User login
Psoriatic arthritis and axial spondyloarthritis patients succeed with reduced TNF inhibitor dosing
Reducing the dose of tumor necrosis factor inhibitors by approximately one-third did not increase disease activity in adults with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) in a stable low–disease activity state, according to findings from two parallel controlled retrospective cohort studies.
Disease activity–guided dose optimization (DAGDO) can reduce drug exposure in patients with PsA or axSpA who have low disease activity, but its impact on increased disease activity has not been as well studied as full-dose continuation, Celia A.J. Michielsens, MD, of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues wrote.
“DAGDO or discontinuation of bDMARDs [biologic disease-modifying antirheumatic drugs] as a standard of care in adults with stable axSpA is currently discouraged by” the American College of Rheumatology, the researchers said. However, guidelines from the European Alliance of Associations for Rheumatology allow for the slow tapering of bDMARDs in patients with sustained remission.
In a controlled, retrospective cohort study published in Rheumatology, the researchers analyzed data from their outpatient clinic, which initiated a specific TNF inhibitor DAGDO protocol in 2010 for patients with RA, PsA, and axSpA. Disease activity was measured using the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) for patients with PsA and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axSpA.
The study population included 153 patients with PsA who had a mean DAS28-CRP of 6.5 and 171 with axSpA who had a similar mean number of disease activity measurements (6.5 with DAS28-CRP and 6.4 with BASDAI). Median follow-up time was several months short of 4 years in each group. Treatment was divided into three periods: continuation of full TNF inhibitor dose, TNF inhibitor DAGDO, and a period with stable TNF inhibitor dose after DAGDO.
Overall, no significant differences appeared in mean DAS28-CRP and BASDAI over the course of the study between the period of the full TNF inhibitor dose continuation and both the TNF inhibitor DAGDO period and the stable TNF inhibitor dose period. Among PsA patients, the mean DAS28-CRP was 1.94 for the full-dose period, 2.0 in the TNF inhibitor DAGDO period, and 1.97 in the stable TNF inhibitor dose after DAGDO period. For axSpA patients, the mean BASDAI was 3.44, 3.47, and 3.48, respectively, for the three periods. Older age, longer disease duration, and longer follow-up were significantly associated with higher DAS28-CRP scores in patients with PsA, and older age and female gender were significantly associated with higher BASDAI scores in patients with axSpA.
The mean percentage of daily defined dose (%DDD) for patients with PsA was 108% during the full dose period, 62% in the TNF inhibitor DAGDO period, and 78% with stable TNF inhibitor after DAGDO, and nearly the same for patients with axSPA at 108%, 62%, and 72%, respectively.
The %DDD represents “a modest degree of tapering,” compared with studies in RA patients, the researchers noted. “Explanations for this difference could be that the full dose-reduction potential was not met due to suboptimal execution of the local protocol, whereas in prospective intervention trials, protocol adherence is likely higher.”
The study findings were limited by several factors including the open-label design and potential for nocebo effects, possible incorrect attribution, and information bias, as well as the use of DAS28-CRP and BASDAI rather than more modern measurement tools, the researchers noted.
However, the results were strengthened by the large sample size and real-world clinical setting, frequent assessment of disease activity, long-term follow-up, and the performance of DAGDO by rheumatologists familiar with the measuring tools, they said. The results suggest that DAGDO is safe and effective for patients with low disease activity in either condition, but randomized, prospective studies can provide more definitive evidence.
The study received no outside funding. One author disclosed relationships with multiple pharmaceutical companies.
Reducing the dose of tumor necrosis factor inhibitors by approximately one-third did not increase disease activity in adults with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) in a stable low–disease activity state, according to findings from two parallel controlled retrospective cohort studies.
Disease activity–guided dose optimization (DAGDO) can reduce drug exposure in patients with PsA or axSpA who have low disease activity, but its impact on increased disease activity has not been as well studied as full-dose continuation, Celia A.J. Michielsens, MD, of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues wrote.
“DAGDO or discontinuation of bDMARDs [biologic disease-modifying antirheumatic drugs] as a standard of care in adults with stable axSpA is currently discouraged by” the American College of Rheumatology, the researchers said. However, guidelines from the European Alliance of Associations for Rheumatology allow for the slow tapering of bDMARDs in patients with sustained remission.
In a controlled, retrospective cohort study published in Rheumatology, the researchers analyzed data from their outpatient clinic, which initiated a specific TNF inhibitor DAGDO protocol in 2010 for patients with RA, PsA, and axSpA. Disease activity was measured using the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) for patients with PsA and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axSpA.
The study population included 153 patients with PsA who had a mean DAS28-CRP of 6.5 and 171 with axSpA who had a similar mean number of disease activity measurements (6.5 with DAS28-CRP and 6.4 with BASDAI). Median follow-up time was several months short of 4 years in each group. Treatment was divided into three periods: continuation of full TNF inhibitor dose, TNF inhibitor DAGDO, and a period with stable TNF inhibitor dose after DAGDO.
Overall, no significant differences appeared in mean DAS28-CRP and BASDAI over the course of the study between the period of the full TNF inhibitor dose continuation and both the TNF inhibitor DAGDO period and the stable TNF inhibitor dose period. Among PsA patients, the mean DAS28-CRP was 1.94 for the full-dose period, 2.0 in the TNF inhibitor DAGDO period, and 1.97 in the stable TNF inhibitor dose after DAGDO period. For axSpA patients, the mean BASDAI was 3.44, 3.47, and 3.48, respectively, for the three periods. Older age, longer disease duration, and longer follow-up were significantly associated with higher DAS28-CRP scores in patients with PsA, and older age and female gender were significantly associated with higher BASDAI scores in patients with axSpA.
