User login
Oncologist accused of inappropriate treatment ‘provided exceptional care’
Leading oncologist
Prof. Stebbing, a cancer medicine and oncology professor at Imperial College London with a private practice on Harley Street, claimed the patient would have died without the chemotherapy, and immunotherapy treatment led to him living for another 2 years.
He’s appearing before a Medical Practitioners Tribunal Service (MPTS) fitness-to-practice hearing and is accused of failing to provide good clinical care to 12 patients between March 2014 and March 2017.
In some cases, Prof. Stebbing is accused of inappropriately treating patients given their advanced cancer or poor prognosis, overstating life expectancy and the benefits of chemotherapy, and continuing to treat patients when it was futile, and they had just weeks to live.
The 36 charges – 21 of which he’s admitted – also include failing to keep proper records and failing to gain informed consent for treatment from patients.
Patient B
Prof. Stebbing’s international reputation for innovative treatments has led to wealthy, terminally ill cancer patients from around the world turning to him in the hope of extending their lives.
The tribunal heard about one lung cancer patient – known only as Patient B – from Spain he treated between May 2014 and October 2015.
Prof. Stebbing is accused of offering doublet chemotherapy to the patient beyond six cycles, despite evidence emerging that he was developing impaired renal function.
He’s also accused of continuing the treatment at a higher dose after 10 cycles despite a “lack of efficacy” and “evidence of harm emerging.”
It’s alleged the chemotherapy would have exposed the patient to risks “without any conceivable prospect of improving health.”
However, Prof. Stebbing defended his actions, saying he’d explained to the patient that if he stopped chemotherapy at any time, “his disease would progress rapidly and he would die.”
He said immunotherapy “typically took 3 months to work,” and because the patient’s lung cancer hadn’t progressed, it was evident that the chemotherapy had worked.
It was possible to provide chemotherapy in cases of renal failure, he said, and he’d only given it in small doses.
“This is one of two patients in the bundle who has an exceptional standard of care,” he said.
“If you look at the problem with his kidneys, this was the minimus in my terms.
“I think I made some very, very difficult decisions that other people may not have made, but I got them right and, as a result, he lived very happily for another 2 years.”
‘Guidelines are a guide’
But Sharon Beattie, for the General Medical Council (GMC), claimed he’d ignored guidelines, and there was no data to support the position he’d taken.
Prof. Stebbing replied: “The guidelines are a guide; they are helpful. They do not replace the skill of an individual doctor.”
“There were no guidelines for a patient like this. I’m absolutely amazed you’re saying, ‘You should have just let him die because there were no guidelines.’”
Ms. Beattie pointed out that Prof. Stebbing had accepted that he’d stopped the chemotherapy treatment in October 2015 because it was clear there was evidence of “toxicity and waning efficacy.”
But he claimed there were only “grade one” levels of toxicity and “mild” disease progression.
At that stage, he said, he realized he was approaching the “end of the line” with the treatment and he was “thinking out of the box” to get immunotherapy for the patient.
Earlier, Prof. Stebbing said the chemotherapy had been “a bridge” to the patient’s immunotherapy treatment, but it had “never been clear” it would be available.
He said: “The whole point of the extended duration chemotherapy was to try to get him to immunotherapy if it was available.”
“It was a very exciting, new possibility. I didn’t know if it was going to be available, but I wanted the patient to have every chance of it being available.”
“The longer he lived for with stable disease the more likelihood it had of becoming available.”
Prof. Stebbing denies failing to discuss the risks and benefits of chemotherapy with the patient and failing to maintain adequate records.
He told the tribunal that he had discussed both the chemotherapy and immunotherapy, but he accepted he’d had “problems” with documenting his decisions.
The tribunal continues.
A version of this article first appeared on Medscape.com.
Leading oncologist
Prof. Stebbing, a cancer medicine and oncology professor at Imperial College London with a private practice on Harley Street, claimed the patient would have died without the chemotherapy, and immunotherapy treatment led to him living for another 2 years.
He’s appearing before a Medical Practitioners Tribunal Service (MPTS) fitness-to-practice hearing and is accused of failing to provide good clinical care to 12 patients between March 2014 and March 2017.
In some cases, Prof. Stebbing is accused of inappropriately treating patients given their advanced cancer or poor prognosis, overstating life expectancy and the benefits of chemotherapy, and continuing to treat patients when it was futile, and they had just weeks to live.
The 36 charges – 21 of which he’s admitted – also include failing to keep proper records and failing to gain informed consent for treatment from patients.
Patient B
Prof. Stebbing’s international reputation for innovative treatments has led to wealthy, terminally ill cancer patients from around the world turning to him in the hope of extending their lives.
The tribunal heard about one lung cancer patient – known only as Patient B – from Spain he treated between May 2014 and October 2015.
Prof. Stebbing is accused of offering doublet chemotherapy to the patient beyond six cycles, despite evidence emerging that he was developing impaired renal function.
He’s also accused of continuing the treatment at a higher dose after 10 cycles despite a “lack of efficacy” and “evidence of harm emerging.”
It’s alleged the chemotherapy would have exposed the patient to risks “without any conceivable prospect of improving health.”
However, Prof. Stebbing defended his actions, saying he’d explained to the patient that if he stopped chemotherapy at any time, “his disease would progress rapidly and he would die.”
He said immunotherapy “typically took 3 months to work,” and because the patient’s lung cancer hadn’t progressed, it was evident that the chemotherapy had worked.
It was possible to provide chemotherapy in cases of renal failure, he said, and he’d only given it in small doses.
“This is one of two patients in the bundle who has an exceptional standard of care,” he said.
“If you look at the problem with his kidneys, this was the minimus in my terms.
“I think I made some very, very difficult decisions that other people may not have made, but I got them right and, as a result, he lived very happily for another 2 years.”
‘Guidelines are a guide’
But Sharon Beattie, for the General Medical Council (GMC), claimed he’d ignored guidelines, and there was no data to support the position he’d taken.
Prof. Stebbing replied: “The guidelines are a guide; they are helpful. They do not replace the skill of an individual doctor.”
“There were no guidelines for a patient like this. I’m absolutely amazed you’re saying, ‘You should have just let him die because there were no guidelines.’”
Ms. Beattie pointed out that Prof. Stebbing had accepted that he’d stopped the chemotherapy treatment in October 2015 because it was clear there was evidence of “toxicity and waning efficacy.”
But he claimed there were only “grade one” levels of toxicity and “mild” disease progression.
At that stage, he said, he realized he was approaching the “end of the line” with the treatment and he was “thinking out of the box” to get immunotherapy for the patient.
Earlier, Prof. Stebbing said the chemotherapy had been “a bridge” to the patient’s immunotherapy treatment, but it had “never been clear” it would be available.
He said: “The whole point of the extended duration chemotherapy was to try to get him to immunotherapy if it was available.”
“It was a very exciting, new possibility. I didn’t know if it was going to be available, but I wanted the patient to have every chance of it being available.”
“The longer he lived for with stable disease the more likelihood it had of becoming available.”
Prof. Stebbing denies failing to discuss the risks and benefits of chemotherapy with the patient and failing to maintain adequate records.
He told the tribunal that he had discussed both the chemotherapy and immunotherapy, but he accepted he’d had “problems” with documenting his decisions.
The tribunal continues.
A version of this article first appeared on Medscape.com.
Leading oncologist
Prof. Stebbing, a cancer medicine and oncology professor at Imperial College London with a private practice on Harley Street, claimed the patient would have died without the chemotherapy, and immunotherapy treatment led to him living for another 2 years.
He’s appearing before a Medical Practitioners Tribunal Service (MPTS) fitness-to-practice hearing and is accused of failing to provide good clinical care to 12 patients between March 2014 and March 2017.
In some cases, Prof. Stebbing is accused of inappropriately treating patients given their advanced cancer or poor prognosis, overstating life expectancy and the benefits of chemotherapy, and continuing to treat patients when it was futile, and they had just weeks to live.
The 36 charges – 21 of which he’s admitted – also include failing to keep proper records and failing to gain informed consent for treatment from patients.
Patient B
Prof. Stebbing’s international reputation for innovative treatments has led to wealthy, terminally ill cancer patients from around the world turning to him in the hope of extending their lives.
The tribunal heard about one lung cancer patient – known only as Patient B – from Spain he treated between May 2014 and October 2015.
Prof. Stebbing is accused of offering doublet chemotherapy to the patient beyond six cycles, despite evidence emerging that he was developing impaired renal function.
He’s also accused of continuing the treatment at a higher dose after 10 cycles despite a “lack of efficacy” and “evidence of harm emerging.”
It’s alleged the chemotherapy would have exposed the patient to risks “without any conceivable prospect of improving health.”
However, Prof. Stebbing defended his actions, saying he’d explained to the patient that if he stopped chemotherapy at any time, “his disease would progress rapidly and he would die.”
He said immunotherapy “typically took 3 months to work,” and because the patient’s lung cancer hadn’t progressed, it was evident that the chemotherapy had worked.
It was possible to provide chemotherapy in cases of renal failure, he said, and he’d only given it in small doses.
“This is one of two patients in the bundle who has an exceptional standard of care,” he said.
“If you look at the problem with his kidneys, this was the minimus in my terms.
“I think I made some very, very difficult decisions that other people may not have made, but I got them right and, as a result, he lived very happily for another 2 years.”
‘Guidelines are a guide’
But Sharon Beattie, for the General Medical Council (GMC), claimed he’d ignored guidelines, and there was no data to support the position he’d taken.
Prof. Stebbing replied: “The guidelines are a guide; they are helpful. They do not replace the skill of an individual doctor.”
“There were no guidelines for a patient like this. I’m absolutely amazed you’re saying, ‘You should have just let him die because there were no guidelines.’”
Ms. Beattie pointed out that Prof. Stebbing had accepted that he’d stopped the chemotherapy treatment in October 2015 because it was clear there was evidence of “toxicity and waning efficacy.”
But he claimed there were only “grade one” levels of toxicity and “mild” disease progression.
At that stage, he said, he realized he was approaching the “end of the line” with the treatment and he was “thinking out of the box” to get immunotherapy for the patient.
Earlier, Prof. Stebbing said the chemotherapy had been “a bridge” to the patient’s immunotherapy treatment, but it had “never been clear” it would be available.
He said: “The whole point of the extended duration chemotherapy was to try to get him to immunotherapy if it was available.”
“It was a very exciting, new possibility. I didn’t know if it was going to be available, but I wanted the patient to have every chance of it being available.”
“The longer he lived for with stable disease the more likelihood it had of becoming available.”
Prof. Stebbing denies failing to discuss the risks and benefits of chemotherapy with the patient and failing to maintain adequate records.
He told the tribunal that he had discussed both the chemotherapy and immunotherapy, but he accepted he’d had “problems” with documenting his decisions.
The tribunal continues.
A version of this article first appeared on Medscape.com.
Hospitalist movers and shakers – July 2021
Vineet Arora, MD, MHM, has been appointed dean of medical education for the University of Chicago’s biological sciences division. She began her assignment on July 1, 2021, taking over for the retiring Halina Brukner, MD, a 36-year veteran in medicine.
Dr. Arora will take charge of undergraduate, graduate, and continuing education for the University of Chicago’s medical education program, with a focus on simulation-based training. She also will represent the medical school within the university proper, as well as with outside organizations such as the Association of American Colleges, the Liaison Committee on Medical Education, and the Accreditation Council for Graduate Medical Education.
Dr. Arora has been a faculty member at Chicago Medicine since 2005. She is a professor of medicine, assistant dean for scholarship and discovery, associate chief medical officer for clinical learning, and Master of the Academy of Distinguished Medical Educators. Dr. Arora is a member of the National Academy of Medicine and is on the board of directors for the American Board of Internal Medicine.
Zeshan Anwar, MD, SFHM, was named new chief of the section of inpatient internal medicine and director of hospitalist services at Reading Hospital–Tower Health (West Reading, Pa.) in January 2021. He provides support to hospitalists, nurses, pharmacists, care managers, support service professionals and others.
Previously, Dr. Anwar worked as vice chair of the department of medicine and medical director of the hospitalist program at Evangelical Community Hospital (Lewisburg, Pa.). He has a background in education, having taught as an assistant professor of clinical medicine at Geisinger Commonwealth School of Medicine (Scranton, Pa.) since 2014.
Katherine Hochman, MD, FHM, has been appointed the first director of the newly established division of hospital medicine at NYU Langone Health in New York. Dr. Hochman is the founder of NYU Langone’s hospitalist program (2004), and the new division was established this year in the wake of the COVID-19 pandemic.
Dr. Hochman will be charged with expanding on the hospitalist program, analyzing best practices, and educating residents, clinicians, and other health care professionals. She plans to emphasize mentorship and creating career pathways for the program’s students.
Dr. Hochman was NYU Langone’s first hospitalist and later became associate program director of medicine at Langone’s Tisch Hospital. She helped grow the hospitalist program to 40 professionals in 2020.
Daniel Asher, MD, recently was named a Top Hospitalist by Continental Who’s Who. Dr. Asher is a night hospitalist at Piedmont Columbus Regional (Columbus, Ga.), where he works with residents and consults with other physicians regarding patients at the facility.
Dr. Asher has spent his entire post–medical school career at Piedmont, serving as a family medicine resident from 2018 to 2020. He was named chief resident in 2019-20, and has continued his efforts at the hospital since then, including front-line work with COVID-19 patients.
Nicholas O’Dell, MD, has been selected as medical director of the Murray Medical Associates hospitalist program at Murray-Calloway County Hospital (Murray, Ky.). Dr. O’Dell, who has been a hospitalist at the facility since 2014, has served as chief medical officer at the hospital since February 2020. He will continue in his role as CMO, but will no longer see clinical patients.
