3 previously described cases of KAs in tattoos that may represent PEH.
When treating lesions with this histologic appearance, consider the clinical and histologic overlap between KAs and PEH. Our patient was managed with clobetasol ointment 0.05% under occlusion followed by intralesional triamcinolone acetonide with notable improvement of the verrucous plaques on the left lateral malleolus (Figure 2). He also noted near resolution of the papules on the pretibial shin and complete resolution of all associated pruritus and burning. Calcineurin inhibitors, photochemotherapy, CO2 laser, excimer laser, and surgical removal with interval grafting also were considered.

It is important to recognize PEH in the differential of eruptions occurring within tattoos to avoid unnecessary invasive surgical procedures such as complete surgical excision of a KA to avoid malignant transformation.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
The Diagnosis: Lichenoid Reaction With Pseudoepitheliomatous Hyperplasia
A shave biopsy of the left ankle and a punch biopsy of the left medial calf were performed and sent for histologic examination and acid-fast stain. Bacterial, fungal, and mycobacterial tissue cultures also were sent for testing. The findings from direct examination were negative, and tissue cultures exhibited no growth. The shave and punch biopsies and histology revealed pseudoepitheliomatous hyperplasia (PEH) with keratinocyte necrosis, satellitosis, and areas of acute folliculitis (Figure 1). A lichenoid hypersensitivity mixed infiltrate that included histiocytes admixed with anthracotic and red-orange pigment, lymphocytes, plasma cells, neutrophils, and rare eosinophils was noted in the dermis. Given these clinical and histopathologic findings, the patient was diagnosed with red-pigment tattoo lichenoid reaction with PEH.

Tattoo-related inflammatory reactions can manifest clinically as allergic contact dermatitis, photodermatitis, infection, malignancy, foreign body granulomas, and delayed hypersensitivity reactions with myriad associated histopathologic patterns including spongiotic, psoriasiform, granulomatous, and lichenoid (as seen in our patient). Lichenoid tattoo reactions are the most common histopathologic variants of delayed hypersensitivity seen, mostly with cinnabar or red dye.1 However, there is a paucity of cases in the literature of PEH following tattooing with red dye. Interestingly, lichenoid tissue reaction accompanies PEH in all reported cases.2
Pseudoepitheliomatous hyperplasia can mimic squamous cell carcinoma and keratoacanthoma (KA) both clinically and histologically. All 3 conditions may exhibit epithelial hyperplasia with prominent dilated hyperplastic infundibula. In a case series of 11 presumed KAs within tattoos, Fraga and Prossick2 reported 82% (9/11) of the lesions were located strictly in areas with red pigment, and many were associated with a lichenoid tissue reaction. Kazlouskaya and Junkins-Hopkins3 previously described cases of KAs in tattoos that may represent PEH.
When treating lesions with this histologic appearance, consider the clinical and histologic overlap between KAs and PEH. Our patient was managed with clobetasol ointment 0.05% under occlusion followed by intralesional triamcinolone acetonide with notable improvement of the verrucous plaques on the left lateral malleolus (Figure 2). He also noted near resolution of the papules on the pretibial shin and complete resolution of all associated pruritus and burning. Calcineurin inhibitors, photochemotherapy, CO2 laser, excimer laser, and surgical removal with interval grafting also were considered.

It is important to recognize PEH in the differential of eruptions occurring within tattoos to avoid unnecessary invasive surgical procedures such as complete surgical excision of a KA to avoid malignant transformation.
The Diagnosis: Lichenoid Reaction With Pseudoepitheliomatous Hyperplasia
A shave biopsy of the left ankle and a punch biopsy of the left medial calf were performed and sent for histologic examination and acid-fast stain. Bacterial, fungal, and mycobacterial tissue cultures also were sent for testing. The findings from direct examination were negative, and tissue cultures exhibited no growth. The shave and punch biopsies and histology revealed pseudoepitheliomatous hyperplasia (PEH) with keratinocyte necrosis, satellitosis, and areas of acute folliculitis (Figure 1). A lichenoid hypersensitivity mixed infiltrate that included histiocytes admixed with anthracotic and red-orange pigment, lymphocytes, plasma cells, neutrophils, and rare eosinophils was noted in the dermis. Given these clinical and histopathologic findings, the patient was diagnosed with red-pigment tattoo lichenoid reaction with PEH.

Tattoo-related inflammatory reactions can manifest clinically as allergic contact dermatitis, photodermatitis, infection, malignancy, foreign body granulomas, and delayed hypersensitivity reactions with myriad associated histopathologic patterns including spongiotic, psoriasiform, granulomatous, and lichenoid (as seen in our patient). Lichenoid tattoo reactions are the most common histopathologic variants of delayed hypersensitivity seen, mostly with cinnabar or red dye.1 However, there is a paucity of cases in the literature of PEH following tattooing with red dye. Interestingly, lichenoid tissue reaction accompanies PEH in all reported cases.2
Pseudoepitheliomatous hyperplasia can mimic squamous cell carcinoma and keratoacanthoma (KA) both clinically and histologically. All 3 conditions may exhibit epithelial hyperplasia with prominent dilated hyperplastic infundibula. In a case series of 11 presumed KAs within tattoos, Fraga and Prossick2 reported 82% (9/11) of the lesions were located strictly in areas with red pigment, and many were associated with a lichenoid tissue reaction. Kazlouskaya and Junkins-Hopkins3 previously described cases of KAs in tattoos that may represent PEH.
When treating lesions with this histologic appearance, consider the clinical and histologic overlap between KAs and PEH. Our patient was managed with clobetasol ointment 0.05% under occlusion followed by intralesional triamcinolone acetonide with notable improvement of the verrucous plaques on the left lateral malleolus (Figure 2). He also noted near resolution of the papules on the pretibial shin and complete resolution of all associated pruritus and burning. Calcineurin inhibitors, photochemotherapy, CO2 laser, excimer laser, and surgical removal with interval grafting also were considered.

