User login
Complying with the Americans With Disabilities Act
. And 7 years ago, the government raised the penalties for failing to do so. So it might be time to re-educate yourself on what the ADA requires.
ADA compliance is not an issue that we talk about or provide training for in medical schools or with our professional organizations. Since fines for small businesses are now $75,000 for a first offense and $150,000 for each subsequent violation, this could be an expensive oversight that malpractice and other liability policies will not cover.
A 2019 study in Boston examined physicians’ knowledge of legal obligations when caring for patients with disabilities. Researchers concluded that most physicians interviewed “exhibited a superficial or incorrect understanding of their legal responsibilities to patients with a disability.” If you feel you’re in that boat, you might want to consult federal guidance with information and common questions physicians ask about their ADA obligations.
The ADA defines a person with a disability as someone with “a physical or mental impairment that substantially limits one or more life activities”; someone with a record of such an impairment; or someone who is “regarded as having such an impairment.” Among the ADA standards required for accessible exam rooms, according to the guidance:
- The entry door to the exam room should be a minimum width of 32 inches when the door is opened at a 90-degree angle.
- There should be a minimum of 30 by 48 inches of clear floor space next to the exam table.
- An accessible exam table should be able to be lowered to the height of the patient’s wheelchair seat, 17 to 19 inches from the floor.
This does not mean that all of your exam rooms must meet these standards, of course; but if you see any patients with disabilities – and who doesn’t? – you need at least one room that meets the criteria.
Federal guidance also includes requirements on removal of architectural barriers, accessible parking, and entrance and maneuvering spaces – which apply to both for-profit and nonprofit organizations. Among them:
- Designated accessible parking spaces must be included among any parking the business provides for the public “if doing so is readily achievable.” Those parking spaces should be the closest to the accessible entrance, on level ground. The spaces should be at least eight feet wide, with an access aisle on either side.
- For accessible spaces for cars, the adjacent access aisle must be at least five feet wide; for van spaces, eight feet wide.
- “If achievable,” an accessible service counter must have a maximum height of 36 inches, with a clear floor space of 30 by 48 inches to permit the use of a wheelchair.
A common misconception is that only new construction and alterations need to be accessible, and that older facilities are “grandfathered,” but that’s not true. Because the ADA is a civil rights law and not a building code, ADA rules apply equally to all facilities, young and old. This is particularly important to remember in light of the long-standing cottage industry of attorneys who sue small businesses for alleged ADA violations.
Another common mistake made by physicians who lease their office space is to assume that their landlord is responsible for meeting all ADA obligations. In fact, The ADA places the legal obligation on both the landlord and the tenant. The landlord and the tenant may decide among themselves who will actually make the changes and provide the aids and services, but both remain legally responsible.
Another aspect that you might not have thought of is access to your website. While ADA applicability to online services remains vague, lawsuits have been filed, and are likely to increase. Online accessibility issues that have been identified include:
- Ability to find and process information on a website (e.g., providing audio descriptions for video content, for the sight-impaired).
- Ability to navigate and use a website (e.g., ensuring that all site functions are easily accessible with only a keyboard).
- Ability to comprehend all information (including clearly understandable error messages).
Hearing-impaired patients present their own considerations for delivering adequate care, which I will discuss in my next column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
. And 7 years ago, the government raised the penalties for failing to do so. So it might be time to re-educate yourself on what the ADA requires.
ADA compliance is not an issue that we talk about or provide training for in medical schools or with our professional organizations. Since fines for small businesses are now $75,000 for a first offense and $150,000 for each subsequent violation, this could be an expensive oversight that malpractice and other liability policies will not cover.
A 2019 study in Boston examined physicians’ knowledge of legal obligations when caring for patients with disabilities. Researchers concluded that most physicians interviewed “exhibited a superficial or incorrect understanding of their legal responsibilities to patients with a disability.” If you feel you’re in that boat, you might want to consult federal guidance with information and common questions physicians ask about their ADA obligations.
The ADA defines a person with a disability as someone with “a physical or mental impairment that substantially limits one or more life activities”; someone with a record of such an impairment; or someone who is “regarded as having such an impairment.” Among the ADA standards required for accessible exam rooms, according to the guidance:
- The entry door to the exam room should be a minimum width of 32 inches when the door is opened at a 90-degree angle.
- There should be a minimum of 30 by 48 inches of clear floor space next to the exam table.
- An accessible exam table should be able to be lowered to the height of the patient’s wheelchair seat, 17 to 19 inches from the floor.
