User login
Vaping marijuana gaining traction among U.S. teens
Monitoring the Future survey asked about daily vaping this year for first time
Vaping has expanded as a popular method of drug delivery for U.S. teenagers, and one in five students in grades 10 and 12 reported vaping marijuana in the past year, according to results of the 2019 Monitoring the Future survey conducted by the National Institute on Drug Abuse (NIDA).
This year’s findings, announced Dec. 18, continue to illustrate “a clear shift in the pattern of drug taking among teenagers,” said NIDA Director Nora D. Volkow, MD, in a teleconference held to review the results.
Use of alcohol and drugs – including opioids and stimulants – continues to decline among teens, but vaping continues its significant rise, with a surge in marijuana vaping this year.
The increase in past-month marijuana vaping among 12th graders, from 7.5% in 2018 to 14% in 2019, represents the second-largest 1-year jump tracked for any substance in the survey’s history, Dr. Volkow said. The largest jump was the increase in past-month nicotine vaping among 12th-graders from 2017-2018.
“It is very unfortunate that we are seeing the steep rise in the use of vaping devices” because the devices deliver drugs in very high concentration, Dr. Volkow said. The growing popularity of vaping “threatens to undo years of progress protecting the health of adolescents in the U.S.,” Dr. Volkow said in a statement. The Monitoring the Future survey began including vaping questions in 2017.
Monitoring the Future is a national tool to assess drug and alcohol use and related attitudes among adolescent students across the United States. This year’s self-reported survey included 42,531 in grades 8, 10, and 12 from 396 public and private schools.
Nicotine vaping increased from 2018 to 2019 across all three grades; past-month nicotine use equated to 1 in 4, 1 in 5, and 1 in 10 (26%, 20%, and 10%) among 12th, 10th, and 8th graders, respectively, according to the survey. Daily nicotine vaping, measured for the first time this year because of public health concerns, was approximately 12% for 12th graders, 7% for 10th graders, and 2% for 8th graders. Daily marijuana vaping, also measured for the first time this year, was approximately 4%, 3%, and 1% among 12th, 10th, and 8th graders, respectively. Additional findings on the rise of vaping by U.S. teenagers were released Dec. 17 in a research letter published online in JAMA (doi: 10.1001/jama.2019.20185).
Meanwhile, positive trends in this year’s survey included a reduction in the misuse of prescription drugs, including OxyContin, Vicodin, and Adderall, and in the use of traditional cigarettes and other tobacco products, as well as alcohol, noted Richard A. Miech, PhD, MPH, of the University of Michigan, Ann Arbor, principal investigator for Monitoring the Future. However, the challenge of preventing and reducing vaping in teens remains “a whole new uncharted territory,” in part because the design of the vaping devices facilitates discreet use at home and at school, he said.
Physicians and parents have important roles to play in screening for vaping among teens, Dr. Volkow said in a question and answer session. Health care clinicians, including pediatricians and family physicians, “are in a unique position to communicate with their young patients” by educating them about the dangers of vaping, encouraging them to stop if they have started using these devices, and referring them for further treatment if they are showing signs of addiction, she said.
Monitoring the Future was funded by NIDA. The researchers had no disclosures.
Monitoring the Future survey asked about daily vaping this year for first time
Monitoring the Future survey asked about daily vaping this year for first time
Vaping has expanded as a popular method of drug delivery for U.S. teenagers, and one in five students in grades 10 and 12 reported vaping marijuana in the past year, according to results of the 2019 Monitoring the Future survey conducted by the National Institute on Drug Abuse (NIDA).
This year’s findings, announced Dec. 18, continue to illustrate “a clear shift in the pattern of drug taking among teenagers,” said NIDA Director Nora D. Volkow, MD, in a teleconference held to review the results.
Use of alcohol and drugs – including opioids and stimulants – continues to decline among teens, but vaping continues its significant rise, with a surge in marijuana vaping this year.
The increase in past-month marijuana vaping among 12th graders, from 7.5% in 2018 to 14% in 2019, represents the second-largest 1-year jump tracked for any substance in the survey’s history, Dr. Volkow said. The largest jump was the increase in past-month nicotine vaping among 12th-graders from 2017-2018.
“It is very unfortunate that we are seeing the steep rise in the use of vaping devices” because the devices deliver drugs in very high concentration, Dr. Volkow said. The growing popularity of vaping “threatens to undo years of progress protecting the health of adolescents in the U.S.,” Dr. Volkow said in a statement. The Monitoring the Future survey began including vaping questions in 2017.
Monitoring the Future is a national tool to assess drug and alcohol use and related attitudes among adolescent students across the United States. This year’s self-reported survey included 42,531 in grades 8, 10, and 12 from 396 public and private schools.
Nicotine vaping increased from 2018 to 2019 across all three grades; past-month nicotine use equated to 1 in 4, 1 in 5, and 1 in 10 (26%, 20%, and 10%) among 12th, 10th, and 8th graders, respectively, according to the survey. Daily nicotine vaping, measured for the first time this year because of public health concerns, was approximately 12% for 12th graders, 7% for 10th graders, and 2% for 8th graders. Daily marijuana vaping, also measured for the first time this year, was approximately 4%, 3%, and 1% among 12th, 10th, and 8th graders, respectively. Additional findings on the rise of vaping by U.S. teenagers were released Dec. 17 in a research letter published online in JAMA (doi: 10.1001/jama.2019.20185).
Meanwhile, positive trends in this year’s survey included a reduction in the misuse of prescription drugs, including OxyContin, Vicodin, and Adderall, and in the use of traditional cigarettes and other tobacco products, as well as alcohol, noted Richard A. Miech, PhD, MPH, of the University of Michigan, Ann Arbor, principal investigator for Monitoring the Future. However, the challenge of preventing and reducing vaping in teens remains “a whole new uncharted territory,” in part because the design of the vaping devices facilitates discreet use at home and at school, he said.
Physicians and parents have important roles to play in screening for vaping among teens, Dr. Volkow said in a question and answer session. Health care clinicians, including pediatricians and family physicians, “are in a unique position to communicate with their young patients” by educating them about the dangers of vaping, encouraging them to stop if they have started using these devices, and referring them for further treatment if they are showing signs of addiction, she said.
Monitoring the Future was funded by NIDA. The researchers had no disclosures.
Vaping has expanded as a popular method of drug delivery for U.S. teenagers, and one in five students in grades 10 and 12 reported vaping marijuana in the past year, according to results of the 2019 Monitoring the Future survey conducted by the National Institute on Drug Abuse (NIDA).
This year’s findings, announced Dec. 18, continue to illustrate “a clear shift in the pattern of drug taking among teenagers,” said NIDA Director Nora D. Volkow, MD, in a teleconference held to review the results.
Use of alcohol and drugs – including opioids and stimulants – continues to decline among teens, but vaping continues its significant rise, with a surge in marijuana vaping this year.
The increase in past-month marijuana vaping among 12th graders, from 7.5% in 2018 to 14% in 2019, represents the second-largest 1-year jump tracked for any substance in the survey’s history, Dr. Volkow said. The largest jump was the increase in past-month nicotine vaping among 12th-graders from 2017-2018.
“It is very unfortunate that we are seeing the steep rise in the use of vaping devices” because the devices deliver drugs in very high concentration, Dr. Volkow said. The growing popularity of vaping “threatens to undo years of progress protecting the health of adolescents in the U.S.,” Dr. Volkow said in a statement. The Monitoring the Future survey began including vaping questions in 2017.
Monitoring the Future is a national tool to assess drug and alcohol use and related attitudes among adolescent students across the United States. This year’s self-reported survey included 42,531 in grades 8, 10, and 12 from 396 public and private schools.
Nicotine vaping increased from 2018 to 2019 across all three grades; past-month nicotine use equated to 1 in 4, 1 in 5, and 1 in 10 (26%, 20%, and 10%) among 12th, 10th, and 8th graders, respectively, according to the survey. Daily nicotine vaping, measured for the first time this year because of public health concerns, was approximately 12% for 12th graders, 7% for 10th graders, and 2% for 8th graders. Daily marijuana vaping, also measured for the first time this year, was approximately 4%, 3%, and 1% among 12th, 10th, and 8th graders, respectively. Additional findings on the rise of vaping by U.S. teenagers were released Dec. 17 in a research letter published online in JAMA (doi: 10.1001/jama.2019.20185).
Meanwhile, positive trends in this year’s survey included a reduction in the misuse of prescription drugs, including OxyContin, Vicodin, and Adderall, and in the use of traditional cigarettes and other tobacco products, as well as alcohol, noted Richard A. Miech, PhD, MPH, of the University of Michigan, Ann Arbor, principal investigator for Monitoring the Future. However, the challenge of preventing and reducing vaping in teens remains “a whole new uncharted territory,” in part because the design of the vaping devices facilitates discreet use at home and at school, he said.
Physicians and parents have important roles to play in screening for vaping among teens, Dr. Volkow said in a question and answer session. Health care clinicians, including pediatricians and family physicians, “are in a unique position to communicate with their young patients” by educating them about the dangers of vaping, encouraging them to stop if they have started using these devices, and referring them for further treatment if they are showing signs of addiction, she said.
Monitoring the Future was funded by NIDA. The researchers had no disclosures.
PEARL lacks luster in metastatic breast cancer progressing on AIs
SAN ANTONIO – For postmenopausal women with hormone receptor–positive, HER2-negative breast cancer that has progressed on aromatase inhibitor therapy, the combination of palbociclib with either exemestane or fulvestrant was not better than capecitabine at delaying progression or inducing clinical responses, results of the phase 3 PEARL trial showed.
At a median follow-up of 13.45 months there was no significant difference in progression-free survival (PFS) for patients treated with either fulvestrant (Faslodex) or exemestane (Aromasin) plus palbociclib (Ibrance) or with capecitabine (Xeloda) alone, nor was there a difference in PFS between patients with mutated or wild-type ESR1, reported Miguel Martin, MD, PhD, from the Gregorio Marañón Health Research Institute in Madrid.
“Palbociclib plus endocrine therapy is one of the standards of care today for patients with prior aromatase inhibitor [therapy] in the metastatic setting, and capecitabine as well is another option for this population, since it produces significant activity and a significant proportion of responses in patients with metastatic breast cancer. But we don’t know actually the relative efficacy of each therapy versus the other,” he said at the San Antonio Breast Cancer Symposium.
The phase 3 PEARL study was a head-to-head comparison of the two regimens in women with hormone receptor (HR)–positive, HER2-negative metastatic breast cancer that had progressed on aromatase inhibitors (AIs).
The study was originally designed to test the combination of palbociclib and exemestane versus capecitabine, and 296 patients were enrolled (cohort 1).
The study was modified in 2016, however, following evidence that mutations in ESR1 are a major mechanism of resistance to AIs in patients with metastatic disease, and that fulvestrant, a selective estrogen receptor down-regulator, did not appear to have cross-resistance to either tamoxifen or AIs, and may be active against tumors with ESR1 mutations. Therefore, a second cohort of 305 patients was enrolled to test palbociclib plus fulvestrant versus capecitabine.
