User login
Study confirms it’s possible to catch COVID-19 twice
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Researchers in Hong Kong say they’ve confirmed that a person can be infected with COVID-19 twice.
The new proof comes from a 33-year-old man in Hong Kong who first caught COVID-19 in March. He was tested for the coronavirus after he developed a cough, sore throat, fever, and a headache for 3 days. He stayed in the hospital until he twice tested negative for the virus in mid-April.
On Aug. 15, the man returned to Hong Kong from a recent trip to Spain and the United Kingdom, areas that have recently seen a resurgence of COVID-19 cases. At the airport, he was screened for COVID-19 with a test that checks saliva for the virus. He tested positive, but this time, had no symptoms. He was taken to the hospital for monitoring. His viral load – the amount of virus he had in his body – went down over time, suggesting that his immune system was taking care of the intrusion on its own.
The special thing about his case is that each time he was hospitalized, doctors sequenced the genome of the virus that infected him. It was slightly different from one infection to the next, suggesting that the virus had mutated – or changed – in the 4 months between his infections. It also proves that it’s possible for this coronavirus to infect the same person twice.
Experts with the World Health Organization responded to the case at a news briefing.
“What we are learning about infection is that people do develop an immune response. What is not completely clear yet is how strong that immune response is and for how long that immune response lasts,” said Maria Van Kerkhove, PhD, an infectious disease epidemiologist with the World Health Organization in Geneva, Switzerland.
A study on the man’s case is being prepared for publication in the journal Clinical Infectious Diseases. Experts say the finding shouldn’t cause alarm, but it does have important implications for the development of herd immunity and efforts to come up with vaccines and treatments.
“This appears to be pretty clear-cut evidence of reinfection because of sequencing and isolation of two different viruses,” said Gregory Poland, MD, an expert on vaccine development and immunology at the Mayo Clinic in Rochester, Minn. “The big unknown is how often is this happening,” he said. More studies are needed to learn whether this was a rare case or something that is happening often.
Past experience guides present
Until we know more, Dr. Poland said, the possibility of getting COVID-19 twice shouldn’t make anyone worry.
This also happens with other kinds of coronaviruses – the ones that cause common colds. Those coronaviruses change slightly each year as they circle the globe, which allows them to keep spreading and causing their more run-of-the-mill kind of misery.
It also happens with seasonal flu. It is the reason people have to get vaccinated against the flu year after year, and why the flu vaccine has to change slightly each year in an effort to keep up with the ever-evolving influenza virus.
“We’ve been making flu vaccines for 80 years, and there are clinical trials happening as we speak to find new and better influenza vaccines,” Dr. Poland said.
There has been other evidence the virus that causes COVID-19 can change this way, too. Researchers at Howard Hughes Medical Center, at Rockefeller University in New York, recently used a key piece of the SARS-CoV-2 virus – the genetic instructions for its spike protein – to repeatedly infect human cells. Scientists watched as each new generation of the virus went on to infect a new batch of cells. Over time, as it copied itself, some of the copies changed their genes to allow them to survive after scientists attacked them with neutralizing antibodies. Those antibodies are among the main weapons used by the immune system to recognize and disable a virus.
Though that study is still a preprint, which means it hasn’t yet been reviewed by outside experts, the authors wrote that their findings suggest the virus can change in ways that help it evade our immune system. If true, they wrote in mid-July, it means reinfection is possible, especially in people who have a weak immune response to the virus the first time they encounter it.
Good news
That seems to be true in the case of the man from Hong Kong. When doctors tested his blood to look for antibodies to the virus, they didn’t find any. That could mean that he either had a weak immune response to the virus the first time around, or that the antibodies he made during his first infection diminished over time. But during his second infection, he quickly developed more antibodies, suggesting that the second infection acted a little bit like a booster to fire up his immune system. That’s probably the reason he didn’t have any symptoms the second time, too.
That’s good news, Dr. Poland said. It means our bodies can get better at fighting off the COVID-19 virus and that catching it once means the second time might not be so bad.
But the fact that the virus can change quickly this way does have some impact on the effort to come up with a vaccine that works well.
“I think a potential implication of this is that we will have to give booster doses. The question is how frequently,” Dr. Poland said. That will depend on how fast the virus is changing, and how often reinfection is happening in the real world.
“I’m a little surprised at 4½ months,” Dr. Poland said, referencing the time between the Hong Kong man’s infections. “I’m not surprised by, you know, I got infected last winter and I got infected again this winter,” he said.
It also suggests that immune-based therapies such as convalescent plasma and monoclonal antibodies may be of limited help over time, since the virus might be changing in ways that help it outsmart those treatments.
Convalescent plasma is essentially a concentrated dose of antibodies from people who have recovered from a COVID-19 infection. As the virus changes, the antibodies in that plasma may not work as well for future infections.
Drug companies have learned to harness the power of monoclonal antibodies as powerful treatments against cancer and other diseases. Monoclonal antibodies, which are mass-produced in a lab, mimic the body’s natural defenses against a pathogen. Just like the virus can become resistant to natural immunity, it can change in ways that help it outsmart lab-created treatments. Some drug companies that are developing monoclonal antibodies to fight COVID-19 have already prepared for that possibility by making antibody cocktails that are designed to disable the virus by locking onto it in different places, which may help prevent it from developing resistance to those therapies.
“We have a lot to learn,” Dr. Poland said. “Now that the proof of principle has been established, and I would say it has with this man, and with our knowledge of seasonal coronaviruses, we need to look more aggressively to define how often this occurs.”
A version of this article originally appeared on WebMD.com.
Research examines links between ‘long COVID’ and ME/CFS
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports – one published in JAMA in July and another published in Morbidity and Mortality Weekly Report in August – chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset.
Although some of the reported persistent symptoms appear specific to SARS-CoV-2 – such as cough, chest pain, and dyspnea – others overlap with the diagnostic criteria for ME/CFS, which is defined by substantial, profound fatigue for at least 6 months, postexertional malaise, unrefreshing sleep, and one or both of orthostatic intolerance and/or cognitive impairment. Although the etiology of ME/CFS is unclear, the condition commonly arises following a viral illness.
At the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis August 21, the opening session was devoted to research documenting the extent to which COVID-19 survivors subsequently meet ME/CFS criteria, and to exploring underlying mechanisms.
“It offers a lot of opportunities for us to study potentially early ME/CFS and how it develops, but in addition, a lot of the research that has been done on ME/CFS may also provide answers for COVID-19,” IACFS/ME vice president Lily Chu, MD, said in an interview.
A hint from the SARS outbreak
This isn’t the first time researchers have seen a possible link between a coronavirus and ME/CFS, Harvey Moldofsky, MD, told attendees. To illustrate that point, Dr. Moldofsky, of the department of psychiatry (emeritus) at the University of Toronto, reviewed data from a previously published case-controlled study, which included 22 health care workers who had been infected in 2003 with SARS-CoV-1 and continued to report chronic fatigue, musculoskeletal pain, and disturbed and unrefreshing sleep with EEG-documented sleep disturbances 1-3 years following the illness. None had been able to return to work by 1 year.
“We’re looking at similar symptoms now” among survivors of COVID-19, Dr. Moldofsky said. “[T]he key issue is that we have no idea of its prevalence. … We need epidemiologic studies.”
Distinguishing ME/CFS from other post–COVID-19 symptoms
Not everyone who has persistent symptoms after COVID-19 will develop ME/CFS, and distinguishing between cases may be important.
Clinically, Dr. Chu said, one way to assess whether a patient with persistent COVID-19 symptoms might be progressing to ME/CFS is to ask him or her specifically about the level of fatigue following physical exertion and the timing of any fatigue. With ME/CFS, postexertional malaise often involves a dramatic exacerbation of symptoms such as fatigue, pain, and cognitive impairment a day or 2 after exertion rather than immediately following it. In contrast, shortness of breath during exertion isn’t typical of ME/CFS.
Objective measures of ME/CFS include low natural killer cell function (the test can be ordered from commercial labs but requires rapid transport of the blood sample), and autonomic dysfunction assessed by a tilt-table test.
While there is currently no cure for ME/CFS, diagnosing it allows for the patient to be taught “pacing” in which the person conserves his or her energy by balancing activity with rest. “That type of behavioral technique is valuable for everyone who suffers from a chronic disease with fatigue. It can help them be more functional,” Dr. Chu said.
If a patient appears to be exhibiting signs of ME/CFS, “don’t wait until they hit the 6-month mark to start helping them manage their symptoms,” she said. “Teaching pacing to COVID-19 patients who have a lot of fatigue isn’t going to harm them. As they get better they’re going to just naturally do more. But if they do have ME/CFS, [pacing] stresses their system less, since the data seem to be pointing to deficiencies in producing energy.”
Will COVID-19 unleash a new wave of ME/CFS patients?
Much of the session at the virtual meeting was devoted to ongoing studies. For example, Leonard Jason, PhD, of the Center for Community Research at DePaul University, Chicago, described a prospective study launched in 2014 that looked at risk factors for developing ME/CFS in college students who contracted infectious mononucleosis as a result of Epstein-Barr virus. Now, his team is also following students from the same cohort who develop COVID-19.
Because the study included collection of baseline biological samples, the results could help reveal predisposing factors associated with long-term illness from either virus.
Another project, funded by the Open Medicine Foundation, will follow patients who are discharged from the ICU following severe COVID-19 illness. Blood, urine, and cerebrospinal fluid will be collected from those with persistent symptoms at 6 months, along with questionnaire data. At 18-24 months, those who continue to report symptoms will undergo more intensive evaluation using genomics, metabolomics, and proteomics.
“We’re taking advantage of this horrible situation, hoping to understand how a serious viral infection might lead to ME/CFS,” said lead investigator Ronald Tompkins, MD, ScD, chief medical officer at the Open Medicine Foundation and a faculty member at Harvard Medical School, Boston. The results, he said, “might give us insight into potential drug targets or biomarkers useful for prevention and treatment strategies.”
Meanwhile, Sadie Whittaker, PhD, head of the Solve ME/CFS initiative, described her organization’s new plan to use their registry to prospectively track the impact of COVID-19 on people with ME/CFS.
She noted that they’ve also teamed up with “long-COVID” communities including Body Politic. “Our goal is to form a coalition to study together or at least harmonize data … and understand what’s going on through the power of bigger sample sizes,” Dr. Whittaker said.
None of the speakers disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
TNF inhibitors linked to inflammatory CNS events
, new research suggests
The nested case-control study included more than 200 participants with diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. Results showed that exposure to TNF inhibitors was significantly associated with increased risk for demyelinating CNS events, such as multiple sclerosis, and nondemyelinating events, such as meningitis and encephalitis.
Interestingly, disease-specific secondary analyses showed that the strongest association for inflammatory events was in patients with rheumatoid arthritis.
Lead author Amy Kunchok, MD, of Mayo Clinic, Rochester, Minn., noted that “these are highly effective therapies for patients” and that these CNS events are likely uncommon.
“Our study has observed an association, but this does not imply causality. Therefore, we are not cautioning against using these therapies in appropriate patients,” Dr. Kunchok said in an interview.
“Rather, we recommend that clinicians assessing patients with both inflammatory demyelinating and nondemyelinating CNS events consider a detailed evaluation of the medication history, particularly in patients with coexistent autoimmune diseases who may have a current or past history of biological therapies,” she said.
The findings were published in JAMA Neurology.
Poorly understood
TNF inhibitors “are common therapies for certain autoimmune diseases,” the investigators noted.
Previously, a link between exposure to these inhibitors and inflammatory CNS events “has been postulated but is poorly understood,” they wrote.
In the current study, they examined records for 106 patients who were treated at Mayo clinics in Minnesota, Arizona, or Florida from January 2003 through February 2019. All participants had been diagnosed with an autoimmune disease that the Food and Drug Administration has listed as an indication for TNF inhibitor use. This included rheumatoid arthritis (n = 48), ankylosing spondylitis (n = 4), psoriasis and psoriatic arthritis (n = 21), Crohn’s disease (n = 27), and ulcerative colitis (n = 6). Their records also showed diagnostic codes for the inflammatory demyelinating CNS events of relapsing-remitting or primary progressive MS, clinically isolated syndrome, radiologically isolated syndrome, neuromyelitis optica spectrum disorder, and transverse myelitis or for the inflammatory nondemyelinating CNS events of meningitis, meningoencephalitis, encephalitis, neurosarcoidosis, and CNS vasculitis. The investigators also included 106 age-, sex-, and autoimmune disease–matched participants 1:1 to act as the control group.
In the total study population, 64% were women and the median age at disease onset was 52 years. In addition, 60% of the patient group and 40% of the control group were exposed to TNF inhibitors.
Novel finding?
Results showed that TNF inhibitor exposure was significantly linked to increased risk for developing any inflammatory CNS event (adjusted odds ratio, 3.01; 95% CI, 1.55-5.82; P = .001). When the outcomes were stratified by class of inflammatory event, these results were similar. The aOR was 3.09 (95% CI, 1.19-8.04; P = .02) for inflammatory demyelinating CNS events and was 2.97 (95% CI, 1.15-7.65; P = .02) for inflammatory nondemyelinating events.
Dr. Kunchok noted that the association between the inhibitors and nondemyelinating events was “a novel finding from this study.”
In secondary analyses, patients with rheumatoid arthritis and exposure to TNF inhibitors had the strongest association with any inflammatory CNS event (aOR, 4.82; 95% CI, 1.62-14.36; P = .005).
A pooled cohort comprising only the participants with the other autoimmune diseases did not show a significant association between exposure to TNF inhibitors and development of CNS events (P = .09).
“Because of the lack of power, further stratification by individual autoimmune diseases was not analyzed,” the investigators reported.
Although the overall findings showed that exposure to TNF inhibitors was linked to increased risk for inflammatory events, whether this association “represents de novo or exacerbated inflammatory pathways requires further research,” the authors wrote.
Dr. Kunchok added that more research, especially population-based studies, is also needed to examine the incidence of these inflammatory CNS events in patients exposed to TNF-alpha inhibitors.