This does not mean that all of your exam rooms must meet these standards, of course; but if you see any patients with disabilities – and who doesn’t? – you need at least one room that meets the criteria.
Federal guidance also includes requirements on removal of architectural barriers, accessible parking, and entrance and maneuvering spaces – which apply to both for-profit and nonprofit organizations. Among them:
- Designated accessible parking spaces must be included among any parking the business provides for the public “if doing so is readily achievable.” Those parking spaces should be the closest to the accessible entrance, on level ground. The spaces should be at least eight feet wide, with an access aisle on either side.
- For accessible spaces for cars, the adjacent access aisle must be at least five feet wide; for van spaces, eight feet wide.
- “If achievable,” an accessible service counter must have a maximum height of 36 inches, with a clear floor space of 30 by 48 inches to permit the use of a wheelchair.
A common misconception is that only new construction and alterations need to be accessible, and that older facilities are “grandfathered,” but that’s not true. Because the ADA is a civil rights law and not a building code, ADA rules apply equally to all facilities, young and old. This is particularly important to remember in light of the long-standing cottage industry of attorneys who sue small businesses for alleged ADA violations.
Another common mistake made by physicians who lease their office space is to assume that their landlord is responsible for meeting all ADA obligations. In fact, The ADA places the legal obligation on both the landlord and the tenant. The landlord and the tenant may decide among themselves who will actually make the changes and provide the aids and services, but both remain legally responsible.
Another aspect that you might not have thought of is access to your website. While ADA applicability to online services remains vague, lawsuits have been filed, and are likely to increase. Online accessibility issues that have been identified include:
- Ability to find and process information on a website (e.g., providing audio descriptions for video content, for the sight-impaired).
- Ability to navigate and use a website (e.g., ensuring that all site functions are easily accessible with only a keyboard).
- Ability to comprehend all information (including clearly understandable error messages).
Hearing-impaired patients present their own considerations for delivering adequate care, which I will discuss in my next column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
. And 7 years ago, the government raised the penalties for failing to do so. So it might be time to re-educate yourself on what the ADA requires.
ADA compliance is not an issue that we talk about or provide training for in medical schools or with our professional organizations. Since fines for small businesses are now $75,000 for a first offense and $150,000 for each subsequent violation, this could be an expensive oversight that malpractice and other liability policies will not cover.
A 2019 study in Boston examined physicians’ knowledge of legal obligations when caring for patients with disabilities. Researchers concluded that most physicians interviewed “exhibited a superficial or incorrect understanding of their legal responsibilities to patients with a disability.” If you feel you’re in that boat, you might want to consult federal guidance with information and common questions physicians ask about their ADA obligations.
The ADA defines a person with a disability as someone with “a physical or mental impairment that substantially limits one or more life activities”; someone with a record of such an impairment; or someone who is “regarded as having such an impairment.” Among the ADA standards required for accessible exam rooms, according to the guidance:
- The entry door to the exam room should be a minimum width of 32 inches when the door is opened at a 90-degree angle.
- There should be a minimum of 30 by 48 inches of clear floor space next to the exam table.
- An accessible exam table should be able to be lowered to the height of the patient’s wheelchair seat, 17 to 19 inches from the floor.
This does not mean that all of your exam rooms must meet these standards, of course; but if you see any patients with disabilities – and who doesn’t? – you need at least one room that meets the criteria.
Federal guidance also includes requirements on removal of architectural barriers, accessible parking, and entrance and maneuvering spaces – which apply to both for-profit and nonprofit organizations. Among them:
- Designated accessible parking spaces must be included among any parking the business provides for the public “if doing so is readily achievable.” Those parking spaces should be the closest to the accessible entrance, on level ground. The spaces should be at least eight feet wide, with an access aisle on either side.
- For accessible spaces for cars, the adjacent access aisle must be at least five feet wide; for van spaces, eight feet wide.
- “If achievable,” an accessible service counter must have a maximum height of 36 inches, with a clear floor space of 30 by 48 inches to permit the use of a wheelchair.
A common misconception is that only new construction and alterations need to be accessible, and that older facilities are “grandfathered,” but that’s not true. Because the ADA is a civil rights law and not a building code, ADA rules apply equally to all facilities, young and old. This is particularly important to remember in light of the long-standing cottage industry of attorneys who sue small businesses for alleged ADA violations.
