User login
Beyond the headlines: A closer look at the USPSTF draft recs on aspirin
REFERENCES
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medication. Published October 12, 2021. Accessed October 25, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication
- National Center for Health Statistics. Figure 4. Number of deaths, percentage of total deaths, and age-adjusted death rates for the 10 leading causes of death in 2019: United States, 2018 and 2019. In: Data Brief 395: Mortality in the United States 2019. Published December 2020. Accessed October 25, 2021. www.cdc.gov/nchs/data/databriefs/db395-tables-508.pdf
- American College of Cardiology/American Heart Association. Heart risk calculator. Updated November 12, 2017. Accessed October 25, 2021. www.cvriskcalculator.com/
REFERENCES
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medication. Published October 12, 2021. Accessed October 25, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication
- National Center for Health Statistics. Figure 4. Number of deaths, percentage of total deaths, and age-adjusted death rates for the 10 leading causes of death in 2019: United States, 2018 and 2019. In: Data Brief 395: Mortality in the United States 2019. Published December 2020. Accessed October 25, 2021. www.cdc.gov/nchs/data/databriefs/db395-tables-508.pdf
- American College of Cardiology/American Heart Association. Heart risk calculator. Updated November 12, 2017. Accessed October 25, 2021. www.cvriskcalculator.com/
REFERENCES
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medication. Published October 12, 2021. Accessed October 25, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication
- National Center for Health Statistics. Figure 4. Number of deaths, percentage of total deaths, and age-adjusted death rates for the 10 leading causes of death in 2019: United States, 2018 and 2019. In: Data Brief 395: Mortality in the United States 2019. Published December 2020. Accessed October 25, 2021. www.cdc.gov/nchs/data/databriefs/db395-tables-508.pdf
- American College of Cardiology/American Heart Association. Heart risk calculator. Updated November 12, 2017. Accessed October 25, 2021. www.cvriskcalculator.com/
Updated MELD score adds serum albumin, female sex
This article was updated Nov. 5, 2021.
A newly updated version of the Model for End-Stage Liver Disease (MELD) score was effective for predicting short-term mortality in patients with end-stage liver disease and addressed important determinants of wait list outcomes that haven’t been addressed in previous versions, according to findings from a recent study. The new model, termed MELD 3.0, includes new variables such as female sex, serum albumin, and updated creatinine cutoffs.
“We believe that the new model represents an opportunity to lower wait list mortality in the United States and propose it to be considered to replace the current version of MELD in determining allocation priorities in liver transplantation,” wrote study authors W. Ray Kim, MD, of Stanford (Calif.) University and colleagues in Gastroenterology.
In patients with end-stage liver disease, the MELD score was shown to be a reliable predictor of short-term survival, according to the researchers. The original version of MELD consists of international normalized ratio of prothrombin time and serum concentrations of bilirubin and creatinine; MELDNa consists of the same with the addition of serum concentrations of total sodium. Since 2016, MELDNa has been utilized in the United States to allocate livers for transplant.
Despite the utility of the current MELD score, questions have been raised concerning the accuracy of the tool’s ability to predict mortality, including a study by Sumeet K. Asrani, MD, MSc, and colleagues. Changes in liver disease epidemiology, the introduction of newer therapies that alter prognosis, as well as increasing age and prevalence of comorbidities in transplant-eligible patients are several drivers for these concerns, according to Dr. Kim and colleagues. Also, there is an increasing concern regarding women and their potential disadvantages in the current system: At least one study has suggested that serum creatinine may overestimate renal function and consequently underestimate mortality risk in female patients, compared with men with the same creatinine level.
Dr. Kim and colleagues sought to further optimize the fit of the current MELD score by considering alternative interactions and including other variables relevant to predicting short-term mortality in patients awaiting liver transplant. The study included patients who are registered on the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research files newly wait-listed from 2016 through 2018. The full cohort was divided 70:30 into a development set (n = 20,587) and a validation set (n = 8,823); there were no significant differences between the sets in respect to age, sex, race, or liver disease severity.
The investigators used univariable and multivariable regression models to predict 90-day survival following wait list registration. The 90-day Kaplan-Meier survival rate in the development set was 91.3%. Additionally, model fit was tested, and the investigators used the Liver Simulated Allocation Model to estimate the impact of replacing the current version of the MELD with MELD 3.0.
In the final MELD 3.0 model, the researchers included several additional variables such as female sex and serum albumin. Additionally, the final model was characterized by interactions between bilirubin and sodium as well as between albumin and creatinine. Also, an adjustment to the current version of MELD lowering the upper bound for creatinine from 4.0 mg/dL to 3.0 mg/dL.
The MELD 3.0 featured significantly better discrimination, compared with the MELDNa (C-statistic = 0.8693 vs. 0.8622, respectively; P < .01). In addition, the researchers wrote that the new MELD 3.0 score “correctly reclassified a net of 8.8% of decedents to a higher MELD tier, affording them a meaningfully higher chance of transplantation, particularly in women.” The MELD 3.0 score with albumin also led to fewer wait-list deaths, compared with the MELDNa, according to the Liver Simulated Allocation Model analysis (P = .02); the number for MELD 3.0 without albumin was not statistically significant.
According to the investigators, a cause of concern for the MELD 3.0 was the addition of albumin, as this variable may be vulnerable to manipulation. In addition, the researchers note that, while differences in wait list mortality and survival based on race/ethnicity were observed in the study, they were unable to describe the exact root causes of worse outcomes among patients belonging to minority groups. “Thus, inclusion in a risk prediction score without fully understanding the underlying reasons for the racial disparity may have unintended consequences,” the researchers wrote.
“Based on recent data consisting of liver transplant candidates in the United States, we identify additional variables that are meaningfully associated with short-term mortality, including female sex and serum albumin. We also found evidence to support lowering the serum creatinine ceiling to 3 mg/dL,” they wrote. “Based on these data, we created an updated version of the MELD score, which improves mortality prediction compared to the current MELDNa model, including the recognition of female sex as a risk factor for death.”
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
This article was updated Nov. 5, 2021.
A newly updated version of the Model for End-Stage Liver Disease (MELD) score was effective for predicting short-term mortality in patients with end-stage liver disease and addressed important determinants of wait list outcomes that haven’t been addressed in previous versions, according to findings from a recent study. The new model, termed MELD 3.0, includes new variables such as female sex, serum albumin, and updated creatinine cutoffs.
“We believe that the new model represents an opportunity to lower wait list mortality in the United States and propose it to be considered to replace the current version of MELD in determining allocation priorities in liver transplantation,” wrote study authors W. Ray Kim, MD, of Stanford (Calif.) University and colleagues in Gastroenterology.
In patients with end-stage liver disease, the MELD score was shown to be a reliable predictor of short-term survival, according to the researchers. The original version of MELD consists of international normalized ratio of prothrombin time and serum concentrations of bilirubin and creatinine; MELDNa consists of the same with the addition of serum concentrations of total sodium. Since 2016, MELDNa has been utilized in the United States to allocate livers for transplant.
Despite the utility of the current MELD score, questions have been raised concerning the accuracy of the tool’s ability to predict mortality, including a study by Sumeet K. Asrani, MD, MSc, and colleagues. Changes in liver disease epidemiology, the introduction of newer therapies that alter prognosis, as well as increasing age and prevalence of comorbidities in transplant-eligible patients are several drivers for these concerns, according to Dr. Kim and colleagues. Also, there is an increasing concern regarding women and their potential disadvantages in the current system: At least one study has suggested that serum creatinine may overestimate renal function and consequently underestimate mortality risk in female patients, compared with men with the same creatinine level.
Dr. Kim and colleagues sought to further optimize the fit of the current MELD score by considering alternative interactions and including other variables relevant to predicting short-term mortality in patients awaiting liver transplant. The study included patients who are registered on the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research files newly wait-listed from 2016 through 2018. The full cohort was divided 70:30 into a development set (n = 20,587) and a validation set (n = 8,823); there were no significant differences between the sets in respect to age, sex, race, or liver disease severity.
The investigators used univariable and multivariable regression models to predict 90-day survival following wait list registration. The 90-day Kaplan-Meier survival rate in the development set was 91.3%. Additionally, model fit was tested, and the investigators used the Liver Simulated Allocation Model to estimate the impact of replacing the current version of the MELD with MELD 3.0.
In the final MELD 3.0 model, the researchers included several additional variables such as female sex and serum albumin. Additionally, the final model was characterized by interactions between bilirubin and sodium as well as between albumin and creatinine. Also, an adjustment to the current version of MELD lowering the upper bound for creatinine from 4.0 mg/dL to 3.0 mg/dL.
