User login
Complete PCI beats culprit-lesion-only PCI in STEMI patients with multivessel CAD
Background: Previous trials have shown a reduction in composite outcomes if STEMI patients undergo staged PCI of nonculprit lesions discovered incidentally at the time of primary PCI for STEMI. However, no randomized trial has had the power to assess if staged PCI of nonculprit lesions reduces cardiovascular death or MI.
Study design: Prospective randomized clinical trial.
Setting: PCI-capable centers in 31 countries.
Synopsis: In this study, if multivessel disease was identified during primary PCI for STEMI, patients were randomized to either culprit-lesion-only PCI or complete revascularization with staged PCI of all suitable nonculprit lesions (either during the index hospitalization or up to 45 days after randomization).
Overall, 4,041 patients from 140 centers were randomized with median 3-year follow-up. The complete revascularization group had lower rates of the primary composite outcome of death from cardiovascular disease or new MI (absolute reduction, 2.7%; 7.8% vs. 10.5%; number needed to treat, 37; hazard ratio, 0.74; 95% confidence interval, 0.60-0.91; P = .004). This finding was driven by lower incidence of new MI in the complete revascularization group – the incidence of death was similar between the groups. A coprimary composite outcome of death from cardiovascular causes, new MI, or ischemia-driven revascularization also favored complete revascularization, with an absolute risk reduction of 7.8% (8.9% vs. 16.7%; NNT, 13; HR, 0.51; 95% CI, 0.43-0.61; P less than .001). No statistically significant differences between groups were noted for the safety outcomes of major bleeding, stroke, stent thrombosis, or contrast-induced kidney injury.
Bottom line: Patients with STEMI who have multivessel disease incidentally discovered during primary PCI have a lower incidence of new MI and ischemia-driven revascularization when they undergo complete revascularization of all suitable lesions, as opposed to PCI of only their culprit lesion.
Citation: Mehta SR et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019 Oct 10;381:1411-21.
Dr. Porter is chief quality and safety resident at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Previous trials have shown a reduction in composite outcomes if STEMI patients undergo staged PCI of nonculprit lesions discovered incidentally at the time of primary PCI for STEMI. However, no randomized trial has had the power to assess if staged PCI of nonculprit lesions reduces cardiovascular death or MI.
Study design: Prospective randomized clinical trial.
Setting: PCI-capable centers in 31 countries.
Synopsis: In this study, if multivessel disease was identified during primary PCI for STEMI, patients were randomized to either culprit-lesion-only PCI or complete revascularization with staged PCI of all suitable nonculprit lesions (either during the index hospitalization or up to 45 days after randomization).
Overall, 4,041 patients from 140 centers were randomized with median 3-year follow-up. The complete revascularization group had lower rates of the primary composite outcome of death from cardiovascular disease or new MI (absolute reduction, 2.7%; 7.8% vs. 10.5%; number needed to treat, 37; hazard ratio, 0.74; 95% confidence interval, 0.60-0.91; P = .004). This finding was driven by lower incidence of new MI in the complete revascularization group – the incidence of death was similar between the groups. A coprimary composite outcome of death from cardiovascular causes, new MI, or ischemia-driven revascularization also favored complete revascularization, with an absolute risk reduction of 7.8% (8.9% vs. 16.7%; NNT, 13; HR, 0.51; 95% CI, 0.43-0.61; P less than .001). No statistically significant differences between groups were noted for the safety outcomes of major bleeding, stroke, stent thrombosis, or contrast-induced kidney injury.
Bottom line: Patients with STEMI who have multivessel disease incidentally discovered during primary PCI have a lower incidence of new MI and ischemia-driven revascularization when they undergo complete revascularization of all suitable lesions, as opposed to PCI of only their culprit lesion.
Citation: Mehta SR et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019 Oct 10;381:1411-21.
Dr. Porter is chief quality and safety resident at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Previous trials have shown a reduction in composite outcomes if STEMI patients undergo staged PCI of nonculprit lesions discovered incidentally at the time of primary PCI for STEMI. However, no randomized trial has had the power to assess if staged PCI of nonculprit lesions reduces cardiovascular death or MI.
Study design: Prospective randomized clinical trial.
Setting: PCI-capable centers in 31 countries.
Synopsis: In this study, if multivessel disease was identified during primary PCI for STEMI, patients were randomized to either culprit-lesion-only PCI or complete revascularization with staged PCI of all suitable nonculprit lesions (either during the index hospitalization or up to 45 days after randomization).
Overall, 4,041 patients from 140 centers were randomized with median 3-year follow-up. The complete revascularization group had lower rates of the primary composite outcome of death from cardiovascular disease or new MI (absolute reduction, 2.7%; 7.8% vs. 10.5%; number needed to treat, 37; hazard ratio, 0.74; 95% confidence interval, 0.60-0.91; P = .004). This finding was driven by lower incidence of new MI in the complete revascularization group – the incidence of death was similar between the groups. A coprimary composite outcome of death from cardiovascular causes, new MI, or ischemia-driven revascularization also favored complete revascularization, with an absolute risk reduction of 7.8% (8.9% vs. 16.7%; NNT, 13; HR, 0.51; 95% CI, 0.43-0.61; P less than .001). No statistically significant differences between groups were noted for the safety outcomes of major bleeding, stroke, stent thrombosis, or contrast-induced kidney injury.
Bottom line: Patients with STEMI who have multivessel disease incidentally discovered during primary PCI have a lower incidence of new MI and ischemia-driven revascularization when they undergo complete revascularization of all suitable lesions, as opposed to PCI of only their culprit lesion.
Citation: Mehta SR et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019 Oct 10;381:1411-21.
Dr. Porter is chief quality and safety resident at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Hair Follicle Bulb Region: A Potential Nidus for the Formation of Osteoma Cutis
The term osteoma cutis (OC) is defined as the ossification or bone formation either in the dermis or hypodermis. 1 It is heterotopic in nature, referring to extraneous bone formation in soft tissue. Osteoma cutis was first described in 1858 2,3 ; in 1868, the multiple miliary form on the face was described. 4 Cutaneous ossification can take many forms, ranging from occurrence in a nevus (nevus of Nanta) to its association with rare genetic disorders, such as fibrodysplasia ossificans progressiva and Albright hereditary osteodystrophy.
Some of these ossifications are classified as primary; others are secondary, depending on the presence of a preexisting lesion (eg, pilomatricoma, basal cell carcinoma). However, certain conditions, such as multiple miliary osteoma of the face, can be difficult to classify due to the presence or absence of a history of acne or dermabrasion, or both. The secondary forms more commonly are encountered due to their incidental association with an excised lesion, such as pilomatricoma.
A precursor of OC has been neglected in the literature despite its common occurrence. It may have been peripherally alluded to in the literature in reference to the miliary form of OC.5,6 The cases reported here demonstrate small round nodules of calcification or ossification, or both, in punch biopsies and excision specimens from hair-bearing areas of skin, especially from the head and neck. These lesions are mainly observed in the peripilar location or more specifically in the approximate location of the hair bulb.
This article reviews a possible mechanism of formation of these osteocalcific micronodules. These often-encountered micronodules are small osteocalcific lesions without typical bone or well-formed OC, such as trabeculae formation or fatty marrow, and may represent earliest stages in the formation of OC.
Clinical Observations
During routine dermatopathologic practice, I observed incidental small osteocalcific micronodules in close proximity to the lower part of the hair follicle in multiple cases. These nodules were not related to the main lesion in the specimen and were not the reason for the biopsy or excision. Most of the time, these micronodules were noted in excision or re-excision specimens or in a punch biopsy.
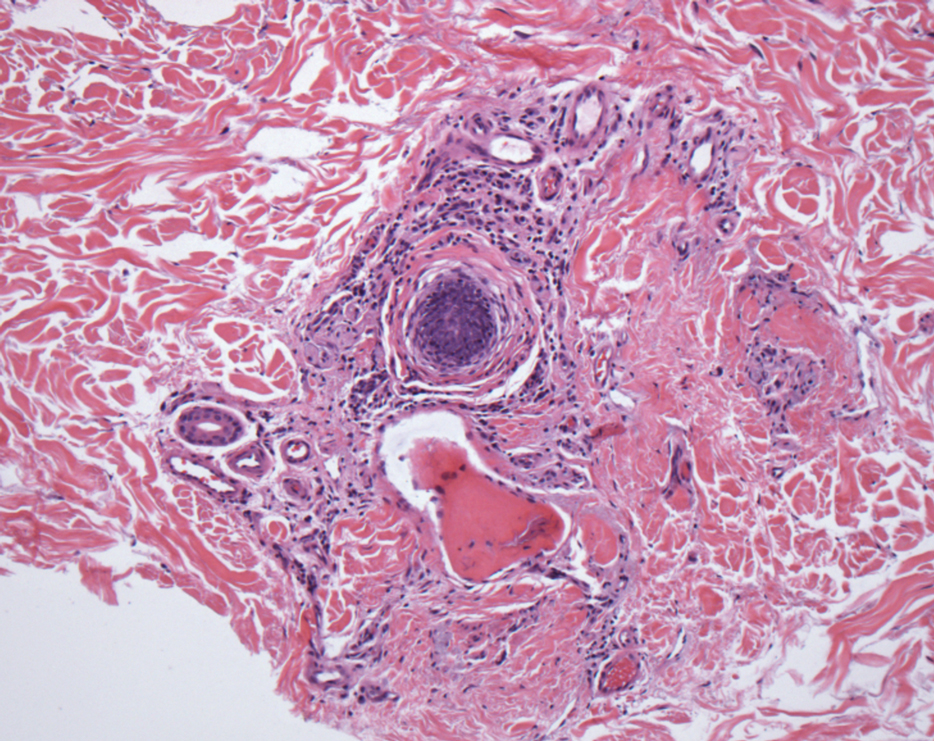
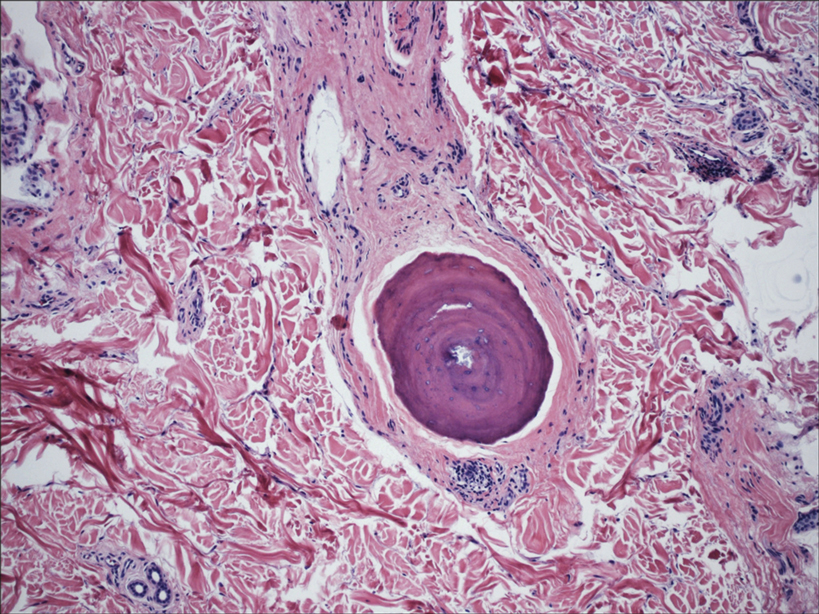
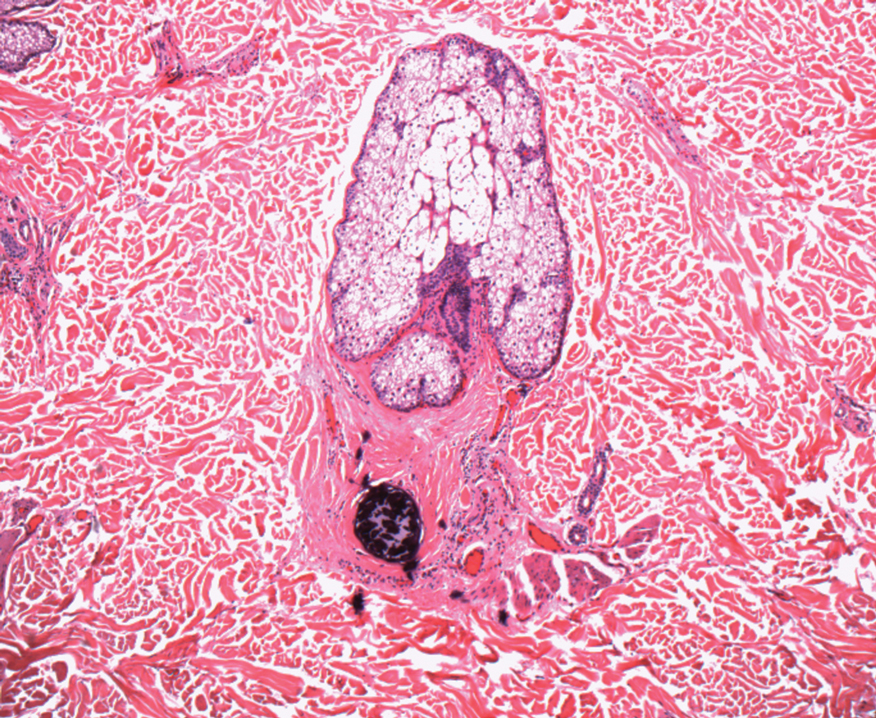
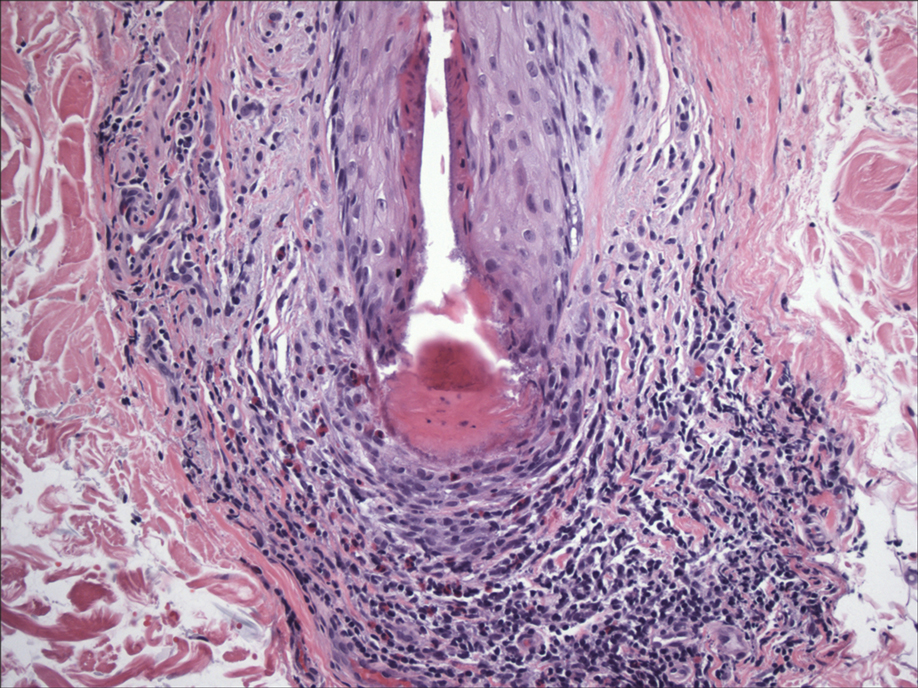
In my review of multiple unrelated cases over time, incidental osteocalcific micronodules were observed occasionally in punch biopsies and excision specimens during routine practice. These micronodules were mainly located in the vicinity of a hair bulb (Figure 1). If the hair bulb was not present in the sections, these micronodules were noted near or within the fibrous tract (Figure 2) or beneath a sebaceous lobule (Figure 3). In an exceptional case, a small round deposit of osteoid was seen forming just above the dermal papilla of the hair bulb (Figure 4).
