User login
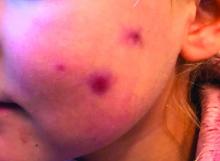
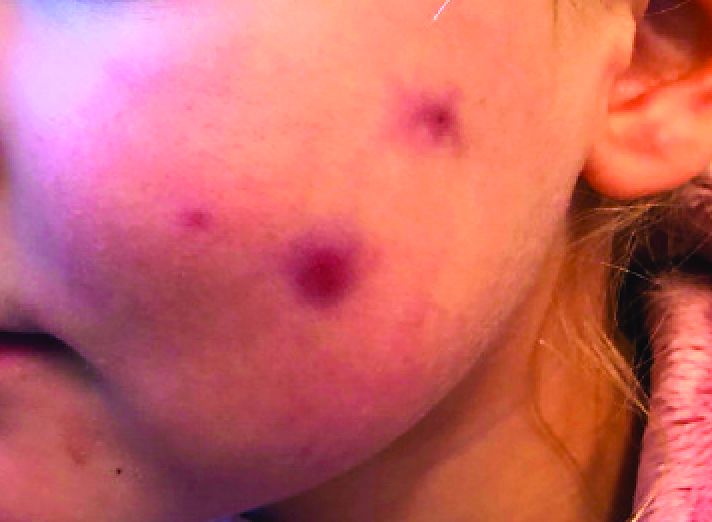
A 4-year-old with a lesion on her cheek, which grew and became firmer over two months
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
A 4-year-old female is brought to our pediatric dermatology clinic for evaluation of a persistent lesion on the cheek.
The mother of the child reports that the lesion started as a small "bug bite" and then started growing and getting firmer for the past 2 months. The girl has developed other smaller red, pimple-like lesions on the cheeks and one of them is starting to increase in size.
She denies any tenderness on the area or any purulent discharge. She has had no fevers, chills, weight loss, nose bleeds, fatigue, or any other symptoms. The mother has not noted any changes on the child's body odor, any rapid growth, or hair on her axillary or pubic area. She was treated with three different courses of oral antibiotics including cephalexin, trimethoprim/sulfamethoxazole, and clindamycin, as well as topical mupirocin, with no improvement.
Her past medical history is significant for several episodes of eyelid cysts that were treated with warm compresses and topical erythromycin ointment. The family history is significant for the father having severe acne as a teenager. She has two cats, she has not traveled, and she has an older sister who has no lesions.
On physical examination she is a lovely 4-year-old female in no acute distress. Her height is on the 70th percentile and weight on the 40th percentile for her age. Her blood pressure is 95/84 with a heart rate of 96. On skin examination she has several pink macules and papules on her bilateral cheeks. On the left cheek there are two pink nodules: One is 1 cm, and the other is 7 mm. The nodules are not tender. There is no warmth, fluctuance, or discharge from the lesions.
She has no cervical lymphadenopathy. She has no axillary or pubic hair. She is Tanner stage I.
PHM20 Virtual: Can’t miss heart disease for hospitalists
PHM20 Virtual session title
Can’t Miss Heart Disease for Hospitalists
Presenter
Erich Maul, DO, MPH, FAAP, SFHM
Session summary
Dr. Erich Maul, professor of pediatrics, medical director for progressive care and acute care, and chief of hospital pediatrics at Kentucky Children’s Hospital, Lexington, presented an engaging, case-based approach to evaluate heart disease when “on call.” He iterated the importance of recognizing congenital heart disease, especially since 25% of these patients usually need surgical intervention within the first month of diagnosis and about 50% of congenital heart disease patients do not have a murmur.
Presenting cases seen during a busy hospitalist call night, Dr. Maul highlighted that patients can present with signs of heart failure, cyanosis, sepsis or hypoperfusion, failure to thrive, and respiratory distress or failure. He discussed the presentation, epidemiology, diagnosis, treatment, and prognosis. He also provided examples of common arrhythmias and provided refreshers on management using basic life support (BLS) and pediatric advanced life support.
Key takeaways
- Always start with the nine steps to resuscitation: ABC (airway, breathing, circulation), ABC, oxygen, access, monitoring.
- Early BLS is important.
- Congenital heart disease often presents with either cyanosis, hypoperfusion, failure to thrive, or respiratory distress.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
PHM20 Virtual session title
Can’t Miss Heart Disease for Hospitalists
Presenter
Erich Maul, DO, MPH, FAAP, SFHM
Session summary
Dr. Erich Maul, professor of pediatrics, medical director for progressive care and acute care, and chief of hospital pediatrics at Kentucky Children’s Hospital, Lexington, presented an engaging, case-based approach to evaluate heart disease when “on call.” He iterated the importance of recognizing congenital heart disease, especially since 25% of these patients usually need surgical intervention within the first month of diagnosis and about 50% of congenital heart disease patients do not have a murmur.
Presenting cases seen during a busy hospitalist call night, Dr. Maul highlighted that patients can present with signs of heart failure, cyanosis, sepsis or hypoperfusion, failure to thrive, and respiratory distress or failure. He discussed the presentation, epidemiology, diagnosis, treatment, and prognosis. He also provided examples of common arrhythmias and provided refreshers on management using basic life support (BLS) and pediatric advanced life support.
Key takeaways
- Always start with the nine steps to resuscitation: ABC (airway, breathing, circulation), ABC, oxygen, access, monitoring.
- Early BLS is important.
- Congenital heart disease often presents with either cyanosis, hypoperfusion, failure to thrive, or respiratory distress.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
PHM20 Virtual session title
Can’t Miss Heart Disease for Hospitalists
Presenter
Erich Maul, DO, MPH, FAAP, SFHM
Session summary
Dr. Erich Maul, professor of pediatrics, medical director for progressive care and acute care, and chief of hospital pediatrics at Kentucky Children’s Hospital, Lexington, presented an engaging, case-based approach to evaluate heart disease when “on call.” He iterated the importance of recognizing congenital heart disease, especially since 25% of these patients usually need surgical intervention within the first month of diagnosis and about 50% of congenital heart disease patients do not have a murmur.
Presenting cases seen during a busy hospitalist call night, Dr. Maul highlighted that patients can present with signs of heart failure, cyanosis, sepsis or hypoperfusion, failure to thrive, and respiratory distress or failure. He discussed the presentation, epidemiology, diagnosis, treatment, and prognosis. He also provided examples of common arrhythmias and provided refreshers on management using basic life support (BLS) and pediatric advanced life support.
Key takeaways
- Always start with the nine steps to resuscitation: ABC (airway, breathing, circulation), ABC, oxygen, access, monitoring.
- Early BLS is important.
- Congenital heart disease often presents with either cyanosis, hypoperfusion, failure to thrive, or respiratory distress.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
SGLT2 inhibitors with metformin look safe for bone
The combination of sodium-glucose transporter-2 (SGLT-2) inhibitors and metformin is not associated with an increase in fracture risk among patients with type 2 diabetes (T2D), according to a new meta-analysis of 25 randomized, controlled trials.
Researchers at The Second Clinical College of Dalian Medical University in Jiangsu, China, compared fracture risk associated with the metformin/SLGT2 combination to metformin alone as well as other T2D therapeutics, and found no differences in risk. The study was published online Aug. 11 in Osteoporosis International.
T2D is associated with an increased risk of fracture, though causative mechanisms remain uncertain. Some lines of evidence suggest multiple factors may contribute to fractures, including hyperglycemia, oxidative stress, toxic effects of advanced glycosylation end-products, altered insulin levels, and treatment-induced hypoglycemia, as well as an association between T2D and increased risk of falls.
Antidiabetes drugs can have positive or negative effects on bone. thiazolidinediones, insulin, and sulfonylureas may increase risk of fractures, while dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-2 (GLP-2) receptor agonists may be protective. Metformin may also reduce fracture risk.
SGLT-2 inhibitors interrupt glucose reabsorption in the kidney, leading to improved glycemic control. Other benefits include improved renal and cardiovascular outcomes, weight loss, and reduced blood pressure, liver fat, and serum uric acid levels.
These properties have made SGLT-2 inhibitors combined with metformin an important therapy for patients at high risk of atherosclerotic disease, or who have heart failure or chronic kidney disease.
But SGLT-2 inhibition increases osmotic diuresis, and this could alter the mineral balance within bone. Some studies also showed that SGLT-2 inhibitors led to changes in bone turnover markers, bone mineral density, and bone microarchitecture. Observational studies of the SGLT-2 inhibitor canagliflozin found associations with a higher rate of fracture risk in patients taking the drug.
Such studies carry the risk of confounding factors, so the researchers took advantage of the fact that many recent clinical trials have examined the impact of SGLT-2 inhibitors on T2D. They pooled data from 25 clinical trials with a total of 19,500 participants, 9,662 of whom received SGLT-2 inhibitors plus metformin; 9,838 received other active comparators.
The fracture rate was 0.91% in the SGLT-2 inhibitors/metformin group, and 0.80% among controls (odds ratio, 0.97; 95% CI, 0.71-1.32), with no heterogeneity. Metformin alone was not associated with a change in fracture rate (OR, 0.95; 95% CI, 0.44-2.08), nor were other forms of diabetes control (OR, 0.95; 95% CI, 0.69-1.31).
