User login
COVID-19 may damage blood vessels in the brain
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Patients fend for themselves to access highly touted COVID antibody treatments
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Allo-HSCT improves disease-free, but not overall survival in adults with ALL, compared with ped-inspired chemo
Allogeneic hematopoietic stem-cell transplantation (AHSCT) improved disease-free survival (DFS), compared with pediatric-inspired Berlin-Frankfurt-Münster (BFM-95) chemotherapy in adults with acute lymphoblastic leukemia (ALL), according to the results of retrospective study published in Clinical Lymphoma, Myeloma & Leukemia. However, overall survival (OS) was not significantly different between the two groups, as reported by Elifcan Aladag, MD, of the Hacettepe University Faculty of Medicine, Ankara, Turkey, and colleagues.
Despite this, “AHSCT is recommended for all patients with suitable donors, but the risk of transplant-related mortality should be kept in mind,” according to the researchers.
The multicenter study compared two different treatment approaches (BFM-95 chemotherapy regimen and AHSCT). The BFM-95 chemotherapy group comprised 47 newly diagnosed ALL patients. The transplant cohort comprised 83 patients with ALL in first complete remission who received AHSCT from fully matched human leukocyte antigen (HLA)-identical siblings. Thirty-five of the AHSCT patients (42.1%) received chemotherapy at least until the M stage of the BFM-95 protocol.
The primary endpoints of the study were OS and duration of DFS. OS was defined from the day of starting BFM-95 chemotherapy until death from any cause, and DFS was calculated from the date of complete remission until the date of first relapse or death from any cause, whichever occurred first, according to the authors.
Study results
The median OS was 68 months in patients who underwent AHSCT and 46 months in patients treated only with BFM-95 (P = .3). Two- and 5-year OS rates were 78% and 60% , respectively, in the AHSCT group, and 69% and 64% in the BFM-95 group (P = .06 and .13, respectively).
The median DFS was 36.6 months in patients who underwent AHSCT and 28 months in patients treated with BFM-95 (P = .033). Two- and 5-year DFS rates were 68.5% and 57%, respectively, in the AHSCT group, and 63% and 38% respectively, in the BFM-95 group (P = .12 and .029, respectively).
Mortality in the BFM-95 group was the result of sepsis due to infections (fungal infection in two patients, resistant bacterial infections in four patients). In the AHSCT group, respectively, three and seven patients died of graft-versus-host disease and bacterial infections (with fungal infections in four patients and resistant bacterial infections in three patients), according to the researchers.
“In our study, no 2-year OS and DFS difference was observed in any treatment group; however, a significant difference occurred in 5-year DFS in favor of AHSCT. This may be due to transplant-related mortality in the first 2 years, which led to no statistically significant difference,” the authors stated.
“In order to further elucidate the role of AHSCT when pediatric-derived regimens are used for the treatment of adult lymphoblastic leukemia, higher-powered randomized prospective studies are needed,” they concluded.
The authors reported that they had no conflicts of interest.
Allogeneic hematopoietic stem-cell transplantation (AHSCT) improved disease-free survival (DFS), compared with pediatric-inspired Berlin-Frankfurt-Münster (BFM-95) chemotherapy in adults with acute lymphoblastic leukemia (ALL), according to the results of retrospective study published in Clinical Lymphoma, Myeloma & Leukemia. However, overall survival (OS) was not significantly different between the two groups, as reported by Elifcan Aladag, MD, of the Hacettepe University Faculty of Medicine, Ankara, Turkey, and colleagues.
Despite this, “AHSCT is recommended for all patients with suitable donors, but the risk of transplant-related mortality should be kept in mind,” according to the researchers.
The multicenter study compared two different treatment approaches (BFM-95 chemotherapy regimen and AHSCT). The BFM-95 chemotherapy group comprised 47 newly diagnosed ALL patients. The transplant cohort comprised 83 patients with ALL in first complete remission who received AHSCT from fully matched human leukocyte antigen (HLA)-identical siblings. Thirty-five of the AHSCT patients (42.1%) received chemotherapy at least until the M stage of the BFM-95 protocol.
The primary endpoints of the study were OS and duration of DFS. OS was defined from the day of starting BFM-95 chemotherapy until death from any cause, and DFS was calculated from the date of complete remission until the date of first relapse or death from any cause, whichever occurred first, according to the authors.
Study results
The median OS was 68 months in patients who underwent AHSCT and 46 months in patients treated only with BFM-95 (P = .3). Two- and 5-year OS rates were 78% and 60% , respectively, in the AHSCT group, and 69% and 64% in the BFM-95 group (P = .06 and .13, respectively).
The median DFS was 36.6 months in patients who underwent AHSCT and 28 months in patients treated with BFM-95 (P = .033). Two- and 5-year DFS rates were 68.5% and 57%, respectively, in the AHSCT group, and 63% and 38% respectively, in the BFM-95 group (P = .12 and .029, respectively).
Mortality in the BFM-95 group was the result of sepsis due to infections (fungal infection in two patients, resistant bacterial infections in four patients). In the AHSCT group, respectively, three and seven patients died of graft-versus-host disease and bacterial infections (with fungal infections in four patients and resistant bacterial infections in three patients), according to the researchers.
“In our study, no 2-year OS and DFS difference was observed in any treatment group; however, a significant difference occurred in 5-year DFS in favor of AHSCT. This may be due to transplant-related mortality in the first 2 years, which led to no statistically significant difference,” the authors stated.
“In order to further elucidate the role of AHSCT when pediatric-derived regimens are used for the treatment of adult lymphoblastic leukemia, higher-powered randomized prospective studies are needed,” they concluded.
The authors reported that they had no conflicts of interest.
Allogeneic hematopoietic stem-cell transplantation (AHSCT) improved disease-free survival (DFS), compared with pediatric-inspired Berlin-Frankfurt-Münster (BFM-95) chemotherapy in adults with acute lymphoblastic leukemia (ALL), according to the results of retrospective study published in Clinical Lymphoma, Myeloma & Leukemia. However, overall survival (OS) was not significantly different between the two groups, as reported by Elifcan Aladag, MD, of the Hacettepe University Faculty of Medicine, Ankara, Turkey, and colleagues.
Despite this, “AHSCT is recommended for all patients with suitable donors, but the risk of transplant-related mortality should be kept in mind,” according to the researchers.
The multicenter study compared two different treatment approaches (BFM-95 chemotherapy regimen and AHSCT). The BFM-95 chemotherapy group comprised 47 newly diagnosed ALL patients. The transplant cohort comprised 83 patients with ALL in first complete remission who received AHSCT from fully matched human leukocyte antigen (HLA)-identical siblings. Thirty-five of the AHSCT patients (42.1%) received chemotherapy at least until the M stage of the BFM-95 protocol.
The primary endpoints of the study were OS and duration of DFS. OS was defined from the day of starting BFM-95 chemotherapy until death from any cause, and DFS was calculated from the date of complete remission until the date of first relapse or death from any cause, whichever occurred first, according to the authors.
Study results
The median OS was 68 months in patients who underwent AHSCT and 46 months in patients treated only with BFM-95 (P = .3). Two- and 5-year OS rates were 78% and 60% , respectively, in the AHSCT group, and 69% and 64% in the BFM-95 group (P = .06 and .13, respectively).
The median DFS was 36.6 months in patients who underwent AHSCT and 28 months in patients treated with BFM-95 (P = .033). Two- and 5-year DFS rates were 68.5% and 57%, respectively, in the AHSCT group, and 63% and 38% respectively, in the BFM-95 group (P = .12 and .029, respectively).
Mortality in the BFM-95 group was the result of sepsis due to infections (fungal infection in two patients, resistant bacterial infections in four patients). In the AHSCT group, respectively, three and seven patients died of graft-versus-host disease and bacterial infections (with fungal infections in four patients and resistant bacterial infections in three patients), according to the researchers.
“In our study, no 2-year OS and DFS difference was observed in any treatment group; however, a significant difference occurred in 5-year DFS in favor of AHSCT. This may be due to transplant-related mortality in the first 2 years, which led to no statistically significant difference,” the authors stated.
“In order to further elucidate the role of AHSCT when pediatric-derived regimens are used for the treatment of adult lymphoblastic leukemia, higher-powered randomized prospective studies are needed,” they concluded.
The authors reported that they had no conflicts of interest.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Higher intensity therapy doesn’t increase surgical risk in esophageal cancer
The trial included patients with clinical stage IB, II, or III (non-T4) thoracic esophageal cancer randomly assigned to cisplatin plus 5-fluorouracil (CF), CF plus radiotherapy (CF-RT), or docetaxel plus CF (DCF) prior to surgery.
Results showed the type of therapy did not significantly affect risk for either perioperative complications or deaths. There was also evidence to suggest that a lower risk of postoperative complications with DCF compared with CF might translate into improved prognosis with the addition of docetaxel, said Kazuo Koyanagi, MD, PhD, of National Cancer Center Hospital in Tokyo.
Dr. Koyanagi presented these results at the 2021 Gastrointestinal Cancers Symposium.
“Based on these results, we could say that preoperative chemotherapy with DCF and CF-RT didn’t increase the risk of postoperative complications when compared with standard CF, and whether the decrease in the risk in the DCF would be reflected in the improvement of prognosis should be examined in the future,” Dr. Koyanagi said.
Trial details
The JCOG1109 trial is a three-arm, phase 3 trial designed to see whether adding docetaxel or radiation to CF could improve outcomes. In the analysis presented here, the investigators examined whether the choice of regimen could affect the safety of esophagectomy, and they looked for risk factors for postoperative complications.
Patients with histologically proven squamous cell, adenosquamous, or basaloid carcinoma with locally advanced lesions in the thoracic esophagus were eligible.
The patients had to have good performance status (Eastern Cooperative Oncology Group 0 or 1) and could not have had chemotherapy, radiotherapy, or hormonal therapy for any cancer, or prior therapy for esophageal cancer except for complete endoscopic mucosal or submucosal dissection.
