User login
In the Future, a Robot Intensivist May Save Your Life
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.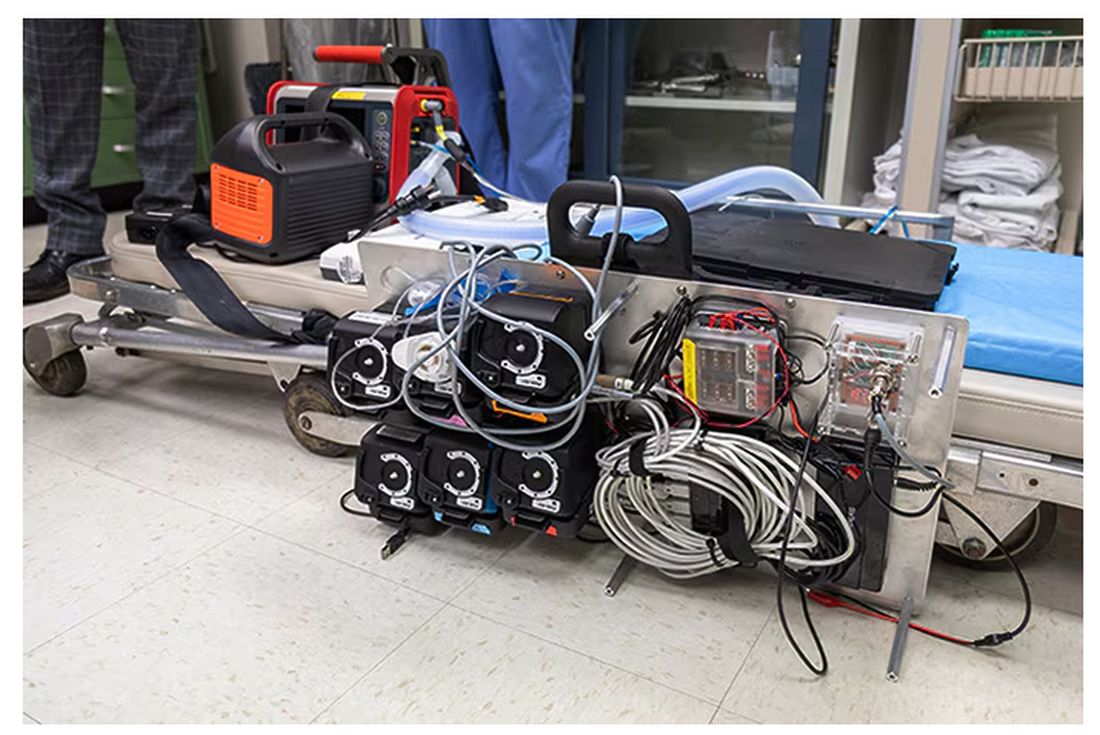
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.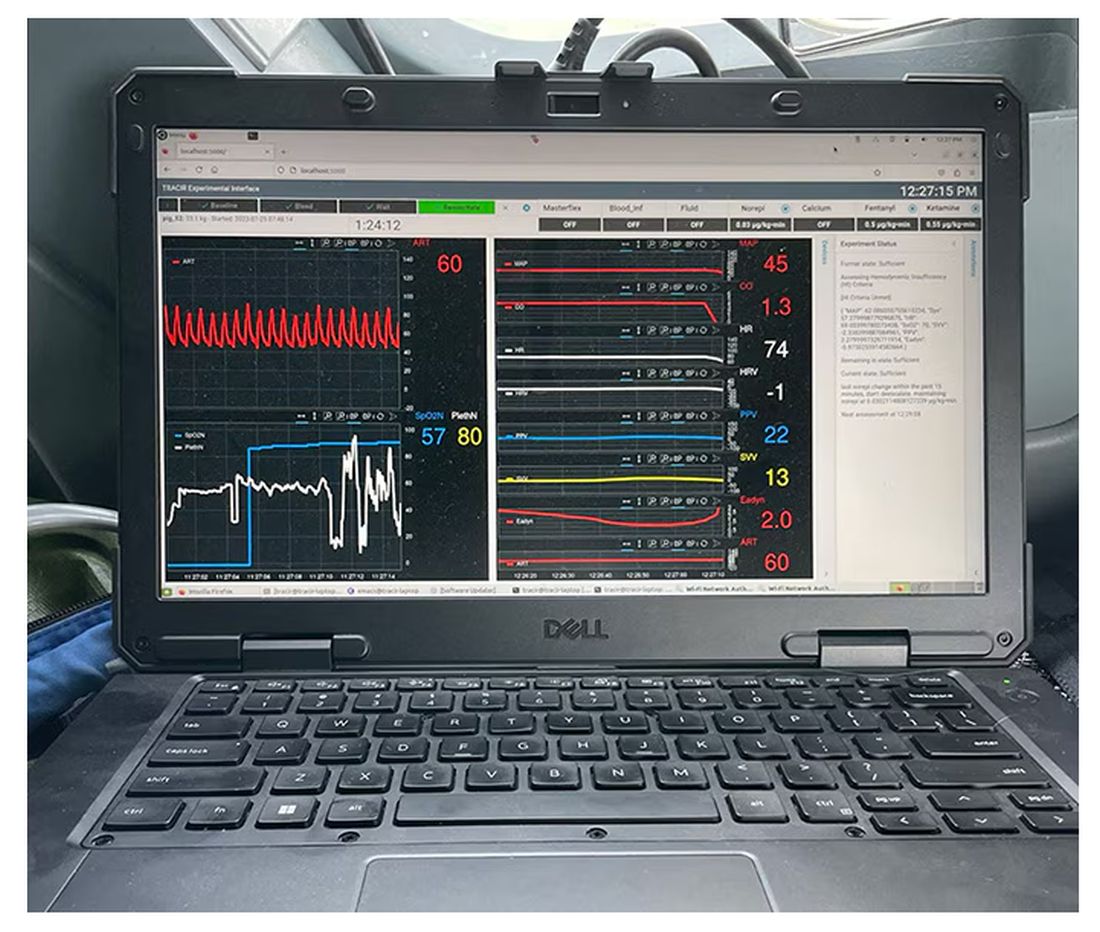
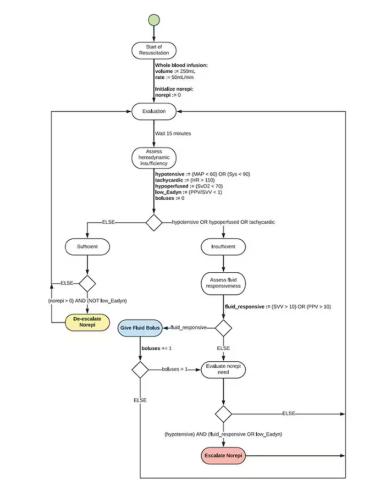
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.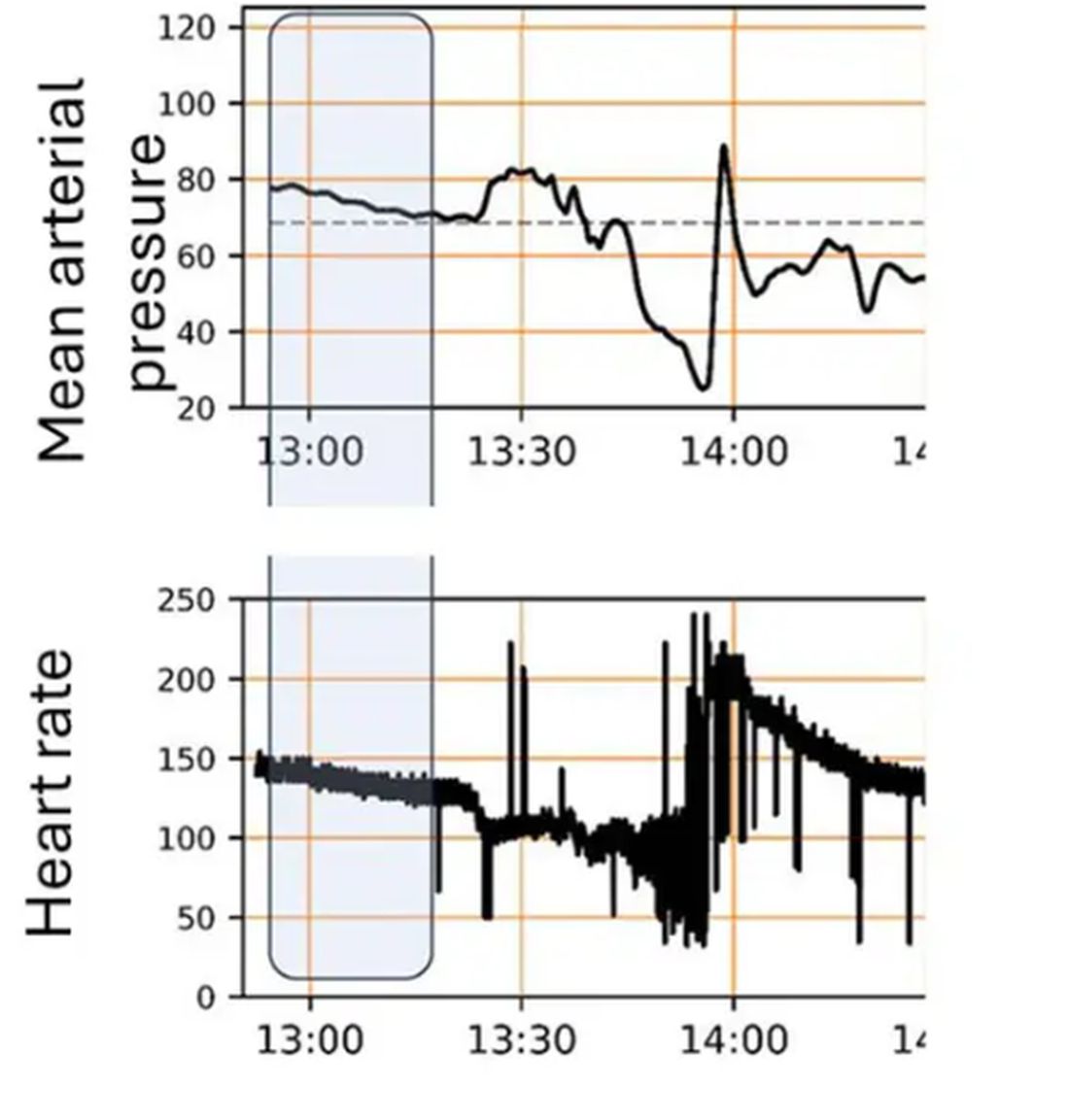
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 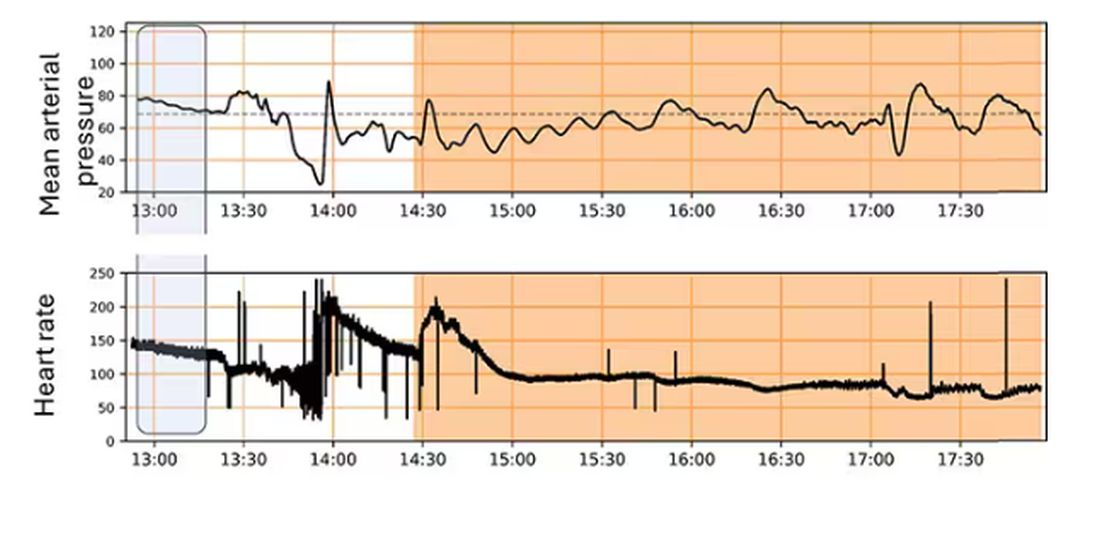
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Neoadjuvant Checkpoint Inhibition Study Sets New Standard of Care in Melanoma
These results set a new standard of care in this patient population, the study’s lead author, Christian U. Blank, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology in Chicago.
Dr. Blank, a hematologist/oncologist from the Netherlands Cancer Institute in Amsterdam, called the result “very special,” noting that the trial included an active comparator, rather than a placebo control.
“When we treat these patients with surgery only, the outcome … is very bad: The 5-year relapse-free survival is only 30% and the overall survival is only 50%. Adjuvant therapy improves relapse-free survival but not overall survival ...Thus, there is an urgent need for these patients for novel therapy approaches,” he said during a press conference at the meeting.
Study Methods and Results
The study included 423 patients with stage III de novo or recurrent pathologically proven resectable melanoma with at least 1 lymph node metastasis. Patients were randomized to either the experimental neoadjuvant arm (n = 212), or the standard treatment control arm (n = 211), which consisted of therapeutic lymph node dissection (TLND) followed by 12 cycles of adjuvant nivolumab (NIVO 480 mg every 4 weeks).
Patients in the experimental arm received two cycles of neoadjuvant ipilimumab (IPI 80 mg every 3 weeks) plus NIVO 240 mg for 3 weeks followed by TLND. Those with a major pathologic response (MPR), defined as less than 10% vital tumor cells in the post-neoadjuvant resection specimen, went straight to follow-up.
Those without an MPR received adjuvant therapy. For patients with BRAF wild-type, this involved 11 cycles of adjuvant NIVO (480 mg every 4 weeks), while BRAF-mutated patients received dabrafenib plus trametinib (150 mg b.i.d./2 mg once a day; 46 weeks).
The study met its primary endpoint — event-free survival (EFS) — at the first interim analysis. After a median follow-up of 9.9 months, the estimated EFS was 83.7% for neoadjuvant immunotherapy versus 57.2% for standard of care, (P less than .0001, hazard ratio [HR] = 0.32).
“When we look into the subgroups, for example BRAF-mutated status or BRAF-wild-type status ... you see for both groups also a highly statistically significant outcome favoring the neoadjuvant therapy with hazard ratios of 0.29 and 0.35,” said Dr. Blank.
In total, 59% of patients in the experimental arm had an MPR needing no further treatment. “This is important, because the patients that achieve a major pathologic response have excellent outcomes, with an EFS of 95%,” said Dr. Blank.
He added that those with a partial response had an EFS of 76%, and among those who had “nonresponse,” the EFS was 57% — the same as that of patients in the control arm.
Toxicities were considered transient and acceptable, with systemic treatment-related grade 3 or 4 events in 29.7% of the neoadjuvant arm and 14.7% of the adjuvant arm.
NADINA is the first neoadjuvant checkpoint inhibitor phase 3 study in melanoma and the first phase 3 trial in oncology testing a checkpoint inhibitor without chemotherapy, noted Dr. Blank.
“At the moment we see only additions of immunotherapy to the chemotherapy neoadjuvant arms, but here you see that we can also treat patients with pure immunotherapy.”
Neoadjuvant Therapy Defined as Standard of Care
When considered along with evidence from the phase 2 SWOG 1801 study (N Engl J Med. 2023;388:813-8), “NADINA defines neoadjuvant therapy as the new standard of care for macroscopic stage III melanoma “which means that all trials currently ongoing need to be amended from adjuvant comparators to neoadjuvant comparators,” he said.
Dr. Blank called the trial a “new template for other malignancies implementing a neoadjuvant immunotherapy regimen followed by a response-driven adjuvant therapy.
“I think we see at the moment only sandwich designs, and this is more sales driven than patient driven, because what we have seen is that if a patient achieves a really deep response, the patient doesn’t need an adjuvant part,” he said.
Commenting during the press conference, Michael Lowe, MD, said the result “confirms and shows for the first time in a phase 3 study that giving immunotherapy before surgery results in superior outcomes to giving immunotherapy only after surgery.”