There were some differences in fracture risk among SGLT-2 inhibitors when studied individually, though none differed significantly from controls. The highest risk was associated with the canagliflozin/metformin (OR, 2.19; 95% CI, 0.66-7.27), followed by dapagliflozin/metformin (OR, 0.91; 95% CI, 0.50-1.64), empagliflozin/metformin (OR, 0.94; 95% CI, 0.59-1.50), and ertugliflozin/metformin (OR, 0.76; 95% CI, 0.38-1.54).
There were no differences with respect to hip or lumbar spine fractures, or other fractures. The researchers found no differences in bone mineral density or bone turnover markers.
The meta-analysis is limited by the relatively short average follow-up in the included studies, which was 61 weeks. Bone damage may occur over longer time periods. Bone fractures were also not a prespecified adverse event in most included studies.
The studies also did not provide detailed information on the types of fractures experienced, such as whether they were result of a fall, or the location of the fracture, or bone health parameters. Although the results support a belief that SGLT-2 inhibitors do not adversely affect bone health, “given limited information on bone health outcomes, further work is needed to validate this conclusion,” the authors wrote.
The authors did not disclose any funding and had no relevant conflicts of interest.
SOURCE: B-B Qian et al. Osteoporosis Int. 2020 Aug 11. doi: 10.1007/s00198-020-05590-y.
The combination of sodium-glucose transporter-2 (SGLT-2) inhibitors and metformin is not associated with an increase in fracture risk among patients with type 2 diabetes (T2D), according to a new meta-analysis of 25 randomized, controlled trials.
Researchers at The Second Clinical College of Dalian Medical University in Jiangsu, China, compared fracture risk associated with the metformin/SLGT2 combination to metformin alone as well as other T2D therapeutics, and found no differences in risk. The study was published online Aug. 11 in Osteoporosis International.
T2D is associated with an increased risk of fracture, though causative mechanisms remain uncertain. Some lines of evidence suggest multiple factors may contribute to fractures, including hyperglycemia, oxidative stress, toxic effects of advanced glycosylation end-products, altered insulin levels, and treatment-induced hypoglycemia, as well as an association between T2D and increased risk of falls.
Antidiabetes drugs can have positive or negative effects on bone. thiazolidinediones, insulin, and sulfonylureas may increase risk of fractures, while dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-2 (GLP-2) receptor agonists may be protective. Metformin may also reduce fracture risk.
SGLT-2 inhibitors interrupt glucose reabsorption in the kidney, leading to improved glycemic control. Other benefits include improved renal and cardiovascular outcomes, weight loss, and reduced blood pressure, liver fat, and serum uric acid levels.
These properties have made SGLT-2 inhibitors combined with metformin an important therapy for patients at high risk of atherosclerotic disease, or who have heart failure or chronic kidney disease.
But SGLT-2 inhibition increases osmotic diuresis, and this could alter the mineral balance within bone. Some studies also showed that SGLT-2 inhibitors led to changes in bone turnover markers, bone mineral density, and bone microarchitecture. Observational studies of the SGLT-2 inhibitor canagliflozin found associations with a higher rate of fracture risk in patients taking the drug.
Such studies carry the risk of confounding factors, so the researchers took advantage of the fact that many recent clinical trials have examined the impact of SGLT-2 inhibitors on T2D. They pooled data from 25 clinical trials with a total of 19,500 participants, 9,662 of whom received SGLT-2 inhibitors plus metformin; 9,838 received other active comparators.
The fracture rate was 0.91% in the SGLT-2 inhibitors/metformin group, and 0.80% among controls (odds ratio, 0.97; 95% CI, 0.71-1.32), with no heterogeneity. Metformin alone was not associated with a change in fracture rate (OR, 0.95; 95% CI, 0.44-2.08), nor were other forms of diabetes control (OR, 0.95; 95% CI, 0.69-1.31).
There were some differences in fracture risk among SGLT-2 inhibitors when studied individually, though none differed significantly from controls. The highest risk was associated with the canagliflozin/metformin (OR, 2.19; 95% CI, 0.66-7.27), followed by dapagliflozin/metformin (OR, 0.91; 95% CI, 0.50-1.64), empagliflozin/metformin (OR, 0.94; 95% CI, 0.59-1.50), and ertugliflozin/metformin (OR, 0.76; 95% CI, 0.38-1.54).
There were no differences with respect to hip or lumbar spine fractures, or other fractures. The researchers found no differences in bone mineral density or bone turnover markers.
The meta-analysis is limited by the relatively short average follow-up in the included studies, which was 61 weeks. Bone damage may occur over longer time periods. Bone fractures were also not a prespecified adverse event in most included studies.
The studies also did not provide detailed information on the types of fractures experienced, such as whether they were result of a fall, or the location of the fracture, or bone health parameters. Although the results support a belief that SGLT-2 inhibitors do not adversely affect bone health, “given limited information on bone health outcomes, further work is needed to validate this conclusion,” the authors wrote.
The authors did not disclose any funding and had no relevant conflicts of interest.
SOURCE: B-B Qian et al. Osteoporosis Int. 2020 Aug 11. doi: 10.1007/s00198-020-05590-y.
The combination of sodium-glucose transporter-2 (SGLT-2) inhibitors and metformin is not associated with an increase in fracture risk among patients with type 2 diabetes (T2D), according to a new meta-analysis of 25 randomized, controlled trials.
Researchers at The Second Clinical College of Dalian Medical University in Jiangsu, China, compared fracture risk associated with the metformin/SLGT2 combination to metformin alone as well as other T2D therapeutics, and found no differences in risk. The study was published online Aug. 11 in Osteoporosis International.
T2D is associated with an increased risk of fracture, though causative mechanisms remain uncertain. Some lines of evidence suggest multiple factors may contribute to fractures, including hyperglycemia, oxidative stress, toxic effects of advanced glycosylation end-products, altered insulin levels, and treatment-induced hypoglycemia, as well as an association between T2D and increased risk of falls.
Antidiabetes drugs can have positive or negative effects on bone. thiazolidinediones, insulin, and sulfonylureas may increase risk of fractures, while dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-2 (GLP-2) receptor agonists may be protective. Metformin may also reduce fracture risk.
SGLT-2 inhibitors interrupt glucose reabsorption in the kidney, leading to improved glycemic control. Other benefits include improved renal and cardiovascular outcomes, weight loss, and reduced blood pressure, liver fat, and serum uric acid levels.
These properties have made SGLT-2 inhibitors combined with metformin an important therapy for patients at high risk of atherosclerotic disease, or who have heart failure or chronic kidney disease.
But SGLT-2 inhibition increases osmotic diuresis, and this could alter the mineral balance within bone. Some studies also showed that SGLT-2 inhibitors led to changes in bone turnover markers, bone mineral density, and bone microarchitecture. Observational studies of the SGLT-2 inhibitor canagliflozin found associations with a higher rate of fracture risk in patients taking the drug.
Such studies carry the risk of confounding factors, so the researchers took advantage of the fact that many recent clinical trials have examined the impact of SGLT-2 inhibitors on T2D. They pooled data from 25 clinical trials with a total of 19,500 participants, 9,662 of whom received SGLT-2 inhibitors plus metformin; 9,838 received other active comparators.
The fracture rate was 0.91% in the SGLT-2 inhibitors/metformin group, and 0.80% among controls (odds ratio, 0.97; 95% CI, 0.71-1.32), with no heterogeneity. Metformin alone was not associated with a change in fracture rate (OR, 0.95; 95% CI, 0.44-2.08), nor were other forms of diabetes control (OR, 0.95; 95% CI, 0.69-1.31).
There were some differences in fracture risk among SGLT-2 inhibitors when studied individually, though none differed significantly from controls. The highest risk was associated with the canagliflozin/metformin (OR, 2.19; 95% CI, 0.66-7.27), followed by dapagliflozin/metformin (OR, 0.91; 95% CI, 0.50-1.64), empagliflozin/metformin (OR, 0.94; 95% CI, 0.59-1.50), and ertugliflozin/metformin (OR, 0.76; 95% CI, 0.38-1.54).
There were no differences with respect to hip or lumbar spine fractures, or other fractures. The researchers found no differences in bone mineral density or bone turnover markers.
The meta-analysis is limited by the relatively short average follow-up in the included studies, which was 61 weeks. Bone damage may occur over longer time periods. Bone fractures were also not a prespecified adverse event in most included studies.
The studies also did not provide detailed information on the types of fractures experienced, such as whether they were result of a fall, or the location of the fracture, or bone health parameters. Although the results support a belief that SGLT-2 inhibitors do not adversely affect bone health, “given limited information on bone health outcomes, further work is needed to validate this conclusion,” the authors wrote.
The authors did not disclose any funding and had no relevant conflicts of interest.
SOURCE: B-B Qian et al. Osteoporosis Int. 2020 Aug 11. doi: 10.1007/s00198-020-05590-y.
FROM OSTEOPOROSIS INTERNATIONAL
COVID-19 child case count now over 400,000
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
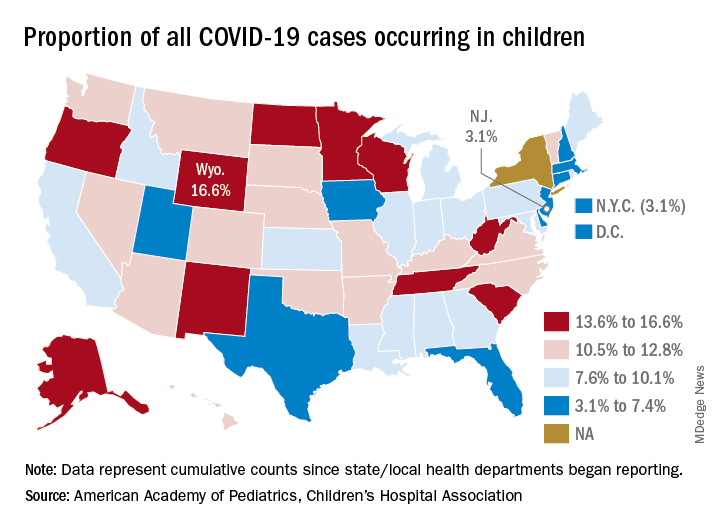
The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
Are aging physicians a burden?
