User login
Who Receives Care in VA Medical Foster Homes?
New models are needed for delivering long-term care (LTC) that are home-based, cost-effective, and appropriate for older adults with a range of care needs.1,2 In fiscal year (FY) 2015, the US Department of Veterans Affairs (VA) spent $7.4 billion on LTC, accounting for 13% of total VA health care spending. Overall, 71% of LTC spending in FY 2015 was allocated to institutional care.3 Beyond cost, 95% of older adults prefer to remain in community rather than institutional LTC settings, such as nursing homes.4 The COVID-19 pandemic created additional concerns related to the spread of infectious disease, with > 37% of COVID-19 deaths in the United States occurring in nursing homes irrespective of facility quality.5,6
One community-based LTC alternative developed within the VA is the Medical Foster Home (MFH) program. The MFH program is an adult foster care program in which veterans who are unable to live independently receive round-the-clock care in the home of a community-based caregiver.7 MFH caregivers usually have previous experience caring for family, working in a nursing home, or working as a caregiver in another capacity. These caregivers are responsible for providing 24-hour supervision and support to residents in their MFH and can care for up to 3 adults. In the MFH program, VA home-based primary care (HBPC) teams composed of physicians, registered nurses, physical and occupational therapists, social workers, pharmacists, dieticians, and psychologists, provide primary care for MFH veterans and oversee care in the caregiver’s home.
The goal of the VA HBPC program is to improve veterans’ access to medical care and shift LTC services from institutional to noninstitutional settings by providing in-home care for those who are too sick or disabled to go to a clinic for care. On average, veterans pay the MFH caregiver $2,500 out-of-pocket per month for their care.8 In 2016, there were 992 veterans residing in MFHs across the country.9 Since MFH program implementation expanded nationwide in 2008, more than 4,000 veterans have resided in MFHs in 45 states and territories.10
The VA is required to pay for nursing home care for veterans who have a qualifying VA service-connected disability or who meet a specific threshold of disability.11 Currently, the VA is not authorized to pay for MFH care for veterans who meet the eligibility criteria for VA-paid nursing home care. Over the past decade, the VA has introduced and expanded several initiatives and programs to help veterans who require LTC remain in their homes and communities. These include but are not limited to the Veteran Directed Care program, the Choose Home Initiative, and the Caregiver Support Program.12-14 Additionally, attempts have been made to pass legislation to authorize the VA to pay for MFH for veterans’ care whose military benefits include coverage for nursing home care.15 This legislation and VA initiatives are clear signs that the VA is committed to supporting programs such as the MFH program. Given this commitment, demand for the MFH program will likely increase.
Therefore, VA practitioners need to better identify which veterans are currently in the MFH program. While veterans are expected to need nursing home level care to qualify for MFH enrollment, little has been published about the physical and mental health care needs of veterans currently receiving MFH care. One previous study compared the demographics, diagnostic characteristics, and care utilization of MFH veterans with that of veterans receiving LTC in VA community living centers (CLCs), and found that veterans in MFHs had similar levels of frailty and comorbidity and had a higher mean age when compared with veterans in CLCs.16
Our study assessed a sample of veterans living in MFHs and describes these veterans’ clinical and functional characteristics. We used the Minimum Data Set 3.0 (MDS) to complete the assessments to allow comparisons with other populations residing in long-term care.17,18 While MDS assessments are required for Medicare/Medicaid-certified nursing home residents and for residents in VA CLCs, this study was the first attempt to perform in-home MDS data assessments in MFHs. This collection of descriptive clinical data is an important first step in providing VA practitioners with information about the characteristics of veterans currently cared for in MFHs and policymakers with data to think critically about which veterans are willing to pay for the MFH program.
Methods
This study was part of a larger research project assessing the impact of the MFH program on veterans’ outcomes and health care spending as well as factors influencing program growth.7,9,10,16,19-23 We report on the characteristics of veterans staying in MFHs, using data from the MDS, including a clinical assessment of patients’ cognitive, function, and health care–related needs, collected from participants recruited for this study.
Five research nurses were trained to administer the MDS assessment to veterans in MFHs. Data were collected between April 2014 and December 2015 from veterans at MFH sites associated with 4 urban VA medical centers in 4 different Veterans Integrated Service Networks (58 total homes). While the VA medical centers (VAMCs)were urban, many of the MFHs were in rural areas, given that MFHs can be up to 50 miles from the associated VAMC. We selected MFH sites for this study based on MFH program veteran census. Specifically, we identified MFH sites with high veteran enrollment to ensure we would have a sufficiently large sample for participant recruitment.
Veterans who had resided in an MFH for at least 90 days were eligible to participate. Of the 155 veterans mailed a letter of invitation to participate, 92 (59%) completed the in-home MDS assessment. Reasons for not participating included: 13 veterans died prior to data collection, 18 veterans declined to participate, 18 family members or legal guardians of cognitively impaired veterans did not want the veteran to participate, and 14 veterans left the MFH program or were hospitalized at the time of data collection.
Family members and legal guardians who declined participation on behalf of a veteran reported that they felt the veteran was too frail to participate or that participating would be an added burden on the veteran. Based on the census of veterans residing in all MFHs nationally in November 2015 (N = 972), 9.5% of MFH veterans were included in this study.7This study was approved by the VA Central Institutional Review Board (CIRB #12–31), in addition to the local VA research and development review boards where MFH MDS assessments were collected.
Assessment Instrument and Variables
The MDS 3.0 assesses numerous aspects of clinical and functional status. Several resident-level characteristics from the MDS 3.0 were included in this study. The Cognitive Function Scale (CFS) was used to categorize cognitive function. The CFS is a categorical variable that is created from MDS 3.0 data. The CFS integrates self- and staff-reported data to classify individuals as cognitively intact, mildly impaired, moderately impaired, or severely impaired based on respondents’ Brief Interview for Mental Status (BIMS) assessment or staff-reported cognitive function collected as part of the MDS 3.0.24 We explored depression by calculating a mean summary severity score for all respondents from the Patient Health Questionnaire-9 item interview (PHQ-9).25 PHQ-9 summary scores range from 0 to 27, with mean scores of ≤ 4 indicating no or minimal depression, and higher scores corresponding to more severe depression as scores increase. For respondents who were unable to complete the PHQ-9, we calculated mean PHQ Observational Version (PHQ-9-OV) scores.
We included 2 variables to characterize behaviors: wandering frequency and presence and frequency of aggressive behaviors. We summarized aggressive behaviors using the Aggressive and Reactive Behavior Scale, which characterizes whether a resident has none, mild, moderate, or severe behavioral symptoms based on the presence and frequency of physical and verbal behaviors and resistance to care.26,27 We included items that described pain, number of falls since admission or prior assessment, degree of urinary and bowel continence (always continent vs not always continent) and mobility device use to describe respondents’ health conditions and functional status. To characterize pain, we used veteran’s self-reported frequency and intensity of pain experienced in the prior 5 days and classified the experienced pain as none, mild, moderate, or severe. Finally, demographic characteristics included age and gender.
To determine functional status, we included measures of needing help to perform activities of daily living (ADLs). The MDS allows us to understand functional status ranging from ADLs lost early in the trajectory of functional decline (ie, bathing, hygiene) to those lost in the middle (ie, walking, dressing, toileting, transferring) to those lost late in the trajectory of functional decline (ie, bed mobility and eating).28,29 To assess MFH veterans’ independence in mobility, we considered the veteran’s ability to walk without supervision or assistance in the hallway outside of their room, ability to move between their room and hallway, and ability to move throughout the house. Mobility includes use of an assistive device such as a cane, walker, or wheelchair if the veteran can use it without assistance. We summarized dependency in ADLs, using a combined score of dependence in bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene that ranges from 0 (independent) to 28 (completely dependent).30 Additionally, we created 3-category variables to indicate the degree of dependence in performing ADLs (independent, supervision or assistance, and completely dependent).
Finally, we included diagnoses identified as active to explore differences in neurologic, mood, psychiatric, and chronic disease morbidity. In the MDS 3.0 assessment, an active diagnosis is defined as a diagnosis documented by a licensed independent practitioner in the prior 60 days that has affected the resident or their care in the prior 7 days.
Analysis
We conducted statistical analyses using Stata MP version 15.1 (StataCorp). We summarized demographic characteristics, cognitive function scores, depression scores, pain status, behavioral symptoms, incidence of falls, degree of continence, functional status, and comorbidities, using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Results
Of the 92 MFH veterans in our sample, 85% were male and 83% were aged ≥ 65 years (Table 1). Veterans had an average length of stay of 927 days at the time of MDS assessment. More than half (55%) of MFH veterans had cognitive impairment (ranging from mild to severe). The mean (SD) depression score was 3.3 (3.9), indicating minimal depression. For veterans who could not complete the depression questionnaire, the mean (SD) staff-assessed depression score was 5.9 (5.5), suggesting mild depression. Overall, 22% of the sample had aggressive behaviors but only 7 were noted to be severe. Few residents had caregiver-reported wandering. Self-reported pain intensity indicated that 45% of the sample had mild, moderate, or severe pain. While more than half the cohort had complete bowel continence (53%), only 36% had complete urinary continence. Use of mobility devices was common, with 56% of residents using a wheelchair, 42% using a walker, and 14% using a cane. One-fourth of veterans had fallen at least once since admission to the MFH.
Of the 11 ADLs assessed, the percentage of MFH veterans requiring assistance with early and mid-loss ADLs ranged from 63% for transferring to 84% for bathing (Table 2). Even for the late-loss ADL of eating, 57% of the MFH cohort required assistance. Overall, MFH veterans had an average ADL dependency score of 11.
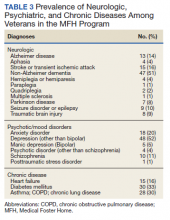
Physicians documented a diagnosis of either Alzheimer disease or non-Alzheimer dementia comorbidity for 65% of the cohort and traumatic brain injury for 9% (Table 3). Based on psychiatric comorbidities recorded in veterans’ health records, over half of MFH residents had depression (52%). Additionally, 1 in 5 MFH veterans had an anxiety disorder diagnosis. Chronic diseases were prevalent among veterans in MFHs, with 33% diagnosed with diabetes mellitus, 30% with asthma, chronic obstructive pulmonary disease, or chronic lung disease, and 16% with heart failure.
Discussion
In this study, we describe the characteristics of veterans receiving LTC in a sample of MFHs. This is the first study to assess veteran health and function across a group of MFHs. To help provide context for the description of MFH residents, we compared demographic characteristics, cognitive impairment, depression, pain, behaviors, functional status, and morbidity of veterans in the MFH program to long-stay residents in community nursing homes (eAppendix 1-3 available at doi:10.12788/fp.0102). A comparison with this reference population suggests that these MFH and nursing home cohorts are similar in terms of age, wandering behavior, incidence of falls, and prevalence of neurologic, psychiatric, and chronic diseases. Compared with nursing home residents, veterans in the MFH cohort had slightly higher mood symptom scores, were more likely to display aggressive behavior, and were more likely to report experiencing moderate and severe pain.
Additionally, MFH veterans displayed a lower level of cognitive impairment, fewer functional impairments, measured by the ADL dependency score, and were less likely to be bowel or bladder incontinent. Despite an overall lower ADL dependency score, a similar proportion of MFH veterans and nursing home residents were totally dependent in performing 7 of 11 ADLs and a higher proportion of MFH veterans were completely dependent for toileting (22% long-stay nursing home vs 31% MFH). The only ADLs for which there was a higher proportion of long-stay nursing home residents who were totally dependent compared with MFH residents were walking in room (54% long-stay nursing home vs 38% MFH), walking in the corridor (57% long-stay nursing home vs 33% MFH), and locomotion off the unit (36% long-stay nursing home vs 22% MFH).