It is important to recognize PEH in the differential of eruptions occurring within tattoos to avoid unnecessary invasive surgical procedures such as complete surgical excision of a KA to avoid malignant transformation.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
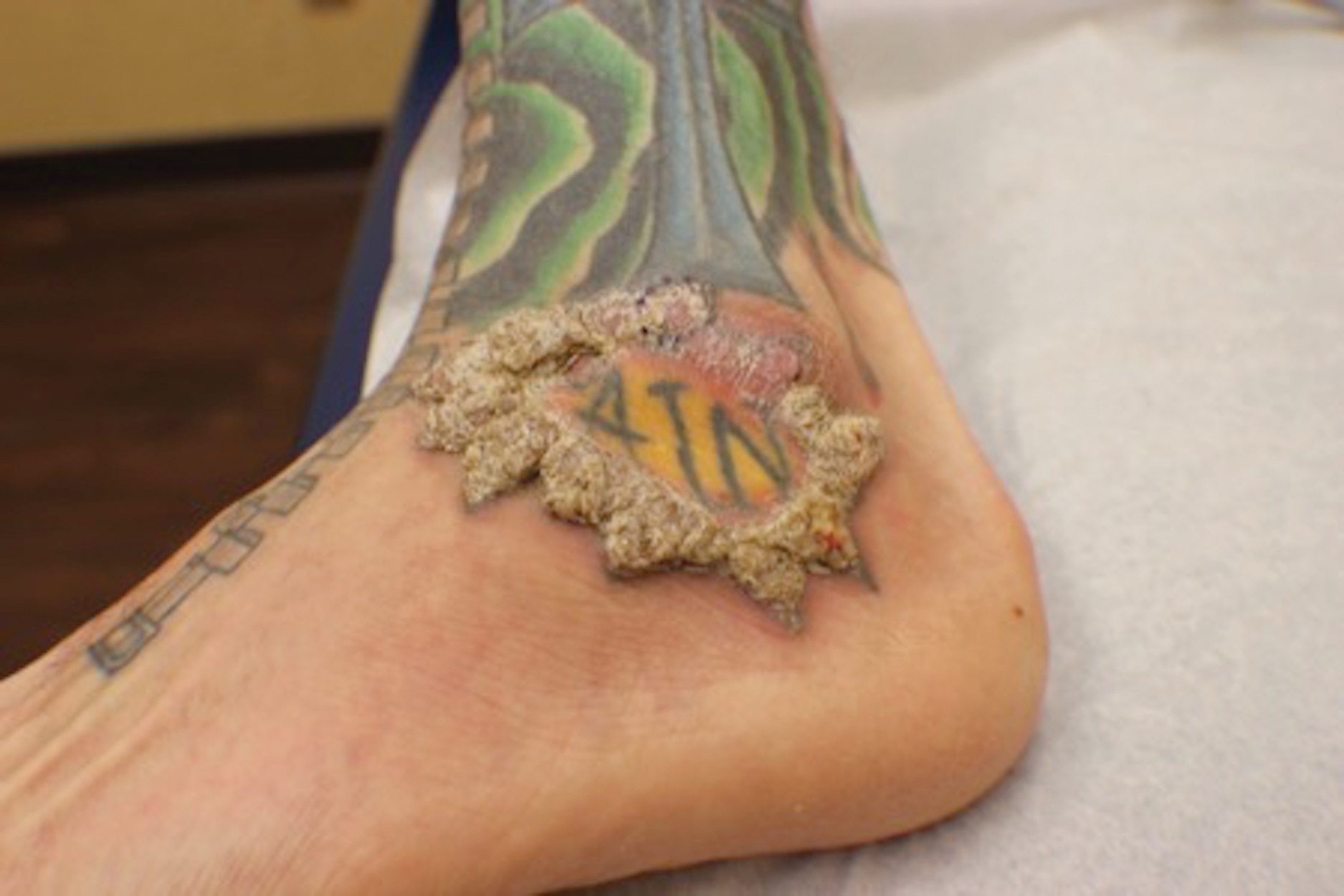
A 40-year-old man with a medical history of human immunodeficiency virus infection managed with highly active antiretroviral therapy (CD4 count, 888 cells/mm3 and an undetectable viral load), psoriasis, and recurrent condyloma acuminatum presented with exophytic, annular, hyperkeratotic, verrucous plaques on the left lateral malleolus with multiple erythematous hyperkeratotic papules on the pretibial shin of the left leg of 6 months' duration. These plaques and papules were localized to areas where red dye was used in a tattoo the patient had received 2 years prior to presentation. There was no associated fluctuance or drainage. The patient reported paroxysmal pruritus and burning pain.
Human hair’s super strength and boiled diabetes
Stop us if you’ve heard this one
An elephant, a capybara, and a horse walk into a bar …
What? You’ve heard it? Really? … No, not really? You were just pulling our leg. Very funny.
Can we go on now? You’re sure? Because if there’s one thing we love, it’s reader participation.
Anyway, there’s this bar, and a bunch of researchers from the University of California’s San Diego and Berkeley campuses are hanging out with a bear, a boar, a javelina (looks and sounds like a pig, about the size of a border collie), and a giraffe when the aforementioned trio shows up.
They see all these mammals together and get an idea. Why not compare the tensile strength of their hair with that of a human adult and child? The next day, after sobering up, they started collecting hair samples.[Legal note: The situation at the bar is a literary device. At no time did the investigators conduct any of their work in such a place.]
Once that was done, uniaxial tension tests were conducted, as you would expect, using an Instron 3342 universal testing system equipped with a 500-N load cell at a strain rate of 10–2s–1.
The results, however, were not expected. Thin hairs are stronger than thick hairs. Hair from a human, with an average diameter of about 60 mcm, is stronger than hair from a boar (average diameter, 235 mcm) or a horse (average diameter, 200 mcm), and the weakest hair of all came from an elephant (diameter of 345 mcm) and a giraffe (diameter of 370 mcm). Also, the hair from the 9-year-old child is stronger than the thicker hair of the 30-year-old adult.
The results show that the inherent strength of human hair’s hierarchical structure – an outer layer, or cuticle, “wraps around an inner cortex made of many small fibers linked by chemical bonds” – makes it resistant to deformation, the investigators explained. They hope that synthetic materials with a similar structure could become lighter and stronger in the future.
Now what? The punchline? All this valuable hair information isn’t enough? Fine.
The shocked bartender, who’s already got the makings of a small zoo in his establishment, points a finger in alarm at the latest arrivals and yells, “Hey!” The horse says, “You read my mind, buddy.”
Boiled type 2 diabetes
Ah, coffee. Is there anything it can’t do? From speeding up delivery of this tardy column (if not boosting its comedy quotient) to reducing the risk of type 2 diabetes, the magic bean seems nearly omnipotent. But java’s magical metabolic properties may be entirely dependent on how you brew your brown elixir.
Coffee-fueled Scandinavians have determined that the stimulating liquid’s antidiabetes effect is only possible when you don’t boil your beans. For those eager to ward off type 2 diabetes, filtered coffee – not boiled coffee – is the ticket to lower insulin resistance.
Researchers at Chalmers University of Technology in Gothenburg, Sweden, and Umeå (Sweden) University identified biomarkers that distinguished the consumption of filtered coffee from its boiled cousin. They found that people who drank two to three cups of filtered coffee a day enjoyed a 60% lower risk of type 2 diabetes than did those who subsisted on less than one cup of filtered coffee per day. But drinking boiled coffee did nothing to dent drinkers’ diabetes risk.
Why the difference? The coffee-sipping Swedish investigators say boiled coffee contains diterpenes, which don’t make it past a coffee filter. Other studies have shown diterpenes inflate the risk of heart and vascular diseases. And the researchers say those unfiltered diterpenes may sap your morning cuppa joe’s antidiabetes powers.
We know what you’re thinking: Who boils powdered coffee? Is Sanka even a thing anymore? It’s an approach that’s unfamiliar to most Mr. Coffee–owning Scandinavians and American java junkies alike. But before you, like us, take another smug sip from that cup you just made in the Keurig machine, know this: Coffee pod machines deal out hot, unfiltered brew.
Maybe just add two sugar packets next time. Your beta cells will thank you.
Let my people poop!
This is the way the world ends, this is the way the world ends: Not with a bang, but a tilted toilet.
The latest piece of late-stage capitalistic horror comes from Great Britain, and has lovingly been called the “StandardToilet.” No dystopian overtones there, absolutely not. The toilet’s “genius” lies in the seat’s 13-degree downward slope, which increases strain on the legs just like a squat thrust would. Sit on one for more than 5 minutes, and the pain becomes too much to handle.