A total of 601 patients were enrolled and randomized to receive one of the following treatments:
- CF, with cisplatin at a dose of 80 mg/m2 on day 1 and 5-fluorouracil at 800 mg/m2 on days 1-5 every 3 weeks for two cycles (199 assigned; 185 had surgery)
- DCF, with cisplatin at 70 mg/m2 on day 1, 5-fluorouracil at 750 mg/m2 on days 1-5, and docetaxel at 70 mg/m2 on day 1 every 3 weeks for three cycles (202 assigned; 183 had surgery)
- CF-RT, with cisplatin at 75 mg/m2 on day 1, 5-fluorouracil at 1,000 mg/m2 on days 1-4 every 4 weeks for two cycles, plus 1.8 Gy radiation divided into 23 fractions for a total of 41.4 Gy (200 assigned; 178 had surgery).
Patient age, body mass index, tumor location, clinical stage and node status were comparable among the treatment groups.
Operative characteristics (duration, blood loss, approach, extent of lymph node dissection) were generally similar between the arms as well, except that significantly fewer lymph nodes were harvested with CF-RT compared with either CF or DCF (median of 49, 58, and 59, respectively).
Results
Incidence rates of major postoperative complications – pneumonia, leakage, and recurrent laryngeal nerve paralysis – were generally similar among the groups.
The cumulative rate of grade 2 or greater postoperative complications was significantly lower for DCF than for CF (P = .02), but not for DCF compared with CF-RT (P = .11). The rates were 43.7% with DCF, 47.8% with CF-RT, and 56.2% with CF.
The rate of grade 2 or greater chylothorax (leakage of lymphatics into the pleural space) was significantly higher with CF-RT than CF (5.1% vs. 1.1%, P = .03) but not with DCF vs. CF (3.8% vs. 1.1%, P = .10)
In multivariable analysis controlling for demographic, clinical, and operative characteristics, factors associated with lower risk for complications included:
- Middle esophageal tumor location vs. upper esophageal tumors (relative risk [RR], 0.79; P = .03)
- DCF (RR, 0.79; P = .02)
- A thoracoscopic vs. open approach (RR, 0.77; P = .002).
The only factor associated with higher risk was operative time longer than 492 minutes (RR, 1.26; P = .008).
Dr. Koyanagi said the reasons for the lower lymph node yield and more frequent chylothorax with CF-RT are unclear but may be related to tissue fibrosis from radiation exposure.
CROSS talk
“As a North American surgeon, I generally look to CROSS induction chemotherapy for the majority of my patients for both adenocarcinoma and squamous cell carcinoma of the esophagus,” said invited discussant Jonathan Yeung, MD, PhD, of the Princess Margaret Cancer Centre in Toronto.
The CROSS regimen consists of carboplatin titrated to an area under the curve of 2 mg/mL per minute and paclitaxel at 50 mg/m2 for 5 weeks with concurrent radiotherapy to a total dose of 41.4 Gy delivered in 23 fractions, 5 days per week.
Dr. Yeung noted that, of the eligible patients in JCOG1109, 92% of those assigned to DCF actually underwent surgery, and 90% of those assigned to CF-RT went on to surgery, compared with 98% of patients who had surgery in the CROSS trial, suggesting that the DCF and CF-RT regimens may be more toxic.
He also noted that the lower lymph node harvest seen with CF-RT was seen in other studies.
“I must say I’m always impressed by the lymph node yields that our Japanese colleagues can obtain at surgery, but this lower lymph node yield is also borne out in the CROSS data, where there are less lymph nodes harvested following chemoradiotherapy,” he said.
A higher rate of chylothorax with CF-RT was also seen in patients in the CROSS trial who were randomized to receive radiation compared with those who received chemotherapy alone.
“I await the final results to see if there is ultimately better survival,” Dr. Yeung said.
JCOG1109 was supported by grants from the National Cancer Center Research and Development Funds and Agency for Medical Research and Development of Japan. Dr. Koyanagi and Dr. Yeung reported no conflicts of interest.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The trial included patients with clinical stage IB, II, or III (non-T4) thoracic esophageal cancer randomly assigned to cisplatin plus 5-fluorouracil (CF), CF plus radiotherapy (CF-RT), or docetaxel plus CF (DCF) prior to surgery.
Results showed the type of therapy did not significantly affect risk for either perioperative complications or deaths. There was also evidence to suggest that a lower risk of postoperative complications with DCF compared with CF might translate into improved prognosis with the addition of docetaxel, said Kazuo Koyanagi, MD, PhD, of National Cancer Center Hospital in Tokyo.
Dr. Koyanagi presented these results at the 2021 Gastrointestinal Cancers Symposium.
“Based on these results, we could say that preoperative chemotherapy with DCF and CF-RT didn’t increase the risk of postoperative complications when compared with standard CF, and whether the decrease in the risk in the DCF would be reflected in the improvement of prognosis should be examined in the future,” Dr. Koyanagi said.
Trial details
The JCOG1109 trial is a three-arm, phase 3 trial designed to see whether adding docetaxel or radiation to CF could improve outcomes. In the analysis presented here, the investigators examined whether the choice of regimen could affect the safety of esophagectomy, and they looked for risk factors for postoperative complications.
Patients with histologically proven squamous cell, adenosquamous, or basaloid carcinoma with locally advanced lesions in the thoracic esophagus were eligible.
The patients had to have good performance status (Eastern Cooperative Oncology Group 0 or 1) and could not have had chemotherapy, radiotherapy, or hormonal therapy for any cancer, or prior therapy for esophageal cancer except for complete endoscopic mucosal or submucosal dissection.
A total of 601 patients were enrolled and randomized to receive one of the following treatments:
- CF, with cisplatin at a dose of 80 mg/m2 on day 1 and 5-fluorouracil at 800 mg/m2 on days 1-5 every 3 weeks for two cycles (199 assigned; 185 had surgery)
- DCF, with cisplatin at 70 mg/m2 on day 1, 5-fluorouracil at 750 mg/m2 on days 1-5, and docetaxel at 70 mg/m2 on day 1 every 3 weeks for three cycles (202 assigned; 183 had surgery)
- CF-RT, with cisplatin at 75 mg/m2 on day 1, 5-fluorouracil at 1,000 mg/m2 on days 1-4 every 4 weeks for two cycles, plus 1.8 Gy radiation divided into 23 fractions for a total of 41.4 Gy (200 assigned; 178 had surgery).
Patient age, body mass index, tumor location, clinical stage and node status were comparable among the treatment groups.
Operative characteristics (duration, blood loss, approach, extent of lymph node dissection) were generally similar between the arms as well, except that significantly fewer lymph nodes were harvested with CF-RT compared with either CF or DCF (median of 49, 58, and 59, respectively).
Results
Incidence rates of major postoperative complications – pneumonia, leakage, and recurrent laryngeal nerve paralysis – were generally similar among the groups.
The cumulative rate of grade 2 or greater postoperative complications was significantly lower for DCF than for CF (P = .02), but not for DCF compared with CF-RT (P = .11). The rates were 43.7% with DCF, 47.8% with CF-RT, and 56.2% with CF.
The rate of grade 2 or greater chylothorax (leakage of lymphatics into the pleural space) was significantly higher with CF-RT than CF (5.1% vs. 1.1%, P = .03) but not with DCF vs. CF (3.8% vs. 1.1%, P = .10)
In multivariable analysis controlling for demographic, clinical, and operative characteristics, factors associated with lower risk for complications included:
- Middle esophageal tumor location vs. upper esophageal tumors (relative risk [RR], 0.79; P = .03)
- DCF (RR, 0.79; P = .02)
- A thoracoscopic vs. open approach (RR, 0.77; P = .002).
The only factor associated with higher risk was operative time longer than 492 minutes (RR, 1.26; P = .008).
Dr. Koyanagi said the reasons for the lower lymph node yield and more frequent chylothorax with CF-RT are unclear but may be related to tissue fibrosis from radiation exposure.
CROSS talk
“As a North American surgeon, I generally look to CROSS induction chemotherapy for the majority of my patients for both adenocarcinoma and squamous cell carcinoma of the esophagus,” said invited discussant Jonathan Yeung, MD, PhD, of the Princess Margaret Cancer Centre in Toronto.
The CROSS regimen consists of carboplatin titrated to an area under the curve of 2 mg/mL per minute and paclitaxel at 50 mg/m2 for 5 weeks with concurrent radiotherapy to a total dose of 41.4 Gy delivered in 23 fractions, 5 days per week.
Dr. Yeung noted that, of the eligible patients in JCOG1109, 92% of those assigned to DCF actually underwent surgery, and 90% of those assigned to CF-RT went on to surgery, compared with 98% of patients who had surgery in the CROSS trial, suggesting that the DCF and CF-RT regimens may be more toxic.
He also noted that the lower lymph node harvest seen with CF-RT was seen in other studies.
“I must say I’m always impressed by the lymph node yields that our Japanese colleagues can obtain at surgery, but this lower lymph node yield is also borne out in the CROSS data, where there are less lymph nodes harvested following chemoradiotherapy,” he said.
A higher rate of chylothorax with CF-RT was also seen in patients in the CROSS trial who were randomized to receive radiation compared with those who received chemotherapy alone.
“I await the final results to see if there is ultimately better survival,” Dr. Yeung said.
JCOG1109 was supported by grants from the National Cancer Center Research and Development Funds and Agency for Medical Research and Development of Japan. Dr. Koyanagi and Dr. Yeung reported no conflicts of interest.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The trial included patients with clinical stage IB, II, or III (non-T4) thoracic esophageal cancer randomly assigned to cisplatin plus 5-fluorouracil (CF), CF plus radiotherapy (CF-RT), or docetaxel plus CF (DCF) prior to surgery.
Results showed the type of therapy did not significantly affect risk for either perioperative complications or deaths. There was also evidence to suggest that a lower risk of postoperative complications with DCF compared with CF might translate into improved prognosis with the addition of docetaxel, said Kazuo Koyanagi, MD, PhD, of National Cancer Center Hospital in Tokyo.
Dr. Koyanagi presented these results at the 2021 Gastrointestinal Cancers Symposium.
“Based on these results, we could say that preoperative chemotherapy with DCF and CF-RT didn’t increase the risk of postoperative complications when compared with standard CF, and whether the decrease in the risk in the DCF would be reflected in the improvement of prognosis should be examined in the future,” Dr. Koyanagi said.
Trial details
The JCOG1109 trial is a three-arm, phase 3 trial designed to see whether adding docetaxel or radiation to CF could improve outcomes. In the analysis presented here, the investigators examined whether the choice of regimen could affect the safety of esophagectomy, and they looked for risk factors for postoperative complications.
