User login
Clostridioides difficile: Two sets of guidelines disagree
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
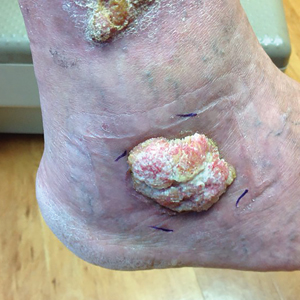
Isotretinoin benefits similar in overweight, obese adolescents, and those in normal weight range
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
FROM SPD 2021
Extra COVID-19 vaccine could help immunocompromised people
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual immunotherapy: Where does it stand?
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Direct-care allergy clinic specializes in sublingual immunotherapy
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
There’s a much safer food allergy immunotherapy – why don’t more doctors offer it?
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
Antibiotic linked to rise in early onset colon cancer?
Exposure to antibiotics appears to be associated with the development of colon cancer, particularly in younger people, and could be contributing to the increase in early-onset colorectal cancer (CRC) that is being documented, say U.K. researchers.
The team conducted a nested case-control study using data from primary care in Scotland, which involved almost 8,000 cases of CRC and over 30,000 healthy controls.
The analysis suggests that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer (but not rectal) by 49%.
“To our knowledge, this is the first study to link antibiotic use with the growing risk of early onset colon cancer, a disease which has been increasing at a rate of at least 3% per year over the last two decades,” said study presenter Sarah Perrott, a medical student at the University of Aberdeen, Scotland.
“Junk food, sugary drinks, obesity, and alcohol are likely to have played a part in that rise, but our data stress the importance of avoiding unnecessary antibiotics, especially in children and young adults,” Ms. Perrott said in a statement.
“We now want to find out if there is a link between antibiotic use and changes in the microbiome which can make the colon more susceptible to cancer, especially in younger people,” added senior author Leslie Samuel, MD, consultant oncologist at Aberdeen Royal Infirmary.
“It’s a complex situation, as we know that the microbiome can quickly revert to its previous state, even when the bowel has been cleared out for a diagnostic procedure,” Dr. Samuel continued.
The research was presented on July 2 at the European Society for Medical Oncology Congress 2021.
Commenting for ESMO, Alberto Sobrero, MD, PhD, Medical Oncology Unit, Ospedale San Martino, Genoa, Italy, said that younger patients with colon cancer typically have a worse prognosis than older people because they are generally diagnosed later.
“Physicians are less likely to investigate a patient with abdominal discomfort for colon cancer if they are in their 30s than if they are in their 70s, and younger patients are not eligible for bowel cancer screening,” he explained.
However, Dr. Sobrero believes it is “too early to say if excessive use of antibiotics could be a causative factor, and we need to understand more about the possible role of the microbiome in bowel cancer before we consider the impact of antibiotics on the intestinal flora.”
The results, nevertheless, “remind us that antibiotics should not be given unless they are really needed, and we cannot exclude the possibility that unnecessary use of antibiotics may be exposing people to an increased risk of cancer,” he concluded.
Similar comments were made by Thomas Seufferlein, MD, department of internal medicine, Ulm University, Germany, who discussed the findings.
He agreed with the authors “that careful use of antibiotics is sensible and paramount” but added that more studies are needed on this suggestion of a link between antibiotic use and the observed increase in early CRC.
Study details
Previous studies have demonstrated that, in older adults, significant alterations in the structure and diversity of the gut microbiome induced by antibiotic therapy influence the development of colorectal cancer.
However, Ms. Perrott said that the impact of antibiotic use on early onset colorectal cancer has not been investigated.
The researchers therefore conducted a nested case-control study of primary care records to identify colorectal cancer cases diagnosed in Scotland between 1999 and 2011.
Patients were divided into those diagnosed before 50 years of age and those diagnosed at 50 years and older and matched with up to five healthy controls.
The study included 7,903 CRC cases, of which 5,281 were colon cancer and 2,622 rectal cancer, alongside 30,418 controls.
Among the CRC patients, 445 (5.6%) were under 50 years of age at diagnosis.
The team also analyzed antibiotic use history. Prescriptions for oral antibiotics, stratified by drug class and by anaerobic/nonanaerobic effect, were extracted, and the total antibiotic exposure period was calculated and categorized as 0, 1-15, 16-60, and >60 days.
Overall, 45% of the patients were prescribed antibiotics. Any antibiotic use was associated with a significantly increased risk of colon cancer, but this was most pronounced in patients aged less than 50 years at diagnosis.
Specifically, any antibiotic use was associated with an adjusted odds ratio of colon cancer of 1.49 (P = .018) in patients aged less than 50 years versus 1.09 (P = .029) in those aged 50 years and over.
In younger patients, the largest association between antibiotic use and colon cancer was seen in patients with a total antibiotic exposure of 1-15 days (at an adjusted odds ratio of 1.55), falling to 1.46 with 16-60 days of exposure, and no association for >60 days exposure.
No such relationship was seen in patients with colon cancer aged 50 years and over at diagnosis.
There was also no significant relationship between any antibiotic use and the occurrence of rectal cancer, at an adjusted odds ratio of 1.17 (P = .493) in those aged under 50 years at diagnosis and 1.07 (P = .698) in older patients.