Another common mistake made by physicians who lease their office space is to assume that their landlord is responsible for meeting all ADA obligations. In fact, The ADA places the legal obligation on both the landlord and the tenant. The landlord and the tenant may decide among themselves who will actually make the changes and provide the aids and services, but both remain legally responsible.
Another aspect that you might not have thought of is access to your website. While ADA applicability to online services remains vague, lawsuits have been filed, and are likely to increase. Online accessibility issues that have been identified include:
- Ability to find and process information on a website (e.g., providing audio descriptions for video content, for the sight-impaired).
- Ability to navigate and use a website (e.g., ensuring that all site functions are easily accessible with only a keyboard).
- Ability to comprehend all information (including clearly understandable error messages).
Hearing-impaired patients present their own considerations for delivering adequate care, which I will discuss in my next column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
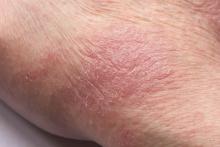
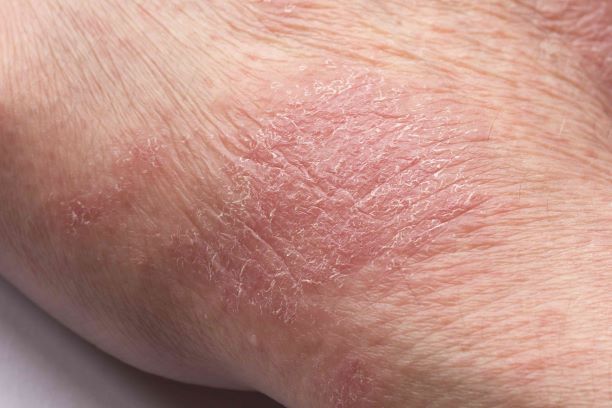
Man presents with diffuse pruritus
This patient has atopic dermatitis (AD), but on the basis of the image and description above, by no means would this be an intuitive diagnosis; the findings are not characteristic of those in younger patients with AD. Further, clinicians might find it difficult to diagnose AD in an older patient because older patients generally tend to have more comorbidities and medication side effects, including chronic pruritus of unknown origin and xerosis, which could confound the diagnosis.
Finally, specific guidelines are lacking for clinicians to distinguish AD from other pruritic skin conditions in the older patient. Currently, according to one report, older patients are diagnosed with AD after at least 6 months of symptom assessment and exclusion of other conditions, including cutaneous T-cell lymphoma, allergic contact dermatitis, psoriasis, drug reactions, and chronic idiopathic or secondary erythroderma.
AD arising de novo in older persons is a discrete form of the disease that characteristically involves the face, neck, trunk, and hands, while sparing the flexural areas, which are prominently involved in younger patients. The eczema can become erythrodermic. Older men are affected threefold more often than older women.
Skin manifestations in older patients with AD generally match those of adolescents and young adults with AD, but the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is more common than the classic sign of localized lichenified eczema at those folds.
Factors rendering older people susceptible to AD include innate physiologic changes of aging, notably a decline in skin barrier function, dysregulation of innate immune cells, and skewing of adaptive immunity to a Th2 response.
Much about how to best treat AD in older patients remains unclear. It is a challenge to treat older patients according to standardized guidelines for general AD treatment because dermatologists and others need to consider comorbidities and the medications that these patients might already be taking. Some examples: Dermatologists might limit cyclosporine use in patients with hypertension and reduced kidney function, or limit systemic steroid use in patients with osteoporosis. Older patients have a greater propensity for infection, which might cause dermatologists to limit systemic immunosuppressant drugs. And skin thinning and diffuse photoaging might cause doctors to limit even topical steroid treatment in these patients.
As in other age groups, regular application of moisturizers in combination with calcineurin inhibitors, adjunctive administration of oral antihistamines and avoidance of exacerbating factors comprise basic treatments for AD in older patients.
Although antihistamines such as hydroxyzine can work for itching in some individuals, they are generally lacking in efficacy in most patients with AD.
Brian S. Kim, MD, is Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has atopic dermatitis (AD), but on the basis of the image and description above, by no means would this be an intuitive diagnosis; the findings are not characteristic of those in younger patients with AD. Further, clinicians might find it difficult to diagnose AD in an older patient because older patients generally tend to have more comorbidities and medication side effects, including chronic pruritus of unknown origin and xerosis, which could confound the diagnosis.