Patients with histologically proven squamous cell, adenosquamous, or basaloid carcinoma with locally advanced lesions in the thoracic esophagus were eligible.
The patients had to have good performance status (Eastern Cooperative Oncology Group 0 or 1) and could not have had chemotherapy, radiotherapy, or hormonal therapy for any cancer, or prior therapy for esophageal cancer except for complete endoscopic mucosal or submucosal dissection.
A total of 601 patients were enrolled and randomized to receive one of the following treatments:
- CF, with cisplatin at a dose of 80 mg/m2 on day 1 and 5-fluorouracil at 800 mg/m2 on days 1-5 every 3 weeks for two cycles (199 assigned; 185 had surgery)
- DCF, with cisplatin at 70 mg/m2 on day 1, 5-fluorouracil at 750 mg/m2 on days 1-5, and docetaxel at 70 mg/m2 on day 1 every 3 weeks for three cycles (202 assigned; 183 had surgery)
- CF-RT, with cisplatin at 75 mg/m2 on day 1, 5-fluorouracil at 1,000 mg/m2 on days 1-4 every 4 weeks for two cycles, plus 1.8 Gy radiation divided into 23 fractions for a total of 41.4 Gy (200 assigned; 178 had surgery).
Patient age, body mass index, tumor location, clinical stage and node status were comparable among the treatment groups.
Operative characteristics (duration, blood loss, approach, extent of lymph node dissection) were generally similar between the arms as well, except that significantly fewer lymph nodes were harvested with CF-RT compared with either CF or DCF (median of 49, 58, and 59, respectively).
Results
Incidence rates of major postoperative complications – pneumonia, leakage, and recurrent laryngeal nerve paralysis – were generally similar among the groups.
The cumulative rate of grade 2 or greater postoperative complications was significantly lower for DCF than for CF (P = .02), but not for DCF compared with CF-RT (P = .11). The rates were 43.7% with DCF, 47.8% with CF-RT, and 56.2% with CF.
The rate of grade 2 or greater chylothorax (leakage of lymphatics into the pleural space) was significantly higher with CF-RT than CF (5.1% vs. 1.1%, P = .03) but not with DCF vs. CF (3.8% vs. 1.1%, P = .10)
In multivariable analysis controlling for demographic, clinical, and operative characteristics, factors associated with lower risk for complications included:
- Middle esophageal tumor location vs. upper esophageal tumors (relative risk [RR], 0.79; P = .03)
- DCF (RR, 0.79; P = .02)
- A thoracoscopic vs. open approach (RR, 0.77; P = .002).
The only factor associated with higher risk was operative time longer than 492 minutes (RR, 1.26; P = .008).
Dr. Koyanagi said the reasons for the lower lymph node yield and more frequent chylothorax with CF-RT are unclear but may be related to tissue fibrosis from radiation exposure.
CROSS talk
“As a North American surgeon, I generally look to CROSS induction chemotherapy for the majority of my patients for both adenocarcinoma and squamous cell carcinoma of the esophagus,” said invited discussant Jonathan Yeung, MD, PhD, of the Princess Margaret Cancer Centre in Toronto.
The CROSS regimen consists of carboplatin titrated to an area under the curve of 2 mg/mL per minute and paclitaxel at 50 mg/m2 for 5 weeks with concurrent radiotherapy to a total dose of 41.4 Gy delivered in 23 fractions, 5 days per week.
Dr. Yeung noted that, of the eligible patients in JCOG1109, 92% of those assigned to DCF actually underwent surgery, and 90% of those assigned to CF-RT went on to surgery, compared with 98% of patients who had surgery in the CROSS trial, suggesting that the DCF and CF-RT regimens may be more toxic.
He also noted that the lower lymph node harvest seen with CF-RT was seen in other studies.
“I must say I’m always impressed by the lymph node yields that our Japanese colleagues can obtain at surgery, but this lower lymph node yield is also borne out in the CROSS data, where there are less lymph nodes harvested following chemoradiotherapy,” he said.
A higher rate of chylothorax with CF-RT was also seen in patients in the CROSS trial who were randomized to receive radiation compared with those who received chemotherapy alone.
“I await the final results to see if there is ultimately better survival,” Dr. Yeung said.
JCOG1109 was supported by grants from the National Cancer Center Research and Development Funds and Agency for Medical Research and Development of Japan. Dr. Koyanagi and Dr. Yeung reported no conflicts of interest.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM GI Cancers Symposium 2021
No benefit seen with radiotherapy in borderline resectable pancreatic cancer
Patients with borderline resectable pancreatic ductal adenocarcinoma (PDAC) are often treated with chemotherapy, radiotherapy, or both before undergoing surgery, but the optimal regimen in this setting is controversial.
Results from the Alliance A021501 study suggest the reference regimen should be neoadjuvant therapy with modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, leucovorin 400 mg/m2, and infusional 5-fluorouracil 2,400 mg/m2 over 46 hours), according to Matthew Katz, MD, FACS, chief of the pancreatic surgery service at the University of Texas MD Anderson Cancer Center, Houston.
This regimen improved survival for patients with PDAC, relative to historical data. For patients who received mFOLFIRINOX, the overall 18-month survival rate was 66.4%. However, when this regimen was combined with hypofractionated radiotherapy, the survival benefit was significantly lower, at 47.3%.
These findings were presented at the 2021 Gastrointestinal Cancers Symposium (GICS), which was held online this year.
“ASCO guidelines recommend that preoperative therapy be administered to patients with localized pancreatic adenocarcinoma who have tumors that have a significant radiographic interface with the major mesenteric blood vessels, as these patients are at high risk for a margin-positive operation and short survival when pancreatectomy is performed de novo,” Dr. Katz explained.
Although both chemotherapy and radiotherapy are used in these patients, there is no consensus as to the best approach, he commented.
The goal of Alliance A021501 “was to define a reference preoperative regimen for future trials of preoperative therapy,” he said.
The cohort included 126 patients who were randomly assigned to receive either mFOLFIRINOX (arm A) or mFOLFIRINOX plus radiotherapy (arm B).
Patients in arm A received eight cycles of neoadjuvant mFOLFIRINOX. Patients in arm B received seven cycles of mFOLFIRINOX followed by 5 days of hypofractionated radiotherapy with either stereotactic body radiotherapy or hypofractionated image-guided radiotherapy.
In either arm, patients who did not experience disease progression underwent pancreatectomy followed by four cycles of adjuvant mFOLFOX6 (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and infusional 5-fluorouracil 2,400 mg/m2 over 46 hours).
The study’s primary endpoint was 18-month overall survival in comparison to a historical control of 50%. “An interim futility analysis was scheduled to be conducted following treatment of 30 patients on each arm,” Dr. Katz said. “Either arm in which 11 or fewer patients underwent [curative] R0 resection on protocol was to be declared futile and closed to further enrollment.
“With 62 evaluable patients in each arm, the final efficacy analysis was powered to detect an improvement in 18-month overall survival of 13% over the historical rate of 50%,” said Dr. Katz. “The two arms would be compared only if both were declared efficacious.”
At the interim analysis, 57% of the first 30 patients in arm A had undergone a resection, vs. 33% in arm B. Therefore, arm B was considered futile and was closed to accrual.
The median overall survival in arm A was 29.8 months. The median event-free survival was 15 months. Nearly half (49%) of patients proceeded to pancreatectomy following neoadjuvant therapy. The R0 resection rate was 88%, the pathologic complete response rate was 0%, and the 18-month overall survival rate was 93.1%.
For the patients in arm B, which had been closed to accrual, the median overall survival was 17.1 months, and the median event-free survival was 10.2 months. About one-third (35%) of patients were able to proceed to pancreatectomy. The R0 resection rate was 74%, the pathologic complete response rate was 11%, and the 18-month overall survival rate was 78.9%.
“Preoperative mFOLFIRINOX was associated with favorable overall survival relative to historical criteria in patients with borderline resectable PDAC, and mFOLFIRINOX with radiation therapy met the predefined futility boundary for R0 resection at interim analysis,” Dr. Katz concluded. “Therefore, mFOLFIRINOX represents a reference perioperative regimen for patients with borderline resectable PDAC.”
Further investigation needed
The paper’s discussant, Rebecca A. Snyder, MD, MPH, of the Brody School of Medicine, East Carolina University, Greenville, N.C., emphasized that this study was not designed or powered to directly compare chemotherapy alone with chemotherapy plus radiotherapy in the neoadjuvant setting.
She noted that it is surprising that the radiotherapy arm was closed early. She said the reasons for this remain unclear, although a few possible reasons can be speculated.
“Based on multiple studies in both the adjuvant and neoadjuvant setting, including the Alliance [A021501] trial presented today, there is no convincing randomized data that radiation therapy prolongs survival in any population of unselected patients with pancreatic cancer,” said Dr. Snyder.
“Certainly, follow-up questions still remain, specifically regarding the role for non-SBRT radiation in the preoperative setting, which may be answered by the ongoing PREOPANC-2 and PRODIGE 44 trials,” she said.
She added that the “addition of SBRT in the preoperative setting does not appear to be justified.”
The role of radiotherapy for subsets of patients remains unknown, and future investigation should focus on patient-centered endpoints, such as symptomatic local control rates, Dr. Snyder concluded.
The study was funded by the National Institutes of Health. Dr. Katz has had a consulting or advisory role for AbbVie and Alcresta Therapeutics. Dr. Snyder has disclosed no relevant financial relationships.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
Patients with borderline resectable pancreatic ductal adenocarcinoma (PDAC) are often treated with chemotherapy, radiotherapy, or both before undergoing surgery, but the optimal regimen in this setting is controversial.
Results from the Alliance A021501 study suggest the reference regimen should be neoadjuvant therapy with modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, leucovorin 400 mg/m2, and infusional 5-fluorouracil 2,400 mg/m2 over 46 hours), according to Matthew Katz, MD, FACS, chief of the pancreatic surgery service at the University of Texas MD Anderson Cancer Center, Houston.
This regimen improved survival for patients with PDAC, relative to historical data. For patients who received mFOLFIRINOX, the overall 18-month survival rate was 66.4%. However, when this regimen was combined with hypofractionated radiotherapy, the survival benefit was significantly lower, at 47.3%.
