User login
Implementation of a Protocol to Manage Patients at Risk for Hospitalization Due to an Ambulatory Care Sensitive Condition
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
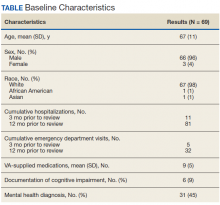
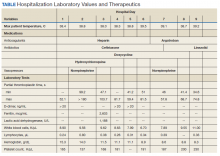
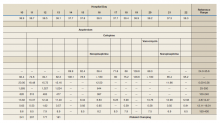
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
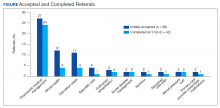
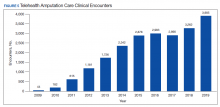
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Immunotherapies Targeting α -Synuclein in Parkinson Disease
Parkinson disease (PD) is a progressive neurodegenerative disorder, characterized by diverse clinical symptoms. PD can present with rest tremor, bradykinesia, rigidity, falls, postural instability, and multiple nonmotor symptoms. Marras and colleagues estimated in a comprehensive meta-analysis that there were 680,000 individuals with PD in the US in 2010; this number is expected to double by 2030 based on the US Census Bureau population projections.1 An estimated 110,000 veterans may be affected by PD; hence, understanding of PD pathology, clinical progression, and effective treatment strategies is of paramount importance to the Veterans Health Administration (VHA).2
The exact pathogenesis underlying clinical features is still being studied. Pathologic diagnosis of PD relies on loss of dopamine neurons in the substantia nigra and accumulation of the abnormal protein, α-synuclein, in the form of Lewy bodies and Lewy neurites. Lewy bodies and neurites accumulate predominantly in the substantia nigra in addition to other brain stem nuclei and cerebral cortex. Lewy bodies are intraneuronal inclusions with a hyaline core and a pale peripheral halo. Central core stains positive for α-synuclein.3,4 Lewy neurites are widespread and are believed to play a larger role in the pathogenesis of PD compared with those of Lewy bodies.5
α-Synuclein
α-synuclein is a small 140 amino-acid protein with a N-terminal region that can interact with cell membranes and a highly acidic unstructured C-terminal region.6 α-synuclein is physiologically present in the presynaptic terminals of neurons and involved in synaptic plasticity and vesicle trafficking.7 There are different hypotheses about the native structure of α-synuclein. The first suggests that it exists in tetrameric form and may be broken down to monomer, which is the pathogenic form of α-synuclein. The second hypothesis suggests that it exists primarily in monomeric form, whereas other studies have shown that both forms exist and with pathologic changes, monomer accumulates in abundance and is neurotoxic.8-11 Work by Burré and colleagues shows that native α-synuclein exists in 2 forms: a soluble, cytosolic α-synuclein, which is monomeric, and a membrane-bound multimeric form.12,13
Alteration in aggregation properties of this protein is believed to play a central role in the pathogenesis of PD.14,15 Pathologic α-synuclein exists in insoluble forms that can aggregate into oligomers and fibrillar structures.16 Lysosomal dysfunction may promote accumulation of insoluble α-synuclein. Prior work has shown that several degradation pathways in lysosomes, including the ubiquitin-proteasome system and autophagy-lysosomal pathway, are down regulated, thus contributing to the accumulation of abnormal α-synuclein.17,18 Accumulation of pathologic α-synuclein leads to mitochondrial dysfunction in PD animal models, contributing further to neurotoxicity.19,20 Aggregates of phosphorylated α-synuclein have been demonstrated in dementia with Lewy body.21
In addition, α-synuclein aggregates may be released into extracellular spaces to be taken up by adjacent cells, where they can cause further misfolding and aggregation of protein.22 Previous work in animal models suggested a prion proteinlike spread of α-synuclein.23 This finding can have long-term therapeutic implications, as preventing extracellular release of abnormal form of α-synuclein will prevent the spread of pathologic protein. This can form the basis of neuroprotection in patients with PD.24
It has been proposed that α-synuclein accumulation and extracellular release initiates an immune response that leads to activation of microglia. This has been shown in PD animal models, overexpressing α-synuclein. In 2008 Park and colleagues demonstrated that microglial activation is enhanced by monomeric α-synuclein, not by the aggregated variant.25 Other studies have reported activated microglia around dopaminergic cells in substantia nigra.26 Sulzer and colleagues showed that peptides from α-synuclein can act as antigens and trigger an autoimmune reaction via T cells.27 PD may be associated with certain HLA-haplotypes.28 In other words, α-synuclein can induce neurodegeneration via different mechanisms, including alteration in synaptic vesicle transmission, mitochondrial dysfunction, neuroinflammation, and induction of humoral immunity.
Immunization
Due to these observations, there had been huge interest in developing antibody-based therapies for PD. A similar approach had been tested in Alzheimer disease (AD). Intracellular tangles of tau protein and extracellular aggregates of amyloid are the pathologic substrates in AD. Clinical trials utilizing antibodies targeting amyloid showed reduction in abnormal protein accumulation but no significant improvement in cognition.29 In addition, adverse events (AEs), such as vasogenic edema and intracerebral hemorrhage, were reported.30 Careful analysis of the data suggested that inadequate patient selection or targeting only amyloid, may have contributed to unfavorable results.31 Since then, more recent clinical trials have focused on careful patient selection, use of second generation anti-amyloid antibodies and immunotherapies targeting tau.32
Several studies have tested immunotherapies in PD animal models with the aim of targeting α-synuclein. Immunotherapies can be instituted in 2 ways: active immunization in which the immune system is stimulated to produce antibodies against α-synuclein or passive immunization in which antibodies against α-synuclein are administered directly. Once α-synuclein antibodies have crossed the blood-brain barrier, they are hypothesized to clear the existing α-synuclein. Animal studies have demonstrated the presence of these antibodies within the neurons. The mechanism of entry is unknown. Once inside the cells, the antibodies activate the lysosomal clearance, affecting intracellular accumulation of α-synuclein. Extracellularly, they can bind to receptors on scavenger cells, mainly microglia, activating them to facilitate uptake of extracellular α-synuclein. Binding of the antibodies to α-synuclein directly prevents the uptake of toxic protein by the cells, blocking the transfer and spread of PD pathology.33
Active Immunization
Active immunization against α-synuclein was demonstrated by Masliah and colleagues almost a decade ago. They administered recombinant human α-synuclein in transgenic mice expressing α-synuclein under the control of platelet-derived growth factor β. Reduction of accumulated α-synuclein in neurons with mild microglia activation was noted. It was proposed that the antibodies produced were able to bind to abnormal α-synuclein, were recognized by the lysosomal pathways, and degraded.34 Ghochikyan and colleagues developed vaccines by using α-synuclein-derived peptides. This induced formation of antibodies against α-synuclein in Lewy-bodies and neurites.35 Over time, other animal studies have been able to expand on these results.36
AFFiRiS, an Austrian biotechnology company, has developed 2 peptide vaccines PD01A and PD03A. Both peptides when administered to PD animal models caused antibody-based immune response against aggregated α-synuclein. Humoral autoimmune response was not observed in these studies; no neuroinflammation or neurotoxicity was noted. These peptides did not affect levels of physiologic α-synuclein, targeting only the aggregated form.37 These animal models showed improved motor and cognitive function. Similar results were noted in multiple system atrophy (MSA) animal models.38,39
The first human phase 1, randomized, parallel-group, single-center study recruited 32 subjects with early PD. Twelve subjects each were included in low- or high-dose treatment group, and 8 were included in the control group. Test subjects randomly received 4 vaccinations of low- or high-dose PD01A. Both doses were well tolerated, and no drug-related serious AEs were reported. The study confirmed the tolerability and safety of subcutaneous PD01A vaccine administration. These subjects were included in a 12-month, phase 1b follow-up extension study, AFF008E. In 2018, it was reported that administration of 6 doses of PD01A, 4 primary and 2 booster immunization, was safe. The vaccine showed a clear immune response against the peptide and cross-reactivity against α-synuclein targeted epitope. Booster doses stabilized the antibody titers. Significant increase in antibody titers against PD01A was seen over time, which was translated into a humoral immune response against α-synuclein. In addition, PD01A antibodies also were reported in cerebrospinal fluid.40
AFFiRiS presented results of a phase 1 randomized, placebo-controlled trial in 2017, confirming the safety of PD03A in patients with PD. The study showed a clear dose-dependent immune response against the peptide and cross-reactivity against α-synuclein targeted epitope.41 AFFiRiS recently presented results of another phase 1 clinical study assessing the safety and tolerability of vaccines PD01A and PD03A in patients with early MSA. Both vaccines were well tolerated, and PD01A induced an immune response against the peptide and α-synuclein epitope.42 These results have provided hope for further endeavors to develop active immunization strategies for PD.
Passive Immunization
Passive immunization against α-synuclein was first reported by Masliah and colleagues in 2011. A monoclonal antibody against the C-terminus of α-synuclein, 9E4, was injected into a transgenic mouse model of PD. There was reduction in α-synuclein aggregates in the brain along with improvement in motor and cognitive impairment.43 The C-terminus of α-synuclein plays a key role in the pathogenesis of PD. Changes in the C-terminus of α-synuclein induces formation of α-synuclein oligomers and subsequent neuronal spread. Antibody binds to the C-terminus and prevents structural changes that can lead to oligomerization of α-synuclein. Since the first study by Masliah, few other immunization studies utilized different antibodies against the C-terminus of α-synuclein. It was shown in a mouse model that binding of such antibodies promoted clearance of the α-synuclein by microglia.44
Based on these animal studies, Prothena Biosciences (South San Francisco, CA) designed a phase 1, double-blind, randomized, placebo-controlled clinical trial of prasinezumab (investigational monoclonal antibody against C-terminus of α-synuclein), in subjects without PD. The results showed that it was well tolerated, and there was dose-dependent reduction in the levels of free
BIIB054 is another monoclonal antibody that targets the N-terminal of α-synuclein. In animal models, antibodies targeting the N-terminus reduced α-synuclein triggered cell death and reduced the number of activated microglia.48 BIIB054, from Biogen (Cambridge, MA), was studied in 40 healthy subjects and was well tolerated with a favorable safety profile and could cross the blood-brain barrier. Like the prasinezumab study, this also was an ascending-dose study to assess safety and tolerability. In 2018, a randomized, double-blind, placebo-controlled, single-ascending dose study in patients with PD reported that BIIB054 was well tolerated, and the presence of BIIB054-synuclein complexes in the plasma were confirmed.49 A phase 2, multicenter, randomized, double-blind, placebo-controlled study (SPARK) with an active-treatment dose-blinded period, designed to evaluate the safety, pharmacokinetics, and the pharmacodynamics of BIIB054 is currently recruiting patients with PD.
Finally, BioArctic (Stockholm, Sweden) developed antibodies that are selective for oligomeric forms of α-synuclein, which it licensed to AbbVie (North Chicago, Il).50 These antibodies do not target the N- or C-terminus of α-synuclein. Since α-synuclein oligomers play an important role in the pathogenesis of PD, targeting them with antibodies at an early stage may prove to be an effective strategy for removal of pathogenic α-synuclein. Clinical trials are forthcoming.
Conclusions
Immunotherapy against α-synuclein has provided a new therapeutic avenue in the field of neuroprotection. Results from the first human clinical trial are promising, but despite these results, more work is needed to clarify the role of α-synuclein in the pathogenesis of PD in humans. Most of the work concerning α-synuclein aggregation and propagation has been reported in animal models. Whether similar process exists in humans is a debatable question. Similarly, more knowledge is needed about how and where in the human brain antibodies act to give neuroprotective effects. Timing of administration of immunotherapies in real time will be a crucial question.
PD is clinically evident once 80% of dopaminergic neurons in substantia nigra are lost due to neurodegeneration. Should immunotherapy be administered to symptomatic patients with PD, or if it will be beneficial only for presymptomatic, high-risk patients needs to be determined. Like AD trials, not only careful selection of patients, but determination of optimal timing for treatment will be essential. As the understanding of PD pathogenesis and therapeutics evolves, it will become clear whether immunization targeting α-synuclein will modify disease progression.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. doi:10.1038/s41531-018-0058-0
2. Mantri S, Duda JE, Morley JF. Early and accurate identification of Parkinson disease among US veterans. Fed Pract. 2019;36(suppl 4):S18-S23. doi:10.12788/fp.37-0034
3. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(suppl 1):S71-S85. doi:10.3233/JPD-179001
4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. doi:10.1038/42166
5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. doi:10.1016/s0197-4580(02)00065-9
6. Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79(6):1044-1066. doi:10.1016/j.neuron.2013.09.004
7. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663-1667. doi:10.1126/science.1195227
8. Binolfi A, Fernández CO, Sica MP, Delfino JM, Santos J. Recognition between a short unstructured peptide and a partially folded fragment leads to the thioredoxin fold sharing native-like dynamics. Proteins. 2012;80(5):1448-1464. doi:10.1002/prot.24043
9. Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345-15364. doi:10.1074/jbc.M111.318949.
10. Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797-17802. doi:10.1073/pnas.1113260108
11. Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183-202. doi:10.1016/j.brainres.2012.04.014
12. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native α-synuclein. Nature. 2013;498(7453):E4-E7.
13. Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111(40):E4274-E4283. doi:10.1073/pnas.1416598111
14. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1-13. doi:10.1038/nm.4269
15. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221-5232. doi:10.1523/JNEUROSCI.4650-14.2015
16. Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24(8):1888-1896. doi:10.1523/JNEUROSCI.3809-03.2004
17. Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L . α-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279(45):46915-46920. doi:10.1074/jbc.M405146200
18. Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200-210. doi:10.1016/j.tibs.2015.02.003
19. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochem Biophys Acta. 2010;1802(1):29-44. doi:10.1016/j.bbadis.2009.08.013
20. Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92-98. doi:10.1038/nrneurol.2013.275
21. Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204-3214. doi:10.1093/brain/awx275
22. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57-71. doi:10.1016/j.neuron.2011.08.033
23. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(pt 4):1128-1138. doi:10.1093/brain/awt037
24. Hasegawa M, Nonaka T, Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol Ther. 2017;172:22-33. doi:10.1016/j.pharmthera.2016.11.010
25. Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215-1223. doi:10.1002/glia.20691
26. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189-201. doi:10.1007/s11481-013-9435-y
27. Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. doi:10.1038/nature22815
28. Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genetics. 2010;42(9):781-785. doi:10.1038/ng.642
29. Holmes C, Boche D, Wilkinson D, et al. Long term effects of Aβ42 immunisation in Alzheimer’s disease: follow up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216-223. doi:10.1016/S0140-6736(08)61075-2
30. Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241-249. doi:10.1016/S1474-4422(12)70015-7
31. Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499-507. doi:10.1016/j.bcp.2013.12.020
32. Herline K, Drummond E, Wisniewski T. Recent advancements toward therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2018;17(8):707-721. doi:10.1080/14760584.2018.1500905
33. Bergstrom AL, Kallunki P, Fog K. Development of passive immunotherapies for synucleopathies. Mov Disord. 2015;31(2):203-213. doi:10.1002/mds.26481
34. Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857-868. doi:10.1016/j.neuron.2005.05.010
35. Ghochikyan A, Petrushina I, Davtyan H, et al. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett. 2014;560:86-91. doi:10.1016/j.neulet.2013.12.028
36. Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M. α-synuclein vaccination prevents the accumulation of Parkinson’s disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol. 2013;72(7):624-645. doi:10.1097/NEN.0b013e31829768d2
37. Mandler M, Valera E, Rockenstein E, et al. Next generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861-879. doi:10.1007/s00401-014-1256-4
38. Mandler M, Valera E, Rockenstein E, et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multisystem atrophy. Mol Neurodegen. 2015;10:721. doi:10.1186/s13024-015-0008-9
39. Schneeberger A, Tierney L, Mandler M. Active immunization therapies. Mov Disord. 2015;31(2):214-224. doi:10.1002/mds.26377
40. Zella SMA, Metzdorf J, Ciftci E, et al. Emerging immunotherapies for Parkinson disease. Neurol Ther. 2019;8(1):29-44. doi:10.1007/s40120-018-0122-z
41. AFFiRiS AG. AFFiRiS announces top line results of first-in-human clinical study using AFFITOPE PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients. https://affiris.com/wp-content/uploads/2018/10/praff011prefinal0607wo-embargo-1.pdf. Published June 7, 2017. Accessed July 29, 2020.
42. AFFiRiS AG. AFFiRiS announces results of a phase I clinical study using AFFITOPEs PD01A and PD03A, confirming safety and tolerability for both compounds as well as immunogenicity for PD01A in early MSA patients. http://sympath-project.eu/wp-content/uploads/PR_AFF009_V1.pdf Published March 1, 2018. Accessed July 29, 2020.
43. Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alphasynuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi:10.1371/journal.pone.0019338
44. Bae EJ, Lee HJ, Rockenstein E, et al. Antibody aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):1345-13469. doi:10.1523/JNEUROSCI.1292-12.2012
45. Schenk DB, Koller M, Ness DK, et al. First‐in‐human assessment of PRX002, an anti–α‐synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32(2):211-218. doi:10.1002/mds.26878.
46. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α -synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206-1214. doi:10.1001/jamaneurol.2018.1487
47. Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34(1):41-44. doi:10.1002/mds.27534
48. Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10(2):E0116841. doi:10.1371/journal.pone.0116841
49. Brys M, Hung S, Fanning L, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-α-synuclein antibody BIIB054 in patients with Parkinson disease. Neurology. 2018;90(suppl 15):S26.001. doi:10.1002/mds.27738
50. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target α-synuclein pathology. Exp Neurol. 2017;298(pt B):225-235. doi:10.1016/j.expneurol.2017.10.003
Parkinson disease (PD) is a progressive neurodegenerative disorder, characterized by diverse clinical symptoms. PD can present with rest tremor, bradykinesia, rigidity, falls, postural instability, and multiple nonmotor symptoms. Marras and colleagues estimated in a comprehensive meta-analysis that there were 680,000 individuals with PD in the US in 2010; this number is expected to double by 2030 based on the US Census Bureau population projections.1 An estimated 110,000 veterans may be affected by PD; hence, understanding of PD pathology, clinical progression, and effective treatment strategies is of paramount importance to the Veterans Health Administration (VHA).2
The exact pathogenesis underlying clinical features is still being studied. Pathologic diagnosis of PD relies on loss of dopamine neurons in the substantia nigra and accumulation of the abnormal protein, α-synuclein, in the form of Lewy bodies and Lewy neurites. Lewy bodies and neurites accumulate predominantly in the substantia nigra in addition to other brain stem nuclei and cerebral cortex. Lewy bodies are intraneuronal inclusions with a hyaline core and a pale peripheral halo. Central core stains positive for α-synuclein.3,4 Lewy neurites are widespread and are believed to play a larger role in the pathogenesis of PD compared with those of Lewy bodies.5
α-Synuclein
α-synuclein is a small 140 amino-acid protein with a N-terminal region that can interact with cell membranes and a highly acidic unstructured C-terminal region.6 α-synuclein is physiologically present in the presynaptic terminals of neurons and involved in synaptic plasticity and vesicle trafficking.7 There are different hypotheses about the native structure of α-synuclein. The first suggests that it exists in tetrameric form and may be broken down to monomer, which is the pathogenic form of α-synuclein. The second hypothesis suggests that it exists primarily in monomeric form, whereas other studies have shown that both forms exist and with pathologic changes, monomer accumulates in abundance and is neurotoxic.8-11 Work by Burré and colleagues shows that native α-synuclein exists in 2 forms: a soluble, cytosolic α-synuclein, which is monomeric, and a membrane-bound multimeric form.12,13
Alteration in aggregation properties of this protein is believed to play a central role in the pathogenesis of PD.14,15 Pathologic α-synuclein exists in insoluble forms that can aggregate into oligomers and fibrillar structures.16 Lysosomal dysfunction may promote accumulation of insoluble α-synuclein. Prior work has shown that several degradation pathways in lysosomes, including the ubiquitin-proteasome system and autophagy-lysosomal pathway, are down regulated, thus contributing to the accumulation of abnormal α-synuclein.17,18 Accumulation of pathologic α-synuclein leads to mitochondrial dysfunction in PD animal models, contributing further to neurotoxicity.19,20 Aggregates of phosphorylated α-synuclein have been demonstrated in dementia with Lewy body.21
In addition, α-synuclein aggregates may be released into extracellular spaces to be taken up by adjacent cells, where they can cause further misfolding and aggregation of protein.22 Previous work in animal models suggested a prion proteinlike spread of α-synuclein.23 This finding can have long-term therapeutic implications, as preventing extracellular release of abnormal form of α-synuclein will prevent the spread of pathologic protein. This can form the basis of neuroprotection in patients with PD.24
It has been proposed that α-synuclein accumulation and extracellular release initiates an immune response that leads to activation of microglia. This has been shown in PD animal models, overexpressing α-synuclein. In 2008 Park and colleagues demonstrated that microglial activation is enhanced by monomeric α-synuclein, not by the aggregated variant.25 Other studies have reported activated microglia around dopaminergic cells in substantia nigra.26 Sulzer and colleagues showed that peptides from α-synuclein can act as antigens and trigger an autoimmune reaction via T cells.27 PD may be associated with certain HLA-haplotypes.28 In other words, α-synuclein can induce neurodegeneration via different mechanisms, including alteration in synaptic vesicle transmission, mitochondrial dysfunction, neuroinflammation, and induction of humoral immunity.
Immunization
Due to these observations, there had been huge interest in developing antibody-based therapies for PD. A similar approach had been tested in Alzheimer disease (AD). Intracellular tangles of tau protein and extracellular aggregates of amyloid are the pathologic substrates in AD. Clinical trials utilizing antibodies targeting amyloid showed reduction in abnormal protein accumulation but no significant improvement in cognition.29 In addition, adverse events (AEs), such as vasogenic edema and intracerebral hemorrhage, were reported.30 Careful analysis of the data suggested that inadequate patient selection or targeting only amyloid, may have contributed to unfavorable results.31 Since then, more recent clinical trials have focused on careful patient selection, use of second generation anti-amyloid antibodies and immunotherapies targeting tau.32
Several studies have tested immunotherapies in PD animal models with the aim of targeting α-synuclein. Immunotherapies can be instituted in 2 ways: active immunization in which the immune system is stimulated to produce antibodies against α-synuclein or passive immunization in which antibodies against α-synuclein are administered directly. Once α-synuclein antibodies have crossed the blood-brain barrier, they are hypothesized to clear the existing α-synuclein. Animal studies have demonstrated the presence of these antibodies within the neurons. The mechanism of entry is unknown. Once inside the cells, the antibodies activate the lysosomal clearance, affecting intracellular accumulation of α-synuclein. Extracellularly, they can bind to receptors on scavenger cells, mainly microglia, activating them to facilitate uptake of extracellular α-synuclein. Binding of the antibodies to α-synuclein directly prevents the uptake of toxic protein by the cells, blocking the transfer and spread of PD pathology.33
Active Immunization
Active immunization against α-synuclein was demonstrated by Masliah and colleagues almost a decade ago. They administered recombinant human α-synuclein in transgenic mice expressing α-synuclein under the control of platelet-derived growth factor β. Reduction of accumulated α-synuclein in neurons with mild microglia activation was noted. It was proposed that the antibodies produced were able to bind to abnormal α-synuclein, were recognized by the lysosomal pathways, and degraded.34 Ghochikyan and colleagues developed vaccines by using α-synuclein-derived peptides. This induced formation of antibodies against α-synuclein in Lewy-bodies and neurites.35 Over time, other animal studies have been able to expand on these results.36
AFFiRiS, an Austrian biotechnology company, has developed 2 peptide vaccines PD01A and PD03A. Both peptides when administered to PD animal models caused antibody-based immune response against aggregated α-synuclein. Humoral autoimmune response was not observed in these studies; no neuroinflammation or neurotoxicity was noted. These peptides did not affect levels of physiologic α-synuclein, targeting only the aggregated form.37 These animal models showed improved motor and cognitive function. Similar results were noted in multiple system atrophy (MSA) animal models.38,39
The first human phase 1, randomized, parallel-group, single-center study recruited 32 subjects with early PD. Twelve subjects each were included in low- or high-dose treatment group, and 8 were included in the control group. Test subjects randomly received 4 vaccinations of low- or high-dose PD01A. Both doses were well tolerated, and no drug-related serious AEs were reported. The study confirmed the tolerability and safety of subcutaneous PD01A vaccine administration. These subjects were included in a 12-month, phase 1b follow-up extension study, AFF008E. In 2018, it was reported that administration of 6 doses of PD01A, 4 primary and 2 booster immunization, was safe. The vaccine showed a clear immune response against the peptide and cross-reactivity against α-synuclein targeted epitope. Booster doses stabilized the antibody titers. Significant increase in antibody titers against PD01A was seen over time, which was translated into a humoral immune response against α-synuclein. In addition, PD01A antibodies also were reported in cerebrospinal fluid.40
AFFiRiS presented results of a phase 1 randomized, placebo-controlled trial in 2017, confirming the safety of PD03A in patients with PD. The study showed a clear dose-dependent immune response against the peptide and cross-reactivity against α-synuclein targeted epitope.41 AFFiRiS recently presented results of another phase 1 clinical study assessing the safety and tolerability of vaccines PD01A and PD03A in patients with early MSA. Both vaccines were well tolerated, and PD01A induced an immune response against the peptide and α-synuclein epitope.42 These results have provided hope for further endeavors to develop active immunization strategies for PD.
Passive Immunization
Passive immunization against α-synuclein was first reported by Masliah and colleagues in 2011. A monoclonal antibody against the C-terminus of α-synuclein, 9E4, was injected into a transgenic mouse model of PD. There was reduction in α-synuclein aggregates in the brain along with improvement in motor and cognitive impairment.43 The C-terminus of α-synuclein plays a key role in the pathogenesis of PD. Changes in the C-terminus of α-synuclein induces formation of α-synuclein oligomers and subsequent neuronal spread. Antibody binds to the C-terminus and prevents structural changes that can lead to oligomerization of α-synuclein. Since the first study by Masliah, few other immunization studies utilized different antibodies against the C-terminus of α-synuclein. It was shown in a mouse model that binding of such antibodies promoted clearance of the α-synuclein by microglia.44
Based on these animal studies, Prothena Biosciences (South San Francisco, CA) designed a phase 1, double-blind, randomized, placebo-controlled clinical trial of prasinezumab (investigational monoclonal antibody against C-terminus of α-synuclein), in subjects without PD. The results showed that it was well tolerated, and there was dose-dependent reduction in the levels of free
BIIB054 is another monoclonal antibody that targets the N-terminal of α-synuclein. In animal models, antibodies targeting the N-terminus reduced α-synuclein triggered cell death and reduced the number of activated microglia.48 BIIB054, from Biogen (Cambridge, MA), was studied in 40 healthy subjects and was well tolerated with a favorable safety profile and could cross the blood-brain barrier. Like the prasinezumab study, this also was an ascending-dose study to assess safety and tolerability. In 2018, a randomized, double-blind, placebo-controlled, single-ascending dose study in patients with PD reported that BIIB054 was well tolerated, and the presence of BIIB054-synuclein complexes in the plasma were confirmed.49 A phase 2, multicenter, randomized, double-blind, placebo-controlled study (SPARK) with an active-treatment dose-blinded period, designed to evaluate the safety, pharmacokinetics, and the pharmacodynamics of BIIB054 is currently recruiting patients with PD.
Finally, BioArctic (Stockholm, Sweden) developed antibodies that are selective for oligomeric forms of α-synuclein, which it licensed to AbbVie (North Chicago, Il).50 These antibodies do not target the N- or C-terminus of α-synuclein. Since α-synuclein oligomers play an important role in the pathogenesis of PD, targeting them with antibodies at an early stage may prove to be an effective strategy for removal of pathogenic α-synuclein. Clinical trials are forthcoming.
Conclusions
Immunotherapy against α-synuclein has provided a new therapeutic avenue in the field of neuroprotection. Results from the first human clinical trial are promising, but despite these results, more work is needed to clarify the role of α-synuclein in the pathogenesis of PD in humans. Most of the work concerning α-synuclein aggregation and propagation has been reported in animal models. Whether similar process exists in humans is a debatable question. Similarly, more knowledge is needed about how and where in the human brain antibodies act to give neuroprotective effects. Timing of administration of immunotherapies in real time will be a crucial question.
PD is clinically evident once 80% of dopaminergic neurons in substantia nigra are lost due to neurodegeneration. Should immunotherapy be administered to symptomatic patients with PD, or if it will be beneficial only for presymptomatic, high-risk patients needs to be determined. Like AD trials, not only careful selection of patients, but determination of optimal timing for treatment will be essential. As the understanding of PD pathogenesis and therapeutics evolves, it will become clear whether immunization targeting α-synuclein will modify disease progression.
Parkinson disease (PD) is a progressive neurodegenerative disorder, characterized by diverse clinical symptoms. PD can present with rest tremor, bradykinesia, rigidity, falls, postural instability, and multiple nonmotor symptoms. Marras and colleagues estimated in a comprehensive meta-analysis that there were 680,000 individuals with PD in the US in 2010; this number is expected to double by 2030 based on the US Census Bureau population projections.1 An estimated 110,000 veterans may be affected by PD; hence, understanding of PD pathology, clinical progression, and effective treatment strategies is of paramount importance to the Veterans Health Administration (VHA).2
The exact pathogenesis underlying clinical features is still being studied. Pathologic diagnosis of PD relies on loss of dopamine neurons in the substantia nigra and accumulation of the abnormal protein, α-synuclein, in the form of Lewy bodies and Lewy neurites. Lewy bodies and neurites accumulate predominantly in the substantia nigra in addition to other brain stem nuclei and cerebral cortex. Lewy bodies are intraneuronal inclusions with a hyaline core and a pale peripheral halo. Central core stains positive for α-synuclein.3,4 Lewy neurites are widespread and are believed to play a larger role in the pathogenesis of PD compared with those of Lewy bodies.5
α-Synuclein
α-synuclein is a small 140 amino-acid protein with a N-terminal region that can interact with cell membranes and a highly acidic unstructured C-terminal region.6 α-synuclein is physiologically present in the presynaptic terminals of neurons and involved in synaptic plasticity and vesicle trafficking.7 There are different hypotheses about the native structure of α-synuclein. The first suggests that it exists in tetrameric form and may be broken down to monomer, which is the pathogenic form of α-synuclein. The second hypothesis suggests that it exists primarily in monomeric form, whereas other studies have shown that both forms exist and with pathologic changes, monomer accumulates in abundance and is neurotoxic.8-11 Work by Burré and colleagues shows that native α-synuclein exists in 2 forms: a soluble, cytosolic α-synuclein, which is monomeric, and a membrane-bound multimeric form.12,13
Alteration in aggregation properties of this protein is believed to play a central role in the pathogenesis of PD.14,15 Pathologic α-synuclein exists in insoluble forms that can aggregate into oligomers and fibrillar structures.16 Lysosomal dysfunction may promote accumulation of insoluble α-synuclein. Prior work has shown that several degradation pathways in lysosomes, including the ubiquitin-proteasome system and autophagy-lysosomal pathway, are down regulated, thus contributing to the accumulation of abnormal α-synuclein.17,18 Accumulation of pathologic α-synuclein leads to mitochondrial dysfunction in PD animal models, contributing further to neurotoxicity.19,20 Aggregates of phosphorylated α-synuclein have been demonstrated in dementia with Lewy body.21
In addition, α-synuclein aggregates may be released into extracellular spaces to be taken up by adjacent cells, where they can cause further misfolding and aggregation of protein.22 Previous work in animal models suggested a prion proteinlike spread of α-synuclein.23 This finding can have long-term therapeutic implications, as preventing extracellular release of abnormal form of α-synuclein will prevent the spread of pathologic protein. This can form the basis of neuroprotection in patients with PD.24
It has been proposed that α-synuclein accumulation and extracellular release initiates an immune response that leads to activation of microglia. This has been shown in PD animal models, overexpressing α-synuclein. In 2008 Park and colleagues demonstrated that microglial activation is enhanced by monomeric α-synuclein, not by the aggregated variant.25 Other studies have reported activated microglia around dopaminergic cells in substantia nigra.26 Sulzer and colleagues showed that peptides from α-synuclein can act as antigens and trigger an autoimmune reaction via T cells.27 PD may be associated with certain HLA-haplotypes.28 In other words, α-synuclein can induce neurodegeneration via different mechanisms, including alteration in synaptic vesicle transmission, mitochondrial dysfunction, neuroinflammation, and induction of humoral immunity.
Immunization
Due to these observations, there had been huge interest in developing antibody-based therapies for PD. A similar approach had been tested in Alzheimer disease (AD). Intracellular tangles of tau protein and extracellular aggregates of amyloid are the pathologic substrates in AD. Clinical trials utilizing antibodies targeting amyloid showed reduction in abnormal protein accumulation but no significant improvement in cognition.29 In addition, adverse events (AEs), such as vasogenic edema and intracerebral hemorrhage, were reported.30 Careful analysis of the data suggested that inadequate patient selection or targeting only amyloid, may have contributed to unfavorable results.31 Since then, more recent clinical trials have focused on careful patient selection, use of second generation anti-amyloid antibodies and immunotherapies targeting tau.32
Several studies have tested immunotherapies in PD animal models with the aim of targeting α-synuclein. Immunotherapies can be instituted in 2 ways: active immunization in which the immune system is stimulated to produce antibodies against α-synuclein or passive immunization in which antibodies against α-synuclein are administered directly. Once α-synuclein antibodies have crossed the blood-brain barrier, they are hypothesized to clear the existing α-synuclein. Animal studies have demonstrated the presence of these antibodies within the neurons. The mechanism of entry is unknown. Once inside the cells, the antibodies activate the lysosomal clearance, affecting intracellular accumulation of α-synuclein. Extracellularly, they can bind to receptors on scavenger cells, mainly microglia, activating them to facilitate uptake of extracellular α-synuclein. Binding of the antibodies to α-synuclein directly prevents the uptake of toxic protein by the cells, blocking the transfer and spread of PD pathology.33
Active Immunization
Active immunization against α-synuclein was demonstrated by Masliah and colleagues almost a decade ago. They administered recombinant human α-synuclein in transgenic mice expressing α-synuclein under the control of platelet-derived growth factor β. Reduction of accumulated α-synuclein in neurons with mild microglia activation was noted. It was proposed that the antibodies produced were able to bind to abnormal α-synuclein, were recognized by the lysosomal pathways, and degraded.34 Ghochikyan and colleagues developed vaccines by using α-synuclein-derived peptides. This induced formation of antibodies against α-synuclein in Lewy-bodies and neurites.35 Over time, other animal studies have been able to expand on these results.36
AFFiRiS, an Austrian biotechnology company, has developed 2 peptide vaccines PD01A and PD03A. Both peptides when administered to PD animal models caused antibody-based immune response against aggregated α-synuclein. Humoral autoimmune response was not observed in these studies; no neuroinflammation or neurotoxicity was noted. These peptides did not affect levels of physiologic α-synuclein, targeting only the aggregated form.37 These animal models showed improved motor and cognitive function. Similar results were noted in multiple system atrophy (MSA) animal models.38,39
The first human phase 1, randomized, parallel-group, single-center study recruited 32 subjects with early PD. Twelve subjects each were included in low- or high-dose treatment group, and 8 were included in the control group. Test subjects randomly received 4 vaccinations of low- or high-dose PD01A. Both doses were well tolerated, and no drug-related serious AEs were reported. The study confirmed the tolerability and safety of subcutaneous PD01A vaccine administration. These subjects were included in a 12-month, phase 1b follow-up extension study, AFF008E. In 2018, it was reported that administration of 6 doses of PD01A, 4 primary and 2 booster immunization, was safe. The vaccine showed a clear immune response against the peptide and cross-reactivity against α-synuclein targeted epitope. Booster doses stabilized the antibody titers. Significant increase in antibody titers against PD01A was seen over time, which was translated into a humoral immune response against α-synuclein. In addition, PD01A antibodies also were reported in cerebrospinal fluid.40
AFFiRiS presented results of a phase 1 randomized, placebo-controlled trial in 2017, confirming the safety of PD03A in patients with PD. The study showed a clear dose-dependent immune response against the peptide and cross-reactivity against α-synuclein targeted epitope.41 AFFiRiS recently presented results of another phase 1 clinical study assessing the safety and tolerability of vaccines PD01A and PD03A in patients with early MSA. Both vaccines were well tolerated, and PD01A induced an immune response against the peptide and α-synuclein epitope.42 These results have provided hope for further endeavors to develop active immunization strategies for PD.
