User login
Probiotics Emerge as Promising Intervention in Cirrhosis
, according to a systematic review and meta-analysis.
They also improve quality of life and have a favorable safety profile, adding to their potential as a promising intervention for treating cirrhosis, the study authors wrote.
“As currently one of the top 10 leading causes of death globally, cirrhosis imposes a great health burden in many countries,” wrote lead author Xing Yang of the Health Management Research Institute at the People’s Hospital of Guangxi Zhuang Autonomous Region and Guangxi Academy of Medical Sciences in Nanning, China, and colleagues.
“The burden has escalated at the worldwide level since 1990, partly because of population growth and aging,” the authors wrote. “Thus, it is meaningful to explore effective treatments for reversing cirrhosis and preventing severe liver function and even systemic damage.”
The study was published online in Frontiers in Medicine .
Analyzing Probiotic Trials
The researchers conducted a systematic review and meta-analysis of 30 randomized controlled trials among 2084 adults with cirrhosis, comparing the effects of probiotic intervention and control treatments, including placebo, no treatment, standard care, or active controls such as lactulose and rifaximin. The studies spanned 14 countries and included 1049 patients in the probiotic groups and 1035 in the control groups.
The research team calculated risk ratios (RRs) or standardized mean difference (SMD) for outcomes such as HE reversal, Model for End-Stage Liver Disease (MELD) scores, safety and tolerability of probiotics, liver function, and quality of life.
Among 17 studies involving patients with different stages of HE, as compared with the control group, probiotics significantly reversed minimal HE (RR, 1.54) and improved HE (RR, 1.94). In particular, the probiotic VSL#3 — which contains Streptococcus, Bifidobacterium, and Lactobacillus — produced more significant HE improvement (RR, 1.44) compared with other types of probiotics.
In addition, probiotics appeared to improve liver function by reducing MELD scores (SMD, −0.57) but didn’t show a difference in other liver function parameters. There were numerical but not significant reductions in mortality and serum inflammatory cytokine expression, including endotoxin, interleukin-6, and tumor necrosis factor-alpha.
Probiotics also improved quality-of-life scores (SMD, 0.51) and gut flora (SMD, 1.67). For gut flora, the numbers of the Lactobacillus group were significantly higher after probiotic treatment, but there wasn’t a significant difference for Bifidobacterium, Enterococcus, Bacteroidaceae, and Fusobacterium.
Finally, compared with control treatments, including placebo, standard therapy, and active controls such as lactulose and rifaximin, probiotics showed higher safety and tolerability profiles, causing a significantly lower incidence of serious adverse events (RR, 0.71).
Longer intervention times reduced the risk for overt HE development, hospitalization, and infections compared with shorter intervention times.
“Probiotics contribute to the reduction of ammonia levels and the improvement of neuropsychometric or neurophysiological status, leading to the reversal of HE associated with cirrhosis,” the study authors wrote. “Moreover, they induce favorable changes in gut flora and quality of life. Therefore, probiotics emerge as a promising intervention for reversing the onset of cirrhosis and preventing disease progression.”
Considering Variables
The authors noted several limitations, including a high or unclear risk for bias in 28 studies and the lack of data on the intervention effect for various types of probiotics or treatment durations.
“Overall, despite a number of methodological concerns, the study shows that probiotics can improve some disease markers in cirrhosis,” Phillipp Hartmann, MD, assistant professor of pediatric gastroenterology, hepatology, and nutrition at the University of California, San Diego, said in an interview.
“One of the methodological concerns is that the authors compared probiotics with a multitude of different treatments, including fiber and lactulose (which are both prebiotics), rifaximin (which is an antibiotic), standard of care, placebo, or no therapy,” he said. “This might contribute to the sometimes-contradictory findings between the different studies. The ideal comparison would be a specific probiotic formulation versus a placebo to understand what the probiotic actually does.”
Dr. Hartmann, who wasn’t involved with this study, has published a review on the potential of probiotics, prebiotics, and synbiotics in liver disease. He and colleagues noted the mechanisms that improve a disrupted intestinal barrier, microbial translocation, and altered gut microbiome metabolism.
“Over the last few years, we and others have studied the intestinal microbiota in various liver diseases, including alcohol-associated liver disease and metabolic dysfunction-associated steatotic liver disease,” he said. “Essentially, all studies support the notion that probiotics improve the microbial structure in the gut by increasing the beneficial and decreasing the potentially pathogenic microbes.”
However, probiotics and supplements are unregulated, Dr. Hartmann noted. Many different probiotic mixes and dosages have been tested in clinical trials, and additional studies are needed to determine the best formulations and dosages.
“Usually, the best outcomes can be achieved with a higher number of strains included in the probiotic formulation (10-30+) and a higher number of colony-forming units at 30-50+ billion per day,” he said.
The study was supported by funds from the Science and Technology Major Project of Guangxi, Guangxi Key Research and Development Program, and Natural Science Foundation of Guangxi Zhuang Autonomous Region. The authors declared no conflicts of interest. Dr. Hartmann reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a systematic review and meta-analysis.
They also improve quality of life and have a favorable safety profile, adding to their potential as a promising intervention for treating cirrhosis, the study authors wrote.
“As currently one of the top 10 leading causes of death globally, cirrhosis imposes a great health burden in many countries,” wrote lead author Xing Yang of the Health Management Research Institute at the People’s Hospital of Guangxi Zhuang Autonomous Region and Guangxi Academy of Medical Sciences in Nanning, China, and colleagues.
“The burden has escalated at the worldwide level since 1990, partly because of population growth and aging,” the authors wrote. “Thus, it is meaningful to explore effective treatments for reversing cirrhosis and preventing severe liver function and even systemic damage.”
The study was published online in Frontiers in Medicine .
Analyzing Probiotic Trials
The researchers conducted a systematic review and meta-analysis of 30 randomized controlled trials among 2084 adults with cirrhosis, comparing the effects of probiotic intervention and control treatments, including placebo, no treatment, standard care, or active controls such as lactulose and rifaximin. The studies spanned 14 countries and included 1049 patients in the probiotic groups and 1035 in the control groups.
The research team calculated risk ratios (RRs) or standardized mean difference (SMD) for outcomes such as HE reversal, Model for End-Stage Liver Disease (MELD) scores, safety and tolerability of probiotics, liver function, and quality of life.
Among 17 studies involving patients with different stages of HE, as compared with the control group, probiotics significantly reversed minimal HE (RR, 1.54) and improved HE (RR, 1.94). In particular, the probiotic VSL#3 — which contains Streptococcus, Bifidobacterium, and Lactobacillus — produced more significant HE improvement (RR, 1.44) compared with other types of probiotics.
In addition, probiotics appeared to improve liver function by reducing MELD scores (SMD, −0.57) but didn’t show a difference in other liver function parameters. There were numerical but not significant reductions in mortality and serum inflammatory cytokine expression, including endotoxin, interleukin-6, and tumor necrosis factor-alpha.
