User login
More STEP data: Semaglutide cuts weight, cravings, beats liraglutide
The STEP 5 clinical trial extends favorable weight loss from 1 year out to 2 years for the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy, Novo Nordisk), given as a once-weekly 2.4-mg subcutaneous injection, and some food cravings were improved in a subgroup analysis.
In another study, STEP 8, weight loss was greater at 68 weeks with semaglutide subcutaneous injection than with a 3-mg daily subcutaneous injection of another GLP-1 agonist, liraglutide (Saxenda, Novo Nordisk), approved earlier for weight loss.
Researchers presented these promising outcomes, with no new safety signals, at ObesityWeek® 2021.
However, there is more to learn about the drug class, researchers agree. Follow-up is still relatively short for a chronic disease and many patients have gastrointestinal side effects with semaglutide, one expert cautions.
The key findings were:
In STEP 5, combined with lifestyle intervention (a reduced-calorie meal plan and advice about physical activity), weekly injection of 2.4 mg semaglutide led to:
- 15.2% weight loss, compared with 2.6% weight loss with placebo at 2 years (P < .0001);
- 77% of patients losing at least 5% of their weight, compared with 34% of patients in the placebo group at 2 years (P < .0001);
- Significantly greater improvement in overall control of cravings, and craving for savory foods, in a subset of patients, versus placebo, but questionnaire scores for positive mood and craving for sweet foods were similar in both groups.
- In STEP 8, mean body weight at 68 weeks was 15.8% lower with 2.4 mg/week subcutaneous semaglutide plus lifestyle changes versus 6.4% lower with 3.0 mg/day subcutaneous liraglutide plus lifestyle changes (P < .001).
Can treat to a target weight-loss range
The undiminished weight loss efficacy in the 2-year data for STEP 5 “portends well,” said W. Timothy Garvey, MD, following his presentation of the results.
“I think this is a new era in obesity care,” said Dr. Garvey, director of the diabetes research center at the University of Alabama at Birmingham. Semaglutide “essentially doubles weight loss efficacy” compared to the other approved pharmacotherapies for obesity.
With this degree of potential weight loss, clinicians “can use weight as a biomarker and treat to a target [weight-loss] range,” he said.
Expounding on this in an interview, Dr. Garvey noted that, as stated in the 2016 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for medical care of patients with obesity, of which he was lead author, “the objective of care in obesity is to increase health of patients and prevent or treat complications.”
Semaglutide “can treat to a range of weight loss of 10% to 20% in the majority of patients,” which is associated with improvements in cardiovascular and metabolic risk factors.
In STEP 5, of the 51% of patients in the semaglutide group who had prediabetes at enrollment, 80% had normal glycemia at 2 years; however, the trial was not powered nor designed to investigate this.
More data are needed to inform long-term care decisions. The ongoing SELECT cardiovascular outcomes trial of semaglutide, with expected primary study completion on Sept. 28, 2023, should provide more information.
Weight loss plus reduced cravings
In another presentation, Sean Wharton, MD, PharmD, said, “In adults with overweight or obesity, substantial weight loss with semaglutide 2.4 mg was accompanied by short- and long-term improvements in control of eating.”
“Most patients living with obesity who are attempting to decrease calories will have food cravings, based on the biological parameters of weight preservation,” Dr. Wharton, medical director at the Wharton Medical Clinic, in Hamilton, Ont., explained in an email.
The degree of craving varies from patient to patient, likely based on genetics, he added. Research in this field is still emerging.
“I believe that semaglutide 2.4 mg is a game-changer in the field of weight management, and it will change the dialogue for insurance plans and with policymakers regarding coverage for this medication,” said Dr. Wharton.
“The data from the STEP programs are very strong. I am certainly hoping for a change to bias against covering these medications that we have seen in the past,” he said.
Clinically meaningful weight loss
When presenting the STEP 8 findings, Domenica M. Rubino, MD, said: “Participants were significantly more likely to achieve clinically meaningful weight loss thresholds with semaglutide 2.4 mg versus liraglutide 3.0 mg, accompanied by greater improvements in cardiometabolic risk factors.”
For example, patients can have better mobility, which is important for quality of life, Dr. Rubino, director of the Washington Center for Weight Management and Research, Arlington, Virginia, noted.
A smaller percentage of patients respond to liraglutide, she added. Clinicians need to individualize treatment.
When asked, “How do you choose which medical therapy?” Dr. Rubino responded: “We sit and talk.” Finding the medical therapy that fits the patient depends on things such as the patient’s insurance coverage and ability to tolerate side effects such as dehydration, diarrhea, and nausea.
When asked, “How do you switch from liraglutide to semaglutide?” she noted that there are no current guidelines for this. “You have to be careful. Start on the lowest dose of Wegovy. Be cautious, conservative.”
Still early days, caveats remain
“The STEP trials as a group appear to be making the case that obesity may now be considered a medically manageable disease, based on the experience with semaglutide,” Julie R. Ingelfinger, MD, who was not involved with the research, commented in an email.
“STEP 5 and 8 may suggest that weight loss occurs and is sustainable in overweight persons without diabetes with one or more comorbidities or in obese persons without diabetes,” added Dr. Ingelfinger, professor of pediatrics, Harvard Medical School, consultant in pediatric nephrology, Massachusetts General Hospital, Boston, and deputy editor, The New England Journal of Medicine.
However, “even 2 years, in the case of STEP 5, and ~68 weeks in the case of STEP 8, may not be long enough to know whether semaglutide is as promising as these brief summaries (abstracts) suggest,” she cautioned.
“Obesity is a chronic condition, and very long-term therapy and management are required,” Dr. Ingelfinger continued.
“Further, it is hard to generalize when gastrointestinal adverse events are common in a study,” she said. For example, in STEP 8, they were just as common with semaglutide as with the comparator liraglutide, she noted.
“The racial and ethnic representativeness of these studies does not reflect population distributions in the U.S., limiting generalization,” she continued.
“So, there remain caveats in interpreting these data.”
STEP 5 weight loss efficacy and safety at 2 years
Garvey reported that STEP 5 was a phase 3b trial that randomized 304 adults in the United States, Canada, Hungary, Italy, and Spain, who were 18 years and older, with a body mass index (BMI) ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) or a BMI ≥30 kg/m2, without type 2 diabetes, to receive semaglutide or placebo plus lifestyle intervention.
Most participants were women (78%) and White (93%). On average, they were 47 years old, weighed 106 kg (223.7 pounds), had a BMI of 38.5 kg/m2, a waist circumference of 115.7 cm (45.6 inches), and an A1c of 5.7%.
A total of 87% of patients in the semaglutide group and 73% of patients in the placebo group completed the trial.
At 104 weeks, participants were more likely to lose ≥10%, ≥15%, and ≥20% of body weight with semaglutide versus placebo (61.8% vs. 13.3%, 52.1% vs. 7.0%, and 36.1% vs. 2.3%, respectively; P < .0001 for all).
Patients in the semaglutide group had greater health improvements in cardiovascular risk factors (waist circumference, systolic and diastolic blood pressure, and C-reactive protein) and metabolic risk factors (A1c, fasting plasma glucose, fasting serum insulin, and triglycerides) than those in the placebo group (P < .05 for all).
Safety and tolerability were consistent with adverse events seen with this drug class, with no new safety signals.
Control of eating questionnaire findings at 2 years in STEP 5
Dr. Wharton and colleagues assessed changes in responses to the Control of Eating questionnaire at baseline and at 20, 52, and 104 weeks in patients from the U.S. and Canada in the STEP 5 trial (88 patients in the semaglutide group and 86 patients in the placebo group).
The questionnaire consisted of 19 questions grouped into four categories: control of food cravings, craving for savory foods (salty and spicy, dairy, or starchy foods), craving for sweet foods (chocolate, sweet foods, or fruit/fruit juice), and positive mood.
At week 104, patients in the semaglutide group had significantly greater improvements in scores for craving for salty and spicy, dairy, and starchy foods, and resisting cravings.
Semaglutide versus liraglutide, 68-week efficacy and safety in STEP 8
STEP 8 randomized 338 U.S. adults without diabetes and a BMI of ≥27 kg/m2 plus one or more weight-related comorbidities or a BMI of ≥30 kg/m2 3:1 to semaglutide 2.4 mg once weekly (n = 126) or matching placebo, or 3:1 liraglutide 3.0 mg once daily (n = 127) or matching placebo, plus lifestyle intervention.
Most participants were women (78%) and were a mean age of 49, had a mean body weight of 104.5 kg, and had a mean BMI of 37.5 kg/m2.
In STEP 8, more participants achieved ≥10%, ≥15%, and ≥20% weight loss with semaglutide than with liraglutide (70.9% vs. 25.6%, 55.6% vs. 12.0%, and 38.5% vs. 6.0%, respectively; P < .001 for all odds ratios).
Semaglutide improved waist circumference, A1c, and C-reactive protein versus liraglutide (unadjusted P < .001 for all).
Gastrointestinal adverse events were reported by 84% and 83% of participants receiving semaglutide and liraglutide, respectively. Most events were mild/moderate and transient, with prevalence declining over time.
Fewer participants stopped treatment due to adverse events with semaglutide than liraglutide (3.2% vs. 12.6%).
Dr. Garvey has reported serving as a site principal investigator for multicentered clinical trials sponsored by his university and funded by Eli Lilly, Novo Nordisk, and Pfizer. Dr. Wharton has reported financial ties to Novo Nordisk, Bausch Health Canada, Eli Lily, and Boehringer Ingelheim Canada. Dr. Rubino has reported ties to Boehringer Ingelheim and AstraZeneca. Dr. Ingelfinger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The STEP 5 clinical trial extends favorable weight loss from 1 year out to 2 years for the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy, Novo Nordisk), given as a once-weekly 2.4-mg subcutaneous injection, and some food cravings were improved in a subgroup analysis.
In another study, STEP 8, weight loss was greater at 68 weeks with semaglutide subcutaneous injection than with a 3-mg daily subcutaneous injection of another GLP-1 agonist, liraglutide (Saxenda, Novo Nordisk), approved earlier for weight loss.
Researchers presented these promising outcomes, with no new safety signals, at ObesityWeek® 2021.
However, there is more to learn about the drug class, researchers agree. Follow-up is still relatively short for a chronic disease and many patients have gastrointestinal side effects with semaglutide, one expert cautions.
The key findings were:
In STEP 5, combined with lifestyle intervention (a reduced-calorie meal plan and advice about physical activity), weekly injection of 2.4 mg semaglutide led to:
- 15.2% weight loss, compared with 2.6% weight loss with placebo at 2 years (P < .0001);
- 77% of patients losing at least 5% of their weight, compared with 34% of patients in the placebo group at 2 years (P < .0001);
- Significantly greater improvement in overall control of cravings, and craving for savory foods, in a subset of patients, versus placebo, but questionnaire scores for positive mood and craving for sweet foods were similar in both groups.
- In STEP 8, mean body weight at 68 weeks was 15.8% lower with 2.4 mg/week subcutaneous semaglutide plus lifestyle changes versus 6.4% lower with 3.0 mg/day subcutaneous liraglutide plus lifestyle changes (P < .001).
Can treat to a target weight-loss range
The undiminished weight loss efficacy in the 2-year data for STEP 5 “portends well,” said W. Timothy Garvey, MD, following his presentation of the results.
“I think this is a new era in obesity care,” said Dr. Garvey, director of the diabetes research center at the University of Alabama at Birmingham. Semaglutide “essentially doubles weight loss efficacy” compared to the other approved pharmacotherapies for obesity.
With this degree of potential weight loss, clinicians “can use weight as a biomarker and treat to a target [weight-loss] range,” he said.
Expounding on this in an interview, Dr. Garvey noted that, as stated in the 2016 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for medical care of patients with obesity, of which he was lead author, “the objective of care in obesity is to increase health of patients and prevent or treat complications.”
Semaglutide “can treat to a range of weight loss of 10% to 20% in the majority of patients,” which is associated with improvements in cardiovascular and metabolic risk factors.
In STEP 5, of the 51% of patients in the semaglutide group who had prediabetes at enrollment, 80% had normal glycemia at 2 years; however, the trial was not powered nor designed to investigate this.
More data are needed to inform long-term care decisions. The ongoing SELECT cardiovascular outcomes trial of semaglutide, with expected primary study completion on Sept. 28, 2023, should provide more information.
Weight loss plus reduced cravings
In another presentation, Sean Wharton, MD, PharmD, said, “In adults with overweight or obesity, substantial weight loss with semaglutide 2.4 mg was accompanied by short- and long-term improvements in control of eating.”
“Most patients living with obesity who are attempting to decrease calories will have food cravings, based on the biological parameters of weight preservation,” Dr. Wharton, medical director at the Wharton Medical Clinic, in Hamilton, Ont., explained in an email.
The degree of craving varies from patient to patient, likely based on genetics, he added. Research in this field is still emerging.
“I believe that semaglutide 2.4 mg is a game-changer in the field of weight management, and it will change the dialogue for insurance plans and with policymakers regarding coverage for this medication,” said Dr. Wharton.
“The data from the STEP programs are very strong. I am certainly hoping for a change to bias against covering these medications that we have seen in the past,” he said.
Clinically meaningful weight loss
When presenting the STEP 8 findings, Domenica M. Rubino, MD, said: “Participants were significantly more likely to achieve clinically meaningful weight loss thresholds with semaglutide 2.4 mg versus liraglutide 3.0 mg, accompanied by greater improvements in cardiometabolic risk factors.”
For example, patients can have better mobility, which is important for quality of life, Dr. Rubino, director of the Washington Center for Weight Management and Research, Arlington, Virginia, noted.
A smaller percentage of patients respond to liraglutide, she added. Clinicians need to individualize treatment.
When asked, “How do you choose which medical therapy?” Dr. Rubino responded: “We sit and talk.” Finding the medical therapy that fits the patient depends on things such as the patient’s insurance coverage and ability to tolerate side effects such as dehydration, diarrhea, and nausea.
When asked, “How do you switch from liraglutide to semaglutide?” she noted that there are no current guidelines for this. “You have to be careful. Start on the lowest dose of Wegovy. Be cautious, conservative.”
Still early days, caveats remain
“The STEP trials as a group appear to be making the case that obesity may now be considered a medically manageable disease, based on the experience with semaglutide,” Julie R. Ingelfinger, MD, who was not involved with the research, commented in an email.
“STEP 5 and 8 may suggest that weight loss occurs and is sustainable in overweight persons without diabetes with one or more comorbidities or in obese persons without diabetes,” added Dr. Ingelfinger, professor of pediatrics, Harvard Medical School, consultant in pediatric nephrology, Massachusetts General Hospital, Boston, and deputy editor, The New England Journal of Medicine.
However, “even 2 years, in the case of STEP 5, and ~68 weeks in the case of STEP 8, may not be long enough to know whether semaglutide is as promising as these brief summaries (abstracts) suggest,” she cautioned.
“Obesity is a chronic condition, and very long-term therapy and management are required,” Dr. Ingelfinger continued.
“Further, it is hard to generalize when gastrointestinal adverse events are common in a study,” she said. For example, in STEP 8, they were just as common with semaglutide as with the comparator liraglutide, she noted.
“The racial and ethnic representativeness of these studies does not reflect population distributions in the U.S., limiting generalization,” she continued.
“So, there remain caveats in interpreting these data.”
STEP 5 weight loss efficacy and safety at 2 years
Garvey reported that STEP 5 was a phase 3b trial that randomized 304 adults in the United States, Canada, Hungary, Italy, and Spain, who were 18 years and older, with a body mass index (BMI) ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) or a BMI ≥30 kg/m2, without type 2 diabetes, to receive semaglutide or placebo plus lifestyle intervention.
Most participants were women (78%) and White (93%). On average, they were 47 years old, weighed 106 kg (223.7 pounds), had a BMI of 38.5 kg/m2, a waist circumference of 115.7 cm (45.6 inches), and an A1c of 5.7%.
A total of 87% of patients in the semaglutide group and 73% of patients in the placebo group completed the trial.
At 104 weeks, participants were more likely to lose ≥10%, ≥15%, and ≥20% of body weight with semaglutide versus placebo (61.8% vs. 13.3%, 52.1% vs. 7.0%, and 36.1% vs. 2.3%, respectively; P < .0001 for all).
Patients in the semaglutide group had greater health improvements in cardiovascular risk factors (waist circumference, systolic and diastolic blood pressure, and C-reactive protein) and metabolic risk factors (A1c, fasting plasma glucose, fasting serum insulin, and triglycerides) than those in the placebo group (P < .05 for all).
Safety and tolerability were consistent with adverse events seen with this drug class, with no new safety signals.
Control of eating questionnaire findings at 2 years in STEP 5
Dr. Wharton and colleagues assessed changes in responses to the Control of Eating questionnaire at baseline and at 20, 52, and 104 weeks in patients from the U.S. and Canada in the STEP 5 trial (88 patients in the semaglutide group and 86 patients in the placebo group).
The questionnaire consisted of 19 questions grouped into four categories: control of food cravings, craving for savory foods (salty and spicy, dairy, or starchy foods), craving for sweet foods (chocolate, sweet foods, or fruit/fruit juice), and positive mood.
At week 104, patients in the semaglutide group had significantly greater improvements in scores for craving for salty and spicy, dairy, and starchy foods, and resisting cravings.
Semaglutide versus liraglutide, 68-week efficacy and safety in STEP 8
STEP 8 randomized 338 U.S. adults without diabetes and a BMI of ≥27 kg/m2 plus one or more weight-related comorbidities or a BMI of ≥30 kg/m2 3:1 to semaglutide 2.4 mg once weekly (n = 126) or matching placebo, or 3:1 liraglutide 3.0 mg once daily (n = 127) or matching placebo, plus lifestyle intervention.
Most participants were women (78%) and were a mean age of 49, had a mean body weight of 104.5 kg, and had a mean BMI of 37.5 kg/m2.
In STEP 8, more participants achieved ≥10%, ≥15%, and ≥20% weight loss with semaglutide than with liraglutide (70.9% vs. 25.6%, 55.6% vs. 12.0%, and 38.5% vs. 6.0%, respectively; P < .001 for all odds ratios).
Semaglutide improved waist circumference, A1c, and C-reactive protein versus liraglutide (unadjusted P < .001 for all).
Gastrointestinal adverse events were reported by 84% and 83% of participants receiving semaglutide and liraglutide, respectively. Most events were mild/moderate and transient, with prevalence declining over time.
Fewer participants stopped treatment due to adverse events with semaglutide than liraglutide (3.2% vs. 12.6%).
Dr. Garvey has reported serving as a site principal investigator for multicentered clinical trials sponsored by his university and funded by Eli Lilly, Novo Nordisk, and Pfizer. Dr. Wharton has reported financial ties to Novo Nordisk, Bausch Health Canada, Eli Lily, and Boehringer Ingelheim Canada. Dr. Rubino has reported ties to Boehringer Ingelheim and AstraZeneca. Dr. Ingelfinger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The STEP 5 clinical trial extends favorable weight loss from 1 year out to 2 years for the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy, Novo Nordisk), given as a once-weekly 2.4-mg subcutaneous injection, and some food cravings were improved in a subgroup analysis.
In another study, STEP 8, weight loss was greater at 68 weeks with semaglutide subcutaneous injection than with a 3-mg daily subcutaneous injection of another GLP-1 agonist, liraglutide (Saxenda, Novo Nordisk), approved earlier for weight loss.
Researchers presented these promising outcomes, with no new safety signals, at ObesityWeek® 2021.
However, there is more to learn about the drug class, researchers agree. Follow-up is still relatively short for a chronic disease and many patients have gastrointestinal side effects with semaglutide, one expert cautions.
The key findings were:
In STEP 5, combined with lifestyle intervention (a reduced-calorie meal plan and advice about physical activity), weekly injection of 2.4 mg semaglutide led to:
- 15.2% weight loss, compared with 2.6% weight loss with placebo at 2 years (P < .0001);
- 77% of patients losing at least 5% of their weight, compared with 34% of patients in the placebo group at 2 years (P < .0001);
- Significantly greater improvement in overall control of cravings, and craving for savory foods, in a subset of patients, versus placebo, but questionnaire scores for positive mood and craving for sweet foods were similar in both groups.
- In STEP 8, mean body weight at 68 weeks was 15.8% lower with 2.4 mg/week subcutaneous semaglutide plus lifestyle changes versus 6.4% lower with 3.0 mg/day subcutaneous liraglutide plus lifestyle changes (P < .001).
Can treat to a target weight-loss range
The undiminished weight loss efficacy in the 2-year data for STEP 5 “portends well,” said W. Timothy Garvey, MD, following his presentation of the results.
“I think this is a new era in obesity care,” said Dr. Garvey, director of the diabetes research center at the University of Alabama at Birmingham. Semaglutide “essentially doubles weight loss efficacy” compared to the other approved pharmacotherapies for obesity.
With this degree of potential weight loss, clinicians “can use weight as a biomarker and treat to a target [weight-loss] range,” he said.
Expounding on this in an interview, Dr. Garvey noted that, as stated in the 2016 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for medical care of patients with obesity, of which he was lead author, “the objective of care in obesity is to increase health of patients and prevent or treat complications.”
Semaglutide “can treat to a range of weight loss of 10% to 20% in the majority of patients,” which is associated with improvements in cardiovascular and metabolic risk factors.
In STEP 5, of the 51% of patients in the semaglutide group who had prediabetes at enrollment, 80% had normal glycemia at 2 years; however, the trial was not powered nor designed to investigate this.
More data are needed to inform long-term care decisions. The ongoing SELECT cardiovascular outcomes trial of semaglutide, with expected primary study completion on Sept. 28, 2023, should provide more information.
Weight loss plus reduced cravings
In another presentation, Sean Wharton, MD, PharmD, said, “In adults with overweight or obesity, substantial weight loss with semaglutide 2.4 mg was accompanied by short- and long-term improvements in control of eating.”
“Most patients living with obesity who are attempting to decrease calories will have food cravings, based on the biological parameters of weight preservation,” Dr. Wharton, medical director at the Wharton Medical Clinic, in Hamilton, Ont., explained in an email.
The degree of craving varies from patient to patient, likely based on genetics, he added. Research in this field is still emerging.
“I believe that semaglutide 2.4 mg is a game-changer in the field of weight management, and it will change the dialogue for insurance plans and with policymakers regarding coverage for this medication,” said Dr. Wharton.
“The data from the STEP programs are very strong. I am certainly hoping for a change to bias against covering these medications that we have seen in the past,” he said.
Clinically meaningful weight loss
When presenting the STEP 8 findings, Domenica M. Rubino, MD, said: “Participants were significantly more likely to achieve clinically meaningful weight loss thresholds with semaglutide 2.4 mg versus liraglutide 3.0 mg, accompanied by greater improvements in cardiometabolic risk factors.”
For example, patients can have better mobility, which is important for quality of life, Dr. Rubino, director of the Washington Center for Weight Management and Research, Arlington, Virginia, noted.
A smaller percentage of patients respond to liraglutide, she added. Clinicians need to individualize treatment.
When asked, “How do you choose which medical therapy?” Dr. Rubino responded: “We sit and talk.” Finding the medical therapy that fits the patient depends on things such as the patient’s insurance coverage and ability to tolerate side effects such as dehydration, diarrhea, and nausea.
When asked, “How do you switch from liraglutide to semaglutide?” she noted that there are no current guidelines for this. “You have to be careful. Start on the lowest dose of Wegovy. Be cautious, conservative.”
Still early days, caveats remain
“The STEP trials as a group appear to be making the case that obesity may now be considered a medically manageable disease, based on the experience with semaglutide,” Julie R. Ingelfinger, MD, who was not involved with the research, commented in an email.
“STEP 5 and 8 may suggest that weight loss occurs and is sustainable in overweight persons without diabetes with one or more comorbidities or in obese persons without diabetes,” added Dr. Ingelfinger, professor of pediatrics, Harvard Medical School, consultant in pediatric nephrology, Massachusetts General Hospital, Boston, and deputy editor, The New England Journal of Medicine.
However, “even 2 years, in the case of STEP 5, and ~68 weeks in the case of STEP 8, may not be long enough to know whether semaglutide is as promising as these brief summaries (abstracts) suggest,” she cautioned.
“Obesity is a chronic condition, and very long-term therapy and management are required,” Dr. Ingelfinger continued.
“Further, it is hard to generalize when gastrointestinal adverse events are common in a study,” she said. For example, in STEP 8, they were just as common with semaglutide as with the comparator liraglutide, she noted.
“The racial and ethnic representativeness of these studies does not reflect population distributions in the U.S., limiting generalization,” she continued.
“So, there remain caveats in interpreting these data.”
STEP 5 weight loss efficacy and safety at 2 years
Garvey reported that STEP 5 was a phase 3b trial that randomized 304 adults in the United States, Canada, Hungary, Italy, and Spain, who were 18 years and older, with a body mass index (BMI) ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) or a BMI ≥30 kg/m2, without type 2 diabetes, to receive semaglutide or placebo plus lifestyle intervention.
Most participants were women (78%) and White (93%). On average, they were 47 years old, weighed 106 kg (223.7 pounds), had a BMI of 38.5 kg/m2, a waist circumference of 115.7 cm (45.6 inches), and an A1c of 5.7%.
A total of 87% of patients in the semaglutide group and 73% of patients in the placebo group completed the trial.
At 104 weeks, participants were more likely to lose ≥10%, ≥15%, and ≥20% of body weight with semaglutide versus placebo (61.8% vs. 13.3%, 52.1% vs. 7.0%, and 36.1% vs. 2.3%, respectively; P < .0001 for all).