Finally, specific guidelines are lacking for clinicians to distinguish AD from other pruritic skin conditions in the older patient. Currently, according to one report, older patients are diagnosed with AD after at least 6 months of symptom assessment and exclusion of other conditions, including cutaneous T-cell lymphoma, allergic contact dermatitis, psoriasis, drug reactions, and chronic idiopathic or secondary erythroderma.
AD arising de novo in older persons is a discrete form of the disease that characteristically involves the face, neck, trunk, and hands, while sparing the flexural areas, which are prominently involved in younger patients. The eczema can become erythrodermic. Older men are affected threefold more often than older women.
Skin manifestations in older patients with AD generally match those of adolescents and young adults with AD, but the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is more common than the classic sign of localized lichenified eczema at those folds.
Factors rendering older people susceptible to AD include innate physiologic changes of aging, notably a decline in skin barrier function, dysregulation of innate immune cells, and skewing of adaptive immunity to a Th2 response.
Much about how to best treat AD in older patients remains unclear. It is a challenge to treat older patients according to standardized guidelines for general AD treatment because dermatologists and others need to consider comorbidities and the medications that these patients might already be taking. Some examples: Dermatologists might limit cyclosporine use in patients with hypertension and reduced kidney function, or limit systemic steroid use in patients with osteoporosis. Older patients have a greater propensity for infection, which might cause dermatologists to limit systemic immunosuppressant drugs. And skin thinning and diffuse photoaging might cause doctors to limit even topical steroid treatment in these patients.
As in other age groups, regular application of moisturizers in combination with calcineurin inhibitors, adjunctive administration of oral antihistamines and avoidance of exacerbating factors comprise basic treatments for AD in older patients.
Although antihistamines such as hydroxyzine can work for itching in some individuals, they are generally lacking in efficacy in most patients with AD.
Brian S. Kim, MD, is Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has atopic dermatitis (AD), but on the basis of the image and description above, by no means would this be an intuitive diagnosis; the findings are not characteristic of those in younger patients with AD. Further, clinicians might find it difficult to diagnose AD in an older patient because older patients generally tend to have more comorbidities and medication side effects, including chronic pruritus of unknown origin and xerosis, which could confound the diagnosis.
Finally, specific guidelines are lacking for clinicians to distinguish AD from other pruritic skin conditions in the older patient. Currently, according to one report, older patients are diagnosed with AD after at least 6 months of symptom assessment and exclusion of other conditions, including cutaneous T-cell lymphoma, allergic contact dermatitis, psoriasis, drug reactions, and chronic idiopathic or secondary erythroderma.
AD arising de novo in older persons is a discrete form of the disease that characteristically involves the face, neck, trunk, and hands, while sparing the flexural areas, which are prominently involved in younger patients. The eczema can become erythrodermic. Older men are affected threefold more often than older women.
Skin manifestations in older patients with AD generally match those of adolescents and young adults with AD, but the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is more common than the classic sign of localized lichenified eczema at those folds.
Factors rendering older people susceptible to AD include innate physiologic changes of aging, notably a decline in skin barrier function, dysregulation of innate immune cells, and skewing of adaptive immunity to a Th2 response.
Much about how to best treat AD in older patients remains unclear. It is a challenge to treat older patients according to standardized guidelines for general AD treatment because dermatologists and others need to consider comorbidities and the medications that these patients might already be taking. Some examples: Dermatologists might limit cyclosporine use in patients with hypertension and reduced kidney function, or limit systemic steroid use in patients with osteoporosis. Older patients have a greater propensity for infection, which might cause dermatologists to limit systemic immunosuppressant drugs. And skin thinning and diffuse photoaging might cause doctors to limit even topical steroid treatment in these patients.
As in other age groups, regular application of moisturizers in combination with calcineurin inhibitors, adjunctive administration of oral antihistamines and avoidance of exacerbating factors comprise basic treatments for AD in older patients.
Although antihistamines such as hydroxyzine can work for itching in some individuals, they are generally lacking in efficacy in most patients with AD.
Brian S. Kim, MD, is Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
A 59-year-old man presents with diffuse pruritus that first appeared 6 months earlier. He developed worsening eczematous, erythematous papules on his hands and face which waxed and waned. The areas of eczema on his hands and face were erythrodermic. The pruritus was intermittently controlled with the antihistamine hydroxyzine, a potent topical corticosteroid, and over-the-counter skin lotions. The patient said he had no history of allergies or asthma.