Each cohort included patients with HR-positive, HER2-negative metastatic breast cancer that had recurred within 1 year of completed adjuvant therapy with nonsteroidal AIs, or progression within 1 month of completing adjuvant AIs for advanced disease. Patients could have received one prior line of chemotherapy for metastatic disease, but no prior capecitabine, exemestane (in cohort 1), or fulvestrant (in cohort 2)
In each cohort, patients were stratified by visceral or nonvisceral metastases, prior sensitivity to hormonal therapy (yes or no), and prior chemotherapy for metastatic breast cancer, and then randomized on a 1:1 basis to capecitabine 1,250 mg/m2 (1,000 mg/m2 for patients older than 70 years) twice daily 2 weeks on, 1 week off every 21 days; to palbociclib 125 mg 3 weeks on, 1 week off every 28 days plus exemestane 25 mg daily in cohort 1; or to fulvestrant 500 mg on days 1 and 15 of cycle 1 and then once every 28 days.
The trial did not meet its coprimary endpoints of superior PFS with palbociclib/fulvestrant regardless of ESR1 mutational status, or superior PFS with palbociclib and either partners in patients with ESR1 wild-type tumors.
In cohort 2, after a median follow-up of 13.47 months, the median PFS with palbociclib/fulvestrant was 7.5 months versus 10 months with capecitabine, with a nonsignificant hazard ratio.
Similarly, in cohort 1 (patients with wild-type ESR1), the median PFS at a median follow-up of 18.89 months was 8.0 months with palbociclib plus endocrine therapy versus 10.6 months with capecitabine.
For the secondary endpoint of PFS in the combined cohorts, the median overall PFS after a median 17.64 months of follow-up was 7.4 versus 9.4 months, respectively.
There were no significant differences by intrinsic breast cancer subtypes expect for nonluminal breast cancer, for which capecitabine had a significantly better benefit (P = .008 in cohort 2, and .002 for patients with ESR1 wild type).
Objective response rates in both cohorts trended in favor of capecitabine, but neither trend was statistically significant.
The palbociclib-containing regimens were, however, generally better tolerated than capecitabine, with a lower frequency of treatment discontinuations (3.7% with palbociclib plus endocrine therapy vs. 12.8% with capecitabine) and a smaller proportion of patients with serious adverse events (3.7% vs. 10.4%, respectively).
In the question and response following his presentation, perennial SABCS provocateur Steven “Vogl New York” Vogl, MD, of Montefiore Medical Center asked Dr. Martin: “Did you really give the capecitabine for a median of 18 months because that was the time to progression?”
Dr. Vogl commented that 18 months “is a very long time to keep a patient on drugs that make their palms sore, give them diarrhea, give them rashes, and sore mouths. So were the Spanish doctors particularly smart about reducing the doses?”
Dr. Martin replied that in his experience patients could be maintained on capecitabine for more than 4 years, with dose adjustments for those who develop palmar or plantar problems or other side effects, but “many patients prefer that to alopecia, to vomiting, to IV injections, so in my view capecitabine is a great drug for luminal metastatic breast cancer cases, and we can keep the drug going for many, many months in most patients.”
The study was funded by Pfizer. Dr. Martin disclosed speaker honoraria and consulting fees from Pfizer and others.
SOURCE: Martin M et al. SABCS 2019. Abstract GS2-07
SAN ANTONIO – For postmenopausal women with hormone receptor–positive, HER2-negative breast cancer that has progressed on aromatase inhibitor therapy, the combination of palbociclib with either exemestane or fulvestrant was not better than capecitabine at delaying progression or inducing clinical responses, results of the phase 3 PEARL trial showed.
At a median follow-up of 13.45 months there was no significant difference in progression-free survival (PFS) for patients treated with either fulvestrant (Faslodex) or exemestane (Aromasin) plus palbociclib (Ibrance) or with capecitabine (Xeloda) alone, nor was there a difference in PFS between patients with mutated or wild-type ESR1, reported Miguel Martin, MD, PhD, from the Gregorio Marañón Health Research Institute in Madrid.
“Palbociclib plus endocrine therapy is one of the standards of care today for patients with prior aromatase inhibitor [therapy] in the metastatic setting, and capecitabine as well is another option for this population, since it produces significant activity and a significant proportion of responses in patients with metastatic breast cancer. But we don’t know actually the relative efficacy of each therapy versus the other,” he said at the San Antonio Breast Cancer Symposium.
The phase 3 PEARL study was a head-to-head comparison of the two regimens in women with hormone receptor (HR)–positive, HER2-negative metastatic breast cancer that had progressed on aromatase inhibitors (AIs).
The study was originally designed to test the combination of palbociclib and exemestane versus capecitabine, and 296 patients were enrolled (cohort 1).
The study was modified in 2016, however, following evidence that mutations in ESR1 are a major mechanism of resistance to AIs in patients with metastatic disease, and that fulvestrant, a selective estrogen receptor down-regulator, did not appear to have cross-resistance to either tamoxifen or AIs, and may be active against tumors with ESR1 mutations. Therefore, a second cohort of 305 patients was enrolled to test palbociclib plus fulvestrant versus capecitabine.
Each cohort included patients with HR-positive, HER2-negative metastatic breast cancer that had recurred within 1 year of completed adjuvant therapy with nonsteroidal AIs, or progression within 1 month of completing adjuvant AIs for advanced disease. Patients could have received one prior line of chemotherapy for metastatic disease, but no prior capecitabine, exemestane (in cohort 1), or fulvestrant (in cohort 2)
In each cohort, patients were stratified by visceral or nonvisceral metastases, prior sensitivity to hormonal therapy (yes or no), and prior chemotherapy for metastatic breast cancer, and then randomized on a 1:1 basis to capecitabine 1,250 mg/m2 (1,000 mg/m2 for patients older than 70 years) twice daily 2 weeks on, 1 week off every 21 days; to palbociclib 125 mg 3 weeks on, 1 week off every 28 days plus exemestane 25 mg daily in cohort 1; or to fulvestrant 500 mg on days 1 and 15 of cycle 1 and then once every 28 days.
The trial did not meet its coprimary endpoints of superior PFS with palbociclib/fulvestrant regardless of ESR1 mutational status, or superior PFS with palbociclib and either partners in patients with ESR1 wild-type tumors.
In cohort 2, after a median follow-up of 13.47 months, the median PFS with palbociclib/fulvestrant was 7.5 months versus 10 months with capecitabine, with a nonsignificant hazard ratio.
Similarly, in cohort 1 (patients with wild-type ESR1), the median PFS at a median follow-up of 18.89 months was 8.0 months with palbociclib plus endocrine therapy versus 10.6 months with capecitabine.
For the secondary endpoint of PFS in the combined cohorts, the median overall PFS after a median 17.64 months of follow-up was 7.4 versus 9.4 months, respectively.
There were no significant differences by intrinsic breast cancer subtypes expect for nonluminal breast cancer, for which capecitabine had a significantly better benefit (P = .008 in cohort 2, and .002 for patients with ESR1 wild type).
Objective response rates in both cohorts trended in favor of capecitabine, but neither trend was statistically significant.
The palbociclib-containing regimens were, however, generally better tolerated than capecitabine, with a lower frequency of treatment discontinuations (3.7% with palbociclib plus endocrine therapy vs. 12.8% with capecitabine) and a smaller proportion of patients with serious adverse events (3.7% vs. 10.4%, respectively).
In the question and response following his presentation, perennial SABCS provocateur Steven “Vogl New York” Vogl, MD, of Montefiore Medical Center asked Dr. Martin: “Did you really give the capecitabine for a median of 18 months because that was the time to progression?”
Dr. Vogl commented that 18 months “is a very long time to keep a patient on drugs that make their palms sore, give them diarrhea, give them rashes, and sore mouths. So were the Spanish doctors particularly smart about reducing the doses?”
Dr. Martin replied that in his experience patients could be maintained on capecitabine for more than 4 years, with dose adjustments for those who develop palmar or plantar problems or other side effects, but “many patients prefer that to alopecia, to vomiting, to IV injections, so in my view capecitabine is a great drug for luminal metastatic breast cancer cases, and we can keep the drug going for many, many months in most patients.”
The study was funded by Pfizer. Dr. Martin disclosed speaker honoraria and consulting fees from Pfizer and others.
SOURCE: Martin M et al. SABCS 2019. Abstract GS2-07
SAN ANTONIO – For postmenopausal women with hormone receptor–positive, HER2-negative breast cancer that has progressed on aromatase inhibitor therapy, the combination of palbociclib with either exemestane or fulvestrant was not better than capecitabine at delaying progression or inducing clinical responses, results of the phase 3 PEARL trial showed.
At a median follow-up of 13.45 months there was no significant difference in progression-free survival (PFS) for patients treated with either fulvestrant (Faslodex) or exemestane (Aromasin) plus palbociclib (Ibrance) or with capecitabine (Xeloda) alone, nor was there a difference in PFS between patients with mutated or wild-type ESR1, reported Miguel Martin, MD, PhD, from the Gregorio Marañón Health Research Institute in Madrid.
“Palbociclib plus endocrine therapy is one of the standards of care today for patients with prior aromatase inhibitor [therapy] in the metastatic setting, and capecitabine as well is another option for this population, since it produces significant activity and a significant proportion of responses in patients with metastatic breast cancer. But we don’t know actually the relative efficacy of each therapy versus the other,” he said at the San Antonio Breast Cancer Symposium.
The phase 3 PEARL study was a head-to-head comparison of the two regimens in women with hormone receptor (HR)–positive, HER2-negative metastatic breast cancer that had progressed on aromatase inhibitors (AIs).
The study was originally designed to test the combination of palbociclib and exemestane versus capecitabine, and 296 patients were enrolled (cohort 1).
The study was modified in 2016, however, following evidence that mutations in ESR1 are a major mechanism of resistance to AIs in patients with metastatic disease, and that fulvestrant, a selective estrogen receptor down-regulator, did not appear to have cross-resistance to either tamoxifen or AIs, and may be active against tumors with ESR1 mutations. Therefore, a second cohort of 305 patients was enrolled to test palbociclib plus fulvestrant versus capecitabine.
Each cohort included patients with HR-positive, HER2-negative metastatic breast cancer that had recurred within 1 year of completed adjuvant therapy with nonsteroidal AIs, or progression within 1 month of completing adjuvant AIs for advanced disease. Patients could have received one prior line of chemotherapy for metastatic disease, but no prior capecitabine, exemestane (in cohort 1), or fulvestrant (in cohort 2)
In each cohort, patients were stratified by visceral or nonvisceral metastases, prior sensitivity to hormonal therapy (yes or no), and prior chemotherapy for metastatic breast cancer, and then randomized on a 1:1 basis to capecitabine 1,250 mg/m2 (1,000 mg/m2 for patients older than 70 years) twice daily 2 weeks on, 1 week off every 21 days; to palbociclib 125 mg 3 weeks on, 1 week off every 28 days plus exemestane 25 mg daily in cohort 1; or to fulvestrant 500 mg on days 1 and 15 of cycle 1 and then once every 28 days.