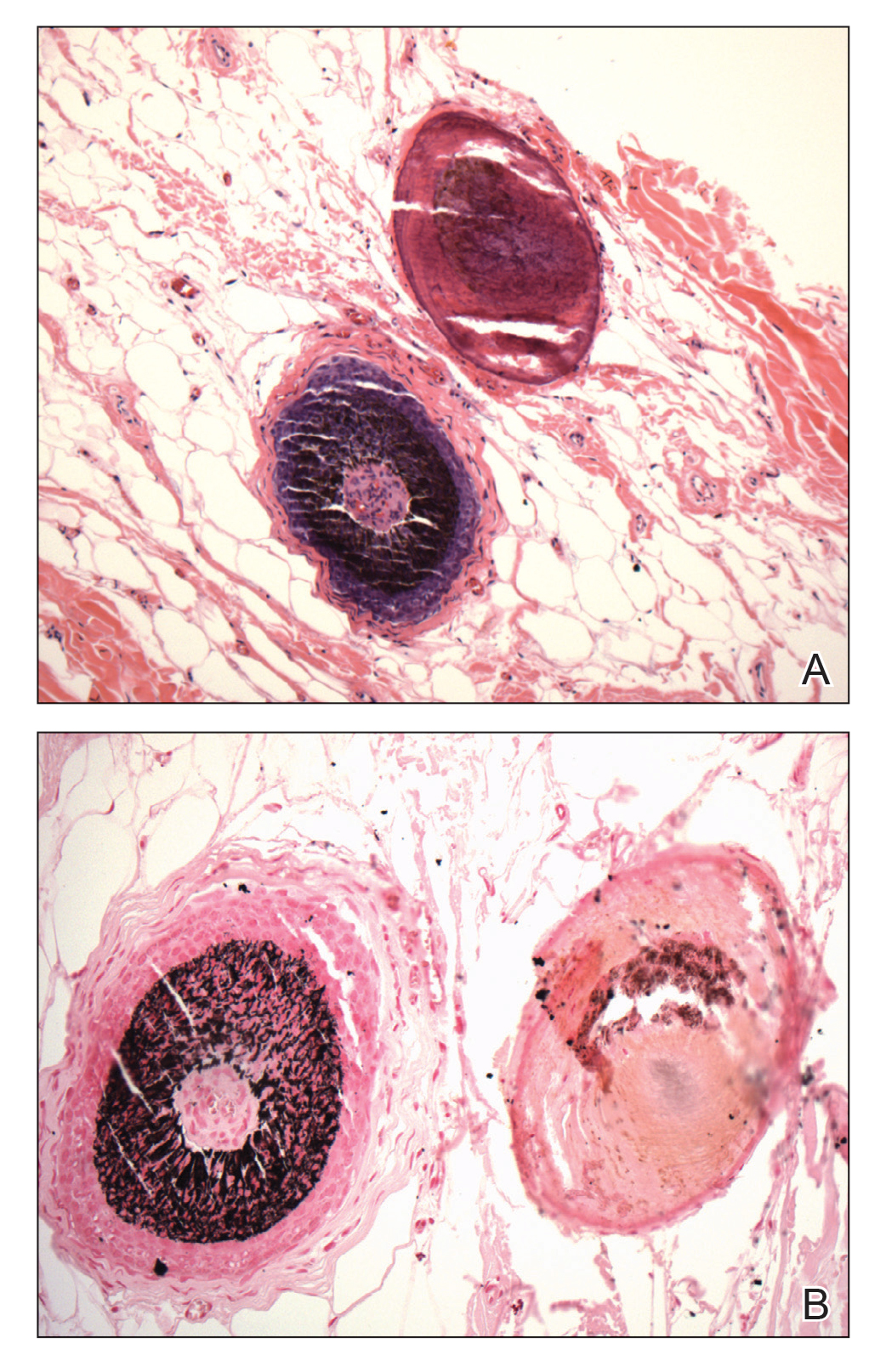
Multiple osteocalcific micronodules were identified in a case of cicatricial alopecia. These micronodules were observed in sections taken at the levels of hair bulbs, and more or less corresponded to the size of the bulb (Figure 5A). Fortuitously, the patient was dark-skinned; the remnants of melanin within the micronodules provided evidence that the micronodules were formed within hair bulbs. Melanin staining confirmed the presence of melanin within some of the micronodules (Figure 5B).
Comment
Skeletogenesis in humans takes place by 2 methods: endochondral ossification and intramembranous ossification. In contrast to endochondral ossification, intramembranous ossification does not require a preexisting cartilaginous template. Instead, there is condensation of mesenchymal cells, which differentiate into osteoblasts and lay down osteoid, thus forming an ossification center. Little is known about the mechanism of formation of OC or the nidus of formation of the primary form.
Incidental micronodules of calcification and ossification are routinely encountered during histopathologic review of specimens from hair-bearing areas of the skin in dermatopathology practice. A review of the literature, however, does not reveal any specific dermatopathologic term ascribed to this phenomenon. These lesions might be similar to those described by Hopkins5 in 1928 in the setting of miliary OC of the face secondary to acne. Rossman and Freeman6 also described the same lesions when referring to facial OC as a “stage of pre-osseous calcification.”
When these osteocalcific micronodules are encountered, it usually is in close proximity to a hair follicle bulb. When a hair bulb is not seen in the sections, the micronodules are noted near fibrous tracts, arrector pili muscles, or sebaceous lobules, suggesting a close peripilar or peribulbar location. The micronodules are approximately 0.5 mm in diameter—roughly the size of a hair bulb. Due to the close anatomic association of micronodules and the hair bulb, these lesions can be called pilar osteocalcific nodules (PONs).
The role of bone morphogenetic protein (BMP) signaling in the maintenance of the hair cycle is well established. Bone morphogenetic proteins are extracellular cytokines that belong to the transforming growth factor β family. The hair bulb microenvironment is rich in BMPs
As the name implies, BMPs were discovered in relation to their important role in osteogenesis and tissue homeostasis. More than 20 BMPs have been identified, many of which promote bone formation and repair of bone fracture. Osteoinductive BMPs include BMP-2 and BMP-4 through BMP-10; BMP-2 and BMP-4 are expressed in the hair matrix and BMP-4 and BMP-6 are expressed in the FDP.8,9 All bone-inducing BMPs can cause mesenchymal stem cells to differentiate into osteoblasts in vitro.10
Overactive BMP signaling has been shown to cause heterotopic ossification in patients with fibrodysplasia ossificans progressiva.8 Immunohistochemical expression of BMP-2 has been demonstrated in shadow cells of pilomatricoma.11 Calcification and ossification are seen in as many as 20% of pilomatricomas. Both BMP-2 and BMP-4 have been shown to induce osteogenic differentiation of mouse skin−derived fibroblasts and FDP cells.12
Myllylä et al13 described 4 cases of multiple miliary osteoma cutis (MMOC). They also found 47 reported cases of MMOC, in which there was a history of acne in 55% (26/47). Only 15% (7/47) of these cases were extrafacial on the neck, chest, back, and arms. Osteomas in these cases were not associated with folliculosebaceous units or other adnexal structures, which may have been due to replacement by acne scarring, as all 4 patients had a history of acne vulgaris. The authors postulated a role for the GNAS gene mutation in the morphogenesis of MMOC; however, no supporting evidence was found for this claim. They also postulated a role for BMPs in the formation of MMOC.13
Some disturbance or imbalance in hair bulb homeostasis leads to overactivity of BMP signaling, causing osteoinduction in the hair bulb region and formation of PONs. The cause of the disturbance could be a traumatic or inflammatory injury to the hair follicle, as in the case of the secondary form of MMOC in association with chronic acne. In the primary form of osteoma cutis, the trigger could be more subtle or subclinical.
Trauma and inflammation are the main initiating factors involved in ossification in patients with fibrodysplasia ossificans progressiva due to ectopic activity of BMPs.9 The primary form of ossification appears to be similar to the mechanism by which intramembranous ossification is laid down (ie, by differentiation of mesenchymal cells into osteoblasts). In the proposed scenario, the cells of FDP, under the influence of BMPs, differentiate into osteoblasts and lay down osteoid, forming a limited-capacity “ossification center” or pilar osteocalcific nodule.
It is difficult to know the exact relationship of PONs or OC to the hair bulb due to the 2-dimensional nature of histologic sections. However, considering the finding of a rare case of osteoid forming within the bulb and in another the presence of melanin within the osteocalcific nodule, it is likely that these lesions are formed within the hair bulb or in situations in which the conditions replicate the biochemical characteristics of the hair bulb (eg, pilomatricoma).
The formation of PONs might act as a terminal phase in the hair cycle that is rarely induced to provide an exit for damaged hair follicles from cyclical perpetuity. An unspecified event or injury might render a hair follicle unable to continue its cyclical growth and cause BMPs to induce premature calcification in or around the hair bulb, which would probably be the only known quasiphysiological mechanism for a damaged hair follicle to exit the hair cycle.
Another interesting aspect of osteoma formation in human skin is the similarity to osteoderms or the integumentary skeleton of vertebrates.14 Early in evolution, the dermal skeleton was the predominant skeletal system in some lineages. Phylogenetically, osteoderms are not uniformly distributed, and show a latent ability to manifest in some groups or lay dormant or disappear in others. The occurrence of primary osteomas in the human integument might be a vestigial manifestation of deep homology,15 a latent ability to form structures that have been lost. The embryologic formation of osteoderms in the dermis of vertebrates is thought to depend on the interaction or cross-talk between ectomesenchymal cells of neural crest origin and cells of the stratum basalis of epidermis, which is somewhat similar to the formation of the hair follicles.
Conclusion
Under certain conditions, the bulb region of a hair follicle might provide a nidus for the formation of OC. The hair bulb region contains both the precursor cellular element (mesenchymal cells of FDP) and the trigger cytokine (BMP) for the induction of osteogenic metaplasia.
- Burgdorf W, Nasemann T. Cutaneous osteomas: a clinical and histopathologic review. Arch Dermatol Res. 1977;260:121-135.
- Essing M. Osteoma cutis of the forehead. HNO. 1985;33:548-550.
- Bouraoui S, Mlika M, Kort R, et al. Miliary osteoma cutis of the face. J Dermatol Case Rep. 2011;5:77-81.
- Virchow R. Die krankhaften Geschwülste. Vol 2. Hirschwald; 1864.
- Hopkins JG. Multiple miliary osteomas of the skin: report of a case. Arch Derm Syphilol. 1928;18:706-715.
- Rossman RE, Freeman RG. Osteoma cutis, a stage of preosseous calcification. Arch Dermatol. 1964;89:68-73.
- Guha U, Mecklenburg L, Cowin P, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729-740.
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543-557.
- Shi S, de Gorter DJJ, Hoogaars WMH, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013;70:407-423.
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51.
- Kurokawa I, Kusumoto K, Bessho K. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
- Myllylä RM, Haapasaari K-M, Lehenkari P, et al. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014;355:463-470.
- Myllylä RM, Haapasaari KM, Palatsi R, et al. Multiple miliary osteoma cutis is a distinct disease entity: four case reports and review of the literature. Br J Dermatol. 2011;164:544-552.
- Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441-464.
- Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269:398-422.
The term osteoma cutis (OC) is defined as the ossification or bone formation either in the dermis or hypodermis. 1 It is heterotopic in nature, referring to extraneous bone formation in soft tissue. Osteoma cutis was first described in 1858 2,3 ; in 1868, the multiple miliary form on the face was described. 4 Cutaneous ossification can take many forms, ranging from occurrence in a nevus (nevus of Nanta) to its association with rare genetic disorders, such as fibrodysplasia ossificans progressiva and Albright hereditary osteodystrophy.
Some of these ossifications are classified as primary; others are secondary, depending on the presence of a preexisting lesion (eg, pilomatricoma, basal cell carcinoma). However, certain conditions, such as multiple miliary osteoma of the face, can be difficult to classify due to the presence or absence of a history of acne or dermabrasion, or both. The secondary forms more commonly are encountered due to their incidental association with an excised lesion, such as pilomatricoma.
A precursor of OC has been neglected in the literature despite its common occurrence. It may have been peripherally alluded to in the literature in reference to the miliary form of OC.5,6 The cases reported here demonstrate small round nodules of calcification or ossification, or both, in punch biopsies and excision specimens from hair-bearing areas of skin, especially from the head and neck. These lesions are mainly observed in the peripilar location or more specifically in the approximate location of the hair bulb.
This article reviews a possible mechanism of formation of these osteocalcific micronodules. These often-encountered micronodules are small osteocalcific lesions without typical bone or well-formed OC, such as trabeculae formation or fatty marrow, and may represent earliest stages in the formation of OC.
Clinical Observations
During routine dermatopathologic practice, I observed incidental small osteocalcific micronodules in close proximity to the lower part of the hair follicle in multiple cases. These nodules were not related to the main lesion in the specimen and were not the reason for the biopsy or excision. Most of the time, these micronodules were noted in excision or re-excision specimens or in a punch biopsy.
In my review of multiple unrelated cases over time, incidental osteocalcific micronodules were observed occasionally in punch biopsies and excision specimens during routine practice. These micronodules were mainly located in the vicinity of a hair bulb (Figure 1). If the hair bulb was not present in the sections, these micronodules were noted near or within the fibrous tract (Figure 2) or beneath a sebaceous lobule (Figure 3). In an exceptional case, a small round deposit of osteoid was seen forming just above the dermal papilla of the hair bulb (Figure 4).




Multiple osteocalcific micronodules were identified in a case of cicatricial alopecia. These micronodules were observed in sections taken at the levels of hair bulbs, and more or less corresponded to the size of the bulb (Figure 5A). Fortuitously, the patient was dark-skinned; the remnants of melanin within the micronodules provided evidence that the micronodules were formed within hair bulbs. Melanin staining confirmed the presence of melanin within some of the micronodules (Figure 5B).

Comment
Skeletogenesis in humans takes place by 2 methods: endochondral ossification and intramembranous ossification. In contrast to endochondral ossification, intramembranous ossification does not require a preexisting cartilaginous template. Instead, there is condensation of mesenchymal cells, which differentiate into osteoblasts and lay down osteoid, thus forming an ossification center. Little is known about the mechanism of formation of OC or the nidus of formation of the primary form.