Dr. Lowe, associate professor in the Division of Surgical Oncology, at Emory University School of Medicine, Atlanta, added that the study “also confirms that giving two immunotherapy drugs before surgery results in excellent responses.”
However, he cautioned that “we cannot make comparisons to trials in which patients only got one immunotherapy. But this study confirms that consistency that patients who receive ipilimumab and nivolumab have superior responses compared to single-agent immunotherapy.”
He noted that all of the patients in the new study had all of their lymph nodes removed and called for doing that to remain the standard of care in terms of surgical approach.
“With short follow-up, it is too early to tell if some patients may have benefited from that adjuvant therapy. However, NADINA confirms that immunotherapy should be given to all patients with advanced melanoma before surgery, when possible, and establishes dual therapy with nivolumab and ipilimumab, as the standard of care in the appropriate patient,” Dr. Lowe said.
EFS Improvement Exceeds Expectations
In an interview, Rodabe N. Amaria, MD, a medical oncologist and professor at The University of Texas MD Anderson Cancer Center in Houston, agreed with Dr. Lowe’s assessment of the findings.
“For years we have been doing neoadjuvant immunotherapy trials, all with favorable results, but all relatively small, with data that was intriguing, but not necessarily definitive,” she said. “I see the data from the NADINA trial as being definitive and true evidence of the many advantages of neoadjuvant immunotherapy for clinical stage 3 melanoma ... This work builds on the data from the SWOG 1801 trial but also exceeds expectations with the 68% improvement in EFS appreciated with the dual combination immunotherapy regimen compared to adjuvant nivolumab.”
Additionally, the approximately 30% grade 3 or higher immune-mediated toxicity is reasonable and in keeping with known data, and this trial demonstrates clearly that neoadjuvant immunotherapy does not increase the rate of surgical complications, she said.
Dr. Amaria also considered that 59% of patients who achieved a major pathologic response were observed in the neoadjuvant setting to be a key finding.
This indicates thats “over half the patients could be spared additional immunotherapy and risk of further immune-mediated toxicities by having only two doses of neoadjuvant immunotherapy, she said.
The results “demonstrate the superiority of a neoadjuvant combination immunotherapy approach for patients with clinical stage III melanoma,” she added.
The study was funded by Bristol Myers-Squibb and the Australian government.
Dr. Blank disclosed ties with Immagene, Signature Oncology, AstraZeneca, Bristol-Myers Squibb, GenMab, GlaxoSmithKline, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Third Rock Ventures, 4SC, NanoString Technologies, WO 2021/177822 A1, and Freshfields Bruckhaus Deringer. No other experts reported any relevant disclosures.
These results set a new standard of care in this patient population, the study’s lead author, Christian U. Blank, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology in Chicago.
Dr. Blank, a hematologist/oncologist from the Netherlands Cancer Institute in Amsterdam, called the result “very special,” noting that the trial included an active comparator, rather than a placebo control.
“When we treat these patients with surgery only, the outcome … is very bad: The 5-year relapse-free survival is only 30% and the overall survival is only 50%. Adjuvant therapy improves relapse-free survival but not overall survival ...Thus, there is an urgent need for these patients for novel therapy approaches,” he said during a press conference at the meeting.
Study Methods and Results
The study included 423 patients with stage III de novo or recurrent pathologically proven resectable melanoma with at least 1 lymph node metastasis. Patients were randomized to either the experimental neoadjuvant arm (n = 212), or the standard treatment control arm (n = 211), which consisted of therapeutic lymph node dissection (TLND) followed by 12 cycles of adjuvant nivolumab (NIVO 480 mg every 4 weeks).
Patients in the experimental arm received two cycles of neoadjuvant ipilimumab (IPI 80 mg every 3 weeks) plus NIVO 240 mg for 3 weeks followed by TLND. Those with a major pathologic response (MPR), defined as less than 10% vital tumor cells in the post-neoadjuvant resection specimen, went straight to follow-up.
Those without an MPR received adjuvant therapy. For patients with BRAF wild-type, this involved 11 cycles of adjuvant NIVO (480 mg every 4 weeks), while BRAF-mutated patients received dabrafenib plus trametinib (150 mg b.i.d./2 mg once a day; 46 weeks).
The study met its primary endpoint — event-free survival (EFS) — at the first interim analysis. After a median follow-up of 9.9 months, the estimated EFS was 83.7% for neoadjuvant immunotherapy versus 57.2% for standard of care, (P less than .0001, hazard ratio [HR] = 0.32).
“When we look into the subgroups, for example BRAF-mutated status or BRAF-wild-type status ... you see for both groups also a highly statistically significant outcome favoring the neoadjuvant therapy with hazard ratios of 0.29 and 0.35,” said Dr. Blank.
In total, 59% of patients in the experimental arm had an MPR needing no further treatment. “This is important, because the patients that achieve a major pathologic response have excellent outcomes, with an EFS of 95%,” said Dr. Blank.
He added that those with a partial response had an EFS of 76%, and among those who had “nonresponse,” the EFS was 57% — the same as that of patients in the control arm.
Toxicities were considered transient and acceptable, with systemic treatment-related grade 3 or 4 events in 29.7% of the neoadjuvant arm and 14.7% of the adjuvant arm.
NADINA is the first neoadjuvant checkpoint inhibitor phase 3 study in melanoma and the first phase 3 trial in oncology testing a checkpoint inhibitor without chemotherapy, noted Dr. Blank.
“At the moment we see only additions of immunotherapy to the chemotherapy neoadjuvant arms, but here you see that we can also treat patients with pure immunotherapy.”
Neoadjuvant Therapy Defined as Standard of Care
When considered along with evidence from the phase 2 SWOG 1801 study (N Engl J Med. 2023;388:813-8), “NADINA defines neoadjuvant therapy as the new standard of care for macroscopic stage III melanoma “which means that all trials currently ongoing need to be amended from adjuvant comparators to neoadjuvant comparators,” he said.
Dr. Blank called the trial a “new template for other malignancies implementing a neoadjuvant immunotherapy regimen followed by a response-driven adjuvant therapy.
“I think we see at the moment only sandwich designs, and this is more sales driven than patient driven, because what we have seen is that if a patient achieves a really deep response, the patient doesn’t need an adjuvant part,” he said.
Commenting during the press conference, Michael Lowe, MD, said the result “confirms and shows for the first time in a phase 3 study that giving immunotherapy before surgery results in superior outcomes to giving immunotherapy only after surgery.”
Dr. Lowe, associate professor in the Division of Surgical Oncology, at Emory University School of Medicine, Atlanta, added that the study “also confirms that giving two immunotherapy drugs before surgery results in excellent responses.”
However, he cautioned that “we cannot make comparisons to trials in which patients only got one immunotherapy. But this study confirms that consistency that patients who receive ipilimumab and nivolumab have superior responses compared to single-agent immunotherapy.”
He noted that all of the patients in the new study had all of their lymph nodes removed and called for doing that to remain the standard of care in terms of surgical approach.
“With short follow-up, it is too early to tell if some patients may have benefited from that adjuvant therapy. However, NADINA confirms that immunotherapy should be given to all patients with advanced melanoma before surgery, when possible, and establishes dual therapy with nivolumab and ipilimumab, as the standard of care in the appropriate patient,” Dr. Lowe said.
EFS Improvement Exceeds Expectations
In an interview, Rodabe N. Amaria, MD, a medical oncologist and professor at The University of Texas MD Anderson Cancer Center in Houston, agreed with Dr. Lowe’s assessment of the findings.
“For years we have been doing neoadjuvant immunotherapy trials, all with favorable results, but all relatively small, with data that was intriguing, but not necessarily definitive,” she said. “I see the data from the NADINA trial as being definitive and true evidence of the many advantages of neoadjuvant immunotherapy for clinical stage 3 melanoma ... This work builds on the data from the SWOG 1801 trial but also exceeds expectations with the 68% improvement in EFS appreciated with the dual combination immunotherapy regimen compared to adjuvant nivolumab.”
Additionally, the approximately 30% grade 3 or higher immune-mediated toxicity is reasonable and in keeping with known data, and this trial demonstrates clearly that neoadjuvant immunotherapy does not increase the rate of surgical complications, she said.
Dr. Amaria also considered that 59% of patients who achieved a major pathologic response were observed in the neoadjuvant setting to be a key finding.
This indicates thats “over half the patients could be spared additional immunotherapy and risk of further immune-mediated toxicities by having only two doses of neoadjuvant immunotherapy, she said.
The results “demonstrate the superiority of a neoadjuvant combination immunotherapy approach for patients with clinical stage III melanoma,” she added.
The study was funded by Bristol Myers-Squibb and the Australian government.
Dr. Blank disclosed ties with Immagene, Signature Oncology, AstraZeneca, Bristol-Myers Squibb, GenMab, GlaxoSmithKline, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Third Rock Ventures, 4SC, NanoString Technologies, WO 2021/177822 A1, and Freshfields Bruckhaus Deringer. No other experts reported any relevant disclosures.
These results set a new standard of care in this patient population, the study’s lead author, Christian U. Blank, MD, PhD, reported at the annual meeting of the American Society of Clinical Oncology in Chicago.
Dr. Blank, a hematologist/oncologist from the Netherlands Cancer Institute in Amsterdam, called the result “very special,” noting that the trial included an active comparator, rather than a placebo control.
“When we treat these patients with surgery only, the outcome … is very bad: The 5-year relapse-free survival is only 30% and the overall survival is only 50%. Adjuvant therapy improves relapse-free survival but not overall survival ...Thus, there is an urgent need for these patients for novel therapy approaches,” he said during a press conference at the meeting.
Study Methods and Results
The study included 423 patients with stage III de novo or recurrent pathologically proven resectable melanoma with at least 1 lymph node metastasis. Patients were randomized to either the experimental neoadjuvant arm (n = 212), or the standard treatment control arm (n = 211), which consisted of therapeutic lymph node dissection (TLND) followed by 12 cycles of adjuvant nivolumab (NIVO 480 mg every 4 weeks).
Patients in the experimental arm received two cycles of neoadjuvant ipilimumab (IPI 80 mg every 3 weeks) plus NIVO 240 mg for 3 weeks followed by TLND. Those with a major pathologic response (MPR), defined as less than 10% vital tumor cells in the post-neoadjuvant resection specimen, went straight to follow-up.
Those without an MPR received adjuvant therapy. For patients with BRAF wild-type, this involved 11 cycles of adjuvant NIVO (480 mg every 4 weeks), while BRAF-mutated patients received dabrafenib plus trametinib (150 mg b.i.d./2 mg once a day; 46 weeks).
The study met its primary endpoint — event-free survival (EFS) — at the first interim analysis. After a median follow-up of 9.9 months, the estimated EFS was 83.7% for neoadjuvant immunotherapy versus 57.2% for standard of care, (P less than .0001, hazard ratio [HR] = 0.32).
“When we look into the subgroups, for example BRAF-mutated status or BRAF-wild-type status ... you see for both groups also a highly statistically significant outcome favoring the neoadjuvant therapy with hazard ratios of 0.29 and 0.35,” said Dr. Blank.
In total, 59% of patients in the experimental arm had an MPR needing no further treatment. “This is important, because the patients that achieve a major pathologic response have excellent outcomes, with an EFS of 95%,” said Dr. Blank.
He added that those with a partial response had an EFS of 76%, and among those who had “nonresponse,” the EFS was 57% — the same as that of patients in the control arm.
Toxicities were considered transient and acceptable, with systemic treatment-related grade 3 or 4 events in 29.7% of the neoadjuvant arm and 14.7% of the adjuvant arm.
NADINA is the first neoadjuvant checkpoint inhibitor phase 3 study in melanoma and the first phase 3 trial in oncology testing a checkpoint inhibitor without chemotherapy, noted Dr. Blank.
“At the moment we see only additions of immunotherapy to the chemotherapy neoadjuvant arms, but here you see that we can also treat patients with pure immunotherapy.”
Neoadjuvant Therapy Defined as Standard of Care
When considered along with evidence from the phase 2 SWOG 1801 study (N Engl J Med. 2023;388:813-8), “NADINA defines neoadjuvant therapy as the new standard of care for macroscopic stage III melanoma “which means that all trials currently ongoing need to be amended from adjuvant comparators to neoadjuvant comparators,” he said.
Dr. Blank called the trial a “new template for other malignancies implementing a neoadjuvant immunotherapy regimen followed by a response-driven adjuvant therapy.
“I think we see at the moment only sandwich designs, and this is more sales driven than patient driven, because what we have seen is that if a patient achieves a really deep response, the patient doesn’t need an adjuvant part,” he said.
Commenting during the press conference, Michael Lowe, MD, said the result “confirms and shows for the first time in a phase 3 study that giving immunotherapy before surgery results in superior outcomes to giving immunotherapy only after surgery.”
Dr. Lowe, associate professor in the Division of Surgical Oncology, at Emory University School of Medicine, Atlanta, added that the study “also confirms that giving two immunotherapy drugs before surgery results in excellent responses.”
However, he cautioned that “we cannot make comparisons to trials in which patients only got one immunotherapy. But this study confirms that consistency that patients who receive ipilimumab and nivolumab have superior responses compared to single-agent immunotherapy.”
He noted that all of the patients in the new study had all of their lymph nodes removed and called for doing that to remain the standard of care in terms of surgical approach.
“With short follow-up, it is too early to tell if some patients may have benefited from that adjuvant therapy. However, NADINA confirms that immunotherapy should be given to all patients with advanced melanoma before surgery, when possible, and establishes dual therapy with nivolumab and ipilimumab, as the standard of care in the appropriate patient,” Dr. Lowe said.
EFS Improvement Exceeds Expectations
In an interview, Rodabe N. Amaria, MD, a medical oncologist and professor at The University of Texas MD Anderson Cancer Center in Houston, agreed with Dr. Lowe’s assessment of the findings.
“For years we have been doing neoadjuvant immunotherapy trials, all with favorable results, but all relatively small, with data that was intriguing, but not necessarily definitive,” she said. “I see the data from the NADINA trial as being definitive and true evidence of the many advantages of neoadjuvant immunotherapy for clinical stage 3 melanoma ... This work builds on the data from the SWOG 1801 trial but also exceeds expectations with the 68% improvement in EFS appreciated with the dual combination immunotherapy regimen compared to adjuvant nivolumab.”
Additionally, the approximately 30% grade 3 or higher immune-mediated toxicity is reasonable and in keeping with known data, and this trial demonstrates clearly that neoadjuvant immunotherapy does not increase the rate of surgical complications, she said.
Dr. Amaria also considered that 59% of patients who achieved a major pathologic response were observed in the neoadjuvant setting to be a key finding.
This indicates thats “over half the patients could be spared additional immunotherapy and risk of further immune-mediated toxicities by having only two doses of neoadjuvant immunotherapy, she said.
The results “demonstrate the superiority of a neoadjuvant combination immunotherapy approach for patients with clinical stage III melanoma,” she added.
The study was funded by Bristol Myers-Squibb and the Australian government.
Dr. Blank disclosed ties with Immagene, Signature Oncology, AstraZeneca, Bristol-Myers Squibb, GenMab, GlaxoSmithKline, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Third Rock Ventures, 4SC, NanoString Technologies, WO 2021/177822 A1, and Freshfields Bruckhaus Deringer. No other experts reported any relevant disclosures.
FROM ASCO 2024
Colchicine: A New Tool for Ischemic Stroke, CVD Event Recurrence?
BASEL, SWITZERLAND — However, the results did reveal a significant reduction in recurrent stroke and cardiovascular events in the per-protocol analysis and in the subgroup of patients with coronary artery disease.
“Although the primary endpoint was neutral, the CONVINCE results support the hypothesis that long-term anti-inflammatory therapy with colchicine may reduce recurrent stroke and cardiovascular events, specifically in stroke patients with atherosclerosis,” lead investigator Peter Kelly, MD, University College Dublin School of Medicine, Dublin, Ireland, concluded.
The results were presented at the European Stroke Organization Conference (ESOC) 2024.
Inflammation, Dr. Kelly said, plays an important role in the pathophysiology of atherosclerotic plaque, a major cause of cardiovascular events and ischemic strokes.
Colchicine, an established, widely available, low-cost drug that reduces inflammatory response, has been shown to reduce recurrent vascular events in patients with coronary artery disease.
The CONVINCE trial was conducted to see whether colchicine could show similar benefits in patients with non-severe, non-cardioembolic stroke or transient ischemic attack.
