User login
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
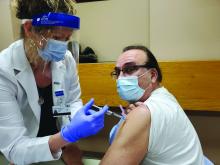
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Bacteriotherapy passes early test in phase 1 atopic dermatitis study
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
that also demonstrated “encouraging clinical and mechanistic results,” Richard L. Gallo, MD, PhD, and his coinvestigators have reported in Nature Medicine.
Findings from the 1-week, 54-patient trial of a topical formulation containing Staphylococcus hominis A9 (ShA9) offer evidence that the strain directly kills S. aureus, inhibits the production of S. aureus–generated toxins, and enables expansion of a healthy bacterial community, “allowing the rest of the microbiome to start to recover to normal,” Dr. Gallo, professor and chairman of the department of dermatology at the University of California, San Diego, said in an interview.
“And perhaps most exciting,” Dr. Gallo added, is the finding that the subset of patients with AD who were most responsive to the ShA9 compound – approximately two-thirds of the participants who were randomized to receive it – showed improvement in local EASI (Eczema Area and Severity Index) and SCORAD (Scoring Atopic Dermatitis) scores used to assess inflammation. Plans are underway for a larger and longer trial, he said.
S. aureus commonly colonizes patients with AD and exacerbates disease by causing inflammation. In recent years, Dr. Gallo and other investigators have come to believe that AD is a cyclic disease in which the skin’s microbiome affects the host, and the host affects the microbiome. The goal of bacteriotherapy is to break the cycle of S. aureus colonization and improve the skin immune and barrier dysfunction characteristics of AD, Dr. Gallo said.
ShA9, a bacterium isolated from healthy human skin, was chosen as a potential topical therapy for AD based on its capacity both to selectively kill S. aureus and to inhibit toxin production by S. aureus. Dr. Gallo’s team’s preclinical work involved screening thousands of isolates of coagulase-negative staphylococci for gene products that perform these two functions by expressing both antimicrobial peptides (AMPs) and autoinducing peptides (AIPs), the latter of which inhibit the S. aureus quorum-sending system that leads to toxin production. Most patients with AD lack protective strains of coagulase-negative staphylococci, including S. hominis, prior research has found.
The double-blind phase 1 trial randomized 54 adults with moderate-severe AD affecting the ventral forearms in a 2:1 fashion to receive the proprietary lyophilized preparation of ShA9 or an ShA9-free formulation twice daily for 1 week. All participants were culture positive for S. aureus.
Clinical assessments and skin swabs were obtained before and within an hour after the first application of day 1, and swabs were collected on days 4 and 7 within 4 hours of the first application.
Blinded physician assessments and skin swabs were also obtained at 24, 48, and 96 hours after the final dose on day 7.
Based on structured daily diaries, there were no serious adverse events, and significantly fewer adverse events in those treated with ShA9, compared with the vehicle alone; 55.6% versus 83.3%, respectively, were considered to have adverse events.
The adverse event–reporting system captured the normal fluctuation of eczema and considered any report of fluctuation above baseline to be an adverse event. “Patients treated with the [placebo formulation] had the expected high frequency of itching, burning, and pain that you see with AD but it was encouraging that the frequency of reporting these events was significantly less in those treated with the active [formulation],” Dr. Gallo said in the interview.
Their report describes a decrease in S. aureus in participants treated with ShA9, and increases in ShA9 DNA. Not all S. aureus strains were directly killed by ShA9, but all strains had reduced expression of mRNA for psm-alpha, an important virulence factor. That reduced expression correlated with ShA9 AIPs and improved EASI scores, the latter of which was observed in a post-hoc analysis. “Participants with S. aureus not killed by ShA9 were still sensitive to inhibition of toxin production, a mechanistic outcome that predicted clinical improvement in mice and may require longer therapy to observe clinical improvement in humans,” the investigators wrote.
Local eczema severity was not significantly different between the bacteriotherapy and control groups. But the post-hoc analysis showed that after 7 days of treatment, and up to 4 days after treatment was discontinued, the patients with S. aureus that was sensitive to killing by ShA9 (21 out of 35 total who received the bacteriotherapy) showed improvement in EASI and SCORAD scores, compared with control patients.
Future research will assess the compound in both S. aureus culture-positive and culture-negative patients, and in patients with mild disease, Dr. Gallo said.
The trial was conducted at USCD and the National Jewish Health General Clinical Research Center in Denver, and was sponsored by the National Institute of Allergy and Infectious Diseases. The ShA9 formulation and related technology are licensed to MatriSys Bioscience, of which Dr. Gallo is the cofounder and an advisory board member. Dr. Gallo holds equity interest in the company.
FROM NATURE MEDICINE
ASCO, CSCO outline ‘best practices’ for nasopharyngeal carcinoma
The guidelines, based on data from more than 100 studies, support offering intensity-modulated radiotherapy to all patients with stage II-IVA nasopharyngeal carcinoma. Recommendations for chemotherapy vary according to disease stage, tumor size, number of nodes, and contraindications.
The guidelines, released jointly by the Chinese Society of Clinical Oncology (CSCO) and the American Society of Clinical Oncology (ASCO), were published in the Journal of Clinical Oncology.
“For practicing oncologists in the United States, who often lack experience treating nasopharyngeal cancer, this guideline provides a useful, succinct summary of available evidence and expert recommendations. Nasopharyngeal cancer can be a technically and medically challenging disease to manage, and a multidisciplinary approach should be strongly encouraged,” said ASCO expert Randall J. Kimple, MD, PhD, of the University of Wisconsin–Madison.
“Much of the data guiding our treatment of these patients comes from endemic regions,” he continued. “How these data apply to patients in the U.S. remains a subject of ongoing study. Patients and providers should be encouraged to seek the opinion of a provider with expertise in the management of nasopharyngeal cancer.”
To compile “best practices” for treating nasopharyngeal carcinoma, the ASCO/CSCO expert panel conducted a literature search that included systematic reviews, meta-analyses, and randomized controlled trials published from 1990 through 2020. The panel identified 108 relevant studies and formulated their guidelines based on the evidence.
Recommendations
For all patients with stage II-IVA nasopharyngeal carcinoma, the guidelines recommend intensity-modulated radiation therapy with daily image guidance. The recommended dose is 70 Gy in 33-35 fractions over 7 weeks.
“This has been the standard approach at most institutions that treat a sufficient number of nasopharyngeal cancer patients each year,” Dr. Kimple said.
When adding chemotherapy to radiotherapy, two approaches are recommended. The first is induction chemotherapy followed by chemoradiation, and the second is chemoradiation followed by adjuvant chemotherapy.