Passive Immunization
Passive immunization against α-synuclein was first reported by Masliah and colleagues in 2011. A monoclonal antibody against the C-terminus of α-synuclein, 9E4, was injected into a transgenic mouse model of PD. There was reduction in α-synuclein aggregates in the brain along with improvement in motor and cognitive impairment.43 The C-terminus of α-synuclein plays a key role in the pathogenesis of PD. Changes in the C-terminus of α-synuclein induces formation of α-synuclein oligomers and subsequent neuronal spread. Antibody binds to the C-terminus and prevents structural changes that can lead to oligomerization of α-synuclein. Since the first study by Masliah, few other immunization studies utilized different antibodies against the C-terminus of α-synuclein. It was shown in a mouse model that binding of such antibodies promoted clearance of the α-synuclein by microglia.44
Based on these animal studies, Prothena Biosciences (South San Francisco, CA) designed a phase 1, double-blind, randomized, placebo-controlled clinical trial of prasinezumab (investigational monoclonal antibody against C-terminus of α-synuclein), in subjects without PD. The results showed that it was well tolerated, and there was dose-dependent reduction in the levels of free
BIIB054 is another monoclonal antibody that targets the N-terminal of α-synuclein. In animal models, antibodies targeting the N-terminus reduced α-synuclein triggered cell death and reduced the number of activated microglia.48 BIIB054, from Biogen (Cambridge, MA), was studied in 40 healthy subjects and was well tolerated with a favorable safety profile and could cross the blood-brain barrier. Like the prasinezumab study, this also was an ascending-dose study to assess safety and tolerability. In 2018, a randomized, double-blind, placebo-controlled, single-ascending dose study in patients with PD reported that BIIB054 was well tolerated, and the presence of BIIB054-synuclein complexes in the plasma were confirmed.49 A phase 2, multicenter, randomized, double-blind, placebo-controlled study (SPARK) with an active-treatment dose-blinded period, designed to evaluate the safety, pharmacokinetics, and the pharmacodynamics of BIIB054 is currently recruiting patients with PD.
Finally, BioArctic (Stockholm, Sweden) developed antibodies that are selective for oligomeric forms of α-synuclein, which it licensed to AbbVie (North Chicago, Il).50 These antibodies do not target the N- or C-terminus of α-synuclein. Since α-synuclein oligomers play an important role in the pathogenesis of PD, targeting them with antibodies at an early stage may prove to be an effective strategy for removal of pathogenic α-synuclein. Clinical trials are forthcoming.
Conclusions
Immunotherapy against α-synuclein has provided a new therapeutic avenue in the field of neuroprotection. Results from the first human clinical trial are promising, but despite these results, more work is needed to clarify the role of α-synuclein in the pathogenesis of PD in humans. Most of the work concerning α-synuclein aggregation and propagation has been reported in animal models. Whether similar process exists in humans is a debatable question. Similarly, more knowledge is needed about how and where in the human brain antibodies act to give neuroprotective effects. Timing of administration of immunotherapies in real time will be a crucial question.
PD is clinically evident once 80% of dopaminergic neurons in substantia nigra are lost due to neurodegeneration. Should immunotherapy be administered to symptomatic patients with PD, or if it will be beneficial only for presymptomatic, high-risk patients needs to be determined. Like AD trials, not only careful selection of patients, but determination of optimal timing for treatment will be essential. As the understanding of PD pathogenesis and therapeutics evolves, it will become clear whether immunization targeting α-synuclein will modify disease progression.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. doi:10.1038/s41531-018-0058-0
2. Mantri S, Duda JE, Morley JF. Early and accurate identification of Parkinson disease among US veterans. Fed Pract. 2019;36(suppl 4):S18-S23. doi:10.12788/fp.37-0034
3. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(suppl 1):S71-S85. doi:10.3233/JPD-179001
4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. doi:10.1038/42166
5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. doi:10.1016/s0197-4580(02)00065-9
6. Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79(6):1044-1066. doi:10.1016/j.neuron.2013.09.004
7. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663-1667. doi:10.1126/science.1195227
8. Binolfi A, Fernández CO, Sica MP, Delfino JM, Santos J. Recognition between a short unstructured peptide and a partially folded fragment leads to the thioredoxin fold sharing native-like dynamics. Proteins. 2012;80(5):1448-1464. doi:10.1002/prot.24043
9. Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345-15364. doi:10.1074/jbc.M111.318949.
10. Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797-17802. doi:10.1073/pnas.1113260108
11. Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183-202. doi:10.1016/j.brainres.2012.04.014
12. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native α-synuclein. Nature. 2013;498(7453):E4-E7.
13. Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111(40):E4274-E4283. doi:10.1073/pnas.1416598111
14. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1-13. doi:10.1038/nm.4269
15. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221-5232. doi:10.1523/JNEUROSCI.4650-14.2015
16. Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24(8):1888-1896. doi:10.1523/JNEUROSCI.3809-03.2004
17. Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L . α-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279(45):46915-46920. doi:10.1074/jbc.M405146200
18. Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200-210. doi:10.1016/j.tibs.2015.02.003
19. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochem Biophys Acta. 2010;1802(1):29-44. doi:10.1016/j.bbadis.2009.08.013
20. Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92-98. doi:10.1038/nrneurol.2013.275
21. Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204-3214. doi:10.1093/brain/awx275
22. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57-71. doi:10.1016/j.neuron.2011.08.033
23. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(pt 4):1128-1138. doi:10.1093/brain/awt037
24. Hasegawa M, Nonaka T, Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol Ther. 2017;172:22-33. doi:10.1016/j.pharmthera.2016.11.010
25. Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215-1223. doi:10.1002/glia.20691
26. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189-201. doi:10.1007/s11481-013-9435-y
27. Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. doi:10.1038/nature22815
28. Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genetics. 2010;42(9):781-785. doi:10.1038/ng.642
29. Holmes C, Boche D, Wilkinson D, et al. Long term effects of Aβ42 immunisation in Alzheimer’s disease: follow up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216-223. doi:10.1016/S0140-6736(08)61075-2
30. Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241-249. doi:10.1016/S1474-4422(12)70015-7
31. Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499-507. doi:10.1016/j.bcp.2013.12.020
32. Herline K, Drummond E, Wisniewski T. Recent advancements toward therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2018;17(8):707-721. doi:10.1080/14760584.2018.1500905
33. Bergstrom AL, Kallunki P, Fog K. Development of passive immunotherapies for synucleopathies. Mov Disord. 2015;31(2):203-213. doi:10.1002/mds.26481
34. Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857-868. doi:10.1016/j.neuron.2005.05.010
35. Ghochikyan A, Petrushina I, Davtyan H, et al. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett. 2014;560:86-91. doi:10.1016/j.neulet.2013.12.028
36. Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M. α-synuclein vaccination prevents the accumulation of Parkinson’s disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol. 2013;72(7):624-645. doi:10.1097/NEN.0b013e31829768d2
37. Mandler M, Valera E, Rockenstein E, et al. Next generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861-879. doi:10.1007/s00401-014-1256-4
38. Mandler M, Valera E, Rockenstein E, et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multisystem atrophy. Mol Neurodegen. 2015;10:721. doi:10.1186/s13024-015-0008-9
39. Schneeberger A, Tierney L, Mandler M. Active immunization therapies. Mov Disord. 2015;31(2):214-224. doi:10.1002/mds.26377
40. Zella SMA, Metzdorf J, Ciftci E, et al. Emerging immunotherapies for Parkinson disease. Neurol Ther. 2019;8(1):29-44. doi:10.1007/s40120-018-0122-z
41. AFFiRiS AG. AFFiRiS announces top line results of first-in-human clinical study using AFFITOPE PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients. https://affiris.com/wp-content/uploads/2018/10/praff011prefinal0607wo-embargo-1.pdf. Published June 7, 2017. Accessed July 29, 2020.
42. AFFiRiS AG. AFFiRiS announces results of a phase I clinical study using AFFITOPEs PD01A and PD03A, confirming safety and tolerability for both compounds as well as immunogenicity for PD01A in early MSA patients. http://sympath-project.eu/wp-content/uploads/PR_AFF009_V1.pdf Published March 1, 2018. Accessed July 29, 2020.
43. Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alphasynuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi:10.1371/journal.pone.0019338
44. Bae EJ, Lee HJ, Rockenstein E, et al. Antibody aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):1345-13469. doi:10.1523/JNEUROSCI.1292-12.2012
45. Schenk DB, Koller M, Ness DK, et al. First‐in‐human assessment of PRX002, an anti–α‐synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32(2):211-218. doi:10.1002/mds.26878.
46. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α -synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206-1214. doi:10.1001/jamaneurol.2018.1487
47. Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34(1):41-44. doi:10.1002/mds.27534
48. Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10(2):E0116841. doi:10.1371/journal.pone.0116841
49. Brys M, Hung S, Fanning L, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-α-synuclein antibody BIIB054 in patients with Parkinson disease. Neurology. 2018;90(suppl 15):S26.001. doi:10.1002/mds.27738
50. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target α-synuclein pathology. Exp Neurol. 2017;298(pt B):225-235. doi:10.1016/j.expneurol.2017.10.003
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. doi:10.1038/s41531-018-0058-0
2. Mantri S, Duda JE, Morley JF. Early and accurate identification of Parkinson disease among US veterans. Fed Pract. 2019;36(suppl 4):S18-S23. doi:10.12788/fp.37-0034
3. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(suppl 1):S71-S85. doi:10.3233/JPD-179001
4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. doi:10.1038/42166
5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. doi:10.1016/s0197-4580(02)00065-9
6. Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79(6):1044-1066. doi:10.1016/j.neuron.2013.09.004
7. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663-1667. doi:10.1126/science.1195227
8. Binolfi A, Fernández CO, Sica MP, Delfino JM, Santos J. Recognition between a short unstructured peptide and a partially folded fragment leads to the thioredoxin fold sharing native-like dynamics. Proteins. 2012;80(5):1448-1464. doi:10.1002/prot.24043
9. Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345-15364. doi:10.1074/jbc.M111.318949.
10. Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797-17802. doi:10.1073/pnas.1113260108
11. Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183-202. doi:10.1016/j.brainres.2012.04.014
12. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native α-synuclein. Nature. 2013;498(7453):E4-E7.
13. Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111(40):E4274-E4283. doi:10.1073/pnas.1416598111
14. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1-13. doi:10.1038/nm.4269
15. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221-5232. doi:10.1523/JNEUROSCI.4650-14.2015
16. Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24(8):1888-1896. doi:10.1523/JNEUROSCI.3809-03.2004
17. Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L . α-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279(45):46915-46920. doi:10.1074/jbc.M405146200
18. Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200-210. doi:10.1016/j.tibs.2015.02.003
19. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochem Biophys Acta. 2010;1802(1):29-44. doi:10.1016/j.bbadis.2009.08.013
20. Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92-98. doi:10.1038/nrneurol.2013.275
21. Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204-3214. doi:10.1093/brain/awx275
22. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57-71. doi:10.1016/j.neuron.2011.08.033
23. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(pt 4):1128-1138. doi:10.1093/brain/awt037
24. Hasegawa M, Nonaka T, Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol Ther. 2017;172:22-33. doi:10.1016/j.pharmthera.2016.11.010
25. Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215-1223. doi:10.1002/glia.20691
26. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189-201. doi:10.1007/s11481-013-9435-y
27. Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. doi:10.1038/nature22815
28. Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genetics. 2010;42(9):781-785. doi:10.1038/ng.642
29. Holmes C, Boche D, Wilkinson D, et al. Long term effects of Aβ42 immunisation in Alzheimer’s disease: follow up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216-223. doi:10.1016/S0140-6736(08)61075-2
30. Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241-249. doi:10.1016/S1474-4422(12)70015-7
31. Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499-507. doi:10.1016/j.bcp.2013.12.020
32. Herline K, Drummond E, Wisniewski T. Recent advancements toward therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2018;17(8):707-721. doi:10.1080/14760584.2018.1500905
33. Bergstrom AL, Kallunki P, Fog K. Development of passive immunotherapies for synucleopathies. Mov Disord. 2015;31(2):203-213. doi:10.1002/mds.26481
34. Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857-868. doi:10.1016/j.neuron.2005.05.010
35. Ghochikyan A, Petrushina I, Davtyan H, et al. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett. 2014;560:86-91. doi:10.1016/j.neulet.2013.12.028
36. Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M. α-synuclein vaccination prevents the accumulation of Parkinson’s disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol. 2013;72(7):624-645. doi:10.1097/NEN.0b013e31829768d2
37. Mandler M, Valera E, Rockenstein E, et al. Next generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861-879. doi:10.1007/s00401-014-1256-4
38. Mandler M, Valera E, Rockenstein E, et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multisystem atrophy. Mol Neurodegen. 2015;10:721. doi:10.1186/s13024-015-0008-9
39. Schneeberger A, Tierney L, Mandler M. Active immunization therapies. Mov Disord. 2015;31(2):214-224. doi:10.1002/mds.26377
40. Zella SMA, Metzdorf J, Ciftci E, et al. Emerging immunotherapies for Parkinson disease. Neurol Ther. 2019;8(1):29-44. doi:10.1007/s40120-018-0122-z
41. AFFiRiS AG. AFFiRiS announces top line results of first-in-human clinical study using AFFITOPE PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients. https://affiris.com/wp-content/uploads/2018/10/praff011prefinal0607wo-embargo-1.pdf. Published June 7, 2017. Accessed July 29, 2020.
42. AFFiRiS AG. AFFiRiS announces results of a phase I clinical study using AFFITOPEs PD01A and PD03A, confirming safety and tolerability for both compounds as well as immunogenicity for PD01A in early MSA patients. http://sympath-project.eu/wp-content/uploads/PR_AFF009_V1.pdf Published March 1, 2018. Accessed July 29, 2020.
43. Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alphasynuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi:10.1371/journal.pone.0019338
44. Bae EJ, Lee HJ, Rockenstein E, et al. Antibody aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):1345-13469. doi:10.1523/JNEUROSCI.1292-12.2012
45. Schenk DB, Koller M, Ness DK, et al. First‐in‐human assessment of PRX002, an anti–α‐synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32(2):211-218. doi:10.1002/mds.26878.
46. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α -synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206-1214. doi:10.1001/jamaneurol.2018.1487
47. Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34(1):41-44. doi:10.1002/mds.27534
48. Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10(2):E0116841. doi:10.1371/journal.pone.0116841
49. Brys M, Hung S, Fanning L, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-α-synuclein antibody BIIB054 in patients with Parkinson disease. Neurology. 2018;90(suppl 15):S26.001. doi:10.1002/mds.27738
50. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target α-synuclein pathology. Exp Neurol. 2017;298(pt B):225-235. doi:10.1016/j.expneurol.2017.10.003
Screening criteria for diabetes in youth won’t capture all at high risk
and therefore “may miss high-risk youth who should be targeted for diabetes prevention,” according to the investigators of a cross-sectional analysis of youth in the 1999-2016 National Health and Nutrition Examination Survey (NHANES) database.
Regardless of whether or not youth meet screening eligibility, they say, hemoglobin A1c appears to be a “specific and useful test” for detecting high-risk youth.
Those with prediabetic levels of A1c or fasting plasma glucose (FPG) – A1c especially – had a high burden of other cardiometabolic risk factors that could benefit from lifestyle interventions to prevent diabetes and cardiovascular risk in adulthood, wrote Amelia S. Wallace and coinvestigators at the Johns Hopkins Bloomberg School of Public Health, Baltimore. The report is in Pediatrics.Their epidemiologic study had two aims: To assess the performance of the American Diabetes Association guidelines for screening in youth, and to evaluate how well various clinical definitions of diabetes and prediabetes identify U.S. youth at high cardiometabolic risk.