Conducted in 16 European countries and Canada, the CONVINCE trial included 3154 patients with a recent non-cardioembolic nondisabling ischemic stroke or high-risk transient ischemic attack. They were randomly assigned to receive colchicine (0.5 mg/d) or placebo.
Key exclusion criteria included evidence of atrial fibrillation or other source of cardioembolism, a defined cause of stroke other than atherosclerosis or small vessel disease, a glomerular filtration rate below 50 mL/min, and the use of drugs that interact with colchicine.
The primary endpoint was a composite of first recurrent ischemic stroke, myocardial infarction, cardiac arrest, or hospitalization for unstable angina. Study participants were followed-up over 36 months.
Results of the primary intention-to-treat analysis showed that the primary endpoint occurred in 153 patients randomized to low-dose colchicine (9.8%) versus 185 in the placebo group (11.8%). This translated into a hazard ratio (HR) of 0.84 (95% CI, 0.68-1.05; P = .12) — a nonsignificant result.
Reduced levels of C-reactive protein in the colchicine group showed the anti-inflammatory effect of treatment with colchicine, Dr. Kelly reported.
In a prespecified on-treatment analysis (excluding patients with major protocol violations), colchicine did show a significant benefit in the primary endpoint (HR, 0.80; 95% CI, 0.63-0.99).
A Novel Target for Stroke Treatment
In addition, significantly reduced rates of recurrent stroke or cardiovascular events were observed in the subgroup of patients with a history of coronary artery disease.
In an updated meta-analysis of existing colchicine studies including CONVINCE, there was a significant reduction in the risk for ischemic stroke (risk ratio, 0.73; 95% CI, 0.58-0.90).
“The signals of benefit of colchicine in secondary analyses are in line with findings from previous trials and indicate the potential of colchicine in prevention after stroke,” Dr. Kelly said.
He pointed out that the COVID pandemic reduced the planned follow-up time in the CONVINCE trial, which led to the study being underpowered for the primary analysis.
“Further trials are needed in all stroke subtypes, but with particular focus on patients with objective evidence of atherosclerosis,” he said.
Commenting on the findings, Mira Katan, MD, University Hospital of Basel, Switzerland, noted that inflammation represents a novel target for stroke treatment.
“We have never before looked at treating inflammation in stroke. Although the primary endpoint was not reached in the CONVINCE study, the on-treatment analysis and meta-analysis showed a risk reduction, and we know colchicine works in cardiology. I think this is a fantastic trial, giving us a new target for stroke therapy,” Dr. Katan said.
“I think we have a new tool, but of course we need further trials to confirm that,” she added.
The CONVINCE trial was supported by Health Research Board Ireland, Deutsche Forschungsgesellschaft, Fonds Wetenschappelijk Onderzoek (FWO), and the Irish Heart Foundation. Dr. Kelly received funding from the Irish Heart Foundation. Dr. Katan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
BASEL, SWITZERLAND — However, the results did reveal a significant reduction in recurrent stroke and cardiovascular events in the per-protocol analysis and in the subgroup of patients with coronary artery disease.
“Although the primary endpoint was neutral, the CONVINCE results support the hypothesis that long-term anti-inflammatory therapy with colchicine may reduce recurrent stroke and cardiovascular events, specifically in stroke patients with atherosclerosis,” lead investigator Peter Kelly, MD, University College Dublin School of Medicine, Dublin, Ireland, concluded.
The results were presented at the European Stroke Organization Conference (ESOC) 2024.
Inflammation, Dr. Kelly said, plays an important role in the pathophysiology of atherosclerotic plaque, a major cause of cardiovascular events and ischemic strokes.
Colchicine, an established, widely available, low-cost drug that reduces inflammatory response, has been shown to reduce recurrent vascular events in patients with coronary artery disease.
The CONVINCE trial was conducted to see whether colchicine could show similar benefits in patients with non-severe, non-cardioembolic stroke or transient ischemic attack.
Conducted in 16 European countries and Canada, the CONVINCE trial included 3154 patients with a recent non-cardioembolic nondisabling ischemic stroke or high-risk transient ischemic attack. They were randomly assigned to receive colchicine (0.5 mg/d) or placebo.
Key exclusion criteria included evidence of atrial fibrillation or other source of cardioembolism, a defined cause of stroke other than atherosclerosis or small vessel disease, a glomerular filtration rate below 50 mL/min, and the use of drugs that interact with colchicine.
The primary endpoint was a composite of first recurrent ischemic stroke, myocardial infarction, cardiac arrest, or hospitalization for unstable angina. Study participants were followed-up over 36 months.
Results of the primary intention-to-treat analysis showed that the primary endpoint occurred in 153 patients randomized to low-dose colchicine (9.8%) versus 185 in the placebo group (11.8%). This translated into a hazard ratio (HR) of 0.84 (95% CI, 0.68-1.05; P = .12) — a nonsignificant result.
Reduced levels of C-reactive protein in the colchicine group showed the anti-inflammatory effect of treatment with colchicine, Dr. Kelly reported.
In a prespecified on-treatment analysis (excluding patients with major protocol violations), colchicine did show a significant benefit in the primary endpoint (HR, 0.80; 95% CI, 0.63-0.99).
A Novel Target for Stroke Treatment
In addition, significantly reduced rates of recurrent stroke or cardiovascular events were observed in the subgroup of patients with a history of coronary artery disease.
In an updated meta-analysis of existing colchicine studies including CONVINCE, there was a significant reduction in the risk for ischemic stroke (risk ratio, 0.73; 95% CI, 0.58-0.90).
“The signals of benefit of colchicine in secondary analyses are in line with findings from previous trials and indicate the potential of colchicine in prevention after stroke,” Dr. Kelly said.
He pointed out that the COVID pandemic reduced the planned follow-up time in the CONVINCE trial, which led to the study being underpowered for the primary analysis.
“Further trials are needed in all stroke subtypes, but with particular focus on patients with objective evidence of atherosclerosis,” he said.
Commenting on the findings, Mira Katan, MD, University Hospital of Basel, Switzerland, noted that inflammation represents a novel target for stroke treatment.
“We have never before looked at treating inflammation in stroke. Although the primary endpoint was not reached in the CONVINCE study, the on-treatment analysis and meta-analysis showed a risk reduction, and we know colchicine works in cardiology. I think this is a fantastic trial, giving us a new target for stroke therapy,” Dr. Katan said.
“I think we have a new tool, but of course we need further trials to confirm that,” she added.
The CONVINCE trial was supported by Health Research Board Ireland, Deutsche Forschungsgesellschaft, Fonds Wetenschappelijk Onderzoek (FWO), and the Irish Heart Foundation. Dr. Kelly received funding from the Irish Heart Foundation. Dr. Katan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
BASEL, SWITZERLAND — However, the results did reveal a significant reduction in recurrent stroke and cardiovascular events in the per-protocol analysis and in the subgroup of patients with coronary artery disease.
“Although the primary endpoint was neutral, the CONVINCE results support the hypothesis that long-term anti-inflammatory therapy with colchicine may reduce recurrent stroke and cardiovascular events, specifically in stroke patients with atherosclerosis,” lead investigator Peter Kelly, MD, University College Dublin School of Medicine, Dublin, Ireland, concluded.
The results were presented at the European Stroke Organization Conference (ESOC) 2024.
Inflammation, Dr. Kelly said, plays an important role in the pathophysiology of atherosclerotic plaque, a major cause of cardiovascular events and ischemic strokes.
Colchicine, an established, widely available, low-cost drug that reduces inflammatory response, has been shown to reduce recurrent vascular events in patients with coronary artery disease.
The CONVINCE trial was conducted to see whether colchicine could show similar benefits in patients with non-severe, non-cardioembolic stroke or transient ischemic attack.
Conducted in 16 European countries and Canada, the CONVINCE trial included 3154 patients with a recent non-cardioembolic nondisabling ischemic stroke or high-risk transient ischemic attack. They were randomly assigned to receive colchicine (0.5 mg/d) or placebo.
Key exclusion criteria included evidence of atrial fibrillation or other source of cardioembolism, a defined cause of stroke other than atherosclerosis or small vessel disease, a glomerular filtration rate below 50 mL/min, and the use of drugs that interact with colchicine.
The primary endpoint was a composite of first recurrent ischemic stroke, myocardial infarction, cardiac arrest, or hospitalization for unstable angina. Study participants were followed-up over 36 months.
Results of the primary intention-to-treat analysis showed that the primary endpoint occurred in 153 patients randomized to low-dose colchicine (9.8%) versus 185 in the placebo group (11.8%). This translated into a hazard ratio (HR) of 0.84 (95% CI, 0.68-1.05; P = .12) — a nonsignificant result.
Reduced levels of C-reactive protein in the colchicine group showed the anti-inflammatory effect of treatment with colchicine, Dr. Kelly reported.
In a prespecified on-treatment analysis (excluding patients with major protocol violations), colchicine did show a significant benefit in the primary endpoint (HR, 0.80; 95% CI, 0.63-0.99).
A Novel Target for Stroke Treatment
In addition, significantly reduced rates of recurrent stroke or cardiovascular events were observed in the subgroup of patients with a history of coronary artery disease.
In an updated meta-analysis of existing colchicine studies including CONVINCE, there was a significant reduction in the risk for ischemic stroke (risk ratio, 0.73; 95% CI, 0.58-0.90).
“The signals of benefit of colchicine in secondary analyses are in line with findings from previous trials and indicate the potential of colchicine in prevention after stroke,” Dr. Kelly said.
He pointed out that the COVID pandemic reduced the planned follow-up time in the CONVINCE trial, which led to the study being underpowered for the primary analysis.
“Further trials are needed in all stroke subtypes, but with particular focus on patients with objective evidence of atherosclerosis,” he said.
Commenting on the findings, Mira Katan, MD, University Hospital of Basel, Switzerland, noted that inflammation represents a novel target for stroke treatment.
“We have never before looked at treating inflammation in stroke. Although the primary endpoint was not reached in the CONVINCE study, the on-treatment analysis and meta-analysis showed a risk reduction, and we know colchicine works in cardiology. I think this is a fantastic trial, giving us a new target for stroke therapy,” Dr. Katan said.
“I think we have a new tool, but of course we need further trials to confirm that,” she added.
The CONVINCE trial was supported by Health Research Board Ireland, Deutsche Forschungsgesellschaft, Fonds Wetenschappelijk Onderzoek (FWO), and the Irish Heart Foundation. Dr. Kelly received funding from the Irish Heart Foundation. Dr. Katan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ESOC 2024
CARACO: Study Shows Safety of Leaving Uninvolved Lymph Nodes in Ovarian Cancer
Retroperitoneal pelvic and para-aortic lymphadenectomy (RPPL) in patients undergoing either primary or interval surgery during ovarian cancer treatment for advanced epithelial ovarian cancer (AEOC) resulted in no benefit and significant harm, the new study found.
“[RPPL] brings only toxicity and it does not increase survival ... because we have a lot of improvement with other treatments than surgery,” lead author Jean-Marc Classe, MD, PhD, said during a press conference at the annual meeting of the American Society of Clinical Oncology. “It’s a surgical de-escalation, because it is not useful.”
Enrollment for the multicenter, phase III CARACO trial stagnated after the LION trial (N Engl J Med . 2019 Feb 28;380[9]:822-832) showed no benefit to doing RPPL in patients undergoing primary surgery for AEOC. Ultimately, the CARACO trial did not enroll the prespecified sample size needed in order for the researchers to show that not doing RPPL was superior.
Dr. Classe, a surgical oncologist at Nantes Université, in Nantes, France, explained that primary surgery is currently much less common than interval surgery for AEOC , thus it was important to design the CARACO trial to explore the risks and benefits of RPPL in a patient population that included more interval surgery.
CARACO enrolled 379 patients, with median age 64-65 years, and was closed prematurely due to stagnation of enrollment. Patients were randomized to no-RPPL (n = 193) or RPPL (n = 186), with about 75% in each arm receiving interval surgery (neoadjuvant chemotherapy, followed by cytoreductive surgery and adjuvant chemotherapy), and about 25% receiving primary surgery (initial cytoreductive surgery followed by adjuvant chemotherapy). There was a similar postsurgical rate of no residual disease (85.6% and 88.3% in the no-RPPL and RPPL groups, respectively), and lymph node metastases were diagnosed in 43% of the patients in the RPPL arm, with a median of 3 involved lymph nodes.
After a median follow-up of 9 years both the primary endpoint of progression-free survival (PFS), and secondary endpoint of overall survival (OS) showed no advantage to RPPL, with a median PFS of 14.8 months in the no-RPPL arm and 18.5 months in the RPPL arm (HR 0.96), and a median OS of 48.9 months and 58.0 months respectively (HR 0.92).
Surgery in the lymphadenectomy arm was 300 minutes versus 240 minutes.
“We observed statistically significant more morbidity in the lymphadenectomy arm with more transfusion (72 vs 57 patients), more re-intervention (15 vs 6 patients), more urinary injury (7 vs zero patients),” said Dr. Classe. Mortality was the same in both arms.
There were 314 events observed in the trial, which was 22 events fewer than the required sample size to show superiority. However, Dr. Classe said, a “worst-case scenario” calculation, assuming that all events would have favored lymphadenectomy, did not change the overall result.
The discussant for the trial, Shitanshu Uppal, MD, assistant professor in the division of gynecologic oncology at the University of Michigan in Ann Arbor, agreed that the study investigators adequately addressed this concern with the “counterfactual scenario.” He added that “as utilization of neoadjuvant chemotherapy goes up these results are really helpful in consolidating the results of the prior LION study, that lymph node dissection has no role in interval debulking surgery as well.”
Commenting on the study during the press conference, Michael Lowe, MD, associate professor in the division of surgical oncology at Emory University School of Medicine in Atlanta, said the CARACO study investigators’ efforts “underscore the difficulty of designing and accruing surgical clinical trials, especially clinical trials in which patients are offered less surgery.” He said the trial’s findings are consistent with other clinical trials in breast cancer and melanoma “that likewise showed similar outcomes for patients that did not undergo removal of clinically normal appearing lymph nodes.”
He pointed out that all of these studies highlight that the focus should turn to improving medical therapies.
Echoing this sentiment, Julie Gralow, MD, ASCO chief medical officer and executive vice-president, said, “it is very clear that lymph node dissection has significant morbidity ... and it’s very clear that we should not be doing more surgery than is needed ... In advanced ovarian cancer where the majority already have distant disease [focusing] on systemic therapy is probably what will have the most impact.”
Christina Annunziata, MD, PhD, senior vice president, Extramural Discovery Science, at the American Cancer Society, and an expert in ovarian cancer, said the analysis “leaves little doubt that there would be a statistically significant difference between the two arms. The numbers are small, but since this study results were consistent with the similar LION trial, I think that this study will tip the balance further towards omitting the lymphadenectomy in both primary and interval surgeries,” she said in an interview.
Dr. Annunziata added that surgeons are already omitting the dissection based on the LION study.
The study was funded by the French National Institute of Cancer. Dr. Classe disclosed consulting or advisory roles for GlaxoSmithKline, Myriad Genetics, and Roche.
None of the other experts interviewed for this piece declared having any relevant disclosures.
Retroperitoneal pelvic and para-aortic lymphadenectomy (RPPL) in patients undergoing either primary or interval surgery during ovarian cancer treatment for advanced epithelial ovarian cancer (AEOC) resulted in no benefit and significant harm, the new study found.
“[RPPL] brings only toxicity and it does not increase survival ... because we have a lot of improvement with other treatments than surgery,” lead author Jean-Marc Classe, MD, PhD, said during a press conference at the annual meeting of the American Society of Clinical Oncology. “It’s a surgical de-escalation, because it is not useful.”
Enrollment for the multicenter, phase III CARACO trial stagnated after the LION trial (N Engl J Med . 2019 Feb 28;380[9]:822-832) showed no benefit to doing RPPL in patients undergoing primary surgery for AEOC. Ultimately, the CARACO trial did not enroll the prespecified sample size needed in order for the researchers to show that not doing RPPL was superior.
Dr. Classe, a surgical oncologist at Nantes Université, in Nantes, France, explained that primary surgery is currently much less common than interval surgery for AEOC , thus it was important to design the CARACO trial to explore the risks and benefits of RPPL in a patient population that included more interval surgery.
