User login
Home Phototherapy During the COVID-19 Pandemic
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Practice Points
- Home phototherapy is a safe and effective option for patients with psoriasis during the coronavirus disease 2019 (COVID-19) pandemic.
- Although a consensus has not been reached with systemic immunosuppressive therapies for patients with psoriasis and the risk of COVID-19, we continue to recommend caution and careful monitoring of clinical outcomes for patients.
What’s Eating You? Black Butterfly (Hylesia nigricans)
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
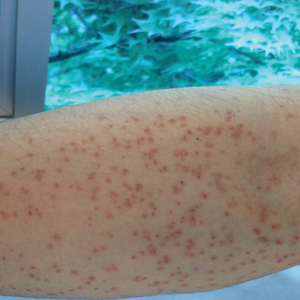
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.
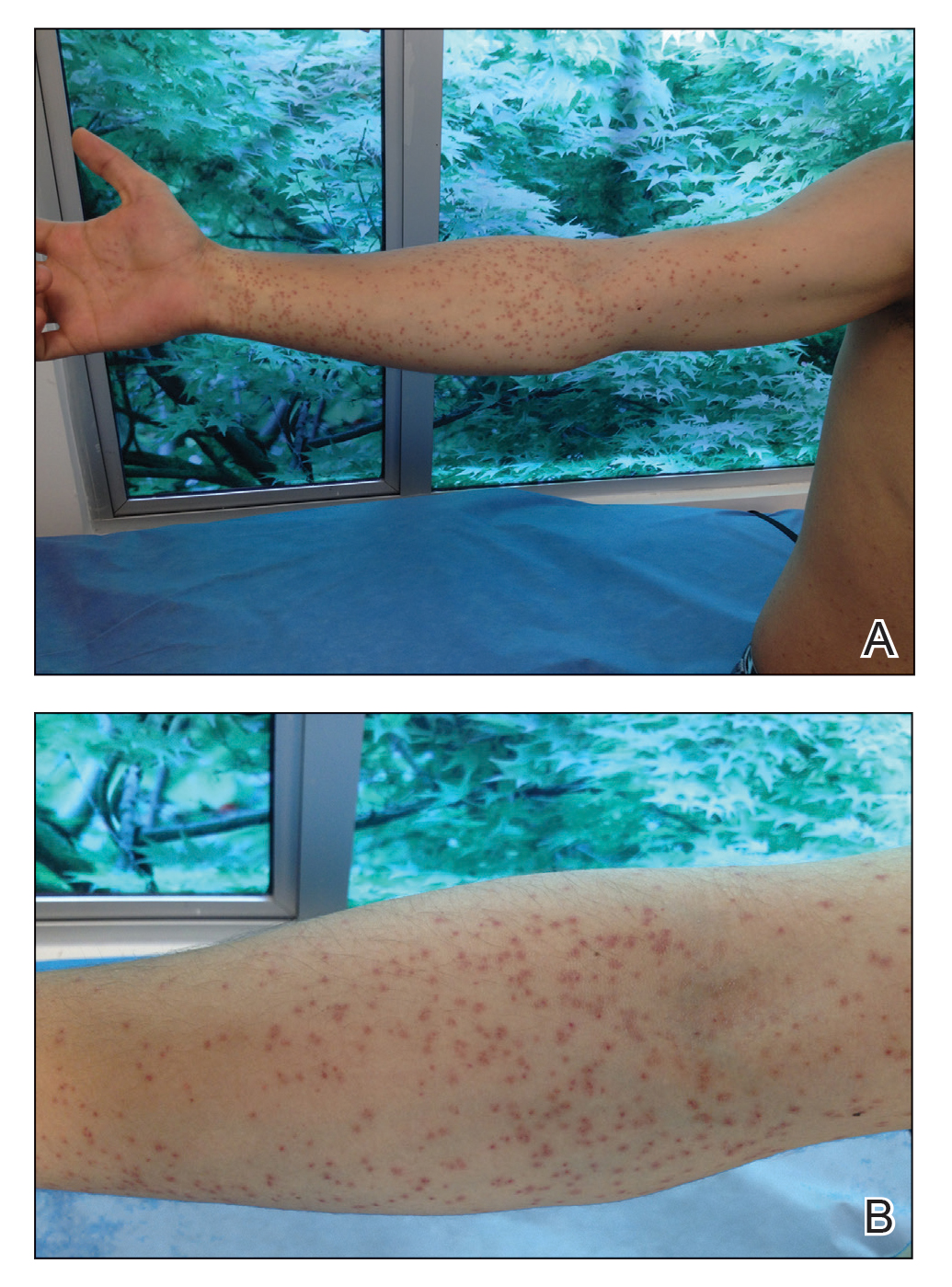
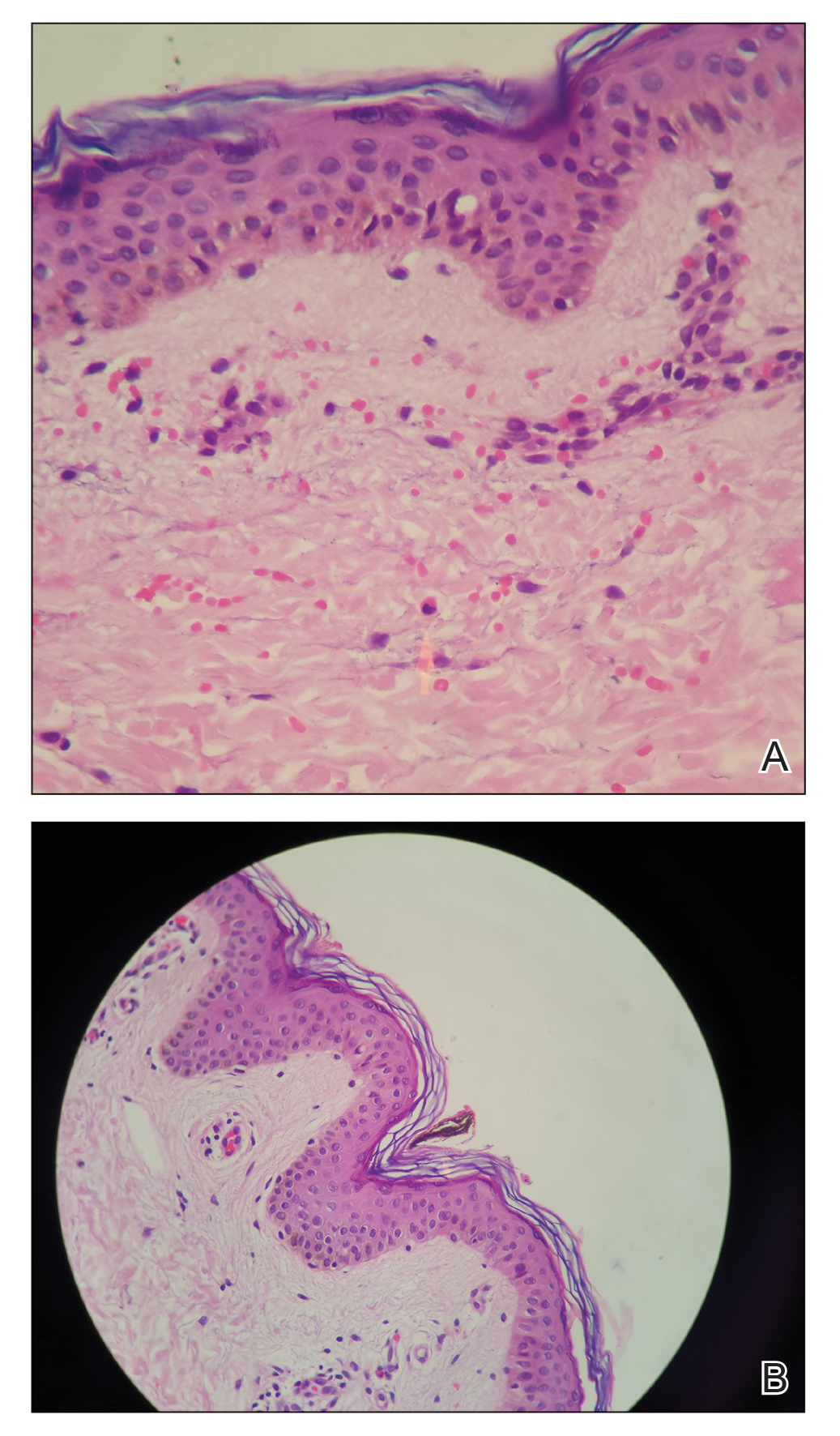
Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.


Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.


Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
Practice Points
- When contact with caterpillars, butterflies, or moths occurs, patients should be advised not to rub or scratch the area or attempt to remove or crush the insect with a bare hand, as this may further spread irritating setae or spines.
- Careful removal of the larva with forceps or a similar instrument, combined with tape stripping of the area and immediate washing with soap and water, can be effective in minimizing exposure.
- Contaminated clothing should be removed and laundered thoroughly.
Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis With Systemic Nonbiologics to Clinical Practice
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
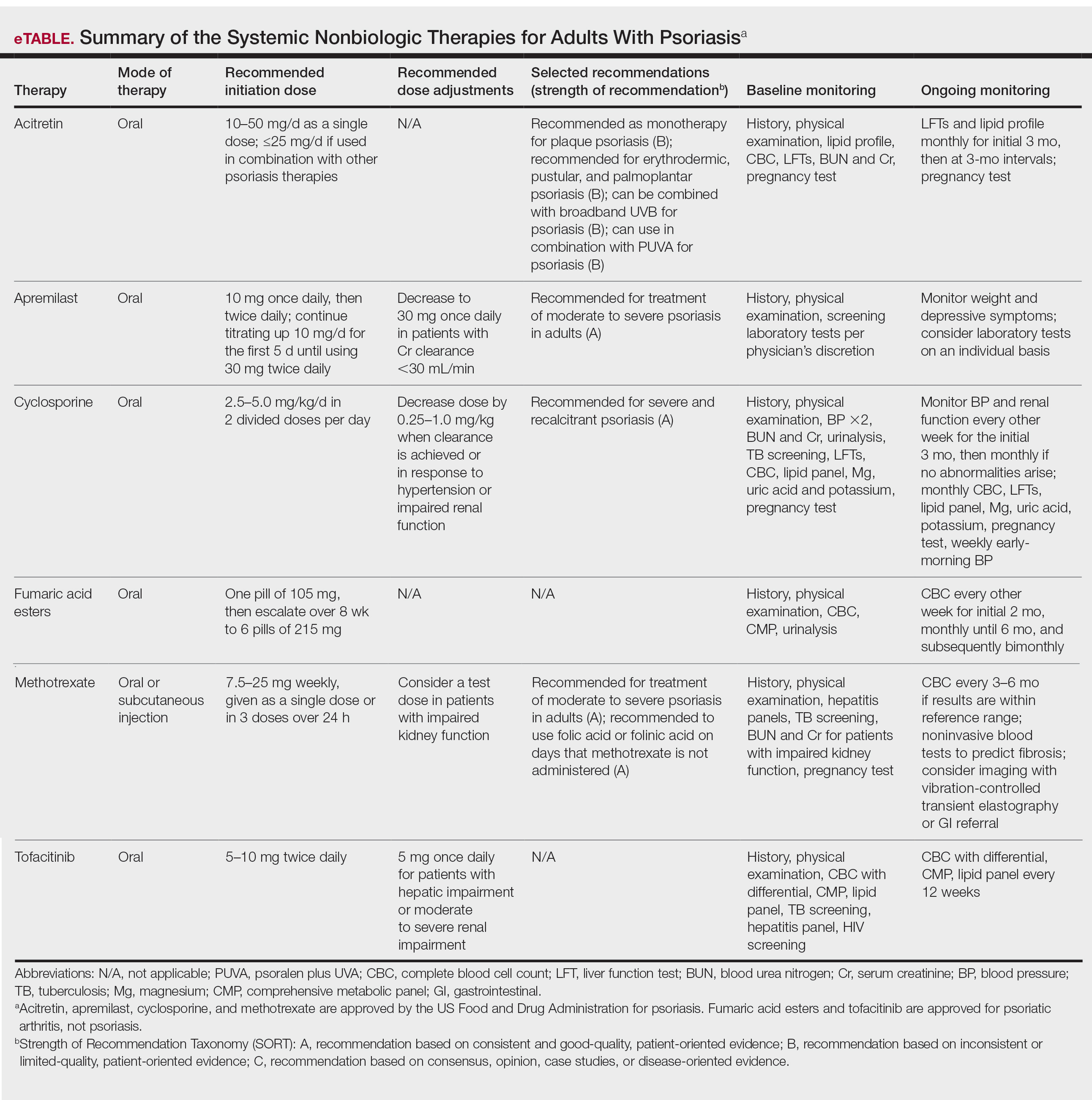
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Practice Points
- Systemic nonbiologic therapies are effective treatments for adults with psoriasis. The benefits of these treatments include ease of administration and the ability to control widespread disease.
- When selecting a therapy, a thorough evaluation of patient characteristics and commitment to lifestyle adjustments is necessary, including careful consideration in women of childbearing potential and those with plans of starting a family.
- Regular drug monitoring and patient follow-up is crucial to ensure safe dosing adjustments and to mitigate potential adverse effects.