Brad Tate, MD, has been elevated to associate chief medical officer at Children’s Medical Center Plano (Texas), starting in the new leadership role in June 2021.
Dr. Tate has been affiliated with Children’s Health since 2010, when he was a hospitalist in Plano, as well as medical director of the Children’s Health Medical Group Hospitalist Group. He advanced that program from Plano to the network’s Dallas campus.
Touchette Regional Hospital (Centreville, Ill.) has contracted with MEDS Emergency Physician Staffing and Management (O’Fallon, Ill.) to provide inpatient physician and nurse practitioner staffing. The move is an extension of the existing relationship between the two entities, as MEDS has provided emergency room staffing services at Touchette since 2019.
Vineet Arora, MD, MHM, has been appointed dean of medical education for the University of Chicago’s biological sciences division. She began her assignment on July 1, 2021, taking over for the retiring Halina Brukner, MD, a 36-year veteran in medicine.
Dr. Arora will take charge of undergraduate, graduate, and continuing education for the University of Chicago’s medical education program, with a focus on simulation-based training. She also will represent the medical school within the university proper, as well as with outside organizations such as the Association of American Colleges, the Liaison Committee on Medical Education, and the Accreditation Council for Graduate Medical Education.
Dr. Arora has been a faculty member at Chicago Medicine since 2005. She is a professor of medicine, assistant dean for scholarship and discovery, associate chief medical officer for clinical learning, and Master of the Academy of Distinguished Medical Educators. Dr. Arora is a member of the National Academy of Medicine and is on the board of directors for the American Board of Internal Medicine.
Zeshan Anwar, MD, SFHM, was named new chief of the section of inpatient internal medicine and director of hospitalist services at Reading Hospital–Tower Health (West Reading, Pa.) in January 2021. He provides support to hospitalists, nurses, pharmacists, care managers, support service professionals and others.
Previously, Dr. Anwar worked as vice chair of the department of medicine and medical director of the hospitalist program at Evangelical Community Hospital (Lewisburg, Pa.). He has a background in education, having taught as an assistant professor of clinical medicine at Geisinger Commonwealth School of Medicine (Scranton, Pa.) since 2014.
Katherine Hochman, MD, FHM, has been appointed the first director of the newly established division of hospital medicine at NYU Langone Health in New York. Dr. Hochman is the founder of NYU Langone’s hospitalist program (2004), and the new division was established this year in the wake of the COVID-19 pandemic.
Dr. Hochman will be charged with expanding on the hospitalist program, analyzing best practices, and educating residents, clinicians, and other health care professionals. She plans to emphasize mentorship and creating career pathways for the program’s students.
Dr. Hochman was NYU Langone’s first hospitalist and later became associate program director of medicine at Langone’s Tisch Hospital. She helped grow the hospitalist program to 40 professionals in 2020.
Daniel Asher, MD, recently was named a Top Hospitalist by Continental Who’s Who. Dr. Asher is a night hospitalist at Piedmont Columbus Regional (Columbus, Ga.), where he works with residents and consults with other physicians regarding patients at the facility.
Dr. Asher has spent his entire post–medical school career at Piedmont, serving as a family medicine resident from 2018 to 2020. He was named chief resident in 2019-20, and has continued his efforts at the hospital since then, including front-line work with COVID-19 patients.
Nicholas O’Dell, MD, has been selected as medical director of the Murray Medical Associates hospitalist program at Murray-Calloway County Hospital (Murray, Ky.). Dr. O’Dell, who has been a hospitalist at the facility since 2014, has served as chief medical officer at the hospital since February 2020. He will continue in his role as CMO, but will no longer see clinical patients.
Brad Tate, MD, has been elevated to associate chief medical officer at Children’s Medical Center Plano (Texas), starting in the new leadership role in June 2021.
Dr. Tate has been affiliated with Children’s Health since 2010, when he was a hospitalist in Plano, as well as medical director of the Children’s Health Medical Group Hospitalist Group. He advanced that program from Plano to the network’s Dallas campus.
Touchette Regional Hospital (Centreville, Ill.) has contracted with MEDS Emergency Physician Staffing and Management (O’Fallon, Ill.) to provide inpatient physician and nurse practitioner staffing. The move is an extension of the existing relationship between the two entities, as MEDS has provided emergency room staffing services at Touchette since 2019.
Vineet Arora, MD, MHM, has been appointed dean of medical education for the University of Chicago’s biological sciences division. She began her assignment on July 1, 2021, taking over for the retiring Halina Brukner, MD, a 36-year veteran in medicine.
Dr. Arora will take charge of undergraduate, graduate, and continuing education for the University of Chicago’s medical education program, with a focus on simulation-based training. She also will represent the medical school within the university proper, as well as with outside organizations such as the Association of American Colleges, the Liaison Committee on Medical Education, and the Accreditation Council for Graduate Medical Education.
Dr. Arora has been a faculty member at Chicago Medicine since 2005. She is a professor of medicine, assistant dean for scholarship and discovery, associate chief medical officer for clinical learning, and Master of the Academy of Distinguished Medical Educators. Dr. Arora is a member of the National Academy of Medicine and is on the board of directors for the American Board of Internal Medicine.
Zeshan Anwar, MD, SFHM, was named new chief of the section of inpatient internal medicine and director of hospitalist services at Reading Hospital–Tower Health (West Reading, Pa.) in January 2021. He provides support to hospitalists, nurses, pharmacists, care managers, support service professionals and others.
Previously, Dr. Anwar worked as vice chair of the department of medicine and medical director of the hospitalist program at Evangelical Community Hospital (Lewisburg, Pa.). He has a background in education, having taught as an assistant professor of clinical medicine at Geisinger Commonwealth School of Medicine (Scranton, Pa.) since 2014.
Katherine Hochman, MD, FHM, has been appointed the first director of the newly established division of hospital medicine at NYU Langone Health in New York. Dr. Hochman is the founder of NYU Langone’s hospitalist program (2004), and the new division was established this year in the wake of the COVID-19 pandemic.
Dr. Hochman will be charged with expanding on the hospitalist program, analyzing best practices, and educating residents, clinicians, and other health care professionals. She plans to emphasize mentorship and creating career pathways for the program’s students.
Dr. Hochman was NYU Langone’s first hospitalist and later became associate program director of medicine at Langone’s Tisch Hospital. She helped grow the hospitalist program to 40 professionals in 2020.
Daniel Asher, MD, recently was named a Top Hospitalist by Continental Who’s Who. Dr. Asher is a night hospitalist at Piedmont Columbus Regional (Columbus, Ga.), where he works with residents and consults with other physicians regarding patients at the facility.
Dr. Asher has spent his entire post–medical school career at Piedmont, serving as a family medicine resident from 2018 to 2020. He was named chief resident in 2019-20, and has continued his efforts at the hospital since then, including front-line work with COVID-19 patients.
Nicholas O’Dell, MD, has been selected as medical director of the Murray Medical Associates hospitalist program at Murray-Calloway County Hospital (Murray, Ky.). Dr. O’Dell, who has been a hospitalist at the facility since 2014, has served as chief medical officer at the hospital since February 2020. He will continue in his role as CMO, but will no longer see clinical patients.
Brad Tate, MD, has been elevated to associate chief medical officer at Children’s Medical Center Plano (Texas), starting in the new leadership role in June 2021.
Dr. Tate has been affiliated with Children’s Health since 2010, when he was a hospitalist in Plano, as well as medical director of the Children’s Health Medical Group Hospitalist Group. He advanced that program from Plano to the network’s Dallas campus.
Touchette Regional Hospital (Centreville, Ill.) has contracted with MEDS Emergency Physician Staffing and Management (O’Fallon, Ill.) to provide inpatient physician and nurse practitioner staffing. The move is an extension of the existing relationship between the two entities, as MEDS has provided emergency room staffing services at Touchette since 2019.
Study spanning 2 decades offers insights into pediatric psoriasis trends
, while predictors of moderate to severe disease include morphology, non-White race, and culture-confirmed infection.
Those are among the key findings from a retrospective analysis of pediatric psoriasis patients who were seen at the University of California, San Francisco, over a 24-year period.
“Overall, our data support prior findings of age- and sex-based differences in location and morphology and presents new information demonstrating associations with severity,” presenting study author Carmel Aghdasi said during the annual meeting of the Society for Pediatric Dermatology. “We provide evidence of the increased use of systemic and biologic therapies over time, an important step in ensuring pediatric patients are adequately treated.”
To characterize the demographics, clinical features, comorbidities, and treatments, and to determine predictors of severity and changes in treatment patterns over 2 decades in a large cohort of pediatric psoriasis patients, Ms. Aghdasi, a 4th-year medical student at the University of California, San Francisco, and colleagues retrospectively evaluated the records of 754 pediatric patients up to 18 years of age who were seen at UCSF for psoriasis from 1997 to 2021. They collected demographic, clinical, familial, comorbidity, and treatment data and divided the cohort into two groups by date of last visit.
Group 1 consisted of 332 patients whose last visit was between 2001 and 2011, while the second group included 422 patients whose last visit was between 2012 and 2021. The researchers also divided the cohort into three age groups: infants (0-2 years of age), children (3-12 years of age), and adolescents (13-18 years of age).
Slightly more than half of the patients (55%) were female and 67% presented between ages 3 and 12. (Seventy-four patients were in the youngest category, 0-2 years, when they presented.) The average age of disease onset was 7 years, the average age at presentation to pediatric dermatology was 8.8 years, and 37% of the total cohort were overweight or obese. The top four comorbidities were being overweight or obese (37%), followed by atopic dermatitis (19%), psychiatric disease (7%), and arthritis (4%).
Plaque was the most common morphology (56%), while the most common sites of involvement were the head and neck (69%), extremities (61%), and trunk (44%). About half of the cohort (51%) had mild disease, 15% had culture-confirmed infections (9% had Streptococcal infections), and 66% of patients reported itch as a symptom.
The researchers observed that inverse psoriasis was significantly more common in infants and decreased with age. Anogenital involvement was more common in males and in those aged 0-2, while head and neck involvement was more common in females. Nail involvement was more common in childhood.
Topical therapy was the most common treatment overall and by far the most common among those in the 0-2 age category. “Overall, phototherapy was used in childhood and adolescents but almost never in infancy,” Ms. Aghdasi said. “Looking at changes in systemic treatment over time, conventional systemic use increased in infants and children and decreased in adolescents. Biologic use increased in all ages, most notably in children aged 3-12 years old.”
Multivariate regression analyses revealed that the following independent variables predicted moderate to severe psoriasis: adolescent age (adjusted odds ratio, 1.9; P = .03), guttate morphology (aOR, 2.2; P = .006), plaque and guttate morphology (aOR, 7.6; P less than .001), pustular or erythrodermic morphology (aOR, 5; P = .003), culture-confirmed infection (aOR, 2; P = .007), Black race (aOR, 3.3; P = .007), Asian race (aOR, 1.8; P = .04, and Hispanic race (aOR, 1.9; P = .03).
“Further analysis is needed to elucidate the influence of race on severity and of the clinical utility of infection as a marker of severity,” Ms. Aghdasi said. “Interestingly, we did not find that obesity was a marker of severity in our cohort.”
In an interview, senior study author Kelly M. Cordoro, MD, professor of dermatology and pediatrics at UCSF, noted that this finding conflicts with prior studies showing an association between obesity and severe psoriasis in children.
“Though methodologies and patient populations differ among studies, what is striking,” she said, is the percentage of overweight/obese patients (37%; defined as a body mass index ≥ 85th percentile) “in our 2-decade single institution dataset.” This “is nearly identical” to the percentage of patients with excess adiposity – 37.9% (also defined as BMI ≥ 85th percentile) – in an international cross-sectional study, which also identified an association between obesity (BMI ≥ 95th percentile) and psoriasis severity in children, she noted.
“What is clear is the strong association between obesity and childhood psoriasis, as multiple studies, including ours, confirm obesity as a major comorbidity of pediatric psoriasis,” Dr. Cordoro said. “Both conditions must be adequately managed to reduce the risk of adverse health outcomes for obese patients with psoriasis.”
The other study coauthors were Dana Feigenbaum, MD, and Alana Ju, MD. The work was supported by the UCSF Yearlong Inquiry Program. The researchers reported having no relevant financial disclosures.
, while predictors of moderate to severe disease include morphology, non-White race, and culture-confirmed infection.
Those are among the key findings from a retrospective analysis of pediatric psoriasis patients who were seen at the University of California, San Francisco, over a 24-year period.
“Overall, our data support prior findings of age- and sex-based differences in location and morphology and presents new information demonstrating associations with severity,” presenting study author Carmel Aghdasi said during the annual meeting of the Society for Pediatric Dermatology. “We provide evidence of the increased use of systemic and biologic therapies over time, an important step in ensuring pediatric patients are adequately treated.”
To characterize the demographics, clinical features, comorbidities, and treatments, and to determine predictors of severity and changes in treatment patterns over 2 decades in a large cohort of pediatric psoriasis patients, Ms. Aghdasi, a 4th-year medical student at the University of California, San Francisco, and colleagues retrospectively evaluated the records of 754 pediatric patients up to 18 years of age who were seen at UCSF for psoriasis from 1997 to 2021. They collected demographic, clinical, familial, comorbidity, and treatment data and divided the cohort into two groups by date of last visit.
Group 1 consisted of 332 patients whose last visit was between 2001 and 2011, while the second group included 422 patients whose last visit was between 2012 and 2021. The researchers also divided the cohort into three age groups: infants (0-2 years of age), children (3-12 years of age), and adolescents (13-18 years of age).