The mean percentage of daily defined dose (%DDD) for patients with PsA was 108% during the full dose period, 62% in the TNF inhibitor DAGDO period, and 78% with stable TNF inhibitor after DAGDO, and nearly the same for patients with axSPA at 108%, 62%, and 72%, respectively.
The %DDD represents “a modest degree of tapering,” compared with studies in RA patients, the researchers noted. “Explanations for this difference could be that the full dose-reduction potential was not met due to suboptimal execution of the local protocol, whereas in prospective intervention trials, protocol adherence is likely higher.”
The study findings were limited by several factors including the open-label design and potential for nocebo effects, possible incorrect attribution, and information bias, as well as the use of DAS28-CRP and BASDAI rather than more modern measurement tools, the researchers noted.
However, the results were strengthened by the large sample size and real-world clinical setting, frequent assessment of disease activity, long-term follow-up, and the performance of DAGDO by rheumatologists familiar with the measuring tools, they said. The results suggest that DAGDO is safe and effective for patients with low disease activity in either condition, but randomized, prospective studies can provide more definitive evidence.
The study received no outside funding. One author disclosed relationships with multiple pharmaceutical companies.
Reducing the dose of tumor necrosis factor inhibitors by approximately one-third did not increase disease activity in adults with psoriatic arthritis (PsA) or axial spondyloarthritis (axSpA) in a stable low–disease activity state, according to findings from two parallel controlled retrospective cohort studies.
Disease activity–guided dose optimization (DAGDO) can reduce drug exposure in patients with PsA or axSpA who have low disease activity, but its impact on increased disease activity has not been as well studied as full-dose continuation, Celia A.J. Michielsens, MD, of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues wrote.
“DAGDO or discontinuation of bDMARDs [biologic disease-modifying antirheumatic drugs] as a standard of care in adults with stable axSpA is currently discouraged by” the American College of Rheumatology, the researchers said. However, guidelines from the European Alliance of Associations for Rheumatology allow for the slow tapering of bDMARDs in patients with sustained remission.
In a controlled, retrospective cohort study published in Rheumatology, the researchers analyzed data from their outpatient clinic, which initiated a specific TNF inhibitor DAGDO protocol in 2010 for patients with RA, PsA, and axSpA. Disease activity was measured using the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) for patients with PsA and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for patients with axSpA.
The study population included 153 patients with PsA who had a mean DAS28-CRP of 6.5 and 171 with axSpA who had a similar mean number of disease activity measurements (6.5 with DAS28-CRP and 6.4 with BASDAI). Median follow-up time was several months short of 4 years in each group. Treatment was divided into three periods: continuation of full TNF inhibitor dose, TNF inhibitor DAGDO, and a period with stable TNF inhibitor dose after DAGDO.
Overall, no significant differences appeared in mean DAS28-CRP and BASDAI over the course of the study between the period of the full TNF inhibitor dose continuation and both the TNF inhibitor DAGDO period and the stable TNF inhibitor dose period. Among PsA patients, the mean DAS28-CRP was 1.94 for the full-dose period, 2.0 in the TNF inhibitor DAGDO period, and 1.97 in the stable TNF inhibitor dose after DAGDO period. For axSpA patients, the mean BASDAI was 3.44, 3.47, and 3.48, respectively, for the three periods. Older age, longer disease duration, and longer follow-up were significantly associated with higher DAS28-CRP scores in patients with PsA, and older age and female gender were significantly associated with higher BASDAI scores in patients with axSpA.
The mean percentage of daily defined dose (%DDD) for patients with PsA was 108% during the full dose period, 62% in the TNF inhibitor DAGDO period, and 78% with stable TNF inhibitor after DAGDO, and nearly the same for patients with axSPA at 108%, 62%, and 72%, respectively.
The %DDD represents “a modest degree of tapering,” compared with studies in RA patients, the researchers noted. “Explanations for this difference could be that the full dose-reduction potential was not met due to suboptimal execution of the local protocol, whereas in prospective intervention trials, protocol adherence is likely higher.”
The study findings were limited by several factors including the open-label design and potential for nocebo effects, possible incorrect attribution, and information bias, as well as the use of DAS28-CRP and BASDAI rather than more modern measurement tools, the researchers noted.
However, the results were strengthened by the large sample size and real-world clinical setting, frequent assessment of disease activity, long-term follow-up, and the performance of DAGDO by rheumatologists familiar with the measuring tools, they said. The results suggest that DAGDO is safe and effective for patients with low disease activity in either condition, but randomized, prospective studies can provide more definitive evidence.
The study received no outside funding. One author disclosed relationships with multiple pharmaceutical companies.
FROM RHEUMATOLOGY
Risankizumab outperforms placebo at 6 months for psoriatic arthritis
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
FROM ACR 2021
Abatacept shows signal to delay onset of rheumatoid arthritis
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
FROM ACR 2021
Firm Digital Papulonodules in an Infant
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
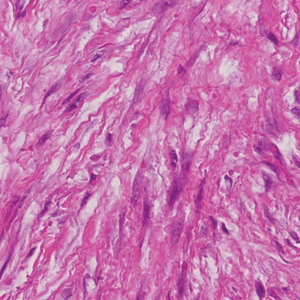
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.
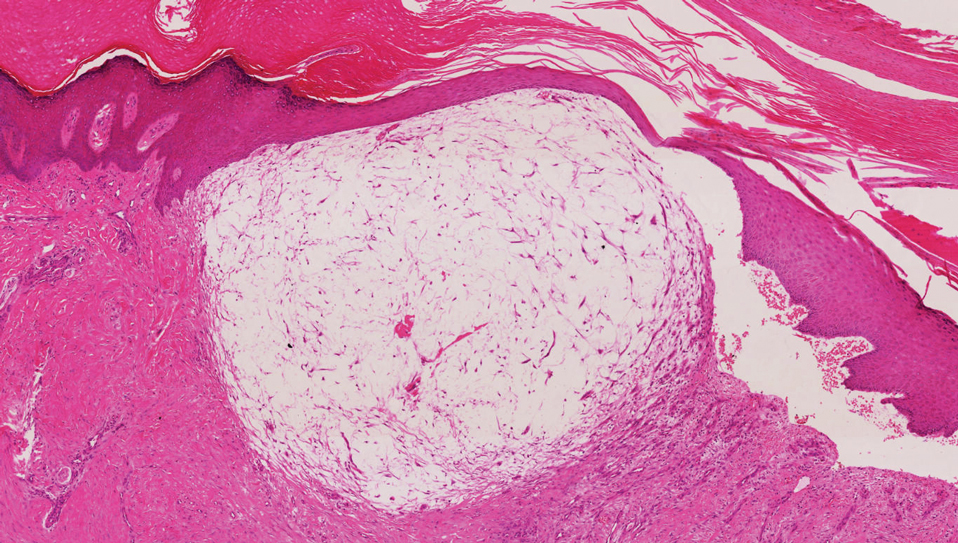
A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).
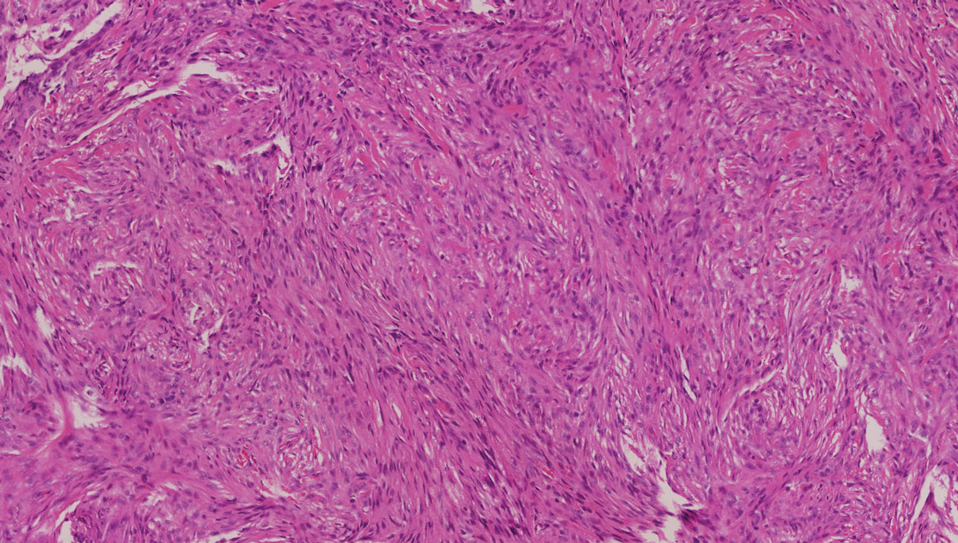
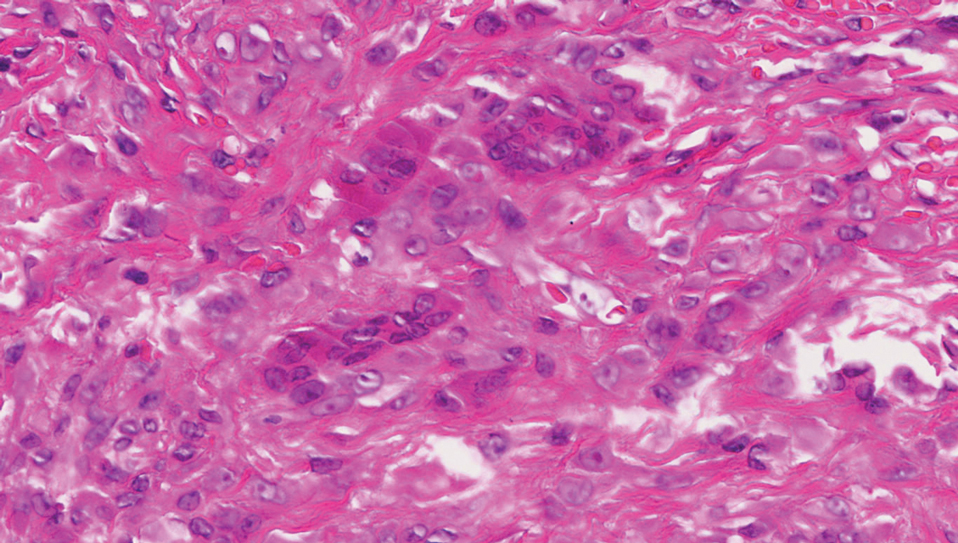
A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.
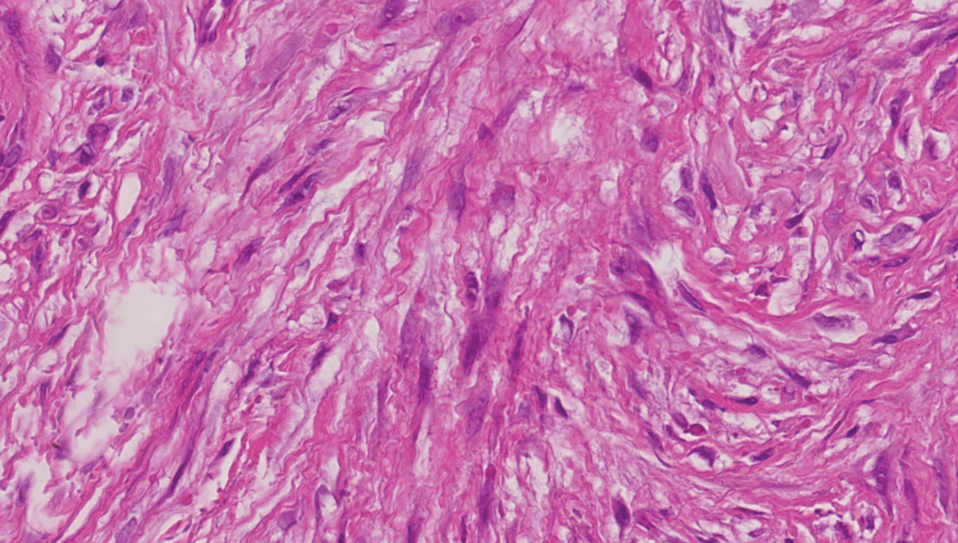
Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.