Incidental micronodules of calcification and ossification are routinely encountered during histopathologic review of specimens from hair-bearing areas of the skin in dermatopathology practice. A review of the literature, however, does not reveal any specific dermatopathologic term ascribed to this phenomenon. These lesions might be similar to those described by Hopkins5 in 1928 in the setting of miliary OC of the face secondary to acne. Rossman and Freeman6 also described the same lesions when referring to facial OC as a “stage of pre-osseous calcification.”
When these osteocalcific micronodules are encountered, it usually is in close proximity to a hair follicle bulb. When a hair bulb is not seen in the sections, the micronodules are noted near fibrous tracts, arrector pili muscles, or sebaceous lobules, suggesting a close peripilar or peribulbar location. The micronodules are approximately 0.5 mm in diameter—roughly the size of a hair bulb. Due to the close anatomic association of micronodules and the hair bulb, these lesions can be called pilar osteocalcific nodules (PONs).
The role of bone morphogenetic protein (BMP) signaling in the maintenance of the hair cycle is well established. Bone morphogenetic proteins are extracellular cytokines that belong to the transforming growth factor β family. The hair bulb microenvironment is rich in BMPs
As the name implies, BMPs were discovered in relation to their important role in osteogenesis and tissue homeostasis. More than 20 BMPs have been identified, many of which promote bone formation and repair of bone fracture. Osteoinductive BMPs include BMP-2 and BMP-4 through BMP-10; BMP-2 and BMP-4 are expressed in the hair matrix and BMP-4 and BMP-6 are expressed in the FDP.8,9 All bone-inducing BMPs can cause mesenchymal stem cells to differentiate into osteoblasts in vitro.10
Overactive BMP signaling has been shown to cause heterotopic ossification in patients with fibrodysplasia ossificans progressiva.8 Immunohistochemical expression of BMP-2 has been demonstrated in shadow cells of pilomatricoma.11 Calcification and ossification are seen in as many as 20% of pilomatricomas. Both BMP-2 and BMP-4 have been shown to induce osteogenic differentiation of mouse skin−derived fibroblasts and FDP cells.12
Myllylä et al13 described 4 cases of multiple miliary osteoma cutis (MMOC). They also found 47 reported cases of MMOC, in which there was a history of acne in 55% (26/47). Only 15% (7/47) of these cases were extrafacial on the neck, chest, back, and arms. Osteomas in these cases were not associated with folliculosebaceous units or other adnexal structures, which may have been due to replacement by acne scarring, as all 4 patients had a history of acne vulgaris. The authors postulated a role for the GNAS gene mutation in the morphogenesis of MMOC; however, no supporting evidence was found for this claim. They also postulated a role for BMPs in the formation of MMOC.13
Some disturbance or imbalance in hair bulb homeostasis leads to overactivity of BMP signaling, causing osteoinduction in the hair bulb region and formation of PONs. The cause of the disturbance could be a traumatic or inflammatory injury to the hair follicle, as in the case of the secondary form of MMOC in association with chronic acne. In the primary form of osteoma cutis, the trigger could be more subtle or subclinical.
Trauma and inflammation are the main initiating factors involved in ossification in patients with fibrodysplasia ossificans progressiva due to ectopic activity of BMPs.9 The primary form of ossification appears to be similar to the mechanism by which intramembranous ossification is laid down (ie, by differentiation of mesenchymal cells into osteoblasts). In the proposed scenario, the cells of FDP, under the influence of BMPs, differentiate into osteoblasts and lay down osteoid, forming a limited-capacity “ossification center” or pilar osteocalcific nodule.
It is difficult to know the exact relationship of PONs or OC to the hair bulb due to the 2-dimensional nature of histologic sections. However, considering the finding of a rare case of osteoid forming within the bulb and in another the presence of melanin within the osteocalcific nodule, it is likely that these lesions are formed within the hair bulb or in situations in which the conditions replicate the biochemical characteristics of the hair bulb (eg, pilomatricoma).
The formation of PONs might act as a terminal phase in the hair cycle that is rarely induced to provide an exit for damaged hair follicles from cyclical perpetuity. An unspecified event or injury might render a hair follicle unable to continue its cyclical growth and cause BMPs to induce premature calcification in or around the hair bulb, which would probably be the only known quasiphysiological mechanism for a damaged hair follicle to exit the hair cycle.
Another interesting aspect of osteoma formation in human skin is the similarity to osteoderms or the integumentary skeleton of vertebrates.14 Early in evolution, the dermal skeleton was the predominant skeletal system in some lineages. Phylogenetically, osteoderms are not uniformly distributed, and show a latent ability to manifest in some groups or lay dormant or disappear in others. The occurrence of primary osteomas in the human integument might be a vestigial manifestation of deep homology,15 a latent ability to form structures that have been lost. The embryologic formation of osteoderms in the dermis of vertebrates is thought to depend on the interaction or cross-talk between ectomesenchymal cells of neural crest origin and cells of the stratum basalis of epidermis, which is somewhat similar to the formation of the hair follicles.
Conclusion
Under certain conditions, the bulb region of a hair follicle might provide a nidus for the formation of OC. The hair bulb region contains both the precursor cellular element (mesenchymal cells of FDP) and the trigger cytokine (BMP) for the induction of osteogenic metaplasia.
The term osteoma cutis (OC) is defined as the ossification or bone formation either in the dermis or hypodermis. 1 It is heterotopic in nature, referring to extraneous bone formation in soft tissue. Osteoma cutis was first described in 1858 2,3 ; in 1868, the multiple miliary form on the face was described. 4 Cutaneous ossification can take many forms, ranging from occurrence in a nevus (nevus of Nanta) to its association with rare genetic disorders, such as fibrodysplasia ossificans progressiva and Albright hereditary osteodystrophy.
Some of these ossifications are classified as primary; others are secondary, depending on the presence of a preexisting lesion (eg, pilomatricoma, basal cell carcinoma). However, certain conditions, such as multiple miliary osteoma of the face, can be difficult to classify due to the presence or absence of a history of acne or dermabrasion, or both. The secondary forms more commonly are encountered due to their incidental association with an excised lesion, such as pilomatricoma.
A precursor of OC has been neglected in the literature despite its common occurrence. It may have been peripherally alluded to in the literature in reference to the miliary form of OC.5,6 The cases reported here demonstrate small round nodules of calcification or ossification, or both, in punch biopsies and excision specimens from hair-bearing areas of skin, especially from the head and neck. These lesions are mainly observed in the peripilar location or more specifically in the approximate location of the hair bulb.
This article reviews a possible mechanism of formation of these osteocalcific micronodules. These often-encountered micronodules are small osteocalcific lesions without typical bone or well-formed OC, such as trabeculae formation or fatty marrow, and may represent earliest stages in the formation of OC.
Clinical Observations
During routine dermatopathologic practice, I observed incidental small osteocalcific micronodules in close proximity to the lower part of the hair follicle in multiple cases. These nodules were not related to the main lesion in the specimen and were not the reason for the biopsy or excision. Most of the time, these micronodules were noted in excision or re-excision specimens or in a punch biopsy.
In my review of multiple unrelated cases over time, incidental osteocalcific micronodules were observed occasionally in punch biopsies and excision specimens during routine practice. These micronodules were mainly located in the vicinity of a hair bulb (Figure 1). If the hair bulb was not present in the sections, these micronodules were noted near or within the fibrous tract (Figure 2) or beneath a sebaceous lobule (Figure 3). In an exceptional case, a small round deposit of osteoid was seen forming just above the dermal papilla of the hair bulb (Figure 4).




Multiple osteocalcific micronodules were identified in a case of cicatricial alopecia. These micronodules were observed in sections taken at the levels of hair bulbs, and more or less corresponded to the size of the bulb (Figure 5A). Fortuitously, the patient was dark-skinned; the remnants of melanin within the micronodules provided evidence that the micronodules were formed within hair bulbs. Melanin staining confirmed the presence of melanin within some of the micronodules (Figure 5B).

Comment
Skeletogenesis in humans takes place by 2 methods: endochondral ossification and intramembranous ossification. In contrast to endochondral ossification, intramembranous ossification does not require a preexisting cartilaginous template. Instead, there is condensation of mesenchymal cells, which differentiate into osteoblasts and lay down osteoid, thus forming an ossification center. Little is known about the mechanism of formation of OC or the nidus of formation of the primary form.
Incidental micronodules of calcification and ossification are routinely encountered during histopathologic review of specimens from hair-bearing areas of the skin in dermatopathology practice. A review of the literature, however, does not reveal any specific dermatopathologic term ascribed to this phenomenon. These lesions might be similar to those described by Hopkins5 in 1928 in the setting of miliary OC of the face secondary to acne. Rossman and Freeman6 also described the same lesions when referring to facial OC as a “stage of pre-osseous calcification.”
When these osteocalcific micronodules are encountered, it usually is in close proximity to a hair follicle bulb. When a hair bulb is not seen in the sections, the micronodules are noted near fibrous tracts, arrector pili muscles, or sebaceous lobules, suggesting a close peripilar or peribulbar location. The micronodules are approximately 0.5 mm in diameter—roughly the size of a hair bulb. Due to the close anatomic association of micronodules and the hair bulb, these lesions can be called pilar osteocalcific nodules (PONs).
The role of bone morphogenetic protein (BMP) signaling in the maintenance of the hair cycle is well established. Bone morphogenetic proteins are extracellular cytokines that belong to the transforming growth factor β family. The hair bulb microenvironment is rich in BMPs
As the name implies, BMPs were discovered in relation to their important role in osteogenesis and tissue homeostasis. More than 20 BMPs have been identified, many of which promote bone formation and repair of bone fracture. Osteoinductive BMPs include BMP-2 and BMP-4 through BMP-10; BMP-2 and BMP-4 are expressed in the hair matrix and BMP-4 and BMP-6 are expressed in the FDP.8,9 All bone-inducing BMPs can cause mesenchymal stem cells to differentiate into osteoblasts in vitro.10
Overactive BMP signaling has been shown to cause heterotopic ossification in patients with fibrodysplasia ossificans progressiva.8 Immunohistochemical expression of BMP-2 has been demonstrated in shadow cells of pilomatricoma.11 Calcification and ossification are seen in as many as 20% of pilomatricomas. Both BMP-2 and BMP-4 have been shown to induce osteogenic differentiation of mouse skin−derived fibroblasts and FDP cells.12
Myllylä et al13 described 4 cases of multiple miliary osteoma cutis (MMOC). They also found 47 reported cases of MMOC, in which there was a history of acne in 55% (26/47). Only 15% (7/47) of these cases were extrafacial on the neck, chest, back, and arms. Osteomas in these cases were not associated with folliculosebaceous units or other adnexal structures, which may have been due to replacement by acne scarring, as all 4 patients had a history of acne vulgaris. The authors postulated a role for the GNAS gene mutation in the morphogenesis of MMOC; however, no supporting evidence was found for this claim. They also postulated a role for BMPs in the formation of MMOC.13
Some disturbance or imbalance in hair bulb homeostasis leads to overactivity of BMP signaling, causing osteoinduction in the hair bulb region and formation of PONs. The cause of the disturbance could be a traumatic or inflammatory injury to the hair follicle, as in the case of the secondary form of MMOC in association with chronic acne. In the primary form of osteoma cutis, the trigger could be more subtle or subclinical.
Trauma and inflammation are the main initiating factors involved in ossification in patients with fibrodysplasia ossificans progressiva due to ectopic activity of BMPs.9 The primary form of ossification appears to be similar to the mechanism by which intramembranous ossification is laid down (ie, by differentiation of mesenchymal cells into osteoblasts). In the proposed scenario, the cells of FDP, under the influence of BMPs, differentiate into osteoblasts and lay down osteoid, forming a limited-capacity “ossification center” or pilar osteocalcific nodule.
It is difficult to know the exact relationship of PONs or OC to the hair bulb due to the 2-dimensional nature of histologic sections. However, considering the finding of a rare case of osteoid forming within the bulb and in another the presence of melanin within the osteocalcific nodule, it is likely that these lesions are formed within the hair bulb or in situations in which the conditions replicate the biochemical characteristics of the hair bulb (eg, pilomatricoma).
The formation of PONs might act as a terminal phase in the hair cycle that is rarely induced to provide an exit for damaged hair follicles from cyclical perpetuity. An unspecified event or injury might render a hair follicle unable to continue its cyclical growth and cause BMPs to induce premature calcification in or around the hair bulb, which would probably be the only known quasiphysiological mechanism for a damaged hair follicle to exit the hair cycle.
Another interesting aspect of osteoma formation in human skin is the similarity to osteoderms or the integumentary skeleton of vertebrates.14 Early in evolution, the dermal skeleton was the predominant skeletal system in some lineages. Phylogenetically, osteoderms are not uniformly distributed, and show a latent ability to manifest in some groups or lay dormant or disappear in others. The occurrence of primary osteomas in the human integument might be a vestigial manifestation of deep homology,15 a latent ability to form structures that have been lost. The embryologic formation of osteoderms in the dermis of vertebrates is thought to depend on the interaction or cross-talk between ectomesenchymal cells of neural crest origin and cells of the stratum basalis of epidermis, which is somewhat similar to the formation of the hair follicles.
Conclusion
Under certain conditions, the bulb region of a hair follicle might provide a nidus for the formation of OC. The hair bulb region contains both the precursor cellular element (mesenchymal cells of FDP) and the trigger cytokine (BMP) for the induction of osteogenic metaplasia.
- Burgdorf W, Nasemann T. Cutaneous osteomas: a clinical and histopathologic review. Arch Dermatol Res. 1977;260:121-135.
- Essing M. Osteoma cutis of the forehead. HNO. 1985;33:548-550.
- Bouraoui S, Mlika M, Kort R, et al. Miliary osteoma cutis of the face. J Dermatol Case Rep. 2011;5:77-81.
- Virchow R. Die krankhaften Geschwülste. Vol 2. Hirschwald; 1864.
- Hopkins JG. Multiple miliary osteomas of the skin: report of a case. Arch Derm Syphilol. 1928;18:706-715.
- Rossman RE, Freeman RG. Osteoma cutis, a stage of preosseous calcification. Arch Dermatol. 1964;89:68-73.
- Guha U, Mecklenburg L, Cowin P, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729-740.
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543-557.
- Shi S, de Gorter DJJ, Hoogaars WMH, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013;70:407-423.
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51.
- Kurokawa I, Kusumoto K, Bessho K. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
- Myllylä RM, Haapasaari K-M, Lehenkari P, et al. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014;355:463-470.
- Myllylä RM, Haapasaari KM, Palatsi R, et al. Multiple miliary osteoma cutis is a distinct disease entity: four case reports and review of the literature. Br J Dermatol. 2011;164:544-552.
- Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441-464.
- Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269:398-422.
- Burgdorf W, Nasemann T. Cutaneous osteomas: a clinical and histopathologic review. Arch Dermatol Res. 1977;260:121-135.