Patients in the semaglutide group had greater health improvements in cardiovascular risk factors (waist circumference, systolic and diastolic blood pressure, and C-reactive protein) and metabolic risk factors (A1c, fasting plasma glucose, fasting serum insulin, and triglycerides) than those in the placebo group (P < .05 for all).
Safety and tolerability were consistent with adverse events seen with this drug class, with no new safety signals.
Control of eating questionnaire findings at 2 years in STEP 5
Dr. Wharton and colleagues assessed changes in responses to the Control of Eating questionnaire at baseline and at 20, 52, and 104 weeks in patients from the U.S. and Canada in the STEP 5 trial (88 patients in the semaglutide group and 86 patients in the placebo group).
The questionnaire consisted of 19 questions grouped into four categories: control of food cravings, craving for savory foods (salty and spicy, dairy, or starchy foods), craving for sweet foods (chocolate, sweet foods, or fruit/fruit juice), and positive mood.
At week 104, patients in the semaglutide group had significantly greater improvements in scores for craving for salty and spicy, dairy, and starchy foods, and resisting cravings.
Semaglutide versus liraglutide, 68-week efficacy and safety in STEP 8
STEP 8 randomized 338 U.S. adults without diabetes and a BMI of ≥27 kg/m2 plus one or more weight-related comorbidities or a BMI of ≥30 kg/m2 3:1 to semaglutide 2.4 mg once weekly (n = 126) or matching placebo, or 3:1 liraglutide 3.0 mg once daily (n = 127) or matching placebo, plus lifestyle intervention.
Most participants were women (78%) and were a mean age of 49, had a mean body weight of 104.5 kg, and had a mean BMI of 37.5 kg/m2.
In STEP 8, more participants achieved ≥10%, ≥15%, and ≥20% weight loss with semaglutide than with liraglutide (70.9% vs. 25.6%, 55.6% vs. 12.0%, and 38.5% vs. 6.0%, respectively; P < .001 for all odds ratios).
Semaglutide improved waist circumference, A1c, and C-reactive protein versus liraglutide (unadjusted P < .001 for all).
Gastrointestinal adverse events were reported by 84% and 83% of participants receiving semaglutide and liraglutide, respectively. Most events were mild/moderate and transient, with prevalence declining over time.
Fewer participants stopped treatment due to adverse events with semaglutide than liraglutide (3.2% vs. 12.6%).
Dr. Garvey has reported serving as a site principal investigator for multicentered clinical trials sponsored by his university and funded by Eli Lilly, Novo Nordisk, and Pfizer. Dr. Wharton has reported financial ties to Novo Nordisk, Bausch Health Canada, Eli Lily, and Boehringer Ingelheim Canada. Dr. Rubino has reported ties to Boehringer Ingelheim and AstraZeneca. Dr. Ingelfinger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New Mexico oncologist faces legal woes once again
A New Mexico oncologist has once again found himself at odds with the law.
Mohamed Aswad, MD, was still under probation for a past misdemeanor involving misbranded cancer drugs when he allegedly underdosed chemotherapy in a patient with colon cancer. The patient later died.
In a wrongful death case, a jury has awarded $2.3 million in damages to the patient’s wife. The patient, James Hoag, was diagnosed with stage IIIB colon cancer in 2015. He underwent surgery and then went for chemotherapy to Dr. Aswad, who was the only oncologist in the area.
Mr. Hoag’s attorneys alleged that
“It was statistically likely that James would beat his cancer with proper treatment,” said his lawyers during the trial, according to a report in the Albuquerque Journal.
The jury deliberated for only 4 hours, and found that negligence by Dr. Aswad was a proximate cause of the patient’s “lost chance to avoid the loss of his life and resulting damages,” and the jury further described Dr. Aswad’s actions as “wanton,” according to a verdict form filed in the case.
The form also shows that the jury decided Dr. Aswad had obtained Mr. Hoag’s informed consent as to the treatment, but still felt that negligence was a cause of Mr. Hoag’s injury and damages. The original judgment of $2.9 million in damages was subsequently reduced to $2.3 million as the jury found Mr. Hoag was 30% responsible for his injuries.
Dr. Aswad could not be reached for comment. The Albuquerque Journal reports that he plans to appeal the verdict.
Only oncologist in the county
The patient had lived in the rural community of Deming, N.M. following his retirement from a career in law enforcement in Michigan. He was diagnosed with colon cancer in 2015, underwent surgical resection, and then went to Dr. Aswad for follow-up chemotherapy.
Dr. Aswad was the only oncologist in the area. Like many other rural communities throughout the United States, this region has a severe shortage of medical specialists.
Sheila Hoag, the patient’s widow, testified during the trial that following her husband’s surgery in August 2015, which removed the tumor, his “prognosis was very good because they had caught it before it spread.” He had an estimated 5-year survival of about 69%.
However, even though Dr. Aswad followed the National Comprehensive Cancer Network (NCCN) guidelines for adjuvant chemotherapy, he did not use the proper dosing. According to trial testimony, he administered “woefully lower doses” than are recommended, and also exceeded the recommended 6-month duration of chemotherapy by more than double. In addition, Dr. Aswad also failed to adequately monitor the disease during treatment, said Hoag’s lawyers.
Aswad has denied the charges and says that he prescribed a modified regimen spread out over a longer period of time because his patient had requested it. He said that Mr. Hoag did not want to undergo chemotherapy that could cause significant side effects, and this was the reason for the altered treatment plan.
However, the chemotherapy failed to slow the cancer’s progression, and Mr. Hoag never returned to see him after November 2016.
By December 2017, Mr. Hoag had been hospitalized and was being treated by another oncologist, located in Las Cruces, 62 miles away from his home. The new oncologist administered the standard treatment, but by then his cancer had metastasized. Mr. Hoag died in 2020 at age 59.
Previous misdemeanor with misbranded drugs
At the time he was treating Mr. Hoag, Dr. Aswad was on federal probation after pleading guilty in 2014 to one misdemeanor count of the unlawful introduction of misbranded drugs into interstate commerce.
Dr. Aswad had treated cancer patients with a “misbranded” drug imported from abroad and not approved by the U.S. Food and Drug Administration (FDA). Altuzan is a form of bevacizumab that is approved for use in Turkey but not the United States. Between July 2010 and April 2012, Dr. Aswad ordered prescription cancer drugs, including Altuzan, from a Canadian company and then administered the “misbranded” drugs to his patients.
A prescription drug is considered to be “misbranded” if it is manufactured in a facility that has not been registered with the FDA for commercial distribution within the United States.
According to various media reports, Dr. Aswad paid significantly less for these drugs than he would have if had he purchased the U.S.-approved product bearing the same brand or generic name, and then billed federal programs such as Medicare for the full price. He has admitted to making just under $1.3 million in profits.
Under the terms of the plea agreement, he was sentenced to three years of probation, required to pay just under $1.3 million in restitution to Medicare and Tricare, the victims of his criminal conduct, and also to forfeit $750,000, which represented part of his net criminal proceeds, to the United States.
At the trial, Dr. Aswad denied that there was any profit motive in extending Mr. Hoag’s treatment, and in a 2019 deposition, he also denied he was under financial pressure to increase his billings because of the $2 million debt that he owed because of the misbranded drugs fine.
Allowed to resume practice
In late 2015, Dr. Aswad was essentially barred by the federal government from accepting any reimbursement from Medicaid or Medicare for a minimum of 13 years. But because of the urgent need for medical specialists, the New Mexico Human Services Department requested a waiver that would permit him to continuing treating cancer patients since he is the only oncologist in Luna County.
He is currently allowed to treat Medicaid and Medicare patients in four other medically underserved New Mexico counties.
Dr. Aswad, a graduate of the University of Aleppo, in Syria, had an internal medicine residency at Our Lady of Mercy Hospital in New York City, where he also had a fellowship in oncology and hematology. He then settled in Deming and has been licensed in New Mexico since 2003.
With the onset of the COVID-19 pandemic, which caused major disruption to healthcare services, Medicare & Medicaid Services officials requested that Dr. Aswad also be allowed to provide internal medicine services for Medicare patients in Catron and Hildalgo counties for the “duration of the Coronavirus Disease 2019 public health emergency declared by the federal government in January 2020.”
A version of this article first appeared on Medscape.com.
A New Mexico oncologist has once again found himself at odds with the law.
Mohamed Aswad, MD, was still under probation for a past misdemeanor involving misbranded cancer drugs when he allegedly underdosed chemotherapy in a patient with colon cancer. The patient later died.
In a wrongful death case, a jury has awarded $2.3 million in damages to the patient’s wife. The patient, James Hoag, was diagnosed with stage IIIB colon cancer in 2015. He underwent surgery and then went for chemotherapy to Dr. Aswad, who was the only oncologist in the area.
Mr. Hoag’s attorneys alleged that
“It was statistically likely that James would beat his cancer with proper treatment,” said his lawyers during the trial, according to a report in the Albuquerque Journal.
The jury deliberated for only 4 hours, and found that negligence by Dr. Aswad was a proximate cause of the patient’s “lost chance to avoid the loss of his life and resulting damages,” and the jury further described Dr. Aswad’s actions as “wanton,” according to a verdict form filed in the case.
The form also shows that the jury decided Dr. Aswad had obtained Mr. Hoag’s informed consent as to the treatment, but still felt that negligence was a cause of Mr. Hoag’s injury and damages. The original judgment of $2.9 million in damages was subsequently reduced to $2.3 million as the jury found Mr. Hoag was 30% responsible for his injuries.
Dr. Aswad could not be reached for comment. The Albuquerque Journal reports that he plans to appeal the verdict.
Only oncologist in the county
The patient had lived in the rural community of Deming, N.M. following his retirement from a career in law enforcement in Michigan. He was diagnosed with colon cancer in 2015, underwent surgical resection, and then went to Dr. Aswad for follow-up chemotherapy.
Dr. Aswad was the only oncologist in the area. Like many other rural communities throughout the United States, this region has a severe shortage of medical specialists.
Sheila Hoag, the patient’s widow, testified during the trial that following her husband’s surgery in August 2015, which removed the tumor, his “prognosis was very good because they had caught it before it spread.” He had an estimated 5-year survival of about 69%.
However, even though Dr. Aswad followed the National Comprehensive Cancer Network (NCCN) guidelines for adjuvant chemotherapy, he did not use the proper dosing. According to trial testimony, he administered “woefully lower doses” than are recommended, and also exceeded the recommended 6-month duration of chemotherapy by more than double. In addition, Dr. Aswad also failed to adequately monitor the disease during treatment, said Hoag’s lawyers.
Aswad has denied the charges and says that he prescribed a modified regimen spread out over a longer period of time because his patient had requested it. He said that Mr. Hoag did not want to undergo chemotherapy that could cause significant side effects, and this was the reason for the altered treatment plan.
However, the chemotherapy failed to slow the cancer’s progression, and Mr. Hoag never returned to see him after November 2016.
By December 2017, Mr. Hoag had been hospitalized and was being treated by another oncologist, located in Las Cruces, 62 miles away from his home. The new oncologist administered the standard treatment, but by then his cancer had metastasized. Mr. Hoag died in 2020 at age 59.
Previous misdemeanor with misbranded drugs
At the time he was treating Mr. Hoag, Dr. Aswad was on federal probation after pleading guilty in 2014 to one misdemeanor count of the unlawful introduction of misbranded drugs into interstate commerce.
Dr. Aswad had treated cancer patients with a “misbranded” drug imported from abroad and not approved by the U.S. Food and Drug Administration (FDA). Altuzan is a form of bevacizumab that is approved for use in Turkey but not the United States. Between July 2010 and April 2012, Dr. Aswad ordered prescription cancer drugs, including Altuzan, from a Canadian company and then administered the “misbranded” drugs to his patients.
A prescription drug is considered to be “misbranded” if it is manufactured in a facility that has not been registered with the FDA for commercial distribution within the United States.
According to various media reports, Dr. Aswad paid significantly less for these drugs than he would have if had he purchased the U.S.-approved product bearing the same brand or generic name, and then billed federal programs such as Medicare for the full price. He has admitted to making just under $1.3 million in profits.
Under the terms of the plea agreement, he was sentenced to three years of probation, required to pay just under $1.3 million in restitution to Medicare and Tricare, the victims of his criminal conduct, and also to forfeit $750,000, which represented part of his net criminal proceeds, to the United States.
At the trial, Dr. Aswad denied that there was any profit motive in extending Mr. Hoag’s treatment, and in a 2019 deposition, he also denied he was under financial pressure to increase his billings because of the $2 million debt that he owed because of the misbranded drugs fine.
Allowed to resume practice
In late 2015, Dr. Aswad was essentially barred by the federal government from accepting any reimbursement from Medicaid or Medicare for a minimum of 13 years. But because of the urgent need for medical specialists, the New Mexico Human Services Department requested a waiver that would permit him to continuing treating cancer patients since he is the only oncologist in Luna County.
He is currently allowed to treat Medicaid and Medicare patients in four other medically underserved New Mexico counties.
Dr. Aswad, a graduate of the University of Aleppo, in Syria, had an internal medicine residency at Our Lady of Mercy Hospital in New York City, where he also had a fellowship in oncology and hematology. He then settled in Deming and has been licensed in New Mexico since 2003.
With the onset of the COVID-19 pandemic, which caused major disruption to healthcare services, Medicare & Medicaid Services officials requested that Dr. Aswad also be allowed to provide internal medicine services for Medicare patients in Catron and Hildalgo counties for the “duration of the Coronavirus Disease 2019 public health emergency declared by the federal government in January 2020.”
A version of this article first appeared on Medscape.com.
A New Mexico oncologist has once again found himself at odds with the law.
Mohamed Aswad, MD, was still under probation for a past misdemeanor involving misbranded cancer drugs when he allegedly underdosed chemotherapy in a patient with colon cancer. The patient later died.
In a wrongful death case, a jury has awarded $2.3 million in damages to the patient’s wife. The patient, James Hoag, was diagnosed with stage IIIB colon cancer in 2015. He underwent surgery and then went for chemotherapy to Dr. Aswad, who was the only oncologist in the area.
Mr. Hoag’s attorneys alleged that
“It was statistically likely that James would beat his cancer with proper treatment,” said his lawyers during the trial, according to a report in the Albuquerque Journal.
The jury deliberated for only 4 hours, and found that negligence by Dr. Aswad was a proximate cause of the patient’s “lost chance to avoid the loss of his life and resulting damages,” and the jury further described Dr. Aswad’s actions as “wanton,” according to a verdict form filed in the case.
The form also shows that the jury decided Dr. Aswad had obtained Mr. Hoag’s informed consent as to the treatment, but still felt that negligence was a cause of Mr. Hoag’s injury and damages. The original judgment of $2.9 million in damages was subsequently reduced to $2.3 million as the jury found Mr. Hoag was 30% responsible for his injuries.
Dr. Aswad could not be reached for comment. The Albuquerque Journal reports that he plans to appeal the verdict.
Only oncologist in the county
The patient had lived in the rural community of Deming, N.M. following his retirement from a career in law enforcement in Michigan. He was diagnosed with colon cancer in 2015, underwent surgical resection, and then went to Dr. Aswad for follow-up chemotherapy.
Dr. Aswad was the only oncologist in the area. Like many other rural communities throughout the United States, this region has a severe shortage of medical specialists.
Sheila Hoag, the patient’s widow, testified during the trial that following her husband’s surgery in August 2015, which removed the tumor, his “prognosis was very good because they had caught it before it spread.” He had an estimated 5-year survival of about 69%.
However, even though Dr. Aswad followed the National Comprehensive Cancer Network (NCCN) guidelines for adjuvant chemotherapy, he did not use the proper dosing. According to trial testimony, he administered “woefully lower doses” than are recommended, and also exceeded the recommended 6-month duration of chemotherapy by more than double. In addition, Dr. Aswad also failed to adequately monitor the disease during treatment, said Hoag’s lawyers.
Aswad has denied the charges and says that he prescribed a modified regimen spread out over a longer period of time because his patient had requested it. He said that Mr. Hoag did not want to undergo chemotherapy that could cause significant side effects, and this was the reason for the altered treatment plan.
However, the chemotherapy failed to slow the cancer’s progression, and Mr. Hoag never returned to see him after November 2016.
By December 2017, Mr. Hoag had been hospitalized and was being treated by another oncologist, located in Las Cruces, 62 miles away from his home. The new oncologist administered the standard treatment, but by then his cancer had metastasized. Mr. Hoag died in 2020 at age 59.
Previous misdemeanor with misbranded drugs
At the time he was treating Mr. Hoag, Dr. Aswad was on federal probation after pleading guilty in 2014 to one misdemeanor count of the unlawful introduction of misbranded drugs into interstate commerce.
Dr. Aswad had treated cancer patients with a “misbranded” drug imported from abroad and not approved by the U.S. Food and Drug Administration (FDA). Altuzan is a form of bevacizumab that is approved for use in Turkey but not the United States. Between July 2010 and April 2012, Dr. Aswad ordered prescription cancer drugs, including Altuzan, from a Canadian company and then administered the “misbranded” drugs to his patients.
A prescription drug is considered to be “misbranded” if it is manufactured in a facility that has not been registered with the FDA for commercial distribution within the United States.
According to various media reports, Dr. Aswad paid significantly less for these drugs than he would have if had he purchased the U.S.-approved product bearing the same brand or generic name, and then billed federal programs such as Medicare for the full price. He has admitted to making just under $1.3 million in profits.
Under the terms of the plea agreement, he was sentenced to three years of probation, required to pay just under $1.3 million in restitution to Medicare and Tricare, the victims of his criminal conduct, and also to forfeit $750,000, which represented part of his net criminal proceeds, to the United States.
At the trial, Dr. Aswad denied that there was any profit motive in extending Mr. Hoag’s treatment, and in a 2019 deposition, he also denied he was under financial pressure to increase his billings because of the $2 million debt that he owed because of the misbranded drugs fine.
Allowed to resume practice
In late 2015, Dr. Aswad was essentially barred by the federal government from accepting any reimbursement from Medicaid or Medicare for a minimum of 13 years. But because of the urgent need for medical specialists, the New Mexico Human Services Department requested a waiver that would permit him to continuing treating cancer patients since he is the only oncologist in Luna County.
He is currently allowed to treat Medicaid and Medicare patients in four other medically underserved New Mexico counties.
Dr. Aswad, a graduate of the University of Aleppo, in Syria, had an internal medicine residency at Our Lady of Mercy Hospital in New York City, where he also had a fellowship in oncology and hematology. He then settled in Deming and has been licensed in New Mexico since 2003.
With the onset of the COVID-19 pandemic, which caused major disruption to healthcare services, Medicare & Medicaid Services officials requested that Dr. Aswad also be allowed to provide internal medicine services for Medicare patients in Catron and Hildalgo counties for the “duration of the Coronavirus Disease 2019 public health emergency declared by the federal government in January 2020.”
A version of this article first appeared on Medscape.com.
What to do about pandemic PTSD
When the COVID-19 pandemic engulfed the nation well over a year ago, Rebecca Hendrickson, MD, PhD, immersed herself in the shell-shocking revelations that clinicians began posting on social media. The accounts offered just a snapshot of the pandemic’s heavy psychological toll, and Dr. Hendrickson, a psychiatrist at the University of Washington in Seattle and an expert in posttraumatic stress disorder (PTSD), wanted to know more.
She and her colleagues devised a survey to assess the impact of several pandemic-related factors, including increased work hours, social distancing restrictions, and lack of adequate personal protective equipment.
What began as a survey of health care workers soon expanded in scope. Of the more than 600 survey respondents to date, health care workers account for about 60%, while the rest are first responders – police officers, firefighters, paramedics, and emergency medical technicians – and nonclinical personnel, such as security guards and office staff, in health care settings. The respondents range in age from 19 to 72, and hail from all regions of the country.
“Our findings were really striking,” Dr. Hendrickson said, “including very high rates of thoughts of suicide and thoughts of leaving one’s current field, which were both strongly linked to COVID-19–related occupational stress exposure.”
The distress stemmed from a multitude of factors. Among the most demoralizing: witnessing patients die in isolation and being stretched thin to provide optimal care for all patients amid an unrelenting onslaught of COVID-19 cases, she said. For some health care workers, living in the garage or basement – to avoid infecting family members with the virus – also wore on their psyches.
Of all health care workers in the study, more than three-quarters reported symptoms that fell within the clinical range for depression (76%) and anxiety (78%). More than 25% noted that they had lost a family member or close colleague to the virus.
Dr. Hendrickson, who works with military veterans at the VA Puget Sound Hospital System’s Mental Illness Research, Education, and Clinical Center and its PTSD outpatient clinic, hadn’t expected the experience of loss to be so pervasive. She said the sheer number of people who “crossed the threshold” into despair concerned her deeply.
Signs and symptoms of PTSD
PTSD’s prevalence among health care workers has always been variable, said Jessica Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry at Washington University in St. Louis.
As a psychiatrist who sees health care workers in her clinical practice, Dr. Gold has noted poor baseline mental health, including depression and trauma. Significant data have pointed to a relatively higher suicide rate among physicians than among the general population. These problems have been compounded by COVID-19.
“It has been an unrelenting series of new stressors,” she said, citing lack of resources; a feeling of being unable to help; and the high frequency of risk of death to patients, family and friends, and the caregivers themselves as just as few examples. “It is very likely going to increase our baseline trauma, and honestly, I don’t know that we can predict how. To me, ”
PTSD can manifest itself in health care workers in several different ways. A few commonalities Dr. Gold has observed are sleep disruption (including insomnia and nightmares), work avoidance by taking disability or quitting, irritability or other changes in mood, trouble concentrating, and hypervigilance.
She said she has seen physical manifestations of trauma – such as body pain, stomachaches, and teeth grinding, which “you might not realize are at all related to trauma but ultimately are.” Sometimes, she added, “people have panic attacks on the way to work or right when they get to work, or are thinking about work.”
Dr. Gold noted that different types of treatment, such as cognitive-behavioral therapy and eye movement desensitization and reprocessing (EMDR), can be effective for PTSD. Medication is often necessary because of comorbid anxiety, depression, or eating disorders, said Dr. Gold, who is conducting a study on the pandemic’s effects on medical students.
The difficulties in isolating COVID-19 as a contributor
Not all researchers are convinced that a causal relationship has been established between the pandemic and worsening mental health among those in the health care sector.
With provider burnout being a long-standing concern in medicine, Ankur A. Butala, MD, assistant professor of neurology, psychiatry, and behavioral sciences at the Johns Hopkins University, Baltimore, said he remains a bit skeptical that acute stressors during the pandemic amounted to a uniquely potent driving force that can be extrapolated and quantified in a study.
“It’s hard to interpret a chronic, rolling, ongoing trauma like COVID-19 against tools or scales developed to investigate symptoms from a singular and acute trauma, like a school shooting or a [military] firefight,” Dr. Butala said.
In addition, he noted a reluctance to generalizing results from a study in which participants were recruited via social media as opposed to research methods involving more rigorous selection protocols.
Although Dr. Hendrickson acknowledged the study’s limitations, she said her team nonetheless found strong correlations between COVID-19-related stressors and self-reported struggles in completing work-related tasks, as well as increasing thoughts of leaving one’s current field. They adjusted for previous lifetime trauma exposure, age, gender, and a personal history of contracting COVID-19.
The underlying premise of the study could be confirmed with repeated surveys over time, Dr. Butala said, as the COVID-19 pandemic evolves and the vaccination effort unfolds.
Follow-up surveys are being sent to participants every 2 weeks and every 3 months to gauge their mood, for a total follow-up period of 9 months per individual. New participants are still welcome. “We will continue to enroll as long as it seems relevant,” Dr. Hendrickson said.
Carol S. North, MD, MPE, who has added to the growing research on the pandemic’s toll on mental health, noted that because symptom scales do not provide psychiatric diagnoses, it is difficult to attribute the prevalence of psychiatric disorders to the pandemic. Dr. North is chair and professor of crisis psychiatry at UT Southwestern Medical Center in Dallas, and director of the program in trauma and disaster at VA North Texas Health Care System.
The DSM-5 criteria exclude naturally occurring illness, such as a virus (even during a pandemic) as a qualifying trauma for the diagnosis of PTSD. According to current criteria by the American Psychiatric Association, COVID-19 and the pandemic are not defined as trauma, Dr. North said, while noting that “just because it’s not trauma or PTSD does not mean that the pandemic should be discounted as not stressful; people are finding it very stressful.”
Identifying the exact source of distress would still be difficult, Dr. North said, as the pandemic has produced severe economic consequences and prolonged social isolation, as well as occurring alongside nationwide protests over racial and ethnic divisions. Studies to date haven’t effectively separated out for these stressors, making it impossible to weigh their relative impact.
Furthermore, “most of us face many other stressors in our daily lives, such as grief, losses, broken relationships, and personal failures,” she said. “All of these may contribute to psychological distress, and research is needed to determine how much was a product of the virus, other aspects of the pandemic, or unrelated life stressors.”
A rallying cry for new interventions
Despite such doubts, a growing number of studies are reporting that health care workers and first responders are experiencing intensified PTSD, depression, anxiety, and insomnia as a result of the pandemic, said Hrayr Pierre Attarian, MD, professor of neurology at Northwestern University, Chicago. These results should act as a rallying cry for implementing more policies tailored to prevent burnout, he said.
“What we are seeing during this terrible pandemic is burnout on steroids,” said Dr. Attarian, medical director of Northwestern’s Center for Sleep Disorders. There are already high burnout rates, “so this should be doubly important.”
Rooting out this problem starts at the institutional level, but merely advising providers to “be well” wouldn’t make inroads. “There needs to be fluid dialogue between health care workers and the leadership,” he said.
Among his proposed remedies: Access to confidential and free mental health resources, increased administrative support, flexible hours, respect for work-life balance, and forgiveness for occasional errors that don’t result in harm.
