User login
New Americans: Considerations for culturally collaborative care
Adam is a 14-year-old who presents for “behavioral concerns” as recommended by his teacher. He is in the eighth grade and is struggling academically and socially. He has intermittent outbursts and poor engagement with other children, and often refuses to do schoolwork. He is seen in the outpatient primary care clinic, usually with his mother and two older siblings, one of whom typically translates for his Arabic-speaking mother. Adam is bilingual, although he prefers Arabic. It is difficult to understand the presenting concern as Adam states that he is doing well and is unsure why the teacher would have made such a report. Mother notes that she does not see these behaviors at home either.
What must we consider? Are there potential barriers, alternate ways to engage, and what role may culture have?
There are many things to consider in the above case, including language barriers, nuanced interactions, and cultural expectations and norms. To understand the scope, statistics reveal that the United States leads the world in its immigrant population with about 44.8 million foreign-born persons in 2018, which accounts for approximately 13.7% of the U.S. population.1 In 2019, 30,000 refugees were resettled in the United States.2 In 2017, immigrant children made up 27% (19.6 million) of U.S. children, of which second-generation children (born in the United States to immigrant parents) were the vast majority at 16.7 million.3 Given this information,
Culture is defined as a set of shared beliefs, norms, values, and behaviors exhibited by a group. Culture plays a role and impacts children in various ways throughout their development. Health care providers would benefit from aspiring to exude cultural humility – learning with and from patients and their families with openness, kindness, and a desire for collaboration. The provider also must consider a family’s history of migration as the response to migration may vary based on age, personal experiences, age at which migration occurred, language abilities, and amount of cultural engagement in the new country (i.e. acculturation).4,5
Cultural framework model
One example of a potential framework to use to engage within a cultural context includes the LEARN (Listen, Explain, Acknowledge, Recommend, Negotiate) model,6,7 which initially was developed to be used within a family medicine clinic. It includes the following:
Listen with sympathy and understanding to the patient’s perception of the problem. Try to understand their perspective of symptoms through considering their thoughts regarding etiology and treatment options.
Explain your perception of the problem. Have a dialogue about what you perceive is the likely cause based on a medical perspective.
Acknowledge and discuss the differences and similarities. Engage in open conversation while being cognizant that there may be similarities and differences in the perception you may have versus your patient’s perception. Try to find areas that can be engaged in and an alliance built upon, as well as respectfully and humbly addressing any concerns about potentially harmful patient understandings.
Recommend treatment. Present a treatment recommendation that considers both yours and the patient’s perspectives.
Negotiate agreement. Discuss, collaborate, and finalize a treatment plan that considers a biopsychosocial and spiritual/religious model of care that is patient-centered and personalized such that the main goal is optimal health and wellness for the patient/family.
The following are tips to consider in the life-long process of becoming more culturally aware:
- Be willing to learn with your patients and be thoughtful about your own feelings/thoughts/behaviors that may be positively or negatively impacting those interactions.
- Be aware of your own identity and what that may contribute to the clinical space.
- Recognize that you are not meant to know everything, but being open to the journey and learning process will go a long way.
- Try to shift the focus from paternalistic medicine to collaborative and patient-centered approaches.
The case at hand
In returning to our case and applying the LEARN model and cultural humility, we may be able to uncover more of the story. Adam is seen at a subsequent appointment, and you determine it best to obtain an in-person interpreter for this appointment. As you listen to the story, you learn that his father was killed early in Adam’s life, his mother has suffered from depression, and they moved here 3 years ago from a refugee camp, where most of their family continues to reside. He notes that at times he feels that he is back in that space and that he also feels frustrated. He is accustomed to doing well academically, but English has been difficult to learn.
You explain your understanding and acknowledge concerns for his past experiences playing a role, the importance of having community supports, and that learning a new language is challenging. You recommend that the school offer culturally appropriate interventions, trauma-informed assessments, and English-language opportunities. Adam and his mother note willingness to engage in this plan but would like to speak to their local religious leader as well.
Collaborating in a manner similar to this will likely build a therapeutic alliance between the patient, their family, and caretakers, thus leading to improved outcomes.
For further reading, consider AACAP Finding Mental Healthcare for Children of Immigrants and the American Academy of Pediatrics Providing Culturally Effective Care Toolkit.
Dr. Abdul-Karim, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. “Key findings about U.S. immigrants.” Pew Research Center, Washington, D.C. (2020)
2. “Key facts about refugees to the U.S.” Pew Research Center, Washington, D.C. (2019)
3. “Immigrant Children.” Child Trends, Bethesda, MD (2018).
4. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 11th ed. (Philadelphia: Lippincott Williams & Wilkins, 2015, pp. 139-45).
5. Lewis’sChild and Adolescent Psychiatry: A Comprehensive Textbook, 5th ed. (Philadelphia: Lippincott Williams & Wilkins, 2017, pp. 111-22).
6. Berlin EA, Fowkes WA Jr.A teaching framework for cross-cultural health care. Application in family practice. West J Med 1983;139(6):934-8.
7. Paediatr Child Health. 2018 Feb;23(1):66-9.
Adam is a 14-year-old who presents for “behavioral concerns” as recommended by his teacher. He is in the eighth grade and is struggling academically and socially. He has intermittent outbursts and poor engagement with other children, and often refuses to do schoolwork. He is seen in the outpatient primary care clinic, usually with his mother and two older siblings, one of whom typically translates for his Arabic-speaking mother. Adam is bilingual, although he prefers Arabic. It is difficult to understand the presenting concern as Adam states that he is doing well and is unsure why the teacher would have made such a report. Mother notes that she does not see these behaviors at home either.
What must we consider? Are there potential barriers, alternate ways to engage, and what role may culture have?
There are many things to consider in the above case, including language barriers, nuanced interactions, and cultural expectations and norms. To understand the scope, statistics reveal that the United States leads the world in its immigrant population with about 44.8 million foreign-born persons in 2018, which accounts for approximately 13.7% of the U.S. population.1 In 2019, 30,000 refugees were resettled in the United States.2 In 2017, immigrant children made up 27% (19.6 million) of U.S. children, of which second-generation children (born in the United States to immigrant parents) were the vast majority at 16.7 million.3 Given this information,
Culture is defined as a set of shared beliefs, norms, values, and behaviors exhibited by a group. Culture plays a role and impacts children in various ways throughout their development. Health care providers would benefit from aspiring to exude cultural humility – learning with and from patients and their families with openness, kindness, and a desire for collaboration. The provider also must consider a family’s history of migration as the response to migration may vary based on age, personal experiences, age at which migration occurred, language abilities, and amount of cultural engagement in the new country (i.e. acculturation).4,5
Cultural framework model
One example of a potential framework to use to engage within a cultural context includes the LEARN (Listen, Explain, Acknowledge, Recommend, Negotiate) model,6,7 which initially was developed to be used within a family medicine clinic. It includes the following:
Listen with sympathy and understanding to the patient’s perception of the problem. Try to understand their perspective of symptoms through considering their thoughts regarding etiology and treatment options.
Explain your perception of the problem. Have a dialogue about what you perceive is the likely cause based on a medical perspective.
Acknowledge and discuss the differences and similarities. Engage in open conversation while being cognizant that there may be similarities and differences in the perception you may have versus your patient’s perception. Try to find areas that can be engaged in and an alliance built upon, as well as respectfully and humbly addressing any concerns about potentially harmful patient understandings.
Recommend treatment. Present a treatment recommendation that considers both yours and the patient’s perspectives.
Negotiate agreement. Discuss, collaborate, and finalize a treatment plan that considers a biopsychosocial and spiritual/religious model of care that is patient-centered and personalized such that the main goal is optimal health and wellness for the patient/family.
The following are tips to consider in the life-long process of becoming more culturally aware:
- Be willing to learn with your patients and be thoughtful about your own feelings/thoughts/behaviors that may be positively or negatively impacting those interactions.
- Be aware of your own identity and what that may contribute to the clinical space.
- Recognize that you are not meant to know everything, but being open to the journey and learning process will go a long way.
- Try to shift the focus from paternalistic medicine to collaborative and patient-centered approaches.
The case at hand
In returning to our case and applying the LEARN model and cultural humility, we may be able to uncover more of the story. Adam is seen at a subsequent appointment, and you determine it best to obtain an in-person interpreter for this appointment. As you listen to the story, you learn that his father was killed early in Adam’s life, his mother has suffered from depression, and they moved here 3 years ago from a refugee camp, where most of their family continues to reside. He notes that at times he feels that he is back in that space and that he also feels frustrated. He is accustomed to doing well academically, but English has been difficult to learn.
You explain your understanding and acknowledge concerns for his past experiences playing a role, the importance of having community supports, and that learning a new language is challenging. You recommend that the school offer culturally appropriate interventions, trauma-informed assessments, and English-language opportunities. Adam and his mother note willingness to engage in this plan but would like to speak to their local religious leader as well.
Collaborating in a manner similar to this will likely build a therapeutic alliance between the patient, their family, and caretakers, thus leading to improved outcomes.
For further reading, consider AACAP Finding Mental Healthcare for Children of Immigrants and the American Academy of Pediatrics Providing Culturally Effective Care Toolkit.
Dr. Abdul-Karim, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. “Key findings about U.S. immigrants.” Pew Research Center, Washington, D.C. (2020)
2. “Key facts about refugees to the U.S.” Pew Research Center, Washington, D.C. (2019)
3. “Immigrant Children.” Child Trends, Bethesda, MD (2018).
4. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 11th ed. (Philadelphia: Lippincott Williams & Wilkins, 2015, pp. 139-45).
5. Lewis’sChild and Adolescent Psychiatry: A Comprehensive Textbook, 5th ed. (Philadelphia: Lippincott Williams & Wilkins, 2017, pp. 111-22).
6. Berlin EA, Fowkes WA Jr.A teaching framework for cross-cultural health care. Application in family practice. West J Med 1983;139(6):934-8.
7. Paediatr Child Health. 2018 Feb;23(1):66-9.
Adam is a 14-year-old who presents for “behavioral concerns” as recommended by his teacher. He is in the eighth grade and is struggling academically and socially. He has intermittent outbursts and poor engagement with other children, and often refuses to do schoolwork. He is seen in the outpatient primary care clinic, usually with his mother and two older siblings, one of whom typically translates for his Arabic-speaking mother. Adam is bilingual, although he prefers Arabic. It is difficult to understand the presenting concern as Adam states that he is doing well and is unsure why the teacher would have made such a report. Mother notes that she does not see these behaviors at home either.
What must we consider? Are there potential barriers, alternate ways to engage, and what role may culture have?