The study was supported by Cancer Research UK. Ms. Perrott, Dr. Sobrero, and Dr. Samuels declared having no conflicts of interest. Dr. Seufferlien has reported relationships with Amgen, Bayer, Merck, Sanofi, Celgene, Shire, Roche, Falk Foundation, AstraZeneca, Lilly, Merck-Serono, Servier, Pierre Fabre, Cantargia, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Exposure to antibiotics appears to be associated with the development of colon cancer, particularly in younger people, and could be contributing to the increase in early-onset colorectal cancer (CRC) that is being documented, say U.K. researchers.
The team conducted a nested case-control study using data from primary care in Scotland, which involved almost 8,000 cases of CRC and over 30,000 healthy controls.
The analysis suggests that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer (but not rectal) by 49%.
“To our knowledge, this is the first study to link antibiotic use with the growing risk of early onset colon cancer, a disease which has been increasing at a rate of at least 3% per year over the last two decades,” said study presenter Sarah Perrott, a medical student at the University of Aberdeen, Scotland.
“Junk food, sugary drinks, obesity, and alcohol are likely to have played a part in that rise, but our data stress the importance of avoiding unnecessary antibiotics, especially in children and young adults,” Ms. Perrott said in a statement.
“We now want to find out if there is a link between antibiotic use and changes in the microbiome which can make the colon more susceptible to cancer, especially in younger people,” added senior author Leslie Samuel, MD, consultant oncologist at Aberdeen Royal Infirmary.
“It’s a complex situation, as we know that the microbiome can quickly revert to its previous state, even when the bowel has been cleared out for a diagnostic procedure,” Dr. Samuel continued.
The research was presented on July 2 at the European Society for Medical Oncology Congress 2021.
Commenting for ESMO, Alberto Sobrero, MD, PhD, Medical Oncology Unit, Ospedale San Martino, Genoa, Italy, said that younger patients with colon cancer typically have a worse prognosis than older people because they are generally diagnosed later.
“Physicians are less likely to investigate a patient with abdominal discomfort for colon cancer if they are in their 30s than if they are in their 70s, and younger patients are not eligible for bowel cancer screening,” he explained.
However, Dr. Sobrero believes it is “too early to say if excessive use of antibiotics could be a causative factor, and we need to understand more about the possible role of the microbiome in bowel cancer before we consider the impact of antibiotics on the intestinal flora.”
The results, nevertheless, “remind us that antibiotics should not be given unless they are really needed, and we cannot exclude the possibility that unnecessary use of antibiotics may be exposing people to an increased risk of cancer,” he concluded.
Similar comments were made by Thomas Seufferlein, MD, department of internal medicine, Ulm University, Germany, who discussed the findings.
He agreed with the authors “that careful use of antibiotics is sensible and paramount” but added that more studies are needed on this suggestion of a link between antibiotic use and the observed increase in early CRC.
Study details
Previous studies have demonstrated that, in older adults, significant alterations in the structure and diversity of the gut microbiome induced by antibiotic therapy influence the development of colorectal cancer.
However, Ms. Perrott said that the impact of antibiotic use on early onset colorectal cancer has not been investigated.
The researchers therefore conducted a nested case-control study of primary care records to identify colorectal cancer cases diagnosed in Scotland between 1999 and 2011.
Patients were divided into those diagnosed before 50 years of age and those diagnosed at 50 years and older and matched with up to five healthy controls.
The study included 7,903 CRC cases, of which 5,281 were colon cancer and 2,622 rectal cancer, alongside 30,418 controls.
Among the CRC patients, 445 (5.6%) were under 50 years of age at diagnosis.
The team also analyzed antibiotic use history. Prescriptions for oral antibiotics, stratified by drug class and by anaerobic/nonanaerobic effect, were extracted, and the total antibiotic exposure period was calculated and categorized as 0, 1-15, 16-60, and >60 days.
Overall, 45% of the patients were prescribed antibiotics. Any antibiotic use was associated with a significantly increased risk of colon cancer, but this was most pronounced in patients aged less than 50 years at diagnosis.
Specifically, any antibiotic use was associated with an adjusted odds ratio of colon cancer of 1.49 (P = .018) in patients aged less than 50 years versus 1.09 (P = .029) in those aged 50 years and over.
In younger patients, the largest association between antibiotic use and colon cancer was seen in patients with a total antibiotic exposure of 1-15 days (at an adjusted odds ratio of 1.55), falling to 1.46 with 16-60 days of exposure, and no association for >60 days exposure.
No such relationship was seen in patients with colon cancer aged 50 years and over at diagnosis.
There was also no significant relationship between any antibiotic use and the occurrence of rectal cancer, at an adjusted odds ratio of 1.17 (P = .493) in those aged under 50 years at diagnosis and 1.07 (P = .698) in older patients.
The study was supported by Cancer Research UK. Ms. Perrott, Dr. Sobrero, and Dr. Samuels declared having no conflicts of interest. Dr. Seufferlien has reported relationships with Amgen, Bayer, Merck, Sanofi, Celgene, Shire, Roche, Falk Foundation, AstraZeneca, Lilly, Merck-Serono, Servier, Pierre Fabre, Cantargia, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Exposure to antibiotics appears to be associated with the development of colon cancer, particularly in younger people, and could be contributing to the increase in early-onset colorectal cancer (CRC) that is being documented, say U.K. researchers.
The team conducted a nested case-control study using data from primary care in Scotland, which involved almost 8,000 cases of CRC and over 30,000 healthy controls.
The analysis suggests that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer (but not rectal) by 49%.
