User login
Transgender individuals twice as likely to die as general population
Mortality is consistently twice as high in transgender people receiving hormone treatment, compared with cisgender individuals in the general population and has not decreased over time, results of a 5 decades–long study from the Netherlands indicate.
Particularly concerning is that trans women (male to female) had a mortality risk nearly double that of cis men (born and remain male) in the general Dutch population (standardized mortality ratio, 1.8), while it was nearly triple that of cis women (SMR, 2.8).
Compared with cisgender women, transgender women were more than twice as likely to die from heart disease, three times more likely to die from lung cancer, and almost nine times more likely to die from infection. HIV-related disease mortality risk was nearly 50 times higher for trans women than cis women, and the risk of suicide was almost seven times greater.
Suicide and other nonnatural causes of death were more common in trans men, compared with cis women.
The report, by Christel J.M. de Blok, MD, of Amsterdam University Medical Center and colleagues, was published online Sept. 2 in The Lancet Diabetes & Endocrinology.
The study included trans men who received testosterone to transition from female to male and trans women who received estrogen plus an antiandrogen to transition from male to female.
Is gender-affirming hormone therapy associated with increased mortality?
Senior author Martin den Heijer, MD, also of Amsterdam University Medical Center, said: “The findings of our large, nationwide study highlight a substantially increased mortality risk among transgender people that has persisted for decades.”
But he pointed out that, overall, the data do not appear to suggest the premature deaths were related to gender-affirming hormone treatment.
However, he conceded that more work is needed on this aspect of care. “There is insufficient evidence at present to determine long-term safety of [gender-affirming hormone treatment]. More research is needed to fully establish whether it in any way affects mortality risk for transgender people,” said Dr. den Heijer.
Endocrinologist Will Malone, MD, of Twin Falls, Idaho, told this news organization, “The study confirms, like others before it, that individuals taking cross-sex hormones are more likely to die prematurely from a number of causes.”
“While the authors speculate that this higher mortality rate is not connected to cross-sex hormones, the study was not designed to be able to make such a claim,” he said, pointing to limited follow-up times.
In an accompanying commentary, Vin Tangpricha, MD, PhD, an endocrinologist from Emory University, Atlanta, noted: “Transgender men do not appear to have as significantly increased comorbidity following receipt of gender-affirming hormone therapy when compared with transgender women.”
Dr. Tangpricha added future studies should examine which factors – hormone regimen, hormone concentrations, access to health care, or other biological factors – explain the higher increased risk of morbidity and mortality observed in trans women as opposed to trans men.
However, Dr. de Blok and colleagues note that, as there were relatively few deaths among transgender men in the cohort, analysis on cause of death in this group is limited.
Transgender individuals more likely to die younger
For their study, Dutch researchers retrospectively examined data from 4,568 transgender people attending their clinic (2,927 transgender women and 1,641 transgender men) treated in 1972-2018. People were excluded if they started treatment before the age of 17 or if they had received puberty-blocking drugs.
Data on age at start of hormone treatment, type of treatment, smoking habits, medical history, and last date of follow-up were gathered from medical records. Where possible, SMRs were determined for deaths among trans men and trans women, compared with rates for the adult Dutch general population.
Median age at the start of cross-sex hormone treatment was 30 years in transgender women and 23 years in transgender men. But the median follow-up time was only 11 years in transgender women and 5 years in transgender men.
A total of 317 (10.8%) trans women died, and 44 (2.7%) trans men died. The findings were higher than expected, compared with the general population of cisgender women (SMR, 1.8) but not cisgender men (SMR 1.2).
Mortality risk did increase more in transgender people who started gender-affirming hormone treatment in the past 2 decades compared with earlier, a fact that Dr. de Blok said was surprising.
Trans men, for example, compared with cis women, had an SMR of 2.1-2.4 in 2000-2018 (compared with 1.8 overall).
“This may be due to changes in clinical practice. ... In the past, health care providers were reluctant to provide hormone treatment to people with a history of comorbidities such as cardiovascular disease. However, because of the many benefits of enabling people to access hormone therapy, nowadays this rarely results in treatment being denied,” Dr. de Blok noted.
More research needed, especially in trans-identifying youth
Dr. Malone remarked that previous studies have shown associations between taking cross-sex hormones and elevated mortality, while also “not designed to detect causality,” have “generally accepted that natal males who take estrogen have estrogen-related increases in the rates of heart disease, stroke, and deep venous thrombosis.”
He added that the risks of testosterone use in natal females were less well established, “but testosterone is also felt to increase their risk of heart disease.”
He stressed the limited follow-up times in the study by Dr. de Blok and colleagues.
This “strongly suggests that the rate of elevated mortality far exceeds the doubling measured by the study, especially for natal females.”
Dr. Malone is one of several clinicians and researchers who has formed the Society for Evidence-Based Gender Medicine, a nonprofit organization that now has at least 100 physician members. SEGM is concerned about the lack of quality evidence for the use of hormonal and surgical interventions as first-line treatment, especially for young people with gender dysphoria.
Dr. Tangpricha also highlighted that the findings do not apply to transgender people who began treatment before age 17 years or those who had taken puberty blockers before gender-affirming hormone treatment.
There are no long-term data on transgender individuals who have received gender-affirming hormone therapies close to the time of puberty.
These data, such as those from the Trans Youth Care study, should be available in the future, he added.
The authors have reported no relevant financial relationships. Dr. Tangpricha has reported receiving funding from the National Institutes of Health and served as past president of the World Professional Association for Transgender Health. He is editor-in-chief of Endocrine Practice and has provided expert testimony for Kirkland and Ellis.
A version of this article first appeared on Medscape.com.
Mortality is consistently twice as high in transgender people receiving hormone treatment, compared with cisgender individuals in the general population and has not decreased over time, results of a 5 decades–long study from the Netherlands indicate.
Particularly concerning is that trans women (male to female) had a mortality risk nearly double that of cis men (born and remain male) in the general Dutch population (standardized mortality ratio, 1.8), while it was nearly triple that of cis women (SMR, 2.8).
Compared with cisgender women, transgender women were more than twice as likely to die from heart disease, three times more likely to die from lung cancer, and almost nine times more likely to die from infection. HIV-related disease mortality risk was nearly 50 times higher for trans women than cis women, and the risk of suicide was almost seven times greater.
Suicide and other nonnatural causes of death were more common in trans men, compared with cis women.
The report, by Christel J.M. de Blok, MD, of Amsterdam University Medical Center and colleagues, was published online Sept. 2 in The Lancet Diabetes & Endocrinology.
The study included trans men who received testosterone to transition from female to male and trans women who received estrogen plus an antiandrogen to transition from male to female.
Is gender-affirming hormone therapy associated with increased mortality?
Senior author Martin den Heijer, MD, also of Amsterdam University Medical Center, said: “The findings of our large, nationwide study highlight a substantially increased mortality risk among transgender people that has persisted for decades.”
But he pointed out that, overall, the data do not appear to suggest the premature deaths were related to gender-affirming hormone treatment.
However, he conceded that more work is needed on this aspect of care. “There is insufficient evidence at present to determine long-term safety of [gender-affirming hormone treatment]. More research is needed to fully establish whether it in any way affects mortality risk for transgender people,” said Dr. den Heijer.
Endocrinologist Will Malone, MD, of Twin Falls, Idaho, told this news organization, “The study confirms, like others before it, that individuals taking cross-sex hormones are more likely to die prematurely from a number of causes.”
“While the authors speculate that this higher mortality rate is not connected to cross-sex hormones, the study was not designed to be able to make such a claim,” he said, pointing to limited follow-up times.
In an accompanying commentary, Vin Tangpricha, MD, PhD, an endocrinologist from Emory University, Atlanta, noted: “Transgender men do not appear to have as significantly increased comorbidity following receipt of gender-affirming hormone therapy when compared with transgender women.”
Dr. Tangpricha added future studies should examine which factors – hormone regimen, hormone concentrations, access to health care, or other biological factors – explain the higher increased risk of morbidity and mortality observed in trans women as opposed to trans men.
However, Dr. de Blok and colleagues note that, as there were relatively few deaths among transgender men in the cohort, analysis on cause of death in this group is limited.
Transgender individuals more likely to die younger
For their study, Dutch researchers retrospectively examined data from 4,568 transgender people attending their clinic (2,927 transgender women and 1,641 transgender men) treated in 1972-2018. People were excluded if they started treatment before the age of 17 or if they had received puberty-blocking drugs.
Data on age at start of hormone treatment, type of treatment, smoking habits, medical history, and last date of follow-up were gathered from medical records. Where possible, SMRs were determined for deaths among trans men and trans women, compared with rates for the adult Dutch general population.
Median age at the start of cross-sex hormone treatment was 30 years in transgender women and 23 years in transgender men. But the median follow-up time was only 11 years in transgender women and 5 years in transgender men.
A total of 317 (10.8%) trans women died, and 44 (2.7%) trans men died. The findings were higher than expected, compared with the general population of cisgender women (SMR, 1.8) but not cisgender men (SMR 1.2).
Mortality risk did increase more in transgender people who started gender-affirming hormone treatment in the past 2 decades compared with earlier, a fact that Dr. de Blok said was surprising.
Trans men, for example, compared with cis women, had an SMR of 2.1-2.4 in 2000-2018 (compared with 1.8 overall).
“This may be due to changes in clinical practice. ... In the past, health care providers were reluctant to provide hormone treatment to people with a history of comorbidities such as cardiovascular disease. However, because of the many benefits of enabling people to access hormone therapy, nowadays this rarely results in treatment being denied,” Dr. de Blok noted.
More research needed, especially in trans-identifying youth
Dr. Malone remarked that previous studies have shown associations between taking cross-sex hormones and elevated mortality, while also “not designed to detect causality,” have “generally accepted that natal males who take estrogen have estrogen-related increases in the rates of heart disease, stroke, and deep venous thrombosis.”
He added that the risks of testosterone use in natal females were less well established, “but testosterone is also felt to increase their risk of heart disease.”
He stressed the limited follow-up times in the study by Dr. de Blok and colleagues.
This “strongly suggests that the rate of elevated mortality far exceeds the doubling measured by the study, especially for natal females.”
Dr. Malone is one of several clinicians and researchers who has formed the Society for Evidence-Based Gender Medicine, a nonprofit organization that now has at least 100 physician members. SEGM is concerned about the lack of quality evidence for the use of hormonal and surgical interventions as first-line treatment, especially for young people with gender dysphoria.
Dr. Tangpricha also highlighted that the findings do not apply to transgender people who began treatment before age 17 years or those who had taken puberty blockers before gender-affirming hormone treatment.
There are no long-term data on transgender individuals who have received gender-affirming hormone therapies close to the time of puberty.
These data, such as those from the Trans Youth Care study, should be available in the future, he added.
The authors have reported no relevant financial relationships. Dr. Tangpricha has reported receiving funding from the National Institutes of Health and served as past president of the World Professional Association for Transgender Health. He is editor-in-chief of Endocrine Practice and has provided expert testimony for Kirkland and Ellis.
A version of this article first appeared on Medscape.com.
Mortality is consistently twice as high in transgender people receiving hormone treatment, compared with cisgender individuals in the general population and has not decreased over time, results of a 5 decades–long study from the Netherlands indicate.
Particularly concerning is that trans women (male to female) had a mortality risk nearly double that of cis men (born and remain male) in the general Dutch population (standardized mortality ratio, 1.8), while it was nearly triple that of cis women (SMR, 2.8).
Compared with cisgender women, transgender women were more than twice as likely to die from heart disease, three times more likely to die from lung cancer, and almost nine times more likely to die from infection. HIV-related disease mortality risk was nearly 50 times higher for trans women than cis women, and the risk of suicide was almost seven times greater.
Suicide and other nonnatural causes of death were more common in trans men, compared with cis women.
The report, by Christel J.M. de Blok, MD, of Amsterdam University Medical Center and colleagues, was published online Sept. 2 in The Lancet Diabetes & Endocrinology.
The study included trans men who received testosterone to transition from female to male and trans women who received estrogen plus an antiandrogen to transition from male to female.
Is gender-affirming hormone therapy associated with increased mortality?
Senior author Martin den Heijer, MD, also of Amsterdam University Medical Center, said: “The findings of our large, nationwide study highlight a substantially increased mortality risk among transgender people that has persisted for decades.”
But he pointed out that, overall, the data do not appear to suggest the premature deaths were related to gender-affirming hormone treatment.
However, he conceded that more work is needed on this aspect of care. “There is insufficient evidence at present to determine long-term safety of [gender-affirming hormone treatment]. More research is needed to fully establish whether it in any way affects mortality risk for transgender people,” said Dr. den Heijer.
Endocrinologist Will Malone, MD, of Twin Falls, Idaho, told this news organization, “The study confirms, like others before it, that individuals taking cross-sex hormones are more likely to die prematurely from a number of causes.”
“While the authors speculate that this higher mortality rate is not connected to cross-sex hormones, the study was not designed to be able to make such a claim,” he said, pointing to limited follow-up times.
In an accompanying commentary, Vin Tangpricha, MD, PhD, an endocrinologist from Emory University, Atlanta, noted: “Transgender men do not appear to have as significantly increased comorbidity following receipt of gender-affirming hormone therapy when compared with transgender women.”
Dr. Tangpricha added future studies should examine which factors – hormone regimen, hormone concentrations, access to health care, or other biological factors – explain the higher increased risk of morbidity and mortality observed in trans women as opposed to trans men.
However, Dr. de Blok and colleagues note that, as there were relatively few deaths among transgender men in the cohort, analysis on cause of death in this group is limited.
Transgender individuals more likely to die younger
For their study, Dutch researchers retrospectively examined data from 4,568 transgender people attending their clinic (2,927 transgender women and 1,641 transgender men) treated in 1972-2018. People were excluded if they started treatment before the age of 17 or if they had received puberty-blocking drugs.
Data on age at start of hormone treatment, type of treatment, smoking habits, medical history, and last date of follow-up were gathered from medical records. Where possible, SMRs were determined for deaths among trans men and trans women, compared with rates for the adult Dutch general population.
Median age at the start of cross-sex hormone treatment was 30 years in transgender women and 23 years in transgender men. But the median follow-up time was only 11 years in transgender women and 5 years in transgender men.
A total of 317 (10.8%) trans women died, and 44 (2.7%) trans men died. The findings were higher than expected, compared with the general population of cisgender women (SMR, 1.8) but not cisgender men (SMR 1.2).
Mortality risk did increase more in transgender people who started gender-affirming hormone treatment in the past 2 decades compared with earlier, a fact that Dr. de Blok said was surprising.
Trans men, for example, compared with cis women, had an SMR of 2.1-2.4 in 2000-2018 (compared with 1.8 overall).
“This may be due to changes in clinical practice. ... In the past, health care providers were reluctant to provide hormone treatment to people with a history of comorbidities such as cardiovascular disease. However, because of the many benefits of enabling people to access hormone therapy, nowadays this rarely results in treatment being denied,” Dr. de Blok noted.
More research needed, especially in trans-identifying youth
Dr. Malone remarked that previous studies have shown associations between taking cross-sex hormones and elevated mortality, while also “not designed to detect causality,” have “generally accepted that natal males who take estrogen have estrogen-related increases in the rates of heart disease, stroke, and deep venous thrombosis.”
He added that the risks of testosterone use in natal females were less well established, “but testosterone is also felt to increase their risk of heart disease.”
He stressed the limited follow-up times in the study by Dr. de Blok and colleagues.
This “strongly suggests that the rate of elevated mortality far exceeds the doubling measured by the study, especially for natal females.”
Dr. Malone is one of several clinicians and researchers who has formed the Society for Evidence-Based Gender Medicine, a nonprofit organization that now has at least 100 physician members. SEGM is concerned about the lack of quality evidence for the use of hormonal and surgical interventions as first-line treatment, especially for young people with gender dysphoria.
Dr. Tangpricha also highlighted that the findings do not apply to transgender people who began treatment before age 17 years or those who had taken puberty blockers before gender-affirming hormone treatment.
There are no long-term data on transgender individuals who have received gender-affirming hormone therapies close to the time of puberty.
These data, such as those from the Trans Youth Care study, should be available in the future, he added.
The authors have reported no relevant financial relationships. Dr. Tangpricha has reported receiving funding from the National Institutes of Health and served as past president of the World Professional Association for Transgender Health. He is editor-in-chief of Endocrine Practice and has provided expert testimony for Kirkland and Ellis.
A version of this article first appeared on Medscape.com.
2021 Update on pelvic floor disorders
With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.

With the increasing prevalence of pelvic floor disorders among our aging population, women’s health clinicians should be prepared to counsel patients on treatment options and posttreatment expectations. In this Update, we will review recent literature on surgical treatments for pelvic organ prolapse (POP) and stress urinary incontinence (SUI). We also include our review of an award-winning and practice-changing study on office-based pessary care. Lastly, we will finish with a summary of a recent Society of Gynecologic Surgeons collaborative systematic review on sexual function after surgery.
5-year RCT data on hysteropexy vs hysterectomy for POP
Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153. e1-153.e31. doi: 10.1016/j.ajog.2021.03.012.
The Pelvic Floor Disorders Network conducted a multisite randomized superiority trial comparing sacrospinous hysteropexy with mesh graft to vaginal hysterectomy with uterosacral ligament suspension for POP.
Study details
Postmenopausal women who desired surgery for symptomatic uterovaginal prolapse were randomly assigned to sacrospinous hysteropexy with polypropylene mesh graft using the Uphold-LITE device (Boston Scientific) versus vaginal hysterectomy with uterosacral ligament suspension. Participants were masked to treatment allocation and completed study visits at 6-month intervals through 60 months. Quantitative prolapse POP-Q exams were performed and patients completed multiple validated questionnaires regarding the presence; severity; and impact of prolapse, urinary, bowel, and pelvic pain symptoms.
Results
A total of 183 postmenopausal women were randomized, and 156 (81 hysteropexy and 75 hysterectomy) patients completed 5-year follow up with no demographic differences between the 2 intervention groups. Operative time was statistically less in the hysteropexy group (111.5 min vs 156.7 min). There were fewer treatment failures (a composite including retreatment for prolapse, prolapse beyond the hymen, and/or bothersome bulge symptoms) in the hysteropexy than in the hysterectomy group (37% vs 54%, respectively) at 5 years of follow up. However, most patients with treatment failure were classified as an intermittent failure, with only 16% of hysteropexy patients and 22% of hysterectomy patients classified as persistent failures. There were no meaningful differences between patient-reported outcomes. Hysteropexy had an 8% mesh exposure risk, with none requiring surgical management.
This study represents the highest quality randomized trial design and boasts high patient retention rates and 5-year follow up. Findings support further investigation on the use of polypropylene mesh for POP. In April of 2019, the US Food and Drug Administration halted the selling and distribution of vaginal mesh products for prolapse repair given the lack of safety outcomes, concerns about mesh exposure rates, and possible increased rates of pelvic pain and adverse events. This study invites pelvic reconstructive surgeons to revisit the debate of hysteropexy versus hysterectomy and synthetic mesh versus native tissue repairs. The 8% mesh exposure rate represents a challenge for the future design and development of vaginal implant materials, weighing the balancing of improved long-term efficacy with the safety and complication concerns.
Continue to: Preliminary 12-month data for a single-incision sling for surgical management of SUI...
Preliminary 12-month data for a single-incision sling for surgical management of SUI
Erickson T, Roovers JP, Gheiler E, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol. 2021;28:93-99. doi: 10.1016/j.jmig.2020.04.014.
In this industry-sponsored study, researchers compared a novel single-incision sling to currently available midurethral slings for SUI with 12-month outcomes and adverse event details. However, results are primarily descriptive with no statistical testing.
Study details
Patients were eligible for inclusion in this prospective, nonrandomized cohort study if SUI was their primary incontinence symptom, with confirmatory office testing. Exclusion criteria included POP greater than stage 2, prior SUI surgery, plans for future pregnancy, elevated postvoid residuals, or concomitant surgical procedures. The single-incision Altis (Coloplast) sling was compared to all commercially available transobturator and retropubic midurethral slings. The primary outcome of this study was reduction in 24-hour pad weights, and secondary outcomes included negative cough-stress test and subjective patient-reported outcomes via validated questionnaires.
Results
A total of 184 women were enrolled in the Altis group and 171 in the comparator other sling group. Symptom severity was similar between groups, but more patients in the comparator group had mixed urinary incontinence, and more patients in the Altis group had intrinsic sphincter deficiency. The Altis group had a higher proportion of “dry patients,” but otherwise the outcomes were similar between the 2 groups, including negative cough-stress test and patientreported outcomes. Two patients in the Altis group and 7 patients in the comparator group underwent device revisions. Again, statistical analysis was not performed.
Single-incision slings may reduce the risk of groin pain associated with transobturator slings and may be a good option for patients who desire less mesh burden than the traditional retropubic slings or who are not good candidates. This trial suggests that the Altis single-incision sling may be similar in outcomes and adverse events to currently available midurethral slings, but further, more rigorous trials are underway to fully evaluate this—including a US-based multicenter randomized trial of Altis single-incision slings versus retropubic slings (ClinicalTrials.gov Identifier: NCT03520114).
Office-based pessary care can be safely spaced out to 24 weeks without an increase in erosions
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2020;135:100-105. doi: 10.1097 /AOG.0000000000003580.
For women already using a pessary without issues, extending office visits to every 6 months does not increase rates of vaginal epithelial abnormalities, according to results of this randomized controlled trial.
Study details
Women already using a Gelhorn, ring, or incontinence dish pessary for POP, SUI, or both were randomized to continue routine care with office evaluation every 12 weeks versus the extended-care cohort (with office evaluation every 24 weeks). Women were excluded if they removed and replaced the pessary themselves or if there was a presence of vaginal epithelial abnormalities, such as erosion or granulation tissue.
Results
The rate of vaginal epithelium erosion was 7.4% in the routine arm and 1.7% in the extended-care arm, meeting criteria for noninferiority of extended care. The majority of patients with office visits every 24 weeks preferred the less frequent examinations, and there was no difference in degree of bother due to vaginal discharge. There was also no difference in the percentage of patients with unscheduled visits. The only factors associated with vaginal epithelium abnormalities were prior abnormalities and lifetime duration of pessary use.
As there are currently no evidenced-based guidelines for pessary care, this study contributes data to support extended office-based care up to 24 weeks, a common practice in the United Kingdom. During the COVID-19 pandemic, with reduced health care access, these findings should be reassuring to clinicians and patients.
Continue to: How can we counsel patients regarding changes in sexual activity and function after surgery for POP?...
How can we counsel patients regarding changes in sexual activity and function after surgery for POP?
Antosh DD, Dieter AA, Balk EM, et al. Sexual function after pelvic organ prolapse surgery: a systematic review comparing different approaches to pelvic floor repair. Am J Obstet Gynecol. 2021;2:S0002-9378(21)00610-4. doi: 10.1016/j.ajog.2021.05.042.
A secondary analysis of a recent systematic review found overall moderate- to high-quality evidence that were no differences in total dyspareunia, de novo dyspareunia, and scores on a validated sexual function questionnaire (PISQ12) when comparing postoperative sexual function outcomes of native tissue repair to sacrocolpopexy, transvaginal mesh, or biologic graft. Rates of postoperative dyspareunia were higher for transvaginal mesh than for sacrocolpopexy.
Study details
The Society of Gynecologic Surgeons Systematic Review Group identified 43 original prospective, comparative studies of reconstructive prolapse surgery that reported sexual function outcomes when comparing 2 different types of POP procedures. Thirty-seven of those studies were randomized controlled trials. Specifically, they looked at data comparing outcomes for native tissue versus sacrocolpopexy, native tissue versus transvaginal mesh, native tissue versus biologic graft, and transvaginal mesh versus sacrocolpopexy.