CARACO enrolled 379 patients, with median age 64-65 years, and was closed prematurely due to stagnation of enrollment. Patients were randomized to no-RPPL (n = 193) or RPPL (n = 186), with about 75% in each arm receiving interval surgery (neoadjuvant chemotherapy, followed by cytoreductive surgery and adjuvant chemotherapy), and about 25% receiving primary surgery (initial cytoreductive surgery followed by adjuvant chemotherapy). There was a similar postsurgical rate of no residual disease (85.6% and 88.3% in the no-RPPL and RPPL groups, respectively), and lymph node metastases were diagnosed in 43% of the patients in the RPPL arm, with a median of 3 involved lymph nodes.
After a median follow-up of 9 years both the primary endpoint of progression-free survival (PFS), and secondary endpoint of overall survival (OS) showed no advantage to RPPL, with a median PFS of 14.8 months in the no-RPPL arm and 18.5 months in the RPPL arm (HR 0.96), and a median OS of 48.9 months and 58.0 months respectively (HR 0.92).
Surgery in the lymphadenectomy arm was 300 minutes versus 240 minutes.
“We observed statistically significant more morbidity in the lymphadenectomy arm with more transfusion (72 vs 57 patients), more re-intervention (15 vs 6 patients), more urinary injury (7 vs zero patients),” said Dr. Classe. Mortality was the same in both arms.
There were 314 events observed in the trial, which was 22 events fewer than the required sample size to show superiority. However, Dr. Classe said, a “worst-case scenario” calculation, assuming that all events would have favored lymphadenectomy, did not change the overall result.
The discussant for the trial, Shitanshu Uppal, MD, assistant professor in the division of gynecologic oncology at the University of Michigan in Ann Arbor, agreed that the study investigators adequately addressed this concern with the “counterfactual scenario.” He added that “as utilization of neoadjuvant chemotherapy goes up these results are really helpful in consolidating the results of the prior LION study, that lymph node dissection has no role in interval debulking surgery as well.”
Commenting on the study during the press conference, Michael Lowe, MD, associate professor in the division of surgical oncology at Emory University School of Medicine in Atlanta, said the CARACO study investigators’ efforts “underscore the difficulty of designing and accruing surgical clinical trials, especially clinical trials in which patients are offered less surgery.” He said the trial’s findings are consistent with other clinical trials in breast cancer and melanoma “that likewise showed similar outcomes for patients that did not undergo removal of clinically normal appearing lymph nodes.”
He pointed out that all of these studies highlight that the focus should turn to improving medical therapies.
Echoing this sentiment, Julie Gralow, MD, ASCO chief medical officer and executive vice-president, said, “it is very clear that lymph node dissection has significant morbidity ... and it’s very clear that we should not be doing more surgery than is needed ... In advanced ovarian cancer where the majority already have distant disease [focusing] on systemic therapy is probably what will have the most impact.”
Christina Annunziata, MD, PhD, senior vice president, Extramural Discovery Science, at the American Cancer Society, and an expert in ovarian cancer, said the analysis “leaves little doubt that there would be a statistically significant difference between the two arms. The numbers are small, but since this study results were consistent with the similar LION trial, I think that this study will tip the balance further towards omitting the lymphadenectomy in both primary and interval surgeries,” she said in an interview.
Dr. Annunziata added that surgeons are already omitting the dissection based on the LION study.
The study was funded by the French National Institute of Cancer. Dr. Classe disclosed consulting or advisory roles for GlaxoSmithKline, Myriad Genetics, and Roche.
None of the other experts interviewed for this piece declared having any relevant disclosures.
Retroperitoneal pelvic and para-aortic lymphadenectomy (RPPL) in patients undergoing either primary or interval surgery during ovarian cancer treatment for advanced epithelial ovarian cancer (AEOC) resulted in no benefit and significant harm, the new study found.
“[RPPL] brings only toxicity and it does not increase survival ... because we have a lot of improvement with other treatments than surgery,” lead author Jean-Marc Classe, MD, PhD, said during a press conference at the annual meeting of the American Society of Clinical Oncology. “It’s a surgical de-escalation, because it is not useful.”
Enrollment for the multicenter, phase III CARACO trial stagnated after the LION trial (N Engl J Med . 2019 Feb 28;380[9]:822-832) showed no benefit to doing RPPL in patients undergoing primary surgery for AEOC. Ultimately, the CARACO trial did not enroll the prespecified sample size needed in order for the researchers to show that not doing RPPL was superior.
Dr. Classe, a surgical oncologist at Nantes Université, in Nantes, France, explained that primary surgery is currently much less common than interval surgery for AEOC , thus it was important to design the CARACO trial to explore the risks and benefits of RPPL in a patient population that included more interval surgery.
CARACO enrolled 379 patients, with median age 64-65 years, and was closed prematurely due to stagnation of enrollment. Patients were randomized to no-RPPL (n = 193) or RPPL (n = 186), with about 75% in each arm receiving interval surgery (neoadjuvant chemotherapy, followed by cytoreductive surgery and adjuvant chemotherapy), and about 25% receiving primary surgery (initial cytoreductive surgery followed by adjuvant chemotherapy). There was a similar postsurgical rate of no residual disease (85.6% and 88.3% in the no-RPPL and RPPL groups, respectively), and lymph node metastases were diagnosed in 43% of the patients in the RPPL arm, with a median of 3 involved lymph nodes.
After a median follow-up of 9 years both the primary endpoint of progression-free survival (PFS), and secondary endpoint of overall survival (OS) showed no advantage to RPPL, with a median PFS of 14.8 months in the no-RPPL arm and 18.5 months in the RPPL arm (HR 0.96), and a median OS of 48.9 months and 58.0 months respectively (HR 0.92).
Surgery in the lymphadenectomy arm was 300 minutes versus 240 minutes.
“We observed statistically significant more morbidity in the lymphadenectomy arm with more transfusion (72 vs 57 patients), more re-intervention (15 vs 6 patients), more urinary injury (7 vs zero patients),” said Dr. Classe. Mortality was the same in both arms.
There were 314 events observed in the trial, which was 22 events fewer than the required sample size to show superiority. However, Dr. Classe said, a “worst-case scenario” calculation, assuming that all events would have favored lymphadenectomy, did not change the overall result.
The discussant for the trial, Shitanshu Uppal, MD, assistant professor in the division of gynecologic oncology at the University of Michigan in Ann Arbor, agreed that the study investigators adequately addressed this concern with the “counterfactual scenario.” He added that “as utilization of neoadjuvant chemotherapy goes up these results are really helpful in consolidating the results of the prior LION study, that lymph node dissection has no role in interval debulking surgery as well.”
Commenting on the study during the press conference, Michael Lowe, MD, associate professor in the division of surgical oncology at Emory University School of Medicine in Atlanta, said the CARACO study investigators’ efforts “underscore the difficulty of designing and accruing surgical clinical trials, especially clinical trials in which patients are offered less surgery.” He said the trial’s findings are consistent with other clinical trials in breast cancer and melanoma “that likewise showed similar outcomes for patients that did not undergo removal of clinically normal appearing lymph nodes.”
He pointed out that all of these studies highlight that the focus should turn to improving medical therapies.
Echoing this sentiment, Julie Gralow, MD, ASCO chief medical officer and executive vice-president, said, “it is very clear that lymph node dissection has significant morbidity ... and it’s very clear that we should not be doing more surgery than is needed ... In advanced ovarian cancer where the majority already have distant disease [focusing] on systemic therapy is probably what will have the most impact.”
Christina Annunziata, MD, PhD, senior vice president, Extramural Discovery Science, at the American Cancer Society, and an expert in ovarian cancer, said the analysis “leaves little doubt that there would be a statistically significant difference between the two arms. The numbers are small, but since this study results were consistent with the similar LION trial, I think that this study will tip the balance further towards omitting the lymphadenectomy in both primary and interval surgeries,” she said in an interview.
Dr. Annunziata added that surgeons are already omitting the dissection based on the LION study.
The study was funded by the French National Institute of Cancer. Dr. Classe disclosed consulting or advisory roles for GlaxoSmithKline, Myriad Genetics, and Roche.
None of the other experts interviewed for this piece declared having any relevant disclosures.
FROM ASCO 2024
Biologics May Improve Outcomes in Overlapping COPD and Asthma
Use of biologics significantly reduced exacerbations and hospitalizations in adults with chronic obstructive pulmonary disease (COPD) and overlapping type 2 asthma inflammation, based on data from a new study presented at the American Thoracic Society’s international conference.
Patients diagnosed with COPD on maximum medical therapy may continue to have disease exacerbations that are highly morbid and are associated with worsening lung function, increased hospitalizations, and worsened mortality, said lead author Stephen Dachert, MD, Temple University Hospital, Philadelphia, in an interview.
Previous research has examined the association between use of individual biologics and reduction in acute exacerbations of COPD, but real-world data on the use of biologics for COPD and asthma-COPD overlap syndrome (ACOS) are lacking, Dr. Dachert and colleagues wrote in their abstract.
In the current study, the researchers reviewed data from 53 adults with COPD who were seen at a single center; 30 had ACOS, and 23 had COPD only. The mean age of the participants was 68.2 years, approximately half were White/Caucasian individuals, 26% were Black/African American individuals, 17% were Hispanic individuals, 4% were Asian individuals/Pacific Islanders, and 2% were from other races/ethnicities; 62% were women. The study population included patients with prior diagnosis codes for COPD and dupilumab, mepolizumab, benralizumab, or tezepelumab; the mean eosinophil count before biologics initiation was 471.
Reduction in Exacerbations and Hospitalizations
The researchers assessed change in exacerbations, hospitalizations, and spirometry from 1 year before to 1 year after initiation of treatment with biologics. Overall, after the use of biologics, patients experienced a significant mean reduction in exacerbations and hospitalizations of 1.780 and 0.944, respectively (both P < .001, using a paired T-test).
In addition, the researchers found a mean reduction of forced expiratory volume per second percent predicted of 0.57% and a mean increase in forced vital capacity percent predicted of 1.3% after the initiation of biologics.
Increases also occurred in total lung capacity percent predicted, residual volume percent predicted, and diffusing capacity of the lungs for carbon monoxide (DLCO) percent predicted (3.37%, 9.90%, and 4.58%, respectively). Of these, only DLCO percent predicted approached statistical significance, the researchers wrote.
The study findings make sense physiologically, Dr. Dachert said in an interview. “If large, randomized trials have shown a reduction in exacerbations in patients with type 2 inflammation asthma, it makes sense that we would see similar results in patients with COPD and type 2 inflammation,” he said. However, as yet only one of several large randomized trials has shown reductions in exacerbations and COPD with type 2 inflammation, he added.
“In our real-world cohort, we saw both a reduction in exacerbations and hospitalizations in the year following initiation of biologic therapy,” Dr. Dachert said. A reduction in hospitalizations, in particular, had not previously been shown in this population, he noted.
The findings were limited by the retrospective design and use of data from a single center; moreover, larger real-world studies are needed to confirm the results, said Dr. Dachert. “As we add patients to our cohort, we may be able to identify which clinical characteristics/risk factors may be associated with an even more robust reduction in exacerbations or hospitalizations,” he said.
“Our cohort of patients was more diverse than those included in prior randomized clinical trials and also has high rates of emphysema and airflow obstruction, populations typically excluded in large randomized trials,” he said.
Data Support the Potential of Biologics for COPD
Biologic agents have been effective in reducing asthma exacerbations, and understanding their effectiveness in reducing COPD exacerbations in a real-world setting is important, said Arianne K. Baldomero, MD, assistant professor of medicine at Minneapolis VA Health Care System, Minneapolis, in an interview.
Dr, Baldomero said she was not surprised by the current study results “as clinical trials are showing similar findings among this group of patients with elevated eosinophil counts.”
The current study adds to the growing evidence supporting the use of biologics to reduce COPD exacerbations, Dr. Baldomero told this news organization. “I anticipate that we will soon begin using biologics to manage frequent exacerbations in patients with COPD,” she said.
“For both asthma and COPD, more research is needed to guide clinicians in tapering or weaning down biologic treatment and determining whether patients still need to use inhalers,” Dr. Baldomero added.
The study received no outside funding. The researchers and Dr. Baldomero had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Use of biologics significantly reduced exacerbations and hospitalizations in adults with chronic obstructive pulmonary disease (COPD) and overlapping type 2 asthma inflammation, based on data from a new study presented at the American Thoracic Society’s international conference.
Patients diagnosed with COPD on maximum medical therapy may continue to have disease exacerbations that are highly morbid and are associated with worsening lung function, increased hospitalizations, and worsened mortality, said lead author Stephen Dachert, MD, Temple University Hospital, Philadelphia, in an interview.
Previous research has examined the association between use of individual biologics and reduction in acute exacerbations of COPD, but real-world data on the use of biologics for COPD and asthma-COPD overlap syndrome (ACOS) are lacking, Dr. Dachert and colleagues wrote in their abstract.
In the current study, the researchers reviewed data from 53 adults with COPD who were seen at a single center; 30 had ACOS, and 23 had COPD only. The mean age of the participants was 68.2 years, approximately half were White/Caucasian individuals, 26% were Black/African American individuals, 17% were Hispanic individuals, 4% were Asian individuals/Pacific Islanders, and 2% were from other races/ethnicities; 62% were women. The study population included patients with prior diagnosis codes for COPD and dupilumab, mepolizumab, benralizumab, or tezepelumab; the mean eosinophil count before biologics initiation was 471.
Reduction in Exacerbations and Hospitalizations
The researchers assessed change in exacerbations, hospitalizations, and spirometry from 1 year before to 1 year after initiation of treatment with biologics. Overall, after the use of biologics, patients experienced a significant mean reduction in exacerbations and hospitalizations of 1.780 and 0.944, respectively (both P < .001, using a paired T-test).
In addition, the researchers found a mean reduction of forced expiratory volume per second percent predicted of 0.57% and a mean increase in forced vital capacity percent predicted of 1.3% after the initiation of biologics.
Increases also occurred in total lung capacity percent predicted, residual volume percent predicted, and diffusing capacity of the lungs for carbon monoxide (DLCO) percent predicted (3.37%, 9.90%, and 4.58%, respectively). Of these, only DLCO percent predicted approached statistical significance, the researchers wrote.
The study findings make sense physiologically, Dr. Dachert said in an interview. “If large, randomized trials have shown a reduction in exacerbations in patients with type 2 inflammation asthma, it makes sense that we would see similar results in patients with COPD and type 2 inflammation,” he said. However, as yet only one of several large randomized trials has shown reductions in exacerbations and COPD with type 2 inflammation, he added.
“In our real-world cohort, we saw both a reduction in exacerbations and hospitalizations in the year following initiation of biologic therapy,” Dr. Dachert said. A reduction in hospitalizations, in particular, had not previously been shown in this population, he noted.
The findings were limited by the retrospective design and use of data from a single center; moreover, larger real-world studies are needed to confirm the results, said Dr. Dachert. “As we add patients to our cohort, we may be able to identify which clinical characteristics/risk factors may be associated with an even more robust reduction in exacerbations or hospitalizations,” he said.
“Our cohort of patients was more diverse than those included in prior randomized clinical trials and also has high rates of emphysema and airflow obstruction, populations typically excluded in large randomized trials,” he said.
Data Support the Potential of Biologics for COPD
Biologic agents have been effective in reducing asthma exacerbations, and understanding their effectiveness in reducing COPD exacerbations in a real-world setting is important, said Arianne K. Baldomero, MD, assistant professor of medicine at Minneapolis VA Health Care System, Minneapolis, in an interview.
Dr, Baldomero said she was not surprised by the current study results “as clinical trials are showing similar findings among this group of patients with elevated eosinophil counts.”
The current study adds to the growing evidence supporting the use of biologics to reduce COPD exacerbations, Dr. Baldomero told this news organization. “I anticipate that we will soon begin using biologics to manage frequent exacerbations in patients with COPD,” she said.
“For both asthma and COPD, more research is needed to guide clinicians in tapering or weaning down biologic treatment and determining whether patients still need to use inhalers,” Dr. Baldomero added.
The study received no outside funding. The researchers and Dr. Baldomero had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Use of biologics significantly reduced exacerbations and hospitalizations in adults with chronic obstructive pulmonary disease (COPD) and overlapping type 2 asthma inflammation, based on data from a new study presented at the American Thoracic Society’s international conference.
Patients diagnosed with COPD on maximum medical therapy may continue to have disease exacerbations that are highly morbid and are associated with worsening lung function, increased hospitalizations, and worsened mortality, said lead author Stephen Dachert, MD, Temple University Hospital, Philadelphia, in an interview.
