User login
Antihypertensives tied to lower Alzheimer’s disease pathology
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANA 2021
Maraviroc, metformin fail to control NAFLD in people with HIV
The MAVMET study, the first randomized controlled trial of – and in some cases, prolonged use actually increased liver fat.
And that means clinicians like Yvonne Gilleece, MB BCh, who was not involved in the study but does run a liver clinic in England for people living with HIV, are returning to the one intervention proven to work. “As yet, the only thing that is proven to have a very positive effect that is published is weight loss,” said Dr. Gilleece, who runs the clinic at Brighton and Sussex University Hospital. “You don’t put someone on these particular drugs, particularly this combination, easily. MAVMET has really demonstrated that, actually, it’s not effective, and it’s not particularly beneficial to patients.”
The MAVMET trial data was presented at the 18th European AIDS Conference,
There was good reason to think maraviroc might work. A 2018 study in the journal Hepatology found that one of maraviroc’s molecular cousins, cenicriviroc, significantly reduced fibrosis in people with NAFLD. Dr. Gilleece is co-investigator of another study of maraviroc in NAFLD, the HEPMARC trial, which is wrapping up now. In addition to those studies, there are other potential treatments in ongoing trials, including semaglutide, which is being studied in the United States under the study name SLIM LIVER.
MAVMET enrolled 90 people living with HIV from six clinical sites in London who were 35 or older and who had at least one marker for NAFLD, such as abnormal liver lab results. But 70% qualified via imaging- and/or biopsy-confirmed NAFLD. Almost all participants (93%) were men and 81% were White. The trial excluded people who were pregnant or breastfeeding. The median age was 52, and the participants met the criteria for overweight but not obesity, with a median BMI of 28.
In other words, participants generally had fatty livers without the inflammation that characterizes the more aggressive nonalcoholic steatohepatitis (NASH). Clinicians can’t yet differentiate between those who will continue to have asymptomatic fatty liver and those who will progress to NASH and potentially need a liver transplant.
All people living with HIV in the trial had undetectable viral loads and were on HIV treatment. Nearly 1 in 5 (19%) were using a treatment regimen containing tenofovir alafenamide (TAF), which has been associated with weight gain. Nearly half were on integrase strand inhibitors.
Investigators divided the participants up into four groups: 24 people stayed on their HIV treatment and added nothing else; 23 people took maraviroc only; 21 took metformin only; and the final group took both maraviroc and metformin. Across groups, liver fat at baseline was 8.9%, and 78% had mild hepatic steatosis.
After taking the medications for 48 weeks, participants returned to clinic to be scanned via MRI proton density fat fraction (MRI-PDFF), which has been found to successfully measure liver fat. However, because of the COVID-19 pandemic, 20 of the 83 people who returned to the clinic came later than 48 weeks after the trial began.
When investigators looked at the results, they didn’t see what they hypothesized, said Sarah Pett, professor of infectious diseases at University College, London: The scatter plot graph of change in weight looked, well, scattershot: People who didn’t take any additional treatment sometimes lost more liver fat than those on treatment. In fact, the mean liver fat percentage rose by 2.2% in the maraviroc group, 1.3% in the metformin group, and 0.8% in the combination group. The control group saw an increase of 1.4% – meaning that there was no difference between the change in fat between those on treatment and those not.
What’s more, those who had delayed scans – and stayed on their treatment for a median of an additional 16 weeks – saw their liver fat increase even more.
In an interview, Dr. Pett called the results “disappointing.” “The numbers are quite small, but we still didn’t expect this,” she said. “It’s not explained by lockdown weight gain, although we still have to look in detail at how alcohol consumption could have contributed.”
There were also some limits to what the design of this particular trial could tell the researchers. For instance, nearly half of the participants in the maraviroc group, a third of the people in the metformin group, and 36% of those in the combination group had hepatic steatosis grades of 0, meaning that their livers were healthy. And MRI-PDFF becomes less reliable at that level.
“One of the regrets is that perhaps we should have done FibroScan [ultrasound], as well,” Dr. Pett said. The consequence is that the study may have undercounted the fat level by using MRI-PFDD.
“This suggests that the surrogate markers of NAFLD used in MAVMET were not very sensitive to those with a higher percentage of fat,” Dr. Pett said during her presentation. “We were really trying to be pragmatic and not require an MRI at screening.”
Whatever the case, she said that the failure of this particular treatment just highlights the growing need to look more seriously, and more collaboratively, at fat and liver health in people living with HIV.
“We need to really focus on setting up large cohorts of people living with HIV to look in a rigorous way at weight gain, changes in waist circumference, ectopic fat, capture fatty liver disease index scores, and cardiovascular risk, to acquire some longitudinal data,” she said. “And [we need to] join with our fellow researchers in overweight and obesity medicine and hepatology to make sure that people living with HIV are included in new treatments for NASH, as several large RCTs have excluded [people living with HIV].”
From Dr. Gilleece’s perspective, it also just speaks to how far the field has to go in identifying those with asymptomatic fatty livers from those who will progress to fibrosis and potentially need liver transplants.
“MAVMET shows the difficulty in managing NAFLD,” she said. “It seems quite an innocuous disease, because for the majority of people it’s not going to cause a problem in their lifetime. But the reality is, for some it will, and we don’t really know how to treat it.”
Dr. Gilleece has disclosed no relevant financial relationships. Dr. Pett reported receiving funding for trials from Gilead Sciences and Janssen-Cilag. ViiV Healthcare funded the MAVMET trial.
A version of this article first appeared on Medscape.com.
The MAVMET study, the first randomized controlled trial of – and in some cases, prolonged use actually increased liver fat.
And that means clinicians like Yvonne Gilleece, MB BCh, who was not involved in the study but does run a liver clinic in England for people living with HIV, are returning to the one intervention proven to work. “As yet, the only thing that is proven to have a very positive effect that is published is weight loss,” said Dr. Gilleece, who runs the clinic at Brighton and Sussex University Hospital. “You don’t put someone on these particular drugs, particularly this combination, easily. MAVMET has really demonstrated that, actually, it’s not effective, and it’s not particularly beneficial to patients.”
The MAVMET trial data was presented at the 18th European AIDS Conference,
There was good reason to think maraviroc might work. A 2018 study in the journal Hepatology found that one of maraviroc’s molecular cousins, cenicriviroc, significantly reduced fibrosis in people with NAFLD. Dr. Gilleece is co-investigator of another study of maraviroc in NAFLD, the HEPMARC trial, which is wrapping up now. In addition to those studies, there are other potential treatments in ongoing trials, including semaglutide, which is being studied in the United States under the study name SLIM LIVER.
MAVMET enrolled 90 people living with HIV from six clinical sites in London who were 35 or older and who had at least one marker for NAFLD, such as abnormal liver lab results. But 70% qualified via imaging- and/or biopsy-confirmed NAFLD. Almost all participants (93%) were men and 81% were White. The trial excluded people who were pregnant or breastfeeding. The median age was 52, and the participants met the criteria for overweight but not obesity, with a median BMI of 28.
In other words, participants generally had fatty livers without the inflammation that characterizes the more aggressive nonalcoholic steatohepatitis (NASH). Clinicians can’t yet differentiate between those who will continue to have asymptomatic fatty liver and those who will progress to NASH and potentially need a liver transplant.
All people living with HIV in the trial had undetectable viral loads and were on HIV treatment. Nearly 1 in 5 (19%) were using a treatment regimen containing tenofovir alafenamide (TAF), which has been associated with weight gain. Nearly half were on integrase strand inhibitors.
Investigators divided the participants up into four groups: 24 people stayed on their HIV treatment and added nothing else; 23 people took maraviroc only; 21 took metformin only; and the final group took both maraviroc and metformin. Across groups, liver fat at baseline was 8.9%, and 78% had mild hepatic steatosis.
After taking the medications for 48 weeks, participants returned to clinic to be scanned via MRI proton density fat fraction (MRI-PDFF), which has been found to successfully measure liver fat. However, because of the COVID-19 pandemic, 20 of the 83 people who returned to the clinic came later than 48 weeks after the trial began.
When investigators looked at the results, they didn’t see what they hypothesized, said Sarah Pett, professor of infectious diseases at University College, London: The scatter plot graph of change in weight looked, well, scattershot: People who didn’t take any additional treatment sometimes lost more liver fat than those on treatment. In fact, the mean liver fat percentage rose by 2.2% in the maraviroc group, 1.3% in the metformin group, and 0.8% in the combination group. The control group saw an increase of 1.4% – meaning that there was no difference between the change in fat between those on treatment and those not.
What’s more, those who had delayed scans – and stayed on their treatment for a median of an additional 16 weeks – saw their liver fat increase even more.
In an interview, Dr. Pett called the results “disappointing.” “The numbers are quite small, but we still didn’t expect this,” she said. “It’s not explained by lockdown weight gain, although we still have to look in detail at how alcohol consumption could have contributed.”
There were also some limits to what the design of this particular trial could tell the researchers. For instance, nearly half of the participants in the maraviroc group, a third of the people in the metformin group, and 36% of those in the combination group had hepatic steatosis grades of 0, meaning that their livers were healthy. And MRI-PDFF becomes less reliable at that level.
“One of the regrets is that perhaps we should have done FibroScan [ultrasound], as well,” Dr. Pett said. The consequence is that the study may have undercounted the fat level by using MRI-PFDD.
“This suggests that the surrogate markers of NAFLD used in MAVMET were not very sensitive to those with a higher percentage of fat,” Dr. Pett said during her presentation. “We were really trying to be pragmatic and not require an MRI at screening.”
Whatever the case, she said that the failure of this particular treatment just highlights the growing need to look more seriously, and more collaboratively, at fat and liver health in people living with HIV.
“We need to really focus on setting up large cohorts of people living with HIV to look in a rigorous way at weight gain, changes in waist circumference, ectopic fat, capture fatty liver disease index scores, and cardiovascular risk, to acquire some longitudinal data,” she said. “And [we need to] join with our fellow researchers in overweight and obesity medicine and hepatology to make sure that people living with HIV are included in new treatments for NASH, as several large RCTs have excluded [people living with HIV].”
From Dr. Gilleece’s perspective, it also just speaks to how far the field has to go in identifying those with asymptomatic fatty livers from those who will progress to fibrosis and potentially need liver transplants.
“MAVMET shows the difficulty in managing NAFLD,” she said. “It seems quite an innocuous disease, because for the majority of people it’s not going to cause a problem in their lifetime. But the reality is, for some it will, and we don’t really know how to treat it.”
Dr. Gilleece has disclosed no relevant financial relationships. Dr. Pett reported receiving funding for trials from Gilead Sciences and Janssen-Cilag. ViiV Healthcare funded the MAVMET trial.
A version of this article first appeared on Medscape.com.
The MAVMET study, the first randomized controlled trial of – and in some cases, prolonged use actually increased liver fat.
And that means clinicians like Yvonne Gilleece, MB BCh, who was not involved in the study but does run a liver clinic in England for people living with HIV, are returning to the one intervention proven to work. “As yet, the only thing that is proven to have a very positive effect that is published is weight loss,” said Dr. Gilleece, who runs the clinic at Brighton and Sussex University Hospital. “You don’t put someone on these particular drugs, particularly this combination, easily. MAVMET has really demonstrated that, actually, it’s not effective, and it’s not particularly beneficial to patients.”
The MAVMET trial data was presented at the 18th European AIDS Conference,
There was good reason to think maraviroc might work. A 2018 study in the journal Hepatology found that one of maraviroc’s molecular cousins, cenicriviroc, significantly reduced fibrosis in people with NAFLD. Dr. Gilleece is co-investigator of another study of maraviroc in NAFLD, the HEPMARC trial, which is wrapping up now. In addition to those studies, there are other potential treatments in ongoing trials, including semaglutide, which is being studied in the United States under the study name SLIM LIVER.
MAVMET enrolled 90 people living with HIV from six clinical sites in London who were 35 or older and who had at least one marker for NAFLD, such as abnormal liver lab results. But 70% qualified via imaging- and/or biopsy-confirmed NAFLD. Almost all participants (93%) were men and 81% were White. The trial excluded people who were pregnant or breastfeeding. The median age was 52, and the participants met the criteria for overweight but not obesity, with a median BMI of 28.
In other words, participants generally had fatty livers without the inflammation that characterizes the more aggressive nonalcoholic steatohepatitis (NASH). Clinicians can’t yet differentiate between those who will continue to have asymptomatic fatty liver and those who will progress to NASH and potentially need a liver transplant.
