User login
Action and awareness are needed to increase immunization rates
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
FDA approves viltolarsen (Viltepso) for Duchenne muscular dystrophy
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
PHM20 Virtual: Common incidental findings seen on pediatric imaging
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
A ‘foolproof’ way to diagnose narrow complex tachycardias on EKGs
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.
she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
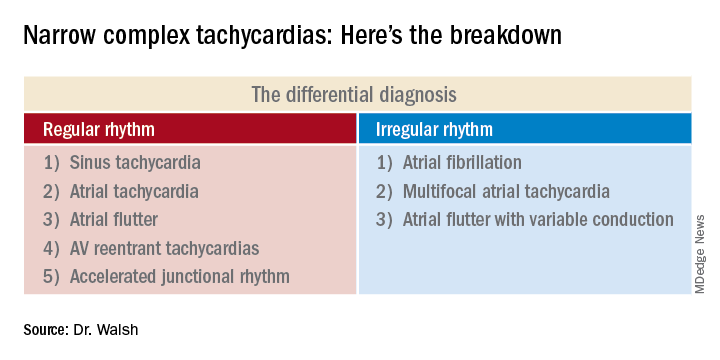
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.

she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.

she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
FROM HM20 VIRTUAL
Determining cause of skin lesions in COVID-19 patients remains challenging
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Multiple traits more common in difficult-to-treat patients with migraine
Overall, insufficient responders—patients less likely to get relief shortly after acute treatment—are “more medically and psychosocially complex,” wrote the authors of the study, which appeared in the July/August issue of Headache.
Common characteristics of insufficient responders
The researchers, led by Louise Lombard, M Nutr, of Eli Lilly and Company, analyzed data from a 2014 cross-sectional survey. They tracked 583 patients with migraine, including 200 (34%) who were considered insufficient responders because they failed to achieve freedom from pain within 2 hours of acute treatment in at least four of five attacks.
The insufficient and sufficient responder groups were similar in age (mean = 40 for both) and gender (80% and 75% female, respectively, P = .170) and race (72% and 77% white, P = .279).
However, insufficient responders were clearly more affected by headaches, multiple treatments, and other burdens. Compared with those who had better responses to treatment, they were more likely to have four or more migraine headache days per month (46% vs. 31%), rebound or medication-overuse headaches (16% vs. 7%) and chronic migraine (12% vs. 5%, all P < .05).
They were also more likely have comorbid depression (38% vs. 22%) and psychological conditions other than depression and anxiety (8% vs. 4%, all P < .05).
As for treatment, insufficient response was higher in patients who waited until the appearance of pain to take medication (odds ratio = 1.83, 95% confidence interval [CI] 1.15–2.92, P = .011, after adjustment for covariates). And insufficient responders were more likely to have been prescribed at least three unique preventive regimens (12% vs. 6%), to take over-the-counter medications (50% vs. 38%) and to take opioid painkillers (16% vs. 8%, all P < .05).
The authors, who caution that the study does not prove cause and effect, wrote that insufficient responders “may benefit from education on how and when to use current treatments.”
Managing insufficient responders
Neurology Reviews editor-in-chief Alan M. Rapoport, MD, said the study “confirms a lot of what we knew.” Dr, Rapoport, who was not involved in the study, is clinical professor of neurology at the University of California, Los Angeles.
“As expected, the insufficient responders used more opioids and over-the-counter medications, which is not the ideal way to treat migraine,” he said. “That probably caused them to have medication-overuse headache, which might have caused them to respond poorly to even the best treatment regimen. They also had more severe symptoms, more comorbidities, and a poorer quality of life. They also had more impairment and greater impact on work, with more of them unemployed.”
The insufficient responders also “took medication at the time or after the pain began, rather than before it when they thought the attack was beginning due to premonitory symptoms,” he said.
Dr. Rapoport also noted a surprising and unusual finding: Patients who did not report sensitivity to light as their most bothersome symptom were more likely to be insufficient responders (OR = 2.3, 95% CI [1.21–4.37], P = .011). “In all recent migraine studies,” he said, “the majority of patients selected photophobia as their most bothersome symptom.”
In the big picture, he said, the study suggests that “a third triptan does not seem to work better than the first two, patients with medication-overuse headache and chronic migraine and those not on preventive medication do not respond that well to acute care treatment, and the same is true when depression is present.”
No study funding was reported. Four study authors reported ties with Eli Lilly, and two reported employment by Adelphi Real World, which provided the survey results..
SOURCE: Lombard L et al. Headache. 2020;60(7):1325-39. doi: 10.1111/head.13835.
Overall, insufficient responders—patients less likely to get relief shortly after acute treatment—are “more medically and psychosocially complex,” wrote the authors of the study, which appeared in the July/August issue of Headache.
Common characteristics of insufficient responders
The researchers, led by Louise Lombard, M Nutr, of Eli Lilly and Company, analyzed data from a 2014 cross-sectional survey. They tracked 583 patients with migraine, including 200 (34%) who were considered insufficient responders because they failed to achieve freedom from pain within 2 hours of acute treatment in at least four of five attacks.
The insufficient and sufficient responder groups were similar in age (mean = 40 for both) and gender (80% and 75% female, respectively, P = .170) and race (72% and 77% white, P = .279).
However, insufficient responders were clearly more affected by headaches, multiple treatments, and other burdens. Compared with those who had better responses to treatment, they were more likely to have four or more migraine headache days per month (46% vs. 31%), rebound or medication-overuse headaches (16% vs. 7%) and chronic migraine (12% vs. 5%, all P < .05).
They were also more likely have comorbid depression (38% vs. 22%) and psychological conditions other than depression and anxiety (8% vs. 4%, all P < .05).
As for treatment, insufficient response was higher in patients who waited until the appearance of pain to take medication (odds ratio = 1.83, 95% confidence interval [CI] 1.15–2.92, P = .011, after adjustment for covariates). And insufficient responders were more likely to have been prescribed at least three unique preventive regimens (12% vs. 6%), to take over-the-counter medications (50% vs. 38%) and to take opioid painkillers (16% vs. 8%, all P < .05).
The authors, who caution that the study does not prove cause and effect, wrote that insufficient responders “may benefit from education on how and when to use current treatments.”
Managing insufficient responders
Neurology Reviews editor-in-chief Alan M. Rapoport, MD, said the study “confirms a lot of what we knew.” Dr, Rapoport, who was not involved in the study, is clinical professor of neurology at the University of California, Los Angeles.
“As expected, the insufficient responders used more opioids and over-the-counter medications, which is not the ideal way to treat migraine,” he said. “That probably caused them to have medication-overuse headache, which might have caused them to respond poorly to even the best treatment regimen. They also had more severe symptoms, more comorbidities, and a poorer quality of life. They also had more impairment and greater impact on work, with more of them unemployed.”
The insufficient responders also “took medication at the time or after the pain began, rather than before it when they thought the attack was beginning due to premonitory symptoms,” he said.
Dr. Rapoport also noted a surprising and unusual finding: Patients who did not report sensitivity to light as their most bothersome symptom were more likely to be insufficient responders (OR = 2.3, 95% CI [1.21–4.37], P = .011). “In all recent migraine studies,” he said, “the majority of patients selected photophobia as their most bothersome symptom.”
In the big picture, he said, the study suggests that “a third triptan does not seem to work better than the first two, patients with medication-overuse headache and chronic migraine and those not on preventive medication do not respond that well to acute care treatment, and the same is true when depression is present.”
No study funding was reported. Four study authors reported ties with Eli Lilly, and two reported employment by Adelphi Real World, which provided the survey results..
SOURCE: Lombard L et al. Headache. 2020;60(7):1325-39. doi: 10.1111/head.13835.
Overall, insufficient responders—patients less likely to get relief shortly after acute treatment—are “more medically and psychosocially complex,” wrote the authors of the study, which appeared in the July/August issue of Headache.
Common characteristics of insufficient responders
The researchers, led by Louise Lombard, M Nutr, of Eli Lilly and Company, analyzed data from a 2014 cross-sectional survey. They tracked 583 patients with migraine, including 200 (34%) who were considered insufficient responders because they failed to achieve freedom from pain within 2 hours of acute treatment in at least four of five attacks.
The insufficient and sufficient responder groups were similar in age (mean = 40 for both) and gender (80% and 75% female, respectively, P = .170) and race (72% and 77% white, P = .279).
However, insufficient responders were clearly more affected by headaches, multiple treatments, and other burdens. Compared with those who had better responses to treatment, they were more likely to have four or more migraine headache days per month (46% vs. 31%), rebound or medication-overuse headaches (16% vs. 7%) and chronic migraine (12% vs. 5%, all P < .05).
They were also more likely have comorbid depression (38% vs. 22%) and psychological conditions other than depression and anxiety (8% vs. 4%, all P < .05).
As for treatment, insufficient response was higher in patients who waited until the appearance of pain to take medication (odds ratio = 1.83, 95% confidence interval [CI] 1.15–2.92, P = .011, after adjustment for covariates). And insufficient responders were more likely to have been prescribed at least three unique preventive regimens (12% vs. 6%), to take over-the-counter medications (50% vs. 38%) and to take opioid painkillers (16% vs. 8%, all P < .05).
The authors, who caution that the study does not prove cause and effect, wrote that insufficient responders “may benefit from education on how and when to use current treatments.”
Managing insufficient responders
Neurology Reviews editor-in-chief Alan M. Rapoport, MD, said the study “confirms a lot of what we knew.” Dr, Rapoport, who was not involved in the study, is clinical professor of neurology at the University of California, Los Angeles.
“As expected, the insufficient responders used more opioids and over-the-counter medications, which is not the ideal way to treat migraine,” he said. “That probably caused them to have medication-overuse headache, which might have caused them to respond poorly to even the best treatment regimen. They also had more severe symptoms, more comorbidities, and a poorer quality of life. They also had more impairment and greater impact on work, with more of them unemployed.”
The insufficient responders also “took medication at the time or after the pain began, rather than before it when they thought the attack was beginning due to premonitory symptoms,” he said.
Dr. Rapoport also noted a surprising and unusual finding: Patients who did not report sensitivity to light as their most bothersome symptom were more likely to be insufficient responders (OR = 2.3, 95% CI [1.21–4.37], P = .011). “In all recent migraine studies,” he said, “the majority of patients selected photophobia as their most bothersome symptom.”
In the big picture, he said, the study suggests that “a third triptan does not seem to work better than the first two, patients with medication-overuse headache and chronic migraine and those not on preventive medication do not respond that well to acute care treatment, and the same is true when depression is present.”
No study funding was reported. Four study authors reported ties with Eli Lilly, and two reported employment by Adelphi Real World, which provided the survey results..
SOURCE: Lombard L et al. Headache. 2020;60(7):1325-39. doi: 10.1111/head.13835.
FROM HEADACHE
Persistent hair loss after radiation improved with minoxidil
The first study to systematically address the problem of persistent hair loss in patients who undergo radiation to the scalp for central nervous system or head and neck tumors has found that treatment with topical minoxidil leads to improvement in hair loss.
The study was published online on Aug. 5 in JAMA Dermatology.
Minoxidil has been used for many years to treat age-associated baldness in men, noted the authors. It was used off label in this study to treat radiation-associated persistent hair loss; 82% of patients showed at least some improvement.
For patients who do not improve with minoxidil, hair transplant and scalp reconstruction with plastic surgery were other options, the authors comment.
“Almost in all instances, there is something that can be done to improve persistent hair loss after radiation and give patients a sense of control,” senior author Mario E. Lacouture, MD, said in an interview. He is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York City.
About 60% of people with CNS tumors and 30% with head and neck cancer receive radiation to the head, and 75%-100% of these patients experience acute hair loss. For many, hair grows back in 2-3 months. However, for about 60%, hair loss persists for 6 or more months after completion of radiotherapy, the authors note.
In past work, Dr. Lacouture and colleagues found that persistent hair loss in cancer survivors is associated with depression, anxiety, and psychosocial distress.
“There are therapies and procedures that may mitigate persistent radiation therapy hair loss that can bring back psychosocial well-being to many of these patients,” Dr. Lacouture said. “These approaches are likely underutilized because patients are not being referred to specialists in hair or scalp reconstruction.”
Specialists can be found through the International Society for Hair Restoration Surgery and the American Academy of Dermatology, he added.
