User login
Undeterred during COVID-19, hospital chaplains transform delivery of spiritual care
The first time that the Rev. Michael Mercier, BCC (a board-certified chaplain), provided spiritual care for a patient hospitalized with COVID-19 in March, he found himself engaged in a bit of soul-searching. Even though he donned a mask, gloves, and gown, he could get no closer than the hospital room doorway to interact with the patient because of infection-control measures.
“It went against all my natural instincts and my experience as a chaplain,” said Rev. Mercier, who serves as director of spiritual care for Rhode Island Hospital, Hasbro Children’s Hospital, Miriam Hospital, and Newport Hospital, which are operated by Lifespan, Rhode Island’s largest health system. “The first instinct is to be physically present in the room with the person who’s dying, to have the family gathered around the bedside.”
Prior to standing in the doorway that day, he’d been on the phone with family members, “just listening to their fear and their anxiety that they could not be with their loved one when their loved one was dying,” he said. “I validated their feelings. I also urged them to work with me and the nurse to bring a phone into the room, hold it to the patient’s ear, and they were able to say their goodbyes and how much they loved the person.”
The patient was a devout Roman Catholic, he added, and the family requested that the Prayer of Commendation and the Apostolic Pardon be performed. Rev. Mercier arranged for a Catholic priest to carry out this request. “The nurse told the patient what was going on, and the priest offered the prayers and the rituals from the doorway,” Rev. Mercier said. “It was a surreal experience. For me, it was almost entirely phone based, and it was mostly with the family because the patient couldn’t talk too much.”
To add to the sense of detachment in a situation like that, doctors, nurses, and chaplains caring for COVID-19 patients are wearing masks and face shields, and sometimes the sickest patients are intubated, which can complicate efforts to communicate. “I’m surprised at how we find the mask as somewhat of a barrier,” said Carolanne B. Hauck, BCC, director of chaplaincy care & education and volunteer services at Lancaster (Pa.) General Hospital, which is part of the Penn Medicine system. “By that I mean, often for us, sitting at the bedside and really being able to see someone’s face and have them see our face – with our masks, that’s just not happening. We’re also having briefer visits when we’re visiting with COVID patients.”
COVID-19 may have quarantined some traditional ways of providing spiritual care, but hospital chaplains are relying on technology more than ever in their efforts to meet the needs of patients and their families, including the use of iPads, FaceTime, and video conferencing programs like Zoom and BlueJeans.
“We’ve used Zoom to talk with family members that live out of state,” Rev. Mercier said. “Most of the time, I get an invitation to join a Zoom meeting, but now I need to become proficient in utilizing Zoom to set up those end-of-life family meetings. There’s a lot of learning on the fly, how to use these technologies in a way that’s helpful for everybody. That’s the biggest thing I’m learning: Connection is connection during this time of high stress and anxiety, and we just have to get creative.”
Despite the “disembodied” nature of technology, patients and their families have expressed gratitude to chaplains for their efforts to facilitate connections between loved ones and to be “a guide on the side,” as Mary Wetsch-Johnson, BCC, put it. She recalled one phone conversation with the daughter of a man with COVID-19 who was placed on comfort measures. “She said her dad was like the dad on the TV series Father Knows Best, just a kind-hearted, loving, wonderful man,” said Ms. Wetsch-Johnson, a chaplain at CHI Franciscan Health, which operates 10 acute-care hospitals in the Puget Sound region of Washington state. “She was able to describe him in a way that I felt like I knew him. She talked about the discord they had in their family and how they’re processing through that, and about her own personal journey with grief and loss. She then asked me for information about funeral homes, and I provided her with information. At the end of it, she said, ‘I did not know that I needed you today, but you are exactly what I needed.’ ”
Hospital chaplains may be using smartphones and other gadgets to communicate with patients and their families more than they did in the pre-COVID-19 world, but their basic job has not changed, said Rabbi Neal J. Loevinger, BCC, director of spiritual care services at Vassar Brothers Medical Center in Poughkeepsie, N.Y., part of a seven-hospital system operated by Nuvance Health. “We offer the hope of a caring presence,” said Rabbi Loevinger, who is also a member of the board of directors for Neshama: Association of Jewish Chaplains. “If someone is in a hole, our job is to climb down into the hole with them and say, ‘We’re going to get out of this hole together.’ We can’t promise that someone’s going to get better. We can’t promise that everything’s going to be all right. What we can promise is that we will not abandon you. We can promise that there will be someone accompanying you in any way we can through this crisis.”
Ms. Hauck remembered a phone conversation with the granddaughter of a patient hospitalized with COVID-19 who was nearing the end of her life. The granddaughter told her a story about how her grandmother and her best friend made a pact with each other that, when one was dying, the other would come to her side and pray the Rosary with her. “The granddaughter got tearful and said, ‘That can’t happen now,’ ” said Ms. Hauck, who oversees a staff of 9 chaplains and 10 per diem chaplains. “I made a promise that I would do my best to be at the bedside and pray the Rosary with her grandmother.”
The nurses were aware of the request, and about a day later, Ms. Hauck received a call at 1 a.m., indicating that the patient was close to dying. She drove to Lancaster General, put on her personal protective equipment, made it to the patient’s bedside, and began to pray the Rosary with her, with a nurse in the room. “The nurse said to me, ‘Carolanne, all of her stats are going up,’ and the patient actually became a little more alert,” she recalled. “We talked a little bit, and I asked, ‘Would you like to pray the Rosary now?’ She shook her head yes, and said, ‘Hail Mary, full of grace ...’ and those were the last words that she spoke. I finished the prayers for her, and then she died. It was very meaningful knowing that I could honor that wish for her, but more importantly, that I could do that for the family, who otherwise would have been at her side saying the Rosary with her. We have a recognition of how hard it is to leave someone at the hospital and not be at their bedside.”

Hospital chaplains are also supporting interdisciplinary teams of physicians, nurses, and other staff, as they navigate the provision of care in the wake of a pandemic. “They are under a great deal of stress – not only from being at work but with all the role changes that have happened in their home life,” Ms. Wetsch-Johnson said. “Some of them now are being the teacher at home and having to care for children. They have a lot that they come in with. My job is to help them so that they can go do their job. Regularly what I do is check in with the units and ask, ‘How are you doing today? What’s going on for you?’ Because people need to know that someone’s there to be with them and walk with them and listen to them.”
In the spirit of being present for their staff, she and her colleagues established “respite rooms” at CHI Franciscan hospitals, where workers can decompress and get recentered before returning to work. “We usually have water and snacks in there for them, and some type of soothing music,” Ms. Wetsch-Johnson said. “There is also literature on breathing exercises and stretching exercises. We’re also inviting people to write little notes of hope and gratitude, and they’re putting those up for each other. It’s important that we keep supporting them as they support the patients. Personally, I also round with our physicians, because they carry a lot with them, just as much as any other staff. I check in with dietary and environmental services. Everybody’s giving in their own unique way; that helps this whole health care system keep going.”
On any given day, it’s not uncommon for hospital staff members to spontaneously pull aside chaplains to vent, pray, or just to talk. “They process their own fears and anxieties about working in this kind of environment,” Rev. Mercier said. “They’re scared for themselves. They think, ‘Could I get the virus? Could I spread the virus to my family?’ Or, they may express the care and concern they have for their patients. Oftentimes, it’s a mixture of both. Those spontaneous conversations are often the most powerful.”
Ms. Hauck noted that some nurses and clinicians at Lancaster General Hospital “are doing work they may have not done before,” she said. “Some of them are experiencing death for the first time, so we help them to navigate that. One of the best things we can do is hear the anxiety they have or the sadness they have when a patient dies. Also, maybe the frustration that they couldn’t do more in some cases and helping them to see that sometimes their best is good enough.”
She recalled one younger patient with COVID-19 who fell seriously ill. “It was really affecting a lot of people on the unit because of the patient’s age,” she said. “When we saw that the patient was getting better and would be discharged, there was such a sense of relief. I’m not sure that patient will ever understand how that helped us. It was comforting to us to know that people are getting better. It is something we celebrate.”
As chaplains adjust to their “new normal,” carving out time for self-care is key. Ms. Hauck and her staff periodically meet on Zoom with a psychotherapist “who understands what we do, asks us really good questions, and reminds us to take care of ourselves,” she said. “Personally, I’m making sure I get my exercise in, I pack a healthy lunch. We do check in with each other. Part of our handoff at every shift provides for an opportunity to debrief about how your day was.”
Rev. Mercier’s self check-in includes deep-breathing meditation and reciting certain prayers throughout the day. “The deep breathing helps me center and refocus with my body, while the prayers remind me of my connection to the Divine,” he said. “It also reminds me that in the midst of the fear and the anxiety, I fear for myself. It’s hard not to be concerned that I could be infected. I have a family at home and could spread this to them. The prayer practices are a reminder to me that it’s okay to feel those fears and anxieties. Sometimes the spiritual practice helps me find that place of acceptance. That enables me to keep moving forward.”
Ms. Wetsch-Johnson described the sense of upendedness caused by the COVID-19 pandemic as a “ripple in the water that’s going to have long-lasting effects on the delivery of health care. People are taking the time to listen to one another. I’ve seen people in all departments be more compassionate with one another. I’ve seen managers go out of their way to make sure their staff are deeply cared for. I think that will have a ripple effect. That’s my hope, that we will continue to be more compassionate, more loving, and more understanding.”
Rabbi Loevinger hopes that even the most reticent physicians remember that chaplains serve as their advocate, too, especially during times of crisis. “This has been a time of unprecedented ethical wrestling in our hospitals, where there’s been a real concern that doctors, nurses, and respiratory therapists are going to be faced with morally distressing situations regarding insufficient PPE, or insufficient ventilator or dialysis machine supply to support everybody that needs to be supported,” he said. “Chaplains are a key part of the process of making ethical decisions, but also supporting physicians who are in distress over [being in] situations they never had imagined. Physicians don’t like to talk about the fact that a lot of the decisions they make are really heartbreaking. But if chaplains understand anything, it’s that being brokenhearted is part of the human condition, and that we can be part of the answer for keeping physicians morally and spiritually grounded in their work. We always invite that conversation.”
For Rev. Mercier, serving in a time of crisis reminds him of the importance of providing care as a team, “not just for patients and families, but for one another,” he said. “One of the lessons we can learn is, how can we build that connection with one another, to support and care for one another? How can we make sure that no one feels alone while working in the hospital?”
He draws inspiration from a saying credited to St. John of the Cross, which reads, “I saw the river through which every soul must pass, and the name of that river is suffering. I saw the boat that carries each soul across that river, and the name of that boat is love.”
“It’s that image that’s sticking with me, not just for myself as a chaplain but for all of my colleagues in the hospital,” said Rev. Mercier, who also pastors Tabernacle Baptist Church in Hope, R.I. “We’re in that river with the patients right now, suffering, and we’re doing our best to help them get to the other side – whatever the other side may look like.”
Correction, 4/30/20: An earlier version of the caption for the photo with Mary Wetsch-Johnson misstated the location. The photo was taken outside St. Elizabeth Hospital in Enumclaw, Wash.