All people living with HIV in the trial had undetectable viral loads and were on HIV treatment. Nearly 1 in 5 (19%) were using a treatment regimen containing tenofovir alafenamide (TAF), which has been associated with weight gain. Nearly half were on integrase strand inhibitors.
Investigators divided the participants up into four groups: 24 people stayed on their HIV treatment and added nothing else; 23 people took maraviroc only; 21 took metformin only; and the final group took both maraviroc and metformin. Across groups, liver fat at baseline was 8.9%, and 78% had mild hepatic steatosis.
After taking the medications for 48 weeks, participants returned to clinic to be scanned via MRI proton density fat fraction (MRI-PDFF), which has been found to successfully measure liver fat. However, because of the COVID-19 pandemic, 20 of the 83 people who returned to the clinic came later than 48 weeks after the trial began.
When investigators looked at the results, they didn’t see what they hypothesized, said Sarah Pett, professor of infectious diseases at University College, London: The scatter plot graph of change in weight looked, well, scattershot: People who didn’t take any additional treatment sometimes lost more liver fat than those on treatment. In fact, the mean liver fat percentage rose by 2.2% in the maraviroc group, 1.3% in the metformin group, and 0.8% in the combination group. The control group saw an increase of 1.4% – meaning that there was no difference between the change in fat between those on treatment and those not.
What’s more, those who had delayed scans – and stayed on their treatment for a median of an additional 16 weeks – saw their liver fat increase even more.
In an interview, Dr. Pett called the results “disappointing.” “The numbers are quite small, but we still didn’t expect this,” she said. “It’s not explained by lockdown weight gain, although we still have to look in detail at how alcohol consumption could have contributed.”
There were also some limits to what the design of this particular trial could tell the researchers. For instance, nearly half of the participants in the maraviroc group, a third of the people in the metformin group, and 36% of those in the combination group had hepatic steatosis grades of 0, meaning that their livers were healthy. And MRI-PDFF becomes less reliable at that level.
“One of the regrets is that perhaps we should have done FibroScan [ultrasound], as well,” Dr. Pett said. The consequence is that the study may have undercounted the fat level by using MRI-PFDD.
“This suggests that the surrogate markers of NAFLD used in MAVMET were not very sensitive to those with a higher percentage of fat,” Dr. Pett said during her presentation. “We were really trying to be pragmatic and not require an MRI at screening.”
Whatever the case, she said that the failure of this particular treatment just highlights the growing need to look more seriously, and more collaboratively, at fat and liver health in people living with HIV.
“We need to really focus on setting up large cohorts of people living with HIV to look in a rigorous way at weight gain, changes in waist circumference, ectopic fat, capture fatty liver disease index scores, and cardiovascular risk, to acquire some longitudinal data,” she said. “And [we need to] join with our fellow researchers in overweight and obesity medicine and hepatology to make sure that people living with HIV are included in new treatments for NASH, as several large RCTs have excluded [people living with HIV].”
From Dr. Gilleece’s perspective, it also just speaks to how far the field has to go in identifying those with asymptomatic fatty livers from those who will progress to fibrosis and potentially need liver transplants.
“MAVMET shows the difficulty in managing NAFLD,” she said. “It seems quite an innocuous disease, because for the majority of people it’s not going to cause a problem in their lifetime. But the reality is, for some it will, and we don’t really know how to treat it.”
Dr. Gilleece has disclosed no relevant financial relationships. Dr. Pett reported receiving funding for trials from Gilead Sciences and Janssen-Cilag. ViiV Healthcare funded the MAVMET trial.
A version of this article first appeared on Medscape.com.
Literature review highlights benefits of chemical peels for field AK treatment
including 88 patients.
AKs remain an ongoing health concern because of their potential to become malignant, and chemical peels are among the recommended options for field therapy, wrote Angela J. Jiang, MD, from the department of dermatology at the Henry Ford Health System, Detroit, and colleagues. “Although most dermatologists agree on the importance of field treatment, cryotherapy still remains the standard of care for treatment of AKs,” they noted, adding that the safety and efficacy of chemical peels for AK field therapy have not been well studied.
Chemical peels offer the benefit of a single treatment for patients, which eliminates the patient compliance issue needed for successful topical therapy, the researchers said. In fact, “patients report preference for the tolerability of treatment with chemical peels and the shorter downtime, compared with other field treatments,” they added.
In the study published in Dermatologic Surgery, they reviewed data from five prospective studies on the safety and efficacy of chemical peels as AK field treatments published from 1946 to March 2020 in the National Library of Medicine’s PubMed database. Of the 151 articles on the use of chemical peels for AKs, the 5 studies met the criteria for their review.
One split-face study evaluated glycolic acid peels (published in 1998), two split-face studies evaluated a combination of Jessner’s and 35% trichloroacetic acid (TCA) peels (published in 1995 and 1997), and two randomized studies evaluated TCA peels alone (published in 2006 and 2016).
Overall, the studies showed efficacy of peels in reducing AK counts, with minimal adverse events. In the glycolic acid study, 70% glycolic acid plus 5-fluorouracil (5-FU) yielded a 91.9% mean reduction in AKs at 6 months’ follow-up. A combination of Jessner’s solution and 35% TCA showed a significant reduction in AKs at 12 and at 32 months post treatment – a 75% reduction at 12 months in one study and 78% at 32 months in the other – similar to results achieved with 5-FU.
In studies of TCA alone, 30% TCA peels were similar in AK reduction (89%) to 5-FU (83%) and carbon dioxide laser resurfacing (92%). In another TCA study, 35% TCA was less effective at AK reduction at 12 months, compared with aminolevulinic acid photodynamic therapy (ALA-PDT), but the 35% peel was applied at a more superficial level than in the study of 30% TCA, the authors wrote.
Chemical peels also demonstrated effectiveness in preventing keratinocytic carcinomas, the researchers wrote. In the 30% TCA study, the rate of keratinocyte carcinoma development was 3.75-5.25 times lower in patients treated with 30% TCA peels, compared with 5-FU and carbon dioxide laser resurfacing (CO2) after 5 years.
Chemical peels were well tolerated overall, although side effects varied among the studies. Patients in one study reported no side effects, while patients in other studies reported transient erythema and discomfort. In the study comparing TCA with PDT treatment, PDT was associated with greater pain, erythema, and pustules, the researchers wrote; however, patients treated with 35% TCA reported scarring.
From patients’ perspectives, chemical peels were preferable because of the single application, brief downtime, and minimal adverse effects. From the provider perspective, chemical peels are a more cost-effective way to treat large surface areas for AKs, compared with 5-FU or lasers, the researchers said.
The study findings were limited by several factors including the small number of prospective studies and relatively small number of patients, they noted. “The small number of included studies is partially due to the lack of studies that performed AK counts before and after treatments,” they said. The dearth of literature on chemical peels for AKs may stem from lack of residency training on the use of peels, they added.
However, the results support the use of chemical peels as an effective option for field treatment of AKs, with the added benefits of convenience and cost-effectiveness for patients, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
including 88 patients.
AKs remain an ongoing health concern because of their potential to become malignant, and chemical peels are among the recommended options for field therapy, wrote Angela J. Jiang, MD, from the department of dermatology at the Henry Ford Health System, Detroit, and colleagues. “Although most dermatologists agree on the importance of field treatment, cryotherapy still remains the standard of care for treatment of AKs,” they noted, adding that the safety and efficacy of chemical peels for AK field therapy have not been well studied.
Chemical peels offer the benefit of a single treatment for patients, which eliminates the patient compliance issue needed for successful topical therapy, the researchers said. In fact, “patients report preference for the tolerability of treatment with chemical peels and the shorter downtime, compared with other field treatments,” they added.
In the study published in Dermatologic Surgery, they reviewed data from five prospective studies on the safety and efficacy of chemical peels as AK field treatments published from 1946 to March 2020 in the National Library of Medicine’s PubMed database. Of the 151 articles on the use of chemical peels for AKs, the 5 studies met the criteria for their review.
One split-face study evaluated glycolic acid peels (published in 1998), two split-face studies evaluated a combination of Jessner’s and 35% trichloroacetic acid (TCA) peels (published in 1995 and 1997), and two randomized studies evaluated TCA peels alone (published in 2006 and 2016).
Overall, the studies showed efficacy of peels in reducing AK counts, with minimal adverse events. In the glycolic acid study, 70% glycolic acid plus 5-fluorouracil (5-FU) yielded a 91.9% mean reduction in AKs at 6 months’ follow-up. A combination of Jessner’s solution and 35% TCA showed a significant reduction in AKs at 12 and at 32 months post treatment – a 75% reduction at 12 months in one study and 78% at 32 months in the other – similar to results achieved with 5-FU.
In studies of TCA alone, 30% TCA peels were similar in AK reduction (89%) to 5-FU (83%) and carbon dioxide laser resurfacing (92%). In another TCA study, 35% TCA was less effective at AK reduction at 12 months, compared with aminolevulinic acid photodynamic therapy (ALA-PDT), but the 35% peel was applied at a more superficial level than in the study of 30% TCA, the authors wrote.
Chemical peels also demonstrated effectiveness in preventing keratinocytic carcinomas, the researchers wrote. In the 30% TCA study, the rate of keratinocyte carcinoma development was 3.75-5.25 times lower in patients treated with 30% TCA peels, compared with 5-FU and carbon dioxide laser resurfacing (CO2) after 5 years.
Chemical peels were well tolerated overall, although side effects varied among the studies. Patients in one study reported no side effects, while patients in other studies reported transient erythema and discomfort. In the study comparing TCA with PDT treatment, PDT was associated with greater pain, erythema, and pustules, the researchers wrote; however, patients treated with 35% TCA reported scarring.
From patients’ perspectives, chemical peels were preferable because of the single application, brief downtime, and minimal adverse effects. From the provider perspective, chemical peels are a more cost-effective way to treat large surface areas for AKs, compared with 5-FU or lasers, the researchers said.
The study findings were limited by several factors including the small number of prospective studies and relatively small number of patients, they noted. “The small number of included studies is partially due to the lack of studies that performed AK counts before and after treatments,” they said. The dearth of literature on chemical peels for AKs may stem from lack of residency training on the use of peels, they added.
However, the results support the use of chemical peels as an effective option for field treatment of AKs, with the added benefits of convenience and cost-effectiveness for patients, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
including 88 patients.
AKs remain an ongoing health concern because of their potential to become malignant, and chemical peels are among the recommended options for field therapy, wrote Angela J. Jiang, MD, from the department of dermatology at the Henry Ford Health System, Detroit, and colleagues. “Although most dermatologists agree on the importance of field treatment, cryotherapy still remains the standard of care for treatment of AKs,” they noted, adding that the safety and efficacy of chemical peels for AK field therapy have not been well studied.
Chemical peels offer the benefit of a single treatment for patients, which eliminates the patient compliance issue needed for successful topical therapy, the researchers said. In fact, “patients report preference for the tolerability of treatment with chemical peels and the shorter downtime, compared with other field treatments,” they added.
In the study published in Dermatologic Surgery, they reviewed data from five prospective studies on the safety and efficacy of chemical peels as AK field treatments published from 1946 to March 2020 in the National Library of Medicine’s PubMed database. Of the 151 articles on the use of chemical peels for AKs, the 5 studies met the criteria for their review.
One split-face study evaluated glycolic acid peels (published in 1998), two split-face studies evaluated a combination of Jessner’s and 35% trichloroacetic acid (TCA) peels (published in 1995 and 1997), and two randomized studies evaluated TCA peels alone (published in 2006 and 2016).
Overall, the studies showed efficacy of peels in reducing AK counts, with minimal adverse events. In the glycolic acid study, 70% glycolic acid plus 5-fluorouracil (5-FU) yielded a 91.9% mean reduction in AKs at 6 months’ follow-up. A combination of Jessner’s solution and 35% TCA showed a significant reduction in AKs at 12 and at 32 months post treatment – a 75% reduction at 12 months in one study and 78% at 32 months in the other – similar to results achieved with 5-FU.
In studies of TCA alone, 30% TCA peels were similar in AK reduction (89%) to 5-FU (83%) and carbon dioxide laser resurfacing (92%). In another TCA study, 35% TCA was less effective at AK reduction at 12 months, compared with aminolevulinic acid photodynamic therapy (ALA-PDT), but the 35% peel was applied at a more superficial level than in the study of 30% TCA, the authors wrote.