Study details
The retrospective cohort study included 71 children and adults who developed persistent hair loss after radiotherapy for primary CNS tumors (90%, n = 64) or head and neck sarcoma (10%, n = 7). The median age of the patients was 27 years (range, 4-75 years); 72% (n = 51) were female and 82% (n = 58) were White.
These patients had been treated at Memorial Sloan Kettering Cancer Center in New York City or St. Jude Children’s Hospital in Memphis from January 2011 to January 2019.
The team analyzed standardized clinical photographs of the scalp using the Common Terminology Criteria for Adverse Events version 5.0. Grade 1 alopecia was defined as hair loss of less than 50% of normal that does not require camouflage with hair pieces, scarves, or similar items. Grade 2 alopecia was defined as hair loss greater than 50% of normal that requires camouflage and is associated with negative psychosocial effects.
Over half of patients (56%, 40/70) had grade 1 hair loss. Clinical images were available for 54 patients; for most of these patients, hair loss was attributable to radiation alone (74%, n = 40). Evaluation of clinical imaging showed three variants of hair loss: localized (54%, 29/54), diffuse (24%, 13/54), and mixed pattern (22%, 12/54). Data on dermatologic imaging of the scalp (trichoscopy) were available for 28 patients; the main finding was white patches (57%, 16/28).
The median scalp radiation dose was 39.6 Gy (range, 15.1-50.0 Gy). The researchers estimate that a dose of 36.1 Gy (95% CI, 33.7-39.6 Gy) was sufficient to induce grade 2 hair loss in 50% of patients.
Severity of hair loss appeared to increase with radiation dose. For every 1-unit increase in radiation dose, the odds of grade 2 hair loss increased by 15% (odds ratio, 1.15; 95% CI, 1.04-1.28; P < .001). Proton irradiation was associated with even higher odds of severe hair loss (OR, 5.7; 95% CI, 1.05-30.8; P < .001). Results remained significant when analyses were controlled for sex, age at time of radiotherapy, and concurrent chemotherapy.
The majority of evaluable patients who were treated with topical minoxidil (5%) twice daily showed a response (82%, 28/34) during a median follow-up of 61 weeks (interquartile range, 21-105 weeks).
Among 25 of these patients for whom clinical images were available, 16% (4/25) showed complete response. Two patients improved with hair transplant, and one showed complete response with plastic surgery reconstruction of the hair.
The study had several limitations, including its retrospective design and a lack of complete data for certain variables, such as standardized clinical photographs, trichoscopy images, and radiotherapy treatment plans.
On the basis of these results, the authors are seeking funding for a prospective study of the use of minoxidil for persistent radiation-induced alopecia.
The study was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center. One or more authors has relationships with pharmaceutical companies, as listed in the original article.
This article first appeared on Medscape.com.
The first study to systematically address the problem of persistent hair loss in patients who undergo radiation to the scalp for central nervous system or head and neck tumors has found that treatment with topical minoxidil leads to improvement in hair loss.
The study was published online on Aug. 5 in JAMA Dermatology.
Minoxidil has been used for many years to treat age-associated baldness in men, noted the authors. It was used off label in this study to treat radiation-associated persistent hair loss; 82% of patients showed at least some improvement.
For patients who do not improve with minoxidil, hair transplant and scalp reconstruction with plastic surgery were other options, the authors comment.
“Almost in all instances, there is something that can be done to improve persistent hair loss after radiation and give patients a sense of control,” senior author Mario E. Lacouture, MD, said in an interview. He is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York City.
About 60% of people with CNS tumors and 30% with head and neck cancer receive radiation to the head, and 75%-100% of these patients experience acute hair loss. For many, hair grows back in 2-3 months. However, for about 60%, hair loss persists for 6 or more months after completion of radiotherapy, the authors note.
In past work, Dr. Lacouture and colleagues found that persistent hair loss in cancer survivors is associated with depression, anxiety, and psychosocial distress.
“There are therapies and procedures that may mitigate persistent radiation therapy hair loss that can bring back psychosocial well-being to many of these patients,” Dr. Lacouture said. “These approaches are likely underutilized because patients are not being referred to specialists in hair or scalp reconstruction.”
Specialists can be found through the International Society for Hair Restoration Surgery and the American Academy of Dermatology, he added.
Study details
The retrospective cohort study included 71 children and adults who developed persistent hair loss after radiotherapy for primary CNS tumors (90%, n = 64) or head and neck sarcoma (10%, n = 7). The median age of the patients was 27 years (range, 4-75 years); 72% (n = 51) were female and 82% (n = 58) were White.
These patients had been treated at Memorial Sloan Kettering Cancer Center in New York City or St. Jude Children’s Hospital in Memphis from January 2011 to January 2019.
The team analyzed standardized clinical photographs of the scalp using the Common Terminology Criteria for Adverse Events version 5.0. Grade 1 alopecia was defined as hair loss of less than 50% of normal that does not require camouflage with hair pieces, scarves, or similar items. Grade 2 alopecia was defined as hair loss greater than 50% of normal that requires camouflage and is associated with negative psychosocial effects.
Over half of patients (56%, 40/70) had grade 1 hair loss. Clinical images were available for 54 patients; for most of these patients, hair loss was attributable to radiation alone (74%, n = 40). Evaluation of clinical imaging showed three variants of hair loss: localized (54%, 29/54), diffuse (24%, 13/54), and mixed pattern (22%, 12/54). Data on dermatologic imaging of the scalp (trichoscopy) were available for 28 patients; the main finding was white patches (57%, 16/28).
The median scalp radiation dose was 39.6 Gy (range, 15.1-50.0 Gy). The researchers estimate that a dose of 36.1 Gy (95% CI, 33.7-39.6 Gy) was sufficient to induce grade 2 hair loss in 50% of patients.
Severity of hair loss appeared to increase with radiation dose. For every 1-unit increase in radiation dose, the odds of grade 2 hair loss increased by 15% (odds ratio, 1.15; 95% CI, 1.04-1.28; P < .001). Proton irradiation was associated with even higher odds of severe hair loss (OR, 5.7; 95% CI, 1.05-30.8; P < .001). Results remained significant when analyses were controlled for sex, age at time of radiotherapy, and concurrent chemotherapy.
The majority of evaluable patients who were treated with topical minoxidil (5%) twice daily showed a response (82%, 28/34) during a median follow-up of 61 weeks (interquartile range, 21-105 weeks).
Among 25 of these patients for whom clinical images were available, 16% (4/25) showed complete response. Two patients improved with hair transplant, and one showed complete response with plastic surgery reconstruction of the hair.
The study had several limitations, including its retrospective design and a lack of complete data for certain variables, such as standardized clinical photographs, trichoscopy images, and radiotherapy treatment plans.
On the basis of these results, the authors are seeking funding for a prospective study of the use of minoxidil for persistent radiation-induced alopecia.
The study was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center. One or more authors has relationships with pharmaceutical companies, as listed in the original article.
This article first appeared on Medscape.com.
The first study to systematically address the problem of persistent hair loss in patients who undergo radiation to the scalp for central nervous system or head and neck tumors has found that treatment with topical minoxidil leads to improvement in hair loss.
The study was published online on Aug. 5 in JAMA Dermatology.
Minoxidil has been used for many years to treat age-associated baldness in men, noted the authors. It was used off label in this study to treat radiation-associated persistent hair loss; 82% of patients showed at least some improvement.
For patients who do not improve with minoxidil, hair transplant and scalp reconstruction with plastic surgery were other options, the authors comment.
“Almost in all instances, there is something that can be done to improve persistent hair loss after radiation and give patients a sense of control,” senior author Mario E. Lacouture, MD, said in an interview. He is director of the Oncodermatology Program at Memorial Sloan Kettering Cancer Center in New York City.
About 60% of people with CNS tumors and 30% with head and neck cancer receive radiation to the head, and 75%-100% of these patients experience acute hair loss. For many, hair grows back in 2-3 months. However, for about 60%, hair loss persists for 6 or more months after completion of radiotherapy, the authors note.
In past work, Dr. Lacouture and colleagues found that persistent hair loss in cancer survivors is associated with depression, anxiety, and psychosocial distress.
“There are therapies and procedures that may mitigate persistent radiation therapy hair loss that can bring back psychosocial well-being to many of these patients,” Dr. Lacouture said. “These approaches are likely underutilized because patients are not being referred to specialists in hair or scalp reconstruction.”
Specialists can be found through the International Society for Hair Restoration Surgery and the American Academy of Dermatology, he added.
Study details
The retrospective cohort study included 71 children and adults who developed persistent hair loss after radiotherapy for primary CNS tumors (90%, n = 64) or head and neck sarcoma (10%, n = 7). The median age of the patients was 27 years (range, 4-75 years); 72% (n = 51) were female and 82% (n = 58) were White.
These patients had been treated at Memorial Sloan Kettering Cancer Center in New York City or St. Jude Children’s Hospital in Memphis from January 2011 to January 2019.
The team analyzed standardized clinical photographs of the scalp using the Common Terminology Criteria for Adverse Events version 5.0. Grade 1 alopecia was defined as hair loss of less than 50% of normal that does not require camouflage with hair pieces, scarves, or similar items. Grade 2 alopecia was defined as hair loss greater than 50% of normal that requires camouflage and is associated with negative psychosocial effects.
Over half of patients (56%, 40/70) had grade 1 hair loss. Clinical images were available for 54 patients; for most of these patients, hair loss was attributable to radiation alone (74%, n = 40). Evaluation of clinical imaging showed three variants of hair loss: localized (54%, 29/54), diffuse (24%, 13/54), and mixed pattern (22%, 12/54). Data on dermatologic imaging of the scalp (trichoscopy) were available for 28 patients; the main finding was white patches (57%, 16/28).
The median scalp radiation dose was 39.6 Gy (range, 15.1-50.0 Gy). The researchers estimate that a dose of 36.1 Gy (95% CI, 33.7-39.6 Gy) was sufficient to induce grade 2 hair loss in 50% of patients.
Severity of hair loss appeared to increase with radiation dose. For every 1-unit increase in radiation dose, the odds of grade 2 hair loss increased by 15% (odds ratio, 1.15; 95% CI, 1.04-1.28; P < .001). Proton irradiation was associated with even higher odds of severe hair loss (OR, 5.7; 95% CI, 1.05-30.8; P < .001). Results remained significant when analyses were controlled for sex, age at time of radiotherapy, and concurrent chemotherapy.
The majority of evaluable patients who were treated with topical minoxidil (5%) twice daily showed a response (82%, 28/34) during a median follow-up of 61 weeks (interquartile range, 21-105 weeks).
Among 25 of these patients for whom clinical images were available, 16% (4/25) showed complete response. Two patients improved with hair transplant, and one showed complete response with plastic surgery reconstruction of the hair.
The study had several limitations, including its retrospective design and a lack of complete data for certain variables, such as standardized clinical photographs, trichoscopy images, and radiotherapy treatment plans.
On the basis of these results, the authors are seeking funding for a prospective study of the use of minoxidil for persistent radiation-induced alopecia.
The study was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center. One or more authors has relationships with pharmaceutical companies, as listed in the original article.
This article first appeared on Medscape.com.
RA patients show decreased risk for new-onset type 2 diabetes
Patients with RA were at lower risk for developing incident type 2 diabetes mellitus (T2DM) in comparison with patients with hypertension, psoriatic arthritis (PsA), or osteoarthritis, as well as the general population without RA in a retrospective cohort study of a large, nationwide, commercial health insurance claims database.
This result goes against what the study researchers from the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, initially hypothesized: The “risk of incident T2DM in RA patients would be similar to or less than PsA and [hypertension] patients, but higher, compared to general non-RA and OA patients.”
Prior epidemiologic studies of the relationship between RA and incident diabetes have yielded inconclusive results suggesting a small increase or no increase in risk of T2DM in patients with RA, possibly because of differences in the risk of T2DM in comparison groups used by previous studies to calculate relative risk, first author Yinzhu Jin and colleagues noted in their report published in Arthritis Care & Research.
After mining a nationwide U.S. commercial health insurance claims database, the Optum Clinformatics Data Mart, for claims data from Jan. 1, 2005, to Dec. 31, 2017, the researchers matched a total of 108,568 patients in RA, general population non-RA, hypertension, and OA cohorts based on age, sex, and index date (the date of disease-specific medication dispensing). Overall, 77% of those patients were female and had a mean age of nearly 56 years, whereas 48% of patients with PsA were female and their mean age was nearly 49 years. (PsA patients were not matched because of smaller numbers.)