Probiotics also improved quality-of-life scores (SMD, 0.51) and gut flora (SMD, 1.67). For gut flora, the numbers of the Lactobacillus group were significantly higher after probiotic treatment, but there wasn’t a significant difference for Bifidobacterium, Enterococcus, Bacteroidaceae, and Fusobacterium.
Finally, compared with control treatments, including placebo, standard therapy, and active controls such as lactulose and rifaximin, probiotics showed higher safety and tolerability profiles, causing a significantly lower incidence of serious adverse events (RR, 0.71).
Longer intervention times reduced the risk for overt HE development, hospitalization, and infections compared with shorter intervention times.
“Probiotics contribute to the reduction of ammonia levels and the improvement of neuropsychometric or neurophysiological status, leading to the reversal of HE associated with cirrhosis,” the study authors wrote. “Moreover, they induce favorable changes in gut flora and quality of life. Therefore, probiotics emerge as a promising intervention for reversing the onset of cirrhosis and preventing disease progression.”
Considering Variables
The authors noted several limitations, including a high or unclear risk for bias in 28 studies and the lack of data on the intervention effect for various types of probiotics or treatment durations.
“Overall, despite a number of methodological concerns, the study shows that probiotics can improve some disease markers in cirrhosis,” Phillipp Hartmann, MD, assistant professor of pediatric gastroenterology, hepatology, and nutrition at the University of California, San Diego, said in an interview.
“One of the methodological concerns is that the authors compared probiotics with a multitude of different treatments, including fiber and lactulose (which are both prebiotics), rifaximin (which is an antibiotic), standard of care, placebo, or no therapy,” he said. “This might contribute to the sometimes-contradictory findings between the different studies. The ideal comparison would be a specific probiotic formulation versus a placebo to understand what the probiotic actually does.”
Dr. Hartmann, who wasn’t involved with this study, has published a review on the potential of probiotics, prebiotics, and synbiotics in liver disease. He and colleagues noted the mechanisms that improve a disrupted intestinal barrier, microbial translocation, and altered gut microbiome metabolism.
“Over the last few years, we and others have studied the intestinal microbiota in various liver diseases, including alcohol-associated liver disease and metabolic dysfunction-associated steatotic liver disease,” he said. “Essentially, all studies support the notion that probiotics improve the microbial structure in the gut by increasing the beneficial and decreasing the potentially pathogenic microbes.”
However, probiotics and supplements are unregulated, Dr. Hartmann noted. Many different probiotic mixes and dosages have been tested in clinical trials, and additional studies are needed to determine the best formulations and dosages.
“Usually, the best outcomes can be achieved with a higher number of strains included in the probiotic formulation (10-30+) and a higher number of colony-forming units at 30-50+ billion per day,” he said.
The study was supported by funds from the Science and Technology Major Project of Guangxi, Guangxi Key Research and Development Program, and Natural Science Foundation of Guangxi Zhuang Autonomous Region. The authors declared no conflicts of interest. Dr. Hartmann reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a systematic review and meta-analysis.
They also improve quality of life and have a favorable safety profile, adding to their potential as a promising intervention for treating cirrhosis, the study authors wrote.
“As currently one of the top 10 leading causes of death globally, cirrhosis imposes a great health burden in many countries,” wrote lead author Xing Yang of the Health Management Research Institute at the People’s Hospital of Guangxi Zhuang Autonomous Region and Guangxi Academy of Medical Sciences in Nanning, China, and colleagues.
“The burden has escalated at the worldwide level since 1990, partly because of population growth and aging,” the authors wrote. “Thus, it is meaningful to explore effective treatments for reversing cirrhosis and preventing severe liver function and even systemic damage.”
The study was published online in Frontiers in Medicine .
Analyzing Probiotic Trials
The researchers conducted a systematic review and meta-analysis of 30 randomized controlled trials among 2084 adults with cirrhosis, comparing the effects of probiotic intervention and control treatments, including placebo, no treatment, standard care, or active controls such as lactulose and rifaximin. The studies spanned 14 countries and included 1049 patients in the probiotic groups and 1035 in the control groups.
The research team calculated risk ratios (RRs) or standardized mean difference (SMD) for outcomes such as HE reversal, Model for End-Stage Liver Disease (MELD) scores, safety and tolerability of probiotics, liver function, and quality of life.
Among 17 studies involving patients with different stages of HE, as compared with the control group, probiotics significantly reversed minimal HE (RR, 1.54) and improved HE (RR, 1.94). In particular, the probiotic VSL#3 — which contains Streptococcus, Bifidobacterium, and Lactobacillus — produced more significant HE improvement (RR, 1.44) compared with other types of probiotics.
In addition, probiotics appeared to improve liver function by reducing MELD scores (SMD, −0.57) but didn’t show a difference in other liver function parameters. There were numerical but not significant reductions in mortality and serum inflammatory cytokine expression, including endotoxin, interleukin-6, and tumor necrosis factor-alpha.
Probiotics also improved quality-of-life scores (SMD, 0.51) and gut flora (SMD, 1.67). For gut flora, the numbers of the Lactobacillus group were significantly higher after probiotic treatment, but there wasn’t a significant difference for Bifidobacterium, Enterococcus, Bacteroidaceae, and Fusobacterium.
Finally, compared with control treatments, including placebo, standard therapy, and active controls such as lactulose and rifaximin, probiotics showed higher safety and tolerability profiles, causing a significantly lower incidence of serious adverse events (RR, 0.71).
Longer intervention times reduced the risk for overt HE development, hospitalization, and infections compared with shorter intervention times.
“Probiotics contribute to the reduction of ammonia levels and the improvement of neuropsychometric or neurophysiological status, leading to the reversal of HE associated with cirrhosis,” the study authors wrote. “Moreover, they induce favorable changes in gut flora and quality of life. Therefore, probiotics emerge as a promising intervention for reversing the onset of cirrhosis and preventing disease progression.”
Considering Variables
The authors noted several limitations, including a high or unclear risk for bias in 28 studies and the lack of data on the intervention effect for various types of probiotics or treatment durations.
“Overall, despite a number of methodological concerns, the study shows that probiotics can improve some disease markers in cirrhosis,” Phillipp Hartmann, MD, assistant professor of pediatric gastroenterology, hepatology, and nutrition at the University of California, San Diego, said in an interview.
“One of the methodological concerns is that the authors compared probiotics with a multitude of different treatments, including fiber and lactulose (which are both prebiotics), rifaximin (which is an antibiotic), standard of care, placebo, or no therapy,” he said. “This might contribute to the sometimes-contradictory findings between the different studies. The ideal comparison would be a specific probiotic formulation versus a placebo to understand what the probiotic actually does.”
Dr. Hartmann, who wasn’t involved with this study, has published a review on the potential of probiotics, prebiotics, and synbiotics in liver disease. He and colleagues noted the mechanisms that improve a disrupted intestinal barrier, microbial translocation, and altered gut microbiome metabolism.
