User login
Commentary: Multifocal Hepatocellular Carcinoma, November 2022
Orimo and colleagues addressed the use of liver resection in patients with more than one HCC in the liver. Patients with no or Child-Pugh A/B cirrhosis were included in this single-center retrospective study of 1088 patients who underwent hepatectomy for Barcelona Clinic Liver Cancer (BCLC) stage 0 (n = 88), A (n = 750), or B (n = 250) HCC, with stages A and B subcategorized into A1 (single nodule 2-5 cm or ≤ 3 nodules ≤ 3 cm), A2 (single nodule 5-10 cm), A3 (single nodule ≥ 10 cm), B1 (2-3 nodules > 3 cm), and B2 (≥ 4 nodules). The 5-year overall survival (OS) rates for stage 0, A1, A2, A3, B1, and B2 patients were 70.4%, 74.2%, 63.8%, 47.7%, 47.5%, and 31.9%, respectively (P < .0001). Significant differences in overall survival (OS) were found between stages A1 and A2 (P = .0118), A2 and A3 (P = .0013), and B1 and B2 (P = .0050), but not between stages A3 and B1 (P = .4742). In stage B1 patients, Child-Pugh B cirrhosis was the only independent prognostic factor for OS. The authors concluded that hepatectomy is beneficial in patients with three or fewer hepatocellular carcinomas and either no or Child-Pugh class A cirrhosis, with the long-term results being comparable to those in patients who underwent a resection of a single HCC. Therefore, resection of up to three HCC is safe and should be considered in clinically appropriate patients.
Many patients with multifocal HCC are not eligible for liver-directed therapies. The standard of care for first-line systemic therapy is the combination of atezolizumab and bevacizumab, as reported in the IMbrave150 clinical trial. Fulgenzi and colleagues published the results of a multicenter prospective observational study, AB-Real, that included 433 patients who received atezolizumab and bevacizumab in routine clinical practice. The investigators confirmed the efficacy of the combination and found that portal vein tumor thrombosis and worse albumin-bilirubin grade were independent prognostic factors for poor OS and were associated with an increased risk for hemorrhagic events. In addition, the authors reported that the overall response rate (ORR) predicted better outcomes, including longer OS. Therefore, atezolizumab and bevacizumab remains a safe and effective first-line treatment for many patients with unresectable HCC.
Finally, Finn and colleagues reported the results of an open-label, noncomparative cohort of the REACH-2 study of ramucirumab in 47 patients with advanced HCC and an alpha-fetoprotein (AFP) level ≥ 400 ng/mL. These patients had previously received one to two lines of systemic therapy, excluding sorafenib or chemotherapy. Lenvatinib was the most common prior systemic therapy (n = 20; 43%). Others included immune checkpoint inhibitor (CPI) monotherapies (n = 11), CPI/antiangiogenic therapy (n = 14), or dual CPI therapy (n = 5). The ORR was 10.6% (95% CI 1.8-19.5) and disease control rate was 46.8% (95% CI 32.5-61.1), with a median duration of response of 8.3 months [95% CI 2.4 to not reached). The grade 3 or more adverse event rate was 57%, with hypertension (11%) being the most common, allowing the authors to conclude that ramucirumab offers clinically significant efficacy with no new safety signals in this setting. Therefore, ramucirumab remains as a safe and effective later-line treatment option for patients with unresectable HCC and an AFP ≥ 400 ng/mL.
Orimo and colleagues addressed the use of liver resection in patients with more than one HCC in the liver. Patients with no or Child-Pugh A/B cirrhosis were included in this single-center retrospective study of 1088 patients who underwent hepatectomy for Barcelona Clinic Liver Cancer (BCLC) stage 0 (n = 88), A (n = 750), or B (n = 250) HCC, with stages A and B subcategorized into A1 (single nodule 2-5 cm or ≤ 3 nodules ≤ 3 cm), A2 (single nodule 5-10 cm), A3 (single nodule ≥ 10 cm), B1 (2-3 nodules > 3 cm), and B2 (≥ 4 nodules). The 5-year overall survival (OS) rates for stage 0, A1, A2, A3, B1, and B2 patients were 70.4%, 74.2%, 63.8%, 47.7%, 47.5%, and 31.9%, respectively (P < .0001). Significant differences in overall survival (OS) were found between stages A1 and A2 (P = .0118), A2 and A3 (P = .0013), and B1 and B2 (P = .0050), but not between stages A3 and B1 (P = .4742). In stage B1 patients, Child-Pugh B cirrhosis was the only independent prognostic factor for OS. The authors concluded that hepatectomy is beneficial in patients with three or fewer hepatocellular carcinomas and either no or Child-Pugh class A cirrhosis, with the long-term results being comparable to those in patients who underwent a resection of a single HCC. Therefore, resection of up to three HCC is safe and should be considered in clinically appropriate patients.
Many patients with multifocal HCC are not eligible for liver-directed therapies. The standard of care for first-line systemic therapy is the combination of atezolizumab and bevacizumab, as reported in the IMbrave150 clinical trial. Fulgenzi and colleagues published the results of a multicenter prospective observational study, AB-Real, that included 433 patients who received atezolizumab and bevacizumab in routine clinical practice. The investigators confirmed the efficacy of the combination and found that portal vein tumor thrombosis and worse albumin-bilirubin grade were independent prognostic factors for poor OS and were associated with an increased risk for hemorrhagic events. In addition, the authors reported that the overall response rate (ORR) predicted better outcomes, including longer OS. Therefore, atezolizumab and bevacizumab remains a safe and effective first-line treatment for many patients with unresectable HCC.
Finally, Finn and colleagues reported the results of an open-label, noncomparative cohort of the REACH-2 study of ramucirumab in 47 patients with advanced HCC and an alpha-fetoprotein (AFP) level ≥ 400 ng/mL. These patients had previously received one to two lines of systemic therapy, excluding sorafenib or chemotherapy. Lenvatinib was the most common prior systemic therapy (n = 20; 43%). Others included immune checkpoint inhibitor (CPI) monotherapies (n = 11), CPI/antiangiogenic therapy (n = 14), or dual CPI therapy (n = 5). The ORR was 10.6% (95% CI 1.8-19.5) and disease control rate was 46.8% (95% CI 32.5-61.1), with a median duration of response of 8.3 months [95% CI 2.4 to not reached). The grade 3 or more adverse event rate was 57%, with hypertension (11%) being the most common, allowing the authors to conclude that ramucirumab offers clinically significant efficacy with no new safety signals in this setting. Therefore, ramucirumab remains as a safe and effective later-line treatment option for patients with unresectable HCC and an AFP ≥ 400 ng/mL.
Orimo and colleagues addressed the use of liver resection in patients with more than one HCC in the liver. Patients with no or Child-Pugh A/B cirrhosis were included in this single-center retrospective study of 1088 patients who underwent hepatectomy for Barcelona Clinic Liver Cancer (BCLC) stage 0 (n = 88), A (n = 750), or B (n = 250) HCC, with stages A and B subcategorized into A1 (single nodule 2-5 cm or ≤ 3 nodules ≤ 3 cm), A2 (single nodule 5-10 cm), A3 (single nodule ≥ 10 cm), B1 (2-3 nodules > 3 cm), and B2 (≥ 4 nodules). The 5-year overall survival (OS) rates for stage 0, A1, A2, A3, B1, and B2 patients were 70.4%, 74.2%, 63.8%, 47.7%, 47.5%, and 31.9%, respectively (P < .0001). Significant differences in overall survival (OS) were found between stages A1 and A2 (P = .0118), A2 and A3 (P = .0013), and B1 and B2 (P = .0050), but not between stages A3 and B1 (P = .4742). In stage B1 patients, Child-Pugh B cirrhosis was the only independent prognostic factor for OS. The authors concluded that hepatectomy is beneficial in patients with three or fewer hepatocellular carcinomas and either no or Child-Pugh class A cirrhosis, with the long-term results being comparable to those in patients who underwent a resection of a single HCC. Therefore, resection of up to three HCC is safe and should be considered in clinically appropriate patients.
Many patients with multifocal HCC are not eligible for liver-directed therapies. The standard of care for first-line systemic therapy is the combination of atezolizumab and bevacizumab, as reported in the IMbrave150 clinical trial. Fulgenzi and colleagues published the results of a multicenter prospective observational study, AB-Real, that included 433 patients who received atezolizumab and bevacizumab in routine clinical practice. The investigators confirmed the efficacy of the combination and found that portal vein tumor thrombosis and worse albumin-bilirubin grade were independent prognostic factors for poor OS and were associated with an increased risk for hemorrhagic events. In addition, the authors reported that the overall response rate (ORR) predicted better outcomes, including longer OS. Therefore, atezolizumab and bevacizumab remains a safe and effective first-line treatment for many patients with unresectable HCC.
Finally, Finn and colleagues reported the results of an open-label, noncomparative cohort of the REACH-2 study of ramucirumab in 47 patients with advanced HCC and an alpha-fetoprotein (AFP) level ≥ 400 ng/mL. These patients had previously received one to two lines of systemic therapy, excluding sorafenib or chemotherapy. Lenvatinib was the most common prior systemic therapy (n = 20; 43%). Others included immune checkpoint inhibitor (CPI) monotherapies (n = 11), CPI/antiangiogenic therapy (n = 14), or dual CPI therapy (n = 5). The ORR was 10.6% (95% CI 1.8-19.5) and disease control rate was 46.8% (95% CI 32.5-61.1), with a median duration of response of 8.3 months [95% CI 2.4 to not reached). The grade 3 or more adverse event rate was 57%, with hypertension (11%) being the most common, allowing the authors to conclude that ramucirumab offers clinically significant efficacy with no new safety signals in this setting. Therefore, ramucirumab remains as a safe and effective later-line treatment option for patients with unresectable HCC and an AFP ≥ 400 ng/mL.
Commentary: Multifocal Hepatocellular Carcinoma, November 2022
Orimo and colleagues addressed the use of liver resection in patients with more than one HCC in the liver. Patients with no or Child-Pugh A/B cirrhosis were included in this single-center retrospective study of 1088 patients who underwent hepatectomy for Barcelona Clinic Liver Cancer (BCLC) stage 0 (n = 88), A (n = 750), or B (n = 250) HCC, with stages A and B subcategorized into A1 (single nodule 2-5 cm or ≤ 3 nodules ≤ 3 cm), A2 (single nodule 5-10 cm), A3 (single nodule ≥ 10 cm), B1 (2-3 nodules > 3 cm), and B2 (≥ 4 nodules). The 5-year overall survival (OS) rates for stage 0, A1, A2, A3, B1, and B2 patients were 70.4%, 74.2%, 63.8%, 47.7%, 47.5%, and 31.9%, respectively (P < .0001). Significant differences in overall survival (OS) were found between stages A1 and A2 (P = .0118), A2 and A3 (P = .0013), and B1 and B2 (P = .0050), but not between stages A3 and B1 (P = .4742). In stage B1 patients, Child-Pugh B cirrhosis was the only independent prognostic factor for OS. The authors concluded that hepatectomy is beneficial in patients with three or fewer hepatocellular carcinomas and either no or Child-Pugh class A cirrhosis, with the long-term results being comparable to those in patients who underwent a resection of a single HCC. Therefore, resection of up to three HCC is safe and should be considered in clinically appropriate patients.
Many patients with multifocal HCC are not eligible for liver-directed therapies. The standard of care for first-line systemic therapy is the combination of atezolizumab and bevacizumab, as reported in the IMbrave150 clinical trial. Fulgenzi and colleagues published the results of a multicenter prospective observational study, AB-Real, that included 433 patients who received atezolizumab and bevacizumab in routine clinical practice. The investigators confirmed the efficacy of the combination and found that portal vein tumor thrombosis and worse albumin-bilirubin grade were independent prognostic factors for poor OS and were associated with an increased risk for hemorrhagic events. In addition, the authors reported that the overall response rate (ORR) predicted better outcomes, including longer OS. Therefore, atezolizumab and bevacizumab remains a safe and effective first-line treatment for many patients with unresectable HCC.
Finally, Finn and colleagues reported the results of an open-label, noncomparative cohort of the REACH-2 study of ramucirumab in 47 patients with advanced HCC and an alpha-fetoprotein (AFP) level ≥ 400 ng/mL. These patients had previously received one to two lines of systemic therapy, excluding sorafenib or chemotherapy. Lenvatinib was the most common prior systemic therapy (n = 20; 43%). Others included immune checkpoint inhibitor (CPI) monotherapies (n = 11), CPI/antiangiogenic therapy (n = 14), or dual CPI therapy (n = 5). The ORR was 10.6% (95% CI 1.8-19.5) and disease control rate was 46.8% (95% CI 32.5-61.1), with a median duration of response of 8.3 months (95% CI 2.4 to not reached). The grade 3 or more adverse event rate was 57%, with hypertension (11%) being the most common, allowing the authors to conclude that ramucirumab offers clinically significant efficacy with no new safety signals in this setting. Therefore, ramucirumab remains as a safe and effective later-line treatment option for patients with unresectable HCC and an AFP ≥ 400 ng/mL.
Orimo and colleagues addressed the use of liver resection in patients with more than one HCC in the liver. Patients with no or Child-Pugh A/B cirrhosis were included in this single-center retrospective study of 1088 patients who underwent hepatectomy for Barcelona Clinic Liver Cancer (BCLC) stage 0 (n = 88), A (n = 750), or B (n = 250) HCC, with stages A and B subcategorized into A1 (single nodule 2-5 cm or ≤ 3 nodules ≤ 3 cm), A2 (single nodule 5-10 cm), A3 (single nodule ≥ 10 cm), B1 (2-3 nodules > 3 cm), and B2 (≥ 4 nodules). The 5-year overall survival (OS) rates for stage 0, A1, A2, A3, B1, and B2 patients were 70.4%, 74.2%, 63.8%, 47.7%, 47.5%, and 31.9%, respectively (P < .0001). Significant differences in overall survival (OS) were found between stages A1 and A2 (P = .0118), A2 and A3 (P = .0013), and B1 and B2 (P = .0050), but not between stages A3 and B1 (P = .4742). In stage B1 patients, Child-Pugh B cirrhosis was the only independent prognostic factor for OS. The authors concluded that hepatectomy is beneficial in patients with three or fewer hepatocellular carcinomas and either no or Child-Pugh class A cirrhosis, with the long-term results being comparable to those in patients who underwent a resection of a single HCC. Therefore, resection of up to three HCC is safe and should be considered in clinically appropriate patients.
Many patients with multifocal HCC are not eligible for liver-directed therapies. The standard of care for first-line systemic therapy is the combination of atezolizumab and bevacizumab, as reported in the IMbrave150 clinical trial. Fulgenzi and colleagues published the results of a multicenter prospective observational study, AB-Real, that included 433 patients who received atezolizumab and bevacizumab in routine clinical practice. The investigators confirmed the efficacy of the combination and found that portal vein tumor thrombosis and worse albumin-bilirubin grade were independent prognostic factors for poor OS and were associated with an increased risk for hemorrhagic events. In addition, the authors reported that the overall response rate (ORR) predicted better outcomes, including longer OS. Therefore, atezolizumab and bevacizumab remains a safe and effective first-line treatment for many patients with unresectable HCC.
Finally, Finn and colleagues reported the results of an open-label, noncomparative cohort of the REACH-2 study of ramucirumab in 47 patients with advanced HCC and an alpha-fetoprotein (AFP) level ≥ 400 ng/mL. These patients had previously received one to two lines of systemic therapy, excluding sorafenib or chemotherapy. Lenvatinib was the most common prior systemic therapy (n = 20; 43%). Others included immune checkpoint inhibitor (CPI) monotherapies (n = 11), CPI/antiangiogenic therapy (n = 14), or dual CPI therapy (n = 5). The ORR was 10.6% (95% CI 1.8-19.5) and disease control rate was 46.8% (95% CI 32.5-61.1), with a median duration of response of 8.3 months (95% CI 2.4 to not reached). The grade 3 or more adverse event rate was 57%, with hypertension (11%) being the most common, allowing the authors to conclude that ramucirumab offers clinically significant efficacy with no new safety signals in this setting. Therefore, ramucirumab remains as a safe and effective later-line treatment option for patients with unresectable HCC and an AFP ≥ 400 ng/mL.
Orimo and colleagues addressed the use of liver resection in patients with more than one HCC in the liver. Patients with no or Child-Pugh A/B cirrhosis were included in this single-center retrospective study of 1088 patients who underwent hepatectomy for Barcelona Clinic Liver Cancer (BCLC) stage 0 (n = 88), A (n = 750), or B (n = 250) HCC, with stages A and B subcategorized into A1 (single nodule 2-5 cm or ≤ 3 nodules ≤ 3 cm), A2 (single nodule 5-10 cm), A3 (single nodule ≥ 10 cm), B1 (2-3 nodules > 3 cm), and B2 (≥ 4 nodules). The 5-year overall survival (OS) rates for stage 0, A1, A2, A3, B1, and B2 patients were 70.4%, 74.2%, 63.8%, 47.7%, 47.5%, and 31.9%, respectively (P < .0001). Significant differences in overall survival (OS) were found between stages A1 and A2 (P = .0118), A2 and A3 (P = .0013), and B1 and B2 (P = .0050), but not between stages A3 and B1 (P = .4742). In stage B1 patients, Child-Pugh B cirrhosis was the only independent prognostic factor for OS. The authors concluded that hepatectomy is beneficial in patients with three or fewer hepatocellular carcinomas and either no or Child-Pugh class A cirrhosis, with the long-term results being comparable to those in patients who underwent a resection of a single HCC. Therefore, resection of up to three HCC is safe and should be considered in clinically appropriate patients.
Many patients with multifocal HCC are not eligible for liver-directed therapies. The standard of care for first-line systemic therapy is the combination of atezolizumab and bevacizumab, as reported in the IMbrave150 clinical trial. Fulgenzi and colleagues published the results of a multicenter prospective observational study, AB-Real, that included 433 patients who received atezolizumab and bevacizumab in routine clinical practice. The investigators confirmed the efficacy of the combination and found that portal vein tumor thrombosis and worse albumin-bilirubin grade were independent prognostic factors for poor OS and were associated with an increased risk for hemorrhagic events. In addition, the authors reported that the overall response rate (ORR) predicted better outcomes, including longer OS. Therefore, atezolizumab and bevacizumab remains a safe and effective first-line treatment for many patients with unresectable HCC.
