User login
Home-grown apps for ObGyn clerkship students
Technology has revolutionized how we access information. One example is the increased use of mobile applications (apps). On the surface, building a new app may seem a daunting and intimidating task. However, new software—such as Glide (glideapps.com)—make it easy for anyone to design, build, and launch a custom web app within hours. This software is free for basic apps but does offer an upgrade for those wanting more professional services (glide-apps.com/pro). Here, by way of example, we identify an area of need and walk the reader
through the process of making an app.
Although there are many apps aimed at educating users on different aspects of obstetrics and gynecology, few are focused on undergraduate medical education (UME). With the assistance of Glide app building software, we created an app focused on providing rapid access to resources aimed at fulfilling medical student objectives from the Association of Professors of Gynecology and Obstetrics (APGO).1 We included 16 of the APGO objectives. On clicking an objective, the user is taken to a screen with links to associated APGO Educational Topic Video and Basic Science Videos. Basic Science Video links were included in order to provide longitudinal learning between the pre-clinical and clinical years of UME. We also created a tab for additional educational resources (including excerpts from the APGO Basic Clinical Skills Curriculum).2 We eventually added two other tabs: one for clerkship schedules that allows students to organize their daily schedule and another that facilitates quick contact with members of the clerkship team. As expected, the app was well-received by our students.
The steps needed for you to make your own app are listed in the FIGURE along with accompanying images for easy navigation. ●
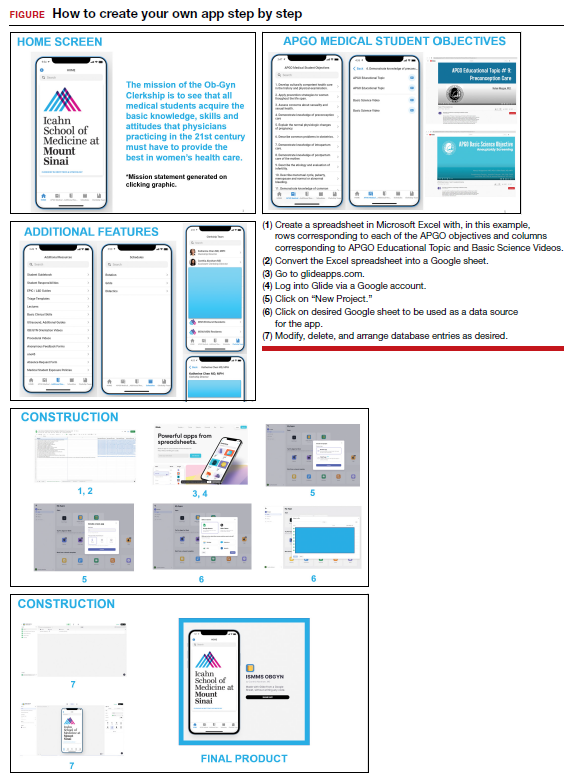
- Association of Professors of Gynecology and Obstetrics (APGO) Medical Student Educational Objectives, 11th ed;2019.
- Association of Professors of Gynecology and Obstetrics (APGO) Basic Clinical Skills Curriculum. Updated 2017.
Technology has revolutionized how we access information. One example is the increased use of mobile applications (apps). On the surface, building a new app may seem a daunting and intimidating task. However, new software—such as Glide (glideapps.com)—make it easy for anyone to design, build, and launch a custom web app within hours. This software is free for basic apps but does offer an upgrade for those wanting more professional services (glide-apps.com/pro). Here, by way of example, we identify an area of need and walk the reader
through the process of making an app.
Although there are many apps aimed at educating users on different aspects of obstetrics and gynecology, few are focused on undergraduate medical education (UME). With the assistance of Glide app building software, we created an app focused on providing rapid access to resources aimed at fulfilling medical student objectives from the Association of Professors of Gynecology and Obstetrics (APGO).1 We included 16 of the APGO objectives. On clicking an objective, the user is taken to a screen with links to associated APGO Educational Topic Video and Basic Science Videos. Basic Science Video links were included in order to provide longitudinal learning between the pre-clinical and clinical years of UME. We also created a tab for additional educational resources (including excerpts from the APGO Basic Clinical Skills Curriculum).2 We eventually added two other tabs: one for clerkship schedules that allows students to organize their daily schedule and another that facilitates quick contact with members of the clerkship team. As expected, the app was well-received by our students.
The steps needed for you to make your own app are listed in the FIGURE along with accompanying images for easy navigation. ●

Technology has revolutionized how we access information. One example is the increased use of mobile applications (apps). On the surface, building a new app may seem a daunting and intimidating task. However, new software—such as Glide (glideapps.com)—make it easy for anyone to design, build, and launch a custom web app within hours. This software is free for basic apps but does offer an upgrade for those wanting more professional services (glide-apps.com/pro). Here, by way of example, we identify an area of need and walk the reader
through the process of making an app.
Although there are many apps aimed at educating users on different aspects of obstetrics and gynecology, few are focused on undergraduate medical education (UME). With the assistance of Glide app building software, we created an app focused on providing rapid access to resources aimed at fulfilling medical student objectives from the Association of Professors of Gynecology and Obstetrics (APGO).1 We included 16 of the APGO objectives. On clicking an objective, the user is taken to a screen with links to associated APGO Educational Topic Video and Basic Science Videos. Basic Science Video links were included in order to provide longitudinal learning between the pre-clinical and clinical years of UME. We also created a tab for additional educational resources (including excerpts from the APGO Basic Clinical Skills Curriculum).2 We eventually added two other tabs: one for clerkship schedules that allows students to organize their daily schedule and another that facilitates quick contact with members of the clerkship team. As expected, the app was well-received by our students.
The steps needed for you to make your own app are listed in the FIGURE along with accompanying images for easy navigation. ●

- Association of Professors of Gynecology and Obstetrics (APGO) Medical Student Educational Objectives, 11th ed;2019.
- Association of Professors of Gynecology and Obstetrics (APGO) Basic Clinical Skills Curriculum. Updated 2017.
- Association of Professors of Gynecology and Obstetrics (APGO) Medical Student Educational Objectives, 11th ed;2019.
- Association of Professors of Gynecology and Obstetrics (APGO) Basic Clinical Skills Curriculum. Updated 2017.
Immunosuppressed rheumatic patients not at high risk of breakthrough COVID-19
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
AT THE EULAR 2022 CONGRESS
Lupus Erythematosus Tumidus Clinical Characteristics and Treatment: A Retrospective Review of 25 Patients
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
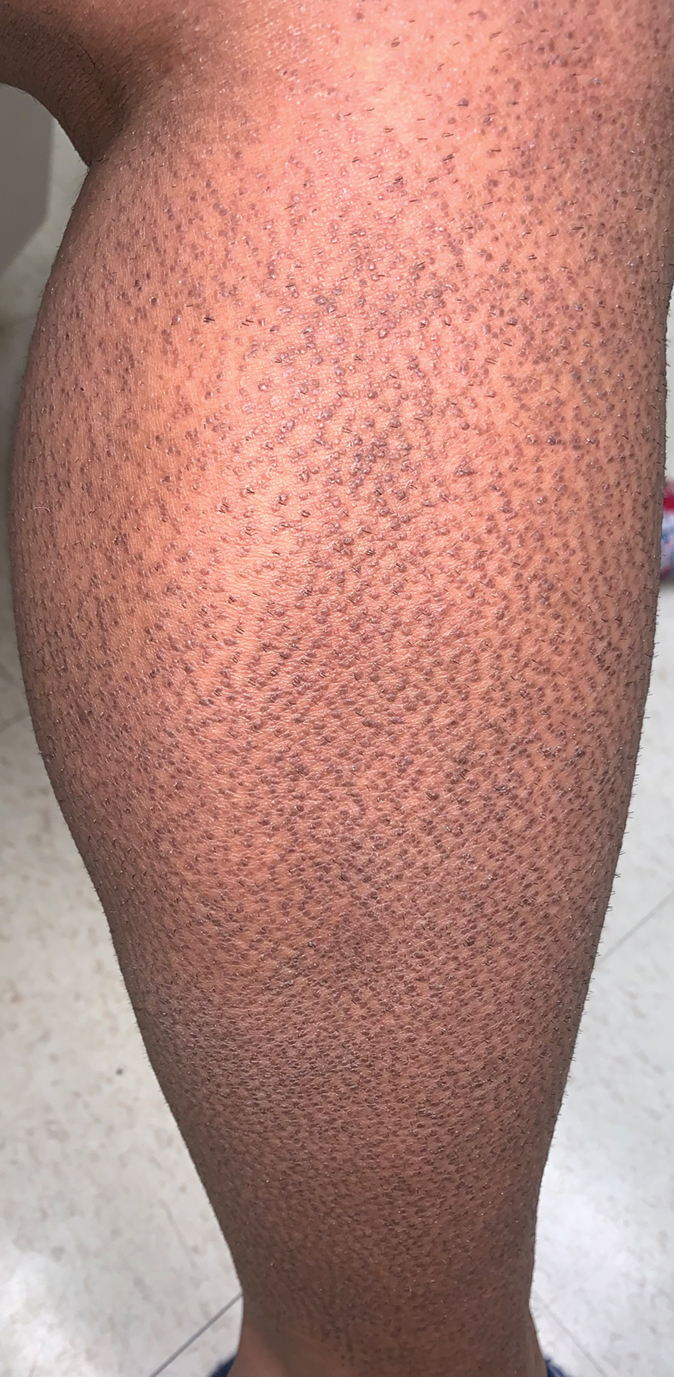
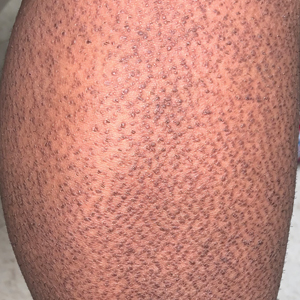
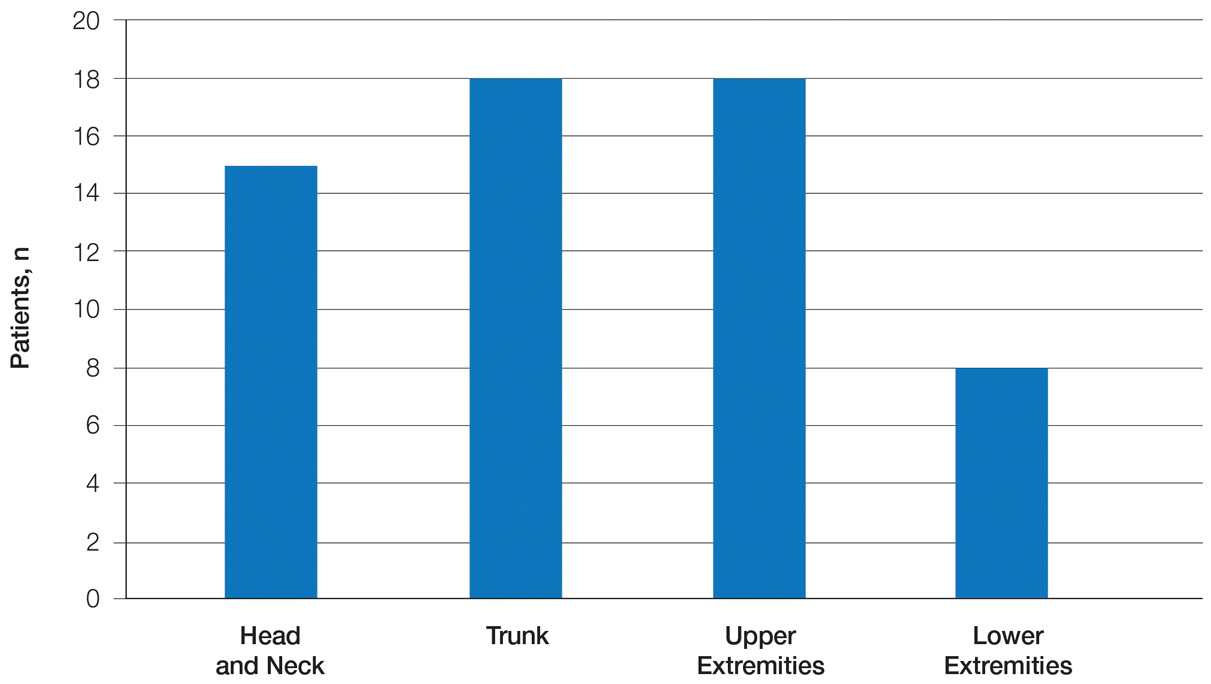
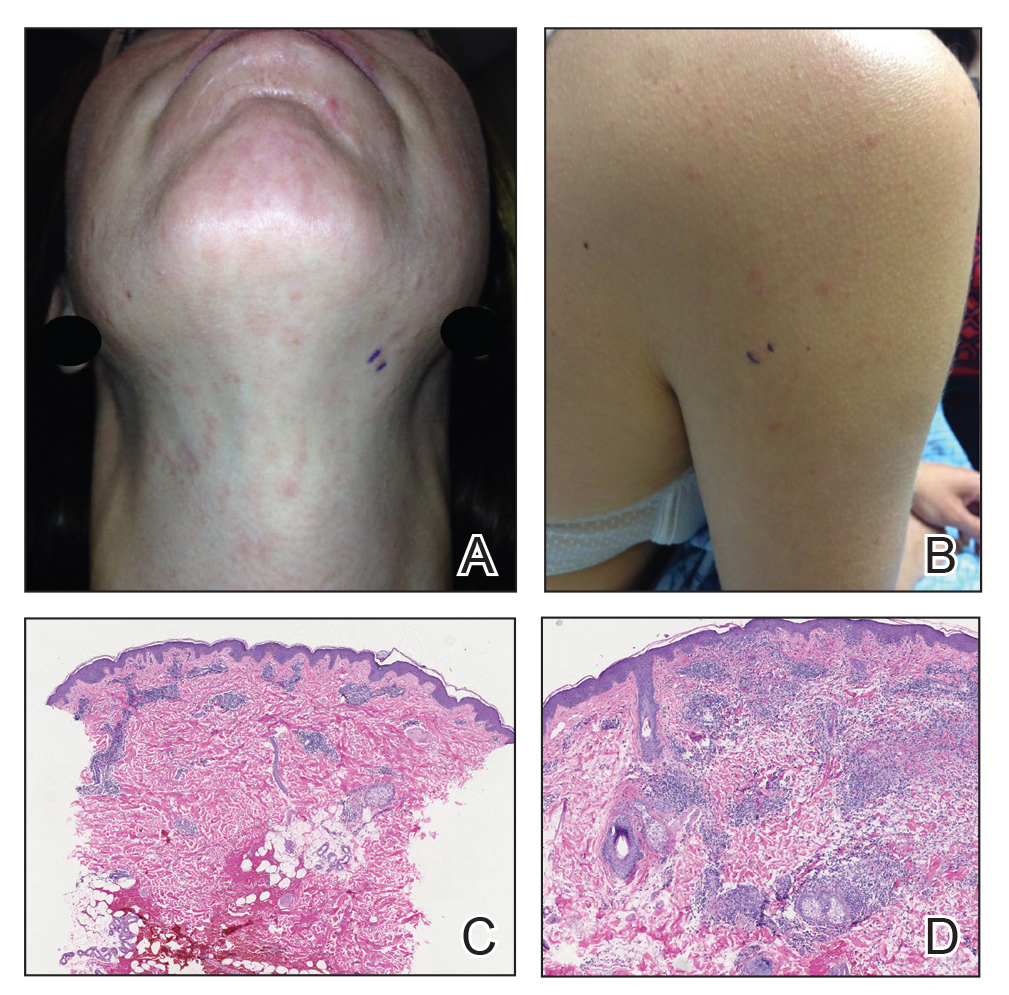
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
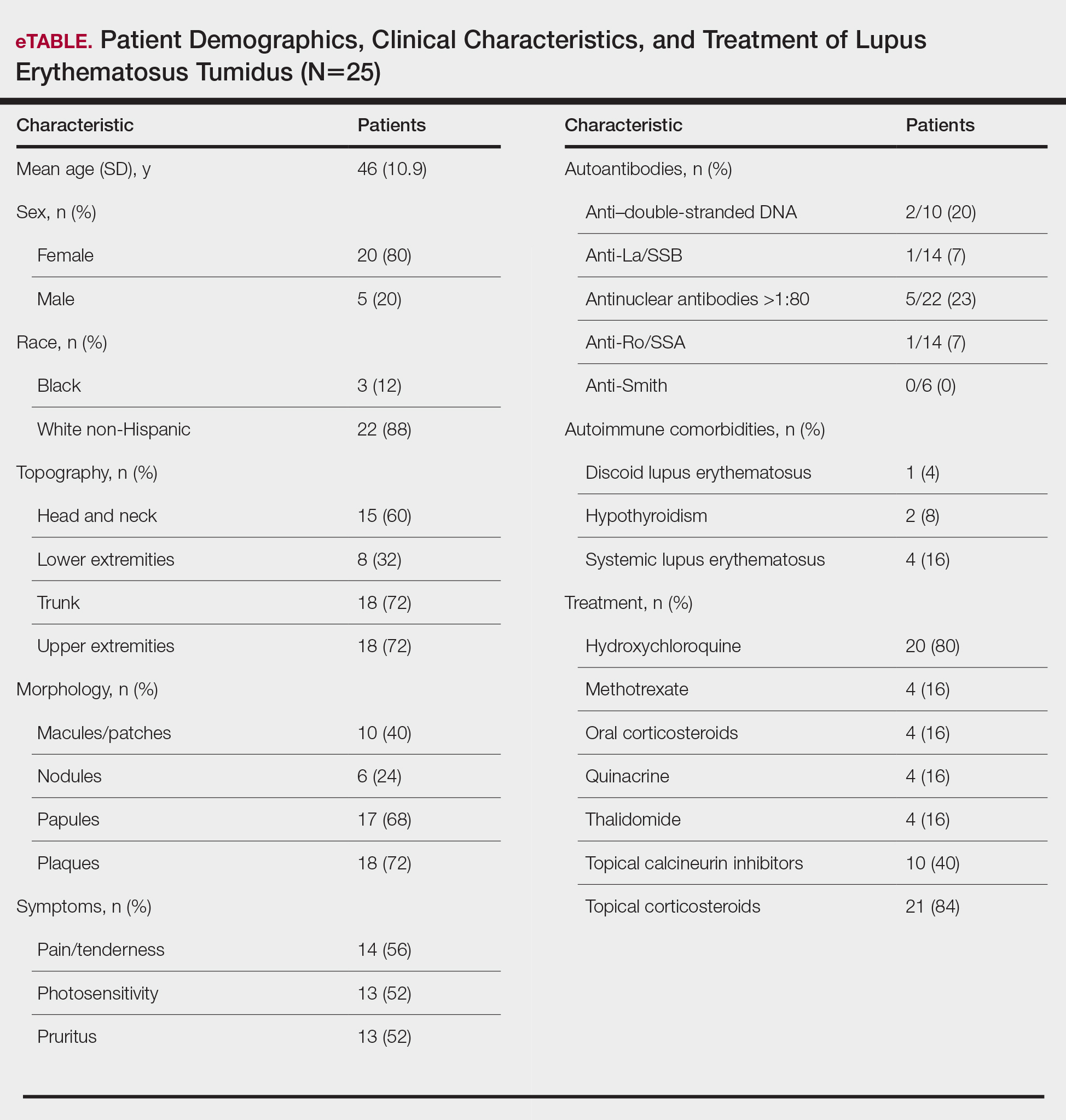
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7

Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.

Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.

Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).

Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7

Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.

Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.

Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).

Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7

Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Practice Points
- Approximately 20% of patients with lupus erythematosus tumidus (LET) will have positive antinuclear antibody titers.
- Along with cutaneous manifestations, approximately 50% of patients with LET also will have pruritus, tenderness, and photosensitivity.
- If LET is resistant to hydroxychloroquine, consider using quinacrine, methotrexate, or thalidomide.
Deployed Airbag Causes Bullous Reaction Following a Motor Vehicle Accident
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
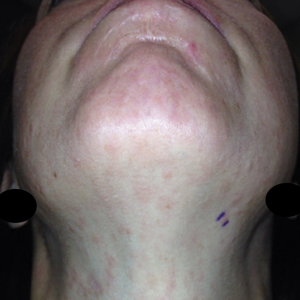
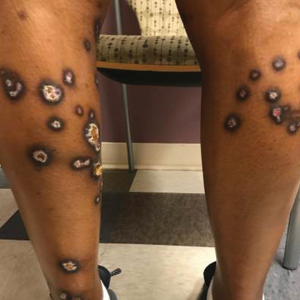
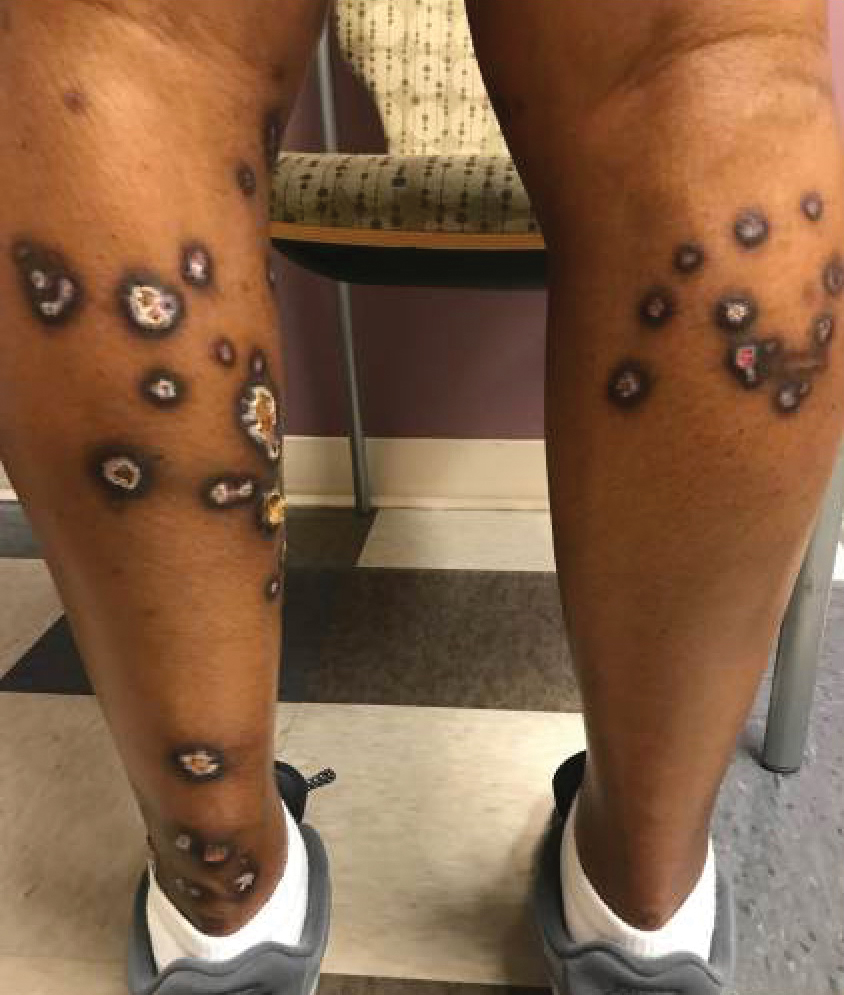
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

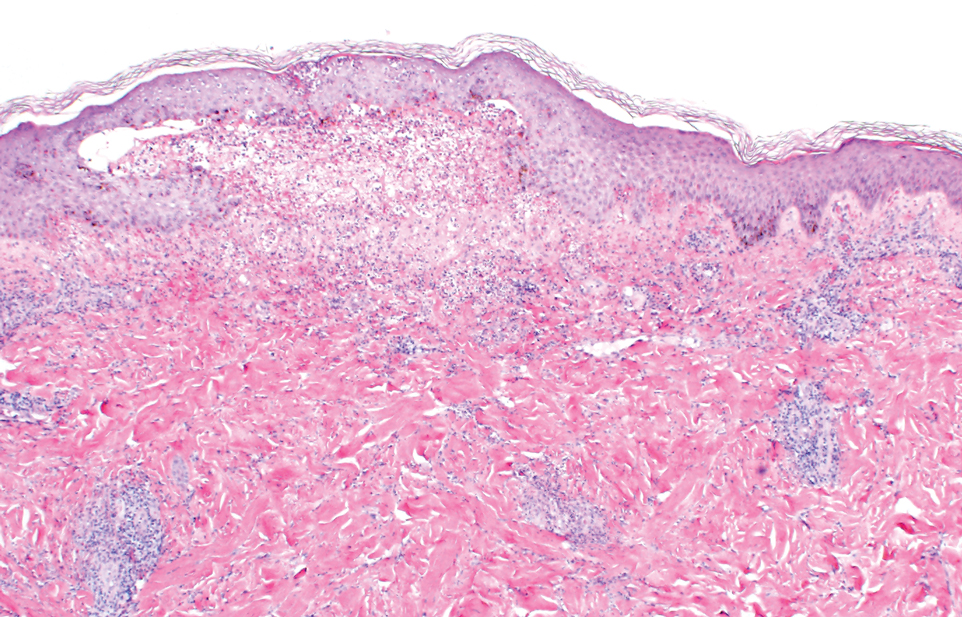
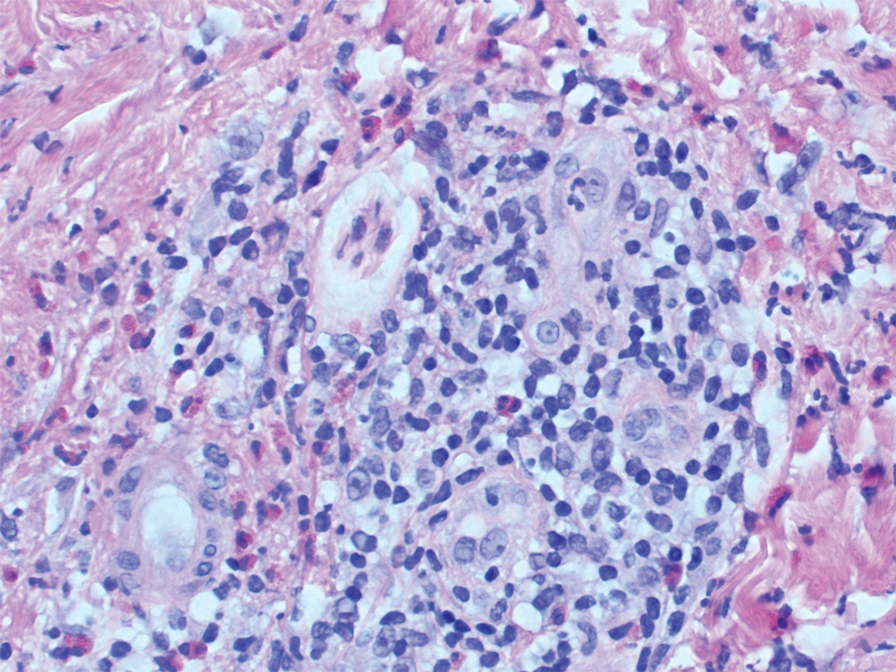
Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.
At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).

Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.


At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).


Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.


At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).


Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Practice Points
- This case highlights the potential for a bullous reaction following airbag deployment.
- After airbag deployment, it is important to immediately cleanse the affected areas of skin with soap and water.
The ERAS Supplemental Application: Current Status and Recommendations for Dermatology Applicants and Programs
In the 2021-2022 residency application cycle, the Association of American Medical Colleges (AAMC) piloted a supplemental application to accompany the standard residency application submitted via the AAMC’s Electronic Residency Application Service (ERAS).1 Dermatology was 1 of 3 specialties to participate in the pilot alongside internal medicine and general surgery. The goal was to develop a tool that could align applicants with programs that best matched their career goals as well as program and geographic preferences. The Association of Professors of Dermatology Residency Program Directors Section was an early advocate for the supplemental application, and members of our leadership were involved in the design, implementation, and evaluation of the pilot supplemental application.
Participating in the supplemental application was optional for both applicants and programs. The supplemental application included a Past Experiences section, which allowed applicants to highlight their 5 most meaningful research, work, and/or volunteer experiences and to describe a challenging life event that might not otherwise be included with their application. The geographic preferences section permitted applicants to select up to 3 regions of interest as well as to indicate an urban vs rural preference. Lastly, a preference-signaling section allowed dermatology applicants to send signals to up to 3 programs of particular interest.
With the close of another application cycle, applicants and programs will begin preparing for the 2022-2023 recruitment season. In this column, we present dermatology-specific data regarding the supplemental application, highlight tentative changes for the upcoming application cycle, and offer tips for applicants and programs as we approach year 2 of the supplemental application.
Results of Supplemental Application Evaluation Surveys
During the 2021-2022 recruitment season, 93% (950/1019) of dermatology applicants submitted the supplemental application, and 87% (117/135) of dermatology residency programs participated in the pilot.2 Surveys conducted by the AAMC between October 2021 and January 2022 showed that a large majority of dermatology programs used supplemental application data during initial application review when deciding who to interview. Eighty-three percent (40/48) of program directors felt that preference signals in particular helped them identify applicants they would have otherwise overlooked. Fifty-seven percent (4288/7516) of applicants across all specialties that participated in the pilot felt that preference signals would help them be noticed by their preferred programs.2 Preference signals were not evenly distributed among dermatology programs. Programs received an average of 23 signals, with a range of 2 to 87 (AAMC, unpublished data, February 2022).
Additional questions remain to be answered: How does the number of signals received affect application review? How often do geographic and program signals convert to interview offers and matches? Regardless, enthusiasm among dermatology programs for the supplemental application remains. In a recent survey of Association of Professors of Dermatology program directors, all 43 respondents planned to participate in the supplemental application again in the upcoming year (Ilana Rosman, MD; unpublished data; February 2022). The pilot will be expanded to include at least 12 other specialties.1As many who reviewed residency applications in 2021-2022 will attest, there was difficulty accessing the supplemental application data because it was not integrated into the Program Directors’ Work Station, the ERAS platform for programs to access applications, which will be remedied for the 2022-2023 iteration. Other tentative changes include modifications to the past experiences sections and timeline of the application.2
Utilizing the Supplemental Application: Recommendations to Applicants
Format of the Application—Applicants should familiarize themselves with the format of the supplemental application in advance and give themselves sufficient time to complete the application. In general, 3 to 4 hours of focused work should be enough time. Applicants should proofread for grammar and spelling before submitting.
Past Experiences—The past experiences section is intended to provide a focused snapshot of an applicant’s most meaningful activities and unique path to residency. Applicants should answer honestly based on their interests. If a student’s focus has been on volunteerism, the bulk of their 5 experiences listed may be related to service. Similarly, a student who has focused on research may preferentially highlight those experiences. In place of the long list of research, volunteer, and work experiences in the traditional ERAS application, applicants can highlight those activities in which they have been most invested. Applicants are encouraged to reflect on all genres of activities at any stage of their careers, even those not medical in nature, including work experience, military service, college athletics, or sustained musical or artistic achievement. Applicants should explain why each experience is meaningful rather than simply describing the activity.
Applicants also have the option to share a notable challenge they have overcome. It is not expected that each applicant will complete this question; in general, applicants who have not faced notable personal or professional obstacles should avoid answering. Additionally, if these challenges have been discussed in other areas of the application—for example, in the personal statement or medical student performance evaluation—it is not necessary to restate them here, though applicants can choose to do so. Examples of topics a student might discuss include being a first-generation college or medical student, growing up in poverty, facing notable personal or family health challenges, or having limited educational opportunities. It is important to share how this experience impacted an applicant’s journey to dermatology residency.
Geographic Preferences—The geographic preferences section can be difficult for applicants to navigate, as it may involve balancing a desire to attend a residency program in a particular region vs a greater desire to simply match in dermatology. In the past, programs may have made assumptions about geographic preferences based on an applicant’s birthplace, hometown, or medical school. In the supplemental application, applicants have the opportunity to directly reveal their preferences. We encourage applicants to be candid. Selecting a geographic region will not necessarily exclude applicants from consideration at other programs. For some applicants, program qualities may be more important than geography, or there may be no regional preferences. Those applicants can choose “no geographic preference.” There is considerable variability in how programs use geographic preferences. For this reason, it is in the best interest of applicants to simply respond honestly.
Preference Signaling—Preference signaling allows applicants to signal up to 3 preferred programs. Dermatology program directors agree that applicants should not signal their home program or programs at which they did in-person away rotations, as those programs would already be aware of the applicant’s interest. Although a signal increases the chances that the application will be reviewed holistically, it does not guarantee an interview offer. Programs may differentially utilize signals depending on multiple factors, including the number of signals received. We encourage applicants to discuss preference signaling strategies with advisors and focus on signaling programs in which they have genuine interest.
Recommendations to Selection Committees and Program Directors
The intent of the supplemental application is to provide a more meaningful picture of applicants and their experiences and preferences, with the goal of optimizing applicant-program fit. Programs should explicitly define for themselves the applicant characteristics and experiences they prioritize as well as their program goals. The supplemental application offers the potential to streamline holistic application review based on these elements. The short essay answers in the past experiences section permit reviewers to quickly scan for important experiences that align with the program’s recruitment goals. Importantly, reviewers should not penalize applicants who have not completed the question regarding other impactful life experiences, as not all applicants will have relevant information to share.
Some programs may find the geographic preferences section more valuable than others. Multiple factors affect how much weight will be given to geographic preferences, including program location and other characteristics that affect the desirability of the program to applicants. The competitiveness of the field, relatively low match rate, and limited number of programs may lead to less emphasis on geographic preferences in dermatology compared to other specialties. The purpose of this section is not to exclude applicants but to give programs more information that may help with alignment.
Anecdotally, many dermatology program directors were most interested in the preference signaling section of the supplemental application. Programs should consider signals to be evidence of strong preliminary interest. Programs may utilize signals differently depending on many factors such as the overall competitiveness of the program, program location, and the total number of signals the program receives. We recommend that programs holistically review all applications accompanied by a signal. Programs that utilize a points system may choose to award a certain number of points for a signal to their program. A signal might have a higher value at a program that receives only a few signals; conversely, a program that receives a large number of signals might not place tremendous value on the signal but may use it as a tiebreaker between similarly qualified applicants. Preference signaling is solely a tool for application review; because applicants’ preferences may change after the interview process, signals should not be utilized during ranking.
Next Steps
For program directors who have excitedly awaited residency application reform, the supplemental ERAS application is an important first step. Ultimately, we hope the supplemental application supplants much of the current residency application, serving as an efficient high-yield tool for holistically evaluating applicants’ academic and service records, accomplishments, and training preferences. Arriving at a new application will undoubtedly take time and discussion among the various stakeholders. Please continue to complete surveys from the AAMC, as feedback is the best method for refining the tool to serve its intended purpose.
Optimization of the application content is only one component of the reforms needed to improve the application process. Even with a revamped application tool, holistic review is challenging when programs are inundated with an ever-increasing number of applications. As such, we encourage stakeholders to simultaneously consider other potential reforms, such as caps on the number of applications, to allow programs and applicants the best opportunity for a mutually successful match.
- Supplemental ERAS application. Association of American Medical Colleges website. Accessed May 9, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplemental-eras-application-eras-2023-cycle
- Association of American Medical Colleges. Supplemental application data and reports. Accessed May 9, 2022. https://www.aamc.org/data-reports/students-residents/report/supplemental-eras-application-data-and-reports
In the 2021-2022 residency application cycle, the Association of American Medical Colleges (AAMC) piloted a supplemental application to accompany the standard residency application submitted via the AAMC’s Electronic Residency Application Service (ERAS).1 Dermatology was 1 of 3 specialties to participate in the pilot alongside internal medicine and general surgery. The goal was to develop a tool that could align applicants with programs that best matched their career goals as well as program and geographic preferences. The Association of Professors of Dermatology Residency Program Directors Section was an early advocate for the supplemental application, and members of our leadership were involved in the design, implementation, and evaluation of the pilot supplemental application.
Participating in the supplemental application was optional for both applicants and programs. The supplemental application included a Past Experiences section, which allowed applicants to highlight their 5 most meaningful research, work, and/or volunteer experiences and to describe a challenging life event that might not otherwise be included with their application. The geographic preferences section permitted applicants to select up to 3 regions of interest as well as to indicate an urban vs rural preference. Lastly, a preference-signaling section allowed dermatology applicants to send signals to up to 3 programs of particular interest.
With the close of another application cycle, applicants and programs will begin preparing for the 2022-2023 recruitment season. In this column, we present dermatology-specific data regarding the supplemental application, highlight tentative changes for the upcoming application cycle, and offer tips for applicants and programs as we approach year 2 of the supplemental application.
Results of Supplemental Application Evaluation Surveys
During the 2021-2022 recruitment season, 93% (950/1019) of dermatology applicants submitted the supplemental application, and 87% (117/135) of dermatology residency programs participated in the pilot.2 Surveys conducted by the AAMC between October 2021 and January 2022 showed that a large majority of dermatology programs used supplemental application data during initial application review when deciding who to interview. Eighty-three percent (40/48) of program directors felt that preference signals in particular helped them identify applicants they would have otherwise overlooked. Fifty-seven percent (4288/7516) of applicants across all specialties that participated in the pilot felt that preference signals would help them be noticed by their preferred programs.2 Preference signals were not evenly distributed among dermatology programs. Programs received an average of 23 signals, with a range of 2 to 87 (AAMC, unpublished data, February 2022).
Additional questions remain to be answered: How does the number of signals received affect application review? How often do geographic and program signals convert to interview offers and matches? Regardless, enthusiasm among dermatology programs for the supplemental application remains. In a recent survey of Association of Professors of Dermatology program directors, all 43 respondents planned to participate in the supplemental application again in the upcoming year (Ilana Rosman, MD; unpublished data; February 2022). The pilot will be expanded to include at least 12 other specialties.1As many who reviewed residency applications in 2021-2022 will attest, there was difficulty accessing the supplemental application data because it was not integrated into the Program Directors’ Work Station, the ERAS platform for programs to access applications, which will be remedied for the 2022-2023 iteration. Other tentative changes include modifications to the past experiences sections and timeline of the application.2
Utilizing the Supplemental Application: Recommendations to Applicants
Format of the Application—Applicants should familiarize themselves with the format of the supplemental application in advance and give themselves sufficient time to complete the application. In general, 3 to 4 hours of focused work should be enough time. Applicants should proofread for grammar and spelling before submitting.
Past Experiences—The past experiences section is intended to provide a focused snapshot of an applicant’s most meaningful activities and unique path to residency. Applicants should answer honestly based on their interests. If a student’s focus has been on volunteerism, the bulk of their 5 experiences listed may be related to service. Similarly, a student who has focused on research may preferentially highlight those experiences. In place of the long list of research, volunteer, and work experiences in the traditional ERAS application, applicants can highlight those activities in which they have been most invested. Applicants are encouraged to reflect on all genres of activities at any stage of their careers, even those not medical in nature, including work experience, military service, college athletics, or sustained musical or artistic achievement. Applicants should explain why each experience is meaningful rather than simply describing the activity.
Applicants also have the option to share a notable challenge they have overcome. It is not expected that each applicant will complete this question; in general, applicants who have not faced notable personal or professional obstacles should avoid answering. Additionally, if these challenges have been discussed in other areas of the application—for example, in the personal statement or medical student performance evaluation—it is not necessary to restate them here, though applicants can choose to do so. Examples of topics a student might discuss include being a first-generation college or medical student, growing up in poverty, facing notable personal or family health challenges, or having limited educational opportunities. It is important to share how this experience impacted an applicant’s journey to dermatology residency.
Geographic Preferences—The geographic preferences section can be difficult for applicants to navigate, as it may involve balancing a desire to attend a residency program in a particular region vs a greater desire to simply match in dermatology. In the past, programs may have made assumptions about geographic preferences based on an applicant’s birthplace, hometown, or medical school. In the supplemental application, applicants have the opportunity to directly reveal their preferences. We encourage applicants to be candid. Selecting a geographic region will not necessarily exclude applicants from consideration at other programs. For some applicants, program qualities may be more important than geography, or there may be no regional preferences. Those applicants can choose “no geographic preference.” There is considerable variability in how programs use geographic preferences. For this reason, it is in the best interest of applicants to simply respond honestly.