Results
Overall, the prevalence of postoperative dyspareunia was lower than preoperatively after all surgery types. The only statistical difference in this review demonstrated higher postoperative prevalence of dyspareunia after transvaginal mesh than sacrocolpopexy, based on 2 studies. When comparing native tissue prolapse repair to transvaginal mesh, sacrocolpopexy, or biologic grafts, there were no significant differences in sexual activity, baseline, or postoperative total dyspareunia, de-novo dyspareunia, or sexual function changes as measured by the PISQ12 validated questionnaire. ●
This systematic review further contributes to the growing evidence that, regardless of surgical approach to POP, sexual function generally improves and dyspareunia rates generally decrease postoperatively, with overall low rates of de novo dyspareunia. This will help patients and providers select the best-fit surgical approach without concern for worsened sexual function. It also underscores the need for inclusion of standardized sexual function terminology use and sexual health outcomes in future prolapse surgery research.
Innovative therapies in gynecology: The evidence and your practice
As more and more gynecologic therapies move to the outpatient setting, keeping up on the latest data regarding emerging options can be challenging. Furthermore, it can be difficult to justify purchasing expensive equipment for the office when a therapy is not covered by medical insurance plans. However, if a therapy is efficacious and patients are willing to pay out of pocket, clinicians may want to have these options available for their patients.
In an effort to work through these complex issues, a panel of experts was convened at the 47th Annual Scientific Meeting of the Society of Gynecologic Surgeons in Palm Springs, California, on June 29, 2021. This article includes the salient points from that panel discussion.
Fractionated CO2 laser therapy
Fractionated CO2 laser therapy is considered second-line therapy for the treatment of genitourinary syndrome of menopause (GSM). In 2018, the US Food and Drug Administration (FDA) issued a safety warning about the use of CO2 laser therapy and warned patients and clinicians that the FDA had not approved the treatment for vaginal rejuvenation or treatment of vaginal symptoms related to menopause, urinary incontinence, or sexual function. Despite this warning, laser treatments are still performed in many practices.
In 2019, the International Continence Society (ICS) and the International Society for the Study of Vulvovaginal Disease (ISSVD) put out a joint practice consensus statement that essentially did not recommend the routine use of laser treatment for GSM, urinary incontinence, or lichen sclerosus.1 Conversely, the 2020 American Urogynecologic Society (AUGS) published a clinical consensus statement that spoke to the promising results of laser therapy for the treatment of vulvovaginal atrophy, vaginal dryness, and menopausal dyspareunia, with benefits lasting up to 1 year.2 This statement also suggested that the short-term safety profile of the CO2 laser device was favorable.
How CO2 lasers work
Fractionated CO2 laser therapy differs from unfractionated treatment (which often is used in the treatment of condyloma) in that it is not ablative. The laser works by using fractionated beams of light to penetrate the affected tissue to create small wounds in the epithelium and underlying lamina propria, which leads to collagen remodeling and regeneration that then results in the restoration of the superficial epithelium, vaginal rugae, and lubrication.3 Most clinicians perform 3 applications of the laser treatment 6 weeks apart, a recommendation that is based on manufacturer-sponsored studies in menopausal women.
Study results of patient outcomes with laser therapy
GSM. Several retrospective4,5 and prospective studies6-10 have looked at short- and longer-term outcomes in patients undergoing treatment with the CO2 laser. All of these studies showed improvement in patient symptoms related to GSM.
The VeLVET trial, conducted by Paraiso and colleagues, was a randomized trial that compared CO2 laser treatment with vaginal estrogen in women with GSM.11 While the study was underpowered due to cessation of enrollment once the FDA safety warning was issued, the authors reported that at 6 months, both the fractionated CO2 laser therapy group and the vaginal estrogen group had similar improvements, with 70% to 80% of participants reporting satisfaction with treatment. The authors concluded that laser therapy is likely to be as efficacious as vaginal estrogen and may be a good option for patients who cannot use vaginal estrogen to treat GSM.11
Lichen sclerosus. Some data exist on the efficacy of laser therapy for the treatment of lichen sclerosus. One recently published randomized trial showed that at 6 months, fractionated CO2 laser treatment and prior treatment with high potency topical corticosteroids was associated with higher improvement in subjective symptoms and objective measures compared with clobetasol propionate treatment.12 Another trial, however, revealed that laser treatment was not an effective monotherapy treatment for lichen sclerosus when compared with placebo.13 Fewer studies have examined the effect of laser therapy on urinary incontinence.
More prospective data are emerging, evidenced by trials currently registered in ClinicalTrials.gov. While some studies provide evidence that laser therapy may be efficacious in the treatment of vulvovaginal atrophy, additional data are needed to confirm the favorable outcomes observed with laser therapy for the treatment of lichen sclerosus, and a significant amount of data are needed to evaluate the efficacy of laser treatment for urinary incontinence.
Until such evidence is available, fractionated CO2 vaginal laser therapy will remain a fee-for-service treatment option and will be inaccessible to patients who cannot afford the cost of treatment.
Continue to: Hydrogel urethral bulking...
Hydrogel urethral bulking
Urethral bulking agents have been used for 5 decades in the treatment of stress urinary incontinence (SUI) in women. Unlike midurethral slings, in which many medical device companies use the same implant material (microporous, monofilament polypropylene mesh), the material for bulking agents has varied greatly. A 2017 Cochrane review of urethral bulking listed these agents used for this indication: autologous fat, carbon beads, calcium hydroxylapatite, ethylene vinyl alcohol copolymer, glutaraldehyde cross-linked bovine collagen, hyaluronic acid with dextranomer, porcine dermal implant, polytetrafluoroethylene, and silicone particles.14 These agents can be injected through a transurethral or periurethral technique. The review failed to find superiority of one material or injection technique over another.
New bulking agent available
In January 2020, the FDA approved the premarket application for a new bulking agent. This new agent is a permanently implanted, nonresorbable hydrogel that consists of cross-linked polyacrylamide (2.5%) and water (97.5%). It is intended to be used with a transurethral bulking system that includes a rotatable sheath and two 23-guage needles; a total of 1.5 to 2.0 mL of the hydrogel is injected in 3 locations in the proximal urethra per session. Patients may undergo an additional 2 sessions, if needed, at least 4 weeks after the previous session.
Polyacrylamide hydrogel has been used as a bulking agent in cosmetic and ophthalmic surgery for many years, and it was first approved for medical use in Europe in 2001. The initial European data on its use as a urethral bulking agent was published in 2006.15 The first North American data came in 2014 from a multicenter, randomized trial that compared polyacrylamide hydrogel with collagen gel.16 This investigation followed 345 women for 12 months and concluded that the safety and efficacy of polyacrylamide hydrogel was not inferior to collagen, with a little over half of both cohorts demonstrating a 50% or greater decrease in incontinence episodes.
Since these initial studies, 3-year17 and 7-year safety and efficacy data18 have been reported, with reassuring findings, but both studies experienced significant attrition of the original group of patients. The most commonly reported adverse events associated with the procedure are pain at the injection site (4%–14%) and urinary tract infection (3%–7%); transient urinary retention rates range in incidence from 1.5% to 15%.19
Short procedure, long-term results
Given that a urethral bulking procedure can be done in less than 10 minutes in the office under local analgesia, this treatment may lend itself to use in more brittle patient populations. One study of women aged 80 or older showed a greater than 50% decrease in the number of daily pads used for up to 2 years after initial injection.20 Another study found the greatest treatment success in women aged 60 years or older with fewer than 2.5 episodes of SUI per day.21
Platelet-rich plasma therapy
Platelet-rich plasma (PRP) therapy has been used in multiple disciplines for more than 2 decades as a treatment to regenerate damaged tissue, particularly in sports medicine for treating tendonitis as well as in plastic surgery, gynecology, urology, and ophthalmology, and good outcomes have been demonstrated with no serious adverse effects. PRP is a natural product in which high levels of platelets are concentrated through centrifugation with bioactive growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and insulin growth factor (IGF).22 The activated platelets are then injected autologously back into the patient’s tissue. This process releases activated growth factors that accelerate tissue healing by stimulating the number of reparative cells to create collagen production, angiogenesis, and neurogenesis while fighting infection and downregulating the autoimmune system.
Continue to: Uses for PRP in gynecology...
Uses for PRP in gynecology
In gynecology, dating back to 2007 PRP was shown to facilitate wound healing, when Fanning and colleagues reported PRP applications in gynecologic operative wounds, such as hysterectomies and urogynecologic procedures, to reduce postoperative pain.23 In the last decade, there has been a dramatic increasing trend in the application of PRP injections as an alternative therapy in gynecology to improve intimate health. PRP has been used to treat lichen sclerosus, atrophic vaginitis, SUI, and female sexual dysfunction; however, there is a dearth of studies that compare PRP with traditional therapies.
Runels and colleagues described the effects of localized injections of autologous PRP for the treatment of sexual dysfunction early in 2014.24 Those authors pioneered PRP use in women with dyspareunia and other symptoms related to sexual dysfunction. Women were offered PRP injections into the periurethral area of the Skene glands and the clitoris. Sexual satisfaction and pain were improved but results did not reach statistical significance. The results of this pilot study of 11 patients suggested that PRP injections could perhaps be an effective method to treat certain types of female sexual dysfunction, including desire, arousal, lubrication, and orgasm.
In another pilot study, Long and colleagues looked at the effectiveness of local injection of PRP for treating women with SUI.25 In that study, younger patients with mild severity of SUI had promising results, with up to 75% cured or improved. Results in the older group, with 50% cured or improved, did not reach statistical significance. Other small, limited studies have been conducted under the hypothesis that PRP as an “O-shot” may be a promising treatment that is a safe, effective, nonsurgical, and nonhormonal option for women with dyspareunia from lack of lubrication and related sexual dysfunction, such as decreased libido or arousal.26-29 A pilot study by Behnia-Willison and colleagues demonstrated clinical improvement in PRP use as an alternative to topical steroids for lichen sclerosus.30 Several other studies also have shown efficacy for the treatment of lichen sclerosus.31-34
More evidence of efficacy needed
To date, preliminary studies suggest that PRP holds promise for a host of gynecologic conditions. Since PRP is autologous, there are no significant contraindications, and thus far there have been no known serious adverse effects. However, most health insurers still do not cover this therapy, so for now patients must pay out-of-pocket fees for these treatments.
As we continue to investigate therapies in regenerative medicine, the continued efforts of our discipline are required to conduct well-designed prospective, randomized controlled studies. While initial series suggest that PRP is safe, it is unlikely that this therapy will be embraced widely in the paradigm as an alternative treatment option for many genitourinary symptoms of menopause and vulvar disorders until efficacy is better established.
Radiofrequency therapy
For the past 20 years, radiofrequency (RF) energy has been used through the vagina, urethra, and periurethral tissues for the treatment of genitourinary symptoms, with limited success. More recently, because some patients hesitate to receive mesh implants for treatment of urinary incontinence,35 there has been gravitation to office-based procedures.
In contrast to lasers, which transmit energy through light, RF waves (measured in hertz) transform the kinetic energy of the intracellular atoms, which move and collide, generating thermal energy.36,37 RF therapy has been shown to increase the proportion of smooth muscle and connective tissue; stimulate proliferation of the epithelium, neovascularization, and collagen formation in the lamina propria; and improve natural lubrication.36,38 In addition, RF is:
- ablative when the heat is capable of generating ablation and/or necrosis of the epidermis and dermis
- microablative when energy fractionation produces microscopic columns of ablative thermal lesions in the epidermis and upper dermis, resulting in microscopic columns of treated tissue interspersed with areas of untreated skin,39 and
- nonablative when trauma occurs only in the dermis by heating without causing ablation of the epidermis.39
The RF devices discussed below are used with settings for microablation in the treatment of SUI and sexual health/vaginal laxity, and with nonablative settings in the treatment of GSM.
RF for the treatment of urinary incontinence
Studies with RF have shown its benefits in urinary symptoms as secondary outcomes, such as improvement of SUI.38,40 One theory that favors energy devices as a treatment for SUI is that the treatment strengthens suburethral and pubocervical support, thereby decreasing urethral mobility.41
In 2016, the Viveve system (Viveve) received FDA 510(k) clearance for “use in general surgical procedures for electrocoagulation and hemostasis.” A single-site, randomized, nonblinded pilot study compared 1 treatment (group 1) versus 2 treatments (group 2) with the Viveve system for SUI in 35 participants.42 At 12 months, only for group 2 did mean scores on the Incontinence Impact Questionnaire Short Form (IIQ-7) and the International Consultation on Incontinence Modular Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) decrease by the minimum clinically important difference of 16 and 2.52 points, respectively, compared with baseline.
The ThermiVa device (ThermiGen, LLC) received FDA clearance for “use in dermatological and general surgical procedures for electrocoagulation and hemostasis” in 2017. A single-site, prospective, double-blind, randomized controlled pilot trial evaluated the efficacy of this device for the treatment of SUI in 20 participants randomly assigned in a 1:1 fashion to active and sham groups.43 At 12 weeks, mean scores of the Urogenital Distress Inventory (UDI-6) and the ICIQ-UI-SF decreased by the minimal clinically important difference only in the treatment group arm. Additionally, 70% of treatment group participants had a negative stress test at 12 weeks compared with 0% of control group participants.43 In another study of 48 patients who were followed longitudinally for 5 months, a substantial improvement in genital appearance was observed.44 Assessment based on validated instruments demonstrated significant improvements in sexual function and SUI.44
A microablative RF device (Wavetronic 6000 Touch Device, Megapulse HF FRAXX system; Loktal Medical Electronics) consists of a vaginal probe with 64 microneedles at the tip, each capable of penetrating to a depth of 1 mm. During activation, delivery of RF energy, which results in vaporization of tissue at 100 °C, occurs in a preset sequence of 8 needles at a time, preventing the overheating of intervening tissue between adjacent needles.
Slongo and colleagues conducted a 3-arm randomized clinical trial that included 117 climacteric women with SUI.45 In group 1, treatment consisted of 3 monthly sessions of RF; group 2 received 12 weekly sessions of pelvic floor muscle training (PFMT); and group 3 received RF treatment plus PFMT simultaneously. Assessments were conducted at baseline and 30 days after the end of therapy using validated questionnaires and scales for urinary, vaginal, and sexual functions, and cytology was used to assess vaginal atrophy. The association between RF and PFMT showed significant improvement in the SUI symptoms assessed by questionnaire. The vaginal symptoms and dryness showed more substantial improvement with the RF treatment, and vaginal laxity showed similar improvement in the 3 treatment groups.45
Continue to: RF for the treatment of GSM...
RF for the treatment of GSM
For women who are not candidates for localized hormone therapy, as well as others who simply do not wish to use hormones, nonablative RF laser therapy may be an alternative for the management of GSM.
The VIVEVE I trial was one of the largest randomized, sham-controlled trials performed to determine the efficacy of vaginal rejuvenation using surface-cooled RF; 174 women received either RF treatment (90 J/cm2) or sham treatment (1 J/cm2).46 Treated participants had a significant improvement in perception of vaginal laxity/looseness and sexual function up to 6 months posttreatment.46 Overall, participants were satisfied with the treatment (77.8%–100%) and reported significant improvements in vaginal laxity and symptoms of atrophy. RF was well tolerated with minimal adverse effects, such as procedure-related erythema and edema of treated tissue, and vaginal discharge. One patient discontinued treatment because of procedural pain.47,48
The ThermiVa system also was evaluated for efficacy in the treatment of GSM in a single-site, double-blind randomized controlled pilot study, the methods of which were previously described above.43 GSM symptoms were evaluated at baseline and 12 weeks using the Vaginal Health Index (VHI) and visual analog scale (VAS). At the 12-week follow-up, compared with baseline scores, VHI scores were unchanged in the control group and improved in the treatment group. Additionally, VAS scores for dyspareunia decreased in the treatment group compared with baseline while VAS for dyspareunia in the sham group did not change from baseline to 12 weeks.
RF treatment for sexual health
The efficacy of the Viveve RF system for female sexual dysfunction was evaluated in an international, randomized, controlled, single-blinded study (n = 154) that compared 6-month outcomes of RF treatment versus sham treatment.46 Although there was a statistically significant improvement in patient-reported sexual dysfunction on validated instruments, it is essential to note that the study was powered for the primary outcome of vaginal laxity. In addition, the study was not adequately powered to evaluate safety; however, the adverse events reported were mild, and the most frequently reported adverse event was vaginal discharge.
Microablative monopolar RF treatment for GSM has been evaluated in 2 single-arm clinical trials that included a total of 70 patients.39,49 Pre- and posttreatment outcomes were analyzed after delivery of 3 treatment sessions 28 to 40 days apart. Although the only significant improvement in quality of life was in the health domain of the World Health Organization Quality of Life Adapted Questionnaire (P = .04), significant improvements in sexual functioning were seen in terms of the desire (P = .002), lubrication (P = .001), satisfaction (P = .003), and pain (P = .007) domains of the Female Sexual Function Index (FSFI) questionnaire except for excitation and orgasm.39 Overall, 100% of participants reported being satisfied or very satisfied with treatments, and 13 of 14 women felt “cured” or “much better.”39 After treatment, significant increases in vaginal Lactobacillus (P<.001), decreases in vaginal pH (P<.001), improvements in maturation of vaginal cellularity (decreased parabasal cells, P<.001; increased superficial cells, P<.001), and increased VHI score (P<.001) alone occurred.49 No adverse events beyond self-limited vaginal burning and redness were reported.39,49 In another study mentioned above, the combination of RF and PFMT in sexual function does not offer benefits superior to those achieved by the therapies alone.45
Evidence on RF treatment does not support marketing efforts
Radiofrequency devices have been marketed for a variety of genitourinary problems in women, with limited high-quality, randomized, comparative evidence of efficacy and durability in the literature. It is unfortunate that RF treatment continues to be promoted by practitioners around the world who cite small, short-term studies that lack biostatistical rigor in their reporting of protocols and results. Statements from both AUGS and the International Urogynecological Association have heeded caution on the use of lasers but they could not even evaluate RF devices due to lack of evidence.2,41
Informed counseling and shared decision making remain the bottom line
By the year 2025, all members of the Baby Boom generation will be aged 60 or older. While in the past there has been a reluctance to discuss women’s sexual health, urinary incontinence, and GSM, the need for open discussion and a variety of treatment options for these conditions has never been more critical.
Many patients prefer office-based therapies over hospital-based procedures, and others are leery of synthetic implants. These concerns are leading toward great interest in the types of treatments covered in this article. However, it is paramount that clinicians are aware of the evidence-based data behind these emerging options so that we can openly and accurately counsel our patients.
As we have shown, the quality of the data behind these officed-based therapies varies significantly. Until a greater body of research data is available, we must carefully balance our desire to meet patient wishes with solid, informed counseling and shared decision making. ●
- Preti M, Viera-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. J Lower Genital Tract Dis. 2019;23:151-160.
- Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices: AUGS clinical consensus statement. Female Pelvic Med Reconstr Surg. 2020;26:287-298.
- Streicher LF. Vulvar and vaginal fractional CO2 laser treatments for genitourinary syndrome of menopause: NAMS practice pearl. Menopause. 2018;25:571-573.
- Gardner AN, Aschkenazi SO. The short-term efficacy and safety of fractional CO2 laser therapy for vulvovaginal symptoms in menopause, breast cancer, and lichen sclerosus. Menopause. 2021; 28:511-516.
- Balchander D, Nyirjesy P. Fractionated CO2 laser therapy in recalcitrant lichen sclerosus. J Lower Genital Tract Disease. 2020;24:225-228.
- Pieralli A, Fallani MG, Becorpi A, et al. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch Gynecol Obstet. 2016;294:841-846.
- Pieralli A, Bianchi C, Longinotti M, et al. Long-term reliability of fractionated CO2 laser as a treatment of vulvovaginal atrophy (VVA) symptoms. Arch Gynecol Obstet. 2017; 296:973-978.
- Sokol ER, Karram MM. Use of novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24: 810-814.
- Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422.
- Sindou-Faurie T, Louis-Vahdat C, Oueld Es Cheikh E, et al. Evaluation of the efficacy of fractional CO2 laser in the treatment of vulvar and vaginal menopausal symptoms. Arch Gynecol Obstet. 2021;303:955-963.
- Paraiso MFR, Ferrando CA, Sokol ER, at al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2019;27:50-56.
- Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978.
- Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;136:979-987.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lose G, Mouritsen L, Nielson JB. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 2006;98:100-104.
- Sokol ER, Karram MM, Dmochowski R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol. 2014;192:843-849.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol. 2015;68:428-433.
- Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508.
- Kasi AD, Pergialiotis V, Perrea DN, et al. Polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016;27:367-375.
- Vecchioli-Scaldazza CV, Smaali C, Morosetti C, et al. Polyacrylamide hydrogel (Bulkamid) in female patients of 80 or more years with urinary incontinence. Int Braz J Urol. 2014;40:37-43.
- Elmelund M, Sokol ER, Darram MM, et al. Patient characteristics that may influence the effect of urethral injection therapy for female stress urinary incontinence. J Urol. 2019;202:125-131.
- Sanoulis V, Nikolettos N, Vlahos N. The use of platelet-rich plasma in the gynecological clinical setting: a review. HJOG. 2019;18:55-65.
- Fanning J, Murrain L, Flora R, et al. Phase I/II prospective trial of autologous platelet tissue graft in gynecologic surgery. J Minim Invasive Gynecol. 2007;14:633-637.
- Runels CE, Melnick H, DeBourbon E, et al. A pilot study of the effect of localized injections of autologous platelet rich plasma (PRP) for the treatment of female sexual dysfunction. J Womens Health Care. 2014;3:4.
- Long CY, Lin KL, Shen CR, et al. A pilot study: effectiveness of local injection of autologous platelet-rich plasma in treating women with stress urinary incontinence. Sci Rep. 2021;11:1584.
- Matz EJ, Pearlman AM, Terlecki RP. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol. 2018;59:61-65.
- Neto JB. O-Shot: platelets rich plasma in intimate female treatment. J Womens Health Care. 2017;6:5.
- Nikolopoulos KI, Pergialiotis V, Perrea D, et al. Restoration of the pubourethral ligament with platelet rich plasma for the treatment of stress urinary incontinence. Med Hypotheses. 2016;90:29-31.
- Hersant B, SidAhmed-Mezi M, Belkacemi Y, et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with a history of breast cancer: a phase 2 pilot study. Menopause. 2018;25:1124-1130.
- Behnia-Willison F, Pour NR, Mohamadi B, et al. Use of platelet-rich plasma for vulvovaginal autoimmune conditions like lichen sclerosus. Plast Reconstr Surg Glob Open. 2016;4:e1124.
- Goldstein AT, King M, Runels C, et al. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2017;76:158-160.
- Casabona F, Priano V, Vallerino V, et al. New surgical approach to lichen sclerosus of the vulva: the role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast Reconstr Surg. 2010;126:210e-211e.
- Franic D, Iternica Z, Franic-Ivanisevic M. Platelet-rich plasma (PRP) for the treatment of vulvar lichen sclerosus in a premenopausal woman: a case report. Case Rep Womens Health. 2018;18: e0062.
- Posey LK, Runels C. In office surgery and use of platelet rich plasma for the treatment of vulvar lichen sclerosus to alleviate painful sexual intercourse. J Lower Genital Tract Dis. 2017;21(4S):S14.
- Stachowicz AM, Hoover ML, Karram MM. Clinical utility of radiofrequency energy for female genitourinary dysfunction: past, present, and future. Int Urogynecol J. 2021;32:1345-1350.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- US Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, MD, on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” https://www.fda.gov/news-events/pressannouncements/statement-fda-commissioner-scott-gottlieb-mdefforts-safeguard-womens-health-deceptive-health-claims. Updated August 2, 2018. Accessed August 13, 2021.