“To our knowledge, this is the first study to link antibiotic use with the growing risk of early onset colon cancer, a disease which has been increasing at a rate of at least 3% per year over the last two decades,” said study presenter Sarah Perrott, a medical student at the University of Aberdeen, Scotland.
“Junk food, sugary drinks, obesity, and alcohol are likely to have played a part in that rise, but our data stress the importance of avoiding unnecessary antibiotics, especially in children and young adults,” Ms. Perrott said in a statement.
“We now want to find out if there is a link between antibiotic use and changes in the microbiome which can make the colon more susceptible to cancer, especially in younger people,” added senior author Leslie Samuel, MD, consultant oncologist at Aberdeen Royal Infirmary.
“It’s a complex situation, as we know that the microbiome can quickly revert to its previous state, even when the bowel has been cleared out for a diagnostic procedure,” Dr. Samuel continued.
The research was presented on July 2 at the European Society for Medical Oncology Congress 2021.
Commenting for ESMO, Alberto Sobrero, MD, PhD, Medical Oncology Unit, Ospedale San Martino, Genoa, Italy, said that younger patients with colon cancer typically have a worse prognosis than older people because they are generally diagnosed later.
“Physicians are less likely to investigate a patient with abdominal discomfort for colon cancer if they are in their 30s than if they are in their 70s, and younger patients are not eligible for bowel cancer screening,” he explained.
However, Dr. Sobrero believes it is “too early to say if excessive use of antibiotics could be a causative factor, and we need to understand more about the possible role of the microbiome in bowel cancer before we consider the impact of antibiotics on the intestinal flora.”
The results, nevertheless, “remind us that antibiotics should not be given unless they are really needed, and we cannot exclude the possibility that unnecessary use of antibiotics may be exposing people to an increased risk of cancer,” he concluded.
Similar comments were made by Thomas Seufferlein, MD, department of internal medicine, Ulm University, Germany, who discussed the findings.
He agreed with the authors “that careful use of antibiotics is sensible and paramount” but added that more studies are needed on this suggestion of a link between antibiotic use and the observed increase in early CRC.
Study details
Previous studies have demonstrated that, in older adults, significant alterations in the structure and diversity of the gut microbiome induced by antibiotic therapy influence the development of colorectal cancer.
However, Ms. Perrott said that the impact of antibiotic use on early onset colorectal cancer has not been investigated.
The researchers therefore conducted a nested case-control study of primary care records to identify colorectal cancer cases diagnosed in Scotland between 1999 and 2011.
Patients were divided into those diagnosed before 50 years of age and those diagnosed at 50 years and older and matched with up to five healthy controls.
The study included 7,903 CRC cases, of which 5,281 were colon cancer and 2,622 rectal cancer, alongside 30,418 controls.
Among the CRC patients, 445 (5.6%) were under 50 years of age at diagnosis.
The team also analyzed antibiotic use history. Prescriptions for oral antibiotics, stratified by drug class and by anaerobic/nonanaerobic effect, were extracted, and the total antibiotic exposure period was calculated and categorized as 0, 1-15, 16-60, and >60 days.
Overall, 45% of the patients were prescribed antibiotics. Any antibiotic use was associated with a significantly increased risk of colon cancer, but this was most pronounced in patients aged less than 50 years at diagnosis.
Specifically, any antibiotic use was associated with an adjusted odds ratio of colon cancer of 1.49 (P = .018) in patients aged less than 50 years versus 1.09 (P = .029) in those aged 50 years and over.
In younger patients, the largest association between antibiotic use and colon cancer was seen in patients with a total antibiotic exposure of 1-15 days (at an adjusted odds ratio of 1.55), falling to 1.46 with 16-60 days of exposure, and no association for >60 days exposure.
No such relationship was seen in patients with colon cancer aged 50 years and over at diagnosis.
There was also no significant relationship between any antibiotic use and the occurrence of rectal cancer, at an adjusted odds ratio of 1.17 (P = .493) in those aged under 50 years at diagnosis and 1.07 (P = .698) in older patients.
The study was supported by Cancer Research UK. Ms. Perrott, Dr. Sobrero, and Dr. Samuels declared having no conflicts of interest. Dr. Seufferlien has reported relationships with Amgen, Bayer, Merck, Sanofi, Celgene, Shire, Roche, Falk Foundation, AstraZeneca, Lilly, Merck-Serono, Servier, Pierre Fabre, Cantargia, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Understanding the alpha hydroxy acids: Glycolic acid
. The extent of exfoliation with any of the alpha hydroxy acids depends on the type of acid, its concentration, and the pH of the preparations. Glycolic acid inhibits tyrosinase and chelates calcium ion concentration between the cells in the epidermis, which results in exfoliation of the skin.
Over-the-counter glycolic acid is available in concentrations up to 30%, and in professional products up to 70%. Clinically, glycolic acid above a concentration of 30% causes local burning, erythema, and dryness.
However, overuse of glycolic acid among consumers has increased the incidence of skin reactions and hyperpigmentation. Professional-grade products containing up to 70% glycolic acid are widely available on the Internet and without proper guidelines on use and sun avoidance, adverse events and long term scarring are becoming prevalent.
The overuse of acids and overexfoliation of the skin in patients with skin types I-IV is a growing problem as consumers are purchasing more “at-home peels,” peel pads, glow pads, and at-home exfoliation regimens. This overexfoliation of the skin and the resulting erythema induces rapid postinflammatory hyperpigmentation. Consumers then often mistakenly try to self-treat the hyperpigmentation with increasing concentrations of acids, retinols, and/or hydroquinone on top of an already compromised skin barrier, further worsening the problem. In addition, these acids increase sensitivity to UV light and increase the risk of sunburns in all skin types.