The trial did not meet its coprimary endpoints of superior PFS with palbociclib/fulvestrant regardless of ESR1 mutational status, or superior PFS with palbociclib and either partners in patients with ESR1 wild-type tumors.
In cohort 2, after a median follow-up of 13.47 months, the median PFS with palbociclib/fulvestrant was 7.5 months versus 10 months with capecitabine, with a nonsignificant hazard ratio.
Similarly, in cohort 1 (patients with wild-type ESR1), the median PFS at a median follow-up of 18.89 months was 8.0 months with palbociclib plus endocrine therapy versus 10.6 months with capecitabine.
For the secondary endpoint of PFS in the combined cohorts, the median overall PFS after a median 17.64 months of follow-up was 7.4 versus 9.4 months, respectively.
There were no significant differences by intrinsic breast cancer subtypes expect for nonluminal breast cancer, for which capecitabine had a significantly better benefit (P = .008 in cohort 2, and .002 for patients with ESR1 wild type).
Objective response rates in both cohorts trended in favor of capecitabine, but neither trend was statistically significant.
The palbociclib-containing regimens were, however, generally better tolerated than capecitabine, with a lower frequency of treatment discontinuations (3.7% with palbociclib plus endocrine therapy vs. 12.8% with capecitabine) and a smaller proportion of patients with serious adverse events (3.7% vs. 10.4%, respectively).
In the question and response following his presentation, perennial SABCS provocateur Steven “Vogl New York” Vogl, MD, of Montefiore Medical Center asked Dr. Martin: “Did you really give the capecitabine for a median of 18 months because that was the time to progression?”
Dr. Vogl commented that 18 months “is a very long time to keep a patient on drugs that make their palms sore, give them diarrhea, give them rashes, and sore mouths. So were the Spanish doctors particularly smart about reducing the doses?”
Dr. Martin replied that in his experience patients could be maintained on capecitabine for more than 4 years, with dose adjustments for those who develop palmar or plantar problems or other side effects, but “many patients prefer that to alopecia, to vomiting, to IV injections, so in my view capecitabine is a great drug for luminal metastatic breast cancer cases, and we can keep the drug going for many, many months in most patients.”
The study was funded by Pfizer. Dr. Martin disclosed speaker honoraria and consulting fees from Pfizer and others.
SOURCE: Martin M et al. SABCS 2019. Abstract GS2-07
REPORTING FROM SABCS 2019
The vaping problem
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Envisioning the future of hospital medicine
I have written frequently over the last few years on topics related to the sustainability of the hospital medicine practice model. I continue to be concerned by what I see as a confluence of significant trends that are conspiring to challenge hospital medicine’s status quo.
On one hand, the financial pressures on U.S. hospitals are unrelenting, and their willingness or even ability to continue providing significant funding to support their hospital medicine groups is in question. Combine this with hospitalists’ rapidly evolving clinical scope and the ever-increasing demands of physicians in other specialties for hospitalist support, and the result is hospital medicine groups that will continue to grow in size, complexity, and the demand for ever more financial support.
On the other hand, the hospitalists I interact with in my work all over the country seem more stressed out than ever, and many are questioning whether this is a job that can be satisfying and sustainable for a career. Increasing patient complexity, productivity pressures, a lack of resources to address patients’ social issues, a systole-diastole schedule, the frustration of EHRs and other documentation responsibilities, and feeling “dumped on” by physicians in other specialties all contribute to hospitalist job stress.
A quick look at the literature confirms that in 2019 hospitalist burnout is definitely “a thing.” Interestingly, it’s been a thing for a while; the risk of hospitalist burnout was first identified by Hoff, et al., in 2002 (doi: 10.2307/30902462002). My colleague, John Nelson, MD, MHM, has written a number of times about strategies for preventing or mitigating hospitalist burnout.
As these trends converge, the hospital medicine practice model as we know it may be facing an existential crisis. If that sounds overly dramatic, let me say instead that the hospital medicine practice model will need to evolve significantly over the next decade in order to continue to meet patient and institutional needs while remaining both affordable and sustainable for the clinicians who work in it.
In September 2019, SHM’s Multi-Site Leaders Special Interest Group met in Chicago for their second annual Multi-Site Leaders Summit to explore the theme of sustainability in hospital medicine. The participants held robust discussions about coping with our changing practice environment, issues relating to hospitalist burnout and resiliency, innovative staffing models, the role of technology in HM sustainability, and financial sustainability
At the end of the meeting, the group engaged in a visioning exercise designed to move beyond what we are doing today by envisioning what the future of hospital medicine will look like and what interventions will be necessary for us to get from here to there. I’d like to share this visioning exercise with you and encourage you to “play along” by thinking seriously about the questions it poses.
Visioning exercise
Feel free to jot down some thoughts as we go through this exercise. But otherwise, just close your eyes and come along for the ride. Imagine yourself sitting at your desk looking at a desk calendar showing today’s date. Watch the pages flip from today, to tomorrow, to the next day, then to next month, and the next, and then to the next year and so on, until we arrive at December 2029.
Imagine that you look up from your desk, and suddenly realize that you aren’t in your office at all, but instead in a huge auditorium where someone is speaking about an award that is going to be announced. It’s crowded and a little stuffy in the auditorium, but people around you are whispering to each other with an air of eager anticipation, their eyes glued to the stage. You realize that the person being introduced up on the podium is the President of the United States, and the award is the Presidential Medal of Freedom, which is only awarded to people or groups who have made “an especially meritorious contribution to the security or national interests of the United States, world peace, cultural, or other significant public or private endeavors.”
Today, the Medal is being awarded to the Society of Hospital Medicine on behalf of all hospital medicine leaders nationally, for their collective accomplishments in saving the specialty of hospital medicine and, by doing so, ensuring that sick people are able to continue receiving the care they need in our nation’s hospitals – and that the hospitals themselves have become reliably safe, efficient, and effective in achieving high quality outcomes.
The President says, “At no time in the history of this award until now have we given this, the highest civilian award in the land, to a whole group of physician leaders across an entire specialty. But the achievements of this group of people in preserving and even enhancing the presence of highly energized, dedicated, capable clinicians in our nation’s hospitals against the significant odds they have faced over the last 10 years is nothing short of extraordinary.” There is a standing ovation, as people jump up out of their chairs to cheer and applaud. When the applause finally dies down, the President goes on to list all the accomplishments that made this group of leaders deserving. Listen to what she is saying. Fill it in in your own mind. What is it that this group has accomplished?
[Brief silence]
Up on a huge screen beside the stage, a video starts. In it, there are several hospital and physician executives in a focus group, and one exec says, “The thing that is great about what these leaders have accomplished in the field of hospital medicine is…” Fill it in – what did that executive say? Another leader jumps in: “That’s all fine and wonderful, but the thing that really makes hospital medicine stand out today compared to where they were 10 years ago is…” Listen to what these executives are saying. What accomplishments are they praising?
The video then moves on to show a focus group of recent hospital patients. One patient says, “10 years ago when my mom was in the hospital, the poor hospitalists caring for her seemed completely overwhelmed and burnt out, and the whole care system seemed fragmented and inefficient; but my own recent hospital experience was so different because…” Additional patients chime in, talking about how confident they felt about the care they received in the hospital and the reasons for that. What is it these patients are describing?
SHM’s CEO gets up to accept the award and explains that 10 years ago, a group of multi-site hospital medicine leaders from across the country came together to begin addressing the issue of sustainability; this led to a formal process for developing a vision and a plan for the future of hospital medicine, and the execution of that plan eventually resulted in the outcomes recognized by this award. She acknowledges that over the years many people questioned whether the hospital medicine model should even continue to exist or whether some other model for inpatient care should be adopted. She talks about all the compelling reasons that supported the continued existence of the specialty of hospital medicine. What are some of the reasons she listed? The SHM CEO goes on to describe some of the key things that were done to address the issues associated with sustainability of the hospital medicine practice model. Listen to what she says; what was it that SHM and the hospital leaders it represents did?
As you are leaving the auditorium, you overhear a group of mid-career staff hospitalists talking. They are saying that they didn’t originally believe the specialty would actually change, and they weren’t sure if they could do this job for a career – but that it did change. They begin talking about what it feels like to work as a hospitalist now, and how these changes have improved their lives. Listen to what they are saying. How does it feel to work as a hospitalist?
As you leave the auditorium and go back to your desk, you sit down to record some of the things you heard. What was it the President of the US said as she presented the Presidential Medal of Freedom? Why did SHM and the hospital medicine leaders it represents deserve the award? What was it that the SHM CEO said was done to bring about the successful changes? What did the staff hospitalists say about working in the specialty?
Whenever you are ready, take a minute to jot down the specifics that came to mind as you read through this exercise. If you are willing to share your thoughts about sustainability in hospital medicine, I’d love to hear from you. Feel free to email me directly at [email protected].
Let’s build the foundation for a sustainable future for our specialty.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif. She serves on SHM’s Practice Analysis Committee, and helps to coordinate SHM’s bi-annual State of Hospital Medicine Survey. This article appeared originally in SHM’s official blog The Hospital Leader.
I have written frequently over the last few years on topics related to the sustainability of the hospital medicine practice model. I continue to be concerned by what I see as a confluence of significant trends that are conspiring to challenge hospital medicine’s status quo.
On one hand, the financial pressures on U.S. hospitals are unrelenting, and their willingness or even ability to continue providing significant funding to support their hospital medicine groups is in question. Combine this with hospitalists’ rapidly evolving clinical scope and the ever-increasing demands of physicians in other specialties for hospitalist support, and the result is hospital medicine groups that will continue to grow in size, complexity, and the demand for ever more financial support.
On the other hand, the hospitalists I interact with in my work all over the country seem more stressed out than ever, and many are questioning whether this is a job that can be satisfying and sustainable for a career. Increasing patient complexity, productivity pressures, a lack of resources to address patients’ social issues, a systole-diastole schedule, the frustration of EHRs and other documentation responsibilities, and feeling “dumped on” by physicians in other specialties all contribute to hospitalist job stress.
A quick look at the literature confirms that in 2019 hospitalist burnout is definitely “a thing.” Interestingly, it’s been a thing for a while; the risk of hospitalist burnout was first identified by Hoff, et al., in 2002 (doi: 10.2307/30902462002). My colleague, John Nelson, MD, MHM, has written a number of times about strategies for preventing or mitigating hospitalist burnout.
As these trends converge, the hospital medicine practice model as we know it may be facing an existential crisis. If that sounds overly dramatic, let me say instead that the hospital medicine practice model will need to evolve significantly over the next decade in order to continue to meet patient and institutional needs while remaining both affordable and sustainable for the clinicians who work in it.
