User login
ECHO-CT: An Interdisciplinary Videoconference Model for Identifying Potential Postdischarge Transition-of-Care Events
As the population of the United States continues to age, hospitals are seeing an increasing number of older patients with significant medical and social complexity. Medicare data have shown that an increasing number require post–acute care after a hospitalization.1 Discharges to post–acute care settings are often longer and more costly compared with discharges to other settings, which suggests that targeting quality improvement efforts at this transition period may improve the value of care.2
The transition from the hospital setting to a post–acute care facility can be dangerous and complicated due to lapses in communication, medication errors, and the complexity of medical treatment plans. Suboptimal transitions in care can result in adverse events for the patient, as well as confusion in medication regimens or incomplete plans for follow-up care.3
The Project ECHO (Extension for Community Healthcare Outcomes) model was first developed and launched by Sanjeev Arora, MD, in New Mexico in 2003 to expand access to subspecialist care using videoconferencing.4 We first applied this model in 2013 to evaluate the impact of this interdisciplinary videoconferencing tool on the care of patients discharged to post–acute settings.5 We found that patients participating in the Extension for Community Healthcare Outcomes–Care Transitions (ECHO-CT) model experienced decreased risk of rehospitalization, decreased skilled nursing facility (SNF) length of stay, and reduced 30-day healthcare costs, compared with those patients not enrolled in this program; these outcomes were likely due to identification and correction of medication-related errors, improved care coordination, improved disease management, and clarification of goals of care.6 Though these investigations did identify some issues arising during the care transition process, they did not fully describe the types of problems uncovered. We sought to better characterize the clinical and operational issues identified through the ECHO-CT conference, hereafter known as transition-of-care events (TCEs). These issues may include new or evolving medical concerns, an adverse event, or a “near miss.” Identification and classification of TCEs that may contribute to unsafe or fractured care transitions are critical in developing systematic solutions to improve transitions of care, which can ultimately improve patient safety and potentially avoid preventable errors.
METHODS
ECHO-CT Multidisciplinary Video Conference
We conducted ECHO-CT at a large, tertiary care academic medical center. The project design for the ECHO-CT program has been previously described.5 In brief, the program is a weekly, multidisciplinary videoconference between a hospital-based team and post–acute care providers to discuss patients discharged from inpatient services to post–acute care sites, including SNFs and long-term acute care hospitals (LTACHs), during the preceding week. All patients discharged from the tertiary care inpatient site to one of the eight participating SNFs or LTACHs, from either a medical or surgical service, are eligible to be discussed at this weekly interdisciplinary conference. Long-term care facilities were not included in this study. The ECHO-CT program used HIPAA (Health Insurance Portability and Accountability Act)-compliant videoconferencing technology to connect hospital and post–acute care providers.
During the videoconferences, each patient’s hospital course and discharge documentation are reviewed by a hospitalist, and a pharmacist performs a medication reconciliation of each patient’s admission, discharge, and post–acute care medication list. The discharging attending, primary care providers, residents, other trainees, and subspecialist providers are invited to attend. Typically, the interdisciplinary team at the post–acute care sites includes physicians, nurse practitioners, physical therapists, social workers, and case managers. Between 10 and 20 patients are discussed in a case-based format, which includes a summary of the patient’s hospital course, an update from the post–acute care team on the patient’s care, and an opportunity for a discussion regarding any concerns or questions raised by the post–acute care or inpatient care teams. The content and duration of discussion typically lasts approximately 3 to 10 minutes, depending on the needs of the patient and the care team. Each of the eight post–acute care sites participating in the project are assigned a 10- to 15-minute block. A copy of the ECHO-CT session process document is included in the Appendix.
Data Collection
At each interdisciplinary patient review, TCEs were identified and recorded. These events were categorized in real time by the ECHO-CT data collection team into the following categories: medication related, medical, discharge communication/coordination, or other, and recorded in a secured, deidentified database. For individuals whose TCEs could represent more than one category, authors reviewed the available information about the TCEs and determined the most appropriate category; if more than one category was felt to be applicable to a patient’s situation, the events were reclassified into all applicable categories. Data about individual patients, including gender, age at the time of discharge, and other demographic information, were obtained from hospital databases. Number of diagnoses included any diagnosis billed during the patient’s hospital stay, and these data were obtained from a hospital billing database. Average number of medications at discharge was obtained from a hospital pharmacy database.
RESULTS
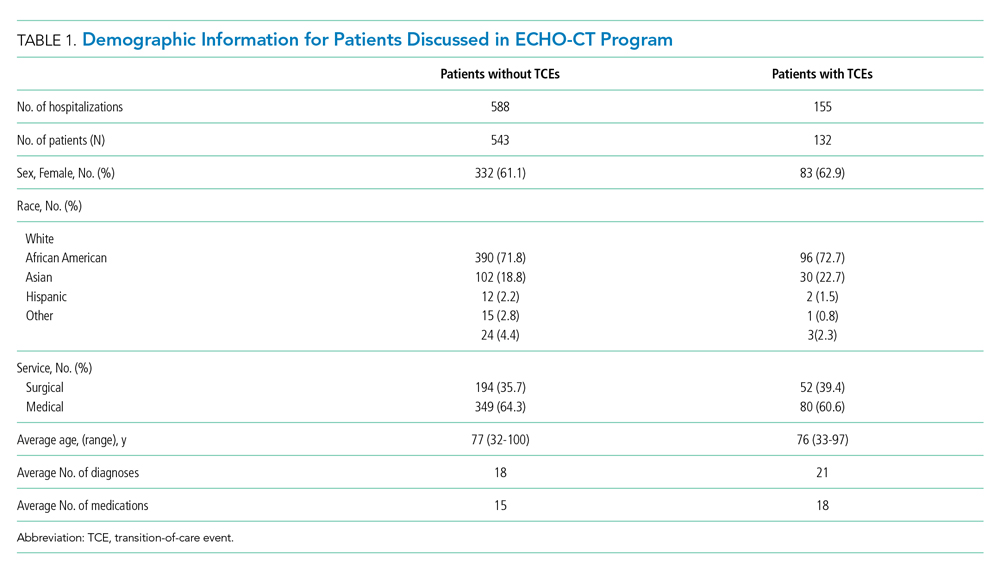
A total of 675 patients (experiencing 743 hospitalizations) were discharged from a medical or surgical service to one of the participating post–acute care sites from January 2016 to October 2018, and were discussed at the interdisciplinary conference. During that time, 139 TCEs were recorded for review, involving 132 patients (Table 1). Patients who experienced TCEs were noted to have a slightly higher average number of diagnoses than did those in the non-TCE group (21 vs 18, respectively) and number of medications (18 vs 15).
Representative examples of TCEs are provided in Table 2. Fifty-eight issues were identified as discharge communication or coordination issues (eg, discharge summary was late or missing at time of discharge to facility, transitional issues were unclear, follow-up appointments were not appropriately scheduled or documented). An additional 52 TCEs were identified as pharmacy or medication issues (eg, medications were inadvertently omitted from discharge medication list, prehospital medication list was incorrect). Medical issues accounted for an additional 27 concerns (eg, patient was hypoglycemic on arrival, inadequate pain control, discovery of new acute medical issues or medical diagnoses that were not clearly documented or communicated by the inpatient team). “Other” issues (two) included unaddressed social concerns, such as insurance issues.

DISCUSSION
The ECHO-CT model unites hospital and post–acute care providers to improve transitions of care and is unique in its focus on the transition from hospital to post–acute care rather than to home care. In 2 years of data collection, we identified several TCEs encompassing a range of concerns. Of the 675 patients discussed, 132 (20%) were noted to have a TCE. When these percentages are applied to the 140 million Medicare hospital discharges that took place during 2000 to 2015, we would estimate nearly 5.5 million TCEs, or 375,000 TCEs per year, that may have affected this population.
The majority of TCEs were communication and coordination errors. Missing or incomplete discharge paperwork, inadequate documentation of inpatient care, and confusion about medical devices or postoperative needs (eg, slings, braces, wound care, drains) were commonly reported. Follow-up appointments with specialists were often not appropriately scheduled or communicated. This may have resulted from unstandardized discharge documentation and a lower priority given to documentation in the setting of multiple clinical demands (eg, direct patient care, complex care coordination, and clinical paperwork and charting). Studies have demonstrated that fewer than one-third of discharge summaries are received by outpatient providers before postdischarge follow-up, and additionally that nearly 40% of patients did not undergo recommended workups for medical issues identified during their hospital stay.7,8 All of this is problematic because appropriate documentation in discharge summaries is associated with a decreased risk of hospital readmission.9
Pharmacy issues were the second most common TCE identified. One member of the post–acute care team noted that “omissions, additions, and replacements” relating to medications were common occurrences. Additionally, it was noted that medications were inadvertently continued for longer than planned or not adjusted appropriately with changing clinical parameters, such as renal function. The results of our analysis are consistent with current literature, which suggests that up to 60% of all medication errors occur during the period surrounding transitions of care.10
There were several limitations to this investigation. Though recording of identified TCEs occurred in real time, analysis of these identified events occurred retrospectively; therefore, investigators had limited ability to retroactively review or recategorize recorded issues, which potentially could have resulted in misclassification or misinterpretation. Additionally, the data were intended to be descriptive; therefore, outcomes such as hospital readmission and patient harm could not be linked to specific TCEs. Furthermore, it is possible that events were not detected by either the postdischarge team or the hospital-based team and, therefore, not captured in this analysis. Further work would be helpful to determine the root causes underlying the identified issues in care transitions, with the goal of improving patient safety and avoiding preventable errors during transitions of care. Although there is comprehensive literature related to errors and medication-related adverse events,11 there is not a consensus of how to classify and report, in a standardized fashion, events arising during the transition period. A validated structure for systematically identifying, monitoring, recording, and reporting issues arising during care transitions will be critical in preventing errors and ensuring patient safety during this high-risk period.
CONCLUSION
Our model is a unique intervention that uses the expertise and engagement of an interdisciplinary team and seeks to identify and remedy issues arising during transitions of care—in real time—to prevent direct harm to vulnerable patients. We have already implemented interventions to improve care based on our experiences with this videoconference-based program. For example, direct feedback was given to discharging teams to improve the discharge summary and associated documentation, and changes to the medication-ordering system were implemented to address specific medication errors discovered. The TCEs identified in this investigation highlight specific areas for improvement with the goal of providing high-quality care for patients and seamless transitions to post–acute care. As health systems and hospitals face new challenges in communication and care coordination, especially due to the recent COVID-19 pandemic, the technology and communication methods used in the ECHO-CT model may become even more relevant for promoting clear communication and patient safety during transitions of care.
Acknowledgment
The ECHO CT team thanks Sabrina Carretie for her contributions in data collection and analysis.
1. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. https://doi.org/10.1001/jama.2018.2408
2. Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. Statistical Brief #205. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; May 2016. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.pdf
3. Kessler C, Williams MC, Moustoukas JN, Pappas C. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49-69. https://doi.org/10.1016/j.cger.2012.10.005
4. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74-77. https://doi.org/10.1177/00333549071220s214
5. Farris G, Sircar M, Bortinger J, et al. Extension for community healthcare outcomes–care transitions: enhancing geriatric care transitions through a multidisciplinary videoconference. J Am Geriatr Soc. 2017;65(3):598-602. https://doi.org/10.1111/jgs.14690
6. Moore AB, Krupp JE, Dufour AB, et al. Improving transitions to postacute care for elderly patients using a novel video-conferencing program: ECHO-Care transitions. Am J Med. 2017;130(10):1199-1204. https://doi.org/10.1016/j.amjmed.2017.04.041
7. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. https://doi.org/10.1001/jama.297.8.831
8. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305-1311. https://doi.org/10.1001/archinte.167.12.1305
9. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x
10. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
11. Claeys C, Nève J, Tulkens PM, Spinewine A. Content validity and inter-rater reliability of an instrument to characterize unintentional medication discrepancies. Drugs Aging. 2012;29(7):577-591. https://doi.org/10.1007/bf03262275
As the population of the United States continues to age, hospitals are seeing an increasing number of older patients with significant medical and social complexity. Medicare data have shown that an increasing number require post–acute care after a hospitalization.1 Discharges to post–acute care settings are often longer and more costly compared with discharges to other settings, which suggests that targeting quality improvement efforts at this transition period may improve the value of care.2
The transition from the hospital setting to a post–acute care facility can be dangerous and complicated due to lapses in communication, medication errors, and the complexity of medical treatment plans. Suboptimal transitions in care can result in adverse events for the patient, as well as confusion in medication regimens or incomplete plans for follow-up care.3
The Project ECHO (Extension for Community Healthcare Outcomes) model was first developed and launched by Sanjeev Arora, MD, in New Mexico in 2003 to expand access to subspecialist care using videoconferencing.4 We first applied this model in 2013 to evaluate the impact of this interdisciplinary videoconferencing tool on the care of patients discharged to post–acute settings.5 We found that patients participating in the Extension for Community Healthcare Outcomes–Care Transitions (ECHO-CT) model experienced decreased risk of rehospitalization, decreased skilled nursing facility (SNF) length of stay, and reduced 30-day healthcare costs, compared with those patients not enrolled in this program; these outcomes were likely due to identification and correction of medication-related errors, improved care coordination, improved disease management, and clarification of goals of care.6 Though these investigations did identify some issues arising during the care transition process, they did not fully describe the types of problems uncovered. We sought to better characterize the clinical and operational issues identified through the ECHO-CT conference, hereafter known as transition-of-care events (TCEs). These issues may include new or evolving medical concerns, an adverse event, or a “near miss.” Identification and classification of TCEs that may contribute to unsafe or fractured care transitions are critical in developing systematic solutions to improve transitions of care, which can ultimately improve patient safety and potentially avoid preventable errors.
METHODS
ECHO-CT Multidisciplinary Video Conference
We conducted ECHO-CT at a large, tertiary care academic medical center. The project design for the ECHO-CT program has been previously described.5 In brief, the program is a weekly, multidisciplinary videoconference between a hospital-based team and post–acute care providers to discuss patients discharged from inpatient services to post–acute care sites, including SNFs and long-term acute care hospitals (LTACHs), during the preceding week. All patients discharged from the tertiary care inpatient site to one of the eight participating SNFs or LTACHs, from either a medical or surgical service, are eligible to be discussed at this weekly interdisciplinary conference. Long-term care facilities were not included in this study. The ECHO-CT program used HIPAA (Health Insurance Portability and Accountability Act)-compliant videoconferencing technology to connect hospital and post–acute care providers.
During the videoconferences, each patient’s hospital course and discharge documentation are reviewed by a hospitalist, and a pharmacist performs a medication reconciliation of each patient’s admission, discharge, and post–acute care medication list. The discharging attending, primary care providers, residents, other trainees, and subspecialist providers are invited to attend. Typically, the interdisciplinary team at the post–acute care sites includes physicians, nurse practitioners, physical therapists, social workers, and case managers. Between 10 and 20 patients are discussed in a case-based format, which includes a summary of the patient’s hospital course, an update from the post–acute care team on the patient’s care, and an opportunity for a discussion regarding any concerns or questions raised by the post–acute care or inpatient care teams. The content and duration of discussion typically lasts approximately 3 to 10 minutes, depending on the needs of the patient and the care team. Each of the eight post–acute care sites participating in the project are assigned a 10- to 15-minute block. A copy of the ECHO-CT session process document is included in the Appendix.
Data Collection
At each interdisciplinary patient review, TCEs were identified and recorded. These events were categorized in real time by the ECHO-CT data collection team into the following categories: medication related, medical, discharge communication/coordination, or other, and recorded in a secured, deidentified database. For individuals whose TCEs could represent more than one category, authors reviewed the available information about the TCEs and determined the most appropriate category; if more than one category was felt to be applicable to a patient’s situation, the events were reclassified into all applicable categories. Data about individual patients, including gender, age at the time of discharge, and other demographic information, were obtained from hospital databases. Number of diagnoses included any diagnosis billed during the patient’s hospital stay, and these data were obtained from a hospital billing database. Average number of medications at discharge was obtained from a hospital pharmacy database.
RESULTS
A total of 675 patients (experiencing 743 hospitalizations) were discharged from a medical or surgical service to one of the participating post–acute care sites from January 2016 to October 2018, and were discussed at the interdisciplinary conference. During that time, 139 TCEs were recorded for review, involving 132 patients (Table 1). Patients who experienced TCEs were noted to have a slightly higher average number of diagnoses than did those in the non-TCE group (21 vs 18, respectively) and number of medications (18 vs 15).

Representative examples of TCEs are provided in Table 2. Fifty-eight issues were identified as discharge communication or coordination issues (eg, discharge summary was late or missing at time of discharge to facility, transitional issues were unclear, follow-up appointments were not appropriately scheduled or documented). An additional 52 TCEs were identified as pharmacy or medication issues (eg, medications were inadvertently omitted from discharge medication list, prehospital medication list was incorrect). Medical issues accounted for an additional 27 concerns (eg, patient was hypoglycemic on arrival, inadequate pain control, discovery of new acute medical issues or medical diagnoses that were not clearly documented or communicated by the inpatient team). “Other” issues (two) included unaddressed social concerns, such as insurance issues.

DISCUSSION
The ECHO-CT model unites hospital and post–acute care providers to improve transitions of care and is unique in its focus on the transition from hospital to post–acute care rather than to home care. In 2 years of data collection, we identified several TCEs encompassing a range of concerns. Of the 675 patients discussed, 132 (20%) were noted to have a TCE. When these percentages are applied to the 140 million Medicare hospital discharges that took place during 2000 to 2015, we would estimate nearly 5.5 million TCEs, or 375,000 TCEs per year, that may have affected this population.
The majority of TCEs were communication and coordination errors. Missing or incomplete discharge paperwork, inadequate documentation of inpatient care, and confusion about medical devices or postoperative needs (eg, slings, braces, wound care, drains) were commonly reported. Follow-up appointments with specialists were often not appropriately scheduled or communicated. This may have resulted from unstandardized discharge documentation and a lower priority given to documentation in the setting of multiple clinical demands (eg, direct patient care, complex care coordination, and clinical paperwork and charting). Studies have demonstrated that fewer than one-third of discharge summaries are received by outpatient providers before postdischarge follow-up, and additionally that nearly 40% of patients did not undergo recommended workups for medical issues identified during their hospital stay.7,8 All of this is problematic because appropriate documentation in discharge summaries is associated with a decreased risk of hospital readmission.9
Pharmacy issues were the second most common TCE identified. One member of the post–acute care team noted that “omissions, additions, and replacements” relating to medications were common occurrences. Additionally, it was noted that medications were inadvertently continued for longer than planned or not adjusted appropriately with changing clinical parameters, such as renal function. The results of our analysis are consistent with current literature, which suggests that up to 60% of all medication errors occur during the period surrounding transitions of care.10
There were several limitations to this investigation. Though recording of identified TCEs occurred in real time, analysis of these identified events occurred retrospectively; therefore, investigators had limited ability to retroactively review or recategorize recorded issues, which potentially could have resulted in misclassification or misinterpretation. Additionally, the data were intended to be descriptive; therefore, outcomes such as hospital readmission and patient harm could not be linked to specific TCEs. Furthermore, it is possible that events were not detected by either the postdischarge team or the hospital-based team and, therefore, not captured in this analysis. Further work would be helpful to determine the root causes underlying the identified issues in care transitions, with the goal of improving patient safety and avoiding preventable errors during transitions of care. Although there is comprehensive literature related to errors and medication-related adverse events,11 there is not a consensus of how to classify and report, in a standardized fashion, events arising during the transition period. A validated structure for systematically identifying, monitoring, recording, and reporting issues arising during care transitions will be critical in preventing errors and ensuring patient safety during this high-risk period.
CONCLUSION
Our model is a unique intervention that uses the expertise and engagement of an interdisciplinary team and seeks to identify and remedy issues arising during transitions of care—in real time—to prevent direct harm to vulnerable patients. We have already implemented interventions to improve care based on our experiences with this videoconference-based program. For example, direct feedback was given to discharging teams to improve the discharge summary and associated documentation, and changes to the medication-ordering system were implemented to address specific medication errors discovered. The TCEs identified in this investigation highlight specific areas for improvement with the goal of providing high-quality care for patients and seamless transitions to post–acute care. As health systems and hospitals face new challenges in communication and care coordination, especially due to the recent COVID-19 pandemic, the technology and communication methods used in the ECHO-CT model may become even more relevant for promoting clear communication and patient safety during transitions of care.
Acknowledgment
The ECHO CT team thanks Sabrina Carretie for her contributions in data collection and analysis.
As the population of the United States continues to age, hospitals are seeing an increasing number of older patients with significant medical and social complexity. Medicare data have shown that an increasing number require post–acute care after a hospitalization.1 Discharges to post–acute care settings are often longer and more costly compared with discharges to other settings, which suggests that targeting quality improvement efforts at this transition period may improve the value of care.2
The transition from the hospital setting to a post–acute care facility can be dangerous and complicated due to lapses in communication, medication errors, and the complexity of medical treatment plans. Suboptimal transitions in care can result in adverse events for the patient, as well as confusion in medication regimens or incomplete plans for follow-up care.3
The Project ECHO (Extension for Community Healthcare Outcomes) model was first developed and launched by Sanjeev Arora, MD, in New Mexico in 2003 to expand access to subspecialist care using videoconferencing.4 We first applied this model in 2013 to evaluate the impact of this interdisciplinary videoconferencing tool on the care of patients discharged to post–acute settings.5 We found that patients participating in the Extension for Community Healthcare Outcomes–Care Transitions (ECHO-CT) model experienced decreased risk of rehospitalization, decreased skilled nursing facility (SNF) length of stay, and reduced 30-day healthcare costs, compared with those patients not enrolled in this program; these outcomes were likely due to identification and correction of medication-related errors, improved care coordination, improved disease management, and clarification of goals of care.6 Though these investigations did identify some issues arising during the care transition process, they did not fully describe the types of problems uncovered. We sought to better characterize the clinical and operational issues identified through the ECHO-CT conference, hereafter known as transition-of-care events (TCEs). These issues may include new or evolving medical concerns, an adverse event, or a “near miss.” Identification and classification of TCEs that may contribute to unsafe or fractured care transitions are critical in developing systematic solutions to improve transitions of care, which can ultimately improve patient safety and potentially avoid preventable errors.
METHODS
ECHO-CT Multidisciplinary Video Conference
We conducted ECHO-CT at a large, tertiary care academic medical center. The project design for the ECHO-CT program has been previously described.5 In brief, the program is a weekly, multidisciplinary videoconference between a hospital-based team and post–acute care providers to discuss patients discharged from inpatient services to post–acute care sites, including SNFs and long-term acute care hospitals (LTACHs), during the preceding week. All patients discharged from the tertiary care inpatient site to one of the eight participating SNFs or LTACHs, from either a medical or surgical service, are eligible to be discussed at this weekly interdisciplinary conference. Long-term care facilities were not included in this study. The ECHO-CT program used HIPAA (Health Insurance Portability and Accountability Act)-compliant videoconferencing technology to connect hospital and post–acute care providers.
During the videoconferences, each patient’s hospital course and discharge documentation are reviewed by a hospitalist, and a pharmacist performs a medication reconciliation of each patient’s admission, discharge, and post–acute care medication list. The discharging attending, primary care providers, residents, other trainees, and subspecialist providers are invited to attend. Typically, the interdisciplinary team at the post–acute care sites includes physicians, nurse practitioners, physical therapists, social workers, and case managers. Between 10 and 20 patients are discussed in a case-based format, which includes a summary of the patient’s hospital course, an update from the post–acute care team on the patient’s care, and an opportunity for a discussion regarding any concerns or questions raised by the post–acute care or inpatient care teams. The content and duration of discussion typically lasts approximately 3 to 10 minutes, depending on the needs of the patient and the care team. Each of the eight post–acute care sites participating in the project are assigned a 10- to 15-minute block. A copy of the ECHO-CT session process document is included in the Appendix.
Data Collection
At each interdisciplinary patient review, TCEs were identified and recorded. These events were categorized in real time by the ECHO-CT data collection team into the following categories: medication related, medical, discharge communication/coordination, or other, and recorded in a secured, deidentified database. For individuals whose TCEs could represent more than one category, authors reviewed the available information about the TCEs and determined the most appropriate category; if more than one category was felt to be applicable to a patient’s situation, the events were reclassified into all applicable categories. Data about individual patients, including gender, age at the time of discharge, and other demographic information, were obtained from hospital databases. Number of diagnoses included any diagnosis billed during the patient’s hospital stay, and these data were obtained from a hospital billing database. Average number of medications at discharge was obtained from a hospital pharmacy database.
RESULTS
A total of 675 patients (experiencing 743 hospitalizations) were discharged from a medical or surgical service to one of the participating post–acute care sites from January 2016 to October 2018, and were discussed at the interdisciplinary conference. During that time, 139 TCEs were recorded for review, involving 132 patients (Table 1). Patients who experienced TCEs were noted to have a slightly higher average number of diagnoses than did those in the non-TCE group (21 vs 18, respectively) and number of medications (18 vs 15).

Representative examples of TCEs are provided in Table 2. Fifty-eight issues were identified as discharge communication or coordination issues (eg, discharge summary was late or missing at time of discharge to facility, transitional issues were unclear, follow-up appointments were not appropriately scheduled or documented). An additional 52 TCEs were identified as pharmacy or medication issues (eg, medications were inadvertently omitted from discharge medication list, prehospital medication list was incorrect). Medical issues accounted for an additional 27 concerns (eg, patient was hypoglycemic on arrival, inadequate pain control, discovery of new acute medical issues or medical diagnoses that were not clearly documented or communicated by the inpatient team). “Other” issues (two) included unaddressed social concerns, such as insurance issues.

DISCUSSION
The ECHO-CT model unites hospital and post–acute care providers to improve transitions of care and is unique in its focus on the transition from hospital to post–acute care rather than to home care. In 2 years of data collection, we identified several TCEs encompassing a range of concerns. Of the 675 patients discussed, 132 (20%) were noted to have a TCE. When these percentages are applied to the 140 million Medicare hospital discharges that took place during 2000 to 2015, we would estimate nearly 5.5 million TCEs, or 375,000 TCEs per year, that may have affected this population.
The majority of TCEs were communication and coordination errors. Missing or incomplete discharge paperwork, inadequate documentation of inpatient care, and confusion about medical devices or postoperative needs (eg, slings, braces, wound care, drains) were commonly reported. Follow-up appointments with specialists were often not appropriately scheduled or communicated. This may have resulted from unstandardized discharge documentation and a lower priority given to documentation in the setting of multiple clinical demands (eg, direct patient care, complex care coordination, and clinical paperwork and charting). Studies have demonstrated that fewer than one-third of discharge summaries are received by outpatient providers before postdischarge follow-up, and additionally that nearly 40% of patients did not undergo recommended workups for medical issues identified during their hospital stay.7,8 All of this is problematic because appropriate documentation in discharge summaries is associated with a decreased risk of hospital readmission.9
Pharmacy issues were the second most common TCE identified. One member of the post–acute care team noted that “omissions, additions, and replacements” relating to medications were common occurrences. Additionally, it was noted that medications were inadvertently continued for longer than planned or not adjusted appropriately with changing clinical parameters, such as renal function. The results of our analysis are consistent with current literature, which suggests that up to 60% of all medication errors occur during the period surrounding transitions of care.10
There were several limitations to this investigation. Though recording of identified TCEs occurred in real time, analysis of these identified events occurred retrospectively; therefore, investigators had limited ability to retroactively review or recategorize recorded issues, which potentially could have resulted in misclassification or misinterpretation. Additionally, the data were intended to be descriptive; therefore, outcomes such as hospital readmission and patient harm could not be linked to specific TCEs. Furthermore, it is possible that events were not detected by either the postdischarge team or the hospital-based team and, therefore, not captured in this analysis. Further work would be helpful to determine the root causes underlying the identified issues in care transitions, with the goal of improving patient safety and avoiding preventable errors during transitions of care. Although there is comprehensive literature related to errors and medication-related adverse events,11 there is not a consensus of how to classify and report, in a standardized fashion, events arising during the transition period. A validated structure for systematically identifying, monitoring, recording, and reporting issues arising during care transitions will be critical in preventing errors and ensuring patient safety during this high-risk period.