- Essing M. Osteoma cutis of the forehead. HNO. 1985;33:548-550.
- Bouraoui S, Mlika M, Kort R, et al. Miliary osteoma cutis of the face. J Dermatol Case Rep. 2011;5:77-81.
- Virchow R. Die krankhaften Geschwülste. Vol 2. Hirschwald; 1864.
- Hopkins JG. Multiple miliary osteomas of the skin: report of a case. Arch Derm Syphilol. 1928;18:706-715.
- Rossman RE, Freeman RG. Osteoma cutis, a stage of preosseous calcification. Arch Dermatol. 1964;89:68-73.
- Guha U, Mecklenburg L, Cowin P, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729-740.
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543-557.
- Shi S, de Gorter DJJ, Hoogaars WMH, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013;70:407-423.
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51.
- Kurokawa I, Kusumoto K, Bessho K. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
- Myllylä RM, Haapasaari K-M, Lehenkari P, et al. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014;355:463-470.
- Myllylä RM, Haapasaari KM, Palatsi R, et al. Multiple miliary osteoma cutis is a distinct disease entity: four case reports and review of the literature. Br J Dermatol. 2011;164:544-552.
- Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441-464.
- Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269:398-422.
Practice Points
- Understanding the pathogenesis of osteoma cutis (OC) can help physicians devise management of these disfiguring lesions.
- Small osteocalcific nodules in close proximity to the lower aspect of the hair bulb may be an important precursor to OC.
Few outcome differences for younger adolescents after bariatric surgery
Younger adolescents who underwent metabolic and bariatric surgery had outcomes similar to those of older adolescents undergoing the same procedure, according to recent research in Pediatrics.
Five years after metabolic and bariatric surgery (MBS), adolescents between ages 13 and 15 years had similar outcomes with regard to reduction in body mass index percentage, hypertension and dyslipidemia, and improved quality of life, compared with adolescents between ages 16 and 19 years, according to Sarah B. Ogle, DO, MS, of Children’s Hospital Colorado at the University of Colorado at Denver, Aurora, and colleagues.
“These results appear promising for the treatment of severe obesity in young patients,” Dr. Ogle and colleagues wrote, “however, further controlled studies are needed to fully evaluate the timing of surgery and extended long-term durability.”
The researchers analyzed the outcomes of adolescents enrolled in the Teen–Longitudinal Assessment of Bariatric Surgery who were aged 19 years or younger and underwent MBS between March 2007 and December 2011 at five U.S. centers. In the group of younger adolescents (66 participants), the mean age at surgery was 15.1 years, while the group of older adolescents (162 participants) had a mean age of 17.7 years at the time of surgery. Both groups consisted mostly of White (71.6%-72.7%) girls (72.7%-75.9%) who were morbidly obese (mean BMI, 52.4-53.1 kg/m2). With regard to baseline comorbidities, about three-quarters of participants in the younger (72.4%) and older (77.0%) adolescent groups had dyslipidemia. More than one-quarter of younger adolescents had hypertension (27.3%) compared with more than one-third of older adolescents (37.1%). The prevalence of type 2 diabetes was 10.6% in the younger adolescent group and 13.6% among older adolescents.
At 5-year follow-up, there was a similar BMI reduction maintained from baseline in the younger adolescent group (–22.2%; 95% confidence interval, –26.2% to –18.2%) and the older adolescent group (–24.6%; 95% CI, –27.7% to –22.5%; P = .59). There was a similar number of participants who had remission of dyslipidemia at 5 years in the younger adolescent group (61%; 95% CI, 46.3%-81.1%) and older adolescent group (58%; 95% CI, 48.0%-68.9%; P = .74). In participants with hypertension, 77% of younger adolescents (95% CI, 57.1%-100.0%) and 67% of older adolescents (95% CI, 54.5%-81.5%) achieved remission at 5 years after MBS, which showed no significant differences after adjustment (P = .84). For participants with type 2 diabetes at baseline, 83% of younger adolescents (6 participants) and 87% of older adolescents (15 participants) experienced remission by 5 years after surgery. Participants in both younger and older adolescent groups had similar quality of life scores at 5 years after surgery. When analyzing nutritional abnormalities, the researchers found younger adolescents in the group were less at risk for elevated transferrin levels (prevalence ratio, 0.52; P = .048) as well as less likely to have low vitamin D levels (prevalence ratio, 0.8; P = .034).
Pediatricians still concerned about safety
In an interview, Kelly A. Curran, MD, MA, assistant professor of pediatrics at University of Oklahoma Children’s Hospital in Oklahoma City, said that the findings by Dr. Ogle and colleagues add to a “growing body of literature about the importance of bariatric surgery for both younger and older adolescents.
“While many often see bariatric surgery as a ‘last resort,’ this study shows good outcomes in resolving obesity-related health conditions in both young and older teens over time – and something that should be considered more frequently than it is currently being used,” she said.
Guidelines from the American Society for Metabolic and Bariatric Surgery removed a restriction for younger age before a patient undergoes MBS, and a policy statement from the American Academy of Pediatrics encouraged increased use and access to MBS for younger adolescents. However, Dr. Curran noted that many pediatricians are still concerned about performing MBS on younger adolescents.
“Despite growing evidence of safety, I think many pediatricians worry about the potential for unintended consequences and potential impact on adolescent development or for lifelong micronutrition deficiencies – especially as there are no longitudinal studies over a lifetime,” she said.
“[W]ith the growing obesity epidemic and the long-term consequences of obesity on health and quality of life – the potential to help impact adolescents’ lives – for now and for the future – is impressive,” Dr. Curran said, acknowledging the ethical challenges involved with performing MBS on a patient who may be too young to understand the full risks and benefits of surgery.
“There are always inherent ethical challenges in providing surgery for patients too young to understand – we are asking parents to act in their child’s best interests, which may be murky to elucidate,” she explained. “While there is [a] growing body of literature around the safety and efficacy in bariatric surgery for children and adolescents, there are still many unanswered questions that remain – especially for parents. Parents can feel trapped in between these two choices – have children undergo surgery or stick with potentially less effective medical management.”
The limitations of the study include its observational nature, small sample size of some comorbidities, and a lack of diversity among participants, most of whom were White and female. In addition, “long-term studies examining the impact of bariatric surgery during adolescence would be important to give more perspective and guidance on the risks and benefits for teens,” Dr. Curran said.
The study was funded by the National Institutes of Health and grants from the National Institute of Diabetes and Digestive and Kidney Diseases as well as grants from Cincinnati Children’s Hospital Medical Center, Nationwide Children’s Hospital, Texas Children’s Hospital and Baylor College of Medicine, University of Pittsburgh, and the University of Alabama at Birmingham. The authors and Dr. Curran reported no conflicts of interest.
Younger adolescents who underwent metabolic and bariatric surgery had outcomes similar to those of older adolescents undergoing the same procedure, according to recent research in Pediatrics.
Five years after metabolic and bariatric surgery (MBS), adolescents between ages 13 and 15 years had similar outcomes with regard to reduction in body mass index percentage, hypertension and dyslipidemia, and improved quality of life, compared with adolescents between ages 16 and 19 years, according to Sarah B. Ogle, DO, MS, of Children’s Hospital Colorado at the University of Colorado at Denver, Aurora, and colleagues.
“These results appear promising for the treatment of severe obesity in young patients,” Dr. Ogle and colleagues wrote, “however, further controlled studies are needed to fully evaluate the timing of surgery and extended long-term durability.”
The researchers analyzed the outcomes of adolescents enrolled in the Teen–Longitudinal Assessment of Bariatric Surgery who were aged 19 years or younger and underwent MBS between March 2007 and December 2011 at five U.S. centers. In the group of younger adolescents (66 participants), the mean age at surgery was 15.1 years, while the group of older adolescents (162 participants) had a mean age of 17.7 years at the time of surgery. Both groups consisted mostly of White (71.6%-72.7%) girls (72.7%-75.9%) who were morbidly obese (mean BMI, 52.4-53.1 kg/m2). With regard to baseline comorbidities, about three-quarters of participants in the younger (72.4%) and older (77.0%) adolescent groups had dyslipidemia. More than one-quarter of younger adolescents had hypertension (27.3%) compared with more than one-third of older adolescents (37.1%). The prevalence of type 2 diabetes was 10.6% in the younger adolescent group and 13.6% among older adolescents.
At 5-year follow-up, there was a similar BMI reduction maintained from baseline in the younger adolescent group (–22.2%; 95% confidence interval, –26.2% to –18.2%) and the older adolescent group (–24.6%; 95% CI, –27.7% to –22.5%; P = .59). There was a similar number of participants who had remission of dyslipidemia at 5 years in the younger adolescent group (61%; 95% CI, 46.3%-81.1%) and older adolescent group (58%; 95% CI, 48.0%-68.9%; P = .74). In participants with hypertension, 77% of younger adolescents (95% CI, 57.1%-100.0%) and 67% of older adolescents (95% CI, 54.5%-81.5%) achieved remission at 5 years after MBS, which showed no significant differences after adjustment (P = .84). For participants with type 2 diabetes at baseline, 83% of younger adolescents (6 participants) and 87% of older adolescents (15 participants) experienced remission by 5 years after surgery. Participants in both younger and older adolescent groups had similar quality of life scores at 5 years after surgery. When analyzing nutritional abnormalities, the researchers found younger adolescents in the group were less at risk for elevated transferrin levels (prevalence ratio, 0.52; P = .048) as well as less likely to have low vitamin D levels (prevalence ratio, 0.8; P = .034).
Pediatricians still concerned about safety
In an interview, Kelly A. Curran, MD, MA, assistant professor of pediatrics at University of Oklahoma Children’s Hospital in Oklahoma City, said that the findings by Dr. Ogle and colleagues add to a “growing body of literature about the importance of bariatric surgery for both younger and older adolescents.
“While many often see bariatric surgery as a ‘last resort,’ this study shows good outcomes in resolving obesity-related health conditions in both young and older teens over time – and something that should be considered more frequently than it is currently being used,” she said.
Guidelines from the American Society for Metabolic and Bariatric Surgery removed a restriction for younger age before a patient undergoes MBS, and a policy statement from the American Academy of Pediatrics encouraged increased use and access to MBS for younger adolescents. However, Dr. Curran noted that many pediatricians are still concerned about performing MBS on younger adolescents.
“Despite growing evidence of safety, I think many pediatricians worry about the potential for unintended consequences and potential impact on adolescent development or for lifelong micronutrition deficiencies – especially as there are no longitudinal studies over a lifetime,” she said.
“[W]ith the growing obesity epidemic and the long-term consequences of obesity on health and quality of life – the potential to help impact adolescents’ lives – for now and for the future – is impressive,” Dr. Curran said, acknowledging the ethical challenges involved with performing MBS on a patient who may be too young to understand the full risks and benefits of surgery.
“There are always inherent ethical challenges in providing surgery for patients too young to understand – we are asking parents to act in their child’s best interests, which may be murky to elucidate,” she explained. “While there is [a] growing body of literature around the safety and efficacy in bariatric surgery for children and adolescents, there are still many unanswered questions that remain – especially for parents. Parents can feel trapped in between these two choices – have children undergo surgery or stick with potentially less effective medical management.”
The limitations of the study include its observational nature, small sample size of some comorbidities, and a lack of diversity among participants, most of whom were White and female. In addition, “long-term studies examining the impact of bariatric surgery during adolescence would be important to give more perspective and guidance on the risks and benefits for teens,” Dr. Curran said.
The study was funded by the National Institutes of Health and grants from the National Institute of Diabetes and Digestive and Kidney Diseases as well as grants from Cincinnati Children’s Hospital Medical Center, Nationwide Children’s Hospital, Texas Children’s Hospital and Baylor College of Medicine, University of Pittsburgh, and the University of Alabama at Birmingham. The authors and Dr. Curran reported no conflicts of interest.
Younger adolescents who underwent metabolic and bariatric surgery had outcomes similar to those of older adolescents undergoing the same procedure, according to recent research in Pediatrics.
Five years after metabolic and bariatric surgery (MBS), adolescents between ages 13 and 15 years had similar outcomes with regard to reduction in body mass index percentage, hypertension and dyslipidemia, and improved quality of life, compared with adolescents between ages 16 and 19 years, according to Sarah B. Ogle, DO, MS, of Children’s Hospital Colorado at the University of Colorado at Denver, Aurora, and colleagues.
“These results appear promising for the treatment of severe obesity in young patients,” Dr. Ogle and colleagues wrote, “however, further controlled studies are needed to fully evaluate the timing of surgery and extended long-term durability.”
The researchers analyzed the outcomes of adolescents enrolled in the Teen–Longitudinal Assessment of Bariatric Surgery who were aged 19 years or younger and underwent MBS between March 2007 and December 2011 at five U.S. centers. In the group of younger adolescents (66 participants), the mean age at surgery was 15.1 years, while the group of older adolescents (162 participants) had a mean age of 17.7 years at the time of surgery. Both groups consisted mostly of White (71.6%-72.7%) girls (72.7%-75.9%) who were morbidly obese (mean BMI, 52.4-53.1 kg/m2). With regard to baseline comorbidities, about three-quarters of participants in the younger (72.4%) and older (77.0%) adolescent groups had dyslipidemia. More than one-quarter of younger adolescents had hypertension (27.3%) compared with more than one-third of older adolescents (37.1%). The prevalence of type 2 diabetes was 10.6% in the younger adolescent group and 13.6% among older adolescents.
At 5-year follow-up, there was a similar BMI reduction maintained from baseline in the younger adolescent group (–22.2%; 95% confidence interval, –26.2% to –18.2%) and the older adolescent group (–24.6%; 95% CI, –27.7% to –22.5%; P = .59). There was a similar number of participants who had remission of dyslipidemia at 5 years in the younger adolescent group (61%; 95% CI, 46.3%-81.1%) and older adolescent group (58%; 95% CI, 48.0%-68.9%; P = .74). In participants with hypertension, 77% of younger adolescents (95% CI, 57.1%-100.0%) and 67% of older adolescents (95% CI, 54.5%-81.5%) achieved remission at 5 years after MBS, which showed no significant differences after adjustment (P = .84). For participants with type 2 diabetes at baseline, 83% of younger adolescents (6 participants) and 87% of older adolescents (15 participants) experienced remission by 5 years after surgery. Participants in both younger and older adolescent groups had similar quality of life scores at 5 years after surgery. When analyzing nutritional abnormalities, the researchers found younger adolescents in the group were less at risk for elevated transferrin levels (prevalence ratio, 0.52; P = .048) as well as less likely to have low vitamin D levels (prevalence ratio, 0.8; P = .034).
Pediatricians still concerned about safety
In an interview, Kelly A. Curran, MD, MA, assistant professor of pediatrics at University of Oklahoma Children’s Hospital in Oklahoma City, said that the findings by Dr. Ogle and colleagues add to a “growing body of literature about the importance of bariatric surgery for both younger and older adolescents.
“While many often see bariatric surgery as a ‘last resort,’ this study shows good outcomes in resolving obesity-related health conditions in both young and older teens over time – and something that should be considered more frequently than it is currently being used,” she said.