While the rates of total ADL dependence among veterans in MFHs suggest that MFHs are providing care to a subset of veterans with high levels of functional impairment and care needs, MFHs are also providing care to veterans who are more independent in performing ADLs and who resemble low-care nursing home residents. A low-care nursing home resident is broadly defined as an one who does not need assistance performing late-loss ADLs (bed mobility, transferring, toileting, and eating) and who does not have the Resource Utilization Group classification of special rehab or clinically complex.31,32 Due to their overall higher functional capacity, low-care residents, even those with chronic medical care needs, may be more appropriately cared for in less intensive care settings than in nursing homes. About 5% to 30% of long-stay nursing home residents can be classified as low care.31,33-37 Additionally, a majority of newly admitted nursing home patients report a preference for or support community discharge rather than long-stay nursing home care, suggesting that many nursing home residents have the potential and desire to transition to a community-based setting.33
Based on the prevalence of veterans in our sample who are similar to low-care nursing home residents and the national focus on shifting LTC to community-based settings, MFHs may be an ideal setting for both low-care nursing home residents and those seeking community-based alternatives to traditional, institutionalized LTC. Additionally, given that we observed greater behavioral and pain needs and similar rates of comorbidities in MFH veterans relative to long-stay nursing home residents, our results indicate that MFHs also have the capacity to care for veterans with higher care needs who desire community-based LTC.
Previous research identified barriers to program MFH growth that may contribute to referral of veterans with fewer ADL dependencies compared with long-stay nursing home residents. A key barrier to MFH referral is that nursing home referral requires selection of a home, whereas MFH referral involves matching veterans with appropriate caregivers, which requires time to align the veteran’s needs with the right caregiver in the right home.7 Given the rigors of finding a match, VA staff who refer veterans may preferentially refer veterans with greater ADL impairments to nursing homes, assuming that higher levels of care needs will complicate the matching process and reserve MFH referral for only the highest functioning candidates.19 However, the ADL data presented here indicate that many MFH residents with significant levels of ADL dependence are living in MFHs. Meeting the care needs of those who have higher ADL dependencies is possible because MFH coordinators and HBPC providers deliver individual, ongoing education to MFH caregivers about caring for MFH veterans and provide available resources needed to safely care for MFH veterans across the spectrum of ADL dependency.7
Veterans with higher levels of functional dependence may also be referred to nursing homes rather than to MFHs because of payment issues. Independent of the VA, veterans or their families negotiate a contract with their caregiver to pay out-of-pocket for MFH caregiving as well as room and board. Particularly for veterans who have military benefits to cover nursing home care costs, the out-of-pocket payment for veterans with high degrees of functional dependence increase as needs increase. These out-of-pocket payments may serve as a barrier to MFH enrollment. The proposed Long-Term Care Veterans Choice Act, which would allow the VA to pay for MFH care for eligible veterans may address this barrier.15
Another possible explanation for the higher rates of functional independence in the MFH cohort is that veterans with functional impairment are not being referred to MFHs. A previous study of the MFH program found that health care providers were often unaware of the program and as a result did not refer eligible veterans to this alternative LTC option.7 The changes proposed by the Long-Term Care Veterans Choice Act may result in an increase in demand in MFH care and thus increase awareness of the program among VA physicians.15
Limitations
There are several potential limitations in this study. First, there are limits to the generalizability of the MFH sample given that the sample of veterans was not randomly selected and that weights were not applied to account for nonresponse bias. Second, charting requirements in MFHs are less intensive compared with nursing home tracking. While the training for research nurses on how to conduct MDS assessments in MFHs was designed to simulate the process in nursing homes, MDS data were likely impacted by differences in charting practices. In addition, MFH caregivers may report certain items, such as aggressive behaviors, more often because they observe MFH veterans round-the-clock compared with NH caregivers who work in shifts and have a lower caregiver to resident ratio. The current data suggest differences in prevalence of behavioral symptoms.
Future studies should examine whether this reflects differences in the populations served or differences in how MFH caregivers track and manage behavioral symptoms. Third, this study was conducted at only MFH sites associated with 4 VAMCs, thus our findings may not be generalizable to veterans in other areas. Finally, there may be differences in the veterans who agreed to participate in the study compared with those who declined to participate. For example, it is possible that the eligible MFH veterans who declined to participate in this study were more functionally impaired than those who did participate. More than one-third (39%) of the family members of cognitively impaired MFH veterans who did not participate cited concerns about the veteran’s frailty as a primary reason for declining to participate. Consequently, the high level of functional status among veterans included in this study compared to nursing home residents may be in part a result of selection bias from more ADL-impaired veterans declining to participate in the study.
Conclusions
Although the MFH program has provided LTC nationally to veterans for nearly 2 decades, this study is the first to administer in-home MDS assessments to veterans in MFHs, allowing for a detailed description of cognitive, functional, and behavioral characteristics of MFH residents. In this study, we found that veterans currently receiving care in MFHs have a wide range of care needs. Our findings indicate that MFHs are caring for some veterans with high functional impairment as well as those who are completely independent in performing ADLs.
Moreover, these results are a preliminary attempt to assist VA health care providers in determining which veterans can be cared for in an MFH such that they can make informed referrals to this alternative LTC setting. To improve the generalizability of these findings, future studies should collect MDS 3.0 assessments longitudinally from a representative sample of veterans in MFHs. Further research is needed to explore how VA providers make the decision to refer a veteran to an MFH compared to a nursing home. Additionally, the percentage of veterans in this study who reported experiencing pain may indicate the need to identify innovative, integrated pain management programs for home settings.
1. Rowe JW, Fulmer T, Fried L. Preparing for better health and health care for an aging population. JAMA. 2016;316(16):1643. doi:10.1001/jama.2016.12335
2. Reaves E, Musumeci M. Medicaid and long-term services and supports: a primer. kaiser family foundation. Published December 15, 2015. Accessed February 12, 2021. https://www.kff.org/medicaid/report/medicaid-and-long-term-services-and-supports-a-primer
3. Collelo KJ, Panangala SV. Long-term care services for veterans. Congressional Research Service Report No. R44697. Published February 14, 2017. Accessed February 12, 2021. https://fas.org/sgp/crs/misc/R44697.pdf
4. American Association of Retired Persons. Beyond 50.05: a report to the nation on livable communities creating environments for successful aging. Published online 2005. Accessed February 12, 2021. https://assets.aarp.org/rgcenter/il/beyond_50_communities.pdf
5. Kaiser Family Foundation. State data and policy actions to address coronavirus. Updated February 11, 2021. Accessed February 12, 2021. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/
6. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 Cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
7. Haverhals LM, Manheim CE, Jones J, Levy C. Launching medical foster home programs: key components to growing this alternative to nursing home placement. J Hous Elderly. 2017;31(1):14-33. doi:10.1080/01634372.2016.1268556
8. US Department of Veterans Affairs. Medical Foster Home Program Procedures- VHA Directive 1141.02(1). Published August 9, 2017. Accessed February 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5447.
9. Haverhals LM, Manheim CE, Gilman CV, Jones J, Levy C. Caregivers create a veteran-centric community in VHA medical foster homes. J Gerontol Soc Work. 2016;59(6):441-457. doi:10.1080/01634372.2016.1231730
10. Jones J, Haverhals LM, Manheim CE, Levy C. Fostering excellence: an examination of high-enrollment VHA Medical Foster Home programs. Home Health Care Manag Pract. 2017;30(1):16-22. doi:10.1177/1084822317736795
11. US Department of Veterans Affairs. Veterans Health Administration. Veterans Health Benefits Handbook. Published 2017. Accessed February 17, 2021. https://www. va.gov/healthbenefits/vhbh/publications/vhbh_sample_handb ook_2014.pdf
12. Duan-Porter W, Ullman K, Rosebush C, McKenzie L, et al; Evidence Synthesis Program. Risk factors and interventions to prevent or delay long term nursing home placement for adults with impairments. Published May 2019. Accessed March 2, 2021. https://www.hsrd.research.va.gov/publications/esp/nursing-home-delay.pdf
13. US Department of Veterans Affairs. Caregiver Support Program- VHA NOTICE 2020-31. Published October 1, 2020. Accessed February 2, 2021. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=9048
14. US Department of Veterans Affairs. Geriatrics and extended care. Published June 10, 2020. Accessed February 22, 2021. https://www.va.gov/geriatrics/pages/Veteran-Directed_Care.asp
15. HR 1527, 116th Cong (2019). Accessed March 1, 2021. congress.gov/bill/116th-congress/house-bill/1527
16. Levy C, Whitfield EA. Medical foster homes: can the adult foster care model substitute for nursing home care? J Am Geriatr Soc. 2016;64(12):2585-2592. doi:10.1111/jgs.14517
17. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602-610. doi:10.1016/j.jamda.2012.06.002
18. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi:10.1016/j.jamda.2012.06.001
19. Gilman C, Haverhals L, Manheim C, Levy C. A qualitative exploration of veteran and family perspectives on medical foster homes. Home Health Care Serv Q. 2018;37(1):1-24. doi:10.1080/01621424.2017.1419156
20. Levy CR, Alemi F, Williams AE, et al. Shared homes as an alternative to nursing home care: impact of VA’s Medical Foster Home program on hospitalization. Gerontologist. 2016;56(1):62-71. doi:10.1093/geront/gnv092
21. Levy CR, Jones J, Haverhals LM, Nowels CT. A qualitative evaluation of a new community living model: medical foster home placement. J Nurs Educ Pract. 2013;4(1):p162. doi:10.5430/jnep.v4n1p162
22. Levy C, Whitfield EA, Gutman R. Medical foster home is less costly than traditional nursing home care. Health Serv Res. 2019;54(6):1346-1356. doi:10.1111/1475-6773.13195
23. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. doi:10.1080/15524256.2016.1156603
24. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi:10.1097/MLR.0000000000000334
25. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618-625. doi:10.1016/j.jamda.2012.06.003
26. Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298-2303. doi:10.1111/j.1532-5415.2008.02048.x
27. McCreedy E, Ogarek JA, Thomas KS, Mor V. The minimum data set agitated and reactive behavior scale: measuring behaviors in nursing home residents with dementia. J Am Med Dir Assoc. 2019;20(12):1548-1552. doi:10.1016/j.jamda.2019.08.030
28. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2016;56(1):52-61. doi:10.1093/geront/gnv099
29. Wysocki A, Thomas KS, Mor V. Functional improvement among short-stay nursing home residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470-474. doi:10.1016/j.jamda.2014.11.018
30. Morris JN, Pries B, Morris’ S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546-M553. doi:10.1093/gerona/54.11.m546
31. Mor V, Zinn J, Gozalo P, Feng Z, Intrator O, Grabowski DC. Prospects for transferring nursing home residents to the community. Health Aff (Millwood). 2007;26(6):1762-1771. doi:10.1377/hlthaff.26.6.1762
32. Ikegami N, Morris JN, Fries BE. Low-care cases in long-term care settings: variation among nations. Age Ageing. 1997;26(suppl 2):67-71. doi:10.1093/ageing/26.suppl_2.67
33. Arling G, Kane RL, Cooke V, Lewis T. Targeting residents for transitions from nursing home to community. Health Serv Res. 2010;45(3):691-711. doi:10.1111/j.1475-6773.2010.01105.x
34. Castle NG. Low-care residents in nursing homes: the impact of market characteristics. J Health Soc Policy. 2002;14(3):41-58. doi:10.1300/J045v14n03_03
35. Grando VT, Rantz MJ, Petroski GF, et al. Prevalence and characteristics of nursing homes residents requiring light-care. Res Nurs Health. 2005;28(3):210-219. doi:10.1002/nur.20079
36. Hahn EA, Thomas KS, Hyer K, Andel R, Meng H. Predictors of low-care prevalence in Florida nursing homes: the role of Medicaid waiver programs. Gerontologist. 2011;51(4):495-503. doi:10.1093/geront/gnr020
37. Thomas KS. The relationship between older Americans act in-home services and low-care residents in nursing homes. J Aging Health. 2014;26(2):250-260. doi:10.1177/0898264313513611
New models are needed for delivering long-term care (LTC) that are home-based, cost-effective, and appropriate for older adults with a range of care needs.1,2 In fiscal year (FY) 2015, the US Department of Veterans Affairs (VA) spent $7.4 billion on LTC, accounting for 13% of total VA health care spending. Overall, 71% of LTC spending in FY 2015 was allocated to institutional care.3 Beyond cost, 95% of older adults prefer to remain in community rather than institutional LTC settings, such as nursing homes.4 The COVID-19 pandemic created additional concerns related to the spread of infectious disease, with > 37% of COVID-19 deaths in the United States occurring in nursing homes irrespective of facility quality.5,6
One community-based LTC alternative developed within the VA is the Medical Foster Home (MFH) program. The MFH program is an adult foster care program in which veterans who are unable to live independently receive round-the-clock care in the home of a community-based caregiver.7 MFH caregivers usually have previous experience caring for family, working in a nursing home, or working as a caregiver in another capacity. These caregivers are responsible for providing 24-hour supervision and support to residents in their MFH and can care for up to 3 adults. In the MFH program, VA home-based primary care (HBPC) teams composed of physicians, registered nurses, physical and occupational therapists, social workers, pharmacists, dieticians, and psychologists, provide primary care for MFH veterans and oversee care in the caregiver’s home.