Previous research has examined the association between use of individual biologics and reduction in acute exacerbations of COPD, but real-world data on the use of biologics for COPD and asthma-COPD overlap syndrome (ACOS) are lacking, Dr. Dachert and colleagues wrote in their abstract.
In the current study, the researchers reviewed data from 53 adults with COPD who were seen at a single center; 30 had ACOS, and 23 had COPD only. The mean age of the participants was 68.2 years, approximately half were White/Caucasian individuals, 26% were Black/African American individuals, 17% were Hispanic individuals, 4% were Asian individuals/Pacific Islanders, and 2% were from other races/ethnicities; 62% were women. The study population included patients with prior diagnosis codes for COPD and dupilumab, mepolizumab, benralizumab, or tezepelumab; the mean eosinophil count before biologics initiation was 471.
Reduction in Exacerbations and Hospitalizations
The researchers assessed change in exacerbations, hospitalizations, and spirometry from 1 year before to 1 year after initiation of treatment with biologics. Overall, after the use of biologics, patients experienced a significant mean reduction in exacerbations and hospitalizations of 1.780 and 0.944, respectively (both P < .001, using a paired T-test).
In addition, the researchers found a mean reduction of forced expiratory volume per second percent predicted of 0.57% and a mean increase in forced vital capacity percent predicted of 1.3% after the initiation of biologics.
Increases also occurred in total lung capacity percent predicted, residual volume percent predicted, and diffusing capacity of the lungs for carbon monoxide (DLCO) percent predicted (3.37%, 9.90%, and 4.58%, respectively). Of these, only DLCO percent predicted approached statistical significance, the researchers wrote.
The study findings make sense physiologically, Dr. Dachert said in an interview. “If large, randomized trials have shown a reduction in exacerbations in patients with type 2 inflammation asthma, it makes sense that we would see similar results in patients with COPD and type 2 inflammation,” he said. However, as yet only one of several large randomized trials has shown reductions in exacerbations and COPD with type 2 inflammation, he added.
“In our real-world cohort, we saw both a reduction in exacerbations and hospitalizations in the year following initiation of biologic therapy,” Dr. Dachert said. A reduction in hospitalizations, in particular, had not previously been shown in this population, he noted.
The findings were limited by the retrospective design and use of data from a single center; moreover, larger real-world studies are needed to confirm the results, said Dr. Dachert. “As we add patients to our cohort, we may be able to identify which clinical characteristics/risk factors may be associated with an even more robust reduction in exacerbations or hospitalizations,” he said.
“Our cohort of patients was more diverse than those included in prior randomized clinical trials and also has high rates of emphysema and airflow obstruction, populations typically excluded in large randomized trials,” he said.
Data Support the Potential of Biologics for COPD
Biologic agents have been effective in reducing asthma exacerbations, and understanding their effectiveness in reducing COPD exacerbations in a real-world setting is important, said Arianne K. Baldomero, MD, assistant professor of medicine at Minneapolis VA Health Care System, Minneapolis, in an interview.
Dr, Baldomero said she was not surprised by the current study results “as clinical trials are showing similar findings among this group of patients with elevated eosinophil counts.”
The current study adds to the growing evidence supporting the use of biologics to reduce COPD exacerbations, Dr. Baldomero told this news organization. “I anticipate that we will soon begin using biologics to manage frequent exacerbations in patients with COPD,” she said.
“For both asthma and COPD, more research is needed to guide clinicians in tapering or weaning down biologic treatment and determining whether patients still need to use inhalers,” Dr. Baldomero added.
The study received no outside funding. The researchers and Dr. Baldomero had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ATS 2024
Abemaciclib Plus Fulvestrant Improves Survival in Advanced Breast Cancer
Disease progression is common in these patients, for whom first-line treatment is cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors plus endocrine therapy, Kevin Kalinsky, MD, of the Winship Cancer Institute of Emory University, Atlanta, said in a presentation at the American Society of Clinical Oncology (ASCO) annual meeting.
A need exists for additional targeted therapies for patients with advanced hormone receptor (HR)+, HER2- breast cancer whose tumors have progressed on endocrine therapy plus a CDK4/6 inhibitor, he said.
Data on the benefits of continuing CDK4/6 inhibitor therapy after progression have been mixed in phase 2 trials, Dr. Kalinsky noted in his presentation. Abemaciclib, an oral CDK4/6 inhibitor, has shown more selectivity for CDK 4 than CDK 6, and is approved in combination with fulvestrant or an aromatase inhibitor for advanced breast cancer, he said.
In a phase 3 study known as postMONARCH, the researchers randomized 182 patients to abemaciclib plus fulvestrant and 186 to placebo plus fulvestrant. The primary endpoint was progression-free survival (PFS) based on investigator assessment; secondary endpoints included PFS based on blinded independent central review (BICR), objective response rate (ORR), and safety.
The PFS rates at 6 months were 50% and 37% for the abemaciclib and placebo arms, respectively.
In the primary analysis, abemaciclib led to a 27% reduction in risk of investigator-assessed progression-free survival events compared with the placebo (117 vs. 141 events, hazard ratio 0.73, P = 0.02).
The study population included men and pre- and postmenopausal women with advanced HR+, HER2- breast cancer and progression after initial CDK4/6 plus endocrine therapy from 96 centers in 16 countries, enrolled between March 2022 and June 2023. The median age of the patients in the abemaciclib and placebo groups was 58 years and 61 years, respectively. Patients underwent scans every 8 weeks for the first 12 months, then every 12 weeks. Most of the patients were enrolled immediately after CDK4/6i + ET as initial therapy for advanced breast cancer. The most common previous CDK4/6 inhibitor therapy was palbociclib (59%), followed by ribociclib (33%) and abemaciclib (8%).
Secondary Endpoints Also Favor Abemaciclib
The effects in favor of abemaciclib were consistent across subgroups, regardless of the presence or absence of baseline genetic mutations (ESR1 or PIK3CA), Dr. Kalinsky said in his presentation.
Overall response rate was significantly improved in the abemaciclib group compared with the placebo group in patients with measurable disease (17% vs. 7%) and PFS according to BICR also significantly improved (HR 0.55).
The magnitude of benefit was less in the subgroup of patients with visceral metastases, Dr. Kalinsky noted.
“Safety was consistent with what is known about the abemaciclib profile,” he added. Six percent of abemaciclib patients discontinued treatment because of adverse events.
The study is the first phase 3 trial to show improvement with CDK4/6 inhibition therapy with a combination of abemaciclib and fulvestrant and offers a new option for patients with HR+, HER2- advanced breast cancer not selected for biomarker status, Dr. Kalinsky concluded.
Data Support Switching CDK Inhibitors in Absence of Mutations
Switching CDK inhibitors to abemaciclib plus endocrine therapy significantly prolonged progression-free survival compared with endocrine therapy alone, with especially pronounced improvement in those without visceral metastases and those with longer durations of first-line CKD4/6 inhibitor therapy, said Ruth O’Regan, MD, of the University of Rochester, New York, who served as the discussant for the new research.
Dr. Regan referenced the improvement with abemaciclib in the BICR, a technique used to identify potential bias introduced by the assessment of local investigators. This can result in more favorable PFS on a treatment arm as seen in this study, but its use generally does not impact overall trial results, she said.
In the context of other studies involving switching CDK 4/6 inhibitors post-progression, the difference of 0.7 months in PFS between the abemaciclib and placebo groups was less than the 2.5 months difference seen in the MAINTAIN trial and the 1.3 months difference seen in the PALMIRA trial, Dr. O’Regan said in her presentation. Conversely, in the PACE trial, the intervention group did worse (4.6 months) than the control group in terms of the PFS (4.8 months), she said. Overall, the results of the postMONARCH trial support the use abemaciclib in patients with no actionable genetic mutation, she said.
In a question-and-answer session, Dr. Kalinsky was asked whether clinicians should still bother with genetic testing, since patients in the current study showed benefits regardless of the presence or absence of a mutation.
“I would still recommend that we check for mutations,” he emphasized. The current study “is one chapter in a much larger book,” and the field continues to evolve, he said.
A Clinician’s Take
“Currently, no standard second-line treatment after progression on first line CDK4/6 inhibitor plus endocrine therapy exists,” Malinda T. West, MD, of the University of Wisconsin, Madison, said in an interview. “Using a different CDK4/6 inhibitor after progression on a first CDK4/6 inhibitor has mixed data,” she said.
“If benefit with a second CDK4/6 inhibitor is confirmed, it may represent an additional low toxicity, chemotherapy-sparing regimen,” she noted.
Earlier data from the MAINTAIN trial had shown benefit with using ribociclib after progression on a primarily first line palbociclib, though other trials looking at use of palbociclib after progression on CDK 4/6 inhibitor [including the PACE and PALMIRA trials] had not, she said.
Overall, the results from postMONARCH support that switching the CDK4/6 inhibitor at progression to ribociclib or abemaciclib may be another treatment option, and reasonable for patients who don’t have other actionable mutations, Dr. West told this news organization.
The study was supported by Eli Lilly. Dr. Kalinsky disclosed that immediate family members are employed by EQRx and GRAIL, with stock or other ownership interests in these companies. He disclosed consulting or advisory roles with 4D Pharma; AstraZeneca; Cullinan Oncology; Daiichi Sankyo/AstraZeneca; eFFECTOR Therapeutics; Genentech/Roche; Immunomedics; Lilly; Menarini Silicon Biosystems; Merck; Mersana; Myovant Sciences; Novartis; Oncosec; Prelude Therapeutics; Puma Biotechnology; RayzeBio; Seagen; and Takeda. Dr. Kalinsky further disclosed research funding to his institution from Ascentage Pharma; AstraZeneca; Daiichi Sankyo; Genentech/Roche; Lilly; Novartis; and Seagen, and relationships with Genentech and Immunomedics.
Dr. O’Regan disclosed honoraria from AstraZeneca/MedImmune; bioTheranostics; Gilead Sciences; Novartis; Pfizer; Puma Biotechnology; and Seagen, serving as a consultant or adviser for AstraZeneca/MedImmune; bioTheranostics; Lilly; Novartis; Puma Biotechnology; and Seagen, and funding to her institution from Novartis and Puma Biotechnology.
Dr. West, who was not involved in the new research or other studies mentioned in this article, had no financial conflicts to disclose.
Disease progression is common in these patients, for whom first-line treatment is cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors plus endocrine therapy, Kevin Kalinsky, MD, of the Winship Cancer Institute of Emory University, Atlanta, said in a presentation at the American Society of Clinical Oncology (ASCO) annual meeting.
A need exists for additional targeted therapies for patients with advanced hormone receptor (HR)+, HER2- breast cancer whose tumors have progressed on endocrine therapy plus a CDK4/6 inhibitor, he said.
Data on the benefits of continuing CDK4/6 inhibitor therapy after progression have been mixed in phase 2 trials, Dr. Kalinsky noted in his presentation. Abemaciclib, an oral CDK4/6 inhibitor, has shown more selectivity for CDK 4 than CDK 6, and is approved in combination with fulvestrant or an aromatase inhibitor for advanced breast cancer, he said.
In a phase 3 study known as postMONARCH, the researchers randomized 182 patients to abemaciclib plus fulvestrant and 186 to placebo plus fulvestrant. The primary endpoint was progression-free survival (PFS) based on investigator assessment; secondary endpoints included PFS based on blinded independent central review (BICR), objective response rate (ORR), and safety.
The PFS rates at 6 months were 50% and 37% for the abemaciclib and placebo arms, respectively.
In the primary analysis, abemaciclib led to a 27% reduction in risk of investigator-assessed progression-free survival events compared with the placebo (117 vs. 141 events, hazard ratio 0.73, P = 0.02).
The study population included men and pre- and postmenopausal women with advanced HR+, HER2- breast cancer and progression after initial CDK4/6 plus endocrine therapy from 96 centers in 16 countries, enrolled between March 2022 and June 2023. The median age of the patients in the abemaciclib and placebo groups was 58 years and 61 years, respectively. Patients underwent scans every 8 weeks for the first 12 months, then every 12 weeks. Most of the patients were enrolled immediately after CDK4/6i + ET as initial therapy for advanced breast cancer. The most common previous CDK4/6 inhibitor therapy was palbociclib (59%), followed by ribociclib (33%) and abemaciclib (8%).
Secondary Endpoints Also Favor Abemaciclib
The effects in favor of abemaciclib were consistent across subgroups, regardless of the presence or absence of baseline genetic mutations (ESR1 or PIK3CA), Dr. Kalinsky said in his presentation.
Overall response rate was significantly improved in the abemaciclib group compared with the placebo group in patients with measurable disease (17% vs. 7%) and PFS according to BICR also significantly improved (HR 0.55).
The magnitude of benefit was less in the subgroup of patients with visceral metastases, Dr. Kalinsky noted.
“Safety was consistent with what is known about the abemaciclib profile,” he added. Six percent of abemaciclib patients discontinued treatment because of adverse events.
The study is the first phase 3 trial to show improvement with CDK4/6 inhibition therapy with a combination of abemaciclib and fulvestrant and offers a new option for patients with HR+, HER2- advanced breast cancer not selected for biomarker status, Dr. Kalinsky concluded.
Data Support Switching CDK Inhibitors in Absence of Mutations
Switching CDK inhibitors to abemaciclib plus endocrine therapy significantly prolonged progression-free survival compared with endocrine therapy alone, with especially pronounced improvement in those without visceral metastases and those with longer durations of first-line CKD4/6 inhibitor therapy, said Ruth O’Regan, MD, of the University of Rochester, New York, who served as the discussant for the new research.
Dr. Regan referenced the improvement with abemaciclib in the BICR, a technique used to identify potential bias introduced by the assessment of local investigators. This can result in more favorable PFS on a treatment arm as seen in this study, but its use generally does not impact overall trial results, she said.
In the context of other studies involving switching CDK 4/6 inhibitors post-progression, the difference of 0.7 months in PFS between the abemaciclib and placebo groups was less than the 2.5 months difference seen in the MAINTAIN trial and the 1.3 months difference seen in the PALMIRA trial, Dr. O’Regan said in her presentation. Conversely, in the PACE trial, the intervention group did worse (4.6 months) than the control group in terms of the PFS (4.8 months), she said. Overall, the results of the postMONARCH trial support the use abemaciclib in patients with no actionable genetic mutation, she said.
In a question-and-answer session, Dr. Kalinsky was asked whether clinicians should still bother with genetic testing, since patients in the current study showed benefits regardless of the presence or absence of a mutation.
“I would still recommend that we check for mutations,” he emphasized. The current study “is one chapter in a much larger book,” and the field continues to evolve, he said.
A Clinician’s Take
“Currently, no standard second-line treatment after progression on first line CDK4/6 inhibitor plus endocrine therapy exists,” Malinda T. West, MD, of the University of Wisconsin, Madison, said in an interview. “Using a different CDK4/6 inhibitor after progression on a first CDK4/6 inhibitor has mixed data,” she said.
“If benefit with a second CDK4/6 inhibitor is confirmed, it may represent an additional low toxicity, chemotherapy-sparing regimen,” she noted.
Earlier data from the MAINTAIN trial had shown benefit with using ribociclib after progression on a primarily first line palbociclib, though other trials looking at use of palbociclib after progression on CDK 4/6 inhibitor [including the PACE and PALMIRA trials] had not, she said.
Overall, the results from postMONARCH support that switching the CDK4/6 inhibitor at progression to ribociclib or abemaciclib may be another treatment option, and reasonable for patients who don’t have other actionable mutations, Dr. West told this news organization.
The study was supported by Eli Lilly. Dr. Kalinsky disclosed that immediate family members are employed by EQRx and GRAIL, with stock or other ownership interests in these companies. He disclosed consulting or advisory roles with 4D Pharma; AstraZeneca; Cullinan Oncology; Daiichi Sankyo/AstraZeneca; eFFECTOR Therapeutics; Genentech/Roche; Immunomedics; Lilly; Menarini Silicon Biosystems; Merck; Mersana; Myovant Sciences; Novartis; Oncosec; Prelude Therapeutics; Puma Biotechnology; RayzeBio; Seagen; and Takeda. Dr. Kalinsky further disclosed research funding to his institution from Ascentage Pharma; AstraZeneca; Daiichi Sankyo; Genentech/Roche; Lilly; Novartis; and Seagen, and relationships with Genentech and Immunomedics.
