User login
What are the cardiorenal differences between type 1 and type 2 diabetes?
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
FROM EASD 2021
Transgender use of dermatologic procedures has strong gender tilt
, according to the results of a recent survey.
Transfeminine persons – those assigned male at birth – were much more likely to report a previous dermatologic procedure, compared with transmasculine respondents, by a margin of 64.9%-7.5%, Laura Ragmanauskaite, MD, and associates reported.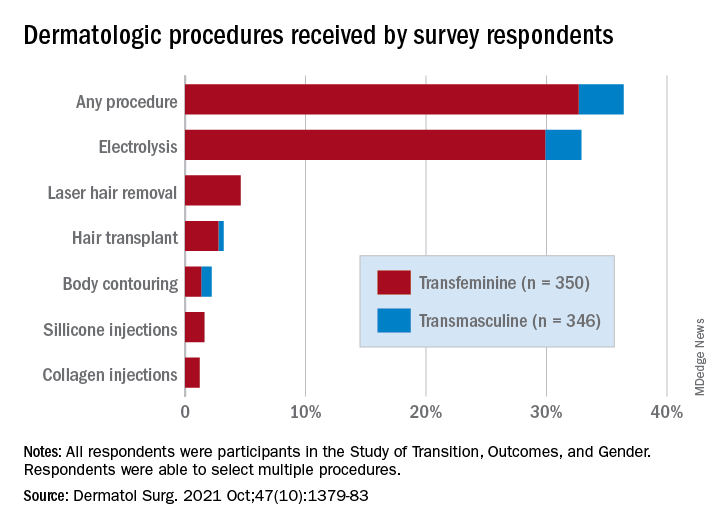
“Hair removal was the most frequently reported procedure type, with electrolysis being more common than laser hair removal,” they said, noting that “previous research on hair removal treatments among gender minority persons did not detect differences in the use of electrolysis and laser hair removal.”
Just under one-third of all respondents (32.9%) said that they had undergone electrolysis and 4.6% reported previous laser hair removal. For electrolysis, that works out to 59.4% of transfeminine and 6.1% of transmasculine respondents, while 9.1% of all transfeminine and no transmasculine persons had received laser hair removal, Dr. Ragmanauskaite of the department of dermatology, Emory University, Atlanta, and her coauthors said.
Those who had undergone gender-affirming surgery were significantly more likely to report electrolysis (78.6%) than were persons who had received no gender-affirming surgery or hormone therapy alone (47.4%), a statistically significant difference (P < .01). All of the other, less common procedures included in the online survey – 696 responses were received from 350 transfeminine and 346 transmasculine persons participating in the Study of Transition, Outcomes, and Gender – were reported more often by the transfeminine respondents. The procedure with the closest gender distribution was body contouring, reported by nine transfeminine and six transmasculine persons, the researchers said.
Use of dermal fillers was even less common (2.8% among all respondents, all transfeminine persons), with just 11 reporting having received silicone and 8 reporting having received collagen, although the survey did not ask about how the injections were obtained. In a previous study, the prevalence of illicit filler injection in transgender women was 16.9%, they pointed out.
These types of noninvasive, gender-affirming procedures “may contribute to higher levels of self-confidence and [reduce] gender dysphoria. Future studies should examine motivations, barriers, and optimal timing” for such procedures in transgender persons, Dr. Ragmanauskaite and associates wrote.
The authors reported that they had no relevant disclosures.
, according to the results of a recent survey.
Transfeminine persons – those assigned male at birth – were much more likely to report a previous dermatologic procedure, compared with transmasculine respondents, by a margin of 64.9%-7.5%, Laura Ragmanauskaite, MD, and associates reported.
“Hair removal was the most frequently reported procedure type, with electrolysis being more common than laser hair removal,” they said, noting that “previous research on hair removal treatments among gender minority persons did not detect differences in the use of electrolysis and laser hair removal.”
Just under one-third of all respondents (32.9%) said that they had undergone electrolysis and 4.6% reported previous laser hair removal. For electrolysis, that works out to 59.4% of transfeminine and 6.1% of transmasculine respondents, while 9.1% of all transfeminine and no transmasculine persons had received laser hair removal, Dr. Ragmanauskaite of the department of dermatology, Emory University, Atlanta, and her coauthors said.
Those who had undergone gender-affirming surgery were significantly more likely to report electrolysis (78.6%) than were persons who had received no gender-affirming surgery or hormone therapy alone (47.4%), a statistically significant difference (P < .01). All of the other, less common procedures included in the online survey – 696 responses were received from 350 transfeminine and 346 transmasculine persons participating in the Study of Transition, Outcomes, and Gender – were reported more often by the transfeminine respondents. The procedure with the closest gender distribution was body contouring, reported by nine transfeminine and six transmasculine persons, the researchers said.
Use of dermal fillers was even less common (2.8% among all respondents, all transfeminine persons), with just 11 reporting having received silicone and 8 reporting having received collagen, although the survey did not ask about how the injections were obtained. In a previous study, the prevalence of illicit filler injection in transgender women was 16.9%, they pointed out.
These types of noninvasive, gender-affirming procedures “may contribute to higher levels of self-confidence and [reduce] gender dysphoria. Future studies should examine motivations, barriers, and optimal timing” for such procedures in transgender persons, Dr. Ragmanauskaite and associates wrote.
The authors reported that they had no relevant disclosures.
, according to the results of a recent survey.
Transfeminine persons – those assigned male at birth – were much more likely to report a previous dermatologic procedure, compared with transmasculine respondents, by a margin of 64.9%-7.5%, Laura Ragmanauskaite, MD, and associates reported.
“Hair removal was the most frequently reported procedure type, with electrolysis being more common than laser hair removal,” they said, noting that “previous research on hair removal treatments among gender minority persons did not detect differences in the use of electrolysis and laser hair removal.”
Just under one-third of all respondents (32.9%) said that they had undergone electrolysis and 4.6% reported previous laser hair removal. For electrolysis, that works out to 59.4% of transfeminine and 6.1% of transmasculine respondents, while 9.1% of all transfeminine and no transmasculine persons had received laser hair removal, Dr. Ragmanauskaite of the department of dermatology, Emory University, Atlanta, and her coauthors said.
Those who had undergone gender-affirming surgery were significantly more likely to report electrolysis (78.6%) than were persons who had received no gender-affirming surgery or hormone therapy alone (47.4%), a statistically significant difference (P < .01). All of the other, less common procedures included in the online survey – 696 responses were received from 350 transfeminine and 346 transmasculine persons participating in the Study of Transition, Outcomes, and Gender – were reported more often by the transfeminine respondents. The procedure with the closest gender distribution was body contouring, reported by nine transfeminine and six transmasculine persons, the researchers said.
Use of dermal fillers was even less common (2.8% among all respondents, all transfeminine persons), with just 11 reporting having received silicone and 8 reporting having received collagen, although the survey did not ask about how the injections were obtained. In a previous study, the prevalence of illicit filler injection in transgender women was 16.9%, they pointed out.
These types of noninvasive, gender-affirming procedures “may contribute to higher levels of self-confidence and [reduce] gender dysphoria. Future studies should examine motivations, barriers, and optimal timing” for such procedures in transgender persons, Dr. Ragmanauskaite and associates wrote.
The authors reported that they had no relevant disclosures.
FROM DERMATOLOGIC SURGERY
Overview of guidelines for patients seeking gender-affirmation surgery
Gender-affirmation surgery refers to a collection of procedures by which a transgender individual physically alters characteristics to align with their gender identity. While not all patients who identify as transgender will choose to undergo surgery, the surgeries are considered medically necessary and lead to significant improvements in emotional and psychological well-being.1 With increasing insurance coverage and improved access to care, more and more patients are seeking gender-affirming surgery, and it is incumbent for providers to familiarize themselves with preoperative recommendations and requirements.
Ob.gyns. play a key role in patients seeking surgical treatment as patients may inquire about available procedures and what steps are necessary prior to scheduling a visit with the appropriate surgeon. The World Professional Association of Transgender Health has established standards of care that provide multidisciplinary, evidence-based guidance for patients seeking a variety of gender-affirming services ranging from mental health, hormone therapy, and surgery.
Basic preoperative surgical prerequisites set forth by WPATH include being a patient with well-documented gender dysphoria, being the age of majority, and having the ability to provide informed consent.1
As with any surgical candidate, it is also equally important for a patient to have well-controlled medical and psychiatric comorbidities, which should also include smoking cessation. A variety of surgical procedures are available to patients and include breast/chest surgery, genital (bottom) surgery, and nongenital surgery (facial feminization, pectoral implant placement, thyroid chondroplasty, lipofilling/liposuction, body contouring, and voice modification). Patients may choose to undergo chest/breast surgery and/or bottom surgery or forgo surgical procedures altogether.
For transmasculine patients, breast/chest surgery, otherwise known as top surgery, is the most common and desired procedure. According to a recent survey, approximately 97% of transmasculine patients had or wanted masculinizing chest surgery.2 In addition to patients meeting the basic requirements set forth by WPATH, one referral from a mental health provider specializing in gender-affirming care is also needed prior to this procedure. It is also important to note that testosterone use is no longer a needed prior to masculinizing chest surgery.
Transmasculine bottom surgery, which includes hysterectomy, bilateral salpingo-oophorectomy, metoidioplasty, vaginectomy, scrotoplasty, testicular implant placement, and/or phalloplasty have additional nuances. Compared with transmasculine individuals seeking top surgery, the number of patients who have had or desire metoidioplasty and phalloplasty is much lower, which is mainly because of the high complication rates of these procedures. In the same survey, only 4% of patients had undergone a metoidioplasty procedure and 2% of patients had undergone a phalloplasty.2
In evaluating rates of hysterectomy with or without salpingo-oophorectomy, approximately 21% of transgender men underwent hysterectomy, with 58% desiring it in the future.2 Unlike patients pursuing top surgery, patients who desire any form of bottom surgery need to be on 12 months of continuous hormone therapy.1 They also must provide two letters from two different mental health providers, one of whom must have either an MD/DO or PhD. In cases in which a patient requests a hysterectomy for reasons other than gender dysphoria, such as pelvic pain or abnormal uterine bleeding, these criteria do not apply.
For transfeminine individuals, augmentation mammoplasty is performed following 12 months of continuous hormone therapy. This is to allow maximum breast growth, which occurs approximately 2-3 months after hormone initiation and peaks at 1-2 years.3 Rates of transfeminine individuals seeking augmentation mammoplasty is similar to that of their transmasculine counterparts at 74%.2 One referral letter from a mental health provider is also needed prior to augmentation mammoplasty.
Transfeminine patients who desire bottom surgery, which can involve an orchiectomy or vaginoplasty (single-stage penile inversion, peritoneal, or colonic interposition), have the same additional requirements as transmasculine individuals seeking bottom surgery. Furthermore, it is interesting to note that 25% of transfeminine individuals had already undergone orchiectomy and 87% had either undergone or desired a vaginoplasty in the future.2 This is in stark contrast to transmasculine patients and rates of bottom surgery.
Unless there is a specific medical contraindication to hormone therapy, emphasis is placed on 12 months of continuous hormone usage. Additional emphasis is placed on patients seeking bottom surgery to live for a minimum of 12 months in their congruent gender role. This also allows patients to further explore their gender identity and make appropriate preparations for surgery.
As with any surgical procedure, obtaining informed consent and reviewing patient expectations are key. In my clinical practice, I discuss with patients that the general surgical goals are to achieve both function and good aesthetic outcome but that their results are also tailored to their individual bodies. Assessing a patient’s support system and social factors is also equally important in the preoperative planning period. As this field continues to grow, it is essential for providers to understand the evolving distinctions in surgical care to improve access to patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She has no conflicts. Email her at [email protected].
References
1. The World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. https://www.wpath.org/publications/soc.
2. James SE et al. The report of the 2015 U.S. Transgender survey. Washington, D.C.: National Center for Transgender Equality. 2016.
3. Thomas TN. Overview of surgery for transgender patients, in “Comprehensive care for the transgender patient.” Philadelphia: Elsevier, 2020. pp. 48-53.
Gender-affirmation surgery refers to a collection of procedures by which a transgender individual physically alters characteristics to align with their gender identity. While not all patients who identify as transgender will choose to undergo surgery, the surgeries are considered medically necessary and lead to significant improvements in emotional and psychological well-being.1 With increasing insurance coverage and improved access to care, more and more patients are seeking gender-affirming surgery, and it is incumbent for providers to familiarize themselves with preoperative recommendations and requirements.
Ob.gyns. play a key role in patients seeking surgical treatment as patients may inquire about available procedures and what steps are necessary prior to scheduling a visit with the appropriate surgeon. The World Professional Association of Transgender Health has established standards of care that provide multidisciplinary, evidence-based guidance for patients seeking a variety of gender-affirming services ranging from mental health, hormone therapy, and surgery.
Basic preoperative surgical prerequisites set forth by WPATH include being a patient with well-documented gender dysphoria, being the age of majority, and having the ability to provide informed consent.1
As with any surgical candidate, it is also equally important for a patient to have well-controlled medical and psychiatric comorbidities, which should also include smoking cessation. A variety of surgical procedures are available to patients and include breast/chest surgery, genital (bottom) surgery, and nongenital surgery (facial feminization, pectoral implant placement, thyroid chondroplasty, lipofilling/liposuction, body contouring, and voice modification). Patients may choose to undergo chest/breast surgery and/or bottom surgery or forgo surgical procedures altogether.
For transmasculine patients, breast/chest surgery, otherwise known as top surgery, is the most common and desired procedure. According to a recent survey, approximately 97% of transmasculine patients had or wanted masculinizing chest surgery.2 In addition to patients meeting the basic requirements set forth by WPATH, one referral from a mental health provider specializing in gender-affirming care is also needed prior to this procedure. It is also important to note that testosterone use is no longer a needed prior to masculinizing chest surgery.
Transmasculine bottom surgery, which includes hysterectomy, bilateral salpingo-oophorectomy, metoidioplasty, vaginectomy, scrotoplasty, testicular implant placement, and/or phalloplasty have additional nuances. Compared with transmasculine individuals seeking top surgery, the number of patients who have had or desire metoidioplasty and phalloplasty is much lower, which is mainly because of the high complication rates of these procedures. In the same survey, only 4% of patients had undergone a metoidioplasty procedure and 2% of patients had undergone a phalloplasty.2
In evaluating rates of hysterectomy with or without salpingo-oophorectomy, approximately 21% of transgender men underwent hysterectomy, with 58% desiring it in the future.2 Unlike patients pursuing top surgery, patients who desire any form of bottom surgery need to be on 12 months of continuous hormone therapy.1 They also must provide two letters from two different mental health providers, one of whom must have either an MD/DO or PhD. In cases in which a patient requests a hysterectomy for reasons other than gender dysphoria, such as pelvic pain or abnormal uterine bleeding, these criteria do not apply.
For transfeminine individuals, augmentation mammoplasty is performed following 12 months of continuous hormone therapy. This is to allow maximum breast growth, which occurs approximately 2-3 months after hormone initiation and peaks at 1-2 years.3 Rates of transfeminine individuals seeking augmentation mammoplasty is similar to that of their transmasculine counterparts at 74%.2 One referral letter from a mental health provider is also needed prior to augmentation mammoplasty.
Transfeminine patients who desire bottom surgery, which can involve an orchiectomy or vaginoplasty (single-stage penile inversion, peritoneal, or colonic interposition), have the same additional requirements as transmasculine individuals seeking bottom surgery. Furthermore, it is interesting to note that 25% of transfeminine individuals had already undergone orchiectomy and 87% had either undergone or desired a vaginoplasty in the future.2 This is in stark contrast to transmasculine patients and rates of bottom surgery.
Unless there is a specific medical contraindication to hormone therapy, emphasis is placed on 12 months of continuous hormone usage. Additional emphasis is placed on patients seeking bottom surgery to live for a minimum of 12 months in their congruent gender role. This also allows patients to further explore their gender identity and make appropriate preparations for surgery.
As with any surgical procedure, obtaining informed consent and reviewing patient expectations are key. In my clinical practice, I discuss with patients that the general surgical goals are to achieve both function and good aesthetic outcome but that their results are also tailored to their individual bodies. Assessing a patient’s support system and social factors is also equally important in the preoperative planning period. As this field continues to grow, it is essential for providers to understand the evolving distinctions in surgical care to improve access to patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She has no conflicts. Email her at [email protected].
References
1. The World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. https://www.wpath.org/publications/soc.
2. James SE et al. The report of the 2015 U.S. Transgender survey. Washington, D.C.: National Center for Transgender Equality. 2016.
3. Thomas TN. Overview of surgery for transgender patients, in “Comprehensive care for the transgender patient.” Philadelphia: Elsevier, 2020. pp. 48-53.
Gender-affirmation surgery refers to a collection of procedures by which a transgender individual physically alters characteristics to align with their gender identity. While not all patients who identify as transgender will choose to undergo surgery, the surgeries are considered medically necessary and lead to significant improvements in emotional and psychological well-being.1 With increasing insurance coverage and improved access to care, more and more patients are seeking gender-affirming surgery, and it is incumbent for providers to familiarize themselves with preoperative recommendations and requirements.
Ob.gyns. play a key role in patients seeking surgical treatment as patients may inquire about available procedures and what steps are necessary prior to scheduling a visit with the appropriate surgeon. The World Professional Association of Transgender Health has established standards of care that provide multidisciplinary, evidence-based guidance for patients seeking a variety of gender-affirming services ranging from mental health, hormone therapy, and surgery.
Basic preoperative surgical prerequisites set forth by WPATH include being a patient with well-documented gender dysphoria, being the age of majority, and having the ability to provide informed consent.1
As with any surgical candidate, it is also equally important for a patient to have well-controlled medical and psychiatric comorbidities, which should also include smoking cessation. A variety of surgical procedures are available to patients and include breast/chest surgery, genital (bottom) surgery, and nongenital surgery (facial feminization, pectoral implant placement, thyroid chondroplasty, lipofilling/liposuction, body contouring, and voice modification). Patients may choose to undergo chest/breast surgery and/or bottom surgery or forgo surgical procedures altogether.
For transmasculine patients, breast/chest surgery, otherwise known as top surgery, is the most common and desired procedure. According to a recent survey, approximately 97% of transmasculine patients had or wanted masculinizing chest surgery.2 In addition to patients meeting the basic requirements set forth by WPATH, one referral from a mental health provider specializing in gender-affirming care is also needed prior to this procedure. It is also important to note that testosterone use is no longer a needed prior to masculinizing chest surgery.
Transmasculine bottom surgery, which includes hysterectomy, bilateral salpingo-oophorectomy, metoidioplasty, vaginectomy, scrotoplasty, testicular implant placement, and/or phalloplasty have additional nuances. Compared with transmasculine individuals seeking top surgery, the number of patients who have had or desire metoidioplasty and phalloplasty is much lower, which is mainly because of the high complication rates of these procedures. In the same survey, only 4% of patients had undergone a metoidioplasty procedure and 2% of patients had undergone a phalloplasty.2
In evaluating rates of hysterectomy with or without salpingo-oophorectomy, approximately 21% of transgender men underwent hysterectomy, with 58% desiring it in the future.2 Unlike patients pursuing top surgery, patients who desire any form of bottom surgery need to be on 12 months of continuous hormone therapy.1 They also must provide two letters from two different mental health providers, one of whom must have either an MD/DO or PhD. In cases in which a patient requests a hysterectomy for reasons other than gender dysphoria, such as pelvic pain or abnormal uterine bleeding, these criteria do not apply.
For transfeminine individuals, augmentation mammoplasty is performed following 12 months of continuous hormone therapy. This is to allow maximum breast growth, which occurs approximately 2-3 months after hormone initiation and peaks at 1-2 years.3 Rates of transfeminine individuals seeking augmentation mammoplasty is similar to that of their transmasculine counterparts at 74%.2 One referral letter from a mental health provider is also needed prior to augmentation mammoplasty.
Transfeminine patients who desire bottom surgery, which can involve an orchiectomy or vaginoplasty (single-stage penile inversion, peritoneal, or colonic interposition), have the same additional requirements as transmasculine individuals seeking bottom surgery. Furthermore, it is interesting to note that 25% of transfeminine individuals had already undergone orchiectomy and 87% had either undergone or desired a vaginoplasty in the future.2 This is in stark contrast to transmasculine patients and rates of bottom surgery.
Unless there is a specific medical contraindication to hormone therapy, emphasis is placed on 12 months of continuous hormone usage. Additional emphasis is placed on patients seeking bottom surgery to live for a minimum of 12 months in their congruent gender role. This also allows patients to further explore their gender identity and make appropriate preparations for surgery.
As with any surgical procedure, obtaining informed consent and reviewing patient expectations are key. In my clinical practice, I discuss with patients that the general surgical goals are to achieve both function and good aesthetic outcome but that their results are also tailored to their individual bodies. Assessing a patient’s support system and social factors is also equally important in the preoperative planning period. As this field continues to grow, it is essential for providers to understand the evolving distinctions in surgical care to improve access to patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She has no conflicts. Email her at [email protected].
References
1. The World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. https://www.wpath.org/publications/soc.
2. James SE et al. The report of the 2015 U.S. Transgender survey. Washington, D.C.: National Center for Transgender Equality. 2016.
3. Thomas TN. Overview of surgery for transgender patients, in “Comprehensive care for the transgender patient.” Philadelphia: Elsevier, 2020. pp. 48-53.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
Tiny worms sniff out early-stage pancreatic cancer
Research shows Caenorhabditis elegans are attracted to the odor certain chemicals give off – a behavior known as attractive chemotaxis – and early evidence indicates these scents may include human cancer cell secretions, cancer tissues, and urine from patients with colorectal, gastric, and breast cancers.
According to the recent analysis, published in Oncotarget, these small worms may be hot on the trail of pancreatic cancer–related compounds too. The researchers found that C. elegans were significantly more attracted to patients with early-stage pancreatic cancer versus healthy controls.
There is a huge need for research like this that explores strategies to detect pancreatic cancer early, but it’s far too soon to tell how, or if, this particular approach will be clinically relevant, according to Neeha Zaidi, MD, assistant professor of oncology and a medical oncologist specializing in pancreatic cancer at John Hopkins Medicine, Baltimore, who was not involved in the current analysis.
Right now, few diagnostic markers exist for identifying pancreatic ductal adenocarcinomas (PDACs), which account for 90% of pancreatic cancers. PDACs remain one of the deadliest cancers, with a 5-year survival rate of 9%.
A combination of surgical resection and chemotherapy is the only curative treatment, and just 20% of patients are eligible, Dr. Zaidi said. The majority are identified after the disease has metastasized.
However, patients’ 5-year survival rate improves markedly – as high as 85% – if the condition is caught sooner.
In the current study, the researchers first exposed C. elegans to the urine of 83 patients from cancer centers across Japan who had various stages of pancreatic cancer before and after undergoing surgical resection. Using an assay, which takes 30 minutes and 50-100 nematodes per test, C. elegans showed significantly higher chemotaxis toward preoperative urine, compared with postoperative urine.
In a second, closed-labeled arm, the nematodes were exposed to the urine of 28 randomized participants – 11 of whom had early-stage pancreatic cancer (0 or IA), plus 17 healthy volunteers. In this instance as well, C. elegans showed significantly higher chemotaxis in patients with early-stage pancreatic cancer, compared with healthy volunteers (P = .034).
According to the authors, C. elegans “had a higher sensitivity for detecting early pancreatic cancer compared to existing diagnostic markers.” And while this strategy needs to be further validated, they believe early detection of pancreatic cancer using C. elegans “can certainly be expected in the near future.”
The study aligns with previous research, showing that wild-type C. elegans are sensitive to scent and that these critters can smell cancer. Other studies have also found that sniffer canines can detect volatile organic compounds – including biomarkers of certain cancers – in the urine and breath of cancer patients. But training an adequate number of these canines for the clinic isn’t feasible, while C. elegans are far more compact and affordable.
According to Dr. Zaidi, a scent test using C. elegans “seems pretty feasible” and cost effective, but whether this approach will “change our care has yet to be determined.”
The authors, for instance, don’t specify how the scent test will be used, though Dr. Zaidi suspects it would be most relevant for following patients with a higher risk of pancreatic cancer. Alternatively, it could be used as a screening test, but that’s a massive undertaking and “this is way too early to tell if it’s going to be helpful to use this test on a broad scale,” Dr. Zaidi said.
To validate the approach, researchers would also need to know what exactly the C. elegans are smelling and to test it in a much larger number of patients, Dr. Zaidi said. The mere 11 patients with cancer in the blinded portion of the study are not sufficient to draw any major conclusions.
