User login
Does fertility preservation in patients with breast cancer impact relapse rates and disease-specific mortality?

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.
Breast conservation safe option in multisite breast cancer
SAN ANTONIO – Women with breast cancer at more than one site can undergo breast-conserving therapy and still have local recurrence rates well under the acceptable threshold of risk, suggest the results of first prospective study of this issue.
The ACOSOG-Z11102 trial involved more than 200 women with primarily endocrine receptor–positive (ER+), human epidermal growth factor receptor 2–negative (HER2-) breast cancer and up to three disease foci, all of whom underwent lumpectomy with nodal staging followed by whole-breast irradiation, then systemic therapy at the oncologist’s discretion.
After 5 years of follow-up, just 3% of women experienced a local recurrence, with none having a local or distant recurrence and one dying of the disease.
The new findings were presented at the San Antonio Breast Cancer Symposium on Dec. 9.
“This study provides important information for clinicians to discuss with patients who have two or three foci of breast cancer in one breast, as it may allow more patients to consider breast-conserving therapy as an option,” said study presenter Judy C. Boughey, MD, chair of the division of breast and melanoma surgical oncology at the Mayo Clinic, Rochester, Minn.
“Lumpectomy with radiation therapy is often preferred to mastectomy, as it is a smaller operation with quicker recovery, resulting in better patient satisfaction and cosmetic outcomes,” Dr. Boughey said in a statement.
“We’ve all been anxiously awaiting the results of this trial,” Andrea V. Barrio, MD, associate attending surgeon, Memorial Sloan Kettering Cancer Center, New York, told this news organization. “We knew that in patients who have a single site tumor in the breast, that outcomes between lumpectomy and mastectomy are the same ... But none of those trials have enrolled women with multiple sites.”
“There were no prospective data out there telling us that doing two lumpectomies in the breast was safe, so a lot of times, women were getting mastectomy for these multiple tumors, even if women had two small tumors in the breast and could easily undergo a lumpectomy with a good cosmetic result,” she said.
“So this data provides very strong evidence that we can begin treating women with small tumors in the breast who can undergo lumpectomy with a good cosmetic results without needing a mastectomy,” Dr. Barrio continued. “From a long-term quality of life standpoint, this is a big deal for women moving forward who really want to keep their breasts.”
Dr. Barrio did highlight, however, that “not everybody routinely does MRI” in women with breast cancer, including her institution, although generally she feels that “our standard imaging has gotten better,” with screening ultrasound identifying more lesions than previously.
She also believes that the numbers of women in the study who did not receive MRI are too small to “draw any definitive conclusions.
“Personally, when I have a patient with multisite disease and I’m going to keep their breasts, that to me is one indication that I would consider an MRI, to make sure that I wasn’t missing intervening disease between the two sites – that there wasn’t something else that would change my mind about doing a two-site lumpectomy,” Dr. Barrio said.
Linda M. Pak, MD, a breast cancer surgeon and surgical oncologist at NYU Langone’s Breast Cancer Center, New York, who was not involved in the study, said that the new study provides “importation information regarding the oncologic safety” of lumpectomy.
These results are “exciting to see, as they provide important information that breast-conserving surgery is safe in these patients, and that we can now share the results of this study with patients when we discuss with them their surgical options.
“I hope this will make more breast surgeons and patients comfortable with this approach and that it will increase the use of breast conservation among these patients,” Dr. Pak said.
Study details
In recent years, there has been increased diagnosis of multiple foci of ipsilateral breast cancer, Dr. Boughey said in her presentation. “This is both as a result of improvements in screening imaging, as well as diagnostic imaging and an increased use of preoperative breast MRI.”
Although historical, retrospective studies have shown high rates of local regional recurrences with breast-conserving therapy in women with more than one foci of breast cancer, more recent analyses have indicated that the approach is associated with “acceptable” recurrence rates.
This, Dr. Boughey explained, is due not only to improvements in breast imaging but also to better pathologic margin assessment, and improved systematic and radiation therapy.
Nevertheless, “most patients who present with two or three sites of cancer in one breast are recommended to undergo a mastectomy,” she noted.
To examine the safety of breast-conserving therapy in such patients, the team conducted a single-arm, phase 2 trial in women at least 40 years of age who had two or three foci of breast cancer, of which at least one site was invasive disease.
“While a randomized trial design would have provided stronger data, we felt that accrual to such a design would be problematic, as many patients and surgeons would not be willing to randomize,” Dr. Boughey explained.
Participants were required to have at least 2 cm of normal tissue between the lesions and disease in no more than two quadrants of the breast. They could have node-negative or N1 disease.
Women were excluded if they had foci > 5 cm on imaging; had bilateral breast cancer; had known BRCA1/2 mutations; had had prior ipsilateral breast cancer; or had received neoadjuvant therapy.
All women in the trial underwent lumpectomy with nodal staging, with adjuvant chemotherapy at the physician’s discretion, followed by whole-breast irradiation, with regional nodal irradiation again at the physician’s discretion. This was followed by systemic therapy, at the discretion of the medical oncologist.
The women were then followed up every 6 months until 5 years after the completion of whole-breast irradiation.
Details of the results
Dr. Boughey said that previously presented data from this study revealed that 67.6% of women achieved a margin-negative excision in a single operation, whereas 7.1% converted to mastectomy. The cosmetic outcome was rated as good or excellent at 2 years by 70.6% of women.
For the current analysis, a total of 204 women were evaluable, who had a median age of 61.1 years. Just over half (59.3%) had T1 stage disease, and 95.6% were node-negative. The majority (83.5%) had ER+/HER2- breast cancer, whereas 5.0% had ER-/HER2- disease and 11.5% had HER2+ positive tumors.
Adjuvant chemotherapy was given to 28.9% of women, whereas 89.7% of those with ER+ disease received adjuvant endocrine therapy.
The primary outcome was local recurrence rate at 5 years, which had a prespecified acceptable rate of less than 8%.
Dr. Boughey showed that, in their series, the 5-year recurrence rate was just 3.1% (95% confidence interval [CI], 1.3%-6.4%), which was “well below” the predefined “clinically significantly threshold.” This involved four cases in the ipsilateral breast, one in the skin, and one in the chest wall.
In addition to the six women with local regional recurrence, six developed contralateral breast cancer and four patients developed distant disease. There were no cases of local and distant recurrence. There were three non–breast cancer primary cancers: one gastric, one lung, and one ovarian.
Eight women died during follow-up; only one of the deaths was related to breast cancer.
Dr. Boughey explained that the small number of local recurrences was too small to identify predictive factors via multivariate analysis.
However, univariate analysis indicated that there were numerical but nonsignificant associations between local recurrence and pathologic stage T2-3 disease, pathologic nodal involvement, and surgical margins just under the negative threshold.
Among the 10 cases of ER–/HER2– breast cancer, there was one local recurrence, giving a 5-year rate of 10.0% vs. 2.6% for women with ER+/HER2– disease.
To examine the role of MRI, Dr. Boughey highlighted that although the imaging modality was initially a requirement for study entry, an amendment to the protocol in 2015 allowed 15 women who had not had MRI to take part.
The local recurrence rate in women who had undergone MRI was 1.7% vs. 22.6% in those who had not, for a hazard ratio of 13.5 (P = .002).
“While this was statistically significant, we need to bear in mind that this was a secondary unplanned analysis,” Dr. Boughey underlined.
Next, the team analyzed the impact of adjuvant endocrine therapy in the 195 women with at least one ER+ lesion, finding that it was associated with a 5-year recurrence rate of 1.9% vs. 12.5% in those who did not receive endocrine therapy, for a hazard ratio of 7.7 (P = .025).
Dr. Boughey highlighted that the study is limited by being single-arm and having only a small subset of patients without preoperative MRI, with HER2+ or ER–/HER2– disease, and with three foci of disease.
She also emphasized that “there is concern that the 5-year follow up on this protocol may be shorter than needed,” especially in women with ER+ disease.
The study was supported by the National Institutes of Health. Dr. Boughey declared relationships with Eli Lilly and Company, Symbiosis Pharma, CairnSurgical, UpToDate, and PeerView.
A version of this article first appeared on Medscape.com.
SAN ANTONIO – Women with breast cancer at more than one site can undergo breast-conserving therapy and still have local recurrence rates well under the acceptable threshold of risk, suggest the results of first prospective study of this issue.
The ACOSOG-Z11102 trial involved more than 200 women with primarily endocrine receptor–positive (ER+), human epidermal growth factor receptor 2–negative (HER2-) breast cancer and up to three disease foci, all of whom underwent lumpectomy with nodal staging followed by whole-breast irradiation, then systemic therapy at the oncologist’s discretion.
After 5 years of follow-up, just 3% of women experienced a local recurrence, with none having a local or distant recurrence and one dying of the disease.
The new findings were presented at the San Antonio Breast Cancer Symposium on Dec. 9.