There are many things to consider in the above case, including language barriers, nuanced interactions, and cultural expectations and norms. To understand the scope, statistics reveal that the United States leads the world in its immigrant population with about 44.8 million foreign-born persons in 2018, which accounts for approximately 13.7% of the U.S. population.1 In 2019, 30,000 refugees were resettled in the United States.2 In 2017, immigrant children made up 27% (19.6 million) of U.S. children, of which second-generation children (born in the United States to immigrant parents) were the vast majority at 16.7 million.3 Given this information,
Culture is defined as a set of shared beliefs, norms, values, and behaviors exhibited by a group. Culture plays a role and impacts children in various ways throughout their development. Health care providers would benefit from aspiring to exude cultural humility – learning with and from patients and their families with openness, kindness, and a desire for collaboration. The provider also must consider a family’s history of migration as the response to migration may vary based on age, personal experiences, age at which migration occurred, language abilities, and amount of cultural engagement in the new country (i.e. acculturation).4,5
Cultural framework model
One example of a potential framework to use to engage within a cultural context includes the LEARN (Listen, Explain, Acknowledge, Recommend, Negotiate) model,6,7 which initially was developed to be used within a family medicine clinic. It includes the following:
Listen with sympathy and understanding to the patient’s perception of the problem. Try to understand their perspective of symptoms through considering their thoughts regarding etiology and treatment options.
Explain your perception of the problem. Have a dialogue about what you perceive is the likely cause based on a medical perspective.
Acknowledge and discuss the differences and similarities. Engage in open conversation while being cognizant that there may be similarities and differences in the perception you may have versus your patient’s perception. Try to find areas that can be engaged in and an alliance built upon, as well as respectfully and humbly addressing any concerns about potentially harmful patient understandings.
Recommend treatment. Present a treatment recommendation that considers both yours and the patient’s perspectives.
Negotiate agreement. Discuss, collaborate, and finalize a treatment plan that considers a biopsychosocial and spiritual/religious model of care that is patient-centered and personalized such that the main goal is optimal health and wellness for the patient/family.
The following are tips to consider in the life-long process of becoming more culturally aware:
- Be willing to learn with your patients and be thoughtful about your own feelings/thoughts/behaviors that may be positively or negatively impacting those interactions.
- Be aware of your own identity and what that may contribute to the clinical space.
- Recognize that you are not meant to know everything, but being open to the journey and learning process will go a long way.
- Try to shift the focus from paternalistic medicine to collaborative and patient-centered approaches.
The case at hand
In returning to our case and applying the LEARN model and cultural humility, we may be able to uncover more of the story. Adam is seen at a subsequent appointment, and you determine it best to obtain an in-person interpreter for this appointment. As you listen to the story, you learn that his father was killed early in Adam’s life, his mother has suffered from depression, and they moved here 3 years ago from a refugee camp, where most of their family continues to reside. He notes that at times he feels that he is back in that space and that he also feels frustrated. He is accustomed to doing well academically, but English has been difficult to learn.
You explain your understanding and acknowledge concerns for his past experiences playing a role, the importance of having community supports, and that learning a new language is challenging. You recommend that the school offer culturally appropriate interventions, trauma-informed assessments, and English-language opportunities. Adam and his mother note willingness to engage in this plan but would like to speak to their local religious leader as well.
Collaborating in a manner similar to this will likely build a therapeutic alliance between the patient, their family, and caretakers, thus leading to improved outcomes.
For further reading, consider AACAP Finding Mental Healthcare for Children of Immigrants and the American Academy of Pediatrics Providing Culturally Effective Care Toolkit.
Dr. Abdul-Karim, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. “Key findings about U.S. immigrants.” Pew Research Center, Washington, D.C. (2020)
2. “Key facts about refugees to the U.S.” Pew Research Center, Washington, D.C. (2019)
3. “Immigrant Children.” Child Trends, Bethesda, MD (2018).
4. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 11th ed. (Philadelphia: Lippincott Williams & Wilkins, 2015, pp. 139-45).
5. Lewis’sChild and Adolescent Psychiatry: A Comprehensive Textbook, 5th ed. (Philadelphia: Lippincott Williams & Wilkins, 2017, pp. 111-22).
6. Berlin EA, Fowkes WA Jr.A teaching framework for cross-cultural health care. Application in family practice. West J Med 1983;139(6):934-8.
7. Paediatr Child Health. 2018 Feb;23(1):66-9.
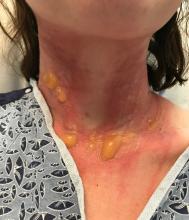
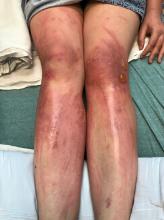
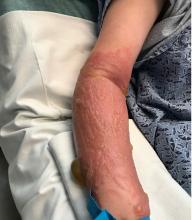
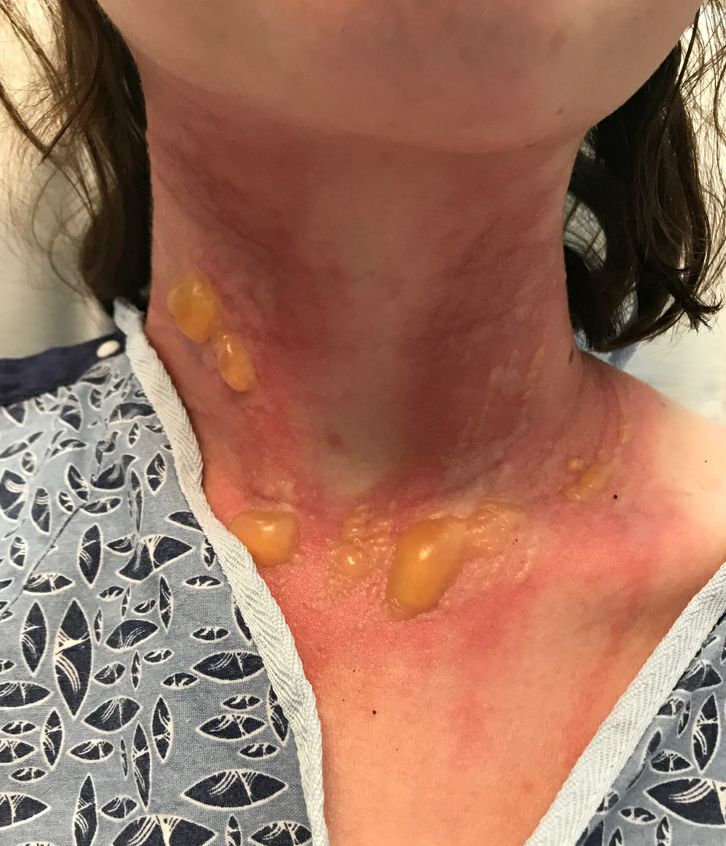
A teen presents with a severe, tender rash on the extremities
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
She started taking lithium for depression and anxiety 3 weeks prior to her developing the rash. She denies taking any other medications, supplements, or recreational drugs.
She denied any prior history of photosensitivity, no history of mouth ulcers, joint pain, muscle weakness, hair loss, or any other symptoms.
Besides her brother, there are no other affected family members, and no history of immune bullous disorders or other skin conditions.
On physical exam, the girl appears in a lot of pain and is uncomfortable. The skin is red and hot, and there are tense bullae on the neck, arms, and legs. There are no ocular or mucosal lesions.
Substance in tears could be used for diabetes monitoring
Measuring glycated albumin (glycoalbumin, GA) in tears could be a future way for those with diabetes to monitor their blood sugar levels noninvasively.
In a 100-patient trial, levels of GA in tears were found to be strongly correlated (r = .722; P < .001) with those in the blood.
“GA levels in blood are widely measured in clinical practice in Japan,” said study investigator Masakazu Aihara, MD, PhD, in an interview.
“It’s a biomarker that reflects the 2-week average blood glucose level like fructosamine,” explained the researcher from the department of diabetes and metabolic diseases in the Graduate School of Medicine at the University of Tokyo.
This could make it a better biomarker for detecting earlier changes in blood glucose than glycated hemoglobin (HbA1c), which reflects changes in blood glucose over the preceding 2-3 months.
Prior studies had shown that glucose levels can be measured in tear samples and that tear glucose levels correlated with blood glucose levels, Dr. Aihara and fellow researchers observed in a poster presentation at the virtual annual meeting of the European Association for the Study of Diabetes.
“While looking for noninvasive diabetes-related markers, we found that tears contained albumin. Based on this fact, we thought that GA could be measured in tears,” Dr. Aihara explained.
Using tears to test for biomarkers is not a new idea – tears not only protect the eye, they contain a variety of large proteins, and their composition can change with disease. Indeed, researchers have been looking at their usefulness in helping find biomarkers for Parkinson’s disease and diabetic peripheral neuropathy.
During their study, Dr. Aihara and associates collected tear and blood samples at the same time. Tear samples were assessed using liquid chromatography (LC) and mass spectrometry (MS). An enzymic method was used to measure GA levels in blood. Several diagnosis assay kits for GA are sold in Japan, Dr. Aihara said, and at least one of these has U.S. Food and Drug Administration approval.
Multiple regression analysis revealed that the correlation between GA levels in tears and in blood was maintained even after adjustment for age, gender, nephropathy stage, and obesity (P < .001). The results obtained from the tests were thought unlikely to be affected by any changes in the concentration or dilution of tear samples.
“Since GA levels in blood are clinically used in all types of diabetes, GA levels in tears is also expected to be useful in all types of diabetes,” Dr. Aihara said, noting that the effects of receiving treatment on GA levels in tears is something that he would like to look at.
The team would also like to optimize how tear samples are collected and reduce the volume of tears that are required for analysis. At the moment tears are collected via a dropper and about 100 mcL of tear fluid is required for measurement.
“At present, it is difficult to measure for dry eye patients because sufficient tears cannot be collected, but if the required amount of tears decreases in the future, it may be indicated for dry eye patients,” Dr. Aihara noted.
Discussing further research plans, he added: “We would like to examine the conditions of LC-MS/MS so that the correlation coefficient with GA in blood can be improved.
“Since LC-MS/MS is a large equipment in the laboratory, I would like to develop a device that can measure at the clinic or at home in the future.”
The study was funded by a grant from the Japan Agency for Medical Research and Development. Dr. Aihara had no conflicts of interest.
SOURCE: Aihara M et al. EASD 2020, poster presentation 624.
Measuring glycated albumin (glycoalbumin, GA) in tears could be a future way for those with diabetes to monitor their blood sugar levels noninvasively.