The study also claims a high sensitivity, but what about specificity, Dr. Zaidi said. In other words, are there a lot of false positives?
In addition, a deeper look at the participants shows the two groups – early PDAC and healthy volunteers – were not adequately balanced. The median age of the diseased patients was 70, and the healthy volunteers was 39.
“This is a big difference,” Eithne Costello, PhD, professor of molecular oncology at Liverpool (England) University, said in an interview. “It [also] appears the controls are all of one sex (either all male or all female), while the cancer group is mixed.”
The authors attributed these shortcomings to the small population they had to work with: There simply aren’t many patients whose pancreatic cancer is detected early. Dr. Zaidi agreed that patients with pancreatic cancer stage 0 or IA are extremely difficult to come by.
Even still, researchers need to understand the mechanisms behind this approach and see it work in a much larger group of patients, Dr. Zaidi said.
The study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology. The authors reported institutional endowments received from Hirotsu Bio Science, Kinshu-kai Medical, IDEA Consultants, Ono Pharmaceutical, and others. Two coauthors are employees of Hirotsu Bio Science.
A version of this article first appeared on Medscape.com.
Research shows Caenorhabditis elegans are attracted to the odor certain chemicals give off – a behavior known as attractive chemotaxis – and early evidence indicates these scents may include human cancer cell secretions, cancer tissues, and urine from patients with colorectal, gastric, and breast cancers.
According to the recent analysis, published in Oncotarget, these small worms may be hot on the trail of pancreatic cancer–related compounds too. The researchers found that C. elegans were significantly more attracted to patients with early-stage pancreatic cancer versus healthy controls.
There is a huge need for research like this that explores strategies to detect pancreatic cancer early, but it’s far too soon to tell how, or if, this particular approach will be clinically relevant, according to Neeha Zaidi, MD, assistant professor of oncology and a medical oncologist specializing in pancreatic cancer at John Hopkins Medicine, Baltimore, who was not involved in the current analysis.
Right now, few diagnostic markers exist for identifying pancreatic ductal adenocarcinomas (PDACs), which account for 90% of pancreatic cancers. PDACs remain one of the deadliest cancers, with a 5-year survival rate of 9%.
A combination of surgical resection and chemotherapy is the only curative treatment, and just 20% of patients are eligible, Dr. Zaidi said. The majority are identified after the disease has metastasized.
However, patients’ 5-year survival rate improves markedly – as high as 85% – if the condition is caught sooner.
In the current study, the researchers first exposed C. elegans to the urine of 83 patients from cancer centers across Japan who had various stages of pancreatic cancer before and after undergoing surgical resection. Using an assay, which takes 30 minutes and 50-100 nematodes per test, C. elegans showed significantly higher chemotaxis toward preoperative urine, compared with postoperative urine.
In a second, closed-labeled arm, the nematodes were exposed to the urine of 28 randomized participants – 11 of whom had early-stage pancreatic cancer (0 or IA), plus 17 healthy volunteers. In this instance as well, C. elegans showed significantly higher chemotaxis in patients with early-stage pancreatic cancer, compared with healthy volunteers (P = .034).
According to the authors, C. elegans “had a higher sensitivity for detecting early pancreatic cancer compared to existing diagnostic markers.” And while this strategy needs to be further validated, they believe early detection of pancreatic cancer using C. elegans “can certainly be expected in the near future.”
The study aligns with previous research, showing that wild-type C. elegans are sensitive to scent and that these critters can smell cancer. Other studies have also found that sniffer canines can detect volatile organic compounds – including biomarkers of certain cancers – in the urine and breath of cancer patients. But training an adequate number of these canines for the clinic isn’t feasible, while C. elegans are far more compact and affordable.
According to Dr. Zaidi, a scent test using C. elegans “seems pretty feasible” and cost effective, but whether this approach will “change our care has yet to be determined.”
The authors, for instance, don’t specify how the scent test will be used, though Dr. Zaidi suspects it would be most relevant for following patients with a higher risk of pancreatic cancer. Alternatively, it could be used as a screening test, but that’s a massive undertaking and “this is way too early to tell if it’s going to be helpful to use this test on a broad scale,” Dr. Zaidi said.
To validate the approach, researchers would also need to know what exactly the C. elegans are smelling and to test it in a much larger number of patients, Dr. Zaidi said. The mere 11 patients with cancer in the blinded portion of the study are not sufficient to draw any major conclusions.
The study also claims a high sensitivity, but what about specificity, Dr. Zaidi said. In other words, are there a lot of false positives?
In addition, a deeper look at the participants shows the two groups – early PDAC and healthy volunteers – were not adequately balanced. The median age of the diseased patients was 70, and the healthy volunteers was 39.
“This is a big difference,” Eithne Costello, PhD, professor of molecular oncology at Liverpool (England) University, said in an interview. “It [also] appears the controls are all of one sex (either all male or all female), while the cancer group is mixed.”
The authors attributed these shortcomings to the small population they had to work with: There simply aren’t many patients whose pancreatic cancer is detected early. Dr. Zaidi agreed that patients with pancreatic cancer stage 0 or IA are extremely difficult to come by.
Even still, researchers need to understand the mechanisms behind this approach and see it work in a much larger group of patients, Dr. Zaidi said.
The study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology. The authors reported institutional endowments received from Hirotsu Bio Science, Kinshu-kai Medical, IDEA Consultants, Ono Pharmaceutical, and others. Two coauthors are employees of Hirotsu Bio Science.
A version of this article first appeared on Medscape.com.
Research shows Caenorhabditis elegans are attracted to the odor certain chemicals give off – a behavior known as attractive chemotaxis – and early evidence indicates these scents may include human cancer cell secretions, cancer tissues, and urine from patients with colorectal, gastric, and breast cancers.
According to the recent analysis, published in Oncotarget, these small worms may be hot on the trail of pancreatic cancer–related compounds too. The researchers found that C. elegans were significantly more attracted to patients with early-stage pancreatic cancer versus healthy controls.
There is a huge need for research like this that explores strategies to detect pancreatic cancer early, but it’s far too soon to tell how, or if, this particular approach will be clinically relevant, according to Neeha Zaidi, MD, assistant professor of oncology and a medical oncologist specializing in pancreatic cancer at John Hopkins Medicine, Baltimore, who was not involved in the current analysis.
Right now, few diagnostic markers exist for identifying pancreatic ductal adenocarcinomas (PDACs), which account for 90% of pancreatic cancers. PDACs remain one of the deadliest cancers, with a 5-year survival rate of 9%.
A combination of surgical resection and chemotherapy is the only curative treatment, and just 20% of patients are eligible, Dr. Zaidi said. The majority are identified after the disease has metastasized.
However, patients’ 5-year survival rate improves markedly – as high as 85% – if the condition is caught sooner.
In the current study, the researchers first exposed C. elegans to the urine of 83 patients from cancer centers across Japan who had various stages of pancreatic cancer before and after undergoing surgical resection. Using an assay, which takes 30 minutes and 50-100 nematodes per test, C. elegans showed significantly higher chemotaxis toward preoperative urine, compared with postoperative urine.
In a second, closed-labeled arm, the nematodes were exposed to the urine of 28 randomized participants – 11 of whom had early-stage pancreatic cancer (0 or IA), plus 17 healthy volunteers. In this instance as well, C. elegans showed significantly higher chemotaxis in patients with early-stage pancreatic cancer, compared with healthy volunteers (P = .034).
According to the authors, C. elegans “had a higher sensitivity for detecting early pancreatic cancer compared to existing diagnostic markers.” And while this strategy needs to be further validated, they believe early detection of pancreatic cancer using C. elegans “can certainly be expected in the near future.”
The study aligns with previous research, showing that wild-type C. elegans are sensitive to scent and that these critters can smell cancer. Other studies have also found that sniffer canines can detect volatile organic compounds – including biomarkers of certain cancers – in the urine and breath of cancer patients. But training an adequate number of these canines for the clinic isn’t feasible, while C. elegans are far more compact and affordable.
According to Dr. Zaidi, a scent test using C. elegans “seems pretty feasible” and cost effective, but whether this approach will “change our care has yet to be determined.”
The authors, for instance, don’t specify how the scent test will be used, though Dr. Zaidi suspects it would be most relevant for following patients with a higher risk of pancreatic cancer. Alternatively, it could be used as a screening test, but that’s a massive undertaking and “this is way too early to tell if it’s going to be helpful to use this test on a broad scale,” Dr. Zaidi said.
To validate the approach, researchers would also need to know what exactly the C. elegans are smelling and to test it in a much larger number of patients, Dr. Zaidi said. The mere 11 patients with cancer in the blinded portion of the study are not sufficient to draw any major conclusions.
The study also claims a high sensitivity, but what about specificity, Dr. Zaidi said. In other words, are there a lot of false positives?
In addition, a deeper look at the participants shows the two groups – early PDAC and healthy volunteers – were not adequately balanced. The median age of the diseased patients was 70, and the healthy volunteers was 39.
“This is a big difference,” Eithne Costello, PhD, professor of molecular oncology at Liverpool (England) University, said in an interview. “It [also] appears the controls are all of one sex (either all male or all female), while the cancer group is mixed.”
The authors attributed these shortcomings to the small population they had to work with: There simply aren’t many patients whose pancreatic cancer is detected early. Dr. Zaidi agreed that patients with pancreatic cancer stage 0 or IA are extremely difficult to come by.
Even still, researchers need to understand the mechanisms behind this approach and see it work in a much larger group of patients, Dr. Zaidi said.
The study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology. The authors reported institutional endowments received from Hirotsu Bio Science, Kinshu-kai Medical, IDEA Consultants, Ono Pharmaceutical, and others. Two coauthors are employees of Hirotsu Bio Science.
A version of this article first appeared on Medscape.com.
Novel light therapy helmet boosts brain function
Near-infrared light delivered to the brain using a specially designed helmet appears to improve memory, motor function, and processing skills in cognitively healthy older adults, in new findings that suggest potential benefit in patients with dementia.
Studies in animals and people have shown “many positive effects” with near-infrared transcranial photobiomodulation therapy (PBM-T), study investigator Paul Chazot, PhD, department of biosciences, Durham University, United Kingdom, told this news organization.
For example, PBM-T has been shown to increase blood circulation (which keeps the brain well oxygenated), boost mitochondria function in neurons, protect neurons from oxidative stress, and help maintain neuronal connectivity, Dr. Chazot explained.
PBM-T has also been shown to reduce amyloid and phosphorylated tau load, pathological signs of Alzheimer’s disease.
“All these in combination improve memory performance and mobility,” Dr. Chazot said.
The study was published online October 18 in Photobiomodulation, Photomedicine and Laser Surgery.
Promising early data
In the study, 14 healthy adults, aged 45 to 70 years, received 6 minutes of transcranial PBM-T twice daily at a wavelength of 1,068 nanometers over 4 weeks. PBM-T was delivered via a helmet that comprised 14 air-cooled light emitting diode panel arrays. A control group of 13 adults used a sham PBM-T helmet.
Before and after active and sham treatment, all participants completed the automated neuropsychological assessment metrics (ANAM) – a computer-based tool designed to detect speed and accuracy of attention, memory, and thinking ability.
According to the research team, compared with sham PBM-T, those receiving active PBM-T showed significant improvement in motor function (finger tapping), working memory, delayed memory, and brain processing speed, the research team reports. No adverse effects were reported.
“This study complements our other recent studies, which showed improvement in memory performance with no obvious side effects,” said Dr. Chazot.
“While this is a pilot study and more research is needed, there are promising indications that therapy involving infrared light might also be beneficial for people living with dementia, and this is worth exploring,” Dr. Chazot added in a news release.
The PBM-T helmet was devised by first author Gordon Dougal, MBChB, of Maculume in the U.K., and a general practitioner based in Durham.
A recent study by Mr. Dougal, Dr. Chazot, and collaborators in the United States provides early evidence that PBM-T can improve memory in adults with dementia.
In that study, 39 patients received 6 minutes of PBM-T twice a day for 8 weeks, alongside a control group of 17 patients who received sham PBM-T.
After 8 weeks, there was about a 20% improvement in Mini-Mental State Exam (MMSE) scores in the active PBM-T group compared with roughly a 6% improvement in the control group, the researchers report in the journal Cureus.
More research needed
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said using light to stimulate the brain is “an emerging technology.”
“However, ,” Dr. Edelmayer told this news organization.
“That being said, we’re starting to see companies looking at similar, noninvasive methods of stimulating the brain. For example, brain stimulation devices have been applied to other neurodegenerative diseases like Parkinson’s to try to prevent degeneration of brain cells,” Dr. Edelmayer noted.
She said more research is needed to understand how photobiomodulation might be used as a therapy or prevention for cognitive decline and dementia.
“Specifically, we need to understand what parts of the brain need to be targeted and at what point(s) in the disease course this treatment would be most impactful. If proven to be effective, this could possibly be part of an approach that’s combined with other treatments, like drugs and lifestyle interventions,” said Dr. Edelmayer.
The Alzheimer’s Association is funding a number of projects looking at noninvasive treatments for Alzheimer’s disease, including two clinical trials looking at deep brain stimulation and photobiomodulation.
Maculume provided funding for the study. Mr. Dougal is a majority shareholder in the company, which manufactures the helmet device used in the study. Dr. Chazot, study co-authors, and Dr. Edelmayer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Near-infrared light delivered to the brain using a specially designed helmet appears to improve memory, motor function, and processing skills in cognitively healthy older adults, in new findings that suggest potential benefit in patients with dementia.
Studies in animals and people have shown “many positive effects” with near-infrared transcranial photobiomodulation therapy (PBM-T), study investigator Paul Chazot, PhD, department of biosciences, Durham University, United Kingdom, told this news organization.
For example, PBM-T has been shown to increase blood circulation (which keeps the brain well oxygenated), boost mitochondria function in neurons, protect neurons from oxidative stress, and help maintain neuronal connectivity, Dr. Chazot explained.
PBM-T has also been shown to reduce amyloid and phosphorylated tau load, pathological signs of Alzheimer’s disease.
“All these in combination improve memory performance and mobility,” Dr. Chazot said.
The study was published online October 18 in Photobiomodulation, Photomedicine and Laser Surgery.
Promising early data
In the study, 14 healthy adults, aged 45 to 70 years, received 6 minutes of transcranial PBM-T twice daily at a wavelength of 1,068 nanometers over 4 weeks. PBM-T was delivered via a helmet that comprised 14 air-cooled light emitting diode panel arrays. A control group of 13 adults used a sham PBM-T helmet.
Before and after active and sham treatment, all participants completed the automated neuropsychological assessment metrics (ANAM) – a computer-based tool designed to detect speed and accuracy of attention, memory, and thinking ability.
According to the research team, compared with sham PBM-T, those receiving active PBM-T showed significant improvement in motor function (finger tapping), working memory, delayed memory, and brain processing speed, the research team reports. No adverse effects were reported.
“This study complements our other recent studies, which showed improvement in memory performance with no obvious side effects,” said Dr. Chazot.
“While this is a pilot study and more research is needed, there are promising indications that therapy involving infrared light might also be beneficial for people living with dementia, and this is worth exploring,” Dr. Chazot added in a news release.
The PBM-T helmet was devised by first author Gordon Dougal, MBChB, of Maculume in the U.K., and a general practitioner based in Durham.
A recent study by Mr. Dougal, Dr. Chazot, and collaborators in the United States provides early evidence that PBM-T can improve memory in adults with dementia.
In that study, 39 patients received 6 minutes of PBM-T twice a day for 8 weeks, alongside a control group of 17 patients who received sham PBM-T.
After 8 weeks, there was about a 20% improvement in Mini-Mental State Exam (MMSE) scores in the active PBM-T group compared with roughly a 6% improvement in the control group, the researchers report in the journal Cureus.
More research needed
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said using light to stimulate the brain is “an emerging technology.”
“However, ,” Dr. Edelmayer told this news organization.
“That being said, we’re starting to see companies looking at similar, noninvasive methods of stimulating the brain. For example, brain stimulation devices have been applied to other neurodegenerative diseases like Parkinson’s to try to prevent degeneration of brain cells,” Dr. Edelmayer noted.
She said more research is needed to understand how photobiomodulation might be used as a therapy or prevention for cognitive decline and dementia.
“Specifically, we need to understand what parts of the brain need to be targeted and at what point(s) in the disease course this treatment would be most impactful. If proven to be effective, this could possibly be part of an approach that’s combined with other treatments, like drugs and lifestyle interventions,” said Dr. Edelmayer.
The Alzheimer’s Association is funding a number of projects looking at noninvasive treatments for Alzheimer’s disease, including two clinical trials looking at deep brain stimulation and photobiomodulation.
Maculume provided funding for the study. Mr. Dougal is a majority shareholder in the company, which manufactures the helmet device used in the study. Dr. Chazot, study co-authors, and Dr. Edelmayer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Near-infrared light delivered to the brain using a specially designed helmet appears to improve memory, motor function, and processing skills in cognitively healthy older adults, in new findings that suggest potential benefit in patients with dementia.
Studies in animals and people have shown “many positive effects” with near-infrared transcranial photobiomodulation therapy (PBM-T), study investigator Paul Chazot, PhD, department of biosciences, Durham University, United Kingdom, told this news organization.
For example, PBM-T has been shown to increase blood circulation (which keeps the brain well oxygenated), boost mitochondria function in neurons, protect neurons from oxidative stress, and help maintain neuronal connectivity, Dr. Chazot explained.
PBM-T has also been shown to reduce amyloid and phosphorylated tau load, pathological signs of Alzheimer’s disease.
“All these in combination improve memory performance and mobility,” Dr. Chazot said.
The study was published online October 18 in Photobiomodulation, Photomedicine and Laser Surgery.
Promising early data
In the study, 14 healthy adults, aged 45 to 70 years, received 6 minutes of transcranial PBM-T twice daily at a wavelength of 1,068 nanometers over 4 weeks. PBM-T was delivered via a helmet that comprised 14 air-cooled light emitting diode panel arrays. A control group of 13 adults used a sham PBM-T helmet.
Before and after active and sham treatment, all participants completed the automated neuropsychological assessment metrics (ANAM) – a computer-based tool designed to detect speed and accuracy of attention, memory, and thinking ability.
According to the research team, compared with sham PBM-T, those receiving active PBM-T showed significant improvement in motor function (finger tapping), working memory, delayed memory, and brain processing speed, the research team reports. No adverse effects were reported.
“This study complements our other recent studies, which showed improvement in memory performance with no obvious side effects,” said Dr. Chazot.
“While this is a pilot study and more research is needed, there are promising indications that therapy involving infrared light might also be beneficial for people living with dementia, and this is worth exploring,” Dr. Chazot added in a news release.
The PBM-T helmet was devised by first author Gordon Dougal, MBChB, of Maculume in the U.K., and a general practitioner based in Durham.
A recent study by Mr. Dougal, Dr. Chazot, and collaborators in the United States provides early evidence that PBM-T can improve memory in adults with dementia.
In that study, 39 patients received 6 minutes of PBM-T twice a day for 8 weeks, alongside a control group of 17 patients who received sham PBM-T.
After 8 weeks, there was about a 20% improvement in Mini-Mental State Exam (MMSE) scores in the active PBM-T group compared with roughly a 6% improvement in the control group, the researchers report in the journal Cureus.
More research needed
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said using light to stimulate the brain is “an emerging technology.”
“However, ,” Dr. Edelmayer told this news organization.
“That being said, we’re starting to see companies looking at similar, noninvasive methods of stimulating the brain. For example, brain stimulation devices have been applied to other neurodegenerative diseases like Parkinson’s to try to prevent degeneration of brain cells,” Dr. Edelmayer noted.
She said more research is needed to understand how photobiomodulation might be used as a therapy or prevention for cognitive decline and dementia.
“Specifically, we need to understand what parts of the brain need to be targeted and at what point(s) in the disease course this treatment would be most impactful. If proven to be effective, this could possibly be part of an approach that’s combined with other treatments, like drugs and lifestyle interventions,” said Dr. Edelmayer.
The Alzheimer’s Association is funding a number of projects looking at noninvasive treatments for Alzheimer’s disease, including two clinical trials looking at deep brain stimulation and photobiomodulation.
Maculume provided funding for the study. Mr. Dougal is a majority shareholder in the company, which manufactures the helmet device used in the study. Dr. Chazot, study co-authors, and Dr. Edelmayer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Molluscum Contagiosum Superimposed on Lymphangioma Circumscriptum
To the Editor:
Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system.1 It is postulated to arise from abnormal lymphatic cisterns, and it grows separately from the normal lymphatic system. These cisterns are connected to malformed dermal lymphatic channels, and the contraction of smooth muscles lining cisterns will cause dilatation of connected lymphatic channels in the papillary dermis due to back pressure,1,2 which causes a classic LC manifestation characterized by multiple translucent, sometimes red-brown, small vesicles grouped together. Lymphangioma circumscriptum can be difficult to differentiate from molluscum contagiosum (MC) due to the similar morphology.1 We present a notable case of MC superimposed on LC.
A 6-year-old girl presented with multiple grouped, clear, vesicular papules on the right buttock of 18 months’ duration. Some of the papules showed tiny whitish pearl-like particles on the top (Figure 1). Similar lesions were not present elsewhere on the body. She had no underlying disease and did not have a history of procedure, edema, or malformation of the lower extremities. Histopathology from one of the lesions showed dilated cystic lymphatic spaces in the papillary dermis lined with flattened endothelium and cup-shaped downward proliferation of the epidermis with presence of large intracytoplasmic inclusion bodies—features of both LC and MC (Figure 2). We waited 4 additional months for the MC lesions to self-resolve, but they persisted. The patient’s mother strongly requested for their removal, and the residual MC lesions were carefully removed by CO2 laser. To prevent unnecessary physical damage to underlying LC lesions and minimize scarring, we opted to use the CO2 laser and not simple curettage. She currently is under periodic observation with no signs of clinical recurrence of MC, but the LC lesions naturally persisted.
Due to its vesicular and sometimes warty appearance, LC can sometimes be hard to differentiate from MC. In one report, a vesicular plaquelike lesion on the trunk initially was misdiagnosed and treated as MC but was histologically confirmed as LC several years later.3 Our case demonstrates the coexistence of MC and LC. Although this phenomenon may be coincidental, we have not noticed any additional MC lesions on the body and MC only existed over the LC lesions, implying a possible pathophysiologic relationship. It is unlikely that MC might have preceded the development of LC. Although acquired LC exists, it has mostly been reported in the genital region of patients with conditions leading to lymphatic obstruction such as surgery, radiation therapy, malignancy, or serious infections.4 Because our patient developed lesions at an early age without any remarkable medical history, it is likely that she had congenital LC that was secondarily infected by the MC virus. Vesicular lesions in LC are known to rupture easily and may serve as a vulnerable entry site for pathogens. Subsequent secondary bacterial infections are common, with Staphylococcus aureus being the most prominent entity.1 However, secondary viral infection rarely is reported. It is possible that the abnormally dilated lymphatic channels of LC that lack communication with the normal lymphatic system have contributed to an LC site-specific vulnerability to MC virus. Further studies and subsequent reports are required to confirm this hypothesis.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111/j.1365-4632.2009.04226.x
- Fatima S, Uddin N, Idrees R, et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. J Coll Physicians Surg Pak. 2015;25:658-661. doi:09.2015/JCPSP.658661
- Patel GA, Siperstein RD, Ragi G, Schwartz RA. Zosteriform lymphangioma circumscriptum. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:179-182.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487. doi:10.1111/ijd.13264
To the Editor:
Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system.1 It is postulated to arise from abnormal lymphatic cisterns, and it grows separately from the normal lymphatic system. These cisterns are connected to malformed dermal lymphatic channels, and the contraction of smooth muscles lining cisterns will cause dilatation of connected lymphatic channels in the papillary dermis due to back pressure,1,2 which causes a classic LC manifestation characterized by multiple translucent, sometimes red-brown, small vesicles grouped together. Lymphangioma circumscriptum can be difficult to differentiate from molluscum contagiosum (MC) due to the similar morphology.1 We present a notable case of MC superimposed on LC.