Although glycolic acids are generally safe, standardized recommendations for their use in the skin care market are necessary. More is not always better. In our clinic, we do not treat patients with postinflammatory hyperpigmentation from acids or peels with more exfoliation. We focus on repairing the barrier for 1-3 months, which includes use of gentle cleansers and occlusive moisturizers, and avoidance of acids, retinols, or scrubs, and aggressive sun protection, and then using gentle fade ingredients – such as kojic acid, licorice root extract, and vitamin C – at low concentrations to slowly decrease melanin production. Barrier repair is the first and most important step and it is often overlooked when clinicians try to lighten the skin in haste.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
. The extent of exfoliation with any of the alpha hydroxy acids depends on the type of acid, its concentration, and the pH of the preparations. Glycolic acid inhibits tyrosinase and chelates calcium ion concentration between the cells in the epidermis, which results in exfoliation of the skin.
Over-the-counter glycolic acid is available in concentrations up to 30%, and in professional products up to 70%. Clinically, glycolic acid above a concentration of 30% causes local burning, erythema, and dryness.
However, overuse of glycolic acid among consumers has increased the incidence of skin reactions and hyperpigmentation. Professional-grade products containing up to 70% glycolic acid are widely available on the Internet and without proper guidelines on use and sun avoidance, adverse events and long term scarring are becoming prevalent.
The overuse of acids and overexfoliation of the skin in patients with skin types I-IV is a growing problem as consumers are purchasing more “at-home peels,” peel pads, glow pads, and at-home exfoliation regimens. This overexfoliation of the skin and the resulting erythema induces rapid postinflammatory hyperpigmentation. Consumers then often mistakenly try to self-treat the hyperpigmentation with increasing concentrations of acids, retinols, and/or hydroquinone on top of an already compromised skin barrier, further worsening the problem. In addition, these acids increase sensitivity to UV light and increase the risk of sunburns in all skin types.
Although glycolic acids are generally safe, standardized recommendations for their use in the skin care market are necessary. More is not always better. In our clinic, we do not treat patients with postinflammatory hyperpigmentation from acids or peels with more exfoliation. We focus on repairing the barrier for 1-3 months, which includes use of gentle cleansers and occlusive moisturizers, and avoidance of acids, retinols, or scrubs, and aggressive sun protection, and then using gentle fade ingredients – such as kojic acid, licorice root extract, and vitamin C – at low concentrations to slowly decrease melanin production. Barrier repair is the first and most important step and it is often overlooked when clinicians try to lighten the skin in haste.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
. The extent of exfoliation with any of the alpha hydroxy acids depends on the type of acid, its concentration, and the pH of the preparations. Glycolic acid inhibits tyrosinase and chelates calcium ion concentration between the cells in the epidermis, which results in exfoliation of the skin.
Over-the-counter glycolic acid is available in concentrations up to 30%, and in professional products up to 70%. Clinically, glycolic acid above a concentration of 30% causes local burning, erythema, and dryness.
However, overuse of glycolic acid among consumers has increased the incidence of skin reactions and hyperpigmentation. Professional-grade products containing up to 70% glycolic acid are widely available on the Internet and without proper guidelines on use and sun avoidance, adverse events and long term scarring are becoming prevalent.
The overuse of acids and overexfoliation of the skin in patients with skin types I-IV is a growing problem as consumers are purchasing more “at-home peels,” peel pads, glow pads, and at-home exfoliation regimens. This overexfoliation of the skin and the resulting erythema induces rapid postinflammatory hyperpigmentation. Consumers then often mistakenly try to self-treat the hyperpigmentation with increasing concentrations of acids, retinols, and/or hydroquinone on top of an already compromised skin barrier, further worsening the problem. In addition, these acids increase sensitivity to UV light and increase the risk of sunburns in all skin types.