Brodalumab in an Organ Transplant Recipient With Psoriasis
The treatment landscape for psoriasis has evolved rapidly over the last decade. Biologic therapies have demonstrated robust efficacy and acceptable safety profiles among many patients with moderate to severe plaque psoriasis. However, the use of biologics among immunocompromised patients with psoriasis rarely is discussed in the literature. As new biologics for psoriasis are being developed, a critical gap exists in the literature regarding the safety and efficacy of these medications in immunocompromised patients. Per American Academy of Dermatology–National Psoriasis Foundation guidelines, caution should be exercised when using biologics in patients with immunocompromising conditions.1 In organ transplant recipients, the potential risks of combining systemic medications used for organ transplantation and biologic treatments for psoriasis are unknown.2
In the posttransplant period, the immunosuppressive regimens for transplantation likely will improve psoriasis. However, patients with organ transplant and psoriasis still experience flares that can be challenging to treat.3 Prior treatment modalities to prevent psoriasis flares in organ transplant recipients have relied largely on topical therapies, posttransplant immunosuppressive medications (eg, cyclosporine, tacrolimus, mycophenolate mofetil) that prevent graft rejection, and systemic corticosteroids. We report a case of a 50-year-old man with a recent history of liver transplantation who presented with severe plaque psoriasis and psoriatic arthritis.
Case Report
A 50-year-old man presented to the dermatology clinic with moderate to severe plaque psoriasis and psoriatic arthritis that had been present for 15 years. His plaque psoriasis covered approximately 40% of the body surface area, including the scalp, trunk, arms, and legs. In addition, he had diffuse joint pain in the hands and feet; a radiograph revealed active psoriatic arthritis involving the joints of the fingers and toes.
One year prior to presentation to our dermatology clinic, the patient underwent an an orthotopic liver transplant for history of Child-Pugh class C liver cirrhosis secondary to untreated hepatitis C virus (HCV) and alcohol use that was complicated by hepatocellular carcinoma. He acquired a high-risk donor liver that was HCV positive with HCV genotype 1a. Starting 2 months after the transplant, he underwent 12 weeks of treatment for HCV with glecaprevir-pibrentasvir. Once his HCV treatment course was completed, he achieved a sustained virologic response with an undetectable viral load. To prevent transplant rejection, he was on chronic immunosuppression with tacrolimus, a calcineurin inhibitor, and mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase whose action leads to decreased proliferation of T cells and B cells.
The patient’s psoriasis initially was treated with triamcinolone acetonide ointment 0.1% applied twice daily to the psoriasis lesions for 1 year by another dermatologist. However, his psoriasis progressed to involve 40% of the body surface area. Following our evaluation 1 year posttransplant, the patient was started on subcutaneous brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks thereafter. Approximately 10 weeks after initiation of brodalumab, the patient’s psoriasis was completely clear, and he was asymptomatic from psoriatic arthritis. The patient’s improvement persisted at 6 months, and his liver enzymes, including alkaline phosphatase, total bilirubin, alanine transaminase, and aspartate transaminase, continued to be within reference range. To date, there has been no evidence of posttransplant complications such as graft-vs-host disease, serious infections, or skin cancers.
Comment
Increased Risk for Infection and Malignancies in Transplant Patients
Transplant patients are on immunosuppressive regimens that increase their risk for infection and malignancies. For example, high doses of immunosuppresants predispose these patients to reactivation of viral infections, including BK and JC viruses.4 In addition, the incidence of squamous cell carcinoma is 65- to 250-fold higher in transplant patients compared to the general population.5 The risk for Merkel cell carcinoma is increased after solid organ transplantation compared to the general population.6 Importantly, transplant patients have a higher mortality from skin cancers than other types of cancers, including breast and colon cancer.7
Psoriasis in Organ Transplant Recipients
Psoriasis is a chronic, immune-mediated, inflammatory disease with a prevalence of approximately 3% in the United States.8 Approximately one-third of patients with psoriasis develop psoriatic arthritis.9 Organ transplant recipients with psoriasis and psoriatic arthritis represent a unique patient population whereby their use of chronic immunosuppressive medications to prevent graft rejection may put them at risk for developing infections and malignancies.
Special Considerations for Brodalumab
Brodalumab is an immunomodulatory biologic that binds to and inhibits IL-17RA, thereby inhibiting the actions of IL-17A, F, E, and C.2 The blockade of IL-17RA by brodalumab has been shown to result in reversal of psoriatic phenotype and gene expression patterns.10 Brodalumab was chosen as the treatment in our patient because it has a rapid onset of action, sustained efficacy, and an acceptable safety profile.11 Brodalumab is well tolerated, with approximately 60% of patients achieving clearance long-term.12 Candidal infections can occur in patients with brodalumab, but the rates are low and they are reversible with antifungal treatment.13 The increased mucocutaneous candidal infections are consistent with medications whose mechanism of action is IL-17 inhibition.14,15 The most common adverse reactions found were nasopharyngitis and headache.16 The causal link between brodalumab and suicidality has not been established.17
The use of brodalumab for psoriasis in organ transplant recipients has not been previously reported in the literature. A few case reports have been published on the successful use of etanercept and ixekizumab as biologic treatment options for psoriasis in transplant patients.18-23 In addition to choosing an appropriate biologic for psoriasis in transplant patients, transplant providers may evaluate the choice of immunosuppression regimen for the organ transplant in the context of psoriasis. In a retrospective analysis of liver transplant patients with psoriasis, Foroncewicz et al3 found cyclosporine, which was used as an antirejection immunosuppressive agent in the posttransplant period, to be more effective than tacrolimus in treating recurrent psoriasis in liver transplant recipients.
Our case illustrates one example of the successful use of brodalumab in a patient with a solid organ transplant. Our patient’s psoriasis and symptoms of psoriatic arthritis greatly improved after initiation of brodalumab. In the posttransplant period, the patient did not develop graft-vs-host disease, infections, malignancies, depression, or suicidal ideation while taking brodalumab.
Conclusion
It is important that the patient, dermatology team, and transplant team work together to navigate the challenges and relatively unknown landscape of psoriasis treatment in organ transplant recipients. As the number of organ transplant recipients continues to increase, this issue will become more clinically relevant. Case reports and future prospective studies will continue to inform us regarding the role of biologics in psoriasis treatment posttransplantation.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Prussick R, Wu JJ, Armstrong AW, et al. Psoriasis in solid organ transplant patients: best practice recommendations from The Medical Board of the National Psoriasis Foundation. J Dermatol Treat. 2018;29:329-333.
- Foroncewicz B, Mucha K, Lerut J, et al. Cyclosporine is superior to tacrolimus in liver transplant recipients with recurrent psoriasis. Ann Transplant. 2014;19:427-433.
- Boukoum H, Nahdi I, Sahtout W, et al. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microbial Pathogenesis. 2016;97:204-208.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647-1656.
- Clark CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after transplant. Clin Oncol. 2019;31:779-788.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828-3836.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82:352-359.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab and ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299-311.
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
- Farahnik B, Beroukhim B, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2016;6:111-124.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.
- DeSimone C, Perino F, Caldarola G, et al. Treatment of psoriasis with etanercept in immunocompromised patients: two case reports. J Int Med Res. 2016;44:67-71.
- Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep. 2015;1:S36-S37.
- Collazo MH, González JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J. 2008;27:346-347.
- Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis. 2007;80:211-214.
- Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc. 2012;44:2776-2777.
- Lora V, Graceffa D, De Felice C, et al. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:E12909.
The treatment landscape for psoriasis has evolved rapidly over the last decade. Biologic therapies have demonstrated robust efficacy and acceptable safety profiles among many patients with moderate to severe plaque psoriasis. However, the use of biologics among immunocompromised patients with psoriasis rarely is discussed in the literature. As new biologics for psoriasis are being developed, a critical gap exists in the literature regarding the safety and efficacy of these medications in immunocompromised patients. Per American Academy of Dermatology–National Psoriasis Foundation guidelines, caution should be exercised when using biologics in patients with immunocompromising conditions.1 In organ transplant recipients, the potential risks of combining systemic medications used for organ transplantation and biologic treatments for psoriasis are unknown.2
In the posttransplant period, the immunosuppressive regimens for transplantation likely will improve psoriasis. However, patients with organ transplant and psoriasis still experience flares that can be challenging to treat.3 Prior treatment modalities to prevent psoriasis flares in organ transplant recipients have relied largely on topical therapies, posttransplant immunosuppressive medications (eg, cyclosporine, tacrolimus, mycophenolate mofetil) that prevent graft rejection, and systemic corticosteroids. We report a case of a 50-year-old man with a recent history of liver transplantation who presented with severe plaque psoriasis and psoriatic arthritis.
Case Report
A 50-year-old man presented to the dermatology clinic with moderate to severe plaque psoriasis and psoriatic arthritis that had been present for 15 years. His plaque psoriasis covered approximately 40% of the body surface area, including the scalp, trunk, arms, and legs. In addition, he had diffuse joint pain in the hands and feet; a radiograph revealed active psoriatic arthritis involving the joints of the fingers and toes.
One year prior to presentation to our dermatology clinic, the patient underwent an an orthotopic liver transplant for history of Child-Pugh class C liver cirrhosis secondary to untreated hepatitis C virus (HCV) and alcohol use that was complicated by hepatocellular carcinoma. He acquired a high-risk donor liver that was HCV positive with HCV genotype 1a. Starting 2 months after the transplant, he underwent 12 weeks of treatment for HCV with glecaprevir-pibrentasvir. Once his HCV treatment course was completed, he achieved a sustained virologic response with an undetectable viral load. To prevent transplant rejection, he was on chronic immunosuppression with tacrolimus, a calcineurin inhibitor, and mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase whose action leads to decreased proliferation of T cells and B cells.
The patient’s psoriasis initially was treated with triamcinolone acetonide ointment 0.1% applied twice daily to the psoriasis lesions for 1 year by another dermatologist. However, his psoriasis progressed to involve 40% of the body surface area. Following our evaluation 1 year posttransplant, the patient was started on subcutaneous brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks thereafter. Approximately 10 weeks after initiation of brodalumab, the patient’s psoriasis was completely clear, and he was asymptomatic from psoriatic arthritis. The patient’s improvement persisted at 6 months, and his liver enzymes, including alkaline phosphatase, total bilirubin, alanine transaminase, and aspartate transaminase, continued to be within reference range. To date, there has been no evidence of posttransplant complications such as graft-vs-host disease, serious infections, or skin cancers.
Comment
Increased Risk for Infection and Malignancies in Transplant Patients
Transplant patients are on immunosuppressive regimens that increase their risk for infection and malignancies. For example, high doses of immunosuppresants predispose these patients to reactivation of viral infections, including BK and JC viruses.4 In addition, the incidence of squamous cell carcinoma is 65- to 250-fold higher in transplant patients compared to the general population.5 The risk for Merkel cell carcinoma is increased after solid organ transplantation compared to the general population.6 Importantly, transplant patients have a higher mortality from skin cancers than other types of cancers, including breast and colon cancer.7
Psoriasis in Organ Transplant Recipients
Psoriasis is a chronic, immune-mediated, inflammatory disease with a prevalence of approximately 3% in the United States.8 Approximately one-third of patients with psoriasis develop psoriatic arthritis.9 Organ transplant recipients with psoriasis and psoriatic arthritis represent a unique patient population whereby their use of chronic immunosuppressive medications to prevent graft rejection may put them at risk for developing infections and malignancies.
Special Considerations for Brodalumab
Brodalumab is an immunomodulatory biologic that binds to and inhibits IL-17RA, thereby inhibiting the actions of IL-17A, F, E, and C.2 The blockade of IL-17RA by brodalumab has been shown to result in reversal of psoriatic phenotype and gene expression patterns.10 Brodalumab was chosen as the treatment in our patient because it has a rapid onset of action, sustained efficacy, and an acceptable safety profile.11 Brodalumab is well tolerated, with approximately 60% of patients achieving clearance long-term.12 Candidal infections can occur in patients with brodalumab, but the rates are low and they are reversible with antifungal treatment.13 The increased mucocutaneous candidal infections are consistent with medications whose mechanism of action is IL-17 inhibition.14,15 The most common adverse reactions found were nasopharyngitis and headache.16 The causal link between brodalumab and suicidality has not been established.17
The use of brodalumab for psoriasis in organ transplant recipients has not been previously reported in the literature. A few case reports have been published on the successful use of etanercept and ixekizumab as biologic treatment options for psoriasis in transplant patients.18-23 In addition to choosing an appropriate biologic for psoriasis in transplant patients, transplant providers may evaluate the choice of immunosuppression regimen for the organ transplant in the context of psoriasis. In a retrospective analysis of liver transplant patients with psoriasis, Foroncewicz et al3 found cyclosporine, which was used as an antirejection immunosuppressive agent in the posttransplant period, to be more effective than tacrolimus in treating recurrent psoriasis in liver transplant recipients.
Our case illustrates one example of the successful use of brodalumab in a patient with a solid organ transplant. Our patient’s psoriasis and symptoms of psoriatic arthritis greatly improved after initiation of brodalumab. In the posttransplant period, the patient did not develop graft-vs-host disease, infections, malignancies, depression, or suicidal ideation while taking brodalumab.
Conclusion
It is important that the patient, dermatology team, and transplant team work together to navigate the challenges and relatively unknown landscape of psoriasis treatment in organ transplant recipients. As the number of organ transplant recipients continues to increase, this issue will become more clinically relevant. Case reports and future prospective studies will continue to inform us regarding the role of biologics in psoriasis treatment posttransplantation.