In a 100-patient trial, levels of GA in tears were found to be strongly correlated (r = .722; P < .001) with those in the blood.
“GA levels in blood are widely measured in clinical practice in Japan,” said study investigator Masakazu Aihara, MD, PhD, in an interview.
“It’s a biomarker that reflects the 2-week average blood glucose level like fructosamine,” explained the researcher from the department of diabetes and metabolic diseases in the Graduate School of Medicine at the University of Tokyo.
This could make it a better biomarker for detecting earlier changes in blood glucose than glycated hemoglobin (HbA1c), which reflects changes in blood glucose over the preceding 2-3 months.
Prior studies had shown that glucose levels can be measured in tear samples and that tear glucose levels correlated with blood glucose levels, Dr. Aihara and fellow researchers observed in a poster presentation at the virtual annual meeting of the European Association for the Study of Diabetes.
“While looking for noninvasive diabetes-related markers, we found that tears contained albumin. Based on this fact, we thought that GA could be measured in tears,” Dr. Aihara explained.
Using tears to test for biomarkers is not a new idea – tears not only protect the eye, they contain a variety of large proteins, and their composition can change with disease. Indeed, researchers have been looking at their usefulness in helping find biomarkers for Parkinson’s disease and diabetic peripheral neuropathy.
During their study, Dr. Aihara and associates collected tear and blood samples at the same time. Tear samples were assessed using liquid chromatography (LC) and mass spectrometry (MS). An enzymic method was used to measure GA levels in blood. Several diagnosis assay kits for GA are sold in Japan, Dr. Aihara said, and at least one of these has U.S. Food and Drug Administration approval.
Multiple regression analysis revealed that the correlation between GA levels in tears and in blood was maintained even after adjustment for age, gender, nephropathy stage, and obesity (P < .001). The results obtained from the tests were thought unlikely to be affected by any changes in the concentration or dilution of tear samples.
“Since GA levels in blood are clinically used in all types of diabetes, GA levels in tears is also expected to be useful in all types of diabetes,” Dr. Aihara said, noting that the effects of receiving treatment on GA levels in tears is something that he would like to look at.
The team would also like to optimize how tear samples are collected and reduce the volume of tears that are required for analysis. At the moment tears are collected via a dropper and about 100 mcL of tear fluid is required for measurement.
“At present, it is difficult to measure for dry eye patients because sufficient tears cannot be collected, but if the required amount of tears decreases in the future, it may be indicated for dry eye patients,” Dr. Aihara noted.
Discussing further research plans, he added: “We would like to examine the conditions of LC-MS/MS so that the correlation coefficient with GA in blood can be improved.
“Since LC-MS/MS is a large equipment in the laboratory, I would like to develop a device that can measure at the clinic or at home in the future.”
The study was funded by a grant from the Japan Agency for Medical Research and Development. Dr. Aihara had no conflicts of interest.
SOURCE: Aihara M et al. EASD 2020, poster presentation 624.
Measuring glycated albumin (glycoalbumin, GA) in tears could be a future way for those with diabetes to monitor their blood sugar levels noninvasively.
In a 100-patient trial, levels of GA in tears were found to be strongly correlated (r = .722; P < .001) with those in the blood.
“GA levels in blood are widely measured in clinical practice in Japan,” said study investigator Masakazu Aihara, MD, PhD, in an interview.
“It’s a biomarker that reflects the 2-week average blood glucose level like fructosamine,” explained the researcher from the department of diabetes and metabolic diseases in the Graduate School of Medicine at the University of Tokyo.
This could make it a better biomarker for detecting earlier changes in blood glucose than glycated hemoglobin (HbA1c), which reflects changes in blood glucose over the preceding 2-3 months.
Prior studies had shown that glucose levels can be measured in tear samples and that tear glucose levels correlated with blood glucose levels, Dr. Aihara and fellow researchers observed in a poster presentation at the virtual annual meeting of the European Association for the Study of Diabetes.
“While looking for noninvasive diabetes-related markers, we found that tears contained albumin. Based on this fact, we thought that GA could be measured in tears,” Dr. Aihara explained.
Using tears to test for biomarkers is not a new idea – tears not only protect the eye, they contain a variety of large proteins, and their composition can change with disease. Indeed, researchers have been looking at their usefulness in helping find biomarkers for Parkinson’s disease and diabetic peripheral neuropathy.
During their study, Dr. Aihara and associates collected tear and blood samples at the same time. Tear samples were assessed using liquid chromatography (LC) and mass spectrometry (MS). An enzymic method was used to measure GA levels in blood. Several diagnosis assay kits for GA are sold in Japan, Dr. Aihara said, and at least one of these has U.S. Food and Drug Administration approval.
Multiple regression analysis revealed that the correlation between GA levels in tears and in blood was maintained even after adjustment for age, gender, nephropathy stage, and obesity (P < .001). The results obtained from the tests were thought unlikely to be affected by any changes in the concentration or dilution of tear samples.
“Since GA levels in blood are clinically used in all types of diabetes, GA levels in tears is also expected to be useful in all types of diabetes,” Dr. Aihara said, noting that the effects of receiving treatment on GA levels in tears is something that he would like to look at.
The team would also like to optimize how tear samples are collected and reduce the volume of tears that are required for analysis. At the moment tears are collected via a dropper and about 100 mcL of tear fluid is required for measurement.
“At present, it is difficult to measure for dry eye patients because sufficient tears cannot be collected, but if the required amount of tears decreases in the future, it may be indicated for dry eye patients,” Dr. Aihara noted.
Discussing further research plans, he added: “We would like to examine the conditions of LC-MS/MS so that the correlation coefficient with GA in blood can be improved.
“Since LC-MS/MS is a large equipment in the laboratory, I would like to develop a device that can measure at the clinic or at home in the future.”
The study was funded by a grant from the Japan Agency for Medical Research and Development. Dr. Aihara had no conflicts of interest.
SOURCE: Aihara M et al. EASD 2020, poster presentation 624.
FROM EASD 2020
Severe Asthma: Changing the Game
In this supplement to CHEST Physician, Dr. Sandra Adams investigates the following topics:
- Difficult-to-control vs severe asthma
- T2-high inflammatory endotype
- T2-low endotype
- Biologic therapies in severe asthma
- Treatment follow-up and assessment
Click here to read.
Author
Sandra G. Adams, MD, MS, FCCP
Division of Pulmonary
Diseases and Critical Care Medicine
UT Health San Antonio
Staff Physician,
Care System
San Antonio, TX
In this supplement to CHEST Physician, Dr. Sandra Adams investigates the following topics:
- Difficult-to-control vs severe asthma
- T2-high inflammatory endotype
- T2-low endotype
- Biologic therapies in severe asthma
- Treatment follow-up and assessment
Click here to read.
Author
Sandra G. Adams, MD, MS, FCCP
Division of Pulmonary
Diseases and Critical Care Medicine
UT Health San Antonio
Staff Physician,
Care System
San Antonio, TX
In this supplement to CHEST Physician, Dr. Sandra Adams investigates the following topics:
- Difficult-to-control vs severe asthma
- T2-high inflammatory endotype
- T2-low endotype
- Biologic therapies in severe asthma
- Treatment follow-up and assessment
Click here to read.
Author
Sandra G. Adams, MD, MS, FCCP
Division of Pulmonary
Diseases and Critical Care Medicine
UT Health San Antonio
Staff Physician,
Care System
San Antonio, TX
The unsteady state
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Diarrhea prevalent among COVID-19 patients with IBD
Diarrhea affected one in every five patients with inflammatory bowel disease (IBD) and COVID-19, compared with only 7%-10% of all patients with COVID-19 in prior studies, researchers reported in Clinical Gastroenterology and Hepatology.
In a systematic review and meta-analysis of 23 studies incorporating data from 449 patients with IBD and COVID-19, their most common symptoms were fever (affecting 48.3% of patients), cough (46.5%), and diarrhea (20.5%), and diarrhea was approximately twice as prevalent as dyspnea, nausea, abdominal pain, and fatigue, wrote Ferdinando D’Amico of Humanitas University in Milan and his associates. “[S]ymptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea,” they wrote. This increased prevalence might result from IBD itself or from inflammatory effects of viral gut tropism, they noted. “Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.”
To characterize the clinical presentation and course of patients with IBD and COVID-19, the researchers searched PubMed, Embase, Web of Science, and MedRxiv through July 29, 2020, for keywords related to COVID-19, Crohn’s disease, ulcerative colitis, and IBD. They identified 23 studies presenting clinical data from adults or children with a confirmed IBD diagnosis and least one case of COVID-19. Among 243,760 patients with IBD, 1,028 patients had COVID-19 infection, including 509 patients with Crohn’s disease, 428 patients with ulcerative colitis, 49 patients with indeterminate colitis, and 42 patients for whom the IBD subtype was not recorded.
In all, 0.4% of patients with IBD had COVID-19. Nearly all had been diagnosed by polymerase chain reaction of nasopharyngeal swabs, and approximately 40% also had received chest CT scans. Most were male (56.5%), and 43.5% were older than 65 years. Patients were receiving a wide range of IBD therapies, most commonly anti–tumor necrosis factor (TNF) agents, mesalamine, thiopurine (alone or in combination with biologics), vedolizumab, ustekinumab, steroids, methotrexate, and tofacitinib. Results from six studies indicated that patients with IBD were significantly more likely to be diagnosed with COVID-19 if they were older than 66 years (odds ratio, 21.3) or had other comorbidities (OR, 1.24). The most commonly used drugs for managing COVID-19 were hydroxychloroquine, lopinavir/ritonavir, steroids, antibiotics, chloroquine, tofacitinib, and infliximab.
A total of 30.6% of patients with IBD and COVID-19 were hospitalized, 11.4% stayed in the ICU, 3.7% required mechanical ventilation, and 3.8% died from COVID-19. Significant risk factors for death from COVID-19 included older age, active IBD, and a Charlson Comorbidity Index score above 1. Similarly, risk factors for severe COVID-19 included older age, having two or more comorbidities, receiving systemic steroids, and receiving mesalamine/sulfasalazine. In one study, a recent (3-month) history of corticosteroid treatment was associated with a 60% increase in the risk for severe COVID-19. Other immune-mediated therapies did not show this association. Patients with ulcerative colitis were significantly more likely to be seen in the ED or hospitalized, compared with patients with other forms of IBD (adjusted OR, 12.7).
No funding sources were disclosed. Dr. D’Amico reported having no conflicts of interest. Two coinvestigators disclosed ties to AbbVie, MSD, Schering-Plough, UCB Pharma, and several other pharmaceutical companies.