Adds to the literature
In an accompanying editorial, Jeffrey M. Gelfand, MD, department of neurology at the University of California, San Francisco, and Jinoos Yazdany, MD, Zuckerberg San Francisco General Hospital at UCSF, noted that although the study adds to the literature, the magnitude of the risk found “remains unclear.”
“Randomized clinical trials are not suited to the study of rare adverse events,” Dr. Gelfand and Dr. Yazdany wrote. They agree with Dr. Kunchok that “next steps should include population-based observational studies that control for disease severity.”
Still, the current study provides additional evidence of rare adverse events in patients receiving TNF inhibitors, they noted. So how should prescribers proceed?
“As with all treatments, the risk-benefit ratio for the individual patient’s situation must be weighed and appropriate counseling must be given to facilitate shared decision-making discussions,” wrote the editorialists.
“Given what is known about the risk of harm, avoiding TNF inhibitors is advisable in patients with known MS,” they wrote.
In addition, neurologic consultation can be helpful for clarifying diagnoses and providing advice on monitoring strategies for TNF inhibitor treatment in those with possible MS or other demyelinating conditions, noted the editorialists.
“In patients who develop new concerning neurological symptoms while receiving TNF inhibitor treatment, timely evaluation is indicated, including consideration of neuroinflammatory, infectious, and neurological diagnoses that may be unrelated to treatment,” they added.
“Broader awareness of risks that studies such as this one by Kunchok et al provide can ... encourage timelier recognition of potential TNF inhibitor–associated neuroinflammatory events and may improve outcomes for patients,” Dr. Gelfand and Dr. Yazdany concluded.
The study was funded by a grant from the National Center for Advancing Translational Sciences. Dr. Kunchok reports having received research funding from Biogen outside this study. A full list of disclosures for the other study authors is in the original article. Dr. Gelfand reports having received g rants for a clinical trial from Genentech and consulting fees from Biogen, Alexion, Theranica, Impel Neuropharma, Advanced Clinical, Biohaven, and Satsuma. Dr. Yazdany reports having received grants from Pfizer and consulting fees from AstraZeneca and Eli Lilly outside the submitted work.
A version of this article originally appeared on Medscape.com.
, new research suggests
The nested case-control study included more than 200 participants with diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. Results showed that exposure to TNF inhibitors was significantly associated with increased risk for demyelinating CNS events, such as multiple sclerosis, and nondemyelinating events, such as meningitis and encephalitis.
Interestingly, disease-specific secondary analyses showed that the strongest association for inflammatory events was in patients with rheumatoid arthritis.
Lead author Amy Kunchok, MD, of Mayo Clinic, Rochester, Minn., noted that “these are highly effective therapies for patients” and that these CNS events are likely uncommon.
“Our study has observed an association, but this does not imply causality. Therefore, we are not cautioning against using these therapies in appropriate patients,” Dr. Kunchok said in an interview.
“Rather, we recommend that clinicians assessing patients with both inflammatory demyelinating and nondemyelinating CNS events consider a detailed evaluation of the medication history, particularly in patients with coexistent autoimmune diseases who may have a current or past history of biological therapies,” she said.
The findings were published in JAMA Neurology.
Poorly understood
TNF inhibitors “are common therapies for certain autoimmune diseases,” the investigators noted.
Previously, a link between exposure to these inhibitors and inflammatory CNS events “has been postulated but is poorly understood,” they wrote.
In the current study, they examined records for 106 patients who were treated at Mayo clinics in Minnesota, Arizona, or Florida from January 2003 through February 2019. All participants had been diagnosed with an autoimmune disease that the Food and Drug Administration has listed as an indication for TNF inhibitor use. This included rheumatoid arthritis (n = 48), ankylosing spondylitis (n = 4), psoriasis and psoriatic arthritis (n = 21), Crohn’s disease (n = 27), and ulcerative colitis (n = 6). Their records also showed diagnostic codes for the inflammatory demyelinating CNS events of relapsing-remitting or primary progressive MS, clinically isolated syndrome, radiologically isolated syndrome, neuromyelitis optica spectrum disorder, and transverse myelitis or for the inflammatory nondemyelinating CNS events of meningitis, meningoencephalitis, encephalitis, neurosarcoidosis, and CNS vasculitis. The investigators also included 106 age-, sex-, and autoimmune disease–matched participants 1:1 to act as the control group.
In the total study population, 64% were women and the median age at disease onset was 52 years. In addition, 60% of the patient group and 40% of the control group were exposed to TNF inhibitors.
Novel finding?
Results showed that TNF inhibitor exposure was significantly linked to increased risk for developing any inflammatory CNS event (adjusted odds ratio, 3.01; 95% CI, 1.55-5.82; P = .001). When the outcomes were stratified by class of inflammatory event, these results were similar. The aOR was 3.09 (95% CI, 1.19-8.04; P = .02) for inflammatory demyelinating CNS events and was 2.97 (95% CI, 1.15-7.65; P = .02) for inflammatory nondemyelinating events.
Dr. Kunchok noted that the association between the inhibitors and nondemyelinating events was “a novel finding from this study.”
In secondary analyses, patients with rheumatoid arthritis and exposure to TNF inhibitors had the strongest association with any inflammatory CNS event (aOR, 4.82; 95% CI, 1.62-14.36; P = .005).
A pooled cohort comprising only the participants with the other autoimmune diseases did not show a significant association between exposure to TNF inhibitors and development of CNS events (P = .09).
“Because of the lack of power, further stratification by individual autoimmune diseases was not analyzed,” the investigators reported.
Although the overall findings showed that exposure to TNF inhibitors was linked to increased risk for inflammatory events, whether this association “represents de novo or exacerbated inflammatory pathways requires further research,” the authors wrote.
Dr. Kunchok added that more research, especially population-based studies, is also needed to examine the incidence of these inflammatory CNS events in patients exposed to TNF-alpha inhibitors.
Adds to the literature
In an accompanying editorial, Jeffrey M. Gelfand, MD, department of neurology at the University of California, San Francisco, and Jinoos Yazdany, MD, Zuckerberg San Francisco General Hospital at UCSF, noted that although the study adds to the literature, the magnitude of the risk found “remains unclear.”
“Randomized clinical trials are not suited to the study of rare adverse events,” Dr. Gelfand and Dr. Yazdany wrote. They agree with Dr. Kunchok that “next steps should include population-based observational studies that control for disease severity.”
Still, the current study provides additional evidence of rare adverse events in patients receiving TNF inhibitors, they noted. So how should prescribers proceed?
“As with all treatments, the risk-benefit ratio for the individual patient’s situation must be weighed and appropriate counseling must be given to facilitate shared decision-making discussions,” wrote the editorialists.
“Given what is known about the risk of harm, avoiding TNF inhibitors is advisable in patients with known MS,” they wrote.
In addition, neurologic consultation can be helpful for clarifying diagnoses and providing advice on monitoring strategies for TNF inhibitor treatment in those with possible MS or other demyelinating conditions, noted the editorialists.
“In patients who develop new concerning neurological symptoms while receiving TNF inhibitor treatment, timely evaluation is indicated, including consideration of neuroinflammatory, infectious, and neurological diagnoses that may be unrelated to treatment,” they added.
“Broader awareness of risks that studies such as this one by Kunchok et al provide can ... encourage timelier recognition of potential TNF inhibitor–associated neuroinflammatory events and may improve outcomes for patients,” Dr. Gelfand and Dr. Yazdany concluded.
The study was funded by a grant from the National Center for Advancing Translational Sciences. Dr. Kunchok reports having received research funding from Biogen outside this study. A full list of disclosures for the other study authors is in the original article. Dr. Gelfand reports having received g rants for a clinical trial from Genentech and consulting fees from Biogen, Alexion, Theranica, Impel Neuropharma, Advanced Clinical, Biohaven, and Satsuma. Dr. Yazdany reports having received grants from Pfizer and consulting fees from AstraZeneca and Eli Lilly outside the submitted work.
A version of this article originally appeared on Medscape.com.
, new research suggests
The nested case-control study included more than 200 participants with diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. Results showed that exposure to TNF inhibitors was significantly associated with increased risk for demyelinating CNS events, such as multiple sclerosis, and nondemyelinating events, such as meningitis and encephalitis.
Interestingly, disease-specific secondary analyses showed that the strongest association for inflammatory events was in patients with rheumatoid arthritis.
Lead author Amy Kunchok, MD, of Mayo Clinic, Rochester, Minn., noted that “these are highly effective therapies for patients” and that these CNS events are likely uncommon.
“Our study has observed an association, but this does not imply causality. Therefore, we are not cautioning against using these therapies in appropriate patients,” Dr. Kunchok said in an interview.
“Rather, we recommend that clinicians assessing patients with both inflammatory demyelinating and nondemyelinating CNS events consider a detailed evaluation of the medication history, particularly in patients with coexistent autoimmune diseases who may have a current or past history of biological therapies,” she said.
The findings were published in JAMA Neurology.
Poorly understood
TNF inhibitors “are common therapies for certain autoimmune diseases,” the investigators noted.
Previously, a link between exposure to these inhibitors and inflammatory CNS events “has been postulated but is poorly understood,” they wrote.
In the current study, they examined records for 106 patients who were treated at Mayo clinics in Minnesota, Arizona, or Florida from January 2003 through February 2019. All participants had been diagnosed with an autoimmune disease that the Food and Drug Administration has listed as an indication for TNF inhibitor use. This included rheumatoid arthritis (n = 48), ankylosing spondylitis (n = 4), psoriasis and psoriatic arthritis (n = 21), Crohn’s disease (n = 27), and ulcerative colitis (n = 6). Their records also showed diagnostic codes for the inflammatory demyelinating CNS events of relapsing-remitting or primary progressive MS, clinically isolated syndrome, radiologically isolated syndrome, neuromyelitis optica spectrum disorder, and transverse myelitis or for the inflammatory nondemyelinating CNS events of meningitis, meningoencephalitis, encephalitis, neurosarcoidosis, and CNS vasculitis. The investigators also included 106 age-, sex-, and autoimmune disease–matched participants 1:1 to act as the control group.
In the total study population, 64% were women and the median age at disease onset was 52 years. In addition, 60% of the patient group and 40% of the control group were exposed to TNF inhibitors.
Novel finding?
Results showed that TNF inhibitor exposure was significantly linked to increased risk for developing any inflammatory CNS event (adjusted odds ratio, 3.01; 95% CI, 1.55-5.82; P = .001). When the outcomes were stratified by class of inflammatory event, these results were similar. The aOR was 3.09 (95% CI, 1.19-8.04; P = .02) for inflammatory demyelinating CNS events and was 2.97 (95% CI, 1.15-7.65; P = .02) for inflammatory nondemyelinating events.
Dr. Kunchok noted that the association between the inhibitors and nondemyelinating events was “a novel finding from this study.”
In secondary analyses, patients with rheumatoid arthritis and exposure to TNF inhibitors had the strongest association with any inflammatory CNS event (aOR, 4.82; 95% CI, 1.62-14.36; P = .005).
A pooled cohort comprising only the participants with the other autoimmune diseases did not show a significant association between exposure to TNF inhibitors and development of CNS events (P = .09).
“Because of the lack of power, further stratification by individual autoimmune diseases was not analyzed,” the investigators reported.
Although the overall findings showed that exposure to TNF inhibitors was linked to increased risk for inflammatory events, whether this association “represents de novo or exacerbated inflammatory pathways requires further research,” the authors wrote.
Dr. Kunchok added that more research, especially population-based studies, is also needed to examine the incidence of these inflammatory CNS events in patients exposed to TNF-alpha inhibitors.
Adds to the literature
In an accompanying editorial, Jeffrey M. Gelfand, MD, department of neurology at the University of California, San Francisco, and Jinoos Yazdany, MD, Zuckerberg San Francisco General Hospital at UCSF, noted that although the study adds to the literature, the magnitude of the risk found “remains unclear.”
“Randomized clinical trials are not suited to the study of rare adverse events,” Dr. Gelfand and Dr. Yazdany wrote. They agree with Dr. Kunchok that “next steps should include population-based observational studies that control for disease severity.”
Still, the current study provides additional evidence of rare adverse events in patients receiving TNF inhibitors, they noted. So how should prescribers proceed?
“As with all treatments, the risk-benefit ratio for the individual patient’s situation must be weighed and appropriate counseling must be given to facilitate shared decision-making discussions,” wrote the editorialists.
“Given what is known about the risk of harm, avoiding TNF inhibitors is advisable in patients with known MS,” they wrote.
In addition, neurologic consultation can be helpful for clarifying diagnoses and providing advice on monitoring strategies for TNF inhibitor treatment in those with possible MS or other demyelinating conditions, noted the editorialists.
“In patients who develop new concerning neurological symptoms while receiving TNF inhibitor treatment, timely evaluation is indicated, including consideration of neuroinflammatory, infectious, and neurological diagnoses that may be unrelated to treatment,” they added.
“Broader awareness of risks that studies such as this one by Kunchok et al provide can ... encourage timelier recognition of potential TNF inhibitor–associated neuroinflammatory events and may improve outcomes for patients,” Dr. Gelfand and Dr. Yazdany concluded.
The study was funded by a grant from the National Center for Advancing Translational Sciences. Dr. Kunchok reports having received research funding from Biogen outside this study. A full list of disclosures for the other study authors is in the original article. Dr. Gelfand reports having received g rants for a clinical trial from Genentech and consulting fees from Biogen, Alexion, Theranica, Impel Neuropharma, Advanced Clinical, Biohaven, and Satsuma. Dr. Yazdany reports having received grants from Pfizer and consulting fees from AstraZeneca and Eli Lilly outside the submitted work.
A version of this article originally appeared on Medscape.com.
FDA clears first brain stimulation device to help smokers quit
The Food and Drug Administration has granted marketing approval for the BrainsWay deep transcranial magnetic stimulation (TMS) system to help adult smokers kick tobacco.
in a press release.
As previously reported, the system has already been approved by the FDA as a treatment for patients suffering from obsessive-compulsive disorder and major depressive disorder.
The BrainsWay deep TMS system with H4-coil is designed to target addiction-related brain circuits.