CONCLUSION
Our model is a unique intervention that uses the expertise and engagement of an interdisciplinary team and seeks to identify and remedy issues arising during transitions of care—in real time—to prevent direct harm to vulnerable patients. We have already implemented interventions to improve care based on our experiences with this videoconference-based program. For example, direct feedback was given to discharging teams to improve the discharge summary and associated documentation, and changes to the medication-ordering system were implemented to address specific medication errors discovered. The TCEs identified in this investigation highlight specific areas for improvement with the goal of providing high-quality care for patients and seamless transitions to post–acute care. As health systems and hospitals face new challenges in communication and care coordination, especially due to the recent COVID-19 pandemic, the technology and communication methods used in the ECHO-CT model may become even more relevant for promoting clear communication and patient safety during transitions of care.
Acknowledgment
The ECHO CT team thanks Sabrina Carretie for her contributions in data collection and analysis.
1. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. https://doi.org/10.1001/jama.2018.2408
2. Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. Statistical Brief #205. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; May 2016. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.pdf
3. Kessler C, Williams MC, Moustoukas JN, Pappas C. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49-69. https://doi.org/10.1016/j.cger.2012.10.005
4. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74-77. https://doi.org/10.1177/00333549071220s214
5. Farris G, Sircar M, Bortinger J, et al. Extension for community healthcare outcomes–care transitions: enhancing geriatric care transitions through a multidisciplinary videoconference. J Am Geriatr Soc. 2017;65(3):598-602. https://doi.org/10.1111/jgs.14690
6. Moore AB, Krupp JE, Dufour AB, et al. Improving transitions to postacute care for elderly patients using a novel video-conferencing program: ECHO-Care transitions. Am J Med. 2017;130(10):1199-1204. https://doi.org/10.1016/j.amjmed.2017.04.041
7. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. https://doi.org/10.1001/jama.297.8.831
8. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305-1311. https://doi.org/10.1001/archinte.167.12.1305
9. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x
10. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
11. Claeys C, Nève J, Tulkens PM, Spinewine A. Content validity and inter-rater reliability of an instrument to characterize unintentional medication discrepancies. Drugs Aging. 2012;29(7):577-591. https://doi.org/10.1007/bf03262275
1. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616–1617. https://doi.org/10.1001/jama.2018.2408
2. Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. Statistical Brief #205. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; May 2016. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.pdf
3. Kessler C, Williams MC, Moustoukas JN, Pappas C. Transitions of care for the geriatric patient in the emergency department. Clin Geriatr Med. 2013;29(1):49-69. https://doi.org/10.1016/j.cger.2012.10.005
4. Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74-77. https://doi.org/10.1177/00333549071220s214
5. Farris G, Sircar M, Bortinger J, et al. Extension for community healthcare outcomes–care transitions: enhancing geriatric care transitions through a multidisciplinary videoconference. J Am Geriatr Soc. 2017;65(3):598-602. https://doi.org/10.1111/jgs.14690
6. Moore AB, Krupp JE, Dufour AB, et al. Improving transitions to postacute care for elderly patients using a novel video-conferencing program: ECHO-Care transitions. Am J Med. 2017;130(10):1199-1204. https://doi.org/10.1016/j.amjmed.2017.04.041
7. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. https://doi.org/10.1001/jama.297.8.831
8. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305-1311. https://doi.org/10.1001/archinte.167.12.1305
9. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x
10. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
11. Claeys C, Nève J, Tulkens PM, Spinewine A. Content validity and inter-rater reliability of an instrument to characterize unintentional medication discrepancies. Drugs Aging. 2012;29(7):577-591. https://doi.org/10.1007/bf03262275
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Anaphylaxis Management in Adults and Children
Anaphylaxis, an acute, life-threatening allergic response, affects multiple organ systems and manifests variably. Anaphylaxis is likely taking place if one or more of the following occurs: (a) sudden- onset skin and mucosal tissue swelling, (b) skin and mucosal abnormalities or respiratory or gastrointestinal symptoms after exposure to an allergen, or (c) reduced blood pressure after exposure to an allergen. With an estimated lifetime prevalence of up to 5.1%, it is a significant cause of morbidity in adults and children.1 The 2020 anaphylaxis practice parameter update provides recommendations on treatment, prevention, and assessment of biphasic symptom risk in patients experiencing anaphylaxis.2 The guideline provides five key recommendations and four good-practice statements, which we have consolidated into five recommendations for this update.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. All patients with suspected or confirmed anaphylaxis should be treated with epinephrine. (Good-practice statement)
Self-injectable epinephrine is the first-line treatment for anaphylaxis, with weight-based dosing of 0.15 mg/kg for children weighing less than 30 kg and 0.30 mg/kg for children weighing more than 30 kg and adults. Delayed administration of epinephrine can increase anaphylaxis-associated morbidity and mortality. After epinephrine administration, patients should be observed in a healthcare setting for symptom resolution.
Recommendation 2. For all patients, clinicians should assess the risk for developing biphasic anaphylaxis. (Conditional recommendation, very low quality of evidence)
Biphasic anaphylaxis is defined as the return of anaphylaxis symptoms after an asymptomatic period of at least 1 hour, all during a single instance of anaphylaxis. Biphasic anaphylaxis occurs in up to 20% of patients.3 Biphasic anaphylaxis is more likely among patients receiving repeated doses of epinephrine (odds ratio [OR], 4.82; 95% CI, 2.70-8.58), delayed epinephrine administration greater than 60 minutes (OR, 2.29; 95% CI, 1.09-4.79), or a severe initial presentation (OR, 4.82; 95% CI, 1.23-3.61).2 The presence of any of these risk factors raises the risk for developing biphasic anaphylaxis by 17%.4 Severe anaphylaxis is characterized by life-threatening symptoms, including loss of consciousness, syncope or dizziness, hypotension, cardiovascular system collapse, or neurologic dysfunction from hypoperfusion or hypoxia after exposure to an allergen.5
Other risk factors for biphasic anaphylaxis in all ages include a widened pulse pressure, unknown anaphylaxis trigger, and cutaneous signs and symptoms. Drug triggers are also a risk factor in pediatric patients.2
Recommendation 3. All patients with anaphylaxis and risk factors for biphasic anaphylaxis should undergo extended clinical observation in a setting capable of managing anaphylaxis. (Conditional recommendation, very low quality of evidence)
All patients should be monitored for resolution of symptoms prior to discharge, regardless of age or severity at onset. Patients with all three of the following can be discharged 1 hour after symptom resolution because these three factors together have a 95% negative predictive value for biphasic anaphylaxis: nonsevere anaphylaxis, prompt response to epinephrine, and access to medical care.5 In contrast, extended observation of at least 6 hours should be offered to patients with increased risk of biphasic reactions. Patients who have potentially fatal underlying illnesses (eg, severe respiratory or cardiac disease), poor access to emergency medical services, poor self-management skills, or inability to access epinephrine should also be considered for extended observation or hospitalization. Evidence is lacking to define the optimal observation time because extended biphasic reactions can occur from 1 to 78 hours after initial anaphylaxis symptoms.6
Given the lack of specific evidence around length of observation, there is an opportunity for shared decision-making. Every patient should receive education regarding trigger avoidance, reasons to seek care or activate emergency medical services, and warning signs of biphasic anaphylaxis. Additionally, self-injectable epinephrine and an action plan detailing how and when to administer the epinephrine should be provided. Patients with anaphylaxis should follow up with an allergist.
Recommendation 4. Administration of glucocorticoids or antihistamines for prevention of biphasic anaphylaxis is not recommended. (Conditional recommendation, very low quality of evidence)
This guideline discourages glucocorticoids and antihistamines as a primary treatment as it may delay epinephrine administration. Despite treating the cutaneous manifestations of anaphylaxis, antihistamines fail to treat the life-threatening cardiovascular and respiratory symptoms. No clear evidence exists on whether antihistamines or glucocorticoids prevent biphasic anaphylaxis.
Recommendation 5. In adult patients receiving chemotherapy, premedication with antihistamine and/or glucocorticoid should be used to prevent anaphylaxis or infusion-related reactions for some chemotherapeutic agents in patients with no previous reaction to the drug. (Conditional recommendation, very low quality of evidence)
Premedication with antihistamines and/or glucocorticoids was associated with 51% reduced odds for anaphylaxis and infusion-related reactions to certain chemotherapy agents (pegaspargase, docetaxel, carboplatin, oxaliplatin, and rituximab) in adults who had not previously experienced a reaction to the drug (OR, 0.49; 95% CI, 0.37-0.66).2 However, this same benefit was not found with other chemotherapy agents for patients without a prior allergic reaction to the agent, which allows clinicians to defer premedication. The benefit of premedication with antihistamines and/or glucocorticoids to patients with prior anaphylactic reactions to chemotherapy agents was not evaluated in this guideline, nor was the role premedication plays in desensitization to chemotherapy.
CRITIQUE
This guideline was created by a panel of allergists, clinical immunologists, and methodologists using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest (COI) were disclosed by all panel members according to the American Academy of Allergy, Asthma, and Immunology (AAAAI) guidelines. The inclusion of many observational studies and meta-analyses improves the generalizability of the guideline. The authors highlighted the low certainty of evidence due to the lack of randomized controlled trials and significant heterogeneity of the included studies.
Some recommendations in the guideline have implications for costs of care. A recent economic analysis looked at cost-effectiveness for extended observation for anaphylaxis and found it was cost-effective only when patients were at increased risk for biphasic anaphylaxis.7 Although Recommendation 4 advises against the use of glucocorticoids for prevention of biphasic anaphylaxis, one retrospective cohort study demonstrated that glucocorticoid use was associated with decreased length of stay in children admitted with anaphylaxis.8 Therefore, the recommendation to avoid glucocorticoids for prevention of biphasic anaphylaxis could possibly increase hospital length of stay for children. The usefulness of dexamethasone to prevent biphasic anaphylaxis in children 3 to 14 months old is being evaluated in a randomized trial (ClinicalTrials.gov, NCT03523221).
AREAS OF FUTURE STUDY
Future research should better characterize risk factors for biphasic reactions to aid in clinical triage and diagnosis. Additional studies are needed to determine the optimal observation duration for patients experiencing anaphylactic reactions or requiring multiple doses of epinephrine. The role of premedication in patients receiving chemotherapy is poorly described, with few studies evaluating the benefit of premedication in patients with previous anaphylactic reactions.
1. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
2. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
3. Lieberman P, Camargo CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596-602. https://doi.org/10.1016/s1081-1206(10)61086-1
4. Kim TH, Yoon SH, Hong H, Kang HR, Cho SH, Lee SY. Duration of observation for detecting a biphasic reaction in anaphylaxis: a meta-analysis. Int Arch Allergy Immunol. 2019;179(1):31-36. https://doi.org/10.1159/000496092
5. Brown AF, Mckinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108(5):861-866. https://doi.org/10.1067/mai.2001.119028
6. Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: a review of the literature and implications for emergency management. Am J Emerg Med. 2018;36(8):1480-1485. https://doi.org/10.1016/j.ajem.2018.05.009
7. Shaker M, Wallace D, Golden DBK, Oppenheimer J, Greenhawt M. Simulation of health and economic benefits of extended observation of resolved anaphylaxis. JAMA Netw Open. 2019;2(10):e1913951. https://doi.org/10.1001/jamanetworkopen.2019.13951
8. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-724.e3. https://doi.org/10.1016/j.jpeds.2015.05.033
Anaphylaxis, an acute, life-threatening allergic response, affects multiple organ systems and manifests variably. Anaphylaxis is likely taking place if one or more of the following occurs: (a) sudden- onset skin and mucosal tissue swelling, (b) skin and mucosal abnormalities or respiratory or gastrointestinal symptoms after exposure to an allergen, or (c) reduced blood pressure after exposure to an allergen. With an estimated lifetime prevalence of up to 5.1%, it is a significant cause of morbidity in adults and children.1 The 2020 anaphylaxis practice parameter update provides recommendations on treatment, prevention, and assessment of biphasic symptom risk in patients experiencing anaphylaxis.2 The guideline provides five key recommendations and four good-practice statements, which we have consolidated into five recommendations for this update.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. All patients with suspected or confirmed anaphylaxis should be treated with epinephrine. (Good-practice statement)
Self-injectable epinephrine is the first-line treatment for anaphylaxis, with weight-based dosing of 0.15 mg/kg for children weighing less than 30 kg and 0.30 mg/kg for children weighing more than 30 kg and adults. Delayed administration of epinephrine can increase anaphylaxis-associated morbidity and mortality. After epinephrine administration, patients should be observed in a healthcare setting for symptom resolution.
Recommendation 2. For all patients, clinicians should assess the risk for developing biphasic anaphylaxis. (Conditional recommendation, very low quality of evidence)
Biphasic anaphylaxis is defined as the return of anaphylaxis symptoms after an asymptomatic period of at least 1 hour, all during a single instance of anaphylaxis. Biphasic anaphylaxis occurs in up to 20% of patients.3 Biphasic anaphylaxis is more likely among patients receiving repeated doses of epinephrine (odds ratio [OR], 4.82; 95% CI, 2.70-8.58), delayed epinephrine administration greater than 60 minutes (OR, 2.29; 95% CI, 1.09-4.79), or a severe initial presentation (OR, 4.82; 95% CI, 1.23-3.61).2 The presence of any of these risk factors raises the risk for developing biphasic anaphylaxis by 17%.4 Severe anaphylaxis is characterized by life-threatening symptoms, including loss of consciousness, syncope or dizziness, hypotension, cardiovascular system collapse, or neurologic dysfunction from hypoperfusion or hypoxia after exposure to an allergen.5
Other risk factors for biphasic anaphylaxis in all ages include a widened pulse pressure, unknown anaphylaxis trigger, and cutaneous signs and symptoms. Drug triggers are also a risk factor in pediatric patients.2
Recommendation 3. All patients with anaphylaxis and risk factors for biphasic anaphylaxis should undergo extended clinical observation in a setting capable of managing anaphylaxis. (Conditional recommendation, very low quality of evidence)
All patients should be monitored for resolution of symptoms prior to discharge, regardless of age or severity at onset. Patients with all three of the following can be discharged 1 hour after symptom resolution because these three factors together have a 95% negative predictive value for biphasic anaphylaxis: nonsevere anaphylaxis, prompt response to epinephrine, and access to medical care.5 In contrast, extended observation of at least 6 hours should be offered to patients with increased risk of biphasic reactions. Patients who have potentially fatal underlying illnesses (eg, severe respiratory or cardiac disease), poor access to emergency medical services, poor self-management skills, or inability to access epinephrine should also be considered for extended observation or hospitalization. Evidence is lacking to define the optimal observation time because extended biphasic reactions can occur from 1 to 78 hours after initial anaphylaxis symptoms.6
Given the lack of specific evidence around length of observation, there is an opportunity for shared decision-making. Every patient should receive education regarding trigger avoidance, reasons to seek care or activate emergency medical services, and warning signs of biphasic anaphylaxis. Additionally, self-injectable epinephrine and an action plan detailing how and when to administer the epinephrine should be provided. Patients with anaphylaxis should follow up with an allergist.
Recommendation 4. Administration of glucocorticoids or antihistamines for prevention of biphasic anaphylaxis is not recommended. (Conditional recommendation, very low quality of evidence)
This guideline discourages glucocorticoids and antihistamines as a primary treatment as it may delay epinephrine administration. Despite treating the cutaneous manifestations of anaphylaxis, antihistamines fail to treat the life-threatening cardiovascular and respiratory symptoms. No clear evidence exists on whether antihistamines or glucocorticoids prevent biphasic anaphylaxis.
Recommendation 5. In adult patients receiving chemotherapy, premedication with antihistamine and/or glucocorticoid should be used to prevent anaphylaxis or infusion-related reactions for some chemotherapeutic agents in patients with no previous reaction to the drug. (Conditional recommendation, very low quality of evidence)
Premedication with antihistamines and/or glucocorticoids was associated with 51% reduced odds for anaphylaxis and infusion-related reactions to certain chemotherapy agents (pegaspargase, docetaxel, carboplatin, oxaliplatin, and rituximab) in adults who had not previously experienced a reaction to the drug (OR, 0.49; 95% CI, 0.37-0.66).2 However, this same benefit was not found with other chemotherapy agents for patients without a prior allergic reaction to the agent, which allows clinicians to defer premedication. The benefit of premedication with antihistamines and/or glucocorticoids to patients with prior anaphylactic reactions to chemotherapy agents was not evaluated in this guideline, nor was the role premedication plays in desensitization to chemotherapy.
CRITIQUE
This guideline was created by a panel of allergists, clinical immunologists, and methodologists using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest (COI) were disclosed by all panel members according to the American Academy of Allergy, Asthma, and Immunology (AAAAI) guidelines. The inclusion of many observational studies and meta-analyses improves the generalizability of the guideline. The authors highlighted the low certainty of evidence due to the lack of randomized controlled trials and significant heterogeneity of the included studies.
Some recommendations in the guideline have implications for costs of care. A recent economic analysis looked at cost-effectiveness for extended observation for anaphylaxis and found it was cost-effective only when patients were at increased risk for biphasic anaphylaxis.7 Although Recommendation 4 advises against the use of glucocorticoids for prevention of biphasic anaphylaxis, one retrospective cohort study demonstrated that glucocorticoid use was associated with decreased length of stay in children admitted with anaphylaxis.8 Therefore, the recommendation to avoid glucocorticoids for prevention of biphasic anaphylaxis could possibly increase hospital length of stay for children. The usefulness of dexamethasone to prevent biphasic anaphylaxis in children 3 to 14 months old is being evaluated in a randomized trial (ClinicalTrials.gov, NCT03523221).
AREAS OF FUTURE STUDY
Future research should better characterize risk factors for biphasic reactions to aid in clinical triage and diagnosis. Additional studies are needed to determine the optimal observation duration for patients experiencing anaphylactic reactions or requiring multiple doses of epinephrine. The role of premedication in patients receiving chemotherapy is poorly described, with few studies evaluating the benefit of premedication in patients with previous anaphylactic reactions.
Anaphylaxis, an acute, life-threatening allergic response, affects multiple organ systems and manifests variably. Anaphylaxis is likely taking place if one or more of the following occurs: (a) sudden- onset skin and mucosal tissue swelling, (b) skin and mucosal abnormalities or respiratory or gastrointestinal symptoms after exposure to an allergen, or (c) reduced blood pressure after exposure to an allergen. With an estimated lifetime prevalence of up to 5.1%, it is a significant cause of morbidity in adults and children.1 The 2020 anaphylaxis practice parameter update provides recommendations on treatment, prevention, and assessment of biphasic symptom risk in patients experiencing anaphylaxis.2 The guideline provides five key recommendations and four good-practice statements, which we have consolidated into five recommendations for this update.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. All patients with suspected or confirmed anaphylaxis should be treated with epinephrine. (Good-practice statement)
Self-injectable epinephrine is the first-line treatment for anaphylaxis, with weight-based dosing of 0.15 mg/kg for children weighing less than 30 kg and 0.30 mg/kg for children weighing more than 30 kg and adults. Delayed administration of epinephrine can increase anaphylaxis-associated morbidity and mortality. After epinephrine administration, patients should be observed in a healthcare setting for symptom resolution.
Recommendation 2. For all patients, clinicians should assess the risk for developing biphasic anaphylaxis. (Conditional recommendation, very low quality of evidence)
Biphasic anaphylaxis is defined as the return of anaphylaxis symptoms after an asymptomatic period of at least 1 hour, all during a single instance of anaphylaxis. Biphasic anaphylaxis occurs in up to 20% of patients.3 Biphasic anaphylaxis is more likely among patients receiving repeated doses of epinephrine (odds ratio [OR], 4.82; 95% CI, 2.70-8.58), delayed epinephrine administration greater than 60 minutes (OR, 2.29; 95% CI, 1.09-4.79), or a severe initial presentation (OR, 4.82; 95% CI, 1.23-3.61).2 The presence of any of these risk factors raises the risk for developing biphasic anaphylaxis by 17%.4 Severe anaphylaxis is characterized by life-threatening symptoms, including loss of consciousness, syncope or dizziness, hypotension, cardiovascular system collapse, or neurologic dysfunction from hypoperfusion or hypoxia after exposure to an allergen.5
Other risk factors for biphasic anaphylaxis in all ages include a widened pulse pressure, unknown anaphylaxis trigger, and cutaneous signs and symptoms. Drug triggers are also a risk factor in pediatric patients.2
Recommendation 3. All patients with anaphylaxis and risk factors for biphasic anaphylaxis should undergo extended clinical observation in a setting capable of managing anaphylaxis. (Conditional recommendation, very low quality of evidence)
All patients should be monitored for resolution of symptoms prior to discharge, regardless of age or severity at onset. Patients with all three of the following can be discharged 1 hour after symptom resolution because these three factors together have a 95% negative predictive value for biphasic anaphylaxis: nonsevere anaphylaxis, prompt response to epinephrine, and access to medical care.5 In contrast, extended observation of at least 6 hours should be offered to patients with increased risk of biphasic reactions. Patients who have potentially fatal underlying illnesses (eg, severe respiratory or cardiac disease), poor access to emergency medical services, poor self-management skills, or inability to access epinephrine should also be considered for extended observation or hospitalization. Evidence is lacking to define the optimal observation time because extended biphasic reactions can occur from 1 to 78 hours after initial anaphylaxis symptoms.6
Given the lack of specific evidence around length of observation, there is an opportunity for shared decision-making. Every patient should receive education regarding trigger avoidance, reasons to seek care or activate emergency medical services, and warning signs of biphasic anaphylaxis. Additionally, self-injectable epinephrine and an action plan detailing how and when to administer the epinephrine should be provided. Patients with anaphylaxis should follow up with an allergist.
Recommendation 4. Administration of glucocorticoids or antihistamines for prevention of biphasic anaphylaxis is not recommended. (Conditional recommendation, very low quality of evidence)
This guideline discourages glucocorticoids and antihistamines as a primary treatment as it may delay epinephrine administration. Despite treating the cutaneous manifestations of anaphylaxis, antihistamines fail to treat the life-threatening cardiovascular and respiratory symptoms. No clear evidence exists on whether antihistamines or glucocorticoids prevent biphasic anaphylaxis.
Recommendation 5. In adult patients receiving chemotherapy, premedication with antihistamine and/or glucocorticoid should be used to prevent anaphylaxis or infusion-related reactions for some chemotherapeutic agents in patients with no previous reaction to the drug. (Conditional recommendation, very low quality of evidence)
Premedication with antihistamines and/or glucocorticoids was associated with 51% reduced odds for anaphylaxis and infusion-related reactions to certain chemotherapy agents (pegaspargase, docetaxel, carboplatin, oxaliplatin, and rituximab) in adults who had not previously experienced a reaction to the drug (OR, 0.49; 95% CI, 0.37-0.66).2 However, this same benefit was not found with other chemotherapy agents for patients without a prior allergic reaction to the agent, which allows clinicians to defer premedication. The benefit of premedication with antihistamines and/or glucocorticoids to patients with prior anaphylactic reactions to chemotherapy agents was not evaluated in this guideline, nor was the role premedication plays in desensitization to chemotherapy.
CRITIQUE
This guideline was created by a panel of allergists, clinical immunologists, and methodologists using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest (COI) were disclosed by all panel members according to the American Academy of Allergy, Asthma, and Immunology (AAAAI) guidelines. The inclusion of many observational studies and meta-analyses improves the generalizability of the guideline. The authors highlighted the low certainty of evidence due to the lack of randomized controlled trials and significant heterogeneity of the included studies.
Some recommendations in the guideline have implications for costs of care. A recent economic analysis looked at cost-effectiveness for extended observation for anaphylaxis and found it was cost-effective only when patients were at increased risk for biphasic anaphylaxis.7 Although Recommendation 4 advises against the use of glucocorticoids for prevention of biphasic anaphylaxis, one retrospective cohort study demonstrated that glucocorticoid use was associated with decreased length of stay in children admitted with anaphylaxis.8 Therefore, the recommendation to avoid glucocorticoids for prevention of biphasic anaphylaxis could possibly increase hospital length of stay for children. The usefulness of dexamethasone to prevent biphasic anaphylaxis in children 3 to 14 months old is being evaluated in a randomized trial (ClinicalTrials.gov, NCT03523221).
AREAS OF FUTURE STUDY
Future research should better characterize risk factors for biphasic reactions to aid in clinical triage and diagnosis. Additional studies are needed to determine the optimal observation duration for patients experiencing anaphylactic reactions or requiring multiple doses of epinephrine. The role of premedication in patients receiving chemotherapy is poorly described, with few studies evaluating the benefit of premedication in patients with previous anaphylactic reactions.
1. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
2. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
3. Lieberman P, Camargo CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596-602. https://doi.org/10.1016/s1081-1206(10)61086-1
4. Kim TH, Yoon SH, Hong H, Kang HR, Cho SH, Lee SY. Duration of observation for detecting a biphasic reaction in anaphylaxis: a meta-analysis. Int Arch Allergy Immunol. 2019;179(1):31-36. https://doi.org/10.1159/000496092
5. Brown AF, Mckinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108(5):861-866. https://doi.org/10.1067/mai.2001.119028
6. Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: a review of the literature and implications for emergency management. Am J Emerg Med. 2018;36(8):1480-1485. https://doi.org/10.1016/j.ajem.2018.05.009
7. Shaker M, Wallace D, Golden DBK, Oppenheimer J, Greenhawt M. Simulation of health and economic benefits of extended observation of resolved anaphylaxis. JAMA Netw Open. 2019;2(10):e1913951. https://doi.org/10.1001/jamanetworkopen.2019.13951
8. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-724.e3. https://doi.org/10.1016/j.jpeds.2015.05.033
1. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
2. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
3. Lieberman P, Camargo CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596-602. https://doi.org/10.1016/s1081-1206(10)61086-1
4. Kim TH, Yoon SH, Hong H, Kang HR, Cho SH, Lee SY. Duration of observation for detecting a biphasic reaction in anaphylaxis: a meta-analysis. Int Arch Allergy Immunol. 2019;179(1):31-36. https://doi.org/10.1159/000496092
5. Brown AF, Mckinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108(5):861-866. https://doi.org/10.1067/mai.2001.119028
6. Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: a review of the literature and implications for emergency management. Am J Emerg Med. 2018;36(8):1480-1485. https://doi.org/10.1016/j.ajem.2018.05.009
7. Shaker M, Wallace D, Golden DBK, Oppenheimer J, Greenhawt M. Simulation of health and economic benefits of extended observation of resolved anaphylaxis. JAMA Netw Open. 2019;2(10):e1913951. https://doi.org/10.1001/jamanetworkopen.2019.13951
8. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-724.e3. https://doi.org/10.1016/j.jpeds.2015.05.033
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Secondary Fracture Prevention for Hospitalized Patients
Osteoporosis is the most prevalent bone disease and a leading cause of morbidity and mortality in older people. According to the National Health and Nutrition Examination Survey, from 2005-2010, there were an estimated 10.2 million adults 50 years and older with osteoporosis and 43.4 million more with low bone mass in the United States.1 Osteoporotic fracture is a leading cause of hospitalization in the United States for women 55 years or older, ahead of heart attacks, stroke, and breast cancer.2 Despite elucidation of the pathogenesis of osteoporosis and the advent of effective and widely available therapies, a “treatment gap” separates the many patients who warrant therapy from the few who receive it. Systematic improvement strategies, such as coordinator-based fracture liaison services, have had a positive impact on addressing this treatment gap.3 There is an opportunity for hospitalists to further narrow this treatment gap.