The goal of the VA HBPC program is to improve veterans’ access to medical care and shift LTC services from institutional to noninstitutional settings by providing in-home care for those who are too sick or disabled to go to a clinic for care. On average, veterans pay the MFH caregiver $2,500 out-of-pocket per month for their care.8 In 2016, there were 992 veterans residing in MFHs across the country.9 Since MFH program implementation expanded nationwide in 2008, more than 4,000 veterans have resided in MFHs in 45 states and territories.10
The VA is required to pay for nursing home care for veterans who have a qualifying VA service-connected disability or who meet a specific threshold of disability.11 Currently, the VA is not authorized to pay for MFH care for veterans who meet the eligibility criteria for VA-paid nursing home care. Over the past decade, the VA has introduced and expanded several initiatives and programs to help veterans who require LTC remain in their homes and communities. These include but are not limited to the Veteran Directed Care program, the Choose Home Initiative, and the Caregiver Support Program.12-14 Additionally, attempts have been made to pass legislation to authorize the VA to pay for MFH for veterans’ care whose military benefits include coverage for nursing home care.15 This legislation and VA initiatives are clear signs that the VA is committed to supporting programs such as the MFH program. Given this commitment, demand for the MFH program will likely increase.
Therefore, VA practitioners need to better identify which veterans are currently in the MFH program. While veterans are expected to need nursing home level care to qualify for MFH enrollment, little has been published about the physical and mental health care needs of veterans currently receiving MFH care. One previous study compared the demographics, diagnostic characteristics, and care utilization of MFH veterans with that of veterans receiving LTC in VA community living centers (CLCs), and found that veterans in MFHs had similar levels of frailty and comorbidity and had a higher mean age when compared with veterans in CLCs.16
Our study assessed a sample of veterans living in MFHs and describes these veterans’ clinical and functional characteristics. We used the Minimum Data Set 3.0 (MDS) to complete the assessments to allow comparisons with other populations residing in long-term care.17,18 While MDS assessments are required for Medicare/Medicaid-certified nursing home residents and for residents in VA CLCs, this study was the first attempt to perform in-home MDS data assessments in MFHs. This collection of descriptive clinical data is an important first step in providing VA practitioners with information about the characteristics of veterans currently cared for in MFHs and policymakers with data to think critically about which veterans are willing to pay for the MFH program.
Methods
This study was part of a larger research project assessing the impact of the MFH program on veterans’ outcomes and health care spending as well as factors influencing program growth.7,9,10,16,19-23 We report on the characteristics of veterans staying in MFHs, using data from the MDS, including a clinical assessment of patients’ cognitive, function, and health care–related needs, collected from participants recruited for this study.
Five research nurses were trained to administer the MDS assessment to veterans in MFHs. Data were collected between April 2014 and December 2015 from veterans at MFH sites associated with 4 urban VA medical centers in 4 different Veterans Integrated Service Networks (58 total homes). While the VA medical centers (VAMCs)were urban, many of the MFHs were in rural areas, given that MFHs can be up to 50 miles from the associated VAMC. We selected MFH sites for this study based on MFH program veteran census. Specifically, we identified MFH sites with high veteran enrollment to ensure we would have a sufficiently large sample for participant recruitment.
Veterans who had resided in an MFH for at least 90 days were eligible to participate. Of the 155 veterans mailed a letter of invitation to participate, 92 (59%) completed the in-home MDS assessment. Reasons for not participating included: 13 veterans died prior to data collection, 18 veterans declined to participate, 18 family members or legal guardians of cognitively impaired veterans did not want the veteran to participate, and 14 veterans left the MFH program or were hospitalized at the time of data collection.
Family members and legal guardians who declined participation on behalf of a veteran reported that they felt the veteran was too frail to participate or that participating would be an added burden on the veteran. Based on the census of veterans residing in all MFHs nationally in November 2015 (N = 972), 9.5% of MFH veterans were included in this study.7This study was approved by the VA Central Institutional Review Board (CIRB #12–31), in addition to the local VA research and development review boards where MFH MDS assessments were collected.
Assessment Instrument and Variables
The MDS 3.0 assesses numerous aspects of clinical and functional status. Several resident-level characteristics from the MDS 3.0 were included in this study. The Cognitive Function Scale (CFS) was used to categorize cognitive function. The CFS is a categorical variable that is created from MDS 3.0 data. The CFS integrates self- and staff-reported data to classify individuals as cognitively intact, mildly impaired, moderately impaired, or severely impaired based on respondents’ Brief Interview for Mental Status (BIMS) assessment or staff-reported cognitive function collected as part of the MDS 3.0.24 We explored depression by calculating a mean summary severity score for all respondents from the Patient Health Questionnaire-9 item interview (PHQ-9).25 PHQ-9 summary scores range from 0 to 27, with mean scores of ≤ 4 indicating no or minimal depression, and higher scores corresponding to more severe depression as scores increase. For respondents who were unable to complete the PHQ-9, we calculated mean PHQ Observational Version (PHQ-9-OV) scores.
We included 2 variables to characterize behaviors: wandering frequency and presence and frequency of aggressive behaviors. We summarized aggressive behaviors using the Aggressive and Reactive Behavior Scale, which characterizes whether a resident has none, mild, moderate, or severe behavioral symptoms based on the presence and frequency of physical and verbal behaviors and resistance to care.26,27 We included items that described pain, number of falls since admission or prior assessment, degree of urinary and bowel continence (always continent vs not always continent) and mobility device use to describe respondents’ health conditions and functional status. To characterize pain, we used veteran’s self-reported frequency and intensity of pain experienced in the prior 5 days and classified the experienced pain as none, mild, moderate, or severe. Finally, demographic characteristics included age and gender.
To determine functional status, we included measures of needing help to perform activities of daily living (ADLs). The MDS allows us to understand functional status ranging from ADLs lost early in the trajectory of functional decline (ie, bathing, hygiene) to those lost in the middle (ie, walking, dressing, toileting, transferring) to those lost late in the trajectory of functional decline (ie, bed mobility and eating).28,29 To assess MFH veterans’ independence in mobility, we considered the veteran’s ability to walk without supervision or assistance in the hallway outside of their room, ability to move between their room and hallway, and ability to move throughout the house. Mobility includes use of an assistive device such as a cane, walker, or wheelchair if the veteran can use it without assistance. We summarized dependency in ADLs, using a combined score of dependence in bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene that ranges from 0 (independent) to 28 (completely dependent).30 Additionally, we created 3-category variables to indicate the degree of dependence in performing ADLs (independent, supervision or assistance, and completely dependent).
Finally, we included diagnoses identified as active to explore differences in neurologic, mood, psychiatric, and chronic disease morbidity. In the MDS 3.0 assessment, an active diagnosis is defined as a diagnosis documented by a licensed independent practitioner in the prior 60 days that has affected the resident or their care in the prior 7 days.
Analysis
We conducted statistical analyses using Stata MP version 15.1 (StataCorp). We summarized demographic characteristics, cognitive function scores, depression scores, pain status, behavioral symptoms, incidence of falls, degree of continence, functional status, and comorbidities, using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Results
Of the 92 MFH veterans in our sample, 85% were male and 83% were aged ≥ 65 years (Table 1). Veterans had an average length of stay of 927 days at the time of MDS assessment. More than half (55%) of MFH veterans had cognitive impairment (ranging from mild to severe). The mean (SD) depression score was 3.3 (3.9), indicating minimal depression. For veterans who could not complete the depression questionnaire, the mean (SD) staff-assessed depression score was 5.9 (5.5), suggesting mild depression. Overall, 22% of the sample had aggressive behaviors but only 7 were noted to be severe. Few residents had caregiver-reported wandering. Self-reported pain intensity indicated that 45% of the sample had mild, moderate, or severe pain. While more than half the cohort had complete bowel continence (53%), only 36% had complete urinary continence. Use of mobility devices was common, with 56% of residents using a wheelchair, 42% using a walker, and 14% using a cane. One-fourth of veterans had fallen at least once since admission to the MFH.
Of the 11 ADLs assessed, the percentage of MFH veterans requiring assistance with early and mid-loss ADLs ranged from 63% for transferring to 84% for bathing (Table 2). Even for the late-loss ADL of eating, 57% of the MFH cohort required assistance. Overall, MFH veterans had an average ADL dependency score of 11.
Physicians documented a diagnosis of either Alzheimer disease or non-Alzheimer dementia comorbidity for 65% of the cohort and traumatic brain injury for 9% (Table 3). Based on psychiatric comorbidities recorded in veterans’ health records, over half of MFH residents had depression (52%). Additionally, 1 in 5 MFH veterans had an anxiety disorder diagnosis. Chronic diseases were prevalent among veterans in MFHs, with 33% diagnosed with diabetes mellitus, 30% with asthma, chronic obstructive pulmonary disease, or chronic lung disease, and 16% with heart failure.
Discussion
In this study, we describe the characteristics of veterans receiving LTC in a sample of MFHs. This is the first study to assess veteran health and function across a group of MFHs. To help provide context for the description of MFH residents, we compared demographic characteristics, cognitive impairment, depression, pain, behaviors, functional status, and morbidity of veterans in the MFH program to long-stay residents in community nursing homes (eAppendix 1-3 available at doi:10.12788/fp.0102). A comparison with this reference population suggests that these MFH and nursing home cohorts are similar in terms of age, wandering behavior, incidence of falls, and prevalence of neurologic, psychiatric, and chronic diseases. Compared with nursing home residents, veterans in the MFH cohort had slightly higher mood symptom scores, were more likely to display aggressive behavior, and were more likely to report experiencing moderate and severe pain.
Additionally, MFH veterans displayed a lower level of cognitive impairment, fewer functional impairments, measured by the ADL dependency score, and were less likely to be bowel or bladder incontinent. Despite an overall lower ADL dependency score, a similar proportion of MFH veterans and nursing home residents were totally dependent in performing 7 of 11 ADLs and a higher proportion of MFH veterans were completely dependent for toileting (22% long-stay nursing home vs 31% MFH). The only ADLs for which there was a higher proportion of long-stay nursing home residents who were totally dependent compared with MFH residents were walking in room (54% long-stay nursing home vs 38% MFH), walking in the corridor (57% long-stay nursing home vs 33% MFH), and locomotion off the unit (36% long-stay nursing home vs 22% MFH).