- Vicariotto F, Raichi M. Technological evolution in the radiofrequency treatment of vaginal laxity and menopausal vulvo-vaginal atrophy and other genitourinary symptoms: first experiences with a novel dynamic quadripolar device. Minerva Ginecol. 2016;68:225-236.
- Kamilos MF, Borrelli CL. New therapeutic option in genitourinary syndrome of menopause: pilot study using microablative fractional radiofrequency. Einstein (Sao Paulo). 2017;15:445-551.
- Caruth JC. Evaluation of the safety and efficacy of a novel radiofrequency device for vaginal treatment. Surg Technol Int. 2018;32:145-149.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Allan BB, Bell S, Husarek K. Early feasibility study to evaluate the Viveve system for female stress urinary incontinence: interim 6-month report. J Womens Health (Larchmt). 2020;29:383-389.
- Leibaschoff G, Izasa PG, Cardona JL, et al. Transcutaneous temperature controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Desai SA, Vakil Z, Kroumpouzos G. Transcutaneous temperature-controlled radiofrequency treatment: improvement in female genital appearance, sexual dysfunction, and stress urinary incontinence. Aesthet Surg J. 2021;sjab174. doi: 10.1093/asj/sjab174.
- Slongo H, Lunardi AL, Riccetto CL, et al. Microablative radiofrequency versus pelvic floor muscle training for stress urinary incontinence: a randomized controlled trial. Int Urogynecol J. 2021. doi: 10.1007 /s00192-021-04758-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30: 429-436.
- Juhasz ML, Korta DZ, Mesinkovska NA. Vaginal rejuvenation: a retrospective review of lasers and radiofrequency devices. Dermatol Surg. 2021;47:489-494.
- Sarmento AC, Fernandes FS, Marconi C, et al. Impact of microablative fractional radiofrequency on the vaginal health, microbiota, and cellularity of postmenopausal women. Clinics (Sao Paulo). 2020;75:e1750.
As more and more gynecologic therapies move to the outpatient setting, keeping up on the latest data regarding emerging options can be challenging. Furthermore, it can be difficult to justify purchasing expensive equipment for the office when a therapy is not covered by medical insurance plans. However, if a therapy is efficacious and patients are willing to pay out of pocket, clinicians may want to have these options available for their patients.
In an effort to work through these complex issues, a panel of experts was convened at the 47th Annual Scientific Meeting of the Society of Gynecologic Surgeons in Palm Springs, California, on June 29, 2021. This article includes the salient points from that panel discussion.
Fractionated CO2 laser therapy
Fractionated CO2 laser therapy is considered second-line therapy for the treatment of genitourinary syndrome of menopause (GSM). In 2018, the US Food and Drug Administration (FDA) issued a safety warning about the use of CO2 laser therapy and warned patients and clinicians that the FDA had not approved the treatment for vaginal rejuvenation or treatment of vaginal symptoms related to menopause, urinary incontinence, or sexual function. Despite this warning, laser treatments are still performed in many practices.
In 2019, the International Continence Society (ICS) and the International Society for the Study of Vulvovaginal Disease (ISSVD) put out a joint practice consensus statement that essentially did not recommend the routine use of laser treatment for GSM, urinary incontinence, or lichen sclerosus.1 Conversely, the 2020 American Urogynecologic Society (AUGS) published a clinical consensus statement that spoke to the promising results of laser therapy for the treatment of vulvovaginal atrophy, vaginal dryness, and menopausal dyspareunia, with benefits lasting up to 1 year.2 This statement also suggested that the short-term safety profile of the CO2 laser device was favorable.
How CO2 lasers work
Fractionated CO2 laser therapy differs from unfractionated treatment (which often is used in the treatment of condyloma) in that it is not ablative. The laser works by using fractionated beams of light to penetrate the affected tissue to create small wounds in the epithelium and underlying lamina propria, which leads to collagen remodeling and regeneration that then results in the restoration of the superficial epithelium, vaginal rugae, and lubrication.3 Most clinicians perform 3 applications of the laser treatment 6 weeks apart, a recommendation that is based on manufacturer-sponsored studies in menopausal women.
Study results of patient outcomes with laser therapy
GSM. Several retrospective4,5 and prospective studies6-10 have looked at short- and longer-term outcomes in patients undergoing treatment with the CO2 laser. All of these studies showed improvement in patient symptoms related to GSM.
The VeLVET trial, conducted by Paraiso and colleagues, was a randomized trial that compared CO2 laser treatment with vaginal estrogen in women with GSM.11 While the study was underpowered due to cessation of enrollment once the FDA safety warning was issued, the authors reported that at 6 months, both the fractionated CO2 laser therapy group and the vaginal estrogen group had similar improvements, with 70% to 80% of participants reporting satisfaction with treatment. The authors concluded that laser therapy is likely to be as efficacious as vaginal estrogen and may be a good option for patients who cannot use vaginal estrogen to treat GSM.11
Lichen sclerosus. Some data exist on the efficacy of laser therapy for the treatment of lichen sclerosus. One recently published randomized trial showed that at 6 months, fractionated CO2 laser treatment and prior treatment with high potency topical corticosteroids was associated with higher improvement in subjective symptoms and objective measures compared with clobetasol propionate treatment.12 Another trial, however, revealed that laser treatment was not an effective monotherapy treatment for lichen sclerosus when compared with placebo.13 Fewer studies have examined the effect of laser therapy on urinary incontinence.
More prospective data are emerging, evidenced by trials currently registered in ClinicalTrials.gov. While some studies provide evidence that laser therapy may be efficacious in the treatment of vulvovaginal atrophy, additional data are needed to confirm the favorable outcomes observed with laser therapy for the treatment of lichen sclerosus, and a significant amount of data are needed to evaluate the efficacy of laser treatment for urinary incontinence.
Until such evidence is available, fractionated CO2 vaginal laser therapy will remain a fee-for-service treatment option and will be inaccessible to patients who cannot afford the cost of treatment.
Continue to: Hydrogel urethral bulking...
Hydrogel urethral bulking
Urethral bulking agents have been used for 5 decades in the treatment of stress urinary incontinence (SUI) in women. Unlike midurethral slings, in which many medical device companies use the same implant material (microporous, monofilament polypropylene mesh), the material for bulking agents has varied greatly. A 2017 Cochrane review of urethral bulking listed these agents used for this indication: autologous fat, carbon beads, calcium hydroxylapatite, ethylene vinyl alcohol copolymer, glutaraldehyde cross-linked bovine collagen, hyaluronic acid with dextranomer, porcine dermal implant, polytetrafluoroethylene, and silicone particles.14 These agents can be injected through a transurethral or periurethral technique. The review failed to find superiority of one material or injection technique over another.
New bulking agent available
In January 2020, the FDA approved the premarket application for a new bulking agent. This new agent is a permanently implanted, nonresorbable hydrogel that consists of cross-linked polyacrylamide (2.5%) and water (97.5%). It is intended to be used with a transurethral bulking system that includes a rotatable sheath and two 23-guage needles; a total of 1.5 to 2.0 mL of the hydrogel is injected in 3 locations in the proximal urethra per session. Patients may undergo an additional 2 sessions, if needed, at least 4 weeks after the previous session.
Polyacrylamide hydrogel has been used as a bulking agent in cosmetic and ophthalmic surgery for many years, and it was first approved for medical use in Europe in 2001. The initial European data on its use as a urethral bulking agent was published in 2006.15 The first North American data came in 2014 from a multicenter, randomized trial that compared polyacrylamide hydrogel with collagen gel.16 This investigation followed 345 women for 12 months and concluded that the safety and efficacy of polyacrylamide hydrogel was not inferior to collagen, with a little over half of both cohorts demonstrating a 50% or greater decrease in incontinence episodes.
Since these initial studies, 3-year17 and 7-year safety and efficacy data18 have been reported, with reassuring findings, but both studies experienced significant attrition of the original group of patients. The most commonly reported adverse events associated with the procedure are pain at the injection site (4%–14%) and urinary tract infection (3%–7%); transient urinary retention rates range in incidence from 1.5% to 15%.19
Short procedure, long-term results
Given that a urethral bulking procedure can be done in less than 10 minutes in the office under local analgesia, this treatment may lend itself to use in more brittle patient populations. One study of women aged 80 or older showed a greater than 50% decrease in the number of daily pads used for up to 2 years after initial injection.20 Another study found the greatest treatment success in women aged 60 years or older with fewer than 2.5 episodes of SUI per day.21
Platelet-rich plasma therapy
Platelet-rich plasma (PRP) therapy has been used in multiple disciplines for more than 2 decades as a treatment to regenerate damaged tissue, particularly in sports medicine for treating tendonitis as well as in plastic surgery, gynecology, urology, and ophthalmology, and good outcomes have been demonstrated with no serious adverse effects. PRP is a natural product in which high levels of platelets are concentrated through centrifugation with bioactive growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and insulin growth factor (IGF).22 The activated platelets are then injected autologously back into the patient’s tissue. This process releases activated growth factors that accelerate tissue healing by stimulating the number of reparative cells to create collagen production, angiogenesis, and neurogenesis while fighting infection and downregulating the autoimmune system.
Continue to: Uses for PRP in gynecology...
Uses for PRP in gynecology
In gynecology, dating back to 2007 PRP was shown to facilitate wound healing, when Fanning and colleagues reported PRP applications in gynecologic operative wounds, such as hysterectomies and urogynecologic procedures, to reduce postoperative pain.23 In the last decade, there has been a dramatic increasing trend in the application of PRP injections as an alternative therapy in gynecology to improve intimate health. PRP has been used to treat lichen sclerosus, atrophic vaginitis, SUI, and female sexual dysfunction; however, there is a dearth of studies that compare PRP with traditional therapies.
Runels and colleagues described the effects of localized injections of autologous PRP for the treatment of sexual dysfunction early in 2014.24 Those authors pioneered PRP use in women with dyspareunia and other symptoms related to sexual dysfunction. Women were offered PRP injections into the periurethral area of the Skene glands and the clitoris. Sexual satisfaction and pain were improved but results did not reach statistical significance. The results of this pilot study of 11 patients suggested that PRP injections could perhaps be an effective method to treat certain types of female sexual dysfunction, including desire, arousal, lubrication, and orgasm.
In another pilot study, Long and colleagues looked at the effectiveness of local injection of PRP for treating women with SUI.25 In that study, younger patients with mild severity of SUI had promising results, with up to 75% cured or improved. Results in the older group, with 50% cured or improved, did not reach statistical significance. Other small, limited studies have been conducted under the hypothesis that PRP as an “O-shot” may be a promising treatment that is a safe, effective, nonsurgical, and nonhormonal option for women with dyspareunia from lack of lubrication and related sexual dysfunction, such as decreased libido or arousal.26-29 A pilot study by Behnia-Willison and colleagues demonstrated clinical improvement in PRP use as an alternative to topical steroids for lichen sclerosus.30 Several other studies also have shown efficacy for the treatment of lichen sclerosus.31-34
More evidence of efficacy needed
To date, preliminary studies suggest that PRP holds promise for a host of gynecologic conditions. Since PRP is autologous, there are no significant contraindications, and thus far there have been no known serious adverse effects. However, most health insurers still do not cover this therapy, so for now patients must pay out-of-pocket fees for these treatments.
As we continue to investigate therapies in regenerative medicine, the continued efforts of our discipline are required to conduct well-designed prospective, randomized controlled studies. While initial series suggest that PRP is safe, it is unlikely that this therapy will be embraced widely in the paradigm as an alternative treatment option for many genitourinary symptoms of menopause and vulvar disorders until efficacy is better established.
Radiofrequency therapy
For the past 20 years, radiofrequency (RF) energy has been used through the vagina, urethra, and periurethral tissues for the treatment of genitourinary symptoms, with limited success. More recently, because some patients hesitate to receive mesh implants for treatment of urinary incontinence,35 there has been gravitation to office-based procedures.
In contrast to lasers, which transmit energy through light, RF waves (measured in hertz) transform the kinetic energy of the intracellular atoms, which move and collide, generating thermal energy.36,37 RF therapy has been shown to increase the proportion of smooth muscle and connective tissue; stimulate proliferation of the epithelium, neovascularization, and collagen formation in the lamina propria; and improve natural lubrication.36,38 In addition, RF is:
- ablative when the heat is capable of generating ablation and/or necrosis of the epidermis and dermis
- microablative when energy fractionation produces microscopic columns of ablative thermal lesions in the epidermis and upper dermis, resulting in microscopic columns of treated tissue interspersed with areas of untreated skin,39 and
- nonablative when trauma occurs only in the dermis by heating without causing ablation of the epidermis.39
The RF devices discussed below are used with settings for microablation in the treatment of SUI and sexual health/vaginal laxity, and with nonablative settings in the treatment of GSM.
RF for the treatment of urinary incontinence
Studies with RF have shown its benefits in urinary symptoms as secondary outcomes, such as improvement of SUI.38,40 One theory that favors energy devices as a treatment for SUI is that the treatment strengthens suburethral and pubocervical support, thereby decreasing urethral mobility.41
In 2016, the Viveve system (Viveve) received FDA 510(k) clearance for “use in general surgical procedures for electrocoagulation and hemostasis.” A single-site, randomized, nonblinded pilot study compared 1 treatment (group 1) versus 2 treatments (group 2) with the Viveve system for SUI in 35 participants.42 At 12 months, only for group 2 did mean scores on the Incontinence Impact Questionnaire Short Form (IIQ-7) and the International Consultation on Incontinence Modular Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) decrease by the minimum clinically important difference of 16 and 2.52 points, respectively, compared with baseline.
The ThermiVa device (ThermiGen, LLC) received FDA clearance for “use in dermatological and general surgical procedures for electrocoagulation and hemostasis” in 2017. A single-site, prospective, double-blind, randomized controlled pilot trial evaluated the efficacy of this device for the treatment of SUI in 20 participants randomly assigned in a 1:1 fashion to active and sham groups.43 At 12 weeks, mean scores of the Urogenital Distress Inventory (UDI-6) and the ICIQ-UI-SF decreased by the minimal clinically important difference only in the treatment group arm. Additionally, 70% of treatment group participants had a negative stress test at 12 weeks compared with 0% of control group participants.43 In another study of 48 patients who were followed longitudinally for 5 months, a substantial improvement in genital appearance was observed.44 Assessment based on validated instruments demonstrated significant improvements in sexual function and SUI.44
A microablative RF device (Wavetronic 6000 Touch Device, Megapulse HF FRAXX system; Loktal Medical Electronics) consists of a vaginal probe with 64 microneedles at the tip, each capable of penetrating to a depth of 1 mm. During activation, delivery of RF energy, which results in vaporization of tissue at 100 °C, occurs in a preset sequence of 8 needles at a time, preventing the overheating of intervening tissue between adjacent needles.
Slongo and colleagues conducted a 3-arm randomized clinical trial that included 117 climacteric women with SUI.45 In group 1, treatment consisted of 3 monthly sessions of RF; group 2 received 12 weekly sessions of pelvic floor muscle training (PFMT); and group 3 received RF treatment plus PFMT simultaneously. Assessments were conducted at baseline and 30 days after the end of therapy using validated questionnaires and scales for urinary, vaginal, and sexual functions, and cytology was used to assess vaginal atrophy. The association between RF and PFMT showed significant improvement in the SUI symptoms assessed by questionnaire. The vaginal symptoms and dryness showed more substantial improvement with the RF treatment, and vaginal laxity showed similar improvement in the 3 treatment groups.45
Continue to: RF for the treatment of GSM...
RF for the treatment of GSM
For women who are not candidates for localized hormone therapy, as well as others who simply do not wish to use hormones, nonablative RF laser therapy may be an alternative for the management of GSM.
The VIVEVE I trial was one of the largest randomized, sham-controlled trials performed to determine the efficacy of vaginal rejuvenation using surface-cooled RF; 174 women received either RF treatment (90 J/cm2) or sham treatment (1 J/cm2).46 Treated participants had a significant improvement in perception of vaginal laxity/looseness and sexual function up to 6 months posttreatment.46 Overall, participants were satisfied with the treatment (77.8%–100%) and reported significant improvements in vaginal laxity and symptoms of atrophy. RF was well tolerated with minimal adverse effects, such as procedure-related erythema and edema of treated tissue, and vaginal discharge. One patient discontinued treatment because of procedural pain.47,48
The ThermiVa system also was evaluated for efficacy in the treatment of GSM in a single-site, double-blind randomized controlled pilot study, the methods of which were previously described above.43 GSM symptoms were evaluated at baseline and 12 weeks using the Vaginal Health Index (VHI) and visual analog scale (VAS). At the 12-week follow-up, compared with baseline scores, VHI scores were unchanged in the control group and improved in the treatment group. Additionally, VAS scores for dyspareunia decreased in the treatment group compared with baseline while VAS for dyspareunia in the sham group did not change from baseline to 12 weeks.
RF treatment for sexual health
The efficacy of the Viveve RF system for female sexual dysfunction was evaluated in an international, randomized, controlled, single-blinded study (n = 154) that compared 6-month outcomes of RF treatment versus sham treatment.46 Although there was a statistically significant improvement in patient-reported sexual dysfunction on validated instruments, it is essential to note that the study was powered for the primary outcome of vaginal laxity. In addition, the study was not adequately powered to evaluate safety; however, the adverse events reported were mild, and the most frequently reported adverse event was vaginal discharge.
Microablative monopolar RF treatment for GSM has been evaluated in 2 single-arm clinical trials that included a total of 70 patients.39,49 Pre- and posttreatment outcomes were analyzed after delivery of 3 treatment sessions 28 to 40 days apart. Although the only significant improvement in quality of life was in the health domain of the World Health Organization Quality of Life Adapted Questionnaire (P = .04), significant improvements in sexual functioning were seen in terms of the desire (P = .002), lubrication (P = .001), satisfaction (P = .003), and pain (P = .007) domains of the Female Sexual Function Index (FSFI) questionnaire except for excitation and orgasm.39 Overall, 100% of participants reported being satisfied or very satisfied with treatments, and 13 of 14 women felt “cured” or “much better.”39 After treatment, significant increases in vaginal Lactobacillus (P<.001), decreases in vaginal pH (P<.001), improvements in maturation of vaginal cellularity (decreased parabasal cells, P<.001; increased superficial cells, P<.001), and increased VHI score (P<.001) alone occurred.49 No adverse events beyond self-limited vaginal burning and redness were reported.39,49 In another study mentioned above, the combination of RF and PFMT in sexual function does not offer benefits superior to those achieved by the therapies alone.45
Evidence on RF treatment does not support marketing efforts
Radiofrequency devices have been marketed for a variety of genitourinary problems in women, with limited high-quality, randomized, comparative evidence of efficacy and durability in the literature. It is unfortunate that RF treatment continues to be promoted by practitioners around the world who cite small, short-term studies that lack biostatistical rigor in their reporting of protocols and results. Statements from both AUGS and the International Urogynecological Association have heeded caution on the use of lasers but they could not even evaluate RF devices due to lack of evidence.2,41
Informed counseling and shared decision making remain the bottom line
By the year 2025, all members of the Baby Boom generation will be aged 60 or older. While in the past there has been a reluctance to discuss women’s sexual health, urinary incontinence, and GSM, the need for open discussion and a variety of treatment options for these conditions has never been more critical.
Many patients prefer office-based therapies over hospital-based procedures, and others are leery of synthetic implants. These concerns are leading toward great interest in the types of treatments covered in this article. However, it is paramount that clinicians are aware of the evidence-based data behind these emerging options so that we can openly and accurately counsel our patients.
As we have shown, the quality of the data behind these officed-based therapies varies significantly. Until a greater body of research data is available, we must carefully balance our desire to meet patient wishes with solid, informed counseling and shared decision making. ●
As more and more gynecologic therapies move to the outpatient setting, keeping up on the latest data regarding emerging options can be challenging. Furthermore, it can be difficult to justify purchasing expensive equipment for the office when a therapy is not covered by medical insurance plans. However, if a therapy is efficacious and patients are willing to pay out of pocket, clinicians may want to have these options available for their patients.
In an effort to work through these complex issues, a panel of experts was convened at the 47th Annual Scientific Meeting of the Society of Gynecologic Surgeons in Palm Springs, California, on June 29, 2021. This article includes the salient points from that panel discussion.
Fractionated CO2 laser therapy
Fractionated CO2 laser therapy is considered second-line therapy for the treatment of genitourinary syndrome of menopause (GSM). In 2018, the US Food and Drug Administration (FDA) issued a safety warning about the use of CO2 laser therapy and warned patients and clinicians that the FDA had not approved the treatment for vaginal rejuvenation or treatment of vaginal symptoms related to menopause, urinary incontinence, or sexual function. Despite this warning, laser treatments are still performed in many practices.
In 2019, the International Continence Society (ICS) and the International Society for the Study of Vulvovaginal Disease (ISSVD) put out a joint practice consensus statement that essentially did not recommend the routine use of laser treatment for GSM, urinary incontinence, or lichen sclerosus.1 Conversely, the 2020 American Urogynecologic Society (AUGS) published a clinical consensus statement that spoke to the promising results of laser therapy for the treatment of vulvovaginal atrophy, vaginal dryness, and menopausal dyspareunia, with benefits lasting up to 1 year.2 This statement also suggested that the short-term safety profile of the CO2 laser device was favorable.
How CO2 lasers work
Fractionated CO2 laser therapy differs from unfractionated treatment (which often is used in the treatment of condyloma) in that it is not ablative. The laser works by using fractionated beams of light to penetrate the affected tissue to create small wounds in the epithelium and underlying lamina propria, which leads to collagen remodeling and regeneration that then results in the restoration of the superficial epithelium, vaginal rugae, and lubrication.3 Most clinicians perform 3 applications of the laser treatment 6 weeks apart, a recommendation that is based on manufacturer-sponsored studies in menopausal women.
Study results of patient outcomes with laser therapy
GSM. Several retrospective4,5 and prospective studies6-10 have looked at short- and longer-term outcomes in patients undergoing treatment with the CO2 laser. All of these studies showed improvement in patient symptoms related to GSM.
The VeLVET trial, conducted by Paraiso and colleagues, was a randomized trial that compared CO2 laser treatment with vaginal estrogen in women with GSM.11 While the study was underpowered due to cessation of enrollment once the FDA safety warning was issued, the authors reported that at 6 months, both the fractionated CO2 laser therapy group and the vaginal estrogen group had similar improvements, with 70% to 80% of participants reporting satisfaction with treatment. The authors concluded that laser therapy is likely to be as efficacious as vaginal estrogen and may be a good option for patients who cannot use vaginal estrogen to treat GSM.11
Lichen sclerosus. Some data exist on the efficacy of laser therapy for the treatment of lichen sclerosus. One recently published randomized trial showed that at 6 months, fractionated CO2 laser treatment and prior treatment with high potency topical corticosteroids was associated with higher improvement in subjective symptoms and objective measures compared with clobetasol propionate treatment.12 Another trial, however, revealed that laser treatment was not an effective monotherapy treatment for lichen sclerosus when compared with placebo.13 Fewer studies have examined the effect of laser therapy on urinary incontinence.
More prospective data are emerging, evidenced by trials currently registered in ClinicalTrials.gov. While some studies provide evidence that laser therapy may be efficacious in the treatment of vulvovaginal atrophy, additional data are needed to confirm the favorable outcomes observed with laser therapy for the treatment of lichen sclerosus, and a significant amount of data are needed to evaluate the efficacy of laser treatment for urinary incontinence.
Until such evidence is available, fractionated CO2 vaginal laser therapy will remain a fee-for-service treatment option and will be inaccessible to patients who cannot afford the cost of treatment.
Continue to: Hydrogel urethral bulking...