Chemical peels also demonstrated effectiveness in preventing keratinocytic carcinomas, the researchers wrote. In the 30% TCA study, the rate of keratinocyte carcinoma development was 3.75-5.25 times lower in patients treated with 30% TCA peels, compared with 5-FU and carbon dioxide laser resurfacing (CO2) after 5 years.
Chemical peels were well tolerated overall, although side effects varied among the studies. Patients in one study reported no side effects, while patients in other studies reported transient erythema and discomfort. In the study comparing TCA with PDT treatment, PDT was associated with greater pain, erythema, and pustules, the researchers wrote; however, patients treated with 35% TCA reported scarring.
From patients’ perspectives, chemical peels were preferable because of the single application, brief downtime, and minimal adverse effects. From the provider perspective, chemical peels are a more cost-effective way to treat large surface areas for AKs, compared with 5-FU or lasers, the researchers said.
The study findings were limited by several factors including the small number of prospective studies and relatively small number of patients, they noted. “The small number of included studies is partially due to the lack of studies that performed AK counts before and after treatments,” they said. The dearth of literature on chemical peels for AKs may stem from lack of residency training on the use of peels, they added.
However, the results support the use of chemical peels as an effective option for field treatment of AKs, with the added benefits of convenience and cost-effectiveness for patients, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM DERMATOLOGIC SURGERY
Universal depression screening in schools doubles odds for teen treatment
Universal screening for adolescent depression in schools, compared with the usual process of targeting students for referral after observing behaviors, resulted in significantly higher odds of identifying major depressive disorder (MDD) and of starting treatment for it, a study of more than 12,000 students suggests. Findings were published online in JAMA Network Open.
Deepa L. Sekhar, MD, MSc, with the department of pediatrics at Pennsylvania State College of Medicine in Hershey, Pa., and colleagues conducted a randomized clinical trial comparing the two screening methods from November 2018 to November 2020.
The trial included students in grades 9 through 12 enrolled at any of the 14 participating Pennsylvania public high schools. Researchers compared the two groups using mixed-effects logistic regression.
They found that adolescents in the universal screening intervention group had 5.92 times higher odds (95% confidence interval [CI], 5.07-6.93) of being identified with MDD symptoms, 3.30 times higher odds (95% CI, 2.49-4.38) of the Student Assistance Program (SAP) confirming follow-up needs, and 2.07 times higher odds (95% CI, 1.39-3.10) of starting MDD treatment.
The study comprised 12,909 students, with an average age of 16 years. Of those students, 2,687 (20.8%) were Hispanic; 2,891 (22.4%) were non-Hispanic Black, 5,842 (45.3%) were non-Hispanic White; and 1,489 (11.5%) were multiracial or of other race or ethnicity.
In the universal screening intervention (n = 6,473) all students completed the Patient Health Questionnaire–9 (PHQ-9). Students who screened positive proceeded to the Student Assistance Program. Students could receive a targeted referral to SAP if they had concerning behavior beyond the PHQ-9.
In the targeted screening group (n = 6,436), students with behaviors prompting concern for MDD were referred to the Student Assistance Program (SAP), mandated in all Pennsylvania schools. The SAP determined follow-up.
The U.S. Preventive Services Task Force (USPSTF) endorsed primary care screening in 2009 and again in 2016 for all adolescents 12-18 years old.
However, the study authors wrote, most U.S. adolescents (more than 60%) don’t have routine access to preventive health care, which limits primary care offices’ ability to properly address the growing numbers.
“[S]creening is inconsistent, with inequalities by race and ethnicity and region, and potential worsening with the COVID-19 pandemic,” they noted.
Depression rates see sharp increase
Meanwhile, the prevalence of adolescents reporting MDD symptoms has “nearly doubled in the last decade, increasing from 8.3% in 2008 to 14.4% in 2018.”
The American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and Children’s Hospital Association recently declared a national emergency in children’s mental health, citing COVID-19’s toll on top of existing challenges.
This study provides further evidence that universal screening is the better approach to identify and treat adolescent depression to save lives, Andres Pumariega, MD, a child and adolescent psychiatrist at University of Florida in Gainesville, told this news organization.
“If you catch these kids early, you can prevent suicide attempts and suicide. You can also prevent complicating costs of care,” he said.
He noted the universal screening removes the potential for bias.
“Relying purely on referral and clinical identification means a lot of kids in minority groups will not be identified and will not be treated accurately. Many clinicians have a problem identifying depression in diverse kids,” he said.
Pushback for universal screening likely
However, he said he has been part of such efforts to implement such programs in Mexico and the United States and said in the Unites States, the political climate will guarantee pushback from having schools more involved in health care and prevention. Recent controversy around COVID-19 vaccines for children illustrates the potential backlash, he said.
Parents often fight such programs as attempts to “label” their children, he said.
“If I have cancer, I sure want to be labeled. A label is used to get them help. We need to find ways to educate parents and support them in facing these issues,” he added.
One concern he has with this intervention is having the SAPs, composed largely of nonclinicians, be the triage point “instead of doing that objectively through objective criteria and by clinicians,” he said. “If we are to have a comprehensive health system where we can serve all kids and manage costs, schools need to be a major part of it.”
School settings offer the chance to see more children, collaborate with teachers and counselors, and integrate results with educational outcomes, he added.
In the study by Sekhar and colleagues, 7 of the 14 schools were classified as urban, with a median size of 370 students.
Researchers noted that the benefit of the universal screening is likely understated because of COVID-19–related school closures during the study period. The closures meant screening wasn’t completed for 7% of students.
The authors concluded that universal screening finds teens living with depression who otherwise would not be found. They said such a program likely works best in schools with strong SAP.
“Adolescents’ consistent contact with schools has been used to support physical health screenings that affect academic success,” the authors wrote. “Major depressive disorder similarly affects academic success, suggesting school-based screening may be especially beneficial.”
In the past 3 years, Dr. Sekhar reported receiving funding from Pfizer through the American Academy of Pediatrics, the Penn State Clinical and Translational Science Awards Program, and a Eugene Washington Patient-Centered Outcomes Research Institute Engagement Award. Full disclosures for coauthors are available in the journal article.
This work was supported in part by the Patient-Centered Outcomes Research Institute. The use of REDCap (Research Electronic Data Capture) in this project was supported by the National Institutes of Health.
Universal screening for adolescent depression in schools, compared with the usual process of targeting students for referral after observing behaviors, resulted in significantly higher odds of identifying major depressive disorder (MDD) and of starting treatment for it, a study of more than 12,000 students suggests. Findings were published online in JAMA Network Open.
Deepa L. Sekhar, MD, MSc, with the department of pediatrics at Pennsylvania State College of Medicine in Hershey, Pa., and colleagues conducted a randomized clinical trial comparing the two screening methods from November 2018 to November 2020.
The trial included students in grades 9 through 12 enrolled at any of the 14 participating Pennsylvania public high schools. Researchers compared the two groups using mixed-effects logistic regression.
They found that adolescents in the universal screening intervention group had 5.92 times higher odds (95% confidence interval [CI], 5.07-6.93) of being identified with MDD symptoms, 3.30 times higher odds (95% CI, 2.49-4.38) of the Student Assistance Program (SAP) confirming follow-up needs, and 2.07 times higher odds (95% CI, 1.39-3.10) of starting MDD treatment.
The study comprised 12,909 students, with an average age of 16 years. Of those students, 2,687 (20.8%) were Hispanic; 2,891 (22.4%) were non-Hispanic Black, 5,842 (45.3%) were non-Hispanic White; and 1,489 (11.5%) were multiracial or of other race or ethnicity.
In the universal screening intervention (n = 6,473) all students completed the Patient Health Questionnaire–9 (PHQ-9). Students who screened positive proceeded to the Student Assistance Program. Students could receive a targeted referral to SAP if they had concerning behavior beyond the PHQ-9.
In the targeted screening group (n = 6,436), students with behaviors prompting concern for MDD were referred to the Student Assistance Program (SAP), mandated in all Pennsylvania schools. The SAP determined follow-up.
The U.S. Preventive Services Task Force (USPSTF) endorsed primary care screening in 2009 and again in 2016 for all adolescents 12-18 years old.
However, the study authors wrote, most U.S. adolescents (more than 60%) don’t have routine access to preventive health care, which limits primary care offices’ ability to properly address the growing numbers.
“[S]creening is inconsistent, with inequalities by race and ethnicity and region, and potential worsening with the COVID-19 pandemic,” they noted.
Depression rates see sharp increase
Meanwhile, the prevalence of adolescents reporting MDD symptoms has “nearly doubled in the last decade, increasing from 8.3% in 2008 to 14.4% in 2018.”
The American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and Children’s Hospital Association recently declared a national emergency in children’s mental health, citing COVID-19’s toll on top of existing challenges.
This study provides further evidence that universal screening is the better approach to identify and treat adolescent depression to save lives, Andres Pumariega, MD, a child and adolescent psychiatrist at University of Florida in Gainesville, told this news organization.
“If you catch these kids early, you can prevent suicide attempts and suicide. You can also prevent complicating costs of care,” he said.
He noted the universal screening removes the potential for bias.
“Relying purely on referral and clinical identification means a lot of kids in minority groups will not be identified and will not be treated accurately. Many clinicians have a problem identifying depression in diverse kids,” he said.
Pushback for universal screening likely
However, he said he has been part of such efforts to implement such programs in Mexico and the United States and said in the Unites States, the political climate will guarantee pushback from having schools more involved in health care and prevention. Recent controversy around COVID-19 vaccines for children illustrates the potential backlash, he said.
Parents often fight such programs as attempts to “label” their children, he said.
“If I have cancer, I sure want to be labeled. A label is used to get them help. We need to find ways to educate parents and support them in facing these issues,” he added.
One concern he has with this intervention is having the SAPs, composed largely of nonclinicians, be the triage point “instead of doing that objectively through objective criteria and by clinicians,” he said. “If we are to have a comprehensive health system where we can serve all kids and manage costs, schools need to be a major part of it.”
School settings offer the chance to see more children, collaborate with teachers and counselors, and integrate results with educational outcomes, he added.
In the study by Sekhar and colleagues, 7 of the 14 schools were classified as urban, with a median size of 370 students.
Researchers noted that the benefit of the universal screening is likely understated because of COVID-19–related school closures during the study period. The closures meant screening wasn’t completed for 7% of students.
The authors concluded that universal screening finds teens living with depression who otherwise would not be found. They said such a program likely works best in schools with strong SAP.
“Adolescents’ consistent contact with schools has been used to support physical health screenings that affect academic success,” the authors wrote. “Major depressive disorder similarly affects academic success, suggesting school-based screening may be especially beneficial.”
In the past 3 years, Dr. Sekhar reported receiving funding from Pfizer through the American Academy of Pediatrics, the Penn State Clinical and Translational Science Awards Program, and a Eugene Washington Patient-Centered Outcomes Research Institute Engagement Award. Full disclosures for coauthors are available in the journal article.
This work was supported in part by the Patient-Centered Outcomes Research Institute. The use of REDCap (Research Electronic Data Capture) in this project was supported by the National Institutes of Health.
Universal screening for adolescent depression in schools, compared with the usual process of targeting students for referral after observing behaviors, resulted in significantly higher odds of identifying major depressive disorder (MDD) and of starting treatment for it, a study of more than 12,000 students suggests. Findings were published online in JAMA Network Open.
Deepa L. Sekhar, MD, MSc, with the department of pediatrics at Pennsylvania State College of Medicine in Hershey, Pa., and colleagues conducted a randomized clinical trial comparing the two screening methods from November 2018 to November 2020.
The trial included students in grades 9 through 12 enrolled at any of the 14 participating Pennsylvania public high schools. Researchers compared the two groups using mixed-effects logistic regression.
They found that adolescents in the universal screening intervention group had 5.92 times higher odds (95% confidence interval [CI], 5.07-6.93) of being identified with MDD symptoms, 3.30 times higher odds (95% CI, 2.49-4.38) of the Student Assistance Program (SAP) confirming follow-up needs, and 2.07 times higher odds (95% CI, 1.39-3.10) of starting MDD treatment.
The study comprised 12,909 students, with an average age of 16 years. Of those students, 2,687 (20.8%) were Hispanic; 2,891 (22.4%) were non-Hispanic Black, 5,842 (45.3%) were non-Hispanic White; and 1,489 (11.5%) were multiracial or of other race or ethnicity.