During a median follow-up period of 1.4-1.8 years across the comparison groups, the crude incidence rate for diabetes per 1,000 person-years in the cohorts was 7.0 for RA, 7.4 for general non-RA, 12.3 for hypertension, 7.8 for OA, and 9.9 for PsA. The hazard ratios and 95% confidence interval for risk of diabetes in patients with RA – after adjustment for more than 40 baseline covariates that included demographics, comorbidities, medication use, and health care utilization – was 0.72 (0.66-0.78) in comparison withh the general non-RA cohort, 0.65 (0.60-0.71) in comparison with the hypertension cohort, 0.75 (0.69-0.81) in comparison with the OA cohort, and 0.76 (0.67-0.86) in comparison with the PsA cohort. These values correspond to RA patients having a 24%-35% lower risk of incident diabetes versus the comparison groups, the researchers noted. They observed results consistent to these when they conducted a sensitivity analysis using a 1-year lag time from the index date before starting follow-up.
The lower risk of T2DM in patients with RA in comparison with patients in the non-RA cohort “may be, in part, due to the effect of biologic DMARD [disease-modifying antirheumatic drug] treatment in RA which likely modifies the risk of DM,” the researchers wrote. “Both the increasing use of biologic DMARDs for RA in the U.S. over the last decade and our cohort entry criteria for the RA cohort (i.e., at least one dispensing of a DMARD) may explain the finding of the lower risk of DM in RA.”
The results found with the other three cohorts did not surprise the researchers. The reduced risk of diabetes among RA patients versus those with OA jibes with “higher rates of obesity and other comorbidities in patients with OA” as well as findings from a recent study that found a higher incidence rate of diabetes in OA, compared with RA. Ms. Jin and colleagues also acknowledged it is well known that “hypertension and PsA are associated with metabolic dysregulation and increase the risk of diabetes.”
The researchers defined patients with RA as having at least twoinpatient or outpatient ICD-9 or ICD-10 diagnosis codes of RA, separated by 7-365 days and having at least one dispensing for DMARDs within 1 year from the first RA diagnosis date, and defined the primary outcome of incident T2DM as at least one inpatient or outpatient diagnosis of T2DM plus at least one dispensing of an antidiabetic drug. They set the general non-RA cohort by selecting patients with any inpatient or outpatient diagnosis codes and a dispensing of any medications, and the hypertension, PsA, and OA comparator groups as having at least two inpatient or outpatient disease-specific ICD-9/ICD-10 codes separated by 7-365 days and at least one dispensing of disease-specific medication within 1 year from the first diagnosis date. They excluded patients with RA, PsA, or psoriasis diagnosis or disease-specific medication dispensing any time prior to or on the index date (the date of disease-specific medication dispensing).
The researchers recognized that the conclusions that can be drawn from the study are limited by the “potential misclassification of cohorts and covariates” because they “mainly used diagnosis codes and pharmacy dispensing records in claims data,” and some “important covariates such as baseline obesity are likely underreported and not adequately captured in claims data.” The level of covariate misclassification also may have been different across the study cohorts on “unmeasured covariates such as body mass index, diet, and physical activity, as well as disease specific measures,” thus introducing residual confounding. They also could not “examine potential difference in the risk of T2DM in untreated or undertreated RA patients” because “RA and all the non-RA comparator cohorts were required to use a disease-specific drug,” they wrote.
“While systemic inflammation in RA is thought to increase the risk of [cardiovascular disease] and cardiovascular risk factors such as DM, our findings suggest having RA itself does not confer an increased risk of DM. Future study should determine whether untreated RA or undertreated RA is associated with a greater risk of developing DM,” the researchers concluded.
The study was supported by a research grant from Bristol-Myers Squibb, which “played no role in the study design, data analysis or interpretation of data or presentation of results,” the researchers said. The company was “given the opportunity to make nonbinding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.” One author reported receiving research grants from Brigham and Women’s Hospital from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche for unrelated topics.
SOURCE: Jin Y et al. Arthritis Care Res. 2020 Aug 4. doi: 10.1002/acr.24343.
Patients with RA were at lower risk for developing incident type 2 diabetes mellitus (T2DM) in comparison with patients with hypertension, psoriatic arthritis (PsA), or osteoarthritis, as well as the general population without RA in a retrospective cohort study of a large, nationwide, commercial health insurance claims database.
This result goes against what the study researchers from the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, initially hypothesized: The “risk of incident T2DM in RA patients would be similar to or less than PsA and [hypertension] patients, but higher, compared to general non-RA and OA patients.”
Prior epidemiologic studies of the relationship between RA and incident diabetes have yielded inconclusive results suggesting a small increase or no increase in risk of T2DM in patients with RA, possibly because of differences in the risk of T2DM in comparison groups used by previous studies to calculate relative risk, first author Yinzhu Jin and colleagues noted in their report published in Arthritis Care & Research.
After mining a nationwide U.S. commercial health insurance claims database, the Optum Clinformatics Data Mart, for claims data from Jan. 1, 2005, to Dec. 31, 2017, the researchers matched a total of 108,568 patients in RA, general population non-RA, hypertension, and OA cohorts based on age, sex, and index date (the date of disease-specific medication dispensing). Overall, 77% of those patients were female and had a mean age of nearly 56 years, whereas 48% of patients with PsA were female and their mean age was nearly 49 years. (PsA patients were not matched because of smaller numbers.)
During a median follow-up period of 1.4-1.8 years across the comparison groups, the crude incidence rate for diabetes per 1,000 person-years in the cohorts was 7.0 for RA, 7.4 for general non-RA, 12.3 for hypertension, 7.8 for OA, and 9.9 for PsA. The hazard ratios and 95% confidence interval for risk of diabetes in patients with RA – after adjustment for more than 40 baseline covariates that included demographics, comorbidities, medication use, and health care utilization – was 0.72 (0.66-0.78) in comparison withh the general non-RA cohort, 0.65 (0.60-0.71) in comparison with the hypertension cohort, 0.75 (0.69-0.81) in comparison with the OA cohort, and 0.76 (0.67-0.86) in comparison with the PsA cohort. These values correspond to RA patients having a 24%-35% lower risk of incident diabetes versus the comparison groups, the researchers noted. They observed results consistent to these when they conducted a sensitivity analysis using a 1-year lag time from the index date before starting follow-up.
The lower risk of T2DM in patients with RA in comparison with patients in the non-RA cohort “may be, in part, due to the effect of biologic DMARD [disease-modifying antirheumatic drug] treatment in RA which likely modifies the risk of DM,” the researchers wrote. “Both the increasing use of biologic DMARDs for RA in the U.S. over the last decade and our cohort entry criteria for the RA cohort (i.e., at least one dispensing of a DMARD) may explain the finding of the lower risk of DM in RA.”
The results found with the other three cohorts did not surprise the researchers. The reduced risk of diabetes among RA patients versus those with OA jibes with “higher rates of obesity and other comorbidities in patients with OA” as well as findings from a recent study that found a higher incidence rate of diabetes in OA, compared with RA. Ms. Jin and colleagues also acknowledged it is well known that “hypertension and PsA are associated with metabolic dysregulation and increase the risk of diabetes.”
The researchers defined patients with RA as having at least twoinpatient or outpatient ICD-9 or ICD-10 diagnosis codes of RA, separated by 7-365 days and having at least one dispensing for DMARDs within 1 year from the first RA diagnosis date, and defined the primary outcome of incident T2DM as at least one inpatient or outpatient diagnosis of T2DM plus at least one dispensing of an antidiabetic drug. They set the general non-RA cohort by selecting patients with any inpatient or outpatient diagnosis codes and a dispensing of any medications, and the hypertension, PsA, and OA comparator groups as having at least two inpatient or outpatient disease-specific ICD-9/ICD-10 codes separated by 7-365 days and at least one dispensing of disease-specific medication within 1 year from the first diagnosis date. They excluded patients with RA, PsA, or psoriasis diagnosis or disease-specific medication dispensing any time prior to or on the index date (the date of disease-specific medication dispensing).
The researchers recognized that the conclusions that can be drawn from the study are limited by the “potential misclassification of cohorts and covariates” because they “mainly used diagnosis codes and pharmacy dispensing records in claims data,” and some “important covariates such as baseline obesity are likely underreported and not adequately captured in claims data.” The level of covariate misclassification also may have been different across the study cohorts on “unmeasured covariates such as body mass index, diet, and physical activity, as well as disease specific measures,” thus introducing residual confounding. They also could not “examine potential difference in the risk of T2DM in untreated or undertreated RA patients” because “RA and all the non-RA comparator cohorts were required to use a disease-specific drug,” they wrote.
“While systemic inflammation in RA is thought to increase the risk of [cardiovascular disease] and cardiovascular risk factors such as DM, our findings suggest having RA itself does not confer an increased risk of DM. Future study should determine whether untreated RA or undertreated RA is associated with a greater risk of developing DM,” the researchers concluded.
The study was supported by a research grant from Bristol-Myers Squibb, which “played no role in the study design, data analysis or interpretation of data or presentation of results,” the researchers said. The company was “given the opportunity to make nonbinding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.” One author reported receiving research grants from Brigham and Women’s Hospital from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche for unrelated topics.
SOURCE: Jin Y et al. Arthritis Care Res. 2020 Aug 4. doi: 10.1002/acr.24343.
Patients with RA were at lower risk for developing incident type 2 diabetes mellitus (T2DM) in comparison with patients with hypertension, psoriatic arthritis (PsA), or osteoarthritis, as well as the general population without RA in a retrospective cohort study of a large, nationwide, commercial health insurance claims database.
This result goes against what the study researchers from the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, initially hypothesized: The “risk of incident T2DM in RA patients would be similar to or less than PsA and [hypertension] patients, but higher, compared to general non-RA and OA patients.”
Prior epidemiologic studies of the relationship between RA and incident diabetes have yielded inconclusive results suggesting a small increase or no increase in risk of T2DM in patients with RA, possibly because of differences in the risk of T2DM in comparison groups used by previous studies to calculate relative risk, first author Yinzhu Jin and colleagues noted in their report published in Arthritis Care & Research.
After mining a nationwide U.S. commercial health insurance claims database, the Optum Clinformatics Data Mart, for claims data from Jan. 1, 2005, to Dec. 31, 2017, the researchers matched a total of 108,568 patients in RA, general population non-RA, hypertension, and OA cohorts based on age, sex, and index date (the date of disease-specific medication dispensing). Overall, 77% of those patients were female and had a mean age of nearly 56 years, whereas 48% of patients with PsA were female and their mean age was nearly 49 years. (PsA patients were not matched because of smaller numbers.)
During a median follow-up period of 1.4-1.8 years across the comparison groups, the crude incidence rate for diabetes per 1,000 person-years in the cohorts was 7.0 for RA, 7.4 for general non-RA, 12.3 for hypertension, 7.8 for OA, and 9.9 for PsA. The hazard ratios and 95% confidence interval for risk of diabetes in patients with RA – after adjustment for more than 40 baseline covariates that included demographics, comorbidities, medication use, and health care utilization – was 0.72 (0.66-0.78) in comparison withh the general non-RA cohort, 0.65 (0.60-0.71) in comparison with the hypertension cohort, 0.75 (0.69-0.81) in comparison with the OA cohort, and 0.76 (0.67-0.86) in comparison with the PsA cohort. These values correspond to RA patients having a 24%-35% lower risk of incident diabetes versus the comparison groups, the researchers noted. They observed results consistent to these when they conducted a sensitivity analysis using a 1-year lag time from the index date before starting follow-up.
The lower risk of T2DM in patients with RA in comparison with patients in the non-RA cohort “may be, in part, due to the effect of biologic DMARD [disease-modifying antirheumatic drug] treatment in RA which likely modifies the risk of DM,” the researchers wrote. “Both the increasing use of biologic DMARDs for RA in the U.S. over the last decade and our cohort entry criteria for the RA cohort (i.e., at least one dispensing of a DMARD) may explain the finding of the lower risk of DM in RA.”
The results found with the other three cohorts did not surprise the researchers. The reduced risk of diabetes among RA patients versus those with OA jibes with “higher rates of obesity and other comorbidities in patients with OA” as well as findings from a recent study that found a higher incidence rate of diabetes in OA, compared with RA. Ms. Jin and colleagues also acknowledged it is well known that “hypertension and PsA are associated with metabolic dysregulation and increase the risk of diabetes.”