The American Society of Bone and Mineral Research, in conjunction with the Center for Medical Technology Policy, developed consensus clinical recommendations to address secondary fracture prevention for people 65 years or older who have experienced a hip or vertebral fracture.4 We address six of the fundamental and two of the supplemental recommendations as they apply to the practice of hospital medicine.
KEY RECOMMENDATIONS FOR HOSPITALISTs
Recommendations 1 and 2
Communicate key information to the patient and their usual healthcare provider. Patients 65 years or older with a hip or vertebral fracture likely have osteoporosis and are at high risk for subsequent fractures, which can lead to a decline in function and an increase in mortality. Patients must be counseled regarding their diagnosis, their risks, and the actions they can take to manage their disease. Primary care providers must be notified of the occurrence of the fracture, the diagnosis of osteoporosis, and the plans for management.
We recommend hospitalists act as leading advocates for at-risk patients to ensure that this communication occurs during hospitalization. We encourage hospitals and institutions to adopt systematic interventions to facilitate postdischarge care for these patients. These may include implementing a fracture liaison service, with multidisciplinary secondary fracture–prevention strategies using physicians, pharmacists, nurses, social workers, and case managers for care coordination and treatment initiation.
Elderly patients with osteoporotic fragility fractures are at risk for further morbidity and mortality. Coordination of care between the inpatient care team and the primary care provider is necessary to reduce this risk. In addition to verbal communication and especially when verbal communication is not feasible, discharge documents provided to patients and outpatient providers should clearly identify the occurrence of a hip or vertebral fracture and a discharge diagnosis of osteoporosis if not previously documented, regardless of bone mineral density (BMD) results or lack of testing.
Recommendation 3
Regularly assess fall risk. Patients 65 years or older with a current or prior hip or vertebral fracture must be regularly assessed for risk of falls. Hospitalists can assess patients’ ongoing risk for falls at time of admission or during hospitalization. Risk factors include prior falls; advanced age; visual, auditory, or cognitive impairment; decreased muscle strength; gait and balance impairment; diabetes mellitus; use of multiple medications, and others.5 Specialist evaluation by a physical therapist or a physiatrist should be considered. Active medications should be reviewed for adverse effects and interactions. The use of diuretics, antipsychotics, antidepressants, benzodiazepines, antiepileptics, and opioids should be minimized.
Recommendations 4, 5, 6, and 11
Offer pharmacologic therapy and initiate calcium and vitamin D supplementation. Recommendations 4 through 6 and 11 advocate pharmacologic interventions including bisphosphonates, denosumab, vitamin D, and/or calcium to reduce the risk of future fractures. Bisphosphonates are the cornerstone of pharmacologic therapy for secondary fracture prevention. The efficacy of these agents for prevention of subsequent fractures outweighs the potential for interference in healing of surgically repaired bones.6 Oral bisphosphonate therapy should be initiated in the hospital or at discharge. Parenteral bisphosphonates and denosumab may be utilized in patients unable to tolerate or absorb oral bisphosphonates due to esophageal or other gastrointestinal disease. Initiation of these agents should be delayed until after vitamin D and calcium supplementation have been administered for 2 weeks after the fracture to reduce the risk of precipitating hypocalcemia, and they should not be used in patients with confirmed hypocalcemia until that is resolved. BMD measurement is not necessary prior to pharmacologic therapy initiation because the risk of fracture is elevated for these patients regardless of BMD. Patients without significant dental disease or planned oral or maxillofacial procedures may begin bisphosphonate therapy prior to a full dental assessment because risk of osteonecrosis of the jaw is low.
The guidelines recommend people 65 years or older with a hip or vertebral fracture receive daily supplementation of at least 800 IU vitamin D. Patients unable to achieve an intake of 1,200 mg/day of calcium from food sources should receive daily calcium supplementation. The effect of vitamin D monotherapy on fracture risk is not clear; however, strong evidence suggests that fracture risk is reduced when individuals at high risk of deficiency receive supplementation with vitamin D and calcium. Calcium supplementation alone has not demonstrated reduction in fracture risk. Total daily calcium intake above 1,500 mg has not been shown to provide additional benefit and is potentially harmful.
Recommendation 9
Counsel patients on lifestyle modifications and consider physical therapy. Tobacco has a deleterious effect on bone density and increases risk for osteoporotic fragility fracture.7 Hospitalists should obtain tobacco use history from all patients with an osteoporotic fracture and provide tobacco cessation counseling when appropriate. Excessive alcohol consumption increases the risk of fall injuries.8 Hospitalists should counsel patients to limit alcohol intake to a maximum of two drinks a day for men and one drink a day for women.
Weight-bearing and strength-training exercises, particularly those involving balance and trunk muscle strength, are associated with reduction in fall-risk. Exercise must be tailored to the patient’s physical capacity. Hospitalists may partner with physical therapists or physiatrists to facilitate development of an exercise plan to maximize benefit and minimize risk of injury.
CRITIQUE
We found this document to be highly informative and well cited, with ample evidence to support the recommendations.
Methods in Preparing Guidelines
The multistakeholder coalition did not employ a rigorous and standardized methodology for the guideline, such as GRADE (Grading of Recommendations Assessment, Development, and Evaluation); hence, no assessment of evidence quality, benefits and harms of an intervention, or resource use was provided.
Potential Conflicts for Guideline Authors
Eight guideline authors have pharmaceutical relationships with the manufacturer of one of the medications listed on the guidelines (Amgen-denosumab, Novartis-zoledronic acid). There are no disclosures reported from the multistakeholder coalition members who are not listed as guideline authors.
AREAS IN NEED OF FUTURE STUDY
We anticipate future studies may report outcomes focused on secondary prevention of fractures. Additionally, we would like to see new studies investigating patient-centered outcomes such as improvement in functional status and ambulatory independence based on improved postfracture medical therapies. We see an opportunity for studies assessing real-world outcomes to inform future recommendations, particularly after widespread implementation of secondary fracture prevention therapy either initiated during hospitalization or purposefully planned for after discharge.
We would like to see more trial data comparing the safety and cost-effectiveness of first-line therapy, namely oral bisphosphonates, to alternative treatments, particularly parenteral agents, which may improve treatment compliance because of the convenience in dosing frequency.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526. https://doi.org/10.1002/jbmr.2269
2. Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53-62. https://doi.org/10.1016/j.mayocp.2014.09.011
3. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028-1034. https://doi.org/10.1007/s00198-003-1507-z
4. Conley RB, Adib G, Adler RA, et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35(1):36-52. https://doi.org/10.1002/jbmr.3877
5. Bueno-Cavanillas A, Padilla-Ruiz F, Jiménez-Moleón JJ, Peinado-Alonso CA, Gálvez-Vargas R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur J Epidemiol. 2000;16(9):849-859. https://doi.org/10.1023/a:1007636531965
6. Vannucci L, Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2016;17(17):2267-2272. https://doi.org/10.1080/14656566.2016.1241765
7. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841-846. https://doi.org/10.1136/bmj.315.7112.841
8. Chen CM, Yoon YH. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004-2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120-1132. https://doi.org/10.1080/10826084.2017.1293101
Osteoporosis is the most prevalent bone disease and a leading cause of morbidity and mortality in older people. According to the National Health and Nutrition Examination Survey, from 2005-2010, there were an estimated 10.2 million adults 50 years and older with osteoporosis and 43.4 million more with low bone mass in the United States.1 Osteoporotic fracture is a leading cause of hospitalization in the United States for women 55 years or older, ahead of heart attacks, stroke, and breast cancer.2 Despite elucidation of the pathogenesis of osteoporosis and the advent of effective and widely available therapies, a “treatment gap” separates the many patients who warrant therapy from the few who receive it. Systematic improvement strategies, such as coordinator-based fracture liaison services, have had a positive impact on addressing this treatment gap.3 There is an opportunity for hospitalists to further narrow this treatment gap.
The American Society of Bone and Mineral Research, in conjunction with the Center for Medical Technology Policy, developed consensus clinical recommendations to address secondary fracture prevention for people 65 years or older who have experienced a hip or vertebral fracture.4 We address six of the fundamental and two of the supplemental recommendations as they apply to the practice of hospital medicine.
KEY RECOMMENDATIONS FOR HOSPITALISTs
Recommendations 1 and 2
Communicate key information to the patient and their usual healthcare provider. Patients 65 years or older with a hip or vertebral fracture likely have osteoporosis and are at high risk for subsequent fractures, which can lead to a decline in function and an increase in mortality. Patients must be counseled regarding their diagnosis, their risks, and the actions they can take to manage their disease. Primary care providers must be notified of the occurrence of the fracture, the diagnosis of osteoporosis, and the plans for management.
We recommend hospitalists act as leading advocates for at-risk patients to ensure that this communication occurs during hospitalization. We encourage hospitals and institutions to adopt systematic interventions to facilitate postdischarge care for these patients. These may include implementing a fracture liaison service, with multidisciplinary secondary fracture–prevention strategies using physicians, pharmacists, nurses, social workers, and case managers for care coordination and treatment initiation.
Elderly patients with osteoporotic fragility fractures are at risk for further morbidity and mortality. Coordination of care between the inpatient care team and the primary care provider is necessary to reduce this risk. In addition to verbal communication and especially when verbal communication is not feasible, discharge documents provided to patients and outpatient providers should clearly identify the occurrence of a hip or vertebral fracture and a discharge diagnosis of osteoporosis if not previously documented, regardless of bone mineral density (BMD) results or lack of testing.
Recommendation 3
Regularly assess fall risk. Patients 65 years or older with a current or prior hip or vertebral fracture must be regularly assessed for risk of falls. Hospitalists can assess patients’ ongoing risk for falls at time of admission or during hospitalization. Risk factors include prior falls; advanced age; visual, auditory, or cognitive impairment; decreased muscle strength; gait and balance impairment; diabetes mellitus; use of multiple medications, and others.5 Specialist evaluation by a physical therapist or a physiatrist should be considered. Active medications should be reviewed for adverse effects and interactions. The use of diuretics, antipsychotics, antidepressants, benzodiazepines, antiepileptics, and opioids should be minimized.
Recommendations 4, 5, 6, and 11
Offer pharmacologic therapy and initiate calcium and vitamin D supplementation. Recommendations 4 through 6 and 11 advocate pharmacologic interventions including bisphosphonates, denosumab, vitamin D, and/or calcium to reduce the risk of future fractures. Bisphosphonates are the cornerstone of pharmacologic therapy for secondary fracture prevention. The efficacy of these agents for prevention of subsequent fractures outweighs the potential for interference in healing of surgically repaired bones.6 Oral bisphosphonate therapy should be initiated in the hospital or at discharge. Parenteral bisphosphonates and denosumab may be utilized in patients unable to tolerate or absorb oral bisphosphonates due to esophageal or other gastrointestinal disease. Initiation of these agents should be delayed until after vitamin D and calcium supplementation have been administered for 2 weeks after the fracture to reduce the risk of precipitating hypocalcemia, and they should not be used in patients with confirmed hypocalcemia until that is resolved. BMD measurement is not necessary prior to pharmacologic therapy initiation because the risk of fracture is elevated for these patients regardless of BMD. Patients without significant dental disease or planned oral or maxillofacial procedures may begin bisphosphonate therapy prior to a full dental assessment because risk of osteonecrosis of the jaw is low.
The guidelines recommend people 65 years or older with a hip or vertebral fracture receive daily supplementation of at least 800 IU vitamin D. Patients unable to achieve an intake of 1,200 mg/day of calcium from food sources should receive daily calcium supplementation. The effect of vitamin D monotherapy on fracture risk is not clear; however, strong evidence suggests that fracture risk is reduced when individuals at high risk of deficiency receive supplementation with vitamin D and calcium. Calcium supplementation alone has not demonstrated reduction in fracture risk. Total daily calcium intake above 1,500 mg has not been shown to provide additional benefit and is potentially harmful.
Recommendation 9
Counsel patients on lifestyle modifications and consider physical therapy. Tobacco has a deleterious effect on bone density and increases risk for osteoporotic fragility fracture.7 Hospitalists should obtain tobacco use history from all patients with an osteoporotic fracture and provide tobacco cessation counseling when appropriate. Excessive alcohol consumption increases the risk of fall injuries.8 Hospitalists should counsel patients to limit alcohol intake to a maximum of two drinks a day for men and one drink a day for women.
Weight-bearing and strength-training exercises, particularly those involving balance and trunk muscle strength, are associated with reduction in fall-risk. Exercise must be tailored to the patient’s physical capacity. Hospitalists may partner with physical therapists or physiatrists to facilitate development of an exercise plan to maximize benefit and minimize risk of injury.
CRITIQUE
We found this document to be highly informative and well cited, with ample evidence to support the recommendations.
Methods in Preparing Guidelines
The multistakeholder coalition did not employ a rigorous and standardized methodology for the guideline, such as GRADE (Grading of Recommendations Assessment, Development, and Evaluation); hence, no assessment of evidence quality, benefits and harms of an intervention, or resource use was provided.
Potential Conflicts for Guideline Authors
Eight guideline authors have pharmaceutical relationships with the manufacturer of one of the medications listed on the guidelines (Amgen-denosumab, Novartis-zoledronic acid). There are no disclosures reported from the multistakeholder coalition members who are not listed as guideline authors.
AREAS IN NEED OF FUTURE STUDY
We anticipate future studies may report outcomes focused on secondary prevention of fractures. Additionally, we would like to see new studies investigating patient-centered outcomes such as improvement in functional status and ambulatory independence based on improved postfracture medical therapies. We see an opportunity for studies assessing real-world outcomes to inform future recommendations, particularly after widespread implementation of secondary fracture prevention therapy either initiated during hospitalization or purposefully planned for after discharge.
We would like to see more trial data comparing the safety and cost-effectiveness of first-line therapy, namely oral bisphosphonates, to alternative treatments, particularly parenteral agents, which may improve treatment compliance because of the convenience in dosing frequency.
Osteoporosis is the most prevalent bone disease and a leading cause of morbidity and mortality in older people. According to the National Health and Nutrition Examination Survey, from 2005-2010, there were an estimated 10.2 million adults 50 years and older with osteoporosis and 43.4 million more with low bone mass in the United States.1 Osteoporotic fracture is a leading cause of hospitalization in the United States for women 55 years or older, ahead of heart attacks, stroke, and breast cancer.2 Despite elucidation of the pathogenesis of osteoporosis and the advent of effective and widely available therapies, a “treatment gap” separates the many patients who warrant therapy from the few who receive it. Systematic improvement strategies, such as coordinator-based fracture liaison services, have had a positive impact on addressing this treatment gap.3 There is an opportunity for hospitalists to further narrow this treatment gap.
The American Society of Bone and Mineral Research, in conjunction with the Center for Medical Technology Policy, developed consensus clinical recommendations to address secondary fracture prevention for people 65 years or older who have experienced a hip or vertebral fracture.4 We address six of the fundamental and two of the supplemental recommendations as they apply to the practice of hospital medicine.
KEY RECOMMENDATIONS FOR HOSPITALISTs
Recommendations 1 and 2
Communicate key information to the patient and their usual healthcare provider. Patients 65 years or older with a hip or vertebral fracture likely have osteoporosis and are at high risk for subsequent fractures, which can lead to a decline in function and an increase in mortality. Patients must be counseled regarding their diagnosis, their risks, and the actions they can take to manage their disease. Primary care providers must be notified of the occurrence of the fracture, the diagnosis of osteoporosis, and the plans for management.
We recommend hospitalists act as leading advocates for at-risk patients to ensure that this communication occurs during hospitalization. We encourage hospitals and institutions to adopt systematic interventions to facilitate postdischarge care for these patients. These may include implementing a fracture liaison service, with multidisciplinary secondary fracture–prevention strategies using physicians, pharmacists, nurses, social workers, and case managers for care coordination and treatment initiation.
Elderly patients with osteoporotic fragility fractures are at risk for further morbidity and mortality. Coordination of care between the inpatient care team and the primary care provider is necessary to reduce this risk. In addition to verbal communication and especially when verbal communication is not feasible, discharge documents provided to patients and outpatient providers should clearly identify the occurrence of a hip or vertebral fracture and a discharge diagnosis of osteoporosis if not previously documented, regardless of bone mineral density (BMD) results or lack of testing.
Recommendation 3
Regularly assess fall risk. Patients 65 years or older with a current or prior hip or vertebral fracture must be regularly assessed for risk of falls. Hospitalists can assess patients’ ongoing risk for falls at time of admission or during hospitalization. Risk factors include prior falls; advanced age; visual, auditory, or cognitive impairment; decreased muscle strength; gait and balance impairment; diabetes mellitus; use of multiple medications, and others.5 Specialist evaluation by a physical therapist or a physiatrist should be considered. Active medications should be reviewed for adverse effects and interactions. The use of diuretics, antipsychotics, antidepressants, benzodiazepines, antiepileptics, and opioids should be minimized.
Recommendations 4, 5, 6, and 11
Offer pharmacologic therapy and initiate calcium and vitamin D supplementation. Recommendations 4 through 6 and 11 advocate pharmacologic interventions including bisphosphonates, denosumab, vitamin D, and/or calcium to reduce the risk of future fractures. Bisphosphonates are the cornerstone of pharmacologic therapy for secondary fracture prevention. The efficacy of these agents for prevention of subsequent fractures outweighs the potential for interference in healing of surgically repaired bones.6 Oral bisphosphonate therapy should be initiated in the hospital or at discharge. Parenteral bisphosphonates and denosumab may be utilized in patients unable to tolerate or absorb oral bisphosphonates due to esophageal or other gastrointestinal disease. Initiation of these agents should be delayed until after vitamin D and calcium supplementation have been administered for 2 weeks after the fracture to reduce the risk of precipitating hypocalcemia, and they should not be used in patients with confirmed hypocalcemia until that is resolved. BMD measurement is not necessary prior to pharmacologic therapy initiation because the risk of fracture is elevated for these patients regardless of BMD. Patients without significant dental disease or planned oral or maxillofacial procedures may begin bisphosphonate therapy prior to a full dental assessment because risk of osteonecrosis of the jaw is low.
The guidelines recommend people 65 years or older with a hip or vertebral fracture receive daily supplementation of at least 800 IU vitamin D. Patients unable to achieve an intake of 1,200 mg/day of calcium from food sources should receive daily calcium supplementation. The effect of vitamin D monotherapy on fracture risk is not clear; however, strong evidence suggests that fracture risk is reduced when individuals at high risk of deficiency receive supplementation with vitamin D and calcium. Calcium supplementation alone has not demonstrated reduction in fracture risk. Total daily calcium intake above 1,500 mg has not been shown to provide additional benefit and is potentially harmful.
Recommendation 9
Counsel patients on lifestyle modifications and consider physical therapy. Tobacco has a deleterious effect on bone density and increases risk for osteoporotic fragility fracture.7 Hospitalists should obtain tobacco use history from all patients with an osteoporotic fracture and provide tobacco cessation counseling when appropriate. Excessive alcohol consumption increases the risk of fall injuries.8 Hospitalists should counsel patients to limit alcohol intake to a maximum of two drinks a day for men and one drink a day for women.
Weight-bearing and strength-training exercises, particularly those involving balance and trunk muscle strength, are associated with reduction in fall-risk. Exercise must be tailored to the patient’s physical capacity. Hospitalists may partner with physical therapists or physiatrists to facilitate development of an exercise plan to maximize benefit and minimize risk of injury.
CRITIQUE
We found this document to be highly informative and well cited, with ample evidence to support the recommendations.
Methods in Preparing Guidelines
The multistakeholder coalition did not employ a rigorous and standardized methodology for the guideline, such as GRADE (Grading of Recommendations Assessment, Development, and Evaluation); hence, no assessment of evidence quality, benefits and harms of an intervention, or resource use was provided.
Potential Conflicts for Guideline Authors
Eight guideline authors have pharmaceutical relationships with the manufacturer of one of the medications listed on the guidelines (Amgen-denosumab, Novartis-zoledronic acid). There are no disclosures reported from the multistakeholder coalition members who are not listed as guideline authors.
AREAS IN NEED OF FUTURE STUDY
We anticipate future studies may report outcomes focused on secondary prevention of fractures. Additionally, we would like to see new studies investigating patient-centered outcomes such as improvement in functional status and ambulatory independence based on improved postfracture medical therapies. We see an opportunity for studies assessing real-world outcomes to inform future recommendations, particularly after widespread implementation of secondary fracture prevention therapy either initiated during hospitalization or purposefully planned for after discharge.
We would like to see more trial data comparing the safety and cost-effectiveness of first-line therapy, namely oral bisphosphonates, to alternative treatments, particularly parenteral agents, which may improve treatment compliance because of the convenience in dosing frequency.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526. https://doi.org/10.1002/jbmr.2269
2. Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53-62. https://doi.org/10.1016/j.mayocp.2014.09.011
3. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028-1034. https://doi.org/10.1007/s00198-003-1507-z
4. Conley RB, Adib G, Adler RA, et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35(1):36-52. https://doi.org/10.1002/jbmr.3877
5. Bueno-Cavanillas A, Padilla-Ruiz F, Jiménez-Moleón JJ, Peinado-Alonso CA, Gálvez-Vargas R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur J Epidemiol. 2000;16(9):849-859. https://doi.org/10.1023/a:1007636531965
6. Vannucci L, Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2016;17(17):2267-2272. https://doi.org/10.1080/14656566.2016.1241765
7. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841-846. https://doi.org/10.1136/bmj.315.7112.841
8. Chen CM, Yoon YH. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004-2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120-1132. https://doi.org/10.1080/10826084.2017.1293101
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520-2526. https://doi.org/10.1002/jbmr.2269
2. Singer A, Exuzides A, Spangler L, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53-62. https://doi.org/10.1016/j.mayocp.2014.09.011
3. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003;14(12):1028-1034. https://doi.org/10.1007/s00198-003-1507-z
4. Conley RB, Adib G, Adler RA, et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35(1):36-52. https://doi.org/10.1002/jbmr.3877
5. Bueno-Cavanillas A, Padilla-Ruiz F, Jiménez-Moleón JJ, Peinado-Alonso CA, Gálvez-Vargas R. Risk factors in falls among the elderly according to extrinsic and intrinsic precipitating causes. Eur J Epidemiol. 2000;16(9):849-859. https://doi.org/10.1023/a:1007636531965
6. Vannucci L, Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2016;17(17):2267-2272. https://doi.org/10.1080/14656566.2016.1241765
7. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841-846. https://doi.org/10.1136/bmj.315.7112.841
8. Chen CM, Yoon YH. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004-2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120-1132. https://doi.org/10.1080/10826084.2017.1293101
© 2021 Society of Hospital Medicine
Protecting Children by Healing Their Caregivers
It was a busy night in the emergency department. EMS called to give a heads up—they were on their way with a girl who was “pretty banged up.” They warned us that the story seemed a little fishy. We thought we were ready. The trauma bay was organized; supplies were at the ready and everyone had a role. Within seconds of her arrival, it was clear that no one could ever have been truly prepared. She was unresponsive and unstable. Her injuries were widespread, brutal, and long term. My seasoned attendings would describe it as no less than horrific. There was no question—someone had done this to her.
After she was stabilized, her wounds were gently tended, her body was bathed, her hair was combed. She died several days later. While distressed, many members of her team took consolation in the idea that, after years of torture, she finally got to be loved.
It’s no wonder that every person involved with her care during her hospitalization was so deeply affected by her. How could anyone do this to another person? Or even worse, to an innocent child? “What a monster,” we said. “Only a monster could have done this.”
While anyone would agree that what this abuser—the girl’s mother—did was brutal and wrong, I would also argue that the underlying danger is much more systemic. We call her the “monster,” but I sense that the real monster is still lurking in the shadows, unnamed. I can’t help but try to understand this woman; it is unfair to condemn her without first learning her story. How were her actions guided by her own history of trauma, abuse, and violent discipline as a child? We preach to each other and to our learners that trauma-informed care is essential; you must not ask what’s wrong with you, but what happened to you. Founder and Director of the Equal Justice Initiative Bryan Stevenson has said that “each of us is more than the worst thing we’ve ever done.”1 It’s inhumane of us to dehumanize her for this atrocity, especially without pausing to ask how her environment, personal trauma, and understanding of child development set the risk.
It is important to understand the cycle of trauma and abuse. Traumatic experiences have been shown to alter neurodevelopment and the body’s stress response, particularly when experiences take place early in life, when they are repeated and long term, and when they are severe. We know that adverse childhood experiences are cumulative and result in adverse outcomes as adults, including increased likelihood of violent or criminal activity. We know that prior history of trauma, specifically child abuse, sexual abuse, or domestic violence, is associated with higher potential for child abuse later on.
The effect of experiencing trauma is such that only 22% of adults who experienced abuse or neglect as children will achieve resiliency.2 In a world in which social distancing and isolation have become the new normal, we must be even more aware of the effects of trauma on families. The COVID-19 pandemic has increased known risk factors for child abuse, including financial hardship, unemployment, increased anxiety, increased caregiver responsibilities, and decreased access to mental health services and community resources.3 Furthermore, virtual learning environments may have significant implications on the reporting of child abuse. Among cases of maltreatment of children that received an investigation or alternative response in 2018, 20.5% were reported by education personnel.4 While remote learning options may be necessary to minimize risk of viral spread, fewer interactions between children and mandatory reporters may result in child maltreatment going undetected. In the face of these challenges, I urge our healthcare system to use current constraints as fuel for creative interventions including the following:
- Applying advances in telemedicine to create a new opportunity to interface with families, provide mental health support, connect them with resources, and offer gentle guidance about safe parenting.
- Improving both screening methods for parental trauma and distress and referrals for support services.
- Advocating for adequate access to life-sustaining resources including shelter, food, and healthcare for all families. This is a necessary foundation for building resilience.
- Providing bias training for mandatory reporters to ensure that all children and their families are approached with respect and compassion.
- Prioritizing innovation that provides long-lasting, sustainable, and equitable access to support and healing.
To best protect our children, we must heal their adult caregivers; we must help them to conquer their monsters.
Our patient and her family have since visited me in my thoughts and dreams, less often now than before. While I never truly knew her, she has left an open void where there should have been the promise of a healthy, growing, and developing child. Within that void resides fear. I fear for other “hidden children” and the abuse they are at risk for experiencing. I fear that her siblings, now living without their mother, will become victims of the instability of being “in the system.” I fear that by turning to punishment as our only solution, we miss opportunities to prevent such tragedy. Despite the darkness, she also brings me hope. I hope that her siblings can rely on each other as a foundation for resilience. I hope that we as a healthcare system can continue to love our patients without question or condition. I hope that we as a society can invest in breaking the cycle of trauma and in supporting parents. I hope that we can create a system in which children can grow up free from abuse.
1. Stevenson B. Just Mercy: A Story of Justice and Redemption. Spiegel & Grau; 2015.
2. De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23(2):185-222. https://doi.org/10.1016/j.chc.2014.01.002
3. Schneider W, Waldfogel J, Brooks-Gunn J. The Great Recession and risk for child abuse and neglect. Child Youth Serv Rev. 2017;72:71-81. https://doi.org/10.1016/j.childyouth.2016.10.016
4. Child Maltreatment 2018. Children’s Bureau, Youth and Families, Administration on Children, Administration for Children and Families, U.S. Department of Health & Human Services; January 15, 2020. Accessed May 10, 2020. https://www.acf.hhs.gov/cb/resource/child-maltreatment-2018
It was a busy night in the emergency department. EMS called to give a heads up—they were on their way with a girl who was “pretty banged up.” They warned us that the story seemed a little fishy. We thought we were ready. The trauma bay was organized; supplies were at the ready and everyone had a role. Within seconds of her arrival, it was clear that no one could ever have been truly prepared. She was unresponsive and unstable. Her injuries were widespread, brutal, and long term. My seasoned attendings would describe it as no less than horrific. There was no question—someone had done this to her.
