User login
Enfortumab vedotin shows promise as new option for urothelial carcinoma
The antibody-drug conjugate enfortumab vedotin is superior to chemotherapy in patients with previously treated advanced urothelial carcinoma, primary results of the EV-301 trial show.
Findings were reported at the 2021 Genitourinary Cancers Symposium (Abstract 393).
“Treatment after platinum-based chemotherapy and immune checkpoint inhibitors is challenging. Overall survival is short, and therapeutic options are also limited,” noted first author Thomas Powles, MD, a professor of genitourinary oncology and director of the Barts Cancer Centre, Queen Mary University of London. “Chemotherapy is being used as the global standard of care, but randomized trials supporting these treatment choices are actually lacking. In this setting, new therapeutic agents supported by randomized trials are needed.”
Patients enrolled in EV-301 (NCT03474107), an international open-label, phase 3 trial, had locally advanced or metastatic urothelial carcinoma, had received platinum-based chemotherapy, and had experienced progression during or after immune checkpoint inhibitor therapy (anti–PD1/PD-L1 therapy).
The trial met its primary endpoint, showing that, relative to chemotherapy, enfortumab vedotin reduced the risk of death by 30%, giving patients nearly 4 additional months of life. The toxicity profile was similar to that seen in earlier trials and was manageable.
“Enfortumab vedotin is the first drug, beyond chemotherapy and immunotherapy, to show a significant survival advantage in previously treated urothelial cancer. This is a big step in the right direction for patients with advanced urothelial cancer, where treatment options remain quite limited,” Dr. Powles maintained.
The drug is also showing promising activity when used in the immunotherapy-treated but cisplatin-ineligible patients in the second cohort of the predecessor EV-201 trial, reported at the symposium as well (Abstract 394), he noted. “I hope that, as we move it earlier, we will show better efficacy.”
The Food and Drug Administration granted enfortumab vedotin accelerated approval as third-line therapy in 2019 on the basis of data from the EV-201 trial’s first cohort. With these new data from both trials, the manufacturers have submitted applications to convert the accelerated approval to regular approval, and to expand the current label to include cisplatin-ineligible patients.
Trial details
In EV-301, a total of 608 patients were randomized evenly to enfortumab vedotin (an antibody-drug conjugate that targets nectin-4, a cell-adhesion molecule highly expressed in urothelial carcinoma) or the physician’s choice among three standard chemotherapy options having similar efficacy (docetaxel, paclitaxel, or vinflunine).
“None of these chemotherapy drugs have spectacular response rates, and the overall survival is best described as modest,” Dr. Powles said.
He reported results of the trial’s planned interim analysis, which became the primary analysis because the primary endpoint was positive. Specifically, median overall survival was 12.9 months with enfortumab vedotin and 9.0 months with chemotherapy (hazard ratio, 0.70; P = .00142). Benefit was similar across most patient subgroups.
The enfortumab vedotin group also had a better median progression-free survival (5.6 vs. 3.7 months; HR, 0.62; P < .00001) and investigator-assessed overall response rate (40.6% vs. 17.9%; P < .001).
The rate of grade 3 or worse treatment-related adverse events was 51% with enfortumab vedotin and 50% with chemotherapy. The former was associated with a higher rate of grade 3 or worse maculopapular rash (7% vs. 0%), whereas the latter was associated with higher rates of grade 3 or worse decreased neutrophil count (13% vs. 6%), decreased white blood cell count (7% vs. 1%), and febrile neutropenia (6% vs. 1%).
Regarding events of special interest, enfortumab vedotin led to more grade 3 or worse skin reactions of any type (15% vs. 1%), peripheral neuropathy (5% vs. 2%), and hyperglycemia (4% vs. 0%). However, the majority of all treatment-related adverse events of special interest were mild to moderate in severity and consistent with those previously reported.
“There is a skill associated with the management of toxicity, and there is going to be a learning curve for people who haven’t used the drug before,” Dr. Powles acknowledged. “But my experience is, it’s a manageable drug, and delays and dose interruptions actually make it a relatively straightforward drug to give in the context of the profile that we’ve seen today.”
Level 1 evidence
“We now know that enfortumab vedotin is here to stay in the armamentarium for the treatment of urothelial cancer, adding its name to the ranks of others which have shown level 1 evidence, proof of a survival benefit in metastatic urothelial carcinoma,” commented invited discussant Arlene O. Siefker-Radtke, MD, a professor in the department of genitourinary medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“I’ve been impressed not only by the activity of enfortumab vedotin in visceral and liver metastases, but also by the impact in patients with bone metastases as this appears very helpful in controlling bone pain in many patients,” she noted.
Preventing and managing toxicity requires appropriate patient selection, careful monitoring, and dose modifications, with knowledge of the agent’s adverse event profile and of factors conferring elevated risk for events, Dr. Siefker-Radtke said.
“The early evidence for enfortumab vedotin in the postimmunotherapy, platinum-ineligible group suggests that this can help treat patients with an unmet need due to their inability to receive platinum-based therapy,” she concluded. “And while it’s currently approved in the third-line setting, we are all eagerly awaiting the outcomes of the frontline studies of enfortumab vedotin combined with pembrolizumab, which showed such a promising objective response rate, as has been presented at earlier meetings.”
The trial was sponsored by Astellas Pharma and Seagen. Dr. Powles disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and numerous other pharmaceutical and biotechnology companies. Dr. Siefker-Radtke disclosed relationships with AstraZeneca, Bavarian Nordic, Bristol-Myers Squibb, and a variety of other pharmaceutical and biotechnology companies, as well as patents, royalties, and/or other intellectual property pertaining to methods of characterizing and treating molecular subsets of muscle-invasive bladder cancer.
The antibody-drug conjugate enfortumab vedotin is superior to chemotherapy in patients with previously treated advanced urothelial carcinoma, primary results of the EV-301 trial show.
Findings were reported at the 2021 Genitourinary Cancers Symposium (Abstract 393).
“Treatment after platinum-based chemotherapy and immune checkpoint inhibitors is challenging. Overall survival is short, and therapeutic options are also limited,” noted first author Thomas Powles, MD, a professor of genitourinary oncology and director of the Barts Cancer Centre, Queen Mary University of London. “Chemotherapy is being used as the global standard of care, but randomized trials supporting these treatment choices are actually lacking. In this setting, new therapeutic agents supported by randomized trials are needed.”
Patients enrolled in EV-301 (NCT03474107), an international open-label, phase 3 trial, had locally advanced or metastatic urothelial carcinoma, had received platinum-based chemotherapy, and had experienced progression during or after immune checkpoint inhibitor therapy (anti–PD1/PD-L1 therapy).
The trial met its primary endpoint, showing that, relative to chemotherapy, enfortumab vedotin reduced the risk of death by 30%, giving patients nearly 4 additional months of life. The toxicity profile was similar to that seen in earlier trials and was manageable.
“Enfortumab vedotin is the first drug, beyond chemotherapy and immunotherapy, to show a significant survival advantage in previously treated urothelial cancer. This is a big step in the right direction for patients with advanced urothelial cancer, where treatment options remain quite limited,” Dr. Powles maintained.
The drug is also showing promising activity when used in the immunotherapy-treated but cisplatin-ineligible patients in the second cohort of the predecessor EV-201 trial, reported at the symposium as well (Abstract 394), he noted. “I hope that, as we move it earlier, we will show better efficacy.”
The Food and Drug Administration granted enfortumab vedotin accelerated approval as third-line therapy in 2019 on the basis of data from the EV-201 trial’s first cohort. With these new data from both trials, the manufacturers have submitted applications to convert the accelerated approval to regular approval, and to expand the current label to include cisplatin-ineligible patients.
Trial details
In EV-301, a total of 608 patients were randomized evenly to enfortumab vedotin (an antibody-drug conjugate that targets nectin-4, a cell-adhesion molecule highly expressed in urothelial carcinoma) or the physician’s choice among three standard chemotherapy options having similar efficacy (docetaxel, paclitaxel, or vinflunine).
“None of these chemotherapy drugs have spectacular response rates, and the overall survival is best described as modest,” Dr. Powles said.
He reported results of the trial’s planned interim analysis, which became the primary analysis because the primary endpoint was positive. Specifically, median overall survival was 12.9 months with enfortumab vedotin and 9.0 months with chemotherapy (hazard ratio, 0.70; P = .00142). Benefit was similar across most patient subgroups.
The enfortumab vedotin group also had a better median progression-free survival (5.6 vs. 3.7 months; HR, 0.62; P < .00001) and investigator-assessed overall response rate (40.6% vs. 17.9%; P < .001).
The rate of grade 3 or worse treatment-related adverse events was 51% with enfortumab vedotin and 50% with chemotherapy. The former was associated with a higher rate of grade 3 or worse maculopapular rash (7% vs. 0%), whereas the latter was associated with higher rates of grade 3 or worse decreased neutrophil count (13% vs. 6%), decreased white blood cell count (7% vs. 1%), and febrile neutropenia (6% vs. 1%).
Regarding events of special interest, enfortumab vedotin led to more grade 3 or worse skin reactions of any type (15% vs. 1%), peripheral neuropathy (5% vs. 2%), and hyperglycemia (4% vs. 0%). However, the majority of all treatment-related adverse events of special interest were mild to moderate in severity and consistent with those previously reported.
“There is a skill associated with the management of toxicity, and there is going to be a learning curve for people who haven’t used the drug before,” Dr. Powles acknowledged. “But my experience is, it’s a manageable drug, and delays and dose interruptions actually make it a relatively straightforward drug to give in the context of the profile that we’ve seen today.”
Level 1 evidence
“We now know that enfortumab vedotin is here to stay in the armamentarium for the treatment of urothelial cancer, adding its name to the ranks of others which have shown level 1 evidence, proof of a survival benefit in metastatic urothelial carcinoma,” commented invited discussant Arlene O. Siefker-Radtke, MD, a professor in the department of genitourinary medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“I’ve been impressed not only by the activity of enfortumab vedotin in visceral and liver metastases, but also by the impact in patients with bone metastases as this appears very helpful in controlling bone pain in many patients,” she noted.
Preventing and managing toxicity requires appropriate patient selection, careful monitoring, and dose modifications, with knowledge of the agent’s adverse event profile and of factors conferring elevated risk for events, Dr. Siefker-Radtke said.
“The early evidence for enfortumab vedotin in the postimmunotherapy, platinum-ineligible group suggests that this can help treat patients with an unmet need due to their inability to receive platinum-based therapy,” she concluded. “And while it’s currently approved in the third-line setting, we are all eagerly awaiting the outcomes of the frontline studies of enfortumab vedotin combined with pembrolizumab, which showed such a promising objective response rate, as has been presented at earlier meetings.”
The trial was sponsored by Astellas Pharma and Seagen. Dr. Powles disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and numerous other pharmaceutical and biotechnology companies. Dr. Siefker-Radtke disclosed relationships with AstraZeneca, Bavarian Nordic, Bristol-Myers Squibb, and a variety of other pharmaceutical and biotechnology companies, as well as patents, royalties, and/or other intellectual property pertaining to methods of characterizing and treating molecular subsets of muscle-invasive bladder cancer.
The antibody-drug conjugate enfortumab vedotin is superior to chemotherapy in patients with previously treated advanced urothelial carcinoma, primary results of the EV-301 trial show.
Findings were reported at the 2021 Genitourinary Cancers Symposium (Abstract 393).
“Treatment after platinum-based chemotherapy and immune checkpoint inhibitors is challenging. Overall survival is short, and therapeutic options are also limited,” noted first author Thomas Powles, MD, a professor of genitourinary oncology and director of the Barts Cancer Centre, Queen Mary University of London. “Chemotherapy is being used as the global standard of care, but randomized trials supporting these treatment choices are actually lacking. In this setting, new therapeutic agents supported by randomized trials are needed.”
Patients enrolled in EV-301 (NCT03474107), an international open-label, phase 3 trial, had locally advanced or metastatic urothelial carcinoma, had received platinum-based chemotherapy, and had experienced progression during or after immune checkpoint inhibitor therapy (anti–PD1/PD-L1 therapy).
The trial met its primary endpoint, showing that, relative to chemotherapy, enfortumab vedotin reduced the risk of death by 30%, giving patients nearly 4 additional months of life. The toxicity profile was similar to that seen in earlier trials and was manageable.
“Enfortumab vedotin is the first drug, beyond chemotherapy and immunotherapy, to show a significant survival advantage in previously treated urothelial cancer. This is a big step in the right direction for patients with advanced urothelial cancer, where treatment options remain quite limited,” Dr. Powles maintained.
The drug is also showing promising activity when used in the immunotherapy-treated but cisplatin-ineligible patients in the second cohort of the predecessor EV-201 trial, reported at the symposium as well (Abstract 394), he noted. “I hope that, as we move it earlier, we will show better efficacy.”