Finally, Finn and colleagues reported the results of an open-label, noncomparative cohort of the REACH-2 study of ramucirumab in 47 patients with advanced HCC and an alpha-fetoprotein (AFP) level ≥ 400 ng/mL. These patients had previously received one to two lines of systemic therapy, excluding sorafenib or chemotherapy. Lenvatinib was the most common prior systemic therapy (n = 20; 43%). Others included immune checkpoint inhibitor (CPI) monotherapies (n = 11), CPI/antiangiogenic therapy (n = 14), or dual CPI therapy (n = 5). The ORR was 10.6% (95% CI 1.8-19.5) and disease control rate was 46.8% (95% CI 32.5-61.1), with a median duration of response of 8.3 months (95% CI 2.4 to not reached). The grade 3 or more adverse event rate was 57%, with hypertension (11%) being the most common, allowing the authors to conclude that ramucirumab offers clinically significant efficacy with no new safety signals in this setting. Therefore, ramucirumab remains as a safe and effective later-line treatment option for patients with unresectable HCC and an AFP ≥ 400 ng/mL.
Strategies to treat food allergy with oral immunotherapy
according to a new review.
In OIT, a patient who is allergic to a specific food consumes increasing amounts of the allergen over time to reduce their risk for allergic reaction.
“OIT is an elective, usually noncurative procedure with inherent risks that require families to function as amateur medical professionals. Preparing them for this role is essential to protect patients and ensure the long-term success of this life-changing procedure,” lead author Douglas P. Mack, MD, MSc, a pediatric allergy, asthma, and immunology specialist at McMaster University in Hamilton, Ont., and colleagues write in Clinical & Experimental Allergy.
From strict avoidance to desensitization
Food allergy treatment has traditionally involved avoiding accidental exposure that may lead to anaphylaxis and providing rescue medication. In recent years, OIT “has been recommended by several guidelines as a primary option,” Dr. Mack and coauthors write. And with the “approval by European and USA regulators of [peanut allergen powder] Palforzia [Aimmune Therapeutics], there are now commercial and noncommercial forms of OIT available for use in several countries.”
They advise physicians to take a proactive, educational, supportive approach to patients and their families throughout the therapy.
“Ultimately, the decision to pursue OIT or continue avoidance strategies remains the responsibility of the family and the patients,” they write. “Some families may not be prepared for the role that they have to play in actively managing their child’s food allergy treatment.”
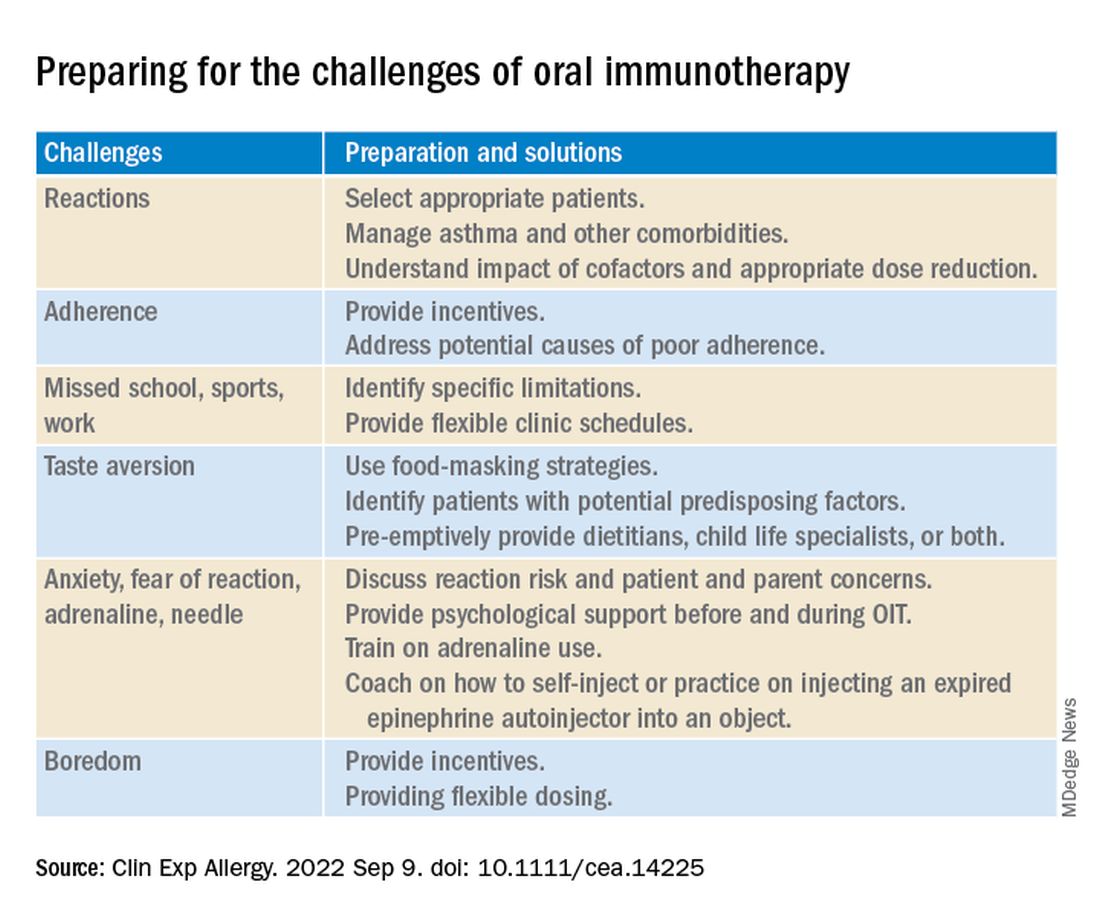
Strategies to overcome OIT challenges
Reviewing the literature about OIT for food allergy, the authors suggest various strategies physicians can use to help OIT patients and their families prepare for and overcome common treatment-related challenges.
Two experts welcome the report
Rita Kachru, MD, a specialist in allergy and immunology and a codirector of the food allergy program at UCLA Health in Los Angeles, called this “an excellent report about a wonderful, individualized option in food allergy management.
“The authors did an excellent job delineating OIT terminology, outlining the goals, risks, and benefits of OIT, noting that it’s not a cure, and emphasizing the crucial importance of discussions with each family throughout the process,” Dr. Kachru, who was not involved in developing the report, told this news organization.
“I thoroughly agree with their assessment,” she added. “The more you do OIT research and clinical care, the more you realize the pitfalls, the benefits, and the importance of patient goals and family dynamics. Discussing the goals, risks, benefits, and alternatives to OIT in detail with the family is crucial so they fully understand the process.”
Basil M. Kahwash, MD, an allergy, pulmonary, and critical care medicine specialist at Vanderbilt University Medical Center in Nashville, Tenn., said that providers in the immunology community have been discussing OIT for years and that he welcomes the well-written report that summarizes the evidence.
“It’s important to periodically summarize the evidence, as well as the consensus expert opinion about the evidence, so we may better inform our colleagues and patients,” said Dr. Kahwash, who was not involved in developing the report. “The authors are well-known experts in our field who have experience with OIT and with reviewing the evidence of food allergy.
“OIT can be a fantastic option for some patients, especially those who are very highly motivated and understand the process from start to finish. But OIT is not the best option for every child, and it’s not much of an option for adults,” he explained. “Patients need to be chosen carefully and understand the level of motivation required to safely follow through with the treatment.
“The report will hopefully affect patient care positively and allow patients to understand the limitations around OIT when they consider their candidacy for it,” he added. “In most cases, OIT patients will still need to avoid the allergen, but if a small amount accidentally gets into their food, they probably won’t have a very severe reaction to it.”
Dr. Kahwash would like to see data on patients who have seen long-term remission with OIT.
“Clearly, some patients benefit from OIT. What differentiates patients who benefit from OIT from those who do not?” he asked. “In the future, we need to consider possible biomarkers of patients who are and who aren’t good candidates for OIT.
“Regardless of OIT’s limitations, the potential for desensitization rather than strict avoidance represents a big step in the evolution of food allergy treatment,” Dr. Kahwash noted.
No funding details were provided. Dr. Mack and three coauthors report financial relationships with Aimmune. Aimmune Therapeutics is the manufacturer of Palforzia OIT (AR101 powder provided in capsules and sachets). Most coauthors also report financial relationships with other pharmaceutical companies. The full list can be found with the original article. Dr. Kachru was an investigator in the PALISADES clinical trial of AR101. Dr. Kahwash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new review.
In OIT, a patient who is allergic to a specific food consumes increasing amounts of the allergen over time to reduce their risk for allergic reaction.
“OIT is an elective, usually noncurative procedure with inherent risks that require families to function as amateur medical professionals. Preparing them for this role is essential to protect patients and ensure the long-term success of this life-changing procedure,” lead author Douglas P. Mack, MD, MSc, a pediatric allergy, asthma, and immunology specialist at McMaster University in Hamilton, Ont., and colleagues write in Clinical & Experimental Allergy.
From strict avoidance to desensitization
Food allergy treatment has traditionally involved avoiding accidental exposure that may lead to anaphylaxis and providing rescue medication. In recent years, OIT “has been recommended by several guidelines as a primary option,” Dr. Mack and coauthors write. And with the “approval by European and USA regulators of [peanut allergen powder] Palforzia [Aimmune Therapeutics], there are now commercial and noncommercial forms of OIT available for use in several countries.”
They advise physicians to take a proactive, educational, supportive approach to patients and their families throughout the therapy.
“Ultimately, the decision to pursue OIT or continue avoidance strategies remains the responsibility of the family and the patients,” they write. “Some families may not be prepared for the role that they have to play in actively managing their child’s food allergy treatment.”
Strategies to overcome OIT challenges
Reviewing the literature about OIT for food allergy, the authors suggest various strategies physicians can use to help OIT patients and their families prepare for and overcome common treatment-related challenges.

Two experts welcome the report
Rita Kachru, MD, a specialist in allergy and immunology and a codirector of the food allergy program at UCLA Health in Los Angeles, called this “an excellent report about a wonderful, individualized option in food allergy management.
“The authors did an excellent job delineating OIT terminology, outlining the goals, risks, and benefits of OIT, noting that it’s not a cure, and emphasizing the crucial importance of discussions with each family throughout the process,” Dr. Kachru, who was not involved in developing the report, told this news organization.
“I thoroughly agree with their assessment,” she added. “The more you do OIT research and clinical care, the more you realize the pitfalls, the benefits, and the importance of patient goals and family dynamics. Discussing the goals, risks, benefits, and alternatives to OIT in detail with the family is crucial so they fully understand the process.”
Basil M. Kahwash, MD, an allergy, pulmonary, and critical care medicine specialist at Vanderbilt University Medical Center in Nashville, Tenn., said that providers in the immunology community have been discussing OIT for years and that he welcomes the well-written report that summarizes the evidence.
“It’s important to periodically summarize the evidence, as well as the consensus expert opinion about the evidence, so we may better inform our colleagues and patients,” said Dr. Kahwash, who was not involved in developing the report. “The authors are well-known experts in our field who have experience with OIT and with reviewing the evidence of food allergy.
“OIT can be a fantastic option for some patients, especially those who are very highly motivated and understand the process from start to finish. But OIT is not the best option for every child, and it’s not much of an option for adults,” he explained. “Patients need to be chosen carefully and understand the level of motivation required to safely follow through with the treatment.
“The report will hopefully affect patient care positively and allow patients to understand the limitations around OIT when they consider their candidacy for it,” he added. “In most cases, OIT patients will still need to avoid the allergen, but if a small amount accidentally gets into their food, they probably won’t have a very severe reaction to it.”
Dr. Kahwash would like to see data on patients who have seen long-term remission with OIT.
“Clearly, some patients benefit from OIT. What differentiates patients who benefit from OIT from those who do not?” he asked. “In the future, we need to consider possible biomarkers of patients who are and who aren’t good candidates for OIT.
“Regardless of OIT’s limitations, the potential for desensitization rather than strict avoidance represents a big step in the evolution of food allergy treatment,” Dr. Kahwash noted.
No funding details were provided. Dr. Mack and three coauthors report financial relationships with Aimmune. Aimmune Therapeutics is the manufacturer of Palforzia OIT (AR101 powder provided in capsules and sachets). Most coauthors also report financial relationships with other pharmaceutical companies. The full list can be found with the original article. Dr. Kachru was an investigator in the PALISADES clinical trial of AR101. Dr. Kahwash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new review.
In OIT, a patient who is allergic to a specific food consumes increasing amounts of the allergen over time to reduce their risk for allergic reaction.
“OIT is an elective, usually noncurative procedure with inherent risks that require families to function as amateur medical professionals. Preparing them for this role is essential to protect patients and ensure the long-term success of this life-changing procedure,” lead author Douglas P. Mack, MD, MSc, a pediatric allergy, asthma, and immunology specialist at McMaster University in Hamilton, Ont., and colleagues write in Clinical & Experimental Allergy.
From strict avoidance to desensitization
Food allergy treatment has traditionally involved avoiding accidental exposure that may lead to anaphylaxis and providing rescue medication. In recent years, OIT “has been recommended by several guidelines as a primary option,” Dr. Mack and coauthors write. And with the “approval by European and USA regulators of [peanut allergen powder] Palforzia [Aimmune Therapeutics], there are now commercial and noncommercial forms of OIT available for use in several countries.”
They advise physicians to take a proactive, educational, supportive approach to patients and their families throughout the therapy.
“Ultimately, the decision to pursue OIT or continue avoidance strategies remains the responsibility of the family and the patients,” they write. “Some families may not be prepared for the role that they have to play in actively managing their child’s food allergy treatment.”
Strategies to overcome OIT challenges
Reviewing the literature about OIT for food allergy, the authors suggest various strategies physicians can use to help OIT patients and their families prepare for and overcome common treatment-related challenges.

Two experts welcome the report
Rita Kachru, MD, a specialist in allergy and immunology and a codirector of the food allergy program at UCLA Health in Los Angeles, called this “an excellent report about a wonderful, individualized option in food allergy management.
“The authors did an excellent job delineating OIT terminology, outlining the goals, risks, and benefits of OIT, noting that it’s not a cure, and emphasizing the crucial importance of discussions with each family throughout the process,” Dr. Kachru, who was not involved in developing the report, told this news organization.
“I thoroughly agree with their assessment,” she added. “The more you do OIT research and clinical care, the more you realize the pitfalls, the benefits, and the importance of patient goals and family dynamics. Discussing the goals, risks, benefits, and alternatives to OIT in detail with the family is crucial so they fully understand the process.”
Basil M. Kahwash, MD, an allergy, pulmonary, and critical care medicine specialist at Vanderbilt University Medical Center in Nashville, Tenn., said that providers in the immunology community have been discussing OIT for years and that he welcomes the well-written report that summarizes the evidence.
“It’s important to periodically summarize the evidence, as well as the consensus expert opinion about the evidence, so we may better inform our colleagues and patients,” said Dr. Kahwash, who was not involved in developing the report. “The authors are well-known experts in our field who have experience with OIT and with reviewing the evidence of food allergy.
“OIT can be a fantastic option for some patients, especially those who are very highly motivated and understand the process from start to finish. But OIT is not the best option for every child, and it’s not much of an option for adults,” he explained. “Patients need to be chosen carefully and understand the level of motivation required to safely follow through with the treatment.
“The report will hopefully affect patient care positively and allow patients to understand the limitations around OIT when they consider their candidacy for it,” he added. “In most cases, OIT patients will still need to avoid the allergen, but if a small amount accidentally gets into their food, they probably won’t have a very severe reaction to it.”
Dr. Kahwash would like to see data on patients who have seen long-term remission with OIT.
“Clearly, some patients benefit from OIT. What differentiates patients who benefit from OIT from those who do not?” he asked. “In the future, we need to consider possible biomarkers of patients who are and who aren’t good candidates for OIT.
“Regardless of OIT’s limitations, the potential for desensitization rather than strict avoidance represents a big step in the evolution of food allergy treatment,” Dr. Kahwash noted.
No funding details were provided. Dr. Mack and three coauthors report financial relationships with Aimmune. Aimmune Therapeutics is the manufacturer of Palforzia OIT (AR101 powder provided in capsules and sachets). Most coauthors also report financial relationships with other pharmaceutical companies. The full list can be found with the original article. Dr. Kachru was an investigator in the PALISADES clinical trial of AR101. Dr. Kahwash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL & EXPERIMENTAL ALLERGY
Legal and malpractice risks when taking call
Taking call is one of the more challenging - and annoying - aspects of the job for many physicians. Calls may wake them up in the middle of the night and can interfere with their at-home activities. In Medscape’s Employed Physicians Report, 37% of respondents said they have from 1 to 5 hours of call per month; 19% said they have 6 to 10 hours; and 12% have 11 hours or more.
“Even if you don’t have to come in to the ED, you can get calls in the middle of the night, and you may get paid very little, if anything,” said Robert Bitterman MD, JD, an emergency physician and attorney in Harbor Springs, Mich.
And responding to the calls is not optional. Dr. Bitterman said
On-call activities are regulated by the federal Emergency Medical Treatment and Active Labor Act (EMTALA). Dr. Bitterman said it’s rare for the federal government to prosecute on-call physicians for violating EMTALA. Instead, it’s more likely that the hospital will be fined for EMTALA violations committed by on-call physicians.
However, the hospital passes the on-call obligation on to individual physicians through medical staff bylaws. Physicians who violate the bylaws may have their privileges restricted or removed, Dr. Bitterman said. Physicians could also be sued for malpractice, even if they never treated the patient, he added.
After-hours call duty in physicians’ practices
A very different type of call duty is having to respond to calls from one’s own patients after regular hours. Unlike doctors on ED call, who usually deal with patients they have never met, these physicians deal with their established patients or those of a colleague in their practice.
Courts have established that physicians have to provide an answering service or other means for their patients to contact them after hours, and the doctor must respond to these calls in a timely manner.
In a 2015 Louisiana ruling, a cardiologist was found liable for malpractice because he didn’t respond to an after-hours call from his patient. The patient tried several times to contact the cardiologist but got no reply.
Physicians may also be responsible if their answering service does not send critical messages to them immediately, if it fails to make appropriate documentation, or if it sends inaccurate data to the doctor.
Cases when on-call doctors didn’t respond
The Office of the Inspector General (OIG) of the U.S. Health and Human Services Administration oversees federal EMTALA violations and regularly reports them.