Slightly more than half of the patients (55%) were female and 67% presented between ages 3 and 12. (Seventy-four patients were in the youngest category, 0-2 years, when they presented.) The average age of disease onset was 7 years, the average age at presentation to pediatric dermatology was 8.8 years, and 37% of the total cohort were overweight or obese. The top four comorbidities were being overweight or obese (37%), followed by atopic dermatitis (19%), psychiatric disease (7%), and arthritis (4%).
Plaque was the most common morphology (56%), while the most common sites of involvement were the head and neck (69%), extremities (61%), and trunk (44%). About half of the cohort (51%) had mild disease, 15% had culture-confirmed infections (9% had Streptococcal infections), and 66% of patients reported itch as a symptom.
The researchers observed that inverse psoriasis was significantly more common in infants and decreased with age. Anogenital involvement was more common in males and in those aged 0-2, while head and neck involvement was more common in females. Nail involvement was more common in childhood.
Topical therapy was the most common treatment overall and by far the most common among those in the 0-2 age category. “Overall, phototherapy was used in childhood and adolescents but almost never in infancy,” Ms. Aghdasi said. “Looking at changes in systemic treatment over time, conventional systemic use increased in infants and children and decreased in adolescents. Biologic use increased in all ages, most notably in children aged 3-12 years old.”
Multivariate regression analyses revealed that the following independent variables predicted moderate to severe psoriasis: adolescent age (adjusted odds ratio, 1.9; P = .03), guttate morphology (aOR, 2.2; P = .006), plaque and guttate morphology (aOR, 7.6; P less than .001), pustular or erythrodermic morphology (aOR, 5; P = .003), culture-confirmed infection (aOR, 2; P = .007), Black race (aOR, 3.3; P = .007), Asian race (aOR, 1.8; P = .04, and Hispanic race (aOR, 1.9; P = .03).
“Further analysis is needed to elucidate the influence of race on severity and of the clinical utility of infection as a marker of severity,” Ms. Aghdasi said. “Interestingly, we did not find that obesity was a marker of severity in our cohort.”
In an interview, senior study author Kelly M. Cordoro, MD, professor of dermatology and pediatrics at UCSF, noted that this finding conflicts with prior studies showing an association between obesity and severe psoriasis in children.
“Though methodologies and patient populations differ among studies, what is striking,” she said, is the percentage of overweight/obese patients (37%; defined as a body mass index ≥ 85th percentile) “in our 2-decade single institution dataset.” This “is nearly identical” to the percentage of patients with excess adiposity – 37.9% (also defined as BMI ≥ 85th percentile) – in an international cross-sectional study, which also identified an association between obesity (BMI ≥ 95th percentile) and psoriasis severity in children, she noted.
“What is clear is the strong association between obesity and childhood psoriasis, as multiple studies, including ours, confirm obesity as a major comorbidity of pediatric psoriasis,” Dr. Cordoro said. “Both conditions must be adequately managed to reduce the risk of adverse health outcomes for obese patients with psoriasis.”
The other study coauthors were Dana Feigenbaum, MD, and Alana Ju, MD. The work was supported by the UCSF Yearlong Inquiry Program. The researchers reported having no relevant financial disclosures.
, while predictors of moderate to severe disease include morphology, non-White race, and culture-confirmed infection.
Those are among the key findings from a retrospective analysis of pediatric psoriasis patients who were seen at the University of California, San Francisco, over a 24-year period.
“Overall, our data support prior findings of age- and sex-based differences in location and morphology and presents new information demonstrating associations with severity,” presenting study author Carmel Aghdasi said during the annual meeting of the Society for Pediatric Dermatology. “We provide evidence of the increased use of systemic and biologic therapies over time, an important step in ensuring pediatric patients are adequately treated.”
To characterize the demographics, clinical features, comorbidities, and treatments, and to determine predictors of severity and changes in treatment patterns over 2 decades in a large cohort of pediatric psoriasis patients, Ms. Aghdasi, a 4th-year medical student at the University of California, San Francisco, and colleagues retrospectively evaluated the records of 754 pediatric patients up to 18 years of age who were seen at UCSF for psoriasis from 1997 to 2021. They collected demographic, clinical, familial, comorbidity, and treatment data and divided the cohort into two groups by date of last visit.
Group 1 consisted of 332 patients whose last visit was between 2001 and 2011, while the second group included 422 patients whose last visit was between 2012 and 2021. The researchers also divided the cohort into three age groups: infants (0-2 years of age), children (3-12 years of age), and adolescents (13-18 years of age).
Slightly more than half of the patients (55%) were female and 67% presented between ages 3 and 12. (Seventy-four patients were in the youngest category, 0-2 years, when they presented.) The average age of disease onset was 7 years, the average age at presentation to pediatric dermatology was 8.8 years, and 37% of the total cohort were overweight or obese. The top four comorbidities were being overweight or obese (37%), followed by atopic dermatitis (19%), psychiatric disease (7%), and arthritis (4%).
Plaque was the most common morphology (56%), while the most common sites of involvement were the head and neck (69%), extremities (61%), and trunk (44%). About half of the cohort (51%) had mild disease, 15% had culture-confirmed infections (9% had Streptococcal infections), and 66% of patients reported itch as a symptom.
The researchers observed that inverse psoriasis was significantly more common in infants and decreased with age. Anogenital involvement was more common in males and in those aged 0-2, while head and neck involvement was more common in females. Nail involvement was more common in childhood.
Topical therapy was the most common treatment overall and by far the most common among those in the 0-2 age category. “Overall, phototherapy was used in childhood and adolescents but almost never in infancy,” Ms. Aghdasi said. “Looking at changes in systemic treatment over time, conventional systemic use increased in infants and children and decreased in adolescents. Biologic use increased in all ages, most notably in children aged 3-12 years old.”
Multivariate regression analyses revealed that the following independent variables predicted moderate to severe psoriasis: adolescent age (adjusted odds ratio, 1.9; P = .03), guttate morphology (aOR, 2.2; P = .006), plaque and guttate morphology (aOR, 7.6; P less than .001), pustular or erythrodermic morphology (aOR, 5; P = .003), culture-confirmed infection (aOR, 2; P = .007), Black race (aOR, 3.3; P = .007), Asian race (aOR, 1.8; P = .04, and Hispanic race (aOR, 1.9; P = .03).
“Further analysis is needed to elucidate the influence of race on severity and of the clinical utility of infection as a marker of severity,” Ms. Aghdasi said. “Interestingly, we did not find that obesity was a marker of severity in our cohort.”
In an interview, senior study author Kelly M. Cordoro, MD, professor of dermatology and pediatrics at UCSF, noted that this finding conflicts with prior studies showing an association between obesity and severe psoriasis in children.
“Though methodologies and patient populations differ among studies, what is striking,” she said, is the percentage of overweight/obese patients (37%; defined as a body mass index ≥ 85th percentile) “in our 2-decade single institution dataset.” This “is nearly identical” to the percentage of patients with excess adiposity – 37.9% (also defined as BMI ≥ 85th percentile) – in an international cross-sectional study, which also identified an association between obesity (BMI ≥ 95th percentile) and psoriasis severity in children, she noted.
“What is clear is the strong association between obesity and childhood psoriasis, as multiple studies, including ours, confirm obesity as a major comorbidity of pediatric psoriasis,” Dr. Cordoro said. “Both conditions must be adequately managed to reduce the risk of adverse health outcomes for obese patients with psoriasis.”
The other study coauthors were Dana Feigenbaum, MD, and Alana Ju, MD. The work was supported by the UCSF Yearlong Inquiry Program. The researchers reported having no relevant financial disclosures.
FROM SPD 2021
Cutaneous Carcinomatous Arteriopathy and Retiform Purpura Secondary to Metastatic Penile Carcinoma
To the Editor:
A 56-year-old man with a history of stage IV metastatic penile squamous cell carcinoma treated with penectomy and chemotherapy with 5-fluorouracil and cisplatin presented with several painful ulcerations in the groin, abdomen, and thighs. The lesions initially appeared in the groin and were treated as bacterial abscesses with antibiotics. Over the next few weeks, new lesions appeared on the abdomen and thighs. An additional cycle of chemotherapy led to a reduction in number; however, they again increased within a few weeks. Medications included enoxaparin followed by 3 weeks of warfarin use due to a right leg deep vein thrombosis.
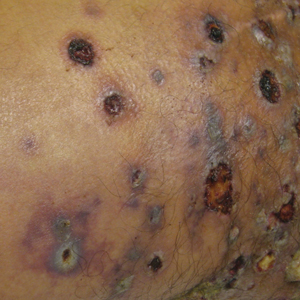
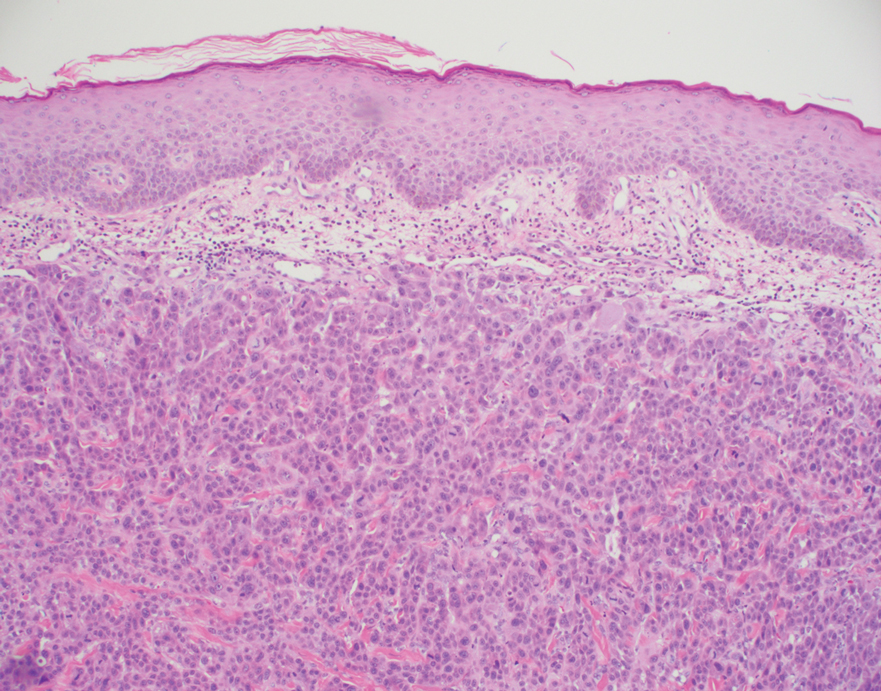
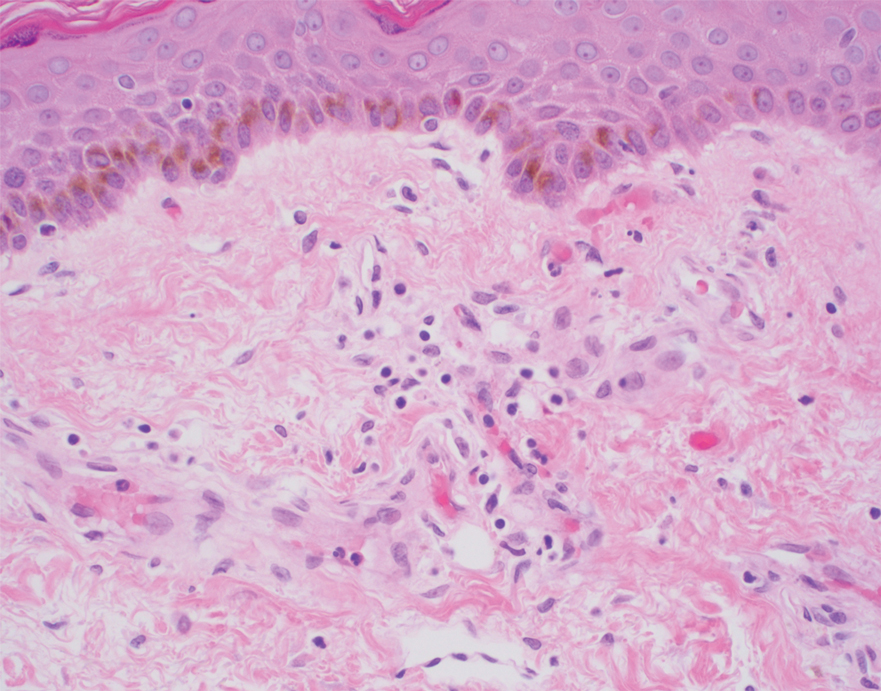
Physical examination revealed multiple 1- to 4-cm, firm, ulcerated nodules on the bilateral inguinal folds, abdomen, and upper thighs, as well as areas of livedo racemosa and noninflammatory retiform purpura with central ulceration (Figures 1 and 2). This retiform purpura was both perilesional and in areas without ulcerations. Laboratory values included the following: sodium, 127 mmol/L (reference range, 136–145 mmol/L); prothrombin time, 16.1 seconds (reference range, 11–15 seconds); white blood cell count, 20.69×109/L (reference range, 4.5–11.0×109/L) with 87% neutrophils (reference range, 54%–62%); hemoglobin, 6.1 g/dL (reference range, 13.5–17.5 g/dL); hematocrit, 18.8% (reference range, 41%–53%); platelets, 474×109/L (reference range, 150–400×109/L); D-dimer, 0.77 mg/L (reference range, ≤0.50 mg/L); fibrinogen, 489 mg/dL (reference range, 150–400 mg/dL); prior urine culture positive for Pseudomonas aeruginosa. He was negative for hepatitis B and hepatitis C viruses as well as HIV, and the lesions were not clinically consistent with herpes simplex virus, as they were not scalloped or circinate. Punch biopsies were obtained from a nodule on the left leg and a purpuric patch on the right leg.