In September 2019, SHM’s Multi-Site Leaders Special Interest Group met in Chicago for their second annual Multi-Site Leaders Summit to explore the theme of sustainability in hospital medicine. The participants held robust discussions about coping with our changing practice environment, issues relating to hospitalist burnout and resiliency, innovative staffing models, the role of technology in HM sustainability, and financial sustainability
At the end of the meeting, the group engaged in a visioning exercise designed to move beyond what we are doing today by envisioning what the future of hospital medicine will look like and what interventions will be necessary for us to get from here to there. I’d like to share this visioning exercise with you and encourage you to “play along” by thinking seriously about the questions it poses.
Visioning exercise
Feel free to jot down some thoughts as we go through this exercise. But otherwise, just close your eyes and come along for the ride. Imagine yourself sitting at your desk looking at a desk calendar showing today’s date. Watch the pages flip from today, to tomorrow, to the next day, then to next month, and the next, and then to the next year and so on, until we arrive at December 2029.
Imagine that you look up from your desk, and suddenly realize that you aren’t in your office at all, but instead in a huge auditorium where someone is speaking about an award that is going to be announced. It’s crowded and a little stuffy in the auditorium, but people around you are whispering to each other with an air of eager anticipation, their eyes glued to the stage. You realize that the person being introduced up on the podium is the President of the United States, and the award is the Presidential Medal of Freedom, which is only awarded to people or groups who have made “an especially meritorious contribution to the security or national interests of the United States, world peace, cultural, or other significant public or private endeavors.”
Today, the Medal is being awarded to the Society of Hospital Medicine on behalf of all hospital medicine leaders nationally, for their collective accomplishments in saving the specialty of hospital medicine and, by doing so, ensuring that sick people are able to continue receiving the care they need in our nation’s hospitals – and that the hospitals themselves have become reliably safe, efficient, and effective in achieving high quality outcomes.
The President says, “At no time in the history of this award until now have we given this, the highest civilian award in the land, to a whole group of physician leaders across an entire specialty. But the achievements of this group of people in preserving and even enhancing the presence of highly energized, dedicated, capable clinicians in our nation’s hospitals against the significant odds they have faced over the last 10 years is nothing short of extraordinary.” There is a standing ovation, as people jump up out of their chairs to cheer and applaud. When the applause finally dies down, the President goes on to list all the accomplishments that made this group of leaders deserving. Listen to what she is saying. Fill it in in your own mind. What is it that this group has accomplished?
[Brief silence]
Up on a huge screen beside the stage, a video starts. In it, there are several hospital and physician executives in a focus group, and one exec says, “The thing that is great about what these leaders have accomplished in the field of hospital medicine is…” Fill it in – what did that executive say? Another leader jumps in: “That’s all fine and wonderful, but the thing that really makes hospital medicine stand out today compared to where they were 10 years ago is…” Listen to what these executives are saying. What accomplishments are they praising?
The video then moves on to show a focus group of recent hospital patients. One patient says, “10 years ago when my mom was in the hospital, the poor hospitalists caring for her seemed completely overwhelmed and burnt out, and the whole care system seemed fragmented and inefficient; but my own recent hospital experience was so different because…” Additional patients chime in, talking about how confident they felt about the care they received in the hospital and the reasons for that. What is it these patients are describing?
SHM’s CEO gets up to accept the award and explains that 10 years ago, a group of multi-site hospital medicine leaders from across the country came together to begin addressing the issue of sustainability; this led to a formal process for developing a vision and a plan for the future of hospital medicine, and the execution of that plan eventually resulted in the outcomes recognized by this award. She acknowledges that over the years many people questioned whether the hospital medicine model should even continue to exist or whether some other model for inpatient care should be adopted. She talks about all the compelling reasons that supported the continued existence of the specialty of hospital medicine. What are some of the reasons she listed? The SHM CEO goes on to describe some of the key things that were done to address the issues associated with sustainability of the hospital medicine practice model. Listen to what she says; what was it that SHM and the hospital leaders it represents did?
As you are leaving the auditorium, you overhear a group of mid-career staff hospitalists talking. They are saying that they didn’t originally believe the specialty would actually change, and they weren’t sure if they could do this job for a career – but that it did change. They begin talking about what it feels like to work as a hospitalist now, and how these changes have improved their lives. Listen to what they are saying. How does it feel to work as a hospitalist?
As you leave the auditorium and go back to your desk, you sit down to record some of the things you heard. What was it the President of the US said as she presented the Presidential Medal of Freedom? Why did SHM and the hospital medicine leaders it represents deserve the award? What was it that the SHM CEO said was done to bring about the successful changes? What did the staff hospitalists say about working in the specialty?
Whenever you are ready, take a minute to jot down the specifics that came to mind as you read through this exercise. If you are willing to share your thoughts about sustainability in hospital medicine, I’d love to hear from you. Feel free to email me directly at [email protected].
Let’s build the foundation for a sustainable future for our specialty.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif. She serves on SHM’s Practice Analysis Committee, and helps to coordinate SHM’s bi-annual State of Hospital Medicine Survey. This article appeared originally in SHM’s official blog The Hospital Leader.
I have written frequently over the last few years on topics related to the sustainability of the hospital medicine practice model. I continue to be concerned by what I see as a confluence of significant trends that are conspiring to challenge hospital medicine’s status quo.
On one hand, the financial pressures on U.S. hospitals are unrelenting, and their willingness or even ability to continue providing significant funding to support their hospital medicine groups is in question. Combine this with hospitalists’ rapidly evolving clinical scope and the ever-increasing demands of physicians in other specialties for hospitalist support, and the result is hospital medicine groups that will continue to grow in size, complexity, and the demand for ever more financial support.
On the other hand, the hospitalists I interact with in my work all over the country seem more stressed out than ever, and many are questioning whether this is a job that can be satisfying and sustainable for a career. Increasing patient complexity, productivity pressures, a lack of resources to address patients’ social issues, a systole-diastole schedule, the frustration of EHRs and other documentation responsibilities, and feeling “dumped on” by physicians in other specialties all contribute to hospitalist job stress.
A quick look at the literature confirms that in 2019 hospitalist burnout is definitely “a thing.” Interestingly, it’s been a thing for a while; the risk of hospitalist burnout was first identified by Hoff, et al., in 2002 (doi: 10.2307/30902462002). My colleague, John Nelson, MD, MHM, has written a number of times about strategies for preventing or mitigating hospitalist burnout.
As these trends converge, the hospital medicine practice model as we know it may be facing an existential crisis. If that sounds overly dramatic, let me say instead that the hospital medicine practice model will need to evolve significantly over the next decade in order to continue to meet patient and institutional needs while remaining both affordable and sustainable for the clinicians who work in it.
In September 2019, SHM’s Multi-Site Leaders Special Interest Group met in Chicago for their second annual Multi-Site Leaders Summit to explore the theme of sustainability in hospital medicine. The participants held robust discussions about coping with our changing practice environment, issues relating to hospitalist burnout and resiliency, innovative staffing models, the role of technology in HM sustainability, and financial sustainability
At the end of the meeting, the group engaged in a visioning exercise designed to move beyond what we are doing today by envisioning what the future of hospital medicine will look like and what interventions will be necessary for us to get from here to there. I’d like to share this visioning exercise with you and encourage you to “play along” by thinking seriously about the questions it poses.
Visioning exercise
Feel free to jot down some thoughts as we go through this exercise. But otherwise, just close your eyes and come along for the ride. Imagine yourself sitting at your desk looking at a desk calendar showing today’s date. Watch the pages flip from today, to tomorrow, to the next day, then to next month, and the next, and then to the next year and so on, until we arrive at December 2029.
Imagine that you look up from your desk, and suddenly realize that you aren’t in your office at all, but instead in a huge auditorium where someone is speaking about an award that is going to be announced. It’s crowded and a little stuffy in the auditorium, but people around you are whispering to each other with an air of eager anticipation, their eyes glued to the stage. You realize that the person being introduced up on the podium is the President of the United States, and the award is the Presidential Medal of Freedom, which is only awarded to people or groups who have made “an especially meritorious contribution to the security or national interests of the United States, world peace, cultural, or other significant public or private endeavors.”
Today, the Medal is being awarded to the Society of Hospital Medicine on behalf of all hospital medicine leaders nationally, for their collective accomplishments in saving the specialty of hospital medicine and, by doing so, ensuring that sick people are able to continue receiving the care they need in our nation’s hospitals – and that the hospitals themselves have become reliably safe, efficient, and effective in achieving high quality outcomes.
The President says, “At no time in the history of this award until now have we given this, the highest civilian award in the land, to a whole group of physician leaders across an entire specialty. But the achievements of this group of people in preserving and even enhancing the presence of highly energized, dedicated, capable clinicians in our nation’s hospitals against the significant odds they have faced over the last 10 years is nothing short of extraordinary.” There is a standing ovation, as people jump up out of their chairs to cheer and applaud. When the applause finally dies down, the President goes on to list all the accomplishments that made this group of leaders deserving. Listen to what she is saying. Fill it in in your own mind. What is it that this group has accomplished?
[Brief silence]
Up on a huge screen beside the stage, a video starts. In it, there are several hospital and physician executives in a focus group, and one exec says, “The thing that is great about what these leaders have accomplished in the field of hospital medicine is…” Fill it in – what did that executive say? Another leader jumps in: “That’s all fine and wonderful, but the thing that really makes hospital medicine stand out today compared to where they were 10 years ago is…” Listen to what these executives are saying. What accomplishments are they praising?
The video then moves on to show a focus group of recent hospital patients. One patient says, “10 years ago when my mom was in the hospital, the poor hospitalists caring for her seemed completely overwhelmed and burnt out, and the whole care system seemed fragmented and inefficient; but my own recent hospital experience was so different because…” Additional patients chime in, talking about how confident they felt about the care they received in the hospital and the reasons for that. What is it these patients are describing?
SHM’s CEO gets up to accept the award and explains that 10 years ago, a group of multi-site hospital medicine leaders from across the country came together to begin addressing the issue of sustainability; this led to a formal process for developing a vision and a plan for the future of hospital medicine, and the execution of that plan eventually resulted in the outcomes recognized by this award. She acknowledges that over the years many people questioned whether the hospital medicine model should even continue to exist or whether some other model for inpatient care should be adopted. She talks about all the compelling reasons that supported the continued existence of the specialty of hospital medicine. What are some of the reasons she listed? The SHM CEO goes on to describe some of the key things that were done to address the issues associated with sustainability of the hospital medicine practice model. Listen to what she says; what was it that SHM and the hospital leaders it represents did?
As you are leaving the auditorium, you overhear a group of mid-career staff hospitalists talking. They are saying that they didn’t originally believe the specialty would actually change, and they weren’t sure if they could do this job for a career – but that it did change. They begin talking about what it feels like to work as a hospitalist now, and how these changes have improved their lives. Listen to what they are saying. How does it feel to work as a hospitalist?