“Sometimes even the perception that a mistake has been made is taken as proof of guilt,” Dr. Attarian said. “It is not conducive to wellness. Extra income does not replace a nurturing work environment.”
Furthermore, “as a profession, we must stop glorifying ‘overwork.’ We must stop wearing ‘lack of sleep’ as badge of honor,” he said. “Sleep is a biological imperative like self-preservation, hunger, and thirst. When we don’t sleep anxiety, pain, and depression get amplified. Our perception of distress is off, as is our judgment.”
The Federation of State Physician Health Programs provides a directory that physicians can use for referrals to confidential consultation or treatment.
Christopher Bundy, MD, MPH, executive medical director of Washington Physicians Health Program in Seattle, has been following Dr. Hendrickson’s longitudinal study with keen interest. As president of the Federation of State Physician Health Programs, he hopes to translate the findings into practice.
“Obviously, the COVID-19 pandemic has been a ‘black swan’ in terms of workforce sustainability issues,” Dr. Bundy said, citing “high rates of burnout, disillusionment, and dissatisfaction.” He sees some similarities with his former role in treating war veterans.
“The invisible wounds of combat, the psychological scars don’t really become apparent until after you’re out of the war zone,” said Dr. Bundy, clinical associate professor of psychiatry at the University of Washington.
Likewise, he expects the “emotional chickens will come home to roost as the pandemic subsides.” Until then, “people are just focused on survival, and in doing their jobs and protecting their patients.” Eventually, “their own wounds inside the pandemic will take hold.”
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic engulfed the nation well over a year ago, Rebecca Hendrickson, MD, PhD, immersed herself in the shell-shocking revelations that clinicians began posting on social media. The accounts offered just a snapshot of the pandemic’s heavy psychological toll, and Dr. Hendrickson, a psychiatrist at the University of Washington in Seattle and an expert in posttraumatic stress disorder (PTSD), wanted to know more.
She and her colleagues devised a survey to assess the impact of several pandemic-related factors, including increased work hours, social distancing restrictions, and lack of adequate personal protective equipment.
What began as a survey of health care workers soon expanded in scope. Of the more than 600 survey respondents to date, health care workers account for about 60%, while the rest are first responders – police officers, firefighters, paramedics, and emergency medical technicians – and nonclinical personnel, such as security guards and office staff, in health care settings. The respondents range in age from 19 to 72, and hail from all regions of the country.
“Our findings were really striking,” Dr. Hendrickson said, “including very high rates of thoughts of suicide and thoughts of leaving one’s current field, which were both strongly linked to COVID-19–related occupational stress exposure.”
The distress stemmed from a multitude of factors. Among the most demoralizing: witnessing patients die in isolation and being stretched thin to provide optimal care for all patients amid an unrelenting onslaught of COVID-19 cases, she said. For some health care workers, living in the garage or basement – to avoid infecting family members with the virus – also wore on their psyches.
Of all health care workers in the study, more than three-quarters reported symptoms that fell within the clinical range for depression (76%) and anxiety (78%). More than 25% noted that they had lost a family member or close colleague to the virus.
Dr. Hendrickson, who works with military veterans at the VA Puget Sound Hospital System’s Mental Illness Research, Education, and Clinical Center and its PTSD outpatient clinic, hadn’t expected the experience of loss to be so pervasive. She said the sheer number of people who “crossed the threshold” into despair concerned her deeply.
Signs and symptoms of PTSD
PTSD’s prevalence among health care workers has always been variable, said Jessica Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry at Washington University in St. Louis.
As a psychiatrist who sees health care workers in her clinical practice, Dr. Gold has noted poor baseline mental health, including depression and trauma. Significant data have pointed to a relatively higher suicide rate among physicians than among the general population. These problems have been compounded by COVID-19.
“It has been an unrelenting series of new stressors,” she said, citing lack of resources; a feeling of being unable to help; and the high frequency of risk of death to patients, family and friends, and the caregivers themselves as just as few examples. “It is very likely going to increase our baseline trauma, and honestly, I don’t know that we can predict how. To me, ”
PTSD can manifest itself in health care workers in several different ways. A few commonalities Dr. Gold has observed are sleep disruption (including insomnia and nightmares), work avoidance by taking disability or quitting, irritability or other changes in mood, trouble concentrating, and hypervigilance.
She said she has seen physical manifestations of trauma – such as body pain, stomachaches, and teeth grinding, which “you might not realize are at all related to trauma but ultimately are.” Sometimes, she added, “people have panic attacks on the way to work or right when they get to work, or are thinking about work.”
Dr. Gold noted that different types of treatment, such as cognitive-behavioral therapy and eye movement desensitization and reprocessing (EMDR), can be effective for PTSD. Medication is often necessary because of comorbid anxiety, depression, or eating disorders, said Dr. Gold, who is conducting a study on the pandemic’s effects on medical students.
The difficulties in isolating COVID-19 as a contributor
Not all researchers are convinced that a causal relationship has been established between the pandemic and worsening mental health among those in the health care sector.
With provider burnout being a long-standing concern in medicine, Ankur A. Butala, MD, assistant professor of neurology, psychiatry, and behavioral sciences at the Johns Hopkins University, Baltimore, said he remains a bit skeptical that acute stressors during the pandemic amounted to a uniquely potent driving force that can be extrapolated and quantified in a study.
“It’s hard to interpret a chronic, rolling, ongoing trauma like COVID-19 against tools or scales developed to investigate symptoms from a singular and acute trauma, like a school shooting or a [military] firefight,” Dr. Butala said.
In addition, he noted a reluctance to generalizing results from a study in which participants were recruited via social media as opposed to research methods involving more rigorous selection protocols.
Although Dr. Hendrickson acknowledged the study’s limitations, she said her team nonetheless found strong correlations between COVID-19-related stressors and self-reported struggles in completing work-related tasks, as well as increasing thoughts of leaving one’s current field. They adjusted for previous lifetime trauma exposure, age, gender, and a personal history of contracting COVID-19.
The underlying premise of the study could be confirmed with repeated surveys over time, Dr. Butala said, as the COVID-19 pandemic evolves and the vaccination effort unfolds.
Follow-up surveys are being sent to participants every 2 weeks and every 3 months to gauge their mood, for a total follow-up period of 9 months per individual. New participants are still welcome. “We will continue to enroll as long as it seems relevant,” Dr. Hendrickson said.
Carol S. North, MD, MPE, who has added to the growing research on the pandemic’s toll on mental health, noted that because symptom scales do not provide psychiatric diagnoses, it is difficult to attribute the prevalence of psychiatric disorders to the pandemic. Dr. North is chair and professor of crisis psychiatry at UT Southwestern Medical Center in Dallas, and director of the program in trauma and disaster at VA North Texas Health Care System.
The DSM-5 criteria exclude naturally occurring illness, such as a virus (even during a pandemic) as a qualifying trauma for the diagnosis of PTSD. According to current criteria by the American Psychiatric Association, COVID-19 and the pandemic are not defined as trauma, Dr. North said, while noting that “just because it’s not trauma or PTSD does not mean that the pandemic should be discounted as not stressful; people are finding it very stressful.”
Identifying the exact source of distress would still be difficult, Dr. North said, as the pandemic has produced severe economic consequences and prolonged social isolation, as well as occurring alongside nationwide protests over racial and ethnic divisions. Studies to date haven’t effectively separated out for these stressors, making it impossible to weigh their relative impact.
Furthermore, “most of us face many other stressors in our daily lives, such as grief, losses, broken relationships, and personal failures,” she said. “All of these may contribute to psychological distress, and research is needed to determine how much was a product of the virus, other aspects of the pandemic, or unrelated life stressors.”
A rallying cry for new interventions
Despite such doubts, a growing number of studies are reporting that health care workers and first responders are experiencing intensified PTSD, depression, anxiety, and insomnia as a result of the pandemic, said Hrayr Pierre Attarian, MD, professor of neurology at Northwestern University, Chicago. These results should act as a rallying cry for implementing more policies tailored to prevent burnout, he said.
“What we are seeing during this terrible pandemic is burnout on steroids,” said Dr. Attarian, medical director of Northwestern’s Center for Sleep Disorders. There are already high burnout rates, “so this should be doubly important.”
Rooting out this problem starts at the institutional level, but merely advising providers to “be well” wouldn’t make inroads. “There needs to be fluid dialogue between health care workers and the leadership,” he said.
Among his proposed remedies: Access to confidential and free mental health resources, increased administrative support, flexible hours, respect for work-life balance, and forgiveness for occasional errors that don’t result in harm.
“Sometimes even the perception that a mistake has been made is taken as proof of guilt,” Dr. Attarian said. “It is not conducive to wellness. Extra income does not replace a nurturing work environment.”
Furthermore, “as a profession, we must stop glorifying ‘overwork.’ We must stop wearing ‘lack of sleep’ as badge of honor,” he said. “Sleep is a biological imperative like self-preservation, hunger, and thirst. When we don’t sleep anxiety, pain, and depression get amplified. Our perception of distress is off, as is our judgment.”
The Federation of State Physician Health Programs provides a directory that physicians can use for referrals to confidential consultation or treatment.
Christopher Bundy, MD, MPH, executive medical director of Washington Physicians Health Program in Seattle, has been following Dr. Hendrickson’s longitudinal study with keen interest. As president of the Federation of State Physician Health Programs, he hopes to translate the findings into practice.
“Obviously, the COVID-19 pandemic has been a ‘black swan’ in terms of workforce sustainability issues,” Dr. Bundy said, citing “high rates of burnout, disillusionment, and dissatisfaction.” He sees some similarities with his former role in treating war veterans.
“The invisible wounds of combat, the psychological scars don’t really become apparent until after you’re out of the war zone,” said Dr. Bundy, clinical associate professor of psychiatry at the University of Washington.
Likewise, he expects the “emotional chickens will come home to roost as the pandemic subsides.” Until then, “people are just focused on survival, and in doing their jobs and protecting their patients.” Eventually, “their own wounds inside the pandemic will take hold.”
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic engulfed the nation well over a year ago, Rebecca Hendrickson, MD, PhD, immersed herself in the shell-shocking revelations that clinicians began posting on social media. The accounts offered just a snapshot of the pandemic’s heavy psychological toll, and Dr. Hendrickson, a psychiatrist at the University of Washington in Seattle and an expert in posttraumatic stress disorder (PTSD), wanted to know more.
She and her colleagues devised a survey to assess the impact of several pandemic-related factors, including increased work hours, social distancing restrictions, and lack of adequate personal protective equipment.
What began as a survey of health care workers soon expanded in scope. Of the more than 600 survey respondents to date, health care workers account for about 60%, while the rest are first responders – police officers, firefighters, paramedics, and emergency medical technicians – and nonclinical personnel, such as security guards and office staff, in health care settings. The respondents range in age from 19 to 72, and hail from all regions of the country.
“Our findings were really striking,” Dr. Hendrickson said, “including very high rates of thoughts of suicide and thoughts of leaving one’s current field, which were both strongly linked to COVID-19–related occupational stress exposure.”
The distress stemmed from a multitude of factors. Among the most demoralizing: witnessing patients die in isolation and being stretched thin to provide optimal care for all patients amid an unrelenting onslaught of COVID-19 cases, she said. For some health care workers, living in the garage or basement – to avoid infecting family members with the virus – also wore on their psyches.
Of all health care workers in the study, more than three-quarters reported symptoms that fell within the clinical range for depression (76%) and anxiety (78%). More than 25% noted that they had lost a family member or close colleague to the virus.
Dr. Hendrickson, who works with military veterans at the VA Puget Sound Hospital System’s Mental Illness Research, Education, and Clinical Center and its PTSD outpatient clinic, hadn’t expected the experience of loss to be so pervasive. She said the sheer number of people who “crossed the threshold” into despair concerned her deeply.
Signs and symptoms of PTSD
PTSD’s prevalence among health care workers has always been variable, said Jessica Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry at Washington University in St. Louis.
As a psychiatrist who sees health care workers in her clinical practice, Dr. Gold has noted poor baseline mental health, including depression and trauma. Significant data have pointed to a relatively higher suicide rate among physicians than among the general population. These problems have been compounded by COVID-19.
“It has been an unrelenting series of new stressors,” she said, citing lack of resources; a feeling of being unable to help; and the high frequency of risk of death to patients, family and friends, and the caregivers themselves as just as few examples. “It is very likely going to increase our baseline trauma, and honestly, I don’t know that we can predict how. To me, ”
PTSD can manifest itself in health care workers in several different ways. A few commonalities Dr. Gold has observed are sleep disruption (including insomnia and nightmares), work avoidance by taking disability or quitting, irritability or other changes in mood, trouble concentrating, and hypervigilance.
She said she has seen physical manifestations of trauma – such as body pain, stomachaches, and teeth grinding, which “you might not realize are at all related to trauma but ultimately are.” Sometimes, she added, “people have panic attacks on the way to work or right when they get to work, or are thinking about work.”
Dr. Gold noted that different types of treatment, such as cognitive-behavioral therapy and eye movement desensitization and reprocessing (EMDR), can be effective for PTSD. Medication is often necessary because of comorbid anxiety, depression, or eating disorders, said Dr. Gold, who is conducting a study on the pandemic’s effects on medical students.
The difficulties in isolating COVID-19 as a contributor
Not all researchers are convinced that a causal relationship has been established between the pandemic and worsening mental health among those in the health care sector.
With provider burnout being a long-standing concern in medicine, Ankur A. Butala, MD, assistant professor of neurology, psychiatry, and behavioral sciences at the Johns Hopkins University, Baltimore, said he remains a bit skeptical that acute stressors during the pandemic amounted to a uniquely potent driving force that can be extrapolated and quantified in a study.
“It’s hard to interpret a chronic, rolling, ongoing trauma like COVID-19 against tools or scales developed to investigate symptoms from a singular and acute trauma, like a school shooting or a [military] firefight,” Dr. Butala said.
In addition, he noted a reluctance to generalizing results from a study in which participants were recruited via social media as opposed to research methods involving more rigorous selection protocols.
Although Dr. Hendrickson acknowledged the study’s limitations, she said her team nonetheless found strong correlations between COVID-19-related stressors and self-reported struggles in completing work-related tasks, as well as increasing thoughts of leaving one’s current field. They adjusted for previous lifetime trauma exposure, age, gender, and a personal history of contracting COVID-19.
The underlying premise of the study could be confirmed with repeated surveys over time, Dr. Butala said, as the COVID-19 pandemic evolves and the vaccination effort unfolds.
Follow-up surveys are being sent to participants every 2 weeks and every 3 months to gauge their mood, for a total follow-up period of 9 months per individual. New participants are still welcome. “We will continue to enroll as long as it seems relevant,” Dr. Hendrickson said.
Carol S. North, MD, MPE, who has added to the growing research on the pandemic’s toll on mental health, noted that because symptom scales do not provide psychiatric diagnoses, it is difficult to attribute the prevalence of psychiatric disorders to the pandemic. Dr. North is chair and professor of crisis psychiatry at UT Southwestern Medical Center in Dallas, and director of the program in trauma and disaster at VA North Texas Health Care System.
The DSM-5 criteria exclude naturally occurring illness, such as a virus (even during a pandemic) as a qualifying trauma for the diagnosis of PTSD. According to current criteria by the American Psychiatric Association, COVID-19 and the pandemic are not defined as trauma, Dr. North said, while noting that “just because it’s not trauma or PTSD does not mean that the pandemic should be discounted as not stressful; people are finding it very stressful.”
Identifying the exact source of distress would still be difficult, Dr. North said, as the pandemic has produced severe economic consequences and prolonged social isolation, as well as occurring alongside nationwide protests over racial and ethnic divisions. Studies to date haven’t effectively separated out for these stressors, making it impossible to weigh their relative impact.
Furthermore, “most of us face many other stressors in our daily lives, such as grief, losses, broken relationships, and personal failures,” she said. “All of these may contribute to psychological distress, and research is needed to determine how much was a product of the virus, other aspects of the pandemic, or unrelated life stressors.”
A rallying cry for new interventions
Despite such doubts, a growing number of studies are reporting that health care workers and first responders are experiencing intensified PTSD, depression, anxiety, and insomnia as a result of the pandemic, said Hrayr Pierre Attarian, MD, professor of neurology at Northwestern University, Chicago. These results should act as a rallying cry for implementing more policies tailored to prevent burnout, he said.
“What we are seeing during this terrible pandemic is burnout on steroids,” said Dr. Attarian, medical director of Northwestern’s Center for Sleep Disorders. There are already high burnout rates, “so this should be doubly important.”
Rooting out this problem starts at the institutional level, but merely advising providers to “be well” wouldn’t make inroads. “There needs to be fluid dialogue between health care workers and the leadership,” he said.
Among his proposed remedies: Access to confidential and free mental health resources, increased administrative support, flexible hours, respect for work-life balance, and forgiveness for occasional errors that don’t result in harm.
“Sometimes even the perception that a mistake has been made is taken as proof of guilt,” Dr. Attarian said. “It is not conducive to wellness. Extra income does not replace a nurturing work environment.”
Furthermore, “as a profession, we must stop glorifying ‘overwork.’ We must stop wearing ‘lack of sleep’ as badge of honor,” he said. “Sleep is a biological imperative like self-preservation, hunger, and thirst. When we don’t sleep anxiety, pain, and depression get amplified. Our perception of distress is off, as is our judgment.”
The Federation of State Physician Health Programs provides a directory that physicians can use for referrals to confidential consultation or treatment.
Christopher Bundy, MD, MPH, executive medical director of Washington Physicians Health Program in Seattle, has been following Dr. Hendrickson’s longitudinal study with keen interest. As president of the Federation of State Physician Health Programs, he hopes to translate the findings into practice.
“Obviously, the COVID-19 pandemic has been a ‘black swan’ in terms of workforce sustainability issues,” Dr. Bundy said, citing “high rates of burnout, disillusionment, and dissatisfaction.” He sees some similarities with his former role in treating war veterans.
“The invisible wounds of combat, the psychological scars don’t really become apparent until after you’re out of the war zone,” said Dr. Bundy, clinical associate professor of psychiatry at the University of Washington.
Likewise, he expects the “emotional chickens will come home to roost as the pandemic subsides.” Until then, “people are just focused on survival, and in doing their jobs and protecting their patients.” Eventually, “their own wounds inside the pandemic will take hold.”
A version of this article first appeared on Medscape.com.
How to meet the challenges of managing patients with IBS
Irritable bowel syndrome (IBS) continues to pose a diagnostic and therapeutic challenge to clinicians and patients—a challenge that arises from the varying manifestations of the condition, its complex pathophysiology, lack of effective treatment, and psychological consequences for patients. In this article, I explore new findings related to the pathophysiology, diagnosis, and management of IBS subtypes.
Start with the Rome IV classification of IBS
The Rome Foundation published its latest IBS classification and diagnostic criteria (known as Rome IV) in 2016.1 IBS is defined as abdominal pain that (1) has recurred, on average, ≥ 1 time per week during the past 3 months and (2) is associated with ≥ 2 of these criteria1:
- related to defecation
- associated with a change in stool frequency
- associated with a change in the appearance of stool.
Onset of symptoms should be present for 6 months before a diagnosis of IBS is made.1
IBS subtypes—constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unclassified (IBS-U) (TABLE 1)1—are based on the frequency of specific stool forms, as described and illustrated in the Bristol Stool Scale (www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale).2
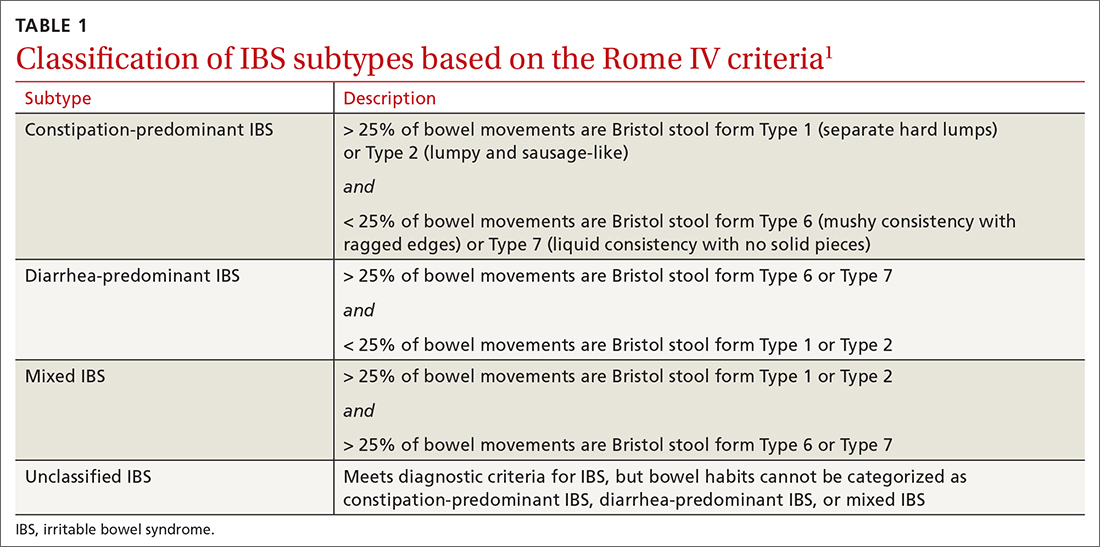
A widespread, costly, potentially debilitating disorder
IBS affects 10% to 12% of adults worldwide. The condition is more common among women and people younger than 50 years.1,3 Women with IBS tend to have more constipation symptoms (IBS-C); men with IBS, more diarrhea symptoms (IBS-D).4
The financial burden of IBS on the health care system and patients is significant. In a 2013 appraisal of 35 studies, the authors note that estimates of the direct cost of IBS care in the United States vary considerably—from $1562 to $7547 for a patient annually.5
A recent study found that almost 25% of IBS patients report absenteeism from work due to IBS symptoms.6 A Danish study that followed 7278 patients for 5 years found that IBS patients utilized more health care, sick days, and disability pension benefits than non-IBS patients, and had increased utilization of medical resources because of psychiatric conditions.7
Continue to: IBS patients also have comorbidities
IBS patients also have comorbidities:
- More than 20% of IBS patients have functional dyspepsia, gastroesophageal reflux disease, incontinence, or pelvic floor dyssynergia.4
- The frequency of fibromyalgia syndrome in IBS patients is reported to be 20% to 65%.8
- 14% of IBS patients meet criteria for chronic fatigue syndrome.8
- Interstitial cystitis and dyspareunia are common among IBS patients.9
Pathophysiology is complex
Models describing the pathophysiology of IBS have evolved through the years. Recent models describe it as a combination of altered gastrointestinal motility, visceral hyperalgesia, increased intestinal permeability, immune activation, altered intestinal microbiota, and dysfunction in the brain–gut axis. Certain environmental and psychological variables (eg, previous gastroenteritis, food intolerance, chronic stress, diverticulitis, and surgery) increase the risk of IBS.1,10,11
In the past several years, considerable attention has been paid to the roles played by the immune system, brain–gut axis function, and intestinal microbiota in IBS manifestations. Research focus in these areas might assist in the development of specific treatment modalities targeting IBS subtypes.
Immune system. A recent meta-analysis of the records of 706 IBS patients found an increased number of mast cells and CD3 T cells in biopsy specimens from the rectosigmoid and descending colon of IBS patients.12 Another study found a significant increase in mast cells in the ileum of IBS patients13; this increase is evident not only on intestinal biopsy but also at the serologic level. IBS-D patients have a higher plasma interleukin (IL)-6 level than the general population.14 Another meta-analysis found an imbalance in the serum level of tumor necrosis factor-α and IL-10 in IBS patients.15
Brain–gut axis. A 2016 meta-analysis showed that patients with anxiety and depression have a 2-fold increased risk of IBS.16 A more recent study, using data from the National Health Insurance Research Database that included 22,356 patients with IBS, found a 3.6-fold increased risk of psychiatric disorders in IBS.17 These findings reflect the complex interaction between the brain and the intestinal tract in IBS.
Continue to: Intestinal microbiota
Intestinal microbiota. Research evaluating the role of altered intestinal microbiota in IBS has yielded mixed results. A meta-analysis of 777 IBS patients showed an increase in Firmicutes spp, a decrease in Bacteroidetes spp, and an increase in the ratio of Firmicutes spp to Bacteroidetes spp in subjects’ fecal specimens.18 Another study, of 1340 patients, found no difference in Bacteroides spp and Enterococcus spp between healthy controls and IBS patients, but did find (1) lower fecal counts of Lactobacillus spp and Bifidobacterium spp and (2) higher fecal counts of Escherichia coli and Enterobacter spp in IBS patients.19
Postinfectious IBS. The Rome Foundation introduced the diagnosis of postinfectious IBS (PI-IBS) in 2019. PI-IBS develops in 10% of patients who have had infectious enteritis. Female gender, younger age, psychological distress during or before the enteritis episode, and severity of the acute episode are risk factors for this IBS variant.20 A study of 21,421 enteritis patients found that 42% with protozoal or parasitic infection and 14% with bacterial infection developed IBS.
Patients with nonviral enteritis often have a more severe course of enteritis, typically requiring antibiotics. It is believed that the resulting irregularities in the intestinal microbiota make these patients more likely to develop PI-IBS.21 PI-IBS patients are likely to improve or fully recover over time. Symptoms of PI-IBS are managed in a manner similar to how non-PI-IBS patients are managed.20
Challenges in making the IBS diagnosis
Historically, the diagnosis of IBS has been made clinically after excluding red flags (ie, signs or symptoms that might reflect other underlying medical problems) in the clinical presentation. For this reason, obtain a thorough clinical history that includes the course of symptoms, triggers, and alleviating factors. Any of the following are considered red flags1,22,23:
- age > 50 years at onset of symptoms
- new-onset constipation in the elderly
- rectal bleeding
- unexplained weight loss or anemia
- family history of organic gastrointestinal disease
- palpable abdominal or rectal mass
- nocturnal symptoms.