While the rates of total ADL dependence among veterans in MFHs suggest that MFHs are providing care to a subset of veterans with high levels of functional impairment and care needs, MFHs are also providing care to veterans who are more independent in performing ADLs and who resemble low-care nursing home residents. A low-care nursing home resident is broadly defined as an one who does not need assistance performing late-loss ADLs (bed mobility, transferring, toileting, and eating) and who does not have the Resource Utilization Group classification of special rehab or clinically complex.31,32 Due to their overall higher functional capacity, low-care residents, even those with chronic medical care needs, may be more appropriately cared for in less intensive care settings than in nursing homes. About 5% to 30% of long-stay nursing home residents can be classified as low care.31,33-37 Additionally, a majority of newly admitted nursing home patients report a preference for or support community discharge rather than long-stay nursing home care, suggesting that many nursing home residents have the potential and desire to transition to a community-based setting.33
Based on the prevalence of veterans in our sample who are similar to low-care nursing home residents and the national focus on shifting LTC to community-based settings, MFHs may be an ideal setting for both low-care nursing home residents and those seeking community-based alternatives to traditional, institutionalized LTC. Additionally, given that we observed greater behavioral and pain needs and similar rates of comorbidities in MFH veterans relative to long-stay nursing home residents, our results indicate that MFHs also have the capacity to care for veterans with higher care needs who desire community-based LTC.
Previous research identified barriers to program MFH growth that may contribute to referral of veterans with fewer ADL dependencies compared with long-stay nursing home residents. A key barrier to MFH referral is that nursing home referral requires selection of a home, whereas MFH referral involves matching veterans with appropriate caregivers, which requires time to align the veteran’s needs with the right caregiver in the right home.7 Given the rigors of finding a match, VA staff who refer veterans may preferentially refer veterans with greater ADL impairments to nursing homes, assuming that higher levels of care needs will complicate the matching process and reserve MFH referral for only the highest functioning candidates.19 However, the ADL data presented here indicate that many MFH residents with significant levels of ADL dependence are living in MFHs. Meeting the care needs of those who have higher ADL dependencies is possible because MFH coordinators and HBPC providers deliver individual, ongoing education to MFH caregivers about caring for MFH veterans and provide available resources needed to safely care for MFH veterans across the spectrum of ADL dependency.7
Veterans with higher levels of functional dependence may also be referred to nursing homes rather than to MFHs because of payment issues. Independent of the VA, veterans or their families negotiate a contract with their caregiver to pay out-of-pocket for MFH caregiving as well as room and board. Particularly for veterans who have military benefits to cover nursing home care costs, the out-of-pocket payment for veterans with high degrees of functional dependence increase as needs increase. These out-of-pocket payments may serve as a barrier to MFH enrollment. The proposed Long-Term Care Veterans Choice Act, which would allow the VA to pay for MFH care for eligible veterans may address this barrier.15
Another possible explanation for the higher rates of functional independence in the MFH cohort is that veterans with functional impairment are not being referred to MFHs. A previous study of the MFH program found that health care providers were often unaware of the program and as a result did not refer eligible veterans to this alternative LTC option.7 The changes proposed by the Long-Term Care Veterans Choice Act may result in an increase in demand in MFH care and thus increase awareness of the program among VA physicians.15
Limitations
There are several potential limitations in this study. First, there are limits to the generalizability of the MFH sample given that the sample of veterans was not randomly selected and that weights were not applied to account for nonresponse bias. Second, charting requirements in MFHs are less intensive compared with nursing home tracking. While the training for research nurses on how to conduct MDS assessments in MFHs was designed to simulate the process in nursing homes, MDS data were likely impacted by differences in charting practices. In addition, MFH caregivers may report certain items, such as aggressive behaviors, more often because they observe MFH veterans round-the-clock compared with NH caregivers who work in shifts and have a lower caregiver to resident ratio. The current data suggest differences in prevalence of behavioral symptoms.
Future studies should examine whether this reflects differences in the populations served or differences in how MFH caregivers track and manage behavioral symptoms. Third, this study was conducted at only MFH sites associated with 4 VAMCs, thus our findings may not be generalizable to veterans in other areas. Finally, there may be differences in the veterans who agreed to participate in the study compared with those who declined to participate. For example, it is possible that the eligible MFH veterans who declined to participate in this study were more functionally impaired than those who did participate. More than one-third (39%) of the family members of cognitively impaired MFH veterans who did not participate cited concerns about the veteran’s frailty as a primary reason for declining to participate. Consequently, the high level of functional status among veterans included in this study compared to nursing home residents may be in part a result of selection bias from more ADL-impaired veterans declining to participate in the study.
Conclusions
Although the MFH program has provided LTC nationally to veterans for nearly 2 decades, this study is the first to administer in-home MDS assessments to veterans in MFHs, allowing for a detailed description of cognitive, functional, and behavioral characteristics of MFH residents. In this study, we found that veterans currently receiving care in MFHs have a wide range of care needs. Our findings indicate that MFHs are caring for some veterans with high functional impairment as well as those who are completely independent in performing ADLs.
Moreover, these results are a preliminary attempt to assist VA health care providers in determining which veterans can be cared for in an MFH such that they can make informed referrals to this alternative LTC setting. To improve the generalizability of these findings, future studies should collect MDS 3.0 assessments longitudinally from a representative sample of veterans in MFHs. Further research is needed to explore how VA providers make the decision to refer a veteran to an MFH compared to a nursing home. Additionally, the percentage of veterans in this study who reported experiencing pain may indicate the need to identify innovative, integrated pain management programs for home settings.
New models are needed for delivering long-term care (LTC) that are home-based, cost-effective, and appropriate for older adults with a range of care needs.1,2 In fiscal year (FY) 2015, the US Department of Veterans Affairs (VA) spent $7.4 billion on LTC, accounting for 13% of total VA health care spending. Overall, 71% of LTC spending in FY 2015 was allocated to institutional care.3 Beyond cost, 95% of older adults prefer to remain in community rather than institutional LTC settings, such as nursing homes.4 The COVID-19 pandemic created additional concerns related to the spread of infectious disease, with > 37% of COVID-19 deaths in the United States occurring in nursing homes irrespective of facility quality.5,6
One community-based LTC alternative developed within the VA is the Medical Foster Home (MFH) program. The MFH program is an adult foster care program in which veterans who are unable to live independently receive round-the-clock care in the home of a community-based caregiver.7 MFH caregivers usually have previous experience caring for family, working in a nursing home, or working as a caregiver in another capacity. These caregivers are responsible for providing 24-hour supervision and support to residents in their MFH and can care for up to 3 adults. In the MFH program, VA home-based primary care (HBPC) teams composed of physicians, registered nurses, physical and occupational therapists, social workers, pharmacists, dieticians, and psychologists, provide primary care for MFH veterans and oversee care in the caregiver’s home.
The goal of the VA HBPC program is to improve veterans’ access to medical care and shift LTC services from institutional to noninstitutional settings by providing in-home care for those who are too sick or disabled to go to a clinic for care. On average, veterans pay the MFH caregiver $2,500 out-of-pocket per month for their care.8 In 2016, there were 992 veterans residing in MFHs across the country.9 Since MFH program implementation expanded nationwide in 2008, more than 4,000 veterans have resided in MFHs in 45 states and territories.10
The VA is required to pay for nursing home care for veterans who have a qualifying VA service-connected disability or who meet a specific threshold of disability.11 Currently, the VA is not authorized to pay for MFH care for veterans who meet the eligibility criteria for VA-paid nursing home care. Over the past decade, the VA has introduced and expanded several initiatives and programs to help veterans who require LTC remain in their homes and communities. These include but are not limited to the Veteran Directed Care program, the Choose Home Initiative, and the Caregiver Support Program.12-14 Additionally, attempts have been made to pass legislation to authorize the VA to pay for MFH for veterans’ care whose military benefits include coverage for nursing home care.15 This legislation and VA initiatives are clear signs that the VA is committed to supporting programs such as the MFH program. Given this commitment, demand for the MFH program will likely increase.
Therefore, VA practitioners need to better identify which veterans are currently in the MFH program. While veterans are expected to need nursing home level care to qualify for MFH enrollment, little has been published about the physical and mental health care needs of veterans currently receiving MFH care. One previous study compared the demographics, diagnostic characteristics, and care utilization of MFH veterans with that of veterans receiving LTC in VA community living centers (CLCs), and found that veterans in MFHs had similar levels of frailty and comorbidity and had a higher mean age when compared with veterans in CLCs.16
Our study assessed a sample of veterans living in MFHs and describes these veterans’ clinical and functional characteristics. We used the Minimum Data Set 3.0 (MDS) to complete the assessments to allow comparisons with other populations residing in long-term care.17,18 While MDS assessments are required for Medicare/Medicaid-certified nursing home residents and for residents in VA CLCs, this study was the first attempt to perform in-home MDS data assessments in MFHs. This collection of descriptive clinical data is an important first step in providing VA practitioners with information about the characteristics of veterans currently cared for in MFHs and policymakers with data to think critically about which veterans are willing to pay for the MFH program.
Methods
This study was part of a larger research project assessing the impact of the MFH program on veterans’ outcomes and health care spending as well as factors influencing program growth.7,9,10,16,19-23 We report on the characteristics of veterans staying in MFHs, using data from the MDS, including a clinical assessment of patients’ cognitive, function, and health care–related needs, collected from participants recruited for this study.
Five research nurses were trained to administer the MDS assessment to veterans in MFHs. Data were collected between April 2014 and December 2015 from veterans at MFH sites associated with 4 urban VA medical centers in 4 different Veterans Integrated Service Networks (58 total homes). While the VA medical centers (VAMCs)were urban, many of the MFHs were in rural areas, given that MFHs can be up to 50 miles from the associated VAMC. We selected MFH sites for this study based on MFH program veteran census. Specifically, we identified MFH sites with high veteran enrollment to ensure we would have a sufficiently large sample for participant recruitment.
Veterans who had resided in an MFH for at least 90 days were eligible to participate. Of the 155 veterans mailed a letter of invitation to participate, 92 (59%) completed the in-home MDS assessment. Reasons for not participating included: 13 veterans died prior to data collection, 18 veterans declined to participate, 18 family members or legal guardians of cognitively impaired veterans did not want the veteran to participate, and 14 veterans left the MFH program or were hospitalized at the time of data collection.
Family members and legal guardians who declined participation on behalf of a veteran reported that they felt the veteran was too frail to participate or that participating would be an added burden on the veteran. Based on the census of veterans residing in all MFHs nationally in November 2015 (N = 972), 9.5% of MFH veterans were included in this study.7This study was approved by the VA Central Institutional Review Board (CIRB #12–31), in addition to the local VA research and development review boards where MFH MDS assessments were collected.
Assessment Instrument and Variables
The MDS 3.0 assesses numerous aspects of clinical and functional status. Several resident-level characteristics from the MDS 3.0 were included in this study. The Cognitive Function Scale (CFS) was used to categorize cognitive function. The CFS is a categorical variable that is created from MDS 3.0 data. The CFS integrates self- and staff-reported data to classify individuals as cognitively intact, mildly impaired, moderately impaired, or severely impaired based on respondents’ Brief Interview for Mental Status (BIMS) assessment or staff-reported cognitive function collected as part of the MDS 3.0.24 We explored depression by calculating a mean summary severity score for all respondents from the Patient Health Questionnaire-9 item interview (PHQ-9).25 PHQ-9 summary scores range from 0 to 27, with mean scores of ≤ 4 indicating no or minimal depression, and higher scores corresponding to more severe depression as scores increase. For respondents who were unable to complete the PHQ-9, we calculated mean PHQ Observational Version (PHQ-9-OV) scores.