Emerging drugs for schizophrenia targeting negative symptoms
Late-stage trials of new antipsychotic drugs are showing promise in the control of negative symptoms, according to an overview presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The progress in these trials deserves attention, because control of negative symptoms is “a major unmet need in schizophrenia,” according to Henry A. Nasrallah, MD, director of the schizophrenia program at the University of Cincinnati.
The novel mechanisms of the agents in development are credited with the promise. Not least, several antipsychotic agents with activity against both positive and negative symptoms “are completely devoid of dopamine receptor blockade,” Dr. Nasrallah said at the virtual meeting, presented by MedscapeLive!
The xanomeline portion of the investigational treatment xanomeline-trospium is one example. Xanomeline is a muscarinic receptor agonist with no activity on dopamine D2 receptors. The role of trospium, a muscarinic receptor antagonist, is to reduce peripheral cholinergic side effects.
Xanomeline-trospium: Negative vs. positive symptoms
In a recently published placebo-controlled, double-blind, phase 2 trial, the reductions relative to placebo after 5 weeks on the negative subscale of the Positive and Negative Syndrome Scale (PANSS) tool (–3.9 vs. –1.3; P < .001) was about as robust as that achieved on the positive subscale (–5.6 vs. –3.2; P < .001).
These subscales were secondary endpoints. Relative to placebo, xanomeline-trospium was also effective on the primary endpoint of the PANSS total score (–17.9 vs. –5.9; P < .001).
The presence of trospium did not eliminate cholinergic side effects, which included constipation, dry mouth, and nausea, but the therapy strengthens the evidence that newer agents with novel mechanisms of action, including those without dopamine blockade, can achieve meaningful clinical effects.
SEP-363856, another example of an experimental agent without direct dopamine blockade, was also recently tested in placebo-controlled, double-blind study.
“This is the first agonist of the TAAR1 [trace amine-associated receptor 1] and 5-HT1A [serotonin 5–hydroxytryptamine type 1A receptor] to reach clinical trials,” said Dr. Nasrallah, calling this an interesting agent for its range of clinical activity, which appears to include antianxiety effects.
SEP-363856: Negative vs. positive symptoms
It also appears to include activity against negative symptoms. While the primary endpoint of total PANSS score favored SEP-363856 over placebo at the end of 4 weeks (–17.2 points vs. –9.7; P = .001), the reductions in the subscales for negative (–3.1 vs. –1.6) and positive (–5.5 vs. 3.9) symptoms were also substantial even if statistical differences were not calculated.
The rates of side effects on SEP-3638656 were low, according to Dr. Nasrallah. The most common complaints, such as somnolence, agitation, and nausea, were observed in fewer than 10% of patients.
Roluperidone, another agent with no direct dopamine blockade, has reached phase 3 trials. The activity of this agent is attributed to antagonist activity on the serotonin 5-HT2A and sigma2 receptors. In a multinational, phase 2b study cited by Dr. Nasrallah, both of two study doses of roluperidone were superior to placebo for the negative symptom dimensions of expressive deficit and experiential deficit. Patients enrolled in the trial were required to have baseline PANSS negative symptom subscale scores of 20 points or greater.
Pimavanserin, an inverse agonist of 5-HT2A receptors, is already approved for the treatment of psychosis in Parkinson’s disease, but it is now attracting interest for its potential efficacy against negative symptoms in schizophrenia, according to Dr. Nasrallah, who cited a poster presented last November at the Psych Congress 2020.
The poster provided results of ADVANCE, a double-blind, placebo-controlled, phase 2 study that associated pimavanserin with significant improvement across several types of negative symptoms, Dr. Nasrallah said. The drug was well tolerated with a side-effect profile “similar to placebo.”
Traditional antipsychotic therapies are generally associated with limited effect against negative symptoms, but it has never been proven that the interaction of treatments on the dopaminergic system is the reason. Indeed, in his list of therapies being pursued for potential benefit against negative symptoms, Dr. Nasrallah cited a clinical study with cariprazine, an agent with multiple effects on dopamine and serotonin signaling.
“Cariprazine is a partial agonist at D2, D3, and 5-HT1A receptors and an antagonist at 5-HT2c and 5-HT7 receptors, but it has the highest affinity to the D3 receptor,” Dr. Nasrallah reported.
Cariprazine is already approved for schizophrenia, acute mania, and bipolar depression, but the authors of a recent review claim evidence of activity against negative symptoms. Furthermore, they speculate that this activity might be mediated by agonism of the D3 receptor.
