User login
mRNA COVID vaccine response found mostly robust in RA, SLE patients
Immunosuppressed patients with autoimmune diseases who received the Moderna mRNA-1273 SARS-CoV-2 two-dose vaccine series had a frequency of adverse events similar to the general population albeit with a somewhat reduced, but still significant, antibody response with no severe vaccine-related disease flares, results of a prospective, nonrandomized open-label comparative trial in Canada demonstrated.
At the same time, patients with RA who were taking rituximab and patients with systemic lupus erythematosus (SLE) who were taking mycophenolate mofetil seemed to have reduced humoral responses after receiving the vaccine, said Ines Colmegna, MD, reporting results of the COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Disease (COVIAAD) study as a late-breaking poster abstract at the virtual annual meeting of the American College of Rheumatology. Dr. Colmegna is an associate professor of rheumatology in the division of experimental medicine at McGill University, Montreal.
“The frequency of adverse events, specifically the reactogenicity in people with comorbid conditions regardless of their diagnosis, was similar to healthy controls in this study, and their frequency was similar also the initial studies in the general population,” Dr. Colmegna said.
COVIAAD prospectively enrolled 220 fully vaccinated patients, 162 with rheumatic disease (131 with RA, 23 with SLE, and 8 with other diseases) and 58 controls. Adverse events a week and a month after each dose was the primary outcome. The postvaccine presence of the IgG antibody against the SARS-CoV-2 spike protein and the receptor binding domain (IgG-RBD) was the secondary outcome. Dr. Colmegna said that the study will continue evaluating participants after they get a third dose.
The Canadian trial appears to validate the ACR’s COVID-19 vaccine guidance, the fourth version of which was issued in October, said Jeffrey Curtis, MD, MS, MPH, professor of immunology and rheumatology at the University of Alabama at Birmingham and lead of the ACR COVID-19 Vaccine Guidance Task Force. Specifically, the guidance recommends that patients on rituximab or other anti-CD20 B-cell–depleting agents discuss vaccine timing with their rheumatologist.
“A few things changed over time when there was a paucity of evidence for any vaccine, but as time has gone on, mostly we were more correct than we weren’t,” Dr. Curtis said of the task force’s work. “The evidence that now is in this poster with regard to systemic lupus erythematosus and mycophenolate mofetil is [that] you have impaired vaccine response. If you’re on a B-cell drug like rituximab, you really have impaired vaccine response.”
In the study, 100% of controls had immunogenicity in terms of anti-spike and anti-RBD levels after the first and second dose. The rate of immunogenicity after the first and second dose were 67% and 88% in all patients with RA, and 35% and 78% in patients with SLE who were taking mycophenolate mofetil. The subset of patients with RA on rituximab (n = 17) had rates of immunogenicity of 5.9% and 17.6%, respectively.
“Measured antibody response is not the only way in which people develop a response to a vaccine, and there are also similar responses that occur even in people who are on rituximab and have not developed antibodies,” Dr. Colmegna said. “That’s a very important message also that we need to convey to patients: The immune response really extends beyond antibody protection.”
Overall, disease activity in both patients with RA and SLE did not appreciably change from baseline within 7 days and 28 days of each vaccine dose.
The study raises important questions about the timing of the vaccine, particularly in patients on rituximab, Dr. Colmegna said in an interview. “In theory, there is no element to suggest that, if you would schedule the vaccine a month prior to the next dose of rituximab, the effect of the drug would have decreased the number of B cells, and that the possibility of developing antibodies in response to the vaccine might be better if you give rituximab a month later when the amount of the drug and the effect of the drug is maximal,” she said. The average interval between patients receiving rituximab and vaccines was 4.5 months, Dr. Colmegna said in answering a question after her presentation.
Dr. Curtis said that the effect of holding rituximab or the vaccine to boost antibodies “is somewhat yet unknown. We think it will help, but that’s not a guarantee,” he said. “We don’t have direct evidence that just because the drug impairs vaccine response, that holding that drug for a week or 2 is going to take care of the problem.”
The study does arm rheumatologists with more information for discussing COVID vaccines with vaccine-hesitant patients with autoimmune diseases, Dr. Curtis said.
“It gives them evidence that for most of our immunomodulatory drugs the vaccine works pretty well,” he said. “The poster provides evidence that, compared to healthy controls, the vaccine doesn’t work quite as well in some patients, but for most people it actually did work pretty well. That reinforces the message: Go get vaccinated because [you] will mount [an immune] response, even, if that response isn’t quite as brisk as it is in healthy people.”
Dr. Colmegna and Dr. Curtis have no relevant relationships to disclose. The study received funding from Health and Social Services Quebec.
Immunosuppressed patients with autoimmune diseases who received the Moderna mRNA-1273 SARS-CoV-2 two-dose vaccine series had a frequency of adverse events similar to the general population albeit with a somewhat reduced, but still significant, antibody response with no severe vaccine-related disease flares, results of a prospective, nonrandomized open-label comparative trial in Canada demonstrated.
At the same time, patients with RA who were taking rituximab and patients with systemic lupus erythematosus (SLE) who were taking mycophenolate mofetil seemed to have reduced humoral responses after receiving the vaccine, said Ines Colmegna, MD, reporting results of the COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Disease (COVIAAD) study as a late-breaking poster abstract at the virtual annual meeting of the American College of Rheumatology. Dr. Colmegna is an associate professor of rheumatology in the division of experimental medicine at McGill University, Montreal.
“The frequency of adverse events, specifically the reactogenicity in people with comorbid conditions regardless of their diagnosis, was similar to healthy controls in this study, and their frequency was similar also the initial studies in the general population,” Dr. Colmegna said.
COVIAAD prospectively enrolled 220 fully vaccinated patients, 162 with rheumatic disease (131 with RA, 23 with SLE, and 8 with other diseases) and 58 controls. Adverse events a week and a month after each dose was the primary outcome. The postvaccine presence of the IgG antibody against the SARS-CoV-2 spike protein and the receptor binding domain (IgG-RBD) was the secondary outcome. Dr. Colmegna said that the study will continue evaluating participants after they get a third dose.
The Canadian trial appears to validate the ACR’s COVID-19 vaccine guidance, the fourth version of which was issued in October, said Jeffrey Curtis, MD, MS, MPH, professor of immunology and rheumatology at the University of Alabama at Birmingham and lead of the ACR COVID-19 Vaccine Guidance Task Force. Specifically, the guidance recommends that patients on rituximab or other anti-CD20 B-cell–depleting agents discuss vaccine timing with their rheumatologist.
“A few things changed over time when there was a paucity of evidence for any vaccine, but as time has gone on, mostly we were more correct than we weren’t,” Dr. Curtis said of the task force’s work. “The evidence that now is in this poster with regard to systemic lupus erythematosus and mycophenolate mofetil is [that] you have impaired vaccine response. If you’re on a B-cell drug like rituximab, you really have impaired vaccine response.”
In the study, 100% of controls had immunogenicity in terms of anti-spike and anti-RBD levels after the first and second dose. The rate of immunogenicity after the first and second dose were 67% and 88% in all patients with RA, and 35% and 78% in patients with SLE who were taking mycophenolate mofetil. The subset of patients with RA on rituximab (n = 17) had rates of immunogenicity of 5.9% and 17.6%, respectively.
“Measured antibody response is not the only way in which people develop a response to a vaccine, and there are also similar responses that occur even in people who are on rituximab and have not developed antibodies,” Dr. Colmegna said. “That’s a very important message also that we need to convey to patients: The immune response really extends beyond antibody protection.”
Overall, disease activity in both patients with RA and SLE did not appreciably change from baseline within 7 days and 28 days of each vaccine dose.
The study raises important questions about the timing of the vaccine, particularly in patients on rituximab, Dr. Colmegna said in an interview. “In theory, there is no element to suggest that, if you would schedule the vaccine a month prior to the next dose of rituximab, the effect of the drug would have decreased the number of B cells, and that the possibility of developing antibodies in response to the vaccine might be better if you give rituximab a month later when the amount of the drug and the effect of the drug is maximal,” she said. The average interval between patients receiving rituximab and vaccines was 4.5 months, Dr. Colmegna said in answering a question after her presentation.
Dr. Curtis said that the effect of holding rituximab or the vaccine to boost antibodies “is somewhat yet unknown. We think it will help, but that’s not a guarantee,” he said. “We don’t have direct evidence that just because the drug impairs vaccine response, that holding that drug for a week or 2 is going to take care of the problem.”
The study does arm rheumatologists with more information for discussing COVID vaccines with vaccine-hesitant patients with autoimmune diseases, Dr. Curtis said.
“It gives them evidence that for most of our immunomodulatory drugs the vaccine works pretty well,” he said. “The poster provides evidence that, compared to healthy controls, the vaccine doesn’t work quite as well in some patients, but for most people it actually did work pretty well. That reinforces the message: Go get vaccinated because [you] will mount [an immune] response, even, if that response isn’t quite as brisk as it is in healthy people.”
Dr. Colmegna and Dr. Curtis have no relevant relationships to disclose. The study received funding from Health and Social Services Quebec.
Immunosuppressed patients with autoimmune diseases who received the Moderna mRNA-1273 SARS-CoV-2 two-dose vaccine series had a frequency of adverse events similar to the general population albeit with a somewhat reduced, but still significant, antibody response with no severe vaccine-related disease flares, results of a prospective, nonrandomized open-label comparative trial in Canada demonstrated.
At the same time, patients with RA who were taking rituximab and patients with systemic lupus erythematosus (SLE) who were taking mycophenolate mofetil seemed to have reduced humoral responses after receiving the vaccine, said Ines Colmegna, MD, reporting results of the COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Disease (COVIAAD) study as a late-breaking poster abstract at the virtual annual meeting of the American College of Rheumatology. Dr. Colmegna is an associate professor of rheumatology in the division of experimental medicine at McGill University, Montreal.
“The frequency of adverse events, specifically the reactogenicity in people with comorbid conditions regardless of their diagnosis, was similar to healthy controls in this study, and their frequency was similar also the initial studies in the general population,” Dr. Colmegna said.
COVIAAD prospectively enrolled 220 fully vaccinated patients, 162 with rheumatic disease (131 with RA, 23 with SLE, and 8 with other diseases) and 58 controls. Adverse events a week and a month after each dose was the primary outcome. The postvaccine presence of the IgG antibody against the SARS-CoV-2 spike protein and the receptor binding domain (IgG-RBD) was the secondary outcome. Dr. Colmegna said that the study will continue evaluating participants after they get a third dose.
The Canadian trial appears to validate the ACR’s COVID-19 vaccine guidance, the fourth version of which was issued in October, said Jeffrey Curtis, MD, MS, MPH, professor of immunology and rheumatology at the University of Alabama at Birmingham and lead of the ACR COVID-19 Vaccine Guidance Task Force. Specifically, the guidance recommends that patients on rituximab or other anti-CD20 B-cell–depleting agents discuss vaccine timing with their rheumatologist.
“A few things changed over time when there was a paucity of evidence for any vaccine, but as time has gone on, mostly we were more correct than we weren’t,” Dr. Curtis said of the task force’s work. “The evidence that now is in this poster with regard to systemic lupus erythematosus and mycophenolate mofetil is [that] you have impaired vaccine response. If you’re on a B-cell drug like rituximab, you really have impaired vaccine response.”
In the study, 100% of controls had immunogenicity in terms of anti-spike and anti-RBD levels after the first and second dose. The rate of immunogenicity after the first and second dose were 67% and 88% in all patients with RA, and 35% and 78% in patients with SLE who were taking mycophenolate mofetil. The subset of patients with RA on rituximab (n = 17) had rates of immunogenicity of 5.9% and 17.6%, respectively.
“Measured antibody response is not the only way in which people develop a response to a vaccine, and there are also similar responses that occur even in people who are on rituximab and have not developed antibodies,” Dr. Colmegna said. “That’s a very important message also that we need to convey to patients: The immune response really extends beyond antibody protection.”
Overall, disease activity in both patients with RA and SLE did not appreciably change from baseline within 7 days and 28 days of each vaccine dose.
The study raises important questions about the timing of the vaccine, particularly in patients on rituximab, Dr. Colmegna said in an interview. “In theory, there is no element to suggest that, if you would schedule the vaccine a month prior to the next dose of rituximab, the effect of the drug would have decreased the number of B cells, and that the possibility of developing antibodies in response to the vaccine might be better if you give rituximab a month later when the amount of the drug and the effect of the drug is maximal,” she said. The average interval between patients receiving rituximab and vaccines was 4.5 months, Dr. Colmegna said in answering a question after her presentation.
Dr. Curtis said that the effect of holding rituximab or the vaccine to boost antibodies “is somewhat yet unknown. We think it will help, but that’s not a guarantee,” he said. “We don’t have direct evidence that just because the drug impairs vaccine response, that holding that drug for a week or 2 is going to take care of the problem.”
The study does arm rheumatologists with more information for discussing COVID vaccines with vaccine-hesitant patients with autoimmune diseases, Dr. Curtis said.
“It gives them evidence that for most of our immunomodulatory drugs the vaccine works pretty well,” he said. “The poster provides evidence that, compared to healthy controls, the vaccine doesn’t work quite as well in some patients, but for most people it actually did work pretty well. That reinforces the message: Go get vaccinated because [you] will mount [an immune] response, even, if that response isn’t quite as brisk as it is in healthy people.”
Dr. Colmegna and Dr. Curtis have no relevant relationships to disclose. The study received funding from Health and Social Services Quebec.
FROM ACR 2021
AHA 2021 puts scientific dialogue, health equity center stage
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Itchy belly
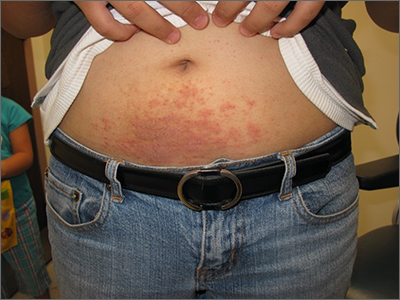
Examination revealed diffusely bordered periumbilical pink to violet scaly plaques consistent with nickel allergic contact dermatitis (Ni-ACD). The patient was wearing a belt with a buckle containing nickel, which had begun dispersing nickel. Her earlobes were also pierced and had similar scale and erythema around the metal earring studs
Ni-ACD is the most common, delayed-type hypersensitivity reaction worldwide. It affects 10% of people in the United States with a strong female predominance and a 4-fold increase in the last 30 years.1 The induction of nickel delayed-type hypersensitivity has been well-studied and includes nickel corrosion dissolving into a solution and exceeding an immunogenic threshold. Piercing practices, sweat, and friction facilitate this process.
Gold jewelry that’s less than 24 karat, “white gold,” and stainless steel all contain nickel and may cause allergy in sensitized individuals. It’s wise to assume that any shiny metal fashion accessory contains nickel, unless proven otherwise. Items can be tested for the presence of nickel with an inexpensive kit containing dimethylglyoxime.
Symptoms of Ni-ACD may range from mild erythema to thickened and weepy lichenified plaques. Distribution is often present at the site of exposure but may also be seen on the eyelids or hands from nickel transfer. A systematized reaction or id reaction is uncommon but can occur. Allergic contact dermatitis can be distinguished from psoriasis by a fading border rather than a sharp, well-demarcated border.
The patient in this case switched to a nonmetallic belt and earrings with plastic studs. She was prescribed topical triamcinolone cream 0.1% bid for 3 weeks, which led to clearance of her rash.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Silverberg N, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:e20200628. doi: 10.1542/peds.2020-0628

Examination revealed diffusely bordered periumbilical pink to violet scaly plaques consistent with nickel allergic contact dermatitis (Ni-ACD). The patient was wearing a belt with a buckle containing nickel, which had begun dispersing nickel. Her earlobes were also pierced and had similar scale and erythema around the metal earring studs
Ni-ACD is the most common, delayed-type hypersensitivity reaction worldwide. It affects 10% of people in the United States with a strong female predominance and a 4-fold increase in the last 30 years.1 The induction of nickel delayed-type hypersensitivity has been well-studied and includes nickel corrosion dissolving into a solution and exceeding an immunogenic threshold. Piercing practices, sweat, and friction facilitate this process.
Gold jewelry that’s less than 24 karat, “white gold,” and stainless steel all contain nickel and may cause allergy in sensitized individuals. It’s wise to assume that any shiny metal fashion accessory contains nickel, unless proven otherwise. Items can be tested for the presence of nickel with an inexpensive kit containing dimethylglyoxime.
Symptoms of Ni-ACD may range from mild erythema to thickened and weepy lichenified plaques. Distribution is often present at the site of exposure but may also be seen on the eyelids or hands from nickel transfer. A systematized reaction or id reaction is uncommon but can occur. Allergic contact dermatitis can be distinguished from psoriasis by a fading border rather than a sharp, well-demarcated border.
The patient in this case switched to a nonmetallic belt and earrings with plastic studs. She was prescribed topical triamcinolone cream 0.1% bid for 3 weeks, which led to clearance of her rash.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Examination revealed diffusely bordered periumbilical pink to violet scaly plaques consistent with nickel allergic contact dermatitis (Ni-ACD). The patient was wearing a belt with a buckle containing nickel, which had begun dispersing nickel. Her earlobes were also pierced and had similar scale and erythema around the metal earring studs
Ni-ACD is the most common, delayed-type hypersensitivity reaction worldwide. It affects 10% of people in the United States with a strong female predominance and a 4-fold increase in the last 30 years.1 The induction of nickel delayed-type hypersensitivity has been well-studied and includes nickel corrosion dissolving into a solution and exceeding an immunogenic threshold. Piercing practices, sweat, and friction facilitate this process.
Gold jewelry that’s less than 24 karat, “white gold,” and stainless steel all contain nickel and may cause allergy in sensitized individuals. It’s wise to assume that any shiny metal fashion accessory contains nickel, unless proven otherwise. Items can be tested for the presence of nickel with an inexpensive kit containing dimethylglyoxime.