A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).

A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.

Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.

A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).

A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.

Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
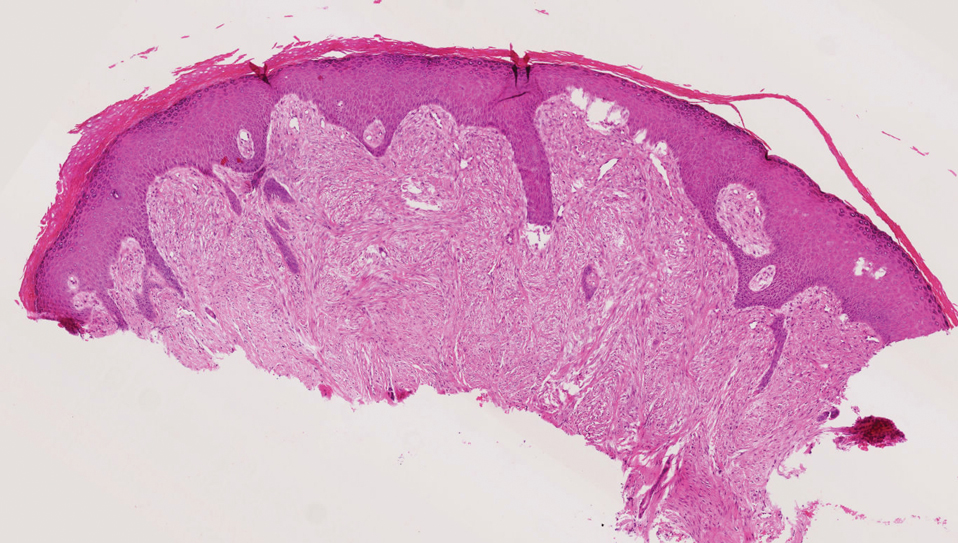
A 3-month-old girl presented with papulonodules on the distal left ring finger. Initially the lesions were thought to be insect bites but became firm over the course of 3 weeks and then gradually increased in size over 2 months. Physical examination revealed a 0.5×0.5-cm firm nodule and a 0.2×0.3-cm firm papule on the radial aspect of the left ring finger over the distal interphalangeal joint. There was no deformity or dysfunction of the finger. Radiography showed soft tissue swelling without bony abnormalities. The lesions were excised; however, a new fleshy nodule reappeared 1 month postoperatively on the radial aspect of the left ring finger over the distal interphalangeal joint. The patient did not seem bothered by the lesions and was in good general health.
Contact Allergy to Topical Medicaments, Part 1: A Double-edged Sword
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
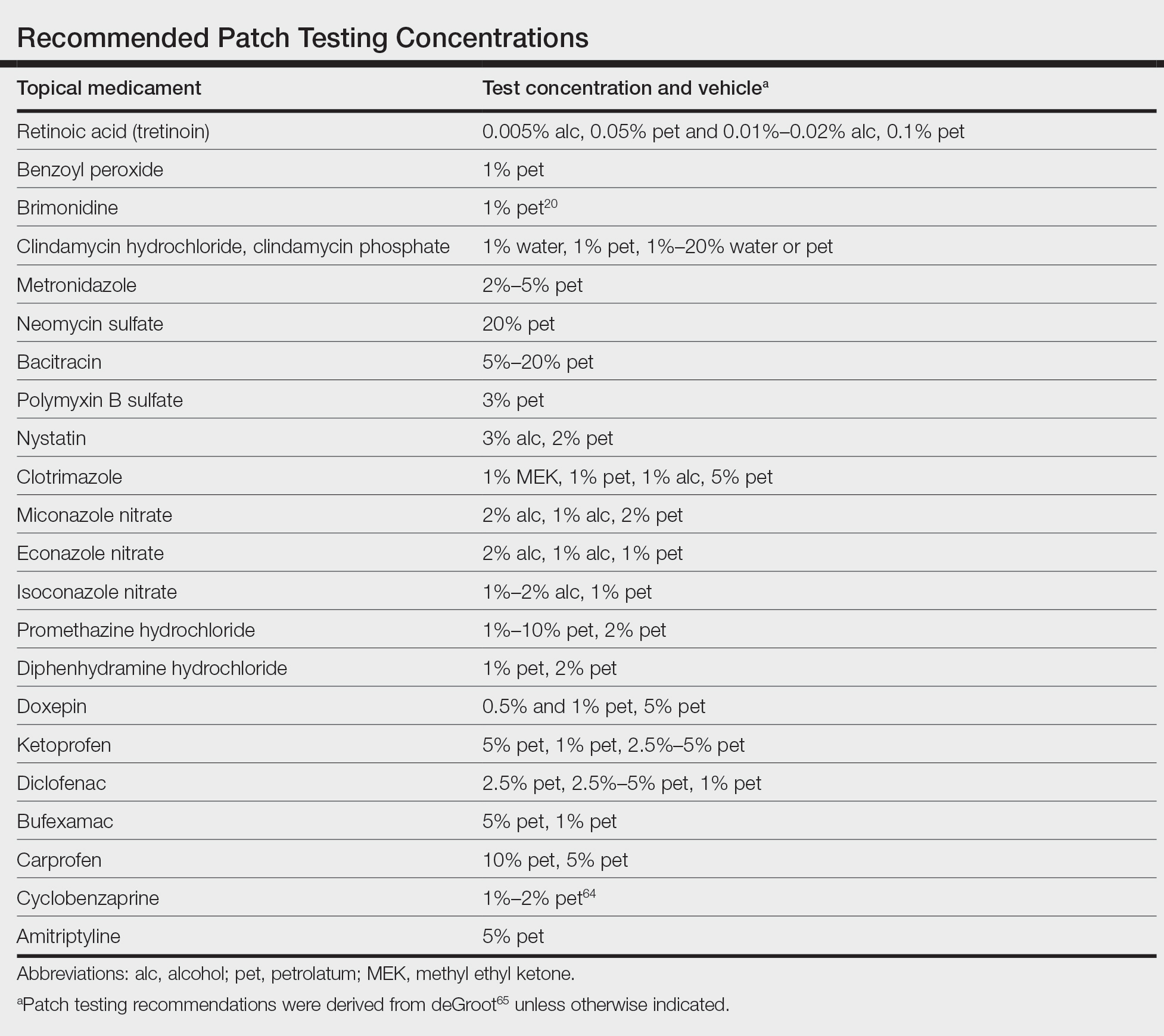
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Practice Points
- Allergic contact dermatitis should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Prior sensitization is not always apparent, and cross-reactions may occur between structurally similar compounds.
- Although most medicaments can be patch tested as is, patch testing to the individual components may be necessary to identify the causative allergen.
Advocacy Update: Is Your Practice Equipped to Handle Looming Changes in Dermatopathology?
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
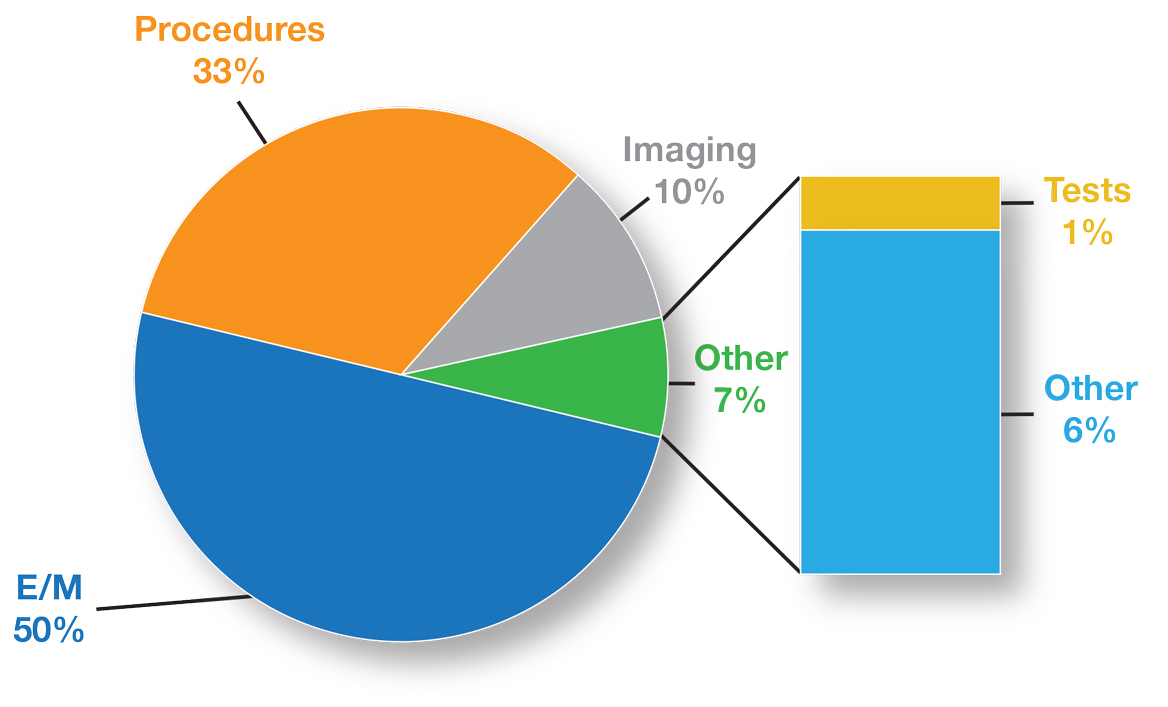
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
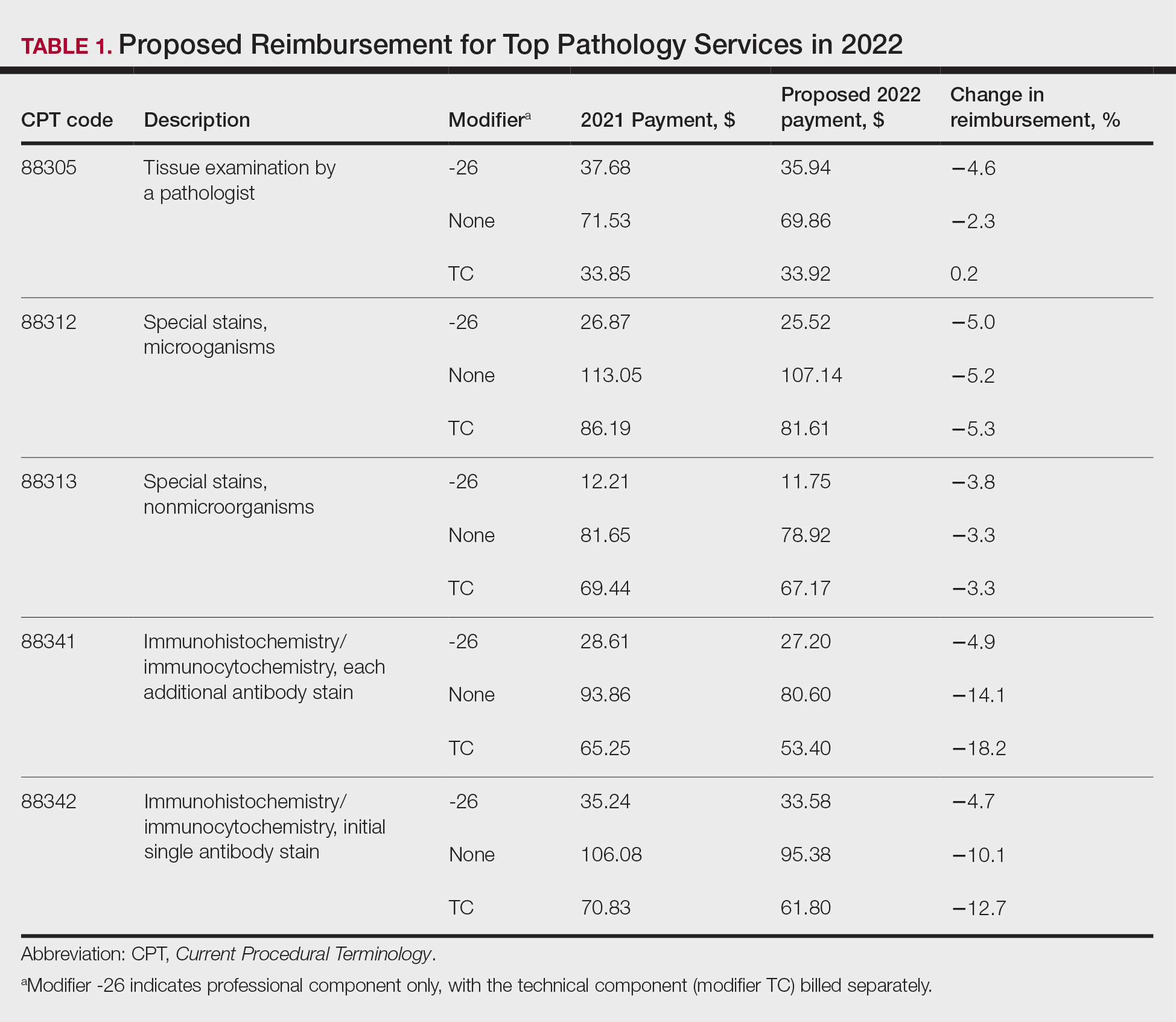
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
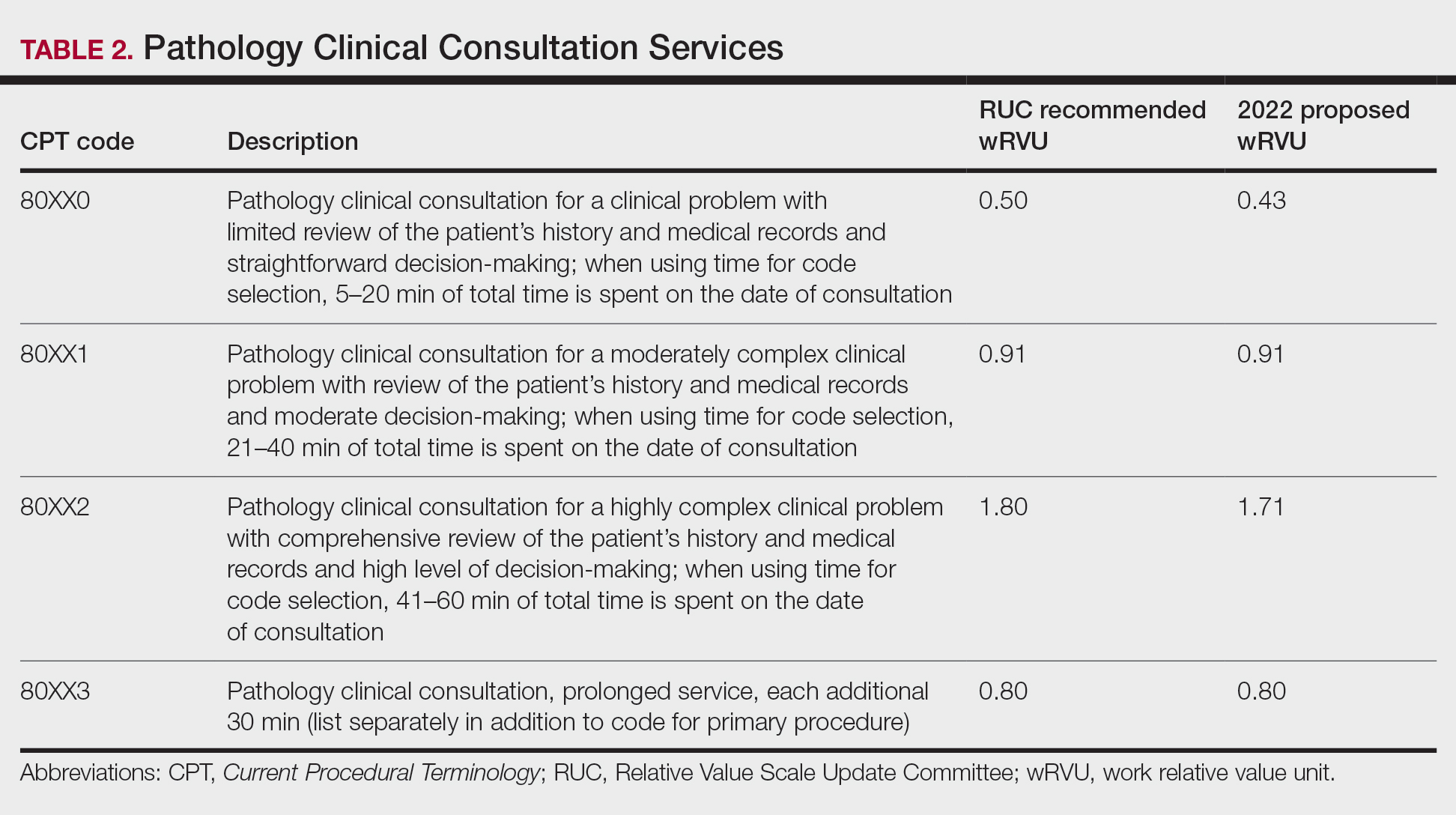
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3

Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4

The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4

According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3

Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4

The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4

According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
Practice Points
- A proposed 2022 fee schedule negatively impacting dermatopathology practices has been published by the Centers for Medicare & Medicaid Services (CMS) in July 2021.
- New pathology consultation codes with new payment rates proposed by CMS can be used starting January 1, 2022.
- The 21st Century Cures Act Final Rule has information blocking provisions.
Botanical Briefs: Phytophotodermatitis Caused by Giant Hogweed (Heracleum mantegazzianum)
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.
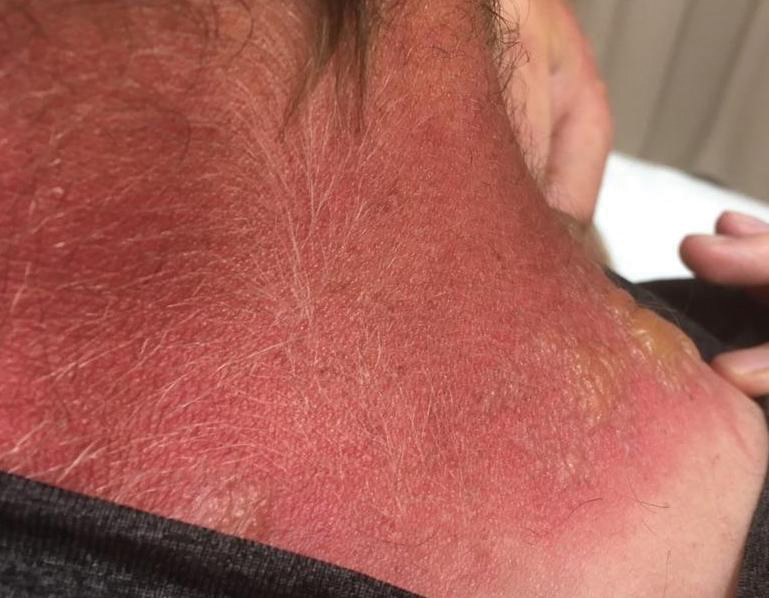
The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.
Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
Giant hogweed (Heracleum mantegazzianum) is an invasive flowering weed of the family Apiaceae that typically reaches a height of 13 feet, with thick stems; large green leaves; and umbrella-shaped, flat-topped, radial clusters (umbels) of small individual white flowers1 (Figure 1). Because of the size and beauty of giant hogweed, it was widely planted in 19th century ornamental gardens in the United Kingdom and has since naturalized and spread throughout central Europe, Canada, and the United States.1,2 The plant most commonly is found in shady areas near rivers and woodlands.1

Due to the invasive nature of the giant hogweed, its prevalence continues to grow, its eradication remains difficult, and reports of phytophotodermatitis are increasing in number and distribution. In fact, there has been widespread media coverage of the dangers of giant hogweed in the United Kingdom since 20161 and in the United States in 2018 and 2019.3-6
Transmission
Phytophotodermatitis is a type of nonimmunologic dermatitis caused by UV light reacting with a plant-based photosensitizing agent. In the case of giant hogweed, sap from the plant’s fruits, leaves, and stem contain furocoumarins or psoralens.7 Upon activation by UVA radiation, furan rings of these compounds create reactive oxygen species and intercalate with DNA pyrimidine bases, which results in cellular death, damage to successive skin layers, and reduced wound healing at the cellular level.8 This effect is intensified with increased percutaneous absorption of furocoumarin, which can result from high temperature, humidity, skin infection, lack of protective clothing, and moist conditions.9
The highest concentration of phototoxic compounds is found in giant hogweed from June through August,7 which, in combination with people increasing their outdoor activity in the summer, results in a greater prevalence and severity of H mantegazzianum phytophotodermatitis during summer months.
Presentation
Phytophotodermatitis caused by giant hogweed can range from burning and erythema to full-thickness chemical burns that require surgical debridement and skin grafting.8 After exposure to the offending agent, a harmful skin reaction can start within 15 minutes. After a latent period of approximately 24 hours, erythema, edema, and bullae can appear and generally peak by 72 hours.10 In addition to cutaneous injury, inhalation of giant hogweed traces can result in obstructive pulmonary symptoms. Eye contact can result in blindness.9
In addition to the rash caused by giant hogweed, a “weed-wacker dermatitis” or “strimmer rash” can be caused by the similar-appearing but smaller common hogweed (Heracleum sphondylium). Common hogweed is highly prevalent in the United States and often is confused with the larger giant hogweed because of tall stems and white, flat-topped flowers.
Management
Following contact with giant hogweed, a person should immediately avoid UV exposure and rinse the area with soap and water. UV radiation must be avoided for at least 48 hours. If erythema occurs, a topical steroid can be applied to the affected area; pain can be alleviated by a nonsteroidal anti-inflammatory drug.9
Further treatment might be required if bullous lesions are present. Small blisters can be punctured and drained; however, large blisters, extensive epidermal-dermal separation, and large areas of detached epidermis should simply be cleansed and dressed. An oral steroid also can be used to reduce inflammation in moderate and severe cases. Full-thickness injury might require surgical intervention.8
Clinical Case
A 27-year-old male landscaper presented to the emergency department with an increasingly painful blistering rash on the arms and neck of 1 day’s duration. He noticed bright red skin and blisters 18 to 24 hours after trimming what he identified as shoulder-high giant hogweed plants. Neither he nor his coworkers were wearing sunscreen or protective clothing as they cleared the plants for several hours. His coworkers developed similar rashes, but his rash was the most severe, requiring treatment in the emergency department.
Physical examination showed innumerable 2- to 10-mm, tense vesicles and bullae on a background of blanching erythema in a striking photodistribution along the neck (Figure 2) and arms (Figure 3). He had notable edema of both arms and several large 3- to 4-cm bullae on the ventral aspects of the forearms.