After she was stabilized, her wounds were gently tended, her body was bathed, her hair was combed. She died several days later. While distressed, many members of her team took consolation in the idea that, after years of torture, she finally got to be loved.
It’s no wonder that every person involved with her care during her hospitalization was so deeply affected by her. How could anyone do this to another person? Or even worse, to an innocent child? “What a monster,” we said. “Only a monster could have done this.”
While anyone would agree that what this abuser—the girl’s mother—did was brutal and wrong, I would also argue that the underlying danger is much more systemic. We call her the “monster,” but I sense that the real monster is still lurking in the shadows, unnamed. I can’t help but try to understand this woman; it is unfair to condemn her without first learning her story. How were her actions guided by her own history of trauma, abuse, and violent discipline as a child? We preach to each other and to our learners that trauma-informed care is essential; you must not ask what’s wrong with you, but what happened to you. Founder and Director of the Equal Justice Initiative Bryan Stevenson has said that “each of us is more than the worst thing we’ve ever done.”1 It’s inhumane of us to dehumanize her for this atrocity, especially without pausing to ask how her environment, personal trauma, and understanding of child development set the risk.
It is important to understand the cycle of trauma and abuse. Traumatic experiences have been shown to alter neurodevelopment and the body’s stress response, particularly when experiences take place early in life, when they are repeated and long term, and when they are severe. We know that adverse childhood experiences are cumulative and result in adverse outcomes as adults, including increased likelihood of violent or criminal activity. We know that prior history of trauma, specifically child abuse, sexual abuse, or domestic violence, is associated with higher potential for child abuse later on.
The effect of experiencing trauma is such that only 22% of adults who experienced abuse or neglect as children will achieve resiliency.2 In a world in which social distancing and isolation have become the new normal, we must be even more aware of the effects of trauma on families. The COVID-19 pandemic has increased known risk factors for child abuse, including financial hardship, unemployment, increased anxiety, increased caregiver responsibilities, and decreased access to mental health services and community resources.3 Furthermore, virtual learning environments may have significant implications on the reporting of child abuse. Among cases of maltreatment of children that received an investigation or alternative response in 2018, 20.5% were reported by education personnel.4 While remote learning options may be necessary to minimize risk of viral spread, fewer interactions between children and mandatory reporters may result in child maltreatment going undetected. In the face of these challenges, I urge our healthcare system to use current constraints as fuel for creative interventions including the following:
- Applying advances in telemedicine to create a new opportunity to interface with families, provide mental health support, connect them with resources, and offer gentle guidance about safe parenting.
- Improving both screening methods for parental trauma and distress and referrals for support services.
- Advocating for adequate access to life-sustaining resources including shelter, food, and healthcare for all families. This is a necessary foundation for building resilience.
- Providing bias training for mandatory reporters to ensure that all children and their families are approached with respect and compassion.
- Prioritizing innovation that provides long-lasting, sustainable, and equitable access to support and healing.
To best protect our children, we must heal their adult caregivers; we must help them to conquer their monsters.
Our patient and her family have since visited me in my thoughts and dreams, less often now than before. While I never truly knew her, she has left an open void where there should have been the promise of a healthy, growing, and developing child. Within that void resides fear. I fear for other “hidden children” and the abuse they are at risk for experiencing. I fear that her siblings, now living without their mother, will become victims of the instability of being “in the system.” I fear that by turning to punishment as our only solution, we miss opportunities to prevent such tragedy. Despite the darkness, she also brings me hope. I hope that her siblings can rely on each other as a foundation for resilience. I hope that we as a healthcare system can continue to love our patients without question or condition. I hope that we as a society can invest in breaking the cycle of trauma and in supporting parents. I hope that we can create a system in which children can grow up free from abuse.
It was a busy night in the emergency department. EMS called to give a heads up—they were on their way with a girl who was “pretty banged up.” They warned us that the story seemed a little fishy. We thought we were ready. The trauma bay was organized; supplies were at the ready and everyone had a role. Within seconds of her arrival, it was clear that no one could ever have been truly prepared. She was unresponsive and unstable. Her injuries were widespread, brutal, and long term. My seasoned attendings would describe it as no less than horrific. There was no question—someone had done this to her.
After she was stabilized, her wounds were gently tended, her body was bathed, her hair was combed. She died several days later. While distressed, many members of her team took consolation in the idea that, after years of torture, she finally got to be loved.
It’s no wonder that every person involved with her care during her hospitalization was so deeply affected by her. How could anyone do this to another person? Or even worse, to an innocent child? “What a monster,” we said. “Only a monster could have done this.”
While anyone would agree that what this abuser—the girl’s mother—did was brutal and wrong, I would also argue that the underlying danger is much more systemic. We call her the “monster,” but I sense that the real monster is still lurking in the shadows, unnamed. I can’t help but try to understand this woman; it is unfair to condemn her without first learning her story. How were her actions guided by her own history of trauma, abuse, and violent discipline as a child? We preach to each other and to our learners that trauma-informed care is essential; you must not ask what’s wrong with you, but what happened to you. Founder and Director of the Equal Justice Initiative Bryan Stevenson has said that “each of us is more than the worst thing we’ve ever done.”1 It’s inhumane of us to dehumanize her for this atrocity, especially without pausing to ask how her environment, personal trauma, and understanding of child development set the risk.
It is important to understand the cycle of trauma and abuse. Traumatic experiences have been shown to alter neurodevelopment and the body’s stress response, particularly when experiences take place early in life, when they are repeated and long term, and when they are severe. We know that adverse childhood experiences are cumulative and result in adverse outcomes as adults, including increased likelihood of violent or criminal activity. We know that prior history of trauma, specifically child abuse, sexual abuse, or domestic violence, is associated with higher potential for child abuse later on.
The effect of experiencing trauma is such that only 22% of adults who experienced abuse or neglect as children will achieve resiliency.2 In a world in which social distancing and isolation have become the new normal, we must be even more aware of the effects of trauma on families. The COVID-19 pandemic has increased known risk factors for child abuse, including financial hardship, unemployment, increased anxiety, increased caregiver responsibilities, and decreased access to mental health services and community resources.3 Furthermore, virtual learning environments may have significant implications on the reporting of child abuse. Among cases of maltreatment of children that received an investigation or alternative response in 2018, 20.5% were reported by education personnel.4 While remote learning options may be necessary to minimize risk of viral spread, fewer interactions between children and mandatory reporters may result in child maltreatment going undetected. In the face of these challenges, I urge our healthcare system to use current constraints as fuel for creative interventions including the following:
- Applying advances in telemedicine to create a new opportunity to interface with families, provide mental health support, connect them with resources, and offer gentle guidance about safe parenting.
- Improving both screening methods for parental trauma and distress and referrals for support services.
- Advocating for adequate access to life-sustaining resources including shelter, food, and healthcare for all families. This is a necessary foundation for building resilience.
- Providing bias training for mandatory reporters to ensure that all children and their families are approached with respect and compassion.
- Prioritizing innovation that provides long-lasting, sustainable, and equitable access to support and healing.
To best protect our children, we must heal their adult caregivers; we must help them to conquer their monsters.
Our patient and her family have since visited me in my thoughts and dreams, less often now than before. While I never truly knew her, she has left an open void where there should have been the promise of a healthy, growing, and developing child. Within that void resides fear. I fear for other “hidden children” and the abuse they are at risk for experiencing. I fear that her siblings, now living without their mother, will become victims of the instability of being “in the system.” I fear that by turning to punishment as our only solution, we miss opportunities to prevent such tragedy. Despite the darkness, she also brings me hope. I hope that her siblings can rely on each other as a foundation for resilience. I hope that we as a healthcare system can continue to love our patients without question or condition. I hope that we as a society can invest in breaking the cycle of trauma and in supporting parents. I hope that we can create a system in which children can grow up free from abuse.
1. Stevenson B. Just Mercy: A Story of Justice and Redemption. Spiegel & Grau; 2015.
2. De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23(2):185-222. https://doi.org/10.1016/j.chc.2014.01.002
3. Schneider W, Waldfogel J, Brooks-Gunn J. The Great Recession and risk for child abuse and neglect. Child Youth Serv Rev. 2017;72:71-81. https://doi.org/10.1016/j.childyouth.2016.10.016
4. Child Maltreatment 2018. Children’s Bureau, Youth and Families, Administration on Children, Administration for Children and Families, U.S. Department of Health & Human Services; January 15, 2020. Accessed May 10, 2020. https://www.acf.hhs.gov/cb/resource/child-maltreatment-2018
1. Stevenson B. Just Mercy: A Story of Justice and Redemption. Spiegel & Grau; 2015.
2. De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23(2):185-222. https://doi.org/10.1016/j.chc.2014.01.002
3. Schneider W, Waldfogel J, Brooks-Gunn J. The Great Recession and risk for child abuse and neglect. Child Youth Serv Rev. 2017;72:71-81. https://doi.org/10.1016/j.childyouth.2016.10.016
4. Child Maltreatment 2018. Children’s Bureau, Youth and Families, Administration on Children, Administration for Children and Families, U.S. Department of Health & Human Services; January 15, 2020. Accessed May 10, 2020. https://www.acf.hhs.gov/cb/resource/child-maltreatment-2018
© 2021 Society of Hospital Medicine
Prioritizing High-Value, Equitable Care After the COVID-19 Shutdown: An Opportunity for a Healthcare Renaissance
The day after Memorial Day 2020 marked an important transition in the United States’ experience with coronavirus disease 2019 (COVID-19), with many states making initial plans to reopen.
This year’s widespread healthcare closures were necessary to reduce COVID-19 transmission and prepare for a future patient surge, but these closures had unintended consequences. Nearly half of adults polled said they or someone in their household had foregone or delayed care since the outbreak began.1 This was especially true for visits to emergency departments and doctors’ offices for strokes, heart attacks, and routine medical care.2 In a survey across 49 states, only 7% of primary care practices considered scheduling preventive visits as a high priority.3 Eleven percent of polled adults reported delaying care worsened their condition,1 and in hard-hit areas such as New York City, non-COVID mortality was 22% higher than expected.4
Avoidance of the medical system decreased not only use of necessary, high-value care but also use of low-value care. Low-value services are those in which the “potential for harms exceed the potential benefits,”5 such as unnecessary hospitalizations, avoidable emergency department or clinic visits, unwarranted or excessive diagnostic testing (eg, annual physicals), and certain procedures (eg, spinal fusion surgery for low-back pain).
KEEP PATIENTS CENTRAL IN REOPENING SERVICES TO DELIVER HIGH-VALUE CARE
Medical centers can better focus on high-value care by defining their high-risk patient populations; high-value treatments, procedures, and preventive care; and phases of reopening. During the first pandemic wave, medical centers tried to reassure patients about emergency care, such as coming in for chest pain or neurologic symptoms, through personal outreach and media campaigns. Outpatient virtual visits also continued, including primary care, specialty services, mental health treatment, and physical therapy. While reopening, some medical centers have assessed disparities by relying on their data analytics and, if available, embedded health services researchers to understand what care was stopped and what populations were most affected.
The University of California health systems, for example, had a learning collaborative focused on sharing methods to restore care delivery that prioritizes patient needs. Some campuses conducted analyses using both electronic health record data and input from patients and their care teams to identify clinical needs and determine patient outreach plans. Some approaches used machine learning models to identify patients at highest risk of hospitalization or emergency department visits over the next 12 months and to conduct additional outreach to schedule these patients in primary and specialty care if clinically appropriate. Similarly, surgical specialties identified the highest-priority nonemergent surgeries for scheduling, including cancer resection, radiation therapy, and pain-management procedures. Similar guidance toward the most meaningful care has been prioritized within the United States Department of Veterans Affairs.
The rapid deployment of telehealth and payment models that reimburse video and in-clinic visits equally created new opportunities for medical centers to expand high-value care in lower-cost home settings. Similarly, new infrastructure is being developed to help define smarter use of virtual visits and home-based lab collection and monitoring.
Medical centers also must pay careful attention to redeploying service capacity for underused, high-value services. The pandemic uncovered existing staff that could be redeployed to support these changes. For example, with an “all hands on deck” mentality during the pandemic, in some medical centers, analysts or care managers from less-prioritized or duplicative areas were reassigned to vital COVID-19 efforts. Medical centers may realize that this staff can provide more value in the future by supporting increased high-value, affordable healthcare.
DELIBERATELY AVOID LOW-VALUE CARE
During the intial wave of the pandemic, medical centers greatly reduced the care they provided, often focusing on delivering essential care. This preparation for a surge of COVID patients had the effect of halting many unnecessary services by moving care from the clinic to home under new reimbursement changes, such as those affecting telehealth payments. The experience of reducing low-value medical services and visits can be extended to limiting unnecessary diagnostic testing. Medical centers could, for example, focus only on tests that advance care plans; reduce unnecessary blood draws, procedures, and vital sign checks on stable patients; shift to medications with less-frequent dosing intervals; and consolidate visits by treatment teams.10,11
Medical centers, however, now face continued pressures to increase revenue because 75% report their organization’s top priority is focused on increasing patient volume.12
PROACTIVELY AVOID WORSENING HEALTHCARE DISPARITIES
As medical centers reboot, operational and clinical leaders must proactively view changes through an equity lens to avoid exacerbating health disparities among vulnerable populations. The pandemic has focused national attention on the severity and pervasiveness of disparities and created an imperative for substantive action to evaluate how every decision will affect health equity. For example, medical centers are expanding use of telehealth to improve patient outreach. However, in a survey of primary care physicians, 72% said they have patients who are unable to access telehealth because they do not have access to technology.3 Exclusion of these patients from programs risks worsening health disparities. In a recent survey, nearly 65% of medical centers report reexamining existing policies, protocols, and practices for patients at risk of disparities.12
REFORM TO SUPPORT HIGH-VALUE CARE DELIVERY
Medical centers nationwide will need payment reform that provides greater financial stability beyond the pandemic to support high-value care delivery. They also will need flexibility to invest in prevention and to deliver the appropriate intensity of care to meet patients’ and communities’ needs.15-17 Options to provide this support include prospective population-based payments that may create more resilience in protecting access to care when it is most needed. Models can include fully capitated payment for physician practices.19,20 For example, after Vermont entered a single accountable care organization (ACO) model with the Centers for Medicare & Medicaid Services (CMS) in 2018, they not only generated a $97 million Medicaid savings, but also had a financial cushion that was later used in their COVID-19 response.21,22 The advanced payments allowed primary care practices and community agencies to invest in a digital tool to support outreach to patient at high risk for virus complications.
Hospitals similarly can adapt global budgets that incentivize financial stewardship by encouraging clinicians to resume necessary services and not unnecessary ones.16 For example, CMS partnered with Pennsylvania’s Department of Health to provide prospective all-payer global budgets for rural hospitals and Maryland’s Health Services Cost Review Commission that negotiates a budget with each hospital. During the COVID-19 pandemic, hospitals in these programs have had more financial protection from fluctuating finances by allowing for easier shifts in service delivery location and adjustments in rates to compensate for declines in visits and procedures.23
Policy makers and payers also can hold medical centers accountable to evidence-based guidelines and appropriate use of care, especially when necessary but expensive (eg, percutaneous coronary interventions, spinal surgeries, or cancer care). Funding agencies, additionally, can support these efforts by focusing on research, dissemination, and reliable implementation of these practices.
CONCLUSION
The COVID-19 crisis presents a tremendous opportunity for each medical center to revitalize healthcare. This opportunity can be seized only with reform by policy makers, payers, and regulatory agencies who encourage restarting high-value care without low-value services. We must take deliberate action so the nation’s medical centers can better meet patients’ needs to make healthcare more resilient, efficient, and fair.
1. Hamel L, Kearney A, Kirzinger A, Lopes L, Muñana C, Brodie M. KFF Health Tracking Poll - May 2020: Impact of Coronavirus on Personal Health, Economic and Food Security, and Medicaid. Kaiser Family Foundation; May 27, 2020. Accessed August 9, 2020. https://www.kff.org/report-section/kff-health-tracking-poll-may-2020-health-and-economic-impacts/
2. McFarling UL. ‘Where are all our patients?’: Covid phobia is keeping people with serious heart symptoms away from ERs. STAT. April 23, 2020. Accessed August 9, 2020. https://www.statnews.com/2020/04/23/coronavirus-phobia-keeping-heart-patients-away-from-er/
3. Primary Care & COVID-19: Week 4 Survey. Primary Care Collaborative; April 9, 2020. Accessed August 9, 2020. https://www.pcpcc.org/2020/04/08/primary-care-covid-19-week-4-survey
4. Olson DR, Huynh M, Fine A, et al. New York City Department of Health and Mental Hygiene (DOHMH) COVID-19 Response Team. Preliminary estimate of excess mortality during the COVID-19 outbreak — New York City, March 11–May 2, 2020. Morbidity and Mortality Weekly Report. May 11, 2020. Accessed August 9, 2020. https://stacks.cdc.gov/view/cdc/87858
5. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280(11):1000-1005. https://doi.org/10.1001/jama.280.11.1000
6. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
7. Collins SR, Gunja MZ, Doty MM, Bhupal HK. Americans’ confidence in their ability to pay for health care is falling. To The Point blog. May 10, 2018. The Commonwealth Fund. Accessed August 9, 2020. https://www.commonwealthfund.org/blog/2018/americans-confidence-their-ability-pay-health-care-falling
8. Saad L. More Americans delaying medical treatment due to cost. Gallup News. December 9, 2019. Accessed August 9, 2020. https://news.gallup.com/poll/269138/americans-delaying-medical-treatment-due-cost.aspx
9. Lee VS. Fee for service is a terrible way to pay for health care. Try a subscription model instead. STAT. June 12, 2020. Accessed August 9, 2020. https://www.statnews.com/2020/06/12/fee-for-service-is-a-terrible-way-to-pay-for-health-care-try-a-subscription-model-instead/
10. Moriates C, Shah NT, Arora VM. A framework for the frontline: how hospitalists can improve healthcare value. J Hosp Med. 2016;11(4):297-302. https://doi.org/10.1002/jhm.2494
11. Seymann G, Komsoukaniants A, Bouland D, Jenkins I. The Silver Linings Playbook for Covid-19. KevinMD. June 12, 2020. Accessed August 9, 2020. https://www.kevinmd.com/blog/2020/06/the-silver-linings-playbook-for-covid-19.html
12. Advis In The News. Industry Professionals Weigh In: Future of Healthcare Survey. Advis. Accessed August 9, 2020. https://advis.com/advis-in-the-news/post-pandemic-survey-june2020/
13. APM Measurement Effort. Healthcare Learning Payment and Action Network; 2019. Accessed August 9, 2020. https://hcp-lan.org/workproducts/apm-infographic-2019.pdf
14. Mosley D, DeBehnke D. Rural hospital sustainability: new analysis shows worsening situation for rural hospitals, residents. Navigant; February 2019. Accessed August 9, 2020. https://guidehouse.com/-/media/www/site/insights/healthcare/2019/navigant-rural-hospital-analysis-22019.pdf
15. Gondi S, Chokshi DA. Financial stability as a goal of payment reform—a lesson from COVID-19. JAMA Health Forum. August 6, 2020. Accessed August 9, 2020. https://jamanetwork.com/channels/health-forum/fullarticle/2769307
16. Murphy K, Koski-Vacirca R, Sharfstein J. Resilience in health care financing. JAMA. 2020;324(2):126-127. https://doi.org/10.1001/jama.2020.10417
17. Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127-2128. https://doi.org/10.1001/jama.2020.6269
18. Weiss AJ, Elixhauser A, Andrews RM. Statistical Brief #170: Characteristics of Operating Room Procedures in U.S. Hospitals, 2011. Healthcare Costs and Utilization Project, Agency for Healthcare Research and Quality; February 2014. Accessed August 9, 2020: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.pdf
19. Crook HL, Saunders RS, Bleser WK, Broome T, Muhlestein D, McLellan MB. Leveraging Payment Reforms For COVID-19 And Beyond: Recommendations For Medicare ACOs And CMS’s Interim Final Rule. Health Affairs Blog. May 29, 2020. Accessed August 9, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200528.402208/full/
20. Blue Cross NC Launches Comprehensive Program to Help Independent Primary Care Practices Stay in Business. Press release. BlueCross BlueShield of North Carolina; June 24, 2020. Accessed August 9, 2020. https://mediacenter.bcbsnc.com/news/blue-cross-nc-launches-comprehensive-program-to-help-independent-primary-care-practices-stay-in-business
21. RTI International. State Innovation Models (SIM) Initiative Evaluation: Model Test Year Five Annual Report. Centers for Medicaid & Medicare Services; December 2019. Accessed August 9, 2020. https://downloads.cms.gov/files/cmmi/sim-rd1-mt-fifthannrpt.pdf
22. Wack A. A Message from OneCare CEO Vicki Loner: OneCare’s Response to the Pandemic. OneCare Vermont. May 1, 2020. Accessed August 9, 2020. https://www.onecarevt.org/20200501-covid19/
23. Haber S, Bell H, Morrison M, et al. Evaluation of the Maryland All-Payer Model: Vol 1: Final Report. Centers for Medicare & Medicaid Services; November 2019. Accessed August 9, 2020. https://downloads.cms.gov/files/md-allpayer-finalevalrpt.pdf
The day after Memorial Day 2020 marked an important transition in the United States’ experience with coronavirus disease 2019 (COVID-19), with many states making initial plans to reopen.
This year’s widespread healthcare closures were necessary to reduce COVID-19 transmission and prepare for a future patient surge, but these closures had unintended consequences. Nearly half of adults polled said they or someone in their household had foregone or delayed care since the outbreak began.1 This was especially true for visits to emergency departments and doctors’ offices for strokes, heart attacks, and routine medical care.2 In a survey across 49 states, only 7% of primary care practices considered scheduling preventive visits as a high priority.3 Eleven percent of polled adults reported delaying care worsened their condition,1 and in hard-hit areas such as New York City, non-COVID mortality was 22% higher than expected.4
Avoidance of the medical system decreased not only use of necessary, high-value care but also use of low-value care. Low-value services are those in which the “potential for harms exceed the potential benefits,”5 such as unnecessary hospitalizations, avoidable emergency department or clinic visits, unwarranted or excessive diagnostic testing (eg, annual physicals), and certain procedures (eg, spinal fusion surgery for low-back pain).
KEEP PATIENTS CENTRAL IN REOPENING SERVICES TO DELIVER HIGH-VALUE CARE
Medical centers can better focus on high-value care by defining their high-risk patient populations; high-value treatments, procedures, and preventive care; and phases of reopening. During the first pandemic wave, medical centers tried to reassure patients about emergency care, such as coming in for chest pain or neurologic symptoms, through personal outreach and media campaigns. Outpatient virtual visits also continued, including primary care, specialty services, mental health treatment, and physical therapy. While reopening, some medical centers have assessed disparities by relying on their data analytics and, if available, embedded health services researchers to understand what care was stopped and what populations were most affected.
The University of California health systems, for example, had a learning collaborative focused on sharing methods to restore care delivery that prioritizes patient needs. Some campuses conducted analyses using both electronic health record data and input from patients and their care teams to identify clinical needs and determine patient outreach plans. Some approaches used machine learning models to identify patients at highest risk of hospitalization or emergency department visits over the next 12 months and to conduct additional outreach to schedule these patients in primary and specialty care if clinically appropriate. Similarly, surgical specialties identified the highest-priority nonemergent surgeries for scheduling, including cancer resection, radiation therapy, and pain-management procedures. Similar guidance toward the most meaningful care has been prioritized within the United States Department of Veterans Affairs.
The rapid deployment of telehealth and payment models that reimburse video and in-clinic visits equally created new opportunities for medical centers to expand high-value care in lower-cost home settings. Similarly, new infrastructure is being developed to help define smarter use of virtual visits and home-based lab collection and monitoring.
Medical centers also must pay careful attention to redeploying service capacity for underused, high-value services. The pandemic uncovered existing staff that could be redeployed to support these changes. For example, with an “all hands on deck” mentality during the pandemic, in some medical centers, analysts or care managers from less-prioritized or duplicative areas were reassigned to vital COVID-19 efforts. Medical centers may realize that this staff can provide more value in the future by supporting increased high-value, affordable healthcare.
DELIBERATELY AVOID LOW-VALUE CARE
During the intial wave of the pandemic, medical centers greatly reduced the care they provided, often focusing on delivering essential care. This preparation for a surge of COVID patients had the effect of halting many unnecessary services by moving care from the clinic to home under new reimbursement changes, such as those affecting telehealth payments. The experience of reducing low-value medical services and visits can be extended to limiting unnecessary diagnostic testing. Medical centers could, for example, focus only on tests that advance care plans; reduce unnecessary blood draws, procedures, and vital sign checks on stable patients; shift to medications with less-frequent dosing intervals; and consolidate visits by treatment teams.10,11
Medical centers, however, now face continued pressures to increase revenue because 75% report their organization’s top priority is focused on increasing patient volume.12
PROACTIVELY AVOID WORSENING HEALTHCARE DISPARITIES
As medical centers reboot, operational and clinical leaders must proactively view changes through an equity lens to avoid exacerbating health disparities among vulnerable populations. The pandemic has focused national attention on the severity and pervasiveness of disparities and created an imperative for substantive action to evaluate how every decision will affect health equity. For example, medical centers are expanding use of telehealth to improve patient outreach. However, in a survey of primary care physicians, 72% said they have patients who are unable to access telehealth because they do not have access to technology.3 Exclusion of these patients from programs risks worsening health disparities. In a recent survey, nearly 65% of medical centers report reexamining existing policies, protocols, and practices for patients at risk of disparities.12
REFORM TO SUPPORT HIGH-VALUE CARE DELIVERY
Medical centers nationwide will need payment reform that provides greater financial stability beyond the pandemic to support high-value care delivery. They also will need flexibility to invest in prevention and to deliver the appropriate intensity of care to meet patients’ and communities’ needs.15-17 Options to provide this support include prospective population-based payments that may create more resilience in protecting access to care when it is most needed. Models can include fully capitated payment for physician practices.19,20 For example, after Vermont entered a single accountable care organization (ACO) model with the Centers for Medicare & Medicaid Services (CMS) in 2018, they not only generated a $97 million Medicaid savings, but also had a financial cushion that was later used in their COVID-19 response.21,22 The advanced payments allowed primary care practices and community agencies to invest in a digital tool to support outreach to patient at high risk for virus complications.
Hospitals similarly can adapt global budgets that incentivize financial stewardship by encouraging clinicians to resume necessary services and not unnecessary ones.16 For example, CMS partnered with Pennsylvania’s Department of Health to provide prospective all-payer global budgets for rural hospitals and Maryland’s Health Services Cost Review Commission that negotiates a budget with each hospital. During the COVID-19 pandemic, hospitals in these programs have had more financial protection from fluctuating finances by allowing for easier shifts in service delivery location and adjustments in rates to compensate for declines in visits and procedures.23
Policy makers and payers also can hold medical centers accountable to evidence-based guidelines and appropriate use of care, especially when necessary but expensive (eg, percutaneous coronary interventions, spinal surgeries, or cancer care). Funding agencies, additionally, can support these efforts by focusing on research, dissemination, and reliable implementation of these practices.
CONCLUSION
The COVID-19 crisis presents a tremendous opportunity for each medical center to revitalize healthcare. This opportunity can be seized only with reform by policy makers, payers, and regulatory agencies who encourage restarting high-value care without low-value services. We must take deliberate action so the nation’s medical centers can better meet patients’ needs to make healthcare more resilient, efficient, and fair.