Despite the evidence that these agents might control negative symptoms, the relative roles will be defined by clinical experience, not just clinical trials, Dr. Nasrallah said. However, he did indicate that there appears to be meaningful progress in this area.
Potential progress in this area is important, because “negative symptoms are a largely unaddressed treatment target in people with schizophrenia,” reported Christoph U. Correll, MD, professor of psychiatry, Hofstra University, Hempstead, N.Y. These symptoms deserve attention for their “important potential to improve interpersonal, educational, and work function.”
Dr. Correll agreed that the newer drugs with mechanisms other than postsynaptic dopamine blockade could be a very important advance in the treatment of schizophrenia.
“Promising new medications with potential efficacy for negative symptoms, either based on their pharmacological profile and/or emerging data, include cariprazine, lumateperone, ulotaront [SEP-363856], and xanomeline plus trospium,” he said. Efficacy for negative symptoms, if proven, will address an “elusive goal.”
MedscapeLive! and this news organization are owned by the same parent company. Dr. Nasrallah reported financial relationships with Acadia Pharmaceuticals, Alkermes, Avanir, Intra-Cellular Therapies, Indivior, Janssen, Neurocrine, and Teva. Dr. Correll listed financial relationships with more than 25 pharmaceutical companies, including several developing medications with potential activity against negative symptoms.
Late-stage trials of new antipsychotic drugs are showing promise in the control of negative symptoms, according to an overview presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The progress in these trials deserves attention, because control of negative symptoms is “a major unmet need in schizophrenia,” according to Henry A. Nasrallah, MD, director of the schizophrenia program at the University of Cincinnati.
The novel mechanisms of the agents in development are credited with the promise. Not least, several antipsychotic agents with activity against both positive and negative symptoms “are completely devoid of dopamine receptor blockade,” Dr. Nasrallah said at the virtual meeting, presented by MedscapeLive!
The xanomeline portion of the investigational treatment xanomeline-trospium is one example. Xanomeline is a muscarinic receptor agonist with no activity on dopamine D2 receptors. The role of trospium, a muscarinic receptor antagonist, is to reduce peripheral cholinergic side effects.
Xanomeline-trospium: Negative vs. positive symptoms
In a recently published placebo-controlled, double-blind, phase 2 trial, the reductions relative to placebo after 5 weeks on the negative subscale of the Positive and Negative Syndrome Scale (PANSS) tool (–3.9 vs. –1.3; P < .001) was about as robust as that achieved on the positive subscale (–5.6 vs. –3.2; P < .001).
These subscales were secondary endpoints. Relative to placebo, xanomeline-trospium was also effective on the primary endpoint of the PANSS total score (–17.9 vs. –5.9; P < .001).
The presence of trospium did not eliminate cholinergic side effects, which included constipation, dry mouth, and nausea, but the therapy strengthens the evidence that newer agents with novel mechanisms of action, including those without dopamine blockade, can achieve meaningful clinical effects.
SEP-363856, another example of an experimental agent without direct dopamine blockade, was also recently tested in placebo-controlled, double-blind study.
“This is the first agonist of the TAAR1 [trace amine-associated receptor 1] and 5-HT1A [serotonin 5–hydroxytryptamine type 1A receptor] to reach clinical trials,” said Dr. Nasrallah, calling this an interesting agent for its range of clinical activity, which appears to include antianxiety effects.
SEP-363856: Negative vs. positive symptoms
It also appears to include activity against negative symptoms. While the primary endpoint of total PANSS score favored SEP-363856 over placebo at the end of 4 weeks (–17.2 points vs. –9.7; P = .001), the reductions in the subscales for negative (–3.1 vs. –1.6) and positive (–5.5 vs. 3.9) symptoms were also substantial even if statistical differences were not calculated.
The rates of side effects on SEP-3638656 were low, according to Dr. Nasrallah. The most common complaints, such as somnolence, agitation, and nausea, were observed in fewer than 10% of patients.
Roluperidone, another agent with no direct dopamine blockade, has reached phase 3 trials. The activity of this agent is attributed to antagonist activity on the serotonin 5-HT2A and sigma2 receptors. In a multinational, phase 2b study cited by Dr. Nasrallah, both of two study doses of roluperidone were superior to placebo for the negative symptom dimensions of expressive deficit and experiential deficit. Patients enrolled in the trial were required to have baseline PANSS negative symptom subscale scores of 20 points or greater.