A 6-year-old girl presented with multiple grouped, clear, vesicular papules on the right buttock of 18 months’ duration. Some of the papules showed tiny whitish pearl-like particles on the top (Figure 1). Similar lesions were not present elsewhere on the body. She had no underlying disease and did not have a history of procedure, edema, or malformation of the lower extremities. Histopathology from one of the lesions showed dilated cystic lymphatic spaces in the papillary dermis lined with flattened endothelium and cup-shaped downward proliferation of the epidermis with presence of large intracytoplasmic inclusion bodies—features of both LC and MC (Figure 2). We waited 4 additional months for the MC lesions to self-resolve, but they persisted. The patient’s mother strongly requested for their removal, and the residual MC lesions were carefully removed by CO2 laser. To prevent unnecessary physical damage to underlying LC lesions and minimize scarring, we opted to use the CO2 laser and not simple curettage. She currently is under periodic observation with no signs of clinical recurrence of MC, but the LC lesions naturally persisted.

Due to its vesicular and sometimes warty appearance, LC can sometimes be hard to differentiate from MC. In one report, a vesicular plaquelike lesion on the trunk initially was misdiagnosed and treated as MC but was histologically confirmed as LC several years later.3 Our case demonstrates the coexistence of MC and LC. Although this phenomenon may be coincidental, we have not noticed any additional MC lesions on the body and MC only existed over the LC lesions, implying a possible pathophysiologic relationship. It is unlikely that MC might have preceded the development of LC. Although acquired LC exists, it has mostly been reported in the genital region of patients with conditions leading to lymphatic obstruction such as surgery, radiation therapy, malignancy, or serious infections.4 Because our patient developed lesions at an early age without any remarkable medical history, it is likely that she had congenital LC that was secondarily infected by the MC virus. Vesicular lesions in LC are known to rupture easily and may serve as a vulnerable entry site for pathogens. Subsequent secondary bacterial infections are common, with Staphylococcus aureus being the most prominent entity.1 However, secondary viral infection rarely is reported. It is possible that the abnormally dilated lymphatic channels of LC that lack communication with the normal lymphatic system have contributed to an LC site-specific vulnerability to MC virus. Further studies and subsequent reports are required to confirm this hypothesis.
To the Editor:
Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system.1 It is postulated to arise from abnormal lymphatic cisterns, and it grows separately from the normal lymphatic system. These cisterns are connected to malformed dermal lymphatic channels, and the contraction of smooth muscles lining cisterns will cause dilatation of connected lymphatic channels in the papillary dermis due to back pressure,1,2 which causes a classic LC manifestation characterized by multiple translucent, sometimes red-brown, small vesicles grouped together. Lymphangioma circumscriptum can be difficult to differentiate from molluscum contagiosum (MC) due to the similar morphology.1 We present a notable case of MC superimposed on LC.

A 6-year-old girl presented with multiple grouped, clear, vesicular papules on the right buttock of 18 months’ duration. Some of the papules showed tiny whitish pearl-like particles on the top (Figure 1). Similar lesions were not present elsewhere on the body. She had no underlying disease and did not have a history of procedure, edema, or malformation of the lower extremities. Histopathology from one of the lesions showed dilated cystic lymphatic spaces in the papillary dermis lined with flattened endothelium and cup-shaped downward proliferation of the epidermis with presence of large intracytoplasmic inclusion bodies—features of both LC and MC (Figure 2). We waited 4 additional months for the MC lesions to self-resolve, but they persisted. The patient’s mother strongly requested for their removal, and the residual MC lesions were carefully removed by CO2 laser. To prevent unnecessary physical damage to underlying LC lesions and minimize scarring, we opted to use the CO2 laser and not simple curettage. She currently is under periodic observation with no signs of clinical recurrence of MC, but the LC lesions naturally persisted.