It was evaluated as an aid to short-term smoking cessation in a prospective, double-blind, randomized, sham-controlled, multicenter study that involved 262 adults who had a history of smoking an average of more than 26 years and had attempted to quit multiple times but failed.
Active and sham treatments were performed daily 5 days a week for 3 weeks, followed by an additional three sessions once weekly for 3 weeks, for a total of 18 sessions over 6 weeks.
In the full intention-to-treat population (all 262 participants), the 4-week continuous quit rate (CQR, the primary endpoint) was higher in the active deep TMS group than in the sham TMS group (17.1% vs. 7.9%; P = .0238).
Among participants who completed the study, that is, those who underwent treatment for 4 weeks, who kept daily records, and for whom confirmatory urine samples were available, the CQR was 28.4% in the active deep TMS group, compared with 11.7% in the sham treatment group (P = .0063).
The average number of cigarettes smoked per day, as determined on the basis of daily records (secondary endpoint), was statistically significantly lower in the active deep TMS group, compared with the sham treatment group (P = .0311).
No patient suffered a seizure. The most common adverse event was headache, for which there was no statistical difference between the active and sham treatment groups. Other side effects included application site discomfort, back pain, muscle twitching, and discomfort.
“This FDA clearance represents a significant milestone for BrainsWay and our deep TMS platform technology,” Christopher von Jako, PhD, president and CEO of the company, said in the release.
“While other therapies are currently available, a substantial medical need continues to exist for treatments that can increase the continuous quit rate among smokers,” Dr. von Jako noted.
“Based on the compelling data from our large, randomized pivotal study of 262 subjects, we are confident that our deep TMS technology can play an important role in treating cigarette smokers who seek to quit,” he added.
The company plans a “controlled” U.S. market release of the system for this indication early next year.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has granted marketing approval for the BrainsWay deep transcranial magnetic stimulation (TMS) system to help adult smokers kick tobacco.
in a press release.
As previously reported, the system has already been approved by the FDA as a treatment for patients suffering from obsessive-compulsive disorder and major depressive disorder.
The BrainsWay deep TMS system with H4-coil is designed to target addiction-related brain circuits.
It was evaluated as an aid to short-term smoking cessation in a prospective, double-blind, randomized, sham-controlled, multicenter study that involved 262 adults who had a history of smoking an average of more than 26 years and had attempted to quit multiple times but failed.
Active and sham treatments were performed daily 5 days a week for 3 weeks, followed by an additional three sessions once weekly for 3 weeks, for a total of 18 sessions over 6 weeks.
In the full intention-to-treat population (all 262 participants), the 4-week continuous quit rate (CQR, the primary endpoint) was higher in the active deep TMS group than in the sham TMS group (17.1% vs. 7.9%; P = .0238).
Among participants who completed the study, that is, those who underwent treatment for 4 weeks, who kept daily records, and for whom confirmatory urine samples were available, the CQR was 28.4% in the active deep TMS group, compared with 11.7% in the sham treatment group (P = .0063).
The average number of cigarettes smoked per day, as determined on the basis of daily records (secondary endpoint), was statistically significantly lower in the active deep TMS group, compared with the sham treatment group (P = .0311).
No patient suffered a seizure. The most common adverse event was headache, for which there was no statistical difference between the active and sham treatment groups. Other side effects included application site discomfort, back pain, muscle twitching, and discomfort.
“This FDA clearance represents a significant milestone for BrainsWay and our deep TMS platform technology,” Christopher von Jako, PhD, president and CEO of the company, said in the release.
“While other therapies are currently available, a substantial medical need continues to exist for treatments that can increase the continuous quit rate among smokers,” Dr. von Jako noted.
“Based on the compelling data from our large, randomized pivotal study of 262 subjects, we are confident that our deep TMS technology can play an important role in treating cigarette smokers who seek to quit,” he added.
The company plans a “controlled” U.S. market release of the system for this indication early next year.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has granted marketing approval for the BrainsWay deep transcranial magnetic stimulation (TMS) system to help adult smokers kick tobacco.
in a press release.
As previously reported, the system has already been approved by the FDA as a treatment for patients suffering from obsessive-compulsive disorder and major depressive disorder.
The BrainsWay deep TMS system with H4-coil is designed to target addiction-related brain circuits.
It was evaluated as an aid to short-term smoking cessation in a prospective, double-blind, randomized, sham-controlled, multicenter study that involved 262 adults who had a history of smoking an average of more than 26 years and had attempted to quit multiple times but failed.
Active and sham treatments were performed daily 5 days a week for 3 weeks, followed by an additional three sessions once weekly for 3 weeks, for a total of 18 sessions over 6 weeks.
In the full intention-to-treat population (all 262 participants), the 4-week continuous quit rate (CQR, the primary endpoint) was higher in the active deep TMS group than in the sham TMS group (17.1% vs. 7.9%; P = .0238).
Among participants who completed the study, that is, those who underwent treatment for 4 weeks, who kept daily records, and for whom confirmatory urine samples were available, the CQR was 28.4% in the active deep TMS group, compared with 11.7% in the sham treatment group (P = .0063).
The average number of cigarettes smoked per day, as determined on the basis of daily records (secondary endpoint), was statistically significantly lower in the active deep TMS group, compared with the sham treatment group (P = .0311).
No patient suffered a seizure. The most common adverse event was headache, for which there was no statistical difference between the active and sham treatment groups. Other side effects included application site discomfort, back pain, muscle twitching, and discomfort.
“This FDA clearance represents a significant milestone for BrainsWay and our deep TMS platform technology,” Christopher von Jako, PhD, president and CEO of the company, said in the release.
“While other therapies are currently available, a substantial medical need continues to exist for treatments that can increase the continuous quit rate among smokers,” Dr. von Jako noted.
“Based on the compelling data from our large, randomized pivotal study of 262 subjects, we are confident that our deep TMS technology can play an important role in treating cigarette smokers who seek to quit,” he added.
The company plans a “controlled” U.S. market release of the system for this indication early next year.
A version of this article originally appeared on Medscape.com.
Staying financially well in the time of COVID-19
As COVID-19 continues to threaten the United States and the world, individuals in every profession have been challenged to examine their financial situation. At Fidelity Investments, we recently conducted a national survey asking people how current events have affected their opinions and behaviors when it comes to their money. The results showed that six in 10 Americans are concerned about household finances over the next 6 months. Unfortunately, we’ve seen that even health care professionals have not been financially spared, with salaries or benefits cut or, worse, furloughs and layoffs as hospital systems struggle. I work with many physicians, including gastroenterologists, in my role as a wealth planner for Fidelity Investments and have received quite a few questions related to shoring up family finances during these difficult times.
Luckily, the financial best practices that I share in “good” times ring true even in today’s world, with a few additions given the health and economic risks created by COVID-19.
1. Review your budget. It’s one thing to know that your budget is generally balanced (the dollars you spend are less than the dollars you earn). But it’s worth taking a closer look to see just where those dollars are going. In times of uncertainty, cutting back on expenses that aren’t necessary or don’t provide meaningful value to your life can be worthwhile. If you or your family have lost income because of the pandemic, you might consider these seven simple tips to help boost your cash flow.
2. Tackle (or find relief from) student loan debt. Doctors today graduate medical school with a median debt of just under $195,000.1 Repaying these loans is daunting, particularly during the COVID-19 crisis. The recent passing of the CARES Act recognizes these difficult times: in fact, it automatically suspended required minimum loan payments and interest accrual on federal student loans until Sept. 30, 2020. This only applies to federal student loans, not private student loans. Beyond this period, if you are still struggling with payments, you may explore the possibility of refinancing, by taking out a lower-interest private loan and using that to pay off student loans (although this may extend the life of your loan). Borrowers could also consider other programs, such as REPAYE (Revised Pay As You Earn) through which your monthly payment tops out at 10% of your monthly income, or Public Service Loan Forgiveness (PSLF) if you work for a not-for-profit hospital or other qualifying employer. This program forgives the remaining balance on your direct loans after you have made 120 qualifying monthly payments while working full-time for a qualifying employer.
Additionally, borrowers could look for opportunities to reduce accrued interest, either by refinancing to a lower rate or making payments every 2 weeks rather than once each month.
3. Evaluate your emergency fund. It’s a good idea to keep 3-6 months’ of essential expenses in cash or cash-like investments. If you don’t yet have this 3- to 6-month cushion saved, now is a good time to work to reduce your expenses and stash away any extra cash.
4. Save early and often for retirement. You can borrow money to support many of life’s needs, from housing, to cars, to college. But you can’t borrow for retirement. That is why I encourage clients to put retirement savings at the top of the list, after accounting for day-to-day needs of their families. People often ask me whether it makes sense to continue saving for retirement, often a far-off goal for younger doctors, especially in these uncertain times. My answer? Yes. If you are able to save, continue to save: the earlier you begin to make contributions to your retirement account, and the longer you continue to do so, the more your retirement account(s) have the potential to grow over time.
Another question I receive is whether to take distributions from a retirement account early if you find yourself in a precarious financial situation because of the COVID-19 crisis. The CARES Act provides options allowing Americans to take a withdrawal or loan from a participating retirement plan if you, your spouse, or your dependent have a COVID-19 related illness or you’re experiencing a loss of income related to the COVID-19 pandemic. Try to look at alternative sources of income before tapping your hard-earned retirement savings. If you can find a way to continue saving and avoid drawing down your retirement accounts, your future self will thank you.
5. If you have a high-deductible health plan that offers it, explore a Health Savings Account (HSA). One of the most important factors in a solid financial plan is knowing how to pay for health care expenses, both now and as we age. HSAs are a tax-advantaged account that can be used to save money for qualified medical expenses. They are considered to provide a “triple-tax advantage” since contributions, qualified withdrawals, and investment growth are all tax-free.2 The dollars in these accounts can stay there over time, so in years with low expenses you could use these to save for health care in retirement, while in other years they can be used to pay necessary medical bills. HSAs require the participant to be enrolled in a high-deductible health plan, so you would first need to verify that your employer provides this option.
6. Be prepared to protect yourself, your practice, and your family. Typically, I encourage the medical professionals I work with to review their current insurance plans (such as disability, life, and malpractice) to determine whether they have the right levels of coverage for their situation. With COVID-19 layered on top of the usual level of risk, it’s important to consider reviewing or updating other key elements of your family’s plan, like your health care proxies and a living will.
7. Put your income to work. When your disposable income grows, and you’ve covered all of the foundational elements of a financial plan (a rainy-day fund, contingency planning for health care costs, and so on), it might be the right time to consider investing for something other than retirement. As you do that, be sure you are invested in a diversified strategy with a balance of risk and return that is comfortable for you.
Recent market volatility can bring nerves that make it difficult to stay invested. However, as long as your risk tolerance and time horizon reflect your asset allocation – the mix of stock, bonds, and cash (which a financial planner can help with) – you can take comfort in knowing that historically every severe downturn has eventually given way to further growth.
During uncertain times like these, I think the best guidance is to focus on what you can control. The considerations above are a great place to start building a financial plan to solidify you and your family’s future. A Fidelity survey found that 44% of Americans are now working to build up their emergency savings, and one-third (34%) are rethinking how they manage their money because of the COVID-19 crisis.3 Despite the stresses we all face, there is no time like the present to start or revisit your financial plan.
Footnotes
1. Barron D. Why Doctors Are Drowning in Medical School Debt. Scientific American. July 15, 2019.
2. With respect to federal taxation only. Contributions, investment earnings, and distributions may or may not be subject to state taxation. The triple tax advantages are only applicable if the money is used to pay for qualified medical expenses as described in IRS Publication 969.
3. Fidelity Market Sentiment Study presents the findings of a nationwide online survey consisting of 3,012 adults, at least 18 years of age, from which 1,591 respondents qualified as having at least one investment account. The study was fielded April 1-8, 2020, by ENGINE INSIGHTS, an independent research firm not affiliated with Fidelity Investments. The results of this survey may not be representative of all adults meeting the same criteria as those surveyed for this study. For the purposes of this study, the generations are defined as follows: Millennials (aged 24-39 years); Generation X (aged 40-55 years); Baby Boomers (aged 56-74 years).
Mr. Tudor is Vice President, Wealth Planning Consultant at Fidelity Investments.
As COVID-19 continues to threaten the United States and the world, individuals in every profession have been challenged to examine their financial situation. At Fidelity Investments, we recently conducted a national survey asking people how current events have affected their opinions and behaviors when it comes to their money. The results showed that six in 10 Americans are concerned about household finances over the next 6 months. Unfortunately, we’ve seen that even health care professionals have not been financially spared, with salaries or benefits cut or, worse, furloughs and layoffs as hospital systems struggle. I work with many physicians, including gastroenterologists, in my role as a wealth planner for Fidelity Investments and have received quite a few questions related to shoring up family finances during these difficult times.
Luckily, the financial best practices that I share in “good” times ring true even in today’s world, with a few additions given the health and economic risks created by COVID-19.
1. Review your budget. It’s one thing to know that your budget is generally balanced (the dollars you spend are less than the dollars you earn). But it’s worth taking a closer look to see just where those dollars are going. In times of uncertainty, cutting back on expenses that aren’t necessary or don’t provide meaningful value to your life can be worthwhile. If you or your family have lost income because of the pandemic, you might consider these seven simple tips to help boost your cash flow.
2. Tackle (or find relief from) student loan debt. Doctors today graduate medical school with a median debt of just under $195,000.1 Repaying these loans is daunting, particularly during the COVID-19 crisis. The recent passing of the CARES Act recognizes these difficult times: in fact, it automatically suspended required minimum loan payments and interest accrual on federal student loans until Sept. 30, 2020. This only applies to federal student loans, not private student loans. Beyond this period, if you are still struggling with payments, you may explore the possibility of refinancing, by taking out a lower-interest private loan and using that to pay off student loans (although this may extend the life of your loan). Borrowers could also consider other programs, such as REPAYE (Revised Pay As You Earn) through which your monthly payment tops out at 10% of your monthly income, or Public Service Loan Forgiveness (PSLF) if you work for a not-for-profit hospital or other qualifying employer. This program forgives the remaining balance on your direct loans after you have made 120 qualifying monthly payments while working full-time for a qualifying employer.