The first time that the Rev. Michael Mercier, BCC (a board-certified chaplain), provided spiritual care for a patient hospitalized with COVID-19 in March, he found himself engaged in a bit of soul-searching. Even though he donned a mask, gloves, and gown, he could get no closer than the hospital room doorway to interact with the patient because of infection-control measures.

“It went against all my natural instincts and my experience as a chaplain,” said Rev. Mercier, who serves as director of spiritual care for Rhode Island Hospital, Hasbro Children’s Hospital, Miriam Hospital, and Newport Hospital, which are operated by Lifespan, Rhode Island’s largest health system. “The first instinct is to be physically present in the room with the person who’s dying, to have the family gathered around the bedside.”
Prior to standing in the doorway that day, he’d been on the phone with family members, “just listening to their fear and their anxiety that they could not be with their loved one when their loved one was dying,” he said. “I validated their feelings. I also urged them to work with me and the nurse to bring a phone into the room, hold it to the patient’s ear, and they were able to say their goodbyes and how much they loved the person.”
The patient was a devout Roman Catholic, he added, and the family requested that the Prayer of Commendation and the Apostolic Pardon be performed. Rev. Mercier arranged for a Catholic priest to carry out this request. “The nurse told the patient what was going on, and the priest offered the prayers and the rituals from the doorway,” Rev. Mercier said. “It was a surreal experience. For me, it was almost entirely phone based, and it was mostly with the family because the patient couldn’t talk too much.”
To add to the sense of detachment in a situation like that, doctors, nurses, and chaplains caring for COVID-19 patients are wearing masks and face shields, and sometimes the sickest patients are intubated, which can complicate efforts to communicate. “I’m surprised at how we find the mask as somewhat of a barrier,” said Carolanne B. Hauck, BCC, director of chaplaincy care & education and volunteer services at Lancaster (Pa.) General Hospital, which is part of the Penn Medicine system. “By that I mean, often for us, sitting at the bedside and really being able to see someone’s face and have them see our face – with our masks, that’s just not happening. We’re also having briefer visits when we’re visiting with COVID patients.”
COVID-19 may have quarantined some traditional ways of providing spiritual care, but hospital chaplains are relying on technology more than ever in their efforts to meet the needs of patients and their families, including the use of iPads, FaceTime, and video conferencing programs like Zoom and BlueJeans.
“We’ve used Zoom to talk with family members that live out of state,” Rev. Mercier said. “Most of the time, I get an invitation to join a Zoom meeting, but now I need to become proficient in utilizing Zoom to set up those end-of-life family meetings. There’s a lot of learning on the fly, how to use these technologies in a way that’s helpful for everybody. That’s the biggest thing I’m learning: Connection is connection during this time of high stress and anxiety, and we just have to get creative.”
Despite the “disembodied” nature of technology, patients and their families have expressed gratitude to chaplains for their efforts to facilitate connections between loved ones and to be “a guide on the side,” as Mary Wetsch-Johnson, BCC, put it. She recalled one phone conversation with the daughter of a man with COVID-19 who was placed on comfort measures. “She said her dad was like the dad on the TV series Father Knows Best, just a kind-hearted, loving, wonderful man,” said Ms. Wetsch-Johnson, a chaplain at CHI Franciscan Health, which operates 10 acute-care hospitals in the Puget Sound region of Washington state. “She was able to describe him in a way that I felt like I knew him. She talked about the discord they had in their family and how they’re processing through that, and about her own personal journey with grief and loss. She then asked me for information about funeral homes, and I provided her with information. At the end of it, she said, ‘I did not know that I needed you today, but you are exactly what I needed.’ ”
Hospital chaplains may be using smartphones and other gadgets to communicate with patients and their families more than they did in the pre-COVID-19 world, but their basic job has not changed, said Rabbi Neal J. Loevinger, BCC, director of spiritual care services at Vassar Brothers Medical Center in Poughkeepsie, N.Y., part of a seven-hospital system operated by Nuvance Health. “We offer the hope of a caring presence,” said Rabbi Loevinger, who is also a member of the board of directors for Neshama: Association of Jewish Chaplains. “If someone is in a hole, our job is to climb down into the hole with them and say, ‘We’re going to get out of this hole together.’ We can’t promise that someone’s going to get better. We can’t promise that everything’s going to be all right. What we can promise is that we will not abandon you. We can promise that there will be someone accompanying you in any way we can through this crisis.”
Ms. Hauck remembered a phone conversation with the granddaughter of a patient hospitalized with COVID-19 who was nearing the end of her life. The granddaughter told her a story about how her grandmother and her best friend made a pact with each other that, when one was dying, the other would come to her side and pray the Rosary with her. “The granddaughter got tearful and said, ‘That can’t happen now,’ ” said Ms. Hauck, who oversees a staff of 9 chaplains and 10 per diem chaplains. “I made a promise that I would do my best to be at the bedside and pray the Rosary with her grandmother.”
The nurses were aware of the request, and about a day later, Ms. Hauck received a call at 1 a.m., indicating that the patient was close to dying. She drove to Lancaster General, put on her personal protective equipment, made it to the patient’s bedside, and began to pray the Rosary with her, with a nurse in the room. “The nurse said to me, ‘Carolanne, all of her stats are going up,’ and the patient actually became a little more alert,” she recalled. “We talked a little bit, and I asked, ‘Would you like to pray the Rosary now?’ She shook her head yes, and said, ‘Hail Mary, full of grace ...’ and those were the last words that she spoke. I finished the prayers for her, and then she died. It was very meaningful knowing that I could honor that wish for her, but more importantly, that I could do that for the family, who otherwise would have been at her side saying the Rosary with her. We have a recognition of how hard it is to leave someone at the hospital and not be at their bedside.”

Hospital chaplains are also supporting interdisciplinary teams of physicians, nurses, and other staff, as they navigate the provision of care in the wake of a pandemic. “They are under a great deal of stress – not only from being at work but with all the role changes that have happened in their home life,” Ms. Wetsch-Johnson said. “Some of them now are being the teacher at home and having to care for children. They have a lot that they come in with. My job is to help them so that they can go do their job. Regularly what I do is check in with the units and ask, ‘How are you doing today? What’s going on for you?’ Because people need to know that someone’s there to be with them and walk with them and listen to them.”
In the spirit of being present for their staff, she and her colleagues established “respite rooms” at CHI Franciscan hospitals, where workers can decompress and get recentered before returning to work. “We usually have water and snacks in there for them, and some type of soothing music,” Ms. Wetsch-Johnson said. “There is also literature on breathing exercises and stretching exercises. We’re also inviting people to write little notes of hope and gratitude, and they’re putting those up for each other. It’s important that we keep supporting them as they support the patients. Personally, I also round with our physicians, because they carry a lot with them, just as much as any other staff. I check in with dietary and environmental services. Everybody’s giving in their own unique way; that helps this whole health care system keep going.”
On any given day, it’s not uncommon for hospital staff members to spontaneously pull aside chaplains to vent, pray, or just to talk. “They process their own fears and anxieties about working in this kind of environment,” Rev. Mercier said. “They’re scared for themselves. They think, ‘Could I get the virus? Could I spread the virus to my family?’ Or, they may express the care and concern they have for their patients. Oftentimes, it’s a mixture of both. Those spontaneous conversations are often the most powerful.”
Ms. Hauck noted that some nurses and clinicians at Lancaster General Hospital “are doing work they may have not done before,” she said. “Some of them are experiencing death for the first time, so we help them to navigate that. One of the best things we can do is hear the anxiety they have or the sadness they have when a patient dies. Also, maybe the frustration that they couldn’t do more in some cases and helping them to see that sometimes their best is good enough.”
She recalled one younger patient with COVID-19 who fell seriously ill. “It was really affecting a lot of people on the unit because of the patient’s age,” she said. “When we saw that the patient was getting better and would be discharged, there was such a sense of relief. I’m not sure that patient will ever understand how that helped us. It was comforting to us to know that people are getting better. It is something we celebrate.”
As chaplains adjust to their “new normal,” carving out time for self-care is key. Ms. Hauck and her staff periodically meet on Zoom with a psychotherapist “who understands what we do, asks us really good questions, and reminds us to take care of ourselves,” she said. “Personally, I’m making sure I get my exercise in, I pack a healthy lunch. We do check in with each other. Part of our handoff at every shift provides for an opportunity to debrief about how your day was.”
Rev. Mercier’s self check-in includes deep-breathing meditation and reciting certain prayers throughout the day. “The deep breathing helps me center and refocus with my body, while the prayers remind me of my connection to the Divine,” he said. “It also reminds me that in the midst of the fear and the anxiety, I fear for myself. It’s hard not to be concerned that I could be infected. I have a family at home and could spread this to them. The prayer practices are a reminder to me that it’s okay to feel those fears and anxieties. Sometimes the spiritual practice helps me find that place of acceptance. That enables me to keep moving forward.”
Ms. Wetsch-Johnson described the sense of upendedness caused by the COVID-19 pandemic as a “ripple in the water that’s going to have long-lasting effects on the delivery of health care. People are taking the time to listen to one another. I’ve seen people in all departments be more compassionate with one another. I’ve seen managers go out of their way to make sure their staff are deeply cared for. I think that will have a ripple effect. That’s my hope, that we will continue to be more compassionate, more loving, and more understanding.”
Rabbi Loevinger hopes that even the most reticent physicians remember that chaplains serve as their advocate, too, especially during times of crisis. “This has been a time of unprecedented ethical wrestling in our hospitals, where there’s been a real concern that doctors, nurses, and respiratory therapists are going to be faced with morally distressing situations regarding insufficient PPE, or insufficient ventilator or dialysis machine supply to support everybody that needs to be supported,” he said. “Chaplains are a key part of the process of making ethical decisions, but also supporting physicians who are in distress over [being in] situations they never had imagined. Physicians don’t like to talk about the fact that a lot of the decisions they make are really heartbreaking. But if chaplains understand anything, it’s that being brokenhearted is part of the human condition, and that we can be part of the answer for keeping physicians morally and spiritually grounded in their work. We always invite that conversation.”
For Rev. Mercier, serving in a time of crisis reminds him of the importance of providing care as a team, “not just for patients and families, but for one another,” he said. “One of the lessons we can learn is, how can we build that connection with one another, to support and care for one another? How can we make sure that no one feels alone while working in the hospital?”
He draws inspiration from a saying credited to St. John of the Cross, which reads, “I saw the river through which every soul must pass, and the name of that river is suffering. I saw the boat that carries each soul across that river, and the name of that boat is love.”
“It’s that image that’s sticking with me, not just for myself as a chaplain but for all of my colleagues in the hospital,” said Rev. Mercier, who also pastors Tabernacle Baptist Church in Hope, R.I. “We’re in that river with the patients right now, suffering, and we’re doing our best to help them get to the other side – whatever the other side may look like.”
Correction, 4/30/20: An earlier version of the caption for the photo with Mary Wetsch-Johnson misstated the location. The photo was taken outside St. Elizabeth Hospital in Enumclaw, Wash.
The first time that the Rev. Michael Mercier, BCC (a board-certified chaplain), provided spiritual care for a patient hospitalized with COVID-19 in March, he found himself engaged in a bit of soul-searching. Even though he donned a mask, gloves, and gown, he could get no closer than the hospital room doorway to interact with the patient because of infection-control measures.