Guidelines from the American Society for Metabolic and Bariatric Surgery removed a restriction for younger age before a patient undergoes MBS, and a policy statement from the American Academy of Pediatrics encouraged increased use and access to MBS for younger adolescents. However, Dr. Curran noted that many pediatricians are still concerned about performing MBS on younger adolescents.
“Despite growing evidence of safety, I think many pediatricians worry about the potential for unintended consequences and potential impact on adolescent development or for lifelong micronutrition deficiencies – especially as there are no longitudinal studies over a lifetime,” she said.
“[W]ith the growing obesity epidemic and the long-term consequences of obesity on health and quality of life – the potential to help impact adolescents’ lives – for now and for the future – is impressive,” Dr. Curran said, acknowledging the ethical challenges involved with performing MBS on a patient who may be too young to understand the full risks and benefits of surgery.
“There are always inherent ethical challenges in providing surgery for patients too young to understand – we are asking parents to act in their child’s best interests, which may be murky to elucidate,” she explained. “While there is [a] growing body of literature around the safety and efficacy in bariatric surgery for children and adolescents, there are still many unanswered questions that remain – especially for parents. Parents can feel trapped in between these two choices – have children undergo surgery or stick with potentially less effective medical management.”
The limitations of the study include its observational nature, small sample size of some comorbidities, and a lack of diversity among participants, most of whom were White and female. In addition, “long-term studies examining the impact of bariatric surgery during adolescence would be important to give more perspective and guidance on the risks and benefits for teens,” Dr. Curran said.
The study was funded by the National Institutes of Health and grants from the National Institute of Diabetes and Digestive and Kidney Diseases as well as grants from Cincinnati Children’s Hospital Medical Center, Nationwide Children’s Hospital, Texas Children’s Hospital and Baylor College of Medicine, University of Pittsburgh, and the University of Alabama at Birmingham. The authors and Dr. Curran reported no conflicts of interest.
FROM PEDIATRICS
Expert shares hyperhidrosis treatment pearls
Even though over-the-counter topical antiperspirants are a common go-to treatment for primary axillary hyperhidrosis, a large survey commissioned by the International Hyperhidrosis Society showed that, while OTC aluminum products are the most recommended, they offer the least satisfaction to patients.
Of the 1,985 survey respondents who self-identified as having excessive sweating, those who received treatment were most satisfied with injections and least satisfied with prescription and OTC antiperspirants and liposuction. “It’s important to recognize that, while these are not invasive, they’re simple, you need to keep up with it, and they’re really not that effective for primary hyperhidrosis,” Adam Friedman, MD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference.
A major development came in 2018, when the Food and Drug Administration approved topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis in adults and in children as young as age 9. It marked the first topical anticholinergic approved for the condition. Results from the pivotal phase 2 ATMOS-1 and ATMOS-2 randomized, controlled trials found that, after 4 weeks of daily use, 53%-66% of patients reported a 4-point improvement or greater on the ASDD item 2, which is defined as the worst sweating they experienced in a 24-hour period on an 11-point scale.
“Patients want to know: How quickly am I going to see improvement? The answer to this can be central to treatment compliance,” said Dr. Friedman, professor and interim chair of dermatology at the George Washington University, Washington. “We have data showing that 23%-29% of patients using glycopyrronium tosylate met that primary outcome within 1 week of use. So, you can tell patients: ‘Help is on the way. You may see a response relatively soon.’ ”
The most common adverse events in the two trials were dry mouth, which affected 24% of patients, followed by mydriasis (7%), and oropharyngeal pain (6%). He advises patients to apply it once at night. “I tell my patients make this the last thing you do during your nighttime routine,” said Dr. Friedman, who coauthored a case-based clinical algorithm for approaching primary hyperhidrosis patients.
“Open it up, one swipe to the right [underarm], flip it over, one wipe of the left [underarm], toss the towelette, and wash your hands thoroughly. You don’t need to remove axillary hair or occlude the area. I tell them they may find some improvement within one week of daily use, but I give realistic expectations, usually 2-3 weeks. Tell them about the potential for side effects, which certainly can happen,” he said.
Investigators are evaluating how this product could be delivered to other body sites. Dr. Friedman said that he uses glycopyrronium tosylate off label for palmar and plantar hyperhidrosis. He advises patients to rub their hands or feet the cloth until it dries, toss the towelette, apply an occlusive agent like Aquaphor followed by gloves/socks for at least an hour, and then wash their hands or feet. “If they can keep the gloves or socks on overnight, that’s fine, but that’s very rare,” Dr. Friedman added.
“Typically, an hour or 2 of occlusive covering will get the product in where it needs to be. The upside of this product is that it’s noninvasive, there’s minimal irritation, it’s effective, and FDA approved. On the downside, it’s a long-term therapy. This is forever, so cost can be an issue, and you have to think about the anticholinergic effects as well.”
Iontophoresis is a first-line treatment for moderate to severe palmar and plantar hyperhidrosis. It’s also effective for mild hyperhidrosis with limited side effects, but it’s cumbersome, he said, requiring thrice-weekly treatment of each palm or sole for approximately 30 minutes to a controlled electric current at 15-20 mA with tap water.
There are no systemic agents approved for hyperhidrosis, only case reports or small case series. For now, the two commonly used anticholinergics are glycopyrrolate and oxybutynin. Glycopyrrolate comes in 1- and 2-mg capsules. “You can break the tablets easily and it’s pretty cheap, with an estimated cost of 2 mg/day at $756 per year,” Dr. Friedman said. “I typically start patients on 1 mg twice per day for a week, then ask how they’re doing. If they notice improvement, have minimal side effects but think they can do better, then I increase it by 1 mg and reassess. I give them autonomy, and at most, want them to max out at 6 mg per day. There is an oral solution for kids, which can make this a little more accessible.”
He prescribes oxybutynin infrequently but considers it effective. “Most patients respond to 5- to 10-mg/day dosing, but doses up to 15 or 20 mg daily may be required,” he noted.
For persistent flushing with hyperhidrosis, Dr. Friedman typically recommends treatment with clonidine. “I start patients pretty low, sometimes 0.05 mg twice per day.”
For patients who sweat because of social phobias and performance anxiety, he typically recommends treatment with a beta-adrenergic blocker. “These are highly lipophilic, so I advise patients not to take them with food,” he said. “The peak concentration is 1-1.5 hours. Usually, I start at 10 mg and I have people do a test run at home. I also take a baseline blood pressure in the office to make sure they’re not hypotensive.” The use of beta-adrenergic blockers is contraindicated in patients with bradycardia, atrioventricular block, and asthma. They can also exacerbate psoriasis.
On Sept. 20, 2020, Brickell Biotech announced the approval of sofpironium bromide gel, 5%, in Japan for the treatment of primary axillary hyperhidrosis. Sofpironium bromide is an analog of glycopyrrolate “that gets metabolized very quickly in order to limit systemic absorption of the active agent and therefore mitigate side effects,” Dr. Friedman said.
A recently published Japanese study found that 54% of patients with primary axillary hyperhidrosis who received sofpironium bromide experienced a 1- or 2-point improvement on the Hyperhidrosis Disease Severity Scale and a 50% or greater reduction in gravimetric sweat production from baseline to week 6 of treatment, compared with 36% of patients in the control group (P = .003). According to Dr. Friedman, a 15% formulation of this product is being studied in the United States, “but the experience in Japan with the 5% formulation should give us some real-world information about this product,” he said. “Out of the gate, we’re going to know something about how it’s being used.”
Dr. Friedman reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies, including some that produce cannabinoids. He is also a speaker for Regeneron, Abbvie, Novartis, LRP, Dermira, and Brickel Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, and Janssen.
Even though over-the-counter topical antiperspirants are a common go-to treatment for primary axillary hyperhidrosis, a large survey commissioned by the International Hyperhidrosis Society showed that, while OTC aluminum products are the most recommended, they offer the least satisfaction to patients.
Of the 1,985 survey respondents who self-identified as having excessive sweating, those who received treatment were most satisfied with injections and least satisfied with prescription and OTC antiperspirants and liposuction. “It’s important to recognize that, while these are not invasive, they’re simple, you need to keep up with it, and they’re really not that effective for primary hyperhidrosis,” Adam Friedman, MD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference.
A major development came in 2018, when the Food and Drug Administration approved topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis in adults and in children as young as age 9. It marked the first topical anticholinergic approved for the condition. Results from the pivotal phase 2 ATMOS-1 and ATMOS-2 randomized, controlled trials found that, after 4 weeks of daily use, 53%-66% of patients reported a 4-point improvement or greater on the ASDD item 2, which is defined as the worst sweating they experienced in a 24-hour period on an 11-point scale.
“Patients want to know: How quickly am I going to see improvement? The answer to this can be central to treatment compliance,” said Dr. Friedman, professor and interim chair of dermatology at the George Washington University, Washington. “We have data showing that 23%-29% of patients using glycopyrronium tosylate met that primary outcome within 1 week of use. So, you can tell patients: ‘Help is on the way. You may see a response relatively soon.’ ”
The most common adverse events in the two trials were dry mouth, which affected 24% of patients, followed by mydriasis (7%), and oropharyngeal pain (6%). He advises patients to apply it once at night. “I tell my patients make this the last thing you do during your nighttime routine,” said Dr. Friedman, who coauthored a case-based clinical algorithm for approaching primary hyperhidrosis patients.
“Open it up, one swipe to the right [underarm], flip it over, one wipe of the left [underarm], toss the towelette, and wash your hands thoroughly. You don’t need to remove axillary hair or occlude the area. I tell them they may find some improvement within one week of daily use, but I give realistic expectations, usually 2-3 weeks. Tell them about the potential for side effects, which certainly can happen,” he said.
Investigators are evaluating how this product could be delivered to other body sites. Dr. Friedman said that he uses glycopyrronium tosylate off label for palmar and plantar hyperhidrosis. He advises patients to rub their hands or feet the cloth until it dries, toss the towelette, apply an occlusive agent like Aquaphor followed by gloves/socks for at least an hour, and then wash their hands or feet. “If they can keep the gloves or socks on overnight, that’s fine, but that’s very rare,” Dr. Friedman added.
“Typically, an hour or 2 of occlusive covering will get the product in where it needs to be. The upside of this product is that it’s noninvasive, there’s minimal irritation, it’s effective, and FDA approved. On the downside, it’s a long-term therapy. This is forever, so cost can be an issue, and you have to think about the anticholinergic effects as well.”
Iontophoresis is a first-line treatment for moderate to severe palmar and plantar hyperhidrosis. It’s also effective for mild hyperhidrosis with limited side effects, but it’s cumbersome, he said, requiring thrice-weekly treatment of each palm or sole for approximately 30 minutes to a controlled electric current at 15-20 mA with tap water.
There are no systemic agents approved for hyperhidrosis, only case reports or small case series. For now, the two commonly used anticholinergics are glycopyrrolate and oxybutynin. Glycopyrrolate comes in 1- and 2-mg capsules. “You can break the tablets easily and it’s pretty cheap, with an estimated cost of 2 mg/day at $756 per year,” Dr. Friedman said. “I typically start patients on 1 mg twice per day for a week, then ask how they’re doing. If they notice improvement, have minimal side effects but think they can do better, then I increase it by 1 mg and reassess. I give them autonomy, and at most, want them to max out at 6 mg per day. There is an oral solution for kids, which can make this a little more accessible.”
He prescribes oxybutynin infrequently but considers it effective. “Most patients respond to 5- to 10-mg/day dosing, but doses up to 15 or 20 mg daily may be required,” he noted.
For persistent flushing with hyperhidrosis, Dr. Friedman typically recommends treatment with clonidine. “I start patients pretty low, sometimes 0.05 mg twice per day.”
For patients who sweat because of social phobias and performance anxiety, he typically recommends treatment with a beta-adrenergic blocker. “These are highly lipophilic, so I advise patients not to take them with food,” he said. “The peak concentration is 1-1.5 hours. Usually, I start at 10 mg and I have people do a test run at home. I also take a baseline blood pressure in the office to make sure they’re not hypotensive.” The use of beta-adrenergic blockers is contraindicated in patients with bradycardia, atrioventricular block, and asthma. They can also exacerbate psoriasis.
On Sept. 20, 2020, Brickell Biotech announced the approval of sofpironium bromide gel, 5%, in Japan for the treatment of primary axillary hyperhidrosis. Sofpironium bromide is an analog of glycopyrrolate “that gets metabolized very quickly in order to limit systemic absorption of the active agent and therefore mitigate side effects,” Dr. Friedman said.
A recently published Japanese study found that 54% of patients with primary axillary hyperhidrosis who received sofpironium bromide experienced a 1- or 2-point improvement on the Hyperhidrosis Disease Severity Scale and a 50% or greater reduction in gravimetric sweat production from baseline to week 6 of treatment, compared with 36% of patients in the control group (P = .003). According to Dr. Friedman, a 15% formulation of this product is being studied in the United States, “but the experience in Japan with the 5% formulation should give us some real-world information about this product,” he said. “Out of the gate, we’re going to know something about how it’s being used.”
Dr. Friedman reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies, including some that produce cannabinoids. He is also a speaker for Regeneron, Abbvie, Novartis, LRP, Dermira, and Brickel Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, and Janssen.
Even though over-the-counter topical antiperspirants are a common go-to treatment for primary axillary hyperhidrosis, a large survey commissioned by the International Hyperhidrosis Society showed that, while OTC aluminum products are the most recommended, they offer the least satisfaction to patients.
Of the 1,985 survey respondents who self-identified as having excessive sweating, those who received treatment were most satisfied with injections and least satisfied with prescription and OTC antiperspirants and liposuction. “It’s important to recognize that, while these are not invasive, they’re simple, you need to keep up with it, and they’re really not that effective for primary hyperhidrosis,” Adam Friedman, MD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference.
A major development came in 2018, when the Food and Drug Administration approved topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis in adults and in children as young as age 9. It marked the first topical anticholinergic approved for the condition. Results from the pivotal phase 2 ATMOS-1 and ATMOS-2 randomized, controlled trials found that, after 4 weeks of daily use, 53%-66% of patients reported a 4-point improvement or greater on the ASDD item 2, which is defined as the worst sweating they experienced in a 24-hour period on an 11-point scale.
“Patients want to know: How quickly am I going to see improvement? The answer to this can be central to treatment compliance,” said Dr. Friedman, professor and interim chair of dermatology at the George Washington University, Washington. “We have data showing that 23%-29% of patients using glycopyrronium tosylate met that primary outcome within 1 week of use. So, you can tell patients: ‘Help is on the way. You may see a response relatively soon.’ ”
The most common adverse events in the two trials were dry mouth, which affected 24% of patients, followed by mydriasis (7%), and oropharyngeal pain (6%). He advises patients to apply it once at night. “I tell my patients make this the last thing you do during your nighttime routine,” said Dr. Friedman, who coauthored a case-based clinical algorithm for approaching primary hyperhidrosis patients.