As you leave the auditorium and go back to your desk, you sit down to record some of the things you heard. What was it the President of the US said as she presented the Presidential Medal of Freedom? Why did SHM and the hospital medicine leaders it represents deserve the award? What was it that the SHM CEO said was done to bring about the successful changes? What did the staff hospitalists say about working in the specialty?
Whenever you are ready, take a minute to jot down the specifics that came to mind as you read through this exercise. If you are willing to share your thoughts about sustainability in hospital medicine, I’d love to hear from you. Feel free to email me directly at [email protected].
Let’s build the foundation for a sustainable future for our specialty.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif. She serves on SHM’s Practice Analysis Committee, and helps to coordinate SHM’s bi-annual State of Hospital Medicine Survey. This article appeared originally in SHM’s official blog The Hospital Leader.
Breakfast or not?
In North America, breakfast is the most personal of all the traditional daily meals and usually the one at which people show the least amount of day-to-day variation.
For example, since retiring from active practice I eat three scrambled eggs and bowl of fresh fruit every morning (yes, I have my lipid screen done annually and it’s fine). When I was a child there were stretches measuring in years during which I would eat the same cold cereal and drink a glass of orange juice. As an adolescent trying to bulk up for football, there was a breakfast-in-a-glass that I drank along with the cereal every morning. There was the frozen waffle decade.
When I was a busy general pediatrician, the meals were short on preparation and equally short on variety. But I always had something to eat before heading out for the day. That’s what my folks did, and that’s the pattern my wife and I programmed into our children. I think my dietary history is not unique. Most people don’t have time for a complex breakfast, and in many cases, they aren’t feeling terribly adventuresome when it comes to food at 6 or 7 in the morning. Breakfast is more of a habit than an event to satisfy one’s hunger. Several generations ago, breakfast was a big deal. Men (and occasionally women) were headed out for a day of demanding physical labor and stoking the furnace at the beginning of the day made sense. In farm families, breakfast was a major meal after the morning chores were completed. Those Norman Rockwellesque days are behind us, and breakfast has receded into a minor nutritional role.
For many adults, it’s just something to chew on with a cup of a stimulant liquid. In some families, breakfast has disappeared completely. For as long as there have been dietitians and nutritionists, we have been told that breakfast can be the most important meal of the day. And for a child, the failure to eat breakfast could jeopardize his or her ability to perform in school. I guess at face value this dictum makes sense, but I’ve never been terribly impressed with the evidence supporting it. A recent study from England has gotten me thinking about the whole issue of breakfast and school performance again (“associations between habitual school-day breakfast consumption frequency and academic performance in British adolescents.” Front Public Health. 2019 Nov 20. doi. 10.3389/fpubh.2019.00283). A trio of researchers at the Human Appetite Research Unit of the School of Psychology, University of Leeds (England), found that in the study group of nearly 300 adolescents aged 16-18 years, the students who frequently skipped breakfast performed more poorly on a battery of standardized national tests. Well, I guess we have to chalk another one up for the dietitians and nutritionists. But let’s think this through again. The authors observe in the discussion of their results that “breakfast quality was not considered in the analysis and therefore conclusions regarding what aspects of breakfast are correlated with academic performance cannot be drawn.”
Could it be that families who don’t give breakfast a priority also don’t prioritize school work? Maybe teenagers with poor sleep hygiene who are habitually difficult to awaken in the morning don’t have time to eat breakfast. It is likely their sleep deprivation is more of a factor in their school performance than the small nutritional deficit that they have incurred by not eating breakfast. The study that might answer these questions hasn’t been done yet. And maybe it doesn’t need to be done. We don’t need to be asking children what they have for breakfast. But we should be entering into a dialogue that begins with “Why don’t you have breakfast?” The answers may lead into a productive discussion with the family about more important contributors to poor school performance.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In North America, breakfast is the most personal of all the traditional daily meals and usually the one at which people show the least amount of day-to-day variation.
For example, since retiring from active practice I eat three scrambled eggs and bowl of fresh fruit every morning (yes, I have my lipid screen done annually and it’s fine). When I was a child there were stretches measuring in years during which I would eat the same cold cereal and drink a glass of orange juice. As an adolescent trying to bulk up for football, there was a breakfast-in-a-glass that I drank along with the cereal every morning. There was the frozen waffle decade.
When I was a busy general pediatrician, the meals were short on preparation and equally short on variety. But I always had something to eat before heading out for the day. That’s what my folks did, and that’s the pattern my wife and I programmed into our children. I think my dietary history is not unique. Most people don’t have time for a complex breakfast, and in many cases, they aren’t feeling terribly adventuresome when it comes to food at 6 or 7 in the morning. Breakfast is more of a habit than an event to satisfy one’s hunger. Several generations ago, breakfast was a big deal. Men (and occasionally women) were headed out for a day of demanding physical labor and stoking the furnace at the beginning of the day made sense. In farm families, breakfast was a major meal after the morning chores were completed. Those Norman Rockwellesque days are behind us, and breakfast has receded into a minor nutritional role.
For many adults, it’s just something to chew on with a cup of a stimulant liquid. In some families, breakfast has disappeared completely. For as long as there have been dietitians and nutritionists, we have been told that breakfast can be the most important meal of the day. And for a child, the failure to eat breakfast could jeopardize his or her ability to perform in school. I guess at face value this dictum makes sense, but I’ve never been terribly impressed with the evidence supporting it. A recent study from England has gotten me thinking about the whole issue of breakfast and school performance again (“associations between habitual school-day breakfast consumption frequency and academic performance in British adolescents.” Front Public Health. 2019 Nov 20. doi. 10.3389/fpubh.2019.00283). A trio of researchers at the Human Appetite Research Unit of the School of Psychology, University of Leeds (England), found that in the study group of nearly 300 adolescents aged 16-18 years, the students who frequently skipped breakfast performed more poorly on a battery of standardized national tests. Well, I guess we have to chalk another one up for the dietitians and nutritionists. But let’s think this through again. The authors observe in the discussion of their results that “breakfast quality was not considered in the analysis and therefore conclusions regarding what aspects of breakfast are correlated with academic performance cannot be drawn.”
Could it be that families who don’t give breakfast a priority also don’t prioritize school work? Maybe teenagers with poor sleep hygiene who are habitually difficult to awaken in the morning don’t have time to eat breakfast. It is likely their sleep deprivation is more of a factor in their school performance than the small nutritional deficit that they have incurred by not eating breakfast. The study that might answer these questions hasn’t been done yet. And maybe it doesn’t need to be done. We don’t need to be asking children what they have for breakfast. But we should be entering into a dialogue that begins with “Why don’t you have breakfast?” The answers may lead into a productive discussion with the family about more important contributors to poor school performance.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In North America, breakfast is the most personal of all the traditional daily meals and usually the one at which people show the least amount of day-to-day variation.
For example, since retiring from active practice I eat three scrambled eggs and bowl of fresh fruit every morning (yes, I have my lipid screen done annually and it’s fine). When I was a child there were stretches measuring in years during which I would eat the same cold cereal and drink a glass of orange juice. As an adolescent trying to bulk up for football, there was a breakfast-in-a-glass that I drank along with the cereal every morning. There was the frozen waffle decade.
When I was a busy general pediatrician, the meals were short on preparation and equally short on variety. But I always had something to eat before heading out for the day. That’s what my folks did, and that’s the pattern my wife and I programmed into our children. I think my dietary history is not unique. Most people don’t have time for a complex breakfast, and in many cases, they aren’t feeling terribly adventuresome when it comes to food at 6 or 7 in the morning. Breakfast is more of a habit than an event to satisfy one’s hunger. Several generations ago, breakfast was a big deal. Men (and occasionally women) were headed out for a day of demanding physical labor and stoking the furnace at the beginning of the day made sense. In farm families, breakfast was a major meal after the morning chores were completed. Those Norman Rockwellesque days are behind us, and breakfast has receded into a minor nutritional role.
For many adults, it’s just something to chew on with a cup of a stimulant liquid. In some families, breakfast has disappeared completely. For as long as there have been dietitians and nutritionists, we have been told that breakfast can be the most important meal of the day. And for a child, the failure to eat breakfast could jeopardize his or her ability to perform in school. I guess at face value this dictum makes sense, but I’ve never been terribly impressed with the evidence supporting it. A recent study from England has gotten me thinking about the whole issue of breakfast and school performance again (“associations between habitual school-day breakfast consumption frequency and academic performance in British adolescents.” Front Public Health. 2019 Nov 20. doi. 10.3389/fpubh.2019.00283). A trio of researchers at the Human Appetite Research Unit of the School of Psychology, University of Leeds (England), found that in the study group of nearly 300 adolescents aged 16-18 years, the students who frequently skipped breakfast performed more poorly on a battery of standardized national tests. Well, I guess we have to chalk another one up for the dietitians and nutritionists. But let’s think this through again. The authors observe in the discussion of their results that “breakfast quality was not considered in the analysis and therefore conclusions regarding what aspects of breakfast are correlated with academic performance cannot be drawn.”
Could it be that families who don’t give breakfast a priority also don’t prioritize school work? Maybe teenagers with poor sleep hygiene who are habitually difficult to awaken in the morning don’t have time to eat breakfast. It is likely their sleep deprivation is more of a factor in their school performance than the small nutritional deficit that they have incurred by not eating breakfast. The study that might answer these questions hasn’t been done yet. And maybe it doesn’t need to be done. We don’t need to be asking children what they have for breakfast. But we should be entering into a dialogue that begins with “Why don’t you have breakfast?” The answers may lead into a productive discussion with the family about more important contributors to poor school performance.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Seven things I am grateful for
It’s almost a New Year and .
The wildlife in my backyard provides endless entertainment. Not only the birds, rabbits, squirrels, raccoons, and skunks, but turkey, red fox, coyote, hawk, and even a mink. At some point, I quit buying koi for the pond and substituted dime gold fish. Of course, we cannot overlook the deer, who trash my shrubs, eat and grind the bark off my baby trees, consume my garden (don’t they know tomato plants are related to deadly nightshade?), and shed ticks. They watch me with calm indifference, even when I shout at them.
Most folks hate cold weather, but it kills the mosquitoes and stink bugs, and allows me to build mesmerizing fires. It is time to clean the yard, turn over the garden, plant new things, all without breaking much of a sweat. It is almost time to empty the compost pile onto the garden mixed with the ashes from the fire pit.
Last year’s tomato crop started late but was a blockbuster. I plant heirlooms grafted onto resistant rootstock (territorial seed company) placed under walls of water in April. I cage them up high. I like to stand in the tomato jungle in high summer, invisible for a few minutes, and eat the little cherry tomatoes and think about nothing but how perfectly the sweetness and tartness is balanced. I still have a few on the kitchen counter making that crucial, very late, decision on whether to ripen or rot.
The U.S. Navy cannot be thanked enough for taking my defiant teenage boy and molding him into what is starting to resemble a fine young man. The Navy is what he needed.