Symptoms of Ni-ACD may range from mild erythema to thickened and weepy lichenified plaques. Distribution is often present at the site of exposure but may also be seen on the eyelids or hands from nickel transfer. A systematized reaction or id reaction is uncommon but can occur. Allergic contact dermatitis can be distinguished from psoriasis by a fading border rather than a sharp, well-demarcated border.
The patient in this case switched to a nonmetallic belt and earrings with plastic studs. She was prescribed topical triamcinolone cream 0.1% bid for 3 weeks, which led to clearance of her rash.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Silverberg N, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:e20200628. doi: 10.1542/peds.2020-0628
1. Silverberg N, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:e20200628. doi: 10.1542/peds.2020-0628
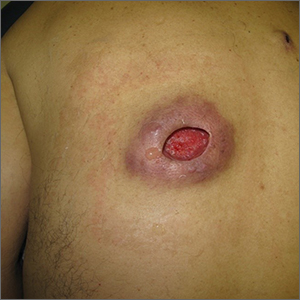
Nonhealing incision and drainage site
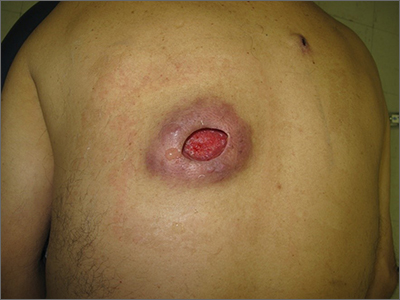
Additional history from the family revealed that they were cleaning the wound with copious amounts of hydrogen peroxide twice a week—a practice that impedes wound healing and was ultimately the cause of this patient’s wound closure delay.
A nonhealing wound should be carefully evaluated to rule out malignancy, infection, or an inflammatory disorder such as pyoderma gangrenosum (PG). In this case, punch biopsies were performed to exclude PG and malignancy, particularly squamous cell carcinoma. Additionally, punch biopsies were performed for bacterial and fungal tissue culture. A complete blood count with differential was obtained to evaluate for signs of infection or hematologic malignancy. All work-ups and biopsies were consistent with a noninfected surgical wound.
Widely available over the counter in 3% to 5% solutions, hydrogen peroxide is used as a low-cost antiseptic for minor cuts and wounds. Data are mixed as to whether hydrogen peroxide improves or impedes wound healing when used outside of initial first aid or postoperatively.1 At higher concentrations, it uniformly causes skin necrosis. Owing to its debriding effect, it is FDA approved to treat seborrheic keratoses as an alternative to cryotherapy or electrodessication and curettage.
At the time of this work-up, and in the absence of other signs of infection, the patient and family were told to stop using hydrogen peroxide. Care instructions were changed to daily topical petroleum jelly and wound occlusion. Four weeks after wound care changes were made, the wound had re-epithelialized completely and reduced in size by two-thirds.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Murphy EC, Friedman AJ. Hydrogen peroxide and cutaneous biology: translational applications, benefits, and risks. J Am Acad Dermatol. 2019;81:1379-1386. doi: 10.1016/j.jaad.2019.05.030

Additional history from the family revealed that they were cleaning the wound with copious amounts of hydrogen peroxide twice a week—a practice that impedes wound healing and was ultimately the cause of this patient’s wound closure delay.
A nonhealing wound should be carefully evaluated to rule out malignancy, infection, or an inflammatory disorder such as pyoderma gangrenosum (PG). In this case, punch biopsies were performed to exclude PG and malignancy, particularly squamous cell carcinoma. Additionally, punch biopsies were performed for bacterial and fungal tissue culture. A complete blood count with differential was obtained to evaluate for signs of infection or hematologic malignancy. All work-ups and biopsies were consistent with a noninfected surgical wound.
Widely available over the counter in 3% to 5% solutions, hydrogen peroxide is used as a low-cost antiseptic for minor cuts and wounds. Data are mixed as to whether hydrogen peroxide improves or impedes wound healing when used outside of initial first aid or postoperatively.1 At higher concentrations, it uniformly causes skin necrosis. Owing to its debriding effect, it is FDA approved to treat seborrheic keratoses as an alternative to cryotherapy or electrodessication and curettage.
At the time of this work-up, and in the absence of other signs of infection, the patient and family were told to stop using hydrogen peroxide. Care instructions were changed to daily topical petroleum jelly and wound occlusion. Four weeks after wound care changes were made, the wound had re-epithelialized completely and reduced in size by two-thirds.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Additional history from the family revealed that they were cleaning the wound with copious amounts of hydrogen peroxide twice a week—a practice that impedes wound healing and was ultimately the cause of this patient’s wound closure delay.
A nonhealing wound should be carefully evaluated to rule out malignancy, infection, or an inflammatory disorder such as pyoderma gangrenosum (PG). In this case, punch biopsies were performed to exclude PG and malignancy, particularly squamous cell carcinoma. Additionally, punch biopsies were performed for bacterial and fungal tissue culture. A complete blood count with differential was obtained to evaluate for signs of infection or hematologic malignancy. All work-ups and biopsies were consistent with a noninfected surgical wound.
Widely available over the counter in 3% to 5% solutions, hydrogen peroxide is used as a low-cost antiseptic for minor cuts and wounds. Data are mixed as to whether hydrogen peroxide improves or impedes wound healing when used outside of initial first aid or postoperatively.1 At higher concentrations, it uniformly causes skin necrosis. Owing to its debriding effect, it is FDA approved to treat seborrheic keratoses as an alternative to cryotherapy or electrodessication and curettage.
At the time of this work-up, and in the absence of other signs of infection, the patient and family were told to stop using hydrogen peroxide. Care instructions were changed to daily topical petroleum jelly and wound occlusion. Four weeks after wound care changes were made, the wound had re-epithelialized completely and reduced in size by two-thirds.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Murphy EC, Friedman AJ. Hydrogen peroxide and cutaneous biology: translational applications, benefits, and risks. J Am Acad Dermatol. 2019;81:1379-1386. doi: 10.1016/j.jaad.2019.05.030
1. Murphy EC, Friedman AJ. Hydrogen peroxide and cutaneous biology: translational applications, benefits, and risks. J Am Acad Dermatol. 2019;81:1379-1386. doi: 10.1016/j.jaad.2019.05.030
Pandemic and sleep: Increased stress, lack of exercise and insomnia
While working as a registered nurse on inpatient Stroke and Generalized Rehabilitation unit, she pursued for a degree in Adult and Gerontology Primary Care degree. She currently practices at UW Medicine/Harborview Medical Center for Sleep Medicine treating a variety of sleep disorders. She strives to provide quality and safe care to her patients.
1. According to the American Academy of Sleep Medicine, even in normal times, 30 to 35 % of the US population contends with acute, or short-term insomnia. As a board-certified nurse practitioner focusing on treating sleep disorders among older adults, can you discuss whether that percentage has increased during the coronavirus (COVID-19) pandemic, and if so, what would you say are the underlying reasons or causes?
As a sleep medicine nurse practitioner at UW (University of Washington) Medicine, I have seen quite a few patients with sleep disorders including acute and chronic insomnia. Since the start of the COVID-19 pandemic there has been a noticeable increase in poor-sleep complaints -- the data indicate a 37% increase in the rate of clinical insomnia since the pandemic started.
Stress can worsen insomnia, and the pandemic has negatively affected most if not everyone’s life. It has changed lifestyles through social distancing, mask mandates, and stay-at-home orders. Many have been forced to balance working from home with household duties; parents are supervising their children’s schooling. This disruption in the workday environment and workload can be hard to manage. The uncertainty of the pandemic has increased worries – health related and financially related. Ready access to media can also increase stress. Moreover, the lack of structure in a person’s day can cause many problems. Working from home, quarantining, living a more sedentary lifestyle, losing a job, losing socialization, including attending events, all can cause a disruption in a person’s daily routine and induce later bed- and wake-up times. This disruption to the body’s biological or circadian rhythm can reduce sleep quality and INCREASE phase-delay insomnia. Moreover, the pandemic has been especially hard on people’s mental health. One CDC study showed that 40% of adults are struggling with adverse mental health and substance-use issues due to COVID. Also, 13.3% of adults have responded to surveys saying they’ve started or increased their use of substances. As the pandemic continues, acute insomnia will likely turn into chronic insomnia.
2. How can increased stress and lack of exercise cause insomnia? What risk factors contribute to lack of sleep and impact our overall health?
The incidence of anxiety disorder and depressive disorder has increased significantly as compared to pre-pandemic rates. Psychological stress, especially at bedtime, increases psychophysiological arousal. The hypothalamic- pituitary- adrenal (HPA) axis responds to stress by releasing cortisol. HPA activation is associated with poorer sleep quality – it increases sleep latency, frequency of awakening, decreases in slow-wave sleep, and degrades overall sleep efficiency. The result of poor quality and fragmented sleep can further activate the HPA axis, causing a positive feedback loop.
A deterrent to poor sleep is physical activity. It greatly improves sleep by improving sleep efficiency, decreasing light sleep, increasing REM sleep, and regulating circadian rhythm. Lack of physical activity has been associated with increased sleep problems such as daytime sleepiness, an insufficient amount of sleep, snoring, sleep apnea symptoms, and restless sleep. And poor sleep further reduces physical activity which perpetuates the problem. The pandemic’s effect on physical activity is significant. It has caused people to stay home more often and therefore decreases in levels of exercise. and increased sedentary lifestyle. More than half of the adults in this country do not meet federal guidelines for aerobic physical activity.
Sleep deprivation can be dangerous, as sleepiness increases the likelihood of major occupational and road traffic accidents. Being awake for at least 18 hours is equivalent to having a blood alcohol content of 0.05% to 0.10% for 24 hours. Chronic sleep deprivation, defined as getting, on average, fewer than 7 hours per night negatively affects all systems of the body. Sleep deprivation therefore reduces quality of life and can reduce life expectancy.
Cardiovascular – Sleep deprivation can increase excessive heart age and reduce heart rate recovery after exercise. It is also linked to increases in heart rate, blood pressure, and death from cardiovascular issues.
Respiratory – Even one night of sleep deprivation can increase respiratory load. Studies have shown an association between sleep apnea and sleep deprivation. Sleep deprivation and respiratory disorders can perpetuate each other.
Neurologic – Sleep is crucial in brain development. Lack of sleep is associated with low grade neuroinflammation, memory and cognitive function decline, and acceleration of Alzheimer’s disease. Sleep deprivation can increase pain sensitivity, the risk of stroke, aggressive behavior, cognitive instability, hyperactivity, and socialization problems.
Endocrine – Sleep deprivation increases appetite stimulation causing excessive food intake and weight gain. It can also impair metabolism, which leads to obesity and insulin resistance.
Reproductive – Studies on sleep deprivation and the human reproduction system are limited. A study in male rats shows a relation between less sleep and overall lower reproductive health such as alteration of spermatic function, “decreased sexual behavior, lower testosterone level, and lower sperm viability level”. Studies also show renal dysfunction and high blood pressure in the offspring of sleep deprived rats in the last week of pregnancy.
3. Please discuss coronasomnia and its symptoms. Also, will you discuss your thoughts on the diagnosis and provide examples of the types of stressors associated with coronasomnia.
Coronasomnia is the term used to describe the increase in sleep problems associated with the COVID-19 pandemic. Coronasomnia is associated with increased sleep onset, maintenance insomnia, delayed sleep schedule, nocturnal awakening, sleep deprivation, and worsened pre-existing sleep issues. The worst insomnia and psychological symptoms are among those who are in the center of the pandemic, such as frontline workers and people living in areas more impacted by COVID-19.
During the pandemic, anxiety, depression, stress, and poor sleep have significantly increased. Anxiety and depression can be accompanied by intrusive thoughts which interfere with falling asleep. Patients with depression have a twofold risk of sleep disruption. Lack of daily routine may be associated with an increase in poor dental hygiene, such as lower rates of flossing and brushing. There’s also an increased rate in snacking (weight gain) and avoidance of visits to the dentists.
More time at home leads to more time spent on TV or social media. Increased screen time and media use at night, especially close to bedtime, are linked to poorer sleep. Blue light emitted by electronic devices can suppress the release of melatonin, making it more difficult to fall asleep. In addition, viewing or listening to content that is distressing or exciting right before bedtime negatively affects sleep quality. Following pandemic news for more than 3 hours a day has been found to be associated with increased levels of anxiety.
Health care providers are especially susceptible to coronasomnia. Those who work directly with COVID -19 patients are twice as likely to report disrupted sleep, anxiety, and depression. An increased work and patient load, the shortage of both fellow providers and supplies, all contribute to increased anxiety and disrupted sleep. Poor sleep, especially coupled with longer work hours and shift work, are associated with a worsened immune system and poor work performance.
4. In looking at the overall challenges pertaining to pandemic-induced sleep problems, what are your guideline recommendations to help ensure we sleep well during this outbreak?
Poor sleep can be detrimental to physical and mental health, and poor sleep hygiene practices can significantly impact sleep quality. Below are some general sleep-hygiene recommendations.
Caffeine – Caffeine consumed close to bedtime can disrupt sleep. Caffeine should be avoided 6 hours prior to bedtime. Everyone’s tolerance to caffeine is different so timing and caffeine dosage may need to be individually tailored.
Alcohol – Alcohol consumed close to bedtime can decrease sleep latency. However, it increases arousal during the second half of the night. It can also worsen snoring and sleep apnea. The effect can be alcohol level dependent.
Exercise – Regular exercise, as already discussed, is linked to better sleep quality. It is typically recommended to exercise earlier in the day; research has shown conflicting results on nighttime exercise. One study of patients with insomnia who exercised at night showed that aerobic exercise of moderate intensity improved polysomnography patient-reported sleep latency, and total sleep time.
Routine – An irregular sleep schedule is associated with poor sleep and daytime sleepiness. Following a consistent sleep schedule promotes stable circadian rhythm. A familiar relaxing routine should be established before bedtime.
Stress – To lower stress, patients should be advised to schedule brief meditation sessions so they can reflect on stressful situations. Patients also should limit the amount of exposure to pandemic news. Writing down and talking about stress, relaxation, and mindfulness techniques may reduce stress. However, stress and anxiety significantly differ case by case and interventions from health care providers may be needed.
Time in bed – Limit the amount of time in bed only for sleep and sex. Limit the use of electronics before bed and avoid use of electronics in bed. Turning off devices or silencing notifications can all help in reducing sleep disruption.
Cognitive behavioral therapy for insomnia (CBT-I) should be considered for patients with chronic insomnia. This therapy often includes sleep hygiene education, sleep restriction therapy, and relaxation training. Benefits of CBT-I treatment are long-term and reduce the need for additional pharmacologic therapies.
While many patients are experiencing insomnia these days, other underlying sleep disorders also should be considered. Patients should be evaluated to see if a sleep specialist is needed to diagnose and treat their sleep disorders.
Sleep Foundation. Sleep Guidelines and Help During the COVID-19 Pandemic. .Apr 7, 2021.
Morin CM, Carrier C. The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021: 77: 346–347. doi: 10.1016/j.sleep.2020.06.005
Pengpid S, Peltzer K. Sedentary Behaviour and 12 Sleep Problem Indicators among Middle-Aged and Elderly Adults in South Africa. Int J Environ Res Public Health. 2019 Apr; 16(8): 1422.
Czeisler M É, Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic — United States, June 24–30, 2020 | MMWR Weekly. Aug 14, 2020. 69(32);1049–1057.
van Dalfsen JH, Markus, CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA)axis reactivity: A systematic review. Sleep Medicine Reviews. June 2018, 187-194. doi.org/10.1016/j.smrv.2017.10.002
Nicolaides NC, et al, eds. Axis and Sleep. Endotext - NCBI Bookshelf. South Dartmouth, MA. 2000- https://www.ncbi.nlm.nih.gov/books/NBK278943/
Issa FG and Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982 Apr; 45: pp 353–359.
Liewa SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Medicine. January 2021, pp 192-204.
Sleep Foundation. Coronasomnia: Definition, Symptoms, and Solutions | Sleep Foundation. Apr 14, 2021. https://www.sleepfoundation.org/covid-19-and-sleep/coronasomnia
American Association of Endodontists. Survey Reveals COVID-19 is a Major Factor in Americans’ Failing Dental Health | American Association of Endodontists (aae.org). Mar 4, 2021.
Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. April 4, 2020. doi.org/10.1111/jsr.13052 https://onlinelibrary.wiley.com/doi/10.1111/jsr.13052
CDC. Drowsy Driving- Sleep and Sleep Disorders. Mar 17, 2017. https://www.cdc.gov/sleep/about_sleep/drowsy_driving.html
Dolezal, BA, Neufeld, EV, Boland DM. Interrelationship between Sleep and Exercise: A Systematic Review. Adv Prev Med. 2017; 2017: 1364387. doi: 10.1155/2017/1364387
Irish LA, Kline, CE, Heather E. Gunn HE, et al. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence.Sleep Med Rev. 2015 Aug; 22: 23–36.doi: 10.1016/j.smrv.2014.10.001
Edinger JD, Arnedt JT, Suzanne M. Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. Feb. 1, 2021.
While working as a registered nurse on inpatient Stroke and Generalized Rehabilitation unit, she pursued for a degree in Adult and Gerontology Primary Care degree. She currently practices at UW Medicine/Harborview Medical Center for Sleep Medicine treating a variety of sleep disorders. She strives to provide quality and safe care to her patients.
1. According to the American Academy of Sleep Medicine, even in normal times, 30 to 35 % of the US population contends with acute, or short-term insomnia. As a board-certified nurse practitioner focusing on treating sleep disorders among older adults, can you discuss whether that percentage has increased during the coronavirus (COVID-19) pandemic, and if so, what would you say are the underlying reasons or causes?
As a sleep medicine nurse practitioner at UW (University of Washington) Medicine, I have seen quite a few patients with sleep disorders including acute and chronic insomnia. Since the start of the COVID-19 pandemic there has been a noticeable increase in poor-sleep complaints -- the data indicate a 37% increase in the rate of clinical insomnia since the pandemic started.