“This study provides important information for clinicians to discuss with patients who have two or three foci of breast cancer in one breast, as it may allow more patients to consider breast-conserving therapy as an option,” said study presenter Judy C. Boughey, MD, chair of the division of breast and melanoma surgical oncology at the Mayo Clinic, Rochester, Minn.
“Lumpectomy with radiation therapy is often preferred to mastectomy, as it is a smaller operation with quicker recovery, resulting in better patient satisfaction and cosmetic outcomes,” Dr. Boughey said in a statement.
“We’ve all been anxiously awaiting the results of this trial,” Andrea V. Barrio, MD, associate attending surgeon, Memorial Sloan Kettering Cancer Center, New York, told this news organization. “We knew that in patients who have a single site tumor in the breast, that outcomes between lumpectomy and mastectomy are the same ... But none of those trials have enrolled women with multiple sites.”
“There were no prospective data out there telling us that doing two lumpectomies in the breast was safe, so a lot of times, women were getting mastectomy for these multiple tumors, even if women had two small tumors in the breast and could easily undergo a lumpectomy with a good cosmetic result,” she said.
“So this data provides very strong evidence that we can begin treating women with small tumors in the breast who can undergo lumpectomy with a good cosmetic results without needing a mastectomy,” Dr. Barrio continued. “From a long-term quality of life standpoint, this is a big deal for women moving forward who really want to keep their breasts.”
Dr. Barrio did highlight, however, that “not everybody routinely does MRI” in women with breast cancer, including her institution, although generally she feels that “our standard imaging has gotten better,” with screening ultrasound identifying more lesions than previously.
She also believes that the numbers of women in the study who did not receive MRI are too small to “draw any definitive conclusions.
“Personally, when I have a patient with multisite disease and I’m going to keep their breasts, that to me is one indication that I would consider an MRI, to make sure that I wasn’t missing intervening disease between the two sites – that there wasn’t something else that would change my mind about doing a two-site lumpectomy,” Dr. Barrio said.
Linda M. Pak, MD, a breast cancer surgeon and surgical oncologist at NYU Langone’s Breast Cancer Center, New York, who was not involved in the study, said that the new study provides “importation information regarding the oncologic safety” of lumpectomy.
These results are “exciting to see, as they provide important information that breast-conserving surgery is safe in these patients, and that we can now share the results of this study with patients when we discuss with them their surgical options.
“I hope this will make more breast surgeons and patients comfortable with this approach and that it will increase the use of breast conservation among these patients,” Dr. Pak said.
Study details
In recent years, there has been increased diagnosis of multiple foci of ipsilateral breast cancer, Dr. Boughey said in her presentation. “This is both as a result of improvements in screening imaging, as well as diagnostic imaging and an increased use of preoperative breast MRI.”
Although historical, retrospective studies have shown high rates of local regional recurrences with breast-conserving therapy in women with more than one foci of breast cancer, more recent analyses have indicated that the approach is associated with “acceptable” recurrence rates.
This, Dr. Boughey explained, is due not only to improvements in breast imaging but also to better pathologic margin assessment, and improved systematic and radiation therapy.
Nevertheless, “most patients who present with two or three sites of cancer in one breast are recommended to undergo a mastectomy,” she noted.
To examine the safety of breast-conserving therapy in such patients, the team conducted a single-arm, phase 2 trial in women at least 40 years of age who had two or three foci of breast cancer, of which at least one site was invasive disease.
“While a randomized trial design would have provided stronger data, we felt that accrual to such a design would be problematic, as many patients and surgeons would not be willing to randomize,” Dr. Boughey explained.
Participants were required to have at least 2 cm of normal tissue between the lesions and disease in no more than two quadrants of the breast. They could have node-negative or N1 disease.
Women were excluded if they had foci > 5 cm on imaging; had bilateral breast cancer; had known BRCA1/2 mutations; had had prior ipsilateral breast cancer; or had received neoadjuvant therapy.
All women in the trial underwent lumpectomy with nodal staging, with adjuvant chemotherapy at the physician’s discretion, followed by whole-breast irradiation, with regional nodal irradiation again at the physician’s discretion. This was followed by systemic therapy, at the discretion of the medical oncologist.
The women were then followed up every 6 months until 5 years after the completion of whole-breast irradiation.
Details of the results
Dr. Boughey said that previously presented data from this study revealed that 67.6% of women achieved a margin-negative excision in a single operation, whereas 7.1% converted to mastectomy. The cosmetic outcome was rated as good or excellent at 2 years by 70.6% of women.
For the current analysis, a total of 204 women were evaluable, who had a median age of 61.1 years. Just over half (59.3%) had T1 stage disease, and 95.6% were node-negative. The majority (83.5%) had ER+/HER2- breast cancer, whereas 5.0% had ER-/HER2- disease and 11.5% had HER2+ positive tumors.
Adjuvant chemotherapy was given to 28.9% of women, whereas 89.7% of those with ER+ disease received adjuvant endocrine therapy.
The primary outcome was local recurrence rate at 5 years, which had a prespecified acceptable rate of less than 8%.
Dr. Boughey showed that, in their series, the 5-year recurrence rate was just 3.1% (95% confidence interval [CI], 1.3%-6.4%), which was “well below” the predefined “clinically significantly threshold.” This involved four cases in the ipsilateral breast, one in the skin, and one in the chest wall.
In addition to the six women with local regional recurrence, six developed contralateral breast cancer and four patients developed distant disease. There were no cases of local and distant recurrence. There were three non–breast cancer primary cancers: one gastric, one lung, and one ovarian.
Eight women died during follow-up; only one of the deaths was related to breast cancer.
Dr. Boughey explained that the small number of local recurrences was too small to identify predictive factors via multivariate analysis.
However, univariate analysis indicated that there were numerical but nonsignificant associations between local recurrence and pathologic stage T2-3 disease, pathologic nodal involvement, and surgical margins just under the negative threshold.
Among the 10 cases of ER–/HER2– breast cancer, there was one local recurrence, giving a 5-year rate of 10.0% vs. 2.6% for women with ER+/HER2– disease.
To examine the role of MRI, Dr. Boughey highlighted that although the imaging modality was initially a requirement for study entry, an amendment to the protocol in 2015 allowed 15 women who had not had MRI to take part.
The local recurrence rate in women who had undergone MRI was 1.7% vs. 22.6% in those who had not, for a hazard ratio of 13.5 (P = .002).
“While this was statistically significant, we need to bear in mind that this was a secondary unplanned analysis,” Dr. Boughey underlined.
Next, the team analyzed the impact of adjuvant endocrine therapy in the 195 women with at least one ER+ lesion, finding that it was associated with a 5-year recurrence rate of 1.9% vs. 12.5% in those who did not receive endocrine therapy, for a hazard ratio of 7.7 (P = .025).
Dr. Boughey highlighted that the study is limited by being single-arm and having only a small subset of patients without preoperative MRI, with HER2+ or ER–/HER2– disease, and with three foci of disease.
She also emphasized that “there is concern that the 5-year follow up on this protocol may be shorter than needed,” especially in women with ER+ disease.
The study was supported by the National Institutes of Health. Dr. Boughey declared relationships with Eli Lilly and Company, Symbiosis Pharma, CairnSurgical, UpToDate, and PeerView.
A version of this article first appeared on Medscape.com.
SAN ANTONIO – Women with breast cancer at more than one site can undergo breast-conserving therapy and still have local recurrence rates well under the acceptable threshold of risk, suggest the results of first prospective study of this issue.
The ACOSOG-Z11102 trial involved more than 200 women with primarily endocrine receptor–positive (ER+), human epidermal growth factor receptor 2–negative (HER2-) breast cancer and up to three disease foci, all of whom underwent lumpectomy with nodal staging followed by whole-breast irradiation, then systemic therapy at the oncologist’s discretion.
After 5 years of follow-up, just 3% of women experienced a local recurrence, with none having a local or distant recurrence and one dying of the disease.
The new findings were presented at the San Antonio Breast Cancer Symposium on Dec. 9.
“This study provides important information for clinicians to discuss with patients who have two or three foci of breast cancer in one breast, as it may allow more patients to consider breast-conserving therapy as an option,” said study presenter Judy C. Boughey, MD, chair of the division of breast and melanoma surgical oncology at the Mayo Clinic, Rochester, Minn.
“Lumpectomy with radiation therapy is often preferred to mastectomy, as it is a smaller operation with quicker recovery, resulting in better patient satisfaction and cosmetic outcomes,” Dr. Boughey said in a statement.
“We’ve all been anxiously awaiting the results of this trial,” Andrea V. Barrio, MD, associate attending surgeon, Memorial Sloan Kettering Cancer Center, New York, told this news organization. “We knew that in patients who have a single site tumor in the breast, that outcomes between lumpectomy and mastectomy are the same ... But none of those trials have enrolled women with multiple sites.”