The inventor of the StandardToilet claims that his product will cut time employees spend on the toilet by 25%, saving businesses as much as 4.8 billion pounds sterling a year. Not only that, he suggests that there are numerous health benefits, such as reduced hemorrhoid risk and a reduction of musculoskeletal disorders.
Critics were quick to point out that people with disabilities would likely have accessibility issues (and let’s not forget people with GI diseases), and a bathroom space expert (yes, that’s a thing) suggested that, if employees are spending too much time on the toilet, the issue is likely not laziness but the poor state of affairs in the workplace itself.
As purveyors of quality toilet- and poop-themed humor, we here at Livin’ on the MDedge world headquarters think it would be a shame if the giant corporations of the world took away normal toilets. So we say to the downtrodden proletariat: Workers of the world, unite! You have nothing to lose but your toilets!
Livin’ on the MDedge will be taking a holiday break, but we’ll be back to advance medical science in January!

Stop us if you’ve heard this one
An elephant, a capybara, and a horse walk into a bar …
What? You’ve heard it? Really? … No, not really? You were just pulling our leg. Very funny.
Can we go on now? You’re sure? Because if there’s one thing we love, it’s reader participation.
Anyway, there’s this bar, and a bunch of researchers from the University of California’s San Diego and Berkeley campuses are hanging out with a bear, a boar, a javelina (looks and sounds like a pig, about the size of a border collie), and a giraffe when the aforementioned trio shows up.
They see all these mammals together and get an idea. Why not compare the tensile strength of their hair with that of a human adult and child? The next day, after sobering up, they started collecting hair samples.[Legal note: The situation at the bar is a literary device. At no time did the investigators conduct any of their work in such a place.]
Once that was done, uniaxial tension tests were conducted, as you would expect, using an Instron 3342 universal testing system equipped with a 500-N load cell at a strain rate of 10–2s–1.
The results, however, were not expected. Thin hairs are stronger than thick hairs. Hair from a human, with an average diameter of about 60 mcm, is stronger than hair from a boar (average diameter, 235 mcm) or a horse (average diameter, 200 mcm), and the weakest hair of all came from an elephant (diameter of 345 mcm) and a giraffe (diameter of 370 mcm). Also, the hair from the 9-year-old child is stronger than the thicker hair of the 30-year-old adult.
The results show that the inherent strength of human hair’s hierarchical structure – an outer layer, or cuticle, “wraps around an inner cortex made of many small fibers linked by chemical bonds” – makes it resistant to deformation, the investigators explained. They hope that synthetic materials with a similar structure could become lighter and stronger in the future.
Now what? The punchline? All this valuable hair information isn’t enough? Fine.
The shocked bartender, who’s already got the makings of a small zoo in his establishment, points a finger in alarm at the latest arrivals and yells, “Hey!” The horse says, “You read my mind, buddy.”
Boiled type 2 diabetes
Ah, coffee. Is there anything it can’t do? From speeding up delivery of this tardy column (if not boosting its comedy quotient) to reducing the risk of type 2 diabetes, the magic bean seems nearly omnipotent. But java’s magical metabolic properties may be entirely dependent on how you brew your brown elixir.
Coffee-fueled Scandinavians have determined that the stimulating liquid’s antidiabetes effect is only possible when you don’t boil your beans. For those eager to ward off type 2 diabetes, filtered coffee – not boiled coffee – is the ticket to lower insulin resistance.
Researchers at Chalmers University of Technology in Gothenburg, Sweden, and Umeå (Sweden) University identified biomarkers that distinguished the consumption of filtered coffee from its boiled cousin. They found that people who drank two to three cups of filtered coffee a day enjoyed a 60% lower risk of type 2 diabetes than did those who subsisted on less than one cup of filtered coffee per day. But drinking boiled coffee did nothing to dent drinkers’ diabetes risk.
Why the difference? The coffee-sipping Swedish investigators say boiled coffee contains diterpenes, which don’t make it past a coffee filter. Other studies have shown diterpenes inflate the risk of heart and vascular diseases. And the researchers say those unfiltered diterpenes may sap your morning cuppa joe’s antidiabetes powers.
We know what you’re thinking: Who boils powdered coffee? Is Sanka even a thing anymore? It’s an approach that’s unfamiliar to most Mr. Coffee–owning Scandinavians and American java junkies alike. But before you, like us, take another smug sip from that cup you just made in the Keurig machine, know this: Coffee pod machines deal out hot, unfiltered brew.
Maybe just add two sugar packets next time. Your beta cells will thank you.
Let my people poop!
This is the way the world ends, this is the way the world ends: Not with a bang, but a tilted toilet.
The latest piece of late-stage capitalistic horror comes from Great Britain, and has lovingly been called the “StandardToilet.” No dystopian overtones there, absolutely not. The toilet’s “genius” lies in the seat’s 13-degree downward slope, which increases strain on the legs just like a squat thrust would. Sit on one for more than 5 minutes, and the pain becomes too much to handle.
The inventor of the StandardToilet claims that his product will cut time employees spend on the toilet by 25%, saving businesses as much as 4.8 billion pounds sterling a year. Not only that, he suggests that there are numerous health benefits, such as reduced hemorrhoid risk and a reduction of musculoskeletal disorders.
Critics were quick to point out that people with disabilities would likely have accessibility issues (and let’s not forget people with GI diseases), and a bathroom space expert (yes, that’s a thing) suggested that, if employees are spending too much time on the toilet, the issue is likely not laziness but the poor state of affairs in the workplace itself.
As purveyors of quality toilet- and poop-themed humor, we here at Livin’ on the MDedge world headquarters think it would be a shame if the giant corporations of the world took away normal toilets. So we say to the downtrodden proletariat: Workers of the world, unite! You have nothing to lose but your toilets!
Livin’ on the MDedge will be taking a holiday break, but we’ll be back to advance medical science in January!

Stop us if you’ve heard this one
An elephant, a capybara, and a horse walk into a bar …
What? You’ve heard it? Really? … No, not really? You were just pulling our leg. Very funny.
Can we go on now? You’re sure? Because if there’s one thing we love, it’s reader participation.
Anyway, there’s this bar, and a bunch of researchers from the University of California’s San Diego and Berkeley campuses are hanging out with a bear, a boar, a javelina (looks and sounds like a pig, about the size of a border collie), and a giraffe when the aforementioned trio shows up.
They see all these mammals together and get an idea. Why not compare the tensile strength of their hair with that of a human adult and child? The next day, after sobering up, they started collecting hair samples.[Legal note: The situation at the bar is a literary device. At no time did the investigators conduct any of their work in such a place.]
Once that was done, uniaxial tension tests were conducted, as you would expect, using an Instron 3342 universal testing system equipped with a 500-N load cell at a strain rate of 10–2s–1.
The results, however, were not expected. Thin hairs are stronger than thick hairs. Hair from a human, with an average diameter of about 60 mcm, is stronger than hair from a boar (average diameter, 235 mcm) or a horse (average diameter, 200 mcm), and the weakest hair of all came from an elephant (diameter of 345 mcm) and a giraffe (diameter of 370 mcm). Also, the hair from the 9-year-old child is stronger than the thicker hair of the 30-year-old adult.