“There are divergent opinions regarding the optimal approach in these patients, with slightly stronger data supporting the use of induction chemotherapy,” Dr. Kimple said. “For patients with earlier stage nasopharyngeal cancer (T1-2N1 or T2N0), chemotherapy can be offered, and is more strongly recommended for those with more advanced disease (T2N1, bulky disease, high EBV load).”
For patients receiving concurrent chemotherapy and radiotherapy, the recommended regimen is cisplatin given either weekly (40 mg/m2) or triweekly (100 mg/m2).
“The stated goal is to achieve a cumulative cisplatin dose in excess of 200 mg/m2 regardless of the approach taken. Several options were provided for patients with a contraindication to cisplatin,” Dr. Kimple said.
Patients with contraindications can receive nedaplatin (100 mg/m2 triweekly), carboplatin (area under curve, 5-6 triweekly), oxaliplatin (70 mg/m2 weekly), or fluoropyrimidines (capecitabine, 5-fluorouracil, or tegafur).
For induction, the guidelines recommend platinum-based chemotherapy. Options include gemcitabine plus cisplatin, cisplatin plus 5-fluorouracil, cisplatin plus capecitabine, docetaxel plus cisplatin, and docetaxel plus cisplatin and 5-fluorouracil.
“[T]here is less strong evidence regarding the optimal induction chemotherapy approach for patients with nasopharyngeal cancer. Several possible regimens (doublet or triplet) are offered, with the use of platinum-based regimens being the common theme,” Dr. Kimple said.
For adjuvant chemotherapy, the guidelines recommend cisplatin plus 5-fluorouracil or carboplatin plus 5-fluorouracil.
The guidelines also suggest that clinicians take into account a patient’s other chronic conditions when formulating the treatment and follow-up plan.
“Patients with multiple chronic conditions pose a particular challenge to guideline-based care due to being commonly excluded from clinical trials,” Dr. Kimple said. “Shared decision-making plays a key role in the recommendations for patients with multiple chronic conditions. In addition, nasopharyngeal cancer patients often have long-term toxicity associated with their care, and, thus, the availability of expertise and resources in management of this disease is important.”
Dr. Kimple disclosed relationships with Galera Therapeutics, Mele Associates, and Guidepoint Global. The guideline authors disclosed relationships with a range of pharmaceutical companies, as listed in the article.
The guidelines, based on data from more than 100 studies, support offering intensity-modulated radiotherapy to all patients with stage II-IVA nasopharyngeal carcinoma. Recommendations for chemotherapy vary according to disease stage, tumor size, number of nodes, and contraindications.
The guidelines, released jointly by the Chinese Society of Clinical Oncology (CSCO) and the American Society of Clinical Oncology (ASCO), were published in the Journal of Clinical Oncology.
“For practicing oncologists in the United States, who often lack experience treating nasopharyngeal cancer, this guideline provides a useful, succinct summary of available evidence and expert recommendations. Nasopharyngeal cancer can be a technically and medically challenging disease to manage, and a multidisciplinary approach should be strongly encouraged,” said ASCO expert Randall J. Kimple, MD, PhD, of the University of Wisconsin–Madison.
“Much of the data guiding our treatment of these patients comes from endemic regions,” he continued. “How these data apply to patients in the U.S. remains a subject of ongoing study. Patients and providers should be encouraged to seek the opinion of a provider with expertise in the management of nasopharyngeal cancer.”
To compile “best practices” for treating nasopharyngeal carcinoma, the ASCO/CSCO expert panel conducted a literature search that included systematic reviews, meta-analyses, and randomized controlled trials published from 1990 through 2020. The panel identified 108 relevant studies and formulated their guidelines based on the evidence.
Recommendations
For all patients with stage II-IVA nasopharyngeal carcinoma, the guidelines recommend intensity-modulated radiation therapy with daily image guidance. The recommended dose is 70 Gy in 33-35 fractions over 7 weeks.
“This has been the standard approach at most institutions that treat a sufficient number of nasopharyngeal cancer patients each year,” Dr. Kimple said.
When adding chemotherapy to radiotherapy, two approaches are recommended. The first is induction chemotherapy followed by chemoradiation, and the second is chemoradiation followed by adjuvant chemotherapy.
“There are divergent opinions regarding the optimal approach in these patients, with slightly stronger data supporting the use of induction chemotherapy,” Dr. Kimple said. “For patients with earlier stage nasopharyngeal cancer (T1-2N1 or T2N0), chemotherapy can be offered, and is more strongly recommended for those with more advanced disease (T2N1, bulky disease, high EBV load).”
For patients receiving concurrent chemotherapy and radiotherapy, the recommended regimen is cisplatin given either weekly (40 mg/m2) or triweekly (100 mg/m2).
“The stated goal is to achieve a cumulative cisplatin dose in excess of 200 mg/m2 regardless of the approach taken. Several options were provided for patients with a contraindication to cisplatin,” Dr. Kimple said.
Patients with contraindications can receive nedaplatin (100 mg/m2 triweekly), carboplatin (area under curve, 5-6 triweekly), oxaliplatin (70 mg/m2 weekly), or fluoropyrimidines (capecitabine, 5-fluorouracil, or tegafur).
For induction, the guidelines recommend platinum-based chemotherapy. Options include gemcitabine plus cisplatin, cisplatin plus 5-fluorouracil, cisplatin plus capecitabine, docetaxel plus cisplatin, and docetaxel plus cisplatin and 5-fluorouracil.
“[T]here is less strong evidence regarding the optimal induction chemotherapy approach for patients with nasopharyngeal cancer. Several possible regimens (doublet or triplet) are offered, with the use of platinum-based regimens being the common theme,” Dr. Kimple said.
For adjuvant chemotherapy, the guidelines recommend cisplatin plus 5-fluorouracil or carboplatin plus 5-fluorouracil.
The guidelines also suggest that clinicians take into account a patient’s other chronic conditions when formulating the treatment and follow-up plan.
“Patients with multiple chronic conditions pose a particular challenge to guideline-based care due to being commonly excluded from clinical trials,” Dr. Kimple said. “Shared decision-making plays a key role in the recommendations for patients with multiple chronic conditions. In addition, nasopharyngeal cancer patients often have long-term toxicity associated with their care, and, thus, the availability of expertise and resources in management of this disease is important.”
Dr. Kimple disclosed relationships with Galera Therapeutics, Mele Associates, and Guidepoint Global. The guideline authors disclosed relationships with a range of pharmaceutical companies, as listed in the article.