The researchers defined patients with RA as having at least twoinpatient or outpatient ICD-9 or ICD-10 diagnosis codes of RA, separated by 7-365 days and having at least one dispensing for DMARDs within 1 year from the first RA diagnosis date, and defined the primary outcome of incident T2DM as at least one inpatient or outpatient diagnosis of T2DM plus at least one dispensing of an antidiabetic drug. They set the general non-RA cohort by selecting patients with any inpatient or outpatient diagnosis codes and a dispensing of any medications, and the hypertension, PsA, and OA comparator groups as having at least two inpatient or outpatient disease-specific ICD-9/ICD-10 codes separated by 7-365 days and at least one dispensing of disease-specific medication within 1 year from the first diagnosis date. They excluded patients with RA, PsA, or psoriasis diagnosis or disease-specific medication dispensing any time prior to or on the index date (the date of disease-specific medication dispensing).
The researchers recognized that the conclusions that can be drawn from the study are limited by the “potential misclassification of cohorts and covariates” because they “mainly used diagnosis codes and pharmacy dispensing records in claims data,” and some “important covariates such as baseline obesity are likely underreported and not adequately captured in claims data.” The level of covariate misclassification also may have been different across the study cohorts on “unmeasured covariates such as body mass index, diet, and physical activity, as well as disease specific measures,” thus introducing residual confounding. They also could not “examine potential difference in the risk of T2DM in untreated or undertreated RA patients” because “RA and all the non-RA comparator cohorts were required to use a disease-specific drug,” they wrote.
“While systemic inflammation in RA is thought to increase the risk of [cardiovascular disease] and cardiovascular risk factors such as DM, our findings suggest having RA itself does not confer an increased risk of DM. Future study should determine whether untreated RA or undertreated RA is associated with a greater risk of developing DM,” the researchers concluded.
The study was supported by a research grant from Bristol-Myers Squibb, which “played no role in the study design, data analysis or interpretation of data or presentation of results,” the researchers said. The company was “given the opportunity to make nonbinding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.” One author reported receiving research grants from Brigham and Women’s Hospital from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche for unrelated topics.
SOURCE: Jin Y et al. Arthritis Care Res. 2020 Aug 4. doi: 10.1002/acr.24343.
FROM ARTHRITIS CARE & RESEARCH
Valproate-Induced Lower Extremity Swelling
Bilateral lower extremity edema is a common condition with a broad differential diagnosis. New, severe peripheral edema implies a more nefarious underlying etiology than chronic venous insufficiency and should prompt a thorough evaluation for underlying conditions, such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, hypoalbuminemia, or lymphatic or venous obstruction. We present a case of a patient with sudden onset new bilateral lower extremity edema due to a rare adverse drug reaction (ADR) from valproate.
Case Presentation
A 63-year-old male with a history of seizures, bipolar disorder type I, and memory impairment due to traumatic brain injury (TBI) from a gunshot wound 24 years prior presented to the emergency department for witnessed seizure activity in the community. The patient had been incarcerated for the past 20 years, throughout which he had been taking the antiepileptic drugs (AEDs) phenytoin and divalproex and did not have any seizure activity. No records prior to his incarceration were available for review.
The patient recently had been released from prison and was nonadherent with his AEDs, leading to a witnessed seizure. This episode was described as preceded by an electric sensation, followed by rhythmic shaking of the right upper extremity without loss of consciousness. His regimen prior to admission included divalproex 1,000 mg daily and phenytoin 200 mg daily. His only other medication was folic acid.
Neurology was consulted on admission. An awake and asleep 4-hour electroencephalogram showed intermittent focal slowing of the right frontocentral region and frequent epileptiform discharges in the right prefrontal region during sleep, corresponding to areas of chronic right anterior frontal and temporal encephalomalacia seen on brain imaging. His seizures were thought likely to be secondary to prior head trauma. While the described seizure activity involving the right upper extremity was not consistent with the location of his prior TBI, neurology considered that he might have simple partial seizures with multiple foci or that his seizure event prior to admission was not accurately described. The neurology consult recommended switching from phenytoin 200 mg daily to lacosamide 100 mg twice daily on admission. His prior dose of divalproex 1,000 mg daily also was resumed for its antiepileptic effect and the added benefit of mood stabilization, as the patient reported elevated mood and decreased need for sleep on admission.
Eight days after changing his AED regimen, the patient was found to have new onset bilateral grade 1+ pitting edema to the level of his shins. He had no history of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysuria, or changes in his urination. Although medical records from his incarceration were not available for review, the patient reported that he had never had peripheral edema.
On physical examination, the patient had no periorbital edema, jugular venous pressure of 8 cm H2O, negative hepatojugular reflex, unremarkable cardiac and lung examination, and grade 2+ posterior tibial and dorsalis pedis pulses bilaterally. He underwent extensive laboratory evaluation for potential underlying causes, including nephrotic syndrome, cirrhosis, hypothyroidism, and CHF (Table). Valproate levels were initially subtherapeutic on admission (< 10 µg/mL, reference range 50-125 µg/mL) then rose to within therapeutic range (54 µg/mL-80 µg/mL throughout admission) after neurology recommended increasing the dose from 1,000 mg daily to 1,500 mg daily. His measured valproate levels were never supratherapeutic.
An electrocardiogram showed normal sinus rhythm unchanged from admission. Transthoracic echocardiogram showed normal left ventricular (LV) size and estimated LV ejection fraction of 55 to 60%. Abdominal ultrasound showed no evidence of cirrhosis and normal portal vein flow. Ultrasound of the lower extremities showed no deep venous thrombosis or valvular insufficiency. The patient was prescribed compression stockings. However, due to memory impairment, he was relatively nonadherent, and his lower extremity edema worsened to grade 3+ over several days. Due to the progressive swelling with no identified cause, a computed tomographic venogram of the abdomen and pelvis was performed to determine whether an inferior vena cava (IVC) thrombus was present. This study was unremarkable and did not show any external IVC compression.
After extensive evaluation did not reveal any other cause, the temporal course of events suggested an association between the patient’s peripheral edema and resumption of divalproex. His swelling remained stable. Discontinuation of divalproex was considered, but the patient’s mood remained euthymic, and he had no further seizure activity while on this medication, so the benefit of continuation was felt to outweigh any risks of switching to another agent.
Discussion
Valproate and its related forms, such as divalproex, often are used in the treatment of generalized or partial seizures, psychiatric disorders, and the prophylaxis of migraine headaches. Common ADRs include gastrointestinal symptoms, sedation, and dose-related thrombocytopenia, among many others. Rare ADRs include fulminant hepatitis, pancreatitis, hyperammonemia, and peripheral edema.1 There have been case reports of valproate-induced peripheral edema, which seems to be an idiosyncratic ADR that occurs after long-term administration of the medication.2,3 Early studies reported valproate-related edema in the context of valproate-induced hepatic injury.4 However, in more recent case reports, valproate-related edema has been found in patients without hepatotoxicity or supratherapeutic drug levels.1,2
The exact mechanism by which valproate causes peripheral edema is unknown. It has been reported that medications affecting the γ-aminobutyric acid (GABA) system such as benzodiazepines, for example, can cause this rare ADR.5 Unlike benzodiazepines, valproate has an indirect effect on the GABA system, through increasing availability of GABA.6 GABA receptors have been identified on peripheral tissues, suggesting that GABAergic medications also may have an effect on regional vascular resistance.7 This mechanism was proposed by prior case reports but has yet to be proven in studies.2
In this case, initiation of lacosamide temporally coinciding with development of the patient’s edema leads one to question whether lacosamide may have caused this ADR. Other medications commonly used in seizure management (such as benzodiazepines and gabapentin) have been reported to cause new onset peripheral edema.5,8 To date, however, there are no reported cases of peripheral edema due to lacosamide. While there are known interactions between various AEDs that may impact drug levels of valproate, there are no reported drug-drug interactions between lacosamide and valproate.9
Conclusions
Our case adds to the small but growing body of literature that suggests peripheral edema is a rare but clinically significant ADR of valproate. With its broad differential diagnosis, new onset peripheral edema is a concern that often warrants an extensive evaluation for underlying causes. Clinicians should be aware of this ADR as use of valproate becomes increasingly common so that an extensive workup is not always performed on patients with peripheral edema.
1. Prajapati H, Kansal D, Negi R. Magnesium valproate-induced pedal edema on chronic therapy: a rare adverse drug reaction. Indian J Pharmacol. 2017;49(5):399. doi:10.4103/ijp.IJP_239_17
2. Lin ST, Chen CS, Yen CF, Tsei JH, Wang SY. Valproate-related peripheral oedema: a manageable but probably neglected condition. Int J Neuropsychopharmacol. 2009;12(7):991-993. doi:10.1017/S1461145709000509
3. Ettinger A, Moshe S, Shinnar S. Edema associated with long‐term valproate therapy. Epilepsia. 1990;31(2):211-213. doi:10.1111/j.1528-1167.1990.tb06308.x
4. Zimmerman HJ, Ishak KG. Valproate‐induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2(5):591S-597S. doi:10.1002/hep.1840020513
5. Mathew T, D’Souza D, Nadimpally US, Nadig R. Clobazam‐induced pedal edema: “an unrecognized side effect of a common antiepileptic drug.” Epilepsia. 2016;57(3): 524-525. doi:10.1111/epi.13316
6. Bourin M, Chenu F, Hascoët M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr Drug Targets. 2009;10(11):1052-1060. doi:10.2174/138945009789735138
7. Takemoto Y. Effects of gamma‐aminobutyric acid on regional vascular resistances of conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22(suppl):S102-Sl04. doi:10.1111/j.1440-1681.1995.tb02839.x
8. Bidaki R, Sadeghi Z, Shafizadegan S, et al. Gabapentin induces edema, hyperesthesia and scaling in a depressed patient; a diagnostic challenge. Adv Biomed Res. 2016;5:1. doi:10.4103/2277-9175.174955
9. Cawello W, Nickel B, Eggert‐Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459-471. doi:10.1177/0091270009347675
Bilateral lower extremity edema is a common condition with a broad differential diagnosis. New, severe peripheral edema implies a more nefarious underlying etiology than chronic venous insufficiency and should prompt a thorough evaluation for underlying conditions, such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, hypoalbuminemia, or lymphatic or venous obstruction. We present a case of a patient with sudden onset new bilateral lower extremity edema due to a rare adverse drug reaction (ADR) from valproate.
Case Presentation
A 63-year-old male with a history of seizures, bipolar disorder type I, and memory impairment due to traumatic brain injury (TBI) from a gunshot wound 24 years prior presented to the emergency department for witnessed seizure activity in the community. The patient had been incarcerated for the past 20 years, throughout which he had been taking the antiepileptic drugs (AEDs) phenytoin and divalproex and did not have any seizure activity. No records prior to his incarceration were available for review.
The patient recently had been released from prison and was nonadherent with his AEDs, leading to a witnessed seizure. This episode was described as preceded by an electric sensation, followed by rhythmic shaking of the right upper extremity without loss of consciousness. His regimen prior to admission included divalproex 1,000 mg daily and phenytoin 200 mg daily. His only other medication was folic acid.
Neurology was consulted on admission. An awake and asleep 4-hour electroencephalogram showed intermittent focal slowing of the right frontocentral region and frequent epileptiform discharges in the right prefrontal region during sleep, corresponding to areas of chronic right anterior frontal and temporal encephalomalacia seen on brain imaging. His seizures were thought likely to be secondary to prior head trauma. While the described seizure activity involving the right upper extremity was not consistent with the location of his prior TBI, neurology considered that he might have simple partial seizures with multiple foci or that his seizure event prior to admission was not accurately described. The neurology consult recommended switching from phenytoin 200 mg daily to lacosamide 100 mg twice daily on admission. His prior dose of divalproex 1,000 mg daily also was resumed for its antiepileptic effect and the added benefit of mood stabilization, as the patient reported elevated mood and decreased need for sleep on admission.
Eight days after changing his AED regimen, the patient was found to have new onset bilateral grade 1+ pitting edema to the level of his shins. He had no history of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysuria, or changes in his urination. Although medical records from his incarceration were not available for review, the patient reported that he had never had peripheral edema.