The 2018 ADA guidelines recommend screening for type 2 diabetes and prediabetes in all asymptomatic youth ages 10 years and older who are overweight or obese and who have at least one risk factor for diabetes: nonwhite race, family history of type 2 diabetes, maternal gestational diabetes, or signs of insulin resistance or conditions associated with insulin resistance (Diabetes Care. 2018:41[suppl 1:S13-S37]).
Approximately one-quarter of U.S. youth were found to be eligible for screening under the current ADA criteria, but there were few cases of confirmed diabetes (A1c greater than or equal to 6.5% and fasting plasma glucose greater than or equal to 126 mg/dL) that had gone undiagnosed (less than 0.5%), said Ms. Wallace and her associates.
Considering all hyperglycemia (undiagnosed diabetes or prediabetes) in the NHANES youth population, the sensitivity and specificity of the ADA criteria for detecting A1c-defined hyperglycemia (greater than or equal to 5.7%) were 56% and 76%, respectively, and the sensitivity and specificity for detecting FBG-defined hyperglycemia (greater than or equal to 100 mg/dL) were 36% and 77%.
The prevalence of any hyperglycemia was higher in youth who met ADA screening criteria than in those who didn’t, but there were also “a substantial number of youth with hyperglycemia in the non–screening eligible population,” they wrote. “In fact, the absolute number of youth with elevated FPG was larger in the non–screening eligible population, and the majority (88.5%) of these youth were of normal weight.”
Across all youth (irrespective of screening eligibility), both FPG and A1c-defined hyperglycemia effectively identified children and adolescents who had a high burden of cardiometabolic risk (obesity, metabolic syndrome, and hypercholesterolemia). Using a confirmatory definition of elevations in both FPG and A1c “provided the highest discrimination for cardiometabolic risk,” Ms. Wallace and her associates said.
But in comparing the single tests, risk factor associations with hyperglycemia were consistently stronger with A1c-defined hyperglycemia (odds ratios of 2.6-4.1) than FBG-defined hyperglycemia (ORs of 1.5-3.0). A1c-defined hyperglycemia “identifies a smaller, but higher-risk, population than FPG-defined hyperglycemia,” they said.
In an accompanying commentary, Tamara S. Hannon, MD, MS, of the division of pediatric endocrinology and diabetology at Indiana University in Indianapolis, said that more effective algorithms to determine who should have laboratory testing “could be useful.” Still, “for youth with obesity and multiple risk factors for developing type 2 diabetes, the principal challenge is how to effectively prevent or delay this disease for them and future generations.”
Pediatricians, she said, should screen for prediabetes and type 2 diabetes “according to professional recommendations with simple clinical tests, such as A1c. Screening and education about prediabetes alone can lead to better rates of follow-up for obesity,” she noted (Pediatrics. August 2020. doi: 10.1542/peds.2020-010272).
Sheela N. Magge, MD, MSCE, who directs the division of pediatric endocrinology and diabetes at John Hopkins University, Baltimore, and was asked to comment on the study, similarly said that the findings should not discourage use of the ADA guidelines.
While the guidelines may not have optimal sensitivity and specificity, “neither HbA1c nor fasting glucose are perfect screening tools for prediabetes and likely give us different mechanistic information,” she said. (The ADA guidelines also allow the use of a 2-hour oral glucose tolerance test, but this is not often used by pediatricians, she noted.)
The measurements are “only tools used to identify children who have prediabetes and are therefore at increased risk for type 2 diabetes,” said Dr. Magge, the Lawson Wilkins Endowed Chair of Pediatric Endocrinology at the university. “These children then need to be managed and followed to try to prevent worsening glycemia.”
Both she and Dr. Hannon stressed that youth with type 2 diabetes have more rapidly progressive disease compared with adults.
Microvascular complications are seen even at diagnosis, Dr. Magge said, and “youth may face serious complications such as cardiovascular disease decades earlier than previous generations.”
Dr. Hannon also noted in her commentary that oral diabetes medications often fail in youth with type 2 diabetes, leading to insulin therapy early on.
The prevalence of youth-onset type 2 diabetes has increased because of rising rates of pediatric overweight and obesity, Dr. Magge emphasized. In her experience, the diabetes risk factors that guide the ADA’s screening approach “are so common in overweight and obese youth that they all have at least one.”
The NHANES data did not contain information on all the variables that make up the current diabetes screening criteria in youth; there was no explicit information on history of maternal gestational diabetes and family history of type 2 diabetes, for instance, or the presence of acanthosis nigricans or polycystic ovarian syndrome – conditions associated with insulin resistance. The investigators said it’s likely, therefore, that the study underestimated the number of U.S. youth who would be eligible for diabetes screening.
And, as Dr. Magge said, “it is difficult to determine which risk factors [in the ADA guidelines] were less predictive.”
The NHANES analysis covered 14,119 youth in the 1999-2016 NHANES surveys, which consisted of interviews and standardized physical exams, including laboratory tests, in home and at a mobile examination center. Analyses involving any fasting lab tests were limited to a random subsample of participants aged 12-19 years without diagnosed diabetes who were asked to fast the night before; 6,225 youth properly followed instructions and were included in this subsample.
The surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study authors and the editorial author indicated that they have no relevant financial disclosures or conflicts of interest. Dr. Magge also said she has no relevant disclosures.
SOURCE: Wallace AS et al. Pediatrics. August 2020. doi: 10.1542/peds.2020-0265.
and therefore “may miss high-risk youth who should be targeted for diabetes prevention,” according to the investigators of a cross-sectional analysis of youth in the 1999-2016 National Health and Nutrition Examination Survey (NHANES) database.
Regardless of whether or not youth meet screening eligibility, they say, hemoglobin A1c appears to be a “specific and useful test” for detecting high-risk youth.
Those with prediabetic levels of A1c or fasting plasma glucose (FPG) – A1c especially – had a high burden of other cardiometabolic risk factors that could benefit from lifestyle interventions to prevent diabetes and cardiovascular risk in adulthood, wrote Amelia S. Wallace and coinvestigators at the Johns Hopkins Bloomberg School of Public Health, Baltimore. The report is in Pediatrics.Their epidemiologic study had two aims: To assess the performance of the American Diabetes Association guidelines for screening in youth, and to evaluate how well various clinical definitions of diabetes and prediabetes identify U.S. youth at high cardiometabolic risk.
The 2018 ADA guidelines recommend screening for type 2 diabetes and prediabetes in all asymptomatic youth ages 10 years and older who are overweight or obese and who have at least one risk factor for diabetes: nonwhite race, family history of type 2 diabetes, maternal gestational diabetes, or signs of insulin resistance or conditions associated with insulin resistance (Diabetes Care. 2018:41[suppl 1:S13-S37]).
Approximately one-quarter of U.S. youth were found to be eligible for screening under the current ADA criteria, but there were few cases of confirmed diabetes (A1c greater than or equal to 6.5% and fasting plasma glucose greater than or equal to 126 mg/dL) that had gone undiagnosed (less than 0.5%), said Ms. Wallace and her associates.
Considering all hyperglycemia (undiagnosed diabetes or prediabetes) in the NHANES youth population, the sensitivity and specificity of the ADA criteria for detecting A1c-defined hyperglycemia (greater than or equal to 5.7%) were 56% and 76%, respectively, and the sensitivity and specificity for detecting FBG-defined hyperglycemia (greater than or equal to 100 mg/dL) were 36% and 77%.
The prevalence of any hyperglycemia was higher in youth who met ADA screening criteria than in those who didn’t, but there were also “a substantial number of youth with hyperglycemia in the non–screening eligible population,” they wrote. “In fact, the absolute number of youth with elevated FPG was larger in the non–screening eligible population, and the majority (88.5%) of these youth were of normal weight.”
Across all youth (irrespective of screening eligibility), both FPG and A1c-defined hyperglycemia effectively identified children and adolescents who had a high burden of cardiometabolic risk (obesity, metabolic syndrome, and hypercholesterolemia). Using a confirmatory definition of elevations in both FPG and A1c “provided the highest discrimination for cardiometabolic risk,” Ms. Wallace and her associates said.
But in comparing the single tests, risk factor associations with hyperglycemia were consistently stronger with A1c-defined hyperglycemia (odds ratios of 2.6-4.1) than FBG-defined hyperglycemia (ORs of 1.5-3.0). A1c-defined hyperglycemia “identifies a smaller, but higher-risk, population than FPG-defined hyperglycemia,” they said.
In an accompanying commentary, Tamara S. Hannon, MD, MS, of the division of pediatric endocrinology and diabetology at Indiana University in Indianapolis, said that more effective algorithms to determine who should have laboratory testing “could be useful.” Still, “for youth with obesity and multiple risk factors for developing type 2 diabetes, the principal challenge is how to effectively prevent or delay this disease for them and future generations.”
Pediatricians, she said, should screen for prediabetes and type 2 diabetes “according to professional recommendations with simple clinical tests, such as A1c. Screening and education about prediabetes alone can lead to better rates of follow-up for obesity,” she noted (Pediatrics. August 2020. doi: 10.1542/peds.2020-010272).
Sheela N. Magge, MD, MSCE, who directs the division of pediatric endocrinology and diabetes at John Hopkins University, Baltimore, and was asked to comment on the study, similarly said that the findings should not discourage use of the ADA guidelines.
While the guidelines may not have optimal sensitivity and specificity, “neither HbA1c nor fasting glucose are perfect screening tools for prediabetes and likely give us different mechanistic information,” she said. (The ADA guidelines also allow the use of a 2-hour oral glucose tolerance test, but this is not often used by pediatricians, she noted.)
The measurements are “only tools used to identify children who have prediabetes and are therefore at increased risk for type 2 diabetes,” said Dr. Magge, the Lawson Wilkins Endowed Chair of Pediatric Endocrinology at the university. “These children then need to be managed and followed to try to prevent worsening glycemia.”
Both she and Dr. Hannon stressed that youth with type 2 diabetes have more rapidly progressive disease compared with adults.
Microvascular complications are seen even at diagnosis, Dr. Magge said, and “youth may face serious complications such as cardiovascular disease decades earlier than previous generations.”
Dr. Hannon also noted in her commentary that oral diabetes medications often fail in youth with type 2 diabetes, leading to insulin therapy early on.
The prevalence of youth-onset type 2 diabetes has increased because of rising rates of pediatric overweight and obesity, Dr. Magge emphasized. In her experience, the diabetes risk factors that guide the ADA’s screening approach “are so common in overweight and obese youth that they all have at least one.”
The NHANES data did not contain information on all the variables that make up the current diabetes screening criteria in youth; there was no explicit information on history of maternal gestational diabetes and family history of type 2 diabetes, for instance, or the presence of acanthosis nigricans or polycystic ovarian syndrome – conditions associated with insulin resistance. The investigators said it’s likely, therefore, that the study underestimated the number of U.S. youth who would be eligible for diabetes screening.
And, as Dr. Magge said, “it is difficult to determine which risk factors [in the ADA guidelines] were less predictive.”
The NHANES analysis covered 14,119 youth in the 1999-2016 NHANES surveys, which consisted of interviews and standardized physical exams, including laboratory tests, in home and at a mobile examination center. Analyses involving any fasting lab tests were limited to a random subsample of participants aged 12-19 years without diagnosed diabetes who were asked to fast the night before; 6,225 youth properly followed instructions and were included in this subsample.
The surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study authors and the editorial author indicated that they have no relevant financial disclosures or conflicts of interest. Dr. Magge also said she has no relevant disclosures.
SOURCE: Wallace AS et al. Pediatrics. August 2020. doi: 10.1542/peds.2020-0265.
and therefore “may miss high-risk youth who should be targeted for diabetes prevention,” according to the investigators of a cross-sectional analysis of youth in the 1999-2016 National Health and Nutrition Examination Survey (NHANES) database.
Regardless of whether or not youth meet screening eligibility, they say, hemoglobin A1c appears to be a “specific and useful test” for detecting high-risk youth.
Those with prediabetic levels of A1c or fasting plasma glucose (FPG) – A1c especially – had a high burden of other cardiometabolic risk factors that could benefit from lifestyle interventions to prevent diabetes and cardiovascular risk in adulthood, wrote Amelia S. Wallace and coinvestigators at the Johns Hopkins Bloomberg School of Public Health, Baltimore. The report is in Pediatrics.Their epidemiologic study had two aims: To assess the performance of the American Diabetes Association guidelines for screening in youth, and to evaluate how well various clinical definitions of diabetes and prediabetes identify U.S. youth at high cardiometabolic risk.
The 2018 ADA guidelines recommend screening for type 2 diabetes and prediabetes in all asymptomatic youth ages 10 years and older who are overweight or obese and who have at least one risk factor for diabetes: nonwhite race, family history of type 2 diabetes, maternal gestational diabetes, or signs of insulin resistance or conditions associated with insulin resistance (Diabetes Care. 2018:41[suppl 1:S13-S37]).
Approximately one-quarter of U.S. youth were found to be eligible for screening under the current ADA criteria, but there were few cases of confirmed diabetes (A1c greater than or equal to 6.5% and fasting plasma glucose greater than or equal to 126 mg/dL) that had gone undiagnosed (less than 0.5%), said Ms. Wallace and her associates.
Considering all hyperglycemia (undiagnosed diabetes or prediabetes) in the NHANES youth population, the sensitivity and specificity of the ADA criteria for detecting A1c-defined hyperglycemia (greater than or equal to 5.7%) were 56% and 76%, respectively, and the sensitivity and specificity for detecting FBG-defined hyperglycemia (greater than or equal to 100 mg/dL) were 36% and 77%.
The prevalence of any hyperglycemia was higher in youth who met ADA screening criteria than in those who didn’t, but there were also “a substantial number of youth with hyperglycemia in the non–screening eligible population,” they wrote. “In fact, the absolute number of youth with elevated FPG was larger in the non–screening eligible population, and the majority (88.5%) of these youth were of normal weight.”
Across all youth (irrespective of screening eligibility), both FPG and A1c-defined hyperglycemia effectively identified children and adolescents who had a high burden of cardiometabolic risk (obesity, metabolic syndrome, and hypercholesterolemia). Using a confirmatory definition of elevations in both FPG and A1c “provided the highest discrimination for cardiometabolic risk,” Ms. Wallace and her associates said.
But in comparing the single tests, risk factor associations with hyperglycemia were consistently stronger with A1c-defined hyperglycemia (odds ratios of 2.6-4.1) than FBG-defined hyperglycemia (ORs of 1.5-3.0). A1c-defined hyperglycemia “identifies a smaller, but higher-risk, population than FPG-defined hyperglycemia,” they said.
In an accompanying commentary, Tamara S. Hannon, MD, MS, of the division of pediatric endocrinology and diabetology at Indiana University in Indianapolis, said that more effective algorithms to determine who should have laboratory testing “could be useful.” Still, “for youth with obesity and multiple risk factors for developing type 2 diabetes, the principal challenge is how to effectively prevent or delay this disease for them and future generations.”
Pediatricians, she said, should screen for prediabetes and type 2 diabetes “according to professional recommendations with simple clinical tests, such as A1c. Screening and education about prediabetes alone can lead to better rates of follow-up for obesity,” she noted (Pediatrics. August 2020. doi: 10.1542/peds.2020-010272).
Sheela N. Magge, MD, MSCE, who directs the division of pediatric endocrinology and diabetes at John Hopkins University, Baltimore, and was asked to comment on the study, similarly said that the findings should not discourage use of the ADA guidelines.
While the guidelines may not have optimal sensitivity and specificity, “neither HbA1c nor fasting glucose are perfect screening tools for prediabetes and likely give us different mechanistic information,” she said. (The ADA guidelines also allow the use of a 2-hour oral glucose tolerance test, but this is not often used by pediatricians, she noted.)
The measurements are “only tools used to identify children who have prediabetes and are therefore at increased risk for type 2 diabetes,” said Dr. Magge, the Lawson Wilkins Endowed Chair of Pediatric Endocrinology at the university. “These children then need to be managed and followed to try to prevent worsening glycemia.”
Both she and Dr. Hannon stressed that youth with type 2 diabetes have more rapidly progressive disease compared with adults.
Microvascular complications are seen even at diagnosis, Dr. Magge said, and “youth may face serious complications such as cardiovascular disease decades earlier than previous generations.”
Dr. Hannon also noted in her commentary that oral diabetes medications often fail in youth with type 2 diabetes, leading to insulin therapy early on.
The prevalence of youth-onset type 2 diabetes has increased because of rising rates of pediatric overweight and obesity, Dr. Magge emphasized. In her experience, the diabetes risk factors that guide the ADA’s screening approach “are so common in overweight and obese youth that they all have at least one.”
The NHANES data did not contain information on all the variables that make up the current diabetes screening criteria in youth; there was no explicit information on history of maternal gestational diabetes and family history of type 2 diabetes, for instance, or the presence of acanthosis nigricans or polycystic ovarian syndrome – conditions associated with insulin resistance. The investigators said it’s likely, therefore, that the study underestimated the number of U.S. youth who would be eligible for diabetes screening.
And, as Dr. Magge said, “it is difficult to determine which risk factors [in the ADA guidelines] were less predictive.”
The NHANES analysis covered 14,119 youth in the 1999-2016 NHANES surveys, which consisted of interviews and standardized physical exams, including laboratory tests, in home and at a mobile examination center. Analyses involving any fasting lab tests were limited to a random subsample of participants aged 12-19 years without diagnosed diabetes who were asked to fast the night before; 6,225 youth properly followed instructions and were included in this subsample.
The surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study authors and the editorial author indicated that they have no relevant financial disclosures or conflicts of interest. Dr. Magge also said she has no relevant disclosures.