These findings were presented at the 2021 Gastrointestinal Cancers Symposium (GICS), which was held online this year.
“ASCO guidelines recommend that preoperative therapy be administered to patients with localized pancreatic adenocarcinoma who have tumors that have a significant radiographic interface with the major mesenteric blood vessels, as these patients are at high risk for a margin-positive operation and short survival when pancreatectomy is performed de novo,” Dr. Katz explained.
Although both chemotherapy and radiotherapy are used in these patients, there is no consensus as to the best approach, he commented.
The goal of Alliance A021501 “was to define a reference preoperative regimen for future trials of preoperative therapy,” he said.
The cohort included 126 patients who were randomly assigned to receive either mFOLFIRINOX (arm A) or mFOLFIRINOX plus radiotherapy (arm B).
Patients in arm A received eight cycles of neoadjuvant mFOLFIRINOX. Patients in arm B received seven cycles of mFOLFIRINOX followed by 5 days of hypofractionated radiotherapy with either stereotactic body radiotherapy or hypofractionated image-guided radiotherapy.
In either arm, patients who did not experience disease progression underwent pancreatectomy followed by four cycles of adjuvant mFOLFOX6 (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and infusional 5-fluorouracil 2,400 mg/m2 over 46 hours).
The study’s primary endpoint was 18-month overall survival in comparison to a historical control of 50%. “An interim futility analysis was scheduled to be conducted following treatment of 30 patients on each arm,” Dr. Katz said. “Either arm in which 11 or fewer patients underwent [curative] R0 resection on protocol was to be declared futile and closed to further enrollment.
“With 62 evaluable patients in each arm, the final efficacy analysis was powered to detect an improvement in 18-month overall survival of 13% over the historical rate of 50%,” said Dr. Katz. “The two arms would be compared only if both were declared efficacious.”
At the interim analysis, 57% of the first 30 patients in arm A had undergone a resection, vs. 33% in arm B. Therefore, arm B was considered futile and was closed to accrual.
The median overall survival in arm A was 29.8 months. The median event-free survival was 15 months. Nearly half (49%) of patients proceeded to pancreatectomy following neoadjuvant therapy. The R0 resection rate was 88%, the pathologic complete response rate was 0%, and the 18-month overall survival rate was 93.1%.
For the patients in arm B, which had been closed to accrual, the median overall survival was 17.1 months, and the median event-free survival was 10.2 months. About one-third (35%) of patients were able to proceed to pancreatectomy. The R0 resection rate was 74%, the pathologic complete response rate was 11%, and the 18-month overall survival rate was 78.9%.
“Preoperative mFOLFIRINOX was associated with favorable overall survival relative to historical criteria in patients with borderline resectable PDAC, and mFOLFIRINOX with radiation therapy met the predefined futility boundary for R0 resection at interim analysis,” Dr. Katz concluded. “Therefore, mFOLFIRINOX represents a reference perioperative regimen for patients with borderline resectable PDAC.”
Further investigation needed
The paper’s discussant, Rebecca A. Snyder, MD, MPH, of the Brody School of Medicine, East Carolina University, Greenville, N.C., emphasized that this study was not designed or powered to directly compare chemotherapy alone with chemotherapy plus radiotherapy in the neoadjuvant setting.
She noted that it is surprising that the radiotherapy arm was closed early. She said the reasons for this remain unclear, although a few possible reasons can be speculated.
“Based on multiple studies in both the adjuvant and neoadjuvant setting, including the Alliance [A021501] trial presented today, there is no convincing randomized data that radiation therapy prolongs survival in any population of unselected patients with pancreatic cancer,” said Dr. Snyder.
“Certainly, follow-up questions still remain, specifically regarding the role for non-SBRT radiation in the preoperative setting, which may be answered by the ongoing PREOPANC-2 and PRODIGE 44 trials,” she said.
She added that the “addition of SBRT in the preoperative setting does not appear to be justified.”
The role of radiotherapy for subsets of patients remains unknown, and future investigation should focus on patient-centered endpoints, such as symptomatic local control rates, Dr. Snyder concluded.
The study was funded by the National Institutes of Health. Dr. Katz has had a consulting or advisory role for AbbVie and Alcresta Therapeutics. Dr. Snyder has disclosed no relevant financial relationships.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
Patients with borderline resectable pancreatic ductal adenocarcinoma (PDAC) are often treated with chemotherapy, radiotherapy, or both before undergoing surgery, but the optimal regimen in this setting is controversial.
Results from the Alliance A021501 study suggest the reference regimen should be neoadjuvant therapy with modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, leucovorin 400 mg/m2, and infusional 5-fluorouracil 2,400 mg/m2 over 46 hours), according to Matthew Katz, MD, FACS, chief of the pancreatic surgery service at the University of Texas MD Anderson Cancer Center, Houston.
This regimen improved survival for patients with PDAC, relative to historical data. For patients who received mFOLFIRINOX, the overall 18-month survival rate was 66.4%. However, when this regimen was combined with hypofractionated radiotherapy, the survival benefit was significantly lower, at 47.3%.
These findings were presented at the 2021 Gastrointestinal Cancers Symposium (GICS), which was held online this year.
“ASCO guidelines recommend that preoperative therapy be administered to patients with localized pancreatic adenocarcinoma who have tumors that have a significant radiographic interface with the major mesenteric blood vessels, as these patients are at high risk for a margin-positive operation and short survival when pancreatectomy is performed de novo,” Dr. Katz explained.
Although both chemotherapy and radiotherapy are used in these patients, there is no consensus as to the best approach, he commented.
The goal of Alliance A021501 “was to define a reference preoperative regimen for future trials of preoperative therapy,” he said.
The cohort included 126 patients who were randomly assigned to receive either mFOLFIRINOX (arm A) or mFOLFIRINOX plus radiotherapy (arm B).
Patients in arm A received eight cycles of neoadjuvant mFOLFIRINOX. Patients in arm B received seven cycles of mFOLFIRINOX followed by 5 days of hypofractionated radiotherapy with either stereotactic body radiotherapy or hypofractionated image-guided radiotherapy.
In either arm, patients who did not experience disease progression underwent pancreatectomy followed by four cycles of adjuvant mFOLFOX6 (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and infusional 5-fluorouracil 2,400 mg/m2 over 46 hours).
The study’s primary endpoint was 18-month overall survival in comparison to a historical control of 50%. “An interim futility analysis was scheduled to be conducted following treatment of 30 patients on each arm,” Dr. Katz said. “Either arm in which 11 or fewer patients underwent [curative] R0 resection on protocol was to be declared futile and closed to further enrollment.
“With 62 evaluable patients in each arm, the final efficacy analysis was powered to detect an improvement in 18-month overall survival of 13% over the historical rate of 50%,” said Dr. Katz. “The two arms would be compared only if both were declared efficacious.”
At the interim analysis, 57% of the first 30 patients in arm A had undergone a resection, vs. 33% in arm B. Therefore, arm B was considered futile and was closed to accrual.
The median overall survival in arm A was 29.8 months. The median event-free survival was 15 months. Nearly half (49%) of patients proceeded to pancreatectomy following neoadjuvant therapy. The R0 resection rate was 88%, the pathologic complete response rate was 0%, and the 18-month overall survival rate was 93.1%.
For the patients in arm B, which had been closed to accrual, the median overall survival was 17.1 months, and the median event-free survival was 10.2 months. About one-third (35%) of patients were able to proceed to pancreatectomy. The R0 resection rate was 74%, the pathologic complete response rate was 11%, and the 18-month overall survival rate was 78.9%.
“Preoperative mFOLFIRINOX was associated with favorable overall survival relative to historical criteria in patients with borderline resectable PDAC, and mFOLFIRINOX with radiation therapy met the predefined futility boundary for R0 resection at interim analysis,” Dr. Katz concluded. “Therefore, mFOLFIRINOX represents a reference perioperative regimen for patients with borderline resectable PDAC.”
Further investigation needed
The paper’s discussant, Rebecca A. Snyder, MD, MPH, of the Brody School of Medicine, East Carolina University, Greenville, N.C., emphasized that this study was not designed or powered to directly compare chemotherapy alone with chemotherapy plus radiotherapy in the neoadjuvant setting.
She noted that it is surprising that the radiotherapy arm was closed early. She said the reasons for this remain unclear, although a few possible reasons can be speculated.
“Based on multiple studies in both the adjuvant and neoadjuvant setting, including the Alliance [A021501] trial presented today, there is no convincing randomized data that radiation therapy prolongs survival in any population of unselected patients with pancreatic cancer,” said Dr. Snyder.
“Certainly, follow-up questions still remain, specifically regarding the role for non-SBRT radiation in the preoperative setting, which may be answered by the ongoing PREOPANC-2 and PRODIGE 44 trials,” she said.
She added that the “addition of SBRT in the preoperative setting does not appear to be justified.”
The role of radiotherapy for subsets of patients remains unknown, and future investigation should focus on patient-centered endpoints, such as symptomatic local control rates, Dr. Snyder concluded.
The study was funded by the National Institutes of Health. Dr. Katz has had a consulting or advisory role for AbbVie and Alcresta Therapeutics. Dr. Snyder has disclosed no relevant financial relationships.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A version of this article first appeared on Medscape.com.