The MELD 3.0 featured significantly better discrimination, compared with the MELDNa (C-statistic = 0.8693 vs. 0.8622, respectively; P < .01). In addition, the researchers wrote that the new MELD 3.0 score “correctly reclassified a net of 8.8% of decedents to a higher MELD tier, affording them a meaningfully higher chance of transplantation, particularly in women.” The MELD 3.0 score with albumin also led to fewer wait-list deaths, compared with the MELDNa, according to the Liver Simulated Allocation Model analysis (P = .02); the number for MELD 3.0 without albumin was not statistically significant.
According to the investigators, a cause of concern for the MELD 3.0 was the addition of albumin, as this variable may be vulnerable to manipulation. In addition, the researchers note that, while differences in wait list mortality and survival based on race/ethnicity were observed in the study, they were unable to describe the exact root causes of worse outcomes among patients belonging to minority groups. “Thus, inclusion in a risk prediction score without fully understanding the underlying reasons for the racial disparity may have unintended consequences,” the researchers wrote.
“Based on recent data consisting of liver transplant candidates in the United States, we identify additional variables that are meaningfully associated with short-term mortality, including female sex and serum albumin. We also found evidence to support lowering the serum creatinine ceiling to 3 mg/dL,” they wrote. “Based on these data, we created an updated version of the MELD score, which improves mortality prediction compared to the current MELDNa model, including the recognition of female sex as a risk factor for death.”
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
This article was updated Nov. 5, 2021.
A newly updated version of the Model for End-Stage Liver Disease (MELD) score was effective for predicting short-term mortality in patients with end-stage liver disease and addressed important determinants of wait list outcomes that haven’t been addressed in previous versions, according to findings from a recent study. The new model, termed MELD 3.0, includes new variables such as female sex, serum albumin, and updated creatinine cutoffs.
“We believe that the new model represents an opportunity to lower wait list mortality in the United States and propose it to be considered to replace the current version of MELD in determining allocation priorities in liver transplantation,” wrote study authors W. Ray Kim, MD, of Stanford (Calif.) University and colleagues in Gastroenterology.
In patients with end-stage liver disease, the MELD score was shown to be a reliable predictor of short-term survival, according to the researchers. The original version of MELD consists of international normalized ratio of prothrombin time and serum concentrations of bilirubin and creatinine; MELDNa consists of the same with the addition of serum concentrations of total sodium. Since 2016, MELDNa has been utilized in the United States to allocate livers for transplant.
Despite the utility of the current MELD score, questions have been raised concerning the accuracy of the tool’s ability to predict mortality, including a study by Sumeet K. Asrani, MD, MSc, and colleagues. Changes in liver disease epidemiology, the introduction of newer therapies that alter prognosis, as well as increasing age and prevalence of comorbidities in transplant-eligible patients are several drivers for these concerns, according to Dr. Kim and colleagues. Also, there is an increasing concern regarding women and their potential disadvantages in the current system: At least one study has suggested that serum creatinine may overestimate renal function and consequently underestimate mortality risk in female patients, compared with men with the same creatinine level.
Dr. Kim and colleagues sought to further optimize the fit of the current MELD score by considering alternative interactions and including other variables relevant to predicting short-term mortality in patients awaiting liver transplant. The study included patients who are registered on the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research files newly wait-listed from 2016 through 2018. The full cohort was divided 70:30 into a development set (n = 20,587) and a validation set (n = 8,823); there were no significant differences between the sets in respect to age, sex, race, or liver disease severity.
The investigators used univariable and multivariable regression models to predict 90-day survival following wait list registration. The 90-day Kaplan-Meier survival rate in the development set was 91.3%. Additionally, model fit was tested, and the investigators used the Liver Simulated Allocation Model to estimate the impact of replacing the current version of the MELD with MELD 3.0.
In the final MELD 3.0 model, the researchers included several additional variables such as female sex and serum albumin. Additionally, the final model was characterized by interactions between bilirubin and sodium as well as between albumin and creatinine. Also, an adjustment to the current version of MELD lowering the upper bound for creatinine from 4.0 mg/dL to 3.0 mg/dL.
The MELD 3.0 featured significantly better discrimination, compared with the MELDNa (C-statistic = 0.8693 vs. 0.8622, respectively; P < .01). In addition, the researchers wrote that the new MELD 3.0 score “correctly reclassified a net of 8.8% of decedents to a higher MELD tier, affording them a meaningfully higher chance of transplantation, particularly in women.” The MELD 3.0 score with albumin also led to fewer wait-list deaths, compared with the MELDNa, according to the Liver Simulated Allocation Model analysis (P = .02); the number for MELD 3.0 without albumin was not statistically significant.
According to the investigators, a cause of concern for the MELD 3.0 was the addition of albumin, as this variable may be vulnerable to manipulation. In addition, the researchers note that, while differences in wait list mortality and survival based on race/ethnicity were observed in the study, they were unable to describe the exact root causes of worse outcomes among patients belonging to minority groups. “Thus, inclusion in a risk prediction score without fully understanding the underlying reasons for the racial disparity may have unintended consequences,” the researchers wrote.
“Based on recent data consisting of liver transplant candidates in the United States, we identify additional variables that are meaningfully associated with short-term mortality, including female sex and serum albumin. We also found evidence to support lowering the serum creatinine ceiling to 3 mg/dL,” they wrote. “Based on these data, we created an updated version of the MELD score, which improves mortality prediction compared to the current MELDNa model, including the recognition of female sex as a risk factor for death.”
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
FROM GASTROENTEROLOGY
Updated MS guidelines advocate earlier, more aggressive treatment
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
and include a recommendation for siponimod (Mayzent) in progressive MS, as well as a general emphasis toward earlier and more aggressive treatment.
The updated guidelines were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and are the result of a collaboration between ECTRIMS and the European Academy of Neurology (EAN).
Maria Pia Amato, MD, ECTRIMS president and co-chair of the guidelines steering committee, noted that the European MS treatment guidelines were last published in 2018. “Since then more trials have been published, and we felt this was a good time to incorporate the new evidence into updated guidelines,” she said.
“As before, the updated guidelines contain a number of core questions that address the efficacy of disease-modifying therapies, early treatment decisions, disease/treatment response monitoring and treatment modifications, treatment suspension and disease reactivation, and pregnancy and breastfeeding,” Dr. Amato said.
New recommendations
New features of the updated guidelines include a recommendation for siponimod for secondary progressive MS with evidence of disease inflammatory activity; in addition, there is more emphasis on starting treatment early, with greater consideration of higher efficacy drugs, depending on the characteristics of the disease and the patient, Dr. Amato commented.
“We also provided more detailed information on disease-modifying therapy use in pregnancy and breastfeeding and also for women with high disease activity who desire to become pregnant,” she added.
Other new features include the introduction of clinical questions dealing with treatment safety and monitoring (for example, for natalizumab) and also considering the current COVID-19 pandemic scenario; switching strategies with more detailed practical indications on timing; and long lasting effects of drugs such as alemtuzumab and cladribine, Dr. Amato said.
The updated guidelines include the following recommendations:
- The entire spectrum of disease-modifying drugs should be prescribed by a neurologist with expertise in MS and ready access to adequate infrastructure to provide proper monitoring of patents, comprehensive assessment, early detection of side effects, and the capacity to address those side effects promptly.
- Offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) highly suggestive of MS and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS.
- For patients with relapsing-remitting MS, the choice between a wide range of available drugs (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, cladribine, fingolimod, ozanimod, ponesimod, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab), from modestly to highly effective, will depend on factors including: underlying disability progression, disease severity/clinical or radiological activity, patient characteristics and morbidity, drug safety profile, family planning, and patient preferences.
Progressive MS
- For patients with secondary progressive MS with evidence of inflammatory activity (relapses and/or MRI activity), offer treatment with siponimod. Treatment with other therapies used for relapsing remitting MS may also be considered.
- For secondary progressive MS without evidence of inflammatory activity, particularly in young patients and those in whom progression has started recently, consider treatment with siponimod or anti-CD20 monoclonal antibodies, taking into account that there is scarce evidence to support their use in this setting.
- For patients with active secondary progressive MS when there is no other therapy available, consider treatment with mitoxantrone, taking into account the safety concerns and tolerability issues of this agent.
- Consider ocrelizumab for patients with primary progressive MS, particularly early and active (clinically and/or radiologically) disease.
Emphasis toward higher-efficacy drugs
- Consider choosing a higher-efficacy disease-modifying drug early on, according to disease activity (either clinically or on MRI).
- Offer a more efficacious drug to patients who show evidence of disease activity with their current treatment.