In the universal screening intervention (n = 6,473) all students completed the Patient Health Questionnaire–9 (PHQ-9). Students who screened positive proceeded to the Student Assistance Program. Students could receive a targeted referral to SAP if they had concerning behavior beyond the PHQ-9.
In the targeted screening group (n = 6,436), students with behaviors prompting concern for MDD were referred to the Student Assistance Program (SAP), mandated in all Pennsylvania schools. The SAP determined follow-up.
The U.S. Preventive Services Task Force (USPSTF) endorsed primary care screening in 2009 and again in 2016 for all adolescents 12-18 years old.
However, the study authors wrote, most U.S. adolescents (more than 60%) don’t have routine access to preventive health care, which limits primary care offices’ ability to properly address the growing numbers.
“[S]creening is inconsistent, with inequalities by race and ethnicity and region, and potential worsening with the COVID-19 pandemic,” they noted.
Depression rates see sharp increase
Meanwhile, the prevalence of adolescents reporting MDD symptoms has “nearly doubled in the last decade, increasing from 8.3% in 2008 to 14.4% in 2018.”
The American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and Children’s Hospital Association recently declared a national emergency in children’s mental health, citing COVID-19’s toll on top of existing challenges.
This study provides further evidence that universal screening is the better approach to identify and treat adolescent depression to save lives, Andres Pumariega, MD, a child and adolescent psychiatrist at University of Florida in Gainesville, told this news organization.
“If you catch these kids early, you can prevent suicide attempts and suicide. You can also prevent complicating costs of care,” he said.
He noted the universal screening removes the potential for bias.
“Relying purely on referral and clinical identification means a lot of kids in minority groups will not be identified and will not be treated accurately. Many clinicians have a problem identifying depression in diverse kids,” he said.
Pushback for universal screening likely
However, he said he has been part of such efforts to implement such programs in Mexico and the United States and said in the Unites States, the political climate will guarantee pushback from having schools more involved in health care and prevention. Recent controversy around COVID-19 vaccines for children illustrates the potential backlash, he said.
Parents often fight such programs as attempts to “label” their children, he said.
“If I have cancer, I sure want to be labeled. A label is used to get them help. We need to find ways to educate parents and support them in facing these issues,” he added.
One concern he has with this intervention is having the SAPs, composed largely of nonclinicians, be the triage point “instead of doing that objectively through objective criteria and by clinicians,” he said. “If we are to have a comprehensive health system where we can serve all kids and manage costs, schools need to be a major part of it.”
School settings offer the chance to see more children, collaborate with teachers and counselors, and integrate results with educational outcomes, he added.
In the study by Sekhar and colleagues, 7 of the 14 schools were classified as urban, with a median size of 370 students.
Researchers noted that the benefit of the universal screening is likely understated because of COVID-19–related school closures during the study period. The closures meant screening wasn’t completed for 7% of students.
The authors concluded that universal screening finds teens living with depression who otherwise would not be found. They said such a program likely works best in schools with strong SAP.
“Adolescents’ consistent contact with schools has been used to support physical health screenings that affect academic success,” the authors wrote. “Major depressive disorder similarly affects academic success, suggesting school-based screening may be especially beneficial.”
In the past 3 years, Dr. Sekhar reported receiving funding from Pfizer through the American Academy of Pediatrics, the Penn State Clinical and Translational Science Awards Program, and a Eugene Washington Patient-Centered Outcomes Research Institute Engagement Award. Full disclosures for coauthors are available in the journal article.
This work was supported in part by the Patient-Centered Outcomes Research Institute. The use of REDCap (Research Electronic Data Capture) in this project was supported by the National Institutes of Health.
JAMA NETWORK OPEN
ACIP recommends universal HBV vaccination for adults under 60, expands recommendations for vaccines against orthopoxviruses and Ebola
The group also voted to expand recommendations for vaccinating people at risk for occupational exposure to Ebola and to recommend Jynneos, a smallpox and monkeypox vaccine, for at-risk populations.
The recommendations were approved Nov. 3.
Previously, ACIP recommended HBV vaccination for unvaccinated adults at increased risk for infection because of sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, HIV infection, and travel to areas with high to intermediate levels of HBV infection. But experts agreed a new strategy was needed, as previously falling rates of HBV have plateaued. “The past decade has illustrated that risk-based screening has got us as far as it can take us,” Mark Weng, MD, a lieutenant commander in the U.S. Public Health Service and lead of the ACIP Hepatitis Vaccine Working Group, said during the meeting.
There are 1.9 million people living with chronic HBV in the United States, with over 20,000 new acute infections every year. Rates are highest among those in their 40s and 50s, Dr. Weng noted.
The group debated whether to apply the universal recommendation to all ages, but in a close vote (eight yes, seven no), ACIP included an age cutoff of 59. The majority argued that adults 60 and older are at lower risk for infection and vaccination efforts targeting younger adults would be more effective. Those 60 and older would continue to follow the risk-based guidelines, but anyone, regardless of age, can receive the vaccine if they wish to be protected, the group added.
The CDC director as well as several professional societies need to approve the recommendation before it becomes public policy.
ACIP also voted to recommend the following:
- Adding updated recommendations to the 2022 immunization schedules for children, adolescents, and adults, including dengue vaccination for children aged 9-16 years in endemic areas and in adults over 65 and those aged 19-64 with certain chronic conditions.
- The use of Jynneos, a smallpox and monkeypox vaccine, as an alternative to ACAM2000 for those at risk for occupational exposure.
- Pre-exposure vaccination of health care personnel involved in the transport and treatment of suspected Ebola patients at special treatment centers, or lab and support staff working with or handling specimens that may contain the Ebola virus.
A version of this article first appeared on Medscape.com.
The group also voted to expand recommendations for vaccinating people at risk for occupational exposure to Ebola and to recommend Jynneos, a smallpox and monkeypox vaccine, for at-risk populations.
The recommendations were approved Nov. 3.
Previously, ACIP recommended HBV vaccination for unvaccinated adults at increased risk for infection because of sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, HIV infection, and travel to areas with high to intermediate levels of HBV infection. But experts agreed a new strategy was needed, as previously falling rates of HBV have plateaued. “The past decade has illustrated that risk-based screening has got us as far as it can take us,” Mark Weng, MD, a lieutenant commander in the U.S. Public Health Service and lead of the ACIP Hepatitis Vaccine Working Group, said during the meeting.
There are 1.9 million people living with chronic HBV in the United States, with over 20,000 new acute infections every year. Rates are highest among those in their 40s and 50s, Dr. Weng noted.
The group debated whether to apply the universal recommendation to all ages, but in a close vote (eight yes, seven no), ACIP included an age cutoff of 59. The majority argued that adults 60 and older are at lower risk for infection and vaccination efforts targeting younger adults would be more effective. Those 60 and older would continue to follow the risk-based guidelines, but anyone, regardless of age, can receive the vaccine if they wish to be protected, the group added.
The CDC director as well as several professional societies need to approve the recommendation before it becomes public policy.
ACIP also voted to recommend the following:
- Adding updated recommendations to the 2022 immunization schedules for children, adolescents, and adults, including dengue vaccination for children aged 9-16 years in endemic areas and in adults over 65 and those aged 19-64 with certain chronic conditions.
- The use of Jynneos, a smallpox and monkeypox vaccine, as an alternative to ACAM2000 for those at risk for occupational exposure.
- Pre-exposure vaccination of health care personnel involved in the transport and treatment of suspected Ebola patients at special treatment centers, or lab and support staff working with or handling specimens that may contain the Ebola virus.
A version of this article first appeared on Medscape.com.
The group also voted to expand recommendations for vaccinating people at risk for occupational exposure to Ebola and to recommend Jynneos, a smallpox and monkeypox vaccine, for at-risk populations.
The recommendations were approved Nov. 3.
Previously, ACIP recommended HBV vaccination for unvaccinated adults at increased risk for infection because of sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, HIV infection, and travel to areas with high to intermediate levels of HBV infection. But experts agreed a new strategy was needed, as previously falling rates of HBV have plateaued. “The past decade has illustrated that risk-based screening has got us as far as it can take us,” Mark Weng, MD, a lieutenant commander in the U.S. Public Health Service and lead of the ACIP Hepatitis Vaccine Working Group, said during the meeting.
There are 1.9 million people living with chronic HBV in the United States, with over 20,000 new acute infections every year. Rates are highest among those in their 40s and 50s, Dr. Weng noted.
The group debated whether to apply the universal recommendation to all ages, but in a close vote (eight yes, seven no), ACIP included an age cutoff of 59. The majority argued that adults 60 and older are at lower risk for infection and vaccination efforts targeting younger adults would be more effective. Those 60 and older would continue to follow the risk-based guidelines, but anyone, regardless of age, can receive the vaccine if they wish to be protected, the group added.
The CDC director as well as several professional societies need to approve the recommendation before it becomes public policy.
ACIP also voted to recommend the following:
- Adding updated recommendations to the 2022 immunization schedules for children, adolescents, and adults, including dengue vaccination for children aged 9-16 years in endemic areas and in adults over 65 and those aged 19-64 with certain chronic conditions.
- The use of Jynneos, a smallpox and monkeypox vaccine, as an alternative to ACAM2000 for those at risk for occupational exposure.
- Pre-exposure vaccination of health care personnel involved in the transport and treatment of suspected Ebola patients at special treatment centers, or lab and support staff working with or handling specimens that may contain the Ebola virus.
A version of this article first appeared on Medscape.com.
Pfizer says its COVID-19 pill is highly effective
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
How applicable is ISCHEMIA trial to U.S. clinical practice?
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
Patients went into the hospital for care. After testing positive there for COVID, some never came out.
They went into hospitals with heart attacks, kidney failure or in a psychiatric crisis.
They left with COVID-19 — if they left at all.
More than 10,000 patients were diagnosed with COVID in a U.S. hospital last year after they were admitted for something else, according to federal and state records analyzed exclusively for KHN. The number is certainly an undercount, since it includes mostly patients 65 and older, plus California and Florida patients of all ages.
Yet in the scheme of things that can go wrong in a hospital, it is catastrophic: About 21% of the patients who contracted COVID in the hospital from April to September last year died, the data shows. In contrast, nearly 8% of other Medicare patients died in the hospital at the time.
Steven Johnson, 66, was expecting to get an infection cut out of his hip flesh and bone at Blake Medical Center in Bradenton, Fla., last November. The retired pharmacist had survived colon cancer and was meticulous to avoid contracting COVID. He could not have known that, from April through September, 8% of that hospital’s Medicare COVID patients were diagnosed with the virus after they were admitted for another concern.
Mr. Johnson had tested negative for COVID two days before he was admitted. After 13 days in the hospital, he tested positive, said his wife, Cindy Johnson, also a retired pharmacist.
Soon he was struggling to clear a glue-like phlegm from his lungs. A medical team could hardly control his pain. They prompted Cindy to share his final wishes. She asked: “Honey, do you want to be intubated?” He responded with an emphatic “no.” He died three days later.
After her husband tested positive, Cindy Johnson, trained in contact tracing, quickly got a COVID test. She tested negative. Then she thought about the large number of hospital staffers flowing into and out of his room — where he was often unmasked — and suspected a staff member had infected him. That the hospital, part of the HCA Healthcare chain, still has not mandated staff vaccinations is “appalling,” she said.
“I’m furious,” she said.
“How can they say on their website,” she asked, “that the safety precautions ‘we’ve put into place make our facilities among the safest possible places to receive healthcare at this time’?”
Blake Medical Center spokesperson Lisa Kirkland said the hospital is “strongly encouraging vaccination” and noted that it follows Centers for Disease Control and Prevention and federal and state guidelines to protect patients. President Joe Biden has called for all hospital employees to be vaccinated, but the requirement could face resistance in a dozen states, including Florida, that have banned vaccine mandates.
Overall, the rate of in-hospital spread among Medicare and other patients was lower than in other countries, including the United Kingdom, which makes such data public and openly discusses it. On average, about 1.7% of U.S. hospitalized COVID patients were diagnosed with the virus in U.S. hospitals, according to an analysis of Medicare records from April 1 to Sept. 30, 2020, provided by Dr. James Kennedy, founder of CDIMD, a Nashville-based consulting and data analytics company.
Yet the rate of infection was far higher in 38 hospitals where 5% or more of the Medicare COVID cases were documented as hospital-acquired. The data is from a challenging stretch last year when protective gear was in short supply and tests were scarce or slow to produce results. The Medicare data for the fourth quarter of 2020 and this year isn’t available yet, and the state data reflects April 1 through Dec. 31, 2020.