Think twice before intensifying BP regimen in older hospitalized patients
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Biden’s COVID-19 challenge: 100 million vaccinations in the first 100 days. It won’t be easy.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
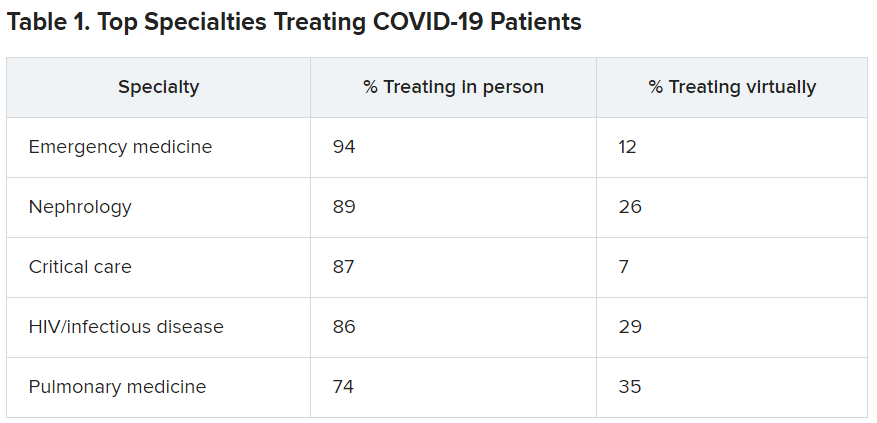
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
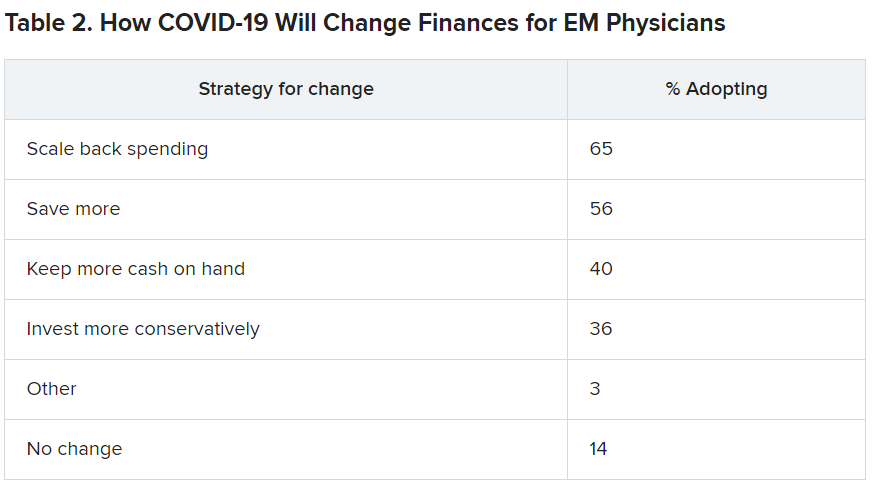
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Cardiometabolic Center Alliance promotes multiorgan, integrated T2D treatment
A one-stop shop approach to managing the spectrum of complications in patients with type 2 diabetes with a coordinated, multidisciplinary team of clinicians has taken root in at least two U.S. medical centers, and their efforts have now joined to take this concept national through the Cardiometabolic Center Alliance, which hopes to have at least 20 such centers running by the end of 2022.
In patients with type 2 diabetes (T2D), “multiple organs are affected by the same disease process,” notably the heart, kidneys, vasculature, and liver, but the care these patients often receive today is “fragmented, and typically without good coordination,” explained Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Saint Luke’s Michael & Marlys Haverty Cardiometabolic Center of Excellence in Kansas City, Mo.
“We need to depart from the outdated idea that each medical specialty focuses on an organ system. It’s one patient with one disease that affects multiple organs and needs comprehensive, multidisciplinary care,” he said.
Historically, “we’ve looked to primary care physicians to ‘conduct the orchestra’ for complex, multispecialty care” for patients with T2D, but a recent “avalanche” of new treatments with new data and recommendations has made coordination by a single, generalist physician essentially impossible. “It isn’t realistic” to expect a single primary care physician to coordinate all the care a patient with T2D now needs to receive, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City. Plus, “patients can get lost” when they try to navigate on their own among several physicians, possibly in disparate locations, and without fully understanding what each physician is responsible for managing.
Application of recommended treatments ‘lagging’
“The data are there, and the recommendations are there for T2D and cardiovascular disease, heart failure, and diabetic kidney disease, but the problem has been implementation,” said Dr. Kosiborod. “Application in practice is lagging way behind the recommendations.” That led him and his associates to devise a “new model of care for patients with T2D,” the cardiometabolic center (CMC), as a status quo alternative.
The CMC paradigm is that patients with T2D, especially those with existing cardiovascular or chronic kidney disease or at high risk for these complications, undergo assessment and treatment at one site from a multidisciplinary staff of physicians and allied caregivers including nurse practitioners, nurse coordinators, pharmacists, dieticians, and diabetes educators who are cross-trained for managing both T2D and cardiovascular diseases.
The Cardiometabolic Center Alliance builds on the idea that this care model is defined by a set of detailed treatment protocols and processes of care that other sites can adopt to boost the number of patients aided by this approach, to gather data from a larger patient pool in a dedicated registry to better document the program’s impact, and to form a quality-improvement network that can collectively improve performance.
“It’s absolutely replicable,” maintained Dr. Kosiborod, who is also executive director of the Cardiometabolic Center Alliance. “We’ve codified all of the care and medications into an impressive package. We now have something that works, and many other centers are interested in building programs like this. By establishing a base of well-defined protocols and operating procedures we can train a cadre of allied professionals who can effectively implement the program across wider populations of patients, while using the brick and mortar center to manage more complex patients,” he added.
“We’re not taking patients” from primary care physicians, Dr. Kosiborod stressed. “We’re helping generalists give better care. They already have their hands full. We’re here to help physicians do better.”
He cited a recent study of 1,735 patients with atherosclerotic cardiovascular disease and diabetes (96% with T2D) enrolled in a registry at 119 U.S. sites during 2016-2018 that found less than 7% were on the full range of guideline-directed medical therapy that they qualified for based on existing treatment guidelines. “This is not acceptable,” Dr. Kosiborod declared.
“It’s so obvious that this needs to be a combined approach. It’s very difficult to have one provider take care of all of the T2D complications. There needs to be a new approach, and [the Cardiometabolic Center program at Saint Luke’s] has done a great job getting their initiative underway to take a more global approach,” commented Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio.
Early results show improved metrics
The Saint Luke’s Haverty CMC launched in 2019, and data from its first 129 patients with at least one follow-up visit documented early efficacy for the program, as reported at the American Heart Association’s Quality of Care and Outcome’s Research meeting, held virtually on May 15 and 16, 2020.
That analysis from Dr. Kosiborod and associates compared various short-term metrics among their CMC patients against a control cohort of 387 similar patients with T2D who also received care in the Saint Luke’s Health System during 2019 but outside of the CMC. This observational study involved no patient randomization, but the researchers used propensity scoring to match the control patients with those managed in the CMC by several demographic and clinical parameters.
During follow-up that was generally less than 6 months, patients managed in the CMC averaged 12 pounds of weight loss, a 0.5% reduction in their average hemoglobin A1c, a mean 4.6–mm Hg cut in their systolic blood pressure, an average drop in their LDL cholesterol of 11.4 mg/dL, and among those receiving insulin the daily, average insulin dose fell by a relative 43%, compared with baseline. Among the controls, averages for these five parameters were weight loss of 2 pounds, a cut in A1c of 0.2%, a systolic blood pressure reduction of 0.6 mm Hg, a drop in LDL cholesterol of 4.9 mg/dL, and a relative rise in insulin dose of 6%. All of these between group differences were statistically significant except for LDL cholesterol.
Additional analysis of the medications that patients in the CMC and control group received also showed striking differences. Combined prescriptions for all components of guideline-directed medical therapy went to 41% of the CMC patients, compared with 2% of the controls, a statistically significant difference. Contributing to this difference were significantly increased rates of prescriptions for ACE inhibitors and statins.
The CMC staff also started 57% of their patients on a SGLT2 inhibitor and 90% on a GLP-1 receptor agonist (GLP-1 RA), compared with rates of 18% and 13%, respectively, among controls. Both of these between-group differences were also significant, and they highlighted the willingness and success of the CMC clinicians to put a large number of their patients on agents from both of these beneficial drug classes. This is a notable change from usual recent practice that limits most patients who actually receive these medications to a drug from just one of these two classes, often because of real or perceived limits on insurance coverage.
The data from these initial patients in the Saint Luke’s CMC show that the program was “very successful; it looks very promising,” said Dr. Kosiborod. The results show “transformational improvement in the quality of care.” Subsequent to this initial cohort from 2019, the Saint Luke’s CMC has seen “hundreds” of additional patients with T2D.
The Cardiometabolic Center Alliance gets started
The second member of the Cardiometabolic Center Alliance is a program run by the University Hospitals system based in Cleveland that had begun earlier in 2020. The University Hospitals’ Center for Integrated and Novel Approaches in Vascular-Metabolic Disease (CINEMA) uses a comprehensive, multidisciplinary-care model developed independently of but very similar to the Saint Luke’s CMC. By the end of 2020, the CINEMA program had managed about 150 patients, said Sanjay Rajagopalan, MD, director of CINEMA and a professor of medicine at Case Western Reserve University, Cleveland.
“Our outcomes have been quite similar” to what the Saint Luke’s program reported, he said. “We had better use of guideline-directed therapies, more weight loss, and better control of metabolic parameters.” The CINEMA program entered the Cardiometabolic Center Alliance as a “key strategic partner,” which means it will have a role in shaping the alliance going forward. One issue the alliance faces is how to leverage its growth to improve management of patients with T2D who do not have access to a CMC.
The CMCs “are not meant for every patient with T2D, but for those with high risk for cardiovascular complications who require extra attention,” Dr. Rajagopalan said in an interview. Both he and Dr. Kosiborod acknowledged that, even if 200 CMCs were to eventually open, and even if each center averaged 5,000 managed patients, those 1 million patients would be a small fraction of the total number of U.S. patients with T2D.
“Having these centers will produce a ripple effect. The protocols will percolate to primary care physicians,” Dr. Rajagopalan predicted. Once that happens, “not all patients will need to go to a cardiometabolic center.” In addition, leveraging established protocols via nurse coordinators and virtual care could bring this model to many more patients, Dr. Kosiborod noted.
By the end of 2020, a total of three additional U.S. centers had joined Saint Luke’s and University Hospitals in the alliance, but Dr. Kosiborod said that none of the three had yet been officially announced. The alliance has also started a national cardiometabolic registry, which will be “instrumental for its mission to track, benchmark, and improve quality of care and outcomes; enable mechanisms for “learning health care systems”; and can be used to answer important research questions,” Dr. Kosiborod said.
Combined SGLT2 inhibitor and GLP-1 RA treatment takes off
A key element of the more aggressive, risk-driven management emphasized in the CMC approach is frequent use of combined treatment with an SGLT2 inhibitor and a GLP-1 RA. Both classes of glucose-lowering drugs have well-documented, risk-reducing benefits, notably reduced atherosclerotic cardiovascular events and weight loss produced by the GLP1-RAs, and cuts in heart failure onset and hospitalizations and slowing of chronic kidney disease progression by the SGLT2 inhibitors.