The Food and Drug Administration granted enfortumab vedotin accelerated approval as third-line therapy in 2019 on the basis of data from the EV-201 trial’s first cohort. With these new data from both trials, the manufacturers have submitted applications to convert the accelerated approval to regular approval, and to expand the current label to include cisplatin-ineligible patients.
Trial details
In EV-301, a total of 608 patients were randomized evenly to enfortumab vedotin (an antibody-drug conjugate that targets nectin-4, a cell-adhesion molecule highly expressed in urothelial carcinoma) or the physician’s choice among three standard chemotherapy options having similar efficacy (docetaxel, paclitaxel, or vinflunine).
“None of these chemotherapy drugs have spectacular response rates, and the overall survival is best described as modest,” Dr. Powles said.
He reported results of the trial’s planned interim analysis, which became the primary analysis because the primary endpoint was positive. Specifically, median overall survival was 12.9 months with enfortumab vedotin and 9.0 months with chemotherapy (hazard ratio, 0.70; P = .00142). Benefit was similar across most patient subgroups.
The enfortumab vedotin group also had a better median progression-free survival (5.6 vs. 3.7 months; HR, 0.62; P < .00001) and investigator-assessed overall response rate (40.6% vs. 17.9%; P < .001).
The rate of grade 3 or worse treatment-related adverse events was 51% with enfortumab vedotin and 50% with chemotherapy. The former was associated with a higher rate of grade 3 or worse maculopapular rash (7% vs. 0%), whereas the latter was associated with higher rates of grade 3 or worse decreased neutrophil count (13% vs. 6%), decreased white blood cell count (7% vs. 1%), and febrile neutropenia (6% vs. 1%).
Regarding events of special interest, enfortumab vedotin led to more grade 3 or worse skin reactions of any type (15% vs. 1%), peripheral neuropathy (5% vs. 2%), and hyperglycemia (4% vs. 0%). However, the majority of all treatment-related adverse events of special interest were mild to moderate in severity and consistent with those previously reported.
“There is a skill associated with the management of toxicity, and there is going to be a learning curve for people who haven’t used the drug before,” Dr. Powles acknowledged. “But my experience is, it’s a manageable drug, and delays and dose interruptions actually make it a relatively straightforward drug to give in the context of the profile that we’ve seen today.”
Level 1 evidence
“We now know that enfortumab vedotin is here to stay in the armamentarium for the treatment of urothelial cancer, adding its name to the ranks of others which have shown level 1 evidence, proof of a survival benefit in metastatic urothelial carcinoma,” commented invited discussant Arlene O. Siefker-Radtke, MD, a professor in the department of genitourinary medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“I’ve been impressed not only by the activity of enfortumab vedotin in visceral and liver metastases, but also by the impact in patients with bone metastases as this appears very helpful in controlling bone pain in many patients,” she noted.
Preventing and managing toxicity requires appropriate patient selection, careful monitoring, and dose modifications, with knowledge of the agent’s adverse event profile and of factors conferring elevated risk for events, Dr. Siefker-Radtke said.
“The early evidence for enfortumab vedotin in the postimmunotherapy, platinum-ineligible group suggests that this can help treat patients with an unmet need due to their inability to receive platinum-based therapy,” she concluded. “And while it’s currently approved in the third-line setting, we are all eagerly awaiting the outcomes of the frontline studies of enfortumab vedotin combined with pembrolizumab, which showed such a promising objective response rate, as has been presented at earlier meetings.”
The trial was sponsored by Astellas Pharma and Seagen. Dr. Powles disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and numerous other pharmaceutical and biotechnology companies. Dr. Siefker-Radtke disclosed relationships with AstraZeneca, Bavarian Nordic, Bristol-Myers Squibb, and a variety of other pharmaceutical and biotechnology companies, as well as patents, royalties, and/or other intellectual property pertaining to methods of characterizing and treating molecular subsets of muscle-invasive bladder cancer.
FROM GUCS 2021
Heavier girls hit hormonal puberty earlier, but develop breasts later
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
ASDS issues first filler safety recommendations
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The lasting effects of childhood trauma
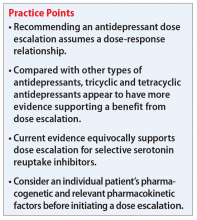
Childhood trauma, which is also called adverse childhood experiences (ACEs), can have lasting detrimental effects on individuals as they grow and mature into adulthood. ACEs may occur in children age ≤18 years if they experience abuse or neglect, violence, or other traumatic losses. More than 60% of people experience at least 1 ACE, and 1 in 6 individuals reported that they had experienced ≥4 ACEs.1 Subsequent additional ACEs have a cumulative deteriorating impact on the brain. This predisposes individuals to mental health disorders, substance use disorders, and other psychosocial problems. The efficacy of current therapeutic approaches provides only partial symptom resolution. For such individuals, the illness load and health care costs typically remain high across the lifespan.1,2
In this article, we discuss types of ACEs, protective factors and risk factors that influence the development of posttraumatic stress disorder (PTSD) in individuals who experience ACEs, how ACEs can negatively impact mental health in adulthood, and approaches to prevent or treat PTSD and other symptoms.
Types of trauma and correlation with PTSD
ACEs can be indexed as neglect or emotional, physical, or sexual abuse. Physical and sexual abuse strongly correlate with an increased risk of PTSD.3 Although neglect and emotional abuse do not directly predict the development of PTSD, these experiences foretell high rates of lifelong trauma exposure and are indirectly related to late PTSD symptoms.4,5 ACEs can impede an individual’s cognitive, social, and emotional development, diminish quality of life, and lead to an early death.6 The lifetime prevalence of PTSD is 6.1% to 9.2%.7 Compared with men, women are 4 times more likely to develop PTSD following a traumatic event.7
The development of PTSD is influenced by the nature, duration, and degree of trauma, and age at the time of exposure to trauma. Children who survive complex trauma (≥2 types of trauma) have a higher likelihood of developing PTSD.8 Prolonged trauma exposure has a more substantial negative impact than a one-time occurrence. However, it is an erroneous oversimplification to assume that each type of ACE has an equally traumatic effect.6
Factors that protect against PTSD
Factors that can protect against developing PTSD are listed in Table 1.7 Two of these are resilience and hope.
Resilience is defined as an individual’s strength to cope with difficulties in life.9 Resilience has internal psychological characteristics and external factors that aid in protecting against childhood adversities.10,11 The Brief Resilience Scale is a self-assessment that measures innate abilities to cope, including optimism, self-efficacy, patience, faith, and humor.12,13 External factors associated with resilience are family, friends, and community support.11,13
Hope can help in surmounting ACEs. The Adult Hope Scale has been used in many studies to assess this construct in individuals who have survived trauma.13 Some studies have found decreased hope in individuals who sustained early trauma and were diagnosed with PTSD in adulthood.14 A study examining children exposed to domestic violence found that children who showed high hope, endurance, and curiosity were better able to cope with adversities.15
Continue to: PTSD risk factors
PTSD risk factors
Many individual and societal risk factors can influence the likelihood of developing PTSD. Some of these factors are outlined in Table 1.7
Pathophysiology of PTSD
Multiple brain regions, pathways, and neurotransmitters are involved in the development of PTSD. Neuroimaging has identified volume and activity changes of the hippocampus, prefrontal cortex, and amygdala in patients with early trauma and PTSD. Some researchers have suggested a gross reduction in locus coeruleus neuronal volume in war veterans with a likely diagnosis of PTSD compared with controls.16,17 In other studies, chronic stress exposure has been found to cause neuronal cell death and affect neuronal plasticity in the limbic area of the brain.18
Diagnosing PTSD
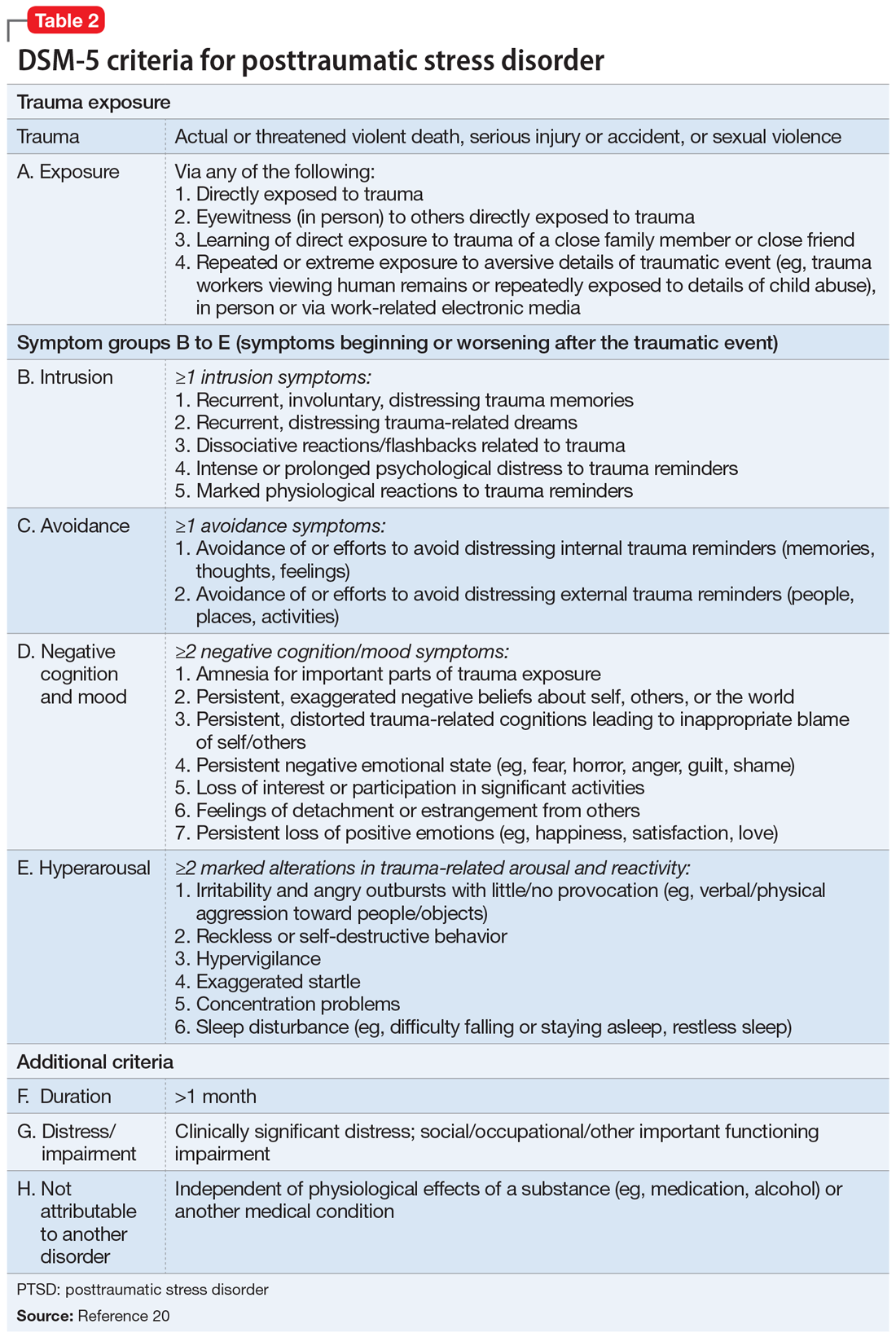
More than 30% of individuals who experience ACEs develop PTSD.19 The DSM-5 diagnostic criteria for PTSD are outlined in Table 2.20 Several instruments are used to determine the diagnosis and assess the severity of PTSD. These include the Clinician-Administered PTSD Scale for DSM-5,21 which is a 30-item structured interview that can be administered in 45 to 60 minutes; the PTSD Symptom Scale Self-Report Version, which is a 17-item, Likert scale, self-report questionnaire; and the Structured Clinical Interview: PTSD Module, which is a semi-structured interview that can take up to several hours to administer.21
Other disorders. In addition to PTSD, individuals with ACEs are at high risk for other mental health issues throughout their lifetime. Individuals with ACE often experience depressive symptoms (approximately 40%); anxiety (approximately 30%); anger; guilt or shame; negative self-cognition; interpersonal difficulties; rumination; and thoughts of self-harm and suicide.22 Epidemiological studies suggest that patients who experience childhood sexual abuse are more likely to develop mood, anxiety, and substance use disorders in adulthood.23,24
Psychotherapeutic treatments for PTSD
Cognitive-behavioral therapy (CBT) addresses the relationship between an individual’s thoughts, emotions, and behaviors. CBT can be used to treat adults and children with PTSD. Before starting CBT, assess the patient’s current safety to ensure that they have the coping skills to manage distress related to their ACEs, and address any coexisting substance use.25
Continue to: According to the American Psychological Association...
According to the American Psychological Association, several CBT-based psychotherapies are recommended for treating PTSD26:
Trauma-focused–CBT includes psychoeducation, trauma narrative, processing, exposure, and relaxation skills training. It consists of approximately 12 to 16 sessions and incorporates elements of family therapy.