Stress can worsen insomnia, and the pandemic has negatively affected most if not everyone’s life. It has changed lifestyles through social distancing, mask mandates, and stay-at-home orders. Many have been forced to balance working from home with household duties; parents are supervising their children’s schooling. This disruption in the workday environment and workload can be hard to manage. The uncertainty of the pandemic has increased worries – health related and financially related. Ready access to media can also increase stress. Moreover, the lack of structure in a person’s day can cause many problems. Working from home, quarantining, living a more sedentary lifestyle, losing a job, losing socialization, including attending events, all can cause a disruption in a person’s daily routine and induce later bed- and wake-up times. This disruption to the body’s biological or circadian rhythm can reduce sleep quality and INCREASE phase-delay insomnia. Moreover, the pandemic has been especially hard on people’s mental health. One CDC study showed that 40% of adults are struggling with adverse mental health and substance-use issues due to COVID. Also, 13.3% of adults have responded to surveys saying they’ve started or increased their use of substances. As the pandemic continues, acute insomnia will likely turn into chronic insomnia.
2. How can increased stress and lack of exercise cause insomnia? What risk factors contribute to lack of sleep and impact our overall health?
The incidence of anxiety disorder and depressive disorder has increased significantly as compared to pre-pandemic rates. Psychological stress, especially at bedtime, increases psychophysiological arousal. The hypothalamic- pituitary- adrenal (HPA) axis responds to stress by releasing cortisol. HPA activation is associated with poorer sleep quality – it increases sleep latency, frequency of awakening, decreases in slow-wave sleep, and degrades overall sleep efficiency. The result of poor quality and fragmented sleep can further activate the HPA axis, causing a positive feedback loop.
A deterrent to poor sleep is physical activity. It greatly improves sleep by improving sleep efficiency, decreasing light sleep, increasing REM sleep, and regulating circadian rhythm. Lack of physical activity has been associated with increased sleep problems such as daytime sleepiness, an insufficient amount of sleep, snoring, sleep apnea symptoms, and restless sleep. And poor sleep further reduces physical activity which perpetuates the problem. The pandemic’s effect on physical activity is significant. It has caused people to stay home more often and therefore decreases in levels of exercise. and increased sedentary lifestyle. More than half of the adults in this country do not meet federal guidelines for aerobic physical activity.
Sleep deprivation can be dangerous, as sleepiness increases the likelihood of major occupational and road traffic accidents. Being awake for at least 18 hours is equivalent to having a blood alcohol content of 0.05% to 0.10% for 24 hours. Chronic sleep deprivation, defined as getting, on average, fewer than 7 hours per night negatively affects all systems of the body. Sleep deprivation therefore reduces quality of life and can reduce life expectancy.
Cardiovascular – Sleep deprivation can increase excessive heart age and reduce heart rate recovery after exercise. It is also linked to increases in heart rate, blood pressure, and death from cardiovascular issues.
Respiratory – Even one night of sleep deprivation can increase respiratory load. Studies have shown an association between sleep apnea and sleep deprivation. Sleep deprivation and respiratory disorders can perpetuate each other.
Neurologic – Sleep is crucial in brain development. Lack of sleep is associated with low grade neuroinflammation, memory and cognitive function decline, and acceleration of Alzheimer’s disease. Sleep deprivation can increase pain sensitivity, the risk of stroke, aggressive behavior, cognitive instability, hyperactivity, and socialization problems.
Endocrine – Sleep deprivation increases appetite stimulation causing excessive food intake and weight gain. It can also impair metabolism, which leads to obesity and insulin resistance.
Reproductive – Studies on sleep deprivation and the human reproduction system are limited. A study in male rats shows a relation between less sleep and overall lower reproductive health such as alteration of spermatic function, “decreased sexual behavior, lower testosterone level, and lower sperm viability level”. Studies also show renal dysfunction and high blood pressure in the offspring of sleep deprived rats in the last week of pregnancy.
3. Please discuss coronasomnia and its symptoms. Also, will you discuss your thoughts on the diagnosis and provide examples of the types of stressors associated with coronasomnia.
Coronasomnia is the term used to describe the increase in sleep problems associated with the COVID-19 pandemic. Coronasomnia is associated with increased sleep onset, maintenance insomnia, delayed sleep schedule, nocturnal awakening, sleep deprivation, and worsened pre-existing sleep issues. The worst insomnia and psychological symptoms are among those who are in the center of the pandemic, such as frontline workers and people living in areas more impacted by COVID-19.
During the pandemic, anxiety, depression, stress, and poor sleep have significantly increased. Anxiety and depression can be accompanied by intrusive thoughts which interfere with falling asleep. Patients with depression have a twofold risk of sleep disruption. Lack of daily routine may be associated with an increase in poor dental hygiene, such as lower rates of flossing and brushing. There’s also an increased rate in snacking (weight gain) and avoidance of visits to the dentists.
More time at home leads to more time spent on TV or social media. Increased screen time and media use at night, especially close to bedtime, are linked to poorer sleep. Blue light emitted by electronic devices can suppress the release of melatonin, making it more difficult to fall asleep. In addition, viewing or listening to content that is distressing or exciting right before bedtime negatively affects sleep quality. Following pandemic news for more than 3 hours a day has been found to be associated with increased levels of anxiety.
Health care providers are especially susceptible to coronasomnia. Those who work directly with COVID -19 patients are twice as likely to report disrupted sleep, anxiety, and depression. An increased work and patient load, the shortage of both fellow providers and supplies, all contribute to increased anxiety and disrupted sleep. Poor sleep, especially coupled with longer work hours and shift work, are associated with a worsened immune system and poor work performance.
4. In looking at the overall challenges pertaining to pandemic-induced sleep problems, what are your guideline recommendations to help ensure we sleep well during this outbreak?
Poor sleep can be detrimental to physical and mental health, and poor sleep hygiene practices can significantly impact sleep quality. Below are some general sleep-hygiene recommendations.
Caffeine – Caffeine consumed close to bedtime can disrupt sleep. Caffeine should be avoided 6 hours prior to bedtime. Everyone’s tolerance to caffeine is different so timing and caffeine dosage may need to be individually tailored.
Alcohol – Alcohol consumed close to bedtime can decrease sleep latency. However, it increases arousal during the second half of the night. It can also worsen snoring and sleep apnea. The effect can be alcohol level dependent.
Exercise – Regular exercise, as already discussed, is linked to better sleep quality. It is typically recommended to exercise earlier in the day; research has shown conflicting results on nighttime exercise. One study of patients with insomnia who exercised at night showed that aerobic exercise of moderate intensity improved polysomnography patient-reported sleep latency, and total sleep time.
Routine – An irregular sleep schedule is associated with poor sleep and daytime sleepiness. Following a consistent sleep schedule promotes stable circadian rhythm. A familiar relaxing routine should be established before bedtime.
Stress – To lower stress, patients should be advised to schedule brief meditation sessions so they can reflect on stressful situations. Patients also should limit the amount of exposure to pandemic news. Writing down and talking about stress, relaxation, and mindfulness techniques may reduce stress. However, stress and anxiety significantly differ case by case and interventions from health care providers may be needed.
Time in bed – Limit the amount of time in bed only for sleep and sex. Limit the use of electronics before bed and avoid use of electronics in bed. Turning off devices or silencing notifications can all help in reducing sleep disruption.
Cognitive behavioral therapy for insomnia (CBT-I) should be considered for patients with chronic insomnia. This therapy often includes sleep hygiene education, sleep restriction therapy, and relaxation training. Benefits of CBT-I treatment are long-term and reduce the need for additional pharmacologic therapies.
While many patients are experiencing insomnia these days, other underlying sleep disorders also should be considered. Patients should be evaluated to see if a sleep specialist is needed to diagnose and treat their sleep disorders.
While working as a registered nurse on inpatient Stroke and Generalized Rehabilitation unit, she pursued for a degree in Adult and Gerontology Primary Care degree. She currently practices at UW Medicine/Harborview Medical Center for Sleep Medicine treating a variety of sleep disorders. She strives to provide quality and safe care to her patients.
1. According to the American Academy of Sleep Medicine, even in normal times, 30 to 35 % of the US population contends with acute, or short-term insomnia. As a board-certified nurse practitioner focusing on treating sleep disorders among older adults, can you discuss whether that percentage has increased during the coronavirus (COVID-19) pandemic, and if so, what would you say are the underlying reasons or causes?
As a sleep medicine nurse practitioner at UW (University of Washington) Medicine, I have seen quite a few patients with sleep disorders including acute and chronic insomnia. Since the start of the COVID-19 pandemic there has been a noticeable increase in poor-sleep complaints -- the data indicate a 37% increase in the rate of clinical insomnia since the pandemic started.
Stress can worsen insomnia, and the pandemic has negatively affected most if not everyone’s life. It has changed lifestyles through social distancing, mask mandates, and stay-at-home orders. Many have been forced to balance working from home with household duties; parents are supervising their children’s schooling. This disruption in the workday environment and workload can be hard to manage. The uncertainty of the pandemic has increased worries – health related and financially related. Ready access to media can also increase stress. Moreover, the lack of structure in a person’s day can cause many problems. Working from home, quarantining, living a more sedentary lifestyle, losing a job, losing socialization, including attending events, all can cause a disruption in a person’s daily routine and induce later bed- and wake-up times. This disruption to the body’s biological or circadian rhythm can reduce sleep quality and INCREASE phase-delay insomnia. Moreover, the pandemic has been especially hard on people’s mental health. One CDC study showed that 40% of adults are struggling with adverse mental health and substance-use issues due to COVID. Also, 13.3% of adults have responded to surveys saying they’ve started or increased their use of substances. As the pandemic continues, acute insomnia will likely turn into chronic insomnia.
2. How can increased stress and lack of exercise cause insomnia? What risk factors contribute to lack of sleep and impact our overall health?
The incidence of anxiety disorder and depressive disorder has increased significantly as compared to pre-pandemic rates. Psychological stress, especially at bedtime, increases psychophysiological arousal. The hypothalamic- pituitary- adrenal (HPA) axis responds to stress by releasing cortisol. HPA activation is associated with poorer sleep quality – it increases sleep latency, frequency of awakening, decreases in slow-wave sleep, and degrades overall sleep efficiency. The result of poor quality and fragmented sleep can further activate the HPA axis, causing a positive feedback loop.
A deterrent to poor sleep is physical activity. It greatly improves sleep by improving sleep efficiency, decreasing light sleep, increasing REM sleep, and regulating circadian rhythm. Lack of physical activity has been associated with increased sleep problems such as daytime sleepiness, an insufficient amount of sleep, snoring, sleep apnea symptoms, and restless sleep. And poor sleep further reduces physical activity which perpetuates the problem. The pandemic’s effect on physical activity is significant. It has caused people to stay home more often and therefore decreases in levels of exercise. and increased sedentary lifestyle. More than half of the adults in this country do not meet federal guidelines for aerobic physical activity.
Sleep deprivation can be dangerous, as sleepiness increases the likelihood of major occupational and road traffic accidents. Being awake for at least 18 hours is equivalent to having a blood alcohol content of 0.05% to 0.10% for 24 hours. Chronic sleep deprivation, defined as getting, on average, fewer than 7 hours per night negatively affects all systems of the body. Sleep deprivation therefore reduces quality of life and can reduce life expectancy.
Cardiovascular – Sleep deprivation can increase excessive heart age and reduce heart rate recovery after exercise. It is also linked to increases in heart rate, blood pressure, and death from cardiovascular issues.
Respiratory – Even one night of sleep deprivation can increase respiratory load. Studies have shown an association between sleep apnea and sleep deprivation. Sleep deprivation and respiratory disorders can perpetuate each other.
Neurologic – Sleep is crucial in brain development. Lack of sleep is associated with low grade neuroinflammation, memory and cognitive function decline, and acceleration of Alzheimer’s disease. Sleep deprivation can increase pain sensitivity, the risk of stroke, aggressive behavior, cognitive instability, hyperactivity, and socialization problems.
Endocrine – Sleep deprivation increases appetite stimulation causing excessive food intake and weight gain. It can also impair metabolism, which leads to obesity and insulin resistance.
Reproductive – Studies on sleep deprivation and the human reproduction system are limited. A study in male rats shows a relation between less sleep and overall lower reproductive health such as alteration of spermatic function, “decreased sexual behavior, lower testosterone level, and lower sperm viability level”. Studies also show renal dysfunction and high blood pressure in the offspring of sleep deprived rats in the last week of pregnancy.
3. Please discuss coronasomnia and its symptoms. Also, will you discuss your thoughts on the diagnosis and provide examples of the types of stressors associated with coronasomnia.
Coronasomnia is the term used to describe the increase in sleep problems associated with the COVID-19 pandemic. Coronasomnia is associated with increased sleep onset, maintenance insomnia, delayed sleep schedule, nocturnal awakening, sleep deprivation, and worsened pre-existing sleep issues. The worst insomnia and psychological symptoms are among those who are in the center of the pandemic, such as frontline workers and people living in areas more impacted by COVID-19.
During the pandemic, anxiety, depression, stress, and poor sleep have significantly increased. Anxiety and depression can be accompanied by intrusive thoughts which interfere with falling asleep. Patients with depression have a twofold risk of sleep disruption. Lack of daily routine may be associated with an increase in poor dental hygiene, such as lower rates of flossing and brushing. There’s also an increased rate in snacking (weight gain) and avoidance of visits to the dentists.
More time at home leads to more time spent on TV or social media. Increased screen time and media use at night, especially close to bedtime, are linked to poorer sleep. Blue light emitted by electronic devices can suppress the release of melatonin, making it more difficult to fall asleep. In addition, viewing or listening to content that is distressing or exciting right before bedtime negatively affects sleep quality. Following pandemic news for more than 3 hours a day has been found to be associated with increased levels of anxiety.
Health care providers are especially susceptible to coronasomnia. Those who work directly with COVID -19 patients are twice as likely to report disrupted sleep, anxiety, and depression. An increased work and patient load, the shortage of both fellow providers and supplies, all contribute to increased anxiety and disrupted sleep. Poor sleep, especially coupled with longer work hours and shift work, are associated with a worsened immune system and poor work performance.
4. In looking at the overall challenges pertaining to pandemic-induced sleep problems, what are your guideline recommendations to help ensure we sleep well during this outbreak?
Poor sleep can be detrimental to physical and mental health, and poor sleep hygiene practices can significantly impact sleep quality. Below are some general sleep-hygiene recommendations.
Caffeine – Caffeine consumed close to bedtime can disrupt sleep. Caffeine should be avoided 6 hours prior to bedtime. Everyone’s tolerance to caffeine is different so timing and caffeine dosage may need to be individually tailored.
Alcohol – Alcohol consumed close to bedtime can decrease sleep latency. However, it increases arousal during the second half of the night. It can also worsen snoring and sleep apnea. The effect can be alcohol level dependent.
Exercise – Regular exercise, as already discussed, is linked to better sleep quality. It is typically recommended to exercise earlier in the day; research has shown conflicting results on nighttime exercise. One study of patients with insomnia who exercised at night showed that aerobic exercise of moderate intensity improved polysomnography patient-reported sleep latency, and total sleep time.
Routine – An irregular sleep schedule is associated with poor sleep and daytime sleepiness. Following a consistent sleep schedule promotes stable circadian rhythm. A familiar relaxing routine should be established before bedtime.
Stress – To lower stress, patients should be advised to schedule brief meditation sessions so they can reflect on stressful situations. Patients also should limit the amount of exposure to pandemic news. Writing down and talking about stress, relaxation, and mindfulness techniques may reduce stress. However, stress and anxiety significantly differ case by case and interventions from health care providers may be needed.
Time in bed – Limit the amount of time in bed only for sleep and sex. Limit the use of electronics before bed and avoid use of electronics in bed. Turning off devices or silencing notifications can all help in reducing sleep disruption.
Cognitive behavioral therapy for insomnia (CBT-I) should be considered for patients with chronic insomnia. This therapy often includes sleep hygiene education, sleep restriction therapy, and relaxation training. Benefits of CBT-I treatment are long-term and reduce the need for additional pharmacologic therapies.
While many patients are experiencing insomnia these days, other underlying sleep disorders also should be considered. Patients should be evaluated to see if a sleep specialist is needed to diagnose and treat their sleep disorders.
Sleep Foundation. Sleep Guidelines and Help During the COVID-19 Pandemic. .Apr 7, 2021.
Morin CM, Carrier C. The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021: 77: 346–347. doi: 10.1016/j.sleep.2020.06.005
Pengpid S, Peltzer K. Sedentary Behaviour and 12 Sleep Problem Indicators among Middle-Aged and Elderly Adults in South Africa. Int J Environ Res Public Health. 2019 Apr; 16(8): 1422.
Czeisler M É, Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic — United States, June 24–30, 2020 | MMWR Weekly. Aug 14, 2020. 69(32);1049–1057.
van Dalfsen JH, Markus, CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA)axis reactivity: A systematic review. Sleep Medicine Reviews. June 2018, 187-194. doi.org/10.1016/j.smrv.2017.10.002
Nicolaides NC, et al, eds. Axis and Sleep. Endotext - NCBI Bookshelf. South Dartmouth, MA. 2000- https://www.ncbi.nlm.nih.gov/books/NBK278943/
Issa FG and Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982 Apr; 45: pp 353–359.
Liewa SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Medicine. January 2021, pp 192-204.
Sleep Foundation. Coronasomnia: Definition, Symptoms, and Solutions | Sleep Foundation. Apr 14, 2021. https://www.sleepfoundation.org/covid-19-and-sleep/coronasomnia
American Association of Endodontists. Survey Reveals COVID-19 is a Major Factor in Americans’ Failing Dental Health | American Association of Endodontists (aae.org). Mar 4, 2021.
Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. April 4, 2020. doi.org/10.1111/jsr.13052 https://onlinelibrary.wiley.com/doi/10.1111/jsr.13052
CDC. Drowsy Driving- Sleep and Sleep Disorders. Mar 17, 2017. https://www.cdc.gov/sleep/about_sleep/drowsy_driving.html
Dolezal, BA, Neufeld, EV, Boland DM. Interrelationship between Sleep and Exercise: A Systematic Review. Adv Prev Med. 2017; 2017: 1364387. doi: 10.1155/2017/1364387
Irish LA, Kline, CE, Heather E. Gunn HE, et al. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence.Sleep Med Rev. 2015 Aug; 22: 23–36.doi: 10.1016/j.smrv.2014.10.001
Edinger JD, Arnedt JT, Suzanne M. Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. Feb. 1, 2021.