In 2018, the OIG fined a hospital in Waterloo, Iowa, $90,000 when an on-call cardiologist failed to implant a pacemaker for an ED patient. According to the OIG’s report, the patient arrived at the hospital with heart problems. Reached by phone, the cardiologist directed the ED physician to begin transcutaneous pacing but asked that the patient be transferred to another hospital for placement of the pacemaker. The patient died after transfer.
The OIG found that the original cardiologist could have placed the pacemaker, but, as often happens, it only fined the hospital, not the on-call physician for the EMTALA violation.
EMTALA requires that hospitals provide on-call specialists to assist emergency physicians with care of patients who arrive in the ED. In specialties for which there are few doctors to choose from, the on-call specialist may be on duty every third night and every third weekend. This can be daunting, especially for specialists who’ve had a grueling day of work.
Occasionally, on-call physicians, fearful they could make a medical error, request that the patient be transferred to another hospital for treatment. This is what a neurosurgeon who was on call at a Topeka, Kan., hospital did in 2001. Transferred to another hospital, the patient underwent an operation but lost sensation in his lower extremities. The patient sued the on-call neurosurgeon for negligence.
During the trial, the on-call neurosurgeon testified that he was “feeling run-down because he had been an on-call physician every third night for more than 10 years.” He also said this was the first time he had refused to see a patient because of fatigue, and he had decided that the patient “would be better off at a trauma center that had a trauma team and a fresher surgeon.”
The neurosurgeon successfully defended the malpractice suit, but Dr. Bitterman said he might have lost had there not been some unusual circumstances in the case. The court ruled that the hospital had not clearly defined the duties of on-call physicians, and the lawsuit didn’t cite the neurosurgeon’s EMTALA duty.
On-call duties defined by EMTALA
EMTALA sets the overall rules for on-call duties, which each hospital is expected to fine-tune on the basis of its own particular circumstances. Here are some of those rules, issued by the Centers for Medicare & Medicaid Services and the OIG.
Only an individual physician can be on call. The hospital’s on-call schedule cannot name a physician practice.
Call applies to all ED patients. Physicians cannot limit their on-call responsibilities to their own patients, to patients in their insurance network, or to paying patients.
There may be some gaps in the call schedule. The OIG is not specific as to how many gaps are allowed, said Nick Healey, an attorney in Cheyenne, Wyo., who has written about on-call duties. Among other things, adequate coverage depends on the number of available physicians and the demand for their services. Mr. Healey added that states may require more extensive availability of on-call physicians at high-level trauma centers.
Hospitals must have made arrangements for transfer. Whenever there is a gap in the schedule, hospitals need to have a designated hospital to send the patient to. Hospitals that unnecessarily transfer patients will be penalized.
The ED physician calls the shots. The emergency physician handling the case decides if the on-call doctor has to come in and treat the patient firsthand.
The on-call physician may delegate the work to others. On-call physicians may designate a nurse practitioner or physician assistant, but the on-call physician is ultimately responsible. The ED doctor may require the physician to come in anyway, according to Todd B. Taylor, MD, an emergency physician in Phoenix, who has written about on-call duties. Dr. Bitterman noted that the physician may designate a colleague to take their call, but the substitute has to have privileges at the hospital.
Physicians may do their own work while on call. Physicians can perform elective surgery while on call, provided they have made arrangements if they then become unavailable for duty, Dr. Taylor said. He added that physicians can also have simultaneous call at other hospitals, provided they make arrangements.
The hospital fine-tunes call obligations
The hospital is expected to further define the federal rules. For instance, the CMS says physicians should respond to calls within a “reasonable period of time” and requires hospitals to specify response times, which may be 15-30 minutes for responding to phone calls and traveling to the ED, Dr. Bitterman said.
The CMS says older physicians can be exempted from call. The hospital determines the age at which physicians can be exempted. “Hospitals typically exempt physicians over age 65 or 70, or when they have certain medical conditions,” said Lowell Brown, a Los Angeles attorney who deals with on-call duties.
The hospital also sets the call schedule, which may result in uncovered periods in specialties in which there are few physicians to draw from, according to Mr. Healey. He said many hospitals still use a simple rule of thumb, even though it has been dismissed by the CMS. Under this so-called “rule of three,” hospitals that have three doctors or fewer in a specialty do not have to provide constant call coverage.
On-call rules are part of the medical staff bylaws, and they have to be approved by the medical staff. This may require delicate negotiations between the staff’s leadership and administrators, Dr. Bitterman said.
It is often up to the emergency physician on duty to enforce the hospital’s on-call rules, Dr. Taylor said. “If the ED physician is having trouble, he or she may contact the on-call physician’s department chairman or, if necessary, the chief of the medical staff and ask that person to deal with the physician,” Dr. Taylor said.
The ED physician has to determine whether the patient needs to be transferred to another hospital. Dr. Taylor said the ED physician must fill out a transfer form and obtain consent from the receiving hospital.
If a patient has to be transferred because an on-call physician failed to appear, the originating hospital has to report this to the CMS, and the physician and the hospital can be cited for an inappropriate transfer and fined, Mr. Brown said. “The possibility of being identified in this way should be a powerful incentive to accept call duty,” he added.
Malpractice exposure of on-call physicians
When on-call doctors provide medical advice regarding an ED patient, that advice may be subject to malpractice litigation, Dr. Taylor said. “Even if you only give the ED doctor advice over the phone, that may establish a patient-physician relationship and a duty that patient can cite in a malpractice case,” he noted.
Refusing to take call may also be grounds for a malpractice lawsuit, Dr. Bitterman said. Refusing to see a patient would not be considered medical negligence, he continued, because no medical decision is made. Rather, it involves general negligence, which occurs when physicians fail to carry out duties expected of them.
Dr. Bitterman cited a 2006 malpractice judgment in which an on-call neurosurgeon in Missouri was found to be generally negligent. The neurosurgeon had arranged for a colleague in his practice to take his call, but the colleague did not have privileges at the hospital.
A patient with a brain bleed came in and the substitute was on duty. The patient had to be transferred to another hospital, where the patient died. The court ordered that the on-call doctor and the originating hospital had to split a fine of $400,800.
On-call physicians can be charged with abandonment
Dr. Bitterman said that if on-call physicians do not provide expected follow-up treatment for an ED patient, they could be charged with abandonment, which is a matter of state law and involves filing a malpractice lawsuit.
Abandonment involves unilaterally terminating the patient relationship without providing notice. There must be an established relationship, which, in the case of call, is formed when the doctor comes to the ED to examine or admit the patient, Dr. Bitterman said. He added that the on-call doctor’s obligation applies only to the medical condition the patient came in for.
Even when an on-call doctor does not see a patient, a relationship can be established if the hospital requires its on-call doctors to make follow-up visits for ED patients, Dr. Taylor said. At some hospitals, he said, on-call doctors have blanket agreements to provide follow-up care in return for not having to arrive in the middle of the night during the ED visit.
Dr. Taylor gave an example of the on-call doctor’s obligation: “The ED doctor puts a splint on the patient’s ankle fracture, and the orthopedic surgeon on call agrees to follow up with the patient within the next few days. If the orthopedic surgeon refuses to follow up without making a reasonable accommodation, it may become an issue of patient abandonment.”
Now everyone has a good grasp of the rules
Fifteen years ago, many doctors were in open revolt against on-call duties, but they are more accepting now and understand the rules better, Mr. Healey said.
“Many hospitals have begun paying some specialists for call and designating hospitalists and surgicalists to do at least some of the work that used to be expected of on-call doctors,” he said.
According to Dr. Taylor, today’s on-call doctors often have less to do than in the past. “For example,” he said, “the hospitalist may admit an orthopedic patient at night, and then the orthopedic surgeon does the operation the next day. We’ve had EMTALA for 36 years now, and hospitals and doctors know how call works.”
A version of this article first appeared on Medscape.com.
Taking call is one of the more challenging - and annoying - aspects of the job for many physicians. Calls may wake them up in the middle of the night and can interfere with their at-home activities. In Medscape’s Employed Physicians Report, 37% of respondents said they have from 1 to 5 hours of call per month; 19% said they have 6 to 10 hours; and 12% have 11 hours or more.
“Even if you don’t have to come in to the ED, you can get calls in the middle of the night, and you may get paid very little, if anything,” said Robert Bitterman MD, JD, an emergency physician and attorney in Harbor Springs, Mich.
And responding to the calls is not optional. Dr. Bitterman said
On-call activities are regulated by the federal Emergency Medical Treatment and Active Labor Act (EMTALA). Dr. Bitterman said it’s rare for the federal government to prosecute on-call physicians for violating EMTALA. Instead, it’s more likely that the hospital will be fined for EMTALA violations committed by on-call physicians.
However, the hospital passes the on-call obligation on to individual physicians through medical staff bylaws. Physicians who violate the bylaws may have their privileges restricted or removed, Dr. Bitterman said. Physicians could also be sued for malpractice, even if they never treated the patient, he added.
After-hours call duty in physicians’ practices
A very different type of call duty is having to respond to calls from one’s own patients after regular hours. Unlike doctors on ED call, who usually deal with patients they have never met, these physicians deal with their established patients or those of a colleague in their practice.
Courts have established that physicians have to provide an answering service or other means for their patients to contact them after hours, and the doctor must respond to these calls in a timely manner.
In a 2015 Louisiana ruling, a cardiologist was found liable for malpractice because he didn’t respond to an after-hours call from his patient. The patient tried several times to contact the cardiologist but got no reply.
Physicians may also be responsible if their answering service does not send critical messages to them immediately, if it fails to make appropriate documentation, or if it sends inaccurate data to the doctor.
Cases when on-call doctors didn’t respond
The Office of the Inspector General (OIG) of the U.S. Health and Human Services Administration oversees federal EMTALA violations and regularly reports them.
In 2018, the OIG fined a hospital in Waterloo, Iowa, $90,000 when an on-call cardiologist failed to implant a pacemaker for an ED patient. According to the OIG’s report, the patient arrived at the hospital with heart problems. Reached by phone, the cardiologist directed the ED physician to begin transcutaneous pacing but asked that the patient be transferred to another hospital for placement of the pacemaker. The patient died after transfer.
The OIG found that the original cardiologist could have placed the pacemaker, but, as often happens, it only fined the hospital, not the on-call physician for the EMTALA violation.
EMTALA requires that hospitals provide on-call specialists to assist emergency physicians with care of patients who arrive in the ED. In specialties for which there are few doctors to choose from, the on-call specialist may be on duty every third night and every third weekend. This can be daunting, especially for specialists who’ve had a grueling day of work.
Occasionally, on-call physicians, fearful they could make a medical error, request that the patient be transferred to another hospital for treatment. This is what a neurosurgeon who was on call at a Topeka, Kan., hospital did in 2001. Transferred to another hospital, the patient underwent an operation but lost sensation in his lower extremities. The patient sued the on-call neurosurgeon for negligence.
During the trial, the on-call neurosurgeon testified that he was “feeling run-down because he had been an on-call physician every third night for more than 10 years.” He also said this was the first time he had refused to see a patient because of fatigue, and he had decided that the patient “would be better off at a trauma center that had a trauma team and a fresher surgeon.”
The neurosurgeon successfully defended the malpractice suit, but Dr. Bitterman said he might have lost had there not been some unusual circumstances in the case. The court ruled that the hospital had not clearly defined the duties of on-call physicians, and the lawsuit didn’t cite the neurosurgeon’s EMTALA duty.
On-call duties defined by EMTALA
EMTALA sets the overall rules for on-call duties, which each hospital is expected to fine-tune on the basis of its own particular circumstances. Here are some of those rules, issued by the Centers for Medicare & Medicaid Services and the OIG.
Only an individual physician can be on call. The hospital’s on-call schedule cannot name a physician practice.
Call applies to all ED patients. Physicians cannot limit their on-call responsibilities to their own patients, to patients in their insurance network, or to paying patients.
There may be some gaps in the call schedule. The OIG is not specific as to how many gaps are allowed, said Nick Healey, an attorney in Cheyenne, Wyo., who has written about on-call duties. Among other things, adequate coverage depends on the number of available physicians and the demand for their services. Mr. Healey added that states may require more extensive availability of on-call physicians at high-level trauma centers.
Hospitals must have made arrangements for transfer. Whenever there is a gap in the schedule, hospitals need to have a designated hospital to send the patient to. Hospitals that unnecessarily transfer patients will be penalized.
The ED physician calls the shots. The emergency physician handling the case decides if the on-call doctor has to come in and treat the patient firsthand.
The on-call physician may delegate the work to others. On-call physicians may designate a nurse practitioner or physician assistant, but the on-call physician is ultimately responsible. The ED doctor may require the physician to come in anyway, according to Todd B. Taylor, MD, an emergency physician in Phoenix, who has written about on-call duties. Dr. Bitterman noted that the physician may designate a colleague to take their call, but the substitute has to have privileges at the hospital.
Physicians may do their own work while on call. Physicians can perform elective surgery while on call, provided they have made arrangements if they then become unavailable for duty, Dr. Taylor said. He added that physicians can also have simultaneous call at other hospitals, provided they make arrangements.
The hospital fine-tunes call obligations
The hospital is expected to further define the federal rules. For instance, the CMS says physicians should respond to calls within a “reasonable period of time” and requires hospitals to specify response times, which may be 15-30 minutes for responding to phone calls and traveling to the ED, Dr. Bitterman said.
The CMS says older physicians can be exempted from call. The hospital determines the age at which physicians can be exempted. “Hospitals typically exempt physicians over age 65 or 70, or when they have certain medical conditions,” said Lowell Brown, a Los Angeles attorney who deals with on-call duties.
The hospital also sets the call schedule, which may result in uncovered periods in specialties in which there are few physicians to draw from, according to Mr. Healey. He said many hospitals still use a simple rule of thumb, even though it has been dismissed by the CMS. Under this so-called “rule of three,” hospitals that have three doctors or fewer in a specialty do not have to provide constant call coverage.
On-call rules are part of the medical staff bylaws, and they have to be approved by the medical staff. This may require delicate negotiations between the staff’s leadership and administrators, Dr. Bitterman said.
It is often up to the emergency physician on duty to enforce the hospital’s on-call rules, Dr. Taylor said. “If the ED physician is having trouble, he or she may contact the on-call physician’s department chairman or, if necessary, the chief of the medical staff and ask that person to deal with the physician,” Dr. Taylor said.
The ED physician has to determine whether the patient needs to be transferred to another hospital. Dr. Taylor said the ED physician must fill out a transfer form and obtain consent from the receiving hospital.
If a patient has to be transferred because an on-call physician failed to appear, the originating hospital has to report this to the CMS, and the physician and the hospital can be cited for an inappropriate transfer and fined, Mr. Brown said. “The possibility of being identified in this way should be a powerful incentive to accept call duty,” he added.
Malpractice exposure of on-call physicians
When on-call doctors provide medical advice regarding an ED patient, that advice may be subject to malpractice litigation, Dr. Taylor said. “Even if you only give the ED doctor advice over the phone, that may establish a patient-physician relationship and a duty that patient can cite in a malpractice case,” he noted.
Refusing to take call may also be grounds for a malpractice lawsuit, Dr. Bitterman said. Refusing to see a patient would not be considered medical negligence, he continued, because no medical decision is made. Rather, it involves general negligence, which occurs when physicians fail to carry out duties expected of them.
Dr. Bitterman cited a 2006 malpractice judgment in which an on-call neurosurgeon in Missouri was found to be generally negligent. The neurosurgeon had arranged for a colleague in his practice to take his call, but the colleague did not have privileges at the hospital.
A patient with a brain bleed came in and the substitute was on duty. The patient had to be transferred to another hospital, where the patient died. The court ordered that the on-call doctor and the originating hospital had to split a fine of $400,800.
On-call physicians can be charged with abandonment
Dr. Bitterman said that if on-call physicians do not provide expected follow-up treatment for an ED patient, they could be charged with abandonment, which is a matter of state law and involves filing a malpractice lawsuit.
Abandonment involves unilaterally terminating the patient relationship without providing notice. There must be an established relationship, which, in the case of call, is formed when the doctor comes to the ED to examine or admit the patient, Dr. Bitterman said. He added that the on-call doctor’s obligation applies only to the medical condition the patient came in for.
Even when an on-call doctor does not see a patient, a relationship can be established if the hospital requires its on-call doctors to make follow-up visits for ED patients, Dr. Taylor said. At some hospitals, he said, on-call doctors have blanket agreements to provide follow-up care in return for not having to arrive in the middle of the night during the ED visit.
Dr. Taylor gave an example of the on-call doctor’s obligation: “The ED doctor puts a splint on the patient’s ankle fracture, and the orthopedic surgeon on call agrees to follow up with the patient within the next few days. If the orthopedic surgeon refuses to follow up without making a reasonable accommodation, it may become an issue of patient abandonment.”
Now everyone has a good grasp of the rules
Fifteen years ago, many doctors were in open revolt against on-call duties, but they are more accepting now and understand the rules better, Mr. Healey said.
“Many hospitals have begun paying some specialists for call and designating hospitalists and surgicalists to do at least some of the work that used to be expected of on-call doctors,” he said.
According to Dr. Taylor, today’s on-call doctors often have less to do than in the past. “For example,” he said, “the hospitalist may admit an orthopedic patient at night, and then the orthopedic surgeon does the operation the next day. We’ve had EMTALA for 36 years now, and hospitals and doctors know how call works.”
A version of this article first appeared on Medscape.com.
Taking call is one of the more challenging - and annoying - aspects of the job for many physicians. Calls may wake them up in the middle of the night and can interfere with their at-home activities. In Medscape’s Employed Physicians Report, 37% of respondents said they have from 1 to 5 hours of call per month; 19% said they have 6 to 10 hours; and 12% have 11 hours or more.
“Even if you don’t have to come in to the ED, you can get calls in the middle of the night, and you may get paid very little, if anything,” said Robert Bitterman MD, JD, an emergency physician and attorney in Harbor Springs, Mich.
And responding to the calls is not optional. Dr. Bitterman said
On-call activities are regulated by the federal Emergency Medical Treatment and Active Labor Act (EMTALA). Dr. Bitterman said it’s rare for the federal government to prosecute on-call physicians for violating EMTALA. Instead, it’s more likely that the hospital will be fined for EMTALA violations committed by on-call physicians.
However, the hospital passes the on-call obligation on to individual physicians through medical staff bylaws. Physicians who violate the bylaws may have their privileges restricted or removed, Dr. Bitterman said. Physicians could also be sued for malpractice, even if they never treated the patient, he added.