“There were no prospective data out there telling us that doing two lumpectomies in the breast was safe, so a lot of times, women were getting mastectomy for these multiple tumors, even if women had two small tumors in the breast and could easily undergo a lumpectomy with a good cosmetic result,” she said.
“So this data provides very strong evidence that we can begin treating women with small tumors in the breast who can undergo lumpectomy with a good cosmetic results without needing a mastectomy,” Dr. Barrio continued. “From a long-term quality of life standpoint, this is a big deal for women moving forward who really want to keep their breasts.”
Dr. Barrio did highlight, however, that “not everybody routinely does MRI” in women with breast cancer, including her institution, although generally she feels that “our standard imaging has gotten better,” with screening ultrasound identifying more lesions than previously.
She also believes that the numbers of women in the study who did not receive MRI are too small to “draw any definitive conclusions.
“Personally, when I have a patient with multisite disease and I’m going to keep their breasts, that to me is one indication that I would consider an MRI, to make sure that I wasn’t missing intervening disease between the two sites – that there wasn’t something else that would change my mind about doing a two-site lumpectomy,” Dr. Barrio said.
Linda M. Pak, MD, a breast cancer surgeon and surgical oncologist at NYU Langone’s Breast Cancer Center, New York, who was not involved in the study, said that the new study provides “importation information regarding the oncologic safety” of lumpectomy.
These results are “exciting to see, as they provide important information that breast-conserving surgery is safe in these patients, and that we can now share the results of this study with patients when we discuss with them their surgical options.
“I hope this will make more breast surgeons and patients comfortable with this approach and that it will increase the use of breast conservation among these patients,” Dr. Pak said.
Study details
In recent years, there has been increased diagnosis of multiple foci of ipsilateral breast cancer, Dr. Boughey said in her presentation. “This is both as a result of improvements in screening imaging, as well as diagnostic imaging and an increased use of preoperative breast MRI.”
Although historical, retrospective studies have shown high rates of local regional recurrences with breast-conserving therapy in women with more than one foci of breast cancer, more recent analyses have indicated that the approach is associated with “acceptable” recurrence rates.
This, Dr. Boughey explained, is due not only to improvements in breast imaging but also to better pathologic margin assessment, and improved systematic and radiation therapy.
Nevertheless, “most patients who present with two or three sites of cancer in one breast are recommended to undergo a mastectomy,” she noted.
To examine the safety of breast-conserving therapy in such patients, the team conducted a single-arm, phase 2 trial in women at least 40 years of age who had two or three foci of breast cancer, of which at least one site was invasive disease.
“While a randomized trial design would have provided stronger data, we felt that accrual to such a design would be problematic, as many patients and surgeons would not be willing to randomize,” Dr. Boughey explained.
Participants were required to have at least 2 cm of normal tissue between the lesions and disease in no more than two quadrants of the breast. They could have node-negative or N1 disease.
Women were excluded if they had foci > 5 cm on imaging; had bilateral breast cancer; had known BRCA1/2 mutations; had had prior ipsilateral breast cancer; or had received neoadjuvant therapy.
All women in the trial underwent lumpectomy with nodal staging, with adjuvant chemotherapy at the physician’s discretion, followed by whole-breast irradiation, with regional nodal irradiation again at the physician’s discretion. This was followed by systemic therapy, at the discretion of the medical oncologist.
The women were then followed up every 6 months until 5 years after the completion of whole-breast irradiation.
Details of the results
Dr. Boughey said that previously presented data from this study revealed that 67.6% of women achieved a margin-negative excision in a single operation, whereas 7.1% converted to mastectomy. The cosmetic outcome was rated as good or excellent at 2 years by 70.6% of women.
For the current analysis, a total of 204 women were evaluable, who had a median age of 61.1 years. Just over half (59.3%) had T1 stage disease, and 95.6% were node-negative. The majority (83.5%) had ER+/HER2- breast cancer, whereas 5.0% had ER-/HER2- disease and 11.5% had HER2+ positive tumors.
Adjuvant chemotherapy was given to 28.9% of women, whereas 89.7% of those with ER+ disease received adjuvant endocrine therapy.
The primary outcome was local recurrence rate at 5 years, which had a prespecified acceptable rate of less than 8%.
Dr. Boughey showed that, in their series, the 5-year recurrence rate was just 3.1% (95% confidence interval [CI], 1.3%-6.4%), which was “well below” the predefined “clinically significantly threshold.” This involved four cases in the ipsilateral breast, one in the skin, and one in the chest wall.
In addition to the six women with local regional recurrence, six developed contralateral breast cancer and four patients developed distant disease. There were no cases of local and distant recurrence. There were three non–breast cancer primary cancers: one gastric, one lung, and one ovarian.
Eight women died during follow-up; only one of the deaths was related to breast cancer.
Dr. Boughey explained that the small number of local recurrences was too small to identify predictive factors via multivariate analysis.
However, univariate analysis indicated that there were numerical but nonsignificant associations between local recurrence and pathologic stage T2-3 disease, pathologic nodal involvement, and surgical margins just under the negative threshold.
Among the 10 cases of ER–/HER2– breast cancer, there was one local recurrence, giving a 5-year rate of 10.0% vs. 2.6% for women with ER+/HER2– disease.
To examine the role of MRI, Dr. Boughey highlighted that although the imaging modality was initially a requirement for study entry, an amendment to the protocol in 2015 allowed 15 women who had not had MRI to take part.
The local recurrence rate in women who had undergone MRI was 1.7% vs. 22.6% in those who had not, for a hazard ratio of 13.5 (P = .002).
“While this was statistically significant, we need to bear in mind that this was a secondary unplanned analysis,” Dr. Boughey underlined.
Next, the team analyzed the impact of adjuvant endocrine therapy in the 195 women with at least one ER+ lesion, finding that it was associated with a 5-year recurrence rate of 1.9% vs. 12.5% in those who did not receive endocrine therapy, for a hazard ratio of 7.7 (P = .025).
Dr. Boughey highlighted that the study is limited by being single-arm and having only a small subset of patients without preoperative MRI, with HER2+ or ER–/HER2– disease, and with three foci of disease.
She also emphasized that “there is concern that the 5-year follow up on this protocol may be shorter than needed,” especially in women with ER+ disease.
The study was supported by the National Institutes of Health. Dr. Boughey declared relationships with Eli Lilly and Company, Symbiosis Pharma, CairnSurgical, UpToDate, and PeerView.
A version of this article first appeared on Medscape.com.
AT SABCS 2022
Mindfulness, exercise strike out in memory trial
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.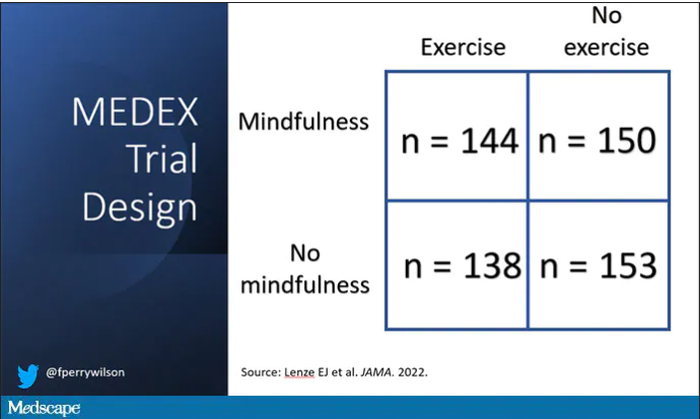
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Patient With Severe Headache After IV Immunoglobulin
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?
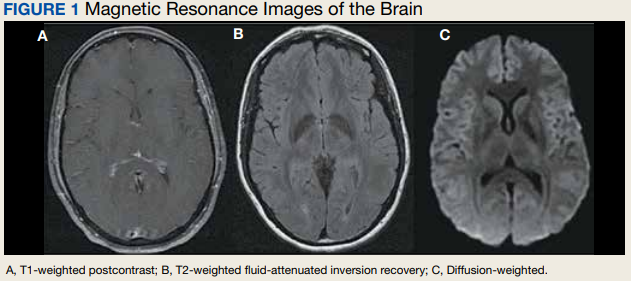
Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
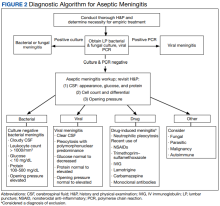
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
Oral Therapy for Aerococcus urinae Bacteremia and Thoracic Spondylodiscitis of Presumed Urinary Origin
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Liver cancer exacts high financial toll on older adults
In the first year after a diagnosis of HCC, median Medicare payments exceed $65,000 and out-of-pocket costs top $10,000.
Even after adjustment for the presence of cirrhosis and its related costs, patients with HCC still have Medicare payments exceeding $50,000 and out-of-pocket costs topping $7000.
Amit Singal, MD, of UT Southwestern Medical Center in Dallas, and colleagues reported their findings in Clinical Gastroenterology and Hepatology.