We included 2 variables to characterize behaviors: wandering frequency and presence and frequency of aggressive behaviors. We summarized aggressive behaviors using the Aggressive and Reactive Behavior Scale, which characterizes whether a resident has none, mild, moderate, or severe behavioral symptoms based on the presence and frequency of physical and verbal behaviors and resistance to care.26,27 We included items that described pain, number of falls since admission or prior assessment, degree of urinary and bowel continence (always continent vs not always continent) and mobility device use to describe respondents’ health conditions and functional status. To characterize pain, we used veteran’s self-reported frequency and intensity of pain experienced in the prior 5 days and classified the experienced pain as none, mild, moderate, or severe. Finally, demographic characteristics included age and gender.
To determine functional status, we included measures of needing help to perform activities of daily living (ADLs). The MDS allows us to understand functional status ranging from ADLs lost early in the trajectory of functional decline (ie, bathing, hygiene) to those lost in the middle (ie, walking, dressing, toileting, transferring) to those lost late in the trajectory of functional decline (ie, bed mobility and eating).28,29 To assess MFH veterans’ independence in mobility, we considered the veteran’s ability to walk without supervision or assistance in the hallway outside of their room, ability to move between their room and hallway, and ability to move throughout the house. Mobility includes use of an assistive device such as a cane, walker, or wheelchair if the veteran can use it without assistance. We summarized dependency in ADLs, using a combined score of dependence in bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene that ranges from 0 (independent) to 28 (completely dependent).30 Additionally, we created 3-category variables to indicate the degree of dependence in performing ADLs (independent, supervision or assistance, and completely dependent).
Finally, we included diagnoses identified as active to explore differences in neurologic, mood, psychiatric, and chronic disease morbidity. In the MDS 3.0 assessment, an active diagnosis is defined as a diagnosis documented by a licensed independent practitioner in the prior 60 days that has affected the resident or their care in the prior 7 days.
Analysis
We conducted statistical analyses using Stata MP version 15.1 (StataCorp). We summarized demographic characteristics, cognitive function scores, depression scores, pain status, behavioral symptoms, incidence of falls, degree of continence, functional status, and comorbidities, using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Results
Of the 92 MFH veterans in our sample, 85% were male and 83% were aged ≥ 65 years (Table 1). Veterans had an average length of stay of 927 days at the time of MDS assessment. More than half (55%) of MFH veterans had cognitive impairment (ranging from mild to severe). The mean (SD) depression score was 3.3 (3.9), indicating minimal depression. For veterans who could not complete the depression questionnaire, the mean (SD) staff-assessed depression score was 5.9 (5.5), suggesting mild depression. Overall, 22% of the sample had aggressive behaviors but only 7 were noted to be severe. Few residents had caregiver-reported wandering. Self-reported pain intensity indicated that 45% of the sample had mild, moderate, or severe pain. While more than half the cohort had complete bowel continence (53%), only 36% had complete urinary continence. Use of mobility devices was common, with 56% of residents using a wheelchair, 42% using a walker, and 14% using a cane. One-fourth of veterans had fallen at least once since admission to the MFH.
Of the 11 ADLs assessed, the percentage of MFH veterans requiring assistance with early and mid-loss ADLs ranged from 63% for transferring to 84% for bathing (Table 2). Even for the late-loss ADL of eating, 57% of the MFH cohort required assistance. Overall, MFH veterans had an average ADL dependency score of 11.
Physicians documented a diagnosis of either Alzheimer disease or non-Alzheimer dementia comorbidity for 65% of the cohort and traumatic brain injury for 9% (Table 3). Based on psychiatric comorbidities recorded in veterans’ health records, over half of MFH residents had depression (52%). Additionally, 1 in 5 MFH veterans had an anxiety disorder diagnosis. Chronic diseases were prevalent among veterans in MFHs, with 33% diagnosed with diabetes mellitus, 30% with asthma, chronic obstructive pulmonary disease, or chronic lung disease, and 16% with heart failure.
Discussion
In this study, we describe the characteristics of veterans receiving LTC in a sample of MFHs. This is the first study to assess veteran health and function across a group of MFHs. To help provide context for the description of MFH residents, we compared demographic characteristics, cognitive impairment, depression, pain, behaviors, functional status, and morbidity of veterans in the MFH program to long-stay residents in community nursing homes (eAppendix 1-3 available at doi:10.12788/fp.0102). A comparison with this reference population suggests that these MFH and nursing home cohorts are similar in terms of age, wandering behavior, incidence of falls, and prevalence of neurologic, psychiatric, and chronic diseases. Compared with nursing home residents, veterans in the MFH cohort had slightly higher mood symptom scores, were more likely to display aggressive behavior, and were more likely to report experiencing moderate and severe pain.
Additionally, MFH veterans displayed a lower level of cognitive impairment, fewer functional impairments, measured by the ADL dependency score, and were less likely to be bowel or bladder incontinent. Despite an overall lower ADL dependency score, a similar proportion of MFH veterans and nursing home residents were totally dependent in performing 7 of 11 ADLs and a higher proportion of MFH veterans were completely dependent for toileting (22% long-stay nursing home vs 31% MFH). The only ADLs for which there was a higher proportion of long-stay nursing home residents who were totally dependent compared with MFH residents were walking in room (54% long-stay nursing home vs 38% MFH), walking in the corridor (57% long-stay nursing home vs 33% MFH), and locomotion off the unit (36% long-stay nursing home vs 22% MFH).
While the rates of total ADL dependence among veterans in MFHs suggest that MFHs are providing care to a subset of veterans with high levels of functional impairment and care needs, MFHs are also providing care to veterans who are more independent in performing ADLs and who resemble low-care nursing home residents. A low-care nursing home resident is broadly defined as an one who does not need assistance performing late-loss ADLs (bed mobility, transferring, toileting, and eating) and who does not have the Resource Utilization Group classification of special rehab or clinically complex.31,32 Due to their overall higher functional capacity, low-care residents, even those with chronic medical care needs, may be more appropriately cared for in less intensive care settings than in nursing homes. About 5% to 30% of long-stay nursing home residents can be classified as low care.31,33-37 Additionally, a majority of newly admitted nursing home patients report a preference for or support community discharge rather than long-stay nursing home care, suggesting that many nursing home residents have the potential and desire to transition to a community-based setting.33
Based on the prevalence of veterans in our sample who are similar to low-care nursing home residents and the national focus on shifting LTC to community-based settings, MFHs may be an ideal setting for both low-care nursing home residents and those seeking community-based alternatives to traditional, institutionalized LTC. Additionally, given that we observed greater behavioral and pain needs and similar rates of comorbidities in MFH veterans relative to long-stay nursing home residents, our results indicate that MFHs also have the capacity to care for veterans with higher care needs who desire community-based LTC.
Previous research identified barriers to program MFH growth that may contribute to referral of veterans with fewer ADL dependencies compared with long-stay nursing home residents. A key barrier to MFH referral is that nursing home referral requires selection of a home, whereas MFH referral involves matching veterans with appropriate caregivers, which requires time to align the veteran’s needs with the right caregiver in the right home.7 Given the rigors of finding a match, VA staff who refer veterans may preferentially refer veterans with greater ADL impairments to nursing homes, assuming that higher levels of care needs will complicate the matching process and reserve MFH referral for only the highest functioning candidates.19 However, the ADL data presented here indicate that many MFH residents with significant levels of ADL dependence are living in MFHs. Meeting the care needs of those who have higher ADL dependencies is possible because MFH coordinators and HBPC providers deliver individual, ongoing education to MFH caregivers about caring for MFH veterans and provide available resources needed to safely care for MFH veterans across the spectrum of ADL dependency.7
Veterans with higher levels of functional dependence may also be referred to nursing homes rather than to MFHs because of payment issues. Independent of the VA, veterans or their families negotiate a contract with their caregiver to pay out-of-pocket for MFH caregiving as well as room and board. Particularly for veterans who have military benefits to cover nursing home care costs, the out-of-pocket payment for veterans with high degrees of functional dependence increase as needs increase. These out-of-pocket payments may serve as a barrier to MFH enrollment. The proposed Long-Term Care Veterans Choice Act, which would allow the VA to pay for MFH care for eligible veterans may address this barrier.15
Another possible explanation for the higher rates of functional independence in the MFH cohort is that veterans with functional impairment are not being referred to MFHs. A previous study of the MFH program found that health care providers were often unaware of the program and as a result did not refer eligible veterans to this alternative LTC option.7 The changes proposed by the Long-Term Care Veterans Choice Act may result in an increase in demand in MFH care and thus increase awareness of the program among VA physicians.15
Limitations
There are several potential limitations in this study. First, there are limits to the generalizability of the MFH sample given that the sample of veterans was not randomly selected and that weights were not applied to account for nonresponse bias. Second, charting requirements in MFHs are less intensive compared with nursing home tracking. While the training for research nurses on how to conduct MDS assessments in MFHs was designed to simulate the process in nursing homes, MDS data were likely impacted by differences in charting practices. In addition, MFH caregivers may report certain items, such as aggressive behaviors, more often because they observe MFH veterans round-the-clock compared with NH caregivers who work in shifts and have a lower caregiver to resident ratio. The current data suggest differences in prevalence of behavioral symptoms.
Future studies should examine whether this reflects differences in the populations served or differences in how MFH caregivers track and manage behavioral symptoms. Third, this study was conducted at only MFH sites associated with 4 VAMCs, thus our findings may not be generalizable to veterans in other areas. Finally, there may be differences in the veterans who agreed to participate in the study compared with those who declined to participate. For example, it is possible that the eligible MFH veterans who declined to participate in this study were more functionally impaired than those who did participate. More than one-third (39%) of the family members of cognitively impaired MFH veterans who did not participate cited concerns about the veteran’s frailty as a primary reason for declining to participate. Consequently, the high level of functional status among veterans included in this study compared to nursing home residents may be in part a result of selection bias from more ADL-impaired veterans declining to participate in the study.
Conclusions
Although the MFH program has provided LTC nationally to veterans for nearly 2 decades, this study is the first to administer in-home MDS assessments to veterans in MFHs, allowing for a detailed description of cognitive, functional, and behavioral characteristics of MFH residents. In this study, we found that veterans currently receiving care in MFHs have a wide range of care needs. Our findings indicate that MFHs are caring for some veterans with high functional impairment as well as those who are completely independent in performing ADLs.
Moreover, these results are a preliminary attempt to assist VA health care providers in determining which veterans can be cared for in an MFH such that they can make informed referrals to this alternative LTC setting. To improve the generalizability of these findings, future studies should collect MDS 3.0 assessments longitudinally from a representative sample of veterans in MFHs. Further research is needed to explore how VA providers make the decision to refer a veteran to an MFH compared to a nursing home. Additionally, the percentage of veterans in this study who reported experiencing pain may indicate the need to identify innovative, integrated pain management programs for home settings.