I give much professional credit to my office staff and my patients. I really haven’t run the office for years; it has its own rhythm and knowledge. You spend more waking hours there than at home, so being fun and entertaining is important. That said, the hiring and management of employees is the most difficult part of running a small office. The patients generally know to come in sooner rather than later if they start growing something ugly. And I have also been blessed with good health, mandatory for maintaining a small office. It’s been a good ride.
My wife is quiet when I am loud, reserved when I am bombastic, an only child matched with a middle. She wears a child’s size bicycle helmet, but her head is packed with brains. She knows millions of things I don’t. She is terribly organized. I float my crazy ideas past her daily and leave with punctured remnants to patch together into a better weave. We spend all our free time together, which is the way it ought to be.
Finally, of course, I am grateful for my specialty of dermatology. I kind of wandered into dermatology after internal medicine, and after seriously considering cardiology. It is a happy and joyous specialty with enough cures and successes to keep gloom and hopelessness at bay. I look forward to going to work and get great satisfaction from my work. I have continued to improve in this field, which gives so much more than it takes.
So enjoy the New Year! Take time to build a roaring fire, buy quirky gifts for your staff, get your spouses or significant others whatever they want, and enjoy your specialty as a dermatologist. You are in one of the best places in the world.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
It’s almost a New Year and .
The wildlife in my backyard provides endless entertainment. Not only the birds, rabbits, squirrels, raccoons, and skunks, but turkey, red fox, coyote, hawk, and even a mink. At some point, I quit buying koi for the pond and substituted dime gold fish. Of course, we cannot overlook the deer, who trash my shrubs, eat and grind the bark off my baby trees, consume my garden (don’t they know tomato plants are related to deadly nightshade?), and shed ticks. They watch me with calm indifference, even when I shout at them.
Most folks hate cold weather, but it kills the mosquitoes and stink bugs, and allows me to build mesmerizing fires. It is time to clean the yard, turn over the garden, plant new things, all without breaking much of a sweat. It is almost time to empty the compost pile onto the garden mixed with the ashes from the fire pit.
Last year’s tomato crop started late but was a blockbuster. I plant heirlooms grafted onto resistant rootstock (territorial seed company) placed under walls of water in April. I cage them up high. I like to stand in the tomato jungle in high summer, invisible for a few minutes, and eat the little cherry tomatoes and think about nothing but how perfectly the sweetness and tartness is balanced. I still have a few on the kitchen counter making that crucial, very late, decision on whether to ripen or rot.
The U.S. Navy cannot be thanked enough for taking my defiant teenage boy and molding him into what is starting to resemble a fine young man. The Navy is what he needed.
I give much professional credit to my office staff and my patients. I really haven’t run the office for years; it has its own rhythm and knowledge. You spend more waking hours there than at home, so being fun and entertaining is important. That said, the hiring and management of employees is the most difficult part of running a small office. The patients generally know to come in sooner rather than later if they start growing something ugly. And I have also been blessed with good health, mandatory for maintaining a small office. It’s been a good ride.
My wife is quiet when I am loud, reserved when I am bombastic, an only child matched with a middle. She wears a child’s size bicycle helmet, but her head is packed with brains. She knows millions of things I don’t. She is terribly organized. I float my crazy ideas past her daily and leave with punctured remnants to patch together into a better weave. We spend all our free time together, which is the way it ought to be.
Finally, of course, I am grateful for my specialty of dermatology. I kind of wandered into dermatology after internal medicine, and after seriously considering cardiology. It is a happy and joyous specialty with enough cures and successes to keep gloom and hopelessness at bay. I look forward to going to work and get great satisfaction from my work. I have continued to improve in this field, which gives so much more than it takes.
So enjoy the New Year! Take time to build a roaring fire, buy quirky gifts for your staff, get your spouses or significant others whatever they want, and enjoy your specialty as a dermatologist. You are in one of the best places in the world.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
It’s almost a New Year and .
The wildlife in my backyard provides endless entertainment. Not only the birds, rabbits, squirrels, raccoons, and skunks, but turkey, red fox, coyote, hawk, and even a mink. At some point, I quit buying koi for the pond and substituted dime gold fish. Of course, we cannot overlook the deer, who trash my shrubs, eat and grind the bark off my baby trees, consume my garden (don’t they know tomato plants are related to deadly nightshade?), and shed ticks. They watch me with calm indifference, even when I shout at them.
Most folks hate cold weather, but it kills the mosquitoes and stink bugs, and allows me to build mesmerizing fires. It is time to clean the yard, turn over the garden, plant new things, all without breaking much of a sweat. It is almost time to empty the compost pile onto the garden mixed with the ashes from the fire pit.
Last year’s tomato crop started late but was a blockbuster. I plant heirlooms grafted onto resistant rootstock (territorial seed company) placed under walls of water in April. I cage them up high. I like to stand in the tomato jungle in high summer, invisible for a few minutes, and eat the little cherry tomatoes and think about nothing but how perfectly the sweetness and tartness is balanced. I still have a few on the kitchen counter making that crucial, very late, decision on whether to ripen or rot.
The U.S. Navy cannot be thanked enough for taking my defiant teenage boy and molding him into what is starting to resemble a fine young man. The Navy is what he needed.
I give much professional credit to my office staff and my patients. I really haven’t run the office for years; it has its own rhythm and knowledge. You spend more waking hours there than at home, so being fun and entertaining is important. That said, the hiring and management of employees is the most difficult part of running a small office. The patients generally know to come in sooner rather than later if they start growing something ugly. And I have also been blessed with good health, mandatory for maintaining a small office. It’s been a good ride.
My wife is quiet when I am loud, reserved when I am bombastic, an only child matched with a middle. She wears a child’s size bicycle helmet, but her head is packed with brains. She knows millions of things I don’t. She is terribly organized. I float my crazy ideas past her daily and leave with punctured remnants to patch together into a better weave. We spend all our free time together, which is the way it ought to be.
Finally, of course, I am grateful for my specialty of dermatology. I kind of wandered into dermatology after internal medicine, and after seriously considering cardiology. It is a happy and joyous specialty with enough cures and successes to keep gloom and hopelessness at bay. I look forward to going to work and get great satisfaction from my work. I have continued to improve in this field, which gives so much more than it takes.
So enjoy the New Year! Take time to build a roaring fire, buy quirky gifts for your staff, get your spouses or significant others whatever they want, and enjoy your specialty as a dermatologist. You are in one of the best places in the world.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
X-Ray Goes Knee Deep
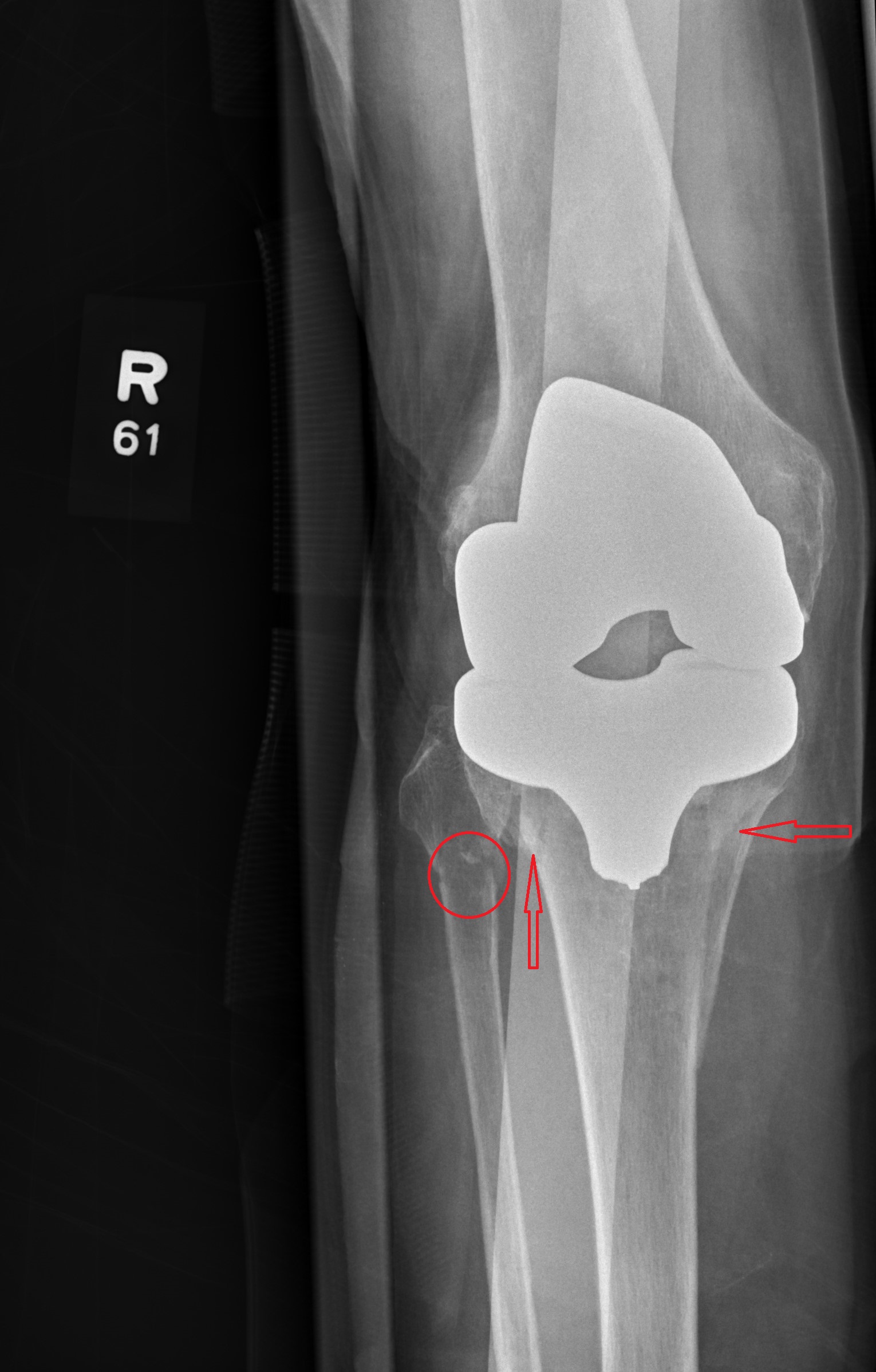
Answer
The radiograph shows evidence of status post arthroplasty. There are no abnormalities within the prosthesis. However, the patient has a fracture of the proximal tibia that extends into the tibial plateau, as well as a nondisplaced fracture of the proximal fibula.
The patient was admitted and orthopedics consulted.

Answer
The radiograph shows evidence of status post arthroplasty. There are no abnormalities within the prosthesis. However, the patient has a fracture of the proximal tibia that extends into the tibial plateau, as well as a nondisplaced fracture of the proximal fibula.
The patient was admitted and orthopedics consulted.

Answer
The radiograph shows evidence of status post arthroplasty. There are no abnormalities within the prosthesis. However, the patient has a fracture of the proximal tibia that extends into the tibial plateau, as well as a nondisplaced fracture of the proximal fibula.
The patient was admitted and orthopedics consulted.