Until now, medical society recommendations as well as opinion leaders have approached these two drug classes with a presumption that physicians would usually prescribe patients an agent from only one of these two classes, largely because the high cost of agents in both classes, all still under patent, often means coverage limits by insurers. Physicians at both the Saint Luke’s and University Hospitals programs have been more proactive, and successful, in prescribing agents from both classes to their high-risk patients with T2D.
“We use combination treatment quite a bit,” said Dr. Kosiborod. “It’s very sensible to use both. Their mechanisms of action are different and likely don’t overlap, so it’s reasonable to presume their activity is complimentary.” But he acknowledged that this has not yet been formally studied for cardiovascular or renal outcomes. Study results have documented complimentary effects between the two classes for weight loss, blood pressure reduction, and to some extent A1c reduction. A key reason for more frequent combined treatment with an SGLT2 inhibitor and GLP-1 RA is increased focus on the ability of both drug classes to lower risk in patients with T2D and high cardiovascular-disease risk, rather than prescribing decisions driven largely by trying to further reduce a patient’s A1c.
Although insurance coverage is not a given, the Saint Luke’s CMC has had good results using patient-assistance programs run by various drug companies. Some patients have received their medications free of charge or with modest copays, depending on their income and insurance coverage. At Saint Luke’s, “many” patients with T2D have been able to get free medications through assistance programs, he said. And for patients with health insurance, getting coverage for an agent from each class “is easier now than it was 3-4 years ago.”
Dr. Kosiborod has been a consultant to several companies, and has received research grants from AstraZeneca and Boehringer Ingelheim. Dr. DeFronzo received research grants from Astra Zeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk. Dr. Rajagopalan has been a consultant to Novo Nordisk and Takeda.
A one-stop shop approach to managing the spectrum of complications in patients with type 2 diabetes with a coordinated, multidisciplinary team of clinicians has taken root in at least two U.S. medical centers, and their efforts have now joined to take this concept national through the Cardiometabolic Center Alliance, which hopes to have at least 20 such centers running by the end of 2022.
In patients with type 2 diabetes (T2D), “multiple organs are affected by the same disease process,” notably the heart, kidneys, vasculature, and liver, but the care these patients often receive today is “fragmented, and typically without good coordination,” explained Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Saint Luke’s Michael & Marlys Haverty Cardiometabolic Center of Excellence in Kansas City, Mo.
“We need to depart from the outdated idea that each medical specialty focuses on an organ system. It’s one patient with one disease that affects multiple organs and needs comprehensive, multidisciplinary care,” he said.
Historically, “we’ve looked to primary care physicians to ‘conduct the orchestra’ for complex, multispecialty care” for patients with T2D, but a recent “avalanche” of new treatments with new data and recommendations has made coordination by a single, generalist physician essentially impossible. “It isn’t realistic” to expect a single primary care physician to coordinate all the care a patient with T2D now needs to receive, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City. Plus, “patients can get lost” when they try to navigate on their own among several physicians, possibly in disparate locations, and without fully understanding what each physician is responsible for managing.
Application of recommended treatments ‘lagging’
“The data are there, and the recommendations are there for T2D and cardiovascular disease, heart failure, and diabetic kidney disease, but the problem has been implementation,” said Dr. Kosiborod. “Application in practice is lagging way behind the recommendations.” That led him and his associates to devise a “new model of care for patients with T2D,” the cardiometabolic center (CMC), as a status quo alternative.
The CMC paradigm is that patients with T2D, especially those with existing cardiovascular or chronic kidney disease or at high risk for these complications, undergo assessment and treatment at one site from a multidisciplinary staff of physicians and allied caregivers including nurse practitioners, nurse coordinators, pharmacists, dieticians, and diabetes educators who are cross-trained for managing both T2D and cardiovascular diseases.
The Cardiometabolic Center Alliance builds on the idea that this care model is defined by a set of detailed treatment protocols and processes of care that other sites can adopt to boost the number of patients aided by this approach, to gather data from a larger patient pool in a dedicated registry to better document the program’s impact, and to form a quality-improvement network that can collectively improve performance.
“It’s absolutely replicable,” maintained Dr. Kosiborod, who is also executive director of the Cardiometabolic Center Alliance. “We’ve codified all of the care and medications into an impressive package. We now have something that works, and many other centers are interested in building programs like this. By establishing a base of well-defined protocols and operating procedures we can train a cadre of allied professionals who can effectively implement the program across wider populations of patients, while using the brick and mortar center to manage more complex patients,” he added.
“We’re not taking patients” from primary care physicians, Dr. Kosiborod stressed. “We’re helping generalists give better care. They already have their hands full. We’re here to help physicians do better.”
He cited a recent study of 1,735 patients with atherosclerotic cardiovascular disease and diabetes (96% with T2D) enrolled in a registry at 119 U.S. sites during 2016-2018 that found less than 7% were on the full range of guideline-directed medical therapy that they qualified for based on existing treatment guidelines. “This is not acceptable,” Dr. Kosiborod declared.
“It’s so obvious that this needs to be a combined approach. It’s very difficult to have one provider take care of all of the T2D complications. There needs to be a new approach, and [the Cardiometabolic Center program at Saint Luke’s] has done a great job getting their initiative underway to take a more global approach,” commented Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio.
Early results show improved metrics
The Saint Luke’s Haverty CMC launched in 2019, and data from its first 129 patients with at least one follow-up visit documented early efficacy for the program, as reported at the American Heart Association’s Quality of Care and Outcome’s Research meeting, held virtually on May 15 and 16, 2020.
That analysis from Dr. Kosiborod and associates compared various short-term metrics among their CMC patients against a control cohort of 387 similar patients with T2D who also received care in the Saint Luke’s Health System during 2019 but outside of the CMC. This observational study involved no patient randomization, but the researchers used propensity scoring to match the control patients with those managed in the CMC by several demographic and clinical parameters.
During follow-up that was generally less than 6 months, patients managed in the CMC averaged 12 pounds of weight loss, a 0.5% reduction in their average hemoglobin A1c, a mean 4.6–mm Hg cut in their systolic blood pressure, an average drop in their LDL cholesterol of 11.4 mg/dL, and among those receiving insulin the daily, average insulin dose fell by a relative 43%, compared with baseline. Among the controls, averages for these five parameters were weight loss of 2 pounds, a cut in A1c of 0.2%, a systolic blood pressure reduction of 0.6 mm Hg, a drop in LDL cholesterol of 4.9 mg/dL, and a relative rise in insulin dose of 6%. All of these between group differences were statistically significant except for LDL cholesterol.
Additional analysis of the medications that patients in the CMC and control group received also showed striking differences. Combined prescriptions for all components of guideline-directed medical therapy went to 41% of the CMC patients, compared with 2% of the controls, a statistically significant difference. Contributing to this difference were significantly increased rates of prescriptions for ACE inhibitors and statins.
The CMC staff also started 57% of their patients on a SGLT2 inhibitor and 90% on a GLP-1 receptor agonist (GLP-1 RA), compared with rates of 18% and 13%, respectively, among controls. Both of these between-group differences were also significant, and they highlighted the willingness and success of the CMC clinicians to put a large number of their patients on agents from both of these beneficial drug classes. This is a notable change from usual recent practice that limits most patients who actually receive these medications to a drug from just one of these two classes, often because of real or perceived limits on insurance coverage.
The data from these initial patients in the Saint Luke’s CMC show that the program was “very successful; it looks very promising,” said Dr. Kosiborod. The results show “transformational improvement in the quality of care.” Subsequent to this initial cohort from 2019, the Saint Luke’s CMC has seen “hundreds” of additional patients with T2D.
The Cardiometabolic Center Alliance gets started
The second member of the Cardiometabolic Center Alliance is a program run by the University Hospitals system based in Cleveland that had begun earlier in 2020. The University Hospitals’ Center for Integrated and Novel Approaches in Vascular-Metabolic Disease (CINEMA) uses a comprehensive, multidisciplinary-care model developed independently of but very similar to the Saint Luke’s CMC. By the end of 2020, the CINEMA program had managed about 150 patients, said Sanjay Rajagopalan, MD, director of CINEMA and a professor of medicine at Case Western Reserve University, Cleveland.
“Our outcomes have been quite similar” to what the Saint Luke’s program reported, he said. “We had better use of guideline-directed therapies, more weight loss, and better control of metabolic parameters.” The CINEMA program entered the Cardiometabolic Center Alliance as a “key strategic partner,” which means it will have a role in shaping the alliance going forward. One issue the alliance faces is how to leverage its growth to improve management of patients with T2D who do not have access to a CMC.
The CMCs “are not meant for every patient with T2D, but for those with high risk for cardiovascular complications who require extra attention,” Dr. Rajagopalan said in an interview. Both he and Dr. Kosiborod acknowledged that, even if 200 CMCs were to eventually open, and even if each center averaged 5,000 managed patients, those 1 million patients would be a small fraction of the total number of U.S. patients with T2D.
“Having these centers will produce a ripple effect. The protocols will percolate to primary care physicians,” Dr. Rajagopalan predicted. Once that happens, “not all patients will need to go to a cardiometabolic center.” In addition, leveraging established protocols via nurse coordinators and virtual care could bring this model to many more patients, Dr. Kosiborod noted.
By the end of 2020, a total of three additional U.S. centers had joined Saint Luke’s and University Hospitals in the alliance, but Dr. Kosiborod said that none of the three had yet been officially announced. The alliance has also started a national cardiometabolic registry, which will be “instrumental for its mission to track, benchmark, and improve quality of care and outcomes; enable mechanisms for “learning health care systems”; and can be used to answer important research questions,” Dr. Kosiborod said.
Combined SGLT2 inhibitor and GLP-1 RA treatment takes off
A key element of the more aggressive, risk-driven management emphasized in the CMC approach is frequent use of combined treatment with an SGLT2 inhibitor and a GLP-1 RA. Both classes of glucose-lowering drugs have well-documented, risk-reducing benefits, notably reduced atherosclerotic cardiovascular events and weight loss produced by the GLP1-RAs, and cuts in heart failure onset and hospitalizations and slowing of chronic kidney disease progression by the SGLT2 inhibitors.