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
The evaluation of physicians with alleged cognitive decline
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
HFNC more comfortable for posthypercapnic patients with COPD
Following invasive ventilation for severe hypercapnic respiratory failure, patients with chronic obstructive pulmonary disease had similar levels of treatment failure if they received high-flow nasal cannula oxygen therapy or noninvasive ventilation, recent research in Critical Care has suggested.
However, for patients with COPD weaned off invasive ventilation, high-flow nasal cannula (HFNC) oxygen therapy was “more comfortable and better tolerated,” compared with noninvasive ventilation (NIV). In addition, “airway care interventions and the incidence of nasofacial skin breakdown associated with HFNC were significantly lower than in NIV,” according to Dingyu Tan of the Clinical Medical College of Yangzhou (China) University, Northern Jiangsu People’s Hospital, and colleagues. “HFNC appears to be an effective means of respiratory support for COPD patients extubated after severe hypercapnic respiratory failure,” they said.
The investigators screened patients with COPD and hypercapnic respiratory failure for enrollment, including those who met Global Initiative for Obstructive Lung Disease (GOLD) criteria, were 85 years old or younger and caring for themselves, had bronchopulmonary infection–induced respiratory failure, and had achieved pulmonary infection control criteria. Exclusion criteria were:
- Patients under age 18 years.
- Presence of oral or facial trauma.
- Poor sputum excretion ability.
- Hemodynamic instability that would contraindicate use of NIV.
- Poor cough during PIC window.
- Poor short-term prognosis.
- Failure of the heart, brain, liver or kidney.
- Patients who could not consent to treatment.
Patients were determined to have failed treatment if they returned to invasive mechanical ventilation or switched from one treatment to another (HFNC to NIV or NIV to HFNC). Investigators also performed an arterial blood gas analysis, recorded the number of duration of airway care interventions, and monitored vital signs at 1 hour, 24 hours, and 48 hours after extubation as secondary analyses.
Overall, 44 patients randomized to receive HFNC and 42 patients randomized for NIV were available for analysis. The investigators found 22.7% of patients in the HFNC group and 28.6% in the NIV group experienced treatment failure (risk difference, –5.8%; 95% confidence interval, −23.8 to 12.4%; P = .535), with patients in the HFNC group experiencing a significantly lower level of treatment intolerance, compared with patients in the NIV group (risk difference, –50.0%; 95% CI, −74.6 to −12.9%; P = .015). There were no significant differences between either group regarding intubation (−0.65%; 95% CI, −16.01 to 14.46%), while rate of switching treatments was lower in the HFNC group but not significant (−5.2%; 95% CI, −19.82 to 9.05%).
Patients in both the HFNC and NIV groups had faster mean respiratory rates 1 hour after extubation (P < .050). After 24 hours, the NIV group had higher-than-baseline respiratory rates, compared with the HFNC group, which had returned to normal (20 vs. 24.5 breaths per minute; P < .050). Both groups had returned to baseline by 48 hours after extubation. At 1 hour after extubation, patients in the HFNC group had lower PaO2/FiO2 (P < .050) and pH values (P < .050), and higher PaCO2 values (P less than .050), compared with baseline. There were no statistically significant differences in PaO2/FiO2, pH, and PaCO2 values in either group at 24 hours or 48 hours after extubation.
Daily airway care interventions were significantly higher on average in the NIV group, compared with the HFNC group (7 vs. 6; P = .0006), and the HFNC group also had significantly better comfort scores (7 vs. 5; P < .001) as measured by a modified visual analog scale, as well as incidence of nasal and facial skin breakdown (0 vs. 9.6%; P = .027), compared with the NIV group.
Results difficult to apply to North American patients
David L. Bowton, MD, FCCP, a professor specializing in critical care at Wake Forest University, Winston-Salem, N.C., said in an interview the results of this trial may not be applicable for patients with infection-related respiratory failure and COPD in North America “due to the differences in common weaning practices between North America and China.”
For example, the trial used the pulmonary infection control (PIC) window criteria for extubation, which requires a significant decrease in radiographic infiltrates, improvement in quality and quantity of sputum, normalizing of leukocyte count, a synchronized intermittent mandatory ventilation (SIMV) rate of 10-12 breaths per minute, and pressure support less than 10-12 cm/H2O (Int J Chron Obstruct Pulmon Dis. 2017;12:1255-67).
“The process used to achieve these measures is not standardized. In North America, daily awakening and screening for spontaneous breathing trials would be usual, but this was not reported in the current trial,” he explained.
Differences in patient population also make the application of the results difficult, Dr. Bowton said. “Only 60% of the patients had spirometrically confirmed COPD and fewer than half were on at least dual inhaled therapy prior to hospitalization with only one-third taking beta agonists or anticholinergic agents,” he noted. “The cause of respiratory failure was infectious, requiring an infiltrate on chest radiograph; thus, patients with hypercarbic respiratory failure without a new infiltrate were excluded from the study. On average, patients were hypercarbic, yet alkalemic at the time of extubation; the PaCO2 and pH at the time of intubation were not reported.
“This study suggests that in some patients with COPD and respiratory failure requiring invasive mechanical ventilation, HFO [high-flow oxygen] may be better tolerated and equally effective as NIPPV [noninvasive positive-pressure ventilation] at mitigating the need for reintubation following extubation. In this patient population where hypoxemia prior to extubation was not severe, the mechanisms by which HFO is beneficial remain speculative,” he said.
This study was funded by the Rui E special fund for emergency medicine research and the Yangzhou Science and Technology Development Plan. The authors report no relevant conflicts of interest. Dr. Bowton reports no relevant conflicts of interest.
SOURCE: Tan D et al. Crit Care. 2020 Aug 6. doi: 10.1186/s13054-020-03214-9.
Following invasive ventilation for severe hypercapnic respiratory failure, patients with chronic obstructive pulmonary disease had similar levels of treatment failure if they received high-flow nasal cannula oxygen therapy or noninvasive ventilation, recent research in Critical Care has suggested.
However, for patients with COPD weaned off invasive ventilation, high-flow nasal cannula (HFNC) oxygen therapy was “more comfortable and better tolerated,” compared with noninvasive ventilation (NIV). In addition, “airway care interventions and the incidence of nasofacial skin breakdown associated with HFNC were significantly lower than in NIV,” according to Dingyu Tan of the Clinical Medical College of Yangzhou (China) University, Northern Jiangsu People’s Hospital, and colleagues. “HFNC appears to be an effective means of respiratory support for COPD patients extubated after severe hypercapnic respiratory failure,” they said.
The investigators screened patients with COPD and hypercapnic respiratory failure for enrollment, including those who met Global Initiative for Obstructive Lung Disease (GOLD) criteria, were 85 years old or younger and caring for themselves, had bronchopulmonary infection–induced respiratory failure, and had achieved pulmonary infection control criteria. Exclusion criteria were:
- Patients under age 18 years.
- Presence of oral or facial trauma.
- Poor sputum excretion ability.
- Hemodynamic instability that would contraindicate use of NIV.
- Poor cough during PIC window.
- Poor short-term prognosis.
- Failure of the heart, brain, liver or kidney.
- Patients who could not consent to treatment.
Patients were determined to have failed treatment if they returned to invasive mechanical ventilation or switched from one treatment to another (HFNC to NIV or NIV to HFNC). Investigators also performed an arterial blood gas analysis, recorded the number of duration of airway care interventions, and monitored vital signs at 1 hour, 24 hours, and 48 hours after extubation as secondary analyses.
Overall, 44 patients randomized to receive HFNC and 42 patients randomized for NIV were available for analysis. The investigators found 22.7% of patients in the HFNC group and 28.6% in the NIV group experienced treatment failure (risk difference, –5.8%; 95% confidence interval, −23.8 to 12.4%; P = .535), with patients in the HFNC group experiencing a significantly lower level of treatment intolerance, compared with patients in the NIV group (risk difference, –50.0%; 95% CI, −74.6 to −12.9%; P = .015). There were no significant differences between either group regarding intubation (−0.65%; 95% CI, −16.01 to 14.46%), while rate of switching treatments was lower in the HFNC group but not significant (−5.2%; 95% CI, −19.82 to 9.05%).
Patients in both the HFNC and NIV groups had faster mean respiratory rates 1 hour after extubation (P < .050). After 24 hours, the NIV group had higher-than-baseline respiratory rates, compared with the HFNC group, which had returned to normal (20 vs. 24.5 breaths per minute; P < .050). Both groups had returned to baseline by 48 hours after extubation. At 1 hour after extubation, patients in the HFNC group had lower PaO2/FiO2 (P < .050) and pH values (P < .050), and higher PaCO2 values (P less than .050), compared with baseline. There were no statistically significant differences in PaO2/FiO2, pH, and PaCO2 values in either group at 24 hours or 48 hours after extubation.