Be proactive in fracture prevention
Several studies published over the last few years have shown declining rates of diagnosis and treatment of osteoporosis in older adults. In part, this may be due to declining ability to diagnose osteoporosis because declining reimbursement for dual x-ray absorptiometry has made it less available to patients and doctors. The study by Curtis et al. from the annual meeting of the American College of Rheumatology confirms prior findings and confirmation is important in driving the message home.
More research is needed to understand the reasons why patients and health care providers are not diagnosing osteoporosis, given that we can easily do so, and not treating osteoporosis or accepting recommended treatments for osteoporosis, given that we have many effective treatments that reduce the risk of fractures, many of them very inexpensive.
The vast majority of Medicare patients have major risk factors for falls, and falls are the most important risk factor for osteoporotic fractures. It is important to be proactive, to ask about drugs and diseases than increase falls, to educate patients about how to prevent falls, and to initiate treatments that strengthen bones so that they are less likely to break as a consequence of falling.
Dr. Shane is an endocrinologist, professor of medicine, and vice chair of medicine for clinical and epidemiological research at Columbia University in New York. She had no conflicts to disclose.
Several studies published over the last few years have shown declining rates of diagnosis and treatment of osteoporosis in older adults. In part, this may be due to declining ability to diagnose osteoporosis because declining reimbursement for dual x-ray absorptiometry has made it less available to patients and doctors. The study by Curtis et al. from the annual meeting of the American College of Rheumatology confirms prior findings and confirmation is important in driving the message home.
More research is needed to understand the reasons why patients and health care providers are not diagnosing osteoporosis, given that we can easily do so, and not treating osteoporosis or accepting recommended treatments for osteoporosis, given that we have many effective treatments that reduce the risk of fractures, many of them very inexpensive.
The vast majority of Medicare patients have major risk factors for falls, and falls are the most important risk factor for osteoporotic fractures. It is important to be proactive, to ask about drugs and diseases than increase falls, to educate patients about how to prevent falls, and to initiate treatments that strengthen bones so that they are less likely to break as a consequence of falling.
Dr. Shane is an endocrinologist, professor of medicine, and vice chair of medicine for clinical and epidemiological research at Columbia University in New York. She had no conflicts to disclose.
Several studies published over the last few years have shown declining rates of diagnosis and treatment of osteoporosis in older adults. In part, this may be due to declining ability to diagnose osteoporosis because declining reimbursement for dual x-ray absorptiometry has made it less available to patients and doctors. The study by Curtis et al. from the annual meeting of the American College of Rheumatology confirms prior findings and confirmation is important in driving the message home.
More research is needed to understand the reasons why patients and health care providers are not diagnosing osteoporosis, given that we can easily do so, and not treating osteoporosis or accepting recommended treatments for osteoporosis, given that we have many effective treatments that reduce the risk of fractures, many of them very inexpensive.
The vast majority of Medicare patients have major risk factors for falls, and falls are the most important risk factor for osteoporotic fractures. It is important to be proactive, to ask about drugs and diseases than increase falls, to educate patients about how to prevent falls, and to initiate treatments that strengthen bones so that they are less likely to break as a consequence of falling.
Dr. Shane is an endocrinologist, professor of medicine, and vice chair of medicine for clinical and epidemiological research at Columbia University in New York. She had no conflicts to disclose.
Osteoporotic fracture risk is undermanaged in older adults
Treatment of osteoporosis in older adults at increased risk for fractures declined from 2010 to 2014, based on a study of nearly 900,000 individuals.
Osteoporotic fractures are associated with morbidity and mortality, functional decline, increased nursing home admissions, and a significant economic burden, Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, said in a presentation at the annual meeting of the American College of Rheumatology.
“The number of Americans at risk for fractures on the basis of having osteoporosis is expected to increase by 32% based on the graying of the population,” he said. “Underdiagnosis and undertreatment may be contributing to the increased burden that we are starting to see,” he added.
To assess the impact of osteoporosis management on patients at increased risk for fractures, Dr. Curtis and his colleagues examined temporal trends over 5 years from 885,676 Medicare fee-for-service members with a closed fragility (or osteoporosis-related) fracture between Jan. 1, 2010, and Dec. 31, 2014. The average age of the patients was 81 years; 91% were white, and 94% were women.
The researchers used diagnosis and procedure codes to create an algorithm with a positive predictive value of more than 90%. Individuals with Paget’s disease or a malignancy other than nonmelanoma skin cancer at baseline were excluded.
Overall, use of dual x-ray absorptiometry (DXA) screening in this high-risk population decreased over the study period, with rates during 2010-2014 of 25%, 24%, 23%, 22%, and 16%, respectively. The presence of an osteoporosis diagnosis in the study population decreased over the same period, with rates of 7%, 6%, 6%, 5%, and 4%, respectively. In addition, the percentage of high-risk patients undergoing osteoporosis treatment at the time of fracture during 2010-2014 was 29%, 24%, 20%, 16%, and 11%, respectively.
Despite their history of fracture, more than half of individuals in each year’s database had a comorbidity or were taking a medication that increased fall risk. The most common comorbidity was impaired mobility (about 20% of each yearly cohort), followed by history of falls, history of stroke, impaired vision, muscle atrophy or weakness, and Parkinson’s disease. Approximately half of the patients in each year’s group were taking opioids, and approximately 20% were taking oral corticosteroids.
The findings were limited by several factors, including those common to studies involving administrative claims databases, such as a lack of complete medical and treatment history, lack of diagnostic validation for osteoporosis-related fractures, and lack of information on why use of DXA decreased over time, Dr. Curtis said. However, the results show the need to improve management of individuals at increased risk for falls and fractures to reduce not only the risk of morbidity and mortality, but also the economic impact.
Dr. Curtis disclosed serving as a consultant for Radius Health and Amgen, and the University of Alabama at Birmingham Medical Center received grants from these companies.
SOURCE: Curtis et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1872.
Treatment of osteoporosis in older adults at increased risk for fractures declined from 2010 to 2014, based on a study of nearly 900,000 individuals.
Osteoporotic fractures are associated with morbidity and mortality, functional decline, increased nursing home admissions, and a significant economic burden, Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, said in a presentation at the annual meeting of the American College of Rheumatology.
“The number of Americans at risk for fractures on the basis of having osteoporosis is expected to increase by 32% based on the graying of the population,” he said. “Underdiagnosis and undertreatment may be contributing to the increased burden that we are starting to see,” he added.
To assess the impact of osteoporosis management on patients at increased risk for fractures, Dr. Curtis and his colleagues examined temporal trends over 5 years from 885,676 Medicare fee-for-service members with a closed fragility (or osteoporosis-related) fracture between Jan. 1, 2010, and Dec. 31, 2014. The average age of the patients was 81 years; 91% were white, and 94% were women.
The researchers used diagnosis and procedure codes to create an algorithm with a positive predictive value of more than 90%. Individuals with Paget’s disease or a malignancy other than nonmelanoma skin cancer at baseline were excluded.
Overall, use of dual x-ray absorptiometry (DXA) screening in this high-risk population decreased over the study period, with rates during 2010-2014 of 25%, 24%, 23%, 22%, and 16%, respectively. The presence of an osteoporosis diagnosis in the study population decreased over the same period, with rates of 7%, 6%, 6%, 5%, and 4%, respectively. In addition, the percentage of high-risk patients undergoing osteoporosis treatment at the time of fracture during 2010-2014 was 29%, 24%, 20%, 16%, and 11%, respectively.
Despite their history of fracture, more than half of individuals in each year’s database had a comorbidity or were taking a medication that increased fall risk. The most common comorbidity was impaired mobility (about 20% of each yearly cohort), followed by history of falls, history of stroke, impaired vision, muscle atrophy or weakness, and Parkinson’s disease. Approximately half of the patients in each year’s group were taking opioids, and approximately 20% were taking oral corticosteroids.
The findings were limited by several factors, including those common to studies involving administrative claims databases, such as a lack of complete medical and treatment history, lack of diagnostic validation for osteoporosis-related fractures, and lack of information on why use of DXA decreased over time, Dr. Curtis said. However, the results show the need to improve management of individuals at increased risk for falls and fractures to reduce not only the risk of morbidity and mortality, but also the economic impact.
Dr. Curtis disclosed serving as a consultant for Radius Health and Amgen, and the University of Alabama at Birmingham Medical Center received grants from these companies.
SOURCE: Curtis et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1872.
Treatment of osteoporosis in older adults at increased risk for fractures declined from 2010 to 2014, based on a study of nearly 900,000 individuals.
Osteoporotic fractures are associated with morbidity and mortality, functional decline, increased nursing home admissions, and a significant economic burden, Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, said in a presentation at the annual meeting of the American College of Rheumatology.
“The number of Americans at risk for fractures on the basis of having osteoporosis is expected to increase by 32% based on the graying of the population,” he said. “Underdiagnosis and undertreatment may be contributing to the increased burden that we are starting to see,” he added.
To assess the impact of osteoporosis management on patients at increased risk for fractures, Dr. Curtis and his colleagues examined temporal trends over 5 years from 885,676 Medicare fee-for-service members with a closed fragility (or osteoporosis-related) fracture between Jan. 1, 2010, and Dec. 31, 2014. The average age of the patients was 81 years; 91% were white, and 94% were women.
The researchers used diagnosis and procedure codes to create an algorithm with a positive predictive value of more than 90%. Individuals with Paget’s disease or a malignancy other than nonmelanoma skin cancer at baseline were excluded.
Overall, use of dual x-ray absorptiometry (DXA) screening in this high-risk population decreased over the study period, with rates during 2010-2014 of 25%, 24%, 23%, 22%, and 16%, respectively. The presence of an osteoporosis diagnosis in the study population decreased over the same period, with rates of 7%, 6%, 6%, 5%, and 4%, respectively. In addition, the percentage of high-risk patients undergoing osteoporosis treatment at the time of fracture during 2010-2014 was 29%, 24%, 20%, 16%, and 11%, respectively.
Despite their history of fracture, more than half of individuals in each year’s database had a comorbidity or were taking a medication that increased fall risk. The most common comorbidity was impaired mobility (about 20% of each yearly cohort), followed by history of falls, history of stroke, impaired vision, muscle atrophy or weakness, and Parkinson’s disease. Approximately half of the patients in each year’s group were taking opioids, and approximately 20% were taking oral corticosteroids.
The findings were limited by several factors, including those common to studies involving administrative claims databases, such as a lack of complete medical and treatment history, lack of diagnostic validation for osteoporosis-related fractures, and lack of information on why use of DXA decreased over time, Dr. Curtis said. However, the results show the need to improve management of individuals at increased risk for falls and fractures to reduce not only the risk of morbidity and mortality, but also the economic impact.
Dr. Curtis disclosed serving as a consultant for Radius Health and Amgen, and the University of Alabama at Birmingham Medical Center received grants from these companies.
SOURCE: Curtis et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1872.