Due to its vesicular and sometimes warty appearance, LC can sometimes be hard to differentiate from MC. In one report, a vesicular plaquelike lesion on the trunk initially was misdiagnosed and treated as MC but was histologically confirmed as LC several years later.3 Our case demonstrates the coexistence of MC and LC. Although this phenomenon may be coincidental, we have not noticed any additional MC lesions on the body and MC only existed over the LC lesions, implying a possible pathophysiologic relationship. It is unlikely that MC might have preceded the development of LC. Although acquired LC exists, it has mostly been reported in the genital region of patients with conditions leading to lymphatic obstruction such as surgery, radiation therapy, malignancy, or serious infections.4 Because our patient developed lesions at an early age without any remarkable medical history, it is likely that she had congenital LC that was secondarily infected by the MC virus. Vesicular lesions in LC are known to rupture easily and may serve as a vulnerable entry site for pathogens. Subsequent secondary bacterial infections are common, with Staphylococcus aureus being the most prominent entity.1 However, secondary viral infection rarely is reported. It is possible that the abnormally dilated lymphatic channels of LC that lack communication with the normal lymphatic system have contributed to an LC site-specific vulnerability to MC virus. Further studies and subsequent reports are required to confirm this hypothesis.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111/j.1365-4632.2009.04226.x
- Fatima S, Uddin N, Idrees R, et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. J Coll Physicians Surg Pak. 2015;25:658-661. doi:09.2015/JCPSP.658661
- Patel GA, Siperstein RD, Ragi G, Schwartz RA. Zosteriform lymphangioma circumscriptum. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:179-182.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487. doi:10.1111/ijd.13264
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111/j.1365-4632.2009.04226.x
- Fatima S, Uddin N, Idrees R, et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. J Coll Physicians Surg Pak. 2015;25:658-661. doi:09.2015/JCPSP.658661
- Patel GA, Siperstein RD, Ragi G, Schwartz RA. Zosteriform lymphangioma circumscriptum. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:179-182.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487. doi:10.1111/ijd.13264
Practice Points
- Lymphangioma circumscriptum (LC) is a benign malformation of the lymphatic system that can be misdiagnosed as molluscum contagiosum (MC).
- Secondary infection of LC is common, with Staphylococcus aureus being the most common entity, but MC virus also can be secondarily infected.
Benefit of combined ascorbic acid, corticosteroids, and thiamine in septic shock remains unproven
Background: Sepsis is a common reason for hospitalization, and studies of the combination of ascorbic acid, corticosteroids, and thiamine have had conflicting results.
Study design: Double-blind randomized controlled trial.
Setting: 14 hospitals in the United States.
Synopsis: A total of 205 patients were randomly assigned to receive parenteral ascorbic acid, hydrocortisone, and thiamine every 6 hours for 4 days or placebo in matching volumes and time points. The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score between enrollment and 72 hours. There was no statistically significant difference in SOFA scores (adjusted mean difference, –0.8; 95% CI, –1.7 to 0.2; P = .12), kidney failure (adjusted risk difference, 0.03; 95% CI, –0.1 to 0.2; P = .58), or 30-day mortality (HR, 1.3; 95% CI 0.8-2.2; P = .26) between the two groups. Adverse effects included hyperglycemia, hypernatremia, and new hospital-acquired infection.
Bottom line: The combination of ascorbic acid, corticosteroids, and thiamine in patients with septic shock does not improve SOFA score.
Citation: Moskowitz A et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA. 2020;324(7):642-50.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Background: Sepsis is a common reason for hospitalization, and studies of the combination of ascorbic acid, corticosteroids, and thiamine have had conflicting results.
Study design: Double-blind randomized controlled trial.
Setting: 14 hospitals in the United States.
Synopsis: A total of 205 patients were randomly assigned to receive parenteral ascorbic acid, hydrocortisone, and thiamine every 6 hours for 4 days or placebo in matching volumes and time points. The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score between enrollment and 72 hours. There was no statistically significant difference in SOFA scores (adjusted mean difference, –0.8; 95% CI, –1.7 to 0.2; P = .12), kidney failure (adjusted risk difference, 0.03; 95% CI, –0.1 to 0.2; P = .58), or 30-day mortality (HR, 1.3; 95% CI 0.8-2.2; P = .26) between the two groups. Adverse effects included hyperglycemia, hypernatremia, and new hospital-acquired infection.
Bottom line: The combination of ascorbic acid, corticosteroids, and thiamine in patients with septic shock does not improve SOFA score.
Citation: Moskowitz A et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA. 2020;324(7):642-50.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Background: Sepsis is a common reason for hospitalization, and studies of the combination of ascorbic acid, corticosteroids, and thiamine have had conflicting results.
Study design: Double-blind randomized controlled trial.
Setting: 14 hospitals in the United States.
Synopsis: A total of 205 patients were randomly assigned to receive parenteral ascorbic acid, hydrocortisone, and thiamine every 6 hours for 4 days or placebo in matching volumes and time points. The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score between enrollment and 72 hours. There was no statistically significant difference in SOFA scores (adjusted mean difference, –0.8; 95% CI, –1.7 to 0.2; P = .12), kidney failure (adjusted risk difference, 0.03; 95% CI, –0.1 to 0.2; P = .58), or 30-day mortality (HR, 1.3; 95% CI 0.8-2.2; P = .26) between the two groups. Adverse effects included hyperglycemia, hypernatremia, and new hospital-acquired infection.
Bottom line: The combination of ascorbic acid, corticosteroids, and thiamine in patients with septic shock does not improve SOFA score.
Citation: Moskowitz A et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: The ACTS randomized clinical trial. JAMA. 2020;324(7):642-50.
Dr. Wallenhorst is a hospitalist and palliative medicine physician at the Lexington (Ky) VA Health Care System.
Enoxaparin-Induced Hemorrhagic Bullae at Sites of Trauma and Endothelial Pathology
To the Editor:
A 67-year-old man with diabetes mellitus was admitted to the hospital for exacerbation of congestive heart failure and atrial flutter with rapid ventricular response. He subsequently developed a non-ST segment elevation myocardial infarction and was started on subcutaneous enoxaparin 110 mg twice daily. On day 9 of hospitalization, small “blood blisters” on the legs were noted by the nurse, and dermatology was consulted.
Physical examination revealed tense hemorrhagic bullae with erythematous haloes scattered over the arms and legs and to a lesser extent on the trunk. The bullae were most concentrated at the surrounding subcutaneous injection sites of insulin and enoxaparin with secondary bruising (Figure 1). The lesions also were present on the legs, where pitting edema and capillaritis also were appreciated (Figure 2).
Laboratory workup for heparin-induced thrombocytopenia was negative. A diagnosis of enoxaparin-associated hemorrhagic bullae was made. Biopsy was recommended, but the patient declined based on anecdotal reports that the bullae typically self-resolve.
The enoxaparin was discontinued 7 days after the dermatology consultation, and the patient was transitioned to apixaban. A review of the medical record during the dermatology consultation revealed he had been on aspirin (81–385 mg/d) for 13 years prior to admission and had received prophylactic enoxaparin (40 mg/d) while hospitalized 2 and 7 years prior to the current episode of hemorrhagic bullae.
The patient declined outpatient dermatology follow-up; however, his cardiologist noted that the skin lesions had resolved at a 3-week postdischarge appointment. Approximately 5 months after discharge, the patient was re-treated by the cardiologist with enoxaparin 110 mg twice daily for 3 days to bridge to warfarin after he developed a deep vein thrombosis while taking apixaban. He did not develop hemorrhagic bullae upon retreatment with enoxaparin.
Heparin-induced hemorrhagic bullous dermatosis (HBD) has been associated with administration of both unfractionated and low-molecular-weight heparin.1 The condition typically develops 5 to 21 days after initiation of heparin as asymptomatic, purple-to-black bullae, sometimes with an erythematous halo.2,3 The arms and legs are the most common location, but the exact pathogenesis of the lesions remains unknown.3,4 Most cases resolve within weeks of discontinuing heparin, although some reports have suggested that discontinuation is unnecessary.3,4
Histopathologic analysis shows intraepidermal or subepidermal bullae with red blood cells and fibrin in the absence of vasculitis and intravascular thrombi.1,4 Immunofluorescence studies are negative.3 In a comprehensive review of HBD, the investigators hypothesized that the pathogenesis may be related to noninflammatory to pauci-inflammatory activation of basement membrane zone proteases or possibly epithelial or endothelial fragility in conjunction with trauma that causes disruption of the vascular endothelium (eg, subcutaneous injections, vasculitis).4
Our case is of particular interest because the bullae were strikingly limited to sites of subcutaneous injection and surrounding areas along with coexistent endothelial pathology on the lower legs (capillaritis and pitting edema). These clinical observations support trauma from the injections and altered endothelia as pathogenetic factors in HBD.
Of interest, our patient had 2 prior hospitalizations during which he received prophylactic enoxaparin and did not develop hemorrhagic bullae. Furthermore, repeat exposure to therapeutic dosing of enoxaparin with a shorter duration did not result in recurrence of HBD. This suggests that heparin dosing and duration of therapy also might be involved in the development of HBD.
Our hope is that future reports of HBD will address the presence or absence of coexistent cutaneous pathology, such as edema, stasis dermatitis, bruising, and capillaritis, along with heparin dosing, duration, and prior exposure to heparin treatment so that risk factors and pathogenesis can be further investigated. We also agree with Snow et al4 that HBD should be included as an outcome in future trials of heparin therapy.
- Komforti MK, Bressler ES, Selim MA, et al. A rare cutaneous manifestation of hemorrhagic bullae to low-molecular-weight heparin and fondaparinux: report of two cases: letter to the editor. J Cutan Pathol. 2017;44:104-106. doi:10.1111/cup.12821
- Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. JAMA Dermatol. 2013;149:871-872. doi:10.1001/jamadermatol.2013.3364a
- Gouveia AI, Lopes L, Soares-Almeida L, et al. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016;35:160-162. doi:10.3109/15569527.2015.1041033
- Snow SC, Pearson DR, Fathi R, et al. Heparin‐induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398. doi:10.1111/ced.13327
To the Editor:
A 67-year-old man with diabetes mellitus was admitted to the hospital for exacerbation of congestive heart failure and atrial flutter with rapid ventricular response. He subsequently developed a non-ST segment elevation myocardial infarction and was started on subcutaneous enoxaparin 110 mg twice daily. On day 9 of hospitalization, small “blood blisters” on the legs were noted by the nurse, and dermatology was consulted.
Physical examination revealed tense hemorrhagic bullae with erythematous haloes scattered over the arms and legs and to a lesser extent on the trunk. The bullae were most concentrated at the surrounding subcutaneous injection sites of insulin and enoxaparin with secondary bruising (Figure 1). The lesions also were present on the legs, where pitting edema and capillaritis also were appreciated (Figure 2).

Laboratory workup for heparin-induced thrombocytopenia was negative. A diagnosis of enoxaparin-associated hemorrhagic bullae was made. Biopsy was recommended, but the patient declined based on anecdotal reports that the bullae typically self-resolve.

The enoxaparin was discontinued 7 days after the dermatology consultation, and the patient was transitioned to apixaban. A review of the medical record during the dermatology consultation revealed he had been on aspirin (81–385 mg/d) for 13 years prior to admission and had received prophylactic enoxaparin (40 mg/d) while hospitalized 2 and 7 years prior to the current episode of hemorrhagic bullae.
The patient declined outpatient dermatology follow-up; however, his cardiologist noted that the skin lesions had resolved at a 3-week postdischarge appointment. Approximately 5 months after discharge, the patient was re-treated by the cardiologist with enoxaparin 110 mg twice daily for 3 days to bridge to warfarin after he developed a deep vein thrombosis while taking apixaban. He did not develop hemorrhagic bullae upon retreatment with enoxaparin.
Heparin-induced hemorrhagic bullous dermatosis (HBD) has been associated with administration of both unfractionated and low-molecular-weight heparin.1 The condition typically develops 5 to 21 days after initiation of heparin as asymptomatic, purple-to-black bullae, sometimes with an erythematous halo.2,3 The arms and legs are the most common location, but the exact pathogenesis of the lesions remains unknown.3,4 Most cases resolve within weeks of discontinuing heparin, although some reports have suggested that discontinuation is unnecessary.3,4
Histopathologic analysis shows intraepidermal or subepidermal bullae with red blood cells and fibrin in the absence of vasculitis and intravascular thrombi.1,4 Immunofluorescence studies are negative.3 In a comprehensive review of HBD, the investigators hypothesized that the pathogenesis may be related to noninflammatory to pauci-inflammatory activation of basement membrane zone proteases or possibly epithelial or endothelial fragility in conjunction with trauma that causes disruption of the vascular endothelium (eg, subcutaneous injections, vasculitis).4
Our case is of particular interest because the bullae were strikingly limited to sites of subcutaneous injection and surrounding areas along with coexistent endothelial pathology on the lower legs (capillaritis and pitting edema). These clinical observations support trauma from the injections and altered endothelia as pathogenetic factors in HBD.
Of interest, our patient had 2 prior hospitalizations during which he received prophylactic enoxaparin and did not develop hemorrhagic bullae. Furthermore, repeat exposure to therapeutic dosing of enoxaparin with a shorter duration did not result in recurrence of HBD. This suggests that heparin dosing and duration of therapy also might be involved in the development of HBD.
Our hope is that future reports of HBD will address the presence or absence of coexistent cutaneous pathology, such as edema, stasis dermatitis, bruising, and capillaritis, along with heparin dosing, duration, and prior exposure to heparin treatment so that risk factors and pathogenesis can be further investigated. We also agree with Snow et al4 that HBD should be included as an outcome in future trials of heparin therapy.
To the Editor:
A 67-year-old man with diabetes mellitus was admitted to the hospital for exacerbation of congestive heart failure and atrial flutter with rapid ventricular response. He subsequently developed a non-ST segment elevation myocardial infarction and was started on subcutaneous enoxaparin 110 mg twice daily. On day 9 of hospitalization, small “blood blisters” on the legs were noted by the nurse, and dermatology was consulted.
Physical examination revealed tense hemorrhagic bullae with erythematous haloes scattered over the arms and legs and to a lesser extent on the trunk. The bullae were most concentrated at the surrounding subcutaneous injection sites of insulin and enoxaparin with secondary bruising (Figure 1). The lesions also were present on the legs, where pitting edema and capillaritis also were appreciated (Figure 2).

Laboratory workup for heparin-induced thrombocytopenia was negative. A diagnosis of enoxaparin-associated hemorrhagic bullae was made. Biopsy was recommended, but the patient declined based on anecdotal reports that the bullae typically self-resolve.