Common and costly
HCC, the most common type of primary liver cancer, is a leading cause of death in patients with cirrhosis and is projected to become the third leading cause of cancer-related death in the United States by 2040, the researchers wrote.
The treatment landscape for HCC has changed over the past decade, with expanded surgical options, introduction of radiation-based therapies, and approval of immunotherapies – all of which are costly.
Yet the magnitude of financial burden of HCC therapy has been understudied, the researchers noted.
To investigate, Dr. Singal and colleagues evaluated Surveillance, Epidemiology, and End Results (SEER)–Medicare data for 4,525 adults with traditional Medicare coverage who were diagnosed with HCC between 2011 and 2015 and a propensity-matched cohort of 4,525 adults with cirrhosis but no HCC as a comparator group to tease out HCC-specific costs beyond those related to cirrhosis. Patients in Medicare managed care were excluded because their cost information is not available in the database.
In the first year after a diagnosis of HCC, the median total Medicare payments were $66,338 (interquartile range [IQR], $30,931-$158,740) and patient liabilities (a proxy for out-of-pocket costs) were $10,008 (IQR, $5,427-$19,669).
First-year costs were higher for patients with HCC than matched patients without HCC; the former group incurred median incremental Medicare payments of $50,110 (IQR, $14,242-$136,239) and patient liabilities of $7,166 (IQR, $2,401-$16,099), the investigators found.
Patients with early-stage HCC had lower incremental patient liabilities (median, $4,195 vs. $8,238) and Medicare payments (median, $28,207 vs. $59,509) than did their peers with larger tumor burden.
NAFLD notably tied to higher costs
Factors associated with higher HCC-related costs were nonalcoholic fatty liver disease (NAFLD) etiology, higher comorbidities, presence of ascites and hepatic encephalopathy, and larger tumor burden.
The researchers said that the link between NAFLD and higher costs is notable, given that NAFLD is an increasingly common underlying cause of HCC.
The link between larger tumor burden and higher costs underscores “another benefit of HCC surveillance and early detection,” they added.
“By separating the financial liabilities borne by patients and Medicare, we provide a clearer outlook of how cancer-related costs are distributed between patients and public payers,” Dr. Singal and colleagues said.
“Our findings will inform policy interventions and will help formulate better financial supports targeting the most vulnerable HCC patients,” they concluded.
The study had no commercial funding. Dr. Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Glycotest, Exact Sciences, Roche, Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, and TARGET RWE.
A version of this article first appeared on Medscape.com.
In the first year after a diagnosis of HCC, median Medicare payments exceed $65,000 and out-of-pocket costs top $10,000.
Even after adjustment for the presence of cirrhosis and its related costs, patients with HCC still have Medicare payments exceeding $50,000 and out-of-pocket costs topping $7000.
Amit Singal, MD, of UT Southwestern Medical Center in Dallas, and colleagues reported their findings in Clinical Gastroenterology and Hepatology.
Common and costly
HCC, the most common type of primary liver cancer, is a leading cause of death in patients with cirrhosis and is projected to become the third leading cause of cancer-related death in the United States by 2040, the researchers wrote.
The treatment landscape for HCC has changed over the past decade, with expanded surgical options, introduction of radiation-based therapies, and approval of immunotherapies – all of which are costly.
Yet the magnitude of financial burden of HCC therapy has been understudied, the researchers noted.
To investigate, Dr. Singal and colleagues evaluated Surveillance, Epidemiology, and End Results (SEER)–Medicare data for 4,525 adults with traditional Medicare coverage who were diagnosed with HCC between 2011 and 2015 and a propensity-matched cohort of 4,525 adults with cirrhosis but no HCC as a comparator group to tease out HCC-specific costs beyond those related to cirrhosis. Patients in Medicare managed care were excluded because their cost information is not available in the database.
In the first year after a diagnosis of HCC, the median total Medicare payments were $66,338 (interquartile range [IQR], $30,931-$158,740) and patient liabilities (a proxy for out-of-pocket costs) were $10,008 (IQR, $5,427-$19,669).
First-year costs were higher for patients with HCC than matched patients without HCC; the former group incurred median incremental Medicare payments of $50,110 (IQR, $14,242-$136,239) and patient liabilities of $7,166 (IQR, $2,401-$16,099), the investigators found.
Patients with early-stage HCC had lower incremental patient liabilities (median, $4,195 vs. $8,238) and Medicare payments (median, $28,207 vs. $59,509) than did their peers with larger tumor burden.
NAFLD notably tied to higher costs
Factors associated with higher HCC-related costs were nonalcoholic fatty liver disease (NAFLD) etiology, higher comorbidities, presence of ascites and hepatic encephalopathy, and larger tumor burden.
The researchers said that the link between NAFLD and higher costs is notable, given that NAFLD is an increasingly common underlying cause of HCC.
The link between larger tumor burden and higher costs underscores “another benefit of HCC surveillance and early detection,” they added.
“By separating the financial liabilities borne by patients and Medicare, we provide a clearer outlook of how cancer-related costs are distributed between patients and public payers,” Dr. Singal and colleagues said.
“Our findings will inform policy interventions and will help formulate better financial supports targeting the most vulnerable HCC patients,” they concluded.
The study had no commercial funding. Dr. Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Glycotest, Exact Sciences, Roche, Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, and TARGET RWE.
A version of this article first appeared on Medscape.com.
In the first year after a diagnosis of HCC, median Medicare payments exceed $65,000 and out-of-pocket costs top $10,000.
Even after adjustment for the presence of cirrhosis and its related costs, patients with HCC still have Medicare payments exceeding $50,000 and out-of-pocket costs topping $7000.
Amit Singal, MD, of UT Southwestern Medical Center in Dallas, and colleagues reported their findings in Clinical Gastroenterology and Hepatology.
Common and costly
HCC, the most common type of primary liver cancer, is a leading cause of death in patients with cirrhosis and is projected to become the third leading cause of cancer-related death in the United States by 2040, the researchers wrote.
The treatment landscape for HCC has changed over the past decade, with expanded surgical options, introduction of radiation-based therapies, and approval of immunotherapies – all of which are costly.
Yet the magnitude of financial burden of HCC therapy has been understudied, the researchers noted.
To investigate, Dr. Singal and colleagues evaluated Surveillance, Epidemiology, and End Results (SEER)–Medicare data for 4,525 adults with traditional Medicare coverage who were diagnosed with HCC between 2011 and 2015 and a propensity-matched cohort of 4,525 adults with cirrhosis but no HCC as a comparator group to tease out HCC-specific costs beyond those related to cirrhosis. Patients in Medicare managed care were excluded because their cost information is not available in the database.
In the first year after a diagnosis of HCC, the median total Medicare payments were $66,338 (interquartile range [IQR], $30,931-$158,740) and patient liabilities (a proxy for out-of-pocket costs) were $10,008 (IQR, $5,427-$19,669).
First-year costs were higher for patients with HCC than matched patients without HCC; the former group incurred median incremental Medicare payments of $50,110 (IQR, $14,242-$136,239) and patient liabilities of $7,166 (IQR, $2,401-$16,099), the investigators found.
Patients with early-stage HCC had lower incremental patient liabilities (median, $4,195 vs. $8,238) and Medicare payments (median, $28,207 vs. $59,509) than did their peers with larger tumor burden.
NAFLD notably tied to higher costs
Factors associated with higher HCC-related costs were nonalcoholic fatty liver disease (NAFLD) etiology, higher comorbidities, presence of ascites and hepatic encephalopathy, and larger tumor burden.
The researchers said that the link between NAFLD and higher costs is notable, given that NAFLD is an increasingly common underlying cause of HCC.
The link between larger tumor burden and higher costs underscores “another benefit of HCC surveillance and early detection,” they added.
“By separating the financial liabilities borne by patients and Medicare, we provide a clearer outlook of how cancer-related costs are distributed between patients and public payers,” Dr. Singal and colleagues said.
“Our findings will inform policy interventions and will help formulate better financial supports targeting the most vulnerable HCC patients,” they concluded.
The study had no commercial funding. Dr. Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Glycotest, Exact Sciences, Roche, Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, and TARGET RWE.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Therapeutic Approaches in Advanced Breast Cancer
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia
Susan M. Domchek, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia
Susan M. Domchek, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia
Susan M. Domchek, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb

CTC-guided therapy beats physician choice in metastatic breast cancer
SAN ANTONIO – When choosing between chemotherapy and endocrine therapy for patients with hormone receptor (HR)+/HER2- metastatic breast cancer, allowing the results from a blood test that measures circulating tumor cell (CTC) count to overrule physician’s choice of therapy can significantly improve overall survival.
But One expert reacting to the findings says probably not, and another pointed out that the CTC count test used in the trial (CellSearch), although approved by the Food and Drug Administration, is not widely used in clinical practice.
The findings comes from updated results from the STIC CTC study.