The treatment landscape for psoriasis has evolved rapidly over the last decade. Biologic therapies have demonstrated robust efficacy and acceptable safety profiles among many patients with moderate to severe plaque psoriasis. However, the use of biologics among immunocompromised patients with psoriasis rarely is discussed in the literature. As new biologics for psoriasis are being developed, a critical gap exists in the literature regarding the safety and efficacy of these medications in immunocompromised patients. Per American Academy of Dermatology–National Psoriasis Foundation guidelines, caution should be exercised when using biologics in patients with immunocompromising conditions.1 In organ transplant recipients, the potential risks of combining systemic medications used for organ transplantation and biologic treatments for psoriasis are unknown.2
In the posttransplant period, the immunosuppressive regimens for transplantation likely will improve psoriasis. However, patients with organ transplant and psoriasis still experience flares that can be challenging to treat.3 Prior treatment modalities to prevent psoriasis flares in organ transplant recipients have relied largely on topical therapies, posttransplant immunosuppressive medications (eg, cyclosporine, tacrolimus, mycophenolate mofetil) that prevent graft rejection, and systemic corticosteroids. We report a case of a 50-year-old man with a recent history of liver transplantation who presented with severe plaque psoriasis and psoriatic arthritis.
Case Report
A 50-year-old man presented to the dermatology clinic with moderate to severe plaque psoriasis and psoriatic arthritis that had been present for 15 years. His plaque psoriasis covered approximately 40% of the body surface area, including the scalp, trunk, arms, and legs. In addition, he had diffuse joint pain in the hands and feet; a radiograph revealed active psoriatic arthritis involving the joints of the fingers and toes.
One year prior to presentation to our dermatology clinic, the patient underwent an an orthotopic liver transplant for history of Child-Pugh class C liver cirrhosis secondary to untreated hepatitis C virus (HCV) and alcohol use that was complicated by hepatocellular carcinoma. He acquired a high-risk donor liver that was HCV positive with HCV genotype 1a. Starting 2 months after the transplant, he underwent 12 weeks of treatment for HCV with glecaprevir-pibrentasvir. Once his HCV treatment course was completed, he achieved a sustained virologic response with an undetectable viral load. To prevent transplant rejection, he was on chronic immunosuppression with tacrolimus, a calcineurin inhibitor, and mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase whose action leads to decreased proliferation of T cells and B cells.
The patient’s psoriasis initially was treated with triamcinolone acetonide ointment 0.1% applied twice daily to the psoriasis lesions for 1 year by another dermatologist. However, his psoriasis progressed to involve 40% of the body surface area. Following our evaluation 1 year posttransplant, the patient was started on subcutaneous brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks thereafter. Approximately 10 weeks after initiation of brodalumab, the patient’s psoriasis was completely clear, and he was asymptomatic from psoriatic arthritis. The patient’s improvement persisted at 6 months, and his liver enzymes, including alkaline phosphatase, total bilirubin, alanine transaminase, and aspartate transaminase, continued to be within reference range. To date, there has been no evidence of posttransplant complications such as graft-vs-host disease, serious infections, or skin cancers.
Comment
Increased Risk for Infection and Malignancies in Transplant Patients
Transplant patients are on immunosuppressive regimens that increase their risk for infection and malignancies. For example, high doses of immunosuppresants predispose these patients to reactivation of viral infections, including BK and JC viruses.4 In addition, the incidence of squamous cell carcinoma is 65- to 250-fold higher in transplant patients compared to the general population.5 The risk for Merkel cell carcinoma is increased after solid organ transplantation compared to the general population.6 Importantly, transplant patients have a higher mortality from skin cancers than other types of cancers, including breast and colon cancer.7
Psoriasis in Organ Transplant Recipients
Psoriasis is a chronic, immune-mediated, inflammatory disease with a prevalence of approximately 3% in the United States.8 Approximately one-third of patients with psoriasis develop psoriatic arthritis.9 Organ transplant recipients with psoriasis and psoriatic arthritis represent a unique patient population whereby their use of chronic immunosuppressive medications to prevent graft rejection may put them at risk for developing infections and malignancies.
Special Considerations for Brodalumab
Brodalumab is an immunomodulatory biologic that binds to and inhibits IL-17RA, thereby inhibiting the actions of IL-17A, F, E, and C.2 The blockade of IL-17RA by brodalumab has been shown to result in reversal of psoriatic phenotype and gene expression patterns.10 Brodalumab was chosen as the treatment in our patient because it has a rapid onset of action, sustained efficacy, and an acceptable safety profile.11 Brodalumab is well tolerated, with approximately 60% of patients achieving clearance long-term.12 Candidal infections can occur in patients with brodalumab, but the rates are low and they are reversible with antifungal treatment.13 The increased mucocutaneous candidal infections are consistent with medications whose mechanism of action is IL-17 inhibition.14,15 The most common adverse reactions found were nasopharyngitis and headache.16 The causal link between brodalumab and suicidality has not been established.17
The use of brodalumab for psoriasis in organ transplant recipients has not been previously reported in the literature. A few case reports have been published on the successful use of etanercept and ixekizumab as biologic treatment options for psoriasis in transplant patients.18-23 In addition to choosing an appropriate biologic for psoriasis in transplant patients, transplant providers may evaluate the choice of immunosuppression regimen for the organ transplant in the context of psoriasis. In a retrospective analysis of liver transplant patients with psoriasis, Foroncewicz et al3 found cyclosporine, which was used as an antirejection immunosuppressive agent in the posttransplant period, to be more effective than tacrolimus in treating recurrent psoriasis in liver transplant recipients.
Our case illustrates one example of the successful use of brodalumab in a patient with a solid organ transplant. Our patient’s psoriasis and symptoms of psoriatic arthritis greatly improved after initiation of brodalumab. In the posttransplant period, the patient did not develop graft-vs-host disease, infections, malignancies, depression, or suicidal ideation while taking brodalumab.
Conclusion
It is important that the patient, dermatology team, and transplant team work together to navigate the challenges and relatively unknown landscape of psoriasis treatment in organ transplant recipients. As the number of organ transplant recipients continues to increase, this issue will become more clinically relevant. Case reports and future prospective studies will continue to inform us regarding the role of biologics in psoriasis treatment posttransplantation.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Prussick R, Wu JJ, Armstrong AW, et al. Psoriasis in solid organ transplant patients: best practice recommendations from The Medical Board of the National Psoriasis Foundation. J Dermatol Treat. 2018;29:329-333.
- Foroncewicz B, Mucha K, Lerut J, et al. Cyclosporine is superior to tacrolimus in liver transplant recipients with recurrent psoriasis. Ann Transplant. 2014;19:427-433.
- Boukoum H, Nahdi I, Sahtout W, et al. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microbial Pathogenesis. 2016;97:204-208.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647-1656.
- Clark CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after transplant. Clin Oncol. 2019;31:779-788.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828-3836.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82:352-359.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab and ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299-311.
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
- Farahnik B, Beroukhim B, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2016;6:111-124.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.
- DeSimone C, Perino F, Caldarola G, et al. Treatment of psoriasis with etanercept in immunocompromised patients: two case reports. J Int Med Res. 2016;44:67-71.
- Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep. 2015;1:S36-S37.
- Collazo MH, González JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J. 2008;27:346-347.
- Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis. 2007;80:211-214.
- Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc. 2012;44:2776-2777.
- Lora V, Graceffa D, De Felice C, et al. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:E12909.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Prussick R, Wu JJ, Armstrong AW, et al. Psoriasis in solid organ transplant patients: best practice recommendations from The Medical Board of the National Psoriasis Foundation. J Dermatol Treat. 2018;29:329-333.
- Foroncewicz B, Mucha K, Lerut J, et al. Cyclosporine is superior to tacrolimus in liver transplant recipients with recurrent psoriasis. Ann Transplant. 2014;19:427-433.
- Boukoum H, Nahdi I, Sahtout W, et al. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microbial Pathogenesis. 2016;97:204-208.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647-1656.
- Clark CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after transplant. Clin Oncol. 2019;31:779-788.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828-3836.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82:352-359.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab and ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299-311.
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
- Farahnik B, Beroukhim B, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2016;6:111-124.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.
- DeSimone C, Perino F, Caldarola G, et al. Treatment of psoriasis with etanercept in immunocompromised patients: two case reports. J Int Med Res. 2016;44:67-71.
- Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep. 2015;1:S36-S37.
- Collazo MH, González JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J. 2008;27:346-347.
- Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis. 2007;80:211-214.
- Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc. 2012;44:2776-2777.
- Lora V, Graceffa D, De Felice C, et al. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:E12909.
Practice Points
- Immunocompromised patients, such as organ transplant recipients, require careful benefit-risk consideration when selecting a systemic agent for psoriasis.
- Brodalumab, an IL-17RA antagonist, was used to treat a patient with psoriasis who had undergone solid organ transplant with excellent response and good tolerability.
- Further studies are needed to evaluate the benefits and risks of using biologic treatments in patients with psoriasis who are organ transplant recipients.
Incidence of autoimmune hepatitis may be rising
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
Historically, autoimmune hepatitis (AIH) was a rare disease in reproductive-age women with chronic active hepatitis and autoantibodies. Today with worldwide information available at our fingertips, autoimmune liver diseases such as AIH and variants are in our armamentarium of differential diagnosis for patients with chronic hepatitis. Autoimmune liver conditions are now diagnosed in a wide range of ethnic groups and age groups.
Unlike highly prevalent chronic liver diseases such as alcohol-related and viral hepatitis, we do not know the trigger for AIH in predisposed patients. It could be difficult to explain to patients how they became susceptible to and acquired AIH. In this geographically defined population with centralized access to health care, it would be curious to know triggers, such as infections, medications, personal habits, dietary and gut microbiome changes, or emerging comorbid conditions that may influence the occurrence of AIH. Population studies helped identify common epidemiologic traits and combined with serologies and clinical criteria, we have become more adept at diagnosis of AIH. Future studies could look at clustering in communities and susceptibility patterns in ethnic groups that may implicate etiologic factors.
Avegail Flores, MD, is with the section of gastroenterology and hepatology at Baylor College of Medicine, Houston, and is the medical director of liver transplant at Michael E. DeBakey Houston Veterans Affairs Medical Center. She has nothing to disclose.
Historically, autoimmune hepatitis (AIH) was a rare disease in reproductive-age women with chronic active hepatitis and autoantibodies. Today with worldwide information available at our fingertips, autoimmune liver diseases such as AIH and variants are in our armamentarium of differential diagnosis for patients with chronic hepatitis. Autoimmune liver conditions are now diagnosed in a wide range of ethnic groups and age groups.
Unlike highly prevalent chronic liver diseases such as alcohol-related and viral hepatitis, we do not know the trigger for AIH in predisposed patients. It could be difficult to explain to patients how they became susceptible to and acquired AIH. In this geographically defined population with centralized access to health care, it would be curious to know triggers, such as infections, medications, personal habits, dietary and gut microbiome changes, or emerging comorbid conditions that may influence the occurrence of AIH. Population studies helped identify common epidemiologic traits and combined with serologies and clinical criteria, we have become more adept at diagnosis of AIH. Future studies could look at clustering in communities and susceptibility patterns in ethnic groups that may implicate etiologic factors.
Avegail Flores, MD, is with the section of gastroenterology and hepatology at Baylor College of Medicine, Houston, and is the medical director of liver transplant at Michael E. DeBakey Houston Veterans Affairs Medical Center. She has nothing to disclose.
Historically, autoimmune hepatitis (AIH) was a rare disease in reproductive-age women with chronic active hepatitis and autoantibodies. Today with worldwide information available at our fingertips, autoimmune liver diseases such as AIH and variants are in our armamentarium of differential diagnosis for patients with chronic hepatitis. Autoimmune liver conditions are now diagnosed in a wide range of ethnic groups and age groups.
Unlike highly prevalent chronic liver diseases such as alcohol-related and viral hepatitis, we do not know the trigger for AIH in predisposed patients. It could be difficult to explain to patients how they became susceptible to and acquired AIH. In this geographically defined population with centralized access to health care, it would be curious to know triggers, such as infections, medications, personal habits, dietary and gut microbiome changes, or emerging comorbid conditions that may influence the occurrence of AIH. Population studies helped identify common epidemiologic traits and combined with serologies and clinical criteria, we have become more adept at diagnosis of AIH. Future studies could look at clustering in communities and susceptibility patterns in ethnic groups that may implicate etiologic factors.
Avegail Flores, MD, is with the section of gastroenterology and hepatology at Baylor College of Medicine, Houston, and is the medical director of liver transplant at Michael E. DeBakey Houston Veterans Affairs Medical Center. She has nothing to disclose.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Unilateral Verrucous Psoriasis
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.