The day after Memorial Day 2020 marked an important transition in the United States’ experience with coronavirus disease 2019 (COVID-19), with many states making initial plans to reopen.
This year’s widespread healthcare closures were necessary to reduce COVID-19 transmission and prepare for a future patient surge, but these closures had unintended consequences. Nearly half of adults polled said they or someone in their household had foregone or delayed care since the outbreak began.1 This was especially true for visits to emergency departments and doctors’ offices for strokes, heart attacks, and routine medical care.2 In a survey across 49 states, only 7% of primary care practices considered scheduling preventive visits as a high priority.3 Eleven percent of polled adults reported delaying care worsened their condition,1 and in hard-hit areas such as New York City, non-COVID mortality was 22% higher than expected.4
Avoidance of the medical system decreased not only use of necessary, high-value care but also use of low-value care. Low-value services are those in which the “potential for harms exceed the potential benefits,”5 such as unnecessary hospitalizations, avoidable emergency department or clinic visits, unwarranted or excessive diagnostic testing (eg, annual physicals), and certain procedures (eg, spinal fusion surgery for low-back pain).
KEEP PATIENTS CENTRAL IN REOPENING SERVICES TO DELIVER HIGH-VALUE CARE
Medical centers can better focus on high-value care by defining their high-risk patient populations; high-value treatments, procedures, and preventive care; and phases of reopening. During the first pandemic wave, medical centers tried to reassure patients about emergency care, such as coming in for chest pain or neurologic symptoms, through personal outreach and media campaigns. Outpatient virtual visits also continued, including primary care, specialty services, mental health treatment, and physical therapy. While reopening, some medical centers have assessed disparities by relying on their data analytics and, if available, embedded health services researchers to understand what care was stopped and what populations were most affected.
The University of California health systems, for example, had a learning collaborative focused on sharing methods to restore care delivery that prioritizes patient needs. Some campuses conducted analyses using both electronic health record data and input from patients and their care teams to identify clinical needs and determine patient outreach plans. Some approaches used machine learning models to identify patients at highest risk of hospitalization or emergency department visits over the next 12 months and to conduct additional outreach to schedule these patients in primary and specialty care if clinically appropriate. Similarly, surgical specialties identified the highest-priority nonemergent surgeries for scheduling, including cancer resection, radiation therapy, and pain-management procedures. Similar guidance toward the most meaningful care has been prioritized within the United States Department of Veterans Affairs.
The rapid deployment of telehealth and payment models that reimburse video and in-clinic visits equally created new opportunities for medical centers to expand high-value care in lower-cost home settings. Similarly, new infrastructure is being developed to help define smarter use of virtual visits and home-based lab collection and monitoring.
Medical centers also must pay careful attention to redeploying service capacity for underused, high-value services. The pandemic uncovered existing staff that could be redeployed to support these changes. For example, with an “all hands on deck” mentality during the pandemic, in some medical centers, analysts or care managers from less-prioritized or duplicative areas were reassigned to vital COVID-19 efforts. Medical centers may realize that this staff can provide more value in the future by supporting increased high-value, affordable healthcare.
DELIBERATELY AVOID LOW-VALUE CARE
During the intial wave of the pandemic, medical centers greatly reduced the care they provided, often focusing on delivering essential care. This preparation for a surge of COVID patients had the effect of halting many unnecessary services by moving care from the clinic to home under new reimbursement changes, such as those affecting telehealth payments. The experience of reducing low-value medical services and visits can be extended to limiting unnecessary diagnostic testing. Medical centers could, for example, focus only on tests that advance care plans; reduce unnecessary blood draws, procedures, and vital sign checks on stable patients; shift to medications with less-frequent dosing intervals; and consolidate visits by treatment teams.10,11
Medical centers, however, now face continued pressures to increase revenue because 75% report their organization’s top priority is focused on increasing patient volume.12
PROACTIVELY AVOID WORSENING HEALTHCARE DISPARITIES
As medical centers reboot, operational and clinical leaders must proactively view changes through an equity lens to avoid exacerbating health disparities among vulnerable populations. The pandemic has focused national attention on the severity and pervasiveness of disparities and created an imperative for substantive action to evaluate how every decision will affect health equity. For example, medical centers are expanding use of telehealth to improve patient outreach. However, in a survey of primary care physicians, 72% said they have patients who are unable to access telehealth because they do not have access to technology.3 Exclusion of these patients from programs risks worsening health disparities. In a recent survey, nearly 65% of medical centers report reexamining existing policies, protocols, and practices for patients at risk of disparities.12
REFORM TO SUPPORT HIGH-VALUE CARE DELIVERY
Medical centers nationwide will need payment reform that provides greater financial stability beyond the pandemic to support high-value care delivery. They also will need flexibility to invest in prevention and to deliver the appropriate intensity of care to meet patients’ and communities’ needs.15-17 Options to provide this support include prospective population-based payments that may create more resilience in protecting access to care when it is most needed. Models can include fully capitated payment for physician practices.19,20 For example, after Vermont entered a single accountable care organization (ACO) model with the Centers for Medicare & Medicaid Services (CMS) in 2018, they not only generated a $97 million Medicaid savings, but also had a financial cushion that was later used in their COVID-19 response.21,22 The advanced payments allowed primary care practices and community agencies to invest in a digital tool to support outreach to patient at high risk for virus complications.
Hospitals similarly can adapt global budgets that incentivize financial stewardship by encouraging clinicians to resume necessary services and not unnecessary ones.16 For example, CMS partnered with Pennsylvania’s Department of Health to provide prospective all-payer global budgets for rural hospitals and Maryland’s Health Services Cost Review Commission that negotiates a budget with each hospital. During the COVID-19 pandemic, hospitals in these programs have had more financial protection from fluctuating finances by allowing for easier shifts in service delivery location and adjustments in rates to compensate for declines in visits and procedures.23
Policy makers and payers also can hold medical centers accountable to evidence-based guidelines and appropriate use of care, especially when necessary but expensive (eg, percutaneous coronary interventions, spinal surgeries, or cancer care). Funding agencies, additionally, can support these efforts by focusing on research, dissemination, and reliable implementation of these practices.
CONCLUSION
The COVID-19 crisis presents a tremendous opportunity for each medical center to revitalize healthcare. This opportunity can be seized only with reform by policy makers, payers, and regulatory agencies who encourage restarting high-value care without low-value services. We must take deliberate action so the nation’s medical centers can better meet patients’ needs to make healthcare more resilient, efficient, and fair.
1. Hamel L, Kearney A, Kirzinger A, Lopes L, Muñana C, Brodie M. KFF Health Tracking Poll - May 2020: Impact of Coronavirus on Personal Health, Economic and Food Security, and Medicaid. Kaiser Family Foundation; May 27, 2020. Accessed August 9, 2020. https://www.kff.org/report-section/kff-health-tracking-poll-may-2020-health-and-economic-impacts/
2. McFarling UL. ‘Where are all our patients?’: Covid phobia is keeping people with serious heart symptoms away from ERs. STAT. April 23, 2020. Accessed August 9, 2020. https://www.statnews.com/2020/04/23/coronavirus-phobia-keeping-heart-patients-away-from-er/
3. Primary Care & COVID-19: Week 4 Survey. Primary Care Collaborative; April 9, 2020. Accessed August 9, 2020. https://www.pcpcc.org/2020/04/08/primary-care-covid-19-week-4-survey
4. Olson DR, Huynh M, Fine A, et al. New York City Department of Health and Mental Hygiene (DOHMH) COVID-19 Response Team. Preliminary estimate of excess mortality during the COVID-19 outbreak — New York City, March 11–May 2, 2020. Morbidity and Mortality Weekly Report. May 11, 2020. Accessed August 9, 2020. https://stacks.cdc.gov/view/cdc/87858
5. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280(11):1000-1005. https://doi.org/10.1001/jama.280.11.1000
6. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
7. Collins SR, Gunja MZ, Doty MM, Bhupal HK. Americans’ confidence in their ability to pay for health care is falling. To The Point blog. May 10, 2018. The Commonwealth Fund. Accessed August 9, 2020. https://www.commonwealthfund.org/blog/2018/americans-confidence-their-ability-pay-health-care-falling
8. Saad L. More Americans delaying medical treatment due to cost. Gallup News. December 9, 2019. Accessed August 9, 2020. https://news.gallup.com/poll/269138/americans-delaying-medical-treatment-due-cost.aspx
9. Lee VS. Fee for service is a terrible way to pay for health care. Try a subscription model instead. STAT. June 12, 2020. Accessed August 9, 2020. https://www.statnews.com/2020/06/12/fee-for-service-is-a-terrible-way-to-pay-for-health-care-try-a-subscription-model-instead/
10. Moriates C, Shah NT, Arora VM. A framework for the frontline: how hospitalists can improve healthcare value. J Hosp Med. 2016;11(4):297-302. https://doi.org/10.1002/jhm.2494
11. Seymann G, Komsoukaniants A, Bouland D, Jenkins I. The Silver Linings Playbook for Covid-19. KevinMD. June 12, 2020. Accessed August 9, 2020. https://www.kevinmd.com/blog/2020/06/the-silver-linings-playbook-for-covid-19.html
12. Advis In The News. Industry Professionals Weigh In: Future of Healthcare Survey. Advis. Accessed August 9, 2020. https://advis.com/advis-in-the-news/post-pandemic-survey-june2020/
13. APM Measurement Effort. Healthcare Learning Payment and Action Network; 2019. Accessed August 9, 2020. https://hcp-lan.org/workproducts/apm-infographic-2019.pdf
14. Mosley D, DeBehnke D. Rural hospital sustainability: new analysis shows worsening situation for rural hospitals, residents. Navigant; February 2019. Accessed August 9, 2020. https://guidehouse.com/-/media/www/site/insights/healthcare/2019/navigant-rural-hospital-analysis-22019.pdf
15. Gondi S, Chokshi DA. Financial stability as a goal of payment reform—a lesson from COVID-19. JAMA Health Forum. August 6, 2020. Accessed August 9, 2020. https://jamanetwork.com/channels/health-forum/fullarticle/2769307
16. Murphy K, Koski-Vacirca R, Sharfstein J. Resilience in health care financing. JAMA. 2020;324(2):126-127. https://doi.org/10.1001/jama.2020.10417
17. Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127-2128. https://doi.org/10.1001/jama.2020.6269
18. Weiss AJ, Elixhauser A, Andrews RM. Statistical Brief #170: Characteristics of Operating Room Procedures in U.S. Hospitals, 2011. Healthcare Costs and Utilization Project, Agency for Healthcare Research and Quality; February 2014. Accessed August 9, 2020: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.pdf
19. Crook HL, Saunders RS, Bleser WK, Broome T, Muhlestein D, McLellan MB. Leveraging Payment Reforms For COVID-19 And Beyond: Recommendations For Medicare ACOs And CMS’s Interim Final Rule. Health Affairs Blog. May 29, 2020. Accessed August 9, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200528.402208/full/
20. Blue Cross NC Launches Comprehensive Program to Help Independent Primary Care Practices Stay in Business. Press release. BlueCross BlueShield of North Carolina; June 24, 2020. Accessed August 9, 2020. https://mediacenter.bcbsnc.com/news/blue-cross-nc-launches-comprehensive-program-to-help-independent-primary-care-practices-stay-in-business
21. RTI International. State Innovation Models (SIM) Initiative Evaluation: Model Test Year Five Annual Report. Centers for Medicaid & Medicare Services; December 2019. Accessed August 9, 2020. https://downloads.cms.gov/files/cmmi/sim-rd1-mt-fifthannrpt.pdf
22. Wack A. A Message from OneCare CEO Vicki Loner: OneCare’s Response to the Pandemic. OneCare Vermont. May 1, 2020. Accessed August 9, 2020. https://www.onecarevt.org/20200501-covid19/
23. Haber S, Bell H, Morrison M, et al. Evaluation of the Maryland All-Payer Model: Vol 1: Final Report. Centers for Medicare & Medicaid Services; November 2019. Accessed August 9, 2020. https://downloads.cms.gov/files/md-allpayer-finalevalrpt.pdf
1. Hamel L, Kearney A, Kirzinger A, Lopes L, Muñana C, Brodie M. KFF Health Tracking Poll - May 2020: Impact of Coronavirus on Personal Health, Economic and Food Security, and Medicaid. Kaiser Family Foundation; May 27, 2020. Accessed August 9, 2020. https://www.kff.org/report-section/kff-health-tracking-poll-may-2020-health-and-economic-impacts/
2. McFarling UL. ‘Where are all our patients?’: Covid phobia is keeping people with serious heart symptoms away from ERs. STAT. April 23, 2020. Accessed August 9, 2020. https://www.statnews.com/2020/04/23/coronavirus-phobia-keeping-heart-patients-away-from-er/
3. Primary Care & COVID-19: Week 4 Survey. Primary Care Collaborative; April 9, 2020. Accessed August 9, 2020. https://www.pcpcc.org/2020/04/08/primary-care-covid-19-week-4-survey
4. Olson DR, Huynh M, Fine A, et al. New York City Department of Health and Mental Hygiene (DOHMH) COVID-19 Response Team. Preliminary estimate of excess mortality during the COVID-19 outbreak — New York City, March 11–May 2, 2020. Morbidity and Mortality Weekly Report. May 11, 2020. Accessed August 9, 2020. https://stacks.cdc.gov/view/cdc/87858
5. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280(11):1000-1005. https://doi.org/10.1001/jama.280.11.1000
6. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. https://doi.org/10.1001/jama.2019.13978
7. Collins SR, Gunja MZ, Doty MM, Bhupal HK. Americans’ confidence in their ability to pay for health care is falling. To The Point blog. May 10, 2018. The Commonwealth Fund. Accessed August 9, 2020. https://www.commonwealthfund.org/blog/2018/americans-confidence-their-ability-pay-health-care-falling
8. Saad L. More Americans delaying medical treatment due to cost. Gallup News. December 9, 2019. Accessed August 9, 2020. https://news.gallup.com/poll/269138/americans-delaying-medical-treatment-due-cost.aspx
9. Lee VS. Fee for service is a terrible way to pay for health care. Try a subscription model instead. STAT. June 12, 2020. Accessed August 9, 2020. https://www.statnews.com/2020/06/12/fee-for-service-is-a-terrible-way-to-pay-for-health-care-try-a-subscription-model-instead/
10. Moriates C, Shah NT, Arora VM. A framework for the frontline: how hospitalists can improve healthcare value. J Hosp Med. 2016;11(4):297-302. https://doi.org/10.1002/jhm.2494
11. Seymann G, Komsoukaniants A, Bouland D, Jenkins I. The Silver Linings Playbook for Covid-19. KevinMD. June 12, 2020. Accessed August 9, 2020. https://www.kevinmd.com/blog/2020/06/the-silver-linings-playbook-for-covid-19.html
12. Advis In The News. Industry Professionals Weigh In: Future of Healthcare Survey. Advis. Accessed August 9, 2020. https://advis.com/advis-in-the-news/post-pandemic-survey-june2020/
13. APM Measurement Effort. Healthcare Learning Payment and Action Network; 2019. Accessed August 9, 2020. https://hcp-lan.org/workproducts/apm-infographic-2019.pdf
14. Mosley D, DeBehnke D. Rural hospital sustainability: new analysis shows worsening situation for rural hospitals, residents. Navigant; February 2019. Accessed August 9, 2020. https://guidehouse.com/-/media/www/site/insights/healthcare/2019/navigant-rural-hospital-analysis-22019.pdf
15. Gondi S, Chokshi DA. Financial stability as a goal of payment reform—a lesson from COVID-19. JAMA Health Forum. August 6, 2020. Accessed August 9, 2020. https://jamanetwork.com/channels/health-forum/fullarticle/2769307
16. Murphy K, Koski-Vacirca R, Sharfstein J. Resilience in health care financing. JAMA. 2020;324(2):126-127. https://doi.org/10.1001/jama.2020.10417
17. Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127-2128. https://doi.org/10.1001/jama.2020.6269
18. Weiss AJ, Elixhauser A, Andrews RM. Statistical Brief #170: Characteristics of Operating Room Procedures in U.S. Hospitals, 2011. Healthcare Costs and Utilization Project, Agency for Healthcare Research and Quality; February 2014. Accessed August 9, 2020: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.pdf
19. Crook HL, Saunders RS, Bleser WK, Broome T, Muhlestein D, McLellan MB. Leveraging Payment Reforms For COVID-19 And Beyond: Recommendations For Medicare ACOs And CMS’s Interim Final Rule. Health Affairs Blog. May 29, 2020. Accessed August 9, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200528.402208/full/
20. Blue Cross NC Launches Comprehensive Program to Help Independent Primary Care Practices Stay in Business. Press release. BlueCross BlueShield of North Carolina; June 24, 2020. Accessed August 9, 2020. https://mediacenter.bcbsnc.com/news/blue-cross-nc-launches-comprehensive-program-to-help-independent-primary-care-practices-stay-in-business
21. RTI International. State Innovation Models (SIM) Initiative Evaluation: Model Test Year Five Annual Report. Centers for Medicaid & Medicare Services; December 2019. Accessed August 9, 2020. https://downloads.cms.gov/files/cmmi/sim-rd1-mt-fifthannrpt.pdf
22. Wack A. A Message from OneCare CEO Vicki Loner: OneCare’s Response to the Pandemic. OneCare Vermont. May 1, 2020. Accessed August 9, 2020. https://www.onecarevt.org/20200501-covid19/
23. Haber S, Bell H, Morrison M, et al. Evaluation of the Maryland All-Payer Model: Vol 1: Final Report. Centers for Medicare & Medicaid Services; November 2019. Accessed August 9, 2020. https://downloads.cms.gov/files/md-allpayer-finalevalrpt.pdf
© 2021 Society of Hospital Medicine
Leveling the Playing Field: Accounting for Academic Productivity During the COVID-19 Pandemic
Professional upheavals caused by the coronavirus disease 2019 (COVID-19) pandemic have affected the academic productivity of many physicians. This is due in part to rapid changes in clinical care and medical education: physician-researchers have been redeployed to frontline clinical care; clinician-educators have been forced to rapidly transition in-person curricula to virtual platforms; and primary care physicians and subspecialists have been forced to transition to telehealth-based practices. In addition to these changes in clinical and educational responsibilities, the COVID-19 pandemic has substantially altered the personal lives of physicians. During the height of the pandemic, clinicians simultaneously wrestled with a lack of available childcare, unexpected home-schooling responsibilities, decreased income, and many other COVID-19-related stresses.1 Additionally, the ever-present “second pandemic” of structural racism, persistent health disparities, and racial inequity has further increased the personal and professional demands facing academic faculty.2
In particular, the pandemic has placed personal and professional pressure on female and minority faculty members. In spite of these pressures, however, the academic promotions process still requires rigid accounting of scholarly productivity. As the focus of academic practices has shifted to support clinical care during the pandemic, scholarly productivity has suffered for clinicians on the frontline. As a result, academic clinical faculty have expressed significant stress and concerns about failing to meet benchmarks for promotion (eg, publications, curricula development, national presentations). To counter these shifts (and the inherent inequity that they create for female clinicians and for men and women who are Black, Indigenous, and/or of color), academic institutions should not only recognize the effects the COVID-19 pandemic has had on faculty, but also adopt immediate solutions to more equitably account for such disruptions to academic portfolios. In this paper, we explore populations whose career trajectories are most at-risk and propose a framework to capture novel and nontraditional contributions while also acknowledging the rapid changes the COVID-19 pandemic has brought to academic medicine.
POPULATIONS AT RISK FOR CAREER DISRUPTION
Even before the COVID-19 pandemic, physician mothers, underrepresented racial/ethnic minority groups, and junior faculty were most at-risk for career disruptions. The closure of daycare facilities and schools and shift to online learning resulting from the pandemic, along with the common challenges of parenting, have taken a significant toll on the lives of working parents. Because women tend to carry a disproportionate share of childcare and household responsibilities, these changes have inequitably leveraged themselves as a “mommy tax” on working women.3,4
As underrepresented medicine faculty (particularly Black, Hispanic, Latino, and Native American clinicians) comprise only 8% of the academic medical workforce,they currently face a variety of personal and professional challenges.5 This is especially true for Black and Latinx physicians who have been experiencing an increased COVID-19 burden in their communities, while concurrently fighting entrenched structural racism and police violence. In academia, these challenges have worsened because of the “minority tax”—the toll of often uncompensated extra responsibilities (time or money) placed on minority faculty in the name of achieving diversity. The unintended consequences of these responsibilities result in having fewer mentors,6 caring for underserved populations,7 and performing more clinical care8 than non-underrepresented minority faculty. Because minority faculty are unlikely to be in leadership positions, it is reasonable to conclude they have been shouldering heavier clinical obligations and facing greater career disruption of scholarly work due to the COVID-19 pandemic.
Junior faculty (eg, instructors and assistant professors) also remain professionally vulnerable during the COVID-19 pandemic. Because junior faculty are often more clinically focused and less likely to hold leadership positions than senior faculty, they are more likely to have assumed frontline clinical positions, which come at the expense of academic work. Junior faculty are also at a critical building phase in their academic career—a time when they benefit from the opportunity to share their scholarly work and network at conferences. Unfortunately, many conferences have been canceled or moved to a virtual platform. Given that some institutions may be freezing academic funding for conferences due to budgetary shortfalls from the pandemic, junior faculty may be particularly at risk if they are not able to present their work. In addition, junior faculty often face disproportionate struggles at home, trying to balance demands of work and caring for young children. Considering the unique needs of each of these groups, it is especially important to consider intersectionality, or the compounded issues for individuals who exist in multiple disproportionately affected groups (eg, a Black female junior faculty member who is also a mother).
THE COVID-19-CURRICULUM VITAE MATRIX
The typical format of a professional curriculum vitae (CV) at most academic institutions does not allow one to document potential disruptions or novel contributions, including those that occurred during the COVID-19 pandemic. As a group of academic clinicians, educators, and researchers whose careers have been affected by the pandemic, we created a COVID-19 CV matrix, a potential framework to serve as a supplement for faculty. In this matrix, faculty members may document their contributions, disruptions that affected their work, and caregiving responsibilities during this time period, while also providing a rubric for promotions and tenure committees to equitably evaluate the pandemic period on an academic CV. Our COVID-19 CV matrix consists of six domains: (1) clinical care, (2) research, (3) education, (4) service, (5) advocacy/media, and (6) social media. These domains encompass traditional and nontraditional contributions made by healthcare professionals during the pandemic (Table). This matrix broadens the ability of both faculty and institutions to determine the actual impact of individuals during the pandemic.
ACCOUNT FOR YOUR (NEW) IMPACT
Throughout the COVID-19 pandemic, academic faculty have been innovative, contributing in novel ways not routinely captured by promotions committees—eg, the digital health researcher who now directs the telemedicine response for their institution and the health disparities researcher who now leads daily webinar sessions on structural racism to medical students. Other novel contributions include advancing COVID-19 innovations and engaging in media and community advocacy (eg, organizing large-scale donations of equipment and funds to support organizations in need). While such nontraditional contributions may not have been readily captured or thought “CV worthy” in the past, faculty should now account for them. More importantly, promotions committees need to recognize that these pivots or alterations in career paths are not signals of professional failure, but rather evidence of a shifting landscape and the respective response of the individual. Furthermore, because these pivots often help fulfill an institutional mission, they are impactful.
ACKNOWLEDGE THE DISRUPTION
It is important for promotions and tenure committees to recognize the impact and disruption COVID-19 has had on traditional academic work, acknowledging the time and energy required for a faculty member to make needed work adjustments. This enables a leader to better assess how a faculty member’s academic portfolio has been affected. For example, researchers have had to halt studies, medical educators have had to redevelop and transition curricula to virtual platforms, and physicians have had to discontinue clinician quality improvement initiatives due to competing hospital priorities. Faculty members who document such unintentional alterations in their academic career path can explain to their institution how they have continued to positively influence their field and the community during the pandemic. This approach is analogous to the current model of accounting for clinical time when judging faculty members’ contributions in scholarly achievement.
The COVID-19 CV matrix has the potential to be annotated to explain the burden of one’s personal situation, which is often “invisible” in the professional environment. For example, many physicians have had to assume additional childcare responsibilities, tend to sick family members, friends, and even themselves. It is also possible that a faculty member has a partner who is also an essential worker, one who had to self-isolate due to COVID-19 exposure or illness, or who has been working overtime due to high patient volumes.
INSTITUTIONAL RESPONSE
How can institutions respond to the altered academic landscape caused by the COVID-19 pandemic? Promotions committees typically have two main tools at their disposal: adjusting the tenure clock or the benchmarks. Extending the period of time available to qualify for tenure is commonplace in the “publish-or-perish” academic tracks of university research professors. Clock adjustments are typically granted to faculty following the birth of a child or for other specific family- or health-related hardships, in accordance with the Family and Medical Leave Act. Unfortunately, tenure-clock extensions for female faculty members can exacerbate gender inequity: Data on tenure-clock extensions show a higher rate of tenure granted to male faculty compared to female faculty.9 For this reason, it is also important to explore adjustments or modifications to benchmark criteria. This could be accomplished by broadening the criteria for promotion, recognizing that impact occurs in many forms, thereby enabling meeting a benchmark. It can also occur by examining the trajectory of an individual within a promotion pathway before it was disrupted to determine impact. To avoid exacerbating social and gender inequities within academia, institutions should use these professional levers and create new ones to provide parity and equality across the promotional playing field. While the CV matrix openly acknowledges the disruptions and tangents the COVID-19 pandemic has had on academic careers, it remains important for academic institutions to recognize these disruptions and innovate the manner in which they acknowledge scholarly contributions.
Conclusion
While academic rigidity and known social taxes (minority and mommy taxes) are particularly problematic in the current climate, these issues have always been at play in evaluating academic success. Improved documentation of novel contributions, disruptions, caregiving, and other challenges can enable more holistic and timely professional advancement for all faculty, regardless of their sex, race, ethnicity, or social background. Ultimately, we hope this framework initiates further conversations among academic institutions on how to define productivity in an age where journal impact factor or number of publications is not the fullest measure of one’s impact in their field.
1. Jones Y, Durand V, Morton K, et al; ADVANCE PHM Steering Committee. Collateral damage: how covid-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
2. Manning KD. When grief and crises intersect: perspectives of a black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
3. Cohen P, Hsu T. Pandemic could scar a generation of working mothers. New York Times. Published June 3, 2020. Updated June 30, 2020. Accessed November 11, 2020. https://www.nytimes.com/2020/06/03/business/economy/coronavirus-working-women.html
4. Cain Miller C. Nearly half of men say they do most of the home schooling. 3 percent of women agree. Published May 6, 2020. Updated May 8, 2020. Accessed November 11, 2020. New York Times. https://www.nytimes.com/2020/05/06/upshot/pandemic-chores-homeschooling-gender.html
5. Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https://doi.org/10.1186/s12909-015-0290-9
6. Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med. 2006;81(3):275-279. https://doi.org/10.1097/00001888-200603000-00020
7. Pololi LH, Evans AT, Gibbs BK, Krupat E, Brennan RT, Civian JT. The experience of minority faculty who are underrepresented in medicine, at 26 representative U.S. medical schools. Acad Med. 2013;88(9):1308-1314. https://doi.org/10.1097/acm.0b013e31829eefff
8. Richert A, Campbell K, Rodríguez J, Borowsky IW, Parikh R, Colwell A. ACU workforce column: expanding and supporting the health care workforce. J Health Care Poor Underserved. 2013;24(4):1423-1431. https://doi.org/10.1353/hpu.2013.0162
9. Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 threatens progress toward gender equity within academic medicine. Acad Med. 2020;29:10.1097/ACM.0000000000003782. https://doi.org/10.1097/acm.0000000000003782
Professional upheavals caused by the coronavirus disease 2019 (COVID-19) pandemic have affected the academic productivity of many physicians. This is due in part to rapid changes in clinical care and medical education: physician-researchers have been redeployed to frontline clinical care; clinician-educators have been forced to rapidly transition in-person curricula to virtual platforms; and primary care physicians and subspecialists have been forced to transition to telehealth-based practices. In addition to these changes in clinical and educational responsibilities, the COVID-19 pandemic has substantially altered the personal lives of physicians. During the height of the pandemic, clinicians simultaneously wrestled with a lack of available childcare, unexpected home-schooling responsibilities, decreased income, and many other COVID-19-related stresses.1 Additionally, the ever-present “second pandemic” of structural racism, persistent health disparities, and racial inequity has further increased the personal and professional demands facing academic faculty.2
In particular, the pandemic has placed personal and professional pressure on female and minority faculty members. In spite of these pressures, however, the academic promotions process still requires rigid accounting of scholarly productivity. As the focus of academic practices has shifted to support clinical care during the pandemic, scholarly productivity has suffered for clinicians on the frontline. As a result, academic clinical faculty have expressed significant stress and concerns about failing to meet benchmarks for promotion (eg, publications, curricula development, national presentations). To counter these shifts (and the inherent inequity that they create for female clinicians and for men and women who are Black, Indigenous, and/or of color), academic institutions should not only recognize the effects the COVID-19 pandemic has had on faculty, but also adopt immediate solutions to more equitably account for such disruptions to academic portfolios. In this paper, we explore populations whose career trajectories are most at-risk and propose a framework to capture novel and nontraditional contributions while also acknowledging the rapid changes the COVID-19 pandemic has brought to academic medicine.