- When treatment with a high-efficacy drug is stopped, whether because of inefficacy or risk of adverse effects, consider starting another high-efficacy drug, taking into account clinical and MRI disease activity before and during treatment, pharmacokinetics and biological activity of the previous drug, and the potential for resumed disease activity or even rebound syndrome (particularly with natalizumab and S1P modulators).
- In the stable patient (clinically and on MRI) who shows no safety or tolerability issues, consider continuing treatment with disease-modifying therapy, taking into account patient characteristics and comorbidities, drug safety profile, family planning, and patient preferences.
Recommendations for pregnancy and breastfeeding
Recommendations for pregnant women and mothers who choose to breastfeed include:
- Advise women who wish to become pregnant to plan their pregnancy beforehand.
- Advise women of childbearing potential that MS disease-modifying therapies are not licensed during pregnancy, with the exception of interferons and glatiramer acetate.
- For women planning a pregnancy, offer interferons and glatiramer acetate and consider continuing these agents during pregnancy after assessment of risk and benefits. Consider using dimethyl fumarate until pregnancy is confirmed and stopping during pregnancy after assessment of the risks and benefits.
- For women with highly active disease who wish to become pregnant, there are a number of therapeutic options:
1) treatment with long lasting effects such as alemtuzumab or cladribine provided that at least 4 or 6 months respectively have elapsed between the last dose and conception2) treatment with anti-CD20 drugs before pregnancy with advice to wait for 2-6 months after the last infusion before becoming pregnant and to avoid further infusions during pregnancy, or3) for patients treated with natalizumab, consider continuing treatment during pregnancy using a 6-week extended dosage regimen until the end of the second trimester or up until week 34 and resuming after delivery (in newborns exposed to natalizumab, check for hematological abnormalities and liver function)
- Only interferons and ofatumumab are currently approved during breastfeeding.
Treatment safety/monitoring
- When treating patients with natalizumab and after a period of stability, consider switching to a 6-week interval regimen in order to minimize the risk of progressive multifocal leukoencephalopathy (PML).
- Consider treatment with high-efficacy drugs including natalizumab in patients with high disease activity, in whom a quick therapeutic effect is required, taking into account the risk of PML in John Cunningham virus (JCV)-positive patients, as well as the therapeutic lag of the different disease-modifying drugs.
- Ideally, prioritize vaccination against COVID-19 before starting immunosuppressive disease-modifying treatments to achieve the highest protection rate possible.
Long-lasting treatments
- When using long-lasting treatments (alemtuzumab or cladribine) in patients who experience disease activity before the treatment is completed (between the first and second cycles), consider waiting until completion of the therapeutic regimen before switching to other drugs.
- Consider offering additional courses of alemtuzumab after the first two cycles at least 1 year apart from each other when disease activity has not remitted completely or reappears after a period of stability, taking into account the balance between the potential benefits and side effects.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2021
DMTs linked to better pediatric MS outcomes
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
FROM ECTRIMS 2021
How 100 years of insulin have changed pregnancy for women with type 1 diabetes
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
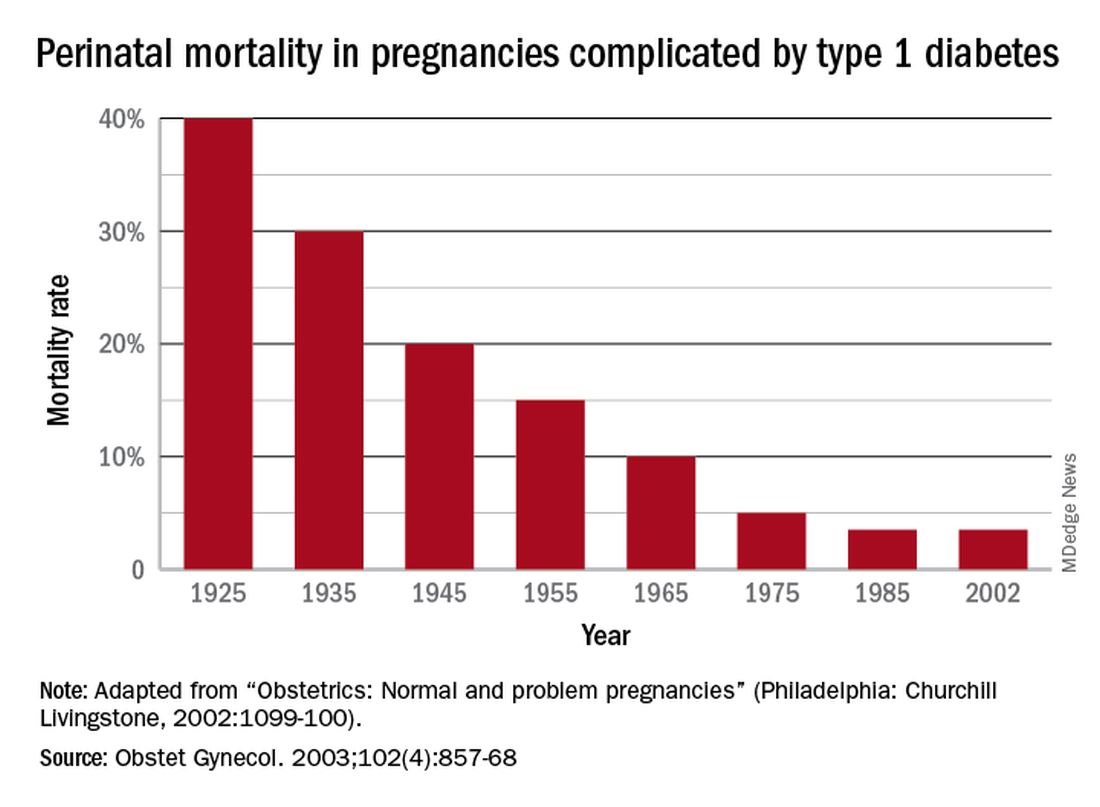
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%
In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.
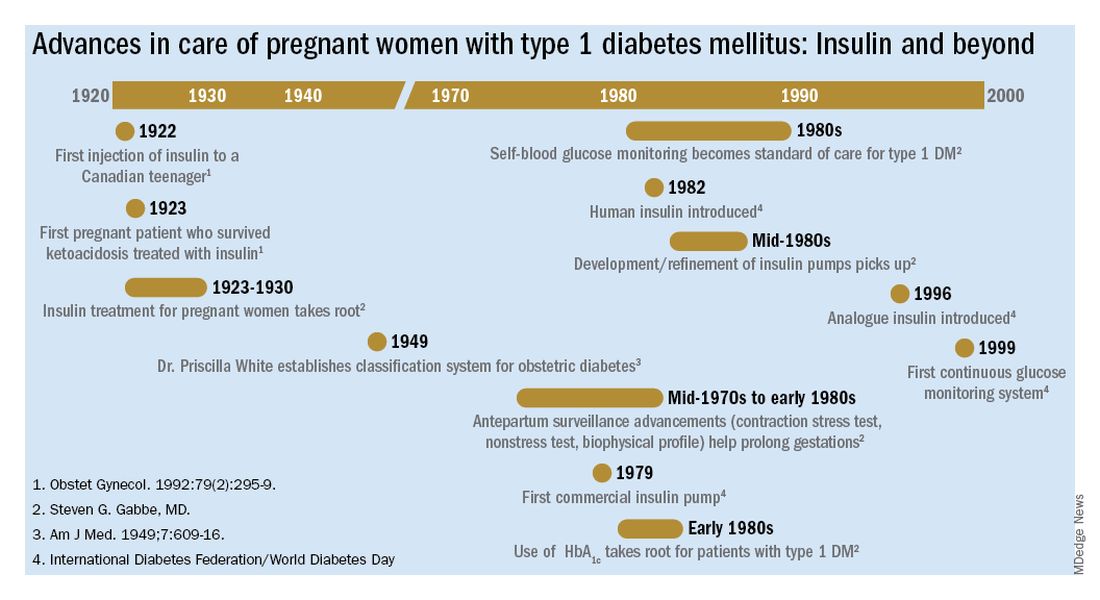
Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Mark B. Landon, MD: The discovery of insulin in 1921 by Dr. Frederick Banting and Dr. Charles Best and its introduction into clinical practice may well be the most significant achievement in the care of pregnant women with diabetes mellitus in the last century. Why was this advance so monumental?
Steven G. Gabbe, MD: Insulin is the single most important drug we use in taking care of diabetes in pregnancy. It is required not only by all patients with type 1 diabetes, but also by the majority of patients with type 2 diabetes. Moreover, at least a third of our patients with gestational diabetes require more than lifestyle change. The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend that insulin be considered as the first-line pharmacologic therapy.
Before insulin, the most prudent option for women who had glucose in their urine early in pregnancy, which was called “true diabetes,” was deemed to be termination of the pregnancy. The chances of surviving a pregnancy, and of having a surviving infant, were low.