“It went against all my natural instincts and my experience as a chaplain,” said Rev. Mercier, who serves as director of spiritual care for Rhode Island Hospital, Hasbro Children’s Hospital, Miriam Hospital, and Newport Hospital, which are operated by Lifespan, Rhode Island’s largest health system. “The first instinct is to be physically present in the room with the person who’s dying, to have the family gathered around the bedside.”
Prior to standing in the doorway that day, he’d been on the phone with family members, “just listening to their fear and their anxiety that they could not be with their loved one when their loved one was dying,” he said. “I validated their feelings. I also urged them to work with me and the nurse to bring a phone into the room, hold it to the patient’s ear, and they were able to say their goodbyes and how much they loved the person.”
The patient was a devout Roman Catholic, he added, and the family requested that the Prayer of Commendation and the Apostolic Pardon be performed. Rev. Mercier arranged for a Catholic priest to carry out this request. “The nurse told the patient what was going on, and the priest offered the prayers and the rituals from the doorway,” Rev. Mercier said. “It was a surreal experience. For me, it was almost entirely phone based, and it was mostly with the family because the patient couldn’t talk too much.”
To add to the sense of detachment in a situation like that, doctors, nurses, and chaplains caring for COVID-19 patients are wearing masks and face shields, and sometimes the sickest patients are intubated, which can complicate efforts to communicate. “I’m surprised at how we find the mask as somewhat of a barrier,” said Carolanne B. Hauck, BCC, director of chaplaincy care & education and volunteer services at Lancaster (Pa.) General Hospital, which is part of the Penn Medicine system. “By that I mean, often for us, sitting at the bedside and really being able to see someone’s face and have them see our face – with our masks, that’s just not happening. We’re also having briefer visits when we’re visiting with COVID patients.”
COVID-19 may have quarantined some traditional ways of providing spiritual care, but hospital chaplains are relying on technology more than ever in their efforts to meet the needs of patients and their families, including the use of iPads, FaceTime, and video conferencing programs like Zoom and BlueJeans.
“We’ve used Zoom to talk with family members that live out of state,” Rev. Mercier said. “Most of the time, I get an invitation to join a Zoom meeting, but now I need to become proficient in utilizing Zoom to set up those end-of-life family meetings. There’s a lot of learning on the fly, how to use these technologies in a way that’s helpful for everybody. That’s the biggest thing I’m learning: Connection is connection during this time of high stress and anxiety, and we just have to get creative.”
Despite the “disembodied” nature of technology, patients and their families have expressed gratitude to chaplains for their efforts to facilitate connections between loved ones and to be “a guide on the side,” as Mary Wetsch-Johnson, BCC, put it. She recalled one phone conversation with the daughter of a man with COVID-19 who was placed on comfort measures. “She said her dad was like the dad on the TV series Father Knows Best, just a kind-hearted, loving, wonderful man,” said Ms. Wetsch-Johnson, a chaplain at CHI Franciscan Health, which operates 10 acute-care hospitals in the Puget Sound region of Washington state. “She was able to describe him in a way that I felt like I knew him. She talked about the discord they had in their family and how they’re processing through that, and about her own personal journey with grief and loss. She then asked me for information about funeral homes, and I provided her with information. At the end of it, she said, ‘I did not know that I needed you today, but you are exactly what I needed.’ ”
Hospital chaplains may be using smartphones and other gadgets to communicate with patients and their families more than they did in the pre-COVID-19 world, but their basic job has not changed, said Rabbi Neal J. Loevinger, BCC, director of spiritual care services at Vassar Brothers Medical Center in Poughkeepsie, N.Y., part of a seven-hospital system operated by Nuvance Health. “We offer the hope of a caring presence,” said Rabbi Loevinger, who is also a member of the board of directors for Neshama: Association of Jewish Chaplains. “If someone is in a hole, our job is to climb down into the hole with them and say, ‘We’re going to get out of this hole together.’ We can’t promise that someone’s going to get better. We can’t promise that everything’s going to be all right. What we can promise is that we will not abandon you. We can promise that there will be someone accompanying you in any way we can through this crisis.”
Ms. Hauck remembered a phone conversation with the granddaughter of a patient hospitalized with COVID-19 who was nearing the end of her life. The granddaughter told her a story about how her grandmother and her best friend made a pact with each other that, when one was dying, the other would come to her side and pray the Rosary with her. “The granddaughter got tearful and said, ‘That can’t happen now,’ ” said Ms. Hauck, who oversees a staff of 9 chaplains and 10 per diem chaplains. “I made a promise that I would do my best to be at the bedside and pray the Rosary with her grandmother.”
The nurses were aware of the request, and about a day later, Ms. Hauck received a call at 1 a.m., indicating that the patient was close to dying. She drove to Lancaster General, put on her personal protective equipment, made it to the patient’s bedside, and began to pray the Rosary with her, with a nurse in the room. “The nurse said to me, ‘Carolanne, all of her stats are going up,’ and the patient actually became a little more alert,” she recalled. “We talked a little bit, and I asked, ‘Would you like to pray the Rosary now?’ She shook her head yes, and said, ‘Hail Mary, full of grace ...’ and those were the last words that she spoke. I finished the prayers for her, and then she died. It was very meaningful knowing that I could honor that wish for her, but more importantly, that I could do that for the family, who otherwise would have been at her side saying the Rosary with her. We have a recognition of how hard it is to leave someone at the hospital and not be at their bedside.”

Hospital chaplains are also supporting interdisciplinary teams of physicians, nurses, and other staff, as they navigate the provision of care in the wake of a pandemic. “They are under a great deal of stress – not only from being at work but with all the role changes that have happened in their home life,” Ms. Wetsch-Johnson said. “Some of them now are being the teacher at home and having to care for children. They have a lot that they come in with. My job is to help them so that they can go do their job. Regularly what I do is check in with the units and ask, ‘How are you doing today? What’s going on for you?’ Because people need to know that someone’s there to be with them and walk with them and listen to them.”
In the spirit of being present for their staff, she and her colleagues established “respite rooms” at CHI Franciscan hospitals, where workers can decompress and get recentered before returning to work. “We usually have water and snacks in there for them, and some type of soothing music,” Ms. Wetsch-Johnson said. “There is also literature on breathing exercises and stretching exercises. We’re also inviting people to write little notes of hope and gratitude, and they’re putting those up for each other. It’s important that we keep supporting them as they support the patients. Personally, I also round with our physicians, because they carry a lot with them, just as much as any other staff. I check in with dietary and environmental services. Everybody’s giving in their own unique way; that helps this whole health care system keep going.”
On any given day, it’s not uncommon for hospital staff members to spontaneously pull aside chaplains to vent, pray, or just to talk. “They process their own fears and anxieties about working in this kind of environment,” Rev. Mercier said. “They’re scared for themselves. They think, ‘Could I get the virus? Could I spread the virus to my family?’ Or, they may express the care and concern they have for their patients. Oftentimes, it’s a mixture of both. Those spontaneous conversations are often the most powerful.”
Ms. Hauck noted that some nurses and clinicians at Lancaster General Hospital “are doing work they may have not done before,” she said. “Some of them are experiencing death for the first time, so we help them to navigate that. One of the best things we can do is hear the anxiety they have or the sadness they have when a patient dies. Also, maybe the frustration that they couldn’t do more in some cases and helping them to see that sometimes their best is good enough.”
She recalled one younger patient with COVID-19 who fell seriously ill. “It was really affecting a lot of people on the unit because of the patient’s age,” she said. “When we saw that the patient was getting better and would be discharged, there was such a sense of relief. I’m not sure that patient will ever understand how that helped us. It was comforting to us to know that people are getting better. It is something we celebrate.”
As chaplains adjust to their “new normal,” carving out time for self-care is key. Ms. Hauck and her staff periodically meet on Zoom with a psychotherapist “who understands what we do, asks us really good questions, and reminds us to take care of ourselves,” she said. “Personally, I’m making sure I get my exercise in, I pack a healthy lunch. We do check in with each other. Part of our handoff at every shift provides for an opportunity to debrief about how your day was.”
Rev. Mercier’s self check-in includes deep-breathing meditation and reciting certain prayers throughout the day. “The deep breathing helps me center and refocus with my body, while the prayers remind me of my connection to the Divine,” he said. “It also reminds me that in the midst of the fear and the anxiety, I fear for myself. It’s hard not to be concerned that I could be infected. I have a family at home and could spread this to them. The prayer practices are a reminder to me that it’s okay to feel those fears and anxieties. Sometimes the spiritual practice helps me find that place of acceptance. That enables me to keep moving forward.”
Ms. Wetsch-Johnson described the sense of upendedness caused by the COVID-19 pandemic as a “ripple in the water that’s going to have long-lasting effects on the delivery of health care. People are taking the time to listen to one another. I’ve seen people in all departments be more compassionate with one another. I’ve seen managers go out of their way to make sure their staff are deeply cared for. I think that will have a ripple effect. That’s my hope, that we will continue to be more compassionate, more loving, and more understanding.”
Rabbi Loevinger hopes that even the most reticent physicians remember that chaplains serve as their advocate, too, especially during times of crisis. “This has been a time of unprecedented ethical wrestling in our hospitals, where there’s been a real concern that doctors, nurses, and respiratory therapists are going to be faced with morally distressing situations regarding insufficient PPE, or insufficient ventilator or dialysis machine supply to support everybody that needs to be supported,” he said. “Chaplains are a key part of the process of making ethical decisions, but also supporting physicians who are in distress over [being in] situations they never had imagined. Physicians don’t like to talk about the fact that a lot of the decisions they make are really heartbreaking. But if chaplains understand anything, it’s that being brokenhearted is part of the human condition, and that we can be part of the answer for keeping physicians morally and spiritually grounded in their work. We always invite that conversation.”
For Rev. Mercier, serving in a time of crisis reminds him of the importance of providing care as a team, “not just for patients and families, but for one another,” he said. “One of the lessons we can learn is, how can we build that connection with one another, to support and care for one another? How can we make sure that no one feels alone while working in the hospital?”
He draws inspiration from a saying credited to St. John of the Cross, which reads, “I saw the river through which every soul must pass, and the name of that river is suffering. I saw the boat that carries each soul across that river, and the name of that boat is love.”
“It’s that image that’s sticking with me, not just for myself as a chaplain but for all of my colleagues in the hospital,” said Rev. Mercier, who also pastors Tabernacle Baptist Church in Hope, R.I. “We’re in that river with the patients right now, suffering, and we’re doing our best to help them get to the other side – whatever the other side may look like.”
Correction, 4/30/20: An earlier version of the caption for the photo with Mary Wetsch-Johnson misstated the location. The photo was taken outside St. Elizabeth Hospital in Enumclaw, Wash.
Menstrual cup use with copper IUDs linked to higher expulsion rates
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
FROM ACOG 2020
Visa worries besiege immigrant physicians fighting COVID-19
Physicians and their sponsoring health care facilities shouldn’t have to worry about visa technicalities as they work on the front lines during the COVID-19 pandemic, said health care leaders and immigration reform advocates.
In a press call hosted by the National Immigration Forum, speakers highlighted the need for fast and flexible solutions to enable health care workers, including physicians, to contribute to efforts to combat the pandemic.
Nationwide, over one in five physicians are immigrants, according to data from the Forum. That figure is over one in three in New York, New Jersey, and California, three states hard-hit by COVID-19 cases.