Additionally, borrowers could look for opportunities to reduce accrued interest, either by refinancing to a lower rate or making payments every 2 weeks rather than once each month.
3. Evaluate your emergency fund. It’s a good idea to keep 3-6 months’ of essential expenses in cash or cash-like investments. If you don’t yet have this 3- to 6-month cushion saved, now is a good time to work to reduce your expenses and stash away any extra cash.
4. Save early and often for retirement. You can borrow money to support many of life’s needs, from housing, to cars, to college. But you can’t borrow for retirement. That is why I encourage clients to put retirement savings at the top of the list, after accounting for day-to-day needs of their families. People often ask me whether it makes sense to continue saving for retirement, often a far-off goal for younger doctors, especially in these uncertain times. My answer? Yes. If you are able to save, continue to save: the earlier you begin to make contributions to your retirement account, and the longer you continue to do so, the more your retirement account(s) have the potential to grow over time.
Another question I receive is whether to take distributions from a retirement account early if you find yourself in a precarious financial situation because of the COVID-19 crisis. The CARES Act provides options allowing Americans to take a withdrawal or loan from a participating retirement plan if you, your spouse, or your dependent have a COVID-19 related illness or you’re experiencing a loss of income related to the COVID-19 pandemic. Try to look at alternative sources of income before tapping your hard-earned retirement savings. If you can find a way to continue saving and avoid drawing down your retirement accounts, your future self will thank you.
5. If you have a high-deductible health plan that offers it, explore a Health Savings Account (HSA). One of the most important factors in a solid financial plan is knowing how to pay for health care expenses, both now and as we age. HSAs are a tax-advantaged account that can be used to save money for qualified medical expenses. They are considered to provide a “triple-tax advantage” since contributions, qualified withdrawals, and investment growth are all tax-free.2 The dollars in these accounts can stay there over time, so in years with low expenses you could use these to save for health care in retirement, while in other years they can be used to pay necessary medical bills. HSAs require the participant to be enrolled in a high-deductible health plan, so you would first need to verify that your employer provides this option.
6. Be prepared to protect yourself, your practice, and your family. Typically, I encourage the medical professionals I work with to review their current insurance plans (such as disability, life, and malpractice) to determine whether they have the right levels of coverage for their situation. With COVID-19 layered on top of the usual level of risk, it’s important to consider reviewing or updating other key elements of your family’s plan, like your health care proxies and a living will.
7. Put your income to work. When your disposable income grows, and you’ve covered all of the foundational elements of a financial plan (a rainy-day fund, contingency planning for health care costs, and so on), it might be the right time to consider investing for something other than retirement. As you do that, be sure you are invested in a diversified strategy with a balance of risk and return that is comfortable for you.
Recent market volatility can bring nerves that make it difficult to stay invested. However, as long as your risk tolerance and time horizon reflect your asset allocation – the mix of stock, bonds, and cash (which a financial planner can help with) – you can take comfort in knowing that historically every severe downturn has eventually given way to further growth.
During uncertain times like these, I think the best guidance is to focus on what you can control. The considerations above are a great place to start building a financial plan to solidify you and your family’s future. A Fidelity survey found that 44% of Americans are now working to build up their emergency savings, and one-third (34%) are rethinking how they manage their money because of the COVID-19 crisis.3 Despite the stresses we all face, there is no time like the present to start or revisit your financial plan.
Footnotes
1. Barron D. Why Doctors Are Drowning in Medical School Debt. Scientific American. July 15, 2019.
2. With respect to federal taxation only. Contributions, investment earnings, and distributions may or may not be subject to state taxation. The triple tax advantages are only applicable if the money is used to pay for qualified medical expenses as described in IRS Publication 969.
3. Fidelity Market Sentiment Study presents the findings of a nationwide online survey consisting of 3,012 adults, at least 18 years of age, from which 1,591 respondents qualified as having at least one investment account. The study was fielded April 1-8, 2020, by ENGINE INSIGHTS, an independent research firm not affiliated with Fidelity Investments. The results of this survey may not be representative of all adults meeting the same criteria as those surveyed for this study. For the purposes of this study, the generations are defined as follows: Millennials (aged 24-39 years); Generation X (aged 40-55 years); Baby Boomers (aged 56-74 years).
Mr. Tudor is Vice President, Wealth Planning Consultant at Fidelity Investments.
As COVID-19 continues to threaten the United States and the world, individuals in every profession have been challenged to examine their financial situation. At Fidelity Investments, we recently conducted a national survey asking people how current events have affected their opinions and behaviors when it comes to their money. The results showed that six in 10 Americans are concerned about household finances over the next 6 months. Unfortunately, we’ve seen that even health care professionals have not been financially spared, with salaries or benefits cut or, worse, furloughs and layoffs as hospital systems struggle. I work with many physicians, including gastroenterologists, in my role as a wealth planner for Fidelity Investments and have received quite a few questions related to shoring up family finances during these difficult times.
Luckily, the financial best practices that I share in “good” times ring true even in today’s world, with a few additions given the health and economic risks created by COVID-19.
1. Review your budget. It’s one thing to know that your budget is generally balanced (the dollars you spend are less than the dollars you earn). But it’s worth taking a closer look to see just where those dollars are going. In times of uncertainty, cutting back on expenses that aren’t necessary or don’t provide meaningful value to your life can be worthwhile. If you or your family have lost income because of the pandemic, you might consider these seven simple tips to help boost your cash flow.
2. Tackle (or find relief from) student loan debt. Doctors today graduate medical school with a median debt of just under $195,000.1 Repaying these loans is daunting, particularly during the COVID-19 crisis. The recent passing of the CARES Act recognizes these difficult times: in fact, it automatically suspended required minimum loan payments and interest accrual on federal student loans until Sept. 30, 2020. This only applies to federal student loans, not private student loans. Beyond this period, if you are still struggling with payments, you may explore the possibility of refinancing, by taking out a lower-interest private loan and using that to pay off student loans (although this may extend the life of your loan). Borrowers could also consider other programs, such as REPAYE (Revised Pay As You Earn) through which your monthly payment tops out at 10% of your monthly income, or Public Service Loan Forgiveness (PSLF) if you work for a not-for-profit hospital or other qualifying employer. This program forgives the remaining balance on your direct loans after you have made 120 qualifying monthly payments while working full-time for a qualifying employer.
Additionally, borrowers could look for opportunities to reduce accrued interest, either by refinancing to a lower rate or making payments every 2 weeks rather than once each month.
3. Evaluate your emergency fund. It’s a good idea to keep 3-6 months’ of essential expenses in cash or cash-like investments. If you don’t yet have this 3- to 6-month cushion saved, now is a good time to work to reduce your expenses and stash away any extra cash.
4. Save early and often for retirement. You can borrow money to support many of life’s needs, from housing, to cars, to college. But you can’t borrow for retirement. That is why I encourage clients to put retirement savings at the top of the list, after accounting for day-to-day needs of their families. People often ask me whether it makes sense to continue saving for retirement, often a far-off goal for younger doctors, especially in these uncertain times. My answer? Yes. If you are able to save, continue to save: the earlier you begin to make contributions to your retirement account, and the longer you continue to do so, the more your retirement account(s) have the potential to grow over time.
Another question I receive is whether to take distributions from a retirement account early if you find yourself in a precarious financial situation because of the COVID-19 crisis. The CARES Act provides options allowing Americans to take a withdrawal or loan from a participating retirement plan if you, your spouse, or your dependent have a COVID-19 related illness or you’re experiencing a loss of income related to the COVID-19 pandemic. Try to look at alternative sources of income before tapping your hard-earned retirement savings. If you can find a way to continue saving and avoid drawing down your retirement accounts, your future self will thank you.
5. If you have a high-deductible health plan that offers it, explore a Health Savings Account (HSA). One of the most important factors in a solid financial plan is knowing how to pay for health care expenses, both now and as we age. HSAs are a tax-advantaged account that can be used to save money for qualified medical expenses. They are considered to provide a “triple-tax advantage” since contributions, qualified withdrawals, and investment growth are all tax-free.2 The dollars in these accounts can stay there over time, so in years with low expenses you could use these to save for health care in retirement, while in other years they can be used to pay necessary medical bills. HSAs require the participant to be enrolled in a high-deductible health plan, so you would first need to verify that your employer provides this option.
6. Be prepared to protect yourself, your practice, and your family. Typically, I encourage the medical professionals I work with to review their current insurance plans (such as disability, life, and malpractice) to determine whether they have the right levels of coverage for their situation. With COVID-19 layered on top of the usual level of risk, it’s important to consider reviewing or updating other key elements of your family’s plan, like your health care proxies and a living will.
7. Put your income to work. When your disposable income grows, and you’ve covered all of the foundational elements of a financial plan (a rainy-day fund, contingency planning for health care costs, and so on), it might be the right time to consider investing for something other than retirement. As you do that, be sure you are invested in a diversified strategy with a balance of risk and return that is comfortable for you.
Recent market volatility can bring nerves that make it difficult to stay invested. However, as long as your risk tolerance and time horizon reflect your asset allocation – the mix of stock, bonds, and cash (which a financial planner can help with) – you can take comfort in knowing that historically every severe downturn has eventually given way to further growth.
During uncertain times like these, I think the best guidance is to focus on what you can control. The considerations above are a great place to start building a financial plan to solidify you and your family’s future. A Fidelity survey found that 44% of Americans are now working to build up their emergency savings, and one-third (34%) are rethinking how they manage their money because of the COVID-19 crisis.3 Despite the stresses we all face, there is no time like the present to start or revisit your financial plan.
Footnotes
1. Barron D. Why Doctors Are Drowning in Medical School Debt. Scientific American. July 15, 2019.
2. With respect to federal taxation only. Contributions, investment earnings, and distributions may or may not be subject to state taxation. The triple tax advantages are only applicable if the money is used to pay for qualified medical expenses as described in IRS Publication 969.
3. Fidelity Market Sentiment Study presents the findings of a nationwide online survey consisting of 3,012 adults, at least 18 years of age, from which 1,591 respondents qualified as having at least one investment account. The study was fielded April 1-8, 2020, by ENGINE INSIGHTS, an independent research firm not affiliated with Fidelity Investments. The results of this survey may not be representative of all adults meeting the same criteria as those surveyed for this study. For the purposes of this study, the generations are defined as follows: Millennials (aged 24-39 years); Generation X (aged 40-55 years); Baby Boomers (aged 56-74 years).
Mr. Tudor is Vice President, Wealth Planning Consultant at Fidelity Investments.
FDA authorizes convalescent plasma for COVID-19
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Convalescent plasma contains antibodies from the blood of recovered COVID-19 patients, which can be used to treat people with severe infections. Convalescent plasma has been used to treat patients for other infectious diseases. The authorization allows the plasma to be distributed in the United States and administered by health care providers.
“COVID-19 convalescent plasma is safe and shows promising efficacy,” Stephen Hahn, MD, commissioner of the FDA, said during a press briefing with President Donald Trump.
In April, the FDA approved a program to test convalescent plasma in COVID-19 patients at the Mayo Clinic, followed by other institutions. More than 90,000 patients have enrolled in the program, and 70,000 have received the treatment, Dr. Hahn said.
The data indicate that the plasma can reduce mortality in patients by 35%, particularly if patients are treated within 3 days of being diagnosed. Those who have benefited the most were under age 80 and not on artificial respiration, Alex Azar, the secretary for the Department of Health & Human Services, said during the briefing.
“We dream, in drug development, of something like a 35% mortality reduction,” he said.
But top scientists pushed back against the announcement.
Eric Topol, MD, director of the Scripps Research Translational Institute, professor of molecular medicine, and executive vice president of Scripps Research, said the data the FDA are relying on did not come from the rigorous randomized, double-blind placebo trials that best determine if a treatment is successful.
Still, convalescent plasma is “one more tool added to the arsenal” of combating COVID-19, Mr. Azar said. The FDA will continue to study convalescent plasma as a COVID-19 treatment, Dr. Hahn added.
“We’re waiting for more data. We’re going to continue to gather data,” Dr. Hahn said during the briefing, but the current results meet FDA criteria for issuing an emergency use authorization.
Convalescent plasma “may be effective in lessening the severity or shortening the length of COVID-19 illness in some hospitalized patients,” according to the FDA announcement. Potential side effects include allergic reactions, transfusion-transmitted infections, and transfusion-associated lung injury.
“We’ve seen a great deal of demand for this from doctors around the country,” Dr. Hahn said during the briefing. “The EUA … allows us to continue that and meet that demand.”
Dr. Topol, however, said it appears Trump and the FDA are playing politics with science.
“There’s no evidence to support any survival benefit,” Dr. Topol said on Twitter. “Two days ago [the] FDA’s website stated there was no evidence for an EUA.”
The American Red Cross and other blood centers put out a national call for blood donors in July, especially for patients who have recovered from COVID-19. Mr. Azar and Dr. Hahn emphasized the need for blood donors during the press briefing.
“If you donate plasma, you could save a life,” Mr. Azar said.
The study has not been peer reviewed and did not include a placebo group for comparison, STAT reported.
Last week several health officials warned that the scientific data were too weak to warrant an emergency authorization, the New York Times reported.
A version of this originally appeared on WebMD.com.
Methotrexate as a Treatment of Palmoplantar Lichen Planus
To the Editor:
Palmoplantar lichen planus (LP) is an uncommon variant of LP that involves the palms and soles. The prevalence of LP is approximately 0.1% to 2% in the general population. It can affect both mucosal and cutaneous surfaces.1 A study of 36 patients with LP showed that 25% (9/36) had palmar and/or plantar involvement.2 Palmoplantar LP is more commonly found in men than women, with an average age of onset of 38 to 65 years.3 It tends to affect the soles more often than the palms, with the most common site being the plantar arch. Itching generally is the most common symptom reported. Lesions often resolve over a few months, but relapses can occur in 10% to 29% of patients.2 The clinical morphology commonly is characterized as erythematous scaly plaques, hyperkeratotic plaques, or ulcerations.4 Due to its rare occurrence, palmoplantar LP often is misdiagnosed as psoriasis, eczematous dermatitis, tinea nigra, or secondary syphilis, making pathology extremely helpful in making the diagnosis.1 Darker skin types can obscure defining characteristics, further impeding a timely diagnosis. We describe a novel case of palmoplantar LP that was successfully treated with methotrexate.