Pregnancies were a rarity to begin with because most women of reproductive age died within a year or two of the onset of their illness. Moreover, most women with what we now know as type 1 diabetes were amenorrheic and infertile. In fact, before insulin, there were few cases of pregnancy complicated by diabetes reported in the literature. A summary of the world literature published in 1909 in the American Journal of the Medical Sciences reported: 66 pregnancies in 43 women; 50% maternal mortality (27% immediate; 23% in next 2 years); and a 41% pregnancy loss (Obstet Gynecol. 1992;79:295-9, Cited Am J Med Sci. 1909;137:1).
The first injection of insulin was administered in 1922 to a 13-year-old Canadian boy, and for several years the focus was on children. (Some of them had been kept alive with 450 calories/day long enough to benefit from the new treatment.)
For women with what we now know as type 1 diabetes, insulin kept them alive, restored their fertility, and enabled them to survive a pregnancy. Maternal mortality dropped dramatically, down to a few percent, once pregnant women became beneficiaries of insulin therapy.
Perinatal outcomes remained poor, however. In the early years of insulin therapy, more than half of the babies died. Some were stillbirths, which had been the primary cause of perinatal deaths in the pre-insulin era. Others were spontaneous preterm births, and still others were delivered prematurely in order to avert a stillbirth, and subsequently died.
Dr. Landon: A significant improvement in perinatal outcomes was eventually realized about two decades after insulin was introduced. By then Dr. Priscilla White of the Joslin Clinic had recorded that women who had so-called ‘normal hormonal balance’ – basically good glucose control – had very low rates of fetal demise and fetal loss compared with those who did not have good control. You had the opportunity to work alongside Dr. White. How did she achieve these results without all the tools we have today?
Dr. Gabbe: In 1925, the perinatal mortality in pregnancies complicated by type 1 diabetes was about 40%. By 1965 it was 10%, and when I began my residency at the Joslin Clinic and Boston Hospital for Women in 1972 it was closer to 5%

In those days we didn’t have accurate methods for dating pregnancies or assessing fetal size or well-being. We didn’t have tools to monitor blood glucose levels, and our insulins were limited to regular insulins and NPH (neutral protamine Hagedorn) as a basal insulin.
Dr. White had concluded early on, and wrote in a 1928 paper, that controlling diabetes was essential to fetal welfare and that the “high glucose content of placental blood” was probably linked to excessive fetal growth. She also wrote about the importance of “close and persistent supervision” of the patient by both an internist and obstetrician.
When I began working with her in the 1970s, her program involved antepartum visits every week or two and a team approach. Patients would be seen by Dr. White and other diabetologists, by head obstetrician Dr. Luke Gillespie, and by nurses and nutritionists. At the end of each day, after all the patients had been seen, we’d gather in Dr. White’s office and look at each patient’s single morning blood glucose measurement and the histories we’d obtained, and we’d make adjustments to their insulin regimens.
Dr. White’s solution to the problem of monitoring blood glucose was a program of hospitalization throughout pregnancy. Patients were hospitalized for a week initially to achieve blood glucose control, and then again around 20 weeks of gestation for monitoring and improvement. Hospitalizations later in the pregnancy were timed according to her classification of obstetric diabetes, which had been published in a landmark paper in 1949. In that paper Dr. Priscilla White wrote: “It is evident that age at onset of diabetes, duration, severity, and degree of maternal vascular disease all influence the fetal survival unfavorably”(Obstet Gynecol. 1992;79:295-9 / Am J Med. 1949;7:609-16).
The classification system considered age of onset, duration of diabetes, need for insulin, and presence of vascular disease. Women in higher classes and at greater risk for intrauterine death were admitted at 32 weeks, while those at less risk could wait until about 34 weeks. The timing of delivery was somewhat arbitrary, but the goal was to choose a time at which the fetus could survive in the nursery and, as Dr. White had written, “before the dreaded late intrauterine accident could occur.” (In the early ’70s, approximately half of newborns admitted to [newborn intensive care unites] at 32 weeks would survive.)
We did measure estriol levels through 24-hour urine collections as a marker for fetal and placental well-being, but as we subsequently learned, a sharp drop was often too late an indicator that something was wrong.
Dr. Landon: Dr. White and others trying to manage diabetes in pregnancy during the initial decades after insulin’s discovery were indeed significantly handicapped by a lack of tools for assessing glucose control. However, the 1970s then ushered in a “Golden Era” of fetal testing. How did advances in antepartum fetal monitoring complement the use of insulin?
Dr. Gabbe: By the mid-1970s, researchers had recognized that fetal heart rate decelerations in labor signaled fetal hypoxemia, and Dr. Roger Freeman had applied these findings to the antepartum setting, pioneering development of the contraction stress test, or oxytocin stress test. The absence of late decelerations during 10 minutes of contractions meant that the fetus was unlikely to be compromised.
When the test was administered to high-risk patients at Los Angeles County Women’s Hospital, including women with diabetes, a negative result predicted that a baby would not die within the next week. The contraction stress test was a major breakthrough. It was the first biophysical test for fetal compromise and was important for pregnancies complicated by diabetes. However, it had to be done on the labor and delivery floor, it could take hours, and it might not be definitive if one couldn’t produce enough contractions.
In the mid-1970s, the nonstress test, which relied on the presence of fetal heart rate accelerations in response to fetal movement, was found to be as reliable as the contraction stress test. It became another important tool for prolonging gestation in women with type 1 diabetes.
Even more predictive and reliable was the biophysical profile described several years later. It combined the nonstress test with an assessment using real-time fetal ultrasound of fetal movements, fetal tone and breathing movements, and amniotic fluid.
So, in a relatively short period of time, antepartum surveillance progressed from the contraction stress test to the nonstress test to the biophysical profile. These advances, along with advances in neonatal intensive care, all contributed to the continued decline in perinatal mortality.
Dr. Landon: You have taught for many years that the principal benefit of these tests of fetal surveillance is not necessarily the results identifying a fetus at risk, but the reassuring normal results that allow further maturation of the fetus that is not at risk in the pregnancy complicated by type 1 diabetes.
You also taught – as I experienced some 40 years ago when training with you at the University of Pennsylvania – that hospitalization later in pregnancy allowed for valuable optimization of our patients’ insulin regimens prior to their scheduled deliveries. This optimization helped to reduce complications such as neonatal hypoglycemia.
The introduction of the first reflectance meters to the antepartum unit eliminated the need for so many blood draws. Subsequently, came portable self-monitoring blood glucose units, which I’d argue were the second greatest achievement after the introduction of insulin because they eliminated the need for routine antepartum admissions. What are your thoughts?
Dr. Gabbe: The reflectance meters as first developed were in-hospital devices. They needed frequent calibration, and readings took several minutes. Once introduced, however, there was rapid advancement in their accuracy, size, and speed of providing results.

Other important advances were the development of rapid-acting insulins and new basal insulins and, in the late 1980s and early 1990s, the development of insulin pumps. At Penn, we studied an early pump that we called the “blue brick” because of its size. Today, of course, smaller and safer pumps paired with continuous glucose monitors are making an enormous difference for our patients with type 1 diabetes, providing them with much better outcomes.
Dr. Landon: A century after the discovery of insulin, congenital malformations remain a problem. We have seen a reduction overall, but recent data here and in Sweden show that the rate of malformations in pregnancy complicated by diabetes still is several-fold greater than in the general population.
The data also support what we’ve known for decades – that the level of glucose control during the periconceptual period is directly correlated with the risk of malformations. Can you speak to our efforts, which have been somewhat, but not completely, successful?
Dr. Gabbe: This is one of our remaining challenges. Malformations are now the leading cause of perinatal mortality in pregnancies involving type 1 and type 2 diabetes. We’ve seen these tragic outcomes over the years. While there were always questions about what caused malformations, our concerns focused on hyperglycemia early in pregnancy as a risk factor.
Knowing now that it is an abnormal intrauterine milieu during the period of organogenesis that leads to the malformations, we have improved by having patients come to us before pregnancy. Studies have shown that we can reduce malformations to a level comparable to the general population, or perhaps a bit higher, through intensive control as a result of prepregnancy care.
The challenge is that many obstetric patients don’t have a planned pregnancy. Our efforts to improve glucose control don’t always go the way we’d like them to. Still, considering where we’ve come from since the introduction of insulin to the modern management of diabetes in pregnancy, our progress has been truly remarkable.
Insulin in pregnancy: A look back at history for Diabetes Awareness Month
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at [email protected].
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at [email protected].
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at [email protected].
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Your patient’s medication label lacks human safety information: What now?