Many physicians stand willing and able to serve where they’re needed, but visa restrictions often block the ability of immigrant physicians to meet COVID-19 surges across the country, said Amit Vashist, MD, senior vice president and chief clinical officer for Ballad Health, Johnson City, Tenn., and a member of the public policy committee of the Society of Hospital Medicine. Ballad Health is an integrated health care system that serves 29 counties in the rural Southeast.
“This pandemic is a war with an invisible enemy, and immigrant physicians have been absolutely critical to providing quality care, especially on the front lines – but current visa restrictions have limited the ability to deploy these physicians in communities with the greatest need,” said Dr. Vashist during the press conference.
Visa requirements currently tie a non-US citizen resident physician to a particular institution and facility, limiting the ability to meet demand flexibly. “Federal agencies and Congress should provide additional flexibility in visa processing to allow for automatic renewals and expediting processing so immigrant medical workers can focus on treating the sick and not on their visa requirements,” said Dr. Vashist.
Dr. Vashist noted that, when he speaks with the many Ballad Health hospitalists who are waiting on permanent residency or citizenship, many of them also cite worries about the fate of their families should they themselves fall ill. Depending on the physician’s visa status, the family may face deportation without recourse if the physician should die.
“Tens of thousands of our physicians continue to endure years, even decades of waiting to obtain a permanent residency in the United States and at the same time, relentlessly and fearlessly serve their communities including in this COVID-19 pandemic,” said Dr. Vashist. “It’s time we take care of them and their long-term immigration needs, and give them the peace of mind that they so desperately deserve,” he added.
Frank Trinity, chief legal officer for the Association of American Medical Colleges, also participated in the call. “For decades,” he said, the United States “has relied on physicians from other countries, especially in rural and underserved areas.”
One of these physicians, Mihir Patel, MD, FHM, a hospitalist at Ballad Health, came to the United States in 2005, but 15 years later is still waiting for the green card that signifies U.S. permanent residency status. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the health system’s COVID-10 Strike Team.
“During the COVID crisis, these restrictions can cause significant negative impact for small rural hospitals,” Dr. Patel said. “There are physicians on a visa who cannot legally work outside their primary facilities – even though they are willing to do so.”
Regarding the pandemic, Mr. Trinity expressed concerns about whether the surge of patients would “outstrip our workforce.” He noted that, with an unprecedented number of desperately ill patients needing emergency care all across the country, “now is the time for our government to take every possible action to ensure that these highly qualified and courageous health professionals are available in the fight against the coronavirus.”
Mr. Trinity outlined five governmental actions AAMC is proposing to allow immigrant physicians to participate fully in the battle against COVID-19. The first would be to approve a blanket extension of visa deadlines. The second would be to expedite processing of visa extension applications, including reinstating expedited processing of physicians currently holding H-1B visa status.
The third action proposed by AAMC is to provide flexibility to visa sponsors during the emergency so that an individual whose visa is currently limited to a particular program can provide care at another location or by means of telehealth.
Fourth, AAMC proposes streamlined entry for the 4,200 physicians who are matched into residency programs so that they may begin their residencies on time or early.
Finally, Mr. Trinity said that AAMC is proposing that work authorizations be maintained for the 29,000 physicians who are currently not U.S. citizens and actively participating in the health care workforce.
Jacinta Ma, the Forum’s vice president of policy and advocacy, said immigrants are a critical component of the U.S. health care workforce as a whole.
“With immigrants accounting for 17% of health care workers amid the COVID-19 pandemic, it’s clear that they are vital to our communities,” she said. “Congress and the Trump administration both have an opportunity to advance solutions that protect immigrants, and remove immigration-related barriers for immigrant medical professionals by ensuring that immigrant doctors, nurses, home health care workers, researchers, and others can continue their vital work during this pandemic while being afforded adequate protection from COVID-19.”
Physicians and their sponsoring health care facilities shouldn’t have to worry about visa technicalities as they work on the front lines during the COVID-19 pandemic, said health care leaders and immigration reform advocates.
In a press call hosted by the National Immigration Forum, speakers highlighted the need for fast and flexible solutions to enable health care workers, including physicians, to contribute to efforts to combat the pandemic.
Nationwide, over one in five physicians are immigrants, according to data from the Forum. That figure is over one in three in New York, New Jersey, and California, three states hard-hit by COVID-19 cases.
Many physicians stand willing and able to serve where they’re needed, but visa restrictions often block the ability of immigrant physicians to meet COVID-19 surges across the country, said Amit Vashist, MD, senior vice president and chief clinical officer for Ballad Health, Johnson City, Tenn., and a member of the public policy committee of the Society of Hospital Medicine. Ballad Health is an integrated health care system that serves 29 counties in the rural Southeast.
“This pandemic is a war with an invisible enemy, and immigrant physicians have been absolutely critical to providing quality care, especially on the front lines – but current visa restrictions have limited the ability to deploy these physicians in communities with the greatest need,” said Dr. Vashist during the press conference.
Visa requirements currently tie a non-US citizen resident physician to a particular institution and facility, limiting the ability to meet demand flexibly. “Federal agencies and Congress should provide additional flexibility in visa processing to allow for automatic renewals and expediting processing so immigrant medical workers can focus on treating the sick and not on their visa requirements,” said Dr. Vashist.
Dr. Vashist noted that, when he speaks with the many Ballad Health hospitalists who are waiting on permanent residency or citizenship, many of them also cite worries about the fate of their families should they themselves fall ill. Depending on the physician’s visa status, the family may face deportation without recourse if the physician should die.
“Tens of thousands of our physicians continue to endure years, even decades of waiting to obtain a permanent residency in the United States and at the same time, relentlessly and fearlessly serve their communities including in this COVID-19 pandemic,” said Dr. Vashist. “It’s time we take care of them and their long-term immigration needs, and give them the peace of mind that they so desperately deserve,” he added.
Frank Trinity, chief legal officer for the Association of American Medical Colleges, also participated in the call. “For decades,” he said, the United States “has relied on physicians from other countries, especially in rural and underserved areas.”
One of these physicians, Mihir Patel, MD, FHM, a hospitalist at Ballad Health, came to the United States in 2005, but 15 years later is still waiting for the green card that signifies U.S. permanent residency status. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the health system’s COVID-10 Strike Team.
“During the COVID crisis, these restrictions can cause significant negative impact for small rural hospitals,” Dr. Patel said. “There are physicians on a visa who cannot legally work outside their primary facilities – even though they are willing to do so.”
Regarding the pandemic, Mr. Trinity expressed concerns about whether the surge of patients would “outstrip our workforce.” He noted that, with an unprecedented number of desperately ill patients needing emergency care all across the country, “now is the time for our government to take every possible action to ensure that these highly qualified and courageous health professionals are available in the fight against the coronavirus.”
Mr. Trinity outlined five governmental actions AAMC is proposing to allow immigrant physicians to participate fully in the battle against COVID-19. The first would be to approve a blanket extension of visa deadlines. The second would be to expedite processing of visa extension applications, including reinstating expedited processing of physicians currently holding H-1B visa status.
The third action proposed by AAMC is to provide flexibility to visa sponsors during the emergency so that an individual whose visa is currently limited to a particular program can provide care at another location or by means of telehealth.
Fourth, AAMC proposes streamlined entry for the 4,200 physicians who are matched into residency programs so that they may begin their residencies on time or early.
Finally, Mr. Trinity said that AAMC is proposing that work authorizations be maintained for the 29,000 physicians who are currently not U.S. citizens and actively participating in the health care workforce.
Jacinta Ma, the Forum’s vice president of policy and advocacy, said immigrants are a critical component of the U.S. health care workforce as a whole.
“With immigrants accounting for 17% of health care workers amid the COVID-19 pandemic, it’s clear that they are vital to our communities,” she said. “Congress and the Trump administration both have an opportunity to advance solutions that protect immigrants, and remove immigration-related barriers for immigrant medical professionals by ensuring that immigrant doctors, nurses, home health care workers, researchers, and others can continue their vital work during this pandemic while being afforded adequate protection from COVID-19.”
Physicians and their sponsoring health care facilities shouldn’t have to worry about visa technicalities as they work on the front lines during the COVID-19 pandemic, said health care leaders and immigration reform advocates.
In a press call hosted by the National Immigration Forum, speakers highlighted the need for fast and flexible solutions to enable health care workers, including physicians, to contribute to efforts to combat the pandemic.
Nationwide, over one in five physicians are immigrants, according to data from the Forum. That figure is over one in three in New York, New Jersey, and California, three states hard-hit by COVID-19 cases.
Many physicians stand willing and able to serve where they’re needed, but visa restrictions often block the ability of immigrant physicians to meet COVID-19 surges across the country, said Amit Vashist, MD, senior vice president and chief clinical officer for Ballad Health, Johnson City, Tenn., and a member of the public policy committee of the Society of Hospital Medicine. Ballad Health is an integrated health care system that serves 29 counties in the rural Southeast.
“This pandemic is a war with an invisible enemy, and immigrant physicians have been absolutely critical to providing quality care, especially on the front lines – but current visa restrictions have limited the ability to deploy these physicians in communities with the greatest need,” said Dr. Vashist during the press conference.
Visa requirements currently tie a non-US citizen resident physician to a particular institution and facility, limiting the ability to meet demand flexibly. “Federal agencies and Congress should provide additional flexibility in visa processing to allow for automatic renewals and expediting processing so immigrant medical workers can focus on treating the sick and not on their visa requirements,” said Dr. Vashist.
Dr. Vashist noted that, when he speaks with the many Ballad Health hospitalists who are waiting on permanent residency or citizenship, many of them also cite worries about the fate of their families should they themselves fall ill. Depending on the physician’s visa status, the family may face deportation without recourse if the physician should die.
“Tens of thousands of our physicians continue to endure years, even decades of waiting to obtain a permanent residency in the United States and at the same time, relentlessly and fearlessly serve their communities including in this COVID-19 pandemic,” said Dr. Vashist. “It’s time we take care of them and their long-term immigration needs, and give them the peace of mind that they so desperately deserve,” he added.
Frank Trinity, chief legal officer for the Association of American Medical Colleges, also participated in the call. “For decades,” he said, the United States “has relied on physicians from other countries, especially in rural and underserved areas.”
One of these physicians, Mihir Patel, MD, FHM, a hospitalist at Ballad Health, came to the United States in 2005, but 15 years later is still waiting for the green card that signifies U.S. permanent residency status. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the health system’s COVID-10 Strike Team.
“During the COVID crisis, these restrictions can cause significant negative impact for small rural hospitals,” Dr. Patel said. “There are physicians on a visa who cannot legally work outside their primary facilities – even though they are willing to do so.”
Regarding the pandemic, Mr. Trinity expressed concerns about whether the surge of patients would “outstrip our workforce.” He noted that, with an unprecedented number of desperately ill patients needing emergency care all across the country, “now is the time for our government to take every possible action to ensure that these highly qualified and courageous health professionals are available in the fight against the coronavirus.”
Mr. Trinity outlined five governmental actions AAMC is proposing to allow immigrant physicians to participate fully in the battle against COVID-19. The first would be to approve a blanket extension of visa deadlines. The second would be to expedite processing of visa extension applications, including reinstating expedited processing of physicians currently holding H-1B visa status.