The enoxaparin was discontinued 7 days after the dermatology consultation, and the patient was transitioned to apixaban. A review of the medical record during the dermatology consultation revealed he had been on aspirin (81–385 mg/d) for 13 years prior to admission and had received prophylactic enoxaparin (40 mg/d) while hospitalized 2 and 7 years prior to the current episode of hemorrhagic bullae.
The patient declined outpatient dermatology follow-up; however, his cardiologist noted that the skin lesions had resolved at a 3-week postdischarge appointment. Approximately 5 months after discharge, the patient was re-treated by the cardiologist with enoxaparin 110 mg twice daily for 3 days to bridge to warfarin after he developed a deep vein thrombosis while taking apixaban. He did not develop hemorrhagic bullae upon retreatment with enoxaparin.
Heparin-induced hemorrhagic bullous dermatosis (HBD) has been associated with administration of both unfractionated and low-molecular-weight heparin.1 The condition typically develops 5 to 21 days after initiation of heparin as asymptomatic, purple-to-black bullae, sometimes with an erythematous halo.2,3 The arms and legs are the most common location, but the exact pathogenesis of the lesions remains unknown.3,4 Most cases resolve within weeks of discontinuing heparin, although some reports have suggested that discontinuation is unnecessary.3,4
Histopathologic analysis shows intraepidermal or subepidermal bullae with red blood cells and fibrin in the absence of vasculitis and intravascular thrombi.1,4 Immunofluorescence studies are negative.3 In a comprehensive review of HBD, the investigators hypothesized that the pathogenesis may be related to noninflammatory to pauci-inflammatory activation of basement membrane zone proteases or possibly epithelial or endothelial fragility in conjunction with trauma that causes disruption of the vascular endothelium (eg, subcutaneous injections, vasculitis).4
Our case is of particular interest because the bullae were strikingly limited to sites of subcutaneous injection and surrounding areas along with coexistent endothelial pathology on the lower legs (capillaritis and pitting edema). These clinical observations support trauma from the injections and altered endothelia as pathogenetic factors in HBD.
Of interest, our patient had 2 prior hospitalizations during which he received prophylactic enoxaparin and did not develop hemorrhagic bullae. Furthermore, repeat exposure to therapeutic dosing of enoxaparin with a shorter duration did not result in recurrence of HBD. This suggests that heparin dosing and duration of therapy also might be involved in the development of HBD.
Our hope is that future reports of HBD will address the presence or absence of coexistent cutaneous pathology, such as edema, stasis dermatitis, bruising, and capillaritis, along with heparin dosing, duration, and prior exposure to heparin treatment so that risk factors and pathogenesis can be further investigated. We also agree with Snow et al4 that HBD should be included as an outcome in future trials of heparin therapy.
- Komforti MK, Bressler ES, Selim MA, et al. A rare cutaneous manifestation of hemorrhagic bullae to low-molecular-weight heparin and fondaparinux: report of two cases: letter to the editor. J Cutan Pathol. 2017;44:104-106. doi:10.1111/cup.12821
- Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. JAMA Dermatol. 2013;149:871-872. doi:10.1001/jamadermatol.2013.3364a
- Gouveia AI, Lopes L, Soares-Almeida L, et al. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016;35:160-162. doi:10.3109/15569527.2015.1041033
- Snow SC, Pearson DR, Fathi R, et al. Heparin‐induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398. doi:10.1111/ced.13327
- Komforti MK, Bressler ES, Selim MA, et al. A rare cutaneous manifestation of hemorrhagic bullae to low-molecular-weight heparin and fondaparinux: report of two cases: letter to the editor. J Cutan Pathol. 2017;44:104-106. doi:10.1111/cup.12821
- Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. JAMA Dermatol. 2013;149:871-872. doi:10.1001/jamadermatol.2013.3364a
- Gouveia AI, Lopes L, Soares-Almeida L, et al. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016;35:160-162. doi:10.3109/15569527.2015.1041033
- Snow SC, Pearson DR, Fathi R, et al. Heparin‐induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398. doi:10.1111/ced.13327
Granulomatous Facial Dermatoses
Cutaneous granulomatous diseases encompass many entities that are skin-limited or systemic. The prototypical cutaneous granuloma is a painless, rounded, well-defined, red-pink or flesh-colored papule1 and is smooth, owing to minimal epidermal involvement. Examples of conditions that present with such lesions include granulomatous periorificial dermatitis (GPD), granulomatous rosacea (GR), lupus miliaris disseminatus faciei (LMDF), and papular sarcoidosis. These entities commonly are seen on the face and can be a source of distress to patients when they are extensive. Several reports have raised the possibility that these conditions lie on a spectrum.2-4 We present 2 cases of patients with facial papular granulomas, discuss potential causes of the lesions, review historical aspects from the literature, and highlight the challenges that these lesions can pose to the clinician.
Case Reports
Patient 1—A 10-year-old Ethiopian girl with a history of atopic dermatitis presented with a facial rash of 4 months’ duration. Her pediatrician initially treated the rash as pityriasis alba and prescribed hydrocortisone cream. Two months into treatment, the patient developed an otherwise asymptomatic, unilateral, papular dermatosis on the right cheek. She subsequently was switched to treatment with benzoyl peroxide and topical clindamycin, which she had been using for 2 months with no improvement at the time of the current presentation. The lesions then spread bilaterally and periorally.
At the current presentation, physical examination demonstrated fine, diffuse, follicular-based, flesh-colored papules over both cheeks, the right side of the nose, and the perioral region (Figure 1). A biopsy of a papular lesion from the right cheek revealed well-formed, noncaseating granulomas in the superficial and mid dermis with an associated lymphocytic infiltrate (Figure 2). No organisms were identified on acid-fast, Fite, or periodic acid–Schiff staining. A tuberculin skin test was negative. A chest radiograph showed small calcified hilar lymph nodes bilaterally. Pulmonary function tests were unremarkable. Calcium and angiotensin-converting enzyme levels were normal.
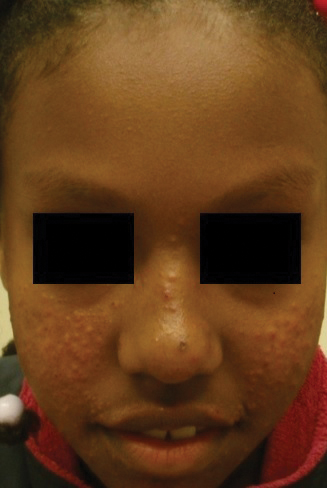
The patient denied any fever, chills, hemoptysis, cough, dyspnea, lymphadenopathy, scleral or conjunctival pain or erythema, visual disturbances, or arthralgias. Hydroxychloroquine 200 mg twice daily was started with minimal improvement after 5 months. Methotrexate 20 mg once weekly was then added. Topical fluocinonide 0.05% also was started at this time, as the patient had required several prednisone tapers over the past 3 months for symptomatic relief. The lesions improved minimally after 5 more months of treatment, at which time she had developed inflammatory papules, pustules, and open comedones in the same areas as well as the glabella.
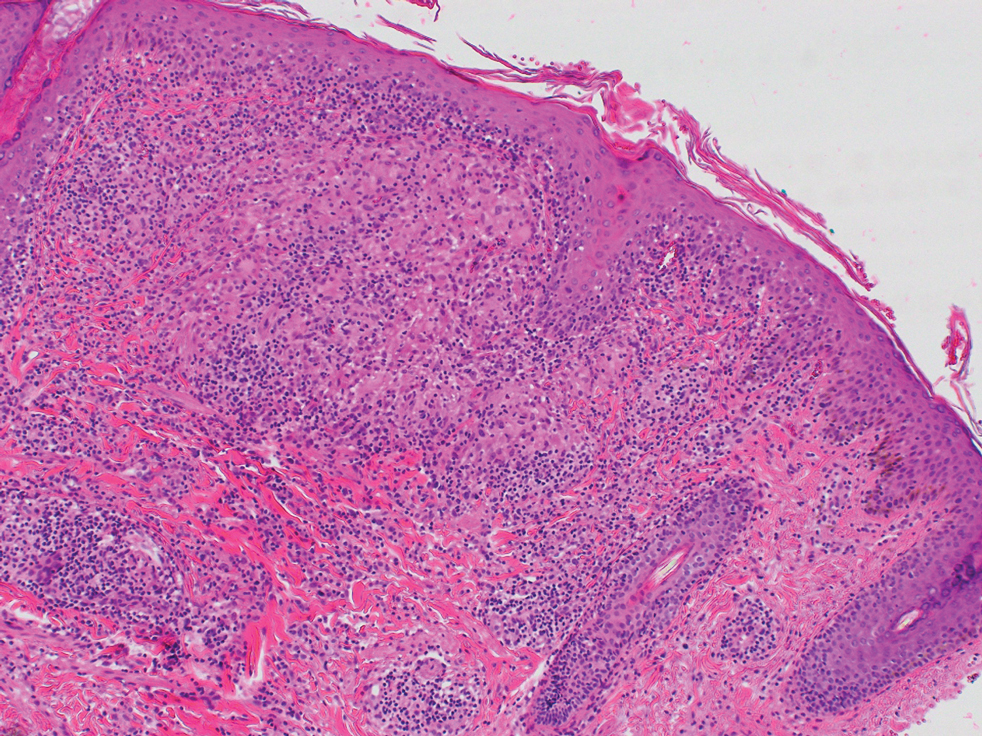
Repeat biopsy of a papular lesion demonstrated noncaseating granulomas and an associated chronic lymphocytic infiltrate in a follicular and perifollicular distribution (Figure 3). Biopsy of a pustule demonstrated acute Demodex folliculitis. Fluocinonide was stopped, and anti-mite therapy with ivermectin, permethrin cream 5%, and selenium sulfide lotion 2.5% was started, with good response from the pustular lesions.
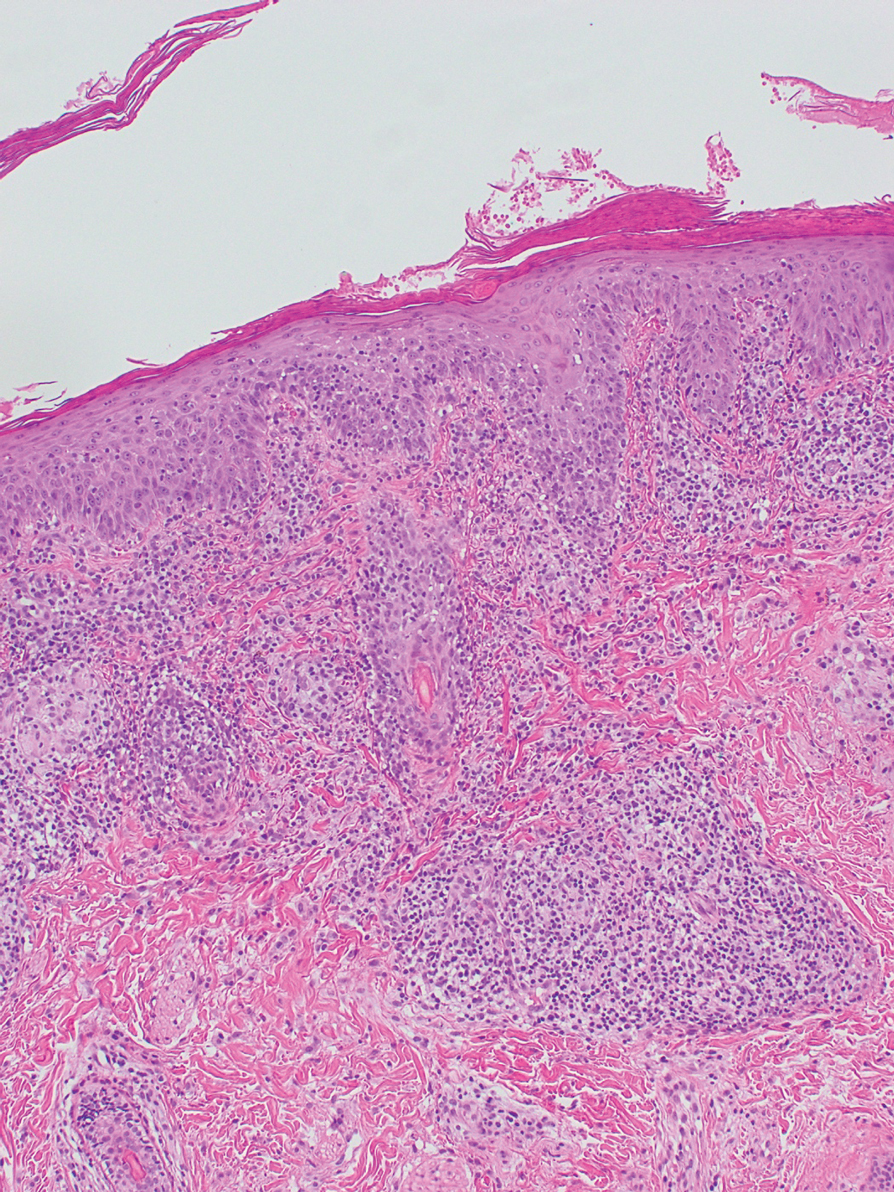
The patient continued taking methotrexate 20 mg once weekly during this time, with improvement in the papular lesions. She discontinued methotrexate after 12 months with complete resolution. At follow-up 12 months after stopping the methotrexate (roughly 2 years after initial presentation), she showed sustained resolution, with small pitted scars on both cheeks and the nasal tip.
Patient 2—A 33-year-old Ethiopian woman presented with a facial rash of 15 years’ duration. The lesions had been accumulating slowly and were asymptomatic. Physical examination revealed multiple follicular-based, flesh-colored, and erythematous papules on the cheeks, chin, perioral area, and forehead (Figure 4). There were no pustules or telangiectasias. Treatment with tretinoin cream 0.05% for 6 months offered minimal relief.
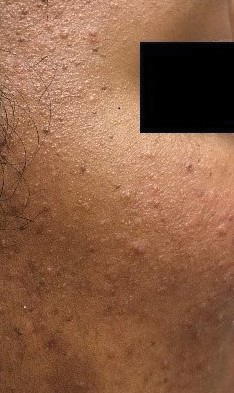
Biopsy of a papule from the left mandible showed superficial vascular telangiectasias, noncaseating granulomas comprising epithelioid histiocytes and lymphocytes in the superficial dermis, and a perifollicular lymphocytic infiltrate (Figure 5). No organisms were identified on Fite or Gomori methenamine silver staining.
Comment
The first step in differentiating cutaneous granulomatous lesions should be to distinguish infectious from noninfectious causes.1 Noninfectious cutaneous granulomas can appear nearly anywhere; however, certain processes have a predilection for the face, including GPD, GR, LMDF, and papular sarcoidosis.5-7 These conditions generally present with papular granulomas with features as described above.
Granulomatous Periorificial Dermatitis—In 1970, Gianotti and colleagues8 briefly described the first possible cases of GPD in 5 children. The eruption comprised numerous yellow, dome-shaped papules in a mostly perioral distribution. Tuberculin and the Kveim tests were nonreactive; histopathology was described as sarcoid-type and not necessarily follicular or perifollicular.8 In 1974, Marten et al9 described 22 Afro-Caribbean children with flesh-colored, papular eruptions on the face that did not show histologic granulomatous changes but were morphologically similar to the reports by Gianotti et al.8 By 1989, Frieden and colleagues10 described this facial eruption as “granulomatous perioral dermatitis in children”. Additionally, the investigators observed granulomatous infiltrates in a perifollicular distribution and suggested follicular disruption as a possible cause. It was clear from the case discussions that these eruptions were not uncommonly diagnosed as papular sarcoidosis.10 The following year, Williams et al11 reported 5 cases of similar papular eruptions in 5 Afro-Caribbean children, coining the term facial Afro-Caribbean eruption.11 Knautz and Lesher12 referred to this entity as “childhood GPD” in 1996 to avoid limiting the diagnosis to Afro-Caribbean patients and to a perioral distribution; this is the most popular current terminology.12 Since then, reports of extrafacial involvement and disease in adults have been published.13,14
Granulomatous periorificial dermatitis often is seen in the perinasal, periocular, and perioral regions of the face.2 It is associated with topical steroid exposure.5 Histologically, noncaseating granulomas around the upper half of undisrupted hair follicles with a lymphocytic infiltrate are typical.13 Treatment should begin with cessation of any topical steroids; first-line agents are oral tetracycline or macrolide antibiotics.5 These agents can be used alone or in combination with topical erythromycin, metronidazole, or sulfur-based lotions.13 Rarely, GPD presents extrafacially.13 Even so, it usually resolves within 2 weeks to 6 months, especially with therapy; scarring is unusual.5,13,15
Granulomatous Rosacea—A report in the early 20th century described patients with tuberculoid granulomas resembling papular rosacea; the initial belief was that this finding represented a rosacealike tuberculid eruption.5 However, this belief was questioned by Snapp,16 among others, who demonstrated near universal lack of reactivity to tuberculin among 20 of these patients in 1949; more recent evidence has substantiated these findings.17 Still, Snapp16 postulated that these rosacealike granulomatous lesions were distinct from classic rosacea because they lacked vascular symptoms and pustules and were recalcitrant to rosacea treatment modalities.
In 1970, Mullanax and colleagues18 introduced the term granulomatous rosacea, reiterating that this entity was not tuberculous. They documented papulopustular lesions as well as telangiectasias, raising the possibility that GR does overlap with acne rosacea. More recent studies have established the current theory that GR is a histologic variant of acne rosacea because, in addition to typical granulomatous papules, its microscopic features can be seen across subtypes of acne rosacea.19,20
Various causes have been proposed for GR. Demodex mites have been reported in association with GR for nearly 30 years.19,20 In the past 10 years, molecular studies have started to define the role of metalloproteinases, UV radiation, and cutaneous peptides in the pathogenesis of acne rosacea and GR.21,22
Granulomatous rosacea typically is seen in middle-aged women.20,23 Hallmarks of rosacea, such as facial erythema, flushing, telangiectasias, pustules, and rhinophyma, are not always present in GR.5,20,23 Lesions usually are distributed around the central face, although extension to the cheeks, total facial involvement, and extrafacial lesions are possible.5,20 Histologically, perifollicular and follicular-based noncaseating granulomas with dilatation of the dermal papillary vasculature are seen.17,23 As a whole, rosacea is comparatively uncommon in dark-skinned patients; when it does occur, GR is a frequent presentation.24
First-line treatment for GR is tetracycline antibiotics.5 Unresponsive cases have been treated—largely anecdotally—with topical modalities (eg, metronidazole, steroids, immunomodulators), systemic agents (eg, dapsone, erythromycin, isotretinoin), and other therapies.5 Granulomatous rosacea tends to have a chronic course.5,23
Lupus Miliaris Disseminatus Faciei—Classic LMDF demonstrates caseating perifollicular granulomas histologically.6,17,25 Lesions tend to appear on the central face, particularly the eyelids, and can be seen extrafacially.3,6,25,26 Although LMDF originally was categorized as a tuberculid eruption, this no longer is thought to be the case.27 It is now regarded by some as a variant of GR25; however, LMDF responds poorly to tetracyclines, is more common in males, and lacks rosacealike vascular abnormalities, leading some to question this association.3,6,17 In the past 20 years, some have proposed renaming LMDF to better reflect its clinical course and to consider it independent of tuberculosis and GR.28 It usually resolves spontaneously after 1 to 3 years, leaving pitted scars.3,6
Papular Sarcoidosis—The first potential documented case of sarcoidosis was by Hutchinson29 in 1869 in a patient seen in London. The author labeled purple plaques on the index patient’s legs and hands as “livid papillary psoriasis.” In 1889, Besnier30 described a patient with violaceous swellings on the nose, ears, and fingers, which he called “lupus pernio”; his contemporary, Tenneson,31 published a case of lupus pernio and described its histologic profile as comprising epithelioid cells and giant cells. It was not until 1899 that the term sarkoid was used to describe these cutaneous lesions by Boeck,32 who thought they were reminiscent of sarcoma. In 1915, Kuznitsky and Bittorf33 described a patient with cutaneous lesions histologically consistent with Boeck’s sarkoid but additionally with hilar lymphadenopathy and pulmonary infiltrates. Around 1916 or 1917, Schaumann34 described patients with cutaneous lesions and additionally with involvement of pulmonary, osseous, hepatosplenic, and tonsillar tissue. These reports are among the first to recognize the multisystemic nature of sarcoidosis. The first possible case of childhood sarcoidosis might have been reported by Osler35 in the United States in 1898.
In the past century or so, an ongoing effort by researchers has focused on identifying etiologic triggers for sarcoidosis. Microbial agents have been considered in this role, with Mycobacterium and Propionibacterium organisms the most intensively studied; the possibility that foreign material contributes to the formation of granulomas also has been raised.36 Current models of the pathogenesis of sarcoidosis involve an interplay between the immune system in genetically predisposed patients and an infection that leads to a hyperimmune type 1 T–helper cell response that clears the infection but not antigens generated by the microbes and the acute host response, including proteins such as serum amyloid A and vimentin.36,37 These antigens aggregate and serve as a nidus for granuloma formation and maintenance long after infection has resolved.
Cutaneous lesions of sarcoidosis include macules, papules, plaques, and lupus pernio, as well as lesions arising within scars or tattoos, with many less common presentations.7,38 Papular sarcoidosis is common on the face but also can involve the extremities.4,7 Strictly, at least 2 organ systems must be involved to diagnose sarcoidosis, but this is debatable.4,7 Among 41 patients with cutaneous sarcoidosis, 24 (58.5%) had systemic disease; cutaneous lesions were the presenting sign in 87.5% (21/24) of patients.38 Histologic analysis, regardless of the lesion, usually shows noncaseating so-called “naked” granulomas, which have minimal lymphocytic infiltrate associated with the epithelioid histiocytes.38,39 Perifollicular granulomas are possible but unusual.40
Treatment depends on the extent of cutaneous and systemic involvement. Pharmacotherapeutic modalities include topical steroids, immunomodulators, and retinoids; systemic immunomodulators and immunosuppressants; and biologic agents.7 Isolated cutaneous sarcoidosis, particularly the papular variant, usually is associated with acute disease lasting less than 2 years, with resolution of skin lesions.7,38 That said, a recent report suggested that cutaneous sarcoidosis can progress to multisystemic disease as long as 7 years after the initial diagnosis.41
Clinical and Histologic Overlap—Despite this categorization of noninfectious facial granulomatous conditions, each has some clinical and histologic overlap with the others, which must be considered when encountering a granulomatous facial dermatosis. Both GPD and GR tend to present with lesions near the eyes, mouth, and nose, although GR can extend to lateral aspects of the face, below the mandible, and the forehead and has different demographic features.15,20,23 Granulomas in both GPD and GR generally are noncaseating and form in a follicular or perifollicular distribution within the dermis.2,15,23 Lupus miliaris disseminatus faciei and GR share a similar facial distribution in some cases.17,20 Even papular cutaneous sarcoidosis has masqueraded as GR clinically and histologically.4
Diagnostic and Treatment Difficulty—Our cases illustrate the range of difficulty in evaluating and managing patients with facial papular granulomas. On one hand, our adult patient’s clinical and histologic findings were highly consistent with GR; on the other hand, our younger patient had clinicopathologic features of both sarcoidosis and GPD at varying times. Both conditions are more common in dark-skinned patients.11,42
Juvenile sarcoidosis is comparatively rare, with a reported annual incidence of 0.22 to 0.27 for every 100,000 children younger than 15 years; however, juvenile sarcoidosis commonly presents around 8 to 15 years of age.43
It is unusual for sarcoid granulomas to be isolated to the skin, much less to the face.4,7,43,44 Patient 1 initially presented in this manner and lacked convincing laboratory or radiographic evidence of systemic sarcoidosis. Bilateral hilar calcifications in sarcoidosis are more typical among adults after 5 to 20 years; there were no signs or symptoms of active infection that could account for the pulmonary and cutaneous lesions.45
The presence of perifollicular granulomas with associated lymphocytic infiltrates on repeat biopsy, coupled with the use of topical steroids, made it difficult to rule out a contribution by GPD to her clinical course. That her lesions resolved with pitted scarring while she was taking methotrexate and after topical steroids had been stopped could be the result of successful management or spontaneous resolution of her dermatosis; both papular sarcoidosis and GPD tend to have a self-limited course.7,13
Conclusion
We present 2 cases of papular facial granulomas in patients with similar skin types who had different clinical courses. Evaluation of such lesions remains challenging given the similarity between specific entities that present in this manner. Certainly, it is reasonable to consider a spectrum upon which all of these conditions fall, in light of the findings of these cases and those reported previously.
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146. doi:10.1007/s12016-017-8666-8
- Lucas CR, Korman NJ, Gilliam AC. Granulomatous periorificial dermatitis: a variant of granulomatous rosacea in children? J Cutan Med Surg. 2009;13:115-118. doi:10.2310/7750.2008.07088
- van de Scheur MR, van der Waal RIF, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120-123. doi:10.1159/000068457
- Simonart T, Lowy M, Rasquin F, et al. Overlap of sarcoidosis and rosacea. Dermatology. 1997;194:416-418. doi:10.1159/000246165
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management. Dermatol Clin. 2015;33:447-455. doi:10.1016/j.det.2015.03.009
- Michaels JD, Cook-Norris RH, Lehman JS, et al. Adult with papular eruption of the central aspect of the face. J Am Acad Dermatol. 2014;71:410-412. doi:10.1016/j.jaad.2012.06.039
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;38:685-702. doi:10.1016/j.ccm.2015.08.010
- Gianotti F, Ermacora E, Benelli MG, et al. Particulière dermatite peri-orale infantile. observations sur 5 cas. Bull Soc Fr Dermatol Syphiligr. 1970;77:341.
- Marten RH, Presbury DG, Adamson JE, et al. An unusual papular and acneiform facial eruption in the negro child. Br J Dermatol. 1974;91:435-438. doi:10.1111/j.1365-2133.1974.tb13083.x
- Frieden IJ, Prose NS, Fletcher V, et al. Granulomatous perioral dermatitis in children. Arch Dermatol. 1989;125:369-373.
- Williams HC, Ashworth J, Pembroke AC, et al. FACE—facial Afro-Caribbean childhood eruption. Clin Exp Dermatol. 1990;15:163-166. doi:10.1111/j.1365-2230.1990.tb02063.x
- Knautz MA, Lesher JL Jr. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13:131-134. doi:10.1111/j.1525-1470.1996.tb01419.x
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358. doi:10.1001/archderm.138.10.1354
- Vincenzi C, Parente G, Tosti A. Perioral granulomatous dermatitis: two cases treated with clarithromycin. J Dermatol Treat. 2000;11:57-61.
- Kim YJ, Shin JW, Lee JS, et al. Childhood granulomatous periorificial dermatitis. Ann Dermatol. 2011;23:386-388. doi:10.5021/ad.2011.23.3.386
- Snapp RH. Lewandowsky’s rosacea-like eruption; a clinical study. J Invest Dermatol. 1949;13:175-190. doi:10.1038/jid.1949.86
- Chougule A, Chatterjee D, Sethi S, et al. Granulomatous rosacea versus lupus miliaris disseminatus faciei—2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819-823. doi:10.1097/DAD.0000000000001243
- Mullanax MG, Kierland RR. Granulomatous rosacea. Arch Dermatol. 1970;101:206-211.
- Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6-9. doi:10.1097/DAD.0b013e31815bc191
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25:1038-1043. doi:10.1016/0190-9622(91)70304-k
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435-1442. doi:10.1038/jid.2012.14
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544-548. doi:10.1111/j.1468-3083.2010.03825.x
- Khokhar O, Khachemoune A. A case of granulomatous rosacea: sorting granulomatous rosacea from other granulomatous diseases that affect the face. Dermatol Online J. 2004;10:6.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73. doi:10.1016/s0190-9622(87)70173-x
- Adams AK, Davis JL, Davis MDP, et al. What is your diagnosis? granulomatous rosacea (lupus miliaris disseminatus faciei, acne agminata). Cutis. 2008;82:103-112.
- Shitara A. Lupus miliaris disseminatus faciei. Int J Dermatol. 1984;23:542-544. doi:10.1111/j.1365-4362.1984.tb04206.x
- Hodak E, Trattner A, Feuerman H, et al. Lupus miliaris disseminatus faciei—the DNA of Mycobacterium tuberculosis is not detectable in active lesions by polymerase chain reaction. Br J Dermatol. 1997;137:614-619. doi: 10.1111/j.1365-2133.1997.tb03797.x
- Skowron F, Causeret AS, Pabion C, et al. F.I.GU.R.E.: facial idiopathic granulomas with regressive evolution. Dermatology. 2000;201:287-289. doi:10.1159/000051539
- Hutchinson J. Case of livid papillary psoriasis. In: London J, Churchill A, eds. Illustrations of Clinical Surgery. J&A Churchill; 1877:42-43.
- Besnier E. Lupus pernio of the face [in French]. Ann Dermatol Syphiligr (Paris). 1889;10:33-36.
- Tenneson H. Lupus pernio. Ann Dermatol Syphiligr (Paris). 1889;10:333-336.
- Boeck C. Multiple benign sarkoid of the skin [in Norwegian]. Norsk Mag Laegevidensk. 1899;14:1321-1334.
- Kuznitsky E, Bittorf A. Sarkoid mit beteiligung innerer organe. Münch Med Wochenschr. 1915;62:1349-1353.
- Schaumann J. Etude sur le lupus pernio et ses rapports avec les sarcoides et la tuberculose. Ann Dermatol Syphiligr. 1916-1917;6:357-373.
- Osler W. On chronic symmetrical enlargement of the salivary and lacrimal glands. Am J Med Sci. 1898;115:27-30.
- Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. doi:10.1007/s12016-015-8481-z
- Eberhardt C, Thillai M, Parker R, et al. Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One. 2017;12:e0170285. doi:10.1371/journal.pone.0170285
- Esteves TC, Aparicio G, Ferrer B, et al. Prognostic value of skin lesions in sarcoidosis: clinical and histopathological clues. Eur J Dermatol. 2015;25:556-562. doi:10.1684/ejd.2015.2666
- Cardoso JC, Cravo M, Reis JP, et al. Cutaneous sarcoidosis: a histopathological study. J Eur Acad Dermatol Venereol. 2009;23:678-682. doi:10.1111/j.1468-3083.2009.03153.x
- Mangas C, Fernández-Figueras M-T, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772-777. doi:10.1111/j.1600-0560.2006.00563.x
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, et al. The natural history of cutaneous sarcoidosis. clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178-184. doi: 10.1111/ijd.14218
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16. doi:10.1186/1546-0096-6-16
- Milman N, Hoffmann AL, Byg KE. Sarcoidosis in children. epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998;87:871-878. doi:10.1080/08035259875001366244. A, H, Yapıcı I. Isolated cutaneous sarcoidosis. Arch Bronconeumol. 2016;52:220.
- Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. 1970;46:530-536. doi:10.1136/pgmj.46.538.530
Cutaneous granulomatous diseases encompass many entities that are skin-limited or systemic. The prototypical cutaneous granuloma is a painless, rounded, well-defined, red-pink or flesh-colored papule1 and is smooth, owing to minimal epidermal involvement. Examples of conditions that present with such lesions include granulomatous periorificial dermatitis (GPD), granulomatous rosacea (GR), lupus miliaris disseminatus faciei (LMDF), and papular sarcoidosis. These entities commonly are seen on the face and can be a source of distress to patients when they are extensive. Several reports have raised the possibility that these conditions lie on a spectrum.2-4 We present 2 cases of patients with facial papular granulomas, discuss potential causes of the lesions, review historical aspects from the literature, and highlight the challenges that these lesions can pose to the clinician.
Case Reports
Patient 1—A 10-year-old Ethiopian girl with a history of atopic dermatitis presented with a facial rash of 4 months’ duration. Her pediatrician initially treated the rash as pityriasis alba and prescribed hydrocortisone cream. Two months into treatment, the patient developed an otherwise asymptomatic, unilateral, papular dermatosis on the right cheek. She subsequently was switched to treatment with benzoyl peroxide and topical clindamycin, which she had been using for 2 months with no improvement at the time of the current presentation. The lesions then spread bilaterally and periorally.
At the current presentation, physical examination demonstrated fine, diffuse, follicular-based, flesh-colored papules over both cheeks, the right side of the nose, and the perioral region (Figure 1). A biopsy of a papular lesion from the right cheek revealed well-formed, noncaseating granulomas in the superficial and mid dermis with an associated lymphocytic infiltrate (Figure 2). No organisms were identified on acid-fast, Fite, or periodic acid–Schiff staining. A tuberculin skin test was negative. A chest radiograph showed small calcified hilar lymph nodes bilaterally. Pulmonary function tests were unremarkable. Calcium and angiotensin-converting enzyme levels were normal.