POPULATIONS AT RISK FOR CAREER DISRUPTION
Even before the COVID-19 pandemic, physician mothers, underrepresented racial/ethnic minority groups, and junior faculty were most at-risk for career disruptions. The closure of daycare facilities and schools and shift to online learning resulting from the pandemic, along with the common challenges of parenting, have taken a significant toll on the lives of working parents. Because women tend to carry a disproportionate share of childcare and household responsibilities, these changes have inequitably leveraged themselves as a “mommy tax” on working women.3,4
As underrepresented medicine faculty (particularly Black, Hispanic, Latino, and Native American clinicians) comprise only 8% of the academic medical workforce,they currently face a variety of personal and professional challenges.5 This is especially true for Black and Latinx physicians who have been experiencing an increased COVID-19 burden in their communities, while concurrently fighting entrenched structural racism and police violence. In academia, these challenges have worsened because of the “minority tax”—the toll of often uncompensated extra responsibilities (time or money) placed on minority faculty in the name of achieving diversity. The unintended consequences of these responsibilities result in having fewer mentors,6 caring for underserved populations,7 and performing more clinical care8 than non-underrepresented minority faculty. Because minority faculty are unlikely to be in leadership positions, it is reasonable to conclude they have been shouldering heavier clinical obligations and facing greater career disruption of scholarly work due to the COVID-19 pandemic.
Junior faculty (eg, instructors and assistant professors) also remain professionally vulnerable during the COVID-19 pandemic. Because junior faculty are often more clinically focused and less likely to hold leadership positions than senior faculty, they are more likely to have assumed frontline clinical positions, which come at the expense of academic work. Junior faculty are also at a critical building phase in their academic career—a time when they benefit from the opportunity to share their scholarly work and network at conferences. Unfortunately, many conferences have been canceled or moved to a virtual platform. Given that some institutions may be freezing academic funding for conferences due to budgetary shortfalls from the pandemic, junior faculty may be particularly at risk if they are not able to present their work. In addition, junior faculty often face disproportionate struggles at home, trying to balance demands of work and caring for young children. Considering the unique needs of each of these groups, it is especially important to consider intersectionality, or the compounded issues for individuals who exist in multiple disproportionately affected groups (eg, a Black female junior faculty member who is also a mother).
THE COVID-19-CURRICULUM VITAE MATRIX
The typical format of a professional curriculum vitae (CV) at most academic institutions does not allow one to document potential disruptions or novel contributions, including those that occurred during the COVID-19 pandemic. As a group of academic clinicians, educators, and researchers whose careers have been affected by the pandemic, we created a COVID-19 CV matrix, a potential framework to serve as a supplement for faculty. In this matrix, faculty members may document their contributions, disruptions that affected their work, and caregiving responsibilities during this time period, while also providing a rubric for promotions and tenure committees to equitably evaluate the pandemic period on an academic CV. Our COVID-19 CV matrix consists of six domains: (1) clinical care, (2) research, (3) education, (4) service, (5) advocacy/media, and (6) social media. These domains encompass traditional and nontraditional contributions made by healthcare professionals during the pandemic (Table). This matrix broadens the ability of both faculty and institutions to determine the actual impact of individuals during the pandemic.

ACCOUNT FOR YOUR (NEW) IMPACT
Throughout the COVID-19 pandemic, academic faculty have been innovative, contributing in novel ways not routinely captured by promotions committees—eg, the digital health researcher who now directs the telemedicine response for their institution and the health disparities researcher who now leads daily webinar sessions on structural racism to medical students. Other novel contributions include advancing COVID-19 innovations and engaging in media and community advocacy (eg, organizing large-scale donations of equipment and funds to support organizations in need). While such nontraditional contributions may not have been readily captured or thought “CV worthy” in the past, faculty should now account for them. More importantly, promotions committees need to recognize that these pivots or alterations in career paths are not signals of professional failure, but rather evidence of a shifting landscape and the respective response of the individual. Furthermore, because these pivots often help fulfill an institutional mission, they are impactful.
ACKNOWLEDGE THE DISRUPTION
It is important for promotions and tenure committees to recognize the impact and disruption COVID-19 has had on traditional academic work, acknowledging the time and energy required for a faculty member to make needed work adjustments. This enables a leader to better assess how a faculty member’s academic portfolio has been affected. For example, researchers have had to halt studies, medical educators have had to redevelop and transition curricula to virtual platforms, and physicians have had to discontinue clinician quality improvement initiatives due to competing hospital priorities. Faculty members who document such unintentional alterations in their academic career path can explain to their institution how they have continued to positively influence their field and the community during the pandemic. This approach is analogous to the current model of accounting for clinical time when judging faculty members’ contributions in scholarly achievement.
The COVID-19 CV matrix has the potential to be annotated to explain the burden of one’s personal situation, which is often “invisible” in the professional environment. For example, many physicians have had to assume additional childcare responsibilities, tend to sick family members, friends, and even themselves. It is also possible that a faculty member has a partner who is also an essential worker, one who had to self-isolate due to COVID-19 exposure or illness, or who has been working overtime due to high patient volumes.
INSTITUTIONAL RESPONSE
How can institutions respond to the altered academic landscape caused by the COVID-19 pandemic? Promotions committees typically have two main tools at their disposal: adjusting the tenure clock or the benchmarks. Extending the period of time available to qualify for tenure is commonplace in the “publish-or-perish” academic tracks of university research professors. Clock adjustments are typically granted to faculty following the birth of a child or for other specific family- or health-related hardships, in accordance with the Family and Medical Leave Act. Unfortunately, tenure-clock extensions for female faculty members can exacerbate gender inequity: Data on tenure-clock extensions show a higher rate of tenure granted to male faculty compared to female faculty.9 For this reason, it is also important to explore adjustments or modifications to benchmark criteria. This could be accomplished by broadening the criteria for promotion, recognizing that impact occurs in many forms, thereby enabling meeting a benchmark. It can also occur by examining the trajectory of an individual within a promotion pathway before it was disrupted to determine impact. To avoid exacerbating social and gender inequities within academia, institutions should use these professional levers and create new ones to provide parity and equality across the promotional playing field. While the CV matrix openly acknowledges the disruptions and tangents the COVID-19 pandemic has had on academic careers, it remains important for academic institutions to recognize these disruptions and innovate the manner in which they acknowledge scholarly contributions.
Conclusion
While academic rigidity and known social taxes (minority and mommy taxes) are particularly problematic in the current climate, these issues have always been at play in evaluating academic success. Improved documentation of novel contributions, disruptions, caregiving, and other challenges can enable more holistic and timely professional advancement for all faculty, regardless of their sex, race, ethnicity, or social background. Ultimately, we hope this framework initiates further conversations among academic institutions on how to define productivity in an age where journal impact factor or number of publications is not the fullest measure of one’s impact in their field.
Professional upheavals caused by the coronavirus disease 2019 (COVID-19) pandemic have affected the academic productivity of many physicians. This is due in part to rapid changes in clinical care and medical education: physician-researchers have been redeployed to frontline clinical care; clinician-educators have been forced to rapidly transition in-person curricula to virtual platforms; and primary care physicians and subspecialists have been forced to transition to telehealth-based practices. In addition to these changes in clinical and educational responsibilities, the COVID-19 pandemic has substantially altered the personal lives of physicians. During the height of the pandemic, clinicians simultaneously wrestled with a lack of available childcare, unexpected home-schooling responsibilities, decreased income, and many other COVID-19-related stresses.1 Additionally, the ever-present “second pandemic” of structural racism, persistent health disparities, and racial inequity has further increased the personal and professional demands facing academic faculty.2
In particular, the pandemic has placed personal and professional pressure on female and minority faculty members. In spite of these pressures, however, the academic promotions process still requires rigid accounting of scholarly productivity. As the focus of academic practices has shifted to support clinical care during the pandemic, scholarly productivity has suffered for clinicians on the frontline. As a result, academic clinical faculty have expressed significant stress and concerns about failing to meet benchmarks for promotion (eg, publications, curricula development, national presentations). To counter these shifts (and the inherent inequity that they create for female clinicians and for men and women who are Black, Indigenous, and/or of color), academic institutions should not only recognize the effects the COVID-19 pandemic has had on faculty, but also adopt immediate solutions to more equitably account for such disruptions to academic portfolios. In this paper, we explore populations whose career trajectories are most at-risk and propose a framework to capture novel and nontraditional contributions while also acknowledging the rapid changes the COVID-19 pandemic has brought to academic medicine.
POPULATIONS AT RISK FOR CAREER DISRUPTION
Even before the COVID-19 pandemic, physician mothers, underrepresented racial/ethnic minority groups, and junior faculty were most at-risk for career disruptions. The closure of daycare facilities and schools and shift to online learning resulting from the pandemic, along with the common challenges of parenting, have taken a significant toll on the lives of working parents. Because women tend to carry a disproportionate share of childcare and household responsibilities, these changes have inequitably leveraged themselves as a “mommy tax” on working women.3,4
As underrepresented medicine faculty (particularly Black, Hispanic, Latino, and Native American clinicians) comprise only 8% of the academic medical workforce,they currently face a variety of personal and professional challenges.5 This is especially true for Black and Latinx physicians who have been experiencing an increased COVID-19 burden in their communities, while concurrently fighting entrenched structural racism and police violence. In academia, these challenges have worsened because of the “minority tax”—the toll of often uncompensated extra responsibilities (time or money) placed on minority faculty in the name of achieving diversity. The unintended consequences of these responsibilities result in having fewer mentors,6 caring for underserved populations,7 and performing more clinical care8 than non-underrepresented minority faculty. Because minority faculty are unlikely to be in leadership positions, it is reasonable to conclude they have been shouldering heavier clinical obligations and facing greater career disruption of scholarly work due to the COVID-19 pandemic.
Junior faculty (eg, instructors and assistant professors) also remain professionally vulnerable during the COVID-19 pandemic. Because junior faculty are often more clinically focused and less likely to hold leadership positions than senior faculty, they are more likely to have assumed frontline clinical positions, which come at the expense of academic work. Junior faculty are also at a critical building phase in their academic career—a time when they benefit from the opportunity to share their scholarly work and network at conferences. Unfortunately, many conferences have been canceled or moved to a virtual platform. Given that some institutions may be freezing academic funding for conferences due to budgetary shortfalls from the pandemic, junior faculty may be particularly at risk if they are not able to present their work. In addition, junior faculty often face disproportionate struggles at home, trying to balance demands of work and caring for young children. Considering the unique needs of each of these groups, it is especially important to consider intersectionality, or the compounded issues for individuals who exist in multiple disproportionately affected groups (eg, a Black female junior faculty member who is also a mother).
THE COVID-19-CURRICULUM VITAE MATRIX
The typical format of a professional curriculum vitae (CV) at most academic institutions does not allow one to document potential disruptions or novel contributions, including those that occurred during the COVID-19 pandemic. As a group of academic clinicians, educators, and researchers whose careers have been affected by the pandemic, we created a COVID-19 CV matrix, a potential framework to serve as a supplement for faculty. In this matrix, faculty members may document their contributions, disruptions that affected their work, and caregiving responsibilities during this time period, while also providing a rubric for promotions and tenure committees to equitably evaluate the pandemic period on an academic CV. Our COVID-19 CV matrix consists of six domains: (1) clinical care, (2) research, (3) education, (4) service, (5) advocacy/media, and (6) social media. These domains encompass traditional and nontraditional contributions made by healthcare professionals during the pandemic (Table). This matrix broadens the ability of both faculty and institutions to determine the actual impact of individuals during the pandemic.

ACCOUNT FOR YOUR (NEW) IMPACT
Throughout the COVID-19 pandemic, academic faculty have been innovative, contributing in novel ways not routinely captured by promotions committees—eg, the digital health researcher who now directs the telemedicine response for their institution and the health disparities researcher who now leads daily webinar sessions on structural racism to medical students. Other novel contributions include advancing COVID-19 innovations and engaging in media and community advocacy (eg, organizing large-scale donations of equipment and funds to support organizations in need). While such nontraditional contributions may not have been readily captured or thought “CV worthy” in the past, faculty should now account for them. More importantly, promotions committees need to recognize that these pivots or alterations in career paths are not signals of professional failure, but rather evidence of a shifting landscape and the respective response of the individual. Furthermore, because these pivots often help fulfill an institutional mission, they are impactful.
ACKNOWLEDGE THE DISRUPTION
It is important for promotions and tenure committees to recognize the impact and disruption COVID-19 has had on traditional academic work, acknowledging the time and energy required for a faculty member to make needed work adjustments. This enables a leader to better assess how a faculty member’s academic portfolio has been affected. For example, researchers have had to halt studies, medical educators have had to redevelop and transition curricula to virtual platforms, and physicians have had to discontinue clinician quality improvement initiatives due to competing hospital priorities. Faculty members who document such unintentional alterations in their academic career path can explain to their institution how they have continued to positively influence their field and the community during the pandemic. This approach is analogous to the current model of accounting for clinical time when judging faculty members’ contributions in scholarly achievement.
The COVID-19 CV matrix has the potential to be annotated to explain the burden of one’s personal situation, which is often “invisible” in the professional environment. For example, many physicians have had to assume additional childcare responsibilities, tend to sick family members, friends, and even themselves. It is also possible that a faculty member has a partner who is also an essential worker, one who had to self-isolate due to COVID-19 exposure or illness, or who has been working overtime due to high patient volumes.
INSTITUTIONAL RESPONSE
How can institutions respond to the altered academic landscape caused by the COVID-19 pandemic? Promotions committees typically have two main tools at their disposal: adjusting the tenure clock or the benchmarks. Extending the period of time available to qualify for tenure is commonplace in the “publish-or-perish” academic tracks of university research professors. Clock adjustments are typically granted to faculty following the birth of a child or for other specific family- or health-related hardships, in accordance with the Family and Medical Leave Act. Unfortunately, tenure-clock extensions for female faculty members can exacerbate gender inequity: Data on tenure-clock extensions show a higher rate of tenure granted to male faculty compared to female faculty.9 For this reason, it is also important to explore adjustments or modifications to benchmark criteria. This could be accomplished by broadening the criteria for promotion, recognizing that impact occurs in many forms, thereby enabling meeting a benchmark. It can also occur by examining the trajectory of an individual within a promotion pathway before it was disrupted to determine impact. To avoid exacerbating social and gender inequities within academia, institutions should use these professional levers and create new ones to provide parity and equality across the promotional playing field. While the CV matrix openly acknowledges the disruptions and tangents the COVID-19 pandemic has had on academic careers, it remains important for academic institutions to recognize these disruptions and innovate the manner in which they acknowledge scholarly contributions.
Conclusion
While academic rigidity and known social taxes (minority and mommy taxes) are particularly problematic in the current climate, these issues have always been at play in evaluating academic success. Improved documentation of novel contributions, disruptions, caregiving, and other challenges can enable more holistic and timely professional advancement for all faculty, regardless of their sex, race, ethnicity, or social background. Ultimately, we hope this framework initiates further conversations among academic institutions on how to define productivity in an age where journal impact factor or number of publications is not the fullest measure of one’s impact in their field.
1. Jones Y, Durand V, Morton K, et al; ADVANCE PHM Steering Committee. Collateral damage: how covid-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
2. Manning KD. When grief and crises intersect: perspectives of a black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
3. Cohen P, Hsu T. Pandemic could scar a generation of working mothers. New York Times. Published June 3, 2020. Updated June 30, 2020. Accessed November 11, 2020. https://www.nytimes.com/2020/06/03/business/economy/coronavirus-working-women.html
4. Cain Miller C. Nearly half of men say they do most of the home schooling. 3 percent of women agree. Published May 6, 2020. Updated May 8, 2020. Accessed November 11, 2020. New York Times. https://www.nytimes.com/2020/05/06/upshot/pandemic-chores-homeschooling-gender.html
5. Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https://doi.org/10.1186/s12909-015-0290-9
6. Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med. 2006;81(3):275-279. https://doi.org/10.1097/00001888-200603000-00020
7. Pololi LH, Evans AT, Gibbs BK, Krupat E, Brennan RT, Civian JT. The experience of minority faculty who are underrepresented in medicine, at 26 representative U.S. medical schools. Acad Med. 2013;88(9):1308-1314. https://doi.org/10.1097/acm.0b013e31829eefff
8. Richert A, Campbell K, Rodríguez J, Borowsky IW, Parikh R, Colwell A. ACU workforce column: expanding and supporting the health care workforce. J Health Care Poor Underserved. 2013;24(4):1423-1431. https://doi.org/10.1353/hpu.2013.0162
9. Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 threatens progress toward gender equity within academic medicine. Acad Med. 2020;29:10.1097/ACM.0000000000003782. https://doi.org/10.1097/acm.0000000000003782
1. Jones Y, Durand V, Morton K, et al; ADVANCE PHM Steering Committee. Collateral damage: how covid-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
2. Manning KD. When grief and crises intersect: perspectives of a black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
3. Cohen P, Hsu T. Pandemic could scar a generation of working mothers. New York Times. Published June 3, 2020. Updated June 30, 2020. Accessed November 11, 2020. https://www.nytimes.com/2020/06/03/business/economy/coronavirus-working-women.html
4. Cain Miller C. Nearly half of men say they do most of the home schooling. 3 percent of women agree. Published May 6, 2020. Updated May 8, 2020. Accessed November 11, 2020. New York Times. https://www.nytimes.com/2020/05/06/upshot/pandemic-chores-homeschooling-gender.html
5. Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https://doi.org/10.1186/s12909-015-0290-9
6. Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med. 2006;81(3):275-279. https://doi.org/10.1097/00001888-200603000-00020
7. Pololi LH, Evans AT, Gibbs BK, Krupat E, Brennan RT, Civian JT. The experience of minority faculty who are underrepresented in medicine, at 26 representative U.S. medical schools. Acad Med. 2013;88(9):1308-1314. https://doi.org/10.1097/acm.0b013e31829eefff
8. Richert A, Campbell K, Rodríguez J, Borowsky IW, Parikh R, Colwell A. ACU workforce column: expanding and supporting the health care workforce. J Health Care Poor Underserved. 2013;24(4):1423-1431. https://doi.org/10.1353/hpu.2013.0162
9. Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 threatens progress toward gender equity within academic medicine. Acad Med. 2020;29:10.1097/ACM.0000000000003782. https://doi.org/10.1097/acm.0000000000003782
© 2021 Society of Hospital Medicine
Capitol siege presents new challenges for psychiatry to help prevent domestic terrorism
On Jan. 6, 2021, Americans and the world witnessed a violent insurrection at the U.S. Capitol inspired by a president and other elected leaders and driven by lies, conspiracy theories, militias, and white supremacy. The violent insurrection was carried out by thousands of citizens, including many with weapons.
Psychiatric organizations condemned the attack and warned about the potential traumatic impact of these events on those directly involved as well as for others in the United States already living under anxiety and fear tied to the surging COVID pandemic.
A major challenge for U.S. society is to prevent other potential future violent attacks. For those who didn’t already know, the Capitol attack made it apparent that the United States faces major problems with white supremacists and domestic terrorism. FBI Director Christopher Wray stipulated that those involved in the Jan. 6 events were violent agitators and extremists.
Addressing the causes and preventing domestic terrorism is also a challenge and opportunity for psychiatry and other mental health professionals. I write as a psychiatrist in academic medicine who has spent more than 10 years advocating for public health approaches to the causes and consequences of violence, especially involving violent extremism. and that psychiatrists have a role to play as part of a whole-of-society coalition with other multidisciplinary practitioners and stakeholders.
Day by day, we learn more and more about those responsible for the insurrection and how to understand their motivations, intentions, and actions. Seditionists incite or commit acts of violence against a lawful authority with the goal of destroying or overthrowing it. Domestic terrorists commit violent, criminal acts to further ideological goals stemming from domestic influences, such as those of a political, religious, social, racial, or environmental nature. The mob that attacked the Capitol contained both. What’s more, the Capitol insurrection might inspire others to take similar actions. The risk for even broader and deeper radicalization to violence is a grave concern.
Aided by more than 100,000 tips, the FBI is conducting a massive nationwide manhunt and thus far, dozens of people have been charged with crimes. Given that the United States has no law that makes domestic terrorism a crime, they are being charged with other crimes. Upholding the rule of law is necessary, but it should not be regarded as sufficient to deal with the white supremacism and domestic terrorism threats.
In many countries all over the world, and to a much lesser extent in the United States, there are successful non–law enforcement programs helping people move away from domestic terrorism and other forms of violence. One example in the United States is Life After Hate, a nongovernmental organization that uses former white supremacist extremists to counsel people to leave the movement. Another example is the Colorado Resilience Collaborative, which takes a socioecological approach to prevent terrorism and targeted violence. At Boston Children’s Hospital, a regional prevention initiative is focused on reducing youth risk for targeted violence and terrorism by reducing mental health problems and increasing social belonging among adolescents. These are but three of several initiatives currently being conducted throughout the United States.
Over the past decade, I have had the opportunity to become familiar with several of these programs domestically and internationally. These include programs aimed at rehabilitating and reintegrating repatriated foreign fighters and their children and other family members all over the world, including in Kazakhstan. I would like to share some of the lessons learned from these programs to aid in preventing domestic terrorism in the United States.
One lesson learned from combating international terrorism is that intelligence and law enforcement strategies (hard counterterrorism) need to be balanced with civil society–led prevention strategies. Overreliance on hard strategies can harm individuals and communities through oversecuritization. Alternatively, we need to build civil society–led initiatives that focus on other levers, such as addressing the underlying conditions, including individual psychosocial and mental health dimensions, or social dimensions (for example, lack of opportunity), that mitigate a person’s involvement in violent extremism.
A second lesson is not to focus exclusively on ideology and deradicalization. Yes, we need to challenge extremist ideology and disinformation, but a wide range of different factors explains involvement in violent extremism and the many pathways into it. Using a socioecological model, we can identify modifiable risk and protective factors that mitigate for or against extremist violence (for example, family support, job prospects, untreated mental health problems). In addition, it is well-established that prevention programs should seek to disengage, not deradicalize, potential violent extremists.
Third, we should leverage existing evidence-based interventions and best practices in mental health and public health, but we should also invest in building and evaluating new models through research approaches, especially for secondary and tertiary prevention. As much as possible, these should be integrated into broader programs to improve individual and community mental health and health.
A fourth lesson is we must vigorously protect the human rights and civil liberties of individuals and communities involved in these programs, and uphold racial equity. We can learn from public health experts about how to engage vulnerable individuals and communities without adding to their stigmatization. One way is to not focus on single communities, and not just on ideologically motivated violence, but to build violence prevention programs that are broad enough to address multiple forms of violence.
Fifth, if we expect community-based organizations to do the work, then they need adequate resources, capacity building, training and supervision, and quality improvement activities to succeed. For example, psychiatrists and other mental health professionals will require additional training to learn how to work effectively and ethically in this space.
Psychiatrists can start by building their knowledge and skills in understanding violent extremism and how it can be assessed and addressed, which is not the same as for suicidality. Psychiatrists can also become involved in established or emerging violence prevention programs, such as threat assessment programs in schools, workplaces, and communities. Across the country, there is a need for building new secondary and tertiary violence prevention initiatives, and they will need psychiatrists to work with them. Academic psychiatrists can become involved in building the models, developing and delivering training, and designing and conducting the program evaluations.
Finally, I suggest that psychiatrists look at domestic terrorism prevention through the lens of public health and not overly “psychiatrize” the issue. A public health approach uses evidence-based programs and policies, addresses underlying causes, and focuses on prevention. Public health builds programs with teams of experts from across disciplines – educators, health care workers, mental health professionals, faith leaders, youth leaders, community advocates, peers, and law enforcement.
As part of a public health–oriented team, psychiatrists can contribute to addressing the grave challenges of domestic terrorism facing our nation today.
Dr. Weine is professor of psychiatry, director of global medicine, and director of the Center for Global Health at the University of Illinois at Chicago. He has no conflicts of interest.
On Jan. 6, 2021, Americans and the world witnessed a violent insurrection at the U.S. Capitol inspired by a president and other elected leaders and driven by lies, conspiracy theories, militias, and white supremacy. The violent insurrection was carried out by thousands of citizens, including many with weapons.
Psychiatric organizations condemned the attack and warned about the potential traumatic impact of these events on those directly involved as well as for others in the United States already living under anxiety and fear tied to the surging COVID pandemic.
A major challenge for U.S. society is to prevent other potential future violent attacks. For those who didn’t already know, the Capitol attack made it apparent that the United States faces major problems with white supremacists and domestic terrorism. FBI Director Christopher Wray stipulated that those involved in the Jan. 6 events were violent agitators and extremists.
Addressing the causes and preventing domestic terrorism is also a challenge and opportunity for psychiatry and other mental health professionals. I write as a psychiatrist in academic medicine who has spent more than 10 years advocating for public health approaches to the causes and consequences of violence, especially involving violent extremism. and that psychiatrists have a role to play as part of a whole-of-society coalition with other multidisciplinary practitioners and stakeholders.
Day by day, we learn more and more about those responsible for the insurrection and how to understand their motivations, intentions, and actions. Seditionists incite or commit acts of violence against a lawful authority with the goal of destroying or overthrowing it. Domestic terrorists commit violent, criminal acts to further ideological goals stemming from domestic influences, such as those of a political, religious, social, racial, or environmental nature. The mob that attacked the Capitol contained both. What’s more, the Capitol insurrection might inspire others to take similar actions. The risk for even broader and deeper radicalization to violence is a grave concern.
Aided by more than 100,000 tips, the FBI is conducting a massive nationwide manhunt and thus far, dozens of people have been charged with crimes. Given that the United States has no law that makes domestic terrorism a crime, they are being charged with other crimes. Upholding the rule of law is necessary, but it should not be regarded as sufficient to deal with the white supremacism and domestic terrorism threats.
In many countries all over the world, and to a much lesser extent in the United States, there are successful non–law enforcement programs helping people move away from domestic terrorism and other forms of violence. One example in the United States is Life After Hate, a nongovernmental organization that uses former white supremacist extremists to counsel people to leave the movement. Another example is the Colorado Resilience Collaborative, which takes a socioecological approach to prevent terrorism and targeted violence. At Boston Children’s Hospital, a regional prevention initiative is focused on reducing youth risk for targeted violence and terrorism by reducing mental health problems and increasing social belonging among adolescents. These are but three of several initiatives currently being conducted throughout the United States.