The guidelines, based on data from more than 100 studies, support offering intensity-modulated radiotherapy to all patients with stage II-IVA nasopharyngeal carcinoma. Recommendations for chemotherapy vary according to disease stage, tumor size, number of nodes, and contraindications.
The guidelines, released jointly by the Chinese Society of Clinical Oncology (CSCO) and the American Society of Clinical Oncology (ASCO), were published in the Journal of Clinical Oncology.
“For practicing oncologists in the United States, who often lack experience treating nasopharyngeal cancer, this guideline provides a useful, succinct summary of available evidence and expert recommendations. Nasopharyngeal cancer can be a technically and medically challenging disease to manage, and a multidisciplinary approach should be strongly encouraged,” said ASCO expert Randall J. Kimple, MD, PhD, of the University of Wisconsin–Madison.
“Much of the data guiding our treatment of these patients comes from endemic regions,” he continued. “How these data apply to patients in the U.S. remains a subject of ongoing study. Patients and providers should be encouraged to seek the opinion of a provider with expertise in the management of nasopharyngeal cancer.”
To compile “best practices” for treating nasopharyngeal carcinoma, the ASCO/CSCO expert panel conducted a literature search that included systematic reviews, meta-analyses, and randomized controlled trials published from 1990 through 2020. The panel identified 108 relevant studies and formulated their guidelines based on the evidence.
Recommendations
For all patients with stage II-IVA nasopharyngeal carcinoma, the guidelines recommend intensity-modulated radiation therapy with daily image guidance. The recommended dose is 70 Gy in 33-35 fractions over 7 weeks.
“This has been the standard approach at most institutions that treat a sufficient number of nasopharyngeal cancer patients each year,” Dr. Kimple said.
When adding chemotherapy to radiotherapy, two approaches are recommended. The first is induction chemotherapy followed by chemoradiation, and the second is chemoradiation followed by adjuvant chemotherapy.
“There are divergent opinions regarding the optimal approach in these patients, with slightly stronger data supporting the use of induction chemotherapy,” Dr. Kimple said. “For patients with earlier stage nasopharyngeal cancer (T1-2N1 or T2N0), chemotherapy can be offered, and is more strongly recommended for those with more advanced disease (T2N1, bulky disease, high EBV load).”
For patients receiving concurrent chemotherapy and radiotherapy, the recommended regimen is cisplatin given either weekly (40 mg/m2) or triweekly (100 mg/m2).
“The stated goal is to achieve a cumulative cisplatin dose in excess of 200 mg/m2 regardless of the approach taken. Several options were provided for patients with a contraindication to cisplatin,” Dr. Kimple said.
Patients with contraindications can receive nedaplatin (100 mg/m2 triweekly), carboplatin (area under curve, 5-6 triweekly), oxaliplatin (70 mg/m2 weekly), or fluoropyrimidines (capecitabine, 5-fluorouracil, or tegafur).
For induction, the guidelines recommend platinum-based chemotherapy. Options include gemcitabine plus cisplatin, cisplatin plus 5-fluorouracil, cisplatin plus capecitabine, docetaxel plus cisplatin, and docetaxel plus cisplatin and 5-fluorouracil.
“[T]here is less strong evidence regarding the optimal induction chemotherapy approach for patients with nasopharyngeal cancer. Several possible regimens (doublet or triplet) are offered, with the use of platinum-based regimens being the common theme,” Dr. Kimple said.
For adjuvant chemotherapy, the guidelines recommend cisplatin plus 5-fluorouracil or carboplatin plus 5-fluorouracil.
The guidelines also suggest that clinicians take into account a patient’s other chronic conditions when formulating the treatment and follow-up plan.
“Patients with multiple chronic conditions pose a particular challenge to guideline-based care due to being commonly excluded from clinical trials,” Dr. Kimple said. “Shared decision-making plays a key role in the recommendations for patients with multiple chronic conditions. In addition, nasopharyngeal cancer patients often have long-term toxicity associated with their care, and, thus, the availability of expertise and resources in management of this disease is important.”
Dr. Kimple disclosed relationships with Galera Therapeutics, Mele Associates, and Guidepoint Global. The guideline authors disclosed relationships with a range of pharmaceutical companies, as listed in the article.
FROM JOURNAL OF CLINICAL ONCOLOGY
Joint pain in patients with hemophilia may be neuropathic
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
FROM EAHAD 2021
Automated software accurately generates ERCP quality reports
Modified endoscopy documentation software can automatically generate endoscopic retrograde cholangiopancreatography (ERCP) quality metrics, based on a trial at two referral centers.
Providers were prompted during procedures, and inputting any missed data took providers less than 30 additional seconds per patient. The approach led to highly accurate quality reports, lead author Gregory A. Coté, MD, MS, of the Medical University of South Carolina, Charleston, and colleagues wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
The investigators suggested that these findings may lead to the kind of quality reports already used for colonoscopy, which are easier to produce. Such reports are important, they wrote, as the U.S. health care system shifts to value-based reimbursement models, which in turn puts greater scrutiny on the quality of endoscopic procedures. However, doing so with ERCP isn’t entirely straightforward.
“Measuring adherence to ERCP quality indicators is especially challenging given: variance in indications, intraprocedural maneuvers, potential outcomes of a complex procedure, and variability in physician report documentation,” Dr. Coté and colleagues wrote. “In order to operationalize robust tracking of clinically relevant adherence to ERCP quality indicators in clinical practice – that is, to provide real-time feedback to providers, health systems, payors, and patients – an automated system of measurement must be developed.”
The quality indicators used in the study were largely drawn from an American Society for Gastrointestinal Endoscopy/American College of Gastroenterology task force document, with exclusion of those that were subjective or required systematic follow-up. The investigators modified existing endoscopy documentation software at two referral centers to include mandatory, structured data fields, principally with inclusion of quality improvements deemed high priority by the society consensus document, study authors, or both. For instance, providers were obligated to select a specific indication instead of various, synonymous terms (for example, “biliary stricture” vs. “common bile duct stricture”). Examples of quality indicators included successful cannulation of the desired duct, successful retrieval of stone less than 10 mm, or successful placement of a bile duct stent when indicated. Endoscopists were also required to note the presence of postoperative foregut anatomy or presence of existing sphincterotomy, variables which serve to stratefy the quality indicator outcome for degree of difficulty and allow appropriate comparisons of data. In addition, the study authors included inquiries about use of rectal indomethacin, use of prophylactic pancreatic duct stent, and documentation of need for repeat ERCP, follow-up x-ray, or both.