New studies demonstrate that several inflammatory markers can help exclude inflammatory bowel disease from the differential diagnosis in patients in whom IBS is suspected and being investigated.24 In 2019, the American Gastroenterological Association published a clinical practice guideline updating the laboratory evaluation of functional diarrhea and IBS-D in adults,25 and made several recommendations:
- Obtain the level of fecal calprotectin (normal level, ≤ 50 mcg/g) or fecal lactoferrin (≤ 4.0-7.25 mcg/g); if these tests are not available or results are not accessible, the C-reactive protein level is a reasonable option.
- Do not routinely use the erythrocyte sedimentation rate or C-reactive protein level to screen for inflammatory bowel disease.
- Test for Giardia lamblia with an antigen or polymerase chain reaction test.
- Do not test for ova and parasites (other than Giardia) in patients who do not have a history of travel or who have not emigrated from a high-risk area recently.
- Obtain testing for celiac disease with immunoglobulin A (IgA) tissue transglutaminase and with a second test, of immunoglobulin G (IgG) tissue transglutaminase and IgG or IgA deaminated gliadin peptides, to detect celiac disease in IgA-deficient patients.
- Order testing for bile-acid diarrhea with selenium homotaurocholic acid nuclear medicine scanning (if available in your region; the test is available in Europe); measurement of bile acid from a 48-hour stool collection; or an assay of fibroblast growth factor 19, which measures defective feedback of bile-acid synthesis. If these tests are unavailable, consider an empiric trial of a bile-acid binder.
- Do not use available serologic IBS testing.
Continue to: Continue to obtain a...
Continue to obtain a complete blood count for the evaluation of anemia. Endoscopic procedures are indicated in patients with a red flag.1
Treat based on subtype
The first step in the treatment of all IBS patients (TABLE 21,3,4,9,26,27) is for you to develop a strong relationship with the patient: You must acknowledge the disease and empower the patient to manage their symptoms. A strong physician–patient relationship leads to more effective outcomes.4
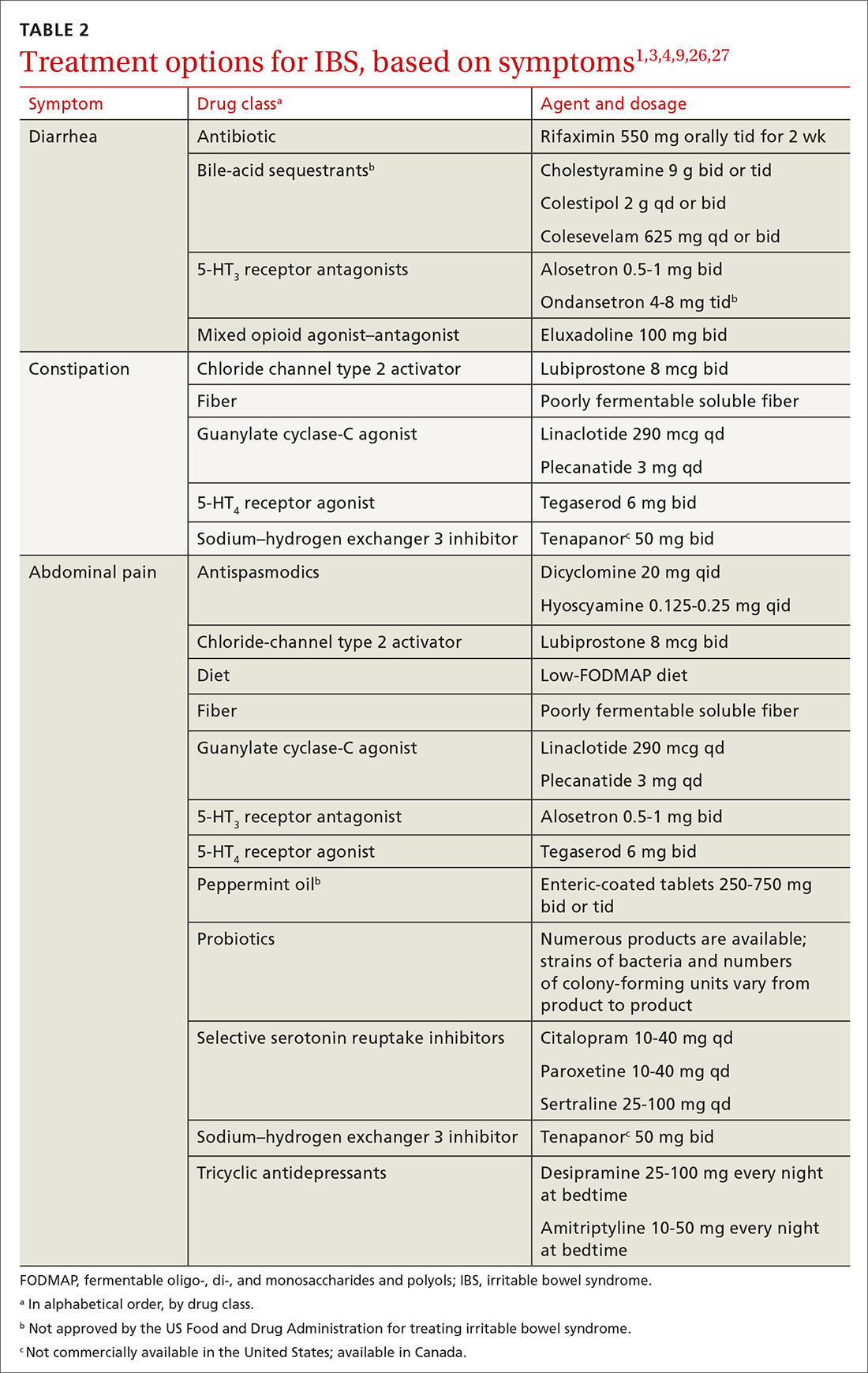
IBS treatment modalities target abdominal pain, bloating, abdominal distention, and altered bowel function—described in the literature as global symptoms. IBS-M patients should direct their treatment to the predominant symptom (constipation or diarrhea). The following sections describe available treatment options. The FIGURE1,3,4,9,25 shows a treatment workflow based on IBS subtype and symptom severity.
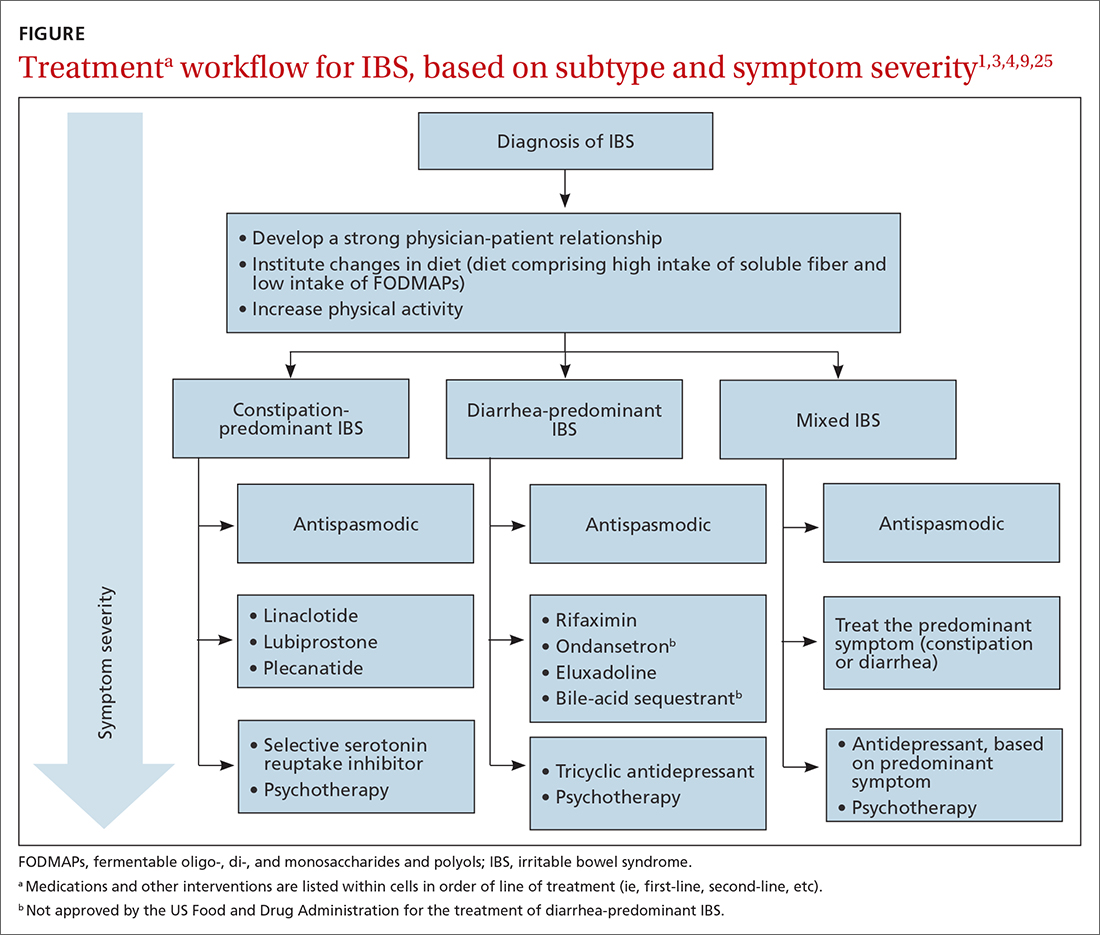
Treatments for all IBS subtypes
Lifestyle modification. Exercise provides overall positive health benefits. With such a variety of exercise forms, however, it is difficult to identify specific exercises that are better for IBS patients.28 A study of 305 IBS patients found that exercise alleviated constipation but not other IBS symptoms, and did not improve quality of life.3 Based on low cost and low risk of adverse effects, exercise should be recommended to all IBS patients.
Dietary restriction therapies have become an area of focus for patients, clinicians, and researchers. Modification of the diet is thought to improve global symptoms and intestinal health through modification of gut microbiota, immune activation, and a decrease in levels of fecal short-chain fatty acids.29
Continue to: The 2 main diets...
The 2 main diets studied for the treatment of IBS are a diet low in fermentable oligo-, di- and monosaccharides and polyols—the so-called low-FODMAP diet (TABLE 330)—and a gluten-free diet. Evidence behind the benefits of both diets conflicts; trials of the low-FODMAP diet are more favorable.
A small study with 20 patients with IBS-D and IBS-M who followed a low-FODMAP diet found improvement in IBS symptoms and a reduction in serum levels of proinflammatory cytokines, fecal bacteria, and total fecal short-chain fatty acid levels.29 Several meta-analyses have shown improvement in overall IBS symptoms for patients who follow a low-FODMAP diet. Because of the heterogeneity of the studies, however, the quality of the data is low.31-34
Data supporting the use of a gluten-free diet for IBS patients are insufficient.31
The American College of Gastroenterology (ACG) gave a weak recommendation for the low-FODMAP diet and recommended against the gluten-free diet in IBS patients.3 More data are needed regarding the safety profile of using a low-FODMAP diet for an extended period: There is concern about the risk of nutritional deficiencies associated with long-term use of this diet.3
Supplementation with poorly fermentable soluble fiber has been shown to alleviate global IBS symptoms; insoluble fiber does not yield improvement of symptoms. Psyllium fiber is recommended over wheat bran.3,35
Continue to: Consider a low-FODMAP diet...
Consider a low-FODMAP diet and soluble fiber as initial treatment for all IBS patients.
Modification of intestinal microbiota. Understanding the difference between prebiotics and probiotics is important when considering treatment for IBS. Prebiotics are foods or dietary supplements that generate changes in the composition and activity of intestinal microbiota. Probiotics are live microorganisms that can improve intestinal health.3
A meta-analysis of 729 IBS patients found that prebiotics do not reduce gastrointestinal symptoms or improve the quality of life of IBS patients.36 Evidence supporting the benefit of probiotics is favorable; however, data in these studies have significant heterogeneity. Several meta-analyses studied the benefits of Lactobacillus spp and Bifidobacterium spp in alleviating IBS symptoms. The studies found improvement in abdominal pain, bloating and distention, and flatulence.3,37-40 Consider recommending probiotics for all IBS patients; for some, however, the high cost of some of these products might be an obstacle.
Researchers are also studying the use of fecal microbiota transplantation (FMT) to treat IBS. Studies have evaluated the delivery of FMT orally (as capsules) and endoscopically. Evidence does not show improvement in global IBS symptoms with FMT. More studies, with larger sample populations, are needed.41-43
Antispasmodic medications and peppermint oil. Antispasmodic medications have been considered a mainstay therapy for IBS because of their effect on intestinal dysmotility. Hyoscine and dicyclomine are commonly used. Meta-analyses have shown improvement in global symptoms and abdominal pain, but effects were modest.3,44 Use this class of drugs as first-line treatment for mild IBS symptoms.
Continue to: Peppermint oil has been...
Peppermint oil has been found useful in improving IBS global symptoms and abdominal pain in several studies.44-46 A common adverse effect of peppermint oil is heartburn, resulting from relaxation of esophageal muscle.3 Peppermint oil can be considered an adjuvant agent in treating IBS.
Antidepressants. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been studied for the treatment of IBS. Meta-analyses show that both are effective in reducing pain and overall IBS symptoms.1,3,47 The number needed to treat (NNT) for TCAs is 4.5; for SSRIs, 5.47 Data do not show that either drug class is superior to the other for IBS. Based on the adverse effect profile, TCAs are more suitable for IBS-D patients; SSRIs are better for IBS-C patients.47
New data show that serotonin-norepinephrine reuptake inhibitors, such as duloxetine and milnacipran, can alleviate IBS symptoms through their pain-modifying properties.47
Based on the adverse effect profile and stigma associated with antidepressant medications, patients might be less likely to take them for IBS symptoms than for these drugs’ primary indications. Clinicians should still consider this drug class if other first-line treatments do not provide full resolution of symptoms.
Psychotherapy. Several psychotherapeutic modalities have been evaluated for efficacy in reducing global IBS symptoms. The approaches studied most often were provider-directed cognitive behavioral therapy, relaxation therapy, hypnotherapy, and multicomponent psychological therapy. The NNT for these modalities is 4, but studies had significant heterogeneity.3 Consider referring patients for psychotherapeutic intervention if they have not responded to medical therapy after 12 months.4
Continue to Treatment of IBS-C
Treatment of IBS-C
Prosecretory agents. Linaclotide and plecanatide are amino-acid peptides that act as a guanylate cyclase C agonist. Both increase gastrointestinal transit rate by increasing electrolyte and fluid transport into the intestinal lumen. They also decrease the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3'5'-monophosphate levels.3,48 In a recent meta-analysis, both treatments produced improvement in global symptoms. However, linaclotide showed superior improvement in abdominal pain and global symptoms compared to other secretory agents.48,49 Diarrhea was the most common adverse effect of linaclotide and plecanatide, although less so with plecanatide.49
Lubiprostone activates the intestinal chloride channel type 2 on the small intestine, leading to an increase in chloride and water efflux into the intestinal lumen, in turn accelerating gastrointestinal transit.3 A meta-analysis with 1468 IBS patients found that lubiprostone improved constipation, stool consistency, abdominal pain, degree of straining, and abdominal bloating.50 Diarrhea and nausea are commonly reported adverse effects of lubiprostone.49,50
Linaclotide, plecanatide, and lubiprostone should be considered first-line therapies for patients with IBS-C. High cost is still a roadblock to the use of these agents.
The US Food and Drug Administration (FDA) approved tenapanor in September 2019; however, the drug is not commercially available in the United States (it is available in Canada). Tenapanor is a sodium–hydrogen exchanger 3 inhibitor that reduces sodium absorption from the intestine and colon. The drug increases water secretion into the intestinal lumen, thus accelerating gut transit time. It also inhibits active absorption of phosphate in the intestine.
Tenapanor was approved for treating both IBS-C and hyperphosphatemia in patients with chronic kidney disease on dialysis or end-stage renal disease.26 In a recent meta-analysis, the drug showed benefit in alleviating global IBS symptoms, and ranked first in reducing bloating.49 It is too soon to know if tenapanor will perform clinically better than other prosecretory agents.
Continue to: Serotonergic agents
Serotonergic agents. Serotonin (5-hydroxytryptamine [5-HT]) modulates gastrointestinal secretions, gut motility, and visceral sensation. Researchers have developed IBS treatments that target receptors involved in these functions.
Tegaserod is a partial, selective 5-HT4 agonist indicated for the treatment of IBS-C in women. A study with 661 women with IBS-M and IBS-C showed that tegaserod increased the number of bowel movement episodes. Patients also reported higher stool consistency scores and fewer days with straining compared to placebo.27 The medication was removed from the market in 2007 because of its potential for cardiovascular adverse effects3; however, it was reintroduced in 2019 for women < 65 years of age with IBS-C. Consider prescribing tegaserod if other treatment options fail to alleviate symptoms.
Treatment of IBS-D
Antibiotics. The nonabsorbable antibiotic rifaximin is approved by the FDA for IBS-D at a dosage of 550 mg tid for 2 weeks.1 Several studies show improvement in IBS global symptoms with the recommended treatment course51-53; benefit persisted for the 10-week follow-up study period.1 A meta-analysis found that the NNT for rifaximin is 8-11.54 Preliminary data indicate that the rates of Clostridioides difficile infection and microbial resistance among rifaximin users are low.3 Consider using rifaximin as a first-line treatment option for patients with IBS-D. Retreatment might be necessary because the drug’s effect gradually disappears.9
Antidiarrheals. Eluxadoline is a µ-opioid and κ-opioid receptor agonist and δ-opioid receptor antagonist with effects on the intestinal nervous system.3 Several meta-analyses demonstrated that eluxadoline improves abdominal pain scores and daily stool consistency in IBS-D patients.53,54 Eluxadoline should be considered early in the management of IBS-D patients. The most common adverse effect is constipation.
The FDA issued a safety warning in 2017 regarding an increased risk of pancreatitis in patients taking eluxadoline who do not have a gallbladder. In addition, eluxadoline should be avoided in patients with a history of sphincter of Oddi dysfunction, alcohol abuse, or severe liver problems.3,54
Continue to: The high cost of...
The high cost of eluxadoline can be a significant barrier to use.
Serotonergic agents. Alosetron is a selective
Ondansetron has also been used to treat IBS-D. In a meta-analysis with 294 patients, ondansetron showed improvement in stool consistency.55 Ondansetron does not improve abdominal pain.4 It can be used in patients who have mild-to-moderate symptoms.9 Ondansetron is not FDA approved for the treatment of IBS-D.
Bile-acid sequestrants. Traditionally, bile-acid sequestrants have been used to treat bile-acid diarrhea. A meta-analysis of 6 studies of 908 patients with IBS-D found that 28.1% were affected by bile-acid malabsorption. Two small studies that evaluated the benefits of colesevelam for IBS-D found significant improvement in stool consistency.54 Another study, which evaluated the benefits of cholestyramine, found improvement in stool consistency, but findings were not significant.54 Many patients taking a bile-acid sequestrant stop taking the medication because of considerable adverse effects (constipation, nausea, bloating, flatulence, and abdominal pain).54 For that reason, this class of medication is not recommended as first-line treatment for IBS-D and is not FDA approved for IBS-D.
SIDEBAR
KEY POINTS The challenge of, and a needed framework for, managing IBS
- IBS is a complex, chronic condition affecting a considerable number of people worldwide.
- Because of the substantial disease burden associated with IBS, patients are at higher risk of mental health disorders.
- Physicians who care for IBS patients must build a strong physician–patient relationship; their mutual trust will ensure development of an effective treatment plan.
- Family physicians and other primary care providers are equipped to help IBS patients navigate the complex health care system and the IBS disease process. They can help coordinate care with specialists and behavioral health clinicians, which will help patients improve quality of life and manage symptoms appropriately.
A role for complementaryand integrative medicine?
Recently, complementary and integrative modalities for treating IBS have sparked the interest of researchers.
Continue to: Acupuncture
Acupuncture. In a meta-analysis with 3440 patients, acupuncture was more effective than Western medicine in alleviating IBS symptoms for as long as 3 months. The authors concluded that acupuncture could be used in combination with other therapies to reduce the severity of IBS symptoms.56
Concomitant acupuncture and Chinese herbal medicine. In a systematic review and meta-analysis comprising 21 randomized controlled trials, researchers reported that acupuncture combined with Chinese herbal medicine improved IBS symptoms, compared to what was noted in matched controls who were treated with Western medicine or with Western medicine combined with Chinese herbal medicine. The authors were cautious about the results of the meta-analysis, however, because the studies examined were small and of low quality, and presented a high risk of bias.57
Agents not to be used routinely for IBS
Loperamide. This peripheral µ-opioid receptor agonist controls diarrhea. However, recent studies showed no significant benefit to loperamide over placebo in IBS-M and IBS-D. In 2018, the FDA issued a safety alert regarding an elevated risk of serious cardiac adverse effects in patients taking loperamide. The ACG recommends against using loperamide to treat IBS symptoms.3,54
Polyethylene glycol. An osmotic laxative that is not absorbed in the intestinal lumen, polyethylene glycol is highly efficacious for alleviating constipation, but it does not reduce pain or other IBS symptoms. For that reason, the ACG recommends against its use.3
CORRESPONDENCE
Jose M. Villalon-Gomez, MD, MPH, Emory Healthcare Family Medicine, 4500 North Shallowford Road, Dunwoody, GA 30338; [email protected]
1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5. doi:10.1053/j.gastro.2016.02.031
2. What kind of poop do I have? WebMD. January 16, 2020. Accessed September 20, 2021. www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale
3. Ford AC, Moayyedi P, Chey WD, et al; . American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18. doi:10.1038/s41395-018-0084-x
4. Ferreira AI, Garrido M, Castro-Poças F. Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol. 2020;27:255-268. doi:10.1159/000503757
5. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. doi: 10.1038/s41575-020-0286-8
6. Frändemark Å, Törnblom H, Jakobsson S, et al. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem: Am J Gastroenterol. 2018;113:1540-1549. doi:10.1038/s41395-018-0262-x
7. Poulsen CH, Eplov LF, Hjorthøj C, et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand J Public Health. 2019;47:867-875. doi:10.1177/1403494818776168
8. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. doi:10.3748/wjg.v20.i20.6024
9. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773-788. doi:10.1177/2050640617731968
10. Zhu S, Wang B, Jia Q, et al. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a [systematic] review and meta-analysis. BMC Gastroenterology. 2019;19:165. doi:10.1186/s12876-019-1084-z
11. Simrén M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. doi:10.1136/gutjnl-2016-312361
12. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13192. doi:10.1111/nmo.13192
13. Robles A, Ingles DP, Myneedu K, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2019;31:e13718. doi:10.1111/nmo.13718
14. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132-138. doi:10.1016/j.cyto.2017.08.017
15. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036-1048. doi:10.1111/nmo.12358
16. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-3080. doi:10.1017/S0033291716001987
17. Yeh H-W, Chien W-C, Chung C-H, et al. Risk of psychiatric disorders in irritable bowel syndrome—a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. doi:10.1111/ijcp.13212
18. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10:e00012. doi:10.14309/ctg.0000000000000012
19. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-controlled studies. J Acad Nutr Diet. 2020;120:565-586. doi:10.1016/j.jand.2019.05.015
20. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46-58.e7. doi:10.1053/j.gastro.2018.07.011
21. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. doi:10.1053/j.gastro.2016.12.039
22. Heidelbaugh JJ. These 3 tools can help you streamline management of IBS. J Fam Pract. 2017;66:346-353.
23. American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35. doi:10.1038/ajg.2008.122
24. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. doi:10.1038/ajg.2015.6
25. Smalley W, Falck-Ytter C, Carrasco-Labra A, et al. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology. 2019;157:851-854. doi:10.1053/j.gastro.2019.07.004
26. Markham A. Tenapanor: first approval. Drugs. 2019;79:1897-1903. doi:10.1007/s40265-019-01215-9
27. Chey WD, Paré P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217-1225. doi:10.1111/j.1572-0241.2008.01808.x
28. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil. 2019;31:e13461. doi:10.1111/nmo.13461
29. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e12969. doi:10.1111/nmo.12969
30. Zegarac JP. The low-FODMAP diet for IBS: what you need to know. Medscape. August 13, 2019. Accessed September 20, 2021. www.medscape.com/viewarticle/917069
31. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low
32. Su H, Li Y-T, Heitkemper MM, et al. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome: Gastroenterol Nurs. 2019;42:150-158. doi:10.1097/SGA.0000000000000428
33. Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2020;59:2237-2248. doi: 10.1007/s00394-019-02074-6
34. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9:940. doi:10.3390/nu9090940
35. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1002-1010. doi:10.1097/MEG.0000000000000425
36. Wilson B, Rossi M, Dimidi E, et al. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:1098-1111. doi:10.1093/ajcn/nqy376
37. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191-1197. doi:10.1080/03007995.2017.1292230
38. Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:16068. doi:10.1097/MD.0000000000016068
39. Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11:2048. doi:10.3390/nu11092048
40. Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiotics Antimicrob Proteins. 2020;12:834-839. doi:10.1007/s12602-019-09609-7
41. Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. doi:10.1111/apt.15330
42. Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1033-1041. doi:10.1177/2050640619866990
43. Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043-1050. doi:10.14309/ajg.0000000000000198
44. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117-131. doi:10.1016/S2468-1253(19)30324-3
45. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. doi:10.1097/MCG.0b013e3182a88357
46. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi:10.1186/s12906-018-2409-0
47. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21-39. doi: 10.1038/s41395-018-0222-5
48. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113:329-338. doi:10.1038/ajg.2017.495
49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018;155:1753-1763. doi:10.1053/j.gastro.2018.08.021
50. Li F, Fu T, Tong W-D, et al. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2016;91:456-468. doi:10.1016/j.mayocp.2016.01.015
51. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. doi:10.1111/apt.15001
52. Yoon K, Kim N, Lee JY, et al. Clinical response of rifaximin treatment in patients with abdominal bloating. Korean J Gastroenterol. 2018;72:121-127. doi:10.4166/kjg.2018.72.3.121
53. Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. doi:10.1136/gutjnl-2018-318160
54. Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817-830. doi:10.1111/apt.14948
55. Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2017;12:e0172846. doi:10.1371/journal.pone.0172846
56. Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:1-13. doi:https://doi.org/10.1155/2019/2871505
57. Yan J, Miao Z-W, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:1-16. https://doi.org/10.1155/2019/7680963
Irritable bowel syndrome (IBS) continues to pose a diagnostic and therapeutic challenge to clinicians and patients—a challenge that arises from the varying manifestations of the condition, its complex pathophysiology, lack of effective treatment, and psychological consequences for patients. In this article, I explore new findings related to the pathophysiology, diagnosis, and management of IBS subtypes.