“When the trial was designed, the question related to the choice between single-agent endocrine therapy and chemotherapy [in] first-line therapy,” explained study presenter François-Clément Bidard, MD, PhD, professor of medical oncology at Institut Curie and Versailles Saint-Quentin University, Paris.
Since then, the first-line treatment has changed and can now can also include cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, but Dr. Bidard said the results are still clinically relevant.
Nowadays, endocrine therapy plus CDK4/6 inhibitors is the “preferred option for treatment-naive patients, but the dilemma between endocrine therapy and chemotherapy remains after disease progression on adjuvant or first-line therapy with CDK4/6 inhibitors, where current guidelines advocate in favor of endocrine therapy, despite its short-lived efficacy.”
“In that scenario, based on the STIC CTC trial results, the CTC count in combination with predictive biomarkers, whenever available, may help customize the early use of chemotherapy or antibody-drug conjugates, which are becoming more and more attractive,” Dr. Bidard said.
The research was presented here at the San Antonio Breast Cancer Symposium (SABCS).
The study involved more than 750 patients with HR+/HER2- metastatic breast cancer randomly assigned to physician choice or CTC-guided therapy, although the physician decision and the recommendation based on the CTC count was recorded in both groups.
Using the CellSearch (Menarini Silicon Biosystems) to perform the CTC count at baseline only, the team defined patients as low or high risk, with low-risk patients deemed to need only endocrine therapy and high-risk patients recommended chemotherapy.
Physicians based their decisions on current guidelines and their clinical experience.
In the 25% of cases where CTC count would recommend chemotherapy while the physician would recommend endocrine therapy, following the CTC count–based choice resulted in a 35% improvement in progression-free survival (PFS) and a 47% increase in overall survival.
In all other situations, including those when the CTC count recommended endocrine therapy in contrast to the physicians, or the approximately 60% of cases in which the two were in agreement, there was no difference in survival outcomes between the approaches.
Reacting to the findings, Nancy Chan, MD, medical oncologist and the director of breast cancer clinical research at NYU Langone’s Perlmutter Cancer Center, said that the “goal is really to understand how we can personalize treatment options for patients.”
Another aim is to avoid performing a tumor biopsy, if possible, “as that has increased morbidity for patients.”
She noted also that choosing between endocrine therapy and chemotherapy is a “big decision.” These researchers “really wanted to help some patients get less chemotherapy,” as they felt that “some patients are getting too much” as they are not really that high risk and should get endocrine therapy instead.
However, Dr. Chan said that the CTC count is a “complicated concept” and is “not something we’re all using in our clinical practice yet.”
With regard to the approximately 40% discordance between the CTC- and physician-guided choices, Dr. Chan said that clinicians are perhaps not as accurate as they believed in predicting risk when relying on the clinical or pathological features of the tumor.
On Twitter, Guilherme Nader-Marta, MD, Jules Bordet Institute, Université Libre de Bruxelles, Belgium, commented that the question behind the study was whether CTC measurement is a “clinically useful strategy for first-line treatment decision-making.”
“Amazingly,” he continued, the trial went “straight to the point” to answer the question and showed that CTC-based decisions can offer a survival benefit.
Daniel F. Hayes, MD, co-director of the Breast Oncology Program at the University of Michigan Comprehensive Cancer Center, Ann Arbor, echoed these thoughts, saying that the goals of therapy are to make patients live longer and “better.”
He said that the point of any clinical biomarker is not only to show that testing for it offers “analytical validity” but that it also provides “clinical utility” in that it can guide treatment decisions to improve outcomes.
Dr. Hayes, who was not involved in the study but has worked for many years on the development of CellSearch, said that the results do not make it clear whether measuring CTC counts meets the definition of clinical utility, but it’s “very close.”
On the other hand, the analytical validity of the test is “excellent,” and, in that context, was well-chosen, he said, adding that the endpoint of the trial “is the one most important to us: improvement in overall survival.”
Dr. Hayes noted that the magnitude of benefit from CTC-guided therapy was “moderate,” although that is a “matter of perception,” and the “level of evidence is probably 2 or 3.” Although the trial was prospective, he said, the key results were in a “relatively small” subgroup.
The question is, Dr. Hayes continued: “Is this enough to change practice? My conclusions are: probably not.”
Although patients rated as low risk based on their low CTC count avoided chemotherapy, “it’s not clear to me that this whole thing is sufficient for clinical utility in context of what we know today.” The key issue, however, is who decides whether CTC counts are measured and whether they will be used to guide therapy decisions – will it be the patient, the caregiver, an expert guidelines panel, or third party payers/society?
Study details
In his presentation, Dr. Bidard explained that CTC count is an FDA-approved standardized liquid biopsy biomarker, with a count of greater than or equal to 5 cells per 7.5 mL of blood deemed an adverse prognostic marker, regardless of the line of therapy, with a grade 1 level of evidence.
Previous studies have indicated that a high CTC count is strongly associated with overall survival, at a hazard ratio of 2.78.
Crucially, the CTC count “complements” and does not duplicate standard clinicopathological prognostic factors, Dr. Bidard said.
To determine the potential of the CTC count as an aid to treatment decisions, Dr. Bidard and colleagues conducted a trial in pre- and postmenopausal women with untreated HR+/HER2- metastatic breast cancer who were able to receive either endocrine therapy or chemotherapy.
They were randomly assigned to either a standard group, in which the treatment decision followed the physician’s choice, regardless of their CTC count, or to a CTC group, in which the physicians made a treatment recommendation but the choice was driven by the CTC count.
Dr. Bidard reminded the audience that the primary endpoint of PFS to demonstrate the non-inferiority of CTC versus physician treatment decisions has already been met, with the results published in 2020. Those results came from an analysis of 788 patients enrolled between February 2012 and July 2016 at 17 sites in France and showed after 42 months of follow-up that the median PFS in the CTC arm was 15.6 months versus 14 months in the physician choice arm, at a hazard ratio of 0.92.
The current pre-planned analysis involved 755 patients who were followed up for a median of 57 months by the time the trial was stopped in 2021.
In the standard treatment arm, endocrine therapy was favored by physicians in 72.7% of cases (Clin-low), while 27.3% were given chemotherapy (Clin-high).
In the CTC group, 73.5% of patients were recommended to have endocrine therapy by their physician based on their clinical characteristics (Clin-low), whereas 26.5% were suggested to have chemotherapy (Clin-high).
In contrast, 60.1% of patients in the standard arm would have received endocrine therapy based on their CTC count (CTC-low), and 39.9% chemotherapy (CTC-high), while 63.4% of those in the CTC arm were given endocrine therapy based on their CTC count (CTC-low), and 36.6% were assigned to chemotherapy (CTC-high).
Once the allocated treatment was known in both treatment groups, the physicians were free to choose between endocrine therapy (mostly a single-agent aromatase inhibitor or fulvestrant) and chemotherapy (mostly paclitaxel or capecitabine).
Although CDK4/6 inhibitors were not approved at the time of enrollment, 42.2% of patients across both treatment groups received one of these drugs as a second-line or later therapy.
Guiding treatment decisions
Dr. Bidard said that, overall, more patients in the CTC arm were assigned to chemotherapy, at a difference of 9.7%. There was approximately 60% concordance between physician- and CTC-guided treatment choices; in other words, patients were recommended the same treatment by the two approaches in both treatment groups.
In these patients, there was no significant difference in overall survival between the physician choice and CTC groups, at a median of 45.5 months versus 51.3 months (hazard ratio, 0.85; P = .11).
The updated PFS data revealed a median PFS of 15.7 months in the CTC group versus 13.8 months, again at a nonsignificant HR of 0.94.
These results, Dr. Bidard said, indicate that CTC-based treatment choices are “safe.”
However, there was discordance between physician and CTC-based treatment choices in around 40% of cases, meaning that the two approaches recommended different therapies.
The physician recommended endocrine therapy, in contrast to the CTC count indicating chemotherapy, in 25% of patients (Clin-low/CTC-high), whereas 13.6% of cases were recommended chemotherapy while their CTC count indicated otherwise (Clin-high/CTC-low).
In Clin-low/CTC-high patients, this resulted in 26.1% of patients in the standard group receiving endocrine therapy when their CTC count indicated chemotherapy, while 23.9% of patients in the CTC group received chemotherapy even though their physician did not recommended it.
Comparing these two groups, the researchers found that patients in the CTC group had a significantly longer PFS, at 15.7 months versus 10 months (HR, 0.65; P = .005). They also had a significantly longer median overall survival, at a median of 51.8 months versus 35.4 months with physician choice (HR, 0.53; P = .001).
Among Clin-high/CTC-low, there was no benefit from physician’s choice of chemotherapy over the CTC-guided recommendation of endocrine therapy, at an HR for PFS of 1.14 for CTC- versus physician-guided therapy (P = .54), and an HR for overall survival of 0.88 (P = .64).
Dr. Bidard highlighted that the treatment effects were seen across prespecified subgroups.