Sleep Foundation. Sleep Guidelines and Help During the COVID-19 Pandemic. .Apr 7, 2021.
Morin CM, Carrier C. The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021: 77: 346–347. doi: 10.1016/j.sleep.2020.06.005
Pengpid S, Peltzer K. Sedentary Behaviour and 12 Sleep Problem Indicators among Middle-Aged and Elderly Adults in South Africa. Int J Environ Res Public Health. 2019 Apr; 16(8): 1422.
Czeisler M É, Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic — United States, June 24–30, 2020 | MMWR Weekly. Aug 14, 2020. 69(32);1049–1057.
van Dalfsen JH, Markus, CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA)axis reactivity: A systematic review. Sleep Medicine Reviews. June 2018, 187-194. doi.org/10.1016/j.smrv.2017.10.002
Nicolaides NC, et al, eds. Axis and Sleep. Endotext - NCBI Bookshelf. South Dartmouth, MA. 2000- https://www.ncbi.nlm.nih.gov/books/NBK278943/
Issa FG and Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982 Apr; 45: pp 353–359.
Liewa SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Medicine. January 2021, pp 192-204.
Sleep Foundation. Coronasomnia: Definition, Symptoms, and Solutions | Sleep Foundation. Apr 14, 2021. https://www.sleepfoundation.org/covid-19-and-sleep/coronasomnia
American Association of Endodontists. Survey Reveals COVID-19 is a Major Factor in Americans’ Failing Dental Health | American Association of Endodontists (aae.org). Mar 4, 2021.
Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. April 4, 2020. doi.org/10.1111/jsr.13052 https://onlinelibrary.wiley.com/doi/10.1111/jsr.13052
CDC. Drowsy Driving- Sleep and Sleep Disorders. Mar 17, 2017. https://www.cdc.gov/sleep/about_sleep/drowsy_driving.html
Dolezal, BA, Neufeld, EV, Boland DM. Interrelationship between Sleep and Exercise: A Systematic Review. Adv Prev Med. 2017; 2017: 1364387. doi: 10.1155/2017/1364387
Irish LA, Kline, CE, Heather E. Gunn HE, et al. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence.Sleep Med Rev. 2015 Aug; 22: 23–36.doi: 10.1016/j.smrv.2014.10.001
Edinger JD, Arnedt JT, Suzanne M. Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. Feb. 1, 2021.
Treating endometriosis: maximizing all options for medical management, from hormones to new medical therapies
Stephanie J. Estes, MD is a board certified Obstetrician/Gynecologist and Professor of Reproductive Endocrinology and Infertility at the Penn State Hershey Medical center in Hershey, Pennsylvania. As a subspecialist, she has a focus on endometriosis and fibroid research and also advances the care of women through advanced reproductive surgery techniques and robotic surgery.
Q: At what point do you consider medical therapies in your approach to a patient with endometriosis?
Dr. Estes: I consider medical therapies for every patient that I see. There are 3 categories that I think about:
- Are they a candidate for medical therapy?
- Are they a candidate for surgical therapy?
- Lastly, are they a candidate for fertility treatment?
The difference between the plans is if the patient desires fertility. Next, I consider the advantages and disadvantages of medical therapy. The pros of medical therapy are avoiding the risks of surgery, with known and unknown complications or adhesions. And the cons are side effects of medical therapy. Also, medical therapy does not address treating hydrosalpinges, endometriomas, or other deep infiltrating nodules and, clearly, it also inhibits fertility treatment. So, my overall process is looking at the patient’s goal of symptom management and how best to limit their number of surgical procedures. My approach spans many options, and I look at all of those to make an appropriate decision for each patient.
Q: How do you determine what options would be best suited for the patient?
Dr. Estes: The first line of symptom treatment best suited for the patient is always NSAIDs, which are nonsteroidal anti-inflammatory drugs. These are an appropriate first start in managing the dysmenorrhea and pelvic pain that can be associated with endometriosis. If there are no contraindications, the next most common way to manage endometriosis symptoms is combined oral contraceptive pills, transdermal patches, or rings. A key principle, especially when selecting a pill, is to look at the type of estrogen and progestin you’re choosing. Some practitioners may see these pills as equals, but there are differences. I always select a 20-µm ethinyl estradiol pill for my endometriosis patients. Then, I select a progestin, such as norethindrone or levonorgestrel, which provide good suppressive treatment.
The preferred hormonal therapy for endometriosis symptoms not only should be easy for the patient to use but also accomplish management of the symptoms that they are coming in for. Hormonal therapies have a low side-effect profile, which allows the patient to feel well for a long time. We know that endometriosis is a chronic disease, so this is something that the patient is going to need to manage for a long time. I really like to help my patients because they have other things to do in life. They want to take care of their kids, or they’re in school, or have other goals. Patients want to feel well while doing all these activities of life, and so an individualized approach is important. Some of my patients love taking pills, and they are perfectly happy to do so. Other people would prefer a more long-acting treatment so that they do not have to deal with remembering to take a medication every day.
Q: Explain how you would apply the use of an intrauterine device (IUD) to manage endometriosis.
Dr. Estes: I use many IUDs with progestin because evidence has shown them to be effective in managing endometriosis symptoms, specifically by decreasing dysmenorrhea. I will usually insert them during a patient’s surgery in the operating room. If I identify endometriosis and can place the IUD at the same time of the surgery, this see-and-treat philosophy maximizes the efficiency of patient care—and the patient avoids the discomfort of an in-office insertion. During the patient’s post-operative check, I evaluate how they feel and how the IUD is working for them. I think many patients are candidates for progestin IUDs, especially those who cannot take an estrogen-containing compound.
Q: Where do newly available therapies for endometriosis fit into your overall management approach?
Dr. Estes: Norethindrone 5 mg is an effective treatment for endometriosis and it is US Food and Drug Administration (FDA)‒approved for that cause. And then, the other medications that Penn State Hershey was part of the clinical trials for include Gonadotropin-releasing hormone (GnRH) antagonists such as elagolix. I saw the improvement in symptoms not only in the trial but also in continued follow-up of patients. GnRH antagonists are a group of medications that have a very good side-effect profile. Patients typically do not have significant side effects, however, hot flashes can me common. Hot flashes that can be ameliorated with some add back hormone therapy. The other drugs in the pipeline are relugolix and linzagolix. Relugolix is FDA approved for prostate cancer treatment. Endometriosis trials are expensive, and they are long and hard to do because of the pain factor—people do not want to stop their other medications to do the trial; however, the use of these medications will continue to be studied, and I look further to continuing to fine tune treatment options
Q: Is cost to the patient a consideration during management counseling, and should it be?
Dr. Estes: Absolutely. It comes up in every conversation because endometriosis is a long-term disease process that needs to be managed throughout the life cycle of a woman; you cannot have something that is going to be so expensive that will have to be taken for years and years or is not going to be continued. Because cost is critical, I use Lupron Depot as well as letrozole, and goserelin implants are also approved for endometriosis treatment. I also occasionally use danazol, which is a very different mechanism of action in select patients, so multiple options are all present. We have streamlined our pre-approval process for the GnRH antagonists to make it fairly easy.
It used to be a little bit harder, but now, if a patient has found that other medications did not offer relief for her endometriosis, then GnRH antagonists are much easier to obtain.
Q: Is there anything else you’d like to add?
Dr. Estes: Our patient involvement in clinical trials is just so valuable for endometriosis disease research. So, anyone out there living with endometriosis who would like to help medical science—wherever you live, wherever you are—get involved because this can help not only you but also the next person who comes after you. We are currently participating in a study right now on quinagolide. It is a vaginal ring that is not hormonal.
Again, we want to keep all options open and see what works and does not work. Science is so fantastic, and it is one of those summary points to note that we always need more information in terms of endometriosis. We really are aspiring to develop and apply treatment options that are effective throughout the lifespan of those affected by endometriosis.
Stephanie J. Estes, MD is a board certified Obstetrician/Gynecologist and Professor of Reproductive Endocrinology and Infertility at the Penn State Hershey Medical center in Hershey, Pennsylvania. As a subspecialist, she has a focus on endometriosis and fibroid research and also advances the care of women through advanced reproductive surgery techniques and robotic surgery.
Q: At what point do you consider medical therapies in your approach to a patient with endometriosis?
Dr. Estes: I consider medical therapies for every patient that I see. There are 3 categories that I think about:
- Are they a candidate for medical therapy?
- Are they a candidate for surgical therapy?
- Lastly, are they a candidate for fertility treatment?
The difference between the plans is if the patient desires fertility. Next, I consider the advantages and disadvantages of medical therapy. The pros of medical therapy are avoiding the risks of surgery, with known and unknown complications or adhesions. And the cons are side effects of medical therapy. Also, medical therapy does not address treating hydrosalpinges, endometriomas, or other deep infiltrating nodules and, clearly, it also inhibits fertility treatment. So, my overall process is looking at the patient’s goal of symptom management and how best to limit their number of surgical procedures. My approach spans many options, and I look at all of those to make an appropriate decision for each patient.
Q: How do you determine what options would be best suited for the patient?
Dr. Estes: The first line of symptom treatment best suited for the patient is always NSAIDs, which are nonsteroidal anti-inflammatory drugs. These are an appropriate first start in managing the dysmenorrhea and pelvic pain that can be associated with endometriosis. If there are no contraindications, the next most common way to manage endometriosis symptoms is combined oral contraceptive pills, transdermal patches, or rings. A key principle, especially when selecting a pill, is to look at the type of estrogen and progestin you’re choosing. Some practitioners may see these pills as equals, but there are differences. I always select a 20-µm ethinyl estradiol pill for my endometriosis patients. Then, I select a progestin, such as norethindrone or levonorgestrel, which provide good suppressive treatment.
The preferred hormonal therapy for endometriosis symptoms not only should be easy for the patient to use but also accomplish management of the symptoms that they are coming in for. Hormonal therapies have a low side-effect profile, which allows the patient to feel well for a long time. We know that endometriosis is a chronic disease, so this is something that the patient is going to need to manage for a long time. I really like to help my patients because they have other things to do in life. They want to take care of their kids, or they’re in school, or have other goals. Patients want to feel well while doing all these activities of life, and so an individualized approach is important. Some of my patients love taking pills, and they are perfectly happy to do so. Other people would prefer a more long-acting treatment so that they do not have to deal with remembering to take a medication every day.
Q: Explain how you would apply the use of an intrauterine device (IUD) to manage endometriosis.
Dr. Estes: I use many IUDs with progestin because evidence has shown them to be effective in managing endometriosis symptoms, specifically by decreasing dysmenorrhea. I will usually insert them during a patient’s surgery in the operating room. If I identify endometriosis and can place the IUD at the same time of the surgery, this see-and-treat philosophy maximizes the efficiency of patient care—and the patient avoids the discomfort of an in-office insertion. During the patient’s post-operative check, I evaluate how they feel and how the IUD is working for them. I think many patients are candidates for progestin IUDs, especially those who cannot take an estrogen-containing compound.
Q: Where do newly available therapies for endometriosis fit into your overall management approach?
Dr. Estes: Norethindrone 5 mg is an effective treatment for endometriosis and it is US Food and Drug Administration (FDA)‒approved for that cause. And then, the other medications that Penn State Hershey was part of the clinical trials for include Gonadotropin-releasing hormone (GnRH) antagonists such as elagolix. I saw the improvement in symptoms not only in the trial but also in continued follow-up of patients. GnRH antagonists are a group of medications that have a very good side-effect profile. Patients typically do not have significant side effects, however, hot flashes can me common. Hot flashes that can be ameliorated with some add back hormone therapy. The other drugs in the pipeline are relugolix and linzagolix. Relugolix is FDA approved for prostate cancer treatment. Endometriosis trials are expensive, and they are long and hard to do because of the pain factor—people do not want to stop their other medications to do the trial; however, the use of these medications will continue to be studied, and I look further to continuing to fine tune treatment options
Q: Is cost to the patient a consideration during management counseling, and should it be?
Dr. Estes: Absolutely. It comes up in every conversation because endometriosis is a long-term disease process that needs to be managed throughout the life cycle of a woman; you cannot have something that is going to be so expensive that will have to be taken for years and years or is not going to be continued. Because cost is critical, I use Lupron Depot as well as letrozole, and goserelin implants are also approved for endometriosis treatment. I also occasionally use danazol, which is a very different mechanism of action in select patients, so multiple options are all present. We have streamlined our pre-approval process for the GnRH antagonists to make it fairly easy.
It used to be a little bit harder, but now, if a patient has found that other medications did not offer relief for her endometriosis, then GnRH antagonists are much easier to obtain.
Q: Is there anything else you’d like to add?
Dr. Estes: Our patient involvement in clinical trials is just so valuable for endometriosis disease research. So, anyone out there living with endometriosis who would like to help medical science—wherever you live, wherever you are—get involved because this can help not only you but also the next person who comes after you. We are currently participating in a study right now on quinagolide. It is a vaginal ring that is not hormonal.
Again, we want to keep all options open and see what works and does not work. Science is so fantastic, and it is one of those summary points to note that we always need more information in terms of endometriosis. We really are aspiring to develop and apply treatment options that are effective throughout the lifespan of those affected by endometriosis.
Stephanie J. Estes, MD is a board certified Obstetrician/Gynecologist and Professor of Reproductive Endocrinology and Infertility at the Penn State Hershey Medical center in Hershey, Pennsylvania. As a subspecialist, she has a focus on endometriosis and fibroid research and also advances the care of women through advanced reproductive surgery techniques and robotic surgery.
Q: At what point do you consider medical therapies in your approach to a patient with endometriosis?
Dr. Estes: I consider medical therapies for every patient that I see. There are 3 categories that I think about:
- Are they a candidate for medical therapy?
- Are they a candidate for surgical therapy?
- Lastly, are they a candidate for fertility treatment?
The difference between the plans is if the patient desires fertility. Next, I consider the advantages and disadvantages of medical therapy. The pros of medical therapy are avoiding the risks of surgery, with known and unknown complications or adhesions. And the cons are side effects of medical therapy. Also, medical therapy does not address treating hydrosalpinges, endometriomas, or other deep infiltrating nodules and, clearly, it also inhibits fertility treatment. So, my overall process is looking at the patient’s goal of symptom management and how best to limit their number of surgical procedures. My approach spans many options, and I look at all of those to make an appropriate decision for each patient.
Q: How do you determine what options would be best suited for the patient?
Dr. Estes: The first line of symptom treatment best suited for the patient is always NSAIDs, which are nonsteroidal anti-inflammatory drugs. These are an appropriate first start in managing the dysmenorrhea and pelvic pain that can be associated with endometriosis. If there are no contraindications, the next most common way to manage endometriosis symptoms is combined oral contraceptive pills, transdermal patches, or rings. A key principle, especially when selecting a pill, is to look at the type of estrogen and progestin you’re choosing. Some practitioners may see these pills as equals, but there are differences. I always select a 20-µm ethinyl estradiol pill for my endometriosis patients. Then, I select a progestin, such as norethindrone or levonorgestrel, which provide good suppressive treatment.
The preferred hormonal therapy for endometriosis symptoms not only should be easy for the patient to use but also accomplish management of the symptoms that they are coming in for. Hormonal therapies have a low side-effect profile, which allows the patient to feel well for a long time. We know that endometriosis is a chronic disease, so this is something that the patient is going to need to manage for a long time. I really like to help my patients because they have other things to do in life. They want to take care of their kids, or they’re in school, or have other goals. Patients want to feel well while doing all these activities of life, and so an individualized approach is important. Some of my patients love taking pills, and they are perfectly happy to do so. Other people would prefer a more long-acting treatment so that they do not have to deal with remembering to take a medication every day.
Q: Explain how you would apply the use of an intrauterine device (IUD) to manage endometriosis.
Dr. Estes: I use many IUDs with progestin because evidence has shown them to be effective in managing endometriosis symptoms, specifically by decreasing dysmenorrhea. I will usually insert them during a patient’s surgery in the operating room. If I identify endometriosis and can place the IUD at the same time of the surgery, this see-and-treat philosophy maximizes the efficiency of patient care—and the patient avoids the discomfort of an in-office insertion. During the patient’s post-operative check, I evaluate how they feel and how the IUD is working for them. I think many patients are candidates for progestin IUDs, especially those who cannot take an estrogen-containing compound.
Q: Where do newly available therapies for endometriosis fit into your overall management approach?
Dr. Estes: Norethindrone 5 mg is an effective treatment for endometriosis and it is US Food and Drug Administration (FDA)‒approved for that cause. And then, the other medications that Penn State Hershey was part of the clinical trials for include Gonadotropin-releasing hormone (GnRH) antagonists such as elagolix. I saw the improvement in symptoms not only in the trial but also in continued follow-up of patients. GnRH antagonists are a group of medications that have a very good side-effect profile. Patients typically do not have significant side effects, however, hot flashes can me common. Hot flashes that can be ameliorated with some add back hormone therapy. The other drugs in the pipeline are relugolix and linzagolix. Relugolix is FDA approved for prostate cancer treatment. Endometriosis trials are expensive, and they are long and hard to do because of the pain factor—people do not want to stop their other medications to do the trial; however, the use of these medications will continue to be studied, and I look further to continuing to fine tune treatment options
Q: Is cost to the patient a consideration during management counseling, and should it be?
Dr. Estes: Absolutely. It comes up in every conversation because endometriosis is a long-term disease process that needs to be managed throughout the life cycle of a woman; you cannot have something that is going to be so expensive that will have to be taken for years and years or is not going to be continued. Because cost is critical, I use Lupron Depot as well as letrozole, and goserelin implants are also approved for endometriosis treatment. I also occasionally use danazol, which is a very different mechanism of action in select patients, so multiple options are all present. We have streamlined our pre-approval process for the GnRH antagonists to make it fairly easy.
