User login
The History of Pediatric Hospital Medicine in the United States, 1996-2019
In 1996, internists Robert Wachter, MD, and Lee Goldman, MD, MPH, coined the term “hospitalist” and predicted an “emerging role in the American health care system.”1 Pediatrics was not far behind: In 1999, Dr Wachter joined Paul Bellet, MD, in authoring an article describing the movement within pediatrics.2 An accompanying editorial, coauthored by a pediatric hospitalist and an office-based practitioner, attempted to answer which was “better” for a hospitalized child: A practitioner who knew the child and family or a hospitalist who might be more knowledgeable about the disease, its inpatient management, and how to get things done in the hospital?3 The authors could not answer which model was better for an individual child with an invested primary pediatrician, but concluded that hospitalists have the potential to improve care for all children in the hospital—the future promise of Pediatric Hospital Medicine (PHM). This article traces the growth of PHM from 1996 to the present, highlighting developments that fueled the hospital movement in general and PHM in particular (Table).
REGULATIONS FOSTER OPPORTUNITIES FOR HOSPITALISTS
In the 7 years after the article by Drs Wachter and Goldman, a series of regulations fostered the adoption of hospitalists in teaching hospitals. The first was the reissuance in 1997 of Intermediary Letter 372, which specifies the requirements for attending physicians to bill Medicare.4 The common practice of jotting “agree with above” and cosigning resident notes was no longer sufficient: Attendings had to document that they personally provided services to patients beyond those of residents. As a demonstration of enforcement, records at the Hospital of the University of Pennsylvania in Philadelphia were audited, and a bill for $30 million for overpayments and penalties was issued.4 Teaching hospitals took notice and instituted mechanisms to assure compliance with IL-372, not limited to patients insured by Medicare. The obvious effect on faculty was the requirement of considerably more time and involvement in direct patient care.
Later in the 1990s, the Accreditation Council for Graduate Medical Education (ACGME) introduced a new direction termed the Outcome Project, which led to two novel trainee competency domains: practice-based improvement and systems-based practice.5 The focus on quality improvement, patient safety, and systems was reinforced by two Institute of Medicine publications, To Err Is Human: Building a Safer Health System6 and Crossing the Quality Chasm: A New Health Care System for the 21st Century.7 Hospitalists had the opportunity to impact both patient care and the education of learners in two ways: Directly, by more actively participating in and closely supervising clinical care (per IL-372) and, indirectly, by improving hospital systems.
In 2003, the ACGME extended work hour restrictions implemented in New York State to the national level.8 The new requirements were intended to improve patient safety and increase trainee supervision, but also had the effect of reducing trainees’ clinical experience. While responses of teaching institutions varied, training program changes generated an increased role for hospitalists.9
These changes occurred on a backdrop of changing models of healthcare payment that provided incentive to shorten length of stay (LOS) and shift care from inpatient to ambulatory settings, which increased the acuity and complexity of hospitalized patients. The pressure to increase efficiency and decrease LOS affected faculty, residents, and practitioners in the community. Managing care of inpatients from a distance became more difficult; rounding more than once a day was often required and was disruptive and inefficient, particularly for community practitioners who might have only one or two patients in the hospital. Moreover, the hospital electronic medical record (EMR) became an additional barrier for many practitioners to continue to provide hospital-based care. Systems often differed from those used in their offices, and even when this was not the case, using and maintaining efficiency in the different components of the EMR was difficult. The conversion from paper to electronic documentation and ordering may have contributed to some practitioners relinquishing care of their patients to hospitalists.
PEDIATRIC HOSPITAL MEDICINE: THREE PARENT ORGANIZATIONS
The development of PHM was aided by support from three separate organizations, each with a different role: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA). SHM was founded the year after the article by Drs Wachter and Goldman as the “National Association of Inpatient Physicians.” The name was changed to Society of Hospital Medicine in 2003 to reflect the evolving field of hospital medicine. While the organization is largely comprised of internists, a pediatrician has been on its board since 1998, and a pediatrics committee (now Special Interest Group, SIG) has been in existence since 1999. (Appendix Tables 1a and 1b; Appendix Figures 1a and 1b). In 2005, an SHM task force was formed to define PHM-specific Core Competencies that could serve as a basis for curriculum building and a definition of the field. These inaugural PHM Core Competencies were endorsed by all three societies; published in 2010 in SHM’s flagship journal, the Journal of Hospital Medicine10; and were recently revised to reflect changes to the field in the past decade.11 SHM has provided valuable opportunities for hospitalists to develop knowledge and skills, particularly in matters related to healthcare operations and leadership, and it serves as a way to keep PHM connected with the larger hospital medicine community.
The AAP initiated its efforts to engage hospitalists in 1998 with the creation of a Provisional Section on Hospital Medicine (SOHM) that became a full section a year later. (Appendix Table 2; Appendix Figure 2) The SOHM listserv®, created in 2000, became a major vehicle for communication among hospitalists—including individuals who are not members of the SOHM—with more than 4,000 subscribers currently. Of the SOHM achievements noted in the Table, one deserves special mention: In 2006, SOHM formally recognized the large number of hospitalists in community hospitals and established a subsection with Karen Kingry Olson, MD, as inaugural leader. Many of the hospitalists in these sites provide care not only to children on inpatient units but also in areas such as the nursery, delivery room, and emergency department, functioning “like water on pavement—filling all the cracks in the hospital,” as Eric Biondi, MD, MS, puts it.12 It is a credit to the AAP and the PHM community that individuals from community hospitals have specifically been afforded leadership roles. SOHM membership has grown considerably from around 100 at inception to 2,700 in 2019. Participation in the AAP keeps PHM connected to the larger pediatrics community.
The APA established a Hospital and Inpatient Medicine SIG in 2001, the name of which was changed to Hospital Medicine SIG in 2004 (Appendix Table 3; Appendix Figure 3; Note: There had been an Inpatient General Pediatricians SIG in 1992, before the term hospitalist was coined, but it only met once.) In 2003, APA was the first national pediatrics organization to sponsor a PHM meeting. The meeting attracted 130 registrants and was considered successful enough to warrant another meeting in 2005, this time with SHM and AAP joining as co
CONSOLIDATION OF PEDIATRIC HOSPITAL MEDICINE
In 2009, PHM leaders within SHM, APA, and AAP held a pivotal strategic planning “roundtable” to discuss the future of the field.14 A vision statement was developed, serving as a guide to the tasks needed to achieve the vision: “Pediatric hospitalists will transform the delivery of hospital care for children.” Five areas were considered: clinical, quality, research, workforce, and structure. Clinical practice was defined as including both “direct patient care and leadership of the inpatient service.” It was recognized that standardizing, disseminating, and increasing knowledge to improve clinical care was important, but so, too, was taking on leadership roles to improve systems and extend into areas such as sedation. Quality improvement was identified as the measure by which the value of PHM would be assessed. To further efforts in this area, a PHM Quality Improvement (QI) Collaborative work group was created. Research was clearly a necessary component to establish and advance the field. The Children’s Hospital Association had launched the Pediatric Health Information System (PHIS) database in 1993, and PHIS began to flourish as a research database when Samir Shah, MD, MSCE, and Matt Hall, PhD, headed the Research Groups in 2007. Discussions to form an independent research network began in 2001, and, in 2002, the Pediatric Research in Inpatient Settings network (PRIS) was launched, led by Christopher Landrigan, MD, MPH.15 The APA provided organization support in 2006, but a redesign was considered necessary to further move the research initiative forward.15 A Research Leadership Task Force was created, resulting in a new PRIS Network Executive Council, chaired by Rajendu Srivastava, MD, MPH, until 2016, when Karen Wilson, MD, MPH, became chair. Clinical and workforce issues focused on the need to supplement residency training with added skills and knowledge to practice as a pediatric hospitalist. An Education Task Force was created, charged with developing “an educational plan supporting the PHM Core Competencies and addressing hospitalist training needs, including the role as formal educators.” The task force was headed by Mary Ottolini, MD, MPH, MEd, who was aided by Jennifer Maniscalco, MD, MPH, MAcM. Regarding structure of PHM, the decision was made not to develop an independent society but to continue to function within and benefit from the resources of SHM, AAP, and APA, with a Joint Council on Pediatric Hospital Medicine (JCPHM). Established in 2011, the JCPHM included representatives of the AAP, APA, SHM, PRIS, VIP, community hospitals, and the Education Task Force. Erin Stucky Fisher, MD, MHM, served as the first chair. The JCPHM was replaced in the fall of 2016 by a Consortium on PHM, which consists of the chairs and chair elects of the AAP SOHM, the APA Hospital Medicine SIG, and the SHM pediatrics committee. The leadership rotates annually among the three organizations.
PATH TO SUBSPECIALTY STATUS
The American Board of Pediatrics (ABP) recognized the growing field of PHM and, through its foundation, commissioned a series of studies, the first of which was published in 2006 entitled “Hospitalists in children’s hospitals: What we know now and what we need to know.”16 It was not clear whether the PHM community would pursue subspecialty certification. The leaders of the 2009 “roundtable” meeting commissioned a Strategic Planning Committee (STP) led by Christopher Maloney, MD, PhD, and Suzanne Swanson Mendez, MD, to evaluate the best course of action: traditional ABP subspecialty certification, hospital medicine residency track (with or without additional fellowship), Recognition of Focused Practice (as implemented by the American Board of Internal Medicine and American Board of Family Medicine), mandatory mentorship program, or status quo with option for specialized training. There was considerable discussion of the alternatives in the PHM community. In 2012, the STP shared the results of Strengths-Weaknesses-Opportunities-Threats analyses—but did not issue a recommendation.17 The following year, a National PHM Leaders Conference was held to consider the various options. Participants concluded that the best path forward was to pursue subspecialty certification with a requirement for 2 years of fellowship (after a time-limited period for practice pathway eligibility). Two years of fellowship was a departure from the ABP’s standard 3 years, but seemed acceptable based on the expectation that the research component would be integrated with clinical activities (eg, QI), rather than separate bench research. The ABP Initiative on Subspecialty Clinical Training and Certification had recommended flexibility in the duration of fellowships,18 and PHM became the first discipline to take advantage of such flexibility. Following an 18-month review of multiple considerations, the ABP concluded that “children will be better served by establishing the discipline as a new subspecialty.”19
The decision to pursue subspecialty certification was not unanimously embraced by the PHM community, with particular concerns expressed regarding the impact on Med-Peds hospitalists and the future in community hospitals. These were considered by the individuals writing the formal proposal to the ABP, but have not been resolved. Moreover, criteria for eligibility for the certifying examination under the Practice Pathway (“grandparenting”) evoked controversy,20 addressed by the ABP. 21 The first subspecialty certifying examination was ultimately administered to ~1,500 pediatric hospitalists in 2019.
THE ONGOING EVOLUTION OF PEDIATRIC HOSPITAL MEDICINE
It is clear that PHM has established itself as a field, with networks for research and quality improvement, more than 50 fellowship programs, divisions in prestigious departments of pediatrics and children’s hospitals, devoted journals and textbooks, and a well-attended annual meeting. PHM has set standards for the core competencies in PHM,11, 12 for pediatric hospitalist programs,22, 23 for coordinating the hospital care of children,24, 25 for the curricular framework of fellowships,26 and for the Entrustable Professional Activities expected of a hospitalist.27 The vision for the future is that continued efforts in research, quality and systems improvement, and clinical care will, in fact, result in significant benefits for all hospitalized children. Such was the promise of PHM in the 1990s and remains so in 2019.
Acknowledgments
For prompting the project: Rachel Marek. For additions, corrections, and confirmations: David Alexander, Niccole Alexander, Paul Bellet, David Bertoch, Douglas Carlson, Laura Degnon, Kimberly Durham, Barrett Fromme, Sandy Gage, Matthew Garber, Karen Jerardi, Christopher Landrigan, Gail McGuinness, Jennifer Maniscalco, Sandy Melzer, Vineeta Mittal, Karen Kingry Olson, Mary Ottolini, Jack Percelay, Kris Rehm, Michael Ruhlen, Samir Shah, Suzanne Woods, and David Zipes.
Disclosures
The authors have nothing to disclose.
1. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-517. https://doi.org/10.1056/NEJM199608153350713.
2. Bellet PS, Wachter RM. The hospitalist movement and its implications for the care of hospitalized children. Pediatrics. 1999;103(2):473-477. https://doi.org/10.1542/peds.103.2.473.
3. Roberts KB, Rappo P. A hospitalist movement? Where to? Pediatrics. 1999;103(2):497. https://doi.org/10.1542/peds.103.2.497.
4. Cohen JJ, Dickler RM. Auditing the Medicare-billing practices of teaching physicians—Welcome accountability, unfair approach. N Engl J Med. 1997;336(18):1317-1320. https://doi.org/10.1056/NEJM199705013361811.
5. Swing SR. The ACGME outcome project: Retrospective and prospective. Med Teach. 2007;29(7):648-654. https://doi.org/10.1080/01421590701392903.
6. Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
7. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
8. Accreditation Council for Graduate Medical Education. History of Duty Hours. Available at https://www.acgme.org/What-We-Do/Accreditation/Clinical-Experience-and-Education-formerly-Duty-Hours/History-of-Duty-Hours. Accessed January 16, 2020.
9. Oshimura JM, Sperring J, Bauer BD, Carroll AE, Rauch DA. Changes in inpatient staffing following implementation of new residency work hours. J Hosp Med. 2014;9(10):640-645. https://doi.org/10.1002/jhm.2242.
10. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies supplement: A framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(Suppl 2):i-xv, 1-114. https://doi.org/10.1002/jhm.776.
11. Gage S, Maniscalco J, Fisher E. The Pediatric Hospital Medicine Core Competencies [published online first ahead of print April XX, 2020].
12. Blum K. Raising the profile of hospital medicine. Hopkins Children’s. 2018 Spring, p 32. https://www.hopkinsmedicine.org/johns-hopkins-childrens-center/_documents/_publications/hopkins_childrens_magazine_spring2018.pdf. Accessed January 16, 2020.
13. Roberts K, Stein R, Cheng T. The Academic Pediatric Association: The first fifty years. Acad Pediatr. 2011;11:173-180. https://doi.org/10.1016/j.acap.2011.02.001.
14. Rauch DA, Lye PS, Carlson D, et al. Pediatric Hospital Medicine: A strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
15. Srivastava R, Landrigan CP. Development of the Pediatric Research in Inpatient Settings (PRIS) Network: Lessons learned. J Hosp Med. 2012;7(8)661-664. https://doi.org/10.1002/jhm.1972.
16. Freed GL, Uren RL. Hospitalists in children’s hospitals: What we know now and what we need to know. J Pediatr. 2006;148(3):296-299. https://doi.org/10.1016/j.jpeds.2005.12.048.
17. Maloney CG, Mendez SS, Quinonez RA, et al. The Strategic Planning Committee report: The first step in a journey to recognize pediatric hospital medicine as a distinct discipline. Hosp Pediatr. 2012;2(4):187-190. https://doi.org/10.1542/hpeds.2012-0048.
18. Stevenson DK, McGuiness GA, Bancroft JD, et al. The Initiative on Subspecialty Clinical Training and Certification (SCTC): Background and recommendations. Pediatrics. 2014;133(Suppl 2):S53-S57. https://doi.org/10.1542/peds.2013-3861C.
19. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
20. Chang WW, Hopkins AM, Rehm KP, Gage SL, Shen M. Society of Hospital Medicine position on the American Board of Pediatrics response to the hospital medicine petition. J Hosp Med. 2019;14(10):589-590. https://doi.org/10.12788/jhm.3326.
21. Nichols DG, Woods SK. The American Board of Pediatrics response to the pediatric hospital medicine petition. J Hosp Med. 2019:14:E1-E3. https://doi.org/10.12788/jhm.3322.
22. American Academy of Pediatrics Section on Hospital Medicine. Guiding principles for pediatric hospitalist programs. Pediatrics. 2005;115:1101-1102.
23. American Academy of Pediatrics Section on Hospital Medicine. Guiding principles for pediatric hospitalist programs. Pediatrics. 2013;132(4):782-786. https://doi.org/10.1542/peds.2013-2269.
24. Lye PS, American Academy of Pediatrics Committee on Hospital Care, Section on Hospital Medicine. Clinical report—physicians’ roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829-832.
25. Rauch DA, American Academy of Pediatrics Committee on Hospital Care, Section on Hospital Medicine. Physician’s role in coordinating care of hospitalized children. Pediatrics. 2018;142(2):e20181503. https://doi.org/10.1542/peds.2018-1503.
26. Jerardi KE, Fisher ER, Rassbach C, et al; on behalf of the Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2019;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
27. American Board of Pediatrics. Pediatric hospital medicine entrustable professional activities. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed August 31, 2019.
28. Perkin RM, Swift JD, Newton DA (Eds). Pediatric Hospital Medicine: Textbook of Inpatient Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
29. Frank F, Shah SS, Catallozzi M, Zaoutis L (Eds). The Philadelphia Guide: Inpatient Pediatrics. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
30. Zaoutis L, Chiang V (Eds). Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Mosby; 2007.
31. Rauch DA, Gershel J (Eds). Caring for the Hospitalized Child: A Handbook of Inpatient Pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; 2013.
32. Rauch DA. Tribute to Jennifer Daru, MD. Hosp Pediatr. 2011;4(4):267-268.
In 1996, internists Robert Wachter, MD, and Lee Goldman, MD, MPH, coined the term “hospitalist” and predicted an “emerging role in the American health care system.”1 Pediatrics was not far behind: In 1999, Dr Wachter joined Paul Bellet, MD, in authoring an article describing the movement within pediatrics.2 An accompanying editorial, coauthored by a pediatric hospitalist and an office-based practitioner, attempted to answer which was “better” for a hospitalized child: A practitioner who knew the child and family or a hospitalist who might be more knowledgeable about the disease, its inpatient management, and how to get things done in the hospital?3 The authors could not answer which model was better for an individual child with an invested primary pediatrician, but concluded that hospitalists have the potential to improve care for all children in the hospital—the future promise of Pediatric Hospital Medicine (PHM). This article traces the growth of PHM from 1996 to the present, highlighting developments that fueled the hospital movement in general and PHM in particular (Table).
REGULATIONS FOSTER OPPORTUNITIES FOR HOSPITALISTS
In the 7 years after the article by Drs Wachter and Goldman, a series of regulations fostered the adoption of hospitalists in teaching hospitals. The first was the reissuance in 1997 of Intermediary Letter 372, which specifies the requirements for attending physicians to bill Medicare.4 The common practice of jotting “agree with above” and cosigning resident notes was no longer sufficient: Attendings had to document that they personally provided services to patients beyond those of residents. As a demonstration of enforcement, records at the Hospital of the University of Pennsylvania in Philadelphia were audited, and a bill for $30 million for overpayments and penalties was issued.4 Teaching hospitals took notice and instituted mechanisms to assure compliance with IL-372, not limited to patients insured by Medicare. The obvious effect on faculty was the requirement of considerably more time and involvement in direct patient care.
Later in the 1990s, the Accreditation Council for Graduate Medical Education (ACGME) introduced a new direction termed the Outcome Project, which led to two novel trainee competency domains: practice-based improvement and systems-based practice.5 The focus on quality improvement, patient safety, and systems was reinforced by two Institute of Medicine publications, To Err Is Human: Building a Safer Health System6 and Crossing the Quality Chasm: A New Health Care System for the 21st Century.7 Hospitalists had the opportunity to impact both patient care and the education of learners in two ways: Directly, by more actively participating in and closely supervising clinical care (per IL-372) and, indirectly, by improving hospital systems.
In 2003, the ACGME extended work hour restrictions implemented in New York State to the national level.8 The new requirements were intended to improve patient safety and increase trainee supervision, but also had the effect of reducing trainees’ clinical experience. While responses of teaching institutions varied, training program changes generated an increased role for hospitalists.9
These changes occurred on a backdrop of changing models of healthcare payment that provided incentive to shorten length of stay (LOS) and shift care from inpatient to ambulatory settings, which increased the acuity and complexity of hospitalized patients. The pressure to increase efficiency and decrease LOS affected faculty, residents, and practitioners in the community. Managing care of inpatients from a distance became more difficult; rounding more than once a day was often required and was disruptive and inefficient, particularly for community practitioners who might have only one or two patients in the hospital. Moreover, the hospital electronic medical record (EMR) became an additional barrier for many practitioners to continue to provide hospital-based care. Systems often differed from those used in their offices, and even when this was not the case, using and maintaining efficiency in the different components of the EMR was difficult. The conversion from paper to electronic documentation and ordering may have contributed to some practitioners relinquishing care of their patients to hospitalists.
PEDIATRIC HOSPITAL MEDICINE: THREE PARENT ORGANIZATIONS
The development of PHM was aided by support from three separate organizations, each with a different role: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA). SHM was founded the year after the article by Drs Wachter and Goldman as the “National Association of Inpatient Physicians.” The name was changed to Society of Hospital Medicine in 2003 to reflect the evolving field of hospital medicine. While the organization is largely comprised of internists, a pediatrician has been on its board since 1998, and a pediatrics committee (now Special Interest Group, SIG) has been in existence since 1999. (Appendix Tables 1a and 1b; Appendix Figures 1a and 1b). In 2005, an SHM task force was formed to define PHM-specific Core Competencies that could serve as a basis for curriculum building and a definition of the field. These inaugural PHM Core Competencies were endorsed by all three societies; published in 2010 in SHM’s flagship journal, the Journal of Hospital Medicine10; and were recently revised to reflect changes to the field in the past decade.11 SHM has provided valuable opportunities for hospitalists to develop knowledge and skills, particularly in matters related to healthcare operations and leadership, and it serves as a way to keep PHM connected with the larger hospital medicine community.
The AAP initiated its efforts to engage hospitalists in 1998 with the creation of a Provisional Section on Hospital Medicine (SOHM) that became a full section a year later. (Appendix Table 2; Appendix Figure 2) The SOHM listserv®, created in 2000, became a major vehicle for communication among hospitalists—including individuals who are not members of the SOHM—with more than 4,000 subscribers currently. Of the SOHM achievements noted in the Table, one deserves special mention: In 2006, SOHM formally recognized the large number of hospitalists in community hospitals and established a subsection with Karen Kingry Olson, MD, as inaugural leader. Many of the hospitalists in these sites provide care not only to children on inpatient units but also in areas such as the nursery, delivery room, and emergency department, functioning “like water on pavement—filling all the cracks in the hospital,” as Eric Biondi, MD, MS, puts it.12 It is a credit to the AAP and the PHM community that individuals from community hospitals have specifically been afforded leadership roles. SOHM membership has grown considerably from around 100 at inception to 2,700 in 2019. Participation in the AAP keeps PHM connected to the larger pediatrics community.
The APA established a Hospital and Inpatient Medicine SIG in 2001, the name of which was changed to Hospital Medicine SIG in 2004 (Appendix Table 3; Appendix Figure 3; Note: There had been an Inpatient General Pediatricians SIG in 1992, before the term hospitalist was coined, but it only met once.) In 2003, APA was the first national pediatrics organization to sponsor a PHM meeting. The meeting attracted 130 registrants and was considered successful enough to warrant another meeting in 2005, this time with SHM and AAP joining as co
CONSOLIDATION OF PEDIATRIC HOSPITAL MEDICINE
In 2009, PHM leaders within SHM, APA, and AAP held a pivotal strategic planning “roundtable” to discuss the future of the field.14 A vision statement was developed, serving as a guide to the tasks needed to achieve the vision: “Pediatric hospitalists will transform the delivery of hospital care for children.” Five areas were considered: clinical, quality, research, workforce, and structure. Clinical practice was defined as including both “direct patient care and leadership of the inpatient service.” It was recognized that standardizing, disseminating, and increasing knowledge to improve clinical care was important, but so, too, was taking on leadership roles to improve systems and extend into areas such as sedation. Quality improvement was identified as the measure by which the value of PHM would be assessed. To further efforts in this area, a PHM Quality Improvement (QI) Collaborative work group was created. Research was clearly a necessary component to establish and advance the field. The Children’s Hospital Association had launched the Pediatric Health Information System (PHIS) database in 1993, and PHIS began to flourish as a research database when Samir Shah, MD, MSCE, and Matt Hall, PhD, headed the Research Groups in 2007. Discussions to form an independent research network began in 2001, and, in 2002, the Pediatric Research in Inpatient Settings network (PRIS) was launched, led by Christopher Landrigan, MD, MPH.15 The APA provided organization support in 2006, but a redesign was considered necessary to further move the research initiative forward.15 A Research Leadership Task Force was created, resulting in a new PRIS Network Executive Council, chaired by Rajendu Srivastava, MD, MPH, until 2016, when Karen Wilson, MD, MPH, became chair. Clinical and workforce issues focused on the need to supplement residency training with added skills and knowledge to practice as a pediatric hospitalist. An Education Task Force was created, charged with developing “an educational plan supporting the PHM Core Competencies and addressing hospitalist training needs, including the role as formal educators.” The task force was headed by Mary Ottolini, MD, MPH, MEd, who was aided by Jennifer Maniscalco, MD, MPH, MAcM. Regarding structure of PHM, the decision was made not to develop an independent society but to continue to function within and benefit from the resources of SHM, AAP, and APA, with a Joint Council on Pediatric Hospital Medicine (JCPHM). Established in 2011, the JCPHM included representatives of the AAP, APA, SHM, PRIS, VIP, community hospitals, and the Education Task Force. Erin Stucky Fisher, MD, MHM, served as the first chair. The JCPHM was replaced in the fall of 2016 by a Consortium on PHM, which consists of the chairs and chair elects of the AAP SOHM, the APA Hospital Medicine SIG, and the SHM pediatrics committee. The leadership rotates annually among the three organizations.
PATH TO SUBSPECIALTY STATUS
The American Board of Pediatrics (ABP) recognized the growing field of PHM and, through its foundation, commissioned a series of studies, the first of which was published in 2006 entitled “Hospitalists in children’s hospitals: What we know now and what we need to know.”16 It was not clear whether the PHM community would pursue subspecialty certification. The leaders of the 2009 “roundtable” meeting commissioned a Strategic Planning Committee (STP) led by Christopher Maloney, MD, PhD, and Suzanne Swanson Mendez, MD, to evaluate the best course of action: traditional ABP subspecialty certification, hospital medicine residency track (with or without additional fellowship), Recognition of Focused Practice (as implemented by the American Board of Internal Medicine and American Board of Family Medicine), mandatory mentorship program, or status quo with option for specialized training. There was considerable discussion of the alternatives in the PHM community. In 2012, the STP shared the results of Strengths-Weaknesses-Opportunities-Threats analyses—but did not issue a recommendation.17 The following year, a National PHM Leaders Conference was held to consider the various options. Participants concluded that the best path forward was to pursue subspecialty certification with a requirement for 2 years of fellowship (after a time-limited period for practice pathway eligibility). Two years of fellowship was a departure from the ABP’s standard 3 years, but seemed acceptable based on the expectation that the research component would be integrated with clinical activities (eg, QI), rather than separate bench research. The ABP Initiative on Subspecialty Clinical Training and Certification had recommended flexibility in the duration of fellowships,18 and PHM became the first discipline to take advantage of such flexibility. Following an 18-month review of multiple considerations, the ABP concluded that “children will be better served by establishing the discipline as a new subspecialty.”19
The decision to pursue subspecialty certification was not unanimously embraced by the PHM community, with particular concerns expressed regarding the impact on Med-Peds hospitalists and the future in community hospitals. These were considered by the individuals writing the formal proposal to the ABP, but have not been resolved. Moreover, criteria for eligibility for the certifying examination under the Practice Pathway (“grandparenting”) evoked controversy,20 addressed by the ABP. 21 The first subspecialty certifying examination was ultimately administered to ~1,500 pediatric hospitalists in 2019.
THE ONGOING EVOLUTION OF PEDIATRIC HOSPITAL MEDICINE
It is clear that PHM has established itself as a field, with networks for research and quality improvement, more than 50 fellowship programs, divisions in prestigious departments of pediatrics and children’s hospitals, devoted journals and textbooks, and a well-attended annual meeting. PHM has set standards for the core competencies in PHM,11, 12 for pediatric hospitalist programs,22, 23 for coordinating the hospital care of children,24, 25 for the curricular framework of fellowships,26 and for the Entrustable Professional Activities expected of a hospitalist.27 The vision for the future is that continued efforts in research, quality and systems improvement, and clinical care will, in fact, result in significant benefits for all hospitalized children. Such was the promise of PHM in the 1990s and remains so in 2019.
Acknowledgments
For prompting the project: Rachel Marek. For additions, corrections, and confirmations: David Alexander, Niccole Alexander, Paul Bellet, David Bertoch, Douglas Carlson, Laura Degnon, Kimberly Durham, Barrett Fromme, Sandy Gage, Matthew Garber, Karen Jerardi, Christopher Landrigan, Gail McGuinness, Jennifer Maniscalco, Sandy Melzer, Vineeta Mittal, Karen Kingry Olson, Mary Ottolini, Jack Percelay, Kris Rehm, Michael Ruhlen, Samir Shah, Suzanne Woods, and David Zipes.
Disclosures
The authors have nothing to disclose.
In 1996, internists Robert Wachter, MD, and Lee Goldman, MD, MPH, coined the term “hospitalist” and predicted an “emerging role in the American health care system.”1 Pediatrics was not far behind: In 1999, Dr Wachter joined Paul Bellet, MD, in authoring an article describing the movement within pediatrics.2 An accompanying editorial, coauthored by a pediatric hospitalist and an office-based practitioner, attempted to answer which was “better” for a hospitalized child: A practitioner who knew the child and family or a hospitalist who might be more knowledgeable about the disease, its inpatient management, and how to get things done in the hospital?3 The authors could not answer which model was better for an individual child with an invested primary pediatrician, but concluded that hospitalists have the potential to improve care for all children in the hospital—the future promise of Pediatric Hospital Medicine (PHM). This article traces the growth of PHM from 1996 to the present, highlighting developments that fueled the hospital movement in general and PHM in particular (Table).