On physical examination, the patient had no periorbital edema, jugular venous pressure of 8 cm H2O, negative hepatojugular reflex, unremarkable cardiac and lung examination, and grade 2+ posterior tibial and dorsalis pedis pulses bilaterally. He underwent extensive laboratory evaluation for potential underlying causes, including nephrotic syndrome, cirrhosis, hypothyroidism, and CHF (Table). Valproate levels were initially subtherapeutic on admission (< 10 µg/mL, reference range 50-125 µg/mL) then rose to within therapeutic range (54 µg/mL-80 µg/mL throughout admission) after neurology recommended increasing the dose from 1,000 mg daily to 1,500 mg daily. His measured valproate levels were never supratherapeutic.
An electrocardiogram showed normal sinus rhythm unchanged from admission. Transthoracic echocardiogram showed normal left ventricular (LV) size and estimated LV ejection fraction of 55 to 60%. Abdominal ultrasound showed no evidence of cirrhosis and normal portal vein flow. Ultrasound of the lower extremities showed no deep venous thrombosis or valvular insufficiency. The patient was prescribed compression stockings. However, due to memory impairment, he was relatively nonadherent, and his lower extremity edema worsened to grade 3+ over several days. Due to the progressive swelling with no identified cause, a computed tomographic venogram of the abdomen and pelvis was performed to determine whether an inferior vena cava (IVC) thrombus was present. This study was unremarkable and did not show any external IVC compression.
After extensive evaluation did not reveal any other cause, the temporal course of events suggested an association between the patient’s peripheral edema and resumption of divalproex. His swelling remained stable. Discontinuation of divalproex was considered, but the patient’s mood remained euthymic, and he had no further seizure activity while on this medication, so the benefit of continuation was felt to outweigh any risks of switching to another agent.
Discussion
Valproate and its related forms, such as divalproex, often are used in the treatment of generalized or partial seizures, psychiatric disorders, and the prophylaxis of migraine headaches. Common ADRs include gastrointestinal symptoms, sedation, and dose-related thrombocytopenia, among many others. Rare ADRs include fulminant hepatitis, pancreatitis, hyperammonemia, and peripheral edema.1 There have been case reports of valproate-induced peripheral edema, which seems to be an idiosyncratic ADR that occurs after long-term administration of the medication.2,3 Early studies reported valproate-related edema in the context of valproate-induced hepatic injury.4 However, in more recent case reports, valproate-related edema has been found in patients without hepatotoxicity or supratherapeutic drug levels.1,2
The exact mechanism by which valproate causes peripheral edema is unknown. It has been reported that medications affecting the γ-aminobutyric acid (GABA) system such as benzodiazepines, for example, can cause this rare ADR.5 Unlike benzodiazepines, valproate has an indirect effect on the GABA system, through increasing availability of GABA.6 GABA receptors have been identified on peripheral tissues, suggesting that GABAergic medications also may have an effect on regional vascular resistance.7 This mechanism was proposed by prior case reports but has yet to be proven in studies.2
In this case, initiation of lacosamide temporally coinciding with development of the patient’s edema leads one to question whether lacosamide may have caused this ADR. Other medications commonly used in seizure management (such as benzodiazepines and gabapentin) have been reported to cause new onset peripheral edema.5,8 To date, however, there are no reported cases of peripheral edema due to lacosamide. While there are known interactions between various AEDs that may impact drug levels of valproate, there are no reported drug-drug interactions between lacosamide and valproate.9
Conclusions
Our case adds to the small but growing body of literature that suggests peripheral edema is a rare but clinically significant ADR of valproate. With its broad differential diagnosis, new onset peripheral edema is a concern that often warrants an extensive evaluation for underlying causes. Clinicians should be aware of this ADR as use of valproate becomes increasingly common so that an extensive workup is not always performed on patients with peripheral edema.
Bilateral lower extremity edema is a common condition with a broad differential diagnosis. New, severe peripheral edema implies a more nefarious underlying etiology than chronic venous insufficiency and should prompt a thorough evaluation for underlying conditions, such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, hypoalbuminemia, or lymphatic or venous obstruction. We present a case of a patient with sudden onset new bilateral lower extremity edema due to a rare adverse drug reaction (ADR) from valproate.
Case Presentation
A 63-year-old male with a history of seizures, bipolar disorder type I, and memory impairment due to traumatic brain injury (TBI) from a gunshot wound 24 years prior presented to the emergency department for witnessed seizure activity in the community. The patient had been incarcerated for the past 20 years, throughout which he had been taking the antiepileptic drugs (AEDs) phenytoin and divalproex and did not have any seizure activity. No records prior to his incarceration were available for review.
The patient recently had been released from prison and was nonadherent with his AEDs, leading to a witnessed seizure. This episode was described as preceded by an electric sensation, followed by rhythmic shaking of the right upper extremity without loss of consciousness. His regimen prior to admission included divalproex 1,000 mg daily and phenytoin 200 mg daily. His only other medication was folic acid.
Neurology was consulted on admission. An awake and asleep 4-hour electroencephalogram showed intermittent focal slowing of the right frontocentral region and frequent epileptiform discharges in the right prefrontal region during sleep, corresponding to areas of chronic right anterior frontal and temporal encephalomalacia seen on brain imaging. His seizures were thought likely to be secondary to prior head trauma. While the described seizure activity involving the right upper extremity was not consistent with the location of his prior TBI, neurology considered that he might have simple partial seizures with multiple foci or that his seizure event prior to admission was not accurately described. The neurology consult recommended switching from phenytoin 200 mg daily to lacosamide 100 mg twice daily on admission. His prior dose of divalproex 1,000 mg daily also was resumed for its antiepileptic effect and the added benefit of mood stabilization, as the patient reported elevated mood and decreased need for sleep on admission.
Eight days after changing his AED regimen, the patient was found to have new onset bilateral grade 1+ pitting edema to the level of his shins. He had no history of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysuria, or changes in his urination. Although medical records from his incarceration were not available for review, the patient reported that he had never had peripheral edema.
On physical examination, the patient had no periorbital edema, jugular venous pressure of 8 cm H2O, negative hepatojugular reflex, unremarkable cardiac and lung examination, and grade 2+ posterior tibial and dorsalis pedis pulses bilaterally. He underwent extensive laboratory evaluation for potential underlying causes, including nephrotic syndrome, cirrhosis, hypothyroidism, and CHF (Table). Valproate levels were initially subtherapeutic on admission (< 10 µg/mL, reference range 50-125 µg/mL) then rose to within therapeutic range (54 µg/mL-80 µg/mL throughout admission) after neurology recommended increasing the dose from 1,000 mg daily to 1,500 mg daily. His measured valproate levels were never supratherapeutic.
An electrocardiogram showed normal sinus rhythm unchanged from admission. Transthoracic echocardiogram showed normal left ventricular (LV) size and estimated LV ejection fraction of 55 to 60%. Abdominal ultrasound showed no evidence of cirrhosis and normal portal vein flow. Ultrasound of the lower extremities showed no deep venous thrombosis or valvular insufficiency. The patient was prescribed compression stockings. However, due to memory impairment, he was relatively nonadherent, and his lower extremity edema worsened to grade 3+ over several days. Due to the progressive swelling with no identified cause, a computed tomographic venogram of the abdomen and pelvis was performed to determine whether an inferior vena cava (IVC) thrombus was present. This study was unremarkable and did not show any external IVC compression.
After extensive evaluation did not reveal any other cause, the temporal course of events suggested an association between the patient’s peripheral edema and resumption of divalproex. His swelling remained stable. Discontinuation of divalproex was considered, but the patient’s mood remained euthymic, and he had no further seizure activity while on this medication, so the benefit of continuation was felt to outweigh any risks of switching to another agent.
Discussion
Valproate and its related forms, such as divalproex, often are used in the treatment of generalized or partial seizures, psychiatric disorders, and the prophylaxis of migraine headaches. Common ADRs include gastrointestinal symptoms, sedation, and dose-related thrombocytopenia, among many others. Rare ADRs include fulminant hepatitis, pancreatitis, hyperammonemia, and peripheral edema.1 There have been case reports of valproate-induced peripheral edema, which seems to be an idiosyncratic ADR that occurs after long-term administration of the medication.2,3 Early studies reported valproate-related edema in the context of valproate-induced hepatic injury.4 However, in more recent case reports, valproate-related edema has been found in patients without hepatotoxicity or supratherapeutic drug levels.1,2
The exact mechanism by which valproate causes peripheral edema is unknown. It has been reported that medications affecting the γ-aminobutyric acid (GABA) system such as benzodiazepines, for example, can cause this rare ADR.5 Unlike benzodiazepines, valproate has an indirect effect on the GABA system, through increasing availability of GABA.6 GABA receptors have been identified on peripheral tissues, suggesting that GABAergic medications also may have an effect on regional vascular resistance.7 This mechanism was proposed by prior case reports but has yet to be proven in studies.2
In this case, initiation of lacosamide temporally coinciding with development of the patient’s edema leads one to question whether lacosamide may have caused this ADR. Other medications commonly used in seizure management (such as benzodiazepines and gabapentin) have been reported to cause new onset peripheral edema.5,8 To date, however, there are no reported cases of peripheral edema due to lacosamide. While there are known interactions between various AEDs that may impact drug levels of valproate, there are no reported drug-drug interactions between lacosamide and valproate.9
Conclusions
Our case adds to the small but growing body of literature that suggests peripheral edema is a rare but clinically significant ADR of valproate. With its broad differential diagnosis, new onset peripheral edema is a concern that often warrants an extensive evaluation for underlying causes. Clinicians should be aware of this ADR as use of valproate becomes increasingly common so that an extensive workup is not always performed on patients with peripheral edema.
1. Prajapati H, Kansal D, Negi R. Magnesium valproate-induced pedal edema on chronic therapy: a rare adverse drug reaction. Indian J Pharmacol. 2017;49(5):399. doi:10.4103/ijp.IJP_239_17
2. Lin ST, Chen CS, Yen CF, Tsei JH, Wang SY. Valproate-related peripheral oedema: a manageable but probably neglected condition. Int J Neuropsychopharmacol. 2009;12(7):991-993. doi:10.1017/S1461145709000509
3. Ettinger A, Moshe S, Shinnar S. Edema associated with long‐term valproate therapy. Epilepsia. 1990;31(2):211-213. doi:10.1111/j.1528-1167.1990.tb06308.x
4. Zimmerman HJ, Ishak KG. Valproate‐induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2(5):591S-597S. doi:10.1002/hep.1840020513
5. Mathew T, D’Souza D, Nadimpally US, Nadig R. Clobazam‐induced pedal edema: “an unrecognized side effect of a common antiepileptic drug.” Epilepsia. 2016;57(3): 524-525. doi:10.1111/epi.13316
6. Bourin M, Chenu F, Hascoët M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr Drug Targets. 2009;10(11):1052-1060. doi:10.2174/138945009789735138
7. Takemoto Y. Effects of gamma‐aminobutyric acid on regional vascular resistances of conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22(suppl):S102-Sl04. doi:10.1111/j.1440-1681.1995.tb02839.x
8. Bidaki R, Sadeghi Z, Shafizadegan S, et al. Gabapentin induces edema, hyperesthesia and scaling in a depressed patient; a diagnostic challenge. Adv Biomed Res. 2016;5:1. doi:10.4103/2277-9175.174955
9. Cawello W, Nickel B, Eggert‐Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459-471. doi:10.1177/0091270009347675
1. Prajapati H, Kansal D, Negi R. Magnesium valproate-induced pedal edema on chronic therapy: a rare adverse drug reaction. Indian J Pharmacol. 2017;49(5):399. doi:10.4103/ijp.IJP_239_17
2. Lin ST, Chen CS, Yen CF, Tsei JH, Wang SY. Valproate-related peripheral oedema: a manageable but probably neglected condition. Int J Neuropsychopharmacol. 2009;12(7):991-993. doi:10.1017/S1461145709000509
3. Ettinger A, Moshe S, Shinnar S. Edema associated with long‐term valproate therapy. Epilepsia. 1990;31(2):211-213. doi:10.1111/j.1528-1167.1990.tb06308.x
4. Zimmerman HJ, Ishak KG. Valproate‐induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2(5):591S-597S. doi:10.1002/hep.1840020513
5. Mathew T, D’Souza D, Nadimpally US, Nadig R. Clobazam‐induced pedal edema: “an unrecognized side effect of a common antiepileptic drug.” Epilepsia. 2016;57(3): 524-525. doi:10.1111/epi.13316
6. Bourin M, Chenu F, Hascoët M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr Drug Targets. 2009;10(11):1052-1060. doi:10.2174/138945009789735138
7. Takemoto Y. Effects of gamma‐aminobutyric acid on regional vascular resistances of conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22(suppl):S102-Sl04. doi:10.1111/j.1440-1681.1995.tb02839.x
8. Bidaki R, Sadeghi Z, Shafizadegan S, et al. Gabapentin induces edema, hyperesthesia and scaling in a depressed patient; a diagnostic challenge. Adv Biomed Res. 2016;5:1. doi:10.4103/2277-9175.174955
9. Cawello W, Nickel B, Eggert‐Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459-471. doi:10.1177/0091270009347675
Chronic Microaspiration and Frailty: A Geriatric Smoking Gun?