“Over the last few years, we and others have studied the intestinal microbiota in various liver diseases, including alcohol-associated liver disease and metabolic dysfunction-associated steatotic liver disease,” he said. “Essentially, all studies support the notion that probiotics improve the microbial structure in the gut by increasing the beneficial and decreasing the potentially pathogenic microbes.”
However, probiotics and supplements are unregulated, Dr. Hartmann noted. Many different probiotic mixes and dosages have been tested in clinical trials, and additional studies are needed to determine the best formulations and dosages.
“Usually, the best outcomes can be achieved with a higher number of strains included in the probiotic formulation (10-30+) and a higher number of colony-forming units at 30-50+ billion per day,” he said.
The study was supported by funds from the Science and Technology Major Project of Guangxi, Guangxi Key Research and Development Program, and Natural Science Foundation of Guangxi Zhuang Autonomous Region. The authors declared no conflicts of interest. Dr. Hartmann reported no relevant disclosures.
A version of this article appeared on Medscape.com .
Energy-Restricted Diet Twice Weekly Tops Exercise in T2D
TOPLINE:
Two days a week of a medically supervised energy-restricted diet may lower blood glucose levels in adults with overweight or obesity and type 2 diabetes (T2D).
METHODOLOGY:
- Daily calorie restrictions and increased physical activity improve glycemic control and induce diabetes remission in patients with T2D, but these approaches are challenging to adhere to.
- Researchers tested whether 2 days a week (a 5:2 regimen) of either a very low-calorie formula diet or a “weekend warrior” physical activity pattern would be effective and more convenient.
- The three-arm IDEATE study enrolled 326 Asian participants with overweight or mild obesity (body mass index, 25.0-39.9) and T2D (diagnosed within prior 2 years; A1c, 7.0-8.9%; not on insulin) and randomly assigned them to receive a diet intervention, an exercise intervention, or routine lifestyle education (control group) for 12 weeks.
- The diet intervention group received an energy-restricted diet of 790 kcal/d on 2 days each week, and the exercise intervention group performed high-intensity interval training (4 minutes of aerobic activity, with a 10-minute total warm-up and cool-down) and resistance training twice a week (four exercises, two sets of eight to 12 repetitions).
- The primary outcome was the change in glycemic control between the diet or exercise intervention group and the control group after 12 weeks. Follow-up continued up to 1 year after intervention.
TAKEAWAY:
- Compared with the control group, patients in the diet intervention group achieved greater reductions in A1c after 12 weeks (difference, -0.34; P =.007), whereas A1c reductions in the exercise intervention group did not differ significantly from the control group.
- The likelihood of achieving diabetes remission was higher in the diet intervention vs the control group (adjusted odds ratio, 3.60; P = .008) but not in the exercise intervention group (P =.52).
- Body weight, body mass index, and high-density lipoprtein cholesterol levels were more effectively controlled in the diet intervention group only.
- However, participants in both the diet and exercise intervention groups showed reduced adiposity, liver fat content, and diastolic blood pressure compared with those in the control group.
IN PRACTICE:
“The diet intervention group experienced a greater energy deficit with a more pronounced metabolic benefit,” the authors wrote. “Our study suggests that a medically supervised 5:2 energy-restricted diet could serve as an alternative strategy for improving glycemic control.”
SOURCE:
Mian Li, of the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, led the study, which was published online in Diabetes Care.
LIMITATIONS:
Body composition was analyzed using bioelectrical impedance analysis, which is a less accurate technique than dual-energy x-ray absorptiometry. The study used finger-prick tests to monitor blood glucose levels, which could have underestimated both hyperglycemic and hypoglycemic episodes. No information was collected on whether the participants maintained the diet or exercise regimen during the postintervention follow-up period.
DISCLOSURES:
This study was supported by the National Key Research and Development Program of China, National Natural Science Foundation of China, Shanghai Rising Star Program grant, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Two days a week of a medically supervised energy-restricted diet may lower blood glucose levels in adults with overweight or obesity and type 2 diabetes (T2D).
METHODOLOGY:
- Daily calorie restrictions and increased physical activity improve glycemic control and induce diabetes remission in patients with T2D, but these approaches are challenging to adhere to.
- Researchers tested whether 2 days a week (a 5:2 regimen) of either a very low-calorie formula diet or a “weekend warrior” physical activity pattern would be effective and more convenient.
- The three-arm IDEATE study enrolled 326 Asian participants with overweight or mild obesity (body mass index, 25.0-39.9) and T2D (diagnosed within prior 2 years; A1c, 7.0-8.9%; not on insulin) and randomly assigned them to receive a diet intervention, an exercise intervention, or routine lifestyle education (control group) for 12 weeks.
- The diet intervention group received an energy-restricted diet of 790 kcal/d on 2 days each week, and the exercise intervention group performed high-intensity interval training (4 minutes of aerobic activity, with a 10-minute total warm-up and cool-down) and resistance training twice a week (four exercises, two sets of eight to 12 repetitions).
- The primary outcome was the change in glycemic control between the diet or exercise intervention group and the control group after 12 weeks. Follow-up continued up to 1 year after intervention.
TAKEAWAY:
- Compared with the control group, patients in the diet intervention group achieved greater reductions in A1c after 12 weeks (difference, -0.34; P =.007), whereas A1c reductions in the exercise intervention group did not differ significantly from the control group.
- The likelihood of achieving diabetes remission was higher in the diet intervention vs the control group (adjusted odds ratio, 3.60; P = .008) but not in the exercise intervention group (P =.52).
- Body weight, body mass index, and high-density lipoprtein cholesterol levels were more effectively controlled in the diet intervention group only.
- However, participants in both the diet and exercise intervention groups showed reduced adiposity, liver fat content, and diastolic blood pressure compared with those in the control group.
IN PRACTICE:
“The diet intervention group experienced a greater energy deficit with a more pronounced metabolic benefit,” the authors wrote. “Our study suggests that a medically supervised 5:2 energy-restricted diet could serve as an alternative strategy for improving glycemic control.”
SOURCE:
Mian Li, of the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, led the study, which was published online in Diabetes Care.
LIMITATIONS:
Body composition was analyzed using bioelectrical impedance analysis, which is a less accurate technique than dual-energy x-ray absorptiometry. The study used finger-prick tests to monitor blood glucose levels, which could have underestimated both hyperglycemic and hypoglycemic episodes. No information was collected on whether the participants maintained the diet or exercise regimen during the postintervention follow-up period.
DISCLOSURES:
This study was supported by the National Key Research and Development Program of China, National Natural Science Foundation of China, Shanghai Rising Star Program grant, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Two days a week of a medically supervised energy-restricted diet may lower blood glucose levels in adults with overweight or obesity and type 2 diabetes (T2D).
METHODOLOGY:
- Daily calorie restrictions and increased physical activity improve glycemic control and induce diabetes remission in patients with T2D, but these approaches are challenging to adhere to.