After 9 months, the system recorded 1,376 ERCP procedures conducted by eight providers, with a median annualized volume of 237 procedures (range, 37-336). Almost one-third (29%) of the patients had not had prior sphincterotomy.
Automated reporting of ERCP was compared with manual record review, which confirmed high (98%-100%) accuracy. This high level of accuracy “obviates the need for manual adjudication of medical records,” the investigators wrote.
They used data from one provider to create a template report card, and while exact comparisons across providers and institutions were not published, an example report card that was published with the study showed how such comparisons could be generated in the real world.
“The tool presented in this study allows for an objective assessment of ERCP performance which can provide explicit feedback to providers and allow transparent assessment of quality outcomes; it has the potential to improve the quality of ERCP akin to what has been demonstrated using colonoscopy report cards,” the investigators wrote. “Importantly, this can be achieved with minimal alteration to providers’ routine procedure documentation.”
Dr. Coté and colleagues also noted that the software modifications “can be implemented in other endoscopy units using the same or similar software.”
Taking the project to the next level would require widespread collaboration, according to the investigators.
“A key next step is to operationalize the transfer of data across multiple institutions, allowing for the creation of interim, standard-quality indicator reports that could be disseminated to providers, health systems, and payors,” they wrote. “If applied to a national cohort, this tool could accurately assess the current landscape of ERCP quality and provide tremendous opportunities for systematic improvement.”
One author disclosed a relationship with Provation Medical, but the remaining authors declared no relevant conflicts.
Quality indicators have been proposed to improve the outcome of patients undergoing endoscopic procedures. The path toward quality improvement begins with selection of parameters, which matters a great deal and have wide performance variation. Endoscopists then track their own performance, compare it with targets based on community standards, and improve their patients’ outcomes using this feedback. Great progress has been made in the area of tracking and improving adenoma detection rate, an indicator closely tied to reduction in colorectal cancer mortality.
Endoscopic retrograde cholangiopancreatography (ERCP) is a high-stakes procedure with great potential therapeutic benefit and with a small but significant risk of life-threatening complications such as pancreatitis. This study by Coté and colleagues illuminates an effective and straightforward step to making ERCP quality improvement feasible. The report card concept is not new, but the novel innovation is to leverage the use of required fields in the electronic report generator. Seamlessly, this produces nuanced reports that link provider performance to patient characteristics and indication. The authors have shown extremely high accuracy of automatic electronic ERCP quality indicator recording, compared with manual data collection. Such data have clear and immediate utility in the credentialing process and quality improvement arena. With this means of recording outcomes, deidentified ERCP quality data might soon join colonoscopy data in national data repositories such as the GI Quality Improvement Consortium, and government quality reporting on ERCP outcomes would become much more feasible. Fellow self-assessment and logging of progress could also be facilitated if report generators were further amended to require recording of fellow participation.
Jonathan Cohen, MD, FASGE, is a clinical professor of medicine at New York University Langone Health. He reported having no relevant conflicts of interest.
Quality indicators have been proposed to improve the outcome of patients undergoing endoscopic procedures. The path toward quality improvement begins with selection of parameters, which matters a great deal and have wide performance variation. Endoscopists then track their own performance, compare it with targets based on community standards, and improve their patients’ outcomes using this feedback. Great progress has been made in the area of tracking and improving adenoma detection rate, an indicator closely tied to reduction in colorectal cancer mortality.
Endoscopic retrograde cholangiopancreatography (ERCP) is a high-stakes procedure with great potential therapeutic benefit and with a small but significant risk of life-threatening complications such as pancreatitis. This study by Coté and colleagues illuminates an effective and straightforward step to making ERCP quality improvement feasible. The report card concept is not new, but the novel innovation is to leverage the use of required fields in the electronic report generator. Seamlessly, this produces nuanced reports that link provider performance to patient characteristics and indication. The authors have shown extremely high accuracy of automatic electronic ERCP quality indicator recording, compared with manual data collection. Such data have clear and immediate utility in the credentialing process and quality improvement arena. With this means of recording outcomes, deidentified ERCP quality data might soon join colonoscopy data in national data repositories such as the GI Quality Improvement Consortium, and government quality reporting on ERCP outcomes would become much more feasible. Fellow self-assessment and logging of progress could also be facilitated if report generators were further amended to require recording of fellow participation.
Jonathan Cohen, MD, FASGE, is a clinical professor of medicine at New York University Langone Health. He reported having no relevant conflicts of interest.
Quality indicators have been proposed to improve the outcome of patients undergoing endoscopic procedures. The path toward quality improvement begins with selection of parameters, which matters a great deal and have wide performance variation. Endoscopists then track their own performance, compare it with targets based on community standards, and improve their patients’ outcomes using this feedback. Great progress has been made in the area of tracking and improving adenoma detection rate, an indicator closely tied to reduction in colorectal cancer mortality.
Endoscopic retrograde cholangiopancreatography (ERCP) is a high-stakes procedure with great potential therapeutic benefit and with a small but significant risk of life-threatening complications such as pancreatitis. This study by Coté and colleagues illuminates an effective and straightforward step to making ERCP quality improvement feasible. The report card concept is not new, but the novel innovation is to leverage the use of required fields in the electronic report generator. Seamlessly, this produces nuanced reports that link provider performance to patient characteristics and indication. The authors have shown extremely high accuracy of automatic electronic ERCP quality indicator recording, compared with manual data collection. Such data have clear and immediate utility in the credentialing process and quality improvement arena. With this means of recording outcomes, deidentified ERCP quality data might soon join colonoscopy data in national data repositories such as the GI Quality Improvement Consortium, and government quality reporting on ERCP outcomes would become much more feasible. Fellow self-assessment and logging of progress could also be facilitated if report generators were further amended to require recording of fellow participation.
Jonathan Cohen, MD, FASGE, is a clinical professor of medicine at New York University Langone Health. He reported having no relevant conflicts of interest.
Modified endoscopy documentation software can automatically generate endoscopic retrograde cholangiopancreatography (ERCP) quality metrics, based on a trial at two referral centers.
Providers were prompted during procedures, and inputting any missed data took providers less than 30 additional seconds per patient. The approach led to highly accurate quality reports, lead author Gregory A. Coté, MD, MS, of the Medical University of South Carolina, Charleston, and colleagues wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
The investigators suggested that these findings may lead to the kind of quality reports already used for colonoscopy, which are easier to produce. Such reports are important, they wrote, as the U.S. health care system shifts to value-based reimbursement models, which in turn puts greater scrutiny on the quality of endoscopic procedures. However, doing so with ERCP isn’t entirely straightforward.