The patient was diagnosed with severe phytophotodermatitis secondary to contact with H mantegazzianum and was started on oral prednisone 70 mg daily (1 mg/kg/d), which was decreased by 10 mg every 3 days until the course of treatment was complete. He also was instructed to apply mupirocin ointment to open areas and petroleum jelly to intact skin. Additionally, he was advised to practice strict photoprotection for the near and distant future.

Within several days after treatment began, the phytophotodermatitis dramatically improved, with complete resolution in 1 week. Postinflammatory hyperpigmentation resolved after several weeks.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
- Baker B, Bedford J, Kanitkar S. Keeping pace with the media; giant hogweed burns—a case series and comprehensive review [published online December 26, 2016]. Burns. 2017;13:933-938. doi:10.1016/j.burns.2016.10.018
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144. doi:10.2478/s13382-014-0238-z
- Zaveria M, Hauser C. Giant hogweed: a plant that can burn and blind you. but don’t panic. New York Times. July 2, 2018. Accessed October 18, 2021. https://www.nytimes.com/2018/07/02/us/giant-hogweed-nyt.html
- Hignett K. Giant hogweed: New York officials warn residents about dangerous plant that causes serious burns, blisters and scars. Newsweek. June 25, 2019. Accessed October 18, 2021. https://www.newsweek.com/giant-hogweed-new-york-dangerous-plant-burns-skin-sunlight-1445785
- Eastman J. Toxic giant hogweed sap that burns, blisters skin found in Clark County. The Oregonian. Updated July 16, 2019. Accessed October 18, 2021. https://www.oregonlive.com/news/2019/07/toxic-giant-hogweed-plant-that-burns-blisters-skin-found-in-clark-county.html
- O’Kane C. Giant hogweed, plant that causes blindness and third-degree burns, discovered in Virginia. CBS News. June 18, 2018. Accessed October 18, 2021. https://www.cbsnews.com/news/giant-hogweed-plant-causes-blindness-third-degree-burns-discovered-in-virginia-otherstates/
- Pira E, Romano C, Sulotto F, et al. Heracleum mantegazzianum growth phases and furocoumarin content. Contact Dermatitis. 1989;21:300-303. doi:10.1111/j.1600-0536.1989.tb04747.x
- Chan JCY, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130. doi:10.1016/j.bjps.2010.03.030
- Pfurtscheller K, Trop M. Phototoxic plant burns: report of a case and review of topical wound treatment in children. Pediatr Dermatol. 2014;31:E156-E159. doi:10.1111/pde.12396
- Kavli G, Volden G: Phytophotodermatitis. Photodermatol. 1984;1:65-75.
PRACTICE POINTS
- The public should be educated, especially during summer months, about how to identify giant hogweed, reduce exposure to the plant’s phototoxin, and thus reduce the risk for severe phytophotodermatitis.
- Phytophotodermatitis should be included in the differential diagnosis when a patient presents with acute erythema and bullae in sun-exposed areas.
- Phytophotodermatitis can be treated by promptly washing the skin with soap and water, protecting the skin from exposure to UV light, and utilizing topical and oral steroids.
Management of Acute and Chronic Pain Associated With Hidradenitis Suppurativa: A Comprehensive Review of Pharmacologic and Therapeutic Considerations in Clinical Practice
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
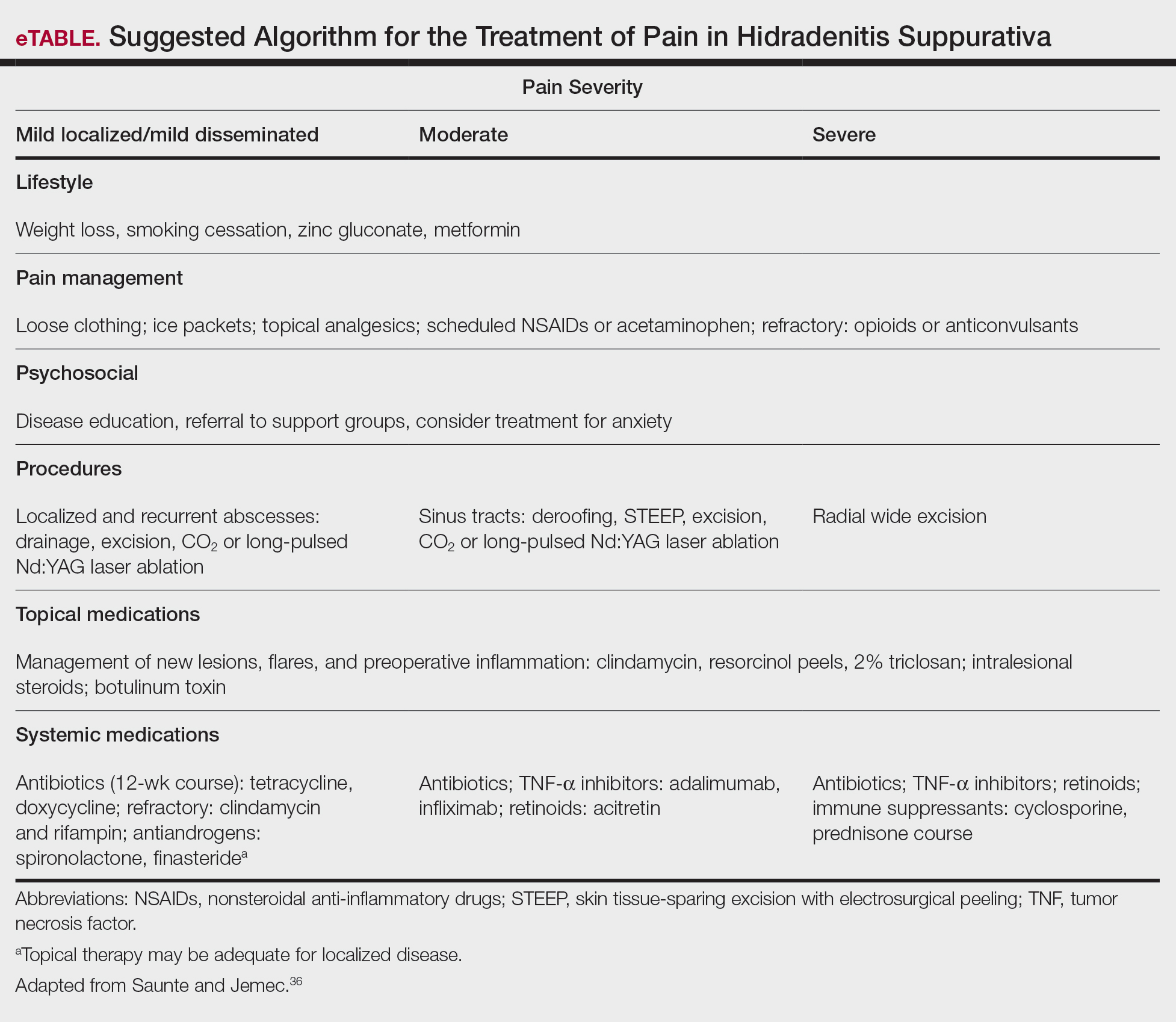
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36

Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36

Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Practice Points
- First-line therapies may not provide adequate pain control in many patients with hidradenitis suppurativa.
- Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.
- Long-term management involves lifestyle modifications and pharmacologic agents.
- The most effective pain remedies developed thus far are limited to surgery and tumor necrosis factor α inhibitors.
The No Judgment Zone: Building Trust Through Trustworthiness
The collective struggle felt by healthcare workers simultaneously learning about and caring for patients impacted by SARS-CoV2 infections throughout 2020 was physically and emotionally exhausting. The majority of us had never experienced a global pandemic. Beyond our work in the professional arena of ambulatory practices and hospitals, we also felt the soul-crushing impact of the pandemic in every other aspect of our lives. Preexisting health disparities were amplified by COVID-19. Some of the most affected communities also bore the weight of an additional tsunami of ongoing racial injustice.1 And as healthcare workers, we did our best to process and navigate it all while trying to avoid burnout—as well as being infected with COVID-19 ourselves. When the news of the highly effective vaccines against SARS-CoV2 receiving emergency use authorization broke late in 2020, it felt like a light at the end of a very dark tunnel.
In the weeks preceding wide availability of the vaccines, it became apparent that significant numbers of people lacked confidence in the vaccines. Given the disproportionate impact of COVID-19 on racial minorities, much of the discussion centered around “vaccine hesitancy” in these communities. Reasons such as historical mistrust, belief in conspiracy theories, and misinformation emerged as the leading explanations.2 Campaigns and educational programs targeting Black Americans were quickly developed to counter this widely distributed narrative.
Vaccine uptake also became politicized, which created additional challenges. As schools and businesses reopened, the voices of those opposing pandemic mitigation mandates such as masking and vaccination were highlighted by media outlets. And though a large movement of individuals who had opted against vaccines existed well before the pandemic, with few exceptions, that number had never been great enough to impact public health to this extent.3 This primarily nonminority group of unvaccinated individuals also morphed into another monolithic identity: the “anti-vaxxer.”
The lion’s share of discussions around vaccine uptake centered on these two groups: the “vaccine hesitant” minority and the “anti-vaxxer.” The perspectives and frustration around these two stereotypical unvaccinated groups were underscored in journals and the lay press. But those working in communities and in direct care came into contact with countless COVID-19-positive patients who were unvaccinated and fell into neither of these categories. There was a large swath of vulnerable people who still had unanswered questions and mistrust in the medical system standing in their way. Awareness of health disparities among racial minorities is something that was discussed among providers, but it was something experienced and felt by patients daily in regard to so much more than just COVID-19.
With broader access to vaccines through retail, community-based, and clinical facilities, more patients who desired vaccination had the opportunity. After an initial rise in vaccine uptake, the numbers plateaued. But what remained was the repetitive messaging and sustained focus directed toward Black people and their “vaccine hesitancy.”
Grady Memorial Hospital, a public safety net hospital in Atlanta, serves a predominantly Black and uninsured patient population. We found that a “FAQ” approach with a narrow range of hypothetical ideas about unvaccinated minorities clashed with the reality of what we encountered in clinical environments and the community. While misinformation did appear to be prevalent, we appreciated that the context and level of conviction were heterogenous. We appreciated that each individual conversation could reveal something new to us about that unique patient and their personal concerns about vaccination. As time moved forward, it became clear that there was no playbook for any group, especially for historically disadvantaged communities. Importantly, it was recognized that attempts to anticipate what may be a person’s barrier to vaccination often worked to further erode trust. However, when we focused on creating a space for dialogue, we found we were able to move beyond information-sharing and instead were able to co-construct interpretations of information and co-create solutions that matched patients’ values and lived experiences.4 Through dialogue, we were better able to be transparent about our own experiences, which ultimately facilitated authentic conversations with patients.
In September 2021, we approached our hospital leadership with a patient-centered strategy aimed at providing our patients, staff, and visitors a psychologically safe place to discuss vaccine-related concerns without judgment. With their support, we set up a table in the busiest part of our hospital atrium between the information desk and vaccine-administration site. Beside it was a folding board sign with an image and these words:
“Still unsure about being vaccinated? Let’s talk about it.”
We aptly called the area the “No Judgment Zone.”
The No Judgment Zone is collaboratively staffed in 1- to 2-hour voluntary increments by physician faculty and resident physicians at Emory University School of Medicine and Morehouse School of Medicine. Our goal is to increase patient trust by honoring individual vaccine-related concerns without shame or ridicule. We also work to increase patient trust by being transparent around our own experiences with COVID-19; by sharing our own journeys, concerns, and challenges, we are better able to engage in meaningful dialogue. Also, recognizing the power of logistical barriers, in addition to answering questions, we offer physical assistance with check-in, forms, and escorts to our administration areas. The numbers of unique visits have varied from day to day, but the impact of each individual encounter cannot be overstated.
Here, we describe our approach to interactions at the No Judgment Zone. These are the instructions offered to our volunteers. Though we offer some explicit examples, each talking point is designed to open the door to a patient-centered individual dialogue. We believe that these strategies can be applied to clinical settings as well as any conversation surrounding vaccination with those who have not yet decided to be vaccinated.
THE GRADY “NO JUDGMENT ZONE” INTERACTION APPROACH
No Labels
Try to think of all who are not yet vaccinated as “on a spectrum of deliberation” about their decision—not “hesitant” or “anti-vaxxer.”
Step 1: Gratitude
- “Thank you for stopping to talk to us today.”
- “I appreciate you taking the time.”
- “Before we start—I’m glad you’re here. Thanks.”
Step 2: Determine Where They Are
- Has the person you’re speaking with been vaccinated yet?
- If no, ask: “On a scale of 0 to 10—zero being “I will never get vaccinated under any circumstances” and 10 being ‘I will definitely get vaccinated’—what number would you give yourself?”
- If the person is a firm zero: “Is there anything I might be able to share with you or tell you about that might move you away from that perspective?”
- If the answer is NO: “It sounds like you’ve thought a lot about this and are no longer deliberating about whether you will be vaccinated. If you find yourself considering it, come back to talk with us, okay?” We are not here to debate or argue. We also need to avail ourselves to those who are open to changing their mind.
- If they say anything other than zero, move to an open-ended question about #WhatsYourWhy.
Step 3: #WhatsYourWhy
- “What would you say has been your main reason for not getting vaccinated yet?”
- “Tell me what has stood in the way of you getting vaccinated.”
- Remember: Assume nothing. It may have nothing to do with misinformation, fear, or perceived threat. It could be logistics or many other things. You will not know unless you ask.
- Providers should feel encouraged to also share their why as well and the reasons they encouraged their parents/kids/loved ones to get vaccinated. Making it personal can help establish connection and be more compelling.
Step 4: Listen Completely
- Give full eye contact. Slow all body movements. Use facilitative gestures to let the person know you are listening.
- Do not plan what you wish to say next.
- Limit reactions to misinformation. Shame and judgment can be subtle. Be mindful.
- Repeat the concern back if you are not sure or want to confirm that you’ve heard correctly.
- Ask questions for clarity if you aren’t sure.
Step 5: Affirm All Concerns and Find Common Ground
- “I can only imagine how scary it must be to take a shot that you believe could cause you to not be able to have babies.”
- “You aren’t alone. That’s a concern that many of my patients have had, too. May I share some information about that with you?”
- “When I first heard about the vaccine, I worried it was too new, too. Can I share what I learned?”
Step 6: Provide Factual Information
- Without excessive medical jargon, offer factual information aimed at each concern or question. Probe to be certain your patient understands through a teach-back or question.
- If you are unsure about the answer to their question, admit that you don’t know. You can also ask a colleague or the attending with you. Another option is to call someone or say “Let’s pull this up together.” Then share your answer.
- It is okay to acknowledge that the healthcare system has not and does not always do right by minority populations, especially Black people. Use that as a pivot to why these truths make vaccination that much more important
- Have FAQ information sheets available. Confirm that the patient is comfortable with the information sheet by asking.
Step 7: Offer to Help Them Get Vaccinated Today
- “Would you like me to help you get vaccinated today?”
- “What can I do to assist you with getting vaccinated? Is today a good day?”
- Escort those who agree to the registration area.
- Affirm those plans to get vaccinated or those who feel closer to getting vaccinated after speaking with you.
Step 8: Gratitude
- Close with gratitude and an affirmation.
- “I’m so glad you took the time to talk with us today. You didn’t have to stop.”
- “Feel free to come back to talk to us if you think of any more questions. I’m grateful that you stopped.”
- We are planting seeds. Do not feel pressure to get a person to say yes. Our secret sauce is kindness, respect, and empathy.
- We do not think of our unvaccinated community members as “hesitant.” We approach all as if they are on a spectrum of deliberation.
Step 9: Reflect
- Understand the importance of your service and the potential impact each encounter has.
- Recognize the unique lived experiences of individual patients and how this may impact their deliberation process. While there is urgency and we may feel frustrated, the ultimate goal is to engender trust through respectful interactions.
- Pause for moments of quiet gratitude and self-check-ins.
Conclusion
Just as SARS-CoV2 spreads from one person to many, we recognize that information—factual and otherwise—has the potential to move quickly as well. It is important to realize that providing an opportunity for people to ask questions or receive clarification and confirmation in a safe space is critical. The No Judgement Zone, as the name indicates, offers this opportunity. The conversations that we have in this space are valuable to those who are still considering the vaccine as an option for themselves. The trust required for such conversations is less about the transmission of information and more about the social act of engaging in bidirectional dialogue. The foundation upon which trust is built is consistent trustworthy actions. One such action is respectful communication without shame or ridicule. Another is our willingness to be transparent about our own concerns, experiences, and journeys. Assumptions based upon single-story narratives of the unvaccinated—particularly those from historically marginalized groups—fracture an already fragile confidence in medical authorities.
While we understand that mitigating the ongoing spread of the virus and getting more people vaccinated will call for more than just individual conversations, we believe that respecting the unique perspectives of community members is an equally critical piece to moving forward. Throughout a healthcare worker’s typical day, we work to create personal moments of connection with patients among the immense bustle of other work that has to be done. Initiatives like this one have a focused intentionality behind creating space for patients to feel heard that is not only helpful for vaccine uptake and addressing mistrust, but can also be restorative for providers as well.
1. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
2. Young S. Black vaccine hesitancy rooted in mistrust, doubts. WebMD. February 2, 2021. Accessed November 1, 2021. https://www.webmd.com/vaccines/covid-19-vaccine/news/20210202/black-vaccine-hesitancy-rooted-in-mistrust-doubts
3. Sanyaolu A, Okorie C, Marinkovic A, et al. Measles outbreak in unvaccinated and partially vaccinated children and adults in the United States and Canada (2018-2019): a narrative review of cases. Inquiry. 2019;56:46958019894098. https://doi.org/10.1177/0046958019894098