After-hours call duty in physicians’ practices
A very different type of call duty is having to respond to calls from one’s own patients after regular hours. Unlike doctors on ED call, who usually deal with patients they have never met, these physicians deal with their established patients or those of a colleague in their practice.
Courts have established that physicians have to provide an answering service or other means for their patients to contact them after hours, and the doctor must respond to these calls in a timely manner.
In a 2015 Louisiana ruling, a cardiologist was found liable for malpractice because he didn’t respond to an after-hours call from his patient. The patient tried several times to contact the cardiologist but got no reply.
Physicians may also be responsible if their answering service does not send critical messages to them immediately, if it fails to make appropriate documentation, or if it sends inaccurate data to the doctor.
Cases when on-call doctors didn’t respond
The Office of the Inspector General (OIG) of the U.S. Health and Human Services Administration oversees federal EMTALA violations and regularly reports them.
In 2018, the OIG fined a hospital in Waterloo, Iowa, $90,000 when an on-call cardiologist failed to implant a pacemaker for an ED patient. According to the OIG’s report, the patient arrived at the hospital with heart problems. Reached by phone, the cardiologist directed the ED physician to begin transcutaneous pacing but asked that the patient be transferred to another hospital for placement of the pacemaker. The patient died after transfer.
The OIG found that the original cardiologist could have placed the pacemaker, but, as often happens, it only fined the hospital, not the on-call physician for the EMTALA violation.
EMTALA requires that hospitals provide on-call specialists to assist emergency physicians with care of patients who arrive in the ED. In specialties for which there are few doctors to choose from, the on-call specialist may be on duty every third night and every third weekend. This can be daunting, especially for specialists who’ve had a grueling day of work.
Occasionally, on-call physicians, fearful they could make a medical error, request that the patient be transferred to another hospital for treatment. This is what a neurosurgeon who was on call at a Topeka, Kan., hospital did in 2001. Transferred to another hospital, the patient underwent an operation but lost sensation in his lower extremities. The patient sued the on-call neurosurgeon for negligence.
During the trial, the on-call neurosurgeon testified that he was “feeling run-down because he had been an on-call physician every third night for more than 10 years.” He also said this was the first time he had refused to see a patient because of fatigue, and he had decided that the patient “would be better off at a trauma center that had a trauma team and a fresher surgeon.”
The neurosurgeon successfully defended the malpractice suit, but Dr. Bitterman said he might have lost had there not been some unusual circumstances in the case. The court ruled that the hospital had not clearly defined the duties of on-call physicians, and the lawsuit didn’t cite the neurosurgeon’s EMTALA duty.
On-call duties defined by EMTALA
EMTALA sets the overall rules for on-call duties, which each hospital is expected to fine-tune on the basis of its own particular circumstances. Here are some of those rules, issued by the Centers for Medicare & Medicaid Services and the OIG.
Only an individual physician can be on call. The hospital’s on-call schedule cannot name a physician practice.
Call applies to all ED patients. Physicians cannot limit their on-call responsibilities to their own patients, to patients in their insurance network, or to paying patients.
There may be some gaps in the call schedule. The OIG is not specific as to how many gaps are allowed, said Nick Healey, an attorney in Cheyenne, Wyo., who has written about on-call duties. Among other things, adequate coverage depends on the number of available physicians and the demand for their services. Mr. Healey added that states may require more extensive availability of on-call physicians at high-level trauma centers.
Hospitals must have made arrangements for transfer. Whenever there is a gap in the schedule, hospitals need to have a designated hospital to send the patient to. Hospitals that unnecessarily transfer patients will be penalized.
The ED physician calls the shots. The emergency physician handling the case decides if the on-call doctor has to come in and treat the patient firsthand.
The on-call physician may delegate the work to others. On-call physicians may designate a nurse practitioner or physician assistant, but the on-call physician is ultimately responsible. The ED doctor may require the physician to come in anyway, according to Todd B. Taylor, MD, an emergency physician in Phoenix, who has written about on-call duties. Dr. Bitterman noted that the physician may designate a colleague to take their call, but the substitute has to have privileges at the hospital.
Physicians may do their own work while on call. Physicians can perform elective surgery while on call, provided they have made arrangements if they then become unavailable for duty, Dr. Taylor said. He added that physicians can also have simultaneous call at other hospitals, provided they make arrangements.
The hospital fine-tunes call obligations
The hospital is expected to further define the federal rules. For instance, the CMS says physicians should respond to calls within a “reasonable period of time” and requires hospitals to specify response times, which may be 15-30 minutes for responding to phone calls and traveling to the ED, Dr. Bitterman said.
The CMS says older physicians can be exempted from call. The hospital determines the age at which physicians can be exempted. “Hospitals typically exempt physicians over age 65 or 70, or when they have certain medical conditions,” said Lowell Brown, a Los Angeles attorney who deals with on-call duties.
The hospital also sets the call schedule, which may result in uncovered periods in specialties in which there are few physicians to draw from, according to Mr. Healey. He said many hospitals still use a simple rule of thumb, even though it has been dismissed by the CMS. Under this so-called “rule of three,” hospitals that have three doctors or fewer in a specialty do not have to provide constant call coverage.
On-call rules are part of the medical staff bylaws, and they have to be approved by the medical staff. This may require delicate negotiations between the staff’s leadership and administrators, Dr. Bitterman said.
It is often up to the emergency physician on duty to enforce the hospital’s on-call rules, Dr. Taylor said. “If the ED physician is having trouble, he or she may contact the on-call physician’s department chairman or, if necessary, the chief of the medical staff and ask that person to deal with the physician,” Dr. Taylor said.
The ED physician has to determine whether the patient needs to be transferred to another hospital. Dr. Taylor said the ED physician must fill out a transfer form and obtain consent from the receiving hospital.
If a patient has to be transferred because an on-call physician failed to appear, the originating hospital has to report this to the CMS, and the physician and the hospital can be cited for an inappropriate transfer and fined, Mr. Brown said. “The possibility of being identified in this way should be a powerful incentive to accept call duty,” he added.
Malpractice exposure of on-call physicians
When on-call doctors provide medical advice regarding an ED patient, that advice may be subject to malpractice litigation, Dr. Taylor said. “Even if you only give the ED doctor advice over the phone, that may establish a patient-physician relationship and a duty that patient can cite in a malpractice case,” he noted.
Refusing to take call may also be grounds for a malpractice lawsuit, Dr. Bitterman said. Refusing to see a patient would not be considered medical negligence, he continued, because no medical decision is made. Rather, it involves general negligence, which occurs when physicians fail to carry out duties expected of them.
Dr. Bitterman cited a 2006 malpractice judgment in which an on-call neurosurgeon in Missouri was found to be generally negligent. The neurosurgeon had arranged for a colleague in his practice to take his call, but the colleague did not have privileges at the hospital.
A patient with a brain bleed came in and the substitute was on duty. The patient had to be transferred to another hospital, where the patient died. The court ordered that the on-call doctor and the originating hospital had to split a fine of $400,800.
On-call physicians can be charged with abandonment
Dr. Bitterman said that if on-call physicians do not provide expected follow-up treatment for an ED patient, they could be charged with abandonment, which is a matter of state law and involves filing a malpractice lawsuit.
Abandonment involves unilaterally terminating the patient relationship without providing notice. There must be an established relationship, which, in the case of call, is formed when the doctor comes to the ED to examine or admit the patient, Dr. Bitterman said. He added that the on-call doctor’s obligation applies only to the medical condition the patient came in for.
Even when an on-call doctor does not see a patient, a relationship can be established if the hospital requires its on-call doctors to make follow-up visits for ED patients, Dr. Taylor said. At some hospitals, he said, on-call doctors have blanket agreements to provide follow-up care in return for not having to arrive in the middle of the night during the ED visit.
Dr. Taylor gave an example of the on-call doctor’s obligation: “The ED doctor puts a splint on the patient’s ankle fracture, and the orthopedic surgeon on call agrees to follow up with the patient within the next few days. If the orthopedic surgeon refuses to follow up without making a reasonable accommodation, it may become an issue of patient abandonment.”
Now everyone has a good grasp of the rules
Fifteen years ago, many doctors were in open revolt against on-call duties, but they are more accepting now and understand the rules better, Mr. Healey said.
“Many hospitals have begun paying some specialists for call and designating hospitalists and surgicalists to do at least some of the work that used to be expected of on-call doctors,” he said.
According to Dr. Taylor, today’s on-call doctors often have less to do than in the past. “For example,” he said, “the hospitalist may admit an orthopedic patient at night, and then the orthopedic surgeon does the operation the next day. We’ve had EMTALA for 36 years now, and hospitals and doctors know how call works.”
A version of this article first appeared on Medscape.com.
Collateral flow flags stroke patients for late thrombectomy
Patients with acute ischemic stroke presenting late at the hospital can be selected for endovascular thrombectomy by the presence of collateral flow on CT angiography (CTA), a new study shows.
The MR CLEAN-LATE trial found that patients selected for thrombectomy in this way had a greater chance of a better functional outcome than patients who did not receive endovascular therapy.
The study was presented at the 14th World Stroke Congress in Singapore by study investigator Susanne Olthuis, MD, of Maastricht (the Netherlands) University Medical Center.
Patients in the intervention group were more likely to show a benefit on the primary endpoint of modified Rankin Scale (mRS) score at 90 days with a significant common odds ratio of 1.68, a finding that received applause from attendees of the plenary WSC session at which the study was presented.
“This means that patients treated with endovascular therapy in this trial had about a 1.7 times higher chance of achieving a better functional outcome at 90 days,” Dr. Olthuis said.
“Selection based on collateral flow identifies an additional group of patients eligible for late-window endovascular therapy in addition to those eligible based on perfusion and clinical criteria,” Dr. Olthuis concluded.
“We recommend implementation of collateral selection in routine clinical practice as it is time efficient. The CTA is already available, and it involves a low-complexity assessment. The only distinction that needs to be made is whether or not there are any collaterals visible on CTA. If collaterals are absent or there is any doubt, then CT perfusion [CTP] imaging can still be used,” she added.
Co–principal investigator Wim H. van Zwam, MD, interventional radiologist at Maastricht, said in a comment:“My take-home message is that now in the late window we can select patients based on the presence of collaterals on CT angiography, which makes selection easier and faster and more widely available.
“If any collaterals are seen – and that is easily done just by looking at the CTA scan – then the patient can be selected for endovascular treatment,” Dr. van Zwam added. “We don’t need to wait for calculations of core and penumbra volumes from the CTP scan. There will also be additional patients who can benefit from endovascular therapy who do not fulfill the CTP criteria but do have visible collaterals.”
Explaining the background to the study, Dr. Olthuis noted that endovascular thrombectomy for large vessel occlusion stroke is safe and effective if performed within 6 hours and the effect then diminishes over time. In the original trial of endovascular treatment, MR CLEAN, patients with higher collateral grades had more treatment benefit, leading to the hypothesis that the assessment of collateral blood flow could help identify patients who would still benefit in the late time window.
The current MR CLEAN-LATE trial therefore set out to compare safety and efficacy of endovascular therapy in patients with acute ischemic stroke in the anterior circulation presenting within 6-24 hours from symptom onset with patients selected based on the presence of collateral flow on CTA.
At the time the trial was starting, the DAWN and DEFUSE 3 trials reported showing benefit of endovascular therapy in patients presenting in the late window who had been selected for endovascular treatment based on a combination of perfusion imaging and clinical criteria, so patients who fitted these criteria were also excluded from MR CLEAN-LATE as they would now be eligible for endovascular therapy under the latest clinical guidelines.
But the study continued, as “we believed collateral selection may still be able to identify an additional group of patients that may benefit from endovascular therapy in the late window,” Dr. Olthuis said.
The trial randomly assigned 502 such patients with a National Institutes of Health Stroke Scale (NIHSS) score of at least 2 and with collateral flow grades of 1-3 to receive endovascular therapy (intervention) or control.
Safety data showed a slightly but nonsignificantly higher mortality rate at 90 days in the control group (30%) versus 24% in the intervention group.
The rate of symptomatic intracranial hemorrhage was higher in the intervention group (6.7%) versus 1.6% in the control group, but Dr. Olthuis pointed out that the rate of sICH in the intervention group was similar to that in the endovascular groups of the DAWN and DEFUSE 3 trials.
The primary endpoint – mRS score at 90 days – showed a shift toward better outcome in the intervention group, with an adjusted common OR of 1.68 (95% confidence interval, 1.21-2.33).
The median mRS score in the intervention group was 3 (95% CI, 2-5) versus 4 (95% CI, 2-6) in the control group.
Secondary outcomes also showed benefits for the intervention group for the endpoints of mRS score 0-1 versus 2-6 (OR, 1.63); mRS 0-2 versus 3-6 (OR 1.54); and mRS 0-3 versus 4-6 (OR, 1.74).
In addition, NIHSS score was reduced by 17% at 24 hours and by 27% by 5-7 days or discharge in the intervention group. Recanalization at 24 hours was also improved in the intervention group (81% vs. 52%) and infarct size was reduced by 32%.
Dr. Olthuis explained that collateral grade was defined as the amount of collateral flow in the affected hemisphere as a percentage of the contralateral site, with grade 0 correlating to an absence of collaterals (and these were the only patients excluded).
Grade 1 included patients with 50% or less collaterals, grade 2 more than 50%, and grade 3 excellent collaterals – around 100%. “We included grade 1, 2 and 3, and subgroup analysis suggested no treatment interactions between different collateral grades in the patients included,” she said.
Dr. van Zwam noted that there has been evidence from other studies suggesting that the presence of collateral flow could be used to select patients for late thrombectomy, but MR CLEAN-LATE is the first randomized trial to show this and provides confirmation that this strategy is valid.
“Our results show that patients can be selected with just standard CT angiography imaging and that CT perfusion is not necessary. This will make it easier and faster to select patients especially for centers in low-resource areas who do not yet have CT perfusion imaging,” he commented.
“But even in centers where CT perfusion imaging is performed, these results should mean that we do not have to wait to analyze these results before going ahead with thrombectomy. It will also give us an additional tool, as some patients do not meet the criteria on perfusion imaging but still have identifiable collaterals and thus would now qualify for endovascular thrombectomy,” he added.
Could collateral assessment replace CT perfusion?
Commenting on the MR CLEAN-LATE trial, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the WSC scientific committee, said it was an “excellent study.”
“This study does not rely on advanced imaging (e.g., mismatch) and criteria can easily be interpreted on CT/CTA. If the study is published and all details are available this study may substantially ease endovascular therapy in the late time window,” Dr. Kiechl told this news organization.
Also commenting, Urs Fischer, MD, chairman of the department of neurology at the University Hospital Basel (Switzerland), who was not involved with MR CLEAN-LATE, said: “This is another study that has nicely shown that endovascular therapy in patients in the later time window is highly effective.”
Dr. Fischer said he was not surprised by the results.
“I was expecting the trial to be positive,” he said. “What we can say is that endovascular therapy in patients with proximal vessel occlusion is a very effective intervention – probably one of the most important interventions in the history of medicine – and now we have another subgroup to whom we can offer this therapy. So, this is an important study that will improve the outcome of many further patients.”
Yvo Roos, MD, professor of acute neurology at University Medical Center, Amsterdam, who was a MR CLEAN-LATE investigator, agreed that the trial has the potential to increase number of patients who can be treated with endovascular therapy.
But both Dr. Roos and Dr. Fischer were not convinced that collateral assessment would replace CT perfusion as the first-line choice in selecting patients for endovascular treatment.
“We need to see what kind of patients were included in the trial and what kind of perfusion imaging characteristics they had, to see how they compare with patients selected by perfusion imaging,” Dr. Roos noted. “I think CT perfusion is here. But if the data shows that collateral score is better able to identify patients for endovascular treatment than CT perfusion, then this has the potential to change practice. But that needs to be shown.”
All patients screened for the MR CLEAN-LATE trial also received CT perfusion imaging as part of the standard imaging protocol, and many were selected for endovascular therapy directly on this basis, so would not have entered the trial. The researchers plan to analyze these results and to compare how the two approaches differ.
MR CLEAN-LATE is an investigator-driven study, funded by the Dutch Heart Foundation, the Brain Foundation Netherlands, and Medtronic. The study was designed and conducted, analyzed, and interpreted by the investigators independently of all sponsors. Dr. Olthuis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with acute ischemic stroke presenting late at the hospital can be selected for endovascular thrombectomy by the presence of collateral flow on CT angiography (CTA), a new study shows.
The MR CLEAN-LATE trial found that patients selected for thrombectomy in this way had a greater chance of a better functional outcome than patients who did not receive endovascular therapy.
The study was presented at the 14th World Stroke Congress in Singapore by study investigator Susanne Olthuis, MD, of Maastricht (the Netherlands) University Medical Center.
Patients in the intervention group were more likely to show a benefit on the primary endpoint of modified Rankin Scale (mRS) score at 90 days with a significant common odds ratio of 1.68, a finding that received applause from attendees of the plenary WSC session at which the study was presented.
“This means that patients treated with endovascular therapy in this trial had about a 1.7 times higher chance of achieving a better functional outcome at 90 days,” Dr. Olthuis said.
“Selection based on collateral flow identifies an additional group of patients eligible for late-window endovascular therapy in addition to those eligible based on perfusion and clinical criteria,” Dr. Olthuis concluded.
“We recommend implementation of collateral selection in routine clinical practice as it is time efficient. The CTA is already available, and it involves a low-complexity assessment. The only distinction that needs to be made is whether or not there are any collaterals visible on CTA. If collaterals are absent or there is any doubt, then CT perfusion [CTP] imaging can still be used,” she added.
Co–principal investigator Wim H. van Zwam, MD, interventional radiologist at Maastricht, said in a comment:“My take-home message is that now in the late window we can select patients based on the presence of collaterals on CT angiography, which makes selection easier and faster and more widely available.
“If any collaterals are seen – and that is easily done just by looking at the CTA scan – then the patient can be selected for endovascular treatment,” Dr. van Zwam added. “We don’t need to wait for calculations of core and penumbra volumes from the CTP scan. There will also be additional patients who can benefit from endovascular therapy who do not fulfill the CTP criteria but do have visible collaterals.”
Explaining the background to the study, Dr. Olthuis noted that endovascular thrombectomy for large vessel occlusion stroke is safe and effective if performed within 6 hours and the effect then diminishes over time. In the original trial of endovascular treatment, MR CLEAN, patients with higher collateral grades had more treatment benefit, leading to the hypothesis that the assessment of collateral blood flow could help identify patients who would still benefit in the late time window.