Start with the Rome IV classification of IBS
The Rome Foundation published its latest IBS classification and diagnostic criteria (known as Rome IV) in 2016.1 IBS is defined as abdominal pain that (1) has recurred, on average, ≥ 1 time per week during the past 3 months and (2) is associated with ≥ 2 of these criteria1:
- related to defecation
- associated with a change in stool frequency
- associated with a change in the appearance of stool.
Onset of symptoms should be present for 6 months before a diagnosis of IBS is made.1
IBS subtypes—constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unclassified (IBS-U) (TABLE 1)1—are based on the frequency of specific stool forms, as described and illustrated in the Bristol Stool Scale (www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale).2

A widespread, costly, potentially debilitating disorder
IBS affects 10% to 12% of adults worldwide. The condition is more common among women and people younger than 50 years.1,3 Women with IBS tend to have more constipation symptoms (IBS-C); men with IBS, more diarrhea symptoms (IBS-D).4
The financial burden of IBS on the health care system and patients is significant. In a 2013 appraisal of 35 studies, the authors note that estimates of the direct cost of IBS care in the United States vary considerably—from $1562 to $7547 for a patient annually.5
A recent study found that almost 25% of IBS patients report absenteeism from work due to IBS symptoms.6 A Danish study that followed 7278 patients for 5 years found that IBS patients utilized more health care, sick days, and disability pension benefits than non-IBS patients, and had increased utilization of medical resources because of psychiatric conditions.7
Continue to: IBS patients also have comorbidities
IBS patients also have comorbidities:
- More than 20% of IBS patients have functional dyspepsia, gastroesophageal reflux disease, incontinence, or pelvic floor dyssynergia.4
- The frequency of fibromyalgia syndrome in IBS patients is reported to be 20% to 65%.8
- 14% of IBS patients meet criteria for chronic fatigue syndrome.8
- Interstitial cystitis and dyspareunia are common among IBS patients.9
Pathophysiology is complex
Models describing the pathophysiology of IBS have evolved through the years. Recent models describe it as a combination of altered gastrointestinal motility, visceral hyperalgesia, increased intestinal permeability, immune activation, altered intestinal microbiota, and dysfunction in the brain–gut axis. Certain environmental and psychological variables (eg, previous gastroenteritis, food intolerance, chronic stress, diverticulitis, and surgery) increase the risk of IBS.1,10,11
In the past several years, considerable attention has been paid to the roles played by the immune system, brain–gut axis function, and intestinal microbiota in IBS manifestations. Research focus in these areas might assist in the development of specific treatment modalities targeting IBS subtypes.
Immune system. A recent meta-analysis of the records of 706 IBS patients found an increased number of mast cells and CD3 T cells in biopsy specimens from the rectosigmoid and descending colon of IBS patients.12 Another study found a significant increase in mast cells in the ileum of IBS patients13; this increase is evident not only on intestinal biopsy but also at the serologic level. IBS-D patients have a higher plasma interleukin (IL)-6 level than the general population.14 Another meta-analysis found an imbalance in the serum level of tumor necrosis factor-α and IL-10 in IBS patients.15
Brain–gut axis. A 2016 meta-analysis showed that patients with anxiety and depression have a 2-fold increased risk of IBS.16 A more recent study, using data from the National Health Insurance Research Database that included 22,356 patients with IBS, found a 3.6-fold increased risk of psychiatric disorders in IBS.17 These findings reflect the complex interaction between the brain and the intestinal tract in IBS.
Continue to: Intestinal microbiota
Intestinal microbiota. Research evaluating the role of altered intestinal microbiota in IBS has yielded mixed results. A meta-analysis of 777 IBS patients showed an increase in Firmicutes spp, a decrease in Bacteroidetes spp, and an increase in the ratio of Firmicutes spp to Bacteroidetes spp in subjects’ fecal specimens.18 Another study, of 1340 patients, found no difference in Bacteroides spp and Enterococcus spp between healthy controls and IBS patients, but did find (1) lower fecal counts of Lactobacillus spp and Bifidobacterium spp and (2) higher fecal counts of Escherichia coli and Enterobacter spp in IBS patients.19
Postinfectious IBS. The Rome Foundation introduced the diagnosis of postinfectious IBS (PI-IBS) in 2019. PI-IBS develops in 10% of patients who have had infectious enteritis. Female gender, younger age, psychological distress during or before the enteritis episode, and severity of the acute episode are risk factors for this IBS variant.20 A study of 21,421 enteritis patients found that 42% with protozoal or parasitic infection and 14% with bacterial infection developed IBS.
Patients with nonviral enteritis often have a more severe course of enteritis, typically requiring antibiotics. It is believed that the resulting irregularities in the intestinal microbiota make these patients more likely to develop PI-IBS.21 PI-IBS patients are likely to improve or fully recover over time. Symptoms of PI-IBS are managed in a manner similar to how non-PI-IBS patients are managed.20
Challenges in making the IBS diagnosis
Historically, the diagnosis of IBS has been made clinically after excluding red flags (ie, signs or symptoms that might reflect other underlying medical problems) in the clinical presentation. For this reason, obtain a thorough clinical history that includes the course of symptoms, triggers, and alleviating factors. Any of the following are considered red flags1,22,23:
- age > 50 years at onset of symptoms
- new-onset constipation in the elderly
- rectal bleeding
- unexplained weight loss or anemia
- family history of organic gastrointestinal disease
- palpable abdominal or rectal mass
- nocturnal symptoms.
New studies demonstrate that several inflammatory markers can help exclude inflammatory bowel disease from the differential diagnosis in patients in whom IBS is suspected and being investigated.24 In 2019, the American Gastroenterological Association published a clinical practice guideline updating the laboratory evaluation of functional diarrhea and IBS-D in adults,25 and made several recommendations:
- Obtain the level of fecal calprotectin (normal level, ≤ 50 mcg/g) or fecal lactoferrin (≤ 4.0-7.25 mcg/g); if these tests are not available or results are not accessible, the C-reactive protein level is a reasonable option.
- Do not routinely use the erythrocyte sedimentation rate or C-reactive protein level to screen for inflammatory bowel disease.
- Test for Giardia lamblia with an antigen or polymerase chain reaction test.
- Do not test for ova and parasites (other than Giardia) in patients who do not have a history of travel or who have not emigrated from a high-risk area recently.
- Obtain testing for celiac disease with immunoglobulin A (IgA) tissue transglutaminase and with a second test, of immunoglobulin G (IgG) tissue transglutaminase and IgG or IgA deaminated gliadin peptides, to detect celiac disease in IgA-deficient patients.
- Order testing for bile-acid diarrhea with selenium homotaurocholic acid nuclear medicine scanning (if available in your region; the test is available in Europe); measurement of bile acid from a 48-hour stool collection; or an assay of fibroblast growth factor 19, which measures defective feedback of bile-acid synthesis. If these tests are unavailable, consider an empiric trial of a bile-acid binder.
- Do not use available serologic IBS testing.
Continue to: Continue to obtain a...
Continue to obtain a complete blood count for the evaluation of anemia. Endoscopic procedures are indicated in patients with a red flag.1
Treat based on subtype
The first step in the treatment of all IBS patients (TABLE 21,3,4,9,26,27) is for you to develop a strong relationship with the patient: You must acknowledge the disease and empower the patient to manage their symptoms. A strong physician–patient relationship leads to more effective outcomes.4

IBS treatment modalities target abdominal pain, bloating, abdominal distention, and altered bowel function—described in the literature as global symptoms. IBS-M patients should direct their treatment to the predominant symptom (constipation or diarrhea). The following sections describe available treatment options. The FIGURE1,3,4,9,25 shows a treatment workflow based on IBS subtype and symptom severity.

Treatments for all IBS subtypes
Lifestyle modification. Exercise provides overall positive health benefits. With such a variety of exercise forms, however, it is difficult to identify specific exercises that are better for IBS patients.28 A study of 305 IBS patients found that exercise alleviated constipation but not other IBS symptoms, and did not improve quality of life.3 Based on low cost and low risk of adverse effects, exercise should be recommended to all IBS patients.
Dietary restriction therapies have become an area of focus for patients, clinicians, and researchers. Modification of the diet is thought to improve global symptoms and intestinal health through modification of gut microbiota, immune activation, and a decrease in levels of fecal short-chain fatty acids.29
Continue to: The 2 main diets...
The 2 main diets studied for the treatment of IBS are a diet low in fermentable oligo-, di- and monosaccharides and polyols—the so-called low-FODMAP diet (TABLE 330)—and a gluten-free diet. Evidence behind the benefits of both diets conflicts; trials of the low-FODMAP diet are more favorable.

A small study with 20 patients with IBS-D and IBS-M who followed a low-FODMAP diet found improvement in IBS symptoms and a reduction in serum levels of proinflammatory cytokines, fecal bacteria, and total fecal short-chain fatty acid levels.29 Several meta-analyses have shown improvement in overall IBS symptoms for patients who follow a low-FODMAP diet. Because of the heterogeneity of the studies, however, the quality of the data is low.31-34
Data supporting the use of a gluten-free diet for IBS patients are insufficient.31
The American College of Gastroenterology (ACG) gave a weak recommendation for the low-FODMAP diet and recommended against the gluten-free diet in IBS patients.3 More data are needed regarding the safety profile of using a low-FODMAP diet for an extended period: There is concern about the risk of nutritional deficiencies associated with long-term use of this diet.3
Supplementation with poorly fermentable soluble fiber has been shown to alleviate global IBS symptoms; insoluble fiber does not yield improvement of symptoms. Psyllium fiber is recommended over wheat bran.3,35
Continue to: Consider a low-FODMAP diet...
Consider a low-FODMAP diet and soluble fiber as initial treatment for all IBS patients.
Modification of intestinal microbiota. Understanding the difference between prebiotics and probiotics is important when considering treatment for IBS. Prebiotics are foods or dietary supplements that generate changes in the composition and activity of intestinal microbiota. Probiotics are live microorganisms that can improve intestinal health.3
A meta-analysis of 729 IBS patients found that prebiotics do not reduce gastrointestinal symptoms or improve the quality of life of IBS patients.36 Evidence supporting the benefit of probiotics is favorable; however, data in these studies have significant heterogeneity. Several meta-analyses studied the benefits of Lactobacillus spp and Bifidobacterium spp in alleviating IBS symptoms. The studies found improvement in abdominal pain, bloating and distention, and flatulence.3,37-40 Consider recommending probiotics for all IBS patients; for some, however, the high cost of some of these products might be an obstacle.
Researchers are also studying the use of fecal microbiota transplantation (FMT) to treat IBS. Studies have evaluated the delivery of FMT orally (as capsules) and endoscopically. Evidence does not show improvement in global IBS symptoms with FMT. More studies, with larger sample populations, are needed.41-43
Antispasmodic medications and peppermint oil. Antispasmodic medications have been considered a mainstay therapy for IBS because of their effect on intestinal dysmotility. Hyoscine and dicyclomine are commonly used. Meta-analyses have shown improvement in global symptoms and abdominal pain, but effects were modest.3,44 Use this class of drugs as first-line treatment for mild IBS symptoms.
Continue to: Peppermint oil has been...
Peppermint oil has been found useful in improving IBS global symptoms and abdominal pain in several studies.44-46 A common adverse effect of peppermint oil is heartburn, resulting from relaxation of esophageal muscle.3 Peppermint oil can be considered an adjuvant agent in treating IBS.
Antidepressants. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been studied for the treatment of IBS. Meta-analyses show that both are effective in reducing pain and overall IBS symptoms.1,3,47 The number needed to treat (NNT) for TCAs is 4.5; for SSRIs, 5.47 Data do not show that either drug class is superior to the other for IBS. Based on the adverse effect profile, TCAs are more suitable for IBS-D patients; SSRIs are better for IBS-C patients.47
New data show that serotonin-norepinephrine reuptake inhibitors, such as duloxetine and milnacipran, can alleviate IBS symptoms through their pain-modifying properties.47
Based on the adverse effect profile and stigma associated with antidepressant medications, patients might be less likely to take them for IBS symptoms than for these drugs’ primary indications. Clinicians should still consider this drug class if other first-line treatments do not provide full resolution of symptoms.
Psychotherapy. Several psychotherapeutic modalities have been evaluated for efficacy in reducing global IBS symptoms. The approaches studied most often were provider-directed cognitive behavioral therapy, relaxation therapy, hypnotherapy, and multicomponent psychological therapy. The NNT for these modalities is 4, but studies had significant heterogeneity.3 Consider referring patients for psychotherapeutic intervention if they have not responded to medical therapy after 12 months.4
Continue to Treatment of IBS-C
Treatment of IBS-C
Prosecretory agents. Linaclotide and plecanatide are amino-acid peptides that act as a guanylate cyclase C agonist. Both increase gastrointestinal transit rate by increasing electrolyte and fluid transport into the intestinal lumen. They also decrease the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3'5'-monophosphate levels.3,48 In a recent meta-analysis, both treatments produced improvement in global symptoms. However, linaclotide showed superior improvement in abdominal pain and global symptoms compared to other secretory agents.48,49 Diarrhea was the most common adverse effect of linaclotide and plecanatide, although less so with plecanatide.49
Lubiprostone activates the intestinal chloride channel type 2 on the small intestine, leading to an increase in chloride and water efflux into the intestinal lumen, in turn accelerating gastrointestinal transit.3 A meta-analysis with 1468 IBS patients found that lubiprostone improved constipation, stool consistency, abdominal pain, degree of straining, and abdominal bloating.50 Diarrhea and nausea are commonly reported adverse effects of lubiprostone.49,50
Linaclotide, plecanatide, and lubiprostone should be considered first-line therapies for patients with IBS-C. High cost is still a roadblock to the use of these agents.
The US Food and Drug Administration (FDA) approved tenapanor in September 2019; however, the drug is not commercially available in the United States (it is available in Canada). Tenapanor is a sodium–hydrogen exchanger 3 inhibitor that reduces sodium absorption from the intestine and colon. The drug increases water secretion into the intestinal lumen, thus accelerating gut transit time. It also inhibits active absorption of phosphate in the intestine.
Tenapanor was approved for treating both IBS-C and hyperphosphatemia in patients with chronic kidney disease on dialysis or end-stage renal disease.26 In a recent meta-analysis, the drug showed benefit in alleviating global IBS symptoms, and ranked first in reducing bloating.49 It is too soon to know if tenapanor will perform clinically better than other prosecretory agents.
Continue to: Serotonergic agents
Serotonergic agents. Serotonin (5-hydroxytryptamine [5-HT]) modulates gastrointestinal secretions, gut motility, and visceral sensation. Researchers have developed IBS treatments that target receptors involved in these functions.
Tegaserod is a partial, selective 5-HT4 agonist indicated for the treatment of IBS-C in women. A study with 661 women with IBS-M and IBS-C showed that tegaserod increased the number of bowel movement episodes. Patients also reported higher stool consistency scores and fewer days with straining compared to placebo.27 The medication was removed from the market in 2007 because of its potential for cardiovascular adverse effects3; however, it was reintroduced in 2019 for women < 65 years of age with IBS-C. Consider prescribing tegaserod if other treatment options fail to alleviate symptoms.
Treatment of IBS-D
Antibiotics. The nonabsorbable antibiotic rifaximin is approved by the FDA for IBS-D at a dosage of 550 mg tid for 2 weeks.1 Several studies show improvement in IBS global symptoms with the recommended treatment course51-53; benefit persisted for the 10-week follow-up study period.1 A meta-analysis found that the NNT for rifaximin is 8-11.54 Preliminary data indicate that the rates of Clostridioides difficile infection and microbial resistance among rifaximin users are low.3 Consider using rifaximin as a first-line treatment option for patients with IBS-D. Retreatment might be necessary because the drug’s effect gradually disappears.9
Antidiarrheals. Eluxadoline is a µ-opioid and κ-opioid receptor agonist and δ-opioid receptor antagonist with effects on the intestinal nervous system.3 Several meta-analyses demonstrated that eluxadoline improves abdominal pain scores and daily stool consistency in IBS-D patients.53,54 Eluxadoline should be considered early in the management of IBS-D patients. The most common adverse effect is constipation.
The FDA issued a safety warning in 2017 regarding an increased risk of pancreatitis in patients taking eluxadoline who do not have a gallbladder. In addition, eluxadoline should be avoided in patients with a history of sphincter of Oddi dysfunction, alcohol abuse, or severe liver problems.3,54
Continue to: The high cost of...
The high cost of eluxadoline can be a significant barrier to use.
Serotonergic agents. Alosetron is a selective
Ondansetron has also been used to treat IBS-D. In a meta-analysis with 294 patients, ondansetron showed improvement in stool consistency.55 Ondansetron does not improve abdominal pain.4 It can be used in patients who have mild-to-moderate symptoms.9 Ondansetron is not FDA approved for the treatment of IBS-D.
Bile-acid sequestrants. Traditionally, bile-acid sequestrants have been used to treat bile-acid diarrhea. A meta-analysis of 6 studies of 908 patients with IBS-D found that 28.1% were affected by bile-acid malabsorption. Two small studies that evaluated the benefits of colesevelam for IBS-D found significant improvement in stool consistency.54 Another study, which evaluated the benefits of cholestyramine, found improvement in stool consistency, but findings were not significant.54 Many patients taking a bile-acid sequestrant stop taking the medication because of considerable adverse effects (constipation, nausea, bloating, flatulence, and abdominal pain).54 For that reason, this class of medication is not recommended as first-line treatment for IBS-D and is not FDA approved for IBS-D.
SIDEBAR
KEY POINTS The challenge of, and a needed framework for, managing IBS
- IBS is a complex, chronic condition affecting a considerable number of people worldwide.
- Because of the substantial disease burden associated with IBS, patients are at higher risk of mental health disorders.
- Physicians who care for IBS patients must build a strong physician–patient relationship; their mutual trust will ensure development of an effective treatment plan.
- Family physicians and other primary care providers are equipped to help IBS patients navigate the complex health care system and the IBS disease process. They can help coordinate care with specialists and behavioral health clinicians, which will help patients improve quality of life and manage symptoms appropriately.
A role for complementaryand integrative medicine?
Recently, complementary and integrative modalities for treating IBS have sparked the interest of researchers.
Continue to: Acupuncture
Acupuncture. In a meta-analysis with 3440 patients, acupuncture was more effective than Western medicine in alleviating IBS symptoms for as long as 3 months. The authors concluded that acupuncture could be used in combination with other therapies to reduce the severity of IBS symptoms.56
Concomitant acupuncture and Chinese herbal medicine. In a systematic review and meta-analysis comprising 21 randomized controlled trials, researchers reported that acupuncture combined with Chinese herbal medicine improved IBS symptoms, compared to what was noted in matched controls who were treated with Western medicine or with Western medicine combined with Chinese herbal medicine. The authors were cautious about the results of the meta-analysis, however, because the studies examined were small and of low quality, and presented a high risk of bias.57
Agents not to be used routinely for IBS
Loperamide. This peripheral µ-opioid receptor agonist controls diarrhea. However, recent studies showed no significant benefit to loperamide over placebo in IBS-M and IBS-D. In 2018, the FDA issued a safety alert regarding an elevated risk of serious cardiac adverse effects in patients taking loperamide. The ACG recommends against using loperamide to treat IBS symptoms.3,54
Polyethylene glycol. An osmotic laxative that is not absorbed in the intestinal lumen, polyethylene glycol is highly efficacious for alleviating constipation, but it does not reduce pain or other IBS symptoms. For that reason, the ACG recommends against its use.3
CORRESPONDENCE
Jose M. Villalon-Gomez, MD, MPH, Emory Healthcare Family Medicine, 4500 North Shallowford Road, Dunwoody, GA 30338; [email protected]
Irritable bowel syndrome (IBS) continues to pose a diagnostic and therapeutic challenge to clinicians and patients—a challenge that arises from the varying manifestations of the condition, its complex pathophysiology, lack of effective treatment, and psychological consequences for patients. In this article, I explore new findings related to the pathophysiology, diagnosis, and management of IBS subtypes.
Start with the Rome IV classification of IBS
The Rome Foundation published its latest IBS classification and diagnostic criteria (known as Rome IV) in 2016.1 IBS is defined as abdominal pain that (1) has recurred, on average, ≥ 1 time per week during the past 3 months and (2) is associated with ≥ 2 of these criteria1:
- related to defecation
- associated with a change in stool frequency
- associated with a change in the appearance of stool.
Onset of symptoms should be present for 6 months before a diagnosis of IBS is made.1
IBS subtypes—constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed (IBS-M), and unclassified (IBS-U) (TABLE 1)1—are based on the frequency of specific stool forms, as described and illustrated in the Bristol Stool Scale (www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale).2

A widespread, costly, potentially debilitating disorder
IBS affects 10% to 12% of adults worldwide. The condition is more common among women and people younger than 50 years.1,3 Women with IBS tend to have more constipation symptoms (IBS-C); men with IBS, more diarrhea symptoms (IBS-D).4
The financial burden of IBS on the health care system and patients is significant. In a 2013 appraisal of 35 studies, the authors note that estimates of the direct cost of IBS care in the United States vary considerably—from $1562 to $7547 for a patient annually.5
A recent study found that almost 25% of IBS patients report absenteeism from work due to IBS symptoms.6 A Danish study that followed 7278 patients for 5 years found that IBS patients utilized more health care, sick days, and disability pension benefits than non-IBS patients, and had increased utilization of medical resources because of psychiatric conditions.7
Continue to: IBS patients also have comorbidities
IBS patients also have comorbidities:
- More than 20% of IBS patients have functional dyspepsia, gastroesophageal reflux disease, incontinence, or pelvic floor dyssynergia.4
- The frequency of fibromyalgia syndrome in IBS patients is reported to be 20% to 65%.8
- 14% of IBS patients meet criteria for chronic fatigue syndrome.8
- Interstitial cystitis and dyspareunia are common among IBS patients.9
Pathophysiology is complex
Models describing the pathophysiology of IBS have evolved through the years. Recent models describe it as a combination of altered gastrointestinal motility, visceral hyperalgesia, increased intestinal permeability, immune activation, altered intestinal microbiota, and dysfunction in the brain–gut axis. Certain environmental and psychological variables (eg, previous gastroenteritis, food intolerance, chronic stress, diverticulitis, and surgery) increase the risk of IBS.1,10,11
In the past several years, considerable attention has been paid to the roles played by the immune system, brain–gut axis function, and intestinal microbiota in IBS manifestations. Research focus in these areas might assist in the development of specific treatment modalities targeting IBS subtypes.
Immune system. A recent meta-analysis of the records of 706 IBS patients found an increased number of mast cells and CD3 T cells in biopsy specimens from the rectosigmoid and descending colon of IBS patients.12 Another study found a significant increase in mast cells in the ileum of IBS patients13; this increase is evident not only on intestinal biopsy but also at the serologic level. IBS-D patients have a higher plasma interleukin (IL)-6 level than the general population.14 Another meta-analysis found an imbalance in the serum level of tumor necrosis factor-α and IL-10 in IBS patients.15
Brain–gut axis. A 2016 meta-analysis showed that patients with anxiety and depression have a 2-fold increased risk of IBS.16 A more recent study, using data from the National Health Insurance Research Database that included 22,356 patients with IBS, found a 3.6-fold increased risk of psychiatric disorders in IBS.17 These findings reflect the complex interaction between the brain and the intestinal tract in IBS.
Continue to: Intestinal microbiota
Intestinal microbiota. Research evaluating the role of altered intestinal microbiota in IBS has yielded mixed results. A meta-analysis of 777 IBS patients showed an increase in Firmicutes spp, a decrease in Bacteroidetes spp, and an increase in the ratio of Firmicutes spp to Bacteroidetes spp in subjects’ fecal specimens.18 Another study, of 1340 patients, found no difference in Bacteroides spp and Enterococcus spp between healthy controls and IBS patients, but did find (1) lower fecal counts of Lactobacillus spp and Bifidobacterium spp and (2) higher fecal counts of Escherichia coli and Enterobacter spp in IBS patients.19
Postinfectious IBS. The Rome Foundation introduced the diagnosis of postinfectious IBS (PI-IBS) in 2019. PI-IBS develops in 10% of patients who have had infectious enteritis. Female gender, younger age, psychological distress during or before the enteritis episode, and severity of the acute episode are risk factors for this IBS variant.20 A study of 21,421 enteritis patients found that 42% with protozoal or parasitic infection and 14% with bacterial infection developed IBS.