SOURCE: D’Amico F et al. Clin Gastroenterol Hepatol. 2020 Aug 7. doi: 10.1016/j.cgh.2020.08.003.
Diarrhea is one of the hallmark features in inflammatory bowel disease (IBD). The systematic review and meta-analysis by D’Amico and colleagues highlights an increased prevalence of diarrhea in IBD patients with COVID-19. We have learned that SARS-CoV-2 enters the gastrointestinal tract through angiotensin converting enzyme 2, which has been found in absorptive enterocytes of the ileum and colon. The subsequent invasion can cause a change in intestinal microbiota (dysbiosis) and trigger diarrhea. Prior studies also reported SARS-CoV-2 being isolated in the duodenum and rectum while showing RNA shedding in approximately 40% of patients. Clinicians may now face the diagnostic challenge of distinguishing the cause of diarrhea as an exacerbation from underlying IBD versus viral superinfection. The authors astutely hypothesized that having access to fecal polymerase chain reaction tests may be particularly useful to guiding clinical treatment decisions.
Lukasz Kwapisz, MD, FRCPC, is assistant professor of medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Diarrhea is one of the hallmark features in inflammatory bowel disease (IBD). The systematic review and meta-analysis by D’Amico and colleagues highlights an increased prevalence of diarrhea in IBD patients with COVID-19. We have learned that SARS-CoV-2 enters the gastrointestinal tract through angiotensin converting enzyme 2, which has been found in absorptive enterocytes of the ileum and colon. The subsequent invasion can cause a change in intestinal microbiota (dysbiosis) and trigger diarrhea. Prior studies also reported SARS-CoV-2 being isolated in the duodenum and rectum while showing RNA shedding in approximately 40% of patients. Clinicians may now face the diagnostic challenge of distinguishing the cause of diarrhea as an exacerbation from underlying IBD versus viral superinfection. The authors astutely hypothesized that having access to fecal polymerase chain reaction tests may be particularly useful to guiding clinical treatment decisions.
Lukasz Kwapisz, MD, FRCPC, is assistant professor of medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Diarrhea is one of the hallmark features in inflammatory bowel disease (IBD). The systematic review and meta-analysis by D’Amico and colleagues highlights an increased prevalence of diarrhea in IBD patients with COVID-19. We have learned that SARS-CoV-2 enters the gastrointestinal tract through angiotensin converting enzyme 2, which has been found in absorptive enterocytes of the ileum and colon. The subsequent invasion can cause a change in intestinal microbiota (dysbiosis) and trigger diarrhea. Prior studies also reported SARS-CoV-2 being isolated in the duodenum and rectum while showing RNA shedding in approximately 40% of patients. Clinicians may now face the diagnostic challenge of distinguishing the cause of diarrhea as an exacerbation from underlying IBD versus viral superinfection. The authors astutely hypothesized that having access to fecal polymerase chain reaction tests may be particularly useful to guiding clinical treatment decisions.
Lukasz Kwapisz, MD, FRCPC, is assistant professor of medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Diarrhea affected one in every five patients with inflammatory bowel disease (IBD) and COVID-19, compared with only 7%-10% of all patients with COVID-19 in prior studies, researchers reported in Clinical Gastroenterology and Hepatology.
In a systematic review and meta-analysis of 23 studies incorporating data from 449 patients with IBD and COVID-19, their most common symptoms were fever (affecting 48.3% of patients), cough (46.5%), and diarrhea (20.5%), and diarrhea was approximately twice as prevalent as dyspnea, nausea, abdominal pain, and fatigue, wrote Ferdinando D’Amico of Humanitas University in Milan and his associates. “[S]ymptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea,” they wrote. This increased prevalence might result from IBD itself or from inflammatory effects of viral gut tropism, they noted. “Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.”
To characterize the clinical presentation and course of patients with IBD and COVID-19, the researchers searched PubMed, Embase, Web of Science, and MedRxiv through July 29, 2020, for keywords related to COVID-19, Crohn’s disease, ulcerative colitis, and IBD. They identified 23 studies presenting clinical data from adults or children with a confirmed IBD diagnosis and least one case of COVID-19. Among 243,760 patients with IBD, 1,028 patients had COVID-19 infection, including 509 patients with Crohn’s disease, 428 patients with ulcerative colitis, 49 patients with indeterminate colitis, and 42 patients for whom the IBD subtype was not recorded.
In all, 0.4% of patients with IBD had COVID-19. Nearly all had been diagnosed by polymerase chain reaction of nasopharyngeal swabs, and approximately 40% also had received chest CT scans. Most were male (56.5%), and 43.5% were older than 65 years. Patients were receiving a wide range of IBD therapies, most commonly anti–tumor necrosis factor (TNF) agents, mesalamine, thiopurine (alone or in combination with biologics), vedolizumab, ustekinumab, steroids, methotrexate, and tofacitinib. Results from six studies indicated that patients with IBD were significantly more likely to be diagnosed with COVID-19 if they were older than 66 years (odds ratio, 21.3) or had other comorbidities (OR, 1.24). The most commonly used drugs for managing COVID-19 were hydroxychloroquine, lopinavir/ritonavir, steroids, antibiotics, chloroquine, tofacitinib, and infliximab.
A total of 30.6% of patients with IBD and COVID-19 were hospitalized, 11.4% stayed in the ICU, 3.7% required mechanical ventilation, and 3.8% died from COVID-19. Significant risk factors for death from COVID-19 included older age, active IBD, and a Charlson Comorbidity Index score above 1. Similarly, risk factors for severe COVID-19 included older age, having two or more comorbidities, receiving systemic steroids, and receiving mesalamine/sulfasalazine. In one study, a recent (3-month) history of corticosteroid treatment was associated with a 60% increase in the risk for severe COVID-19. Other immune-mediated therapies did not show this association. Patients with ulcerative colitis were significantly more likely to be seen in the ED or hospitalized, compared with patients with other forms of IBD (adjusted OR, 12.7).
No funding sources were disclosed. Dr. D’Amico reported having no conflicts of interest. Two coinvestigators disclosed ties to AbbVie, MSD, Schering-Plough, UCB Pharma, and several other pharmaceutical companies.
SOURCE: D’Amico F et al. Clin Gastroenterol Hepatol. 2020 Aug 7. doi: 10.1016/j.cgh.2020.08.003.
Diarrhea affected one in every five patients with inflammatory bowel disease (IBD) and COVID-19, compared with only 7%-10% of all patients with COVID-19 in prior studies, researchers reported in Clinical Gastroenterology and Hepatology.
In a systematic review and meta-analysis of 23 studies incorporating data from 449 patients with IBD and COVID-19, their most common symptoms were fever (affecting 48.3% of patients), cough (46.5%), and diarrhea (20.5%), and diarrhea was approximately twice as prevalent as dyspnea, nausea, abdominal pain, and fatigue, wrote Ferdinando D’Amico of Humanitas University in Milan and his associates. “[S]ymptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea,” they wrote. This increased prevalence might result from IBD itself or from inflammatory effects of viral gut tropism, they noted. “Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.”
To characterize the clinical presentation and course of patients with IBD and COVID-19, the researchers searched PubMed, Embase, Web of Science, and MedRxiv through July 29, 2020, for keywords related to COVID-19, Crohn’s disease, ulcerative colitis, and IBD. They identified 23 studies presenting clinical data from adults or children with a confirmed IBD diagnosis and least one case of COVID-19. Among 243,760 patients with IBD, 1,028 patients had COVID-19 infection, including 509 patients with Crohn’s disease, 428 patients with ulcerative colitis, 49 patients with indeterminate colitis, and 42 patients for whom the IBD subtype was not recorded.
In all, 0.4% of patients with IBD had COVID-19. Nearly all had been diagnosed by polymerase chain reaction of nasopharyngeal swabs, and approximately 40% also had received chest CT scans. Most were male (56.5%), and 43.5% were older than 65 years. Patients were receiving a wide range of IBD therapies, most commonly anti–tumor necrosis factor (TNF) agents, mesalamine, thiopurine (alone or in combination with biologics), vedolizumab, ustekinumab, steroids, methotrexate, and tofacitinib. Results from six studies indicated that patients with IBD were significantly more likely to be diagnosed with COVID-19 if they were older than 66 years (odds ratio, 21.3) or had other comorbidities (OR, 1.24). The most commonly used drugs for managing COVID-19 were hydroxychloroquine, lopinavir/ritonavir, steroids, antibiotics, chloroquine, tofacitinib, and infliximab.
A total of 30.6% of patients with IBD and COVID-19 were hospitalized, 11.4% stayed in the ICU, 3.7% required mechanical ventilation, and 3.8% died from COVID-19. Significant risk factors for death from COVID-19 included older age, active IBD, and a Charlson Comorbidity Index score above 1. Similarly, risk factors for severe COVID-19 included older age, having two or more comorbidities, receiving systemic steroids, and receiving mesalamine/sulfasalazine. In one study, a recent (3-month) history of corticosteroid treatment was associated with a 60% increase in the risk for severe COVID-19. Other immune-mediated therapies did not show this association. Patients with ulcerative colitis were significantly more likely to be seen in the ED or hospitalized, compared with patients with other forms of IBD (adjusted OR, 12.7).
No funding sources were disclosed. Dr. D’Amico reported having no conflicts of interest. Two coinvestigators disclosed ties to AbbVie, MSD, Schering-Plough, UCB Pharma, and several other pharmaceutical companies.
SOURCE: D’Amico F et al. Clin Gastroenterol Hepatol. 2020 Aug 7. doi: 10.1016/j.cgh.2020.08.003.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Prodrug infusion beats oral Parkinson’s disease therapy for motor symptoms
, according to a new study. The beneficial effects of these phosphate prodrugs of levodopa and carbidopa were most noticeable in the early morning, results of the phase 1B study showed.
As Parkinson’s disease progresses and dosing of oral levodopa/carbidopa (LD/CD) increases, its therapeutic window narrows, resulting in troublesome dyskinesia at peak drug levels and tremors and rigidity when levels fall.
“Foslevodopa/foscarbidopa shows lower ‘off’ time than oral levodopa/carbidopa, and this was statistically significant. Also, foslevodopa/foscarbidopa (fosL/fosC) showed more ‘on’ time without dyskinesia, compared with oral levodopa/carbidopa. This was also statistically significant,” lead author Sven Stodtmann, PhD, of AbbVie GmbH, Ludwigshafen, Germany, reported in his recorded presentation at the Movement Disorders Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorder (Virtual) 2020.
Continuous infusion versus oral therapy
The analysis included 20 patients, and all data from these individuals were collected between 4:30 a.m. and 9:30 p.m.