Preference Signaling—Preference signaling allows applicants to signal up to 3 preferred programs. Dermatology program directors agree that applicants should not signal their home program or programs at which they did in-person away rotations, as those programs would already be aware of the applicant’s interest. Although a signal increases the chances that the application will be reviewed holistically, it does not guarantee an interview offer. Programs may differentially utilize signals depending on multiple factors, including the number of signals received. We encourage applicants to discuss preference signaling strategies with advisors and focus on signaling programs in which they have genuine interest.
Recommendations to Selection Committees and Program Directors
The intent of the supplemental application is to provide a more meaningful picture of applicants and their experiences and preferences, with the goal of optimizing applicant-program fit. Programs should explicitly define for themselves the applicant characteristics and experiences they prioritize as well as their program goals. The supplemental application offers the potential to streamline holistic application review based on these elements. The short essay answers in the past experiences section permit reviewers to quickly scan for important experiences that align with the program’s recruitment goals. Importantly, reviewers should not penalize applicants who have not completed the question regarding other impactful life experiences, as not all applicants will have relevant information to share.
Some programs may find the geographic preferences section more valuable than others. Multiple factors affect how much weight will be given to geographic preferences, including program location and other characteristics that affect the desirability of the program to applicants. The competitiveness of the field, relatively low match rate, and limited number of programs may lead to less emphasis on geographic preferences in dermatology compared to other specialties. The purpose of this section is not to exclude applicants but to give programs more information that may help with alignment.
Anecdotally, many dermatology program directors were most interested in the preference signaling section of the supplemental application. Programs should consider signals to be evidence of strong preliminary interest. Programs may utilize signals differently depending on many factors such as the overall competitiveness of the program, program location, and the total number of signals the program receives. We recommend that programs holistically review all applications accompanied by a signal. Programs that utilize a points system may choose to award a certain number of points for a signal to their program. A signal might have a higher value at a program that receives only a few signals; conversely, a program that receives a large number of signals might not place tremendous value on the signal but may use it as a tiebreaker between similarly qualified applicants. Preference signaling is solely a tool for application review; because applicants’ preferences may change after the interview process, signals should not be utilized during ranking.
Next Steps
For program directors who have excitedly awaited residency application reform, the supplemental ERAS application is an important first step. Ultimately, we hope the supplemental application supplants much of the current residency application, serving as an efficient high-yield tool for holistically evaluating applicants’ academic and service records, accomplishments, and training preferences. Arriving at a new application will undoubtedly take time and discussion among the various stakeholders. Please continue to complete surveys from the AAMC, as feedback is the best method for refining the tool to serve its intended purpose.
Optimization of the application content is only one component of the reforms needed to improve the application process. Even with a revamped application tool, holistic review is challenging when programs are inundated with an ever-increasing number of applications. As such, we encourage stakeholders to simultaneously consider other potential reforms, such as caps on the number of applications, to allow programs and applicants the best opportunity for a mutually successful match.
In the 2021-2022 residency application cycle, the Association of American Medical Colleges (AAMC) piloted a supplemental application to accompany the standard residency application submitted via the AAMC’s Electronic Residency Application Service (ERAS).1 Dermatology was 1 of 3 specialties to participate in the pilot alongside internal medicine and general surgery. The goal was to develop a tool that could align applicants with programs that best matched their career goals as well as program and geographic preferences. The Association of Professors of Dermatology Residency Program Directors Section was an early advocate for the supplemental application, and members of our leadership were involved in the design, implementation, and evaluation of the pilot supplemental application.
Participating in the supplemental application was optional for both applicants and programs. The supplemental application included a Past Experiences section, which allowed applicants to highlight their 5 most meaningful research, work, and/or volunteer experiences and to describe a challenging life event that might not otherwise be included with their application. The geographic preferences section permitted applicants to select up to 3 regions of interest as well as to indicate an urban vs rural preference. Lastly, a preference-signaling section allowed dermatology applicants to send signals to up to 3 programs of particular interest.
With the close of another application cycle, applicants and programs will begin preparing for the 2022-2023 recruitment season. In this column, we present dermatology-specific data regarding the supplemental application, highlight tentative changes for the upcoming application cycle, and offer tips for applicants and programs as we approach year 2 of the supplemental application.
Results of Supplemental Application Evaluation Surveys
During the 2021-2022 recruitment season, 93% (950/1019) of dermatology applicants submitted the supplemental application, and 87% (117/135) of dermatology residency programs participated in the pilot.2 Surveys conducted by the AAMC between October 2021 and January 2022 showed that a large majority of dermatology programs used supplemental application data during initial application review when deciding who to interview. Eighty-three percent (40/48) of program directors felt that preference signals in particular helped them identify applicants they would have otherwise overlooked. Fifty-seven percent (4288/7516) of applicants across all specialties that participated in the pilot felt that preference signals would help them be noticed by their preferred programs.2 Preference signals were not evenly distributed among dermatology programs. Programs received an average of 23 signals, with a range of 2 to 87 (AAMC, unpublished data, February 2022).
Additional questions remain to be answered: How does the number of signals received affect application review? How often do geographic and program signals convert to interview offers and matches? Regardless, enthusiasm among dermatology programs for the supplemental application remains. In a recent survey of Association of Professors of Dermatology program directors, all 43 respondents planned to participate in the supplemental application again in the upcoming year (Ilana Rosman, MD; unpublished data; February 2022). The pilot will be expanded to include at least 12 other specialties.1As many who reviewed residency applications in 2021-2022 will attest, there was difficulty accessing the supplemental application data because it was not integrated into the Program Directors’ Work Station, the ERAS platform for programs to access applications, which will be remedied for the 2022-2023 iteration. Other tentative changes include modifications to the past experiences sections and timeline of the application.2
Utilizing the Supplemental Application: Recommendations to Applicants
Format of the Application—Applicants should familiarize themselves with the format of the supplemental application in advance and give themselves sufficient time to complete the application. In general, 3 to 4 hours of focused work should be enough time. Applicants should proofread for grammar and spelling before submitting.
Past Experiences—The past experiences section is intended to provide a focused snapshot of an applicant’s most meaningful activities and unique path to residency. Applicants should answer honestly based on their interests. If a student’s focus has been on volunteerism, the bulk of their 5 experiences listed may be related to service. Similarly, a student who has focused on research may preferentially highlight those experiences. In place of the long list of research, volunteer, and work experiences in the traditional ERAS application, applicants can highlight those activities in which they have been most invested. Applicants are encouraged to reflect on all genres of activities at any stage of their careers, even those not medical in nature, including work experience, military service, college athletics, or sustained musical or artistic achievement. Applicants should explain why each experience is meaningful rather than simply describing the activity.
Applicants also have the option to share a notable challenge they have overcome. It is not expected that each applicant will complete this question; in general, applicants who have not faced notable personal or professional obstacles should avoid answering. Additionally, if these challenges have been discussed in other areas of the application—for example, in the personal statement or medical student performance evaluation—it is not necessary to restate them here, though applicants can choose to do so. Examples of topics a student might discuss include being a first-generation college or medical student, growing up in poverty, facing notable personal or family health challenges, or having limited educational opportunities. It is important to share how this experience impacted an applicant’s journey to dermatology residency.
Geographic Preferences—The geographic preferences section can be difficult for applicants to navigate, as it may involve balancing a desire to attend a residency program in a particular region vs a greater desire to simply match in dermatology. In the past, programs may have made assumptions about geographic preferences based on an applicant’s birthplace, hometown, or medical school. In the supplemental application, applicants have the opportunity to directly reveal their preferences. We encourage applicants to be candid. Selecting a geographic region will not necessarily exclude applicants from consideration at other programs. For some applicants, program qualities may be more important than geography, or there may be no regional preferences. Those applicants can choose “no geographic preference.” There is considerable variability in how programs use geographic preferences. For this reason, it is in the best interest of applicants to simply respond honestly.
Preference Signaling—Preference signaling allows applicants to signal up to 3 preferred programs. Dermatology program directors agree that applicants should not signal their home program or programs at which they did in-person away rotations, as those programs would already be aware of the applicant’s interest. Although a signal increases the chances that the application will be reviewed holistically, it does not guarantee an interview offer. Programs may differentially utilize signals depending on multiple factors, including the number of signals received. We encourage applicants to discuss preference signaling strategies with advisors and focus on signaling programs in which they have genuine interest.
Recommendations to Selection Committees and Program Directors
The intent of the supplemental application is to provide a more meaningful picture of applicants and their experiences and preferences, with the goal of optimizing applicant-program fit. Programs should explicitly define for themselves the applicant characteristics and experiences they prioritize as well as their program goals. The supplemental application offers the potential to streamline holistic application review based on these elements. The short essay answers in the past experiences section permit reviewers to quickly scan for important experiences that align with the program’s recruitment goals. Importantly, reviewers should not penalize applicants who have not completed the question regarding other impactful life experiences, as not all applicants will have relevant information to share.
Some programs may find the geographic preferences section more valuable than others. Multiple factors affect how much weight will be given to geographic preferences, including program location and other characteristics that affect the desirability of the program to applicants. The competitiveness of the field, relatively low match rate, and limited number of programs may lead to less emphasis on geographic preferences in dermatology compared to other specialties. The purpose of this section is not to exclude applicants but to give programs more information that may help with alignment.
Anecdotally, many dermatology program directors were most interested in the preference signaling section of the supplemental application. Programs should consider signals to be evidence of strong preliminary interest. Programs may utilize signals differently depending on many factors such as the overall competitiveness of the program, program location, and the total number of signals the program receives. We recommend that programs holistically review all applications accompanied by a signal. Programs that utilize a points system may choose to award a certain number of points for a signal to their program. A signal might have a higher value at a program that receives only a few signals; conversely, a program that receives a large number of signals might not place tremendous value on the signal but may use it as a tiebreaker between similarly qualified applicants. Preference signaling is solely a tool for application review; because applicants’ preferences may change after the interview process, signals should not be utilized during ranking.
Next Steps
For program directors who have excitedly awaited residency application reform, the supplemental ERAS application is an important first step. Ultimately, we hope the supplemental application supplants much of the current residency application, serving as an efficient high-yield tool for holistically evaluating applicants’ academic and service records, accomplishments, and training preferences. Arriving at a new application will undoubtedly take time and discussion among the various stakeholders. Please continue to complete surveys from the AAMC, as feedback is the best method for refining the tool to serve its intended purpose.
Optimization of the application content is only one component of the reforms needed to improve the application process. Even with a revamped application tool, holistic review is challenging when programs are inundated with an ever-increasing number of applications. As such, we encourage stakeholders to simultaneously consider other potential reforms, such as caps on the number of applications, to allow programs and applicants the best opportunity for a mutually successful match.
- Supplemental ERAS application. Association of American Medical Colleges website. Accessed May 9, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplemental-eras-application-eras-2023-cycle
- Association of American Medical Colleges. Supplemental application data and reports. Accessed May 9, 2022. https://www.aamc.org/data-reports/students-residents/report/supplemental-eras-application-data-and-reports
- Supplemental ERAS application. Association of American Medical Colleges website. Accessed May 9, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplemental-eras-application-eras-2023-cycle
- Association of American Medical Colleges. Supplemental application data and reports. Accessed May 9, 2022. https://www.aamc.org/data-reports/students-residents/report/supplemental-eras-application-data-and-reports
Practice Points
- The Electronic Residency Application Service (ERAS) Supplemental Application was piloted in the 2021-2022 residency application cycle and was utilized by the vast majority of dermatology applicants and programs.
- Survey data suggested that both applicants and programs found the supplemental application useful, particularly the preference signaling portion.
- The supplemental application will return for the 2022-2023 application cycle and will be integrated into the MyERAS workstation platform for easier access by programs.
Going Beyond Hydroquinone: Alternative Skin Lightening Agents
Disorders of hyperpigmentation—melasma, postinflammatory hyperpigmentation, lichen planus pigmentosus, erythema dyschromicum perstans, and pigmented contact dermatitis, among others—are common and challenging to treat. Although they can affect individuals of all skin types, they most commonly are seen in skin of color; in fact, dyspigmentation is one of the most common chief concerns for which individuals of color see a dermatologist.1,2
For many years, hydroquinone (HQ) was one of the main options available for use as a lightening agent. Although effective, it has the risk of causing irritant dermatitis, potentially leading to further dyspigmentation, in addition to the risk of ochronosis with long-term use. It remains an important and useful treatment for pigmentary disorders, but there are numerous other lightening agents that also can be considered in the treatment of disorders of hyperpigmentation.
Herein, we provide recommendations for traditional and newer non-HQ lightening agents that can be considered when treating disorders of hyperpigmentation.
Traditional Non-HQ Lightening Agents
Retinoids—Retinoids are topical vitamin A derivatives that have been used safely and effectively for decades in the treatment of pigmentary disorders. Retinoids have multiple mechanisms of action in improving pigmentation. In addition to impeding tyrosinase induction, they inhibit pigment transfer to keratinocytes and lead to accelerated pigment loss due to epidermal shedding.3 Over-the-counter formulations include retinol, retinaldehyde, and adapalene. Prescription formulations include tretinoin and tazarotene in different strengths and vehicle formulations.4