A 38-year-old man with no notable medical history presented for dermatologic evaluation of a palmar and plantar rash of 4 months' duration. The rash was accompanied by intense burning pain and pruritus. Prior to presentation, he had been treated with multiple prednisone tapers starting at 40 mg daily as well as combination therapy of a 2-week course of minocycline 100 mg twice daily and clobetasol ointment twice daily for 4 months, with no notable improvement. Workup prior to presentation included a negative potassium hydroxide fungal preparation and a normal antinuclear antibody titer. A review of symptoms was negative for arthralgia, myalgia, photosensitivity, malar rash, Raynaud phenomenon, pleuritic pain, seizures, and psychosis.
Physical examination revealed focal areas of mildly thick, hyperkeratotic scale with desquamation on the plantar and palmar surfaces of the feet and hands. The underlying skin of the feet consisted of dyspigmented patches of dark brown and hypopigmented skin with erythema, profound scaling, and sparing of the internal plantar arches (Figure 1A). On the palms, thin hyperkeratotic plaques with desquamation and erythematous maceration of the surrounding skin were observed (Figure 2A). Thin white plaques of the posterior bilateral buccal mucosa were appreciated as well as an erosion that extended to the lower lip.
The differential diagnosis included LP, psoriasis, acquired palmoplantar keratoderma, and discoid lupus erythematosus. Tinea pedis and tinea manuum were less likely in the setting of a negative potassium hydroxide fungal preparation.

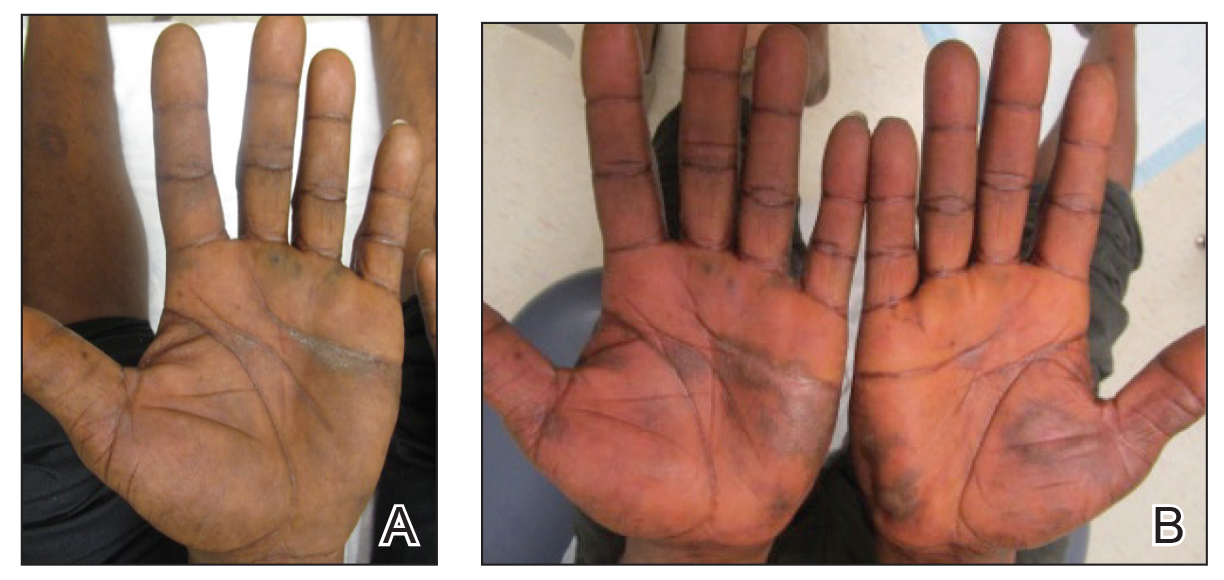
A biopsy of the lateral aspect of the left foot showed a cell-poor interface dermatitis that could resemble partially treated LP or a lichenoid hypersensitivity reaction (Figure 3). Given the clinical and pathologic findings, a diagnosis of palmoplantar LP was favored. The patient was on no medications or over-the-counter supplements prior to the appearance of the rash, making a lichenoid hypersensitivity rash less likely. The histology findings likely were muted, as they were done at the end of the prednisone taper.
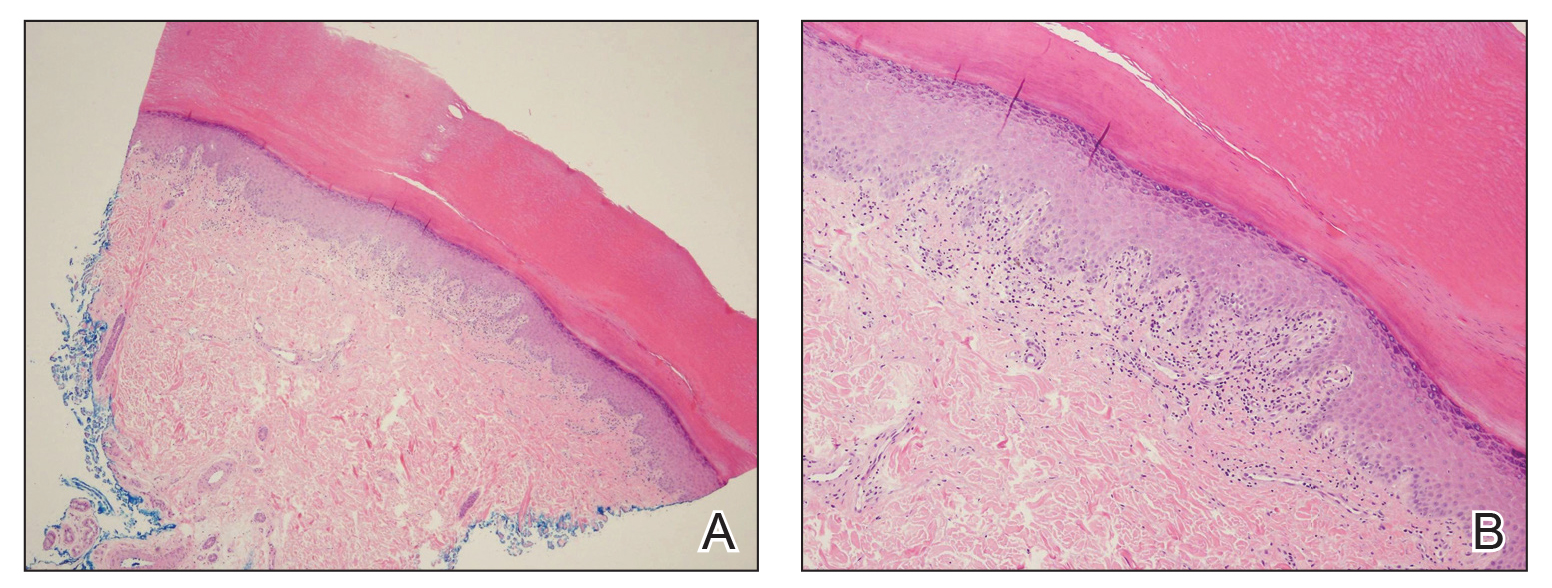
Minocycline and clobetasol ointment were discontinued, and the prednisone taper was completed as originally prescribed. The patient was started on 25 mg daily of acitretin for 4 weeks, then increased to 35 mg daily. Notable improvement in the palmar and plantar lesions was noted after the initial 4 weeks of therapy; however, acitretin treatment was discontinued due to lack of adequate insurance coverage for the medication. The patient became symptomatic several weeks following acitretin cessation and was started on methotrexate 15 mg weekly with triamcinolone acetonide paste 0.1% for the oral lesions. Once again, improvement was seen on both the palmar and plantar surfaces after 4 weeks of therapy (Figures 1B and 2B).
Evidence for treatment of palmoplantar LP is limited to a few case reports and case series. Documented treatments for palmoplantar LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive medications.4 One case report described a patient who responded well to prednisone therapy (1 mg/kg daily for 3 weeks, then reduced to 5 mg daily).5 Another report described a patient who responded favorably to cyclosporine 3.5 mg/kg daily for 4 weeks, then tapered over another 4 weeks for a total of 8 weeks of treatment.4 Although the most common treatments described in the literature consist of acitretin as well as topical and systemic steroids, few have discussed the efficacy of methotrexate. In one study, acitretin did not result in clearance, but the patient saw profound improvement with methotrexate (titrated up to 25 mg weekly) over 2 months.1
In our case, treatment with methotrexate was proven successful in a patient who responded to acitretin but was unable to afford treatment. This case highlights a rare variant of a common disease and the possibility of methotrexate as a cost-effective and useful treatment option for LP.
- Rieder E, Hale CS, Meehan SA, et al. Palmoplantar lichen planus. Dermatol Online J. 2015;20:13030/qt1vn9s55z.
- Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
- Gutte R, Khopkar U. Predominant palmoplantar lichen planus: a diagnostic challenge. Indian J Dermatol. 2014;59:343-347.
- Karakatsanis G, Patsatsi A, Kastoridou C, et al Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
- Goucha S, Khaled A, Bennani Z, et al. Erosive lichen planus of the soles: Effective response to prednisone. Dermatol Ther. 2011;1:20-24.
To the Editor:
Palmoplantar lichen planus (LP) is an uncommon variant of LP that involves the palms and soles. The prevalence of LP is approximately 0.1% to 2% in the general population. It can affect both mucosal and cutaneous surfaces.1 A study of 36 patients with LP showed that 25% (9/36) had palmar and/or plantar involvement.2 Palmoplantar LP is more commonly found in men than women, with an average age of onset of 38 to 65 years.3 It tends to affect the soles more often than the palms, with the most common site being the plantar arch. Itching generally is the most common symptom reported. Lesions often resolve over a few months, but relapses can occur in 10% to 29% of patients.2 The clinical morphology commonly is characterized as erythematous scaly plaques, hyperkeratotic plaques, or ulcerations.4 Due to its rare occurrence, palmoplantar LP often is misdiagnosed as psoriasis, eczematous dermatitis, tinea nigra, or secondary syphilis, making pathology extremely helpful in making the diagnosis.1 Darker skin types can obscure defining characteristics, further impeding a timely diagnosis. We describe a novel case of palmoplantar LP that was successfully treated with methotrexate.
A 38-year-old man with no notable medical history presented for dermatologic evaluation of a palmar and plantar rash of 4 months' duration. The rash was accompanied by intense burning pain and pruritus. Prior to presentation, he had been treated with multiple prednisone tapers starting at 40 mg daily as well as combination therapy of a 2-week course of minocycline 100 mg twice daily and clobetasol ointment twice daily for 4 months, with no notable improvement. Workup prior to presentation included a negative potassium hydroxide fungal preparation and a normal antinuclear antibody titer. A review of symptoms was negative for arthralgia, myalgia, photosensitivity, malar rash, Raynaud phenomenon, pleuritic pain, seizures, and psychosis.
Physical examination revealed focal areas of mildly thick, hyperkeratotic scale with desquamation on the plantar and palmar surfaces of the feet and hands. The underlying skin of the feet consisted of dyspigmented patches of dark brown and hypopigmented skin with erythema, profound scaling, and sparing of the internal plantar arches (Figure 1A). On the palms, thin hyperkeratotic plaques with desquamation and erythematous maceration of the surrounding skin were observed (Figure 2A). Thin white plaques of the posterior bilateral buccal mucosa were appreciated as well as an erosion that extended to the lower lip.
The differential diagnosis included LP, psoriasis, acquired palmoplantar keratoderma, and discoid lupus erythematosus. Tinea pedis and tinea manuum were less likely in the setting of a negative potassium hydroxide fungal preparation.


A biopsy of the lateral aspect of the left foot showed a cell-poor interface dermatitis that could resemble partially treated LP or a lichenoid hypersensitivity reaction (Figure 3). Given the clinical and pathologic findings, a diagnosis of palmoplantar LP was favored. The patient was on no medications or over-the-counter supplements prior to the appearance of the rash, making a lichenoid hypersensitivity rash less likely. The histology findings likely were muted, as they were done at the end of the prednisone taper.

Minocycline and clobetasol ointment were discontinued, and the prednisone taper was completed as originally prescribed. The patient was started on 25 mg daily of acitretin for 4 weeks, then increased to 35 mg daily. Notable improvement in the palmar and plantar lesions was noted after the initial 4 weeks of therapy; however, acitretin treatment was discontinued due to lack of adequate insurance coverage for the medication. The patient became symptomatic several weeks following acitretin cessation and was started on methotrexate 15 mg weekly with triamcinolone acetonide paste 0.1% for the oral lesions. Once again, improvement was seen on both the palmar and plantar surfaces after 4 weeks of therapy (Figures 1B and 2B).
Evidence for treatment of palmoplantar LP is limited to a few case reports and case series. Documented treatments for palmoplantar LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive medications.4 One case report described a patient who responded well to prednisone therapy (1 mg/kg daily for 3 weeks, then reduced to 5 mg daily).5 Another report described a patient who responded favorably to cyclosporine 3.5 mg/kg daily for 4 weeks, then tapered over another 4 weeks for a total of 8 weeks of treatment.4 Although the most common treatments described in the literature consist of acitretin as well as topical and systemic steroids, few have discussed the efficacy of methotrexate. In one study, acitretin did not result in clearance, but the patient saw profound improvement with methotrexate (titrated up to 25 mg weekly) over 2 months.1
In our case, treatment with methotrexate was proven successful in a patient who responded to acitretin but was unable to afford treatment. This case highlights a rare variant of a common disease and the possibility of methotrexate as a cost-effective and useful treatment option for LP.