Patients with nonviral enteritis often have a more severe course of enteritis, typically requiring antibiotics. It is believed that the resulting irregularities in the intestinal microbiota make these patients more likely to develop PI-IBS.21 PI-IBS patients are likely to improve or fully recover over time. Symptoms of PI-IBS are managed in a manner similar to how non-PI-IBS patients are managed.20
Challenges in making the IBS diagnosis
Historically, the diagnosis of IBS has been made clinically after excluding red flags (ie, signs or symptoms that might reflect other underlying medical problems) in the clinical presentation. For this reason, obtain a thorough clinical history that includes the course of symptoms, triggers, and alleviating factors. Any of the following are considered red flags1,22,23:
- age > 50 years at onset of symptoms
- new-onset constipation in the elderly
- rectal bleeding
- unexplained weight loss or anemia
- family history of organic gastrointestinal disease
- palpable abdominal or rectal mass
- nocturnal symptoms.
New studies demonstrate that several inflammatory markers can help exclude inflammatory bowel disease from the differential diagnosis in patients in whom IBS is suspected and being investigated.24 In 2019, the American Gastroenterological Association published a clinical practice guideline updating the laboratory evaluation of functional diarrhea and IBS-D in adults,25 and made several recommendations:
- Obtain the level of fecal calprotectin (normal level, ≤ 50 mcg/g) or fecal lactoferrin (≤ 4.0-7.25 mcg/g); if these tests are not available or results are not accessible, the C-reactive protein level is a reasonable option.
- Do not routinely use the erythrocyte sedimentation rate or C-reactive protein level to screen for inflammatory bowel disease.
- Test for Giardia lamblia with an antigen or polymerase chain reaction test.
- Do not test for ova and parasites (other than Giardia) in patients who do not have a history of travel or who have not emigrated from a high-risk area recently.
- Obtain testing for celiac disease with immunoglobulin A (IgA) tissue transglutaminase and with a second test, of immunoglobulin G (IgG) tissue transglutaminase and IgG or IgA deaminated gliadin peptides, to detect celiac disease in IgA-deficient patients.
- Order testing for bile-acid diarrhea with selenium homotaurocholic acid nuclear medicine scanning (if available in your region; the test is available in Europe); measurement of bile acid from a 48-hour stool collection; or an assay of fibroblast growth factor 19, which measures defective feedback of bile-acid synthesis. If these tests are unavailable, consider an empiric trial of a bile-acid binder.
- Do not use available serologic IBS testing.
Continue to: Continue to obtain a...
Continue to obtain a complete blood count for the evaluation of anemia. Endoscopic procedures are indicated in patients with a red flag.1
Treat based on subtype
The first step in the treatment of all IBS patients (TABLE 21,3,4,9,26,27) is for you to develop a strong relationship with the patient: You must acknowledge the disease and empower the patient to manage their symptoms. A strong physician–patient relationship leads to more effective outcomes.4

IBS treatment modalities target abdominal pain, bloating, abdominal distention, and altered bowel function—described in the literature as global symptoms. IBS-M patients should direct their treatment to the predominant symptom (constipation or diarrhea). The following sections describe available treatment options. The FIGURE1,3,4,9,25 shows a treatment workflow based on IBS subtype and symptom severity.

Treatments for all IBS subtypes
Lifestyle modification. Exercise provides overall positive health benefits. With such a variety of exercise forms, however, it is difficult to identify specific exercises that are better for IBS patients.28 A study of 305 IBS patients found that exercise alleviated constipation but not other IBS symptoms, and did not improve quality of life.3 Based on low cost and low risk of adverse effects, exercise should be recommended to all IBS patients.
Dietary restriction therapies have become an area of focus for patients, clinicians, and researchers. Modification of the diet is thought to improve global symptoms and intestinal health through modification of gut microbiota, immune activation, and a decrease in levels of fecal short-chain fatty acids.29
Continue to: The 2 main diets...
The 2 main diets studied for the treatment of IBS are a diet low in fermentable oligo-, di- and monosaccharides and polyols—the so-called low-FODMAP diet (TABLE 330)—and a gluten-free diet. Evidence behind the benefits of both diets conflicts; trials of the low-FODMAP diet are more favorable.

A small study with 20 patients with IBS-D and IBS-M who followed a low-FODMAP diet found improvement in IBS symptoms and a reduction in serum levels of proinflammatory cytokines, fecal bacteria, and total fecal short-chain fatty acid levels.29 Several meta-analyses have shown improvement in overall IBS symptoms for patients who follow a low-FODMAP diet. Because of the heterogeneity of the studies, however, the quality of the data is low.31-34
Data supporting the use of a gluten-free diet for IBS patients are insufficient.31
The American College of Gastroenterology (ACG) gave a weak recommendation for the low-FODMAP diet and recommended against the gluten-free diet in IBS patients.3 More data are needed regarding the safety profile of using a low-FODMAP diet for an extended period: There is concern about the risk of nutritional deficiencies associated with long-term use of this diet.3
Supplementation with poorly fermentable soluble fiber has been shown to alleviate global IBS symptoms; insoluble fiber does not yield improvement of symptoms. Psyllium fiber is recommended over wheat bran.3,35
Continue to: Consider a low-FODMAP diet...
Consider a low-FODMAP diet and soluble fiber as initial treatment for all IBS patients.
Modification of intestinal microbiota. Understanding the difference between prebiotics and probiotics is important when considering treatment for IBS. Prebiotics are foods or dietary supplements that generate changes in the composition and activity of intestinal microbiota. Probiotics are live microorganisms that can improve intestinal health.3
A meta-analysis of 729 IBS patients found that prebiotics do not reduce gastrointestinal symptoms or improve the quality of life of IBS patients.36 Evidence supporting the benefit of probiotics is favorable; however, data in these studies have significant heterogeneity. Several meta-analyses studied the benefits of Lactobacillus spp and Bifidobacterium spp in alleviating IBS symptoms. The studies found improvement in abdominal pain, bloating and distention, and flatulence.3,37-40 Consider recommending probiotics for all IBS patients; for some, however, the high cost of some of these products might be an obstacle.
Researchers are also studying the use of fecal microbiota transplantation (FMT) to treat IBS. Studies have evaluated the delivery of FMT orally (as capsules) and endoscopically. Evidence does not show improvement in global IBS symptoms with FMT. More studies, with larger sample populations, are needed.41-43
Antispasmodic medications and peppermint oil. Antispasmodic medications have been considered a mainstay therapy for IBS because of their effect on intestinal dysmotility. Hyoscine and dicyclomine are commonly used. Meta-analyses have shown improvement in global symptoms and abdominal pain, but effects were modest.3,44 Use this class of drugs as first-line treatment for mild IBS symptoms.
Continue to: Peppermint oil has been...
Peppermint oil has been found useful in improving IBS global symptoms and abdominal pain in several studies.44-46 A common adverse effect of peppermint oil is heartburn, resulting from relaxation of esophageal muscle.3 Peppermint oil can be considered an adjuvant agent in treating IBS.
Antidepressants. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have been studied for the treatment of IBS. Meta-analyses show that both are effective in reducing pain and overall IBS symptoms.1,3,47 The number needed to treat (NNT) for TCAs is 4.5; for SSRIs, 5.47 Data do not show that either drug class is superior to the other for IBS. Based on the adverse effect profile, TCAs are more suitable for IBS-D patients; SSRIs are better for IBS-C patients.47
New data show that serotonin-norepinephrine reuptake inhibitors, such as duloxetine and milnacipran, can alleviate IBS symptoms through their pain-modifying properties.47
Based on the adverse effect profile and stigma associated with antidepressant medications, patients might be less likely to take them for IBS symptoms than for these drugs’ primary indications. Clinicians should still consider this drug class if other first-line treatments do not provide full resolution of symptoms.
Psychotherapy. Several psychotherapeutic modalities have been evaluated for efficacy in reducing global IBS symptoms. The approaches studied most often were provider-directed cognitive behavioral therapy, relaxation therapy, hypnotherapy, and multicomponent psychological therapy. The NNT for these modalities is 4, but studies had significant heterogeneity.3 Consider referring patients for psychotherapeutic intervention if they have not responded to medical therapy after 12 months.4
Continue to Treatment of IBS-C
Treatment of IBS-C
Prosecretory agents. Linaclotide and plecanatide are amino-acid peptides that act as a guanylate cyclase C agonist. Both increase gastrointestinal transit rate by increasing electrolyte and fluid transport into the intestinal lumen. They also decrease the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3'5'-monophosphate levels.3,48 In a recent meta-analysis, both treatments produced improvement in global symptoms. However, linaclotide showed superior improvement in abdominal pain and global symptoms compared to other secretory agents.48,49 Diarrhea was the most common adverse effect of linaclotide and plecanatide, although less so with plecanatide.49
Lubiprostone activates the intestinal chloride channel type 2 on the small intestine, leading to an increase in chloride and water efflux into the intestinal lumen, in turn accelerating gastrointestinal transit.3 A meta-analysis with 1468 IBS patients found that lubiprostone improved constipation, stool consistency, abdominal pain, degree of straining, and abdominal bloating.50 Diarrhea and nausea are commonly reported adverse effects of lubiprostone.49,50
Linaclotide, plecanatide, and lubiprostone should be considered first-line therapies for patients with IBS-C. High cost is still a roadblock to the use of these agents.
The US Food and Drug Administration (FDA) approved tenapanor in September 2019; however, the drug is not commercially available in the United States (it is available in Canada). Tenapanor is a sodium–hydrogen exchanger 3 inhibitor that reduces sodium absorption from the intestine and colon. The drug increases water secretion into the intestinal lumen, thus accelerating gut transit time. It also inhibits active absorption of phosphate in the intestine.
Tenapanor was approved for treating both IBS-C and hyperphosphatemia in patients with chronic kidney disease on dialysis or end-stage renal disease.26 In a recent meta-analysis, the drug showed benefit in alleviating global IBS symptoms, and ranked first in reducing bloating.49 It is too soon to know if tenapanor will perform clinically better than other prosecretory agents.
Continue to: Serotonergic agents
Serotonergic agents. Serotonin (5-hydroxytryptamine [5-HT]) modulates gastrointestinal secretions, gut motility, and visceral sensation. Researchers have developed IBS treatments that target receptors involved in these functions.
Tegaserod is a partial, selective 5-HT4 agonist indicated for the treatment of IBS-C in women. A study with 661 women with IBS-M and IBS-C showed that tegaserod increased the number of bowel movement episodes. Patients also reported higher stool consistency scores and fewer days with straining compared to placebo.27 The medication was removed from the market in 2007 because of its potential for cardiovascular adverse effects3; however, it was reintroduced in 2019 for women < 65 years of age with IBS-C. Consider prescribing tegaserod if other treatment options fail to alleviate symptoms.
Treatment of IBS-D
Antibiotics. The nonabsorbable antibiotic rifaximin is approved by the FDA for IBS-D at a dosage of 550 mg tid for 2 weeks.1 Several studies show improvement in IBS global symptoms with the recommended treatment course51-53; benefit persisted for the 10-week follow-up study period.1 A meta-analysis found that the NNT for rifaximin is 8-11.54 Preliminary data indicate that the rates of Clostridioides difficile infection and microbial resistance among rifaximin users are low.3 Consider using rifaximin as a first-line treatment option for patients with IBS-D. Retreatment might be necessary because the drug’s effect gradually disappears.9
Antidiarrheals. Eluxadoline is a µ-opioid and κ-opioid receptor agonist and δ-opioid receptor antagonist with effects on the intestinal nervous system.3 Several meta-analyses demonstrated that eluxadoline improves abdominal pain scores and daily stool consistency in IBS-D patients.53,54 Eluxadoline should be considered early in the management of IBS-D patients. The most common adverse effect is constipation.
The FDA issued a safety warning in 2017 regarding an increased risk of pancreatitis in patients taking eluxadoline who do not have a gallbladder. In addition, eluxadoline should be avoided in patients with a history of sphincter of Oddi dysfunction, alcohol abuse, or severe liver problems.3,54
Continue to: The high cost of...
The high cost of eluxadoline can be a significant barrier to use.
Serotonergic agents. Alosetron is a selective
Ondansetron has also been used to treat IBS-D. In a meta-analysis with 294 patients, ondansetron showed improvement in stool consistency.55 Ondansetron does not improve abdominal pain.4 It can be used in patients who have mild-to-moderate symptoms.9 Ondansetron is not FDA approved for the treatment of IBS-D.
Bile-acid sequestrants. Traditionally, bile-acid sequestrants have been used to treat bile-acid diarrhea. A meta-analysis of 6 studies of 908 patients with IBS-D found that 28.1% were affected by bile-acid malabsorption. Two small studies that evaluated the benefits of colesevelam for IBS-D found significant improvement in stool consistency.54 Another study, which evaluated the benefits of cholestyramine, found improvement in stool consistency, but findings were not significant.54 Many patients taking a bile-acid sequestrant stop taking the medication because of considerable adverse effects (constipation, nausea, bloating, flatulence, and abdominal pain).54 For that reason, this class of medication is not recommended as first-line treatment for IBS-D and is not FDA approved for IBS-D.
SIDEBAR
KEY POINTS The challenge of, and a needed framework for, managing IBS
- IBS is a complex, chronic condition affecting a considerable number of people worldwide.
- Because of the substantial disease burden associated with IBS, patients are at higher risk of mental health disorders.
- Physicians who care for IBS patients must build a strong physician–patient relationship; their mutual trust will ensure development of an effective treatment plan.
- Family physicians and other primary care providers are equipped to help IBS patients navigate the complex health care system and the IBS disease process. They can help coordinate care with specialists and behavioral health clinicians, which will help patients improve quality of life and manage symptoms appropriately.
A role for complementaryand integrative medicine?
Recently, complementary and integrative modalities for treating IBS have sparked the interest of researchers.
Continue to: Acupuncture
Acupuncture. In a meta-analysis with 3440 patients, acupuncture was more effective than Western medicine in alleviating IBS symptoms for as long as 3 months. The authors concluded that acupuncture could be used in combination with other therapies to reduce the severity of IBS symptoms.56
Concomitant acupuncture and Chinese herbal medicine. In a systematic review and meta-analysis comprising 21 randomized controlled trials, researchers reported that acupuncture combined with Chinese herbal medicine improved IBS symptoms, compared to what was noted in matched controls who were treated with Western medicine or with Western medicine combined with Chinese herbal medicine. The authors were cautious about the results of the meta-analysis, however, because the studies examined were small and of low quality, and presented a high risk of bias.57
Agents not to be used routinely for IBS
Loperamide. This peripheral µ-opioid receptor agonist controls diarrhea. However, recent studies showed no significant benefit to loperamide over placebo in IBS-M and IBS-D. In 2018, the FDA issued a safety alert regarding an elevated risk of serious cardiac adverse effects in patients taking loperamide. The ACG recommends against using loperamide to treat IBS symptoms.3,54
Polyethylene glycol. An osmotic laxative that is not absorbed in the intestinal lumen, polyethylene glycol is highly efficacious for alleviating constipation, but it does not reduce pain or other IBS symptoms. For that reason, the ACG recommends against its use.3
CORRESPONDENCE
Jose M. Villalon-Gomez, MD, MPH, Emory Healthcare Family Medicine, 4500 North Shallowford Road, Dunwoody, GA 30338; [email protected]
1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5. doi:10.1053/j.gastro.2016.02.031
2. What kind of poop do I have? WebMD. January 16, 2020. Accessed September 20, 2021. www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale
3. Ford AC, Moayyedi P, Chey WD, et al; . American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18. doi:10.1038/s41395-018-0084-x
4. Ferreira AI, Garrido M, Castro-Poças F. Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol. 2020;27:255-268. doi:10.1159/000503757
5. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. doi: 10.1038/s41575-020-0286-8
6. Frändemark Å, Törnblom H, Jakobsson S, et al. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem: Am J Gastroenterol. 2018;113:1540-1549. doi:10.1038/s41395-018-0262-x
7. Poulsen CH, Eplov LF, Hjorthøj C, et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand J Public Health. 2019;47:867-875. doi:10.1177/1403494818776168
8. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. doi:10.3748/wjg.v20.i20.6024
9. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773-788. doi:10.1177/2050640617731968
10. Zhu S, Wang B, Jia Q, et al. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a [systematic] review and meta-analysis. BMC Gastroenterology. 2019;19:165. doi:10.1186/s12876-019-1084-z
11. Simrén M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. doi:10.1136/gutjnl-2016-312361
12. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13192. doi:10.1111/nmo.13192
13. Robles A, Ingles DP, Myneedu K, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2019;31:e13718. doi:10.1111/nmo.13718
14. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132-138. doi:10.1016/j.cyto.2017.08.017
15. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036-1048. doi:10.1111/nmo.12358
16. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-3080. doi:10.1017/S0033291716001987
17. Yeh H-W, Chien W-C, Chung C-H, et al. Risk of psychiatric disorders in irritable bowel syndrome—a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. doi:10.1111/ijcp.13212
18. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10:e00012. doi:10.14309/ctg.0000000000000012
19. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-controlled studies. J Acad Nutr Diet. 2020;120:565-586. doi:10.1016/j.jand.2019.05.015
20. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46-58.e7. doi:10.1053/j.gastro.2018.07.011
21. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. doi:10.1053/j.gastro.2016.12.039
22. Heidelbaugh JJ. These 3 tools can help you streamline management of IBS. J Fam Pract. 2017;66:346-353.
23. American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35. doi:10.1038/ajg.2008.122
24. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. doi:10.1038/ajg.2015.6
25. Smalley W, Falck-Ytter C, Carrasco-Labra A, et al. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology. 2019;157:851-854. doi:10.1053/j.gastro.2019.07.004
26. Markham A. Tenapanor: first approval. Drugs. 2019;79:1897-1903. doi:10.1007/s40265-019-01215-9
27. Chey WD, Paré P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217-1225. doi:10.1111/j.1572-0241.2008.01808.x
28. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil. 2019;31:e13461. doi:10.1111/nmo.13461
29. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e12969. doi:10.1111/nmo.12969
30. Zegarac JP. The low-FODMAP diet for IBS: what you need to know. Medscape. August 13, 2019. Accessed September 20, 2021. www.medscape.com/viewarticle/917069
31. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low
32. Su H, Li Y-T, Heitkemper MM, et al. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome: Gastroenterol Nurs. 2019;42:150-158. doi:10.1097/SGA.0000000000000428
33. Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2020;59:2237-2248. doi: 10.1007/s00394-019-02074-6
34. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9:940. doi:10.3390/nu9090940
35. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1002-1010. doi:10.1097/MEG.0000000000000425
36. Wilson B, Rossi M, Dimidi E, et al. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:1098-1111. doi:10.1093/ajcn/nqy376
37. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191-1197. doi:10.1080/03007995.2017.1292230
38. Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:16068. doi:10.1097/MD.0000000000016068
39. Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11:2048. doi:10.3390/nu11092048
40. Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiotics Antimicrob Proteins. 2020;12:834-839. doi:10.1007/s12602-019-09609-7
41. Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. doi:10.1111/apt.15330
42. Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1033-1041. doi:10.1177/2050640619866990
43. Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043-1050. doi:10.14309/ajg.0000000000000198
44. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117-131. doi:10.1016/S2468-1253(19)30324-3
45. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. doi:10.1097/MCG.0b013e3182a88357
46. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi:10.1186/s12906-018-2409-0
47. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21-39. doi: 10.1038/s41395-018-0222-5
48. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113:329-338. doi:10.1038/ajg.2017.495
49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018;155:1753-1763. doi:10.1053/j.gastro.2018.08.021
50. Li F, Fu T, Tong W-D, et al. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2016;91:456-468. doi:10.1016/j.mayocp.2016.01.015
51. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. doi:10.1111/apt.15001
52. Yoon K, Kim N, Lee JY, et al. Clinical response of rifaximin treatment in patients with abdominal bloating. Korean J Gastroenterol. 2018;72:121-127. doi:10.4166/kjg.2018.72.3.121
53. Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. doi:10.1136/gutjnl-2018-318160
54. Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817-830. doi:10.1111/apt.14948
55. Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2017;12:e0172846. doi:10.1371/journal.pone.0172846
56. Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:1-13. doi:https://doi.org/10.1155/2019/2871505
57. Yan J, Miao Z-W, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:1-16. https://doi.org/10.1155/2019/7680963
1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.e5. doi:10.1053/j.gastro.2016.02.031
2. What kind of poop do I have? WebMD. January 16, 2020. Accessed September 20, 2021. www.webmd.com/digestive-disorders/poop-chart-bristol-stool-scale
3. Ford AC, Moayyedi P, Chey WD, et al; . American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18. doi:10.1038/s41395-018-0084-x
4. Ferreira AI, Garrido M, Castro-Poças F. Irritable bowel syndrome: news from an old disorder. GE Port J Gastroenterol. 2020;27:255-268. doi:10.1159/000503757
5. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. doi: 10.1038/s41575-020-0286-8
6. Frändemark Å, Törnblom H, Jakobsson S, et al. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem: Am J Gastroenterol. 2018;113:1540-1549. doi:10.1038/s41395-018-0262-x
7. Poulsen CH, Eplov LF, Hjorthøj C, et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand J Public Health. 2019;47:867-875. doi:10.1177/1403494818776168
8. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20:6024-6030. doi:10.3748/wjg.v20.i20.6024
9. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5:773-788. doi:10.1177/2050640617731968
10. Zhu S, Wang B, Jia Q, et al. Candidate single nucleotide polymorphisms of irritable bowel syndrome: a [systematic] review and meta-analysis. BMC Gastroenterology. 2019;19:165. doi:10.1186/s12876-019-1084-z
11. Simrén M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. doi:10.1136/gutjnl-2016-312361
12. Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13192. doi:10.1111/nmo.13192
13. Robles A, Ingles DP, Myneedu K, et al. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2019;31:e13718. doi:10.1111/nmo.13718
14. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: A systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132-138. doi:10.1016/j.cyto.2017.08.017
15. Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26:1036-1048. doi:10.1111/nmo.12358
16. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-3080. doi:10.1017/S0033291716001987
17. Yeh H-W, Chien W-C, Chung C-H, et al. Risk of psychiatric disorders in irritable bowel syndrome—a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72:e13212. doi:10.1111/ijcp.13212
18. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10:e00012. doi:10.14309/ctg.0000000000000012
19. Wang L, Alammar N, Singh R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-controlled studies. J Acad Nutr Diet. 2020;120:565-586. doi:10.1016/j.jand.2019.05.015
20. Barbara G, Grover M, Bercik P, et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46-58.e7. doi:10.1053/j.gastro.2018.07.011
21. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. doi:10.1053/j.gastro.2016.12.039
22. Heidelbaugh JJ. These 3 tools can help you streamline management of IBS. J Fam Pract. 2017;66:346-353.
23. American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35. doi:10.1038/ajg.2008.122
24. Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444-454. doi:10.1038/ajg.2015.6
25. Smalley W, Falck-Ytter C, Carrasco-Labra A, et al. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-D). Gastroenterology. 2019;157:851-854. doi:10.1053/j.gastro.2019.07.004
26. Markham A. Tenapanor: first approval. Drugs. 2019;79:1897-1903. doi:10.1007/s40265-019-01215-9
27. Chey WD, Paré P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217-1225. doi:10.1111/j.1572-0241.2008.01808.x
28. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil. 2019;31:e13461. doi:10.1111/nmo.13461
29. Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e12969. doi:10.1111/nmo.12969
30. Zegarac JP. The low-FODMAP diet for IBS: what you need to know. Medscape. August 13, 2019. Accessed September 20, 2021. www.medscape.com/viewarticle/917069
31. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low
32. Su H, Li Y-T, Heitkemper MM, et al. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome: Gastroenterol Nurs. 2019;42:150-158. doi:10.1097/SGA.0000000000000428
33. Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2020;59:2237-2248. doi: 10.1007/s00394-019-02074-6
34. Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9:940. doi:10.3390/nu9090940
35. Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27:1002-1010. doi:10.1097/MEG.0000000000000425
36. Wilson B, Rossi M, Dimidi E, et al. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:1098-1111. doi:10.1093/ajcn/nqy376
37. Yuan F, Ni H, Asche CV, et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191-1197. doi:10.1080/03007995.2017.1292230
38. Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: a network meta-analysis. Medicine (Baltimore). 2019;98:16068. doi:10.1097/MD.0000000000016068
39. Dale HF, Rasmussen SH, Asiller ÖÖ, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11:2048. doi:10.3390/nu11092048
40. Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiotics Antimicrob Proteins. 2020;12:834-839. doi:10.1007/s12602-019-09609-7
41. Ianiro G, Eusebi LH, Black CJ, et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. doi:10.1111/apt.15330
42. Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7:1033-1041. doi:10.1177/2050640619866990
43. Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1043-1050. doi:10.14309/ajg.0000000000000198
44. Black CJ, Yuan Y, Selinger CP, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117-131. doi:10.1016/S2468-1253(19)30324-3
45. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. doi:10.1097/MCG.0b013e3182a88357
46. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi:10.1186/s12906-018-2409-0
47. Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114:21-39. doi: 10.1038/s41395-018-0222-5
48. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113:329-338. doi:10.1038/ajg.2017.495
49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018;155:1753-1763. doi:10.1053/j.gastro.2018.08.021
50. Li F, Fu T, Tong W-D, et al. Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2016;91:456-468. doi:10.1016/j.mayocp.2016.01.015
51. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. doi:10.1111/apt.15001
52. Yoon K, Kim N, Lee JY, et al. Clinical response of rifaximin treatment in patients with abdominal bloating. Korean J Gastroenterol. 2018;72:121-127. doi:10.4166/kjg.2018.72.3.121
53. Black CJ, Burr NE, Camilleri M, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. doi:10.1136/gutjnl-2018-318160
54. Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817-830. doi:10.1111/apt.14948
55. Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE. 2017;12:e0172846. doi:10.1371/journal.pone.0172846
56. Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:1-13. doi:https://doi.org/10.1155/2019/2871505
57. Yan J, Miao Z-W, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:1-16. https://doi.org/10.1155/2019/7680963
PRACTICE RECOMMENDATIONS
› Make the diagnosis of irritable bowel syndrome (IBS) based on clinical findings, after excluding red flags in the presentation. C
› Screen patients with diarrhea-predominant IBS with fecal and serologic studies to rule out inflammatory bowel disease and celiac disease. B
› Counsel all IBS patients to increase their intake of soluble fiber, follow a low-FODMAP (fermentable oligo-, di-, and monosaccharide, and polyol) diet, and increase physical activity. B
› Prescribe an antispasmodic to treat mild IBS of all subtypes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Pulmonary rehabilitation: Similar benefit in both IPF and COPD patients
Patients with idiopathic pulmonary fibrosis (IPF) complete and respond to pulmonary rehabilitation at rates similar to patients with chronic obstructive pulmonary disease (COPD), according to results of a real-world study. The findings reported in an article published in the journal CHEST® reinforce pulmonary rehabilitation’s benefits for this population.