Until now, medical society recommendations as well as opinion leaders have approached these two drug classes with a presumption that physicians would usually prescribe patients an agent from only one of these two classes, largely because the high cost of agents in both classes, all still under patent, often means coverage limits by insurers. Physicians at both the Saint Luke’s and University Hospitals programs have been more proactive, and successful, in prescribing agents from both classes to their high-risk patients with T2D.
“We use combination treatment quite a bit,” said Dr. Kosiborod. “It’s very sensible to use both. Their mechanisms of action are different and likely don’t overlap, so it’s reasonable to presume their activity is complimentary.” But he acknowledged that this has not yet been formally studied for cardiovascular or renal outcomes. Study results have documented complimentary effects between the two classes for weight loss, blood pressure reduction, and to some extent A1c reduction. A key reason for more frequent combined treatment with an SGLT2 inhibitor and GLP-1 RA is increased focus on the ability of both drug classes to lower risk in patients with T2D and high cardiovascular-disease risk, rather than prescribing decisions driven largely by trying to further reduce a patient’s A1c.
Although insurance coverage is not a given, the Saint Luke’s CMC has had good results using patient-assistance programs run by various drug companies. Some patients have received their medications free of charge or with modest copays, depending on their income and insurance coverage. At Saint Luke’s, “many” patients with T2D have been able to get free medications through assistance programs, he said. And for patients with health insurance, getting coverage for an agent from each class “is easier now than it was 3-4 years ago.”
Dr. Kosiborod has been a consultant to several companies, and has received research grants from AstraZeneca and Boehringer Ingelheim. Dr. DeFronzo received research grants from Astra Zeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk. Dr. Rajagopalan has been a consultant to Novo Nordisk and Takeda.
A one-stop shop approach to managing the spectrum of complications in patients with type 2 diabetes with a coordinated, multidisciplinary team of clinicians has taken root in at least two U.S. medical centers, and their efforts have now joined to take this concept national through the Cardiometabolic Center Alliance, which hopes to have at least 20 such centers running by the end of 2022.
In patients with type 2 diabetes (T2D), “multiple organs are affected by the same disease process,” notably the heart, kidneys, vasculature, and liver, but the care these patients often receive today is “fragmented, and typically without good coordination,” explained Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Saint Luke’s Michael & Marlys Haverty Cardiometabolic Center of Excellence in Kansas City, Mo.
“We need to depart from the outdated idea that each medical specialty focuses on an organ system. It’s one patient with one disease that affects multiple organs and needs comprehensive, multidisciplinary care,” he said.
Historically, “we’ve looked to primary care physicians to ‘conduct the orchestra’ for complex, multispecialty care” for patients with T2D, but a recent “avalanche” of new treatments with new data and recommendations has made coordination by a single, generalist physician essentially impossible. “It isn’t realistic” to expect a single primary care physician to coordinate all the care a patient with T2D now needs to receive, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City. Plus, “patients can get lost” when they try to navigate on their own among several physicians, possibly in disparate locations, and without fully understanding what each physician is responsible for managing.
Application of recommended treatments ‘lagging’
“The data are there, and the recommendations are there for T2D and cardiovascular disease, heart failure, and diabetic kidney disease, but the problem has been implementation,” said Dr. Kosiborod. “Application in practice is lagging way behind the recommendations.” That led him and his associates to devise a “new model of care for patients with T2D,” the cardiometabolic center (CMC), as a status quo alternative.
The CMC paradigm is that patients with T2D, especially those with existing cardiovascular or chronic kidney disease or at high risk for these complications, undergo assessment and treatment at one site from a multidisciplinary staff of physicians and allied caregivers including nurse practitioners, nurse coordinators, pharmacists, dieticians, and diabetes educators who are cross-trained for managing both T2D and cardiovascular diseases.
The Cardiometabolic Center Alliance builds on the idea that this care model is defined by a set of detailed treatment protocols and processes of care that other sites can adopt to boost the number of patients aided by this approach, to gather data from a larger patient pool in a dedicated registry to better document the program’s impact, and to form a quality-improvement network that can collectively improve performance.
“It’s absolutely replicable,” maintained Dr. Kosiborod, who is also executive director of the Cardiometabolic Center Alliance. “We’ve codified all of the care and medications into an impressive package. We now have something that works, and many other centers are interested in building programs like this. By establishing a base of well-defined protocols and operating procedures we can train a cadre of allied professionals who can effectively implement the program across wider populations of patients, while using the brick and mortar center to manage more complex patients,” he added.
“We’re not taking patients” from primary care physicians, Dr. Kosiborod stressed. “We’re helping generalists give better care. They already have their hands full. We’re here to help physicians do better.”
He cited a recent study of 1,735 patients with atherosclerotic cardiovascular disease and diabetes (96% with T2D) enrolled in a registry at 119 U.S. sites during 2016-2018 that found less than 7% were on the full range of guideline-directed medical therapy that they qualified for based on existing treatment guidelines. “This is not acceptable,” Dr. Kosiborod declared.
“It’s so obvious that this needs to be a combined approach. It’s very difficult to have one provider take care of all of the T2D complications. There needs to be a new approach, and [the Cardiometabolic Center program at Saint Luke’s] has done a great job getting their initiative underway to take a more global approach,” commented Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio.
Early results show improved metrics
The Saint Luke’s Haverty CMC launched in 2019, and data from its first 129 patients with at least one follow-up visit documented early efficacy for the program, as reported at the American Heart Association’s Quality of Care and Outcome’s Research meeting, held virtually on May 15 and 16, 2020.
That analysis from Dr. Kosiborod and associates compared various short-term metrics among their CMC patients against a control cohort of 387 similar patients with T2D who also received care in the Saint Luke’s Health System during 2019 but outside of the CMC. This observational study involved no patient randomization, but the researchers used propensity scoring to match the control patients with those managed in the CMC by several demographic and clinical parameters.
During follow-up that was generally less than 6 months, patients managed in the CMC averaged 12 pounds of weight loss, a 0.5% reduction in their average hemoglobin A1c, a mean 4.6–mm Hg cut in their systolic blood pressure, an average drop in their LDL cholesterol of 11.4 mg/dL, and among those receiving insulin the daily, average insulin dose fell by a relative 43%, compared with baseline. Among the controls, averages for these five parameters were weight loss of 2 pounds, a cut in A1c of 0.2%, a systolic blood pressure reduction of 0.6 mm Hg, a drop in LDL cholesterol of 4.9 mg/dL, and a relative rise in insulin dose of 6%. All of these between group differences were statistically significant except for LDL cholesterol.
Additional analysis of the medications that patients in the CMC and control group received also showed striking differences. Combined prescriptions for all components of guideline-directed medical therapy went to 41% of the CMC patients, compared with 2% of the controls, a statistically significant difference. Contributing to this difference were significantly increased rates of prescriptions for ACE inhibitors and statins.
The CMC staff also started 57% of their patients on a SGLT2 inhibitor and 90% on a GLP-1 receptor agonist (GLP-1 RA), compared with rates of 18% and 13%, respectively, among controls. Both of these between-group differences were also significant, and they highlighted the willingness and success of the CMC clinicians to put a large number of their patients on agents from both of these beneficial drug classes. This is a notable change from usual recent practice that limits most patients who actually receive these medications to a drug from just one of these two classes, often because of real or perceived limits on insurance coverage.
The data from these initial patients in the Saint Luke’s CMC show that the program was “very successful; it looks very promising,” said Dr. Kosiborod. The results show “transformational improvement in the quality of care.” Subsequent to this initial cohort from 2019, the Saint Luke’s CMC has seen “hundreds” of additional patients with T2D.
The Cardiometabolic Center Alliance gets started
The second member of the Cardiometabolic Center Alliance is a program run by the University Hospitals system based in Cleveland that had begun earlier in 2020. The University Hospitals’ Center for Integrated and Novel Approaches in Vascular-Metabolic Disease (CINEMA) uses a comprehensive, multidisciplinary-care model developed independently of but very similar to the Saint Luke’s CMC. By the end of 2020, the CINEMA program had managed about 150 patients, said Sanjay Rajagopalan, MD, director of CINEMA and a professor of medicine at Case Western Reserve University, Cleveland.
“Our outcomes have been quite similar” to what the Saint Luke’s program reported, he said. “We had better use of guideline-directed therapies, more weight loss, and better control of metabolic parameters.” The CINEMA program entered the Cardiometabolic Center Alliance as a “key strategic partner,” which means it will have a role in shaping the alliance going forward. One issue the alliance faces is how to leverage its growth to improve management of patients with T2D who do not have access to a CMC.
The CMCs “are not meant for every patient with T2D, but for those with high risk for cardiovascular complications who require extra attention,” Dr. Rajagopalan said in an interview. Both he and Dr. Kosiborod acknowledged that, even if 200 CMCs were to eventually open, and even if each center averaged 5,000 managed patients, those 1 million patients would be a small fraction of the total number of U.S. patients with T2D.
“Having these centers will produce a ripple effect. The protocols will percolate to primary care physicians,” Dr. Rajagopalan predicted. Once that happens, “not all patients will need to go to a cardiometabolic center.” In addition, leveraging established protocols via nurse coordinators and virtual care could bring this model to many more patients, Dr. Kosiborod noted.
By the end of 2020, a total of three additional U.S. centers had joined Saint Luke’s and University Hospitals in the alliance, but Dr. Kosiborod said that none of the three had yet been officially announced. The alliance has also started a national cardiometabolic registry, which will be “instrumental for its mission to track, benchmark, and improve quality of care and outcomes; enable mechanisms for “learning health care systems”; and can be used to answer important research questions,” Dr. Kosiborod said.
Combined SGLT2 inhibitor and GLP-1 RA treatment takes off
A key element of the more aggressive, risk-driven management emphasized in the CMC approach is frequent use of combined treatment with an SGLT2 inhibitor and a GLP-1 RA. Both classes of glucose-lowering drugs have well-documented, risk-reducing benefits, notably reduced atherosclerotic cardiovascular events and weight loss produced by the GLP1-RAs, and cuts in heart failure onset and hospitalizations and slowing of chronic kidney disease progression by the SGLT2 inhibitors.