“Measuring adherence to ERCP quality indicators is especially challenging given: variance in indications, intraprocedural maneuvers, potential outcomes of a complex procedure, and variability in physician report documentation,” Dr. Coté and colleagues wrote. “In order to operationalize robust tracking of clinically relevant adherence to ERCP quality indicators in clinical practice – that is, to provide real-time feedback to providers, health systems, payors, and patients – an automated system of measurement must be developed.”
The quality indicators used in the study were largely drawn from an American Society for Gastrointestinal Endoscopy/American College of Gastroenterology task force document, with exclusion of those that were subjective or required systematic follow-up. The investigators modified existing endoscopy documentation software at two referral centers to include mandatory, structured data fields, principally with inclusion of quality improvements deemed high priority by the society consensus document, study authors, or both. For instance, providers were obligated to select a specific indication instead of various, synonymous terms (for example, “biliary stricture” vs. “common bile duct stricture”). Examples of quality indicators included successful cannulation of the desired duct, successful retrieval of stone less than 10 mm, or successful placement of a bile duct stent when indicated. Endoscopists were also required to note the presence of postoperative foregut anatomy or presence of existing sphincterotomy, variables which serve to stratefy the quality indicator outcome for degree of difficulty and allow appropriate comparisons of data. In addition, the study authors included inquiries about use of rectal indomethacin, use of prophylactic pancreatic duct stent, and documentation of need for repeat ERCP, follow-up x-ray, or both.
After 9 months, the system recorded 1,376 ERCP procedures conducted by eight providers, with a median annualized volume of 237 procedures (range, 37-336). Almost one-third (29%) of the patients had not had prior sphincterotomy.
Automated reporting of ERCP was compared with manual record review, which confirmed high (98%-100%) accuracy. This high level of accuracy “obviates the need for manual adjudication of medical records,” the investigators wrote.
They used data from one provider to create a template report card, and while exact comparisons across providers and institutions were not published, an example report card that was published with the study showed how such comparisons could be generated in the real world.
“The tool presented in this study allows for an objective assessment of ERCP performance which can provide explicit feedback to providers and allow transparent assessment of quality outcomes; it has the potential to improve the quality of ERCP akin to what has been demonstrated using colonoscopy report cards,” the investigators wrote. “Importantly, this can be achieved with minimal alteration to providers’ routine procedure documentation.”
Dr. Coté and colleagues also noted that the software modifications “can be implemented in other endoscopy units using the same or similar software.”
Taking the project to the next level would require widespread collaboration, according to the investigators.
“A key next step is to operationalize the transfer of data across multiple institutions, allowing for the creation of interim, standard-quality indicator reports that could be disseminated to providers, health systems, and payors,” they wrote. “If applied to a national cohort, this tool could accurately assess the current landscape of ERCP quality and provide tremendous opportunities for systematic improvement.”
One author disclosed a relationship with Provation Medical, but the remaining authors declared no relevant conflicts.
Modified endoscopy documentation software can automatically generate endoscopic retrograde cholangiopancreatography (ERCP) quality metrics, based on a trial at two referral centers.
Providers were prompted during procedures, and inputting any missed data took providers less than 30 additional seconds per patient. The approach led to highly accurate quality reports, lead author Gregory A. Coté, MD, MS, of the Medical University of South Carolina, Charleston, and colleagues wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
The investigators suggested that these findings may lead to the kind of quality reports already used for colonoscopy, which are easier to produce. Such reports are important, they wrote, as the U.S. health care system shifts to value-based reimbursement models, which in turn puts greater scrutiny on the quality of endoscopic procedures. However, doing so with ERCP isn’t entirely straightforward.
“Measuring adherence to ERCP quality indicators is especially challenging given: variance in indications, intraprocedural maneuvers, potential outcomes of a complex procedure, and variability in physician report documentation,” Dr. Coté and colleagues wrote. “In order to operationalize robust tracking of clinically relevant adherence to ERCP quality indicators in clinical practice – that is, to provide real-time feedback to providers, health systems, payors, and patients – an automated system of measurement must be developed.”
The quality indicators used in the study were largely drawn from an American Society for Gastrointestinal Endoscopy/American College of Gastroenterology task force document, with exclusion of those that were subjective or required systematic follow-up. The investigators modified existing endoscopy documentation software at two referral centers to include mandatory, structured data fields, principally with inclusion of quality improvements deemed high priority by the society consensus document, study authors, or both. For instance, providers were obligated to select a specific indication instead of various, synonymous terms (for example, “biliary stricture” vs. “common bile duct stricture”). Examples of quality indicators included successful cannulation of the desired duct, successful retrieval of stone less than 10 mm, or successful placement of a bile duct stent when indicated. Endoscopists were also required to note the presence of postoperative foregut anatomy or presence of existing sphincterotomy, variables which serve to stratefy the quality indicator outcome for degree of difficulty and allow appropriate comparisons of data. In addition, the study authors included inquiries about use of rectal indomethacin, use of prophylactic pancreatic duct stent, and documentation of need for repeat ERCP, follow-up x-ray, or both.
After 9 months, the system recorded 1,376 ERCP procedures conducted by eight providers, with a median annualized volume of 237 procedures (range, 37-336). Almost one-third (29%) of the patients had not had prior sphincterotomy.
Automated reporting of ERCP was compared with manual record review, which confirmed high (98%-100%) accuracy. This high level of accuracy “obviates the need for manual adjudication of medical records,” the investigators wrote.
They used data from one provider to create a template report card, and while exact comparisons across providers and institutions were not published, an example report card that was published with the study showed how such comparisons could be generated in the real world.
“The tool presented in this study allows for an objective assessment of ERCP performance which can provide explicit feedback to providers and allow transparent assessment of quality outcomes; it has the potential to improve the quality of ERCP akin to what has been demonstrated using colonoscopy report cards,” the investigators wrote. “Importantly, this can be achieved with minimal alteration to providers’ routine procedure documentation.”
Dr. Coté and colleagues also noted that the software modifications “can be implemented in other endoscopy units using the same or similar software.”
Taking the project to the next level would require widespread collaboration, according to the investigators.
“A key next step is to operationalize the transfer of data across multiple institutions, allowing for the creation of interim, standard-quality indicator reports that could be disseminated to providers, health systems, and payors,” they wrote. “If applied to a national cohort, this tool could accurately assess the current landscape of ERCP quality and provide tremendous opportunities for systematic improvement.”