Hydrogel urethral bulking
Urethral bulking agents have been used for 5 decades in the treatment of stress urinary incontinence (SUI) in women. Unlike midurethral slings, in which many medical device companies use the same implant material (microporous, monofilament polypropylene mesh), the material for bulking agents has varied greatly. A 2017 Cochrane review of urethral bulking listed these agents used for this indication: autologous fat, carbon beads, calcium hydroxylapatite, ethylene vinyl alcohol copolymer, glutaraldehyde cross-linked bovine collagen, hyaluronic acid with dextranomer, porcine dermal implant, polytetrafluoroethylene, and silicone particles.14 These agents can be injected through a transurethral or periurethral technique. The review failed to find superiority of one material or injection technique over another.
New bulking agent available
In January 2020, the FDA approved the premarket application for a new bulking agent. This new agent is a permanently implanted, nonresorbable hydrogel that consists of cross-linked polyacrylamide (2.5%) and water (97.5%). It is intended to be used with a transurethral bulking system that includes a rotatable sheath and two 23-guage needles; a total of 1.5 to 2.0 mL of the hydrogel is injected in 3 locations in the proximal urethra per session. Patients may undergo an additional 2 sessions, if needed, at least 4 weeks after the previous session.
Polyacrylamide hydrogel has been used as a bulking agent in cosmetic and ophthalmic surgery for many years, and it was first approved for medical use in Europe in 2001. The initial European data on its use as a urethral bulking agent was published in 2006.15 The first North American data came in 2014 from a multicenter, randomized trial that compared polyacrylamide hydrogel with collagen gel.16 This investigation followed 345 women for 12 months and concluded that the safety and efficacy of polyacrylamide hydrogel was not inferior to collagen, with a little over half of both cohorts demonstrating a 50% or greater decrease in incontinence episodes.
Since these initial studies, 3-year17 and 7-year safety and efficacy data18 have been reported, with reassuring findings, but both studies experienced significant attrition of the original group of patients. The most commonly reported adverse events associated with the procedure are pain at the injection site (4%–14%) and urinary tract infection (3%–7%); transient urinary retention rates range in incidence from 1.5% to 15%.19
Short procedure, long-term results
Given that a urethral bulking procedure can be done in less than 10 minutes in the office under local analgesia, this treatment may lend itself to use in more brittle patient populations. One study of women aged 80 or older showed a greater than 50% decrease in the number of daily pads used for up to 2 years after initial injection.20 Another study found the greatest treatment success in women aged 60 years or older with fewer than 2.5 episodes of SUI per day.21
Platelet-rich plasma therapy
Platelet-rich plasma (PRP) therapy has been used in multiple disciplines for more than 2 decades as a treatment to regenerate damaged tissue, particularly in sports medicine for treating tendonitis as well as in plastic surgery, gynecology, urology, and ophthalmology, and good outcomes have been demonstrated with no serious adverse effects. PRP is a natural product in which high levels of platelets are concentrated through centrifugation with bioactive growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and insulin growth factor (IGF).22 The activated platelets are then injected autologously back into the patient’s tissue. This process releases activated growth factors that accelerate tissue healing by stimulating the number of reparative cells to create collagen production, angiogenesis, and neurogenesis while fighting infection and downregulating the autoimmune system.
Continue to: Uses for PRP in gynecology...
Uses for PRP in gynecology
In gynecology, dating back to 2007 PRP was shown to facilitate wound healing, when Fanning and colleagues reported PRP applications in gynecologic operative wounds, such as hysterectomies and urogynecologic procedures, to reduce postoperative pain.23 In the last decade, there has been a dramatic increasing trend in the application of PRP injections as an alternative therapy in gynecology to improve intimate health. PRP has been used to treat lichen sclerosus, atrophic vaginitis, SUI, and female sexual dysfunction; however, there is a dearth of studies that compare PRP with traditional therapies.
Runels and colleagues described the effects of localized injections of autologous PRP for the treatment of sexual dysfunction early in 2014.24 Those authors pioneered PRP use in women with dyspareunia and other symptoms related to sexual dysfunction. Women were offered PRP injections into the periurethral area of the Skene glands and the clitoris. Sexual satisfaction and pain were improved but results did not reach statistical significance. The results of this pilot study of 11 patients suggested that PRP injections could perhaps be an effective method to treat certain types of female sexual dysfunction, including desire, arousal, lubrication, and orgasm.
In another pilot study, Long and colleagues looked at the effectiveness of local injection of PRP for treating women with SUI.25 In that study, younger patients with mild severity of SUI had promising results, with up to 75% cured or improved. Results in the older group, with 50% cured or improved, did not reach statistical significance. Other small, limited studies have been conducted under the hypothesis that PRP as an “O-shot” may be a promising treatment that is a safe, effective, nonsurgical, and nonhormonal option for women with dyspareunia from lack of lubrication and related sexual dysfunction, such as decreased libido or arousal.26-29 A pilot study by Behnia-Willison and colleagues demonstrated clinical improvement in PRP use as an alternative to topical steroids for lichen sclerosus.30 Several other studies also have shown efficacy for the treatment of lichen sclerosus.31-34
More evidence of efficacy needed
To date, preliminary studies suggest that PRP holds promise for a host of gynecologic conditions. Since PRP is autologous, there are no significant contraindications, and thus far there have been no known serious adverse effects. However, most health insurers still do not cover this therapy, so for now patients must pay out-of-pocket fees for these treatments.
As we continue to investigate therapies in regenerative medicine, the continued efforts of our discipline are required to conduct well-designed prospective, randomized controlled studies. While initial series suggest that PRP is safe, it is unlikely that this therapy will be embraced widely in the paradigm as an alternative treatment option for many genitourinary symptoms of menopause and vulvar disorders until efficacy is better established.
Radiofrequency therapy
For the past 20 years, radiofrequency (RF) energy has been used through the vagina, urethra, and periurethral tissues for the treatment of genitourinary symptoms, with limited success. More recently, because some patients hesitate to receive mesh implants for treatment of urinary incontinence,35 there has been gravitation to office-based procedures.
In contrast to lasers, which transmit energy through light, RF waves (measured in hertz) transform the kinetic energy of the intracellular atoms, which move and collide, generating thermal energy.36,37 RF therapy has been shown to increase the proportion of smooth muscle and connective tissue; stimulate proliferation of the epithelium, neovascularization, and collagen formation in the lamina propria; and improve natural lubrication.36,38 In addition, RF is:
- ablative when the heat is capable of generating ablation and/or necrosis of the epidermis and dermis
- microablative when energy fractionation produces microscopic columns of ablative thermal lesions in the epidermis and upper dermis, resulting in microscopic columns of treated tissue interspersed with areas of untreated skin,39 and
- nonablative when trauma occurs only in the dermis by heating without causing ablation of the epidermis.39
The RF devices discussed below are used with settings for microablation in the treatment of SUI and sexual health/vaginal laxity, and with nonablative settings in the treatment of GSM.
RF for the treatment of urinary incontinence
Studies with RF have shown its benefits in urinary symptoms as secondary outcomes, such as improvement of SUI.38,40 One theory that favors energy devices as a treatment for SUI is that the treatment strengthens suburethral and pubocervical support, thereby decreasing urethral mobility.41
In 2016, the Viveve system (Viveve) received FDA 510(k) clearance for “use in general surgical procedures for electrocoagulation and hemostasis.” A single-site, randomized, nonblinded pilot study compared 1 treatment (group 1) versus 2 treatments (group 2) with the Viveve system for SUI in 35 participants.42 At 12 months, only for group 2 did mean scores on the Incontinence Impact Questionnaire Short Form (IIQ-7) and the International Consultation on Incontinence Modular Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) decrease by the minimum clinically important difference of 16 and 2.52 points, respectively, compared with baseline.
The ThermiVa device (ThermiGen, LLC) received FDA clearance for “use in dermatological and general surgical procedures for electrocoagulation and hemostasis” in 2017. A single-site, prospective, double-blind, randomized controlled pilot trial evaluated the efficacy of this device for the treatment of SUI in 20 participants randomly assigned in a 1:1 fashion to active and sham groups.43 At 12 weeks, mean scores of the Urogenital Distress Inventory (UDI-6) and the ICIQ-UI-SF decreased by the minimal clinically important difference only in the treatment group arm. Additionally, 70% of treatment group participants had a negative stress test at 12 weeks compared with 0% of control group participants.43 In another study of 48 patients who were followed longitudinally for 5 months, a substantial improvement in genital appearance was observed.44 Assessment based on validated instruments demonstrated significant improvements in sexual function and SUI.44
A microablative RF device (Wavetronic 6000 Touch Device, Megapulse HF FRAXX system; Loktal Medical Electronics) consists of a vaginal probe with 64 microneedles at the tip, each capable of penetrating to a depth of 1 mm. During activation, delivery of RF energy, which results in vaporization of tissue at 100 °C, occurs in a preset sequence of 8 needles at a time, preventing the overheating of intervening tissue between adjacent needles.
Slongo and colleagues conducted a 3-arm randomized clinical trial that included 117 climacteric women with SUI.45 In group 1, treatment consisted of 3 monthly sessions of RF; group 2 received 12 weekly sessions of pelvic floor muscle training (PFMT); and group 3 received RF treatment plus PFMT simultaneously. Assessments were conducted at baseline and 30 days after the end of therapy using validated questionnaires and scales for urinary, vaginal, and sexual functions, and cytology was used to assess vaginal atrophy. The association between RF and PFMT showed significant improvement in the SUI symptoms assessed by questionnaire. The vaginal symptoms and dryness showed more substantial improvement with the RF treatment, and vaginal laxity showed similar improvement in the 3 treatment groups.45
Continue to: RF for the treatment of GSM...
RF for the treatment of GSM
For women who are not candidates for localized hormone therapy, as well as others who simply do not wish to use hormones, nonablative RF laser therapy may be an alternative for the management of GSM.
The VIVEVE I trial was one of the largest randomized, sham-controlled trials performed to determine the efficacy of vaginal rejuvenation using surface-cooled RF; 174 women received either RF treatment (90 J/cm2) or sham treatment (1 J/cm2).46 Treated participants had a significant improvement in perception of vaginal laxity/looseness and sexual function up to 6 months posttreatment.46 Overall, participants were satisfied with the treatment (77.8%–100%) and reported significant improvements in vaginal laxity and symptoms of atrophy. RF was well tolerated with minimal adverse effects, such as procedure-related erythema and edema of treated tissue, and vaginal discharge. One patient discontinued treatment because of procedural pain.47,48
The ThermiVa system also was evaluated for efficacy in the treatment of GSM in a single-site, double-blind randomized controlled pilot study, the methods of which were previously described above.43 GSM symptoms were evaluated at baseline and 12 weeks using the Vaginal Health Index (VHI) and visual analog scale (VAS). At the 12-week follow-up, compared with baseline scores, VHI scores were unchanged in the control group and improved in the treatment group. Additionally, VAS scores for dyspareunia decreased in the treatment group compared with baseline while VAS for dyspareunia in the sham group did not change from baseline to 12 weeks.
RF treatment for sexual health
The efficacy of the Viveve RF system for female sexual dysfunction was evaluated in an international, randomized, controlled, single-blinded study (n = 154) that compared 6-month outcomes of RF treatment versus sham treatment.46 Although there was a statistically significant improvement in patient-reported sexual dysfunction on validated instruments, it is essential to note that the study was powered for the primary outcome of vaginal laxity. In addition, the study was not adequately powered to evaluate safety; however, the adverse events reported were mild, and the most frequently reported adverse event was vaginal discharge.
Microablative monopolar RF treatment for GSM has been evaluated in 2 single-arm clinical trials that included a total of 70 patients.39,49 Pre- and posttreatment outcomes were analyzed after delivery of 3 treatment sessions 28 to 40 days apart. Although the only significant improvement in quality of life was in the health domain of the World Health Organization Quality of Life Adapted Questionnaire (P = .04), significant improvements in sexual functioning were seen in terms of the desire (P = .002), lubrication (P = .001), satisfaction (P = .003), and pain (P = .007) domains of the Female Sexual Function Index (FSFI) questionnaire except for excitation and orgasm.39 Overall, 100% of participants reported being satisfied or very satisfied with treatments, and 13 of 14 women felt “cured” or “much better.”39 After treatment, significant increases in vaginal Lactobacillus (P<.001), decreases in vaginal pH (P<.001), improvements in maturation of vaginal cellularity (decreased parabasal cells, P<.001; increased superficial cells, P<.001), and increased VHI score (P<.001) alone occurred.49 No adverse events beyond self-limited vaginal burning and redness were reported.39,49 In another study mentioned above, the combination of RF and PFMT in sexual function does not offer benefits superior to those achieved by the therapies alone.45
Evidence on RF treatment does not support marketing efforts
Radiofrequency devices have been marketed for a variety of genitourinary problems in women, with limited high-quality, randomized, comparative evidence of efficacy and durability in the literature. It is unfortunate that RF treatment continues to be promoted by practitioners around the world who cite small, short-term studies that lack biostatistical rigor in their reporting of protocols and results. Statements from both AUGS and the International Urogynecological Association have heeded caution on the use of lasers but they could not even evaluate RF devices due to lack of evidence.2,41
Informed counseling and shared decision making remain the bottom line
By the year 2025, all members of the Baby Boom generation will be aged 60 or older. While in the past there has been a reluctance to discuss women’s sexual health, urinary incontinence, and GSM, the need for open discussion and a variety of treatment options for these conditions has never been more critical.
Many patients prefer office-based therapies over hospital-based procedures, and others are leery of synthetic implants. These concerns are leading toward great interest in the types of treatments covered in this article. However, it is paramount that clinicians are aware of the evidence-based data behind these emerging options so that we can openly and accurately counsel our patients.
As we have shown, the quality of the data behind these officed-based therapies varies significantly. Until a greater body of research data is available, we must carefully balance our desire to meet patient wishes with solid, informed counseling and shared decision making. ●
- Preti M, Viera-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. J Lower Genital Tract Dis. 2019;23:151-160.
- Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices: AUGS clinical consensus statement. Female Pelvic Med Reconstr Surg. 2020;26:287-298.
- Streicher LF. Vulvar and vaginal fractional CO2 laser treatments for genitourinary syndrome of menopause: NAMS practice pearl. Menopause. 2018;25:571-573.
- Gardner AN, Aschkenazi SO. The short-term efficacy and safety of fractional CO2 laser therapy for vulvovaginal symptoms in menopause, breast cancer, and lichen sclerosus. Menopause. 2021; 28:511-516.
- Balchander D, Nyirjesy P. Fractionated CO2 laser therapy in recalcitrant lichen sclerosus. J Lower Genital Tract Disease. 2020;24:225-228.
- Pieralli A, Fallani MG, Becorpi A, et al. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch Gynecol Obstet. 2016;294:841-846.
- Pieralli A, Bianchi C, Longinotti M, et al. Long-term reliability of fractionated CO2 laser as a treatment of vulvovaginal atrophy (VVA) symptoms. Arch Gynecol Obstet. 2017; 296:973-978.
- Sokol ER, Karram MM. Use of novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24: 810-814.
- Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422.
- Sindou-Faurie T, Louis-Vahdat C, Oueld Es Cheikh E, et al. Evaluation of the efficacy of fractional CO2 laser in the treatment of vulvar and vaginal menopausal symptoms. Arch Gynecol Obstet. 2021;303:955-963.
- Paraiso MFR, Ferrando CA, Sokol ER, at al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2019;27:50-56.
- Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978.
- Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;136:979-987.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lose G, Mouritsen L, Nielson JB. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 2006;98:100-104.
- Sokol ER, Karram MM, Dmochowski R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol. 2014;192:843-849.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol. 2015;68:428-433.
- Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508.
- Kasi AD, Pergialiotis V, Perrea DN, et al. Polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016;27:367-375.
- Vecchioli-Scaldazza CV, Smaali C, Morosetti C, et al. Polyacrylamide hydrogel (Bulkamid) in female patients of 80 or more years with urinary incontinence. Int Braz J Urol. 2014;40:37-43.
- Elmelund M, Sokol ER, Darram MM, et al. Patient characteristics that may influence the effect of urethral injection therapy for female stress urinary incontinence. J Urol. 2019;202:125-131.
- Sanoulis V, Nikolettos N, Vlahos N. The use of platelet-rich plasma in the gynecological clinical setting: a review. HJOG. 2019;18:55-65.
- Fanning J, Murrain L, Flora R, et al. Phase I/II prospective trial of autologous platelet tissue graft in gynecologic surgery. J Minim Invasive Gynecol. 2007;14:633-637.
- Runels CE, Melnick H, DeBourbon E, et al. A pilot study of the effect of localized injections of autologous platelet rich plasma (PRP) for the treatment of female sexual dysfunction. J Womens Health Care. 2014;3:4.
- Long CY, Lin KL, Shen CR, et al. A pilot study: effectiveness of local injection of autologous platelet-rich plasma in treating women with stress urinary incontinence. Sci Rep. 2021;11:1584.
- Matz EJ, Pearlman AM, Terlecki RP. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol. 2018;59:61-65.
- Neto JB. O-Shot: platelets rich plasma in intimate female treatment. J Womens Health Care. 2017;6:5.
- Nikolopoulos KI, Pergialiotis V, Perrea D, et al. Restoration of the pubourethral ligament with platelet rich plasma for the treatment of stress urinary incontinence. Med Hypotheses. 2016;90:29-31.
- Hersant B, SidAhmed-Mezi M, Belkacemi Y, et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with a history of breast cancer: a phase 2 pilot study. Menopause. 2018;25:1124-1130.
- Behnia-Willison F, Pour NR, Mohamadi B, et al. Use of platelet-rich plasma for vulvovaginal autoimmune conditions like lichen sclerosus. Plast Reconstr Surg Glob Open. 2016;4:e1124.
- Goldstein AT, King M, Runels C, et al. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2017;76:158-160.
- Casabona F, Priano V, Vallerino V, et al. New surgical approach to lichen sclerosus of the vulva: the role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast Reconstr Surg. 2010;126:210e-211e.
- Franic D, Iternica Z, Franic-Ivanisevic M. Platelet-rich plasma (PRP) for the treatment of vulvar lichen sclerosus in a premenopausal woman: a case report. Case Rep Womens Health. 2018;18: e0062.
- Posey LK, Runels C. In office surgery and use of platelet rich plasma for the treatment of vulvar lichen sclerosus to alleviate painful sexual intercourse. J Lower Genital Tract Dis. 2017;21(4S):S14.
- Stachowicz AM, Hoover ML, Karram MM. Clinical utility of radiofrequency energy for female genitourinary dysfunction: past, present, and future. Int Urogynecol J. 2021;32:1345-1350.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- US Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, MD, on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” https://www.fda.gov/news-events/pressannouncements/statement-fda-commissioner-scott-gottlieb-mdefforts-safeguard-womens-health-deceptive-health-claims. Updated August 2, 2018. Accessed August 13, 2021.
- Vicariotto F, Raichi M. Technological evolution in the radiofrequency treatment of vaginal laxity and menopausal vulvo-vaginal atrophy and other genitourinary symptoms: first experiences with a novel dynamic quadripolar device. Minerva Ginecol. 2016;68:225-236.
- Kamilos MF, Borrelli CL. New therapeutic option in genitourinary syndrome of menopause: pilot study using microablative fractional radiofrequency. Einstein (Sao Paulo). 2017;15:445-551.
- Caruth JC. Evaluation of the safety and efficacy of a novel radiofrequency device for vaginal treatment. Surg Technol Int. 2018;32:145-149.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Allan BB, Bell S, Husarek K. Early feasibility study to evaluate the Viveve system for female stress urinary incontinence: interim 6-month report. J Womens Health (Larchmt). 2020;29:383-389.
- Leibaschoff G, Izasa PG, Cardona JL, et al. Transcutaneous temperature controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Desai SA, Vakil Z, Kroumpouzos G. Transcutaneous temperature-controlled radiofrequency treatment: improvement in female genital appearance, sexual dysfunction, and stress urinary incontinence. Aesthet Surg J. 2021;sjab174. doi: 10.1093/asj/sjab174.
- Slongo H, Lunardi AL, Riccetto CL, et al. Microablative radiofrequency versus pelvic floor muscle training for stress urinary incontinence: a randomized controlled trial. Int Urogynecol J. 2021. doi: 10.1007 /s00192-021-04758-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30: 429-436.
- Juhasz ML, Korta DZ, Mesinkovska NA. Vaginal rejuvenation: a retrospective review of lasers and radiofrequency devices. Dermatol Surg. 2021;47:489-494.
- Sarmento AC, Fernandes FS, Marconi C, et al. Impact of microablative fractional radiofrequency on the vaginal health, microbiota, and cellularity of postmenopausal women. Clinics (Sao Paulo). 2020;75:e1750.
- Preti M, Viera-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. J Lower Genital Tract Dis. 2019;23:151-160.
- Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices: AUGS clinical consensus statement. Female Pelvic Med Reconstr Surg. 2020;26:287-298.
- Streicher LF. Vulvar and vaginal fractional CO2 laser treatments for genitourinary syndrome of menopause: NAMS practice pearl. Menopause. 2018;25:571-573.
- Gardner AN, Aschkenazi SO. The short-term efficacy and safety of fractional CO2 laser therapy for vulvovaginal symptoms in menopause, breast cancer, and lichen sclerosus. Menopause. 2021; 28:511-516.
- Balchander D, Nyirjesy P. Fractionated CO2 laser therapy in recalcitrant lichen sclerosus. J Lower Genital Tract Disease. 2020;24:225-228.
- Pieralli A, Fallani MG, Becorpi A, et al. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch Gynecol Obstet. 2016;294:841-846.
- Pieralli A, Bianchi C, Longinotti M, et al. Long-term reliability of fractionated CO2 laser as a treatment of vulvovaginal atrophy (VVA) symptoms. Arch Gynecol Obstet. 2017; 296:973-978.
- Sokol ER, Karram MM. Use of novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24: 810-814.
- Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422.
- Sindou-Faurie T, Louis-Vahdat C, Oueld Es Cheikh E, et al. Evaluation of the efficacy of fractional CO2 laser in the treatment of vulvar and vaginal menopausal symptoms. Arch Gynecol Obstet. 2021;303:955-963.
- Paraiso MFR, Ferrando CA, Sokol ER, at al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2019;27:50-56.
- Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978.
- Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;136:979-987.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lose G, Mouritsen L, Nielson JB. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 2006;98:100-104.
- Sokol ER, Karram MM, Dmochowski R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol. 2014;192:843-849.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol. 2015;68:428-433.
- Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508.
- Kasi AD, Pergialiotis V, Perrea DN, et al. Polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016;27:367-375.
- Vecchioli-Scaldazza CV, Smaali C, Morosetti C, et al. Polyacrylamide hydrogel (Bulkamid) in female patients of 80 or more years with urinary incontinence. Int Braz J Urol. 2014;40:37-43.
- Elmelund M, Sokol ER, Darram MM, et al. Patient characteristics that may influence the effect of urethral injection therapy for female stress urinary incontinence. J Urol. 2019;202:125-131.
- Sanoulis V, Nikolettos N, Vlahos N. The use of platelet-rich plasma in the gynecological clinical setting: a review. HJOG. 2019;18:55-65.
- Fanning J, Murrain L, Flora R, et al. Phase I/II prospective trial of autologous platelet tissue graft in gynecologic surgery. J Minim Invasive Gynecol. 2007;14:633-637.
- Runels CE, Melnick H, DeBourbon E, et al. A pilot study of the effect of localized injections of autologous platelet rich plasma (PRP) for the treatment of female sexual dysfunction. J Womens Health Care. 2014;3:4.