A KHN review of work-safety records, medical literature and interviews with staff at high-spread hospitals points to why the virus took hold: Hospital leaders were slow to appreciate its airborne nature, which made coughing patients hazardous to roommates and staff members, who often wore less-protective surgical masks instead of N95s. Hospitals failed to test every admitted patient, enabled by CDC guidance that leaves such testing to the “discretion of the facility.” Management often failed to inform workers when they’d been exposed to COVID and so were at risk of spreading it themselves.
Spread among patients and staffers seemed to go hand in hand. At Beaumont Hospital, Taylor, in Michigan, 139 employee COVID infections were logged between April 6 to Oct. 20 last year, a hospital inspection report shows. Nearly 7% of the Medicare patients with COVID tested positive after they were admitted to that hospital for something else, the federal data shows. A hospital spokesperson said tests were not available to screen all patients last year, resulting in some late diagnoses. He said all incoming patients are tested now.
Tracking COVID inside health facilities is no new task to federal officials, who publicly report new staff and resident cases weekly for each U.S. nursing home. Yet the Department of Health and Human Services reports data on COVID’s spread in hospitals only on a statewide basis, so patients are in the dark about which facilities have cases.
KHN commissioned analyses of hospital billing records, which are also used more broadly to spot various hospital-acquired infections. For COVID, the data has limitations. It can pick up some community-acquired cases that were slow to show up, as it can take two to 14 days from exposure to the virus for symptoms to appear, with the average being four to five days. The records do not account for cases picked up in an emergency room or diagnosed after a hospital patient was discharged.
Linda Moore, 71, tested positive at least 15 days into a hospital stay for spinal surgery, according to her daughter Trisha Tavolazzi. Her mother was at Havasu Regional Medical Center in Lake Havasu City, Ariz., which did not have a higher-than-average rate of internal spread last summer.
The hospital implemented “rigorous health and safety protocols to protect all of our patients” during the pandemic, said hospital spokesperson Corey Santoriello, who would not comment on Ms. Moore’s case, citing privacy laws.
Ms. Moore was airlifted to another hospital, where her condition only declined further, her daughter said. After the ventilator was removed, she clung to life fitfully for 5½ hours, as her daughter prayed for her mother to find her way to heaven.
“I asked her mom and her dad and her family and prayed to God, ‘Please just come show her the way,’” Ms. Tavolazzi said. “I relive it every day.”
When Ms. Tavolazzi sought answers from the hospital about where her mom got the virus, she said, she got none: “No one ever called me back.”
Two negative COVID tests, then ‘patient zero’
As the second surge of COVID subsided last September, doctors from the prestigious Brigham and Women’s Hospital published a reassuringstudy: With careful infection control, only two of 697 COVID patients acquired the virus within the Boston hospital. That is about 0.3% of patients --about six times lower than the overall Medicare rate. Brigham tested every patient it admitted, exceeding CDC recommendations. It was transparent and open about safety concerns.
But the study, published in the high-profile JAMA Network Open journal, conveyed the wrong message, according to Dr. Manoj Jain, an infectious-disease physician and adjunct professor at the Rollins School of Public Health at Emory University. COVID was spreading in hospitals, he said, and the study buried “the problem under the rug.”
Before the virtual ink on the study was dry, the virus began a stealthy streak through the elite hospital. It slipped in with a patient who tested negative twice -- but turned out to be positive. She was “patient zero” in an outbreak affecting 38 staffers and 14 patients, according to a study in Annals of Internal Medicine initially published Feb. 9.
That study’s authors sequenced the genome of the virus to confirm which cases were related and precisely how it traveled through the hospital.
As patients were moved from room to room in the early days of the outbreak, COVID spread among roommates 8 out of 9 times, likely through aerosol transmission, the study says. A survey of staff members revealed that those caring for coughing patients were more likely to get sick.
The virus also appeared to have breached the CDC-OK’d protective gear. Two staff members who had close patient contact while wearing a surgical mask and face shield still wound up infected. The findings suggested that more-protective N95 respirators could help safeguard staff.
Brigham and Women’s now tests every patient upon admission and again soon after. Nurses are encouraged to test again if they see a subtle sign of COVID, said Dr. Erica Shenoy, associate chief of the Infection Control Unit at Massachusetts General Hospital, who helped craft policy at Brigham.
She said nurses and environmental services workers are at the table for policymaking: “I personally make it a point to say, ‘Tell me what you’re thinking,’” Dr. Shenoy said. “‘There’s no retribution because we need to know.’”
CDC guidelines, though, left wide latitude on protective gear and testing. To this day, Dr. Shenoy said, hospitals employ a wide range of policies.
The CDC said in a statement that its guidelines “provide a comprehensive and layered approach to preventing transmission of SARS-CoV-2 in healthcare settings,” and include testing patients with “even mild symptoms” or recent exposure to someone with COVID.
Infection control policies are rarely apparent to patients or visitors, beyond whether they’re asked to wear a mask. But reviews of public records and interviews with more than a dozen people show that at hospitals with high rates of COVID spread, staff members were often alarmed by the lack of safety practices.
Nurses sound the alarm on COVID spread
As COVID crept into Florida in spring 2020, nurse Victoria Holland clashed with managers at Blake Medical Center in Bradenton, where Steven Johnson died.
She said managers suspended her early in the pandemic after taking part in a protest and “having a hissy fit” when she was denied a new N95 respirator before an “aerosol-generating” procedure. The CDC warns that such procedures can spread the virus through the air. Before the pandemic, nurses were trained to dispose of an N95 after each patient encounter.
When the suspension was over, Ms. Holland said, she felt unsafe. “They told us nothing,” she said. “It was all a little whisper between the doctors. You had potential COVIDs and you’d get a little surgical mask because [they didn’t] want to waste” an N95 unless they knew the patient was positive.
Ms. Holland said she quit in mid-April. Her nursing colleagues lodged a complaint with the Occupational Safety and Health Administration in late June alleging that staff “working around possible COVID-19 positive cases” had been denied PPE. Staff members protested outside the hospital in July and filed another OSHA complaint that said the hospital was allowing COVID-exposed employees to keep working.
Ms. Kirkland, the Blake spokesperson, said the hospital responded to OSHA and “no deficiencies were identified.”
The Medicare analysis shows that 22 of 273 patients with COVID, or 8%, were diagnosed with the virus after they were admitted to Blake. That’s about five times as high as the national average.
Ms. Kirkland said “there is no standard way for measuring COVID-19 hospital-associated transmissions” and “there is no evidence to suggest the risk of transmission at Blake Medical Center is different than what you would find at other hospitals.”
In Washington, D.C., 34 Medicare COVID patients contracted the virus at MedStar Washington Hospital Center, or nearly 6% of its total, the analysis shows.
Unhappy with the safety practices — which included gas sterilization and reuse of N95s — National Nurses United members protested on the hospital lawn in July 2020. At the protest, nurse Zoe Bendixen said one nurse had died of the virus and 50 had gotten sick: “[Nurses] can become a source for spreading the disease to other patients, co-workers and family members.”
Nurse Yuhana Gidey said she caught COVID after treating a patient who turned out to be infected. Another nurse, not managers doing contact tracing, told her she’d been exposed, she said.
Nurse Kimberly Walsh said in an interview there was an outbreak in a geriatric unit where she worked in September 2020. She said management blamed nurses for bringing the virus into the unit. But Ms. Walsh pointed to another problem: The hospital wasn’t COVID-testing patients coming in from nursing homes, where spread was rampant last year.
MedStar declined a request for an interview about its infection control practices and did not respond to specific questions.
While hospitals must track and publicly report rates of persistent infections like C. diff, antibiotic-resistant staph and surgical site infections, similar hospital-acquired COVID rates are not reported.
KHN examined a different source of data that Congress required hospitals to document about “hospital-acquired conditions.” The Medicare data, which notes whether each COVID case was “present on admission” or not, becomes available months after a hospitalization in obscure files that require a data-use agreement typically granted to researchers. KHN counted cases, as federal officials do, in some instances in which the documentation is deemed insufficient to categorize a case (see data methodology on the KHN website).
For this data, whether to deem a COVID case hospital-acquired lies with medical coders who review doctors’ notes and discharge summaries and ask doctors questions if the status is unclear, said Sue Bowman, senior director of coding policy and compliance at American Health Information Management Association.
She said medical coders are aware that the data is used for hospital quality measures and would be careful to review the contract tracing or other information in the medical record.
If a case was in the data KHN used, “that would mean it was acquired during the hospital stay either from a health care worker or another patient or maybe if a hospital allowed visitors, from a visitor,” Ms. Bowman said. “That would be a fair interpretation of the data.”
The high death rate for those diagnosed with COVID during a hospital stay — about 21% — mirrors the death rate for other Medicare COVID patients last year, when doctors had few proven methods to help patients. It also highlights the hazard unvaccinated staffers pose to patients, said Dr. Jain, the infectious-disease doctor. The American Hospital Association estimates that about 42% of U.S. hospitals have mandated that all staff members be vaccinated.
“We don’t need [unvaccinated staff] to be a threat to patients,” Dr. Jain said. “[Hospital] administration is too afraid to push the nursing staff, and the general public is clueless at what a threat a non-vaccinated person poses to a vulnerable population.”
Cindy Johnson said the hospital where she believes her husband contracted COVID faced minimal scrutiny in a state inspection, even after she said she reported that he caught COVID there. She explored suing, but an attorney told her it would be nearly impossible to win such a case. A 2021 state law requires proof of “at least gross negligence” to prevail in court.
Ms. Johnson did ask a doctor who sees patients at the hospital for this: Please take down the big “OPEN & SAFE” sign outside.
Within days, the sign was gone.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
They went into hospitals with heart attacks, kidney failure or in a psychiatric crisis.
They left with COVID-19 — if they left at all.
More than 10,000 patients were diagnosed with COVID in a U.S. hospital last year after they were admitted for something else, according to federal and state records analyzed exclusively for KHN. The number is certainly an undercount, since it includes mostly patients 65 and older, plus California and Florida patients of all ages.
Yet in the scheme of things that can go wrong in a hospital, it is catastrophic: About 21% of the patients who contracted COVID in the hospital from April to September last year died, the data shows. In contrast, nearly 8% of other Medicare patients died in the hospital at the time.
Steven Johnson, 66, was expecting to get an infection cut out of his hip flesh and bone at Blake Medical Center in Bradenton, Fla., last November. The retired pharmacist had survived colon cancer and was meticulous to avoid contracting COVID. He could not have known that, from April through September, 8% of that hospital’s Medicare COVID patients were diagnosed with the virus after they were admitted for another concern.
Mr. Johnson had tested negative for COVID two days before he was admitted. After 13 days in the hospital, he tested positive, said his wife, Cindy Johnson, also a retired pharmacist.
Soon he was struggling to clear a glue-like phlegm from his lungs. A medical team could hardly control his pain. They prompted Cindy to share his final wishes. She asked: “Honey, do you want to be intubated?” He responded with an emphatic “no.” He died three days later.
After her husband tested positive, Cindy Johnson, trained in contact tracing, quickly got a COVID test. She tested negative. Then she thought about the large number of hospital staffers flowing into and out of his room — where he was often unmasked — and suspected a staff member had infected him. That the hospital, part of the HCA Healthcare chain, still has not mandated staff vaccinations is “appalling,” she said.
“I’m furious,” she said.
“How can they say on their website,” she asked, “that the safety precautions ‘we’ve put into place make our facilities among the safest possible places to receive healthcare at this time’?”
Blake Medical Center spokesperson Lisa Kirkland said the hospital is “strongly encouraging vaccination” and noted that it follows Centers for Disease Control and Prevention and federal and state guidelines to protect patients. President Joe Biden has called for all hospital employees to be vaccinated, but the requirement could face resistance in a dozen states, including Florida, that have banned vaccine mandates.
Overall, the rate of in-hospital spread among Medicare and other patients was lower than in other countries, including the United Kingdom, which makes such data public and openly discusses it. On average, about 1.7% of U.S. hospitalized COVID patients were diagnosed with the virus in U.S. hospitals, according to an analysis of Medicare records from April 1 to Sept. 30, 2020, provided by Dr. James Kennedy, founder of CDIMD, a Nashville-based consulting and data analytics company.