REGULATIONS FOSTER OPPORTUNITIES FOR HOSPITALISTS
In the 7 years after the article by Drs Wachter and Goldman, a series of regulations fostered the adoption of hospitalists in teaching hospitals. The first was the reissuance in 1997 of Intermediary Letter 372, which specifies the requirements for attending physicians to bill Medicare.4 The common practice of jotting “agree with above” and cosigning resident notes was no longer sufficient: Attendings had to document that they personally provided services to patients beyond those of residents. As a demonstration of enforcement, records at the Hospital of the University of Pennsylvania in Philadelphia were audited, and a bill for $30 million for overpayments and penalties was issued.4 Teaching hospitals took notice and instituted mechanisms to assure compliance with IL-372, not limited to patients insured by Medicare. The obvious effect on faculty was the requirement of considerably more time and involvement in direct patient care.
Later in the 1990s, the Accreditation Council for Graduate Medical Education (ACGME) introduced a new direction termed the Outcome Project, which led to two novel trainee competency domains: practice-based improvement and systems-based practice.5 The focus on quality improvement, patient safety, and systems was reinforced by two Institute of Medicine publications, To Err Is Human: Building a Safer Health System6 and Crossing the Quality Chasm: A New Health Care System for the 21st Century.7 Hospitalists had the opportunity to impact both patient care and the education of learners in two ways: Directly, by more actively participating in and closely supervising clinical care (per IL-372) and, indirectly, by improving hospital systems.
In 2003, the ACGME extended work hour restrictions implemented in New York State to the national level.8 The new requirements were intended to improve patient safety and increase trainee supervision, but also had the effect of reducing trainees’ clinical experience. While responses of teaching institutions varied, training program changes generated an increased role for hospitalists.9
These changes occurred on a backdrop of changing models of healthcare payment that provided incentive to shorten length of stay (LOS) and shift care from inpatient to ambulatory settings, which increased the acuity and complexity of hospitalized patients. The pressure to increase efficiency and decrease LOS affected faculty, residents, and practitioners in the community. Managing care of inpatients from a distance became more difficult; rounding more than once a day was often required and was disruptive and inefficient, particularly for community practitioners who might have only one or two patients in the hospital. Moreover, the hospital electronic medical record (EMR) became an additional barrier for many practitioners to continue to provide hospital-based care. Systems often differed from those used in their offices, and even when this was not the case, using and maintaining efficiency in the different components of the EMR was difficult. The conversion from paper to electronic documentation and ordering may have contributed to some practitioners relinquishing care of their patients to hospitalists.
PEDIATRIC HOSPITAL MEDICINE: THREE PARENT ORGANIZATIONS
The development of PHM was aided by support from three separate organizations, each with a different role: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA). SHM was founded the year after the article by Drs Wachter and Goldman as the “National Association of Inpatient Physicians.” The name was changed to Society of Hospital Medicine in 2003 to reflect the evolving field of hospital medicine. While the organization is largely comprised of internists, a pediatrician has been on its board since 1998, and a pediatrics committee (now Special Interest Group, SIG) has been in existence since 1999. (Appendix Tables 1a and 1b; Appendix Figures 1a and 1b). In 2005, an SHM task force was formed to define PHM-specific Core Competencies that could serve as a basis for curriculum building and a definition of the field. These inaugural PHM Core Competencies were endorsed by all three societies; published in 2010 in SHM’s flagship journal, the Journal of Hospital Medicine10; and were recently revised to reflect changes to the field in the past decade.11 SHM has provided valuable opportunities for hospitalists to develop knowledge and skills, particularly in matters related to healthcare operations and leadership, and it serves as a way to keep PHM connected with the larger hospital medicine community.
The AAP initiated its efforts to engage hospitalists in 1998 with the creation of a Provisional Section on Hospital Medicine (SOHM) that became a full section a year later. (Appendix Table 2; Appendix Figure 2) The SOHM listserv®, created in 2000, became a major vehicle for communication among hospitalists—including individuals who are not members of the SOHM—with more than 4,000 subscribers currently. Of the SOHM achievements noted in the Table, one deserves special mention: In 2006, SOHM formally recognized the large number of hospitalists in community hospitals and established a subsection with Karen Kingry Olson, MD, as inaugural leader. Many of the hospitalists in these sites provide care not only to children on inpatient units but also in areas such as the nursery, delivery room, and emergency department, functioning “like water on pavement—filling all the cracks in the hospital,” as Eric Biondi, MD, MS, puts it.12 It is a credit to the AAP and the PHM community that individuals from community hospitals have specifically been afforded leadership roles. SOHM membership has grown considerably from around 100 at inception to 2,700 in 2019. Participation in the AAP keeps PHM connected to the larger pediatrics community.
The APA established a Hospital and Inpatient Medicine SIG in 2001, the name of which was changed to Hospital Medicine SIG in 2004 (Appendix Table 3; Appendix Figure 3; Note: There had been an Inpatient General Pediatricians SIG in 1992, before the term hospitalist was coined, but it only met once.) In 2003, APA was the first national pediatrics organization to sponsor a PHM meeting. The meeting attracted 130 registrants and was considered successful enough to warrant another meeting in 2005, this time with SHM and AAP joining as co
CONSOLIDATION OF PEDIATRIC HOSPITAL MEDICINE
In 2009, PHM leaders within SHM, APA, and AAP held a pivotal strategic planning “roundtable” to discuss the future of the field.14 A vision statement was developed, serving as a guide to the tasks needed to achieve the vision: “Pediatric hospitalists will transform the delivery of hospital care for children.” Five areas were considered: clinical, quality, research, workforce, and structure. Clinical practice was defined as including both “direct patient care and leadership of the inpatient service.” It was recognized that standardizing, disseminating, and increasing knowledge to improve clinical care was important, but so, too, was taking on leadership roles to improve systems and extend into areas such as sedation. Quality improvement was identified as the measure by which the value of PHM would be assessed. To further efforts in this area, a PHM Quality Improvement (QI) Collaborative work group was created. Research was clearly a necessary component to establish and advance the field. The Children’s Hospital Association had launched the Pediatric Health Information System (PHIS) database in 1993, and PHIS began to flourish as a research database when Samir Shah, MD, MSCE, and Matt Hall, PhD, headed the Research Groups in 2007. Discussions to form an independent research network began in 2001, and, in 2002, the Pediatric Research in Inpatient Settings network (PRIS) was launched, led by Christopher Landrigan, MD, MPH.15 The APA provided organization support in 2006, but a redesign was considered necessary to further move the research initiative forward.15 A Research Leadership Task Force was created, resulting in a new PRIS Network Executive Council, chaired by Rajendu Srivastava, MD, MPH, until 2016, when Karen Wilson, MD, MPH, became chair. Clinical and workforce issues focused on the need to supplement residency training with added skills and knowledge to practice as a pediatric hospitalist. An Education Task Force was created, charged with developing “an educational plan supporting the PHM Core Competencies and addressing hospitalist training needs, including the role as formal educators.” The task force was headed by Mary Ottolini, MD, MPH, MEd, who was aided by Jennifer Maniscalco, MD, MPH, MAcM. Regarding structure of PHM, the decision was made not to develop an independent society but to continue to function within and benefit from the resources of SHM, AAP, and APA, with a Joint Council on Pediatric Hospital Medicine (JCPHM). Established in 2011, the JCPHM included representatives of the AAP, APA, SHM, PRIS, VIP, community hospitals, and the Education Task Force. Erin Stucky Fisher, MD, MHM, served as the first chair. The JCPHM was replaced in the fall of 2016 by a Consortium on PHM, which consists of the chairs and chair elects of the AAP SOHM, the APA Hospital Medicine SIG, and the SHM pediatrics committee. The leadership rotates annually among the three organizations.
PATH TO SUBSPECIALTY STATUS
The American Board of Pediatrics (ABP) recognized the growing field of PHM and, through its foundation, commissioned a series of studies, the first of which was published in 2006 entitled “Hospitalists in children’s hospitals: What we know now and what we need to know.”16 It was not clear whether the PHM community would pursue subspecialty certification. The leaders of the 2009 “roundtable” meeting commissioned a Strategic Planning Committee (STP) led by Christopher Maloney, MD, PhD, and Suzanne Swanson Mendez, MD, to evaluate the best course of action: traditional ABP subspecialty certification, hospital medicine residency track (with or without additional fellowship), Recognition of Focused Practice (as implemented by the American Board of Internal Medicine and American Board of Family Medicine), mandatory mentorship program, or status quo with option for specialized training. There was considerable discussion of the alternatives in the PHM community. In 2012, the STP shared the results of Strengths-Weaknesses-Opportunities-Threats analyses—but did not issue a recommendation.17 The following year, a National PHM Leaders Conference was held to consider the various options. Participants concluded that the best path forward was to pursue subspecialty certification with a requirement for 2 years of fellowship (after a time-limited period for practice pathway eligibility). Two years of fellowship was a departure from the ABP’s standard 3 years, but seemed acceptable based on the expectation that the research component would be integrated with clinical activities (eg, QI), rather than separate bench research. The ABP Initiative on Subspecialty Clinical Training and Certification had recommended flexibility in the duration of fellowships,18 and PHM became the first discipline to take advantage of such flexibility. Following an 18-month review of multiple considerations, the ABP concluded that “children will be better served by establishing the discipline as a new subspecialty.”19
The decision to pursue subspecialty certification was not unanimously embraced by the PHM community, with particular concerns expressed regarding the impact on Med-Peds hospitalists and the future in community hospitals. These were considered by the individuals writing the formal proposal to the ABP, but have not been resolved. Moreover, criteria for eligibility for the certifying examination under the Practice Pathway (“grandparenting”) evoked controversy,20 addressed by the ABP. 21 The first subspecialty certifying examination was ultimately administered to ~1,500 pediatric hospitalists in 2019.
THE ONGOING EVOLUTION OF PEDIATRIC HOSPITAL MEDICINE
It is clear that PHM has established itself as a field, with networks for research and quality improvement, more than 50 fellowship programs, divisions in prestigious departments of pediatrics and children’s hospitals, devoted journals and textbooks, and a well-attended annual meeting. PHM has set standards for the core competencies in PHM,11, 12 for pediatric hospitalist programs,22, 23 for coordinating the hospital care of children,24, 25 for the curricular framework of fellowships,26 and for the Entrustable Professional Activities expected of a hospitalist.27 The vision for the future is that continued efforts in research, quality and systems improvement, and clinical care will, in fact, result in significant benefits for all hospitalized children. Such was the promise of PHM in the 1990s and remains so in 2019.
Acknowledgments
For prompting the project: Rachel Marek. For additions, corrections, and confirmations: David Alexander, Niccole Alexander, Paul Bellet, David Bertoch, Douglas Carlson, Laura Degnon, Kimberly Durham, Barrett Fromme, Sandy Gage, Matthew Garber, Karen Jerardi, Christopher Landrigan, Gail McGuinness, Jennifer Maniscalco, Sandy Melzer, Vineeta Mittal, Karen Kingry Olson, Mary Ottolini, Jack Percelay, Kris Rehm, Michael Ruhlen, Samir Shah, Suzanne Woods, and David Zipes.
Disclosures
The authors have nothing to disclose.
1. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-517. https://doi.org/10.1056/NEJM199608153350713.
2. Bellet PS, Wachter RM. The hospitalist movement and its implications for the care of hospitalized children. Pediatrics. 1999;103(2):473-477. https://doi.org/10.1542/peds.103.2.473.
3. Roberts KB, Rappo P. A hospitalist movement? Where to? Pediatrics. 1999;103(2):497. https://doi.org/10.1542/peds.103.2.497.
4. Cohen JJ, Dickler RM. Auditing the Medicare-billing practices of teaching physicians—Welcome accountability, unfair approach. N Engl J Med. 1997;336(18):1317-1320. https://doi.org/10.1056/NEJM199705013361811.
5. Swing SR. The ACGME outcome project: Retrospective and prospective. Med Teach. 2007;29(7):648-654. https://doi.org/10.1080/01421590701392903.
6. Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
7. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
8. Accreditation Council for Graduate Medical Education. History of Duty Hours. Available at https://www.acgme.org/What-We-Do/Accreditation/Clinical-Experience-and-Education-formerly-Duty-Hours/History-of-Duty-Hours. Accessed January 16, 2020.
9. Oshimura JM, Sperring J, Bauer BD, Carroll AE, Rauch DA. Changes in inpatient staffing following implementation of new residency work hours. J Hosp Med. 2014;9(10):640-645. https://doi.org/10.1002/jhm.2242.
10. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies supplement: A framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(Suppl 2):i-xv, 1-114. https://doi.org/10.1002/jhm.776.
11. Gage S, Maniscalco J, Fisher E. The Pediatric Hospital Medicine Core Competencies [published online first ahead of print April XX, 2020].
12. Blum K. Raising the profile of hospital medicine. Hopkins Children’s. 2018 Spring, p 32. https://www.hopkinsmedicine.org/johns-hopkins-childrens-center/_documents/_publications/hopkins_childrens_magazine_spring2018.pdf. Accessed January 16, 2020.
13. Roberts K, Stein R, Cheng T. The Academic Pediatric Association: The first fifty years. Acad Pediatr. 2011;11:173-180. https://doi.org/10.1016/j.acap.2011.02.001.
14. Rauch DA, Lye PS, Carlson D, et al. Pediatric Hospital Medicine: A strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
15. Srivastava R, Landrigan CP. Development of the Pediatric Research in Inpatient Settings (PRIS) Network: Lessons learned. J Hosp Med. 2012;7(8)661-664. https://doi.org/10.1002/jhm.1972.
16. Freed GL, Uren RL. Hospitalists in children’s hospitals: What we know now and what we need to know. J Pediatr. 2006;148(3):296-299. https://doi.org/10.1016/j.jpeds.2005.12.048.
17. Maloney CG, Mendez SS, Quinonez RA, et al. The Strategic Planning Committee report: The first step in a journey to recognize pediatric hospital medicine as a distinct discipline. Hosp Pediatr. 2012;2(4):187-190. https://doi.org/10.1542/hpeds.2012-0048.
18. Stevenson DK, McGuiness GA, Bancroft JD, et al. The Initiative on Subspecialty Clinical Training and Certification (SCTC): Background and recommendations. Pediatrics. 2014;133(Suppl 2):S53-S57. https://doi.org/10.1542/peds.2013-3861C.
19. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
20. Chang WW, Hopkins AM, Rehm KP, Gage SL, Shen M. Society of Hospital Medicine position on the American Board of Pediatrics response to the hospital medicine petition. J Hosp Med. 2019;14(10):589-590. https://doi.org/10.12788/jhm.3326.
21. Nichols DG, Woods SK. The American Board of Pediatrics response to the pediatric hospital medicine petition. J Hosp Med. 2019:14:E1-E3. https://doi.org/10.12788/jhm.3322.
22. American Academy of Pediatrics Section on Hospital Medicine. Guiding principles for pediatric hospitalist programs. Pediatrics. 2005;115:1101-1102.
23. American Academy of Pediatrics Section on Hospital Medicine. Guiding principles for pediatric hospitalist programs. Pediatrics. 2013;132(4):782-786. https://doi.org/10.1542/peds.2013-2269.
24. Lye PS, American Academy of Pediatrics Committee on Hospital Care, Section on Hospital Medicine. Clinical report—physicians’ roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829-832.
25. Rauch DA, American Academy of Pediatrics Committee on Hospital Care, Section on Hospital Medicine. Physician’s role in coordinating care of hospitalized children. Pediatrics. 2018;142(2):e20181503. https://doi.org/10.1542/peds.2018-1503.
26. Jerardi KE, Fisher ER, Rassbach C, et al; on behalf of the Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2019;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
27. American Board of Pediatrics. Pediatric hospital medicine entrustable professional activities. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed August 31, 2019.
28. Perkin RM, Swift JD, Newton DA (Eds). Pediatric Hospital Medicine: Textbook of Inpatient Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
29. Frank F, Shah SS, Catallozzi M, Zaoutis L (Eds). The Philadelphia Guide: Inpatient Pediatrics. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
30. Zaoutis L, Chiang V (Eds). Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Mosby; 2007.
31. Rauch DA, Gershel J (Eds). Caring for the Hospitalized Child: A Handbook of Inpatient Pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; 2013.
32. Rauch DA. Tribute to Jennifer Daru, MD. Hosp Pediatr. 2011;4(4):267-268.
1. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-517. https://doi.org/10.1056/NEJM199608153350713.
2. Bellet PS, Wachter RM. The hospitalist movement and its implications for the care of hospitalized children. Pediatrics. 1999;103(2):473-477. https://doi.org/10.1542/peds.103.2.473.
3. Roberts KB, Rappo P. A hospitalist movement? Where to? Pediatrics. 1999;103(2):497. https://doi.org/10.1542/peds.103.2.497.
4. Cohen JJ, Dickler RM. Auditing the Medicare-billing practices of teaching physicians—Welcome accountability, unfair approach. N Engl J Med. 1997;336(18):1317-1320. https://doi.org/10.1056/NEJM199705013361811.
5. Swing SR. The ACGME outcome project: Retrospective and prospective. Med Teach. 2007;29(7):648-654. https://doi.org/10.1080/01421590701392903.
6. Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
7. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
8. Accreditation Council for Graduate Medical Education. History of Duty Hours. Available at https://www.acgme.org/What-We-Do/Accreditation/Clinical-Experience-and-Education-formerly-Duty-Hours/History-of-Duty-Hours. Accessed January 16, 2020.
9. Oshimura JM, Sperring J, Bauer BD, Carroll AE, Rauch DA. Changes in inpatient staffing following implementation of new residency work hours. J Hosp Med. 2014;9(10):640-645. https://doi.org/10.1002/jhm.2242.
10. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies supplement: A framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(Suppl 2):i-xv, 1-114. https://doi.org/10.1002/jhm.776.
11. Gage S, Maniscalco J, Fisher E. The Pediatric Hospital Medicine Core Competencies [published online first ahead of print April XX, 2020].
12. Blum K. Raising the profile of hospital medicine. Hopkins Children’s. 2018 Spring, p 32. https://www.hopkinsmedicine.org/johns-hopkins-childrens-center/_documents/_publications/hopkins_childrens_magazine_spring2018.pdf. Accessed January 16, 2020.
13. Roberts K, Stein R, Cheng T. The Academic Pediatric Association: The first fifty years. Acad Pediatr. 2011;11:173-180. https://doi.org/10.1016/j.acap.2011.02.001.
14. Rauch DA, Lye PS, Carlson D, et al. Pediatric Hospital Medicine: A strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
15. Srivastava R, Landrigan CP. Development of the Pediatric Research in Inpatient Settings (PRIS) Network: Lessons learned. J Hosp Med. 2012;7(8)661-664. https://doi.org/10.1002/jhm.1972.
16. Freed GL, Uren RL. Hospitalists in children’s hospitals: What we know now and what we need to know. J Pediatr. 2006;148(3):296-299. https://doi.org/10.1016/j.jpeds.2005.12.048.
17. Maloney CG, Mendez SS, Quinonez RA, et al. The Strategic Planning Committee report: The first step in a journey to recognize pediatric hospital medicine as a distinct discipline. Hosp Pediatr. 2012;2(4):187-190. https://doi.org/10.1542/hpeds.2012-0048.
18. Stevenson DK, McGuiness GA, Bancroft JD, et al. The Initiative on Subspecialty Clinical Training and Certification (SCTC): Background and recommendations. Pediatrics. 2014;133(Suppl 2):S53-S57. https://doi.org/10.1542/peds.2013-3861C.
19. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3):e20161823. https://doi.org/10.1542/peds.2016-1823.
20. Chang WW, Hopkins AM, Rehm KP, Gage SL, Shen M. Society of Hospital Medicine position on the American Board of Pediatrics response to the hospital medicine petition. J Hosp Med. 2019;14(10):589-590. https://doi.org/10.12788/jhm.3326.
21. Nichols DG, Woods SK. The American Board of Pediatrics response to the pediatric hospital medicine petition. J Hosp Med. 2019:14:E1-E3. https://doi.org/10.12788/jhm.3322.
22. American Academy of Pediatrics Section on Hospital Medicine. Guiding principles for pediatric hospitalist programs. Pediatrics. 2005;115:1101-1102.
23. American Academy of Pediatrics Section on Hospital Medicine. Guiding principles for pediatric hospitalist programs. Pediatrics. 2013;132(4):782-786. https://doi.org/10.1542/peds.2013-2269.
24. Lye PS, American Academy of Pediatrics Committee on Hospital Care, Section on Hospital Medicine. Clinical report—physicians’ roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829-832.
25. Rauch DA, American Academy of Pediatrics Committee on Hospital Care, Section on Hospital Medicine. Physician’s role in coordinating care of hospitalized children. Pediatrics. 2018;142(2):e20181503. https://doi.org/10.1542/peds.2018-1503.
26. Jerardi KE, Fisher ER, Rassbach C, et al; on behalf of the Council of Pediatric Hospital Medicine Fellowship Directors. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2019;140(1):e20170698. https://doi.org/10.1542/peds.2017-0698.
27. American Board of Pediatrics. Pediatric hospital medicine entrustable professional activities. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed August 31, 2019.
28. Perkin RM, Swift JD, Newton DA (Eds). Pediatric Hospital Medicine: Textbook of Inpatient Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
29. Frank F, Shah SS, Catallozzi M, Zaoutis L (Eds). The Philadelphia Guide: Inpatient Pediatrics. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
30. Zaoutis L, Chiang V (Eds). Comprehensive Pediatric Hospital Medicine. Philadelphia, PA: Mosby; 2007.
31. Rauch DA, Gershel J (Eds). Caring for the Hospitalized Child: A Handbook of Inpatient Pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; 2013.
32. Rauch DA. Tribute to Jennifer Daru, MD. Hosp Pediatr. 2011;4(4):267-268.
© 2020 Society of Hospital Medicine
Methodological Progress Note: Classification and Regression Tree Analysis
Machine-learning is a type of artificial intelligence in which systems automatically learn and improve from experience without being explicitly programmed. Classification and Regression Tree (CART) analysis is a machine-learning algorithm that was developed to visually classify or segment populations into subgroups with similar characteristics and outcomes. CART analysis is a decision tree methodology that was initially developed in the 1960s for use in product marketing.1 Since then, a number of health disciplines have used it to isolate patient subgroups from larger populations to guide clinical decision-making by better identifying those most likely to benefit.2 The clinical utility of CART mirrors how most clinicians think, which is not in terms of coefficients (ie, regression output) but rather in terms of categories or classifications (eg, low vs high risk).
In this issue of the Journal of Hospital Medicine, Young and colleagues use classification trees to predict discharge placement (postacute care facility vs home) based on a patient’s hospital admission characteristics and mobility score. The resulting decision tree indicates that patients with the lowest mobility scores, as well as those 65 years and older, were most likely to be discharged to postacute care facilities.3 In this review, we orient the reader to the basics of CART analysis, discuss important intricacies, and weigh its pros, cons, and application as a statistical tool.
WHAT IS CART ANALYSIS?
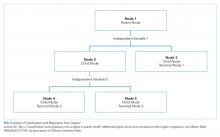
CART is a nonparametric (ie, makes no assumptions about data distribution) statistical tool that identifies subgroups within a population whose members share common characteristics as defined by the independent variables included in the model. CART analysis is unique in that it yields a visual output of the data in the form of a multisegmented structure that resembles the branches of a tree (Figure). CART analysis consists of four basic steps: (1) tree-building (including splitting criteria and estimation of classification error), (2) stopping the tree-building process, (3) tree “pruning,” and (4) tree selection.
In general, CART analysis begins with a single “node” or group, which contains the entire sample population. This is referred to as the “parent node.” The CART procedure simultaneously examines all available independent variables and selects one that results in two groups that are the most distinct with respect to the outcome variable of interest. In Young et al’s example, posthospital discharge placement is the outcome.3 This parent node then branches into two “child nodes” according to the independent variable that was selected. Within each of these “child nodes,” the tree-growing methodology recursively assesses each of the remaining independent variables to determine which will result in the best split according to the chosen splitting criterion.2 Each subsequent “child node” will become a “parent node” to the two groups in which it splits. This process is repeated on the data in each subsequent “child node” and is stopped once a predefined stopping point is reached. Notably, while division into two groups is the most common application of CART modeling, there are models that can split data into more than two child nodes.
Since CART outcomes can be heavily dependent on the data being used (eg, electronic health records or administrative data), it is important to attempt to confirm results in a similar, but different, study cohort. Because obtaining separate data sources with similar cohorts can be difficult, many investigators using CART will utilize a “split sample approach” in which study data are split into separate training and validation sets.4 In the training set, which frequently comprises two-thirds of the available data, the algorithm is tested in exploratory analysis. Once the algorithm is defined and agreed upon, it is retested within a validation set, constructed from the remaining one-third of data. This approach, which Young et al utilize,3 allows for improved confidence and reduced risk of bias in the findings and allows for some degree of external validation. Further, the split sample approach supports more reliable measures of predictive accuracy: in Young et al’s case, the proportion of correctly classified patients discharged to a postacute care facility (sensitivity: 58%, 95% CI 49-68%) and the proportion of correctly classified patients discharged home (specificity: 84%, 95% CI 78-90%). Despite these advantages, the split sample approach is not universally used.
Classification Versus Regression Trees
While commonly grouped together, CARTs can be distinguished from one another based on the dependent, or outcome, variable. Categorical outcome variables require the use of a classification tree, while continuous outcomes utilize regression trees. Of note, the independent, or predictor, variables can be any combination of categorical or continuous variables. However, splitting at each node creates categorical output when using CART algorithms.
Splitting Criteria
The splitting of each node is based on reducing the degree of “impurity” (heterogeneity with respect to the outcome variable) within each node. For example, a node that has no impurity will have a zero error rate labeling its binary outcomes. While CART works well with categorical variables, continuous variables (eg, age) can also be assessed, though only with certain algorithms. Several different splitting criteria exist, each of which attempt to maximize the differences within each child node. While beyond the scope of this review, examples of popular splitting criteria are Gini, entropy, and minimum error.5
Stopping Rules
To manage the size of a tree, CART analysis allows for predefined stopping rules to minimize the extent of growth while also establishing a minimal degree of statistical difference between nodes that is considered meaningful. To accomplish this task, two stopping rules are often used. The first defines the minimum number of observations in child, or “terminal,” nodes. The second defines the maximum number of levels a tree may grow, thus allowing the investigator to decide the total number of predictor variables that can define a terminal node. While several other stopping rules exist, these are the most commonly utilized.
Pruning
To avoid missing important associations due to premature stoppage, investigators may use another mechanism to limit tree growth called “pruning.” For pruning, the first step is to grow a considerably large tree that includes many levels or nodes, possibly to the point where there are just a few observations per terminal node. Then, similar to the residual sum of squares in a regression, the investigator can calculate a misclassification cost (ie, goodness of fit) and select the tree with the smallest cost.2 Of note, stopping rules and pruning can be used simultaneously.
Classification Error
Similar to other forms of statistical inference it remains important to understand the uncertainty within the inference. In regression modeling, for example, classification errors can be calculated using standard errors of the parameter estimates. In CART analysis, because random samples from a population may produce different trees, measures of variability can be more complicated. One strategy is to generate a tree from a test sample and then use the remaining data to calculate a measure of the misclassification cost (a measure of how much additional accuracy a split must add to the entire tree to warrant the additional complexity). Alternatively, a “k-fold cross-validation” can be performed in which the data is broken down into k subsets from which a tree is created using all data except for one of the subsets. The computed tree is then applied to the remaining subset to determine a misclassification cost. These classification costs are important as they also impact the stopping and pruning processes. Ultimately, a final tree, which best limits classification errors, is selected.
WHEN WOULD YOU USE CART ANALYSIS?
This method can be useful in multiple settings in which an investigator wants to characterize a subpopulation from a larger cohort. Adaptation of this could include, but is not limited to, risk stratification,6 diagnostics,7 and patient identification for medical interventions.8 Moreover, CART analysis has the added benefit of creating visually interpretable predictive models that can be utilized for front-line clinical decision making.9,10
STRENGTHS OF CART ANALYSIS
CART analysis has been shown to have several advantages over other commonly used modeling methods. First, it is a nonparametric model that can handle highly skewed data and does not require that the predictor, or predictors, takes on a predetermined form (allowing them to be constructed from the data). This is helpful as many clinical variables can have wide degrees of variance.
Unlike other modeling techniques, CART can identify higher-order interactions between multiple variables, meaning it can handle interactions that occur whenever one variable affects the nature of an interaction between two other variables. Further, CART can handle multiple correlated independent variables, something logistic regression models classically cannot do.
From a clinical standpoint, the “logic” of the visual-based CART output can be easier to interpret than the probabilistic output (eg, odds ratio) associated with logistic regression modeling, making it more practical, applicable, and easier for clinicians to adopt.10,12 Finally, CART software is easy to use for those who do not have strong statistical backgrounds, and it is less resource intensive than other statistical methods.2
LIMITATIONS OF CART ANALYSIS
Despite these features, CART does have several disadvantages. First, due to the ease with which CART analysis can be performed, “data dredging” can be a significant concern. Its ideal use is with a priori consideration of independent variables.2 Second, while CART is most beneficial in describing links and cutoffs between variables, it may not be useful for hypothesis testing.2 Third, large data sets are needed to perform CART, especially if the investigator is using the split sample approach mentioned above.11 Finally, while CART is the most utilized decision tree methodology, several other types of decision tree methods exist: C4.5, CRUISE, Quick, Unbiased, Efficient Statistical Trees, Chi-square-Automatic-Interaction-Detection, and others. Many of these allow for splitting into more than two groups and have other features that may be more advantageous to one’s analysis.13
WHY DID THE AUTHORS USE CART?
Decision trees offer simple, interpretable results of multiple factors that can be easily applied to clinical scenarios. In this case, the authors specifically used classification tree analysis to take advantage of CART’s machine-learning ability to consider higher-order interactions to build their model—as they lacked a priori evidence to help guide them in traditional (ie, logistic regression) model construction. Furthermore, CART analysis created an output that logically and visually illustrates which combination of characteristics is most associated with discharge placement and can potentially be utilized to help facilitate discharge planning in future hospitalized patients. To sum up, this machine-learning methodology allowed the investigators to determine which variables taken together were the most suitable in predicting their outcome of interest and present these findings in a manner that busy clinicians can interpret and apply.
1. Magee JF. Decision Trees for Decision Making. Harvard Business Review. 1964. https://hbr.org/1964/07/decision-trees-for-decision-making. Accessed August 26, 2019.
2. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172-181. https://doi.org/10.1207/S15324796ABM2603_02
3. Young D, Colantuoni E, Seltzer D, et al. Prediction of disposition within 48-hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9):540-543. https://doi.org/10.12788/jhm.3332
4. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-1358. https://doi.org/10.1056/NEJMra1814259
5. Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer-Verlag; 1999. https://www.springer.com/gp/book/9781475730272. Accessed August 24, 2019.
6. Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, for the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. https://doi.org/10.1001/jama.293.5.572
7. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5(11):3403-3410.
8. Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23(19):4322-4329. https://doi.org/10.1200/JCO.2005.11.136
9. Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci. 2011;56(12):3664-3671. https://doi.org/10.1007/s10620-011-1849-x
10. Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Proceedings of Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, May 22-25, 2000; pp. 1–14.
11. Perlich C, Provost F, Simonoff JS. Tree induction vs logistic regression: a learning-curve analysis. J Mach Learn Res. 2003;4(Jun):211-255. https://doi.org/10.1162/153244304322972694
12. Woolever D. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31-36.
13. Loh WY. Classification and regression trees. Wires Data Min Know Disc. 2011;1(1):14-23. https://doi.org/10.1002/widm.8
Machine-learning is a type of artificial intelligence in which systems automatically learn and improve from experience without being explicitly programmed. Classification and Regression Tree (CART) analysis is a machine-learning algorithm that was developed to visually classify or segment populations into subgroups with similar characteristics and outcomes. CART analysis is a decision tree methodology that was initially developed in the 1960s for use in product marketing.1 Since then, a number of health disciplines have used it to isolate patient subgroups from larger populations to guide clinical decision-making by better identifying those most likely to benefit.2 The clinical utility of CART mirrors how most clinicians think, which is not in terms of coefficients (ie, regression output) but rather in terms of categories or classifications (eg, low vs high risk).
In this issue of the Journal of Hospital Medicine, Young and colleagues use classification trees to predict discharge placement (postacute care facility vs home) based on a patient’s hospital admission characteristics and mobility score. The resulting decision tree indicates that patients with the lowest mobility scores, as well as those 65 years and older, were most likely to be discharged to postacute care facilities.3 In this review, we orient the reader to the basics of CART analysis, discuss important intricacies, and weigh its pros, cons, and application as a statistical tool.
WHAT IS CART ANALYSIS?
CART is a nonparametric (ie, makes no assumptions about data distribution) statistical tool that identifies subgroups within a population whose members share common characteristics as defined by the independent variables included in the model. CART analysis is unique in that it yields a visual output of the data in the form of a multisegmented structure that resembles the branches of a tree (Figure). CART analysis consists of four basic steps: (1) tree-building (including splitting criteria and estimation of classification error), (2) stopping the tree-building process, (3) tree “pruning,” and (4) tree selection.
In general, CART analysis begins with a single “node” or group, which contains the entire sample population. This is referred to as the “parent node.” The CART procedure simultaneously examines all available independent variables and selects one that results in two groups that are the most distinct with respect to the outcome variable of interest. In Young et al’s example, posthospital discharge placement is the outcome.3 This parent node then branches into two “child nodes” according to the independent variable that was selected. Within each of these “child nodes,” the tree-growing methodology recursively assesses each of the remaining independent variables to determine which will result in the best split according to the chosen splitting criterion.2 Each subsequent “child node” will become a “parent node” to the two groups in which it splits. This process is repeated on the data in each subsequent “child node” and is stopped once a predefined stopping point is reached. Notably, while division into two groups is the most common application of CART modeling, there are models that can split data into more than two child nodes.
Since CART outcomes can be heavily dependent on the data being used (eg, electronic health records or administrative data), it is important to attempt to confirm results in a similar, but different, study cohort. Because obtaining separate data sources with similar cohorts can be difficult, many investigators using CART will utilize a “split sample approach” in which study data are split into separate training and validation sets.4 In the training set, which frequently comprises two-thirds of the available data, the algorithm is tested in exploratory analysis. Once the algorithm is defined and agreed upon, it is retested within a validation set, constructed from the remaining one-third of data. This approach, which Young et al utilize,3 allows for improved confidence and reduced risk of bias in the findings and allows for some degree of external validation. Further, the split sample approach supports more reliable measures of predictive accuracy: in Young et al’s case, the proportion of correctly classified patients discharged to a postacute care facility (sensitivity: 58%, 95% CI 49-68%) and the proportion of correctly classified patients discharged home (specificity: 84%, 95% CI 78-90%). Despite these advantages, the split sample approach is not universally used.
Classification Versus Regression Trees
While commonly grouped together, CARTs can be distinguished from one another based on the dependent, or outcome, variable. Categorical outcome variables require the use of a classification tree, while continuous outcomes utilize regression trees. Of note, the independent, or predictor, variables can be any combination of categorical or continuous variables. However, splitting at each node creates categorical output when using CART algorithms.
Splitting Criteria
The splitting of each node is based on reducing the degree of “impurity” (heterogeneity with respect to the outcome variable) within each node. For example, a node that has no impurity will have a zero error rate labeling its binary outcomes. While CART works well with categorical variables, continuous variables (eg, age) can also be assessed, though only with certain algorithms. Several different splitting criteria exist, each of which attempt to maximize the differences within each child node. While beyond the scope of this review, examples of popular splitting criteria are Gini, entropy, and minimum error.5
Stopping Rules
To manage the size of a tree, CART analysis allows for predefined stopping rules to minimize the extent of growth while also establishing a minimal degree of statistical difference between nodes that is considered meaningful. To accomplish this task, two stopping rules are often used. The first defines the minimum number of observations in child, or “terminal,” nodes. The second defines the maximum number of levels a tree may grow, thus allowing the investigator to decide the total number of predictor variables that can define a terminal node. While several other stopping rules exist, these are the most commonly utilized.
Pruning
To avoid missing important associations due to premature stoppage, investigators may use another mechanism to limit tree growth called “pruning.” For pruning, the first step is to grow a considerably large tree that includes many levels or nodes, possibly to the point where there are just a few observations per terminal node. Then, similar to the residual sum of squares in a regression, the investigator can calculate a misclassification cost (ie, goodness of fit) and select the tree with the smallest cost.2 Of note, stopping rules and pruning can be used simultaneously.
Classification Error
Similar to other forms of statistical inference it remains important to understand the uncertainty within the inference. In regression modeling, for example, classification errors can be calculated using standard errors of the parameter estimates. In CART analysis, because random samples from a population may produce different trees, measures of variability can be more complicated. One strategy is to generate a tree from a test sample and then use the remaining data to calculate a measure of the misclassification cost (a measure of how much additional accuracy a split must add to the entire tree to warrant the additional complexity). Alternatively, a “k-fold cross-validation” can be performed in which the data is broken down into k subsets from which a tree is created using all data except for one of the subsets. The computed tree is then applied to the remaining subset to determine a misclassification cost. These classification costs are important as they also impact the stopping and pruning processes. Ultimately, a final tree, which best limits classification errors, is selected.
WHEN WOULD YOU USE CART ANALYSIS?
This method can be useful in multiple settings in which an investigator wants to characterize a subpopulation from a larger cohort. Adaptation of this could include, but is not limited to, risk stratification,6 diagnostics,7 and patient identification for medical interventions.8 Moreover, CART analysis has the added benefit of creating visually interpretable predictive models that can be utilized for front-line clinical decision making.9,10
STRENGTHS OF CART ANALYSIS
CART analysis has been shown to have several advantages over other commonly used modeling methods. First, it is a nonparametric model that can handle highly skewed data and does not require that the predictor, or predictors, takes on a predetermined form (allowing them to be constructed from the data). This is helpful as many clinical variables can have wide degrees of variance.
Unlike other modeling techniques, CART can identify higher-order interactions between multiple variables, meaning it can handle interactions that occur whenever one variable affects the nature of an interaction between two other variables. Further, CART can handle multiple correlated independent variables, something logistic regression models classically cannot do.
From a clinical standpoint, the “logic” of the visual-based CART output can be easier to interpret than the probabilistic output (eg, odds ratio) associated with logistic regression modeling, making it more practical, applicable, and easier for clinicians to adopt.10,12 Finally, CART software is easy to use for those who do not have strong statistical backgrounds, and it is less resource intensive than other statistical methods.2
LIMITATIONS OF CART ANALYSIS
Despite these features, CART does have several disadvantages. First, due to the ease with which CART analysis can be performed, “data dredging” can be a significant concern. Its ideal use is with a priori consideration of independent variables.2 Second, while CART is most beneficial in describing links and cutoffs between variables, it may not be useful for hypothesis testing.2 Third, large data sets are needed to perform CART, especially if the investigator is using the split sample approach mentioned above.11 Finally, while CART is the most utilized decision tree methodology, several other types of decision tree methods exist: C4.5, CRUISE, Quick, Unbiased, Efficient Statistical Trees, Chi-square-Automatic-Interaction-Detection, and others. Many of these allow for splitting into more than two groups and have other features that may be more advantageous to one’s analysis.13
WHY DID THE AUTHORS USE CART?
Decision trees offer simple, interpretable results of multiple factors that can be easily applied to clinical scenarios. In this case, the authors specifically used classification tree analysis to take advantage of CART’s machine-learning ability to consider higher-order interactions to build their model—as they lacked a priori evidence to help guide them in traditional (ie, logistic regression) model construction. Furthermore, CART analysis created an output that logically and visually illustrates which combination of characteristics is most associated with discharge placement and can potentially be utilized to help facilitate discharge planning in future hospitalized patients. To sum up, this machine-learning methodology allowed the investigators to determine which variables taken together were the most suitable in predicting their outcome of interest and present these findings in a manner that busy clinicians can interpret and apply.
Machine-learning is a type of artificial intelligence in which systems automatically learn and improve from experience without being explicitly programmed. Classification and Regression Tree (CART) analysis is a machine-learning algorithm that was developed to visually classify or segment populations into subgroups with similar characteristics and outcomes. CART analysis is a decision tree methodology that was initially developed in the 1960s for use in product marketing.1 Since then, a number of health disciplines have used it to isolate patient subgroups from larger populations to guide clinical decision-making by better identifying those most likely to benefit.2 The clinical utility of CART mirrors how most clinicians think, which is not in terms of coefficients (ie, regression output) but rather in terms of categories or classifications (eg, low vs high risk).
In this issue of the Journal of Hospital Medicine, Young and colleagues use classification trees to predict discharge placement (postacute care facility vs home) based on a patient’s hospital admission characteristics and mobility score. The resulting decision tree indicates that patients with the lowest mobility scores, as well as those 65 years and older, were most likely to be discharged to postacute care facilities.3 In this review, we orient the reader to the basics of CART analysis, discuss important intricacies, and weigh its pros, cons, and application as a statistical tool.
WHAT IS CART ANALYSIS?
CART is a nonparametric (ie, makes no assumptions about data distribution) statistical tool that identifies subgroups within a population whose members share common characteristics as defined by the independent variables included in the model. CART analysis is unique in that it yields a visual output of the data in the form of a multisegmented structure that resembles the branches of a tree (Figure). CART analysis consists of four basic steps: (1) tree-building (including splitting criteria and estimation of classification error), (2) stopping the tree-building process, (3) tree “pruning,” and (4) tree selection.
In general, CART analysis begins with a single “node” or group, which contains the entire sample population. This is referred to as the “parent node.” The CART procedure simultaneously examines all available independent variables and selects one that results in two groups that are the most distinct with respect to the outcome variable of interest. In Young et al’s example, posthospital discharge placement is the outcome.3 This parent node then branches into two “child nodes” according to the independent variable that was selected. Within each of these “child nodes,” the tree-growing methodology recursively assesses each of the remaining independent variables to determine which will result in the best split according to the chosen splitting criterion.2 Each subsequent “child node” will become a “parent node” to the two groups in which it splits. This process is repeated on the data in each subsequent “child node” and is stopped once a predefined stopping point is reached. Notably, while division into two groups is the most common application of CART modeling, there are models that can split data into more than two child nodes.
Since CART outcomes can be heavily dependent on the data being used (eg, electronic health records or administrative data), it is important to attempt to confirm results in a similar, but different, study cohort. Because obtaining separate data sources with similar cohorts can be difficult, many investigators using CART will utilize a “split sample approach” in which study data are split into separate training and validation sets.4 In the training set, which frequently comprises two-thirds of the available data, the algorithm is tested in exploratory analysis. Once the algorithm is defined and agreed upon, it is retested within a validation set, constructed from the remaining one-third of data. This approach, which Young et al utilize,3 allows for improved confidence and reduced risk of bias in the findings and allows for some degree of external validation. Further, the split sample approach supports more reliable measures of predictive accuracy: in Young et al’s case, the proportion of correctly classified patients discharged to a postacute care facility (sensitivity: 58%, 95% CI 49-68%) and the proportion of correctly classified patients discharged home (specificity: 84%, 95% CI 78-90%). Despite these advantages, the split sample approach is not universally used.
Classification Versus Regression Trees
While commonly grouped together, CARTs can be distinguished from one another based on the dependent, or outcome, variable. Categorical outcome variables require the use of a classification tree, while continuous outcomes utilize regression trees. Of note, the independent, or predictor, variables can be any combination of categorical or continuous variables. However, splitting at each node creates categorical output when using CART algorithms.
Splitting Criteria
The splitting of each node is based on reducing the degree of “impurity” (heterogeneity with respect to the outcome variable) within each node. For example, a node that has no impurity will have a zero error rate labeling its binary outcomes. While CART works well with categorical variables, continuous variables (eg, age) can also be assessed, though only with certain algorithms. Several different splitting criteria exist, each of which attempt to maximize the differences within each child node. While beyond the scope of this review, examples of popular splitting criteria are Gini, entropy, and minimum error.5
Stopping Rules
To manage the size of a tree, CART analysis allows for predefined stopping rules to minimize the extent of growth while also establishing a minimal degree of statistical difference between nodes that is considered meaningful. To accomplish this task, two stopping rules are often used. The first defines the minimum number of observations in child, or “terminal,” nodes. The second defines the maximum number of levels a tree may grow, thus allowing the investigator to decide the total number of predictor variables that can define a terminal node. While several other stopping rules exist, these are the most commonly utilized.
Pruning
To avoid missing important associations due to premature stoppage, investigators may use another mechanism to limit tree growth called “pruning.” For pruning, the first step is to grow a considerably large tree that includes many levels or nodes, possibly to the point where there are just a few observations per terminal node. Then, similar to the residual sum of squares in a regression, the investigator can calculate a misclassification cost (ie, goodness of fit) and select the tree with the smallest cost.2 Of note, stopping rules and pruning can be used simultaneously.
Classification Error
Similar to other forms of statistical inference it remains important to understand the uncertainty within the inference. In regression modeling, for example, classification errors can be calculated using standard errors of the parameter estimates. In CART analysis, because random samples from a population may produce different trees, measures of variability can be more complicated. One strategy is to generate a tree from a test sample and then use the remaining data to calculate a measure of the misclassification cost (a measure of how much additional accuracy a split must add to the entire tree to warrant the additional complexity). Alternatively, a “k-fold cross-validation” can be performed in which the data is broken down into k subsets from which a tree is created using all data except for one of the subsets. The computed tree is then applied to the remaining subset to determine a misclassification cost. These classification costs are important as they also impact the stopping and pruning processes. Ultimately, a final tree, which best limits classification errors, is selected.
WHEN WOULD YOU USE CART ANALYSIS?
This method can be useful in multiple settings in which an investigator wants to characterize a subpopulation from a larger cohort. Adaptation of this could include, but is not limited to, risk stratification,6 diagnostics,7 and patient identification for medical interventions.8 Moreover, CART analysis has the added benefit of creating visually interpretable predictive models that can be utilized for front-line clinical decision making.9,10
STRENGTHS OF CART ANALYSIS
CART analysis has been shown to have several advantages over other commonly used modeling methods. First, it is a nonparametric model that can handle highly skewed data and does not require that the predictor, or predictors, takes on a predetermined form (allowing them to be constructed from the data). This is helpful as many clinical variables can have wide degrees of variance.
Unlike other modeling techniques, CART can identify higher-order interactions between multiple variables, meaning it can handle interactions that occur whenever one variable affects the nature of an interaction between two other variables. Further, CART can handle multiple correlated independent variables, something logistic regression models classically cannot do.
From a clinical standpoint, the “logic” of the visual-based CART output can be easier to interpret than the probabilistic output (eg, odds ratio) associated with logistic regression modeling, making it more practical, applicable, and easier for clinicians to adopt.10,12 Finally, CART software is easy to use for those who do not have strong statistical backgrounds, and it is less resource intensive than other statistical methods.2
LIMITATIONS OF CART ANALYSIS
Despite these features, CART does have several disadvantages. First, due to the ease with which CART analysis can be performed, “data dredging” can be a significant concern. Its ideal use is with a priori consideration of independent variables.2 Second, while CART is most beneficial in describing links and cutoffs between variables, it may not be useful for hypothesis testing.2 Third, large data sets are needed to perform CART, especially if the investigator is using the split sample approach mentioned above.11 Finally, while CART is the most utilized decision tree methodology, several other types of decision tree methods exist: C4.5, CRUISE, Quick, Unbiased, Efficient Statistical Trees, Chi-square-Automatic-Interaction-Detection, and others. Many of these allow for splitting into more than two groups and have other features that may be more advantageous to one’s analysis.13
WHY DID THE AUTHORS USE CART?
Decision trees offer simple, interpretable results of multiple factors that can be easily applied to clinical scenarios. In this case, the authors specifically used classification tree analysis to take advantage of CART’s machine-learning ability to consider higher-order interactions to build their model—as they lacked a priori evidence to help guide them in traditional (ie, logistic regression) model construction. Furthermore, CART analysis created an output that logically and visually illustrates which combination of characteristics is most associated with discharge placement and can potentially be utilized to help facilitate discharge planning in future hospitalized patients. To sum up, this machine-learning methodology allowed the investigators to determine which variables taken together were the most suitable in predicting their outcome of interest and present these findings in a manner that busy clinicians can interpret and apply.
1. Magee JF. Decision Trees for Decision Making. Harvard Business Review. 1964. https://hbr.org/1964/07/decision-trees-for-decision-making. Accessed August 26, 2019.
2. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172-181. https://doi.org/10.1207/S15324796ABM2603_02
3. Young D, Colantuoni E, Seltzer D, et al. Prediction of disposition within 48-hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9):540-543. https://doi.org/10.12788/jhm.3332
4. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-1358. https://doi.org/10.1056/NEJMra1814259
5. Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer-Verlag; 1999. https://www.springer.com/gp/book/9781475730272. Accessed August 24, 2019.
6. Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, for the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. https://doi.org/10.1001/jama.293.5.572
7. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5(11):3403-3410.
8. Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23(19):4322-4329. https://doi.org/10.1200/JCO.2005.11.136
9. Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci. 2011;56(12):3664-3671. https://doi.org/10.1007/s10620-011-1849-x
10. Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Proceedings of Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, May 22-25, 2000; pp. 1–14.
11. Perlich C, Provost F, Simonoff JS. Tree induction vs logistic regression: a learning-curve analysis. J Mach Learn Res. 2003;4(Jun):211-255. https://doi.org/10.1162/153244304322972694
12. Woolever D. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31-36.
13. Loh WY. Classification and regression trees. Wires Data Min Know Disc. 2011;1(1):14-23. https://doi.org/10.1002/widm.8
1. Magee JF. Decision Trees for Decision Making. Harvard Business Review. 1964. https://hbr.org/1964/07/decision-trees-for-decision-making. Accessed August 26, 2019.
2. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172-181. https://doi.org/10.1207/S15324796ABM2603_02
3. Young D, Colantuoni E, Seltzer D, et al. Prediction of disposition within 48-hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9):540-543. https://doi.org/10.12788/jhm.3332
4. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-1358. https://doi.org/10.1056/NEJMra1814259
5. Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer-Verlag; 1999. https://www.springer.com/gp/book/9781475730272. Accessed August 24, 2019.
6. Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, for the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. https://doi.org/10.1001/jama.293.5.572
7. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5(11):3403-3410.
8. Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23(19):4322-4329. https://doi.org/10.1200/JCO.2005.11.136
9. Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci. 2011;56(12):3664-3671. https://doi.org/10.1007/s10620-011-1849-x
10. Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Proceedings of Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, May 22-25, 2000; pp. 1–14.
11. Perlich C, Provost F, Simonoff JS. Tree induction vs logistic regression: a learning-curve analysis. J Mach Learn Res. 2003;4(Jun):211-255. https://doi.org/10.1162/153244304322972694
12. Woolever D. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31-36.
13. Loh WY. Classification and regression trees. Wires Data Min Know Disc. 2011;1(1):14-23. https://doi.org/10.1002/widm.8
© 2020 Society of Hospital Medicine
Two-Year Experience of 14 French Pigtail Catheters Placed by Procedure-Focused Hospitalists
Over the last 15 years, studies have demonstrated the efficacy of small-bore chest tubes (SBCTs), or pigtail catheters (PCs, most commonly ≤14 French), in treating pneumothorax (PTX),1-5 traumatic hemothorax (THTX), hemopneumothorax (HPTX),6,7 parapneumonic effusions (PPEs),8,9 pleural infections,10 and symptomatic malignant pleural effusions.11 A randomized, controlled trial also showed that PC placement resulted in better pain scores, compared with large-bore chest tubes (LBCTs), for traumatic PTX.5 The British Thoracic Society does state that LBCTs may be needed for PTXs with very large air leaks, especially postoperatively. Further, LBCTs may be indicated if small-bore drainage fails, but otherwise they recommend PCs as first-line therapy for PTX, free flowing pleural effusions, and pleural infections.12
BEDSIDE PROCEDURE SERVICE DEVELOPMENT
The Medical College of Wisconsin (MCW) provides hospitalist services to
BPS Pigtail Catheter Training
CT surgery initially trained the BPS director in PC placement using the Seldinger technique in 2015. The director’s training period with CT surgery included direct observation by CT surgery providers for 5 PC placements. Prior to placing PCs, the director had performed approximately 400 ultrasound-guided thoracenteses. The BPS director then independently trained the remaining BPS and has placed or supervised over half of the service’s 124 PCs. Initial credentialing for each BPS physician requires 5 PC placements and 20 thoracenteses under direct supervision of credentialed BPS members. Credentialing is maintained by BPS physicians completing 3 PCs and 15 thoracenteses per year.
Newly credentialed providers are capable of independently placing most PCs. However, the requirements for credentialing are minimal and newly credentialed physicians still encounter PC placements with challenging factors not addressed in their training, such as anterior approach, small effusions, atypical effusion location, mild to moderate coagulopathy, recent therapeutic anticoagulation, and large body habitus. To address these challenges, the BPS has instituted an “on call” system. This system is typically staffed by the BPS director or associate director, already attending on a separate medical service. When needed, the “on call” physician will supervise the newer BPS members to ensure safety while the less experienced physician places the PC. Although rare, if an “on call” member is not available, then it is the practice of the BPS to recommend IR for PC placement.
BPS Operation
Daily BPS operation consists of one attending hospitalist, two internal medicine residents, and a third-year medical student. PCs are placed primarily (95%) by the attending on service under ultrasound guidance using the Seldinger technique with lidocaine for anesthetic. For all PC consults, the attending BPS physician reviews the indication prior to placement. If not a direct consult from surgical services, most PC consults are appropriate referrals to the service after the primary medicine service has consulted CT-surgery or p ulmonary consult teams. After review, the primary role of the BPS is assessing safety of PC placement, including whether the patient can tolerate PC placement without procedural sedation. The BPS’s additional standards for safe PC placement are listed in Table 1.
Additionally, it is not routine practice of the BPS to recommend PC placement when consulted for a thoracentesis. The exception to this rule is patients whose PPE sonographic imaging demonstrates loculation or septations. This is consistent with the latest review on pleural disease.13 In addition, the institution’s CT surgery services prefer to initially treat septated PPEs with PCs and fibrinolytic therapy rather than immediate video-assisted thoracoscopic surgery (VATS).
The BPS operates a partnership with CT surgery in which, after successful PC placement, CT surgery manages the PC immediately and until removal including the negative pressure applied and need for fibrinolytic therapy. CT surgery also determines if secondary therapy, commonly second PC or VATS, is required. After PC placement, a portable chest x-ray (CXR) is taken and then BPS follows the patient in person the following day to note any insertion-related complications (IRCs).
In this paper, data on the consults to the BPS for PC placement over a 2-year period are presented. Primary outcomes included numbers of and indications for PCs consulted—attempted or not attempted—consulting services, IRCs, unsuccessful attempts (UAs), and adverse outcomes (AOs). PC duration, fluid drainage, need for fibrinolytic therapy, or need for secondary therapy were not measured because these decisions were managed by the CT surgery service.
PATIENTS AND METHODS
Institutional review board approval of this retrospective study was granted by MCW/Froedtert Hospital Institutional Review Board #5 on January 14, 2019 (MCW IRB #PRO00033496). Adult patients hospitalized at Froedtert Hospital whose primary team determined they would clinically benefit from a PC and consulted the BPS service for placement were included. There were no exclusion criteria.
The authors conducted a retrospective review of two secure BPS databases. The first database is a record of all procedure consults, while the second database contains information about all attempted PCs. Initial review of the BPS’s consult database found 142 PC consults. Consults were classified as “declined” or “attempted.”
RESULTS
Over a 2-year period, the BPS was consulted to place 142 PCs. After resolution of the 3 discrepancies, total consults remained 142, PC attempts totaled 124 (87.3%), and declined consults totaled 18 (12.7%).
The 18 declined consults were not performed for reasons relating to procedural safety. These included 15 (83.3%) for insufficient fluid depth, 1 (5.6%) poor window for PTX, and 1 (5.6%) patient unstable per BPS attending judgement. One (5.6%) final consult had a previous drain in same hemithorax that resumed functioning.
The manual chart review of procedures performed 48 hours after declined PC consults found only 3 of 17 (17.6%) patients received a PC within the subsequent 48 hours. The 18th patient was unable to be followed in our electronic medical record because his medical record number was recorded incorrectly.
The remaining 124 consults were deemed safe for PC placement. Indications for PC placement varied; the most common indications were complicated effusion (36.3%), large or recurrent effusions (21.8%), PTX (17%), and hemothorax (HTX; 17%). The most common teams who consulted the BPS for PC were medicine/hospitalists (42.7%) and CT surgery (40.3%).
Three UAs were charted in the database, but on review it was determined that only 2 (1.6%) qualified as UAs (Table 3). A PC was attempted with the UA patient No. 3 for a loculated apical PTX. It is clear in the procedure note that the pleural space was accessed, air was appropriately drained, and a PC was advanced safely into the pleural space; however, the PC then stopped draining air. CXR interpretation also noted “pneumothorax described on prior exam is less evident.” Because the pleural space was accessed safely and had a partially therapeutic response, we do not count this PC placement as a UA. The PC may count as “failed,” but determination of a “failure rate” is not the intent of this paper. This point is further discussed in the Discussion section.
In addition, chart review demonstrated that UA patient No. 3 required intubation within the 24-hour period after our PC attempt, which is an AO. Approximately 10 hours after our PC was placed and removed, CT surgery placed a second PC, and 3 hours after their PC placement, the patient was intubated with subsequent bronchoscopy. The patient was extubated after only 17 hours. This sequence of events suggests mucus plugging as a more likely cause for respiratory failure than our PC attempt, but we have included it as an AO given the time frame.
Overall, the AO rate was low. Out of 124 attempted PC placements only 3 (2.4%) had an AO, and as noted above, it is believed that 2 of these patients had an AO caused by other medical problems rather than by PC placement.
DISCUSSION
To our knowledge, this is the first report of the experience of procedure-focused hospitalists with PC placement in a partnership with CT surgery. We believe that, at high volume, tertiary care centers similar to Froedtert Hospital, internal medicine–trained, procedure-focused hospitalists can serve as adjuncts to surgery, pulmonary, and IR services in the placement of PCs in hospitalized patients that do not require procedural sedation.
Given the development of this service and the nature of its shared operations with CT surgery, we do not believe that the BPS has an appropriate comparison in the literature; however, the IRCs are similar to previous papers describing PC placement.5-7,14 Notably, the IRC and AO rates were low, both 2.4%, which indicates safe placement of PCs. Kulvatunyou et al and Bauman et al reported on PC placement from a surgical perspective and reported IRC rates of 4%-10%.5-7,14 These higher IRC rates likely have a few reasons. First, Kulvatunyou et al and Bauman et al did not use ultrasound guidance. Use of ultrasound guidance may have significantly lowered their IRC rate. Second, the definition of IRC used by Kulvatunyou et al and Bauman et al included dislodgements, but we do not believe this to be an IRC. Dislodgements can happen for several reasons, frequently a result of patient movement or forgetfulness, not because of improper placement. Third, the PCs with this BPS are placed primarily by attending physicians. Resident roles on our BPS in PC placement are primarily as assistants, whereas Kulvatunyou et al and Bauman et al note that both attendings and residents, under attending supervision, placed PCs; however, it is not clear what percentage of PCs were placed by attendings or residents in their studies. Finally, this BPS’s IRCs are self-reported, so they could be perceived as falsely low, but given the small number of physicians involved in the group and its standardized follow-up, we do not suspect this is truly contributing to the low rates.
Other complication rates regarding the use of wire-guided SBCTs and PCs range from 0% to 42%15-20; however, several differences including tube size, physician training, and PC indication make these studies imperfect comparisons. The most notable difference in our opinion is the variable definition, or lack of definition, of a complication. One study did not define their complications,19 while other studies list subjective measures like pain,16,20 cough,16 bleeding, 16,20 and hematomas4,15 as complications. We believe that the lack of consensus definition for PC complication or IRC contributes to the large range of complication rates in the literature. This problem is likely not unique to PC placement, but is instead true across all bedside procedures. In a shared-practice model between hospitalists and CT surgeons, we believe the definition of IRC in this paper is adequate in capturing most complications. The only complication we are currently unable to track well is infection. We consider other items discussed previously, such as pain, cough (often from lung re-expansion), minor bleeding, and even small hematomas, to be a part of the procedure and not a complication.