The results show that the inherent strength of human hair’s hierarchical structure – an outer layer, or cuticle, “wraps around an inner cortex made of many small fibers linked by chemical bonds” – makes it resistant to deformation, the investigators explained. They hope that synthetic materials with a similar structure could become lighter and stronger in the future.
Now what? The punchline? All this valuable hair information isn’t enough? Fine.
The shocked bartender, who’s already got the makings of a small zoo in his establishment, points a finger in alarm at the latest arrivals and yells, “Hey!” The horse says, “You read my mind, buddy.”
Boiled type 2 diabetes
Ah, coffee. Is there anything it can’t do? From speeding up delivery of this tardy column (if not boosting its comedy quotient) to reducing the risk of type 2 diabetes, the magic bean seems nearly omnipotent. But java’s magical metabolic properties may be entirely dependent on how you brew your brown elixir.
Coffee-fueled Scandinavians have determined that the stimulating liquid’s antidiabetes effect is only possible when you don’t boil your beans. For those eager to ward off type 2 diabetes, filtered coffee – not boiled coffee – is the ticket to lower insulin resistance.
Researchers at Chalmers University of Technology in Gothenburg, Sweden, and Umeå (Sweden) University identified biomarkers that distinguished the consumption of filtered coffee from its boiled cousin. They found that people who drank two to three cups of filtered coffee a day enjoyed a 60% lower risk of type 2 diabetes than did those who subsisted on less than one cup of filtered coffee per day. But drinking boiled coffee did nothing to dent drinkers’ diabetes risk.
Why the difference? The coffee-sipping Swedish investigators say boiled coffee contains diterpenes, which don’t make it past a coffee filter. Other studies have shown diterpenes inflate the risk of heart and vascular diseases. And the researchers say those unfiltered diterpenes may sap your morning cuppa joe’s antidiabetes powers.
We know what you’re thinking: Who boils powdered coffee? Is Sanka even a thing anymore? It’s an approach that’s unfamiliar to most Mr. Coffee–owning Scandinavians and American java junkies alike. But before you, like us, take another smug sip from that cup you just made in the Keurig machine, know this: Coffee pod machines deal out hot, unfiltered brew.
Maybe just add two sugar packets next time. Your beta cells will thank you.
Let my people poop!
This is the way the world ends, this is the way the world ends: Not with a bang, but a tilted toilet.
The latest piece of late-stage capitalistic horror comes from Great Britain, and has lovingly been called the “StandardToilet.” No dystopian overtones there, absolutely not. The toilet’s “genius” lies in the seat’s 13-degree downward slope, which increases strain on the legs just like a squat thrust would. Sit on one for more than 5 minutes, and the pain becomes too much to handle.
The inventor of the StandardToilet claims that his product will cut time employees spend on the toilet by 25%, saving businesses as much as 4.8 billion pounds sterling a year. Not only that, he suggests that there are numerous health benefits, such as reduced hemorrhoid risk and a reduction of musculoskeletal disorders.
Critics were quick to point out that people with disabilities would likely have accessibility issues (and let’s not forget people with GI diseases), and a bathroom space expert (yes, that’s a thing) suggested that, if employees are spending too much time on the toilet, the issue is likely not laziness but the poor state of affairs in the workplace itself.
As purveyors of quality toilet- and poop-themed humor, we here at Livin’ on the MDedge world headquarters think it would be a shame if the giant corporations of the world took away normal toilets. So we say to the downtrodden proletariat: Workers of the world, unite! You have nothing to lose but your toilets!
Livin’ on the MDedge will be taking a holiday break, but we’ll be back to advance medical science in January!

FDA warns gabapentin, pregabalin may cause serious breathing problems
Elderly patients who take these drugs also are at increased risk of breathing problems, the announcement said.
Gabapentin (marketed as Neurontin, Gralise, and Horizant) and pregabalin (Lyrica and Lyrica CR) are used to treat seizures, nerve pain, and restless legs syndrome. Physicians increasingly are prescribing these medications, and people are misusing and abusing these drugs more frequently, the agency said. Gabapentin and pregabalin often are combined with central nervous system depressants such as opioids, antianxiety medicines, antidepressants, and antihistamines, which increases the risk of respiratory depression.
Conditions that reduce lung function, including chronic obstructive pulmonary disease (COPD), also increase the likelihood of breathing problems when taking gabapentin and pregabalin.
“There is less evidence supporting the risk of serious breathing difficulties in healthy individuals taking gabapentinoids alone. We will continue to monitor these medicines as part of our routine monitoring of all FDA-approved drugs,” the announcement said.
The FDA is requiring new warnings about the risk of respiratory depression in the prescribing information of gabapentinoids. In addition, drug manufacturers must further assess the abuse potential of these drugs, particularly in combination with opioids.
Patients and caregivers should seek immediate medical attention for respiratory problems, which can be life threatening. Symptoms include confusion or disorientation; unusual dizziness or lightheadedness; extreme sleepiness or lethargy; slowed, shallow, or difficult breathing; unresponsiveness; and bluish-colored or tinted skin, especially on the lips, fingers, and toes.
Physicians should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation when coprescribing these drugs with an opioid or other central nervous system depressant such as a benzodiazepine, according to the FDA.
The agency reviewed 49 case reports that were submitted between 2012 and 2017. Among these cases, 12 people died from respiratory depression with gabapentinoids. All of the patients who died had at least one risk factor.
Gabapentin first was approved in 1993, and pregabalin was approved in 2004. Drug adverse events and side effects can be reported online, the agency noted.
Elderly patients who take these drugs also are at increased risk of breathing problems, the announcement said.
Gabapentin (marketed as Neurontin, Gralise, and Horizant) and pregabalin (Lyrica and Lyrica CR) are used to treat seizures, nerve pain, and restless legs syndrome. Physicians increasingly are prescribing these medications, and people are misusing and abusing these drugs more frequently, the agency said. Gabapentin and pregabalin often are combined with central nervous system depressants such as opioids, antianxiety medicines, antidepressants, and antihistamines, which increases the risk of respiratory depression.
Conditions that reduce lung function, including chronic obstructive pulmonary disease (COPD), also increase the likelihood of breathing problems when taking gabapentin and pregabalin.
“There is less evidence supporting the risk of serious breathing difficulties in healthy individuals taking gabapentinoids alone. We will continue to monitor these medicines as part of our routine monitoring of all FDA-approved drugs,” the announcement said.
The FDA is requiring new warnings about the risk of respiratory depression in the prescribing information of gabapentinoids. In addition, drug manufacturers must further assess the abuse potential of these drugs, particularly in combination with opioids.
Patients and caregivers should seek immediate medical attention for respiratory problems, which can be life threatening. Symptoms include confusion or disorientation; unusual dizziness or lightheadedness; extreme sleepiness or lethargy; slowed, shallow, or difficult breathing; unresponsiveness; and bluish-colored or tinted skin, especially on the lips, fingers, and toes.
Physicians should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation when coprescribing these drugs with an opioid or other central nervous system depressant such as a benzodiazepine, according to the FDA.
The agency reviewed 49 case reports that were submitted between 2012 and 2017. Among these cases, 12 people died from respiratory depression with gabapentinoids. All of the patients who died had at least one risk factor.
Gabapentin first was approved in 1993, and pregabalin was approved in 2004. Drug adverse events and side effects can be reported online, the agency noted.