Nearly 9 in 10 U.S. women take a medication at some point in their pregnancy, with approximately 50% of women taking at least one prescription medication.1 These medications may be prescribed without the benefit of knowledge gained through clinical trials. Knowledge is gained after market, often after multiple years, and potentially following widespread use. The situation is similar for vaccines, as was recently seen with the SARS-CoV2 pandemic. Early in the pandemic, evidence emerged that pregnancy increased the risk for severe illness from COVID-19, yet pregnant people and their providers were forced to make a difficult decision of risk/benefit with little data to guide them.
The FDA product label provides a summary and narrative of animal and human safety studies relating to pregnancy. But what if that label contains little to no information, or reports studies with conflicting results? Perhaps the product is new on the market or is infrequently used during pregnancy. Regardless, health care providers and pregnant patients still need to make decisions about medication use. The following list outlines information that can be found, and strategies to support providers and patients in making informed choices for a treatment plan.
Taking stock of the available information:
- If possible, connect with the specialist who prescribed the patient’s medication in question. They may have already assembled information regarding use of that medication in pregnancy.
- The sponsor may have published useful information from the phase 3 trials, including the outcomes of enrolled patients who inadvertently became pregnant.
- Review the animal data in the product label. Regulators require the careful selection of animal models, and this data can present a source of adjunct information regarding the medication’s effects on pregnancy, reproduction, and development. Negative results can be as revealing as positive results.
- Pharmacologic data in the label can also be informative. Although most labels have pharmacologic data based on trials in healthy nonpregnant individuals, understanding pregnancy physiology and the patient’s preexisting or pregnancy-specific condition(s) can provide insights.2 Close patient monitoring and follow-up are of key importance.
- Consider viable alternatives that may address the patient’s needs. There may be effective alternatives that have been better studied and shown to have low reproductive toxicity.
- Consider the risks to the patient as well as the developing fetus if the preexisting or pregnancy-specific condition is uncontrolled.
- Consult a teratogen specialist who can provide information to both patients and health care providers on the reproductive hazards or safety of many exposures, even those with limited data regarding use in pregnancy. For example, MotherToBaby provides a network of teratogen specialists.
Understanding perceptions of risk, decision-making, and strategies to support informed choices:
- Perceptions of risk: Each person perceives risk and benefit differently. The few studies that have attempted to investigate perception of teratogenic risk have found that many pregnant people overestimate the magnitude of teratogenic risk associated with a particular exposure.3 Alternatively, a medication’s benefit in controlling the maternal condition is often not considered sufficiently. Health care providers may have their own distorted perceptions of risk, even in the presence of evidence.
- Decision-making: Most teratogen data inherently involve uncertainty; it is rare to have completely nonconflicting data with which to make a decision. This makes decisions about whether or not to utilize a particular medication or other agent in pregnancy very difficult. For example, a patient would prefer to be told a black and white answer such as vaccines are either 100% safe or 100% harmful. However, no medical treatment is held to that standard of certainty. Even though it may be more comfortable to avoid an action and “just let things happen,” the lack of a decision is still a decision. The decision to not take medication may have risks inherent in not treating a condition and may result in adverse outcomes in the developing fetus. Lastly, presenting teratogen information often involves challenges in portraying and interpreting numerical risk. For example, when considering data presented in fraction format, patients and some health care providers may focus on the numerator or count of adverse events, while ignoring the magnitude of the denominator.
- Strategies: Health literacy “best practice” strategies are useful whether there is a lot of data or very little. These include the of use plain language and messages delivered in a clear and respectful voice, the use of visual aids, and the use effective teaching methods such as asking open-ended questions to assess understanding. Other strategies include using caution in framing information: for example, discussing a 1% increase in risk for a baby to have a medication-associated birth defect should also be presented as a 99% chance the medication will not cause a birth defect. Numeracy challenges can also be addressed by using natural numbers rather than fractions or percentages: for example, if there were 100 women in this room, one would have a baby with a birth defect after taking this medication in pregnancy, but 99 of these women would not.
In today’s medical world, shared decision-making is the preferred approach to choices. Communicating and appropriately utilizing information to make choices about medication safety in pregnancy are vital undertakings. An important provider responsibility is helping patients understand that science is built on evidence that amasses and changes over time and that it represents rich shades of gray rather than “black and white” options.
Contributing to evidence: A pregnancy exposure registry is a study that collects health information from women who take prescription medicines or vaccines when they are pregnant. Information is also collected on the neonate. This information is compared with women who have not taken medicine during pregnancy. Enrolling in a pregnancy exposure registry can help improve safety information for medication used during pregnancy and can be used to update drug labeling. Please consult the Food and Drug Administration listing below to learn if there is an ongoing registry for the patient’s medication in question. If there is and the patient is eligible, provide her with the information. If she is interested and willing, help her enroll. It’s a great step toward building the scientific evidence on medication safety in pregnancy.
For further information about health literacy, consult:
https://www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
https://www.cdc.gov/ncbddd/birthdefects/index.html
https://mothertobaby.org
The MotherToBaby web page has hundreds of fact sheets written in a way that patients can understand, and available in English and Spanish. MotherToBaby coordinates research studies on specific agents. The toll-free number is 866-626-6847.
For a listing of pregnancy registries, consult:
https://www.fda.gov/science-research/womens-health-research/pregnancy-registries
Dr. Hardy is executive director, head of pharmacoepidemiology, Biohaven Pharmaceuticals. She serves as a member of Council for the Society for Birth Defects Research and Prevention (BDRP), represents the BDRP on the Coalition to Advance Maternal Therapeutics, and is a member of the North American Board for Amandla Development, South Africa. Dr. Conover is the director of Nebraska MotherToBaby. She is assistant professor at the Munroe Meyer Institute, University of Nebraska Medical Center.
References
1. Mitchell AA et al. Am J Obstet Gynecol. 2011;205(1):51:e1-e8.
2. Feghali M et al. Semin Perinatol 2015;39:512-9.
3. Conover EA, Polifka JE. Am J Med Genet Part C Semin Med Genet 2011;157:227-33.
Nearly 9 in 10 U.S. women take a medication at some point in their pregnancy, with approximately 50% of women taking at least one prescription medication.1 These medications may be prescribed without the benefit of knowledge gained through clinical trials. Knowledge is gained after market, often after multiple years, and potentially following widespread use. The situation is similar for vaccines, as was recently seen with the SARS-CoV2 pandemic. Early in the pandemic, evidence emerged that pregnancy increased the risk for severe illness from COVID-19, yet pregnant people and their providers were forced to make a difficult decision of risk/benefit with little data to guide them.
The FDA product label provides a summary and narrative of animal and human safety studies relating to pregnancy. But what if that label contains little to no information, or reports studies with conflicting results? Perhaps the product is new on the market or is infrequently used during pregnancy. Regardless, health care providers and pregnant patients still need to make decisions about medication use. The following list outlines information that can be found, and strategies to support providers and patients in making informed choices for a treatment plan.
Taking stock of the available information:
- If possible, connect with the specialist who prescribed the patient’s medication in question. They may have already assembled information regarding use of that medication in pregnancy.
- The sponsor may have published useful information from the phase 3 trials, including the outcomes of enrolled patients who inadvertently became pregnant.
- Review the animal data in the product label. Regulators require the careful selection of animal models, and this data can present a source of adjunct information regarding the medication’s effects on pregnancy, reproduction, and development. Negative results can be as revealing as positive results.
- Pharmacologic data in the label can also be informative. Although most labels have pharmacologic data based on trials in healthy nonpregnant individuals, understanding pregnancy physiology and the patient’s preexisting or pregnancy-specific condition(s) can provide insights.2 Close patient monitoring and follow-up are of key importance.
- Consider viable alternatives that may address the patient’s needs. There may be effective alternatives that have been better studied and shown to have low reproductive toxicity.
- Consider the risks to the patient as well as the developing fetus if the preexisting or pregnancy-specific condition is uncontrolled.
- Consult a teratogen specialist who can provide information to both patients and health care providers on the reproductive hazards or safety of many exposures, even those with limited data regarding use in pregnancy. For example, MotherToBaby provides a network of teratogen specialists.
Understanding perceptions of risk, decision-making, and strategies to support informed choices:
- Perceptions of risk: Each person perceives risk and benefit differently. The few studies that have attempted to investigate perception of teratogenic risk have found that many pregnant people overestimate the magnitude of teratogenic risk associated with a particular exposure.3 Alternatively, a medication’s benefit in controlling the maternal condition is often not considered sufficiently. Health care providers may have their own distorted perceptions of risk, even in the presence of evidence.