“Open it up, one swipe to the right [underarm], flip it over, one wipe of the left [underarm], toss the towelette, and wash your hands thoroughly. You don’t need to remove axillary hair or occlude the area. I tell them they may find some improvement within one week of daily use, but I give realistic expectations, usually 2-3 weeks. Tell them about the potential for side effects, which certainly can happen,” he said.
Investigators are evaluating how this product could be delivered to other body sites. Dr. Friedman said that he uses glycopyrronium tosylate off label for palmar and plantar hyperhidrosis. He advises patients to rub their hands or feet the cloth until it dries, toss the towelette, apply an occlusive agent like Aquaphor followed by gloves/socks for at least an hour, and then wash their hands or feet. “If they can keep the gloves or socks on overnight, that’s fine, but that’s very rare,” Dr. Friedman added.
“Typically, an hour or 2 of occlusive covering will get the product in where it needs to be. The upside of this product is that it’s noninvasive, there’s minimal irritation, it’s effective, and FDA approved. On the downside, it’s a long-term therapy. This is forever, so cost can be an issue, and you have to think about the anticholinergic effects as well.”
Iontophoresis is a first-line treatment for moderate to severe palmar and plantar hyperhidrosis. It’s also effective for mild hyperhidrosis with limited side effects, but it’s cumbersome, he said, requiring thrice-weekly treatment of each palm or sole for approximately 30 minutes to a controlled electric current at 15-20 mA with tap water.
There are no systemic agents approved for hyperhidrosis, only case reports or small case series. For now, the two commonly used anticholinergics are glycopyrrolate and oxybutynin. Glycopyrrolate comes in 1- and 2-mg capsules. “You can break the tablets easily and it’s pretty cheap, with an estimated cost of 2 mg/day at $756 per year,” Dr. Friedman said. “I typically start patients on 1 mg twice per day for a week, then ask how they’re doing. If they notice improvement, have minimal side effects but think they can do better, then I increase it by 1 mg and reassess. I give them autonomy, and at most, want them to max out at 6 mg per day. There is an oral solution for kids, which can make this a little more accessible.”
He prescribes oxybutynin infrequently but considers it effective. “Most patients respond to 5- to 10-mg/day dosing, but doses up to 15 or 20 mg daily may be required,” he noted.
For persistent flushing with hyperhidrosis, Dr. Friedman typically recommends treatment with clonidine. “I start patients pretty low, sometimes 0.05 mg twice per day.”
For patients who sweat because of social phobias and performance anxiety, he typically recommends treatment with a beta-adrenergic blocker. “These are highly lipophilic, so I advise patients not to take them with food,” he said. “The peak concentration is 1-1.5 hours. Usually, I start at 10 mg and I have people do a test run at home. I also take a baseline blood pressure in the office to make sure they’re not hypotensive.” The use of beta-adrenergic blockers is contraindicated in patients with bradycardia, atrioventricular block, and asthma. They can also exacerbate psoriasis.
On Sept. 20, 2020, Brickell Biotech announced the approval of sofpironium bromide gel, 5%, in Japan for the treatment of primary axillary hyperhidrosis. Sofpironium bromide is an analog of glycopyrrolate “that gets metabolized very quickly in order to limit systemic absorption of the active agent and therefore mitigate side effects,” Dr. Friedman said.
A recently published Japanese study found that 54% of patients with primary axillary hyperhidrosis who received sofpironium bromide experienced a 1- or 2-point improvement on the Hyperhidrosis Disease Severity Scale and a 50% or greater reduction in gravimetric sweat production from baseline to week 6 of treatment, compared with 36% of patients in the control group (P = .003). According to Dr. Friedman, a 15% formulation of this product is being studied in the United States, “but the experience in Japan with the 5% formulation should give us some real-world information about this product,” he said. “Out of the gate, we’re going to know something about how it’s being used.”
Dr. Friedman reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies, including some that produce cannabinoids. He is also a speaker for Regeneron, Abbvie, Novartis, LRP, Dermira, and Brickel Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, and Janssen.
FROM ODAC 2021
How to choose the best aesthetic devices when launching your career
When a new body contouring device hit the market a few years ago, Nazanin Saedi, MD, had an opportunity to become the first Philadelphia area dermatologist to add the technology to her practice.
“I thought about it, but it didn’t make sense because it wasn’t something important to my patient population,” Dr. Saedi, who directs the Jefferson Laser Surgery and Cosmetic Dermatology Center in Philadelphia, said during the Orlando Dermatology Aesthetic and Clinical Conference. “If I’m not going to have the patient demand and make money from it, then it just doesn’t make sense.”
That experience illustrates one of many pearls of advice that Dr. Saedi shared during . “Include additional questions in new patient intake forms or online forms to get a sense of what your patient population is interested in,” she advised. “It’s important to understand that before you start to offer new services. Don’t just depend on social media to inform you of the latest trends and what people are doing across the country, because if you purchase something that is very popular on social media for people in New York or L.A., that might not be the best for your practice.”
According to market trends from the American Society for Dermatologic Surgery, 3.5 million laser-, light-, and energy-based procedures were performed in 2018. The top five were for wrinkles (809,166), sun damage (786,856), facial redness (612,367), excess hair (385,466), and melasma (226,007). “Considering this data, when you start a practice, do you buy something for wrinkles or for sun damage right away?” Dr. Saedi asked. “Maybe, but you really need to gauge the market that you practice in. You also want to consider your own skill set and what other dermatologists in your area are offering. If you don’t want to do aggressive procedures, then purchasing a fractional CO2 laser might not be the best device to start off with. If you are not comfortable dealing with those patients, and potential infections and scarring, then that’s not the right treatment for you. You have to reflect on and identify what you’re comfortable learning and doing and managing.”
Taking time to investigate the services offered by dermatologists and med spas within a few miles of your practice can help you avoid redundancy. “Learn the techniques and the small nuances that will give you a little bit of finesse and make you an expert, to set you apart from other practices,” said Dr. Saedi, who coauthored a chapter in the book, “The Business of Dermatology” (New York: Thieme Medical Publishers, 2020). “I always recommend treating your staff and members of your family, to understand how you can tweak treatments to get the most out of them. Once you treat your staff, they are walking advertisements for what you do. They can also counsel patients, walking them through the healing process after a procedure, so they can know what to expect.”
Appropriate planning and preparation can help avoid acquiring the wrong device, she continued. This includes patient demand, scheduling availability, office space, overhead costs, and the level of staff training. She recommends buying one device at a time and clearing profitability from that device before purchasing another, “because it can be a burden on your practice to have multiple devices all at once,” she said. “You also have to think about the hidden costs – the maintenance and the service contracts. That can exceed $10,000 per year, so consider that when you’re looking to purchase a new device.”
Most people buy laser-, light-, and energy-based devices, but renting for a stretch can help you test the waters without a significant long-term investment. “It might not be the newest laser, but it can help you gauge how much of demand you have for that service to see if you have the patient base to make that larger step of purchasing the device,” she said. “If you buy a new device, make sure that it’s not a counterfeit and that you still have a company service contract. There are many third-party companies selling pre-owned laser aesthetics. Make sure you’re getting the authentic device and that there is some kind of a service contract with the actual manufacturer so they can help fix it when things break down.”
When Dr. Saedi counsels residents about purchasing devices, she typically recommends these five categories in order of preference: vascular, pigment, hair, resurfacing, and body contouring/skin tightening. “If you can cover vascular, pigment, and some kind of textural improvement, you can treat about 90% of aesthetic patients who come through your door,” she said. “Sure, there are some who may want skin tightening that you may not be able to offer with laser resurfacing, but you’re going to be able capture a high patient population by offering these services,” she added. That is why a lot of people end up getting a platform with attachable handpieces, “where you can have one system that is able to offer many different services right off the bat.”
She advised factoring in the amount of time it takes for a procedure and how much time it will take up in a certain room. “That will affect your revenue as well. Are you going to delegate this, or is this something you will do on your own? Take that into account.”
Above all, don’t rush your device purchase. “Some laser company sales representatives may pressure you at the end of a quarter by saying, ‘This is the best deal I’m going to offer you. You’re never going to get a deal like this ever again,’ ” she said. “I advise people to do multiple demos so you’re not just doing a demo for a day and seeing one or two patients. Treat the same patients again a month later. Do multiple demos so that you can feel comfortable. Talk to dermatologists who have the device, who have real experience with it, so you can have the most amount of information moving forward.”
Dr. Saedi reported that she has received equipment from Alma, Aerolase, Cartessa, and Cynosure. She is a consultant to and/or an advisory board member for those companies, as well as for Alastin.
When a new body contouring device hit the market a few years ago, Nazanin Saedi, MD, had an opportunity to become the first Philadelphia area dermatologist to add the technology to her practice.
“I thought about it, but it didn’t make sense because it wasn’t something important to my patient population,” Dr. Saedi, who directs the Jefferson Laser Surgery and Cosmetic Dermatology Center in Philadelphia, said during the Orlando Dermatology Aesthetic and Clinical Conference. “If I’m not going to have the patient demand and make money from it, then it just doesn’t make sense.”
That experience illustrates one of many pearls of advice that Dr. Saedi shared during . “Include additional questions in new patient intake forms or online forms to get a sense of what your patient population is interested in,” she advised. “It’s important to understand that before you start to offer new services. Don’t just depend on social media to inform you of the latest trends and what people are doing across the country, because if you purchase something that is very popular on social media for people in New York or L.A., that might not be the best for your practice.”
According to market trends from the American Society for Dermatologic Surgery, 3.5 million laser-, light-, and energy-based procedures were performed in 2018. The top five were for wrinkles (809,166), sun damage (786,856), facial redness (612,367), excess hair (385,466), and melasma (226,007). “Considering this data, when you start a practice, do you buy something for wrinkles or for sun damage right away?” Dr. Saedi asked. “Maybe, but you really need to gauge the market that you practice in. You also want to consider your own skill set and what other dermatologists in your area are offering. If you don’t want to do aggressive procedures, then purchasing a fractional CO2 laser might not be the best device to start off with. If you are not comfortable dealing with those patients, and potential infections and scarring, then that’s not the right treatment for you. You have to reflect on and identify what you’re comfortable learning and doing and managing.”
Taking time to investigate the services offered by dermatologists and med spas within a few miles of your practice can help you avoid redundancy. “Learn the techniques and the small nuances that will give you a little bit of finesse and make you an expert, to set you apart from other practices,” said Dr. Saedi, who coauthored a chapter in the book, “The Business of Dermatology” (New York: Thieme Medical Publishers, 2020). “I always recommend treating your staff and members of your family, to understand how you can tweak treatments to get the most out of them. Once you treat your staff, they are walking advertisements for what you do. They can also counsel patients, walking them through the healing process after a procedure, so they can know what to expect.”
Appropriate planning and preparation can help avoid acquiring the wrong device, she continued. This includes patient demand, scheduling availability, office space, overhead costs, and the level of staff training. She recommends buying one device at a time and clearing profitability from that device before purchasing another, “because it can be a burden on your practice to have multiple devices all at once,” she said. “You also have to think about the hidden costs – the maintenance and the service contracts. That can exceed $10,000 per year, so consider that when you’re looking to purchase a new device.”
Most people buy laser-, light-, and energy-based devices, but renting for a stretch can help you test the waters without a significant long-term investment. “It might not be the newest laser, but it can help you gauge how much of demand you have for that service to see if you have the patient base to make that larger step of purchasing the device,” she said. “If you buy a new device, make sure that it’s not a counterfeit and that you still have a company service contract. There are many third-party companies selling pre-owned laser aesthetics. Make sure you’re getting the authentic device and that there is some kind of a service contract with the actual manufacturer so they can help fix it when things break down.”
When Dr. Saedi counsels residents about purchasing devices, she typically recommends these five categories in order of preference: vascular, pigment, hair, resurfacing, and body contouring/skin tightening. “If you can cover vascular, pigment, and some kind of textural improvement, you can treat about 90% of aesthetic patients who come through your door,” she said. “Sure, there are some who may want skin tightening that you may not be able to offer with laser resurfacing, but you’re going to be able capture a high patient population by offering these services,” she added. That is why a lot of people end up getting a platform with attachable handpieces, “where you can have one system that is able to offer many different services right off the bat.”
She advised factoring in the amount of time it takes for a procedure and how much time it will take up in a certain room. “That will affect your revenue as well. Are you going to delegate this, or is this something you will do on your own? Take that into account.”
Above all, don’t rush your device purchase. “Some laser company sales representatives may pressure you at the end of a quarter by saying, ‘This is the best deal I’m going to offer you. You’re never going to get a deal like this ever again,’ ” she said. “I advise people to do multiple demos so you’re not just doing a demo for a day and seeing one or two patients. Treat the same patients again a month later. Do multiple demos so that you can feel comfortable. Talk to dermatologists who have the device, who have real experience with it, so you can have the most amount of information moving forward.”
Dr. Saedi reported that she has received equipment from Alma, Aerolase, Cartessa, and Cynosure. She is a consultant to and/or an advisory board member for those companies, as well as for Alastin.
When a new body contouring device hit the market a few years ago, Nazanin Saedi, MD, had an opportunity to become the first Philadelphia area dermatologist to add the technology to her practice.
“I thought about it, but it didn’t make sense because it wasn’t something important to my patient population,” Dr. Saedi, who directs the Jefferson Laser Surgery and Cosmetic Dermatology Center in Philadelphia, said during the Orlando Dermatology Aesthetic and Clinical Conference. “If I’m not going to have the patient demand and make money from it, then it just doesn’t make sense.”
That experience illustrates one of many pearls of advice that Dr. Saedi shared during . “Include additional questions in new patient intake forms or online forms to get a sense of what your patient population is interested in,” she advised. “It’s important to understand that before you start to offer new services. Don’t just depend on social media to inform you of the latest trends and what people are doing across the country, because if you purchase something that is very popular on social media for people in New York or L.A., that might not be the best for your practice.”
According to market trends from the American Society for Dermatologic Surgery, 3.5 million laser-, light-, and energy-based procedures were performed in 2018. The top five were for wrinkles (809,166), sun damage (786,856), facial redness (612,367), excess hair (385,466), and melasma (226,007). “Considering this data, when you start a practice, do you buy something for wrinkles or for sun damage right away?” Dr. Saedi asked. “Maybe, but you really need to gauge the market that you practice in. You also want to consider your own skill set and what other dermatologists in your area are offering. If you don’t want to do aggressive procedures, then purchasing a fractional CO2 laser might not be the best device to start off with. If you are not comfortable dealing with those patients, and potential infections and scarring, then that’s not the right treatment for you. You have to reflect on and identify what you’re comfortable learning and doing and managing.”
Taking time to investigate the services offered by dermatologists and med spas within a few miles of your practice can help you avoid redundancy. “Learn the techniques and the small nuances that will give you a little bit of finesse and make you an expert, to set you apart from other practices,” said Dr. Saedi, who coauthored a chapter in the book, “The Business of Dermatology” (New York: Thieme Medical Publishers, 2020). “I always recommend treating your staff and members of your family, to understand how you can tweak treatments to get the most out of them. Once you treat your staff, they are walking advertisements for what you do. They can also counsel patients, walking them through the healing process after a procedure, so they can know what to expect.”