SOURCE: Wallace AS et al. Pediatrics. August 2020. doi: 10.1542/peds.2020-0265.
FROM PEDIATRICS
Effects of Computer-Based Documentation Procedures on Health Care Workload Assessment and Resource Allocation: An Example From VA Sleep Medicine Programs
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Ten-Year Outcomes of a Systems-Based Approach to Longitudinal Amputation Care in the US Department of Veteran Affairs
The US Department of Veterans Affairs (VA) established a formal Amputation System of Care (ASoC) in 2008 with the goal of enhancing the quality and consistency of amputation rehabilitation care for veterans with limb loss.1,2 Throughout its history, the VA has placed a high priority on the care that is provided to veterans with limb amputation.1,3 Amputations have medical, physical, social, and psychological ramifications for the veteran and his or her family. Therefore, management of veterans with limb loss requires a comprehensive, coordinated, transdisciplinary program of services throughout the continuum of care. This includes offering the latest practices in medical interventions, artificial limbs, assistive technologies, and rehabilitation strategies to restore function and thereby optimize quality of life.
Amputation System of Care
The ASoC is an integrated system within the Veterans Health Administration (VHA) that provides specialized expertise in amputation rehabilitation incorporating the latest practices in medical management, rehabilitation therapies, artificial limbs, and assistive technologies. The system facilitates patient-centered, gender-sensitive, lifelong care and care coordination across the entire health continuum from acute inpatient hospitalization through a spectrum of inpatient, residential, and outpatient rehabilitation care settings. Through the provision of quality rehabilitation and prosthetic limb care, the ASoC strives to minimize disability and enable the highest level of social, vocational, and recreational success for veterans with an amputation.1-3
The policy and procedures for the ASoC have been detailed in prior VA Handbooks and in the ASoC Directive.1 This article highlights the background, population served, and organizational structure of the ASoC by detailing the outcomes and accomplishments of this systems-based approach to longitudinal amputation care between 2009 and 2019. Four core areas of activities and accomplishments are highlighted: (1) learning organization creation; (2) trust in VA care; (3) system modernization; and (4) customer service. This analysis and description of the VA amputation care program serves as a model of amputation care that can be used in the civilian sector. There also is potential for the ASoC to serve as a care model example for other populations within the VA.
Organizational Structure
The ASoC is an integrated, national health care delivery system in which each VA medical center (VAMC) has a specific designation that reflects the level of expertise and accessibility across the system based on an individual veteran’s needs and the specific capabilities of each VAMC.1-3 The organizational structure for the ASoC is similar to the Polytrauma System of Care in that facilities are divided into 4 tiers.1,4
For the ASoC, the 4 tiers are Regional Amputation Centers (RAC) at 7 VAMCs, Polytrauma Amputation Network Sites (PANS) at 18 VAMCs, Amputation Clinic Teams (ACT) at 106 VAMCs, and Amputation Points of Contact (APoC) at 22 VAMCs. The RAC locations provide the highest level of specialized expertise in clinical care and prosthetic limb technology and have rehabilitation capabilities to manage the most complicated cases. Like the RAC facilities, PANS provide a full range of clinical and ancillary services to veterans within their catchment area and serve as referral locations for veterans with needs that are more complex. ACT sites have a core amputation specialty team that provides regular follow-up and address ongoing care needs. ACT sites may or may not have full ancillary services, such as surgical subspecialties or an in-house prosthetics laboratory. APoC facilities have at least 1 person on staff who serves as the point of contact for consultation, assessment, and referral of a veteran with an amputation to a facility capable of providing the level of services required.1
The VA also places a high priority on both primary and secondary amputation prevention. The Preventing Amputations in Veterans Everywhere (PAVE) program and the ASoC coordinate efforts in order to address the prevention of an initial amputation, the rehabilitation of veterans who have had an amputation, and the prevention of a second amputation in those with an amputation.1,5
Population Served
The ASoC serves veterans with limb loss regardless of the etiology. This includes care of individuals with complex limb trauma and those with other injuries or disease processes resulting in a high likelihood of requiring a limb amputation. In 2019, the VA provided care to 96,519 veterans with amputation, and about half (46,214) had at least 1 major limb amputation, which is defined as an amputation at or proximal to the wrist or ankle.6 The majority of veterans with amputation treated within the VA have limb loss resulting from disease processes, such as diabetes mellitus (DM) and peripheral vascular disease (PVD). Amputations caused by these diseases generally occur in the older veteran population and are associated with comorbidities, such as cardiovascular disease, hypertension, and end-stage renal disease. Veterans with amputation due to trauma, including conflict-related injuries, are commonly younger at the time of their amputation. Although the number of conflict-related amputations is small compared with the number of amputations associated with disease processes, both groups require high-quality, comprehensive, lifelong care.
Between 2009 and 2019, the number of veterans with limb loss receiving care in the VA increased 34%.6 With advances in vascular surgery and limb-sparing procedures, minor amputations are more common than major limb amputations and more below-knee rather than above-knee amputations have been noted over the same time. However, the high prevalence of DM in the overall veteran population places about 1.8 million veterans at risk for amputation, and it is anticipated that the volume of limb loss in the veteran population will continue to grow and possibly accelerate.5
Performance Metrics
During this same period, the amputation specialty clinic encounter to unique ratio (a measure of how frequently patients return to the clinic each year) rose from 1.8 in 2009 to 2.3 in 2019 for both the total amputation population and for those with major limb amputation. When looking more specifically at the RAC facilities, the encounter to unique ratio increased from 1.5 to 3.0 over the same time, reflecting the added benefit of having dedicated resources for the amputation specialty program.6
Comparing the percentage of veterans with amputation who are seen in the VA for any service with those who also are seen in the amputation specialty clinic in the same year is a performance metric that reflects the penetration of amputation specialty services across the system. Between 2009 and 2019, this increased from 2.9 to 12.7% for the overall amputation population and from 4.8 to 26% for those with major limb amputation (Figure 2). This metric improved to a greater extent in RAC facilities; 44% of veterans with major limb amputation seen at a RAC were also seen in the amputation specialty clinic in 2019.6
System Hallmarks
One of the primary hallmarks of the ASoC is the interdisciplinary team approach addressing all aspects of management across the continuum of care (Table). The core team consists of a physician, therapist, and prosthetist, and may include a variety of other disciplines based on a veteran’s individual needs. This model promotes veteran-centric care. Comprehensive management of veterans with limb loss includes addressing medical considerations such as residual limb skin health to the prescription of artificial limbs and the provision of therapy services for prosthetic limb gait training.1,2
Lifelong care for veterans living with limb loss is another hallmark of the ASoC. The provision of care coordination across the continuum of care from acute hospitalization following an amputation to long-term follow-up in the outpatient setting for veteran’s lifespan is essential. Care coordination is provided across the system of care, which assures that a veteran with limb loss can obtain the required services through consultation or referral to a RAC or PANS as needed. Care coordination for the ASoC is facilitated by amputation rehabilitation coordinators at each of the RAC and PANS designated VAMCs.
Integration of services and resource collaboration are additional key aspects of the ASoC (Figure 3). In order to be successful, care of the veteran facing potential amputation or living with the challenges postamputation must be well-integrated into the broader care of the individual. Many veterans who undergo amputation have significant medical comorbidities, including a high prevalence of DM and peripheral vascular disease. Management of these conditions in collaboration with primary care and other medical specialties promotes the achievement of rehabilitation goals. Integration of surgical services and amputation prevention strategies is critical. Another essential element of the system is maintaining amputation specialty care team contact with all veterans with limb loss on at least an annual basis. A clinical practice guideline published in 2017 on lower Limb amputation rehabilitation emphasizes this need for an annual contact and includes a management and referral algorithm to assist primary care providers in the management of veterans with amputation (Figure 4).7
Collaboration with external partners has been an important element in the system of care development. The VA has partnered extensively with the US Department of Defense (DoD) to transition service members with amputation from the military health care system to the VA. The VA and DoD also have collaborated through the development and provision of joint provider trainings, clinical practice guidelines, incentive funding programs, and patient education materials. Congress authorized the Extremity Trauma and Amputation Center of Excellence (EACE) in 2009 with the mission to serve as the joint DoD and VA lead element focused on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations. The EACE has several lines of effort, including clinical affairs, research, and global outreach focused on building partnerships and fostering collaboration to optimize quality of life for those with extremity trauma and amputation. The Amputee Coalition, the largest nonprofit consumer-based amputee advocacy organization in the US, has been an important strategic partner for the dissemination of guideline recommendations and patient education as well as the development and provision of peer support services.
Methods
The ASoC created a learning organization to develop and maintain a knowledgeable and highly skilled clinical workforce through the identification of best practices related to amputation rehabilitation and the use of innovative education delivery models. During the past 10 years, the ASoC conducted 9 national, live health care provider training events in conjunction with the DoD. In conjunction with the EACE, the ASoC holds 6 national Grand Rounds sessions each year. Dissemination of information and trainings across both the VA and DoD has been facilitated through a national listserv referred to as the Federal Amputation Interest Group (FAIG), which has > 800 members. Since 2009, the VA, in collaboration with the DoD, has produced 3 clinical practice guidelines (CPGs) related to amputation care. The Lower Limb Amputation CPG was published in 2007 and updated in 2017, and a CPG and associated clinician resources focused on upper extremity amputation were published in 2014.7,8 In addition to these formal, comprehensive, and evidence-driven guidelines, the ASoC has developed other clinical support documents covering a range of topics from prosthesis prescription candidacy determination to osseointegration. In conjunction with the EACE, The ASoC also has published guidance for clinical implementation of new technologies such as the Mobius Bionics LUKE arm and Dynamic Response Ankle-Foot Orthoses.
The ASoC strives to improve the psychosocial welfare of veterans with amputation and enhance trust in VA amputation care services through sharing results on the quality and timeliness of care. The Commission on Accreditation for Rehabilitation Facilities (CARF) provides an international, independent, peer-reviewed system of accreditation that is widely recognized by federal agencies, state governments, major insurers, and professional organizations.1,2 CARF offers amputation specialty accreditation for inpatient and outpatient programs that signifies the attainment of a distinguished level of expertise and the provision of a comprehensive spectrum of services related to amputation care and rehabilitation. During its development, the ASoC established the expectation that each of the RAC and PANS designated VAMCs would attain and maintain CARF amputation specialty accreditation. The ASoC has achieved 100% success on this metric.
In addition, the ASoC has completed many other initiatives focused on enhancing trust in VA amputation care services. These include assuring compliance with implementation of the Mission Act as it relates to the provision of amputation care and prosthetic limb delivery so that any services provided in the community are well integrated and at the direction of the amputation specialty team. The ASoC has maintained a strong relationship with the Amputee Coalition to provide veterans with high-quality patient education materials as well as integrated peer support services.
ASoC virtual and face-to-face training events incorporate suicide prevention training for providers. Special focus has been placed on care provision for Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans with conflict-related multiple limb amputations. Although relatively small, this cohort is recognized as a unique and important population due to their unique care needs and increased risk for secondary complications. In 2019, 83% of these individuals were contacted to assure their amputation care needs were being adequately addressed.
Discussion
Over the past 10 years, the ASoC has built a modern, high-performance network of care to best serve veterans with amputation. Maturation of the system has included the addition of 3 new PANS locations to improve access to services as well as to better support geographic regions near large DoD military treatment facilities. The number of ACT designated VAMCs also has grown from 101 to 106 locations. The regional organization of sites has been modified to enhance the availability of referral and consultative services across the system. In addition, the ASoC has supported the development of an upper extremity amputation specialty program for consultation or referral to a highly specialized team of providers well versed in the significant technology advances that have taken place with upper extremity prostheses.9
One of the key components to high-performance network development is attaining a clear picture of the clinical demands and service delivery needs of the population served. The Amputee Data Repository was developed with the support of the VHA Support Service Center (VSSC) in order to better understand and track the population of veterans with amputation.6 The development and implementation of the Amputee Data Repository took place over several years, and the product was officially released into publication in 2015. The overall goals of this resource are to provide a data system for the ASoC to identify clinical care volumes and patterns of treatment; better understand the demographics of the veteran amputee population; assess the effectiveness of new treatment strategies; and utilize data analysis outcomes to influence clinical practice. The acquisition and analysis of this information will provide justification for the modification of clinical practice and will enhance the quality of care for all veterans with amputation.
Although the ASoC focuses primarily on the provision of clinical services, the system has been leveraged to support research activities and the advancement of artificial limb technologies. For example, ASoC providers and investigators supported the clinical research required to test and optimize the development of the DEKA arm. These research efforts resulted in the US Food and Drug Administration approval and commercialization of this device. Once the device became commercially available as the LUKE arm, the ASoC developed a clinical implementation strategy that assured availability and appropriate prescription and training with the new technology. The VA also has supported research and program development in osseointegration with further investigations and clinical implementation being planned.
Telehealth
The goal of the ASoC is to provide timely access and greater choice to specialty amputation rehabilitation services for veterans as determined by their clinical needs. One key strategy used to achieve this goal has been the expansion of virtual communication tools to enhance access to clinical expertise. Telehealth (Virtual Care) amputation services afford the opportunity to provide specialized clinical expertise to veterans who otherwise may not have access to this level of service or consultation.1,2 For others, virtual care services reduce the need for travel. The ASoC has leveraged these services effectively to enhance specialty amputation care for veterans in rural areas. Over time, the scope of virtual care services has expanded to provide virtual peer support services as well as care in the veteran’s home.
Another unique example is the use of virtual care to see veterans when they are being provided services by a community prosthetist. This service improves the timeliness of care and reduces the travel burden for the veteran. Between 2009 and 2019, total virtual care encounters to provide amputation-related services grew from 44 encounters to 3,905 encounters (Figure 5). In 2019, 13.8% of veterans seen in a VA outpatient amputation specialty clinic had at least 1 virtual encounter in the same year.6
In addition to the expansion of virtual care and building capacity through increasing the number of amputation specialty clinics and providers, the ASoC has used a host of other strategies to improve care access. The development of provider expertise in amputation care has been achieved through the methods of extensive provider training. Implementation of Patient Self-Referral Direct Scheduling allows veterans to access the outpatient amputation specialty clinic without a referral and without having to be seen by their primary care provider. This initiative provides easier and more timely access to amputation specialty services while reducing burden on primary care services. The amputation outpatient specialty clinic was one of a few specialty programs to be an early adopter of national online scheduling. The implementation of this service is still ongoing, but this program gives veterans greater control over scheduling, canceling, and rescheduling appointments.
Conclusions
During the 10 years following its implementation, the VA ASoC has successfully enhanced the quality and consistency of care and rehabilitation services provided to veterans with limb loss through the provision of highly specialized services in the areas of medical care, rehabilitation services, and prosthetic technology. This mission has been accomplished through prioritization and implementation of key strategic initiatives in learning organization creation, trust in VA care, development of a modern, high-performance network, and customer service. Collaborative partnerships both internally within the VA and externally with key stakeholders has facilitated this development, and these will need to be enhanced for future success. Evolving trends in amputation surgery, limb transplantation, artificial limb control and suspension strategies as well as advances in assistive technology also will need to be integrated into best practices and program development.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.03(1): Amputation system of care. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7482. Published August 3, 2018. Accessed July 31, 2020.
2. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs Amputations System of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
3. Reiber GE, Smith DG. VA paradigm shift in care of veterans with limb loss. J Rehabil Res Dev. 2010;47(4):vii-x. doi:10.1682/jrrd.2010.03.0030
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.01: Polytrauma system of care. https://www.va.gov/OPTOMETRY/docs/VHA_Directive_1172-01_Polytrauma_System_of_Care_1172_01_D_2019-01-24.pdf. Published January 24, 2019. Accessed July 31, 2020.
5. VHA Directive 1410, Prevention of amputation in veterans everywhere (PAVE) program, https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed July 31, 2020.
6. VHA Amputee Data Repository. VHA Support Service Center. http://vssc.med.va.gov. [Nonpublic source, not verified.]
7. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf. Accessed July 16, 2020.
8. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: The Management of upper extremity amputation rehabilitation.Version 1-2014. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf. Accessed July 16, 2020.
9. Resnik L, Meucci MR, Lieberman-Klinger S, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710-717. doi:10.1016/j.apmr.2011.11.010
10. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. Pocket card. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPGPocketCard092817.pdf. Accessed July 31, 2020.
The US Department of Veterans Affairs (VA) established a formal Amputation System of Care (ASoC) in 2008 with the goal of enhancing the quality and consistency of amputation rehabilitation care for veterans with limb loss.1,2 Throughout its history, the VA has placed a high priority on the care that is provided to veterans with limb amputation.1,3 Amputations have medical, physical, social, and psychological ramifications for the veteran and his or her family. Therefore, management of veterans with limb loss requires a comprehensive, coordinated, transdisciplinary program of services throughout the continuum of care. This includes offering the latest practices in medical interventions, artificial limbs, assistive technologies, and rehabilitation strategies to restore function and thereby optimize quality of life.