Daily airway care interventions were significantly higher on average in the NIV group, compared with the HFNC group (7 vs. 6; P = .0006), and the HFNC group also had significantly better comfort scores (7 vs. 5; P < .001) as measured by a modified visual analog scale, as well as incidence of nasal and facial skin breakdown (0 vs. 9.6%; P = .027), compared with the NIV group.
Results difficult to apply to North American patients
David L. Bowton, MD, FCCP, a professor specializing in critical care at Wake Forest University, Winston-Salem, N.C., said in an interview the results of this trial may not be applicable for patients with infection-related respiratory failure and COPD in North America “due to the differences in common weaning practices between North America and China.”
For example, the trial used the pulmonary infection control (PIC) window criteria for extubation, which requires a significant decrease in radiographic infiltrates, improvement in quality and quantity of sputum, normalizing of leukocyte count, a synchronized intermittent mandatory ventilation (SIMV) rate of 10-12 breaths per minute, and pressure support less than 10-12 cm/H2O (Int J Chron Obstruct Pulmon Dis. 2017;12:1255-67).
“The process used to achieve these measures is not standardized. In North America, daily awakening and screening for spontaneous breathing trials would be usual, but this was not reported in the current trial,” he explained.
Differences in patient population also make the application of the results difficult, Dr. Bowton said. “Only 60% of the patients had spirometrically confirmed COPD and fewer than half were on at least dual inhaled therapy prior to hospitalization with only one-third taking beta agonists or anticholinergic agents,” he noted. “The cause of respiratory failure was infectious, requiring an infiltrate on chest radiograph; thus, patients with hypercarbic respiratory failure without a new infiltrate were excluded from the study. On average, patients were hypercarbic, yet alkalemic at the time of extubation; the PaCO2 and pH at the time of intubation were not reported.
“This study suggests that in some patients with COPD and respiratory failure requiring invasive mechanical ventilation, HFO [high-flow oxygen] may be better tolerated and equally effective as NIPPV [noninvasive positive-pressure ventilation] at mitigating the need for reintubation following extubation. In this patient population where hypoxemia prior to extubation was not severe, the mechanisms by which HFO is beneficial remain speculative,” he said.
This study was funded by the Rui E special fund for emergency medicine research and the Yangzhou Science and Technology Development Plan. The authors report no relevant conflicts of interest. Dr. Bowton reports no relevant conflicts of interest.
SOURCE: Tan D et al. Crit Care. 2020 Aug 6. doi: 10.1186/s13054-020-03214-9.
Following invasive ventilation for severe hypercapnic respiratory failure, patients with chronic obstructive pulmonary disease had similar levels of treatment failure if they received high-flow nasal cannula oxygen therapy or noninvasive ventilation, recent research in Critical Care has suggested.
However, for patients with COPD weaned off invasive ventilation, high-flow nasal cannula (HFNC) oxygen therapy was “more comfortable and better tolerated,” compared with noninvasive ventilation (NIV). In addition, “airway care interventions and the incidence of nasofacial skin breakdown associated with HFNC were significantly lower than in NIV,” according to Dingyu Tan of the Clinical Medical College of Yangzhou (China) University, Northern Jiangsu People’s Hospital, and colleagues. “HFNC appears to be an effective means of respiratory support for COPD patients extubated after severe hypercapnic respiratory failure,” they said.
The investigators screened patients with COPD and hypercapnic respiratory failure for enrollment, including those who met Global Initiative for Obstructive Lung Disease (GOLD) criteria, were 85 years old or younger and caring for themselves, had bronchopulmonary infection–induced respiratory failure, and had achieved pulmonary infection control criteria. Exclusion criteria were:
- Patients under age 18 years.
- Presence of oral or facial trauma.
- Poor sputum excretion ability.
- Hemodynamic instability that would contraindicate use of NIV.
- Poor cough during PIC window.
- Poor short-term prognosis.
- Failure of the heart, brain, liver or kidney.
- Patients who could not consent to treatment.
Patients were determined to have failed treatment if they returned to invasive mechanical ventilation or switched from one treatment to another (HFNC to NIV or NIV to HFNC). Investigators also performed an arterial blood gas analysis, recorded the number of duration of airway care interventions, and monitored vital signs at 1 hour, 24 hours, and 48 hours after extubation as secondary analyses.
Overall, 44 patients randomized to receive HFNC and 42 patients randomized for NIV were available for analysis. The investigators found 22.7% of patients in the HFNC group and 28.6% in the NIV group experienced treatment failure (risk difference, –5.8%; 95% confidence interval, −23.8 to 12.4%; P = .535), with patients in the HFNC group experiencing a significantly lower level of treatment intolerance, compared with patients in the NIV group (risk difference, –50.0%; 95% CI, −74.6 to −12.9%; P = .015). There were no significant differences between either group regarding intubation (−0.65%; 95% CI, −16.01 to 14.46%), while rate of switching treatments was lower in the HFNC group but not significant (−5.2%; 95% CI, −19.82 to 9.05%).
Patients in both the HFNC and NIV groups had faster mean respiratory rates 1 hour after extubation (P < .050). After 24 hours, the NIV group had higher-than-baseline respiratory rates, compared with the HFNC group, which had returned to normal (20 vs. 24.5 breaths per minute; P < .050). Both groups had returned to baseline by 48 hours after extubation. At 1 hour after extubation, patients in the HFNC group had lower PaO2/FiO2 (P < .050) and pH values (P < .050), and higher PaCO2 values (P less than .050), compared with baseline. There were no statistically significant differences in PaO2/FiO2, pH, and PaCO2 values in either group at 24 hours or 48 hours after extubation.
Daily airway care interventions were significantly higher on average in the NIV group, compared with the HFNC group (7 vs. 6; P = .0006), and the HFNC group also had significantly better comfort scores (7 vs. 5; P < .001) as measured by a modified visual analog scale, as well as incidence of nasal and facial skin breakdown (0 vs. 9.6%; P = .027), compared with the NIV group.
Results difficult to apply to North American patients
David L. Bowton, MD, FCCP, a professor specializing in critical care at Wake Forest University, Winston-Salem, N.C., said in an interview the results of this trial may not be applicable for patients with infection-related respiratory failure and COPD in North America “due to the differences in common weaning practices between North America and China.”
For example, the trial used the pulmonary infection control (PIC) window criteria for extubation, which requires a significant decrease in radiographic infiltrates, improvement in quality and quantity of sputum, normalizing of leukocyte count, a synchronized intermittent mandatory ventilation (SIMV) rate of 10-12 breaths per minute, and pressure support less than 10-12 cm/H2O (Int J Chron Obstruct Pulmon Dis. 2017;12:1255-67).
“The process used to achieve these measures is not standardized. In North America, daily awakening and screening for spontaneous breathing trials would be usual, but this was not reported in the current trial,” he explained.
Differences in patient population also make the application of the results difficult, Dr. Bowton said. “Only 60% of the patients had spirometrically confirmed COPD and fewer than half were on at least dual inhaled therapy prior to hospitalization with only one-third taking beta agonists or anticholinergic agents,” he noted. “The cause of respiratory failure was infectious, requiring an infiltrate on chest radiograph; thus, patients with hypercarbic respiratory failure without a new infiltrate were excluded from the study. On average, patients were hypercarbic, yet alkalemic at the time of extubation; the PaCO2 and pH at the time of intubation were not reported.
“This study suggests that in some patients with COPD and respiratory failure requiring invasive mechanical ventilation, HFO [high-flow oxygen] may be better tolerated and equally effective as NIPPV [noninvasive positive-pressure ventilation] at mitigating the need for reintubation following extubation. In this patient population where hypoxemia prior to extubation was not severe, the mechanisms by which HFO is beneficial remain speculative,” he said.
This study was funded by the Rui E special fund for emergency medicine research and the Yangzhou Science and Technology Development Plan. The authors report no relevant conflicts of interest. Dr. Bowton reports no relevant conflicts of interest.
SOURCE: Tan D et al. Crit Care. 2020 Aug 6. doi: 10.1186/s13054-020-03214-9.
FROM CRITICAL CARE
Diabetes plus weight loss equals increased risk of pancreatic cancer
A new study has linked recent-onset diabetes and subsequent weight loss to an increased risk of pancreatic cancer, indicating a distinct group of individuals to screen early for this deadly disease.
“The likelihood of a pancreatic cancer diagnosis was even further elevated among individuals with older age, healthy weight before weight loss, and unintentional weight loss,” wrote Chen Yuan, ScD, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston. The study was published in JAMA Oncology.
To determine whether an association exists between diabetes plus weight change and pancreatic cancer, the researchers analyzed decades of medical history data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The study population from the NHS included 112,818 women with a mean age of 59 years; the population from the HPFS included 46,207 men with a mean age of 65 years. Since enrollment – the baseline was 1978 for the NHS and 1988 for the HPFS – participants have provided follow-up information via biennial questionnaires.
Recent diabetes onset, weight loss boost cancer risk
From those combined groups, 1,116 incident cases of pancreatic cancer (0.7%) were identified. Compared with patients with no diabetes, patients with recent-onset diabetes had triple the risk of pancreatic cancer (age-adjusted hazard ratio, 2.97; 95% confidence interval, 2.31-3.82) and patients with longstanding diabetes had more than double the risk (HR, 2.16; 95% CI, 1.78-2.60). Patients with longer disease duration also had more than twice the risk of pancreatic cancer, with HRs of 2.25 for those with diabetes for 4-10 years (95% CI, 1.74-2.92) and 2.07 for more than 10 years (95% CI, 1.61-2.66).