The study was funded by the Institut National du Cancer, the Institut Curie SIRIC2 program, and Menarini Silicon Biosystems. Dr. Chan reports no relevant financial relationships. Dr. Hayes and Dr. Bidard reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SAN ANTONIO – When choosing between chemotherapy and endocrine therapy for patients with hormone receptor (HR)+/HER2- metastatic breast cancer, allowing the results from a blood test that measures circulating tumor cell (CTC) count to overrule physician’s choice of therapy can significantly improve overall survival.
But One expert reacting to the findings says probably not, and another pointed out that the CTC count test used in the trial (CellSearch), although approved by the Food and Drug Administration, is not widely used in clinical practice.
The findings comes from updated results from the STIC CTC study.
“When the trial was designed, the question related to the choice between single-agent endocrine therapy and chemotherapy [in] first-line therapy,” explained study presenter François-Clément Bidard, MD, PhD, professor of medical oncology at Institut Curie and Versailles Saint-Quentin University, Paris.
Since then, the first-line treatment has changed and can now can also include cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, but Dr. Bidard said the results are still clinically relevant.
Nowadays, endocrine therapy plus CDK4/6 inhibitors is the “preferred option for treatment-naive patients, but the dilemma between endocrine therapy and chemotherapy remains after disease progression on adjuvant or first-line therapy with CDK4/6 inhibitors, where current guidelines advocate in favor of endocrine therapy, despite its short-lived efficacy.”
“In that scenario, based on the STIC CTC trial results, the CTC count in combination with predictive biomarkers, whenever available, may help customize the early use of chemotherapy or antibody-drug conjugates, which are becoming more and more attractive,” Dr. Bidard said.
The research was presented here at the San Antonio Breast Cancer Symposium (SABCS).
The study involved more than 750 patients with HR+/HER2- metastatic breast cancer randomly assigned to physician choice or CTC-guided therapy, although the physician decision and the recommendation based on the CTC count was recorded in both groups.
Using the CellSearch (Menarini Silicon Biosystems) to perform the CTC count at baseline only, the team defined patients as low or high risk, with low-risk patients deemed to need only endocrine therapy and high-risk patients recommended chemotherapy.
Physicians based their decisions on current guidelines and their clinical experience.
In the 25% of cases where CTC count would recommend chemotherapy while the physician would recommend endocrine therapy, following the CTC count–based choice resulted in a 35% improvement in progression-free survival (PFS) and a 47% increase in overall survival.
In all other situations, including those when the CTC count recommended endocrine therapy in contrast to the physicians, or the approximately 60% of cases in which the two were in agreement, there was no difference in survival outcomes between the approaches.
Reacting to the findings, Nancy Chan, MD, medical oncologist and the director of breast cancer clinical research at NYU Langone’s Perlmutter Cancer Center, said that the “goal is really to understand how we can personalize treatment options for patients.”
Another aim is to avoid performing a tumor biopsy, if possible, “as that has increased morbidity for patients.”
She noted also that choosing between endocrine therapy and chemotherapy is a “big decision.” These researchers “really wanted to help some patients get less chemotherapy,” as they felt that “some patients are getting too much” as they are not really that high risk and should get endocrine therapy instead.
However, Dr. Chan said that the CTC count is a “complicated concept” and is “not something we’re all using in our clinical practice yet.”
With regard to the approximately 40% discordance between the CTC- and physician-guided choices, Dr. Chan said that clinicians are perhaps not as accurate as they believed in predicting risk when relying on the clinical or pathological features of the tumor.
On Twitter, Guilherme Nader-Marta, MD, Jules Bordet Institute, Université Libre de Bruxelles, Belgium, commented that the question behind the study was whether CTC measurement is a “clinically useful strategy for first-line treatment decision-making.”
“Amazingly,” he continued, the trial went “straight to the point” to answer the question and showed that CTC-based decisions can offer a survival benefit.
Daniel F. Hayes, MD, co-director of the Breast Oncology Program at the University of Michigan Comprehensive Cancer Center, Ann Arbor, echoed these thoughts, saying that the goals of therapy are to make patients live longer and “better.”
He said that the point of any clinical biomarker is not only to show that testing for it offers “analytical validity” but that it also provides “clinical utility” in that it can guide treatment decisions to improve outcomes.
Dr. Hayes, who was not involved in the study but has worked for many years on the development of CellSearch, said that the results do not make it clear whether measuring CTC counts meets the definition of clinical utility, but it’s “very close.”
On the other hand, the analytical validity of the test is “excellent,” and, in that context, was well-chosen, he said, adding that the endpoint of the trial “is the one most important to us: improvement in overall survival.”
Dr. Hayes noted that the magnitude of benefit from CTC-guided therapy was “moderate,” although that is a “matter of perception,” and the “level of evidence is probably 2 or 3.” Although the trial was prospective, he said, the key results were in a “relatively small” subgroup.
The question is, Dr. Hayes continued: “Is this enough to change practice? My conclusions are: probably not.”
Although patients rated as low risk based on their low CTC count avoided chemotherapy, “it’s not clear to me that this whole thing is sufficient for clinical utility in context of what we know today.” The key issue, however, is who decides whether CTC counts are measured and whether they will be used to guide therapy decisions – will it be the patient, the caregiver, an expert guidelines panel, or third party payers/society?
Study details
In his presentation, Dr. Bidard explained that CTC count is an FDA-approved standardized liquid biopsy biomarker, with a count of greater than or equal to 5 cells per 7.5 mL of blood deemed an adverse prognostic marker, regardless of the line of therapy, with a grade 1 level of evidence.
Previous studies have indicated that a high CTC count is strongly associated with overall survival, at a hazard ratio of 2.78.
Crucially, the CTC count “complements” and does not duplicate standard clinicopathological prognostic factors, Dr. Bidard said.
To determine the potential of the CTC count as an aid to treatment decisions, Dr. Bidard and colleagues conducted a trial in pre- and postmenopausal women with untreated HR+/HER2- metastatic breast cancer who were able to receive either endocrine therapy or chemotherapy.
They were randomly assigned to either a standard group, in which the treatment decision followed the physician’s choice, regardless of their CTC count, or to a CTC group, in which the physicians made a treatment recommendation but the choice was driven by the CTC count.
Dr. Bidard reminded the audience that the primary endpoint of PFS to demonstrate the non-inferiority of CTC versus physician treatment decisions has already been met, with the results published in 2020. Those results came from an analysis of 788 patients enrolled between February 2012 and July 2016 at 17 sites in France and showed after 42 months of follow-up that the median PFS in the CTC arm was 15.6 months versus 14 months in the physician choice arm, at a hazard ratio of 0.92.
The current pre-planned analysis involved 755 patients who were followed up for a median of 57 months by the time the trial was stopped in 2021.
In the standard treatment arm, endocrine therapy was favored by physicians in 72.7% of cases (Clin-low), while 27.3% were given chemotherapy (Clin-high).
In the CTC group, 73.5% of patients were recommended to have endocrine therapy by their physician based on their clinical characteristics (Clin-low), whereas 26.5% were suggested to have chemotherapy (Clin-high).
In contrast, 60.1% of patients in the standard arm would have received endocrine therapy based on their CTC count (CTC-low), and 39.9% chemotherapy (CTC-high), while 63.4% of those in the CTC arm were given endocrine therapy based on their CTC count (CTC-low), and 36.6% were assigned to chemotherapy (CTC-high).
Once the allocated treatment was known in both treatment groups, the physicians were free to choose between endocrine therapy (mostly a single-agent aromatase inhibitor or fulvestrant) and chemotherapy (mostly paclitaxel or capecitabine).
Although CDK4/6 inhibitors were not approved at the time of enrollment, 42.2% of patients across both treatment groups received one of these drugs as a second-line or later therapy.
Guiding treatment decisions
Dr. Bidard said that, overall, more patients in the CTC arm were assigned to chemotherapy, at a difference of 9.7%. There was approximately 60% concordance between physician- and CTC-guided treatment choices; in other words, patients were recommended the same treatment by the two approaches in both treatment groups.
In these patients, there was no significant difference in overall survival between the physician choice and CTC groups, at a median of 45.5 months versus 51.3 months (hazard ratio, 0.85; P = .11).
The updated PFS data revealed a median PFS of 15.7 months in the CTC group versus 13.8 months, again at a nonsignificant HR of 0.94.
These results, Dr. Bidard said, indicate that CTC-based treatment choices are “safe.”
However, there was discordance between physician and CTC-based treatment choices in around 40% of cases, meaning that the two approaches recommended different therapies.
The physician recommended endocrine therapy, in contrast to the CTC count indicating chemotherapy, in 25% of patients (Clin-low/CTC-high), whereas 13.6% of cases were recommended chemotherapy while their CTC count indicated otherwise (Clin-high/CTC-low).
In Clin-low/CTC-high patients, this resulted in 26.1% of patients in the standard group receiving endocrine therapy when their CTC count indicated chemotherapy, while 23.9% of patients in the CTC group received chemotherapy even though their physician did not recommended it.