REPORTING FROM ACR 2019
Platinum-based therapy superior in study of upper GI tumors
A study that aimed to validate intratumoral ERCC1 levels as a predictive marker of platinum sensitivity in upper GI tumors failed to do so, but it did reach one firm conclusion: That platinum therapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) was superior in efficacy to a non–platinum-containing regimen of irinotecan and docetaxel (IT).
Approximately 200 untreated patients with unresectable advanced or metastatic HER2-negative adenocarcinoma of the esophagus, stomach or gastroesophageal junction were evaluated in the phase 2 study for mRNA expression of ERCC1 level and then randomized to either a platinum-containing or non–platinum-containing treatment arm with stratification for ERCC1 level (either low, with levels less than 1.7, or high, meaning 1.7 or more).
In retrospective studies of patients with gastric cancer, levels of gene expression of ERCC1 within the primary tumor have had a significant inverse relationship to response to platinum compounds and overall survival; low ERCC1 expression, in other words, has been associated with higher response rates and better survival. This inverse relationship of expression of the ERCC1 gene and platinum sensitivity has been demonstrated in colon cancer and other tumor types as well.
The problem is, approximately 86% of the patients in this phase 2, randomized study had ERCC1 values lower than 1.7 – many more than the investigators anticipated based on data from prior studies. (They expected a 50-50 distribution, roughly.) The predominance of low ERCC1 mRNA expression meant that evaluation of the ERCC1-high subgroup – and evaluation of interactive effects between ERCC1 expression and treatment type – was limited, said Syma Iqbal, MD, of the University of Southern California, Los Angeles, and coinvestigators.
“Unfortunately, this study did not validate or identify ERCC1 as a predictive marker of platinum sensitivity in upper GI tumors,” they wrote in the Journal of Clinical Oncology. However, “it did support the use of FOLFOX, a platinum-containing regimen, as a standard and superior frontline regimen, compared with the non–platinum-containing IT.”
Across all patients in the FOLFOX arm, the median progression-free survival (PFS) was significantly longer – 5.7 months versus 2.9 months in the IT arm (hazard ratio, 0.71; P = .02). The median overall survival was greater with FOLFOX as well, though this difference – 11.4 months versus 8.7 months – did not reach statistical significance. Similarly, in the ERCC1-low subgroup, the median PFS in patients receiving FOLFOX was statistically superior to IT – 5.9 months versus 2.8 months – and overall survival was better as well, though this latter difference was not statistically significant.
In the ERCC1-high subgroup, the median PFS was similar in the FOLFOX and IT arms (4.7 months vs. 5.3 months), the investigators noted. They plotted PFS within ERCC1 quartiles and found a consistent pattern of improved PFS in the FOLFOX versus IT arm, and “thus, little evidence of differential treatment effects on PFS across ERCC1 levels in this population.”
Regarding safety, the investigators noted, of 91 patients who completed protocol therapy in the FOLFOX arm and were analyzed for adverse events, 3 treatment-related deaths were reported and 9 additional patients experienced grade 4 adverse events. Of 98 patients assessed for adverse events in the IT arm, 3 treatment-related deaths were reported and 14 additional patients experienced grade 4 adverse events.
To be eligible for the study patients had to have a Zubrod performance status of 0-1 and been either treatment naive or have completed adjuvant therapy at least 180 days prior to enrollment.
The study was supported by the National Cancer Institute. Dr. Iqbal reported receiving honoraria from Celgene, Eisai, and F. Hoffmann–La Roche; serving a consulting or advisory role for F. Hoffmann–La Roche; serving on the speakers’ bureau for Celgene and Eisai; and receiving research funding from Bayer and Onyx.
SOURCE: Iqbal S et al. J Clin Oncol. 2019 Dec 9. doi: 10.1200/JCO.19.00925.
A study that aimed to validate intratumoral ERCC1 levels as a predictive marker of platinum sensitivity in upper GI tumors failed to do so, but it did reach one firm conclusion: That platinum therapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) was superior in efficacy to a non–platinum-containing regimen of irinotecan and docetaxel (IT).
Approximately 200 untreated patients with unresectable advanced or metastatic HER2-negative adenocarcinoma of the esophagus, stomach or gastroesophageal junction were evaluated in the phase 2 study for mRNA expression of ERCC1 level and then randomized to either a platinum-containing or non–platinum-containing treatment arm with stratification for ERCC1 level (either low, with levels less than 1.7, or high, meaning 1.7 or more).
In retrospective studies of patients with gastric cancer, levels of gene expression of ERCC1 within the primary tumor have had a significant inverse relationship to response to platinum compounds and overall survival; low ERCC1 expression, in other words, has been associated with higher response rates and better survival. This inverse relationship of expression of the ERCC1 gene and platinum sensitivity has been demonstrated in colon cancer and other tumor types as well.
The problem is, approximately 86% of the patients in this phase 2, randomized study had ERCC1 values lower than 1.7 – many more than the investigators anticipated based on data from prior studies. (They expected a 50-50 distribution, roughly.) The predominance of low ERCC1 mRNA expression meant that evaluation of the ERCC1-high subgroup – and evaluation of interactive effects between ERCC1 expression and treatment type – was limited, said Syma Iqbal, MD, of the University of Southern California, Los Angeles, and coinvestigators.
“Unfortunately, this study did not validate or identify ERCC1 as a predictive marker of platinum sensitivity in upper GI tumors,” they wrote in the Journal of Clinical Oncology. However, “it did support the use of FOLFOX, a platinum-containing regimen, as a standard and superior frontline regimen, compared with the non–platinum-containing IT.”
Across all patients in the FOLFOX arm, the median progression-free survival (PFS) was significantly longer – 5.7 months versus 2.9 months in the IT arm (hazard ratio, 0.71; P = .02). The median overall survival was greater with FOLFOX as well, though this difference – 11.4 months versus 8.7 months – did not reach statistical significance. Similarly, in the ERCC1-low subgroup, the median PFS in patients receiving FOLFOX was statistically superior to IT – 5.9 months versus 2.8 months – and overall survival was better as well, though this latter difference was not statistically significant.
In the ERCC1-high subgroup, the median PFS was similar in the FOLFOX and IT arms (4.7 months vs. 5.3 months), the investigators noted. They plotted PFS within ERCC1 quartiles and found a consistent pattern of improved PFS in the FOLFOX versus IT arm, and “thus, little evidence of differential treatment effects on PFS across ERCC1 levels in this population.”
Regarding safety, the investigators noted, of 91 patients who completed protocol therapy in the FOLFOX arm and were analyzed for adverse events, 3 treatment-related deaths were reported and 9 additional patients experienced grade 4 adverse events. Of 98 patients assessed for adverse events in the IT arm, 3 treatment-related deaths were reported and 14 additional patients experienced grade 4 adverse events.
To be eligible for the study patients had to have a Zubrod performance status of 0-1 and been either treatment naive or have completed adjuvant therapy at least 180 days prior to enrollment.
The study was supported by the National Cancer Institute. Dr. Iqbal reported receiving honoraria from Celgene, Eisai, and F. Hoffmann–La Roche; serving a consulting or advisory role for F. Hoffmann–La Roche; serving on the speakers’ bureau for Celgene and Eisai; and receiving research funding from Bayer and Onyx.
SOURCE: Iqbal S et al. J Clin Oncol. 2019 Dec 9. doi: 10.1200/JCO.19.00925.
A study that aimed to validate intratumoral ERCC1 levels as a predictive marker of platinum sensitivity in upper GI tumors failed to do so, but it did reach one firm conclusion: That platinum therapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) was superior in efficacy to a non–platinum-containing regimen of irinotecan and docetaxel (IT).
Approximately 200 untreated patients with unresectable advanced or metastatic HER2-negative adenocarcinoma of the esophagus, stomach or gastroesophageal junction were evaluated in the phase 2 study for mRNA expression of ERCC1 level and then randomized to either a platinum-containing or non–platinum-containing treatment arm with stratification for ERCC1 level (either low, with levels less than 1.7, or high, meaning 1.7 or more).
In retrospective studies of patients with gastric cancer, levels of gene expression of ERCC1 within the primary tumor have had a significant inverse relationship to response to platinum compounds and overall survival; low ERCC1 expression, in other words, has been associated with higher response rates and better survival. This inverse relationship of expression of the ERCC1 gene and platinum sensitivity has been demonstrated in colon cancer and other tumor types as well.
The problem is, approximately 86% of the patients in this phase 2, randomized study had ERCC1 values lower than 1.7 – many more than the investigators anticipated based on data from prior studies. (They expected a 50-50 distribution, roughly.) The predominance of low ERCC1 mRNA expression meant that evaluation of the ERCC1-high subgroup – and evaluation of interactive effects between ERCC1 expression and treatment type – was limited, said Syma Iqbal, MD, of the University of Southern California, Los Angeles, and coinvestigators.
“Unfortunately, this study did not validate or identify ERCC1 as a predictive marker of platinum sensitivity in upper GI tumors,” they wrote in the Journal of Clinical Oncology. However, “it did support the use of FOLFOX, a platinum-containing regimen, as a standard and superior frontline regimen, compared with the non–platinum-containing IT.”
Across all patients in the FOLFOX arm, the median progression-free survival (PFS) was significantly longer – 5.7 months versus 2.9 months in the IT arm (hazard ratio, 0.71; P = .02). The median overall survival was greater with FOLFOX as well, though this difference – 11.4 months versus 8.7 months – did not reach statistical significance. Similarly, in the ERCC1-low subgroup, the median PFS in patients receiving FOLFOX was statistically superior to IT – 5.9 months versus 2.8 months – and overall survival was better as well, though this latter difference was not statistically significant.
In the ERCC1-high subgroup, the median PFS was similar in the FOLFOX and IT arms (4.7 months vs. 5.3 months), the investigators noted. They plotted PFS within ERCC1 quartiles and found a consistent pattern of improved PFS in the FOLFOX versus IT arm, and “thus, little evidence of differential treatment effects on PFS across ERCC1 levels in this population.”
Regarding safety, the investigators noted, of 91 patients who completed protocol therapy in the FOLFOX arm and were analyzed for adverse events, 3 treatment-related deaths were reported and 9 additional patients experienced grade 4 adverse events. Of 98 patients assessed for adverse events in the IT arm, 3 treatment-related deaths were reported and 14 additional patients experienced grade 4 adverse events.
To be eligible for the study patients had to have a Zubrod performance status of 0-1 and been either treatment naive or have completed adjuvant therapy at least 180 days prior to enrollment.
The study was supported by the National Cancer Institute. Dr. Iqbal reported receiving honoraria from Celgene, Eisai, and F. Hoffmann–La Roche; serving a consulting or advisory role for F. Hoffmann–La Roche; serving on the speakers’ bureau for Celgene and Eisai; and receiving research funding from Bayer and Onyx.
SOURCE: Iqbal S et al. J Clin Oncol. 2019 Dec 9. doi: 10.1200/JCO.19.00925.
FROM THE JOURNAL OF CLINICAL ONCOLOGY