Elderly patients who take these drugs also are at increased risk of breathing problems, the announcement said.
Gabapentin (marketed as Neurontin, Gralise, and Horizant) and pregabalin (Lyrica and Lyrica CR) are used to treat seizures, nerve pain, and restless legs syndrome. Physicians increasingly are prescribing these medications, and people are misusing and abusing these drugs more frequently, the agency said. Gabapentin and pregabalin often are combined with central nervous system depressants such as opioids, antianxiety medicines, antidepressants, and antihistamines, which increases the risk of respiratory depression.
Conditions that reduce lung function, including chronic obstructive pulmonary disease (COPD), also increase the likelihood of breathing problems when taking gabapentin and pregabalin.
“There is less evidence supporting the risk of serious breathing difficulties in healthy individuals taking gabapentinoids alone. We will continue to monitor these medicines as part of our routine monitoring of all FDA-approved drugs,” the announcement said.
The FDA is requiring new warnings about the risk of respiratory depression in the prescribing information of gabapentinoids. In addition, drug manufacturers must further assess the abuse potential of these drugs, particularly in combination with opioids.
Patients and caregivers should seek immediate medical attention for respiratory problems, which can be life threatening. Symptoms include confusion or disorientation; unusual dizziness or lightheadedness; extreme sleepiness or lethargy; slowed, shallow, or difficult breathing; unresponsiveness; and bluish-colored or tinted skin, especially on the lips, fingers, and toes.
Physicians should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation when coprescribing these drugs with an opioid or other central nervous system depressant such as a benzodiazepine, according to the FDA.
The agency reviewed 49 case reports that were submitted between 2012 and 2017. Among these cases, 12 people died from respiratory depression with gabapentinoids. All of the patients who died had at least one risk factor.
Gabapentin first was approved in 1993, and pregabalin was approved in 2004. Drug adverse events and side effects can be reported online, the agency noted.
FDA approves antibody-drug conjugate for advanced urothelial cancer
The Food and Drug Administration has granted accelerated approval to enfortumab vedotin-ejfv (Padcev) for the treatment of adult patients with locally advanced or metastatic urothelial cancer that has previously been treated with a programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.
The conjugate was approved based on overall response rate in a trial of 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, the FDA said in a press statement.
The overall response rate was 44%, with 12% having a complete response and 32% having a partial response. The median duration of response was 7.6 months.
The most common side effects for patients were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, altered taste, diarrhea, dry eye, pruritis, and dry skin. Patients may experience hyperglycemia, and blood sugar levels should be monitored closely in patients receiving enfortumab vedotin-ejfv, the FDA said.
Patients may experience eye disorders, and health care professionals may consider prophylactic artificial tears for dry eyes and referral to an ophthalmologist for any new symptoms related to the eye, the agency said. The FDA also advises telling patients of reproductive age to use effective contraception during treatment, and for a period of time thereafter. Women who are pregnant or breastfeeding should not take the antibody-drug conjugate because it may cause harm to a developing fetus or newborn baby or cause delivery complications.
The Food and Drug Administration has granted accelerated approval to enfortumab vedotin-ejfv (Padcev) for the treatment of adult patients with locally advanced or metastatic urothelial cancer that has previously been treated with a programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.
The conjugate was approved based on overall response rate in a trial of 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, the FDA said in a press statement.
The overall response rate was 44%, with 12% having a complete response and 32% having a partial response. The median duration of response was 7.6 months.
The most common side effects for patients were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, altered taste, diarrhea, dry eye, pruritis, and dry skin. Patients may experience hyperglycemia, and blood sugar levels should be monitored closely in patients receiving enfortumab vedotin-ejfv, the FDA said.
Patients may experience eye disorders, and health care professionals may consider prophylactic artificial tears for dry eyes and referral to an ophthalmologist for any new symptoms related to the eye, the agency said. The FDA also advises telling patients of reproductive age to use effective contraception during treatment, and for a period of time thereafter. Women who are pregnant or breastfeeding should not take the antibody-drug conjugate because it may cause harm to a developing fetus or newborn baby or cause delivery complications.
The Food and Drug Administration has granted accelerated approval to enfortumab vedotin-ejfv (Padcev) for the treatment of adult patients with locally advanced or metastatic urothelial cancer that has previously been treated with a programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.
The conjugate was approved based on overall response rate in a trial of 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy, the FDA said in a press statement.
The overall response rate was 44%, with 12% having a complete response and 32% having a partial response. The median duration of response was 7.6 months.
The most common side effects for patients were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, altered taste, diarrhea, dry eye, pruritis, and dry skin. Patients may experience hyperglycemia, and blood sugar levels should be monitored closely in patients receiving enfortumab vedotin-ejfv, the FDA said.
Patients may experience eye disorders, and health care professionals may consider prophylactic artificial tears for dry eyes and referral to an ophthalmologist for any new symptoms related to the eye, the agency said. The FDA also advises telling patients of reproductive age to use effective contraception during treatment, and for a period of time thereafter. Women who are pregnant or breastfeeding should not take the antibody-drug conjugate because it may cause harm to a developing fetus or newborn baby or cause delivery complications.
What do the new AGA guidelines recommend for opioid-induced constipation?
References
- Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the Medical Management of Opioid-Induced Constipation. Gastroenterology. 2019;156:218-226.
- Rao VL, Micic D, Davis AM. Medical management of opioid-induced constipation. JAMA. 2019;322:2241-2242.
- Naldemedine (Symproic) for opioid-induced constipation. Med Lett Drugs Ther. 2017;59:196-198.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
References
- Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the Medical Management of Opioid-Induced Constipation. Gastroenterology. 2019;156:218-226.
- Rao VL, Micic D, Davis AM. Medical management of opioid-induced constipation. JAMA. 2019;322:2241-2242.
- Naldemedine (Symproic) for opioid-induced constipation. Med Lett Drugs Ther. 2017;59:196-198.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
References
- Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the Medical Management of Opioid-Induced Constipation. Gastroenterology. 2019;156:218-226.
- Rao VL, Micic D, Davis AM. Medical management of opioid-induced constipation. JAMA. 2019;322:2241-2242.
- Naldemedine (Symproic) for opioid-induced constipation. Med Lett Drugs Ther. 2017;59:196-198.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
Out-of-network billing in in-network hospitals adds $40 billion in spending
As the debate over how best to address surprise billing continues, new research shows that billing from out-of-network physicians at in-network facilities is adding $40 billion in costs.
Researchers focused on four different types of physicians that account for out-of-network billing: anesthesiologists, pathologists, radiologists, and cases involving an assistant surgeon, which had out-of-network bills in about 10% of claims that were examined as part of the research.
“To give a rough estimate of the savings that could be achieved by eliminating the ability of these four types of specialists to readily bill out of network, we simulated what would happen if all of these specialists received the same average payments as orthopedic surgeons did (164% of Medicare rates),” Zack Cooper, PhD, associate professor of health policy at Yale University, New Haven, Conn., and colleagues wrote in a research report published in Health Affairs.
“We estimated that if these physicians were paid the same average rate as orthopedists for all of the services that they delivered in our sample, spending would be lowered on anesthesiologists by 53.5%, on pathologists by 47.4%, on radiologists by 16.3%, and on assistant surgeons by 46.2%,” the authors wrote.