Cognitive processing therapy (CPT) focuses on helping patients develop adaptive cognitive domains about the self, the people around them, and the world. CPT therapists assist in information processing by accessing the traumatic memory and trying to eliminate emotions tied to it.25,27 CPT consists of 12 to 16 structured individual, group, or combined sessions.
Prolonged exposure (PE) targets fear-related emotions and works on the principles of habituation to extinguish trauma and fear response to the trigger. This increases self-reliance and competence and decreases the generalization of anxiety to innocuous triggers. PE typically consists of 9 to 12 sessions. PE alone or in combination with cognitive restructuring is successful in treating patients with PTSD, but cognitive restructuring has limited utility in young children.25,27
Cognitive exposure can be individual or group therapy delivered over 3 months, where negative self-evaluation and traumatic memories are challenged with the goal of interrupting maladaptive behaviors and thoughts.27
Continue to: Stress inoculation training
Stress inoculation training (SIT) provides psychoeducation, skills training, role-playing, deep muscle relaxation, paced breathing, and thought stopping. Emphasis is on coaching skills to alleviate anxiety, fear, and symptoms of depression associated with trauma. In SIT, exposures to traumatic memories are indirect (eg, role play), compared with PE, where the exposures are direct.25
The American Psychological Association conditionally recommended several other forms for psychotherapy for treating patients with PTSD26:
Brief eclectic psychotherapy uses CBT and psychodynamic approaches to target feelings of guilt and shame in 16 sessions.27
Narrative exposure therapy consists of 4 to 10 group sessions in which individuals provide detailed narration of the events; the focus is on self-respect and personal rights.27
Eye movement desensitization and reprocessing (EMDR) is a 6- to 12-session, 8-phase treatment that uses principles of accelerated information processing to target nonverbal expression of trauma and dissociative experiences. Patients with PTSD are suggested to have disrupted rapid eye movements. In EMDR, patients follow rhythmic movements of the therapist’s hands or flashed light. This is designed to decrease stress associated with accessing trauma memories, the emotional/physiologic response from the memories, and negative cognitive distortions about self, and to replace negative cognition distortions with positive thoughts about self.25,27
Continue to: Accelerated resolution therapy
Accelerated resolution therapy is a derivative of EMDR. It helps to reconsolidate the emotional and physical experiences associated with distressing memories by replacing them with positive ones or decreasing physiological arousal and anxiety related to the recall of traumatic memories.28
Pharmacologic treatments
Selective serotonin reuptake inhibitors (SSRIs). Multiple studies using different scales have found that paroxetine, sertraline, and fluoxetine can decrease PTSD symptoms. Approximately 60% of patients treated with SSRIs experience partial remission of symptoms, and 20% to 30% experience complete symptom resolution.29 Davidson et al30 found that 22% of patients with PTSD who received fluoxetine had a relapse of symptoms, compared with 50% of patients who received placebo.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and other antidepressants. The SNRIs venlafaxine and duloxetine can help reduce hyperarousal symptoms and improve mood, anxiety, and sleep.26 Mirtazapine, an alpha 2A/2C adrenoceptor antagonist/5-HT 2A/2C/3 antagonist, can address PTSD symptoms from both serotonergic pathways and increase norepinephrine release by blocking autoreceptors and enhancing alpha-1 receptor activity. This alleviates hyperarousal symptoms and promotes sleep.29 In addition to having monoaminergic effects, antidepressant medications also regulate the hypothalamic–pituitary–adrenal (HPA) axis response to stress and promote neurogenesis in the hippocampal region.29
Adrenergic agents
Adrenergic receptor antagonists. Prazosin, an alpha-1 adrenoceptor antagonist, decreases hyperarousal symptoms, improves sleep, and decreases nightmares related to PTSD by decreasing noradrenergic hyperactivity.29
Beta-blockers such as propranolol can decrease physiological response to trauma but have mixed results in the prevention or improvement of PTSD symptoms.29,31
Continue to: Glucocorticoid receptor agonists
Glucocorticoid receptor agonists. In a very small study, low-dose cortisol decreased the severity of traumatic memory (consolidation phase).32 Glucocorticoid receptor agonists can also diminish memory retrieval (reconsolidation phase) through intrusive thoughts and flashbacks.29
Anticonvulsants, benzodiazepines, and antipsychotics
These medications have had a limited role in the treatment of PTSD.26,29
Future directions: Preventive treatments
Because PTSD has a profound impact on an individual’s quality of life and the development of other illnesses, there is strong interest in finding treatments that can prevent PTSD. Based on limited evidence primarily from animal studies, some researchers have suggested that certain agents may someday be helpful for PTSD prevention29:
Glucocorticoid antagonists such as corticotropin-releasing factor 1 (CRF1) antagonists or cholecystokinin 2 (CCK2) receptor antagonists might promote resilience to stress by inhibiting the HPA axis and influencing the amygdala by decreasing fear conditioning, as observed in animal models. Similarly, in animal models, CRF1 and CCK2 are predicted to decrease memory consolidation in response to exposure to stress.
Adrenoceptor antagonists and agonists also might have a role in preventive treatment, but the evidence is scarce. Prazosin, an alpha-1 adrenoceptor antagonist, was ineffective in animal models.29,31 Propranolol, a beta-adrenoceptor blocker, has had mixed results but can decrease trauma-induced physiological arousal when administered soon after exposure.29
Continue to: N-methyl-
N-methyl-
Bottom Line
Adverse childhood experiences (ACEs) are strong predictors for the development of posttraumatic stress disorder (PTSD) and other mental health or medical issues in late adolescence and adulthood. Experiencing a higher number of ACEs increases the risk of developing PTSD as an adult. Timely psychotherapeutic and pharmacologic interventions can help limit symptoms and reduce the severity of PTSD.
Related Resources
- Smith P, Dalglesih T, Meiser-Stedman R. Practitioner review: posttraumatic stress disorder and its treatment in children and adolescents. J Child Psychol Psychiatry. 2019;60(5):500-515.
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Duloxetine • Cymbalta
Fluoxetine • Prozac
Mirtazapine • Remeron
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal, Pronol
Sertraline • Zoloft
Venlafaxine • Effexor
1. Centers for Disease Control and Prevention. Preventing adverse childhood experiences. Published April 3, 2020. Accessed January 26, 2021. https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html
2. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378-385.
3. Norman RE, Byambaa M, De R, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349
4. Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27(11):1247-1258.
5. Glück TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. 2017;8(suppl 3):1372543. doi: 10.1080/20008198.2017.1372543
6. Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460.
7. Sareen J. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. UpToDate. Updated December 3, 2020. Accessed January 26, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
8. Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999:156;1223-1229.
9. Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316-331.
10. Ahern NR, Kiehl EM, Sole ML, et al. A review of instruments measuring resilience. Issues Compr Pediatr Nurs. 2006;29(2):103-125.
11. Zimmerman MA. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ Behav. 2013;40(4):381-383.
12. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Munoz RT, Hanks H, Hellman CM. Hope and resilience as distinct contributors to psychological flourishing among childhood trauma survivors. Traumatology. 2020;26(2):177-184.
14. Baxter MA, Hemming EJ, McIntosh HC, et al. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus. 2017;26(8):948-956.
15. Hellman CM, Gwinn C. Camp HOPE as an intervention for children exposed to domestic violence: a program evaluation of hope, and strength of character. Child Adolesc Soc Work J. 2017;34:269-276.
16. Bracha HS, Garcia-Rill E, Mrak RE, et al. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503-9.
17. de Lange GM. Understanding the cellular and molecular alterations in PTSD brains: the necessity of post-mortem brain tissue. Eur J Psychotraumatol. 2017;8(1):1341824. doi: 10.1080/20008198.2017.1341824
18. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722-729.
19. Greeson JKP, Briggs EC, Kisiel CL, et al. Complex trauma and mental health in children and adolescents placed in foster care: findings from the national child traumatic stress network. Child Welfare. 2011;90(6):91-108.
20. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
21. American Psychological Association. PTSD assessment instruments. Updated September 26, 2018. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/assessment/
22. Bellis MA, Hughes K, Ford K, et al. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517-e528. doi: 10.1016/S2468-2667(19)30145-8
23. Mullen PE, Martin JL, Anderson JC, et al. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721-732.
24. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
25. Chard KM, Gilman R. Counseling trauma victims: 4 brief therapies meet the test. Current Psychiatry. 2005;4(8):50,55-58,61-62.
26. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychol. 2019;74(5):596-607.
27. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder. PTSD treatments. Updated June 2020. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/treatments/
28. Kip KE, Elk CA, Sullivan KL, et al. Brief treatment of symptoms of post-traumatic stress disorder (PTSD) by use of accelerated resolution therapy (ART(®)). Behav Sci (Basel). 2012;2(2):115-134.
29. Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617-627.
30. Davidson JR, Connor KM, Hertzberg MA, et al. Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol. 2005;25(2):166-169.
31. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
32. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiat. 2004;161(8):1488-1490.
33. McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 suppl):S195-S198. doi: 10.1097/TA.0b013e318160ba1d
Childhood trauma, which is also called adverse childhood experiences (ACEs), can have lasting detrimental effects on individuals as they grow and mature into adulthood. ACEs may occur in children age ≤18 years if they experience abuse or neglect, violence, or other traumatic losses. More than 60% of people experience at least 1 ACE, and 1 in 6 individuals reported that they had experienced ≥4 ACEs.1 Subsequent additional ACEs have a cumulative deteriorating impact on the brain. This predisposes individuals to mental health disorders, substance use disorders, and other psychosocial problems. The efficacy of current therapeutic approaches provides only partial symptom resolution. For such individuals, the illness load and health care costs typically remain high across the lifespan.1,2
In this article, we discuss types of ACEs, protective factors and risk factors that influence the development of posttraumatic stress disorder (PTSD) in individuals who experience ACEs, how ACEs can negatively impact mental health in adulthood, and approaches to prevent or treat PTSD and other symptoms.
Types of trauma and correlation with PTSD
ACEs can be indexed as neglect or emotional, physical, or sexual abuse. Physical and sexual abuse strongly correlate with an increased risk of PTSD.3 Although neglect and emotional abuse do not directly predict the development of PTSD, these experiences foretell high rates of lifelong trauma exposure and are indirectly related to late PTSD symptoms.4,5 ACEs can impede an individual’s cognitive, social, and emotional development, diminish quality of life, and lead to an early death.6 The lifetime prevalence of PTSD is 6.1% to 9.2%.7 Compared with men, women are 4 times more likely to develop PTSD following a traumatic event.7
The development of PTSD is influenced by the nature, duration, and degree of trauma, and age at the time of exposure to trauma. Children who survive complex trauma (≥2 types of trauma) have a higher likelihood of developing PTSD.8 Prolonged trauma exposure has a more substantial negative impact than a one-time occurrence. However, it is an erroneous oversimplification to assume that each type of ACE has an equally traumatic effect.6
Factors that protect against PTSD
Factors that can protect against developing PTSD are listed in Table 1.7 Two of these are resilience and hope.
Resilience is defined as an individual’s strength to cope with difficulties in life.9 Resilience has internal psychological characteristics and external factors that aid in protecting against childhood adversities.10,11 The Brief Resilience Scale is a self-assessment that measures innate abilities to cope, including optimism, self-efficacy, patience, faith, and humor.12,13 External factors associated with resilience are family, friends, and community support.11,13
Hope can help in surmounting ACEs. The Adult Hope Scale has been used in many studies to assess this construct in individuals who have survived trauma.13 Some studies have found decreased hope in individuals who sustained early trauma and were diagnosed with PTSD in adulthood.14 A study examining children exposed to domestic violence found that children who showed high hope, endurance, and curiosity were better able to cope with adversities.15
Continue to: PTSD risk factors
PTSD risk factors
Many individual and societal risk factors can influence the likelihood of developing PTSD. Some of these factors are outlined in Table 1.7
Pathophysiology of PTSD
Multiple brain regions, pathways, and neurotransmitters are involved in the development of PTSD. Neuroimaging has identified volume and activity changes of the hippocampus, prefrontal cortex, and amygdala in patients with early trauma and PTSD. Some researchers have suggested a gross reduction in locus coeruleus neuronal volume in war veterans with a likely diagnosis of PTSD compared with controls.16,17 In other studies, chronic stress exposure has been found to cause neuronal cell death and affect neuronal plasticity in the limbic area of the brain.18
Diagnosing PTSD
More than 30% of individuals who experience ACEs develop PTSD.19 The DSM-5 diagnostic criteria for PTSD are outlined in Table 2.20 Several instruments are used to determine the diagnosis and assess the severity of PTSD. These include the Clinician-Administered PTSD Scale for DSM-5,21 which is a 30-item structured interview that can be administered in 45 to 60 minutes; the PTSD Symptom Scale Self-Report Version, which is a 17-item, Likert scale, self-report questionnaire; and the Structured Clinical Interview: PTSD Module, which is a semi-structured interview that can take up to several hours to administer.21

Other disorders. In addition to PTSD, individuals with ACEs are at high risk for other mental health issues throughout their lifetime. Individuals with ACE often experience depressive symptoms (approximately 40%); anxiety (approximately 30%); anger; guilt or shame; negative self-cognition; interpersonal difficulties; rumination; and thoughts of self-harm and suicide.22 Epidemiological studies suggest that patients who experience childhood sexual abuse are more likely to develop mood, anxiety, and substance use disorders in adulthood.23,24
Psychotherapeutic treatments for PTSD
Cognitive-behavioral therapy (CBT) addresses the relationship between an individual’s thoughts, emotions, and behaviors. CBT can be used to treat adults and children with PTSD. Before starting CBT, assess the patient’s current safety to ensure that they have the coping skills to manage distress related to their ACEs, and address any coexisting substance use.25
Continue to: According to the American Psychological Association...