The third action proposed by AAMC is to provide flexibility to visa sponsors during the emergency so that an individual whose visa is currently limited to a particular program can provide care at another location or by means of telehealth.
Fourth, AAMC proposes streamlined entry for the 4,200 physicians who are matched into residency programs so that they may begin their residencies on time or early.
Finally, Mr. Trinity said that AAMC is proposing that work authorizations be maintained for the 29,000 physicians who are currently not U.S. citizens and actively participating in the health care workforce.
Jacinta Ma, the Forum’s vice president of policy and advocacy, said immigrants are a critical component of the U.S. health care workforce as a whole.
“With immigrants accounting for 17% of health care workers amid the COVID-19 pandemic, it’s clear that they are vital to our communities,” she said. “Congress and the Trump administration both have an opportunity to advance solutions that protect immigrants, and remove immigration-related barriers for immigrant medical professionals by ensuring that immigrant doctors, nurses, home health care workers, researchers, and others can continue their vital work during this pandemic while being afforded adequate protection from COVID-19.”
Increased risk of lung cancer with COPD, even in never smokers
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.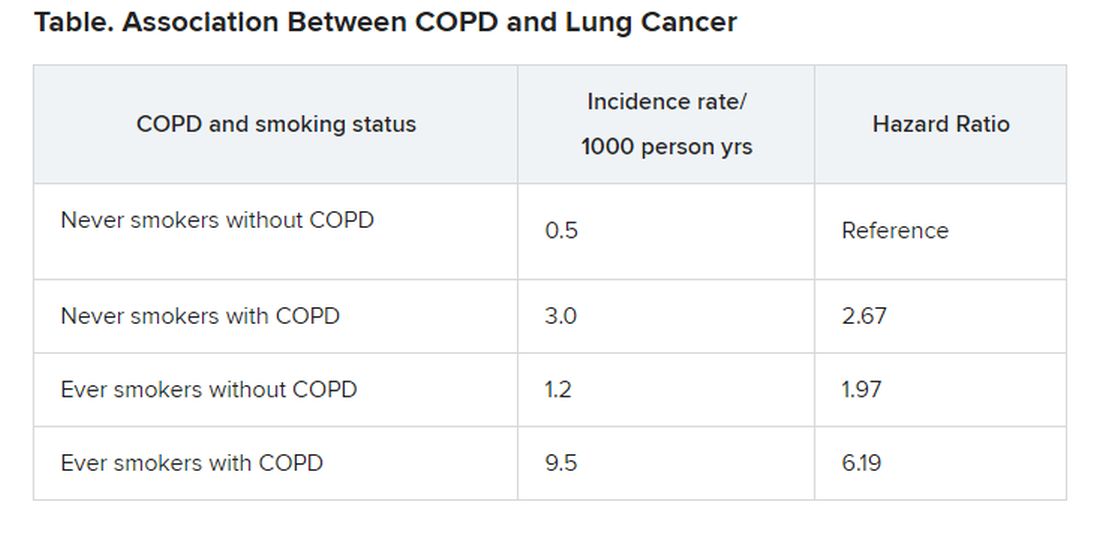
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19 registry tracks pregnant women, newborns
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Certain anaerobic bacteria linked to increased CRC risk
Certain species of anaerobic bacteria have been linked to dramatic increases in colorectal cancer (CRC), often within a year of infection, although whether or not the bacteria are causal has yet to be determined, say Danish researchers.
“We are not convinced that all the bacteria are directly involved in CRC development — they could just be innocent bystanders that invade the blood stream when the cancer [itself] has caused a breach in the intestinal wall,” lead author Ulrik Justesen, MD, Odense University Hospital, Denmark, told Medscape Medical News in an email.
“But an algorithm for colonoscopy based on the [infecting] species, which could then be supplemented with specific characteristics [of the bacteria] along with age, is certainly a realistic perspective,” he added.
The study was to have be presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Paris, France, but the conference was canceled due to COVID-19.
Another study suggesting a link between bacteria and CRC was published earlier this year in Nature, as reported at the time by Medscape Medical News. That study, from the Netherlands, suggests that a strain of Escherichia coli may be involved in the development of CRC.
Population-Based Study
The latest study from Denmark was a population-based study involving over 2 million people.
From this large cohort, blood culture data from the years 2007 and 2016 were analyzed.
“We combined blood culture data with the national register for colorectal cancer — the Danish Colorectal Cancer Group Database — and identified incident CRC after bacteraemia,” the investigators state.
The risk for incident CRC was investigated specifically for the anaerobic bacteria Bacteroides spp, Clostridium spp, and Fusobacterium spp.
Incident rates were then compared to those from nonanaerobic bacteria, including the Streptococcus bovis group, Escherichia coli, and Staphylococcus aureus, as well as from negative blood samples.
“We included 45,760 bacteraemia episodes, of which 492 or 1.1% were diagnosed with CRC after the bacteraemia; 241 ― 0.5% ― within 1 year,” the researchers report.
The risk for CRC was notably increased in association with most anaerobic species, compared with negative blood cultures and with E coli and S aureus cultures, for which the risk was similar to that of negative blood cultures.
For example, infection with C septicum was associated with a 42 times greater risk for CRC within 1 year of infection and a 21 times greater risk overall with no time limitation.
Infection with B ovatus was linked to a 13 times greater risk for CRC within 1 year and a six times greater risk overall with no time limitation.
Justesen noted that their group will now focus on specific bacteria from cancer patients in an effort to identify characteristics of the bacteria that could be implicated in cancer development.
“If this is the case, it could be of great importance when it comes to screening and treatment of CRC,” he said in a statement.
For example, if there was evidence that a patient had been infected with C septicum, the anaerobic species associated with the highest risk for CRC within 1 year of infection, “we would immediately inform the treating physician about this risk and that the [patient] should be investigated further,” he told Medscape Medical News.
Justesen also noted that if there was evidence that a patient was infected with any of these high-risk bacteria and the patient was elderly, “then it would definitely be worth screening the patient for CRC,” he said. However, more research is needed before specific recommendations can be made for CRC screening in the context of any anaerobic infection, he stressed.
Justesen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Certain species of anaerobic bacteria have been linked to dramatic increases in colorectal cancer (CRC), often within a year of infection, although whether or not the bacteria are causal has yet to be determined, say Danish researchers.
“We are not convinced that all the bacteria are directly involved in CRC development — they could just be innocent bystanders that invade the blood stream when the cancer [itself] has caused a breach in the intestinal wall,” lead author Ulrik Justesen, MD, Odense University Hospital, Denmark, told Medscape Medical News in an email.
“But an algorithm for colonoscopy based on the [infecting] species, which could then be supplemented with specific characteristics [of the bacteria] along with age, is certainly a realistic perspective,” he added.
The study was to have be presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Paris, France, but the conference was canceled due to COVID-19.
Another study suggesting a link between bacteria and CRC was published earlier this year in Nature, as reported at the time by Medscape Medical News. That study, from the Netherlands, suggests that a strain of Escherichia coli may be involved in the development of CRC.
Population-Based Study
The latest study from Denmark was a population-based study involving over 2 million people.
From this large cohort, blood culture data from the years 2007 and 2016 were analyzed.
“We combined blood culture data with the national register for colorectal cancer — the Danish Colorectal Cancer Group Database — and identified incident CRC after bacteraemia,” the investigators state.
The risk for incident CRC was investigated specifically for the anaerobic bacteria Bacteroides spp, Clostridium spp, and Fusobacterium spp.
Incident rates were then compared to those from nonanaerobic bacteria, including the Streptococcus bovis group, Escherichia coli, and Staphylococcus aureus, as well as from negative blood samples.
“We included 45,760 bacteraemia episodes, of which 492 or 1.1% were diagnosed with CRC after the bacteraemia; 241 ― 0.5% ― within 1 year,” the researchers report.
The risk for CRC was notably increased in association with most anaerobic species, compared with negative blood cultures and with E coli and S aureus cultures, for which the risk was similar to that of negative blood cultures.
For example, infection with C septicum was associated with a 42 times greater risk for CRC within 1 year of infection and a 21 times greater risk overall with no time limitation.
Infection with B ovatus was linked to a 13 times greater risk for CRC within 1 year and a six times greater risk overall with no time limitation.
Justesen noted that their group will now focus on specific bacteria from cancer patients in an effort to identify characteristics of the bacteria that could be implicated in cancer development.
“If this is the case, it could be of great importance when it comes to screening and treatment of CRC,” he said in a statement.
For example, if there was evidence that a patient had been infected with C septicum, the anaerobic species associated with the highest risk for CRC within 1 year of infection, “we would immediately inform the treating physician about this risk and that the [patient] should be investigated further,” he told Medscape Medical News.
Justesen also noted that if there was evidence that a patient was infected with any of these high-risk bacteria and the patient was elderly, “then it would definitely be worth screening the patient for CRC,” he said. However, more research is needed before specific recommendations can be made for CRC screening in the context of any anaerobic infection, he stressed.
Justesen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Certain species of anaerobic bacteria have been linked to dramatic increases in colorectal cancer (CRC), often within a year of infection, although whether or not the bacteria are causal has yet to be determined, say Danish researchers.
“We are not convinced that all the bacteria are directly involved in CRC development — they could just be innocent bystanders that invade the blood stream when the cancer [itself] has caused a breach in the intestinal wall,” lead author Ulrik Justesen, MD, Odense University Hospital, Denmark, told Medscape Medical News in an email.
“But an algorithm for colonoscopy based on the [infecting] species, which could then be supplemented with specific characteristics [of the bacteria] along with age, is certainly a realistic perspective,” he added.
The study was to have be presented at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Paris, France, but the conference was canceled due to COVID-19.
Another study suggesting a link between bacteria and CRC was published earlier this year in Nature, as reported at the time by Medscape Medical News. That study, from the Netherlands, suggests that a strain of Escherichia coli may be involved in the development of CRC.
Population-Based Study
The latest study from Denmark was a population-based study involving over 2 million people.
From this large cohort, blood culture data from the years 2007 and 2016 were analyzed.
“We combined blood culture data with the national register for colorectal cancer — the Danish Colorectal Cancer Group Database — and identified incident CRC after bacteraemia,” the investigators state.
The risk for incident CRC was investigated specifically for the anaerobic bacteria Bacteroides spp, Clostridium spp, and Fusobacterium spp.
Incident rates were then compared to those from nonanaerobic bacteria, including the Streptococcus bovis group, Escherichia coli, and Staphylococcus aureus, as well as from negative blood samples.
“We included 45,760 bacteraemia episodes, of which 492 or 1.1% were diagnosed with CRC after the bacteraemia; 241 ― 0.5% ― within 1 year,” the researchers report.
The risk for CRC was notably increased in association with most anaerobic species, compared with negative blood cultures and with E coli and S aureus cultures, for which the risk was similar to that of negative blood cultures.
For example, infection with C septicum was associated with a 42 times greater risk for CRC within 1 year of infection and a 21 times greater risk overall with no time limitation.
Infection with B ovatus was linked to a 13 times greater risk for CRC within 1 year and a six times greater risk overall with no time limitation.
Justesen noted that their group will now focus on specific bacteria from cancer patients in an effort to identify characteristics of the bacteria that could be implicated in cancer development.