The current MR CLEAN-LATE trial therefore set out to compare safety and efficacy of endovascular therapy in patients with acute ischemic stroke in the anterior circulation presenting within 6-24 hours from symptom onset with patients selected based on the presence of collateral flow on CTA.
At the time the trial was starting, the DAWN and DEFUSE 3 trials reported showing benefit of endovascular therapy in patients presenting in the late window who had been selected for endovascular treatment based on a combination of perfusion imaging and clinical criteria, so patients who fitted these criteria were also excluded from MR CLEAN-LATE as they would now be eligible for endovascular therapy under the latest clinical guidelines.
But the study continued, as “we believed collateral selection may still be able to identify an additional group of patients that may benefit from endovascular therapy in the late window,” Dr. Olthuis said.
The trial randomly assigned 502 such patients with a National Institutes of Health Stroke Scale (NIHSS) score of at least 2 and with collateral flow grades of 1-3 to receive endovascular therapy (intervention) or control.
Safety data showed a slightly but nonsignificantly higher mortality rate at 90 days in the control group (30%) versus 24% in the intervention group.
The rate of symptomatic intracranial hemorrhage was higher in the intervention group (6.7%) versus 1.6% in the control group, but Dr. Olthuis pointed out that the rate of sICH in the intervention group was similar to that in the endovascular groups of the DAWN and DEFUSE 3 trials.
The primary endpoint – mRS score at 90 days – showed a shift toward better outcome in the intervention group, with an adjusted common OR of 1.68 (95% confidence interval, 1.21-2.33).
The median mRS score in the intervention group was 3 (95% CI, 2-5) versus 4 (95% CI, 2-6) in the control group.
Secondary outcomes also showed benefits for the intervention group for the endpoints of mRS score 0-1 versus 2-6 (OR, 1.63); mRS 0-2 versus 3-6 (OR 1.54); and mRS 0-3 versus 4-6 (OR, 1.74).
In addition, NIHSS score was reduced by 17% at 24 hours and by 27% by 5-7 days or discharge in the intervention group. Recanalization at 24 hours was also improved in the intervention group (81% vs. 52%) and infarct size was reduced by 32%.
Dr. Olthuis explained that collateral grade was defined as the amount of collateral flow in the affected hemisphere as a percentage of the contralateral site, with grade 0 correlating to an absence of collaterals (and these were the only patients excluded).
Grade 1 included patients with 50% or less collaterals, grade 2 more than 50%, and grade 3 excellent collaterals – around 100%. “We included grade 1, 2 and 3, and subgroup analysis suggested no treatment interactions between different collateral grades in the patients included,” she said.
Dr. van Zwam noted that there has been evidence from other studies suggesting that the presence of collateral flow could be used to select patients for late thrombectomy, but MR CLEAN-LATE is the first randomized trial to show this and provides confirmation that this strategy is valid.
“Our results show that patients can be selected with just standard CT angiography imaging and that CT perfusion is not necessary. This will make it easier and faster to select patients especially for centers in low-resource areas who do not yet have CT perfusion imaging,” he commented.
“But even in centers where CT perfusion imaging is performed, these results should mean that we do not have to wait to analyze these results before going ahead with thrombectomy. It will also give us an additional tool, as some patients do not meet the criteria on perfusion imaging but still have identifiable collaterals and thus would now qualify for endovascular thrombectomy,” he added.
Could collateral assessment replace CT perfusion?
Commenting on the MR CLEAN-LATE trial, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the WSC scientific committee, said it was an “excellent study.”
“This study does not rely on advanced imaging (e.g., mismatch) and criteria can easily be interpreted on CT/CTA. If the study is published and all details are available this study may substantially ease endovascular therapy in the late time window,” Dr. Kiechl told this news organization.
Also commenting, Urs Fischer, MD, chairman of the department of neurology at the University Hospital Basel (Switzerland), who was not involved with MR CLEAN-LATE, said: “This is another study that has nicely shown that endovascular therapy in patients in the later time window is highly effective.”
Dr. Fischer said he was not surprised by the results.
“I was expecting the trial to be positive,” he said. “What we can say is that endovascular therapy in patients with proximal vessel occlusion is a very effective intervention – probably one of the most important interventions in the history of medicine – and now we have another subgroup to whom we can offer this therapy. So, this is an important study that will improve the outcome of many further patients.”
Yvo Roos, MD, professor of acute neurology at University Medical Center, Amsterdam, who was a MR CLEAN-LATE investigator, agreed that the trial has the potential to increase number of patients who can be treated with endovascular therapy.
But both Dr. Roos and Dr. Fischer were not convinced that collateral assessment would replace CT perfusion as the first-line choice in selecting patients for endovascular treatment.
“We need to see what kind of patients were included in the trial and what kind of perfusion imaging characteristics they had, to see how they compare with patients selected by perfusion imaging,” Dr. Roos noted. “I think CT perfusion is here. But if the data shows that collateral score is better able to identify patients for endovascular treatment than CT perfusion, then this has the potential to change practice. But that needs to be shown.”
All patients screened for the MR CLEAN-LATE trial also received CT perfusion imaging as part of the standard imaging protocol, and many were selected for endovascular therapy directly on this basis, so would not have entered the trial. The researchers plan to analyze these results and to compare how the two approaches differ.
MR CLEAN-LATE is an investigator-driven study, funded by the Dutch Heart Foundation, the Brain Foundation Netherlands, and Medtronic. The study was designed and conducted, analyzed, and interpreted by the investigators independently of all sponsors. Dr. Olthuis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with acute ischemic stroke presenting late at the hospital can be selected for endovascular thrombectomy by the presence of collateral flow on CT angiography (CTA), a new study shows.
The MR CLEAN-LATE trial found that patients selected for thrombectomy in this way had a greater chance of a better functional outcome than patients who did not receive endovascular therapy.
The study was presented at the 14th World Stroke Congress in Singapore by study investigator Susanne Olthuis, MD, of Maastricht (the Netherlands) University Medical Center.
Patients in the intervention group were more likely to show a benefit on the primary endpoint of modified Rankin Scale (mRS) score at 90 days with a significant common odds ratio of 1.68, a finding that received applause from attendees of the plenary WSC session at which the study was presented.
“This means that patients treated with endovascular therapy in this trial had about a 1.7 times higher chance of achieving a better functional outcome at 90 days,” Dr. Olthuis said.
“Selection based on collateral flow identifies an additional group of patients eligible for late-window endovascular therapy in addition to those eligible based on perfusion and clinical criteria,” Dr. Olthuis concluded.
“We recommend implementation of collateral selection in routine clinical practice as it is time efficient. The CTA is already available, and it involves a low-complexity assessment. The only distinction that needs to be made is whether or not there are any collaterals visible on CTA. If collaterals are absent or there is any doubt, then CT perfusion [CTP] imaging can still be used,” she added.
Co–principal investigator Wim H. van Zwam, MD, interventional radiologist at Maastricht, said in a comment:“My take-home message is that now in the late window we can select patients based on the presence of collaterals on CT angiography, which makes selection easier and faster and more widely available.
“If any collaterals are seen – and that is easily done just by looking at the CTA scan – then the patient can be selected for endovascular treatment,” Dr. van Zwam added. “We don’t need to wait for calculations of core and penumbra volumes from the CTP scan. There will also be additional patients who can benefit from endovascular therapy who do not fulfill the CTP criteria but do have visible collaterals.”
Explaining the background to the study, Dr. Olthuis noted that endovascular thrombectomy for large vessel occlusion stroke is safe and effective if performed within 6 hours and the effect then diminishes over time. In the original trial of endovascular treatment, MR CLEAN, patients with higher collateral grades had more treatment benefit, leading to the hypothesis that the assessment of collateral blood flow could help identify patients who would still benefit in the late time window.
The current MR CLEAN-LATE trial therefore set out to compare safety and efficacy of endovascular therapy in patients with acute ischemic stroke in the anterior circulation presenting within 6-24 hours from symptom onset with patients selected based on the presence of collateral flow on CTA.
At the time the trial was starting, the DAWN and DEFUSE 3 trials reported showing benefit of endovascular therapy in patients presenting in the late window who had been selected for endovascular treatment based on a combination of perfusion imaging and clinical criteria, so patients who fitted these criteria were also excluded from MR CLEAN-LATE as they would now be eligible for endovascular therapy under the latest clinical guidelines.
But the study continued, as “we believed collateral selection may still be able to identify an additional group of patients that may benefit from endovascular therapy in the late window,” Dr. Olthuis said.
The trial randomly assigned 502 such patients with a National Institutes of Health Stroke Scale (NIHSS) score of at least 2 and with collateral flow grades of 1-3 to receive endovascular therapy (intervention) or control.
Safety data showed a slightly but nonsignificantly higher mortality rate at 90 days in the control group (30%) versus 24% in the intervention group.
The rate of symptomatic intracranial hemorrhage was higher in the intervention group (6.7%) versus 1.6% in the control group, but Dr. Olthuis pointed out that the rate of sICH in the intervention group was similar to that in the endovascular groups of the DAWN and DEFUSE 3 trials.
The primary endpoint – mRS score at 90 days – showed a shift toward better outcome in the intervention group, with an adjusted common OR of 1.68 (95% confidence interval, 1.21-2.33).
The median mRS score in the intervention group was 3 (95% CI, 2-5) versus 4 (95% CI, 2-6) in the control group.
Secondary outcomes also showed benefits for the intervention group for the endpoints of mRS score 0-1 versus 2-6 (OR, 1.63); mRS 0-2 versus 3-6 (OR 1.54); and mRS 0-3 versus 4-6 (OR, 1.74).
In addition, NIHSS score was reduced by 17% at 24 hours and by 27% by 5-7 days or discharge in the intervention group. Recanalization at 24 hours was also improved in the intervention group (81% vs. 52%) and infarct size was reduced by 32%.
Dr. Olthuis explained that collateral grade was defined as the amount of collateral flow in the affected hemisphere as a percentage of the contralateral site, with grade 0 correlating to an absence of collaterals (and these were the only patients excluded).
Grade 1 included patients with 50% or less collaterals, grade 2 more than 50%, and grade 3 excellent collaterals – around 100%. “We included grade 1, 2 and 3, and subgroup analysis suggested no treatment interactions between different collateral grades in the patients included,” she said.
Dr. van Zwam noted that there has been evidence from other studies suggesting that the presence of collateral flow could be used to select patients for late thrombectomy, but MR CLEAN-LATE is the first randomized trial to show this and provides confirmation that this strategy is valid.
“Our results show that patients can be selected with just standard CT angiography imaging and that CT perfusion is not necessary. This will make it easier and faster to select patients especially for centers in low-resource areas who do not yet have CT perfusion imaging,” he commented.
“But even in centers where CT perfusion imaging is performed, these results should mean that we do not have to wait to analyze these results before going ahead with thrombectomy. It will also give us an additional tool, as some patients do not meet the criteria on perfusion imaging but still have identifiable collaterals and thus would now qualify for endovascular thrombectomy,” he added.
Could collateral assessment replace CT perfusion?
Commenting on the MR CLEAN-LATE trial, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the WSC scientific committee, said it was an “excellent study.”
“This study does not rely on advanced imaging (e.g., mismatch) and criteria can easily be interpreted on CT/CTA. If the study is published and all details are available this study may substantially ease endovascular therapy in the late time window,” Dr. Kiechl told this news organization.
Also commenting, Urs Fischer, MD, chairman of the department of neurology at the University Hospital Basel (Switzerland), who was not involved with MR CLEAN-LATE, said: “This is another study that has nicely shown that endovascular therapy in patients in the later time window is highly effective.”
Dr. Fischer said he was not surprised by the results.
“I was expecting the trial to be positive,” he said. “What we can say is that endovascular therapy in patients with proximal vessel occlusion is a very effective intervention – probably one of the most important interventions in the history of medicine – and now we have another subgroup to whom we can offer this therapy. So, this is an important study that will improve the outcome of many further patients.”
Yvo Roos, MD, professor of acute neurology at University Medical Center, Amsterdam, who was a MR CLEAN-LATE investigator, agreed that the trial has the potential to increase number of patients who can be treated with endovascular therapy.
But both Dr. Roos and Dr. Fischer were not convinced that collateral assessment would replace CT perfusion as the first-line choice in selecting patients for endovascular treatment.
“We need to see what kind of patients were included in the trial and what kind of perfusion imaging characteristics they had, to see how they compare with patients selected by perfusion imaging,” Dr. Roos noted. “I think CT perfusion is here. But if the data shows that collateral score is better able to identify patients for endovascular treatment than CT perfusion, then this has the potential to change practice. But that needs to be shown.”
All patients screened for the MR CLEAN-LATE trial also received CT perfusion imaging as part of the standard imaging protocol, and many were selected for endovascular therapy directly on this basis, so would not have entered the trial. The researchers plan to analyze these results and to compare how the two approaches differ.
MR CLEAN-LATE is an investigator-driven study, funded by the Dutch Heart Foundation, the Brain Foundation Netherlands, and Medtronic. The study was designed and conducted, analyzed, and interpreted by the investigators independently of all sponsors. Dr. Olthuis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Thyroid dysfunction may linger a year after severe COVID-19
MONTREAL – Patients hospitalized with severe COVID-19 and no prior history of thyroid dysfunction show signs of thyroiditis that, although asymptomatic, continue to persist for up to a year after infection, according to research that adds to evidence on the complex involvement of the thyroid in COVID-19.
“To our knowledge these findings are novel,” first author Ilaria Muller, MD, PhD, an assistant professor in endocrinology in the department of clinical sciences and community health, University of Milan, told this news organization.
“Little has been written about the long-term follow-up of thyroid function after severe COVID-19 disease, and we have followed patients up to 1 year after infection.”
The effects are seen in about 10%-15% of patients, and “[while] the thyroid dysfunction is transient, ultrasound areas of thyroiditis may persist after 1 year, even if they progressively shrink,” said Dr. Muller, who presented the findings at the American Thyroid Association annual meeting.
Immunological scars? Clinical implications unclear
The nature and implications of the persistent thyroiditis areas are uncertain, Dr. Muller noted. “These areas of thyroiditis are likely a sort of ‘immunologic scar’ of the previous SARS-CoV-2 infection,” she explained. “We still don’t know if there are clinical implications, even if they seem unlikely.”
Of note, increases in autoimmune processes or a higher incidence of thyroid dysfunction after COVID-19 have not been observed, and the shrinkage of the areas of thyroiditis over time is encouraging, she said.
The reasons why some patients develop atypical thyroiditis and others don’t are also unclear, with Dr. Muller’s team investigating further. Importantly, similar effects have been associated with other severe infections, not just COVID-19. “It is well known that in classic subacute thyroiditis due to other viral infections, the areas of thyroiditis persist for months, so this phenomenon might not be unique to COVID-19,” she explained.
Commenting on the story, Jeffrey R. Garber, MD, also noted that such thyroiditis areas stemming from other types of infection may persist – but go unnoticed.
“Resolution is the clinical rule, [and] we generally do not restudy in detail those who clinically recover,” he said in an interview. “However, there is evidence of impaired thyroid reserve in those who recover from viral thyroiditis due to other sources.”
“Thyroid symptoms are often not specific, so ‘atypical’ [cases] are common, [and] resolution with restoring thyroid status to normal is mixed,” noted Dr. Garber, an associate professor of medicine at Harvard Medical School and chief of the division of endocrinology at Atrius Health, Boston.
In terms of clinical practice, while such issues should be kept in mind when evaluating abnormal thyroid tests during severe COVID-19, “it is not a call for routinely checking it in the absence of clinical suspicion,” he observed.
Study details
Dr. Muller and her team previously observed that patients hospitalized in intensive care with COVID-19 often had low or suppressed serum thyroid-stimulating hormone (TSH) levels, with and without elevated free thyroxine (FT4) concentrations, suggestive of thyrotoxicosis.
Upon investigating those cases, they found, as in their previous study reported by this news organization, that a painless, atypical thyroiditis occurs with nonthyroidal illness syndrome among patients hospitalized with severe COVID-19. The atypical thyroiditis was slightly more common in men and was associated with lymphopenia.
To further investigate those cases and follow patients up to 1 year, the team conducted a longitudinal study of 183 patients hospitalized with severe COVID-19 in Italy. The patients, who had no known prior history of thyroid dysfunction, were assessed for serum thyroid function, autoantibodies, and inflammatory markers.
At baseline, 10% of the patients were found to have thyrotoxicosis, and ultrasound performed within 2-3 months postinfection on 65 patients showed that 18 (28%) had areas of thyroiditis.
Importantly, 60% of those patients with the areas of thyroiditis had low TSH levels, while 25% had normal TSH levels (P = .034).
In addition, those showing the presence of thyroiditis on ultrasound at 23 months were more likely to have elevated serum concentrations of FT4 (P = .018) and higher levels of interleukin-26 (P = .016), compared with those with normal ultrasound readings.
In a longitudinal analysis further following patients post infection, among 15 patients who were evaluated at 6 months, most, 13 (87%), still had areas of thyroiditis, and 6 of 12 (50%) had thyroiditis areas that, though reduced in size, still persisted even at 12 months.
In terms of thyroid uptake, at 3 months, 14 of 17 patients (82%) had diffused or focal areas of a reduction of uptake. After 6 months, there was a recovery, with a median of 28% of thyroid uptake recovered, however, 67% of patients still had some focal or diffused reduction in thyroid uptake.
Of note, the indications of thyroiditis on imaging persisted even though patients’ TSH levels had quickly normalized at the end of infection and remained normal up to 1 year of follow-up.
The patients showed no apparent development of thyroglobulin antibody, thyroid peroxidase antibodies, or TSH receptor antibodies.
A further fine needle aspiration analysis of eight patients with atypical thyroiditis at 3 months after infection showed that those patients had tissue resident memory T cells (CD4+/CD8+/CD103+/CD69+) within the thyroid, but not in the blood as expected.
Additional assessments at 8 months after infection showed those tissue resident memory T cells continued to be present on imaging.
The results showed “SARS-CoV-2–specific T cells were enriched within the thyroid compared with the blood, many with a tissue resident phenotype,” Dr. Muller explained.
The findings are notable in that “such an in-depth characterization of areas of thyroiditis triggered by SARS-CoV-2 infection combining ultrasound, scintigraphy, and immunological phenotyping has not been performed so far,” she said.
“In particular, SARS-CoV-2–specific tissue-resident memory T lymphocytes have not been described before in the thyroid gland.”