It used to be a little bit harder, but now, if a patient has found that other medications did not offer relief for her endometriosis, then GnRH antagonists are much easier to obtain.
Q: Is there anything else you’d like to add?
Dr. Estes: Our patient involvement in clinical trials is just so valuable for endometriosis disease research. So, anyone out there living with endometriosis who would like to help medical science—wherever you live, wherever you are—get involved because this can help not only you but also the next person who comes after you. We are currently participating in a study right now on quinagolide. It is a vaginal ring that is not hormonal.
Again, we want to keep all options open and see what works and does not work. Science is so fantastic, and it is one of those summary points to note that we always need more information in terms of endometriosis. We really are aspiring to develop and apply treatment options that are effective throughout the lifespan of those affected by endometriosis.
Enriched infant formula offers no academic benefit later: Study
Infants who are given nutrient- or supplement-enriched formula milk do not later have higher academic scores as adolescents than those fed with standard formula, a study published online in the BMJ suggests.
One goal of modifying infant formula has been to make long-term cognitive outcomes similar to those for breast-fed infants, the authors noted. Rates for breastfeeding beyond 6 weeks are low in many parts of the world and more than 60% of babies worldwide under the age of 6 months are given formula to replace or supplement breast milk, the paper states.
So far, research has been inconclusive on benefits, though enhancements continue to be added and claims have been made as to their benefits on cognition in advertising. Long-term trials are difficult as researchers move on and participants are lost to follow-up.
In a new study, however, researchers led by Maximiliane L. Verfürden, MsC, with the University College of London’s Great Ormond Street Institute of Child Health, linked data from seven dormant, randomized, controlled infant formula trials to participants’ performance later as adolescents in the United Kingdom on mandatory national school math and English exams at ages 11 and 16 and found no difference in scores.
They followed 1,763 adolescents who had been participants in the formula trials, which were conducted between 1993 and 2001, and were able to link 91.2% (1,607) to academic records.
They found “no benefit of the infant formula modifications on cognitive outcomes.”
Three types of formula studied
In this study, the researchers discuss three widely available types of modified infant formulas that have been promoted as benefiting cognitive development: formula enriched with nutrients; formula supplemented with long-chain polyunsaturated fatty acids (LCPUFAs); and follow-on formula fortified with iron.
In one supplement group the academic results were worse than for those given standard formula. At age 11, children who had been given the LCPUFA-enhanced formula scored lower in both English and math.
“Given the potential associations between the source of LCPUFAs and adverse cognitive outcomes, long-term follow-up of trials testing infant formulas from other sources of LCPUFAs is recommended,” the authors wrote.
Nutrients can harm, editorialist says
Charlotte Wright, BM BCH, MSc, a pediatrician and epidemiologist with the Glasgow Royal Hospital for Children in Glasgow, who was not part of the study, coauthored an editorial that accompanied the article in the BMJ.
Dr. Wright and nutritionist Ada L. Gargia, PhD, at the University of Glasgow, wrote that nutrients in some formula enhancements can harm and that infant milk trials often have been poorly conducted.
The editorialists point to a large systematic review of formula milk trials published this year in the BMJ by Helfer et al. that found that most were funded by industry.
“Helfer and colleagues’ review found that 80% of studies were at high risk of bias, mainly because of selective reporting, with 92% of abstracts mentioning positive findings, despite only 42% of trials finding statistically significant differences in a stated primary outcome,” they wrote.
Dr. Wright, who runs a specialist feeding clinic for children, said in an interview that the study is valuable in that it has follow-up “to an age when adult cognition can be robustly assessed.”
She noted that the authors say additives that have been shown to be harmful are still routinely added.
“There is now evidence that adding LCPUFAs results in lower cognition and that giving extra iron to healthy children increases their risk of infection and may even slow their growth,” she said.
But advertisements to the contrary are quickly found in an Internet search, she said, even if no specific claims are made for them.
She gave an example of an advertisement for a commonly used enhanced formula, which reads: “Our formulation contains our highest levels of DHA (Omega 3 LCPs) and is enriched with iron to support normal cognitive development.”
The formula studies were done more than 20 years ago, but Dr. Wright said that does not downplay their relevance.
The basic formulation of the formulas hasn’t changed much, she said, and the additives are still present.
This work was supported by the Economic and Social Research Council UCL, Bloomsbury and East London Doctoral Training Partnership and a Great Ormond Street Hospital Charity Research grant. Full disclosures for all authors are available with the full text of the paper. Dr. Wright and Dr. Garcia declared no relevant financial relationships.
Infants who are given nutrient- or supplement-enriched formula milk do not later have higher academic scores as adolescents than those fed with standard formula, a study published online in the BMJ suggests.
One goal of modifying infant formula has been to make long-term cognitive outcomes similar to those for breast-fed infants, the authors noted. Rates for breastfeeding beyond 6 weeks are low in many parts of the world and more than 60% of babies worldwide under the age of 6 months are given formula to replace or supplement breast milk, the paper states.
So far, research has been inconclusive on benefits, though enhancements continue to be added and claims have been made as to their benefits on cognition in advertising. Long-term trials are difficult as researchers move on and participants are lost to follow-up.
In a new study, however, researchers led by Maximiliane L. Verfürden, MsC, with the University College of London’s Great Ormond Street Institute of Child Health, linked data from seven dormant, randomized, controlled infant formula trials to participants’ performance later as adolescents in the United Kingdom on mandatory national school math and English exams at ages 11 and 16 and found no difference in scores.
They followed 1,763 adolescents who had been participants in the formula trials, which were conducted between 1993 and 2001, and were able to link 91.2% (1,607) to academic records.
They found “no benefit of the infant formula modifications on cognitive outcomes.”
Three types of formula studied
In this study, the researchers discuss three widely available types of modified infant formulas that have been promoted as benefiting cognitive development: formula enriched with nutrients; formula supplemented with long-chain polyunsaturated fatty acids (LCPUFAs); and follow-on formula fortified with iron.
In one supplement group the academic results were worse than for those given standard formula. At age 11, children who had been given the LCPUFA-enhanced formula scored lower in both English and math.
“Given the potential associations between the source of LCPUFAs and adverse cognitive outcomes, long-term follow-up of trials testing infant formulas from other sources of LCPUFAs is recommended,” the authors wrote.
Nutrients can harm, editorialist says
Charlotte Wright, BM BCH, MSc, a pediatrician and epidemiologist with the Glasgow Royal Hospital for Children in Glasgow, who was not part of the study, coauthored an editorial that accompanied the article in the BMJ.
Dr. Wright and nutritionist Ada L. Gargia, PhD, at the University of Glasgow, wrote that nutrients in some formula enhancements can harm and that infant milk trials often have been poorly conducted.
The editorialists point to a large systematic review of formula milk trials published this year in the BMJ by Helfer et al. that found that most were funded by industry.
“Helfer and colleagues’ review found that 80% of studies were at high risk of bias, mainly because of selective reporting, with 92% of abstracts mentioning positive findings, despite only 42% of trials finding statistically significant differences in a stated primary outcome,” they wrote.
Dr. Wright, who runs a specialist feeding clinic for children, said in an interview that the study is valuable in that it has follow-up “to an age when adult cognition can be robustly assessed.”
She noted that the authors say additives that have been shown to be harmful are still routinely added.
“There is now evidence that adding LCPUFAs results in lower cognition and that giving extra iron to healthy children increases their risk of infection and may even slow their growth,” she said.
But advertisements to the contrary are quickly found in an Internet search, she said, even if no specific claims are made for them.
She gave an example of an advertisement for a commonly used enhanced formula, which reads: “Our formulation contains our highest levels of DHA (Omega 3 LCPs) and is enriched with iron to support normal cognitive development.”
The formula studies were done more than 20 years ago, but Dr. Wright said that does not downplay their relevance.
The basic formulation of the formulas hasn’t changed much, she said, and the additives are still present.
This work was supported by the Economic and Social Research Council UCL, Bloomsbury and East London Doctoral Training Partnership and a Great Ormond Street Hospital Charity Research grant. Full disclosures for all authors are available with the full text of the paper. Dr. Wright and Dr. Garcia declared no relevant financial relationships.
Infants who are given nutrient- or supplement-enriched formula milk do not later have higher academic scores as adolescents than those fed with standard formula, a study published online in the BMJ suggests.
One goal of modifying infant formula has been to make long-term cognitive outcomes similar to those for breast-fed infants, the authors noted. Rates for breastfeeding beyond 6 weeks are low in many parts of the world and more than 60% of babies worldwide under the age of 6 months are given formula to replace or supplement breast milk, the paper states.
So far, research has been inconclusive on benefits, though enhancements continue to be added and claims have been made as to their benefits on cognition in advertising. Long-term trials are difficult as researchers move on and participants are lost to follow-up.
In a new study, however, researchers led by Maximiliane L. Verfürden, MsC, with the University College of London’s Great Ormond Street Institute of Child Health, linked data from seven dormant, randomized, controlled infant formula trials to participants’ performance later as adolescents in the United Kingdom on mandatory national school math and English exams at ages 11 and 16 and found no difference in scores.
They followed 1,763 adolescents who had been participants in the formula trials, which were conducted between 1993 and 2001, and were able to link 91.2% (1,607) to academic records.
They found “no benefit of the infant formula modifications on cognitive outcomes.”
Three types of formula studied
In this study, the researchers discuss three widely available types of modified infant formulas that have been promoted as benefiting cognitive development: formula enriched with nutrients; formula supplemented with long-chain polyunsaturated fatty acids (LCPUFAs); and follow-on formula fortified with iron.
In one supplement group the academic results were worse than for those given standard formula. At age 11, children who had been given the LCPUFA-enhanced formula scored lower in both English and math.
“Given the potential associations between the source of LCPUFAs and adverse cognitive outcomes, long-term follow-up of trials testing infant formulas from other sources of LCPUFAs is recommended,” the authors wrote.
Nutrients can harm, editorialist says
Charlotte Wright, BM BCH, MSc, a pediatrician and epidemiologist with the Glasgow Royal Hospital for Children in Glasgow, who was not part of the study, coauthored an editorial that accompanied the article in the BMJ.
Dr. Wright and nutritionist Ada L. Gargia, PhD, at the University of Glasgow, wrote that nutrients in some formula enhancements can harm and that infant milk trials often have been poorly conducted.
The editorialists point to a large systematic review of formula milk trials published this year in the BMJ by Helfer et al. that found that most were funded by industry.
“Helfer and colleagues’ review found that 80% of studies were at high risk of bias, mainly because of selective reporting, with 92% of abstracts mentioning positive findings, despite only 42% of trials finding statistically significant differences in a stated primary outcome,” they wrote.
Dr. Wright, who runs a specialist feeding clinic for children, said in an interview that the study is valuable in that it has follow-up “to an age when adult cognition can be robustly assessed.”
She noted that the authors say additives that have been shown to be harmful are still routinely added.
“There is now evidence that adding LCPUFAs results in lower cognition and that giving extra iron to healthy children increases their risk of infection and may even slow their growth,” she said.
But advertisements to the contrary are quickly found in an Internet search, she said, even if no specific claims are made for them.
She gave an example of an advertisement for a commonly used enhanced formula, which reads: “Our formulation contains our highest levels of DHA (Omega 3 LCPs) and is enriched with iron to support normal cognitive development.”
The formula studies were done more than 20 years ago, but Dr. Wright said that does not downplay their relevance.
The basic formulation of the formulas hasn’t changed much, she said, and the additives are still present.
This work was supported by the Economic and Social Research Council UCL, Bloomsbury and East London Doctoral Training Partnership and a Great Ormond Street Hospital Charity Research grant. Full disclosures for all authors are available with the full text of the paper. Dr. Wright and Dr. Garcia declared no relevant financial relationships.
FROM THE BMJ
When a JAK inhibitor fails for a patient with RA, what’s next?
For patients with rheumatoid arthritis (RA) for whom a first Janus kinase inhibitor (JAKi) has failed, there appears to be no difference in treatment effectiveness whether the patient is cycled to a second JAKi or receives a biologic disease-modifying antirheumatic drug (bDMARD), a study of international patient registry data suggests.
However, patients who are prescribed a different JAKi after the first has failed them tend to have conditions that are more difficult to treat than do patients who are switched to a bDMARD after JAKi failure. In addition, adverse events that occur with the first JAKi are likely to occur again if a different agent in the same class is used, reported Manuel Pombo-Suarez, MD, PhD, adjunct professor of medicine at the University Hospital of Santiago de Compostela, Spain.
“When the first JAK inhibitor was stopped due to an adverse event, it was also more likely that the second JAK inhibitor would be stopped for the same reason,” he said in an oral abstract presentation during the American College of Rheumatology (ACR) 2021 Annual Meeting, which was held online.
The 2019 update of the European Alliance of Associations for Rheumatology (EULAR) guidelines for RA recommend that for patients for whom a first JAKi has failed, clinicians can consider a different JAKi or switch to a bDMARD. But at the time the guidelines were published, no data were available from studies in which a second JAKi was used after the failure of a first JAKi, Dr. Pombo-Suarez noted.
“We are trying to shed a light on this growing population of patients, as prescription of these drugs is increasing and new JAK inhibitors come into play, meaning that this scenario, we propose, is becoming more and more frequent in real life. We must provide a solution for these patients,” he said.
Pooled registry data
The investigators compared the effectiveness of the two approaches with respect to rates of drug retention and Disease Activity Score in 28 joints (DAS28).
They conducted a nested cohort study using data from 14 national registries that are part of the JAK-pot collaboration.
They pooled data from each registry on patients with RA for whom a first JAKi had failed and who were then treated with either a second JAKi or a bDMARD.
They identified a total of 708 patients for whom a JAKi had failed initially. Of these patients, 154 were given a different JAKi, and 554 were switched to a bDMARD. In each group, women accounted for a large majority of patients.
The mean age was slightly older among those who received a second JAKi (58.41 years vs. 54.74 years for patients who were given a bDMARD). The mean disease duration was 13.95 years and 11.37 years, respectively.
In each group, approximately 77% of patients received tofacitinib (Xeljanz).
At baseline, the mean DAS28 scores were similar between the groups: 4.10 in the group that received a second JAKi, and 4.17 in the group given a bDMARD.
Reasons for initially stopping use of a JAKi were as follows: adverse events (27.3% of those who took a second JAKi after they had stopped taking one initially, and 17.9% of patients who received a bDMARD); lack of efficacy (61% and 65%, respectively), and other reasons (11.7% and 17.1%, respectively).
At 2 years’ follow-up, drug survival rates were similar between the two treatment arms, although there was a nonsignificant trend toward a higher rate of discontinuation among patients who were given a second JAKi after they stopped taking the first JAKi because of adverse events. In contrast, there was also a nonsignificant trend toward lower discontinuation rates among patients who were given a second JAKi after they had stopped taking the first JAKi because of lack of efficacy.
As noted before, patients who stopped taking the first JAKi because of an adverse event were more likely to stop taking the second JAKi because of they experienced either the same or a different adverse event, whereas patients who started taking a bDMARD were equally likely to stop taking the second therapy because of either adverse events or lack of efficacy.
The treatment strategies were virtually identical with respect to improvement of DAS28 at 7 months after the start of therapy.
Dr. Pombo-Suarez acknowledged that the study was limited by the fact that heterogeneity between countries could not be assessed, owing to the small sample sizes in each nation’s registry. Other limitations include short follow-up and the fact that tofacitinib was used as the first JAKi by the large majority of patients.
What’s your practice?
In a media briefing during which Dr. Pombo-Suarez discussed the study findings, this news organization polled other speakers who were not involved in the study about their go-to strategies when JAKi therapy fails.
Silje Watterdal Syversen, MD, PhD, a consultant rheumatologist and researcher at Diakonhjemmet Hospital, Oslo, said that she would choose to switch to a tumor necrosis factor [TNF] inhibitor.
“I think it would depend on what prior treatment the patient had received,” said April Jorge, MD, a rheumatologist at Massachusetts General Hospital, Boston. “In my practice, patients receiving a JAK inhibitor typically failed on their biologics. I haven’t had many fail a JAK inhibitor – a small sample size.”
“That’s what we see in our study,” Dr. Pombo-Suarez said. “Most of the patients that cycled JAK inhibitors had higher numbers of biologics compared with switchers.”
“I can share my experience, which is a greater comfort level with cycling a TNF antagonist. I agree with Dr Jorge: I don’t use JAK inhibitors in the first line for rheumatoid arthritis, but based on the work that’s been described here and future data, I might have a greater comfort level cycling JAK inhibitors once the data support such an approach,” commented H. Michael Belmont, MD, professor of medicine at New York University, co-director of the NYU Lupus Center, and medical director of Bellevue Hospital Lupus Center, New York.
The JAK-pot study is supported by unrestricted research grants from AbbVie and Galapagos. Dr. Pombo-Suarez has received adviser and speaker honoraria from several companies other than the funders. Dr. Syversen has received honoraria from Thermo Fisher. Dr. Jorge has disclosed no relevant financial relationships. Dr. Belmont has received honoraria from Alexion.
A version of this article first appeared on Medscape.com.
For patients with rheumatoid arthritis (RA) for whom a first Janus kinase inhibitor (JAKi) has failed, there appears to be no difference in treatment effectiveness whether the patient is cycled to a second JAKi or receives a biologic disease-modifying antirheumatic drug (bDMARD), a study of international patient registry data suggests.
However, patients who are prescribed a different JAKi after the first has failed them tend to have conditions that are more difficult to treat than do patients who are switched to a bDMARD after JAKi failure. In addition, adverse events that occur with the first JAKi are likely to occur again if a different agent in the same class is used, reported Manuel Pombo-Suarez, MD, PhD, adjunct professor of medicine at the University Hospital of Santiago de Compostela, Spain.
“When the first JAK inhibitor was stopped due to an adverse event, it was also more likely that the second JAK inhibitor would be stopped for the same reason,” he said in an oral abstract presentation during the American College of Rheumatology (ACR) 2021 Annual Meeting, which was held online.