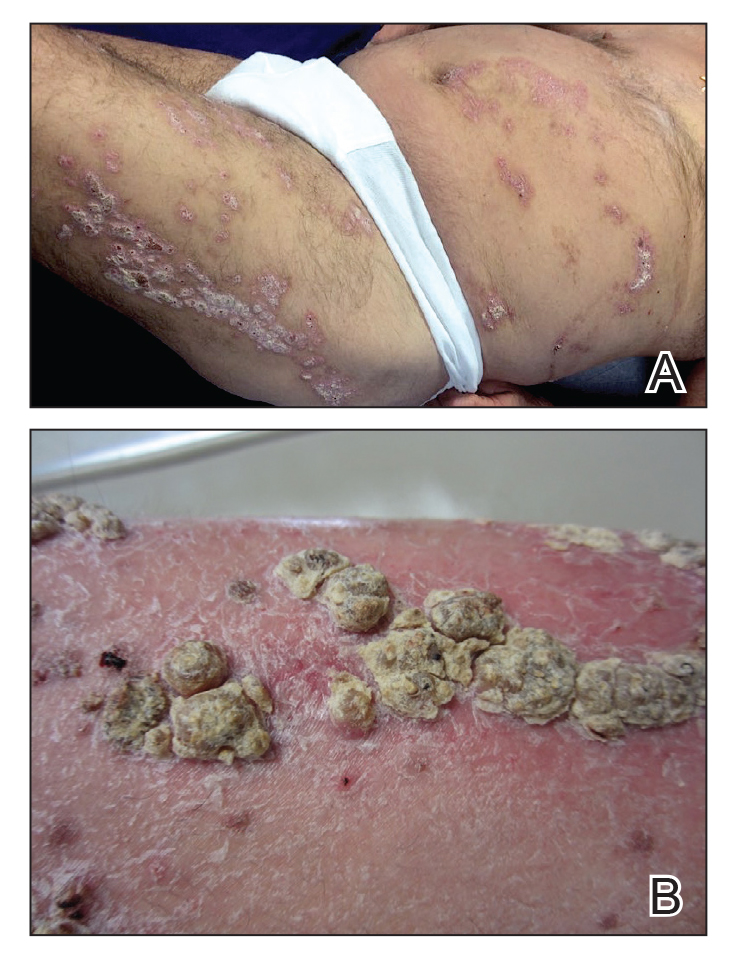
At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.
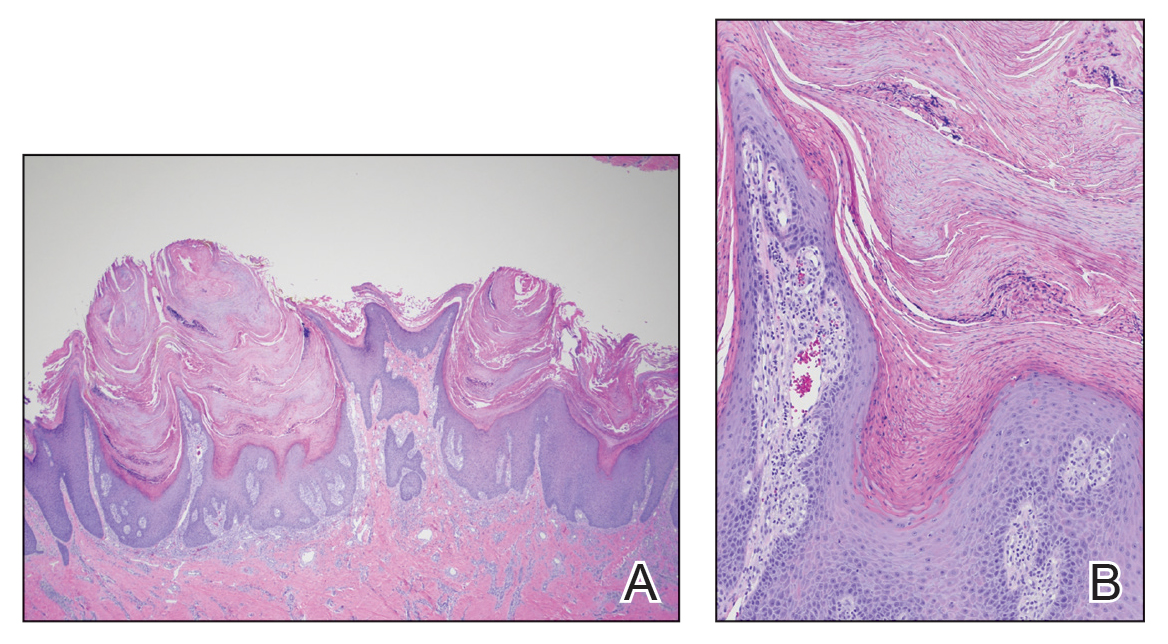
Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.

At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.

Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.

At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.

Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
Practice Points
- Verrucous psoriasis is a rare variant of psoriasis characterized by hypertrophic verrucous papules and plaques on an erythematous base.
- Histologically, verrucous psoriasis presents with overlapping features of verruca and psoriasis.
- Although psoriasis typically presents in a symmetric distribution, unilateral psoriasis can occur either de novo in younger patients or after surgical trauma in older patients.
Cutaneous Manifestations of Nutritional Excess: Pathophysiologic Effects of Hyperglycemia and Hyperinsulinemia on the Skin
Nutritional dermatoses are classically associated with dietary nutrient deficiencies; however, cutaneous disease as a consequence of nutrient excess often is overlooked. Chronic hyperglycemia and hyperinsulinemia resulting from excess carbohydrate intake may be implicated in a number of cutaneous pathologies, of which every dermatologist should be aware.1-3
Although diabetic patients exhibit many cutaneous manifestations of excess carbohydrate consumption, the absence of a diagnosis of type 2 diabetes mellitus (T2DM) does not necessarily preclude them.4-6 Emerging evidence now highlights the development of insulin resistance well before a patient ever meets the diagnostic criteria for T2DM.7,8 Cutaneous disease can provide early insight into a patient’s glucose tolerance and may be the first sign of metabolic derangement. Prompt recognition of these cutaneous alterations and management of the patient’s underlying systemic disease can improve their quality of life and help prevent severe systemic complications associated with insulin resistance and impaired glucose tolerance.
The aim of this review is to highlight both common and rare cutaneous manifestations associated with the persistent consumption of high glycemic load diets, resultant hyperglycemic and hyperinsulinemic states, and the pathophysiologic mechanisms that underlie them.
Acanthosis Nigricans
Acanthosis nigricans (AN) is a highly prevalent cutaneous finding in individuals with insulin resistance that clinically presents as thickened, hyperpigmented, velvety plaques on the intertriginous and flexural surfaces. The most frequently involved sites include the neck, axillae (Figure), and inframammary and inguinal folds. Black and Hispanic patients most commonly are affected. Although classically associated with T2DM, AN also can be observed in normoglycemic individuals.7-9 One recent study reported the rate of AN to be 36% in a cohort of middle-aged patients (N=320) with normal fasting blood glucose levels, while the rate of AN in matched patients with hyperglycemia (prediabetes and T2DM) was approximately 50%.7 Quantification of insulin resistance was performed using the homeostatic model assessment of insulin resistance index. Interestingly, the specificity for insulin resistance in normoglycemic and hyperglycemic subjects with AN was 85% and 90%, respectively.7 These findings suggest that AN may serve as a convenient surrogate marker for subclinical insulin resistance, a conclusion that has been reported in a series of previous studies.8-10
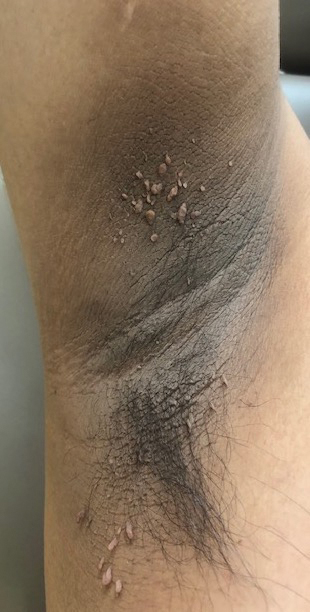
Although the pathogenesis of AN has not been fully elucidated, it is known that persistently elevated blood glucose triggers continual secretion of insulin and insulinlike growth factor 1 (IGF-1), which results in the overstimulation of insulin and IGF-1 receptors on keratinocytes and dermal fibroblasts through direct and indirect pathways.11,12 The resultant cellular proliferation can be observed histologically in the forms of orthokeratotic hyperkeratosis and papillomatosis, as occurs in AN.11,13 Further supporting the association between elevated insulin and AN are reports of AN developing at sites of repeated insulin injection as well as genetic mutations in the insulin receptor resulting in severe AN in children.14-16
The treatment of AN ultimately focuses on improving glycemic control and reducing insulin resistance through lifestyle modification and pharmacotherapy with agents such as metformin.11,13 Dermatologic treatment with oral and topical keratolytic agents such as isotretinoin and other retinoids, salicylic acid, urea, or ammonium lactate may be used, but their efficacy generally has been limited.11,13,17,18
Diabetic Dermopathy
Diabetic dermopathy (DD), commonly known as shin spots, refers to the red-brown, atrophic, circinate macules and patches that often appear on the lower extremities in patients with T2DM. Although the pretibial area is the most frequently involved site, other areas of bony prominence such as the forearms can be affected. The prevalence of DD in the diabetic population can be exceedingly high, with some studies reporting incidence rates greater than 50%, particularly in those with poorly controlled T2DM.19-21 Interestingly, DD also has been documented in patients without T2DM and has been postulated to be an early sign of insulin resistance.20,22
The pathogenesis of DD remains uncertain, but one proposed mechanism is through microvascular damage caused by hyperglycemia-induced, nonenzymatic glycation, possibly in conjunction with mild trauma, that leads to the deposition of hemosiderin and melanin in the skin.20,23 A recent study identified increased vascularization of dermopathy lesions when compared with surrounding tissue.24 Subcutaneous nerve ischemia and degeneration secondary to diabetic neuropathy also have been postulated as causative.20,23 Given the lack of effective therapies and the asymptomatic nature of DD, treatment typically is not pursued. However, DD is associated with other diabetic microvascular complications, including diabetic nephropathy, retinopathy, and neuropathy. For this reason, identification of DD warrants further characterization and management of a patient’s underlying diabetes.19,20
Scleredema Diabeticorum
Scleredema diabeticorum (SD) refers to the slowly progressive, painless thickening and woody induration of the neck, shoulders, and upper back in individuals with long-standing, poorly controlled diabetes. The condition is almost exclusively seen in the diabetic population, with prevalence rates reported to be as high as 14%.25-27 Although SD generally is asymptomatic, some individuals may experience restricted mobility and decreased sensation in affected areas.25,27,28 The diagnosis of SD frequently is missed or ignored clinically. Biopsy can provide diagnostic confirmation of this entity, as histopathology reveals a thickened reticular dermis with an accumulation of collagen and adjacent mucinous infiltrate with no edema or sclerosis.28,29
Although the pathogenesis of SD is not well established, it is theorized that the binding of advanced glycation end products (AGEs) to collagen fibers impairs proper cross-linking and degradation by collagenase.29-31 It is well known that hyperglycemic conditions can promote endogenous formation of AGEs, which occur when reducing sugar molecules become glycated through a nonenzymatic reaction.30-32 The Western diet also is high in preformed AGEs, which are created primarily through certain high-heat cooking methods such as frying and grilling.31,32 Hyperglycemia-induced stimulation of fibroblasts also has been proposed as a driver of increased collagen deposition observed histologically in SD.28,29,33 Treatment of SD can be difficult, as there are no consistently reported therapies, and even improvement in glycemic control does not appear to reverse this condition.29 Case reports have demonstrated some efficacy with various phototherapeutic modalities, including psoralen plus UVA and narrowband UVB phototherapy.34-36
Ichthyosiform Skin Changes
Ichthyosiform skin changes refer to areas of xerosis and scaling that classically present on the anterior distal lower extremities. Although ichthyosiform alterations have been associated with numerous systemic diseases, they often represent an early finding in diabetic patients.27,37 The development of ichthyosiform skin changes has been linked to the formation and accumulation of AGEs, which can cause defective cell adhesion in the stratum corneum.37,38 Treatment with topical emollients and keratolytics may prove beneficial for the skin but do not improve the underlying systemic condition.39
Acrochordons
Acrochordons (skin tags) are common benign fibroepithelial polyps that classically present on the face, neck, and trunk. The underlying mechanism responsible for the development of acrochordons is uncertain, but the association with insulin resistance and impaired carbohydrate metabolism is well validated.40-46 Several large cross-sectional and case-control studies have reported rates of T2DM ranging from 23% to 72% in patients with acrochordons.41,42,47 The pathophysiology may involve an increase in tissue and epidermal growth factors driven by elevated serum insulin levels, stimulation of IGF-1 receptors, and a localized proliferation of cutaneous tissue in elastin-poor areas.45,48,49 Interestingly, the quantity of acrochordons has been positively correlated with fasting blood glucose levels. Additionally, the presence of 30 or more acrochordons was found to increase the risk of developing T2DM.41 Therefore, the presence and number of acrochordons may serve as a convenient indicator of systemic glycemic control and insulin resistance. Screening for T2DM is warranted in individuals without a prior diagnosis who present with multiple acrochordons.