To the Editor:
Palmoplantar lichen planus (LP) is an uncommon variant of LP that involves the palms and soles. The prevalence of LP is approximately 0.1% to 2% in the general population. It can affect both mucosal and cutaneous surfaces.1 A study of 36 patients with LP showed that 25% (9/36) had palmar and/or plantar involvement.2 Palmoplantar LP is more commonly found in men than women, with an average age of onset of 38 to 65 years.3 It tends to affect the soles more often than the palms, with the most common site being the plantar arch. Itching generally is the most common symptom reported. Lesions often resolve over a few months, but relapses can occur in 10% to 29% of patients.2 The clinical morphology commonly is characterized as erythematous scaly plaques, hyperkeratotic plaques, or ulcerations.4 Due to its rare occurrence, palmoplantar LP often is misdiagnosed as psoriasis, eczematous dermatitis, tinea nigra, or secondary syphilis, making pathology extremely helpful in making the diagnosis.1 Darker skin types can obscure defining characteristics, further impeding a timely diagnosis. We describe a novel case of palmoplantar LP that was successfully treated with methotrexate.
A 38-year-old man with no notable medical history presented for dermatologic evaluation of a palmar and plantar rash of 4 months' duration. The rash was accompanied by intense burning pain and pruritus. Prior to presentation, he had been treated with multiple prednisone tapers starting at 40 mg daily as well as combination therapy of a 2-week course of minocycline 100 mg twice daily and clobetasol ointment twice daily for 4 months, with no notable improvement. Workup prior to presentation included a negative potassium hydroxide fungal preparation and a normal antinuclear antibody titer. A review of symptoms was negative for arthralgia, myalgia, photosensitivity, malar rash, Raynaud phenomenon, pleuritic pain, seizures, and psychosis.
Physical examination revealed focal areas of mildly thick, hyperkeratotic scale with desquamation on the plantar and palmar surfaces of the feet and hands. The underlying skin of the feet consisted of dyspigmented patches of dark brown and hypopigmented skin with erythema, profound scaling, and sparing of the internal plantar arches (Figure 1A). On the palms, thin hyperkeratotic plaques with desquamation and erythematous maceration of the surrounding skin were observed (Figure 2A). Thin white plaques of the posterior bilateral buccal mucosa were appreciated as well as an erosion that extended to the lower lip.
The differential diagnosis included LP, psoriasis, acquired palmoplantar keratoderma, and discoid lupus erythematosus. Tinea pedis and tinea manuum were less likely in the setting of a negative potassium hydroxide fungal preparation.


A biopsy of the lateral aspect of the left foot showed a cell-poor interface dermatitis that could resemble partially treated LP or a lichenoid hypersensitivity reaction (Figure 3). Given the clinical and pathologic findings, a diagnosis of palmoplantar LP was favored. The patient was on no medications or over-the-counter supplements prior to the appearance of the rash, making a lichenoid hypersensitivity rash less likely. The histology findings likely were muted, as they were done at the end of the prednisone taper.

Minocycline and clobetasol ointment were discontinued, and the prednisone taper was completed as originally prescribed. The patient was started on 25 mg daily of acitretin for 4 weeks, then increased to 35 mg daily. Notable improvement in the palmar and plantar lesions was noted after the initial 4 weeks of therapy; however, acitretin treatment was discontinued due to lack of adequate insurance coverage for the medication. The patient became symptomatic several weeks following acitretin cessation and was started on methotrexate 15 mg weekly with triamcinolone acetonide paste 0.1% for the oral lesions. Once again, improvement was seen on both the palmar and plantar surfaces after 4 weeks of therapy (Figures 1B and 2B).
Evidence for treatment of palmoplantar LP is limited to a few case reports and case series. Documented treatments for palmoplantar LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive medications.4 One case report described a patient who responded well to prednisone therapy (1 mg/kg daily for 3 weeks, then reduced to 5 mg daily).5 Another report described a patient who responded favorably to cyclosporine 3.5 mg/kg daily for 4 weeks, then tapered over another 4 weeks for a total of 8 weeks of treatment.4 Although the most common treatments described in the literature consist of acitretin as well as topical and systemic steroids, few have discussed the efficacy of methotrexate. In one study, acitretin did not result in clearance, but the patient saw profound improvement with methotrexate (titrated up to 25 mg weekly) over 2 months.1
In our case, treatment with methotrexate was proven successful in a patient who responded to acitretin but was unable to afford treatment. This case highlights a rare variant of a common disease and the possibility of methotrexate as a cost-effective and useful treatment option for LP.
- Rieder E, Hale CS, Meehan SA, et al. Palmoplantar lichen planus. Dermatol Online J. 2015;20:13030/qt1vn9s55z.
- Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
- Gutte R, Khopkar U. Predominant palmoplantar lichen planus: a diagnostic challenge. Indian J Dermatol. 2014;59:343-347.
- Karakatsanis G, Patsatsi A, Kastoridou C, et al Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
- Goucha S, Khaled A, Bennani Z, et al. Erosive lichen planus of the soles: Effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Rieder E, Hale CS, Meehan SA, et al. Palmoplantar lichen planus. Dermatol Online J. 2015;20:13030/qt1vn9s55z.
- Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
- Gutte R, Khopkar U. Predominant palmoplantar lichen planus: a diagnostic challenge. Indian J Dermatol. 2014;59:343-347.
- Karakatsanis G, Patsatsi A, Kastoridou C, et al Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
- Goucha S, Khaled A, Bennani Z, et al. Erosive lichen planus of the soles: Effective response to prednisone. Dermatol Ther. 2011;1:20-24.
Practice Points
- Palmoplantar lichen planus (LP) is a rare variant of LP that is resistant to most treatments.
- Methotrexate may be a cost-effective option in patients who cannot tolerate systemic retinoids.
Serum cortisol testing for suspected adrenal insufficiency
Evaluating the hospitalized adult patient
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2
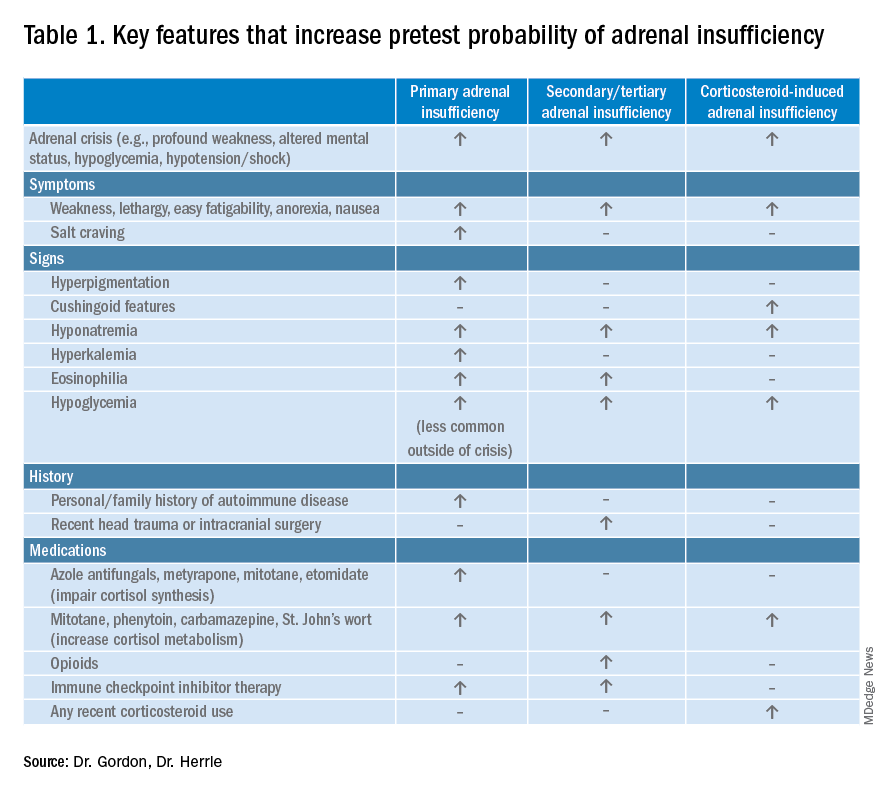
AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).
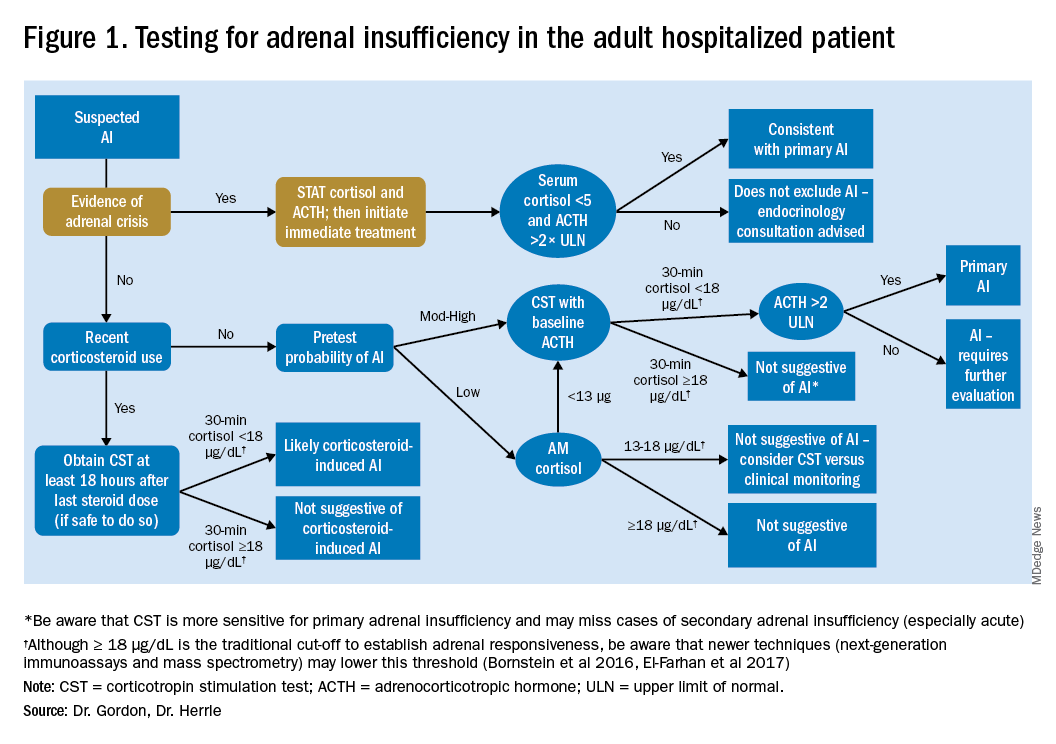
For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).
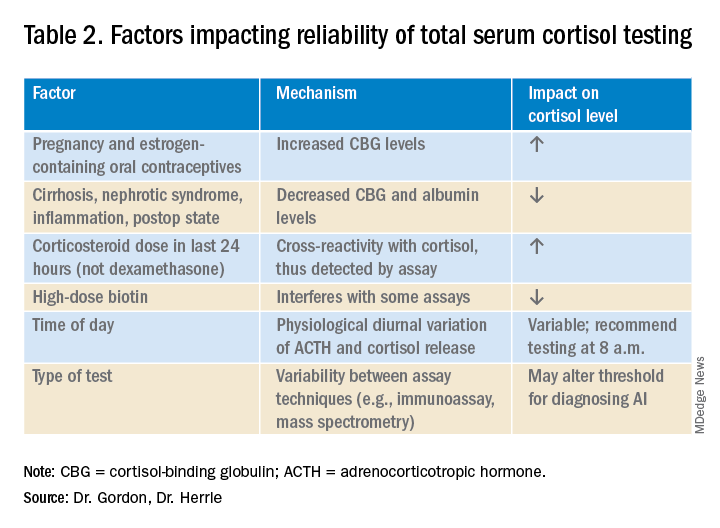
Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Evaluating the hospitalized adult patient
Evaluating the hospitalized adult patient
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2

AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).

For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).

Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2

AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).

For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).

Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Near-hanging injuries: Critical care, psychiatric management
Suicide by hanging results in many deaths, and half of those survivors who are admitted later die from cardiac arrest.
Although hanging is a common form of suicide, studies of the clinical outcomes of near-hanging injury are rare. To address this void, Louise de Charentenay, MD, of the Medical-Surgical Intensive Care Unit, Centre Hospitalier de Versailles (France) and colleagues examined the vital and functional outcomes of more than 800 patients with suicidal near-hanging injury over 2 decades. Despite the high in-hospital mortality rate among survivors, those who do survive have an excellent chance of a full neurocognitive recovery. The investigators published their findings in Chest.
New data on near-hanging injuries
Near hanging refers to strangulation or hanging that doesn’t immediately lead to death. Little data have been available on this subject, particularly on the morbidity and mortality of patients admitted to the ICU following near-hanging injuries. In a retrospective analysis spanning 23 years (1992-2014), researchers looked at outcomes and early predictors of hospital deaths in patients with this injury. The study included 886 adult patients who were admitted to 31 university or university-affiliated ICUs in France and Belgium following successful resuscitation of suicidal near-hanging injury.
Investigators used logistic multivariate regression to report vital and functional outcomes at hospital discharge as a primary objective. They also aimed to identify predictors of hospital mortality in these patients.
Among all patients, 450 (50.8%) had hanging-induced cardiac arrest and of these, 371 (95.4%) eventually died. Although the rate of crude hospital deaths decreased over the 23-year period, hanging-induced cardiac arrest emerged as the strongest predictor of hospital mortality, followed by high blood lactate and hyperglycemia at ICU admission. “Hanging-induced cardiac arrest and worse consciousness impairment at ICU admission are directly related to the hanging, whereas higher glycemia and lactate levels at ICU admission represent biochemical markers of physiologic perturbation and injury severity that may suggest avenues for improvement in prehospital care,” wrote the investigators.
More than 56% of the patients survived to discharge, with a majority achieving favorable outcomes (a Glasgow Outcome Scale scores of 4 or 5 at discharge).