Although glycolic acids are generally safe, standardized recommendations for their use in the skin care market are necessary. More is not always better. In our clinic, we do not treat patients with postinflammatory hyperpigmentation from acids or peels with more exfoliation. We focus on repairing the barrier for 1-3 months, which includes use of gentle cleansers and occlusive moisturizers, and avoidance of acids, retinols, or scrubs, and aggressive sun protection, and then using gentle fade ingredients – such as kojic acid, licorice root extract, and vitamin C – at low concentrations to slowly decrease melanin production. Barrier repair is the first and most important step and it is often overlooked when clinicians try to lighten the skin in haste.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Etanercept-Induced Squamous Proliferations in a Patient With Porokeratosis
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
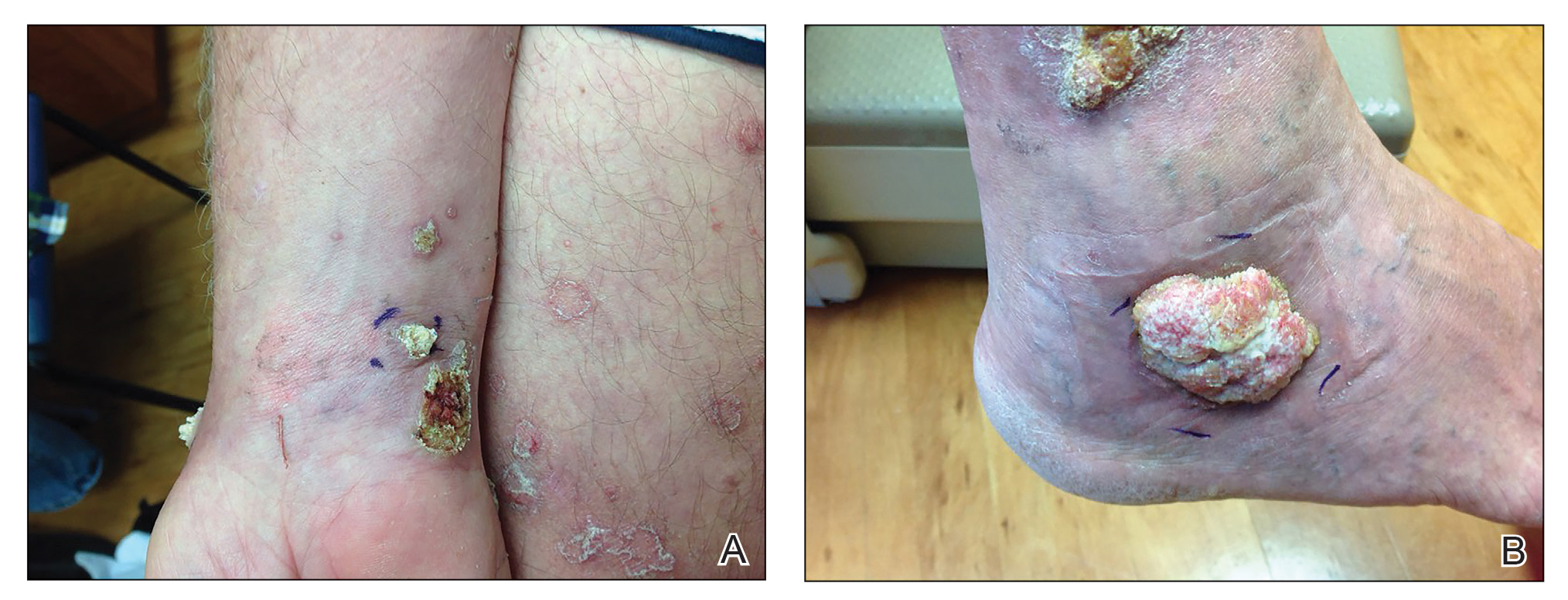
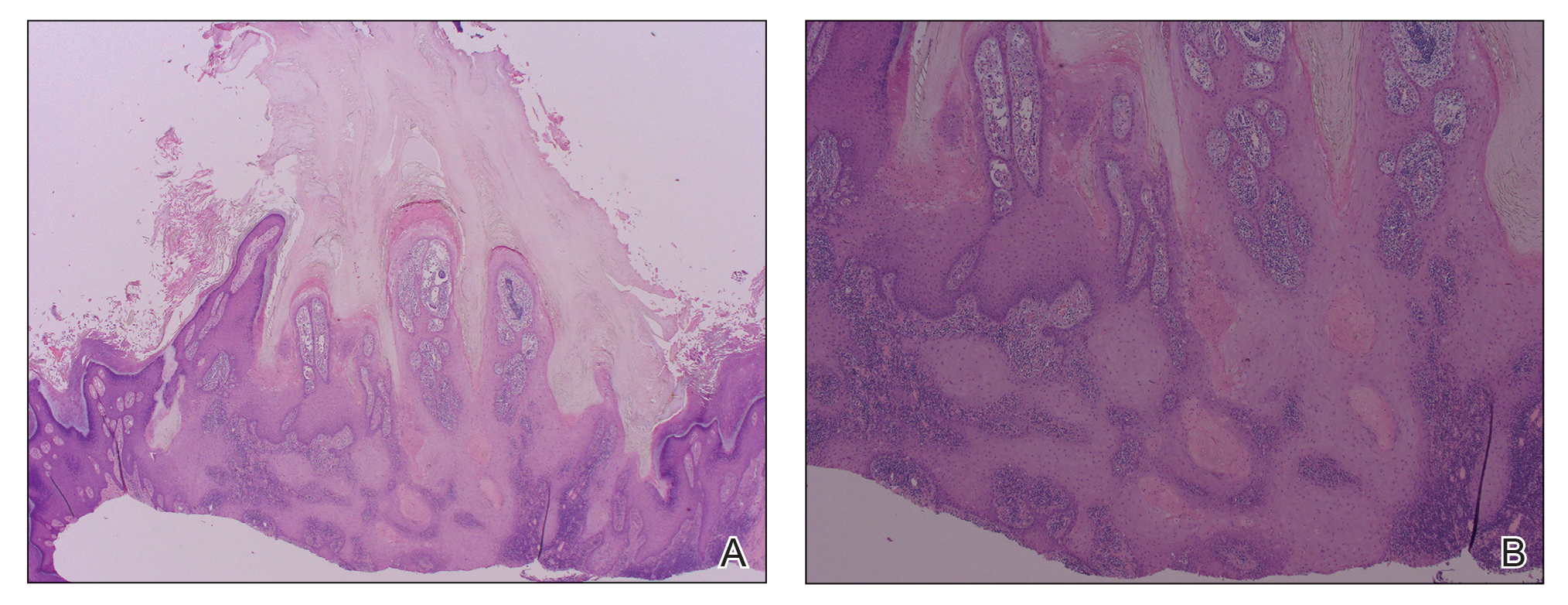
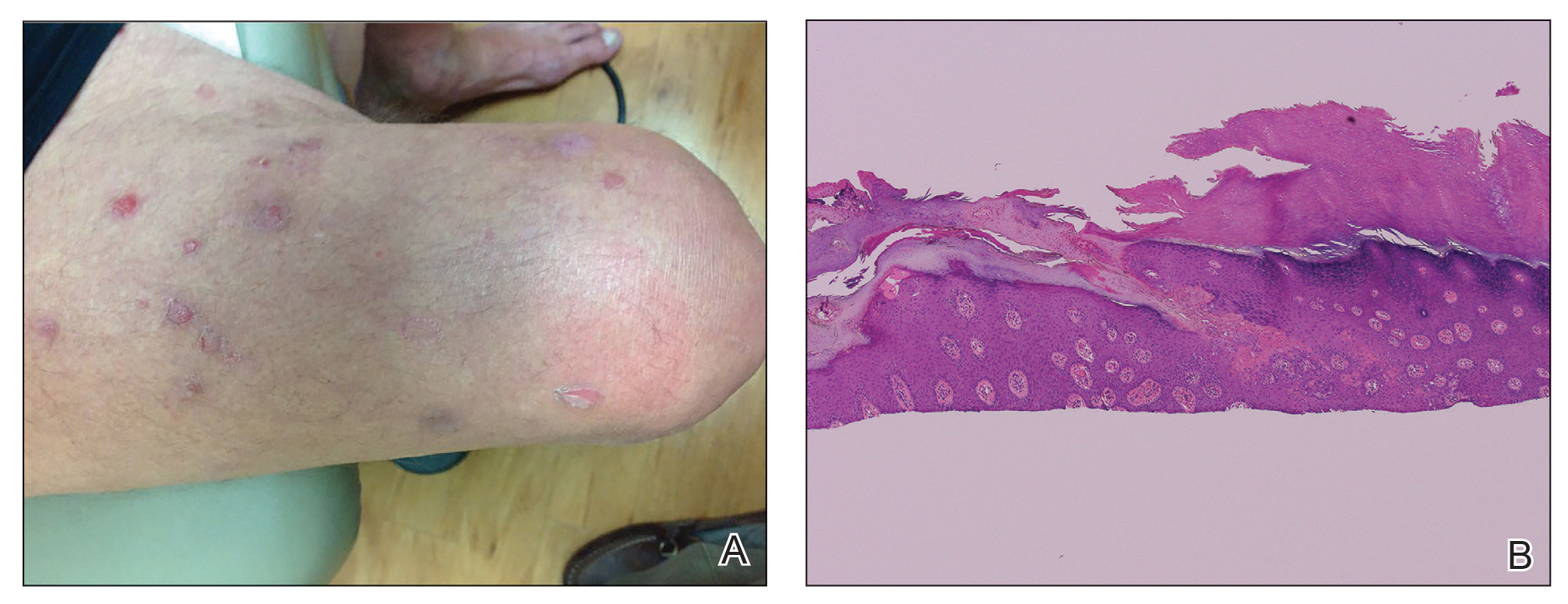
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).



The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).



The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
Practice Points
- The use of biologics, particularly tumor necrosis factor α blockers, rarely are reported to induce skin cancer.
- Squamous cell carcinoma in the setting of biologic treatment would warrant a change of systemic medication.
The case for suicide-risk screening in primary care
Suicide-risk screening may identify cases that typically fall through the cracks during depression screening, new research suggests.
The study, published in Pediatrics, found that the Ask Suicide-Screening Questions (ASQ) identified 2.2% of additional cases compared with those screened for any type of depression or other mental illnesses, and 8.3% of additional cases compared with those who screened positive for major depressive disorder.