Compared with patients who hadn’t lost any weight, patients who reported a 1- to 4-pound weight loss (HR, 1.25; 95% CI, 1.03-1.52), a 5- to 8-pound weight loss (HR, 1.33; 95% CI, 1.06-1.66), and a more than 8-pound weight loss (HR, 1.92; 95% CI, 1.58-2.32) had higher risks of pancreatic cancer. Patients with recent-onset diabetes and a 1- to 8-pound weight loss (91 incident cases per 100,000 person-years; 95% CI, 55-151) or a weight loss of more than 8 pounds (164 incident cases per 100,000 person years; 95% CI, 114-238) had a much higher incidence of pancreatic cancer, compared with patients with neither (16 incident cases per 100,000 person-years; 95% CI, 14-17).
After stratified analyses of patients with both recent-onset diabetes and weight loss, rates of pancreatic cancer were also notably high in those 70 years or older (234 cases per 100,000 person years), those with a body mass index of less than 25 kg/m2 before weight loss (400 cases per 100,000 person years), and those with a low likelihood of intentional weight loss (334 cases per 100,000 person years).
“I like the study because it reminds us of the importance of not thinking everyone that presents with type 2 diabetes necessarily has garden-variety diabetes,” Paul Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “I have always been concerned when a new-onset diabetic individual presents with no family history of diabetes or prediabetes, especially if they’re neither overweight nor obese. I have sometimes screened those individuals for pancreatic abnormalities.”
A call for screening
“This study highlights the consideration for further screening to those with weight loss at the time of diabetes diagnosis, which is very sensible given how unusual weight loss is as a presenting symptom at the time of diagnosis of typical type 2 diabetes,” Dr. Jellinger added. “The combination of weight loss and no family history of diabetes at the time of diagnosis should be an even stronger signal for pancreatic cancer screening and potential detection at a much earlier stage.”
The authors acknowledged their study’s limitations, including some patients with pancreatic cancer not returning their questionnaires and the timing of the questionnaires meaning that patients could’ve developed diabetes after returning it. In addition, they recognized that the participants were “predominantly White health professionals” and recommended a study of “additional patient populations” in the future.
The authors noted numerous potential conflicts of interest, including receiving grants and personal fees from various initiatives, organizations, and pharmaceutical companies.
SOURCE: Yuan C et al. JAMA Oncol. 2020 Aug 13. doi: 10.1001/jamaoncol.2020.2948.
A new study has linked recent-onset diabetes and subsequent weight loss to an increased risk of pancreatic cancer, indicating a distinct group of individuals to screen early for this deadly disease.
“The likelihood of a pancreatic cancer diagnosis was even further elevated among individuals with older age, healthy weight before weight loss, and unintentional weight loss,” wrote Chen Yuan, ScD, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston. The study was published in JAMA Oncology.
To determine whether an association exists between diabetes plus weight change and pancreatic cancer, the researchers analyzed decades of medical history data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The study population from the NHS included 112,818 women with a mean age of 59 years; the population from the HPFS included 46,207 men with a mean age of 65 years. Since enrollment – the baseline was 1978 for the NHS and 1988 for the HPFS – participants have provided follow-up information via biennial questionnaires.
Recent diabetes onset, weight loss boost cancer risk
From those combined groups, 1,116 incident cases of pancreatic cancer (0.7%) were identified. Compared with patients with no diabetes, patients with recent-onset diabetes had triple the risk of pancreatic cancer (age-adjusted hazard ratio, 2.97; 95% confidence interval, 2.31-3.82) and patients with longstanding diabetes had more than double the risk (HR, 2.16; 95% CI, 1.78-2.60). Patients with longer disease duration also had more than twice the risk of pancreatic cancer, with HRs of 2.25 for those with diabetes for 4-10 years (95% CI, 1.74-2.92) and 2.07 for more than 10 years (95% CI, 1.61-2.66).
Compared with patients who hadn’t lost any weight, patients who reported a 1- to 4-pound weight loss (HR, 1.25; 95% CI, 1.03-1.52), a 5- to 8-pound weight loss (HR, 1.33; 95% CI, 1.06-1.66), and a more than 8-pound weight loss (HR, 1.92; 95% CI, 1.58-2.32) had higher risks of pancreatic cancer. Patients with recent-onset diabetes and a 1- to 8-pound weight loss (91 incident cases per 100,000 person-years; 95% CI, 55-151) or a weight loss of more than 8 pounds (164 incident cases per 100,000 person years; 95% CI, 114-238) had a much higher incidence of pancreatic cancer, compared with patients with neither (16 incident cases per 100,000 person-years; 95% CI, 14-17).
After stratified analyses of patients with both recent-onset diabetes and weight loss, rates of pancreatic cancer were also notably high in those 70 years or older (234 cases per 100,000 person years), those with a body mass index of less than 25 kg/m2 before weight loss (400 cases per 100,000 person years), and those with a low likelihood of intentional weight loss (334 cases per 100,000 person years).
“I like the study because it reminds us of the importance of not thinking everyone that presents with type 2 diabetes necessarily has garden-variety diabetes,” Paul Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “I have always been concerned when a new-onset diabetic individual presents with no family history of diabetes or prediabetes, especially if they’re neither overweight nor obese. I have sometimes screened those individuals for pancreatic abnormalities.”
A call for screening
“This study highlights the consideration for further screening to those with weight loss at the time of diabetes diagnosis, which is very sensible given how unusual weight loss is as a presenting symptom at the time of diagnosis of typical type 2 diabetes,” Dr. Jellinger added. “The combination of weight loss and no family history of diabetes at the time of diagnosis should be an even stronger signal for pancreatic cancer screening and potential detection at a much earlier stage.”
The authors acknowledged their study’s limitations, including some patients with pancreatic cancer not returning their questionnaires and the timing of the questionnaires meaning that patients could’ve developed diabetes after returning it. In addition, they recognized that the participants were “predominantly White health professionals” and recommended a study of “additional patient populations” in the future.
The authors noted numerous potential conflicts of interest, including receiving grants and personal fees from various initiatives, organizations, and pharmaceutical companies.
SOURCE: Yuan C et al. JAMA Oncol. 2020 Aug 13. doi: 10.1001/jamaoncol.2020.2948.
A new study has linked recent-onset diabetes and subsequent weight loss to an increased risk of pancreatic cancer, indicating a distinct group of individuals to screen early for this deadly disease.
“The likelihood of a pancreatic cancer diagnosis was even further elevated among individuals with older age, healthy weight before weight loss, and unintentional weight loss,” wrote Chen Yuan, ScD, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston. The study was published in JAMA Oncology.
To determine whether an association exists between diabetes plus weight change and pancreatic cancer, the researchers analyzed decades of medical history data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The study population from the NHS included 112,818 women with a mean age of 59 years; the population from the HPFS included 46,207 men with a mean age of 65 years. Since enrollment – the baseline was 1978 for the NHS and 1988 for the HPFS – participants have provided follow-up information via biennial questionnaires.
Recent diabetes onset, weight loss boost cancer risk
From those combined groups, 1,116 incident cases of pancreatic cancer (0.7%) were identified. Compared with patients with no diabetes, patients with recent-onset diabetes had triple the risk of pancreatic cancer (age-adjusted hazard ratio, 2.97; 95% confidence interval, 2.31-3.82) and patients with longstanding diabetes had more than double the risk (HR, 2.16; 95% CI, 1.78-2.60). Patients with longer disease duration also had more than twice the risk of pancreatic cancer, with HRs of 2.25 for those with diabetes for 4-10 years (95% CI, 1.74-2.92) and 2.07 for more than 10 years (95% CI, 1.61-2.66).
Compared with patients who hadn’t lost any weight, patients who reported a 1- to 4-pound weight loss (HR, 1.25; 95% CI, 1.03-1.52), a 5- to 8-pound weight loss (HR, 1.33; 95% CI, 1.06-1.66), and a more than 8-pound weight loss (HR, 1.92; 95% CI, 1.58-2.32) had higher risks of pancreatic cancer. Patients with recent-onset diabetes and a 1- to 8-pound weight loss (91 incident cases per 100,000 person-years; 95% CI, 55-151) or a weight loss of more than 8 pounds (164 incident cases per 100,000 person years; 95% CI, 114-238) had a much higher incidence of pancreatic cancer, compared with patients with neither (16 incident cases per 100,000 person-years; 95% CI, 14-17).
After stratified analyses of patients with both recent-onset diabetes and weight loss, rates of pancreatic cancer were also notably high in those 70 years or older (234 cases per 100,000 person years), those with a body mass index of less than 25 kg/m2 before weight loss (400 cases per 100,000 person years), and those with a low likelihood of intentional weight loss (334 cases per 100,000 person years).
“I like the study because it reminds us of the importance of not thinking everyone that presents with type 2 diabetes necessarily has garden-variety diabetes,” Paul Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “I have always been concerned when a new-onset diabetic individual presents with no family history of diabetes or prediabetes, especially if they’re neither overweight nor obese. I have sometimes screened those individuals for pancreatic abnormalities.”