Over the past decade, I have had the opportunity to become familiar with several of these programs domestically and internationally. These include programs aimed at rehabilitating and reintegrating repatriated foreign fighters and their children and other family members all over the world, including in Kazakhstan. I would like to share some of the lessons learned from these programs to aid in preventing domestic terrorism in the United States.
One lesson learned from combating international terrorism is that intelligence and law enforcement strategies (hard counterterrorism) need to be balanced with civil society–led prevention strategies. Overreliance on hard strategies can harm individuals and communities through oversecuritization. Alternatively, we need to build civil society–led initiatives that focus on other levers, such as addressing the underlying conditions, including individual psychosocial and mental health dimensions, or social dimensions (for example, lack of opportunity), that mitigate a person’s involvement in violent extremism.
A second lesson is not to focus exclusively on ideology and deradicalization. Yes, we need to challenge extremist ideology and disinformation, but a wide range of different factors explains involvement in violent extremism and the many pathways into it. Using a socioecological model, we can identify modifiable risk and protective factors that mitigate for or against extremist violence (for example, family support, job prospects, untreated mental health problems). In addition, it is well-established that prevention programs should seek to disengage, not deradicalize, potential violent extremists.
Third, we should leverage existing evidence-based interventions and best practices in mental health and public health, but we should also invest in building and evaluating new models through research approaches, especially for secondary and tertiary prevention. As much as possible, these should be integrated into broader programs to improve individual and community mental health and health.
A fourth lesson is we must vigorously protect the human rights and civil liberties of individuals and communities involved in these programs, and uphold racial equity. We can learn from public health experts about how to engage vulnerable individuals and communities without adding to their stigmatization. One way is to not focus on single communities, and not just on ideologically motivated violence, but to build violence prevention programs that are broad enough to address multiple forms of violence.
Fifth, if we expect community-based organizations to do the work, then they need adequate resources, capacity building, training and supervision, and quality improvement activities to succeed. For example, psychiatrists and other mental health professionals will require additional training to learn how to work effectively and ethically in this space.
Psychiatrists can start by building their knowledge and skills in understanding violent extremism and how it can be assessed and addressed, which is not the same as for suicidality. Psychiatrists can also become involved in established or emerging violence prevention programs, such as threat assessment programs in schools, workplaces, and communities. Across the country, there is a need for building new secondary and tertiary violence prevention initiatives, and they will need psychiatrists to work with them. Academic psychiatrists can become involved in building the models, developing and delivering training, and designing and conducting the program evaluations.
Finally, I suggest that psychiatrists look at domestic terrorism prevention through the lens of public health and not overly “psychiatrize” the issue. A public health approach uses evidence-based programs and policies, addresses underlying causes, and focuses on prevention. Public health builds programs with teams of experts from across disciplines – educators, health care workers, mental health professionals, faith leaders, youth leaders, community advocates, peers, and law enforcement.
As part of a public health–oriented team, psychiatrists can contribute to addressing the grave challenges of domestic terrorism facing our nation today.
Dr. Weine is professor of psychiatry, director of global medicine, and director of the Center for Global Health at the University of Illinois at Chicago. He has no conflicts of interest.
On Jan. 6, 2021, Americans and the world witnessed a violent insurrection at the U.S. Capitol inspired by a president and other elected leaders and driven by lies, conspiracy theories, militias, and white supremacy. The violent insurrection was carried out by thousands of citizens, including many with weapons.
Psychiatric organizations condemned the attack and warned about the potential traumatic impact of these events on those directly involved as well as for others in the United States already living under anxiety and fear tied to the surging COVID pandemic.
A major challenge for U.S. society is to prevent other potential future violent attacks. For those who didn’t already know, the Capitol attack made it apparent that the United States faces major problems with white supremacists and domestic terrorism. FBI Director Christopher Wray stipulated that those involved in the Jan. 6 events were violent agitators and extremists.
Addressing the causes and preventing domestic terrorism is also a challenge and opportunity for psychiatry and other mental health professionals. I write as a psychiatrist in academic medicine who has spent more than 10 years advocating for public health approaches to the causes and consequences of violence, especially involving violent extremism. and that psychiatrists have a role to play as part of a whole-of-society coalition with other multidisciplinary practitioners and stakeholders.
Day by day, we learn more and more about those responsible for the insurrection and how to understand their motivations, intentions, and actions. Seditionists incite or commit acts of violence against a lawful authority with the goal of destroying or overthrowing it. Domestic terrorists commit violent, criminal acts to further ideological goals stemming from domestic influences, such as those of a political, religious, social, racial, or environmental nature. The mob that attacked the Capitol contained both. What’s more, the Capitol insurrection might inspire others to take similar actions. The risk for even broader and deeper radicalization to violence is a grave concern.
Aided by more than 100,000 tips, the FBI is conducting a massive nationwide manhunt and thus far, dozens of people have been charged with crimes. Given that the United States has no law that makes domestic terrorism a crime, they are being charged with other crimes. Upholding the rule of law is necessary, but it should not be regarded as sufficient to deal with the white supremacism and domestic terrorism threats.
In many countries all over the world, and to a much lesser extent in the United States, there are successful non–law enforcement programs helping people move away from domestic terrorism and other forms of violence. One example in the United States is Life After Hate, a nongovernmental organization that uses former white supremacist extremists to counsel people to leave the movement. Another example is the Colorado Resilience Collaborative, which takes a socioecological approach to prevent terrorism and targeted violence. At Boston Children’s Hospital, a regional prevention initiative is focused on reducing youth risk for targeted violence and terrorism by reducing mental health problems and increasing social belonging among adolescents. These are but three of several initiatives currently being conducted throughout the United States.
Over the past decade, I have had the opportunity to become familiar with several of these programs domestically and internationally. These include programs aimed at rehabilitating and reintegrating repatriated foreign fighters and their children and other family members all over the world, including in Kazakhstan. I would like to share some of the lessons learned from these programs to aid in preventing domestic terrorism in the United States.
One lesson learned from combating international terrorism is that intelligence and law enforcement strategies (hard counterterrorism) need to be balanced with civil society–led prevention strategies. Overreliance on hard strategies can harm individuals and communities through oversecuritization. Alternatively, we need to build civil society–led initiatives that focus on other levers, such as addressing the underlying conditions, including individual psychosocial and mental health dimensions, or social dimensions (for example, lack of opportunity), that mitigate a person’s involvement in violent extremism.
A second lesson is not to focus exclusively on ideology and deradicalization. Yes, we need to challenge extremist ideology and disinformation, but a wide range of different factors explains involvement in violent extremism and the many pathways into it. Using a socioecological model, we can identify modifiable risk and protective factors that mitigate for or against extremist violence (for example, family support, job prospects, untreated mental health problems). In addition, it is well-established that prevention programs should seek to disengage, not deradicalize, potential violent extremists.
Third, we should leverage existing evidence-based interventions and best practices in mental health and public health, but we should also invest in building and evaluating new models through research approaches, especially for secondary and tertiary prevention. As much as possible, these should be integrated into broader programs to improve individual and community mental health and health.
A fourth lesson is we must vigorously protect the human rights and civil liberties of individuals and communities involved in these programs, and uphold racial equity. We can learn from public health experts about how to engage vulnerable individuals and communities without adding to their stigmatization. One way is to not focus on single communities, and not just on ideologically motivated violence, but to build violence prevention programs that are broad enough to address multiple forms of violence.
Fifth, if we expect community-based organizations to do the work, then they need adequate resources, capacity building, training and supervision, and quality improvement activities to succeed. For example, psychiatrists and other mental health professionals will require additional training to learn how to work effectively and ethically in this space.
Psychiatrists can start by building their knowledge and skills in understanding violent extremism and how it can be assessed and addressed, which is not the same as for suicidality. Psychiatrists can also become involved in established or emerging violence prevention programs, such as threat assessment programs in schools, workplaces, and communities. Across the country, there is a need for building new secondary and tertiary violence prevention initiatives, and they will need psychiatrists to work with them. Academic psychiatrists can become involved in building the models, developing and delivering training, and designing and conducting the program evaluations.
Finally, I suggest that psychiatrists look at domestic terrorism prevention through the lens of public health and not overly “psychiatrize” the issue. A public health approach uses evidence-based programs and policies, addresses underlying causes, and focuses on prevention. Public health builds programs with teams of experts from across disciplines – educators, health care workers, mental health professionals, faith leaders, youth leaders, community advocates, peers, and law enforcement.
As part of a public health–oriented team, psychiatrists can contribute to addressing the grave challenges of domestic terrorism facing our nation today.
Dr. Weine is professor of psychiatry, director of global medicine, and director of the Center for Global Health at the University of Illinois at Chicago. He has no conflicts of interest.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
Better survival with S-1 plus docetaxel in stage III gastric cancer
A new recommendation for the treatment of patients with gastric cancer has been proposed on the basis of final results from the phase 3 trial GC-07, which showed survival benefit. The trial was conducted by the Japan Clinical Cancer Research Organization.
, say the researchers.
The 3-year relapse-free survival (RFS) and 3-year overall survival rates were significantly superior among patients treated with S-1/docetaxel, compared with those treated with S-1 alone, commented lead study author Kazuhiro Yoshida, PhD, MD, director of Gifu University Hospital and professor and chairman of the department of surgical oncology, Gifu (Japan) University.
“The study met its primary endpoint and improved the RFS [recurrence-free survival],” he said. “Postoperative S-1 plus docetaxel was safe and manageable.”
Dr. Yoshida presented the updated findings of the GC-07 trial at the Gastrointestinal Cancers Symposium (GICS) 2021, which was held online this year.
S-1 widely used in Asia
S-1 is a novel oral dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine. The drug, which is a biochemical modulation of 5-fluorouracil, comprises tegafur and two types of enzyme inhibitor. It is widely used to treat various solid tumors in Asia.
“S1 is a standard postoperative adjuvant chemotherapy for patients with p-stage II/III gastric cancer in Asia,” said Dr. Yoshida, but the “outcome in p-stage III is unsatisfactory,” he added.
The GC-07 trial set out to further investigate the use of this drug in this patient population. Dr. Yoshida and colleagues included 915 patients with stage III gastric cancer who had undergone R0 resection and D2 lymphadenectomy and who tested negative on peritoneal-washing cytology. The patients were randomly assigned to receive either S-1 plus docetaxel or S-1 alone for up to 1 year in the postoperative setting.
The data presented at the meeting are the final results from GC-07. They confirm earlier data.
Previously, a second interim analysis showed that the trial had met its primary endpoint. As a result of that analysis, the study was terminated.
That interim analysis showed that the 3-year RFS of the S-1/docetaxel arm was significantly superior to that of the S-1 arm (65.9% vs. 49.6%; hazard ratio, 0.632; P = .0007).
Now, the final results, at a median follow-up of 48.2 months, show that there were 400 recurrences and 324 deaths. The 3-year RFS was 67.7% in the S-1/docetaxel group, which was significantly superior to the 57.4% reported in the S-1 group (HR, 0.715; P = .0008). Similarly, 3-year overall survival was 77.7% in the S-1/docetaxel group, vs. 71.2% in the S-1 group (HR, 0.742; P = .0076).
At 12 months, 62.7% of patients in the S-1 group had experienced treatment failure, compared with 56.2% in the combination-therapy group.
In addition to reducing overall relapse, treatment with combination therapy also decreased the incidence of relapse at specific sites, compared with S-1 alone. These included reductions in lymphatic recurrence (6.4% vs. 15.0%), hematogenous recurrence (9.7% vs. 15.5%), local recurrence (2.9% vs. 4.4%), and peritoneal recurrence (18.8% vs. 21.4%).
No new safety signals were observed, Dr. Yoshida commented. Grade 3/4 adverse events that occurred more frequently with S-1/docetaxel than with S-1 alone included neutropenia (39.2% vs. 16.4%), leukopenia (22.4% vs. 2.7%), and febrile neutropenia (5.7% vs. 0.4%).
However, the authors noted that, in a subgroup analysis, patients with stage IIIB disease did not derive the same benefit in RFS and overall survival with combination therapy as the patients with stage IIIA or IIIC disease.
The discussant for this paper, Rutika Mehta, MD, MPH, of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla., highlighted differences in benefit among the subgroups, as well as the finding that patients with stage IIIB appeared to benefit less.
However, she noted that the seventh edition of the American Joint Committee on Cancer TNM classification, which distinguishes patients on the basis of prognostic subgroups, is inaccurate for stage III disease, and this might have affected the study results. Dr. Mehta pointed to a previous analysis in which more than 33% of individuals with stage IIIB disease, determined in accordance with the seventh edition of the AJCC staging system, were reclassified as having stage IIIC disease, as determined using the more recent eighth edition.
“There were also few T2, N0, and N1 patients, making meaningful deductions in these subgroups not possible,” she said.
She said that despite these limitations, these “results are meaningful and impactful, and the combination of docetaxel and S-1 showed better RFS and overall survival than S-1 alone.
“These results do favor a new recommendation for the use of docetaxel plus S-1 for stage III gastric cancer patients after D2 lymphadenectomy,” she concluded.
The study was funded by the Japan Clinical Cancer Research Organization. Dr. Yoshida has received honoraria and research funding from many pharmaceutical companies, as listed in the abstract.
A version of this article first appeared on Medscape.com.
A new recommendation for the treatment of patients with gastric cancer has been proposed on the basis of final results from the phase 3 trial GC-07, which showed survival benefit. The trial was conducted by the Japan Clinical Cancer Research Organization.
, say the researchers.
The 3-year relapse-free survival (RFS) and 3-year overall survival rates were significantly superior among patients treated with S-1/docetaxel, compared with those treated with S-1 alone, commented lead study author Kazuhiro Yoshida, PhD, MD, director of Gifu University Hospital and professor and chairman of the department of surgical oncology, Gifu (Japan) University.
“The study met its primary endpoint and improved the RFS [recurrence-free survival],” he said. “Postoperative S-1 plus docetaxel was safe and manageable.”
Dr. Yoshida presented the updated findings of the GC-07 trial at the Gastrointestinal Cancers Symposium (GICS) 2021, which was held online this year.
S-1 widely used in Asia
S-1 is a novel oral dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine. The drug, which is a biochemical modulation of 5-fluorouracil, comprises tegafur and two types of enzyme inhibitor. It is widely used to treat various solid tumors in Asia.
“S1 is a standard postoperative adjuvant chemotherapy for patients with p-stage II/III gastric cancer in Asia,” said Dr. Yoshida, but the “outcome in p-stage III is unsatisfactory,” he added.
The GC-07 trial set out to further investigate the use of this drug in this patient population. Dr. Yoshida and colleagues included 915 patients with stage III gastric cancer who had undergone R0 resection and D2 lymphadenectomy and who tested negative on peritoneal-washing cytology. The patients were randomly assigned to receive either S-1 plus docetaxel or S-1 alone for up to 1 year in the postoperative setting.
The data presented at the meeting are the final results from GC-07. They confirm earlier data.
Previously, a second interim analysis showed that the trial had met its primary endpoint. As a result of that analysis, the study was terminated.
That interim analysis showed that the 3-year RFS of the S-1/docetaxel arm was significantly superior to that of the S-1 arm (65.9% vs. 49.6%; hazard ratio, 0.632; P = .0007).
Now, the final results, at a median follow-up of 48.2 months, show that there were 400 recurrences and 324 deaths. The 3-year RFS was 67.7% in the S-1/docetaxel group, which was significantly superior to the 57.4% reported in the S-1 group (HR, 0.715; P = .0008). Similarly, 3-year overall survival was 77.7% in the S-1/docetaxel group, vs. 71.2% in the S-1 group (HR, 0.742; P = .0076).
At 12 months, 62.7% of patients in the S-1 group had experienced treatment failure, compared with 56.2% in the combination-therapy group.
In addition to reducing overall relapse, treatment with combination therapy also decreased the incidence of relapse at specific sites, compared with S-1 alone. These included reductions in lymphatic recurrence (6.4% vs. 15.0%), hematogenous recurrence (9.7% vs. 15.5%), local recurrence (2.9% vs. 4.4%), and peritoneal recurrence (18.8% vs. 21.4%).
No new safety signals were observed, Dr. Yoshida commented. Grade 3/4 adverse events that occurred more frequently with S-1/docetaxel than with S-1 alone included neutropenia (39.2% vs. 16.4%), leukopenia (22.4% vs. 2.7%), and febrile neutropenia (5.7% vs. 0.4%).
However, the authors noted that, in a subgroup analysis, patients with stage IIIB disease did not derive the same benefit in RFS and overall survival with combination therapy as the patients with stage IIIA or IIIC disease.
The discussant for this paper, Rutika Mehta, MD, MPH, of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla., highlighted differences in benefit among the subgroups, as well as the finding that patients with stage IIIB appeared to benefit less.
However, she noted that the seventh edition of the American Joint Committee on Cancer TNM classification, which distinguishes patients on the basis of prognostic subgroups, is inaccurate for stage III disease, and this might have affected the study results. Dr. Mehta pointed to a previous analysis in which more than 33% of individuals with stage IIIB disease, determined in accordance with the seventh edition of the AJCC staging system, were reclassified as having stage IIIC disease, as determined using the more recent eighth edition.
“There were also few T2, N0, and N1 patients, making meaningful deductions in these subgroups not possible,” she said.
She said that despite these limitations, these “results are meaningful and impactful, and the combination of docetaxel and S-1 showed better RFS and overall survival than S-1 alone.
“These results do favor a new recommendation for the use of docetaxel plus S-1 for stage III gastric cancer patients after D2 lymphadenectomy,” she concluded.
The study was funded by the Japan Clinical Cancer Research Organization. Dr. Yoshida has received honoraria and research funding from many pharmaceutical companies, as listed in the abstract.
A version of this article first appeared on Medscape.com.
A new recommendation for the treatment of patients with gastric cancer has been proposed on the basis of final results from the phase 3 trial GC-07, which showed survival benefit. The trial was conducted by the Japan Clinical Cancer Research Organization.
, say the researchers.
The 3-year relapse-free survival (RFS) and 3-year overall survival rates were significantly superior among patients treated with S-1/docetaxel, compared with those treated with S-1 alone, commented lead study author Kazuhiro Yoshida, PhD, MD, director of Gifu University Hospital and professor and chairman of the department of surgical oncology, Gifu (Japan) University.
“The study met its primary endpoint and improved the RFS [recurrence-free survival],” he said. “Postoperative S-1 plus docetaxel was safe and manageable.”
Dr. Yoshida presented the updated findings of the GC-07 trial at the Gastrointestinal Cancers Symposium (GICS) 2021, which was held online this year.
S-1 widely used in Asia
S-1 is a novel oral dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine. The drug, which is a biochemical modulation of 5-fluorouracil, comprises tegafur and two types of enzyme inhibitor. It is widely used to treat various solid tumors in Asia.
“S1 is a standard postoperative adjuvant chemotherapy for patients with p-stage II/III gastric cancer in Asia,” said Dr. Yoshida, but the “outcome in p-stage III is unsatisfactory,” he added.
The GC-07 trial set out to further investigate the use of this drug in this patient population. Dr. Yoshida and colleagues included 915 patients with stage III gastric cancer who had undergone R0 resection and D2 lymphadenectomy and who tested negative on peritoneal-washing cytology. The patients were randomly assigned to receive either S-1 plus docetaxel or S-1 alone for up to 1 year in the postoperative setting.
The data presented at the meeting are the final results from GC-07. They confirm earlier data.
Previously, a second interim analysis showed that the trial had met its primary endpoint. As a result of that analysis, the study was terminated.
That interim analysis showed that the 3-year RFS of the S-1/docetaxel arm was significantly superior to that of the S-1 arm (65.9% vs. 49.6%; hazard ratio, 0.632; P = .0007).
Now, the final results, at a median follow-up of 48.2 months, show that there were 400 recurrences and 324 deaths. The 3-year RFS was 67.7% in the S-1/docetaxel group, which was significantly superior to the 57.4% reported in the S-1 group (HR, 0.715; P = .0008). Similarly, 3-year overall survival was 77.7% in the S-1/docetaxel group, vs. 71.2% in the S-1 group (HR, 0.742; P = .0076).
At 12 months, 62.7% of patients in the S-1 group had experienced treatment failure, compared with 56.2% in the combination-therapy group.
In addition to reducing overall relapse, treatment with combination therapy also decreased the incidence of relapse at specific sites, compared with S-1 alone. These included reductions in lymphatic recurrence (6.4% vs. 15.0%), hematogenous recurrence (9.7% vs. 15.5%), local recurrence (2.9% vs. 4.4%), and peritoneal recurrence (18.8% vs. 21.4%).
No new safety signals were observed, Dr. Yoshida commented. Grade 3/4 adverse events that occurred more frequently with S-1/docetaxel than with S-1 alone included neutropenia (39.2% vs. 16.4%), leukopenia (22.4% vs. 2.7%), and febrile neutropenia (5.7% vs. 0.4%).
However, the authors noted that, in a subgroup analysis, patients with stage IIIB disease did not derive the same benefit in RFS and overall survival with combination therapy as the patients with stage IIIA or IIIC disease.
The discussant for this paper, Rutika Mehta, MD, MPH, of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla., highlighted differences in benefit among the subgroups, as well as the finding that patients with stage IIIB appeared to benefit less.
However, she noted that the seventh edition of the American Joint Committee on Cancer TNM classification, which distinguishes patients on the basis of prognostic subgroups, is inaccurate for stage III disease, and this might have affected the study results. Dr. Mehta pointed to a previous analysis in which more than 33% of individuals with stage IIIB disease, determined in accordance with the seventh edition of the AJCC staging system, were reclassified as having stage IIIC disease, as determined using the more recent eighth edition.
“There were also few T2, N0, and N1 patients, making meaningful deductions in these subgroups not possible,” she said.
She said that despite these limitations, these “results are meaningful and impactful, and the combination of docetaxel and S-1 showed better RFS and overall survival than S-1 alone.
“These results do favor a new recommendation for the use of docetaxel plus S-1 for stage III gastric cancer patients after D2 lymphadenectomy,” she concluded.
The study was funded by the Japan Clinical Cancer Research Organization. Dr. Yoshida has received honoraria and research funding from many pharmaceutical companies, as listed in the abstract.
A version of this article first appeared on Medscape.com.
Women psychiatrists struggle to balance work-life demands during COVID-19
Daily life is now a juggling act for Misty Richards, MD, MS. As the program director of a rigorous child psychiatry fellowship, a psychiatrist caring for women with perinatal psychiatric disorders, and the mother of three young children, Dr. Richards tries to view these tasks as an opportunity for growth. But some days it feels as if she’s navigating a storm in the middle of the ocean without a life jacket.
In the age of COVID, “the wave of demands has morphed into one giant tidal wave of desperate need,” Dr. Richards, of the department of psychiatry & biobehavioral sciences, University of California, Los Angeles, Semel Institute of Neuroscience & Human Behavior, said in an interview. “The painfully loud and clear message is that our patients need us, and our children – who have been stripped from healthy routines and peer interactions that nourish social-emotional development – rely on us. We cannot turn our backs for even a moment, or else they will suffer.”
Tasked with caring for a much sicker and distressed population, navigating home duties such as child care, online school, and taking care of certain family members, women psychiatrists are feeling the impact of COVID-19.
Many have seamlessly transferred their practices online, maintaining a lifeline with their patients through telehealth visits. Even with this convenience, the emotional labor of being a psychiatrist is still very stressful, Pooja Lakshmin, MD, of the department of psychiatry and behavioral sciences at George Washington University, Washington, said in an interview. Because the nature of work has changed, and many are doing things virtually at home, separating home from work life can be a challenge. “It’s harder to disconnect,” admitted Dr. Lakshmin. “Even my patients tell me that they have no time to themselves anymore.”
– a moving target that remains nowhere in sight, Dr. Richards said. “In this process, we are expected to fill the emotional cups of a broken nation, to provide answers that do not exist, and to do so with never-ending gratitude for a demanding system that has no ‘off’ switch,” she noted.
‘In two places at once’
COVID-19’s physical and emotional toll has swept across the various subspecialties of clinical psychiatry. As some navigate outpatient/telehealth work, inpatient psychiatrists directly interact with COVID patients.
“Our inpatient psychiatry unit regularly takes care of COVID patients, including perinatal patients who are COVID positive,” Samantha Meltzer-Brody, MD, MPH, distinguished professor and chair, University of North Carolina, Chapel Hill, department of psychiatry and director of medical school’s Center for Women’s Mood Disorders, said in an interview. A psychiatry consultation-liaison service also provides psychiatry care to medical and surgical patients, including medically ill COVID patients across the hospital.
“We are on the front lines in the sense that we are dealing with the trauma of the general population and having to be present for that emotional distress,” Dr. Meltzer-Brody said.
The struggle to balance rising caseloads and home responsibilities makes things difficult, she continued. “There’s a never-ending onslaught of patient referrals,” reflecting the anxiety and depression issues people are experiencing in the wake of a global pandemic, frenetic political situation in the United States, and job uncertainty.
Child care and elder care responsibilities affect both men and women, yet research shows that caregiving demands disproportionately affect women, observed Dr. Meltzer-Brody.
Overall, the stress of caregiving and parenting responsibilities for men and women has been markedly higher during the pandemic. Most clinical psychiatrists “have been extraordinarily busy for a very long time,” she added.
Tiffani L. Bell, MD, a psychiatrist in Winston-Salem, N.C., has seen an increase in anxiety and depression in people with no previous history of diagnosed mental illness. “The impact of the pandemic has truly been multifaceted. People are struggling with loss of jobs, loss of wages, and loss of loved ones, along with grieving the loss of the usual way of life,” she said in an interview.
Many of her colleagues report feeling overburdened at work with increased admissions and patient loads, decreased time to see each patient, and the feeling of “needing to be in two places at once.”
“As a female psychiatrist, I do believe that we can sometimes have an increased mental burden due to the emotional and physical burnout that can occur when our routines are shaken,” added Dr. Bell, who specializes in adult, child, and adolescent psychiatry, and obesity and lifestyle medicine. Even in the early months of the pandemic, Dr. Bell said she heard people joke that “they don’t know if they are working from home or living at work.”
Physicians aren’t the only ones who are overwhelmed. “We’re also hearing stories from our patients – those at risk for partner violence, dealing with kids out of school, working full time while providing support at home,” Ludmila De Faria, MD, chair of the American Psychiatric Association’s Committee on Women’s Mental Health, said in an interview.
American mothers in particular spend nearly twice as much time caring for their children and cooking than their spouses, said Dr. Bell, citing recent studies. “Even if one is not a mom, if you couple the increased housework at baseline with the added responsibilities of working as a front-line physician and/or working from home while managing a household, it can lead to increased stress for all involved.”
Women leaving the workforce
Nationally, a growing number of women are either reducing their hours or leaving the workforce in response to the pandemic. Fidelity Investments, which surveyed 1,902 U.S. adults in mid-2020 projected that 4 in 10 women were mulling such options. Among 951 women surveyed, 42% were considering stepping back from their jobs because of their children’s homeschooling needs, and 27% cited difficulties of balancing home and job responsibilities.
Interruptions caused by child care affect women more than men, according to a report from the Century Foundation and the Center for American Progress. “Study after study has shown that, in response to school, child care, and camp closings, as well as reduced hours and reduced class sizes, significantly more women than men have reduced their work hours, left work to care for children, and spent more time on education and household tasks,” the authors noted.
They estimated that the American economy could incur $64.5 billion per year in lost wages and economic activity from the fallout of these trends. In September 2020, four times as many women as men left the workforce, nearly 865,000 women in comparison to 216,000 men.