4. O’Brien BC. Do you see what I see? Reflections on the relationship between transparency and trust. Acad Med. 2019;94(6):757-759. https://doi.org/10.1097/ACM.0000000000002710
The collective struggle felt by healthcare workers simultaneously learning about and caring for patients impacted by SARS-CoV2 infections throughout 2020 was physically and emotionally exhausting. The majority of us had never experienced a global pandemic. Beyond our work in the professional arena of ambulatory practices and hospitals, we also felt the soul-crushing impact of the pandemic in every other aspect of our lives. Preexisting health disparities were amplified by COVID-19. Some of the most affected communities also bore the weight of an additional tsunami of ongoing racial injustice.1 And as healthcare workers, we did our best to process and navigate it all while trying to avoid burnout—as well as being infected with COVID-19 ourselves. When the news of the highly effective vaccines against SARS-CoV2 receiving emergency use authorization broke late in 2020, it felt like a light at the end of a very dark tunnel.
In the weeks preceding wide availability of the vaccines, it became apparent that significant numbers of people lacked confidence in the vaccines. Given the disproportionate impact of COVID-19 on racial minorities, much of the discussion centered around “vaccine hesitancy” in these communities. Reasons such as historical mistrust, belief in conspiracy theories, and misinformation emerged as the leading explanations.2 Campaigns and educational programs targeting Black Americans were quickly developed to counter this widely distributed narrative.
Vaccine uptake also became politicized, which created additional challenges. As schools and businesses reopened, the voices of those opposing pandemic mitigation mandates such as masking and vaccination were highlighted by media outlets. And though a large movement of individuals who had opted against vaccines existed well before the pandemic, with few exceptions, that number had never been great enough to impact public health to this extent.3 This primarily nonminority group of unvaccinated individuals also morphed into another monolithic identity: the “anti-vaxxer.”
The lion’s share of discussions around vaccine uptake centered on these two groups: the “vaccine hesitant” minority and the “anti-vaxxer.” The perspectives and frustration around these two stereotypical unvaccinated groups were underscored in journals and the lay press. But those working in communities and in direct care came into contact with countless COVID-19-positive patients who were unvaccinated and fell into neither of these categories. There was a large swath of vulnerable people who still had unanswered questions and mistrust in the medical system standing in their way. Awareness of health disparities among racial minorities is something that was discussed among providers, but it was something experienced and felt by patients daily in regard to so much more than just COVID-19.
With broader access to vaccines through retail, community-based, and clinical facilities, more patients who desired vaccination had the opportunity. After an initial rise in vaccine uptake, the numbers plateaued. But what remained was the repetitive messaging and sustained focus directed toward Black people and their “vaccine hesitancy.”
Grady Memorial Hospital, a public safety net hospital in Atlanta, serves a predominantly Black and uninsured patient population. We found that a “FAQ” approach with a narrow range of hypothetical ideas about unvaccinated minorities clashed with the reality of what we encountered in clinical environments and the community. While misinformation did appear to be prevalent, we appreciated that the context and level of conviction were heterogenous. We appreciated that each individual conversation could reveal something new to us about that unique patient and their personal concerns about vaccination. As time moved forward, it became clear that there was no playbook for any group, especially for historically disadvantaged communities. Importantly, it was recognized that attempts to anticipate what may be a person’s barrier to vaccination often worked to further erode trust. However, when we focused on creating a space for dialogue, we found we were able to move beyond information-sharing and instead were able to co-construct interpretations of information and co-create solutions that matched patients’ values and lived experiences.4 Through dialogue, we were better able to be transparent about our own experiences, which ultimately facilitated authentic conversations with patients.
In September 2021, we approached our hospital leadership with a patient-centered strategy aimed at providing our patients, staff, and visitors a psychologically safe place to discuss vaccine-related concerns without judgment. With their support, we set up a table in the busiest part of our hospital atrium between the information desk and vaccine-administration site. Beside it was a folding board sign with an image and these words:
“Still unsure about being vaccinated? Let’s talk about it.”
We aptly called the area the “No Judgment Zone.”
The No Judgment Zone is collaboratively staffed in 1- to 2-hour voluntary increments by physician faculty and resident physicians at Emory University School of Medicine and Morehouse School of Medicine. Our goal is to increase patient trust by honoring individual vaccine-related concerns without shame or ridicule. We also work to increase patient trust by being transparent around our own experiences with COVID-19; by sharing our own journeys, concerns, and challenges, we are better able to engage in meaningful dialogue. Also, recognizing the power of logistical barriers, in addition to answering questions, we offer physical assistance with check-in, forms, and escorts to our administration areas. The numbers of unique visits have varied from day to day, but the impact of each individual encounter cannot be overstated.
Here, we describe our approach to interactions at the No Judgment Zone. These are the instructions offered to our volunteers. Though we offer some explicit examples, each talking point is designed to open the door to a patient-centered individual dialogue. We believe that these strategies can be applied to clinical settings as well as any conversation surrounding vaccination with those who have not yet decided to be vaccinated.
THE GRADY “NO JUDGMENT ZONE” INTERACTION APPROACH
No Labels
Try to think of all who are not yet vaccinated as “on a spectrum of deliberation” about their decision—not “hesitant” or “anti-vaxxer.”
Step 1: Gratitude
- “Thank you for stopping to talk to us today.”
- “I appreciate you taking the time.”
- “Before we start—I’m glad you’re here. Thanks.”
Step 2: Determine Where They Are
- Has the person you’re speaking with been vaccinated yet?
- If no, ask: “On a scale of 0 to 10—zero being “I will never get vaccinated under any circumstances” and 10 being ‘I will definitely get vaccinated’—what number would you give yourself?”
- If the person is a firm zero: “Is there anything I might be able to share with you or tell you about that might move you away from that perspective?”
- If the answer is NO: “It sounds like you’ve thought a lot about this and are no longer deliberating about whether you will be vaccinated. If you find yourself considering it, come back to talk with us, okay?” We are not here to debate or argue. We also need to avail ourselves to those who are open to changing their mind.
- If they say anything other than zero, move to an open-ended question about #WhatsYourWhy.
Step 3: #WhatsYourWhy
- “What would you say has been your main reason for not getting vaccinated yet?”
- “Tell me what has stood in the way of you getting vaccinated.”
- Remember: Assume nothing. It may have nothing to do with misinformation, fear, or perceived threat. It could be logistics or many other things. You will not know unless you ask.
- Providers should feel encouraged to also share their why as well and the reasons they encouraged their parents/kids/loved ones to get vaccinated. Making it personal can help establish connection and be more compelling.
Step 4: Listen Completely
- Give full eye contact. Slow all body movements. Use facilitative gestures to let the person know you are listening.
- Do not plan what you wish to say next.
- Limit reactions to misinformation. Shame and judgment can be subtle. Be mindful.
- Repeat the concern back if you are not sure or want to confirm that you’ve heard correctly.
- Ask questions for clarity if you aren’t sure.
Step 5: Affirm All Concerns and Find Common Ground
- “I can only imagine how scary it must be to take a shot that you believe could cause you to not be able to have babies.”
- “You aren’t alone. That’s a concern that many of my patients have had, too. May I share some information about that with you?”
- “When I first heard about the vaccine, I worried it was too new, too. Can I share what I learned?”
Step 6: Provide Factual Information
- Without excessive medical jargon, offer factual information aimed at each concern or question. Probe to be certain your patient understands through a teach-back or question.
- If you are unsure about the answer to their question, admit that you don’t know. You can also ask a colleague or the attending with you. Another option is to call someone or say “Let’s pull this up together.” Then share your answer.
- It is okay to acknowledge that the healthcare system has not and does not always do right by minority populations, especially Black people. Use that as a pivot to why these truths make vaccination that much more important
- Have FAQ information sheets available. Confirm that the patient is comfortable with the information sheet by asking.
Step 7: Offer to Help Them Get Vaccinated Today
- “Would you like me to help you get vaccinated today?”
- “What can I do to assist you with getting vaccinated? Is today a good day?”
- Escort those who agree to the registration area.
- Affirm those plans to get vaccinated or those who feel closer to getting vaccinated after speaking with you.
Step 8: Gratitude
- Close with gratitude and an affirmation.
- “I’m so glad you took the time to talk with us today. You didn’t have to stop.”
- “Feel free to come back to talk to us if you think of any more questions. I’m grateful that you stopped.”
- We are planting seeds. Do not feel pressure to get a person to say yes. Our secret sauce is kindness, respect, and empathy.
- We do not think of our unvaccinated community members as “hesitant.” We approach all as if they are on a spectrum of deliberation.
Step 9: Reflect
- Understand the importance of your service and the potential impact each encounter has.
- Recognize the unique lived experiences of individual patients and how this may impact their deliberation process. While there is urgency and we may feel frustrated, the ultimate goal is to engender trust through respectful interactions.
- Pause for moments of quiet gratitude and self-check-ins.
Conclusion
Just as SARS-CoV2 spreads from one person to many, we recognize that information—factual and otherwise—has the potential to move quickly as well. It is important to realize that providing an opportunity for people to ask questions or receive clarification and confirmation in a safe space is critical. The No Judgement Zone, as the name indicates, offers this opportunity. The conversations that we have in this space are valuable to those who are still considering the vaccine as an option for themselves. The trust required for such conversations is less about the transmission of information and more about the social act of engaging in bidirectional dialogue. The foundation upon which trust is built is consistent trustworthy actions. One such action is respectful communication without shame or ridicule. Another is our willingness to be transparent about our own concerns, experiences, and journeys. Assumptions based upon single-story narratives of the unvaccinated—particularly those from historically marginalized groups—fracture an already fragile confidence in medical authorities.
While we understand that mitigating the ongoing spread of the virus and getting more people vaccinated will call for more than just individual conversations, we believe that respecting the unique perspectives of community members is an equally critical piece to moving forward. Throughout a healthcare worker’s typical day, we work to create personal moments of connection with patients among the immense bustle of other work that has to be done. Initiatives like this one have a focused intentionality behind creating space for patients to feel heard that is not only helpful for vaccine uptake and addressing mistrust, but can also be restorative for providers as well.
The collective struggle felt by healthcare workers simultaneously learning about and caring for patients impacted by SARS-CoV2 infections throughout 2020 was physically and emotionally exhausting. The majority of us had never experienced a global pandemic. Beyond our work in the professional arena of ambulatory practices and hospitals, we also felt the soul-crushing impact of the pandemic in every other aspect of our lives. Preexisting health disparities were amplified by COVID-19. Some of the most affected communities also bore the weight of an additional tsunami of ongoing racial injustice.1 And as healthcare workers, we did our best to process and navigate it all while trying to avoid burnout—as well as being infected with COVID-19 ourselves. When the news of the highly effective vaccines against SARS-CoV2 receiving emergency use authorization broke late in 2020, it felt like a light at the end of a very dark tunnel.