About 3.2% of U.S. children between the ages of 3 and 17 have been diagnosed with depression, according to the Centers for Disease Control and Prevention. The American Academy of Pediatrics and the U.S. Preventive Services Task Force recommends that all teens be routinely screened for depression. However, there’s no specific recommendation that adolescents should also be screened for suicide in addition to depression screening.
The study highlights the high baseline rates of depression and suicide risk and the need for pediatric practices to plan for them and develop strategies about how they’re going to provide follow-up care, including treatment for suicidal teens.
“We began this project because we were concerned that we might be missing teens with increased risk of suicide by screening only for depression,” study author Alex Kemper, MD, said in an interview. “Our goal with this project was really to compare standard depression screening tools that we’ve used for a long time with a suicide-specific instrument just to see if we would identify additional cases with a suicide-risk instrument.”
Dr. Kemper and colleagues collected data from 803 mostly Medicaid-enrolled adolescents across 12 primary care practices. The subjects were between the ages of 12 and 20 years, with no recent history of depression or self-harm, who were screened with the Patient Health Questionnaire–9 Modified for Adolescents (PHQ-9A) and ASQ. For the study, two PHQ-9A screening strategies were evaluated: screening for any type of depression or other mental illness (positive on any item) or screening for major depressive disorder.
In addition, the researchers found that 56.4% of patients had a positive PHQ-9A screen for any type of depression and 24.7% had a positive PHQ-9A screen for major depressive disorder. Meanwhile, 21.1% of the population received a positive screen result. Of those who responded on the PHQ-9A that they did not have suicidal thoughts in the past month, 13.2% had a positive ASQ result.
Dr. Kemper, division chief of primary care pediatrics at Nationwide Children’s Hospital and professor of pediatrics at the Ohio State University, both in Columbus, said the suicide-risk screening questions were more direct and clear than were the two suicide questions included in the PHQ-9A screening.