Glycolic Acid—Glycolic acid is derived from sugarcane and is considered an α-hydroxy acid that leads to rapid desquamation of pigmented keratinocytes.5 Glycolic acid can not only be used in chemical peels but also in topical creams. It is the most common α-hydroxy acid peel and is sometimes paired with HQ and other topical lightening agents for increased penetration. Glycolic acid peels are available in concentrations of 20% to 70% and can be used at various depths. When used incorrectly, it can cause redness, burning, and even skin discoloration; however, when used at the proper concentrations and depth according to Fitzpatrick skin type, there typically are no notable adverse effects, and clinical results are favorable.
Kojic Acid—Kojic acid is a natural metabolite derived from fungi and is widely used in Asian countries. It works by inhibiting the catecholase activity of tyrosinase6 and typically is available in concentrations of 1% to 4%. A study suggested that a concentration of 1% or less typically is safe to use for prolonged periods without adverse effects. Although not more effective than HQ as a monotherapy, kojic acid has been shown to haveimproved efficacy when used in combination with other lightening agents.7
Azelaic Acid—Azelaic acid works by inhibiting tyrosinase, mitochondrial oxidoreductase activation, and DNA synthesis. It preferentially targets heavily pigmented melanocytes and possesses anti-inflammatory and antibacterial properties.8 A 20% concentration of azelaic acid was compared to HQ 4% for the treatment of melasma, and results revealed that the liposomal form of azelaic acid was considerably more tolerable than HQ 4% and also more effective.9
Licorice Extracts—Licorice extracts have been safely used in several cosmeceutical skin lightening products.10 The main active compounds in licorice root are glabridin and liquiritin, which work to disperse melanin. These compounds often are used topically at concentrations of 10% to 40%. A study by Amer and Metwalli11 found that topical liquiritin produced a reduction of pigmentary intensity, with 80% of patients showing an excellent response, which was described as no difference between the previously pigmented area and the normal skin surrounding it.
Aloesin—Aloesin is a low-molecular-weight glycoprotein found in aloe vera plants. Its mechanism of action includes competitive inhibition of the dihydroxyphenylalanine oxidation site, resulting in the inhibition of tyrosinase.12 It often is combined with arbutin for an enhanced lightening effect.
Niacinamide—Niacinamide is a form of vitamin B3 that works by suppressing the transfer of melanosomes to keratinocytes.13 In addition to its skin lightening effects, it also is photoprotective and antimicrobial, and its tolerability and safety have led to its inclusion in many cosmeceutical and prescription products.14
Ascorbic Acid—Ascorbic acid affects the monopherase activity of tyrosinase, thus reducing the synthesis of melanin. It also serves as an antioxidant in the skin by preventing the production of free radicals that can induce melanogenesis.15 Although it tends to be well tolerated with a low adverse effect profile, its relative instability and varying permeability can present a challenge. It is less effective as a monotherapy, so it often is combined with other lightening ingredients for greater efficacy.
Corticosteroids—Topical corticosteroids are anti-inflammatory and impact melanogenesis, though the mechanism of action of the latter has not been fully elucidated.16,17 Low- to mid-potency topical steroids often are used in conjunction with skin lightening products to diminish irritation and decrease inflammation.18 However, prolonged use of corticosteroids can lead to cutaneous adverse effects such as striae, hypopigmentation, and acne, as well as systemic side effects if there is sufficient absorption over time.
Soybean Extracts—Soybean extracts contain serine protease inhibitors that reduce the transfer of melanosomes into keratinocytes by inhibiting the PAR-2 (protease-activated receptor 2) pathway.19,20
Ellagic Acid—Ellagic acid is found in common plants such as eucalyptus and strawberry as well as green tea.21 It works as an antioxidant and decreases melanogenesis through inhibition of tyrosinase activity.
Paper Mulberry—Paper mulberry extract comes from the roots of the Broussonetia papyrifera tree and functions by inhibiting tyrosinase activity. It is widely used in South Africa and Europe.22
Resveratrol—Resveratrol is an ingredient extracted from Morus alba L and functions as an antimelanogentic agent by directly inhibiting tyrosinase as well as transcriptional and posttranscriptional processing of tyrosinase.23 It also holds antiproliferative, anti-inflammatory, and antioxidant properties and has widely been used for antiaging and skin lightening purposes.24
Newer Non-HQ Lightening Agents
Silymarin—Silymarin (also known as milk thistle [Silybum marianum]), is a polyphenolic flavonoid that possesses anticarcinogenic, antioxidant, and anti-inflammatory properties. It prevents melanin production in a dose-dependent manner by inhibiting levodopa (L-dopa) oxidation activity of tyrosinase and also reduces the expression of tyrosinase protein.25 In combination with vitamins C and E and hexylresorcinol, silymarin has been found to reduce the effects of photodamage, brighten skin, improve evenness and lines, as well as improve global facial appearance.26
Malassezin—Malassezin is an indole produced by Malessezia furfur yeast and has recently been investigated for melanogenesis suppression. Grimes et al27 assessed the efficacy of topical malassezin in 7 patients with facial hyperpigmentation applied twice daily for 14 weeks. Punch biopsies were taken at weeks 0, 8, 14, and 22. Biopsies from weeks 8 and 14 demonstrated reduced epidermal melanin compared to baseline in all participants; however, at 22 weeks, biopsies showed no difference in melanin content compared to baseline, indicating a temporary process induced by the malassezin.27 More clinical studies are needed to investigate this further.
N-acetyl-glucosamine—N-acetyl-glucosamine is an aminosaccharide that inhibits the glycosylation of tyrosinase as well as its function in melanogenesis.28 It is synthesized and included in topical products for wound healing, rhytides, moisturization, and pigmentation disorders.
Topical Tranexamic Acid—Tranexamic acid traditionally has been used orally for the treatment of menorrhagia but also has been found to be beneficial as a therapy for hyperpigmentation and erythema. Tranexamic acid interferes with plasmin activity, thus indirectly inhibiting melanogenesis while also inhibiting angiogenesis by targeting vascular endothelial growth factor (VEGF) receptors.29 It also leads to an increase in the levels of β-endorphin and μ-opioid receptors as well as the expression of estrogen receptor β on the surface of mast cells.30 Its oral benefit led to the development of topical formulations, typically in 2% to 5% concentrations. It has proven particularly beneficial in the treatment of melasma due to its effects on improving pigmentation, erythema, and skin barrier function.31 Topical tranexamic acid has a relatively high safety profile, with minor side effects such as transient skin irritation and erythema being reported.32
Cysteamine—Cysteamine inhibits tyrosinase, peroxidase, and chelating copper ions necessary for melanogenesis. It has proven to be effective in treating melasma and chronic severe postinflammatory hyperpigmentation when used in a 5% cream formulation.33,34 Lima et al35 were the first to compare the effects of topical cysteamine to HQ in the treatment of facial melasma. They found that the mean reduction in modified Melasma Area and Severity Index score was 24% for cysteamine and 41% for HQ after 60 days. There were no severe adverse effects with either treatment group.35
Final Thoughts
Hydroquinone remains the gold standard for treatment of hyperpigmentation; however, its side-effect profile and risk of ochronosis with long-term use has ushered in various other safe and effective skin lightening agents that can be used as monotherapies or in combination with other lightening agents. Many of these products also can be used effectively with procedural treatments such as chemical peels, lasers, and microneedling for enhanced absorption and efficacy. As newer agents are developed, additional well-designed studies will be needed to determine their safety and efficacy in different skin types as well as their role in the treatment of pigmentary disorders.
- Woolery-Lloyd H, Kammer JN. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171-175. doi:10.1016/j.sder.2011.06.004
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman AM, Grove GL, Hirose R, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 pt 2):836-859. doi:10.1016/s0190-9622(86)70242-9
- Sharad J. Glycolic acid peel therapy—a current review. Clin Cosmet Investig Dermatol. 2013;6:281-288. doi:10.2147/CCID.S34029
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34:1000-1014. doi:10.1111/pcmr.12986
- Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582-593. doi:10.1016/j.biopha.2018.12.006
- Schulte BC, Wu W, Rosen T. Azelaic acid: evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14:964-968.
- Akl EM. Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study [published online April 7, 2021]. J Dermatol Treat. doi:10.1080/09546634.2021.1905765
- Holloway VL. Ethnic cosmetic products. Dermatol Clin. 2003;21:743-749. doi:10.1016/s0733-8635(03)00089-5
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299-301. doi:10.1046/j.1365-4362.2000.00943.x
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-340. doi:10.1034/j.1600-0749.2002.02014.x
- Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147:20-31. doi:10.1046/j.1365-2133.2002.04834.x
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27:311-315. doi:10.1159/000359974
- Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. doi:10.1046/j.1524-4725.2002.01129.x
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378. doi:10.4103/0378-6323.178903
- Petit L, Piérard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169-181. doi:10.1046/j.1467-2494.2003.00182.x
- Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatol Basel Switz. 1994;188:170. doi:10.1159/000247129
- Paine C, Sharlow E, Liebel F, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587-595. doi:10.1046/j.1523-1747.2001.01291.x
- Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162-167. doi:10.1046/j.1523-1747.2000.00035.x
- Shimogaki H, Tanaka Y, Tamai H, et al. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291-303. doi:10.1046/j.1467-2494.2000.00023.x
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 pt 2):886-889; discussion 889. doi:10.1111/j.1524-4725.2005.31736
- Na JI, Shin JW, Choi HR, et al. Resveratrol as a multifunctional topical hypopigmenting agent [published online February 22, 2019]. Int J Mol Sci. 2019;20:956. doi:10.3390/ijms20040956
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21:84-90. doi:10.1080/14764172.2018.1469767
- Choo SJ, Ryoo IJ, Kim YH, et al. Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 2009;61:663-667. doi:10.1211/jpp/61.05.0016
- Draelos ZD, Diaz I, Cohen A, et al. A novel skin brightening topical technology. J Cosmet Dermatol. 2020;19:3280-3285. doi:10.1111/jocd.13741
- Grimes P, Bhawan J, Howell M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21:141-145. doi:10.36849/jdd.6596
- Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309-315. doi:10.1111/j.1473-2165.2006.00277.x
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911. doi:10.7150/ijms.44188
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid inhibits the plasma and non-irradiated skin markers of photoaging induced by long-term UVA eye irradiation in female mice. Biomed Pharmacother. 2018;107:54-58. doi:10.1016/j.biopha.2018.07.146
- da Silva Souza ID, Lampe L, Winn D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J Cosmet Dermatol. 2021;20:561-565. doi:10.1111/jocd.13545
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781. doi:10.2340/00015555-2668
- Mathe N, Balogun M, Yoo J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J Cosmet Dermatol. 2021;20:204-206. doi:10.1111/jocd.13755
- Mansouri P, Farshi S, Hashemi Z, et al. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173:209-217. doi:10.1111/bjd.13424
- Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59:1531-1536. doi:10.1111/ijd.15146
Disorders of hyperpigmentation—melasma, postinflammatory hyperpigmentation, lichen planus pigmentosus, erythema dyschromicum perstans, and pigmented contact dermatitis, among others—are common and challenging to treat. Although they can affect individuals of all skin types, they most commonly are seen in skin of color; in fact, dyspigmentation is one of the most common chief concerns for which individuals of color see a dermatologist.1,2
For many years, hydroquinone (HQ) was one of the main options available for use as a lightening agent. Although effective, it has the risk of causing irritant dermatitis, potentially leading to further dyspigmentation, in addition to the risk of ochronosis with long-term use. It remains an important and useful treatment for pigmentary disorders, but there are numerous other lightening agents that also can be considered in the treatment of disorders of hyperpigmentation.
Herein, we provide recommendations for traditional and newer non-HQ lightening agents that can be considered when treating disorders of hyperpigmentation.
Traditional Non-HQ Lightening Agents
Retinoids—Retinoids are topical vitamin A derivatives that have been used safely and effectively for decades in the treatment of pigmentary disorders. Retinoids have multiple mechanisms of action in improving pigmentation. In addition to impeding tyrosinase induction, they inhibit pigment transfer to keratinocytes and lead to accelerated pigment loss due to epidermal shedding.3 Over-the-counter formulations include retinol, retinaldehyde, and adapalene. Prescription formulations include tretinoin and tazarotene in different strengths and vehicle formulations.4
Glycolic Acid—Glycolic acid is derived from sugarcane and is considered an α-hydroxy acid that leads to rapid desquamation of pigmented keratinocytes.5 Glycolic acid can not only be used in chemical peels but also in topical creams. It is the most common α-hydroxy acid peel and is sometimes paired with HQ and other topical lightening agents for increased penetration. Glycolic acid peels are available in concentrations of 20% to 70% and can be used at various depths. When used incorrectly, it can cause redness, burning, and even skin discoloration; however, when used at the proper concentrations and depth according to Fitzpatrick skin type, there typically are no notable adverse effects, and clinical results are favorable.
Kojic Acid—Kojic acid is a natural metabolite derived from fungi and is widely used in Asian countries. It works by inhibiting the catecholase activity of tyrosinase6 and typically is available in concentrations of 1% to 4%. A study suggested that a concentration of 1% or less typically is safe to use for prolonged periods without adverse effects. Although not more effective than HQ as a monotherapy, kojic acid has been shown to haveimproved efficacy when used in combination with other lightening agents.7
Azelaic Acid—Azelaic acid works by inhibiting tyrosinase, mitochondrial oxidoreductase activation, and DNA synthesis. It preferentially targets heavily pigmented melanocytes and possesses anti-inflammatory and antibacterial properties.8 A 20% concentration of azelaic acid was compared to HQ 4% for the treatment of melasma, and results revealed that the liposomal form of azelaic acid was considerably more tolerable than HQ 4% and also more effective.9
Licorice Extracts—Licorice extracts have been safely used in several cosmeceutical skin lightening products.10 The main active compounds in licorice root are glabridin and liquiritin, which work to disperse melanin. These compounds often are used topically at concentrations of 10% to 40%. A study by Amer and Metwalli11 found that topical liquiritin produced a reduction of pigmentary intensity, with 80% of patients showing an excellent response, which was described as no difference between the previously pigmented area and the normal skin surrounding it.
Aloesin—Aloesin is a low-molecular-weight glycoprotein found in aloe vera plants. Its mechanism of action includes competitive inhibition of the dihydroxyphenylalanine oxidation site, resulting in the inhibition of tyrosinase.12 It often is combined with arbutin for an enhanced lightening effect.
Niacinamide—Niacinamide is a form of vitamin B3 that works by suppressing the transfer of melanosomes to keratinocytes.13 In addition to its skin lightening effects, it also is photoprotective and antimicrobial, and its tolerability and safety have led to its inclusion in many cosmeceutical and prescription products.14
Ascorbic Acid—Ascorbic acid affects the monopherase activity of tyrosinase, thus reducing the synthesis of melanin. It also serves as an antioxidant in the skin by preventing the production of free radicals that can induce melanogenesis.15 Although it tends to be well tolerated with a low adverse effect profile, its relative instability and varying permeability can present a challenge. It is less effective as a monotherapy, so it often is combined with other lightening ingredients for greater efficacy.
Corticosteroids—Topical corticosteroids are anti-inflammatory and impact melanogenesis, though the mechanism of action of the latter has not been fully elucidated.16,17 Low- to mid-potency topical steroids often are used in conjunction with skin lightening products to diminish irritation and decrease inflammation.18 However, prolonged use of corticosteroids can lead to cutaneous adverse effects such as striae, hypopigmentation, and acne, as well as systemic side effects if there is sufficient absorption over time.
Soybean Extracts—Soybean extracts contain serine protease inhibitors that reduce the transfer of melanosomes into keratinocytes by inhibiting the PAR-2 (protease-activated receptor 2) pathway.19,20
Ellagic Acid—Ellagic acid is found in common plants such as eucalyptus and strawberry as well as green tea.21 It works as an antioxidant and decreases melanogenesis through inhibition of tyrosinase activity.
Paper Mulberry—Paper mulberry extract comes from the roots of the Broussonetia papyrifera tree and functions by inhibiting tyrosinase activity. It is widely used in South Africa and Europe.22
Resveratrol—Resveratrol is an ingredient extracted from Morus alba L and functions as an antimelanogentic agent by directly inhibiting tyrosinase as well as transcriptional and posttranscriptional processing of tyrosinase.23 It also holds antiproliferative, anti-inflammatory, and antioxidant properties and has widely been used for antiaging and skin lightening purposes.24
Newer Non-HQ Lightening Agents
Silymarin—Silymarin (also known as milk thistle [Silybum marianum]), is a polyphenolic flavonoid that possesses anticarcinogenic, antioxidant, and anti-inflammatory properties. It prevents melanin production in a dose-dependent manner by inhibiting levodopa (L-dopa) oxidation activity of tyrosinase and also reduces the expression of tyrosinase protein.25 In combination with vitamins C and E and hexylresorcinol, silymarin has been found to reduce the effects of photodamage, brighten skin, improve evenness and lines, as well as improve global facial appearance.26