Yet the rate of infection was far higher in 38 hospitals where 5% or more of the Medicare COVID cases were documented as hospital-acquired. The data is from a challenging stretch last year when protective gear was in short supply and tests were scarce or slow to produce results. The Medicare data for the fourth quarter of 2020 and this year isn’t available yet, and the state data reflects April 1 through Dec. 31, 2020.
A KHN review of work-safety records, medical literature and interviews with staff at high-spread hospitals points to why the virus took hold: Hospital leaders were slow to appreciate its airborne nature, which made coughing patients hazardous to roommates and staff members, who often wore less-protective surgical masks instead of N95s. Hospitals failed to test every admitted patient, enabled by CDC guidance that leaves such testing to the “discretion of the facility.” Management often failed to inform workers when they’d been exposed to COVID and so were at risk of spreading it themselves.
Spread among patients and staffers seemed to go hand in hand. At Beaumont Hospital, Taylor, in Michigan, 139 employee COVID infections were logged between April 6 to Oct. 20 last year, a hospital inspection report shows. Nearly 7% of the Medicare patients with COVID tested positive after they were admitted to that hospital for something else, the federal data shows. A hospital spokesperson said tests were not available to screen all patients last year, resulting in some late diagnoses. He said all incoming patients are tested now.
Tracking COVID inside health facilities is no new task to federal officials, who publicly report new staff and resident cases weekly for each U.S. nursing home. Yet the Department of Health and Human Services reports data on COVID’s spread in hospitals only on a statewide basis, so patients are in the dark about which facilities have cases.
KHN commissioned analyses of hospital billing records, which are also used more broadly to spot various hospital-acquired infections. For COVID, the data has limitations. It can pick up some community-acquired cases that were slow to show up, as it can take two to 14 days from exposure to the virus for symptoms to appear, with the average being four to five days. The records do not account for cases picked up in an emergency room or diagnosed after a hospital patient was discharged.
Linda Moore, 71, tested positive at least 15 days into a hospital stay for spinal surgery, according to her daughter Trisha Tavolazzi. Her mother was at Havasu Regional Medical Center in Lake Havasu City, Ariz., which did not have a higher-than-average rate of internal spread last summer.
The hospital implemented “rigorous health and safety protocols to protect all of our patients” during the pandemic, said hospital spokesperson Corey Santoriello, who would not comment on Ms. Moore’s case, citing privacy laws.
Ms. Moore was airlifted to another hospital, where her condition only declined further, her daughter said. After the ventilator was removed, she clung to life fitfully for 5½ hours, as her daughter prayed for her mother to find her way to heaven.
“I asked her mom and her dad and her family and prayed to God, ‘Please just come show her the way,’” Ms. Tavolazzi said. “I relive it every day.”
When Ms. Tavolazzi sought answers from the hospital about where her mom got the virus, she said, she got none: “No one ever called me back.”
Two negative COVID tests, then ‘patient zero’
As the second surge of COVID subsided last September, doctors from the prestigious Brigham and Women’s Hospital published a reassuringstudy: With careful infection control, only two of 697 COVID patients acquired the virus within the Boston hospital. That is about 0.3% of patients --about six times lower than the overall Medicare rate. Brigham tested every patient it admitted, exceeding CDC recommendations. It was transparent and open about safety concerns.
But the study, published in the high-profile JAMA Network Open journal, conveyed the wrong message, according to Dr. Manoj Jain, an infectious-disease physician and adjunct professor at the Rollins School of Public Health at Emory University. COVID was spreading in hospitals, he said, and the study buried “the problem under the rug.”
Before the virtual ink on the study was dry, the virus began a stealthy streak through the elite hospital. It slipped in with a patient who tested negative twice -- but turned out to be positive. She was “patient zero” in an outbreak affecting 38 staffers and 14 patients, according to a study in Annals of Internal Medicine initially published Feb. 9.
That study’s authors sequenced the genome of the virus to confirm which cases were related and precisely how it traveled through the hospital.
As patients were moved from room to room in the early days of the outbreak, COVID spread among roommates 8 out of 9 times, likely through aerosol transmission, the study says. A survey of staff members revealed that those caring for coughing patients were more likely to get sick.
The virus also appeared to have breached the CDC-OK’d protective gear. Two staff members who had close patient contact while wearing a surgical mask and face shield still wound up infected. The findings suggested that more-protective N95 respirators could help safeguard staff.
Brigham and Women’s now tests every patient upon admission and again soon after. Nurses are encouraged to test again if they see a subtle sign of COVID, said Dr. Erica Shenoy, associate chief of the Infection Control Unit at Massachusetts General Hospital, who helped craft policy at Brigham.
She said nurses and environmental services workers are at the table for policymaking: “I personally make it a point to say, ‘Tell me what you’re thinking,’” Dr. Shenoy said. “‘There’s no retribution because we need to know.’”
CDC guidelines, though, left wide latitude on protective gear and testing. To this day, Dr. Shenoy said, hospitals employ a wide range of policies.
The CDC said in a statement that its guidelines “provide a comprehensive and layered approach to preventing transmission of SARS-CoV-2 in healthcare settings,” and include testing patients with “even mild symptoms” or recent exposure to someone with COVID.
Infection control policies are rarely apparent to patients or visitors, beyond whether they’re asked to wear a mask. But reviews of public records and interviews with more than a dozen people show that at hospitals with high rates of COVID spread, staff members were often alarmed by the lack of safety practices.
Nurses sound the alarm on COVID spread
As COVID crept into Florida in spring 2020, nurse Victoria Holland clashed with managers at Blake Medical Center in Bradenton, where Steven Johnson died.
She said managers suspended her early in the pandemic after taking part in a protest and “having a hissy fit” when she was denied a new N95 respirator before an “aerosol-generating” procedure. The CDC warns that such procedures can spread the virus through the air. Before the pandemic, nurses were trained to dispose of an N95 after each patient encounter.
When the suspension was over, Ms. Holland said, she felt unsafe. “They told us nothing,” she said. “It was all a little whisper between the doctors. You had potential COVIDs and you’d get a little surgical mask because [they didn’t] want to waste” an N95 unless they knew the patient was positive.
Ms. Holland said she quit in mid-April. Her nursing colleagues lodged a complaint with the Occupational Safety and Health Administration in late June alleging that staff “working around possible COVID-19 positive cases” had been denied PPE. Staff members protested outside the hospital in July and filed another OSHA complaint that said the hospital was allowing COVID-exposed employees to keep working.
Ms. Kirkland, the Blake spokesperson, said the hospital responded to OSHA and “no deficiencies were identified.”
The Medicare analysis shows that 22 of 273 patients with COVID, or 8%, were diagnosed with the virus after they were admitted to Blake. That’s about five times as high as the national average.
Ms. Kirkland said “there is no standard way for measuring COVID-19 hospital-associated transmissions” and “there is no evidence to suggest the risk of transmission at Blake Medical Center is different than what you would find at other hospitals.”
In Washington, D.C., 34 Medicare COVID patients contracted the virus at MedStar Washington Hospital Center, or nearly 6% of its total, the analysis shows.
Unhappy with the safety practices — which included gas sterilization and reuse of N95s — National Nurses United members protested on the hospital lawn in July 2020. At the protest, nurse Zoe Bendixen said one nurse had died of the virus and 50 had gotten sick: “[Nurses] can become a source for spreading the disease to other patients, co-workers and family members.”
Nurse Yuhana Gidey said she caught COVID after treating a patient who turned out to be infected. Another nurse, not managers doing contact tracing, told her she’d been exposed, she said.
Nurse Kimberly Walsh said in an interview there was an outbreak in a geriatric unit where she worked in September 2020. She said management blamed nurses for bringing the virus into the unit. But Ms. Walsh pointed to another problem: The hospital wasn’t COVID-testing patients coming in from nursing homes, where spread was rampant last year.
MedStar declined a request for an interview about its infection control practices and did not respond to specific questions.
While hospitals must track and publicly report rates of persistent infections like C. diff, antibiotic-resistant staph and surgical site infections, similar hospital-acquired COVID rates are not reported.
KHN examined a different source of data that Congress required hospitals to document about “hospital-acquired conditions.” The Medicare data, which notes whether each COVID case was “present on admission” or not, becomes available months after a hospitalization in obscure files that require a data-use agreement typically granted to researchers. KHN counted cases, as federal officials do, in some instances in which the documentation is deemed insufficient to categorize a case (see data methodology on the KHN website).
For this data, whether to deem a COVID case hospital-acquired lies with medical coders who review doctors’ notes and discharge summaries and ask doctors questions if the status is unclear, said Sue Bowman, senior director of coding policy and compliance at American Health Information Management Association.
She said medical coders are aware that the data is used for hospital quality measures and would be careful to review the contract tracing or other information in the medical record.
If a case was in the data KHN used, “that would mean it was acquired during the hospital stay either from a health care worker or another patient or maybe if a hospital allowed visitors, from a visitor,” Ms. Bowman said. “That would be a fair interpretation of the data.”
The high death rate for those diagnosed with COVID during a hospital stay — about 21% — mirrors the death rate for other Medicare COVID patients last year, when doctors had few proven methods to help patients. It also highlights the hazard unvaccinated staffers pose to patients, said Dr. Jain, the infectious-disease doctor. The American Hospital Association estimates that about 42% of U.S. hospitals have mandated that all staff members be vaccinated.
“We don’t need [unvaccinated staff] to be a threat to patients,” Dr. Jain said. “[Hospital] administration is too afraid to push the nursing staff, and the general public is clueless at what a threat a non-vaccinated person poses to a vulnerable population.”
Cindy Johnson said the hospital where she believes her husband contracted COVID faced minimal scrutiny in a state inspection, even after she said she reported that he caught COVID there. She explored suing, but an attorney told her it would be nearly impossible to win such a case. A 2021 state law requires proof of “at least gross negligence” to prevail in court.
Ms. Johnson did ask a doctor who sees patients at the hospital for this: Please take down the big “OPEN & SAFE” sign outside.
Within days, the sign was gone.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
They went into hospitals with heart attacks, kidney failure or in a psychiatric crisis.
They left with COVID-19 — if they left at all.
More than 10,000 patients were diagnosed with COVID in a U.S. hospital last year after they were admitted for something else, according to federal and state records analyzed exclusively for KHN. The number is certainly an undercount, since it includes mostly patients 65 and older, plus California and Florida patients of all ages.
Yet in the scheme of things that can go wrong in a hospital, it is catastrophic: About 21% of the patients who contracted COVID in the hospital from April to September last year died, the data shows. In contrast, nearly 8% of other Medicare patients died in the hospital at the time.
Steven Johnson, 66, was expecting to get an infection cut out of his hip flesh and bone at Blake Medical Center in Bradenton, Fla., last November. The retired pharmacist had survived colon cancer and was meticulous to avoid contracting COVID. He could not have known that, from April through September, 8% of that hospital’s Medicare COVID patients were diagnosed with the virus after they were admitted for another concern.
Mr. Johnson had tested negative for COVID two days before he was admitted. After 13 days in the hospital, he tested positive, said his wife, Cindy Johnson, also a retired pharmacist.
Soon he was struggling to clear a glue-like phlegm from his lungs. A medical team could hardly control his pain. They prompted Cindy to share his final wishes. She asked: “Honey, do you want to be intubated?” He responded with an emphatic “no.” He died three days later.
After her husband tested positive, Cindy Johnson, trained in contact tracing, quickly got a COVID test. She tested negative. Then she thought about the large number of hospital staffers flowing into and out of his room — where he was often unmasked — and suspected a staff member had infected him. That the hospital, part of the HCA Healthcare chain, still has not mandated staff vaccinations is “appalling,” she said.
“I’m furious,” she said.
“How can they say on their website,” she asked, “that the safety precautions ‘we’ve put into place make our facilities among the safest possible places to receive healthcare at this time’?”
Blake Medical Center spokesperson Lisa Kirkland said the hospital is “strongly encouraging vaccination” and noted that it follows Centers for Disease Control and Prevention and federal and state guidelines to protect patients. President Joe Biden has called for all hospital employees to be vaccinated, but the requirement could face resistance in a dozen states, including Florida, that have banned vaccine mandates.
Overall, the rate of in-hospital spread among Medicare and other patients was lower than in other countries, including the United Kingdom, which makes such data public and openly discusses it. On average, about 1.7% of U.S. hospitalized COVID patients were diagnosed with the virus in U.S. hospitals, according to an analysis of Medicare records from April 1 to Sept. 30, 2020, provided by Dr. James Kennedy, founder of CDIMD, a Nashville-based consulting and data analytics company.