The 2019 update of the European Alliance of Associations for Rheumatology (EULAR) guidelines for RA recommend that for patients for whom a first JAKi has failed, clinicians can consider a different JAKi or switch to a bDMARD. But at the time the guidelines were published, no data were available from studies in which a second JAKi was used after the failure of a first JAKi, Dr. Pombo-Suarez noted.
“We are trying to shed a light on this growing population of patients, as prescription of these drugs is increasing and new JAK inhibitors come into play, meaning that this scenario, we propose, is becoming more and more frequent in real life. We must provide a solution for these patients,” he said.
Pooled registry data
The investigators compared the effectiveness of the two approaches with respect to rates of drug retention and Disease Activity Score in 28 joints (DAS28).
They conducted a nested cohort study using data from 14 national registries that are part of the JAK-pot collaboration.
They pooled data from each registry on patients with RA for whom a first JAKi had failed and who were then treated with either a second JAKi or a bDMARD.
They identified a total of 708 patients for whom a JAKi had failed initially. Of these patients, 154 were given a different JAKi, and 554 were switched to a bDMARD. In each group, women accounted for a large majority of patients.
The mean age was slightly older among those who received a second JAKi (58.41 years vs. 54.74 years for patients who were given a bDMARD). The mean disease duration was 13.95 years and 11.37 years, respectively.
In each group, approximately 77% of patients received tofacitinib (Xeljanz).
At baseline, the mean DAS28 scores were similar between the groups: 4.10 in the group that received a second JAKi, and 4.17 in the group given a bDMARD.
Reasons for initially stopping use of a JAKi were as follows: adverse events (27.3% of those who took a second JAKi after they had stopped taking one initially, and 17.9% of patients who received a bDMARD); lack of efficacy (61% and 65%, respectively), and other reasons (11.7% and 17.1%, respectively).
At 2 years’ follow-up, drug survival rates were similar between the two treatment arms, although there was a nonsignificant trend toward a higher rate of discontinuation among patients who were given a second JAKi after they stopped taking the first JAKi because of adverse events. In contrast, there was also a nonsignificant trend toward lower discontinuation rates among patients who were given a second JAKi after they had stopped taking the first JAKi because of lack of efficacy.
As noted before, patients who stopped taking the first JAKi because of an adverse event were more likely to stop taking the second JAKi because of they experienced either the same or a different adverse event, whereas patients who started taking a bDMARD were equally likely to stop taking the second therapy because of either adverse events or lack of efficacy.
The treatment strategies were virtually identical with respect to improvement of DAS28 at 7 months after the start of therapy.
Dr. Pombo-Suarez acknowledged that the study was limited by the fact that heterogeneity between countries could not be assessed, owing to the small sample sizes in each nation’s registry. Other limitations include short follow-up and the fact that tofacitinib was used as the first JAKi by the large majority of patients.
What’s your practice?
In a media briefing during which Dr. Pombo-Suarez discussed the study findings, this news organization polled other speakers who were not involved in the study about their go-to strategies when JAKi therapy fails.
Silje Watterdal Syversen, MD, PhD, a consultant rheumatologist and researcher at Diakonhjemmet Hospital, Oslo, said that she would choose to switch to a tumor necrosis factor [TNF] inhibitor.
“I think it would depend on what prior treatment the patient had received,” said April Jorge, MD, a rheumatologist at Massachusetts General Hospital, Boston. “In my practice, patients receiving a JAK inhibitor typically failed on their biologics. I haven’t had many fail a JAK inhibitor – a small sample size.”
“That’s what we see in our study,” Dr. Pombo-Suarez said. “Most of the patients that cycled JAK inhibitors had higher numbers of biologics compared with switchers.”
“I can share my experience, which is a greater comfort level with cycling a TNF antagonist. I agree with Dr Jorge: I don’t use JAK inhibitors in the first line for rheumatoid arthritis, but based on the work that’s been described here and future data, I might have a greater comfort level cycling JAK inhibitors once the data support such an approach,” commented H. Michael Belmont, MD, professor of medicine at New York University, co-director of the NYU Lupus Center, and medical director of Bellevue Hospital Lupus Center, New York.
The JAK-pot study is supported by unrestricted research grants from AbbVie and Galapagos. Dr. Pombo-Suarez has received adviser and speaker honoraria from several companies other than the funders. Dr. Syversen has received honoraria from Thermo Fisher. Dr. Jorge has disclosed no relevant financial relationships. Dr. Belmont has received honoraria from Alexion.
A version of this article first appeared on Medscape.com.
For patients with rheumatoid arthritis (RA) for whom a first Janus kinase inhibitor (JAKi) has failed, there appears to be no difference in treatment effectiveness whether the patient is cycled to a second JAKi or receives a biologic disease-modifying antirheumatic drug (bDMARD), a study of international patient registry data suggests.
However, patients who are prescribed a different JAKi after the first has failed them tend to have conditions that are more difficult to treat than do patients who are switched to a bDMARD after JAKi failure. In addition, adverse events that occur with the first JAKi are likely to occur again if a different agent in the same class is used, reported Manuel Pombo-Suarez, MD, PhD, adjunct professor of medicine at the University Hospital of Santiago de Compostela, Spain.
“When the first JAK inhibitor was stopped due to an adverse event, it was also more likely that the second JAK inhibitor would be stopped for the same reason,” he said in an oral abstract presentation during the American College of Rheumatology (ACR) 2021 Annual Meeting, which was held online.
The 2019 update of the European Alliance of Associations for Rheumatology (EULAR) guidelines for RA recommend that for patients for whom a first JAKi has failed, clinicians can consider a different JAKi or switch to a bDMARD. But at the time the guidelines were published, no data were available from studies in which a second JAKi was used after the failure of a first JAKi, Dr. Pombo-Suarez noted.
“We are trying to shed a light on this growing population of patients, as prescription of these drugs is increasing and new JAK inhibitors come into play, meaning that this scenario, we propose, is becoming more and more frequent in real life. We must provide a solution for these patients,” he said.
Pooled registry data
The investigators compared the effectiveness of the two approaches with respect to rates of drug retention and Disease Activity Score in 28 joints (DAS28).
They conducted a nested cohort study using data from 14 national registries that are part of the JAK-pot collaboration.
They pooled data from each registry on patients with RA for whom a first JAKi had failed and who were then treated with either a second JAKi or a bDMARD.
They identified a total of 708 patients for whom a JAKi had failed initially. Of these patients, 154 were given a different JAKi, and 554 were switched to a bDMARD. In each group, women accounted for a large majority of patients.
The mean age was slightly older among those who received a second JAKi (58.41 years vs. 54.74 years for patients who were given a bDMARD). The mean disease duration was 13.95 years and 11.37 years, respectively.
In each group, approximately 77% of patients received tofacitinib (Xeljanz).
At baseline, the mean DAS28 scores were similar between the groups: 4.10 in the group that received a second JAKi, and 4.17 in the group given a bDMARD.
Reasons for initially stopping use of a JAKi were as follows: adverse events (27.3% of those who took a second JAKi after they had stopped taking one initially, and 17.9% of patients who received a bDMARD); lack of efficacy (61% and 65%, respectively), and other reasons (11.7% and 17.1%, respectively).
At 2 years’ follow-up, drug survival rates were similar between the two treatment arms, although there was a nonsignificant trend toward a higher rate of discontinuation among patients who were given a second JAKi after they stopped taking the first JAKi because of adverse events. In contrast, there was also a nonsignificant trend toward lower discontinuation rates among patients who were given a second JAKi after they had stopped taking the first JAKi because of lack of efficacy.
As noted before, patients who stopped taking the first JAKi because of an adverse event were more likely to stop taking the second JAKi because of they experienced either the same or a different adverse event, whereas patients who started taking a bDMARD were equally likely to stop taking the second therapy because of either adverse events or lack of efficacy.
The treatment strategies were virtually identical with respect to improvement of DAS28 at 7 months after the start of therapy.
Dr. Pombo-Suarez acknowledged that the study was limited by the fact that heterogeneity between countries could not be assessed, owing to the small sample sizes in each nation’s registry. Other limitations include short follow-up and the fact that tofacitinib was used as the first JAKi by the large majority of patients.
What’s your practice?
In a media briefing during which Dr. Pombo-Suarez discussed the study findings, this news organization polled other speakers who were not involved in the study about their go-to strategies when JAKi therapy fails.
Silje Watterdal Syversen, MD, PhD, a consultant rheumatologist and researcher at Diakonhjemmet Hospital, Oslo, said that she would choose to switch to a tumor necrosis factor [TNF] inhibitor.
“I think it would depend on what prior treatment the patient had received,” said April Jorge, MD, a rheumatologist at Massachusetts General Hospital, Boston. “In my practice, patients receiving a JAK inhibitor typically failed on their biologics. I haven’t had many fail a JAK inhibitor – a small sample size.”
“That’s what we see in our study,” Dr. Pombo-Suarez said. “Most of the patients that cycled JAK inhibitors had higher numbers of biologics compared with switchers.”
“I can share my experience, which is a greater comfort level with cycling a TNF antagonist. I agree with Dr Jorge: I don’t use JAK inhibitors in the first line for rheumatoid arthritis, but based on the work that’s been described here and future data, I might have a greater comfort level cycling JAK inhibitors once the data support such an approach,” commented H. Michael Belmont, MD, professor of medicine at New York University, co-director of the NYU Lupus Center, and medical director of Bellevue Hospital Lupus Center, New York.
The JAK-pot study is supported by unrestricted research grants from AbbVie and Galapagos. Dr. Pombo-Suarez has received adviser and speaker honoraria from several companies other than the funders. Dr. Syversen has received honoraria from Thermo Fisher. Dr. Jorge has disclosed no relevant financial relationships. Dr. Belmont has received honoraria from Alexion.
A version of this article first appeared on Medscape.com.
Infected, vaccinated, or both: How protected am I from COVID-19?
As the United States rounds out its second year of the pandemic, many people are trying to figure out just how vulnerable they may be to COVID-19 infection, and whether it’s finally safe to fully return to all the activities they miss.
On an individual basis, the degree and durability of the immunity a person gets after vaccination versus an infection is not an easy question to answer. But it’s one that science is hotly pursing.
“This virus is teaching us a lot about immunology,” says Gregory Poland, MD, who studies how the body responds to vaccines at the Mayo Clinic in Rochester, Minn. Dr. Poland says this moment in science reminds him of a quote attributed to Ralph Waldo Emerson: “We learn about geology the morning after the earthquake.”
“And that’s the case here. It is and will continue to teach us a lot of immunology,” he says.
It’s vital to understand how a COVID-19 infection reshapes the body’s immune defenses so that researchers can tailor vaccines and therapies to do the same or better.
“Because, of course, it’s much more risky to get infected with the actual virus, than with the vaccine,” says Daniela Weiskopf, PhD, a researcher at the La Jolla Institute for Immunology in California.
What is known so far is that how much protection you get and how long you may have it depends on several factors. Those include your age, whether you’ve had COVID-19 before and how severe your symptoms were, your vaccination status, and how long it has been since you were infected or inoculated. Your underlying health matters, too. Immune protection also depends on the virus and how much it is changing as it evolves to evade all our hard-won defenses.
In a new scientific brief, Here’s what we know so far:
Durability of immunity
The agency’s researchers say if you’ve recovered from a COVID-19 infection or are fully vaccinated, you’re probably in good shape for at least 6 months. That’s why this is the recommended interval for people to consider getting a booster dose.
Even though the protection you get after infection and vaccination is generally strong, it’s not perfect.
Getting COVID-19 after you’ve been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you. And if you do happen to catch COVID, if your immune system has already gotten a heads up about the virus, your infection is much less likely to be one that lands you in the hospital or morgue.
According to CDC data, at the height of the Delta surge in August, fully vaccinated people were six times less likely to get a COVID-19 infection compared with unvaccinated people, and 11 times less likely to die if they did get it.
How strong is immunity after a COVID-19 Infection?
About 90% of people develop some number of protective antibodies after a COVID-19 infection, according to the CDC. But how high those levels climb appears to be all over the map. Studies show peak antibody concentrations can vary as much as 200-fold, or 2,000%.
Where you fall within that very large range will depend on your age and how sick you became from your COVID-19 infection. It also depends on whether you have an underlying health condition or take a medication that blunts immune function.
Our immune system slows down with age. Immunosenescence starts to affect a person’s health around the age of 60. But there’s no bright line for failure. People who exercise and are generally healthy will have better immune function than someone who doesn’t, no matter their age. In general, though, the older you are, the less likely you are to get a robust immune response after an infection or a vaccination. That’s why this group has been prioritized both for first vaccine doses and boosters.
Beyond age, your protection from future infection seems to depend on how ill you were with the first. Several studies have shown that blood levels of antibodies rise faster and reach a higher peak in people with more severe infections.
In general, people with cold-like symptoms who tested positive but recovered at home are better protected than people who didn’t get any symptoms. And people who were hospitalized for their infections are better protected over the long term than people with milder infections. They may have paid a steep price for that protection: Many hospitalized patients continue to have debilitating symptoms that last for months after they go home.
On average, though, protection after infection seems to be comparable to vaccination, at least for a while. Six large studies from different countries have looked into this question, and five of them have used the very sensitive real-time polymerase chain reaction test (RT-PCR) to count people as truly being previously infected. These studies found that for 6 to 9 months after recovery, a person was 80% to 93% less likely to get COVID-19 again.
There are some caveats to mention, though. Early in the pandemic when supplies were scarce, it was hard to get tested unless you were so sick you landed in the hospital. Studies have shown that the concentration of antibodies a person makes after an infection seems to depend on how sick they got in the first place.
People who had milder infections, or who didn’t have any symptoms at all, may not develop as much protection as those who have more severe symptoms. So these studies may reflect the immunity developed by people who were pretty ill during their first infections.
One study of 25,000 health care workers, who were all tested every 2 weeks -- whether they had symptoms or not -- may offer a clearer picture. In this study, health care workers who’d previously tested positive for COVID-19 were 84% less likely to test positive for the virus again. They were 93% less likely to get an infection that made them sick, and 52% less likely to get an infection without symptoms, for at least 6 months after they recovered.
How does protection after infection compare to vaccination?
Two weeks after your final vaccine dose, protection against a COVID-19 infection is high -- around 90% for the Pfizer and Moderna mRNA vaccines and 66% for the one-dose Johnson & Johnson shot. Clinical trials conducted by the manufacturer have shown that a second dose of the Johnson & Johnson vaccine given at least 2 months after vaccination boosts protection against illness in the United States to about 94%, which is why another dose has been recommended for all Johnson & Johnson vaccine recipients 2 months after their first shot.
It’s not yet known how long the COVID-19 vaccines remain protective. There’s some evidence that protection against symptomatic infections wanes a bit over time as antibody levels drop. But protection against severe illness, including hospitalization and death, has remained high so far, even without a booster.
Are antibodies different after infection compared to vaccination?
Yes. And researchers don’t yet understand what these differences mean.
It seems to come down to a question of quality versus quantity. Vaccines seem to produce higher peak antibody levels than natural infections do. But these antibodies are highly specialized, able to recognize only the parts of the virus they were designed to target.
“The mRNA vaccine directs all the immune responses to the single spike protein,” says Alice Cho, PhD, who is studying the differences in vaccine and infection-created immunity at the Rockefeller University in New York. “There’s a lot more to respond to with a virus than there is in a vaccine.”
During an infection, the immune system learns to recognize and grab onto many parts of the virus, not just its spike.
The job of remembering the various pieces and parts of a foreign invader, so that it can be quickly recognized and disarmed should it ever return, falls to memory B cells.
Memory B cells, in turn, make plasma cells that then crank out antibodies that are custom tailored to attach to their targets.
Antibody levels gradually fall over a few months’ time as the plasma cells that make them die off. But memory B cells live for extended periods. One study that was attempting to measure the lifespan of individual memory B cells in mice found that these cells probably live as long as the mouse itself. Memory B cells induced by smallpox vaccination may live at least 60 years -- virtually an entire lifetime.
Dr. Cho’s research team has found that when memory B cells are trained by the vaccine, they become one-hit wonders, cranking out copious amounts of the same kinds of antibodies over and over again.
Memory B cells trained by viral infection, however, are more versatile. They continue to evolve over several months and produce higher quality antibodies that appear to become more potent over time and can even develop activity against future variants.
Still, the researchers stress that it’s not smart to wait to get a COVID-19 infection in hopes of getting these more versatile antibodies.
“While a natural infection may induce maturation of antibodies with broader activity than a vaccine does -- a natural infection can also kill you,” says Michel Nussenzweig, MD, PhD, head of Rockefeller’s Laboratory of Molecular Immunology.
Sure, memory B cells generated by infections may be immunological Swiss Army Knives, but maybe, argues Donna Farber, PhD, an immunologist at Columbia University in New York, we really only need a single blade.
“The thing with the vaccine is that it’s really focused,” she says. “It’s not giving you all these other viral proteins. It’s only giving you the spike.”
“It may be even better than the level of neutralizing spike antibodies you’re going to get from the infection,” she says. “With a viral infection, the immune response really has a lot to do. It’s really being distracted by all these other proteins.”
“Whereas with the vaccine, it’s just saying to the immune response, ‘This is the immunity we need,’” Dr. Farber says. “‘Just generate this immunity.’ So it’s focusing the immune response in a way that’s going to guarantee that you’re going to get that protective response.”
What if you had COVID and later got vaccinated?
This is called hybrid immunity, and it’s the best of both worlds.
“You have the benefit of very deep, but narrow, immunity produced by vaccine, and very broad, but not very deep, immunity produced by infection,” Dr. Poland says. He says you’ve effectively cross-trained your immune system.
In studies of people who recovered from COVID-19 and then went on to get an mRNA vaccine, after one dose, their antibodies were as high as someone who had been fully vaccinated. After two doses, their antibody levels were about double the average levels seen in someone who’d only been vaccinated.
Studies have shown this kind of immunity has real benefits, too. A recent study by researchers at the University of Kentucky and the CDC found that people who’d gotten COVID-19 in 2020, but had not been vaccinated, were about twice as likely to be reinfected in May and June compared with those who recovered and went on to get their vaccines.
What antibody level is protective?