- Long CY, Lin KL, Shen CR, et al. A pilot study: effectiveness of local injection of autologous platelet-rich plasma in treating women with stress urinary incontinence. Sci Rep. 2021;11:1584.
- Matz EJ, Pearlman AM, Terlecki RP. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol. 2018;59:61-65.
- Neto JB. O-Shot: platelets rich plasma in intimate female treatment. J Womens Health Care. 2017;6:5.
- Nikolopoulos KI, Pergialiotis V, Perrea D, et al. Restoration of the pubourethral ligament with platelet rich plasma for the treatment of stress urinary incontinence. Med Hypotheses. 2016;90:29-31.
- Hersant B, SidAhmed-Mezi M, Belkacemi Y, et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with a history of breast cancer: a phase 2 pilot study. Menopause. 2018;25:1124-1130.
- Behnia-Willison F, Pour NR, Mohamadi B, et al. Use of platelet-rich plasma for vulvovaginal autoimmune conditions like lichen sclerosus. Plast Reconstr Surg Glob Open. 2016;4:e1124.
- Goldstein AT, King M, Runels C, et al. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2017;76:158-160.
- Casabona F, Priano V, Vallerino V, et al. New surgical approach to lichen sclerosus of the vulva: the role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast Reconstr Surg. 2010;126:210e-211e.
- Franic D, Iternica Z, Franic-Ivanisevic M. Platelet-rich plasma (PRP) for the treatment of vulvar lichen sclerosus in a premenopausal woman: a case report. Case Rep Womens Health. 2018;18: e0062.
- Posey LK, Runels C. In office surgery and use of platelet rich plasma for the treatment of vulvar lichen sclerosus to alleviate painful sexual intercourse. J Lower Genital Tract Dis. 2017;21(4S):S14.
- Stachowicz AM, Hoover ML, Karram MM. Clinical utility of radiofrequency energy for female genitourinary dysfunction: past, present, and future. Int Urogynecol J. 2021;32:1345-1350.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- US Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, MD, on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” https://www.fda.gov/news-events/pressannouncements/statement-fda-commissioner-scott-gottlieb-mdefforts-safeguard-womens-health-deceptive-health-claims. Updated August 2, 2018. Accessed August 13, 2021.
- Vicariotto F, Raichi M. Technological evolution in the radiofrequency treatment of vaginal laxity and menopausal vulvo-vaginal atrophy and other genitourinary symptoms: first experiences with a novel dynamic quadripolar device. Minerva Ginecol. 2016;68:225-236.
- Kamilos MF, Borrelli CL. New therapeutic option in genitourinary syndrome of menopause: pilot study using microablative fractional radiofrequency. Einstein (Sao Paulo). 2017;15:445-551.
- Caruth JC. Evaluation of the safety and efficacy of a novel radiofrequency device for vaginal treatment. Surg Technol Int. 2018;32:145-149.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Allan BB, Bell S, Husarek K. Early feasibility study to evaluate the Viveve system for female stress urinary incontinence: interim 6-month report. J Womens Health (Larchmt). 2020;29:383-389.
- Leibaschoff G, Izasa PG, Cardona JL, et al. Transcutaneous temperature controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Desai SA, Vakil Z, Kroumpouzos G. Transcutaneous temperature-controlled radiofrequency treatment: improvement in female genital appearance, sexual dysfunction, and stress urinary incontinence. Aesthet Surg J. 2021;sjab174. doi: 10.1093/asj/sjab174.
- Slongo H, Lunardi AL, Riccetto CL, et al. Microablative radiofrequency versus pelvic floor muscle training for stress urinary incontinence: a randomized controlled trial. Int Urogynecol J. 2021. doi: 10.1007 /s00192-021-04758-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30: 429-436.
- Juhasz ML, Korta DZ, Mesinkovska NA. Vaginal rejuvenation: a retrospective review of lasers and radiofrequency devices. Dermatol Surg. 2021;47:489-494.
- Sarmento AC, Fernandes FS, Marconi C, et al. Impact of microablative fractional radiofrequency on the vaginal health, microbiota, and cellularity of postmenopausal women. Clinics (Sao Paulo). 2020;75:e1750.
Gender equity and gynecologic surgery: Ensuring a culture of diversity and inclusion
A workplace environment conducive to success includes equal access to resources and opportunities, work-life integration, freedom from gender discrimination and sexual harassment, and supportive leadership. With focused leadership that is accountable for actionable interventions through measurable outcomes, it is possible to create an equitable, safe, and dignified workplace for all ObGyns.
Recently, obstetrics and gynecology has become the only surgical specialty in which a majority of practitioners are women. Since the 1990s, women in ObGyn have composed the majority of trainees, and 2012 marked the first year that more than half of the American College of Obstetricians and Gynecologists (ACOG) Fellows in practice were women.1
Despite the large proportion of women within the specialty, ongoing gender-based inequities continue. Many of these inequities are rooted in our pervasive societal views of behavioral norms based on biologic or perceived sex, otherwise known as “gender,” roles.2 The cultural gender role for men embodies characteristics that are bold, competitive, decisive, analytical; qualities for women include modesty, nurturing, and accommodating in interactions with others. Such male-typed traits and behaviors are termed “agentic” because they involve human agency, whereas female-typed traits and behaviors are termed “communal.”3
Gender biases remain widespread, even among health care providers.4 When gender roles are applied to medical specialties, there is an assumption that women tend toward “communal” specialties, such as pediatrics or family practice, whereas men are better suited for technical or procedural specialties.5 ObGyn is an outlier in this schema because its procedural and surgical aspects would characterize the specialty as “agentic,” yet the majority of ObGyn trainees and physicians are women.
Biases related to gender impact many aspects of practice for the ObGyn, including:
- surgical education and training
- the gender wage gap
- interpersonal interactions and sexual harassment
- advancement and promotion.
Surgical education and training
The message that desirable characteristics for leadership and autonomy are aligned with masculinity is enforced early in medical culture, and it supports the ubiquity of deep-seated stereotypes about gender roles in medicine. For example, the language used for letters of recommendation for women applying to residency and fellowship highlight communal language (nurturing, warm), whereas those for men more typically use agentic terms (decisive, strong, future leader).6 During ObGyn surgical training, women residents receive more negative evaluations than men from nurses throughout training, and they report spending more effort to nurture these relationships, including changing communication in order to engage assistance from nurses.7
Similarly, women trainees receive harsher and more contradictory feedback from attending physicians.8 For example, a woman resident may be criticized for failing to develop independence and execute complete plans for patient care; later, she might be labeled as “rogue” and told that she should engage with and seek input from supervising faculty when independently executing a treatment plan.
Even when attempting to apply feedback in the operating room, women trainees are afforded less surgical autonomy than men trainees.9 These factors contribute to lower surgical confidence in women trainees despite their having the same technical skills as men, as measured by the Fundamentals of Laparoscopic Surgery skills exam.10
Continue to: The gender wage gap...
The gender wage gap
The mean salary for women ObGyns remains lower than that for men at every academic rank, with the differences ranging from $54,700 at the assistant professor rank to $183,200 for the department chair position.11 Notably, the pay discrepancy persists after adjustments are made for common salary-influencing metrics, such as experience, practice construct, and academic productivity.12 The gender salary gap is further identified for women subspecialists, as women reproductive endocrinology and infertility specialists and gynecologic oncologists earn $67,000 and $120,000 less, respectively, than men colleagues.13,14
While the gender wage gap often is attributed to women’s desire to work part time, similar rates of graduating women and men medical students in 2018 ranked schedule flexibility as important, suggesting that work-life balance is related to an individual’s generation rather than gender.11
Parenting status specifically adversely affects women physicians, with an ascribed “motherhood penalty” and “fatherhood bonus” phenomenon: women physicians who became parents lost an additional 6% salary, whereas men physicians saw a salary increase of 4% with parenthood.15
Most worrisome for the specialty is evidence of declining wages for ObGyns relative to other fields. “Occupational segregation”16 refers to the pronounced negative effect on earnings as more women enter a given field, which has been described in other professions.17 Overall, ObGyn salaries are the lowest among surgical specialties18 and show evidence of decline corresponding to the increasing numbers of women in the field.16
Interpersonal interactions and sexual harassment
In the workplace, women in ObGyn face more interpersonal relationship friction than men. Practicing women ObGyns report differing treatment by nurses as compared to men,19 noting that additional time and effort are required to nurture professional relationships. Additionally, nurses and trainees20 evaluate practicing women ObGyns more harshly than they evaluate men. Further, women gynecologic surgeons experience gender bias from patients, as patients endorse a preference to have a woman gynecologist but prefer a man gynecologic surgeon.21
In addition to gender bias, the experience of gender harassment, including sexual harassment, is common, as two-thirds of women gynecologists report workplace harassment, 90% of which is attributed to gender.22 This rate is 3 times higher than that for men, with a senior colleague in a position of power within the same organization reported to be the harasser to women in 91% of occurrences.
Advancement and promotion
Within academia, women faculty face specific career-limiting barriers related to gender. Rates of academic promotion and leadership opportunities remain lower for women than for men faculty. Although there has been more women representation in ObGyn over the past 20 years, the number of women serving as department chairs, cancer center directors, editors-in-chief, or on a board of directors remains lower than what would be expected by representation ratios.23 (Representation ratios were calculated as the proportion of ObGyn department-based leadership roles held by women in 2019 divided by the proportion of women ObGyn residents in 1990; representation ratios <1.0 indicate underrepresentation of women). This lag in attainment of leadership roles is compounded by the difficulties women faculty experience in finding mentorship and sponsorship,24 which are known benefits to career advancement.
Having fewer women hold leadership roles also negatively influences those in training. For example, a survey of emergency medicine and ObGyn residents identified an implicit gender bias that men and women residents favored men for leadership roles.25 This difference, however, was not significant when division chiefs and department chairs were women, which suggests that visibility of women leaders positively influences the stereotype perception of men and women trainees.4
Continue to: Blueprint for change...
Blueprint for change
While the issues surrounding gender bias are widespread, solutions exist to create gender equity within ObGyn. Efforts to change individual behavior and organizational culture should start with an understanding of the current environment.
Multiple studies have promoted the concept of “culture change,”26,27 which parallels a standard change process. A critical aspect of change is that individuals and organizations maintain the status quo until something prompts a desire to achieve a different way of being. As data regarding the breadth and impact of gender bias emerge and awareness is raised, there is recognition that the status quo is not achieving the goals of the department or institution. This may occur through the result of loss of physician talent, reduced access for vulnerable patient populations, or lower financial productivity.
Once change is considered, it must deliberately be pursued through a specific process. The first actionable step is to assess the existing state and then identify prior barriers to and current opportunities for success. A validated instrument that has been applied for this purpose is the Diversity Engagement Survey, a 22-item questionnaire that assesses 8 domains of organizational inclusion on a 5-point Likert scale (see TABLE).28 This tool not only provides a measure of institutional culture but also obtains characteristics of the respondents so that it additionally assesses how engaged specific groups are within the organization. Once baseline data are obtained, an action plan can be formulated and enacted. This cycle of assessment, system influences, plan, and act should be continued until the desired changes are achieved.
It is critically important to identify objective, measurable outcomes to assure that the interventions are moving the culture toward enhanced gender equity. As the ideal state is achieved, development of practices and enforceable policies help to ensure the longevity of cultural changes. Furthermore, periodic re-evaluation of the existing organizational culture will confirm the maintenance of gender equity objectives.
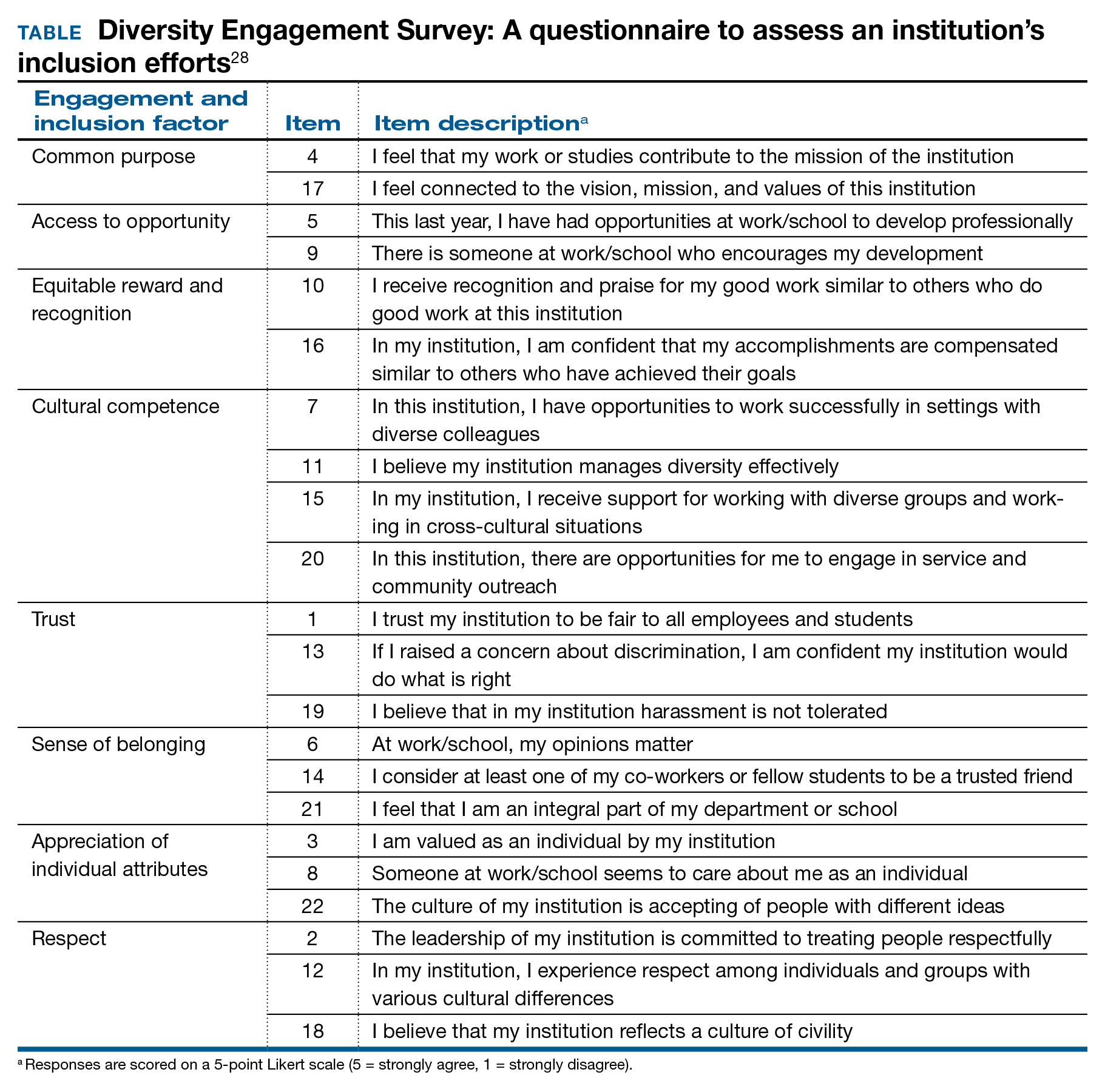
Solutions toward gender equity
Gender inequity may arise from societal gender roles, but it is incumbent on health care organizations to create an environment free from gender bias and gender harassment. An imperative first step is to identify the occurrence of gender discrimination.
The HITS (Hurt, Insulted, Threatened with harm, or Screamed at) screening tool has been used effectively with surgical residents to identify the prevalence of and most common types of abuse.29 This instrument could be adapted and administered to ObGyns in practice or in training. These data should inform the need for system-level antisexist training as well as enforcement of zero-tolerance policies.
Organizations have the ability to create a salary-only compensation model for physicians within the same specialty regardless of academic rank or academic productivity, which has been demonstrated to eliminate gender pay disparity.30 Additional measures to achieve gender equity involve antisexist hiring processes31 and transparency in metrics for job performance, salary, and promotion.32
While health care organizations are obliged to construct a gender-equitable culture, efforts can be made on the individual level. Implicit bias is ascribed to the unconscious attitudes and stereotypes people conclude about groups. The Implicit Association Test (IAT) is a validated instrument that provides the respondent with information about one’s own implicit biases. By uncovering gender bias “blind spots,” an individual can work to consciously overcome these stereotypes. Extending from the mental reframing required for overturning implicit biases, individuals can learn to identify and intervene in real-world situations. This concept of “being an upstander” denotes stepping in and standing up when an inappropriate situation arises33 (see “Case example: Being an upstander”). The targeted individual may not have the ability or safety to navigate through a confrontation, but an upstander might be able to assist the target with empowerment, verbalization of needs, and support.
Lastly, mentorship and sponsorship are critical factors for professional development and career advancement. Bidirectional mentorship identifies benefit for the mentee and the mentor whereby the junior faculty obtain career development and support and the senior faculty may learn new teaching or communication skills.34

A final word
As recognized advocates for women’s health, we must intentionally move toward a workplace that is equitable, safe, and dignified for all ObGyns. Ensuring gender equity within obstetrics and gynecology is everyone’s responsibility. ●
Dr. Bethany Wain is attending a departmental conference and is talking with another member of her division when Dr. Joselle, her division director, approaches. He is accompanied by the Visiting Professor, an internationally reputable and dynamic man, a content expert in the field of work in which Dr. Wain is interested and has published. Dr. Joselle introduces the Visiting Professor formally, using his title of “doctor.” He then introduces Dr. Wain by her abridged first name, Beth.
As an upstander, the Visiting Professor quickly addresses Dr. Wain by her title and uses the situation as a platform to highlight the need to maintain professional address in the professional environment. He then adds that women, who are usually junior in academic rank, confer more benefit to being addressed formally and receiving visibility and respect for their work in a public forum. In this way, the Visiting Professor amplifies Dr. Wain’s work and status and demonstrates the standard of using professional address for women and men.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications, 2017. Washington, DC: American Congress of Obstetricians and Gynecologists; 2017.
- Carnes M. Commentary: deconstructing gender differences. Acad Med. 2010;85:575-577.
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64:644-658.
- Salles A, Awad M, Goldin L, et al. Estimating implicit and explicit gender bias among health care professionals and surgeons. JAMA Netw Open. 2019;2:e196545.
- Carnes M, Bartels CM, Kaatz A, et al. Why is John more likely to become department chair than Jennifer? Trans Am Clin Climatol Assoc. 2015;126:197-214.
- Hoffman A, Grant, W, McCormick, et al. Gendered differences in letters of recommendation for transplant surgery fellowship applicants. J Surg Edu. 2019;76:427-432.
- Galvin SL, Parlier AB, Martino E, et al. Gender bias in nurse evaluations of residents in obstetrics and gynecology. Obstet Gynecol. 2015;126(suppl 4):7S-12S.
- Gerull KM, Loe M, Seiler K, et al. Assessing gender bias in qualitative evaluations of surgical residents. Am J Surg. 2019;217:306-313.
- Meyerson SL, Sternbach JM, Zwischenberger JB, et al. The effect of gender on resident autonomy in the operating room. J Surg Educ. 2017;74:e111-e118.
- Flyckt RL, White EE, Goodman LR, et al. The use of laparoscopy simulation to explore gender differences in resident surgical confidence. Obstet Gynecol Int. 2017;2017:1945801.
- Heisler CA, Mark K, Ton J, et al. Has a critical mass of women resulted in gender equity in gynecologic surgery? Am J Obstet Gynecol. 2020;223:665-673.
- Warner AS, Lehmann LS. Gender wage disparities in medicine: time to close the gap. J Gen Intern Med. 2019;34:1334-1336.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility specialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Croft KM, Rauh LA, Orr JW, et al. Compensation differences by gender in gynecologic oncology. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. 2020. https://sgo.confex.com /sgo/2020/meetingapp.cgi/Paper/15762. 2020. Accessed April 1, 2020.
- Wang SS, Ackerman S. The motherhood penalty: is it alive and well in 2020? J Am Coll Radiol. 2020;17:688-689.
- Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95:1499-1506.
- Hegewisch A, Hartmann H. Occupational segregation and the gender wage gap: a job half done. Institute for Women’s Policy Research. 2014. https://iwpr.org/iwpr-issues/employment-and-earnings /occupational-segregation-and-the-gender-wage-gap-a-job-half -done/. Accessed August 26, 2021.
- Greenberg CC. Association for Academic Surgery presidential address: sticky floors and glass ceilings. J Surg Res. 2017;219:ix-xviii.
- Dossett LA, Vitous CA, Lindquist K, et al. Women surgeons’ experiences of interprofessional workplace conflict. JAMA Netw Open. 2020;3:e2019843.
- Morgan HK, Purkis JA, Porter AC, et al. Student evaluation of faculty physicians: gender differences in teaching evaluations. J Womens Health (Larchmt). 2016;25:453-456.
- Childs AJ, Friedman WH, Schwartz MP, et al. Female patients’ sex preferences in selection of gynecologists and surgeons. South Med J. 2005;98:405-408.
- Brown J, Drury L, Raub K, et al. Workplace harassment and discrimination in gynecology: results of the AAGL Member Survey. J Minim Invasive Gynecol. 2019;26:838-846.
- Temkin AM, Rubinsak L, Benoit MF, et al. Take me to your leader: reporting structures and equity in academic gynecologic oncology. Gynecol Oncol. 2020;157:759-764.
- Shakil S, Redberg RF. Gender disparities in sponsorship—how they perpetuate the glass ceiling. JAMA Intern Med. 2017;177:582.
- Hansen M, Schoonover A, Skarica B, et al. Implicit gender bias among US resident physicians. BMC Med Ed. 2019;19:396.
- Estrada M, Burnett M, Campbell AG, et al. Improving underrepresented minority student persistence in STEM. CBE Life Sci Educ. 2016;15:es5.
- Carnes M, Handelsman J, Sheridan J. Diversity in academic medicine: the stages of change model. J Womens Health (Larchmt). 2005;14:471-475.
- Person SD, Jordan CG, Allison JJ, et al. Measuring diversity and inclusion in academic medicine. The Diversity Engagement Survey. Acad Med. 2015;90:1675-1683.
- Fitzgerald CA, Smith RN, Luo-Owen X, et al. Screening for harassment, abuse, and discrimination among surgery residents: an EAST multicenter trial. Am Surg. 2019;85:456-461.
- Hayes SN, Noseworthy JH, Farrugia G. A structured compensation plan results in equitable physician compensation: a single-center analysis. Mayo Clin Proc. 2020;95:35-43.
- Devine PG, Forscher PS, Cox WT, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215.
- Morgan AU, Chaiyachati KH, Weissman GE, et al. Eliminating genderbased bias in academic medicine: more than naming the “elephant in the room.” J Gen Intern Med. 2018;33:966-968.
- Mello MM, Jagsi R. Standing up against gender bias and harassment— a matter of professional ethics. N Engl J Med. 2020;382:1385-1387.
- Burgess A, van Diggele C, Mellis C. Mentorship in the health profession: a review. Clin Teach. 2018;15:197-202.
A workplace environment conducive to success includes equal access to resources and opportunities, work-life integration, freedom from gender discrimination and sexual harassment, and supportive leadership. With focused leadership that is accountable for actionable interventions through measurable outcomes, it is possible to create an equitable, safe, and dignified workplace for all ObGyns.