Participants were 12 men and 8 women, aged 30-80 years, with advanced, idiopathic Parkinson’s disease responsive to levodopa but inadequately controlled on their current stable therapy, having a minimum of 2.5 off hours/day. Mean age was 61.3 plus or minus 10.5 years (range 35-77 years).
In this single-arm, open-label study, they received subcutaneous infusions of personalized therapeutic doses of fosL/fosC 24 hours/day for 28 days after a 10- to 30-day screening period during which they recorded LD/CD doses in a diary and had motor symptoms monitored using a wearable device.
Following the screening period, fosL/fosC doses were titrated over up to 5 days, with subsequent weekly study visits, for a total time on fosL/fosC of 28 days. Drug titration was aimed at maximizing functional on time and minimizing the number of off episodes while minimizing troublesome dyskinesia.
Continuous infusion of fosL/fosC performed better than oral LD/CD on all counts.
“The off time is much lower in the morning for people on foslevodopa/foscarbidopa [compared with oral LD/CD] because this is a 24-hour infusion product,” Dr. Stodtmann explained.
The effect was maintained over the course of the day with little fluctuation with fosL/fosC, off periods never exceeding about 25% between 4:30 a.m. and 9 p.m. For LD/CD, off periods were highest in the early morning and peaked at about 50% on a 3- to 4-hour cycle during the course of the day.
Increased on time without dyskinesia varied between about 60% and 80% during the day with fosL/fosC, showing the greatest difference between fosL/fosC and oral LD/CD in the early morning hours.
“On time with nontroublesome dyskinesia was lower for foscarbidopa/foslevodopa, compared to oral levodopa/carbidopa, but this was not statistically significant,” Dr. Stodtmann said. On time with troublesome dyskinesia followed the same pattern, again, not statistically significant.
Looking at the data another way, the investigators calculated the odds ratios of motor symptoms using fosL/fosC, compared with oral LD/CD. Use of fosL/fosC was associated with a 59% lower risk of being in the off state during the day, compared with oral LD/CD (odds ratio, 0.4; 95% confidence interval, 0.2-0.7; P < .01). Similarly, the probability of being in the on state without dyskinesia was much greater with fosL/fosC (OR, 2.75; 95% CI, 1.08-6.99; P < .05).
Encouraging, but more data needed
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that the field has been waiting to see data on fosL/fosC.
“It seems like it’s pretty reasonable in terms of what the goals were, which is to improve stability of Parkinson’s symptoms, to improve off time and give on time without troublesome dyskinesia,” she said. “So I think those [goals] have been met.”
Dr. Subramanian, who was not involved with the research, said she would have liked to have seen results concerning safety of this drug formulation, which the presentation lacked, “because historically, there have been issues with nodule formation and skin breakdown, things like that, due to the stability of the product in the subcutaneous form. … So, always to my understanding, there has been this search for things that are tolerated in the subcutaneous delivery.”
If this formulation proves safe and tolerable, Dr. Subramanian sees a potential place for it for some patients with advanced Parkinson’s disease.
“Certainly a subcutaneous formulation will be better than something that requires … deep brain surgery or even a pump insertion like Duopa [carbidopa/levodopa enteral suspension, AbbVie] or something like that,” she said. “I think [it] would be beneficial over something with the gut because the gut historically has been a problem to rely on in advanced Parkinson’s patients due to slower transit times, and the gut itself is affected with Parkinson’s disease.”
Dr. Stodtmann and all coauthors are employees of AbbVie, which was the sponsor of the study and was responsible for all aspects of it. Dr. Subramanian has given talks for Acadia Pharmaceuticals and Acorda Therapeutics in the past.
A version of this article originally appeared on Medscape.com.
, according to a new study. The beneficial effects of these phosphate prodrugs of levodopa and carbidopa were most noticeable in the early morning, results of the phase 1B study showed.
As Parkinson’s disease progresses and dosing of oral levodopa/carbidopa (LD/CD) increases, its therapeutic window narrows, resulting in troublesome dyskinesia at peak drug levels and tremors and rigidity when levels fall.
“Foslevodopa/foscarbidopa shows lower ‘off’ time than oral levodopa/carbidopa, and this was statistically significant. Also, foslevodopa/foscarbidopa (fosL/fosC) showed more ‘on’ time without dyskinesia, compared with oral levodopa/carbidopa. This was also statistically significant,” lead author Sven Stodtmann, PhD, of AbbVie GmbH, Ludwigshafen, Germany, reported in his recorded presentation at the Movement Disorders Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorder (Virtual) 2020.
Continuous infusion versus oral therapy
The analysis included 20 patients, and all data from these individuals were collected between 4:30 a.m. and 9:30 p.m.
Participants were 12 men and 8 women, aged 30-80 years, with advanced, idiopathic Parkinson’s disease responsive to levodopa but inadequately controlled on their current stable therapy, having a minimum of 2.5 off hours/day. Mean age was 61.3 plus or minus 10.5 years (range 35-77 years).
In this single-arm, open-label study, they received subcutaneous infusions of personalized therapeutic doses of fosL/fosC 24 hours/day for 28 days after a 10- to 30-day screening period during which they recorded LD/CD doses in a diary and had motor symptoms monitored using a wearable device.
Following the screening period, fosL/fosC doses were titrated over up to 5 days, with subsequent weekly study visits, for a total time on fosL/fosC of 28 days. Drug titration was aimed at maximizing functional on time and minimizing the number of off episodes while minimizing troublesome dyskinesia.
Continuous infusion of fosL/fosC performed better than oral LD/CD on all counts.
“The off time is much lower in the morning for people on foslevodopa/foscarbidopa [compared with oral LD/CD] because this is a 24-hour infusion product,” Dr. Stodtmann explained.
The effect was maintained over the course of the day with little fluctuation with fosL/fosC, off periods never exceeding about 25% between 4:30 a.m. and 9 p.m. For LD/CD, off periods were highest in the early morning and peaked at about 50% on a 3- to 4-hour cycle during the course of the day.
Increased on time without dyskinesia varied between about 60% and 80% during the day with fosL/fosC, showing the greatest difference between fosL/fosC and oral LD/CD in the early morning hours.
“On time with nontroublesome dyskinesia was lower for foscarbidopa/foslevodopa, compared to oral levodopa/carbidopa, but this was not statistically significant,” Dr. Stodtmann said. On time with troublesome dyskinesia followed the same pattern, again, not statistically significant.
Looking at the data another way, the investigators calculated the odds ratios of motor symptoms using fosL/fosC, compared with oral LD/CD. Use of fosL/fosC was associated with a 59% lower risk of being in the off state during the day, compared with oral LD/CD (odds ratio, 0.4; 95% confidence interval, 0.2-0.7; P < .01). Similarly, the probability of being in the on state without dyskinesia was much greater with fosL/fosC (OR, 2.75; 95% CI, 1.08-6.99; P < .05).
Encouraging, but more data needed
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that the field has been waiting to see data on fosL/fosC.
“It seems like it’s pretty reasonable in terms of what the goals were, which is to improve stability of Parkinson’s symptoms, to improve off time and give on time without troublesome dyskinesia,” she said. “So I think those [goals] have been met.”
Dr. Subramanian, who was not involved with the research, said she would have liked to have seen results concerning safety of this drug formulation, which the presentation lacked, “because historically, there have been issues with nodule formation and skin breakdown, things like that, due to the stability of the product in the subcutaneous form. … So, always to my understanding, there has been this search for things that are tolerated in the subcutaneous delivery.”
If this formulation proves safe and tolerable, Dr. Subramanian sees a potential place for it for some patients with advanced Parkinson’s disease.
“Certainly a subcutaneous formulation will be better than something that requires … deep brain surgery or even a pump insertion like Duopa [carbidopa/levodopa enteral suspension, AbbVie] or something like that,” she said. “I think [it] would be beneficial over something with the gut because the gut historically has been a problem to rely on in advanced Parkinson’s patients due to slower transit times, and the gut itself is affected with Parkinson’s disease.”
Dr. Stodtmann and all coauthors are employees of AbbVie, which was the sponsor of the study and was responsible for all aspects of it. Dr. Subramanian has given talks for Acadia Pharmaceuticals and Acorda Therapeutics in the past.
A version of this article originally appeared on Medscape.com.
, according to a new study. The beneficial effects of these phosphate prodrugs of levodopa and carbidopa were most noticeable in the early morning, results of the phase 1B study showed.
As Parkinson’s disease progresses and dosing of oral levodopa/carbidopa (LD/CD) increases, its therapeutic window narrows, resulting in troublesome dyskinesia at peak drug levels and tremors and rigidity when levels fall.
“Foslevodopa/foscarbidopa shows lower ‘off’ time than oral levodopa/carbidopa, and this was statistically significant. Also, foslevodopa/foscarbidopa (fosL/fosC) showed more ‘on’ time without dyskinesia, compared with oral levodopa/carbidopa. This was also statistically significant,” lead author Sven Stodtmann, PhD, of AbbVie GmbH, Ludwigshafen, Germany, reported in his recorded presentation at the Movement Disorders Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorder (Virtual) 2020.
Continuous infusion versus oral therapy
The analysis included 20 patients, and all data from these individuals were collected between 4:30 a.m. and 9:30 p.m.
Participants were 12 men and 8 women, aged 30-80 years, with advanced, idiopathic Parkinson’s disease responsive to levodopa but inadequately controlled on their current stable therapy, having a minimum of 2.5 off hours/day. Mean age was 61.3 plus or minus 10.5 years (range 35-77 years).
In this single-arm, open-label study, they received subcutaneous infusions of personalized therapeutic doses of fosL/fosC 24 hours/day for 28 days after a 10- to 30-day screening period during which they recorded LD/CD doses in a diary and had motor symptoms monitored using a wearable device.
Following the screening period, fosL/fosC doses were titrated over up to 5 days, with subsequent weekly study visits, for a total time on fosL/fosC of 28 days. Drug titration was aimed at maximizing functional on time and minimizing the number of off episodes while minimizing troublesome dyskinesia.
Continuous infusion of fosL/fosC performed better than oral LD/CD on all counts.
“The off time is much lower in the morning for people on foslevodopa/foscarbidopa [compared with oral LD/CD] because this is a 24-hour infusion product,” Dr. Stodtmann explained.
The effect was maintained over the course of the day with little fluctuation with fosL/fosC, off periods never exceeding about 25% between 4:30 a.m. and 9 p.m. For LD/CD, off periods were highest in the early morning and peaked at about 50% on a 3- to 4-hour cycle during the course of the day.