Dr. Muller and Dr. Garber have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Patients hospitalized with severe COVID-19 and no prior history of thyroid dysfunction show signs of thyroiditis that, although asymptomatic, continue to persist for up to a year after infection, according to research that adds to evidence on the complex involvement of the thyroid in COVID-19.
“To our knowledge these findings are novel,” first author Ilaria Muller, MD, PhD, an assistant professor in endocrinology in the department of clinical sciences and community health, University of Milan, told this news organization.
“Little has been written about the long-term follow-up of thyroid function after severe COVID-19 disease, and we have followed patients up to 1 year after infection.”
The effects are seen in about 10%-15% of patients, and “[while] the thyroid dysfunction is transient, ultrasound areas of thyroiditis may persist after 1 year, even if they progressively shrink,” said Dr. Muller, who presented the findings at the American Thyroid Association annual meeting.
Immunological scars? Clinical implications unclear
The nature and implications of the persistent thyroiditis areas are uncertain, Dr. Muller noted. “These areas of thyroiditis are likely a sort of ‘immunologic scar’ of the previous SARS-CoV-2 infection,” she explained. “We still don’t know if there are clinical implications, even if they seem unlikely.”
Of note, increases in autoimmune processes or a higher incidence of thyroid dysfunction after COVID-19 have not been observed, and the shrinkage of the areas of thyroiditis over time is encouraging, she said.
The reasons why some patients develop atypical thyroiditis and others don’t are also unclear, with Dr. Muller’s team investigating further. Importantly, similar effects have been associated with other severe infections, not just COVID-19. “It is well known that in classic subacute thyroiditis due to other viral infections, the areas of thyroiditis persist for months, so this phenomenon might not be unique to COVID-19,” she explained.
Commenting on the story, Jeffrey R. Garber, MD, also noted that such thyroiditis areas stemming from other types of infection may persist – but go unnoticed.
“Resolution is the clinical rule, [and] we generally do not restudy in detail those who clinically recover,” he said in an interview. “However, there is evidence of impaired thyroid reserve in those who recover from viral thyroiditis due to other sources.”
“Thyroid symptoms are often not specific, so ‘atypical’ [cases] are common, [and] resolution with restoring thyroid status to normal is mixed,” noted Dr. Garber, an associate professor of medicine at Harvard Medical School and chief of the division of endocrinology at Atrius Health, Boston.
In terms of clinical practice, while such issues should be kept in mind when evaluating abnormal thyroid tests during severe COVID-19, “it is not a call for routinely checking it in the absence of clinical suspicion,” he observed.
Study details
Dr. Muller and her team previously observed that patients hospitalized in intensive care with COVID-19 often had low or suppressed serum thyroid-stimulating hormone (TSH) levels, with and without elevated free thyroxine (FT4) concentrations, suggestive of thyrotoxicosis.
Upon investigating those cases, they found, as in their previous study reported by this news organization, that a painless, atypical thyroiditis occurs with nonthyroidal illness syndrome among patients hospitalized with severe COVID-19. The atypical thyroiditis was slightly more common in men and was associated with lymphopenia.
To further investigate those cases and follow patients up to 1 year, the team conducted a longitudinal study of 183 patients hospitalized with severe COVID-19 in Italy. The patients, who had no known prior history of thyroid dysfunction, were assessed for serum thyroid function, autoantibodies, and inflammatory markers.
At baseline, 10% of the patients were found to have thyrotoxicosis, and ultrasound performed within 2-3 months postinfection on 65 patients showed that 18 (28%) had areas of thyroiditis.
Importantly, 60% of those patients with the areas of thyroiditis had low TSH levels, while 25% had normal TSH levels (P = .034).
In addition, those showing the presence of thyroiditis on ultrasound at 23 months were more likely to have elevated serum concentrations of FT4 (P = .018) and higher levels of interleukin-26 (P = .016), compared with those with normal ultrasound readings.
In a longitudinal analysis further following patients post infection, among 15 patients who were evaluated at 6 months, most, 13 (87%), still had areas of thyroiditis, and 6 of 12 (50%) had thyroiditis areas that, though reduced in size, still persisted even at 12 months.
In terms of thyroid uptake, at 3 months, 14 of 17 patients (82%) had diffused or focal areas of a reduction of uptake. After 6 months, there was a recovery, with a median of 28% of thyroid uptake recovered, however, 67% of patients still had some focal or diffused reduction in thyroid uptake.
Of note, the indications of thyroiditis on imaging persisted even though patients’ TSH levels had quickly normalized at the end of infection and remained normal up to 1 year of follow-up.
The patients showed no apparent development of thyroglobulin antibody, thyroid peroxidase antibodies, or TSH receptor antibodies.
A further fine needle aspiration analysis of eight patients with atypical thyroiditis at 3 months after infection showed that those patients had tissue resident memory T cells (CD4+/CD8+/CD103+/CD69+) within the thyroid, but not in the blood as expected.
Additional assessments at 8 months after infection showed those tissue resident memory T cells continued to be present on imaging.
The results showed “SARS-CoV-2–specific T cells were enriched within the thyroid compared with the blood, many with a tissue resident phenotype,” Dr. Muller explained.
The findings are notable in that “such an in-depth characterization of areas of thyroiditis triggered by SARS-CoV-2 infection combining ultrasound, scintigraphy, and immunological phenotyping has not been performed so far,” she said.
“In particular, SARS-CoV-2–specific tissue-resident memory T lymphocytes have not been described before in the thyroid gland.”
Dr. Muller and Dr. Garber have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Patients hospitalized with severe COVID-19 and no prior history of thyroid dysfunction show signs of thyroiditis that, although asymptomatic, continue to persist for up to a year after infection, according to research that adds to evidence on the complex involvement of the thyroid in COVID-19.
“To our knowledge these findings are novel,” first author Ilaria Muller, MD, PhD, an assistant professor in endocrinology in the department of clinical sciences and community health, University of Milan, told this news organization.
“Little has been written about the long-term follow-up of thyroid function after severe COVID-19 disease, and we have followed patients up to 1 year after infection.”
The effects are seen in about 10%-15% of patients, and “[while] the thyroid dysfunction is transient, ultrasound areas of thyroiditis may persist after 1 year, even if they progressively shrink,” said Dr. Muller, who presented the findings at the American Thyroid Association annual meeting.
Immunological scars? Clinical implications unclear
The nature and implications of the persistent thyroiditis areas are uncertain, Dr. Muller noted. “These areas of thyroiditis are likely a sort of ‘immunologic scar’ of the previous SARS-CoV-2 infection,” she explained. “We still don’t know if there are clinical implications, even if they seem unlikely.”
Of note, increases in autoimmune processes or a higher incidence of thyroid dysfunction after COVID-19 have not been observed, and the shrinkage of the areas of thyroiditis over time is encouraging, she said.
The reasons why some patients develop atypical thyroiditis and others don’t are also unclear, with Dr. Muller’s team investigating further. Importantly, similar effects have been associated with other severe infections, not just COVID-19. “It is well known that in classic subacute thyroiditis due to other viral infections, the areas of thyroiditis persist for months, so this phenomenon might not be unique to COVID-19,” she explained.
Commenting on the story, Jeffrey R. Garber, MD, also noted that such thyroiditis areas stemming from other types of infection may persist – but go unnoticed.
“Resolution is the clinical rule, [and] we generally do not restudy in detail those who clinically recover,” he said in an interview. “However, there is evidence of impaired thyroid reserve in those who recover from viral thyroiditis due to other sources.”
“Thyroid symptoms are often not specific, so ‘atypical’ [cases] are common, [and] resolution with restoring thyroid status to normal is mixed,” noted Dr. Garber, an associate professor of medicine at Harvard Medical School and chief of the division of endocrinology at Atrius Health, Boston.
In terms of clinical practice, while such issues should be kept in mind when evaluating abnormal thyroid tests during severe COVID-19, “it is not a call for routinely checking it in the absence of clinical suspicion,” he observed.
Study details
Dr. Muller and her team previously observed that patients hospitalized in intensive care with COVID-19 often had low or suppressed serum thyroid-stimulating hormone (TSH) levels, with and without elevated free thyroxine (FT4) concentrations, suggestive of thyrotoxicosis.
Upon investigating those cases, they found, as in their previous study reported by this news organization, that a painless, atypical thyroiditis occurs with nonthyroidal illness syndrome among patients hospitalized with severe COVID-19. The atypical thyroiditis was slightly more common in men and was associated with lymphopenia.
To further investigate those cases and follow patients up to 1 year, the team conducted a longitudinal study of 183 patients hospitalized with severe COVID-19 in Italy. The patients, who had no known prior history of thyroid dysfunction, were assessed for serum thyroid function, autoantibodies, and inflammatory markers.
At baseline, 10% of the patients were found to have thyrotoxicosis, and ultrasound performed within 2-3 months postinfection on 65 patients showed that 18 (28%) had areas of thyroiditis.
Importantly, 60% of those patients with the areas of thyroiditis had low TSH levels, while 25% had normal TSH levels (P = .034).
In addition, those showing the presence of thyroiditis on ultrasound at 23 months were more likely to have elevated serum concentrations of FT4 (P = .018) and higher levels of interleukin-26 (P = .016), compared with those with normal ultrasound readings.
In a longitudinal analysis further following patients post infection, among 15 patients who were evaluated at 6 months, most, 13 (87%), still had areas of thyroiditis, and 6 of 12 (50%) had thyroiditis areas that, though reduced in size, still persisted even at 12 months.
In terms of thyroid uptake, at 3 months, 14 of 17 patients (82%) had diffused or focal areas of a reduction of uptake. After 6 months, there was a recovery, with a median of 28% of thyroid uptake recovered, however, 67% of patients still had some focal or diffused reduction in thyroid uptake.
Of note, the indications of thyroiditis on imaging persisted even though patients’ TSH levels had quickly normalized at the end of infection and remained normal up to 1 year of follow-up.
The patients showed no apparent development of thyroglobulin antibody, thyroid peroxidase antibodies, or TSH receptor antibodies.
A further fine needle aspiration analysis of eight patients with atypical thyroiditis at 3 months after infection showed that those patients had tissue resident memory T cells (CD4+/CD8+/CD103+/CD69+) within the thyroid, but not in the blood as expected.
Additional assessments at 8 months after infection showed those tissue resident memory T cells continued to be present on imaging.
The results showed “SARS-CoV-2–specific T cells were enriched within the thyroid compared with the blood, many with a tissue resident phenotype,” Dr. Muller explained.
The findings are notable in that “such an in-depth characterization of areas of thyroiditis triggered by SARS-CoV-2 infection combining ultrasound, scintigraphy, and immunological phenotyping has not been performed so far,” she said.
“In particular, SARS-CoV-2–specific tissue-resident memory T lymphocytes have not been described before in the thyroid gland.”
Dr. Muller and Dr. Garber have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATA 2022
Can MS be stopped early in its tracks?
, the earliest detected preclinical phase of multiple sclerosis (MS). Researchers found that dimethyl fumarate reduced the risk of a first acute or progressive event related to CNS demyelination by more than 80%, compared with placebo.
Patients with RIS have incidental MRI abnormalities typical of MS but have no symptoms of the disease. The condition is usually detected when a patient seeks treatment for another issue, such as migraines or head trauma.
The study is the first randomized clinical trial to examine efficacy of a disease-modifying therapy in delaying symptoms in RIS. “It really supports the concept of the benefit of early treatment intervention within this given MS disease spectrum,” lead investigator Darin Okuda, MD, professor of neurology at the University of Texas Southwestern Medical Center in Dallas, told delegates attending the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis.
Topic of debate
RIS was first identified by Dr. Okuda in 2008. Increased use of brain imaging led to the detection of patients with incidental white-matter pathology in the central nervous system on MRI. Some of the findings were nonspecific, but others were highly suggestive of demyelinating pathology based on their location and morphology in the central nervous system.
Although the prevalence of RIS is unknown, incidentally discovered white matter lesions resembling demyelination occur in an estimated 0.1%-0.7% of the general population. Up to half of patients with RIS experience a first clinical MS event within 10 years.
Diagnostic criteria and whether to treat patients with RIS prophylactically has long been a topic of debate among neurologists and radiologists.
Researchers conducted the multicenter, randomized, double-blinded, placebo-controlled ARISE study in 2016, recruiting 87 patients with RIS. The majority of participants were women, and most were diagnosed in their early 40s.
Patients received oral dimethyl fumarate at 240 mg twice daily or placebo and were followed for 96 weeks (1 year, 10 months). Clinical assessments were completed at baseline, at weeks 48 and 96, and at the time of the first clinical event. MRI scans were performed only at the beginning and end of the study.
Those who received dimethyl fumarate had a significantly lower risk of experiencing a first clinical demyelinating event during the study period (adjusted hazard ratio, 0.07; P = .005).
After adjusting for the number of gadolinium-enhancing lesions at baseline, there was a significant reduction in the number of new or newly enlarged T2-weighted hyperintense lesions, a secondary endpoint, in the dimethyl fumarate group, compared with those on placebo (HR, 0.20; P = .042).
“In the future we’d like to see further studies performed to assess the impact on disability outcome measures following treatment for a meaningful amount of time,” Dr. Okuda said.
‘Striking’ findings
Barbara Giesser, MD, a neurologist at Pacific Neuroscience Institute in Santa Monica, Calif., called the results “striking,” but noted that questions remain.
“Up until this study we haven’t had anything like it to guide us in determining whether we should treat RIS or not,” she said. “Obviously, larger studies are needed, but I think this is a very important and well-done study.”
In addition to the small sample size, Dr. Giesser noted that the study lacked data on the presence of risk factors that are known to increase RIS patients’ risk of developing MS, such as evidence of spinal cord lesions. That issue was also raised during discussion of the paper in Amsterdam.
“I don’t think this means you should treat everybody with RIS necessarily,” Dr. Giesser said. “But I think it suggests that in people with RIS, particularly if they do have risk factors, you could consider treatment with dimethyl fumarate.”
The study was funded by Biogen. Dr. Okuda has received support from Biogen and EMD Serono/Merck; and consulting fees from Alexion, Biogen, EMD Serono, Genzyme, Novartis, RVL Pharmaceuticals, TG Therapeutics, Viela Bio, Celgene/Bristol Myers Squibb, Genentech, Janssen Pharmaceuticals, and Osmotica Pharmaceuticals. Dr. Giesser reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the earliest detected preclinical phase of multiple sclerosis (MS). Researchers found that dimethyl fumarate reduced the risk of a first acute or progressive event related to CNS demyelination by more than 80%, compared with placebo.
Patients with RIS have incidental MRI abnormalities typical of MS but have no symptoms of the disease. The condition is usually detected when a patient seeks treatment for another issue, such as migraines or head trauma.
The study is the first randomized clinical trial to examine efficacy of a disease-modifying therapy in delaying symptoms in RIS. “It really supports the concept of the benefit of early treatment intervention within this given MS disease spectrum,” lead investigator Darin Okuda, MD, professor of neurology at the University of Texas Southwestern Medical Center in Dallas, told delegates attending the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis.
Topic of debate
RIS was first identified by Dr. Okuda in 2008. Increased use of brain imaging led to the detection of patients with incidental white-matter pathology in the central nervous system on MRI. Some of the findings were nonspecific, but others were highly suggestive of demyelinating pathology based on their location and morphology in the central nervous system.
Although the prevalence of RIS is unknown, incidentally discovered white matter lesions resembling demyelination occur in an estimated 0.1%-0.7% of the general population. Up to half of patients with RIS experience a first clinical MS event within 10 years.
Diagnostic criteria and whether to treat patients with RIS prophylactically has long been a topic of debate among neurologists and radiologists.
Researchers conducted the multicenter, randomized, double-blinded, placebo-controlled ARISE study in 2016, recruiting 87 patients with RIS. The majority of participants were women, and most were diagnosed in their early 40s.
Patients received oral dimethyl fumarate at 240 mg twice daily or placebo and were followed for 96 weeks (1 year, 10 months). Clinical assessments were completed at baseline, at weeks 48 and 96, and at the time of the first clinical event. MRI scans were performed only at the beginning and end of the study.
Those who received dimethyl fumarate had a significantly lower risk of experiencing a first clinical demyelinating event during the study period (adjusted hazard ratio, 0.07; P = .005).
After adjusting for the number of gadolinium-enhancing lesions at baseline, there was a significant reduction in the number of new or newly enlarged T2-weighted hyperintense lesions, a secondary endpoint, in the dimethyl fumarate group, compared with those on placebo (HR, 0.20; P = .042).
“In the future we’d like to see further studies performed to assess the impact on disability outcome measures following treatment for a meaningful amount of time,” Dr. Okuda said.
‘Striking’ findings
Barbara Giesser, MD, a neurologist at Pacific Neuroscience Institute in Santa Monica, Calif., called the results “striking,” but noted that questions remain.
“Up until this study we haven’t had anything like it to guide us in determining whether we should treat RIS or not,” she said. “Obviously, larger studies are needed, but I think this is a very important and well-done study.”
In addition to the small sample size, Dr. Giesser noted that the study lacked data on the presence of risk factors that are known to increase RIS patients’ risk of developing MS, such as evidence of spinal cord lesions. That issue was also raised during discussion of the paper in Amsterdam.
“I don’t think this means you should treat everybody with RIS necessarily,” Dr. Giesser said. “But I think it suggests that in people with RIS, particularly if they do have risk factors, you could consider treatment with dimethyl fumarate.”
The study was funded by Biogen. Dr. Okuda has received support from Biogen and EMD Serono/Merck; and consulting fees from Alexion, Biogen, EMD Serono, Genzyme, Novartis, RVL Pharmaceuticals, TG Therapeutics, Viela Bio, Celgene/Bristol Myers Squibb, Genentech, Janssen Pharmaceuticals, and Osmotica Pharmaceuticals. Dr. Giesser reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the earliest detected preclinical phase of multiple sclerosis (MS). Researchers found that dimethyl fumarate reduced the risk of a first acute or progressive event related to CNS demyelination by more than 80%, compared with placebo.
Patients with RIS have incidental MRI abnormalities typical of MS but have no symptoms of the disease. The condition is usually detected when a patient seeks treatment for another issue, such as migraines or head trauma.
The study is the first randomized clinical trial to examine efficacy of a disease-modifying therapy in delaying symptoms in RIS. “It really supports the concept of the benefit of early treatment intervention within this given MS disease spectrum,” lead investigator Darin Okuda, MD, professor of neurology at the University of Texas Southwestern Medical Center in Dallas, told delegates attending the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis.