A call for screening
“This study highlights the consideration for further screening to those with weight loss at the time of diabetes diagnosis, which is very sensible given how unusual weight loss is as a presenting symptom at the time of diagnosis of typical type 2 diabetes,” Dr. Jellinger added. “The combination of weight loss and no family history of diabetes at the time of diagnosis should be an even stronger signal for pancreatic cancer screening and potential detection at a much earlier stage.”
The authors acknowledged their study’s limitations, including some patients with pancreatic cancer not returning their questionnaires and the timing of the questionnaires meaning that patients could’ve developed diabetes after returning it. In addition, they recognized that the participants were “predominantly White health professionals” and recommended a study of “additional patient populations” in the future.
The authors noted numerous potential conflicts of interest, including receiving grants and personal fees from various initiatives, organizations, and pharmaceutical companies.
SOURCE: Yuan C et al. JAMA Oncol. 2020 Aug 13. doi: 10.1001/jamaoncol.2020.2948.
FROM JAMA ONCOLOGY
Obesity negatively affects axial spondyloarthritis disease activity
Obesity in adults with axial spondyloarthritis is associated with significantly worse scores on measures of disease activity when compared with normal-weight patients, according to findings from a meta-analysis of 10 studies.
Obesity or overweight could influence axial spondyloarthritis (axSpA) disease activity in several ways, including production of inflammatory mediators by adipose tissue, joint pain caused by excess weight, loss of muscle mass, and increased atherosclerosis, wrote Augusta Ortolan, MD, of the University of Padova (Italy), and colleagues.
However, “it is less clear whether overweight or obesity per se may be a cause of higher disease activity scores,” they added.
In a systematic review published in Arthritis Care & Research, the investigators identified 10 studies to use in the meta-analysis that involved associations between body mass index (BMI) and disease activity in adults with axSpA. The review included three cohort studies and seven cross-sectional studies of patients aged 18 years and older. Disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Ankylosing Spondylitis Disease Activity Score (ASDAS).
Overall, the mean difference in BASDAI between patients with normal BMI and overweight or obese patients was –0.38 (P < .0001), and the mean difference in ASDAS between the same groups was –0.19 (P < .0001). When separated into overweight and obese categories, only the difference between normal weight and obesity remained significantly associated with differences in scores on both measures (–0.78 and –0.42, respectively; P < .0001 for both measures). The difference in scores between normal-weight and overweight/obese patients increased with BMI categories, which suggests a possible “dose-effect” relationship between fat mass and disease activity, the researchers wrote.
The study findings were limited by several factors, including the lack of randomized, controlled trials, and potential bias and inconsistency involving BMI measurements, the researchers noted.
However, “we were able to mitigate such limitations via a strict methodology and consistency in the outcomes, thus allowing us to use mean differences – instead of standardized mean differences – as outcomes, which are much easier to interpret and can be directly related to the measurement unit of the outcome,” they wrote.
The results extend data from previous studies, which “could help interpret disease activity measures and understand the difference we could expect between an axSpA patient with normal BMI and increased BMI,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Ortolan A et al. Arthritis Care Res. 2020 Aug 16. doi: 10.1002/acr.24416.
Obesity in adults with axial spondyloarthritis is associated with significantly worse scores on measures of disease activity when compared with normal-weight patients, according to findings from a meta-analysis of 10 studies.
Obesity or overweight could influence axial spondyloarthritis (axSpA) disease activity in several ways, including production of inflammatory mediators by adipose tissue, joint pain caused by excess weight, loss of muscle mass, and increased atherosclerosis, wrote Augusta Ortolan, MD, of the University of Padova (Italy), and colleagues.
However, “it is less clear whether overweight or obesity per se may be a cause of higher disease activity scores,” they added.
In a systematic review published in Arthritis Care & Research, the investigators identified 10 studies to use in the meta-analysis that involved associations between body mass index (BMI) and disease activity in adults with axSpA. The review included three cohort studies and seven cross-sectional studies of patients aged 18 years and older. Disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Ankylosing Spondylitis Disease Activity Score (ASDAS).
Overall, the mean difference in BASDAI between patients with normal BMI and overweight or obese patients was –0.38 (P < .0001), and the mean difference in ASDAS between the same groups was –0.19 (P < .0001). When separated into overweight and obese categories, only the difference between normal weight and obesity remained significantly associated with differences in scores on both measures (–0.78 and –0.42, respectively; P < .0001 for both measures). The difference in scores between normal-weight and overweight/obese patients increased with BMI categories, which suggests a possible “dose-effect” relationship between fat mass and disease activity, the researchers wrote.
The study findings were limited by several factors, including the lack of randomized, controlled trials, and potential bias and inconsistency involving BMI measurements, the researchers noted.
However, “we were able to mitigate such limitations via a strict methodology and consistency in the outcomes, thus allowing us to use mean differences – instead of standardized mean differences – as outcomes, which are much easier to interpret and can be directly related to the measurement unit of the outcome,” they wrote.
The results extend data from previous studies, which “could help interpret disease activity measures and understand the difference we could expect between an axSpA patient with normal BMI and increased BMI,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Ortolan A et al. Arthritis Care Res. 2020 Aug 16. doi: 10.1002/acr.24416.
Obesity in adults with axial spondyloarthritis is associated with significantly worse scores on measures of disease activity when compared with normal-weight patients, according to findings from a meta-analysis of 10 studies.
Obesity or overweight could influence axial spondyloarthritis (axSpA) disease activity in several ways, including production of inflammatory mediators by adipose tissue, joint pain caused by excess weight, loss of muscle mass, and increased atherosclerosis, wrote Augusta Ortolan, MD, of the University of Padova (Italy), and colleagues.
However, “it is less clear whether overweight or obesity per se may be a cause of higher disease activity scores,” they added.
In a systematic review published in Arthritis Care & Research, the investigators identified 10 studies to use in the meta-analysis that involved associations between body mass index (BMI) and disease activity in adults with axSpA. The review included three cohort studies and seven cross-sectional studies of patients aged 18 years and older. Disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Ankylosing Spondylitis Disease Activity Score (ASDAS).
Overall, the mean difference in BASDAI between patients with normal BMI and overweight or obese patients was –0.38 (P < .0001), and the mean difference in ASDAS between the same groups was –0.19 (P < .0001). When separated into overweight and obese categories, only the difference between normal weight and obesity remained significantly associated with differences in scores on both measures (–0.78 and –0.42, respectively; P < .0001 for both measures). The difference in scores between normal-weight and overweight/obese patients increased with BMI categories, which suggests a possible “dose-effect” relationship between fat mass and disease activity, the researchers wrote.
The study findings were limited by several factors, including the lack of randomized, controlled trials, and potential bias and inconsistency involving BMI measurements, the researchers noted.
However, “we were able to mitigate such limitations via a strict methodology and consistency in the outcomes, thus allowing us to use mean differences – instead of standardized mean differences – as outcomes, which are much easier to interpret and can be directly related to the measurement unit of the outcome,” they wrote.
The results extend data from previous studies, which “could help interpret disease activity measures and understand the difference we could expect between an axSpA patient with normal BMI and increased BMI,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Ortolan A et al. Arthritis Care Res. 2020 Aug 16. doi: 10.1002/acr.24416.
FROM ARTHRITIS CARE & RESEARCH
Evidence mounts for COVID-19 effects on thyroid gland
Rates of thyrotoxicosis are significantly higher among patients who are critically ill with COVID-19 than among patients who are critically ill but who do not not have COVID-19, suggesting an atypical form of thyroiditis related to the novel coronavirus infection, according to new research.
“We suggest routine assessment of thyroid function in patients with COVID-19 requiring high-intensity care because they frequently present with thyrotoxicosis due to a form of subacute thyroiditis related to SARS-CoV-2,” the authors wrote in correspondence published online in The Lancet Diabetes and Endocrinology.
However, notably, the study – which compared critically ill ICU patients who had COVID-19 with those who did not have COVID-19 or who had milder cases of COVID-19 – indicates that thyroid disorders do not appear to increase the risk of developing COVID-19, first author Ilaria Muller, MD, PhD, of the department of endocrinology, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, said in an interview.
“It is important to highlight that we did not find an increased prevalence of preexisting thyroid disorders in COVID-19 patients (contrary to early media reports),” she said. “So far, clinical observations do not support this fear, and we need to reassure people with thyroid disorders, since such disorders are very common among the general population.”
Yet the findings add to emerging evidence of a COVID-19/thyroid relationship, Angela M. Leung, MD, said in an interview.
“Given the health care impacts of the current COVID-19 pandemic worldwide, this study provides some insight on the potential systemic inflammation, as well as thyroid-specific inflammation, of the SARS-Cov-2 virus that is described in some emerging reports,” she said.
“This study joins at least six others that have reported a clinical presentation resembling subacute thyroiditis in critically ill patients with COVID-19,” noted Dr. Leung, of the division of endocrinology, diabetes, and metabolism in the department of medicine at the University of California, Los Angeles.
Thyroid function analysis in those with severe COVID-19
Dr. Muller explained that preliminary data from her institution showed thyroid abnormalities in patients who were severely ill with COVID-19. She and her team extended the evaluation to include thyroid data and other data on 93 patients with COVID-19 who were admitted to high-intensity care units (HICUs) in Italy during the 2020 pandemic.
Those data were compared with data on 101 critically ill patients admitted to the same HICUs in 2019 who did not have COVID-19. A third group of 52 patients with COVID-19 who were admitted to low-intensity care units (LICUs) in Italy in 2020 were also included in the analysis.