1. Rowe JW, Fulmer T, Fried L. Preparing for better health and health care for an aging population. JAMA. 2016;316(16):1643. doi:10.1001/jama.2016.12335
2. Reaves E, Musumeci M. Medicaid and long-term services and supports: a primer. kaiser family foundation. Published December 15, 2015. Accessed February 12, 2021. https://www.kff.org/medicaid/report/medicaid-and-long-term-services-and-supports-a-primer
3. Collelo KJ, Panangala SV. Long-term care services for veterans. Congressional Research Service Report No. R44697. Published February 14, 2017. Accessed February 12, 2021. https://fas.org/sgp/crs/misc/R44697.pdf
4. American Association of Retired Persons. Beyond 50.05: a report to the nation on livable communities creating environments for successful aging. Published online 2005. Accessed February 12, 2021. https://assets.aarp.org/rgcenter/il/beyond_50_communities.pdf
5. Kaiser Family Foundation. State data and policy actions to address coronavirus. Updated February 11, 2021. Accessed February 12, 2021. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/
6. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 Cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
7. Haverhals LM, Manheim CE, Jones J, Levy C. Launching medical foster home programs: key components to growing this alternative to nursing home placement. J Hous Elderly. 2017;31(1):14-33. doi:10.1080/01634372.2016.1268556
8. US Department of Veterans Affairs. Medical Foster Home Program Procedures- VHA Directive 1141.02(1). Published August 9, 2017. Accessed February 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5447.
9. Haverhals LM, Manheim CE, Gilman CV, Jones J, Levy C. Caregivers create a veteran-centric community in VHA medical foster homes. J Gerontol Soc Work. 2016;59(6):441-457. doi:10.1080/01634372.2016.1231730
10. Jones J, Haverhals LM, Manheim CE, Levy C. Fostering excellence: an examination of high-enrollment VHA Medical Foster Home programs. Home Health Care Manag Pract. 2017;30(1):16-22. doi:10.1177/1084822317736795
11. US Department of Veterans Affairs. Veterans Health Administration. Veterans Health Benefits Handbook. Published 2017. Accessed February 17, 2021. https://www. va.gov/healthbenefits/vhbh/publications/vhbh_sample_handb ook_2014.pdf
12. Duan-Porter W, Ullman K, Rosebush C, McKenzie L, et al; Evidence Synthesis Program. Risk factors and interventions to prevent or delay long term nursing home placement for adults with impairments. Published May 2019. Accessed March 2, 2021. https://www.hsrd.research.va.gov/publications/esp/nursing-home-delay.pdf
13. US Department of Veterans Affairs. Caregiver Support Program- VHA NOTICE 2020-31. Published October 1, 2020. Accessed February 2, 2021. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=9048
14. US Department of Veterans Affairs. Geriatrics and extended care. Published June 10, 2020. Accessed February 22, 2021. https://www.va.gov/geriatrics/pages/Veteran-Directed_Care.asp
15. HR 1527, 116th Cong (2019). Accessed March 1, 2021. congress.gov/bill/116th-congress/house-bill/1527
16. Levy C, Whitfield EA. Medical foster homes: can the adult foster care model substitute for nursing home care? J Am Geriatr Soc. 2016;64(12):2585-2592. doi:10.1111/jgs.14517
17. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602-610. doi:10.1016/j.jamda.2012.06.002
18. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi:10.1016/j.jamda.2012.06.001
19. Gilman C, Haverhals L, Manheim C, Levy C. A qualitative exploration of veteran and family perspectives on medical foster homes. Home Health Care Serv Q. 2018;37(1):1-24. doi:10.1080/01621424.2017.1419156
20. Levy CR, Alemi F, Williams AE, et al. Shared homes as an alternative to nursing home care: impact of VA’s Medical Foster Home program on hospitalization. Gerontologist. 2016;56(1):62-71. doi:10.1093/geront/gnv092
21. Levy CR, Jones J, Haverhals LM, Nowels CT. A qualitative evaluation of a new community living model: medical foster home placement. J Nurs Educ Pract. 2013;4(1):p162. doi:10.5430/jnep.v4n1p162
22. Levy C, Whitfield EA, Gutman R. Medical foster home is less costly than traditional nursing home care. Health Serv Res. 2019;54(6):1346-1356. doi:10.1111/1475-6773.13195
23. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. doi:10.1080/15524256.2016.1156603
24. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi:10.1097/MLR.0000000000000334
25. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618-625. doi:10.1016/j.jamda.2012.06.003
26. Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298-2303. doi:10.1111/j.1532-5415.2008.02048.x
27. McCreedy E, Ogarek JA, Thomas KS, Mor V. The minimum data set agitated and reactive behavior scale: measuring behaviors in nursing home residents with dementia. J Am Med Dir Assoc. 2019;20(12):1548-1552. doi:10.1016/j.jamda.2019.08.030
28. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2016;56(1):52-61. doi:10.1093/geront/gnv099
29. Wysocki A, Thomas KS, Mor V. Functional improvement among short-stay nursing home residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470-474. doi:10.1016/j.jamda.2014.11.018
30. Morris JN, Pries B, Morris’ S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546-M553. doi:10.1093/gerona/54.11.m546
31. Mor V, Zinn J, Gozalo P, Feng Z, Intrator O, Grabowski DC. Prospects for transferring nursing home residents to the community. Health Aff (Millwood). 2007;26(6):1762-1771. doi:10.1377/hlthaff.26.6.1762
32. Ikegami N, Morris JN, Fries BE. Low-care cases in long-term care settings: variation among nations. Age Ageing. 1997;26(suppl 2):67-71. doi:10.1093/ageing/26.suppl_2.67
33. Arling G, Kane RL, Cooke V, Lewis T. Targeting residents for transitions from nursing home to community. Health Serv Res. 2010;45(3):691-711. doi:10.1111/j.1475-6773.2010.01105.x
34. Castle NG. Low-care residents in nursing homes: the impact of market characteristics. J Health Soc Policy. 2002;14(3):41-58. doi:10.1300/J045v14n03_03
35. Grando VT, Rantz MJ, Petroski GF, et al. Prevalence and characteristics of nursing homes residents requiring light-care. Res Nurs Health. 2005;28(3):210-219. doi:10.1002/nur.20079
36. Hahn EA, Thomas KS, Hyer K, Andel R, Meng H. Predictors of low-care prevalence in Florida nursing homes: the role of Medicaid waiver programs. Gerontologist. 2011;51(4):495-503. doi:10.1093/geront/gnr020
37. Thomas KS. The relationship between older Americans act in-home services and low-care residents in nursing homes. J Aging Health. 2014;26(2):250-260. doi:10.1177/0898264313513611
1. Rowe JW, Fulmer T, Fried L. Preparing for better health and health care for an aging population. JAMA. 2016;316(16):1643. doi:10.1001/jama.2016.12335
2. Reaves E, Musumeci M. Medicaid and long-term services and supports: a primer. kaiser family foundation. Published December 15, 2015. Accessed February 12, 2021. https://www.kff.org/medicaid/report/medicaid-and-long-term-services-and-supports-a-primer
3. Collelo KJ, Panangala SV. Long-term care services for veterans. Congressional Research Service Report No. R44697. Published February 14, 2017. Accessed February 12, 2021. https://fas.org/sgp/crs/misc/R44697.pdf
4. American Association of Retired Persons. Beyond 50.05: a report to the nation on livable communities creating environments for successful aging. Published online 2005. Accessed February 12, 2021. https://assets.aarp.org/rgcenter/il/beyond_50_communities.pdf
5. Kaiser Family Foundation. State data and policy actions to address coronavirus. Updated February 11, 2021. Accessed February 12, 2021. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/
6. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 Cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
7. Haverhals LM, Manheim CE, Jones J, Levy C. Launching medical foster home programs: key components to growing this alternative to nursing home placement. J Hous Elderly. 2017;31(1):14-33. doi:10.1080/01634372.2016.1268556
8. US Department of Veterans Affairs. Medical Foster Home Program Procedures- VHA Directive 1141.02(1). Published August 9, 2017. Accessed February 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5447.
9. Haverhals LM, Manheim CE, Gilman CV, Jones J, Levy C. Caregivers create a veteran-centric community in VHA medical foster homes. J Gerontol Soc Work. 2016;59(6):441-457. doi:10.1080/01634372.2016.1231730
10. Jones J, Haverhals LM, Manheim CE, Levy C. Fostering excellence: an examination of high-enrollment VHA Medical Foster Home programs. Home Health Care Manag Pract. 2017;30(1):16-22. doi:10.1177/1084822317736795
11. US Department of Veterans Affairs. Veterans Health Administration. Veterans Health Benefits Handbook. Published 2017. Accessed February 17, 2021. https://www. va.gov/healthbenefits/vhbh/publications/vhbh_sample_handb ook_2014.pdf
12. Duan-Porter W, Ullman K, Rosebush C, McKenzie L, et al; Evidence Synthesis Program. Risk factors and interventions to prevent or delay long term nursing home placement for adults with impairments. Published May 2019. Accessed March 2, 2021. https://www.hsrd.research.va.gov/publications/esp/nursing-home-delay.pdf
13. US Department of Veterans Affairs. Caregiver Support Program- VHA NOTICE 2020-31. Published October 1, 2020. Accessed February 2, 2021. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=9048
14. US Department of Veterans Affairs. Geriatrics and extended care. Published June 10, 2020. Accessed February 22, 2021. https://www.va.gov/geriatrics/pages/Veteran-Directed_Care.asp
15. HR 1527, 116th Cong (2019). Accessed March 1, 2021. congress.gov/bill/116th-congress/house-bill/1527
16. Levy C, Whitfield EA. Medical foster homes: can the adult foster care model substitute for nursing home care? J Am Geriatr Soc. 2016;64(12):2585-2592. doi:10.1111/jgs.14517
17. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602-610. doi:10.1016/j.jamda.2012.06.002
18. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi:10.1016/j.jamda.2012.06.001
19. Gilman C, Haverhals L, Manheim C, Levy C. A qualitative exploration of veteran and family perspectives on medical foster homes. Home Health Care Serv Q. 2018;37(1):1-24. doi:10.1080/01621424.2017.1419156
20. Levy CR, Alemi F, Williams AE, et al. Shared homes as an alternative to nursing home care: impact of VA’s Medical Foster Home program on hospitalization. Gerontologist. 2016;56(1):62-71. doi:10.1093/geront/gnv092
21. Levy CR, Jones J, Haverhals LM, Nowels CT. A qualitative evaluation of a new community living model: medical foster home placement. J Nurs Educ Pract. 2013;4(1):p162. doi:10.5430/jnep.v4n1p162
22. Levy C, Whitfield EA, Gutman R. Medical foster home is less costly than traditional nursing home care. Health Serv Res. 2019;54(6):1346-1356. doi:10.1111/1475-6773.13195
23. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. doi:10.1080/15524256.2016.1156603
24. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi:10.1097/MLR.0000000000000334
25. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618-625. doi:10.1016/j.jamda.2012.06.003
26. Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298-2303. doi:10.1111/j.1532-5415.2008.02048.x
27. McCreedy E, Ogarek JA, Thomas KS, Mor V. The minimum data set agitated and reactive behavior scale: measuring behaviors in nursing home residents with dementia. J Am Med Dir Assoc. 2019;20(12):1548-1552. doi:10.1016/j.jamda.2019.08.030
28. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2016;56(1):52-61. doi:10.1093/geront/gnv099
29. Wysocki A, Thomas KS, Mor V. Functional improvement among short-stay nursing home residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470-474. doi:10.1016/j.jamda.2014.11.018
30. Morris JN, Pries B, Morris’ S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546-M553. doi:10.1093/gerona/54.11.m546
31. Mor V, Zinn J, Gozalo P, Feng Z, Intrator O, Grabowski DC. Prospects for transferring nursing home residents to the community. Health Aff (Millwood). 2007;26(6):1762-1771. doi:10.1377/hlthaff.26.6.1762
32. Ikegami N, Morris JN, Fries BE. Low-care cases in long-term care settings: variation among nations. Age Ageing. 1997;26(suppl 2):67-71. doi:10.1093/ageing/26.suppl_2.67
33. Arling G, Kane RL, Cooke V, Lewis T. Targeting residents for transitions from nursing home to community. Health Serv Res. 2010;45(3):691-711. doi:10.1111/j.1475-6773.2010.01105.x
34. Castle NG. Low-care residents in nursing homes: the impact of market characteristics. J Health Soc Policy. 2002;14(3):41-58. doi:10.1300/J045v14n03_03
35. Grando VT, Rantz MJ, Petroski GF, et al. Prevalence and characteristics of nursing homes residents requiring light-care. Res Nurs Health. 2005;28(3):210-219. doi:10.1002/nur.20079
36. Hahn EA, Thomas KS, Hyer K, Andel R, Meng H. Predictors of low-care prevalence in Florida nursing homes: the role of Medicaid waiver programs. Gerontologist. 2011;51(4):495-503. doi:10.1093/geront/gnr020
37. Thomas KS. The relationship between older Americans act in-home services and low-care residents in nursing homes. J Aging Health. 2014;26(2):250-260. doi:10.1177/0898264313513611
Physician Responsiveness to Positive Blood Culture Results at the Minneapolis Veterans Affairs Hospital—Is Anyone Paying Attention?