Topic of debate
RIS was first identified by Dr. Okuda in 2008. Increased use of brain imaging led to the detection of patients with incidental white-matter pathology in the central nervous system on MRI. Some of the findings were nonspecific, but others were highly suggestive of demyelinating pathology based on their location and morphology in the central nervous system.
Although the prevalence of RIS is unknown, incidentally discovered white matter lesions resembling demyelination occur in an estimated 0.1%-0.7% of the general population. Up to half of patients with RIS experience a first clinical MS event within 10 years.
Diagnostic criteria and whether to treat patients with RIS prophylactically has long been a topic of debate among neurologists and radiologists.
Researchers conducted the multicenter, randomized, double-blinded, placebo-controlled ARISE study in 2016, recruiting 87 patients with RIS. The majority of participants were women, and most were diagnosed in their early 40s.
Patients received oral dimethyl fumarate at 240 mg twice daily or placebo and were followed for 96 weeks (1 year, 10 months). Clinical assessments were completed at baseline, at weeks 48 and 96, and at the time of the first clinical event. MRI scans were performed only at the beginning and end of the study.
Those who received dimethyl fumarate had a significantly lower risk of experiencing a first clinical demyelinating event during the study period (adjusted hazard ratio, 0.07; P = .005).
After adjusting for the number of gadolinium-enhancing lesions at baseline, there was a significant reduction in the number of new or newly enlarged T2-weighted hyperintense lesions, a secondary endpoint, in the dimethyl fumarate group, compared with those on placebo (HR, 0.20; P = .042).
“In the future we’d like to see further studies performed to assess the impact on disability outcome measures following treatment for a meaningful amount of time,” Dr. Okuda said.
‘Striking’ findings
Barbara Giesser, MD, a neurologist at Pacific Neuroscience Institute in Santa Monica, Calif., called the results “striking,” but noted that questions remain.
“Up until this study we haven’t had anything like it to guide us in determining whether we should treat RIS or not,” she said. “Obviously, larger studies are needed, but I think this is a very important and well-done study.”
In addition to the small sample size, Dr. Giesser noted that the study lacked data on the presence of risk factors that are known to increase RIS patients’ risk of developing MS, such as evidence of spinal cord lesions. That issue was also raised during discussion of the paper in Amsterdam.
“I don’t think this means you should treat everybody with RIS necessarily,” Dr. Giesser said. “But I think it suggests that in people with RIS, particularly if they do have risk factors, you could consider treatment with dimethyl fumarate.”
The study was funded by Biogen. Dr. Okuda has received support from Biogen and EMD Serono/Merck; and consulting fees from Alexion, Biogen, EMD Serono, Genzyme, Novartis, RVL Pharmaceuticals, TG Therapeutics, Viela Bio, Celgene/Bristol Myers Squibb, Genentech, Janssen Pharmaceuticals, and Osmotica Pharmaceuticals. Dr. Giesser reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2022
NAFLD progresses to cirrhosis in young and old at similar rate
CHARLOTTE, N.C. – Metabolic and genetic risk factors for nonalcoholic fatty liver disease (NAFLD) vary across the age spectrum, but once steatosis has started, the risk of progression to cirrhosis is similar for both young and old, investigators found.
At a large Midwest medical center, younger adults were more likely than older patients to have a high-risk gene variant predisposing carriers to NAFLD. And they were less likely than their senior counterparts to have metabolic risk factors, reported Matthew J. Miller, MD, a 3rd-year resident in the department of internal medicine at the University of Michigan Hospital in Ann Arbor.
“Progression to cirrhosis was similar in patients younger than 40, compared to older patients, suggesting NAFLD in the young should not be considered more benign than in older patients,” he said in an oral abstract presented at the annual meeting of the American College of Gastroenterology.
The prevalence of NAFLD among younger adults is increasing, but it’s still unknown whether the course of NAFLD is more benign in these patients than in older adults.
In addition, the rate of progression to cirrhosis in patients with NAFLD can vary, making it difficult to predict those patients most at risk for advanced liver disease, Dr. Miller said.
He and his colleagues sought to characterize genetic and metabolic risk factors for NAFLD and their effects on disease progression in patients from 18 to 40 years, 40 to 59 years, and 60 and older.
The investigators collected data on patients with documented objective evidence of NAFLD seen at the Michigan Medicine health care system from 2010 through 2021.
They identified NAFLD by hepatic steatosis on imaging, biopsy, or transient elastography in the absence of other chronic liver diseases, with the earliest date of a hepatic steatosis diagnosis determined to be the index date.
The investigators determined the presence of cirrhosis using validated International Classification of Diseases version 9 or 10 codes, with incident cirrhosis defined as any new cirrhosis diagnosis at least 1 year after the index date.
They also looked at the frequency of known NAFLD risk alleles in a subset of patients with available genetic data.
They divided 31,505 patients into three age groups for comparison: 8,252 patients age 18-39 at the time of steatosis identification, 15,035 age 40-59, and 8,218 age 60 or older.
Of the full cohort, 804 had cirrhosis at the index date, and 388 others developed incident cirrhosis during 128,090 person-years of follow-up.
The prevalence of hypertension, hyperlipidemia, and diabetes were significantly lower in the youngest group, compared with the two older groups, but the youngest patients had a higher prevalence of obesity than the other two groups, with a significantly higher prevalence of class 3 (morbid) obesity.
Of the 4,359 patients with genetic data available, the NAFLD-promoting PNPLA3-rs738409-G allele was more common in the young, compared with the other two age groups (P = .016).
When the investigators looked at the ability of three laboratory tests – the AST to Platelet Ratio Index (APRI), Fibrosis-4 (FIB4), and NAFLD fibrosis score for identifying prevalent cirrhosis – they found that the scores performed similarly for patients in the 40-59 group, but the NFS did less well among patients in the 18-39 group. There were no significant differences among the three age groups in the risk for incident cirrhosis over 10 years.
The study helps to answer some of the questions surrounding differences in risk factors across the age spectrum, commented Patricia Jones, MD, MSCR, from the University of Miami.
“We wonder how these people with fatty liver are different. Do younger people have a more malignant course? Are they going to progress more rapidly than others, or not? Because if you think of a disease like fatty liver or for that matter any metabolic syndrome–based disease, it’s a spectrum and a continuum, and by the time you’re diagnosed you’ve already had that condition, so it’s really more interesting to me when people are diagnosed, because diagnosing at a younger age allows you to intervene earlier,” she said in an interview.
Dr. Jones said that she was also interested in exploring how the genetic data might be used to improve care for patients, perhaps by testing for the high-risk allele in routine clinical practice.
“It will be interesting to see how people with this allele progress, independently of whether they’re diagnosed at 40, 50, or 60,” she said.
Dr. Jones was a moderator of the session where Dr. Williams presented his data.
Comoderator Mitchell A. Mah’moud, MD, FACG from Duke University in Durham, N.C., commented in an interview that, “with the medications we have available, maybe we can target these patients and prevent progression to cirrhosis and some of the decompensation that we see.”
The study authors did not disclose a funding source. Dr. Miller, Dr. Jones, and Dr. Mah’moud all reported having no relevant financial disclosures.
CHARLOTTE, N.C. – Metabolic and genetic risk factors for nonalcoholic fatty liver disease (NAFLD) vary across the age spectrum, but once steatosis has started, the risk of progression to cirrhosis is similar for both young and old, investigators found.
At a large Midwest medical center, younger adults were more likely than older patients to have a high-risk gene variant predisposing carriers to NAFLD. And they were less likely than their senior counterparts to have metabolic risk factors, reported Matthew J. Miller, MD, a 3rd-year resident in the department of internal medicine at the University of Michigan Hospital in Ann Arbor.
“Progression to cirrhosis was similar in patients younger than 40, compared to older patients, suggesting NAFLD in the young should not be considered more benign than in older patients,” he said in an oral abstract presented at the annual meeting of the American College of Gastroenterology.
The prevalence of NAFLD among younger adults is increasing, but it’s still unknown whether the course of NAFLD is more benign in these patients than in older adults.
In addition, the rate of progression to cirrhosis in patients with NAFLD can vary, making it difficult to predict those patients most at risk for advanced liver disease, Dr. Miller said.
He and his colleagues sought to characterize genetic and metabolic risk factors for NAFLD and their effects on disease progression in patients from 18 to 40 years, 40 to 59 years, and 60 and older.
The investigators collected data on patients with documented objective evidence of NAFLD seen at the Michigan Medicine health care system from 2010 through 2021.
They identified NAFLD by hepatic steatosis on imaging, biopsy, or transient elastography in the absence of other chronic liver diseases, with the earliest date of a hepatic steatosis diagnosis determined to be the index date.
The investigators determined the presence of cirrhosis using validated International Classification of Diseases version 9 or 10 codes, with incident cirrhosis defined as any new cirrhosis diagnosis at least 1 year after the index date.
They also looked at the frequency of known NAFLD risk alleles in a subset of patients with available genetic data.
They divided 31,505 patients into three age groups for comparison: 8,252 patients age 18-39 at the time of steatosis identification, 15,035 age 40-59, and 8,218 age 60 or older.
Of the full cohort, 804 had cirrhosis at the index date, and 388 others developed incident cirrhosis during 128,090 person-years of follow-up.
The prevalence of hypertension, hyperlipidemia, and diabetes were significantly lower in the youngest group, compared with the two older groups, but the youngest patients had a higher prevalence of obesity than the other two groups, with a significantly higher prevalence of class 3 (morbid) obesity.
Of the 4,359 patients with genetic data available, the NAFLD-promoting PNPLA3-rs738409-G allele was more common in the young, compared with the other two age groups (P = .016).
When the investigators looked at the ability of three laboratory tests – the AST to Platelet Ratio Index (APRI), Fibrosis-4 (FIB4), and NAFLD fibrosis score for identifying prevalent cirrhosis – they found that the scores performed similarly for patients in the 40-59 group, but the NFS did less well among patients in the 18-39 group. There were no significant differences among the three age groups in the risk for incident cirrhosis over 10 years.
The study helps to answer some of the questions surrounding differences in risk factors across the age spectrum, commented Patricia Jones, MD, MSCR, from the University of Miami.
“We wonder how these people with fatty liver are different. Do younger people have a more malignant course? Are they going to progress more rapidly than others, or not? Because if you think of a disease like fatty liver or for that matter any metabolic syndrome–based disease, it’s a spectrum and a continuum, and by the time you’re diagnosed you’ve already had that condition, so it’s really more interesting to me when people are diagnosed, because diagnosing at a younger age allows you to intervene earlier,” she said in an interview.
Dr. Jones said that she was also interested in exploring how the genetic data might be used to improve care for patients, perhaps by testing for the high-risk allele in routine clinical practice.
“It will be interesting to see how people with this allele progress, independently of whether they’re diagnosed at 40, 50, or 60,” she said.
Dr. Jones was a moderator of the session where Dr. Williams presented his data.
Comoderator Mitchell A. Mah’moud, MD, FACG from Duke University in Durham, N.C., commented in an interview that, “with the medications we have available, maybe we can target these patients and prevent progression to cirrhosis and some of the decompensation that we see.”
The study authors did not disclose a funding source. Dr. Miller, Dr. Jones, and Dr. Mah’moud all reported having no relevant financial disclosures.
CHARLOTTE, N.C. – Metabolic and genetic risk factors for nonalcoholic fatty liver disease (NAFLD) vary across the age spectrum, but once steatosis has started, the risk of progression to cirrhosis is similar for both young and old, investigators found.
At a large Midwest medical center, younger adults were more likely than older patients to have a high-risk gene variant predisposing carriers to NAFLD. And they were less likely than their senior counterparts to have metabolic risk factors, reported Matthew J. Miller, MD, a 3rd-year resident in the department of internal medicine at the University of Michigan Hospital in Ann Arbor.
“Progression to cirrhosis was similar in patients younger than 40, compared to older patients, suggesting NAFLD in the young should not be considered more benign than in older patients,” he said in an oral abstract presented at the annual meeting of the American College of Gastroenterology.
The prevalence of NAFLD among younger adults is increasing, but it’s still unknown whether the course of NAFLD is more benign in these patients than in older adults.
In addition, the rate of progression to cirrhosis in patients with NAFLD can vary, making it difficult to predict those patients most at risk for advanced liver disease, Dr. Miller said.
He and his colleagues sought to characterize genetic and metabolic risk factors for NAFLD and their effects on disease progression in patients from 18 to 40 years, 40 to 59 years, and 60 and older.
The investigators collected data on patients with documented objective evidence of NAFLD seen at the Michigan Medicine health care system from 2010 through 2021.
They identified NAFLD by hepatic steatosis on imaging, biopsy, or transient elastography in the absence of other chronic liver diseases, with the earliest date of a hepatic steatosis diagnosis determined to be the index date.
The investigators determined the presence of cirrhosis using validated International Classification of Diseases version 9 or 10 codes, with incident cirrhosis defined as any new cirrhosis diagnosis at least 1 year after the index date.
They also looked at the frequency of known NAFLD risk alleles in a subset of patients with available genetic data.
They divided 31,505 patients into three age groups for comparison: 8,252 patients age 18-39 at the time of steatosis identification, 15,035 age 40-59, and 8,218 age 60 or older.
Of the full cohort, 804 had cirrhosis at the index date, and 388 others developed incident cirrhosis during 128,090 person-years of follow-up.
The prevalence of hypertension, hyperlipidemia, and diabetes were significantly lower in the youngest group, compared with the two older groups, but the youngest patients had a higher prevalence of obesity than the other two groups, with a significantly higher prevalence of class 3 (morbid) obesity.
Of the 4,359 patients with genetic data available, the NAFLD-promoting PNPLA3-rs738409-G allele was more common in the young, compared with the other two age groups (P = .016).
When the investigators looked at the ability of three laboratory tests – the AST to Platelet Ratio Index (APRI), Fibrosis-4 (FIB4), and NAFLD fibrosis score for identifying prevalent cirrhosis – they found that the scores performed similarly for patients in the 40-59 group, but the NFS did less well among patients in the 18-39 group. There were no significant differences among the three age groups in the risk for incident cirrhosis over 10 years.
The study helps to answer some of the questions surrounding differences in risk factors across the age spectrum, commented Patricia Jones, MD, MSCR, from the University of Miami.
“We wonder how these people with fatty liver are different. Do younger people have a more malignant course? Are they going to progress more rapidly than others, or not? Because if you think of a disease like fatty liver or for that matter any metabolic syndrome–based disease, it’s a spectrum and a continuum, and by the time you’re diagnosed you’ve already had that condition, so it’s really more interesting to me when people are diagnosed, because diagnosing at a younger age allows you to intervene earlier,” she said in an interview.
Dr. Jones said that she was also interested in exploring how the genetic data might be used to improve care for patients, perhaps by testing for the high-risk allele in routine clinical practice.
“It will be interesting to see how people with this allele progress, independently of whether they’re diagnosed at 40, 50, or 60,” she said.
Dr. Jones was a moderator of the session where Dr. Williams presented his data.
Comoderator Mitchell A. Mah’moud, MD, FACG from Duke University in Durham, N.C., commented in an interview that, “with the medications we have available, maybe we can target these patients and prevent progression to cirrhosis and some of the decompensation that we see.”
The study authors did not disclose a funding source. Dr. Miller, Dr. Jones, and Dr. Mah’moud all reported having no relevant financial disclosures.
AT ACG 2022
Research ties gout in women to comorbidities more than genetics
Comorbidities may play a greater role than genetics women with gout, although this appears not to be true for men, Nicholas Sumpter, MSc, of the University of Alabama at Birmingham said at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Mr. Sumpter was among the authors of a recent paper in Arthritis & Rheumatology that suggested that earlier gout onset involves the accumulation of certain allelic variants in men. This genetic risk was shared across multiple ancestral groups in the study, conducted with men of European and Polynesian ancestry, Mr. Sumpter and colleagues reported.
“There might be more than one factor in gout in men, but in women we’ve been getting at this idea that comorbidities are the big thing,” he said.
During his presentation, Mr. Sumpter offered a hypothesis that in men there might be a kind of “two-pronged attack,” with increases in serum urate linked to genetic risk, but comorbidities also playing a role. “But that may not be the case for women.”
In his presentation, Mr. Sumpter noted a paper published in March 2022 from his University of Alabama at Birmingham colleagues, Aakash V. Patel, MD, and Angelo L. Gaffo, MD. In the article, Dr. Patel and Dr. Gaffo delved into the challenges of treating women with gout given “the paucity of appropriately well-powered, randomized-controlled trials investigating the efficacy” of commonly used treatments.
“This poses major challenges for the management of female gout patients since they carry a greater burden of cardiovascular and renal morbidity, which is known to modulate the pathophysiology of gout; as such, conclusions regarding the efficacy of treatments for females cannot be extrapolated from investigative studies that are predominantly male,” they wrote, calling for increased efforts to enroll women in studies of treatments for this condition.
There’s increased interest in how gout affects women, including findings in a paper published in September in Arthritis & Rheumatology that found people with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status.
Gout has become more common in women, although this remains a condition that is far more likely to strike men.
The age-standardized prevalence of gout among women rose from 233.52 per 100,000 in 1990 to 253.49 in 2017, a gain of about 9%, according to a systematic analysis of the Global Burden of Disease Study.
That topped the roughly 5% gain seen for men in the same time frame, with the rate going from 747.48 per 100,000 to 790.90. With the aging of the global population, gout’s burden in terms of prevalence and disability is expected to increase.
Impact of obesity and healthy eating patterns
Obesity, or excess adiposity, appears to be of particular concern for women in terms of gout risk.
While obesity and genetic predisposition both are strongly associated with a higher risk of gout, the excess risk of both combined was higher than the sum of each, particularly among women, Natalie McCormick, PhD, of Massachusetts General Hospital, Boston, and coauthors reported in Annals of the Rheumatic Diseases.
These findings suggested that “addressing excess adiposity could prevent a large proportion of female gout cases in particular, as well as its cardiometabolic comorbidities, and the benefit could be greater in genetically predisposed women,” they wrote.
In general, there’s a need to re-examine the advice given by many clinicians in the past that people with gout, or those at risk for it, should follow a low-protein diet to avoid purines, Dr. McCormick said in an interview.
“Now we’re finding that a healthier diet that balances protein as well as fat intake can actually be better both for cardiovascular health and for gout prevention,” she said.
Dr. McCormick’s research on this topic includes a 2022 JAMA Internal Medicine article, and a 2021 article in Current Rheumatology Reports. In the latter article, Dr. McCormick and colleagues examined the benefits of changing habits for patients, such as following one of several well-established healthy eating patterns, including the Mediterranean and DASH diets.