The patient denied any fever, chills, hemoptysis, cough, dyspnea, lymphadenopathy, scleral or conjunctival pain or erythema, visual disturbances, or arthralgias. Hydroxychloroquine 200 mg twice daily was started with minimal improvement after 5 months. Methotrexate 20 mg once weekly was then added. Topical fluocinonide 0.05% also was started at this time, as the patient had required several prednisone tapers over the past 3 months for symptomatic relief. The lesions improved minimally after 5 more months of treatment, at which time she had developed inflammatory papules, pustules, and open comedones in the same areas as well as the glabella.

Repeat biopsy of a papular lesion demonstrated noncaseating granulomas and an associated chronic lymphocytic infiltrate in a follicular and perifollicular distribution (Figure 3). Biopsy of a pustule demonstrated acute Demodex folliculitis. Fluocinonide was stopped, and anti-mite therapy with ivermectin, permethrin cream 5%, and selenium sulfide lotion 2.5% was started, with good response from the pustular lesions.

The patient continued taking methotrexate 20 mg once weekly during this time, with improvement in the papular lesions. She discontinued methotrexate after 12 months with complete resolution. At follow-up 12 months after stopping the methotrexate (roughly 2 years after initial presentation), she showed sustained resolution, with small pitted scars on both cheeks and the nasal tip.
Patient 2—A 33-year-old Ethiopian woman presented with a facial rash of 15 years’ duration. The lesions had been accumulating slowly and were asymptomatic. Physical examination revealed multiple follicular-based, flesh-colored, and erythematous papules on the cheeks, chin, perioral area, and forehead (Figure 4). There were no pustules or telangiectasias. Treatment with tretinoin cream 0.05% for 6 months offered minimal relief.

Biopsy of a papule from the left mandible showed superficial vascular telangiectasias, noncaseating granulomas comprising epithelioid histiocytes and lymphocytes in the superficial dermis, and a perifollicular lymphocytic infiltrate (Figure 5). No organisms were identified on Fite or Gomori methenamine silver staining.