Scientists aren’t exactly sure how high antibody levels need to be for protection, or even which kinds of antibodies or other immune components matter most yet.
But vaccines appear to generate higher antibody levels than infections do. In a recent study published in the journal Science , Dr. Weiskopf and her colleagues at the La Jolla Institute of Immunology detail the findings of a de-escalation study, where they gave people one-quarter of the normal dose of the Moderna mRNA vaccine and then collected blood samples over time to study their immune responses.
Their immune responses were scaled down with the dose.
“We saw that this has the exact same levels as natural infection,” Dr. Weiskopf says. “People who are vaccinated have much higher immune memory than people who are naturally infected,” she says.
Antibody levels are not easy to determine in the real world. Can you take a test to find out how protected you are? The answer is no, because we don’t yet know what antibody level, or even which kind of antibodies, correlate with protection.
Also, there are many different kinds of antibody tests and they all use a slightly different scale, so there’s no broadly agreed upon way to measure them yet. It’s difficult to compare levels test to test.
Weeks or months between doses? Which is best?
Both the Pfizer and Moderna vaccines were tested to be given 3 and 4 weeks apart, respectively. But when the vaccines were first rolling out, shortages prompted some countries to stretch the interval between doses to 4 or more months.
Researchers who have studied the immune responses of people who were inoculated on an extended dosing schedule noticed something interesting: When the interval was stretched, people had better antibody responses. In fact, their antibody responses looked like the sky-high levels people got with hybrid immunity.
Susanna Dunachie, PhD, a global research professor at the University of Oxford in the United Kingdom, wondered why. She’s leading a team of researchers who are doing detailed studies of the immune responses of health care workers after their vaccinations.
“We found that B cells, which are the cells that make antibodies to the viral spike protein after vaccination, carry on increasing in number between 4 and 10 weeks after vaccination,” she says.
Waiting to give the second vaccine 6 to 14 weeks seems to stimulate the immune system when all of its antibody-making factories are finally up and running.
For this reason, giving the second dose at 3 weeks, she says, might be premature.
But there’s a tradeoff involved in waiting. If there are high levels of the virus circulating in a community, you want to get people fully vaccinated as quickly as possible to maximize their protection in the shortest window of time, which is what we decided to do in the United States.
Researchers say it might be a good idea to revisit the dosing interval when it’s less risky to try it.
A version of this article first appeared on WebMD.com.
As the United States rounds out its second year of the pandemic, many people are trying to figure out just how vulnerable they may be to COVID-19 infection, and whether it’s finally safe to fully return to all the activities they miss.
On an individual basis, the degree and durability of the immunity a person gets after vaccination versus an infection is not an easy question to answer. But it’s one that science is hotly pursing.
“This virus is teaching us a lot about immunology,” says Gregory Poland, MD, who studies how the body responds to vaccines at the Mayo Clinic in Rochester, Minn. Dr. Poland says this moment in science reminds him of a quote attributed to Ralph Waldo Emerson: “We learn about geology the morning after the earthquake.”
“And that’s the case here. It is and will continue to teach us a lot of immunology,” he says.
It’s vital to understand how a COVID-19 infection reshapes the body’s immune defenses so that researchers can tailor vaccines and therapies to do the same or better.
“Because, of course, it’s much more risky to get infected with the actual virus, than with the vaccine,” says Daniela Weiskopf, PhD, a researcher at the La Jolla Institute for Immunology in California.
What is known so far is that how much protection you get and how long you may have it depends on several factors. Those include your age, whether you’ve had COVID-19 before and how severe your symptoms were, your vaccination status, and how long it has been since you were infected or inoculated. Your underlying health matters, too. Immune protection also depends on the virus and how much it is changing as it evolves to evade all our hard-won defenses.
In a new scientific brief, Here’s what we know so far:
Durability of immunity
The agency’s researchers say if you’ve recovered from a COVID-19 infection or are fully vaccinated, you’re probably in good shape for at least 6 months. That’s why this is the recommended interval for people to consider getting a booster dose.
Even though the protection you get after infection and vaccination is generally strong, it’s not perfect.
Getting COVID-19 after you’ve been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you. And if you do happen to catch COVID, if your immune system has already gotten a heads up about the virus, your infection is much less likely to be one that lands you in the hospital or morgue.
According to CDC data, at the height of the Delta surge in August, fully vaccinated people were six times less likely to get a COVID-19 infection compared with unvaccinated people, and 11 times less likely to die if they did get it.
How strong is immunity after a COVID-19 Infection?
About 90% of people develop some number of protective antibodies after a COVID-19 infection, according to the CDC. But how high those levels climb appears to be all over the map. Studies show peak antibody concentrations can vary as much as 200-fold, or 2,000%.
Where you fall within that very large range will depend on your age and how sick you became from your COVID-19 infection. It also depends on whether you have an underlying health condition or take a medication that blunts immune function.
Our immune system slows down with age. Immunosenescence starts to affect a person’s health around the age of 60. But there’s no bright line for failure. People who exercise and are generally healthy will have better immune function than someone who doesn’t, no matter their age. In general, though, the older you are, the less likely you are to get a robust immune response after an infection or a vaccination. That’s why this group has been prioritized both for first vaccine doses and boosters.
Beyond age, your protection from future infection seems to depend on how ill you were with the first. Several studies have shown that blood levels of antibodies rise faster and reach a higher peak in people with more severe infections.
In general, people with cold-like symptoms who tested positive but recovered at home are better protected than people who didn’t get any symptoms. And people who were hospitalized for their infections are better protected over the long term than people with milder infections. They may have paid a steep price for that protection: Many hospitalized patients continue to have debilitating symptoms that last for months after they go home.
On average, though, protection after infection seems to be comparable to vaccination, at least for a while. Six large studies from different countries have looked into this question, and five of them have used the very sensitive real-time polymerase chain reaction test (RT-PCR) to count people as truly being previously infected. These studies found that for 6 to 9 months after recovery, a person was 80% to 93% less likely to get COVID-19 again.
There are some caveats to mention, though. Early in the pandemic when supplies were scarce, it was hard to get tested unless you were so sick you landed in the hospital. Studies have shown that the concentration of antibodies a person makes after an infection seems to depend on how sick they got in the first place.
People who had milder infections, or who didn’t have any symptoms at all, may not develop as much protection as those who have more severe symptoms. So these studies may reflect the immunity developed by people who were pretty ill during their first infections.
One study of 25,000 health care workers, who were all tested every 2 weeks -- whether they had symptoms or not -- may offer a clearer picture. In this study, health care workers who’d previously tested positive for COVID-19 were 84% less likely to test positive for the virus again. They were 93% less likely to get an infection that made them sick, and 52% less likely to get an infection without symptoms, for at least 6 months after they recovered.
How does protection after infection compare to vaccination?
Two weeks after your final vaccine dose, protection against a COVID-19 infection is high -- around 90% for the Pfizer and Moderna mRNA vaccines and 66% for the one-dose Johnson & Johnson shot. Clinical trials conducted by the manufacturer have shown that a second dose of the Johnson & Johnson vaccine given at least 2 months after vaccination boosts protection against illness in the United States to about 94%, which is why another dose has been recommended for all Johnson & Johnson vaccine recipients 2 months after their first shot.
It’s not yet known how long the COVID-19 vaccines remain protective. There’s some evidence that protection against symptomatic infections wanes a bit over time as antibody levels drop. But protection against severe illness, including hospitalization and death, has remained high so far, even without a booster.
Are antibodies different after infection compared to vaccination?
Yes. And researchers don’t yet understand what these differences mean.
It seems to come down to a question of quality versus quantity. Vaccines seem to produce higher peak antibody levels than natural infections do. But these antibodies are highly specialized, able to recognize only the parts of the virus they were designed to target.
“The mRNA vaccine directs all the immune responses to the single spike protein,” says Alice Cho, PhD, who is studying the differences in vaccine and infection-created immunity at the Rockefeller University in New York. “There’s a lot more to respond to with a virus than there is in a vaccine.”
During an infection, the immune system learns to recognize and grab onto many parts of the virus, not just its spike.
The job of remembering the various pieces and parts of a foreign invader, so that it can be quickly recognized and disarmed should it ever return, falls to memory B cells.
Memory B cells, in turn, make plasma cells that then crank out antibodies that are custom tailored to attach to their targets.
Antibody levels gradually fall over a few months’ time as the plasma cells that make them die off. But memory B cells live for extended periods. One study that was attempting to measure the lifespan of individual memory B cells in mice found that these cells probably live as long as the mouse itself. Memory B cells induced by smallpox vaccination may live at least 60 years -- virtually an entire lifetime.
Dr. Cho’s research team has found that when memory B cells are trained by the vaccine, they become one-hit wonders, cranking out copious amounts of the same kinds of antibodies over and over again.
Memory B cells trained by viral infection, however, are more versatile. They continue to evolve over several months and produce higher quality antibodies that appear to become more potent over time and can even develop activity against future variants.
Still, the researchers stress that it’s not smart to wait to get a COVID-19 infection in hopes of getting these more versatile antibodies.
“While a natural infection may induce maturation of antibodies with broader activity than a vaccine does -- a natural infection can also kill you,” says Michel Nussenzweig, MD, PhD, head of Rockefeller’s Laboratory of Molecular Immunology.
Sure, memory B cells generated by infections may be immunological Swiss Army Knives, but maybe, argues Donna Farber, PhD, an immunologist at Columbia University in New York, we really only need a single blade.
“The thing with the vaccine is that it’s really focused,” she says. “It’s not giving you all these other viral proteins. It’s only giving you the spike.”
“It may be even better than the level of neutralizing spike antibodies you’re going to get from the infection,” she says. “With a viral infection, the immune response really has a lot to do. It’s really being distracted by all these other proteins.”
“Whereas with the vaccine, it’s just saying to the immune response, ‘This is the immunity we need,’” Dr. Farber says. “‘Just generate this immunity.’ So it’s focusing the immune response in a way that’s going to guarantee that you’re going to get that protective response.”
What if you had COVID and later got vaccinated?
This is called hybrid immunity, and it’s the best of both worlds.
“You have the benefit of very deep, but narrow, immunity produced by vaccine, and very broad, but not very deep, immunity produced by infection,” Dr. Poland says. He says you’ve effectively cross-trained your immune system.
In studies of people who recovered from COVID-19 and then went on to get an mRNA vaccine, after one dose, their antibodies were as high as someone who had been fully vaccinated. After two doses, their antibody levels were about double the average levels seen in someone who’d only been vaccinated.
Studies have shown this kind of immunity has real benefits, too. A recent study by researchers at the University of Kentucky and the CDC found that people who’d gotten COVID-19 in 2020, but had not been vaccinated, were about twice as likely to be reinfected in May and June compared with those who recovered and went on to get their vaccines.
What antibody level is protective?
Scientists aren’t exactly sure how high antibody levels need to be for protection, or even which kinds of antibodies or other immune components matter most yet.
But vaccines appear to generate higher antibody levels than infections do. In a recent study published in the journal Science , Dr. Weiskopf and her colleagues at the La Jolla Institute of Immunology detail the findings of a de-escalation study, where they gave people one-quarter of the normal dose of the Moderna mRNA vaccine and then collected blood samples over time to study their immune responses.
Their immune responses were scaled down with the dose.
“We saw that this has the exact same levels as natural infection,” Dr. Weiskopf says. “People who are vaccinated have much higher immune memory than people who are naturally infected,” she says.
Antibody levels are not easy to determine in the real world. Can you take a test to find out how protected you are? The answer is no, because we don’t yet know what antibody level, or even which kind of antibodies, correlate with protection.
Also, there are many different kinds of antibody tests and they all use a slightly different scale, so there’s no broadly agreed upon way to measure them yet. It’s difficult to compare levels test to test.
Weeks or months between doses? Which is best?
Both the Pfizer and Moderna vaccines were tested to be given 3 and 4 weeks apart, respectively. But when the vaccines were first rolling out, shortages prompted some countries to stretch the interval between doses to 4 or more months.
Researchers who have studied the immune responses of people who were inoculated on an extended dosing schedule noticed something interesting: When the interval was stretched, people had better antibody responses. In fact, their antibody responses looked like the sky-high levels people got with hybrid immunity.
Susanna Dunachie, PhD, a global research professor at the University of Oxford in the United Kingdom, wondered why. She’s leading a team of researchers who are doing detailed studies of the immune responses of health care workers after their vaccinations.
“We found that B cells, which are the cells that make antibodies to the viral spike protein after vaccination, carry on increasing in number between 4 and 10 weeks after vaccination,” she says.
Waiting to give the second vaccine 6 to 14 weeks seems to stimulate the immune system when all of its antibody-making factories are finally up and running.
For this reason, giving the second dose at 3 weeks, she says, might be premature.
But there’s a tradeoff involved in waiting. If there are high levels of the virus circulating in a community, you want to get people fully vaccinated as quickly as possible to maximize their protection in the shortest window of time, which is what we decided to do in the United States.
Researchers say it might be a good idea to revisit the dosing interval when it’s less risky to try it.
A version of this article first appeared on WebMD.com.
As the United States rounds out its second year of the pandemic, many people are trying to figure out just how vulnerable they may be to COVID-19 infection, and whether it’s finally safe to fully return to all the activities they miss.
On an individual basis, the degree and durability of the immunity a person gets after vaccination versus an infection is not an easy question to answer. But it’s one that science is hotly pursing.
“This virus is teaching us a lot about immunology,” says Gregory Poland, MD, who studies how the body responds to vaccines at the Mayo Clinic in Rochester, Minn. Dr. Poland says this moment in science reminds him of a quote attributed to Ralph Waldo Emerson: “We learn about geology the morning after the earthquake.”
“And that’s the case here. It is and will continue to teach us a lot of immunology,” he says.
It’s vital to understand how a COVID-19 infection reshapes the body’s immune defenses so that researchers can tailor vaccines and therapies to do the same or better.
“Because, of course, it’s much more risky to get infected with the actual virus, than with the vaccine,” says Daniela Weiskopf, PhD, a researcher at the La Jolla Institute for Immunology in California.
What is known so far is that how much protection you get and how long you may have it depends on several factors. Those include your age, whether you’ve had COVID-19 before and how severe your symptoms were, your vaccination status, and how long it has been since you were infected or inoculated. Your underlying health matters, too. Immune protection also depends on the virus and how much it is changing as it evolves to evade all our hard-won defenses.
In a new scientific brief, Here’s what we know so far:
Durability of immunity
The agency’s researchers say if you’ve recovered from a COVID-19 infection or are fully vaccinated, you’re probably in good shape for at least 6 months. That’s why this is the recommended interval for people to consider getting a booster dose.
Even though the protection you get after infection and vaccination is generally strong, it’s not perfect.
Getting COVID-19 after you’ve been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you. And if you do happen to catch COVID, if your immune system has already gotten a heads up about the virus, your infection is much less likely to be one that lands you in the hospital or morgue.
According to CDC data, at the height of the Delta surge in August, fully vaccinated people were six times less likely to get a COVID-19 infection compared with unvaccinated people, and 11 times less likely to die if they did get it.
How strong is immunity after a COVID-19 Infection?
About 90% of people develop some number of protective antibodies after a COVID-19 infection, according to the CDC. But how high those levels climb appears to be all over the map. Studies show peak antibody concentrations can vary as much as 200-fold, or 2,000%.
Where you fall within that very large range will depend on your age and how sick you became from your COVID-19 infection. It also depends on whether you have an underlying health condition or take a medication that blunts immune function.
Our immune system slows down with age. Immunosenescence starts to affect a person’s health around the age of 60. But there’s no bright line for failure. People who exercise and are generally healthy will have better immune function than someone who doesn’t, no matter their age. In general, though, the older you are, the less likely you are to get a robust immune response after an infection or a vaccination. That’s why this group has been prioritized both for first vaccine doses and boosters.
Beyond age, your protection from future infection seems to depend on how ill you were with the first. Several studies have shown that blood levels of antibodies rise faster and reach a higher peak in people with more severe infections.
In general, people with cold-like symptoms who tested positive but recovered at home are better protected than people who didn’t get any symptoms. And people who were hospitalized for their infections are better protected over the long term than people with milder infections. They may have paid a steep price for that protection: Many hospitalized patients continue to have debilitating symptoms that last for months after they go home.
On average, though, protection after infection seems to be comparable to vaccination, at least for a while. Six large studies from different countries have looked into this question, and five of them have used the very sensitive real-time polymerase chain reaction test (RT-PCR) to count people as truly being previously infected. These studies found that for 6 to 9 months after recovery, a person was 80% to 93% less likely to get COVID-19 again.
There are some caveats to mention, though. Early in the pandemic when supplies were scarce, it was hard to get tested unless you were so sick you landed in the hospital. Studies have shown that the concentration of antibodies a person makes after an infection seems to depend on how sick they got in the first place.
People who had milder infections, or who didn’t have any symptoms at all, may not develop as much protection as those who have more severe symptoms. So these studies may reflect the immunity developed by people who were pretty ill during their first infections.
One study of 25,000 health care workers, who were all tested every 2 weeks -- whether they had symptoms or not -- may offer a clearer picture. In this study, health care workers who’d previously tested positive for COVID-19 were 84% less likely to test positive for the virus again. They were 93% less likely to get an infection that made them sick, and 52% less likely to get an infection without symptoms, for at least 6 months after they recovered.
How does protection after infection compare to vaccination?
Two weeks after your final vaccine dose, protection against a COVID-19 infection is high -- around 90% for the Pfizer and Moderna mRNA vaccines and 66% for the one-dose Johnson & Johnson shot. Clinical trials conducted by the manufacturer have shown that a second dose of the Johnson & Johnson vaccine given at least 2 months after vaccination boosts protection against illness in the United States to about 94%, which is why another dose has been recommended for all Johnson & Johnson vaccine recipients 2 months after their first shot.
It’s not yet known how long the COVID-19 vaccines remain protective. There’s some evidence that protection against symptomatic infections wanes a bit over time as antibody levels drop. But protection against severe illness, including hospitalization and death, has remained high so far, even without a booster.
Are antibodies different after infection compared to vaccination?
Yes. And researchers don’t yet understand what these differences mean.