- Decision-making: Most teratogen data inherently involve uncertainty; it is rare to have completely nonconflicting data with which to make a decision. This makes decisions about whether or not to utilize a particular medication or other agent in pregnancy very difficult. For example, a patient would prefer to be told a black and white answer such as vaccines are either 100% safe or 100% harmful. However, no medical treatment is held to that standard of certainty. Even though it may be more comfortable to avoid an action and “just let things happen,” the lack of a decision is still a decision. The decision to not take medication may have risks inherent in not treating a condition and may result in adverse outcomes in the developing fetus. Lastly, presenting teratogen information often involves challenges in portraying and interpreting numerical risk. For example, when considering data presented in fraction format, patients and some health care providers may focus on the numerator or count of adverse events, while ignoring the magnitude of the denominator.
- Strategies: Health literacy “best practice” strategies are useful whether there is a lot of data or very little. These include the of use plain language and messages delivered in a clear and respectful voice, the use of visual aids, and the use effective teaching methods such as asking open-ended questions to assess understanding. Other strategies include using caution in framing information: for example, discussing a 1% increase in risk for a baby to have a medication-associated birth defect should also be presented as a 99% chance the medication will not cause a birth defect. Numeracy challenges can also be addressed by using natural numbers rather than fractions or percentages: for example, if there were 100 women in this room, one would have a baby with a birth defect after taking this medication in pregnancy, but 99 of these women would not.
In today’s medical world, shared decision-making is the preferred approach to choices. Communicating and appropriately utilizing information to make choices about medication safety in pregnancy are vital undertakings. An important provider responsibility is helping patients understand that science is built on evidence that amasses and changes over time and that it represents rich shades of gray rather than “black and white” options.
Contributing to evidence: A pregnancy exposure registry is a study that collects health information from women who take prescription medicines or vaccines when they are pregnant. Information is also collected on the neonate. This information is compared with women who have not taken medicine during pregnancy. Enrolling in a pregnancy exposure registry can help improve safety information for medication used during pregnancy and can be used to update drug labeling. Please consult the Food and Drug Administration listing below to learn if there is an ongoing registry for the patient’s medication in question. If there is and the patient is eligible, provide her with the information. If she is interested and willing, help her enroll. It’s a great step toward building the scientific evidence on medication safety in pregnancy.
For further information about health literacy, consult:
https://www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
https://www.cdc.gov/ncbddd/birthdefects/index.html
https://mothertobaby.org
The MotherToBaby web page has hundreds of fact sheets written in a way that patients can understand, and available in English and Spanish. MotherToBaby coordinates research studies on specific agents. The toll-free number is 866-626-6847.
For a listing of pregnancy registries, consult:
https://www.fda.gov/science-research/womens-health-research/pregnancy-registries
Dr. Hardy is executive director, head of pharmacoepidemiology, Biohaven Pharmaceuticals. She serves as a member of Council for the Society for Birth Defects Research and Prevention (BDRP), represents the BDRP on the Coalition to Advance Maternal Therapeutics, and is a member of the North American Board for Amandla Development, South Africa. Dr. Conover is the director of Nebraska MotherToBaby. She is assistant professor at the Munroe Meyer Institute, University of Nebraska Medical Center.
References
1. Mitchell AA et al. Am J Obstet Gynecol. 2011;205(1):51:e1-e8.
2. Feghali M et al. Semin Perinatol 2015;39:512-9.
3. Conover EA, Polifka JE. Am J Med Genet Part C Semin Med Genet 2011;157:227-33.
Nearly 9 in 10 U.S. women take a medication at some point in their pregnancy, with approximately 50% of women taking at least one prescription medication.1 These medications may be prescribed without the benefit of knowledge gained through clinical trials. Knowledge is gained after market, often after multiple years, and potentially following widespread use. The situation is similar for vaccines, as was recently seen with the SARS-CoV2 pandemic. Early in the pandemic, evidence emerged that pregnancy increased the risk for severe illness from COVID-19, yet pregnant people and their providers were forced to make a difficult decision of risk/benefit with little data to guide them.
The FDA product label provides a summary and narrative of animal and human safety studies relating to pregnancy. But what if that label contains little to no information, or reports studies with conflicting results? Perhaps the product is new on the market or is infrequently used during pregnancy. Regardless, health care providers and pregnant patients still need to make decisions about medication use. The following list outlines information that can be found, and strategies to support providers and patients in making informed choices for a treatment plan.
Taking stock of the available information:
- If possible, connect with the specialist who prescribed the patient’s medication in question. They may have already assembled information regarding use of that medication in pregnancy.
- The sponsor may have published useful information from the phase 3 trials, including the outcomes of enrolled patients who inadvertently became pregnant.
- Review the animal data in the product label. Regulators require the careful selection of animal models, and this data can present a source of adjunct information regarding the medication’s effects on pregnancy, reproduction, and development. Negative results can be as revealing as positive results.
- Pharmacologic data in the label can also be informative. Although most labels have pharmacologic data based on trials in healthy nonpregnant individuals, understanding pregnancy physiology and the patient’s preexisting or pregnancy-specific condition(s) can provide insights.2 Close patient monitoring and follow-up are of key importance.
- Consider viable alternatives that may address the patient’s needs. There may be effective alternatives that have been better studied and shown to have low reproductive toxicity.
- Consider the risks to the patient as well as the developing fetus if the preexisting or pregnancy-specific condition is uncontrolled.
- Consult a teratogen specialist who can provide information to both patients and health care providers on the reproductive hazards or safety of many exposures, even those with limited data regarding use in pregnancy. For example, MotherToBaby provides a network of teratogen specialists.
Understanding perceptions of risk, decision-making, and strategies to support informed choices:
- Perceptions of risk: Each person perceives risk and benefit differently. The few studies that have attempted to investigate perception of teratogenic risk have found that many pregnant people overestimate the magnitude of teratogenic risk associated with a particular exposure.3 Alternatively, a medication’s benefit in controlling the maternal condition is often not considered sufficiently. Health care providers may have their own distorted perceptions of risk, even in the presence of evidence.
- Decision-making: Most teratogen data inherently involve uncertainty; it is rare to have completely nonconflicting data with which to make a decision. This makes decisions about whether or not to utilize a particular medication or other agent in pregnancy very difficult. For example, a patient would prefer to be told a black and white answer such as vaccines are either 100% safe or 100% harmful. However, no medical treatment is held to that standard of certainty. Even though it may be more comfortable to avoid an action and “just let things happen,” the lack of a decision is still a decision. The decision to not take medication may have risks inherent in not treating a condition and may result in adverse outcomes in the developing fetus. Lastly, presenting teratogen information often involves challenges in portraying and interpreting numerical risk. For example, when considering data presented in fraction format, patients and some health care providers may focus on the numerator or count of adverse events, while ignoring the magnitude of the denominator.
- Strategies: Health literacy “best practice” strategies are useful whether there is a lot of data or very little. These include the of use plain language and messages delivered in a clear and respectful voice, the use of visual aids, and the use effective teaching methods such as asking open-ended questions to assess understanding. Other strategies include using caution in framing information: for example, discussing a 1% increase in risk for a baby to have a medication-associated birth defect should also be presented as a 99% chance the medication will not cause a birth defect. Numeracy challenges can also be addressed by using natural numbers rather than fractions or percentages: for example, if there were 100 women in this room, one would have a baby with a birth defect after taking this medication in pregnancy, but 99 of these women would not.
In today’s medical world, shared decision-making is the preferred approach to choices. Communicating and appropriately utilizing information to make choices about medication safety in pregnancy are vital undertakings. An important provider responsibility is helping patients understand that science is built on evidence that amasses and changes over time and that it represents rich shades of gray rather than “black and white” options.
Contributing to evidence: A pregnancy exposure registry is a study that collects health information from women who take prescription medicines or vaccines when they are pregnant. Information is also collected on the neonate. This information is compared with women who have not taken medicine during pregnancy. Enrolling in a pregnancy exposure registry can help improve safety information for medication used during pregnancy and can be used to update drug labeling. Please consult the Food and Drug Administration listing below to learn if there is an ongoing registry for the patient’s medication in question. If there is and the patient is eligible, provide her with the information. If she is interested and willing, help her enroll. It’s a great step toward building the scientific evidence on medication safety in pregnancy.
For further information about health literacy, consult:
https://www.cdc.gov/pregnancy/meds/treatingfortwo/index.html
https://www.cdc.gov/ncbddd/birthdefects/index.html
https://mothertobaby.org
The MotherToBaby web page has hundreds of fact sheets written in a way that patients can understand, and available in English and Spanish. MotherToBaby coordinates research studies on specific agents. The toll-free number is 866-626-6847.