Researchers said that physician spending for these four specialties would be lowered by 13.4% and would lower total spending for people with employer-sponsored insurance by about 3.4%, or $40 billion. If spending on these four specialties were lowered to 150% of Medicare rates, it would lower spending on physicians by 15.3%.
To help combat the issues of surprise billing in a way that lowers total commercial health care spending and helps to preserve a competitive price for physician services, Dr. Cooper and colleagues recommended an approach that would regulate the contracts of physicians who work in hospitals and are not chosen by patients. It would establish a bundled package for services that include the emergency department physicians and the four specialists examined as part of the research and would use the fee associated with the package of services to recruit specialists to work at the hospital.
The authors said this kind of policy would eliminate the possibility of patients seeing out-of-network providers at in-network hospitals and, unlike arbitration (a favored solution among physician groups if it is set up in an agreeable manner), patients are protected without being required to take any action. The policy also sets a competitive rate for these services.
“Under this bundled care approach, physicians would compete to offer their services on the basis of price and quality,” Dr. Cooper and colleagues stated. “Hospitals would compete with one another on the price and quality of their care, including the services provided by the physicians they recruited. Hospitals would also need to compete to retain physicians.”
This approach is not included in any current surprise billing legislation. There was hope that surprise billing would be addressed in a government spending bill that would be signed before year’s end. But a second bipartisan plan was introduced in the House Ways and Means Committee after a bipartisan compromise was reached by the House Energy and Commerce and the Senate Health, Education, Labor, and Pensions committees. This has postponed a decision on surprise billing legislation into the coming year.
[email protected]
SOURCE: Cooper Z et al. Health Aff. 2019 Dec 16. doi: 10.1377/hlthaff.2019.00507.
As the debate over how best to address surprise billing continues, new research shows that billing from out-of-network physicians at in-network facilities is adding $40 billion in costs.
Researchers focused on four different types of physicians that account for out-of-network billing: anesthesiologists, pathologists, radiologists, and cases involving an assistant surgeon, which had out-of-network bills in about 10% of claims that were examined as part of the research.
“To give a rough estimate of the savings that could be achieved by eliminating the ability of these four types of specialists to readily bill out of network, we simulated what would happen if all of these specialists received the same average payments as orthopedic surgeons did (164% of Medicare rates),” Zack Cooper, PhD, associate professor of health policy at Yale University, New Haven, Conn., and colleagues wrote in a research report published in Health Affairs.
“We estimated that if these physicians were paid the same average rate as orthopedists for all of the services that they delivered in our sample, spending would be lowered on anesthesiologists by 53.5%, on pathologists by 47.4%, on radiologists by 16.3%, and on assistant surgeons by 46.2%,” the authors wrote.
Researchers said that physician spending for these four specialties would be lowered by 13.4% and would lower total spending for people with employer-sponsored insurance by about 3.4%, or $40 billion. If spending on these four specialties were lowered to 150% of Medicare rates, it would lower spending on physicians by 15.3%.
To help combat the issues of surprise billing in a way that lowers total commercial health care spending and helps to preserve a competitive price for physician services, Dr. Cooper and colleagues recommended an approach that would regulate the contracts of physicians who work in hospitals and are not chosen by patients. It would establish a bundled package for services that include the emergency department physicians and the four specialists examined as part of the research and would use the fee associated with the package of services to recruit specialists to work at the hospital.
The authors said this kind of policy would eliminate the possibility of patients seeing out-of-network providers at in-network hospitals and, unlike arbitration (a favored solution among physician groups if it is set up in an agreeable manner), patients are protected without being required to take any action. The policy also sets a competitive rate for these services.
“Under this bundled care approach, physicians would compete to offer their services on the basis of price and quality,” Dr. Cooper and colleagues stated. “Hospitals would compete with one another on the price and quality of their care, including the services provided by the physicians they recruited. Hospitals would also need to compete to retain physicians.”
This approach is not included in any current surprise billing legislation. There was hope that surprise billing would be addressed in a government spending bill that would be signed before year’s end. But a second bipartisan plan was introduced in the House Ways and Means Committee after a bipartisan compromise was reached by the House Energy and Commerce and the Senate Health, Education, Labor, and Pensions committees. This has postponed a decision on surprise billing legislation into the coming year.
[email protected]
SOURCE: Cooper Z et al. Health Aff. 2019 Dec 16. doi: 10.1377/hlthaff.2019.00507.
As the debate over how best to address surprise billing continues, new research shows that billing from out-of-network physicians at in-network facilities is adding $40 billion in costs.
Researchers focused on four different types of physicians that account for out-of-network billing: anesthesiologists, pathologists, radiologists, and cases involving an assistant surgeon, which had out-of-network bills in about 10% of claims that were examined as part of the research.
“To give a rough estimate of the savings that could be achieved by eliminating the ability of these four types of specialists to readily bill out of network, we simulated what would happen if all of these specialists received the same average payments as orthopedic surgeons did (164% of Medicare rates),” Zack Cooper, PhD, associate professor of health policy at Yale University, New Haven, Conn., and colleagues wrote in a research report published in Health Affairs.
“We estimated that if these physicians were paid the same average rate as orthopedists for all of the services that they delivered in our sample, spending would be lowered on anesthesiologists by 53.5%, on pathologists by 47.4%, on radiologists by 16.3%, and on assistant surgeons by 46.2%,” the authors wrote.
Researchers said that physician spending for these four specialties would be lowered by 13.4% and would lower total spending for people with employer-sponsored insurance by about 3.4%, or $40 billion. If spending on these four specialties were lowered to 150% of Medicare rates, it would lower spending on physicians by 15.3%.
To help combat the issues of surprise billing in a way that lowers total commercial health care spending and helps to preserve a competitive price for physician services, Dr. Cooper and colleagues recommended an approach that would regulate the contracts of physicians who work in hospitals and are not chosen by patients. It would establish a bundled package for services that include the emergency department physicians and the four specialists examined as part of the research and would use the fee associated with the package of services to recruit specialists to work at the hospital.
The authors said this kind of policy would eliminate the possibility of patients seeing out-of-network providers at in-network hospitals and, unlike arbitration (a favored solution among physician groups if it is set up in an agreeable manner), patients are protected without being required to take any action. The policy also sets a competitive rate for these services.
“Under this bundled care approach, physicians would compete to offer their services on the basis of price and quality,” Dr. Cooper and colleagues stated. “Hospitals would compete with one another on the price and quality of their care, including the services provided by the physicians they recruited. Hospitals would also need to compete to retain physicians.”
This approach is not included in any current surprise billing legislation. There was hope that surprise billing would be addressed in a government spending bill that would be signed before year’s end. But a second bipartisan plan was introduced in the House Ways and Means Committee after a bipartisan compromise was reached by the House Energy and Commerce and the Senate Health, Education, Labor, and Pensions committees. This has postponed a decision on surprise billing legislation into the coming year.
[email protected]
SOURCE: Cooper Z et al. Health Aff. 2019 Dec 16. doi: 10.1377/hlthaff.2019.00507.
FROM HEALTH AFFAIRS
RCC: Tivozanib beats sorafenib in later lines
For third- or fourth-line treatment of metastatic renal cell carcinoma, the vascular epidermal growth factor receptor (VEGFR) tyrosine kinase inhibitor tivozanib may offer better progression-free survival than sorafenib, another VEGFR inhibitor, based on results from the TIVO-3 trial.