In the weeks preceding wide availability of the vaccines, it became apparent that significant numbers of people lacked confidence in the vaccines. Given the disproportionate impact of COVID-19 on racial minorities, much of the discussion centered around “vaccine hesitancy” in these communities. Reasons such as historical mistrust, belief in conspiracy theories, and misinformation emerged as the leading explanations.2 Campaigns and educational programs targeting Black Americans were quickly developed to counter this widely distributed narrative.
Vaccine uptake also became politicized, which created additional challenges. As schools and businesses reopened, the voices of those opposing pandemic mitigation mandates such as masking and vaccination were highlighted by media outlets. And though a large movement of individuals who had opted against vaccines existed well before the pandemic, with few exceptions, that number had never been great enough to impact public health to this extent.3 This primarily nonminority group of unvaccinated individuals also morphed into another monolithic identity: the “anti-vaxxer.”
The lion’s share of discussions around vaccine uptake centered on these two groups: the “vaccine hesitant” minority and the “anti-vaxxer.” The perspectives and frustration around these two stereotypical unvaccinated groups were underscored in journals and the lay press. But those working in communities and in direct care came into contact with countless COVID-19-positive patients who were unvaccinated and fell into neither of these categories. There was a large swath of vulnerable people who still had unanswered questions and mistrust in the medical system standing in their way. Awareness of health disparities among racial minorities is something that was discussed among providers, but it was something experienced and felt by patients daily in regard to so much more than just COVID-19.
With broader access to vaccines through retail, community-based, and clinical facilities, more patients who desired vaccination had the opportunity. After an initial rise in vaccine uptake, the numbers plateaued. But what remained was the repetitive messaging and sustained focus directed toward Black people and their “vaccine hesitancy.”
Grady Memorial Hospital, a public safety net hospital in Atlanta, serves a predominantly Black and uninsured patient population. We found that a “FAQ” approach with a narrow range of hypothetical ideas about unvaccinated minorities clashed with the reality of what we encountered in clinical environments and the community. While misinformation did appear to be prevalent, we appreciated that the context and level of conviction were heterogenous. We appreciated that each individual conversation could reveal something new to us about that unique patient and their personal concerns about vaccination. As time moved forward, it became clear that there was no playbook for any group, especially for historically disadvantaged communities. Importantly, it was recognized that attempts to anticipate what may be a person’s barrier to vaccination often worked to further erode trust. However, when we focused on creating a space for dialogue, we found we were able to move beyond information-sharing and instead were able to co-construct interpretations of information and co-create solutions that matched patients’ values and lived experiences.4 Through dialogue, we were better able to be transparent about our own experiences, which ultimately facilitated authentic conversations with patients.
In September 2021, we approached our hospital leadership with a patient-centered strategy aimed at providing our patients, staff, and visitors a psychologically safe place to discuss vaccine-related concerns without judgment. With their support, we set up a table in the busiest part of our hospital atrium between the information desk and vaccine-administration site. Beside it was a folding board sign with an image and these words:
“Still unsure about being vaccinated? Let’s talk about it.”
We aptly called the area the “No Judgment Zone.”
The No Judgment Zone is collaboratively staffed in 1- to 2-hour voluntary increments by physician faculty and resident physicians at Emory University School of Medicine and Morehouse School of Medicine. Our goal is to increase patient trust by honoring individual vaccine-related concerns without shame or ridicule. We also work to increase patient trust by being transparent around our own experiences with COVID-19; by sharing our own journeys, concerns, and challenges, we are better able to engage in meaningful dialogue. Also, recognizing the power of logistical barriers, in addition to answering questions, we offer physical assistance with check-in, forms, and escorts to our administration areas. The numbers of unique visits have varied from day to day, but the impact of each individual encounter cannot be overstated.
Here, we describe our approach to interactions at the No Judgment Zone. These are the instructions offered to our volunteers. Though we offer some explicit examples, each talking point is designed to open the door to a patient-centered individual dialogue. We believe that these strategies can be applied to clinical settings as well as any conversation surrounding vaccination with those who have not yet decided to be vaccinated.
THE GRADY “NO JUDGMENT ZONE” INTERACTION APPROACH
No Labels
Try to think of all who are not yet vaccinated as “on a spectrum of deliberation” about their decision—not “hesitant” or “anti-vaxxer.”
Step 1: Gratitude
- “Thank you for stopping to talk to us today.”
- “I appreciate you taking the time.”
- “Before we start—I’m glad you’re here. Thanks.”
Step 2: Determine Where They Are
- Has the person you’re speaking with been vaccinated yet?
- If no, ask: “On a scale of 0 to 10—zero being “I will never get vaccinated under any circumstances” and 10 being ‘I will definitely get vaccinated’—what number would you give yourself?”
- If the person is a firm zero: “Is there anything I might be able to share with you or tell you about that might move you away from that perspective?”
- If the answer is NO: “It sounds like you’ve thought a lot about this and are no longer deliberating about whether you will be vaccinated. If you find yourself considering it, come back to talk with us, okay?” We are not here to debate or argue. We also need to avail ourselves to those who are open to changing their mind.
- If they say anything other than zero, move to an open-ended question about #WhatsYourWhy.
Step 3: #WhatsYourWhy
- “What would you say has been your main reason for not getting vaccinated yet?”
- “Tell me what has stood in the way of you getting vaccinated.”
- Remember: Assume nothing. It may have nothing to do with misinformation, fear, or perceived threat. It could be logistics or many other things. You will not know unless you ask.
- Providers should feel encouraged to also share their why as well and the reasons they encouraged their parents/kids/loved ones to get vaccinated. Making it personal can help establish connection and be more compelling.
Step 4: Listen Completely
- Give full eye contact. Slow all body movements. Use facilitative gestures to let the person know you are listening.
- Do not plan what you wish to say next.
- Limit reactions to misinformation. Shame and judgment can be subtle. Be mindful.
- Repeat the concern back if you are not sure or want to confirm that you’ve heard correctly.
- Ask questions for clarity if you aren’t sure.
Step 5: Affirm All Concerns and Find Common Ground
- “I can only imagine how scary it must be to take a shot that you believe could cause you to not be able to have babies.”
- “You aren’t alone. That’s a concern that many of my patients have had, too. May I share some information about that with you?”
- “When I first heard about the vaccine, I worried it was too new, too. Can I share what I learned?”
Step 6: Provide Factual Information
- Without excessive medical jargon, offer factual information aimed at each concern or question. Probe to be certain your patient understands through a teach-back or question.
- If you are unsure about the answer to their question, admit that you don’t know. You can also ask a colleague or the attending with you. Another option is to call someone or say “Let’s pull this up together.” Then share your answer.
- It is okay to acknowledge that the healthcare system has not and does not always do right by minority populations, especially Black people. Use that as a pivot to why these truths make vaccination that much more important
- Have FAQ information sheets available. Confirm that the patient is comfortable with the information sheet by asking.
Step 7: Offer to Help Them Get Vaccinated Today
- “Would you like me to help you get vaccinated today?”
- “What can I do to assist you with getting vaccinated? Is today a good day?”
- Escort those who agree to the registration area.
- Affirm those plans to get vaccinated or those who feel closer to getting vaccinated after speaking with you.
Step 8: Gratitude
- Close with gratitude and an affirmation.
- “I’m so glad you took the time to talk with us today. You didn’t have to stop.”
- “Feel free to come back to talk to us if you think of any more questions. I’m grateful that you stopped.”
- We are planting seeds. Do not feel pressure to get a person to say yes. Our secret sauce is kindness, respect, and empathy.
- We do not think of our unvaccinated community members as “hesitant.” We approach all as if they are on a spectrum of deliberation.
Step 9: Reflect
- Understand the importance of your service and the potential impact each encounter has.
- Recognize the unique lived experiences of individual patients and how this may impact their deliberation process. While there is urgency and we may feel frustrated, the ultimate goal is to engender trust through respectful interactions.
- Pause for moments of quiet gratitude and self-check-ins.
Conclusion
Just as SARS-CoV2 spreads from one person to many, we recognize that information—factual and otherwise—has the potential to move quickly as well. It is important to realize that providing an opportunity for people to ask questions or receive clarification and confirmation in a safe space is critical. The No Judgement Zone, as the name indicates, offers this opportunity. The conversations that we have in this space are valuable to those who are still considering the vaccine as an option for themselves. The trust required for such conversations is less about the transmission of information and more about the social act of engaging in bidirectional dialogue. The foundation upon which trust is built is consistent trustworthy actions. One such action is respectful communication without shame or ridicule. Another is our willingness to be transparent about our own concerns, experiences, and journeys. Assumptions based upon single-story narratives of the unvaccinated—particularly those from historically marginalized groups—fracture an already fragile confidence in medical authorities.
While we understand that mitigating the ongoing spread of the virus and getting more people vaccinated will call for more than just individual conversations, we believe that respecting the unique perspectives of community members is an equally critical piece to moving forward. Throughout a healthcare worker’s typical day, we work to create personal moments of connection with patients among the immense bustle of other work that has to be done. Initiatives like this one have a focused intentionality behind creating space for patients to feel heard that is not only helpful for vaccine uptake and addressing mistrust, but can also be restorative for providers as well.
1. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
2. Young S. Black vaccine hesitancy rooted in mistrust, doubts. WebMD. February 2, 2021. Accessed November 1, 2021. https://www.webmd.com/vaccines/covid-19-vaccine/news/20210202/black-vaccine-hesitancy-rooted-in-mistrust-doubts
3. Sanyaolu A, Okorie C, Marinkovic A, et al. Measles outbreak in unvaccinated and partially vaccinated children and adults in the United States and Canada (2018-2019): a narrative review of cases. Inquiry. 2019;56:46958019894098. https://doi.org/10.1177/0046958019894098
4. O’Brien BC. Do you see what I see? Reflections on the relationship between transparency and trust. Acad Med. 2019;94(6):757-759. https://doi.org/10.1097/ACM.0000000000002710
1. Manning KD. When grief and crises intersect: perspectives of a Black physician in the time of two pandemics. J Hosp Med. 2020;15(9):566-567. https://doi.org/10.12788/jhm.3481
2. Young S. Black vaccine hesitancy rooted in mistrust, doubts. WebMD. February 2, 2021. Accessed November 1, 2021. https://www.webmd.com/vaccines/covid-19-vaccine/news/20210202/black-vaccine-hesitancy-rooted-in-mistrust-doubts
3. Sanyaolu A, Okorie C, Marinkovic A, et al. Measles outbreak in unvaccinated and partially vaccinated children and adults in the United States and Canada (2018-2019): a narrative review of cases. Inquiry. 2019;56:46958019894098. https://doi.org/10.1177/0046958019894098
4. O’Brien BC. Do you see what I see? Reflections on the relationship between transparency and trust. Acad Med. 2019;94(6):757-759. https://doi.org/10.1097/ACM.0000000000002710
© 2021 Society of Hospital Medicine
Radiologically Isolated Syndrome: A condition that often precedes an MS diagnosis in children
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227