Comparing these two groups, the researchers found that patients in the CTC group had a significantly longer PFS, at 15.7 months versus 10 months (HR, 0.65; P = .005). They also had a significantly longer median overall survival, at a median of 51.8 months versus 35.4 months with physician choice (HR, 0.53; P = .001).
Among Clin-high/CTC-low, there was no benefit from physician’s choice of chemotherapy over the CTC-guided recommendation of endocrine therapy, at an HR for PFS of 1.14 for CTC- versus physician-guided therapy (P = .54), and an HR for overall survival of 0.88 (P = .64).
Dr. Bidard highlighted that the treatment effects were seen across prespecified subgroups.
The study was funded by the Institut National du Cancer, the Institut Curie SIRIC2 program, and Menarini Silicon Biosystems. Dr. Chan reports no relevant financial relationships. Dr. Hayes and Dr. Bidard reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SAN ANTONIO – When choosing between chemotherapy and endocrine therapy for patients with hormone receptor (HR)+/HER2- metastatic breast cancer, allowing the results from a blood test that measures circulating tumor cell (CTC) count to overrule physician’s choice of therapy can significantly improve overall survival.
But One expert reacting to the findings says probably not, and another pointed out that the CTC count test used in the trial (CellSearch), although approved by the Food and Drug Administration, is not widely used in clinical practice.
The findings comes from updated results from the STIC CTC study.
“When the trial was designed, the question related to the choice between single-agent endocrine therapy and chemotherapy [in] first-line therapy,” explained study presenter François-Clément Bidard, MD, PhD, professor of medical oncology at Institut Curie and Versailles Saint-Quentin University, Paris.
Since then, the first-line treatment has changed and can now can also include cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, but Dr. Bidard said the results are still clinically relevant.
Nowadays, endocrine therapy plus CDK4/6 inhibitors is the “preferred option for treatment-naive patients, but the dilemma between endocrine therapy and chemotherapy remains after disease progression on adjuvant or first-line therapy with CDK4/6 inhibitors, where current guidelines advocate in favor of endocrine therapy, despite its short-lived efficacy.”
“In that scenario, based on the STIC CTC trial results, the CTC count in combination with predictive biomarkers, whenever available, may help customize the early use of chemotherapy or antibody-drug conjugates, which are becoming more and more attractive,” Dr. Bidard said.
The research was presented here at the San Antonio Breast Cancer Symposium (SABCS).
The study involved more than 750 patients with HR+/HER2- metastatic breast cancer randomly assigned to physician choice or CTC-guided therapy, although the physician decision and the recommendation based on the CTC count was recorded in both groups.
Using the CellSearch (Menarini Silicon Biosystems) to perform the CTC count at baseline only, the team defined patients as low or high risk, with low-risk patients deemed to need only endocrine therapy and high-risk patients recommended chemotherapy.
Physicians based their decisions on current guidelines and their clinical experience.
In the 25% of cases where CTC count would recommend chemotherapy while the physician would recommend endocrine therapy, following the CTC count–based choice resulted in a 35% improvement in progression-free survival (PFS) and a 47% increase in overall survival.
In all other situations, including those when the CTC count recommended endocrine therapy in contrast to the physicians, or the approximately 60% of cases in which the two were in agreement, there was no difference in survival outcomes between the approaches.
Reacting to the findings, Nancy Chan, MD, medical oncologist and the director of breast cancer clinical research at NYU Langone’s Perlmutter Cancer Center, said that the “goal is really to understand how we can personalize treatment options for patients.”
Another aim is to avoid performing a tumor biopsy, if possible, “as that has increased morbidity for patients.”
She noted also that choosing between endocrine therapy and chemotherapy is a “big decision.” These researchers “really wanted to help some patients get less chemotherapy,” as they felt that “some patients are getting too much” as they are not really that high risk and should get endocrine therapy instead.
However, Dr. Chan said that the CTC count is a “complicated concept” and is “not something we’re all using in our clinical practice yet.”
With regard to the approximately 40% discordance between the CTC- and physician-guided choices, Dr. Chan said that clinicians are perhaps not as accurate as they believed in predicting risk when relying on the clinical or pathological features of the tumor.
On Twitter, Guilherme Nader-Marta, MD, Jules Bordet Institute, Université Libre de Bruxelles, Belgium, commented that the question behind the study was whether CTC measurement is a “clinically useful strategy for first-line treatment decision-making.”
“Amazingly,” he continued, the trial went “straight to the point” to answer the question and showed that CTC-based decisions can offer a survival benefit.
Daniel F. Hayes, MD, co-director of the Breast Oncology Program at the University of Michigan Comprehensive Cancer Center, Ann Arbor, echoed these thoughts, saying that the goals of therapy are to make patients live longer and “better.”
He said that the point of any clinical biomarker is not only to show that testing for it offers “analytical validity” but that it also provides “clinical utility” in that it can guide treatment decisions to improve outcomes.
Dr. Hayes, who was not involved in the study but has worked for many years on the development of CellSearch, said that the results do not make it clear whether measuring CTC counts meets the definition of clinical utility, but it’s “very close.”
On the other hand, the analytical validity of the test is “excellent,” and, in that context, was well-chosen, he said, adding that the endpoint of the trial “is the one most important to us: improvement in overall survival.”
Dr. Hayes noted that the magnitude of benefit from CTC-guided therapy was “moderate,” although that is a “matter of perception,” and the “level of evidence is probably 2 or 3.” Although the trial was prospective, he said, the key results were in a “relatively small” subgroup.
The question is, Dr. Hayes continued: “Is this enough to change practice? My conclusions are: probably not.”
Although patients rated as low risk based on their low CTC count avoided chemotherapy, “it’s not clear to me that this whole thing is sufficient for clinical utility in context of what we know today.” The key issue, however, is who decides whether CTC counts are measured and whether they will be used to guide therapy decisions – will it be the patient, the caregiver, an expert guidelines panel, or third party payers/society?
Study details
In his presentation, Dr. Bidard explained that CTC count is an FDA-approved standardized liquid biopsy biomarker, with a count of greater than or equal to 5 cells per 7.5 mL of blood deemed an adverse prognostic marker, regardless of the line of therapy, with a grade 1 level of evidence.
Previous studies have indicated that a high CTC count is strongly associated with overall survival, at a hazard ratio of 2.78.
Crucially, the CTC count “complements” and does not duplicate standard clinicopathological prognostic factors, Dr. Bidard said.
To determine the potential of the CTC count as an aid to treatment decisions, Dr. Bidard and colleagues conducted a trial in pre- and postmenopausal women with untreated HR+/HER2- metastatic breast cancer who were able to receive either endocrine therapy or chemotherapy.
They were randomly assigned to either a standard group, in which the treatment decision followed the physician’s choice, regardless of their CTC count, or to a CTC group, in which the physicians made a treatment recommendation but the choice was driven by the CTC count.
Dr. Bidard reminded the audience that the primary endpoint of PFS to demonstrate the non-inferiority of CTC versus physician treatment decisions has already been met, with the results published in 2020. Those results came from an analysis of 788 patients enrolled between February 2012 and July 2016 at 17 sites in France and showed after 42 months of follow-up that the median PFS in the CTC arm was 15.6 months versus 14 months in the physician choice arm, at a hazard ratio of 0.92.
The current pre-planned analysis involved 755 patients who were followed up for a median of 57 months by the time the trial was stopped in 2021.
In the standard treatment arm, endocrine therapy was favored by physicians in 72.7% of cases (Clin-low), while 27.3% were given chemotherapy (Clin-high).
In the CTC group, 73.5% of patients were recommended to have endocrine therapy by their physician based on their clinical characteristics (Clin-low), whereas 26.5% were suggested to have chemotherapy (Clin-high).
In contrast, 60.1% of patients in the standard arm would have received endocrine therapy based on their CTC count (CTC-low), and 39.9% chemotherapy (CTC-high), while 63.4% of those in the CTC arm were given endocrine therapy based on their CTC count (CTC-low), and 36.6% were assigned to chemotherapy (CTC-high).
Once the allocated treatment was known in both treatment groups, the physicians were free to choose between endocrine therapy (mostly a single-agent aromatase inhibitor or fulvestrant) and chemotherapy (mostly paclitaxel or capecitabine).
Although CDK4/6 inhibitors were not approved at the time of enrollment, 42.2% of patients across both treatment groups received one of these drugs as a second-line or later therapy.
Guiding treatment decisions
Dr. Bidard said that, overall, more patients in the CTC arm were assigned to chemotherapy, at a difference of 9.7%. There was approximately 60% concordance between physician- and CTC-guided treatment choices; in other words, patients were recommended the same treatment by the two approaches in both treatment groups.
In these patients, there was no significant difference in overall survival between the physician choice and CTC groups, at a median of 45.5 months versus 51.3 months (hazard ratio, 0.85; P = .11).