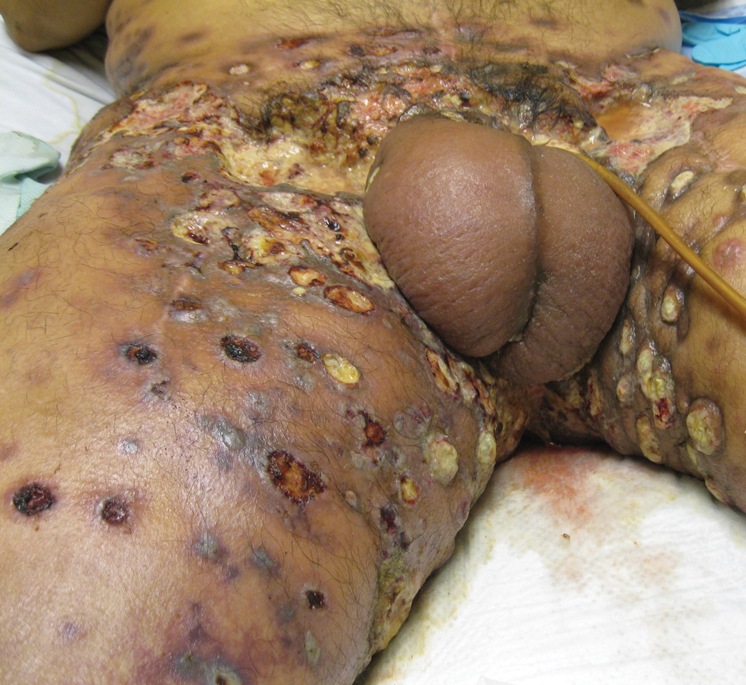
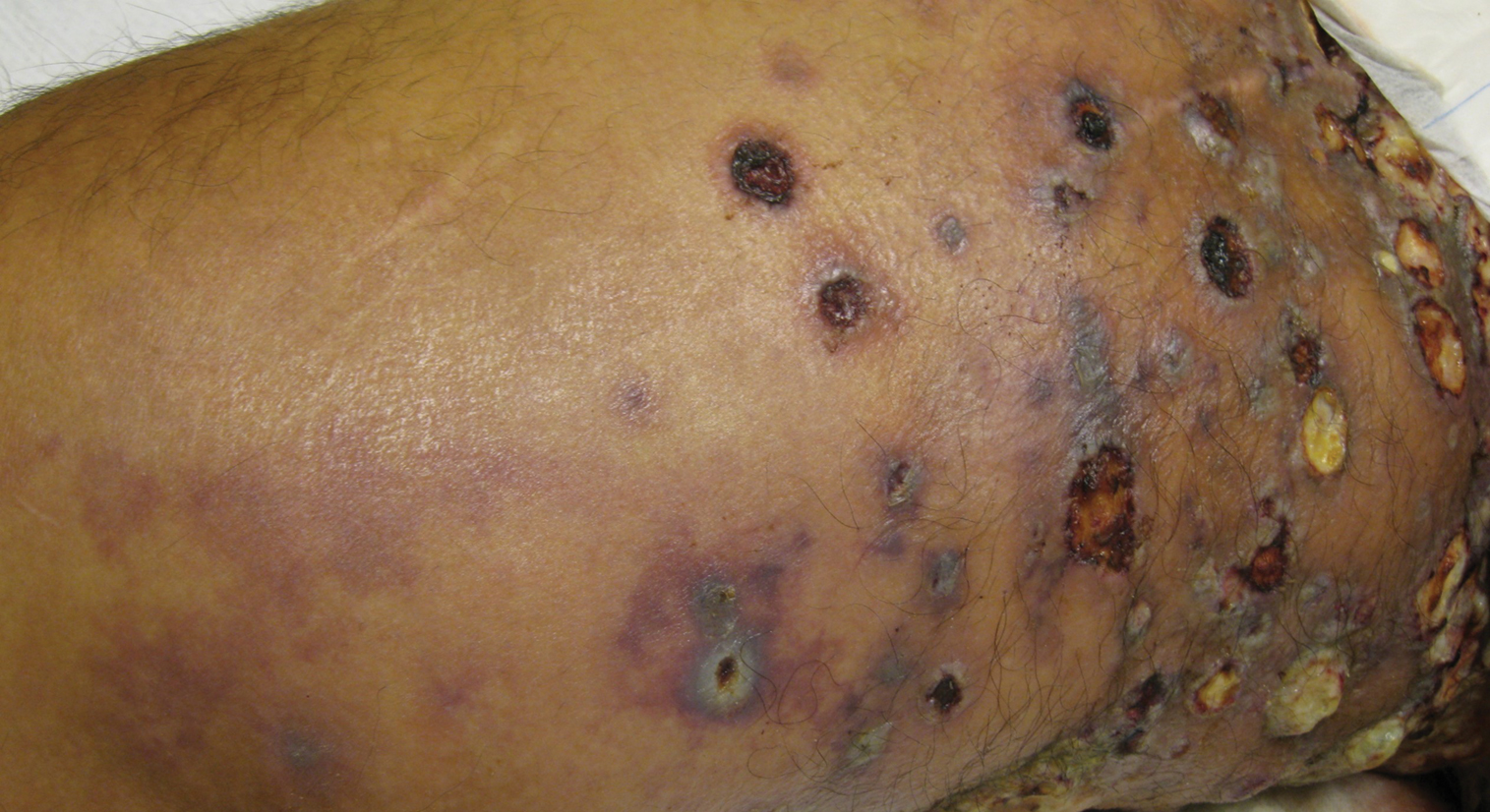
Histopathology of the ulcerated nodule revealed a proliferation of atypical keratinocytes with hyperchromatic and pleomorphic nuclei in the dermis without involvement of the overlying epidermis, consistent with metastatic squamous cell carcinoma (Figure 3). Histopathology of the purpuric patch demonstrated a thrombotic vasculopathy with numerous fibrin thrombi in the lumina of superficial dermal capillaries (Figure 4). No atypical cells, calcifications, or organisms were seen in the vessels. Periodic acid–Schiff, Fite, and Gram stains also were negative. The extent of the disease portended a poor prognosis, and additional vasculopathic workup was not pursued. Following antibiotic treatment and palliative care consultation, he died from subsequent infectious complications 1 month after presentation.
Cutaneous metastases may occur in the setting of multiple malignancies including breast, lung, melanoma, and various gastrointestinal cancers.1 These may present in multiple ways, including firm nontender nodules or as plaques with one of the following morphologies: carcinoma erysipeloides: erythematous, occasionally tender areas resembling cellulitis due to lymphatic obstruction by tumor cells2; carcinoma en cuirasse: indurated sclerotic scarlike plaques due to collagen infiltration3; or carcinoma telangiectoides: telangiectatic, thin erythematous plaques due to dermal capillary infiltration by malignant cells.3
Ischemic cutaneous lesions less commonly occur in the setting of malignancy and can be the result of both direct and indirect systemic effects from the cancer. Malignancies are known to directly trigger vasculopathies in other organs, most commonly the lungs, through 2 primary mechanisms. First, in carcinomatous arteriopathy, metastatic cells promote fibrocellular intimal proliferation of small pulmonary arteries and arterioles leading to stenosis, thrombosis, and obliteration. This mechanism has been described in pulmonary thrombotic microangiopathy secondary to lung carcinoma.4 This pathophysiology likely is also what underlies paraneoplastic acral vascular syndromes, which culminate in digital ischemia. Hypothesized mechanisms for this ischemia also range from vasospasm to thromboembolism.5 Secondly, in vasculitis carcinomatosa, metastatic tumor cells damage or block vessel walls, resulting in end-organ ischemia. Vasculitis carcinomatosa is a well-known phenomenon in angiocentric and intravascular lymphoid malignancies (typically of B-T or natural killer/T-cell origin) but also has been reported in a case of gastric adenocarcinoma with arterial invasion.6 This process is different than carcinoma telangiectoides where malignant cells may be present in the vasculature on histopathology but not trigger thrombosis and ischemic skin necrosis.
Systemic coagulopathies such as disseminated intravascular coagulation (DIC), thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome can occur in the setting of malignancies.7 Clinically, all may present with livedo racemosa, noninflammatory retiform purpura, and widespread skin necrosis. In adult patients, purpura fulminans most often is seen in the setting of sepsis and DIC, with accompanying evidence of microangiopathy.8 Catastrophic antiphospholipid antibody syndrome can be triggered by malignancy and is characterized by central nervous system, renal, pulmonary, and gastrointestinal complications. Skin involvement such as ulcers, livedo reticularis, and gangrene have been reported.9 Other causes of thrombotic vasculopathy include warfarin necrosis, heparin-induced thrombotic thrombocytopenia, calciphylaxis, and angioinvasive infections.8 Warfarin necrosis and heparin-induced thrombotic thrombocytopenia typically present days after initiating therapy with the respective medication. Calciphylaxis typically occurs in patients on dialysis, though it may occur in nonuremic patients including those with malignancy.8 Patients with malignancies on chemotherapy can become neutropenic and are at risk for ecthyma gangrenosum due to P aeruginosa and other gram-negative rods, Staphylococcus aureus, and angioinvasive fungi.10
Based on clinical, histopathological, and laboratory data, we favored a diagnosis of cutaneous carcinomatous arteriopathy. Vasculitis carcinomatosa was a possibility despite the lack of vasculotropism on histopathology, which may have been due to biopsy site selection. Systemic coagulopathies such as DIC, thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome were unlikely, as the ischemic skin lesions and livedo racemosa were limited to areas adjacent to cutaneous metastases, and the patient lacked other common multiorgan manifestations or laboratory findings. Although our patient was on warfarin, he was on a stable dose for weeks and histopathologic features of subcutaneous thrombosis were not seen. The biopsy also was not consistent with calciphylaxis. Ecthyma gangrenosum was unlikely given the lack of organisms on histopathology and negative skin and blood cultures. Although additional laboratory testing in this patient may have included cryoglobulins and cryofibrinogens, both entities were unlikely due to a lack of ischemic acral lesions.
In conclusion, we present a case of localized thrombotic vasculopathy that likely was secondary to cutaneous carcinomatous arteriopathy in the setting of cutaneous metastatic penile squamous cell carcinoma. The differential diagnosis of retiform purpura, livedo racemosa, and other signs of cutaneous ischemia in patients with metastatic cancer is broad and can be the result of both direct and indirect systemic effects from the cancer. Appropriate workup in these cases should include skin biopsies for histopathology and culture, medication review, and laboratory evaluation for systemic coagulopathies.
- Alcaraz I, Cerroni L, Ruetten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Prat L, Chouaid C, Kettaneh A, et al. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma: case report and literature review. Lung Cancer. 2013;79:91-93.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633-638.
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-592.
- Besnerais ML, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer. Medicine. 2014;93:E47.
- Sweeney S, Utzschneider R, Fraire AE. Vasculitis carcinomatosa occurring in association with adenocarcinoma of the stomach. Ann Diagn Pathol. 1998;2:247-249.
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miesbach W, Asherson RA, Cervera R, et al; CAPS Registry Group. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson’s) syndrome. Clin Rheumatol. 2007;26:2109-2114.
- Pozo D. Ecthyma gangrenosum‐like eruption associated with Morganella morganii infection. Br J Dermatol. 1998;139:520-521.
To the Editor:
A 56-year-old man with a history of stage IV metastatic penile squamous cell carcinoma treated with penectomy and chemotherapy with 5-fluorouracil and cisplatin presented with several painful ulcerations in the groin, abdomen, and thighs. The lesions initially appeared in the groin and were treated as bacterial abscesses with antibiotics. Over the next few weeks, new lesions appeared on the abdomen and thighs. An additional cycle of chemotherapy led to a reduction in number; however, they again increased within a few weeks. Medications included enoxaparin followed by 3 weeks of warfarin use due to a right leg deep vein thrombosis.
Physical examination revealed multiple 1- to 4-cm, firm, ulcerated nodules on the bilateral inguinal folds, abdomen, and upper thighs, as well as areas of livedo racemosa and noninflammatory retiform purpura with central ulceration (Figures 1 and 2). This retiform purpura was both perilesional and in areas without ulcerations. Laboratory values included the following: sodium, 127 mmol/L (reference range, 136–145 mmol/L); prothrombin time, 16.1 seconds (reference range, 11–15 seconds); white blood cell count, 20.69×109/L (reference range, 4.5–11.0×109/L) with 87% neutrophils (reference range, 54%–62%); hemoglobin, 6.1 g/dL (reference range, 13.5–17.5 g/dL); hematocrit, 18.8% (reference range, 41%–53%); platelets, 474×109/L (reference range, 150–400×109/L); D-dimer, 0.77 mg/L (reference range, ≤0.50 mg/L); fibrinogen, 489 mg/dL (reference range, 150–400 mg/dL); prior urine culture positive for Pseudomonas aeruginosa. He was negative for hepatitis B and hepatitis C viruses as well as HIV, and the lesions were not clinically consistent with herpes simplex virus, as they were not scalloped or circinate. Punch biopsies were obtained from a nodule on the left leg and a purpuric patch on the right leg.


Histopathology of the ulcerated nodule revealed a proliferation of atypical keratinocytes with hyperchromatic and pleomorphic nuclei in the dermis without involvement of the overlying epidermis, consistent with metastatic squamous cell carcinoma (Figure 3). Histopathology of the purpuric patch demonstrated a thrombotic vasculopathy with numerous fibrin thrombi in the lumina of superficial dermal capillaries (Figure 4). No atypical cells, calcifications, or organisms were seen in the vessels. Periodic acid–Schiff, Fite, and Gram stains also were negative. The extent of the disease portended a poor prognosis, and additional vasculopathic workup was not pursued. Following antibiotic treatment and palliative care consultation, he died from subsequent infectious complications 1 month after presentation.