A progressive decline in respiratory and physical function characterizes IPF, with median survival from diagnosis of 3-5 years, according to Claire Nolan, PhD, of Harefield Hospital, Middlesex, England, and colleagues. The effects of pharmacologic therapies on IPF on symptom burden and quality of life are modest, although lung function decline may be slowed. Supporting evidence for pulmonary rehabilitation benefit in IPF is more modest than it is for COPD, for which exercise capacity, dyspnea, and health-related quality of life improvement have been demonstrated.
“We did not design a randomized, controlled trial,” Dr. Nolan said in an interview, “as it was considered unethical by the local ethics committee to withhold pulmonary rehabilitation based on clinical guidance in the United Kingdom.” She pointed out that initial pulmonary rehabilitation trials in COPD included an intervention (pulmonary rehabilitation) and a control (standard medical care) arm.
The study aims were to compare the effects of pulmonary rehabilitation with real-world data between IPF and COPD with respect to magnitude of effect and survival. The authors’ hypothesis was that IPF patients would have a blunted response to pulmonary rehabilitation with reduced completion rates, compared with a matched COPD group, and with increased mortality.
Study details
Investigators use propensity score matching of 163 IPF patients with a control group of 163 patients with COPD referred to pulmonary rehabilitation. Completion rates, responses, and survival status were recorded for 1-year following pulmonary rehabilitation discharge. The 8-week outpatient program was composed of two supervised exercise and education sessions with additional unsupervised home-based exercise each week.
While spirometry data, as expected, showed a higher proportion of IPF patients using supplemental oxygen, pulmonary rehabilitation completion rates were similar for both groups (IPF, 69%; COPD, 63%; P = .24) and there was no between group difference in the number of sessions attended (P = .39). Medical Research Council (muscle strength) (MRC), incremental shuttle walk test (ISW), and Chronic Respiratory Questionnaire total score (CRQ-T) improved significantly in both groups, again with no significant difference between groups.
Over the study course, there was progressive, significant worsening of forced vital capacity percentage, predicted, prescription supplemental oxygen, resting peripheral oxygen saturation, exercise capacity, health-related quality of life and pulmonary rehabilitation adherence across groups of responders (n = 63; 38%), nonresponders (n = 50; 31%) and noncompleters (n = 50; 31%). Among the IPF patients, 6 died before completing pulmonary rehabilitation, with 42 (27%) dying during follow-up.
Benefits of rehabilitation
Multivariable analyses showed that noncompletion and nonresponse were associated with significantly higher risk of all-cause mortality at 1-year. Also, time to all-cause mortality was shorter (P = .001) for noncompleters and nonresponders, compared with completers. A trend toward higher completion rates in the IPF group, compared with the COPD group, may be explained, the researchers explained, by fewer hospitalizations over the prior 12 months in the IPF group.
“Although many programs are designed for people with COPD,” Dr. Nolan and colleagues concluded, “our study demonstrates that people with IPF have similar clinical benefits and completion rates to those with COPD. These data reinforce the importance of referral to and engagement in pulmonary rehabilitation amongst the IPF population.”
These findings, Dr. Nolan emphasized, emerged from a single center, and validation in other settings is needed.
This study was funded by a National Institute for Health Research Doctoral Research Fellowship (2014-07-089) and a Medical Research Council New Investigator Research Grant (98576).
Patients with idiopathic pulmonary fibrosis (IPF) complete and respond to pulmonary rehabilitation at rates similar to patients with chronic obstructive pulmonary disease (COPD), according to results of a real-world study. The findings reported in an article published in the journal CHEST® reinforce pulmonary rehabilitation’s benefits for this population.
A progressive decline in respiratory and physical function characterizes IPF, with median survival from diagnosis of 3-5 years, according to Claire Nolan, PhD, of Harefield Hospital, Middlesex, England, and colleagues. The effects of pharmacologic therapies on IPF on symptom burden and quality of life are modest, although lung function decline may be slowed. Supporting evidence for pulmonary rehabilitation benefit in IPF is more modest than it is for COPD, for which exercise capacity, dyspnea, and health-related quality of life improvement have been demonstrated.
“We did not design a randomized, controlled trial,” Dr. Nolan said in an interview, “as it was considered unethical by the local ethics committee to withhold pulmonary rehabilitation based on clinical guidance in the United Kingdom.” She pointed out that initial pulmonary rehabilitation trials in COPD included an intervention (pulmonary rehabilitation) and a control (standard medical care) arm.
The study aims were to compare the effects of pulmonary rehabilitation with real-world data between IPF and COPD with respect to magnitude of effect and survival. The authors’ hypothesis was that IPF patients would have a blunted response to pulmonary rehabilitation with reduced completion rates, compared with a matched COPD group, and with increased mortality.
Study details
Investigators use propensity score matching of 163 IPF patients with a control group of 163 patients with COPD referred to pulmonary rehabilitation. Completion rates, responses, and survival status were recorded for 1-year following pulmonary rehabilitation discharge. The 8-week outpatient program was composed of two supervised exercise and education sessions with additional unsupervised home-based exercise each week.
While spirometry data, as expected, showed a higher proportion of IPF patients using supplemental oxygen, pulmonary rehabilitation completion rates were similar for both groups (IPF, 69%; COPD, 63%; P = .24) and there was no between group difference in the number of sessions attended (P = .39). Medical Research Council (muscle strength) (MRC), incremental shuttle walk test (ISW), and Chronic Respiratory Questionnaire total score (CRQ-T) improved significantly in both groups, again with no significant difference between groups.
Over the study course, there was progressive, significant worsening of forced vital capacity percentage, predicted, prescription supplemental oxygen, resting peripheral oxygen saturation, exercise capacity, health-related quality of life and pulmonary rehabilitation adherence across groups of responders (n = 63; 38%), nonresponders (n = 50; 31%) and noncompleters (n = 50; 31%). Among the IPF patients, 6 died before completing pulmonary rehabilitation, with 42 (27%) dying during follow-up.
Benefits of rehabilitation
Multivariable analyses showed that noncompletion and nonresponse were associated with significantly higher risk of all-cause mortality at 1-year. Also, time to all-cause mortality was shorter (P = .001) for noncompleters and nonresponders, compared with completers. A trend toward higher completion rates in the IPF group, compared with the COPD group, may be explained, the researchers explained, by fewer hospitalizations over the prior 12 months in the IPF group.
“Although many programs are designed for people with COPD,” Dr. Nolan and colleagues concluded, “our study demonstrates that people with IPF have similar clinical benefits and completion rates to those with COPD. These data reinforce the importance of referral to and engagement in pulmonary rehabilitation amongst the IPF population.”
These findings, Dr. Nolan emphasized, emerged from a single center, and validation in other settings is needed.
This study was funded by a National Institute for Health Research Doctoral Research Fellowship (2014-07-089) and a Medical Research Council New Investigator Research Grant (98576).
Patients with idiopathic pulmonary fibrosis (IPF) complete and respond to pulmonary rehabilitation at rates similar to patients with chronic obstructive pulmonary disease (COPD), according to results of a real-world study. The findings reported in an article published in the journal CHEST® reinforce pulmonary rehabilitation’s benefits for this population.
A progressive decline in respiratory and physical function characterizes IPF, with median survival from diagnosis of 3-5 years, according to Claire Nolan, PhD, of Harefield Hospital, Middlesex, England, and colleagues. The effects of pharmacologic therapies on IPF on symptom burden and quality of life are modest, although lung function decline may be slowed. Supporting evidence for pulmonary rehabilitation benefit in IPF is more modest than it is for COPD, for which exercise capacity, dyspnea, and health-related quality of life improvement have been demonstrated.
“We did not design a randomized, controlled trial,” Dr. Nolan said in an interview, “as it was considered unethical by the local ethics committee to withhold pulmonary rehabilitation based on clinical guidance in the United Kingdom.” She pointed out that initial pulmonary rehabilitation trials in COPD included an intervention (pulmonary rehabilitation) and a control (standard medical care) arm.
The study aims were to compare the effects of pulmonary rehabilitation with real-world data between IPF and COPD with respect to magnitude of effect and survival. The authors’ hypothesis was that IPF patients would have a blunted response to pulmonary rehabilitation with reduced completion rates, compared with a matched COPD group, and with increased mortality.
Study details
Investigators use propensity score matching of 163 IPF patients with a control group of 163 patients with COPD referred to pulmonary rehabilitation. Completion rates, responses, and survival status were recorded for 1-year following pulmonary rehabilitation discharge. The 8-week outpatient program was composed of two supervised exercise and education sessions with additional unsupervised home-based exercise each week.
While spirometry data, as expected, showed a higher proportion of IPF patients using supplemental oxygen, pulmonary rehabilitation completion rates were similar for both groups (IPF, 69%; COPD, 63%; P = .24) and there was no between group difference in the number of sessions attended (P = .39). Medical Research Council (muscle strength) (MRC), incremental shuttle walk test (ISW), and Chronic Respiratory Questionnaire total score (CRQ-T) improved significantly in both groups, again with no significant difference between groups.
Over the study course, there was progressive, significant worsening of forced vital capacity percentage, predicted, prescription supplemental oxygen, resting peripheral oxygen saturation, exercise capacity, health-related quality of life and pulmonary rehabilitation adherence across groups of responders (n = 63; 38%), nonresponders (n = 50; 31%) and noncompleters (n = 50; 31%). Among the IPF patients, 6 died before completing pulmonary rehabilitation, with 42 (27%) dying during follow-up.
Benefits of rehabilitation
Multivariable analyses showed that noncompletion and nonresponse were associated with significantly higher risk of all-cause mortality at 1-year. Also, time to all-cause mortality was shorter (P = .001) for noncompleters and nonresponders, compared with completers. A trend toward higher completion rates in the IPF group, compared with the COPD group, may be explained, the researchers explained, by fewer hospitalizations over the prior 12 months in the IPF group.
“Although many programs are designed for people with COPD,” Dr. Nolan and colleagues concluded, “our study demonstrates that people with IPF have similar clinical benefits and completion rates to those with COPD. These data reinforce the importance of referral to and engagement in pulmonary rehabilitation amongst the IPF population.”
These findings, Dr. Nolan emphasized, emerged from a single center, and validation in other settings is needed.
This study was funded by a National Institute for Health Research Doctoral Research Fellowship (2014-07-089) and a Medical Research Council New Investigator Research Grant (98576).
FROM THE JOURNAL CHEST®
Direct comparison shows differing strengths for left atrial closure devices
On the basis of outcomes, there was no clear winner from a trial that directly compared two modern devices used in patients undergoing percutaneous left atrial appendage (LAA) closure.
But the devices were not interchangeable for rates of complications or leaks, according to results of the open-label SWISS APERO trial, which compared the Amplatzer Amulet to the Watchman FLX device at eight participating centers in Europe.
At 45 days, the overall rates of leaks and the clinical outcomes in the two randomized groups were not significantly different, but there were differences in secondary endpoints, such as rates of peridevice leak (PDL), which were lower in the Amulet device group, and procedural complications, which were higher, Roberto Galea, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
LAA closure devices were developed as an alternative to oral anticoagulation in patients with nonvalvular atrial fibrillation. Although a similar comparison of LAA closure devices, called Amulet IDE, was recently published, that trial compared Amulet to Watchman 2.5, an earlier generation device.
Started later, SWISS APERO was also a planned comparison of Amulet and the Watchman 2.5, but the comparison switched to the Watchman FLX, when it was released in March of 2019.
First randomized comparison with Watchman FLX
“This is the first multicenter randomized controlled trial to include the Watchman FLX,” said Dr. Galea, a clinical investigator in the department of cardiology, Bern (Switzerland) University Hospital. He noted that Watchman FLX included some adjustments in design with the potential to reduce leak rates.
After preprocedural transesophageal echocardiography confirmed that patients had suitable anatomy to receive either device, the 221 patients who qualified for SWISS APERO were randomized. The primary endpoint was a composite of a justified crossover to a device other than the one to which they were assigned or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days.
The primary endpoint was reached by 67.6% of patients randomized to the Amulet device and 70% of those randomized to Watchman Flex, a statistically nonsignificant difference (P = .71).
Because only one patient in the Amulet group and none in the Watchman group had a justified crossover to a nonrandomized device, most of the differences in the 45-day CCTA involved patency, defined as LAA density of at least 100 Hounsfield units. While the proportion of patients with leaks was similar, the types of leaks, which were stratified by underlying leak mechanism into PDL, mixed leaks (including incomplete side sealing), intradevice leaks, and leaks of unclear origin, were different.
Peridevice leaks twofold greater with Watchman
Those randomized to the Watchman device were more than twice as likely to have PDL (27.5% vs. 13.7%; P = .02), although no visible leak exceeded 5 mm in size. They were also more likely to have mixed leaks (14% vs. 3.8%; P = .01) and patency with no visible leak (21.0% vs. 9.5%; P = .02). There were also more device-related thrombi in the Watchman group even though the difference did not reach statistical significance (9.9% vs. 3.7%; P = .08).
Intradevice leaks (44.8% vs. 23.0%; P = .001) were the only type of patency significantly more common among patients randomized to Amulet, but the difference was relatively large. In addition, procedural complications of any type (32.4% vs. 19.1%; P = .023) were higher in the Amulet group. Most of these involved non–clinically relevant pericardial effusions, Dr. Galea said at the meeting, sponsored by the Cardiovascular Research Foundation.
The proportion of patients with adverse outcomes by 45 days was similar, but the types of complications differed. Of the six deaths, two occurred in the Amulet group as a result of periprocedural complications (one stemming from an air embolism and the other from a series of events following pericardial effusion). Three of the four deaths in the Watchman group were due to fatal bleeding. The fourth was a sudden death that occurred 30 days after the procedure.
Amulet IDE trial generates similar data
The much larger Amulet IDE trial, which compared Amulet to the Watchman 2.5 device, produced generally similar results. Again, the proportion of patients reaching the composite primary endpoints was similar.
The primary safety endpoint, which included death and major bleeding within 12 months of randomization, occurred in 14.5% and 14.7% of the Amulet and Watchman patients, respectively (P < .001 for noninferiority). The primary efficacy endpoint, which included stroke or systemic embolism within 18 months of randomization, occurred in 2.8% of patients in both groups.
As in SWISS APERO, the 1,878-patient Amulet IDE trial showed that the devices are similarly effective and safe but not necessarily interchangeable. Ultimately, the rate of LAA occlusion was higher for Amulet than the older generation Watchman (98.9% vs. 96.8%; P = .003) but procedural complication occurred more frequently among those randomized to the Amulet device (4.5% vs. 2.5%).
“The closure mechanisms are not the same, which might explain why we see differences in some secondary outcomes even when they perform similarly on the primary outcomes,” said Dhanunjaya R. Lakkireddy, MD, executive medical director, Kansas City (Kansas) Heart Rhythm Institute.
The lead investigator of the Amulet IDE trial, Dr. Lakkireddy was referring to both the AMULET IDE and the SWISS APERO study when he said that the currently available data do not allow one device to be considered superior to the other. He did suggest that differences between devices might still be considered meaningful in specific clinical situations or to specific clinicians.
Without studies to show objective differences, Dr. Lakkireddy suggested that training and experience is probably the most important variable in achieving treatment goals. “Operator comfort is certainly important,” he said.
Dr. Galea reports no significant financial relationships. The investigator-initiated study received funding from Abbott, the manufacturer of the Amulet device. Dr. Lakkireddy has financial relationships with Abbott, AltaThera, Medtronic, Biotronik, and Boston Scientific, which makes the Watchman device.
On the basis of outcomes, there was no clear winner from a trial that directly compared two modern devices used in patients undergoing percutaneous left atrial appendage (LAA) closure.
But the devices were not interchangeable for rates of complications or leaks, according to results of the open-label SWISS APERO trial, which compared the Amplatzer Amulet to the Watchman FLX device at eight participating centers in Europe.
At 45 days, the overall rates of leaks and the clinical outcomes in the two randomized groups were not significantly different, but there were differences in secondary endpoints, such as rates of peridevice leak (PDL), which were lower in the Amulet device group, and procedural complications, which were higher, Roberto Galea, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
LAA closure devices were developed as an alternative to oral anticoagulation in patients with nonvalvular atrial fibrillation. Although a similar comparison of LAA closure devices, called Amulet IDE, was recently published, that trial compared Amulet to Watchman 2.5, an earlier generation device.
Started later, SWISS APERO was also a planned comparison of Amulet and the Watchman 2.5, but the comparison switched to the Watchman FLX, when it was released in March of 2019.
First randomized comparison with Watchman FLX
“This is the first multicenter randomized controlled trial to include the Watchman FLX,” said Dr. Galea, a clinical investigator in the department of cardiology, Bern (Switzerland) University Hospital. He noted that Watchman FLX included some adjustments in design with the potential to reduce leak rates.
After preprocedural transesophageal echocardiography confirmed that patients had suitable anatomy to receive either device, the 221 patients who qualified for SWISS APERO were randomized. The primary endpoint was a composite of a justified crossover to a device other than the one to which they were assigned or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days.
The primary endpoint was reached by 67.6% of patients randomized to the Amulet device and 70% of those randomized to Watchman Flex, a statistically nonsignificant difference (P = .71).
Because only one patient in the Amulet group and none in the Watchman group had a justified crossover to a nonrandomized device, most of the differences in the 45-day CCTA involved patency, defined as LAA density of at least 100 Hounsfield units. While the proportion of patients with leaks was similar, the types of leaks, which were stratified by underlying leak mechanism into PDL, mixed leaks (including incomplete side sealing), intradevice leaks, and leaks of unclear origin, were different.
Peridevice leaks twofold greater with Watchman
Those randomized to the Watchman device were more than twice as likely to have PDL (27.5% vs. 13.7%; P = .02), although no visible leak exceeded 5 mm in size. They were also more likely to have mixed leaks (14% vs. 3.8%; P = .01) and patency with no visible leak (21.0% vs. 9.5%; P = .02). There were also more device-related thrombi in the Watchman group even though the difference did not reach statistical significance (9.9% vs. 3.7%; P = .08).
Intradevice leaks (44.8% vs. 23.0%; P = .001) were the only type of patency significantly more common among patients randomized to Amulet, but the difference was relatively large. In addition, procedural complications of any type (32.4% vs. 19.1%; P = .023) were higher in the Amulet group. Most of these involved non–clinically relevant pericardial effusions, Dr. Galea said at the meeting, sponsored by the Cardiovascular Research Foundation.
The proportion of patients with adverse outcomes by 45 days was similar, but the types of complications differed. Of the six deaths, two occurred in the Amulet group as a result of periprocedural complications (one stemming from an air embolism and the other from a series of events following pericardial effusion). Three of the four deaths in the Watchman group were due to fatal bleeding. The fourth was a sudden death that occurred 30 days after the procedure.
Amulet IDE trial generates similar data
The much larger Amulet IDE trial, which compared Amulet to the Watchman 2.5 device, produced generally similar results. Again, the proportion of patients reaching the composite primary endpoints was similar.
The primary safety endpoint, which included death and major bleeding within 12 months of randomization, occurred in 14.5% and 14.7% of the Amulet and Watchman patients, respectively (P < .001 for noninferiority). The primary efficacy endpoint, which included stroke or systemic embolism within 18 months of randomization, occurred in 2.8% of patients in both groups.
As in SWISS APERO, the 1,878-patient Amulet IDE trial showed that the devices are similarly effective and safe but not necessarily interchangeable. Ultimately, the rate of LAA occlusion was higher for Amulet than the older generation Watchman (98.9% vs. 96.8%; P = .003) but procedural complication occurred more frequently among those randomized to the Amulet device (4.5% vs. 2.5%).
“The closure mechanisms are not the same, which might explain why we see differences in some secondary outcomes even when they perform similarly on the primary outcomes,” said Dhanunjaya R. Lakkireddy, MD, executive medical director, Kansas City (Kansas) Heart Rhythm Institute.
The lead investigator of the Amulet IDE trial, Dr. Lakkireddy was referring to both the AMULET IDE and the SWISS APERO study when he said that the currently available data do not allow one device to be considered superior to the other. He did suggest that differences between devices might still be considered meaningful in specific clinical situations or to specific clinicians.
Without studies to show objective differences, Dr. Lakkireddy suggested that training and experience is probably the most important variable in achieving treatment goals. “Operator comfort is certainly important,” he said.
Dr. Galea reports no significant financial relationships. The investigator-initiated study received funding from Abbott, the manufacturer of the Amulet device. Dr. Lakkireddy has financial relationships with Abbott, AltaThera, Medtronic, Biotronik, and Boston Scientific, which makes the Watchman device.
On the basis of outcomes, there was no clear winner from a trial that directly compared two modern devices used in patients undergoing percutaneous left atrial appendage (LAA) closure.
But the devices were not interchangeable for rates of complications or leaks, according to results of the open-label SWISS APERO trial, which compared the Amplatzer Amulet to the Watchman FLX device at eight participating centers in Europe.
At 45 days, the overall rates of leaks and the clinical outcomes in the two randomized groups were not significantly different, but there were differences in secondary endpoints, such as rates of peridevice leak (PDL), which were lower in the Amulet device group, and procedural complications, which were higher, Roberto Galea, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
LAA closure devices were developed as an alternative to oral anticoagulation in patients with nonvalvular atrial fibrillation. Although a similar comparison of LAA closure devices, called Amulet IDE, was recently published, that trial compared Amulet to Watchman 2.5, an earlier generation device.
Started later, SWISS APERO was also a planned comparison of Amulet and the Watchman 2.5, but the comparison switched to the Watchman FLX, when it was released in March of 2019.
First randomized comparison with Watchman FLX
“This is the first multicenter randomized controlled trial to include the Watchman FLX,” said Dr. Galea, a clinical investigator in the department of cardiology, Bern (Switzerland) University Hospital. He noted that Watchman FLX included some adjustments in design with the potential to reduce leak rates.
After preprocedural transesophageal echocardiography confirmed that patients had suitable anatomy to receive either device, the 221 patients who qualified for SWISS APERO were randomized. The primary endpoint was a composite of a justified crossover to a device other than the one to which they were assigned or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days.
The primary endpoint was reached by 67.6% of patients randomized to the Amulet device and 70% of those randomized to Watchman Flex, a statistically nonsignificant difference (P = .71).
Because only one patient in the Amulet group and none in the Watchman group had a justified crossover to a nonrandomized device, most of the differences in the 45-day CCTA involved patency, defined as LAA density of at least 100 Hounsfield units. While the proportion of patients with leaks was similar, the types of leaks, which were stratified by underlying leak mechanism into PDL, mixed leaks (including incomplete side sealing), intradevice leaks, and leaks of unclear origin, were different.
Peridevice leaks twofold greater with Watchman
Those randomized to the Watchman device were more than twice as likely to have PDL (27.5% vs. 13.7%; P = .02), although no visible leak exceeded 5 mm in size. They were also more likely to have mixed leaks (14% vs. 3.8%; P = .01) and patency with no visible leak (21.0% vs. 9.5%; P = .02). There were also more device-related thrombi in the Watchman group even though the difference did not reach statistical significance (9.9% vs. 3.7%; P = .08).
Intradevice leaks (44.8% vs. 23.0%; P = .001) were the only type of patency significantly more common among patients randomized to Amulet, but the difference was relatively large. In addition, procedural complications of any type (32.4% vs. 19.1%; P = .023) were higher in the Amulet group. Most of these involved non–clinically relevant pericardial effusions, Dr. Galea said at the meeting, sponsored by the Cardiovascular Research Foundation.
The proportion of patients with adverse outcomes by 45 days was similar, but the types of complications differed. Of the six deaths, two occurred in the Amulet group as a result of periprocedural complications (one stemming from an air embolism and the other from a series of events following pericardial effusion). Three of the four deaths in the Watchman group were due to fatal bleeding. The fourth was a sudden death that occurred 30 days after the procedure.
Amulet IDE trial generates similar data
The much larger Amulet IDE trial, which compared Amulet to the Watchman 2.5 device, produced generally similar results. Again, the proportion of patients reaching the composite primary endpoints was similar.
The primary safety endpoint, which included death and major bleeding within 12 months of randomization, occurred in 14.5% and 14.7% of the Amulet and Watchman patients, respectively (P < .001 for noninferiority). The primary efficacy endpoint, which included stroke or systemic embolism within 18 months of randomization, occurred in 2.8% of patients in both groups.