Amputation System of Care
The ASoC is an integrated system within the Veterans Health Administration (VHA) that provides specialized expertise in amputation rehabilitation incorporating the latest practices in medical management, rehabilitation therapies, artificial limbs, and assistive technologies. The system facilitates patient-centered, gender-sensitive, lifelong care and care coordination across the entire health continuum from acute inpatient hospitalization through a spectrum of inpatient, residential, and outpatient rehabilitation care settings. Through the provision of quality rehabilitation and prosthetic limb care, the ASoC strives to minimize disability and enable the highest level of social, vocational, and recreational success for veterans with an amputation.1-3
The policy and procedures for the ASoC have been detailed in prior VA Handbooks and in the ASoC Directive.1 This article highlights the background, population served, and organizational structure of the ASoC by detailing the outcomes and accomplishments of this systems-based approach to longitudinal amputation care between 2009 and 2019. Four core areas of activities and accomplishments are highlighted: (1) learning organization creation; (2) trust in VA care; (3) system modernization; and (4) customer service. This analysis and description of the VA amputation care program serves as a model of amputation care that can be used in the civilian sector. There also is potential for the ASoC to serve as a care model example for other populations within the VA.
Organizational Structure
The ASoC is an integrated, national health care delivery system in which each VA medical center (VAMC) has a specific designation that reflects the level of expertise and accessibility across the system based on an individual veteran’s needs and the specific capabilities of each VAMC.1-3 The organizational structure for the ASoC is similar to the Polytrauma System of Care in that facilities are divided into 4 tiers.1,4
For the ASoC, the 4 tiers are Regional Amputation Centers (RAC) at 7 VAMCs, Polytrauma Amputation Network Sites (PANS) at 18 VAMCs, Amputation Clinic Teams (ACT) at 106 VAMCs, and Amputation Points of Contact (APoC) at 22 VAMCs. The RAC locations provide the highest level of specialized expertise in clinical care and prosthetic limb technology and have rehabilitation capabilities to manage the most complicated cases. Like the RAC facilities, PANS provide a full range of clinical and ancillary services to veterans within their catchment area and serve as referral locations for veterans with needs that are more complex. ACT sites have a core amputation specialty team that provides regular follow-up and address ongoing care needs. ACT sites may or may not have full ancillary services, such as surgical subspecialties or an in-house prosthetics laboratory. APoC facilities have at least 1 person on staff who serves as the point of contact for consultation, assessment, and referral of a veteran with an amputation to a facility capable of providing the level of services required.1
The VA also places a high priority on both primary and secondary amputation prevention. The Preventing Amputations in Veterans Everywhere (PAVE) program and the ASoC coordinate efforts in order to address the prevention of an initial amputation, the rehabilitation of veterans who have had an amputation, and the prevention of a second amputation in those with an amputation.1,5
Population Served
The ASoC serves veterans with limb loss regardless of the etiology. This includes care of individuals with complex limb trauma and those with other injuries or disease processes resulting in a high likelihood of requiring a limb amputation. In 2019, the VA provided care to 96,519 veterans with amputation, and about half (46,214) had at least 1 major limb amputation, which is defined as an amputation at or proximal to the wrist or ankle.6 The majority of veterans with amputation treated within the VA have limb loss resulting from disease processes, such as diabetes mellitus (DM) and peripheral vascular disease (PVD). Amputations caused by these diseases generally occur in the older veteran population and are associated with comorbidities, such as cardiovascular disease, hypertension, and end-stage renal disease. Veterans with amputation due to trauma, including conflict-related injuries, are commonly younger at the time of their amputation. Although the number of conflict-related amputations is small compared with the number of amputations associated with disease processes, both groups require high-quality, comprehensive, lifelong care.
Between 2009 and 2019, the number of veterans with limb loss receiving care in the VA increased 34%.6 With advances in vascular surgery and limb-sparing procedures, minor amputations are more common than major limb amputations and more below-knee rather than above-knee amputations have been noted over the same time. However, the high prevalence of DM in the overall veteran population places about 1.8 million veterans at risk for amputation, and it is anticipated that the volume of limb loss in the veteran population will continue to grow and possibly accelerate.5
Performance Metrics
During this same period, the amputation specialty clinic encounter to unique ratio (a measure of how frequently patients return to the clinic each year) rose from 1.8 in 2009 to 2.3 in 2019 for both the total amputation population and for those with major limb amputation. When looking more specifically at the RAC facilities, the encounter to unique ratio increased from 1.5 to 3.0 over the same time, reflecting the added benefit of having dedicated resources for the amputation specialty program.6
Comparing the percentage of veterans with amputation who are seen in the VA for any service with those who also are seen in the amputation specialty clinic in the same year is a performance metric that reflects the penetration of amputation specialty services across the system. Between 2009 and 2019, this increased from 2.9 to 12.7% for the overall amputation population and from 4.8 to 26% for those with major limb amputation (Figure 2). This metric improved to a greater extent in RAC facilities; 44% of veterans with major limb amputation seen at a RAC were also seen in the amputation specialty clinic in 2019.6
System Hallmarks
One of the primary hallmarks of the ASoC is the interdisciplinary team approach addressing all aspects of management across the continuum of care (Table). The core team consists of a physician, therapist, and prosthetist, and may include a variety of other disciplines based on a veteran’s individual needs. This model promotes veteran-centric care. Comprehensive management of veterans with limb loss includes addressing medical considerations such as residual limb skin health to the prescription of artificial limbs and the provision of therapy services for prosthetic limb gait training.1,2
Lifelong care for veterans living with limb loss is another hallmark of the ASoC. The provision of care coordination across the continuum of care from acute hospitalization following an amputation to long-term follow-up in the outpatient setting for veteran’s lifespan is essential. Care coordination is provided across the system of care, which assures that a veteran with limb loss can obtain the required services through consultation or referral to a RAC or PANS as needed. Care coordination for the ASoC is facilitated by amputation rehabilitation coordinators at each of the RAC and PANS designated VAMCs.
Integration of services and resource collaboration are additional key aspects of the ASoC (Figure 3). In order to be successful, care of the veteran facing potential amputation or living with the challenges postamputation must be well-integrated into the broader care of the individual. Many veterans who undergo amputation have significant medical comorbidities, including a high prevalence of DM and peripheral vascular disease. Management of these conditions in collaboration with primary care and other medical specialties promotes the achievement of rehabilitation goals. Integration of surgical services and amputation prevention strategies is critical. Another essential element of the system is maintaining amputation specialty care team contact with all veterans with limb loss on at least an annual basis. A clinical practice guideline published in 2017 on lower Limb amputation rehabilitation emphasizes this need for an annual contact and includes a management and referral algorithm to assist primary care providers in the management of veterans with amputation (Figure 4).7
Collaboration with external partners has been an important element in the system of care development. The VA has partnered extensively with the US Department of Defense (DoD) to transition service members with amputation from the military health care system to the VA. The VA and DoD also have collaborated through the development and provision of joint provider trainings, clinical practice guidelines, incentive funding programs, and patient education materials. Congress authorized the Extremity Trauma and Amputation Center of Excellence (EACE) in 2009 with the mission to serve as the joint DoD and VA lead element focused on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations. The EACE has several lines of effort, including clinical affairs, research, and global outreach focused on building partnerships and fostering collaboration to optimize quality of life for those with extremity trauma and amputation. The Amputee Coalition, the largest nonprofit consumer-based amputee advocacy organization in the US, has been an important strategic partner for the dissemination of guideline recommendations and patient education as well as the development and provision of peer support services.
Methods
The ASoC created a learning organization to develop and maintain a knowledgeable and highly skilled clinical workforce through the identification of best practices related to amputation rehabilitation and the use of innovative education delivery models. During the past 10 years, the ASoC conducted 9 national, live health care provider training events in conjunction with the DoD. In conjunction with the EACE, the ASoC holds 6 national Grand Rounds sessions each year. Dissemination of information and trainings across both the VA and DoD has been facilitated through a national listserv referred to as the Federal Amputation Interest Group (FAIG), which has > 800 members. Since 2009, the VA, in collaboration with the DoD, has produced 3 clinical practice guidelines (CPGs) related to amputation care. The Lower Limb Amputation CPG was published in 2007 and updated in 2017, and a CPG and associated clinician resources focused on upper extremity amputation were published in 2014.7,8 In addition to these formal, comprehensive, and evidence-driven guidelines, the ASoC has developed other clinical support documents covering a range of topics from prosthesis prescription candidacy determination to osseointegration. In conjunction with the EACE, The ASoC also has published guidance for clinical implementation of new technologies such as the Mobius Bionics LUKE arm and Dynamic Response Ankle-Foot Orthoses.
The ASoC strives to improve the psychosocial welfare of veterans with amputation and enhance trust in VA amputation care services through sharing results on the quality and timeliness of care. The Commission on Accreditation for Rehabilitation Facilities (CARF) provides an international, independent, peer-reviewed system of accreditation that is widely recognized by federal agencies, state governments, major insurers, and professional organizations.1,2 CARF offers amputation specialty accreditation for inpatient and outpatient programs that signifies the attainment of a distinguished level of expertise and the provision of a comprehensive spectrum of services related to amputation care and rehabilitation. During its development, the ASoC established the expectation that each of the RAC and PANS designated VAMCs would attain and maintain CARF amputation specialty accreditation. The ASoC has achieved 100% success on this metric.
In addition, the ASoC has completed many other initiatives focused on enhancing trust in VA amputation care services. These include assuring compliance with implementation of the Mission Act as it relates to the provision of amputation care and prosthetic limb delivery so that any services provided in the community are well integrated and at the direction of the amputation specialty team. The ASoC has maintained a strong relationship with the Amputee Coalition to provide veterans with high-quality patient education materials as well as integrated peer support services.
ASoC virtual and face-to-face training events incorporate suicide prevention training for providers. Special focus has been placed on care provision for Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans with conflict-related multiple limb amputations. Although relatively small, this cohort is recognized as a unique and important population due to their unique care needs and increased risk for secondary complications. In 2019, 83% of these individuals were contacted to assure their amputation care needs were being adequately addressed.
Discussion
Over the past 10 years, the ASoC has built a modern, high-performance network of care to best serve veterans with amputation. Maturation of the system has included the addition of 3 new PANS locations to improve access to services as well as to better support geographic regions near large DoD military treatment facilities. The number of ACT designated VAMCs also has grown from 101 to 106 locations. The regional organization of sites has been modified to enhance the availability of referral and consultative services across the system. In addition, the ASoC has supported the development of an upper extremity amputation specialty program for consultation or referral to a highly specialized team of providers well versed in the significant technology advances that have taken place with upper extremity prostheses.9
One of the key components to high-performance network development is attaining a clear picture of the clinical demands and service delivery needs of the population served. The Amputee Data Repository was developed with the support of the VHA Support Service Center (VSSC) in order to better understand and track the population of veterans with amputation.6 The development and implementation of the Amputee Data Repository took place over several years, and the product was officially released into publication in 2015. The overall goals of this resource are to provide a data system for the ASoC to identify clinical care volumes and patterns of treatment; better understand the demographics of the veteran amputee population; assess the effectiveness of new treatment strategies; and utilize data analysis outcomes to influence clinical practice. The acquisition and analysis of this information will provide justification for the modification of clinical practice and will enhance the quality of care for all veterans with amputation.
Although the ASoC focuses primarily on the provision of clinical services, the system has been leveraged to support research activities and the advancement of artificial limb technologies. For example, ASoC providers and investigators supported the clinical research required to test and optimize the development of the DEKA arm. These research efforts resulted in the US Food and Drug Administration approval and commercialization of this device. Once the device became commercially available as the LUKE arm, the ASoC developed a clinical implementation strategy that assured availability and appropriate prescription and training with the new technology. The VA also has supported research and program development in osseointegration with further investigations and clinical implementation being planned.
Telehealth
The goal of the ASoC is to provide timely access and greater choice to specialty amputation rehabilitation services for veterans as determined by their clinical needs. One key strategy used to achieve this goal has been the expansion of virtual communication tools to enhance access to clinical expertise. Telehealth (Virtual Care) amputation services afford the opportunity to provide specialized clinical expertise to veterans who otherwise may not have access to this level of service or consultation.1,2 For others, virtual care services reduce the need for travel. The ASoC has leveraged these services effectively to enhance specialty amputation care for veterans in rural areas. Over time, the scope of virtual care services has expanded to provide virtual peer support services as well as care in the veteran’s home.
Another unique example is the use of virtual care to see veterans when they are being provided services by a community prosthetist. This service improves the timeliness of care and reduces the travel burden for the veteran. Between 2009 and 2019, total virtual care encounters to provide amputation-related services grew from 44 encounters to 3,905 encounters (Figure 5). In 2019, 13.8% of veterans seen in a VA outpatient amputation specialty clinic had at least 1 virtual encounter in the same year.6
In addition to the expansion of virtual care and building capacity through increasing the number of amputation specialty clinics and providers, the ASoC has used a host of other strategies to improve care access. The development of provider expertise in amputation care has been achieved through the methods of extensive provider training. Implementation of Patient Self-Referral Direct Scheduling allows veterans to access the outpatient amputation specialty clinic without a referral and without having to be seen by their primary care provider. This initiative provides easier and more timely access to amputation specialty services while reducing burden on primary care services. The amputation outpatient specialty clinic was one of a few specialty programs to be an early adopter of national online scheduling. The implementation of this service is still ongoing, but this program gives veterans greater control over scheduling, canceling, and rescheduling appointments.
Conclusions
During the 10 years following its implementation, the VA ASoC has successfully enhanced the quality and consistency of care and rehabilitation services provided to veterans with limb loss through the provision of highly specialized services in the areas of medical care, rehabilitation services, and prosthetic technology. This mission has been accomplished through prioritization and implementation of key strategic initiatives in learning organization creation, trust in VA care, development of a modern, high-performance network, and customer service. Collaborative partnerships both internally within the VA and externally with key stakeholders has facilitated this development, and these will need to be enhanced for future success. Evolving trends in amputation surgery, limb transplantation, artificial limb control and suspension strategies as well as advances in assistive technology also will need to be integrated into best practices and program development.
The US Department of Veterans Affairs (VA) established a formal Amputation System of Care (ASoC) in 2008 with the goal of enhancing the quality and consistency of amputation rehabilitation care for veterans with limb loss.1,2 Throughout its history, the VA has placed a high priority on the care that is provided to veterans with limb amputation.1,3 Amputations have medical, physical, social, and psychological ramifications for the veteran and his or her family. Therefore, management of veterans with limb loss requires a comprehensive, coordinated, transdisciplinary program of services throughout the continuum of care. This includes offering the latest practices in medical interventions, artificial limbs, assistive technologies, and rehabilitation strategies to restore function and thereby optimize quality of life.
Amputation System of Care
The ASoC is an integrated system within the Veterans Health Administration (VHA) that provides specialized expertise in amputation rehabilitation incorporating the latest practices in medical management, rehabilitation therapies, artificial limbs, and assistive technologies. The system facilitates patient-centered, gender-sensitive, lifelong care and care coordination across the entire health continuum from acute inpatient hospitalization through a spectrum of inpatient, residential, and outpatient rehabilitation care settings. Through the provision of quality rehabilitation and prosthetic limb care, the ASoC strives to minimize disability and enable the highest level of social, vocational, and recreational success for veterans with an amputation.1-3
The policy and procedures for the ASoC have been detailed in prior VA Handbooks and in the ASoC Directive.1 This article highlights the background, population served, and organizational structure of the ASoC by detailing the outcomes and accomplishments of this systems-based approach to longitudinal amputation care between 2009 and 2019. Four core areas of activities and accomplishments are highlighted: (1) learning organization creation; (2) trust in VA care; (3) system modernization; and (4) customer service. This analysis and description of the VA amputation care program serves as a model of amputation care that can be used in the civilian sector. There also is potential for the ASoC to serve as a care model example for other populations within the VA.
Organizational Structure
The ASoC is an integrated, national health care delivery system in which each VA medical center (VAMC) has a specific designation that reflects the level of expertise and accessibility across the system based on an individual veteran’s needs and the specific capabilities of each VAMC.1-3 The organizational structure for the ASoC is similar to the Polytrauma System of Care in that facilities are divided into 4 tiers.1,4
For the ASoC, the 4 tiers are Regional Amputation Centers (RAC) at 7 VAMCs, Polytrauma Amputation Network Sites (PANS) at 18 VAMCs, Amputation Clinic Teams (ACT) at 106 VAMCs, and Amputation Points of Contact (APoC) at 22 VAMCs. The RAC locations provide the highest level of specialized expertise in clinical care and prosthetic limb technology and have rehabilitation capabilities to manage the most complicated cases. Like the RAC facilities, PANS provide a full range of clinical and ancillary services to veterans within their catchment area and serve as referral locations for veterans with needs that are more complex. ACT sites have a core amputation specialty team that provides regular follow-up and address ongoing care needs. ACT sites may or may not have full ancillary services, such as surgical subspecialties or an in-house prosthetics laboratory. APoC facilities have at least 1 person on staff who serves as the point of contact for consultation, assessment, and referral of a veteran with an amputation to a facility capable of providing the level of services required.1
The VA also places a high priority on both primary and secondary amputation prevention. The Preventing Amputations in Veterans Everywhere (PAVE) program and the ASoC coordinate efforts in order to address the prevention of an initial amputation, the rehabilitation of veterans who have had an amputation, and the prevention of a second amputation in those with an amputation.1,5
Population Served
The ASoC serves veterans with limb loss regardless of the etiology. This includes care of individuals with complex limb trauma and those with other injuries or disease processes resulting in a high likelihood of requiring a limb amputation. In 2019, the VA provided care to 96,519 veterans with amputation, and about half (46,214) had at least 1 major limb amputation, which is defined as an amputation at or proximal to the wrist or ankle.6 The majority of veterans with amputation treated within the VA have limb loss resulting from disease processes, such as diabetes mellitus (DM) and peripheral vascular disease (PVD). Amputations caused by these diseases generally occur in the older veteran population and are associated with comorbidities, such as cardiovascular disease, hypertension, and end-stage renal disease. Veterans with amputation due to trauma, including conflict-related injuries, are commonly younger at the time of their amputation. Although the number of conflict-related amputations is small compared with the number of amputations associated with disease processes, both groups require high-quality, comprehensive, lifelong care.