Cutaneous metastases may occur in the setting of multiple malignancies including breast, lung, melanoma, and various gastrointestinal cancers.1 These may present in multiple ways, including firm nontender nodules or as plaques with one of the following morphologies: carcinoma erysipeloides: erythematous, occasionally tender areas resembling cellulitis due to lymphatic obstruction by tumor cells2; carcinoma en cuirasse: indurated sclerotic scarlike plaques due to collagen infiltration3; or carcinoma telangiectoides: telangiectatic, thin erythematous plaques due to dermal capillary infiltration by malignant cells.3
Ischemic cutaneous lesions less commonly occur in the setting of malignancy and can be the result of both direct and indirect systemic effects from the cancer. Malignancies are known to directly trigger vasculopathies in other organs, most commonly the lungs, through 2 primary mechanisms. First, in carcinomatous arteriopathy, metastatic cells promote fibrocellular intimal proliferation of small pulmonary arteries and arterioles leading to stenosis, thrombosis, and obliteration. This mechanism has been described in pulmonary thrombotic microangiopathy secondary to lung carcinoma.4 This pathophysiology likely is also what underlies paraneoplastic acral vascular syndromes, which culminate in digital ischemia. Hypothesized mechanisms for this ischemia also range from vasospasm to thromboembolism.5 Secondly, in vasculitis carcinomatosa, metastatic tumor cells damage or block vessel walls, resulting in end-organ ischemia. Vasculitis carcinomatosa is a well-known phenomenon in angiocentric and intravascular lymphoid malignancies (typically of B-T or natural killer/T-cell origin) but also has been reported in a case of gastric adenocarcinoma with arterial invasion.6 This process is different than carcinoma telangiectoides where malignant cells may be present in the vasculature on histopathology but not trigger thrombosis and ischemic skin necrosis.
Systemic coagulopathies such as disseminated intravascular coagulation (DIC), thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome can occur in the setting of malignancies.7 Clinically, all may present with livedo racemosa, noninflammatory retiform purpura, and widespread skin necrosis. In adult patients, purpura fulminans most often is seen in the setting of sepsis and DIC, with accompanying evidence of microangiopathy.8 Catastrophic antiphospholipid antibody syndrome can be triggered by malignancy and is characterized by central nervous system, renal, pulmonary, and gastrointestinal complications. Skin involvement such as ulcers, livedo reticularis, and gangrene have been reported.9 Other causes of thrombotic vasculopathy include warfarin necrosis, heparin-induced thrombotic thrombocytopenia, calciphylaxis, and angioinvasive infections.8 Warfarin necrosis and heparin-induced thrombotic thrombocytopenia typically present days after initiating therapy with the respective medication. Calciphylaxis typically occurs in patients on dialysis, though it may occur in nonuremic patients including those with malignancy.8 Patients with malignancies on chemotherapy can become neutropenic and are at risk for ecthyma gangrenosum due to P aeruginosa and other gram-negative rods, Staphylococcus aureus, and angioinvasive fungi.10
Based on clinical, histopathological, and laboratory data, we favored a diagnosis of cutaneous carcinomatous arteriopathy. Vasculitis carcinomatosa was a possibility despite the lack of vasculotropism on histopathology, which may have been due to biopsy site selection. Systemic coagulopathies such as DIC, thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome were unlikely, as the ischemic skin lesions and livedo racemosa were limited to areas adjacent to cutaneous metastases, and the patient lacked other common multiorgan manifestations or laboratory findings. Although our patient was on warfarin, he was on a stable dose for weeks and histopathologic features of subcutaneous thrombosis were not seen. The biopsy also was not consistent with calciphylaxis. Ecthyma gangrenosum was unlikely given the lack of organisms on histopathology and negative skin and blood cultures. Although additional laboratory testing in this patient may have included cryoglobulins and cryofibrinogens, both entities were unlikely due to a lack of ischemic acral lesions.
In conclusion, we present a case of localized thrombotic vasculopathy that likely was secondary to cutaneous carcinomatous arteriopathy in the setting of cutaneous metastatic penile squamous cell carcinoma. The differential diagnosis of retiform purpura, livedo racemosa, and other signs of cutaneous ischemia in patients with metastatic cancer is broad and can be the result of both direct and indirect systemic effects from the cancer. Appropriate workup in these cases should include skin biopsies for histopathology and culture, medication review, and laboratory evaluation for systemic coagulopathies.
To the Editor:
A 56-year-old man with a history of stage IV metastatic penile squamous cell carcinoma treated with penectomy and chemotherapy with 5-fluorouracil and cisplatin presented with several painful ulcerations in the groin, abdomen, and thighs. The lesions initially appeared in the groin and were treated as bacterial abscesses with antibiotics. Over the next few weeks, new lesions appeared on the abdomen and thighs. An additional cycle of chemotherapy led to a reduction in number; however, they again increased within a few weeks. Medications included enoxaparin followed by 3 weeks of warfarin use due to a right leg deep vein thrombosis.
Physical examination revealed multiple 1- to 4-cm, firm, ulcerated nodules on the bilateral inguinal folds, abdomen, and upper thighs, as well as areas of livedo racemosa and noninflammatory retiform purpura with central ulceration (Figures 1 and 2). This retiform purpura was both perilesional and in areas without ulcerations. Laboratory values included the following: sodium, 127 mmol/L (reference range, 136–145 mmol/L); prothrombin time, 16.1 seconds (reference range, 11–15 seconds); white blood cell count, 20.69×109/L (reference range, 4.5–11.0×109/L) with 87% neutrophils (reference range, 54%–62%); hemoglobin, 6.1 g/dL (reference range, 13.5–17.5 g/dL); hematocrit, 18.8% (reference range, 41%–53%); platelets, 474×109/L (reference range, 150–400×109/L); D-dimer, 0.77 mg/L (reference range, ≤0.50 mg/L); fibrinogen, 489 mg/dL (reference range, 150–400 mg/dL); prior urine culture positive for Pseudomonas aeruginosa. He was negative for hepatitis B and hepatitis C viruses as well as HIV, and the lesions were not clinically consistent with herpes simplex virus, as they were not scalloped or circinate. Punch biopsies were obtained from a nodule on the left leg and a purpuric patch on the right leg.


Histopathology of the ulcerated nodule revealed a proliferation of atypical keratinocytes with hyperchromatic and pleomorphic nuclei in the dermis without involvement of the overlying epidermis, consistent with metastatic squamous cell carcinoma (Figure 3). Histopathology of the purpuric patch demonstrated a thrombotic vasculopathy with numerous fibrin thrombi in the lumina of superficial dermal capillaries (Figure 4). No atypical cells, calcifications, or organisms were seen in the vessels. Periodic acid–Schiff, Fite, and Gram stains also were negative. The extent of the disease portended a poor prognosis, and additional vasculopathic workup was not pursued. Following antibiotic treatment and palliative care consultation, he died from subsequent infectious complications 1 month after presentation.


Cutaneous metastases may occur in the setting of multiple malignancies including breast, lung, melanoma, and various gastrointestinal cancers.1 These may present in multiple ways, including firm nontender nodules or as plaques with one of the following morphologies: carcinoma erysipeloides: erythematous, occasionally tender areas resembling cellulitis due to lymphatic obstruction by tumor cells2; carcinoma en cuirasse: indurated sclerotic scarlike plaques due to collagen infiltration3; or carcinoma telangiectoides: telangiectatic, thin erythematous plaques due to dermal capillary infiltration by malignant cells.3
Ischemic cutaneous lesions less commonly occur in the setting of malignancy and can be the result of both direct and indirect systemic effects from the cancer. Malignancies are known to directly trigger vasculopathies in other organs, most commonly the lungs, through 2 primary mechanisms. First, in carcinomatous arteriopathy, metastatic cells promote fibrocellular intimal proliferation of small pulmonary arteries and arterioles leading to stenosis, thrombosis, and obliteration. This mechanism has been described in pulmonary thrombotic microangiopathy secondary to lung carcinoma.4 This pathophysiology likely is also what underlies paraneoplastic acral vascular syndromes, which culminate in digital ischemia. Hypothesized mechanisms for this ischemia also range from vasospasm to thromboembolism.5 Secondly, in vasculitis carcinomatosa, metastatic tumor cells damage or block vessel walls, resulting in end-organ ischemia. Vasculitis carcinomatosa is a well-known phenomenon in angiocentric and intravascular lymphoid malignancies (typically of B-T or natural killer/T-cell origin) but also has been reported in a case of gastric adenocarcinoma with arterial invasion.6 This process is different than carcinoma telangiectoides where malignant cells may be present in the vasculature on histopathology but not trigger thrombosis and ischemic skin necrosis.
Systemic coagulopathies such as disseminated intravascular coagulation (DIC), thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome can occur in the setting of malignancies.7 Clinically, all may present with livedo racemosa, noninflammatory retiform purpura, and widespread skin necrosis. In adult patients, purpura fulminans most often is seen in the setting of sepsis and DIC, with accompanying evidence of microangiopathy.8 Catastrophic antiphospholipid antibody syndrome can be triggered by malignancy and is characterized by central nervous system, renal, pulmonary, and gastrointestinal complications. Skin involvement such as ulcers, livedo reticularis, and gangrene have been reported.9 Other causes of thrombotic vasculopathy include warfarin necrosis, heparin-induced thrombotic thrombocytopenia, calciphylaxis, and angioinvasive infections.8 Warfarin necrosis and heparin-induced thrombotic thrombocytopenia typically present days after initiating therapy with the respective medication. Calciphylaxis typically occurs in patients on dialysis, though it may occur in nonuremic patients including those with malignancy.8 Patients with malignancies on chemotherapy can become neutropenic and are at risk for ecthyma gangrenosum due to P aeruginosa and other gram-negative rods, Staphylococcus aureus, and angioinvasive fungi.10
Based on clinical, histopathological, and laboratory data, we favored a diagnosis of cutaneous carcinomatous arteriopathy. Vasculitis carcinomatosa was a possibility despite the lack of vasculotropism on histopathology, which may have been due to biopsy site selection. Systemic coagulopathies such as DIC, thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome were unlikely, as the ischemic skin lesions and livedo racemosa were limited to areas adjacent to cutaneous metastases, and the patient lacked other common multiorgan manifestations or laboratory findings. Although our patient was on warfarin, he was on a stable dose for weeks and histopathologic features of subcutaneous thrombosis were not seen. The biopsy also was not consistent with calciphylaxis. Ecthyma gangrenosum was unlikely given the lack of organisms on histopathology and negative skin and blood cultures. Although additional laboratory testing in this patient may have included cryoglobulins and cryofibrinogens, both entities were unlikely due to a lack of ischemic acral lesions.
In conclusion, we present a case of localized thrombotic vasculopathy that likely was secondary to cutaneous carcinomatous arteriopathy in the setting of cutaneous metastatic penile squamous cell carcinoma. The differential diagnosis of retiform purpura, livedo racemosa, and other signs of cutaneous ischemia in patients with metastatic cancer is broad and can be the result of both direct and indirect systemic effects from the cancer. Appropriate workup in these cases should include skin biopsies for histopathology and culture, medication review, and laboratory evaluation for systemic coagulopathies.
- Alcaraz I, Cerroni L, Ruetten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Prat L, Chouaid C, Kettaneh A, et al. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma: case report and literature review. Lung Cancer. 2013;79:91-93.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633-638.
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-592.
- Besnerais ML, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer. Medicine. 2014;93:E47.
- Sweeney S, Utzschneider R, Fraire AE. Vasculitis carcinomatosa occurring in association with adenocarcinoma of the stomach. Ann Diagn Pathol. 1998;2:247-249.
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miesbach W, Asherson RA, Cervera R, et al; CAPS Registry Group. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson’s) syndrome. Clin Rheumatol. 2007;26:2109-2114.
- Pozo D. Ecthyma gangrenosum‐like eruption associated with Morganella morganii infection. Br J Dermatol. 1998;139:520-521.
- Alcaraz I, Cerroni L, Ruetten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Prat L, Chouaid C, Kettaneh A, et al. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma: case report and literature review. Lung Cancer. 2013;79:91-93.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633-638.
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-592.
- Besnerais ML, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer. Medicine. 2014;93:E47.
- Sweeney S, Utzschneider R, Fraire AE. Vasculitis carcinomatosa occurring in association with adenocarcinoma of the stomach. Ann Diagn Pathol. 1998;2:247-249.
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miesbach W, Asherson RA, Cervera R, et al; CAPS Registry Group. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson’s) syndrome. Clin Rheumatol. 2007;26:2109-2114.
- Pozo D. Ecthyma gangrenosum‐like eruption associated with Morganella morganii infection. Br J Dermatol. 1998;139:520-521.
Practice Points
- Cutaneous metastases may present in multiple ways, including carcinoma erysipeloides, carcinoma en cuirasse, or carcinoma telangiectoides.
- Ischemic cutaneous lesions, characterized by livedoid skin changes and retiform purpura, occur less commonly in the setting of malignancy.
- Direct mechanisms include carcinomatous arteriopathy and vasculitis carcinomatosa. Indirect systemic processes include coagulopathies such as disseminated intravascular coagulation, thrombotic thrombocytopenia purpura, catastrophic antiphospholipid antibody syndrome, calciphylaxis, and cryoglobulinemia.
Cutaneous Complications Associated With Intraosseous Access Placement
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
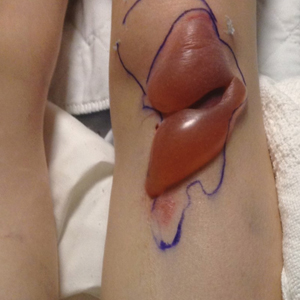
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg

At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.

Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg

At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.

Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg

At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.

Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
Practice Points
- Intraosseous (IO) access provides rapid vascular access for the delivery of fluids, drugs, and blood products in emergent situations.
- Bullae are potential complications from IO line placement.
California’s highest COVID infection rates shift to rural counties
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.
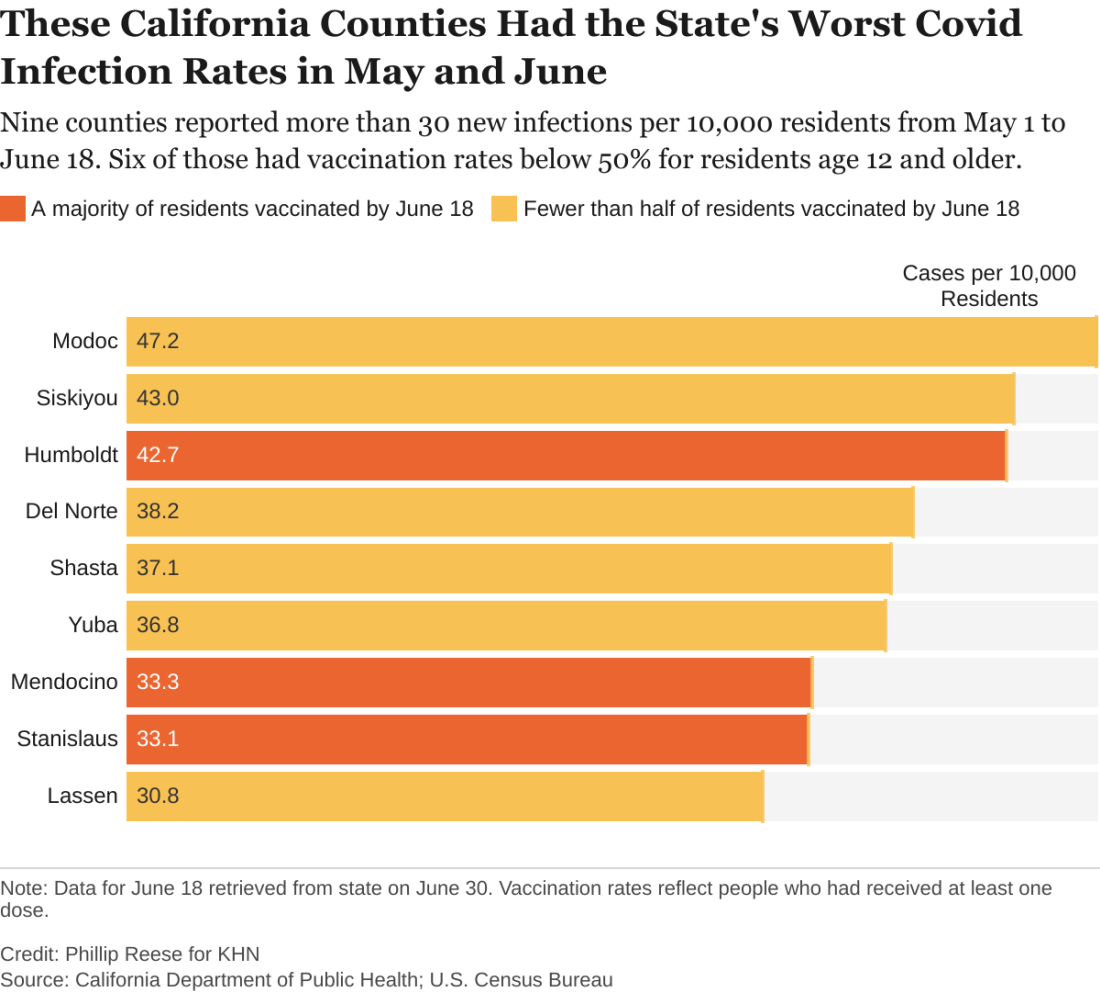
And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.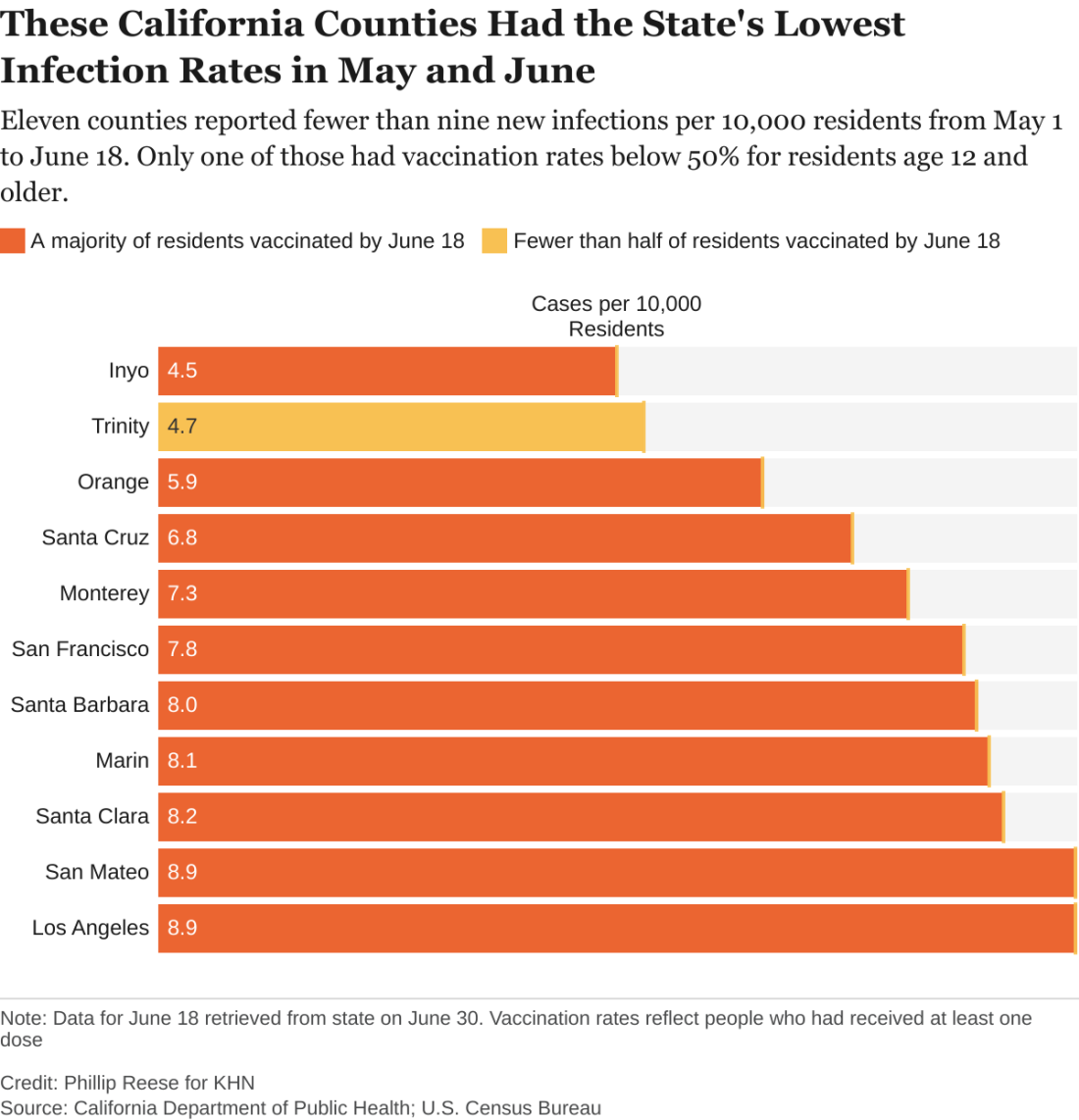
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.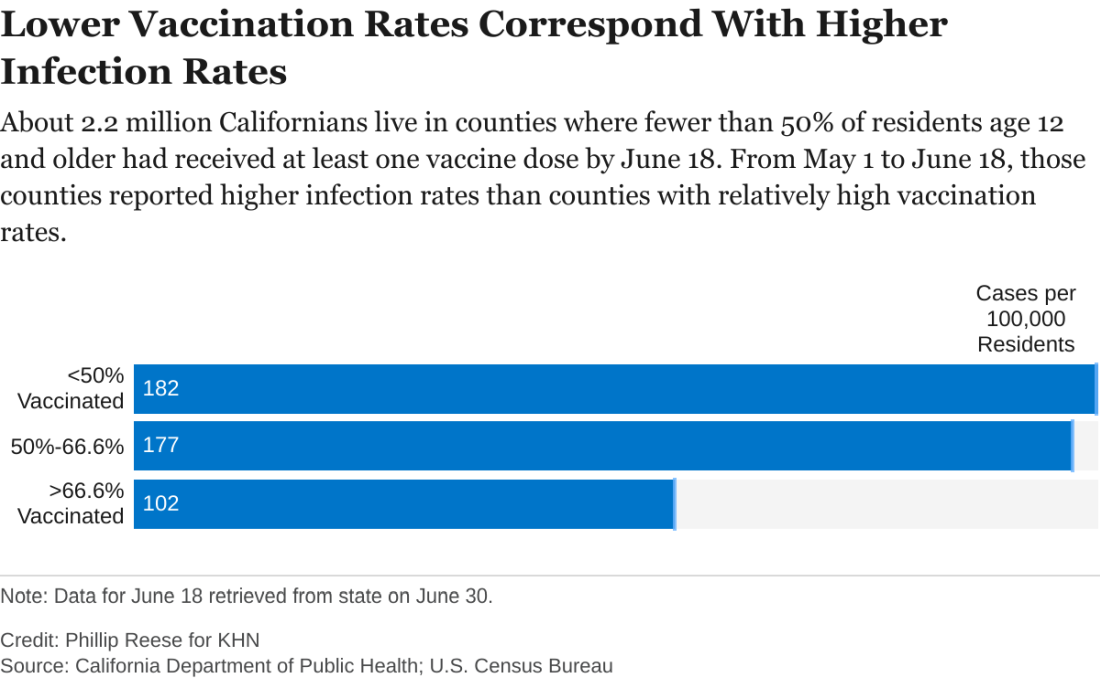
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.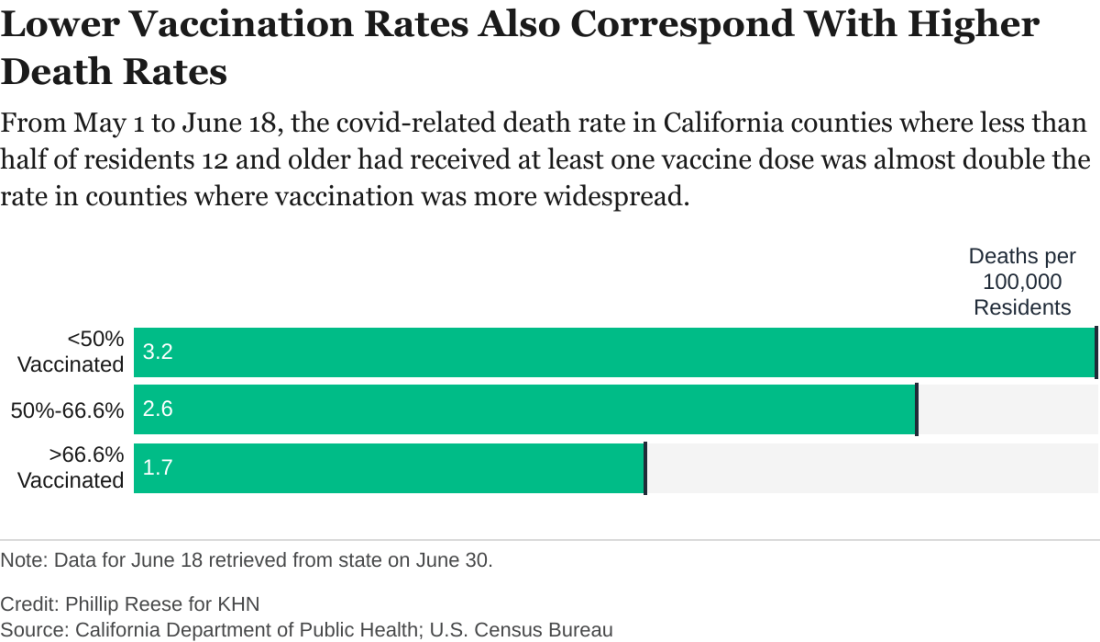
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.

And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.

And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Sleep-disordered breathing in neuromuscular disease: Early noninvasive ventilation needed
Sleep-disordered breathing is common in patients with neuromuscular disease and is increasingly addressed with noninvasive ventilation, but its patterns go beyond obstructive sleep apnea (OSA) and include hypoventilation, hypoxemia, central sleep apnea, pseudocentrals, periodic breathing, and Cheyne-Stokes respiration, Gaurav Singh, MD, MPH said at the virtual annual meeting of the Associated Professional Sleep Societies.
The prevalence of sleep-related disordered breathing surpasses 40% in patients diagnosed with neuromuscular disease, but “sleep disordered breathing [in these patients] does not equal obstructive sleep apnea,” said Dr. Singh, staff physician at the Veteran Affairs Palo Alto (Calif.) Health Care System in the section of pulmonary, critical care and sleep medicine, and an affiliated clinical assistant professor at Stanford (Calif.) University.
“The most common sleep-related breathing disorder in neuromuscular disease is probably hypopnea and hypoventilation with the sawtooth pattern of dips in oxygen saturation that occur during REM sleep,” he said. As neuromuscular diseases progress, hypoventilation may occur during non-REM sleep as well.