- Researchers tested whether 2 days a week (a 5:2 regimen) of either a very low-calorie formula diet or a “weekend warrior” physical activity pattern would be effective and more convenient.
- The three-arm IDEATE study enrolled 326 Asian participants with overweight or mild obesity (body mass index, 25.0-39.9) and T2D (diagnosed within prior 2 years; A1c, 7.0-8.9%; not on insulin) and randomly assigned them to receive a diet intervention, an exercise intervention, or routine lifestyle education (control group) for 12 weeks.
- The diet intervention group received an energy-restricted diet of 790 kcal/d on 2 days each week, and the exercise intervention group performed high-intensity interval training (4 minutes of aerobic activity, with a 10-minute total warm-up and cool-down) and resistance training twice a week (four exercises, two sets of eight to 12 repetitions).
- The primary outcome was the change in glycemic control between the diet or exercise intervention group and the control group after 12 weeks. Follow-up continued up to 1 year after intervention.
TAKEAWAY:
- Compared with the control group, patients in the diet intervention group achieved greater reductions in A1c after 12 weeks (difference, -0.34; P =.007), whereas A1c reductions in the exercise intervention group did not differ significantly from the control group.
- The likelihood of achieving diabetes remission was higher in the diet intervention vs the control group (adjusted odds ratio, 3.60; P = .008) but not in the exercise intervention group (P =.52).
- Body weight, body mass index, and high-density lipoprtein cholesterol levels were more effectively controlled in the diet intervention group only.
- However, participants in both the diet and exercise intervention groups showed reduced adiposity, liver fat content, and diastolic blood pressure compared with those in the control group.
IN PRACTICE:
“The diet intervention group experienced a greater energy deficit with a more pronounced metabolic benefit,” the authors wrote. “Our study suggests that a medically supervised 5:2 energy-restricted diet could serve as an alternative strategy for improving glycemic control.”
SOURCE:
Mian Li, of the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, led the study, which was published online in Diabetes Care.
LIMITATIONS:
Body composition was analyzed using bioelectrical impedance analysis, which is a less accurate technique than dual-energy x-ray absorptiometry. The study used finger-prick tests to monitor blood glucose levels, which could have underestimated both hyperglycemic and hypoglycemic episodes. No information was collected on whether the participants maintained the diet or exercise regimen during the postintervention follow-up period.
DISCLOSURES:
This study was supported by the National Key Research and Development Program of China, National Natural Science Foundation of China, Shanghai Rising Star Program grant, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
New Genetic Variant May Guard Against Alzheimer’s in High-Risk Individuals
, new research suggests.
The variant occurs on the fibronectin 1 (FN1) gene, which expresses fibronectin, an adhesive glycoprotein that lines the blood vessels at the blood-brain barrier and controls substances that move in and out of the brain.
While fibronectin is normally present in the blood-brain barrier in small amounts, individuals with Alzheimer’s disease tend to have it in excess. Normally, patients with Alzheimer’s disease have amyloid deposits that collect in the brain, but those with the FN1 variant appear to have the ability to amyloid from the brain before symptoms begin.
The researchers estimate that 1%-3% of APOE4 carriers in the United States — roughly 200,000-620,000 people — may have the protective mutation.
“Alzheimer’s disease may get started with amyloid deposits in the brain, but the disease manifestations are the result of changes that happen after the deposits appear,” Caghan Kizil, PhD, of Columbia University Vagelos College of Physicians and Surgeons in New York City, and a co-leader of the study, said in a press release.
The findings were published online in Acta Neuropathologica,
Combing Genetic Data
To find potentially protective Alzheimer’s disease variants, the investigators sequenced the genomes of more than 3500 APOE4 carriers older than 70 years with and without Alzheimer’s disease from various ethnic backgrounds.
They identified two variants on the FN1 gene, rs116558455 and rs140926439, present in healthy APOE4 carriers, that protected the APOE4 carriers against Alzheimer’s disease.
After Dr. Kizil and colleagues published their findings in a preprint, another research group that included investigators from Stanford and Washington Universities replicated the Columbia results in an independent sample of more than 7000 APOE4 carriers aged 60 years who were of European descent and identified the same FN1 variant.
The two research groups then combined their data on 11,000 participants and found that the FN1 variant rs140926439 was associated with a significantly reduced risk for Alzheimer’s disease in APOE4 carriers (odds ratio, 0.29; P = .014). A secondary analysis showed that the variant delayed Alzheimer’s disease symptom onset by 3.4 years (P = .025).
The investigators hope to use these findings to develop therapies to protect APOE4 carriers against Alzheimer’s disease.
“Anything that reduces excess fibronectin should provide some protection, and a drug that does this could be a significant step forward in the fight against this debilitating condition,” Dr. Kizil said.
Study limitations included a lack of longitudinal data on the relationship between amyloid concentration and fibronectin and the fact that investigators conducted the studies in clinically assessed individuals. Given the rare occurrence of the FN1 mutation, researchers do not have neuropathological assessments of study participants with the variant.
The study was funded by the National Institute on Aging, the Schaefer Research Scholars Program Award, Taub Institute Grants for Emerging Research, the National Institute of General Medical Sciences, and the Thompson Family Foundation Program for Accelerated Medicine Exploration in Alzheimer’s Disease and Related Disorders of the Nervous System. There were no disclosures reported.
A version of this article appeared on Medscape.com.
, new research suggests.
The variant occurs on the fibronectin 1 (FN1) gene, which expresses fibronectin, an adhesive glycoprotein that lines the blood vessels at the blood-brain barrier and controls substances that move in and out of the brain.
While fibronectin is normally present in the blood-brain barrier in small amounts, individuals with Alzheimer’s disease tend to have it in excess. Normally, patients with Alzheimer’s disease have amyloid deposits that collect in the brain, but those with the FN1 variant appear to have the ability to amyloid from the brain before symptoms begin.
The researchers estimate that 1%-3% of APOE4 carriers in the United States — roughly 200,000-620,000 people — may have the protective mutation.
“Alzheimer’s disease may get started with amyloid deposits in the brain, but the disease manifestations are the result of changes that happen after the deposits appear,” Caghan Kizil, PhD, of Columbia University Vagelos College of Physicians and Surgeons in New York City, and a co-leader of the study, said in a press release.
The findings were published online in Acta Neuropathologica,
Combing Genetic Data
To find potentially protective Alzheimer’s disease variants, the investigators sequenced the genomes of more than 3500 APOE4 carriers older than 70 years with and without Alzheimer’s disease from various ethnic backgrounds.
They identified two variants on the FN1 gene, rs116558455 and rs140926439, present in healthy APOE4 carriers, that protected the APOE4 carriers against Alzheimer’s disease.
After Dr. Kizil and colleagues published their findings in a preprint, another research group that included investigators from Stanford and Washington Universities replicated the Columbia results in an independent sample of more than 7000 APOE4 carriers aged 60 years who were of European descent and identified the same FN1 variant.