For example, the PHQ-9A includes the following suicide-risk questions: “Has there been a time in the past month when you have had serious thoughts about ending your life?” and “Have you EVER, in your WHOLE LIFE, tried to kill yourself or made a suicide attempt?” The teen can respond with “not at all,” “several days,” “more than half the days” or “nearly every day.”
Meanwhile, the ASQ questionnaire focuses on a more narrow time period and includes questions such as “In the past few weeks, have you wished you were dead?” and “Have you ever tried to kill yourself?” Teens respond by answering “yes” or “no.”
“So I think the difference is by asking questions that are really direct and very clear about suicide risk, you end up identifying more cases than this kind of general question about thoughts of killing yourself,” Dr. Kemper explained. “It makes sense when you think about where adolescents are in terms of their development, that the more specific you [are], the more likely you are to find what you’re looking for.”
Kelly Curran, MD, who was not involved in the study, said that because some of the ASQ questions “overlap” with the suicide-risk questions on the PHQ-9A, she didn’t expect the ASQ to identify more positive cases.
However, Dr. Curran said it is possible for suicidal teens to fall through the cracks during a depression screening because some of them may not self-identify as depressed.
“I don’t think we often think about the importance of linguistics or how something is asked,” said Dr. Curran, associate professor in the department of pediatrics at the University of Oklahoma, Oklahoma City.
“So asking [teens] these kind of direct questions about suicide may pick up on these cases of people who don’t necessarily have the insight into their sadness or their general kind of thought process.”
Dr. Kemper said he hopes the study would encourage pediatricians to adopt depression screening if they’re not already doing it and to think about whether they should implement suicide-risk screening in their practice. The study also highlights the importance of following up after a positive screening.
“There are a lot of teens who have depression or increased suicide risk that you wouldn’t identify if you didn’t screen, and a key aspect of any kind of screening is that you need to be prepared to provide follow-up care after a positive screening,” he explained.
Study limitations include the fact that the subjects were recruited from a single health care system that serves mostly urban and low-income communities, and that the study was not designed to determine test accuracy.
Dr. Kemper and Dr. Curran indicated that they have no financial disclosures.
Suicide-risk screening may identify cases that typically fall through the cracks during depression screening, new research suggests.
The study, published in Pediatrics, found that the Ask Suicide-Screening Questions (ASQ) identified 2.2% of additional cases compared with those screened for any type of depression or other mental illnesses, and 8.3% of additional cases compared with those who screened positive for major depressive disorder.
About 3.2% of U.S. children between the ages of 3 and 17 have been diagnosed with depression, according to the Centers for Disease Control and Prevention. The American Academy of Pediatrics and the U.S. Preventive Services Task Force recommends that all teens be routinely screened for depression. However, there’s no specific recommendation that adolescents should also be screened for suicide in addition to depression screening.
The study highlights the high baseline rates of depression and suicide risk and the need for pediatric practices to plan for them and develop strategies about how they’re going to provide follow-up care, including treatment for suicidal teens.
“We began this project because we were concerned that we might be missing teens with increased risk of suicide by screening only for depression,” study author Alex Kemper, MD, said in an interview. “Our goal with this project was really to compare standard depression screening tools that we’ve used for a long time with a suicide-specific instrument just to see if we would identify additional cases with a suicide-risk instrument.”
Dr. Kemper and colleagues collected data from 803 mostly Medicaid-enrolled adolescents across 12 primary care practices. The subjects were between the ages of 12 and 20 years, with no recent history of depression or self-harm, who were screened with the Patient Health Questionnaire–9 Modified for Adolescents (PHQ-9A) and ASQ. For the study, two PHQ-9A screening strategies were evaluated: screening for any type of depression or other mental illness (positive on any item) or screening for major depressive disorder.
In addition, the researchers found that 56.4% of patients had a positive PHQ-9A screen for any type of depression and 24.7% had a positive PHQ-9A screen for major depressive disorder. Meanwhile, 21.1% of the population received a positive screen result. Of those who responded on the PHQ-9A that they did not have suicidal thoughts in the past month, 13.2% had a positive ASQ result.
Dr. Kemper, division chief of primary care pediatrics at Nationwide Children’s Hospital and professor of pediatrics at the Ohio State University, both in Columbus, said the suicide-risk screening questions were more direct and clear than were the two suicide questions included in the PHQ-9A screening.
For example, the PHQ-9A includes the following suicide-risk questions: “Has there been a time in the past month when you have had serious thoughts about ending your life?” and “Have you EVER, in your WHOLE LIFE, tried to kill yourself or made a suicide attempt?” The teen can respond with “not at all,” “several days,” “more than half the days” or “nearly every day.”
Meanwhile, the ASQ questionnaire focuses on a more narrow time period and includes questions such as “In the past few weeks, have you wished you were dead?” and “Have you ever tried to kill yourself?” Teens respond by answering “yes” or “no.”
“So I think the difference is by asking questions that are really direct and very clear about suicide risk, you end up identifying more cases than this kind of general question about thoughts of killing yourself,” Dr. Kemper explained. “It makes sense when you think about where adolescents are in terms of their development, that the more specific you [are], the more likely you are to find what you’re looking for.”
Kelly Curran, MD, who was not involved in the study, said that because some of the ASQ questions “overlap” with the suicide-risk questions on the PHQ-9A, she didn’t expect the ASQ to identify more positive cases.