Keratosis Pilaris
Keratosis pilaris (KP) is a benign skin condition characterized by pink-red, erythematous, monomorphic, follicular papules often seen on the extensor arms, thighs, buttocks, and cheeks. Keratosis pilaris is exceedingly common in the general population but occurs more frequently and with more extensive involvement in those with atopic dermatitis and T2DM.27,50,51 The mechanism underlying the hyperkeratosis and inflammatory change observed in KP is not well understood and is likely multifactorial.52,53 Hyperandrogenism, as a consequence of hyperinsulinemia, may play an important role in KP, as elevated circulating androgens are known drivers of keratinocyte proliferation of the pilosebaceous unit of hair follicles.52,54 Support for this theory includes the clinical exaggeration of KP frequently encountered around puberty when androgen levels peak.55,56 Moreover, one study found a higher incidence of KP among adolescent patients with type 1 diabetes mellitus than among healthy age-matched controls.27 The most effective treatment of KP appears to be laser therapy, particularly the Q-switched Nd:YAG laser. Numerous topical modalities have been employed to treat KP but exhibit limited efficacy, including mineral oil, tacrolimus, azelaic acid, and salicylic acid, among others.57
Necrobiosis Lipoidica
Necrobiosis lipoidica (NL) is a chronic granulomatous skin condition of unknown origin that presents with well-demarcated, yellow-brown, atrophic patches and plaques often found exclusively on the shins. There is a strong association with type 1 diabetes mellitus, with reported rates ranging from 11% to 65% in patients with NL.58-60 In a recent retrospective study of 236 patients with NL, 58.5% of patients had diabetes.61 Nevertheless, NL is a rare entity that affects less than 1% of the diabetic population.60 Given its correlation with diabetes, it has been postulated that the pathogenesis of NL is due to microvascular ischemic changes resulting from prolonged hyperglycemia.60 However, studies revealing an increase in blood flow to NL lesions suggest that the condition may instead be attributed to an inflammatory process.62 Despite the disfiguring appearance, the lesions of NL often are asymptomatic. Pain or pruritus may develop secondary to ulceration, which occurs in approximately one-third of patients. Although many treatment options have been attempted—including topical and intralesional corticosteroids, immunomodulators, platelet inhibitors, and phototherapy—efficacy is limited.60
Bullosis Diabeticorum
Bullosis diabeticorum (BD) is the abrupt onset of noninflammatory vesicles and bullae developing in the setting of diabetes. The prevalence of BD in the diabetic population ranges from 0.16% to 0.5%.63-66 Bullosis diabeticorum occasionally has been reported to occur prior to the onset of diabetes, warranting screening hemoglobin A1c in patients without an established diagnosis of diabetes.67 Bullae most commonly present over the acral surfaces, but the lower extremities also are routinely affected. Bullae typically are large and painless, contain clear fluid, and may progress from tense to flaccid over the course of several days. Although histologic analysis reveals nonspecific findings, biopsy may be useful in excluding other bullous disorders. Because BD is a benign condition that spontaneously resolves over several weeks, treatment rarely is pursued.63,64
Generalized Granuloma Annulare
Generalized granuloma annulare (GA) is an idiopathic inflammatory cutaneous disorder characterized by pink-red, arciform and annular, nonscaly, beaded papules and plaques. Granuloma annulare can be localized or generalized with perforating, patch, and palmoplantar variants. Although the pathogenesis is poorly understood, some studies have demonstrated a correlation between GA and type 1 diabetes mellitus.68-71 Generalized GA appears to be most strongly associated with diabetes, and approximately 10% to 15% of cases occur in this population.70,72 Because GA has been reported to precede the diagnosis of diabetes, patients with generalized or recurrent localized GA should be screened for persistent hyperglycemia with a hemoglobin A1c test.71,73 Although some GA is self-resolving, treatment options for persevering GA include topical and intralesional steroids, isotretinoin, dapsone, tacrolimus, antimalarials, biologic medications, and psoralen plus UVA therapy.74
Final Thoughts
Mechanistic links between common cutaneous conditions and persistent hyperglycemic and hyperinsulinemic states are slowly emerging. Hyperglycemia promotes nonenzymatic glycation of the vascular endothelium as well as formation of AGEs that impair cross-linking of collagen in the skin. The consequent microangiopathic damage may lead to cutaneous conditions such as DD, NL, and BD. In addition to microvascular compromise, impaired collagen cross-linking may result in ichthyosiform skin changes and SD. Hyperinsulinemia causes increased circulating levels of IGF-1, which leads to the overactivation of IGF-1 receptors present on fibroblasts and keratinocytes. This aberrant IGF-1 signaling drives cellular hyperproliferation and differentiation, which may be responsible for cutaneous findings such as AN, KP, and/or acrochordons. An insulin-dependent increase in IGF-1 and androgenic signaling may have implications for hormonally driven inflammatory skin disorders such as acne vulgaris and hidradenitis suppurativa, warranting further investigation.
Physicians should be aware of these dermatologic manifestations and their proposed underlying pathophysiologic mechanisms related to impaired glucose tolerance and insulin resistance. A diagnosis of T2DM is not a prerequisite for metabolic disturbance, and the skin may serve as the first clue to underlying systemic disease. Early identification of these cutaneous conditions may lead to timely patient counseling, lifestyle modification, and/or medical management, preventing the long-term sequelae associated with metabolic disorders.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Thomas DD, Corkey BE, Istfan NW, et al. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3:1727-1747.
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
- Holzer G, Straßegger B, Volc-Platzer B. Cutaneous manifestations of metabolic syndrome. Hautarzt. 2016;67:982-988.
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Álvarez-Villalobos NA, Rodríguez-Gutiérrez R, González-Saldivar G, et al. Acanthosis nigricans in middle-age adults: a highly prevalent and specific clinical sign of insulin resistance. Int J Clin Pract. 2020;74:E13453.
- Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance--an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6:43-46.
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79.
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):82S-85S.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
Tuhan H, Ceylaner S, Nalbantoǧlu Ö, et al. A mutation in INSR in a child presenting with severe acanthosis nigricans. J Clin Res Pediatr Endocrinol. 2017;9:371-374.
- Accili D, Barbetti F, Cama A, et al. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance and acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):S77-S81.
- Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094.
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans [published online January 3, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2019.1708855
- Ragunatha S, Anitha B, Inamadar AC, et al. Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-164.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: a subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14:95-97.
- Bustan RS, Wasim D, Yderstræde KB, et al. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state--a systematic review. Dan Med J. 2017;64:A5316.
- McCash S, Emanuel PO. Defining diabetic dermopathy. J Dermatol. 2011;38:988-992.
- Brugler A, Thompson S, Turner S, et al. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Venencie PY, Powell FC, Su WP, et al. Scleredema: a review of thirty-three cases. J Am Acad Dermatol. 1984;11:128-134.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Martín C, Requena L, Manrique K, et al. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273.
- Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259-270.
- Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20:1-5.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916.e912.
- Tran K, Boyd KP, Robinson MR, et al. Scleredema diabeticorum. Dermatol Online J. 2013;19:20718.
- Nakajima K, Iwagaki M, Ikeda M, et al. Two cases of diabetic scleredema that responded to PUVA therapy. J Dermatol. 2006;33:820-822.
- Xiao T, Yang Z-H, He C-D, et al. Scleredema adultorum treated with narrow-band ultraviolet B phototherapy. J Dermatol. 2007;34:270-272.
- Kokpol C, Rajatanavin N, Rattanakemakorn P. Successful treatment of scleredema diabeticorum by combining local PUVA and colchicine: a case report. Case Rep Dermatol. 2012;4:265-268.
- Sanli H, Akay BN, Sen BB, et al. Acquired ichthyosis associated with type 1 diabetes mellitus. Dermatoendocrinol. 2009;1:34-36.
- Patel N, Spencer LA, English JC 3rd, et al. Acquired ichthyosis. J Am Acad Dermatol. 2006;55:647-656.
- Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351-364.
- Shah R, Jindal A, Patel N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. 2014;4:202-205.
- Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-1159.
- Kahana M, Grossman E, Feinstein A, et al. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-177.
- Tamega Ade A, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25-31.
- Senel E, Salmanoǧlu M, Solmazgül E, et al. Acrochordons as a cutaneous sign of impaired carbohydrate metabolism, hyperlipidemia, liver enzyme abnormalities and hypertension: a case-control study [published online December 21, 2011]. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04396.x
- Köseoǧlu HG, Bozca BC, Basşorgun C, et al. The role of insulin-like growth factor in acrochordon etiopathology. BMC Dermatol. 2020;20:14.
- Singh SK, Agrawal NK, Vishwakarma AK. Association of acanthosis nigricans and acrochordon with insulin resistance: a cross-sectional hospital-based study from North India. Indian J Dermatol. 2020;65:112-117.
- Margolis J, Margolis LS. Letter: skin tags--a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- González-Saldivar G, Rodríguez-Gutiérrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther (Heidelb). 2017;7:37-51.
- Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23(6 pt 1):1070-1077.
- Hirt PA, Castillo DE, Yosipovitch G, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037-1057.
- Yosipovitch G, Mevorah B, Mashiach J, et al. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000;201:34-36.
- Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
- Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185:1012-1021.
- Barth JH, Wojnarowska F, Dawber RP. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240-241.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711-713.
- Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review [published online September 14, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1818678
- O'Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatology. 2019;155:455-459.
- Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47:354-358.
- Zhang AJ, Garret M, Miller S. Bullosis diabeticorum: case report and review. N Z Med J. 2013;126:91-94.
- Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum--a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- El Fekih N, Zéglaoui F, Sioud A, et al. Bullosis diabeticorum: report of ten cases. Tunis Med. 2009;87:747-749.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Lopez PR, Leicht S, Sigmon JR, et al. Bullosis diabeticorum associated with a prediabetic state. South Med J. 2009;102:643-644.
- Muhlemann MF, Williams DR. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
- Haim S, Friedman-Birnbaum R, Haim N, et al. Carbohydrate tolerance in patients with granuloma annulare. Br J Dermatol. 1973;88:447-451.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Agrawal P, Pursnani N, Jose R, et al. Granuloma annulare: a rare dermatological manifestation of diabetes mellitus. J Family Med Prim Care. 2019;8:3419-3421.
- Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
- Spicuzza L, Salafia S, Capizzi A, et al. Granuloma annulare as first clinical manifestation of diabetes mellitus in children: a case report. Diabetes Res Clin Pract. 2012;95:E55-E57.
- Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333-344.
Nutritional dermatoses are classically associated with dietary nutrient deficiencies; however, cutaneous disease as a consequence of nutrient excess often is overlooked. Chronic hyperglycemia and hyperinsulinemia resulting from excess carbohydrate intake may be implicated in a number of cutaneous pathologies, of which every dermatologist should be aware.1-3
Although diabetic patients exhibit many cutaneous manifestations of excess carbohydrate consumption, the absence of a diagnosis of type 2 diabetes mellitus (T2DM) does not necessarily preclude them.4-6 Emerging evidence now highlights the development of insulin resistance well before a patient ever meets the diagnostic criteria for T2DM.7,8 Cutaneous disease can provide early insight into a patient’s glucose tolerance and may be the first sign of metabolic derangement. Prompt recognition of these cutaneous alterations and management of the patient’s underlying systemic disease can improve their quality of life and help prevent severe systemic complications associated with insulin resistance and impaired glucose tolerance.
The aim of this review is to highlight both common and rare cutaneous manifestations associated with the persistent consumption of high glycemic load diets, resultant hyperglycemic and hyperinsulinemic states, and the pathophysiologic mechanisms that underlie them.