Finally, regarding the IRCs and associated death, this was a tragic event. Complications for all of the BPS’s procedures are infrequent (0.35% over the same time period) and reviewed between the BPS director and the attending who performed the procedure; in addition, given this mortality, the case was reviewed immediately in detail with our CT surgery colleagues. On review, it was easy to determine that the operator had found a clear lung tip and sonographic signs of PTX; however, CXR review did demonstrate a medial placement of the PC. This was judged to be a poor placement location (even with imaging demonstrating PTX in that area) given the well-known “triangle of safety” defined by the British Thoracic Society.12
After review, the primary emphasis for PC placement was safe location. The BPS now strives to place PCs for PTX only in the “triangle of safety.” The BPS believe that most PTXs can be addressed with this placement. In the rare case of a PTX requiring an anterior approach, only the BPS director currently places apical PCs for PTX while on service or “on call.” He discusses the placement with pulmonary and CT surgery directly to determine that the PC is of absolute necessity.
Given the focus on appropriate location, no formal changes were made to the procedural imaging practice described in Table 1. We realize that vascular imaging would seem necessary after this patient’s mammary artery laceration; however, safe location, in addition to the BPS’s current image requirements, is believed to minimize this risk. We feel the imaging criteria align with recommendation No. 5 of the Society of Hospital Medicine’s Position Statement for Ultrasound Guidance for Adult Thoracentesis.21 Some BPS members use vascular ultrasound imaging to confirm absence of vascularity, but it is not required and occasionally not possible, such as in the occasional case of PTX with subcutaneous emphysema.
The UA rate is low without a natural comparator in the literature. It is important to clarify the difference between the UAs and the frequently mentioned “failure rate” (FR) in Kulvatunyou et al and Bauman et al6,7,14 We classify UAs as the inability, for any reason, to access the pleural space and insert a PC. At this stage, these UAs appear to reflect the service’s new experience with PC placement and inability to provide procedural sedation. Kulvatunyou et al and Bauman et al’s FR is defined as an initial PC successfully placed into the pleural space that then required a second PC or intervention (frequently VATS) to resolve the PTX or retained HTX.
We believe calculating the failure rate will be helpful in demonstrating the value of our BPS and our shared-practice model. We look forward to publishing this and other future research, including determination of the cost and time saved by the BPS for PCs and other procedures.
Limitations of this study include its retrospective nature, results from a single center’s experience, and lack of a comparison group.
Our institution feels that there is great benefit in having a BPS operated by procedure-focused hospitalists. It would also be important to determine if our model can be replicated by another institution.
Acknowledgments
The authors thank CT surgery for helping to develop this shared-practice model and to both CT surgery and IR physicians here at the Medical College of Wisconsin and Froedtert Hospital who assist us with both IRCs and UAs of pigtail catheters.
The authors also thank Dr. Ricardo Franco-Sadud for his oversight and thoughtful improvements to the paper.
1. Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. https://doi.org/10.1016/j.chest.2018.01.048.
2. Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. https://doi.org/10.1016/j.annemergmed.2013.12.017.
3. Lin YC, Tu CY, Liang SJ, et al. Pigtail catheter for the management of pneumothorax in mechanically ventilated patients. Am J Emerg Med. 2010;28(4):466-471. https://doi.org/10.1016/j.ajem.2009.01.033.
4. Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. https://doi.org/10.1016/j.ajem.2006.04.006.
5. Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. https://doi.org/10.1002/bjs.9377.
6. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg. 2012;73(6):1423-1427. https://doi.org/10.1097/TA.0b013e318271c1c7.
7. Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg. 2018;42(1):107-113. https://doi.org/10.1007/s00268-017-4168-3.
8. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med. 2010;31(6):760-768. https://doi.org/10.1055/s-0030-1269836.
9. Ozkan OS, Ozmen MN, Akhan O. Percutaneous management of parapneumonic effusions. Eur J Radiol. 2005;55(3):311-320. https://doi.org/10.1016/j.ejrad.2005.03.004.
10. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543. https://doi.org/10.1378/chest.09-1044.
11. Saffran L, Ost DE, Fein AM, Schiff MJ. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118(2):417-421. https://doi.org/10.1378/chest.118.2.417.
12. Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. https://doi.org/10.1136/thx.2010.137026.
13. Feller-Kopman D, Light R. Pleural disease. N Engl J Med. 2018;378(8):740-751. https://doi.org/10.1056/NEJMra1403503.
14. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: A changing trend. J Trauma. 2011;71(5):1104-1107; discussion 1107. https://doi.org/10.1097/TA.0b013e31822dd130.
15. Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005;12(1):29-33. https://doi.org/10.1155/2005/498709.
16. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006;130(6):1857-1863. https://doi.org/10.1378/chest.130.6.1857.
17. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA. Radiographically guided percutaneous catheter drainage of pleural fluid collections. Am J Roentgenol. 1988;151(6):1113-1116. https://doi.org/10.2214/ajr.151.6.1113.
18. Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes. Surgery. 2013;154(4):655-660; discussion 660-651. https://doi.org/10.1016/j.surg.2013.04.032.
19. Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999;3(1):57-61.
20. Davies HE, Merchant S, McGown A. A study of the complications of small bore ‘Seldinger’ intercostal chest drains. Respirology. 2008;13(4):603-607. https://doi.org/10.1111/j.1440-1843.2008.01296.x.
21. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
Over the last 15 years, studies have demonstrated the efficacy of small-bore chest tubes (SBCTs), or pigtail catheters (PCs, most commonly ≤14 French), in treating pneumothorax (PTX),1-5 traumatic hemothorax (THTX), hemopneumothorax (HPTX),6,7 parapneumonic effusions (PPEs),8,9 pleural infections,10 and symptomatic malignant pleural effusions.11 A randomized, controlled trial also showed that PC placement resulted in better pain scores, compared with large-bore chest tubes (LBCTs), for traumatic PTX.5 The British Thoracic Society does state that LBCTs may be needed for PTXs with very large air leaks, especially postoperatively. Further, LBCTs may be indicated if small-bore drainage fails, but otherwise they recommend PCs as first-line therapy for PTX, free flowing pleural effusions, and pleural infections.12
BEDSIDE PROCEDURE SERVICE DEVELOPMENT
The Medical College of Wisconsin (MCW) provides hospitalist services to
BPS Pigtail Catheter Training
CT surgery initially trained the BPS director in PC placement using the Seldinger technique in 2015. The director’s training period with CT surgery included direct observation by CT surgery providers for 5 PC placements. Prior to placing PCs, the director had performed approximately 400 ultrasound-guided thoracenteses. The BPS director then independently trained the remaining BPS and has placed or supervised over half of the service’s 124 PCs. Initial credentialing for each BPS physician requires 5 PC placements and 20 thoracenteses under direct supervision of credentialed BPS members. Credentialing is maintained by BPS physicians completing 3 PCs and 15 thoracenteses per year.
Newly credentialed providers are capable of independently placing most PCs. However, the requirements for credentialing are minimal and newly credentialed physicians still encounter PC placements with challenging factors not addressed in their training, such as anterior approach, small effusions, atypical effusion location, mild to moderate coagulopathy, recent therapeutic anticoagulation, and large body habitus. To address these challenges, the BPS has instituted an “on call” system. This system is typically staffed by the BPS director or associate director, already attending on a separate medical service. When needed, the “on call” physician will supervise the newer BPS members to ensure safety while the less experienced physician places the PC. Although rare, if an “on call” member is not available, then it is the practice of the BPS to recommend IR for PC placement.
BPS Operation
Daily BPS operation consists of one attending hospitalist, two internal medicine residents, and a third-year medical student. PCs are placed primarily (95%) by the attending on service under ultrasound guidance using the Seldinger technique with lidocaine for anesthetic. For all PC consults, the attending BPS physician reviews the indication prior to placement. If not a direct consult from surgical services, most PC consults are appropriate referrals to the service after the primary medicine service has consulted CT-surgery or p ulmonary consult teams. After review, the primary role of the BPS is assessing safety of PC placement, including whether the patient can tolerate PC placement without procedural sedation. The BPS’s additional standards for safe PC placement are listed in Table 1.
Additionally, it is not routine practice of the BPS to recommend PC placement when consulted for a thoracentesis. The exception to this rule is patients whose PPE sonographic imaging demonstrates loculation or septations. This is consistent with the latest review on pleural disease.13 In addition, the institution’s CT surgery services prefer to initially treat septated PPEs with PCs and fibrinolytic therapy rather than immediate video-assisted thoracoscopic surgery (VATS).
The BPS operates a partnership with CT surgery in which, after successful PC placement, CT surgery manages the PC immediately and until removal including the negative pressure applied and need for fibrinolytic therapy. CT surgery also determines if secondary therapy, commonly second PC or VATS, is required. After PC placement, a portable chest x-ray (CXR) is taken and then BPS follows the patient in person the following day to note any insertion-related complications (IRCs).
In this paper, data on the consults to the BPS for PC placement over a 2-year period are presented. Primary outcomes included numbers of and indications for PCs consulted—attempted or not attempted—consulting services, IRCs, unsuccessful attempts (UAs), and adverse outcomes (AOs). PC duration, fluid drainage, need for fibrinolytic therapy, or need for secondary therapy were not measured because these decisions were managed by the CT surgery service.
PATIENTS AND METHODS
Institutional review board approval of this retrospective study was granted by MCW/Froedtert Hospital Institutional Review Board #5 on January 14, 2019 (MCW IRB #PRO00033496). Adult patients hospitalized at Froedtert Hospital whose primary team determined they would clinically benefit from a PC and consulted the BPS service for placement were included. There were no exclusion criteria.
The authors conducted a retrospective review of two secure BPS databases. The first database is a record of all procedure consults, while the second database contains information about all attempted PCs. Initial review of the BPS’s consult database found 142 PC consults. Consults were classified as “declined” or “attempted.”
RESULTS
Over a 2-year period, the BPS was consulted to place 142 PCs. After resolution of the 3 discrepancies, total consults remained 142, PC attempts totaled 124 (87.3%), and declined consults totaled 18 (12.7%).
The 18 declined consults were not performed for reasons relating to procedural safety. These included 15 (83.3%) for insufficient fluid depth, 1 (5.6%) poor window for PTX, and 1 (5.6%) patient unstable per BPS attending judgement. One (5.6%) final consult had a previous drain in same hemithorax that resumed functioning.
The manual chart review of procedures performed 48 hours after declined PC consults found only 3 of 17 (17.6%) patients received a PC within the subsequent 48 hours. The 18th patient was unable to be followed in our electronic medical record because his medical record number was recorded incorrectly.
The remaining 124 consults were deemed safe for PC placement. Indications for PC placement varied; the most common indications were complicated effusion (36.3%), large or recurrent effusions (21.8%), PTX (17%), and hemothorax (HTX; 17%). The most common teams who consulted the BPS for PC were medicine/hospitalists (42.7%) and CT surgery (40.3%).
Three UAs were charted in the database, but on review it was determined that only 2 (1.6%) qualified as UAs (Table 3). A PC was attempted with the UA patient No. 3 for a loculated apical PTX. It is clear in the procedure note that the pleural space was accessed, air was appropriately drained, and a PC was advanced safely into the pleural space; however, the PC then stopped draining air. CXR interpretation also noted “pneumothorax described on prior exam is less evident.” Because the pleural space was accessed safely and had a partially therapeutic response, we do not count this PC placement as a UA. The PC may count as “failed,” but determination of a “failure rate” is not the intent of this paper. This point is further discussed in the Discussion section.
In addition, chart review demonstrated that UA patient No. 3 required intubation within the 24-hour period after our PC attempt, which is an AO. Approximately 10 hours after our PC was placed and removed, CT surgery placed a second PC, and 3 hours after their PC placement, the patient was intubated with subsequent bronchoscopy. The patient was extubated after only 17 hours. This sequence of events suggests mucus plugging as a more likely cause for respiratory failure than our PC attempt, but we have included it as an AO given the time frame.
Overall, the AO rate was low. Out of 124 attempted PC placements only 3 (2.4%) had an AO, and as noted above, it is believed that 2 of these patients had an AO caused by other medical problems rather than by PC placement.
DISCUSSION
To our knowledge, this is the first report of the experience of procedure-focused hospitalists with PC placement in a partnership with CT surgery. We believe that, at high volume, tertiary care centers similar to Froedtert Hospital, internal medicine–trained, procedure-focused hospitalists can serve as adjuncts to surgery, pulmonary, and IR services in the placement of PCs in hospitalized patients that do not require procedural sedation.
Given the development of this service and the nature of its shared operations with CT surgery, we do not believe that the BPS has an appropriate comparison in the literature; however, the IRCs are similar to previous papers describing PC placement.5-7,14 Notably, the IRC and AO rates were low, both 2.4%, which indicates safe placement of PCs. Kulvatunyou et al and Bauman et al reported on PC placement from a surgical perspective and reported IRC rates of 4%-10%.5-7,14 These higher IRC rates likely have a few reasons. First, Kulvatunyou et al and Bauman et al did not use ultrasound guidance. Use of ultrasound guidance may have significantly lowered their IRC rate. Second, the definition of IRC used by Kulvatunyou et al and Bauman et al included dislodgements, but we do not believe this to be an IRC. Dislodgements can happen for several reasons, frequently a result of patient movement or forgetfulness, not because of improper placement. Third, the PCs with this BPS are placed primarily by attending physicians. Resident roles on our BPS in PC placement are primarily as assistants, whereas Kulvatunyou et al and Bauman et al note that both attendings and residents, under attending supervision, placed PCs; however, it is not clear what percentage of PCs were placed by attendings or residents in their studies. Finally, this BPS’s IRCs are self-reported, so they could be perceived as falsely low, but given the small number of physicians involved in the group and its standardized follow-up, we do not suspect this is truly contributing to the low rates.
Other complication rates regarding the use of wire-guided SBCTs and PCs range from 0% to 42%15-20; however, several differences including tube size, physician training, and PC indication make these studies imperfect comparisons. The most notable difference in our opinion is the variable definition, or lack of definition, of a complication. One study did not define their complications,19 while other studies list subjective measures like pain,16,20 cough,16 bleeding, 16,20 and hematomas4,15 as complications. We believe that the lack of consensus definition for PC complication or IRC contributes to the large range of complication rates in the literature. This problem is likely not unique to PC placement, but is instead true across all bedside procedures. In a shared-practice model between hospitalists and CT surgeons, we believe the definition of IRC in this paper is adequate in capturing most complications. The only complication we are currently unable to track well is infection. We consider other items discussed previously, such as pain, cough (often from lung re-expansion), minor bleeding, and even small hematomas, to be a part of the procedure and not a complication.
Finally, regarding the IRCs and associated death, this was a tragic event. Complications for all of the BPS’s procedures are infrequent (0.35% over the same time period) and reviewed between the BPS director and the attending who performed the procedure; in addition, given this mortality, the case was reviewed immediately in detail with our CT surgery colleagues. On review, it was easy to determine that the operator had found a clear lung tip and sonographic signs of PTX; however, CXR review did demonstrate a medial placement of the PC. This was judged to be a poor placement location (even with imaging demonstrating PTX in that area) given the well-known “triangle of safety” defined by the British Thoracic Society.12
After review, the primary emphasis for PC placement was safe location. The BPS now strives to place PCs for PTX only in the “triangle of safety.” The BPS believe that most PTXs can be addressed with this placement. In the rare case of a PTX requiring an anterior approach, only the BPS director currently places apical PCs for PTX while on service or “on call.” He discusses the placement with pulmonary and CT surgery directly to determine that the PC is of absolute necessity.
Given the focus on appropriate location, no formal changes were made to the procedural imaging practice described in Table 1. We realize that vascular imaging would seem necessary after this patient’s mammary artery laceration; however, safe location, in addition to the BPS’s current image requirements, is believed to minimize this risk. We feel the imaging criteria align with recommendation No. 5 of the Society of Hospital Medicine’s Position Statement for Ultrasound Guidance for Adult Thoracentesis.21 Some BPS members use vascular ultrasound imaging to confirm absence of vascularity, but it is not required and occasionally not possible, such as in the occasional case of PTX with subcutaneous emphysema.
The UA rate is low without a natural comparator in the literature. It is important to clarify the difference between the UAs and the frequently mentioned “failure rate” (FR) in Kulvatunyou et al and Bauman et al6,7,14 We classify UAs as the inability, for any reason, to access the pleural space and insert a PC. At this stage, these UAs appear to reflect the service’s new experience with PC placement and inability to provide procedural sedation. Kulvatunyou et al and Bauman et al’s FR is defined as an initial PC successfully placed into the pleural space that then required a second PC or intervention (frequently VATS) to resolve the PTX or retained HTX.
We believe calculating the failure rate will be helpful in demonstrating the value of our BPS and our shared-practice model. We look forward to publishing this and other future research, including determination of the cost and time saved by the BPS for PCs and other procedures.
Limitations of this study include its retrospective nature, results from a single center’s experience, and lack of a comparison group.
Our institution feels that there is great benefit in having a BPS operated by procedure-focused hospitalists. It would also be important to determine if our model can be replicated by another institution.
Acknowledgments
The authors thank CT surgery for helping to develop this shared-practice model and to both CT surgery and IR physicians here at the Medical College of Wisconsin and Froedtert Hospital who assist us with both IRCs and UAs of pigtail catheters.
The authors also thank Dr. Ricardo Franco-Sadud for his oversight and thoughtful improvements to the paper.
Over the last 15 years, studies have demonstrated the efficacy of small-bore chest tubes (SBCTs), or pigtail catheters (PCs, most commonly ≤14 French), in treating pneumothorax (PTX),1-5 traumatic hemothorax (THTX), hemopneumothorax (HPTX),6,7 parapneumonic effusions (PPEs),8,9 pleural infections,10 and symptomatic malignant pleural effusions.11 A randomized, controlled trial also showed that PC placement resulted in better pain scores, compared with large-bore chest tubes (LBCTs), for traumatic PTX.5 The British Thoracic Society does state that LBCTs may be needed for PTXs with very large air leaks, especially postoperatively. Further, LBCTs may be indicated if small-bore drainage fails, but otherwise they recommend PCs as first-line therapy for PTX, free flowing pleural effusions, and pleural infections.12
BEDSIDE PROCEDURE SERVICE DEVELOPMENT
The Medical College of Wisconsin (MCW) provides hospitalist services to
BPS Pigtail Catheter Training
CT surgery initially trained the BPS director in PC placement using the Seldinger technique in 2015. The director’s training period with CT surgery included direct observation by CT surgery providers for 5 PC placements. Prior to placing PCs, the director had performed approximately 400 ultrasound-guided thoracenteses. The BPS director then independently trained the remaining BPS and has placed or supervised over half of the service’s 124 PCs. Initial credentialing for each BPS physician requires 5 PC placements and 20 thoracenteses under direct supervision of credentialed BPS members. Credentialing is maintained by BPS physicians completing 3 PCs and 15 thoracenteses per year.
Newly credentialed providers are capable of independently placing most PCs. However, the requirements for credentialing are minimal and newly credentialed physicians still encounter PC placements with challenging factors not addressed in their training, such as anterior approach, small effusions, atypical effusion location, mild to moderate coagulopathy, recent therapeutic anticoagulation, and large body habitus. To address these challenges, the BPS has instituted an “on call” system. This system is typically staffed by the BPS director or associate director, already attending on a separate medical service. When needed, the “on call” physician will supervise the newer BPS members to ensure safety while the less experienced physician places the PC. Although rare, if an “on call” member is not available, then it is the practice of the BPS to recommend IR for PC placement.
BPS Operation
Daily BPS operation consists of one attending hospitalist, two internal medicine residents, and a third-year medical student. PCs are placed primarily (95%) by the attending on service under ultrasound guidance using the Seldinger technique with lidocaine for anesthetic. For all PC consults, the attending BPS physician reviews the indication prior to placement. If not a direct consult from surgical services, most PC consults are appropriate referrals to the service after the primary medicine service has consulted CT-surgery or p ulmonary consult teams. After review, the primary role of the BPS is assessing safety of PC placement, including whether the patient can tolerate PC placement without procedural sedation. The BPS’s additional standards for safe PC placement are listed in Table 1.
Additionally, it is not routine practice of the BPS to recommend PC placement when consulted for a thoracentesis. The exception to this rule is patients whose PPE sonographic imaging demonstrates loculation or septations. This is consistent with the latest review on pleural disease.13 In addition, the institution’s CT surgery services prefer to initially treat septated PPEs with PCs and fibrinolytic therapy rather than immediate video-assisted thoracoscopic surgery (VATS).
The BPS operates a partnership with CT surgery in which, after successful PC placement, CT surgery manages the PC immediately and until removal including the negative pressure applied and need for fibrinolytic therapy. CT surgery also determines if secondary therapy, commonly second PC or VATS, is required. After PC placement, a portable chest x-ray (CXR) is taken and then BPS follows the patient in person the following day to note any insertion-related complications (IRCs).
In this paper, data on the consults to the BPS for PC placement over a 2-year period are presented. Primary outcomes included numbers of and indications for PCs consulted—attempted or not attempted—consulting services, IRCs, unsuccessful attempts (UAs), and adverse outcomes (AOs). PC duration, fluid drainage, need for fibrinolytic therapy, or need for secondary therapy were not measured because these decisions were managed by the CT surgery service.
PATIENTS AND METHODS
Institutional review board approval of this retrospective study was granted by MCW/Froedtert Hospital Institutional Review Board #5 on January 14, 2019 (MCW IRB #PRO00033496). Adult patients hospitalized at Froedtert Hospital whose primary team determined they would clinically benefit from a PC and consulted the BPS service for placement were included. There were no exclusion criteria.
The authors conducted a retrospective review of two secure BPS databases. The first database is a record of all procedure consults, while the second database contains information about all attempted PCs. Initial review of the BPS’s consult database found 142 PC consults. Consults were classified as “declined” or “attempted.”
RESULTS
Over a 2-year period, the BPS was consulted to place 142 PCs. After resolution of the 3 discrepancies, total consults remained 142, PC attempts totaled 124 (87.3%), and declined consults totaled 18 (12.7%).
The 18 declined consults were not performed for reasons relating to procedural safety. These included 15 (83.3%) for insufficient fluid depth, 1 (5.6%) poor window for PTX, and 1 (5.6%) patient unstable per BPS attending judgement. One (5.6%) final consult had a previous drain in same hemithorax that resumed functioning.
The manual chart review of procedures performed 48 hours after declined PC consults found only 3 of 17 (17.6%) patients received a PC within the subsequent 48 hours. The 18th patient was unable to be followed in our electronic medical record because his medical record number was recorded incorrectly.
The remaining 124 consults were deemed safe for PC placement. Indications for PC placement varied; the most common indications were complicated effusion (36.3%), large or recurrent effusions (21.8%), PTX (17%), and hemothorax (HTX; 17%). The most common teams who consulted the BPS for PC were medicine/hospitalists (42.7%) and CT surgery (40.3%).
Three UAs were charted in the database, but on review it was determined that only 2 (1.6%) qualified as UAs (Table 3). A PC was attempted with the UA patient No. 3 for a loculated apical PTX. It is clear in the procedure note that the pleural space was accessed, air was appropriately drained, and a PC was advanced safely into the pleural space; however, the PC then stopped draining air. CXR interpretation also noted “pneumothorax described on prior exam is less evident.” Because the pleural space was accessed safely and had a partially therapeutic response, we do not count this PC placement as a UA. The PC may count as “failed,” but determination of a “failure rate” is not the intent of this paper. This point is further discussed in the Discussion section.
In addition, chart review demonstrated that UA patient No. 3 required intubation within the 24-hour period after our PC attempt, which is an AO. Approximately 10 hours after our PC was placed and removed, CT surgery placed a second PC, and 3 hours after their PC placement, the patient was intubated with subsequent bronchoscopy. The patient was extubated after only 17 hours. This sequence of events suggests mucus plugging as a more likely cause for respiratory failure than our PC attempt, but we have included it as an AO given the time frame.
Overall, the AO rate was low. Out of 124 attempted PC placements only 3 (2.4%) had an AO, and as noted above, it is believed that 2 of these patients had an AO caused by other medical problems rather than by PC placement.
DISCUSSION
To our knowledge, this is the first report of the experience of procedure-focused hospitalists with PC placement in a partnership with CT surgery. We believe that, at high volume, tertiary care centers similar to Froedtert Hospital, internal medicine–trained, procedure-focused hospitalists can serve as adjuncts to surgery, pulmonary, and IR services in the placement of PCs in hospitalized patients that do not require procedural sedation.
Given the development of this service and the nature of its shared operations with CT surgery, we do not believe that the BPS has an appropriate comparison in the literature; however, the IRCs are similar to previous papers describing PC placement.5-7,14 Notably, the IRC and AO rates were low, both 2.4%, which indicates safe placement of PCs. Kulvatunyou et al and Bauman et al reported on PC placement from a surgical perspective and reported IRC rates of 4%-10%.5-7,14 These higher IRC rates likely have a few reasons. First, Kulvatunyou et al and Bauman et al did not use ultrasound guidance. Use of ultrasound guidance may have significantly lowered their IRC rate. Second, the definition of IRC used by Kulvatunyou et al and Bauman et al included dislodgements, but we do not believe this to be an IRC. Dislodgements can happen for several reasons, frequently a result of patient movement or forgetfulness, not because of improper placement. Third, the PCs with this BPS are placed primarily by attending physicians. Resident roles on our BPS in PC placement are primarily as assistants, whereas Kulvatunyou et al and Bauman et al note that both attendings and residents, under attending supervision, placed PCs; however, it is not clear what percentage of PCs were placed by attendings or residents in their studies. Finally, this BPS’s IRCs are self-reported, so they could be perceived as falsely low, but given the small number of physicians involved in the group and its standardized follow-up, we do not suspect this is truly contributing to the low rates.
Other complication rates regarding the use of wire-guided SBCTs and PCs range from 0% to 42%15-20; however, several differences including tube size, physician training, and PC indication make these studies imperfect comparisons. The most notable difference in our opinion is the variable definition, or lack of definition, of a complication. One study did not define their complications,19 while other studies list subjective measures like pain,16,20 cough,16 bleeding, 16,20 and hematomas4,15 as complications. We believe that the lack of consensus definition for PC complication or IRC contributes to the large range of complication rates in the literature. This problem is likely not unique to PC placement, but is instead true across all bedside procedures. In a shared-practice model between hospitalists and CT surgeons, we believe the definition of IRC in this paper is adequate in capturing most complications. The only complication we are currently unable to track well is infection. We consider other items discussed previously, such as pain, cough (often from lung re-expansion), minor bleeding, and even small hematomas, to be a part of the procedure and not a complication.
Finally, regarding the IRCs and associated death, this was a tragic event. Complications for all of the BPS’s procedures are infrequent (0.35% over the same time period) and reviewed between the BPS director and the attending who performed the procedure; in addition, given this mortality, the case was reviewed immediately in detail with our CT surgery colleagues. On review, it was easy to determine that the operator had found a clear lung tip and sonographic signs of PTX; however, CXR review did demonstrate a medial placement of the PC. This was judged to be a poor placement location (even with imaging demonstrating PTX in that area) given the well-known “triangle of safety” defined by the British Thoracic Society.12
After review, the primary emphasis for PC placement was safe location. The BPS now strives to place PCs for PTX only in the “triangle of safety.” The BPS believe that most PTXs can be addressed with this placement. In the rare case of a PTX requiring an anterior approach, only the BPS director currently places apical PCs for PTX while on service or “on call.” He discusses the placement with pulmonary and CT surgery directly to determine that the PC is of absolute necessity.
Given the focus on appropriate location, no formal changes were made to the procedural imaging practice described in Table 1. We realize that vascular imaging would seem necessary after this patient’s mammary artery laceration; however, safe location, in addition to the BPS’s current image requirements, is believed to minimize this risk. We feel the imaging criteria align with recommendation No. 5 of the Society of Hospital Medicine’s Position Statement for Ultrasound Guidance for Adult Thoracentesis.21 Some BPS members use vascular ultrasound imaging to confirm absence of vascularity, but it is not required and occasionally not possible, such as in the occasional case of PTX with subcutaneous emphysema.
The UA rate is low without a natural comparator in the literature. It is important to clarify the difference between the UAs and the frequently mentioned “failure rate” (FR) in Kulvatunyou et al and Bauman et al6,7,14 We classify UAs as the inability, for any reason, to access the pleural space and insert a PC. At this stage, these UAs appear to reflect the service’s new experience with PC placement and inability to provide procedural sedation. Kulvatunyou et al and Bauman et al’s FR is defined as an initial PC successfully placed into the pleural space that then required a second PC or intervention (frequently VATS) to resolve the PTX or retained HTX.
We believe calculating the failure rate will be helpful in demonstrating the value of our BPS and our shared-practice model. We look forward to publishing this and other future research, including determination of the cost and time saved by the BPS for PCs and other procedures.
Limitations of this study include its retrospective nature, results from a single center’s experience, and lack of a comparison group.
Our institution feels that there is great benefit in having a BPS operated by procedure-focused hospitalists. It would also be important to determine if our model can be replicated by another institution.
Acknowledgments
The authors thank CT surgery for helping to develop this shared-practice model and to both CT surgery and IR physicians here at the Medical College of Wisconsin and Froedtert Hospital who assist us with both IRCs and UAs of pigtail catheters.
The authors also thank Dr. Ricardo Franco-Sadud for his oversight and thoughtful improvements to the paper.
1. Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. https://doi.org/10.1016/j.chest.2018.01.048.
2. Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. https://doi.org/10.1016/j.annemergmed.2013.12.017.
3. Lin YC, Tu CY, Liang SJ, et al. Pigtail catheter for the management of pneumothorax in mechanically ventilated patients. Am J Emerg Med. 2010;28(4):466-471. https://doi.org/10.1016/j.ajem.2009.01.033.
4. Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. https://doi.org/10.1016/j.ajem.2006.04.006.
5. Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. https://doi.org/10.1002/bjs.9377.
6. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg. 2012;73(6):1423-1427. https://doi.org/10.1097/TA.0b013e318271c1c7.
7. Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg. 2018;42(1):107-113. https://doi.org/10.1007/s00268-017-4168-3.
8. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med. 2010;31(6):760-768. https://doi.org/10.1055/s-0030-1269836.
9. Ozkan OS, Ozmen MN, Akhan O. Percutaneous management of parapneumonic effusions. Eur J Radiol. 2005;55(3):311-320. https://doi.org/10.1016/j.ejrad.2005.03.004.
10. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543. https://doi.org/10.1378/chest.09-1044.
11. Saffran L, Ost DE, Fein AM, Schiff MJ. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118(2):417-421. https://doi.org/10.1378/chest.118.2.417.
12. Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. https://doi.org/10.1136/thx.2010.137026.
13. Feller-Kopman D, Light R. Pleural disease. N Engl J Med. 2018;378(8):740-751. https://doi.org/10.1056/NEJMra1403503.
14. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: A changing trend. J Trauma. 2011;71(5):1104-1107; discussion 1107. https://doi.org/10.1097/TA.0b013e31822dd130.
15. Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005;12(1):29-33. https://doi.org/10.1155/2005/498709.
16. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006;130(6):1857-1863. https://doi.org/10.1378/chest.130.6.1857.
17. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA. Radiographically guided percutaneous catheter drainage of pleural fluid collections. Am J Roentgenol. 1988;151(6):1113-1116. https://doi.org/10.2214/ajr.151.6.1113.
18. Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes. Surgery. 2013;154(4):655-660; discussion 660-651. https://doi.org/10.1016/j.surg.2013.04.032.
19. Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999;3(1):57-61.
20. Davies HE, Merchant S, McGown A. A study of the complications of small bore ‘Seldinger’ intercostal chest drains. Respirology. 2008;13(4):603-607. https://doi.org/10.1111/j.1440-1843.2008.01296.x.
21. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
1. Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. https://doi.org/10.1016/j.chest.2018.01.048.
2. Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. https://doi.org/10.1016/j.annemergmed.2013.12.017.
3. Lin YC, Tu CY, Liang SJ, et al. Pigtail catheter for the management of pneumothorax in mechanically ventilated patients. Am J Emerg Med. 2010;28(4):466-471. https://doi.org/10.1016/j.ajem.2009.01.033.
4. Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. https://doi.org/10.1016/j.ajem.2006.04.006.
5. Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. https://doi.org/10.1002/bjs.9377.
6. Kulvatunyou N, Joseph B, Friese RS, et al. 14 French pigtail catheters placed by surgeons to drain blood on trauma patients: is 14-Fr too small? J Trauma Acute Care Surg. 2012;73(6):1423-1427. https://doi.org/10.1097/TA.0b013e318271c1c7.
7. Bauman ZM, Kulvatunyou N, Joseph B, et al. A Prospective Study of 7-Year Experience Using Percutaneous 14-French Pigtail Catheters for Traumatic Hemothorax/Hemopneumothorax at a Level-1 Trauma Center: Size Still Does Not Matter. World J Surg. 2018;42(1):107-113. https://doi.org/10.1007/s00268-017-4168-3.
8. Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med. 2010;31(6):760-768. https://doi.org/10.1055/s-0030-1269836.
9. Ozkan OS, Ozmen MN, Akhan O. Percutaneous management of parapneumonic effusions. Eur J Radiol. 2005;55(3):311-320. https://doi.org/10.1016/j.ejrad.2005.03.004.
10. Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010;137(3):536-543. https://doi.org/10.1378/chest.09-1044.
11. Saffran L, Ost DE, Fein AM, Schiff MJ. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118(2):417-421. https://doi.org/10.1378/chest.118.2.417.
12. Havelock T, Teoh R, Laws D, Gleeson F, Group BPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. https://doi.org/10.1136/thx.2010.137026.
13. Feller-Kopman D, Light R. Pleural disease. N Engl J Med. 2018;378(8):740-751. https://doi.org/10.1056/NEJMra1403503.
14. Kulvatunyou N, Vijayasekaran A, Hansen A, et al. Two-year experience of using pigtail catheters to treat traumatic pneumothorax: A changing trend. J Trauma. 2011;71(5):1104-1107; discussion 1107. https://doi.org/10.1097/TA.0b013e31822dd130.
15. Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J. 2005;12(1):29-33. https://doi.org/10.1155/2005/498709.
16. Horsley A, Jones L, White J, Henry M. Efficacy and complications of small-bore, wire-guided chest drains. Chest. 2006;130(6):1857-1863. https://doi.org/10.1378/chest.130.6.1857.
17. Merriam MA, Cronan JJ, Dorfman GS, Lambiase RE, Haas RA. Radiographically guided percutaneous catheter drainage of pleural fluid collections. Am J Roentgenol. 1988;151(6):1113-1116. https://doi.org/10.2214/ajr.151.6.1113.
18. Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes. Surgery. 2013;154(4):655-660; discussion 660-651. https://doi.org/10.1016/j.surg.2013.04.032.
19. Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS. 1999;3(1):57-61.
20. Davies HE, Merchant S, McGown A. A study of the complications of small bore ‘Seldinger’ intercostal chest drains. Respirology. 2008;13(4):603-607. https://doi.org/10.1111/j.1440-1843.2008.01296.x.
21. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
© 2020 Society of Hospital Medicine
A Qualitative Study of Increased Pediatric Reutilization After a Postdischarge Home Nurse Visit
Readmission rates are used as metrics for care quality and reimbursement, with penalties applied to hospitals with higher than expected rates1 and up to 30% of pediatric readmissions deemed potentially preventable.2 There is a paucity of information on how to prevent pediatric readmissions,3 yet pediatric hospitals are tasked with implementing interventions for readmission reduction.
The Hospital to Home Outcomes (H2O) trial was a 2-arm, randomized controlled trial in which patients discharged from hospital medicine and neuroscience services at a single institution were randomized to receive a single home visit from a registered nurse (RN) within 96 hours of discharge.4 RNs completed a structured nurse visit designed specifically for the trial. Lists of “red flags” or warning signs associated with common diagnoses were provided to assist RNs in standardizing education about when to seek additional care. The hypothesis was that the postdischarge visits would result in lower reutilization rates (unplanned readmissions, emergency department [ED] visits, and urgent care visits).5
Unexpectedly, children randomized to receive the postdischarge nurse visit had higher rates of 30-day unplanned healthcare reutilization, with children randomly assigned to the intervention demonstrating higher odds of 30-day healthcare use (OR 1.33; 95% CI 1.003-1.76).4 We sought to understand perspectives on these unanticipated findings by obtaining input from relevant stakeholders. There were 2 goals for the qualitative analysis: first, to understand possible explanations of the increased reutilization finding; second, to elicit suggestions for improving the nurse visit intervention.
METHODS
We selected an in-depth qualitative approach, using interviews and focus groups to explore underlying explanations for the increase in 30-day unplanned healthcare reutilization among those randomized to receive the postdischarge nurse visit during the H2O trial.4 Input was sought from 4 stakeholder groups—parents, primary care physicians (PCPs), hospital medicine physicians, and home care RNs—in an effort to triangulate data sources and elicit rich and diverse opinions. Approval was obtained from the Institutional Review Board prior to conducting the study.
Recruitment
Parents
Because we conducted interviews approximately 1 year after the trial’s conclusion, we purposefully selected families who were enrolled in the latter portion of the H2O trial in order to enhance recall. Beginning with the last families in the study, we sequentially contacted families in reverse order. We contacted 10 families in each of 4 categories (intervention/reutilization, intervention/no reutilization, control/reutilization, control/no reutilization). A total of 3 attempts were made by telephone to contact each family. Participants received a grocery store gift card for participating in the study.
Primary Care Physicians
We conducted focus groups with a purposive sample of physicians recruited from 2 community practices and 1 hospital-owned practice.
Hospital Medicine Physicians
We conducted focus groups with a purposive sample of physicians from our Division of Hospital Medicine. There was a varying level of knowledge of the original trial; however, none of the participants were collaborators in the trial.
Home Care RNs
We conducted focus groups with a subset of RNs who were involved with trial visits. All RNs were members of the pediatric home care division associated with the hospital with specific training in caring for patients at home.
Data Collection
The study team designed question guides for each stakeholder group (Appendix 1). While questions were tailored for specific stakeholders, all guides included the following topics: benefits and challenges of nurse visits, suggestions for improving the intervention in future trials, and reactions to the trial results (once presented to participants). Only the results of the intention-to-treat (ITT) analysis were shared with stakeholders because ITT is considered the gold standard for trial analysis and allows easy understanding of the results.
A single investigator (A.L.) conducted parental interviews by telephone. Focus groups for PCPs, hospital medicine physicians, and RN groups were held at practice locations in private conference rooms and were conducted by trained moderators (S.N.S., A.L., and H.T.C.). Moderators probed responses to the open-ended questions to delve deeply into issues. The question guides were modified in an iterative fashion to include new concepts raised during interviews or focus groups. All interviews and focus groups were recorded and transcribed verbatim with all identifiable information redacted.
Data Analysis
During multiple cycles of inductive thematic analysis,6 we examined, discussed, interpreted, and organized responses to the open-ended questions,6,7 analyzing each stakeholder group separately. First, transcripts were shared with and reviewed by the entire multidisciplinary team (12 members) which included hospital medicine physicians, PCPs, home care nursing leaders, a nurse scientist, a parent representative, research coordinators, and a qualitative research methodologist. Second, team members convened to discuss overall concepts and ideas and created the preliminary coding frameworks. Third, a smaller subgroup (research coordinator [A.L]., hospital medicine physician [S.R.], parent representative [M.M.], and qualitative research methodologist [S.N.S.]), refined the unique coding framework for each stakeholder group and then independently applied codes to participant comments. This subgroup met regularly to reach consensus about the assigned codes and to further refine the codebooks. The codes were organized into major and minor themes based on recurring patterns in the data and the salience or emphasis given by participants. The subgroup’s work was reviewed and discussed on an ongoing basis by the entire multidisciplinary team. Triangulation of the data was achieved in multiple ways. The preliminary results were shared in several forums, and feedback was solicited and incorporated. Two of 4 members of the subgroup analytic team were not part of the trial planning or data collection, providing a potentially broader perspective. All coding decisions were maintained in an electronic database, and an audit trail was created to document codebook revisions.
RESULTS
A total of 33 parents participated in the interviews (intervention/readmit [8], intervention/no readmit [8], control/readmit [8], and control/no readmit [9]). Although we selected families from all 4 categories, we were not able to explore qualitative differences between these groups because of the relatively low numbers of participants. Parent data was very limited as interviews were brief and “control” parents had not received the intervention. Three focus groups were held with PCPs (7 participants in total), 2 focus groups were held with hospital medicine physicians (12 participants), and 2 focus groups were held with RNs (10 participants).
Goal 1: Explanation of Reutilization Rates
During interviews and focus groups, the results of the H2O trial were discussed, and stakeholders were asked to comment on potential explanations of the findings. 4 major themes and 5 minor themes emerged from analysis of the transcripts (summarized in Table 1).
Theme 1: Appropriateness of Patient Reutilization
Hospital medicine physicians and home care RNs questioned whether the reutilization events were clinically indicated. RNs wondered whether children who reutilized the ED were also readmitted to the hospital; many perceived that if the child was ill enough to be readmitted, then the ED revisit was warranted (Table 2). Parents commented on parental decision-making and changes in clinical status of the child leading to reutilization (Table 2).
Theme 2: Impact of Red Flags/Warning Sign Instructions on Family’s Reutilization Decisions
Theme 3: Hospital-Affiliated RNs “Directing Traffic” Back to Hospital
Both physician groups were concerned that, because the study was conducted by hospital-employed nurses, families might have been more likely to reaccess care at the hospital. Thus, the connection with the hospital was strengthened in the H2O model, potentially at the expense of the connection with PCPs. Physicians hypothesized that families might “still feel part of the medical system,” so families would return to the hospital if there was a problem. PCPs emphasized that there may have been straightforward situations that could have been handled appropriately in the outpatient office (Table 2).
Theme 4: Home Visit RNs Had a Low Threshold for Escalating Care
Parents and PCPs hypothesized that RNs are more conservative and, therefore, would have had a low threshold to refer back to the hospital if there were concerns in the home. One parent commented: “I guess, nurses are just by trade accustomed to erring on the side of caution and medical intervention instead of letting time take its course. … They’re more apt to say it’s better off to go to the hospital and have everything be fine” (Table 2).
Minor Themes
Participants also explained reutilization in ways that coalesced into 5 minor themes: (1) families receiving a visit might perceive that their child was sicker; (2) patients in the control group did not reutilize enough; (3) receiving more education on a child’s illness drives reutilization; (4) provider access issues; and (5) variability of RN experience may determine whether escalated care. Supportive quotations found in Appendix 2.
We directly asked parents if they would want a nurse home visit in the future after discussing the results of the study. Almost all of the parents in the intervention group and most of the parents in the control group were in favor of receiving a visit, even knowing that patients who had received a visit were more likely to reutilize care.
Goal 2: Suggestions for Improving Intervention Design
Three major themes and 3 minor themes were related to improving the design of the intervention (Table 1).
Theme 1: Need for Improved Postdischarge Communication
All stakeholder groups highlighted postdischarge communication as an area that could be improved. Parents were frustrated with regard to attempts to connect with inpatient physicians after discharge. PCPs suggested developing pathways for the RN to connect with the primary care office as opposed to the hospital. Hospital medicine physicians discussed a lack of consensus regarding patient ownership following discharge and were uncertain about what types of postdischarge symptoms PCPs would be comfortable managing. RNs described specific situations when they had difficulty contacting a physician to escalate care (Table 3).
Theme 2: Individualizing Home Visits—One Size Does Not Fit All
All stakeholder groups also encouraged “individualization” of home visits according to patient and family characteristics, diagnosis, and both timing and severity of illness. PCPs recommended visits only for certain diagnoses. Hospital medicine physicians voiced similar sentiments as the PCPs and added that worrisome family dynamics during a hospitalization, such as a lack of engagement with the medical team, might also warrant a visit. RNs suggested visits for those families with more concerns, for example, those with young children or children recovering from an acute respiratory illness (Table 3).
Theme 3: Providing Context for and Framing of Red Flags
Physicians and nurses suggested providing more context to “red flag” instructions and education. RNs emphasized that some families seemed to benefit from the opportunity to discuss their postdischarge concerns with a medical professional. Others appreciated concrete written instructions that spelled out how to respond in certain situations (Table 3).
Minor Themes
Three minor themes were revealed regarding intervention design improvement (Table 1): (1) streamlining the discharge process; (2) improving the definition of the scope and goal of intervention; and (3) extending inpatient team expertise post discharge. Supportive quotations can be found in Appendix 3.
DISCUSSION
When stakeholders were asked about why postdischarge RN visits led to increased postdischarge urgent healthcare visits, they questioned the appropriateness of the reutilization events, wondered about the lack of context for the warning signs that nurses provided families as part of the intervention, worried that families were encouraged to return to the hospital because of the ties of the trial to the hospital, and suggested that RNs had a low threshold to refer patients back to the hospital. When asked about how to design an improved nurse visit to better support families, stakeholders emphasized improving communication, individualizing the visit, and providing context around the red-flag discussion, enabling more nuanced instructions about how to respond to specific events.
A synthesis of themes suggests that potential drivers for increased utilization rates may lie in the design and goals of the initial project. The intervention was designed to support families and enhance education after discharge, with components derived from pretrial focus groups with families after a hospital discharge.8 The intervention was not designed to divert patients from the ED nor did it enhance access to the PCP. A second trial of the intervention adapted to a phone call also failed to decrease reutilization rates.9 Both physician stakeholder groups perceived that the intervention directed traffic back to the hospital because of the intervention design. Coupled with the perception that the red flags may have changed a family’s threshold for seeking care and/or that an RN may be more apt to refer back to care, this failure to push utilization to the primary care office may explain the unexpected trial results. Despite the stakeholders’ perception of enhanced connection back to the hospital as a result of the nurse visit, in analysis of visit referral patterns, a referral was made directly back to the ED in only 4 of the 651 trial visits (Tubbs-Cooley H, Riddle SR, Gold JM, et al.; under review. Pediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period 2020).
Both H2O trials demonstrated improved recall of red flags by parents who received the intervention, which may be important given the stakeholders’ perspectives that the red flags may not have been contextualized well enough. Yet neither trial demonstrated any differences in postdischarge coping or time to return to normal routine. In interviews with parents, despite the clearly stated results of increased reutilization, intervention parents endorsed a desire for a home visit in the future, raising the possibility that our outcome measures did not capture parents’ priorities adequately.
When asked to recommend design improvements of the intervention, 2 major themes (improvement in communication and individualization of visits) were discussed by all stakeholder groups, providing actionable information to modify or create new interventions. Focus groups with clinicians suggested that communication challenges may have influenced reutilization likelihood during the postdischarge period. RNs expressed uncertainty about who to call with problems or questions at the time of a home visit. This was compounded by difficulty reaching physicians. Both hospital medicine physicians and PCPs identified system challenges including questions of patient ownership, variable PCP practice communication preferences, and difficulty in identifying a partnered staff member (on either end of the inpatient-outpatient continuum) who was familiar with a specific patient. While the communication issues raised may reflect difficulties in our local healthcare system, there is broad evidence of postdischarge communication challenges. In adults, postdischarge communication failures between home health staff and physicians are associated with an increased risk of readmission.10 The real or perceived lack of communication between inpatient and outpatient providers can add to parental confusion post discharge.11 Although there have been efforts to improve the reliability of communication across this gulf,12,13 it is not clear whether changes to discharge communication could help to avoid pediatric reutilization events.14
The theme of individualization of the home nurse visit is consistent with evidence regarding the impact of focusing the intervention on patients with specific diagnoses or demographics. In adults, reduced reutilization associated with postdischarge home nurse visits has been described in specific populations such as patients with heart failure and chronic obstructive pulmonary disease.15 Impact of home nurse visits on patients within diagnosis-specific populations with certain demographics (such as advanced age) has also been described.16 In the pediatric population, readmission rates vary widely by diagnosis.17 A systematic review of interventions to reduce pediatric readmissions found increased impact of discharge interventions in specific populations (asthma, oncology, and NICU).3
Next steps may lie in interventions in targeted populations that function as part of a care continuum bridging the patient from the inpatient to the outpatient setting. A home nurse visit as part of this discharge structure may prove to have more impact on reducing reutilization. One population which accounts for a large proportion of readmissions and where there has been recent focus on discharge transition of care has been children with medical complexity.18 This group was largely excluded from the H2O trial. Postdischarge home nurse visits in this population have been found to be feasible and address many questions and problems, but the effect on readmission is less clear.19 Family priorities and preferences related to preparation for discharge, including family engagement, respect for discharge readiness, and goal of returning to normal routines, may be areas on which to focus with future interventions in this population.20 In summary, although widespread postdischarge interventions (home nurse visit4 and nurse telephone call9) have not been found to be effective, targeting interventions to specific populations by diagnosis or demographic factors may prove to be more effective in reducing pediatric reutilization.
There were several strengths to this study. This qualitative approach allowed us to elucidate potential explanations for the H2O trial results from multiple perspectives. The multidisciplinary composition of our analytic team and the use of an iterative process sparked diverse contributions in a dynamic, ongoing discussion and interpretation of our data.
This study should be considered in the context of several limitations. For families and RNs, there was a time lag between participation in the trial and participation in the qualitative study call or focus group which could lead to difficulty recalling details. Only families who received the intervention could give opinions on their experience of the nurse visit, while families in the control group were asked to hypothesize. Focus groups with hospital medicine physicians and PCPs were purposive samples, and complete demographic information of participants was not collected.
CONCLUSION
Key stakeholders reflecting on a postdischarge RN visit trial suggested multiple potential explanations for the unexpected increase in reutilization in children randomized to the intervention. Certain participants questioned whether all reutilization events were appropriate or necessary. Others expressed concerns that the H2O intervention lacked context and directed children back to the hospital instead of the PCP. Parents, PCPs, hospital medicine physicians, and RNs all suggested that future transition-focused interventions should enhance postdischarge communication, strengthen connection to the PCP, and be more effectively tailored to the needs of the individual patient and family.
Acknowledgments
Collaborators: H2O Trial Study Group: Joanne Bachus, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Monica L Borell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lenisa V Chang, MA, PhD; Patricia Crawford, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sarah A Ferris, BA, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Judy A Heilman BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Jane C Khoury, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen Lawley, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lynne O’Donnell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Hadley S Sauers-Ford, MPH, Department of Pediatrics, UC Davis Health, Sacramento, California; Anita N Shah, DO, MPH, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lauren G Solan, MD, Med, University of Rochester, Rochester, New York; Heidi J Sucharew, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen P Sullivan, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Christine M White, MD, MAT, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
1. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a Children’s Hospital. Pediatrics. 2016;138(2). https://doi.org/10.1542/peds.2015-4182.
3. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
4. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1). https://doi.org/10.1542/peds.2017-3919.
5. Tubbs-Cooley HL, Pickler RH, Simmons JM, et al. Testing a post-discharge nurse-led transitional home visit in acute care pediatrics: the Hospital-To-Home Outcomes (H2O) study protocol. J Adv Nurs. 2016;72(4):915-925. https://doi.org/10.1111/jan.12882.
6. Guest G. Collecting Qualitative Data: A Field Manual for Applied Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2013.
7. Patton M. Qualitative Research and Evaluation Methods. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
8. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on Hospital to Home Transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
9. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
10. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse-physician communication, patient severity, and hospital readmission. Health Serv Res. 2018;53(2):1008-1024. https://doi.org/10.1111/1475-6773.12667.
11. Solan LG, Beck AF, Shardo SA, et al. Caregiver perspectives on communication during hospitalization at an academic pediatric institution: a qualitative study. J Hosp Med. 2018;13(5):304-311. https://doi.org/10.12788/jhm.2919.
12. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119.
13. Mussman GM, Vossmeyer MT, Brady PW, et al. Improving the reliability of verbal communication between primary care physicians and pediatric hospitalists at hospital discharge. J Hosp Med. 2015;10(9):574-580. https://doi.org/10.1002/jhm.2392.
14. Coller RJ, Klitzner TS, Saenz AA, et al. Discharge handoff communication and pediatric readmissions. J Hosp Med. 2017;12(1):29-35. https://doi.org/10.1002/jhm.2670.
15. Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2017;14(2):251-261. https://doi.org/10.1080/15412555.2016.1256384.
16. Finlayson K, Chang AM, Courtney MD, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. https://doi.org/10.1186/s12913-018-3771-9.
17. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
18. Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628-e1647. https://doi.org/10.1542/peds.2014-1956.
19. Wells S, O’Neill M, Rogers J, et al. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10-16. https://doi.org/10.1016/j.pedn.2017.03.003.
20. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-1581.
Readmission rates are used as metrics for care quality and reimbursement, with penalties applied to hospitals with higher than expected rates1 and up to 30% of pediatric readmissions deemed potentially preventable.2 There is a paucity of information on how to prevent pediatric readmissions,3 yet pediatric hospitals are tasked with implementing interventions for readmission reduction.
The Hospital to Home Outcomes (H2O) trial was a 2-arm, randomized controlled trial in which patients discharged from hospital medicine and neuroscience services at a single institution were randomized to receive a single home visit from a registered nurse (RN) within 96 hours of discharge.4 RNs completed a structured nurse visit designed specifically for the trial. Lists of “red flags” or warning signs associated with common diagnoses were provided to assist RNs in standardizing education about when to seek additional care. The hypothesis was that the postdischarge visits would result in lower reutilization rates (unplanned readmissions, emergency department [ED] visits, and urgent care visits).5
Unexpectedly, children randomized to receive the postdischarge nurse visit had higher rates of 30-day unplanned healthcare reutilization, with children randomly assigned to the intervention demonstrating higher odds of 30-day healthcare use (OR 1.33; 95% CI 1.003-1.76).4 We sought to understand perspectives on these unanticipated findings by obtaining input from relevant stakeholders. There were 2 goals for the qualitative analysis: first, to understand possible explanations of the increased reutilization finding; second, to elicit suggestions for improving the nurse visit intervention.
METHODS
We selected an in-depth qualitative approach, using interviews and focus groups to explore underlying explanations for the increase in 30-day unplanned healthcare reutilization among those randomized to receive the postdischarge nurse visit during the H2O trial.4 Input was sought from 4 stakeholder groups—parents, primary care physicians (PCPs), hospital medicine physicians, and home care RNs—in an effort to triangulate data sources and elicit rich and diverse opinions. Approval was obtained from the Institutional Review Board prior to conducting the study.
Recruitment
Parents
Because we conducted interviews approximately 1 year after the trial’s conclusion, we purposefully selected families who were enrolled in the latter portion of the H2O trial in order to enhance recall. Beginning with the last families in the study, we sequentially contacted families in reverse order. We contacted 10 families in each of 4 categories (intervention/reutilization, intervention/no reutilization, control/reutilization, control/no reutilization). A total of 3 attempts were made by telephone to contact each family. Participants received a grocery store gift card for participating in the study.
Primary Care Physicians
We conducted focus groups with a purposive sample of physicians recruited from 2 community practices and 1 hospital-owned practice.
Hospital Medicine Physicians
We conducted focus groups with a purposive sample of physicians from our Division of Hospital Medicine. There was a varying level of knowledge of the original trial; however, none of the participants were collaborators in the trial.
Home Care RNs
We conducted focus groups with a subset of RNs who were involved with trial visits. All RNs were members of the pediatric home care division associated with the hospital with specific training in caring for patients at home.
Data Collection
The study team designed question guides for each stakeholder group (Appendix 1). While questions were tailored for specific stakeholders, all guides included the following topics: benefits and challenges of nurse visits, suggestions for improving the intervention in future trials, and reactions to the trial results (once presented to participants). Only the results of the intention-to-treat (ITT) analysis were shared with stakeholders because ITT is considered the gold standard for trial analysis and allows easy understanding of the results.
A single investigator (A.L.) conducted parental interviews by telephone. Focus groups for PCPs, hospital medicine physicians, and RN groups were held at practice locations in private conference rooms and were conducted by trained moderators (S.N.S., A.L., and H.T.C.). Moderators probed responses to the open-ended questions to delve deeply into issues. The question guides were modified in an iterative fashion to include new concepts raised during interviews or focus groups. All interviews and focus groups were recorded and transcribed verbatim with all identifiable information redacted.
Data Analysis
During multiple cycles of inductive thematic analysis,6 we examined, discussed, interpreted, and organized responses to the open-ended questions,6,7 analyzing each stakeholder group separately. First, transcripts were shared with and reviewed by the entire multidisciplinary team (12 members) which included hospital medicine physicians, PCPs, home care nursing leaders, a nurse scientist, a parent representative, research coordinators, and a qualitative research methodologist. Second, team members convened to discuss overall concepts and ideas and created the preliminary coding frameworks. Third, a smaller subgroup (research coordinator [A.L]., hospital medicine physician [S.R.], parent representative [M.M.], and qualitative research methodologist [S.N.S.]), refined the unique coding framework for each stakeholder group and then independently applied codes to participant comments. This subgroup met regularly to reach consensus about the assigned codes and to further refine the codebooks. The codes were organized into major and minor themes based on recurring patterns in the data and the salience or emphasis given by participants. The subgroup’s work was reviewed and discussed on an ongoing basis by the entire multidisciplinary team. Triangulation of the data was achieved in multiple ways. The preliminary results were shared in several forums, and feedback was solicited and incorporated. Two of 4 members of the subgroup analytic team were not part of the trial planning or data collection, providing a potentially broader perspective. All coding decisions were maintained in an electronic database, and an audit trail was created to document codebook revisions.
RESULTS
A total of 33 parents participated in the interviews (intervention/readmit [8], intervention/no readmit [8], control/readmit [8], and control/no readmit [9]). Although we selected families from all 4 categories, we were not able to explore qualitative differences between these groups because of the relatively low numbers of participants. Parent data was very limited as interviews were brief and “control” parents had not received the intervention. Three focus groups were held with PCPs (7 participants in total), 2 focus groups were held with hospital medicine physicians (12 participants), and 2 focus groups were held with RNs (10 participants).
Goal 1: Explanation of Reutilization Rates
During interviews and focus groups, the results of the H2O trial were discussed, and stakeholders were asked to comment on potential explanations of the findings. 4 major themes and 5 minor themes emerged from analysis of the transcripts (summarized in Table 1).
Theme 1: Appropriateness of Patient Reutilization
Hospital medicine physicians and home care RNs questioned whether the reutilization events were clinically indicated. RNs wondered whether children who reutilized the ED were also readmitted to the hospital; many perceived that if the child was ill enough to be readmitted, then the ED revisit was warranted (Table 2). Parents commented on parental decision-making and changes in clinical status of the child leading to reutilization (Table 2).
Theme 2: Impact of Red Flags/Warning Sign Instructions on Family’s Reutilization Decisions
Theme 3: Hospital-Affiliated RNs “Directing Traffic” Back to Hospital
Both physician groups were concerned that, because the study was conducted by hospital-employed nurses, families might have been more likely to reaccess care at the hospital. Thus, the connection with the hospital was strengthened in the H2O model, potentially at the expense of the connection with PCPs. Physicians hypothesized that families might “still feel part of the medical system,” so families would return to the hospital if there was a problem. PCPs emphasized that there may have been straightforward situations that could have been handled appropriately in the outpatient office (Table 2).
Theme 4: Home Visit RNs Had a Low Threshold for Escalating Care
Parents and PCPs hypothesized that RNs are more conservative and, therefore, would have had a low threshold to refer back to the hospital if there were concerns in the home. One parent commented: “I guess, nurses are just by trade accustomed to erring on the side of caution and medical intervention instead of letting time take its course. … They’re more apt to say it’s better off to go to the hospital and have everything be fine” (Table 2).
Minor Themes
Participants also explained reutilization in ways that coalesced into 5 minor themes: (1) families receiving a visit might perceive that their child was sicker; (2) patients in the control group did not reutilize enough; (3) receiving more education on a child’s illness drives reutilization; (4) provider access issues; and (5) variability of RN experience may determine whether escalated care. Supportive quotations found in Appendix 2.
We directly asked parents if they would want a nurse home visit in the future after discussing the results of the study. Almost all of the parents in the intervention group and most of the parents in the control group were in favor of receiving a visit, even knowing that patients who had received a visit were more likely to reutilize care.
Goal 2: Suggestions for Improving Intervention Design
Three major themes and 3 minor themes were related to improving the design of the intervention (Table 1).
Theme 1: Need for Improved Postdischarge Communication
All stakeholder groups highlighted postdischarge communication as an area that could be improved. Parents were frustrated with regard to attempts to connect with inpatient physicians after discharge. PCPs suggested developing pathways for the RN to connect with the primary care office as opposed to the hospital. Hospital medicine physicians discussed a lack of consensus regarding patient ownership following discharge and were uncertain about what types of postdischarge symptoms PCPs would be comfortable managing. RNs described specific situations when they had difficulty contacting a physician to escalate care (Table 3).