Recently, obstetrics and gynecology has become the only surgical specialty in which a majority of practitioners are women. Since the 1990s, women in ObGyn have composed the majority of trainees, and 2012 marked the first year that more than half of the American College of Obstetricians and Gynecologists (ACOG) Fellows in practice were women.1
Despite the large proportion of women within the specialty, ongoing gender-based inequities continue. Many of these inequities are rooted in our pervasive societal views of behavioral norms based on biologic or perceived sex, otherwise known as “gender,” roles.2 The cultural gender role for men embodies characteristics that are bold, competitive, decisive, analytical; qualities for women include modesty, nurturing, and accommodating in interactions with others. Such male-typed traits and behaviors are termed “agentic” because they involve human agency, whereas female-typed traits and behaviors are termed “communal.”3
Gender biases remain widespread, even among health care providers.4 When gender roles are applied to medical specialties, there is an assumption that women tend toward “communal” specialties, such as pediatrics or family practice, whereas men are better suited for technical or procedural specialties.5 ObGyn is an outlier in this schema because its procedural and surgical aspects would characterize the specialty as “agentic,” yet the majority of ObGyn trainees and physicians are women.
Biases related to gender impact many aspects of practice for the ObGyn, including:
- surgical education and training
- the gender wage gap
- interpersonal interactions and sexual harassment
- advancement and promotion.
Surgical education and training
The message that desirable characteristics for leadership and autonomy are aligned with masculinity is enforced early in medical culture, and it supports the ubiquity of deep-seated stereotypes about gender roles in medicine. For example, the language used for letters of recommendation for women applying to residency and fellowship highlight communal language (nurturing, warm), whereas those for men more typically use agentic terms (decisive, strong, future leader).6 During ObGyn surgical training, women residents receive more negative evaluations than men from nurses throughout training, and they report spending more effort to nurture these relationships, including changing communication in order to engage assistance from nurses.7
Similarly, women trainees receive harsher and more contradictory feedback from attending physicians.8 For example, a woman resident may be criticized for failing to develop independence and execute complete plans for patient care; later, she might be labeled as “rogue” and told that she should engage with and seek input from supervising faculty when independently executing a treatment plan.
Even when attempting to apply feedback in the operating room, women trainees are afforded less surgical autonomy than men trainees.9 These factors contribute to lower surgical confidence in women trainees despite their having the same technical skills as men, as measured by the Fundamentals of Laparoscopic Surgery skills exam.10
Continue to: The gender wage gap...
The gender wage gap
The mean salary for women ObGyns remains lower than that for men at every academic rank, with the differences ranging from $54,700 at the assistant professor rank to $183,200 for the department chair position.11 Notably, the pay discrepancy persists after adjustments are made for common salary-influencing metrics, such as experience, practice construct, and academic productivity.12 The gender salary gap is further identified for women subspecialists, as women reproductive endocrinology and infertility specialists and gynecologic oncologists earn $67,000 and $120,000 less, respectively, than men colleagues.13,14
While the gender wage gap often is attributed to women’s desire to work part time, similar rates of graduating women and men medical students in 2018 ranked schedule flexibility as important, suggesting that work-life balance is related to an individual’s generation rather than gender.11
Parenting status specifically adversely affects women physicians, with an ascribed “motherhood penalty” and “fatherhood bonus” phenomenon: women physicians who became parents lost an additional 6% salary, whereas men physicians saw a salary increase of 4% with parenthood.15
Most worrisome for the specialty is evidence of declining wages for ObGyns relative to other fields. “Occupational segregation”16 refers to the pronounced negative effect on earnings as more women enter a given field, which has been described in other professions.17 Overall, ObGyn salaries are the lowest among surgical specialties18 and show evidence of decline corresponding to the increasing numbers of women in the field.16
Interpersonal interactions and sexual harassment
In the workplace, women in ObGyn face more interpersonal relationship friction than men. Practicing women ObGyns report differing treatment by nurses as compared to men,19 noting that additional time and effort are required to nurture professional relationships. Additionally, nurses and trainees20 evaluate practicing women ObGyns more harshly than they evaluate men. Further, women gynecologic surgeons experience gender bias from patients, as patients endorse a preference to have a woman gynecologist but prefer a man gynecologic surgeon.21
In addition to gender bias, the experience of gender harassment, including sexual harassment, is common, as two-thirds of women gynecologists report workplace harassment, 90% of which is attributed to gender.22 This rate is 3 times higher than that for men, with a senior colleague in a position of power within the same organization reported to be the harasser to women in 91% of occurrences.
Advancement and promotion
Within academia, women faculty face specific career-limiting barriers related to gender. Rates of academic promotion and leadership opportunities remain lower for women than for men faculty. Although there has been more women representation in ObGyn over the past 20 years, the number of women serving as department chairs, cancer center directors, editors-in-chief, or on a board of directors remains lower than what would be expected by representation ratios.23 (Representation ratios were calculated as the proportion of ObGyn department-based leadership roles held by women in 2019 divided by the proportion of women ObGyn residents in 1990; representation ratios <1.0 indicate underrepresentation of women). This lag in attainment of leadership roles is compounded by the difficulties women faculty experience in finding mentorship and sponsorship,24 which are known benefits to career advancement.
Having fewer women hold leadership roles also negatively influences those in training. For example, a survey of emergency medicine and ObGyn residents identified an implicit gender bias that men and women residents favored men for leadership roles.25 This difference, however, was not significant when division chiefs and department chairs were women, which suggests that visibility of women leaders positively influences the stereotype perception of men and women trainees.4
Continue to: Blueprint for change...
Blueprint for change
While the issues surrounding gender bias are widespread, solutions exist to create gender equity within ObGyn. Efforts to change individual behavior and organizational culture should start with an understanding of the current environment.
Multiple studies have promoted the concept of “culture change,”26,27 which parallels a standard change process. A critical aspect of change is that individuals and organizations maintain the status quo until something prompts a desire to achieve a different way of being. As data regarding the breadth and impact of gender bias emerge and awareness is raised, there is recognition that the status quo is not achieving the goals of the department or institution. This may occur through the result of loss of physician talent, reduced access for vulnerable patient populations, or lower financial productivity.
Once change is considered, it must deliberately be pursued through a specific process. The first actionable step is to assess the existing state and then identify prior barriers to and current opportunities for success. A validated instrument that has been applied for this purpose is the Diversity Engagement Survey, a 22-item questionnaire that assesses 8 domains of organizational inclusion on a 5-point Likert scale (see TABLE).28 This tool not only provides a measure of institutional culture but also obtains characteristics of the respondents so that it additionally assesses how engaged specific groups are within the organization. Once baseline data are obtained, an action plan can be formulated and enacted. This cycle of assessment, system influences, plan, and act should be continued until the desired changes are achieved.
It is critically important to identify objective, measurable outcomes to assure that the interventions are moving the culture toward enhanced gender equity. As the ideal state is achieved, development of practices and enforceable policies help to ensure the longevity of cultural changes. Furthermore, periodic re-evaluation of the existing organizational culture will confirm the maintenance of gender equity objectives.

Solutions toward gender equity
Gender inequity may arise from societal gender roles, but it is incumbent on health care organizations to create an environment free from gender bias and gender harassment. An imperative first step is to identify the occurrence of gender discrimination.
The HITS (Hurt, Insulted, Threatened with harm, or Screamed at) screening tool has been used effectively with surgical residents to identify the prevalence of and most common types of abuse.29 This instrument could be adapted and administered to ObGyns in practice or in training. These data should inform the need for system-level antisexist training as well as enforcement of zero-tolerance policies.
Organizations have the ability to create a salary-only compensation model for physicians within the same specialty regardless of academic rank or academic productivity, which has been demonstrated to eliminate gender pay disparity.30 Additional measures to achieve gender equity involve antisexist hiring processes31 and transparency in metrics for job performance, salary, and promotion.32
While health care organizations are obliged to construct a gender-equitable culture, efforts can be made on the individual level. Implicit bias is ascribed to the unconscious attitudes and stereotypes people conclude about groups. The Implicit Association Test (IAT) is a validated instrument that provides the respondent with information about one’s own implicit biases. By uncovering gender bias “blind spots,” an individual can work to consciously overcome these stereotypes. Extending from the mental reframing required for overturning implicit biases, individuals can learn to identify and intervene in real-world situations. This concept of “being an upstander” denotes stepping in and standing up when an inappropriate situation arises33 (see “Case example: Being an upstander”). The targeted individual may not have the ability or safety to navigate through a confrontation, but an upstander might be able to assist the target with empowerment, verbalization of needs, and support.
Lastly, mentorship and sponsorship are critical factors for professional development and career advancement. Bidirectional mentorship identifies benefit for the mentee and the mentor whereby the junior faculty obtain career development and support and the senior faculty may learn new teaching or communication skills.34

A final word
As recognized advocates for women’s health, we must intentionally move toward a workplace that is equitable, safe, and dignified for all ObGyns. Ensuring gender equity within obstetrics and gynecology is everyone’s responsibility. ●
Dr. Bethany Wain is attending a departmental conference and is talking with another member of her division when Dr. Joselle, her division director, approaches. He is accompanied by the Visiting Professor, an internationally reputable and dynamic man, a content expert in the field of work in which Dr. Wain is interested and has published. Dr. Joselle introduces the Visiting Professor formally, using his title of “doctor.” He then introduces Dr. Wain by her abridged first name, Beth.
As an upstander, the Visiting Professor quickly addresses Dr. Wain by her title and uses the situation as a platform to highlight the need to maintain professional address in the professional environment. He then adds that women, who are usually junior in academic rank, confer more benefit to being addressed formally and receiving visibility and respect for their work in a public forum. In this way, the Visiting Professor amplifies Dr. Wain’s work and status and demonstrates the standard of using professional address for women and men.
A workplace environment conducive to success includes equal access to resources and opportunities, work-life integration, freedom from gender discrimination and sexual harassment, and supportive leadership. With focused leadership that is accountable for actionable interventions through measurable outcomes, it is possible to create an equitable, safe, and dignified workplace for all ObGyns.
Recently, obstetrics and gynecology has become the only surgical specialty in which a majority of practitioners are women. Since the 1990s, women in ObGyn have composed the majority of trainees, and 2012 marked the first year that more than half of the American College of Obstetricians and Gynecologists (ACOG) Fellows in practice were women.1
Despite the large proportion of women within the specialty, ongoing gender-based inequities continue. Many of these inequities are rooted in our pervasive societal views of behavioral norms based on biologic or perceived sex, otherwise known as “gender,” roles.2 The cultural gender role for men embodies characteristics that are bold, competitive, decisive, analytical; qualities for women include modesty, nurturing, and accommodating in interactions with others. Such male-typed traits and behaviors are termed “agentic” because they involve human agency, whereas female-typed traits and behaviors are termed “communal.”3
Gender biases remain widespread, even among health care providers.4 When gender roles are applied to medical specialties, there is an assumption that women tend toward “communal” specialties, such as pediatrics or family practice, whereas men are better suited for technical or procedural specialties.5 ObGyn is an outlier in this schema because its procedural and surgical aspects would characterize the specialty as “agentic,” yet the majority of ObGyn trainees and physicians are women.
Biases related to gender impact many aspects of practice for the ObGyn, including:
- surgical education and training
- the gender wage gap
- interpersonal interactions and sexual harassment
- advancement and promotion.
Surgical education and training
The message that desirable characteristics for leadership and autonomy are aligned with masculinity is enforced early in medical culture, and it supports the ubiquity of deep-seated stereotypes about gender roles in medicine. For example, the language used for letters of recommendation for women applying to residency and fellowship highlight communal language (nurturing, warm), whereas those for men more typically use agentic terms (decisive, strong, future leader).6 During ObGyn surgical training, women residents receive more negative evaluations than men from nurses throughout training, and they report spending more effort to nurture these relationships, including changing communication in order to engage assistance from nurses.7
Similarly, women trainees receive harsher and more contradictory feedback from attending physicians.8 For example, a woman resident may be criticized for failing to develop independence and execute complete plans for patient care; later, she might be labeled as “rogue” and told that she should engage with and seek input from supervising faculty when independently executing a treatment plan.
Even when attempting to apply feedback in the operating room, women trainees are afforded less surgical autonomy than men trainees.9 These factors contribute to lower surgical confidence in women trainees despite their having the same technical skills as men, as measured by the Fundamentals of Laparoscopic Surgery skills exam.10
Continue to: The gender wage gap...
The gender wage gap
The mean salary for women ObGyns remains lower than that for men at every academic rank, with the differences ranging from $54,700 at the assistant professor rank to $183,200 for the department chair position.11 Notably, the pay discrepancy persists after adjustments are made for common salary-influencing metrics, such as experience, practice construct, and academic productivity.12 The gender salary gap is further identified for women subspecialists, as women reproductive endocrinology and infertility specialists and gynecologic oncologists earn $67,000 and $120,000 less, respectively, than men colleagues.13,14
While the gender wage gap often is attributed to women’s desire to work part time, similar rates of graduating women and men medical students in 2018 ranked schedule flexibility as important, suggesting that work-life balance is related to an individual’s generation rather than gender.11
Parenting status specifically adversely affects women physicians, with an ascribed “motherhood penalty” and “fatherhood bonus” phenomenon: women physicians who became parents lost an additional 6% salary, whereas men physicians saw a salary increase of 4% with parenthood.15
Most worrisome for the specialty is evidence of declining wages for ObGyns relative to other fields. “Occupational segregation”16 refers to the pronounced negative effect on earnings as more women enter a given field, which has been described in other professions.17 Overall, ObGyn salaries are the lowest among surgical specialties18 and show evidence of decline corresponding to the increasing numbers of women in the field.16
Interpersonal interactions and sexual harassment
In the workplace, women in ObGyn face more interpersonal relationship friction than men. Practicing women ObGyns report differing treatment by nurses as compared to men,19 noting that additional time and effort are required to nurture professional relationships. Additionally, nurses and trainees20 evaluate practicing women ObGyns more harshly than they evaluate men. Further, women gynecologic surgeons experience gender bias from patients, as patients endorse a preference to have a woman gynecologist but prefer a man gynecologic surgeon.21
In addition to gender bias, the experience of gender harassment, including sexual harassment, is common, as two-thirds of women gynecologists report workplace harassment, 90% of which is attributed to gender.22 This rate is 3 times higher than that for men, with a senior colleague in a position of power within the same organization reported to be the harasser to women in 91% of occurrences.
Advancement and promotion
Within academia, women faculty face specific career-limiting barriers related to gender. Rates of academic promotion and leadership opportunities remain lower for women than for men faculty. Although there has been more women representation in ObGyn over the past 20 years, the number of women serving as department chairs, cancer center directors, editors-in-chief, or on a board of directors remains lower than what would be expected by representation ratios.23 (Representation ratios were calculated as the proportion of ObGyn department-based leadership roles held by women in 2019 divided by the proportion of women ObGyn residents in 1990; representation ratios <1.0 indicate underrepresentation of women). This lag in attainment of leadership roles is compounded by the difficulties women faculty experience in finding mentorship and sponsorship,24 which are known benefits to career advancement.
Having fewer women hold leadership roles also negatively influences those in training. For example, a survey of emergency medicine and ObGyn residents identified an implicit gender bias that men and women residents favored men for leadership roles.25 This difference, however, was not significant when division chiefs and department chairs were women, which suggests that visibility of women leaders positively influences the stereotype perception of men and women trainees.4
Continue to: Blueprint for change...
Blueprint for change
While the issues surrounding gender bias are widespread, solutions exist to create gender equity within ObGyn. Efforts to change individual behavior and organizational culture should start with an understanding of the current environment.
Multiple studies have promoted the concept of “culture change,”26,27 which parallels a standard change process. A critical aspect of change is that individuals and organizations maintain the status quo until something prompts a desire to achieve a different way of being. As data regarding the breadth and impact of gender bias emerge and awareness is raised, there is recognition that the status quo is not achieving the goals of the department or institution. This may occur through the result of loss of physician talent, reduced access for vulnerable patient populations, or lower financial productivity.
Once change is considered, it must deliberately be pursued through a specific process. The first actionable step is to assess the existing state and then identify prior barriers to and current opportunities for success. A validated instrument that has been applied for this purpose is the Diversity Engagement Survey, a 22-item questionnaire that assesses 8 domains of organizational inclusion on a 5-point Likert scale (see TABLE).28 This tool not only provides a measure of institutional culture but also obtains characteristics of the respondents so that it additionally assesses how engaged specific groups are within the organization. Once baseline data are obtained, an action plan can be formulated and enacted. This cycle of assessment, system influences, plan, and act should be continued until the desired changes are achieved.
It is critically important to identify objective, measurable outcomes to assure that the interventions are moving the culture toward enhanced gender equity. As the ideal state is achieved, development of practices and enforceable policies help to ensure the longevity of cultural changes. Furthermore, periodic re-evaluation of the existing organizational culture will confirm the maintenance of gender equity objectives.

Solutions toward gender equity
Gender inequity may arise from societal gender roles, but it is incumbent on health care organizations to create an environment free from gender bias and gender harassment. An imperative first step is to identify the occurrence of gender discrimination.
The HITS (Hurt, Insulted, Threatened with harm, or Screamed at) screening tool has been used effectively with surgical residents to identify the prevalence of and most common types of abuse.29 This instrument could be adapted and administered to ObGyns in practice or in training. These data should inform the need for system-level antisexist training as well as enforcement of zero-tolerance policies.
Organizations have the ability to create a salary-only compensation model for physicians within the same specialty regardless of academic rank or academic productivity, which has been demonstrated to eliminate gender pay disparity.30 Additional measures to achieve gender equity involve antisexist hiring processes31 and transparency in metrics for job performance, salary, and promotion.32
While health care organizations are obliged to construct a gender-equitable culture, efforts can be made on the individual level. Implicit bias is ascribed to the unconscious attitudes and stereotypes people conclude about groups. The Implicit Association Test (IAT) is a validated instrument that provides the respondent with information about one’s own implicit biases. By uncovering gender bias “blind spots,” an individual can work to consciously overcome these stereotypes. Extending from the mental reframing required for overturning implicit biases, individuals can learn to identify and intervene in real-world situations. This concept of “being an upstander” denotes stepping in and standing up when an inappropriate situation arises33 (see “Case example: Being an upstander”). The targeted individual may not have the ability or safety to navigate through a confrontation, but an upstander might be able to assist the target with empowerment, verbalization of needs, and support.
Lastly, mentorship and sponsorship are critical factors for professional development and career advancement. Bidirectional mentorship identifies benefit for the mentee and the mentor whereby the junior faculty obtain career development and support and the senior faculty may learn new teaching or communication skills.34

A final word
As recognized advocates for women’s health, we must intentionally move toward a workplace that is equitable, safe, and dignified for all ObGyns. Ensuring gender equity within obstetrics and gynecology is everyone’s responsibility. ●
Dr. Bethany Wain is attending a departmental conference and is talking with another member of her division when Dr. Joselle, her division director, approaches. He is accompanied by the Visiting Professor, an internationally reputable and dynamic man, a content expert in the field of work in which Dr. Wain is interested and has published. Dr. Joselle introduces the Visiting Professor formally, using his title of “doctor.” He then introduces Dr. Wain by her abridged first name, Beth.
As an upstander, the Visiting Professor quickly addresses Dr. Wain by her title and uses the situation as a platform to highlight the need to maintain professional address in the professional environment. He then adds that women, who are usually junior in academic rank, confer more benefit to being addressed formally and receiving visibility and respect for their work in a public forum. In this way, the Visiting Professor amplifies Dr. Wain’s work and status and demonstrates the standard of using professional address for women and men.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications, 2017. Washington, DC: American Congress of Obstetricians and Gynecologists; 2017.
- Carnes M. Commentary: deconstructing gender differences. Acad Med. 2010;85:575-577.
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64:644-658.
- Salles A, Awad M, Goldin L, et al. Estimating implicit and explicit gender bias among health care professionals and surgeons. JAMA Netw Open. 2019;2:e196545.
- Carnes M, Bartels CM, Kaatz A, et al. Why is John more likely to become department chair than Jennifer? Trans Am Clin Climatol Assoc. 2015;126:197-214.
- Hoffman A, Grant, W, McCormick, et al. Gendered differences in letters of recommendation for transplant surgery fellowship applicants. J Surg Edu. 2019;76:427-432.
- Galvin SL, Parlier AB, Martino E, et al. Gender bias in nurse evaluations of residents in obstetrics and gynecology. Obstet Gynecol. 2015;126(suppl 4):7S-12S.
- Gerull KM, Loe M, Seiler K, et al. Assessing gender bias in qualitative evaluations of surgical residents. Am J Surg. 2019;217:306-313.
- Meyerson SL, Sternbach JM, Zwischenberger JB, et al. The effect of gender on resident autonomy in the operating room. J Surg Educ. 2017;74:e111-e118.
- Flyckt RL, White EE, Goodman LR, et al. The use of laparoscopy simulation to explore gender differences in resident surgical confidence. Obstet Gynecol Int. 2017;2017:1945801.
- Heisler CA, Mark K, Ton J, et al. Has a critical mass of women resulted in gender equity in gynecologic surgery? Am J Obstet Gynecol. 2020;223:665-673.
- Warner AS, Lehmann LS. Gender wage disparities in medicine: time to close the gap. J Gen Intern Med. 2019;34:1334-1336.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility specialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Croft KM, Rauh LA, Orr JW, et al. Compensation differences by gender in gynecologic oncology. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. 2020. https://sgo.confex.com /sgo/2020/meetingapp.cgi/Paper/15762. 2020. Accessed April 1, 2020.
- Wang SS, Ackerman S. The motherhood penalty: is it alive and well in 2020? J Am Coll Radiol. 2020;17:688-689.
- Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95:1499-1506.
- Hegewisch A, Hartmann H. Occupational segregation and the gender wage gap: a job half done. Institute for Women’s Policy Research. 2014. https://iwpr.org/iwpr-issues/employment-and-earnings /occupational-segregation-and-the-gender-wage-gap-a-job-half -done/. Accessed August 26, 2021.
- Greenberg CC. Association for Academic Surgery presidential address: sticky floors and glass ceilings. J Surg Res. 2017;219:ix-xviii.
- Dossett LA, Vitous CA, Lindquist K, et al. Women surgeons’ experiences of interprofessional workplace conflict. JAMA Netw Open. 2020;3:e2019843.
- Morgan HK, Purkis JA, Porter AC, et al. Student evaluation of faculty physicians: gender differences in teaching evaluations. J Womens Health (Larchmt). 2016;25:453-456.
- Childs AJ, Friedman WH, Schwartz MP, et al. Female patients’ sex preferences in selection of gynecologists and surgeons. South Med J. 2005;98:405-408.
- Brown J, Drury L, Raub K, et al. Workplace harassment and discrimination in gynecology: results of the AAGL Member Survey. J Minim Invasive Gynecol. 2019;26:838-846.
- Temkin AM, Rubinsak L, Benoit MF, et al. Take me to your leader: reporting structures and equity in academic gynecologic oncology. Gynecol Oncol. 2020;157:759-764.
- Shakil S, Redberg RF. Gender disparities in sponsorship—how they perpetuate the glass ceiling. JAMA Intern Med. 2017;177:582.
- Hansen M, Schoonover A, Skarica B, et al. Implicit gender bias among US resident physicians. BMC Med Ed. 2019;19:396.
- Estrada M, Burnett M, Campbell AG, et al. Improving underrepresented minority student persistence in STEM. CBE Life Sci Educ. 2016;15:es5.
- Carnes M, Handelsman J, Sheridan J. Diversity in academic medicine: the stages of change model. J Womens Health (Larchmt). 2005;14:471-475.
- Person SD, Jordan CG, Allison JJ, et al. Measuring diversity and inclusion in academic medicine. The Diversity Engagement Survey. Acad Med. 2015;90:1675-1683.
- Fitzgerald CA, Smith RN, Luo-Owen X, et al. Screening for harassment, abuse, and discrimination among surgery residents: an EAST multicenter trial. Am Surg. 2019;85:456-461.
- Hayes SN, Noseworthy JH, Farrugia G. A structured compensation plan results in equitable physician compensation: a single-center analysis. Mayo Clin Proc. 2020;95:35-43.
- Devine PG, Forscher PS, Cox WT, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215.