Tivozanib also had fewer dose reductions and dose interruptions than sorafenib, likely because of less off-target activity, reported lead author Brian I. Rini, MD, of the Cleveland Clinic and Case Western Reserve University, also in Cleveland, and colleagues.
“These results support tivozanib as a treatment option for patients with recurrent and progressive renal cell carcinoma, including those who have progressed after previous immunotherapy,” the investigators wrote in Lancet Oncology.
The open-label, phase 3 trial involved 350 patients with metastatic renal cell carcinoma who had been treated with two or more systemic therapies, at least one of which was a VEGFR inhibitor. Patients were randomized in 1:1 ratio to receive either tivozanib 1.5 mg orally once daily in 4-week cycles or sorafenib 400 mg orally twice daily on a continuous basis. The primary endpoint was progression-free survival. Secondary endpoints included overall survival, objective response rates, and duration of response. Endpoints were evaluated by independent review. Safety was reported for all patients who received one or more doses of therapy.
After a median follow-up of 19 months, tivozanib was associated with a median progression-free survival of 5.6 months, compared with 3.9 months for sorafenib (P = .016). Significantly more patients in the tivozanib group achieved a response, and at 1 year, 71% of patients in the tivozanib group maintained a response, compared with 46% of the patients in the sorafenib group. At data cutoff, median overall survival was not significantly different between groups.
The most common treatment-related grade 3-4 adverse event was hypertension, which occurred in a slightly higher rate among those treated with tivozanib (20% vs. 14%). Serious treatment-related adverse events occurred at comparable rates in each treatment arm, at 11% for tivozanib and 10% for sorafenib. Compared with patients who received sorafenib, those in the tivozanib group had fewer dose reductions (24% vs. 38%) and dose interruptions (48% vs. 63%) because of adverse events, a benefit that the investigators attributed to relatively lower class-related off-target activity.
“To our knowledge, TIVO-3 provides the largest amount of prospective data generated to date regarding the use of VEGFR tyrosine kinase inhibitors after checkpoint inhibitor treatment,” the investigators wrote. “Notably, the benefit of tivozanib extended to patients who had previously received checkpoint inhibitors and those treated with two previous tyrosine kinase inhibitors.”
The study was funded by AVEO Oncology. The investigators reported additional relationships with Pfizer, Eisai, Astellas, and others.
SOURCE: Rini BI et al. Lancet Oncol. 2019 Dec 3. doi: 10.1016/S1470-2045(19)30735-1.
Although tivozanib demonstrated longer progression-free survival and higher tolerability than sorafenib in the recent phase 3 TIVO-3 trial conducted by Rini and colleagues, the role of tivozanib in the treatment of metastatic renal cell carcinoma remains unclear. Critically, treatment with tivozanib in TIVO-3 was not associated with longer median overall survival than treatment with sorafenib.
In contrast, two previous phase 3 trials, METEOR and CheckMate 025, which also involved patients who had received two lines of a vascular epidermal growth factor receptor (VEGFR) tyrosine kinase inhibitor, showed that cabozantinib or nivolumab were associated with better overall survival than standard everolimus. While the comparator was different than the TIVO-3 trial, the evidence of benefit from these previous studies is superior, based on larger population size and overall survival advantage.
Therefore, it is too early to recommend a place for tivozanib, or to say that it should be preferred over other VEGFR tyrosine kinase inhibitors.
Axel Bex, MD, PhD, is with the Royal Free London NHS Foundation Trust at the University College London. Dr. Bex reported financial relationships with Pfizer, Ipsen, Novartis, and others. His remarks are adapted from an accompanying editorial (Lancet Oncol. 2019 Dec 3. doi: 10.1016/S1470-2045(19)30781-8).
Although tivozanib demonstrated longer progression-free survival and higher tolerability than sorafenib in the recent phase 3 TIVO-3 trial conducted by Rini and colleagues, the role of tivozanib in the treatment of metastatic renal cell carcinoma remains unclear. Critically, treatment with tivozanib in TIVO-3 was not associated with longer median overall survival than treatment with sorafenib.
In contrast, two previous phase 3 trials, METEOR and CheckMate 025, which also involved patients who had received two lines of a vascular epidermal growth factor receptor (VEGFR) tyrosine kinase inhibitor, showed that cabozantinib or nivolumab were associated with better overall survival than standard everolimus. While the comparator was different than the TIVO-3 trial, the evidence of benefit from these previous studies is superior, based on larger population size and overall survival advantage.
Therefore, it is too early to recommend a place for tivozanib, or to say that it should be preferred over other VEGFR tyrosine kinase inhibitors.
Axel Bex, MD, PhD, is with the Royal Free London NHS Foundation Trust at the University College London. Dr. Bex reported financial relationships with Pfizer, Ipsen, Novartis, and others. His remarks are adapted from an accompanying editorial (Lancet Oncol. 2019 Dec 3. doi: 10.1016/S1470-2045(19)30781-8).
Although tivozanib demonstrated longer progression-free survival and higher tolerability than sorafenib in the recent phase 3 TIVO-3 trial conducted by Rini and colleagues, the role of tivozanib in the treatment of metastatic renal cell carcinoma remains unclear. Critically, treatment with tivozanib in TIVO-3 was not associated with longer median overall survival than treatment with sorafenib.
In contrast, two previous phase 3 trials, METEOR and CheckMate 025, which also involved patients who had received two lines of a vascular epidermal growth factor receptor (VEGFR) tyrosine kinase inhibitor, showed that cabozantinib or nivolumab were associated with better overall survival than standard everolimus. While the comparator was different than the TIVO-3 trial, the evidence of benefit from these previous studies is superior, based on larger population size and overall survival advantage.
Therefore, it is too early to recommend a place for tivozanib, or to say that it should be preferred over other VEGFR tyrosine kinase inhibitors.
Axel Bex, MD, PhD, is with the Royal Free London NHS Foundation Trust at the University College London. Dr. Bex reported financial relationships with Pfizer, Ipsen, Novartis, and others. His remarks are adapted from an accompanying editorial (Lancet Oncol. 2019 Dec 3. doi: 10.1016/S1470-2045(19)30781-8).
For third- or fourth-line treatment of metastatic renal cell carcinoma, the vascular epidermal growth factor receptor (VEGFR) tyrosine kinase inhibitor tivozanib may offer better progression-free survival than sorafenib, another VEGFR inhibitor, based on results from the TIVO-3 trial.
Tivozanib also had fewer dose reductions and dose interruptions than sorafenib, likely because of less off-target activity, reported lead author Brian I. Rini, MD, of the Cleveland Clinic and Case Western Reserve University, also in Cleveland, and colleagues.
“These results support tivozanib as a treatment option for patients with recurrent and progressive renal cell carcinoma, including those who have progressed after previous immunotherapy,” the investigators wrote in Lancet Oncology.
The open-label, phase 3 trial involved 350 patients with metastatic renal cell carcinoma who had been treated with two or more systemic therapies, at least one of which was a VEGFR inhibitor. Patients were randomized in 1:1 ratio to receive either tivozanib 1.5 mg orally once daily in 4-week cycles or sorafenib 400 mg orally twice daily on a continuous basis. The primary endpoint was progression-free survival. Secondary endpoints included overall survival, objective response rates, and duration of response. Endpoints were evaluated by independent review. Safety was reported for all patients who received one or more doses of therapy.