According to the American Psychological Association, several CBT-based psychotherapies are recommended for treating PTSD26:
Trauma-focused–CBT includes psychoeducation, trauma narrative, processing, exposure, and relaxation skills training. It consists of approximately 12 to 16 sessions and incorporates elements of family therapy.
Cognitive processing therapy (CPT) focuses on helping patients develop adaptive cognitive domains about the self, the people around them, and the world. CPT therapists assist in information processing by accessing the traumatic memory and trying to eliminate emotions tied to it.25,27 CPT consists of 12 to 16 structured individual, group, or combined sessions.
Prolonged exposure (PE) targets fear-related emotions and works on the principles of habituation to extinguish trauma and fear response to the trigger. This increases self-reliance and competence and decreases the generalization of anxiety to innocuous triggers. PE typically consists of 9 to 12 sessions. PE alone or in combination with cognitive restructuring is successful in treating patients with PTSD, but cognitive restructuring has limited utility in young children.25,27
Cognitive exposure can be individual or group therapy delivered over 3 months, where negative self-evaluation and traumatic memories are challenged with the goal of interrupting maladaptive behaviors and thoughts.27
Continue to: Stress inoculation training
Stress inoculation training (SIT) provides psychoeducation, skills training, role-playing, deep muscle relaxation, paced breathing, and thought stopping. Emphasis is on coaching skills to alleviate anxiety, fear, and symptoms of depression associated with trauma. In SIT, exposures to traumatic memories are indirect (eg, role play), compared with PE, where the exposures are direct.25
The American Psychological Association conditionally recommended several other forms for psychotherapy for treating patients with PTSD26:
Brief eclectic psychotherapy uses CBT and psychodynamic approaches to target feelings of guilt and shame in 16 sessions.27
Narrative exposure therapy consists of 4 to 10 group sessions in which individuals provide detailed narration of the events; the focus is on self-respect and personal rights.27
Eye movement desensitization and reprocessing (EMDR) is a 6- to 12-session, 8-phase treatment that uses principles of accelerated information processing to target nonverbal expression of trauma and dissociative experiences. Patients with PTSD are suggested to have disrupted rapid eye movements. In EMDR, patients follow rhythmic movements of the therapist’s hands or flashed light. This is designed to decrease stress associated with accessing trauma memories, the emotional/physiologic response from the memories, and negative cognitive distortions about self, and to replace negative cognition distortions with positive thoughts about self.25,27
Continue to: Accelerated resolution therapy
Accelerated resolution therapy is a derivative of EMDR. It helps to reconsolidate the emotional and physical experiences associated with distressing memories by replacing them with positive ones or decreasing physiological arousal and anxiety related to the recall of traumatic memories.28
Pharmacologic treatments
Selective serotonin reuptake inhibitors (SSRIs). Multiple studies using different scales have found that paroxetine, sertraline, and fluoxetine can decrease PTSD symptoms. Approximately 60% of patients treated with SSRIs experience partial remission of symptoms, and 20% to 30% experience complete symptom resolution.29 Davidson et al30 found that 22% of patients with PTSD who received fluoxetine had a relapse of symptoms, compared with 50% of patients who received placebo.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and other antidepressants. The SNRIs venlafaxine and duloxetine can help reduce hyperarousal symptoms and improve mood, anxiety, and sleep.26 Mirtazapine, an alpha 2A/2C adrenoceptor antagonist/5-HT 2A/2C/3 antagonist, can address PTSD symptoms from both serotonergic pathways and increase norepinephrine release by blocking autoreceptors and enhancing alpha-1 receptor activity. This alleviates hyperarousal symptoms and promotes sleep.29 In addition to having monoaminergic effects, antidepressant medications also regulate the hypothalamic–pituitary–adrenal (HPA) axis response to stress and promote neurogenesis in the hippocampal region.29
Adrenergic agents
Adrenergic receptor antagonists. Prazosin, an alpha-1 adrenoceptor antagonist, decreases hyperarousal symptoms, improves sleep, and decreases nightmares related to PTSD by decreasing noradrenergic hyperactivity.29
Beta-blockers such as propranolol can decrease physiological response to trauma but have mixed results in the prevention or improvement of PTSD symptoms.29,31
Continue to: Glucocorticoid receptor agonists
Glucocorticoid receptor agonists. In a very small study, low-dose cortisol decreased the severity of traumatic memory (consolidation phase).32 Glucocorticoid receptor agonists can also diminish memory retrieval (reconsolidation phase) through intrusive thoughts and flashbacks.29
Anticonvulsants, benzodiazepines, and antipsychotics
These medications have had a limited role in the treatment of PTSD.26,29
Future directions: Preventive treatments
Because PTSD has a profound impact on an individual’s quality of life and the development of other illnesses, there is strong interest in finding treatments that can prevent PTSD. Based on limited evidence primarily from animal studies, some researchers have suggested that certain agents may someday be helpful for PTSD prevention29:
Glucocorticoid antagonists such as corticotropin-releasing factor 1 (CRF1) antagonists or cholecystokinin 2 (CCK2) receptor antagonists might promote resilience to stress by inhibiting the HPA axis and influencing the amygdala by decreasing fear conditioning, as observed in animal models. Similarly, in animal models, CRF1 and CCK2 are predicted to decrease memory consolidation in response to exposure to stress.
Adrenoceptor antagonists and agonists also might have a role in preventive treatment, but the evidence is scarce. Prazosin, an alpha-1 adrenoceptor antagonist, was ineffective in animal models.29,31 Propranolol, a beta-adrenoceptor blocker, has had mixed results but can decrease trauma-induced physiological arousal when administered soon after exposure.29
Continue to: N-methyl-
N-methyl-
Bottom Line
Adverse childhood experiences (ACEs) are strong predictors for the development of posttraumatic stress disorder (PTSD) and other mental health or medical issues in late adolescence and adulthood. Experiencing a higher number of ACEs increases the risk of developing PTSD as an adult. Timely psychotherapeutic and pharmacologic interventions can help limit symptoms and reduce the severity of PTSD.
Related Resources
- Smith P, Dalglesih T, Meiser-Stedman R. Practitioner review: posttraumatic stress disorder and its treatment in children and adolescents. J Child Psychol Psychiatry. 2019;60(5):500-515.
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Duloxetine • Cymbalta
Fluoxetine • Prozac
Mirtazapine • Remeron
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal, Pronol
Sertraline • Zoloft
Venlafaxine • Effexor
Childhood trauma, which is also called adverse childhood experiences (ACEs), can have lasting detrimental effects on individuals as they grow and mature into adulthood. ACEs may occur in children age ≤18 years if they experience abuse or neglect, violence, or other traumatic losses. More than 60% of people experience at least 1 ACE, and 1 in 6 individuals reported that they had experienced ≥4 ACEs.1 Subsequent additional ACEs have a cumulative deteriorating impact on the brain. This predisposes individuals to mental health disorders, substance use disorders, and other psychosocial problems. The efficacy of current therapeutic approaches provides only partial symptom resolution. For such individuals, the illness load and health care costs typically remain high across the lifespan.1,2
In this article, we discuss types of ACEs, protective factors and risk factors that influence the development of posttraumatic stress disorder (PTSD) in individuals who experience ACEs, how ACEs can negatively impact mental health in adulthood, and approaches to prevent or treat PTSD and other symptoms.
Types of trauma and correlation with PTSD
ACEs can be indexed as neglect or emotional, physical, or sexual abuse. Physical and sexual abuse strongly correlate with an increased risk of PTSD.3 Although neglect and emotional abuse do not directly predict the development of PTSD, these experiences foretell high rates of lifelong trauma exposure and are indirectly related to late PTSD symptoms.4,5 ACEs can impede an individual’s cognitive, social, and emotional development, diminish quality of life, and lead to an early death.6 The lifetime prevalence of PTSD is 6.1% to 9.2%.7 Compared with men, women are 4 times more likely to develop PTSD following a traumatic event.7
The development of PTSD is influenced by the nature, duration, and degree of trauma, and age at the time of exposure to trauma. Children who survive complex trauma (≥2 types of trauma) have a higher likelihood of developing PTSD.8 Prolonged trauma exposure has a more substantial negative impact than a one-time occurrence. However, it is an erroneous oversimplification to assume that each type of ACE has an equally traumatic effect.6
Factors that protect against PTSD
Factors that can protect against developing PTSD are listed in Table 1.7 Two of these are resilience and hope.
Resilience is defined as an individual’s strength to cope with difficulties in life.9 Resilience has internal psychological characteristics and external factors that aid in protecting against childhood adversities.10,11 The Brief Resilience Scale is a self-assessment that measures innate abilities to cope, including optimism, self-efficacy, patience, faith, and humor.12,13 External factors associated with resilience are family, friends, and community support.11,13
Hope can help in surmounting ACEs. The Adult Hope Scale has been used in many studies to assess this construct in individuals who have survived trauma.13 Some studies have found decreased hope in individuals who sustained early trauma and were diagnosed with PTSD in adulthood.14 A study examining children exposed to domestic violence found that children who showed high hope, endurance, and curiosity were better able to cope with adversities.15
Continue to: PTSD risk factors
PTSD risk factors
Many individual and societal risk factors can influence the likelihood of developing PTSD. Some of these factors are outlined in Table 1.7
Pathophysiology of PTSD
Multiple brain regions, pathways, and neurotransmitters are involved in the development of PTSD. Neuroimaging has identified volume and activity changes of the hippocampus, prefrontal cortex, and amygdala in patients with early trauma and PTSD. Some researchers have suggested a gross reduction in locus coeruleus neuronal volume in war veterans with a likely diagnosis of PTSD compared with controls.16,17 In other studies, chronic stress exposure has been found to cause neuronal cell death and affect neuronal plasticity in the limbic area of the brain.18
Diagnosing PTSD
More than 30% of individuals who experience ACEs develop PTSD.19 The DSM-5 diagnostic criteria for PTSD are outlined in Table 2.20 Several instruments are used to determine the diagnosis and assess the severity of PTSD. These include the Clinician-Administered PTSD Scale for DSM-5,21 which is a 30-item structured interview that can be administered in 45 to 60 minutes; the PTSD Symptom Scale Self-Report Version, which is a 17-item, Likert scale, self-report questionnaire; and the Structured Clinical Interview: PTSD Module, which is a semi-structured interview that can take up to several hours to administer.21

Other disorders. In addition to PTSD, individuals with ACEs are at high risk for other mental health issues throughout their lifetime. Individuals with ACE often experience depressive symptoms (approximately 40%); anxiety (approximately 30%); anger; guilt or shame; negative self-cognition; interpersonal difficulties; rumination; and thoughts of self-harm and suicide.22 Epidemiological studies suggest that patients who experience childhood sexual abuse are more likely to develop mood, anxiety, and substance use disorders in adulthood.23,24
Psychotherapeutic treatments for PTSD
Cognitive-behavioral therapy (CBT) addresses the relationship between an individual’s thoughts, emotions, and behaviors. CBT can be used to treat adults and children with PTSD. Before starting CBT, assess the patient’s current safety to ensure that they have the coping skills to manage distress related to their ACEs, and address any coexisting substance use.25
Continue to: According to the American Psychological Association...
According to the American Psychological Association, several CBT-based psychotherapies are recommended for treating PTSD26:
Trauma-focused–CBT includes psychoeducation, trauma narrative, processing, exposure, and relaxation skills training. It consists of approximately 12 to 16 sessions and incorporates elements of family therapy.
Cognitive processing therapy (CPT) focuses on helping patients develop adaptive cognitive domains about the self, the people around them, and the world. CPT therapists assist in information processing by accessing the traumatic memory and trying to eliminate emotions tied to it.25,27 CPT consists of 12 to 16 structured individual, group, or combined sessions.