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
Testosterone decline after steroid abuse revealed with new biomarker
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
FDA okays novel dual-action stimulant med for ADHD
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
Semaglutide for meaningful weight loss in obesity and diabetes?
A 2.4-mg weekly injection of the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide led to a clinically meaningful 5% loss in weight for roughly two-thirds of patients with both overweight/obesity and type 2 diabetes, researchers report.
These findings from the Semaglutide Treatment Effect in People With Obesity 2 (STEP 2) trial, one of four phase 3 trials of this drug, which is currently under regulatory review for weight loss, were published March 2 in The Lancet.
More than 1,000 patients (mean initial weight, 100 kg [220 pounds]) were randomly assigned to receive a lifestyle intervention plus a weekly injection of semaglutide 2.4 mg or semaglutide 1.0 mg or placebo. At 68 weeks, they had lost a mean of 9.6%, 7.0%, and 3.4%, respectively, of their starting weight.
In addition, 69% of patients who had received semaglutide 2.4 mg experienced a clinically meaningful 5% loss of weight, compared with 57% of patients who had received the lower dose and 29% of patients who had received placebo.
The higher dose of semaglutide was associated with a greater improvement in cardiometabolic risk factors. The safety profile was similar to that seen with other drugs in this class.
“By far the best results with any weight loss medicine in diabetes”
Importantly, “more than a quarter of participants lost over 15% of their body weight,” senior author Ildiko Lingvay, MD, stressed. This “is by far the best result we had with any weight loss medicine in patients with diabetes,” Dr. Lingvay, of the University of Texas, Dallas, said in a statement from the university.
“The drug works by suppressing appetite centers in the brain to reduce caloric intake,” she explained. “The medication continually tells the body that you just ate, you’re full.”
Similarly, lead author Melanie J. Davies, MD, said that the STEP 2 results “are exciting and represent a new era in weight management in people with type 2 diabetes.
“They mark a real paradigm shift in our ability to treat obesity,” with results closer to those achieved with bariatric surgery, Dr. Davies, of the University of Leicester, England, said in a statement from her institution.
“It is really encouraging,” she continued, “that along with the weight loss we saw real improvements in general health, with significant improvement in physical functioning scores, blood pressure, and blood glucose control.”
Dr. Lingvay noted that on average, patients in the four STEP clinical trials lost 10%-17% of their body weight, “which is a huge step forward compared with all other medications currently available to treat obesity.” She stressed that these results are comparable to the 20%-30% weight loss seen with bariatric surgery.
One of four trials under review
More than 90% of people with type 2 diabetes are overweight or have obesity, and more than 20% of people with obesity have diabetes, wrote Dr. Davies and colleagues.
Semaglutide (Ozempic), administered subcutaneously at a dose of 0.5 mg to 1 mg weekly, is approved by the Food and Drug Administration for the treatment of type 2 diabetes. Dosing studies indicated that it is associated with weight loss.
As previously reported, four trials of the use of semaglutide for weight loss (STEP 1, 2, 3, and 4) have been completed. The combined data were submitted to the FDA on Dec. 4, 2020 (a decision is expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
The STEP 1 and STEP 3 trials of semaglutide 2.4 mg vs. placebo were recently published. The STEP 1 trial involved 1,961 adults with obesity or overweight; the STEP 3 trial, 611 adults with obesity or overweight. In each of the trials, some patients also underwent an intensive lifestyle intervention, and some did not. In both trials, patients with type 2 diabetes were excluded.
Topline results from STEP 2 were reported in June 2020.
STEP 2 enrolled patients with type 2 diabetes
STEP 2 involved 1,210 adults in 149 outpatient clinics in 12 countries in Europe, North America, South America, the Middle East, South Africa, and Asia. All participants had type 2 diabetes.
For all patients, the body mass index was ≥27 kg/m2, and the A1c concentration was 7%-10%. The mean BMI was 35.7 kg/m2, and the mean A1c was 8.1%.
The mean age of the patients was 55 years, and 51% were women; 62% were White, 26% were Asian, 13% were Hispanic, 8% were Black, and 4% were of other ethnicity.
Participants were managed with diet and exercise alone or underwent treatment with a stable dose of up to three oral glucose-lowering agents (metformin, sulfonylureas, SGLT2 inhibitors, or thiazolidinediones) for at least 90 days. They were then randomly assigned in 1:1:1 ratio to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo.
The starting dose of semaglutide was 0.25 mg/wk; the dose was escalated every 4 weeks to reach the target dose.
All patients received monthly counseling from a dietitian about calories (the goal was a 500-calorie/day deficit) and activity (the goal was 150 minutes of walking or stair climbing per week).
The mean A1c dropped by 1.6% and 1.5% in the semaglutide groups and by 0.4% in the placebo group.
Adverse events were more frequent among the patients who received semaglutide (88% and 82%) than in the placebo group (77%).
Gastrointestinal events that were mainly mild to moderate in severity were reported by 64% of patients in the 2.4-mg semaglutide group, 58% in the 1.0-mg semaglutide group, and 34% in the placebo group.
Semaglutide (Rybelsus) is approved in the United States as a once-daily oral agent for use in type 2 diabetes in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
The study was supported by Novo Nordisk. The authors’ relevant financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
A 2.4-mg weekly injection of the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide led to a clinically meaningful 5% loss in weight for roughly two-thirds of patients with both overweight/obesity and type 2 diabetes, researchers report.
These findings from the Semaglutide Treatment Effect in People With Obesity 2 (STEP 2) trial, one of four phase 3 trials of this drug, which is currently under regulatory review for weight loss, were published March 2 in The Lancet.
More than 1,000 patients (mean initial weight, 100 kg [220 pounds]) were randomly assigned to receive a lifestyle intervention plus a weekly injection of semaglutide 2.4 mg or semaglutide 1.0 mg or placebo. At 68 weeks, they had lost a mean of 9.6%, 7.0%, and 3.4%, respectively, of their starting weight.
In addition, 69% of patients who had received semaglutide 2.4 mg experienced a clinically meaningful 5% loss of weight, compared with 57% of patients who had received the lower dose and 29% of patients who had received placebo.
The higher dose of semaglutide was associated with a greater improvement in cardiometabolic risk factors. The safety profile was similar to that seen with other drugs in this class.
“By far the best results with any weight loss medicine in diabetes”
Importantly, “more than a quarter of participants lost over 15% of their body weight,” senior author Ildiko Lingvay, MD, stressed. This “is by far the best result we had with any weight loss medicine in patients with diabetes,” Dr. Lingvay, of the University of Texas, Dallas, said in a statement from the university.
“The drug works by suppressing appetite centers in the brain to reduce caloric intake,” she explained. “The medication continually tells the body that you just ate, you’re full.”
Similarly, lead author Melanie J. Davies, MD, said that the STEP 2 results “are exciting and represent a new era in weight management in people with type 2 diabetes.
“They mark a real paradigm shift in our ability to treat obesity,” with results closer to those achieved with bariatric surgery, Dr. Davies, of the University of Leicester, England, said in a statement from her institution.
“It is really encouraging,” she continued, “that along with the weight loss we saw real improvements in general health, with significant improvement in physical functioning scores, blood pressure, and blood glucose control.”
Dr. Lingvay noted that on average, patients in the four STEP clinical trials lost 10%-17% of their body weight, “which is a huge step forward compared with all other medications currently available to treat obesity.” She stressed that these results are comparable to the 20%-30% weight loss seen with bariatric surgery.
One of four trials under review
More than 90% of people with type 2 diabetes are overweight or have obesity, and more than 20% of people with obesity have diabetes, wrote Dr. Davies and colleagues.
Semaglutide (Ozempic), administered subcutaneously at a dose of 0.5 mg to 1 mg weekly, is approved by the Food and Drug Administration for the treatment of type 2 diabetes. Dosing studies indicated that it is associated with weight loss.
As previously reported, four trials of the use of semaglutide for weight loss (STEP 1, 2, 3, and 4) have been completed. The combined data were submitted to the FDA on Dec. 4, 2020 (a decision is expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
The STEP 1 and STEP 3 trials of semaglutide 2.4 mg vs. placebo were recently published. The STEP 1 trial involved 1,961 adults with obesity or overweight; the STEP 3 trial, 611 adults with obesity or overweight. In each of the trials, some patients also underwent an intensive lifestyle intervention, and some did not. In both trials, patients with type 2 diabetes were excluded.
Topline results from STEP 2 were reported in June 2020.
STEP 2 enrolled patients with type 2 diabetes
STEP 2 involved 1,210 adults in 149 outpatient clinics in 12 countries in Europe, North America, South America, the Middle East, South Africa, and Asia. All participants had type 2 diabetes.
For all patients, the body mass index was ≥27 kg/m2, and the A1c concentration was 7%-10%. The mean BMI was 35.7 kg/m2, and the mean A1c was 8.1%.
The mean age of the patients was 55 years, and 51% were women; 62% were White, 26% were Asian, 13% were Hispanic, 8% were Black, and 4% were of other ethnicity.
Participants were managed with diet and exercise alone or underwent treatment with a stable dose of up to three oral glucose-lowering agents (metformin, sulfonylureas, SGLT2 inhibitors, or thiazolidinediones) for at least 90 days. They were then randomly assigned in 1:1:1 ratio to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo.
The starting dose of semaglutide was 0.25 mg/wk; the dose was escalated every 4 weeks to reach the target dose.
All patients received monthly counseling from a dietitian about calories (the goal was a 500-calorie/day deficit) and activity (the goal was 150 minutes of walking or stair climbing per week).
The mean A1c dropped by 1.6% and 1.5% in the semaglutide groups and by 0.4% in the placebo group.
Adverse events were more frequent among the patients who received semaglutide (88% and 82%) than in the placebo group (77%).
Gastrointestinal events that were mainly mild to moderate in severity were reported by 64% of patients in the 2.4-mg semaglutide group, 58% in the 1.0-mg semaglutide group, and 34% in the placebo group.
Semaglutide (Rybelsus) is approved in the United States as a once-daily oral agent for use in type 2 diabetes in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
The study was supported by Novo Nordisk. The authors’ relevant financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
A 2.4-mg weekly injection of the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide led to a clinically meaningful 5% loss in weight for roughly two-thirds of patients with both overweight/obesity and type 2 diabetes, researchers report.
These findings from the Semaglutide Treatment Effect in People With Obesity 2 (STEP 2) trial, one of four phase 3 trials of this drug, which is currently under regulatory review for weight loss, were published March 2 in The Lancet.