Theme 2: Individualizing Home Visits—One Size Does Not Fit All
All stakeholder groups also encouraged “individualization” of home visits according to patient and family characteristics, diagnosis, and both timing and severity of illness. PCPs recommended visits only for certain diagnoses. Hospital medicine physicians voiced similar sentiments as the PCPs and added that worrisome family dynamics during a hospitalization, such as a lack of engagement with the medical team, might also warrant a visit. RNs suggested visits for those families with more concerns, for example, those with young children or children recovering from an acute respiratory illness (Table 3).
Theme 3: Providing Context for and Framing of Red Flags
Physicians and nurses suggested providing more context to “red flag” instructions and education. RNs emphasized that some families seemed to benefit from the opportunity to discuss their postdischarge concerns with a medical professional. Others appreciated concrete written instructions that spelled out how to respond in certain situations (Table 3).
Minor Themes
Three minor themes were revealed regarding intervention design improvement (Table 1): (1) streamlining the discharge process; (2) improving the definition of the scope and goal of intervention; and (3) extending inpatient team expertise post discharge. Supportive quotations can be found in Appendix 3.
DISCUSSION
When stakeholders were asked about why postdischarge RN visits led to increased postdischarge urgent healthcare visits, they questioned the appropriateness of the reutilization events, wondered about the lack of context for the warning signs that nurses provided families as part of the intervention, worried that families were encouraged to return to the hospital because of the ties of the trial to the hospital, and suggested that RNs had a low threshold to refer patients back to the hospital. When asked about how to design an improved nurse visit to better support families, stakeholders emphasized improving communication, individualizing the visit, and providing context around the red-flag discussion, enabling more nuanced instructions about how to respond to specific events.
A synthesis of themes suggests that potential drivers for increased utilization rates may lie in the design and goals of the initial project. The intervention was designed to support families and enhance education after discharge, with components derived from pretrial focus groups with families after a hospital discharge.8 The intervention was not designed to divert patients from the ED nor did it enhance access to the PCP. A second trial of the intervention adapted to a phone call also failed to decrease reutilization rates.9 Both physician stakeholder groups perceived that the intervention directed traffic back to the hospital because of the intervention design. Coupled with the perception that the red flags may have changed a family’s threshold for seeking care and/or that an RN may be more apt to refer back to care, this failure to push utilization to the primary care office may explain the unexpected trial results. Despite the stakeholders’ perception of enhanced connection back to the hospital as a result of the nurse visit, in analysis of visit referral patterns, a referral was made directly back to the ED in only 4 of the 651 trial visits (Tubbs-Cooley H, Riddle SR, Gold JM, et al.; under review. Pediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period 2020).
Both H2O trials demonstrated improved recall of red flags by parents who received the intervention, which may be important given the stakeholders’ perspectives that the red flags may not have been contextualized well enough. Yet neither trial demonstrated any differences in postdischarge coping or time to return to normal routine. In interviews with parents, despite the clearly stated results of increased reutilization, intervention parents endorsed a desire for a home visit in the future, raising the possibility that our outcome measures did not capture parents’ priorities adequately.
When asked to recommend design improvements of the intervention, 2 major themes (improvement in communication and individualization of visits) were discussed by all stakeholder groups, providing actionable information to modify or create new interventions. Focus groups with clinicians suggested that communication challenges may have influenced reutilization likelihood during the postdischarge period. RNs expressed uncertainty about who to call with problems or questions at the time of a home visit. This was compounded by difficulty reaching physicians. Both hospital medicine physicians and PCPs identified system challenges including questions of patient ownership, variable PCP practice communication preferences, and difficulty in identifying a partnered staff member (on either end of the inpatient-outpatient continuum) who was familiar with a specific patient. While the communication issues raised may reflect difficulties in our local healthcare system, there is broad evidence of postdischarge communication challenges. In adults, postdischarge communication failures between home health staff and physicians are associated with an increased risk of readmission.10 The real or perceived lack of communication between inpatient and outpatient providers can add to parental confusion post discharge.11 Although there have been efforts to improve the reliability of communication across this gulf,12,13 it is not clear whether changes to discharge communication could help to avoid pediatric reutilization events.14
The theme of individualization of the home nurse visit is consistent with evidence regarding the impact of focusing the intervention on patients with specific diagnoses or demographics. In adults, reduced reutilization associated with postdischarge home nurse visits has been described in specific populations such as patients with heart failure and chronic obstructive pulmonary disease.15 Impact of home nurse visits on patients within diagnosis-specific populations with certain demographics (such as advanced age) has also been described.16 In the pediatric population, readmission rates vary widely by diagnosis.17 A systematic review of interventions to reduce pediatric readmissions found increased impact of discharge interventions in specific populations (asthma, oncology, and NICU).3
Next steps may lie in interventions in targeted populations that function as part of a care continuum bridging the patient from the inpatient to the outpatient setting. A home nurse visit as part of this discharge structure may prove to have more impact on reducing reutilization. One population which accounts for a large proportion of readmissions and where there has been recent focus on discharge transition of care has been children with medical complexity.18 This group was largely excluded from the H2O trial. Postdischarge home nurse visits in this population have been found to be feasible and address many questions and problems, but the effect on readmission is less clear.19 Family priorities and preferences related to preparation for discharge, including family engagement, respect for discharge readiness, and goal of returning to normal routines, may be areas on which to focus with future interventions in this population.20 In summary, although widespread postdischarge interventions (home nurse visit4 and nurse telephone call9) have not been found to be effective, targeting interventions to specific populations by diagnosis or demographic factors may prove to be more effective in reducing pediatric reutilization.
There were several strengths to this study. This qualitative approach allowed us to elucidate potential explanations for the H2O trial results from multiple perspectives. The multidisciplinary composition of our analytic team and the use of an iterative process sparked diverse contributions in a dynamic, ongoing discussion and interpretation of our data.
This study should be considered in the context of several limitations. For families and RNs, there was a time lag between participation in the trial and participation in the qualitative study call or focus group which could lead to difficulty recalling details. Only families who received the intervention could give opinions on their experience of the nurse visit, while families in the control group were asked to hypothesize. Focus groups with hospital medicine physicians and PCPs were purposive samples, and complete demographic information of participants was not collected.
CONCLUSION
Key stakeholders reflecting on a postdischarge RN visit trial suggested multiple potential explanations for the unexpected increase in reutilization in children randomized to the intervention. Certain participants questioned whether all reutilization events were appropriate or necessary. Others expressed concerns that the H2O intervention lacked context and directed children back to the hospital instead of the PCP. Parents, PCPs, hospital medicine physicians, and RNs all suggested that future transition-focused interventions should enhance postdischarge communication, strengthen connection to the PCP, and be more effectively tailored to the needs of the individual patient and family.
Acknowledgments
Collaborators: H2O Trial Study Group: Joanne Bachus, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Monica L Borell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lenisa V Chang, MA, PhD; Patricia Crawford, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sarah A Ferris, BA, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Judy A Heilman BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Jane C Khoury, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen Lawley, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lynne O’Donnell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Hadley S Sauers-Ford, MPH, Department of Pediatrics, UC Davis Health, Sacramento, California; Anita N Shah, DO, MPH, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lauren G Solan, MD, Med, University of Rochester, Rochester, New York; Heidi J Sucharew, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen P Sullivan, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Christine M White, MD, MAT, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Readmission rates are used as metrics for care quality and reimbursement, with penalties applied to hospitals with higher than expected rates1 and up to 30% of pediatric readmissions deemed potentially preventable.2 There is a paucity of information on how to prevent pediatric readmissions,3 yet pediatric hospitals are tasked with implementing interventions for readmission reduction.
The Hospital to Home Outcomes (H2O) trial was a 2-arm, randomized controlled trial in which patients discharged from hospital medicine and neuroscience services at a single institution were randomized to receive a single home visit from a registered nurse (RN) within 96 hours of discharge.4 RNs completed a structured nurse visit designed specifically for the trial. Lists of “red flags” or warning signs associated with common diagnoses were provided to assist RNs in standardizing education about when to seek additional care. The hypothesis was that the postdischarge visits would result in lower reutilization rates (unplanned readmissions, emergency department [ED] visits, and urgent care visits).5
Unexpectedly, children randomized to receive the postdischarge nurse visit had higher rates of 30-day unplanned healthcare reutilization, with children randomly assigned to the intervention demonstrating higher odds of 30-day healthcare use (OR 1.33; 95% CI 1.003-1.76).4 We sought to understand perspectives on these unanticipated findings by obtaining input from relevant stakeholders. There were 2 goals for the qualitative analysis: first, to understand possible explanations of the increased reutilization finding; second, to elicit suggestions for improving the nurse visit intervention.
METHODS
We selected an in-depth qualitative approach, using interviews and focus groups to explore underlying explanations for the increase in 30-day unplanned healthcare reutilization among those randomized to receive the postdischarge nurse visit during the H2O trial.4 Input was sought from 4 stakeholder groups—parents, primary care physicians (PCPs), hospital medicine physicians, and home care RNs—in an effort to triangulate data sources and elicit rich and diverse opinions. Approval was obtained from the Institutional Review Board prior to conducting the study.
Recruitment
Parents
Because we conducted interviews approximately 1 year after the trial’s conclusion, we purposefully selected families who were enrolled in the latter portion of the H2O trial in order to enhance recall. Beginning with the last families in the study, we sequentially contacted families in reverse order. We contacted 10 families in each of 4 categories (intervention/reutilization, intervention/no reutilization, control/reutilization, control/no reutilization). A total of 3 attempts were made by telephone to contact each family. Participants received a grocery store gift card for participating in the study.
Primary Care Physicians
We conducted focus groups with a purposive sample of physicians recruited from 2 community practices and 1 hospital-owned practice.
Hospital Medicine Physicians
We conducted focus groups with a purposive sample of physicians from our Division of Hospital Medicine. There was a varying level of knowledge of the original trial; however, none of the participants were collaborators in the trial.
Home Care RNs
We conducted focus groups with a subset of RNs who were involved with trial visits. All RNs were members of the pediatric home care division associated with the hospital with specific training in caring for patients at home.
Data Collection
The study team designed question guides for each stakeholder group (Appendix 1). While questions were tailored for specific stakeholders, all guides included the following topics: benefits and challenges of nurse visits, suggestions for improving the intervention in future trials, and reactions to the trial results (once presented to participants). Only the results of the intention-to-treat (ITT) analysis were shared with stakeholders because ITT is considered the gold standard for trial analysis and allows easy understanding of the results.
A single investigator (A.L.) conducted parental interviews by telephone. Focus groups for PCPs, hospital medicine physicians, and RN groups were held at practice locations in private conference rooms and were conducted by trained moderators (S.N.S., A.L., and H.T.C.). Moderators probed responses to the open-ended questions to delve deeply into issues. The question guides were modified in an iterative fashion to include new concepts raised during interviews or focus groups. All interviews and focus groups were recorded and transcribed verbatim with all identifiable information redacted.
Data Analysis
During multiple cycles of inductive thematic analysis,6 we examined, discussed, interpreted, and organized responses to the open-ended questions,6,7 analyzing each stakeholder group separately. First, transcripts were shared with and reviewed by the entire multidisciplinary team (12 members) which included hospital medicine physicians, PCPs, home care nursing leaders, a nurse scientist, a parent representative, research coordinators, and a qualitative research methodologist. Second, team members convened to discuss overall concepts and ideas and created the preliminary coding frameworks. Third, a smaller subgroup (research coordinator [A.L]., hospital medicine physician [S.R.], parent representative [M.M.], and qualitative research methodologist [S.N.S.]), refined the unique coding framework for each stakeholder group and then independently applied codes to participant comments. This subgroup met regularly to reach consensus about the assigned codes and to further refine the codebooks. The codes were organized into major and minor themes based on recurring patterns in the data and the salience or emphasis given by participants. The subgroup’s work was reviewed and discussed on an ongoing basis by the entire multidisciplinary team. Triangulation of the data was achieved in multiple ways. The preliminary results were shared in several forums, and feedback was solicited and incorporated. Two of 4 members of the subgroup analytic team were not part of the trial planning or data collection, providing a potentially broader perspective. All coding decisions were maintained in an electronic database, and an audit trail was created to document codebook revisions.
RESULTS
A total of 33 parents participated in the interviews (intervention/readmit [8], intervention/no readmit [8], control/readmit [8], and control/no readmit [9]). Although we selected families from all 4 categories, we were not able to explore qualitative differences between these groups because of the relatively low numbers of participants. Parent data was very limited as interviews were brief and “control” parents had not received the intervention. Three focus groups were held with PCPs (7 participants in total), 2 focus groups were held with hospital medicine physicians (12 participants), and 2 focus groups were held with RNs (10 participants).
Goal 1: Explanation of Reutilization Rates
During interviews and focus groups, the results of the H2O trial were discussed, and stakeholders were asked to comment on potential explanations of the findings. 4 major themes and 5 minor themes emerged from analysis of the transcripts (summarized in Table 1).
Theme 1: Appropriateness of Patient Reutilization
Hospital medicine physicians and home care RNs questioned whether the reutilization events were clinically indicated. RNs wondered whether children who reutilized the ED were also readmitted to the hospital; many perceived that if the child was ill enough to be readmitted, then the ED revisit was warranted (Table 2). Parents commented on parental decision-making and changes in clinical status of the child leading to reutilization (Table 2).
Theme 2: Impact of Red Flags/Warning Sign Instructions on Family’s Reutilization Decisions
Theme 3: Hospital-Affiliated RNs “Directing Traffic” Back to Hospital
Both physician groups were concerned that, because the study was conducted by hospital-employed nurses, families might have been more likely to reaccess care at the hospital. Thus, the connection with the hospital was strengthened in the H2O model, potentially at the expense of the connection with PCPs. Physicians hypothesized that families might “still feel part of the medical system,” so families would return to the hospital if there was a problem. PCPs emphasized that there may have been straightforward situations that could have been handled appropriately in the outpatient office (Table 2).
Theme 4: Home Visit RNs Had a Low Threshold for Escalating Care
Parents and PCPs hypothesized that RNs are more conservative and, therefore, would have had a low threshold to refer back to the hospital if there were concerns in the home. One parent commented: “I guess, nurses are just by trade accustomed to erring on the side of caution and medical intervention instead of letting time take its course. … They’re more apt to say it’s better off to go to the hospital and have everything be fine” (Table 2).
Minor Themes
Participants also explained reutilization in ways that coalesced into 5 minor themes: (1) families receiving a visit might perceive that their child was sicker; (2) patients in the control group did not reutilize enough; (3) receiving more education on a child’s illness drives reutilization; (4) provider access issues; and (5) variability of RN experience may determine whether escalated care. Supportive quotations found in Appendix 2.
We directly asked parents if they would want a nurse home visit in the future after discussing the results of the study. Almost all of the parents in the intervention group and most of the parents in the control group were in favor of receiving a visit, even knowing that patients who had received a visit were more likely to reutilize care.
Goal 2: Suggestions for Improving Intervention Design
Three major themes and 3 minor themes were related to improving the design of the intervention (Table 1).
Theme 1: Need for Improved Postdischarge Communication
All stakeholder groups highlighted postdischarge communication as an area that could be improved. Parents were frustrated with regard to attempts to connect with inpatient physicians after discharge. PCPs suggested developing pathways for the RN to connect with the primary care office as opposed to the hospital. Hospital medicine physicians discussed a lack of consensus regarding patient ownership following discharge and were uncertain about what types of postdischarge symptoms PCPs would be comfortable managing. RNs described specific situations when they had difficulty contacting a physician to escalate care (Table 3).
Theme 2: Individualizing Home Visits—One Size Does Not Fit All
All stakeholder groups also encouraged “individualization” of home visits according to patient and family characteristics, diagnosis, and both timing and severity of illness. PCPs recommended visits only for certain diagnoses. Hospital medicine physicians voiced similar sentiments as the PCPs and added that worrisome family dynamics during a hospitalization, such as a lack of engagement with the medical team, might also warrant a visit. RNs suggested visits for those families with more concerns, for example, those with young children or children recovering from an acute respiratory illness (Table 3).
Theme 3: Providing Context for and Framing of Red Flags
Physicians and nurses suggested providing more context to “red flag” instructions and education. RNs emphasized that some families seemed to benefit from the opportunity to discuss their postdischarge concerns with a medical professional. Others appreciated concrete written instructions that spelled out how to respond in certain situations (Table 3).
Minor Themes
Three minor themes were revealed regarding intervention design improvement (Table 1): (1) streamlining the discharge process; (2) improving the definition of the scope and goal of intervention; and (3) extending inpatient team expertise post discharge. Supportive quotations can be found in Appendix 3.
DISCUSSION
When stakeholders were asked about why postdischarge RN visits led to increased postdischarge urgent healthcare visits, they questioned the appropriateness of the reutilization events, wondered about the lack of context for the warning signs that nurses provided families as part of the intervention, worried that families were encouraged to return to the hospital because of the ties of the trial to the hospital, and suggested that RNs had a low threshold to refer patients back to the hospital. When asked about how to design an improved nurse visit to better support families, stakeholders emphasized improving communication, individualizing the visit, and providing context around the red-flag discussion, enabling more nuanced instructions about how to respond to specific events.
A synthesis of themes suggests that potential drivers for increased utilization rates may lie in the design and goals of the initial project. The intervention was designed to support families and enhance education after discharge, with components derived from pretrial focus groups with families after a hospital discharge.8 The intervention was not designed to divert patients from the ED nor did it enhance access to the PCP. A second trial of the intervention adapted to a phone call also failed to decrease reutilization rates.9 Both physician stakeholder groups perceived that the intervention directed traffic back to the hospital because of the intervention design. Coupled with the perception that the red flags may have changed a family’s threshold for seeking care and/or that an RN may be more apt to refer back to care, this failure to push utilization to the primary care office may explain the unexpected trial results. Despite the stakeholders’ perception of enhanced connection back to the hospital as a result of the nurse visit, in analysis of visit referral patterns, a referral was made directly back to the ED in only 4 of the 651 trial visits (Tubbs-Cooley H, Riddle SR, Gold JM, et al.; under review. Pediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period 2020).
Both H2O trials demonstrated improved recall of red flags by parents who received the intervention, which may be important given the stakeholders’ perspectives that the red flags may not have been contextualized well enough. Yet neither trial demonstrated any differences in postdischarge coping or time to return to normal routine. In interviews with parents, despite the clearly stated results of increased reutilization, intervention parents endorsed a desire for a home visit in the future, raising the possibility that our outcome measures did not capture parents’ priorities adequately.
When asked to recommend design improvements of the intervention, 2 major themes (improvement in communication and individualization of visits) were discussed by all stakeholder groups, providing actionable information to modify or create new interventions. Focus groups with clinicians suggested that communication challenges may have influenced reutilization likelihood during the postdischarge period. RNs expressed uncertainty about who to call with problems or questions at the time of a home visit. This was compounded by difficulty reaching physicians. Both hospital medicine physicians and PCPs identified system challenges including questions of patient ownership, variable PCP practice communication preferences, and difficulty in identifying a partnered staff member (on either end of the inpatient-outpatient continuum) who was familiar with a specific patient. While the communication issues raised may reflect difficulties in our local healthcare system, there is broad evidence of postdischarge communication challenges. In adults, postdischarge communication failures between home health staff and physicians are associated with an increased risk of readmission.10 The real or perceived lack of communication between inpatient and outpatient providers can add to parental confusion post discharge.11 Although there have been efforts to improve the reliability of communication across this gulf,12,13 it is not clear whether changes to discharge communication could help to avoid pediatric reutilization events.14
The theme of individualization of the home nurse visit is consistent with evidence regarding the impact of focusing the intervention on patients with specific diagnoses or demographics. In adults, reduced reutilization associated with postdischarge home nurse visits has been described in specific populations such as patients with heart failure and chronic obstructive pulmonary disease.15 Impact of home nurse visits on patients within diagnosis-specific populations with certain demographics (such as advanced age) has also been described.16 In the pediatric population, readmission rates vary widely by diagnosis.17 A systematic review of interventions to reduce pediatric readmissions found increased impact of discharge interventions in specific populations (asthma, oncology, and NICU).3
Next steps may lie in interventions in targeted populations that function as part of a care continuum bridging the patient from the inpatient to the outpatient setting. A home nurse visit as part of this discharge structure may prove to have more impact on reducing reutilization. One population which accounts for a large proportion of readmissions and where there has been recent focus on discharge transition of care has been children with medical complexity.18 This group was largely excluded from the H2O trial. Postdischarge home nurse visits in this population have been found to be feasible and address many questions and problems, but the effect on readmission is less clear.19 Family priorities and preferences related to preparation for discharge, including family engagement, respect for discharge readiness, and goal of returning to normal routines, may be areas on which to focus with future interventions in this population.20 In summary, although widespread postdischarge interventions (home nurse visit4 and nurse telephone call9) have not been found to be effective, targeting interventions to specific populations by diagnosis or demographic factors may prove to be more effective in reducing pediatric reutilization.
There were several strengths to this study. This qualitative approach allowed us to elucidate potential explanations for the H2O trial results from multiple perspectives. The multidisciplinary composition of our analytic team and the use of an iterative process sparked diverse contributions in a dynamic, ongoing discussion and interpretation of our data.
This study should be considered in the context of several limitations. For families and RNs, there was a time lag between participation in the trial and participation in the qualitative study call or focus group which could lead to difficulty recalling details. Only families who received the intervention could give opinions on their experience of the nurse visit, while families in the control group were asked to hypothesize. Focus groups with hospital medicine physicians and PCPs were purposive samples, and complete demographic information of participants was not collected.
CONCLUSION
Key stakeholders reflecting on a postdischarge RN visit trial suggested multiple potential explanations for the unexpected increase in reutilization in children randomized to the intervention. Certain participants questioned whether all reutilization events were appropriate or necessary. Others expressed concerns that the H2O intervention lacked context and directed children back to the hospital instead of the PCP. Parents, PCPs, hospital medicine physicians, and RNs all suggested that future transition-focused interventions should enhance postdischarge communication, strengthen connection to the PCP, and be more effectively tailored to the needs of the individual patient and family.
Acknowledgments
Collaborators: H2O Trial Study Group: Joanne Bachus, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Monica L Borell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lenisa V Chang, MA, PhD; Patricia Crawford, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sarah A Ferris, BA, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Judy A Heilman BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Jane C Khoury, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen Lawley, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lynne O’Donnell, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Hadley S Sauers-Ford, MPH, Department of Pediatrics, UC Davis Health, Sacramento, California; Anita N Shah, DO, MPH, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Lauren G Solan, MD, Med, University of Rochester, Rochester, New York; Heidi J Sucharew, PhD, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Karen P Sullivan, BSN, RN, Department of Patient Services, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Christine M White, MD, MAT, Division of Hospital Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
1. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a Children’s Hospital. Pediatrics. 2016;138(2). https://doi.org/10.1542/peds.2015-4182.
3. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
4. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1). https://doi.org/10.1542/peds.2017-3919.
5. Tubbs-Cooley HL, Pickler RH, Simmons JM, et al. Testing a post-discharge nurse-led transitional home visit in acute care pediatrics: the Hospital-To-Home Outcomes (H2O) study protocol. J Adv Nurs. 2016;72(4):915-925. https://doi.org/10.1111/jan.12882.
6. Guest G. Collecting Qualitative Data: A Field Manual for Applied Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2013.
7. Patton M. Qualitative Research and Evaluation Methods. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
8. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on Hospital to Home Transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
9. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
10. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse-physician communication, patient severity, and hospital readmission. Health Serv Res. 2018;53(2):1008-1024. https://doi.org/10.1111/1475-6773.12667.
11. Solan LG, Beck AF, Shardo SA, et al. Caregiver perspectives on communication during hospitalization at an academic pediatric institution: a qualitative study. J Hosp Med. 2018;13(5):304-311. https://doi.org/10.12788/jhm.2919.
12. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119.
13. Mussman GM, Vossmeyer MT, Brady PW, et al. Improving the reliability of verbal communication between primary care physicians and pediatric hospitalists at hospital discharge. J Hosp Med. 2015;10(9):574-580. https://doi.org/10.1002/jhm.2392.
14. Coller RJ, Klitzner TS, Saenz AA, et al. Discharge handoff communication and pediatric readmissions. J Hosp Med. 2017;12(1):29-35. https://doi.org/10.1002/jhm.2670.
15. Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2017;14(2):251-261. https://doi.org/10.1080/15412555.2016.1256384.
16. Finlayson K, Chang AM, Courtney MD, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. https://doi.org/10.1186/s12913-018-3771-9.
17. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
18. Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628-e1647. https://doi.org/10.1542/peds.2014-1956.
19. Wells S, O’Neill M, Rogers J, et al. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10-16. https://doi.org/10.1016/j.pedn.2017.03.003.
20. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-1581.
1. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a Children’s Hospital. Pediatrics. 2016;138(2). https://doi.org/10.1542/peds.2015-4182.
3. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. https://doi.org/10.1002/jhm.2134.
4. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1). https://doi.org/10.1542/peds.2017-3919.
5. Tubbs-Cooley HL, Pickler RH, Simmons JM, et al. Testing a post-discharge nurse-led transitional home visit in acute care pediatrics: the Hospital-To-Home Outcomes (H2O) study protocol. J Adv Nurs. 2016;72(4):915-925. https://doi.org/10.1111/jan.12882.
6. Guest G. Collecting Qualitative Data: A Field Manual for Applied Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2013.
7. Patton M. Qualitative Research and Evaluation Methods. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.; 2014.
8. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on Hospital to Home Transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. https://doi.org/10.1542/peds.2015-2098.
9. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482.
10. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse-physician communication, patient severity, and hospital readmission. Health Serv Res. 2018;53(2):1008-1024. https://doi.org/10.1111/1475-6773.12667.
11. Solan LG, Beck AF, Shardo SA, et al. Caregiver perspectives on communication during hospitalization at an academic pediatric institution: a qualitative study. J Hosp Med. 2018;13(5):304-311. https://doi.org/10.12788/jhm.2919.
12. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119.
13. Mussman GM, Vossmeyer MT, Brady PW, et al. Improving the reliability of verbal communication between primary care physicians and pediatric hospitalists at hospital discharge. J Hosp Med. 2015;10(9):574-580. https://doi.org/10.1002/jhm.2392.
14. Coller RJ, Klitzner TS, Saenz AA, et al. Discharge handoff communication and pediatric readmissions. J Hosp Med. 2017;12(1):29-35. https://doi.org/10.1002/jhm.2670.
15. Yang F, Xiong ZF, Yang C, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2017;14(2):251-261. https://doi.org/10.1080/15412555.2016.1256384.
16. Finlayson K, Chang AM, Courtney MD, et al. Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res. 2018;18(1):956. https://doi.org/10.1186/s12913-018-3771-9.
17. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
18. Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628-e1647. https://doi.org/10.1542/peds.2014-1956.
19. Wells S, O’Neill M, Rogers J, et al. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10-16. https://doi.org/10.1016/j.pedn.2017.03.003.
20. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-1581.
© 2020 Society of Hospital Medicine
ERRATUM: Decreasing Hypoglycemia following Insulin Administration for Inpatient Hyperkalemia
A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

Things We Do for No Reason™: Obtaining an Abdominal X-ray to Assess for Constipation in Children

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 5-year old boy is admitted to the hospital for a bowel clean-out after presenting with abdominal pain and having an abdominal x-ray that demonstrated a “moderate stool burden.” After ingestion of the bowel prep, he develops worsening abdominal cramping and diarrhea. Upon reviewing the bowel history with his mother afterward, the team learns that he has had a bowel movement every 1-2 days as usual and has been having soft stools without any straining, pain, or blood present.
BACKGROUND
Functional constipation is a common clinical problem in pediatrics and constitutes a large number of admissions into hospitals and visits to clinics and emergency departments. In the United States, up to 36% of children are affected.Associated healthcare costs for children with constipation are estimated at $5.9 billion per year, which is $3.9 billion more than the general pediatric population without constipation.1 In 2011, American children aged 17 years and younger had more than 270,000 visits to the emergency department for constipation.2 As many as 70% of children who are given a diagnosis of constipation in the emergency department have an abdominal x-ray completed.3 The carcinogenic effects of radiation from radiography are well known. Unnecessary imaging places the child at risk for these effects while adding to the overall cost of medical care.4
WHY AN ABDOMINAL X-RAY MAY SEEM HELPFUL
The overall utilization of diagnostic imaging is increasing in pediatric emergency departments.4 When questioning why this is the case, one should consider the method of problem solving used by most physicians. After formulating initial hypotheses based on available information, prior knowledge, and experience, physicians aim to obtain additional data to confirm or reject each hypothesis.5
A 2017 study surveyed 24 pediatric gastroenterologists after 72 patient encounters and found that the most common cause for obtaining an abdominal x-ray was for evaluation of stool burden (70%).5 Other reasons included assessing the need for a bowel clean-out (35%), diagnosing fecal impaction (27%), finding the cause for abdominal pain (24%), and demonstrating stool burden to a family (14%). This same study found that most of the polled providers used an abdominal x-ray to assess for constipation, and nearly half changed their management based on the findings.
WHY ABDOMINAL X-RAYS ARE NOT HELPFUL
Many systematic reviews and retrospective studies have investigated the efficacy of abdominal x-rays for diagnosing constipation. One retrospective review involving 160 children with defecation complaints assessed the accuracy of different radiologic scoring methods in identifying children with constipation.6 Three pediatric gastroenterologists and 1 pediatric radiologist blindly applied 4 scoring methods: colonic transit time, Leech score, Barr score, and fecal loading. The results showed that all x-ray scoring methods had low sensitivity for diagnosing constipation, variable specificity, and low interobserver reproducibility of scores.6 There was also poor ability to differentiate between patients with constipation and nonretentive fecal incontinence. Fecal loading had the worst performance in differentiating between these 2. Greater than 20% of children with clinically diagnosed constipation had normal Barr and Leech scores.6 Another systematic review also found no evidence for a diagnostic association between clinical symptoms of constipation and fecal loading on abdominal x-rays.7 In this study, the sensitivity and specificity of the x-ray were as low as 61% and 55%, respectively, which indicate poor overall diagnostic accuracy. Abdominal x-rays are subjective, not standardized, and represent a single observation in time. The amount of fecal loading seen on imaging is subject to daily variation depending on the timing of last food intake and timing of last defecation. There is a large variance in interpretation of fecal loading, and any stool seen on an x-ray does not rule out another potential diagnosis causing abdominal pain.