Prolonged exposure (PE) targets fear-related emotions and works on the principles of habituation to extinguish trauma and fear response to the trigger. This increases self-reliance and competence and decreases the generalization of anxiety to innocuous triggers. PE typically consists of 9 to 12 sessions. PE alone or in combination with cognitive restructuring is successful in treating patients with PTSD, but cognitive restructuring has limited utility in young children.25,27
Cognitive exposure can be individual or group therapy delivered over 3 months, where negative self-evaluation and traumatic memories are challenged with the goal of interrupting maladaptive behaviors and thoughts.27
Continue to: Stress inoculation training
Stress inoculation training (SIT) provides psychoeducation, skills training, role-playing, deep muscle relaxation, paced breathing, and thought stopping. Emphasis is on coaching skills to alleviate anxiety, fear, and symptoms of depression associated with trauma. In SIT, exposures to traumatic memories are indirect (eg, role play), compared with PE, where the exposures are direct.25
The American Psychological Association conditionally recommended several other forms for psychotherapy for treating patients with PTSD26:
Brief eclectic psychotherapy uses CBT and psychodynamic approaches to target feelings of guilt and shame in 16 sessions.27
Narrative exposure therapy consists of 4 to 10 group sessions in which individuals provide detailed narration of the events; the focus is on self-respect and personal rights.27
Eye movement desensitization and reprocessing (EMDR) is a 6- to 12-session, 8-phase treatment that uses principles of accelerated information processing to target nonverbal expression of trauma and dissociative experiences. Patients with PTSD are suggested to have disrupted rapid eye movements. In EMDR, patients follow rhythmic movements of the therapist’s hands or flashed light. This is designed to decrease stress associated with accessing trauma memories, the emotional/physiologic response from the memories, and negative cognitive distortions about self, and to replace negative cognition distortions with positive thoughts about self.25,27
Continue to: Accelerated resolution therapy
Accelerated resolution therapy is a derivative of EMDR. It helps to reconsolidate the emotional and physical experiences associated with distressing memories by replacing them with positive ones or decreasing physiological arousal and anxiety related to the recall of traumatic memories.28
Pharmacologic treatments
Selective serotonin reuptake inhibitors (SSRIs). Multiple studies using different scales have found that paroxetine, sertraline, and fluoxetine can decrease PTSD symptoms. Approximately 60% of patients treated with SSRIs experience partial remission of symptoms, and 20% to 30% experience complete symptom resolution.29 Davidson et al30 found that 22% of patients with PTSD who received fluoxetine had a relapse of symptoms, compared with 50% of patients who received placebo.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and other antidepressants. The SNRIs venlafaxine and duloxetine can help reduce hyperarousal symptoms and improve mood, anxiety, and sleep.26 Mirtazapine, an alpha 2A/2C adrenoceptor antagonist/5-HT 2A/2C/3 antagonist, can address PTSD symptoms from both serotonergic pathways and increase norepinephrine release by blocking autoreceptors and enhancing alpha-1 receptor activity. This alleviates hyperarousal symptoms and promotes sleep.29 In addition to having monoaminergic effects, antidepressant medications also regulate the hypothalamic–pituitary–adrenal (HPA) axis response to stress and promote neurogenesis in the hippocampal region.29
Adrenergic agents
Adrenergic receptor antagonists. Prazosin, an alpha-1 adrenoceptor antagonist, decreases hyperarousal symptoms, improves sleep, and decreases nightmares related to PTSD by decreasing noradrenergic hyperactivity.29
Beta-blockers such as propranolol can decrease physiological response to trauma but have mixed results in the prevention or improvement of PTSD symptoms.29,31
Continue to: Glucocorticoid receptor agonists
Glucocorticoid receptor agonists. In a very small study, low-dose cortisol decreased the severity of traumatic memory (consolidation phase).32 Glucocorticoid receptor agonists can also diminish memory retrieval (reconsolidation phase) through intrusive thoughts and flashbacks.29
Anticonvulsants, benzodiazepines, and antipsychotics
These medications have had a limited role in the treatment of PTSD.26,29
Future directions: Preventive treatments
Because PTSD has a profound impact on an individual’s quality of life and the development of other illnesses, there is strong interest in finding treatments that can prevent PTSD. Based on limited evidence primarily from animal studies, some researchers have suggested that certain agents may someday be helpful for PTSD prevention29:
Glucocorticoid antagonists such as corticotropin-releasing factor 1 (CRF1) antagonists or cholecystokinin 2 (CCK2) receptor antagonists might promote resilience to stress by inhibiting the HPA axis and influencing the amygdala by decreasing fear conditioning, as observed in animal models. Similarly, in animal models, CRF1 and CCK2 are predicted to decrease memory consolidation in response to exposure to stress.
Adrenoceptor antagonists and agonists also might have a role in preventive treatment, but the evidence is scarce. Prazosin, an alpha-1 adrenoceptor antagonist, was ineffective in animal models.29,31 Propranolol, a beta-adrenoceptor blocker, has had mixed results but can decrease trauma-induced physiological arousal when administered soon after exposure.29
Continue to: N-methyl-
N-methyl-
Bottom Line
Adverse childhood experiences (ACEs) are strong predictors for the development of posttraumatic stress disorder (PTSD) and other mental health or medical issues in late adolescence and adulthood. Experiencing a higher number of ACEs increases the risk of developing PTSD as an adult. Timely psychotherapeutic and pharmacologic interventions can help limit symptoms and reduce the severity of PTSD.
Related Resources
- Smith P, Dalglesih T, Meiser-Stedman R. Practitioner review: posttraumatic stress disorder and its treatment in children and adolescents. J Child Psychol Psychiatry. 2019;60(5):500-515.
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry 2018;17(4):35-43.
Drug Brand Names
Duloxetine • Cymbalta
Fluoxetine • Prozac
Mirtazapine • Remeron
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal, Pronol
Sertraline • Zoloft
Venlafaxine • Effexor
1. Centers for Disease Control and Prevention. Preventing adverse childhood experiences. Published April 3, 2020. Accessed January 26, 2021. https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html
2. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378-385.
3. Norman RE, Byambaa M, De R, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349
4. Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27(11):1247-1258.
5. Glück TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. 2017;8(suppl 3):1372543. doi: 10.1080/20008198.2017.1372543
6. Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460.
7. Sareen J. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. UpToDate. Updated December 3, 2020. Accessed January 26, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
8. Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999:156;1223-1229.
9. Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316-331.
10. Ahern NR, Kiehl EM, Sole ML, et al. A review of instruments measuring resilience. Issues Compr Pediatr Nurs. 2006;29(2):103-125.
11. Zimmerman MA. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ Behav. 2013;40(4):381-383.
12. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Munoz RT, Hanks H, Hellman CM. Hope and resilience as distinct contributors to psychological flourishing among childhood trauma survivors. Traumatology. 2020;26(2):177-184.
14. Baxter MA, Hemming EJ, McIntosh HC, et al. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus. 2017;26(8):948-956.
15. Hellman CM, Gwinn C. Camp HOPE as an intervention for children exposed to domestic violence: a program evaluation of hope, and strength of character. Child Adolesc Soc Work J. 2017;34:269-276.
16. Bracha HS, Garcia-Rill E, Mrak RE, et al. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503-9.
17. de Lange GM. Understanding the cellular and molecular alterations in PTSD brains: the necessity of post-mortem brain tissue. Eur J Psychotraumatol. 2017;8(1):1341824. doi: 10.1080/20008198.2017.1341824
18. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722-729.
19. Greeson JKP, Briggs EC, Kisiel CL, et al. Complex trauma and mental health in children and adolescents placed in foster care: findings from the national child traumatic stress network. Child Welfare. 2011;90(6):91-108.
20. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
21. American Psychological Association. PTSD assessment instruments. Updated September 26, 2018. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/assessment/
22. Bellis MA, Hughes K, Ford K, et al. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517-e528. doi: 10.1016/S2468-2667(19)30145-8
23. Mullen PE, Martin JL, Anderson JC, et al. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721-732.
24. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
25. Chard KM, Gilman R. Counseling trauma victims: 4 brief therapies meet the test. Current Psychiatry. 2005;4(8):50,55-58,61-62.
26. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychol. 2019;74(5):596-607.
27. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder. PTSD treatments. Updated June 2020. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/treatments/
28. Kip KE, Elk CA, Sullivan KL, et al. Brief treatment of symptoms of post-traumatic stress disorder (PTSD) by use of accelerated resolution therapy (ART(®)). Behav Sci (Basel). 2012;2(2):115-134.
29. Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617-627.
30. Davidson JR, Connor KM, Hertzberg MA, et al. Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol. 2005;25(2):166-169.
31. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
32. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiat. 2004;161(8):1488-1490.
33. McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 suppl):S195-S198. doi: 10.1097/TA.0b013e318160ba1d
1. Centers for Disease Control and Prevention. Preventing adverse childhood experiences. Published April 3, 2020. Accessed January 26, 2021. https://www.cdc.gov/violenceprevention/childabuseandneglect/aces/fastfact.html
2. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378-385.
3. Norman RE, Byambaa M, De R, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349
4. Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27(11):1247-1258.
5. Glück TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. 2017;8(suppl 3):1372543. doi: 10.1080/20008198.2017.1372543
6. Edwards VJ, Holden GW, Felitti VJ, et al. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453-1460.
7. Sareen J. Posttraumatic stress disorder in adults: epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. UpToDate. Updated December 3, 2020. Accessed January 26, 2021. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
8. Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999:156;1223-1229.
9. Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316-331.
10. Ahern NR, Kiehl EM, Sole ML, et al. A review of instruments measuring resilience. Issues Compr Pediatr Nurs. 2006;29(2):103-125.
11. Zimmerman MA. Resiliency theory: a strengths-based approach to research and practice for adolescent health. Health Educ Behav. 2013;40(4):381-383.
12. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Munoz RT, Hanks H, Hellman CM. Hope and resilience as distinct contributors to psychological flourishing among childhood trauma survivors. Traumatology. 2020;26(2):177-184.
14. Baxter MA, Hemming EJ, McIntosh HC, et al. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus. 2017;26(8):948-956.
15. Hellman CM, Gwinn C. Camp HOPE as an intervention for children exposed to domestic violence: a program evaluation of hope, and strength of character. Child Adolesc Soc Work J. 2017;34:269-276.
16. Bracha HS, Garcia-Rill E, Mrak RE, et al. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503-9.
17. de Lange GM. Understanding the cellular and molecular alterations in PTSD brains: the necessity of post-mortem brain tissue. Eur J Psychotraumatol. 2017;8(1):1341824. doi: 10.1080/20008198.2017.1341824
18. Zunszain PA, Anacker C, Cattaneo A, et al. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722-729.
19. Greeson JKP, Briggs EC, Kisiel CL, et al. Complex trauma and mental health in children and adolescents placed in foster care: findings from the national child traumatic stress network. Child Welfare. 2011;90(6):91-108.
20. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
21. American Psychological Association. PTSD assessment instruments. Updated September 26, 2018. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/assessment/
22. Bellis MA, Hughes K, Ford K, et al. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517-e528. doi: 10.1016/S2468-2667(19)30145-8
23. Mullen PE, Martin JL, Anderson JC, et al. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721-732.
24. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
25. Chard KM, Gilman R. Counseling trauma victims: 4 brief therapies meet the test. Current Psychiatry. 2005;4(8):50,55-58,61-62.
26. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychol. 2019;74(5):596-607.
27. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder. PTSD treatments. Updated June 2020. Accessed January 27, 2021. https://www.apa.org/ptsd-guideline/treatments/
28. Kip KE, Elk CA, Sullivan KL, et al. Brief treatment of symptoms of post-traumatic stress disorder (PTSD) by use of accelerated resolution therapy (ART(®)). Behav Sci (Basel). 2012;2(2):115-134.
29. Steckler T, Risbrough V. Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology. 2012;62(2):617-627.
30. Davidson JR, Connor KM, Hertzberg MA, et al. Maintenance therapy with fluoxetine in posttraumatic stress disorder: a placebo-controlled discontinuation study. J Clin Psychopharmacol. 2005;25(2):166-169.
31. Benedek DM, Friedman MJ, Zatzick D, et al. Guideline watch (March 2009): Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
32. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiat. 2004;161(8):1488-1490.
33. McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 suppl):S195-S198. doi: 10.1097/TA.0b013e318160ba1d
Sleep disorders in older adults
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24

Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
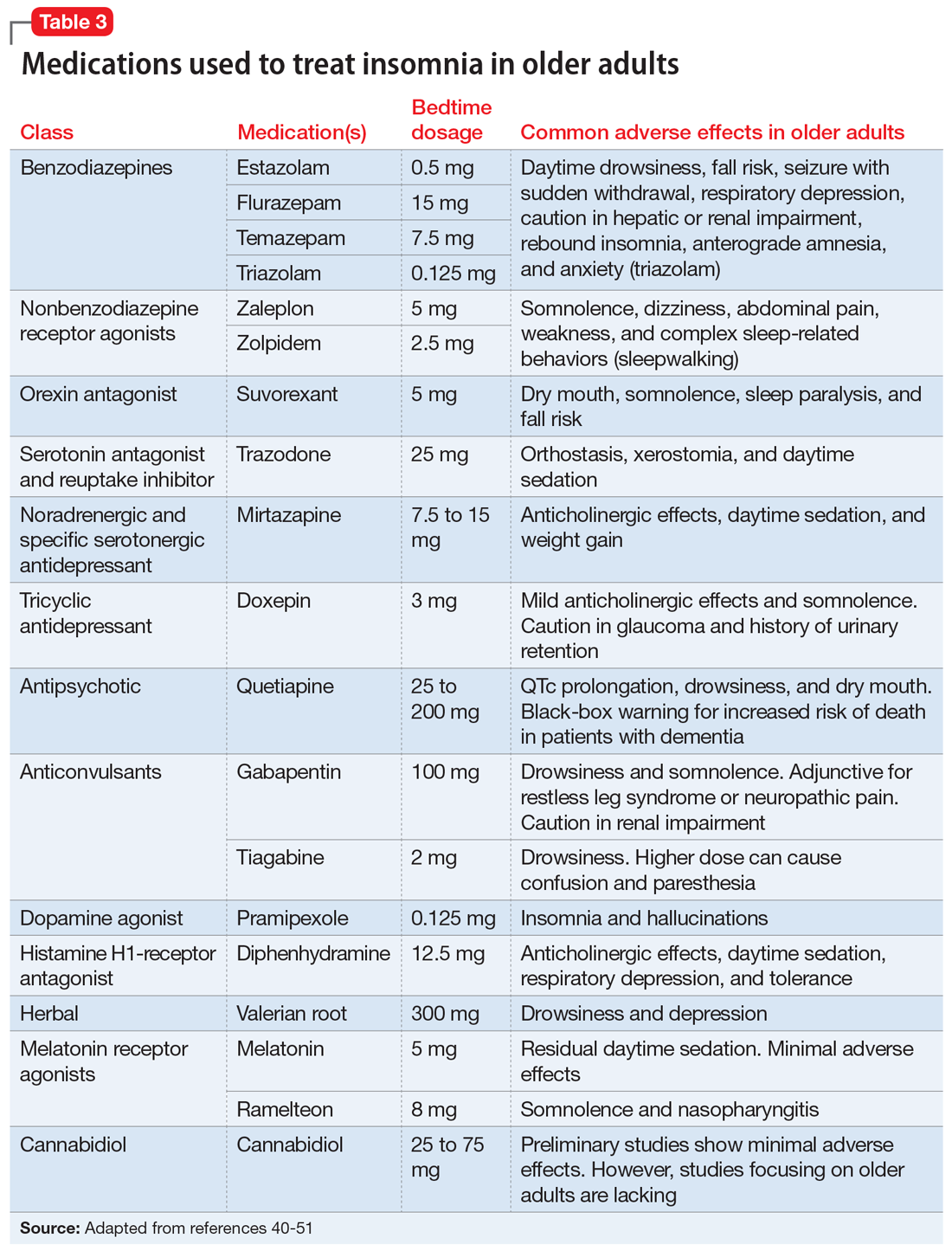
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24

Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.

Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24

Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.

Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
Does L-methylfolate have a role in ADHD management?
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
Metadata, malpractice claims, and making changes to the EHR
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
Addressing structural racism: An update from the APA
The coronavirus disease 2019 pandemic, which as of mid-February 2021 had caused more than 486,000 deaths in the United States, has changed our lives forever. Elders and Black, Indigenous, and People of Color (BIPOC) have been overrepresented among those lost. That, when juxtaposed with the civil unrest that followed the brutal killing of George Floyd, an unarmed Black man, by a White law enforcement officer on May 25, 2020, have compelled us to talk about US race relations in unprecedented ways. These and other traumas disproportionately affect the quality of life and health of minority and underserved individuals. The international outcry about racism, serial trauma, and health disparities left the medical profession well positioned to promulgate changes that are conducive to achieving health equity.
Race is a social construct
In November 2020, the American Medical Association (AMA) Board of Trustees made several public acknowledgments about race.1 First, race is a social, nonbiological classification that is different from biology, ethnicity, or genetic ancestry.1 Next, race contributes to health disparities and poor health outcomes for minorities and members of underserved communities.1 Also, racism, which includes disproportionate police brutality against Black and Indigenous people, is a driver of health inequity for them and people in marginalized communities.1
The AMA also commented on how serial trauma and racism can affect one’s health. The AMA acknowledged that exposure to serial trauma throughout one’s life can have a cumulative effect that is “associated with chronic stress, higher rates of comorbidities and lower life expectancy” and results in increased health care costs and decreased quality of life for those who are affected.1 Also, the AMA proclaimed that racism is a threat to public health and pledged to dismantle discriminatory practices and policies in health care, including medical education and research.2
Diversity and inclusion in psychiatry
While the AMA has been striving to reduce bias in health care systems, psychiatry has been forging its path. In March 2015, the American Psychiatric Association’s (APA) Board of Trustees approved the APA’s Strategic Initiative, which has 4 goals: 1) advancing the integration of psychiatry in health care; 2) supporting research; 3) supporting education; and 4) promoting diversity and inclusion in psychiatry.3 The latter goal includes advocating for antiracist policies that promote cultural competence and health equity in education, research and psychiatric care; increased recruitment and retention of psychiatrists from groups that historically have been underrepresented in medicine and medical leadership; and ensuring representation of these groups in APA governance at all levels.3
The APA’s antiracism agenda
In March 2020, outgoing APA President Bruce Schwartz concurred with Board members that diversity and inclusion in the APA warranted a closer review. On May 5, 2020, APA President Jeff Geller committed to authorizing a systematic study of diversity and inclusion in various branches of the APA, including councils and governance. By the end of May, with civil unrest in full swing in the United States, President Geller decided to expand the APA’s diversity agenda.
President Geller appointed the APA Presidential Task Force to Address Structural Racism Throughout Psychiatry (SR Task Force), which had its virtual inaugural meeting on June 27, 2020.4 The SR Task Force exists to focus on structural racism (aka institutional racism) in organized psychiatry, psychiatric patients, and those who provide psychiatric services to patients. The charge, which is subject to revision, if warranted, is clear: provide resources and education on the history of structural racism in the APA and psychiatry, explain how structural racism impacts psychiatric patients and the profession, craft actionable recommendations to dismantle structural racism in the APA and psychiatry, report those findings to the APA’s Board of Trustees, and implement a quality assurance protocol to ensure that the Task Force’s work is consistent with its charge. President Geller decided to have the Task Force focus on anti-Black racism in its inaugural year and believes that the outcome will benefit all psychiatrists, other mental health professionals, and patients who identify as members of minority and underserved groups in the United States and the profession of psychiatry.5
Presidential Task Forces in APA
Presidential Task Forces report directly to the Board of Trustees, which expedites the review of progress reports and deliberation on and, when favorable, implementation of recommendations. Also, Presidential Task Forces are afforded additional APA resources. For example, the SR Task Force has 16 APA staff members who have been appointed or volunteered to assist the Task Force in some way. Many APA staff have graduate degrees in law, education, and other subjects. The skill sets, networks, institutional memory, and commitment that they bring to the project are conducive to advancing the SR Task Force’s agenda at a brisk pace.
Continue to: The APA President...
The APA President decides whom to appoint to each Task Force. President Geller propitiously appointed subject matter experts and members of the Board of Trustees to serve on the SR Task Force. Subject matter experts contribute historical and contemporary content about racism, including anti-Black racism, to the discussion. The data are used to craft research questions that may yield pertinent data. (Note that not all subject matter experts are Black, nor are all Board members White.) APA staff support the Task Force by sharing their expertise, compiling data, coordinating meetings, collaborating on program development, disseminating the work product to APA members and the media, and other important tasks.
The SR Task Force’s work
The SR Task Force strives for transparency in a process that is informed by APA members. The group immediately set up a web hub that is used to communicate with APA members.5 Individual members also use social media to alert members to SR Task Force activities and events. Member input has been solicited by posting several brief surveys on the SR Task Force web hub. Topics have included the effect of structural racism on patient care, psychiatric practice, and organized psychiatry, including the APA. The responses, which collectively totaled >1,600, were reviewed and used to inform Task Force priorities while working within the scope of the charge.5
Based on member feedback, the first large project of the SR Task Force has been to examine structural racism in the APA. The SR Task Force formed workgroups to study data pertaining to diversity and inclusion in the APA Assembly, governance (the Board of Trustees), Councils and Committees, and Scientific Program Committee. As APA Publishing and the DSM Steering Committee have internal processes to address structural racism, the SR Task Force did not convene workgroups to study this. However, the SR Task Force will be meeting with leaders of those groups to learn about their protocols and will request that information be made available to APA members.
The SR Task Force reviews and interprets data that are compiled by each workgroup, deliberates on its significance, and when appropriate, drafts achievable recommendations to improve diversity and inclusion in the APA. This is where Trustee involvement is invaluable to the SR Task Force, because the report and recommendations will be presented to the Board of Trustees.
There is no guarantee that the recommendations contained in a report that is accepted by the Board of Trustees will be implemented unless they are approved. It is imperative, therefore, that SR Task Force recommendations to the Board take into consideration Board structure, processes, goals, efficiency, history, and other matters. The learning curve can be steep, especially when the first major report was due 3 months after the SR Task Force was appointed. Clarity and efficiency are key in report preparation. For example, during the Winter 2020 Board of Trustees meeting, the SR Task Force presented its report, answered questions, and offered 7 action items to the Board for deliberation and voting. The endeavor, which was completed in 20 minutes, resulted in the Board supporting 6 of the recommendations and deferring the deliberation of the seventh recommendation to the spring Board meeting, due to logistical concerns.
Continue to: Thus far, the SR Task Force Workgroups...
Thus far, the SR Task Force Workgroups on the Assembly and Governance have presented their reports. 5 The SR Task Force reports on the Scientific Program Committee and Councils and Committees are scheduled to be presented to the Board during the Spring 2021 meeting.
The SR Task Force has been fulfilling the commitment to provide relevant educational materials to members in several ways. There have been 4 virtual Structural Racism Town Hall meetings that featured subject matter experts. The first Town Hall session addressed the initial steps towards dismantling structural racism and included President Geller’s announcement about appointing a SR Task Force. The next Town Hall meeting addressed structural racism in medicine and psychiatry, its effect on children and individuals who identify as transgender, and its intersectionality (the cumulative effect of discrimination on a person who belongs to 2 non-dominant groups.) The panel in the third Town Hall meeting reviewed the impact of structural racism, including intersectionality, on transgenerational trauma in several minority groups. The meeting ended with an update of Task Force activities. The February 2021 Town Hall meeting focused on how structural racism affects recruitment and retention of minority psychiatry residents, and how this can undermine efforts to grow a diverse workforce. Recordings of these and other events can be accessed on the SR Task Force web hub.5 The SR Task Force members plan to present a review of the year’s work during the next Town Hall meeting, which is scheduled to occur on Saturday, May 1, 2021, during the APA’s Annual Meeting.
The SR Task Force web hub contains other resources, including APA position statements, press releases, and news articles, and a glossary of relevant terms. It also includes internet links to President Geller’s 9-part series on the history of Structural Racism in the APA. There are CME and other webinars, a curated list of references, videos, podcasts, and other media.4
The SR Task Force believes that much of the antiracism work needs to occur beyond APA headquarters. Consequently, President Geller challenged all APA Councils to work on an antiracism project to support the APA’s antiracism agenda. APA committees and caucuses have been encouraged to do the same. The SR Task Force has asked APA District Branches and Allied Organizations to share information about what they are doing to educate members about structural racism and what they are doing for input regarding their antiracist activities. Additionally, Task Force members have been speaking with these and other groups to inform them about the APA’s antiracism work.
APA’s Board of Trustees actions
It would be inappropriate for the APA to task groups with focusing on antiracism unless the organization was doing its part. In July 2020, the Board of Trustees had a 2-hour round table discussion during which each member spoke about the problem and how the APA should address it. Next, President Geller appointed a Board Workgroup to clarify the definitions of “minority” and “underrepresented.” Although the APA Assembly has defined the terms, the APA has not. Additionally, the APA Board of Trustees retained a consultant to assess all aspects of how it functions as a Board. The Board’s management of matters pertaining to diversity and inclusion was part of the examination. The recommendations are being reviewed and the Board will undergo diversity training.
Continue to: President Geller's study...
President Geller’s study of racism in the APA, which involved a review of past APA presidential addresses, brought to light a long-term pattern of racism in the organization.5 On January 18, 2021, Martin Luther King, Jr. Day, the APA acknowledged and apologized to psychiatrists, patients, and the public for its history of engaging in and passively condoning racist behavior.6 The APA has committed to being better informed about diversity and inclusion at every level. Lastly, hired consultants with expertise in diversity and inclusion are working with APA staff at every level so that the environment can be a welcoming and comfortable workspace for recruiting and retaining a diverse workforce.
Although it may seem that the APA has engaged in many antiracist activities in a brief period, there is much more to accomplish. The Task Force hopes that the work will speak for itself and will be sustained over time. It’s long overdue.