Acanthosis Nigricans
Acanthosis nigricans (AN) is a highly prevalent cutaneous finding in individuals with insulin resistance that clinically presents as thickened, hyperpigmented, velvety plaques on the intertriginous and flexural surfaces. The most frequently involved sites include the neck, axillae (Figure), and inframammary and inguinal folds. Black and Hispanic patients most commonly are affected. Although classically associated with T2DM, AN also can be observed in normoglycemic individuals.7-9 One recent study reported the rate of AN to be 36% in a cohort of middle-aged patients (N=320) with normal fasting blood glucose levels, while the rate of AN in matched patients with hyperglycemia (prediabetes and T2DM) was approximately 50%.7 Quantification of insulin resistance was performed using the homeostatic model assessment of insulin resistance index. Interestingly, the specificity for insulin resistance in normoglycemic and hyperglycemic subjects with AN was 85% and 90%, respectively.7 These findings suggest that AN may serve as a convenient surrogate marker for subclinical insulin resistance, a conclusion that has been reported in a series of previous studies.8-10

Although the pathogenesis of AN has not been fully elucidated, it is known that persistently elevated blood glucose triggers continual secretion of insulin and insulinlike growth factor 1 (IGF-1), which results in the overstimulation of insulin and IGF-1 receptors on keratinocytes and dermal fibroblasts through direct and indirect pathways.11,12 The resultant cellular proliferation can be observed histologically in the forms of orthokeratotic hyperkeratosis and papillomatosis, as occurs in AN.11,13 Further supporting the association between elevated insulin and AN are reports of AN developing at sites of repeated insulin injection as well as genetic mutations in the insulin receptor resulting in severe AN in children.14-16
The treatment of AN ultimately focuses on improving glycemic control and reducing insulin resistance through lifestyle modification and pharmacotherapy with agents such as metformin.11,13 Dermatologic treatment with oral and topical keratolytic agents such as isotretinoin and other retinoids, salicylic acid, urea, or ammonium lactate may be used, but their efficacy generally has been limited.11,13,17,18
Diabetic Dermopathy
Diabetic dermopathy (DD), commonly known as shin spots, refers to the red-brown, atrophic, circinate macules and patches that often appear on the lower extremities in patients with T2DM. Although the pretibial area is the most frequently involved site, other areas of bony prominence such as the forearms can be affected. The prevalence of DD in the diabetic population can be exceedingly high, with some studies reporting incidence rates greater than 50%, particularly in those with poorly controlled T2DM.19-21 Interestingly, DD also has been documented in patients without T2DM and has been postulated to be an early sign of insulin resistance.20,22
The pathogenesis of DD remains uncertain, but one proposed mechanism is through microvascular damage caused by hyperglycemia-induced, nonenzymatic glycation, possibly in conjunction with mild trauma, that leads to the deposition of hemosiderin and melanin in the skin.20,23 A recent study identified increased vascularization of dermopathy lesions when compared with surrounding tissue.24 Subcutaneous nerve ischemia and degeneration secondary to diabetic neuropathy also have been postulated as causative.20,23 Given the lack of effective therapies and the asymptomatic nature of DD, treatment typically is not pursued. However, DD is associated with other diabetic microvascular complications, including diabetic nephropathy, retinopathy, and neuropathy. For this reason, identification of DD warrants further characterization and management of a patient’s underlying diabetes.19,20
Scleredema Diabeticorum
Scleredema diabeticorum (SD) refers to the slowly progressive, painless thickening and woody induration of the neck, shoulders, and upper back in individuals with long-standing, poorly controlled diabetes. The condition is almost exclusively seen in the diabetic population, with prevalence rates reported to be as high as 14%.25-27 Although SD generally is asymptomatic, some individuals may experience restricted mobility and decreased sensation in affected areas.25,27,28 The diagnosis of SD frequently is missed or ignored clinically. Biopsy can provide diagnostic confirmation of this entity, as histopathology reveals a thickened reticular dermis with an accumulation of collagen and adjacent mucinous infiltrate with no edema or sclerosis.28,29
Although the pathogenesis of SD is not well established, it is theorized that the binding of advanced glycation end products (AGEs) to collagen fibers impairs proper cross-linking and degradation by collagenase.29-31 It is well known that hyperglycemic conditions can promote endogenous formation of AGEs, which occur when reducing sugar molecules become glycated through a nonenzymatic reaction.30-32 The Western diet also is high in preformed AGEs, which are created primarily through certain high-heat cooking methods such as frying and grilling.31,32 Hyperglycemia-induced stimulation of fibroblasts also has been proposed as a driver of increased collagen deposition observed histologically in SD.28,29,33 Treatment of SD can be difficult, as there are no consistently reported therapies, and even improvement in glycemic control does not appear to reverse this condition.29 Case reports have demonstrated some efficacy with various phototherapeutic modalities, including psoralen plus UVA and narrowband UVB phototherapy.34-36
Ichthyosiform Skin Changes
Ichthyosiform skin changes refer to areas of xerosis and scaling that classically present on the anterior distal lower extremities. Although ichthyosiform alterations have been associated with numerous systemic diseases, they often represent an early finding in diabetic patients.27,37 The development of ichthyosiform skin changes has been linked to the formation and accumulation of AGEs, which can cause defective cell adhesion in the stratum corneum.37,38 Treatment with topical emollients and keratolytics may prove beneficial for the skin but do not improve the underlying systemic condition.39
Acrochordons
Acrochordons (skin tags) are common benign fibroepithelial polyps that classically present on the face, neck, and trunk. The underlying mechanism responsible for the development of acrochordons is uncertain, but the association with insulin resistance and impaired carbohydrate metabolism is well validated.40-46 Several large cross-sectional and case-control studies have reported rates of T2DM ranging from 23% to 72% in patients with acrochordons.41,42,47 The pathophysiology may involve an increase in tissue and epidermal growth factors driven by elevated serum insulin levels, stimulation of IGF-1 receptors, and a localized proliferation of cutaneous tissue in elastin-poor areas.45,48,49 Interestingly, the quantity of acrochordons has been positively correlated with fasting blood glucose levels. Additionally, the presence of 30 or more acrochordons was found to increase the risk of developing T2DM.41 Therefore, the presence and number of acrochordons may serve as a convenient indicator of systemic glycemic control and insulin resistance. Screening for T2DM is warranted in individuals without a prior diagnosis who present with multiple acrochordons.
Keratosis Pilaris
Keratosis pilaris (KP) is a benign skin condition characterized by pink-red, erythematous, monomorphic, follicular papules often seen on the extensor arms, thighs, buttocks, and cheeks. Keratosis pilaris is exceedingly common in the general population but occurs more frequently and with more extensive involvement in those with atopic dermatitis and T2DM.27,50,51 The mechanism underlying the hyperkeratosis and inflammatory change observed in KP is not well understood and is likely multifactorial.52,53 Hyperandrogenism, as a consequence of hyperinsulinemia, may play an important role in KP, as elevated circulating androgens are known drivers of keratinocyte proliferation of the pilosebaceous unit of hair follicles.52,54 Support for this theory includes the clinical exaggeration of KP frequently encountered around puberty when androgen levels peak.55,56 Moreover, one study found a higher incidence of KP among adolescent patients with type 1 diabetes mellitus than among healthy age-matched controls.27 The most effective treatment of KP appears to be laser therapy, particularly the Q-switched Nd:YAG laser. Numerous topical modalities have been employed to treat KP but exhibit limited efficacy, including mineral oil, tacrolimus, azelaic acid, and salicylic acid, among others.57
Necrobiosis Lipoidica
Necrobiosis lipoidica (NL) is a chronic granulomatous skin condition of unknown origin that presents with well-demarcated, yellow-brown, atrophic patches and plaques often found exclusively on the shins. There is a strong association with type 1 diabetes mellitus, with reported rates ranging from 11% to 65% in patients with NL.58-60 In a recent retrospective study of 236 patients with NL, 58.5% of patients had diabetes.61 Nevertheless, NL is a rare entity that affects less than 1% of the diabetic population.60 Given its correlation with diabetes, it has been postulated that the pathogenesis of NL is due to microvascular ischemic changes resulting from prolonged hyperglycemia.60 However, studies revealing an increase in blood flow to NL lesions suggest that the condition may instead be attributed to an inflammatory process.62 Despite the disfiguring appearance, the lesions of NL often are asymptomatic. Pain or pruritus may develop secondary to ulceration, which occurs in approximately one-third of patients. Although many treatment options have been attempted—including topical and intralesional corticosteroids, immunomodulators, platelet inhibitors, and phototherapy—efficacy is limited.60
Bullosis Diabeticorum
Bullosis diabeticorum (BD) is the abrupt onset of noninflammatory vesicles and bullae developing in the setting of diabetes. The prevalence of BD in the diabetic population ranges from 0.16% to 0.5%.63-66 Bullosis diabeticorum occasionally has been reported to occur prior to the onset of diabetes, warranting screening hemoglobin A1c in patients without an established diagnosis of diabetes.67 Bullae most commonly present over the acral surfaces, but the lower extremities also are routinely affected. Bullae typically are large and painless, contain clear fluid, and may progress from tense to flaccid over the course of several days. Although histologic analysis reveals nonspecific findings, biopsy may be useful in excluding other bullous disorders. Because BD is a benign condition that spontaneously resolves over several weeks, treatment rarely is pursued.63,64
Generalized Granuloma Annulare
Generalized granuloma annulare (GA) is an idiopathic inflammatory cutaneous disorder characterized by pink-red, arciform and annular, nonscaly, beaded papules and plaques. Granuloma annulare can be localized or generalized with perforating, patch, and palmoplantar variants. Although the pathogenesis is poorly understood, some studies have demonstrated a correlation between GA and type 1 diabetes mellitus.68-71 Generalized GA appears to be most strongly associated with diabetes, and approximately 10% to 15% of cases occur in this population.70,72 Because GA has been reported to precede the diagnosis of diabetes, patients with generalized or recurrent localized GA should be screened for persistent hyperglycemia with a hemoglobin A1c test.71,73 Although some GA is self-resolving, treatment options for persevering GA include topical and intralesional steroids, isotretinoin, dapsone, tacrolimus, antimalarials, biologic medications, and psoralen plus UVA therapy.74
Final Thoughts
Mechanistic links between common cutaneous conditions and persistent hyperglycemic and hyperinsulinemic states are slowly emerging. Hyperglycemia promotes nonenzymatic glycation of the vascular endothelium as well as formation of AGEs that impair cross-linking of collagen in the skin. The consequent microangiopathic damage may lead to cutaneous conditions such as DD, NL, and BD. In addition to microvascular compromise, impaired collagen cross-linking may result in ichthyosiform skin changes and SD. Hyperinsulinemia causes increased circulating levels of IGF-1, which leads to the overactivation of IGF-1 receptors present on fibroblasts and keratinocytes. This aberrant IGF-1 signaling drives cellular hyperproliferation and differentiation, which may be responsible for cutaneous findings such as AN, KP, and/or acrochordons. An insulin-dependent increase in IGF-1 and androgenic signaling may have implications for hormonally driven inflammatory skin disorders such as acne vulgaris and hidradenitis suppurativa, warranting further investigation.
Physicians should be aware of these dermatologic manifestations and their proposed underlying pathophysiologic mechanisms related to impaired glucose tolerance and insulin resistance. A diagnosis of T2DM is not a prerequisite for metabolic disturbance, and the skin may serve as the first clue to underlying systemic disease. Early identification of these cutaneous conditions may lead to timely patient counseling, lifestyle modification, and/or medical management, preventing the long-term sequelae associated with metabolic disorders.
Nutritional dermatoses are classically associated with dietary nutrient deficiencies; however, cutaneous disease as a consequence of nutrient excess often is overlooked. Chronic hyperglycemia and hyperinsulinemia resulting from excess carbohydrate intake may be implicated in a number of cutaneous pathologies, of which every dermatologist should be aware.1-3
Although diabetic patients exhibit many cutaneous manifestations of excess carbohydrate consumption, the absence of a diagnosis of type 2 diabetes mellitus (T2DM) does not necessarily preclude them.4-6 Emerging evidence now highlights the development of insulin resistance well before a patient ever meets the diagnostic criteria for T2DM.7,8 Cutaneous disease can provide early insight into a patient’s glucose tolerance and may be the first sign of metabolic derangement. Prompt recognition of these cutaneous alterations and management of the patient’s underlying systemic disease can improve their quality of life and help prevent severe systemic complications associated with insulin resistance and impaired glucose tolerance.
The aim of this review is to highlight both common and rare cutaneous manifestations associated with the persistent consumption of high glycemic load diets, resultant hyperglycemic and hyperinsulinemic states, and the pathophysiologic mechanisms that underlie them.
Acanthosis Nigricans
Acanthosis nigricans (AN) is a highly prevalent cutaneous finding in individuals with insulin resistance that clinically presents as thickened, hyperpigmented, velvety plaques on the intertriginous and flexural surfaces. The most frequently involved sites include the neck, axillae (Figure), and inframammary and inguinal folds. Black and Hispanic patients most commonly are affected. Although classically associated with T2DM, AN also can be observed in normoglycemic individuals.7-9 One recent study reported the rate of AN to be 36% in a cohort of middle-aged patients (N=320) with normal fasting blood glucose levels, while the rate of AN in matched patients with hyperglycemia (prediabetes and T2DM) was approximately 50%.7 Quantification of insulin resistance was performed using the homeostatic model assessment of insulin resistance index. Interestingly, the specificity for insulin resistance in normoglycemic and hyperglycemic subjects with AN was 85% and 90%, respectively.7 These findings suggest that AN may serve as a convenient surrogate marker for subclinical insulin resistance, a conclusion that has been reported in a series of previous studies.8-10

Although the pathogenesis of AN has not been fully elucidated, it is known that persistently elevated blood glucose triggers continual secretion of insulin and insulinlike growth factor 1 (IGF-1), which results in the overstimulation of insulin and IGF-1 receptors on keratinocytes and dermal fibroblasts through direct and indirect pathways.11,12 The resultant cellular proliferation can be observed histologically in the forms of orthokeratotic hyperkeratosis and papillomatosis, as occurs in AN.11,13 Further supporting the association between elevated insulin and AN are reports of AN developing at sites of repeated insulin injection as well as genetic mutations in the insulin receptor resulting in severe AN in children.14-16
The treatment of AN ultimately focuses on improving glycemic control and reducing insulin resistance through lifestyle modification and pharmacotherapy with agents such as metformin.11,13 Dermatologic treatment with oral and topical keratolytic agents such as isotretinoin and other retinoids, salicylic acid, urea, or ammonium lactate may be used, but their efficacy generally has been limited.11,13,17,18
Diabetic Dermopathy
Diabetic dermopathy (DD), commonly known as shin spots, refers to the red-brown, atrophic, circinate macules and patches that often appear on the lower extremities in patients with T2DM. Although the pretibial area is the most frequently involved site, other areas of bony prominence such as the forearms can be affected. The prevalence of DD in the diabetic population can be exceedingly high, with some studies reporting incidence rates greater than 50%, particularly in those with poorly controlled T2DM.19-21 Interestingly, DD also has been documented in patients without T2DM and has been postulated to be an early sign of insulin resistance.20,22
The pathogenesis of DD remains uncertain, but one proposed mechanism is through microvascular damage caused by hyperglycemia-induced, nonenzymatic glycation, possibly in conjunction with mild trauma, that leads to the deposition of hemosiderin and melanin in the skin.20,23 A recent study identified increased vascularization of dermopathy lesions when compared with surrounding tissue.24 Subcutaneous nerve ischemia and degeneration secondary to diabetic neuropathy also have been postulated as causative.20,23 Given the lack of effective therapies and the asymptomatic nature of DD, treatment typically is not pursued. However, DD is associated with other diabetic microvascular complications, including diabetic nephropathy, retinopathy, and neuropathy. For this reason, identification of DD warrants further characterization and management of a patient’s underlying diabetes.19,20
Scleredema Diabeticorum
Scleredema diabeticorum (SD) refers to the slowly progressive, painless thickening and woody induration of the neck, shoulders, and upper back in individuals with long-standing, poorly controlled diabetes. The condition is almost exclusively seen in the diabetic population, with prevalence rates reported to be as high as 14%.25-27 Although SD generally is asymptomatic, some individuals may experience restricted mobility and decreased sensation in affected areas.25,27,28 The diagnosis of SD frequently is missed or ignored clinically. Biopsy can provide diagnostic confirmation of this entity, as histopathology reveals a thickened reticular dermis with an accumulation of collagen and adjacent mucinous infiltrate with no edema or sclerosis.28,29
Although the pathogenesis of SD is not well established, it is theorized that the binding of advanced glycation end products (AGEs) to collagen fibers impairs proper cross-linking and degradation by collagenase.29-31 It is well known that hyperglycemic conditions can promote endogenous formation of AGEs, which occur when reducing sugar molecules become glycated through a nonenzymatic reaction.30-32 The Western diet also is high in preformed AGEs, which are created primarily through certain high-heat cooking methods such as frying and grilling.31,32 Hyperglycemia-induced stimulation of fibroblasts also has been proposed as a driver of increased collagen deposition observed histologically in SD.28,29,33 Treatment of SD can be difficult, as there are no consistently reported therapies, and even improvement in glycemic control does not appear to reverse this condition.29 Case reports have demonstrated some efficacy with various phototherapeutic modalities, including psoralen plus UVA and narrowband UVB phototherapy.34-36
Ichthyosiform Skin Changes
Ichthyosiform skin changes refer to areas of xerosis and scaling that classically present on the anterior distal lower extremities. Although ichthyosiform alterations have been associated with numerous systemic diseases, they often represent an early finding in diabetic patients.27,37 The development of ichthyosiform skin changes has been linked to the formation and accumulation of AGEs, which can cause defective cell adhesion in the stratum corneum.37,38 Treatment with topical emollients and keratolytics may prove beneficial for the skin but do not improve the underlying systemic condition.39
Acrochordons
Acrochordons (skin tags) are common benign fibroepithelial polyps that classically present on the face, neck, and trunk. The underlying mechanism responsible for the development of acrochordons is uncertain, but the association with insulin resistance and impaired carbohydrate metabolism is well validated.40-46 Several large cross-sectional and case-control studies have reported rates of T2DM ranging from 23% to 72% in patients with acrochordons.41,42,47 The pathophysiology may involve an increase in tissue and epidermal growth factors driven by elevated serum insulin levels, stimulation of IGF-1 receptors, and a localized proliferation of cutaneous tissue in elastin-poor areas.45,48,49 Interestingly, the quantity of acrochordons has been positively correlated with fasting blood glucose levels. Additionally, the presence of 30 or more acrochordons was found to increase the risk of developing T2DM.41 Therefore, the presence and number of acrochordons may serve as a convenient indicator of systemic glycemic control and insulin resistance. Screening for T2DM is warranted in individuals without a prior diagnosis who present with multiple acrochordons.