Evaluation is usually performed with polysomnography and pulmonary function testing, he said, but supplementary testing including serum bicarbonate levels, arterial blood gases, and echocardiography to assess for left ventricular ejection fraction and cardiomyopathy may be useful as well.
While a sleep study is not required per Centers for Medicare & Medicaid coverage criteria for the use of respiratory assist devices in patients with neuromuscular disease, polysomnography is valuable for identifying early nocturnal respiratory impairment before the appearance of symptoms and daytime abnormalities in gas exchange, and is better than home testing for distinguishing different types of events (including pseudocentrals). It also is helpful for determining the appropriate pressures needed for ventilatory support and for assessing the need for a backup rate, Dr. Singh said.
Commonly used types of noninvasive ventilation include bilevel positive airway pressure on the spontaneous/timed or pressure control modes, with or without volume-assured pressure support, he said.
Expiratory positive airway pressure (EPAP) is usually set low initially to help decrease the work of breathing and improve triggering, then titrated up to ensure that upper airway obstructive events are treated. Pressure support (the difference between the inspiratory positive airway pressure and EPAP) is set to achieve target tidal volume and to rest the respiratory muscles. And inspiratory time is set “on the longer end” to achieve maximal target volume and ensure appropriate gas exchange, Dr. Singh said.
Data from randomized controlled trials evaluating the effectiveness of NIV are limited, he said. A study published 15 years ago showed a survival benefit and improvement in quality of life measures in patients with amyotrophic lateral sclerosis (ALS) with normal or moderately impaired bulbar function but not in those with severe bulbar weakness.
Regarding the timing of initiating NIV, a retrospective study published several years ago looked at almost 200 ALS patients and evaluated differences in survival amongst those started earlier with NIV (forced vital capacity ≥80%) and those started later (FVC <80%). At 36 months from diagnosis, mortality was 35% for the early group and 53% for the later group. “Improved survival was driven by benefit in patients with non–bulbar-onset ALS, compared with bulbar-onset disease,” Dr. Singh said.
“This study and several other similar studies seem to indicate that the earlier NIV [noninvasive ventilation] is started in patients with neuromuscular disease, the better in terms of improving survival and other relevant measures such as quality of life,” he said.
Asked about Dr. Singh’s presentation, Michelle Cao, DO, clinical associate professor at Stanford University, said that NIV is an “invaluable tool in the treatment of conditions leading to chronic respiratory failure,” such as neuromuscular disease, and that it’s important to incorporate NIV training for future pulmonary, critical care and sleep physicians. Dr. Cao directs the adult NIV program for the neuromuscular medical program at Stanford Health Care.
Saiprakash B. Venkateshiah, MD, of Emory University, Atlanta, also said in introducing Dr. Singh at the meeting that earlier diagnosis and appropriate NIV therapy “may improve quality of life and possibly even lower survival in certain disorders.”
In addition, he noted that sleep disturbances “may be the earliest sign of muscle weakness in [patients with neuromuscular disease], sometimes being detected before their underlying neuromuscular disease is diagnosed.”
Dr. Singh, Dr. Cao, and Dr. Venkateshiah each reported that they had no potential conflicts of interest.
Sleep-disordered breathing is common in patients with neuromuscular disease and is increasingly addressed with noninvasive ventilation, but its patterns go beyond obstructive sleep apnea (OSA) and include hypoventilation, hypoxemia, central sleep apnea, pseudocentrals, periodic breathing, and Cheyne-Stokes respiration, Gaurav Singh, MD, MPH said at the virtual annual meeting of the Associated Professional Sleep Societies.
The prevalence of sleep-related disordered breathing surpasses 40% in patients diagnosed with neuromuscular disease, but “sleep disordered breathing [in these patients] does not equal obstructive sleep apnea,” said Dr. Singh, staff physician at the Veteran Affairs Palo Alto (Calif.) Health Care System in the section of pulmonary, critical care and sleep medicine, and an affiliated clinical assistant professor at Stanford (Calif.) University.
“The most common sleep-related breathing disorder in neuromuscular disease is probably hypopnea and hypoventilation with the sawtooth pattern of dips in oxygen saturation that occur during REM sleep,” he said. As neuromuscular diseases progress, hypoventilation may occur during non-REM sleep as well.
Evaluation is usually performed with polysomnography and pulmonary function testing, he said, but supplementary testing including serum bicarbonate levels, arterial blood gases, and echocardiography to assess for left ventricular ejection fraction and cardiomyopathy may be useful as well.
While a sleep study is not required per Centers for Medicare & Medicaid coverage criteria for the use of respiratory assist devices in patients with neuromuscular disease, polysomnography is valuable for identifying early nocturnal respiratory impairment before the appearance of symptoms and daytime abnormalities in gas exchange, and is better than home testing for distinguishing different types of events (including pseudocentrals). It also is helpful for determining the appropriate pressures needed for ventilatory support and for assessing the need for a backup rate, Dr. Singh said.
Commonly used types of noninvasive ventilation include bilevel positive airway pressure on the spontaneous/timed or pressure control modes, with or without volume-assured pressure support, he said.
Expiratory positive airway pressure (EPAP) is usually set low initially to help decrease the work of breathing and improve triggering, then titrated up to ensure that upper airway obstructive events are treated. Pressure support (the difference between the inspiratory positive airway pressure and EPAP) is set to achieve target tidal volume and to rest the respiratory muscles. And inspiratory time is set “on the longer end” to achieve maximal target volume and ensure appropriate gas exchange, Dr. Singh said.
Data from randomized controlled trials evaluating the effectiveness of NIV are limited, he said. A study published 15 years ago showed a survival benefit and improvement in quality of life measures in patients with amyotrophic lateral sclerosis (ALS) with normal or moderately impaired bulbar function but not in those with severe bulbar weakness.
Regarding the timing of initiating NIV, a retrospective study published several years ago looked at almost 200 ALS patients and evaluated differences in survival amongst those started earlier with NIV (forced vital capacity ≥80%) and those started later (FVC <80%). At 36 months from diagnosis, mortality was 35% for the early group and 53% for the later group. “Improved survival was driven by benefit in patients with non–bulbar-onset ALS, compared with bulbar-onset disease,” Dr. Singh said.
“This study and several other similar studies seem to indicate that the earlier NIV [noninvasive ventilation] is started in patients with neuromuscular disease, the better in terms of improving survival and other relevant measures such as quality of life,” he said.
Asked about Dr. Singh’s presentation, Michelle Cao, DO, clinical associate professor at Stanford University, said that NIV is an “invaluable tool in the treatment of conditions leading to chronic respiratory failure,” such as neuromuscular disease, and that it’s important to incorporate NIV training for future pulmonary, critical care and sleep physicians. Dr. Cao directs the adult NIV program for the neuromuscular medical program at Stanford Health Care.
Saiprakash B. Venkateshiah, MD, of Emory University, Atlanta, also said in introducing Dr. Singh at the meeting that earlier diagnosis and appropriate NIV therapy “may improve quality of life and possibly even lower survival in certain disorders.”
In addition, he noted that sleep disturbances “may be the earliest sign of muscle weakness in [patients with neuromuscular disease], sometimes being detected before their underlying neuromuscular disease is diagnosed.”
Dr. Singh, Dr. Cao, and Dr. Venkateshiah each reported that they had no potential conflicts of interest.
Sleep-disordered breathing is common in patients with neuromuscular disease and is increasingly addressed with noninvasive ventilation, but its patterns go beyond obstructive sleep apnea (OSA) and include hypoventilation, hypoxemia, central sleep apnea, pseudocentrals, periodic breathing, and Cheyne-Stokes respiration, Gaurav Singh, MD, MPH said at the virtual annual meeting of the Associated Professional Sleep Societies.
The prevalence of sleep-related disordered breathing surpasses 40% in patients diagnosed with neuromuscular disease, but “sleep disordered breathing [in these patients] does not equal obstructive sleep apnea,” said Dr. Singh, staff physician at the Veteran Affairs Palo Alto (Calif.) Health Care System in the section of pulmonary, critical care and sleep medicine, and an affiliated clinical assistant professor at Stanford (Calif.) University.
“The most common sleep-related breathing disorder in neuromuscular disease is probably hypopnea and hypoventilation with the sawtooth pattern of dips in oxygen saturation that occur during REM sleep,” he said. As neuromuscular diseases progress, hypoventilation may occur during non-REM sleep as well.
Evaluation is usually performed with polysomnography and pulmonary function testing, he said, but supplementary testing including serum bicarbonate levels, arterial blood gases, and echocardiography to assess for left ventricular ejection fraction and cardiomyopathy may be useful as well.
While a sleep study is not required per Centers for Medicare & Medicaid coverage criteria for the use of respiratory assist devices in patients with neuromuscular disease, polysomnography is valuable for identifying early nocturnal respiratory impairment before the appearance of symptoms and daytime abnormalities in gas exchange, and is better than home testing for distinguishing different types of events (including pseudocentrals). It also is helpful for determining the appropriate pressures needed for ventilatory support and for assessing the need for a backup rate, Dr. Singh said.
Commonly used types of noninvasive ventilation include bilevel positive airway pressure on the spontaneous/timed or pressure control modes, with or without volume-assured pressure support, he said.
Expiratory positive airway pressure (EPAP) is usually set low initially to help decrease the work of breathing and improve triggering, then titrated up to ensure that upper airway obstructive events are treated. Pressure support (the difference between the inspiratory positive airway pressure and EPAP) is set to achieve target tidal volume and to rest the respiratory muscles. And inspiratory time is set “on the longer end” to achieve maximal target volume and ensure appropriate gas exchange, Dr. Singh said.
Data from randomized controlled trials evaluating the effectiveness of NIV are limited, he said. A study published 15 years ago showed a survival benefit and improvement in quality of life measures in patients with amyotrophic lateral sclerosis (ALS) with normal or moderately impaired bulbar function but not in those with severe bulbar weakness.
Regarding the timing of initiating NIV, a retrospective study published several years ago looked at almost 200 ALS patients and evaluated differences in survival amongst those started earlier with NIV (forced vital capacity ≥80%) and those started later (FVC <80%). At 36 months from diagnosis, mortality was 35% for the early group and 53% for the later group. “Improved survival was driven by benefit in patients with non–bulbar-onset ALS, compared with bulbar-onset disease,” Dr. Singh said.
“This study and several other similar studies seem to indicate that the earlier NIV [noninvasive ventilation] is started in patients with neuromuscular disease, the better in terms of improving survival and other relevant measures such as quality of life,” he said.
Asked about Dr. Singh’s presentation, Michelle Cao, DO, clinical associate professor at Stanford University, said that NIV is an “invaluable tool in the treatment of conditions leading to chronic respiratory failure,” such as neuromuscular disease, and that it’s important to incorporate NIV training for future pulmonary, critical care and sleep physicians. Dr. Cao directs the adult NIV program for the neuromuscular medical program at Stanford Health Care.
Saiprakash B. Venkateshiah, MD, of Emory University, Atlanta, also said in introducing Dr. Singh at the meeting that earlier diagnosis and appropriate NIV therapy “may improve quality of life and possibly even lower survival in certain disorders.”
In addition, he noted that sleep disturbances “may be the earliest sign of muscle weakness in [patients with neuromuscular disease], sometimes being detected before their underlying neuromuscular disease is diagnosed.”
Dr. Singh, Dr. Cao, and Dr. Venkateshiah each reported that they had no potential conflicts of interest.
FROM SLEEP 2021
Garlic cloves in the nose and beer dreams and pareidolia faces
Insert clove A into nostril B
Just when you start wondering what crazy and potentially dangerous thing people can do to themselves next, comes a crazy and potentially dangerous new trend. The good folks at TikTok have provided patients a new treatment for stuffed up sinuses.
Dangerous? Well, that’s what doctors say, anyway.
“We typically do not recommend putting anything into the nostril for the obvious fact that it could get dislodged or lodged up into the nasal cavity,” Anthony Del Signore, MD, of Mount Sinai Union Square, New York, told TODAY.
“Not only does it have the potential to rot or cause a nasal obstruction, it can induce an episode of sinusitis,” Omid Mehdizadeh, MD, of Providence Saint John’s Health Center, Santa Monica, Calif., explained to Shape.
But who doesn't want to breathe easier and keep blood-sucking vampires at bay?
TikTokers are posting videos of themselves sticking garlic cloves in their nostrils for several minutes. They, “then, pull the garlic out, followed, typically, by long strands of mucus,” according to The Hill.
That can’t be real, you’re probably saying. Or maybe you think that no one is actually watching this stuff. Well, wake up! This isn’t network television we’re talking about. It’s freakin’ TikTok! One video has been favorited over half a million times. Another is up to 2.2 million.
It’s all true. Really. We couldn’t make this stuff up if we tried.
Seeing faces in random places?
Ever look up at the clouds, at a fast-moving train, or into your morning bowl of cereal and see two eyes, a nose, and a mouth looking back at you? You may shake it off and think you’re imagining something, but it's actually your brain doing what it’s built to do and researchers know why.
The phenomenon is called face pareidolia, and it’s technically an error function of the human brain. Evolution has molded our brains to rapidly identify faces, according to David Alais, PhD, of the University of Sydney, Australia, lead author of the study.
“But the system plays ‘fast and loose’ by applying a crude template of two eyes over a nose and mouth. Lots of things can satisfy that template and thus trigger a face detection response,” he said in a separate statement. But not only are we seeing faces, our brains go one step further and seemingly give those faces feelings.
In the study, Dr. Alais and his team looked for two things about each pareidolia face: Was it analyzed for facial expression or just rejected as a face altogether? The participants were shown a series of faces and then asked to rate the expression on a scale from angry to happy. What the researchers found was that once a face was detected, the brain analyzed the pareidolia face in the same way as a human face. Have you ever seen an angry trash can? Or a smile on an over-easy egg?