Between 2009 and 2019, the number of veterans with limb loss receiving care in the VA increased 34%.6 With advances in vascular surgery and limb-sparing procedures, minor amputations are more common than major limb amputations and more below-knee rather than above-knee amputations have been noted over the same time. However, the high prevalence of DM in the overall veteran population places about 1.8 million veterans at risk for amputation, and it is anticipated that the volume of limb loss in the veteran population will continue to grow and possibly accelerate.5
Performance Metrics
During this same period, the amputation specialty clinic encounter to unique ratio (a measure of how frequently patients return to the clinic each year) rose from 1.8 in 2009 to 2.3 in 2019 for both the total amputation population and for those with major limb amputation. When looking more specifically at the RAC facilities, the encounter to unique ratio increased from 1.5 to 3.0 over the same time, reflecting the added benefit of having dedicated resources for the amputation specialty program.6
Comparing the percentage of veterans with amputation who are seen in the VA for any service with those who also are seen in the amputation specialty clinic in the same year is a performance metric that reflects the penetration of amputation specialty services across the system. Between 2009 and 2019, this increased from 2.9 to 12.7% for the overall amputation population and from 4.8 to 26% for those with major limb amputation (Figure 2). This metric improved to a greater extent in RAC facilities; 44% of veterans with major limb amputation seen at a RAC were also seen in the amputation specialty clinic in 2019.6
System Hallmarks
One of the primary hallmarks of the ASoC is the interdisciplinary team approach addressing all aspects of management across the continuum of care (Table). The core team consists of a physician, therapist, and prosthetist, and may include a variety of other disciplines based on a veteran’s individual needs. This model promotes veteran-centric care. Comprehensive management of veterans with limb loss includes addressing medical considerations such as residual limb skin health to the prescription of artificial limbs and the provision of therapy services for prosthetic limb gait training.1,2
Lifelong care for veterans living with limb loss is another hallmark of the ASoC. The provision of care coordination across the continuum of care from acute hospitalization following an amputation to long-term follow-up in the outpatient setting for veteran’s lifespan is essential. Care coordination is provided across the system of care, which assures that a veteran with limb loss can obtain the required services through consultation or referral to a RAC or PANS as needed. Care coordination for the ASoC is facilitated by amputation rehabilitation coordinators at each of the RAC and PANS designated VAMCs.
Integration of services and resource collaboration are additional key aspects of the ASoC (Figure 3). In order to be successful, care of the veteran facing potential amputation or living with the challenges postamputation must be well-integrated into the broader care of the individual. Many veterans who undergo amputation have significant medical comorbidities, including a high prevalence of DM and peripheral vascular disease. Management of these conditions in collaboration with primary care and other medical specialties promotes the achievement of rehabilitation goals. Integration of surgical services and amputation prevention strategies is critical. Another essential element of the system is maintaining amputation specialty care team contact with all veterans with limb loss on at least an annual basis. A clinical practice guideline published in 2017 on lower Limb amputation rehabilitation emphasizes this need for an annual contact and includes a management and referral algorithm to assist primary care providers in the management of veterans with amputation (Figure 4).7
Collaboration with external partners has been an important element in the system of care development. The VA has partnered extensively with the US Department of Defense (DoD) to transition service members with amputation from the military health care system to the VA. The VA and DoD also have collaborated through the development and provision of joint provider trainings, clinical practice guidelines, incentive funding programs, and patient education materials. Congress authorized the Extremity Trauma and Amputation Center of Excellence (EACE) in 2009 with the mission to serve as the joint DoD and VA lead element focused on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations. The EACE has several lines of effort, including clinical affairs, research, and global outreach focused on building partnerships and fostering collaboration to optimize quality of life for those with extremity trauma and amputation. The Amputee Coalition, the largest nonprofit consumer-based amputee advocacy organization in the US, has been an important strategic partner for the dissemination of guideline recommendations and patient education as well as the development and provision of peer support services.
Methods
The ASoC created a learning organization to develop and maintain a knowledgeable and highly skilled clinical workforce through the identification of best practices related to amputation rehabilitation and the use of innovative education delivery models. During the past 10 years, the ASoC conducted 9 national, live health care provider training events in conjunction with the DoD. In conjunction with the EACE, the ASoC holds 6 national Grand Rounds sessions each year. Dissemination of information and trainings across both the VA and DoD has been facilitated through a national listserv referred to as the Federal Amputation Interest Group (FAIG), which has > 800 members. Since 2009, the VA, in collaboration with the DoD, has produced 3 clinical practice guidelines (CPGs) related to amputation care. The Lower Limb Amputation CPG was published in 2007 and updated in 2017, and a CPG and associated clinician resources focused on upper extremity amputation were published in 2014.7,8 In addition to these formal, comprehensive, and evidence-driven guidelines, the ASoC has developed other clinical support documents covering a range of topics from prosthesis prescription candidacy determination to osseointegration. In conjunction with the EACE, The ASoC also has published guidance for clinical implementation of new technologies such as the Mobius Bionics LUKE arm and Dynamic Response Ankle-Foot Orthoses.
The ASoC strives to improve the psychosocial welfare of veterans with amputation and enhance trust in VA amputation care services through sharing results on the quality and timeliness of care. The Commission on Accreditation for Rehabilitation Facilities (CARF) provides an international, independent, peer-reviewed system of accreditation that is widely recognized by federal agencies, state governments, major insurers, and professional organizations.1,2 CARF offers amputation specialty accreditation for inpatient and outpatient programs that signifies the attainment of a distinguished level of expertise and the provision of a comprehensive spectrum of services related to amputation care and rehabilitation. During its development, the ASoC established the expectation that each of the RAC and PANS designated VAMCs would attain and maintain CARF amputation specialty accreditation. The ASoC has achieved 100% success on this metric.
In addition, the ASoC has completed many other initiatives focused on enhancing trust in VA amputation care services. These include assuring compliance with implementation of the Mission Act as it relates to the provision of amputation care and prosthetic limb delivery so that any services provided in the community are well integrated and at the direction of the amputation specialty team. The ASoC has maintained a strong relationship with the Amputee Coalition to provide veterans with high-quality patient education materials as well as integrated peer support services.
ASoC virtual and face-to-face training events incorporate suicide prevention training for providers. Special focus has been placed on care provision for Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans with conflict-related multiple limb amputations. Although relatively small, this cohort is recognized as a unique and important population due to their unique care needs and increased risk for secondary complications. In 2019, 83% of these individuals were contacted to assure their amputation care needs were being adequately addressed.
Discussion
Over the past 10 years, the ASoC has built a modern, high-performance network of care to best serve veterans with amputation. Maturation of the system has included the addition of 3 new PANS locations to improve access to services as well as to better support geographic regions near large DoD military treatment facilities. The number of ACT designated VAMCs also has grown from 101 to 106 locations. The regional organization of sites has been modified to enhance the availability of referral and consultative services across the system. In addition, the ASoC has supported the development of an upper extremity amputation specialty program for consultation or referral to a highly specialized team of providers well versed in the significant technology advances that have taken place with upper extremity prostheses.9
One of the key components to high-performance network development is attaining a clear picture of the clinical demands and service delivery needs of the population served. The Amputee Data Repository was developed with the support of the VHA Support Service Center (VSSC) in order to better understand and track the population of veterans with amputation.6 The development and implementation of the Amputee Data Repository took place over several years, and the product was officially released into publication in 2015. The overall goals of this resource are to provide a data system for the ASoC to identify clinical care volumes and patterns of treatment; better understand the demographics of the veteran amputee population; assess the effectiveness of new treatment strategies; and utilize data analysis outcomes to influence clinical practice. The acquisition and analysis of this information will provide justification for the modification of clinical practice and will enhance the quality of care for all veterans with amputation.
Although the ASoC focuses primarily on the provision of clinical services, the system has been leveraged to support research activities and the advancement of artificial limb technologies. For example, ASoC providers and investigators supported the clinical research required to test and optimize the development of the DEKA arm. These research efforts resulted in the US Food and Drug Administration approval and commercialization of this device. Once the device became commercially available as the LUKE arm, the ASoC developed a clinical implementation strategy that assured availability and appropriate prescription and training with the new technology. The VA also has supported research and program development in osseointegration with further investigations and clinical implementation being planned.
Telehealth
The goal of the ASoC is to provide timely access and greater choice to specialty amputation rehabilitation services for veterans as determined by their clinical needs. One key strategy used to achieve this goal has been the expansion of virtual communication tools to enhance access to clinical expertise. Telehealth (Virtual Care) amputation services afford the opportunity to provide specialized clinical expertise to veterans who otherwise may not have access to this level of service or consultation.1,2 For others, virtual care services reduce the need for travel. The ASoC has leveraged these services effectively to enhance specialty amputation care for veterans in rural areas. Over time, the scope of virtual care services has expanded to provide virtual peer support services as well as care in the veteran’s home.
Another unique example is the use of virtual care to see veterans when they are being provided services by a community prosthetist. This service improves the timeliness of care and reduces the travel burden for the veteran. Between 2009 and 2019, total virtual care encounters to provide amputation-related services grew from 44 encounters to 3,905 encounters (Figure 5). In 2019, 13.8% of veterans seen in a VA outpatient amputation specialty clinic had at least 1 virtual encounter in the same year.6
In addition to the expansion of virtual care and building capacity through increasing the number of amputation specialty clinics and providers, the ASoC has used a host of other strategies to improve care access. The development of provider expertise in amputation care has been achieved through the methods of extensive provider training. Implementation of Patient Self-Referral Direct Scheduling allows veterans to access the outpatient amputation specialty clinic without a referral and without having to be seen by their primary care provider. This initiative provides easier and more timely access to amputation specialty services while reducing burden on primary care services. The amputation outpatient specialty clinic was one of a few specialty programs to be an early adopter of national online scheduling. The implementation of this service is still ongoing, but this program gives veterans greater control over scheduling, canceling, and rescheduling appointments.
Conclusions
During the 10 years following its implementation, the VA ASoC has successfully enhanced the quality and consistency of care and rehabilitation services provided to veterans with limb loss through the provision of highly specialized services in the areas of medical care, rehabilitation services, and prosthetic technology. This mission has been accomplished through prioritization and implementation of key strategic initiatives in learning organization creation, trust in VA care, development of a modern, high-performance network, and customer service. Collaborative partnerships both internally within the VA and externally with key stakeholders has facilitated this development, and these will need to be enhanced for future success. Evolving trends in amputation surgery, limb transplantation, artificial limb control and suspension strategies as well as advances in assistive technology also will need to be integrated into best practices and program development.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.03(1): Amputation system of care. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7482. Published August 3, 2018. Accessed July 31, 2020.
2. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs Amputations System of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
3. Reiber GE, Smith DG. VA paradigm shift in care of veterans with limb loss. J Rehabil Res Dev. 2010;47(4):vii-x. doi:10.1682/jrrd.2010.03.0030
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.01: Polytrauma system of care. https://www.va.gov/OPTOMETRY/docs/VHA_Directive_1172-01_Polytrauma_System_of_Care_1172_01_D_2019-01-24.pdf. Published January 24, 2019. Accessed July 31, 2020.
5. VHA Directive 1410, Prevention of amputation in veterans everywhere (PAVE) program, https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed July 31, 2020.
6. VHA Amputee Data Repository. VHA Support Service Center. http://vssc.med.va.gov. [Nonpublic source, not verified.]
7. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf. Accessed July 16, 2020.
8. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: The Management of upper extremity amputation rehabilitation.Version 1-2014. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf. Accessed July 16, 2020.
9. Resnik L, Meucci MR, Lieberman-Klinger S, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710-717. doi:10.1016/j.apmr.2011.11.010
10. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. Pocket card. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPGPocketCard092817.pdf. Accessed July 31, 2020.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.03(1): Amputation system of care. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7482. Published August 3, 2018. Accessed July 31, 2020.
2. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs Amputations System of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
3. Reiber GE, Smith DG. VA paradigm shift in care of veterans with limb loss. J Rehabil Res Dev. 2010;47(4):vii-x. doi:10.1682/jrrd.2010.03.0030
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.01: Polytrauma system of care. https://www.va.gov/OPTOMETRY/docs/VHA_Directive_1172-01_Polytrauma_System_of_Care_1172_01_D_2019-01-24.pdf. Published January 24, 2019. Accessed July 31, 2020.
5. VHA Directive 1410, Prevention of amputation in veterans everywhere (PAVE) program, https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed July 31, 2020.
6. VHA Amputee Data Repository. VHA Support Service Center. http://vssc.med.va.gov. [Nonpublic source, not verified.]
7. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf. Accessed July 16, 2020.
8. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: The Management of upper extremity amputation rehabilitation.Version 1-2014. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf. Accessed July 16, 2020.
9. Resnik L, Meucci MR, Lieberman-Klinger S, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710-717. doi:10.1016/j.apmr.2011.11.010
10. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. Pocket card. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPGPocketCard092817.pdf. Accessed July 31, 2020.
Pan-Pseudothrombocytopenia in COVID-19: A Harbinger for Lethal Arterial Thrombosis?
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
Creating an Intensive Care Unit From a Postanesthesia Care Unit for the COVID-19 Surge at the Veterans Affairs Ann Arbor Healthcare System
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
All Hands on Deck: The Federal Health Care Response to the COVID-19 National Emergency
A torrent of blame has deluged the administration’s management of the pandemic. There is though one part of the government that deserves the praise of the nation for its response to this public health crisis—the federal health care system. In this column, we discuss the ways in which the Veterans Health Administration (VHA), the Department of Defense (DoD), and the US Public Health Service (PHS) Commissioned Corps especially have bravely and generously responded to the medical emergency of COVID-19 in the US.
Four missions drive the US Department of Veterans Affairs (VA). Though the fourth of these missions usually is in the background, it has risen to the forefront during the pandemic. To put the fourth mission in its proper perspective, we first should review the other 3 charges given to the largest integrated health care system in the country.
The first mission is to provide the highest quality care possible for the more than 9 million veterans enrolled in that system at each of the 1,255 VHA locations. The second mission is to ensure that the Veterans Benefits Administration delivers the full range of benefits that veterans earned through their service. These including funding for education, loans for homes, and many other types of support that assist service men and women to be successful in their transition from military to civilian life. The third mission is to honor the commitment of those who fought for their country unto death. The National Cemeteries Administration oversees 142 national cemeteries where veterans are buried with dignity and remembered with gratitude for their uniformed service. The purpose of these 3 internally focused missions is to provide a safety net for eligible veterans from the day they separate from the military until the hour they pass from this earth.
The fourth mission is different. This mission looks outside the military family to the civilian world. Its goal is to bolster the ability of the nation as a whole to handle wars, terrorism, national emergencies, and natural disasters. It does this through emergency response plans that preserve the integrity of the 3 other missions to veterans while enhancing the capacity of local and state governments to manage the threat of these public health, safety, or security crises.1
At the same time the VA was aggressively mounting a defense against the threat COVID-19 posed to the other missions, it also launched the fourth mission. In announcing these actions in April 2020, VA Secretary Robert Wilke succinctly summarized the need to balance the fourth mission with the other 3. “VA is committed to helping the nation in this effort to combat COVID-19. Helping veterans is our first mission, but in many locations across the country we’re helping states and local communities. VA is in this fight not only for the millions of veterans we serve each day; we’re in the fight for the people of the United States.”2
During the 2009 H1N1 pandemic I saw firsthand how VA disaster preparedness and emergency training were far superior to many academic and community health care systems. Given VA’s detailed and drilled crisis response plans, its specialized expertise in public health disasters, and its immense resources, it is no wonder that as the virus stretched civilian health care systems, some states turned to the VA for help. At my Albuquerque, New Mexico, VA medical center, 5 medical surgical beds and 3 intensive care beds were opened to the Indian Health Service overwhelmed with cases of COVID-19 in the hard-hit Navajo Nation. In New Jersey where Federal Practitioner is published, the fourth mission reached out to the state-run veterans homes as 90 VA nurses and gerontologists were deployed to 2 of its veterans facilities where close to 150 veterans have died.3 State veterans homes in Massachusetts, Pennsylvania, Alabama, and many other states have received supplies, including direly needed testing and personal protective equipment, staff, technology, and training.4
In July, VA published an impressive summary of fourth mission activities, which I encourage you to read. When you are look at this site, remember with a moment of silent appreciation all the altruistic and courageous VA clinical and administrative staff who volunteered for these assignments many of which put them directly in harm’s way.5
The VA is not alone in answering the call of COVID-19. In March, despite the grave risk to their health, their life, and their families, the USNS Comfort was deployed to New York City to help with its COVID-19 response while the USNS Mercy assisted in the efforts in Los Angeles. More recently, the military deployed > 700 Military Health System medical and support professionals to support COVID-19 operations in both Texas and California. Brooke Army Medical Center in San Antonio has taken on a handful of civilian patients with COVID-19 and increase its level I trauma cases as local hospitals have strained under the caseload.6
For the PHS Commissioned Corps its first mission is to serve as “America’s health responders.”7 This pandemic has intensified the extant health inequities in our country and compounded them with racial injustice and economic disparity. Thus, it is important to recognize that the very purpose of the PHS is to “fight disease, conduct research, and care for patients in underserved communities across the nation.”8 More than 3,900 PHS officers have been deployed nationally and internationally in COVID-19 clinical strike teams. Early in the pandemic the clinical response teams were deployed to a long-term care facility in Kirkland, Washington; convention center-based hospitals in New York City, Detroit, Michigan, and Washington DC, and Navajo Nation facilities. PHS officers also are providing clinical guidance at Bureau of Prison facilities for infection control and personal protective equipment training.