Yet the rate of infection was far higher in 38 hospitals where 5% or more of the Medicare COVID cases were documented as hospital-acquired. The data is from a challenging stretch last year when protective gear was in short supply and tests were scarce or slow to produce results. The Medicare data for the fourth quarter of 2020 and this year isn’t available yet, and the state data reflects April 1 through Dec. 31, 2020.
A KHN review of work-safety records, medical literature and interviews with staff at high-spread hospitals points to why the virus took hold: Hospital leaders were slow to appreciate its airborne nature, which made coughing patients hazardous to roommates and staff members, who often wore less-protective surgical masks instead of N95s. Hospitals failed to test every admitted patient, enabled by CDC guidance that leaves such testing to the “discretion of the facility.” Management often failed to inform workers when they’d been exposed to COVID and so were at risk of spreading it themselves.
Spread among patients and staffers seemed to go hand in hand. At Beaumont Hospital, Taylor, in Michigan, 139 employee COVID infections were logged between April 6 to Oct. 20 last year, a hospital inspection report shows. Nearly 7% of the Medicare patients with COVID tested positive after they were admitted to that hospital for something else, the federal data shows. A hospital spokesperson said tests were not available to screen all patients last year, resulting in some late diagnoses. He said all incoming patients are tested now.
Tracking COVID inside health facilities is no new task to federal officials, who publicly report new staff and resident cases weekly for each U.S. nursing home. Yet the Department of Health and Human Services reports data on COVID’s spread in hospitals only on a statewide basis, so patients are in the dark about which facilities have cases.
KHN commissioned analyses of hospital billing records, which are also used more broadly to spot various hospital-acquired infections. For COVID, the data has limitations. It can pick up some community-acquired cases that were slow to show up, as it can take two to 14 days from exposure to the virus for symptoms to appear, with the average being four to five days. The records do not account for cases picked up in an emergency room or diagnosed after a hospital patient was discharged.
Linda Moore, 71, tested positive at least 15 days into a hospital stay for spinal surgery, according to her daughter Trisha Tavolazzi. Her mother was at Havasu Regional Medical Center in Lake Havasu City, Ariz., which did not have a higher-than-average rate of internal spread last summer.
The hospital implemented “rigorous health and safety protocols to protect all of our patients” during the pandemic, said hospital spokesperson Corey Santoriello, who would not comment on Ms. Moore’s case, citing privacy laws.
Ms. Moore was airlifted to another hospital, where her condition only declined further, her daughter said. After the ventilator was removed, she clung to life fitfully for 5½ hours, as her daughter prayed for her mother to find her way to heaven.
“I asked her mom and her dad and her family and prayed to God, ‘Please just come show her the way,’” Ms. Tavolazzi said. “I relive it every day.”
When Ms. Tavolazzi sought answers from the hospital about where her mom got the virus, she said, she got none: “No one ever called me back.”
Two negative COVID tests, then ‘patient zero’
As the second surge of COVID subsided last September, doctors from the prestigious Brigham and Women’s Hospital published a reassuringstudy: With careful infection control, only two of 697 COVID patients acquired the virus within the Boston hospital. That is about 0.3% of patients --about six times lower than the overall Medicare rate. Brigham tested every patient it admitted, exceeding CDC recommendations. It was transparent and open about safety concerns.
But the study, published in the high-profile JAMA Network Open journal, conveyed the wrong message, according to Dr. Manoj Jain, an infectious-disease physician and adjunct professor at the Rollins School of Public Health at Emory University. COVID was spreading in hospitals, he said, and the study buried “the problem under the rug.”
Before the virtual ink on the study was dry, the virus began a stealthy streak through the elite hospital. It slipped in with a patient who tested negative twice -- but turned out to be positive. She was “patient zero” in an outbreak affecting 38 staffers and 14 patients, according to a study in Annals of Internal Medicine initially published Feb. 9.
That study’s authors sequenced the genome of the virus to confirm which cases were related and precisely how it traveled through the hospital.
As patients were moved from room to room in the early days of the outbreak, COVID spread among roommates 8 out of 9 times, likely through aerosol transmission, the study says. A survey of staff members revealed that those caring for coughing patients were more likely to get sick.
The virus also appeared to have breached the CDC-OK’d protective gear. Two staff members who had close patient contact while wearing a surgical mask and face shield still wound up infected. The findings suggested that more-protective N95 respirators could help safeguard staff.
Brigham and Women’s now tests every patient upon admission and again soon after. Nurses are encouraged to test again if they see a subtle sign of COVID, said Dr. Erica Shenoy, associate chief of the Infection Control Unit at Massachusetts General Hospital, who helped craft policy at Brigham.
She said nurses and environmental services workers are at the table for policymaking: “I personally make it a point to say, ‘Tell me what you’re thinking,’” Dr. Shenoy said. “‘There’s no retribution because we need to know.’”
CDC guidelines, though, left wide latitude on protective gear and testing. To this day, Dr. Shenoy said, hospitals employ a wide range of policies.
The CDC said in a statement that its guidelines “provide a comprehensive and layered approach to preventing transmission of SARS-CoV-2 in healthcare settings,” and include testing patients with “even mild symptoms” or recent exposure to someone with COVID.
Infection control policies are rarely apparent to patients or visitors, beyond whether they’re asked to wear a mask. But reviews of public records and interviews with more than a dozen people show that at hospitals with high rates of COVID spread, staff members were often alarmed by the lack of safety practices.
Nurses sound the alarm on COVID spread
As COVID crept into Florida in spring 2020, nurse Victoria Holland clashed with managers at Blake Medical Center in Bradenton, where Steven Johnson died.
She said managers suspended her early in the pandemic after taking part in a protest and “having a hissy fit” when she was denied a new N95 respirator before an “aerosol-generating” procedure. The CDC warns that such procedures can spread the virus through the air. Before the pandemic, nurses were trained to dispose of an N95 after each patient encounter.
When the suspension was over, Ms. Holland said, she felt unsafe. “They told us nothing,” she said. “It was all a little whisper between the doctors. You had potential COVIDs and you’d get a little surgical mask because [they didn’t] want to waste” an N95 unless they knew the patient was positive.
Ms. Holland said she quit in mid-April. Her nursing colleagues lodged a complaint with the Occupational Safety and Health Administration in late June alleging that staff “working around possible COVID-19 positive cases” had been denied PPE. Staff members protested outside the hospital in July and filed another OSHA complaint that said the hospital was allowing COVID-exposed employees to keep working.
Ms. Kirkland, the Blake spokesperson, said the hospital responded to OSHA and “no deficiencies were identified.”
The Medicare analysis shows that 22 of 273 patients with COVID, or 8%, were diagnosed with the virus after they were admitted to Blake. That’s about five times as high as the national average.
Ms. Kirkland said “there is no standard way for measuring COVID-19 hospital-associated transmissions” and “there is no evidence to suggest the risk of transmission at Blake Medical Center is different than what you would find at other hospitals.”
In Washington, D.C., 34 Medicare COVID patients contracted the virus at MedStar Washington Hospital Center, or nearly 6% of its total, the analysis shows.
Unhappy with the safety practices — which included gas sterilization and reuse of N95s — National Nurses United members protested on the hospital lawn in July 2020. At the protest, nurse Zoe Bendixen said one nurse had died of the virus and 50 had gotten sick: “[Nurses] can become a source for spreading the disease to other patients, co-workers and family members.”
Nurse Yuhana Gidey said she caught COVID after treating a patient who turned out to be infected. Another nurse, not managers doing contact tracing, told her she’d been exposed, she said.
Nurse Kimberly Walsh said in an interview there was an outbreak in a geriatric unit where she worked in September 2020. She said management blamed nurses for bringing the virus into the unit. But Ms. Walsh pointed to another problem: The hospital wasn’t COVID-testing patients coming in from nursing homes, where spread was rampant last year.
MedStar declined a request for an interview about its infection control practices and did not respond to specific questions.
While hospitals must track and publicly report rates of persistent infections like C. diff, antibiotic-resistant staph and surgical site infections, similar hospital-acquired COVID rates are not reported.
KHN examined a different source of data that Congress required hospitals to document about “hospital-acquired conditions.” The Medicare data, which notes whether each COVID case was “present on admission” or not, becomes available months after a hospitalization in obscure files that require a data-use agreement typically granted to researchers. KHN counted cases, as federal officials do, in some instances in which the documentation is deemed insufficient to categorize a case (see data methodology on the KHN website).
For this data, whether to deem a COVID case hospital-acquired lies with medical coders who review doctors’ notes and discharge summaries and ask doctors questions if the status is unclear, said Sue Bowman, senior director of coding policy and compliance at American Health Information Management Association.
She said medical coders are aware that the data is used for hospital quality measures and would be careful to review the contract tracing or other information in the medical record.
If a case was in the data KHN used, “that would mean it was acquired during the hospital stay either from a health care worker or another patient or maybe if a hospital allowed visitors, from a visitor,” Ms. Bowman said. “That would be a fair interpretation of the data.”
The high death rate for those diagnosed with COVID during a hospital stay — about 21% — mirrors the death rate for other Medicare COVID patients last year, when doctors had few proven methods to help patients. It also highlights the hazard unvaccinated staffers pose to patients, said Dr. Jain, the infectious-disease doctor. The American Hospital Association estimates that about 42% of U.S. hospitals have mandated that all staff members be vaccinated.
“We don’t need [unvaccinated staff] to be a threat to patients,” Dr. Jain said. “[Hospital] administration is too afraid to push the nursing staff, and the general public is clueless at what a threat a non-vaccinated person poses to a vulnerable population.”
Cindy Johnson said the hospital where she believes her husband contracted COVID faced minimal scrutiny in a state inspection, even after she said she reported that he caught COVID there. She explored suing, but an attorney told her it would be nearly impossible to win such a case. A 2021 state law requires proof of “at least gross negligence” to prevail in court.
Ms. Johnson did ask a doctor who sees patients at the hospital for this: Please take down the big “OPEN & SAFE” sign outside.
Within days, the sign was gone.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
FFR-guided PCI falls short vs. surgery in multivessel disease: FAME 3
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”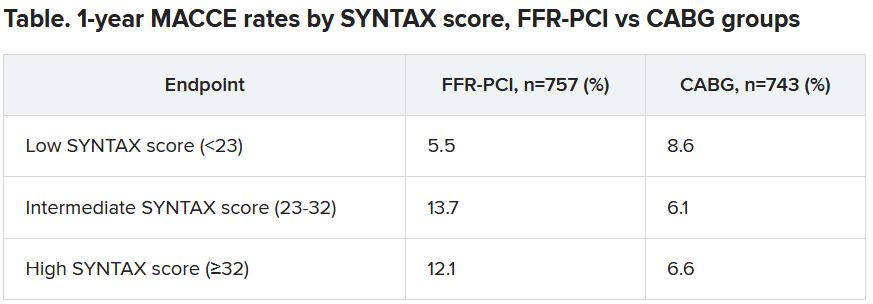
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
HIV prescription mandate controversy reaches the Supreme Court
A firestorm of controversy over access to HIV medications and protection against discriminatory insurance practices has been making its way through U.S. district courts for the past 3 years, pitting HIV patients against pharmacy benefits managers and, ostensibly, the healthcare industry itself.
.
At odds are whether or not mandatory mail-order requirements for specialty medications violate specific provisions of the Patient Protection and Affordable Care Act (ACA) and the Rehabilitation Act of 1973, both of which prohibit discrimination by programs that receive federal funds.
An amicus brief submitted on October 29 by the Center for Health Law and Policy Innovation (CHLPI) of Harvard Law School on behalf of five John Does and a number of medical practitioners and practitioner organizations underscores the degree to which advances in HIV treatment, viral suppression, and care linkage — not to mention the national mandate to end the AIDS epidemic by 2030 — might ultimately be affected.
“We decided to file the brief at the Supreme Court level because we wanted to make sure that the perspectives of people living with HIV, their providers, and advocates were in the record,” Maryanne Tomazic, a clinical instructor at CHLPI, toldthis news organization.
“It’s important for the court to consider why robust access to prescription drug coverage and pharmacy services are so important for people living with HIV, and why it’s not appropriate to compromise access to antiretroviral therapy,” she explained.
A bitter pill, regardless of who swallows it
CVS Pharmacy Inc. v. Doe focuses on a legal concept known as “disparate impact discrimination,” which refers to a policy that appears neutral but unintentionally discriminates against a protected class of people (eg, on the basis of sex, age, or ethnicity).