Malassezin—Malassezin is an indole produced by Malessezia furfur yeast and has recently been investigated for melanogenesis suppression. Grimes et al27 assessed the efficacy of topical malassezin in 7 patients with facial hyperpigmentation applied twice daily for 14 weeks. Punch biopsies were taken at weeks 0, 8, 14, and 22. Biopsies from weeks 8 and 14 demonstrated reduced epidermal melanin compared to baseline in all participants; however, at 22 weeks, biopsies showed no difference in melanin content compared to baseline, indicating a temporary process induced by the malassezin.27 More clinical studies are needed to investigate this further.
N-acetyl-glucosamine—N-acetyl-glucosamine is an aminosaccharide that inhibits the glycosylation of tyrosinase as well as its function in melanogenesis.28 It is synthesized and included in topical products for wound healing, rhytides, moisturization, and pigmentation disorders.
Topical Tranexamic Acid—Tranexamic acid traditionally has been used orally for the treatment of menorrhagia but also has been found to be beneficial as a therapy for hyperpigmentation and erythema. Tranexamic acid interferes with plasmin activity, thus indirectly inhibiting melanogenesis while also inhibiting angiogenesis by targeting vascular endothelial growth factor (VEGF) receptors.29 It also leads to an increase in the levels of β-endorphin and μ-opioid receptors as well as the expression of estrogen receptor β on the surface of mast cells.30 Its oral benefit led to the development of topical formulations, typically in 2% to 5% concentrations. It has proven particularly beneficial in the treatment of melasma due to its effects on improving pigmentation, erythema, and skin barrier function.31 Topical tranexamic acid has a relatively high safety profile, with minor side effects such as transient skin irritation and erythema being reported.32
Cysteamine—Cysteamine inhibits tyrosinase, peroxidase, and chelating copper ions necessary for melanogenesis. It has proven to be effective in treating melasma and chronic severe postinflammatory hyperpigmentation when used in a 5% cream formulation.33,34 Lima et al35 were the first to compare the effects of topical cysteamine to HQ in the treatment of facial melasma. They found that the mean reduction in modified Melasma Area and Severity Index score was 24% for cysteamine and 41% for HQ after 60 days. There were no severe adverse effects with either treatment group.35
Final Thoughts
Hydroquinone remains the gold standard for treatment of hyperpigmentation; however, its side-effect profile and risk of ochronosis with long-term use has ushered in various other safe and effective skin lightening agents that can be used as monotherapies or in combination with other lightening agents. Many of these products also can be used effectively with procedural treatments such as chemical peels, lasers, and microneedling for enhanced absorption and efficacy. As newer agents are developed, additional well-designed studies will be needed to determine their safety and efficacy in different skin types as well as their role in the treatment of pigmentary disorders.
Disorders of hyperpigmentation—melasma, postinflammatory hyperpigmentation, lichen planus pigmentosus, erythema dyschromicum perstans, and pigmented contact dermatitis, among others—are common and challenging to treat. Although they can affect individuals of all skin types, they most commonly are seen in skin of color; in fact, dyspigmentation is one of the most common chief concerns for which individuals of color see a dermatologist.1,2
For many years, hydroquinone (HQ) was one of the main options available for use as a lightening agent. Although effective, it has the risk of causing irritant dermatitis, potentially leading to further dyspigmentation, in addition to the risk of ochronosis with long-term use. It remains an important and useful treatment for pigmentary disorders, but there are numerous other lightening agents that also can be considered in the treatment of disorders of hyperpigmentation.
Herein, we provide recommendations for traditional and newer non-HQ lightening agents that can be considered when treating disorders of hyperpigmentation.
Traditional Non-HQ Lightening Agents
Retinoids—Retinoids are topical vitamin A derivatives that have been used safely and effectively for decades in the treatment of pigmentary disorders. Retinoids have multiple mechanisms of action in improving pigmentation. In addition to impeding tyrosinase induction, they inhibit pigment transfer to keratinocytes and lead to accelerated pigment loss due to epidermal shedding.3 Over-the-counter formulations include retinol, retinaldehyde, and adapalene. Prescription formulations include tretinoin and tazarotene in different strengths and vehicle formulations.4
Glycolic Acid—Glycolic acid is derived from sugarcane and is considered an α-hydroxy acid that leads to rapid desquamation of pigmented keratinocytes.5 Glycolic acid can not only be used in chemical peels but also in topical creams. It is the most common α-hydroxy acid peel and is sometimes paired with HQ and other topical lightening agents for increased penetration. Glycolic acid peels are available in concentrations of 20% to 70% and can be used at various depths. When used incorrectly, it can cause redness, burning, and even skin discoloration; however, when used at the proper concentrations and depth according to Fitzpatrick skin type, there typically are no notable adverse effects, and clinical results are favorable.
Kojic Acid—Kojic acid is a natural metabolite derived from fungi and is widely used in Asian countries. It works by inhibiting the catecholase activity of tyrosinase6 and typically is available in concentrations of 1% to 4%. A study suggested that a concentration of 1% or less typically is safe to use for prolonged periods without adverse effects. Although not more effective than HQ as a monotherapy, kojic acid has been shown to haveimproved efficacy when used in combination with other lightening agents.7
Azelaic Acid—Azelaic acid works by inhibiting tyrosinase, mitochondrial oxidoreductase activation, and DNA synthesis. It preferentially targets heavily pigmented melanocytes and possesses anti-inflammatory and antibacterial properties.8 A 20% concentration of azelaic acid was compared to HQ 4% for the treatment of melasma, and results revealed that the liposomal form of azelaic acid was considerably more tolerable than HQ 4% and also more effective.9
Licorice Extracts—Licorice extracts have been safely used in several cosmeceutical skin lightening products.10 The main active compounds in licorice root are glabridin and liquiritin, which work to disperse melanin. These compounds often are used topically at concentrations of 10% to 40%. A study by Amer and Metwalli11 found that topical liquiritin produced a reduction of pigmentary intensity, with 80% of patients showing an excellent response, which was described as no difference between the previously pigmented area and the normal skin surrounding it.
Aloesin—Aloesin is a low-molecular-weight glycoprotein found in aloe vera plants. Its mechanism of action includes competitive inhibition of the dihydroxyphenylalanine oxidation site, resulting in the inhibition of tyrosinase.12 It often is combined with arbutin for an enhanced lightening effect.
Niacinamide—Niacinamide is a form of vitamin B3 that works by suppressing the transfer of melanosomes to keratinocytes.13 In addition to its skin lightening effects, it also is photoprotective and antimicrobial, and its tolerability and safety have led to its inclusion in many cosmeceutical and prescription products.14
Ascorbic Acid—Ascorbic acid affects the monopherase activity of tyrosinase, thus reducing the synthesis of melanin. It also serves as an antioxidant in the skin by preventing the production of free radicals that can induce melanogenesis.15 Although it tends to be well tolerated with a low adverse effect profile, its relative instability and varying permeability can present a challenge. It is less effective as a monotherapy, so it often is combined with other lightening ingredients for greater efficacy.
Corticosteroids—Topical corticosteroids are anti-inflammatory and impact melanogenesis, though the mechanism of action of the latter has not been fully elucidated.16,17 Low- to mid-potency topical steroids often are used in conjunction with skin lightening products to diminish irritation and decrease inflammation.18 However, prolonged use of corticosteroids can lead to cutaneous adverse effects such as striae, hypopigmentation, and acne, as well as systemic side effects if there is sufficient absorption over time.
Soybean Extracts—Soybean extracts contain serine protease inhibitors that reduce the transfer of melanosomes into keratinocytes by inhibiting the PAR-2 (protease-activated receptor 2) pathway.19,20
Ellagic Acid—Ellagic acid is found in common plants such as eucalyptus and strawberry as well as green tea.21 It works as an antioxidant and decreases melanogenesis through inhibition of tyrosinase activity.
Paper Mulberry—Paper mulberry extract comes from the roots of the Broussonetia papyrifera tree and functions by inhibiting tyrosinase activity. It is widely used in South Africa and Europe.22
Resveratrol—Resveratrol is an ingredient extracted from Morus alba L and functions as an antimelanogentic agent by directly inhibiting tyrosinase as well as transcriptional and posttranscriptional processing of tyrosinase.23 It also holds antiproliferative, anti-inflammatory, and antioxidant properties and has widely been used for antiaging and skin lightening purposes.24
Newer Non-HQ Lightening Agents
Silymarin—Silymarin (also known as milk thistle [Silybum marianum]), is a polyphenolic flavonoid that possesses anticarcinogenic, antioxidant, and anti-inflammatory properties. It prevents melanin production in a dose-dependent manner by inhibiting levodopa (L-dopa) oxidation activity of tyrosinase and also reduces the expression of tyrosinase protein.25 In combination with vitamins C and E and hexylresorcinol, silymarin has been found to reduce the effects of photodamage, brighten skin, improve evenness and lines, as well as improve global facial appearance.26
Malassezin—Malassezin is an indole produced by Malessezia furfur yeast and has recently been investigated for melanogenesis suppression. Grimes et al27 assessed the efficacy of topical malassezin in 7 patients with facial hyperpigmentation applied twice daily for 14 weeks. Punch biopsies were taken at weeks 0, 8, 14, and 22. Biopsies from weeks 8 and 14 demonstrated reduced epidermal melanin compared to baseline in all participants; however, at 22 weeks, biopsies showed no difference in melanin content compared to baseline, indicating a temporary process induced by the malassezin.27 More clinical studies are needed to investigate this further.
N-acetyl-glucosamine—N-acetyl-glucosamine is an aminosaccharide that inhibits the glycosylation of tyrosinase as well as its function in melanogenesis.28 It is synthesized and included in topical products for wound healing, rhytides, moisturization, and pigmentation disorders.
Topical Tranexamic Acid—Tranexamic acid traditionally has been used orally for the treatment of menorrhagia but also has been found to be beneficial as a therapy for hyperpigmentation and erythema. Tranexamic acid interferes with plasmin activity, thus indirectly inhibiting melanogenesis while also inhibiting angiogenesis by targeting vascular endothelial growth factor (VEGF) receptors.29 It also leads to an increase in the levels of β-endorphin and μ-opioid receptors as well as the expression of estrogen receptor β on the surface of mast cells.30 Its oral benefit led to the development of topical formulations, typically in 2% to 5% concentrations. It has proven particularly beneficial in the treatment of melasma due to its effects on improving pigmentation, erythema, and skin barrier function.31 Topical tranexamic acid has a relatively high safety profile, with minor side effects such as transient skin irritation and erythema being reported.32
Cysteamine—Cysteamine inhibits tyrosinase, peroxidase, and chelating copper ions necessary for melanogenesis. It has proven to be effective in treating melasma and chronic severe postinflammatory hyperpigmentation when used in a 5% cream formulation.33,34 Lima et al35 were the first to compare the effects of topical cysteamine to HQ in the treatment of facial melasma. They found that the mean reduction in modified Melasma Area and Severity Index score was 24% for cysteamine and 41% for HQ after 60 days. There were no severe adverse effects with either treatment group.35
Final Thoughts
Hydroquinone remains the gold standard for treatment of hyperpigmentation; however, its side-effect profile and risk of ochronosis with long-term use has ushered in various other safe and effective skin lightening agents that can be used as monotherapies or in combination with other lightening agents. Many of these products also can be used effectively with procedural treatments such as chemical peels, lasers, and microneedling for enhanced absorption and efficacy. As newer agents are developed, additional well-designed studies will be needed to determine their safety and efficacy in different skin types as well as their role in the treatment of pigmentary disorders.
- Woolery-Lloyd H, Kammer JN. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171-175. doi:10.1016/j.sder.2011.06.004
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman AM, Grove GL, Hirose R, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 pt 2):836-859. doi:10.1016/s0190-9622(86)70242-9
- Sharad J. Glycolic acid peel therapy—a current review. Clin Cosmet Investig Dermatol. 2013;6:281-288. doi:10.2147/CCID.S34029
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34:1000-1014. doi:10.1111/pcmr.12986
- Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582-593. doi:10.1016/j.biopha.2018.12.006
- Schulte BC, Wu W, Rosen T. Azelaic acid: evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14:964-968.
- Akl EM. Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study [published online April 7, 2021]. J Dermatol Treat. doi:10.1080/09546634.2021.1905765
- Holloway VL. Ethnic cosmetic products. Dermatol Clin. 2003;21:743-749. doi:10.1016/s0733-8635(03)00089-5
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299-301. doi:10.1046/j.1365-4362.2000.00943.x
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-340. doi:10.1034/j.1600-0749.2002.02014.x
- Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147:20-31. doi:10.1046/j.1365-2133.2002.04834.x
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27:311-315. doi:10.1159/000359974
- Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. doi:10.1046/j.1524-4725.2002.01129.x
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378. doi:10.4103/0378-6323.178903
- Petit L, Piérard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169-181. doi:10.1046/j.1467-2494.2003.00182.x
- Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatol Basel Switz. 1994;188:170. doi:10.1159/000247129
- Paine C, Sharlow E, Liebel F, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587-595. doi:10.1046/j.1523-1747.2001.01291.x
- Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162-167. doi:10.1046/j.1523-1747.2000.00035.x
- Shimogaki H, Tanaka Y, Tamai H, et al. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291-303. doi:10.1046/j.1467-2494.2000.00023.x
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 pt 2):886-889; discussion 889. doi:10.1111/j.1524-4725.2005.31736
- Na JI, Shin JW, Choi HR, et al. Resveratrol as a multifunctional topical hypopigmenting agent [published online February 22, 2019]. Int J Mol Sci. 2019;20:956. doi:10.3390/ijms20040956
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21:84-90. doi:10.1080/14764172.2018.1469767
- Choo SJ, Ryoo IJ, Kim YH, et al. Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 2009;61:663-667. doi:10.1211/jpp/61.05.0016
- Draelos ZD, Diaz I, Cohen A, et al. A novel skin brightening topical technology. J Cosmet Dermatol. 2020;19:3280-3285. doi:10.1111/jocd.13741
- Grimes P, Bhawan J, Howell M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21:141-145. doi:10.36849/jdd.6596
- Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309-315. doi:10.1111/j.1473-2165.2006.00277.x
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911. doi:10.7150/ijms.44188
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid inhibits the plasma and non-irradiated skin markers of photoaging induced by long-term UVA eye irradiation in female mice. Biomed Pharmacother. 2018;107:54-58. doi:10.1016/j.biopha.2018.07.146
- da Silva Souza ID, Lampe L, Winn D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J Cosmet Dermatol. 2021;20:561-565. doi:10.1111/jocd.13545
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781. doi:10.2340/00015555-2668
- Mathe N, Balogun M, Yoo J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J Cosmet Dermatol. 2021;20:204-206. doi:10.1111/jocd.13755
- Mansouri P, Farshi S, Hashemi Z, et al. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173:209-217. doi:10.1111/bjd.13424
- Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59:1531-1536. doi:10.1111/ijd.15146
- Woolery-Lloyd H, Kammer JN. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171-175. doi:10.1016/j.sder.2011.06.004
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman AM, Grove GL, Hirose R, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 pt 2):836-859. doi:10.1016/s0190-9622(86)70242-9
- Sharad J. Glycolic acid peel therapy—a current review. Clin Cosmet Investig Dermatol. 2013;6:281-288. doi:10.2147/CCID.S34029
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34:1000-1014. doi:10.1111/pcmr.12986
- Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582-593. doi:10.1016/j.biopha.2018.12.006
- Schulte BC, Wu W, Rosen T. Azelaic acid: evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14:964-968.
- Akl EM. Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study [published online April 7, 2021]. J Dermatol Treat. doi:10.1080/09546634.2021.1905765
- Holloway VL. Ethnic cosmetic products. Dermatol Clin. 2003;21:743-749. doi:10.1016/s0733-8635(03)00089-5
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299-301. doi:10.1046/j.1365-4362.2000.00943.x
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-340. doi:10.1034/j.1600-0749.2002.02014.x
- Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147:20-31. doi:10.1046/j.1365-2133.2002.04834.x
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27:311-315. doi:10.1159/000359974
- Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. doi:10.1046/j.1524-4725.2002.01129.x
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378. doi:10.4103/0378-6323.178903