“If this is the case, it could be of great importance when it comes to screening and treatment of CRC,” he said in a statement.
For example, if there was evidence that a patient had been infected with C septicum, the anaerobic species associated with the highest risk for CRC within 1 year of infection, “we would immediately inform the treating physician about this risk and that the [patient] should be investigated further,” he told Medscape Medical News.
Justesen also noted that if there was evidence that a patient was infected with any of these high-risk bacteria and the patient was elderly, “then it would definitely be worth screening the patient for CRC,” he said. However, more research is needed before specific recommendations can be made for CRC screening in the context of any anaerobic infection, he stressed.
Justesen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19: Telemedicine boosting access but is not a panacea
The recent surge in telemedicine services fueled by the COVID-19 pandemic has improved access to psychiatry care and may have set the stage for even more dramatic forays into virtual care in the future. However, not all patients want video visits, and it is not clear that the way telepsychiatry is practiced right now will be the best model for clinical practice once the crisis abates, speakers said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
The COVID-19 pandemic has effectively “democratized” telepsychiatry, a mode of health care delivery that previously was thought of as “overly complex” and limited to a few specialists, said Avrim Fishkind, MD, CEO/consultant in emergency psychiatry and psychiatric emergency services design at Empathic Soul Health in Houston.
“In a blink of an eye, every psychiatrist and every mental health professional now can see themselves – and many have been forced into – becoming telepsychiatrists,” Dr. Fishkind said in a presentation at the meeting.
“Access to you is fantastic ... and your no-show rates decrease dramatically when people have flexibility to talk to you when they want to schedule and where they want to schedule.”
On the other hand, telepsychiatry should not be viewed as a panacea, cautioned Patrice A. Harris, MD, a child and adolescent psychiatrist and current president of the American Medical Association. The AMA has advocated for the more flexible federal regulations and payment policies that have helped boost telemedicine adoption during the crisis.
“Not every regulation that was relaxed, and not everything we are doing now in the midst of this pandemic, should be continued,” Dr. Harris said in a question-and-answer session earlier in the conference.
“I don’t want us all to say, ‘Wow, we had this experience, and it worked,’ and then continue to do it in the exact same way,” she added. “I know that we, the APA, and AMA, will be there to have a thoughtful, science-based, data-driven conversation about the next move regarding telemedicine and telehealth after we get through this pandemic.”
Telepsychiatry has nevertheless proven very versatile and applicable to a broad swath of patients during the COVID-19 pandemic, according to Dr. Fishkind. “I start from the position that I can see every patient this way, and I have to find a reason why I can’t,” said Dr. Fishkind, who also is lead telepsychiatrist at the Harris Center in Houston and a past president of the American Association for Emergency Psychiatry.
Telemedicine services can be as good as office visits, if not better, he told attendees at the virtual meeting. For example, a virtual visit can obviate the need for an in-person evaluation of a child with autism for whom an in-person visit would be challenging for the patient and parent alike.
However, Dr. Fishkind acknowledged that telepsychiatry is not for everyone: “I don’t want to say it’s heaven on earth. There are some patients who do refuse to be seen this way.”
What happens next in telepsychiatry is anyone’s guess, though Dr. Fishkind said he envisions an online “wheel of access” model of psychiatric services delivery.
In this portal-style model, the psychiatric patient might log in, answer a few automated questions, and then, based on their responses, they would be routed to a social worker or nurse navigator at the center of that services wheel.
In turn, the navigator might route the patient to one of the services on the spokes of the wheel, such as a psychiatrist consult, video-based or online cognitive-behavioral therapy, peer forums, group therapy, a pharmacist, or to other clinicians and interventions.
“Patients would have instant access to all of the things that we always want them to have access to – but now, by using virtual technologies, they could actually get them,” said Dr. Fishkind.
Dr. Fishkind reported no financial conflicts.
SOURCE: Fishkind A. APA 2020, Abstract.
The recent surge in telemedicine services fueled by the COVID-19 pandemic has improved access to psychiatry care and may have set the stage for even more dramatic forays into virtual care in the future. However, not all patients want video visits, and it is not clear that the way telepsychiatry is practiced right now will be the best model for clinical practice once the crisis abates, speakers said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
The COVID-19 pandemic has effectively “democratized” telepsychiatry, a mode of health care delivery that previously was thought of as “overly complex” and limited to a few specialists, said Avrim Fishkind, MD, CEO/consultant in emergency psychiatry and psychiatric emergency services design at Empathic Soul Health in Houston.
“In a blink of an eye, every psychiatrist and every mental health professional now can see themselves – and many have been forced into – becoming telepsychiatrists,” Dr. Fishkind said in a presentation at the meeting.
“Access to you is fantastic ... and your no-show rates decrease dramatically when people have flexibility to talk to you when they want to schedule and where they want to schedule.”
On the other hand, telepsychiatry should not be viewed as a panacea, cautioned Patrice A. Harris, MD, a child and adolescent psychiatrist and current president of the American Medical Association. The AMA has advocated for the more flexible federal regulations and payment policies that have helped boost telemedicine adoption during the crisis.
“Not every regulation that was relaxed, and not everything we are doing now in the midst of this pandemic, should be continued,” Dr. Harris said in a question-and-answer session earlier in the conference.
“I don’t want us all to say, ‘Wow, we had this experience, and it worked,’ and then continue to do it in the exact same way,” she added. “I know that we, the APA, and AMA, will be there to have a thoughtful, science-based, data-driven conversation about the next move regarding telemedicine and telehealth after we get through this pandemic.”
Telepsychiatry has nevertheless proven very versatile and applicable to a broad swath of patients during the COVID-19 pandemic, according to Dr. Fishkind. “I start from the position that I can see every patient this way, and I have to find a reason why I can’t,” said Dr. Fishkind, who also is lead telepsychiatrist at the Harris Center in Houston and a past president of the American Association for Emergency Psychiatry.
Telemedicine services can be as good as office visits, if not better, he told attendees at the virtual meeting. For example, a virtual visit can obviate the need for an in-person evaluation of a child with autism for whom an in-person visit would be challenging for the patient and parent alike.
However, Dr. Fishkind acknowledged that telepsychiatry is not for everyone: “I don’t want to say it’s heaven on earth. There are some patients who do refuse to be seen this way.”
What happens next in telepsychiatry is anyone’s guess, though Dr. Fishkind said he envisions an online “wheel of access” model of psychiatric services delivery.
In this portal-style model, the psychiatric patient might log in, answer a few automated questions, and then, based on their responses, they would be routed to a social worker or nurse navigator at the center of that services wheel.
In turn, the navigator might route the patient to one of the services on the spokes of the wheel, such as a psychiatrist consult, video-based or online cognitive-behavioral therapy, peer forums, group therapy, a pharmacist, or to other clinicians and interventions.
“Patients would have instant access to all of the things that we always want them to have access to – but now, by using virtual technologies, they could actually get them,” said Dr. Fishkind.
Dr. Fishkind reported no financial conflicts.
SOURCE: Fishkind A. APA 2020, Abstract.
The recent surge in telemedicine services fueled by the COVID-19 pandemic has improved access to psychiatry care and may have set the stage for even more dramatic forays into virtual care in the future. However, not all patients want video visits, and it is not clear that the way telepsychiatry is practiced right now will be the best model for clinical practice once the crisis abates, speakers said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
The COVID-19 pandemic has effectively “democratized” telepsychiatry, a mode of health care delivery that previously was thought of as “overly complex” and limited to a few specialists, said Avrim Fishkind, MD, CEO/consultant in emergency psychiatry and psychiatric emergency services design at Empathic Soul Health in Houston.
“In a blink of an eye, every psychiatrist and every mental health professional now can see themselves – and many have been forced into – becoming telepsychiatrists,” Dr. Fishkind said in a presentation at the meeting.
“Access to you is fantastic ... and your no-show rates decrease dramatically when people have flexibility to talk to you when they want to schedule and where they want to schedule.”
On the other hand, telepsychiatry should not be viewed as a panacea, cautioned Patrice A. Harris, MD, a child and adolescent psychiatrist and current president of the American Medical Association. The AMA has advocated for the more flexible federal regulations and payment policies that have helped boost telemedicine adoption during the crisis.
“Not every regulation that was relaxed, and not everything we are doing now in the midst of this pandemic, should be continued,” Dr. Harris said in a question-and-answer session earlier in the conference.
“I don’t want us all to say, ‘Wow, we had this experience, and it worked,’ and then continue to do it in the exact same way,” she added. “I know that we, the APA, and AMA, will be there to have a thoughtful, science-based, data-driven conversation about the next move regarding telemedicine and telehealth after we get through this pandemic.”
Telepsychiatry has nevertheless proven very versatile and applicable to a broad swath of patients during the COVID-19 pandemic, according to Dr. Fishkind. “I start from the position that I can see every patient this way, and I have to find a reason why I can’t,” said Dr. Fishkind, who also is lead telepsychiatrist at the Harris Center in Houston and a past president of the American Association for Emergency Psychiatry.
Telemedicine services can be as good as office visits, if not better, he told attendees at the virtual meeting. For example, a virtual visit can obviate the need for an in-person evaluation of a child with autism for whom an in-person visit would be challenging for the patient and parent alike.
However, Dr. Fishkind acknowledged that telepsychiatry is not for everyone: “I don’t want to say it’s heaven on earth. There are some patients who do refuse to be seen this way.”
What happens next in telepsychiatry is anyone’s guess, though Dr. Fishkind said he envisions an online “wheel of access” model of psychiatric services delivery.
In this portal-style model, the psychiatric patient might log in, answer a few automated questions, and then, based on their responses, they would be routed to a social worker or nurse navigator at the center of that services wheel.
In turn, the navigator might route the patient to one of the services on the spokes of the wheel, such as a psychiatrist consult, video-based or online cognitive-behavioral therapy, peer forums, group therapy, a pharmacist, or to other clinicians and interventions.
“Patients would have instant access to all of the things that we always want them to have access to – but now, by using virtual technologies, they could actually get them,” said Dr. Fishkind.
Dr. Fishkind reported no financial conflicts.
SOURCE: Fishkind A. APA 2020, Abstract.
FROM APA 2020
COVID-19 linked to large vessel stroke in young adults
In a rapid communication to be published online April 29 in the New England Journal of Medicine, investigators led by Thomas Oxley, MD, PhD, of the department of neurosurgery at Mount Sinai Health System, reported five cases of large vessel stroke over a 2-week period in COVID-19 patients under age 50 years. This represents a sevenfold increase in what would normally be expected.
The five cases had either no, or mild, COVID-19 symptoms.
“It’s been surprising to learn that the virus appears to cause disease through a process of blood clotting,” Dr. Oxley said in an interview.
The message for neurologists and other physicians is “we’re learning that this can disproportionally affect large vessels more than small vessels in terms of presentation of stroke,” he said.
Inflammation in the blood vessel walls may be driving thrombosis formation, Dr. Oxley added. This report joins other research pointing to this emerging phenomenon.
Recently, investigators in the Netherlands found a “remarkably high” 31% rate of thrombotic complications among 184 critical care patients with COVID-19 pneumonia.
Dr. Oxley and colleagues also suggested that, since the onset of the pandemic, fewer patients may be calling emergency services when they experience signs of a stroke. The physicians noted that two of the five cases in the report delayed calling an ambulance.