It seems to come down to a question of quality versus quantity. Vaccines seem to produce higher peak antibody levels than natural infections do. But these antibodies are highly specialized, able to recognize only the parts of the virus they were designed to target.
“The mRNA vaccine directs all the immune responses to the single spike protein,” says Alice Cho, PhD, who is studying the differences in vaccine and infection-created immunity at the Rockefeller University in New York. “There’s a lot more to respond to with a virus than there is in a vaccine.”
During an infection, the immune system learns to recognize and grab onto many parts of the virus, not just its spike.
The job of remembering the various pieces and parts of a foreign invader, so that it can be quickly recognized and disarmed should it ever return, falls to memory B cells.
Memory B cells, in turn, make plasma cells that then crank out antibodies that are custom tailored to attach to their targets.
Antibody levels gradually fall over a few months’ time as the plasma cells that make them die off. But memory B cells live for extended periods. One study that was attempting to measure the lifespan of individual memory B cells in mice found that these cells probably live as long as the mouse itself. Memory B cells induced by smallpox vaccination may live at least 60 years -- virtually an entire lifetime.
Dr. Cho’s research team has found that when memory B cells are trained by the vaccine, they become one-hit wonders, cranking out copious amounts of the same kinds of antibodies over and over again.
Memory B cells trained by viral infection, however, are more versatile. They continue to evolve over several months and produce higher quality antibodies that appear to become more potent over time and can even develop activity against future variants.
Still, the researchers stress that it’s not smart to wait to get a COVID-19 infection in hopes of getting these more versatile antibodies.
“While a natural infection may induce maturation of antibodies with broader activity than a vaccine does -- a natural infection can also kill you,” says Michel Nussenzweig, MD, PhD, head of Rockefeller’s Laboratory of Molecular Immunology.
Sure, memory B cells generated by infections may be immunological Swiss Army Knives, but maybe, argues Donna Farber, PhD, an immunologist at Columbia University in New York, we really only need a single blade.
“The thing with the vaccine is that it’s really focused,” she says. “It’s not giving you all these other viral proteins. It’s only giving you the spike.”
“It may be even better than the level of neutralizing spike antibodies you’re going to get from the infection,” she says. “With a viral infection, the immune response really has a lot to do. It’s really being distracted by all these other proteins.”
“Whereas with the vaccine, it’s just saying to the immune response, ‘This is the immunity we need,’” Dr. Farber says. “‘Just generate this immunity.’ So it’s focusing the immune response in a way that’s going to guarantee that you’re going to get that protective response.”
What if you had COVID and later got vaccinated?
This is called hybrid immunity, and it’s the best of both worlds.
“You have the benefit of very deep, but narrow, immunity produced by vaccine, and very broad, but not very deep, immunity produced by infection,” Dr. Poland says. He says you’ve effectively cross-trained your immune system.
In studies of people who recovered from COVID-19 and then went on to get an mRNA vaccine, after one dose, their antibodies were as high as someone who had been fully vaccinated. After two doses, their antibody levels were about double the average levels seen in someone who’d only been vaccinated.
Studies have shown this kind of immunity has real benefits, too. A recent study by researchers at the University of Kentucky and the CDC found that people who’d gotten COVID-19 in 2020, but had not been vaccinated, were about twice as likely to be reinfected in May and June compared with those who recovered and went on to get their vaccines.
What antibody level is protective?
Scientists aren’t exactly sure how high antibody levels need to be for protection, or even which kinds of antibodies or other immune components matter most yet.
But vaccines appear to generate higher antibody levels than infections do. In a recent study published in the journal Science , Dr. Weiskopf and her colleagues at the La Jolla Institute of Immunology detail the findings of a de-escalation study, where they gave people one-quarter of the normal dose of the Moderna mRNA vaccine and then collected blood samples over time to study their immune responses.
Their immune responses were scaled down with the dose.
“We saw that this has the exact same levels as natural infection,” Dr. Weiskopf says. “People who are vaccinated have much higher immune memory than people who are naturally infected,” she says.
Antibody levels are not easy to determine in the real world. Can you take a test to find out how protected you are? The answer is no, because we don’t yet know what antibody level, or even which kind of antibodies, correlate with protection.
Also, there are many different kinds of antibody tests and they all use a slightly different scale, so there’s no broadly agreed upon way to measure them yet. It’s difficult to compare levels test to test.
Weeks or months between doses? Which is best?
Both the Pfizer and Moderna vaccines were tested to be given 3 and 4 weeks apart, respectively. But when the vaccines were first rolling out, shortages prompted some countries to stretch the interval between doses to 4 or more months.
Researchers who have studied the immune responses of people who were inoculated on an extended dosing schedule noticed something interesting: When the interval was stretched, people had better antibody responses. In fact, their antibody responses looked like the sky-high levels people got with hybrid immunity.
Susanna Dunachie, PhD, a global research professor at the University of Oxford in the United Kingdom, wondered why. She’s leading a team of researchers who are doing detailed studies of the immune responses of health care workers after their vaccinations.
“We found that B cells, which are the cells that make antibodies to the viral spike protein after vaccination, carry on increasing in number between 4 and 10 weeks after vaccination,” she says.
Waiting to give the second vaccine 6 to 14 weeks seems to stimulate the immune system when all of its antibody-making factories are finally up and running.
For this reason, giving the second dose at 3 weeks, she says, might be premature.
But there’s a tradeoff involved in waiting. If there are high levels of the virus circulating in a community, you want to get people fully vaccinated as quickly as possible to maximize their protection in the shortest window of time, which is what we decided to do in the United States.
Researchers say it might be a good idea to revisit the dosing interval when it’s less risky to try it.
A version of this article first appeared on WebMD.com.
Brief, automated cognitive test may offer key advantages in MS
(RRMS), new research shows.
“To our knowledge this is the first psychometric evaluation of the NIH Toolbox Cognition Battery in MS,” said study investigator Heena R. Manglani, MA, a clinical psychology fellow at Massachusetts General Hospital and Harvard Medical School, Boston.
“[The findings] suggest that the NIH Toolbox Cognition Battery may be used as an alternative to other gold-standard measures which may cover limited domains or require manual scoring,” added Ms. Manglani, who is working toward her PhD in clinical psychology.
The study was presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
An indicator of disease activity?
Cognitive deficits affecting a range of functions – including memory, attention and communication – are common in MS and affect 34% to 65% of patients with the disease, and the ability to detect and monitor such deficits has important implications.
Cognitive changes can provide a unique opportunity to identify acute disease activity in patients with MS that might be already occurring before physical manifestations become apparent, said Ms. Manglani. “If we can detect subtle changes in cognition that might foreshadow other symptoms of disease worsening, we can then allocate interventions that might stave off cognitive decline,” she explained.
While there is an array of well-established neuropsychological tests for the assessment of cognitive deficits, each has limitations, so a shorter, computerized, convenient, and reliable test could prove beneficial.
The NIHTB-CB has been validated in a large, nationally representative sample of individuals aged 8 to 85 and represents a potentially attractive option, yielding composite measures and scores corrected for age, gender, education, race, and ethnicity.
Comparative testing
To compare the test with other leading cognition tools used in MS, the investigators recruited 87 patients with RRMS (79% female, mean age 47.3 years). Participants were recruited to perform the full NIHTB-CB (about 30 minutes) and the full Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS), which takes about 90 minutes, as well as some subsets from the Wechsler Adult Intelligence Scale-IV (WAIS-IV) covering processing speed and working memory. All patients had an EDSS of 5.0 or below and, on average, had been living with MS for about a decade.
The results showed the normative scores for NIHTB-CB had significant concordance with the other measures in terms of processing speed (concordance correlation coefficient [CCC] range = 0.28-0.48), working memory (CCC range = 0.27-0.37), and episodic memory (CCC range = 0.21-0.32). However, agreement was not shown for executive function (CCC range = 0.096-0.11).
Ms. Manglani noted executive function included various submeasures such as planning and inhibitory control. “Perhaps our gold standard measures tapped into a different facet of executive function than measured by the NIHTB,” she said.
The investigators found the proportion of participants classified as cognitively impaired was similar between the MACFIMS and the NIHTB tests.
Further assessment of fluid cognition on the NIHTB-CB – a composite of processing speed, working memory, episodic memory, and executive function that is automatically generated by the toolbox – showed the measure was negatively associated with disease severity, as measured by the EDSS (P = .006). However, the measure was not associated with a difference in depression (P = .39) or fatigue (P = .69).
Of note, a similar association with lower disease severity on the EDSS was not observed with MACFIMS.
“Interestingly, we found that only the NIHTB-CB fluid cognition was associated with disease severity, such that it was associated with nearly 11% of the variance in EDSS scores, and we were surprised that we didn’t see this with MACFIMS,” Ms. Manglani said.
Key advantages
The NIHTB-CB was developed as part of the NIH Blueprint for Neuroscience Research initiative and commissioned by 16 NIH Institutes to provide brief, efficient assessment measures of cognitive function.
The battery has been validated in healthy individuals and tested in other populations with neurologic disorders, including patients who have suffered stroke and traumatic brain injury.
Ms. Manglani noted that the NIHTB-CB had key advantages over other tests. “First, it is a 30-minute iPad-based battery, which is shorter than most cognitive batteries available, and one of the few that is completely computerized. In addition, it automatically scores performance and yields a report with both composite scores and scores for each subtest,” she said.
In addition, said Ms. Manglani, “the NIH toolbox has a large validation sample of individuals between 8-85 years of age and provides normative scores that account for age, gender, education, and race/ethnicity, which allows individuals’ performances to be compared with their peers.”
The findings underscore that with further validation, the battery could have an important role in MS, she added.
“The NIH Toolbox needs to be tested in all subtypes of MS, with a full range of disease severity, and in MS clinics to gauge the clinical feasibility. Larger samples and repeated assessments are also needed to assess the test-retest reliability,” she said.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(RRMS), new research shows.
“To our knowledge this is the first psychometric evaluation of the NIH Toolbox Cognition Battery in MS,” said study investigator Heena R. Manglani, MA, a clinical psychology fellow at Massachusetts General Hospital and Harvard Medical School, Boston.
“[The findings] suggest that the NIH Toolbox Cognition Battery may be used as an alternative to other gold-standard measures which may cover limited domains or require manual scoring,” added Ms. Manglani, who is working toward her PhD in clinical psychology.
The study was presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
An indicator of disease activity?
Cognitive deficits affecting a range of functions – including memory, attention and communication – are common in MS and affect 34% to 65% of patients with the disease, and the ability to detect and monitor such deficits has important implications.
Cognitive changes can provide a unique opportunity to identify acute disease activity in patients with MS that might be already occurring before physical manifestations become apparent, said Ms. Manglani. “If we can detect subtle changes in cognition that might foreshadow other symptoms of disease worsening, we can then allocate interventions that might stave off cognitive decline,” she explained.
While there is an array of well-established neuropsychological tests for the assessment of cognitive deficits, each has limitations, so a shorter, computerized, convenient, and reliable test could prove beneficial.
The NIHTB-CB has been validated in a large, nationally representative sample of individuals aged 8 to 85 and represents a potentially attractive option, yielding composite measures and scores corrected for age, gender, education, race, and ethnicity.
Comparative testing
To compare the test with other leading cognition tools used in MS, the investigators recruited 87 patients with RRMS (79% female, mean age 47.3 years). Participants were recruited to perform the full NIHTB-CB (about 30 minutes) and the full Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS), which takes about 90 minutes, as well as some subsets from the Wechsler Adult Intelligence Scale-IV (WAIS-IV) covering processing speed and working memory. All patients had an EDSS of 5.0 or below and, on average, had been living with MS for about a decade.
The results showed the normative scores for NIHTB-CB had significant concordance with the other measures in terms of processing speed (concordance correlation coefficient [CCC] range = 0.28-0.48), working memory (CCC range = 0.27-0.37), and episodic memory (CCC range = 0.21-0.32). However, agreement was not shown for executive function (CCC range = 0.096-0.11).
Ms. Manglani noted executive function included various submeasures such as planning and inhibitory control. “Perhaps our gold standard measures tapped into a different facet of executive function than measured by the NIHTB,” she said.
The investigators found the proportion of participants classified as cognitively impaired was similar between the MACFIMS and the NIHTB tests.
Further assessment of fluid cognition on the NIHTB-CB – a composite of processing speed, working memory, episodic memory, and executive function that is automatically generated by the toolbox – showed the measure was negatively associated with disease severity, as measured by the EDSS (P = .006). However, the measure was not associated with a difference in depression (P = .39) or fatigue (P = .69).
Of note, a similar association with lower disease severity on the EDSS was not observed with MACFIMS.
“Interestingly, we found that only the NIHTB-CB fluid cognition was associated with disease severity, such that it was associated with nearly 11% of the variance in EDSS scores, and we were surprised that we didn’t see this with MACFIMS,” Ms. Manglani said.
Key advantages
The NIHTB-CB was developed as part of the NIH Blueprint for Neuroscience Research initiative and commissioned by 16 NIH Institutes to provide brief, efficient assessment measures of cognitive function.
The battery has been validated in healthy individuals and tested in other populations with neurologic disorders, including patients who have suffered stroke and traumatic brain injury.
Ms. Manglani noted that the NIHTB-CB had key advantages over other tests. “First, it is a 30-minute iPad-based battery, which is shorter than most cognitive batteries available, and one of the few that is completely computerized. In addition, it automatically scores performance and yields a report with both composite scores and scores for each subtest,” she said.
In addition, said Ms. Manglani, “the NIH toolbox has a large validation sample of individuals between 8-85 years of age and provides normative scores that account for age, gender, education, and race/ethnicity, which allows individuals’ performances to be compared with their peers.”
The findings underscore that with further validation, the battery could have an important role in MS, she added.
“The NIH Toolbox needs to be tested in all subtypes of MS, with a full range of disease severity, and in MS clinics to gauge the clinical feasibility. Larger samples and repeated assessments are also needed to assess the test-retest reliability,” she said.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(RRMS), new research shows.
“To our knowledge this is the first psychometric evaluation of the NIH Toolbox Cognition Battery in MS,” said study investigator Heena R. Manglani, MA, a clinical psychology fellow at Massachusetts General Hospital and Harvard Medical School, Boston.
“[The findings] suggest that the NIH Toolbox Cognition Battery may be used as an alternative to other gold-standard measures which may cover limited domains or require manual scoring,” added Ms. Manglani, who is working toward her PhD in clinical psychology.
The study was presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
An indicator of disease activity?
Cognitive deficits affecting a range of functions – including memory, attention and communication – are common in MS and affect 34% to 65% of patients with the disease, and the ability to detect and monitor such deficits has important implications.
Cognitive changes can provide a unique opportunity to identify acute disease activity in patients with MS that might be already occurring before physical manifestations become apparent, said Ms. Manglani. “If we can detect subtle changes in cognition that might foreshadow other symptoms of disease worsening, we can then allocate interventions that might stave off cognitive decline,” she explained.
While there is an array of well-established neuropsychological tests for the assessment of cognitive deficits, each has limitations, so a shorter, computerized, convenient, and reliable test could prove beneficial.
The NIHTB-CB has been validated in a large, nationally representative sample of individuals aged 8 to 85 and represents a potentially attractive option, yielding composite measures and scores corrected for age, gender, education, race, and ethnicity.
Comparative testing
To compare the test with other leading cognition tools used in MS, the investigators recruited 87 patients with RRMS (79% female, mean age 47.3 years). Participants were recruited to perform the full NIHTB-CB (about 30 minutes) and the full Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS), which takes about 90 minutes, as well as some subsets from the Wechsler Adult Intelligence Scale-IV (WAIS-IV) covering processing speed and working memory. All patients had an EDSS of 5.0 or below and, on average, had been living with MS for about a decade.
The results showed the normative scores for NIHTB-CB had significant concordance with the other measures in terms of processing speed (concordance correlation coefficient [CCC] range = 0.28-0.48), working memory (CCC range = 0.27-0.37), and episodic memory (CCC range = 0.21-0.32). However, agreement was not shown for executive function (CCC range = 0.096-0.11).
Ms. Manglani noted executive function included various submeasures such as planning and inhibitory control. “Perhaps our gold standard measures tapped into a different facet of executive function than measured by the NIHTB,” she said.
The investigators found the proportion of participants classified as cognitively impaired was similar between the MACFIMS and the NIHTB tests.
Further assessment of fluid cognition on the NIHTB-CB – a composite of processing speed, working memory, episodic memory, and executive function that is automatically generated by the toolbox – showed the measure was negatively associated with disease severity, as measured by the EDSS (P = .006). However, the measure was not associated with a difference in depression (P = .39) or fatigue (P = .69).
Of note, a similar association with lower disease severity on the EDSS was not observed with MACFIMS.
“Interestingly, we found that only the NIHTB-CB fluid cognition was associated with disease severity, such that it was associated with nearly 11% of the variance in EDSS scores, and we were surprised that we didn’t see this with MACFIMS,” Ms. Manglani said.
Key advantages
The NIHTB-CB was developed as part of the NIH Blueprint for Neuroscience Research initiative and commissioned by 16 NIH Institutes to provide brief, efficient assessment measures of cognitive function.
The battery has been validated in healthy individuals and tested in other populations with neurologic disorders, including patients who have suffered stroke and traumatic brain injury.
Ms. Manglani noted that the NIHTB-CB had key advantages over other tests. “First, it is a 30-minute iPad-based battery, which is shorter than most cognitive batteries available, and one of the few that is completely computerized. In addition, it automatically scores performance and yields a report with both composite scores and scores for each subtest,” she said.
In addition, said Ms. Manglani, “the NIH toolbox has a large validation sample of individuals between 8-85 years of age and provides normative scores that account for age, gender, education, and race/ethnicity, which allows individuals’ performances to be compared with their peers.”
The findings underscore that with further validation, the battery could have an important role in MS, she added.
“The NIH Toolbox needs to be tested in all subtypes of MS, with a full range of disease severity, and in MS clinics to gauge the clinical feasibility. Larger samples and repeated assessments are also needed to assess the test-retest reliability,” she said.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021