After a median follow-up of 19 months, tivozanib was associated with a median progression-free survival of 5.6 months, compared with 3.9 months for sorafenib (P = .016). Significantly more patients in the tivozanib group achieved a response, and at 1 year, 71% of patients in the tivozanib group maintained a response, compared with 46% of the patients in the sorafenib group. At data cutoff, median overall survival was not significantly different between groups.
The most common treatment-related grade 3-4 adverse event was hypertension, which occurred in a slightly higher rate among those treated with tivozanib (20% vs. 14%). Serious treatment-related adverse events occurred at comparable rates in each treatment arm, at 11% for tivozanib and 10% for sorafenib. Compared with patients who received sorafenib, those in the tivozanib group had fewer dose reductions (24% vs. 38%) and dose interruptions (48% vs. 63%) because of adverse events, a benefit that the investigators attributed to relatively lower class-related off-target activity.
“To our knowledge, TIVO-3 provides the largest amount of prospective data generated to date regarding the use of VEGFR tyrosine kinase inhibitors after checkpoint inhibitor treatment,” the investigators wrote. “Notably, the benefit of tivozanib extended to patients who had previously received checkpoint inhibitors and those treated with two previous tyrosine kinase inhibitors.”
The study was funded by AVEO Oncology. The investigators reported additional relationships with Pfizer, Eisai, Astellas, and others.
SOURCE: Rini BI et al. Lancet Oncol. 2019 Dec 3. doi: 10.1016/S1470-2045(19)30735-1.
For third- or fourth-line treatment of metastatic renal cell carcinoma, the vascular epidermal growth factor receptor (VEGFR) tyrosine kinase inhibitor tivozanib may offer better progression-free survival than sorafenib, another VEGFR inhibitor, based on results from the TIVO-3 trial.
Tivozanib also had fewer dose reductions and dose interruptions than sorafenib, likely because of less off-target activity, reported lead author Brian I. Rini, MD, of the Cleveland Clinic and Case Western Reserve University, also in Cleveland, and colleagues.
“These results support tivozanib as a treatment option for patients with recurrent and progressive renal cell carcinoma, including those who have progressed after previous immunotherapy,” the investigators wrote in Lancet Oncology.
The open-label, phase 3 trial involved 350 patients with metastatic renal cell carcinoma who had been treated with two or more systemic therapies, at least one of which was a VEGFR inhibitor. Patients were randomized in 1:1 ratio to receive either tivozanib 1.5 mg orally once daily in 4-week cycles or sorafenib 400 mg orally twice daily on a continuous basis. The primary endpoint was progression-free survival. Secondary endpoints included overall survival, objective response rates, and duration of response. Endpoints were evaluated by independent review. Safety was reported for all patients who received one or more doses of therapy.
After a median follow-up of 19 months, tivozanib was associated with a median progression-free survival of 5.6 months, compared with 3.9 months for sorafenib (P = .016). Significantly more patients in the tivozanib group achieved a response, and at 1 year, 71% of patients in the tivozanib group maintained a response, compared with 46% of the patients in the sorafenib group. At data cutoff, median overall survival was not significantly different between groups.
The most common treatment-related grade 3-4 adverse event was hypertension, which occurred in a slightly higher rate among those treated with tivozanib (20% vs. 14%). Serious treatment-related adverse events occurred at comparable rates in each treatment arm, at 11% for tivozanib and 10% for sorafenib. Compared with patients who received sorafenib, those in the tivozanib group had fewer dose reductions (24% vs. 38%) and dose interruptions (48% vs. 63%) because of adverse events, a benefit that the investigators attributed to relatively lower class-related off-target activity.
“To our knowledge, TIVO-3 provides the largest amount of prospective data generated to date regarding the use of VEGFR tyrosine kinase inhibitors after checkpoint inhibitor treatment,” the investigators wrote. “Notably, the benefit of tivozanib extended to patients who had previously received checkpoint inhibitors and those treated with two previous tyrosine kinase inhibitors.”
The study was funded by AVEO Oncology. The investigators reported additional relationships with Pfizer, Eisai, Astellas, and others.
SOURCE: Rini BI et al. Lancet Oncol. 2019 Dec 3. doi: 10.1016/S1470-2045(19)30735-1.
FROM LANCET ONCOLOGY
Biofeedback corrects dyssynergic constipation in elderly
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
SAN ANTONIO – Biofeedback for treatment of dyssynergic constipation is highly effective in the elderly, just as it is in younger patients, Samantha Spilman, MD, reported at the annual meeting of the American College of Gastroenterology.
“I think the main point of this study is that older adults have a profound burden of constipation with dyssynergic defecation, and we propose that biofeedback be given strong consideration as first-line therapy for this population, in whom overall we’re trying to reduce medication use,” said Dr. Spilman, a gastroenterology fellow at the University of California, San Diego.
The prevalence of constipation in older patients is estimated to be up to 40%. Yet few prior studies have scrutinized how well older patients with constipation actually respond to biofeedback. It’s a legitimate question, since biofeedback training involves operant conditioning and requires learning new techniques. For this reason, she and her coinvestigators conducted a retrospective analysis of 58 patients over age 65 referred from the university’s gastrointestinal motility and physiology program to the biofeedback program for treatment of dyssynergic defection. The patients’ mean age was 74 years, with a 9.5-year history of constipation. The oldest patient was 88. Most of the subjects were high school graduates. Thirteen of the 58 carried a diagnosis of irritable bowel syndrome.
Numerous studies have demonstrated that 70%-80% of younger adults with dyssynergic constipation experience marked improvement in response to biofeedback training, which typically utilizes an inflated rectal balloon to simulate retained stool. The key finding in Dr. Spilman’s study was that the elderly patients did comparably well in terms of both self-reported outcomes and objective high-resolution anorectal manometric parameters upon completing an average of three biofeedback sessions.
Mean global bowel satisfaction on a 1-10 scale nearly doubled from 2.77 at baseline to 5.01 with biofeedback. Moreover, 79% of seniors demonstrated resolution of their dyssynergia on high-resolution anorectal manometry performed with sensors in the rectum and anal canal. The proportion of patients who reported a feeling of incomplete evacuation after stooling – a sensation individuals with constipation find highly bothersome – improved from 95% to 24% with biofeedback.
The strongest response in terms of the defecation index was observed in older patients with type 2 dyssynergia, characterized by defective propulsion coupled with a paradoxical contraction of the sphincter muscles during defecation. Their defecation index score, derived by dividing intrarectal pressure by residual intra-anal pressure during simulated defection, showed a robust improvement from 0.307 at baseline to 0.793. Patients with types 1 and 3 dyssynergia showed lesser improvements on this objective measure.
Dr. Spilman noted as a study limitation that baseline cognitive status wasn’t formally assessed, so the investigators don’t know how many of the older patients had minimal cognitive impairment. However, baseline quality of life assessment via the Short Form-36 indicated that patients scored average or above for physical and social functioning as well as emotional well-being.
Dr. Spilman reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Spilman S. ACG 2019. Abstract 45.
REPORTING FROM ACG 2019