Dr. O’Regan disclosed honoraria from AstraZeneca/MedImmune; bioTheranostics; Gilead Sciences; Novartis; Pfizer; Puma Biotechnology; and Seagen, serving as a consultant or adviser for AstraZeneca/MedImmune; bioTheranostics; Lilly; Novartis; Puma Biotechnology; and Seagen, and funding to her institution from Novartis and Puma Biotechnology.
Dr. West, who was not involved in the new research or other studies mentioned in this article, had no financial conflicts to disclose.
Disease progression is common in these patients, for whom first-line treatment is cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors plus endocrine therapy, Kevin Kalinsky, MD, of the Winship Cancer Institute of Emory University, Atlanta, said in a presentation at the American Society of Clinical Oncology (ASCO) annual meeting.
A need exists for additional targeted therapies for patients with advanced hormone receptor (HR)+, HER2- breast cancer whose tumors have progressed on endocrine therapy plus a CDK4/6 inhibitor, he said.
Data on the benefits of continuing CDK4/6 inhibitor therapy after progression have been mixed in phase 2 trials, Dr. Kalinsky noted in his presentation. Abemaciclib, an oral CDK4/6 inhibitor, has shown more selectivity for CDK 4 than CDK 6, and is approved in combination with fulvestrant or an aromatase inhibitor for advanced breast cancer, he said.
In a phase 3 study known as postMONARCH, the researchers randomized 182 patients to abemaciclib plus fulvestrant and 186 to placebo plus fulvestrant. The primary endpoint was progression-free survival (PFS) based on investigator assessment; secondary endpoints included PFS based on blinded independent central review (BICR), objective response rate (ORR), and safety.
The PFS rates at 6 months were 50% and 37% for the abemaciclib and placebo arms, respectively.
In the primary analysis, abemaciclib led to a 27% reduction in risk of investigator-assessed progression-free survival events compared with the placebo (117 vs. 141 events, hazard ratio 0.73, P = 0.02).
The study population included men and pre- and postmenopausal women with advanced HR+, HER2- breast cancer and progression after initial CDK4/6 plus endocrine therapy from 96 centers in 16 countries, enrolled between March 2022 and June 2023. The median age of the patients in the abemaciclib and placebo groups was 58 years and 61 years, respectively. Patients underwent scans every 8 weeks for the first 12 months, then every 12 weeks. Most of the patients were enrolled immediately after CDK4/6i + ET as initial therapy for advanced breast cancer. The most common previous CDK4/6 inhibitor therapy was palbociclib (59%), followed by ribociclib (33%) and abemaciclib (8%).
Secondary Endpoints Also Favor Abemaciclib
The effects in favor of abemaciclib were consistent across subgroups, regardless of the presence or absence of baseline genetic mutations (ESR1 or PIK3CA), Dr. Kalinsky said in his presentation.
Overall response rate was significantly improved in the abemaciclib group compared with the placebo group in patients with measurable disease (17% vs. 7%) and PFS according to BICR also significantly improved (HR 0.55).
The magnitude of benefit was less in the subgroup of patients with visceral metastases, Dr. Kalinsky noted.
“Safety was consistent with what is known about the abemaciclib profile,” he added. Six percent of abemaciclib patients discontinued treatment because of adverse events.
The study is the first phase 3 trial to show improvement with CDK4/6 inhibition therapy with a combination of abemaciclib and fulvestrant and offers a new option for patients with HR+, HER2- advanced breast cancer not selected for biomarker status, Dr. Kalinsky concluded.
Data Support Switching CDK Inhibitors in Absence of Mutations
Switching CDK inhibitors to abemaciclib plus endocrine therapy significantly prolonged progression-free survival compared with endocrine therapy alone, with especially pronounced improvement in those without visceral metastases and those with longer durations of first-line CKD4/6 inhibitor therapy, said Ruth O’Regan, MD, of the University of Rochester, New York, who served as the discussant for the new research.
Dr. Regan referenced the improvement with abemaciclib in the BICR, a technique used to identify potential bias introduced by the assessment of local investigators. This can result in more favorable PFS on a treatment arm as seen in this study, but its use generally does not impact overall trial results, she said.
In the context of other studies involving switching CDK 4/6 inhibitors post-progression, the difference of 0.7 months in PFS between the abemaciclib and placebo groups was less than the 2.5 months difference seen in the MAINTAIN trial and the 1.3 months difference seen in the PALMIRA trial, Dr. O’Regan said in her presentation. Conversely, in the PACE trial, the intervention group did worse (4.6 months) than the control group in terms of the PFS (4.8 months), she said. Overall, the results of the postMONARCH trial support the use abemaciclib in patients with no actionable genetic mutation, she said.
In a question-and-answer session, Dr. Kalinsky was asked whether clinicians should still bother with genetic testing, since patients in the current study showed benefits regardless of the presence or absence of a mutation.
“I would still recommend that we check for mutations,” he emphasized. The current study “is one chapter in a much larger book,” and the field continues to evolve, he said.
A Clinician’s Take
“Currently, no standard second-line treatment after progression on first line CDK4/6 inhibitor plus endocrine therapy exists,” Malinda T. West, MD, of the University of Wisconsin, Madison, said in an interview. “Using a different CDK4/6 inhibitor after progression on a first CDK4/6 inhibitor has mixed data,” she said.
“If benefit with a second CDK4/6 inhibitor is confirmed, it may represent an additional low toxicity, chemotherapy-sparing regimen,” she noted.
Earlier data from the MAINTAIN trial had shown benefit with using ribociclib after progression on a primarily first line palbociclib, though other trials looking at use of palbociclib after progression on CDK 4/6 inhibitor [including the PACE and PALMIRA trials] had not, she said.
Overall, the results from postMONARCH support that switching the CDK4/6 inhibitor at progression to ribociclib or abemaciclib may be another treatment option, and reasonable for patients who don’t have other actionable mutations, Dr. West told this news organization.
The study was supported by Eli Lilly. Dr. Kalinsky disclosed that immediate family members are employed by EQRx and GRAIL, with stock or other ownership interests in these companies. He disclosed consulting or advisory roles with 4D Pharma; AstraZeneca; Cullinan Oncology; Daiichi Sankyo/AstraZeneca; eFFECTOR Therapeutics; Genentech/Roche; Immunomedics; Lilly; Menarini Silicon Biosystems; Merck; Mersana; Myovant Sciences; Novartis; Oncosec; Prelude Therapeutics; Puma Biotechnology; RayzeBio; Seagen; and Takeda. Dr. Kalinsky further disclosed research funding to his institution from Ascentage Pharma; AstraZeneca; Daiichi Sankyo; Genentech/Roche; Lilly; Novartis; and Seagen, and relationships with Genentech and Immunomedics.
Dr. O’Regan disclosed honoraria from AstraZeneca/MedImmune; bioTheranostics; Gilead Sciences; Novartis; Pfizer; Puma Biotechnology; and Seagen, serving as a consultant or adviser for AstraZeneca/MedImmune; bioTheranostics; Lilly; Novartis; Puma Biotechnology; and Seagen, and funding to her institution from Novartis and Puma Biotechnology.
Dr. West, who was not involved in the new research or other studies mentioned in this article, had no financial conflicts to disclose.
FROM ASCO 2024
Dupilumab May Reduce Exacerbations in COPD, Type 2 Inflammation
Dupilumab significantly reduced exacerbations and improved lung function in adults with uncontrolled chronic obstructive pulmonary disease (COPD) and type 2 inflammation, based on data from more than 900 individuals.
Data from a phase 3 trial known as NOTUS were presented at the American Thoracic Society’s international conference and published simultaneously in The New England Journal of Medicine.
Dupilumab, a fully human monoclonal antibody, works by inhibiting the signaling of the interleukin 4 (IL-4) and IL-13 pathways and is approved for many conditions characterized by type 2 inflammation, wrote Surya P. Bhatt, MD, of The University of Alabama at Birmingham, and colleagues in the NEJM study.
“Last year, we showed in the BOREAS trial that dupilumab was very effective in lowering exacerbation frequency in patients with COPD who continued to have frequent exacerbations despite being on maximal inhaled therapy,” Dr. Bhatt said in an interview.
12 Months of COPD, Triple Inhaler Therapy
In the NOTUS study, the researchers randomized 470 adults with uncontrolled COPD and type 2 inflammation (defined as a blood eosinophil count of ≥ 300 cells/µL) to 300-mg subcutaneous dupilumab and 465 to a placebo every 2 weeks. Patients were enrolled between July 2020 and May 2023.
The study population included adults aged 40-85 years with physician-diagnosed COPD for at least 12 months who had received background triple inhaler therapy (an inhaled glucocorticoid agent plus long-acting muscarinic antagonist [LAMA]–long-acting beta-agonist [LABA] or LAMA-LABA alone) for at least 3 months and at a stable dose for at least 1 month. All participants were current or former smokers with a smoking history of at least 10 pack-years.
The primary endpoint was a reduction in the annualized rate of moderate or severe COPD exacerbations at 52 weeks.
Patients in the dupilumab group also saw a significantly greater improvement in lung function compared with individuals in the placebo group based on prebronchodilator forced expiratory volume in 1 second from baseline to 12 weeks (least squares mean change of 139 mL vs 57 mL). This improvement was sustained at 52 weeks (least squares mean change of 115 mL vs 54 mL).
Improvement in respiratory symptom severity based on the St. George’s Respiratory Questionnaire was another secondary endpoint, and changes in total score were greater in the dupilumab group than in the placebo group (least squares mean change of 9.8 vs 6.4).
Safety outcomes were similar between the dupilumab and placebo groups, with approximately 66% of patients in each group reporting adverse events during the 52-week study period. Serious adverse events occurred in 13% and 15.9% of dupilumab and placebo patients, respectively, and adverse events resulting in death occurred in 2.6% and 1.5%, respectively. The most common adverse events were COVID-19, which occurred in 9.4% and 8.2% of the dupilumab and placebo patients, respectively, followed by headache, COPD, and nasopharyngitis. Major adverse cardiovascular events occurred in three patients in the dupilumab group and seven patients in the placebo group.
The findings were limited by several factors including the reduced sample size for 52-week endpoints because of the earlier analysis and the primarily White study population, the researchers noted. The study was conducted in part during the COVID-19 pandemic period, which contributed to healthcare disruptions and behavior changes that decreased exposure to viral respiratory infections, they wrote in their discussion. However, the results were strengthened by the large numbers and international population without other major pulmonary diseases, such as asthma, and the 34% reduction in exacerbations with dupilumab vs placebo is clinically significant, they said.
Data May Drive US Food and Drug Administration (FDA) Approval
In the BOREAS trial, dupilumab also improved lung function and quality of life, with no notable safety concerns. “As with any trial evaluating the efficacy and safety of a medication, it is important to confirm the findings in a replicative study,” said Dr. Bhatt. “With NOTUS, we confirmed the findings of BOREAS,” and the researchers were reassured by the substantial reduction in exacerbation frequency and the replication of key secondary outcomes, he said.
With the NOTUS study, “two randomized trials have now shown near identical reductions in exacerbation frequency in a difficult-to-treat population of patients with COPD with type 2 inflammation and frequent exacerbations,” as well as a significant and meaningful improvement in lung function, Dr. Bhatt said in an interview. “We hope these trials pave for the way for regulatory body approval of dupilumab for clinical use,” he said. Looking ahead, more studies are needed to test the potential disease modification effects of dupilumab in patients with COPD, he added.
Potential Change in Patient Management
Approximately 20%-40% of patients with COPD have type 2 inflammation with elevated blood eosinophil count, and this subset of patients has an increased risk for exacerbations, with worsening lung function and quality of life, Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
Prior phase 3 studies have shown that dupilumab, a blocker of IL-4 and IL-13 pathways, could effectively reduce exacerbations and improve lung function in these patients, and the NOTUS study aimed to confirm the findings in a larger, more diverse population, said Dr. Narendra, who was not involved in the study.
The NOTUS study represents a paradigm shift in the management of COPD patients with type 2 inflammation, said Narendra. “This study validates the previous BOREAS trial and has shown that dupilumab reduces exacerbations, improves lung function and quality of life, and potentially slows disease progression,” she said.
If approved, potential barriers to the use of dupilumab in practice include cost and insurance coverage, education and dissemination of study findings, and limited data on side effects, said Dr. Narendra.
“While the NOTUS study provides valuable insights into the efficacy and safety of dupilumab over 52 weeks, longer-term studies are needed to understand the sustained benefits and risks of continued treatment,” Dr. Narendra told this news organization. “Studies comparing dupilumab with other biological agents and newer COPD treatments could provide insights into its relative efficacy and position in treatment protocols,” she said.
In addition, further research into dupilumab’s underlying mechanisms could provide deeper insights into the pathophysiology of type 2 inflammation in COPD and inform the development of new treatments, Dr. Narendra said. “These steps will help integrate dupilumab more effectively into clinical practice and optimize its use for COPD patients with type 2 inflammation,” she noted.
Dupilumab is undergoing Priority Review by the FDA as an add-on maintenance therapy for adults with uncontrolled COPD and type 2 inflammation, with a target action date of June 27, 2024, according to a company press release.
The study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Narendra had no financial conflicts to disclose but serves on the Editorial Advisory Board of CHEST Physician.
A version of this article appeared on Medscape.com.
Dupilumab significantly reduced exacerbations and improved lung function in adults with uncontrolled chronic obstructive pulmonary disease (COPD) and type 2 inflammation, based on data from more than 900 individuals.
Data from a phase 3 trial known as NOTUS were presented at the American Thoracic Society’s international conference and published simultaneously in The New England Journal of Medicine.
Dupilumab, a fully human monoclonal antibody, works by inhibiting the signaling of the interleukin 4 (IL-4) and IL-13 pathways and is approved for many conditions characterized by type 2 inflammation, wrote Surya P. Bhatt, MD, of The University of Alabama at Birmingham, and colleagues in the NEJM study.
“Last year, we showed in the BOREAS trial that dupilumab was very effective in lowering exacerbation frequency in patients with COPD who continued to have frequent exacerbations despite being on maximal inhaled therapy,” Dr. Bhatt said in an interview.
12 Months of COPD, Triple Inhaler Therapy
In the NOTUS study, the researchers randomized 470 adults with uncontrolled COPD and type 2 inflammation (defined as a blood eosinophil count of ≥ 300 cells/µL) to 300-mg subcutaneous dupilumab and 465 to a placebo every 2 weeks. Patients were enrolled between July 2020 and May 2023.
The study population included adults aged 40-85 years with physician-diagnosed COPD for at least 12 months who had received background triple inhaler therapy (an inhaled glucocorticoid agent plus long-acting muscarinic antagonist [LAMA]–long-acting beta-agonist [LABA] or LAMA-LABA alone) for at least 3 months and at a stable dose for at least 1 month. All participants were current or former smokers with a smoking history of at least 10 pack-years.
The primary endpoint was a reduction in the annualized rate of moderate or severe COPD exacerbations at 52 weeks.
Patients in the dupilumab group also saw a significantly greater improvement in lung function compared with individuals in the placebo group based on prebronchodilator forced expiratory volume in 1 second from baseline to 12 weeks (least squares mean change of 139 mL vs 57 mL). This improvement was sustained at 52 weeks (least squares mean change of 115 mL vs 54 mL).
Improvement in respiratory symptom severity based on the St. George’s Respiratory Questionnaire was another secondary endpoint, and changes in total score were greater in the dupilumab group than in the placebo group (least squares mean change of 9.8 vs 6.4).
Safety outcomes were similar between the dupilumab and placebo groups, with approximately 66% of patients in each group reporting adverse events during the 52-week study period. Serious adverse events occurred in 13% and 15.9% of dupilumab and placebo patients, respectively, and adverse events resulting in death occurred in 2.6% and 1.5%, respectively. The most common adverse events were COVID-19, which occurred in 9.4% and 8.2% of the dupilumab and placebo patients, respectively, followed by headache, COPD, and nasopharyngitis. Major adverse cardiovascular events occurred in three patients in the dupilumab group and seven patients in the placebo group.
The findings were limited by several factors including the reduced sample size for 52-week endpoints because of the earlier analysis and the primarily White study population, the researchers noted. The study was conducted in part during the COVID-19 pandemic period, which contributed to healthcare disruptions and behavior changes that decreased exposure to viral respiratory infections, they wrote in their discussion. However, the results were strengthened by the large numbers and international population without other major pulmonary diseases, such as asthma, and the 34% reduction in exacerbations with dupilumab vs placebo is clinically significant, they said.