The two research groups then combined their data on 11,000 participants and found that the FN1 variant rs140926439 was associated with a significantly reduced risk for Alzheimer’s disease in APOE4 carriers (odds ratio, 0.29; P = .014). A secondary analysis showed that the variant delayed Alzheimer’s disease symptom onset by 3.4 years (P = .025).
The investigators hope to use these findings to develop therapies to protect APOE4 carriers against Alzheimer’s disease.
“Anything that reduces excess fibronectin should provide some protection, and a drug that does this could be a significant step forward in the fight against this debilitating condition,” Dr. Kizil said.
Study limitations included a lack of longitudinal data on the relationship between amyloid concentration and fibronectin and the fact that investigators conducted the studies in clinically assessed individuals. Given the rare occurrence of the FN1 mutation, researchers do not have neuropathological assessments of study participants with the variant.
The study was funded by the National Institute on Aging, the Schaefer Research Scholars Program Award, Taub Institute Grants for Emerging Research, the National Institute of General Medical Sciences, and the Thompson Family Foundation Program for Accelerated Medicine Exploration in Alzheimer’s Disease and Related Disorders of the Nervous System. There were no disclosures reported.
A version of this article appeared on Medscape.com.
, new research suggests.
The variant occurs on the fibronectin 1 (FN1) gene, which expresses fibronectin, an adhesive glycoprotein that lines the blood vessels at the blood-brain barrier and controls substances that move in and out of the brain.
While fibronectin is normally present in the blood-brain barrier in small amounts, individuals with Alzheimer’s disease tend to have it in excess. Normally, patients with Alzheimer’s disease have amyloid deposits that collect in the brain, but those with the FN1 variant appear to have the ability to amyloid from the brain before symptoms begin.
The researchers estimate that 1%-3% of APOE4 carriers in the United States — roughly 200,000-620,000 people — may have the protective mutation.
“Alzheimer’s disease may get started with amyloid deposits in the brain, but the disease manifestations are the result of changes that happen after the deposits appear,” Caghan Kizil, PhD, of Columbia University Vagelos College of Physicians and Surgeons in New York City, and a co-leader of the study, said in a press release.
The findings were published online in Acta Neuropathologica,
Combing Genetic Data
To find potentially protective Alzheimer’s disease variants, the investigators sequenced the genomes of more than 3500 APOE4 carriers older than 70 years with and without Alzheimer’s disease from various ethnic backgrounds.
They identified two variants on the FN1 gene, rs116558455 and rs140926439, present in healthy APOE4 carriers, that protected the APOE4 carriers against Alzheimer’s disease.
After Dr. Kizil and colleagues published their findings in a preprint, another research group that included investigators from Stanford and Washington Universities replicated the Columbia results in an independent sample of more than 7000 APOE4 carriers aged 60 years who were of European descent and identified the same FN1 variant.
The two research groups then combined their data on 11,000 participants and found that the FN1 variant rs140926439 was associated with a significantly reduced risk for Alzheimer’s disease in APOE4 carriers (odds ratio, 0.29; P = .014). A secondary analysis showed that the variant delayed Alzheimer’s disease symptom onset by 3.4 years (P = .025).
The investigators hope to use these findings to develop therapies to protect APOE4 carriers against Alzheimer’s disease.
“Anything that reduces excess fibronectin should provide some protection, and a drug that does this could be a significant step forward in the fight against this debilitating condition,” Dr. Kizil said.
Study limitations included a lack of longitudinal data on the relationship between amyloid concentration and fibronectin and the fact that investigators conducted the studies in clinically assessed individuals. Given the rare occurrence of the FN1 mutation, researchers do not have neuropathological assessments of study participants with the variant.
The study was funded by the National Institute on Aging, the Schaefer Research Scholars Program Award, Taub Institute Grants for Emerging Research, the National Institute of General Medical Sciences, and the Thompson Family Foundation Program for Accelerated Medicine Exploration in Alzheimer’s Disease and Related Disorders of the Nervous System. There were no disclosures reported.
A version of this article appeared on Medscape.com.
FROM ACTA NEUROPATHOLOGICA
May 2024 – ICYMI
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
The AGA Future Leaders Program: A Mentee-Mentor Triad Perspective
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.
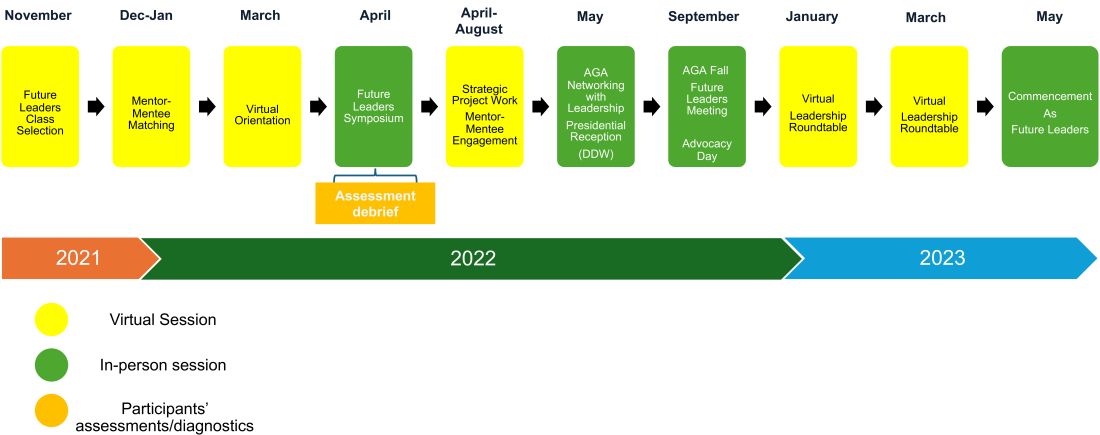
We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.

We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.

We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
‘Autoantibody Signature’ Flags MS Years Before Symptom Onset
, according to a new study.
Investigators screened blood samples from 250 individuals with MS drawn 5 years before and 1 year after symptom onset, profiled MS-related autoantibodies, and compared the sample with 250 matched controls.
A unique cluster of autoantibodies was found in 10% of people with MS, appearing up to 5 years before the onset of clinical symptoms and remaining higher 1 year after diagnosis.
“Our work demonstrates that a subset of MS patients has antibodies that react to a common protein motif, both before, during, and after diagnosis and symptom onset,” said lead investigator Colin R. Zamecnik, PhD, a postdoctoral researcher at UCSF School of Medicine, University of California, San Francisco.
Such a discovery could aid in early diagnosis, Dr. Zamecnik added. MS treatments “have gotten much better in the last 15-20 years and evidence shows early treatment can improve outcomes,” he said.
The study was published online in Nature Medicine.
Seeking Earlier Diagnosis
Previous research shows that nonspecific neurologic episodes occur more frequently in people who received an MS diagnosis later in life, pointing to the possibility of an MS prodrome, the authors noted.