Appropriate planning and preparation can help avoid acquiring the wrong device, she continued. This includes patient demand, scheduling availability, office space, overhead costs, and the level of staff training. She recommends buying one device at a time and clearing profitability from that device before purchasing another, “because it can be a burden on your practice to have multiple devices all at once,” she said. “You also have to think about the hidden costs – the maintenance and the service contracts. That can exceed $10,000 per year, so consider that when you’re looking to purchase a new device.”
Most people buy laser-, light-, and energy-based devices, but renting for a stretch can help you test the waters without a significant long-term investment. “It might not be the newest laser, but it can help you gauge how much of demand you have for that service to see if you have the patient base to make that larger step of purchasing the device,” she said. “If you buy a new device, make sure that it’s not a counterfeit and that you still have a company service contract. There are many third-party companies selling pre-owned laser aesthetics. Make sure you’re getting the authentic device and that there is some kind of a service contract with the actual manufacturer so they can help fix it when things break down.”
When Dr. Saedi counsels residents about purchasing devices, she typically recommends these five categories in order of preference: vascular, pigment, hair, resurfacing, and body contouring/skin tightening. “If you can cover vascular, pigment, and some kind of textural improvement, you can treat about 90% of aesthetic patients who come through your door,” she said. “Sure, there are some who may want skin tightening that you may not be able to offer with laser resurfacing, but you’re going to be able capture a high patient population by offering these services,” she added. That is why a lot of people end up getting a platform with attachable handpieces, “where you can have one system that is able to offer many different services right off the bat.”
She advised factoring in the amount of time it takes for a procedure and how much time it will take up in a certain room. “That will affect your revenue as well. Are you going to delegate this, or is this something you will do on your own? Take that into account.”
Above all, don’t rush your device purchase. “Some laser company sales representatives may pressure you at the end of a quarter by saying, ‘This is the best deal I’m going to offer you. You’re never going to get a deal like this ever again,’ ” she said. “I advise people to do multiple demos so you’re not just doing a demo for a day and seeing one or two patients. Treat the same patients again a month later. Do multiple demos so that you can feel comfortable. Talk to dermatologists who have the device, who have real experience with it, so you can have the most amount of information moving forward.”
Dr. Saedi reported that she has received equipment from Alma, Aerolase, Cartessa, and Cynosure. She is a consultant to and/or an advisory board member for those companies, as well as for Alastin.
FROM ODAC 2021
Cognitive effects seen as transient for Alzheimer’s drug atabecestat
according to follow-up results from a truncated clinical trial.
A blinded, placebo-controlled, manufacturer-sponsored trial that had randomized 557 patients with preclinical Alzheimer’s disease to 25 mg daily oral atabecestat, 5 mg atabecestat, or placebo, was halted in 2018 over concerns about liver toxicity. The main outcome measure of the trial was change on the Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite, while two other scales were used to assess cognitive function and neuropsychological status.
A preliminary analysis found the higher dose of the atabecestat to significantly worsen subjects’ cognition starting at around 3 months of treatment, compared with placebo. Treatment with atabecestat was also seen associated with higher incidence of neuropsychiatric adverse events, including anxiety and depression.
In their follow-up study published Jan. 19, 2021 in JAMA Neurology (doi: 10.1001/jamaneurol.2020.4857), Reisa Sperling, MD, of Brigham and Women’s Hospital, Boston, and colleagues reported that the cognitive worsening and neuropsychiatric adverse effects seen linked to atabecestat treatment reverted to baseline levels within 6 months of halting treatment. Most of the worsening seen in the study was associated with episodic memory tasks, including “list learning, story memory, list recognition, story recall, and figure recall,” Dr. Sperling and colleagues found.
Atabecestat was also associated with “dose-related and duration-related decreases in whole-brain volume, compared with placebo treatment,” the investigators reported. Brain volume loss has been seen in trials of other beta-secretase (BACE) inhibitors and shown with one, umibecestat, to be reversible after stopping treatment.
Dr. Sperling and colleagues acknowledged as a major limitation of their study that just over a third of the cohort received another cognitive composite score after baseline. “The observation that cognitive worsening and neuropsychiatric-related [adverse events] recovered following discontinuation of atabecestat is encouraging but needs replication, given that the observation period after stopping treatment was variable and not preplanned,” the investigators wrote in their analysis. After a median exposure of 21 weeks to the study drug or placebo, subjects were followed off treatment for a median 15 weeks.
Questions surround BACE inhibitors
Development of atabecestat has been discontinued along with others in its class of agents, known as BACE inhibitors, which target an enzyme that initiates production of amyloid-beta, the plaque-forming peptide that is considered a driver of Alzheimer’s disease. In the past few years a number of BACE inhibitors have been shown in trials to worsen cognition in a dose-dependent way, compared with placebo. The reasons for these effects are still unknown.
Dr. Sperling and colleagues concluded that, if BACE investigators like atabecestat are to be studied anew, it must be at low doses, with more modest enzyme inhibition, and alongside careful safety and cognitive monitoring.
While no BACE inhibitor is currently in the pipeline for Alzheimer’s – trials of these agents have been stopped for futility or toxicity –Paul Aisen, MD of the University of Southern California, Los Angeles, and a coauthor of Dr. Sperling and colleagues’ study, commented that it was important that clinical investigation of BACE inhibitors continue.
“This drug class is optimal to correct the metabolic dysregulation that is likely a primary root cause” of Alzheimer’s disease, Dr. Aisen said in an interview. “Evidence from trials such as this suggest that the cognitive toxicity of BACE inhibitors is dose related, nonprogressive, and reversible. We should now focus on establishing the safety of relatively low-dose BACE inhibition so that such regimens can be tested in AD trials.”
Research should continue
Robert Vassar, PhD, of Northwestern University, Chicago, who was not a coauthor on the study, also expressed a desire for BACE inhibitor research to continue.
“It is my view that the cognitive worsening of atabecestat and the other BACE inhibitors was caused by overinhibition of the enzyme related to functions of certain BACE substrates in the brain,” Dr. Vassar commented. “A major question is whether a lower dose of BACE inhibitor – achieving about 30% inhibition – could be safe and lower amyloid-beta enough to delay onset in people still without symptoms. The good news of this study is that the atabecestat-related cognitive worsening is reversible, leaving open the possibility of low-dose prevention trials.”
Dr. Vassar noted that, with both doses of atabecestat, Dr. Sperling and colleagues did not see changes in neurofilament light or total tau, two biomarkers of neurodegeneration, but did report decreases in phosphorylated tau (p181 tau), a marker of disease progression, compared with placebo.
“This indicates that atabecestat did not cause neurodegeneration and in fact moved p181 tau in the beneficial direction for Alzheimer’s disease. Perhaps if it were not for the liver toxicity, the trial may have been completed and other Alzheimer’s disease biomarkers may have changed in the beneficial direction as well,” Dr. Vassar said.
Dr. Sperling and colleagues’ study was sponsored by Janssen, the manufacturer of atabecestat. Dr. Sperling disclosed receiving research funding from Janssen and other drug makers, while nearly all the study’s coauthors reported being directly employed by the sponsor or receiving industry funding. Dr. Aisen disclosed personal fees from several manufacturers and past fees from the sponsor. Dr. Vassar disclosed consulting and other financial relationships with biotechnology companies that did not include this study’s sponsor.
according to follow-up results from a truncated clinical trial.
A blinded, placebo-controlled, manufacturer-sponsored trial that had randomized 557 patients with preclinical Alzheimer’s disease to 25 mg daily oral atabecestat, 5 mg atabecestat, or placebo, was halted in 2018 over concerns about liver toxicity. The main outcome measure of the trial was change on the Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite, while two other scales were used to assess cognitive function and neuropsychological status.
A preliminary analysis found the higher dose of the atabecestat to significantly worsen subjects’ cognition starting at around 3 months of treatment, compared with placebo. Treatment with atabecestat was also seen associated with higher incidence of neuropsychiatric adverse events, including anxiety and depression.
In their follow-up study published Jan. 19, 2021 in JAMA Neurology (doi: 10.1001/jamaneurol.2020.4857), Reisa Sperling, MD, of Brigham and Women’s Hospital, Boston, and colleagues reported that the cognitive worsening and neuropsychiatric adverse effects seen linked to atabecestat treatment reverted to baseline levels within 6 months of halting treatment. Most of the worsening seen in the study was associated with episodic memory tasks, including “list learning, story memory, list recognition, story recall, and figure recall,” Dr. Sperling and colleagues found.
Atabecestat was also associated with “dose-related and duration-related decreases in whole-brain volume, compared with placebo treatment,” the investigators reported. Brain volume loss has been seen in trials of other beta-secretase (BACE) inhibitors and shown with one, umibecestat, to be reversible after stopping treatment.
Dr. Sperling and colleagues acknowledged as a major limitation of their study that just over a third of the cohort received another cognitive composite score after baseline. “The observation that cognitive worsening and neuropsychiatric-related [adverse events] recovered following discontinuation of atabecestat is encouraging but needs replication, given that the observation period after stopping treatment was variable and not preplanned,” the investigators wrote in their analysis. After a median exposure of 21 weeks to the study drug or placebo, subjects were followed off treatment for a median 15 weeks.
Questions surround BACE inhibitors
Development of atabecestat has been discontinued along with others in its class of agents, known as BACE inhibitors, which target an enzyme that initiates production of amyloid-beta, the plaque-forming peptide that is considered a driver of Alzheimer’s disease. In the past few years a number of BACE inhibitors have been shown in trials to worsen cognition in a dose-dependent way, compared with placebo. The reasons for these effects are still unknown.
Dr. Sperling and colleagues concluded that, if BACE investigators like atabecestat are to be studied anew, it must be at low doses, with more modest enzyme inhibition, and alongside careful safety and cognitive monitoring.
While no BACE inhibitor is currently in the pipeline for Alzheimer’s – trials of these agents have been stopped for futility or toxicity –Paul Aisen, MD of the University of Southern California, Los Angeles, and a coauthor of Dr. Sperling and colleagues’ study, commented that it was important that clinical investigation of BACE inhibitors continue.
“This drug class is optimal to correct the metabolic dysregulation that is likely a primary root cause” of Alzheimer’s disease, Dr. Aisen said in an interview. “Evidence from trials such as this suggest that the cognitive toxicity of BACE inhibitors is dose related, nonprogressive, and reversible. We should now focus on establishing the safety of relatively low-dose BACE inhibition so that such regimens can be tested in AD trials.”
Research should continue
Robert Vassar, PhD, of Northwestern University, Chicago, who was not a coauthor on the study, also expressed a desire for BACE inhibitor research to continue.
“It is my view that the cognitive worsening of atabecestat and the other BACE inhibitors was caused by overinhibition of the enzyme related to functions of certain BACE substrates in the brain,” Dr. Vassar commented. “A major question is whether a lower dose of BACE inhibitor – achieving about 30% inhibition – could be safe and lower amyloid-beta enough to delay onset in people still without symptoms. The good news of this study is that the atabecestat-related cognitive worsening is reversible, leaving open the possibility of low-dose prevention trials.”
Dr. Vassar noted that, with both doses of atabecestat, Dr. Sperling and colleagues did not see changes in neurofilament light or total tau, two biomarkers of neurodegeneration, but did report decreases in phosphorylated tau (p181 tau), a marker of disease progression, compared with placebo.
“This indicates that atabecestat did not cause neurodegeneration and in fact moved p181 tau in the beneficial direction for Alzheimer’s disease. Perhaps if it were not for the liver toxicity, the trial may have been completed and other Alzheimer’s disease biomarkers may have changed in the beneficial direction as well,” Dr. Vassar said.
Dr. Sperling and colleagues’ study was sponsored by Janssen, the manufacturer of atabecestat. Dr. Sperling disclosed receiving research funding from Janssen and other drug makers, while nearly all the study’s coauthors reported being directly employed by the sponsor or receiving industry funding. Dr. Aisen disclosed personal fees from several manufacturers and past fees from the sponsor. Dr. Vassar disclosed consulting and other financial relationships with biotechnology companies that did not include this study’s sponsor.
according to follow-up results from a truncated clinical trial.
A blinded, placebo-controlled, manufacturer-sponsored trial that had randomized 557 patients with preclinical Alzheimer’s disease to 25 mg daily oral atabecestat, 5 mg atabecestat, or placebo, was halted in 2018 over concerns about liver toxicity. The main outcome measure of the trial was change on the Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite, while two other scales were used to assess cognitive function and neuropsychological status.
A preliminary analysis found the higher dose of the atabecestat to significantly worsen subjects’ cognition starting at around 3 months of treatment, compared with placebo. Treatment with atabecestat was also seen associated with higher incidence of neuropsychiatric adverse events, including anxiety and depression.
In their follow-up study published Jan. 19, 2021 in JAMA Neurology (doi: 10.1001/jamaneurol.2020.4857), Reisa Sperling, MD, of Brigham and Women’s Hospital, Boston, and colleagues reported that the cognitive worsening and neuropsychiatric adverse effects seen linked to atabecestat treatment reverted to baseline levels within 6 months of halting treatment. Most of the worsening seen in the study was associated with episodic memory tasks, including “list learning, story memory, list recognition, story recall, and figure recall,” Dr. Sperling and colleagues found.
Atabecestat was also associated with “dose-related and duration-related decreases in whole-brain volume, compared with placebo treatment,” the investigators reported. Brain volume loss has been seen in trials of other beta-secretase (BACE) inhibitors and shown with one, umibecestat, to be reversible after stopping treatment.
Dr. Sperling and colleagues acknowledged as a major limitation of their study that just over a third of the cohort received another cognitive composite score after baseline. “The observation that cognitive worsening and neuropsychiatric-related [adverse events] recovered following discontinuation of atabecestat is encouraging but needs replication, given that the observation period after stopping treatment was variable and not preplanned,” the investigators wrote in their analysis. After a median exposure of 21 weeks to the study drug or placebo, subjects were followed off treatment for a median 15 weeks.
Questions surround BACE inhibitors
Development of atabecestat has been discontinued along with others in its class of agents, known as BACE inhibitors, which target an enzyme that initiates production of amyloid-beta, the plaque-forming peptide that is considered a driver of Alzheimer’s disease. In the past few years a number of BACE inhibitors have been shown in trials to worsen cognition in a dose-dependent way, compared with placebo. The reasons for these effects are still unknown.
Dr. Sperling and colleagues concluded that, if BACE investigators like atabecestat are to be studied anew, it must be at low doses, with more modest enzyme inhibition, and alongside careful safety and cognitive monitoring.
While no BACE inhibitor is currently in the pipeline for Alzheimer’s – trials of these agents have been stopped for futility or toxicity –Paul Aisen, MD of the University of Southern California, Los Angeles, and a coauthor of Dr. Sperling and colleagues’ study, commented that it was important that clinical investigation of BACE inhibitors continue.