- Petit L, Piérard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169-181. doi:10.1046/j.1467-2494.2003.00182.x
- Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatol Basel Switz. 1994;188:170. doi:10.1159/000247129
- Paine C, Sharlow E, Liebel F, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587-595. doi:10.1046/j.1523-1747.2001.01291.x
- Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162-167. doi:10.1046/j.1523-1747.2000.00035.x
- Shimogaki H, Tanaka Y, Tamai H, et al. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291-303. doi:10.1046/j.1467-2494.2000.00023.x
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 pt 2):886-889; discussion 889. doi:10.1111/j.1524-4725.2005.31736
- Na JI, Shin JW, Choi HR, et al. Resveratrol as a multifunctional topical hypopigmenting agent [published online February 22, 2019]. Int J Mol Sci. 2019;20:956. doi:10.3390/ijms20040956
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21:84-90. doi:10.1080/14764172.2018.1469767
- Choo SJ, Ryoo IJ, Kim YH, et al. Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 2009;61:663-667. doi:10.1211/jpp/61.05.0016
- Draelos ZD, Diaz I, Cohen A, et al. A novel skin brightening topical technology. J Cosmet Dermatol. 2020;19:3280-3285. doi:10.1111/jocd.13741
- Grimes P, Bhawan J, Howell M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21:141-145. doi:10.36849/jdd.6596
- Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309-315. doi:10.1111/j.1473-2165.2006.00277.x
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911. doi:10.7150/ijms.44188
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid inhibits the plasma and non-irradiated skin markers of photoaging induced by long-term UVA eye irradiation in female mice. Biomed Pharmacother. 2018;107:54-58. doi:10.1016/j.biopha.2018.07.146
- da Silva Souza ID, Lampe L, Winn D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J Cosmet Dermatol. 2021;20:561-565. doi:10.1111/jocd.13545
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781. doi:10.2340/00015555-2668
- Mathe N, Balogun M, Yoo J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J Cosmet Dermatol. 2021;20:204-206. doi:10.1111/jocd.13755
- Mansouri P, Farshi S, Hashemi Z, et al. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173:209-217. doi:10.1111/bjd.13424
- Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59:1531-1536. doi:10.1111/ijd.15146
Stem cell transplants for MS are a ‘reasonable option,’ but questions persist
NATIONAL HARBOR, MD. – .” But, he said, it remains unclear which patients should undergo the procedure.
An especially pressing question is “whether transplant is an alternative to our high-efficacy disease-modifying therapies” (DMTs) in some high-risk patients, Jeffrey A. Cohen, MD, director of experimental therapeutics at Cleveland Clinic’s Mellen Center, said in a presidential lecture at the annual meeting of the Consortium of Multiple Sclerosis Centers.
A handful of ongoing randomized controlled trials will bring answers, he said.
Stem cell therapy exists because there are gaps in MS treatment, Dr. Cohen said. “We have now more than 20 medications. However, none of these therapies is completely effective in all patients. In particular, there are some patients with very active disease who continue to have relapses or new MRI lesion activity despite treatment with all of the available therapies.”
And in progressive MS, the efficacy of a couple of agents is modest and mainly for people with ongoing focal lesion activity, he said. “Finally, all of these currently available therapies are intended primarily to prevent the accumulation of damage, and none of them directly promotes repair. So despite our progress in the field, there still are a number of unmet needs.”
‘Rebooting’ the immune system
Several types of stem cell therapy exist, including remyelination and anti-inflammatory therapy, Dr. Cohen said. In his lecture, he focused on immunoablative or myeloablative therapy followed by autologous hematopoietic stem cell transplantation.
This “complicated, multistep procedure” involves first eliminating the immune system to get rid of pathogenic inflammatory cells. This “big component is actually the therapy for MS. It’s also the step that has the most potential complications,” he said.
According to Dr. Cohen, the next step has been described as “rebooting” the immune system.
Does this procedure help patients with MS? In relapsing MS, reports suggest there’s “almost complete abrogation of disease activity following transplant,” he said, “a benefit that’s lasted 5-10-15 years. In addition, there’s also a benefit on the accumulation of CNS damage as measured by whole brain atrophy.”
Recent data, he said, suggests that MS patients most likely to benefit are young, developed MS relatively recently, are still ambulatory, and have highly active MS despite treatment with first- and second-line agents.
However, there have only been two randomized controlled trials of stem cell transplantation versus DMT, and Dr. Cohen said both studies have limitations. The first one, from 2015, is very small, with just 21 subjects. The second study – from 2019 – is larger (n = 103), but some patients weren’t taking higher-efficacy DMTs.
Other research is more promising: Dr. Cohen highlighted a 2017 analysis that found patients with relapsing/remitting MS who underwent stem-cell transplantation were more likely to be symptom-free at 2 years (78%-83%) than those who took DMTs in clinical trials (13%-46%).
Clinical concerns
As for side effects of stem cell transplantation, Dr. Cohen said, “most patients have some adverse effects during the procedure itself. There may be an MS relapse or pseudorelapses in association with the mobilization and the conditioning regimens.”
A wide range of other adverse effects is possible before the immune system is reconstituted, he said, including reactivation of various virus infections, such as HPV, CMV or EPV (Epstein-Barr virus), secondary autoimmune phenomenon, and secondary malignancy. EPV infection is also common after transplant, and is “probably the most troublesome posttransplant complication from a management point of view,” he said.
“Thankfully, once the patient ... recovers from the transplant procedure, late adverse effects are relatively uncommon, the most common of which would be infertility,” he said. “There have been some reports of successful pregnancies following transplant, but it’s important to advise people who are considering transplant that most men and women have infertility after the procedure. So if they desire to have children afterward, they need to be counseled on necessary preparations to do that.”
What about progressive multifocal leukoencephalopathy (PML), which seems a possible risk because of the suppression of the immune system? Dr. Cohen is aware of one case linked to a stem-cell transplant, and it may not have been caused by the procedure.
Cost is another potential obstacle, he noted. The National Multiple Sclerosis Society estimates that autologous hematopoietic stem cell transplants cost $150,000 on average in the U.S., although the expense varies widely.
Unanswered questions
Moving forward, Dr. Cohen said it remains unclear how these procedures fare against the newest generations of DMTs in MS. Five phase 3 randomized controlled trials are now trying to clarify the matter, he said, by pitting stem-cell transplantation against various MS drugs – alemtuzumab, cladribine, natalizumab, ocrelizumab, and rituximab.
There are also unanswered questions about the best conditioning regimens in the transplants, he said, and lack of clarity about where to draw the line between eligible and ineligible patients with MS. “How many DMTs does the person have to fail? What’s the upper level of disability beyond which it is unlikely to be helpful and more likely to cause harm?”
He added: “A particular profile that we’re seeing increasingly at our center is someone with very active disease and onset who gets started on a high-efficacy therapy as their first therapy and effectively stops relapses and MRI lesion activity. But within a couple of years, we can tell that they’re already starting to accumulate disability. Is this someone for whom transplant might be useful, and by extension, is transplant appropriate as the first therapy in some patients? And beyond MS, is transplant a consideration for other autoimmune CNS disorders? There are lots of unanswered questions, which future studies will hopefully begin to address.”
Dr. Cohen reports consulting for Biogen, Bristol‐Myers Squibb, Convelo, EMD Serono, GlaxoSmithKline (now GSK), Janssen, Mylan, and PSI. He serves as an editor of Multiple Sclerosis Journal.
NATIONAL HARBOR, MD. – .” But, he said, it remains unclear which patients should undergo the procedure.
An especially pressing question is “whether transplant is an alternative to our high-efficacy disease-modifying therapies” (DMTs) in some high-risk patients, Jeffrey A. Cohen, MD, director of experimental therapeutics at Cleveland Clinic’s Mellen Center, said in a presidential lecture at the annual meeting of the Consortium of Multiple Sclerosis Centers.
A handful of ongoing randomized controlled trials will bring answers, he said.
Stem cell therapy exists because there are gaps in MS treatment, Dr. Cohen said. “We have now more than 20 medications. However, none of these therapies is completely effective in all patients. In particular, there are some patients with very active disease who continue to have relapses or new MRI lesion activity despite treatment with all of the available therapies.”
And in progressive MS, the efficacy of a couple of agents is modest and mainly for people with ongoing focal lesion activity, he said. “Finally, all of these currently available therapies are intended primarily to prevent the accumulation of damage, and none of them directly promotes repair. So despite our progress in the field, there still are a number of unmet needs.”
‘Rebooting’ the immune system
Several types of stem cell therapy exist, including remyelination and anti-inflammatory therapy, Dr. Cohen said. In his lecture, he focused on immunoablative or myeloablative therapy followed by autologous hematopoietic stem cell transplantation.
This “complicated, multistep procedure” involves first eliminating the immune system to get rid of pathogenic inflammatory cells. This “big component is actually the therapy for MS. It’s also the step that has the most potential complications,” he said.
According to Dr. Cohen, the next step has been described as “rebooting” the immune system.
Does this procedure help patients with MS? In relapsing MS, reports suggest there’s “almost complete abrogation of disease activity following transplant,” he said, “a benefit that’s lasted 5-10-15 years. In addition, there’s also a benefit on the accumulation of CNS damage as measured by whole brain atrophy.”
Recent data, he said, suggests that MS patients most likely to benefit are young, developed MS relatively recently, are still ambulatory, and have highly active MS despite treatment with first- and second-line agents.
However, there have only been two randomized controlled trials of stem cell transplantation versus DMT, and Dr. Cohen said both studies have limitations. The first one, from 2015, is very small, with just 21 subjects. The second study – from 2019 – is larger (n = 103), but some patients weren’t taking higher-efficacy DMTs.
Other research is more promising: Dr. Cohen highlighted a 2017 analysis that found patients with relapsing/remitting MS who underwent stem-cell transplantation were more likely to be symptom-free at 2 years (78%-83%) than those who took DMTs in clinical trials (13%-46%).
Clinical concerns
As for side effects of stem cell transplantation, Dr. Cohen said, “most patients have some adverse effects during the procedure itself. There may be an MS relapse or pseudorelapses in association with the mobilization and the conditioning regimens.”
A wide range of other adverse effects is possible before the immune system is reconstituted, he said, including reactivation of various virus infections, such as HPV, CMV or EPV (Epstein-Barr virus), secondary autoimmune phenomenon, and secondary malignancy. EPV infection is also common after transplant, and is “probably the most troublesome posttransplant complication from a management point of view,” he said.
“Thankfully, once the patient ... recovers from the transplant procedure, late adverse effects are relatively uncommon, the most common of which would be infertility,” he said. “There have been some reports of successful pregnancies following transplant, but it’s important to advise people who are considering transplant that most men and women have infertility after the procedure. So if they desire to have children afterward, they need to be counseled on necessary preparations to do that.”
What about progressive multifocal leukoencephalopathy (PML), which seems a possible risk because of the suppression of the immune system? Dr. Cohen is aware of one case linked to a stem-cell transplant, and it may not have been caused by the procedure.
Cost is another potential obstacle, he noted. The National Multiple Sclerosis Society estimates that autologous hematopoietic stem cell transplants cost $150,000 on average in the U.S., although the expense varies widely.
Unanswered questions
Moving forward, Dr. Cohen said it remains unclear how these procedures fare against the newest generations of DMTs in MS. Five phase 3 randomized controlled trials are now trying to clarify the matter, he said, by pitting stem-cell transplantation against various MS drugs – alemtuzumab, cladribine, natalizumab, ocrelizumab, and rituximab.
There are also unanswered questions about the best conditioning regimens in the transplants, he said, and lack of clarity about where to draw the line between eligible and ineligible patients with MS. “How many DMTs does the person have to fail? What’s the upper level of disability beyond which it is unlikely to be helpful and more likely to cause harm?”
He added: “A particular profile that we’re seeing increasingly at our center is someone with very active disease and onset who gets started on a high-efficacy therapy as their first therapy and effectively stops relapses and MRI lesion activity. But within a couple of years, we can tell that they’re already starting to accumulate disability. Is this someone for whom transplant might be useful, and by extension, is transplant appropriate as the first therapy in some patients? And beyond MS, is transplant a consideration for other autoimmune CNS disorders? There are lots of unanswered questions, which future studies will hopefully begin to address.”
Dr. Cohen reports consulting for Biogen, Bristol‐Myers Squibb, Convelo, EMD Serono, GlaxoSmithKline (now GSK), Janssen, Mylan, and PSI. He serves as an editor of Multiple Sclerosis Journal.
NATIONAL HARBOR, MD. – .” But, he said, it remains unclear which patients should undergo the procedure.
An especially pressing question is “whether transplant is an alternative to our high-efficacy disease-modifying therapies” (DMTs) in some high-risk patients, Jeffrey A. Cohen, MD, director of experimental therapeutics at Cleveland Clinic’s Mellen Center, said in a presidential lecture at the annual meeting of the Consortium of Multiple Sclerosis Centers.
A handful of ongoing randomized controlled trials will bring answers, he said.
Stem cell therapy exists because there are gaps in MS treatment, Dr. Cohen said. “We have now more than 20 medications. However, none of these therapies is completely effective in all patients. In particular, there are some patients with very active disease who continue to have relapses or new MRI lesion activity despite treatment with all of the available therapies.”
And in progressive MS, the efficacy of a couple of agents is modest and mainly for people with ongoing focal lesion activity, he said. “Finally, all of these currently available therapies are intended primarily to prevent the accumulation of damage, and none of them directly promotes repair. So despite our progress in the field, there still are a number of unmet needs.”
‘Rebooting’ the immune system
Several types of stem cell therapy exist, including remyelination and anti-inflammatory therapy, Dr. Cohen said. In his lecture, he focused on immunoablative or myeloablative therapy followed by autologous hematopoietic stem cell transplantation.
This “complicated, multistep procedure” involves first eliminating the immune system to get rid of pathogenic inflammatory cells. This “big component is actually the therapy for MS. It’s also the step that has the most potential complications,” he said.
According to Dr. Cohen, the next step has been described as “rebooting” the immune system.
Does this procedure help patients with MS? In relapsing MS, reports suggest there’s “almost complete abrogation of disease activity following transplant,” he said, “a benefit that’s lasted 5-10-15 years. In addition, there’s also a benefit on the accumulation of CNS damage as measured by whole brain atrophy.”
Recent data, he said, suggests that MS patients most likely to benefit are young, developed MS relatively recently, are still ambulatory, and have highly active MS despite treatment with first- and second-line agents.
However, there have only been two randomized controlled trials of stem cell transplantation versus DMT, and Dr. Cohen said both studies have limitations. The first one, from 2015, is very small, with just 21 subjects. The second study – from 2019 – is larger (n = 103), but some patients weren’t taking higher-efficacy DMTs.
Other research is more promising: Dr. Cohen highlighted a 2017 analysis that found patients with relapsing/remitting MS who underwent stem-cell transplantation were more likely to be symptom-free at 2 years (78%-83%) than those who took DMTs in clinical trials (13%-46%).
Clinical concerns
As for side effects of stem cell transplantation, Dr. Cohen said, “most patients have some adverse effects during the procedure itself. There may be an MS relapse or pseudorelapses in association with the mobilization and the conditioning regimens.”
A wide range of other adverse effects is possible before the immune system is reconstituted, he said, including reactivation of various virus infections, such as HPV, CMV or EPV (Epstein-Barr virus), secondary autoimmune phenomenon, and secondary malignancy. EPV infection is also common after transplant, and is “probably the most troublesome posttransplant complication from a management point of view,” he said.
“Thankfully, once the patient ... recovers from the transplant procedure, late adverse effects are relatively uncommon, the most common of which would be infertility,” he said. “There have been some reports of successful pregnancies following transplant, but it’s important to advise people who are considering transplant that most men and women have infertility after the procedure. So if they desire to have children afterward, they need to be counseled on necessary preparations to do that.”