Frailty is a highly prevalent syndrome in nursing homes, occurring in at least 50% of patients.1 The frailty phenotype has been described by Fried and colleagues as impairment in ≥ 3 of 5 domains: unintentional weight loss, self-reported exhaustion, muscle weakness, slow gait speed, and low physical activity. By this definition, frailty is highly associated with poor quality of life and mortality.2,3
In recent years, there has been evolving evidence of a relationship between frailty and chronic systemic inflammation.4-6 Some degree of chronic inflammation is likely inherent to the aging process and increases the risk of frailty (so-called inflammaging) but is seen to a greater degree in many pathologic conditions in nursing homes, including cancer, organ failure, and chronic infection.4,6-8
Dysphagia also is highly prevalent in nursing homes, affecting up to 60% of patients and is a strong predictor of hospital utilization and of mortality.9,10 Overt aspiration pneumonitis and pneumonia are perhaps the best studied sequelae, but chronic occult microaspiration also is prevalent in this population.11 Just as normal systemic inflammatory changes in aging may increase vulnerability to frailty with additional illness burden, normal aging changes in swallowing function may increase vulnerability to dysphagia and to microaspiration with additional illness burden.12,13 In older adults, important risk factors for microaspiration include not only overt dysphagia, dementia, and other neurologic illnesses, but also general debility, weakness, and immobility.14
Matsuse and colleagues have described diffuse aspiration bronchiolitis (DAB) in patients with chronic microaspiration.14 DAB often goes undiagnosed.14-16 As in frailty, weight loss and chronic anemia may be seen, and many of these patients are bedridden.14,17 Episodes of macroaspiration and overt lobar pneumonia also may occur.14 Lung biopsy or autopsy reveals chronic bronchiolar inflammation and sometimes pulmonary fibrosis, but to date there have been no reports suggesting chronic systemic inflammation or elevated proinflammatory cytokines.14,15,17 We present 3 patients with progressive weight loss, functional decline, and frailty in whom chronic microaspiration likely played a significant role.
Case 1 Presentation
A 68-year-old man with a 6-year history of rapidly progressive Parkinson disease was admitted to the Haley’s Cove Community Living Center (CLC) on the James A. Haley Veterans’ Hospital campus in Tampa, Florida for long-term care. The patient’s medical history also was significant for bipolar illness and for small cell carcinoma of the lung in sustained remission.
Medications included levodopa/carbidopa 50 mg/200 mg 4 times daily, entacapone 200 mg 4 times daily, lithium carbonate 600 mg every night at bedtime, lamotrigine 150 mg daily, quetiapine 200 mg every night at bedtime, pravastatin 40 mg every night at bedtime, omeprazole 20 mg daily, tamsulosin 0.4 mg every night at bedtime, and aspirin 81 mg daily. He initially did well, but after 6 months the nursing staff began to notice the patient coughing during and after meals. Speech pathology evaluation revealed moderate oropharyngeal dysphagia, and his diet was downgraded to nectar-thickened liquids.
Over the subsequent 10 months, he became progressively weaker in physical therapy and more inactive, with about a 20-lb weight loss and mild hypoalbuminemia of 3.0 gm/dL. He had developed 3 episodes of aspiration pneumonia during this period; a repeat swallow evaluation after the last episode revealed worsened dysphagia, and his physician suggested nil per os (NPO) status and an alternative feeding route. His guardian declined placement of a percutaneous endoscopic gastrostomy (PEG) tube, he was transferred to the inpatient hospice unit, and died 2 weeks later. An autopsy was declined.
Case 2 Presentation
A 66-year-old man with a medical history of multiple traumatic brain injuries (TBIs) was admitted to the CLC for long-term care. Sequelae of the TBIs included moderate dementia, spastic paraparesis with multiple pressure injuries, a well-controlled seizure disorder, and severe oropharyngeal dysphagia with NPO status and a percutaneous endoscopic gastrostomy (PEG) tube. His medical history included TBIs and hepatitis C virus infection; medications included levetiracetam 1,000 mg twice daily, lamotrigine 25 mg twice daily, and cholecalciferol 2,000 U daily. He had multiple stage III pressure injuries and an ischial stage IV injury at the time of admission.
His 11-month stay in the CLC was characterized by progressively worsening weakness and inactivity, with a 25-lb weight loss in spite of adequate tube feeding. Serum albumin remained in the 2.0 to 2.5 gm/dL range, hemoglobin in the 7 to 9 gm/dL range without any obvious source of anemia. Most of the pressure injuries worsened during his stay in spite of aggressive wound care, and he developed a second stage IV sacral wound. A single C-reactive protein (CRP) level 2 months prior to his death was markedly elevated at 19.5 mg/dL. In spite of maintaining NPO status, he developed 3 episodes of aspiration pneumonia, all of which responded well to treatment. Ultimately, he was found pulseless and apneic and resuscitation was unsuccessful. An autopsy revealed purulent material in the small airways.
Case 3 Presentation
A 65-year-old man with a long history of paranoid schizophrenia and severe gastroesophageal reflux disease had resided in the CLC for about 10 years. Medications included risperidone microspheres 37.5 mg every 2 weeks, valproic acid 500 mg 3 times daily and 1,000 mg every night at bedtime, lansoprazole 30 mg twice daily, ranitidine 150 mg every night at bedtime, sucralfate 1,000 mg 3 times daily, simvastatin 20 mg every night at bedtime, and tamsulosin 0.4 mg every night at bedtime. He had done well for many years but developed some drooling and a modest resting tremor (but no other signs of pseudoparkinsonism) about 8 years after admission.
There had been no changes to his risperidone dosage. He also lost about 20 lb over a period of 1 year and became increasingly weak and dependent in gait, serum albumin dropped as low as 1.6 gm/dL, hemoglobin dropped to the 7 to 8 gm/dL range (without any other obvious source of anemia), and he developed a gradually worsening right-sided pleural effusion. CRP was chronically elevated at this point, in the 6 to 15 mg/dL range and as high as 17.2 mg/dL. Ultimately, he developed 3 episodes of aspiration pneumonia over a period of 2 months. Swallowing evaluation at that time revealed severe oropharyngeal dysphagia and a PEG tube was placed. Due to concerns for possible antipsychotic-induced dysphagia, risperidone was discontinued, and quetiapine 400 mg a day was substituted. He did well over the subsequent year with no further pneumonia and advancement back to a regular diet. He regained all of the lost weight and began independent ambulation. Albumin improved to the 3 gm/dL range, hemoglobin to the 12 to 13 gm/dL range, and CRP had decreased to 0.7 mg/dL. The pleural effusion (believed to have been a parapneumonic effusion) had resolved.
Discussion
All 3 patients met the Fried criteria for frailty, although there were several confounding issues.2 All 3 patients lost between 20 and 25 lb; all had clearly become weaker according to nursing and rehabilitation staff (although none were formally assessed for grip strength); and all had clear declines in their activity level. Patient 3 had a clear decrement in gait speed, but patient 1 had severe gait impairment due to Parkinson disease (although his gait in therapy had clearly worsened). Patient 2 was paraparetic and unable to ambulate. There also was evidence of limited biomarkers of systemic inflammation; all 3 patients’ albumin had decreased, and patients 2 and 3 had significant decrease in hemoglobin; but these commonplace clinical biomarkers are obviously multifactorially determined. We have limited data on our patients’ CRP levels; serial levels would have been more specific for systemic inflammation but were infrequently performed on the patients.
Multimorbidity and medical complexity are more the rule than the exception in frail geriatric patients,and it is difficult to separate the role of microaspiration from other confounding conditions that might have contributed to these patients’ evolving systemic inflammation and frailty.18 It might be argued that the decline for patient 1 was related to the underlying Parkinson disease (a progressive neurologic illness in which systemic inflammation has been reported), or that the decline of patient 2 was related to the worsening pressure injuries rather than to covert microaspiration.19 However, the TBIs for patient 2 and the schizophrenia for patient 3 would not be expected to be associated with frailty or with systemic inflammation. Furthermore, the frailty symptoms of patient 3 and inflammatory biomarkers improved after the risperidone, which was likely responsible for his microaspiration, was discontinued. All 3 patients were at risk for oropharyngeal dysphagia (antipsychotic medication is clearly associated with dysphagia20); patient 2 demonstrated pathologic evidence of DAB at autopsy.
There is evolving evidence that chronic systemic inflammation and immune activation are key mechanisms in the pathogenesis of frailty.4-6 It is known that elevated serum levels of proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6, and CRP are directly associated with frailty and are inversely associated with levels of albumin, hemoglobin, insulin-like growth factor-1, and several micronutrients in frail individuals.4-7,21,22 Chronic inflammation contributes to the pathophysiology of frailty through detrimental effects on a broad range of systems, including the musculoskeletal, endocrine, and hematopoietic systems and through nutritional dysregulation.2,4,23 These changes may lead to further deleterious effects, creating a downward spiral of worsening frailty. For example, it seems likely that our patients’ progressive weakness further compromised airway protection, creating a vicious cycle of worsening microaspiration and chronic inflammation.
Conclusions
To date, the role of chronic microaspiration and DAB in chronic systemic inflammation or in frailty has not been explored. Given the prevalence of microaspiration in nursing home residents and the devastating consequences of frailty, though, this seems to be a crucial area of investigation. It is equally crucial for long-term care staff, both providers and nursing staff, to have a heightened awareness of covert microaspiration and a low threshold for referral to speech pathology for further investigation. Staff also should be aware of the utility of the Fried criteria to improve identification of frailty in general. It is probable that covert microaspiration will prove to be an important part of the differential diagnosis of frailty.
1. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940-945. doi:10.1016/j.jamda.2015.06.025
2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. doi:10.1093/gerona/56.3.m146
3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi:10.1016/j.jamda.2013.03.022
4. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. doi:10.2147/CIA.S45300.
5. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi:10.1016/j.arr.2016.08.006
6. Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777-1783. doi:10.1111/jgs.14876
7. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-882. doi:10.1016/j.jamda.2013.05.009
8. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218-1225. doi:10.1093/gerona/glw240
9. Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs. 2000;26(8):35-48. doi:10.3928/0098-9134-20000801-09
10. Altman KW, Yu GP, Schaefer SD. Consequences of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784-789. doi:10.1001/archoto.2010.129
11. Sakai K, Hirano H, Watanabe Y, et al. An examination of factors related to aspiration and silent aspiration in older adults requiring long-term care in rural Japan. J Oral Rehabil. 2016;43(2):103-110. doi:10.1111/joor.12349
12. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180-184. doi:10.1007/BF00366381
13. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270-274. doi:10.1007/s00455-006-9051-6
14. Matsuse T, Oka T, Kida K, Fukuchi Y. Importance of diffuse aspiration bronchiolitis caused by chronic occult aspiration in the elderly. Chest. 1996;110(5):1289-1293. doi:10.1378/chest.110.5.1289
15. Cardasis JJ, MacMahon H, Husain AN. The spectrum of lung disease due to chronic occult aspiration. Ann Am Thorac Soc. 2014;11(6):865-873. doi:10.1513/AnnalsATS.201310-360OC
16. Pereira-Silva JL, Silva CIS, Araujo Neto CA, Andrade TL, Muller NL. Chronic pulmonary microaspiration: high-resolution computed tomographic findings in 13 patients. J Thorac Imaging. 2014;29(5):298-303. doi:10.1097/RTI.0000000000000091
17. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815-823. doi:10.1378/chest.14-1049
18. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Aging. 2017;46(6):882-888. doi:10.1093/ageing/afx150
19. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80-91. doi:10.1016/j.freeradbiomed.2017.10.379
20. Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467-471. doi:10.1007/s00455-017-9802-6
21. Velissaris D, Pantzaris N, Koniari I, et al. C-reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461-465. doi:10.14740/jocmr2959w
22. Hubbard RE, O’Mahoney MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103-3109. doi:10.1111/j.1582-4934.2009.00733.x
23. Argiles JM, Busquets S, Stemmler B, Lotez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. doi:10.1016/j.coph.2015.04.003
Frailty is a highly prevalent syndrome in nursing homes, occurring in at least 50% of patients.1 The frailty phenotype has been described by Fried and colleagues as impairment in ≥ 3 of 5 domains: unintentional weight loss, self-reported exhaustion, muscle weakness, slow gait speed, and low physical activity. By this definition, frailty is highly associated with poor quality of life and mortality.2,3
In recent years, there has been evolving evidence of a relationship between frailty and chronic systemic inflammation.4-6 Some degree of chronic inflammation is likely inherent to the aging process and increases the risk of frailty (so-called inflammaging) but is seen to a greater degree in many pathologic conditions in nursing homes, including cancer, organ failure, and chronic infection.4,6-8
Dysphagia also is highly prevalent in nursing homes, affecting up to 60% of patients and is a strong predictor of hospital utilization and of mortality.9,10 Overt aspiration pneumonitis and pneumonia are perhaps the best studied sequelae, but chronic occult microaspiration also is prevalent in this population.11 Just as normal systemic inflammatory changes in aging may increase vulnerability to frailty with additional illness burden, normal aging changes in swallowing function may increase vulnerability to dysphagia and to microaspiration with additional illness burden.12,13 In older adults, important risk factors for microaspiration include not only overt dysphagia, dementia, and other neurologic illnesses, but also general debility, weakness, and immobility.14
Matsuse and colleagues have described diffuse aspiration bronchiolitis (DAB) in patients with chronic microaspiration.14 DAB often goes undiagnosed.14-16 As in frailty, weight loss and chronic anemia may be seen, and many of these patients are bedridden.14,17 Episodes of macroaspiration and overt lobar pneumonia also may occur.14 Lung biopsy or autopsy reveals chronic bronchiolar inflammation and sometimes pulmonary fibrosis, but to date there have been no reports suggesting chronic systemic inflammation or elevated proinflammatory cytokines.14,15,17 We present 3 patients with progressive weight loss, functional decline, and frailty in whom chronic microaspiration likely played a significant role.
Case 1 Presentation
A 68-year-old man with a 6-year history of rapidly progressive Parkinson disease was admitted to the Haley’s Cove Community Living Center (CLC) on the James A. Haley Veterans’ Hospital campus in Tampa, Florida for long-term care. The patient’s medical history also was significant for bipolar illness and for small cell carcinoma of the lung in sustained remission.
Medications included levodopa/carbidopa 50 mg/200 mg 4 times daily, entacapone 200 mg 4 times daily, lithium carbonate 600 mg every night at bedtime, lamotrigine 150 mg daily, quetiapine 200 mg every night at bedtime, pravastatin 40 mg every night at bedtime, omeprazole 20 mg daily, tamsulosin 0.4 mg every night at bedtime, and aspirin 81 mg daily. He initially did well, but after 6 months the nursing staff began to notice the patient coughing during and after meals. Speech pathology evaluation revealed moderate oropharyngeal dysphagia, and his diet was downgraded to nectar-thickened liquids.
Over the subsequent 10 months, he became progressively weaker in physical therapy and more inactive, with about a 20-lb weight loss and mild hypoalbuminemia of 3.0 gm/dL. He had developed 3 episodes of aspiration pneumonia during this period; a repeat swallow evaluation after the last episode revealed worsened dysphagia, and his physician suggested nil per os (NPO) status and an alternative feeding route. His guardian declined placement of a percutaneous endoscopic gastrostomy (PEG) tube, he was transferred to the inpatient hospice unit, and died 2 weeks later. An autopsy was declined.
Case 2 Presentation
A 66-year-old man with a medical history of multiple traumatic brain injuries (TBIs) was admitted to the CLC for long-term care. Sequelae of the TBIs included moderate dementia, spastic paraparesis with multiple pressure injuries, a well-controlled seizure disorder, and severe oropharyngeal dysphagia with NPO status and a percutaneous endoscopic gastrostomy (PEG) tube. His medical history included TBIs and hepatitis C virus infection; medications included levetiracetam 1,000 mg twice daily, lamotrigine 25 mg twice daily, and cholecalciferol 2,000 U daily. He had multiple stage III pressure injuries and an ischial stage IV injury at the time of admission.
His 11-month stay in the CLC was characterized by progressively worsening weakness and inactivity, with a 25-lb weight loss in spite of adequate tube feeding. Serum albumin remained in the 2.0 to 2.5 gm/dL range, hemoglobin in the 7 to 9 gm/dL range without any obvious source of anemia. Most of the pressure injuries worsened during his stay in spite of aggressive wound care, and he developed a second stage IV sacral wound. A single C-reactive protein (CRP) level 2 months prior to his death was markedly elevated at 19.5 mg/dL. In spite of maintaining NPO status, he developed 3 episodes of aspiration pneumonia, all of which responded well to treatment. Ultimately, he was found pulseless and apneic and resuscitation was unsuccessful. An autopsy revealed purulent material in the small airways.
Case 3 Presentation
A 65-year-old man with a long history of paranoid schizophrenia and severe gastroesophageal reflux disease had resided in the CLC for about 10 years. Medications included risperidone microspheres 37.5 mg every 2 weeks, valproic acid 500 mg 3 times daily and 1,000 mg every night at bedtime, lansoprazole 30 mg twice daily, ranitidine 150 mg every night at bedtime, sucralfate 1,000 mg 3 times daily, simvastatin 20 mg every night at bedtime, and tamsulosin 0.4 mg every night at bedtime. He had done well for many years but developed some drooling and a modest resting tremor (but no other signs of pseudoparkinsonism) about 8 years after admission.
There had been no changes to his risperidone dosage. He also lost about 20 lb over a period of 1 year and became increasingly weak and dependent in gait, serum albumin dropped as low as 1.6 gm/dL, hemoglobin dropped to the 7 to 8 gm/dL range (without any other obvious source of anemia), and he developed a gradually worsening right-sided pleural effusion. CRP was chronically elevated at this point, in the 6 to 15 mg/dL range and as high as 17.2 mg/dL. Ultimately, he developed 3 episodes of aspiration pneumonia over a period of 2 months. Swallowing evaluation at that time revealed severe oropharyngeal dysphagia and a PEG tube was placed. Due to concerns for possible antipsychotic-induced dysphagia, risperidone was discontinued, and quetiapine 400 mg a day was substituted. He did well over the subsequent year with no further pneumonia and advancement back to a regular diet. He regained all of the lost weight and began independent ambulation. Albumin improved to the 3 gm/dL range, hemoglobin to the 12 to 13 gm/dL range, and CRP had decreased to 0.7 mg/dL. The pleural effusion (believed to have been a parapneumonic effusion) had resolved.
Discussion
All 3 patients met the Fried criteria for frailty, although there were several confounding issues.2 All 3 patients lost between 20 and 25 lb; all had clearly become weaker according to nursing and rehabilitation staff (although none were formally assessed for grip strength); and all had clear declines in their activity level. Patient 3 had a clear decrement in gait speed, but patient 1 had severe gait impairment due to Parkinson disease (although his gait in therapy had clearly worsened). Patient 2 was paraparetic and unable to ambulate. There also was evidence of limited biomarkers of systemic inflammation; all 3 patients’ albumin had decreased, and patients 2 and 3 had significant decrease in hemoglobin; but these commonplace clinical biomarkers are obviously multifactorially determined. We have limited data on our patients’ CRP levels; serial levels would have been more specific for systemic inflammation but were infrequently performed on the patients.
Multimorbidity and medical complexity are more the rule than the exception in frail geriatric patients,and it is difficult to separate the role of microaspiration from other confounding conditions that might have contributed to these patients’ evolving systemic inflammation and frailty.18 It might be argued that the decline for patient 1 was related to the underlying Parkinson disease (a progressive neurologic illness in which systemic inflammation has been reported), or that the decline of patient 2 was related to the worsening pressure injuries rather than to covert microaspiration.19 However, the TBIs for patient 2 and the schizophrenia for patient 3 would not be expected to be associated with frailty or with systemic inflammation. Furthermore, the frailty symptoms of patient 3 and inflammatory biomarkers improved after the risperidone, which was likely responsible for his microaspiration, was discontinued. All 3 patients were at risk for oropharyngeal dysphagia (antipsychotic medication is clearly associated with dysphagia20); patient 2 demonstrated pathologic evidence of DAB at autopsy.
There is evolving evidence that chronic systemic inflammation and immune activation are key mechanisms in the pathogenesis of frailty.4-6 It is known that elevated serum levels of proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6, and CRP are directly associated with frailty and are inversely associated with levels of albumin, hemoglobin, insulin-like growth factor-1, and several micronutrients in frail individuals.4-7,21,22 Chronic inflammation contributes to the pathophysiology of frailty through detrimental effects on a broad range of systems, including the musculoskeletal, endocrine, and hematopoietic systems and through nutritional dysregulation.2,4,23 These changes may lead to further deleterious effects, creating a downward spiral of worsening frailty. For example, it seems likely that our patients’ progressive weakness further compromised airway protection, creating a vicious cycle of worsening microaspiration and chronic inflammation.
Conclusions
To date, the role of chronic microaspiration and DAB in chronic systemic inflammation or in frailty has not been explored. Given the prevalence of microaspiration in nursing home residents and the devastating consequences of frailty, though, this seems to be a crucial area of investigation. It is equally crucial for long-term care staff, both providers and nursing staff, to have a heightened awareness of covert microaspiration and a low threshold for referral to speech pathology for further investigation. Staff also should be aware of the utility of the Fried criteria to improve identification of frailty in general. It is probable that covert microaspiration will prove to be an important part of the differential diagnosis of frailty.
Frailty is a highly prevalent syndrome in nursing homes, occurring in at least 50% of patients.1 The frailty phenotype has been described by Fried and colleagues as impairment in ≥ 3 of 5 domains: unintentional weight loss, self-reported exhaustion, muscle weakness, slow gait speed, and low physical activity. By this definition, frailty is highly associated with poor quality of life and mortality.2,3
In recent years, there has been evolving evidence of a relationship between frailty and chronic systemic inflammation.4-6 Some degree of chronic inflammation is likely inherent to the aging process and increases the risk of frailty (so-called inflammaging) but is seen to a greater degree in many pathologic conditions in nursing homes, including cancer, organ failure, and chronic infection.4,6-8
Dysphagia also is highly prevalent in nursing homes, affecting up to 60% of patients and is a strong predictor of hospital utilization and of mortality.9,10 Overt aspiration pneumonitis and pneumonia are perhaps the best studied sequelae, but chronic occult microaspiration also is prevalent in this population.11 Just as normal systemic inflammatory changes in aging may increase vulnerability to frailty with additional illness burden, normal aging changes in swallowing function may increase vulnerability to dysphagia and to microaspiration with additional illness burden.12,13 In older adults, important risk factors for microaspiration include not only overt dysphagia, dementia, and other neurologic illnesses, but also general debility, weakness, and immobility.14
Matsuse and colleagues have described diffuse aspiration bronchiolitis (DAB) in patients with chronic microaspiration.14 DAB often goes undiagnosed.14-16 As in frailty, weight loss and chronic anemia may be seen, and many of these patients are bedridden.14,17 Episodes of macroaspiration and overt lobar pneumonia also may occur.14 Lung biopsy or autopsy reveals chronic bronchiolar inflammation and sometimes pulmonary fibrosis, but to date there have been no reports suggesting chronic systemic inflammation or elevated proinflammatory cytokines.14,15,17 We present 3 patients with progressive weight loss, functional decline, and frailty in whom chronic microaspiration likely played a significant role.