The other question faced: Was there a bias on emotion? Yup, and excuse the dad joke.
The researchers showed a mixed series of human faces and pareidolia faces to participants and found that responses were influenced by the previous face seen, no matter if the face was human or not.
So if someone smiled at you on the way to the grocery store and you see a grinning tomato in the produce section, your mind is playing tricks on you, and it’s totally normal.
Corporate dream manipulation
Advertisements are quite literally everywhere. On billboards, in commercials, in videos, in movies; the list goes on and on. Still, at least you can shut your eyes and be mercifully free of corporate interference inside your own head, right? Right?
Early in 2021, Coors launched an ad campaign that seemed to be a b bit of a gimmick, if not a joke. Coors claimed that if people watched an ad before bed, and played an 8-hour soundscape while sleeping, their dreams would be filled with crisp mountains and cold, thirst-quenching beverages. While, the Coors campaign didn’t go viral, someone was paying attention. A group of 35 leading researchers published an open letter on the subject of corporate dream manipulation, in the journal Dream Engineering.
"Multiple marketing studies are openly testing new ways to alter and motivate purchasing behavior through dream and sleep hacking. The commercial, for-profit use of dream incubation is rapidly becoming a reality," wrote the investigators. "As sleep and dream researchers, we are deeply concerned about marketing plans aimed at generating profits at the cost of interfering with our natural nocturnal memory processing."
People have tried to manipulate their dreams for countless years, but only in recent years have scientists attempted to target or manipulate behavior through dreams. In a 2014 study, smokers exposed to tobacco smoke and rotten egg smell while sleeping reduced their cigarette consumption by 30%.
Most research into dream manipulation has been aimed at positive results, but the experts warn that there’s no reason corporations couldn’t use it for their own purposes, especially given the widespread usage of devices such as Alexa. A company could play a certain sound during a commercial, they suggested, and then replay that sound through a device while people are sleeping to trigger a dream about that product.
And just when our COVID-19–driven anxiety dreams were starting to subside.
The experts said that the Federal Trade Commission could intervene to prevent companies from attempting dream manipulation, and have done so in the past to stop subliminal advertising, but as of right now, there’s nothing stopping big business from messing with your dreams. But hey, at least they’re not directly beaming commercials into our heads with gamma radiation. Yet.
Got breast milk?
As we know, breast milk has endless benefits for newbords and babies, but many things can stand in the way of a mother’s ability to breastfeed. Baby formula has served as a good enough substitute. But now, there might be something that’s even better.
A start-up company called BIOMILQ created a product that could be groundbreaking. Using “breakthrough mammary biotechnology,” BIOMILQ created cell-cultured breast milk.
Leila Strickland, a biologist who is the company’s cofounder and chief science officer, said she’s had her own personal experience with breastfeeding and believes the product could benefit many if just given a chance. "Some of the cells we’ve looked at can produce milk for months and months," according to a company statement
Baby formula has done its job feeding and nourishing babies since 1865, but could BIOMILQ do better?
Time – and babies – will tell.
Insert clove A into nostril B
Just when you start wondering what crazy and potentially dangerous thing people can do to themselves next, comes a crazy and potentially dangerous new trend. The good folks at TikTok have provided patients a new treatment for stuffed up sinuses.
Dangerous? Well, that’s what doctors say, anyway.
“We typically do not recommend putting anything into the nostril for the obvious fact that it could get dislodged or lodged up into the nasal cavity,” Anthony Del Signore, MD, of Mount Sinai Union Square, New York, told TODAY.
“Not only does it have the potential to rot or cause a nasal obstruction, it can induce an episode of sinusitis,” Omid Mehdizadeh, MD, of Providence Saint John’s Health Center, Santa Monica, Calif., explained to Shape.
But who doesn't want to breathe easier and keep blood-sucking vampires at bay?
TikTokers are posting videos of themselves sticking garlic cloves in their nostrils for several minutes. They, “then, pull the garlic out, followed, typically, by long strands of mucus,” according to The Hill.
That can’t be real, you’re probably saying. Or maybe you think that no one is actually watching this stuff. Well, wake up! This isn’t network television we’re talking about. It’s freakin’ TikTok! One video has been favorited over half a million times. Another is up to 2.2 million.
It’s all true. Really. We couldn’t make this stuff up if we tried.
Seeing faces in random places?
Ever look up at the clouds, at a fast-moving train, or into your morning bowl of cereal and see two eyes, a nose, and a mouth looking back at you? You may shake it off and think you’re imagining something, but it's actually your brain doing what it’s built to do and researchers know why.
The phenomenon is called face pareidolia, and it’s technically an error function of the human brain. Evolution has molded our brains to rapidly identify faces, according to David Alais, PhD, of the University of Sydney, Australia, lead author of the study.
“But the system plays ‘fast and loose’ by applying a crude template of two eyes over a nose and mouth. Lots of things can satisfy that template and thus trigger a face detection response,” he said in a separate statement. But not only are we seeing faces, our brains go one step further and seemingly give those faces feelings.
In the study, Dr. Alais and his team looked for two things about each pareidolia face: Was it analyzed for facial expression or just rejected as a face altogether? The participants were shown a series of faces and then asked to rate the expression on a scale from angry to happy. What the researchers found was that once a face was detected, the brain analyzed the pareidolia face in the same way as a human face. Have you ever seen an angry trash can? Or a smile on an over-easy egg?
The other question faced: Was there a bias on emotion? Yup, and excuse the dad joke.
The researchers showed a mixed series of human faces and pareidolia faces to participants and found that responses were influenced by the previous face seen, no matter if the face was human or not.
So if someone smiled at you on the way to the grocery store and you see a grinning tomato in the produce section, your mind is playing tricks on you, and it’s totally normal.
Corporate dream manipulation
Advertisements are quite literally everywhere. On billboards, in commercials, in videos, in movies; the list goes on and on. Still, at least you can shut your eyes and be mercifully free of corporate interference inside your own head, right? Right?
Early in 2021, Coors launched an ad campaign that seemed to be a b bit of a gimmick, if not a joke. Coors claimed that if people watched an ad before bed, and played an 8-hour soundscape while sleeping, their dreams would be filled with crisp mountains and cold, thirst-quenching beverages. While, the Coors campaign didn’t go viral, someone was paying attention. A group of 35 leading researchers published an open letter on the subject of corporate dream manipulation, in the journal Dream Engineering.
"Multiple marketing studies are openly testing new ways to alter and motivate purchasing behavior through dream and sleep hacking. The commercial, for-profit use of dream incubation is rapidly becoming a reality," wrote the investigators. "As sleep and dream researchers, we are deeply concerned about marketing plans aimed at generating profits at the cost of interfering with our natural nocturnal memory processing."
People have tried to manipulate their dreams for countless years, but only in recent years have scientists attempted to target or manipulate behavior through dreams. In a 2014 study, smokers exposed to tobacco smoke and rotten egg smell while sleeping reduced their cigarette consumption by 30%.
Most research into dream manipulation has been aimed at positive results, but the experts warn that there’s no reason corporations couldn’t use it for their own purposes, especially given the widespread usage of devices such as Alexa. A company could play a certain sound during a commercial, they suggested, and then replay that sound through a device while people are sleeping to trigger a dream about that product.
And just when our COVID-19–driven anxiety dreams were starting to subside.
The experts said that the Federal Trade Commission could intervene to prevent companies from attempting dream manipulation, and have done so in the past to stop subliminal advertising, but as of right now, there’s nothing stopping big business from messing with your dreams. But hey, at least they’re not directly beaming commercials into our heads with gamma radiation. Yet.
Got breast milk?
As we know, breast milk has endless benefits for newbords and babies, but many things can stand in the way of a mother’s ability to breastfeed. Baby formula has served as a good enough substitute. But now, there might be something that’s even better.
A start-up company called BIOMILQ created a product that could be groundbreaking. Using “breakthrough mammary biotechnology,” BIOMILQ created cell-cultured breast milk.
Leila Strickland, a biologist who is the company’s cofounder and chief science officer, said she’s had her own personal experience with breastfeeding and believes the product could benefit many if just given a chance. "Some of the cells we’ve looked at can produce milk for months and months," according to a company statement
Baby formula has done its job feeding and nourishing babies since 1865, but could BIOMILQ do better?
Time – and babies – will tell.
Insert clove A into nostril B
Just when you start wondering what crazy and potentially dangerous thing people can do to themselves next, comes a crazy and potentially dangerous new trend. The good folks at TikTok have provided patients a new treatment for stuffed up sinuses.
Dangerous? Well, that’s what doctors say, anyway.
“We typically do not recommend putting anything into the nostril for the obvious fact that it could get dislodged or lodged up into the nasal cavity,” Anthony Del Signore, MD, of Mount Sinai Union Square, New York, told TODAY.
“Not only does it have the potential to rot or cause a nasal obstruction, it can induce an episode of sinusitis,” Omid Mehdizadeh, MD, of Providence Saint John’s Health Center, Santa Monica, Calif., explained to Shape.
But who doesn't want to breathe easier and keep blood-sucking vampires at bay?
TikTokers are posting videos of themselves sticking garlic cloves in their nostrils for several minutes. They, “then, pull the garlic out, followed, typically, by long strands of mucus,” according to The Hill.
That can’t be real, you’re probably saying. Or maybe you think that no one is actually watching this stuff. Well, wake up! This isn’t network television we’re talking about. It’s freakin’ TikTok! One video has been favorited over half a million times. Another is up to 2.2 million.
It’s all true. Really. We couldn’t make this stuff up if we tried.
Seeing faces in random places?
Ever look up at the clouds, at a fast-moving train, or into your morning bowl of cereal and see two eyes, a nose, and a mouth looking back at you? You may shake it off and think you’re imagining something, but it's actually your brain doing what it’s built to do and researchers know why.
The phenomenon is called face pareidolia, and it’s technically an error function of the human brain. Evolution has molded our brains to rapidly identify faces, according to David Alais, PhD, of the University of Sydney, Australia, lead author of the study.
“But the system plays ‘fast and loose’ by applying a crude template of two eyes over a nose and mouth. Lots of things can satisfy that template and thus trigger a face detection response,” he said in a separate statement. But not only are we seeing faces, our brains go one step further and seemingly give those faces feelings.
In the study, Dr. Alais and his team looked for two things about each pareidolia face: Was it analyzed for facial expression or just rejected as a face altogether? The participants were shown a series of faces and then asked to rate the expression on a scale from angry to happy. What the researchers found was that once a face was detected, the brain analyzed the pareidolia face in the same way as a human face. Have you ever seen an angry trash can? Or a smile on an over-easy egg?
The other question faced: Was there a bias on emotion? Yup, and excuse the dad joke.
The researchers showed a mixed series of human faces and pareidolia faces to participants and found that responses were influenced by the previous face seen, no matter if the face was human or not.
So if someone smiled at you on the way to the grocery store and you see a grinning tomato in the produce section, your mind is playing tricks on you, and it’s totally normal.
Corporate dream manipulation
Advertisements are quite literally everywhere. On billboards, in commercials, in videos, in movies; the list goes on and on. Still, at least you can shut your eyes and be mercifully free of corporate interference inside your own head, right? Right?
Early in 2021, Coors launched an ad campaign that seemed to be a b bit of a gimmick, if not a joke. Coors claimed that if people watched an ad before bed, and played an 8-hour soundscape while sleeping, their dreams would be filled with crisp mountains and cold, thirst-quenching beverages. While, the Coors campaign didn’t go viral, someone was paying attention. A group of 35 leading researchers published an open letter on the subject of corporate dream manipulation, in the journal Dream Engineering.
"Multiple marketing studies are openly testing new ways to alter and motivate purchasing behavior through dream and sleep hacking. The commercial, for-profit use of dream incubation is rapidly becoming a reality," wrote the investigators. "As sleep and dream researchers, we are deeply concerned about marketing plans aimed at generating profits at the cost of interfering with our natural nocturnal memory processing."
People have tried to manipulate their dreams for countless years, but only in recent years have scientists attempted to target or manipulate behavior through dreams. In a 2014 study, smokers exposed to tobacco smoke and rotten egg smell while sleeping reduced their cigarette consumption by 30%.
Most research into dream manipulation has been aimed at positive results, but the experts warn that there’s no reason corporations couldn’t use it for their own purposes, especially given the widespread usage of devices such as Alexa. A company could play a certain sound during a commercial, they suggested, and then replay that sound through a device while people are sleeping to trigger a dream about that product.
And just when our COVID-19–driven anxiety dreams were starting to subside.
The experts said that the Federal Trade Commission could intervene to prevent companies from attempting dream manipulation, and have done so in the past to stop subliminal advertising, but as of right now, there’s nothing stopping big business from messing with your dreams. But hey, at least they’re not directly beaming commercials into our heads with gamma radiation. Yet.
Got breast milk?
As we know, breast milk has endless benefits for newbords and babies, but many things can stand in the way of a mother’s ability to breastfeed. Baby formula has served as a good enough substitute. But now, there might be something that’s even better.
A start-up company called BIOMILQ created a product that could be groundbreaking. Using “breakthrough mammary biotechnology,” BIOMILQ created cell-cultured breast milk.
Leila Strickland, a biologist who is the company’s cofounder and chief science officer, said she’s had her own personal experience with breastfeeding and believes the product could benefit many if just given a chance. "Some of the cells we’ve looked at can produce milk for months and months," according to a company statement
Baby formula has done its job feeding and nourishing babies since 1865, but could BIOMILQ do better?
Time – and babies – will tell.
CABANA: Ablation bests drugs for AFib in racial/ethnic minorities
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
Heart failure med undertreatment because of older age common, flouts evidence
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.