What about progressive multifocal leukoencephalopathy (PML), which seems a possible risk because of the suppression of the immune system? Dr. Cohen is aware of one case linked to a stem-cell transplant, and it may not have been caused by the procedure.
Cost is another potential obstacle, he noted. The National Multiple Sclerosis Society estimates that autologous hematopoietic stem cell transplants cost $150,000 on average in the U.S., although the expense varies widely.
Unanswered questions
Moving forward, Dr. Cohen said it remains unclear how these procedures fare against the newest generations of DMTs in MS. Five phase 3 randomized controlled trials are now trying to clarify the matter, he said, by pitting stem-cell transplantation against various MS drugs – alemtuzumab, cladribine, natalizumab, ocrelizumab, and rituximab.
There are also unanswered questions about the best conditioning regimens in the transplants, he said, and lack of clarity about where to draw the line between eligible and ineligible patients with MS. “How many DMTs does the person have to fail? What’s the upper level of disability beyond which it is unlikely to be helpful and more likely to cause harm?”
He added: “A particular profile that we’re seeing increasingly at our center is someone with very active disease and onset who gets started on a high-efficacy therapy as their first therapy and effectively stops relapses and MRI lesion activity. But within a couple of years, we can tell that they’re already starting to accumulate disability. Is this someone for whom transplant might be useful, and by extension, is transplant appropriate as the first therapy in some patients? And beyond MS, is transplant a consideration for other autoimmune CNS disorders? There are lots of unanswered questions, which future studies will hopefully begin to address.”
Dr. Cohen reports consulting for Biogen, Bristol‐Myers Squibb, Convelo, EMD Serono, GlaxoSmithKline (now GSK), Janssen, Mylan, and PSI. He serves as an editor of Multiple Sclerosis Journal.
At CMSC 2022
HIV care continuum conundrum: Challenges of out-of-care patients
Among an estimated 87% of people with HIV (PWH) whose condition has been diagnosed, roughly 66% have received medication. But only half are retained in care, leaving substantial risk for viral rebound and further HIV transmission.
A variety of factors contribute to falling out of care (OOC), a primary reason why a team from the Centers for Disease Control and Prevention reviewed over three decades of studies with the goal of identifying best practices for re-engagement.
The research, which was published in the journal AIDS, underscores the need for more customized strategies, rather than a one-size-fits-all approach, especially for historically underserved communities.
“Many study participants across the studies included in this review represented communities who have the largest challenges with remaining in care,” Darrel H. Higa, PhD, MSW, lead study author and a behavioral scientist at the CDC in Atlanta, told this news organization.
For example, “Some face barriers that may limit their access to care ... including not having health insurance or being unable to pay for doctor visits or medication, HIV-related stigma, racism, homophobia, transphobia, health literacy, and a lack of providers who specialize in HIV care,” he said.
Other challenges relate to personal barriers, such as competing priorities (for example, work or childcare), substance use, mental health disorders, transportation problems, or a lack of social support.
Even with improvements that address some of these barriers, such as expanded access to health care insurance and broader provision of medical care and HIV medications through the national Ryan White program, structural challenges and social barriers persist.
Better versus best practices
In their analysis, the CDC team expanded the scope of prior reviews by including literature published between 2000 and 2020 and further conducted meta-analyses to assess the effectiveness of five common, non mutually exclusive interventions:
- patient navigation
- appointment help/alerts
- psychosocial support
- transportation/appointment reminders
- data-to-care HIV care outcomes (using health department surveillance data and/or patient health records to identify and re-engage OOC PWH)
The majority of the 26,154 participants in 39 included studies (incorporating 42 unique interventions) were male (71%) and Black (64%); the most common time frame for OOC was between 6 and 12 months, but some studies used a time frame of 3-4 months, and others more than 12 months.
Definitions for re-engagement and retention were likewise inconsistent across studies but most commonly involved having an HIV medical visit or viral load test record between 2 and 6 months (re-engagement), and ≥ 1 medical visits in each 6-month period a minimum of 60 days apart for a period of over 2 years (retention).
This is notable, as it points to the role played – at least in part – by the care fragmentation inherent in the United States health care system. Without national indicators or thresholds for clinical outcomes, services are unlikely to reach scale.
said Mary Jane Rotheram-Borus, PhD, distinguished professor of clinical psychology and director of the Global Center for Children and Families at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. (Dr. Rotheram-Borus authored an accompanying editorial but was not involved in the study.)
Nevertheless, review findings highlighted that overall, the five interventions appeared to improve care re-engagement (odds ratio = 1.79, 95% confidence interval, 1.36-2.36), care retention (OR = 2.01; 95% CI, 1.64-2.46), and viral suppression (OR = 2.50; 95% CI, 1.87-2.24).
Overall, the five strategies were associated with optimal re-engagement and retention in care. In addition, four of them were associated with viral suppression for PWH who were OOC during the study time frame. The one exception was data-to-care, for which the evidence supporting an association with viral suppression was unclear.
Because of the similarities between patient navigation and transportation/appointment accompaniment, the researchers also compared PWH who received combined strategies to those who did not.
“The findings suggest that patient navigation services that often include helping with transportation to appointments or accompanying PWH to appointments may be more effective compared to interventions without the combination,” explained Dr. Higa, “especially for communities with the largest challenges remaining in care.”
He added that, moving forward, many of the same strategies that help re-engage out-of-care PWH may be useful for retention. These include co-locating services, outreach, mental health services, clinical care models, telemedicine, and financial incentives.
Despite its financial investments toward ending the HIV epidemic, the United States arguably still has a long way to go to improve retention and care.
Still, Dr. Rotheram-Borus underscores the silver lining.
“The breakthroughs in medication are substantial,” she said, pointing to her own research, which has shown that at least 60% of newly infected, poor, LGBTQ+ young people up to age 24 have been linked to care and are adherent enough to be virally suppressed.
For PWH who are out of care, perhaps treatment advances – including long-acting injectables – may ultimately fill in the gaps.
Dr. Higa and Dr. Rotherum-Borus report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among an estimated 87% of people with HIV (PWH) whose condition has been diagnosed, roughly 66% have received medication. But only half are retained in care, leaving substantial risk for viral rebound and further HIV transmission.
A variety of factors contribute to falling out of care (OOC), a primary reason why a team from the Centers for Disease Control and Prevention reviewed over three decades of studies with the goal of identifying best practices for re-engagement.
The research, which was published in the journal AIDS, underscores the need for more customized strategies, rather than a one-size-fits-all approach, especially for historically underserved communities.
“Many study participants across the studies included in this review represented communities who have the largest challenges with remaining in care,” Darrel H. Higa, PhD, MSW, lead study author and a behavioral scientist at the CDC in Atlanta, told this news organization.
For example, “Some face barriers that may limit their access to care ... including not having health insurance or being unable to pay for doctor visits or medication, HIV-related stigma, racism, homophobia, transphobia, health literacy, and a lack of providers who specialize in HIV care,” he said.
Other challenges relate to personal barriers, such as competing priorities (for example, work or childcare), substance use, mental health disorders, transportation problems, or a lack of social support.
Even with improvements that address some of these barriers, such as expanded access to health care insurance and broader provision of medical care and HIV medications through the national Ryan White program, structural challenges and social barriers persist.
Better versus best practices
In their analysis, the CDC team expanded the scope of prior reviews by including literature published between 2000 and 2020 and further conducted meta-analyses to assess the effectiveness of five common, non mutually exclusive interventions:
- patient navigation
- appointment help/alerts
- psychosocial support
- transportation/appointment reminders
- data-to-care HIV care outcomes (using health department surveillance data and/or patient health records to identify and re-engage OOC PWH)
The majority of the 26,154 participants in 39 included studies (incorporating 42 unique interventions) were male (71%) and Black (64%); the most common time frame for OOC was between 6 and 12 months, but some studies used a time frame of 3-4 months, and others more than 12 months.
Definitions for re-engagement and retention were likewise inconsistent across studies but most commonly involved having an HIV medical visit or viral load test record between 2 and 6 months (re-engagement), and ≥ 1 medical visits in each 6-month period a minimum of 60 days apart for a period of over 2 years (retention).
This is notable, as it points to the role played – at least in part – by the care fragmentation inherent in the United States health care system. Without national indicators or thresholds for clinical outcomes, services are unlikely to reach scale.
said Mary Jane Rotheram-Borus, PhD, distinguished professor of clinical psychology and director of the Global Center for Children and Families at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. (Dr. Rotheram-Borus authored an accompanying editorial but was not involved in the study.)
Nevertheless, review findings highlighted that overall, the five interventions appeared to improve care re-engagement (odds ratio = 1.79, 95% confidence interval, 1.36-2.36), care retention (OR = 2.01; 95% CI, 1.64-2.46), and viral suppression (OR = 2.50; 95% CI, 1.87-2.24).
Overall, the five strategies were associated with optimal re-engagement and retention in care. In addition, four of them were associated with viral suppression for PWH who were OOC during the study time frame. The one exception was data-to-care, for which the evidence supporting an association with viral suppression was unclear.
Because of the similarities between patient navigation and transportation/appointment accompaniment, the researchers also compared PWH who received combined strategies to those who did not.
“The findings suggest that patient navigation services that often include helping with transportation to appointments or accompanying PWH to appointments may be more effective compared to interventions without the combination,” explained Dr. Higa, “especially for communities with the largest challenges remaining in care.”
He added that, moving forward, many of the same strategies that help re-engage out-of-care PWH may be useful for retention. These include co-locating services, outreach, mental health services, clinical care models, telemedicine, and financial incentives.
Despite its financial investments toward ending the HIV epidemic, the United States arguably still has a long way to go to improve retention and care.
Still, Dr. Rotheram-Borus underscores the silver lining.
“The breakthroughs in medication are substantial,” she said, pointing to her own research, which has shown that at least 60% of newly infected, poor, LGBTQ+ young people up to age 24 have been linked to care and are adherent enough to be virally suppressed.
For PWH who are out of care, perhaps treatment advances – including long-acting injectables – may ultimately fill in the gaps.
Dr. Higa and Dr. Rotherum-Borus report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among an estimated 87% of people with HIV (PWH) whose condition has been diagnosed, roughly 66% have received medication. But only half are retained in care, leaving substantial risk for viral rebound and further HIV transmission.
A variety of factors contribute to falling out of care (OOC), a primary reason why a team from the Centers for Disease Control and Prevention reviewed over three decades of studies with the goal of identifying best practices for re-engagement.
The research, which was published in the journal AIDS, underscores the need for more customized strategies, rather than a one-size-fits-all approach, especially for historically underserved communities.
“Many study participants across the studies included in this review represented communities who have the largest challenges with remaining in care,” Darrel H. Higa, PhD, MSW, lead study author and a behavioral scientist at the CDC in Atlanta, told this news organization.
For example, “Some face barriers that may limit their access to care ... including not having health insurance or being unable to pay for doctor visits or medication, HIV-related stigma, racism, homophobia, transphobia, health literacy, and a lack of providers who specialize in HIV care,” he said.
Other challenges relate to personal barriers, such as competing priorities (for example, work or childcare), substance use, mental health disorders, transportation problems, or a lack of social support.
Even with improvements that address some of these barriers, such as expanded access to health care insurance and broader provision of medical care and HIV medications through the national Ryan White program, structural challenges and social barriers persist.
Better versus best practices
In their analysis, the CDC team expanded the scope of prior reviews by including literature published between 2000 and 2020 and further conducted meta-analyses to assess the effectiveness of five common, non mutually exclusive interventions:
- patient navigation
- appointment help/alerts
- psychosocial support
- transportation/appointment reminders
- data-to-care HIV care outcomes (using health department surveillance data and/or patient health records to identify and re-engage OOC PWH)
The majority of the 26,154 participants in 39 included studies (incorporating 42 unique interventions) were male (71%) and Black (64%); the most common time frame for OOC was between 6 and 12 months, but some studies used a time frame of 3-4 months, and others more than 12 months.
Definitions for re-engagement and retention were likewise inconsistent across studies but most commonly involved having an HIV medical visit or viral load test record between 2 and 6 months (re-engagement), and ≥ 1 medical visits in each 6-month period a minimum of 60 days apart for a period of over 2 years (retention).
This is notable, as it points to the role played – at least in part – by the care fragmentation inherent in the United States health care system. Without national indicators or thresholds for clinical outcomes, services are unlikely to reach scale.
said Mary Jane Rotheram-Borus, PhD, distinguished professor of clinical psychology and director of the Global Center for Children and Families at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. (Dr. Rotheram-Borus authored an accompanying editorial but was not involved in the study.)
Nevertheless, review findings highlighted that overall, the five interventions appeared to improve care re-engagement (odds ratio = 1.79, 95% confidence interval, 1.36-2.36), care retention (OR = 2.01; 95% CI, 1.64-2.46), and viral suppression (OR = 2.50; 95% CI, 1.87-2.24).
Overall, the five strategies were associated with optimal re-engagement and retention in care. In addition, four of them were associated with viral suppression for PWH who were OOC during the study time frame. The one exception was data-to-care, for which the evidence supporting an association with viral suppression was unclear.
Because of the similarities between patient navigation and transportation/appointment accompaniment, the researchers also compared PWH who received combined strategies to those who did not.
“The findings suggest that patient navigation services that often include helping with transportation to appointments or accompanying PWH to appointments may be more effective compared to interventions without the combination,” explained Dr. Higa, “especially for communities with the largest challenges remaining in care.”
He added that, moving forward, many of the same strategies that help re-engage out-of-care PWH may be useful for retention. These include co-locating services, outreach, mental health services, clinical care models, telemedicine, and financial incentives.
Despite its financial investments toward ending the HIV epidemic, the United States arguably still has a long way to go to improve retention and care.
Still, Dr. Rotheram-Borus underscores the silver lining.
“The breakthroughs in medication are substantial,” she said, pointing to her own research, which has shown that at least 60% of newly infected, poor, LGBTQ+ young people up to age 24 have been linked to care and are adherent enough to be virally suppressed.
For PWH who are out of care, perhaps treatment advances – including long-acting injectables – may ultimately fill in the gaps.
Dr. Higa and Dr. Rotherum-Borus report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endoscopic management of duodenal and ampullary adenomas
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.
The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.

The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.

The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Rippled Macules and Papules on the Legs
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
A 34-year-old woman presented to our dermatology clinic with an intensely pruritic rash on the legs of 2 years’ duration. The pruritus had waxed and waned in intensity, and the skin lesions were refractory to treatment with low-potency topical steroids. She had no other chronic medical conditions and was not taking any other medications.