Increased on time without dyskinesia varied between about 60% and 80% during the day with fosL/fosC, showing the greatest difference between fosL/fosC and oral LD/CD in the early morning hours.
“On time with nontroublesome dyskinesia was lower for foscarbidopa/foslevodopa, compared to oral levodopa/carbidopa, but this was not statistically significant,” Dr. Stodtmann said. On time with troublesome dyskinesia followed the same pattern, again, not statistically significant.
Looking at the data another way, the investigators calculated the odds ratios of motor symptoms using fosL/fosC, compared with oral LD/CD. Use of fosL/fosC was associated with a 59% lower risk of being in the off state during the day, compared with oral LD/CD (odds ratio, 0.4; 95% confidence interval, 0.2-0.7; P < .01). Similarly, the probability of being in the on state without dyskinesia was much greater with fosL/fosC (OR, 2.75; 95% CI, 1.08-6.99; P < .05).
Encouraging, but more data needed
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that the field has been waiting to see data on fosL/fosC.
“It seems like it’s pretty reasonable in terms of what the goals were, which is to improve stability of Parkinson’s symptoms, to improve off time and give on time without troublesome dyskinesia,” she said. “So I think those [goals] have been met.”
Dr. Subramanian, who was not involved with the research, said she would have liked to have seen results concerning safety of this drug formulation, which the presentation lacked, “because historically, there have been issues with nodule formation and skin breakdown, things like that, due to the stability of the product in the subcutaneous form. … So, always to my understanding, there has been this search for things that are tolerated in the subcutaneous delivery.”
If this formulation proves safe and tolerable, Dr. Subramanian sees a potential place for it for some patients with advanced Parkinson’s disease.
“Certainly a subcutaneous formulation will be better than something that requires … deep brain surgery or even a pump insertion like Duopa [carbidopa/levodopa enteral suspension, AbbVie] or something like that,” she said. “I think [it] would be beneficial over something with the gut because the gut historically has been a problem to rely on in advanced Parkinson’s patients due to slower transit times, and the gut itself is affected with Parkinson’s disease.”
Dr. Stodtmann and all coauthors are employees of AbbVie, which was the sponsor of the study and was responsible for all aspects of it. Dr. Subramanian has given talks for Acadia Pharmaceuticals and Acorda Therapeutics in the past.
A version of this article originally appeared on Medscape.com.
Laparoscopic specimen retrieval bags in gyn surgery: Expert guidance on selection
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.
Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.
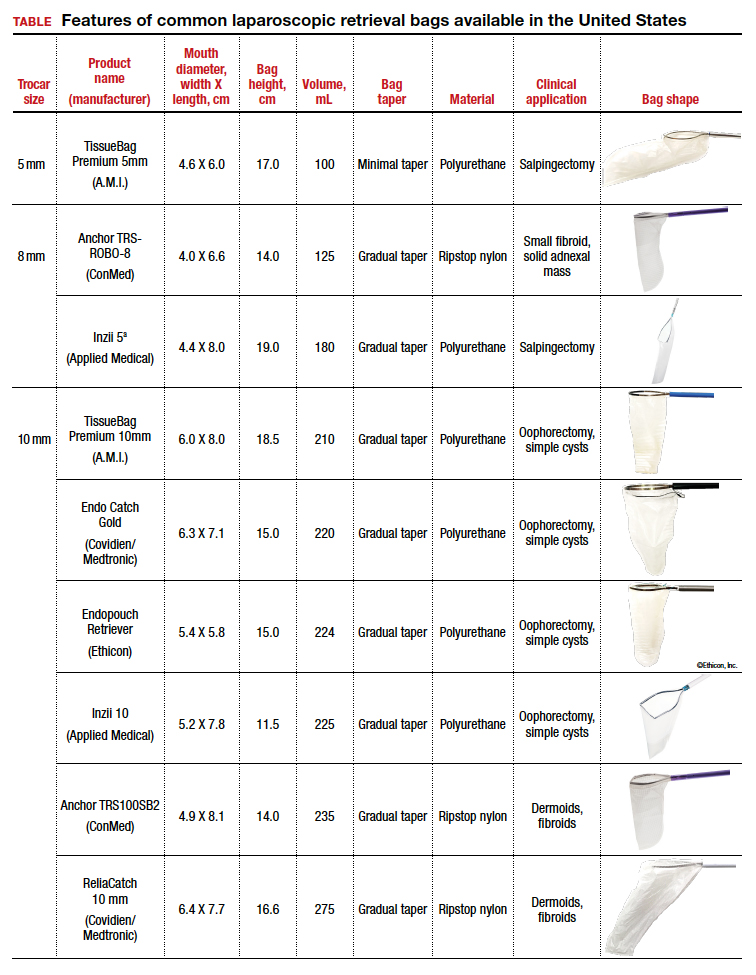
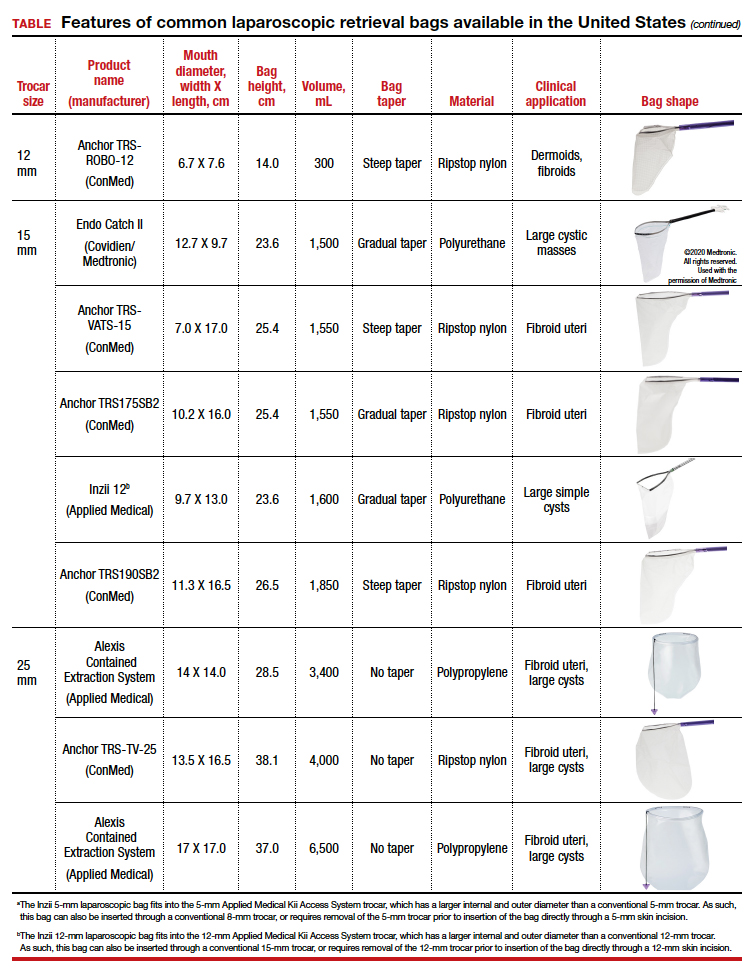
The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).
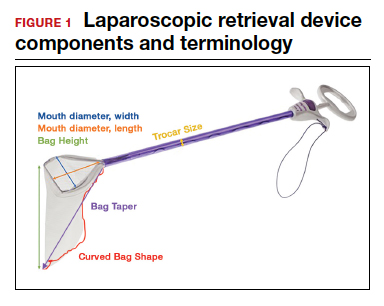

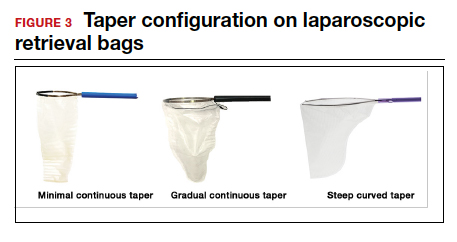
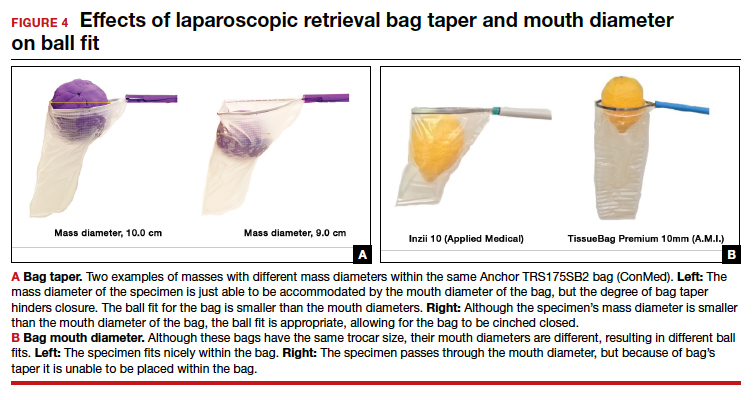
Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.

Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.


The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).




Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.

Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.


The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).




Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
HIT-6 may help track meaningful change in chronic migraine
, recent research suggests.
Using data from the phase 3 PROMISE-2 study, which evaluated intravenous eptinezumab in doses of 100 mg or 300 mg, or placebo every 12 weeks in 1,072 participants for the prevention of chronic migraine, Carrie R. Houts, PhD, director of psychometrics at the Vector Psychometric Group, in Chapel Hill, N.C., and colleagues determined that their finding of 6-point improvement of HIT-6 total score was consistent with other studies. However, they pointed out that little research has been done in evaluating how item-specific scores of HIT-6 impact individuals with chronic migraine. HIT-6 item scores examine whether individuals with headaches experience severe pain, limit their daily activities, have a desire to lie down, feel too tired to do daily activities, felt “fed up or irritated” because of headaches, and feel their headaches limit concentration on work or daily activities.
“The item-specific responder definitions give clinicians and researchers the ability to evaluate and track the impact of headache on specific item-level areas of patients’ lives. These responder definitions provide practical and easily interpreted results that can be used to evaluate treatment benefits over time and to improve clinician-patients communication focus on improvements in key aspects of functioning in individuals with chronic migraine,” Dr. Houts and colleagues wrote in their study, published in the October issue of Headache.
The 6-point value and the 1-2 category improvement values in item-specific scores, they suggested, could be used as a benchmark to help other clinicians and researchers detect meaningful change in individual patients with chronic migraine. Although the user guide for HIT-6 highlights a 5-point change in the total score as clinically meaningful, the authors of the guide do not provide evidence for why the 5-point value signifies clinically meaningful change, they said.