For a listing of pregnancy registries, consult:
https://www.fda.gov/science-research/womens-health-research/pregnancy-registries
Dr. Hardy is executive director, head of pharmacoepidemiology, Biohaven Pharmaceuticals. She serves as a member of Council for the Society for Birth Defects Research and Prevention (BDRP), represents the BDRP on the Coalition to Advance Maternal Therapeutics, and is a member of the North American Board for Amandla Development, South Africa. Dr. Conover is the director of Nebraska MotherToBaby. She is assistant professor at the Munroe Meyer Institute, University of Nebraska Medical Center.
References
1. Mitchell AA et al. Am J Obstet Gynecol. 2011;205(1):51:e1-e8.
2. Feghali M et al. Semin Perinatol 2015;39:512-9.
3. Conover EA, Polifka JE. Am J Med Genet Part C Semin Med Genet 2011;157:227-33.
Fluoroquinolones linked to sudden death risk for those on hemodialysis
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A pill for C. difficile works by increasing microbiome diversity
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021
Most infant formula trials lack transparency, carry high risk of bias: Systematic review
Baby formula trials are not reliable, and have an “almost universal lack of transparency” which could undermine breastfeeding, according to the results of a systematic review published in BMJ. The findings underscore the need for significant change in the way such trials are conducted and reported, concluded lead author Bartosz Helfer, PhD, of the National Heart and Lung Institute at Imperial College London and the University of Wroclaw (Poland) Institute of Psychology and his coauthors. Citing a high risk of bias, selective reporting, and “almost universally favourable conclusions,” the international team of investigators suggested “some trials might have a marketing aim and no robust scientific aim,” concluding “much of the recent information generated about formula products might be misleading.”
The review included a detailed evaluation of 125 trials published since 2015, that compared at least two formula products in 23,757 children less than 3 years of age. The trials were evaluated for how they were conducted and reported, with specific attention paid to their risk of bias and risk of undermining breastfeeding.
Using the Cochrane risk-of-bias assessment 2.0 (ROB2), the analysis found that risk of bias was high in 80% of trials “usually because of inappropriate exclusions of participants from the analysis, and selective reporting,” the investigators noted. “This lack of transparency was complemented by favourable conclusions in more than 90% of recent trials, and evidence of publication bias in recent superiority trials.”
When conflict of interest was assessed, the analysis showed 84% of the trials received support from the formula milk industry, and of these, 77% had at least one author affiliated with a formula company. Overall, only 14% of trials had a low level of conflicts of interest according to the investigators’ definition “that the main source of funding had no commercial interest in the outcome of the trial and all of the authors of the study declared no financial ties to an entity with a commercial interest in the outcome of the trial.”
The investigators also noted that, by providing free formula to parents of breastfed or mixed-fed infants, many of the trials may have contravened the International Code of Marketing of Breast-milk Substitutes – an international agreement used to protect breastfeeding and limit the marketing of formula. “Claims arising from formula trials can contribute to formula marketing by narrowing the perceived benefits of breast milk over formula for consumers,” they wrote, calling for “improved oversight, conduct, and reporting of formula trials to ensure they provide a rigorous evidence base to inform nutrition in infants and young children.”
Asked to comment, Jennifer L. Pomeranz, JD, MPH, who was not involved in the study, told this publication the findings are “very concerning.” Ms. Pomeranz of New York University’s School of Global Public Health, recently reported similar issues in an analysis of baby formula websites. “Infant formula labels in the U.S. are adorned with a plethora of unsupported health and nutrition-related claims, including unregulated structure/function claims and breast milk comparison claims,” she said. “Moreover, infant formula marketing uses these claims to convince new parents that infant formula is necessary and even better for their infants than breast milk. Our research indicates that parents believe the popular claims made by formula companies and some even believe that infant formula is better for their child’s development than breast milk. If these claims are based on trials with no robust scientific basis, as the study suggests might be the case, then they are certainly false, deceptive, unfair, and misleading.”
Ms. Pomeranz called for the Food and Drug Administration’s regulation of infant formula labels, adding that “Congress should grant the FDA the explicit authority to require evidence to support structure/function claims on infant formula and prohibit breast milk comparison claims. ... The Federal Trade Commission and state attorneys general should bring actions against infant formula manufacturers for false and deceptive claims made in marketing materials,” she added.
Jack Newman, MD, another expert not involved in the study told this publication that the findings show how most formula studies “are essentially another marketing tool of the formula companies and are aimed at a very susceptible audience – health care professionals.” According to Dr. Newman, chief pediatrician and founder of the Newman Breastfeeding Clinic in Toronto and a former UNICEF consultant for the Baby Friendly Hospital Initiative, “health care professionals often like to believe they are immune to formula company marketing – yet this study shows that, even if they believed they were relying on scientific evidence, they were in fact being influenced toward formula feeding by studies that are biased, unreliable, and designed to promote formula to begin with.”
However, Stewart Forsyth, MD, honorary professor in child health, at the University of Dundee (Scotland) and retired consultant pediatrician and medical director at NHS Tayside, Scotland, cautioned that this is a delicate issue on all sides of the debate. The possibility of bias “is a potential issue with all aspects of research but is heightened in relation to infant feeding research because of the longstanding conflict involving the World Health Organisation, breastfeeding activist groups, and the infant formula industry, and as a consequence, all three of these organisations frequently resort to overinterpreting the data to favour their arguments,” he told this publication. An example is the suggestion that formula trials might contravene the International Code of Marketing of Breastmilk Substitutes because they provide free formula to participants. “Since when do participants in a research study have to pay for the intervention that is being studied?” he asked.
Dr. Stewart advised three key considerations “to mitigate the damaging effects that this type of inappropriate and misleading information may have on policy, practice, and engagement with parents.” First, it must be acknowledged that there is need for “a product that will provide a safety net for infants who are not offered breast milk,” he said. “It has been argued that to determine optimum nutrient requirements in infants and young children collaboration with nutrition companies is required.” Second, “all researchers need to comply with regulations relating to scientific methods, ethical standards, and financial diligence.” And finally, “there needs to be more effective planning and coordination of research activities to ensure that lessons are learned from the many studies that have design and methodological deficiencies.”
The study was funded by Imperial Health Charity. Ms. Pomeranz and Dr. Newman reported no conflicts of interest. Dr. Forsyth has undertaken consultancy work with governments, health care institutions, academia, and industry and has received research grants and honoraria from governments, charitable organizations and industry, including infant formula companies.
Senior author Robert J. Boyle, MBChB, MRCP, PhD, received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. Coauthor Jo Leonardi-Bee, MSc, PhD, received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work.
Baby formula trials are not reliable, and have an “almost universal lack of transparency” which could undermine breastfeeding, according to the results of a systematic review published in BMJ. The findings underscore the need for significant change in the way such trials are conducted and reported, concluded lead author Bartosz Helfer, PhD, of the National Heart and Lung Institute at Imperial College London and the University of Wroclaw (Poland) Institute of Psychology and his coauthors. Citing a high risk of bias, selective reporting, and “almost universally favourable conclusions,” the international team of investigators suggested “some trials might have a marketing aim and no robust scientific aim,” concluding “much of the recent information generated about formula products might be misleading.”
The review included a detailed evaluation of 125 trials published since 2015, that compared at least two formula products in 23,757 children less than 3 years of age. The trials were evaluated for how they were conducted and reported, with specific attention paid to their risk of bias and risk of undermining breastfeeding.
Using the Cochrane risk-of-bias assessment 2.0 (ROB2), the analysis found that risk of bias was high in 80% of trials “usually because of inappropriate exclusions of participants from the analysis, and selective reporting,” the investigators noted. “This lack of transparency was complemented by favourable conclusions in more than 90% of recent trials, and evidence of publication bias in recent superiority trials.”
When conflict of interest was assessed, the analysis showed 84% of the trials received support from the formula milk industry, and of these, 77% had at least one author affiliated with a formula company. Overall, only 14% of trials had a low level of conflicts of interest according to the investigators’ definition “that the main source of funding had no commercial interest in the outcome of the trial and all of the authors of the study declared no financial ties to an entity with a commercial interest in the outcome of the trial.”
The investigators also noted that, by providing free formula to parents of breastfed or mixed-fed infants, many of the trials may have contravened the International Code of Marketing of Breast-milk Substitutes – an international agreement used to protect breastfeeding and limit the marketing of formula. “Claims arising from formula trials can contribute to formula marketing by narrowing the perceived benefits of breast milk over formula for consumers,” they wrote, calling for “improved oversight, conduct, and reporting of formula trials to ensure they provide a rigorous evidence base to inform nutrition in infants and young children.”