Case 1 Presentation
A 68-year-old man with a 6-year history of rapidly progressive Parkinson disease was admitted to the Haley’s Cove Community Living Center (CLC) on the James A. Haley Veterans’ Hospital campus in Tampa, Florida for long-term care. The patient’s medical history also was significant for bipolar illness and for small cell carcinoma of the lung in sustained remission.
Medications included levodopa/carbidopa 50 mg/200 mg 4 times daily, entacapone 200 mg 4 times daily, lithium carbonate 600 mg every night at bedtime, lamotrigine 150 mg daily, quetiapine 200 mg every night at bedtime, pravastatin 40 mg every night at bedtime, omeprazole 20 mg daily, tamsulosin 0.4 mg every night at bedtime, and aspirin 81 mg daily. He initially did well, but after 6 months the nursing staff began to notice the patient coughing during and after meals. Speech pathology evaluation revealed moderate oropharyngeal dysphagia, and his diet was downgraded to nectar-thickened liquids.
Over the subsequent 10 months, he became progressively weaker in physical therapy and more inactive, with about a 20-lb weight loss and mild hypoalbuminemia of 3.0 gm/dL. He had developed 3 episodes of aspiration pneumonia during this period; a repeat swallow evaluation after the last episode revealed worsened dysphagia, and his physician suggested nil per os (NPO) status and an alternative feeding route. His guardian declined placement of a percutaneous endoscopic gastrostomy (PEG) tube, he was transferred to the inpatient hospice unit, and died 2 weeks later. An autopsy was declined.
Case 2 Presentation
A 66-year-old man with a medical history of multiple traumatic brain injuries (TBIs) was admitted to the CLC for long-term care. Sequelae of the TBIs included moderate dementia, spastic paraparesis with multiple pressure injuries, a well-controlled seizure disorder, and severe oropharyngeal dysphagia with NPO status and a percutaneous endoscopic gastrostomy (PEG) tube. His medical history included TBIs and hepatitis C virus infection; medications included levetiracetam 1,000 mg twice daily, lamotrigine 25 mg twice daily, and cholecalciferol 2,000 U daily. He had multiple stage III pressure injuries and an ischial stage IV injury at the time of admission.
His 11-month stay in the CLC was characterized by progressively worsening weakness and inactivity, with a 25-lb weight loss in spite of adequate tube feeding. Serum albumin remained in the 2.0 to 2.5 gm/dL range, hemoglobin in the 7 to 9 gm/dL range without any obvious source of anemia. Most of the pressure injuries worsened during his stay in spite of aggressive wound care, and he developed a second stage IV sacral wound. A single C-reactive protein (CRP) level 2 months prior to his death was markedly elevated at 19.5 mg/dL. In spite of maintaining NPO status, he developed 3 episodes of aspiration pneumonia, all of which responded well to treatment. Ultimately, he was found pulseless and apneic and resuscitation was unsuccessful. An autopsy revealed purulent material in the small airways.
Case 3 Presentation
A 65-year-old man with a long history of paranoid schizophrenia and severe gastroesophageal reflux disease had resided in the CLC for about 10 years. Medications included risperidone microspheres 37.5 mg every 2 weeks, valproic acid 500 mg 3 times daily and 1,000 mg every night at bedtime, lansoprazole 30 mg twice daily, ranitidine 150 mg every night at bedtime, sucralfate 1,000 mg 3 times daily, simvastatin 20 mg every night at bedtime, and tamsulosin 0.4 mg every night at bedtime. He had done well for many years but developed some drooling and a modest resting tremor (but no other signs of pseudoparkinsonism) about 8 years after admission.
There had been no changes to his risperidone dosage. He also lost about 20 lb over a period of 1 year and became increasingly weak and dependent in gait, serum albumin dropped as low as 1.6 gm/dL, hemoglobin dropped to the 7 to 8 gm/dL range (without any other obvious source of anemia), and he developed a gradually worsening right-sided pleural effusion. CRP was chronically elevated at this point, in the 6 to 15 mg/dL range and as high as 17.2 mg/dL. Ultimately, he developed 3 episodes of aspiration pneumonia over a period of 2 months. Swallowing evaluation at that time revealed severe oropharyngeal dysphagia and a PEG tube was placed. Due to concerns for possible antipsychotic-induced dysphagia, risperidone was discontinued, and quetiapine 400 mg a day was substituted. He did well over the subsequent year with no further pneumonia and advancement back to a regular diet. He regained all of the lost weight and began independent ambulation. Albumin improved to the 3 gm/dL range, hemoglobin to the 12 to 13 gm/dL range, and CRP had decreased to 0.7 mg/dL. The pleural effusion (believed to have been a parapneumonic effusion) had resolved.
Discussion
All 3 patients met the Fried criteria for frailty, although there were several confounding issues.2 All 3 patients lost between 20 and 25 lb; all had clearly become weaker according to nursing and rehabilitation staff (although none were formally assessed for grip strength); and all had clear declines in their activity level. Patient 3 had a clear decrement in gait speed, but patient 1 had severe gait impairment due to Parkinson disease (although his gait in therapy had clearly worsened). Patient 2 was paraparetic and unable to ambulate. There also was evidence of limited biomarkers of systemic inflammation; all 3 patients’ albumin had decreased, and patients 2 and 3 had significant decrease in hemoglobin; but these commonplace clinical biomarkers are obviously multifactorially determined. We have limited data on our patients’ CRP levels; serial levels would have been more specific for systemic inflammation but were infrequently performed on the patients.
Multimorbidity and medical complexity are more the rule than the exception in frail geriatric patients,and it is difficult to separate the role of microaspiration from other confounding conditions that might have contributed to these patients’ evolving systemic inflammation and frailty.18 It might be argued that the decline for patient 1 was related to the underlying Parkinson disease (a progressive neurologic illness in which systemic inflammation has been reported), or that the decline of patient 2 was related to the worsening pressure injuries rather than to covert microaspiration.19 However, the TBIs for patient 2 and the schizophrenia for patient 3 would not be expected to be associated with frailty or with systemic inflammation. Furthermore, the frailty symptoms of patient 3 and inflammatory biomarkers improved after the risperidone, which was likely responsible for his microaspiration, was discontinued. All 3 patients were at risk for oropharyngeal dysphagia (antipsychotic medication is clearly associated with dysphagia20); patient 2 demonstrated pathologic evidence of DAB at autopsy.
There is evolving evidence that chronic systemic inflammation and immune activation are key mechanisms in the pathogenesis of frailty.4-6 It is known that elevated serum levels of proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6, and CRP are directly associated with frailty and are inversely associated with levels of albumin, hemoglobin, insulin-like growth factor-1, and several micronutrients in frail individuals.4-7,21,22 Chronic inflammation contributes to the pathophysiology of frailty through detrimental effects on a broad range of systems, including the musculoskeletal, endocrine, and hematopoietic systems and through nutritional dysregulation.2,4,23 These changes may lead to further deleterious effects, creating a downward spiral of worsening frailty. For example, it seems likely that our patients’ progressive weakness further compromised airway protection, creating a vicious cycle of worsening microaspiration and chronic inflammation.
Conclusions
To date, the role of chronic microaspiration and DAB in chronic systemic inflammation or in frailty has not been explored. Given the prevalence of microaspiration in nursing home residents and the devastating consequences of frailty, though, this seems to be a crucial area of investigation. It is equally crucial for long-term care staff, both providers and nursing staff, to have a heightened awareness of covert microaspiration and a low threshold for referral to speech pathology for further investigation. Staff also should be aware of the utility of the Fried criteria to improve identification of frailty in general. It is probable that covert microaspiration will prove to be an important part of the differential diagnosis of frailty.
1. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940-945. doi:10.1016/j.jamda.2015.06.025
2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. doi:10.1093/gerona/56.3.m146
3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi:10.1016/j.jamda.2013.03.022
4. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. doi:10.2147/CIA.S45300.
5. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi:10.1016/j.arr.2016.08.006
6. Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777-1783. doi:10.1111/jgs.14876
7. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-882. doi:10.1016/j.jamda.2013.05.009
8. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218-1225. doi:10.1093/gerona/glw240
9. Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs. 2000;26(8):35-48. doi:10.3928/0098-9134-20000801-09
10. Altman KW, Yu GP, Schaefer SD. Consequences of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784-789. doi:10.1001/archoto.2010.129
11. Sakai K, Hirano H, Watanabe Y, et al. An examination of factors related to aspiration and silent aspiration in older adults requiring long-term care in rural Japan. J Oral Rehabil. 2016;43(2):103-110. doi:10.1111/joor.12349
12. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180-184. doi:10.1007/BF00366381
13. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270-274. doi:10.1007/s00455-006-9051-6
14. Matsuse T, Oka T, Kida K, Fukuchi Y. Importance of diffuse aspiration bronchiolitis caused by chronic occult aspiration in the elderly. Chest. 1996;110(5):1289-1293. doi:10.1378/chest.110.5.1289
15. Cardasis JJ, MacMahon H, Husain AN. The spectrum of lung disease due to chronic occult aspiration. Ann Am Thorac Soc. 2014;11(6):865-873. doi:10.1513/AnnalsATS.201310-360OC
16. Pereira-Silva JL, Silva CIS, Araujo Neto CA, Andrade TL, Muller NL. Chronic pulmonary microaspiration: high-resolution computed tomographic findings in 13 patients. J Thorac Imaging. 2014;29(5):298-303. doi:10.1097/RTI.0000000000000091
17. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815-823. doi:10.1378/chest.14-1049
18. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Aging. 2017;46(6):882-888. doi:10.1093/ageing/afx150
19. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80-91. doi:10.1016/j.freeradbiomed.2017.10.379
20. Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467-471. doi:10.1007/s00455-017-9802-6
21. Velissaris D, Pantzaris N, Koniari I, et al. C-reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461-465. doi:10.14740/jocmr2959w
22. Hubbard RE, O’Mahoney MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103-3109. doi:10.1111/j.1582-4934.2009.00733.x
23. Argiles JM, Busquets S, Stemmler B, Lotez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. doi:10.1016/j.coph.2015.04.003
1. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940-945. doi:10.1016/j.jamda.2015.06.025
2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. doi:10.1093/gerona/56.3.m146
3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi:10.1016/j.jamda.2013.03.022
4. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. doi:10.2147/CIA.S45300.
5. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi:10.1016/j.arr.2016.08.006
6. Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777-1783. doi:10.1111/jgs.14876
7. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-882. doi:10.1016/j.jamda.2013.05.009
8. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218-1225. doi:10.1093/gerona/glw240
9. Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs. 2000;26(8):35-48. doi:10.3928/0098-9134-20000801-09
10. Altman KW, Yu GP, Schaefer SD. Consequences of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784-789. doi:10.1001/archoto.2010.129
11. Sakai K, Hirano H, Watanabe Y, et al. An examination of factors related to aspiration and silent aspiration in older adults requiring long-term care in rural Japan. J Oral Rehabil. 2016;43(2):103-110. doi:10.1111/joor.12349
12. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180-184. doi:10.1007/BF00366381
13. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270-274. doi:10.1007/s00455-006-9051-6
14. Matsuse T, Oka T, Kida K, Fukuchi Y. Importance of diffuse aspiration bronchiolitis caused by chronic occult aspiration in the elderly. Chest. 1996;110(5):1289-1293. doi:10.1378/chest.110.5.1289
15. Cardasis JJ, MacMahon H, Husain AN. The spectrum of lung disease due to chronic occult aspiration. Ann Am Thorac Soc. 2014;11(6):865-873. doi:10.1513/AnnalsATS.201310-360OC
16. Pereira-Silva JL, Silva CIS, Araujo Neto CA, Andrade TL, Muller NL. Chronic pulmonary microaspiration: high-resolution computed tomographic findings in 13 patients. J Thorac Imaging. 2014;29(5):298-303. doi:10.1097/RTI.0000000000000091
17. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815-823. doi:10.1378/chest.14-1049
18. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Aging. 2017;46(6):882-888. doi:10.1093/ageing/afx150
19. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80-91. doi:10.1016/j.freeradbiomed.2017.10.379
20. Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467-471. doi:10.1007/s00455-017-9802-6
21. Velissaris D, Pantzaris N, Koniari I, et al. C-reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461-465. doi:10.14740/jocmr2959w
22. Hubbard RE, O’Mahoney MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103-3109. doi:10.1111/j.1582-4934.2009.00733.x
23. Argiles JM, Busquets S, Stemmler B, Lotez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. doi:10.1016/j.coph.2015.04.003