Determining thresholds of clinically meaningful change
In their study, Dr. Houts and colleagues used distribution-based methods to gauge responder values for the HIT-6 total score, while item-specific HIT-6 analyses were measured with Patients’ Global Impression of Change (PGIC), reduction in migraine frequency through monthly migraine days (MMDs), and EuroQol 5 dimensions 5 levels visual analog scale (EQ-5D-5L VAS). The researchers also used HIT-6 values from a literature review and from analyses in PROMISE-2 to calculate “a final chronic migraine-specific responder definition value” between baseline and 12 weeks. Participants in the PROMISE-2 study were mostly women (88.2%) and white (91.0%) with a mean age of 40.5 years.
The literature search revealed responder thresholds for the HIT-6 total score in a range between a decrease of 3 points and 8 points. Within PROMISE-2, the HIT-6 total score responder threshold was found to be between –2.6 and –2.2, which the researchers rounded down to a decrease of 3 points. When taking both sets of responder thresholds into account, the researchers calculated the median responder value as –5.5, which was rounded down to a decrease in 6 points in the HIT-6 total score. “[The estimate] appears most appropriate for discriminating between individuals with chronic migraine who have experienced meaningful change over time and those who have not,” Dr. Houts and colleagues said.
For item-specific HIT-6 scores, the mean score changes were –1 points for categories involving severe pain, limiting activities, lying down, and –2 points for categories involving feeling tired, being fed up or irritated, and limiting concentration.
“Taken together, the current chronic migraine-specific results are consistent with values derived from general headache/migraine samples and suggest that a decrease of 6 points or more on the HIT-6 total score would be considered meaningful to chronic migraine patients,” Dr. Houts and colleagues said. “This would translate to approximately a 4-category change on a single item, change on 2 items of approximately 2 and 3 categories, or a 1-category change on 3 or 4 of the 6 items, depending on the initial category.”
The researchers cautioned that the values outlined in the study “should not be used to determine clinically meaningful difference between treatment groups” and that “future work, similar to that reported here, will identify a chronic migraine-specific clinically meaningful difference between treatment groups value.”
A better measure of chronic migraine?
In an interview, J. D. Bartleson Jr., MD, a retired neurologist with the Mayo Clinic in Rochester, Minn., questioned why HIT-6 criteria was used in the initial PROMISE-2 study. “There is not a lot of difference between the significant and insignificant categories. Chronic migraine may be better measured with pain severity and number of headache days per month,” he said.
,“It may be appropriate to use just 1 or 2 symptoms for evaluating a given patient’s headache burden,” in terms of clinical application of the study for neurologists, Dr. Bartleson said. He emphasized that more research is needed.
This study was funded by H. Lundbeck A/S, which also provided funding of medical writing and editorial support for the manuscript. Three authors report being employees of Vector Psychometric Group at the time of the study, and the company received funding from H. Lundbeck A/S for their time conducting study-related research. Three other authors report relationships with pharmaceutical companies, medical societies, government agencies, and industry related to the study in the form of consultancies, advisory board memberships, honoraria, research support, stock or stock options, and employment. Dr. Bartleson reports no relevant conflicts of interest.
, recent research suggests.
Using data from the phase 3 PROMISE-2 study, which evaluated intravenous eptinezumab in doses of 100 mg or 300 mg, or placebo every 12 weeks in 1,072 participants for the prevention of chronic migraine, Carrie R. Houts, PhD, director of psychometrics at the Vector Psychometric Group, in Chapel Hill, N.C., and colleagues determined that their finding of 6-point improvement of HIT-6 total score was consistent with other studies. However, they pointed out that little research has been done in evaluating how item-specific scores of HIT-6 impact individuals with chronic migraine. HIT-6 item scores examine whether individuals with headaches experience severe pain, limit their daily activities, have a desire to lie down, feel too tired to do daily activities, felt “fed up or irritated” because of headaches, and feel their headaches limit concentration on work or daily activities.
“The item-specific responder definitions give clinicians and researchers the ability to evaluate and track the impact of headache on specific item-level areas of patients’ lives. These responder definitions provide practical and easily interpreted results that can be used to evaluate treatment benefits over time and to improve clinician-patients communication focus on improvements in key aspects of functioning in individuals with chronic migraine,” Dr. Houts and colleagues wrote in their study, published in the October issue of Headache.
The 6-point value and the 1-2 category improvement values in item-specific scores, they suggested, could be used as a benchmark to help other clinicians and researchers detect meaningful change in individual patients with chronic migraine. Although the user guide for HIT-6 highlights a 5-point change in the total score as clinically meaningful, the authors of the guide do not provide evidence for why the 5-point value signifies clinically meaningful change, they said.
Determining thresholds of clinically meaningful change
In their study, Dr. Houts and colleagues used distribution-based methods to gauge responder values for the HIT-6 total score, while item-specific HIT-6 analyses were measured with Patients’ Global Impression of Change (PGIC), reduction in migraine frequency through monthly migraine days (MMDs), and EuroQol 5 dimensions 5 levels visual analog scale (EQ-5D-5L VAS). The researchers also used HIT-6 values from a literature review and from analyses in PROMISE-2 to calculate “a final chronic migraine-specific responder definition value” between baseline and 12 weeks. Participants in the PROMISE-2 study were mostly women (88.2%) and white (91.0%) with a mean age of 40.5 years.
The literature search revealed responder thresholds for the HIT-6 total score in a range between a decrease of 3 points and 8 points. Within PROMISE-2, the HIT-6 total score responder threshold was found to be between –2.6 and –2.2, which the researchers rounded down to a decrease of 3 points. When taking both sets of responder thresholds into account, the researchers calculated the median responder value as –5.5, which was rounded down to a decrease in 6 points in the HIT-6 total score. “[The estimate] appears most appropriate for discriminating between individuals with chronic migraine who have experienced meaningful change over time and those who have not,” Dr. Houts and colleagues said.
For item-specific HIT-6 scores, the mean score changes were –1 points for categories involving severe pain, limiting activities, lying down, and –2 points for categories involving feeling tired, being fed up or irritated, and limiting concentration.
“Taken together, the current chronic migraine-specific results are consistent with values derived from general headache/migraine samples and suggest that a decrease of 6 points or more on the HIT-6 total score would be considered meaningful to chronic migraine patients,” Dr. Houts and colleagues said. “This would translate to approximately a 4-category change on a single item, change on 2 items of approximately 2 and 3 categories, or a 1-category change on 3 or 4 of the 6 items, depending on the initial category.”
The researchers cautioned that the values outlined in the study “should not be used to determine clinically meaningful difference between treatment groups” and that “future work, similar to that reported here, will identify a chronic migraine-specific clinically meaningful difference between treatment groups value.”
A better measure of chronic migraine?
In an interview, J. D. Bartleson Jr., MD, a retired neurologist with the Mayo Clinic in Rochester, Minn., questioned why HIT-6 criteria was used in the initial PROMISE-2 study. “There is not a lot of difference between the significant and insignificant categories. Chronic migraine may be better measured with pain severity and number of headache days per month,” he said.
,“It may be appropriate to use just 1 or 2 symptoms for evaluating a given patient’s headache burden,” in terms of clinical application of the study for neurologists, Dr. Bartleson said. He emphasized that more research is needed.
This study was funded by H. Lundbeck A/S, which also provided funding of medical writing and editorial support for the manuscript. Three authors report being employees of Vector Psychometric Group at the time of the study, and the company received funding from H. Lundbeck A/S for their time conducting study-related research. Three other authors report relationships with pharmaceutical companies, medical societies, government agencies, and industry related to the study in the form of consultancies, advisory board memberships, honoraria, research support, stock or stock options, and employment. Dr. Bartleson reports no relevant conflicts of interest.
, recent research suggests.
Using data from the phase 3 PROMISE-2 study, which evaluated intravenous eptinezumab in doses of 100 mg or 300 mg, or placebo every 12 weeks in 1,072 participants for the prevention of chronic migraine, Carrie R. Houts, PhD, director of psychometrics at the Vector Psychometric Group, in Chapel Hill, N.C., and colleagues determined that their finding of 6-point improvement of HIT-6 total score was consistent with other studies. However, they pointed out that little research has been done in evaluating how item-specific scores of HIT-6 impact individuals with chronic migraine. HIT-6 item scores examine whether individuals with headaches experience severe pain, limit their daily activities, have a desire to lie down, feel too tired to do daily activities, felt “fed up or irritated” because of headaches, and feel their headaches limit concentration on work or daily activities.
“The item-specific responder definitions give clinicians and researchers the ability to evaluate and track the impact of headache on specific item-level areas of patients’ lives. These responder definitions provide practical and easily interpreted results that can be used to evaluate treatment benefits over time and to improve clinician-patients communication focus on improvements in key aspects of functioning in individuals with chronic migraine,” Dr. Houts and colleagues wrote in their study, published in the October issue of Headache.
The 6-point value and the 1-2 category improvement values in item-specific scores, they suggested, could be used as a benchmark to help other clinicians and researchers detect meaningful change in individual patients with chronic migraine. Although the user guide for HIT-6 highlights a 5-point change in the total score as clinically meaningful, the authors of the guide do not provide evidence for why the 5-point value signifies clinically meaningful change, they said.
Determining thresholds of clinically meaningful change
In their study, Dr. Houts and colleagues used distribution-based methods to gauge responder values for the HIT-6 total score, while item-specific HIT-6 analyses were measured with Patients’ Global Impression of Change (PGIC), reduction in migraine frequency through monthly migraine days (MMDs), and EuroQol 5 dimensions 5 levels visual analog scale (EQ-5D-5L VAS). The researchers also used HIT-6 values from a literature review and from analyses in PROMISE-2 to calculate “a final chronic migraine-specific responder definition value” between baseline and 12 weeks. Participants in the PROMISE-2 study were mostly women (88.2%) and white (91.0%) with a mean age of 40.5 years.
The literature search revealed responder thresholds for the HIT-6 total score in a range between a decrease of 3 points and 8 points. Within PROMISE-2, the HIT-6 total score responder threshold was found to be between –2.6 and –2.2, which the researchers rounded down to a decrease of 3 points. When taking both sets of responder thresholds into account, the researchers calculated the median responder value as –5.5, which was rounded down to a decrease in 6 points in the HIT-6 total score. “[The estimate] appears most appropriate for discriminating between individuals with chronic migraine who have experienced meaningful change over time and those who have not,” Dr. Houts and colleagues said.
For item-specific HIT-6 scores, the mean score changes were –1 points for categories involving severe pain, limiting activities, lying down, and –2 points for categories involving feeling tired, being fed up or irritated, and limiting concentration.