One author disclosed a relationship with Provation Medical, but the remaining authors declared no relevant conflicts.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Palliative care and hospital medicine partnerships in the pandemic
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Clinical Edge Journal Scan Commentary: Breast Cancer March 2021
Peripheral neuropathy is a well-recognized complication of taxane therapy that can impact functioning and quality of life. Dose-reductions are applied in an effort to continue treatment and minimize risk of worsening neuropathy. In a prospective analysis of breast cancer patients receiving weekly paclitaxel, Timmins et al showed neuropathy symptoms affected 85% with severe symptoms in 38%, and about half of the cohort had persistent symptoms up to 12 months post-chemotherapy. Patients who received dose reductions reported worse neuropathy and symptom burden compared to those who received full dose paclitaxel chemotherapy. It is challenging to predict with certainty which patients may experience significant neuropathy, and important to acknowledge individual patients factors such as age and other medical co-morbidities. Additional research is warranted to refine individual risk assessment as well as prevention and management strategies.
The treatment landscape for metastatic HER2-positive breast cancer is evolving at a rapid pace. Margetuximab is a chimeric antibody with similar epitope specificity to trastuzumab, but with an engineered Fc region that enhances immune activation. The phase 3 SOPHIA trial included 536 patients with pretreated HER2-positive advanced breast cancer and demonstrated modest improvement in progression free-survival with margetuximab plus chemotherapy compared to trastuzumab plus chemotherapy (median PFS 5.8 versus 4.9 months; HR 0.76, p=0.03). The introduction of other therapies in this space (tucatinib, trastuzumab deruxtecan, neratinib) provides patients with many options, but simultaneously creates a complex treatment algorithm when it comes to therapy selection. Toxicity profiles and sites of metastases should be taken into consideration when deciding on best therapy for an individual patient.
Given the impressive outcomes seen with endocrine therapy plus CDK 4/6 inhibitors in the advanced HR+/HER2- population, these combinations are being studied in the curative setting. The phase 3 PALLAS study randomized 5,760 patients with stage I-III HR+/HER2- breast cancer to ongoing endocrine therapy with or without palbociclib for 2 years. Data from the second interim analysis of this trial showed similar invasive disease-free survival rates for the two arms (3 years iDFS 88.2% for palbociclib plus endocrine therapy versus 88.5% for endocrine therapy alone; HR 0.93, p=0.51). In contrast, the phase 3 monarchE trial showed improvement in iDFS with abemaciclib for 2 years with ongoing endocrine therapy compared to endocrine therapy alone (2 year iDFS rate of 92.3% versus 89.3%; HR 0.713, p=0.0009). Differences in study populations, mechanism of action of various CDK 4/6 inhibitors, dosing and drug exposure, may possibly impact results. Long-term follow-up and biomarker studies are desired to further delineate the role of CDK 4/6 inhibitors in this setting.
References:
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18:958.
O'Shaughnessy JA, Johnston S, Harbeck N, Toi M, Im Y-H, Reinisch M, Shao Z, Kellokumpu Lehtinen PL, Huang C-S, Tryakin A, Goetz M, Rugo HS, Senkus E, Testa L, Andersson M, Tamura K, Steger GG, Del Mastro L, Cox J, Forrester T, Sherwood S, Li X, Wei R, Martin M, Rastogi P. Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Presented at: 2020 Virtual San Antonio Breast Cancer Symposium; December 8-11, 2020. Abstract GS1-01.
Peripheral neuropathy is a well-recognized complication of taxane therapy that can impact functioning and quality of life. Dose-reductions are applied in an effort to continue treatment and minimize risk of worsening neuropathy. In a prospective analysis of breast cancer patients receiving weekly paclitaxel, Timmins et al showed neuropathy symptoms affected 85% with severe symptoms in 38%, and about half of the cohort had persistent symptoms up to 12 months post-chemotherapy. Patients who received dose reductions reported worse neuropathy and symptom burden compared to those who received full dose paclitaxel chemotherapy. It is challenging to predict with certainty which patients may experience significant neuropathy, and important to acknowledge individual patients factors such as age and other medical co-morbidities. Additional research is warranted to refine individual risk assessment as well as prevention and management strategies.
The treatment landscape for metastatic HER2-positive breast cancer is evolving at a rapid pace. Margetuximab is a chimeric antibody with similar epitope specificity to trastuzumab, but with an engineered Fc region that enhances immune activation. The phase 3 SOPHIA trial included 536 patients with pretreated HER2-positive advanced breast cancer and demonstrated modest improvement in progression free-survival with margetuximab plus chemotherapy compared to trastuzumab plus chemotherapy (median PFS 5.8 versus 4.9 months; HR 0.76, p=0.03). The introduction of other therapies in this space (tucatinib, trastuzumab deruxtecan, neratinib) provides patients with many options, but simultaneously creates a complex treatment algorithm when it comes to therapy selection. Toxicity profiles and sites of metastases should be taken into consideration when deciding on best therapy for an individual patient.
Given the impressive outcomes seen with endocrine therapy plus CDK 4/6 inhibitors in the advanced HR+/HER2- population, these combinations are being studied in the curative setting. The phase 3 PALLAS study randomized 5,760 patients with stage I-III HR+/HER2- breast cancer to ongoing endocrine therapy with or without palbociclib for 2 years. Data from the second interim analysis of this trial showed similar invasive disease-free survival rates for the two arms (3 years iDFS 88.2% for palbociclib plus endocrine therapy versus 88.5% for endocrine therapy alone; HR 0.93, p=0.51). In contrast, the phase 3 monarchE trial showed improvement in iDFS with abemaciclib for 2 years with ongoing endocrine therapy compared to endocrine therapy alone (2 year iDFS rate of 92.3% versus 89.3%; HR 0.713, p=0.0009). Differences in study populations, mechanism of action of various CDK 4/6 inhibitors, dosing and drug exposure, may possibly impact results. Long-term follow-up and biomarker studies are desired to further delineate the role of CDK 4/6 inhibitors in this setting.
References:
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18:958.
O'Shaughnessy JA, Johnston S, Harbeck N, Toi M, Im Y-H, Reinisch M, Shao Z, Kellokumpu Lehtinen PL, Huang C-S, Tryakin A, Goetz M, Rugo HS, Senkus E, Testa L, Andersson M, Tamura K, Steger GG, Del Mastro L, Cox J, Forrester T, Sherwood S, Li X, Wei R, Martin M, Rastogi P. Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Presented at: 2020 Virtual San Antonio Breast Cancer Symposium; December 8-11, 2020. Abstract GS1-01.