These neurologic episodes may be indicative of ongoing neuroinflammatory processes in the preclinical period, they added. Studies in several other autoimmune diseases show that diagnostic autoantibodies can appear years before symptom onset. However, no such antibodies have previously been identified in MS patients.
To investigate, the researchers turned to data from a large, prospective incident MS cohort assembled during the Gulf War era in more than 10 million US military veterans.
Records of those with the earliest diagnosis (an average of 5 years before symptom onset) and 1 year after the first attack were analyzed, and matched controls were selected.
Investigators used a technique called phage display immunoprecipitation sequencing to screen human blood for antibodies. They conducted a whole-proteome autoantibody screen and serum neurofilament light (sNfL) measurements on these samples in both case patients and controls at the same time points.
Early Signs of Injury
In the preclinical serum samples, sNfL levels were higher nearer the date of diagnosis and significantly higher in post- versus pre-onset samples in people with MS. “Together, these data provide evidence that at least some people with MS exhibit early signs of neuroaxonal injury long before onset of symptoms,” the authors noted.
Analysis of the collection of peptides, described by the investigators as an “autoantibody signature,” was consistent over time and was present regardless of diagnosis.
Further analysis of the autoantibodies revealed a characteristic protein motif found in common viruses, including Epstein-Barr virus (EBV) and hepatitis C virus, among others.
The motif “shares remarkable similarity to those found on many pathogens that infect humans, including EBV, which is known to be a risk factor for development of MS,” Dr. Zamecnik said.
The researchers validated these findings by analyzing serum and cerebrospinal fluid samples from participants in ORIGINS, an MS cohort at the University of California, San Francisco, that enrolled patients at clinical onset. As with the other cohort, 10% of patients had the autoantibody signature.
The investigators added that the findings detail some of the first autoantigen-specific biomarkers found in preclinical MS.
“Taken together, our future work will focus on profiling these patients more closely over time to see how they differ from their counterparts and gives further evidence of viral-host crosstalk as a hallmark of this disease,” Dr. Zamecnik said.
Not Ready for Prime Time
Commenting on the findings, Bruce Bebo, PhD, executive vice president of research, National Multiple Sclerosis Society, said the study corroborates the “growing appreciation that MS has a prodrome.”
Such a discovery might “accelerate progress toward the possibility of treating MS ever-earlier in the course of the disease, or possibly even preventing MS from occurring in the first place,” he added.
Dr. Bebo, who was not involved in this research, noted that it was conducted at a single center, is only preliminary, and “has no immediate clinical applicability.”
Also, because this pattern was identified in only 10% of individuals with MS, “an additional hurdle is whether we can identify other patterns in greater numbers of people,” he added.
This work was supported by the Valhalla Foundation; the Weill Neurohub; the Westridge Foundation; the National Institute of Neurological Disorders and Stroke; the National Institute of Allergy and Infectious Diseases; National Multiple Sclerosis Society; the Department of Defense; the German Society of Multiple Sclerosis; the Water Cove Charitable Foundation; Tim and Laura O’Shaughnessy; the Littera Family; School of Medicine Dean’s Yearlong Fellowship, supported by residual funds from the Howard Hughes Medical Institute Medical Fellows at UCSF; the Chan Zuckerberg Biohub San Francisco; the John A. Watson Scholar Program at UCSF; the Hanna H. Gray Fellowship, Howard Hughes Medical Institute; the National Institutes of Health; and the University of California President’s Postdoctoral Fellowship Program. Dr. Zamecnik received funding toward this study from the National Multiple Sclerosis Society and the Water Cove Charitable Foundation. He declared no competing financial interests. The other authors’ disclosures are listed on the original paper. Dr. Bebo is the executive vice president of the National Multiple Sclerosis Society, which provided support for the study.
A version of this article appeared on Medscape.com.
, according to a new study.
Investigators screened blood samples from 250 individuals with MS drawn 5 years before and 1 year after symptom onset, profiled MS-related autoantibodies, and compared the sample with 250 matched controls.
A unique cluster of autoantibodies was found in 10% of people with MS, appearing up to 5 years before the onset of clinical symptoms and remaining higher 1 year after diagnosis.
“Our work demonstrates that a subset of MS patients has antibodies that react to a common protein motif, both before, during, and after diagnosis and symptom onset,” said lead investigator Colin R. Zamecnik, PhD, a postdoctoral researcher at UCSF School of Medicine, University of California, San Francisco.
Such a discovery could aid in early diagnosis, Dr. Zamecnik added. MS treatments “have gotten much better in the last 15-20 years and evidence shows early treatment can improve outcomes,” he said.
The study was published online in Nature Medicine.
Seeking Earlier Diagnosis
Previous research shows that nonspecific neurologic episodes occur more frequently in people who received an MS diagnosis later in life, pointing to the possibility of an MS prodrome, the authors noted.
These neurologic episodes may be indicative of ongoing neuroinflammatory processes in the preclinical period, they added. Studies in several other autoimmune diseases show that diagnostic autoantibodies can appear years before symptom onset. However, no such antibodies have previously been identified in MS patients.
To investigate, the researchers turned to data from a large, prospective incident MS cohort assembled during the Gulf War era in more than 10 million US military veterans.
Records of those with the earliest diagnosis (an average of 5 years before symptom onset) and 1 year after the first attack were analyzed, and matched controls were selected.
Investigators used a technique called phage display immunoprecipitation sequencing to screen human blood for antibodies. They conducted a whole-proteome autoantibody screen and serum neurofilament light (sNfL) measurements on these samples in both case patients and controls at the same time points.
Early Signs of Injury
In the preclinical serum samples, sNfL levels were higher nearer the date of diagnosis and significantly higher in post- versus pre-onset samples in people with MS. “Together, these data provide evidence that at least some people with MS exhibit early signs of neuroaxonal injury long before onset of symptoms,” the authors noted.
Analysis of the collection of peptides, described by the investigators as an “autoantibody signature,” was consistent over time and was present regardless of diagnosis.
Further analysis of the autoantibodies revealed a characteristic protein motif found in common viruses, including Epstein-Barr virus (EBV) and hepatitis C virus, among others.
The motif “shares remarkable similarity to those found on many pathogens that infect humans, including EBV, which is known to be a risk factor for development of MS,” Dr. Zamecnik said.
The researchers validated these findings by analyzing serum and cerebrospinal fluid samples from participants in ORIGINS, an MS cohort at the University of California, San Francisco, that enrolled patients at clinical onset. As with the other cohort, 10% of patients had the autoantibody signature.
The investigators added that the findings detail some of the first autoantigen-specific biomarkers found in preclinical MS.
“Taken together, our future work will focus on profiling these patients more closely over time to see how they differ from their counterparts and gives further evidence of viral-host crosstalk as a hallmark of this disease,” Dr. Zamecnik said.
Not Ready for Prime Time
Commenting on the findings, Bruce Bebo, PhD, executive vice president of research, National Multiple Sclerosis Society, said the study corroborates the “growing appreciation that MS has a prodrome.”