However, Dr. Curran said it is possible for suicidal teens to fall through the cracks during a depression screening because some of them may not self-identify as depressed.
“I don’t think we often think about the importance of linguistics or how something is asked,” said Dr. Curran, associate professor in the department of pediatrics at the University of Oklahoma, Oklahoma City.
“So asking [teens] these kind of direct questions about suicide may pick up on these cases of people who don’t necessarily have the insight into their sadness or their general kind of thought process.”
Dr. Kemper said he hopes the study would encourage pediatricians to adopt depression screening if they’re not already doing it and to think about whether they should implement suicide-risk screening in their practice. The study also highlights the importance of following up after a positive screening.
“There are a lot of teens who have depression or increased suicide risk that you wouldn’t identify if you didn’t screen, and a key aspect of any kind of screening is that you need to be prepared to provide follow-up care after a positive screening,” he explained.
Study limitations include the fact that the subjects were recruited from a single health care system that serves mostly urban and low-income communities, and that the study was not designed to determine test accuracy.
Dr. Kemper and Dr. Curran indicated that they have no financial disclosures.
Suicide-risk screening may identify cases that typically fall through the cracks during depression screening, new research suggests.
The study, published in Pediatrics, found that the Ask Suicide-Screening Questions (ASQ) identified 2.2% of additional cases compared with those screened for any type of depression or other mental illnesses, and 8.3% of additional cases compared with those who screened positive for major depressive disorder.
About 3.2% of U.S. children between the ages of 3 and 17 have been diagnosed with depression, according to the Centers for Disease Control and Prevention. The American Academy of Pediatrics and the U.S. Preventive Services Task Force recommends that all teens be routinely screened for depression. However, there’s no specific recommendation that adolescents should also be screened for suicide in addition to depression screening.
The study highlights the high baseline rates of depression and suicide risk and the need for pediatric practices to plan for them and develop strategies about how they’re going to provide follow-up care, including treatment for suicidal teens.
“We began this project because we were concerned that we might be missing teens with increased risk of suicide by screening only for depression,” study author Alex Kemper, MD, said in an interview. “Our goal with this project was really to compare standard depression screening tools that we’ve used for a long time with a suicide-specific instrument just to see if we would identify additional cases with a suicide-risk instrument.”
Dr. Kemper and colleagues collected data from 803 mostly Medicaid-enrolled adolescents across 12 primary care practices. The subjects were between the ages of 12 and 20 years, with no recent history of depression or self-harm, who were screened with the Patient Health Questionnaire–9 Modified for Adolescents (PHQ-9A) and ASQ. For the study, two PHQ-9A screening strategies were evaluated: screening for any type of depression or other mental illness (positive on any item) or screening for major depressive disorder.
In addition, the researchers found that 56.4% of patients had a positive PHQ-9A screen for any type of depression and 24.7% had a positive PHQ-9A screen for major depressive disorder. Meanwhile, 21.1% of the population received a positive screen result. Of those who responded on the PHQ-9A that they did not have suicidal thoughts in the past month, 13.2% had a positive ASQ result.
Dr. Kemper, division chief of primary care pediatrics at Nationwide Children’s Hospital and professor of pediatrics at the Ohio State University, both in Columbus, said the suicide-risk screening questions were more direct and clear than were the two suicide questions included in the PHQ-9A screening.
For example, the PHQ-9A includes the following suicide-risk questions: “Has there been a time in the past month when you have had serious thoughts about ending your life?” and “Have you EVER, in your WHOLE LIFE, tried to kill yourself or made a suicide attempt?” The teen can respond with “not at all,” “several days,” “more than half the days” or “nearly every day.”
Meanwhile, the ASQ questionnaire focuses on a more narrow time period and includes questions such as “In the past few weeks, have you wished you were dead?” and “Have you ever tried to kill yourself?” Teens respond by answering “yes” or “no.”
“So I think the difference is by asking questions that are really direct and very clear about suicide risk, you end up identifying more cases than this kind of general question about thoughts of killing yourself,” Dr. Kemper explained. “It makes sense when you think about where adolescents are in terms of their development, that the more specific you [are], the more likely you are to find what you’re looking for.”
Kelly Curran, MD, who was not involved in the study, said that because some of the ASQ questions “overlap” with the suicide-risk questions on the PHQ-9A, she didn’t expect the ASQ to identify more positive cases.
However, Dr. Curran said it is possible for suicidal teens to fall through the cracks during a depression screening because some of them may not self-identify as depressed.
“I don’t think we often think about the importance of linguistics or how something is asked,” said Dr. Curran, associate professor in the department of pediatrics at the University of Oklahoma, Oklahoma City.
“So asking [teens] these kind of direct questions about suicide may pick up on these cases of people who don’t necessarily have the insight into their sadness or their general kind of thought process.”
Dr. Kemper said he hopes the study would encourage pediatricians to adopt depression screening if they’re not already doing it and to think about whether they should implement suicide-risk screening in their practice. The study also highlights the importance of following up after a positive screening.
“There are a lot of teens who have depression or increased suicide risk that you wouldn’t identify if you didn’t screen, and a key aspect of any kind of screening is that you need to be prepared to provide follow-up care after a positive screening,” he explained.
Study limitations include the fact that the subjects were recruited from a single health care system that serves mostly urban and low-income communities, and that the study was not designed to determine test accuracy.
Dr. Kemper and Dr. Curran indicated that they have no financial disclosures.