“This drug class is optimal to correct the metabolic dysregulation that is likely a primary root cause” of Alzheimer’s disease, Dr. Aisen said in an interview. “Evidence from trials such as this suggest that the cognitive toxicity of BACE inhibitors is dose related, nonprogressive, and reversible. We should now focus on establishing the safety of relatively low-dose BACE inhibition so that such regimens can be tested in AD trials.”
Research should continue
Robert Vassar, PhD, of Northwestern University, Chicago, who was not a coauthor on the study, also expressed a desire for BACE inhibitor research to continue.
“It is my view that the cognitive worsening of atabecestat and the other BACE inhibitors was caused by overinhibition of the enzyme related to functions of certain BACE substrates in the brain,” Dr. Vassar commented. “A major question is whether a lower dose of BACE inhibitor – achieving about 30% inhibition – could be safe and lower amyloid-beta enough to delay onset in people still without symptoms. The good news of this study is that the atabecestat-related cognitive worsening is reversible, leaving open the possibility of low-dose prevention trials.”
Dr. Vassar noted that, with both doses of atabecestat, Dr. Sperling and colleagues did not see changes in neurofilament light or total tau, two biomarkers of neurodegeneration, but did report decreases in phosphorylated tau (p181 tau), a marker of disease progression, compared with placebo.
“This indicates that atabecestat did not cause neurodegeneration and in fact moved p181 tau in the beneficial direction for Alzheimer’s disease. Perhaps if it were not for the liver toxicity, the trial may have been completed and other Alzheimer’s disease biomarkers may have changed in the beneficial direction as well,” Dr. Vassar said.
Dr. Sperling and colleagues’ study was sponsored by Janssen, the manufacturer of atabecestat. Dr. Sperling disclosed receiving research funding from Janssen and other drug makers, while nearly all the study’s coauthors reported being directly employed by the sponsor or receiving industry funding. Dr. Aisen disclosed personal fees from several manufacturers and past fees from the sponsor. Dr. Vassar disclosed consulting and other financial relationships with biotechnology companies that did not include this study’s sponsor.
FROM JAMA NEUROLOGY
Afternoon napping associated with better cognition in elderly, study shows
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
according to a new study in General Psychiatry.
The findings add to those seen in other observational studies showing afternoon napping promotes cognitive function, said the authors of the paper, published in General Psychiatry.
“The prevalence of afternoon napping has been increasing in older adults much more than in younger individuals,” wrote Han Cai, MS, of the department of geriatrics at The Fourth People’s Hospital of Wuhu, Anhui, China, and coauthors. “The elderly individuals who took afternoon naps showed significantly higher cognitive performance compared with those who did not nap.”
The researchers enrolled 2,214 people in the study – all Han Chinese and aged 60 or older. Afternoon napping was considered any period of inactivity of at least 5 minutes but less than 2 hours after lunch and outside of the person’s main sleep schedule. Those who reported ever napping – 1,534 subjects – were included in the napping group, and the others – 680 – in the nonnapping group. Patients with major physical conditions were excluded.
The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and the Neuropsychological Test Battery (NTB) were used to measure cognitive function, and 739 patients agreed to blood tests for lipid values.
The average total MMSE score was higher for the napping group at 25.3 points out of 30, than for the nonnapping group, at 24.56 (P = .003). Those in the napping group also had significantly higher scores in the orientation portion of the MoCA test, at 5.55 out of 6 points, compared with 5.41 for the nonnapping group (P = .006).
Those in the napping group scored significantly higher on the digit span and language fluency parts of the Neuropsychological Test Battery (P = .009 and .020, respectively).
Dementia was assessed with face-to-face visits with clinicians, but diagnoses of dementia were not different between the groups.
Triglycerides were found to be higher – though still in the normal range – in the napping group compared with the nonnapping group, 1.80 mmol/L to 1.75 mmol/L, the researchers found (P = .001). No differences were seen for HDL or LDL cholesterol levels, or in hypertension or diabetes, the researchers reported.
The authors noted that inflammation is likely an important feature in the relationship between napping and cognitive function. Inflammatory cytokines have been found to play a role in sleep disorders, and strong inflammatory responses can lead to adverse events, including cognitive impairment.
“Sleep is known to be a regulator of the immune response that counters these inflammatory mediators, whereas napping, in particular, is thought to be an evolved response to inflammation,” they said.
The average age of patients in the napping group was 72.8 years, slightly older than those in the nonnapping group at 71.3 years, and this was a significant difference (P = .016).
The researchers acknowledged that the study “could not show direct causality of napping, whether beneficial or harmful,” and that “a lack of detailed information regarding napping duration ... also limited the description of napping status.”
Junxin Li, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, who has studied napping and cognition, said that previous research generally supports a U-shaped relationship between napping and mental acuity, with shorter or medium-length naps benefiting cognition and no naps or naps that are too long being detrimental.
“This study looked at no nap versus naps of less than 2 hours and may not be able to capture this potential U-shaped association,” she said.
For clinicians, the duration, timing, frequency, and purpose of naps are important factors in making recommendations to patients, she said.
“For example, timing – napping in the early evening close to older adult’s bedtime may delay their bedtime and interfere with their nighttime sleep quality. Taking naps after lunchtime is hypothesized to provide the most therapeutic values to the health and usually recommended,” she said. Regular napping is better than “randomly dozing off,” Dr. Li added.
There are also cultural considerations – in east Asia, napping tends to be considered part of a healthy lifestyle, while in western countries it is not – and this could impact napping behaviors and how these behaviors affect cognition, she said.
Phyllis C. Zee, MD, PhD, director of the Center for Circadian and Sleep Medicine at the Northwestern University, Chicago, said the results are consistent with early cross-sectional studies that showed that regular, scheduled naps in the afternoon were associated with positive cognitive performance and lower cardiometabolic disease risk.
Dr. Zee noted that it’s important to recognize that the positive data are associated with naps that are planned, while older adults napping because of excess sleepiness are at a higher risk for cognitive impairment and other health issues.
The study authors, Dr. Li, and Dr. Zee reported no relevant financial disclosures.
Dan Kastner wins Crafoord Prize in Polyarthritis
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
“for establishing the concept of autoinflammatory diseases.” The prize, named after the donor Holger Crafoord because of his bout with severe rheumatoid arthritis toward the end of his life, is for 6 million Swedish kronor (approximately USD $700,000).
Dr. Kastner, scientific director at the U.S. National Human Genome Research Institute’s division of intramural research, received the award for identifying the mechanisms responsible for familial Mediterranean fever, tumor necrosis factor receptor–associated periodic syndrome, and other diagnoses within the group of autoinflammatory diseases.
“Dan Kastner is often called the father of autoinflammatory diseases, a title that he thoroughly deserves. His discoveries have taught us a great deal about the immune system and its functions, contributing to effective treatments that reduce the symptoms of diseases from which patients previously suffered enormously, sometimes leading to premature death,” Olle Kämpe, chair of the prize committee, said in a press announcement.
While the Crafoord Prize normally is awarded on a 3-year rotating basis for achievements in mathematics and astronomy, geosciences, and biosciences, the prize in polyarthritis is “only awarded when there has been scientific progress that motivates a prize,” according to the press release.
Microthrombi, necrosis seen in COVID-19 hearts on autopsy
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Cutaneous Manifestations of COVID-19
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
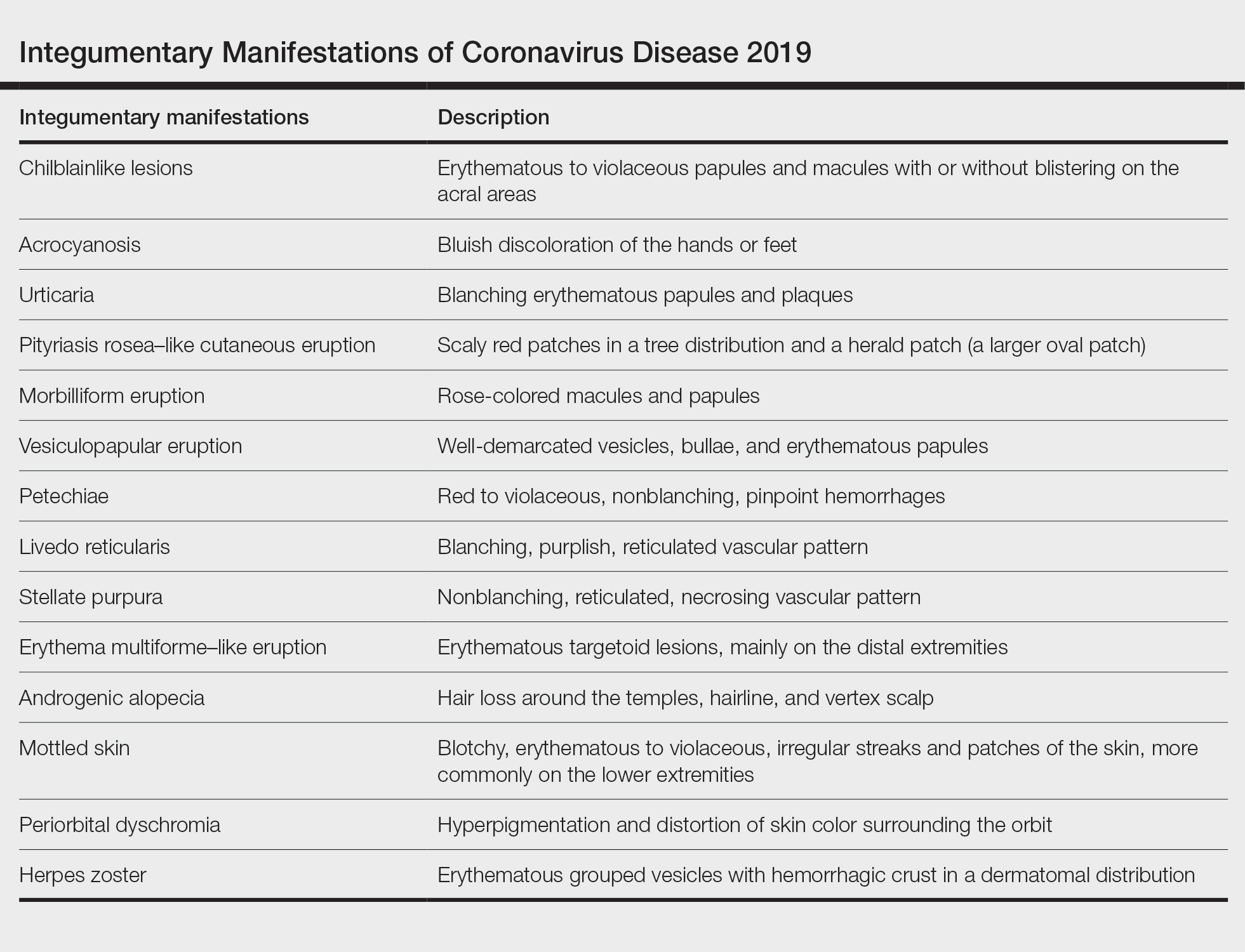
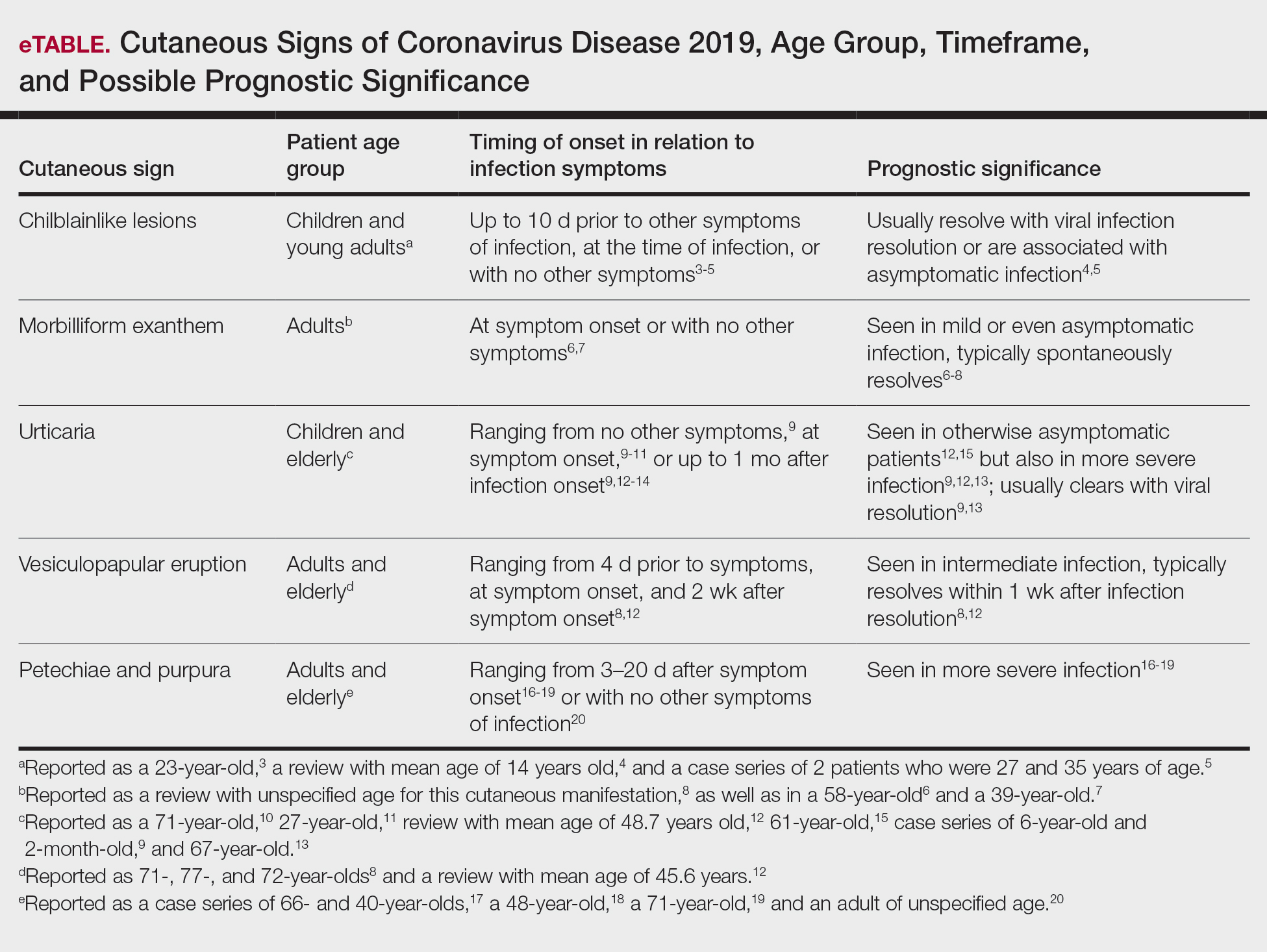
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.


Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.


Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
Practice Points
- Coronavirus disease 2019 (COVID-19) is a worldwide pandemic that affects multiple organ systems via a pathogenesis that is still being elucidated.
- Understanding the various cutaneous manifestations of COVID-19 will aid in early detection and proper treatment, thus increasing patient satisfaction and outcomes.