1. American Medical Association. New AMA policies recognize race as a social, not biological, construct. Published November 16, 2020. Accessed February 1, 2021. https://www.ama-assn.org/press-center/press-releases/new-ama-policies-recognize-race-social-not-biological-construct
2. American Medical Association. New AMA policy recognizes racism as a public health threat. Published November 16, 2020. Accessed February 1, 2021. https://www.ama-assn.org/press-center/press-releases/new-ama-policy-recognizes-racism-public-health-threat
3. American Psychiatric Association. Board-approved recommendation on strategic planning. Published March 2015. Accessed February 1, 2021. https://www.psychiatry.org/about-apa/read-apa-organization-documents-and-policies/strategic-plan
4. Geller J. Structural racism in American psychiatry and APA. Parts 1-9. Published July-November 2020. Accessed February 8, 2021. https://psychnews.psychiatryonline.org/topic/news-president?sortBy=Ppub
5. American Psychiatric Association. Structural Racism Task Force. Accessed February 8, 2021. https://www.psychiatry.org/psychiatrists/structural-racism-task-force
6. American Psychiatric Association. APA’s apology to black, indigenous and people of color for its support of structural racism in psychiatry. Published January 18, 2021. Accessed February 8, 2021. https://www.psychiatry.org/newsroom/apa-apology-for-its-support-of-structural-racism-in-psychiatry
The coronavirus disease 2019 pandemic, which as of mid-February 2021 had caused more than 486,000 deaths in the United States, has changed our lives forever. Elders and Black, Indigenous, and People of Color (BIPOC) have been overrepresented among those lost. That, when juxtaposed with the civil unrest that followed the brutal killing of George Floyd, an unarmed Black man, by a White law enforcement officer on May 25, 2020, have compelled us to talk about US race relations in unprecedented ways. These and other traumas disproportionately affect the quality of life and health of minority and underserved individuals. The international outcry about racism, serial trauma, and health disparities left the medical profession well positioned to promulgate changes that are conducive to achieving health equity.
Race is a social construct
In November 2020, the American Medical Association (AMA) Board of Trustees made several public acknowledgments about race.1 First, race is a social, nonbiological classification that is different from biology, ethnicity, or genetic ancestry.1 Next, race contributes to health disparities and poor health outcomes for minorities and members of underserved communities.1 Also, racism, which includes disproportionate police brutality against Black and Indigenous people, is a driver of health inequity for them and people in marginalized communities.1
The AMA also commented on how serial trauma and racism can affect one’s health. The AMA acknowledged that exposure to serial trauma throughout one’s life can have a cumulative effect that is “associated with chronic stress, higher rates of comorbidities and lower life expectancy” and results in increased health care costs and decreased quality of life for those who are affected.1 Also, the AMA proclaimed that racism is a threat to public health and pledged to dismantle discriminatory practices and policies in health care, including medical education and research.2
Diversity and inclusion in psychiatry
While the AMA has been striving to reduce bias in health care systems, psychiatry has been forging its path. In March 2015, the American Psychiatric Association’s (APA) Board of Trustees approved the APA’s Strategic Initiative, which has 4 goals: 1) advancing the integration of psychiatry in health care; 2) supporting research; 3) supporting education; and 4) promoting diversity and inclusion in psychiatry.3 The latter goal includes advocating for antiracist policies that promote cultural competence and health equity in education, research and psychiatric care; increased recruitment and retention of psychiatrists from groups that historically have been underrepresented in medicine and medical leadership; and ensuring representation of these groups in APA governance at all levels.3
The APA’s antiracism agenda
In March 2020, outgoing APA President Bruce Schwartz concurred with Board members that diversity and inclusion in the APA warranted a closer review. On May 5, 2020, APA President Jeff Geller committed to authorizing a systematic study of diversity and inclusion in various branches of the APA, including councils and governance. By the end of May, with civil unrest in full swing in the United States, President Geller decided to expand the APA’s diversity agenda.
President Geller appointed the APA Presidential Task Force to Address Structural Racism Throughout Psychiatry (SR Task Force), which had its virtual inaugural meeting on June 27, 2020.4 The SR Task Force exists to focus on structural racism (aka institutional racism) in organized psychiatry, psychiatric patients, and those who provide psychiatric services to patients. The charge, which is subject to revision, if warranted, is clear: provide resources and education on the history of structural racism in the APA and psychiatry, explain how structural racism impacts psychiatric patients and the profession, craft actionable recommendations to dismantle structural racism in the APA and psychiatry, report those findings to the APA’s Board of Trustees, and implement a quality assurance protocol to ensure that the Task Force’s work is consistent with its charge. President Geller decided to have the Task Force focus on anti-Black racism in its inaugural year and believes that the outcome will benefit all psychiatrists, other mental health professionals, and patients who identify as members of minority and underserved groups in the United States and the profession of psychiatry.5
Presidential Task Forces in APA
Presidential Task Forces report directly to the Board of Trustees, which expedites the review of progress reports and deliberation on and, when favorable, implementation of recommendations. Also, Presidential Task Forces are afforded additional APA resources. For example, the SR Task Force has 16 APA staff members who have been appointed or volunteered to assist the Task Force in some way. Many APA staff have graduate degrees in law, education, and other subjects. The skill sets, networks, institutional memory, and commitment that they bring to the project are conducive to advancing the SR Task Force’s agenda at a brisk pace.
Continue to: The APA President...
The APA President decides whom to appoint to each Task Force. President Geller propitiously appointed subject matter experts and members of the Board of Trustees to serve on the SR Task Force. Subject matter experts contribute historical and contemporary content about racism, including anti-Black racism, to the discussion. The data are used to craft research questions that may yield pertinent data. (Note that not all subject matter experts are Black, nor are all Board members White.) APA staff support the Task Force by sharing their expertise, compiling data, coordinating meetings, collaborating on program development, disseminating the work product to APA members and the media, and other important tasks.
The SR Task Force’s work
The SR Task Force strives for transparency in a process that is informed by APA members. The group immediately set up a web hub that is used to communicate with APA members.5 Individual members also use social media to alert members to SR Task Force activities and events. Member input has been solicited by posting several brief surveys on the SR Task Force web hub. Topics have included the effect of structural racism on patient care, psychiatric practice, and organized psychiatry, including the APA. The responses, which collectively totaled >1,600, were reviewed and used to inform Task Force priorities while working within the scope of the charge.5
Based on member feedback, the first large project of the SR Task Force has been to examine structural racism in the APA. The SR Task Force formed workgroups to study data pertaining to diversity and inclusion in the APA Assembly, governance (the Board of Trustees), Councils and Committees, and Scientific Program Committee. As APA Publishing and the DSM Steering Committee have internal processes to address structural racism, the SR Task Force did not convene workgroups to study this. However, the SR Task Force will be meeting with leaders of those groups to learn about their protocols and will request that information be made available to APA members.
The SR Task Force reviews and interprets data that are compiled by each workgroup, deliberates on its significance, and when appropriate, drafts achievable recommendations to improve diversity and inclusion in the APA. This is where Trustee involvement is invaluable to the SR Task Force, because the report and recommendations will be presented to the Board of Trustees.
There is no guarantee that the recommendations contained in a report that is accepted by the Board of Trustees will be implemented unless they are approved. It is imperative, therefore, that SR Task Force recommendations to the Board take into consideration Board structure, processes, goals, efficiency, history, and other matters. The learning curve can be steep, especially when the first major report was due 3 months after the SR Task Force was appointed. Clarity and efficiency are key in report preparation. For example, during the Winter 2020 Board of Trustees meeting, the SR Task Force presented its report, answered questions, and offered 7 action items to the Board for deliberation and voting. The endeavor, which was completed in 20 minutes, resulted in the Board supporting 6 of the recommendations and deferring the deliberation of the seventh recommendation to the spring Board meeting, due to logistical concerns.
Continue to: Thus far, the SR Task Force Workgroups...
Thus far, the SR Task Force Workgroups on the Assembly and Governance have presented their reports. 5 The SR Task Force reports on the Scientific Program Committee and Councils and Committees are scheduled to be presented to the Board during the Spring 2021 meeting.
The SR Task Force has been fulfilling the commitment to provide relevant educational materials to members in several ways. There have been 4 virtual Structural Racism Town Hall meetings that featured subject matter experts. The first Town Hall session addressed the initial steps towards dismantling structural racism and included President Geller’s announcement about appointing a SR Task Force. The next Town Hall meeting addressed structural racism in medicine and psychiatry, its effect on children and individuals who identify as transgender, and its intersectionality (the cumulative effect of discrimination on a person who belongs to 2 non-dominant groups.) The panel in the third Town Hall meeting reviewed the impact of structural racism, including intersectionality, on transgenerational trauma in several minority groups. The meeting ended with an update of Task Force activities. The February 2021 Town Hall meeting focused on how structural racism affects recruitment and retention of minority psychiatry residents, and how this can undermine efforts to grow a diverse workforce. Recordings of these and other events can be accessed on the SR Task Force web hub.5 The SR Task Force members plan to present a review of the year’s work during the next Town Hall meeting, which is scheduled to occur on Saturday, May 1, 2021, during the APA’s Annual Meeting.
The SR Task Force web hub contains other resources, including APA position statements, press releases, and news articles, and a glossary of relevant terms. It also includes internet links to President Geller’s 9-part series on the history of Structural Racism in the APA. There are CME and other webinars, a curated list of references, videos, podcasts, and other media.4
The SR Task Force believes that much of the antiracism work needs to occur beyond APA headquarters. Consequently, President Geller challenged all APA Councils to work on an antiracism project to support the APA’s antiracism agenda. APA committees and caucuses have been encouraged to do the same. The SR Task Force has asked APA District Branches and Allied Organizations to share information about what they are doing to educate members about structural racism and what they are doing for input regarding their antiracist activities. Additionally, Task Force members have been speaking with these and other groups to inform them about the APA’s antiracism work.
APA’s Board of Trustees actions
It would be inappropriate for the APA to task groups with focusing on antiracism unless the organization was doing its part. In July 2020, the Board of Trustees had a 2-hour round table discussion during which each member spoke about the problem and how the APA should address it. Next, President Geller appointed a Board Workgroup to clarify the definitions of “minority” and “underrepresented.” Although the APA Assembly has defined the terms, the APA has not. Additionally, the APA Board of Trustees retained a consultant to assess all aspects of how it functions as a Board. The Board’s management of matters pertaining to diversity and inclusion was part of the examination. The recommendations are being reviewed and the Board will undergo diversity training.
Continue to: President Geller's study...
President Geller’s study of racism in the APA, which involved a review of past APA presidential addresses, brought to light a long-term pattern of racism in the organization.5 On January 18, 2021, Martin Luther King, Jr. Day, the APA acknowledged and apologized to psychiatrists, patients, and the public for its history of engaging in and passively condoning racist behavior.6 The APA has committed to being better informed about diversity and inclusion at every level. Lastly, hired consultants with expertise in diversity and inclusion are working with APA staff at every level so that the environment can be a welcoming and comfortable workspace for recruiting and retaining a diverse workforce.
Although it may seem that the APA has engaged in many antiracist activities in a brief period, there is much more to accomplish. The Task Force hopes that the work will speak for itself and will be sustained over time. It’s long overdue.
The coronavirus disease 2019 pandemic, which as of mid-February 2021 had caused more than 486,000 deaths in the United States, has changed our lives forever. Elders and Black, Indigenous, and People of Color (BIPOC) have been overrepresented among those lost. That, when juxtaposed with the civil unrest that followed the brutal killing of George Floyd, an unarmed Black man, by a White law enforcement officer on May 25, 2020, have compelled us to talk about US race relations in unprecedented ways. These and other traumas disproportionately affect the quality of life and health of minority and underserved individuals. The international outcry about racism, serial trauma, and health disparities left the medical profession well positioned to promulgate changes that are conducive to achieving health equity.
Race is a social construct
In November 2020, the American Medical Association (AMA) Board of Trustees made several public acknowledgments about race.1 First, race is a social, nonbiological classification that is different from biology, ethnicity, or genetic ancestry.1 Next, race contributes to health disparities and poor health outcomes for minorities and members of underserved communities.1 Also, racism, which includes disproportionate police brutality against Black and Indigenous people, is a driver of health inequity for them and people in marginalized communities.1
The AMA also commented on how serial trauma and racism can affect one’s health. The AMA acknowledged that exposure to serial trauma throughout one’s life can have a cumulative effect that is “associated with chronic stress, higher rates of comorbidities and lower life expectancy” and results in increased health care costs and decreased quality of life for those who are affected.1 Also, the AMA proclaimed that racism is a threat to public health and pledged to dismantle discriminatory practices and policies in health care, including medical education and research.2
Diversity and inclusion in psychiatry
While the AMA has been striving to reduce bias in health care systems, psychiatry has been forging its path. In March 2015, the American Psychiatric Association’s (APA) Board of Trustees approved the APA’s Strategic Initiative, which has 4 goals: 1) advancing the integration of psychiatry in health care; 2) supporting research; 3) supporting education; and 4) promoting diversity and inclusion in psychiatry.3 The latter goal includes advocating for antiracist policies that promote cultural competence and health equity in education, research and psychiatric care; increased recruitment and retention of psychiatrists from groups that historically have been underrepresented in medicine and medical leadership; and ensuring representation of these groups in APA governance at all levels.3
The APA’s antiracism agenda
In March 2020, outgoing APA President Bruce Schwartz concurred with Board members that diversity and inclusion in the APA warranted a closer review. On May 5, 2020, APA President Jeff Geller committed to authorizing a systematic study of diversity and inclusion in various branches of the APA, including councils and governance. By the end of May, with civil unrest in full swing in the United States, President Geller decided to expand the APA’s diversity agenda.