Pimavanserin, an inverse agonist of 5-HT2A receptors, is already approved for the treatment of psychosis in Parkinson’s disease, but it is now attracting interest for its potential efficacy against negative symptoms in schizophrenia, according to Dr. Nasrallah, who cited a poster presented last November at the Psych Congress 2020.
The poster provided results of ADVANCE, a double-blind, placebo-controlled, phase 2 study that associated pimavanserin with significant improvement across several types of negative symptoms, Dr. Nasrallah said. The drug was well tolerated with a side-effect profile “similar to placebo.”
Traditional antipsychotic therapies are generally associated with limited effect against negative symptoms, but it has never been proven that the interaction of treatments on the dopaminergic system is the reason. Indeed, in his list of therapies being pursued for potential benefit against negative symptoms, Dr. Nasrallah cited a clinical study with cariprazine, an agent with multiple effects on dopamine and serotonin signaling.
“Cariprazine is a partial agonist at D2, D3, and 5-HT1A receptors and an antagonist at 5-HT2c and 5-HT7 receptors, but it has the highest affinity to the D3 receptor,” Dr. Nasrallah reported.
Cariprazine is already approved for schizophrenia, acute mania, and bipolar depression, but the authors of a recent review claim evidence of activity against negative symptoms. Furthermore, they speculate that this activity might be mediated by agonism of the D3 receptor.
Despite the evidence that these agents might control negative symptoms, the relative roles will be defined by clinical experience, not just clinical trials, Dr. Nasrallah said. However, he did indicate that there appears to be meaningful progress in this area.
Potential progress in this area is important, because “negative symptoms are a largely unaddressed treatment target in people with schizophrenia,” reported Christoph U. Correll, MD, professor of psychiatry, Hofstra University, Hempstead, N.Y. These symptoms deserve attention for their “important potential to improve interpersonal, educational, and work function.”
Dr. Correll agreed that the newer drugs with mechanisms other than postsynaptic dopamine blockade could be a very important advance in the treatment of schizophrenia.
“Promising new medications with potential efficacy for negative symptoms, either based on their pharmacological profile and/or emerging data, include cariprazine, lumateperone, ulotaront [SEP-363856], and xanomeline plus trospium,” he said. Efficacy for negative symptoms, if proven, will address an “elusive goal.”
MedscapeLive! and this news organization are owned by the same parent company. Dr. Nasrallah reported financial relationships with Acadia Pharmaceuticals, Alkermes, Avanir, Intra-Cellular Therapies, Indivior, Janssen, Neurocrine, and Teva. Dr. Correll listed financial relationships with more than 25 pharmaceutical companies, including several developing medications with potential activity against negative symptoms.
Late-stage trials of new antipsychotic drugs are showing promise in the control of negative symptoms, according to an overview presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The progress in these trials deserves attention, because control of negative symptoms is “a major unmet need in schizophrenia,” according to Henry A. Nasrallah, MD, director of the schizophrenia program at the University of Cincinnati.
The novel mechanisms of the agents in development are credited with the promise. Not least, several antipsychotic agents with activity against both positive and negative symptoms “are completely devoid of dopamine receptor blockade,” Dr. Nasrallah said at the virtual meeting, presented by MedscapeLive!
The xanomeline portion of the investigational treatment xanomeline-trospium is one example. Xanomeline is a muscarinic receptor agonist with no activity on dopamine D2 receptors. The role of trospium, a muscarinic receptor antagonist, is to reduce peripheral cholinergic side effects.
Xanomeline-trospium: Negative vs. positive symptoms
In a recently published placebo-controlled, double-blind, phase 2 trial, the reductions relative to placebo after 5 weeks on the negative subscale of the Positive and Negative Syndrome Scale (PANSS) tool (–3.9 vs. –1.3; P < .001) was about as robust as that achieved on the positive subscale (–5.6 vs. –3.2; P < .001).
These subscales were secondary endpoints. Relative to placebo, xanomeline-trospium was also effective on the primary endpoint of the PANSS total score (–17.9 vs. –5.9; P < .001).
The presence of trospium did not eliminate cholinergic side effects, which included constipation, dry mouth, and nausea, but the therapy strengthens the evidence that newer agents with novel mechanisms of action, including those without dopamine blockade, can achieve meaningful clinical effects.
SEP-363856, another example of an experimental agent without direct dopamine blockade, was also recently tested in placebo-controlled, double-blind study.