‘COVID-lateral’ damage and ICU management
Casey D. Bryant, MD, of the department of anesthesiology and the department of emergency medicine at Wake Forest Baptist Health, Winston-Salem, N.C., has treated these patients in the ICU and is prepared to see more of them in light of the current situation. He said in an interview, “The “COVID-lateral” damage being unleashed on the population as a result of increased isolation, lack of access to resources, higher unemployment, and increased substance abuse was detailed recently in an article by one of my colleagues, Dr. Seth Hawkins (Emerg Med News. 2020 Jun;42[6]:1,31-2). According to the Centers for Disease Control and Prevention, hanging is the second leading cause of suicide in the United States, and one can only assume that with increased mental health crises there will also be an increased number of hanging attempts.”
Dr. Bryant suggested that the first task of doctors who learn that a near-hanging patient has been admitted is to “recover from the gut-punch you feel when you learn that a fellow human has tried to take their own life.” Once one is composed, he said, the first order of business is to come up with a treatment plan, one that typically begins with the airway. “These patients are at a high risk for cervical vertebrae injury (e.g., hangman’s fracture), spinal cord injury, tracheal injury, and neck vessel injury or dissection, so care must be taken to maintain in-line stabilization and limit movement of the neck during intubation while also being prepared for all manner of airway disasters. After airway management, addressing traumatic injuries, and initial stabilization, the focus then shifts to ‘bread and butter’ critical care, including optimization of ventilator management, titration of analgosedation, providing adequate nutrition, and strict avoidance of hypoxia, hypotension, fever, and either hyper- or hypoglycemia.”
Dr. Bryant noted that targeted temperature management prescriptions remain an area of debate in those with comatose state after hanging, but fever should absolutely be avoided. He added: “As the path to recovery begins to be forged, the full gamut of mental health resources should be provided to the patients in order to give them the best chance for success once they leave the ICU, and ultimately the hospital.”
The different hospitals seemed to have varying degrees of success in saving these patients, which is surprising, Mangala Narasimhan, DO, FCCP, regional director of critical care, director of the acute lung injury/ECMO center at Northwell and a professor of medicine at the Hofstra/Northwell School of Medicine, New York, said in an interview. “Usually, the death rate for cardiac arrest is high and the death rate for hanging is high. But here, it was high in some places and low in others.” Different time frames from presenting from hanging and different treatments may explain this, said Dr. Narasimhan.
Patient characteristics
Consistent with previous research, near-hanging patients are predominantly male, have at least one psychiatric diagnosis and a previous suicidal attempt (rarely by hanging), and abuse substances such as an alcohol, Stéphane Legriel, MD, PhD, the study’s corresponding author, said in an interview. Overall, 67.7% of the patients had a diagnosed mental illness and 30% had previously attempted suicide. Most of the hangings took place at home (79%), while some took place in a hospital ward (6%), a correctional facility (7%), or outside (5%).
The study had several limitations: It applied only to near-hanging patients admitted to the ICU, and its long duration may have resulted in heterogeneity of the population and therapeutic interventions, and in some missing data. “However, the multivariate analysis was adjusted for the time period and we carried out a sensitivity analysis after multiple imputation for missing data by means of chained equations, which reinforces confidence in our findings,” Dr. Legriel said. Next steps are to conduct a prospective data collection.
Postdischarge recovery and psychiatric follow-up
Those left to treat survivors of near-hangings are psychiatrists and other mental health clinicians, Eric M. Plakun, MD, said in an interview.
“Some of these survivors will regret they survived and remain high suicide risks. Some will feel their lives are transformed or at least no longer as intensely drawn to suicide as a solution to a life filled with the impact of adversity, trauma, comorbidity, and other struggles – but even these individuals will still have to face the often complex underlying issues that led them to choose suicide as a solution,” said Dr. Plakun, medical director and CEO of the Austen Riggs Center in Stockbridge, Mass.
Patients with medically serious suicide attempts are seen a lot at Austen Riggs, he said, because acute inpatient settings are designed for brief, crisis-focused treatment of those for whom safety is an issue. After the crisis has been stabilized, patients are discharged, and then must begin to achieve recovery as outpatients, he said.
John Kruse, MD, PhD, a psychiatrist who practices in San Francisco, praised the size and the breath of the study. “One limitation was the reliance on hospital records, without an opportunity to directly evaluate or interview the patients involved.”
The authors disclosed no conflicts of interest. The study received grant support from the French public funding agency, Délégation la Recherche Clinique et de l’Innovation in Versailles, France.
SOURCE: de Charentenay L et al. 2020 Aug 3. doi: 10.1016/j.chest.2020.07.064
Suicide by hanging results in many deaths, and half of those survivors who are admitted later die from cardiac arrest.
Although hanging is a common form of suicide, studies of the clinical outcomes of near-hanging injury are rare. To address this void, Louise de Charentenay, MD, of the Medical-Surgical Intensive Care Unit, Centre Hospitalier de Versailles (France) and colleagues examined the vital and functional outcomes of more than 800 patients with suicidal near-hanging injury over 2 decades. Despite the high in-hospital mortality rate among survivors, those who do survive have an excellent chance of a full neurocognitive recovery. The investigators published their findings in Chest.
New data on near-hanging injuries
Near hanging refers to strangulation or hanging that doesn’t immediately lead to death. Little data have been available on this subject, particularly on the morbidity and mortality of patients admitted to the ICU following near-hanging injuries. In a retrospective analysis spanning 23 years (1992-2014), researchers looked at outcomes and early predictors of hospital deaths in patients with this injury. The study included 886 adult patients who were admitted to 31 university or university-affiliated ICUs in France and Belgium following successful resuscitation of suicidal near-hanging injury.
Investigators used logistic multivariate regression to report vital and functional outcomes at hospital discharge as a primary objective. They also aimed to identify predictors of hospital mortality in these patients.
Among all patients, 450 (50.8%) had hanging-induced cardiac arrest and of these, 371 (95.4%) eventually died. Although the rate of crude hospital deaths decreased over the 23-year period, hanging-induced cardiac arrest emerged as the strongest predictor of hospital mortality, followed by high blood lactate and hyperglycemia at ICU admission. “Hanging-induced cardiac arrest and worse consciousness impairment at ICU admission are directly related to the hanging, whereas higher glycemia and lactate levels at ICU admission represent biochemical markers of physiologic perturbation and injury severity that may suggest avenues for improvement in prehospital care,” wrote the investigators.
More than 56% of the patients survived to discharge, with a majority achieving favorable outcomes (a Glasgow Outcome Scale scores of 4 or 5 at discharge).
‘COVID-lateral’ damage and ICU management
Casey D. Bryant, MD, of the department of anesthesiology and the department of emergency medicine at Wake Forest Baptist Health, Winston-Salem, N.C., has treated these patients in the ICU and is prepared to see more of them in light of the current situation. He said in an interview, “The “COVID-lateral” damage being unleashed on the population as a result of increased isolation, lack of access to resources, higher unemployment, and increased substance abuse was detailed recently in an article by one of my colleagues, Dr. Seth Hawkins (Emerg Med News. 2020 Jun;42[6]:1,31-2). According to the Centers for Disease Control and Prevention, hanging is the second leading cause of suicide in the United States, and one can only assume that with increased mental health crises there will also be an increased number of hanging attempts.”
Dr. Bryant suggested that the first task of doctors who learn that a near-hanging patient has been admitted is to “recover from the gut-punch you feel when you learn that a fellow human has tried to take their own life.” Once one is composed, he said, the first order of business is to come up with a treatment plan, one that typically begins with the airway. “These patients are at a high risk for cervical vertebrae injury (e.g., hangman’s fracture), spinal cord injury, tracheal injury, and neck vessel injury or dissection, so care must be taken to maintain in-line stabilization and limit movement of the neck during intubation while also being prepared for all manner of airway disasters. After airway management, addressing traumatic injuries, and initial stabilization, the focus then shifts to ‘bread and butter’ critical care, including optimization of ventilator management, titration of analgosedation, providing adequate nutrition, and strict avoidance of hypoxia, hypotension, fever, and either hyper- or hypoglycemia.”
Dr. Bryant noted that targeted temperature management prescriptions remain an area of debate in those with comatose state after hanging, but fever should absolutely be avoided. He added: “As the path to recovery begins to be forged, the full gamut of mental health resources should be provided to the patients in order to give them the best chance for success once they leave the ICU, and ultimately the hospital.”
The different hospitals seemed to have varying degrees of success in saving these patients, which is surprising, Mangala Narasimhan, DO, FCCP, regional director of critical care, director of the acute lung injury/ECMO center at Northwell and a professor of medicine at the Hofstra/Northwell School of Medicine, New York, said in an interview. “Usually, the death rate for cardiac arrest is high and the death rate for hanging is high. But here, it was high in some places and low in others.” Different time frames from presenting from hanging and different treatments may explain this, said Dr. Narasimhan.
Patient characteristics
Consistent with previous research, near-hanging patients are predominantly male, have at least one psychiatric diagnosis and a previous suicidal attempt (rarely by hanging), and abuse substances such as an alcohol, Stéphane Legriel, MD, PhD, the study’s corresponding author, said in an interview. Overall, 67.7% of the patients had a diagnosed mental illness and 30% had previously attempted suicide. Most of the hangings took place at home (79%), while some took place in a hospital ward (6%), a correctional facility (7%), or outside (5%).
The study had several limitations: It applied only to near-hanging patients admitted to the ICU, and its long duration may have resulted in heterogeneity of the population and therapeutic interventions, and in some missing data. “However, the multivariate analysis was adjusted for the time period and we carried out a sensitivity analysis after multiple imputation for missing data by means of chained equations, which reinforces confidence in our findings,” Dr. Legriel said. Next steps are to conduct a prospective data collection.
Postdischarge recovery and psychiatric follow-up
Those left to treat survivors of near-hangings are psychiatrists and other mental health clinicians, Eric M. Plakun, MD, said in an interview.
“Some of these survivors will regret they survived and remain high suicide risks. Some will feel their lives are transformed or at least no longer as intensely drawn to suicide as a solution to a life filled with the impact of adversity, trauma, comorbidity, and other struggles – but even these individuals will still have to face the often complex underlying issues that led them to choose suicide as a solution,” said Dr. Plakun, medical director and CEO of the Austen Riggs Center in Stockbridge, Mass.
Patients with medically serious suicide attempts are seen a lot at Austen Riggs, he said, because acute inpatient settings are designed for brief, crisis-focused treatment of those for whom safety is an issue. After the crisis has been stabilized, patients are discharged, and then must begin to achieve recovery as outpatients, he said.
John Kruse, MD, PhD, a psychiatrist who practices in San Francisco, praised the size and the breath of the study. “One limitation was the reliance on hospital records, without an opportunity to directly evaluate or interview the patients involved.”
The authors disclosed no conflicts of interest. The study received grant support from the French public funding agency, Délégation la Recherche Clinique et de l’Innovation in Versailles, France.
SOURCE: de Charentenay L et al. 2020 Aug 3. doi: 10.1016/j.chest.2020.07.064
Suicide by hanging results in many deaths, and half of those survivors who are admitted later die from cardiac arrest.
Although hanging is a common form of suicide, studies of the clinical outcomes of near-hanging injury are rare. To address this void, Louise de Charentenay, MD, of the Medical-Surgical Intensive Care Unit, Centre Hospitalier de Versailles (France) and colleagues examined the vital and functional outcomes of more than 800 patients with suicidal near-hanging injury over 2 decades. Despite the high in-hospital mortality rate among survivors, those who do survive have an excellent chance of a full neurocognitive recovery. The investigators published their findings in Chest.
New data on near-hanging injuries
Near hanging refers to strangulation or hanging that doesn’t immediately lead to death. Little data have been available on this subject, particularly on the morbidity and mortality of patients admitted to the ICU following near-hanging injuries. In a retrospective analysis spanning 23 years (1992-2014), researchers looked at outcomes and early predictors of hospital deaths in patients with this injury. The study included 886 adult patients who were admitted to 31 university or university-affiliated ICUs in France and Belgium following successful resuscitation of suicidal near-hanging injury.
Investigators used logistic multivariate regression to report vital and functional outcomes at hospital discharge as a primary objective. They also aimed to identify predictors of hospital mortality in these patients.
Among all patients, 450 (50.8%) had hanging-induced cardiac arrest and of these, 371 (95.4%) eventually died. Although the rate of crude hospital deaths decreased over the 23-year period, hanging-induced cardiac arrest emerged as the strongest predictor of hospital mortality, followed by high blood lactate and hyperglycemia at ICU admission. “Hanging-induced cardiac arrest and worse consciousness impairment at ICU admission are directly related to the hanging, whereas higher glycemia and lactate levels at ICU admission represent biochemical markers of physiologic perturbation and injury severity that may suggest avenues for improvement in prehospital care,” wrote the investigators.
More than 56% of the patients survived to discharge, with a majority achieving favorable outcomes (a Glasgow Outcome Scale scores of 4 or 5 at discharge).
‘COVID-lateral’ damage and ICU management
Casey D. Bryant, MD, of the department of anesthesiology and the department of emergency medicine at Wake Forest Baptist Health, Winston-Salem, N.C., has treated these patients in the ICU and is prepared to see more of them in light of the current situation. He said in an interview, “The “COVID-lateral” damage being unleashed on the population as a result of increased isolation, lack of access to resources, higher unemployment, and increased substance abuse was detailed recently in an article by one of my colleagues, Dr. Seth Hawkins (Emerg Med News. 2020 Jun;42[6]:1,31-2). According to the Centers for Disease Control and Prevention, hanging is the second leading cause of suicide in the United States, and one can only assume that with increased mental health crises there will also be an increased number of hanging attempts.”
Dr. Bryant suggested that the first task of doctors who learn that a near-hanging patient has been admitted is to “recover from the gut-punch you feel when you learn that a fellow human has tried to take their own life.” Once one is composed, he said, the first order of business is to come up with a treatment plan, one that typically begins with the airway. “These patients are at a high risk for cervical vertebrae injury (e.g., hangman’s fracture), spinal cord injury, tracheal injury, and neck vessel injury or dissection, so care must be taken to maintain in-line stabilization and limit movement of the neck during intubation while also being prepared for all manner of airway disasters. After airway management, addressing traumatic injuries, and initial stabilization, the focus then shifts to ‘bread and butter’ critical care, including optimization of ventilator management, titration of analgosedation, providing adequate nutrition, and strict avoidance of hypoxia, hypotension, fever, and either hyper- or hypoglycemia.”