Peripheral neuropathy is a well-recognized complication of taxane therapy that can impact functioning and quality of life. Dose-reductions are applied in an effort to continue treatment and minimize risk of worsening neuropathy. In a prospective analysis of breast cancer patients receiving weekly paclitaxel, Timmins et al showed neuropathy symptoms affected 85% with severe symptoms in 38%, and about half of the cohort had persistent symptoms up to 12 months post-chemotherapy. Patients who received dose reductions reported worse neuropathy and symptom burden compared to those who received full dose paclitaxel chemotherapy. It is challenging to predict with certainty which patients may experience significant neuropathy, and important to acknowledge individual patients factors such as age and other medical co-morbidities. Additional research is warranted to refine individual risk assessment as well as prevention and management strategies.
The treatment landscape for metastatic HER2-positive breast cancer is evolving at a rapid pace. Margetuximab is a chimeric antibody with similar epitope specificity to trastuzumab, but with an engineered Fc region that enhances immune activation. The phase 3 SOPHIA trial included 536 patients with pretreated HER2-positive advanced breast cancer and demonstrated modest improvement in progression free-survival with margetuximab plus chemotherapy compared to trastuzumab plus chemotherapy (median PFS 5.8 versus 4.9 months; HR 0.76, p=0.03). The introduction of other therapies in this space (tucatinib, trastuzumab deruxtecan, neratinib) provides patients with many options, but simultaneously creates a complex treatment algorithm when it comes to therapy selection. Toxicity profiles and sites of metastases should be taken into consideration when deciding on best therapy for an individual patient.
Given the impressive outcomes seen with endocrine therapy plus CDK 4/6 inhibitors in the advanced HR+/HER2- population, these combinations are being studied in the curative setting. The phase 3 PALLAS study randomized 5,760 patients with stage I-III HR+/HER2- breast cancer to ongoing endocrine therapy with or without palbociclib for 2 years. Data from the second interim analysis of this trial showed similar invasive disease-free survival rates for the two arms (3 years iDFS 88.2% for palbociclib plus endocrine therapy versus 88.5% for endocrine therapy alone; HR 0.93, p=0.51). In contrast, the phase 3 monarchE trial showed improvement in iDFS with abemaciclib for 2 years with ongoing endocrine therapy compared to endocrine therapy alone (2 year iDFS rate of 92.3% versus 89.3%; HR 0.713, p=0.0009). Differences in study populations, mechanism of action of various CDK 4/6 inhibitors, dosing and drug exposure, may possibly impact results. Long-term follow-up and biomarker studies are desired to further delineate the role of CDK 4/6 inhibitors in this setting.
References:
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18:958.
O'Shaughnessy JA, Johnston S, Harbeck N, Toi M, Im Y-H, Reinisch M, Shao Z, Kellokumpu Lehtinen PL, Huang C-S, Tryakin A, Goetz M, Rugo HS, Senkus E, Testa L, Andersson M, Tamura K, Steger GG, Del Mastro L, Cox J, Forrester T, Sherwood S, Li X, Wei R, Martin M, Rastogi P. Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Presented at: 2020 Virtual San Antonio Breast Cancer Symposium; December 8-11, 2020. Abstract GS1-01.
De-escalated radiation and endocrine therapy strategies in older women with breast cancer
Key clinical point: Adjuvant radiation therapy (RT) alone or in combination with endocrine therapy (ET) was associated with a lower risk for recurrence than ET alone in older women with early node-negative, human receptor-positive (HR+) breast cancer (BC). In addition, most older women with stage I HR+ breast cancers continue to receive radiation, at higher rates than patients with node-negative stage II tumors.
Major finding: Compared with ET alone, use of RT+ET (hazard ratio [HR], 0.62; P less than .0001) and RT alone (HR, 0.75; P less than .0001) was associated with a lower risk for recurrence at a median follow-up of 48 months. RT was received by 65.5% of patients, with no decrease over time. However, patients with T2 vs. T1 tumors remained less likely to receive RT (odds ratio, 0.83; P = .0024).
Study details: This study evaluated the use of adjuvant RT (n=2,046), ET (n=2,407), or RT+ET (n=4,643) after breast-conserving therapeutic surgery in older women (age at diagnosis, 66 years or more) with T1-2 node-negative, HR+ BC.
Disclosures: This study was supported by grants from the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, and the American Society for Radiation Oncology. Some of the study investigators reported employment and ownership in various pharmaceutical companies.
Source: Reeder-Hayes KE et al. J Geriatr Oncol. 2021 Feb 4. doi: 10.1016/j.jgo.2021.01.003.
Key clinical point: Adjuvant radiation therapy (RT) alone or in combination with endocrine therapy (ET) was associated with a lower risk for recurrence than ET alone in older women with early node-negative, human receptor-positive (HR+) breast cancer (BC). In addition, most older women with stage I HR+ breast cancers continue to receive radiation, at higher rates than patients with node-negative stage II tumors.
Major finding: Compared with ET alone, use of RT+ET (hazard ratio [HR], 0.62; P less than .0001) and RT alone (HR, 0.75; P less than .0001) was associated with a lower risk for recurrence at a median follow-up of 48 months. RT was received by 65.5% of patients, with no decrease over time. However, patients with T2 vs. T1 tumors remained less likely to receive RT (odds ratio, 0.83; P = .0024).
Study details: This study evaluated the use of adjuvant RT (n=2,046), ET (n=2,407), or RT+ET (n=4,643) after breast-conserving therapeutic surgery in older women (age at diagnosis, 66 years or more) with T1-2 node-negative, HR+ BC.
Disclosures: This study was supported by grants from the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, and the American Society for Radiation Oncology. Some of the study investigators reported employment and ownership in various pharmaceutical companies.
Source: Reeder-Hayes KE et al. J Geriatr Oncol. 2021 Feb 4. doi: 10.1016/j.jgo.2021.01.003.
Key clinical point: Adjuvant radiation therapy (RT) alone or in combination with endocrine therapy (ET) was associated with a lower risk for recurrence than ET alone in older women with early node-negative, human receptor-positive (HR+) breast cancer (BC). In addition, most older women with stage I HR+ breast cancers continue to receive radiation, at higher rates than patients with node-negative stage II tumors.
Major finding: Compared with ET alone, use of RT+ET (hazard ratio [HR], 0.62; P less than .0001) and RT alone (HR, 0.75; P less than .0001) was associated with a lower risk for recurrence at a median follow-up of 48 months. RT was received by 65.5% of patients, with no decrease over time. However, patients with T2 vs. T1 tumors remained less likely to receive RT (odds ratio, 0.83; P = .0024).