Comment
The first step in differentiating cutaneous granulomatous lesions should be to distinguish infectious from noninfectious causes.1 Noninfectious cutaneous granulomas can appear nearly anywhere; however, certain processes have a predilection for the face, including GPD, GR, LMDF, and papular sarcoidosis.5-7 These conditions generally present with papular granulomas with features as described above.
Granulomatous Periorificial Dermatitis—In 1970, Gianotti and colleagues8 briefly described the first possible cases of GPD in 5 children. The eruption comprised numerous yellow, dome-shaped papules in a mostly perioral distribution. Tuberculin and the Kveim tests were nonreactive; histopathology was described as sarcoid-type and not necessarily follicular or perifollicular.8 In 1974, Marten et al9 described 22 Afro-Caribbean children with flesh-colored, papular eruptions on the face that did not show histologic granulomatous changes but were morphologically similar to the reports by Gianotti et al.8 By 1989, Frieden and colleagues10 described this facial eruption as “granulomatous perioral dermatitis in children”. Additionally, the investigators observed granulomatous infiltrates in a perifollicular distribution and suggested follicular disruption as a possible cause. It was clear from the case discussions that these eruptions were not uncommonly diagnosed as papular sarcoidosis.10 The following year, Williams et al11 reported 5 cases of similar papular eruptions in 5 Afro-Caribbean children, coining the term facial Afro-Caribbean eruption.11 Knautz and Lesher12 referred to this entity as “childhood GPD” in 1996 to avoid limiting the diagnosis to Afro-Caribbean patients and to a perioral distribution; this is the most popular current terminology.12 Since then, reports of extrafacial involvement and disease in adults have been published.13,14
Granulomatous periorificial dermatitis often is seen in the perinasal, periocular, and perioral regions of the face.2 It is associated with topical steroid exposure.5 Histologically, noncaseating granulomas around the upper half of undisrupted hair follicles with a lymphocytic infiltrate are typical.13 Treatment should begin with cessation of any topical steroids; first-line agents are oral tetracycline or macrolide antibiotics.5 These agents can be used alone or in combination with topical erythromycin, metronidazole, or sulfur-based lotions.13 Rarely, GPD presents extrafacially.13 Even so, it usually resolves within 2 weeks to 6 months, especially with therapy; scarring is unusual.5,13,15
Granulomatous Rosacea—A report in the early 20th century described patients with tuberculoid granulomas resembling papular rosacea; the initial belief was that this finding represented a rosacealike tuberculid eruption.5 However, this belief was questioned by Snapp,16 among others, who demonstrated near universal lack of reactivity to tuberculin among 20 of these patients in 1949; more recent evidence has substantiated these findings.17 Still, Snapp16 postulated that these rosacealike granulomatous lesions were distinct from classic rosacea because they lacked vascular symptoms and pustules and were recalcitrant to rosacea treatment modalities.
In 1970, Mullanax and colleagues18 introduced the term granulomatous rosacea, reiterating that this entity was not tuberculous. They documented papulopustular lesions as well as telangiectasias, raising the possibility that GR does overlap with acne rosacea. More recent studies have established the current theory that GR is a histologic variant of acne rosacea because, in addition to typical granulomatous papules, its microscopic features can be seen across subtypes of acne rosacea.19,20
Various causes have been proposed for GR. Demodex mites have been reported in association with GR for nearly 30 years.19,20 In the past 10 years, molecular studies have started to define the role of metalloproteinases, UV radiation, and cutaneous peptides in the pathogenesis of acne rosacea and GR.21,22
Granulomatous rosacea typically is seen in middle-aged women.20,23 Hallmarks of rosacea, such as facial erythema, flushing, telangiectasias, pustules, and rhinophyma, are not always present in GR.5,20,23 Lesions usually are distributed around the central face, although extension to the cheeks, total facial involvement, and extrafacial lesions are possible.5,20 Histologically, perifollicular and follicular-based noncaseating granulomas with dilatation of the dermal papillary vasculature are seen.17,23 As a whole, rosacea is comparatively uncommon in dark-skinned patients; when it does occur, GR is a frequent presentation.24
First-line treatment for GR is tetracycline antibiotics.5 Unresponsive cases have been treated—largely anecdotally—with topical modalities (eg, metronidazole, steroids, immunomodulators), systemic agents (eg, dapsone, erythromycin, isotretinoin), and other therapies.5 Granulomatous rosacea tends to have a chronic course.5,23
Lupus Miliaris Disseminatus Faciei—Classic LMDF demonstrates caseating perifollicular granulomas histologically.6,17,25 Lesions tend to appear on the central face, particularly the eyelids, and can be seen extrafacially.3,6,25,26 Although LMDF originally was categorized as a tuberculid eruption, this no longer is thought to be the case.27 It is now regarded by some as a variant of GR25; however, LMDF responds poorly to tetracyclines, is more common in males, and lacks rosacealike vascular abnormalities, leading some to question this association.3,6,17 In the past 20 years, some have proposed renaming LMDF to better reflect its clinical course and to consider it independent of tuberculosis and GR.28 It usually resolves spontaneously after 1 to 3 years, leaving pitted scars.3,6
Papular Sarcoidosis—The first potential documented case of sarcoidosis was by Hutchinson29 in 1869 in a patient seen in London. The author labeled purple plaques on the index patient’s legs and hands as “livid papillary psoriasis.” In 1889, Besnier30 described a patient with violaceous swellings on the nose, ears, and fingers, which he called “lupus pernio”; his contemporary, Tenneson,31 published a case of lupus pernio and described its histologic profile as comprising epithelioid cells and giant cells. It was not until 1899 that the term sarkoid was used to describe these cutaneous lesions by Boeck,32 who thought they were reminiscent of sarcoma. In 1915, Kuznitsky and Bittorf33 described a patient with cutaneous lesions histologically consistent with Boeck’s sarkoid but additionally with hilar lymphadenopathy and pulmonary infiltrates. Around 1916 or 1917, Schaumann34 described patients with cutaneous lesions and additionally with involvement of pulmonary, osseous, hepatosplenic, and tonsillar tissue. These reports are among the first to recognize the multisystemic nature of sarcoidosis. The first possible case of childhood sarcoidosis might have been reported by Osler35 in the United States in 1898.
In the past century or so, an ongoing effort by researchers has focused on identifying etiologic triggers for sarcoidosis. Microbial agents have been considered in this role, with Mycobacterium and Propionibacterium organisms the most intensively studied; the possibility that foreign material contributes to the formation of granulomas also has been raised.36 Current models of the pathogenesis of sarcoidosis involve an interplay between the immune system in genetically predisposed patients and an infection that leads to a hyperimmune type 1 T–helper cell response that clears the infection but not antigens generated by the microbes and the acute host response, including proteins such as serum amyloid A and vimentin.36,37 These antigens aggregate and serve as a nidus for granuloma formation and maintenance long after infection has resolved.
Cutaneous lesions of sarcoidosis include macules, papules, plaques, and lupus pernio, as well as lesions arising within scars or tattoos, with many less common presentations.7,38 Papular sarcoidosis is common on the face but also can involve the extremities.4,7 Strictly, at least 2 organ systems must be involved to diagnose sarcoidosis, but this is debatable.4,7 Among 41 patients with cutaneous sarcoidosis, 24 (58.5%) had systemic disease; cutaneous lesions were the presenting sign in 87.5% (21/24) of patients.38 Histologic analysis, regardless of the lesion, usually shows noncaseating so-called “naked” granulomas, which have minimal lymphocytic infiltrate associated with the epithelioid histiocytes.38,39 Perifollicular granulomas are possible but unusual.40
Treatment depends on the extent of cutaneous and systemic involvement. Pharmacotherapeutic modalities include topical steroids, immunomodulators, and retinoids; systemic immunomodulators and immunosuppressants; and biologic agents.7 Isolated cutaneous sarcoidosis, particularly the papular variant, usually is associated with acute disease lasting less than 2 years, with resolution of skin lesions.7,38 That said, a recent report suggested that cutaneous sarcoidosis can progress to multisystemic disease as long as 7 years after the initial diagnosis.41
Clinical and Histologic Overlap—Despite this categorization of noninfectious facial granulomatous conditions, each has some clinical and histologic overlap with the others, which must be considered when encountering a granulomatous facial dermatosis. Both GPD and GR tend to present with lesions near the eyes, mouth, and nose, although GR can extend to lateral aspects of the face, below the mandible, and the forehead and has different demographic features.15,20,23 Granulomas in both GPD and GR generally are noncaseating and form in a follicular or perifollicular distribution within the dermis.2,15,23 Lupus miliaris disseminatus faciei and GR share a similar facial distribution in some cases.17,20 Even papular cutaneous sarcoidosis has masqueraded as GR clinically and histologically.4
Diagnostic and Treatment Difficulty—Our cases illustrate the range of difficulty in evaluating and managing patients with facial papular granulomas. On one hand, our adult patient’s clinical and histologic findings were highly consistent with GR; on the other hand, our younger patient had clinicopathologic features of both sarcoidosis and GPD at varying times. Both conditions are more common in dark-skinned patients.11,42
Juvenile sarcoidosis is comparatively rare, with a reported annual incidence of 0.22 to 0.27 for every 100,000 children younger than 15 years; however, juvenile sarcoidosis commonly presents around 8 to 15 years of age.43
It is unusual for sarcoid granulomas to be isolated to the skin, much less to the face.4,7,43,44 Patient 1 initially presented in this manner and lacked convincing laboratory or radiographic evidence of systemic sarcoidosis. Bilateral hilar calcifications in sarcoidosis are more typical among adults after 5 to 20 years; there were no signs or symptoms of active infection that could account for the pulmonary and cutaneous lesions.45
The presence of perifollicular granulomas with associated lymphocytic infiltrates on repeat biopsy, coupled with the use of topical steroids, made it difficult to rule out a contribution by GPD to her clinical course. That her lesions resolved with pitted scarring while she was taking methotrexate and after topical steroids had been stopped could be the result of successful management or spontaneous resolution of her dermatosis; both papular sarcoidosis and GPD tend to have a self-limited course.7,13
Conclusion
We present 2 cases of papular facial granulomas in patients with similar skin types who had different clinical courses. Evaluation of such lesions remains challenging given the similarity between specific entities that present in this manner. Certainly, it is reasonable to consider a spectrum upon which all of these conditions fall, in light of the findings of these cases and those reported previously.
Cutaneous granulomatous diseases encompass many entities that are skin-limited or systemic. The prototypical cutaneous granuloma is a painless, rounded, well-defined, red-pink or flesh-colored papule1 and is smooth, owing to minimal epidermal involvement. Examples of conditions that present with such lesions include granulomatous periorificial dermatitis (GPD), granulomatous rosacea (GR), lupus miliaris disseminatus faciei (LMDF), and papular sarcoidosis. These entities commonly are seen on the face and can be a source of distress to patients when they are extensive. Several reports have raised the possibility that these conditions lie on a spectrum.2-4 We present 2 cases of patients with facial papular granulomas, discuss potential causes of the lesions, review historical aspects from the literature, and highlight the challenges that these lesions can pose to the clinician.
Case Reports
Patient 1—A 10-year-old Ethiopian girl with a history of atopic dermatitis presented with a facial rash of 4 months’ duration. Her pediatrician initially treated the rash as pityriasis alba and prescribed hydrocortisone cream. Two months into treatment, the patient developed an otherwise asymptomatic, unilateral, papular dermatosis on the right cheek. She subsequently was switched to treatment with benzoyl peroxide and topical clindamycin, which she had been using for 2 months with no improvement at the time of the current presentation. The lesions then spread bilaterally and periorally.
At the current presentation, physical examination demonstrated fine, diffuse, follicular-based, flesh-colored papules over both cheeks, the right side of the nose, and the perioral region (Figure 1). A biopsy of a papular lesion from the right cheek revealed well-formed, noncaseating granulomas in the superficial and mid dermis with an associated lymphocytic infiltrate (Figure 2). No organisms were identified on acid-fast, Fite, or periodic acid–Schiff staining. A tuberculin skin test was negative. A chest radiograph showed small calcified hilar lymph nodes bilaterally. Pulmonary function tests were unremarkable. Calcium and angiotensin-converting enzyme levels were normal.

The patient denied any fever, chills, hemoptysis, cough, dyspnea, lymphadenopathy, scleral or conjunctival pain or erythema, visual disturbances, or arthralgias. Hydroxychloroquine 200 mg twice daily was started with minimal improvement after 5 months. Methotrexate 20 mg once weekly was then added. Topical fluocinonide 0.05% also was started at this time, as the patient had required several prednisone tapers over the past 3 months for symptomatic relief. The lesions improved minimally after 5 more months of treatment, at which time she had developed inflammatory papules, pustules, and open comedones in the same areas as well as the glabella.

Repeat biopsy of a papular lesion demonstrated noncaseating granulomas and an associated chronic lymphocytic infiltrate in a follicular and perifollicular distribution (Figure 3). Biopsy of a pustule demonstrated acute Demodex folliculitis. Fluocinonide was stopped, and anti-mite therapy with ivermectin, permethrin cream 5%, and selenium sulfide lotion 2.5% was started, with good response from the pustular lesions.

The patient continued taking methotrexate 20 mg once weekly during this time, with improvement in the papular lesions. She discontinued methotrexate after 12 months with complete resolution. At follow-up 12 months after stopping the methotrexate (roughly 2 years after initial presentation), she showed sustained resolution, with small pitted scars on both cheeks and the nasal tip.
Patient 2—A 33-year-old Ethiopian woman presented with a facial rash of 15 years’ duration. The lesions had been accumulating slowly and were asymptomatic. Physical examination revealed multiple follicular-based, flesh-colored, and erythematous papules on the cheeks, chin, perioral area, and forehead (Figure 4). There were no pustules or telangiectasias. Treatment with tretinoin cream 0.05% for 6 months offered minimal relief.

Biopsy of a papule from the left mandible showed superficial vascular telangiectasias, noncaseating granulomas comprising epithelioid histiocytes and lymphocytes in the superficial dermis, and a perifollicular lymphocytic infiltrate (Figure 5). No organisms were identified on Fite or Gomori methenamine silver staining.