More than 1,000 patients (mean initial weight, 100 kg [220 pounds]) were randomly assigned to receive a lifestyle intervention plus a weekly injection of semaglutide 2.4 mg or semaglutide 1.0 mg or placebo. At 68 weeks, they had lost a mean of 9.6%, 7.0%, and 3.4%, respectively, of their starting weight.
In addition, 69% of patients who had received semaglutide 2.4 mg experienced a clinically meaningful 5% loss of weight, compared with 57% of patients who had received the lower dose and 29% of patients who had received placebo.
The higher dose of semaglutide was associated with a greater improvement in cardiometabolic risk factors. The safety profile was similar to that seen with other drugs in this class.
“By far the best results with any weight loss medicine in diabetes”
Importantly, “more than a quarter of participants lost over 15% of their body weight,” senior author Ildiko Lingvay, MD, stressed. This “is by far the best result we had with any weight loss medicine in patients with diabetes,” Dr. Lingvay, of the University of Texas, Dallas, said in a statement from the university.
“The drug works by suppressing appetite centers in the brain to reduce caloric intake,” she explained. “The medication continually tells the body that you just ate, you’re full.”
Similarly, lead author Melanie J. Davies, MD, said that the STEP 2 results “are exciting and represent a new era in weight management in people with type 2 diabetes.
“They mark a real paradigm shift in our ability to treat obesity,” with results closer to those achieved with bariatric surgery, Dr. Davies, of the University of Leicester, England, said in a statement from her institution.
“It is really encouraging,” she continued, “that along with the weight loss we saw real improvements in general health, with significant improvement in physical functioning scores, blood pressure, and blood glucose control.”
Dr. Lingvay noted that on average, patients in the four STEP clinical trials lost 10%-17% of their body weight, “which is a huge step forward compared with all other medications currently available to treat obesity.” She stressed that these results are comparable to the 20%-30% weight loss seen with bariatric surgery.
One of four trials under review
More than 90% of people with type 2 diabetes are overweight or have obesity, and more than 20% of people with obesity have diabetes, wrote Dr. Davies and colleagues.
Semaglutide (Ozempic), administered subcutaneously at a dose of 0.5 mg to 1 mg weekly, is approved by the Food and Drug Administration for the treatment of type 2 diabetes. Dosing studies indicated that it is associated with weight loss.
As previously reported, four trials of the use of semaglutide for weight loss (STEP 1, 2, 3, and 4) have been completed. The combined data were submitted to the FDA on Dec. 4, 2020 (a decision is expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
The STEP 1 and STEP 3 trials of semaglutide 2.4 mg vs. placebo were recently published. The STEP 1 trial involved 1,961 adults with obesity or overweight; the STEP 3 trial, 611 adults with obesity or overweight. In each of the trials, some patients also underwent an intensive lifestyle intervention, and some did not. In both trials, patients with type 2 diabetes were excluded.
Topline results from STEP 2 were reported in June 2020.
STEP 2 enrolled patients with type 2 diabetes
STEP 2 involved 1,210 adults in 149 outpatient clinics in 12 countries in Europe, North America, South America, the Middle East, South Africa, and Asia. All participants had type 2 diabetes.
For all patients, the body mass index was ≥27 kg/m2, and the A1c concentration was 7%-10%. The mean BMI was 35.7 kg/m2, and the mean A1c was 8.1%.
The mean age of the patients was 55 years, and 51% were women; 62% were White, 26% were Asian, 13% were Hispanic, 8% were Black, and 4% were of other ethnicity.
Participants were managed with diet and exercise alone or underwent treatment with a stable dose of up to three oral glucose-lowering agents (metformin, sulfonylureas, SGLT2 inhibitors, or thiazolidinediones) for at least 90 days. They were then randomly assigned in 1:1:1 ratio to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo.
The starting dose of semaglutide was 0.25 mg/wk; the dose was escalated every 4 weeks to reach the target dose.
All patients received monthly counseling from a dietitian about calories (the goal was a 500-calorie/day deficit) and activity (the goal was 150 minutes of walking or stair climbing per week).
The mean A1c dropped by 1.6% and 1.5% in the semaglutide groups and by 0.4% in the placebo group.
Adverse events were more frequent among the patients who received semaglutide (88% and 82%) than in the placebo group (77%).
Gastrointestinal events that were mainly mild to moderate in severity were reported by 64% of patients in the 2.4-mg semaglutide group, 58% in the 1.0-mg semaglutide group, and 34% in the placebo group.
Semaglutide (Rybelsus) is approved in the United States as a once-daily oral agent for use in type 2 diabetes in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
The study was supported by Novo Nordisk. The authors’ relevant financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Decline in weekly child COVID-19 cases has almost stopped
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
Call to action on obesity amid COVID-19 pandemic
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Risdiplam study shows promise for spinal muscular atrophy
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Federal Government Ramps Up COVID-19 Vaccination Programs
The Biden Administration launched the first phase of the Federally Qualified Health Center (FQHC) Program for COVID-19 Vaccination. Beginning February 15, FQHCs (including centers in the Urban Indian Health Program) began directly receiving vaccines.
The announcement coincided with a boost in vaccine supply for states, Tribes, and territories. In early February, the Biden Administration announced it would expand vaccine supply to 11 million doses nationwide, a 28% increase since January 20, when President Biden took office. According to a White House fact sheet, “The Administration is committing to maintaining this as the minimum supply level for the next three weeks, and we will continue to work with manufacturers in their efforts to ramp up supply.”
In February, President Biden and Vice President Harris travelled to Arizona and toured a vaccination site at State Farm Stadium in Glendale. Arizona, one of the first states to reach out for federal help from the new administration, has 15 counties and 22 Tribes with sovereign lands in the state. Those 37 entities work collaboratively with the Federal Emergency Management Agency (FEMA), said Major General Michael McGuire, head of the Arizona National Guard.
In his remarks during the tour, President Biden addressed equity, saying, “[I]t really does matter that we have access to the people who are most in need [and are] most affected by the COVID crisis, dying at faster rates, getting sick at faster rates, …but not being able to get into the mix. …Equity is a big thing.”
To that end, one of the programs under way is to stand up four vaccination centers for the Navajo Nation. Tammy Littrell, Acting Regional Administrator for FEMA, said the centers will help increase tribal members’ access to vaccination, as well as take the burden off from having to drive in “austere winter conditions.”
In addition to more vaccines, Indian Health Services (IHS) is allocating $1 billion it received to help with COVID-19 response. Of the $1 billion, $790 million will go to testing, contact tracing, containment, and mitigation, among other things. Another $210 million will support IHS, tribal, and urban Indian health programs for vaccine-related activities to ensure broad-based distribution, access, and vaccine coverage. The money is part of the fifth round of supplemental COVID-19 funding from the Coronavirus Response and Relief Supplemental Appropriations Act. The funds transferred so far amount to nearly $3 billion.
According to IHS, the money can be used to scale up testing by public health, academic, commercial, and hospital laboratories, as well as community-based testing sites, mobile testing units, healthcare facilities, and other entities engaged in COVID-19 testing. The funds are also legally available to lease or purchase non-federally owned facilities to improve COVID-19 preparedness and response capability.
The Biden Administration launched the first phase of the Federally Qualified Health Center (FQHC) Program for COVID-19 Vaccination. Beginning February 15, FQHCs (including centers in the Urban Indian Health Program) began directly receiving vaccines.
The announcement coincided with a boost in vaccine supply for states, Tribes, and territories. In early February, the Biden Administration announced it would expand vaccine supply to 11 million doses nationwide, a 28% increase since January 20, when President Biden took office. According to a White House fact sheet, “The Administration is committing to maintaining this as the minimum supply level for the next three weeks, and we will continue to work with manufacturers in their efforts to ramp up supply.”
In February, President Biden and Vice President Harris travelled to Arizona and toured a vaccination site at State Farm Stadium in Glendale. Arizona, one of the first states to reach out for federal help from the new administration, has 15 counties and 22 Tribes with sovereign lands in the state. Those 37 entities work collaboratively with the Federal Emergency Management Agency (FEMA), said Major General Michael McGuire, head of the Arizona National Guard.
In his remarks during the tour, President Biden addressed equity, saying, “[I]t really does matter that we have access to the people who are most in need [and are] most affected by the COVID crisis, dying at faster rates, getting sick at faster rates, …but not being able to get into the mix. …Equity is a big thing.”
To that end, one of the programs under way is to stand up four vaccination centers for the Navajo Nation. Tammy Littrell, Acting Regional Administrator for FEMA, said the centers will help increase tribal members’ access to vaccination, as well as take the burden off from having to drive in “austere winter conditions.”
In addition to more vaccines, Indian Health Services (IHS) is allocating $1 billion it received to help with COVID-19 response. Of the $1 billion, $790 million will go to testing, contact tracing, containment, and mitigation, among other things. Another $210 million will support IHS, tribal, and urban Indian health programs for vaccine-related activities to ensure broad-based distribution, access, and vaccine coverage. The money is part of the fifth round of supplemental COVID-19 funding from the Coronavirus Response and Relief Supplemental Appropriations Act. The funds transferred so far amount to nearly $3 billion.
According to IHS, the money can be used to scale up testing by public health, academic, commercial, and hospital laboratories, as well as community-based testing sites, mobile testing units, healthcare facilities, and other entities engaged in COVID-19 testing. The funds are also legally available to lease or purchase non-federally owned facilities to improve COVID-19 preparedness and response capability.
The Biden Administration launched the first phase of the Federally Qualified Health Center (FQHC) Program for COVID-19 Vaccination. Beginning February 15, FQHCs (including centers in the Urban Indian Health Program) began directly receiving vaccines.
The announcement coincided with a boost in vaccine supply for states, Tribes, and territories. In early February, the Biden Administration announced it would expand vaccine supply to 11 million doses nationwide, a 28% increase since January 20, when President Biden took office. According to a White House fact sheet, “The Administration is committing to maintaining this as the minimum supply level for the next three weeks, and we will continue to work with manufacturers in their efforts to ramp up supply.”
In February, President Biden and Vice President Harris travelled to Arizona and toured a vaccination site at State Farm Stadium in Glendale. Arizona, one of the first states to reach out for federal help from the new administration, has 15 counties and 22 Tribes with sovereign lands in the state. Those 37 entities work collaboratively with the Federal Emergency Management Agency (FEMA), said Major General Michael McGuire, head of the Arizona National Guard.
In his remarks during the tour, President Biden addressed equity, saying, “[I]t really does matter that we have access to the people who are most in need [and are] most affected by the COVID crisis, dying at faster rates, getting sick at faster rates, …but not being able to get into the mix. …Equity is a big thing.”
To that end, one of the programs under way is to stand up four vaccination centers for the Navajo Nation. Tammy Littrell, Acting Regional Administrator for FEMA, said the centers will help increase tribal members’ access to vaccination, as well as take the burden off from having to drive in “austere winter conditions.”
In addition to more vaccines, Indian Health Services (IHS) is allocating $1 billion it received to help with COVID-19 response. Of the $1 billion, $790 million will go to testing, contact tracing, containment, and mitigation, among other things. Another $210 million will support IHS, tribal, and urban Indian health programs for vaccine-related activities to ensure broad-based distribution, access, and vaccine coverage. The money is part of the fifth round of supplemental COVID-19 funding from the Coronavirus Response and Relief Supplemental Appropriations Act. The funds transferred so far amount to nearly $3 billion.
According to IHS, the money can be used to scale up testing by public health, academic, commercial, and hospital laboratories, as well as community-based testing sites, mobile testing units, healthcare facilities, and other entities engaged in COVID-19 testing. The funds are also legally available to lease or purchase non-federally owned facilities to improve COVID-19 preparedness and response capability.
Pediatric TB – more work needed, especially with HIV-coinfection
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
FROM CROI 2021