Study details: This study evaluated the use of adjuvant RT (n=2,046), ET (n=2,407), or RT+ET (n=4,643) after breast-conserving therapeutic surgery in older women (age at diagnosis, 66 years or more) with T1-2 node-negative, HR+ BC.
Disclosures: This study was supported by grants from the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, and the American Society for Radiation Oncology. Some of the study investigators reported employment and ownership in various pharmaceutical companies.
Source: Reeder-Hayes KE et al. J Geriatr Oncol. 2021 Feb 4. doi: 10.1016/j.jgo.2021.01.003.
Locoregional surgery improves PFS in de novo stage IV breast cancer
Key clinical point: Locoregional surgery of the primary tumor vs. no surgery significantly improved locoregional progression-free survival (PFS) in patients with de novo stage IV breast cancer.
Major finding: Locoregional PFS was significantly longer with locoregional surgery vs. no surgery (hazard ratio, 0.23; P less than .001).
Study details: Findings are from a meta-analysis of 1,110 patients from 6 prospective clinical trials and 353 patients from a cohort study that assessed effects of locoregional surgery vs. no surgery in de novo stage IV breast cancer.
Disclosures: This study was supported by grants from the National Science and Technology Major Project, Sun Yat-Sen Memorial Hospital, the National Natural Science Foundation of Guangdong Province, Guangzhou Science and Technology Major Program, the Guangdong Science and Technology Department, Sun Yat-Sen University Clinical Research 5010 Program, and Sun Yat-Sen Clinical Research Cultivating Program. The authors declared no conflicts of interest.
Source: Yu Y et al. Ann Surg Oncol. 2021 Feb 3. doi: 10.1245/s10434-021-09650-3.
Key clinical point: Locoregional surgery of the primary tumor vs. no surgery significantly improved locoregional progression-free survival (PFS) in patients with de novo stage IV breast cancer.
Major finding: Locoregional PFS was significantly longer with locoregional surgery vs. no surgery (hazard ratio, 0.23; P less than .001).
Study details: Findings are from a meta-analysis of 1,110 patients from 6 prospective clinical trials and 353 patients from a cohort study that assessed effects of locoregional surgery vs. no surgery in de novo stage IV breast cancer.
Disclosures: This study was supported by grants from the National Science and Technology Major Project, Sun Yat-Sen Memorial Hospital, the National Natural Science Foundation of Guangdong Province, Guangzhou Science and Technology Major Program, the Guangdong Science and Technology Department, Sun Yat-Sen University Clinical Research 5010 Program, and Sun Yat-Sen Clinical Research Cultivating Program. The authors declared no conflicts of interest.
Source: Yu Y et al. Ann Surg Oncol. 2021 Feb 3. doi: 10.1245/s10434-021-09650-3.
Key clinical point: Locoregional surgery of the primary tumor vs. no surgery significantly improved locoregional progression-free survival (PFS) in patients with de novo stage IV breast cancer.
Major finding: Locoregional PFS was significantly longer with locoregional surgery vs. no surgery (hazard ratio, 0.23; P less than .001).
Study details: Findings are from a meta-analysis of 1,110 patients from 6 prospective clinical trials and 353 patients from a cohort study that assessed effects of locoregional surgery vs. no surgery in de novo stage IV breast cancer.
Disclosures: This study was supported by grants from the National Science and Technology Major Project, Sun Yat-Sen Memorial Hospital, the National Natural Science Foundation of Guangdong Province, Guangzhou Science and Technology Major Program, the Guangdong Science and Technology Department, Sun Yat-Sen University Clinical Research 5010 Program, and Sun Yat-Sen Clinical Research Cultivating Program. The authors declared no conflicts of interest.
Source: Yu Y et al. Ann Surg Oncol. 2021 Feb 3. doi: 10.1245/s10434-021-09650-3.
Early breast cancer: Rates of local recurrence higher with APBI than WBI
Key clinical point: Rates of local recurrence were higher with accelerated partial breast irradiation (APBI) than whole breast irradiation (WBI) in patients receiving breast-conservation treatment for early-stage breast cancer. Rate of distant metastasis, overall survival (OS), and disease-free survival (DFS) were similar.
Major finding: Patients receiving APBI vs. WBI had significantly higher rates of local recurrence (hazard ratio [HR], 1.46; P = .0002). DFS (HR, 1.11; P = .09), OS (HR, 1.11; P = .09), and distant metastasis (HR, 1.17; P = .11) were not different between the groups.
Study details: Findings are from a meta-analysis of 10 randomized controlled trials including 15,500 patients with early-stage breast cancer, including 7,758 patients in APBI and 7,742 patients in WBI groups.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Xiang X et al. Radiat Oncol. 2021 Feb 2. doi: 10.1186/s13014-021-01752-2.
Key clinical point: Rates of local recurrence were higher with accelerated partial breast irradiation (APBI) than whole breast irradiation (WBI) in patients receiving breast-conservation treatment for early-stage breast cancer. Rate of distant metastasis, overall survival (OS), and disease-free survival (DFS) were similar.
Major finding: Patients receiving APBI vs. WBI had significantly higher rates of local recurrence (hazard ratio [HR], 1.46; P = .0002). DFS (HR, 1.11; P = .09), OS (HR, 1.11; P = .09), and distant metastasis (HR, 1.17; P = .11) were not different between the groups.
Study details: Findings are from a meta-analysis of 10 randomized controlled trials including 15,500 patients with early-stage breast cancer, including 7,758 patients in APBI and 7,742 patients in WBI groups.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Xiang X et al. Radiat Oncol. 2021 Feb 2. doi: 10.1186/s13014-021-01752-2.
Key clinical point: Rates of local recurrence were higher with accelerated partial breast irradiation (APBI) than whole breast irradiation (WBI) in patients receiving breast-conservation treatment for early-stage breast cancer. Rate of distant metastasis, overall survival (OS), and disease-free survival (DFS) were similar.
Major finding: Patients receiving APBI vs. WBI had significantly higher rates of local recurrence (hazard ratio [HR], 1.46; P = .0002). DFS (HR, 1.11; P = .09), OS (HR, 1.11; P = .09), and distant metastasis (HR, 1.17; P = .11) were not different between the groups.
Study details: Findings are from a meta-analysis of 10 randomized controlled trials including 15,500 patients with early-stage breast cancer, including 7,758 patients in APBI and 7,742 patients in WBI groups.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Xiang X et al. Radiat Oncol. 2021 Feb 2. doi: 10.1186/s13014-021-01752-2.