- Morgan AU, Chaiyachati KH, Weissman GE, et al. Eliminating genderbased bias in academic medicine: more than naming the “elephant in the room.” J Gen Intern Med. 2018;33:966-968.
- Mello MM, Jagsi R. Standing up against gender bias and harassment— a matter of professional ethics. N Engl J Med. 2020;382:1385-1387.
- Burgess A, van Diggele C, Mellis C. Mentorship in the health profession: a review. Clin Teach. 2018;15:197-202.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications, 2017. Washington, DC: American Congress of Obstetricians and Gynecologists; 2017.
- Carnes M. Commentary: deconstructing gender differences. Acad Med. 2010;85:575-577.
- Eagly AH. The his and hers of prosocial behavior: an examination of the social psychology of gender. Am Psychol. 2009;64:644-658.
- Salles A, Awad M, Goldin L, et al. Estimating implicit and explicit gender bias among health care professionals and surgeons. JAMA Netw Open. 2019;2:e196545.
- Carnes M, Bartels CM, Kaatz A, et al. Why is John more likely to become department chair than Jennifer? Trans Am Clin Climatol Assoc. 2015;126:197-214.
- Hoffman A, Grant, W, McCormick, et al. Gendered differences in letters of recommendation for transplant surgery fellowship applicants. J Surg Edu. 2019;76:427-432.
- Galvin SL, Parlier AB, Martino E, et al. Gender bias in nurse evaluations of residents in obstetrics and gynecology. Obstet Gynecol. 2015;126(suppl 4):7S-12S.
- Gerull KM, Loe M, Seiler K, et al. Assessing gender bias in qualitative evaluations of surgical residents. Am J Surg. 2019;217:306-313.
- Meyerson SL, Sternbach JM, Zwischenberger JB, et al. The effect of gender on resident autonomy in the operating room. J Surg Educ. 2017;74:e111-e118.
- Flyckt RL, White EE, Goodman LR, et al. The use of laparoscopy simulation to explore gender differences in resident surgical confidence. Obstet Gynecol Int. 2017;2017:1945801.
- Heisler CA, Mark K, Ton J, et al. Has a critical mass of women resulted in gender equity in gynecologic surgery? Am J Obstet Gynecol. 2020;223:665-673.
- Warner AS, Lehmann LS. Gender wage disparities in medicine: time to close the gap. J Gen Intern Med. 2019;34:1334-1336.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility specialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Croft KM, Rauh LA, Orr JW, et al. Compensation differences by gender in gynecologic oncology. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. 2020. https://sgo.confex.com /sgo/2020/meetingapp.cgi/Paper/15762. 2020. Accessed April 1, 2020.
- Wang SS, Ackerman S. The motherhood penalty: is it alive and well in 2020? J Am Coll Radiol. 2020;17:688-689.
- Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95:1499-1506.
- Hegewisch A, Hartmann H. Occupational segregation and the gender wage gap: a job half done. Institute for Women’s Policy Research. 2014. https://iwpr.org/iwpr-issues/employment-and-earnings /occupational-segregation-and-the-gender-wage-gap-a-job-half -done/. Accessed August 26, 2021.
- Greenberg CC. Association for Academic Surgery presidential address: sticky floors and glass ceilings. J Surg Res. 2017;219:ix-xviii.
- Dossett LA, Vitous CA, Lindquist K, et al. Women surgeons’ experiences of interprofessional workplace conflict. JAMA Netw Open. 2020;3:e2019843.
- Morgan HK, Purkis JA, Porter AC, et al. Student evaluation of faculty physicians: gender differences in teaching evaluations. J Womens Health (Larchmt). 2016;25:453-456.
- Childs AJ, Friedman WH, Schwartz MP, et al. Female patients’ sex preferences in selection of gynecologists and surgeons. South Med J. 2005;98:405-408.
- Brown J, Drury L, Raub K, et al. Workplace harassment and discrimination in gynecology: results of the AAGL Member Survey. J Minim Invasive Gynecol. 2019;26:838-846.
- Temkin AM, Rubinsak L, Benoit MF, et al. Take me to your leader: reporting structures and equity in academic gynecologic oncology. Gynecol Oncol. 2020;157:759-764.
- Shakil S, Redberg RF. Gender disparities in sponsorship—how they perpetuate the glass ceiling. JAMA Intern Med. 2017;177:582.
- Hansen M, Schoonover A, Skarica B, et al. Implicit gender bias among US resident physicians. BMC Med Ed. 2019;19:396.
- Estrada M, Burnett M, Campbell AG, et al. Improving underrepresented minority student persistence in STEM. CBE Life Sci Educ. 2016;15:es5.
- Carnes M, Handelsman J, Sheridan J. Diversity in academic medicine: the stages of change model. J Womens Health (Larchmt). 2005;14:471-475.
- Person SD, Jordan CG, Allison JJ, et al. Measuring diversity and inclusion in academic medicine. The Diversity Engagement Survey. Acad Med. 2015;90:1675-1683.
- Fitzgerald CA, Smith RN, Luo-Owen X, et al. Screening for harassment, abuse, and discrimination among surgery residents: an EAST multicenter trial. Am Surg. 2019;85:456-461.
- Hayes SN, Noseworthy JH, Farrugia G. A structured compensation plan results in equitable physician compensation: a single-center analysis. Mayo Clin Proc. 2020;95:35-43.
- Devine PG, Forscher PS, Cox WT, et al. A gender bias habit-breaking intervention led to increased hiring of female faculty in STEMM departments. J Exp Soc Psychol. 2017;73:211-215.
- Morgan AU, Chaiyachati KH, Weissman GE, et al. Eliminating genderbased bias in academic medicine: more than naming the “elephant in the room.” J Gen Intern Med. 2018;33:966-968.
- Mello MM, Jagsi R. Standing up against gender bias and harassment— a matter of professional ethics. N Engl J Med. 2020;382:1385-1387.
- Burgess A, van Diggele C, Mellis C. Mentorship in the health profession: a review. Clin Teach. 2018;15:197-202.
Is the 52-mg LNG-IUD effective as emergency contraception?
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
EXPERT COMMENTARY
Emergency contraception refers to therapies used to prevent pregnancy after inadequately protected intercourse.1 Evidence-based forms of EC available in the United States include oral LNG, oral ulipristal acetate, and the copper IUD. The copper IUD provides not only EC but also highly effective contraception after placement.2 The LNG-IUD has a favorable side effect profile compared with the copper IUD and is theorized to act as EC through direct interference with sperm and oviduct transport.3 Recently, Turok and colleagues conducted a noninferiority trial designed to investigate the EC effectiveness of the LNG-IUD compared with the copper IUD.3
Details of the study
Turok and colleagues recruited participants aged 18 to 35 who requested EC from 6 family planning clinics in Utah from 2016 to 2019. Participants who reported unprotected intercourse within the past 120 hours and who desired an IUD to prevent pregnancy for at least 1 year were randomly assigned to receive either the LNG-IUD or the copper IUD. Individuals were excluded from the trial if they had contraindications to IUD placement, were breastfeeding, had abnormal uterine bleeding, had irregular menses, were currently using highly effective contraception, or had recent EC use. Researchers determined pregnancy status at 1 month through a pregnancy test or clinical records review.
Results. Of 711 participants randomly assigned, 317 who received the LNG-IUD and 321 who received the copper IUD provided 1-month outcome data. Pregnancy 1 month after IUD placement occurred in 1 participant (0.3%) in the LNG-IUD group and in no participants in the copper IUD group (0%). The between-group difference of 0.3 percentage points was within the margin of noninferiority and was not significant.
Study strengths and limitations
This large, multicenter randomized controlled trial contributes novel information about the effectiveness and noninferiority of the LNG-IUD as EC. Unlike prior studies of oral EC, which commonly limited participants to 1 episode of unprotected intercourse, this trial enrolled women at potentially higher risk of pregnancy with multiple episodes of intercourse and found fewer pregnancies than expected. Randomization ensured equivalence between groups, with the exception of the reason for needing EC.
Study limitations include a higher than expected rate of loss to follow-up, requiring clinical records and survey data to confirm pregnancy status. After randomization, clinicians were unable to place IUDs in more than 5% of participants in both groups; noninferiority was demonstrated nonetheless. This study did not include participants receiving oral EC, so direct comparison of effectiveness is not possible. Pregnancy rates among IUD users in this study were favorable to rates reported in previous studies of oral EC.4
When choosing an IUD for contraception, more women select the LNG-IUD for its favorable side effect profile and reduction in menstrual bleeding. In this randomized IUD study, only 7% of eligible participants enrolled, potentially introducing selection bias. The majority who declined enrollment did not want an IUD. Previous studies that allowed participants to choose their IUD had higher enrollment rates. ●
The study by Turok and colleagues is the largest randomized controlled trial to date of IUDs as EC. It demonstrated that LNG-IUDs are noninferior to copper IUDs in preventing pregnancy when placed within 5 days of unprotected intercourse. IUDs offer advantages over oral EC methods: only IUDs provide ongoing contraception after EC, and IUD efficacy does not vary by body mass index. It is reasonable for clinicians and patients to consider LNG-IUDs among EC options after shared decision making.
This study suggests that quick-start placement of the LNG-IUD at any time in the menstrual cycle is reasonable given its effectiveness as EC. Additionally, there were no pregnancies among 138 study participants who resumed intercourse within 7 days of LNG-IUD placement, most of whom did not use backup contraception.5 While current guidelines still recommend backup contraception after LNG-IUD placement, clinicians may reassure patients with unprotected intercourse following any type of IUD placement about the low risk of pregnancy.
LISA HOFLER, MD, MPH, MBA,
AND SMITA CARROLL, MD, MBA
- ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 152: emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
- Fay KE, Clement AC, Gero A, et al. Rates of pregnancy among levonorgestrel and copper intrauterine emergency contraception initiators: implications for backup contraception recommendations. Contraception. 2021;S0010-7824(21)00210-9.
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
EXPERT COMMENTARY
Emergency contraception refers to therapies used to prevent pregnancy after inadequately protected intercourse.1 Evidence-based forms of EC available in the United States include oral LNG, oral ulipristal acetate, and the copper IUD. The copper IUD provides not only EC but also highly effective contraception after placement.2 The LNG-IUD has a favorable side effect profile compared with the copper IUD and is theorized to act as EC through direct interference with sperm and oviduct transport.3 Recently, Turok and colleagues conducted a noninferiority trial designed to investigate the EC effectiveness of the LNG-IUD compared with the copper IUD.3
Details of the study
Turok and colleagues recruited participants aged 18 to 35 who requested EC from 6 family planning clinics in Utah from 2016 to 2019. Participants who reported unprotected intercourse within the past 120 hours and who desired an IUD to prevent pregnancy for at least 1 year were randomly assigned to receive either the LNG-IUD or the copper IUD. Individuals were excluded from the trial if they had contraindications to IUD placement, were breastfeeding, had abnormal uterine bleeding, had irregular menses, were currently using highly effective contraception, or had recent EC use. Researchers determined pregnancy status at 1 month through a pregnancy test or clinical records review.
Results. Of 711 participants randomly assigned, 317 who received the LNG-IUD and 321 who received the copper IUD provided 1-month outcome data. Pregnancy 1 month after IUD placement occurred in 1 participant (0.3%) in the LNG-IUD group and in no participants in the copper IUD group (0%). The between-group difference of 0.3 percentage points was within the margin of noninferiority and was not significant.
Study strengths and limitations
This large, multicenter randomized controlled trial contributes novel information about the effectiveness and noninferiority of the LNG-IUD as EC. Unlike prior studies of oral EC, which commonly limited participants to 1 episode of unprotected intercourse, this trial enrolled women at potentially higher risk of pregnancy with multiple episodes of intercourse and found fewer pregnancies than expected. Randomization ensured equivalence between groups, with the exception of the reason for needing EC.
Study limitations include a higher than expected rate of loss to follow-up, requiring clinical records and survey data to confirm pregnancy status. After randomization, clinicians were unable to place IUDs in more than 5% of participants in both groups; noninferiority was demonstrated nonetheless. This study did not include participants receiving oral EC, so direct comparison of effectiveness is not possible. Pregnancy rates among IUD users in this study were favorable to rates reported in previous studies of oral EC.4
When choosing an IUD for contraception, more women select the LNG-IUD for its favorable side effect profile and reduction in menstrual bleeding. In this randomized IUD study, only 7% of eligible participants enrolled, potentially introducing selection bias. The majority who declined enrollment did not want an IUD. Previous studies that allowed participants to choose their IUD had higher enrollment rates. ●
The study by Turok and colleagues is the largest randomized controlled trial to date of IUDs as EC. It demonstrated that LNG-IUDs are noninferior to copper IUDs in preventing pregnancy when placed within 5 days of unprotected intercourse. IUDs offer advantages over oral EC methods: only IUDs provide ongoing contraception after EC, and IUD efficacy does not vary by body mass index. It is reasonable for clinicians and patients to consider LNG-IUDs among EC options after shared decision making.
This study suggests that quick-start placement of the LNG-IUD at any time in the menstrual cycle is reasonable given its effectiveness as EC. Additionally, there were no pregnancies among 138 study participants who resumed intercourse within 7 days of LNG-IUD placement, most of whom did not use backup contraception.5 While current guidelines still recommend backup contraception after LNG-IUD placement, clinicians may reassure patients with unprotected intercourse following any type of IUD placement about the low risk of pregnancy.
LISA HOFLER, MD, MPH, MBA,
AND SMITA CARROLL, MD, MBA
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
EXPERT COMMENTARY
Emergency contraception refers to therapies used to prevent pregnancy after inadequately protected intercourse.1 Evidence-based forms of EC available in the United States include oral LNG, oral ulipristal acetate, and the copper IUD. The copper IUD provides not only EC but also highly effective contraception after placement.2 The LNG-IUD has a favorable side effect profile compared with the copper IUD and is theorized to act as EC through direct interference with sperm and oviduct transport.3 Recently, Turok and colleagues conducted a noninferiority trial designed to investigate the EC effectiveness of the LNG-IUD compared with the copper IUD.3
Details of the study
Turok and colleagues recruited participants aged 18 to 35 who requested EC from 6 family planning clinics in Utah from 2016 to 2019. Participants who reported unprotected intercourse within the past 120 hours and who desired an IUD to prevent pregnancy for at least 1 year were randomly assigned to receive either the LNG-IUD or the copper IUD. Individuals were excluded from the trial if they had contraindications to IUD placement, were breastfeeding, had abnormal uterine bleeding, had irregular menses, were currently using highly effective contraception, or had recent EC use. Researchers determined pregnancy status at 1 month through a pregnancy test or clinical records review.
Results. Of 711 participants randomly assigned, 317 who received the LNG-IUD and 321 who received the copper IUD provided 1-month outcome data. Pregnancy 1 month after IUD placement occurred in 1 participant (0.3%) in the LNG-IUD group and in no participants in the copper IUD group (0%). The between-group difference of 0.3 percentage points was within the margin of noninferiority and was not significant.
Study strengths and limitations
This large, multicenter randomized controlled trial contributes novel information about the effectiveness and noninferiority of the LNG-IUD as EC. Unlike prior studies of oral EC, which commonly limited participants to 1 episode of unprotected intercourse, this trial enrolled women at potentially higher risk of pregnancy with multiple episodes of intercourse and found fewer pregnancies than expected. Randomization ensured equivalence between groups, with the exception of the reason for needing EC.
Study limitations include a higher than expected rate of loss to follow-up, requiring clinical records and survey data to confirm pregnancy status. After randomization, clinicians were unable to place IUDs in more than 5% of participants in both groups; noninferiority was demonstrated nonetheless. This study did not include participants receiving oral EC, so direct comparison of effectiveness is not possible. Pregnancy rates among IUD users in this study were favorable to rates reported in previous studies of oral EC.4
When choosing an IUD for contraception, more women select the LNG-IUD for its favorable side effect profile and reduction in menstrual bleeding. In this randomized IUD study, only 7% of eligible participants enrolled, potentially introducing selection bias. The majority who declined enrollment did not want an IUD. Previous studies that allowed participants to choose their IUD had higher enrollment rates. ●
The study by Turok and colleagues is the largest randomized controlled trial to date of IUDs as EC. It demonstrated that LNG-IUDs are noninferior to copper IUDs in preventing pregnancy when placed within 5 days of unprotected intercourse. IUDs offer advantages over oral EC methods: only IUDs provide ongoing contraception after EC, and IUD efficacy does not vary by body mass index. It is reasonable for clinicians and patients to consider LNG-IUDs among EC options after shared decision making.
This study suggests that quick-start placement of the LNG-IUD at any time in the menstrual cycle is reasonable given its effectiveness as EC. Additionally, there were no pregnancies among 138 study participants who resumed intercourse within 7 days of LNG-IUD placement, most of whom did not use backup contraception.5 While current guidelines still recommend backup contraception after LNG-IUD placement, clinicians may reassure patients with unprotected intercourse following any type of IUD placement about the low risk of pregnancy.
LISA HOFLER, MD, MPH, MBA,
AND SMITA CARROLL, MD, MBA
- ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 152: emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
- Fay KE, Clement AC, Gero A, et al. Rates of pregnancy among levonorgestrel and copper intrauterine emergency contraception initiators: implications for backup contraception recommendations. Contraception. 2021;S0010-7824(21)00210-9.
- ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 152: emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
- Fay KE, Clement AC, Gero A, et al. Rates of pregnancy among levonorgestrel and copper intrauterine emergency contraception initiators: implications for backup contraception recommendations. Contraception. 2021;S0010-7824(21)00210-9.
Relugolix combination therapy: A novel hormonal treatment for AUB associated with uterine fibroids
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.
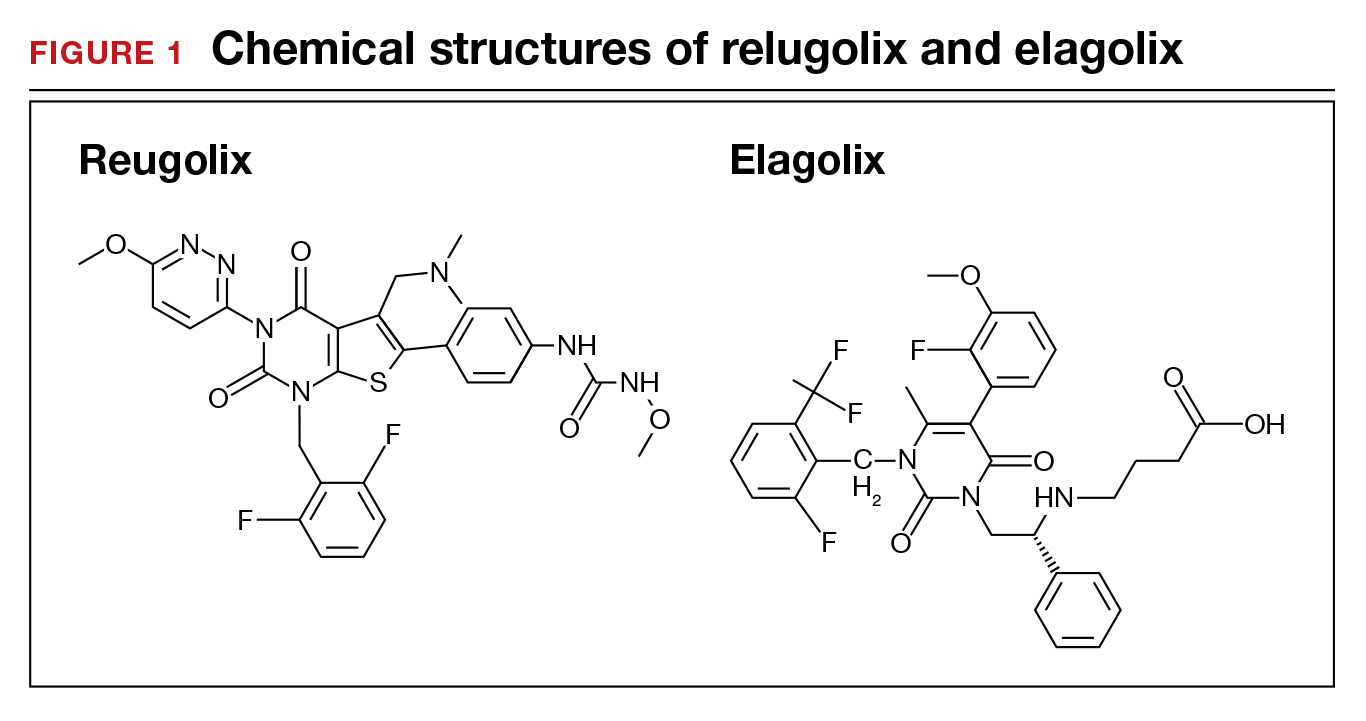
Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.
The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
To scan or not to scan: Routine ultrasonography is not necessary after medication management of early pregnancy loss
CASE Patient finds that follow-up ultrasonography is burdensome
Ms. MB presents to the clinic for dating ultrasonography and is diagnosed with a missed abortion measuring 7 weeks. After reviewing her management options, she elects for medication management. She receives mifepristone 200 mg and misoprostol 800 µg, with a plan to follow-up in clinic for repeat ultrasonography in a week. The day of her follow-up appointment, there is a large snowstorm. She calls her care team to ask if she needs to have a follow-up visit, as she is certain she has passed tissue and her bleeding is now minimal. She is told, however, that a follow-up ultrasonography is required, per clinic policy, to ensure successful management. Despite Ms. MB’s grief and the difficult travel conditions, she makes the arduous journey back to the clinic to complete the ultrasound.
Do all patients need an ultrasound after medication management of early pregnancy loss? Or is there an alternative follow-up option?
Early pregnancy loss (EPL) is a common pregnancy complication, and its management is a routine part of reproductive health care. In the clinically stable patient, EPL may be managed expectantly, surgically, or medically, based on the patient’s preference. For patients who select medication management, clear evidence supports that a combination regimen of mifepristone and misoprostol is more effective than treatment with misoprostol alone.1,2 The data suggest that 91% of patients will experience expulsion of the gestational sac by 30 days with medication management.1 Because a minority of patients will have a retained gestational sac despite medication therapy, follow-up ensures complete expulsion of pregnancy tissue.
In the United States, most follow-up protocols include an ultrasound examination, which often entails transvaginal ultrasonography. Returning to clinic for an additional ultrasound may be costly and inconvenient—and during a global pandemic medically risky. Further, it may undermine a fundamental principle in management of EPL: autonomy. Many patients who select medication management do so out of a desire to minimize interventions or procedures. Follow-up protocols that align with patient preferences for fewer interventions are critically important to the provision of patient-centered care. Additionally, the COVID-19 pandemic highlights the value of offering an alternative follow-up strategy that minimizes the need for additional visits to a clinic or hospital.
Lessons from medication abortion management
In many ways, follow-up after medication management of EPL is analogous to follow-up after medication abortion. In both cases, the goal of follow-up is to ensure that complete expulsion has occurred without complication and to identify patients with incomplete expulsion of pregnancy tissue who may benefit from further treatment with additional medication or uterine aspiration. A key difference in the management of EPL is that there is no concern for ongoing pregnancy.
Historically, follow-up transvaginal ultrasonography was routinely performed after medication abortion to ensure complete expulsion of pregnancy.3 However, requiring patients to return to a health care facility for ultrasonography after abortion can be burdensome, both for patients and clinicians. To provide more accessible, patient-centered care, researchers have investigated alternative follow-up strategies for medication abortion that remove the necessity for ultrasonography. Guidelines from both the National Abortion Federation and the American College of Obstetricians and Gynecologists state that routine ultrasonography is not necessary after medication abortion.4,5
Quantitative serum human chorionic gonadotropin (hCG) testing before treatment and at a follow-up visit is one reasonable strategy to ensure successful treatment. In one study of medication abortion patients, an 80% decrease in serum hCG was predictive of complete expulsion in 98.5% of patients.6 While this strategy avoids ultrasonography, it still necessitates a visit to a health care facility for a blood draw. As an alternative, substantial evidence now demonstrates the safety and feasibility of using a combination of clinical symptoms and urine pregnancy testing to confirm completed medication abortion. The evidence for follow-up using a combination of clinical symptoms and urine pregnancy testing is discussed below.