In 2014, the North American Society for Pediatric Gastroenterology, Hepatology, & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) released joint clinical guidelines that the evidence supports not obtaining an abdominal x-ray to diagnose functional constipation.8 Similarly, the National Institute for Health and Care Excellence (NICE) stated that abdominal x-rays should not be recommended as an additional test for constipation in children.9 These groups advocate for diagnosing functional constipation clinically by using a careful history and physical exam.
WHY ABDOMINAL X-RAYS ARE POTENTIALLY HARMFUL
The primary patient harm associated with abdominal x-rays is radiation exposure. While the amount of radiation in a single x-ray is low, children with constipation tend to have frequent revisits, significantly more than children with other common chronic conditions (eg, asthma and migraine headaches).10
One multicenter retrospective cohort study that included approximately 282,000 children diagnosed with constipation found that children who received an abdominal x-ray were twice as likely to return to the emergency department with a clinically significant alternate diagnosis (0.33% vs 0.17%). The 2 most common missed diagnoses were acute appendicitis and intussusception.3 Another retrospective study that included about 3,700 children also found that x-rays were performed more frequently in children who were misdiagnosed than in those who did not have a significant alternate diagnosis (75% vs 46%).11 In this case, both of these groups had a similar amount of stool on the x-rays as determined by the mean Leech scores. While this study identified an association between abdominal x-ray use and misdiagnoses, a causative effect was not necessarily discovered between the 2. The authors felt that even relatively large amounts of stool on an x-ray should not discount serious causes of abdominal pain or tenderness.11 A third retrospective study determined that children who received an abdominal x-ray and were diagnosed with constipation were significantly more likely to be admitted to the hospital, further raising healthcare costs.12 In this study, having an x-ray reduced the odds of being discharged home by about half. They also found that abdominal x-rays could be avoided if digital rectal exams were performed.12
HOW CONSTIPATION SHOULD BE DIAGNOSED
Functional constipation is a clinical diagnosis based on a thorough collection of history and a complete physical exam in children of all ages, including digital examination of the rectum to assess for fecal impaction, if necessary.
The Rome IV criteria for chronic constipation can be helpful and includes at least 2 of the following features for at least 1 month in infants up to 4 years of age: 2 or fewer stools per week, history of excessive stool retention, history of painful or hard bowel movements, history of large-diameter stools, and presence of a large fecal mass in the rectum.13 In children who are toilet trained, 2 additional criteria may be used: at least 1 episode of fecal incontinence per week after being toilet-trained and history of large-diameter stools that may obstruct the toilet.13
The NASPGHAN and ESPGHAN joint guidelines from 2014 state that, while constipation is based on history and physical exam, a major role of the history and physical exam is to exclude other disorders that also present with difficulty in defecation.8 This can help identify red-flag features or complications and guide further investigation. While evidence did not support routine use of a digital rectal exam in diagnosing constipation, the guidelines stated that a rectal exam (visual and digital) helps to evaluate for anorectal malformations, anal stenosis, rectal tone, distension, erythema, skin tags, anal fissures, or a fecal mass.8
In regard to history, approximately 0.4%-20% of healthy children without constipation have at least 1 clinical feature listed above. Therefore, the use of a single clinical finding to diagnose constipation, such as decreased bowel frequency, can result in an inappropriate diagnosis. Children experience large variations in stool output depending on diet, genetics, and environmental factors.10 The usual pattern of bowel habits in humans range from 3 times daily to every 3 days.14 Importantly, there are times to order an abdominal x-ray for patients with abdominal pain. The NASPGHAN and ESPGHAN joint guidelines recommend obtaining abdominal x-rays to evaluate children who have concerning features, such as previous abdominal surgeries, known genetic conditions or malformations, bilious emesis, or severe abdominal distension.8
RECOMMENDATIONS
- Functional constipation should be diagnosed based purely on a thorough history and physical examination, including a rectal exam
- Abdominal x-rays (ordered for any reason) should not be used to diagnose or assess for functional constipation
CONCLUSIONS
Performing abdominal x-rays to assess for pediatric functional constipation is not beneficial and potentially harmful to patients. Multiple retrospective studies revealed no diagnostic association between clinical symptoms or severity of constipation and findings on abdominal radiography. X-rays have very low sensitivity and specificity for diagnosing constipation. In the pediatric emergency department, abdominal x-rays completed for patients diagnosed with constipation have been associated with missed diagnoses, false reassurance of constipation, more frequent admissions into the hospital, longer hospital stays, higher healthcare costs, and unnecessary radiation exposure. The NICE as well as 2014 NASPGHAN and ESPGHAN clinical guidelines recommend against obtaining x-rays to diagnose constipation. The most effective way to diagnose functional constipation in children is with a thorough collection of history and physical exam. In the introductory case, the boy received an osmotic laxative based on abdominal x-ray findings, which resulted in the adverse effect of diarrhea. This case demonstrates how using abdominal x-rays to assess for constipation can be misleading and emphasizes the importance of collecting a thorough history and physical exam.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Afzal NA, Tighe MP, Thomson MA. (2011, June 13). Constipation in children. Ital J Pediatr. 2011;37:28. https://doi.org/10.1186/1824-7288-37-28.
2. Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110(4):572-579. https://doi.org/10.1038/ajg.2015.64.
3. Freedman SB, Rodean J, Hall M, et al. (2017). Delayed diagnoses in children with constipation: multicenter retrospective cohort study. J Pediatr. 186, 87-94.e16. https://doi.org/10.1016/j.jpeds.2017.03.061.
4. Reed MH. Imaging utilization commentary: A radiology perspective. Pediatr Radiol. 2008;38 (Suppl 4):S660-S663. https://doi.org/10.1007/s00247-008-0982-y.
5. Beinvogl B, Sabharwal S, McSweeney M, Nurko S. Are we using abdominal radiographs appropriately in the management of pediatric constipation? J Pediatr. 2017;191:179-183. https://doi.org/10.1016/j.jpeds.2017.08.075.
6. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. J Pediatr Gastroenterol Nutr. 2010;51(2):155-159. https://doi.org/10.1097/MPG.0b013e3181cb4309.
7. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J Pediatr. 2012;161(1):44–50.e502. https://doi.org/10.1016/j.jpeds.2011.12.045.
8. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258-274. https://doi.org/10.1097/mpg.0000000000000266.
9. Bardisa-Ezcurra L, Ullman R, Gordon J; Guideline Development Group. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 2010;340:c2585. https://doi.org/10.1136/bmj.c2585.
10. Rajindrajith S, Manjuri Devanarayana N, Benninga MA. Defecation Disorders in Children: Constipation and Functional Fecal Incontinence. In: Guandalini S, Dhawan A, Branski D. eds. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice (1st ed.). Basingstoke, England: Springer; 2016:247-260.
11. Freedman SB, Thull-Freedman J, Manson D, et al. Pediatric abdominal radiograph use, constipation, and significant misdiagnoses. J Pediatr. 2014;164(1):83-88.e2. https://doi.org/10.1016/j.jpeds.2013.08.074.
12. Chumpitazi CE, Rees CA, Camp EA, Henkel EB, Valdez KL, Chumpitazi BP. Diagnostic approach to constipation impacts pediatric emergency department disposition. Am J Emerg Med. 2017;35(10):1490-1493. https://doi.org/10.1016/j.ajem.2017.04.060.
13. Benninga MA, Nurko S, Faure C, Hyman PE, St. James Roberts I, Schechter NL. Childhood functional GI disorders: Neonate/toddler. Gastroenterology. 2016;150(6):1443-1455. https://doi.org/10.1053/j.gastro.2016.02.016.
14. Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol. 2010;45(5):556-566. https://doi.org/10.3109/00365520903551332.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 5-year old boy is admitted to the hospital for a bowel clean-out after presenting with abdominal pain and having an abdominal x-ray that demonstrated a “moderate stool burden.” After ingestion of the bowel prep, he develops worsening abdominal cramping and diarrhea. Upon reviewing the bowel history with his mother afterward, the team learns that he has had a bowel movement every 1-2 days as usual and has been having soft stools without any straining, pain, or blood present.
BACKGROUND
Functional constipation is a common clinical problem in pediatrics and constitutes a large number of admissions into hospitals and visits to clinics and emergency departments. In the United States, up to 36% of children are affected.Associated healthcare costs for children with constipation are estimated at $5.9 billion per year, which is $3.9 billion more than the general pediatric population without constipation.1 In 2011, American children aged 17 years and younger had more than 270,000 visits to the emergency department for constipation.2 As many as 70% of children who are given a diagnosis of constipation in the emergency department have an abdominal x-ray completed.3 The carcinogenic effects of radiation from radiography are well known. Unnecessary imaging places the child at risk for these effects while adding to the overall cost of medical care.4
WHY AN ABDOMINAL X-RAY MAY SEEM HELPFUL
The overall utilization of diagnostic imaging is increasing in pediatric emergency departments.4 When questioning why this is the case, one should consider the method of problem solving used by most physicians. After formulating initial hypotheses based on available information, prior knowledge, and experience, physicians aim to obtain additional data to confirm or reject each hypothesis.5
A 2017 study surveyed 24 pediatric gastroenterologists after 72 patient encounters and found that the most common cause for obtaining an abdominal x-ray was for evaluation of stool burden (70%).5 Other reasons included assessing the need for a bowel clean-out (35%), diagnosing fecal impaction (27%), finding the cause for abdominal pain (24%), and demonstrating stool burden to a family (14%). This same study found that most of the polled providers used an abdominal x-ray to assess for constipation, and nearly half changed their management based on the findings.
WHY ABDOMINAL X-RAYS ARE NOT HELPFUL
Many systematic reviews and retrospective studies have investigated the efficacy of abdominal x-rays for diagnosing constipation. One retrospective review involving 160 children with defecation complaints assessed the accuracy of different radiologic scoring methods in identifying children with constipation.6 Three pediatric gastroenterologists and 1 pediatric radiologist blindly applied 4 scoring methods: colonic transit time, Leech score, Barr score, and fecal loading. The results showed that all x-ray scoring methods had low sensitivity for diagnosing constipation, variable specificity, and low interobserver reproducibility of scores.6 There was also poor ability to differentiate between patients with constipation and nonretentive fecal incontinence. Fecal loading had the worst performance in differentiating between these 2. Greater than 20% of children with clinically diagnosed constipation had normal Barr and Leech scores.6 Another systematic review also found no evidence for a diagnostic association between clinical symptoms of constipation and fecal loading on abdominal x-rays.7 In this study, the sensitivity and specificity of the x-ray were as low as 61% and 55%, respectively, which indicate poor overall diagnostic accuracy. Abdominal x-rays are subjective, not standardized, and represent a single observation in time. The amount of fecal loading seen on imaging is subject to daily variation depending on the timing of last food intake and timing of last defecation. There is a large variance in interpretation of fecal loading, and any stool seen on an x-ray does not rule out another potential diagnosis causing abdominal pain.
In 2014, the North American Society for Pediatric Gastroenterology, Hepatology, & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) released joint clinical guidelines that the evidence supports not obtaining an abdominal x-ray to diagnose functional constipation.8 Similarly, the National Institute for Health and Care Excellence (NICE) stated that abdominal x-rays should not be recommended as an additional test for constipation in children.9 These groups advocate for diagnosing functional constipation clinically by using a careful history and physical exam.
WHY ABDOMINAL X-RAYS ARE POTENTIALLY HARMFUL
The primary patient harm associated with abdominal x-rays is radiation exposure. While the amount of radiation in a single x-ray is low, children with constipation tend to have frequent revisits, significantly more than children with other common chronic conditions (eg, asthma and migraine headaches).10
One multicenter retrospective cohort study that included approximately 282,000 children diagnosed with constipation found that children who received an abdominal x-ray were twice as likely to return to the emergency department with a clinically significant alternate diagnosis (0.33% vs 0.17%). The 2 most common missed diagnoses were acute appendicitis and intussusception.3 Another retrospective study that included about 3,700 children also found that x-rays were performed more frequently in children who were misdiagnosed than in those who did not have a significant alternate diagnosis (75% vs 46%).11 In this case, both of these groups had a similar amount of stool on the x-rays as determined by the mean Leech scores. While this study identified an association between abdominal x-ray use and misdiagnoses, a causative effect was not necessarily discovered between the 2. The authors felt that even relatively large amounts of stool on an x-ray should not discount serious causes of abdominal pain or tenderness.11 A third retrospective study determined that children who received an abdominal x-ray and were diagnosed with constipation were significantly more likely to be admitted to the hospital, further raising healthcare costs.12 In this study, having an x-ray reduced the odds of being discharged home by about half. They also found that abdominal x-rays could be avoided if digital rectal exams were performed.12
HOW CONSTIPATION SHOULD BE DIAGNOSED
Functional constipation is a clinical diagnosis based on a thorough collection of history and a complete physical exam in children of all ages, including digital examination of the rectum to assess for fecal impaction, if necessary.
The Rome IV criteria for chronic constipation can be helpful and includes at least 2 of the following features for at least 1 month in infants up to 4 years of age: 2 or fewer stools per week, history of excessive stool retention, history of painful or hard bowel movements, history of large-diameter stools, and presence of a large fecal mass in the rectum.13 In children who are toilet trained, 2 additional criteria may be used: at least 1 episode of fecal incontinence per week after being toilet-trained and history of large-diameter stools that may obstruct the toilet.13
The NASPGHAN and ESPGHAN joint guidelines from 2014 state that, while constipation is based on history and physical exam, a major role of the history and physical exam is to exclude other disorders that also present with difficulty in defecation.8 This can help identify red-flag features or complications and guide further investigation. While evidence did not support routine use of a digital rectal exam in diagnosing constipation, the guidelines stated that a rectal exam (visual and digital) helps to evaluate for anorectal malformations, anal stenosis, rectal tone, distension, erythema, skin tags, anal fissures, or a fecal mass.8
In regard to history, approximately 0.4%-20% of healthy children without constipation have at least 1 clinical feature listed above. Therefore, the use of a single clinical finding to diagnose constipation, such as decreased bowel frequency, can result in an inappropriate diagnosis. Children experience large variations in stool output depending on diet, genetics, and environmental factors.10 The usual pattern of bowel habits in humans range from 3 times daily to every 3 days.14 Importantly, there are times to order an abdominal x-ray for patients with abdominal pain. The NASPGHAN and ESPGHAN joint guidelines recommend obtaining abdominal x-rays to evaluate children who have concerning features, such as previous abdominal surgeries, known genetic conditions or malformations, bilious emesis, or severe abdominal distension.8
RECOMMENDATIONS
- Functional constipation should be diagnosed based purely on a thorough history and physical examination, including a rectal exam
- Abdominal x-rays (ordered for any reason) should not be used to diagnose or assess for functional constipation
CONCLUSIONS
Performing abdominal x-rays to assess for pediatric functional constipation is not beneficial and potentially harmful to patients. Multiple retrospective studies revealed no diagnostic association between clinical symptoms or severity of constipation and findings on abdominal radiography. X-rays have very low sensitivity and specificity for diagnosing constipation. In the pediatric emergency department, abdominal x-rays completed for patients diagnosed with constipation have been associated with missed diagnoses, false reassurance of constipation, more frequent admissions into the hospital, longer hospital stays, higher healthcare costs, and unnecessary radiation exposure. The NICE as well as 2014 NASPGHAN and ESPGHAN clinical guidelines recommend against obtaining x-rays to diagnose constipation. The most effective way to diagnose functional constipation in children is with a thorough collection of history and physical exam. In the introductory case, the boy received an osmotic laxative based on abdominal x-ray findings, which resulted in the adverse effect of diarrhea. This case demonstrates how using abdominal x-rays to assess for constipation can be misleading and emphasizes the importance of collecting a thorough history and physical exam.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 5-year old boy is admitted to the hospital for a bowel clean-out after presenting with abdominal pain and having an abdominal x-ray that demonstrated a “moderate stool burden.” After ingestion of the bowel prep, he develops worsening abdominal cramping and diarrhea. Upon reviewing the bowel history with his mother afterward, the team learns that he has had a bowel movement every 1-2 days as usual and has been having soft stools without any straining, pain, or blood present.
BACKGROUND
Functional constipation is a common clinical problem in pediatrics and constitutes a large number of admissions into hospitals and visits to clinics and emergency departments. In the United States, up to 36% of children are affected.Associated healthcare costs for children with constipation are estimated at $5.9 billion per year, which is $3.9 billion more than the general pediatric population without constipation.1 In 2011, American children aged 17 years and younger had more than 270,000 visits to the emergency department for constipation.2 As many as 70% of children who are given a diagnosis of constipation in the emergency department have an abdominal x-ray completed.3 The carcinogenic effects of radiation from radiography are well known. Unnecessary imaging places the child at risk for these effects while adding to the overall cost of medical care.4
WHY AN ABDOMINAL X-RAY MAY SEEM HELPFUL
The overall utilization of diagnostic imaging is increasing in pediatric emergency departments.4 When questioning why this is the case, one should consider the method of problem solving used by most physicians. After formulating initial hypotheses based on available information, prior knowledge, and experience, physicians aim to obtain additional data to confirm or reject each hypothesis.5
A 2017 study surveyed 24 pediatric gastroenterologists after 72 patient encounters and found that the most common cause for obtaining an abdominal x-ray was for evaluation of stool burden (70%).5 Other reasons included assessing the need for a bowel clean-out (35%), diagnosing fecal impaction (27%), finding the cause for abdominal pain (24%), and demonstrating stool burden to a family (14%). This same study found that most of the polled providers used an abdominal x-ray to assess for constipation, and nearly half changed their management based on the findings.
WHY ABDOMINAL X-RAYS ARE NOT HELPFUL
Many systematic reviews and retrospective studies have investigated the efficacy of abdominal x-rays for diagnosing constipation. One retrospective review involving 160 children with defecation complaints assessed the accuracy of different radiologic scoring methods in identifying children with constipation.6 Three pediatric gastroenterologists and 1 pediatric radiologist blindly applied 4 scoring methods: colonic transit time, Leech score, Barr score, and fecal loading. The results showed that all x-ray scoring methods had low sensitivity for diagnosing constipation, variable specificity, and low interobserver reproducibility of scores.6 There was also poor ability to differentiate between patients with constipation and nonretentive fecal incontinence. Fecal loading had the worst performance in differentiating between these 2. Greater than 20% of children with clinically diagnosed constipation had normal Barr and Leech scores.6 Another systematic review also found no evidence for a diagnostic association between clinical symptoms of constipation and fecal loading on abdominal x-rays.7 In this study, the sensitivity and specificity of the x-ray were as low as 61% and 55%, respectively, which indicate poor overall diagnostic accuracy. Abdominal x-rays are subjective, not standardized, and represent a single observation in time. The amount of fecal loading seen on imaging is subject to daily variation depending on the timing of last food intake and timing of last defecation. There is a large variance in interpretation of fecal loading, and any stool seen on an x-ray does not rule out another potential diagnosis causing abdominal pain.
In 2014, the North American Society for Pediatric Gastroenterology, Hepatology, & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) released joint clinical guidelines that the evidence supports not obtaining an abdominal x-ray to diagnose functional constipation.8 Similarly, the National Institute for Health and Care Excellence (NICE) stated that abdominal x-rays should not be recommended as an additional test for constipation in children.9 These groups advocate for diagnosing functional constipation clinically by using a careful history and physical exam.
WHY ABDOMINAL X-RAYS ARE POTENTIALLY HARMFUL
The primary patient harm associated with abdominal x-rays is radiation exposure. While the amount of radiation in a single x-ray is low, children with constipation tend to have frequent revisits, significantly more than children with other common chronic conditions (eg, asthma and migraine headaches).10
One multicenter retrospective cohort study that included approximately 282,000 children diagnosed with constipation found that children who received an abdominal x-ray were twice as likely to return to the emergency department with a clinically significant alternate diagnosis (0.33% vs 0.17%). The 2 most common missed diagnoses were acute appendicitis and intussusception.3 Another retrospective study that included about 3,700 children also found that x-rays were performed more frequently in children who were misdiagnosed than in those who did not have a significant alternate diagnosis (75% vs 46%).11 In this case, both of these groups had a similar amount of stool on the x-rays as determined by the mean Leech scores. While this study identified an association between abdominal x-ray use and misdiagnoses, a causative effect was not necessarily discovered between the 2. The authors felt that even relatively large amounts of stool on an x-ray should not discount serious causes of abdominal pain or tenderness.11 A third retrospective study determined that children who received an abdominal x-ray and were diagnosed with constipation were significantly more likely to be admitted to the hospital, further raising healthcare costs.12 In this study, having an x-ray reduced the odds of being discharged home by about half. They also found that abdominal x-rays could be avoided if digital rectal exams were performed.12
HOW CONSTIPATION SHOULD BE DIAGNOSED
Functional constipation is a clinical diagnosis based on a thorough collection of history and a complete physical exam in children of all ages, including digital examination of the rectum to assess for fecal impaction, if necessary.
The Rome IV criteria for chronic constipation can be helpful and includes at least 2 of the following features for at least 1 month in infants up to 4 years of age: 2 or fewer stools per week, history of excessive stool retention, history of painful or hard bowel movements, history of large-diameter stools, and presence of a large fecal mass in the rectum.13 In children who are toilet trained, 2 additional criteria may be used: at least 1 episode of fecal incontinence per week after being toilet-trained and history of large-diameter stools that may obstruct the toilet.13
The NASPGHAN and ESPGHAN joint guidelines from 2014 state that, while constipation is based on history and physical exam, a major role of the history and physical exam is to exclude other disorders that also present with difficulty in defecation.8 This can help identify red-flag features or complications and guide further investigation. While evidence did not support routine use of a digital rectal exam in diagnosing constipation, the guidelines stated that a rectal exam (visual and digital) helps to evaluate for anorectal malformations, anal stenosis, rectal tone, distension, erythema, skin tags, anal fissures, or a fecal mass.8
In regard to history, approximately 0.4%-20% of healthy children without constipation have at least 1 clinical feature listed above. Therefore, the use of a single clinical finding to diagnose constipation, such as decreased bowel frequency, can result in an inappropriate diagnosis. Children experience large variations in stool output depending on diet, genetics, and environmental factors.10 The usual pattern of bowel habits in humans range from 3 times daily to every 3 days.14 Importantly, there are times to order an abdominal x-ray for patients with abdominal pain. The NASPGHAN and ESPGHAN joint guidelines recommend obtaining abdominal x-rays to evaluate children who have concerning features, such as previous abdominal surgeries, known genetic conditions or malformations, bilious emesis, or severe abdominal distension.8
RECOMMENDATIONS
- Functional constipation should be diagnosed based purely on a thorough history and physical examination, including a rectal exam
- Abdominal x-rays (ordered for any reason) should not be used to diagnose or assess for functional constipation
CONCLUSIONS
Performing abdominal x-rays to assess for pediatric functional constipation is not beneficial and potentially harmful to patients. Multiple retrospective studies revealed no diagnostic association between clinical symptoms or severity of constipation and findings on abdominal radiography. X-rays have very low sensitivity and specificity for diagnosing constipation. In the pediatric emergency department, abdominal x-rays completed for patients diagnosed with constipation have been associated with missed diagnoses, false reassurance of constipation, more frequent admissions into the hospital, longer hospital stays, higher healthcare costs, and unnecessary radiation exposure. The NICE as well as 2014 NASPGHAN and ESPGHAN clinical guidelines recommend against obtaining x-rays to diagnose constipation. The most effective way to diagnose functional constipation in children is with a thorough collection of history and physical exam. In the introductory case, the boy received an osmotic laxative based on abdominal x-ray findings, which resulted in the adverse effect of diarrhea. This case demonstrates how using abdominal x-rays to assess for constipation can be misleading and emphasizes the importance of collecting a thorough history and physical exam.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Afzal NA, Tighe MP, Thomson MA. (2011, June 13). Constipation in children. Ital J Pediatr. 2011;37:28. https://doi.org/10.1186/1824-7288-37-28.
2. Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110(4):572-579. https://doi.org/10.1038/ajg.2015.64.
3. Freedman SB, Rodean J, Hall M, et al. (2017). Delayed diagnoses in children with constipation: multicenter retrospective cohort study. J Pediatr. 186, 87-94.e16. https://doi.org/10.1016/j.jpeds.2017.03.061.
4. Reed MH. Imaging utilization commentary: A radiology perspective. Pediatr Radiol. 2008;38 (Suppl 4):S660-S663. https://doi.org/10.1007/s00247-008-0982-y.
5. Beinvogl B, Sabharwal S, McSweeney M, Nurko S. Are we using abdominal radiographs appropriately in the management of pediatric constipation? J Pediatr. 2017;191:179-183. https://doi.org/10.1016/j.jpeds.2017.08.075.
6. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. J Pediatr Gastroenterol Nutr. 2010;51(2):155-159. https://doi.org/10.1097/MPG.0b013e3181cb4309.
7. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J Pediatr. 2012;161(1):44–50.e502. https://doi.org/10.1016/j.jpeds.2011.12.045.
8. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258-274. https://doi.org/10.1097/mpg.0000000000000266.
9. Bardisa-Ezcurra L, Ullman R, Gordon J; Guideline Development Group. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 2010;340:c2585. https://doi.org/10.1136/bmj.c2585.
10. Rajindrajith S, Manjuri Devanarayana N, Benninga MA. Defecation Disorders in Children: Constipation and Functional Fecal Incontinence. In: Guandalini S, Dhawan A, Branski D. eds. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice (1st ed.). Basingstoke, England: Springer; 2016:247-260.
11. Freedman SB, Thull-Freedman J, Manson D, et al. Pediatric abdominal radiograph use, constipation, and significant misdiagnoses. J Pediatr. 2014;164(1):83-88.e2. https://doi.org/10.1016/j.jpeds.2013.08.074.
12. Chumpitazi CE, Rees CA, Camp EA, Henkel EB, Valdez KL, Chumpitazi BP. Diagnostic approach to constipation impacts pediatric emergency department disposition. Am J Emerg Med. 2017;35(10):1490-1493. https://doi.org/10.1016/j.ajem.2017.04.060.
13. Benninga MA, Nurko S, Faure C, Hyman PE, St. James Roberts I, Schechter NL. Childhood functional GI disorders: Neonate/toddler. Gastroenterology. 2016;150(6):1443-1455. https://doi.org/10.1053/j.gastro.2016.02.016.
14. Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol. 2010;45(5):556-566. https://doi.org/10.3109/00365520903551332.
1. Afzal NA, Tighe MP, Thomson MA. (2011, June 13). Constipation in children. Ital J Pediatr. 2011;37:28. https://doi.org/10.1186/1824-7288-37-28.
2. Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110(4):572-579. https://doi.org/10.1038/ajg.2015.64.
3. Freedman SB, Rodean J, Hall M, et al. (2017). Delayed diagnoses in children with constipation: multicenter retrospective cohort study. J Pediatr. 186, 87-94.e16. https://doi.org/10.1016/j.jpeds.2017.03.061.
4. Reed MH. Imaging utilization commentary: A radiology perspective. Pediatr Radiol. 2008;38 (Suppl 4):S660-S663. https://doi.org/10.1007/s00247-008-0982-y.
5. Beinvogl B, Sabharwal S, McSweeney M, Nurko S. Are we using abdominal radiographs appropriately in the management of pediatric constipation? J Pediatr. 2017;191:179-183. https://doi.org/10.1016/j.jpeds.2017.08.075.
6. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. J Pediatr Gastroenterol Nutr. 2010;51(2):155-159. https://doi.org/10.1097/MPG.0b013e3181cb4309.
7. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J Pediatr. 2012;161(1):44–50.e502. https://doi.org/10.1016/j.jpeds.2011.12.045.
8. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258-274. https://doi.org/10.1097/mpg.0000000000000266.
9. Bardisa-Ezcurra L, Ullman R, Gordon J; Guideline Development Group. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 2010;340:c2585. https://doi.org/10.1136/bmj.c2585.
10. Rajindrajith S, Manjuri Devanarayana N, Benninga MA. Defecation Disorders in Children: Constipation and Functional Fecal Incontinence. In: Guandalini S, Dhawan A, Branski D. eds. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice (1st ed.). Basingstoke, England: Springer; 2016:247-260.
11. Freedman SB, Thull-Freedman J, Manson D, et al. Pediatric abdominal radiograph use, constipation, and significant misdiagnoses. J Pediatr. 2014;164(1):83-88.e2. https://doi.org/10.1016/j.jpeds.2013.08.074.
12. Chumpitazi CE, Rees CA, Camp EA, Henkel EB, Valdez KL, Chumpitazi BP. Diagnostic approach to constipation impacts pediatric emergency department disposition. Am J Emerg Med. 2017;35(10):1490-1493. https://doi.org/10.1016/j.ajem.2017.04.060.
13. Benninga MA, Nurko S, Faure C, Hyman PE, St. James Roberts I, Schechter NL. Childhood functional GI disorders: Neonate/toddler. Gastroenterology. 2016;150(6):1443-1455. https://doi.org/10.1053/j.gastro.2016.02.016.
14. Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol. 2010;45(5):556-566. https://doi.org/10.3109/00365520903551332.
© 2020 Society of Hospital Medicine
A Traumatic Traveler
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
© 2020 Society of Hospital Medicine
Maternal mortality: A national crisis
This article is the first in a series on maternal mortality.
“You’re in really bad shape, kid. I don’t know if you’re gonna live through the night. I’m going to do everything I can to save your life, but the truth is you might die.”
If Timoria McQueen Saba imagined the words she would hear in the moments after she gave birth, those likely weren’t among them. But then she started to bleed. The energy around her shifted; she felt the urgency and intensity in the room, and she could see it – reflected from the television monitor over her bed – in the faces of her care team. After her husband and newborn daughter were led from the room, she did, in fact, hear those words.
They were spoken by a surgeon called in after efforts to control the bleeding failed – emetic words that joined forces with her hemorrhaging and confusion and fear, and as she began to vomit, her eyelids felt heavy. She fought to keep them open, sensing that if she closed them they might never open again.
In 2018 alone, similar words perhaps were spoken to the 658 U.S. women who suffered maternal complications and whose eyes never did open again. This is the latest official maternal mortality data from the Centers for Disease Control and Prevention.