As in SWISS APERO, the 1,878-patient Amulet IDE trial showed that the devices are similarly effective and safe but not necessarily interchangeable. Ultimately, the rate of LAA occlusion was higher for Amulet than the older generation Watchman (98.9% vs. 96.8%; P = .003) but procedural complication occurred more frequently among those randomized to the Amulet device (4.5% vs. 2.5%).
“The closure mechanisms are not the same, which might explain why we see differences in some secondary outcomes even when they perform similarly on the primary outcomes,” said Dhanunjaya R. Lakkireddy, MD, executive medical director, Kansas City (Kansas) Heart Rhythm Institute.
The lead investigator of the Amulet IDE trial, Dr. Lakkireddy was referring to both the AMULET IDE and the SWISS APERO study when he said that the currently available data do not allow one device to be considered superior to the other. He did suggest that differences between devices might still be considered meaningful in specific clinical situations or to specific clinicians.
Without studies to show objective differences, Dr. Lakkireddy suggested that training and experience is probably the most important variable in achieving treatment goals. “Operator comfort is certainly important,” he said.
Dr. Galea reports no significant financial relationships. The investigator-initiated study received funding from Abbott, the manufacturer of the Amulet device. Dr. Lakkireddy has financial relationships with Abbott, AltaThera, Medtronic, Biotronik, and Boston Scientific, which makes the Watchman device.
FROM TCT 2021
Single infusion of ketamine rapidly reduces suicidal thoughts
A single infusion of ketamine rapidly improves distorted thinking and reasoning to reduce suicidal thoughts, independent of the drug’s effect on severe depression, new research shows.
“Previously it was shown that ketamine rapidly improved depression and that explained part of the rapid improvement in suicidal ideation,” senior author J. John Mann, MD, with Columbia University, New York, said in an interview.
“What was unclear was what else changed that could decrease suicidal ideation and the risk for suicidal behavior. This study identifies a second new domain of improvement – namely rapid improvement in several cognitive functions that can potentially reduce suicide risk,” said Dr. Mann.
The study was published online Nov. 2, 2021, in the Journal of Clinical Psychiatry.
Boosts cognitive function
A total of 78 adults with major depressive disorder and clinically significant suicidal ideation underwent neuropsychological testing before, and 1 day after, double-blind treatment with a single intravenous infusion of ketamine or midazolam.
“Ketamine produced rapid improvement in suicidal ideation and mood” compared with midazolam, the authors reported.
Ketamine was linked to specific improvement in reaction time and cognitive control/interference processing – a measure that has been associated with previous suicide attempt in depression.
A subgroup of patients whose suicidal ideation did not remit on midazolam were later treated with unblinded ketamine and retested. In these individuals, reaction time and cognitive control/interference processing also improved relative to preketamine assessments.
Neurocognitive improvement, however, was not correlated with changes in depression, suicidal thinking, or general mood, the researchers noted.
Nonetheless, they say ketamine had a “positive therapeutic effect” on neurocognition 1 day after treatment on at least one measure associated with suicidal behavior in the context of depression.
The results suggest “additional independent therapeutic effects for ketamine in the treatment of depressed patients at risk for suicidal behavior,” they wrote.
“Ketamine modulates many neurotransmitter systems including glutamate transmission which is crucial for learning and memory. It increases the number of synapses or connections between neurons. These effects are fundamental to cognition and are logical explanations of the beneficial effects observed in this study,” Dr. Mann said in an interview.
“Our study helped us gain a better understanding of how ketamine works in the brain and how quickly it can improve distorted thinking. study investigator Ravi. N. Shah, MD, chief innovation officer, Columbia Psychiatry, said in a news release.
Important research with caveats
In a comment, James Murrough, MD, PhD, director of the Depression and Anxiety Center for Discovery and Treatment at Mount Sinai in New York, said the study is important and “adds to a growing understanding of how ketamine affects brain systems and thinking in the context of depression and suicide risk.”
“One reason this study is significant is that prior studies have shown that ketamine can have harmful effects on cognitive functioning in the context of ketamine misuse and exposures to high doses for long periods of time,” Dr. Murrough, who wasn’t involved in the study, said in an interview.
“In contrast in this study, a single low-dose treatment of ketamine can have the opposite effects, actually boosting some markers of cognitive functioning, at least in the short-term,” he noted.
Dr. Murrough said one caveat to the study is that it only examined the effect of ketamine on cognition once, 1 day after a single treatment.
“While this is an important initial observation, we don’t yet have any understanding of how persistent this effect on cognition is, or how this observed change may be related to any benefit ketamine may have on depression or suicide risk,” Dr. Murrough said.
“In fact, the researchers found that there was no association between change in cognitive functioning following ketamine and change in depression or suicidal thinking. The patients who showed improved cognitive function following ketamine did not differ in terms of mood or suicide risk compared to patients who did not show an improvement in cognition,” Dr. Murrough noted.
“This raises the important question of what is the relevance of change in cognition to the potential benefits of ketamine. This is an important area and should be the focus of future research in order to improve outcomes for patients with depression and who are at risk for suicide,” he added.
Also weighing in, Roger S. McIntyre, MD, professor of psychiatry and pharmacology and head of the mood disorders psychopharmacology unit, University of Toronto, said the study is “very interesting and in keeping” with some previous work that he and his colleagues have done showing that ketamine “seems to benefit aspects of cognition which is a core element in depression.”
“It’s a testable hypothesis that the improvement in cognition now being reported and replicated could play some role in the improved quality of life and functioning with this treatment and as well reduce reducing suicide,” said Dr. McIntyre.
This study was supported by the National Institute of Mental Health. Dr. Mann receives royalties for commercial use of the Columbia-Suicide Severity Rating Scale, which was not used in this study. Dr. Murrough’s institution was involved in research involving esketamine (Spravato) for treatment-resistant depression and receives financial remuneration from the manufacturer of esketamine. Dr. McIntyre has received research grant support from the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
A single infusion of ketamine rapidly improves distorted thinking and reasoning to reduce suicidal thoughts, independent of the drug’s effect on severe depression, new research shows.
“Previously it was shown that ketamine rapidly improved depression and that explained part of the rapid improvement in suicidal ideation,” senior author J. John Mann, MD, with Columbia University, New York, said in an interview.
“What was unclear was what else changed that could decrease suicidal ideation and the risk for suicidal behavior. This study identifies a second new domain of improvement – namely rapid improvement in several cognitive functions that can potentially reduce suicide risk,” said Dr. Mann.
The study was published online Nov. 2, 2021, in the Journal of Clinical Psychiatry.
Boosts cognitive function
A total of 78 adults with major depressive disorder and clinically significant suicidal ideation underwent neuropsychological testing before, and 1 day after, double-blind treatment with a single intravenous infusion of ketamine or midazolam.
“Ketamine produced rapid improvement in suicidal ideation and mood” compared with midazolam, the authors reported.
Ketamine was linked to specific improvement in reaction time and cognitive control/interference processing – a measure that has been associated with previous suicide attempt in depression.
A subgroup of patients whose suicidal ideation did not remit on midazolam were later treated with unblinded ketamine and retested. In these individuals, reaction time and cognitive control/interference processing also improved relative to preketamine assessments.
Neurocognitive improvement, however, was not correlated with changes in depression, suicidal thinking, or general mood, the researchers noted.
Nonetheless, they say ketamine had a “positive therapeutic effect” on neurocognition 1 day after treatment on at least one measure associated with suicidal behavior in the context of depression.
The results suggest “additional independent therapeutic effects for ketamine in the treatment of depressed patients at risk for suicidal behavior,” they wrote.
“Ketamine modulates many neurotransmitter systems including glutamate transmission which is crucial for learning and memory. It increases the number of synapses or connections between neurons. These effects are fundamental to cognition and are logical explanations of the beneficial effects observed in this study,” Dr. Mann said in an interview.
“Our study helped us gain a better understanding of how ketamine works in the brain and how quickly it can improve distorted thinking. study investigator Ravi. N. Shah, MD, chief innovation officer, Columbia Psychiatry, said in a news release.
Important research with caveats
In a comment, James Murrough, MD, PhD, director of the Depression and Anxiety Center for Discovery and Treatment at Mount Sinai in New York, said the study is important and “adds to a growing understanding of how ketamine affects brain systems and thinking in the context of depression and suicide risk.”
“One reason this study is significant is that prior studies have shown that ketamine can have harmful effects on cognitive functioning in the context of ketamine misuse and exposures to high doses for long periods of time,” Dr. Murrough, who wasn’t involved in the study, said in an interview.
“In contrast in this study, a single low-dose treatment of ketamine can have the opposite effects, actually boosting some markers of cognitive functioning, at least in the short-term,” he noted.
Dr. Murrough said one caveat to the study is that it only examined the effect of ketamine on cognition once, 1 day after a single treatment.
“While this is an important initial observation, we don’t yet have any understanding of how persistent this effect on cognition is, or how this observed change may be related to any benefit ketamine may have on depression or suicide risk,” Dr. Murrough said.
“In fact, the researchers found that there was no association between change in cognitive functioning following ketamine and change in depression or suicidal thinking. The patients who showed improved cognitive function following ketamine did not differ in terms of mood or suicide risk compared to patients who did not show an improvement in cognition,” Dr. Murrough noted.
“This raises the important question of what is the relevance of change in cognition to the potential benefits of ketamine. This is an important area and should be the focus of future research in order to improve outcomes for patients with depression and who are at risk for suicide,” he added.
Also weighing in, Roger S. McIntyre, MD, professor of psychiatry and pharmacology and head of the mood disorders psychopharmacology unit, University of Toronto, said the study is “very interesting and in keeping” with some previous work that he and his colleagues have done showing that ketamine “seems to benefit aspects of cognition which is a core element in depression.”
“It’s a testable hypothesis that the improvement in cognition now being reported and replicated could play some role in the improved quality of life and functioning with this treatment and as well reduce reducing suicide,” said Dr. McIntyre.
This study was supported by the National Institute of Mental Health. Dr. Mann receives royalties for commercial use of the Columbia-Suicide Severity Rating Scale, which was not used in this study. Dr. Murrough’s institution was involved in research involving esketamine (Spravato) for treatment-resistant depression and receives financial remuneration from the manufacturer of esketamine. Dr. McIntyre has received research grant support from the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
A single infusion of ketamine rapidly improves distorted thinking and reasoning to reduce suicidal thoughts, independent of the drug’s effect on severe depression, new research shows.
“Previously it was shown that ketamine rapidly improved depression and that explained part of the rapid improvement in suicidal ideation,” senior author J. John Mann, MD, with Columbia University, New York, said in an interview.
“What was unclear was what else changed that could decrease suicidal ideation and the risk for suicidal behavior. This study identifies a second new domain of improvement – namely rapid improvement in several cognitive functions that can potentially reduce suicide risk,” said Dr. Mann.
The study was published online Nov. 2, 2021, in the Journal of Clinical Psychiatry.
Boosts cognitive function
A total of 78 adults with major depressive disorder and clinically significant suicidal ideation underwent neuropsychological testing before, and 1 day after, double-blind treatment with a single intravenous infusion of ketamine or midazolam.
“Ketamine produced rapid improvement in suicidal ideation and mood” compared with midazolam, the authors reported.
Ketamine was linked to specific improvement in reaction time and cognitive control/interference processing – a measure that has been associated with previous suicide attempt in depression.
A subgroup of patients whose suicidal ideation did not remit on midazolam were later treated with unblinded ketamine and retested. In these individuals, reaction time and cognitive control/interference processing also improved relative to preketamine assessments.
Neurocognitive improvement, however, was not correlated with changes in depression, suicidal thinking, or general mood, the researchers noted.
Nonetheless, they say ketamine had a “positive therapeutic effect” on neurocognition 1 day after treatment on at least one measure associated with suicidal behavior in the context of depression.
The results suggest “additional independent therapeutic effects for ketamine in the treatment of depressed patients at risk for suicidal behavior,” they wrote.
“Ketamine modulates many neurotransmitter systems including glutamate transmission which is crucial for learning and memory. It increases the number of synapses or connections between neurons. These effects are fundamental to cognition and are logical explanations of the beneficial effects observed in this study,” Dr. Mann said in an interview.
“Our study helped us gain a better understanding of how ketamine works in the brain and how quickly it can improve distorted thinking. study investigator Ravi. N. Shah, MD, chief innovation officer, Columbia Psychiatry, said in a news release.
Important research with caveats
In a comment, James Murrough, MD, PhD, director of the Depression and Anxiety Center for Discovery and Treatment at Mount Sinai in New York, said the study is important and “adds to a growing understanding of how ketamine affects brain systems and thinking in the context of depression and suicide risk.”
“One reason this study is significant is that prior studies have shown that ketamine can have harmful effects on cognitive functioning in the context of ketamine misuse and exposures to high doses for long periods of time,” Dr. Murrough, who wasn’t involved in the study, said in an interview.
“In contrast in this study, a single low-dose treatment of ketamine can have the opposite effects, actually boosting some markers of cognitive functioning, at least in the short-term,” he noted.
Dr. Murrough said one caveat to the study is that it only examined the effect of ketamine on cognition once, 1 day after a single treatment.
“While this is an important initial observation, we don’t yet have any understanding of how persistent this effect on cognition is, or how this observed change may be related to any benefit ketamine may have on depression or suicide risk,” Dr. Murrough said.
“In fact, the researchers found that there was no association between change in cognitive functioning following ketamine and change in depression or suicidal thinking. The patients who showed improved cognitive function following ketamine did not differ in terms of mood or suicide risk compared to patients who did not show an improvement in cognition,” Dr. Murrough noted.
“This raises the important question of what is the relevance of change in cognition to the potential benefits of ketamine. This is an important area and should be the focus of future research in order to improve outcomes for patients with depression and who are at risk for suicide,” he added.
Also weighing in, Roger S. McIntyre, MD, professor of psychiatry and pharmacology and head of the mood disorders psychopharmacology unit, University of Toronto, said the study is “very interesting and in keeping” with some previous work that he and his colleagues have done showing that ketamine “seems to benefit aspects of cognition which is a core element in depression.”
“It’s a testable hypothesis that the improvement in cognition now being reported and replicated could play some role in the improved quality of life and functioning with this treatment and as well reduce reducing suicide,” said Dr. McIntyre.
This study was supported by the National Institute of Mental Health. Dr. Mann receives royalties for commercial use of the Columbia-Suicide Severity Rating Scale, which was not used in this study. Dr. Murrough’s institution was involved in research involving esketamine (Spravato) for treatment-resistant depression and receives financial remuneration from the manufacturer of esketamine. Dr. McIntyre has received research grant support from the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Taking 2021’s rheumatology advocacy momentum into 2022
As 2021 winds down, I reflect on the achievements the rheumatology community has had in the realm of advocacy throughout the year. And there were many: Seven states signed accumulator program bans into law, five states reformed the use of step therapy protocols, Texas passed an innovative “gold carding” law to reduce the burden of prior authorization, and West Virginia passed a rebate pass-through law to directly assist patients with high out-of-pocket costs – the first of its kind in the nation.
Of course, the close of another year also means gearing up for the year ahead. The majority of state legislatures are getting ready to open legislative sessions in the New Year. This means a year of new opportunities and new challenges. As a community, there are some key areas rheumatology will need to focus on during the course of the upcoming year as policy makers return to the business of policy making.
Many in the rheumatology community are aware that the buy and bill acquisition system has come under threat in recent years, mainly from payer mandates to use the alternative white bagging model. In some cases, payers have gone as far as to mandate the practice of “brown bagging,” or home infusion. These practices can endanger patient safety and overall quality of care. A study led by Matthew Baker, MD, of Stanford (Calif.) University found that biologic infusions administered at home, compared with those administered at a facility, were associated with increased adverse events requiring escalation of care; specifically, home infusions were associated with 25% increased odds of ED or hospital admission on the same or next day after infusion, compared with facility infusions, and 28% increased odds of permanent discontinuation of the biologic after emergency department or hospital admission. Additionally, site-of-care research by Paul Fronstin, PhD, at Employee Benefit Research Institute clearly shows that in-office infusion with physician supervision is far more cost effective than hospital and, in some cases, home infusion as well.
Further, the metastasis of these payer mandates is likely to severely limit availability of and access to care. It is unclear whether outpatient infusion, especially in private rheumatology practices, will prove sustainable in a world of white bagging. The net result of an expansion of the white-bagging requirements may well be broad access challenges that inconvenience patients deeply and irresponsibly.
The expansion of these mandates has not come without pushback, and rheumatologists should be prepared to advocate for policy that prohibits payers from mandating the use of white bagging, brown bagging, and home infusion. It is abundantly clear that arguments of safety and cost effectiveness are sufficient grounds for policy makers to curtail the mandatory use of these practices.
Similar to white bagging, another key issue in the year ahead is formulary construction based on the rebate system and proposed policies to address its attendant problems. Propagated by pharmacy benefit managers (PBMs), the rebate-based, or kickback, system of formulary construction often rewards higher-priced drugs with preferred placement regardless of whether they are the best and most affordable medications for our patients. A few states are beginning to address the affordability issue by mandating that the rebate/kickback acquired by the PBM be passed back to the patient at the point of sale. West Virginia passed a first-of-its-kind law to ensure that patients who generate drug rebates benefit from them by requiring that their cost shares are reduced by an amount equivalent to the rebate received by a health plan. This policy does not get at the root of the formulary construction problem, but if it is adopted more broadly across the country, it will deliver direct relief to patients who are struggling with out-of-pocket costs associated with prescription drugs. I anticipate that this will be a prominent issue nationwide during the upcoming year with opportunity for rheumatologists to lend their voices.
Many of the rheumatology community’s longstanding issues persist, and while progress has been made, more work remains to be done. Whether it’s accumulator programs, prior authorization, nonmedical switching, or step therapy, there will be opportunities in almost every state to engage in improving our ability to provide excellent care to our patients.
We tend to be motivated into action when one of these individual issues appear in our own state’s legislature; however, consistency of engagement is also important. While it is important to talk to your legislators when you need them to vote a certain way on a certain bill, scheduling a meeting with them or sending them a message detailing some of the issues that the rheumatology community faces before legislatures return in full swing is equally important to establish the relationship.
By making “rheum for action” now, you’ll have more impact when legislation relevant to our daily work does appear in the state legislatures. You can find your state representatives at the Coalition for State Rheumatology Organization’s Action Center.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As 2021 winds down, I reflect on the achievements the rheumatology community has had in the realm of advocacy throughout the year. And there were many: Seven states signed accumulator program bans into law, five states reformed the use of step therapy protocols, Texas passed an innovative “gold carding” law to reduce the burden of prior authorization, and West Virginia passed a rebate pass-through law to directly assist patients with high out-of-pocket costs – the first of its kind in the nation.
Of course, the close of another year also means gearing up for the year ahead. The majority of state legislatures are getting ready to open legislative sessions in the New Year. This means a year of new opportunities and new challenges. As a community, there are some key areas rheumatology will need to focus on during the course of the upcoming year as policy makers return to the business of policy making.
Many in the rheumatology community are aware that the buy and bill acquisition system has come under threat in recent years, mainly from payer mandates to use the alternative white bagging model. In some cases, payers have gone as far as to mandate the practice of “brown bagging,” or home infusion. These practices can endanger patient safety and overall quality of care. A study led by Matthew Baker, MD, of Stanford (Calif.) University found that biologic infusions administered at home, compared with those administered at a facility, were associated with increased adverse events requiring escalation of care; specifically, home infusions were associated with 25% increased odds of ED or hospital admission on the same or next day after infusion, compared with facility infusions, and 28% increased odds of permanent discontinuation of the biologic after emergency department or hospital admission. Additionally, site-of-care research by Paul Fronstin, PhD, at Employee Benefit Research Institute clearly shows that in-office infusion with physician supervision is far more cost effective than hospital and, in some cases, home infusion as well.
Further, the metastasis of these payer mandates is likely to severely limit availability of and access to care. It is unclear whether outpatient infusion, especially in private rheumatology practices, will prove sustainable in a world of white bagging. The net result of an expansion of the white-bagging requirements may well be broad access challenges that inconvenience patients deeply and irresponsibly.
The expansion of these mandates has not come without pushback, and rheumatologists should be prepared to advocate for policy that prohibits payers from mandating the use of white bagging, brown bagging, and home infusion. It is abundantly clear that arguments of safety and cost effectiveness are sufficient grounds for policy makers to curtail the mandatory use of these practices.
Similar to white bagging, another key issue in the year ahead is formulary construction based on the rebate system and proposed policies to address its attendant problems. Propagated by pharmacy benefit managers (PBMs), the rebate-based, or kickback, system of formulary construction often rewards higher-priced drugs with preferred placement regardless of whether they are the best and most affordable medications for our patients. A few states are beginning to address the affordability issue by mandating that the rebate/kickback acquired by the PBM be passed back to the patient at the point of sale. West Virginia passed a first-of-its-kind law to ensure that patients who generate drug rebates benefit from them by requiring that their cost shares are reduced by an amount equivalent to the rebate received by a health plan. This policy does not get at the root of the formulary construction problem, but if it is adopted more broadly across the country, it will deliver direct relief to patients who are struggling with out-of-pocket costs associated with prescription drugs. I anticipate that this will be a prominent issue nationwide during the upcoming year with opportunity for rheumatologists to lend their voices.
Many of the rheumatology community’s longstanding issues persist, and while progress has been made, more work remains to be done. Whether it’s accumulator programs, prior authorization, nonmedical switching, or step therapy, there will be opportunities in almost every state to engage in improving our ability to provide excellent care to our patients.
We tend to be motivated into action when one of these individual issues appear in our own state’s legislature; however, consistency of engagement is also important. While it is important to talk to your legislators when you need them to vote a certain way on a certain bill, scheduling a meeting with them or sending them a message detailing some of the issues that the rheumatology community faces before legislatures return in full swing is equally important to establish the relationship.
By making “rheum for action” now, you’ll have more impact when legislation relevant to our daily work does appear in the state legislatures. You can find your state representatives at the Coalition for State Rheumatology Organization’s Action Center.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As 2021 winds down, I reflect on the achievements the rheumatology community has had in the realm of advocacy throughout the year. And there were many: Seven states signed accumulator program bans into law, five states reformed the use of step therapy protocols, Texas passed an innovative “gold carding” law to reduce the burden of prior authorization, and West Virginia passed a rebate pass-through law to directly assist patients with high out-of-pocket costs – the first of its kind in the nation.
Of course, the close of another year also means gearing up for the year ahead. The majority of state legislatures are getting ready to open legislative sessions in the New Year. This means a year of new opportunities and new challenges. As a community, there are some key areas rheumatology will need to focus on during the course of the upcoming year as policy makers return to the business of policy making.
Many in the rheumatology community are aware that the buy and bill acquisition system has come under threat in recent years, mainly from payer mandates to use the alternative white bagging model. In some cases, payers have gone as far as to mandate the practice of “brown bagging,” or home infusion. These practices can endanger patient safety and overall quality of care. A study led by Matthew Baker, MD, of Stanford (Calif.) University found that biologic infusions administered at home, compared with those administered at a facility, were associated with increased adverse events requiring escalation of care; specifically, home infusions were associated with 25% increased odds of ED or hospital admission on the same or next day after infusion, compared with facility infusions, and 28% increased odds of permanent discontinuation of the biologic after emergency department or hospital admission. Additionally, site-of-care research by Paul Fronstin, PhD, at Employee Benefit Research Institute clearly shows that in-office infusion with physician supervision is far more cost effective than hospital and, in some cases, home infusion as well.
Further, the metastasis of these payer mandates is likely to severely limit availability of and access to care. It is unclear whether outpatient infusion, especially in private rheumatology practices, will prove sustainable in a world of white bagging. The net result of an expansion of the white-bagging requirements may well be broad access challenges that inconvenience patients deeply and irresponsibly.
The expansion of these mandates has not come without pushback, and rheumatologists should be prepared to advocate for policy that prohibits payers from mandating the use of white bagging, brown bagging, and home infusion. It is abundantly clear that arguments of safety and cost effectiveness are sufficient grounds for policy makers to curtail the mandatory use of these practices.
Similar to white bagging, another key issue in the year ahead is formulary construction based on the rebate system and proposed policies to address its attendant problems. Propagated by pharmacy benefit managers (PBMs), the rebate-based, or kickback, system of formulary construction often rewards higher-priced drugs with preferred placement regardless of whether they are the best and most affordable medications for our patients. A few states are beginning to address the affordability issue by mandating that the rebate/kickback acquired by the PBM be passed back to the patient at the point of sale. West Virginia passed a first-of-its-kind law to ensure that patients who generate drug rebates benefit from them by requiring that their cost shares are reduced by an amount equivalent to the rebate received by a health plan. This policy does not get at the root of the formulary construction problem, but if it is adopted more broadly across the country, it will deliver direct relief to patients who are struggling with out-of-pocket costs associated with prescription drugs. I anticipate that this will be a prominent issue nationwide during the upcoming year with opportunity for rheumatologists to lend their voices.
Many of the rheumatology community’s longstanding issues persist, and while progress has been made, more work remains to be done. Whether it’s accumulator programs, prior authorization, nonmedical switching, or step therapy, there will be opportunities in almost every state to engage in improving our ability to provide excellent care to our patients.
We tend to be motivated into action when one of these individual issues appear in our own state’s legislature; however, consistency of engagement is also important. While it is important to talk to your legislators when you need them to vote a certain way on a certain bill, scheduling a meeting with them or sending them a message detailing some of the issues that the rheumatology community faces before legislatures return in full swing is equally important to establish the relationship.
By making “rheum for action” now, you’ll have more impact when legislation relevant to our daily work does appear in the state legislatures. You can find your state representatives at the Coalition for State Rheumatology Organization’s Action Center.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Fully endovascular mitral valve replacement a limited success in feasibility study
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
FDA panel slams Endologix response to stent-graft safety issues
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.