Asked to comment, Jennifer L. Pomeranz, JD, MPH, who was not involved in the study, told this publication the findings are “very concerning.” Ms. Pomeranz of New York University’s School of Global Public Health, recently reported similar issues in an analysis of baby formula websites. “Infant formula labels in the U.S. are adorned with a plethora of unsupported health and nutrition-related claims, including unregulated structure/function claims and breast milk comparison claims,” she said. “Moreover, infant formula marketing uses these claims to convince new parents that infant formula is necessary and even better for their infants than breast milk. Our research indicates that parents believe the popular claims made by formula companies and some even believe that infant formula is better for their child’s development than breast milk. If these claims are based on trials with no robust scientific basis, as the study suggests might be the case, then they are certainly false, deceptive, unfair, and misleading.”
Ms. Pomeranz called for the Food and Drug Administration’s regulation of infant formula labels, adding that “Congress should grant the FDA the explicit authority to require evidence to support structure/function claims on infant formula and prohibit breast milk comparison claims. ... The Federal Trade Commission and state attorneys general should bring actions against infant formula manufacturers for false and deceptive claims made in marketing materials,” she added.
Jack Newman, MD, another expert not involved in the study told this publication that the findings show how most formula studies “are essentially another marketing tool of the formula companies and are aimed at a very susceptible audience – health care professionals.” According to Dr. Newman, chief pediatrician and founder of the Newman Breastfeeding Clinic in Toronto and a former UNICEF consultant for the Baby Friendly Hospital Initiative, “health care professionals often like to believe they are immune to formula company marketing – yet this study shows that, even if they believed they were relying on scientific evidence, they were in fact being influenced toward formula feeding by studies that are biased, unreliable, and designed to promote formula to begin with.”
However, Stewart Forsyth, MD, honorary professor in child health, at the University of Dundee (Scotland) and retired consultant pediatrician and medical director at NHS Tayside, Scotland, cautioned that this is a delicate issue on all sides of the debate. The possibility of bias “is a potential issue with all aspects of research but is heightened in relation to infant feeding research because of the longstanding conflict involving the World Health Organisation, breastfeeding activist groups, and the infant formula industry, and as a consequence, all three of these organisations frequently resort to overinterpreting the data to favour their arguments,” he told this publication. An example is the suggestion that formula trials might contravene the International Code of Marketing of Breastmilk Substitutes because they provide free formula to participants. “Since when do participants in a research study have to pay for the intervention that is being studied?” he asked.
Dr. Stewart advised three key considerations “to mitigate the damaging effects that this type of inappropriate and misleading information may have on policy, practice, and engagement with parents.” First, it must be acknowledged that there is need for “a product that will provide a safety net for infants who are not offered breast milk,” he said. “It has been argued that to determine optimum nutrient requirements in infants and young children collaboration with nutrition companies is required.” Second, “all researchers need to comply with regulations relating to scientific methods, ethical standards, and financial diligence.” And finally, “there needs to be more effective planning and coordination of research activities to ensure that lessons are learned from the many studies that have design and methodological deficiencies.”
The study was funded by Imperial Health Charity. Ms. Pomeranz and Dr. Newman reported no conflicts of interest. Dr. Forsyth has undertaken consultancy work with governments, health care institutions, academia, and industry and has received research grants and honoraria from governments, charitable organizations and industry, including infant formula companies.
Senior author Robert J. Boyle, MBChB, MRCP, PhD, received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. Coauthor Jo Leonardi-Bee, MSc, PhD, received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work.
Baby formula trials are not reliable, and have an “almost universal lack of transparency” which could undermine breastfeeding, according to the results of a systematic review published in BMJ. The findings underscore the need for significant change in the way such trials are conducted and reported, concluded lead author Bartosz Helfer, PhD, of the National Heart and Lung Institute at Imperial College London and the University of Wroclaw (Poland) Institute of Psychology and his coauthors. Citing a high risk of bias, selective reporting, and “almost universally favourable conclusions,” the international team of investigators suggested “some trials might have a marketing aim and no robust scientific aim,” concluding “much of the recent information generated about formula products might be misleading.”
The review included a detailed evaluation of 125 trials published since 2015, that compared at least two formula products in 23,757 children less than 3 years of age. The trials were evaluated for how they were conducted and reported, with specific attention paid to their risk of bias and risk of undermining breastfeeding.
Using the Cochrane risk-of-bias assessment 2.0 (ROB2), the analysis found that risk of bias was high in 80% of trials “usually because of inappropriate exclusions of participants from the analysis, and selective reporting,” the investigators noted. “This lack of transparency was complemented by favourable conclusions in more than 90% of recent trials, and evidence of publication bias in recent superiority trials.”
When conflict of interest was assessed, the analysis showed 84% of the trials received support from the formula milk industry, and of these, 77% had at least one author affiliated with a formula company. Overall, only 14% of trials had a low level of conflicts of interest according to the investigators’ definition “that the main source of funding had no commercial interest in the outcome of the trial and all of the authors of the study declared no financial ties to an entity with a commercial interest in the outcome of the trial.”
The investigators also noted that, by providing free formula to parents of breastfed or mixed-fed infants, many of the trials may have contravened the International Code of Marketing of Breast-milk Substitutes – an international agreement used to protect breastfeeding and limit the marketing of formula. “Claims arising from formula trials can contribute to formula marketing by narrowing the perceived benefits of breast milk over formula for consumers,” they wrote, calling for “improved oversight, conduct, and reporting of formula trials to ensure they provide a rigorous evidence base to inform nutrition in infants and young children.”
Asked to comment, Jennifer L. Pomeranz, JD, MPH, who was not involved in the study, told this publication the findings are “very concerning.” Ms. Pomeranz of New York University’s School of Global Public Health, recently reported similar issues in an analysis of baby formula websites. “Infant formula labels in the U.S. are adorned with a plethora of unsupported health and nutrition-related claims, including unregulated structure/function claims and breast milk comparison claims,” she said. “Moreover, infant formula marketing uses these claims to convince new parents that infant formula is necessary and even better for their infants than breast milk. Our research indicates that parents believe the popular claims made by formula companies and some even believe that infant formula is better for their child’s development than breast milk. If these claims are based on trials with no robust scientific basis, as the study suggests might be the case, then they are certainly false, deceptive, unfair, and misleading.”
Ms. Pomeranz called for the Food and Drug Administration’s regulation of infant formula labels, adding that “Congress should grant the FDA the explicit authority to require evidence to support structure/function claims on infant formula and prohibit breast milk comparison claims. ... The Federal Trade Commission and state attorneys general should bring actions against infant formula manufacturers for false and deceptive claims made in marketing materials,” she added.
Jack Newman, MD, another expert not involved in the study told this publication that the findings show how most formula studies “are essentially another marketing tool of the formula companies and are aimed at a very susceptible audience – health care professionals.” According to Dr. Newman, chief pediatrician and founder of the Newman Breastfeeding Clinic in Toronto and a former UNICEF consultant for the Baby Friendly Hospital Initiative, “health care professionals often like to believe they are immune to formula company marketing – yet this study shows that, even if they believed they were relying on scientific evidence, they were in fact being influenced toward formula feeding by studies that are biased, unreliable, and designed to promote formula to begin with.”
However, Stewart Forsyth, MD, honorary professor in child health, at the University of Dundee (Scotland) and retired consultant pediatrician and medical director at NHS Tayside, Scotland, cautioned that this is a delicate issue on all sides of the debate. The possibility of bias “is a potential issue with all aspects of research but is heightened in relation to infant feeding research because of the longstanding conflict involving the World Health Organisation, breastfeeding activist groups, and the infant formula industry, and as a consequence, all three of these organisations frequently resort to overinterpreting the data to favour their arguments,” he told this publication. An example is the suggestion that formula trials might contravene the International Code of Marketing of Breastmilk Substitutes because they provide free formula to participants. “Since when do participants in a research study have to pay for the intervention that is being studied?” he asked.
Dr. Stewart advised three key considerations “to mitigate the damaging effects that this type of inappropriate and misleading information may have on policy, practice, and engagement with parents.” First, it must be acknowledged that there is need for “a product that will provide a safety net for infants who are not offered breast milk,” he said. “It has been argued that to determine optimum nutrient requirements in infants and young children collaboration with nutrition companies is required.” Second, “all researchers need to comply with regulations relating to scientific methods, ethical standards, and financial diligence.” And finally, “there needs to be more effective planning and coordination of research activities to ensure that lessons are learned from the many studies that have design and methodological deficiencies.”
The study was funded by Imperial Health Charity. Ms. Pomeranz and Dr. Newman reported no conflicts of interest. Dr. Forsyth has undertaken consultancy work with governments, health care institutions, academia, and industry and has received research grants and honoraria from governments, charitable organizations and industry, including infant formula companies.
Senior author Robert J. Boyle, MBChB, MRCP, PhD, received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. Coauthor Jo Leonardi-Bee, MSc, PhD, received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work.