“I understand why people do not want to leave the household. I think people are more willing to ignore other [non–COVID-19] symptoms in this environment,” he said.
As previously reported, physicians in hospitals across the United States and elsewhere have reported a significant drop in stroke patients since the COVID-19 pandemic took hold, which suggests that patients may indeed be foregoing emergency care.
The observations from Dr. Oxley and colleagues call for greater awareness of the association between COVID-19 and large vessel strokes in this age group, they add.
One patient in the case series died, one remains hospitalized, two are undergoing rehabilitation, and one was discharged home as of April 24.
Dr. Oxley and colleagues dedicated their report to “our inspiring colleague Gary Sclar, MD, a stroke physician who succumbed to COVID-19 while caring for his patients.”
Dr. Oxley has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a rapid communication to be published online April 29 in the New England Journal of Medicine, investigators led by Thomas Oxley, MD, PhD, of the department of neurosurgery at Mount Sinai Health System, reported five cases of large vessel stroke over a 2-week period in COVID-19 patients under age 50 years. This represents a sevenfold increase in what would normally be expected.
The five cases had either no, or mild, COVID-19 symptoms.
“It’s been surprising to learn that the virus appears to cause disease through a process of blood clotting,” Dr. Oxley said in an interview.
The message for neurologists and other physicians is “we’re learning that this can disproportionally affect large vessels more than small vessels in terms of presentation of stroke,” he said.
Inflammation in the blood vessel walls may be driving thrombosis formation, Dr. Oxley added. This report joins other research pointing to this emerging phenomenon.
Recently, investigators in the Netherlands found a “remarkably high” 31% rate of thrombotic complications among 184 critical care patients with COVID-19 pneumonia.
Dr. Oxley and colleagues also suggested that, since the onset of the pandemic, fewer patients may be calling emergency services when they experience signs of a stroke. The physicians noted that two of the five cases in the report delayed calling an ambulance.
“I understand why people do not want to leave the household. I think people are more willing to ignore other [non–COVID-19] symptoms in this environment,” he said.
As previously reported, physicians in hospitals across the United States and elsewhere have reported a significant drop in stroke patients since the COVID-19 pandemic took hold, which suggests that patients may indeed be foregoing emergency care.
The observations from Dr. Oxley and colleagues call for greater awareness of the association between COVID-19 and large vessel strokes in this age group, they add.
One patient in the case series died, one remains hospitalized, two are undergoing rehabilitation, and one was discharged home as of April 24.
Dr. Oxley and colleagues dedicated their report to “our inspiring colleague Gary Sclar, MD, a stroke physician who succumbed to COVID-19 while caring for his patients.”
Dr. Oxley has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a rapid communication to be published online April 29 in the New England Journal of Medicine, investigators led by Thomas Oxley, MD, PhD, of the department of neurosurgery at Mount Sinai Health System, reported five cases of large vessel stroke over a 2-week period in COVID-19 patients under age 50 years. This represents a sevenfold increase in what would normally be expected.
The five cases had either no, or mild, COVID-19 symptoms.
“It’s been surprising to learn that the virus appears to cause disease through a process of blood clotting,” Dr. Oxley said in an interview.
The message for neurologists and other physicians is “we’re learning that this can disproportionally affect large vessels more than small vessels in terms of presentation of stroke,” he said.
Inflammation in the blood vessel walls may be driving thrombosis formation, Dr. Oxley added. This report joins other research pointing to this emerging phenomenon.
Recently, investigators in the Netherlands found a “remarkably high” 31% rate of thrombotic complications among 184 critical care patients with COVID-19 pneumonia.
Dr. Oxley and colleagues also suggested that, since the onset of the pandemic, fewer patients may be calling emergency services when they experience signs of a stroke. The physicians noted that two of the five cases in the report delayed calling an ambulance.
“I understand why people do not want to leave the household. I think people are more willing to ignore other [non–COVID-19] symptoms in this environment,” he said.
As previously reported, physicians in hospitals across the United States and elsewhere have reported a significant drop in stroke patients since the COVID-19 pandemic took hold, which suggests that patients may indeed be foregoing emergency care.
The observations from Dr. Oxley and colleagues call for greater awareness of the association between COVID-19 and large vessel strokes in this age group, they add.
One patient in the case series died, one remains hospitalized, two are undergoing rehabilitation, and one was discharged home as of April 24.
Dr. Oxley and colleagues dedicated their report to “our inspiring colleague Gary Sclar, MD, a stroke physician who succumbed to COVID-19 while caring for his patients.”
Dr. Oxley has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19: Psychiatrists ‘more than a match’ for crisis moment
Tackling the COVID-19 crisis will require psychiatrists to muster the courage to lead, establish trust, and ultimately provide psychiatric care with competence, honesty, and compassion, said Patrice A. Harris, MD, an Atlanta-based psychiatrist who is president of the American Medical Association.
Leaders in psychiatry are uniquely positioned to combat a wave of disease misinformation, address inequities in care, and meet the logistical challenges of safely meeting patient needs as the outbreak continues, Dr. Harris said at the American Psychiatric Association annual meeting, which was held as a virtual live event.
“I believe you, we, are more than a match for this moment – a moment that requires our leadership and requires us to hold other leaders accountable as we fight this pandemic,” she said in remarks to online attendees.
Using trust to fight myths
Misinformation about COVID-19 has been “spreading rapidly, even intentionally, due to fear or political agendas,” said Dr. Harris, who became the 174th president of the AMA in June 2019.
Others believe the coronavirus crisis is a new way to force vaccinations on people who don’t want them, added Dr. Harris.
Myths, rumors, and conspiracy theories lead to “more illness and death,” she said, at a time when most Americans say they’ve lost trust in the federal government and even in other American citizens.
“Fortunately, people still trust us – their doctors,” she added. “We fight for science, we call out quackery and snake oil when we see it, [and] we are willing to counter the propaganda of the antiscience voice.”
Physicians are ranked among the most trusted professions because they are committed to seeing, acknowledging, and sharing patients’ human experience, “and of course, I believe we do that as psychiatrists more than most,” Dr. Harris said.
Fighting COVID-19 at the AMA level
During the pandemic, the AMA has advocated for adequate testing and supplies, adequate insurance coverage, and changes to current procedural technology (CPT) codes to streamline novel coronavirus testing. The AMA has also developed a free COVID-19 resource center on the JAMA Network website, Dr. Harris said, as well as guidance on protecting medical students responding to the pandemic.
The safety of health care clinicians remains a central issue for the AMA at a time when masks and other personal protective equipment (PPE) remain in short supply.
In a recent letter to Vice President Mike Pence, who is leading the White House’s coronavirus task force, AMA Executive Vice President and CEO James L. Madara, MD, urged the Trump administration to undertake a Manhattan Project–like effort to expand capacity for needed supplies.
“We will continue to call on the White House, and APA has as well, to make sure these needs are met,” Dr. Harris said.
COVID-19 and inequities in care
Because the pandemic has had dramatic effects on African American communities across the United States, AMA Chief Health Equity Officer Aletha Maybank, MD, has made recent media appearances to highlight care inequities and what can be done about them.
Meanwhile, the AMA and other physician associations have urged the Trump Administration to collect, analyze, and make available COVID-19 data by race and ethnicity: “We can’t fix a problem until we identify a problem,” Dr. Harris said in her address to the APA.
Relying on science
In a virtual address hosted by the National Press Club earlier in April, Dr. Harris made an appeal for “relying on the science and evidence” to inform COVID-19–related decisions.
Elected officials need to “affirm science, evidence, and fact in their words and actions,” while media need to be vigilant in citing credible sources and challenging those who “chose to trade in misinformation,” she said in that address.
Speaking at the APA virtual meeting, Dr. Harris spoke of an “assault on science for several years” that inspired the National Press Club address. “We wanted to remind the public of its responsibility to focus on science and the evidence, for us to turn the tide against COVID-19,” she explained.
Physician care and self-care
While the AMA urges social distancing, Dr. Harris used the term “physical distancing” in her APA address. Physical distancing emphasizes the need for stay-at-home and shelter-in-place restrictions, while recognizing the need for maintaining meaningful social interactions, she explained.
Social media use represents one “opportunity” to bridge that gap when physical proximity is not an option, she added.
Dr. Harris also stressed the need for physicians to “take time out and practice self-care” to ensure that they are recharged and able to provide optimal patient care.
“We need to be there for others, but we have to put our own masks on first,” she said.
Dr. Harris reported no financial relationships with commercial interests.
SOURCE: Harris PA. APA 2020 Virtual Meeting.
Tackling the COVID-19 crisis will require psychiatrists to muster the courage to lead, establish trust, and ultimately provide psychiatric care with competence, honesty, and compassion, said Patrice A. Harris, MD, an Atlanta-based psychiatrist who is president of the American Medical Association.
Leaders in psychiatry are uniquely positioned to combat a wave of disease misinformation, address inequities in care, and meet the logistical challenges of safely meeting patient needs as the outbreak continues, Dr. Harris said at the American Psychiatric Association annual meeting, which was held as a virtual live event.
“I believe you, we, are more than a match for this moment – a moment that requires our leadership and requires us to hold other leaders accountable as we fight this pandemic,” she said in remarks to online attendees.
Using trust to fight myths
Misinformation about COVID-19 has been “spreading rapidly, even intentionally, due to fear or political agendas,” said Dr. Harris, who became the 174th president of the AMA in June 2019.
Others believe the coronavirus crisis is a new way to force vaccinations on people who don’t want them, added Dr. Harris.
Myths, rumors, and conspiracy theories lead to “more illness and death,” she said, at a time when most Americans say they’ve lost trust in the federal government and even in other American citizens.
“Fortunately, people still trust us – their doctors,” she added. “We fight for science, we call out quackery and snake oil when we see it, [and] we are willing to counter the propaganda of the antiscience voice.”
Physicians are ranked among the most trusted professions because they are committed to seeing, acknowledging, and sharing patients’ human experience, “and of course, I believe we do that as psychiatrists more than most,” Dr. Harris said.
Fighting COVID-19 at the AMA level
During the pandemic, the AMA has advocated for adequate testing and supplies, adequate insurance coverage, and changes to current procedural technology (CPT) codes to streamline novel coronavirus testing. The AMA has also developed a free COVID-19 resource center on the JAMA Network website, Dr. Harris said, as well as guidance on protecting medical students responding to the pandemic.
The safety of health care clinicians remains a central issue for the AMA at a time when masks and other personal protective equipment (PPE) remain in short supply.
In a recent letter to Vice President Mike Pence, who is leading the White House’s coronavirus task force, AMA Executive Vice President and CEO James L. Madara, MD, urged the Trump administration to undertake a Manhattan Project–like effort to expand capacity for needed supplies.
“We will continue to call on the White House, and APA has as well, to make sure these needs are met,” Dr. Harris said.
COVID-19 and inequities in care
Because the pandemic has had dramatic effects on African American communities across the United States, AMA Chief Health Equity Officer Aletha Maybank, MD, has made recent media appearances to highlight care inequities and what can be done about them.
Meanwhile, the AMA and other physician associations have urged the Trump Administration to collect, analyze, and make available COVID-19 data by race and ethnicity: “We can’t fix a problem until we identify a problem,” Dr. Harris said in her address to the APA.