Comment
The first step in differentiating cutaneous granulomatous lesions should be to distinguish infectious from noninfectious causes.1 Noninfectious cutaneous granulomas can appear nearly anywhere; however, certain processes have a predilection for the face, including GPD, GR, LMDF, and papular sarcoidosis.5-7 These conditions generally present with papular granulomas with features as described above.
Granulomatous Periorificial Dermatitis—In 1970, Gianotti and colleagues8 briefly described the first possible cases of GPD in 5 children. The eruption comprised numerous yellow, dome-shaped papules in a mostly perioral distribution. Tuberculin and the Kveim tests were nonreactive; histopathology was described as sarcoid-type and not necessarily follicular or perifollicular.8 In 1974, Marten et al9 described 22 Afro-Caribbean children with flesh-colored, papular eruptions on the face that did not show histologic granulomatous changes but were morphologically similar to the reports by Gianotti et al.8 By 1989, Frieden and colleagues10 described this facial eruption as “granulomatous perioral dermatitis in children”. Additionally, the investigators observed granulomatous infiltrates in a perifollicular distribution and suggested follicular disruption as a possible cause. It was clear from the case discussions that these eruptions were not uncommonly diagnosed as papular sarcoidosis.10 The following year, Williams et al11 reported 5 cases of similar papular eruptions in 5 Afro-Caribbean children, coining the term facial Afro-Caribbean eruption.11 Knautz and Lesher12 referred to this entity as “childhood GPD” in 1996 to avoid limiting the diagnosis to Afro-Caribbean patients and to a perioral distribution; this is the most popular current terminology.12 Since then, reports of extrafacial involvement and disease in adults have been published.13,14
Granulomatous periorificial dermatitis often is seen in the perinasal, periocular, and perioral regions of the face.2 It is associated with topical steroid exposure.5 Histologically, noncaseating granulomas around the upper half of undisrupted hair follicles with a lymphocytic infiltrate are typical.13 Treatment should begin with cessation of any topical steroids; first-line agents are oral tetracycline or macrolide antibiotics.5 These agents can be used alone or in combination with topical erythromycin, metronidazole, or sulfur-based lotions.13 Rarely, GPD presents extrafacially.13 Even so, it usually resolves within 2 weeks to 6 months, especially with therapy; scarring is unusual.5,13,15
Granulomatous Rosacea—A report in the early 20th century described patients with tuberculoid granulomas resembling papular rosacea; the initial belief was that this finding represented a rosacealike tuberculid eruption.5 However, this belief was questioned by Snapp,16 among others, who demonstrated near universal lack of reactivity to tuberculin among 20 of these patients in 1949; more recent evidence has substantiated these findings.17 Still, Snapp16 postulated that these rosacealike granulomatous lesions were distinct from classic rosacea because they lacked vascular symptoms and pustules and were recalcitrant to rosacea treatment modalities.
In 1970, Mullanax and colleagues18 introduced the term granulomatous rosacea, reiterating that this entity was not tuberculous. They documented papulopustular lesions as well as telangiectasias, raising the possibility that GR does overlap with acne rosacea. More recent studies have established the current theory that GR is a histologic variant of acne rosacea because, in addition to typical granulomatous papules, its microscopic features can be seen across subtypes of acne rosacea.19,20
Various causes have been proposed for GR. Demodex mites have been reported in association with GR for nearly 30 years.19,20 In the past 10 years, molecular studies have started to define the role of metalloproteinases, UV radiation, and cutaneous peptides in the pathogenesis of acne rosacea and GR.21,22
Granulomatous rosacea typically is seen in middle-aged women.20,23 Hallmarks of rosacea, such as facial erythema, flushing, telangiectasias, pustules, and rhinophyma, are not always present in GR.5,20,23 Lesions usually are distributed around the central face, although extension to the cheeks, total facial involvement, and extrafacial lesions are possible.5,20 Histologically, perifollicular and follicular-based noncaseating granulomas with dilatation of the dermal papillary vasculature are seen.17,23 As a whole, rosacea is comparatively uncommon in dark-skinned patients; when it does occur, GR is a frequent presentation.24
First-line treatment for GR is tetracycline antibiotics.5 Unresponsive cases have been treated—largely anecdotally—with topical modalities (eg, metronidazole, steroids, immunomodulators), systemic agents (eg, dapsone, erythromycin, isotretinoin), and other therapies.5 Granulomatous rosacea tends to have a chronic course.5,23
Lupus Miliaris Disseminatus Faciei—Classic LMDF demonstrates caseating perifollicular granulomas histologically.6,17,25 Lesions tend to appear on the central face, particularly the eyelids, and can be seen extrafacially.3,6,25,26 Although LMDF originally was categorized as a tuberculid eruption, this no longer is thought to be the case.27 It is now regarded by some as a variant of GR25; however, LMDF responds poorly to tetracyclines, is more common in males, and lacks rosacealike vascular abnormalities, leading some to question this association.3,6,17 In the past 20 years, some have proposed renaming LMDF to better reflect its clinical course and to consider it independent of tuberculosis and GR.28 It usually resolves spontaneously after 1 to 3 years, leaving pitted scars.3,6
Papular Sarcoidosis—The first potential documented case of sarcoidosis was by Hutchinson29 in 1869 in a patient seen in London. The author labeled purple plaques on the index patient’s legs and hands as “livid papillary psoriasis.” In 1889, Besnier30 described a patient with violaceous swellings on the nose, ears, and fingers, which he called “lupus pernio”; his contemporary, Tenneson,31 published a case of lupus pernio and described its histologic profile as comprising epithelioid cells and giant cells. It was not until 1899 that the term sarkoid was used to describe these cutaneous lesions by Boeck,32 who thought they were reminiscent of sarcoma. In 1915, Kuznitsky and Bittorf33 described a patient with cutaneous lesions histologically consistent with Boeck’s sarkoid but additionally with hilar lymphadenopathy and pulmonary infiltrates. Around 1916 or 1917, Schaumann34 described patients with cutaneous lesions and additionally with involvement of pulmonary, osseous, hepatosplenic, and tonsillar tissue. These reports are among the first to recognize the multisystemic nature of sarcoidosis. The first possible case of childhood sarcoidosis might have been reported by Osler35 in the United States in 1898.
In the past century or so, an ongoing effort by researchers has focused on identifying etiologic triggers for sarcoidosis. Microbial agents have been considered in this role, with Mycobacterium and Propionibacterium organisms the most intensively studied; the possibility that foreign material contributes to the formation of granulomas also has been raised.36 Current models of the pathogenesis of sarcoidosis involve an interplay between the immune system in genetically predisposed patients and an infection that leads to a hyperimmune type 1 T–helper cell response that clears the infection but not antigens generated by the microbes and the acute host response, including proteins such as serum amyloid A and vimentin.36,37 These antigens aggregate and serve as a nidus for granuloma formation and maintenance long after infection has resolved.
Cutaneous lesions of sarcoidosis include macules, papules, plaques, and lupus pernio, as well as lesions arising within scars or tattoos, with many less common presentations.7,38 Papular sarcoidosis is common on the face but also can involve the extremities.4,7 Strictly, at least 2 organ systems must be involved to diagnose sarcoidosis, but this is debatable.4,7 Among 41 patients with cutaneous sarcoidosis, 24 (58.5%) had systemic disease; cutaneous lesions were the presenting sign in 87.5% (21/24) of patients.38 Histologic analysis, regardless of the lesion, usually shows noncaseating so-called “naked” granulomas, which have minimal lymphocytic infiltrate associated with the epithelioid histiocytes.38,39 Perifollicular granulomas are possible but unusual.40
Treatment depends on the extent of cutaneous and systemic involvement. Pharmacotherapeutic modalities include topical steroids, immunomodulators, and retinoids; systemic immunomodulators and immunosuppressants; and biologic agents.7 Isolated cutaneous sarcoidosis, particularly the papular variant, usually is associated with acute disease lasting less than 2 years, with resolution of skin lesions.7,38 That said, a recent report suggested that cutaneous sarcoidosis can progress to multisystemic disease as long as 7 years after the initial diagnosis.41
Clinical and Histologic Overlap—Despite this categorization of noninfectious facial granulomatous conditions, each has some clinical and histologic overlap with the others, which must be considered when encountering a granulomatous facial dermatosis. Both GPD and GR tend to present with lesions near the eyes, mouth, and nose, although GR can extend to lateral aspects of the face, below the mandible, and the forehead and has different demographic features.15,20,23 Granulomas in both GPD and GR generally are noncaseating and form in a follicular or perifollicular distribution within the dermis.2,15,23 Lupus miliaris disseminatus faciei and GR share a similar facial distribution in some cases.17,20 Even papular cutaneous sarcoidosis has masqueraded as GR clinically and histologically.4
Diagnostic and Treatment Difficulty—Our cases illustrate the range of difficulty in evaluating and managing patients with facial papular granulomas. On one hand, our adult patient’s clinical and histologic findings were highly consistent with GR; on the other hand, our younger patient had clinicopathologic features of both sarcoidosis and GPD at varying times. Both conditions are more common in dark-skinned patients.11,42
Juvenile sarcoidosis is comparatively rare, with a reported annual incidence of 0.22 to 0.27 for every 100,000 children younger than 15 years; however, juvenile sarcoidosis commonly presents around 8 to 15 years of age.43
It is unusual for sarcoid granulomas to be isolated to the skin, much less to the face.4,7,43,44 Patient 1 initially presented in this manner and lacked convincing laboratory or radiographic evidence of systemic sarcoidosis. Bilateral hilar calcifications in sarcoidosis are more typical among adults after 5 to 20 years; there were no signs or symptoms of active infection that could account for the pulmonary and cutaneous lesions.45
The presence of perifollicular granulomas with associated lymphocytic infiltrates on repeat biopsy, coupled with the use of topical steroids, made it difficult to rule out a contribution by GPD to her clinical course. That her lesions resolved with pitted scarring while she was taking methotrexate and after topical steroids had been stopped could be the result of successful management or spontaneous resolution of her dermatosis; both papular sarcoidosis and GPD tend to have a self-limited course.7,13
Conclusion
We present 2 cases of papular facial granulomas in patients with similar skin types who had different clinical courses. Evaluation of such lesions remains challenging given the similarity between specific entities that present in this manner. Certainly, it is reasonable to consider a spectrum upon which all of these conditions fall, in light of the findings of these cases and those reported previously.
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146. doi:10.1007/s12016-017-8666-8
- Lucas CR, Korman NJ, Gilliam AC. Granulomatous periorificial dermatitis: a variant of granulomatous rosacea in children? J Cutan Med Surg. 2009;13:115-118. doi:10.2310/7750.2008.07088
- van de Scheur MR, van der Waal RIF, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120-123. doi:10.1159/000068457
- Simonart T, Lowy M, Rasquin F, et al. Overlap of sarcoidosis and rosacea. Dermatology. 1997;194:416-418. doi:10.1159/000246165
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management. Dermatol Clin. 2015;33:447-455. doi:10.1016/j.det.2015.03.009
- Michaels JD, Cook-Norris RH, Lehman JS, et al. Adult with papular eruption of the central aspect of the face. J Am Acad Dermatol. 2014;71:410-412. doi:10.1016/j.jaad.2012.06.039
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;38:685-702. doi:10.1016/j.ccm.2015.08.010
- Gianotti F, Ermacora E, Benelli MG, et al. Particulière dermatite peri-orale infantile. observations sur 5 cas. Bull Soc Fr Dermatol Syphiligr. 1970;77:341.
- Marten RH, Presbury DG, Adamson JE, et al. An unusual papular and acneiform facial eruption in the negro child. Br J Dermatol. 1974;91:435-438. doi:10.1111/j.1365-2133.1974.tb13083.x
- Frieden IJ, Prose NS, Fletcher V, et al. Granulomatous perioral dermatitis in children. Arch Dermatol. 1989;125:369-373.
- Williams HC, Ashworth J, Pembroke AC, et al. FACE—facial Afro-Caribbean childhood eruption. Clin Exp Dermatol. 1990;15:163-166. doi:10.1111/j.1365-2230.1990.tb02063.x
- Knautz MA, Lesher JL Jr. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13:131-134. doi:10.1111/j.1525-1470.1996.tb01419.x
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358. doi:10.1001/archderm.138.10.1354
- Vincenzi C, Parente G, Tosti A. Perioral granulomatous dermatitis: two cases treated with clarithromycin. J Dermatol Treat. 2000;11:57-61.
- Kim YJ, Shin JW, Lee JS, et al. Childhood granulomatous periorificial dermatitis. Ann Dermatol. 2011;23:386-388. doi:10.5021/ad.2011.23.3.386
- Snapp RH. Lewandowsky’s rosacea-like eruption; a clinical study. J Invest Dermatol. 1949;13:175-190. doi:10.1038/jid.1949.86
- Chougule A, Chatterjee D, Sethi S, et al. Granulomatous rosacea versus lupus miliaris disseminatus faciei—2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819-823. doi:10.1097/DAD.0000000000001243
- Mullanax MG, Kierland RR. Granulomatous rosacea. Arch Dermatol. 1970;101:206-211.
- Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6-9. doi:10.1097/DAD.0b013e31815bc191
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25:1038-1043. doi:10.1016/0190-9622(91)70304-k
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435-1442. doi:10.1038/jid.2012.14
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544-548. doi:10.1111/j.1468-3083.2010.03825.x
- Khokhar O, Khachemoune A. A case of granulomatous rosacea: sorting granulomatous rosacea from other granulomatous diseases that affect the face. Dermatol Online J. 2004;10:6.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73. doi:10.1016/s0190-9622(87)70173-x
- Adams AK, Davis JL, Davis MDP, et al. What is your diagnosis? granulomatous rosacea (lupus miliaris disseminatus faciei, acne agminata). Cutis. 2008;82:103-112.
- Shitara A. Lupus miliaris disseminatus faciei. Int J Dermatol. 1984;23:542-544. doi:10.1111/j.1365-4362.1984.tb04206.x
- Hodak E, Trattner A, Feuerman H, et al. Lupus miliaris disseminatus faciei—the DNA of Mycobacterium tuberculosis is not detectable in active lesions by polymerase chain reaction. Br J Dermatol. 1997;137:614-619. doi: 10.1111/j.1365-2133.1997.tb03797.x
- Skowron F, Causeret AS, Pabion C, et al. F.I.GU.R.E.: facial idiopathic granulomas with regressive evolution. Dermatology. 2000;201:287-289. doi:10.1159/000051539
- Hutchinson J. Case of livid papillary psoriasis. In: London J, Churchill A, eds. Illustrations of Clinical Surgery. J&A Churchill; 1877:42-43.
- Besnier E. Lupus pernio of the face [in French]. Ann Dermatol Syphiligr (Paris). 1889;10:33-36.
- Tenneson H. Lupus pernio. Ann Dermatol Syphiligr (Paris). 1889;10:333-336.
- Boeck C. Multiple benign sarkoid of the skin [in Norwegian]. Norsk Mag Laegevidensk. 1899;14:1321-1334.
- Kuznitsky E, Bittorf A. Sarkoid mit beteiligung innerer organe. Münch Med Wochenschr. 1915;62:1349-1353.
- Schaumann J. Etude sur le lupus pernio et ses rapports avec les sarcoides et la tuberculose. Ann Dermatol Syphiligr. 1916-1917;6:357-373.
- Osler W. On chronic symmetrical enlargement of the salivary and lacrimal glands. Am J Med Sci. 1898;115:27-30.
- Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. doi:10.1007/s12016-015-8481-z
- Eberhardt C, Thillai M, Parker R, et al. Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One. 2017;12:e0170285. doi:10.1371/journal.pone.0170285
- Esteves TC, Aparicio G, Ferrer B, et al. Prognostic value of skin lesions in sarcoidosis: clinical and histopathological clues. Eur J Dermatol. 2015;25:556-562. doi:10.1684/ejd.2015.2666
- Cardoso JC, Cravo M, Reis JP, et al. Cutaneous sarcoidosis: a histopathological study. J Eur Acad Dermatol Venereol. 2009;23:678-682. doi:10.1111/j.1468-3083.2009.03153.x
- Mangas C, Fernández-Figueras M-T, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772-777. doi:10.1111/j.1600-0560.2006.00563.x
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, et al. The natural history of cutaneous sarcoidosis. clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178-184. doi: 10.1111/ijd.14218
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16. doi:10.1186/1546-0096-6-16
- Milman N, Hoffmann AL, Byg KE. Sarcoidosis in children. epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998;87:871-878. doi:10.1080/08035259875001366244. A, H, Yapıcı I. Isolated cutaneous sarcoidosis. Arch Bronconeumol. 2016;52:220.
- Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. 1970;46:530-536. doi:10.1136/pgmj.46.538.530
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146. doi:10.1007/s12016-017-8666-8
- Lucas CR, Korman NJ, Gilliam AC. Granulomatous periorificial dermatitis: a variant of granulomatous rosacea in children? J Cutan Med Surg. 2009;13:115-118. doi:10.2310/7750.2008.07088
- van de Scheur MR, van der Waal RIF, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120-123. doi:10.1159/000068457
- Simonart T, Lowy M, Rasquin F, et al. Overlap of sarcoidosis and rosacea. Dermatology. 1997;194:416-418. doi:10.1159/000246165
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management. Dermatol Clin. 2015;33:447-455. doi:10.1016/j.det.2015.03.009
- Michaels JD, Cook-Norris RH, Lehman JS, et al. Adult with papular eruption of the central aspect of the face. J Am Acad Dermatol. 2014;71:410-412. doi:10.1016/j.jaad.2012.06.039
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;38:685-702. doi:10.1016/j.ccm.2015.08.010
- Gianotti F, Ermacora E, Benelli MG, et al. Particulière dermatite peri-orale infantile. observations sur 5 cas. Bull Soc Fr Dermatol Syphiligr. 1970;77:341.
- Marten RH, Presbury DG, Adamson JE, et al. An unusual papular and acneiform facial eruption in the negro child. Br J Dermatol. 1974;91:435-438. doi:10.1111/j.1365-2133.1974.tb13083.x
- Frieden IJ, Prose NS, Fletcher V, et al. Granulomatous perioral dermatitis in children. Arch Dermatol. 1989;125:369-373.
- Williams HC, Ashworth J, Pembroke AC, et al. FACE—facial Afro-Caribbean childhood eruption. Clin Exp Dermatol. 1990;15:163-166. doi:10.1111/j.1365-2230.1990.tb02063.x
- Knautz MA, Lesher JL Jr. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13:131-134. doi:10.1111/j.1525-1470.1996.tb01419.x
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358. doi:10.1001/archderm.138.10.1354
- Vincenzi C, Parente G, Tosti A. Perioral granulomatous dermatitis: two cases treated with clarithromycin. J Dermatol Treat. 2000;11:57-61.
- Kim YJ, Shin JW, Lee JS, et al. Childhood granulomatous periorificial dermatitis. Ann Dermatol. 2011;23:386-388. doi:10.5021/ad.2011.23.3.386
- Snapp RH. Lewandowsky’s rosacea-like eruption; a clinical study. J Invest Dermatol. 1949;13:175-190. doi:10.1038/jid.1949.86
- Chougule A, Chatterjee D, Sethi S, et al. Granulomatous rosacea versus lupus miliaris disseminatus faciei—2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819-823. doi:10.1097/DAD.0000000000001243
- Mullanax MG, Kierland RR. Granulomatous rosacea. Arch Dermatol. 1970;101:206-211.
- Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6-9. doi:10.1097/DAD.0b013e31815bc191
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25:1038-1043. doi:10.1016/0190-9622(91)70304-k
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435-1442. doi:10.1038/jid.2012.14
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544-548. doi:10.1111/j.1468-3083.2010.03825.x
- Khokhar O, Khachemoune A. A case of granulomatous rosacea: sorting granulomatous rosacea from other granulomatous diseases that affect the face. Dermatol Online J. 2004;10:6.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73. doi:10.1016/s0190-9622(87)70173-x
- Adams AK, Davis JL, Davis MDP, et al. What is your diagnosis? granulomatous rosacea (lupus miliaris disseminatus faciei, acne agminata). Cutis. 2008;82:103-112.
- Shitara A. Lupus miliaris disseminatus faciei. Int J Dermatol. 1984;23:542-544. doi:10.1111/j.1365-4362.1984.tb04206.x
- Hodak E, Trattner A, Feuerman H, et al. Lupus miliaris disseminatus faciei—the DNA of Mycobacterium tuberculosis is not detectable in active lesions by polymerase chain reaction. Br J Dermatol. 1997;137:614-619. doi: 10.1111/j.1365-2133.1997.tb03797.x
- Skowron F, Causeret AS, Pabion C, et al. F.I.GU.R.E.: facial idiopathic granulomas with regressive evolution. Dermatology. 2000;201:287-289. doi:10.1159/000051539
- Hutchinson J. Case of livid papillary psoriasis. In: London J, Churchill A, eds. Illustrations of Clinical Surgery. J&A Churchill; 1877:42-43.
- Besnier E. Lupus pernio of the face [in French]. Ann Dermatol Syphiligr (Paris). 1889;10:33-36.
- Tenneson H. Lupus pernio. Ann Dermatol Syphiligr (Paris). 1889;10:333-336.
- Boeck C. Multiple benign sarkoid of the skin [in Norwegian]. Norsk Mag Laegevidensk. 1899;14:1321-1334.
- Kuznitsky E, Bittorf A. Sarkoid mit beteiligung innerer organe. Münch Med Wochenschr. 1915;62:1349-1353.
- Schaumann J. Etude sur le lupus pernio et ses rapports avec les sarcoides et la tuberculose. Ann Dermatol Syphiligr. 1916-1917;6:357-373.
- Osler W. On chronic symmetrical enlargement of the salivary and lacrimal glands. Am J Med Sci. 1898;115:27-30.
- Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. doi:10.1007/s12016-015-8481-z
- Eberhardt C, Thillai M, Parker R, et al. Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One. 2017;12:e0170285. doi:10.1371/journal.pone.0170285
- Esteves TC, Aparicio G, Ferrer B, et al. Prognostic value of skin lesions in sarcoidosis: clinical and histopathological clues. Eur J Dermatol. 2015;25:556-562. doi:10.1684/ejd.2015.2666
- Cardoso JC, Cravo M, Reis JP, et al. Cutaneous sarcoidosis: a histopathological study. J Eur Acad Dermatol Venereol. 2009;23:678-682. doi:10.1111/j.1468-3083.2009.03153.x
- Mangas C, Fernández-Figueras M-T, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772-777. doi:10.1111/j.1600-0560.2006.00563.x
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, et al. The natural history of cutaneous sarcoidosis. clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178-184. doi: 10.1111/ijd.14218
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16. doi:10.1186/1546-0096-6-16
- Milman N, Hoffmann AL, Byg KE. Sarcoidosis in children. epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998;87:871-878. doi:10.1080/08035259875001366244. A, H, Yapıcı I. Isolated cutaneous sarcoidosis. Arch Bronconeumol. 2016;52:220.
- Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. 1970;46:530-536. doi:10.1136/pgmj.46.538.530
Practice Points
- Dermatologists should be aware that noninfectious granulomatous dermatosis of the face can be caused by granulomatous periorificial dermatitis, granulomatous rosacea, lupus miliaris disseminatus faciei, and papular sarcoidosis.
- These conditions lie on a spectrum, suggested by their historical description and clinical and histological features.
- Because their clinical courses can vary considerably from patient to patient, a thorough effort should be made to differentiate these conditions.