The updated PFS data revealed a median PFS of 15.7 months in the CTC group versus 13.8 months, again at a nonsignificant HR of 0.94.
These results, Dr. Bidard said, indicate that CTC-based treatment choices are “safe.”
However, there was discordance between physician and CTC-based treatment choices in around 40% of cases, meaning that the two approaches recommended different therapies.
The physician recommended endocrine therapy, in contrast to the CTC count indicating chemotherapy, in 25% of patients (Clin-low/CTC-high), whereas 13.6% of cases were recommended chemotherapy while their CTC count indicated otherwise (Clin-high/CTC-low).
In Clin-low/CTC-high patients, this resulted in 26.1% of patients in the standard group receiving endocrine therapy when their CTC count indicated chemotherapy, while 23.9% of patients in the CTC group received chemotherapy even though their physician did not recommended it.
Comparing these two groups, the researchers found that patients in the CTC group had a significantly longer PFS, at 15.7 months versus 10 months (HR, 0.65; P = .005). They also had a significantly longer median overall survival, at a median of 51.8 months versus 35.4 months with physician choice (HR, 0.53; P = .001).
Among Clin-high/CTC-low, there was no benefit from physician’s choice of chemotherapy over the CTC-guided recommendation of endocrine therapy, at an HR for PFS of 1.14 for CTC- versus physician-guided therapy (P = .54), and an HR for overall survival of 0.88 (P = .64).
Dr. Bidard highlighted that the treatment effects were seen across prespecified subgroups.
The study was funded by the Institut National du Cancer, the Institut Curie SIRIC2 program, and Menarini Silicon Biosystems. Dr. Chan reports no relevant financial relationships. Dr. Hayes and Dr. Bidard reported relationships with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT SABCS 2022
Cancer researcher banned from federal funding for faking data in nearly 400 images in 16 grant applications
according to a U.S. government research watchdog.
Alice C. Chang, PhD, whose publications and grants listed her name as Chun-Ju Chang, received nearly $700,000 in funding from the National Institutes of Health through grant applications that the U.S. Office of Research Integrity said contained fake data. She will be banned from receiving federal grants for a decade – a more severe sanction than ORI has typically imposed in recent years.
In its findings, ORI said Dr. Chang, who was an associate professor of basic medical sciences at Purdue’s College of Veterinary Medicine, West Lafayette, Ind., “knowingly, intentionally, or recklessly falsified and/or fabricated data from the same mouse models or cell lines by reusing the data, with or without manipulation, to represent unrelated experiments from different mouse models or cell lines with different treatments in three hundred eighty-four (384) figure panels in sixteen (16) grant applications.”
Two of the grant applications were funded. Dr. Chang received $688,196 from the National Cancer Institute, a division of NIH, from 2018 to 2019 for “Targeting metformin-directed stem cell fate in triple negative breast cancer.” The other grant ORI says was submitted in 2014 and funded, “Targeting cell polarity machinery to exhaust breast cancer stem cell pool,” does not show up in NIH RePorter. The rest of the grants were not approved.
We found a Chun-Ju Chang who is dean of the College of Life Sciences at China Medical University in Taiwan and has published papers with a group that Chun-Ju Chang at Purdue also published with. She did not immediately respond to our request for comment.
ORI’s finding also stated Dr. Chang faked data in two papers supported by government funding by reusing figures reporting gene expression in mice and cells after drug treatments, relabeling them to say they showed the results of different experiments. According to the agency, she has agreed to request corrections for the papers:
“Leptin–STAT3–G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression,” published in May 2015 in Cancer Research and cited 83 times, according to Clarivate’s Web of Science.
“Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKC-zeta pathway,” published in February 2017 in Oncogene and cited 26 times.
Between the two papers and 15 of the grant applications, ORI said that Dr. Chang reused gene expression data, sometimes with manipulation, in 119 figure panels. She reused other types of data and images in hundreds of figures across multiple grant applications, ORI found.
As well as correcting the two papers, Dr. Chang agreed to a 10-year ban from all federal contracting, including grant funding. She also agreed not to serve in any advisory or consulting role with the U.S. Public Health Service, which includes the NIH, for that time period.
A version of this article first appeared on Retraction Watch.
according to a U.S. government research watchdog.
Alice C. Chang, PhD, whose publications and grants listed her name as Chun-Ju Chang, received nearly $700,000 in funding from the National Institutes of Health through grant applications that the U.S. Office of Research Integrity said contained fake data. She will be banned from receiving federal grants for a decade – a more severe sanction than ORI has typically imposed in recent years.
In its findings, ORI said Dr. Chang, who was an associate professor of basic medical sciences at Purdue’s College of Veterinary Medicine, West Lafayette, Ind., “knowingly, intentionally, or recklessly falsified and/or fabricated data from the same mouse models or cell lines by reusing the data, with or without manipulation, to represent unrelated experiments from different mouse models or cell lines with different treatments in three hundred eighty-four (384) figure panels in sixteen (16) grant applications.”
Two of the grant applications were funded. Dr. Chang received $688,196 from the National Cancer Institute, a division of NIH, from 2018 to 2019 for “Targeting metformin-directed stem cell fate in triple negative breast cancer.” The other grant ORI says was submitted in 2014 and funded, “Targeting cell polarity machinery to exhaust breast cancer stem cell pool,” does not show up in NIH RePorter. The rest of the grants were not approved.
We found a Chun-Ju Chang who is dean of the College of Life Sciences at China Medical University in Taiwan and has published papers with a group that Chun-Ju Chang at Purdue also published with. She did not immediately respond to our request for comment.
ORI’s finding also stated Dr. Chang faked data in two papers supported by government funding by reusing figures reporting gene expression in mice and cells after drug treatments, relabeling them to say they showed the results of different experiments. According to the agency, she has agreed to request corrections for the papers:
“Leptin–STAT3–G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression,” published in May 2015 in Cancer Research and cited 83 times, according to Clarivate’s Web of Science.
“Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKC-zeta pathway,” published in February 2017 in Oncogene and cited 26 times.
Between the two papers and 15 of the grant applications, ORI said that Dr. Chang reused gene expression data, sometimes with manipulation, in 119 figure panels. She reused other types of data and images in hundreds of figures across multiple grant applications, ORI found.
As well as correcting the two papers, Dr. Chang agreed to a 10-year ban from all federal contracting, including grant funding. She also agreed not to serve in any advisory or consulting role with the U.S. Public Health Service, which includes the NIH, for that time period.
A version of this article first appeared on Retraction Watch.
according to a U.S. government research watchdog.
Alice C. Chang, PhD, whose publications and grants listed her name as Chun-Ju Chang, received nearly $700,000 in funding from the National Institutes of Health through grant applications that the U.S. Office of Research Integrity said contained fake data. She will be banned from receiving federal grants for a decade – a more severe sanction than ORI has typically imposed in recent years.
In its findings, ORI said Dr. Chang, who was an associate professor of basic medical sciences at Purdue’s College of Veterinary Medicine, West Lafayette, Ind., “knowingly, intentionally, or recklessly falsified and/or fabricated data from the same mouse models or cell lines by reusing the data, with or without manipulation, to represent unrelated experiments from different mouse models or cell lines with different treatments in three hundred eighty-four (384) figure panels in sixteen (16) grant applications.”
Two of the grant applications were funded. Dr. Chang received $688,196 from the National Cancer Institute, a division of NIH, from 2018 to 2019 for “Targeting metformin-directed stem cell fate in triple negative breast cancer.” The other grant ORI says was submitted in 2014 and funded, “Targeting cell polarity machinery to exhaust breast cancer stem cell pool,” does not show up in NIH RePorter. The rest of the grants were not approved.
We found a Chun-Ju Chang who is dean of the College of Life Sciences at China Medical University in Taiwan and has published papers with a group that Chun-Ju Chang at Purdue also published with. She did not immediately respond to our request for comment.
ORI’s finding also stated Dr. Chang faked data in two papers supported by government funding by reusing figures reporting gene expression in mice and cells after drug treatments, relabeling them to say they showed the results of different experiments. According to the agency, she has agreed to request corrections for the papers:
“Leptin–STAT3–G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression,” published in May 2015 in Cancer Research and cited 83 times, according to Clarivate’s Web of Science.
“Retinoic acid directs breast cancer cell state changes through regulation of TET2-PKC-zeta pathway,” published in February 2017 in Oncogene and cited 26 times.
Between the two papers and 15 of the grant applications, ORI said that Dr. Chang reused gene expression data, sometimes with manipulation, in 119 figure panels. She reused other types of data and images in hundreds of figures across multiple grant applications, ORI found.
As well as correcting the two papers, Dr. Chang agreed to a 10-year ban from all federal contracting, including grant funding. She also agreed not to serve in any advisory or consulting role with the U.S. Public Health Service, which includes the NIH, for that time period.
A version of this article first appeared on Retraction Watch.
Nitroglycerin’s safety and value examined
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.