Relying on science
In a virtual address hosted by the National Press Club earlier in April, Dr. Harris made an appeal for “relying on the science and evidence” to inform COVID-19–related decisions.
Elected officials need to “affirm science, evidence, and fact in their words and actions,” while media need to be vigilant in citing credible sources and challenging those who “chose to trade in misinformation,” she said in that address.
Speaking at the APA virtual meeting, Dr. Harris spoke of an “assault on science for several years” that inspired the National Press Club address. “We wanted to remind the public of its responsibility to focus on science and the evidence, for us to turn the tide against COVID-19,” she explained.
Physician care and self-care
While the AMA urges social distancing, Dr. Harris used the term “physical distancing” in her APA address. Physical distancing emphasizes the need for stay-at-home and shelter-in-place restrictions, while recognizing the need for maintaining meaningful social interactions, she explained.
Social media use represents one “opportunity” to bridge that gap when physical proximity is not an option, she added.
Dr. Harris also stressed the need for physicians to “take time out and practice self-care” to ensure that they are recharged and able to provide optimal patient care.
“We need to be there for others, but we have to put our own masks on first,” she said.
Dr. Harris reported no financial relationships with commercial interests.
SOURCE: Harris PA. APA 2020 Virtual Meeting.
Tackling the COVID-19 crisis will require psychiatrists to muster the courage to lead, establish trust, and ultimately provide psychiatric care with competence, honesty, and compassion, said Patrice A. Harris, MD, an Atlanta-based psychiatrist who is president of the American Medical Association.
Leaders in psychiatry are uniquely positioned to combat a wave of disease misinformation, address inequities in care, and meet the logistical challenges of safely meeting patient needs as the outbreak continues, Dr. Harris said at the American Psychiatric Association annual meeting, which was held as a virtual live event.
“I believe you, we, are more than a match for this moment – a moment that requires our leadership and requires us to hold other leaders accountable as we fight this pandemic,” she said in remarks to online attendees.
Using trust to fight myths
Misinformation about COVID-19 has been “spreading rapidly, even intentionally, due to fear or political agendas,” said Dr. Harris, who became the 174th president of the AMA in June 2019.
Others believe the coronavirus crisis is a new way to force vaccinations on people who don’t want them, added Dr. Harris.
Myths, rumors, and conspiracy theories lead to “more illness and death,” she said, at a time when most Americans say they’ve lost trust in the federal government and even in other American citizens.
“Fortunately, people still trust us – their doctors,” she added. “We fight for science, we call out quackery and snake oil when we see it, [and] we are willing to counter the propaganda of the antiscience voice.”
Physicians are ranked among the most trusted professions because they are committed to seeing, acknowledging, and sharing patients’ human experience, “and of course, I believe we do that as psychiatrists more than most,” Dr. Harris said.
Fighting COVID-19 at the AMA level
During the pandemic, the AMA has advocated for adequate testing and supplies, adequate insurance coverage, and changes to current procedural technology (CPT) codes to streamline novel coronavirus testing. The AMA has also developed a free COVID-19 resource center on the JAMA Network website, Dr. Harris said, as well as guidance on protecting medical students responding to the pandemic.
The safety of health care clinicians remains a central issue for the AMA at a time when masks and other personal protective equipment (PPE) remain in short supply.
In a recent letter to Vice President Mike Pence, who is leading the White House’s coronavirus task force, AMA Executive Vice President and CEO James L. Madara, MD, urged the Trump administration to undertake a Manhattan Project–like effort to expand capacity for needed supplies.
“We will continue to call on the White House, and APA has as well, to make sure these needs are met,” Dr. Harris said.
COVID-19 and inequities in care
Because the pandemic has had dramatic effects on African American communities across the United States, AMA Chief Health Equity Officer Aletha Maybank, MD, has made recent media appearances to highlight care inequities and what can be done about them.
Meanwhile, the AMA and other physician associations have urged the Trump Administration to collect, analyze, and make available COVID-19 data by race and ethnicity: “We can’t fix a problem until we identify a problem,” Dr. Harris said in her address to the APA.
Relying on science
In a virtual address hosted by the National Press Club earlier in April, Dr. Harris made an appeal for “relying on the science and evidence” to inform COVID-19–related decisions.
Elected officials need to “affirm science, evidence, and fact in their words and actions,” while media need to be vigilant in citing credible sources and challenging those who “chose to trade in misinformation,” she said in that address.
Speaking at the APA virtual meeting, Dr. Harris spoke of an “assault on science for several years” that inspired the National Press Club address. “We wanted to remind the public of its responsibility to focus on science and the evidence, for us to turn the tide against COVID-19,” she explained.
Physician care and self-care
While the AMA urges social distancing, Dr. Harris used the term “physical distancing” in her APA address. Physical distancing emphasizes the need for stay-at-home and shelter-in-place restrictions, while recognizing the need for maintaining meaningful social interactions, she explained.
Social media use represents one “opportunity” to bridge that gap when physical proximity is not an option, she added.
Dr. Harris also stressed the need for physicians to “take time out and practice self-care” to ensure that they are recharged and able to provide optimal patient care.
“We need to be there for others, but we have to put our own masks on first,” she said.
Dr. Harris reported no financial relationships with commercial interests.
SOURCE: Harris PA. APA 2020 Virtual Meeting.
FROM APA 2020
COVID-19 decimates outpatient visits
There has been a massive decline in outpatient office visits as patients have stayed home – likely deferring needed care – because of COVID-19, new research shows.
The number of visits to ambulatory practices dropped by a whopping 60% in mid-March, and continues to be down by at least 50% since early February, according to new data compiled and analyzed by Harvard University and Phreesia, a health care technology company.
Phreesia – which helps medical practices with patient registration, insurance verification, and payments – has data on 50,000 providers in all 50 states; in a typical year, Phreesia tracks 50 million outpatient visits.
The report was published online April 23 by the Commonwealth Fund.
The company captured data on visits from February 1 through April 16. The decline was greatest in New England and the Mid-Atlantic states, where, at the steepest end of the decline in late March, visits were down 66%.
They have rebounded slightly since then but are still down 64%. Practices in the mountain states had the smallest decline, but visits were down by 45% as of April 16.
Many practices have attempted to reach out to patients through telemedicine. As of April 16, about 30% of all visits tracked by Phreesia were provided via telemedicine – by phone or through video. That’s a monumental increase from mid-February, when zero visits were conducted virtually.
However, the Harvard researchers found that telemedicine visits barely made up for the huge decline in office visits.
Decline by specialty
Not surprisingly, declining visits have been steeper in procedure-oriented specialties.
Overall visits – including telemedicine – to ophthalmologists and otolaryngologists had declined by 79% and 75%, respectively, as of the week of April 5. Dermatology saw a 73% decline. Surgery, pulmonology, urology, orthopedics, cardiology, and gastroenterology all experienced declines ranging from 61% to 66%.
Primary care offices, oncology, endocrinology, and obstetrics/gynecology all fared slightly better, with visits down by half. Behavioral health experienced the lowest rate of decline (30%).
School-aged children were skipping care most often. The study showed a 71% drop in visits in 7- to 17-year-olds, and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Nearly two-thirds of Americans over age 65 also stayed away from their doctors. Only half of those aged 18 to 64 reduced their physician visits.
This article first appeared on Medscape.com.
There has been a massive decline in outpatient office visits as patients have stayed home – likely deferring needed care – because of COVID-19, new research shows.
The number of visits to ambulatory practices dropped by a whopping 60% in mid-March, and continues to be down by at least 50% since early February, according to new data compiled and analyzed by Harvard University and Phreesia, a health care technology company.
Phreesia – which helps medical practices with patient registration, insurance verification, and payments – has data on 50,000 providers in all 50 states; in a typical year, Phreesia tracks 50 million outpatient visits.
The report was published online April 23 by the Commonwealth Fund.
The company captured data on visits from February 1 through April 16. The decline was greatest in New England and the Mid-Atlantic states, where, at the steepest end of the decline in late March, visits were down 66%.
They have rebounded slightly since then but are still down 64%. Practices in the mountain states had the smallest decline, but visits were down by 45% as of April 16.
Many practices have attempted to reach out to patients through telemedicine. As of April 16, about 30% of all visits tracked by Phreesia were provided via telemedicine – by phone or through video. That’s a monumental increase from mid-February, when zero visits were conducted virtually.
However, the Harvard researchers found that telemedicine visits barely made up for the huge decline in office visits.
Decline by specialty
Not surprisingly, declining visits have been steeper in procedure-oriented specialties.
Overall visits – including telemedicine – to ophthalmologists and otolaryngologists had declined by 79% and 75%, respectively, as of the week of April 5. Dermatology saw a 73% decline. Surgery, pulmonology, urology, orthopedics, cardiology, and gastroenterology all experienced declines ranging from 61% to 66%.
Primary care offices, oncology, endocrinology, and obstetrics/gynecology all fared slightly better, with visits down by half. Behavioral health experienced the lowest rate of decline (30%).
School-aged children were skipping care most often. The study showed a 71% drop in visits in 7- to 17-year-olds, and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Nearly two-thirds of Americans over age 65 also stayed away from their doctors. Only half of those aged 18 to 64 reduced their physician visits.
This article first appeared on Medscape.com.
There has been a massive decline in outpatient office visits as patients have stayed home – likely deferring needed care – because of COVID-19, new research shows.
The number of visits to ambulatory practices dropped by a whopping 60% in mid-March, and continues to be down by at least 50% since early February, according to new data compiled and analyzed by Harvard University and Phreesia, a health care technology company.
Phreesia – which helps medical practices with patient registration, insurance verification, and payments – has data on 50,000 providers in all 50 states; in a typical year, Phreesia tracks 50 million outpatient visits.
The report was published online April 23 by the Commonwealth Fund.
The company captured data on visits from February 1 through April 16. The decline was greatest in New England and the Mid-Atlantic states, where, at the steepest end of the decline in late March, visits were down 66%.
They have rebounded slightly since then but are still down 64%. Practices in the mountain states had the smallest decline, but visits were down by 45% as of April 16.
Many practices have attempted to reach out to patients through telemedicine. As of April 16, about 30% of all visits tracked by Phreesia were provided via telemedicine – by phone or through video. That’s a monumental increase from mid-February, when zero visits were conducted virtually.
However, the Harvard researchers found that telemedicine visits barely made up for the huge decline in office visits.
Decline by specialty
Not surprisingly, declining visits have been steeper in procedure-oriented specialties.
Overall visits – including telemedicine – to ophthalmologists and otolaryngologists had declined by 79% and 75%, respectively, as of the week of April 5. Dermatology saw a 73% decline. Surgery, pulmonology, urology, orthopedics, cardiology, and gastroenterology all experienced declines ranging from 61% to 66%.
Primary care offices, oncology, endocrinology, and obstetrics/gynecology all fared slightly better, with visits down by half. Behavioral health experienced the lowest rate of decline (30%).
School-aged children were skipping care most often. The study showed a 71% drop in visits in 7- to 17-year-olds, and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Nearly two-thirds of Americans over age 65 also stayed away from their doctors. Only half of those aged 18 to 64 reduced their physician visits.
This article first appeared on Medscape.com.