Ms. Saba’s eyes, however, remained open through her birth trauma and through the PTSD that followed. A fierce advocate for maternal health, she shares her story often, as she did during a panel discussion at the American College of Obstetricians and Gynecologists’ annual meeting in May 2019, in an effort to improve outcomes for other women and families.
But her story unfolded nearly a decade ago and those eyes still are seeing women die from childbirth. Despite her efforts and the efforts of countless other individuals and organizations working to improve maternal outcomes, the new CDC data show that the United States has the highest maternal mortality rate of any similarly wealthy industrialized nation.
“I cannot believe I’m still talking about this issue,” Ms. Saba told a standing-room-only crowd and her copanelists Neel T. Shah, MD, and Charles S. Johnson IV, whose wife, Kira, died in 2016 during surgery for bleeding complications following the birth of their second child. “If all the people who I’d written to had just listened maybe once and tried to propel my message forward back then, Charles would be in a much better situation and so would his children.”
Mr. Johnson said that for 10 hours he and other family members pleaded for help for Kira, a healthy, vibrant women he described as “sunshine personified.”
She showed signs of postpartum bleeding after delivering a healthy baby boy by C-section, but a “STAT CT” order went unheeded for hours before she was finally taken for surgery.
“You’re walking down this corridor, you get to this point, these double doors open, and you just can’t go any further – and that was the last time I saw my wife alive,” he said. “When they took Kira back into the operating room, there were three-and-a-half liters of blood in her abdomen, and her heart stopped immediately.
Kira Johnson died April 13, 2016.
“I’m not here to tell you what I think, I’m here to tell you what I know, and that’s that Kira deserved so much better, and that Kira’s not alone, and that women all over this country deserve so much better.”
The U.S. maternal mortality crisis
Dr. Shah, an ob.gyn. at Beth Israel Deaconess Medical Center and director of the Delivery Decisions Initiative at Harvard Medical School’s Ariadne Labs, both in Boston, where he has “been on this mission to improve safety in childbirth for years now,” echoed Ms. Saba’s dismay regarding the pace of progress.
“It’s not just about the present, it’s about the future, it’s about the pact that every generation ought to have with the next one to leave things at least as well as they found them. And when it comes to the health of our moms in this country, we are not doing that,” he said. “An American mom today is 50% more likely to die in childbirth than her own mother was, and 3-4 times more likely to die if she’s black than if she’s white.”
Indeed, the data released Jan. 30 by the CDC’s National Center for Health Statistics (NCHS) – the first on maternal mortality released by the agency since 2007 – show a U.S. maternal mortality rate of 17.4 maternal deaths per 100,000 live births in 2018.
The rate is higher than the 12.7 per 100,000 live births reported in 2007, but the increase is attributable mostly to changes in data collection and reporting methods. In 2003, “a consensus process recommended that all states add a standardized ‘checkbox’ to improve the identification of maternal deaths,” and implementation wasn’t complete until 2017 as “funding, technology, and state laws allowed,” meaning 2018 was the first year that data were reported in a standardized fashion across states, the CDC explained in a press release.
The data demonstrate ongoing wide racial/ethnic disparities: the maternal mortality rates for non-Hispanic black women, non-Hispanic white women, and Hispanic women were 37.1, 14.7, and 11.8 per 100,000 live births, consistent with earlier data.
Further, the rates for women aged 40 years and over were nearly eightfold higher than for those under age 25 years (81.9 vs. 10.6 per 100,000 live births).
CDC officials noted, however, that inconsistencies in reporting still leave some question about the accuracy of the data, stating in the release that “NCHS has identified instances where application of the checkbox information according to coding rules led to misclassification of maternal deaths.”
The agency is making changes in rules and reporting to ensure greater accuracy, but the numbers nevertheless reveal a startling truth: “The United States is the most dangerous place to deliver a baby in the industrialized world.”
Progress and challenges
Rebekah Gee, MD, an ob.gyn. who served for 4 years as Secretary of the Louisiana Department of Health before leaving the position in January, made that statement during another panel discussion at ACOG 2019 – The President’s Panel: Maternal Mortality: Progress Toward Prevention – which was moderated by Lisa M. Hollier, MD, now the immediate past president of ACOG.
That’s not to say progress hasn’t been or can’t be made, Dr. Gee said.
In fact, quality improvement measures she facilitated in Louisiana led to a 25% reduction in infant mortality and a 10% reduction in neonatal intensive care unit admissions, demonstrating the potential for improvement with such initiatives, but addressing maternal issues is a greater challenge, she said.
“I think part of the sad truth is that we really focus on babies first, not moms ... and that needs to change,” Dr. Gee said.
Dr. Hollier focused much of her attention during her tenure as ACOG president on doing just that, particularly through an emphasis on heart disease, which is the leading cause of U.S. maternal deaths in pregnancy and the postpartum period.
In an interview, she shared her thoughts on the progress achieved and the work that remains.
ACOG was instrumental in the enactment of the Preventing Maternal Deaths Act of 2018, which appropriated funding for Enhancing Reviews and Surveillance to Eliminate Maternal Mortality (ERASE MM), a CDC initiative to support state-based maternal mortality review committees, said Dr. Hollier, professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
“The really great news is that almost immediately after passage of the legislation, the CDC put out the notice of the funding opportunity, and they were able to provide 24 awards supporting 25 states,” she said.
ERASE MM will enhance state data collection and availability and enable a level of data sharing that “will really add strength and depth to reporting from the maternal mortality review committees, which really provides us with the best information we have to truly understand the causes, the contributing factors, and the strategies that can be put in place to prevent future maternal deaths.”
Further, the Alliance for Innovation on Maternal Health (AIM) program, a cooperative agreement with the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau to improve safety and outcomes through evidence-based patient safety bundles, was extended, and in May 2019, ACOG updated its guidance on managing cardiac contributors to maternal mortality, releasing its “Pregnancy and Heart Disease” Practice Bulletin, she said.
Dr. Hollier continues in her quest for improved maternal outcomes. She is slated to deliver a keynote address at the American College of Cardiology/World Congress of Cardiology conference March 28 in Chicago.
“I’m so excited ... to talk about the new guidelines that we’ve put out and to really talk about how cardiologists and ob.gyns. can work together to improve women’s health outcomes,” she said, adding that she already is seeing a strengthening of such partnerships.
A number of academic institutions are developing “pregnancy heart teams” to identify and care for women who have or develop heart disease during pregnancy.
“This type of collaboration ... is going to be essential to address mortality from cardiovascular causes and from cardiomyopathy, which accounts for about 25% of all maternal mortality,” she said. “The next area where we really need some buy-in is from our emergency physicians.”
Enhanced collaboration with emergency physicians and other specialties present opportunities to better identify and address pregnancy-related complications and sequelae, she said.
“Women are dying because they’re not being diagnosed,” she added. “We have to raise that level of awareness – it’s just absolutely critical.”
Identifying and addressing drivers of the crisis
Dr. Gee further emphasized the importance of addressing maternal health, noting that for every woman who dies from maternal causes, 100 experience maternal morbidity.
“It’s startling and it’s scary,” she said. “We are looking at this not just as a problem of outcomes, but a problem of racial inequity and racial bias and implicit bias.”
When she and her team assessed maternal mortality in Louisiana, they looked specifically at whether each death could have been prevented if, for example, blood was given sooner, cardiomyopathy was recognized sooner, or hypertension was treated on time.
“When we looked at these numbers ... when we looked at white women, 9% of the time we could have done better with our medical care; with black women, 59% of the time we could have saved her life with better care,” said Dr Gee, who is a gratis assistant professor of obstetrics and gynecology at Louisiana State University, New Orleans. “And if that doesn’t convince you that racial bias is an incredibly important thing to address – that we need to have a conversation about and address at a national level – I don’t know what would.”
In fact, numerous health, societal, socioeconomic, and other factors – some known, some yet to be identified, and many inter-related – are among the drivers of the U.S. maternal mortality crisis. In the coming months, an Ob.Gyn. News team will examine several of these drivers in depth. We’ll look specifically at the role of racism and bias, and at urban-rural disparities in access and outcomes – especially for women of color and indigenous women. We’ll address the scope and impact of each, successes and failures in addressing the problems, and ongoing initiatives.
Follow us for insights from experts, researchers, practicing physicians, and patients and families affected by the maternal mortality crisis, and stay with us through coverage of ACOG 2020 for perspective on what, specifically, ob.gyns. can do about it.
Mr. Johnson proposed a starting point:
“Here’s the good news – you guys ready for this? We can fix this,” he said, adding that the solution starts with “speaking Timoria’s name ... speaking the name of Kira Dixon Johnson ... speaking the names of these women and then asking the people that are around you, ‘What are we prepared to do to make sure that this doesn’t happen to other women.’ ”
This article is the first in a series on maternal mortality.
“You’re in really bad shape, kid. I don’t know if you’re gonna live through the night. I’m going to do everything I can to save your life, but the truth is you might die.”
If Timoria McQueen Saba imagined the words she would hear in the moments after she gave birth, those likely weren’t among them. But then she started to bleed. The energy around her shifted; she felt the urgency and intensity in the room, and she could see it – reflected from the television monitor over her bed – in the faces of her care team. After her husband and newborn daughter were led from the room, she did, in fact, hear those words.
They were spoken by a surgeon called in after efforts to control the bleeding failed – emetic words that joined forces with her hemorrhaging and confusion and fear, and as she began to vomit, her eyelids felt heavy. She fought to keep them open, sensing that if she closed them they might never open again.
In 2018 alone, similar words perhaps were spoken to the 658 U.S. women who suffered maternal complications and whose eyes never did open again. This is the latest official maternal mortality data from the Centers for Disease Control and Prevention.
Ms. Saba’s eyes, however, remained open through her birth trauma and through the PTSD that followed. A fierce advocate for maternal health, she shares her story often, as she did during a panel discussion at the American College of Obstetricians and Gynecologists’ annual meeting in May 2019, in an effort to improve outcomes for other women and families.
But her story unfolded nearly a decade ago and those eyes still are seeing women die from childbirth. Despite her efforts and the efforts of countless other individuals and organizations working to improve maternal outcomes, the new CDC data show that the United States has the highest maternal mortality rate of any similarly wealthy industrialized nation.
“I cannot believe I’m still talking about this issue,” Ms. Saba told a standing-room-only crowd and her copanelists Neel T. Shah, MD, and Charles S. Johnson IV, whose wife, Kira, died in 2016 during surgery for bleeding complications following the birth of their second child. “If all the people who I’d written to had just listened maybe once and tried to propel my message forward back then, Charles would be in a much better situation and so would his children.”
Mr. Johnson said that for 10 hours he and other family members pleaded for help for Kira, a healthy, vibrant women he described as “sunshine personified.”
She showed signs of postpartum bleeding after delivering a healthy baby boy by C-section, but a “STAT CT” order went unheeded for hours before she was finally taken for surgery.
“You’re walking down this corridor, you get to this point, these double doors open, and you just can’t go any further – and that was the last time I saw my wife alive,” he said. “When they took Kira back into the operating room, there were three-and-a-half liters of blood in her abdomen, and her heart stopped immediately.
Kira Johnson died April 13, 2016.
“I’m not here to tell you what I think, I’m here to tell you what I know, and that’s that Kira deserved so much better, and that Kira’s not alone, and that women all over this country deserve so much better.”
The U.S. maternal mortality crisis
Dr. Shah, an ob.gyn. at Beth Israel Deaconess Medical Center and director of the Delivery Decisions Initiative at Harvard Medical School’s Ariadne Labs, both in Boston, where he has “been on this mission to improve safety in childbirth for years now,” echoed Ms. Saba’s dismay regarding the pace of progress.
“It’s not just about the present, it’s about the future, it’s about the pact that every generation ought to have with the next one to leave things at least as well as they found them. And when it comes to the health of our moms in this country, we are not doing that,” he said. “An American mom today is 50% more likely to die in childbirth than her own mother was, and 3-4 times more likely to die if she’s black than if she’s white.”
Indeed, the data released Jan. 30 by the CDC’s National Center for Health Statistics (NCHS) – the first on maternal mortality released by the agency since 2007 – show a U.S. maternal mortality rate of 17.4 maternal deaths per 100,000 live births in 2018.
The rate is higher than the 12.7 per 100,000 live births reported in 2007, but the increase is attributable mostly to changes in data collection and reporting methods. In 2003, “a consensus process recommended that all states add a standardized ‘checkbox’ to improve the identification of maternal deaths,” and implementation wasn’t complete until 2017 as “funding, technology, and state laws allowed,” meaning 2018 was the first year that data were reported in a standardized fashion across states, the CDC explained in a press release.
The data demonstrate ongoing wide racial/ethnic disparities: the maternal mortality rates for non-Hispanic black women, non-Hispanic white women, and Hispanic women were 37.1, 14.7, and 11.8 per 100,000 live births, consistent with earlier data.
Further, the rates for women aged 40 years and over were nearly eightfold higher than for those under age 25 years (81.9 vs. 10.6 per 100,000 live births).
CDC officials noted, however, that inconsistencies in reporting still leave some question about the accuracy of the data, stating in the release that “NCHS has identified instances where application of the checkbox information according to coding rules led to misclassification of maternal deaths.”
The agency is making changes in rules and reporting to ensure greater accuracy, but the numbers nevertheless reveal a startling truth: “The United States is the most dangerous place to deliver a baby in the industrialized world.”
Progress and challenges
Rebekah Gee, MD, an ob.gyn. who served for 4 years as Secretary of the Louisiana Department of Health before leaving the position in January, made that statement during another panel discussion at ACOG 2019 – The President’s Panel: Maternal Mortality: Progress Toward Prevention – which was moderated by Lisa M. Hollier, MD, now the immediate past president of ACOG.
That’s not to say progress hasn’t been or can’t be made, Dr. Gee said.
In fact, quality improvement measures she facilitated in Louisiana led to a 25% reduction in infant mortality and a 10% reduction in neonatal intensive care unit admissions, demonstrating the potential for improvement with such initiatives, but addressing maternal issues is a greater challenge, she said.
“I think part of the sad truth is that we really focus on babies first, not moms ... and that needs to change,” Dr. Gee said.
Dr. Hollier focused much of her attention during her tenure as ACOG president on doing just that, particularly through an emphasis on heart disease, which is the leading cause of U.S. maternal deaths in pregnancy and the postpartum period.
In an interview, she shared her thoughts on the progress achieved and the work that remains.
ACOG was instrumental in the enactment of the Preventing Maternal Deaths Act of 2018, which appropriated funding for Enhancing Reviews and Surveillance to Eliminate Maternal Mortality (ERASE MM), a CDC initiative to support state-based maternal mortality review committees, said Dr. Hollier, professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
“The really great news is that almost immediately after passage of the legislation, the CDC put out the notice of the funding opportunity, and they were able to provide 24 awards supporting 25 states,” she said.
ERASE MM will enhance state data collection and availability and enable a level of data sharing that “will really add strength and depth to reporting from the maternal mortality review committees, which really provides us with the best information we have to truly understand the causes, the contributing factors, and the strategies that can be put in place to prevent future maternal deaths.”
Further, the Alliance for Innovation on Maternal Health (AIM) program, a cooperative agreement with the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau to improve safety and outcomes through evidence-based patient safety bundles, was extended, and in May 2019, ACOG updated its guidance on managing cardiac contributors to maternal mortality, releasing its “Pregnancy and Heart Disease” Practice Bulletin, she said.
Dr. Hollier continues in her quest for improved maternal outcomes. She is slated to deliver a keynote address at the American College of Cardiology/World Congress of Cardiology conference March 28 in Chicago.
“I’m so excited ... to talk about the new guidelines that we’ve put out and to really talk about how cardiologists and ob.gyns. can work together to improve women’s health outcomes,” she said, adding that she already is seeing a strengthening of such partnerships.
A number of academic institutions are developing “pregnancy heart teams” to identify and care for women who have or develop heart disease during pregnancy.
“This type of collaboration ... is going to be essential to address mortality from cardiovascular causes and from cardiomyopathy, which accounts for about 25% of all maternal mortality,” she said. “The next area where we really need some buy-in is from our emergency physicians.”
Enhanced collaboration with emergency physicians and other specialties present opportunities to better identify and address pregnancy-related complications and sequelae, she said.
“Women are dying because they’re not being diagnosed,” she added. “We have to raise that level of awareness – it’s just absolutely critical.”
Identifying and addressing drivers of the crisis
Dr. Gee further emphasized the importance of addressing maternal health, noting that for every woman who dies from maternal causes, 100 experience maternal morbidity.
“It’s startling and it’s scary,” she said. “We are looking at this not just as a problem of outcomes, but a problem of racial inequity and racial bias and implicit bias.”
When she and her team assessed maternal mortality in Louisiana, they looked specifically at whether each death could have been prevented if, for example, blood was given sooner, cardiomyopathy was recognized sooner, or hypertension was treated on time.
“When we looked at these numbers ... when we looked at white women, 9% of the time we could have done better with our medical care; with black women, 59% of the time we could have saved her life with better care,” said Dr Gee, who is a gratis assistant professor of obstetrics and gynecology at Louisiana State University, New Orleans. “And if that doesn’t convince you that racial bias is an incredibly important thing to address – that we need to have a conversation about and address at a national level – I don’t know what would.”
In fact, numerous health, societal, socioeconomic, and other factors – some known, some yet to be identified, and many inter-related – are among the drivers of the U.S. maternal mortality crisis. In the coming months, an Ob.Gyn. News team will examine several of these drivers in depth. We’ll look specifically at the role of racism and bias, and at urban-rural disparities in access and outcomes – especially for women of color and indigenous women. We’ll address the scope and impact of each, successes and failures in addressing the problems, and ongoing initiatives.
Follow us for insights from experts, researchers, practicing physicians, and patients and families affected by the maternal mortality crisis, and stay with us through coverage of ACOG 2020 for perspective on what, specifically, ob.gyns. can do about it.
Mr. Johnson proposed a starting point:
“Here’s the good news – you guys ready for this? We can fix this,” he said, adding that the solution starts with “speaking Timoria’s name ... speaking the name of Kira Dixon Johnson ... speaking the names of these women and then asking the people that are around you, ‘What are we prepared to do to make sure that this doesn’t happen to other women.’ ”
This article is the first in a series on maternal mortality.
“You’re in really bad shape, kid. I don’t know if you’re gonna live through the night. I’m going to do everything I can to save your life, but the truth is you might die.”
If Timoria McQueen Saba imagined the words she would hear in the moments after she gave birth, those likely weren’t among them. But then she started to bleed. The energy around her shifted; she felt the urgency and intensity in the room, and she could see it – reflected from the television monitor over her bed – in the faces of her care team. After her husband and newborn daughter were led from the room, she did, in fact, hear those words.
They were spoken by a surgeon called in after efforts to control the bleeding failed – emetic words that joined forces with her hemorrhaging and confusion and fear, and as she began to vomit, her eyelids felt heavy. She fought to keep them open, sensing that if she closed them they might never open again.
In 2018 alone, similar words perhaps were spoken to the 658 U.S. women who suffered maternal complications and whose eyes never did open again. This is the latest official maternal mortality data from the Centers for Disease Control and Prevention.
Ms. Saba’s eyes, however, remained open through her birth trauma and through the PTSD that followed. A fierce advocate for maternal health, she shares her story often, as she did during a panel discussion at the American College of Obstetricians and Gynecologists’ annual meeting in May 2019, in an effort to improve outcomes for other women and families.
But her story unfolded nearly a decade ago and those eyes still are seeing women die from childbirth. Despite her efforts and the efforts of countless other individuals and organizations working to improve maternal outcomes, the new CDC data show that the United States has the highest maternal mortality rate of any similarly wealthy industrialized nation.
“I cannot believe I’m still talking about this issue,” Ms. Saba told a standing-room-only crowd and her copanelists Neel T. Shah, MD, and Charles S. Johnson IV, whose wife, Kira, died in 2016 during surgery for bleeding complications following the birth of their second child. “If all the people who I’d written to had just listened maybe once and tried to propel my message forward back then, Charles would be in a much better situation and so would his children.”
Mr. Johnson said that for 10 hours he and other family members pleaded for help for Kira, a healthy, vibrant women he described as “sunshine personified.”
She showed signs of postpartum bleeding after delivering a healthy baby boy by C-section, but a “STAT CT” order went unheeded for hours before she was finally taken for surgery.
“You’re walking down this corridor, you get to this point, these double doors open, and you just can’t go any further – and that was the last time I saw my wife alive,” he said. “When they took Kira back into the operating room, there were three-and-a-half liters of blood in her abdomen, and her heart stopped immediately.
Kira Johnson died April 13, 2016.
“I’m not here to tell you what I think, I’m here to tell you what I know, and that’s that Kira deserved so much better, and that Kira’s not alone, and that women all over this country deserve so much better.”
The U.S. maternal mortality crisis
Dr. Shah, an ob.gyn. at Beth Israel Deaconess Medical Center and director of the Delivery Decisions Initiative at Harvard Medical School’s Ariadne Labs, both in Boston, where he has “been on this mission to improve safety in childbirth for years now,” echoed Ms. Saba’s dismay regarding the pace of progress.
“It’s not just about the present, it’s about the future, it’s about the pact that every generation ought to have with the next one to leave things at least as well as they found them. And when it comes to the health of our moms in this country, we are not doing that,” he said. “An American mom today is 50% more likely to die in childbirth than her own mother was, and 3-4 times more likely to die if she’s black than if she’s white.”
Indeed, the data released Jan. 30 by the CDC’s National Center for Health Statistics (NCHS) – the first on maternal mortality released by the agency since 2007 – show a U.S. maternal mortality rate of 17.4 maternal deaths per 100,000 live births in 2018.
The rate is higher than the 12.7 per 100,000 live births reported in 2007, but the increase is attributable mostly to changes in data collection and reporting methods. In 2003, “a consensus process recommended that all states add a standardized ‘checkbox’ to improve the identification of maternal deaths,” and implementation wasn’t complete until 2017 as “funding, technology, and state laws allowed,” meaning 2018 was the first year that data were reported in a standardized fashion across states, the CDC explained in a press release.
The data demonstrate ongoing wide racial/ethnic disparities: the maternal mortality rates for non-Hispanic black women, non-Hispanic white women, and Hispanic women were 37.1, 14.7, and 11.8 per 100,000 live births, consistent with earlier data.
Further, the rates for women aged 40 years and over were nearly eightfold higher than for those under age 25 years (81.9 vs. 10.6 per 100,000 live births).
CDC officials noted, however, that inconsistencies in reporting still leave some question about the accuracy of the data, stating in the release that “NCHS has identified instances where application of the checkbox information according to coding rules led to misclassification of maternal deaths.”
The agency is making changes in rules and reporting to ensure greater accuracy, but the numbers nevertheless reveal a startling truth: “The United States is the most dangerous place to deliver a baby in the industrialized world.”
Progress and challenges
Rebekah Gee, MD, an ob.gyn. who served for 4 years as Secretary of the Louisiana Department of Health before leaving the position in January, made that statement during another panel discussion at ACOG 2019 – The President’s Panel: Maternal Mortality: Progress Toward Prevention – which was moderated by Lisa M. Hollier, MD, now the immediate past president of ACOG.
That’s not to say progress hasn’t been or can’t be made, Dr. Gee said.
In fact, quality improvement measures she facilitated in Louisiana led to a 25% reduction in infant mortality and a 10% reduction in neonatal intensive care unit admissions, demonstrating the potential for improvement with such initiatives, but addressing maternal issues is a greater challenge, she said.
“I think part of the sad truth is that we really focus on babies first, not moms ... and that needs to change,” Dr. Gee said.
Dr. Hollier focused much of her attention during her tenure as ACOG president on doing just that, particularly through an emphasis on heart disease, which is the leading cause of U.S. maternal deaths in pregnancy and the postpartum period.
In an interview, she shared her thoughts on the progress achieved and the work that remains.
ACOG was instrumental in the enactment of the Preventing Maternal Deaths Act of 2018, which appropriated funding for Enhancing Reviews and Surveillance to Eliminate Maternal Mortality (ERASE MM), a CDC initiative to support state-based maternal mortality review committees, said Dr. Hollier, professor of obstetrics and gynecology at Baylor College of Medicine, Houston.
“The really great news is that almost immediately after passage of the legislation, the CDC put out the notice of the funding opportunity, and they were able to provide 24 awards supporting 25 states,” she said.
ERASE MM will enhance state data collection and availability and enable a level of data sharing that “will really add strength and depth to reporting from the maternal mortality review committees, which really provides us with the best information we have to truly understand the causes, the contributing factors, and the strategies that can be put in place to prevent future maternal deaths.”
Further, the Alliance for Innovation on Maternal Health (AIM) program, a cooperative agreement with the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau to improve safety and outcomes through evidence-based patient safety bundles, was extended, and in May 2019, ACOG updated its guidance on managing cardiac contributors to maternal mortality, releasing its “Pregnancy and Heart Disease” Practice Bulletin, she said.
Dr. Hollier continues in her quest for improved maternal outcomes. She is slated to deliver a keynote address at the American College of Cardiology/World Congress of Cardiology conference March 28 in Chicago.
“I’m so excited ... to talk about the new guidelines that we’ve put out and to really talk about how cardiologists and ob.gyns. can work together to improve women’s health outcomes,” she said, adding that she already is seeing a strengthening of such partnerships.
A number of academic institutions are developing “pregnancy heart teams” to identify and care for women who have or develop heart disease during pregnancy.
“This type of collaboration ... is going to be essential to address mortality from cardiovascular causes and from cardiomyopathy, which accounts for about 25% of all maternal mortality,” she said. “The next area where we really need some buy-in is from our emergency physicians.”
Enhanced collaboration with emergency physicians and other specialties present opportunities to better identify and address pregnancy-related complications and sequelae, she said.
“Women are dying because they’re not being diagnosed,” she added. “We have to raise that level of awareness – it’s just absolutely critical.”
Identifying and addressing drivers of the crisis
Dr. Gee further emphasized the importance of addressing maternal health, noting that for every woman who dies from maternal causes, 100 experience maternal morbidity.
“It’s startling and it’s scary,” she said. “We are looking at this not just as a problem of outcomes, but a problem of racial inequity and racial bias and implicit bias.”
When she and her team assessed maternal mortality in Louisiana, they looked specifically at whether each death could have been prevented if, for example, blood was given sooner, cardiomyopathy was recognized sooner, or hypertension was treated on time.
“When we looked at these numbers ... when we looked at white women, 9% of the time we could have done better with our medical care; with black women, 59% of the time we could have saved her life with better care,” said Dr Gee, who is a gratis assistant professor of obstetrics and gynecology at Louisiana State University, New Orleans. “And if that doesn’t convince you that racial bias is an incredibly important thing to address – that we need to have a conversation about and address at a national level – I don’t know what would.”
In fact, numerous health, societal, socioeconomic, and other factors – some known, some yet to be identified, and many inter-related – are among the drivers of the U.S. maternal mortality crisis. In the coming months, an Ob.Gyn. News team will examine several of these drivers in depth. We’ll look specifically at the role of racism and bias, and at urban-rural disparities in access and outcomes – especially for women of color and indigenous women. We’ll address the scope and impact of each, successes and failures in addressing the problems, and ongoing initiatives.
Follow us for insights from experts, researchers, practicing physicians, and patients and families affected by the maternal mortality crisis, and stay with us through coverage of ACOG 2020 for perspective on what, specifically, ob.gyns. can do about it.
Mr. Johnson proposed a starting point:
“Here’s the good news – you guys ready for this? We can fix this,” he said, adding that the solution starts with “speaking Timoria’s name ... speaking the name of Kira Dixon Johnson ... speaking the names of these women and then asking the people that are around you, ‘What are we prepared to do to make sure that this doesn’t happen to other women.’ ”
White House expands Medicare telehealth services amid COVID-19
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“Medicare beneficiaries across the nation, no matter where they live, will now be able to receive a wide range of services via telehealth without ever having to leave home,” CMS Administrator Seema Verma said during a March 17 White House press briefing on administration actions to contain the spread of COVID-19. “These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more.”
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using the telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health and Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states. CMS also said it will not conduct audits to ensure that an established relationship exists between the provider and the patient – a prior requirement for telehealth billing – during this public health emergency.
Billing for virtual check-ins, which are essentially brief conversations that may not require a full visit to the physician office, needs an established relationship between the practice and the patient. Likewise, for e-visits, which include non–face-to-face communications through online patient portals, billing can occur only when there is an established patient relationship.
Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She noted this was all a part of mitigation efforts to limit the spread of COVID-19.
“As we are encouraging Americans to stay home whenever possible, we don’t want our Medicare policies getting in the way,” she said, adding that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“Medicare beneficiaries across the nation, no matter where they live, will now be able to receive a wide range of services via telehealth without ever having to leave home,” CMS Administrator Seema Verma said during a March 17 White House press briefing on administration actions to contain the spread of COVID-19. “These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more.”
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using the telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health and Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states. CMS also said it will not conduct audits to ensure that an established relationship exists between the provider and the patient – a prior requirement for telehealth billing – during this public health emergency.
Billing for virtual check-ins, which are essentially brief conversations that may not require a full visit to the physician office, needs an established relationship between the practice and the patient. Likewise, for e-visits, which include non–face-to-face communications through online patient portals, billing can occur only when there is an established patient relationship.
Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She noted this was all a part of mitigation efforts to limit the spread of COVID-19.
“As we are encouraging Americans to stay home whenever possible, we don’t want our Medicare policies getting in the way,” she said, adding that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“Medicare beneficiaries across the nation, no matter where they live, will now be able to receive a wide range of services via telehealth without ever having to leave home,” CMS Administrator Seema Verma said during a March 17 White House press briefing on administration actions to contain the spread of COVID-19. “These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more.”
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using the telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health and Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states. CMS also said it will not conduct audits to ensure that an established relationship exists between the provider and the patient – a prior requirement for telehealth billing – during this public health emergency.
Billing for virtual check-ins, which are essentially brief conversations that may not require a full visit to the physician office, needs an established relationship between the practice and the patient. Likewise, for e-visits, which include non–face-to-face communications through online patient portals, billing can occur only when there is an established patient relationship.
Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She noted this was all a part of mitigation efforts to limit the spread of COVID-19.
“As we are encouraging Americans to stay home whenever possible, we don’t want our Medicare policies getting in the way,” she said, adding that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
COVID-19 in pediatric patients: What the hospitalist needs to know
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.