With excess weight and associated cardiovascular and endocrine risks already elevated among people with gout, especially women, the “conventional low-purine (i.e., low-protein) approach to gout dietary guidance is neither helpful nor sustainable and may lead to detrimental effects related to worsening insulin resistance as a result of substitution of healthy proteins with unhealthy carbohydrates or fats,” they wrote. “Rather, by focusing our dietary recommendations on healthy eating patterns which have been proven to reduce cardiometabolic risk factors, as opposed to singular ‘good’ or ‘bad’ food items or groups, the beneficial effects of such diets on relevant gout endpoints should naturally follow for the majority of typical gout cases, mediated through changes in insulin resistance.”
Mr. Sumpter and Dr. McCormick had no competing interests to declare.
Comorbidities may play a greater role than genetics women with gout, although this appears not to be true for men, Nicholas Sumpter, MSc, of the University of Alabama at Birmingham said at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Mr. Sumpter was among the authors of a recent paper in Arthritis & Rheumatology that suggested that earlier gout onset involves the accumulation of certain allelic variants in men. This genetic risk was shared across multiple ancestral groups in the study, conducted with men of European and Polynesian ancestry, Mr. Sumpter and colleagues reported.
“There might be more than one factor in gout in men, but in women we’ve been getting at this idea that comorbidities are the big thing,” he said.
During his presentation, Mr. Sumpter offered a hypothesis that in men there might be a kind of “two-pronged attack,” with increases in serum urate linked to genetic risk, but comorbidities also playing a role. “But that may not be the case for women.”
In his presentation, Mr. Sumpter noted a paper published in March 2022 from his University of Alabama at Birmingham colleagues, Aakash V. Patel, MD, and Angelo L. Gaffo, MD. In the article, Dr. Patel and Dr. Gaffo delved into the challenges of treating women with gout given “the paucity of appropriately well-powered, randomized-controlled trials investigating the efficacy” of commonly used treatments.
“This poses major challenges for the management of female gout patients since they carry a greater burden of cardiovascular and renal morbidity, which is known to modulate the pathophysiology of gout; as such, conclusions regarding the efficacy of treatments for females cannot be extrapolated from investigative studies that are predominantly male,” they wrote, calling for increased efforts to enroll women in studies of treatments for this condition.
There’s increased interest in how gout affects women, including findings in a paper published in September in Arthritis & Rheumatology that found people with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status.
Gout has become more common in women, although this remains a condition that is far more likely to strike men.
The age-standardized prevalence of gout among women rose from 233.52 per 100,000 in 1990 to 253.49 in 2017, a gain of about 9%, according to a systematic analysis of the Global Burden of Disease Study.
That topped the roughly 5% gain seen for men in the same time frame, with the rate going from 747.48 per 100,000 to 790.90. With the aging of the global population, gout’s burden in terms of prevalence and disability is expected to increase.
Impact of obesity and healthy eating patterns
Obesity, or excess adiposity, appears to be of particular concern for women in terms of gout risk.
While obesity and genetic predisposition both are strongly associated with a higher risk of gout, the excess risk of both combined was higher than the sum of each, particularly among women, Natalie McCormick, PhD, of Massachusetts General Hospital, Boston, and coauthors reported in Annals of the Rheumatic Diseases.
These findings suggested that “addressing excess adiposity could prevent a large proportion of female gout cases in particular, as well as its cardiometabolic comorbidities, and the benefit could be greater in genetically predisposed women,” they wrote.
In general, there’s a need to re-examine the advice given by many clinicians in the past that people with gout, or those at risk for it, should follow a low-protein diet to avoid purines, Dr. McCormick said in an interview.
“Now we’re finding that a healthier diet that balances protein as well as fat intake can actually be better both for cardiovascular health and for gout prevention,” she said.
Dr. McCormick’s research on this topic includes a 2022 JAMA Internal Medicine article, and a 2021 article in Current Rheumatology Reports. In the latter article, Dr. McCormick and colleagues examined the benefits of changing habits for patients, such as following one of several well-established healthy eating patterns, including the Mediterranean and DASH diets.
With excess weight and associated cardiovascular and endocrine risks already elevated among people with gout, especially women, the “conventional low-purine (i.e., low-protein) approach to gout dietary guidance is neither helpful nor sustainable and may lead to detrimental effects related to worsening insulin resistance as a result of substitution of healthy proteins with unhealthy carbohydrates or fats,” they wrote. “Rather, by focusing our dietary recommendations on healthy eating patterns which have been proven to reduce cardiometabolic risk factors, as opposed to singular ‘good’ or ‘bad’ food items or groups, the beneficial effects of such diets on relevant gout endpoints should naturally follow for the majority of typical gout cases, mediated through changes in insulin resistance.”
Mr. Sumpter and Dr. McCormick had no competing interests to declare.
Comorbidities may play a greater role than genetics women with gout, although this appears not to be true for men, Nicholas Sumpter, MSc, of the University of Alabama at Birmingham said at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Mr. Sumpter was among the authors of a recent paper in Arthritis & Rheumatology that suggested that earlier gout onset involves the accumulation of certain allelic variants in men. This genetic risk was shared across multiple ancestral groups in the study, conducted with men of European and Polynesian ancestry, Mr. Sumpter and colleagues reported.
“There might be more than one factor in gout in men, but in women we’ve been getting at this idea that comorbidities are the big thing,” he said.
During his presentation, Mr. Sumpter offered a hypothesis that in men there might be a kind of “two-pronged attack,” with increases in serum urate linked to genetic risk, but comorbidities also playing a role. “But that may not be the case for women.”
In his presentation, Mr. Sumpter noted a paper published in March 2022 from his University of Alabama at Birmingham colleagues, Aakash V. Patel, MD, and Angelo L. Gaffo, MD. In the article, Dr. Patel and Dr. Gaffo delved into the challenges of treating women with gout given “the paucity of appropriately well-powered, randomized-controlled trials investigating the efficacy” of commonly used treatments.
“This poses major challenges for the management of female gout patients since they carry a greater burden of cardiovascular and renal morbidity, which is known to modulate the pathophysiology of gout; as such, conclusions regarding the efficacy of treatments for females cannot be extrapolated from investigative studies that are predominantly male,” they wrote, calling for increased efforts to enroll women in studies of treatments for this condition.
There’s increased interest in how gout affects women, including findings in a paper published in September in Arthritis & Rheumatology that found people with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status.
Gout has become more common in women, although this remains a condition that is far more likely to strike men.
The age-standardized prevalence of gout among women rose from 233.52 per 100,000 in 1990 to 253.49 in 2017, a gain of about 9%, according to a systematic analysis of the Global Burden of Disease Study.
That topped the roughly 5% gain seen for men in the same time frame, with the rate going from 747.48 per 100,000 to 790.90. With the aging of the global population, gout’s burden in terms of prevalence and disability is expected to increase.
Impact of obesity and healthy eating patterns
Obesity, or excess adiposity, appears to be of particular concern for women in terms of gout risk.
While obesity and genetic predisposition both are strongly associated with a higher risk of gout, the excess risk of both combined was higher than the sum of each, particularly among women, Natalie McCormick, PhD, of Massachusetts General Hospital, Boston, and coauthors reported in Annals of the Rheumatic Diseases.
These findings suggested that “addressing excess adiposity could prevent a large proportion of female gout cases in particular, as well as its cardiometabolic comorbidities, and the benefit could be greater in genetically predisposed women,” they wrote.
In general, there’s a need to re-examine the advice given by many clinicians in the past that people with gout, or those at risk for it, should follow a low-protein diet to avoid purines, Dr. McCormick said in an interview.
“Now we’re finding that a healthier diet that balances protein as well as fat intake can actually be better both for cardiovascular health and for gout prevention,” she said.
Dr. McCormick’s research on this topic includes a 2022 JAMA Internal Medicine article, and a 2021 article in Current Rheumatology Reports. In the latter article, Dr. McCormick and colleagues examined the benefits of changing habits for patients, such as following one of several well-established healthy eating patterns, including the Mediterranean and DASH diets.
With excess weight and associated cardiovascular and endocrine risks already elevated among people with gout, especially women, the “conventional low-purine (i.e., low-protein) approach to gout dietary guidance is neither helpful nor sustainable and may lead to detrimental effects related to worsening insulin resistance as a result of substitution of healthy proteins with unhealthy carbohydrates or fats,” they wrote. “Rather, by focusing our dietary recommendations on healthy eating patterns which have been proven to reduce cardiometabolic risk factors, as opposed to singular ‘good’ or ‘bad’ food items or groups, the beneficial effects of such diets on relevant gout endpoints should naturally follow for the majority of typical gout cases, mediated through changes in insulin resistance.”
Mr. Sumpter and Dr. McCormick had no competing interests to declare.
FROM G-CAN 2022
Syphilis screening: Who and when
The US Preventive Services Task Force (USPSTF) published updated recommendations on screening for syphilis on September 27.1 The Task Force continues to recommend screening for all adolescents and adults who are at increased risk for infection. (As part of previous recommendations, the USPSTF also advocates screening all pregnant women for syphilis early in their pregnancy to prevent congenital syphilis.2)
Who is at increased risk? Men who have sex with men (MSM), those with HIV or other sexually transmitted infections (STIs), those who use illicit drugs, and those with a history of incarceration, sex work, or military service are considered to be at increased risk for syphilis. Additionally, since state and local health departments collect and publish STI incidence data, it’s important to stay up to date on how common syphilis is in one’s community and tailor screening practices accordingly.
Men account for more than 80% of all primary and secondary syphilis infections, and MSM account for 53% of cases in men.3 The highest rates of syphilis are in men ages 25-29 years and 30-34 years (58.1 and 55.7 cases per 100,000, respectively).3
Why screening is important. Primary and secondary syphilis rates have increased steadily from an all-time low of 2.1 per 100,000 in 2000 to 12.7 per 100,000 in 2020.4 There were 171,074 cases reported in 2021.5
If not detected and treated, syphilis will progress from the primary and secondary stages to a latent form. About one-third of those with latent syphilis will develop tertiary syphilis, which can affect every organ system and cause multiple neurologic disorders.
How to screen. Syphilis screening typically involves a 2-step process. The first test that should be performed is a Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test. This is followed by a treponemal antibody test if the initial test is positive. While the VDRL and RPR tests have high sensitivity, many other conditions can cause a false-positive result, necessitating confirmation with the more specific antibody test.
As far as frequency, the Task Force suggests screening annually for those at continued risk and more frequently (every 3 or 6 months) for those at highest risk.
Treatment for primary, secondary, and early latent syphilis (< 1 year’s duration) is a single intramuscular (IM) injection of benzathine penicillin, 2.4 million units. For late latent syphilis or syphilis of unknown duration, treatment is benzathine penicillin, 2.4 million units, administered in 3 weekly IM doses.
Treatment for those with penicillin allergies depends on the stage of syphilis and whether or not the patient is pregnant. Refer to the STD treatment guidelines for guidance.6
The CDC recommends presumptive treatment for anyone who has had sexual contact in the past 90 days with a person who’s been given a diagnosis of primary, secondary, or early latent syphilis.6
And finally, remember that all STIs are reportable to your local health department, which can assist with contract tracing and treatment follow-up.
1. USPSTF. Syphilis infection in nonpregnant adolescents and adults: Screening. Final recommendation statement. September 27, 2022. Accessed October 25, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening
2. USPSTF. Syphilis infection in pregnant women: screening. Final recommendation statement. September 4, 2018. Accessed October 25, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-pregnancy-screening
3. CDC. Sexually transmitted disease surveillance 2020: syphilis. Updated August 22, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/figures/2020-STD-Surveillance-Syphilis.pptx
4. CDC. Sexually transmitted disease surveillance 2020. Table 1: Sexually transmitted diseases—reported cases and rates of reported cases, United States, 1941-2020. Updated April 12, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/tables/1.htm
5. CDC. Preliminary 2021 STD surveillance data. Updated September 1, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2021/default.htm
6. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Rep. 2021;70:1-187.
The US Preventive Services Task Force (USPSTF) published updated recommendations on screening for syphilis on September 27.1 The Task Force continues to recommend screening for all adolescents and adults who are at increased risk for infection. (As part of previous recommendations, the USPSTF also advocates screening all pregnant women for syphilis early in their pregnancy to prevent congenital syphilis.2)
Who is at increased risk? Men who have sex with men (MSM), those with HIV or other sexually transmitted infections (STIs), those who use illicit drugs, and those with a history of incarceration, sex work, or military service are considered to be at increased risk for syphilis. Additionally, since state and local health departments collect and publish STI incidence data, it’s important to stay up to date on how common syphilis is in one’s community and tailor screening practices accordingly.
Men account for more than 80% of all primary and secondary syphilis infections, and MSM account for 53% of cases in men.3 The highest rates of syphilis are in men ages 25-29 years and 30-34 years (58.1 and 55.7 cases per 100,000, respectively).3
Why screening is important. Primary and secondary syphilis rates have increased steadily from an all-time low of 2.1 per 100,000 in 2000 to 12.7 per 100,000 in 2020.4 There were 171,074 cases reported in 2021.5
If not detected and treated, syphilis will progress from the primary and secondary stages to a latent form. About one-third of those with latent syphilis will develop tertiary syphilis, which can affect every organ system and cause multiple neurologic disorders.
How to screen. Syphilis screening typically involves a 2-step process. The first test that should be performed is a Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test. This is followed by a treponemal antibody test if the initial test is positive. While the VDRL and RPR tests have high sensitivity, many other conditions can cause a false-positive result, necessitating confirmation with the more specific antibody test.
As far as frequency, the Task Force suggests screening annually for those at continued risk and more frequently (every 3 or 6 months) for those at highest risk.
Treatment for primary, secondary, and early latent syphilis (< 1 year’s duration) is a single intramuscular (IM) injection of benzathine penicillin, 2.4 million units. For late latent syphilis or syphilis of unknown duration, treatment is benzathine penicillin, 2.4 million units, administered in 3 weekly IM doses.
Treatment for those with penicillin allergies depends on the stage of syphilis and whether or not the patient is pregnant. Refer to the STD treatment guidelines for guidance.6
The CDC recommends presumptive treatment for anyone who has had sexual contact in the past 90 days with a person who’s been given a diagnosis of primary, secondary, or early latent syphilis.6
And finally, remember that all STIs are reportable to your local health department, which can assist with contract tracing and treatment follow-up.
The US Preventive Services Task Force (USPSTF) published updated recommendations on screening for syphilis on September 27.1 The Task Force continues to recommend screening for all adolescents and adults who are at increased risk for infection. (As part of previous recommendations, the USPSTF also advocates screening all pregnant women for syphilis early in their pregnancy to prevent congenital syphilis.2)
Who is at increased risk? Men who have sex with men (MSM), those with HIV or other sexually transmitted infections (STIs), those who use illicit drugs, and those with a history of incarceration, sex work, or military service are considered to be at increased risk for syphilis. Additionally, since state and local health departments collect and publish STI incidence data, it’s important to stay up to date on how common syphilis is in one’s community and tailor screening practices accordingly.
Men account for more than 80% of all primary and secondary syphilis infections, and MSM account for 53% of cases in men.3 The highest rates of syphilis are in men ages 25-29 years and 30-34 years (58.1 and 55.7 cases per 100,000, respectively).3
Why screening is important. Primary and secondary syphilis rates have increased steadily from an all-time low of 2.1 per 100,000 in 2000 to 12.7 per 100,000 in 2020.4 There were 171,074 cases reported in 2021.5
If not detected and treated, syphilis will progress from the primary and secondary stages to a latent form. About one-third of those with latent syphilis will develop tertiary syphilis, which can affect every organ system and cause multiple neurologic disorders.
How to screen. Syphilis screening typically involves a 2-step process. The first test that should be performed is a Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test. This is followed by a treponemal antibody test if the initial test is positive. While the VDRL and RPR tests have high sensitivity, many other conditions can cause a false-positive result, necessitating confirmation with the more specific antibody test.
As far as frequency, the Task Force suggests screening annually for those at continued risk and more frequently (every 3 or 6 months) for those at highest risk.
Treatment for primary, secondary, and early latent syphilis (< 1 year’s duration) is a single intramuscular (IM) injection of benzathine penicillin, 2.4 million units. For late latent syphilis or syphilis of unknown duration, treatment is benzathine penicillin, 2.4 million units, administered in 3 weekly IM doses.
Treatment for those with penicillin allergies depends on the stage of syphilis and whether or not the patient is pregnant. Refer to the STD treatment guidelines for guidance.6
The CDC recommends presumptive treatment for anyone who has had sexual contact in the past 90 days with a person who’s been given a diagnosis of primary, secondary, or early latent syphilis.6
And finally, remember that all STIs are reportable to your local health department, which can assist with contract tracing and treatment follow-up.
1. USPSTF. Syphilis infection in nonpregnant adolescents and adults: Screening. Final recommendation statement. September 27, 2022. Accessed October 25, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening
2. USPSTF. Syphilis infection in pregnant women: screening. Final recommendation statement. September 4, 2018. Accessed October 25, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-pregnancy-screening
3. CDC. Sexually transmitted disease surveillance 2020: syphilis. Updated August 22, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/figures/2020-STD-Surveillance-Syphilis.pptx
4. CDC. Sexually transmitted disease surveillance 2020. Table 1: Sexually transmitted diseases—reported cases and rates of reported cases, United States, 1941-2020. Updated April 12, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/tables/1.htm
5. CDC. Preliminary 2021 STD surveillance data. Updated September 1, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2021/default.htm
6. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Rep. 2021;70:1-187.
1. USPSTF. Syphilis infection in nonpregnant adolescents and adults: Screening. Final recommendation statement. September 27, 2022. Accessed October 25, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening
2. USPSTF. Syphilis infection in pregnant women: screening. Final recommendation statement. September 4, 2018. Accessed October 25, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-pregnancy-screening
3. CDC. Sexually transmitted disease surveillance 2020: syphilis. Updated August 22, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/figures/2020-STD-Surveillance-Syphilis.pptx
4. CDC. Sexually transmitted disease surveillance 2020. Table 1: Sexually transmitted diseases—reported cases and rates of reported cases, United States, 1941-2020. Updated April 12, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/tables/1.htm
5. CDC. Preliminary 2021 STD surveillance data. Updated September 1, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2021/default.htm
6. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Rep. 2021;70:1-187.