President Geller appointed the APA Presidential Task Force to Address Structural Racism Throughout Psychiatry (SR Task Force), which had its virtual inaugural meeting on June 27, 2020.4 The SR Task Force exists to focus on structural racism (aka institutional racism) in organized psychiatry, psychiatric patients, and those who provide psychiatric services to patients. The charge, which is subject to revision, if warranted, is clear: provide resources and education on the history of structural racism in the APA and psychiatry, explain how structural racism impacts psychiatric patients and the profession, craft actionable recommendations to dismantle structural racism in the APA and psychiatry, report those findings to the APA’s Board of Trustees, and implement a quality assurance protocol to ensure that the Task Force’s work is consistent with its charge. President Geller decided to have the Task Force focus on anti-Black racism in its inaugural year and believes that the outcome will benefit all psychiatrists, other mental health professionals, and patients who identify as members of minority and underserved groups in the United States and the profession of psychiatry.5
Presidential Task Forces in APA
Presidential Task Forces report directly to the Board of Trustees, which expedites the review of progress reports and deliberation on and, when favorable, implementation of recommendations. Also, Presidential Task Forces are afforded additional APA resources. For example, the SR Task Force has 16 APA staff members who have been appointed or volunteered to assist the Task Force in some way. Many APA staff have graduate degrees in law, education, and other subjects. The skill sets, networks, institutional memory, and commitment that they bring to the project are conducive to advancing the SR Task Force’s agenda at a brisk pace.
Continue to: The APA President...
The APA President decides whom to appoint to each Task Force. President Geller propitiously appointed subject matter experts and members of the Board of Trustees to serve on the SR Task Force. Subject matter experts contribute historical and contemporary content about racism, including anti-Black racism, to the discussion. The data are used to craft research questions that may yield pertinent data. (Note that not all subject matter experts are Black, nor are all Board members White.) APA staff support the Task Force by sharing their expertise, compiling data, coordinating meetings, collaborating on program development, disseminating the work product to APA members and the media, and other important tasks.
The SR Task Force’s work
The SR Task Force strives for transparency in a process that is informed by APA members. The group immediately set up a web hub that is used to communicate with APA members.5 Individual members also use social media to alert members to SR Task Force activities and events. Member input has been solicited by posting several brief surveys on the SR Task Force web hub. Topics have included the effect of structural racism on patient care, psychiatric practice, and organized psychiatry, including the APA. The responses, which collectively totaled >1,600, were reviewed and used to inform Task Force priorities while working within the scope of the charge.5
Based on member feedback, the first large project of the SR Task Force has been to examine structural racism in the APA. The SR Task Force formed workgroups to study data pertaining to diversity and inclusion in the APA Assembly, governance (the Board of Trustees), Councils and Committees, and Scientific Program Committee. As APA Publishing and the DSM Steering Committee have internal processes to address structural racism, the SR Task Force did not convene workgroups to study this. However, the SR Task Force will be meeting with leaders of those groups to learn about their protocols and will request that information be made available to APA members.
The SR Task Force reviews and interprets data that are compiled by each workgroup, deliberates on its significance, and when appropriate, drafts achievable recommendations to improve diversity and inclusion in the APA. This is where Trustee involvement is invaluable to the SR Task Force, because the report and recommendations will be presented to the Board of Trustees.
There is no guarantee that the recommendations contained in a report that is accepted by the Board of Trustees will be implemented unless they are approved. It is imperative, therefore, that SR Task Force recommendations to the Board take into consideration Board structure, processes, goals, efficiency, history, and other matters. The learning curve can be steep, especially when the first major report was due 3 months after the SR Task Force was appointed. Clarity and efficiency are key in report preparation. For example, during the Winter 2020 Board of Trustees meeting, the SR Task Force presented its report, answered questions, and offered 7 action items to the Board for deliberation and voting. The endeavor, which was completed in 20 minutes, resulted in the Board supporting 6 of the recommendations and deferring the deliberation of the seventh recommendation to the spring Board meeting, due to logistical concerns.
Continue to: Thus far, the SR Task Force Workgroups...
Thus far, the SR Task Force Workgroups on the Assembly and Governance have presented their reports. 5 The SR Task Force reports on the Scientific Program Committee and Councils and Committees are scheduled to be presented to the Board during the Spring 2021 meeting.
The SR Task Force has been fulfilling the commitment to provide relevant educational materials to members in several ways. There have been 4 virtual Structural Racism Town Hall meetings that featured subject matter experts. The first Town Hall session addressed the initial steps towards dismantling structural racism and included President Geller’s announcement about appointing a SR Task Force. The next Town Hall meeting addressed structural racism in medicine and psychiatry, its effect on children and individuals who identify as transgender, and its intersectionality (the cumulative effect of discrimination on a person who belongs to 2 non-dominant groups.) The panel in the third Town Hall meeting reviewed the impact of structural racism, including intersectionality, on transgenerational trauma in several minority groups. The meeting ended with an update of Task Force activities. The February 2021 Town Hall meeting focused on how structural racism affects recruitment and retention of minority psychiatry residents, and how this can undermine efforts to grow a diverse workforce. Recordings of these and other events can be accessed on the SR Task Force web hub.5 The SR Task Force members plan to present a review of the year’s work during the next Town Hall meeting, which is scheduled to occur on Saturday, May 1, 2021, during the APA’s Annual Meeting.
The SR Task Force web hub contains other resources, including APA position statements, press releases, and news articles, and a glossary of relevant terms. It also includes internet links to President Geller’s 9-part series on the history of Structural Racism in the APA. There are CME and other webinars, a curated list of references, videos, podcasts, and other media.4
The SR Task Force believes that much of the antiracism work needs to occur beyond APA headquarters. Consequently, President Geller challenged all APA Councils to work on an antiracism project to support the APA’s antiracism agenda. APA committees and caucuses have been encouraged to do the same. The SR Task Force has asked APA District Branches and Allied Organizations to share information about what they are doing to educate members about structural racism and what they are doing for input regarding their antiracist activities. Additionally, Task Force members have been speaking with these and other groups to inform them about the APA’s antiracism work.
APA’s Board of Trustees actions
It would be inappropriate for the APA to task groups with focusing on antiracism unless the organization was doing its part. In July 2020, the Board of Trustees had a 2-hour round table discussion during which each member spoke about the problem and how the APA should address it. Next, President Geller appointed a Board Workgroup to clarify the definitions of “minority” and “underrepresented.” Although the APA Assembly has defined the terms, the APA has not. Additionally, the APA Board of Trustees retained a consultant to assess all aspects of how it functions as a Board. The Board’s management of matters pertaining to diversity and inclusion was part of the examination. The recommendations are being reviewed and the Board will undergo diversity training.
Continue to: President Geller's study...
President Geller’s study of racism in the APA, which involved a review of past APA presidential addresses, brought to light a long-term pattern of racism in the organization.5 On January 18, 2021, Martin Luther King, Jr. Day, the APA acknowledged and apologized to psychiatrists, patients, and the public for its history of engaging in and passively condoning racist behavior.6 The APA has committed to being better informed about diversity and inclusion at every level. Lastly, hired consultants with expertise in diversity and inclusion are working with APA staff at every level so that the environment can be a welcoming and comfortable workspace for recruiting and retaining a diverse workforce.
Although it may seem that the APA has engaged in many antiracist activities in a brief period, there is much more to accomplish. The Task Force hopes that the work will speak for itself and will be sustained over time. It’s long overdue.
1. American Medical Association. New AMA policies recognize race as a social, not biological, construct. Published November 16, 2020. Accessed February 1, 2021. https://www.ama-assn.org/press-center/press-releases/new-ama-policies-recognize-race-social-not-biological-construct
2. American Medical Association. New AMA policy recognizes racism as a public health threat. Published November 16, 2020. Accessed February 1, 2021. https://www.ama-assn.org/press-center/press-releases/new-ama-policy-recognizes-racism-public-health-threat
3. American Psychiatric Association. Board-approved recommendation on strategic planning. Published March 2015. Accessed February 1, 2021. https://www.psychiatry.org/about-apa/read-apa-organization-documents-and-policies/strategic-plan
4. Geller J. Structural racism in American psychiatry and APA. Parts 1-9. Published July-November 2020. Accessed February 8, 2021. https://psychnews.psychiatryonline.org/topic/news-president?sortBy=Ppub
5. American Psychiatric Association. Structural Racism Task Force. Accessed February 8, 2021. https://www.psychiatry.org/psychiatrists/structural-racism-task-force
6. American Psychiatric Association. APA’s apology to black, indigenous and people of color for its support of structural racism in psychiatry. Published January 18, 2021. Accessed February 8, 2021. https://www.psychiatry.org/newsroom/apa-apology-for-its-support-of-structural-racism-in-psychiatry
1. American Medical Association. New AMA policies recognize race as a social, not biological, construct. Published November 16, 2020. Accessed February 1, 2021. https://www.ama-assn.org/press-center/press-releases/new-ama-policies-recognize-race-social-not-biological-construct
2. American Medical Association. New AMA policy recognizes racism as a public health threat. Published November 16, 2020. Accessed February 1, 2021. https://www.ama-assn.org/press-center/press-releases/new-ama-policy-recognizes-racism-public-health-threat
3. American Psychiatric Association. Board-approved recommendation on strategic planning. Published March 2015. Accessed February 1, 2021. https://www.psychiatry.org/about-apa/read-apa-organization-documents-and-policies/strategic-plan
4. Geller J. Structural racism in American psychiatry and APA. Parts 1-9. Published July-November 2020. Accessed February 8, 2021. https://psychnews.psychiatryonline.org/topic/news-president?sortBy=Ppub
5. American Psychiatric Association. Structural Racism Task Force. Accessed February 8, 2021. https://www.psychiatry.org/psychiatrists/structural-racism-task-force
6. American Psychiatric Association. APA’s apology to black, indigenous and people of color for its support of structural racism in psychiatry. Published January 18, 2021. Accessed February 8, 2021. https://www.psychiatry.org/newsroom/apa-apology-for-its-support-of-structural-racism-in-psychiatry
Treatment resistance is a myth!
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
Antidepressants: Is a higher dose always better?
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).
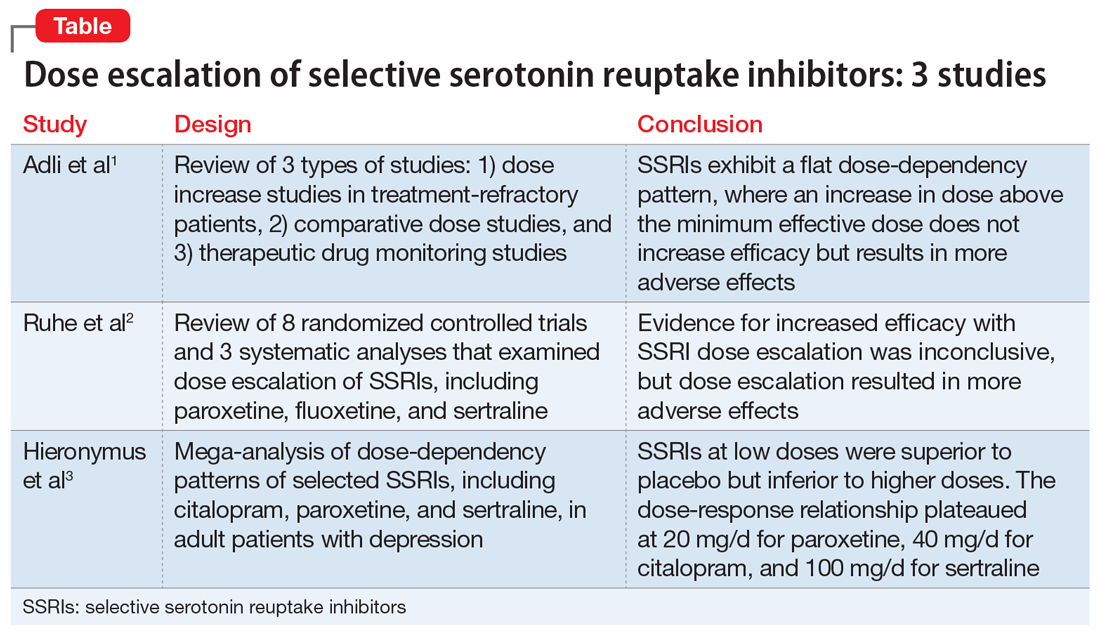
Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).

Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).

Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.