“Taken together, the current chronic migraine-specific results are consistent with values derived from general headache/migraine samples and suggest that a decrease of 6 points or more on the HIT-6 total score would be considered meaningful to chronic migraine patients,” Dr. Houts and colleagues said. “This would translate to approximately a 4-category change on a single item, change on 2 items of approximately 2 and 3 categories, or a 1-category change on 3 or 4 of the 6 items, depending on the initial category.”
The researchers cautioned that the values outlined in the study “should not be used to determine clinically meaningful difference between treatment groups” and that “future work, similar to that reported here, will identify a chronic migraine-specific clinically meaningful difference between treatment groups value.”
A better measure of chronic migraine?
In an interview, J. D. Bartleson Jr., MD, a retired neurologist with the Mayo Clinic in Rochester, Minn., questioned why HIT-6 criteria was used in the initial PROMISE-2 study. “There is not a lot of difference between the significant and insignificant categories. Chronic migraine may be better measured with pain severity and number of headache days per month,” he said.
,“It may be appropriate to use just 1 or 2 symptoms for evaluating a given patient’s headache burden,” in terms of clinical application of the study for neurologists, Dr. Bartleson said. He emphasized that more research is needed.
This study was funded by H. Lundbeck A/S, which also provided funding of medical writing and editorial support for the manuscript. Three authors report being employees of Vector Psychometric Group at the time of the study, and the company received funding from H. Lundbeck A/S for their time conducting study-related research. Three other authors report relationships with pharmaceutical companies, medical societies, government agencies, and industry related to the study in the form of consultancies, advisory board memberships, honoraria, research support, stock or stock options, and employment. Dr. Bartleson reports no relevant conflicts of interest.
FROM HEADACHE
COVID-19: A second wave of mental illness 'imminent'
The mental health consequences of COVID-19 deaths are likely to overwhelm an already tattered U.S. mental health system, leading to a lack of access, particularly for the most vulnerable, experts warn.
“A second wave of devastation is imminent, attributable to mental health consequences of COVID-19,” write Naomi Simon, MD, and coauthors with the department of psychiatry, New York University.
In a Viewpoint article published in JAMA on Oct. 12, physicians offer some sobering statistics.
Since February 2020, COVID-19 has taken the lives of more than 214,000 Americans. The number of deaths currently attributed to the virus is nearly four times the number of Americans killed during the Vietnam War. The magnitude of death over a short period is a tragedy on a “historic scale,” wrote Dr. Simon and colleagues.
The surge in mental health problems related to COVID-19 deaths will bring further challenges to individuals, families, and communities, including a spike in deaths from suicide and drug overdoses, they warned.
It’s important to consider, they noted, that each COVID-19 death leaves an estimated nine family members bereaved, which is projected to lead to an estimated 2 million bereaved individuals in the United States.
The necessary social distancing and quarantine measures implemented to fight the virus have amplified emotional turmoil and have disrupted the ability of personal support networks and communities to come together and grieve.
“Of central concern is the transformation of normal grief and distress into prolonged grief and major depressive disorder and symptoms of posttraumatic stress disorder,” Simon and colleagues said.
“Once established, these conditions can become chronic with additional comorbidities such as substance use disorders. Prolonged grief affects approximately 10% of bereaved individuals, but this is likely an underestimate for grief related to deaths from COVID-19,” they wrote.
As with the first COVID-19 wave, the mental health wave will disproportionately affect Black persons, Hispanic persons, older adults, persons in lower socioeconomic groups of all races and ethnicities, and healthcare workers, they note.
The psychological risks for health care and other essential workers are of particular concern, they say. “Supporting the mental health of these and other essential workforce is critical to readiness for managing recurrent waves of the pandemic,” they stated.
How will the United States manage this impending wave of mental health problems?
“The solution will require increased funding for mental health; widespread screening to identify individuals at highest risk including suicide risk; availability of primary care clinicians and mental health professionals trained to treat those with prolonged grief, depression, traumatic stress, and substance abuse; and a diligent focus on families and communities to creatively restore the approaches by which they have managed tragedy and loss over generations,” the authors wrote.
“History has shown that societies recover from such devastation when leaders and members are joined by a shared purpose, acting in a unified way to facilitate recovery. In such societies, there is a shared understanding that its members must care for one another because the loss of one is a loss for all. Above all, this shared understanding must be restored,” they concluded.
Dr. Simon has received personal fees from Vanda Pharmaceuticals Inc, MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind, and Wiley (deputy editor, Depression and Anxiety). Saxe has received royalties from Guilford Press for the book Trauma Systems Therapy for Children and Teens (2016). Marmar serves on the scientific advisory board and owns equity in Receptor Life Sciences and serves on the PTSD advisory board for Otsuka Pharmaceutical.
A version of this article originally appeared on Medscape.com.
The mental health consequences of COVID-19 deaths are likely to overwhelm an already tattered U.S. mental health system, leading to a lack of access, particularly for the most vulnerable, experts warn.
“A second wave of devastation is imminent, attributable to mental health consequences of COVID-19,” write Naomi Simon, MD, and coauthors with the department of psychiatry, New York University.
In a Viewpoint article published in JAMA on Oct. 12, physicians offer some sobering statistics.
Since February 2020, COVID-19 has taken the lives of more than 214,000 Americans. The number of deaths currently attributed to the virus is nearly four times the number of Americans killed during the Vietnam War. The magnitude of death over a short period is a tragedy on a “historic scale,” wrote Dr. Simon and colleagues.
The surge in mental health problems related to COVID-19 deaths will bring further challenges to individuals, families, and communities, including a spike in deaths from suicide and drug overdoses, they warned.
It’s important to consider, they noted, that each COVID-19 death leaves an estimated nine family members bereaved, which is projected to lead to an estimated 2 million bereaved individuals in the United States.
The necessary social distancing and quarantine measures implemented to fight the virus have amplified emotional turmoil and have disrupted the ability of personal support networks and communities to come together and grieve.
“Of central concern is the transformation of normal grief and distress into prolonged grief and major depressive disorder and symptoms of posttraumatic stress disorder,” Simon and colleagues said.
“Once established, these conditions can become chronic with additional comorbidities such as substance use disorders. Prolonged grief affects approximately 10% of bereaved individuals, but this is likely an underestimate for grief related to deaths from COVID-19,” they wrote.
As with the first COVID-19 wave, the mental health wave will disproportionately affect Black persons, Hispanic persons, older adults, persons in lower socioeconomic groups of all races and ethnicities, and healthcare workers, they note.
The psychological risks for health care and other essential workers are of particular concern, they say. “Supporting the mental health of these and other essential workforce is critical to readiness for managing recurrent waves of the pandemic,” they stated.
How will the United States manage this impending wave of mental health problems?
“The solution will require increased funding for mental health; widespread screening to identify individuals at highest risk including suicide risk; availability of primary care clinicians and mental health professionals trained to treat those with prolonged grief, depression, traumatic stress, and substance abuse; and a diligent focus on families and communities to creatively restore the approaches by which they have managed tragedy and loss over generations,” the authors wrote.
“History has shown that societies recover from such devastation when leaders and members are joined by a shared purpose, acting in a unified way to facilitate recovery. In such societies, there is a shared understanding that its members must care for one another because the loss of one is a loss for all. Above all, this shared understanding must be restored,” they concluded.
Dr. Simon has received personal fees from Vanda Pharmaceuticals Inc, MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind, and Wiley (deputy editor, Depression and Anxiety). Saxe has received royalties from Guilford Press for the book Trauma Systems Therapy for Children and Teens (2016). Marmar serves on the scientific advisory board and owns equity in Receptor Life Sciences and serves on the PTSD advisory board for Otsuka Pharmaceutical.
A version of this article originally appeared on Medscape.com.
The mental health consequences of COVID-19 deaths are likely to overwhelm an already tattered U.S. mental health system, leading to a lack of access, particularly for the most vulnerable, experts warn.
“A second wave of devastation is imminent, attributable to mental health consequences of COVID-19,” write Naomi Simon, MD, and coauthors with the department of psychiatry, New York University.
In a Viewpoint article published in JAMA on Oct. 12, physicians offer some sobering statistics.
Since February 2020, COVID-19 has taken the lives of more than 214,000 Americans. The number of deaths currently attributed to the virus is nearly four times the number of Americans killed during the Vietnam War. The magnitude of death over a short period is a tragedy on a “historic scale,” wrote Dr. Simon and colleagues.
The surge in mental health problems related to COVID-19 deaths will bring further challenges to individuals, families, and communities, including a spike in deaths from suicide and drug overdoses, they warned.
It’s important to consider, they noted, that each COVID-19 death leaves an estimated nine family members bereaved, which is projected to lead to an estimated 2 million bereaved individuals in the United States.
The necessary social distancing and quarantine measures implemented to fight the virus have amplified emotional turmoil and have disrupted the ability of personal support networks and communities to come together and grieve.
“Of central concern is the transformation of normal grief and distress into prolonged grief and major depressive disorder and symptoms of posttraumatic stress disorder,” Simon and colleagues said.
“Once established, these conditions can become chronic with additional comorbidities such as substance use disorders. Prolonged grief affects approximately 10% of bereaved individuals, but this is likely an underestimate for grief related to deaths from COVID-19,” they wrote.
As with the first COVID-19 wave, the mental health wave will disproportionately affect Black persons, Hispanic persons, older adults, persons in lower socioeconomic groups of all races and ethnicities, and healthcare workers, they note.
The psychological risks for health care and other essential workers are of particular concern, they say. “Supporting the mental health of these and other essential workforce is critical to readiness for managing recurrent waves of the pandemic,” they stated.
How will the United States manage this impending wave of mental health problems?
“The solution will require increased funding for mental health; widespread screening to identify individuals at highest risk including suicide risk; availability of primary care clinicians and mental health professionals trained to treat those with prolonged grief, depression, traumatic stress, and substance abuse; and a diligent focus on families and communities to creatively restore the approaches by which they have managed tragedy and loss over generations,” the authors wrote.
“History has shown that societies recover from such devastation when leaders and members are joined by a shared purpose, acting in a unified way to facilitate recovery. In such societies, there is a shared understanding that its members must care for one another because the loss of one is a loss for all. Above all, this shared understanding must be restored,” they concluded.
Dr. Simon has received personal fees from Vanda Pharmaceuticals Inc, MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind, and Wiley (deputy editor, Depression and Anxiety). Saxe has received royalties from Guilford Press for the book Trauma Systems Therapy for Children and Teens (2016). Marmar serves on the scientific advisory board and owns equity in Receptor Life Sciences and serves on the PTSD advisory board for Otsuka Pharmaceutical.
A version of this article originally appeared on Medscape.com.