The mean age of the patients in the HICU 2020 group was 65.3 years; in the HICU 2019 group, it was 73 years; and in the LICU group, it was 70 years (P = .001). In addition, the HICU 2020 group included more men than the other two groups (69% vs. 56% and 48%; P = .03).
Of note, only 9% of patients in the HICU 2020 group had preexisting thyroid disorders, compared with 21% in the LICU group and 23% in the HICU 2019 group (P = .017).
These findings suggest that “such conditions are not a risk factor for SARS-CoV-2 infection or severity of COVID-19,” the authors wrote.
The patients with the preexisting thyroid conditions were excluded from the thyroid function analysis.
A significantly higher proportion of patients in the HICU 2020 group (13; 15%) were thyrotoxic upon admission, compared with just 1 (1%) of 78 patients in the HICU 2019 group (P = .002) and one (2%) of 41 patients in the LICU group (P = .025).
Among the 14 patients in the two COVID-19 groups who had thyrotoxicosis, the majority were male (9; 64%)
Among those in the HICU 2020 group, serum thyroid-stimulating hormone concentrations were lower than in either of the other two groups (P = .018), and serum free thyroxine (free T4) concentrations were higher than in the LICU group (P = .016) but not the HICU 2019 group.
Differences compared with other infection-related thyroiditis
Although thyrotoxicosis relating to subacute viral thyroiditis can result from a wide variety of viral infections, there are some key differences with COVID-19, Dr. Muller said.
“Thyroid dysfunction related to SARS-CoV-2 seems to be milder than that of classic subacute thyroiditis due to other viruses,” she explained. Furthermore, thyroid dysfunction associated with other viral infections is more common in women, whereas there were more male patients with the COVID-19–related atypical thyroiditis.
In addition, the thyroid effects developed early with COVID-19, whereas they usually emerge after the infections by other viruses.
Patients did not demonstrate the neck pain that is common with classic viral thyroiditis, and the thyroid abnormalities appear to correlate with the severity of COVID-19, whereas they are seen even in patients with mild symptoms when other viral infections are the cause.
In addition to the risk for subacute viral thyroiditis, critically ill patients in general are at risk of developing nonthyroidal illness syndrome, with alterations in thyroid function. However, thyroid hormone measures in the patients severely ill with COVID-19 were not consistent with that syndrome.
A subanalysis of eight HICU 2020 patients with thyroid dysfunction who were followed for 55 days after discharge showed that two experienced hyperthyroidism but likely not from COVID-19; in the remaining six, thyroid function normalized.
Muller speculated that, when ill with COVID-19, the patients likely had a combination of SARS-CoV-2–related atypical thyroiditis and nonthyroidal illness syndrome, known as T4 toxicosis.
Will there be any long-term effects?
Importantly, it remains unknown whether the novel coronavirus has longer-term effects on the thyroid, Dr. Muller said.
“We cannot predict what will be the long-lasting thyroid effects after COVID-19,” she said.
With classic subacute viral thyroiditis, “After a few years ... 5%-20% of patients develop permanent hypothyroidism, [and] the same might happen in COVID-19 patients,” she hypothesized. “We will follow our patients long term to answer this question – this study is already ongoing.”
In the meantime, diagnosis of thyroid dysfunction in patients with COVID-19 is important, inasmuch as it could worsen the already critical conditions of patients, Muller stressed.
“The gold-standard treatment for thyroiditis is steroids, so the presence of thyroid dysfunction might represent an additional indication to such treatment in COVID-19 patients, to be verified in properly designed clinical trials,” she advised.
ACE2 cell receptors highly expressed in thyroid
Dr. Muller and colleagues also noted recent research showing that ACE2 – demonstrated to be a key host-cell entry receptor for both SARS-CoV and SARS-CoV-2 – is expressed in even higher levels in the thyroid than the lungs, where it causes COVID-19’s notorious pulmonary effects.
Dr. Muller said the implications of ACE2 expression in the thyroid remain to be elucidated.
“If ACE2 is confirmed to be expressed at higher levels, compared with the lungs in the thyroid gland and other tissues, i.e., small intestine, testis, kidney, heart, etc, dedicated studies will be needed to correlate ACE2 expression with the organs’ susceptibility to SARS-CoV-2 reflected by clinical presentation,” she said.
Dr. Leung added that, as a take-home message from these and the other thyroid/COVID-19 studies, “data are starting to show us that COVID-19 infection may cause thyrotoxicosis that is possibly related to thyroid and systemic inflammation. However, the serum thyroid function test abnormalities seen in COVID-19 patients with subacute thyroiditis are also likely exacerbated to a substantial extent by nonthyroidal illness physiology.”
The authors have disclosed no relevant financial relationships. Dr. Leung is on the advisory board of Medscape Diabetes and Endocrinology.
A version of this article originally appeared on Medscape.com.
Rates of thyrotoxicosis are significantly higher among patients who are critically ill with COVID-19 than among patients who are critically ill but who do not not have COVID-19, suggesting an atypical form of thyroiditis related to the novel coronavirus infection, according to new research.
“We suggest routine assessment of thyroid function in patients with COVID-19 requiring high-intensity care because they frequently present with thyrotoxicosis due to a form of subacute thyroiditis related to SARS-CoV-2,” the authors wrote in correspondence published online in The Lancet Diabetes and Endocrinology.
However, notably, the study – which compared critically ill ICU patients who had COVID-19 with those who did not have COVID-19 or who had milder cases of COVID-19 – indicates that thyroid disorders do not appear to increase the risk of developing COVID-19, first author Ilaria Muller, MD, PhD, of the department of endocrinology, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, said in an interview.
“It is important to highlight that we did not find an increased prevalence of preexisting thyroid disorders in COVID-19 patients (contrary to early media reports),” she said. “So far, clinical observations do not support this fear, and we need to reassure people with thyroid disorders, since such disorders are very common among the general population.”
Yet the findings add to emerging evidence of a COVID-19/thyroid relationship, Angela M. Leung, MD, said in an interview.
“Given the health care impacts of the current COVID-19 pandemic worldwide, this study provides some insight on the potential systemic inflammation, as well as thyroid-specific inflammation, of the SARS-Cov-2 virus that is described in some emerging reports,” she said.
“This study joins at least six others that have reported a clinical presentation resembling subacute thyroiditis in critically ill patients with COVID-19,” noted Dr. Leung, of the division of endocrinology, diabetes, and metabolism in the department of medicine at the University of California, Los Angeles.
Thyroid function analysis in those with severe COVID-19
Dr. Muller explained that preliminary data from her institution showed thyroid abnormalities in patients who were severely ill with COVID-19. She and her team extended the evaluation to include thyroid data and other data on 93 patients with COVID-19 who were admitted to high-intensity care units (HICUs) in Italy during the 2020 pandemic.
Those data were compared with data on 101 critically ill patients admitted to the same HICUs in 2019 who did not have COVID-19. A third group of 52 patients with COVID-19 who were admitted to low-intensity care units (LICUs) in Italy in 2020 were also included in the analysis.
The mean age of the patients in the HICU 2020 group was 65.3 years; in the HICU 2019 group, it was 73 years; and in the LICU group, it was 70 years (P = .001). In addition, the HICU 2020 group included more men than the other two groups (69% vs. 56% and 48%; P = .03).
Of note, only 9% of patients in the HICU 2020 group had preexisting thyroid disorders, compared with 21% in the LICU group and 23% in the HICU 2019 group (P = .017).
These findings suggest that “such conditions are not a risk factor for SARS-CoV-2 infection or severity of COVID-19,” the authors wrote.
The patients with the preexisting thyroid conditions were excluded from the thyroid function analysis.
A significantly higher proportion of patients in the HICU 2020 group (13; 15%) were thyrotoxic upon admission, compared with just 1 (1%) of 78 patients in the HICU 2019 group (P = .002) and one (2%) of 41 patients in the LICU group (P = .025).
Among the 14 patients in the two COVID-19 groups who had thyrotoxicosis, the majority were male (9; 64%)
Among those in the HICU 2020 group, serum thyroid-stimulating hormone concentrations were lower than in either of the other two groups (P = .018), and serum free thyroxine (free T4) concentrations were higher than in the LICU group (P = .016) but not the HICU 2019 group.
Differences compared with other infection-related thyroiditis
Although thyrotoxicosis relating to subacute viral thyroiditis can result from a wide variety of viral infections, there are some key differences with COVID-19, Dr. Muller said.
“Thyroid dysfunction related to SARS-CoV-2 seems to be milder than that of classic subacute thyroiditis due to other viruses,” she explained. Furthermore, thyroid dysfunction associated with other viral infections is more common in women, whereas there were more male patients with the COVID-19–related atypical thyroiditis.
In addition, the thyroid effects developed early with COVID-19, whereas they usually emerge after the infections by other viruses.
Patients did not demonstrate the neck pain that is common with classic viral thyroiditis, and the thyroid abnormalities appear to correlate with the severity of COVID-19, whereas they are seen even in patients with mild symptoms when other viral infections are the cause.
In addition to the risk for subacute viral thyroiditis, critically ill patients in general are at risk of developing nonthyroidal illness syndrome, with alterations in thyroid function. However, thyroid hormone measures in the patients severely ill with COVID-19 were not consistent with that syndrome.
A subanalysis of eight HICU 2020 patients with thyroid dysfunction who were followed for 55 days after discharge showed that two experienced hyperthyroidism but likely not from COVID-19; in the remaining six, thyroid function normalized.