Data May Drive US Food and Drug Administration (FDA) Approval
In the BOREAS trial, dupilumab also improved lung function and quality of life, with no notable safety concerns. “As with any trial evaluating the efficacy and safety of a medication, it is important to confirm the findings in a replicative study,” said Dr. Bhatt. “With NOTUS, we confirmed the findings of BOREAS,” and the researchers were reassured by the substantial reduction in exacerbation frequency and the replication of key secondary outcomes, he said.
With the NOTUS study, “two randomized trials have now shown near identical reductions in exacerbation frequency in a difficult-to-treat population of patients with COPD with type 2 inflammation and frequent exacerbations,” as well as a significant and meaningful improvement in lung function, Dr. Bhatt said in an interview. “We hope these trials pave for the way for regulatory body approval of dupilumab for clinical use,” he said. Looking ahead, more studies are needed to test the potential disease modification effects of dupilumab in patients with COPD, he added.
Potential Change in Patient Management
Approximately 20%-40% of patients with COPD have type 2 inflammation with elevated blood eosinophil count, and this subset of patients has an increased risk for exacerbations, with worsening lung function and quality of life, Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
Prior phase 3 studies have shown that dupilumab, a blocker of IL-4 and IL-13 pathways, could effectively reduce exacerbations and improve lung function in these patients, and the NOTUS study aimed to confirm the findings in a larger, more diverse population, said Dr. Narendra, who was not involved in the study.
The NOTUS study represents a paradigm shift in the management of COPD patients with type 2 inflammation, said Narendra. “This study validates the previous BOREAS trial and has shown that dupilumab reduces exacerbations, improves lung function and quality of life, and potentially slows disease progression,” she said.
If approved, potential barriers to the use of dupilumab in practice include cost and insurance coverage, education and dissemination of study findings, and limited data on side effects, said Dr. Narendra.
“While the NOTUS study provides valuable insights into the efficacy and safety of dupilumab over 52 weeks, longer-term studies are needed to understand the sustained benefits and risks of continued treatment,” Dr. Narendra told this news organization. “Studies comparing dupilumab with other biological agents and newer COPD treatments could provide insights into its relative efficacy and position in treatment protocols,” she said.
In addition, further research into dupilumab’s underlying mechanisms could provide deeper insights into the pathophysiology of type 2 inflammation in COPD and inform the development of new treatments, Dr. Narendra said. “These steps will help integrate dupilumab more effectively into clinical practice and optimize its use for COPD patients with type 2 inflammation,” she noted.
Dupilumab is undergoing Priority Review by the FDA as an add-on maintenance therapy for adults with uncontrolled COPD and type 2 inflammation, with a target action date of June 27, 2024, according to a company press release.
The study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Narendra had no financial conflicts to disclose but serves on the Editorial Advisory Board of CHEST Physician.
A version of this article appeared on Medscape.com.
Dupilumab significantly reduced exacerbations and improved lung function in adults with uncontrolled chronic obstructive pulmonary disease (COPD) and type 2 inflammation, based on data from more than 900 individuals.
Data from a phase 3 trial known as NOTUS were presented at the American Thoracic Society’s international conference and published simultaneously in The New England Journal of Medicine.
Dupilumab, a fully human monoclonal antibody, works by inhibiting the signaling of the interleukin 4 (IL-4) and IL-13 pathways and is approved for many conditions characterized by type 2 inflammation, wrote Surya P. Bhatt, MD, of The University of Alabama at Birmingham, and colleagues in the NEJM study.
“Last year, we showed in the BOREAS trial that dupilumab was very effective in lowering exacerbation frequency in patients with COPD who continued to have frequent exacerbations despite being on maximal inhaled therapy,” Dr. Bhatt said in an interview.
12 Months of COPD, Triple Inhaler Therapy
In the NOTUS study, the researchers randomized 470 adults with uncontrolled COPD and type 2 inflammation (defined as a blood eosinophil count of ≥ 300 cells/µL) to 300-mg subcutaneous dupilumab and 465 to a placebo every 2 weeks. Patients were enrolled between July 2020 and May 2023.
The study population included adults aged 40-85 years with physician-diagnosed COPD for at least 12 months who had received background triple inhaler therapy (an inhaled glucocorticoid agent plus long-acting muscarinic antagonist [LAMA]–long-acting beta-agonist [LABA] or LAMA-LABA alone) for at least 3 months and at a stable dose for at least 1 month. All participants were current or former smokers with a smoking history of at least 10 pack-years.
The primary endpoint was a reduction in the annualized rate of moderate or severe COPD exacerbations at 52 weeks.
Patients in the dupilumab group also saw a significantly greater improvement in lung function compared with individuals in the placebo group based on prebronchodilator forced expiratory volume in 1 second from baseline to 12 weeks (least squares mean change of 139 mL vs 57 mL). This improvement was sustained at 52 weeks (least squares mean change of 115 mL vs 54 mL).
Improvement in respiratory symptom severity based on the St. George’s Respiratory Questionnaire was another secondary endpoint, and changes in total score were greater in the dupilumab group than in the placebo group (least squares mean change of 9.8 vs 6.4).
Safety outcomes were similar between the dupilumab and placebo groups, with approximately 66% of patients in each group reporting adverse events during the 52-week study period. Serious adverse events occurred in 13% and 15.9% of dupilumab and placebo patients, respectively, and adverse events resulting in death occurred in 2.6% and 1.5%, respectively. The most common adverse events were COVID-19, which occurred in 9.4% and 8.2% of the dupilumab and placebo patients, respectively, followed by headache, COPD, and nasopharyngitis. Major adverse cardiovascular events occurred in three patients in the dupilumab group and seven patients in the placebo group.
The findings were limited by several factors including the reduced sample size for 52-week endpoints because of the earlier analysis and the primarily White study population, the researchers noted. The study was conducted in part during the COVID-19 pandemic period, which contributed to healthcare disruptions and behavior changes that decreased exposure to viral respiratory infections, they wrote in their discussion. However, the results were strengthened by the large numbers and international population without other major pulmonary diseases, such as asthma, and the 34% reduction in exacerbations with dupilumab vs placebo is clinically significant, they said.
Data May Drive US Food and Drug Administration (FDA) Approval
In the BOREAS trial, dupilumab also improved lung function and quality of life, with no notable safety concerns. “As with any trial evaluating the efficacy and safety of a medication, it is important to confirm the findings in a replicative study,” said Dr. Bhatt. “With NOTUS, we confirmed the findings of BOREAS,” and the researchers were reassured by the substantial reduction in exacerbation frequency and the replication of key secondary outcomes, he said.
With the NOTUS study, “two randomized trials have now shown near identical reductions in exacerbation frequency in a difficult-to-treat population of patients with COPD with type 2 inflammation and frequent exacerbations,” as well as a significant and meaningful improvement in lung function, Dr. Bhatt said in an interview. “We hope these trials pave for the way for regulatory body approval of dupilumab for clinical use,” he said. Looking ahead, more studies are needed to test the potential disease modification effects of dupilumab in patients with COPD, he added.
Potential Change in Patient Management
Approximately 20%-40% of patients with COPD have type 2 inflammation with elevated blood eosinophil count, and this subset of patients has an increased risk for exacerbations, with worsening lung function and quality of life, Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
Prior phase 3 studies have shown that dupilumab, a blocker of IL-4 and IL-13 pathways, could effectively reduce exacerbations and improve lung function in these patients, and the NOTUS study aimed to confirm the findings in a larger, more diverse population, said Dr. Narendra, who was not involved in the study.
The NOTUS study represents a paradigm shift in the management of COPD patients with type 2 inflammation, said Narendra. “This study validates the previous BOREAS trial and has shown that dupilumab reduces exacerbations, improves lung function and quality of life, and potentially slows disease progression,” she said.
If approved, potential barriers to the use of dupilumab in practice include cost and insurance coverage, education and dissemination of study findings, and limited data on side effects, said Dr. Narendra.
“While the NOTUS study provides valuable insights into the efficacy and safety of dupilumab over 52 weeks, longer-term studies are needed to understand the sustained benefits and risks of continued treatment,” Dr. Narendra told this news organization. “Studies comparing dupilumab with other biological agents and newer COPD treatments could provide insights into its relative efficacy and position in treatment protocols,” she said.
In addition, further research into dupilumab’s underlying mechanisms could provide deeper insights into the pathophysiology of type 2 inflammation in COPD and inform the development of new treatments, Dr. Narendra said. “These steps will help integrate dupilumab more effectively into clinical practice and optimize its use for COPD patients with type 2 inflammation,” she noted.
Dupilumab is undergoing Priority Review by the FDA as an add-on maintenance therapy for adults with uncontrolled COPD and type 2 inflammation, with a target action date of June 27, 2024, according to a company press release.
The study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Narendra had no financial conflicts to disclose but serves on the Editorial Advisory Board of CHEST Physician.
A version of this article appeared on Medscape.com.
FROM ATS 2024
Understudied Patients With COPD Benefit From BLVR
BLVR has shown promising results in previous studies for carefully selected patients with COPD, said Michael J. Nicholson, DO, of Temple University Hospital, Philadelphia. However, those with AATD have often been excluded from large BLVR trials, so data on its effectiveness in this population are limited, he said.
“The distinct pathophysiology of AATD poses challenges in extrapolating findings from trials involving COPD patients without AATD,” Dr. Nicholson noted. “Variations in affected lung lobes and disease progression are major differences between the AATD and non-AATD populations; we sought to examine if BLVR could provide significant, sustained benefit to AATD patients despite their differences from the typical COPD cohort,” he said.
Patients With COPD and AATD
In a study presented at the American Thoracic Society (ATS) 2024 International Conference, Dr. Nicholson and colleagues reviewed data from 238 adults with COPD including 14 with AATD who underwent BLVR at a single center between August 2018 and December 2022. Pulmonary function test data were collected at baseline and at a median of 7 months post-BLVR. The mean age of patients with AATD was 61.5 years, and 79% were men.
The primary outcome was the percentage of patients with forced expiratory volume per second (FEV1) improvement greater than 15%. Half of the patients with AATD achieved this outcome, with a median improvement in FEV1 of 110 mL and a significant difference in pre- and post-BLVR FEV1 volume based on a Wilcoxon signed rank test (W = 11.5; P < .05).
Patients with AATD also showed significant improvement in several secondary outcomes including BODE index, residual volume (RV), total lung capacity (TLC), RV/TLC ratio, and inspiratory capacity/RV ratio between pre- and post-BLVR.
“The sustained improvements seen at 7 months post-BLVR in patients with lower lobe disease were unexpected and promising,” Dr. Nicholson said in an interview. “In contrast to the National Emphysema Treatment Trial (NETT), which found lung volume reduction surgery ineffective for lower lobe disease, our study revealed significant improvements in lower lobe disease following BLVR,” he said. The sustained improvements up to 7 months post-BLVR are encouraging, given clinical concerns that the ongoing destruction of lung tissue in AATD could cause initial BLVR improvements to regress, he added.
Overall, the results suggest that BLVR is an effective therapy for appropriately selected patients with AATD and COPD, and that significant improvement in lung function can be achieved regardless of the affected lobe, Dr. Nicholson said.
“The primary obstacles to widespread BLVR implementation include the scarcity of equipment, as well as insufficient education and training for pulmonologists outside of major academic institutions,” Dr. Nicholson told this news organization. “Successful outcomes in BLVR require clinicians to have a deep understanding of patient selection criteria, extensive training in BLVR techniques, and access to the necessary technology within their facilities,” he said. However, BLVR has been integrated into pulmonary and interventional pulmonary fellowships nationwide, which paves the way for a new generation of pulmonologists to expand the use of the procedure, he said.
Looking ahead, prospective examination of BLVR vs the current standard of care in patients with AATD would provide invaluable data, Dr. Nicholson said. Since the presentation of the study at the meeting, additional patient data have been added to the analysis and increased the power of the findings, he said. “We intend to extend our assessment of pulmonary function testing beyond 7 months post-BLVR to evaluate the persistence of improvements in the long term,” he added.
Study Confirms Benefits for Wider Patient Population
“Lung volume reduction is an important intervention in patients with severe emphysema,” said David M. Mannino, MD, of the University of Kentucky, Lexington, Kentucky, in an interview. Most emphysema is in the upper lobes, but it tends to occur more in the lower lobes in patients with AATD, said Dr. Mannino, who was not involved in the study.
The findings were not especially surprising, but they were reassuring, Dr. Mannino told this news organization. “We know this intervention works in those with severe emphysema,” and it was helpful to confirm similar success in patients with AATD, he said.
The implications for practice are that BLVR is both a safe and an effective intervention for patients with lower or upper lobe emphysema, although longer-term follow-up studies are needed, he said.
The study received no outside funding. Dr. Nicholson and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
BLVR has shown promising results in previous studies for carefully selected patients with COPD, said Michael J. Nicholson, DO, of Temple University Hospital, Philadelphia. However, those with AATD have often been excluded from large BLVR trials, so data on its effectiveness in this population are limited, he said.
“The distinct pathophysiology of AATD poses challenges in extrapolating findings from trials involving COPD patients without AATD,” Dr. Nicholson noted. “Variations in affected lung lobes and disease progression are major differences between the AATD and non-AATD populations; we sought to examine if BLVR could provide significant, sustained benefit to AATD patients despite their differences from the typical COPD cohort,” he said.
Patients With COPD and AATD
In a study presented at the American Thoracic Society (ATS) 2024 International Conference, Dr. Nicholson and colleagues reviewed data from 238 adults with COPD including 14 with AATD who underwent BLVR at a single center between August 2018 and December 2022. Pulmonary function test data were collected at baseline and at a median of 7 months post-BLVR. The mean age of patients with AATD was 61.5 years, and 79% were men.
The primary outcome was the percentage of patients with forced expiratory volume per second (FEV1) improvement greater than 15%. Half of the patients with AATD achieved this outcome, with a median improvement in FEV1 of 110 mL and a significant difference in pre- and post-BLVR FEV1 volume based on a Wilcoxon signed rank test (W = 11.5; P < .05).
Patients with AATD also showed significant improvement in several secondary outcomes including BODE index, residual volume (RV), total lung capacity (TLC), RV/TLC ratio, and inspiratory capacity/RV ratio between pre- and post-BLVR.
“The sustained improvements seen at 7 months post-BLVR in patients with lower lobe disease were unexpected and promising,” Dr. Nicholson said in an interview. “In contrast to the National Emphysema Treatment Trial (NETT), which found lung volume reduction surgery ineffective for lower lobe disease, our study revealed significant improvements in lower lobe disease following BLVR,” he said. The sustained improvements up to 7 months post-BLVR are encouraging, given clinical concerns that the ongoing destruction of lung tissue in AATD could cause initial BLVR improvements to regress, he added.
Overall, the results suggest that BLVR is an effective therapy for appropriately selected patients with AATD and COPD, and that significant improvement in lung function can be achieved regardless of the affected lobe, Dr. Nicholson said.
“The primary obstacles to widespread BLVR implementation include the scarcity of equipment, as well as insufficient education and training for pulmonologists outside of major academic institutions,” Dr. Nicholson told this news organization. “Successful outcomes in BLVR require clinicians to have a deep understanding of patient selection criteria, extensive training in BLVR techniques, and access to the necessary technology within their facilities,” he said. However, BLVR has been integrated into pulmonary and interventional pulmonary fellowships nationwide, which paves the way for a new generation of pulmonologists to expand the use of the procedure, he said.
Looking ahead, prospective examination of BLVR vs the current standard of care in patients with AATD would provide invaluable data, Dr. Nicholson said. Since the presentation of the study at the meeting, additional patient data have been added to the analysis and increased the power of the findings, he said. “We intend to extend our assessment of pulmonary function testing beyond 7 months post-BLVR to evaluate the persistence of improvements in the long term,” he added.
Study Confirms Benefits for Wider Patient Population
“Lung volume reduction is an important intervention in patients with severe emphysema,” said David M. Mannino, MD, of the University of Kentucky, Lexington, Kentucky, in an interview. Most emphysema is in the upper lobes, but it tends to occur more in the lower lobes in patients with AATD, said Dr. Mannino, who was not involved in the study.
The findings were not especially surprising, but they were reassuring, Dr. Mannino told this news organization. “We know this intervention works in those with severe emphysema,” and it was helpful to confirm similar success in patients with AATD, he said.
The implications for practice are that BLVR is both a safe and an effective intervention for patients with lower or upper lobe emphysema, although longer-term follow-up studies are needed, he said.