Until now, medical society recommendations as well as opinion leaders have approached these two drug classes with a presumption that physicians would usually prescribe patients an agent from only one of these two classes, largely because the high cost of agents in both classes, all still under patent, often means coverage limits by insurers. Physicians at both the Saint Luke’s and University Hospitals programs have been more proactive, and successful, in prescribing agents from both classes to their high-risk patients with T2D.
“We use combination treatment quite a bit,” said Dr. Kosiborod. “It’s very sensible to use both. Their mechanisms of action are different and likely don’t overlap, so it’s reasonable to presume their activity is complimentary.” But he acknowledged that this has not yet been formally studied for cardiovascular or renal outcomes. Study results have documented complimentary effects between the two classes for weight loss, blood pressure reduction, and to some extent A1c reduction. A key reason for more frequent combined treatment with an SGLT2 inhibitor and GLP-1 RA is increased focus on the ability of both drug classes to lower risk in patients with T2D and high cardiovascular-disease risk, rather than prescribing decisions driven largely by trying to further reduce a patient’s A1c.
Although insurance coverage is not a given, the Saint Luke’s CMC has had good results using patient-assistance programs run by various drug companies. Some patients have received their medications free of charge or with modest copays, depending on their income and insurance coverage. At Saint Luke’s, “many” patients with T2D have been able to get free medications through assistance programs, he said. And for patients with health insurance, getting coverage for an agent from each class “is easier now than it was 3-4 years ago.”
Dr. Kosiborod has been a consultant to several companies, and has received research grants from AstraZeneca and Boehringer Ingelheim. Dr. DeFronzo received research grants from Astra Zeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk. Dr. Rajagopalan has been a consultant to Novo Nordisk and Takeda.
How COVID-19 will continue to alter patient visits
Finding the current domestic and global situations too disheartening to write about, I have decided for the moment to take the long view in hopes of finding something to stimulate your imaginations. It appears that we have several vaccines effective against SARS-CoV-2 if not in your hands at the moment at least in someone’s freezer or at the very least somewhere near beginning of their journey in the production pipeline. It may be a year of more but thanks to the vaccines and herd immunity there will be a time when parents may feel more comfortable about bringing their children into your office. How are you going to dial back your office routine to something even vaguely familiar?
To keep your office afloat financially you have probably been forced to adopt and adapt telemedicine strategies to your practice style. Prior to the pandemic you may have been among the few who were actively experimenting with practicing remotely. But, it is more likely that you had given little serious thought to how you would manage your patients without them being physically present.
You probably carried in your mind a list of symptoms and complaints which you had promised yourself that you would never treat without first laying eyes and hands on the patient. You may have even codified this list into a set of guidelines that you included in the office manual for your nurses, assistants, and receptionists. You may have looked askance at some of your colleagues whom you felt too often treated their patients (and yours when they were covering) based on what seemed to be scanty information gleaned from a phone call. The impropriety of this kind of clinical behavior may have even come up at staff meetings or at least been the topic of hallway discussions.
How did your list of complaints that demanded an in-person visit evolve? I suspect that in large part it was formed as you modeled the behavior of your mentors and teachers. In some cases you may have heard of tragic cases in which a child had died or suffered serious consequences of being treated without an in-person evaluation. In many cases you were following a tradition or ethic that said treating in certain circumstances without an exam just wasn’t done.
Have the realities of the pandemic forced you to alter your list of must-see-before-I’ll-treat complaints? Have you found yourself calling in antibiotic prescriptions for children with ear pain who 1 year ago you would have told to come in for an office visit? Are you treating “strep throats” without a rapid strep test or culture? How many stimulant prescriptions have you refilled for children who haven’t been reevaluated in the office in over a year? How are you going to manage the tsunami of requests for sports physicals once the junior high and high school teams are allowed to return to action? You probably won’t have the time to examine all of the sports candidates who show up in your office with crumpled forms recently retrieved from crumb-filled backpacks.
Where are you going to reset the bar as the pandemic lifts and the barriers that have prevented patients from coming to your office over the last year or year and a half recede? Have you realized that many of your office visits in prepandemic times were unnecessary? How many children with otitis really needed to be followed up with an ear recheck visit? Which children with sore throats and a fever needed to be examined? Was a yearly exam really necessary for a high school sophomore who wanted to play basketball? Has your comfort zone widened to include more patient complaints that can be managed without a face to face encounter? Where will telemedicine fit into the mix?
At some time in the next 12 months you will have to recalibrate and reset the bar. It will probably be a gradual process that in large part can be molded by the responses of the families who may have also come to realize that seeing you in the office isn’t quite as necessary as you both may have thought it was.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
Finding the current domestic and global situations too disheartening to write about, I have decided for the moment to take the long view in hopes of finding something to stimulate your imaginations. It appears that we have several vaccines effective against SARS-CoV-2 if not in your hands at the moment at least in someone’s freezer or at the very least somewhere near beginning of their journey in the production pipeline. It may be a year of more but thanks to the vaccines and herd immunity there will be a time when parents may feel more comfortable about bringing their children into your office. How are you going to dial back your office routine to something even vaguely familiar?
To keep your office afloat financially you have probably been forced to adopt and adapt telemedicine strategies to your practice style. Prior to the pandemic you may have been among the few who were actively experimenting with practicing remotely. But, it is more likely that you had given little serious thought to how you would manage your patients without them being physically present.
You probably carried in your mind a list of symptoms and complaints which you had promised yourself that you would never treat without first laying eyes and hands on the patient. You may have even codified this list into a set of guidelines that you included in the office manual for your nurses, assistants, and receptionists. You may have looked askance at some of your colleagues whom you felt too often treated their patients (and yours when they were covering) based on what seemed to be scanty information gleaned from a phone call. The impropriety of this kind of clinical behavior may have even come up at staff meetings or at least been the topic of hallway discussions.
How did your list of complaints that demanded an in-person visit evolve? I suspect that in large part it was formed as you modeled the behavior of your mentors and teachers. In some cases you may have heard of tragic cases in which a child had died or suffered serious consequences of being treated without an in-person evaluation. In many cases you were following a tradition or ethic that said treating in certain circumstances without an exam just wasn’t done.
Have the realities of the pandemic forced you to alter your list of must-see-before-I’ll-treat complaints? Have you found yourself calling in antibiotic prescriptions for children with ear pain who 1 year ago you would have told to come in for an office visit? Are you treating “strep throats” without a rapid strep test or culture? How many stimulant prescriptions have you refilled for children who haven’t been reevaluated in the office in over a year? How are you going to manage the tsunami of requests for sports physicals once the junior high and high school teams are allowed to return to action? You probably won’t have the time to examine all of the sports candidates who show up in your office with crumpled forms recently retrieved from crumb-filled backpacks.
Where are you going to reset the bar as the pandemic lifts and the barriers that have prevented patients from coming to your office over the last year or year and a half recede? Have you realized that many of your office visits in prepandemic times were unnecessary? How many children with otitis really needed to be followed up with an ear recheck visit? Which children with sore throats and a fever needed to be examined? Was a yearly exam really necessary for a high school sophomore who wanted to play basketball? Has your comfort zone widened to include more patient complaints that can be managed without a face to face encounter? Where will telemedicine fit into the mix?
At some time in the next 12 months you will have to recalibrate and reset the bar. It will probably be a gradual process that in large part can be molded by the responses of the families who may have also come to realize that seeing you in the office isn’t quite as necessary as you both may have thought it was.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
Finding the current domestic and global situations too disheartening to write about, I have decided for the moment to take the long view in hopes of finding something to stimulate your imaginations. It appears that we have several vaccines effective against SARS-CoV-2 if not in your hands at the moment at least in someone’s freezer or at the very least somewhere near beginning of their journey in the production pipeline. It may be a year of more but thanks to the vaccines and herd immunity there will be a time when parents may feel more comfortable about bringing their children into your office. How are you going to dial back your office routine to something even vaguely familiar?
To keep your office afloat financially you have probably been forced to adopt and adapt telemedicine strategies to your practice style. Prior to the pandemic you may have been among the few who were actively experimenting with practicing remotely. But, it is more likely that you had given little serious thought to how you would manage your patients without them being physically present.
You probably carried in your mind a list of symptoms and complaints which you had promised yourself that you would never treat without first laying eyes and hands on the patient. You may have even codified this list into a set of guidelines that you included in the office manual for your nurses, assistants, and receptionists. You may have looked askance at some of your colleagues whom you felt too often treated their patients (and yours when they were covering) based on what seemed to be scanty information gleaned from a phone call. The impropriety of this kind of clinical behavior may have even come up at staff meetings or at least been the topic of hallway discussions.
How did your list of complaints that demanded an in-person visit evolve? I suspect that in large part it was formed as you modeled the behavior of your mentors and teachers. In some cases you may have heard of tragic cases in which a child had died or suffered serious consequences of being treated without an in-person evaluation. In many cases you were following a tradition or ethic that said treating in certain circumstances without an exam just wasn’t done.
Have the realities of the pandemic forced you to alter your list of must-see-before-I’ll-treat complaints? Have you found yourself calling in antibiotic prescriptions for children with ear pain who 1 year ago you would have told to come in for an office visit? Are you treating “strep throats” without a rapid strep test or culture? How many stimulant prescriptions have you refilled for children who haven’t been reevaluated in the office in over a year? How are you going to manage the tsunami of requests for sports physicals once the junior high and high school teams are allowed to return to action? You probably won’t have the time to examine all of the sports candidates who show up in your office with crumpled forms recently retrieved from crumb-filled backpacks.
Where are you going to reset the bar as the pandemic lifts and the barriers that have prevented patients from coming to your office over the last year or year and a half recede? Have you realized that many of your office visits in prepandemic times were unnecessary? How many children with otitis really needed to be followed up with an ear recheck visit? Which children with sore throats and a fever needed to be examined? Was a yearly exam really necessary for a high school sophomore who wanted to play basketball? Has your comfort zone widened to include more patient complaints that can be managed without a face to face encounter? Where will telemedicine fit into the mix?
At some time in the next 12 months you will have to recalibrate and reset the bar. It will probably be a gradual process that in large part can be molded by the responses of the families who may have also come to realize that seeing you in the office isn’t quite as necessary as you both may have thought it was.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]