Muller speculated that, when ill with COVID-19, the patients likely had a combination of SARS-CoV-2–related atypical thyroiditis and nonthyroidal illness syndrome, known as T4 toxicosis.
Will there be any long-term effects?
Importantly, it remains unknown whether the novel coronavirus has longer-term effects on the thyroid, Dr. Muller said.
“We cannot predict what will be the long-lasting thyroid effects after COVID-19,” she said.
With classic subacute viral thyroiditis, “After a few years ... 5%-20% of patients develop permanent hypothyroidism, [and] the same might happen in COVID-19 patients,” she hypothesized. “We will follow our patients long term to answer this question – this study is already ongoing.”
In the meantime, diagnosis of thyroid dysfunction in patients with COVID-19 is important, inasmuch as it could worsen the already critical conditions of patients, Muller stressed.
“The gold-standard treatment for thyroiditis is steroids, so the presence of thyroid dysfunction might represent an additional indication to such treatment in COVID-19 patients, to be verified in properly designed clinical trials,” she advised.
ACE2 cell receptors highly expressed in thyroid
Dr. Muller and colleagues also noted recent research showing that ACE2 – demonstrated to be a key host-cell entry receptor for both SARS-CoV and SARS-CoV-2 – is expressed in even higher levels in the thyroid than the lungs, where it causes COVID-19’s notorious pulmonary effects.
Dr. Muller said the implications of ACE2 expression in the thyroid remain to be elucidated.
“If ACE2 is confirmed to be expressed at higher levels, compared with the lungs in the thyroid gland and other tissues, i.e., small intestine, testis, kidney, heart, etc, dedicated studies will be needed to correlate ACE2 expression with the organs’ susceptibility to SARS-CoV-2 reflected by clinical presentation,” she said.
Dr. Leung added that, as a take-home message from these and the other thyroid/COVID-19 studies, “data are starting to show us that COVID-19 infection may cause thyrotoxicosis that is possibly related to thyroid and systemic inflammation. However, the serum thyroid function test abnormalities seen in COVID-19 patients with subacute thyroiditis are also likely exacerbated to a substantial extent by nonthyroidal illness physiology.”
The authors have disclosed no relevant financial relationships. Dr. Leung is on the advisory board of Medscape Diabetes and Endocrinology.
A version of this article originally appeared on Medscape.com.
Rates of thyrotoxicosis are significantly higher among patients who are critically ill with COVID-19 than among patients who are critically ill but who do not not have COVID-19, suggesting an atypical form of thyroiditis related to the novel coronavirus infection, according to new research.
“We suggest routine assessment of thyroid function in patients with COVID-19 requiring high-intensity care because they frequently present with thyrotoxicosis due to a form of subacute thyroiditis related to SARS-CoV-2,” the authors wrote in correspondence published online in The Lancet Diabetes and Endocrinology.
However, notably, the study – which compared critically ill ICU patients who had COVID-19 with those who did not have COVID-19 or who had milder cases of COVID-19 – indicates that thyroid disorders do not appear to increase the risk of developing COVID-19, first author Ilaria Muller, MD, PhD, of the department of endocrinology, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, said in an interview.
“It is important to highlight that we did not find an increased prevalence of preexisting thyroid disorders in COVID-19 patients (contrary to early media reports),” she said. “So far, clinical observations do not support this fear, and we need to reassure people with thyroid disorders, since such disorders are very common among the general population.”
Yet the findings add to emerging evidence of a COVID-19/thyroid relationship, Angela M. Leung, MD, said in an interview.
“Given the health care impacts of the current COVID-19 pandemic worldwide, this study provides some insight on the potential systemic inflammation, as well as thyroid-specific inflammation, of the SARS-Cov-2 virus that is described in some emerging reports,” she said.
“This study joins at least six others that have reported a clinical presentation resembling subacute thyroiditis in critically ill patients with COVID-19,” noted Dr. Leung, of the division of endocrinology, diabetes, and metabolism in the department of medicine at the University of California, Los Angeles.
Thyroid function analysis in those with severe COVID-19
Dr. Muller explained that preliminary data from her institution showed thyroid abnormalities in patients who were severely ill with COVID-19. She and her team extended the evaluation to include thyroid data and other data on 93 patients with COVID-19 who were admitted to high-intensity care units (HICUs) in Italy during the 2020 pandemic.
Those data were compared with data on 101 critically ill patients admitted to the same HICUs in 2019 who did not have COVID-19. A third group of 52 patients with COVID-19 who were admitted to low-intensity care units (LICUs) in Italy in 2020 were also included in the analysis.
The mean age of the patients in the HICU 2020 group was 65.3 years; in the HICU 2019 group, it was 73 years; and in the LICU group, it was 70 years (P = .001). In addition, the HICU 2020 group included more men than the other two groups (69% vs. 56% and 48%; P = .03).
Of note, only 9% of patients in the HICU 2020 group had preexisting thyroid disorders, compared with 21% in the LICU group and 23% in the HICU 2019 group (P = .017).
These findings suggest that “such conditions are not a risk factor for SARS-CoV-2 infection or severity of COVID-19,” the authors wrote.
The patients with the preexisting thyroid conditions were excluded from the thyroid function analysis.
A significantly higher proportion of patients in the HICU 2020 group (13; 15%) were thyrotoxic upon admission, compared with just 1 (1%) of 78 patients in the HICU 2019 group (P = .002) and one (2%) of 41 patients in the LICU group (P = .025).
Among the 14 patients in the two COVID-19 groups who had thyrotoxicosis, the majority were male (9; 64%)
Among those in the HICU 2020 group, serum thyroid-stimulating hormone concentrations were lower than in either of the other two groups (P = .018), and serum free thyroxine (free T4) concentrations were higher than in the LICU group (P = .016) but not the HICU 2019 group.
Differences compared with other infection-related thyroiditis
Although thyrotoxicosis relating to subacute viral thyroiditis can result from a wide variety of viral infections, there are some key differences with COVID-19, Dr. Muller said.
“Thyroid dysfunction related to SARS-CoV-2 seems to be milder than that of classic subacute thyroiditis due to other viruses,” she explained. Furthermore, thyroid dysfunction associated with other viral infections is more common in women, whereas there were more male patients with the COVID-19–related atypical thyroiditis.
In addition, the thyroid effects developed early with COVID-19, whereas they usually emerge after the infections by other viruses.
Patients did not demonstrate the neck pain that is common with classic viral thyroiditis, and the thyroid abnormalities appear to correlate with the severity of COVID-19, whereas they are seen even in patients with mild symptoms when other viral infections are the cause.
In addition to the risk for subacute viral thyroiditis, critically ill patients in general are at risk of developing nonthyroidal illness syndrome, with alterations in thyroid function. However, thyroid hormone measures in the patients severely ill with COVID-19 were not consistent with that syndrome.
A subanalysis of eight HICU 2020 patients with thyroid dysfunction who were followed for 55 days after discharge showed that two experienced hyperthyroidism but likely not from COVID-19; in the remaining six, thyroid function normalized.
Muller speculated that, when ill with COVID-19, the patients likely had a combination of SARS-CoV-2–related atypical thyroiditis and nonthyroidal illness syndrome, known as T4 toxicosis.
Will there be any long-term effects?
Importantly, it remains unknown whether the novel coronavirus has longer-term effects on the thyroid, Dr. Muller said.
“We cannot predict what will be the long-lasting thyroid effects after COVID-19,” she said.
With classic subacute viral thyroiditis, “After a few years ... 5%-20% of patients develop permanent hypothyroidism, [and] the same might happen in COVID-19 patients,” she hypothesized. “We will follow our patients long term to answer this question – this study is already ongoing.”
In the meantime, diagnosis of thyroid dysfunction in patients with COVID-19 is important, inasmuch as it could worsen the already critical conditions of patients, Muller stressed.
“The gold-standard treatment for thyroiditis is steroids, so the presence of thyroid dysfunction might represent an additional indication to such treatment in COVID-19 patients, to be verified in properly designed clinical trials,” she advised.
ACE2 cell receptors highly expressed in thyroid
Dr. Muller and colleagues also noted recent research showing that ACE2 – demonstrated to be a key host-cell entry receptor for both SARS-CoV and SARS-CoV-2 – is expressed in even higher levels in the thyroid than the lungs, where it causes COVID-19’s notorious pulmonary effects.
Dr. Muller said the implications of ACE2 expression in the thyroid remain to be elucidated.
“If ACE2 is confirmed to be expressed at higher levels, compared with the lungs in the thyroid gland and other tissues, i.e., small intestine, testis, kidney, heart, etc, dedicated studies will be needed to correlate ACE2 expression with the organs’ susceptibility to SARS-CoV-2 reflected by clinical presentation,” she said.
Dr. Leung added that, as a take-home message from these and the other thyroid/COVID-19 studies, “data are starting to show us that COVID-19 infection may cause thyrotoxicosis that is possibly related to thyroid and systemic inflammation. However, the serum thyroid function test abnormalities seen in COVID-19 patients with subacute thyroiditis are also likely exacerbated to a substantial extent by nonthyroidal illness physiology.”
The authors have disclosed no relevant financial relationships. Dr. Leung is on the advisory board of Medscape Diabetes and Endocrinology.
A version of this article originally appeared on Medscape.com.
Non-COVID-19 clinical trials grind to a halt during pandemic
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.