The study received no outside funding. Dr. Nicholson and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
BLVR has shown promising results in previous studies for carefully selected patients with COPD, said Michael J. Nicholson, DO, of Temple University Hospital, Philadelphia. However, those with AATD have often been excluded from large BLVR trials, so data on its effectiveness in this population are limited, he said.
“The distinct pathophysiology of AATD poses challenges in extrapolating findings from trials involving COPD patients without AATD,” Dr. Nicholson noted. “Variations in affected lung lobes and disease progression are major differences between the AATD and non-AATD populations; we sought to examine if BLVR could provide significant, sustained benefit to AATD patients despite their differences from the typical COPD cohort,” he said.
Patients With COPD and AATD
In a study presented at the American Thoracic Society (ATS) 2024 International Conference, Dr. Nicholson and colleagues reviewed data from 238 adults with COPD including 14 with AATD who underwent BLVR at a single center between August 2018 and December 2022. Pulmonary function test data were collected at baseline and at a median of 7 months post-BLVR. The mean age of patients with AATD was 61.5 years, and 79% were men.
The primary outcome was the percentage of patients with forced expiratory volume per second (FEV1) improvement greater than 15%. Half of the patients with AATD achieved this outcome, with a median improvement in FEV1 of 110 mL and a significant difference in pre- and post-BLVR FEV1 volume based on a Wilcoxon signed rank test (W = 11.5; P < .05).
Patients with AATD also showed significant improvement in several secondary outcomes including BODE index, residual volume (RV), total lung capacity (TLC), RV/TLC ratio, and inspiratory capacity/RV ratio between pre- and post-BLVR.
“The sustained improvements seen at 7 months post-BLVR in patients with lower lobe disease were unexpected and promising,” Dr. Nicholson said in an interview. “In contrast to the National Emphysema Treatment Trial (NETT), which found lung volume reduction surgery ineffective for lower lobe disease, our study revealed significant improvements in lower lobe disease following BLVR,” he said. The sustained improvements up to 7 months post-BLVR are encouraging, given clinical concerns that the ongoing destruction of lung tissue in AATD could cause initial BLVR improvements to regress, he added.
Overall, the results suggest that BLVR is an effective therapy for appropriately selected patients with AATD and COPD, and that significant improvement in lung function can be achieved regardless of the affected lobe, Dr. Nicholson said.
“The primary obstacles to widespread BLVR implementation include the scarcity of equipment, as well as insufficient education and training for pulmonologists outside of major academic institutions,” Dr. Nicholson told this news organization. “Successful outcomes in BLVR require clinicians to have a deep understanding of patient selection criteria, extensive training in BLVR techniques, and access to the necessary technology within their facilities,” he said. However, BLVR has been integrated into pulmonary and interventional pulmonary fellowships nationwide, which paves the way for a new generation of pulmonologists to expand the use of the procedure, he said.
Looking ahead, prospective examination of BLVR vs the current standard of care in patients with AATD would provide invaluable data, Dr. Nicholson said. Since the presentation of the study at the meeting, additional patient data have been added to the analysis and increased the power of the findings, he said. “We intend to extend our assessment of pulmonary function testing beyond 7 months post-BLVR to evaluate the persistence of improvements in the long term,” he added.
Study Confirms Benefits for Wider Patient Population
“Lung volume reduction is an important intervention in patients with severe emphysema,” said David M. Mannino, MD, of the University of Kentucky, Lexington, Kentucky, in an interview. Most emphysema is in the upper lobes, but it tends to occur more in the lower lobes in patients with AATD, said Dr. Mannino, who was not involved in the study.
The findings were not especially surprising, but they were reassuring, Dr. Mannino told this news organization. “We know this intervention works in those with severe emphysema,” and it was helpful to confirm similar success in patients with AATD, he said.
The implications for practice are that BLVR is both a safe and an effective intervention for patients with lower or upper lobe emphysema, although longer-term follow-up studies are needed, he said.
The study received no outside funding. Dr. Nicholson and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Educational Tool Reduces Unnecessary Inhaler Use in ILD
Use of an electronic tool contributed to the deprescribing of unnecessary inhalers in patients with interstitial lung disease (ILD), based on data from nearly 200 individuals.
Patients with ILD often have symptoms that overlap with those of obstructive airways diseases, Stephanie Nevison, MD, of the University of Toronto, and colleagues wrote in a study presented at the American Thoracic Society’s international conference.
, they noted.
“Our aim was twofold: To quantify the extent of inappropriate inhaler use in patients with ILD and to discontinue them where appropriate,” the researchers wrote.
“We hypothesized that inappropriate inhaler use in ILD is common and that an electronic initiative would improve deprescribing rates,” they said.
The researchers conducted a quality improvement project in an ILD clinic at a single center. They reviewed 5 months of baseline data for 191 patients with ILD to assess baseline frequency of inappropriate inhaler use, defined as one or more of the following criteria: Reported asthma history, smoking history of > 15 pack/years, emphysema on chest CT, patient-reported benefits from therapy, airflow obstruction, or bronchodilator response on spirometry.
A total of 48 patients (25.1%) were on inhalers, and 15 (7.8%) had no indication for them (9% of new referrals and 7% of follow-up patients). The most-prescribed inhalers for patients with no indication were corticosteroids (10 patients), short-acting beta-agonists (8 patients), and long-acting beta-agonists (7 patients).
None of the patients on inhalers received counseling about discontinuing their use. The results of the baseline assessment were shared with clinicians along with education about reducing unnecessary inhaler use in the form of a prompt linked to electronic medical records to discuss deprescription of unnecessary inhalers.
The electronic intervention was applied in 400 of 518 patient encounters, and the researchers reviewed data over another 5-month period. A total of 99 patients were on inhalers, and 3.3% had no indication (5.3% of new referrals and 3.0% of follow-up patients). In the wake of the intervention, “all patients on unnecessary inhalers were counseled on deprescribing, representing a significant increase compared to the preintervention period,” the researchers wrote.
Intervention Shows Potential to Curb Unnecessary Inhaler Use
More research is needed as the findings were limited by the relatively small sample size and use of data from a single center, the researchers noted.
However, the results suggest that electronic reminders are effective for prompting a review of inhaler use, and deprescribing inappropriate inhalers for patients with ILD could reduce the potential for adverse events associated with their use, they concluded.
The current study is important because some patients with ILD may not benefit from inhaler use, David Mannino, MD, of the University of Kentucky, Lexington, said in an interview. In the study, “I was a bit surprised that only 3.3% of patients had no indication for them; this seems rather low,” said Dr. Mannino, who was not involved in the study.
Use of an electronic system that evaluates patients and flags inappropriate therapy is an effective way to decrease overprescribing of medications, Dr. Mannino told this news organization.
As for additional research, application of the tool used in this study to other pulmonary populations could be interesting and potentially useful, he said.
The study received no outside funding. The researchers and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Use of an electronic tool contributed to the deprescribing of unnecessary inhalers in patients with interstitial lung disease (ILD), based on data from nearly 200 individuals.
Patients with ILD often have symptoms that overlap with those of obstructive airways diseases, Stephanie Nevison, MD, of the University of Toronto, and colleagues wrote in a study presented at the American Thoracic Society’s international conference.
, they noted.
“Our aim was twofold: To quantify the extent of inappropriate inhaler use in patients with ILD and to discontinue them where appropriate,” the researchers wrote.
“We hypothesized that inappropriate inhaler use in ILD is common and that an electronic initiative would improve deprescribing rates,” they said.
The researchers conducted a quality improvement project in an ILD clinic at a single center. They reviewed 5 months of baseline data for 191 patients with ILD to assess baseline frequency of inappropriate inhaler use, defined as one or more of the following criteria: Reported asthma history, smoking history of > 15 pack/years, emphysema on chest CT, patient-reported benefits from therapy, airflow obstruction, or bronchodilator response on spirometry.
A total of 48 patients (25.1%) were on inhalers, and 15 (7.8%) had no indication for them (9% of new referrals and 7% of follow-up patients). The most-prescribed inhalers for patients with no indication were corticosteroids (10 patients), short-acting beta-agonists (8 patients), and long-acting beta-agonists (7 patients).
None of the patients on inhalers received counseling about discontinuing their use. The results of the baseline assessment were shared with clinicians along with education about reducing unnecessary inhaler use in the form of a prompt linked to electronic medical records to discuss deprescription of unnecessary inhalers.
The electronic intervention was applied in 400 of 518 patient encounters, and the researchers reviewed data over another 5-month period. A total of 99 patients were on inhalers, and 3.3% had no indication (5.3% of new referrals and 3.0% of follow-up patients). In the wake of the intervention, “all patients on unnecessary inhalers were counseled on deprescribing, representing a significant increase compared to the preintervention period,” the researchers wrote.
Intervention Shows Potential to Curb Unnecessary Inhaler Use
More research is needed as the findings were limited by the relatively small sample size and use of data from a single center, the researchers noted.
However, the results suggest that electronic reminders are effective for prompting a review of inhaler use, and deprescribing inappropriate inhalers for patients with ILD could reduce the potential for adverse events associated with their use, they concluded.
The current study is important because some patients with ILD may not benefit from inhaler use, David Mannino, MD, of the University of Kentucky, Lexington, said in an interview. In the study, “I was a bit surprised that only 3.3% of patients had no indication for them; this seems rather low,” said Dr. Mannino, who was not involved in the study.
Use of an electronic system that evaluates patients and flags inappropriate therapy is an effective way to decrease overprescribing of medications, Dr. Mannino told this news organization.
As for additional research, application of the tool used in this study to other pulmonary populations could be interesting and potentially useful, he said.
The study received no outside funding. The researchers and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Use of an electronic tool contributed to the deprescribing of unnecessary inhalers in patients with interstitial lung disease (ILD), based on data from nearly 200 individuals.
Patients with ILD often have symptoms that overlap with those of obstructive airways diseases, Stephanie Nevison, MD, of the University of Toronto, and colleagues wrote in a study presented at the American Thoracic Society’s international conference.
, they noted.
“Our aim was twofold: To quantify the extent of inappropriate inhaler use in patients with ILD and to discontinue them where appropriate,” the researchers wrote.
“We hypothesized that inappropriate inhaler use in ILD is common and that an electronic initiative would improve deprescribing rates,” they said.
The researchers conducted a quality improvement project in an ILD clinic at a single center. They reviewed 5 months of baseline data for 191 patients with ILD to assess baseline frequency of inappropriate inhaler use, defined as one or more of the following criteria: Reported asthma history, smoking history of > 15 pack/years, emphysema on chest CT, patient-reported benefits from therapy, airflow obstruction, or bronchodilator response on spirometry.
A total of 48 patients (25.1%) were on inhalers, and 15 (7.8%) had no indication for them (9% of new referrals and 7% of follow-up patients). The most-prescribed inhalers for patients with no indication were corticosteroids (10 patients), short-acting beta-agonists (8 patients), and long-acting beta-agonists (7 patients).
None of the patients on inhalers received counseling about discontinuing their use. The results of the baseline assessment were shared with clinicians along with education about reducing unnecessary inhaler use in the form of a prompt linked to electronic medical records to discuss deprescription of unnecessary inhalers.
The electronic intervention was applied in 400 of 518 patient encounters, and the researchers reviewed data over another 5-month period. A total of 99 patients were on inhalers, and 3.3% had no indication (5.3% of new referrals and 3.0% of follow-up patients). In the wake of the intervention, “all patients on unnecessary inhalers were counseled on deprescribing, representing a significant increase compared to the preintervention period,” the researchers wrote.
Intervention Shows Potential to Curb Unnecessary Inhaler Use
More research is needed as the findings were limited by the relatively small sample size and use of data from a single center, the researchers noted.
However, the results suggest that electronic reminders are effective for prompting a review of inhaler use, and deprescribing inappropriate inhalers for patients with ILD could reduce the potential for adverse events associated with their use, they concluded.
The current study is important because some patients with ILD may not benefit from inhaler use, David Mannino, MD, of the University of Kentucky, Lexington, said in an interview. In the study, “I was a bit surprised that only 3.3% of patients had no indication for them; this seems rather low,” said Dr. Mannino, who was not involved in the study.
Use of an electronic system that evaluates patients and flags inappropriate therapy is an effective way to decrease overprescribing of medications, Dr. Mannino told this news organization.
As for additional research, application of the tool used in this study to other pulmonary populations could be interesting and potentially useful, he said.
The study received no outside funding. The researchers and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ATS 2024
Analysis Finds Minority of Chronic Wounds Treated by Dermatologists
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
. However, fewer than 8% of chronic wounds were managed by dermatologists during this time.
Those are among key findings from an analysis of National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2019 presented as a late-breaking abstract at the annual meeting of the Society for Investigative Dermatology. “Cutaneous wounds were estimated to account for 28.1 to 96.1 billion dollars in US health care costs in 2014,” one of the study authors, Rithi Chandy, MD, MS, a research fellow at the Center for Dermatology Research at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in an interview following the meeting. “By examining national trends in patient visits and treatment, we may be able to better inform health care utilization for cutaneous wounds.”
Dr. Chandy and colleagues analyzed de-identified patient data from the 2011 to 2019 NAMCS for acute and chronic wound diagnoses, medications prescribed, and physician specialty categories. During the time studied, 5.76 billion patient visits were made, including 45.1 million visits for cutaneous wounds. Of these, the most common diagnoses were open wounds of the thumb without nail damage (7.96%), the lower leg (5.75%), nonpressure chronic ulcers of other parts of the foot (5.08%), and open wounds of the ear (5%).
Among all visits for cutaneous wounds, about one third were chronic cutaneous wounds, with the following descriptions: “Nonpressure chronic ulcer of other part of foot” (17.8%); “nonpressure chronic ulcer of skin, not elsewhere classified” (9.38%); and “ulcer of lower limbs, excluding decubitus, unspecified” (8.72%). “The frequency of patient visits per year during the study period remained stable for both acute and chronic wounds,” Dr. Chandy said. The number of visits for which antimicrobials were used was stable over time for both acute and chronic cutaneous wounds, with the exception of increased use of antivirals for chronic cutaneous wounds, he added.
Specifically, prescriptions were issued in 156 million visits over the time studied, most commonly cephalexin (4.22%), topical silver sulfadiazine (1.59%), topical mupirocin (1.12%), and miscellaneous antibiotics (1.18%).
“Our data shows that topical mupirocin is the most commonly used topical antimicrobial for cutaneous wounds,” Dr. Chandy said. “However, there are reports of emerging bacterial resistance to mupirocin. Our data can inform ongoing efforts to promote antimicrobial stewardship and drug development to provide alternative options that are less likely to induce antimicrobial resistance.”
In findings limited to specialty-specific NAMCS data available from 2011 and from 2013 to 2016, dermatologists managed 3.85% of overall cutaneous wounds, 2.35% of acute wounds, and 7.39% of chronic wounds. By contrast, Dr. Chandy said, 21.1% of chronic wounds were managed by general/family practice physicians, 20.7% by internists, 6.84% by general surgeons, and 5.65% by orthopedic surgeons.
“As dermatologists are experts in the structure and function of the skin and are trained to manage cutaneous disorders including wound healing, we [believe that] dermatologists are equipped with the skill set” for managing wounds, especially for chronic ulcers, he said. The decline in dermatologists who specialize in wound care, he added, “underscores the need for structured dermatology fellowship programs to prepare next-generation dermatologists to address this shortage and ensure dermatology leadership in cutaneous wound healing.”
Dr. Chandy acknowledged certain limitations of the study, including the potential for misclassification of diagnoses or medications prescribed and the fact that the NAMCS database is unable to provide insight into individual patient experiences such as continual cutaneous wound management for the same patient over time.
In the opinion of Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the Nail Division at Weill Cornell Medicine, New York, who was asked to comment on the study, the most interesting finding was that dermatologists cared for a small minority of patients with cutaneous wounds. “It would be interesting to know whether this is due to dermatologist shortages or knowledge gaps on the part of primary care physicians or patients that dermatologists are trained to care for wounds,” Dr. Lipner told this news organization. Other unanswered questions, she noted, “are patient demographics, geographic locations, and comorbidities.”
One of the study authors, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, disclosed that he has received research, speaking and/or consulting support from numerous pharmaceutical companies. No other authors reported having relevant disclosures. Dr. Lipner reported having no disclosures.
A version of this article appeared on Medscape.com .
FROM SID 2024