Dr. Bryant noted that targeted temperature management prescriptions remain an area of debate in those with comatose state after hanging, but fever should absolutely be avoided. He added: “As the path to recovery begins to be forged, the full gamut of mental health resources should be provided to the patients in order to give them the best chance for success once they leave the ICU, and ultimately the hospital.”
The different hospitals seemed to have varying degrees of success in saving these patients, which is surprising, Mangala Narasimhan, DO, FCCP, regional director of critical care, director of the acute lung injury/ECMO center at Northwell and a professor of medicine at the Hofstra/Northwell School of Medicine, New York, said in an interview. “Usually, the death rate for cardiac arrest is high and the death rate for hanging is high. But here, it was high in some places and low in others.” Different time frames from presenting from hanging and different treatments may explain this, said Dr. Narasimhan.
Patient characteristics
Consistent with previous research, near-hanging patients are predominantly male, have at least one psychiatric diagnosis and a previous suicidal attempt (rarely by hanging), and abuse substances such as an alcohol, Stéphane Legriel, MD, PhD, the study’s corresponding author, said in an interview. Overall, 67.7% of the patients had a diagnosed mental illness and 30% had previously attempted suicide. Most of the hangings took place at home (79%), while some took place in a hospital ward (6%), a correctional facility (7%), or outside (5%).
The study had several limitations: It applied only to near-hanging patients admitted to the ICU, and its long duration may have resulted in heterogeneity of the population and therapeutic interventions, and in some missing data. “However, the multivariate analysis was adjusted for the time period and we carried out a sensitivity analysis after multiple imputation for missing data by means of chained equations, which reinforces confidence in our findings,” Dr. Legriel said. Next steps are to conduct a prospective data collection.
Postdischarge recovery and psychiatric follow-up
Those left to treat survivors of near-hangings are psychiatrists and other mental health clinicians, Eric M. Plakun, MD, said in an interview.
“Some of these survivors will regret they survived and remain high suicide risks. Some will feel their lives are transformed or at least no longer as intensely drawn to suicide as a solution to a life filled with the impact of adversity, trauma, comorbidity, and other struggles – but even these individuals will still have to face the often complex underlying issues that led them to choose suicide as a solution,” said Dr. Plakun, medical director and CEO of the Austen Riggs Center in Stockbridge, Mass.
Patients with medically serious suicide attempts are seen a lot at Austen Riggs, he said, because acute inpatient settings are designed for brief, crisis-focused treatment of those for whom safety is an issue. After the crisis has been stabilized, patients are discharged, and then must begin to achieve recovery as outpatients, he said.
John Kruse, MD, PhD, a psychiatrist who practices in San Francisco, praised the size and the breath of the study. “One limitation was the reliance on hospital records, without an opportunity to directly evaluate or interview the patients involved.”
The authors disclosed no conflicts of interest. The study received grant support from the French public funding agency, Délégation la Recherche Clinique et de l’Innovation in Versailles, France.
SOURCE: de Charentenay L et al. 2020 Aug 3. doi: 10.1016/j.chest.2020.07.064
Novel oral drug improves sunlight tolerance in patients with erythropoietic protoporphyria
in a multicenter, phase 2, randomized trial, Kirstine Belongie, PhD, reported at the virtual annual meeting of the American Academy of Dermatology.
Based upon these favorable phase 2 results, a pivotal phase 3 clinical trial is now underway, added Dr. Belongie of Mitsubishi Tanabe Pharma Development America, in Jersey City, N.J., the study sponsor.
Erythropoietic protoporphyria (EPP) is the most common cutaneous porphyria as well as the most common porphyria of any type in children. It’s a rare but devastating disorder, with an incidence estimated at 1 in 75,000-200,000. It involves acute cutaneous photosensitivity to sunlight, which takes the form of incapacitating burning pain that lasts 3-7 days and is then followed by erythema and edema.
“These phototoxic reactions are extremely painful and cause the patients to have extreme fear of the sun. They do everything they can to avoid the sun. It leads to a highly impaired quality of life that’s restricted to the indoors,” she explained.
Current first-line therapy is sun avoidance, the use of zinc oxide sunblock, and protective clothing. It’s inadequate for most patients. “There is a tremendously high unmet medical need for treatment options, especially in the pediatric population,” Dr. Belongie observed.
Patients with EPP experience prodromal symptoms – tingling, itching, and burning – which serve as a signal to get out of the sun immediately. As demonstrated in the phase 2 trial, dersimelagon prolongs the time to onset of these prodromal symptoms by increasing melanin density in the skin in a dose-dependent fashion.
The phase 2 study included 102 EPP patients, with an average age 40 years, at 9 sites, who were randomized double blind to 16 weeks of dersimelagon at 100 mg or 300 mg once daily or placebo. The goal was to increase their pain-free sunlight exposure time.
The primary endpoint was change from baseline to week 16 in the average daily time to first prodromal symptoms. There was a 20-minute increase with placebo, a 74-minute gain with dersimelagon at 100 mg, and an 83-minute gain with dersimelagon at 300 mg. The difference between active medication and placebo became significant at week 6.
Treatment-emergent adverse events leading to study discontinuation occurred in one patient on dersimelagon at 100 mg/day, five patients on the higher dose, and none on placebo. Dr. Belangie said that the drug was well tolerated, with roughly 90% of adverse events being mild or moderate in severity. The frequency of adverse events was dose-related. The most common were headache, nausea, and diarrhea, occurring in 29%, 46%, and 23%, respectively, of patients on dersimelagon at 300 mg/day, compared with 18%, 12% and 12% of those on placebo.
Consistent with the drug’s mechanism of action, there was also a dose-related increase in hyperpigmentation side effects. New freckles were documented in 15% and 31% of patients on low- and high-dose dersimelagon, skin hyperpigmentation in 9% and 31%, and melanocytic nevi in 12% and 20%.
The ongoing double-blind, international, phase 3 trial includes not only patients with EPP, but also individuals with X-linked porphyria, which has similar clinical symptoms. The trial is double blind for the first 26 weeks, followed by another 26 weeks of open-label treatment.
EPP is an inherited metabolic disorder caused by a genetic mutation resulting in deficient activity of the enzyme ferrochelatase. This leads to accumulation of protoporphyrin IX in erythrocytes, skin, and the liver. The excess protoporphyrin is excreted in bile and can cause hepatobiliary disease. Indeed, up to 5% of patients with EPP develop liver failure.
In October 2019, the Food and Drug Administration approved afamelanotide (Scenesse), also a melanocortin-1 receptor agonist, to increase pain-free light exposure in adults with a history of phototoxic reactions EPP; this was the first FDA-approved treatment for helping EPP patients increase their exposure to light, according to the agency. It is administered as an implant every 2 months.
Dr. Belangie is employed by the study sponsor.
in a multicenter, phase 2, randomized trial, Kirstine Belongie, PhD, reported at the virtual annual meeting of the American Academy of Dermatology.
Based upon these favorable phase 2 results, a pivotal phase 3 clinical trial is now underway, added Dr. Belongie of Mitsubishi Tanabe Pharma Development America, in Jersey City, N.J., the study sponsor.
Erythropoietic protoporphyria (EPP) is the most common cutaneous porphyria as well as the most common porphyria of any type in children. It’s a rare but devastating disorder, with an incidence estimated at 1 in 75,000-200,000. It involves acute cutaneous photosensitivity to sunlight, which takes the form of incapacitating burning pain that lasts 3-7 days and is then followed by erythema and edema.
“These phototoxic reactions are extremely painful and cause the patients to have extreme fear of the sun. They do everything they can to avoid the sun. It leads to a highly impaired quality of life that’s restricted to the indoors,” she explained.
Current first-line therapy is sun avoidance, the use of zinc oxide sunblock, and protective clothing. It’s inadequate for most patients. “There is a tremendously high unmet medical need for treatment options, especially in the pediatric population,” Dr. Belongie observed.
Patients with EPP experience prodromal symptoms – tingling, itching, and burning – which serve as a signal to get out of the sun immediately. As demonstrated in the phase 2 trial, dersimelagon prolongs the time to onset of these prodromal symptoms by increasing melanin density in the skin in a dose-dependent fashion.
The phase 2 study included 102 EPP patients, with an average age 40 years, at 9 sites, who were randomized double blind to 16 weeks of dersimelagon at 100 mg or 300 mg once daily or placebo. The goal was to increase their pain-free sunlight exposure time.
The primary endpoint was change from baseline to week 16 in the average daily time to first prodromal symptoms. There was a 20-minute increase with placebo, a 74-minute gain with dersimelagon at 100 mg, and an 83-minute gain with dersimelagon at 300 mg. The difference between active medication and placebo became significant at week 6.
Treatment-emergent adverse events leading to study discontinuation occurred in one patient on dersimelagon at 100 mg/day, five patients on the higher dose, and none on placebo. Dr. Belangie said that the drug was well tolerated, with roughly 90% of adverse events being mild or moderate in severity. The frequency of adverse events was dose-related. The most common were headache, nausea, and diarrhea, occurring in 29%, 46%, and 23%, respectively, of patients on dersimelagon at 300 mg/day, compared with 18%, 12% and 12% of those on placebo.
Consistent with the drug’s mechanism of action, there was also a dose-related increase in hyperpigmentation side effects. New freckles were documented in 15% and 31% of patients on low- and high-dose dersimelagon, skin hyperpigmentation in 9% and 31%, and melanocytic nevi in 12% and 20%.
The ongoing double-blind, international, phase 3 trial includes not only patients with EPP, but also individuals with X-linked porphyria, which has similar clinical symptoms. The trial is double blind for the first 26 weeks, followed by another 26 weeks of open-label treatment.
EPP is an inherited metabolic disorder caused by a genetic mutation resulting in deficient activity of the enzyme ferrochelatase. This leads to accumulation of protoporphyrin IX in erythrocytes, skin, and the liver. The excess protoporphyrin is excreted in bile and can cause hepatobiliary disease. Indeed, up to 5% of patients with EPP develop liver failure.
In October 2019, the Food and Drug Administration approved afamelanotide (Scenesse), also a melanocortin-1 receptor agonist, to increase pain-free light exposure in adults with a history of phototoxic reactions EPP; this was the first FDA-approved treatment for helping EPP patients increase their exposure to light, according to the agency. It is administered as an implant every 2 months.
Dr. Belangie is employed by the study sponsor.
in a multicenter, phase 2, randomized trial, Kirstine Belongie, PhD, reported at the virtual annual meeting of the American Academy of Dermatology.
Based upon these favorable phase 2 results, a pivotal phase 3 clinical trial is now underway, added Dr. Belongie of Mitsubishi Tanabe Pharma Development America, in Jersey City, N.J., the study sponsor.
Erythropoietic protoporphyria (EPP) is the most common cutaneous porphyria as well as the most common porphyria of any type in children. It’s a rare but devastating disorder, with an incidence estimated at 1 in 75,000-200,000. It involves acute cutaneous photosensitivity to sunlight, which takes the form of incapacitating burning pain that lasts 3-7 days and is then followed by erythema and edema.
“These phototoxic reactions are extremely painful and cause the patients to have extreme fear of the sun. They do everything they can to avoid the sun. It leads to a highly impaired quality of life that’s restricted to the indoors,” she explained.
Current first-line therapy is sun avoidance, the use of zinc oxide sunblock, and protective clothing. It’s inadequate for most patients. “There is a tremendously high unmet medical need for treatment options, especially in the pediatric population,” Dr. Belongie observed.
Patients with EPP experience prodromal symptoms – tingling, itching, and burning – which serve as a signal to get out of the sun immediately. As demonstrated in the phase 2 trial, dersimelagon prolongs the time to onset of these prodromal symptoms by increasing melanin density in the skin in a dose-dependent fashion.
The phase 2 study included 102 EPP patients, with an average age 40 years, at 9 sites, who were randomized double blind to 16 weeks of dersimelagon at 100 mg or 300 mg once daily or placebo. The goal was to increase their pain-free sunlight exposure time.
The primary endpoint was change from baseline to week 16 in the average daily time to first prodromal symptoms. There was a 20-minute increase with placebo, a 74-minute gain with dersimelagon at 100 mg, and an 83-minute gain with dersimelagon at 300 mg. The difference between active medication and placebo became significant at week 6.
Treatment-emergent adverse events leading to study discontinuation occurred in one patient on dersimelagon at 100 mg/day, five patients on the higher dose, and none on placebo. Dr. Belangie said that the drug was well tolerated, with roughly 90% of adverse events being mild or moderate in severity. The frequency of adverse events was dose-related. The most common were headache, nausea, and diarrhea, occurring in 29%, 46%, and 23%, respectively, of patients on dersimelagon at 300 mg/day, compared with 18%, 12% and 12% of those on placebo.
Consistent with the drug’s mechanism of action, there was also a dose-related increase in hyperpigmentation side effects. New freckles were documented in 15% and 31% of patients on low- and high-dose dersimelagon, skin hyperpigmentation in 9% and 31%, and melanocytic nevi in 12% and 20%.
The ongoing double-blind, international, phase 3 trial includes not only patients with EPP, but also individuals with X-linked porphyria, which has similar clinical symptoms. The trial is double blind for the first 26 weeks, followed by another 26 weeks of open-label treatment.
EPP is an inherited metabolic disorder caused by a genetic mutation resulting in deficient activity of the enzyme ferrochelatase. This leads to accumulation of protoporphyrin IX in erythrocytes, skin, and the liver. The excess protoporphyrin is excreted in bile and can cause hepatobiliary disease. Indeed, up to 5% of patients with EPP develop liver failure.
In October 2019, the Food and Drug Administration approved afamelanotide (Scenesse), also a melanocortin-1 receptor agonist, to increase pain-free light exposure in adults with a history of phototoxic reactions EPP; this was the first FDA-approved treatment for helping EPP patients increase their exposure to light, according to the agency. It is administered as an implant every 2 months.
Dr. Belangie is employed by the study sponsor.
FROM AAD 2020