Continue to: Symptoms...
Symptoms. An assessment of symptoms alone, by the patient or clinician, is an important indicator of treatment success and can be completed easily via telephone. In one study of medication abortion with mifepristone and misoprostol, patients correctly predicted passage of a gestational sac 85% of the time based on symptoms alone.7 In another study, the combined clinical assessment from the patient and the clinician had a sensitivity of 96% and a specificity of 67% for predicting complete pregnancy expulsion.8 Finally, in an analysis of 931 patients after medication abortion, when both the patient and clinician believed that the gestational sac had passed, ultrasonography demonstrated complete expulsion 99% of the time.9
Urine pregnancy testing. Several studies have demonstrated that the addition of urine pregnancy testing to a clinical assessment of symptoms is a safe and effective follow-up strategy in medication abortion. Contemporary over-the-counter pregnancy tests are high-sensitivity tests that have an hCG detection threshold of 25 to 50 mIU/mL. As these tests are widely and commercially available in the United States, they can be a useful tool in follow-up strategies.
In a study by Chen and colleagues, patients seeking medication abortion were offered a choice of follow-up with ultrasonography at 1 week versus a combination of a 1-week phone call and a 4-week high-sensitivity urine pregnancy test. In this study, approximately 40% of patients opted for phone follow-up. The rates of incomplete abortion and loss to follow-up were similar between the 2 groups, highlighting the significant number of individuals interested in alternative models of follow-up and the efficacy of phone and urine testing specifically.10
In another study that evaluated the feasibility of a telephone and urine testing follow-up strategy, 97% of patients completed follow-up and all continuing pregnancies were diagnosed prior to the 4-week urine pregnancy test.8
In a hospital in Edinburgh, where a telephone-based symptom assessment in combination with a 2-week low-sensitivity pregnancy test (hCG detection threshold of 2,000 mIU/mL; not commercially available in the United States) is the standard of care for medication abortion follow-up, Michie and Cameron reported a sensitivity of 100% and a specificity of 88% to detect ongoing pregnancies.11
Taken together, these data demonstrate that a combination of symptom assessment via telephone and home urine pregnancy testing (in addition to standard patient instructions and return precautions) is an appropriate strategy for medication abortion follow-up, and they suggest that similar strategies can be employed in the medication management of EPL.
To scan or not to scan?
Many published studies of EPL have used ultrasonography to confirm complete expulsion of pregnancy tissue; however, others have relied on either clinical evaluation or urine pregnancy testing to determine treatment success, using ultrasonography only as clinically indicated.12-14 In their evaluation of medication management versus surgical management of miscarriage, Niinimäki and colleagues performed urine hCG testing at a 5- to 6-week follow-up visit to determine treatment success; ultrasonography was obtained only if the urine hCG test was positive. They demonstrated a treatment success rate of 90% with mifepristone and misoprostol treatment,12 congruent with previously published results.
While a follow-up ultrasound scan may be helpful to accurately assess treatment efficacy in research protocols, it should not be considered necessary in clinical practice. Posttreatment imaging in an asymptomatic patient may place additional burden on the patient and health care system and may result in unnecessary intervention. Although treatment success is reliably defined by the absence of a gestational sac,15,16 the finding of a thickened endometrium or presence of vascularity may result in the patient receiving an unnecessary aspiration or other intervention.
The evidence from the medication abortion literature suggests that a combination of a 1-week telephone call to assess patient symptoms in addition to a 4-week high-sensitivity pregnancy test is a reasonable alternative follow-up strategy. A similar strategy is already used in the United Kingdom, where current National Institute for Health and Care Excellence guidelines for follow-up after medication management of EPL recommend home pregnancy testing in 3 weeks unless the patient experiences worsening pain or bleeding symptoms.17
Time to rethink follow-up care
Follow-up care for EPL should be provided in a way that is sensitive to the needs and preferences of the patient and, if desired, minimizes additional health care visits, testing, or procedures. While some patients may prefer ultrasonography follow-up, it is important for the clinician to recognize that there are safe and effective alternatives. Patient preference guides the choice of EPL management; this logic extends to follow-up strategies. As we strive to provide evidence-based, patient-centered EPL care, there is no need for universal follow-up ultrasonography. ●
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LF, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Benson J, Clark KA, Gerhardt A, et al. Early abortion services in the United States: a provider survey. Contraception. 2003;67:287-294.
- Medication abortion up to 70 days of gestation: ACOG practice bulletin summary, No. 225. Obstet Gynecol. 2020;136:855-858.
- National Abortion Federation. 2020 Clinical Policy Guidelines for Abortion Care. Washington, DC; 2020. https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna -ssl.com/wp-content/uploads/2020_CPGs.pdf. Accessed July 19, 2021.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190-195.
- Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception. 2001;64:87-92.
- Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143-149.
- Rossi B, Creinin MD, Meyn LA. Ability of the clinician and patient to predict the outcome of mifepristone and misoprostol medical abortion. Contraception. 2004;70:313-317.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89:440-445.
- Niinimäki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367- 372.
- Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol. 2005;106:540-547.
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563-566.
- Reeves MF, Lohr PA, Harwood B, et al. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstet Gynecol. 2008;111:106-112.
- Reeves MF, Fox MC, Lohr PA, et al. Endometrial thickness following medical abortion is not predictive of subsequent surgical intervention. Ultrasound Obstet Gynecol. 2009;34:104-109.
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guideline NG126. April 17, 2019. ttps:// www.nice.org.uk/guidance/ng126/chapter/Recommen dations#management-of-miscarriage. Accessed July 19, 2021.
CASE Patient finds that follow-up ultrasonography is burdensome
Ms. MB presents to the clinic for dating ultrasonography and is diagnosed with a missed abortion measuring 7 weeks. After reviewing her management options, she elects for medication management. She receives mifepristone 200 mg and misoprostol 800 µg, with a plan to follow-up in clinic for repeat ultrasonography in a week. The day of her follow-up appointment, there is a large snowstorm. She calls her care team to ask if she needs to have a follow-up visit, as she is certain she has passed tissue and her bleeding is now minimal. She is told, however, that a follow-up ultrasonography is required, per clinic policy, to ensure successful management. Despite Ms. MB’s grief and the difficult travel conditions, she makes the arduous journey back to the clinic to complete the ultrasound.
Do all patients need an ultrasound after medication management of early pregnancy loss? Or is there an alternative follow-up option?
Early pregnancy loss (EPL) is a common pregnancy complication, and its management is a routine part of reproductive health care. In the clinically stable patient, EPL may be managed expectantly, surgically, or medically, based on the patient’s preference. For patients who select medication management, clear evidence supports that a combination regimen of mifepristone and misoprostol is more effective than treatment with misoprostol alone.1,2 The data suggest that 91% of patients will experience expulsion of the gestational sac by 30 days with medication management.1 Because a minority of patients will have a retained gestational sac despite medication therapy, follow-up ensures complete expulsion of pregnancy tissue.
In the United States, most follow-up protocols include an ultrasound examination, which often entails transvaginal ultrasonography. Returning to clinic for an additional ultrasound may be costly and inconvenient—and during a global pandemic medically risky. Further, it may undermine a fundamental principle in management of EPL: autonomy. Many patients who select medication management do so out of a desire to minimize interventions or procedures. Follow-up protocols that align with patient preferences for fewer interventions are critically important to the provision of patient-centered care. Additionally, the COVID-19 pandemic highlights the value of offering an alternative follow-up strategy that minimizes the need for additional visits to a clinic or hospital.
Lessons from medication abortion management
In many ways, follow-up after medication management of EPL is analogous to follow-up after medication abortion. In both cases, the goal of follow-up is to ensure that complete expulsion has occurred without complication and to identify patients with incomplete expulsion of pregnancy tissue who may benefit from further treatment with additional medication or uterine aspiration. A key difference in the management of EPL is that there is no concern for ongoing pregnancy.
Historically, follow-up transvaginal ultrasonography was routinely performed after medication abortion to ensure complete expulsion of pregnancy.3 However, requiring patients to return to a health care facility for ultrasonography after abortion can be burdensome, both for patients and clinicians. To provide more accessible, patient-centered care, researchers have investigated alternative follow-up strategies for medication abortion that remove the necessity for ultrasonography. Guidelines from both the National Abortion Federation and the American College of Obstetricians and Gynecologists state that routine ultrasonography is not necessary after medication abortion.4,5
Quantitative serum human chorionic gonadotropin (hCG) testing before treatment and at a follow-up visit is one reasonable strategy to ensure successful treatment. In one study of medication abortion patients, an 80% decrease in serum hCG was predictive of complete expulsion in 98.5% of patients.6 While this strategy avoids ultrasonography, it still necessitates a visit to a health care facility for a blood draw. As an alternative, substantial evidence now demonstrates the safety and feasibility of using a combination of clinical symptoms and urine pregnancy testing to confirm completed medication abortion. The evidence for follow-up using a combination of clinical symptoms and urine pregnancy testing is discussed below.
Continue to: Symptoms...
Symptoms. An assessment of symptoms alone, by the patient or clinician, is an important indicator of treatment success and can be completed easily via telephone. In one study of medication abortion with mifepristone and misoprostol, patients correctly predicted passage of a gestational sac 85% of the time based on symptoms alone.7 In another study, the combined clinical assessment from the patient and the clinician had a sensitivity of 96% and a specificity of 67% for predicting complete pregnancy expulsion.8 Finally, in an analysis of 931 patients after medication abortion, when both the patient and clinician believed that the gestational sac had passed, ultrasonography demonstrated complete expulsion 99% of the time.9
Urine pregnancy testing. Several studies have demonstrated that the addition of urine pregnancy testing to a clinical assessment of symptoms is a safe and effective follow-up strategy in medication abortion. Contemporary over-the-counter pregnancy tests are high-sensitivity tests that have an hCG detection threshold of 25 to 50 mIU/mL. As these tests are widely and commercially available in the United States, they can be a useful tool in follow-up strategies.
In a study by Chen and colleagues, patients seeking medication abortion were offered a choice of follow-up with ultrasonography at 1 week versus a combination of a 1-week phone call and a 4-week high-sensitivity urine pregnancy test. In this study, approximately 40% of patients opted for phone follow-up. The rates of incomplete abortion and loss to follow-up were similar between the 2 groups, highlighting the significant number of individuals interested in alternative models of follow-up and the efficacy of phone and urine testing specifically.10
In another study that evaluated the feasibility of a telephone and urine testing follow-up strategy, 97% of patients completed follow-up and all continuing pregnancies were diagnosed prior to the 4-week urine pregnancy test.8
In a hospital in Edinburgh, where a telephone-based symptom assessment in combination with a 2-week low-sensitivity pregnancy test (hCG detection threshold of 2,000 mIU/mL; not commercially available in the United States) is the standard of care for medication abortion follow-up, Michie and Cameron reported a sensitivity of 100% and a specificity of 88% to detect ongoing pregnancies.11
Taken together, these data demonstrate that a combination of symptom assessment via telephone and home urine pregnancy testing (in addition to standard patient instructions and return precautions) is an appropriate strategy for medication abortion follow-up, and they suggest that similar strategies can be employed in the medication management of EPL.
To scan or not to scan?
Many published studies of EPL have used ultrasonography to confirm complete expulsion of pregnancy tissue; however, others have relied on either clinical evaluation or urine pregnancy testing to determine treatment success, using ultrasonography only as clinically indicated.12-14 In their evaluation of medication management versus surgical management of miscarriage, Niinimäki and colleagues performed urine hCG testing at a 5- to 6-week follow-up visit to determine treatment success; ultrasonography was obtained only if the urine hCG test was positive. They demonstrated a treatment success rate of 90% with mifepristone and misoprostol treatment,12 congruent with previously published results.
While a follow-up ultrasound scan may be helpful to accurately assess treatment efficacy in research protocols, it should not be considered necessary in clinical practice. Posttreatment imaging in an asymptomatic patient may place additional burden on the patient and health care system and may result in unnecessary intervention. Although treatment success is reliably defined by the absence of a gestational sac,15,16 the finding of a thickened endometrium or presence of vascularity may result in the patient receiving an unnecessary aspiration or other intervention.
The evidence from the medication abortion literature suggests that a combination of a 1-week telephone call to assess patient symptoms in addition to a 4-week high-sensitivity pregnancy test is a reasonable alternative follow-up strategy. A similar strategy is already used in the United Kingdom, where current National Institute for Health and Care Excellence guidelines for follow-up after medication management of EPL recommend home pregnancy testing in 3 weeks unless the patient experiences worsening pain or bleeding symptoms.17
Time to rethink follow-up care
Follow-up care for EPL should be provided in a way that is sensitive to the needs and preferences of the patient and, if desired, minimizes additional health care visits, testing, or procedures. While some patients may prefer ultrasonography follow-up, it is important for the clinician to recognize that there are safe and effective alternatives. Patient preference guides the choice of EPL management; this logic extends to follow-up strategies. As we strive to provide evidence-based, patient-centered EPL care, there is no need for universal follow-up ultrasonography. ●
CASE Patient finds that follow-up ultrasonography is burdensome
Ms. MB presents to the clinic for dating ultrasonography and is diagnosed with a missed abortion measuring 7 weeks. After reviewing her management options, she elects for medication management. She receives mifepristone 200 mg and misoprostol 800 µg, with a plan to follow-up in clinic for repeat ultrasonography in a week. The day of her follow-up appointment, there is a large snowstorm. She calls her care team to ask if she needs to have a follow-up visit, as she is certain she has passed tissue and her bleeding is now minimal. She is told, however, that a follow-up ultrasonography is required, per clinic policy, to ensure successful management. Despite Ms. MB’s grief and the difficult travel conditions, she makes the arduous journey back to the clinic to complete the ultrasound.
Do all patients need an ultrasound after medication management of early pregnancy loss? Or is there an alternative follow-up option?
Early pregnancy loss (EPL) is a common pregnancy complication, and its management is a routine part of reproductive health care. In the clinically stable patient, EPL may be managed expectantly, surgically, or medically, based on the patient’s preference. For patients who select medication management, clear evidence supports that a combination regimen of mifepristone and misoprostol is more effective than treatment with misoprostol alone.1,2 The data suggest that 91% of patients will experience expulsion of the gestational sac by 30 days with medication management.1 Because a minority of patients will have a retained gestational sac despite medication therapy, follow-up ensures complete expulsion of pregnancy tissue.
In the United States, most follow-up protocols include an ultrasound examination, which often entails transvaginal ultrasonography. Returning to clinic for an additional ultrasound may be costly and inconvenient—and during a global pandemic medically risky. Further, it may undermine a fundamental principle in management of EPL: autonomy. Many patients who select medication management do so out of a desire to minimize interventions or procedures. Follow-up protocols that align with patient preferences for fewer interventions are critically important to the provision of patient-centered care. Additionally, the COVID-19 pandemic highlights the value of offering an alternative follow-up strategy that minimizes the need for additional visits to a clinic or hospital.
Lessons from medication abortion management
In many ways, follow-up after medication management of EPL is analogous to follow-up after medication abortion. In both cases, the goal of follow-up is to ensure that complete expulsion has occurred without complication and to identify patients with incomplete expulsion of pregnancy tissue who may benefit from further treatment with additional medication or uterine aspiration. A key difference in the management of EPL is that there is no concern for ongoing pregnancy.
Historically, follow-up transvaginal ultrasonography was routinely performed after medication abortion to ensure complete expulsion of pregnancy.3 However, requiring patients to return to a health care facility for ultrasonography after abortion can be burdensome, both for patients and clinicians. To provide more accessible, patient-centered care, researchers have investigated alternative follow-up strategies for medication abortion that remove the necessity for ultrasonography. Guidelines from both the National Abortion Federation and the American College of Obstetricians and Gynecologists state that routine ultrasonography is not necessary after medication abortion.4,5
Quantitative serum human chorionic gonadotropin (hCG) testing before treatment and at a follow-up visit is one reasonable strategy to ensure successful treatment. In one study of medication abortion patients, an 80% decrease in serum hCG was predictive of complete expulsion in 98.5% of patients.6 While this strategy avoids ultrasonography, it still necessitates a visit to a health care facility for a blood draw. As an alternative, substantial evidence now demonstrates the safety and feasibility of using a combination of clinical symptoms and urine pregnancy testing to confirm completed medication abortion. The evidence for follow-up using a combination of clinical symptoms and urine pregnancy testing is discussed below.
Continue to: Symptoms...
Symptoms. An assessment of symptoms alone, by the patient or clinician, is an important indicator of treatment success and can be completed easily via telephone. In one study of medication abortion with mifepristone and misoprostol, patients correctly predicted passage of a gestational sac 85% of the time based on symptoms alone.7 In another study, the combined clinical assessment from the patient and the clinician had a sensitivity of 96% and a specificity of 67% for predicting complete pregnancy expulsion.8 Finally, in an analysis of 931 patients after medication abortion, when both the patient and clinician believed that the gestational sac had passed, ultrasonography demonstrated complete expulsion 99% of the time.9
Urine pregnancy testing. Several studies have demonstrated that the addition of urine pregnancy testing to a clinical assessment of symptoms is a safe and effective follow-up strategy in medication abortion. Contemporary over-the-counter pregnancy tests are high-sensitivity tests that have an hCG detection threshold of 25 to 50 mIU/mL. As these tests are widely and commercially available in the United States, they can be a useful tool in follow-up strategies.
In a study by Chen and colleagues, patients seeking medication abortion were offered a choice of follow-up with ultrasonography at 1 week versus a combination of a 1-week phone call and a 4-week high-sensitivity urine pregnancy test. In this study, approximately 40% of patients opted for phone follow-up. The rates of incomplete abortion and loss to follow-up were similar between the 2 groups, highlighting the significant number of individuals interested in alternative models of follow-up and the efficacy of phone and urine testing specifically.10
In another study that evaluated the feasibility of a telephone and urine testing follow-up strategy, 97% of patients completed follow-up and all continuing pregnancies were diagnosed prior to the 4-week urine pregnancy test.8
In a hospital in Edinburgh, where a telephone-based symptom assessment in combination with a 2-week low-sensitivity pregnancy test (hCG detection threshold of 2,000 mIU/mL; not commercially available in the United States) is the standard of care for medication abortion follow-up, Michie and Cameron reported a sensitivity of 100% and a specificity of 88% to detect ongoing pregnancies.11
Taken together, these data demonstrate that a combination of symptom assessment via telephone and home urine pregnancy testing (in addition to standard patient instructions and return precautions) is an appropriate strategy for medication abortion follow-up, and they suggest that similar strategies can be employed in the medication management of EPL.
To scan or not to scan?
Many published studies of EPL have used ultrasonography to confirm complete expulsion of pregnancy tissue; however, others have relied on either clinical evaluation or urine pregnancy testing to determine treatment success, using ultrasonography only as clinically indicated.12-14 In their evaluation of medication management versus surgical management of miscarriage, Niinimäki and colleagues performed urine hCG testing at a 5- to 6-week follow-up visit to determine treatment success; ultrasonography was obtained only if the urine hCG test was positive. They demonstrated a treatment success rate of 90% with mifepristone and misoprostol treatment,12 congruent with previously published results.
While a follow-up ultrasound scan may be helpful to accurately assess treatment efficacy in research protocols, it should not be considered necessary in clinical practice. Posttreatment imaging in an asymptomatic patient may place additional burden on the patient and health care system and may result in unnecessary intervention. Although treatment success is reliably defined by the absence of a gestational sac,15,16 the finding of a thickened endometrium or presence of vascularity may result in the patient receiving an unnecessary aspiration or other intervention.
The evidence from the medication abortion literature suggests that a combination of a 1-week telephone call to assess patient symptoms in addition to a 4-week high-sensitivity pregnancy test is a reasonable alternative follow-up strategy. A similar strategy is already used in the United Kingdom, where current National Institute for Health and Care Excellence guidelines for follow-up after medication management of EPL recommend home pregnancy testing in 3 weeks unless the patient experiences worsening pain or bleeding symptoms.17
Time to rethink follow-up care
Follow-up care for EPL should be provided in a way that is sensitive to the needs and preferences of the patient and, if desired, minimizes additional health care visits, testing, or procedures. While some patients may prefer ultrasonography follow-up, it is important for the clinician to recognize that there are safe and effective alternatives. Patient preference guides the choice of EPL management; this logic extends to follow-up strategies. As we strive to provide evidence-based, patient-centered EPL care, there is no need for universal follow-up ultrasonography. ●
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LF, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Benson J, Clark KA, Gerhardt A, et al. Early abortion services in the United States: a provider survey. Contraception. 2003;67:287-294.
- Medication abortion up to 70 days of gestation: ACOG practice bulletin summary, No. 225. Obstet Gynecol. 2020;136:855-858.
- National Abortion Federation. 2020 Clinical Policy Guidelines for Abortion Care. Washington, DC; 2020. https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna -ssl.com/wp-content/uploads/2020_CPGs.pdf. Accessed July 19, 2021.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190-195.
- Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception. 2001;64:87-92.
- Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143-149.
- Rossi B, Creinin MD, Meyn LA. Ability of the clinician and patient to predict the outcome of mifepristone and misoprostol medical abortion. Contraception. 2004;70:313-317.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89:440-445.
- Niinimäki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367- 372.
- Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol. 2005;106:540-547.
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563-566.
- Reeves MF, Lohr PA, Harwood B, et al. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstet Gynecol. 2008;111:106-112.
- Reeves MF, Fox MC, Lohr PA, et al. Endometrial thickness following medical abortion is not predictive of subsequent surgical intervention. Ultrasound Obstet Gynecol. 2009;34:104-109.
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guideline NG126. April 17, 2019. ttps:// www.nice.org.uk/guidance/ng126/chapter/Recommen dations#management-of-miscarriage. Accessed July 19, 2021.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LF, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Benson J, Clark KA, Gerhardt A, et al. Early abortion services in the United States: a provider survey. Contraception. 2003;67:287-294.
- Medication abortion up to 70 days of gestation: ACOG practice bulletin summary, No. 225. Obstet Gynecol. 2020;136:855-858.
- National Abortion Federation. 2020 Clinical Policy Guidelines for Abortion Care. Washington, DC; 2020. https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna -ssl.com/wp-content/uploads/2020_CPGs.pdf. Accessed July 19, 2021.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190-195.
- Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception. 2001;64:87-92.
- Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143-149.
- Rossi B, Creinin MD, Meyn LA. Ability of the clinician and patient to predict the outcome of mifepristone and misoprostol medical abortion. Contraception. 2004;70:313-317.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89:440-445.
- Niinimäki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367- 372.
- Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol. 2005;106:540-547.
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563-566.
- Reeves MF, Lohr PA, Harwood B, et al. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstet Gynecol. 2008;111:106-112.
- Reeves MF, Fox MC, Lohr PA, et al. Endometrial thickness following medical abortion is not predictive of subsequent surgical intervention. Ultrasound Obstet Gynecol. 2009;34:104-109.
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guideline NG126. April 17, 2019. ttps:// www.nice.org.uk/guidance/ng126/chapter/Recommen dations#management-of-miscarriage. Accessed July 19, 2021.
COVID-19 linked to rise in suicide-related ED visits among youth
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
FROM JAMA PSYCHIATRY
COVID vaccine response in patients with solid tumors
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID could spell kidney troubles down the line
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.