Such a discovery might “accelerate progress toward the possibility of treating MS ever-earlier in the course of the disease, or possibly even preventing MS from occurring in the first place,” he added.
Dr. Bebo, who was not involved in this research, noted that it was conducted at a single center, is only preliminary, and “has no immediate clinical applicability.”
Also, because this pattern was identified in only 10% of individuals with MS, “an additional hurdle is whether we can identify other patterns in greater numbers of people,” he added.
This work was supported by the Valhalla Foundation; the Weill Neurohub; the Westridge Foundation; the National Institute of Neurological Disorders and Stroke; the National Institute of Allergy and Infectious Diseases; National Multiple Sclerosis Society; the Department of Defense; the German Society of Multiple Sclerosis; the Water Cove Charitable Foundation; Tim and Laura O’Shaughnessy; the Littera Family; School of Medicine Dean’s Yearlong Fellowship, supported by residual funds from the Howard Hughes Medical Institute Medical Fellows at UCSF; the Chan Zuckerberg Biohub San Francisco; the John A. Watson Scholar Program at UCSF; the Hanna H. Gray Fellowship, Howard Hughes Medical Institute; the National Institutes of Health; and the University of California President’s Postdoctoral Fellowship Program. Dr. Zamecnik received funding toward this study from the National Multiple Sclerosis Society and the Water Cove Charitable Foundation. He declared no competing financial interests. The other authors’ disclosures are listed on the original paper. Dr. Bebo is the executive vice president of the National Multiple Sclerosis Society, which provided support for the study.
A version of this article appeared on Medscape.com.
, according to a new study.
Investigators screened blood samples from 250 individuals with MS drawn 5 years before and 1 year after symptom onset, profiled MS-related autoantibodies, and compared the sample with 250 matched controls.
A unique cluster of autoantibodies was found in 10% of people with MS, appearing up to 5 years before the onset of clinical symptoms and remaining higher 1 year after diagnosis.
“Our work demonstrates that a subset of MS patients has antibodies that react to a common protein motif, both before, during, and after diagnosis and symptom onset,” said lead investigator Colin R. Zamecnik, PhD, a postdoctoral researcher at UCSF School of Medicine, University of California, San Francisco.
Such a discovery could aid in early diagnosis, Dr. Zamecnik added. MS treatments “have gotten much better in the last 15-20 years and evidence shows early treatment can improve outcomes,” he said.
The study was published online in Nature Medicine.
Seeking Earlier Diagnosis
Previous research shows that nonspecific neurologic episodes occur more frequently in people who received an MS diagnosis later in life, pointing to the possibility of an MS prodrome, the authors noted.
These neurologic episodes may be indicative of ongoing neuroinflammatory processes in the preclinical period, they added. Studies in several other autoimmune diseases show that diagnostic autoantibodies can appear years before symptom onset. However, no such antibodies have previously been identified in MS patients.
To investigate, the researchers turned to data from a large, prospective incident MS cohort assembled during the Gulf War era in more than 10 million US military veterans.
Records of those with the earliest diagnosis (an average of 5 years before symptom onset) and 1 year after the first attack were analyzed, and matched controls were selected.
Investigators used a technique called phage display immunoprecipitation sequencing to screen human blood for antibodies. They conducted a whole-proteome autoantibody screen and serum neurofilament light (sNfL) measurements on these samples in both case patients and controls at the same time points.
Early Signs of Injury
In the preclinical serum samples, sNfL levels were higher nearer the date of diagnosis and significantly higher in post- versus pre-onset samples in people with MS. “Together, these data provide evidence that at least some people with MS exhibit early signs of neuroaxonal injury long before onset of symptoms,” the authors noted.
Analysis of the collection of peptides, described by the investigators as an “autoantibody signature,” was consistent over time and was present regardless of diagnosis.
Further analysis of the autoantibodies revealed a characteristic protein motif found in common viruses, including Epstein-Barr virus (EBV) and hepatitis C virus, among others.
The motif “shares remarkable similarity to those found on many pathogens that infect humans, including EBV, which is known to be a risk factor for development of MS,” Dr. Zamecnik said.
The researchers validated these findings by analyzing serum and cerebrospinal fluid samples from participants in ORIGINS, an MS cohort at the University of California, San Francisco, that enrolled patients at clinical onset. As with the other cohort, 10% of patients had the autoantibody signature.
The investigators added that the findings detail some of the first autoantigen-specific biomarkers found in preclinical MS.
“Taken together, our future work will focus on profiling these patients more closely over time to see how they differ from their counterparts and gives further evidence of viral-host crosstalk as a hallmark of this disease,” Dr. Zamecnik said.
Not Ready for Prime Time
Commenting on the findings, Bruce Bebo, PhD, executive vice president of research, National Multiple Sclerosis Society, said the study corroborates the “growing appreciation that MS has a prodrome.”
Such a discovery might “accelerate progress toward the possibility of treating MS ever-earlier in the course of the disease, or possibly even preventing MS from occurring in the first place,” he added.
Dr. Bebo, who was not involved in this research, noted that it was conducted at a single center, is only preliminary, and “has no immediate clinical applicability.”
Also, because this pattern was identified in only 10% of individuals with MS, “an additional hurdle is whether we can identify other patterns in greater numbers of people,” he added.
This work was supported by the Valhalla Foundation; the Weill Neurohub; the Westridge Foundation; the National Institute of Neurological Disorders and Stroke; the National Institute of Allergy and Infectious Diseases; National Multiple Sclerosis Society; the Department of Defense; the German Society of Multiple Sclerosis; the Water Cove Charitable Foundation; Tim and Laura O’Shaughnessy; the Littera Family; School of Medicine Dean’s Yearlong Fellowship, supported by residual funds from the Howard Hughes Medical Institute Medical Fellows at UCSF; the Chan Zuckerberg Biohub San Francisco; the John A. Watson Scholar Program at UCSF; the Hanna H. Gray Fellowship, Howard Hughes Medical Institute; the National Institutes of Health; and the University of California President’s Postdoctoral Fellowship Program. Dr. Zamecnik received funding toward this study from the National Multiple Sclerosis Society and the Water Cove Charitable Foundation. He declared no competing financial interests. The other authors’ disclosures are listed on the original paper. Dr. Bebo is the executive vice president of the National Multiple Sclerosis Society, which provided support for the study.
A version of this article appeared on Medscape.com.
FROM NATURE MEDICINE
Artificial Intelligence in GI and Hepatology
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Navigating the Search for a Financial Adviser
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
Achieving Promotion for Junior Faculty in Academic Medicine: An Interview With Experts
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Dramatic Increase in College Student Suicide Rates
TOPLINE:
, a new study by the National Collegiate Athletic Association (NCAA) found.
METHODOLOGY:
- Investigators analyzed deaths between 2002 and 2022, using Poisson regression models to assess changes in incidence rates over time.
- Data were drawn from the NCAA death database, which includes death from any cause, and included demographic characteristics such as age and race and sporting discipline.