Many women psychiatrists have been forced to choose between their careers or child care duties – decisions they don’t want to make, but that may be necessary during these unprecedented circumstances. They may be reducing their work hours to assist at home. Others are leaving their jobs, “a terrible situation given the enormous mental health needs of the pandemic” and the fact that so many areas of the United States already suffer from a shortage of clinical psychiatrists, said Dr. Meltzer-Brody.
She has personally seen the effects of this in the large academic department she supervises. “I’m seeing women reducing their work hours or leave positions,” she continued. In addition to child care needs, these women are tending to aging parents affected by COVID-19 or other illnesses, or dealing with the fact that options for elder care aren’t available.
“I have multiple faculty contending with that situation,” added Dr. Meltzer-Brody. As a result, productivity is going down. “These women are trying to keep all of the balls in the air but find they can’t.”
Dr. Richards believes some changes are in order to take the disproportionate burden off of women in psychiatry, and the workforce as a whole. The health care system “places too much pressure on individuals to compensate for its deficiencies. Those individuals who often step up to the plate are women, and this is not their sole burden to carry.”
A move toward telehealth in clinical psychiatry has made it possible for patients and physicians to meet virtually in their respective homes and discuss treatment options. “Even while this is both a blessing and privilege, it comes with the unique challenges of having to manage Zoom calls, child care, meals, distance learning, cleaning, and work responsibilities, while previously there was a clearer delineation to the day for many,” Dr. Bell said.
Clinical psychiatrists educating the public about the mental stressors of COVID-19 face their own unique challenges.
Dr. Lakshmin, who makes appearances in various media and social media outlets, said this adds more pressure to the job. “One of the challenges for me is to figure out how much outward facing I do. That’s hard when you’re navigating working and living through a pandemic. This is something I do because I enjoy doing it. But it’s still a type of work. And it’s certainly increased because the media has been paying more attention to mental health” since the pandemic started, she added.
The dual stress of COVID and social justice
Some women psychiatrists of color are dealing with social justice issues on top of other COVID stressors, Dr. De Faria said. The focus on addressing institutionalized racism means that minority women are taking on extra work to advocate for their peers.
Michelle Jacobs-Elliott, MD, of the department of psychiatry and assistant dean of the Office of Diversity and Health Equity at the University of Florida, Gainesville, knows of such responsibilities. “I have been in many discussions either with my coworkers in my department or others who work for the University of Florida” on systemic racism, she said in an interview.
Dr. Jacobs-Elliott became a trainer for Bias Reduction in Internal Medicine, a workshop aimed at reducing bias, and prior to 2020 participated in a social justice summit at the University of Florida. “Talking with my medical as well as undergraduate students about their experiences both here in Gainesville and elsewhere, they are all feeling the hurt, disappointment, and disbelief that we are still fighting battles that our grandparents fought in health care, housing, and employment. This adds an extra layer of stress to everyone’s life.”
The tense social climate has made the apparent racial inequalities in COVID-19 deaths and severity of disease hard to ignore, Dr. Bell noted. “It is my sincere hope that the availability of COVID-19 vaccines will help decrease the number of people affected by this horrible disease. The added burden of racism on top of the stressors of this pandemic can feel insurmountable. I hope 2021 will provide a way forward for us all.”
Taking time for self-care
Amid the endless referrals and increasing demands at home, women psychiatrists often don’t have the time to do normal activities, Dr. Meltzer-Brody observed. Like most people, COVID restrictions prevent them from traveling or going to the gym or restaurants. Dr. De Faria has not been able to visit family in Latin America, a trip she used to make twice a year. “That was once my de-stress time. But now, I can’t connect with my roots. My father is elderly and very much at risk.”
This is the time to get creative and resourceful – to make time for self-care, several sources said.
“We need to realize that we cannot be all things to all people, at the same time,” noted Dr. Bell. It’s important to prioritize what’s most important – and keep assessing your priorities. There’s no shame in tending to your own needs. Dr. Bell recommended that women in her profession should pick 1 day a week, put it in their calendar, and stick to this goal of self-care.
“Even if it’s only 15 minutes, it is important to put time aside. Some quick, cheap ideas are to do a quick meditation session, read a chapter in a book, listen to an audiobook, journal, go for a walk and get fresh air. Eat a healthy meal. Even 10 minutes helps,” she urged.
COVID-19 has pushed society to find new ways to do things, Dr. Bell continued. Women psychiatrists, in assessing their work-life balance, may need to reassess their goals. Consider work schedules and see if there’s a place to scale back a task. Delegate tasks at home to family members, if necessary. Most importantly, exercise self-compassion, she stressed. “During this pandemic, I believe it is vital to keep our cups filled so we can pour into others.”
Dr. Lakshmin said she has benefited greatly from having a therapist during the pandemic. “It has been so instrumental in forcing me to take that time for myself, to give me a space to take care of me, and remember it’s okay to take care of me. It’s so important for us as psychiatrists to have that for ourselves. It’s not just for our patients – we need it, too.”
The APA has resources and numerous support groups that meet regularly to address and discuss the stressors of the pandemic. Its College Mental Health Caucus, for example, holds a monthly, hour-long Zoom meeting. Not surprisingly, women comprise the majority of attendees, Dr. De Faria said. “Most women in academic psychiatry are working from home and using telehealth, which isolates people a lot.” Maureen Sayres Van Niel, MD, who is head of the APA’s Women’s Caucus, sends out a regular newsletter that advises on self-care. Women psychiatrists should also contact their local psychiatric organizations to get support from their professional peers.
Sometimes it’s wise to leave work behind and engage with friends. Dr. De Faria regularly Zooms with a group of friends outside of her profession to de-stress and reconnect. “At least I can talk to them about things other than psychiatry.”
Mentally and physically exhausted, Dr. Jacobs-Elliott said she looks forward to the day when society can return to meeting with friends and family “without being afraid that we are an asymptomatic carrier who is infecting our loved ones.”
Daily life is now a juggling act for Misty Richards, MD, MS. As the program director of a rigorous child psychiatry fellowship, a psychiatrist caring for women with perinatal psychiatric disorders, and the mother of three young children, Dr. Richards tries to view these tasks as an opportunity for growth. But some days it feels as if she’s navigating a storm in the middle of the ocean without a life jacket.
In the age of COVID, “the wave of demands has morphed into one giant tidal wave of desperate need,” Dr. Richards, of the department of psychiatry & biobehavioral sciences, University of California, Los Angeles, Semel Institute of Neuroscience & Human Behavior, said in an interview. “The painfully loud and clear message is that our patients need us, and our children – who have been stripped from healthy routines and peer interactions that nourish social-emotional development – rely on us. We cannot turn our backs for even a moment, or else they will suffer.”
Tasked with caring for a much sicker and distressed population, navigating home duties such as child care, online school, and taking care of certain family members, women psychiatrists are feeling the impact of COVID-19.
Many have seamlessly transferred their practices online, maintaining a lifeline with their patients through telehealth visits. Even with this convenience, the emotional labor of being a psychiatrist is still very stressful, Pooja Lakshmin, MD, of the department of psychiatry and behavioral sciences at George Washington University, Washington, said in an interview. Because the nature of work has changed, and many are doing things virtually at home, separating home from work life can be a challenge. “It’s harder to disconnect,” admitted Dr. Lakshmin. “Even my patients tell me that they have no time to themselves anymore.”
– a moving target that remains nowhere in sight, Dr. Richards said. “In this process, we are expected to fill the emotional cups of a broken nation, to provide answers that do not exist, and to do so with never-ending gratitude for a demanding system that has no ‘off’ switch,” she noted.
‘In two places at once’
COVID-19’s physical and emotional toll has swept across the various subspecialties of clinical psychiatry. As some navigate outpatient/telehealth work, inpatient psychiatrists directly interact with COVID patients.
“Our inpatient psychiatry unit regularly takes care of COVID patients, including perinatal patients who are COVID positive,” Samantha Meltzer-Brody, MD, MPH, distinguished professor and chair, University of North Carolina, Chapel Hill, department of psychiatry and director of medical school’s Center for Women’s Mood Disorders, said in an interview. A psychiatry consultation-liaison service also provides psychiatry care to medical and surgical patients, including medically ill COVID patients across the hospital.
“We are on the front lines in the sense that we are dealing with the trauma of the general population and having to be present for that emotional distress,” Dr. Meltzer-Brody said.
The struggle to balance rising caseloads and home responsibilities makes things difficult, she continued. “There’s a never-ending onslaught of patient referrals,” reflecting the anxiety and depression issues people are experiencing in the wake of a global pandemic, frenetic political situation in the United States, and job uncertainty.
Child care and elder care responsibilities affect both men and women, yet research shows that caregiving demands disproportionately affect women, observed Dr. Meltzer-Brody.
Overall, the stress of caregiving and parenting responsibilities for men and women has been markedly higher during the pandemic. Most clinical psychiatrists “have been extraordinarily busy for a very long time,” she added.
Tiffani L. Bell, MD, a psychiatrist in Winston-Salem, N.C., has seen an increase in anxiety and depression in people with no previous history of diagnosed mental illness. “The impact of the pandemic has truly been multifaceted. People are struggling with loss of jobs, loss of wages, and loss of loved ones, along with grieving the loss of the usual way of life,” she said in an interview.
Many of her colleagues report feeling overburdened at work with increased admissions and patient loads, decreased time to see each patient, and the feeling of “needing to be in two places at once.”
“As a female psychiatrist, I do believe that we can sometimes have an increased mental burden due to the emotional and physical burnout that can occur when our routines are shaken,” added Dr. Bell, who specializes in adult, child, and adolescent psychiatry, and obesity and lifestyle medicine. Even in the early months of the pandemic, Dr. Bell said she heard people joke that “they don’t know if they are working from home or living at work.”
Physicians aren’t the only ones who are overwhelmed. “We’re also hearing stories from our patients – those at risk for partner violence, dealing with kids out of school, working full time while providing support at home,” Ludmila De Faria, MD, chair of the American Psychiatric Association’s Committee on Women’s Mental Health, said in an interview.
American mothers in particular spend nearly twice as much time caring for their children and cooking than their spouses, said Dr. Bell, citing recent studies. “Even if one is not a mom, if you couple the increased housework at baseline with the added responsibilities of working as a front-line physician and/or working from home while managing a household, it can lead to increased stress for all involved.”
Women leaving the workforce
Nationally, a growing number of women are either reducing their hours or leaving the workforce in response to the pandemic. Fidelity Investments, which surveyed 1,902 U.S. adults in mid-2020 projected that 4 in 10 women were mulling such options. Among 951 women surveyed, 42% were considering stepping back from their jobs because of their children’s homeschooling needs, and 27% cited difficulties of balancing home and job responsibilities.
Interruptions caused by child care affect women more than men, according to a report from the Century Foundation and the Center for American Progress. “Study after study has shown that, in response to school, child care, and camp closings, as well as reduced hours and reduced class sizes, significantly more women than men have reduced their work hours, left work to care for children, and spent more time on education and household tasks,” the authors noted.
They estimated that the American economy could incur $64.5 billion per year in lost wages and economic activity from the fallout of these trends. In September 2020, four times as many women as men left the workforce, nearly 865,000 women in comparison to 216,000 men.
Many women psychiatrists have been forced to choose between their careers or child care duties – decisions they don’t want to make, but that may be necessary during these unprecedented circumstances. They may be reducing their work hours to assist at home. Others are leaving their jobs, “a terrible situation given the enormous mental health needs of the pandemic” and the fact that so many areas of the United States already suffer from a shortage of clinical psychiatrists, said Dr. Meltzer-Brody.
She has personally seen the effects of this in the large academic department she supervises. “I’m seeing women reducing their work hours or leave positions,” she continued. In addition to child care needs, these women are tending to aging parents affected by COVID-19 or other illnesses, or dealing with the fact that options for elder care aren’t available.
“I have multiple faculty contending with that situation,” added Dr. Meltzer-Brody. As a result, productivity is going down. “These women are trying to keep all of the balls in the air but find they can’t.”
Dr. Richards believes some changes are in order to take the disproportionate burden off of women in psychiatry, and the workforce as a whole. The health care system “places too much pressure on individuals to compensate for its deficiencies. Those individuals who often step up to the plate are women, and this is not their sole burden to carry.”
A move toward telehealth in clinical psychiatry has made it possible for patients and physicians to meet virtually in their respective homes and discuss treatment options. “Even while this is both a blessing and privilege, it comes with the unique challenges of having to manage Zoom calls, child care, meals, distance learning, cleaning, and work responsibilities, while previously there was a clearer delineation to the day for many,” Dr. Bell said.
Clinical psychiatrists educating the public about the mental stressors of COVID-19 face their own unique challenges.
Dr. Lakshmin, who makes appearances in various media and social media outlets, said this adds more pressure to the job. “One of the challenges for me is to figure out how much outward facing I do. That’s hard when you’re navigating working and living through a pandemic. This is something I do because I enjoy doing it. But it’s still a type of work. And it’s certainly increased because the media has been paying more attention to mental health” since the pandemic started, she added.
The dual stress of COVID and social justice
Some women psychiatrists of color are dealing with social justice issues on top of other COVID stressors, Dr. De Faria said. The focus on addressing institutionalized racism means that minority women are taking on extra work to advocate for their peers.
Michelle Jacobs-Elliott, MD, of the department of psychiatry and assistant dean of the Office of Diversity and Health Equity at the University of Florida, Gainesville, knows of such responsibilities. “I have been in many discussions either with my coworkers in my department or others who work for the University of Florida” on systemic racism, she said in an interview.
Dr. Jacobs-Elliott became a trainer for Bias Reduction in Internal Medicine, a workshop aimed at reducing bias, and prior to 2020 participated in a social justice summit at the University of Florida. “Talking with my medical as well as undergraduate students about their experiences both here in Gainesville and elsewhere, they are all feeling the hurt, disappointment, and disbelief that we are still fighting battles that our grandparents fought in health care, housing, and employment. This adds an extra layer of stress to everyone’s life.”
The tense social climate has made the apparent racial inequalities in COVID-19 deaths and severity of disease hard to ignore, Dr. Bell noted. “It is my sincere hope that the availability of COVID-19 vaccines will help decrease the number of people affected by this horrible disease. The added burden of racism on top of the stressors of this pandemic can feel insurmountable. I hope 2021 will provide a way forward for us all.”
Taking time for self-care
Amid the endless referrals and increasing demands at home, women psychiatrists often don’t have the time to do normal activities, Dr. Meltzer-Brody observed. Like most people, COVID restrictions prevent them from traveling or going to the gym or restaurants. Dr. De Faria has not been able to visit family in Latin America, a trip she used to make twice a year. “That was once my de-stress time. But now, I can’t connect with my roots. My father is elderly and very much at risk.”
This is the time to get creative and resourceful – to make time for self-care, several sources said.
“We need to realize that we cannot be all things to all people, at the same time,” noted Dr. Bell. It’s important to prioritize what’s most important – and keep assessing your priorities. There’s no shame in tending to your own needs. Dr. Bell recommended that women in her profession should pick 1 day a week, put it in their calendar, and stick to this goal of self-care.
“Even if it’s only 15 minutes, it is important to put time aside. Some quick, cheap ideas are to do a quick meditation session, read a chapter in a book, listen to an audiobook, journal, go for a walk and get fresh air. Eat a healthy meal. Even 10 minutes helps,” she urged.
COVID-19 has pushed society to find new ways to do things, Dr. Bell continued. Women psychiatrists, in assessing their work-life balance, may need to reassess their goals. Consider work schedules and see if there’s a place to scale back a task. Delegate tasks at home to family members, if necessary. Most importantly, exercise self-compassion, she stressed. “During this pandemic, I believe it is vital to keep our cups filled so we can pour into others.”
Dr. Lakshmin said she has benefited greatly from having a therapist during the pandemic. “It has been so instrumental in forcing me to take that time for myself, to give me a space to take care of me, and remember it’s okay to take care of me. It’s so important for us as psychiatrists to have that for ourselves. It’s not just for our patients – we need it, too.”
The APA has resources and numerous support groups that meet regularly to address and discuss the stressors of the pandemic. Its College Mental Health Caucus, for example, holds a monthly, hour-long Zoom meeting. Not surprisingly, women comprise the majority of attendees, Dr. De Faria said. “Most women in academic psychiatry are working from home and using telehealth, which isolates people a lot.” Maureen Sayres Van Niel, MD, who is head of the APA’s Women’s Caucus, sends out a regular newsletter that advises on self-care. Women psychiatrists should also contact their local psychiatric organizations to get support from their professional peers.
Sometimes it’s wise to leave work behind and engage with friends. Dr. De Faria regularly Zooms with a group of friends outside of her profession to de-stress and reconnect. “At least I can talk to them about things other than psychiatry.”
Mentally and physically exhausted, Dr. Jacobs-Elliott said she looks forward to the day when society can return to meeting with friends and family “without being afraid that we are an asymptomatic carrier who is infecting our loved ones.”
Daily life is now a juggling act for Misty Richards, MD, MS. As the program director of a rigorous child psychiatry fellowship, a psychiatrist caring for women with perinatal psychiatric disorders, and the mother of three young children, Dr. Richards tries to view these tasks as an opportunity for growth. But some days it feels as if she’s navigating a storm in the middle of the ocean without a life jacket.
In the age of COVID, “the wave of demands has morphed into one giant tidal wave of desperate need,” Dr. Richards, of the department of psychiatry & biobehavioral sciences, University of California, Los Angeles, Semel Institute of Neuroscience & Human Behavior, said in an interview. “The painfully loud and clear message is that our patients need us, and our children – who have been stripped from healthy routines and peer interactions that nourish social-emotional development – rely on us. We cannot turn our backs for even a moment, or else they will suffer.”
Tasked with caring for a much sicker and distressed population, navigating home duties such as child care, online school, and taking care of certain family members, women psychiatrists are feeling the impact of COVID-19.
Many have seamlessly transferred their practices online, maintaining a lifeline with their patients through telehealth visits. Even with this convenience, the emotional labor of being a psychiatrist is still very stressful, Pooja Lakshmin, MD, of the department of psychiatry and behavioral sciences at George Washington University, Washington, said in an interview. Because the nature of work has changed, and many are doing things virtually at home, separating home from work life can be a challenge. “It’s harder to disconnect,” admitted Dr. Lakshmin. “Even my patients tell me that they have no time to themselves anymore.”
– a moving target that remains nowhere in sight, Dr. Richards said. “In this process, we are expected to fill the emotional cups of a broken nation, to provide answers that do not exist, and to do so with never-ending gratitude for a demanding system that has no ‘off’ switch,” she noted.
‘In two places at once’
COVID-19’s physical and emotional toll has swept across the various subspecialties of clinical psychiatry. As some navigate outpatient/telehealth work, inpatient psychiatrists directly interact with COVID patients.
“Our inpatient psychiatry unit regularly takes care of COVID patients, including perinatal patients who are COVID positive,” Samantha Meltzer-Brody, MD, MPH, distinguished professor and chair, University of North Carolina, Chapel Hill, department of psychiatry and director of medical school’s Center for Women’s Mood Disorders, said in an interview. A psychiatry consultation-liaison service also provides psychiatry care to medical and surgical patients, including medically ill COVID patients across the hospital.
“We are on the front lines in the sense that we are dealing with the trauma of the general population and having to be present for that emotional distress,” Dr. Meltzer-Brody said.
The struggle to balance rising caseloads and home responsibilities makes things difficult, she continued. “There’s a never-ending onslaught of patient referrals,” reflecting the anxiety and depression issues people are experiencing in the wake of a global pandemic, frenetic political situation in the United States, and job uncertainty.
Child care and elder care responsibilities affect both men and women, yet research shows that caregiving demands disproportionately affect women, observed Dr. Meltzer-Brody.
Overall, the stress of caregiving and parenting responsibilities for men and women has been markedly higher during the pandemic. Most clinical psychiatrists “have been extraordinarily busy for a very long time,” she added.
Tiffani L. Bell, MD, a psychiatrist in Winston-Salem, N.C., has seen an increase in anxiety and depression in people with no previous history of diagnosed mental illness. “The impact of the pandemic has truly been multifaceted. People are struggling with loss of jobs, loss of wages, and loss of loved ones, along with grieving the loss of the usual way of life,” she said in an interview.
Many of her colleagues report feeling overburdened at work with increased admissions and patient loads, decreased time to see each patient, and the feeling of “needing to be in two places at once.”
“As a female psychiatrist, I do believe that we can sometimes have an increased mental burden due to the emotional and physical burnout that can occur when our routines are shaken,” added Dr. Bell, who specializes in adult, child, and adolescent psychiatry, and obesity and lifestyle medicine. Even in the early months of the pandemic, Dr. Bell said she heard people joke that “they don’t know if they are working from home or living at work.”
Physicians aren’t the only ones who are overwhelmed. “We’re also hearing stories from our patients – those at risk for partner violence, dealing with kids out of school, working full time while providing support at home,” Ludmila De Faria, MD, chair of the American Psychiatric Association’s Committee on Women’s Mental Health, said in an interview.
American mothers in particular spend nearly twice as much time caring for their children and cooking than their spouses, said Dr. Bell, citing recent studies. “Even if one is not a mom, if you couple the increased housework at baseline with the added responsibilities of working as a front-line physician and/or working from home while managing a household, it can lead to increased stress for all involved.”
Women leaving the workforce
Nationally, a growing number of women are either reducing their hours or leaving the workforce in response to the pandemic. Fidelity Investments, which surveyed 1,902 U.S. adults in mid-2020 projected that 4 in 10 women were mulling such options. Among 951 women surveyed, 42% were considering stepping back from their jobs because of their children’s homeschooling needs, and 27% cited difficulties of balancing home and job responsibilities.
Interruptions caused by child care affect women more than men, according to a report from the Century Foundation and the Center for American Progress. “Study after study has shown that, in response to school, child care, and camp closings, as well as reduced hours and reduced class sizes, significantly more women than men have reduced their work hours, left work to care for children, and spent more time on education and household tasks,” the authors noted.
They estimated that the American economy could incur $64.5 billion per year in lost wages and economic activity from the fallout of these trends. In September 2020, four times as many women as men left the workforce, nearly 865,000 women in comparison to 216,000 men.
Many women psychiatrists have been forced to choose between their careers or child care duties – decisions they don’t want to make, but that may be necessary during these unprecedented circumstances. They may be reducing their work hours to assist at home. Others are leaving their jobs, “a terrible situation given the enormous mental health needs of the pandemic” and the fact that so many areas of the United States already suffer from a shortage of clinical psychiatrists, said Dr. Meltzer-Brody.
She has personally seen the effects of this in the large academic department she supervises. “I’m seeing women reducing their work hours or leave positions,” she continued. In addition to child care needs, these women are tending to aging parents affected by COVID-19 or other illnesses, or dealing with the fact that options for elder care aren’t available.
“I have multiple faculty contending with that situation,” added Dr. Meltzer-Brody. As a result, productivity is going down. “These women are trying to keep all of the balls in the air but find they can’t.”
Dr. Richards believes some changes are in order to take the disproportionate burden off of women in psychiatry, and the workforce as a whole. The health care system “places too much pressure on individuals to compensate for its deficiencies. Those individuals who often step up to the plate are women, and this is not their sole burden to carry.”
A move toward telehealth in clinical psychiatry has made it possible for patients and physicians to meet virtually in their respective homes and discuss treatment options. “Even while this is both a blessing and privilege, it comes with the unique challenges of having to manage Zoom calls, child care, meals, distance learning, cleaning, and work responsibilities, while previously there was a clearer delineation to the day for many,” Dr. Bell said.
Clinical psychiatrists educating the public about the mental stressors of COVID-19 face their own unique challenges.
Dr. Lakshmin, who makes appearances in various media and social media outlets, said this adds more pressure to the job. “One of the challenges for me is to figure out how much outward facing I do. That’s hard when you’re navigating working and living through a pandemic. This is something I do because I enjoy doing it. But it’s still a type of work. And it’s certainly increased because the media has been paying more attention to mental health” since the pandemic started, she added.
The dual stress of COVID and social justice
Some women psychiatrists of color are dealing with social justice issues on top of other COVID stressors, Dr. De Faria said. The focus on addressing institutionalized racism means that minority women are taking on extra work to advocate for their peers.
Michelle Jacobs-Elliott, MD, of the department of psychiatry and assistant dean of the Office of Diversity and Health Equity at the University of Florida, Gainesville, knows of such responsibilities. “I have been in many discussions either with my coworkers in my department or others who work for the University of Florida” on systemic racism, she said in an interview.
Dr. Jacobs-Elliott became a trainer for Bias Reduction in Internal Medicine, a workshop aimed at reducing bias, and prior to 2020 participated in a social justice summit at the University of Florida. “Talking with my medical as well as undergraduate students about their experiences both here in Gainesville and elsewhere, they are all feeling the hurt, disappointment, and disbelief that we are still fighting battles that our grandparents fought in health care, housing, and employment. This adds an extra layer of stress to everyone’s life.”
The tense social climate has made the apparent racial inequalities in COVID-19 deaths and severity of disease hard to ignore, Dr. Bell noted. “It is my sincere hope that the availability of COVID-19 vaccines will help decrease the number of people affected by this horrible disease. The added burden of racism on top of the stressors of this pandemic can feel insurmountable. I hope 2021 will provide a way forward for us all.”
Taking time for self-care
Amid the endless referrals and increasing demands at home, women psychiatrists often don’t have the time to do normal activities, Dr. Meltzer-Brody observed. Like most people, COVID restrictions prevent them from traveling or going to the gym or restaurants. Dr. De Faria has not been able to visit family in Latin America, a trip she used to make twice a year. “That was once my de-stress time. But now, I can’t connect with my roots. My father is elderly and very much at risk.”
This is the time to get creative and resourceful – to make time for self-care, several sources said.
“We need to realize that we cannot be all things to all people, at the same time,” noted Dr. Bell. It’s important to prioritize what’s most important – and keep assessing your priorities. There’s no shame in tending to your own needs. Dr. Bell recommended that women in her profession should pick 1 day a week, put it in their calendar, and stick to this goal of self-care.
“Even if it’s only 15 minutes, it is important to put time aside. Some quick, cheap ideas are to do a quick meditation session, read a chapter in a book, listen to an audiobook, journal, go for a walk and get fresh air. Eat a healthy meal. Even 10 minutes helps,” she urged.
COVID-19 has pushed society to find new ways to do things, Dr. Bell continued. Women psychiatrists, in assessing their work-life balance, may need to reassess their goals. Consider work schedules and see if there’s a place to scale back a task. Delegate tasks at home to family members, if necessary. Most importantly, exercise self-compassion, she stressed. “During this pandemic, I believe it is vital to keep our cups filled so we can pour into others.”
Dr. Lakshmin said she has benefited greatly from having a therapist during the pandemic. “It has been so instrumental in forcing me to take that time for myself, to give me a space to take care of me, and remember it’s okay to take care of me. It’s so important for us as psychiatrists to have that for ourselves. It’s not just for our patients – we need it, too.”
The APA has resources and numerous support groups that meet regularly to address and discuss the stressors of the pandemic. Its College Mental Health Caucus, for example, holds a monthly, hour-long Zoom meeting. Not surprisingly, women comprise the majority of attendees, Dr. De Faria said. “Most women in academic psychiatry are working from home and using telehealth, which isolates people a lot.” Maureen Sayres Van Niel, MD, who is head of the APA’s Women’s Caucus, sends out a regular newsletter that advises on self-care. Women psychiatrists should also contact their local psychiatric organizations to get support from their professional peers.
Sometimes it’s wise to leave work behind and engage with friends. Dr. De Faria regularly Zooms with a group of friends outside of her profession to de-stress and reconnect. “At least I can talk to them about things other than psychiatry.”
Mentally and physically exhausted, Dr. Jacobs-Elliott said she looks forward to the day when society can return to meeting with friends and family “without being afraid that we are an asymptomatic carrier who is infecting our loved ones.”