The Supreme Court’s decision in the case will address a central question: did CVS Pharmacy, Caremark, and Caremark Specialty Pharmacy (“CVS”) discriminate against the respondents by requiring that they obtain specialty medications (including those for HIV) by mail order or drop shipment for pickup, or, alternatively, pay out-of-network prices for these medications at non-CVS pharmacies?
The decision will also address whether the ACA’s inclusion of clause 504 of the Rehabilitation Act, which prohibits protected class discrimination, allows patients to challenge terms and conditions of their healthcare plans, a decision that has broad and far-reaching implications for insurers’ abilities to set plan restrictions and pricing.
A spokesperson for CVS declined to comment when contacted by this news organization but provided a link to an April 9, 2021 SCOTUS blog post about the filing. In its court filing, CVS contended that the program applies to all specialty medications (not just HIV) and simply reflects the cost/complexity of specialty medications.
Not everyone agrees that cost is the most important issue at play. Indeed, a critical take-away for practitioners is how mandated mail-order pharmacy programs can disrupt coordination of care.
“In the traditional model, the physician is talking to the pharmacists [or] talking with the patient, and you have kind of triangular communication model that helps not only the patient stay engaged in care but [also] allows the healthcare provider team to adjust the medication quickly without delay,” said Ms. Tomazic.
The John Doe statements in the original case highlight these concerns. They focus on how mandatory mail orders restrict highly personable relationships with local specialty pharmacists who are familiar with their patients’ medical histories as well as their medication dosing and adjustments and who regularly communicate with the complete care team on the patients’ behalf.
“JOHN DOE THREE and others depend on these types of long standing relationships with local pharmacists to maximize the benefits of HIV/AIDS medications and treat the complex and ever-changing needs of the HIV/AIDS patients,” wrote attorneys in the 2018 class action filing.
Other issues raised by the suit involve the following: privacy with respect to medication pickup; specialty care customer representatives’ lack of understanding and knowledge of HIV medications; incomplete prescription fills; late medication deliveries; exposure of medications to the elements; work and employment interruptions; and restrictions on early fills and reorders, which increase the risk for missed doses and potentially serious health problems, including interruptions in viral suppression and resistance.
Discrimination issues also raised
CHLPI’s amicus joins several others in support of the unique needs of persons with HIV, especially in Black and Hispanic/Latino communities, which are disproportionately affected by HIV.
A press release distributed by the National Association for the Advancement of Colored People Legal Defense and Educational Fund (LDF) reinforces the idea that not only are Black people more likely to have a disability other groups, owing to the country’s legacy of racial inequality, but also that they are likely to encounter unique forms of discrimination and specific barriers to full participation in society, further underscoring the need for disparate impact liability to address unfair policies and practices.
“Inequity in access to resources, including healthcare, further amplifies the instance and persistence of disabilities among Black people,” LDF attorneys wrote in the brief.
“We saw with COVID-19 that [mail-order prescription] programs can serve in a supportive role in access to care,” said Ms. Tomazic. “But we don’t want those programs to be mandated, and we don’t want to forget about communities where these kinds of programs are simply not a viable option,” she said.
Oral arguments in the case begin on December 7. A decision is expected some months later.
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
A firestorm of controversy over access to HIV medications and protection against discriminatory insurance practices has been making its way through U.S. district courts for the past 3 years, pitting HIV patients against pharmacy benefits managers and, ostensibly, the healthcare industry itself.
.
At odds are whether or not mandatory mail-order requirements for specialty medications violate specific provisions of the Patient Protection and Affordable Care Act (ACA) and the Rehabilitation Act of 1973, both of which prohibit discrimination by programs that receive federal funds.
An amicus brief submitted on October 29 by the Center for Health Law and Policy Innovation (CHLPI) of Harvard Law School on behalf of five John Does and a number of medical practitioners and practitioner organizations underscores the degree to which advances in HIV treatment, viral suppression, and care linkage — not to mention the national mandate to end the AIDS epidemic by 2030 — might ultimately be affected.
“We decided to file the brief at the Supreme Court level because we wanted to make sure that the perspectives of people living with HIV, their providers, and advocates were in the record,” Maryanne Tomazic, a clinical instructor at CHLPI, toldthis news organization.
“It’s important for the court to consider why robust access to prescription drug coverage and pharmacy services are so important for people living with HIV, and why it’s not appropriate to compromise access to antiretroviral therapy,” she explained.
A bitter pill, regardless of who swallows it
CVS Pharmacy Inc. v. Doe focuses on a legal concept known as “disparate impact discrimination,” which refers to a policy that appears neutral but unintentionally discriminates against a protected class of people (eg, on the basis of sex, age, or ethnicity).
The Supreme Court’s decision in the case will address a central question: did CVS Pharmacy, Caremark, and Caremark Specialty Pharmacy (“CVS”) discriminate against the respondents by requiring that they obtain specialty medications (including those for HIV) by mail order or drop shipment for pickup, or, alternatively, pay out-of-network prices for these medications at non-CVS pharmacies?
The decision will also address whether the ACA’s inclusion of clause 504 of the Rehabilitation Act, which prohibits protected class discrimination, allows patients to challenge terms and conditions of their healthcare plans, a decision that has broad and far-reaching implications for insurers’ abilities to set plan restrictions and pricing.
A spokesperson for CVS declined to comment when contacted by this news organization but provided a link to an April 9, 2021 SCOTUS blog post about the filing. In its court filing, CVS contended that the program applies to all specialty medications (not just HIV) and simply reflects the cost/complexity of specialty medications.
Not everyone agrees that cost is the most important issue at play. Indeed, a critical take-away for practitioners is how mandated mail-order pharmacy programs can disrupt coordination of care.
“In the traditional model, the physician is talking to the pharmacists [or] talking with the patient, and you have kind of triangular communication model that helps not only the patient stay engaged in care but [also] allows the healthcare provider team to adjust the medication quickly without delay,” said Ms. Tomazic.
The John Doe statements in the original case highlight these concerns. They focus on how mandatory mail orders restrict highly personable relationships with local specialty pharmacists who are familiar with their patients’ medical histories as well as their medication dosing and adjustments and who regularly communicate with the complete care team on the patients’ behalf.
“JOHN DOE THREE and others depend on these types of long standing relationships with local pharmacists to maximize the benefits of HIV/AIDS medications and treat the complex and ever-changing needs of the HIV/AIDS patients,” wrote attorneys in the 2018 class action filing.
Other issues raised by the suit involve the following: privacy with respect to medication pickup; specialty care customer representatives’ lack of understanding and knowledge of HIV medications; incomplete prescription fills; late medication deliveries; exposure of medications to the elements; work and employment interruptions; and restrictions on early fills and reorders, which increase the risk for missed doses and potentially serious health problems, including interruptions in viral suppression and resistance.
Discrimination issues also raised
CHLPI’s amicus joins several others in support of the unique needs of persons with HIV, especially in Black and Hispanic/Latino communities, which are disproportionately affected by HIV.
A press release distributed by the National Association for the Advancement of Colored People Legal Defense and Educational Fund (LDF) reinforces the idea that not only are Black people more likely to have a disability other groups, owing to the country’s legacy of racial inequality, but also that they are likely to encounter unique forms of discrimination and specific barriers to full participation in society, further underscoring the need for disparate impact liability to address unfair policies and practices.
“Inequity in access to resources, including healthcare, further amplifies the instance and persistence of disabilities among Black people,” LDF attorneys wrote in the brief.
“We saw with COVID-19 that [mail-order prescription] programs can serve in a supportive role in access to care,” said Ms. Tomazic. “But we don’t want those programs to be mandated, and we don’t want to forget about communities where these kinds of programs are simply not a viable option,” she said.
Oral arguments in the case begin on December 7. A decision is expected some months later.
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
A firestorm of controversy over access to HIV medications and protection against discriminatory insurance practices has been making its way through U.S. district courts for the past 3 years, pitting HIV patients against pharmacy benefits managers and, ostensibly, the healthcare industry itself.
.
At odds are whether or not mandatory mail-order requirements for specialty medications violate specific provisions of the Patient Protection and Affordable Care Act (ACA) and the Rehabilitation Act of 1973, both of which prohibit discrimination by programs that receive federal funds.
An amicus brief submitted on October 29 by the Center for Health Law and Policy Innovation (CHLPI) of Harvard Law School on behalf of five John Does and a number of medical practitioners and practitioner organizations underscores the degree to which advances in HIV treatment, viral suppression, and care linkage — not to mention the national mandate to end the AIDS epidemic by 2030 — might ultimately be affected.
“We decided to file the brief at the Supreme Court level because we wanted to make sure that the perspectives of people living with HIV, their providers, and advocates were in the record,” Maryanne Tomazic, a clinical instructor at CHLPI, toldthis news organization.
“It’s important for the court to consider why robust access to prescription drug coverage and pharmacy services are so important for people living with HIV, and why it’s not appropriate to compromise access to antiretroviral therapy,” she explained.
A bitter pill, regardless of who swallows it
CVS Pharmacy Inc. v. Doe focuses on a legal concept known as “disparate impact discrimination,” which refers to a policy that appears neutral but unintentionally discriminates against a protected class of people (eg, on the basis of sex, age, or ethnicity).
The Supreme Court’s decision in the case will address a central question: did CVS Pharmacy, Caremark, and Caremark Specialty Pharmacy (“CVS”) discriminate against the respondents by requiring that they obtain specialty medications (including those for HIV) by mail order or drop shipment for pickup, or, alternatively, pay out-of-network prices for these medications at non-CVS pharmacies?
The decision will also address whether the ACA’s inclusion of clause 504 of the Rehabilitation Act, which prohibits protected class discrimination, allows patients to challenge terms and conditions of their healthcare plans, a decision that has broad and far-reaching implications for insurers’ abilities to set plan restrictions and pricing.
A spokesperson for CVS declined to comment when contacted by this news organization but provided a link to an April 9, 2021 SCOTUS blog post about the filing. In its court filing, CVS contended that the program applies to all specialty medications (not just HIV) and simply reflects the cost/complexity of specialty medications.
Not everyone agrees that cost is the most important issue at play. Indeed, a critical take-away for practitioners is how mandated mail-order pharmacy programs can disrupt coordination of care.
“In the traditional model, the physician is talking to the pharmacists [or] talking with the patient, and you have kind of triangular communication model that helps not only the patient stay engaged in care but [also] allows the healthcare provider team to adjust the medication quickly without delay,” said Ms. Tomazic.
The John Doe statements in the original case highlight these concerns. They focus on how mandatory mail orders restrict highly personable relationships with local specialty pharmacists who are familiar with their patients’ medical histories as well as their medication dosing and adjustments and who regularly communicate with the complete care team on the patients’ behalf.
“JOHN DOE THREE and others depend on these types of long standing relationships with local pharmacists to maximize the benefits of HIV/AIDS medications and treat the complex and ever-changing needs of the HIV/AIDS patients,” wrote attorneys in the 2018 class action filing.
Other issues raised by the suit involve the following: privacy with respect to medication pickup; specialty care customer representatives’ lack of understanding and knowledge of HIV medications; incomplete prescription fills; late medication deliveries; exposure of medications to the elements; work and employment interruptions; and restrictions on early fills and reorders, which increase the risk for missed doses and potentially serious health problems, including interruptions in viral suppression and resistance.
Discrimination issues also raised
CHLPI’s amicus joins several others in support of the unique needs of persons with HIV, especially in Black and Hispanic/Latino communities, which are disproportionately affected by HIV.
A press release distributed by the National Association for the Advancement of Colored People Legal Defense and Educational Fund (LDF) reinforces the idea that not only are Black people more likely to have a disability other groups, owing to the country’s legacy of racial inequality, but also that they are likely to encounter unique forms of discrimination and specific barriers to full participation in society, further underscoring the need for disparate impact liability to address unfair policies and practices.
“Inequity in access to resources, including healthcare, further amplifies the instance and persistence of disabilities among Black people,” LDF attorneys wrote in the brief.
“We saw with COVID-19 that [mail-order prescription] programs can serve in a supportive role in access to care,” said Ms. Tomazic. “But we don’t want those programs to be mandated, and we don’t want to forget about communities where these kinds of programs are simply not a viable option,” she said.
Oral arguments in the case begin on December 7. A decision is expected some months later.
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.