Keratosis Pilaris
Keratosis pilaris (KP) is a benign skin condition characterized by pink-red, erythematous, monomorphic, follicular papules often seen on the extensor arms, thighs, buttocks, and cheeks. Keratosis pilaris is exceedingly common in the general population but occurs more frequently and with more extensive involvement in those with atopic dermatitis and T2DM.27,50,51 The mechanism underlying the hyperkeratosis and inflammatory change observed in KP is not well understood and is likely multifactorial.52,53 Hyperandrogenism, as a consequence of hyperinsulinemia, may play an important role in KP, as elevated circulating androgens are known drivers of keratinocyte proliferation of the pilosebaceous unit of hair follicles.52,54 Support for this theory includes the clinical exaggeration of KP frequently encountered around puberty when androgen levels peak.55,56 Moreover, one study found a higher incidence of KP among adolescent patients with type 1 diabetes mellitus than among healthy age-matched controls.27 The most effective treatment of KP appears to be laser therapy, particularly the Q-switched Nd:YAG laser. Numerous topical modalities have been employed to treat KP but exhibit limited efficacy, including mineral oil, tacrolimus, azelaic acid, and salicylic acid, among others.57
Necrobiosis Lipoidica
Necrobiosis lipoidica (NL) is a chronic granulomatous skin condition of unknown origin that presents with well-demarcated, yellow-brown, atrophic patches and plaques often found exclusively on the shins. There is a strong association with type 1 diabetes mellitus, with reported rates ranging from 11% to 65% in patients with NL.58-60 In a recent retrospective study of 236 patients with NL, 58.5% of patients had diabetes.61 Nevertheless, NL is a rare entity that affects less than 1% of the diabetic population.60 Given its correlation with diabetes, it has been postulated that the pathogenesis of NL is due to microvascular ischemic changes resulting from prolonged hyperglycemia.60 However, studies revealing an increase in blood flow to NL lesions suggest that the condition may instead be attributed to an inflammatory process.62 Despite the disfiguring appearance, the lesions of NL often are asymptomatic. Pain or pruritus may develop secondary to ulceration, which occurs in approximately one-third of patients. Although many treatment options have been attempted—including topical and intralesional corticosteroids, immunomodulators, platelet inhibitors, and phototherapy—efficacy is limited.60
Bullosis Diabeticorum
Bullosis diabeticorum (BD) is the abrupt onset of noninflammatory vesicles and bullae developing in the setting of diabetes. The prevalence of BD in the diabetic population ranges from 0.16% to 0.5%.63-66 Bullosis diabeticorum occasionally has been reported to occur prior to the onset of diabetes, warranting screening hemoglobin A1c in patients without an established diagnosis of diabetes.67 Bullae most commonly present over the acral surfaces, but the lower extremities also are routinely affected. Bullae typically are large and painless, contain clear fluid, and may progress from tense to flaccid over the course of several days. Although histologic analysis reveals nonspecific findings, biopsy may be useful in excluding other bullous disorders. Because BD is a benign condition that spontaneously resolves over several weeks, treatment rarely is pursued.63,64
Generalized Granuloma Annulare
Generalized granuloma annulare (GA) is an idiopathic inflammatory cutaneous disorder characterized by pink-red, arciform and annular, nonscaly, beaded papules and plaques. Granuloma annulare can be localized or generalized with perforating, patch, and palmoplantar variants. Although the pathogenesis is poorly understood, some studies have demonstrated a correlation between GA and type 1 diabetes mellitus.68-71 Generalized GA appears to be most strongly associated with diabetes, and approximately 10% to 15% of cases occur in this population.70,72 Because GA has been reported to precede the diagnosis of diabetes, patients with generalized or recurrent localized GA should be screened for persistent hyperglycemia with a hemoglobin A1c test.71,73 Although some GA is self-resolving, treatment options for persevering GA include topical and intralesional steroids, isotretinoin, dapsone, tacrolimus, antimalarials, biologic medications, and psoralen plus UVA therapy.74
Final Thoughts
Mechanistic links between common cutaneous conditions and persistent hyperglycemic and hyperinsulinemic states are slowly emerging. Hyperglycemia promotes nonenzymatic glycation of the vascular endothelium as well as formation of AGEs that impair cross-linking of collagen in the skin. The consequent microangiopathic damage may lead to cutaneous conditions such as DD, NL, and BD. In addition to microvascular compromise, impaired collagen cross-linking may result in ichthyosiform skin changes and SD. Hyperinsulinemia causes increased circulating levels of IGF-1, which leads to the overactivation of IGF-1 receptors present on fibroblasts and keratinocytes. This aberrant IGF-1 signaling drives cellular hyperproliferation and differentiation, which may be responsible for cutaneous findings such as AN, KP, and/or acrochordons. An insulin-dependent increase in IGF-1 and androgenic signaling may have implications for hormonally driven inflammatory skin disorders such as acne vulgaris and hidradenitis suppurativa, warranting further investigation.
Physicians should be aware of these dermatologic manifestations and their proposed underlying pathophysiologic mechanisms related to impaired glucose tolerance and insulin resistance. A diagnosis of T2DM is not a prerequisite for metabolic disturbance, and the skin may serve as the first clue to underlying systemic disease. Early identification of these cutaneous conditions may lead to timely patient counseling, lifestyle modification, and/or medical management, preventing the long-term sequelae associated with metabolic disorders.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Thomas DD, Corkey BE, Istfan NW, et al. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3:1727-1747.
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
- Holzer G, Straßegger B, Volc-Platzer B. Cutaneous manifestations of metabolic syndrome. Hautarzt. 2016;67:982-988.
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Álvarez-Villalobos NA, Rodríguez-Gutiérrez R, González-Saldivar G, et al. Acanthosis nigricans in middle-age adults: a highly prevalent and specific clinical sign of insulin resistance. Int J Clin Pract. 2020;74:E13453.
- Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance--an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6:43-46.
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79.
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):82S-85S.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
Tuhan H, Ceylaner S, Nalbantoǧlu Ö, et al. A mutation in INSR in a child presenting with severe acanthosis nigricans. J Clin Res Pediatr Endocrinol. 2017;9:371-374.
- Accili D, Barbetti F, Cama A, et al. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance and acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):S77-S81.
- Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094.
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans [published online January 3, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2019.1708855
- Ragunatha S, Anitha B, Inamadar AC, et al. Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-164.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: a subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14:95-97.
- Bustan RS, Wasim D, Yderstræde KB, et al. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state--a systematic review. Dan Med J. 2017;64:A5316.
- McCash S, Emanuel PO. Defining diabetic dermopathy. J Dermatol. 2011;38:988-992.
- Brugler A, Thompson S, Turner S, et al. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Venencie PY, Powell FC, Su WP, et al. Scleredema: a review of thirty-three cases. J Am Acad Dermatol. 1984;11:128-134.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Martín C, Requena L, Manrique K, et al. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273.
- Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259-270.
- Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20:1-5.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916.e912.
- Tran K, Boyd KP, Robinson MR, et al. Scleredema diabeticorum. Dermatol Online J. 2013;19:20718.
- Nakajima K, Iwagaki M, Ikeda M, et al. Two cases of diabetic scleredema that responded to PUVA therapy. J Dermatol. 2006;33:820-822.
- Xiao T, Yang Z-H, He C-D, et al. Scleredema adultorum treated with narrow-band ultraviolet B phototherapy. J Dermatol. 2007;34:270-272.
- Kokpol C, Rajatanavin N, Rattanakemakorn P. Successful treatment of scleredema diabeticorum by combining local PUVA and colchicine: a case report. Case Rep Dermatol. 2012;4:265-268.
- Sanli H, Akay BN, Sen BB, et al. Acquired ichthyosis associated with type 1 diabetes mellitus. Dermatoendocrinol. 2009;1:34-36.
- Patel N, Spencer LA, English JC 3rd, et al. Acquired ichthyosis. J Am Acad Dermatol. 2006;55:647-656.
- Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351-364.
- Shah R, Jindal A, Patel N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. 2014;4:202-205.
- Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-1159.
- Kahana M, Grossman E, Feinstein A, et al. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-177.
- Tamega Ade A, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25-31.
- Senel E, Salmanoǧlu M, Solmazgül E, et al. Acrochordons as a cutaneous sign of impaired carbohydrate metabolism, hyperlipidemia, liver enzyme abnormalities and hypertension: a case-control study [published online December 21, 2011]. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04396.x
- Köseoǧlu HG, Bozca BC, Basşorgun C, et al. The role of insulin-like growth factor in acrochordon etiopathology. BMC Dermatol. 2020;20:14.
- Singh SK, Agrawal NK, Vishwakarma AK. Association of acanthosis nigricans and acrochordon with insulin resistance: a cross-sectional hospital-based study from North India. Indian J Dermatol. 2020;65:112-117.
- Margolis J, Margolis LS. Letter: skin tags--a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- González-Saldivar G, Rodríguez-Gutiérrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther (Heidelb). 2017;7:37-51.
- Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23(6 pt 1):1070-1077.
- Hirt PA, Castillo DE, Yosipovitch G, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037-1057.
- Yosipovitch G, Mevorah B, Mashiach J, et al. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000;201:34-36.
- Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
- Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185:1012-1021.
- Barth JH, Wojnarowska F, Dawber RP. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240-241.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711-713.
- Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review [published online September 14, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1818678
- O'Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatology. 2019;155:455-459.
- Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47:354-358.
- Zhang AJ, Garret M, Miller S. Bullosis diabeticorum: case report and review. N Z Med J. 2013;126:91-94.
- Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum--a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- El Fekih N, Zéglaoui F, Sioud A, et al. Bullosis diabeticorum: report of ten cases. Tunis Med. 2009;87:747-749.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Lopez PR, Leicht S, Sigmon JR, et al. Bullosis diabeticorum associated with a prediabetic state. South Med J. 2009;102:643-644.
- Muhlemann MF, Williams DR. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
- Haim S, Friedman-Birnbaum R, Haim N, et al. Carbohydrate tolerance in patients with granuloma annulare. Br J Dermatol. 1973;88:447-451.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Agrawal P, Pursnani N, Jose R, et al. Granuloma annulare: a rare dermatological manifestation of diabetes mellitus. J Family Med Prim Care. 2019;8:3419-3421.
- Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
- Spicuzza L, Salafia S, Capizzi A, et al. Granuloma annulare as first clinical manifestation of diabetes mellitus in children: a case report. Diabetes Res Clin Pract. 2012;95:E55-E57.
- Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333-344.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Thomas DD, Corkey BE, Istfan NW, et al. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3:1727-1747.
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
- Holzer G, Straßegger B, Volc-Platzer B. Cutaneous manifestations of metabolic syndrome. Hautarzt. 2016;67:982-988.
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Álvarez-Villalobos NA, Rodríguez-Gutiérrez R, González-Saldivar G, et al. Acanthosis nigricans in middle-age adults: a highly prevalent and specific clinical sign of insulin resistance. Int J Clin Pract. 2020;74:E13453.
- Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance--an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6:43-46.
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79.