We know that there are many more examples of heroic service by federal health care professionals and staff than we could locate or celebrate in this brief column. Readers of this journal are well aware of the near constant criticism of the VA and calls for privatization,9 the inadequate funding of the PHS,10 and the recent downsizing of DoD health care11 that threatens to undermine its core functions. The pandemic has powerfully demonstrated that degrading the ability of federal health care to agilely and masterfully mobilize in the event of a public health disaster endangers not just veterans and the military but the health and well-being of a nation, particularly its most vulnerable citizens.
1. US Department of Veterans Affairs. About VA: VA mission statement. https://www.va.gov/about_va. Updated April 8, 2020. Accessed August 3, 2020.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA announces ‘Fourth Mission’ actions to help America respond to COVID-19. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5420. Published April 14, 2020. Accessed August 3, 2020.
3. Dyer J. COVID-19 strikes hard at state-run veterans nursing homes. https://www.mdedge.com/fedprac/article/221098/coronavirus-updates/covid-19-strikes-hard-state-run-veterans-nursing-homes. Published April 21, 2020. Accessed August 3, 2020.
4. Leigh D. Coronavirus news: VA secretary addresses COVID-19 deaths among veterans in the tri-state. https://abc7ny.com/va-secretary-veteran-covid-19-deaths-nursing-homes-veterans-memorial-home/6227770. Published June 3, 2020. Accessed August 3, 2020.
5. US Department of Veterans Affairs, Veterans Health Administration. VA Fourth Mission Summary. https://www.va.gov/health/coronavirus/statesupport.asp. Updated August 3, 2020. Accessed August 3, 2020.
6. Sanchez E. BAMC adapts to support greater San Antonio community during COVID-19 pandemic. https://www.health.mil/News/Articles/2020/07/15/BAMC-adapts-to-support-greater-San-Antonio-community-during-COVID-19-pandemic. Published July 17, 2020. Accessed August 3, 2020.
7. US Public Health Service. Commissioned Corps of the U.S. Public Health Service: America’s health responders. https://www.usphs.gov/default.aspx. Accessed August 3, 2020.
8. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med . 2020;35(8):2441-2442. doi:10.1007/s11606-020-05881-4
9. Gordon S, Craven J. The best health system to react to COVID-19. The American Prospect. March 20, 2020. https://prospect.org/coronavirus/the-best-health-system-to-react-to-covid-19. Accessed August 1, 2020.
10. Lessons from the COVID-19 pandemic: it’s time to invest in public health. Fed Pract . 2020;37(suppl 3):S8-S11.
11. Wright O, Zuegel K. COVID-19 shows why military health care shouldn’t be downsized. https://www.militarytimes.com/opinion/commentary/2020/03/31/covid-19-shows-why-military-health-care-shouldnt-be-downsized. Published March 31, 2020. Accessed August 1,2020.
A torrent of blame has deluged the administration’s management of the pandemic. There is though one part of the government that deserves the praise of the nation for its response to this public health crisis—the federal health care system. In this column, we discuss the ways in which the Veterans Health Administration (VHA), the Department of Defense (DoD), and the US Public Health Service (PHS) Commissioned Corps especially have bravely and generously responded to the medical emergency of COVID-19 in the US.
Four missions drive the US Department of Veterans Affairs (VA). Though the fourth of these missions usually is in the background, it has risen to the forefront during the pandemic. To put the fourth mission in its proper perspective, we first should review the other 3 charges given to the largest integrated health care system in the country.
The first mission is to provide the highest quality care possible for the more than 9 million veterans enrolled in that system at each of the 1,255 VHA locations. The second mission is to ensure that the Veterans Benefits Administration delivers the full range of benefits that veterans earned through their service. These including funding for education, loans for homes, and many other types of support that assist service men and women to be successful in their transition from military to civilian life. The third mission is to honor the commitment of those who fought for their country unto death. The National Cemeteries Administration oversees 142 national cemeteries where veterans are buried with dignity and remembered with gratitude for their uniformed service. The purpose of these 3 internally focused missions is to provide a safety net for eligible veterans from the day they separate from the military until the hour they pass from this earth.
The fourth mission is different. This mission looks outside the military family to the civilian world. Its goal is to bolster the ability of the nation as a whole to handle wars, terrorism, national emergencies, and natural disasters. It does this through emergency response plans that preserve the integrity of the 3 other missions to veterans while enhancing the capacity of local and state governments to manage the threat of these public health, safety, or security crises.1
At the same time the VA was aggressively mounting a defense against the threat COVID-19 posed to the other missions, it also launched the fourth mission. In announcing these actions in April 2020, VA Secretary Robert Wilke succinctly summarized the need to balance the fourth mission with the other 3. “VA is committed to helping the nation in this effort to combat COVID-19. Helping veterans is our first mission, but in many locations across the country we’re helping states and local communities. VA is in this fight not only for the millions of veterans we serve each day; we’re in the fight for the people of the United States.”2
During the 2009 H1N1 pandemic I saw firsthand how VA disaster preparedness and emergency training were far superior to many academic and community health care systems. Given VA’s detailed and drilled crisis response plans, its specialized expertise in public health disasters, and its immense resources, it is no wonder that as the virus stretched civilian health care systems, some states turned to the VA for help. At my Albuquerque, New Mexico, VA medical center, 5 medical surgical beds and 3 intensive care beds were opened to the Indian Health Service overwhelmed with cases of COVID-19 in the hard-hit Navajo Nation. In New Jersey where Federal Practitioner is published, the fourth mission reached out to the state-run veterans homes as 90 VA nurses and gerontologists were deployed to 2 of its veterans facilities where close to 150 veterans have died.3 State veterans homes in Massachusetts, Pennsylvania, Alabama, and many other states have received supplies, including direly needed testing and personal protective equipment, staff, technology, and training.4
In July, VA published an impressive summary of fourth mission activities, which I encourage you to read. When you are look at this site, remember with a moment of silent appreciation all the altruistic and courageous VA clinical and administrative staff who volunteered for these assignments many of which put them directly in harm’s way.5
The VA is not alone in answering the call of COVID-19. In March, despite the grave risk to their health, their life, and their families, the USNS Comfort was deployed to New York City to help with its COVID-19 response while the USNS Mercy assisted in the efforts in Los Angeles. More recently, the military deployed > 700 Military Health System medical and support professionals to support COVID-19 operations in both Texas and California. Brooke Army Medical Center in San Antonio has taken on a handful of civilian patients with COVID-19 and increase its level I trauma cases as local hospitals have strained under the caseload.6
For the PHS Commissioned Corps its first mission is to serve as “America’s health responders.”7 This pandemic has intensified the extant health inequities in our country and compounded them with racial injustice and economic disparity. Thus, it is important to recognize that the very purpose of the PHS is to “fight disease, conduct research, and care for patients in underserved communities across the nation.”8 More than 3,900 PHS officers have been deployed nationally and internationally in COVID-19 clinical strike teams. Early in the pandemic the clinical response teams were deployed to a long-term care facility in Kirkland, Washington; convention center-based hospitals in New York City, Detroit, Michigan, and Washington DC, and Navajo Nation facilities. PHS officers also are providing clinical guidance at Bureau of Prison facilities for infection control and personal protective equipment training.
We know that there are many more examples of heroic service by federal health care professionals and staff than we could locate or celebrate in this brief column. Readers of this journal are well aware of the near constant criticism of the VA and calls for privatization,9 the inadequate funding of the PHS,10 and the recent downsizing of DoD health care11 that threatens to undermine its core functions. The pandemic has powerfully demonstrated that degrading the ability of federal health care to agilely and masterfully mobilize in the event of a public health disaster endangers not just veterans and the military but the health and well-being of a nation, particularly its most vulnerable citizens.
A torrent of blame has deluged the administration’s management of the pandemic. There is though one part of the government that deserves the praise of the nation for its response to this public health crisis—the federal health care system. In this column, we discuss the ways in which the Veterans Health Administration (VHA), the Department of Defense (DoD), and the US Public Health Service (PHS) Commissioned Corps especially have bravely and generously responded to the medical emergency of COVID-19 in the US.
Four missions drive the US Department of Veterans Affairs (VA). Though the fourth of these missions usually is in the background, it has risen to the forefront during the pandemic. To put the fourth mission in its proper perspective, we first should review the other 3 charges given to the largest integrated health care system in the country.
The first mission is to provide the highest quality care possible for the more than 9 million veterans enrolled in that system at each of the 1,255 VHA locations. The second mission is to ensure that the Veterans Benefits Administration delivers the full range of benefits that veterans earned through their service. These including funding for education, loans for homes, and many other types of support that assist service men and women to be successful in their transition from military to civilian life. The third mission is to honor the commitment of those who fought for their country unto death. The National Cemeteries Administration oversees 142 national cemeteries where veterans are buried with dignity and remembered with gratitude for their uniformed service. The purpose of these 3 internally focused missions is to provide a safety net for eligible veterans from the day they separate from the military until the hour they pass from this earth.
The fourth mission is different. This mission looks outside the military family to the civilian world. Its goal is to bolster the ability of the nation as a whole to handle wars, terrorism, national emergencies, and natural disasters. It does this through emergency response plans that preserve the integrity of the 3 other missions to veterans while enhancing the capacity of local and state governments to manage the threat of these public health, safety, or security crises.1
At the same time the VA was aggressively mounting a defense against the threat COVID-19 posed to the other missions, it also launched the fourth mission. In announcing these actions in April 2020, VA Secretary Robert Wilke succinctly summarized the need to balance the fourth mission with the other 3. “VA is committed to helping the nation in this effort to combat COVID-19. Helping veterans is our first mission, but in many locations across the country we’re helping states and local communities. VA is in this fight not only for the millions of veterans we serve each day; we’re in the fight for the people of the United States.”2
During the 2009 H1N1 pandemic I saw firsthand how VA disaster preparedness and emergency training were far superior to many academic and community health care systems. Given VA’s detailed and drilled crisis response plans, its specialized expertise in public health disasters, and its immense resources, it is no wonder that as the virus stretched civilian health care systems, some states turned to the VA for help. At my Albuquerque, New Mexico, VA medical center, 5 medical surgical beds and 3 intensive care beds were opened to the Indian Health Service overwhelmed with cases of COVID-19 in the hard-hit Navajo Nation. In New Jersey where Federal Practitioner is published, the fourth mission reached out to the state-run veterans homes as 90 VA nurses and gerontologists were deployed to 2 of its veterans facilities where close to 150 veterans have died.3 State veterans homes in Massachusetts, Pennsylvania, Alabama, and many other states have received supplies, including direly needed testing and personal protective equipment, staff, technology, and training.4
In July, VA published an impressive summary of fourth mission activities, which I encourage you to read. When you are look at this site, remember with a moment of silent appreciation all the altruistic and courageous VA clinical and administrative staff who volunteered for these assignments many of which put them directly in harm’s way.5
The VA is not alone in answering the call of COVID-19. In March, despite the grave risk to their health, their life, and their families, the USNS Comfort was deployed to New York City to help with its COVID-19 response while the USNS Mercy assisted in the efforts in Los Angeles. More recently, the military deployed > 700 Military Health System medical and support professionals to support COVID-19 operations in both Texas and California. Brooke Army Medical Center in San Antonio has taken on a handful of civilian patients with COVID-19 and increase its level I trauma cases as local hospitals have strained under the caseload.6
For the PHS Commissioned Corps its first mission is to serve as “America’s health responders.”7 This pandemic has intensified the extant health inequities in our country and compounded them with racial injustice and economic disparity. Thus, it is important to recognize that the very purpose of the PHS is to “fight disease, conduct research, and care for patients in underserved communities across the nation.”8 More than 3,900 PHS officers have been deployed nationally and internationally in COVID-19 clinical strike teams. Early in the pandemic the clinical response teams were deployed to a long-term care facility in Kirkland, Washington; convention center-based hospitals in New York City, Detroit, Michigan, and Washington DC, and Navajo Nation facilities. PHS officers also are providing clinical guidance at Bureau of Prison facilities for infection control and personal protective equipment training.
We know that there are many more examples of heroic service by federal health care professionals and staff than we could locate or celebrate in this brief column. Readers of this journal are well aware of the near constant criticism of the VA and calls for privatization,9 the inadequate funding of the PHS,10 and the recent downsizing of DoD health care11 that threatens to undermine its core functions. The pandemic has powerfully demonstrated that degrading the ability of federal health care to agilely and masterfully mobilize in the event of a public health disaster endangers not just veterans and the military but the health and well-being of a nation, particularly its most vulnerable citizens.
1. US Department of Veterans Affairs. About VA: VA mission statement. https://www.va.gov/about_va. Updated April 8, 2020. Accessed August 3, 2020.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA announces ‘Fourth Mission’ actions to help America respond to COVID-19. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5420. Published April 14, 2020. Accessed August 3, 2020.
3. Dyer J. COVID-19 strikes hard at state-run veterans nursing homes. https://www.mdedge.com/fedprac/article/221098/coronavirus-updates/covid-19-strikes-hard-state-run-veterans-nursing-homes. Published April 21, 2020. Accessed August 3, 2020.
4. Leigh D. Coronavirus news: VA secretary addresses COVID-19 deaths among veterans in the tri-state. https://abc7ny.com/va-secretary-veteran-covid-19-deaths-nursing-homes-veterans-memorial-home/6227770. Published June 3, 2020. Accessed August 3, 2020.
5. US Department of Veterans Affairs, Veterans Health Administration. VA Fourth Mission Summary. https://www.va.gov/health/coronavirus/statesupport.asp. Updated August 3, 2020. Accessed August 3, 2020.
6. Sanchez E. BAMC adapts to support greater San Antonio community during COVID-19 pandemic. https://www.health.mil/News/Articles/2020/07/15/BAMC-adapts-to-support-greater-San-Antonio-community-during-COVID-19-pandemic. Published July 17, 2020. Accessed August 3, 2020.
7. US Public Health Service. Commissioned Corps of the U.S. Public Health Service: America’s health responders. https://www.usphs.gov/default.aspx. Accessed August 3, 2020.
8. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med . 2020;35(8):2441-2442. doi:10.1007/s11606-020-05881-4
9. Gordon S, Craven J. The best health system to react to COVID-19. The American Prospect. March 20, 2020. https://prospect.org/coronavirus/the-best-health-system-to-react-to-covid-19. Accessed August 1, 2020.
10. Lessons from the COVID-19 pandemic: it’s time to invest in public health. Fed Pract . 2020;37(suppl 3):S8-S11.
11. Wright O, Zuegel K. COVID-19 shows why military health care shouldn’t be downsized. https://www.militarytimes.com/opinion/commentary/2020/03/31/covid-19-shows-why-military-health-care-shouldnt-be-downsized. Published March 31, 2020. Accessed August 1,2020.
1. US Department of Veterans Affairs. About VA: VA mission statement. https://www.va.gov/about_va. Updated April 8, 2020. Accessed August 3, 2020.
2. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA announces ‘Fourth Mission’ actions to help America respond to COVID-19. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5420. Published April 14, 2020. Accessed August 3, 2020.
3. Dyer J. COVID-19 strikes hard at state-run veterans nursing homes. https://www.mdedge.com/fedprac/article/221098/coronavirus-updates/covid-19-strikes-hard-state-run-veterans-nursing-homes. Published April 21, 2020. Accessed August 3, 2020.
4. Leigh D. Coronavirus news: VA secretary addresses COVID-19 deaths among veterans in the tri-state. https://abc7ny.com/va-secretary-veteran-covid-19-deaths-nursing-homes-veterans-memorial-home/6227770. Published June 3, 2020. Accessed August 3, 2020.
5. US Department of Veterans Affairs, Veterans Health Administration. VA Fourth Mission Summary. https://www.va.gov/health/coronavirus/statesupport.asp. Updated August 3, 2020. Accessed August 3, 2020.
6. Sanchez E. BAMC adapts to support greater San Antonio community during COVID-19 pandemic. https://www.health.mil/News/Articles/2020/07/15/BAMC-adapts-to-support-greater-San-Antonio-community-during-COVID-19-pandemic. Published July 17, 2020. Accessed August 3, 2020.
7. US Public Health Service. Commissioned Corps of the U.S. Public Health Service: America’s health responders. https://www.usphs.gov/default.aspx. Accessed August 3, 2020.
8. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med . 2020;35(8):2441-2442. doi:10.1007/s11606-020-05881-4
9. Gordon S, Craven J. The best health system to react to COVID-19. The American Prospect. March 20, 2020. https://prospect.org/coronavirus/the-best-health-system-to-react-to-covid-19. Accessed August 1, 2020.
10. Lessons from the COVID-19 pandemic: it’s time to invest in public health. Fed Pract . 2020;37(suppl 3):S8-S11.
11. Wright O, Zuegel K. COVID-19 shows why military health care shouldn’t be downsized. https://www.militarytimes.com/opinion/commentary/2020/03/31/covid-19-shows-why-military-health-care-shouldnt-be-downsized. Published March 31, 2020. Accessed August 1,2020.
Since COVID-19 onset, admissions for MI are down, mortality rates are up
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
FROM JAMA CARDIOLOGY
Swallowable ‘sponge on string’ to diagnose esophageal cancer
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
This article first appeared on Medscape.com.