- They utilized linear and quadratic fits between year and suicide incidence for men and women.
- Given the low incidence of suicide deaths per year, the incidence rate was multiplied by 100,000 to calculate the incidence per 100,000 athlete-years (AYs).
TAKEAWAY:
- Of 1102 total deaths, 11.6% were due to suicide (98 men, 30 women).
- Athletes who died by suicide ranged in age from 17 to 24 years (mean, 20 years) were predominantly men (77%) and White (59%), with the highest suicide incidence rate among male cross-country athletes (1:29 per 815 AYs).
- The overall incidence of suicide was 1:71 per 145 AYs.
- Over the last 10 years, suicide was the second most common cause of death after accidents, with the proportion of deaths by suicide doubling from the first to the second decades (7.6% to 15.3%).
- Among men, the suicide incidence rate increased in a linear fashion (5-year incidence rate ratio, 1.32; 95% CI, 1.14-1.53), while among women, a quadratic association was identified (P = .002), with the incidence rate reaching its lowest point in women from 2010 to 2011 and increasing thereafter.
IN PRACTICE:
“Athletes are generally thought of as one of the healthiest populations in our society, yet the pressures of school, internal and external performance expectations, time demands, injury, athletic identity, and physical fatigue can lead to depression, mental health problems, and suicide,” the authors wrote. “Although the rate of suicide among collegiate athletes remains lower than the general population, it is important to recognize the parallel increase to ensure this population is not overlooked when assessing for risk factors and implementing prevention strategies.”
SOURCE:
Bridget M. Whelan, MPH, research scientist in the Department of Family Medicine, Sports Medicine Section, University of Washington School of Medicine, Seattle, was the lead and corresponding author on the study, which was published online in the British Journal of Sports Medicine.
LIMITATIONS:
There is no mandatory reporting system for athlete deaths in the United States, and investigators’ search identified 16 deaths with unknown causes, suggesting reported suicide incidence rates may be underestimated. Additionally, in cases of overdose that were not clearly intentional, the death was listed as “overdose,” possibly resulting in underreporting of suicide.
DISCLOSURES:
No source of study funding was listed. The authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study by the National Collegiate Athletic Association (NCAA) found.
METHODOLOGY:
- Investigators analyzed deaths between 2002 and 2022, using Poisson regression models to assess changes in incidence rates over time.
- Data were drawn from the NCAA death database, which includes death from any cause, and included demographic characteristics such as age and race and sporting discipline.
- They utilized linear and quadratic fits between year and suicide incidence for men and women.
- Given the low incidence of suicide deaths per year, the incidence rate was multiplied by 100,000 to calculate the incidence per 100,000 athlete-years (AYs).
TAKEAWAY:
- Of 1102 total deaths, 11.6% were due to suicide (98 men, 30 women).
- Athletes who died by suicide ranged in age from 17 to 24 years (mean, 20 years) were predominantly men (77%) and White (59%), with the highest suicide incidence rate among male cross-country athletes (1:29 per 815 AYs).
- The overall incidence of suicide was 1:71 per 145 AYs.
- Over the last 10 years, suicide was the second most common cause of death after accidents, with the proportion of deaths by suicide doubling from the first to the second decades (7.6% to 15.3%).
- Among men, the suicide incidence rate increased in a linear fashion (5-year incidence rate ratio, 1.32; 95% CI, 1.14-1.53), while among women, a quadratic association was identified (P = .002), with the incidence rate reaching its lowest point in women from 2010 to 2011 and increasing thereafter.
IN PRACTICE:
“Athletes are generally thought of as one of the healthiest populations in our society, yet the pressures of school, internal and external performance expectations, time demands, injury, athletic identity, and physical fatigue can lead to depression, mental health problems, and suicide,” the authors wrote. “Although the rate of suicide among collegiate athletes remains lower than the general population, it is important to recognize the parallel increase to ensure this population is not overlooked when assessing for risk factors and implementing prevention strategies.”
SOURCE:
Bridget M. Whelan, MPH, research scientist in the Department of Family Medicine, Sports Medicine Section, University of Washington School of Medicine, Seattle, was the lead and corresponding author on the study, which was published online in the British Journal of Sports Medicine.
LIMITATIONS:
There is no mandatory reporting system for athlete deaths in the United States, and investigators’ search identified 16 deaths with unknown causes, suggesting reported suicide incidence rates may be underestimated. Additionally, in cases of overdose that were not clearly intentional, the death was listed as “overdose,” possibly resulting in underreporting of suicide.
DISCLOSURES:
No source of study funding was listed. The authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study by the National Collegiate Athletic Association (NCAA) found.
METHODOLOGY:
- Investigators analyzed deaths between 2002 and 2022, using Poisson regression models to assess changes in incidence rates over time.
- Data were drawn from the NCAA death database, which includes death from any cause, and included demographic characteristics such as age and race and sporting discipline.
- They utilized linear and quadratic fits between year and suicide incidence for men and women.
- Given the low incidence of suicide deaths per year, the incidence rate was multiplied by 100,000 to calculate the incidence per 100,000 athlete-years (AYs).
TAKEAWAY:
- Of 1102 total deaths, 11.6% were due to suicide (98 men, 30 women).
- Athletes who died by suicide ranged in age from 17 to 24 years (mean, 20 years) were predominantly men (77%) and White (59%), with the highest suicide incidence rate among male cross-country athletes (1:29 per 815 AYs).
- The overall incidence of suicide was 1:71 per 145 AYs.
- Over the last 10 years, suicide was the second most common cause of death after accidents, with the proportion of deaths by suicide doubling from the first to the second decades (7.6% to 15.3%).
- Among men, the suicide incidence rate increased in a linear fashion (5-year incidence rate ratio, 1.32; 95% CI, 1.14-1.53), while among women, a quadratic association was identified (P = .002), with the incidence rate reaching its lowest point in women from 2010 to 2011 and increasing thereafter.
IN PRACTICE:
“Athletes are generally thought of as one of the healthiest populations in our society, yet the pressures of school, internal and external performance expectations, time demands, injury, athletic identity, and physical fatigue can lead to depression, mental health problems, and suicide,” the authors wrote. “Although the rate of suicide among collegiate athletes remains lower than the general population, it is important to recognize the parallel increase to ensure this population is not overlooked when assessing for risk factors and implementing prevention strategies.”
SOURCE:
Bridget M. Whelan, MPH, research scientist in the Department of Family Medicine, Sports Medicine Section, University of Washington School of Medicine, Seattle, was the lead and corresponding author on the study, which was published online in the British Journal of Sports Medicine.
LIMITATIONS:
There is no mandatory reporting system for athlete deaths in the United States, and investigators’ search identified 16 deaths with unknown causes, suggesting reported suicide incidence rates may be underestimated. Additionally, in cases of overdose that were not clearly intentional, the death was listed as “overdose,” possibly resulting in underreporting of suicide.
DISCLOSURES:
No source of study funding was listed. The authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.