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):82S-85S.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
Tuhan H, Ceylaner S, Nalbantoǧlu Ö, et al. A mutation in INSR in a child presenting with severe acanthosis nigricans. J Clin Res Pediatr Endocrinol. 2017;9:371-374.
- Accili D, Barbetti F, Cama A, et al. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance and acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):S77-S81.
- Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094.
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans [published online January 3, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2019.1708855
- Ragunatha S, Anitha B, Inamadar AC, et al. Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-164.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: a subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14:95-97.
- Bustan RS, Wasim D, Yderstræde KB, et al. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state--a systematic review. Dan Med J. 2017;64:A5316.
- McCash S, Emanuel PO. Defining diabetic dermopathy. J Dermatol. 2011;38:988-992.
- Brugler A, Thompson S, Turner S, et al. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Venencie PY, Powell FC, Su WP, et al. Scleredema: a review of thirty-three cases. J Am Acad Dermatol. 1984;11:128-134.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Martín C, Requena L, Manrique K, et al. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273.
- Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259-270.
- Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20:1-5.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916.e912.
- Tran K, Boyd KP, Robinson MR, et al. Scleredema diabeticorum. Dermatol Online J. 2013;19:20718.
- Nakajima K, Iwagaki M, Ikeda M, et al. Two cases of diabetic scleredema that responded to PUVA therapy. J Dermatol. 2006;33:820-822.
- Xiao T, Yang Z-H, He C-D, et al. Scleredema adultorum treated with narrow-band ultraviolet B phototherapy. J Dermatol. 2007;34:270-272.
- Kokpol C, Rajatanavin N, Rattanakemakorn P. Successful treatment of scleredema diabeticorum by combining local PUVA and colchicine: a case report. Case Rep Dermatol. 2012;4:265-268.
- Sanli H, Akay BN, Sen BB, et al. Acquired ichthyosis associated with type 1 diabetes mellitus. Dermatoendocrinol. 2009;1:34-36.
- Patel N, Spencer LA, English JC 3rd, et al. Acquired ichthyosis. J Am Acad Dermatol. 2006;55:647-656.
- Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351-364.
- Shah R, Jindal A, Patel N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. 2014;4:202-205.
- Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-1159.
- Kahana M, Grossman E, Feinstein A, et al. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-177.
- Tamega Ade A, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25-31.
- Senel E, Salmanoǧlu M, Solmazgül E, et al. Acrochordons as a cutaneous sign of impaired carbohydrate metabolism, hyperlipidemia, liver enzyme abnormalities and hypertension: a case-control study [published online December 21, 2011]. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04396.x
- Köseoǧlu HG, Bozca BC, Basşorgun C, et al. The role of insulin-like growth factor in acrochordon etiopathology. BMC Dermatol. 2020;20:14.
- Singh SK, Agrawal NK, Vishwakarma AK. Association of acanthosis nigricans and acrochordon with insulin resistance: a cross-sectional hospital-based study from North India. Indian J Dermatol. 2020;65:112-117.
- Margolis J, Margolis LS. Letter: skin tags--a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- González-Saldivar G, Rodríguez-Gutiérrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther (Heidelb). 2017;7:37-51.
- Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23(6 pt 1):1070-1077.
- Hirt PA, Castillo DE, Yosipovitch G, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037-1057.
- Yosipovitch G, Mevorah B, Mashiach J, et al. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000;201:34-36.
- Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
- Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185:1012-1021.
- Barth JH, Wojnarowska F, Dawber RP. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240-241.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711-713.
- Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review [published online September 14, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1818678
- O'Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatology. 2019;155:455-459.
- Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47:354-358.
- Zhang AJ, Garret M, Miller S. Bullosis diabeticorum: case report and review. N Z Med J. 2013;126:91-94.
- Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum--a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- El Fekih N, Zéglaoui F, Sioud A, et al. Bullosis diabeticorum: report of ten cases. Tunis Med. 2009;87:747-749.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Lopez PR, Leicht S, Sigmon JR, et al. Bullosis diabeticorum associated with a prediabetic state. South Med J. 2009;102:643-644.
- Muhlemann MF, Williams DR. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
- Haim S, Friedman-Birnbaum R, Haim N, et al. Carbohydrate tolerance in patients with granuloma annulare. Br J Dermatol. 1973;88:447-451.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Agrawal P, Pursnani N, Jose R, et al. Granuloma annulare: a rare dermatological manifestation of diabetes mellitus. J Family Med Prim Care. 2019;8:3419-3421.
- Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
- Spicuzza L, Salafia S, Capizzi A, et al. Granuloma annulare as first clinical manifestation of diabetes mellitus in children: a case report. Diabetes Res Clin Pract. 2012;95:E55-E57.
- Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333-344.
Practice Points
- Dermatologists should be aware of common cutaneous conditions associated with chronic hyperglycemia and hyperinsulinemia, such as acanthosis nigricans, diabetic dermopathy, scleredema diabeticorum, ichthyosiform skin changes, acrochordons, and keratosis pilaris.
- More rare cutaneous pathologies related to chronically elevated blood glucose and/or insulin levels include necrobiosis lipoidica, bullosis diabeticorum, and generalized granuloma annulare.
- The cutaneous manifestations of persistent hyperglycemia and hyperinsulinemia may precede a formal diagnosis of diabetes mellitus and may be the first signs of metabolic derangement.
- Early recognition and management of these cutaneous conditions can help maximize patient quality of life and avoid long-term sequelae associated with insulin resistance and prolonged hyperglycemia.
Reimbursement for Teledermatology During the COVID-19 Public Health Emergency: Change Has Come, But Will It Stay?
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
Immunoabsorption shows promise as an adjunct treatment for high-risk acquired hemophilia
Despite the high mortality rate for acquired hemophilia and the availability of suggested drug treatments, there are no randomized, controlled studies to inform doctors of the best therapies for their patients.
Immunoabsorption therapy (IA) is one such treatment that has been proposed as valid because of its ability to remove factor VIII clotting inhibitors from the bloodstream, but the data on its effectiveness are limited, according to Michael Esteves Pereira, of the Bern (Switzerland) University Hospital and the University of Bern, and colleagues.
In order to help answer the question of the benefits of IA for treating acquired hemophilia, the researchers performed a retrospective study assessing observational data as well as a systemic review and meta-analysis of published literature. They found evidence that the therapy was effective, but suggest that more confirmatory studies are needed, according to their report published online in Transfusion Medicine Reviews.
Data from the authors’ institution were available for 12 patients with acquired hemophilia treated since 2002. The median age was 76 years and four patients were women. The bleeding phenotype was extensive bruising and/or muscle hematomas in nine patients, gastrointestinal bleeding in two patients, and extensive bleeding after tooth extraction in one patient. Their data were added to the 10 published studies included in the literature review, resulting in a total of 118 patients.
Promising results
The author’s single institution analysis showed that IA treatment stopped bleeding in nine patients, while three patients did not respond. At 3 months, the median factor VIII increased to 80 IU/dL (considered complete remission) and the median inhibitor titer decreased to 0.15 BU/mL.
The pooled proportion of the meta-analysis patients treated with IA who achieved factor VIII recovery defined as complete remission was 86% (95% confidence interval, 76%-94%). The pooled proportion of patients with a reduction of the inhibitor titer was 95% (95% CI, 83%-100%), while the pooled mortality was 7% (95% CI, 0%-18%). Sensitivity analyses did not reveal any significant differences in retrospective studies or in studies using different absorbing agents.
In addition, there were few reported side effects, most of which were considered mild, according to the researchers. These included nausea and vomiting, paresthesia, and mild hypotension. The authors did suggest that, as a central venous catheter is often used, patients were exposed to an added risk of bleeding and infection.
“At our institution, IA is considered on a case-by-case base rather than a strict cutoff level. Strong arguments are life-threatening bleeding complications, inhibitor titers 20 BU/mL or greater, or failed immunosuppressive treatment with corticosteroids and cyclophosphamide using an established dose regimen,” the researchers stated.
“Even though firm evidence is still lacking and the actual ‘added value’ of IA cannot be adequately assessed, we believe that IA might be a beneficial adjunctive treatment modality in some patients with acquired hemophilia. It was associated with a complete remission in the majority of patients, most of whom are at high risk of bleeding,” the researchers added.
The authors reported that they had no relevant disclosures.
Despite the high mortality rate for acquired hemophilia and the availability of suggested drug treatments, there are no randomized, controlled studies to inform doctors of the best therapies for their patients.
Immunoabsorption therapy (IA) is one such treatment that has been proposed as valid because of its ability to remove factor VIII clotting inhibitors from the bloodstream, but the data on its effectiveness are limited, according to Michael Esteves Pereira, of the Bern (Switzerland) University Hospital and the University of Bern, and colleagues.
In order to help answer the question of the benefits of IA for treating acquired hemophilia, the researchers performed a retrospective study assessing observational data as well as a systemic review and meta-analysis of published literature. They found evidence that the therapy was effective, but suggest that more confirmatory studies are needed, according to their report published online in Transfusion Medicine Reviews.
Data from the authors’ institution were available for 12 patients with acquired hemophilia treated since 2002. The median age was 76 years and four patients were women. The bleeding phenotype was extensive bruising and/or muscle hematomas in nine patients, gastrointestinal bleeding in two patients, and extensive bleeding after tooth extraction in one patient. Their data were added to the 10 published studies included in the literature review, resulting in a total of 118 patients.
Promising results
The author’s single institution analysis showed that IA treatment stopped bleeding in nine patients, while three patients did not respond. At 3 months, the median factor VIII increased to 80 IU/dL (considered complete remission) and the median inhibitor titer decreased to 0.15 BU/mL.
The pooled proportion of the meta-analysis patients treated with IA who achieved factor VIII recovery defined as complete remission was 86% (95% confidence interval, 76%-94%). The pooled proportion of patients with a reduction of the inhibitor titer was 95% (95% CI, 83%-100%), while the pooled mortality was 7% (95% CI, 0%-18%). Sensitivity analyses did not reveal any significant differences in retrospective studies or in studies using different absorbing agents.
In addition, there were few reported side effects, most of which were considered mild, according to the researchers. These included nausea and vomiting, paresthesia, and mild hypotension. The authors did suggest that, as a central venous catheter is often used, patients were exposed to an added risk of bleeding and infection.
“At our institution, IA is considered on a case-by-case base rather than a strict cutoff level. Strong arguments are life-threatening bleeding complications, inhibitor titers 20 BU/mL or greater, or failed immunosuppressive treatment with corticosteroids and cyclophosphamide using an established dose regimen,” the researchers stated.
“Even though firm evidence is still lacking and the actual ‘added value’ of IA cannot be adequately assessed, we believe that IA might be a beneficial adjunctive treatment modality in some patients with acquired hemophilia. It was associated with a complete remission in the majority of patients, most of whom are at high risk of bleeding,” the researchers added.
The authors reported that they had no relevant disclosures.
Despite the high mortality rate for acquired hemophilia and the availability of suggested drug treatments, there are no randomized, controlled studies to inform doctors of the best therapies for their patients.
Immunoabsorption therapy (IA) is one such treatment that has been proposed as valid because of its ability to remove factor VIII clotting inhibitors from the bloodstream, but the data on its effectiveness are limited, according to Michael Esteves Pereira, of the Bern (Switzerland) University Hospital and the University of Bern, and colleagues.
In order to help answer the question of the benefits of IA for treating acquired hemophilia, the researchers performed a retrospective study assessing observational data as well as a systemic review and meta-analysis of published literature. They found evidence that the therapy was effective, but suggest that more confirmatory studies are needed, according to their report published online in Transfusion Medicine Reviews.
Data from the authors’ institution were available for 12 patients with acquired hemophilia treated since 2002. The median age was 76 years and four patients were women. The bleeding phenotype was extensive bruising and/or muscle hematomas in nine patients, gastrointestinal bleeding in two patients, and extensive bleeding after tooth extraction in one patient. Their data were added to the 10 published studies included in the literature review, resulting in a total of 118 patients.
Promising results
The author’s single institution analysis showed that IA treatment stopped bleeding in nine patients, while three patients did not respond. At 3 months, the median factor VIII increased to 80 IU/dL (considered complete remission) and the median inhibitor titer decreased to 0.15 BU/mL.
The pooled proportion of the meta-analysis patients treated with IA who achieved factor VIII recovery defined as complete remission was 86% (95% confidence interval, 76%-94%). The pooled proportion of patients with a reduction of the inhibitor titer was 95% (95% CI, 83%-100%), while the pooled mortality was 7% (95% CI, 0%-18%). Sensitivity analyses did not reveal any significant differences in retrospective studies or in studies using different absorbing agents.
In addition, there were few reported side effects, most of which were considered mild, according to the researchers. These included nausea and vomiting, paresthesia, and mild hypotension. The authors did suggest that, as a central venous catheter is often used, patients were exposed to an added risk of bleeding and infection.
“At our institution, IA is considered on a case-by-case base rather than a strict cutoff level. Strong arguments are life-threatening bleeding complications, inhibitor titers 20 BU/mL or greater, or failed immunosuppressive treatment with corticosteroids and cyclophosphamide using an established dose regimen,” the researchers stated.
“Even though firm evidence is still lacking and the actual ‘added value’ of IA cannot be adequately assessed, we believe that IA might be a beneficial adjunctive treatment modality in some patients with acquired hemophilia. It was associated with a complete remission in the majority of patients, most of whom are at high risk of bleeding,” the researchers added.
The authors reported that they had no relevant disclosures.
FROM TRANSFUSION MEDICINE REVIEWS
Opioid-related deaths lower in counties with active cannabis dispensaries
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
FROM BMJ