“This is the first agonist of the TAAR1 [trace amine-associated receptor 1] and 5-HT1A [serotonin 5–hydroxytryptamine type 1A receptor] to reach clinical trials,” said Dr. Nasrallah, calling this an interesting agent for its range of clinical activity, which appears to include antianxiety effects.
SEP-363856: Negative vs. positive symptoms
It also appears to include activity against negative symptoms. While the primary endpoint of total PANSS score favored SEP-363856 over placebo at the end of 4 weeks (–17.2 points vs. –9.7; P = .001), the reductions in the subscales for negative (–3.1 vs. –1.6) and positive (–5.5 vs. 3.9) symptoms were also substantial even if statistical differences were not calculated.
The rates of side effects on SEP-3638656 were low, according to Dr. Nasrallah. The most common complaints, such as somnolence, agitation, and nausea, were observed in fewer than 10% of patients.
Roluperidone, another agent with no direct dopamine blockade, has reached phase 3 trials. The activity of this agent is attributed to antagonist activity on the serotonin 5-HT2A and sigma2 receptors. In a multinational, phase 2b study cited by Dr. Nasrallah, both of two study doses of roluperidone were superior to placebo for the negative symptom dimensions of expressive deficit and experiential deficit. Patients enrolled in the trial were required to have baseline PANSS negative symptom subscale scores of 20 points or greater.
Pimavanserin, an inverse agonist of 5-HT2A receptors, is already approved for the treatment of psychosis in Parkinson’s disease, but it is now attracting interest for its potential efficacy against negative symptoms in schizophrenia, according to Dr. Nasrallah, who cited a poster presented last November at the Psych Congress 2020.
The poster provided results of ADVANCE, a double-blind, placebo-controlled, phase 2 study that associated pimavanserin with significant improvement across several types of negative symptoms, Dr. Nasrallah said. The drug was well tolerated with a side-effect profile “similar to placebo.”
Traditional antipsychotic therapies are generally associated with limited effect against negative symptoms, but it has never been proven that the interaction of treatments on the dopaminergic system is the reason. Indeed, in his list of therapies being pursued for potential benefit against negative symptoms, Dr. Nasrallah cited a clinical study with cariprazine, an agent with multiple effects on dopamine and serotonin signaling.
“Cariprazine is a partial agonist at D2, D3, and 5-HT1A receptors and an antagonist at 5-HT2c and 5-HT7 receptors, but it has the highest affinity to the D3 receptor,” Dr. Nasrallah reported.
Cariprazine is already approved for schizophrenia, acute mania, and bipolar depression, but the authors of a recent review claim evidence of activity against negative symptoms. Furthermore, they speculate that this activity might be mediated by agonism of the D3 receptor.
Despite the evidence that these agents might control negative symptoms, the relative roles will be defined by clinical experience, not just clinical trials, Dr. Nasrallah said. However, he did indicate that there appears to be meaningful progress in this area.
Potential progress in this area is important, because “negative symptoms are a largely unaddressed treatment target in people with schizophrenia,” reported Christoph U. Correll, MD, professor of psychiatry, Hofstra University, Hempstead, N.Y. These symptoms deserve attention for their “important potential to improve interpersonal, educational, and work function.”
Dr. Correll agreed that the newer drugs with mechanisms other than postsynaptic dopamine blockade could be a very important advance in the treatment of schizophrenia.
“Promising new medications with potential efficacy for negative symptoms, either based on their pharmacological profile and/or emerging data, include cariprazine, lumateperone, ulotaront [SEP-363856], and xanomeline plus trospium,” he said. Efficacy for negative symptoms, if proven, will address an “elusive goal.”
MedscapeLive! and this news organization are owned by the same parent company. Dr. Nasrallah reported financial relationships with Acadia Pharmaceuticals, Alkermes, Avanir, Intra-Cellular Therapies, Indivior, Janssen, Neurocrine, and Teva. Dr. Correll listed financial relationships with more than 25 pharmaceutical companies, including several developing medications with potential activity against negative symptoms.
FROM CP/AACP PSYCHIATRY UPDATE
Understanding the grieving process
Loss is inevitable – and understanding essential
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
Loss is inevitable – and understanding essential
Loss is inevitable – and understanding essential
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
Giving flu and COVID-19 shots at same time appears safe, effective: Study
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Reversal agents curb DOAC-related bleeding but deaths still high
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
New AMA president discusses pandemic during inaugural address
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
Supreme Court upholds Affordable Care Act
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
Extensive limb swelling after vaccines – including SARS-CoV-2 vaccine
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.
What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.